Abstract
Background and Objectives
Studies of hypertensive disorders of pregnancy (HDP), including gestational or chronic hypertension (GH/CH) and preeclampsia/eclampsia (PE/E), suggest associations with early-life and mid-life cognition but have been limited by self-report or use of diagnostic codes, exclusion of nulliparous women, and lack of measurement of cognition in later life. We examined the effects of any HDP, GH/CH, PE/E, and nulliparity on cognition in later life.
Methods
Participants included 2,239 women (median age 73) enrolled in the Mayo Clinic Study of Aging with medical record–abstracted pregnancy information. A cognitive battery of 9 tests was conducted every 15 months. Global cognitive and domain-specific z scores (memory, executive/attention, visuospatial, and language) were outcomes. Linear mixed-effect models evaluated associations between pregnancy history (all normotensive, any HPD, HPD subtype [GH/CH, PE/E], or nulliparous) and cognitive decline, adjusting for age and education. Additional models adjusted for APOE, smoking, hypertension, dyslipidemia, body mass index (BMI), diabetes, stroke, and heart disease. Interactions between pregnancy history and age or education on cognitive performance were examined.
Results
Of the 2,239 women, 1,854 (82.8%) had at least 1 pregnancy (1,607 all normotensive, 100 GH/CH, and 147 PE/E); 385 (17.2%) were nulliparous. Cognitive performance did not cross-sectionally differ for women with a history of any HDP, GH/CH, or PE/E vs women with a history of all normotensive pregnancies; women who were nulliparous had lower global and domain-specific cognition (all p < 0.05) in age- and education-adjusted models. There was an interaction (p = 0.015) between nulliparity and education such that the lower cognitive performance was most pronounced among nulliparous women with ≤12 years of education (beta = −0.42, p < 0.001) vs 12 + years (b = −0.11, p = 0.049). Longitudinally, women with any HDP had greater declines in global cognition and attention/executive z scores compared with women with all normotensive pregnancies. When stratified by HDP type, only women with PE/E had greater declines in global cognition (beta = −0.04, p < 0.001), language (beta = −0.03, p = 0.001), and attention (beta = −0.04, p < 0.001) z scores. Adjustment for vascular risk factors, BMI, smoking, and APOE did not attenuate results.
Discussion
Women with a history of HDP, especially PE/E, are at greater risk of cognitive decline in later life.
Hypertensive disorders of pregnancy (HDP) include preeclampsia (PE)/eclampsia (E), gestational hypertension (GH), chronic hypertension (CH), and PE/E superimposed on chronic hypertension. When assessed on a per-women basis, compared with per pregnancy, approximately 15.3% of women have an HDP and 7.5% have PE during their lifetimes.1 HDP have been consistently associated with cardiovascular and cerebrovascular disease,2 as well as renal disease and multimorbidity.1,3,4 Although these outcomes have been associated with dementia, few studies have directly assessed the association between HDP and global and domain-specific cognitive decline.
Studies that have examined the association between HDP and cognition have reported conflicting results. Some studies have suggested that PE is associated with mild cognitive impairment,5 vascular dementia,6,7 Alzheimer disease,8 or all-cause dementia,1 whereas other studies did not find associations.9,10 Some of the observed associations, however, were not significant in models adjusted for cardiovascular risk factors.11
Studies of global and domain-specific cognitive decline have also been mixed, with some suggesting women with a history of GH or PE perform worse in mid-life on measures of psychomotor speed,11,12 attention/executive function,11 or memory.13 Other studies, however, have not found associations with psychomotor speed,13 attention/executive function,12,13 or memory.11,12
Limitations of studies to date include small sample sizes, use of self-report and diagnostic codes for HDP, lack of consideration of both GH and PE (most studies focus on PE only), and lack of examination of cognition in later life. For example, most studies of HDP were cross-sectional and measured cognition among women aged 50–65 years. Although it is important to identify risk factors for mid-life cognitive impairment, cognitive decline significantly increases after age 65 years, and it is also critical to understand the effects of HDP on later-life cognition. It is not known whether any cognitive effects of HDP will appear in later life such that a history of HDP should still be obtained when calculating future risk in this age group. Moreover, it is not clear which cognitive domains are most affected. In addition, most studies have been cross-sectional and have not examined cognitive trajectories for women with a history of HDP. Finally, studies of HDP have generally not examined risk of cognitive decline among nulliparous women. Reasons for nulliparity include infertility, which has been associated with earlier mortality and underlying health,14 and lifestyle choices based on multiple social factors.
In the present study, we assessed the effects of HDP and type (GH vs PE/E) and nulliparity on global and domain-specific cognitive decline among 2,239 women enrolled in the population-based Mayo Clinic Study of Aging (MCSA). We further assessed whether adjustments for cardiovascular risk factors and conditions would attenuate any associations with cognition. We hypothesized that a history of HPD, especially PE/E, would be associated with greater global and domain-specific cognitive decline.
Methods
Population
The MCSA is a community-based study of cognitive aging among a representative sample of individuals aged 50 years and older living in Olmsted County, Minnesota.15 Residents were enumerated using the Rochester Epidemiology Project (REP) medical record linkage system in a random sampling design stratified by age and sex.16,17 The current analysis included 2,239 women who were enrolled in the MCSA between December 2004 and December 2019.
Standard Protocol Approvals, Registrations, and Patient Consents
Olmsted Medical Center and Mayo Clinic Institutional Review Boards approved the study, and participants provided written informed consent.
Study Design
MCSA participants completed study visits every 15 months. These visits included a study coordinator interview, a physician examination, and cognitive testing.15 The study coordinator interview included questions on medical history as well as memory to both the participant and study partner. The physician administered the Short Test of Mental Status and conducted a medical history and neurologic examination.18
All participants had cognitive testing that included 9 tests covering 4 domains15: (1) memory (Auditory Verbal Learning Test [AVLT] Delayed Recall, Wechsler Memory Scale–Revised [WMS‐R] Logical Memory II & Visual Reproduction II), (2) attention/executive function (Trail Making Test B, WAIS‐R Digit Symbol), (3) language (Boston Naming Test, Category Fluency), and (4) visuospatial (Wechsler Adult Intelligence Scale–Revised [WAIS‐R] Picture Completion & Block Design). Global z scores were an average of domain‐specific z scores and referenced to 3,686 MCSA cognitively unimpaired (CU) participants between 2004 and 2012, aged 50–89 years.
All results from study participants were discussed by a consensus committee without knowledge of diagnoses at previous visits. Mayo's Older Americans Normative Studies age-adjusted scores were used as comparisons for cognitive tests.19 A diagnosis of MCI was based on previously published criteria.20 A dementia diagnosis was based on Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria.21
Ascertainment of Hypertensive Pregnancy Disorders
The number of pregnancies lasting more than 20 weeks was determined for each woman using a combination of REP medical record abstraction and a questionnaire.
Medical Record Chart Abstraction
The REP uses a record linkage system to capture medical records from all health care providers in Olmsted County, Minnesota, from 1966 to present. This record linkage system provides health information on virtually all Olmsted County residents; 2% refuse access to medical records for research. More specific details on the REP have been described previously.16,17
For all women in the MCSA, 3 nurses abstracted the medical records. Information including blood pressures, hypertensive medication use, and dipstick protein available from the first prenatal visit through 12 weeks postpartum was recorded, dated, and entered directly into a database for analysis. Abstracted laboratory values included 24-hour urinary protein, platelet count, serum creatinine, and liver function tests. Only pregnancies with at least 1 blood pressure measurement from a prenatal visit and 1 measurement at or after admission for delivery were considered sufficient to determine HDP status.
Algorithm to Determine HDP Status
We used a previously described and published electronic algorithm that used REP data to diagnose HDP.22 It was originally developed as a standardized means of diagnosing HDP using retrospective data abstracted by nonphysicians. The algorithm was validated by comparing obtained diagnoses to physician-made diagnoses, which was considered the gold standard. The algorithm had significantly higher sensitivity and specificity for the diagnosis of HDP compared with the use of diagnostic codes.22 Briefly, the algorithm was based on a strict definition of hypertension that required sustained hypertension, defined as blood pressure elevations in greater than 50% of readings, starting with the first blood pressure greater than 140 mm Hg systolic and/or greater than 90 mm Hg diastolic. The 50% rule was used because it was found, in the development of the algorithm, that the use of 2 blood pressures greater than 140/90 mm Hg on 2 occasions, at least 4 hours apart (i.e., part of the American College of Obstetricians and Gynecologists definition of gestational hypertension at the time of clinical disease), was not suitable for making retrospective diagnoses for research purposes.
After medical record abstraction, we identified 6 women who had a pregnancy with CH (hypertension diagnosis <20 weeks gestation), 93 GH (development of hypertension at 20 weeks or greater gestation), 1 with CH and GH pregnancies, 142 with PE, 2 with PE superimposed on chronic hypertension, and 3 with eclampsia. Given the small numbers of several groups, we combined diagnoses into the following groups: normotensive, GH/CH (includes both GH and CH), PE or E (PE, E, and PE superimposed on CH), and nulliparous (no pregnancies ≥20 weeks). Each woman was classified based on the most severe form of HDP experienced. There were only 2 women with more than 1 GH pregnancy (both had 2 GH pregnancies) and only 14 women with more than 1 pregnancy with PE/E (13 with 2 and 1 with 3). Therefore, women were classified as having any pregnancy with the disorder as opposed to counting the number of pregnancies with the disorder.
Questionnaire Ascertainment of HDP Status and Validation
A previously validated questionnaire23 was administered to all women enrolled in the MCSA from 2018 onward to assess number of pregnancies and HDP status. Some women moved into Olmsted County after or during their reproductive period such that all, or some, of their pregnancy medical records were not available. There were 412 women who completed the questionnaire and had abstracted pregnancy information for all pregnancies. Among these women, there was 89.6% agreement (369/412) on GH and 88.8% agreement (366/412) on PE/E. Because of the high agreement, we classified a woman as having an HDP if either the questionnaire or the medical record abstraction identified the condition. Analyses used the most severe HDP recorded for each woman.
Covariates
Age and education (years) were self-reported. Cardiovascular risk factors or conditions were abstracted from the REP medical records, as previously described.15 Risk factors or conditions included hypertension (HTN), diabetes mellitus (DM), dyslipidemia, coronary heart disease, and stroke. Body mass index (BMI) was measured and calculated as the weight in kilograms divided by the height in meters squared. Ever/never smoking was obtained by self-report. From a blood sample, APOE was genotyped.
Statistical Analyses
Data were summarized using medians (interquartile ranges) for continuous data and frequencies (%) for categorical data. Data distributions by inclusion were compared using chi-square or Fisher exact tests (where appropriate) for categorical data and Wilcoxon rank-sum tests for continuous data. Similar methods were used to compare data distributions across women who had a history of all normotensive pregnancies, GH/CH, PE/E, or were nulliparous (with Kruskal-Wallis used instead of the Wilcoxon rank-sum test). Random effects models with a random intercept and slope were used to examine the cross-sectional and longitudinal associations between baseline HDP and type and cognitive trajectories in models that adjusted for age and education.
Additional sensitivity analyses were conducted. First, models were rerun after additional adjustments for APOE and cardiovascular risk factors and conditions (smoking, BMI, HTN, dyslipidemia, DM, heart disease, and stroke). Second, the results were rerun after excluding individuals with MCI at baseline. Last, we assessed whether there were interactions between pregnancy status and either age or education (less than or equal to 12 years vs greater than 12 years) for baseline cognition and cognitive trajectories. SAS version 9.4 was used to perform the analyses (SAS Institute Inc., Cary, NC). We considered a two-sided p value < 0.05 to be statistically significant.
Data Availability
Deidentified data from the MCSA are available on reasonable request.
Results
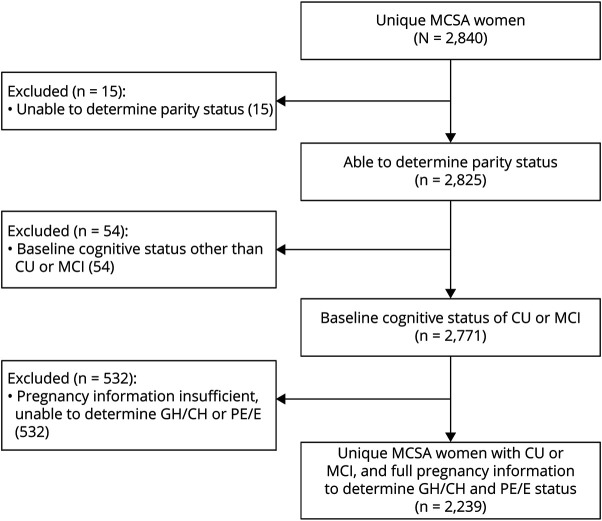
Of the 2,840 women enrolled in the MCSA, 2,825 women had pregnancy information either through manual abstraction or questionnaire. Of these 2,825, 54 had a clinical diagnosis other than CU or MCI at baseline, and 532 were determined to be parous but had insufficient medical records to determine GH/CH or PE/E status and did not complete a questionnaire (Figure 1). This left 2,239 women for the analyses. Compared with the 2,239 women with complete pregnancy information, the 601 women who did not were older (median 79 vs 73 years, p < 0.001), less educated (45% vs 33.6% ≤ 12 years, p < 0.001), and were more frequently diagnosed with DM, dyslipidemia, heart disease, and stroke (eTable 1, links.lww.com/WNL/C660).
Figure 1. Flowchart of the Study Sample.
CU = cognitively unimpaired; GH = gestational hypertension; MCSA = Mayo Clinic Study of Aging; MCI = mild cognitive impairment; PE/E = preeclampsia or eclampsia.
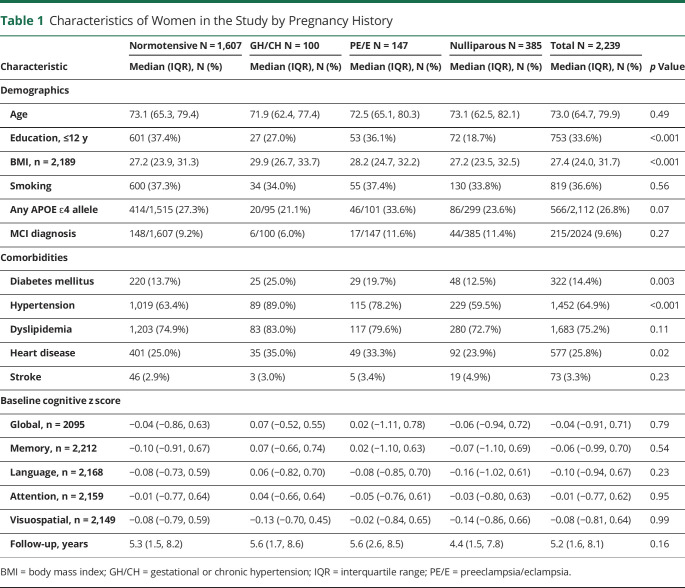
Of the 2,239 women with pregnancy information, 1,854 (82.8%) had at least 1 pregnancy (n = 6,260 total pregnancies) and 385 (17.2%) were nulliparous. Of the 1,854 women with at least 1 pregnancy, 1,607 (86.7%) had all normotensive pregnancies, 100 (5.4%) had GH, and 147 (7.9%) had PE/E. Table 1 shows the characteristics of women at the MCSA baseline enrollment visit for those with all normotensive pregnancies, GH/CH, PE/E, or nulliparity. Women who were nulliparous had more years of education, lower BMI, and a lower frequency of many comorbidities compared with the other 3 groups. Women with a history of GH/CH or PE/E were more frequently diagnosed with DM, HTN, or myocardial infarction and had a greater number of overall comorbidities.
Table 1.
Characteristics of Women in the Study by Pregnancy History
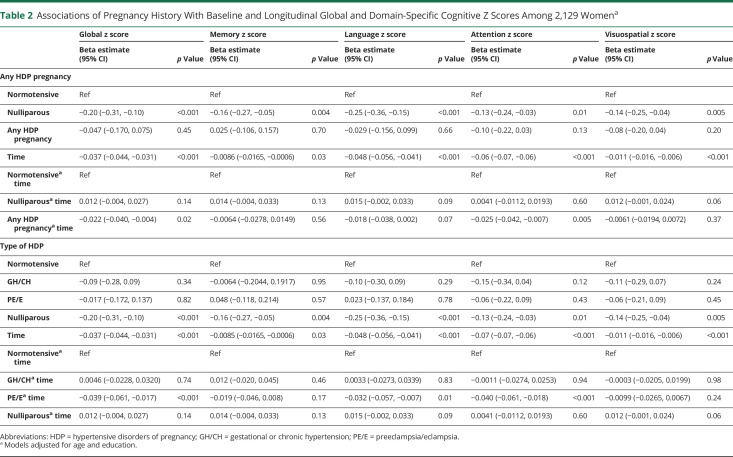
The cross-sectional and longitudinal associations between HDP and cognitive z scores are shown in Table 2 and Figure 2. Cross-sectionally, at the baseline visit (time 0), there were no differences in any cognitive z score for women with a history of any HDP compared with all normotensive pregnancies in unadjusted analyses (eTable 2, links.lww.com/WNL/C660) or after adjustments for age and education (Table 2). When stratified by HDP type, there were also no differences. In contrast, women who were nulliparous had worse performance compared with those with a history of normotensive pregnancies on language (b = −0.14, p = 0.02) in unadjusted analyses (eTable 2, links.lww.com/WNL/C660). After adjustments for age and education, nulliparous women performed significantly worse globally (b = −0.20, p < 0.001) and in all cognitive domains: memory (b = −0.16, p = 0.004), language (b = −0.25, p < 0.001), attention/executive function (b = −0.13, p = 0.02), and visuospatial (b = −0.14, p = 0.005) (Table 2).
Table 2.
Associations of Pregnancy History With Baseline and Longitudinal Global and Domain-Specific Cognitive Z Scores Among 2,129 Womena
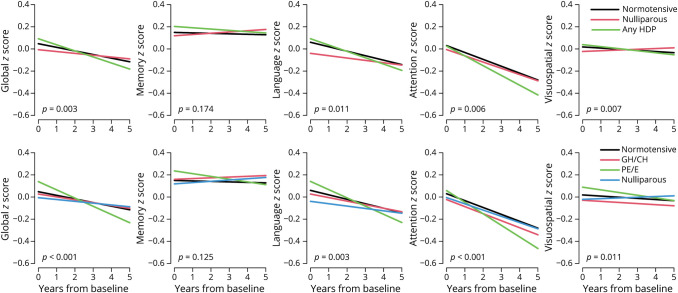
Figure 2. Cross-sectional and Longitudinal Associations Between Pregnancy History and Global and Domain-Specific Cognitive Decline.
Models are adjusted for age and education. GH/CH = gestational or chronic hypertension; HDP = hypertensive disorders of pregnancy; PE/E = preeclampsia or eclampsia.
Longitudinally, women with any HDP showed greater declines in global cognition (b = −0.021, p = 0.02) and attention/executive function (b = −0.022, p = 0.005) z scores compared with women with all normotensive pregnancies after adjusting for age and education. When stratified by HDP type, women with GH/CH did not differ in global or domain-specific cognitive decline compared with women with all normotensive pregnancies. By contrast, women with PE/E had greater declines in global cognition (b = −0.039, p < 0.001), language (b = −0.032, p = 0.01), and attention/executive function (b = −0.040, p < 0.001) z scores but not memory (b = −0.019, p = 0.17) or visuospatial (b = −0.0099, p = 0.24) z scores compared with women with all normotensive pregnancies.
Sensitivity Analyses
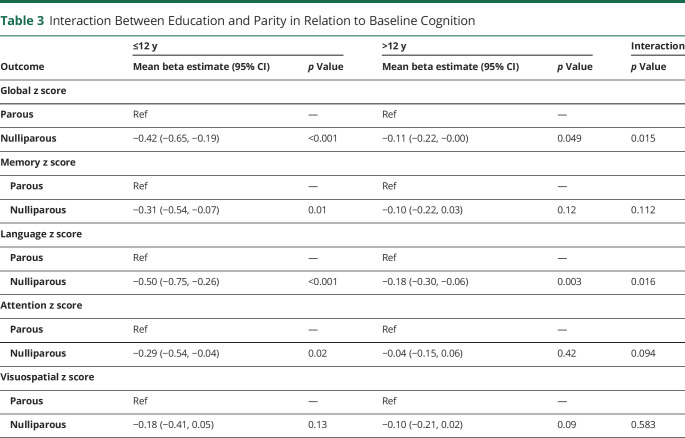
Several sensitivity analyses were conducted. First, we additionally adjusted for APOE, BMI, smoking, and cardiovascular risk factors and conditions, but the results did not change (eTable 3, links.lww.com/WNL/C660). Second, we restricted the analyses to individuals who were cognitively unimpaired at baseline, but the results remained (data not shown). Third, we examined interactions of HDP history with age or education (≤12 years vs >12 years). Age did not modify the associations between HDP history and cognitive outcomes at baseline or longitudinally. However, we observed an interaction between education and HDP history in relation to baseline cognition (p < 0.05). This interaction with education was driven by women who were nulliparous (Table 3). Among women with ≤12 years of education, those who were nulliparous had significantly lower baseline z scores, except for visuospatial. Among women with >12 years of education, women who were nulliparous had lower baseline global and language z scores, but the decline was not as large as the decline among women with ≤12 years education. Finally, in additional analyses, we statistically assessed whether cognitive decline for the PE/E group differed from the GH/CH group. The PE/E group had significantly greater cognitive decline than the GH/CH group for global cognitive (b = −0.044, p = 0.01) and attention (b = −0.039, p = 0.02) z scores, and there was a trend for language (b = −0.036, p = 0.07).
Table 3.
Interaction Between Education and Parity in Relation to Baseline Cognition
Discussion
In this large, population-based study, we examined associations between HDP and type with global and domain-specific cognitive decline in women with a mean age of 73 years at baseline. We report several novel findings. First, although there was no difference in age- and education-adjusted cognitive performance at baseline for women with a history of any HDP vs a history of all normotensive pregnancies, women with a history of any HDP had faster declines in global cognition and attention/executive function. Second, when we examined HDP type, these findings were only observed for women with a history of PE/E and not among women with a history of GH/CH. Third, adjusting for cardiovascular risk factors and conditions did not attenuate any of the results. Last, there was an interaction between nulliparity and education at baseline such that nulliparous women with an education of high school or less had worse cognition at baseline.
Our findings of an association between PE and cognitive decline are consistent with other studies that reported that PE was a risk factor for mild cognitive impairment,5 vascular dementia,6,7 Alzheimer disease,8 or all-cause dementia.1 Two studies that did not find associations between PE and cognition used self-reported exposures, which are less accurate.9,10 Most studies examining HDP and cognition have focused either on any HDP or PE only and have not assessed whether there are differences in risk for those with GH vs PE. Using the Swedish medical birth record and diagnostic codes for both exposure and outcome, One study6 reported that a history of GH or PE PE was associated with vascular dementia but not other dementia types. Notably, PE was a stronger risk factor for vascular dementia compared with GH. In contrast, another study reported that GH, and not PE, drove the association between HDP and cognition.13 In the present study, women with a history of PE/E, but not GH/CH, had greater global and domain-specific cognitive decline. There may be several reasons for these observed differences. First, PE/E is a systemic disease with pronounced endothelial dysfunction and systemic inflammation, which affects cerebral circulation and may result in long-term vascular damage. Second, PE/E has been associated with posterior reversible encephalopathy syndrome (PRES), a diagnosis that encompasses both clinical and radiologic findings. It presents as a complex of neurologic symptoms, including headache, visual changes, lethargy, and seizures.24 Up to 20% of the affected women may demonstrate abnormal neuroradiologic findings 6–8 weeks postpartum,25 presumably caused by gliosis in response to infarction, which can manifest later in life as cognitive decline.
When examining cognitive domains in the current study, we found that women with a history of PE/E had greater declines in global cognition, language, and attention/executive function z scores compared with women with normotensive pregnancies. However, PE/E was not associated with memory or visuospatial decline. The results suggest an underlying vascular pathology and microvascular contribution, which is generally consistent with the literature. In contrast to these findings, another study13 reported associations between HPD and memory but not executive dysfunction. It is difficult to fully compare the 2 studies, though, because different cognitive tests were used and the mean age of the women differed (47 years in the previous study vs 73 years in the current study).
HDP are recognized as risk factors for cardiovascular and cerebrovascular diseases.2 It is therefore possible that the relationship between HDP and cognition could be mediated solely by these vascular factors and conditions and when these factors are adjusted for the results would be fully attenuated. HDP may alternatively contribute to risk of cognitive decline independent of the effects of other vascular risk factors or conditions. In the present study, adjustment for vascular factors and conditions did not attenuate the relationships between HDP and cognitive decline. These results suggest that HDP, especially PE/E, is an independent risk factor for cognitive decline. This finding is consistent with another study in which the associations between HDP and cognition remained after adjustments for demographic and vascular factors.6,12 By contrast, 2 other studies have reported attenuated results after adjustment for demographic, metabolic, and vascular factors.11,13 Notably, the latter 2 studies had much smaller sample sizes and were of younger cohorts (mean ages of women in their 40s compared with 70s), which may have resulted in differences. It is possible, for example, that preexisting vascular damage due to PE/E is further exacerbated or unmasked by other sex-specific factors, such as menopause.
Few studies have addressed nulliparity when examining the relationship between HDP and cognition, but our results highlight the importance of including all women in such analyses. In the current study, women who were nulliparous had worse baseline cognition compared with women with normotensive pregnancies. Nulliparous women represent a mixture of those who might have had normotensive or PE/E pregnancies if they had gotten pregnant. It is possible that women predisposed to both PE/E and cognitive decline are overrepresented in this group, which could explain the poorer cognitive performance. Alternatively, if a woman's nulliparous status was caused by infertility, several underlying conditions associated with impaired cognition (e.g., DM, early menopause, and polycystic ovary syndrome) could have contributed. As such, uncomplicated pregnancies, during which a woman responded with normal blood pressure, may be viewed as a physiologic stress that identifies those with healthy metabolic and vascular profiles. In further examination of the relationship between nulliparity and cognition, we found that the association was primarily among women with 12 years of education or less. This observation further highlights the complicated sociocultural factors for women deciding whether to become pregnant, as well as the need to go beyond examining parity and cognitive outcomes without consideration of socioeconomic and other factors.
There are multiple study strengths including the large sample size, well-defined HDP status compared with the use of codes, and the comprehensive cognitive battery. However, there are also limitations that warrant acknowledgment. First, the women in the study were predominantly White, so the results may not be generalizable to more diverse populations. African American women, for example, have higher rates of HDP than White women and are at greater risk of cardiovascular disease and cognitive decline.26 Second, HDP have been associated with an increased risk of cardiovascular and cerebrovascular disease and mortality.2 Thus, women with the most severe HDP may have died before the study or not participated in the MCSA, which would have led to more conservative results. In addition, it is possible that women with HDP at earlier ages were also less likely to participate in the MCSA if they had comorbidities sooner. Third, reasons for nulliparity were not known and only 3% of pregnancies were multiple births. Therefore, we were not able to assess the effect of infertility, miscarriages, or multiple births on cognitive decline. Last, we did not have information on whether hypertension as a risk factor was controlled or uncontrolled.
Glossary
- BMI
body mass index
- CU
cognitively unimpaired
- DM
diabetes mellitus
- GH/CH
gestational or chronic hypertension
- HDP
hypertensive disorders of pregnancy
- HTN
hypertension
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- PE/E
preeclampsia/eclampsia
- PRES
posterior reversible encephalopathy syndrome
- REP
Rochester Epidemiology Project
Appendix. Authors
Footnotes
See page 893
CME Course: NPub.org/cmelist
Study Funding
Funding for this study was provided by grants from the NIH (U01 AG006786, RF1 AG055151, R01 HL136348, and U54 AG044170) and the GHR Foundation. This study used the resources of the Rochester Epidemiology Project (REP) medical record linkage system, which is supported by the National Institute on Aging (R33 AG058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. However, the content of this article is solely the responsibility of the authors and does not represent the official views of the NIH or the Mayo Clinic. Dr. Rocca was partly funded by the Ralph S. and Beverley E. Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
Disclosure
M.M. Mielke has served on scientific advisory boards and/or has consulted for Biogen, LabCorp, Lilly, and Merck and receives grant support from the NIH and Department of Defense. The other authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Garovic VD, White WM, Vaughan L, et al. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75(18):2323-2334. doi. 10.1016/j.jacc.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Kelly AC, Michos ED, Shufelt CL, et al. Pregnancy and reproductive risk factors for cardiovascular disease in women. Circ Res. 2022;130(4):652-672. doi. 10.1161/CIRCRESAHA.121.319895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kattah AG, Scantlebury DC, Agarwal S, et al. Preeclampsia and ESRD: the role of shared risk factors. Am J Kidney Dis. 2017;69(4):498-505. doi. 10.1053/j.ajkd.2016.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshunbade AA, Lirette ST, Windham BG, et al. Hypertensive diseases in pregnancy and kidney function later in life: the Genetic epidemiology Network of Arteriopathy (GENOA) Study. Mayo Clin Proc. 2022;97(1):78-87. doi. 10.1016/j.mayocp.2021.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields JA, Garovic VD, Mielke MM, et al. Preeclampsia and cognitive impairment later in life. Am J Obstet Gynecol. 2017;217(1):74.e71-74.e11. doi. 10.1016/j.ajog.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andolf E, Bladh M, Moller L, Sydsjo G. Prior placental bed disorders and later dementia: a retrospective Swedish register-based cohort study. BJOG. 2020;127(9):1090-1099. doi. 10.1111/1471-0528.16201 [DOI] [PubMed] [Google Scholar]
- 7.Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018;363:k4109. doi: 10.1136/bmj.k4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theilen LH, Fraser A, Hollingshaus MS, et al. All-cause and cause-specific mortality after hypertensive disease of pregnancy. Obstet Gynecol. 2016;128(2):238-244. doi. 10.1097/AOG.0000000000001534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelander M, Cnattingius S, Akerud H, Wikstrom J, Pedersen NL, Wikstrom AK. Pregnancy hypertensive disease and risk of dementia and cardiovascular disease in women aged 65 years or older: a cohort study. BMJ Open. 2016;6(1):e009880. doi. 10.1136/bmjopen-2015-009880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abheiden CN, van Doornik R, Aukes AM, van der Flier WM, Scheltens P, de Groot CJ. Hypertensive disorders of pregnancy appear not to be associated with Alzheimer's disease later in life. Dement Geriatr Cogn Dis Extra. 2015;5(3):375-385. doi. 10.1159/000439043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayan N, Kaur A, Elharram M, Rossi AM, Pilote L. Impact of preeclampsia on long-term cognitive function. Hypertension. 2018;72(6):1374-1380. doi. 10.1161/HYPERTENSIONAHA.118.11320 [DOI] [PubMed] [Google Scholar]
- 12.Mielke MM, Milic NM, Weissgerber TL, et al. Impaired cognition and brain atrophy decades after hypertensive pregnancy disorders. Circ Cardiovasc Qual Outcomes. 2016;9(1):S70-S76. doi. 10.1161/CIRCOUTCOMES.115.002461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adank MC, Hussainali RF, Oosterveer LC, et al. Hypertensive disorders of pregnancy and cognitive impairment: a prospective cohort study. Neurology. 2021;96(5):e709–e718. doi. 10.1212/WNL.0000000000011363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy E, Kravdal O. Reproductive history and mortality in late middle age among Norwegian men and women. Am J Epidemiol. 2008;167(3):271-279. doi. 10.1093/aje/kwm295 [DOI] [PubMed] [Google Scholar]
- 15.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58-69. doi. 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauver StJL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614-1624. doi. 10.1093/ije/dys195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocca WA, Grossardt BR, Brue SM, et al. Data resource profile: expansion of the Rochester epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol. 2018;47(2):368-368j. doi. 10.1093/ije/dyx268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48(7):725-728. [DOI] [PubMed] [Google Scholar]
- 19.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's Older Americans Normative Studies: updated AVLT norms for ages 56 to 97. Clin Neuropsychologist. 1992;6(Supplement):83-104. [Google Scholar]
- 20.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194; doi:doi. 10.1111/j.1365-2796.2004.01388 [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 4th ed: American Psychiatric Association; 1994. [Google Scholar]
- 22.Milic NM, Codsi E, Butler Tobah YS, et al. Electronic algorithm is superior to hospital discharge codes for diagnoses of hypertensive disorders of pregnancy in historical cohorts. Mayo Clin Proc. 2018;93(12):1707-1719. doi. 10.1016/j.mayocp.2018.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diehl CL, Brost BC, Hogan MC, et al. Preeclampsia as a risk factor for cardiovascular disease later in life: validation of a preeclampsia questionnaire. Am J Obstet Gynecol. 2008;198(5):e11-e13. doi. 10.1016/j.ajog.2007.09.038 [DOI] [PubMed] [Google Scholar]
- 24.Wagner SJ, Acquah LA, Lindell EP, et al. Posterior reversible encephalopathy syndrome and eclampsia: pressing the case for more aggressive blood pressure control. Mayo Clin Proc. 2011;86(9):851-856. doi. 10.4065/mcp.2011.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol. 2004;190(3):714-720. doi. 10.1016/j.ajog.2003.09.015 [DOI] [PubMed] [Google Scholar]
- 26.Ghosh G, Grewal J, Mannisto T, et al. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn Dis. 2014;24(3):283-289. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data from the MCSA are available on reasonable request.