Abstract
Arsenic-induced carcinogenesis is a worldwide health problem. Identifying the molecular mechanisms responsible for the induction of arsenic-induced cancers is important for developing treatment strategies. MicroRNA (miRNA) dysregulation is known to affect development and progression of human cancer. Several studies have identified an association between altered miRNA expression in cancers from individuals chronically exposed to arsenic and in cell models for arsenic-induced carcinogenesis. This chapter provides a comprehensive review for miRNA dysregulation in arsenic-induced cancer.
1. Introduction
Recognized as the “King of Poisons,” arsenic has a long history of being both an intentional and unintentional human poison (Hughes, 2016). Paradoxically, and unbeknownst to most laymen, arsenic is also an effective cancer therapeutic for acute myeloid leukemia (Miller, Schipper, Lee, Singer, & Waxman, 2002). Within the past 25 years, research has also uncovered that in some areas of the world, high levels of arsenic are naturally present in drinking water supplies (Podgorski & Berg, 2020; Shaji et al., 2021). Chronic arsenic exposure, caused primarily by continuous consumption of arsenic-contaminated drinking water, is estimated to affect roughly 220 million people worldwide (Podgorski & Berg, 2020). Arsenic has long been recognized as a human carcinogen and is classified as a class I carcinogen by the International Agency for Research on Cancer (IARC, 2012). However, the exact mechanism of action for arsenic-induced carcinogenesis remains unknown.
Within the last 20 years, aberrant microRNA (miRNA) expression has been identified in many different types of human tumors. miRNAs are proposed to contribute to carcinogenesis because they can function as tumor suppressors or oncogenes. More recently, studies have identified the impact of arsenic exposure on miRNA expression.
The aim of this chapter is to review the contributions of arsenic-induced dysregulation of miRNA expression in the context of arsenic-induced carcinogenesis. The chapter starts with a general overview of arsenicals and their metabolism, reviews exposure risk, and recognizes arsenic-induced cancers associated with chronic arsenic exposure. Next, the chapter reviews miRNA biogenesis and discusses miRNAs that are associated with arsenic-induced carcinogenesis in human populations and those that are dysregulated in arsenic-induced malignant transformation in vitro. A discussion of miRNAs that are dysregulated across in vitro models follows. The chapter concludes with a discussion of future avenues of research to fill current knowledge gaps with respect to miRNAs and arsenic-induced carcinogenesis.
2. Arsenical species and arsenic metabolism
Arsenic is a naturally occurring, ubiquitous, toxic metalloid widely present in the earth’s crust (Polya & Lawson, 2016). Arsenic exists in both inorganic and organic forms and four different valence states: −3, 0, +3 (trivalent, AsIII), and +5 (pentavalent, AsV) (Hughes, 2016; IARC, 2012). Pentavalent or trivalent inorganic arsenic found in the environment is most relevant to modern human exposure.
To better understand the susceptibility of cells, and the overall individual, to arsenic-induced health effects, and define arsenic’s mode of action, understanding cellular uptake, metabolism, and efflux of arsenicals is required. The influx, metabolism, and efflux of arsenic has been extensively reviewed (Garbinski, Rosen, & Chen, 2019; Palma-Lara et al., 2020; Roggenbeck, Banerjee, & Leslie, 2016). Briefly, arsenic enters cells through uptake systems for nutrients such as phosphate permeases (pentavalent inorganic arsenic, arsenate), aquaglyceroporins, or glucose permeases (trivalent inorganic arsenic, arsenite) (Garbinski et al., 2019). Upon entering liver cells, arsenate is reduced to arsenite (Fig. 1A).
Fig. 1.
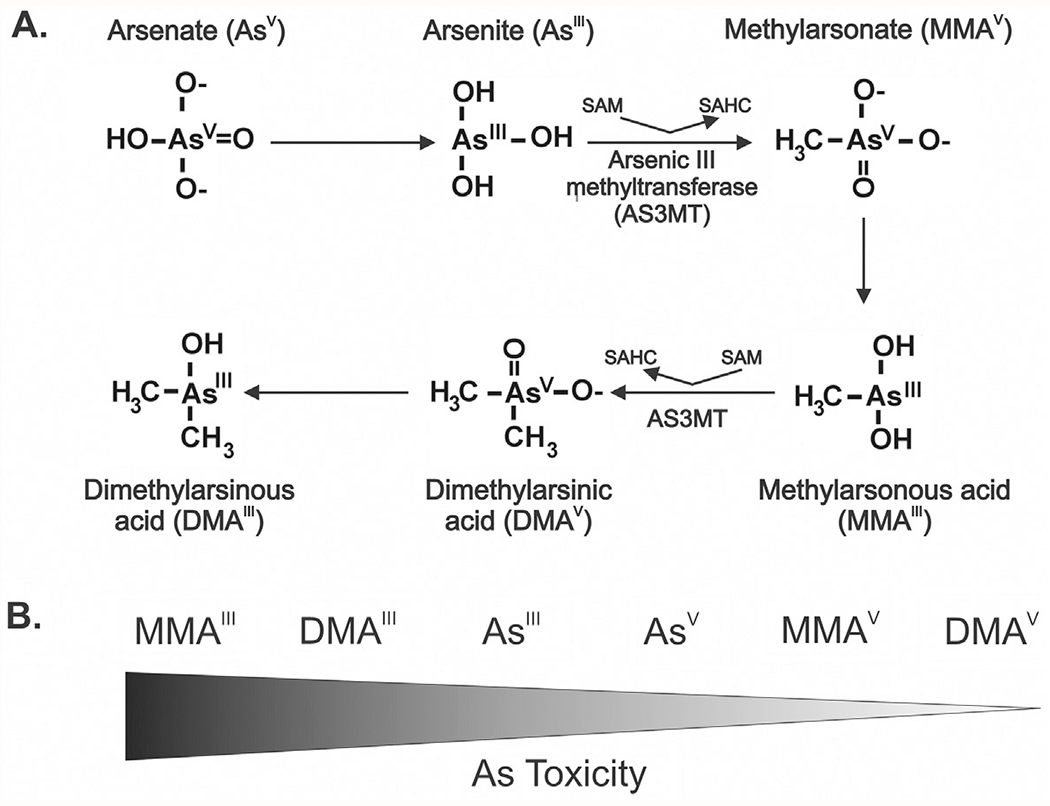
Metabolism and toxicity of arsenic metabolites. (A) Arsenic is predominantly present in the environment as either arsenate or arsenite. Presented is the classical metabolic pathway of arsenic (As) in humans (Thomas, 2016). In liver, arsenate is first reduced to arsenite. Arsenite is then methylated by Arsenic III methyltransferase (AS3MT) producing methylarsonate (MMAV). MMAV is then reduced to methylarsonous acid (MMAIII). MMAIII is methylated by AS3MT to form dimethylarsinous acid (DMAV) which is then reduced to form DMAIII. (B) Toxicity of arsenic metabolites. Trivalent methylated arsenic species are highly cytotoxic and genotoxic compared to arsenite and pentavalent arsenicals.
AsIII methylation is catalyzed by the enzyme arsenic (+3 oxidation state) methyltransferase (AS3MT) (Polya & Lawson, 2016; Thomas, 2016). AS3MT utilizes S-adenosylmethionine (SAM) as a methyl donor for the methylation of AsIII to monomethylarsonate, or monomethylarsonous acid (MMAV). MMAV is then reduced to monomethyl arsonic acid (MMAIII). The catalysis of MMAIII to dimethylarsinous acid (DMAV) is again mediated by AS3MT. DMAV is reduced to form dimethylarsenic acid (DMAIII) that can further be methylated to the trimethyl arsenic oxide (TMAVO). Although generation of TMAVO readily occurs in rodents, its formation in humans is limited unless exposed to very high levels of inorganic arsenic (Tsuji et al., 2019). Differences in arsenic metabolism might account for the lack of a suitable animal model for predicting human toxicity. It is well-known that arsenic metabolism can influence its toxicity (States, 2016; States et al., 2011). In cells, MMAV and DMAV are less toxic than inorganic arsenic and MMAIII is more toxic than AsV and AsIII; MMAIII is the most toxic form of arsenic (Fig. 1B) (Banerjee et al., 2014; Styblo et al., 2000; Thomas, Styblo, & Lin, 2001). Both inorganic arsenicals and organic, or methylated arsenicals, are excreted in urine (Gao, Yu, & Yang, 2011; Tseng, 2007). Most tissues, at least in mice, seem to have arsenic methylating capacity, although the liver is the major site of arsenic methylation (Vahter & Concha, 2001).
To date, both trivalent and pentavalent arsenic efflux systems have been identified (Garbinski et al., 2019; Roggenbeck et al., 2016). Several factors, including genetic polymorphisms in AS3MT, exposure duration, and gender are known to modify susceptibility to the toxic and carcinogenic effects of arsenic (Khairul, Wang, Jiang, Wang, & Naranmandura, 2017; Ozturk et al., 2022; Vahter, 2000; Vahter & Concha, 2001).
3. Arsenic exposure
Routes of arsenic exposure include inhalation, absorption through the skin, and ingestion. The type of arsenical species, dose, and duration of exposure can determine arsenic’s toxic effects. Most cases of acute (short duration) arsenic poisoning occur from accidental ingestion of insecticides or pesticides and less commonly from attempted suicide or homicide. Individuals that consume small amounts of arsenic (<5 mg) typically have nausea, vomiting, and diarrhea. The lethal dose of arsenic (1–3 mg/kg) can result in encephalopathy, peripheral neuropathy, paresthesia in the limbs, and ultimately death (Fee, 2016; Hughes, 2016; Nurchi et al., 2020).
Chronic exposure to arsenic can occur in occupational settings such as mining, smelting, or manufacturing by inhalation (Baker, Cassano, & Murray, 2018). Common industries where arsenic inhalation can occur include coal-fired power plants, or manufacturing that produces metal alloys, battery grids, ammunition, or semiconductor and circuit board chips that are used in electronics (Baker et al., 2018). However, anthropogenic activities such as mining, smelting, pesticide use, and coal ash disposal significantly increase exposure risk for individuals who are not at risk of chronic arsenic exposure from drinking water or occupation. Because arsenic is a natural component of rocks containing copper or lead, mining of these materials can lead to release of arsenic into water or air in mining areas (Martinez, Vucic, Becker-Santos, Gil, & Lam, 2011). Additionally, chronic arsenic exposure can occur from natural geologic sources. For example, groundwater used for drinking in Bangladesh is contaminated with high levels of arsenic due to arsenic-rich geological formations (Martinez et al., 2011).
In today’s world, the majority of people chronically exposed to arsenic are by ingestion of arsenic contaminated water, food, and soil (Ayotte, Medalie, Qi, Backer, & Nolan, 2017). The World Health Organization and U.S. Environmental Protection Agency maximum contaminant level for arsenic in drinking water is 10ppb (10 μg/L). Global prediction maps based on these guidelines suggest that up to 220 million people worldwide are chronically exposed to high arsenic concentrations (>10 μg/L) from groundwater usage (Podgorski & Berg, 2020). In some areas of the world, levels of arsenic in water may range from tens to hundreds or even thousands of micrograms per liter (IARC, 2012; Shaji et al., 2021). Arsenic in drinking water is found almost entirely as pentavalent or trivalent inorganic arsenic.
The use of arsenic-contaminated groundwater for irrigation of crops is a major source for arsenic-contaminated food and soil. Food contains both inorganic and organic arsenic compounds, although the amount of inorganic versus organic arsenic species within food is completely dependent upon the food type. Some sources of food, such as shellfish, contain organic arsenic compounds which have low toxicity (Chung, Yu, & Hong, 2014). However, some foodstuffs such as rice, contain high amounts of inorganic arsenic (Chung et al., 2014). Arsenic in soil is almost entirely in its inorganic form and predominates as pentavalent species due to oxidation of trivalent arsenic (Hughes, Beck, Chen, Lewis, & Thomas, 2011).
The odorless, tasteless, and colorless physical properties of arsenic cause exposure to often go unnoticed, which potentiates the risk of long-term, or chronic, exposure (Ayotte et al., 2017). Arsenic is classified as a class I carcinogen by IARC, meaning that there is sufficient evidence of carcinogenicity in humans (IARC, 2004). Several types of cancers, including skin, lung, bladder, kidney, and prostate are associated with chronic arsenic exposure in humans.
4. Arsenic-induced cancers
Although arsenic is known to be associated with the development of skin, lung, bladder, kidney, and prostate cancer, the susceptibility to the development of arsenic-associated cancers varies among individuals and can be influenced by various factors including age, gender, nutrition, and alterations in genes involved in arsenic biotransformation. Additionally, it has been demonstrated that other environmental carcinogens such as smoking and UV exposure can further potentiate arsenic toxicity (Minatel et al., 2018; Tapio & Grosche, 2006). Due to these confounders or synergisms, directly linking consumption of arsenic to carcinogenesis has proven challenging for large-scale studies in the absence of appropriate animal models. Therefore, the molecular mechanisms that underlie arsenic toxicity and cancer susceptibility are not fully understood and are likely complex. In spite of these confounding factors, significant dose—response relationships between ingestion of high inorganic arsenic concentrations and mortality and/or specific cancers (skin, lung, bladder, kidney, and prostate cancers) have been established (Chen, Chen, Wu, & Kuo, 1992; Smith et al., 2018; Yu, Liao, & Chai, 2006).
Currently, the IARC recognizes that chronic arsenic exposure induces skin, lung, and bladder cancers (IARC, 2004). Only evidence for an association between chronic arsenic ingestion and kidney or prostate cancers has been established; confounding variables have not yet been ruled out from epidemiological studies (IARC, 2004). Later sections include descriptions of associations between miRNAs significantly dysregulated in human populations with known increased cancer incidence as a result of chronic arsenic exposure, and miRNA levels that significantly changed as a result of arsenic-induced malignant transformation of cells related to the abovementioned cancers. Thus, the following sections present evidence for chronic arsenic exposure and skin, lung, bladder, kidney, and prostate cancers to provide better context for later sections.
4.1. Arsenic-induced skin cancer
Skin lesions are a hallmark of chronic arsenic exposure. Clinically detectable signs of arsenicosis can appear within months or up to 10 years after chronic exposure to arsenic contaminated drinking water sources (Sarma, 2016). Skin lesions that result from chronic arsenic exposure can be divided into non-malignant or malignant types. Non-malignant types of arsenic-induced lesions include pigmentary changes, including spotted or diffuse hyper- or hypo- pigmentation, and raindrop pigmentation (well-defined small hyperpigmented macules) in sun-protected areas. Keratoses on palms and soles of feet are also classical non-malignant skin alterations in those exposed chronically to arsenic. Keratosis may give rise to basal and squamous cell carcinoma, however it may take up to two decades of exposure for carcinomas to develop (Sarma, 2016). The association between skin cancer and iatrogenic arsenic exposure was suspected in 1891 by Dr. Jonathan Hutchinson (Hutchinson, 1891). Later reports confirmed an increased frequency of skin cancer cases following treatment with Fowler’s solution (1% potassium arsenite) used to treat various skin and hematological disorders (Antman, 2001; Cuzick, Evans, Gillman, & Price Evans, 1982; Martinez et al., 2011).
There are several distinguishing features of arsenic-induced skin cancer compared to UV-induced skin cancer. Firstly, malignant melanoma is not observed with arsenicosis and is often associated with skin cancer. Secondly, multiple and different types of skin cancers (Bowen’s disease, basal and squamous cell carcinoma) occurring in a patient that has been chronically exposed to arsenic is common. Lastly, and as mentioned previously, arsenic-induced skin lesions most commonly develop in sun-protected areas. Chronic arsenic exposure can induce Bowen’s disease (squamous cell carcinoma in situ), squamous cell carcinoma, and basal cell carcinoma. More recently, it has been discovered that Merkel cell carcinoma, a rare primary neuroendocrine carcinoma of the skin, is associated with chronic arsenic exposure (Choudhury et al., 2018; Ho, Tsai, Lee, & Guo, 2005). Among these, Bowen’s disease is the most common type of cutaneous malignancy caused by chronic arsenic exposure.
4.2. Arsenic-induced lung cancer
Similar to arsenic-induced skin cancer, lung malignancy was first demonstrated in patients following treatment with Fowler’s solution (Martinez et al., 2011; Robson & Jelliffe, 1963; Sommers & McManus, 1953). Additionally, occupational exposure studies in the copper industry have established definitive links between arsenic, a byproduct of copper smelting, and lung cancer via inhalation (Hughes et al., 2011). Early work demonstrated that chronic exposure to arsenic by inhalation was associated with lung cancer and nonmalignant lung diseases (Sherwood & Lantz, 2016). However, later studies showed that ingestion of high levels of arsenic (>100ppb) in drinking water also led to an increased risk of lung cancer (Celik et al., 2008; Chen, Chuang, Lin, & Wu, 1985; D’Ippoliti et al., 2015; Ren, Zhou, Liu, & Wang, 2021; Smith et al., 2018). Additionally, lung cancer mortality rate was found to be higher among those exposed to high doses of arsenic (>100ppb) compared to unexposed people, even when arsenic exposed individuals had not been exposed to arsenic for 30 years or longer (Roh et al., 2018; Smith et al., 2018). Thus, chronic arsenic exposure can have latent detrimental health effects. Although lung adenocarcinoma is the most common type of lung cancer worldwide, the most common types of lung cancer associated with arsenic (in both smokers and non-smokers) are squamous cell carcinoma (SqCC) and small cell carcinoma (SCLC) (Hubaux et al., 2013). Thus, the etiology of arsenic-induced lung cancers is unique.
4.3. Arsenic-induced bladder cancer
The bladder is a primary target organ for arsenic-induced carcinogenicity and metabolites in urine are suspected to be directly involved in carcinogenesis (Khairul et al., 2017). Bladder cancer, also known as urological cancer or urinary bladder cancer, is the ninth most frequent cancer in the world and mostly a cancer of developed countries (Saginala et al., 2020). The bladder’s main purpose is to store urine received from kidneys via the ureter. The urothelial cells lining the bladder and urinary tract are constantly exposed to environmental, and potentially mutagenic agents that are filtered into the urine by the kidneys (Saginala et al., 2020). About 90% of bladder cancer cases arise from urothelial cells mostly in the bladder but sometimes in the urinary tract; squamous cell carcinoma accounts for the remaining 10% of cases (Saginala et al., 2020). Most bladder cancers can be traced back to exposure to environmental or occupational chemicals. For example, men are about four times more likely to develop bladder cancer due to the use of tobacco smoke and occupational exposures (Saginala et al., 2020).
A strong association between arsenic and bladder cancer exists based on epidemiological studies (IARC, 2012; Palma-Lara et al., 2020). Specifically, epidemiological studies have demonstrated an increased risk in bladder cancer incidence, dose–response relationships for inorganic arsenic concentrations in drinking water ranging from <10 to ≥300 μg/L and bladder cancer incidence, and increased risk of death from bladder cancer with arsenic exposure in drinking water (Saint-Jacques, Parker, Brown, & Dummer, 2014). Additionally, a study conducted in Finland found that exposure to arsenic at very low concentrations (0.5 μg/L) in well water, in addition to smoking and nutritional factors, had a synergistic effect on bladder cancer risk (Kurttio, Pukkala, Kahelin, Auvinen, & Pekkanen, 1999).
Interestingly, epidemiological studies indicate that arsenic metabolites in urine vary from person to person even when exposed to the same arsenic levels in drinking water (Khairul et al., 2017). Given that the bladder is the primary organ for urine storage prior to excretion, the amount of biotransformed arsenicals in urine may contribute to arsenic-induced bladder cancer. Indeed, the bioconversion of arsenic to biomethylated metabolites determines its toxicity and carcinogenic potential (Cullen, 2014). Thus, individuals that are less efficient at metabolizing inorganic arsenic within the liver, may have increased levels of inorganic arsenic in the bladder available for biotransformation and potential carcinogenic action.
4.4. Arsenic-induced kidney cancer
Studies have described a dose–response relationship between well water arsenic concentrations and kidney cancer mortality rates, kidney cancer incidence, and associations between kidney cancer, arsenic exposure, and concentrations of arsenic in drinking water in human populations (Saint-Jacques et al., 2014). In a Blackfoot Disease-endemic area in Taiwan, kidney cancer mortality rates, as well as many other cancer mortality rates (skin, lung, bladder, liver, and colon) were significantly higher compared to villages without Blackfoot Disease (Chen et al., 1985). In Chile, a clear dose–response relationship between arsenic in drinking water and kidney cancers was established (Ferreccio et al., 2013). Typically, studies report levels of inorganic arsenic in drinking water that range from 150 to over 1000 μg/L (Saint-Jacques et al., 2014). In general, the risk of death from kidney cancer increases with exposure to arsenic in drinking water (Chen et al., 1992; Saint-Jacques et al., 2014). However, studies have failed to demonstrate risk with lower levels of exposure (<150 μg/L) (Saint-Jacques et al., 2014).
4.5. Arsenic-induced prostate cancer
Multiple studies in humans reveal an association between environmental inorganic arsenic exposure and prostate cancer incidence and mortality (Benbrahim-Tallaa & Waalkes, 2008; Wu, Kuo, Hwang, & Chen, 1989). The first evidence that inorganic arsenic was associated with prostate cancer in humans came from Taiwan in the late 1980s (Benbrahim-Tallaa & Waalkes, 2008; IARC, 2004). Several studies since then have identified a clear dose–response relationship between arsenic-exposed populations (through drinking water sources) and prostate cancer mortality (Benbrahim-Tallaa & Waalkes, 2008). Additionally, in vitro data have shown that chronic exposure of human non-tumorigenic epithelial cells to inorganic arsenic induces malignant transformation (Achanzar, Brambila, Diwan, Webber, & Waalkes, 2002); these data suggest that the prostate is directly susceptible to arsenic-induced carcinogenesis.
5. Considerations for models of arsenic-induced carcinogenesis
Despite known arsenic-induced health effects in humans, animal models have failed to recapitulate arsenic-induced carcinogenesis at doses relevant to human exposure (States et al., 2011). In human populations chronically exposed to iAs, mean blood and serum iAs concentrations are estimated around 100 nanomolar (nM) (Pi et al., 2000; Gonsebatt et al., 1994, 1997), whereas animal models require micromolar (μM) concentrations (Pi et al., 2000; States, 2016). Furthermore, animal models typically require co-administration of arsenic with known carcinogenic agents to induce carcinogenesis. It is noteworthy that in rodent carcinogenesis models, ingestion of high concentrations of arsenic (parts per million, ppm) consistently induces lung and bladder cancers (IARC, 2012).
To provide mechanistic evaluation in order to better define the mode of action (MOA) for arsenic-induced carcinogenesis, many studies utilized cultured cells. However, many in vitro studies used concentrations that are much higher than physiologically relevant for human exposure. Dose becomes critical when defining biological outcomes at the low end of the dose–response curve, where epidemiological studies may lack precision to define thresholds for arsenic-induced mechanisms that malignantly transform cells.
The type of arsenical as well as dose is also important for carcinogenic potential and action mediated by arsenic metabolism. Arsenic toxicity is primarily caused by binding trivalent arsenicals to close-proximity sulfhydryl groups of folded proteins. As a result, higher arsenic doses administered to cells which fall outside a toxicologically relevant range will be difficult to extrapolate to in vivo effects in humans. Furthermore, defining molecular hallmarks of the transformation process requires that multiple endpoints are measured in experimental models. Multiple experimental endpoints allow for the identification of molecular mechanisms that precede transformation and can be used as either biomarkers or potential targets for chemoprevention strategies.
6. miRNAs: A proposed mechanism of arsenic-induced carcinogenesis
In light of potential confounding issues mentioned in the previous section, several mechanisms of arsenic-induced carcinogenesis have been proposed including, but not limited to, DNA damage signaling and repair inhibition, induction of reactive oxygen species, induced cell proliferation, mitotic dysregulation, altered epigenetic changes in DNA methylation, alteration of miRNA expression and alternative mRNA splicing (Banerjee et al., 2021; Cardoso, Al-Eryani, & States, 2018; Mayer & Goldman, 2016; Nail, McCaffrey, Banerjee, Ferragut Cardoso, & States, 2022). In particular, microRNAs (miRNAs) are identified as dysregulated in a number of cancers and are shown to affect several hallmarks of tumor initiation and progression including transcription, cellular proliferation, apoptosis, and epithelial to mesenchymal transition (Aleckovic & Kang, 2015; Peng & Croce, 2016). Furthermore, miRNAs can be released from cells, are stable in biological samples, and can be reliably detected from a multitude of bodily fluids including blood, serum, plasma, bronchial lavage, seminal fluid, and urine. Thus, these aptly named extracellular miRNAs (ECmiRNAs), have remarkable potential to monitor for arsenic-induced cancers. Lastly, the identification of specific aberrant miRNA expression associated with arsenic-induced tumors may lead to the development of arsenic-induced miRNA signatures. These signatures may be important for diagnosing tumor types that otherwise cannot be determined on the basis of tumor biopsy samples (Calin & Croce, 2006).
7. miRNAs
Small RNAs are non-coding RNA molecules that associate with the Argonaute family proteins (AGO family), and range in size from 18 to 30 nucleotides (nt) (Ha & Kim, 2014). miRNA, silencing RNA (siRNA), and PIWI-interacting RNA (piRNA) are the major classes of small RNAs, but miRNAs are by far the most well studied (Watson, Belli, & Di Pietro, 2019). miRNAs were first described in 1993 in the nematode C. elegans (Lee, Feinbaum, & Ambros, 1993). Since then, approximately 2600 mature miRNAs and more than 200,000 transcripts, with slightly variable isoforms, have been identified in humans (Plotnikova, Baranova, & Skoblov, 2019). miRNAs are also detected in all animal models; some are highly conserved between species (O’Brien, Hayder, Zayed, & Peng, 2018). In the human genome, miRNAs are capable of regulating up to 60% of protein coding genes at the translational level (Catalanotto, Cogoni, & Zardo, 2016; Friedman, Farh, Burge, & Bartel, 2009) by hybridizing specific seed sequences (nucleotides 2–7) to the 3’UTR of messenger RNAs (mRNAs) with perfect or imperfect complementarities (Fig. 2).
Fig. 2.
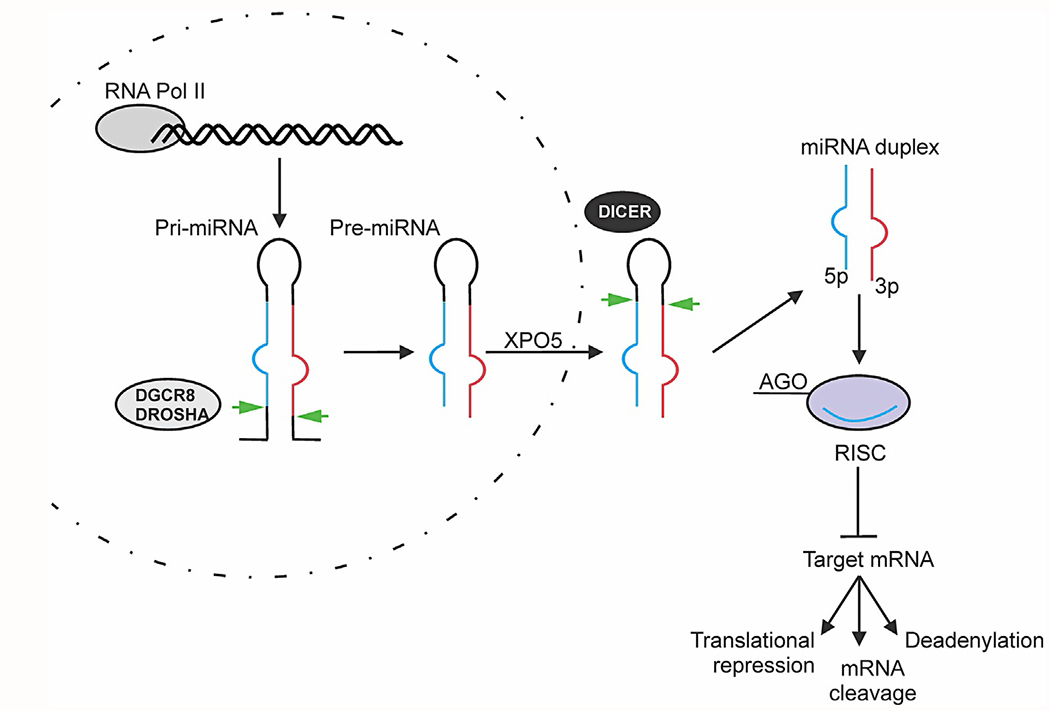
Schematic overview of canonical miRNA biosynthesis. Primary miRNA transcript (pri-miRNA) is transcribed by RNA polymerase II and processed in the nucleus by DROSHA/DGCR8 into pre-miRNAs. Pre-miRNAs are exported to the cytoplasm via Exportin 5 (XPO5), followed by subsequent Dicer cleavage to its mature length. The guide miRNA strand is loaded together with Argonaute (AGO) proteins into the RNA-induced silencing complex (RISC) where it guides RISC to target and silence mRNAs via mRNA cleavage, translational repression, or deadenylation.
Perfect or imperfect miRNA:mRNA base pairing leads to mRNA degradation and decreased translation, respectively (Ha & Kim, 2014; Krol, Loedige, & Filipowicz, 2010). An individual miRNA can target several mRNAs, and a particular mRNA can bind to many miRNAs, sometimes simultaneously (Selbach et al., 2008; Uhlmann et al., 2012). Although interactions between miRNA and mRNA have been reported and predicted, a complete mapping of interactions is still lacking because of challenges in bioinformatic predictions and the widespread use of high throughput sequencing technologies only within the past 10 years, such as cross-linking, ligation, and sequencing of hybrids (CLASH) (Plotnikova et al., 2019; Powell, Zhao, Ipe, Liu, & Skaar, 2021).
miRNAs are transcribed in the nucleus by RNA polymerases II as long primary miRNA (pri-miRNA) (Fig. 2). An individual pri-miRNA can either generate a single miRNA or present clusters of two or more miRNAs that are processed from a common primary transcript (Lin & Gregory, 2015). pri-miRNAs show a double-stranded stem of ~ 30 base pairs, a terminal loop and two flanking single-stranded tails. They are then cleaved by the Microprocessor complex, consisting of the RNase III enzyme DROSHA and its essential cofactor, the double stranded-RNA binding protein, Di George syndrome critical region 8 gene (DGCR8) (Ha & Kim, 2014; Lin & Gregory, 2015; Winter, Jung, Keller, Gregory, & Diederichs, 2009). DROSHA cleaves the pri-mRNA duplex releasing ~70 nt precursor hairpin, or pre-miRNA. The pre-miRNA is exported from the nucleus by Exportin 5 (XPO5) (Ha & Kim, 2014; Lin & Gregory, 2015; Winter et al., 2009). In the cytoplasm, the RNase DICER1 in complex with transactivation responsive RNA-binding protein (TRBP) cleaves the pre-miRNA to its mature length (Winter et al., 2009). The guide strand of the mature miRNA is loaded with Argonaute (Ago2) proteins into the RNA-induced silencing complex (RISC), where it directs the RISC to target mRNAs by base-pairing complementarity and induce mRNA cleavage, translational repression or deadenylation (Ha & Kim, 2014; Lin & Gregory, 2015; Winter et al., 2009).
While a majority of miRNAs can be detected in the cellular microenvironment, extracellular miRNAs (ECmiRNAs), have been detected in the extracellular environment including different biological fluids and cell culture media (Cui et al., 2019). There are two major populations of ECmiRNAs, vesicle-associated and non-vesicle-associated ECmiRNAs. Both vesicle and protein miRNA transporters protect circulating miRNAs from RNase in the extracellular environment (Nail, Ferragut Cardoso, Banerjee, & States, 2022). Previous studies investigating ECmiRNAs secretion into extracellular vesicles have found that certain miRNAs are selectively sorted into extracellular vesicles, lowering their expression levels in the cells that secrete them, while other miRNAs are selectively retained in cells (Luo, Jean-Toussaint, Sacan, & Ajit, 2021). However, the mechanism that determines whether miRNAs will be retained or excreted remains unknown.
7.1. miRNAs and cancer
Dysregulation of the tightly regulated process of miRNA expression and biogenesis is frequently seen in human diseases, including cancer (Ha & Kim, 2014; Lujambio & Lowe, 2012). Chromosomal abnormalities and transcriptional changes of miRNAs can lead to changes in miRNA expression (Peng & Croce, 2016). Altered gene location and genomic miRNA copy number due to translocation, deletion or amplification can cause abnormal miRNA expression in tumorigenesis. For instance, in lung cancers (Hayashita et al., 2005) and B-cell lymphomas (Tagawa & Seto, 2005) the miR-17-92 cluster is amplified. Key transcription factors, such as c-Myc, can also regulate miRNA expression and contribute to carcinogenesis. C-Myc, an oncogenic transcription factor, has been shown to repress expression of miRNAs important for preventing tumor formation, including miR-15a, miR-26, miR-29, miR-30 and let-7 families (Chang et al., 2008).
Recent studies have demonstrated that alterations in miRNA biogenesis is oncogenic (Lin & Gregory, 2015). Upregulation of DROSHA changes global miRNA expression, promoting cell proliferation, migration and invasion (Lin & Gregory, 2015; Muralidhar et al., 2011; Sugito et al., 2006). On the other hand, decreased levels of DROSHA also impact miRNA expression and contributes to metastasis and poor patient survival (Guo et al., 2012; Jafarnejad, Sjoestroem, Martinka, & Li, 2013; Lin & Gregory, 2015). These observations confirm the importance of a tightly regulated miRNA biogenesis process.
Accumulating evidence demonstrates that ECmiRNAs can participate in the invasion and metastasis of cancer via cell communication with recipient cells (Cui et al., 2019). Furthermore, ECmiRNAs may have specific roles that are dependent on their cellular origin. For example, some ECmiRNAs protect cancer cells from immune clearance by decreasing the immunogenicity of cancer cells and downregulating the anti-cancer immune response (Yi et al., 2020; Zhang et al., 2022). It has been hypothesized that ECmiRNAs released from tumor cells and immune cells may work together resulting in poor clinical outcomes (Ma, Jiang, & Kang, 2012).
8. miRNAs and arsenic-induced carcinogenesis
Environmental pollution by arsenic occurs as a result of natural phenomena such as soil erosion, and can occur as a result of anthropogenic activities; several arsenic-containing compounds are produced industrially (Tchounwou, Yedjou, Patlolla, & Sutton, 2012). Chemical carcinogens, such as arsenic, are shown to increase the risk of cancer; however, the underlying mechanisms of arsenic-induced carcinogenesis are not well understood (Li, Huo, Davuljigari, Dai, & Xu, 2019). A growing body of evidence demonstrates that miRNA dysregulation plays an important role in chemical-induced cancers. For example, changes in miRNA induced by air pollution, cigarette smoke, organic pollutants, and heavy metals have been documented in chemical-induced cancers (Doukas et al., 2020; Li et al., 2019; Sima, Rossnerova, Simova, & Rossner, 2021).
The following sections discuss the current knowledge of dysregulated miRNA expression in arsenic-induced cancers. Due to the challenges and confounding issues accompanying the use of animal models to study arsenic-induced carcinogenesis, the following sections focus on identifying aberrant expression of miRNAs both in arsenic-induced cancers from human populations and in cells malignantly transformed by chronic arsenic exposure. Lastly, it is also important to note that we have presented highlights here and a comprehensive list of miRNAs as well as change of expression are provided within each reference cited.
8.1. Skin
Skin is a major target for arsenic. Skin lesions are an early manifestation of arsenic exposure and toxicity, and present a risk for subsequent cancer development (Cui et al., 2021). Recent research has reported changes in skin miRNA expression following chronic arsenic exposure (Cardoso et al., 2018; Ferragut Cardoso, Udoh, & States, 2020; Ren et al., 2011; Wallace et al., 2020). Expression levels of miR-21, miR-145, miR-155 and miR-191 were upregulated in plasma of individuals showing skin damage (Zeng et al., 2019). Participants for this study were recruited from an endemic area for arsenic poisoning caused by coal-burning in China (Zeng et al., 2019). Although this study did not classify the skin lesions as malignant, the involvement of these miRNAs in arsenic-induced tumorigenesis might be important since skin lesions represent a risk factor for cancer.
Microarray analysis for miRNAs using plasma from arsenic-exposed individuals with skin lesions (precancerous and cancerous) and individuals without skin lesions from West Bengal, identified 145 unique miRNAs upregulated and 2 miRNAs downregulated in the plasma of individuals with skin lesions (Banerjee et al., 2019). RT-qPCR validation of miRNAs relevant for carcinogenesis showed increased levels of miR-21, miR-23a, miR-619, miR-126, and miR-3613 and decreased miR-1282 and miR-4530 levels (Banerjee et al., 2019).
In another study, individuals were recruited from West Bengal that were exposed to high levels of arsenic and had arsenic-induced malignant squamous cell carcinoma (SCC) or basal cell carcinoma (BCC), exposed to high levels of arsenic but had no development of skin lesions, or unexposed to high levels of arsenic (Banerjee et al., 2017). Circulating levels of miR-21 was increased in arsenic-exposed compared to unexposed individuals, and much higher in exposed individuals showing skin lesions (Banerjee et al., 2017). Al-Eryani et al. evaluated miRNA expression profiles in arsenic-induced skin lesion samples collected from individuals in West Bengal (Al-Eryani, Jenkins, et al., 2018; Al-Eryani, Waigel, et al., 2018). miR-425-5p and miR-433 were increased in BCC and SCC relative to the premalignant lesion hyperkeratosis (HK) suggesting these miRNAs may be involved with promoting malignancy (Al-Eryani, Jenkins, et al., 2018; Al-Eryani, Waigel, et al., 2018). Two other miRNAs, miR-184 and miR-576-3p, were also overexpressed in SCC relative to both BCC and HK. Lastly, the levels of miR-29c, miR-381, miR-452, miR-487b, miR-494 and miR-590-5p were decreased in BCC compared to SCC and HK (Al-Eryani, Jenkins, et al., 2018; Al-Eryani, Waigel, et al., 2018). These results suggest that changes in miRNA expression are possibly associated with skin tumor phenotypes and stages. A recent study conducted in a highly arsenic-contaminated area of West Bengal also showed down-regulation of miR-663 in arsenic-induced skin cancer tissues compared to control tissues (Sanyal, Paul, Bhattacharjee, & Bhattacharjee, 2020).
Several in vitro studies have sought to better understand the role of miRNA dysregulation in arsenic-induced skin carcinogenesis. The most frequently used cell line for these studies are HaCaT cells. HaCaT cells are a spontaneously immortalized human keratinocyte cell line derived from a distant periphery of a malignant melanoma (Boukamp et al., 1988). The following paragraphs first describe in vitro studies using toxicologically relevant concentrations of inorganic arsenic based on blood arsenic levels reported in arsenic-exposed populations and finish by describing studies using higher arsenic concentrations to induce malignant transformation of HaCaT cells (Pi et al., 2000; Wang, Nioka, Wang, Leigh, & Chance, 1993; Wu et al., 2001).
Recently, Banerjee et al. (2021) induced malignant transformation of HaCaT cells after chronic exposure to 100nM arsenic for 28 weeks. Samples were analyzed by RNAseq at 7, 19 and 28 weeks of exposure, representing early transformation changes, transformation initiation, and full transformation, respectively. At each time point, miRNA and mRNA expression between passage-matched unexposed and arsenic-exposed cells were compared. More than 50 miRNAs were differentially expressed between unexposed and arsenic exposed cells (Banerjee et al., 2021). Interestingly, most of the miRNA changes were uniquely observed in one time point. Only miR-6733 was suppressed in all three time points (Banerjee et al., 2021). In this study, the authors also analyzed observed changes in mRNA that would be explained by differentially expressed miRNAs. In this case, depending on the time point, almost 50% of the mRNAs were being targeted by miRNAs (Banerjee et al., 2021). Although the study observed global changes in miRNA expression, no confirmation of selected miRNAs by RT-PCR was performed. In another study, microarray analysis of arsenic-transformed HaCaT cells (100nM arsenic for 28 weeks) showed 26 differentially expressed miRNAs (12 up- and 14 downregulated) (Zhou et al., 2017). Some of the top miRNAs that were upregulated and confirmed by RT-PCR include miR-4521, miR-6739-5p, miR-181b-5p, miR-100-5p and miR-3919, while mir-513a-5p was downregulated and confirmed by RT-qPCR (Zhou et al., 2017). Al-Eryani et al. (Al-Eryani, Jenkins, et al., 2018; Al-Eryani, Waigel, et al., 2018) also reported upregulation of 2 miRNAs (miR-645 and miR-2682-5) and downregulation 4 miRNAs (miR-548-3p, miR-1254, miR-3618, and miR-8083) in HaCaT cells chronically exposed to 100 nM arsenic at both 3 and 7 week timepoints assayed by microarray hybridization.
In HaCaT cells transformed by chronic exposure to 1 μM arsenic for 40 passages, miR-889 levels are increased (Xiao et al., 2018). In another study, HaCaT transformation was achieved after chronic exposure to arsenic (1 μM) for 30 passages (Jiang et al., 2014). The levels of let-7a, let-7b, and let-7c were decreased in arsenic-transformed cells (Jiang et al., 2014). Using the same experimental conditions, Lu et al. reported increased levels of miR-21 in arsenic transformed cells (Lu et al., 2015). The Locked Nucleic Acid miRNA array system was also used to assess miRNA changes in transformed HaCaT cells (500 nM arsenic for 4 weeks) (Gonzalez et al., 2015). In total, 30 miRNAs were dysregulated; 21 were upregulated whereas 9 were downregulated. Increased levels of miR-21, miR-200a and miR-141 and decreased levels of miRPLUS-A1087 were confirmed by RT-qPCR (Gonzalez et al., 2015). Taken together, a multitude of miRNAs are dysregulated in HaCaT cells malignantly transformed by chronic arsenic exposure.
8.2. Lung
A significant dose–response relationship between arsenic concentration in water and incidence of lung cancer in both men and women has been observed in many parts of the world, including the U.S. (Martinez et al., 2011). Both human population-based and in vivo studies are needed to assess the impact of miRNA dysregulation in arsenic-induced lung cancer. Studies that have assessed miRNA dysregulation in relation to arsenic-induced lung carcinogenesis are focused only in cell model systems. Both human population-based and in vivo studies are still needed to assess the impact of miRNA dysregulation in arsenic-induced lung cancer.
The most commonly used cell line to evaluate mechanisms of arsenic-induced lung carcinogenesis are immortalized human bronchial epithelial cells (BEAS-2B). To properly culture these cells, the use of serum free growth media and coated plates are required (Lechner, Haugen, McClendon, & Pettis, 1982). If these factors are not considered, changes in genes associated with epithelial to mesenchymal transition (EMT) and increase in arsenic sensitivity can occur, which can lead to data misinterpretation (Malm, Amouzougan, & Klimecki, 2018; Zhao & Klimecki, 2015). The following studies reviewed within this section however, failed to use serum free media and coated plates to culture BEAS-2B cells. Furthermore, the majority of studies used micromolar concentrations of arsenic to induce transformation of BEAS-2B cells. Thus, their findings need to be carefully evaluated.
Almutairy et al. used a low concentration of arsenic (250nM) to malignantly transform BEAS-2B cells; BEAS-2B cells transformed by continuous exposure for 24 weeks had cancer stem-like cell characteristics and increased miR-21 expression (Almutairy et al., 2022). In a separate study, Zhong et al. induced cell transformation by exposing BEAS-2B cells to 250nM arsenic for 24 weeks (Zhong et al., 2018). Levels of miR-301a were highly upregulated in transformed BEAS-2B cells compared with non-transformed cells. The level of SMAD family member 4 (SMAD4), a potential target of miR-301a, also was decreased in transformed BEAS-2B cells (Zhong et al., 2018). Inhibition of miR-301a in arsenic-transformed cells suppressed cell proliferation, colony formation and migration, and increased SMAD4 levels (Zhong et al., 2018). Transformation of BEAS-2B cells by chronic exposure to 1 μM arsenic can also occur after 26 weeks (Wang et al., 2016). In these transformed cells, miR-222 was significantly upregulated. Stable overexpression of a miR-222 inhibitor resulted in decreased in cell proliferation, migration, and tumor growth, indicating miR-222 might be an essential player in arsenic-induced lung carcinogenesis (Wang et al., 2016). In a separate study, chronic exposure of BEAS-2B cells to 1 μM arsenic for 26 weeks induced transformation of BEAS-2B cells and led to significantly reduced expression of miR-199a in arsenic-transformed cells (He et al., 2014). Predicted targets of miR-199a, including cyclooxygenase-2 (COX-2) and hypoxia inducible factor 1 alpha (HIF-1α) were increased (He et al., 2014). Finally, down-regulation of miR-31 was observed in BEAS-2B cells transformed by chronic arsenic exposure (2 μM) for 6 weeks (Chen et al., 2018). Additionally, the level of special AT-rich sequence-binding protein 2 (SATB2), a miR-31 target, was increased in BEAS-2B cells (Chen et al., 2018).
In addition to BEAS-2B cells, two other human bronchial epithelial cells lines, HBEC and 16-HBE, are also transformed by chronic arsenic exposure and serve as important cell models for arsenic-induced lung carcinogenesis. Cell transformation of HBECs with TP53 expression stably knocked down (p53lowHBECs) was achieved using 2.5 μM continuous arsenic exposure for 16 weeks (Wang et al., 2011). Along with EMT, arsenic-exposed cells show reduced levels of miR-200. The transformed phenotype was completely reversed when miR-200 was stably re-expressed (Wang et al., 2011).
Chen, Jiang, Gu, & Zhang, (2017) induced 16-HBE transformation by continuous exposure to 2.5 μM arsenic for 13 weeks. miR-155 levels were enhanced and a predicted miR-155 target mRNA, nuclear factor erythroid 2–related factor 2 (Nrf2) had reduced levels in 16-HBE transformed cells. Furthermore, inhibition of miR-155 in these cells attenuated the observed malignant phenotype and promoted apoptotic cell death (Chen et al., 2017). 16-HBE cells exposed to 1 μM arsenic for 15 weeks show changes in EMT markers such as N-cadherin, E-cadherin and vimentin, and increased miR-21 levels (Luo et al., 2013). Arsenic-transformed 16-HBE cells (1 μM, 15 weeks) also induced tumors in nude mice (Xu et al., 2012). miRNA array showed 51 dysregulated miRNAs between normal and transformed 16-HBE cells, 30 miRNAs were found up- and 21 down-regulated (Xu et al., 2015). Validation by RT-qPCR showed upregulation of miR-21, miR-31, miR-125b, and miR-191, and down-regulation of miR-200c in arsenic-transformed 16-HBE cells. Within the upregulated miRNA’s, miR-191 levels showed the highest fold change (Xu et al., 2015). Transformed 16-HBE cells (2.5 μM arsenic, 13 weeks exposure) also formed colonies in soft agar assays confirming their malignant properties (Gu, Sun, Li, & Zhang, 2017). In this study, analysis of miRNA expression by RNA microarray identified 191 differentially expressed miRNAs between transformed and control HBE cells (128 miRNAs downregulated and 63 upregulated). Downregulation of miR-192b-5p, miR-15b-5p, and miR-33b-5p and upregulation of miR-141-3p, miR-106b-5p, and miR-200b-3p were validated by RT-qPCR (Gu et al., 2017).
Lastly, miR-21 was also upregulated in human embryo lung fibroblast (HELF) cells exposed to 1 μM arsenic for 15 weeks that underwent malignant transformation (Ling et al., 2012). In conclusion, all human immortalized lung epithelial cell lines show malignant transformation and miR-21 levels are increased when chronically exposed to arsenic.
8.3. Bladder
Very few studies have shown bladder cell transformation in vivo or in vitro, making the identification of molecular events that lead to urothelial tumorigenesis very challenging. In Bangladesh, decreased miR-205 levels were found in urine samples of arsenic-exposed (> 10 μg/L in drinking water) compared to unexposed individuals (<10 μg/L in drinking water) (Michailidi et al., 2015). Interestingly, individuals with urothelial carcinoma, but without arsenic exposure, have lower miR-205 levels in urine compared individuals without cancer (Michailidi et al., 2015).
Immortalized human urothelial cells (HUC1) exposed to 1 μM arsenic for 8 and 10 months acquire a malignant phenotype (Michailidi et al., 2015). Expression levels of miR-200a, miR-200b, and miR-200c in arsenic-transformed HUC1 cells are reduced compared to unexposed cells (Michailidi et al., 2015). In conclusion, miR-200 family miRNAs are reduced in urine and in an in vitro model for arsenic-induced bladder cancer.
8.4. Kidney
Studies that directly identify miRNA expression in relation to the development of arsenic-induced kidney cancer in human populations are nonexistent. However, candidate miRNAs that may contribute to arsenic-induced kidney carcinogenesis have been identified (Polo et al., 2018). Additionally, due to the absence of animal models for arsenic-induced kidney cancer, studies to investigate the mechanisms underlying arsenic-induced kidney cancer have been conducted using the human epithelial cell line, human kidney 2 (HK – 2) (Fang et al., 2018). HK-2 cells chronically exposed to arsenic (2 or 5 μM) for 30 weeks, undergo malignant transformation (Fang et al., 2018). Fang et al. determined that levels of miR-142-5p were significantly higher in transformed HK-2 cells, whereas levels of miR-182-5p and miR-802 were significantly reduced (Fang et al., 2018). The authors concluded that reduced miR-182-5p expression was likely to contribute to arsenic-induced overexpression of Hypoxia-inducible factor 2 alpha (HIF2α); HIF2α expression was previously shown to be induced by chronic arsenic exposure in other cell types (Fang et al., 2018). To our knowledge, this is the only study that provides a mechanistic assessment for arsenic-induced carcinogenesis in kidney cells related to miRNA expression.
8.5. Prostate
Currently, rodent models of inorganic arsenic-induced prostate carcinogenesis are not available. As such, in vitro models have been important for studies investigating carcinogenic mechanisms that contribute to arsenic-induced prostate carcinogenesis. The immortalized human prostate epithelial cell line, RWPE-1, is non-tumorigenic when inoculated into immunocompromised mice. Achanzar et al. were the first to demonstrate that continuous exposure of RWPE-1 cells to 5 μM arsenic for 29 weeks induced malignant transformation compared to passage-matched controls (Achanzar et al., 2002). Malignantly transformed RWPE-1 cells (chronic arsenic-exposed prostate epithelial [CAsE-PE] cells) have increased mixed metalloproteinase 9 (MMP-9) secretion, produced tumors in nude mice, and overproduced prostate specific antigen (PSA); all are common features of human prostate cancers (Benbrahim-Tallaa & Waalkes, 2008). Additionally, WPE stem cells, a human stem cell progenitor line derived from the heterogeneous mature prostate line, RWPE-1, can also be malignantly transformed by continuous 5 μM arsenic exposure for 18 weeks (Arsenic Cancer Stem cells; As-CSC; Tokar, Diwan, & Waalkes, 2010). Previously, it was demonstrated that RWPE-1 and CAsE-PE cells are poor at methylating arsenic (Benbrahim-Tallaa et al., 2005). Therefore, due to the inability of prostate cell models to metabolize inorganic arsenic measured via methylation, the majority of molecular effects are mediated by inorganic arsenic (Asv and AsIII).
To date, no studies have directly assessed the relationship of miRNA expression and arsenic-induced prostate cancer in human populations. However, aberrant miRNA expression analyzed by microarray or qRT-PCR expression analysis is described in arsenic transformed prostate stem and epithelial cells. Four miRNAs are induced in transformed As-CSC cells including miR-34a, let-29b, miR-193b, and miR-7 (Ngalame, Tokar, Person, Xu, & Waalkes, 2014). Additionally, 9 miRNAs are significantly reduced in As-CSC after chronic arsenic exposure. Comparatively, CAsE-PE cells have a larger number of miRNAs significantly reduced (26 miRNAs), and several miRNAs upregulated after chronic arsenic exposure including miR-9, miR-96, and miR-183. Follow up studies by Ngalame et al. identified differences between cellular and exosomal miRNAs that were upregulated or downregulated after chronic arsenic exposure in CAsE-PE and RWPE-1 cells (Ngalame, Luz, Makia, & Tokar, 2018). Importantly, the majority of miRNAs were downregulated as opposed to upregulated. This study also reported that CAsE-PE cells secrete higher levels of exosomes (>700%) compared to parental RWPE-1 cells and that depletion of exosomes from media lessens the development of cancer stem cell characteristics in CAsE-PE cells. These results suggest that chronic arsenic exposure influences the extracellular tumor microenvironment via export of miRNAs in exosomes as a mechanism of intercellular communication, which ultimately influences malignant transformation of prostate cells.
Interestingly, another study reported that overexpression of a significantly reduced miRNA in exosomes (miR-143-3p) by lentiviral transduction in As-CSCs reduced specific characteristics of malignant transformation including secretion of both MMP-2 and MMP-9, reduced cellular proliferation, and resistance to apoptosis (Ngalame, Makia, Waalkes, & Tokar, 2016). Taken together, these studies demonstrate that chronic arsenic exposure, albeit at high concentration, induces prostate cell malignant transformation, and that a majority of miRNAs are reduced as a result of arsenic exposure.
8.6. Summary of aberrant miRNAs expressed across cell models
In total, 646 miRNAs are differentially expressed following arsenic-induced malignant transformation across cell lines that represent five target tissues for arsenic-induced carcinogenesis in humans. The largest number of differentially expressed miRNAs is in human keratinocytes (skin, n = 276), followed by bronchial epithelial cells (lung, n = 252), prostate epithelial cells (prostate, n = 112), kidney tubular cells (kidney, n = 3) and uroepithelial cells (bladder, n = 3). Fig. 3A depicts the 43 miRNAs that are differentially expressed across cell lines that represent target tissue types for arsenic-induced carcinogenesis; only 5 miRNAs (miR-200a, miR-222, miR-148, miR-18a-5p, and let-7i) are differentially expressed across three cell lines.
Fig. 3.
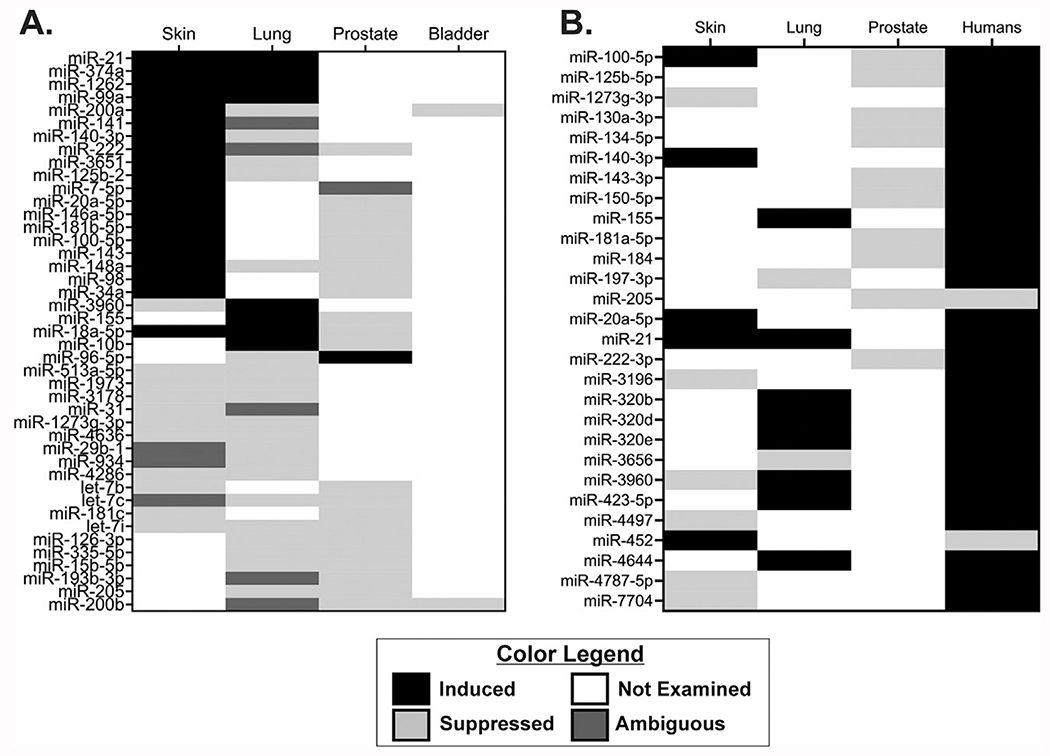
Differential expression of miRNAs dysregulated across arsenic transformed cell models and arsenic-induced human cancer. (A) miRNAs dysregulated across different cell models representing four target tissues of arsenic-induced cancer (Skin, Lung, Prostate, Bladder). (B) miRNAs dysregulated between arsenic transformed cell models (Skin, Lung, Prostate) and arsenic-induced human cancers (Humans). Induced miRNAs are represented in black, suppressed miRNAs are represented in light gray, ambiguous changes in expression (conflicting results between studies) are represented in dark gray, and miRNAs not examined or that were not found to be significantly different in a particular cell model or tumor are represented as white.
miRNAs dysregulated in arsenic-induced kidney cancer models did not have any overlapping miRNAs dysregulated in other tissues. The number of differentially expressed miRNAs within each representative tissue type (skin, lung, prostate, or bladder) is reflective of both the number of studies and the technology used to identify aberrant miRNA expression. For instance, in skin cell models, seven studies have investigated differential miRNA expression using either miRNA microarray, RNA-sequencing, or Quantitative Reverse Transcription PCR (RT-qPCR), whereas in bladder, only one study (Michailidi et al., 2015) used RT-qPCR to identify dysregulation of 3 miRNAs. Thus, high-throughput studies are needed to address which miRNAs are dysregulated in malignantly transformed kidney and bladder cell models.
A limited number of miRNAs were consistently upregulated across arsenic-transformed cell lines; all miRNAs with concomitantly higher expression in two cell lines were from skin or lung cancer models (miR-21, miR-374a, miR-1262, miR-99a, and miR-18a-5p). Previous work also identified that circulating miR-21 is higher in blood and plasma of arsenic exposed individuals (Banerjee et al., 2017; Nail, Ferragut Cardoso, et al., 2022; Sun et al., 2017). Furthermore, circulating miR-21 level are higher in individuals with arsenic-induced skin lesions (Fig. 3B) (Banerjee et al., 2017;). These data suggest a causal link between miR-21 and arsenic-induced skin carcinogenesis. miR-21 is considered an oncogenic miRNA that is highly expressed in most cancers (Bautista-Sanchez et al., 2020; Pfeffer, Yang, & Pfeffer, 2015). Additionally, miR-21 targets mRNAs of proteins in skin malignancies critical for proliferation (PTEN, PI3K, FOX01, TIPE2, P53, cyclin D1), resistance to apoptosis (FOX01, FBOX11, APAF1, TIMP3, TIPE2), genetic instability (MSH2, FBOX11, hTERT), increased oxidative stress (FOX01), angiogenesis (PTEN, HIF1α, TIMP3), and metastatic potential (APAF1, PTEN, PDCD4, TIMP3) (Garcia-Sancha, Corchado-Cobos, Perez-Losada, & Canueto, 2019; Melnik, 2015). In prostate cancer stem cells malignantly transformed by continuous exposure to 5 μM arsenic, PTEN expression is suppressed (Xu, Tokar, Sun, & Waalkes, 2012). Several other miRNAs induced in skin, in addition to miR-21, also had higher circulating levels in blood, plasma, or serum from arsenic-exposed populations (miR-20a-5p, miR-148a, miR-98, miR-155) (Gu et al., 2017; Nail, Ferragut Cardoso, et al., 2022; Sun et al., 2017). However, the cancer status of individuals from these populations was not established.
Alignments of miRNAs dysregulated in arsenic transformed cell lines and in human populations identified that 28 miRNAs were dysregulated between at least one model of arsenic-induced transformation and arsenic-induced cancer from human populations (Fig. 3B). In total, only three miRNAs (miR-100–5p, miR-21, andmiR-3960) were significantly dysregulated between at least two models and populations with arsenic-induced human cancer. It is important to note that the majority of miRNAs studied from human populations are in relation to arsenic-induced skin cancer. Interestingly, five miRNAs were upregulated both in arsenic transformed skin cancer models and from skin cancer in human populations (miR-100-5p, miR-140-3p, miR-20a-5p, miR-21, and miR-452). Furthermore, unique miRNAs (miR-155, miR-320b, miR-320d, miR-320e, miR-423-5p, and miR-4644) between arsenic transformed lung cancer models and human populations, compared to arsenic transformation skin models, were upregulated. Lastly, miR-205 was downregulated in both arsenic transformed prostate cells and bladder cancer from human populations that were not exposed to arsenic suggesting a potential role in genitourinary cancer regardless of exposure.
9. Conclusion
A major challenge for future research is identifying miRNAs that are both dysregulated in individuals that have developed cancer as a result of chronic arsenic exposure, and aberrantly expressed in arsenic-induced carcinogenic models. The assessment of arsenic-induced cancer miRNAs from human populations is limited by availability of biopsy samples from internal cancers for analysis. A potential approach to address this problem could be assessment of both cellular and extracellular miRNAs during the process of malignant transformation in cell models. Ngalame et al. identified extracellular miRNAs dysregulated by chronic arsenic exposure using prostate epithelial cells (Ngalame et al., 2018). However, these types of studies need to be expanded into other arsenic-induced cancer cell models. Additionally, a focus in future human population studies on identifying circulating miRNAs from biofluids that are likely to be directly associated with arsenic-induced cancers such as bronchoalveolar lavage miRNAs for arsenic-induced lung cancer, seminal fluid for arsenic-induced prostate cancer, and urine miRNAs for arsenic-induced kidney and bladder cancer, would be beneficial.
In this chapter, we have begun to address these challenges by aligning miRNAs dysregulated in arsenic transformed cell models and arsenic-induced cancers from human populations, albeit with limitations. For instance, one study identified the majority of miRNAs dysregulated from plasma of individuals either with precancerous or cancerous skin lesions (Banerjee et al., 2019). As such, these dysregulated miRNAs are circulating miRNAs, whereas in both skin and lung arsenic transformed models, miRNA dysregulation was studied only within cells and not extracellularly. Another limitation may lie in the susceptibility of tissues to arsenic based on their ability to metabolize arsenic efficiently. Related to this point, there may be a disconnect between age of onset for certain cancers (i.e., skin versus prostate cancer), and/or whether many cancers are simultaneously present in one individual, or whether only the most obvious (i.e., skin cancer) is diagnosed. These are topics to be addressed in future studies.
Other challenges that remain, such as understanding the longitudinal changes in miRNA expression, can be had by measuring miRNA expression at multiple timepoints during chronic exposure. This approach would identify which miRNAs contribute towards carcinogenic transformation of cells, rather than measuring miRNAs expressed only in malignantly transformed cells. However, this approach is lacking for the majority of studies conducted thus far. Consequently, a significant knowledge gap exists that characterizes differential expression of miRNAs prior to malignant transformation, but whose expression is likely mediated by arsenic exposure. Improvements made to experimental design of studies can identify miRNAs important for carcinogenesis and that can act as potential biomarkers for arsenic-induced cancers.
In recent years, it has been found that dysregulated miRNAs can affect the response of cancer cells to chemotherapy drugs by modulation of their target genes (Si, Shen, Zheng, & Fan, 2019). For instance, miR-21 has been found in many cancers to promote resistance to cisplatin, 5-fluorouracil, and Trastuzumab (Si et al., 2019). Likewise, some miRNAs (i.e., miR-200c) enhance non-small cell lung cancer sensitivity to Vincristine, cisplatin, Cetuximab (Shah & Shah, 2020). Future studies investigating the impact of miRNA dysregulation with respect to cancer treatment strategies could be helpful in designing new treatments. For example, strategies to inhibit miRNAs in combination with chemotherapeutic agents may prove highly promising for treatment of arsenic-induced cancers.
Acknowledgments
The authors were supported by NIH grants R01ES02778, R21ES030334, P30ES030283, T32ES011564 and R25CA134283. The views expressed in this work are those of the authors and not of the National Institutes of Health.
Conflict of interest statement
The authors declare they have no financial conflicts of interest other than grants R01ES02778, R21ES030334, P30ES030283, T32ES011564 and R25CA134283 that supported the authors.
Nonstandard Abbreviations
- AGO family
Argonaute family proteins
- Ago2
Argonaute
- AS3MT
arsenic (+3 oxidation state) methyltransferase
- As-CSC
Arsenic Cancer Stem cells
- AsIII
trivalent
- AsV
pentavalent
- BCC
basal cell carcinoma
- CAsE-PE
chronic arsenic-exposed prostate epithelial cells
- CLASH
cross-linking, ligation, and sequencing of hybrids
- COX-2
cyclooxygenase-2
- DGCR8
Di George syndrome critical region 8 gene
- DMAIII
dimethylarsenic acid
- DMAV
dimethylarsinous acid
- ECmiRNAs
extracellular miRNAs
- EMT
epithelial to mesenchymal transition
- HELF
human embryo lung fibroblast
- HIF-1α
hypoxia inducible factor 1 alpha
- HIF2α
Hypoxia-inducible factor 2 alpha
- HK
hyperkeratosis
- HUC1
human urothelial cells
- miRNA
microRNA
- MMAIII
monomethyl arsonic acid
- MMAV
monomethylarsonous acid
- MMP-9
mixed metalloproteinase 9
- mRNAs
messenger RNAs
- Nrf2
nuclear factor erythroid 2–related factor 2
- piRNA
PIWI-interacting RNA
- PSA
prostate specific antigen
- RISC
RNA-induced silencing complex
- SAM
S-adenosylmethionine
- SATB2
special AT-rich sequence-binding protein 2
- SCC
squamous cell carcinoma
- siRNA
silencing RNA
- SMAD4
SMAD family member 4
- TMAVO
trimethyl arsenic oxide
- TRBP
transactivation responsive RNA-binding protein
- XPO5
Exportin 5
References
- Achanzar WE, Brambila EM, Diwan BA, Webber MM, & Waalkes MP (2002). Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. Journal of the National Cancer Institute, 94(24), 1888–1891. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12488483. [DOI] [PubMed] [Google Scholar]
- Aleckovic M, & Kang Y (2015). Regulation of cancer metastasis by cell-free miRNAs. Biochimica et Biophysica Acta, 1855(1), 24–42. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25450578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Eryani L, Jenkins SF, States VA, Pan J, Malone JC, Rai SN, et al. (2018). miRNA expression profiles of premalignant and malignant arsenic-induced skin lesions. PLoS One, 13(8), e0202579. 10.1371/journal.pone.0202579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Eryani L, Waigel S, Tyagi AH, Peremarti J, Jenkins SF, Damodaran C.l., et al. (2018). Differentially expressed mRNA targets of differentially expressed miRNAs predict changes in the TP53 Axis and carcinogenesis-related pathways in human keratinocytes chronically exposed to arsenic. Toxicological Sciences, 162(2), 645–654. Retrieved from https://academic.oup.com/toxsci/article/162/2/645/4793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutairy B, Fu Y, Bi Z, Zhang W, Wadgaonkar P, Qiu Y, et al. (2022). Arsenic activates STAT3 signaling during the transformation of the human bronchial epithelial cells. Toxicology and Applied Pharmacology, 436, 115884. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/35031324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antman KH (2001). Introduction: The history of arsenic trioxide in cancer therapy. The Oncologist, 6(Suppl 2), 1–2. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11331433. [DOI] [PubMed] [Google Scholar]
- Ayotte JD, Medalie L, Qi SL, Backer LC, & Nolan BT (2017). Estimating the high-arsenic domestic-well population in the conterminous United States. Environmental Science & Technology, 51(21), 12443–12454. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29043784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BA, Cassano VA, & Murray C (2018). Arsenic exposure, assessment, toxicity, diagnosis, and management: Guidance for occupational and environmental physicians. Journal of Occupational and Environmental Medicine, 60(12), e634–e639. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30358658. [DOI] [PubMed] [Google Scholar]
- Banerjee N, Bandyopadhyay AK, Dutta S, Das JK, Roy Chowdhury T, Bandyopadhyay A, et al. (2017). Increased microRNA 21 expression contributes to arsenic induced skin lesions, skin cancers and respiratory distress in chronically exposed individuals. Toxicology, 378, 10–16. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28069514. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Carew MW, Roggenbeck BA, Whitlock BD, Naranmandura H, Le XC, et al. (2014). A novel pathway for arsenic elimination: Human multidrug resistance protein 4 (MRP4/ABCC4) mediates cellular export of dimethylarsinic acid (DMAV) and the diglutathione conjugate of monomethylarsonous acid (MMAIII). Molecular Pharmacology, 86(2), 168–179. 10.1124/mol.113.091314. [DOI] [PubMed] [Google Scholar]
- Banerjee N, Das S, Tripathy S, Bandyopadhyay AK, Sarma N, Bandyopadhyay A, et al. (2019). MicroRNAs play an important role in contributing to arsenic susceptibility in the chronically exposed individuals of West Bengal. India. Environ Sci Pollut Res Int, 26(27), 28052–28061. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Ferragut Cardoso A, Al-Eryani L, Pan J, Kalbfleisch TS, Srivastava S, et al. (2021). Dynamic alteration in miRNA and mRNA expression profiles at different stages of chronic arsenic exposure-induced carcinogenesis in a human cell culture model of skin cancer. Archives of Toxicology, 95(7), 2351–2365. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista-Sanchez D, Arriaga-Canon C, Pedroza-Torres A, De La Rosa-Velazquez IA, Gonzalez-Barrios R, Contreras-Espinosa L, et al. (2020). The promising role of miR-21 as a Cancer biomarker and its importance in RNA-based therapeutics. Molecular Therapy—Nucleic Acids, 20, 409–420. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32244168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, & Waalkes MP (2008). Inorganic arsenic and human prostate cancer. Environmental Health Perspectives, 116(2), 158–164. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18288312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterland RA, Styblo M, Achanzar WE, Webber MM, & Waalkes MP (2005). Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: Aberrant genomic DNA methylation and K-ras oncogene activation. Toxicology and Applied Pharmacology, 206(3), 288–298. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16039940. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, & Fusenig NE (1988). Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. The Journal of Cell Biology, 106(3), 761–771. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2450098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, & Croce CM (2006). MicroRNA signatures in human cancers. Nature Reviews. Cancer, 6(11), 857–866. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17060945. [DOI] [PubMed] [Google Scholar]
- Cardoso APF, Al-Eryani L, & States JC (2018). Arsenic-induced carcinogenesis: The impact of miRNA dysregulation. Toxicological Sciences : An Official Journal of the Society of Toxicology, 165(2), 284–290. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29846715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalanotto C, Cogoni C, & Zardo G (2016). MicroRNA in control of gene expression: An overview of nuclear functions. International Journal of Molecular Sciences, 17(10). Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27754357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik I, Gallicchio L, Boyd K, Lam TK, Matanoski G, Tao X, et al. (2008). Arsenic in drinking water and lung cancer: A systematic review. Environmental Research, 108(1), 48–55. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18511031. [DOI] [PubMed] [Google Scholar]
- Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. (2008). Widespread microRNA repression by Myc contributes to tumorigenesis. Nature Genetics, 40(1), 43–50. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18066065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Chen CW, Wu MM, & Kuo TL (1992). Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. British Journal of Cancer, 66(5), 888–892. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1419632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Chuang YC, Lin TM, & Wu HY (1985). Malignant neoplasms among residents of a Blackfoot disease-endemic area in Taiwan: High-arsenic artesian well water and cancers. Cancer Research, 45(11 Pt 2), 5895–5899. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4053060. [PubMed] [Google Scholar]
- Chen C,Jiang X, Gu S, & Zhang Z (2017). MicroRNA-155 regulates arsenite-induced malignant transformation by targeting Nrf2-mediated oxidative damage in human bronchial epithelial cells. Toxicology Letters, 278, 38–47. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28688901. [DOI] [PubMed] [Google Scholar]
- Chen QY, Li J, Sun H, Wu F, Zhu Y, Kluz T, et al. (2018). Role of miR-31 and SATB2 in arsenic-induced malignant BEAS-2B cell transformation. Molecular Carcinogenesis, 57(8), 968–977. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29603397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury MIM, Shabnam N, Ahsan T, Ahsan SMA, Kabir MS, Khan RM, et al. (2018). Cutaneous malignancy due to Arsenicosis in Bangladesh: 12-year study in tertiary level hospital. BioMed Research International, 2018, 4678362. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30643806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Yu SD, & Hong YS (2014). Environmental source of arsenic exposure. Journal of Preventive Medicine and Public Health, 47(5), 253–257. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25284196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Wang H, Yao X, Zhang D, Xie Y, Cui R, & Zhang X (2019). Circulating microRNAs in cancer: Potential and challenge. Frontiers Genetics, 10, 626. Retrieved from https://pubmed.ncbi.nlm.nih.gov/31379918. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6656856/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YH, Yang S, Wei J, Shea CR, Zhong W, Wang F, et al. (2021). Autophagy of the m(6)a mRNA demethylase FTO is impaired by low-level arsenic exposure to promote tumorigenesis. Nature Communications, 12(1), 2183. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33846348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen WR (2014). Chemical mechanism of arsenic biomethylation. Chemical Research in Toxicology, 27(4), 457–461. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24517124. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Evans S, Gillman M, & Price Evans DA (1982). Medicinal arsenic and internal malignancies. British Journal of Cancer, 45(6), 904–911. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6212076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ippoliti D, Santelli E, De Sario M, Scortichini M, Davoli M, & Michelozzi P (2015). Arsenic in drinking water and mortality for Cancer and chronic diseases in Central Italy, 1990-2010. PLoS One, 10(9), e0138182. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26383851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas SG, Vageli DP, Lazopoulos G, Spandidos DA, Sasaki CT, & Tsatsakis A (2020). The effect of NNK, a tobacco smoke carcinogen, on the miRNA and mismatch DNA repair expression profiles in lung and head and neck squamous cancer cells. Cells, 9(4). Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32326378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Sun R, Hu Y, Wang H, Guo Y, Yang B, et al. (2018). miRNA-182-5p, via HIF2alpha, contributes to arsenic carcinogenesis: Evidence from human renal epithelial cells. Metallomics, 10(11), 1607–1617. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30334557. [DOI] [PubMed] [Google Scholar]
- Fee DB (2016). Neurological effects of arsenic exposure. In States JC (Ed.), Arsenic: Exposure sources, health risks, and mechanisms of toxicity (pp. 193–220). Hoboken: Wiley. [Google Scholar]
- Ferragut Cardoso AP, Udoh KT, & States JC (2020). Arsenic-induced changes in miRNA expression in cancer and other diseases. Toxicology and Applied Pharmacology, 409, 115306. 10.1016/j.taap.2020.115306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C, Smith AH, Duran V, Barlaro T, Benitez href., Valdes R, et al. (2013). Case-control study of arsenic in drinking water and kidney cancer in uniquely exposed northern Chile. American Journal of Epidemiology, 178(5), 813–818. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23764934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, & Bartel DP (2009). Most mammalian mRNAs are conserved targets of microRNAs. Genome Research, 19(1), 92–105. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18955434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Yu J, & Yang L (2011). Urinary arsenic metabolites of subjects exposed to elevated arsenic present in coal in Shaanxi Province, China. International Journal of Environmental Research and Public Health, 8(6), 1991–2008. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21776214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbinski LD, Rosen BP, & Chen J (2019). Pathways of arsenic uptake and efflux. Environment International, 126, 585–597. 10.1016/j.envint.2019.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sancha N, Corchado-Cobos R, Perez-Losada J, & Canueto J (2019). MicroRNA dysregulation in cutaneous squamous cell carcinoma. International Journal of Molecular Sciences, 20(9). 10.3390/ijms20092181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsebatt ME,Vega L, Montero R, Garcia-Vargas G, Del Razo LM, Albores A, et al. (1994). Lymphocyte replicating ability in individuals exposed to arsenic via drinking water. Mutation Research, 313(2—3), 293–299. 10.1016/0165-1161(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Gonsebatt ME, Vega L, Salazar AM, Montero R, Guzmán P, Bias J, et al. (1997). Cytogenetic effects in human exposure to arsenic. Mutation Research, 386(3), 219–228. 10.1016/s1383-5742(97)00009-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez H, Lema C, Kirken RA, Maldonado RA, Varela-Ramirez A, & Aguilera RJ (2015). Arsenic-exposed keratinocytes exhibit differential microRNAs expression profile; potential implication of miR-21, miR-200a and miR-141 in melanoma pathway. Clinical Cancer Drugs, 2(2), 138–147. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27054085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Sun D, Li X, & Zhang Z (2017). Alterations of miRNAs and their potential roles in Arsenite-induced transformation of human bronchial epithelial cells. Genes (Basel), 8(10). Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28972549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Liao Q, Chen P, Li X, Xiong W, Ma J, et al. (2012). The microRNA-processing enzymes: Drosha and dicer can predict prognosis of nasopharyngeal carcinoma. Journal of Cancer Research and Clinical Oncology, 138(1), 49–56. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21953080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, & Kim VN (2014). Regulation of microRNA biogenesis. Nature Reviews. Molecular Cell Biology, 15(8), 509–524. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25027649. [DOI] [PubMed] [Google Scholar]
- Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, et al. (2005). A polycistronic microRNA cluster, miR-17–92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Research, 65(21), 9628–9632. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16266980. [DOI] [PubMed] [Google Scholar]
- He J, Wang M, Jiang Y, Chen Q, Xu S, Xu Q, et al. (2014). Chronic arsenic exposure and angiogenesis in human bronchial epithelial cells via the ROS/miR-199a-5p/HIF-1alpha/COX-2 pathway. Environmental Health Perspectives, 122(3), 255–261. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24413338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SY, Tsai YC, Lee MC, & Guo HR (2005). Merkel cell carcinoma in patients with long-term ingestion of arsenic. Journal of Occupational Health, 47(2), 188–192. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15824485. [DOI] [PubMed] [Google Scholar]
- Hubaux R, Becker-Santos DD, Enfield KS, Rowbotham D, Lam S, Lam WL, et al. (2013). Molecular features in arsenic-induced lung tumors. Molecular Cancer, 12,20. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23510327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF (2016). History of arsenic as a poison and medicinal agent. In States JC (Ed.), Arsenic: Exposure sources, health risks, and mechanisms of toxicity. Hoboken: Wiley. [Google Scholar]
- Hughes MF, Beck BD, Chen Y, Lewis AS, & Thomas DJ (2011). Arsenic exposure and toxicology: A historical perspective. Toxicological Sciences : An Official Journal of the Society of Toxicology, 123(2), 305–332. Retrieved from https://pubmed.ncbi.nlm.nih.gov/21750349. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3179678/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J (1891). A lecture on arsenic as a drug. British Medical Journal, 1(1588), 1213–1215. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20753330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. (2004). Some drinking-water disinfectants and contaminants, including arsenic. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 84, 1–477. [PMC free article] [PubMed] [Google Scholar]
- IARC. (2012). Arsenic, metals, fibres, and dusts. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 100(Pt C), 11–465. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23189751. [PMC free article] [PubMed] [Google Scholar]
- Jafarnejad SM, Sjoestroem C, Martinka M, & Li G (2013). Expression of the RNase III enzyme DROSHA is reduced during progression of human cutaneous melanoma. Modern Pathology, 26(7), 902–910. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23370771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Li Y, Zhang A, Wang B, Xu Y, Xu W, et al. (2014). The acquisition of cancer stem cell-like properties and neoplastic transformation of human keratinocytes induced by arsenite involves epigenetic silencing of let-7c via Ras/NF-kappaB. Toxicology Letters, 227(2), 91–98. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24704393. [DOI] [PubMed] [Google Scholar]
- Khairul I, Wang QQ,Jiang YH, Wang C, & Naranmandura H (2017). Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget, 8(14), 23905–23926. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28108741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Loedige I, & Filipowicz W (2010). The widespread regulation of microRNA biogenesis, function and decay. Nature Reviews. Genetics, 11(9), 597–610. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20661255. [DOI] [PubMed] [Google Scholar]
- Kurttio P, Pukkala E, Kahelin H, Auvinen A, & Pekkanen J (1999). Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland. Environmental Health Perspectives, 107(9), 705–710. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10464069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner JF, Haugen A, McClendon IA, & Pettis EW (1982). Clonal growth of normal adult human bronchial epithelial cells in a serum-free medium. In Vitro, 18(7), 633–642. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7141447. [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, & Ambros V (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell, 75(5), 843–854. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8252621. [DOI] [PubMed] [Google Scholar]
- Li M, Huo X, Davuljigari CB, Dai Q, & Xu X (2019). MicroRNAs and their role in environmental chemical carcinogenesis. Environmental Geochemistry and Health, 41(1), 225–247. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30171477. [DOI] [PubMed] [Google Scholar]
- Lin S, & Gregory RI (2015). MicroRNA biogenesis pathways in cancer. Nature Reviews. Cancer, 15(6), 321–333. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25998712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling M, Li Y, Xu Y, Pang Y, Shen L, Jiang R, et al. (2012). Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-kappaB in arsenite-induced cell transformation. Free Radical Biology & Medicine, 52(9), 1508–1518. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22387281. [DOI] [PubMed] [Google Scholar]
- Lu X, Luo F, Liu Y, Zhang A, Li J, Wang B, et al. (2015). The IL-6/STAT3 pathway via miR-21 is involved in the neoplastic and metastatic properties of arsenite-transformed human keratinocytes. Toxicology Letters, 237(3), 191–199. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26101800. [DOI] [PubMed] [Google Scholar]
- Lujambio A, & Lowe SW (2012). The microcosmos of cancer. Nature, 482(7385), 347–355. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22337054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Jean-Toussaint R, Sacan A, & Ajit SK (2021). Differential RNA packaging into small extracellular vesicles by neurons and astrocytes. Cell Communication and Signaling: CCS, 19(1), 75. Retrieved from 10.1186/s12964-021-00757-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Xu Y, Ling M, Zhao Y, Xu W, Liang X, et al. (2013). Arsenite evokes IL-6 secretion, autocrine regulation of STAT3 signaling, and miR-21 expression, processes involved in the EMT and malignant transformation of human bronchial epithelial cells. Toxicology and Applied Pharmacology, 273(1), 27–34. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24004609. [DOI] [PubMed] [Google Scholar]
- Ma R, Jiang T, & Kang X (2012). Circulating microRNAs in cancer: Origin, function and application. Journal of Experimental & Clinical Cancer Research, 31(1), 38. Retrieved from 10.1186/1756-9966-31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm SW, Amouzougan EA, & Klimecki WT (2018). Fetal bovine serum induces sustained, but reversible, epithelial-mesenchymal transition in the BEAS-2B cell line. Toxicology In Vitro, 50, 383–390. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29678786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez VD, Vucic EA, Becker-Santos DD, Gil L, & Lam WL (2011). Arsenic exposure and the induction of human cancers. Journal of Toxicology, 2011, 431287. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22174709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JE, & Goldman RH (2016). Arsenic and skin cancer in the USA: The current evidence regarding arsenic-contaminated drinking water. International Journal of Dermatology, 55(11), e585–e591. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27420023. [DOI] [PubMed] [Google Scholar]
- Melnik BC (2015). MiR-21: An environmental driver of malignant melanoma? Journal of Translational Medicine, 13, 202. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidi C, Hayashi M, Datta S, Sen T, Zenner K, Oladeru O, et al. (2015). Involvement of epigenetics and EMT-related miRNA in arsenic-induced neoplastic transformation and their potential clinical use. Cancer Prevention Research (Philadelphia, Pa.), 8(3), 208–221. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25586904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WH Jr., Schipper HM, Lee JS, Singer J, &Waxman S (2002). Mechanisms of action of arsenic trioxide. Cancer Research, 62(14), 3893–3903. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12124315. [PubMed] [Google Scholar]
- Minatel BC, Sage AP, Anderson C, Hubaux R, Marshall EA, Lam WL, et al. (2018). Environmental arsenic exposure: From genetic susceptibility to pathogenesis. Environment International, 112, 183–197. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29275244. [DOI] [PubMed] [Google Scholar]
- Muralidhar B, Winder D, Murray M, Palmer R, Barbosa-Morais N, Saini H, et al. (2011). Functional evidence that Drosha overexpression in cervical squamous cell carcinoma affects cell phenotype and microRNA profiles. The Journal of Pathology, 224(4), 496–507. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21590768. [DOI] [PubMed] [Google Scholar]
- Nail AN, Ferragut Cardoso AP, Banerjee M, & States JC (2022). Circulating miRNAs as biomarkers of toxic heavy metal exposure. In Sahu SC (Ed.), Genomic and epigenomic biomarkers of toxicology and disease (pp. 63–87). Hoboken: Wiley. [Google Scholar]
- Nail AN, McCaffrey LM, Banerjee M, Ferragut Cardoso AP, & States JC (2022). Chronic arsenic exposure suppresses ATM pathway activation in human keratinocytes. Toxicology and Applied Pharmacology, 446, 116042. 10.1016/j.taap.2022.116042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngalame NNO, Luz AL, Makia N, & Tokar EJ (2018). Arsenic alters exosome quantity and cargo to mediate stem cell recruitment into a Cancer stem cell-like phenotype. Toxicological Sciences : An Official Journal of the Society of Toxicology, 165(1), 40–49. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21590768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngalame NN, Makia NL, Waalkes MP, & Tokar EJ (2016). Mitigation of arsenic-induced acquired cancer phenotype in prostate cancer stem cells by miR-143 restoration. Toxicology and Applied Pharmacology, 312, 11–18. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26721309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngalame NN, Tokar EJ, Person RJ, Xu Y, & Waalkes MP (2014). Aberrant microRNA expression likely controls RAS oncogene activation during malignant transformation of human prostate epithelial and stem cells by arsenic. Toxicological Sciences : An Official Journal of the Society of Toxicology, 138(2), 268–277. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24431212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurchi VM, Djordjevic AB, Crisponi G, Alexander J, Bjorklund G, & Aaseth J (2020). Arsenic toxicity: Molecular targets and therapeutic agents. Biomolecules, 10(2). Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32033229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Hayder H, Zayed Y, & Peng C (2018). Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne), 9, 402. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30123182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk M, Metin M, Altay V, Bhat RA, Ejaz M, Gul A, et al. (2022). Arsenic and human health: Genotoxicity, epigenomic effects, and Cancer signaling. Biological Trace Element Research, 200(3), 988–1001. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33864199. [DOI] [PubMed] [Google Scholar]
- Palma-Lara I, Martinez-Castillo M, Quintana-Perez JC, Arellano-Mendoza MG, Tamay-Cach F, Valenzuela-Limon OL, et al. (2020). Arsenic exposure: A public health problem leading to several cancers. Regulatory Toxicology and Pharmacology, 110, 104539. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31765675. [DOI] [PubMed] [Google Scholar]
- Peng Y, & Croce CM (2016). The role of MicroRNAs in human cancer. Signal Transduction and Targeted Therapy, 1, 15004. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29263891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR, Yang CH, & Pfeffer LM (2015). The role of miR-21 in Cancer. Drug Development Research, 76(6), 270–277. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26082192. [DOI] [PubMed] [Google Scholar]
- Pi J, Kumagai Y, Sun G, Yamauchi H, Yoshida T, Iso H, et al. (2000). Decreased serum concentrations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in inner Mongolia. Free Radical Biology & Medicine, 28(7), 1137–1142. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10832076. [DOI] [PubMed] [Google Scholar]
- Plotnikova O, Baranova A, & Skoblov M (2019). Comprehensive analysis of human microRNA-mRNA interactome. Frontiers in Genetics, 10, 933. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31649721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorski J, & Berg M (2020). Global threat of arsenic in groundwater. Science, 368(6493), 845–850. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32439786. [DOI] [PubMed] [Google Scholar]
- Polo A, Marchese S,De Petro G, Montella M, Ciliberto G, Budillon A, et al. (2018). Identifying a panel of genes/proteins/miRNAs modulated by arsenicals in bladder, prostate, kidney cancers. Scientific Reports, 8(1), 10395. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29991691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya DA, & Lawson M (2016). Geogenic and anthropogenic arsenic Hazard in groundwaters and Soilds: Distribution, nature, origin, and human exposure routes. In States JC (Ed.), Arsenic: Exposure sources, health risks, and mechanisms of toxicity. Hoboken: Wiley. [Google Scholar]
- Powell NR, Zhao H, Ipe J, Liu Y, & Skaar TC (2021). Mapping the miRNA-mRNA interactome in human hepatocytes and identification of functional mirSNPs in Pharmacogenes. Clinical Pharmacology and Therapeutics, 110(4), 1106–1118. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34314509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, & Zhang L (2011).An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environmental Health Perspectives, 119(1), 11–19. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20682481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Zhou Y, Liu W, & Wang Q (2021). Paradoxical effects of arsenic in the lungs. Environmental Health and Preventive Medicine, 26(1), 80. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34388980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson AO, & Jelliffe AM (1963). Medicinal arsenic poisoning and lung cancer. British Medical Journal, 2(5351), 207–209. Retrieved from https://pubmed.ncbi.nlm.nih.gov/13974488. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1872330/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenbeck BA, Banerjee M, & Leslie EM (2016). Cellular arsenic transport pathways in mammals. Journal of Environmental Sciences (China), 49, 38–58. 10.1016/j.jes.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Roh T, Steinmaus C, Marshall G, Ferreccio C, Liaw J, & Smith AH (2018). Age at exposure to arsenic in water and mortality 30–40 years after exposure cessation. American Journal of Epidemiology, 187(11), 2297–2305. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30084889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, & Barsouk A (2020). Epidemiology of bladder Cancer. Medical Sciences (Basel), 8(1). Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32183076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jacques N, Parker L, Brown P, & Dummer TJ (2014). Arsenic in drinking water and urinary tract cancers: A systematic review of 30 years of epidemiological evidence. Environmental Health, 13, 44. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24889821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal T, Paul M, Bhattacharjee S, & Bhattacharjee P (2020). Epigenetic alteration of mitochondrial biogenesis regulatory genes in arsenic exposed individuals (with and without skin lesions) and in skin cancer tissues: A case control study. Chemosphere, 258, 127305. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32563914. [DOI] [PubMed] [Google Scholar]
- Sarma N (2016). Skin manifestations of chronic Arsenicosis. In States JC (Ed.), Arsenic: Exposure sources, health risks, and mechanisms of toxicity. Hoboken: Wiley. [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, & Rajewsky N (2008). Widespread changes in protein synthesis induced by microRNAs. Nature, 455(7209), 58–63. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18668040. [DOI] [PubMed] [Google Scholar]
- Shah V, & Shah J (2020). Recent trends in targeting miRNAs for cancer therapy. The Journal of Pharmacy and Pharmacology, 72(12), 1732–1749. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32783235. [DOI] [PubMed] [Google Scholar]
- Shaji E, Santosh M, Sarath KV, Prakash P, Deepchand V, & Divya BV (2021). Arsenic contamination of ground water: A global synopsis with focus on the Indian peninsula. Geoscience Frontiers, 12(3), 101079. Retrieved from https://www.sciencedirect.com/science/article/pii/S1674987120302115. [Google Scholar]
- Sherwood CL, & Lantz RC (2016). Lung Cancer and other pulmonary diseases. In States JC (Ed.), Arsenic: Exposure sources, health risks, and mechanisms of toxicity. Hoboken: Wiley. [Google Scholar]
- Si W, Shen J, Zheng H, & Fan W (2019). The role and mechanisms of action of microRNAs in cancer drug resistance. Clinical Epigenetics, 11(1), 25. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30744689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima M, Rossnerova A, Simova Z, & Rossner P Jr. (2021). The impact of air pollution exposure on the MicroRNA machinery and lung Cancer development. Journal of Personalized Medicine, 11(1). Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33477935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Roh T, Ferreccio C, Liaw J, & Steinmaus C (2018). Lung, bladder, and kidney Cancer mortality 40 years after arsenic exposure reduction. Journal of the National Cancer Institute, 110(3), 241–249. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29069505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers SC, & McManus RG (1953). Multiple arsenical cancers of skin and internal organs. Cancer, 6(2), 347–359. [DOI] [PubMed] [Google Scholar]
- States JC (2016). Translating experimental data to human populations. In States JC (Ed.), Arsenic: Exposure sources, health risks, and mechanisms of toxicity. Hoboken: Wiley. [Google Scholar]
- States JC, Barchowsky A, Cartwright IL, Reichard JF, Futscher BW, & Lantz RC (2011). Arsenic toxicology: Translating between experimental models and human pathology. Environmental Health Perspectives, 119(10), 1356–1363. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21684831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, et al. (2000). Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Archives of Toxicology, 74(6), 289–299. 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Sugito N, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Kurehara H, et al. (2006). RNASEN regulates cell proliferation and affects survival in esophageal cancer patients. Clinical Cancer Research, 12(24), 7322–7328. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17121874. [DOI] [PubMed] [Google Scholar]
- Sun B, Xue J, Li J, Luo F, Chen X, Liu Y, et al. (2017). Circulating miRNAs and their target genes associated with arsenism caused by coal-burning. Toxicology Research (Camb), 6(2), 162–172. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30090486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa H, & Seto M (2005). A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia, 19(11), 2013–2016. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16167061. [DOI] [PubMed] [Google Scholar]
- Tapio S, & Grosche B (2006). Arsenic in the aetiology of cancer. Mutation Research, 612(3), 215–246. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16574468. [DOI] [PubMed] [Google Scholar]
- Tchounwou PB, Yedjou CG, Patlolla AK, & Sutton DJ (2012). Heavy metal toxicity and the environment. Experientia. Supplementum, 101, 133–164. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22945569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ (2016). The chemistry and metabolism of arsenic. In States JC (Ed.), Arsenic: Exposure sources, health risks, and mechanisms of toxicity (pp. 81–109). Hoboken: Wiley. [Google Scholar]
- Thomas DJ, Styblo M, & Lin S (2001). The cellular metabolism and systemic toxicity of arsenic. Toxicology and Applied Pharmacology, 176(2), 127–144. 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, & Waalkes MP (2010). Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environmental Health Perspectives, 118(1), 108–115. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20056578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CH (2007). Arsenic methylation, urinary arsenic metabolites and human diseases: Current perspective. Journal of Environmental Science and Health. Part C, Environmental Carcinogenesis & Ecotoxicology Reviews, 25(1), 1–22. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17365340. [DOI] [PubMed] [Google Scholar]
- Tsuji JS, Chang ET, Gentry PR, Clewell HJ, Boffetta P, & Cohen SM (2019). dose–response for assessing the cancer risk of inorganic arsenic in drinking water: The scientific basis for use ofa threshold approach. Critical Reviews in Toxicology, 49(1), 36–84. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30932726. [DOI] [PubMed] [Google Scholar]
- Uhlmann S, Mannsperger H, Zhang JD, Horvat EA, Schmidt C, Kublbeck M, et al. (2012). Global microRNA level regulation of EGFR-driven cell-cycle protein network in breast cancer. Molecular Systems Biology, 8, 570. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22333974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M (2000). Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicology Letters, 112–113, 209–217. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10720733. [DOI] [PubMed] [Google Scholar]
- Vahter M, & Concha G (2001). Role of metabolism in arsenic toxicity. Pharmacology & Toxicology, 89(1), 1–5. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11484904. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Taalab YM, Heinze S, Tariba Lovaković B, Pizent A, Renieri E, et al. (2020). Toxic-metal-induced alteration in miRNA expression profile as a proposed mechanism for disease development. Cell, 9(4), 901. Retrieved from https://www.mdpi.com/2073-4409/9/4/901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ge X, Zheng J, Li D, Liu X, Wang L, et al. (2016). Role and mechanism of miR-222 in arsenic-transformed cells for inducing tumor growth. Oncotarget, 7(14), 17805–17814. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26909602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Nioka S, Wang Z, Leigh JS, & Chance B (1993). NMR visibility studies of N-delta proton of proximal histidine in deoxyhemoglobin in lysed and intact red cells. Magnetic Resonance in Medicine, 30(6), 759–763. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8139460. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhao Y, Smith E, Goodall GJ, Drew PA, Brabletz T, et al. (2011). Reversal and prevention of arsenic-induced human bronchial epithelial cell malignant transformation by microRNA-200b. Toxicological Sciences : An Official Journal of the Society of Toxicology, 121(1), 110–122. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21292642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CN, Belli A, & Di Pietro V (2019). Small non-coding RNAs: New class of biomarkers and potential therapeutic targets in neurodegenerative disease. Frontiers in Genetics, 10. Retrieved from 10.3389/fgene.2019.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Jung S, Keller S, Gregory RI, & Diederichs S (2009). Many roads to maturity: microRNA biogenesis pathways and their regulation. Nature Cell Biology, 11(3), 228–234. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19255566. [DOI] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Wang TW, Hsueh YM, Wang IH, Chen CJ, et al. (2001). Association of blood arsenic levels with increased reactive oxidants and decreased antioxidant capacity in a human population of northeastern Taiwan. Environmental Health Perspectives, 109(10), 1011–1017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11675266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Kuo TL, Hwang YH, & Chen CJ (1989). dose–response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. American Journal of Epidemiology, 130(6), 1123–1132. [DOI] [PubMed] [Google Scholar]
- Xiao T, Xue J, Shi M, Chen C, Luo F, Xu H, et al. (2018). Circ008913, via miR-889 regulation of DAB2IP/ZEB1, is involved in the arsenite-induced acquisition of CSC-like properties by human keratinocytes in carcinogenesis. Metallomics, 10(9), 1328–1338. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30167605. [DOI] [PubMed] [Google Scholar]
- Xu W,Ji J, Xu Y, Liu Y, Shi L, Liu Y, et al. (2015).MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Molecular Carcinogenesis, 54(Suppl 1), E148–E161. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25252218. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li Y, Pang Y, Ling M, Shen L, Jiang R, et al. (2012). Blockade of p53 by HIF-2alpha, but not HIF-1alpha, is involved in arsenite-induced malignant transformation of human bronchial epithelial cells. Archives of Toxicology, 86(6), 947–959. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22447124. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tokar EJ, Sun Y, & Waalkes MP (2012). Arsenic-transformed malignant prostate epithelia can convert noncontiguous normal stem cells into an oncogenic phenotype. Environmental Health Perspectives, 120(6), 865–871. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22472196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Xu L, Jiao Y, Luo S, Li A, & Wu K (2020). The role of cancer-derived microRNAs in cancer immune escape. Journal of Hematology & Oncolology, 13(1), 25. Retrieved from https://pubmed.ncbi.nlm.nih.gov/32222150. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7103070/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Liao WT, & Chai CY (2006). Arsenic carcinogenesis in the skin. Journal of Biomedical Science, 13(5), 657–666. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16807664. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Zou Z, Wang Q, Sun B, Liu Y, Liang B, et al. (2019). Association and risk of five miRNAs with arsenic-induced multiorgan damage. Science of the Total Environment, 680, 1–9. Retrieved from https://linkinghub.elsevier.com/retrieve/pii/S0048969719320534. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Huang Q,Yu L, Zhu D, Li Y, Xue Z, et al. (2022). The role of miRNAin tumor immune escape and miRNA-based therapeutic strategies. Frontiers in Immunology, 12. Retrieved from 10.3389/fimmu.2021.807895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, & Klimecki WT (2015). Culture conditions profoundly impact phenotype in BEAS-2B, a human pulmonary epithelial model. Journal of Applied Toxicology, 35(8), 945–951. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25524072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Huang Z, Wang L, Lin Z, Cao Z, Li X, et al. (2018). Malignant transformation of human bronchial epithelial cells induced by arsenic through STAT3/miR-301a/SMAD4 loop. Scientific Reports, 8(1), 13291. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30185897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Su J, Wu Z, Wang C, Zhong W, et al. (2017). Integration of microRNAome, proteomics and metabolomics to analyze arsenic-induced malignant cell transformation. Oncotarget, 8(53), 90879–90896. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29207610. [DOI] [PMC free article] [PubMed] [Google Scholar]


