Abstract
Objective
To assess the impact of menu calorie labelling on reducing obesity-associated cancer burdens in the USA.
Design
Cost-effectiveness analysis using a Markov cohort state-transition model.
Setting
Policy intervention.
Participants
A modelled population of 235 million adults aged ≥20 years in 2015–2016.
Interventions
The impact of menu calorie labelling on reducing 13 obesity-associated cancers among US adults over a lifetime was evaluated for: (1) effects on consumer behaviours; and (2) additional effects on industry reformulation. The model integrated nationally representative demographics, calorie intake from restaurants, cancer statistics and estimates on associations of policy with calorie intake, dietary change with body mass index (BMI) change, BMI with cancer rates, and policy and healthcare costs from published literature.
Main outcome measures
Averted new cancer cases and cancer deaths and net costs (in 2015 US$) among the total population and demographic subgroups were determined. Incremental cost-effectiveness ratios from societal and healthcare perspectives were assessed and compared with the threshold of US$150 000 per quality-adjusted life year (QALY) gained. Probabilistic sensitivity analyses incorporated uncertainty in input parameters and generated 95% uncertainty intervals (UIs).
Results
Considering consumer behaviour alone, this policy was associated with 28 000 (95% UI 16 300 to 39 100) new cancer cases and 16 700 (9610 to 23 600) cancer deaths averted, 111 000 (64 800 to 158 000) QALYs gained, and US$1480 (884 to 2080) million saved in cancer-related medical costs among US adults. The policy was associated with net cost savings of US$1460 (864 to 2060) million and US$1350 (486 to 2260) million from healthcare and societal perspectives, respectively. Additional industry reformulation would substantially increase policy impact. Greater health gains and cost savings were predicted among young adults, Hispanic and non-Hispanic Black individuals.
Conclusions
Study findings suggest that menu calorie labelling is associated with lower obesity-related cancer burdens and reduced healthcare costs. Policymakers may prioritise nutrition policies for cancer prevention in the USA.
Keywords: health policy, nutrition & dietetics, health economics
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Our study populated a Markov cohort state-transition model among 32 subgroups based on the nationally representative distributions of age, sex and race/ethnicity.
This cost-effectiveness evaluation incorporated data input parameters from established resources, and the evidence was robust to different policy scenarios.
However, given the nature of modelling research, this study does not provide a real-world evaluation of the impact of policy implementation on health and economic outcomes.
We modelled only the impact of menu calorie labelling on calories, although the policy may also result in potential changes in the nutritional quality of the restaurant meals.
Introduction
Obesity affects one in three Americans and is an established risk factor for 13 types of cancer, such as endometrial, liver, breast, prostate and colorectal cancers.1 Obesity-associated cancer represents 40% of all newly diagnosed cancer cases and contributes to 43.5% of total direct cancer care expenditures, estimated at US$35.9 billion in 2015.1–7 Rates of obesity-associated cancers are also rising disproportionately among young adults.5 8 Substantial health and economic burdens highlight the need to prioritise cost-effective strategies to reduce obesity-associated cancers in the USA.
Diet is one of the few modifiable factors for both obesity and obesity-associated cancers.2 9 Restaurant meals account for one in five calories consumed by US adults, including 9% of calories from full-service restaurants and 12% from fast-food restaurants,10 and therefore, can be an important target for improving population diet. Restaurant meals can have very high calories, with a mean energy of 1362 kcal/meal and 969 kcal/meal in popular meals from randomly selected full-service and fast-food restaurants, respectively.11 Consistently, individuals who cook less frequently at home consume more daily calories than those who cook more at home.12 Thus, reducing calories consumed from restaurant meals has the potential to reduce daily calorie intake and subsequent obesity and obesity-related cancer burdens.
To help consumers make lower-calorie choices, the Affordable Care Act mandated that all chain restaurants with 20 or more outlets post calorie information on menus and menu boards for all standard menu items.13 The Food and Drug Administration (FDA) published the final rules for this policy in 2016, which was subsequently implemented in 2018. A meta-analysis of 14 interventional studies, including five randomised controlled trials (RCTs) and a recent quasi-experimental longitudinal study among 104 restaurants, demonstrated that menu calorie labelling resulted in a reduction of 7.3% in caloric intake per meal and a 60 kcal (4%) reduction in calorie purchased per transaction, respectively.14 15 Such policy can also motivate restaurant reformulation to lower calorie contents or introduce healthier food options.16–21 Prior cost-effectiveness analyses suggest that this policy is associated with substantial health gains and is a cost-saving strategy for reducing obesity and obesity-related diseases.22 23 It was estimated that the menu calorie labelling on fast foods was associated with a 25 kJ (6 kcal) reduction in mean daily energy intake, leading to a −0.2 kg change in mean body weight, a gain of 63 492 health-adjusted life-years, and net savings of half a billion (2010 Australian dollars) among Australians aged ≥2 years over their lifetime.22 Researchers in the USA have demonstrated that this policy would prevent a large number of incident cardiovascular diseases (135 781) and type 2 diabetes (99 736) and net savings of over US$10 billion (2018 US dollars) among US adults over a lifetime.22 23 However, the health and economic benefits of the policy for obesity-associated cancers have not been evaluated. This study aimed to address the knowledge gap by evaluating the cost-effectiveness of the federal menu calorie labelling policy and obesity-associated cancer burdens among US adults.
Methods
Study overview
The Diet and Cancer Outcome (DiCOM), a probabilistic cohort state-transition model,24 was used to perform an economic evaluation of the menu calorie labelling and obesity-associated cancer rates among 235 million US adults aged 20 years and older (US census), by comparing a policy scenario (menu calorie label) with the status quo (no policy), over a simulated lifetime starting from 2015. The model consists of (1) four health states: healthy without cancer, initial diagnosis and treatment for 13 types of obesity-related cancer, continuous care for each of the 13 cancers, and death (from 13 cancers or other causes); (2) the annual likelihood of changes in health and (3) the lifetime consequences of such changes on health outcomes and economic cost (online supplemental figure 1). The DiCOM model integrated independent parameters from different data sources, including nationally representative population demographics, dietary intake and cancer statistics, association estimates of policy intervention with diet, diet change with body mass index (BMI) and BMI with cancer risks; and policy and health-related costs from established sources (table 1). This study used de-identified datasets and was exempt from institutional review board review and follows the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines.
Table 1.
Key input parameters and data sources in the Dietary Cancer Outcome Model (DiCOM)
| Model input | Outcome | Estimates | Distribution | Comments | Data source |
| 1. Simulated population | Population | Mean consumption of calories was 332 kcal/day from full-service or fast-food restaurants (online supplemental tables 1, 8 and 9) | Gamma | Stratified by age, sex, race/ethnicity; 32 subgroups | NHANES 2013–2016 |
| 2. Policy effect* | |||||
| a. Consumer behaviour | Policy effect | 7.3% (95% CI 4.4% to 10.1%) (online supplemental appendix 1 and appendix table 1) | Beta | One-time effect | Meta-analysis of labelling interventions on reducing calorie intake, Shangguan et al, 2019 15 |
| b. Industry response | Policy effect | 5% (online supplemental appendix 1 and appendix table 2) | Beta | Assumption: no reformulation in the first year of policy intervention; restaurants will replace the high-calorie menu items with low-calorie options or reformulate the menu items in years 2 to 5 of the intervention to achieve a 5% reduction in calorie content | Calorie changes in large chain restaurants from 2008 to 201518; higher-calorie menu items eliminated in large-chain restaurants19 |
| 3. Effect of change in calorie intake on BMI change (kg/m2)* | Dietary effect | Among individuals with: BMI <25: 0.0015 per kcal BMI ≥25: 0.003 per kcal |
Normal | Assumption: 55 kcal per day reduction in calorie intake would lead to one pound weight loss within 1 year, with no further weight loss in the future | Hall et al, 201830; Hall et al, 201129 |
| 4. Etiologic effect of BMI on cancer outcomes* | Cancer outcome | RRs ranged from 1.05 to 1.50 (online supplemental table 2) | Log normal | BMI change and cancer incidence | Continuous update project (CUP) conducted by the World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) |
| 5. Cancer statistics* | Cancer incidence‡ and survival | online supplemental tables 3 and 4 and appendices 2 and 3, appendix tables 3 and 4 | Beta | Stratified by age, sex and race/ethnicity | NCI’s Surveillance, Epidemiology, and End Results Programme (SEER) Database; CDC’s National Programme of Cancer Registries (NPCR) Database |
| 6. Healthcare-related costs*† | Medical expenditures, productivity loss and patient time costs | online supplemental tables 6 and 7, appendix 6 and appendix table 7 | Gamma | Stratified by age and sex | NCI’s cancer prevalence and cost of care projections; published literature |
| 7. Policy costs*† | For government and industry | online supplemental appendix 5 and appendix tables 5 and 6 | Gamma | Administration and monitoring costs for government; compliance and reformulation costs for industry | FDA’s budget report; Nutrition Review Project; and FDA’s Regulatory Impact Analysis |
| 8. Health-related quality of life (HRQoL)* | For 13 types of cancer | Ranged from 0.64 to 0.86 (online supplemental table 5 and appendix 4) | Beta | EQ-5D§ data from published literature by cancer type | Published literature |
*Uncertainty distributions were incorporated in the probabilistic sensitivity analyses. Uncertainties in each parameter are presented in supplemental materials (online supplemental appendix table 5 and online supplemental tables 2–9).
†If the source did not provide uncertainty estimates, we assumed the standard errors were 20% of the mean estimate to generate gamma distribution.
‡Time-varying input parameter, for which the model accounted for the secular trends. Details are provided in the Supplements.
§EQ-5D is a standardised instrument developed by the EuroQol Group as a measure of health-related quality of life that can be used in a wide range of health conditions and treatments.
BMI, body mass index; CDC, Centers for Disease Control and Prevention; FDA, Food and Drug Administration; NCI, National Cancer Institute; NHANES, National Health and Nutrition Examination Survey.
bmjopen-2022-063614supp001.pdf (261.9KB, pdf)
bmjopen-2022-063614supp002.pdf (3.4MB, pdf)
Simulated US population
Because FDA’s final rules on menu calorie labelling were published in 2016 and implemented in 2018, considering that some restaurants had implemented this policy before 2016 given that the law was passed in 2010, we used 2015–2016 as the baseline and assumed a closed cohort for this analysis. The projected population size of US adults aged ≥20 in 2015–2016 was obtained from the US census data.25 We combined the 2013–2016 National Health and Nutrition Examination Survey (NHANES) to approximate the baseline and simulate the nationally representative US adult population aged ≥20 years in 32 subgroups stratified by age (20–44, 45–54, 55–64, ≥65), sex (male, female), and race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Other) (online supplemental table 1). This closed cohort of US adults was modelled from baseline through their lifetime up to 80 years or until death.
Calorie consumption from restaurants
Mean calorie consumption from full-service and fast-food restaurants, demographics and prevalence of overweight or obesity were estimated using data collected from NHANES participants with at least one valid 24-hour diet recall, in every 32 strata. Following FDA’s estimates,13 we assumed that policy would affect 56.5% of calories consumed at full-service restaurants and 100% at fast-food restaurants. The National Cancer Institute method was used to estimate the usual intake distribution by statistically adjusting for within versus between variance in dietary recalls.26–28 The complex survey design was incorporated in all statistical analyses to ensure the representativeness of study findings to the non-institutionalised US adults.
Policy association with calorie consumption
Policy association with consumer behaviours was obtained from a systematic review and meta-analysis of 13 interventional studies (5 RCTs) with 19 interventions conducted in fast-food, full-service, cafeterias, and laboratories between 2000 and 2015 that evaluated the effectiveness of menu calorie labelling on consumers’ calorie consumption per meal (online supplemental appendix 1 and appendix table 1).15 The study results showed a 7.3% (95% CI 4.4% to 10.1%) reduction in calories consumed per meal following calorie labelling. We assumed that the policy would have a one-time effect over 1 year, with no further change over time.
Policy intervention may stimulate industries to reformulate their products to lower the calorie content. Potential policy impact on industry reformulation was derived from studies of restaurant menu items following the passage and initial period of partial implementation of the final rules (online supplemental appendix table 2). Between 2012 and 2014, among 66 of the 100 largest US chain restaurants, replacing higher-calorie menu items with lower-calorie items led to a 1–5% calorie reduction per menu item.19 20 Among 44 chain restaurants with menu calorie information available in 2008, the calories per menu item fell by 7% between 2008 and 2015.18 Based on the evidence, we chose 5% as the mid-point for the potential policy impact on industry response, which may include discontinuation of existing high-calorie menu items and/or introduction of lower-calorie menu items. We assumed that no reformulation occurs in the first year of policy intervention, and restaurants will replace the high-calorie menu items with low-calorie options or reformulate the menu items in years 2 to 5 of the intervention to achieve a 5% reduction in calorie content, with no change thereafter. Combining the effect on consumer behaviours with the effect on industry response, the policy would lead to a 12.3% reduction in calories consumed per meal.
In addition, we conservatively assumed that there would be some compensatory increased calorie intake outside of restaurants so that only half of all calories reduced from restaurant meals would translate into long-term reductions in daily calories (compensation rate=50%). Therefore, the reduction in calorie consumption from fast-food or full-service restaurants among the simulated population was computed using the baseline consumption times the policy effect estimates, and then times the compensation rate.
Calorie reduction and obesity-associated cancer risk
To estimate the relationships between calorie intake and obesity-associated cancers, we associated the multivariate-adjusted association of change in calorie intake (kcal/day) with change in BMI (kg/m2) and the estimates of BMI and cancer risks. Based on an established energy–weight dynamic model that accounted for the long-term impacts of calorie reduction on weight and metabolic expenditure, we assumed that each 55 kcal/day calorie reduction leads to one pound weight loss over 1 year among overweight or obese adults, with no further reduction thereafter.29 30 Because long-term observational studies suggest that weight change for an equivalent change in dietary intake is about twice as large in overweight or obese adults than normal-weight adults,31 32 we conservatively applied half of this estimate to individuals with normal weight. For each of the 13 obesity-related cancers, the estimated change in risk for each 5 kg/m2 change in BMI was derived from the systematic reviews and meta-analyses of multivariable-adjusted prospective cohort studies conducted by the World Cancer Research Fund/American Institute for Cancer Research Continuous Update Project (CUP) and the International Agency for Research on Cancer (online supplemental table 2).2
Cancer incidence, mortality and health-related quality of life
The incidences of age-adjusted cancer in 2015 were obtained from the National Programme of Cancer Registries and the Surveillance, Epidemiology, and End Results (SEER) programme. We projected the cancer incidence from 2015 to 2030 based on the 2006–2014 trend using the average annual percent change method.33 We then combined the projected incidence rates with the projected US population from the national interim projections34 to account for changes in population age distribution over time. We further applied the cohort-period method to estimate cancer incidence in the closed cohort of US adults in each of the 32 groups as they age (online supplemental table 3, appendix 2 and appendix table 3). The 5-year relative survival rates for each cancer were extracted and converted to an annual probability of death (online supplemental table 4, appendix 3 and appendix table 4).35–37 Health-related quality of life data were obtained from publications that reported the EuroQol-5 dimension utility weights for each cancer among the US patient population (online supplemental table 5 and appendix 4).
Policy and health-related costs
Policy costs included government costs to administer, monitor and evaluate the policy, and industry costs to comply with the policy and reformulate their products (in scenario 2). Government costs were estimated from FDA’s budget report and Nutrition Review Project (online supplemental appendix 5 and appendix tables 5 and 6).38 39 Industry compliance and reformulation costs were based on the FDA’s regulatory impact analysis that included initial and recurring nutrition analysis of standard menu items and menu replacement, provision of nutrition information, employee training and legal review, and accounted for restaurant size and type, reformulation type and compliance period.13
Direct medical costs for cancer care were extracted from the SEER–Medicare linked database for three phases of cancer care: initial (12 months after diagnosis), continuing, and end-of-life (the last year of life) (online supplemental tables 6 and 7, appendix 6 and appendix table 7).33 40 For individuals without cancer, the direct medical costs were estimated based on Medical Expenditure Panel Survey (MEPS) data and insurance claims.24 41 42 Indirect costs including productivity loss due to disability or missed work days and patient time costs were derived from publications using MEPS data.43–46
Cost-effectiveness analysis
Following the guidelines on cost-effectiveness in health and medicine,47 we evaluated the policy impact by projecting the numbers of new cancer cases and cancer deaths averted and quality-adjusted life-years (QALYs) gained and cost-effectiveness from both healthcare and societal perspectives. Net costs from the healthcare perspective were assessed as the difference between government costs for implementing the policy and the direct medical costs of cancer care. Net costs from the societal perspective were assessed as the difference between total policy costs (including both government and industry costs) and health-related costs saved (including direct and indirect costs of cancer care). All costs were inflated to 2015 US dollars using the Consumer Price Index or Personal Healthcare Index, with all costs and QALYs discounted at 3% annually.47 Incremental cost-effectiveness ratios (ICERs) were calculated as net costs divided by the difference in QALYs between policy versus no policy. ICERs falling below a willingness-to-pay threshold of US$150 000 per QALY gained were considered to be cost-effective.48 49 Cost-effectiveness analysis was further conducted among population subgroups by age, sex and race/ethnicity to evaluate policy associations with health disparities.
One-way sensitivity analyses were performed by varying input parameters, including reducing the outside-the-restaurant calorie compensation level to 25% or increasing it to 75%, altering coverage of the FDA’s final rule to all calories from full-service restaurants, reducing the diet–BMI associations to half or doubling the estimates, incorporating an estimated 2% annual increase in medical expenditures associated with cancer care and altering annual discounting rates from 3% to 0% or 5%. We also evaluated impacts at a 10-year time horizon for stakeholders interested in shorter-term health gains and economic benefits. Probabilistic sensitivity analyses were conducted to incorporate uncertainty in all input parameters jointly (table 1). A total of 1000 Monte Carlo simulations were performed, and 95% uncertainty intervals (UIs) were estimated based on the 2.5 and 97.5 percentiles of 1000 simulations. All analyses were conducted using SAS (version 9.4) and R (version 3.3.1).
Patient and public involvement
This study used de-identified datasets and did not involve patients or the public in the design, conduct, reporting or dissemination plans of our research.
Results
Population characteristics
The simulated cohort of US adults in 2015–2016 had a mean age of 47.8 years, with 65.0% being non-Hispanic White adults and 71.4% being overweight or obese (online supplemental tables 8 and 9). A mean of 332 daily calories was consumed from full-service or fast-food restaurants. Higher levels were consumed among younger adults aged 20–44 years (425 kcal/day), men (388 kcal/day), non-Hispanic black (361 kcal/day) and Hispanic (367 kcal/day) adults, in comparison with other corresponding subgroups.
Health gains
The menu calorie labelling was estimated to reduce calories consumed from restaurants by a mean of 24 kcal/day among US adults, and total daily calories by 12 kcal/day. Accounting for potential industry reformulation would reduce the mean intake by an additional 16 kcal/day, and total daily calories by 8 kcal/day.
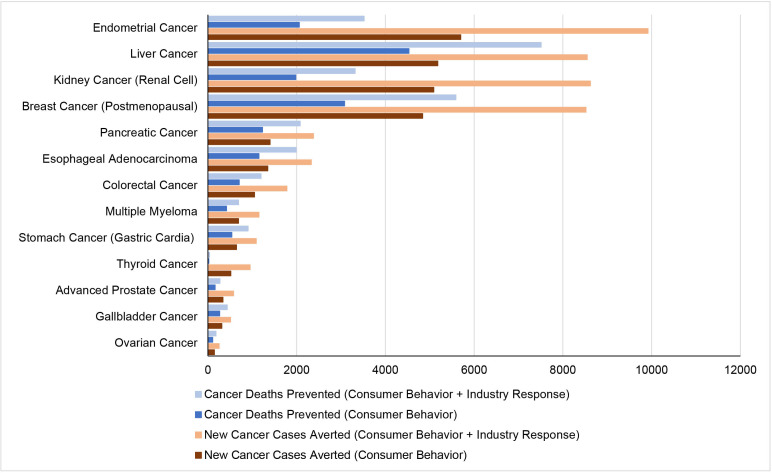
Based on changes in consumer behaviour alone, the policy was associated with a reduction of 28 000 (95% UI 16 300 to 39 100) new cancer cases and 16 700 (9610 to 23 600) cancer deaths, and a gain of 111 000 (64 800 to 158 000) QALYs among 235 million US adults over a median follow-up of 34.4 years (table 2 and figure 1). By cancer type, the greatest numbers of new cancer cases averted were cancers of endometrial (5700 (95% UI 2380 to 9190)), liver (5180 (2800 to 7730)), kidney (5090 (2670 to 7470)), postmenopausal breast (4840 (2010 to 8230)), and pancreas (1400 (756 to 2100)). The greatest numbers of prevented cancer deaths were estimated for cancers of the liver (4530 [2410 to 6760)), postmenopausal breast (3080 (862 to 5650)), endometrial (2060 (957 to 3220)), kidney (1980 (1080 to 2920)), and pancreas (1230 (661 to 1830)).
Table 2.
Estimated health gains and costs of the federal menu calorie labelling policy on reducing the obesity-related cancer burdens in the USA over 10 years and a lifetime (US population=235 162 844)*
| Menu calorie labelling policy | ||||
| 10 Years | Lifetime | |||
| Consumer behaviour Median (2.5% to 97.5%) |
Consumer behaviour+industry response Median (2.5% to 97.5%) |
Consumer behaviour Median (2.5% to 97.5%) |
Consumer behaviour+ industry response Median (2.5% to 97.5%) |
|
| New cancer cases averted, N (95% UI) | ||||
| Endometrial cancer | 692 (276 to 1100) | 1130 (716 to 1550) | 5700 (2380 to 9190) | 9920 (6630 to 13 600) |
| Liver cancer | 366 (144 to 615) | 626 (386 to 887) | 5180 (2800 to 7730) | 8550 (5960 to 11 300) |
| Kidney cancer | 584 (290 to 884) | 980 (689 to 1280) | 5090 (2670 to 7470) | 8620 (6200 to 11 000) |
| Breast cancer (postmenopausal) | 670 (256 to 1110) | 1080 (658 to 1520) | 4840 (2010 to 8230) | 8520 (5610 to 12 200) |
| Pancreatic cancer | 170 (83 to 257) | 273 (183 to 367) | 1400 (756 to 2100) | 2380 (1690 to 3140) |
| Oesophageal adenocarcinoma | 179 (56 to 304) | 286 (159 to 411) | 1350 (485 to 2230) | 2330 (1440 to 3280) |
| Colorectal cancer | 189 (97 to 284) | 319 (225 to 418) | 1050 (561 to 1600) | 1780 (1230 to 2370) |
| Multiple myeloma | 75 (37 to 117) | 122 (81 to 169) | 690 (384 to 1090) | 1150 (775 to 1630) |
| Stomach cancer (cardia) | 54 (6 to 109) | 98 (51 to 165) | 647 (261 to 1140) | 1090 (644 to 1660) |
| Thyroid cancer | 105 (58 to 161) | 176 (123 to 243) | 516 (206 to 914) | 951 (576 to 1420) |
| Advanced prostate cancer | 66 (17 to 118) | 107 (57 to 162) | 339 (138 to 561) | 577 (352 to 836) |
| Gallbladder cancer | 29 (16 to 42) | 46 (34 to 60) | 314 (213 to 438) | 512 (399 to 648) |
| Ovarian cancer | 33 (15 to 56) | 53 (33 to 78) | 147 (44 to 282) | 254 (110 to 420) |
| Total | 3300 (1750 to 4720) | 5230 (3870 to 6790) | 28 000 (16 300 to 39 100) | 47 300 (35 400 to 59 100) |
| Cancer deaths prevented, N (95% UI) | ||||
| Liver cancer | 168 (59 to 287) | 287 (174 to 410) | 4530 (2410 to 6760) | 7510 (5200 to 9980) |
| Breast cancer (postmenopausal) | 68 (33 to 106) | 111 (74 to 149) | 3080 (862 to 5650) | 5590 (3230 to 8310) |
| Endometrial cancer | 52 (20 to 86) | 87 (55 to 121) | 2060 (957 to 3220) | 3520 (2390 to 4700) |
| Kidney cancer | 70 (29 to 110) | 114 (74 to 154) | 1980 (1080 to 2920) | 3320 (2430 to 4300) |
| Pancreatic cancer | 88 (38 to 138) | 143 (93 to 195) | 1230 (661 to 1830) | 2080 (1480 to 2740) |
| Oesophageal adenocarcinoma | 76 (21 to 131) | 122 (69 to 178) | 1150 (403 to 1930) | 1990 (1210 to 2820) |
| Colorectal cancer | 34 (17 to 53) | 57 (40 to 77) | 706 (369 to 1080) | 1200 (839 to 1600) |
| Stomach cancer (cardia) | 22 (2 to 48) | 40 (19 to 68) | 541 (230 to 947) | 907 (538 to 1400) |
| Multiple myeloma | 18 (8 to 30) | 29 (18 to 42) | 420 (239 to 662) | 691 (481 to 980) |
| Gallbladder cancer | 13 (7 to 20) | 21 (15 to 28) | 267 (181 to 369) | 436 (341 to 551) |
| Advanced prostate cancer | 9 (3 to 15) | 13 (7 to 19) | 163 (65 to 280) | 273 (163 to 404) |
| Ovarian cancer | 8 (3 to 15) | 13 (7 to 20) | 107 (39 to 191) | 181 (94 to 290) |
| Thyroid cancer | 1 (1 to 2) | 2 (1 to 3) | 23 (11 to 38) | 38 (24 to 58) |
| Total | 654 (320 to 970) | 1080 (746 to 1400) | 16 700 (9610 to 23 600) | 28 200 (21 100 to 35 300) |
| Life-years gained | 678 (288 to 1040) | 1120 (738 to 1490) | 76 400 (43 400 to 109 000) | 130 000 (96 900 to 162 000) |
| QALYs gained | 4280 (2170 to 6250) | 7030 (4960 to 9090) | 1 11 000 (64 800 to 158 000) | 189 000 (140 000 to 236 000) |
| Changes in health-related costs (US$, millions)ठ| ||||
| Healthcare (medical) cost | −192 (−277 to −100) | −319 (−403 to −227) | −1480 (−2080 to −884) | −2500 (−3090 to −1900) |
| Patient time cost | −7.33 (−10.9 to −3.56) | −12.2 (−15.8 to −8.39) | −102 (−144 to −62.2) | −172 (−216 to −131) |
| Productivity loss | −48.7 (−70.1 to −24.5) | −80.4 (−102 to −56.7) | −608 (−865 to −363) | −1030 (−1290 to −780) |
| Policy implementation costs (US$, millions)ठ| ||||
| Total | 518 (493 to 548) | 644 (612 to 680) | 839 (780 to 908) | 1140 (1060 to 1220) |
| Government cost | 13.2 (11.4 to 15.9) | 13.1 (11.4 to 15.7) | 18.5 (14.5 to 25.1) | 18.5 (14.4 to 25.5) |
| Administration | 9.08 (8.59 to 9.60) | 9.07 (8.64 to 9.50) | 9.07 (8.61 to 9.56) | 9.09 (8.62 to 9.55) |
| Monitoring | 4.09 (2.40 to 6.74) | 4.00 (2.35 to 6.63) | 9.40 (5.45 to 16.1) | 9.38 (5.30 to 16.3) |
| Industry cost | 505 (480 to 535) | 631 (599 to 667) | 820 (762 to 889) | 1120 (1040 to 1210) |
| Compliance | 505 (480 to 535) | 506 (480 to 533) | 820 (762 to 889) | 823 (757 to 889) |
| Reformulation | - | 124 (107 to 146) | - | 296 (249 to 353) |
| Net costs (US$, millions)द | ||||
| Societal perspective | 270 (156 to 389) | 233 (119 to 356) | −1350 (−2260 to −486) | −2570 (−3460 to −1650) |
| Healthcare perspective | −179 (−263 to −86.3) | −305 (−390 to−-214) | −1460 (−2060 to −864) | −2480 (−3070 to −1880) |
| ICER (US$/QALY)† | ||||
| Societal perspective | 64 500 (26 100 to 187 000) | 33 600 (13 300 to 72 400) | Dominant | Dominant |
| Healthcare perspective | Dominant | Dominant | Dominant | Dominant |
*Values are the median estimates (95% uncertainty intervals) of each distribution of 1000 simulations.
†ICER threshold was evaluated at US$150 000/QALY. Dominant represents less costly and more effective than the ”no-policy” intervention.
‡Health-related costs were inflated to 2015 US$ using the Personal Healthcare (PHC) Index. Policy intervention costs were inflated to 2015 US dollars using the Consumer Price Index. Negative costs represent savings.
§Costs are medians from 1000 simulations so may not add up to totals.
¶Net costs were calculated as policy costs minus health-related costs from reduced cancer burden. The societal perspective includes healthcare costs, patient time costs, productivity costs and policy implementation costs; the healthcare perspective included policy costs relevant to policy implementation and programme monitoring and evaluation and medical costs.
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-years; UI, uncertainty interval.
Figure 1.
Estimated new cancer cases and deaths prevented by federal menu calorie labelling policy in the USA by cancer type over a lifetime.
Based on additional industry response, the total estimated health gains approximately doubled, preventing 47 300 (35 400-59 100) new cancer cases and 28 200 (21 100 to 35 300) cancer deaths, and gaining 189 000 (140 000 to 236 000) QALYs, with similar rankings of the types of new cancer cases and cancer deaths prevented.
Economic impacts
Implementing the policy would cost the government US$19 (95% UI 15 to 25) million and the restaurant industry, US$820 (762 to 889) million in compliance costs over a lifetime (table 2). The policy was associated with savings of US$1480 (884 to 2080) million in direct medical costs, US$608 (363 to 865) million in productivity loss costs and US$102 (62 to 144) million in patient time costs. Potential industry reformulation would cost the restaurant industry an additional US$296 (249 to 353) million to implement but would also result in greater healthcare savings, including US$2500 (1900 to 3090) million, US$1030 (780 to 1290) million and US$172 (131 to 216) million in reduced direct medical, productivity loss, and patient time costs, respectively.
From both the healthcare and social perspectives, implementing the menu calorie labelling policy among US adults over a lifetime would be cost saving. With changes in consumer behaviour alone, the net cost savings were estimated to be US$1460 (864 to 2060) million and US$1350 (486 to 2260) million from the healthcare and societal perspective, respectively. With additional industry response, estimated cost savings increased to US$2480 ($1880 to 3070) million from the healthcare perspective and US$2570 (1650 to 3460) million from the societal perspective.
Policy impacts among population subgroups
Among population subgroups, the consumer response to the policy was estimated to result in greater health gains per 100 000 individuals among adults aged 20–44 years (15 new cancer cases averted) and 55–64 years (16 new cancer cases averted) than older age groups (aged ≥65 years; 6 new cancer cases averted); Hispanic and non-Hispanic Black individuals than Non-Hispanic White group (22 vs 9 and 17 vs 9 new cancer cases averted) (table 3). The numbers of cancer deaths averted, life-years and QALYs gained, health-related costs saved and net costs among population subgroups followed a similar pattern (online supplemental tables 10 and 11 and figures 2–5). For instance, the policy was associated with more cancer deaths prevented per 100 000 individuals among younger adults aged 20–44 years than older adults aged ≥65 years (10 vs 3 cancer deaths averted) and Hispanic and non-Hispanic Black adults than non-Hispanic White individuals (14 vs 5 and 11 vs 5 cancer deaths averted). Adding potential industry reformulations resulted in larger health gains among adults aged 45–54 (128% increase in new cancer cases averted) and non-Hispanic White adults (84% increase in new cancer cases averted).
Table 3.
Estimated new cancer cases and deaths prevented by the federal menu calorie labelling project in the USA by age, sex and race/ethnicity, over a lifetime*
| Consumer behaviour | Consumer behaviour+Industry response | |||
| N (95% UI) | Per 100 000 individuals (95% UI) | N (95% UI) | Per 100 000 individuals (95% UI) | |
| New cancer cases averted | ||||
| Age | ||||
| 20–44 | 15 700 (6170 to 25 100) | 15.0 (5.89 to 24.0) | 28 000 (18 000 to 37 500) | 26.7 (17.2 to 35.8) |
| 45–54 | 2810 (−2110 to 8030) | 6.61 (−4.97 to 18.9) | 6420 (1390 to 11 600) | 15.1 (3.27 to 27.2) |
| 55–64 | 6330 (3540 to 9400) | 15.7 (8.76 to 23.3) | 8640 (5790 to 11 800) | 21.4 (14.3 to 29.1) |
| ≥65 | 2740 (795 to 4650) | 5.77 (1.68 to 9.80) | 4060 (2070 to 5950) | 8.55 (4.36 to 12.6) |
| Sex | ||||
| Female | 15 100 (6650 to 24 000) | 12.5 (5.51 to 19.8) | 25 900 (17 400 to 34 900) | 21.4 (14.4 to 28.9) |
| Male | 12 500 (4920 to 20 100) | 10.9 (4.30 to 17.6) | 21 100 (13 500 to 29 100) | 18.4 (11.8 to 25.4) |
| Race/Ethnicity | ||||
| Non-Hispanic White | 14 300 (4310 to 24 500) | 9.16 (2.77 to 15.7) | 26 300 (16 000 to 36 700) | 16.9 (10.3 to 23.6) |
| Non-Hispanic Black | 4720 (1820 to 8100) | 16.6 (6.37 to 28.4) | 7630 (4750 to 11 100) | 26.8 (16.7 to 38.9) |
| Hispanic | 7700 (3560 to 11 500) | 21.5 (9.93 to 32.2) | 11 200 (7060 to 15 300) | 31.3 (19.7 to 42.6) |
| Other | 1150 (−240 to 2440) | 7.60 (−1.59 to 16.2) | 1990 (652 to 3310) | 13.2 (4.33 to 22.0) |
| Cancer deaths prevented | ||||
| Age | ||||
| 20–44 | 10 200 (4170 to 16 400) | 9.73 (3.98 to 15.7) | 18 100 (11 700 to 24 500) | 17.3 (11.2 to 23.4) |
| 45–54 | 1730 (−853 to 4240) | 4.07 (−2.01 to 9.97) | 3650 (1040 to 6240) | 8.58 (2.44 to 14.7) |
| 55–64 | 3320 (1760 to 4930) | 8.21 (4.36 to 12.2) | 4480 (2890 to 6090) | 11.1 (7.15 to 15.1) |
| ≥65 | 1200 (285 to 2130) | 2.53 (0.60 to 4.48) | 1800 (848 to 2720) | 3.79 (1.79 to 5.73) |
| Sex | ||||
| Female | 7810 (3290 to 12 600) | 6.47 (2.73 to 10.5) | 13 400 (8850 to 18 500) | 11.1 (7.33 to 15.3) |
| Male | 8510 (3500 to 13 900) | 7.44 (3.06 to 12.1) | 14 400 (9300 to 20 000) | 12.6 (8.13 to 17.5) |
| Race/ethnicity | ||||
| Non-Hispanic White | 7920 (2180 to 13 900) | 5.08 (1.40 to 8.94) | 14 700 (8770 to 20 900) | 9.45 (5.64 to 13.5) |
| Non-Hispanic Black | 3010 (1000 to 5370) | 10.6 (3.51 to 18.8) | 4990 (2950 to 7380) | 17.5 (10.4 to 25.9) |
| Hispanic | 4960 (2360 to 7560) | 13.8 (6.58 to 21.1) | 7190 (4480 to 9870) | 20.0 (12.5 to 27.5) |
| Other | 565 (−246 to 1350) | 3.75 (−1.63 to 8.97) | 1070 (273 to 1870) | 7.12 (1.81 to 12.4) |
*Values are the median estimates (95% uncertainty intervals) of each distribution of 1000 simulations.
Sensitivity analyses
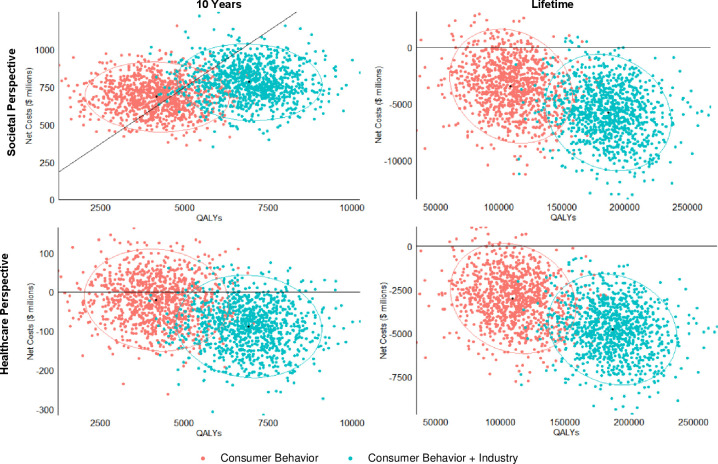
In probabilistic sensitivity analyses, based on consumer responses alone, the menu calorie labelling was cost saving over a lifetime in 93% of 1000 simulations and cost-effective (<$150,000/QALY) in the remaining 7% from the societal perspective, and was cost saving in over 98% of 1000 simulations from the healthcare perspective. Adding the additional industry response increased the probability of cost savings to nearly 100% of the simulations for both the societal and healthcare perspectives (figure 2).
Figure 2.
Probabilistic sensitivity analyses for cost-effectiveness of the federal menu calorie labelling project over 10 years and a lifetime. Values are presented in cost-effectiveness planes of net costs ($millions) versus incremental quality-adjusted life years (QALYs). For each policy scenario, each coloured dot represents one of the 1000 simulations, with the largest dot showing the median incremental cost-effectiveness ratio (ICER, US$/QALY); and the ellipse representing the 95% uncertainty intervals. Results are presented from the societal perspective and the healthcare perspective. Negative values indicate cost savings.
Evaluating health gains, costs and cost-effectiveness at 10 years, the policy remained cost saving from the healthcare perspective and was cost-effective from the societal perspective, with an ICER of US$64 500 (26 100 to 187 000) per QALY based on consumer response alone and US$33 600 (13 300 to 72 400) per QALY with additional industry response. The cost-effectiveness of this policy was most sensitive to varied assumptions of the diet–BMI estimates and annual discounting rates (online supplemental tables 12,13 and figure 6).
Discussion
This study estimated that the federal menu calorie labelling policy, based on consumer response alone, was associated with a reduction of approximately 28 000 new cancer cases and 16 700 cancer deaths among US adults over a lifetime, and net savings of US$1350 and US$1460 million from societal and healthcare perspectives, respectively. Incorporating additional modest industry responses, these health and economic gains were approximately doubled. Greater health gains were expected among younger, middle-aged subgroups, Hispanic, and non-Hispanic Black individuals than for other subgroups. Findings were robust to a range of probabilistic and one-way sensitivity analyses.
Our study findings supported the hypothesis that nutrition policies can have meaningful health and economic impacts on cancer prevention in the USA. In this case, a modest change in mean calorie consumption, distributed across the population, was estimated to achieve important reductions in obesity-related cancer burdens among US adults. Using the best available estimates, our study further suggested that the federal menu calorie labelling policy is cost-effective in the short term and cost saving in the long term in reducing obesity-associated cancer burdens. Many preventive medical screenings are cost-effective, but none of them achieve net savings. For example, among a large cohort of women born in the 1960s over a lifetime, mammography screening starting at age 45 years was estimated to have an ICER of US$40 135/QALY.50 Colonoscopy screening starting at age 45 years among US adults achieved an ICER of US$33 900/QALY.51 Prostate-specific antigen screening had an ICER of US$70 831 to US$136 332/QALY among US men beginning at 40 years of age over a lifetime.52 In contrast, population-based nutrition interventions could be a cost-saving strategy for cancer prevention. Cost-effectiveness analyses showed that a penny-per-ounce tax on sugar-sweetened beverages would be a highly cost-effective strategy for cancer prevention among US adults, with an ICER of US$13 220, the nutrition facts added sugar labelling would prevent 30 000 incident obesity-related cancer cases and 17 100 cancer deaths and be associated with a net saving of US$704 million, and processed meat taxes would avert 77 000 colorectal cancer cases and 12 500 stomach cancer cases and save US$4.5 billion, all from the societal perspective.24 53 54 Thus, while we shall continue the efforts of increasing the screening rates, we also need to consider population-based strategies to improve nutrition for cancer prevention in the USA.
Our findings also indicated the importance of assessing potential industry response, which could nearly double health and economic benefits. The additional impacts of industry reformulation in response to nutrition-related policies have been reported in other studies focused on obesity-associated cancer, diabetes and cardiovascular diseases.23 54–56 Our new findings build on this recent work and highlight the importance of potential strategies to encourage industry reformulation under the federal menu calorie labelling framework to further improve the health benefits and cost-effectiveness of such policies.
In addition, our results showed that population-based nutrition policies such as menu calorie labelling can potentially narrow diet-associated cancer disparities. We found greater health gains and economic impacts among racial/ethnic minorities compared with non-Hispanic Whites, probably due to higher diet-associated cancer burdens among minorities.57 However, labelling policies may have fewer effects on food purchasing behaviours among minorities or socioeconomically disadvantaged groups. Prior studies reported that individuals with higher education and income attainment were more likely to notice and use the menu calorie labels when ordering foods in fast-food or full-service restaurants compared with socioeconomically disadvantaged groups,58–60 and multiracial individuals were less likely to notice and use menu calorie labels in fast food restaurants than non-Hispanic Whites.58 Previous studies also showed that literacy or numeracy could be a barrier to label use.61 62 Thus, it is important for labelling policies to be paired with nutrition education to effectively reduce diet-associated health disparities.
Potential limitations should be considered. First, as a modelling study, our investigation does not provide the impact of real-world policy implementation on the health and economic outcomes of federal menu calorie labelling. However, conducting randomised controlled trials of national nutrition policy interventions is extremely difficult and often implausible, whereas simulation modelling can provide complementary evidence with the flexibility to assess different policy scenarios that help inform policymaking. Second, this evaluation did not include the potential benefits of menu calorie labelling on other health outcomes, such as diabetes and cardiovascular diseases. Considering such outcomes is likely to be associated with greater health gains and cost savings.23 63 64 Third, menu calorie labelling could have a greater effect among subgroups with higher levels of income and education and non-Hispanic White adults58–60 and thus exacerbate health disparities. Owing to the lack of consistent policy effect sizes among populations with different socioeconomic statuses, we were unable to integrate this into our modelling. Fourth, we modelled only the impact of menu calorie labelling on calories, although the policy might also result in potential changes in the nutritional quality of restaurant meals. The majority of current restaurant meals consumed by American adults—70% of meals consumed from fast-food restaurants and 50% consumed from full-service restaurants—are of poor nutritional quality, and the remainder are only of intermediate nutritional quality, with very few being ideal.10 If the policy also improves the quality of restaurant meals, the total reduction in obesity-associated cancer burdens could be greater than our current estimates.
Conclusions
Study findings suggest that menu calorie labelling is associated with lower obesity-related cancer rates and reduced costs. Policymakers may prioritise nutrition policies for cancer prevention in the USA.
Supplementary Material
Footnotes
Twitter: @ddkim62
Contributors: MD contributed to the data curation, formal analysis, visualization, original draft preparation, review, and editing; CFG contributed to the data curation, review, and editing; FC, HE and DDK contributed to software; JBW, PW, DDK, DMi, YCW, and DMo contributed to the review and editing; FFZ contributed the conceptualization, methodology, review and editing, supervision, and funding acquisition. All authors approved the final version. FFZ acts as the guarantor of the study.
Funding: This study was supported by NIH/NIMHD 1R01MD011501. The funding sources had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work. JBW reports leadership or fiduciary role in the US Preventive Services Task Force. DDK reports research funding from the National Institutes of Health, Arnold Ventures, Pharmaceutical Research and Manufacturers of America, Sarepta Therapeutics, and Janssen Therapeutics; consulting fees from Panalgo and the American College of Physicians. DMo reports research funding from the National Institutes of Health, Gates Foundation, Rockefeller Foundation, and Vail Institute for Global Research; consulting fees from Acasti Pharma, Barilla, Danone, and Motif FoodWorks; participating on scientific advisory boards of start-up companies focused on innovations for health including Beren Therapeutics Brightseed, Calibrate, DayTwo, Elysium Health, Filtricine, Foodome, HumanCo, January Inc., Perfect Day, Season, and Tiny Organics; and chapter royalties from UpToDate. All of the above is outside the submitted work. No other relationships or activities could appear to have influenced the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data described in the manuscript, codebook, and analytic code will be made available upon request.
Ethics approval
This study used de-identified datasets and was exempt from institutional review board review.
References
- 1. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer - viewpoint of the IARC working group. N Engl J Med 2016;375:794–8. 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Cancer Research Fund/American Institute for Cancer Research . Continuous update project expert report 2018, body fatness and weight gain and the risk of cancer. 2018. [Google Scholar]
- 3. Steele CB, Thomas CC, Henley SJ, et al. Vital signs: trends in incidence of cancers associated with overweight and obesity-United States, 2005-2014. MMWR Morb Mortal Wkly Rep 2017;66:1052–8. 10.15585/mmwr.mm6639e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2015–2016. 2018. [Google Scholar]
- 5. Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319:1723–5. 10.1001/jama.2018.3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention NCfCDPaHP . Health and economic cost of chronic diseases 2019. Available: https://www.cdc.gov/chronicdisease/about/costs/index.htm [Accessed 26 Jan 2020].
- 7. Hong Y-R, Huo J, Desai R, et al. Excess costs and economic burden of obesity-related cancers in the United States. Value Health 2019;22:1378–86. 10.1016/j.jval.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koroukian SM, Dong W, Berger NA. Changes in age distribution of obesity-associated cancers. JAMA Netw Open 2019;2:e199261. 10.1001/jamanetworkopen.2019.9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin 2020;70:245–71. 10.3322/caac.21591 [DOI] [PubMed] [Google Scholar]
- 10. Liu J, Rehm CD, Micha R, et al. Quality of meals consumed by US adults at full-service and fast-food restaurants, 2003-2016: persistent low quality and widening disparities. J Nutr 2020;150:873–83. 10.1093/jn/nxz299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts SB, Das SK, Suen VMM, et al. Measured energy content of frequently purchased restaurant meals: multi-country cross sectional study [BMJ (Clinical research ed) 2018;363:k4864]. BMJ 2018;363:k4864. 10.1136/bmj.k4864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolfson JA, Bleich SN. Is cooking at home associated with better diet quality or weight-loss intention? Public Health Nutr 2015;18:1397–406. 10.1017/S1368980014001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Food and Drug Administration . Food labeling; nutrition labeling of standard menu items in restaurants and similar retail food establishments; calorie labeling of articles of food in vending machines; final rule in: department of health and human services, ed. 2014. [PubMed] [Google Scholar]
- 14. Petimar J, Zhang F, Cleveland LP, et al. Estimating the effect of calorie menu labeling on calories purchased in a large restaurant franchise in the southern United States: quasi-experimental study. BMJ 2019;367:l5837. 10.1136/bmj.l5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shangguan S, Afshin A, Shulkin M, et al. A meta-analysis of food labeling effects on consumer diet behaviors and industry practices. Am J Prev Med 2019;56:300–14. 10.1016/j.amepre.2018.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Block JP, Roberto CA. Potential benefits of calorie labeling in restaurants. JAMA 2014;312:887–8. 10.1001/jama.2014.9239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Namba A, Auchincloss A, Leonberg BL, et al. Exploratory analysis of fast-food chain restaurant menus before and after implementation of local calorie-labeling policies, 2005-2011. Prev Chronic Dis 2013;10:E101. 10.5888/pcd10.120224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bleich SN, Wolfson JA, Jarlenski MP. Calorie changes in large chain restaurants from 2008 to 2015. Prev Med 2017;100:112–6. 10.1016/j.ypmed.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 19. Bleich SN, Moran AJ, Jarlenski MP, et al. Higher-calorie menu items eliminated in large chain restaurants. Am J Prev Med 2018;54:214–20. 10.1016/j.amepre.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 20. Bleich SN, Wolfson JA, Jarlenski MP. Calorie changes in large chain restaurants: declines in new menu items but room for improvement. Am J Prev Med 2016;50:e1–8. 10.1016/j.amepre.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bleich SN, Wolfson JA, Jarlenski MP, et al. Restaurants with calories displayed on menus had lower calorie counts compared to restaurants without such labels. Health Aff (Millwood) 2015;34:1877–84. 10.1377/hlthaff.2015.0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ananthapavan J, Sacks G, Brown V, et al. Priority-setting for obesity prevention-the assessing cost-effectiveness of obesity prevention policies in Australia (ACE-obesity policy) study. PLoS One 2020;15:e0234804. 10.1371/journal.pone.0234804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Mozaffarian D, Sy S, et al. Health and economic impacts of the national Menu Calorie Labeling Law in the United States: a microsimulation study. Circ Cardiovasc Qual Outcomes 2020;13:e006313. 10.1161/CIRCOUTCOMES.119.006313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim DD, Wilde PE, Michaud DS, et al. Cost effectiveness of nutrition policies on processed meat: implications for cancer burden in the U.S. Am J Prev Med 2019;57:e143–52. 10.1016/j.amepre.2019.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. United States Census Bureau . National population projections tables: main series. 2017. Available: https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html [Accessed 3 Jul 2019].
- 26. Freedman LS, Midthune D, Carroll RJ, et al. Adjustments to improve the estimation of usual dietary intake distributions in the population. J Nutr 2004;134:1836–43. 10.1093/jn/134.7.1836 [DOI] [PubMed] [Google Scholar]
- 27. Herrick KA, Rossen LM, Parsons R, et al. Estimating usual dietary in take from national health and nutrition examination survey data using the National Cancer Institute method. Vital and health statistics series 2, data evaluation and methods research. 2018:1–63. [PubMed]
- 28. Dodd KW, Guenther PM, Freedman LS, et al. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc 2006;106:1640–50. 10.1016/j.jada.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 29. Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–37. 10.1016/S0140-6736(11)60812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hall KD, Schoeller DA, Brown AW. Reducing calories to lose weight. JAMA 2018;319:2336–7. 10.1001/jama.2018.4257 [DOI] [PubMed] [Google Scholar]
- 31. Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Micha R, Peñalvo JL, Cudhea F, et al. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 2017;317:912–24. 10.1001/jama.2017.0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011;103:117–28. 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. United States Census Bureau . National population projections tables. 2014. Available: https://www.census.gov/data/tables/2014/demo/popproj/2014-summary-tables.html [Accessed 3 Jul 2019].
- 35. Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet 2002;360:1131–5. 10.1016/S0140-6736(02)11199-8 [DOI] [PubMed] [Google Scholar]
- 36. Brenner H, Hakulinen T. Up-to-date and precise estimates of cancer patient survival: model-based period analysis. Am J Epidemiol 2006;164:689–96. 10.1093/aje/kwj243 [DOI] [PubMed] [Google Scholar]
- 37. Brenner H, Hakulinen T. Up-to-date cancer survival: period analysis and beyond. Int J Cancer 2009;124:1384–90. 10.1002/ijc.24021 [DOI] [PubMed] [Google Scholar]
- 38. Food and Drug Administration . Justification of estimates for appropriations committees fiscal year. 2012. [Google Scholar]
- 39. Food and Drug Administration . The nutrition review project. report to the director, center for food safety and applied nutrition. 2014. [Google Scholar]
- 40. Martin AB, Hartman M, Washington B, et al. National health care spending in 2017: growth slows to post-great recession rates; share of GDP stabilizes. Health Aff (Millwood) 2019;38:101377hlthaff201805085. 10.1377/hlthaff.2018.05085 [DOI] [PubMed] [Google Scholar]
- 41. French EB, McCauley J, Aragon M, et al. End-of-life medical spending in last twelve months of life is lower than previously reported. Health Aff (Millwood) 2017;36:1211–7. 10.1377/hlthaff.2017.0174 [DOI] [PubMed] [Google Scholar]
- 42. Hogan C, Lunney J, Gabel J, et al. Medicare beneficiaries’ costs of care in the last year of life. Health Aff (Millwood) 2001;20:188–95. 10.1377/hlthaff.20.4.188 [DOI] [PubMed] [Google Scholar]
- 43. Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Nat Cancer Inst 2007;99:14–23. 10.1093/jnci/djk001 [DOI] [PubMed] [Google Scholar]
- 44. Yabroff KR, Guy GP, Ekwueme DU, et al. Annual patient time costs associated with medical care among cancer survivors in the United States. Med Care 2014;52:594–601. 10.1097/MLR.0000000000000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zheng Z, Yabroff KR, Guy GP, et al. Annual medical expenditure and productivity loss among colorectal, female breast, and prostate cancer survivors in the United States. J Natl Cancer Inst 2016;108:djv382. 10.1093/jnci/djv382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guy GP, Ekwueme DU, Yabroff KR, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol 2013;31:3749–57. 10.1200/JCO.2013.49.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093–103. 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 48. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796–7. 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 49. Greenberg D, Earle C, Fang C-H, et al. When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology. J Natl Cancer Inst 2010;102:82–8. 10.1093/jnci/djp472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tina Shih Y-C, Dong W, Xu Y, et al. Assessing the cost-effectiveness of updated breast cancer screening guidelines for average-risk women. Value Health 2019;22:185–93. 10.1016/j.jval.2018.07.880 [DOI] [PubMed] [Google Scholar]
- 51. Ladabaum U, Mannalithara A, Meester RGS, et al. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology 2019;157:137–48. 10.1053/j.gastro.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roth JA, Gulati R, Gore JL, et al. Economic analysis of prostate-specific antigen screening and selective treatment strategies. JAMA Oncol 2016;2:890–8. 10.1001/jamaoncol.2015.6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Du M, Griecci CF, Kim DD, et al. Cost-effectiveness of a national sugar-sweetened beverage tax to reduce cancer burdens and disparities in the United States. JNCI Cancer Spectr 2020;4:pkaa073. 10.1093/jncics/pkaa073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Du M, Griecci CF, Cudhea FF, et al. Cost-effectiveness analysis of nutrition facts added-sugar labeling and obesity-associated cancer rates in the US. JAMA Netw Open 2021;4:e217501. 10.1001/jamanetworkopen.2021.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilde P, Huang Y, Sy S, et al. Cost-effectiveness of a US national sugar-sweetened beverage tax with a multistakeholder approach: who pays and who benefits. Am J Public Health 2019;109:276–84. 10.2105/AJPH.2018.304803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang Y, Kypridemos C, Liu J, et al. Cost-effectiveness of the US Food and Drug Administration added sugar labeling policy for improving diet and health. Circulation 2019;139:2613–24. 10.1161/CIRCULATIONAHA.118.036751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang FF, Cudhea F, Shan Z, et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectr 2019;3:pkz034. 10.1093/jncics/pkz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Feng W, Fox A. Menu labels, for better, and worse? Exploring socio-economic and race-ethnic differences in menu label use in a national sample. Appetite 2018;128:223–32. 10.1016/j.appet.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 59. Green JE, Brown AG, Ohri-Vachaspati P. Sociodemographic disparities among fast-food restaurant customers who notice and use calorie menu labels. J Acad Nutr Diet 2015;115:1093–101. 10.1016/j.jand.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 60. Lee-Kwan SH, Pan L, Maynard LM, et al. Factors associated with self-reported menu-labeling usage among US adults. J Acad Nutr Diet 2016;116:1127–35. 10.1016/j.jand.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Malloy-Weir L, Cooper M. Health literacy, literacy, numeracy and nutrition label understanding and use: a scoping review of the literature. J Hum Nutr Diet 2017;30:309–25. 10.1111/jhn.12428 [DOI] [PubMed] [Google Scholar]
- 62. Nogueira LM, Thai CL, Nelson W, et al. Nutrition label numeracy: disparities and association with health behaviors. Am J Health Behav 2016;40:427–36. 10.5993/AJHB.40.4.4 [DOI] [PubMed] [Google Scholar]
- 63. Gortmaker SL, Wang YC, Long MW, et al. Three interventions that reduce childhood obesity are projected to save more than they cost to implement. Health Aff (Millwood) 2015;34:1932–9. 10.1377/hlthaff.2015.0631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kuo T, Jarosz CJ, Simon P, et al. Menu labeling as a potential strategy for combating the obesity epidemic: a health impact assessment. Am J Public Health 2009;99:1680–6. 10.2105/AJPH.2008.153023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063614supp001.pdf (261.9KB, pdf)
bmjopen-2022-063614supp002.pdf (3.4MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data described in the manuscript, codebook, and analytic code will be made available upon request.