Abstract
Background:
Abnormalities of gamma-aminobutyric acid-ergic (GABAegic) systems may play a role in schizophrenia and mood disorders. Magnetic resonance spectroscopy allows for non-invasive in-vivo quantification of GABA; however, studies of GABA in schizophrenia have yielded inconsistent findings. This may stem from grouping together disparate voxels from functionally heterogeneous regions.
Methods:
We searched PubMed for magnetic resonance spectroscopy studies of medial frontal cortex (MFC) GABA in patients with schizophrenia, bipolar disorder, depression, and individuals meeting ultra-high risk for psychosis criteria. Voxel placements were classified as rostral-, rostral-mid-, mid-, or posterior MFC, and meta-analyses conducted for each group, for each sub-region.
Results:
Of 341 screened articles, 23 studies of schizophrenia, 6 studies of bipolar disorder, 20 studies of depression and 7 studies of ultra-high risk met inclusion criteria. Meta-analysis revealed lower mid- (SMD = −0.28, 95% confidence interval [CI] = −0.48 to −0.07, p < .01) and posterior (SMD = −0.29, 95% CI = −0.49 to −0.09, p <.01) MFC GABA in schizophrenia and increased rostral MFC GABA in bipolar disorder (SMD = 0.76, 95% CI = 0.25 to 1.25, p < .01). In depression, reduced rostral MFC GABA (SMD = −0.36, 95% CI = −0.64 to −0.08, p = .01) did not survive correction for multiple comparisons. We found no evidence for GABA differences in ultra-high risk individuals.
Conclusions:
While limited by small numbers of published studies, these results substantiate the relevance GABA in the pathophysiology of psychosis spectrum and mood disorders and underline the importance of voxel placement.
INTRODUCTION
Substantial evidence from several lines of research has suggested that disturbances in the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) play a role in the pathophysiology of both schizophrenia spectrum and mood disorders. Post-mortem and preclinical studies in schizophrenia suggest abnormalities in fast-spiking, parvalbumin-positive GABAergic interneurons(1), as well as reductions in mRNA and protein levels of the 67-kDa isoform of glutamic acid decarboxylase (GAD67), the GABA-synthesizing enzyme(2–5). Although the bulk of the findings have been reported in schizophrenia/schizoaffective cohorts, similar results have also been found in bipolar patients(6–8). Decreased prefrontal GAD67 expression has been shown in depression(5,9) as well as reduced GABA concentrations in plasma(10,11) and cerebrospinal fluid(12–14), possibly linked to vulnerability of somatostatin-positive GABAergic interneurons(15). An important question is whether the post-mortem findings reflect GABAergic alterations in-vivo. Proton magnetic resonance spectroscopy (1H-MRS) is a powerful way to non-invasively investigate in-vivo GABA concentrations. Measuring GABA poses specific challenges due to its relatively low concentration and high spectral overlap with more abundant metabolites; however, sequences such as MEGA-PRESS(16,17) take advantage of couplings within the GABA molecule, allowing GABA signals to be reliably separated from stronger signals.
While the post-mortem evidence linking schizophrenia spectrum and mood disorders to GABAergic disfunction is well-replicated, 1H-MRS studies have, thus far, yielded inconsistent findings. In schizophrenia for example, 1H-MRS studies have revealed increased(18,19), decreased(20,21), and normal GABA concentrations(22), when patients are compared with controls. Attempts to reach consensus by pooling study data via meta-analysis also appear contradictory, with evidence for reduced GABA concentrations found in some analyses(23–25) , but not others(26,27). The picture for depression is a little clearer, however there is evidence supporting both decreased(28–30) and normal GABA concentrations(31–33).
There are several possible reasons to explain these mixed findings. Individual studies may differ in the clinical characteristics of their samples, in terms of illness duration, symptom profile or medication use. There may be large differences between studies in methodology, e.g., magnet strength or reference metabolite used. Especially critical may be the location of the 1H-MRS voxel. Due to the low concentration of GABA in comparison with other metabolites, large voxels, typically around 3 × 3 × 3cm3, are collected to offset the low signal-to-noise ratio. The time to acquire these voxels is usually approximately 10 minutes per voxel, meaning researchers generally only have enough time to collect one or two voxels per study. When meta-analyses combine data from these studies, often voxels from disparate brain regions are combined – across the whole brain, or entire frontal cortex – sometimes with minimal overlap. In healthy controls, evidence indicates that GABA concentrations vary across the brain, with significant variance in GABA demonstrated between frontal cortex voxels, including those placed in the ventral midcingulate, dorsolateral prefrontal cortex and orbitofrontal cortex(34–36).
The medial frontal cortex (MFC) has been a frequent target of 1H-MRS studies for psychiatric disorders, as it has been strongly implicated in the etiologies of schizophrenia spectrum disorders and mood disorders. However, published studies have placed voxels across a large extent of the MFC, covering a functionally heterogeneous region. Meta-analysis of the MFC shows distinct functional profiles, with rostral MFC implicated in reward, decision making, social processing and episodic memory, the middle MFC with cognitive control, negative affect and pain, and the posterior in motor function(37). These regions likely contribute to symptoms of psychosis spectrum and mood disorders in different ways; thus, combining GABA concentration findings across functionally heterogeneous regions may not be the best strategy to gain the benefit of pooled studies in a meta-analysis.
Given the importance of the various functions of the MFC for psychiatric disorders, as well as the number of studies that focus on the MFC, we performed meta-analyses of 1H-MRS studies of medial frontal GABA concentration in schizophrenia spectrum disorders, bipolar disorder, and depression. Our primary focus was on schizophrenia, given the relative strength of the post-mortem findings and focus of our prior work(38). We also included mood disorders in the analysis, given both the evidence of altered GABA function in these disorders and the involvement of MFC regions in mood regulation, to compare and contrast with schizophrenia. We also included individuals with the ultra-high risk (UHR) syndrome, as this condition is thought to precede the development of schizophrenia. Voxels were classified as being in one of four medial frontal sub-regions – rostral, rostral-mid, mid, and posterior, and each of these sub-regions are examined separately. Our aim was to gain a more nuanced picture of the profile of medial frontal GABA dysfunction in psychiatric disorders.
METHODS AND MATERIALS
Search Strategy
Meta-analyses were conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology. 1H-MRS studies that examined differences in GABA between healthy controls and patients with schizophrenia, bipolar disorder, depression, or UHR individuals were identified through a PubMed search, using the term “(MRS OR Magnetic Resonance Spectroscopy) AND (GABA OR Gamma Aminobutyric Acid) AND (psychosis OR depression OR schizophrenia OR bipolar disorder). Databases were searched for articles published before October 25, 2021. Titles and abstracts were examined to determine suitability for inclusion. Reference sections of the returned articles, as well as review articles(38) and meta-analyses(23,25,27,39–42) were searched for additional articles that fulfilled inclusion criteria. We also searched the online pre-print servers bioAriv, medRxiv and psyArXiv for studies which met our inclusion criteria.
Study Selection
Studies met inclusion criteria if they: (a) were an original research article; (b) used 1H-MRS to study in-vivo GABA; (c) compared groups with schizophrenia spectrum disorders, bipolar disorder, depression, or UHR, with healthy control participants; (d) included 1H-MRS voxels in the medial frontal cortex; (e) were published in, or translated into, English. Articles were screened for overlapping samples, and where present, data from the article reporting the largest sample was included.
Data extraction
From each study that met inclusion criteria, we extracted publication information (authors, year of publication), participant characteristics (diagnosis, sample size, age, gender, illness duration, medication status), methodological characteristics (field strength, acquisition sequence, reference metabolite, analysis software), voxel location, and GABA concentration (mean and SD in each group). This information was extracted by one author (C.J.S.) and independently verified by another (M.S.). Where published articles did not include this information in the text, tables or supplementary materials, the authors were contacted, or where possible, values were estimated using an online tool (https://automeris.io/WebPlotDigitizer/).
Voxel Classification
Using figures and text descriptions from the included publications, voxels were classified as rostral-, mid- and posterior MFC. Several voxels formed a cluster which straddled the rostral- and mid MFC, and we classified these separately as rostral-mid MFC. A fully detailed examination of the classification process is provided in the supplemental materials.
Meta-analysis
Separate meta-analyses were conducted for each MFC subregion (rostral, rostral-mid, mid, and posterior), for each clinical population (schizophrenia, bipolar disorder, depression, and UHR). Studies of schizophrenia frequently included schizoaffective and schizophreniform patients, which we refer to collectively as ‘schizophrenia,’ for simplicity and consistent with common practice in the literature. For comparison with our subregion analyses, we performed meta-analyses for each clinical population in which we included voxels from all MFC subregions. Results of these meta-analyses are described in the Supplementary Materials, including forest plot visualizations in Supplementary Figures 1–4. Data were analyzed using R (version 4.1.1), using the “metafor” package(44). Meta-analyses were conducted when at least three datasets met inclusion criteria for a subregion, for a clinical population. Results were summarized if this number was not met. Schizophrenia samples were classified as acute (average duration of illness < 5 years) and chronic (average duration of illness ≥ 5 years), and depression samples were classified as depressed or remitted at the time of scanning. Analyses of these subgroups were conducted if at least five datasets met inclusion criteria for that subregion. Meta-regressions were performed to determine differences between the subgroups. Effect sizes were described using standardized mean differences (SMD; also known as Hedges’ g) and 95% confidence intervals. Use of SMD allowed comparison of different units of GABA measurement (institutional units, ratios to reference metabolites). Random effects models were used to pool effect sizes, as we assumed heterogeneity in both the clinical profile of patient samples and the methodology employed in each study. Since examining GABA concentrations in schizophrenia was our primary interest, we corrected for multiple comparisons by applying a Bonferroni-corrected threshold of p < 0.0125 (=0.05/4) to determine statistical significance, since we performed 4 independent meta-analyses. For the meta-analyses of depression, bipolar disorder, and UHR, we applied a Bonferroni-corrected threshold of p < 0.0083 (=0.05/6) for statistical significance, since we performed a total of 6 meta-analyses investigating these samples.
Between-study heterogeneity was assessed with the I2 index and Q-statistic. Higher I2 scores indicate higher variation between studies, with values of 25%, 50% and 75% representing small, moderate, and high levels of heterogeneity, respectively. Significant Q-statistics suggest heterogeneity but do not indicate the extent of this heterogeneity (45). Publication bias was assessed by visually examining funnel plots for asymmetry and performing Egger’s regression test for funnel plot asymmetry(46).
RESULTS
Study characteristics
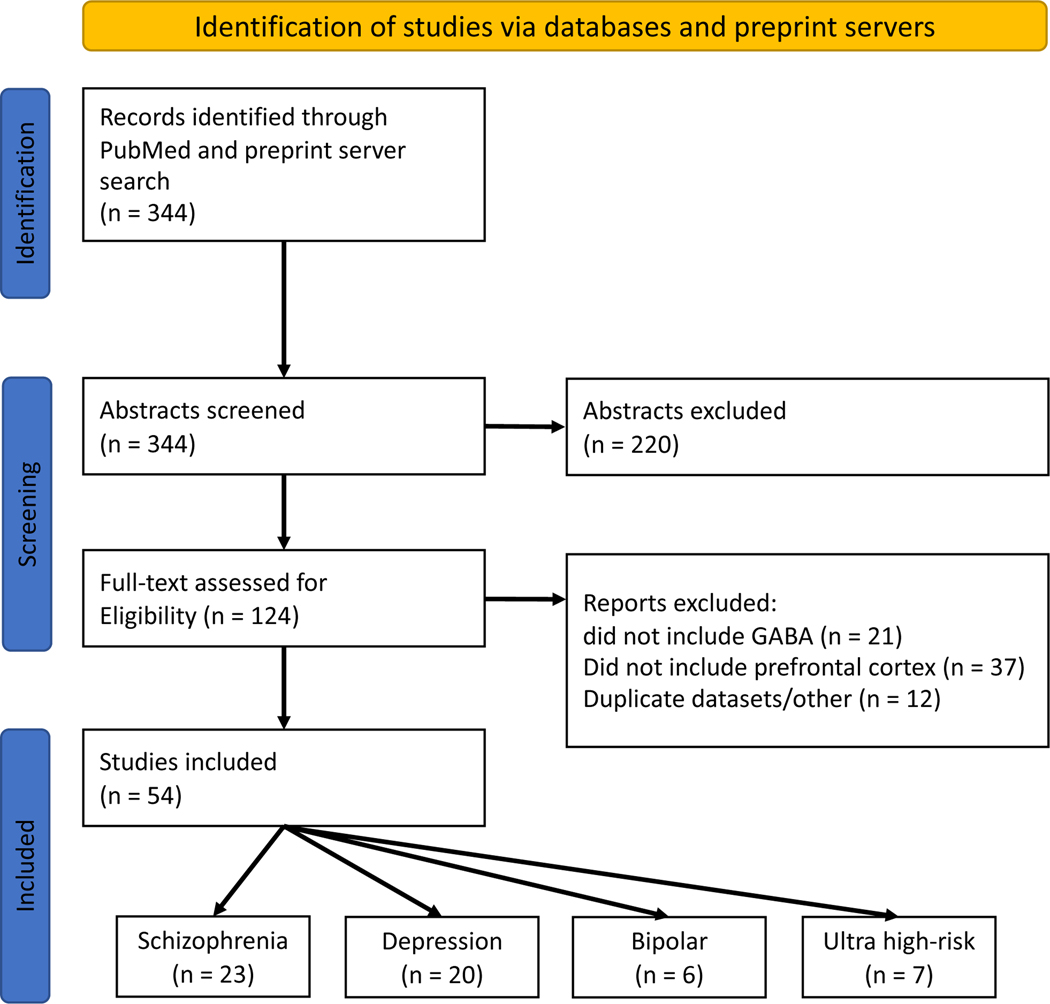
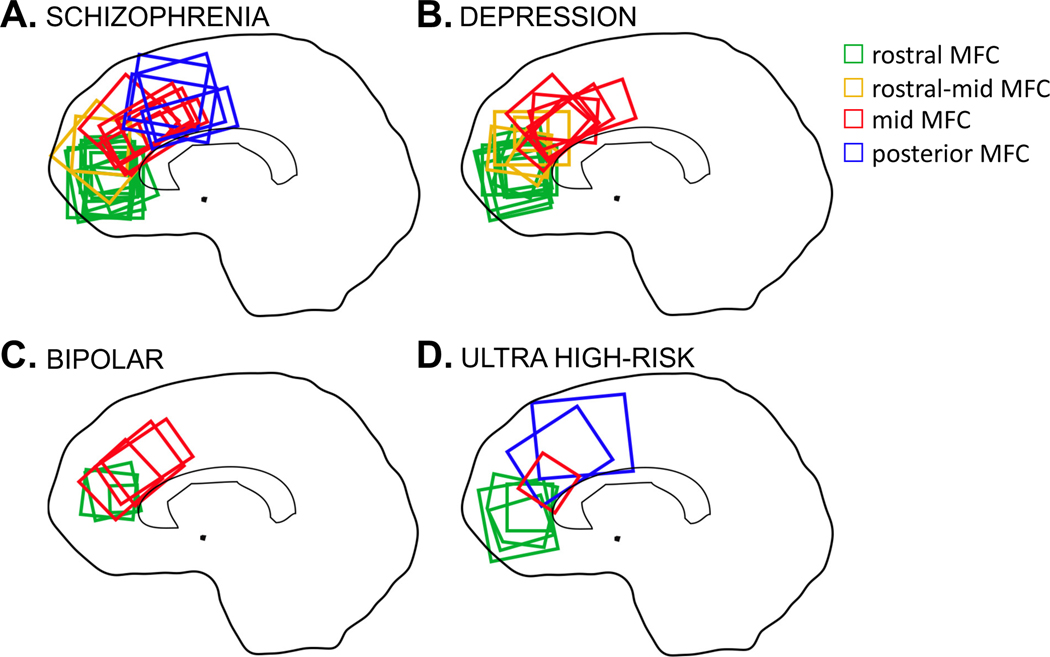
The literature review identified a total of 54 studies meeting inclusion criteria. The PRISMA flow diagram is presented in Figure 1. Of the 54 studies, 23 studies included participants with schizophrenia(18,19,53–62,20,63,64,22,47–52) (752 cases, 856 controls), 7 studies included individuals at UHR(52,64–69) (229 cases, 232 controls), 20 studies included individuals with depression(28,30,77–84,32,70–76) (463 cases, 499 controls), and 6 studies included participants with bipolar disorder(85–90) (129 cases, 94 controls). Two studies included multiple clinical samples(52,64), therefore these numbers sum 56. Detailed study characteristics are presented in Supplementary Tables 1-4. Voxels from each study were classified as rostral-, rostral-mid-, mid-, and posterior MFC, with classifications shown in Figure 2.
Figure 1:
PRISMA flow diagram of search for meta-analyses. Note, two studies included both schizophrenia and ultra-high risk samples, and so the breakdown of included studies totals 56 due to these duplicates.
Figure 2:
Voxel location in medial frontal cortex GABA 1H-MRS studies. A: 1H-MRS studies of schizophrenia; B: 1H-MRS studies of depression; C: 1H-MRS studies of bipolar disorder; and D: 1H-MRS studies of individuals meeting ultra-high risk of developing psychosis criteria.
Medial Frontal GABA concentrations in Psychosis Spectrum and Mood Disorders
Schizophrenia
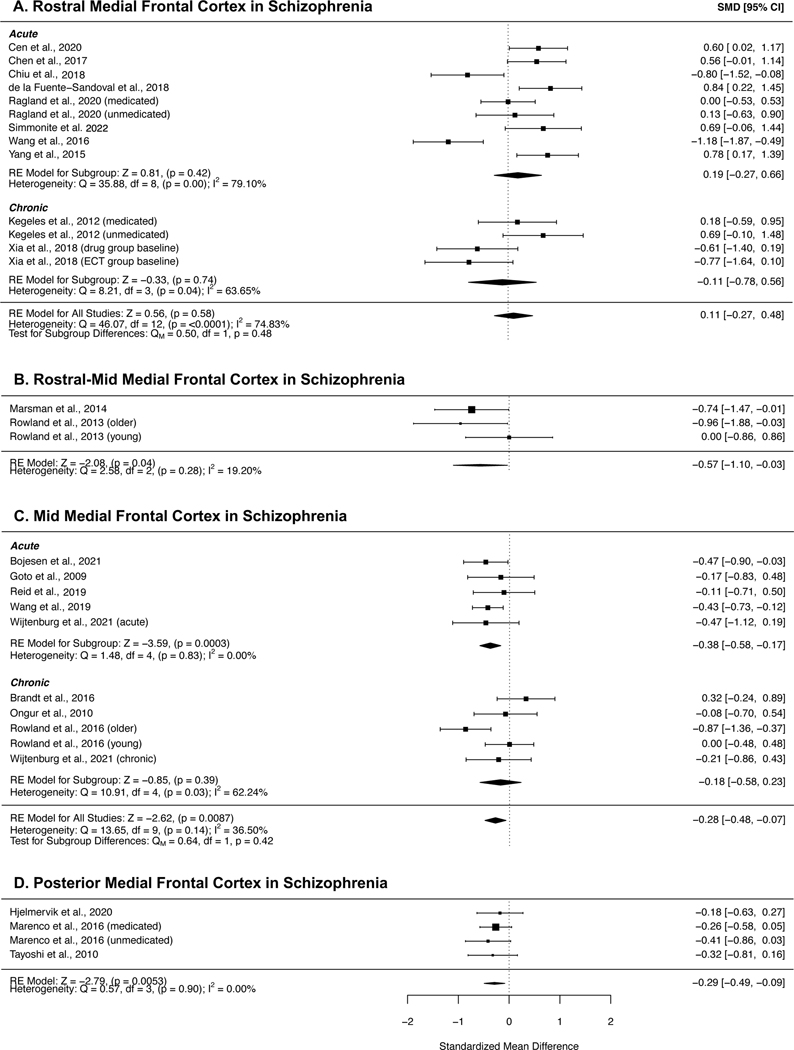
Figure 3 shows results of the meta-analyses of individuals with schizophrenia in MFC subregions. GABA concentrations were significantly reduced in both the mid- (SMD = −0.28, 95% CI = −0.48 to −0.07, p = .0087) and posterior MFC (SMD = −0.29, 95% CI = −0.49 to −0.09, p = .005) in patients with schizophrenia compared with healthy controls. I2 values were 36.50% and 0.00% respectively, suggesting small heterogeneity across studies. Sub-group analyses revealed that mid MFC GABA concentrations were significantly reduced in acute patients (SMD = −0.38, 95% CI = −0.58 to −0.17, p = 0.0003), but not chronic patients (SMD = −0.18, 95% CI = −0.58 to 0.23, p = .39); however meta-regression did not indicate significant differences between the subgroups. GABA in the rostral MFC showed no difference compared to controls (SMD = 0.11, 95% CI = −0.27 to 0.48, p = .58), and likewise no significant effects in the subgroup analyses of acute and chronic patients (acute: SMD = 0.19, 95% CI = −0.27 to 0.66, p = 0.42; chronic: SMD = −0.11, 95% CI = −0.27 to 0.48, p = 0.74). This region was notable for high heterogeneity in the combined group (I2=74.83%), as well as acute (I2=79.10%) and chronic (I2=63.65%) subgroups. There were insufficient studies of posterior MFC GABA to perform subgroup analyses for this region. Reductions in GABA in the rostral-mid MFC in patients with schizophrenia did not survive corrections for multiple comparisons (SMD = −0.57, 95% CI = −1.10 to −0.03, p = .04).
Figure 3:
Forest plots showing summary effect sizes for group differences between individuals with schizophrenia and healthy controls in the A) rostral MFC; B) rostral-mid MFC, C) mid MFC and D) posterior MFC. Negative SMDs denote lower GABA concentrations in patients than healthy controls; positive SMDs denote higher GABA concentrations in patients than healthy controls. Abbreviations: SMD – standardized mean difference, CI – confidence interval, RE – Random effects, df – degrees of freedom.
Depression
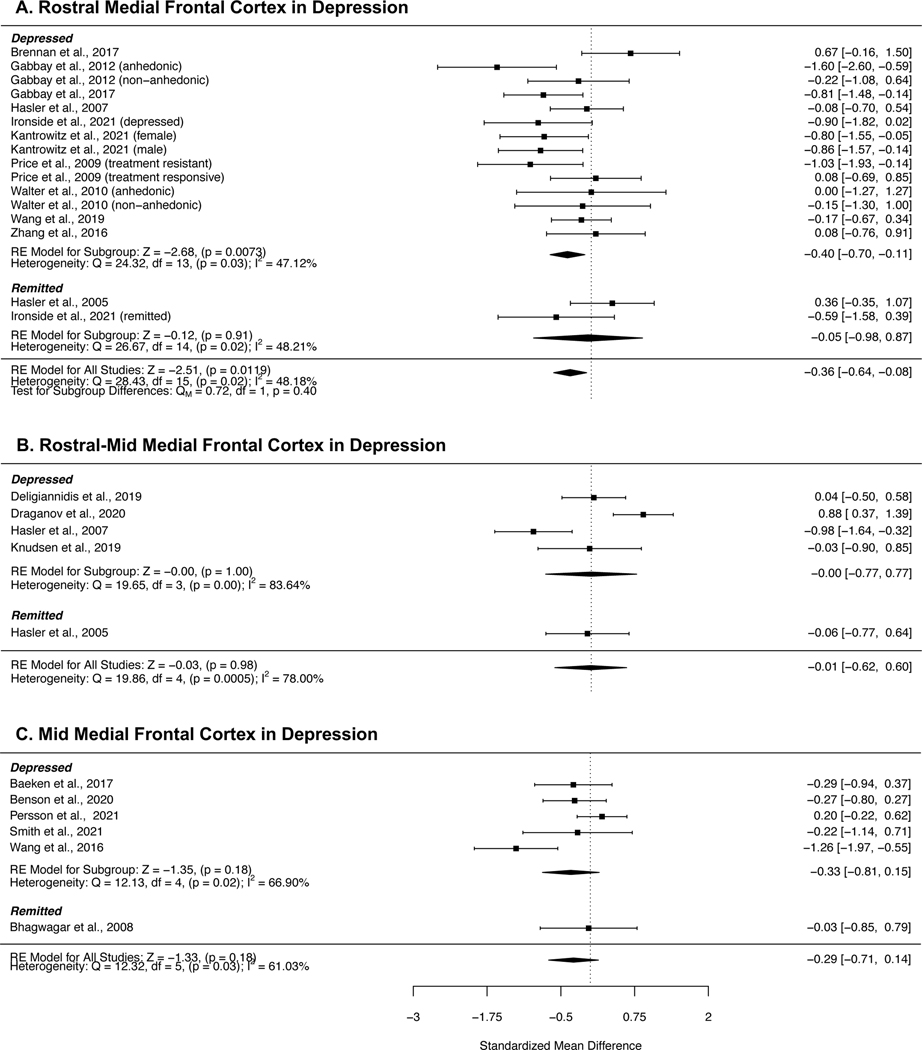
Forest plots detailing the meta-analyses of regional MFC GABA concentrations in studies investigating depression are presented in Figure 4. While the meta-analysis of rostral MFC GABA in depression indicated a reduction compared with controls, the significance of this effect did not survive correction for multiple comparisons (SMD = −0.36, 95% CI = −0.64 to −0.08, p = 0.01). Subgroup analyses of currently depressed patients revealed a similar effect size (SMD = −0.40, 95% CI = −0.70 to −0.11, p = 0.0073), but there were insufficient studies of remitted patients to perform an analysis of this group. Heterogeneity was moderate across the full sample (I2 = 48.15%) and within the currently depressed subgroup (I2 = 47.12%). We did not find any studies which reported posterior GABA in depression.
Figure 4:
Forest plots showing summary effect sizes for group differences between individuals with depression and healthy controls in the A) rostral MFC, B) rostral-mid MFC and C, mid MFC. Negative SMDs denote lower GABA concentrations in patients than healthy controls; positive SMDs denote higher GABA concentrations in patients than healthy controls. Abbreviations: SMD – standardized mean difference, CI – confidence interval, RE – Random effects, df – degrees of freedom.
Bipolar Disorder
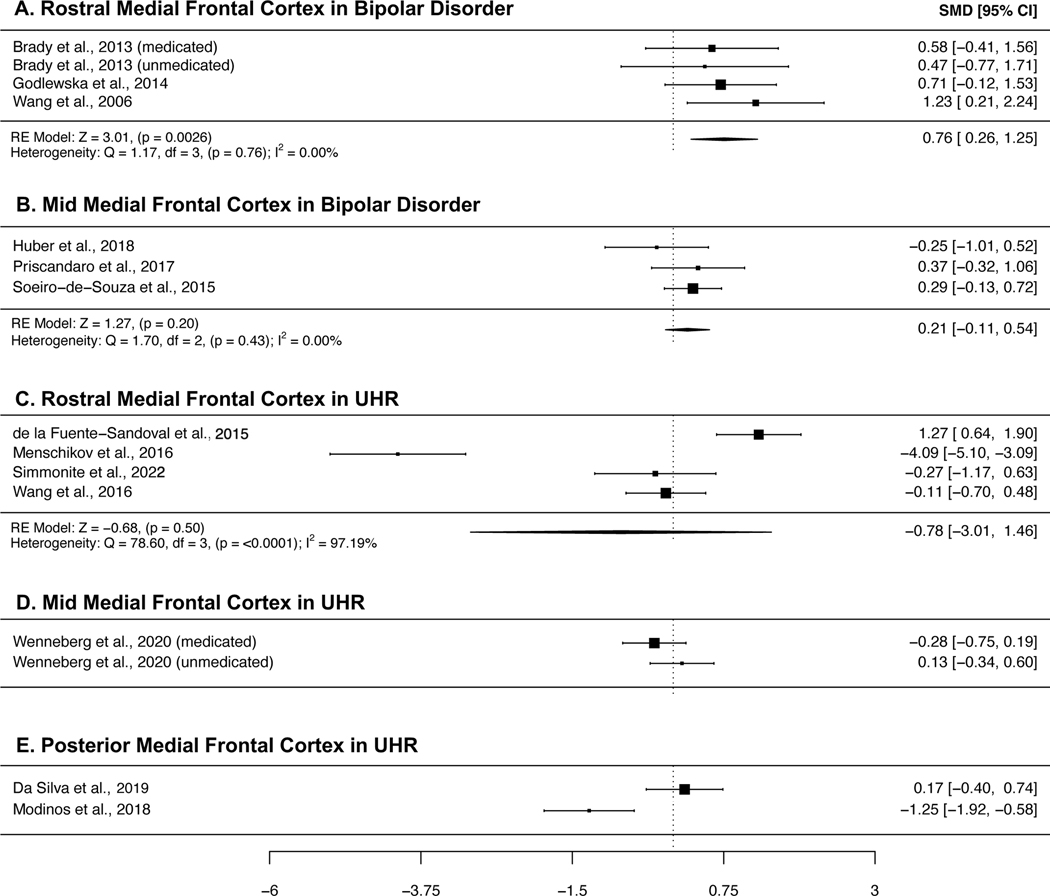
Figure 5a-b present the meta-analyses of studies of bipolar disorder. GABA concentrations in rostral MFC were higher in patients with bipolar disorder compared with controls (SMD = 0.76, 95% CI = 0.26 to 1.25, p = 0.0026), and heterogeneity across studies was small (I2 = 0.00%). We did not find any studies which published analyses of posterior GABA.
Figure 5:
Forest plots showing summary effect sizes for group differences between individuals with bipolar disorder and healthy controls in the A) rostral MFC, and B) mid MFC, and individuals meeting ultra-high risk for psychosis criteria and healthy controls in the C) rostral MFC, D) mid MFC and E) posterior MFC. Negative SMDs denote lower GABA concentrations in patients than healthy controls, positive SMDs denote higher GABA concentrations in patients than healthy controls. Abbreviations: SMD – standardized mean difference, CI – confidence interval, RE – Random effects, df – degrees of freedom.
UHR
Plots summarizing the findings of GABA 1H-MRS studies in UHR samples are presented in Figure 5c-e. Due to the limited number of studies published, we were only able to perform a meta-analysis of rostral MFC GABA, where we found no significant differences between individuals at UHR and controls (SMD = −0.78, 95% CI = −3.01 to 1.46, p = 0.50), and high between-study heterogeneity (I2 = 97.19%). Only two published studies were found for each of the mid and posterior MFC subregions, and inspection of their findings did not reveal consistent GABA abnormalities.
Publication Bias
Visual inspection of funnel plots and results of Eggers test (provided in Supplementary Figures 5-7) did not suggest publication bias.
DISCUSSION
The current study presents several meta-analyses in which regional MFC GABA concentrations are investigated, comparing individuals with schizophrenia spectrum disorders and mood disorders with healthy controls. Our main findings were: (a) patients with schizophrenia have significantly decreased GABA concentrations in the mid- and posterior MFC (b) patients with bipolar disorder have increased GABA concentrations in the rostral MFC (c) reduced GABA concentrations in the rostral MFC in depression did not survive correction for multiple comparisons.
Decreased mid and posterior MFC GABA in schizophrenia
In individuals with schizophrenia, our analysis indicated significantly decreased GABA concentrations in the mid and posterior regions of the MFC, while we found no significant differences in the rostral MFC. Decreases in the rostral-mid MFC did not meet correction for multiple comparisons. Subgroup analysis revealed these declines were significant in acute, but not chronic patients (those with an illness duration of greater than five years), suggesting that GABA abnormalities may be modulated by illness stage or mitigated by prolonged medication use. Our finding is similar to that of Nakahara et al. (24), who recently presented meta-analyses demonstrating reduced GABA concentrations in their midcingulate cortex region (analogous to our mid- and posterior MFC subregions), but not their anterior cingulate cortex region (analogous to our rostral and rostral-mid MFC subregions) in first episode psychosis (FEP) and a patient group comprised of FEP and schizophrenia patients, as well as an unmedicated subgroup. Kumar et al.’s(23) investigation of frontal GABA in schizophrenia revealed reductions restricted to a subregion of frontal cortex they termed ‘ACC’, at first appearance suggesting their findings were in contrast to ours and those of Nakahara et al.(24). While they do not provide a precise anatomical definition of the ACC region they investigate, examination of the studies they included revealed voxels which spanned all four of our MFC sub-regions, and both Nakahara et al.’s(24) ACC and MCC regions,. It is likely that differing terminologies for regions of the frontal cortex may result in differing classification schemes and explain apparent discrepancies. In support of the conclusion that more anatomically focused investigations are more likely to reveal group differences in the MFC, when we combined all voxels in the MFC, we did not find significant group differences (see supplementary materials).
Evidence from functional and structural studies have implicated the MFC as a key region in psychosis spectrum disorders (91). It has been linked with several functions, including cognitive control(92), emotion regulation(93,94) and conflict monitoring(95,96), all of which are impaired in psychosis spectrum disorders. While there has been debate about the precise functional mapping of the MFC, meta-analysis has implicated rostral regions of the MFC in reward, episodic memory and social processing, and more posterior regions in cognitive control(37). Our finding of decreased GABA concentrations in these regions are in line with post-mortem studies of schizophrenia, which have consistently shown reductions in the mRNA and protein levels of the 67-kDa isoform of GAD in the ACC(5,97), which is responsible for the majority of GABA synthesis, as well as findings of impaired cognitive control in functional measures(98,99). While it is important to note that 1H-MRS studies do not distinguish between intra- and extra-cellular GABA pools, making interpretation and reconciliation with post-mortem findings difficult, the findings appear generally consistent.
While we found evidence of GABA reduction in the mid and posterior MFC in schizophrenia, and our subgroup of acute schizophrenia, search of the literature did not uncover enough investigations of GABA in these regions in UHR individuals for us to perform meta-analyses for that sample. Review of the available UHR publications does not indicate robust GABA reductions in either the mid- or posterior MFC, suggesting that the regional GABA reductions we found in patients with schizophrenia are associated with the onset of symptoms, rather than being a trait marker for vulnerability for schizophrenia.
Increased rostral MFC GABA concentrations in bipolar disorder
Prior meta-analyses have found no significant differences in GABA concentrations, when including voxels from across the whole brain(25,39), or in the pregenual anterior cingulate/ventral midcingulate region of the frontal cortex(40). Our analysis focusing on the MFC found significantly increased GABA concentrations in the rostral MFC, a subregion which also included the pregenual anterior cingulate. Increased GABA was not significant in the mid MFC, and we were unable to perform an analysis of the posterior MFC, due to a lack of studies investigating this region. To our knowledge, we are the first meta-analysis of bipolar disorder to investigate GABA concentrations in multiple subregions of the frontal cortex; however, our findings should be interpreted with caution due to the small number of studies available for inclusion in our analysis.
Our finding of significantly increased GABA aligns with prior reports of elevated GABA levels in the plasma of bipolar patients(100,101). Increased GABA could be the result of a primary pathological process, or it could be a compensatory response, e.g., to environmental stressors. In rodents, a chronic unpredictable stress model was observed to increase GABA levels in the ACC, as measured by MRS(102). Increased GABA could also be a secondary response to glutamatergic dysfunction. Ketamine infusion, which blocks excitatory NMDA receptors, has been shown to increase MPFC GABA in humans(103).
As noted, the number of investigations of GABA concentrations in bipolar disorder is limited, and therefore we are unable to perform subgroup analyses based on medication status. Furthermore, often the samples reported are comprised of both medicated and unmedicated participants, meaning meta-analyses cannot untangle the effects of medication. One study included in our meta-analysis considered the use of GABA-modulating medications, such as benzodiazepines (which are used to treat anxiety or insomnia in psychosis spectrum and mood disorders), and found such medications partially correct GABA concentrations to a healthy control level in their sample(85). This finding indicates that medication may obscure GABA concentration increases in bipolar disorder.
Decreased GABA in the rostral MFC in depression did not survive correction for multiple comparisons
Previous meta-analyses have revealed reduced GABA concentrations in depression when considering voxels from across the whole brain(25,41). When focusing on the frontal cortex, Schur et al.(25), found no evidence for abnormal GABA concentrations in depression, while Romeo et al. (39) revealed significantly reduced GABA. Godfrey et al.(41) found significantly reduced GABA in an analysis of ACC GABA concentration, comprised of voxels in the ventromedial prefrontal cortex and pregenual ACC, (which were included in our rostral MFC subregion), While the studies included in Godfrey et al.’s(41) analysis of GABA were a subsection of those included in our investigation of rostral MFC, our finding of reduced GABA in the rostral MFC was suggestive of a GABA deficit, although the failure to survive correction for multiple comparisons suggests caution around any definitive conclusions. Interestingly, when we conducted a meta-analysis which included voxels from across all MFC subregions, we found evidence to suggest reduced GABA concentrations in depression, however, the effect size was smaller than that for rostral MFC voxels alone, suggesting there is indeed merit in considering these subregions separately.
Heterogeneity of findings
We aimed to reduce heterogeneity by pooling effects from overlapping voxels placed in small, more functionally homogeneous regions and performing subgroup analyses based on illness duration in schizophrenia and current symptom profile in depression. We succeeded in uncovering significant findings of GABA concentration across the various psychiatric conditions, but we still found evidence for moderate to large amounts of between-study heterogeneity in some of the meta-analyses we conducted, although it appeared that heterogeneity in significant subregions tended to be slightly lower than when we included all subregions in the same analysis. This heterogeneity likely reflects several factors, including differences in patient sample characteristics, alongside differences in the methodological characteristics of data collection. While analyses of the mid- and posterior-MFC in schizophrenia yielded low heterogeneity and significant group effects, other voxels, such as the rostral-MFC in schizophrenia, still exhibited high heterogeneity. MR spectra are affected by magnetic field inhomogeneities and susceptibility artifacts, and it is difficult to obtain good data in voxels that are close to bone or air-filled sinuses, which may affect some regions, such as the rostral MFC, more than others, a fact that may have accounted for heterogeneous findings.
Another source of heterogeneity could be different medication status across samples. While it is likely that the current medication status of an individual has an impact on GABA concentrations, we did not examine this directly in the present study. For bipolar disorder and UHR individuals, there were not enough eligible studies per region to perform any kind of subgroup analysis. Of the studies of depression which met our inclusion criteria, the vast majority (25 of 27 datasets) were comprised of unmedicated participants or samples which were of mixed medication status, meaning medication status could not be meaningfully probed. In schizophrenia, we opted to perform sub-analyses of acute and chronic schizophrenia. This sub-grouping does not map directly onto a currently medicated vs unmedicated division but investigating different illness stages does give some idea of the impact of prolonged medication use.
Limitations
Due to the limited number of studies available, particularly of bipolar disorder and UHR individuals, we were unable to perform meta-analyses examining GABA concentrations in some regions of the MFC, in some of our clinical populations of interest. Where we were able to perform meta-analyses, some contained only three or four datasets, and many studies that were included had small participant sample sizes. It is likely that some of these analyses were low or underpowered, which can lead to inflated effect sizes and reduce the likelihood that significant results are true effects(104). While we found no evidence of publication bias, i.e., the tendency for studies with positive findings to be more likely to be published, it is possible that the pooled effects from our meta-analyses are inflated. Caution should be exercised when evaluating the results of some analyses we present which are comprised of these small samples. Care should be taken when comparing effects from these smaller analyses, with those for which we were able to identify and include a greater number of samples.
A further limitation is that we were unable to investigate the impact of medication on GABA concentrations. Sample characteristics prevented us from exploring his important issue, however, previous evidence suggests that anti-psychotic medications lead to changes in GABA concentrations, and typical and atypical antipsychotics may perhaps have differing effects(22).
Classification of voxels into the appropriate subregions was performed based on figures included in the articles and written descriptions if figures were not included. Classifications were reviewed, and re-classified if necessary. Despite our best efforts, classifications were somewhat subjective, and accuracy depended on the example image or description provided.
Conclusion
The present study utilizes several 1H-MRS meta-analyses to reveal medial frontal GABA alterations in psychosis spectrum disorders and suggests an avenue for clarifying inconsistencies in the literature. While more studies are required to fully explore the sub-regions of the MFC in bipolar disorder and UHR individuals, these results suggest abnormal GABAergic transmission in psychosis spectrum disorders and support the role of the GABA system in the pathophysiology of these disorders.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference |
|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. |
| Software; Algorithm | R version 4.1.1 | https://cran.r-project.org/ |
| Software; Algorithm | metafor v 3.4–0 | https://cran.r-project.org/web/packages/metafor/index.html |
| Software; Algorithm | web plot digitizer | https://automeris.io/WebPlotDigitizer/ |
ACKNOWLEDGEMENTS
SFT is supported by R01MH118634-01 from NIMH
Footnotes
DISCLOSURES
S.F.T. has received contracted research support from Boehringer-Ingelheim, and he receives consulting fees for membership on scientific advisory boards for NIH- and privately funded projects. M.S., and C.J.S report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lewis DA, Hashimoto T, Volk DW (2005, April): Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience, vol. 6. Nature Publishing Group, pp 312–324. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. (2003): Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 23: 6315–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA (2000): Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical γ-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 57: 237–245. [DOI] [PubMed] [Google Scholar]
- 4.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jones EG (1995): Gene Expression for Glutamic Acid Decarboxylase is Reduced without Loss of Neurons in Prefrontal Cortex of Schizophrenics. Arch Gen Psychiatry 52: 258–266. [DOI] [PubMed] [Google Scholar]
- 5.Thompson M, Weickert CS, Wyatt E, Webster MJ (2009): Decreased glutamic acid decarboxylase67 mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res 43: 970–977. [DOI] [PubMed] [Google Scholar]
- 6.Guidott A, Auta J, Davis JM, Gerevini VD, Dwived Y, Grayson DR, et al. (2000): Decrease in Reelin and Glutamic Acid Decarboxylase67 (GAD67) Expression in Schizophrenia and Bipolar Disorder: A Postmortem Brain Study. Arch Gen Psychiatry 57: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 7.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB (2005): Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. 57: 252–260. [DOI] [PubMed] [Google Scholar]
- 8.Woo TUW, Kim AM, Viscidi E (2008): Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. 1218: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karolewicz B, MacIag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G (2010): Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 13: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petty F (1994): Plasma concentrations of γ-aminobutyric acid (GABA) and mood disorders: A blood test for manic depressive disease? Clinical Chemistry, vol. 40 40: 296–302. [PubMed] [Google Scholar]
- 11.Petty F, Kramer GL, Fulton M, Davis L, Rush AJ (1995): Stability of plasma GABA at four-year follow-up in patients with primary unipolar depression. Biol Psychiatry 37: 806–810. [DOI] [PubMed] [Google Scholar]
- 12.Gold BI, Bowers MB, Roth RH, Sweeney DW (1980): GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry 137: 362–364. [DOI] [PubMed] [Google Scholar]
- 13.Gerner RH, Hare TA (1981): CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am J Psychiatry 138: 1098–1101. [DOI] [PubMed] [Google Scholar]
- 14.Kasa K, Otsuki S, Yamamoto M, Sato M, Kuroda H, Ogawa N (1982): Cerebrospinal fluid γ-aminobutyric acid and homovanillic acid in depressive disorders. Biol Psychiatry 17: 877–883. [PubMed] [Google Scholar]
- 15.Fee C, Banasr M, Sibille E (2017, October 15): Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biological Psychiatry, vol. 82. Elsevier, pp 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mescher M, Tannus A, O’Neil Johnson M, Garwood M (1996): Solvent suppression using selective echo dephasing. J Magn Reson - Ser A 123: 226–229. [Google Scholar]
- 17.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R (1998): Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11: 266–272. [DOI] [PubMed] [Google Scholar]
- 18.Öngür D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF (2010): Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry 68: 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. (2012): Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69: 449–459. [DOI] [PubMed] [Google Scholar]
- 20.Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, et al. (2013): In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull 39: 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelemen O, Kiss I, Benedek G, Kéri S (2013): Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: The potential impact of GABA concentration in the visual cortex. Prog Neuro-Psychopharmacology Biol Psychiatry 47: 13–19. [DOI] [PubMed] [Google Scholar]
- 22.Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, et al. (2010): GABA concentration in schizophrenia patients and the effects of antipsychotic medication: A proton magnetic resonance spectroscopy study. Schizophr Res 117: 83–91. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, Vajawat B, Rao NP (2021): Frontal GABA in schizophrenia: A meta-analysis of 1H-MRS studies. World J Biol Psychiatry 22: 1–13. [DOI] [PubMed] [Google Scholar]
- 24.Nakahara T, Tsugawa S, Noda Y, Ueno F, Honda S, Kinjo M, et al. (2021): Glutamatergic and GABAergic metabolite levels in schizophrenia-spectrum disorders: a meta-analysis of 1H-magnetic resonance spectroscopy studies. Molecular Psychiatry. 10.1038/s41380-021-01297-6 [DOI] [PubMed] [Google Scholar]
- 25.Schür RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MGJC, Joëls M, et al. (2016, September 1): Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of 1H-MRS studies. Human Brain Mapping, vol. 37. John Wiley and Sons Inc., pp 3337–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sydnor VJ, Roalf DR (2020): A meta-analysis of ultra-high field glutamate, glutamine, GABA and glutathione 1HMRS in psychosis: Implications for studies of psychosis risk. Schizophr Res 226: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egerton A, Modinos G, Ferrera D, McGuire P (2017): Neuroimaging studies of GABA in schizophrenia: A systematic review with meta-analysis. Translational Psychiatry, vol. 7. 10.1038/tp.2017.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, Matthews PM, Cowen PJ (2008): Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol 11: 255–260. [DOI] [PubMed] [Google Scholar]
- 29.Song XM, Hu XW, Li Z, Gao Y, Ju X, Liu DY, et al. (2021): Reduction of higher-order occipital GABA and impaired visual perception in acute major depressive disorder. Mol Psychiatry 2021 2611 26: 6747–6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. (2009): Amino Acid Neurotransmitters Assessed by Proton Magnetic Resonance Spectroscopy: Relationship to Treatment Resistance in Major Depressive Disorder. Biol Psychiatry 65: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdallah CG, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL, et al. (2014): Glutamate Metabolism in Major Depressive Disorder. Am J Psychiatry 171: 1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, et al. (2009): The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry 66: 478–486. [DOI] [PubMed] [Google Scholar]
- 33.Shaw A, Brealy J, Richardson H, Muthukumaraswamy SD, Edden RA, John Evans C, et al. (2013): Marked Reductions in Visual Evoked Responses But Not γ-Aminobutyric Acid Concentrations or γ-Band Measures in Remitted Depression. Biol Psychiatry 73: 691–698. [DOI] [PubMed] [Google Scholar]
- 34.Boy F, Evans CJ, Edden RAE, Singh KD, Husain M, Sumner P (2010): Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol 20: 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grachev ID, Vania Apkarian A (2001): Aging alters regional multichemical profile of the human brain: An in vivo 1H-MRS study of young versus middle-aged subjects. J Neurochem 76: 582–593. [DOI] [PubMed] [Google Scholar]
- 36.Greenhouse I, Noah S, Maddock RJ, Ivry RB (2016): Individual differences in GABA content are reliable but are not uniform across the human cortex. Neuroimage 139: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T (2016): Large-Scale Meta-Analysis of Human Medial Frontal Cortex Reveals Tripartite Functional Organization. J Neurosci 36: 6553–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor SF, Tso IF (2015, September 1): GABA abnormalities in schizophrenia: A methodological review of in vivo studies. Schizophrenia Research, vol. 167. Elsevier B.V., pp 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romeo B, Choucha W, Fossati P, Rotge JY (2018): Meta-analysis of central and peripheral γ-aminobutyric acid levels in patients with unipolar and bipolar depression. J Psychiatry Neurosci 43: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scotti-Muzzi E, Umla-Runge K, Soeiro-de-Souza MG (2021): Anterior cingulate cortex neurometabolites in bipolar disorder are influenced by mood state and medication: A meta-analysis of 1H-MRS studies. European Neuropsychopharmacology, vol. 47. pp 62–73. [DOI] [PubMed] [Google Scholar]
- 41.Godfrey KEM, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD (2018): Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. Journal of Psychiatric Research, vol. 105. pp 33–44. [DOI] [PubMed] [Google Scholar]
- 42.Wenneberg C, Glenthøj BY, Hjorthøj C, Buchardt Zingenberg FJ, Glenthøj LB, Rostrup E, et al. (2020): Cerebral glutamate and GABA levels in high-risk of psychosis states: A focused review and meta-analysis of 1H-MRS studies. Schizophrenia Research, vol. 215. pp 38–48. [DOI] [PubMed] [Google Scholar]
- 43.Conn VS, Valentine JC, Cooper HM, Rantz MJ, VS C, JC V, et al. (2003): Grey literature in meta-analyses. Nursing Research, vol. 52. Nurs Res, pp 256–261. [DOI] [PubMed] [Google Scholar]
- 44.Viechtbauer W (2010): Conducting meta-analyses in R with the metafor. J Stat Softw 36: 1–48. [Google Scholar]
- 45.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J (2006): Assessing heterogeneity in meta-analysis: Q statistic or I 2 Index? Psychol Methods 11: 193–206. [DOI] [PubMed] [Google Scholar]
- 46.Eggers HC, Lipa P, Buschbeck B (1997): Sensitive test for models of bose-einstein correlations. Phys Rev Lett 79: 197–200. [Google Scholar]
- 47.Cen H, Xu J, Yang Z, Mei L, Chen T, Zhuo K, et al. (2020): Neurochemical and brain functional changes in the ventromedial prefrontal cortex of first-episode psychosis patients: A combined functional magnetic resonance imaging—proton magnetic resonance spectroscopy study. Aust N Z J Psychiatry 54: 519–527. [DOI] [PubMed] [Google Scholar]
- 48.Chen T, Wang Y, Zhang J, Wang Z, Xu J, Li Y, et al. (2017): Abnormal Concentration of GABA and Glutamate in The Prefrontal Cortex in Schizophrenia.-An in Vivo 1H-MRS Study. Shanghai Arch Psychiatry 29: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiu PW, Lui SSY, Hung KSY, Chan RCK, Chan Q, Sham PC, et al. (2018): In vivo gamma-aminobutyric acid and glutamate levels in people with first-episode schizophrenia: A proton magnetic resonance spectroscopy study. Schizophr Res 193: 295–303. [DOI] [PubMed] [Google Scholar]
- 50.de la Fuente-Sandova C, Reyes-Madriga F, Mao X, León-Ortiz P, Rodríguez-Mayoral O, Jung-Cook H, et al. (2018): Prefrontal and Striatal Gamma-Aminobutyric Acid Levels and the Effect of Antipsychotic Treatment in First-Episode Psychosis Patients. Biol Psychiatry 83: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ragland JD, Maddock RJ, Hurtado MY, Tanase C, Lesh TA, Niendam TA, et al. (2020): Disrupted GABAergic facilitation of working memory performance in people with schizophrenia. NeuroImage Clin 25. 10.1016/j.nicl.2019.102127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang JJ, Wang JJ, Tang Y, Zhang T, Cui H, Xu L, et al. (2016): Reduced γ-Aminobutyric Acid and Glutamate+Glutamine Levels in Drug-Naïve Patients with First-Episode Schizophrenia but Not in Those at Ultrahigh Risk. Neural Plast 2016. 10.1155/2016/3915703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia M, Wang J, Sheng J, Tang Y, Li C, Lim K, et al. (2018): Effect of Electroconvulsive Therapy on Medial Prefrontal γ-Aminobutyric Acid among Schizophrenia Patients: A Proton Magnetic Resonance Spectroscopy Study. J ECT 34: 227–232. [DOI] [PubMed] [Google Scholar]
- 54.Yang Z, Zhu Y, Song Z, Mei L, Zhang J, Chen T, et al. (2015): Comparison of the density of gamma-aminobutyric acid in the ventromedial prefrontal cortex of patients with first-episode psychosis and healthy controls. Shanghai Arch Psychiatry 27: 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marsman A, Mandl RCW, Klomp DWJ, Bohlken MM, Boer VO, Andreychenko A, et al. (2014): GABA and glutamate in schizophrenia: A 7 T 1H-MRS study. NeuroImage Clin 6: 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bojesen KB, Ebdrup BH, Jessen K, Sigvard A, Tangmose K, Edden RAE, et al. (2020): Treatment response after 6 and 26 weeks is related to baseline glutamate and GABA levels in antipsychotic-naïve patients with psychosis. Psychol Med 50: 2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandt AS, Unschuld PG, Pradhan S, Lim IAL, Churchill G, Harris AD, et al. (2016): Age-related changes in anterior cingulate cortex glutamate in schizophrenia: A 1H MRS Study at 7 Tesla. Schizophr Res 172: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, et al. (2009): Reduction of brain γ-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophrenia Research, vol. 112. pp 192–193. [DOI] [PubMed] [Google Scholar]
- 59.Reid MA, Salibi N, White DM, Gawne TJ, Denney TS, Lahti AC (2019): 7T Proton Magnetic Resonance Spectroscopy of the Anterior Cingulate Cortex in First-Episode Schizophrenia. Schizophr Bull 45: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, et al. (2016): Medial frontal GABA is lower in older schizophrenia: A MEGA-PRESS with macromolecule suppression study. Mol Psychiatry 21: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wijtenburg SA, Wang M, Korenic SA, Chen S, Barker PB, Rowland LM (2021): Metabolite Alterations in Adults With Schizophrenia, First Degree Relatives, and Healthy Controls: A Multi-Region 7T MRS Study. Front Psychiatry 12. 10.3389/fpsyt.2021.656459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hjelmervik H, Craven AR, Sinceviciute I, Johnsen E, Kompus K, Bless JJ, et al. (2020): Intra-Regional Glu-GABA vs Inter-Regional Glu-Glu Imbalance: A 1H-MRS Study of the Neurochemistry of Auditory Verbal Hallucinations in Schizophrenia. Schizophr Bull 46: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marenco S, Meyer C, Kuo S, Van Der Veen JW, Shen J, DeJong K, et al. (2016): Prefrontal GABA levels measured with magnetic resonance spectroscopy in patients with psychosis and unaffected siblings. American Journal of Psychiatry, vol. 173 173: 527–534. [DOI] [PubMed] [Google Scholar]
- 64.Simmonite M, Taylor SF (2021): GABA concentrations in first episode psychosis and attenuated psychosis syndrome. Manuscr Prep. [Google Scholar]
- 65.De La Fuente-Sandoval C, Reyes-Madrigal F, Mao X, León-Ortiz P, Rodríguez-Mayoral O, Solís-Vivanco R, et al. (2015): Cortico-striatal GABAergic and glutamatergic dysregulations in subjects at ultra-high risk for psychosis investigated with proton magnetic resonance spectroscopy. Int J Neuropsychopharmacol 19: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menschikov PE, Semenova NA, Ublinskiy MV., Akhadov TA, Keshishyan RA, Lebedeva IS, et al. (2016): 1H-MRS and MEGA-PRESS pulse sequence in the study of balance of inhibitory and excitatory neurotransmitters in the human brain of ultra-high risk of schizophrenia patients. Dokl Biochem Biophys 468: 168–172. [DOI] [PubMed] [Google Scholar]
- 67.Wenneberg C, Glenthøj BY, Glenthøj LB, Fagerlund B, Krakauer K, Kristensen TD, et al. (2020): Baseline measures of cerebral glutamate and GABA levels in individuals at ultrahigh risk for psychosis: Implications for clinical outcome after 12 months. Eur Psychiatry 63. 10.1192/j.eurpsy.2020.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Da Silva T, Hafiz S, Rusjan PM, Houle S, Wilson AA, Prce I, et al. (2019): GABA levels and TSPO expression in people at clinical high risk for psychosis and healthy volunteers: A PET-MRS study. J Psychiatry Neurosci 44: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modinos G, Şimşek F, Azis M, Bossong M, Bonoldi I, Samson C, et al. (2018): Prefrontal GABA levels, hippocampal resting perfusion and the risk of psychosis. Neuropsychopharmacology 43: 2652–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brennan BP, Admon R, Perriello C, LaFlamme EM, Athey AJ, Pizzagalli DA, et al. (2017): Acute change in anterior cingulate cortex GABA, but not glutamine/glutamate, mediates antidepressant response to citalopram. Psychiatry Res - Neuroimaging 269: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. (2012): Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: Relationship to anhedonia. Arch Gen Psychiatry 69: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hasler G, Neumeister A, Van Der Veen JW, Tumonis T, Bain EE, Shen J, et al. (2005): Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry 58: 969–973. [DOI] [PubMed] [Google Scholar]
- 73.Hasler G, Van Der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC (2007): Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64: 193–200. [DOI] [PubMed] [Google Scholar]
- 74.Ironside M, Moser AD, Holsen LM, Zuo CS, Du F, Perlo S, et al. (2021): Reductions in rostral anterior cingulate GABA are associated with stress circuitry in females with major depression: a multimodal imaging investigation. Neuropsychopharmacology 46: 2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kantrowitz JT, Dong Z, Milak MS, Rashid R, Kegeles LS, Javitt DC, et al. (2021): Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl Psychiatry 11. 10.1038/s41398-021-01541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Z, Zhang A, Zhao B, Gan J, Wang G, Gao F, et al. (2016): GABA+ levels in postmenopausal women with mild-to-moderate depression A preliminary study. Med (United States) 95. 10.1097/MD.0000000000004918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z, Fan Q, Bai Y, Wang Z, Zhang H, Xiao Z (2016): Brain gamma-aminobutyric acid (GABA) concentration of the prefrontal lobe in unmedicated patients with Obsessivecompulsive disorder: a research of magnetic resonance spectroscopy. Shanghai Arch Psychiatry 28: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, Shaffer SA, Villamarin V, Tan Y, et al. (2019): Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology 44: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Draganov M, Vives-Gilabert Y, de Diego-Adeliño J, Vicent-Gil M, Puigdemont D, Portella MJ (2020): Glutamatergic and GABA-ergic abnormalities in First-episode depression. A 1-year follow-up 1H-MR spectroscopic study. J Affect Disord 266: 572–577. [DOI] [PubMed] [Google Scholar]
- 80.Knudsen MK, Near J, Blicher AB, Videbech P, Blicher JU (2019): Magnetic resonance (MR) spectroscopic measurement of γ-aminobutyric acid (GABA) in major depression before and after electroconvulsive therapy. Acta Neuropsychiatr 31: 17–26. [DOI] [PubMed] [Google Scholar]
- 81.Baeken C, Lefaucheur JP, Van Schuerbeek P (2017): The impact of accelerated high frequency rTMS on brain neurochemicals in treatment-resistant depression: Insights from 1H MR spectroscopy. Clin Neurophysiol 128: 1664–1672. [DOI] [PubMed] [Google Scholar]
- 82.Persson J, Wall A, Weis J, Gingnell M, Antoni G, Lubberink M, Bodén R (2021): Inhibitory and excitatory neurotransmitter systems in depressed and healthy: A positron emission tomography and magnetic resonance spectroscopy study. Psychiatry Res - Neuroimaging 315. 10.1016/j.pscychresns.2021.111327 [DOI] [PubMed] [Google Scholar]
- 83.Smith GS, Oeltzschner G, Gould NF, Leoutsakos JMS, Nassery N, Joo JH, et al. (2021): Neurotransmitters and Neurometabolites in Late-Life Depression: A Preliminary Magnetic Resonance Spectroscopy Study at 7T. J Affect Disord 279: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benson KL, Bottary R, Schoerning L, Baer L, Gonenc A, Eric Jensen J, Winkelman JW (2020): 1H MRS Measurement of Cortical GABA and Glutamate in Primary Insomnia and Major Depressive Disorder: Relationship to Sleep Quality and Depression Severity. J Affect Disord 274: 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brady RO, Mccarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, et al. (2013): Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord 15: 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Godlewska BR, Yip SW, Near J, Goodwin GM, Cowen PJ (2014): Cortical glutathione levels in young people with bipolar disorder: A pilot study using magnetic resonance spectroscopy. Psychopharmacology (Berl) 231: 327–332. [DOI] [PubMed] [Google Scholar]
- 87.Wang PW, Sailasuta N, Chandler RA, Ketter TA (2006): Magnetic resonance spectroscopic measurement of cerebral gamma-aminobutyric acid concentrations in patients with bipolar disorders. Acta Neuropsychiatr 18: 120–126. [DOI] [PubMed] [Google Scholar]
- 88.Huber RS, Kondo DG, Shi XF, Prescot AP, Clark E, Renshaw PF, Yurgelun-Todd DA (2018): Relationship of executive functioning deficits to N-acetyl aspartate (NAA) and gamma-aminobutyric acid (GABA) in youth with bipolar disorder. J Affect Disord 225: 71–78. [DOI] [PubMed] [Google Scholar]
- 89.Prisciandaro JJ, Tolliver BK, Prescot AP, Brenner HM, Renshaw PF, Brown TR, Anton RF (2017): Unique prefrontal GABA and glutamate disturbances in co-occurring bipolar disorder and alcohol dependence. Transl Psychiatry 7. 10.1038/tp.2017.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soeiro-de-Souza MG, Henning A, Machado-Vieira R, Moreno RA, Pastorello BF, da Costa Leite C, et al. (2015): Anterior cingulate Glutamate-Glutamine cycle metabolites are altered in euthymic bipolar I disorder. Eur Neuropsychopharmacol 25: 2221–2229. [DOI] [PubMed] [Google Scholar]
- 91.Pomarol-Clotet E, Canales-Rodríguez EJ, Salvador R, Sarró S, Gomar JJ, Vila F, et al. (2010): Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry 15: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004, October 15): The role of the medial frontal cortex in cognitive control. Science, vol. 306. Science, pp 443–447. [DOI] [PubMed] [Google Scholar]
- 93.Taylor SF, Liberzon I (2007): Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci 11: 413–418. [DOI] [PubMed] [Google Scholar]
- 94.Waugh C, Lemus M, … IG-C and A, 2014. U (n.d.): The role of the medial frontal cortex in the maintenance of emotional states. academic.oup.com. Retrieved February 1, 2022, from https://academic.oup.com/scan/article-abstract/9/12/2001/1619973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luu P, Flaisch T, Tucker DM (2000): Medial frontal cortex in action monitoring. J Neurosci 20: 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM (2004): Action sets and decisions in the medial frontal cortex. Trends in Cognitive Sciences, vol. 8. pp 410–417. [DOI] [PubMed] [Google Scholar]
- 97.Woo TUW, Walsh JP, Benes FM (2004): Density of Glutamic Acid Decarboxylase 67 Messenger RNA–ContainingNeurons That Express the N-Methyl-D-AspartateReceptor Subunit NR2A in the Anterior Cingulate Cortex in Schizophreniaand Bipolar Disorder. Arch Gen Psychiatry 61: 649–657. [DOI] [PubMed] [Google Scholar]
- 98.Barch DM, Ceaser A (2012, January 1): Cognition in schizophrenia: Core psychological and neural mechanisms. Trends in Cognitive Sciences, vol. 16. Elsevier Current Trends, pp 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009): Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petty F, Sherman AD (1984): Plasma GABA levels in psychiatric illness. J Affect Disord 6: 131–138. [DOI] [PubMed] [Google Scholar]
- 101.Petty F, Schlesser MA (1981): Plasma GABA in affective illness: A preliminary investigation. J Affect Disord 3: 339–343. [DOI] [PubMed] [Google Scholar]
- 102.Perrine SA, Ghoddoussi F, Michaels MS, Sheikh IS, McKelvey G, Galloway MP (2014): Ketamine reverses stress-induced depression-like behavior and increased GABA levels in the anterior cingulate: An 11.7T 1H-MRS study in rats. Prog Neuro-Psychopharmacology Biol Psychiatry 51: 9–15. [DOI] [PubMed] [Google Scholar]
- 103.Rodriguez CI, Kegeles LS, Levinson A, Ogden RT, Mao X, Milak MS, et al. (2015): In vivo effects of ketamine on glutamate-glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder: Proof of concept. Psychiatry Res - Neuroimaging 233: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR (2013): Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14: 365–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.