In the threatening world of dog eat dog, or rather, fish eat fish, marine animals have adopted many strategies for protection against predators (25). The Hawaiian squid Euprymna scolopes is no exception. When startled, this small, shallow-water invertebrate rapidly swims away while releasing a blob of ink, meant to mimic the form of its body, to fool and detain its would-be predator. In addition, E. scolopes hosts a symbiotic colony of the bioluminescent bacterium Vibrio fischeri as an even more sophisticated antipredation measure. During its nocturnal feeding period, the squid emits light downward and modulates it to match the intensity of moonlight, thus preventing the formation of a tell-tale shadow on the ocean floor below.
Maintenance of this bioluminescent bacterial partner is therefore important for survival of the squid, which has evolved a special organ in the center of its body cavity for promoting growth of the bacteria and controlling the emission of light (24). A monospecific culture of V. fischeri inhabits this light-emitting organ (3, 4), or light organ, at a cell density upwards of 1011 cells per cm3 (31). The bacteria are concentrated in crypts, or small spaces within the light organ, which are lined by columnar epithelial cells. Although the bacterial cells are extracellular, they are in intimate contact with the microvilli of the epithelium (16, 23). This bacterium-containing tissue is flanked by reflector, lens, and ink sac tissues that serve to modulate and direct the bacterial bioluminescence (23).
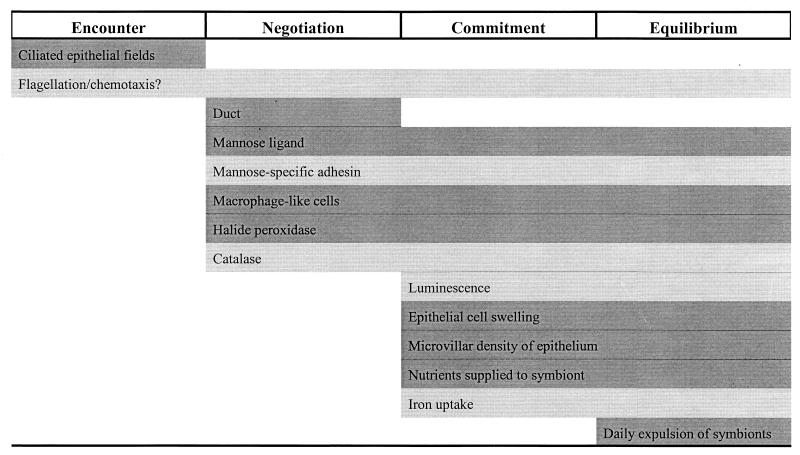
In contrast to the adult light organ, which is exquisitely adapted for the control of luminescence (23), the light organ of a newly hatched juvenile has a completely different morphology (29) and, importantly, contains no bacteria (27, 39). A symbiotic colonization rapidly ensues within hours of hatching, triggering a series of morphological and developmental changes in both organisms that serve to enhance the interaction (16, 28, 33). Specificity in this association is achieved through a reciprocal dialogue between the host and symbiont in a series of stages that ultimately result in the establishment of a stable relationship that endures throughout the lifetime of the host. These stages include the initial encounter between the two organisms, early negotiation for entry and attachment by the correct bacterial species, commitment to the relationship by the partners, and equilibrium associated with a long-term dynamic relationship. To better understand the depth of the challenge associated with achieving specificity in these processes, it is important to grasp the nature of the landscape and the scale over which the process of colonization takes place by putting into context the relationship of the bacterial macroecology to the microecology of the host's body cavity. This minireview seeks to place the role of specificity in the development of this cooperative light organ symbiosis into such a conceptual framework.
STAGE 1. INITIATING A PRODUCTIVE ENCOUNTER
What factors contribute to the ability of the two organisms, V. fischeri and the newly hatched E. scolopes, to find each other? Recent data suggest that mechanisms are in place to promote the initial contact, which requires participation from both organisms. In this section, we will define the scope of the problem by discussing the abundance of V. fischeri in seawater and the rate at which contact occurs between the organisms. We will then describe the surface features of the light organ that promote the encounter and the genetic requirements for the initiation of symbiotic colonization by the bacterium.
The newly hatched E. scolopes encounters an aquatic world teeming with microorganisms. Estimates of bacterial cell numbers in the ocean indicate that there are generally at least 1 million bacteria per ml of seawater. V. fischeri, a common marine bacterium, inhabits the warm coastal waters surrounding the Hawaiian Islands at a concentration of about 0.02 cell per ml, while in the shallow bays inhabited by E. scolopes, the number of culturable V. fischeri cells is 100-fold higher, reaching approximately 2 cells per ml (18). Further analysis of the seawater in the squid habitat under conditions that did not require growth of the V. fischeri cells increased the estimate of this species' abundance to 300 to 700 cells per ml and indicated that V. fischeri cells may enter a cryptically viable state (20). Even at this higher value, however, V. fischeri cells in the environment represent less than 0.1% of the bacterial community. Despite these low numbers of bacteria, not a single aposymbiotic (squid without light organ symbionts) specimen has been noted among the hundreds of E. scolopes of all sizes that have been collected for study (3). This lack of aposymbiotic animals in the field not only underscores the importance of the symbiosis to the biology of the host but also suggests that in nature the symbiosis is established very early in the life history of the squid. This latter conclusion is further supported by laboratory studies of the colonization process: the symbiosis can be experimentally established within the first few hours of hatching, either through inoculation with natural seawater collected from adult squid habitats or by the introduction of cultured V. fischeri cells into V. fischeri-free seawater (32).
The bacterium-containing seawater flushes into and out of the body cavity of E. scolopes as a result of the animal's ventilation (Fig. 1A), a process that both promotes oxygen exchange across the gills and allows the animal to propel itself rapidly through the seawater. In the juvenile, ventilation of approximately 1 to 1.5 μl of fluid occurs at a rate of about three or four times per second (D. Park and M. McFall-Ngai, unpublished data). Thus, given that there are approximately 500 cells of V. fischeri per ml of seawater in the squid's habitat, on average only 1 V. fischeri cell would enter and exit the body cavity every 0.3 second! In this short period of time, a bacterium would have to find one of six 15-μm-wide pores on the surface of the organ to colonize successfully. Perhaps the scale of this challenge is best understood by analogy: the odds of V. fischeri finding the light organ pores is similar to a bee locating, within one second, one of six daisies present in a one-acre field in a strong wind!
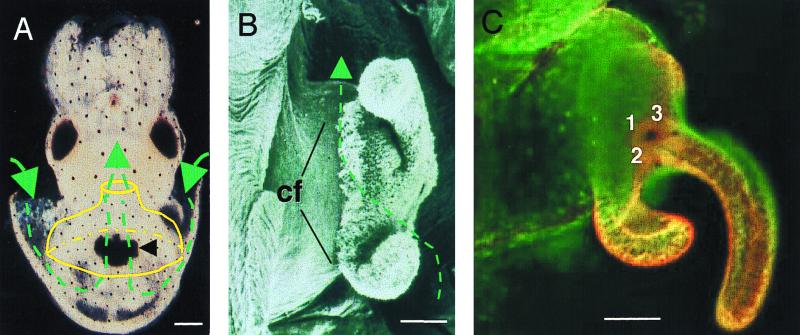
FIG. 1.
Path of V. fischeri to the entrance of the host light organ. (A) Under conditions of anesthesia, the light organ can be seen as a dark mass (black arrow) through the ventral surface of the body wall. During each ventilatory cycle, the body cavity is expanded, water is drawn into the cavity (lateral green arrows) and then the body wall contracts, expelling water out (central green arrow) through the funnel (yellow). Because the light organ is circumscribed by the funnel, the water that is exiting passes over the light organ surface. Bar, 200 μm. (B) A scanning electron micrograph of one half of the ventral surface of a hatching light organ reveals the transparent, complex ciliated fields (cf) on the lateral surfaces of the organ. Water flows across these surfaces as shown by the broken green line and arrow. Bar, 50 μm. (C) A confocal micrograph of the ciliated surface of a living animal demonstrates that the appendages of the field are dynamic, most often forming a ring-like structure lateral to the main body of the light organ. Staining of the light organ with a fluorochrome that delineates the cells reveals the three pores at the base of the appendage, which are labeled 1, 2, and 3 to the left of each pore, designating the locations of the pore leading to the largest, mid-sized, and smallest crypts, respectively. Bar, 50 μm.
The observed frequency of successful colonization in the face of these remarkably poor odds of encounter suggests that mechanisms must exist to increase the probability of successful inoculation of the light organ. Analyses of the morphology of the hatchling light organ provided the first piece of this complex puzzle. During embryogenesis, the nascent light organ develops to the point that it is primed for the interaction with the environmental microbiota that the host encounters upon hatching (29). Specifically, the organ of the hatching has two ciliated epithelial fields, one on each lateral surface of the organ. Each field consists of a layer of cells on the surface of the organ that extends into two long appendages (Fig. 1B). At the base of each set of appendages are three pores (29) (Fig. 1C). Investigation of the activity of these ciliated projections has revealed that they entrain particles in the water surrounding the light organ, but the precise consequences of this activity have not yet been fully elucidated. However, upon successful colonization of the organ by V. fischeri, the ciliated fields are lost (28), an observation that provides evidence that the fields provide a function critical to initiation of the symbiosis and suggests that their sole function is to promote colonization of the host by its specific symbiont. In addition to the clues provided by host morphology, studies of the dynamics of the infection process also suggest that the squid efficiently harvests symbionts from the environment. Seawater containing a newly hatched host and an inoculum of about 1,000 V. fischeri cells became depleted of the introduced bacterial cells within 1 to 2 h (Park and McFall-Ngai, unpublished). These data suggest that there is a high probability that once V. fischeri has entered the body cavity, it will be removed from the seawater and recruited into the symbiosis.
Although E. scolopes actively brings potential symbionts into the vicinity of the colonization site, V. fischeri is not merely a passive bystander in the inoculation process. Neither nonflagellated nor flagellated but nonmotile cells of V. fischeri can initiate colonization (11), indicating that some action is required on the part of the bacterium. Because many bacteria are motile, however, it seems unlikely that this ability by itself contributes much to the specificity of the interaction. The ability of V. fischeri to direct its movement in response to a particular nutrient provided by the squid may be important in specificity, but the contribution of chemotaxis to colonization has not yet been examined. Recently, a V. fischeri mutant that appears to be defective in initiating symbiotic colonization was isolated (K. L. Visick, unpublished data). Because this mutant is not deficient in motility, characterization of this strain may shed light on the genetic basis of specificity in this symbiotic interaction.
STAGE 2. NEGOTIATING A SETTLEMENT
What environmental conditions do V. fischeri cells encounter in the light organ? The challenges presented by colonization do not end at entry into the light organ. In this section, we describe the features of the light organ that may contribute to specificity at the next stage of the association. These features include the duct (the path that the bacteria must traverse to reach their site of colonization), host defense cells, host epithelial cells, and the chemical environment.
The light organ of the newly hatched squid is rapidly colonized exclusively by the V. fischeri cells present in the surrounding seawater (27). Moreover, in the absence of V. fischeri, no other bacteria colonize this space (27), despite the observation that cells of other species enter the crypt spaces (M. F. Claes and P. V. Dunlap, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. N-72, p. 461, 1999; E. V. Stabb and E. G. Ruby, unpublished data). These observations suggest that (i) the light organ environment may include physical and chemical barriers that only V. fischeri can overcome and/or (ii) specific receptor-ligand interactions may be required for the initiation and persistence of the partnership.
Upon entering the light organ through one of the pores, a V. fischeri cell (1 to 2 μm in length) first encounters the environment of the ducts (Fig. 2A). This transition area between the relatively capacious body cavity of the host and the internal crypt space of the light organ is filled with mucus, lined by a densely ciliated epithelium, and covers a distance equivalent to many bacterial cell lengths. The orientation of the ciliary rootlets of the duct epithelial cells, as revealed by transmission electron microscopy (TEM) and the behavior of the fluid in the duct, as viewed by confocal microscopy, indicate that the effective beat of the cilia is outward (26). Thus, upon entering, the bacteria must make their way through the ducts against a current, perhaps by responding to a chemotactic stimulus. Microscopic studies of the duct walls have shown that they are not colonized by bacteria, either because of the activity of the cilia and/or because the chemical environment in the duct is unfavorable. Further studies of the behavior of both specific and nonspecific bacteria in the ducts should reveal whether this portion of the light organ plays a significant role in making the crypts the exclusive domain of V. fischeri.
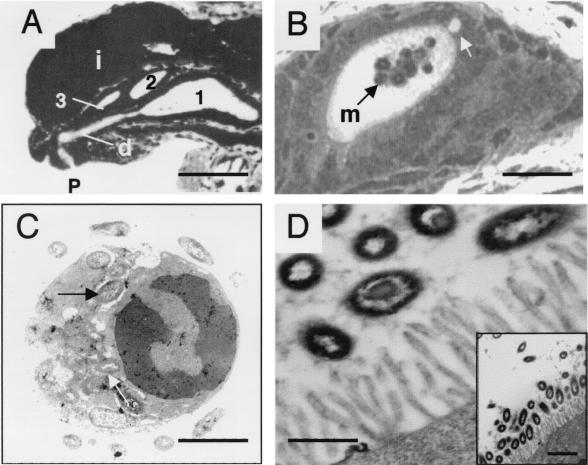
FIG. 2.
Environment of the light organ. (A) A light micrograph of a cross section of a hatchling light organ provides an overview of the tissues that V. fischeri will encounter. Although portions of all three crypts (designated 1, 2, and 3) are visible in this section, the entire path of a colonizing symbiont can be seen only for crypt 1. Here the bacteria enter through the pore (p) and then travel down a lengthy duct (d) and into the crypt space. i, ink sac. Bar, 75 μm. (B) The bacterial symbionts encounter macrophages (indicated by the m and black arrow) within the crypt space. Often disruptions of the epithelium can be observed (white arrow), possible vestiges left by cells that have recently entered the crypt space. Bar, 50 μm. (C) Bacteria that appear to be phagocytosed are depicted in this TEM of a macrophage from a juvenile host. Bar, 5 μm. (D) The symbionts that initially colonize the light organ appear to attach to the microvilli of the host epithelial cells that line the crypts. Views with two magnifications are shown. Bar of the principal micrograph, 1 μm; bar of the insert, 3 μm.
Once the bacteria have navigated the physical hurdle of the ducts, they enter the crypt spaces. There, the bacterial symbionts interact not only with the fluid milieu but also with two cell types: (i) a population of blood cells that act like macrophages (Fig. 2B and C) (31) and (ii) columnar epithelial cells lining the crypts. The behavior of the macrophage-like cells suggests that they may be critical players in the dynamics of the symbiosis. These cells, the principal immune surveillance system of the squid, are produced by the white body (7) (an analogue of the mammalian thymus gland) and released into the bloodstream. The macrophage-like cells appear to enter the light organ crypts by squeezing between the epithelial cells, much like vertebrate white blood cells traverse endothelial cell layers (2). The number of macrophage-like cells in the crypt spaces of the juvenile light organ varies from none to several at any given time. Observations of the behavior of these macrophage-like cells suggest that they may sample the space and convey the information to other parts of the body. For example, when fluorescent dextran particles are introduced into seawater containing a juvenile squid, they enter the light organ and are apparently confined within the crypt spaces, yet hours later, they can be visualized in macrophages located in regions of the squid body remote from the light organ crypts (Park and McFall-Ngai, unpublished).
The macrophage-like cells in the crypt spaces of juvenile squid have also been observed to contain bacteria (Fig. 2C) (31). Thus, these host cells are likely to be responsible for removing any nonspecific interlopers that may enter the crypt space during the early hours of light organ colonization (Claes and Dunlap, Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). However, because the specificity of these cells has not been unequivocally established, we cannot rule out the possibility that they also engulf V. fischeri cells. Indeed, our data indicate that a minimum infective dose of about 500 V. fischeri cells is required to initiate colonization (Park and McFall-Ngai, unpublished), suggesting that the bacteria must overcome some antimicrobial activity of the host, which could be a phagocytic activity, to which the V. fischeri cells are not completely resistant. Interestingly, the macrophages found in the crypts of an adult squid, although surrounded by a dense, monospecific culture of V. fischeri, rarely contain phagocytosed bacteria (S. N. Nyholm and M. J. McFall-Ngai, unpublished data). Whether the developmental biology of the host involves an adaptation of the host immune system, so that it regards V. fischeri as self remains to be determined. However, the persistent presence of macrophage-like cells in the crypts of both the juvenile and adult squids suggests that they protect this special niche from invasion by pathogenic or nonspecific bacteria and/or from overgrowth of the symbionts.
While some V. fischeri cells may have contact with host macrophage cells, the majority of the symbionts in the population are eventually found in intimate association with the microvilli of the crypt epithelial cells (Fig. 2D). The microvilli form a continuous interface, or brush border, between the host cells and the bacterial symbionts. In the first stages of colonization, the founder bacterial cells line the crypt spaces, positioning themselves closely to host epithelial cells. This interaction may be promoted by adhesin-glycan interactions, similar to those of many pathogenic associations, in which bacteria attach to eukaryotic host cells using fimbriae with a mannose-recognizing adhesin at the tip (15, 34). Several lines of evidence support this hypothesis. First, labeling of host cells with specific lectins has revealed that mannose is the most common glycan detected along the membranes of the crypt brush border (22). Second, addition of a mannose analog to seawater containing juvenile E. scolopes and an infective dose of V. fischeri completely inhibited initiation of a symbiotic colonization, while introduction of fucose or a galactose analog had no significant effect (22). Third, V. fischeri cells agglutinate guinea pig red blood cells in a mannose-sensitive reaction, indicating the presence of mannose-recognizing adhesins (22) and mutants that fail to hemagglutinate exhibit a defect in colonization (B. Feliciano and E. Ruby, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. N-75, 1999). Taken together, these data strongly suggest that a receptor-ligand interaction between bacterial mannose adhesins and squid glycans underlies the association of V. fischeri with the crypt microvilli.
In addition to encouraging binding of the correct symbionts by providing surface ligands, the host epithelial cells may deter inappropriate bacteria by secreting an active halide peroxidase (HPO) (37, 40). Evidence suggests that HPO has a bactericidal function similar to its mammalian homolog myeloperoxidase (35, 40), an important component of host defense. These enzymes convert hydrogen peroxide to hypohalous acid, a reactive oxygen species toxic to many bacteria. There is no known mechanism to detoxify hypohalous acid, so one strategy that V. fischeri may use to survive in this unfavorable environment is to remove the substrate of HPO, hydrogen peroxide, using a catalase enzyme. V. fischeri cells growing exponentially in culture express a basal level of catalase activity that is induced three- to fourfold with the addition of a low level of hydrogen peroxide (38). In addition, catalase activity is localized to the periplasm, appropriate for dealing with external sources of hydrogen peroxide. A symbiotic role for catalase was supported by the construction of a catalase mutant, which exhibited a competitive disadvantage in colonization when presented to E. scolopes in a mixed population with the wild type (38). In a single-strain infection, however, the catalase mutant was not noticeably defective in colonization (38), suggesting the possible involvement of other stress proteins, such as those controlled by SoxRS or OxyR (13, 36, 41), in survival of V. fischeri in an environment potentially rich in reactive oxygen species. Alternatively, it is possible that the HPO activity is somehow specifically targeted toward nonsymbionts.
The presence of HPO and phagocytic activity, along with evidence for a specific receptor-ligand interaction, suggests that both the chemical environment of the light organ and cellular contacts are important in providing specificity to the association. Further work must be performed to more clearly determine the exact chemical nature of the light organ environment and additional roles of the host cell types in achieving such an exacting environment where only V. fischeri can flourish.
STAGE 3. UNDERTAKING A COMMITMENT
How is the initial contact between V. fischeri and E. scolopes enhanced? V. fischeri and E. scolopes commit to their relationship through a series of events, including bacterial proliferation and developmental events in both partners, which serve to increase the physical intimacy of the association. At the same time, bioluminescence, an essential consequence of the interaction, is produced. We discuss here these events and what is known about the factors that promote them.
During the first few hours following initial exposure to the symbiont, the bacterial cell population in the light organ increases rapidly, with a doubling time of 20 min, to a cell number of approximately 105 (33). Within 10 to 12 h, the crypt spaces are filled with bacteria, and bacterial growth rate is curtailed to a doubling time of 5.5 h (33). The total volume of the juvenile light organ crypts is about 750 pl (29), resulting in a high density of bacterial cells, approximately 1011 cells per ml of crypt fluid. The rapid growth to such a high cell density indicates the presence of easily obtained nutrients. Two possibilities are currently being considered: (i) the crypt spaces are nutrient rich, and V. fischeri cells, once in the crypts, can avail themselves of this nutrient pool, or (ii) the crypt spaces are devoid of nutrients, a condition that helps inhibit the growth of nonspecific bacteria, and V. fischeri cells are able to induce the host to transport nutrients into the crypt spaces for their use.
Although it is at present difficult to distinguish between these possibilities, the nature of the nutrients provided has been investigated. The results of these studies have suggested that amino acids are a significant source of carbon and nitrogen provided by the squid to the bacterial symbionts. High-pressure liquid chromatography analysis of the crypt contents of adult specimens has demonstrated that the light organ has a high concentration of amino acids, mostly in the form of peptides and/or proteins, that could be utilized as nutrients by the V. fischeri cells. In addition, several amino acid auxotrophs of V. fischeri were shown to achieve relatively high colonization levels and in one case, a level indistinguishable from that achieved by the wild type (12). Host-derived amino acids, however, may not represent the only nutrient source for V. fischeri. This bacterium also produces a proteolytic mucinase that may play dual roles in freeing the bacterium from the sticky mucus filling the ducts and obtaining nutrients for growth (P. M. Fidopiastis and E. G. Ruby, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. N-73, 1999). In addition, as described above, host cell surface molecules are decorated with sugars, specifically mannose, that may provide a source of additional nutrients for V. fischeri. Precedents for such a scenario include experiments showing that the enteric bacteria of mice not only use glycans on the surface of host cells but also induce the host to produce increased levels of these glycans (6).
In addition to sources of carbon and nitrogen, bacteria require sulfur, phosphorus, and trace metals, such as iron. While sulfur in the form of sulfate is abundant in seawater, phosphate and/or iron can be limiting. Because V. fischeri has the unusual ability to grow in culture on cyclic AMP as the sole carbon, nitrogen, and phosphorus source, it has been proposed that a mutant lacking cyclic AMP phosphodiesterase and defective for this activity might be incapable of symbiotic growth (9). This hypothesis is currently under investigation. The importance of iron uptake in the symbiosis has been underscored by the lowered level of colonization exhibited by a glnD mutant of V. fischeri with defective siderophore production and uptake (J. Graf and E. G. Ruby, submitted for publication). Although this gene is a homolog of the E. coli uridyl-removing and uridylytransferase gene important in nitrogen regulation, data suggest that the colonization defect stems from the iron uptake deficiency rather than from an alteration in nitrogen regulation (Graf and Ruby, submitted).
Coincident with rapid colonization, induction of bioluminescence occurs and peaks within 12 h (33). Thus, appearance of the most biologically relevant product of the association, bioluminescence, occurs less than a day after hatching of the juvenile. Although dark mutants commonly arise spontaneously in culture, intriguingly, of the hundreds of analyzed bacterial isolates from the light organs of E. scolopes of all ages, no nonluminescent strains have been found. Luminescence demands about 20% of a cell's metabolic potential (21), suggesting that strong selective pressure must be present to maintain this trait; otherwise, the loss of this capability presumably would confer a distinct competitive advantage on nonluminescent mutants. Consistent with this hypothesis, mutants defective in structural (luxA) or regulatory (luxI and luxR) luminescence genes all exhibit defects in sustaining the wild-type level of colonization within 48 h of the symbiotic association (K. L. Visick, J. A. Doino, J. S. Foster, M. J. McFall-Ngai, and E. G. Ruby, unpublished data). The phenotype of the regulatory mutants likely results from their requirement for expression of the structural genes, although additional roles for these regulators in controlling other genes important in the symbiosis are also possible (S. M. Callahan and P. V. Dunlap, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. H-185, p. 364, 1999). Because luminescence is the raison d'être of the symbiosis from the squid's point of view, it seems likely that a sophisticated and stringent mechanism exists to ensure that only luminescent V. fischeri can establish or continue the symbiotic relationship. Possible mechanisms include a direct sensing of light by the squid and an indirect monitoring of the level of bioluminescence by a biochemical sensing of the oxygen consumption mediated by luciferase.
Within the first hours to days of the symbiotic interaction, an increased intimacy between the bacterial cells and the flanking epithelial cells can be observed, such that nearly every bacterium contacts a host cell, an arrangement that may promote nutrient and/or signal exchange. This increased intimacy results from two bacterium-induced developmental processes: increased microvillar density along the crypt brush border and swelling of the epithelial cells (Fig. 3) (16, 28). The increase in density of the microvilli can be visualized as early as 12 h following the first exposure to V. fischeri and is reversible by curing the light organ of bacteria by antibiotic treatment, demonstrating that persistent interaction with bacteria is required to maintain the dense brush border characteristic of the colonized organ (16). Furthermore, the bacterial signal that induces these changes does not appear to be related to bacterial cell density or to light production. Neither an amino acid auxotroph (10-fold reduction in cell density) nor a luxA mutant (Visick et al., unpublished) (4-fold reduction and nonluminescent) exhibited a defect in stimulating increased microvillar density (16). Host cell swelling occurs within the first 24 h of the symbiosis and also is reversible by antibiotic curing (8). A V. fischeri mutant that fails to induce normal epithelial cell swelling has recently been identified (8). The mechanism by which the genetic defect in this mutant prevents V. fischeri from inducing host cell swelling is currently under investigation.
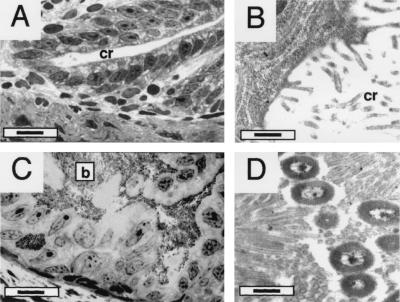
FIG. 3.
Bacterium-induced changes in crypt epithelial cells. (A) A light micrograph of an aposymbiotic juvenile reveals that the epithelial cells lining the crypt space are columnar and vividly stained with histological stains. cr, crypt. Bar, 20 μm. (B) A TEM of the brush border of these aposymbiotic juveniles shows that the apical borders of the epithelial cells have a sparse covering with microvilli. cr, crypt. Bar, 0.5 μm. (C) In contrast, in a low-magnification image of a symbiotic juvenile host, the crypt cells are swollen and stain lightly with histological stains. The bacterial symbionts (b) appear as darkly staining material between the host cells. Bar, 20 μm. (D) A TEM of a 4-day-old symbiotic juvenile shows a dramatically increased density of microvilli along the brush border of the host crypt. The bacterial cell surfaces are nearly entirely flanked by host cell membranes. Bar, 0.5 μm.
The increase in cell contact between the host and the symbiont population is reminiscent of the reinforcement of cell-cell adhesion that occurs during the development of stable contact between eukaryotic cells that comprise animal tissues (10). Underlying these processes is a recruitment of additional cell adhesion molecules to the site of contact. From the behavior of the symbiont cells in relation to host cells, we might expect similar processes to be occurring in the squid-vibrio association. Whether the increase in microvillar density and intimacy of host and symbiont cells involves reinforcement by the recruitment of additional mannose residues to the surface of the host cells and/or the expression of additional mannose-recognizing adhesins on V. fischeri remains to be determined.
STAGE 4. ESTABLISHING A DYNAMIC EQUILIBRIUM
How is a healthy culture of bacteria maintained in the light organ? The V. fischeri-E. scolopes symbiosis is not a static relationship but a dynamic, intimate partnership that lasts many months. In this section, we discuss the morphology and behavior of V. fischeri in the established colonization and the cyclic nature of the symbiotic association.
V. fischeri cells encounter two distinct habitats within the light organ crypts. Early descriptions of the juvenile light organ, which were derived from reconstructions of serial histological sections, had suggested that the crypts are rather simple sacs (29). However, recent analysis using confocal microscopy has revealed that at least the largest and most mature of the three crypts is more complex (Nyholm and McFall-Ngai, unpublished). In this crypt, the duct leads to an expanded space (30 μm) which gives way medially to three, very narrow (10 μm), blind-ended diverticula (Fig. 4). These distinct areas are also reflected in the morphology and behavior of the bacterial symbionts: in the expanded area just inside the duct, the bacteria are larger (approximately 1 by 2 μm) and flagellated, while in the narrow, blind-ended portion of the crypts, they are smaller (approximately 0.5 by 1 μm) and nonflagellated (Nyholm and McFall-Ngai, unpublished) (Fig. 4B). The morphology of V. fischeri cells in the expanded area most closely resembles that of culture-grown bacterial cells. The environmental differences in these regions, the signals to which the bacteria might be responding, and the advantage to the squid or bacteria of the two habitats, or two morphologies, are the subjects of ongoing research.
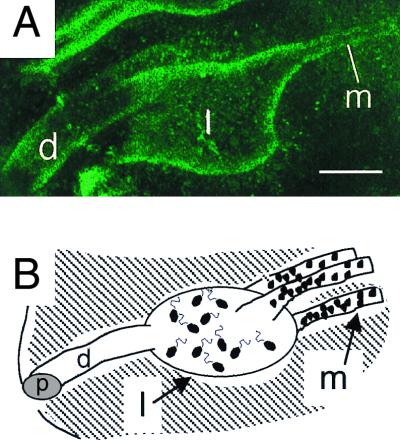
FIG. 4.
Architecture of the host crypt space. (A) A confocal micrograph of the juvenile host crypt, taken using the intrinsic autofluorescence of the tissues, reveals the various regions of the crypt space. The duct (d) leads into a lateral, cavernous space (l), which grades into a narrow, medial set of diverticula (m). Bar, 30 μm. (B) Cartoon of panel A, illustrating the regions of the crypts and the morphology of the bacteria within these regions. While the duct (d) rarely contains symbionts, bacteria occur as larger (1 by 2 μm), motile cells in the lateral, expanded space (l) just inside the duct. In the narrow, medial diverticula (m), the bacteria are more densely packed, smaller, and nonflagellated. p, pore.
Once V. fischeri and E. scolopes have established a relationship, they are challenged to balance the association over the several months that the host lives, such that neither do the symbionts overgrow the host nor does the host completely eliminate the symbiont population. Furthermore, continuous pressure must be exerted to discourage nonspecific bacterial interlopers, because the light organ crypts remain in contact with the environment throughout the lifetime of the host; during development, the three pores on each side of the juvenile organ coalesce into a single pore, and the resulting two pores maintain a conduit between the crypts and the seawater passing through the body cavity of the host.
The nature of this delicate balance remains largely unknown, but the population dynamics of the bacteria in the light organ provide some insight into these issues. Each morning, beginning with the first dawn following colonization of the organ, the squid vents 90% of its crypt contents into the body cavity (Fig. 5A), and the ventilatory movements of the squid carry this material out into the seawater (19, 31). The remaining 5 to 10% of the bacteria multiply and repopulate the light organ over the day. This behavioral pattern is not an entrainable, or circadian, rhythm; the animal responds each morning directly to the cue of an increasing level of ambient light (5, 31). Host-controlled muscular pressure imposed on the crypts results in the release of a thick exudate (Fig. 5B) containing not only bacteria but also macrophages, epithelial cell debris, and the protein-rich matrix of the crypt spaces (12, 31). This expulsion event may serve several functions. For example, this behavior may be a means of controlling symbiont number: while V. fischeri is not considered a pathogen, unchecked bacterial multiplication might harm the host. Also, it may be important for the squid to maintain a fresh culture of V. fischeri cells. V. fischeri cells grown in culture induce luminescence in a density-dependent (autoinducer-dependent) manner (3). Coincident with stationary phase in culture, however, the level of luminescence drops. Furthermore, V. fischeri cells exhibit poor stationary-phase survival in the laboratory. Because bioluminescence is unnecessary in the daylight hours after dawn, during which time the animal is buried in the sand, expulsion of the bacteria may serve to maintain a healthy culture of bacteria that will be ready to bioluminescence the following evening.
FIG. 5.
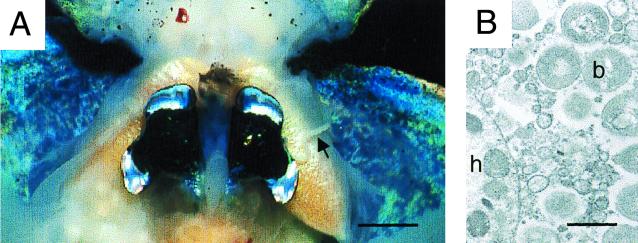
Daily expulsion of crypt contents. (A) A ventral dissection of an adult animal at dawn reveals that the vented crypt matrix is a thick, coherent, string-like mass of material (black arrow). In this animal, only one side of the light organ was caught in the act of venting its crypt contents. Bar, 0.5 cm. (B) An electron micrograph of the vented material demonstrates that it is composed of host cells (h), bacterial cells (b), and numerous membrane bound vesicles and other debris. Bar, 1 μm.
There are two ecological consequences of the daily venting. The first consequence is that the concentration of bacteria in the habitats populated by the host becomes higher than in areas where no E. scolopes are found. Lee and Ruby reported that inoculation of newly hatched juveniles with natural seawater obtained from locations incrementally distant from the squid's natural habitat was increasingly difficult to achieve (20). Thus, the daily venting behavior may serve the important function of seeding the environment for the young. A second, possibly deleterious, consequence is that the now nearly empty light organ may be more susceptible to colonization by outside bacteria: when tagged V. fischeri are introduced into the water surrounding an adult, these V. fischeri can be isolated in low numbers from the crypts, an observation that indicates that the light organ in the mature squid remains accessible to bacteria in the environment (17). However, the rarity of this event suggests that the remnant of the established bacterial population is at an advantage over bacteria in the seawater, either because they have become better adapted or because they are bound to the best sites on the epithelial cell surfaces. The presence of host macrophages and halide peroxidase activity may be a continuing requirement in order to maintain specificity.
EVOLUTION OF SPECIFICITY DETERMINANTS
Specificity in this symbiosis goes deeper than the ability of E. scolopes to distinguish V. fischeri from unrelated bacteria in the seawater. Accumulating evidence suggests that E. scolopes and V. fischeri have coevolved, indicating that they have become uniquely suited for one another. Molecular sequence analysis of related squid species (4 Euprymna spp. and 7 Sepiola spp.) and their bacterial symbionts demonstrated that the phylogenetic trees are congruent between the hosts and symbionts (30). Congruence in host-symbiont phylogenies has been noted in a number of associations (e.g., references 1 and 14), and it has been hypothesized that the congruence signifies coevolution of the host and symbiont. In an experimental test of coevolution, V. fischeri strains were isolated from the light organs of 11 related squid species. When presented alone to E. scolopes, each V. fischeri isolate was able to colonize the juvenile light organ (30). Importantly, when these symbiotic strains were presented in a mixed inoculation with a V. fischeri isolate from E. scolopes, the native E. scolopes symbiont was always able to outcompete bacterial isolates from other Euprymna or Sepiola species. Furthermore, E. scolopes exposed to two nonnative strains of V. fischeri was always colonized by the strain from the host most closely related to E. scolopes (30). Thus, the relative colonization abilities of the V. fischeri isolates mirrored the phylogenetic data. These findings suggest that, in the squid-vibrio symbiosis, there appears to have been incremental change in the specificity determinants over evolutionary time and that these determinants are expressed in the first stages of the symbiosis. Future experiments will determine what bacterial genes contribute to these incremental alterations in specificity.
PERSPECTIVES
The V. fischeri-E. scolopes symbiosis thus offers a complex but accessible puzzle for investigating how specificity is achieved in a monospecific association. At each stage, encounter, negotiation, commitment, and equilibrium, mechanisms are in place to ensure that a productive association is formed only with the correct symbiont (Fig. 6). There exist few environmental conditions that promote complete dominance of a single bacterial species in a niche. Mixtures of bacteria inhabit soil, fresh and salt water, air, and biological surfaces such as in and on animals. Given the richness and diversity of bacteria in seawater, it is surprising that V. fischeri can persist as the sole species inside the squid light organ. Perhaps more surprising is the fact that, in the absence of V. fischeri, no other organism claims the space. Such a pristine environment exists in the bloodstream and internal tissues of animals which, when host defenses are breeched, provide capable pathogenic bacteria with a deluxe habitat, at least temporarily. The progressive steps of colonization in such pathogenic associations resemble the stages that we've described for the light organ symbiosis, with the exception that an equilibrium does not typically occur—usually, either the bacteria are eliminated or the host succumbs to the infection. Long-term, chronic pathogenic associations, such as tuberculosis or Pseudomonas aeruginosa infections of cystic fibrosis patients, do occur and must result from some compromise between the host and the invading pathogen. Understanding how V. fischeri and E. scolopes ultimately arrive at and sustain a persistent relationship may yield insight into treatment of such chronic illnesses.
FIG. 6.
Proposed scheme illustrating where, in the progression of colonization, several bacterial and host specificity determinants operate. The proposed timing and duration of each specificity determinant with respect to the stages of colonization are indicated by the placement of the shaded bars (dark gray, E. scolopes determinants; light gray, V. fischeri determinants).
The similarities between pathogenic and cooperative colonization processes predict that colonization, regardless of the outcome to the health of the host, requires a reciprocal communication between bacteria and their hosts. Investigation of these processes is perhaps simplified in a model system in which one bacterium communicates with one host, although even such a system is not truly simple, as discussed here. With the descriptions of the structural landscape and developmental processes of the E. scolopes host providing a foundation, we anticipate that the continued isolation and characterization of V. fischeri mutants defective for each of these stages of symbiotic association will yield a more thorough understanding of the mechanisms necessary to achieve such a high host-symbiont specificity.
ACKNOWLEDGMENTS
We are grateful to Ned Ruby, Seana Davidson, and Jon Visick for their thoughtful insights and critical review of this manuscript. We thank Spencer Nyholm and Jamie Foster for providing confocal microscopy images and Spencer Nyholm for communicating results in advance of publication.
This work was supported in part by NSF grant IBN9904601 and NIH grant R01 RR10926, shared by M.J.M.-N. and E. G. Ruby.
REFERENCES
- 1.Baumann P, Moran N A, Baumann L. The evolution and genetics of aphid endosymbionts. BioScience. 1997;47:12–20. [Google Scholar]
- 2.Bianchi E, Bender J R, Blasi F, Pardi R. Through and beyond the wall: late steps in leukocyte transendothelial migration. Immunol Today. 1997;18:586–591. doi: 10.1016/s0167-5699(97)01162-6. [DOI] [PubMed] [Google Scholar]
- 3.Boettcher K J, Ruby E G. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boettcher K J, Ruby E G. Occurrence of plasmid DNA in the sepiolid squid symbiont Vibrio fischeri. Curr Microbiol. 1994;29:279–286. [Google Scholar]
- 5.Boettcher K J, Ruby E G, McFall-Ngai M J. Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythm. J Comp Physiol. 1996;179:65–73. [Google Scholar]
- 6.Bry L, Falk P G, Midtvedt T, Gordon J I. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273:1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 7.Cowden R R, Curtis S K. Invertebrate blood cells. In: Ratcliffe N A, Rowley A F, editors. Cephalopods. London, United Kingdom: Academic Press; 1981. pp. 301–323. [Google Scholar]
- 8.Doino J A. The role of light organ symbionts in signaling early morphological and biochemical events in the sepiolid squid Euprymna scolopes. Ph.D. thesis. Los Angeles: University of Southern California; 1998. [Google Scholar]
- 9.Dunlap P V, Callahan S M. Characterization of a periplasmic 3′:5′-cyclic nucleotide phosphodiesterase gene, cpdP, from the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1993;175:4615–4624. doi: 10.1128/jb.175.15.4615-4624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelman G M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- 11.Graf J, Dunlap P V, Ruby E G. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J Bacteriol. 1994;176:6986–6991. doi: 10.1128/jb.176.22.6986-6991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graf J, Ruby E G. Host-derived amino acids support the proliferation of symbiotic bacteria. Proc Natl Acad Sci USA. 1998;95:1818–1822. doi: 10.1073/pnas.95.4.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg J T, Monach P, Chou J H, Josephy P D, Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinkle G, Wetterer J K, Schultz T R, Sogin M L. Phylogeny of the attine ant fungi based on analysis of small subunit rRNA gene sequence. Science. 1994;266:1695–1697. doi: 10.1126/science.7992052. [DOI] [PubMed] [Google Scholar]
- 15.Krogfelt K A, Bergmans H, Klemm P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect Immun. 1990;57:3364–3371. doi: 10.1128/iai.58.6.1995-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamarcq L H, McFall-Ngai M J. Induction of a gradual, reversible morphogenesis of its host's epithelial brush border by Vibrio fischeri. Infect Immun. 1998;66:777–785. doi: 10.1128/iai.66.2.777-785.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee K-H, Ruby E G. Competition between Vibrio fischeri strains during initiation and maintenance of a light organ symbiosis. J Bacteriol. 1994;176:1985–1991. doi: 10.1128/jb.176.7.1985-1991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K-H, Ruby E G. Detection of the light organ symbiont, Vibrio fischeri, in Hawaiian seawater by using lux gene probes. Appl Environ Microbiol. 1992;58:942–947. doi: 10.1128/aem.58.3.942-947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K-H, Ruby E G. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl Environ Microbiol. 1994;60:1565–1571. doi: 10.1128/aem.60.5.1565-1571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K-H, Ruby E G. Symbiotic role of the viable but nonculturable state of Vibrio fischeri in Hawaiian coastal seawater. Appl Environ Microbiol. 1995;61:278–283. doi: 10.1128/aem.61.1.278-283.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makemson J C. Luciferase-dependent oxygen consumption by bioluminescent vibrios. J Bacteriol. 1986;165:461–466. doi: 10.1128/jb.165.2.461-466.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFall-Ngai M, Brennan C, Weis V, Lamarcq L. Mannose adhesin-glycan interactions in the Euprymna scolopes-Vibrio fischeri symbiosis. In: Le Gal Y, Halvorson H O, editors. New developments in marine biotechnology. New York, N.Y: Plenum Press; 1998. pp. 273–276. [Google Scholar]
- 23.McFall-Ngai M, Montgomery M K. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda:Sepiolidae) Biol Bull. 1990;179:332–339. doi: 10.2307/1542325. [DOI] [PubMed] [Google Scholar]
- 24.McFall-Ngai M J. Consequences of evolving with bacterial symbionts: lessons from the squid-vibrio associations. Annu Rev Ecol Syst. 1999;30:235–256. [Google Scholar]
- 25.McFall-Ngai M J. Crypsis in the pelagic environment. Am Zool. 1990;30:175–188. [Google Scholar]
- 26.McFall-Ngai M J, Ruby E G. Sepiolids and vibrios: when first they meet. Reciprocal interactions between host and symbiont lead to the creation of a complex light-emitting organ. BioScience. 1998;48:257–265. [Google Scholar]
- 27.McFall-Ngai M J, Ruby E G. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science. 1991;254:1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery M K, McFall-Ngai M. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development. 1994;120:1719–1729. doi: 10.1242/dev.120.7.1719. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery M K, McFall-Ngai M. Embryonic development of the light organ of the sepiolid squid Euprymna scolopes Berry. Biol Bull. 1993;184:296–308. doi: 10.2307/1542448. [DOI] [PubMed] [Google Scholar]
- 30.Nishiguchi M K, Ruby E G, McFall-Ngai M J. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid-vibrio symbioses. Appl Environ Microbiol. 1998;64:3209–3213. doi: 10.1128/aem.64.9.3209-3213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyholm S V, McFall-Ngai M J. Sampling the light-organ microenvironment of Euprymna scolopes: description of a population of host cells in association with the bacterial symbiont Vibrio fischeri. Biol Bull. 1998;195:89–97. doi: 10.2307/1542815. [DOI] [PubMed] [Google Scholar]
- 32.Ruby E G. Lessons from a cooperative, bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu Rev Microbiol. 1996;50:591–624. doi: 10.1146/annurev.micro.50.1.591. [DOI] [PubMed] [Google Scholar]
- 33.Ruby E G, Asato L M. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch Microbiol. 1993;159:160–167. doi: 10.1007/BF00250277. [DOI] [PubMed] [Google Scholar]
- 34.Sharon N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987;217:145–157. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- 35.Small A, McFall-Ngai M. Halide peroxidase in tissues that interact with bacteria in the host squid Euprymna scolopes. J Cell Biochem. 1999;72:445–457. [PubMed] [Google Scholar]
- 36.Tao K, Makino K, Yonei S, Nakata A, Shinagawa H. Molecular cloning and nucleotide sequencing of oxyR, the positive regulatory gene of a regulon for an adaptive response to oxidative stress in Escherichia coli: homologies between OxyR protein and a family of bacterial activator proteins. Mol Gen Genet. 1989;218:371–376. doi: 10.1007/BF00332397. [DOI] [PubMed] [Google Scholar]
- 37.Tomarev S I, Zinovieva R D, Weis V M, Chepelinsky A B, Piatigorsky J, McFall-Ngai M J. Abundant mRNAs in the squid light organ encode proteins with a high similarity to mammalian peroxidases. Gene. 1993;132:219–226. doi: 10.1016/0378-1119(93)90199-d. [DOI] [PubMed] [Google Scholar]
- 38.Visick K L, Ruby E G. The periplasmic, group III catalase of Vibrio fischeri is required for normal symbiotic competence and is induced both by oxidative stress and by approach to stationary phase. J Bacteriol. 1998;180:2087–2092. doi: 10.1128/jb.180.8.2087-2092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei S L, Young R E. Development of symbiotic bacterial bioluminescence in a nearshore cephalopod, Euprymna scolopes. Mar Biol. 1989;103:541–546. [Google Scholar]
- 40.Weis V M, Small A L, McFall-Ngai M J. A peroxidase related to the mammalian antimicrobial protein myeloperoxidase in the Euprymna-Vibrio mutualism. Proc Natl Acad Sci USA. 1996;93:13683–13688. doi: 10.1073/pnas.93.24.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Weiss B. Two divergently transcribed genes, soxR and soxS, control a superoxide response regulon of Escherichia coli. J Bacteriol. 1991;173:2864–2871. doi: 10.1128/jb.173.9.2864-2871.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]