Abstract
We have cloned a hrp gene cluster from Xanthomonas oryzae pv. oryzae. Bacteria with mutations in the hrp region have reduced growth in rice leaves and lose the ability to elicit a hypersensitive response (HR) on the appropriate resistant cultivars of rice and the nonhost plant tomato. A 12,165-bp portion of nucleotide sequence from the presumed left end and extending through the hrpB operon was determined. The region was most similar to hrp genes from Xanthomonas campestris pv. vesicatoria and Ralstonia solanacearum. Two new hrp-associated loci, named hpa1 and hpa2, were located beyond the hrpA operon. The hpa1 gene encoded a 13-kDa glycine-rich protein with a composition similar to those of harpins and PopA. The product of hpa2 was similar to lysozyme-like proteins. Perfect PIP boxes were present in the hrpB and hpa1 operons, while a variant PIP box was located upstream of hpa2. A strain with a deletion encompassing hpa1 and hpa2 had reduced pathogenicity and elicited a weak HR on nonhost and resistant host plants. Experiments using single mutations in hpa1 and hpa2 indicated that the loss of hpa1 was the principal cause of the reduced pathogenicity of the deletion strain. A 1,519-bp insertion element was located immediately downstream of hpa2. Hybridization with hpa2 indicated that the gene was present in all of the strains of Xanthomonas examined. Hybridization experiments with hpa1 and IS1114 indicated that these sequences were detectable in all strains of X. oryzae pv. oryzae and some other Xanthomonas species.
The hrp (“harp”) genes encode type III secretory pathways and are required by many phytopathogenic bacteria to elicit a hypersensitive response (HR) on nonhost or resistant host plants and for pathogenesis on susceptible hosts. The HR is a rapid localized death of the host cells that occurs upon pathogen infection and, together with the expression of a complex array of defense-related genes, is a component of plant resistance. The hrp genes were first identified in Pseudomonas syringae pv. phaseolicola, a bean pathogen (38). Since then, hrp genes from a variety of plant pathogenic bacteria, including Erwinia, Pseudomonas, Ralstonia, and Xanthomonas, have been characterized (for reviews, see references 2, 9, and 11). The specific functions of the hrp pathway in pathogenesis are not known. However, type III secretion pathways of animal and plant pathogens have been demonstrated to mediate the secretion of virulence factors into the extracellular melieu. Some of the proteins ultimately end up in the host cell cytoplasm (reviewed in references 11, 25, and 37). In mediating the interaction of the bacterium and the host plant, the hrp pathway presumably acts to prevent or inhibit a general resistance response or otherwise enhance the colonization of the plant by the bacteria.
Given the importance of the type III systems to pathogenicity, it can be expected that analysis of the systems in different species will provide insight into the adaptation of the species to their respective host plants. The hrp gene clusters appear to group into two types on the basis of sequence relatedness and operon organization (reviewed in reference 9). The hrp genes of Pseudomonas and Erwinia comprise one group, and the hrp genes of Xanthomonas campestris pv. vesicatoria and Ralstonia solanacearum comprise the second group. Our present understanding of the group 2 hrp genes is based almost entirely on the characterization of two strains representing R. solanacearum and X. campestris pv. vesicatoria. The genus Xanthomonas itself is comprised of a large number of different species and pathovars that colonize over 392 species of plants (36). Xanthomonas oryzae pv. oryzae is the causal pathogen of bacterial leaf blight on rice (54). We report here the cloning of a hrp cluster from X. oryzae pv. oryzae and sequence analysis of the left end of the region. In the process of characterization, we identified two novel loci that are associated with the hrp cluster and an insertion sequence (IS) element not previously characterized in X. oryzae pv. oryzae.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in the experiments are listed in Table 1. Escherichia coli strains were grown in Terrific broth (TB) or on Luria agar plates at 37°C with the appropriate antibiotics. X. oryzae pv. oryzae strains were cultured on tryptone sucrose agar or in TB at 28°C. The genomic library of PXO86 in pHM1 was described previously (24). Carbenicillin, spectinomycin, and kanamycin were used at 100 μg/ml.
TABLE 1.
Strains and plasmids used in study
| Strain, phage, or plasmid | Relevent characteristics | Reference or source |
|---|---|---|
| Strains | ||
| X. oryzae pv. oryzae | ||
| PXO86 | Wild-type strain, race 2 | 24 |
| PXO99A | 5-Azacytidine resistant, race 6 | 24 |
| PXO99A2785mx | GPS-1 insertion in hrpA, Knr | This study |
| PXO99A3230mx | GPS-1 insertion in hrpA, Knr | This study |
| PXO99A8mx | hrpB mutant of PXO99A, Knr | This study |
| PXO99A9mx | hrpC mutant of PXO99A, Knr | This study |
| PXO99A56mx | hrpD mutant of PXO99A, Knr | This study |
| PXO99AΔ2 | PXO99A with 2-kb SalI/XhoI deletion, Knr | This study |
| E. coli | ||
| DH5α | F−recA φ80dlacZ ΔM15 | Gibco-BRL |
| S17-1 | recA Tra+ Spr | 53 |
| TB1 | recA+ C2110, Nalr Rifr polA1 rha his | 35 |
| Phage λA1 | Tn5-gusA1 Knr Tetr | 51 |
| Plasmids | ||
| pBluescript II KS(+) | Phagemid, pUC derivative, Cbr | Stratagene |
| pCR2.1-TOPO | Phagemid, Cbr | Invitrogen |
| pUC4K | pUC7 with nptII | 58 |
| pHM1 | Broad-host-range vector with pUC19 polylinker, Spr | R. Innis |
| p2-2 | Cosmid clone of X. oryzae pv. oryzae hrp region | This study |
| p23-14 | Cosmid clone of X. oryzae pv. oryzae hrp region | This study |
| p23-44 | Cosmid clone of X. oryzae pv. oryzae hrp region | This study |
| pFWX10-F2 | avrXa10 in pHM1 | 65 |
| pK4.0 | 4.0-kb KpnI fragment from p23-44 | This study |
| pK6.0 | 6.0-kb KpnI fragment from p23-44 | This study |
| pK6.0B | BglII/BamHI deletion of pK6.0 | This study |
| pK6.0BΔ2 | 2.0-kb SalI/XhoI fragment replaced by 1.3-kb SalI Knr fragment in pK6.0B | This study |
| p23-44Δ2 | Cosmid with 2.0-kb SalI/XhoI deletion, Knr | This study |
| pK6.0B-479 | GPS-1 insertion at position 479, Knr | This study |
| pK6.0B-1116 | GPS-1 insertion at position 1116, Knr | This study |
| pK6.0B-1139 | 1.3-kb fragment from pUC4K, Knr | This study |
| pK6.0B-1795 | GPS-1 insertion at position 1795, Knr | This study |
Recombinant DNA techniques.
DNA manipulations were performed by standard procedures (6). Restriction enzymes, T4 DNA ligase, Klenow fragment of DNA polymerase I, and Taq polymerase were purchased from Life Technologies, Inc. (Gaithersburg, Md.) and Fisher Scientific (St. Louis, Mo.). Chemicals were purchased from Sigma Chemical (St. Louis, Mo.) and Fisher Scientific. BioTrace HP membrane (Gelman Sciences, Ann Arbor, Mich.) was used for Southern blot hybridization. EcoRI DNA fragments from p23-44, a cosmid clone containing hrp genes of X. oryzae pv. oryzae, were subcloned into pBluescript KS(+) (Stratagene, Inc., La Jolla, Calif.). The DNA sequences of the two strands of these subclones were determined by the DNA Sequencing Facility of Iowa State University. Amino acid alignments were constructed using Clustal W 1.7 (55). Similarity searches were performed using the BLAST program (4). Identity and similarity comparisons among proteins were performed using the Fasta program (45). Potential signal peptides at the N-terminal and transmembrane domains of the proteins were predicted by PSORT (41, 42) and TopPred2 (60), respectively. Specific motif searches were performed using the MOTIF program (43).
Transposon and deletion mutagenesis.
Mutagenesis of p23-44 with Tn5-gusA1 was performed in E. coli strain DH5αMCR. Cells growing exponentially in TB were infected with bacteriophage pλA1 carrying Tn5-gusA1 (51). After 1 h of incubation at 37°C, which allowed the phage to be absorbed and phenotypic expression of kanamycin resistance, bacteria were plated on Luria agar medium supplemented with kanamycin and incubated overnight at 28°C. Plasmid DNA was extracted from single colonies, digested with EcoRI, and analyzed by electrophoresis on 1% agarose gels. Each plasmid with a transposon in the bacterial genomic DNA of the clone was transformed into PXO99A by electroporation or by conjugation using the conjugal helper strain S17-1 (53). Marker exchange mutagenesis was performed using spontaneous homologous recombination and screening for kanamycin-resistant, spectinomycin-sensitive clones. Mutations were confirmed by Southern blot analysis of bacterial genomic DNA using 32P-labeled probes for subclones of p23-44. Four isolates from each marker exchange were tested on tomato and rice plants. The p23-44 cosmid was reintroduced by electroporation into the hrp mutants for complementation tests.
Mutations in hpa1, hpa2, and hrpA were made using GPS-1 of the Genome Priming System (New England BioLabs, Beverly, Mass.). GPS-1 contains a modified Tn7 with the nptII gene for resistance to kanamycin, and insertions were generated in vitro in pK4.0 and pK6.0B according to the instructions of the manufacturer (14). One mutation in hpa1 was generated by introducing the gene for resistance to kanamycin from pUC4K into the EcoRI site of hpa1 (58). Marker exchange mutations of hrpA were created by first introducing the GPS-1 mutations into p23-44 in E. coli. Recombinants were generated by introduction of both plasmids into the Rec+ strain of E. coli TB1. Recombinant plasmids were rescued by electroporation into E. coli strain C2110 (35), which is deficient in polymerase I activity and does not permit replication of ColE1 replicons (pK4.0 or pK6.0B), and selection for resistance to spectinomycin and kanamycin. The resulting recombinant cosmids were transformed into E. coli strain S17-1 and moved from S17-1 into the PXO99A strain of X. oryzae pv. oryzae by biparental mating. Four colonies were selected for marker exchange mutagenesis as described above.
The deletion in the left end of the hrp cluster covering hpa1 and hpa2 was created by replacing a 2.1-kb SalI/XhoI fragment in pK6.0B with 1.3-kb SalI fragment containing a kanamycin resistance gene from pUC4K (58). The resulting plasmid, pK6.0BΔ2, was introduced into p23-44 by homologous recombination in E. coli as described above for the GPS-1 hrpA mutations. The resulting cosmid, p23-44Δ2, was first transformed into E. coli strain S17-1 and then moved from S17-1 into X. oryzae pv. oryzae PXO99A by biparental mating. Twenty colonies that were sensitive to spectinomycin and resistant to kanamycin after growth on nonselective media were obtained and tested for virulence and HR on rice and tomato.
Plant assays.
Pathogenicity and hypersensitivity assays were performed as described previously (24). Tomato cv. VFN8 was used for the nonhost hypersensitivity test. Ten-day-old rice seedlings IRBB10 and IRBB7, containing corresponding resistance genes Xa10 and Xa7, respectively, were used for race-specific resistance assays. IR24 was used for pathogenicity testing. All plants were grown in growth chambers at 28°C (daytime) and 25°C (nighttime) with a 14-h photoperiod and 85% humidity. For hrp phenotype assays, inoculum concentrations were adjusted to an optical density at 600 nm of 1.0 (approximately 2 × 109 CFU/ml) using a DU-64 spectrophotometer (Beckman Instruments). Inoculum concentrations for hpa1 and hpa2 mutation phenotype assays were adjusted to an optical density at 600 nm of 0.3. The differences between wild-type and mutant pathogenicity and hypersensitivity reactions were enhanced at the lower dilution. Growth of bacteria on rice after infiltration was monitored as previously described (24).
Sequence analysis of PXO99A.
A 651-bp region was amplified by PCR from PXO99A using the primers 5′-GATTGTCTGCGGAAAATAG-3′ (IS99FOR) and 5′-GGTACGCAGCAGATCTGGG-3′ (IS99REV), cloned into pCR2.1-TOPO (52), and sequenced. The parameters used for PCR were as follows: step 1, 95°C for 2 min; step 2, 50°C for 30 s; step 3, 72°C for 90 s; step 4, 95°C for 30 s; step 5, 35 cycles from step 2 to step 4; step 6, 72°C for 2 min.
Southern hybridization analysis.
Total DNA isolation and Southern hybridization analysis were as previously described (34). Probes were prepared by either PCR (49) or gel purification and random priming (6). The 408-bp EcoRI/XhoI fragment from hpa1 was used as a probe for hpa1-related sequences. A 321-bp probe for hpa1 was generated by PCR using internal primers 5′-AACAGGATCCAGATTGCTTCGAAGAGGCTGCC-3′ (HPA2F1) and 5′-AACAAGGATCCGCATATTTATCACGCTCC-3′ (HPA2R1). A 1522 base pair probe for IS1114 was amplified using primers 5′-AGTCGCCCCTGAAAAACCCCCAG-3′ (ISHRPF1) and 5′-AAGTCGCCCCTGAAAAACCCTC-3′ (ISHRPR1). PCR conditions for both probes were as described above. Probes were labeled using a Rediprime random-priming labeling kit (Amersham, Arlington Heights, Ill.).
Nucleotide sequence accession numbers.
The DNA sequence for the hpa2-to-hrpB region has GenBank accession no. AF232057. The sequence for the region including IS1114 from PXO86 is under GenBank accession no. AF232058. The sequence of the corresponding region of IS1114 insertion in PXO99A is under GenBank accession no. AF232714.
RESULTS
Isolation of the hrp region of X. oryzae pv. oryzae.
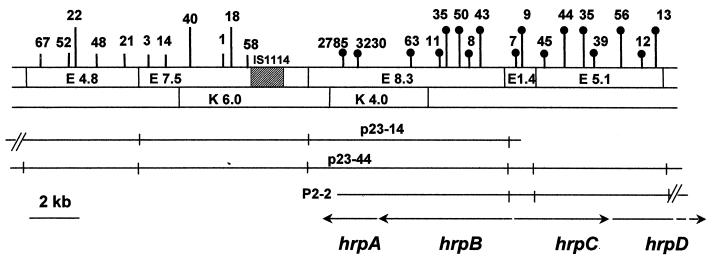
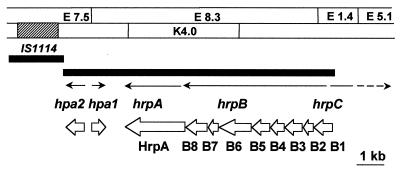
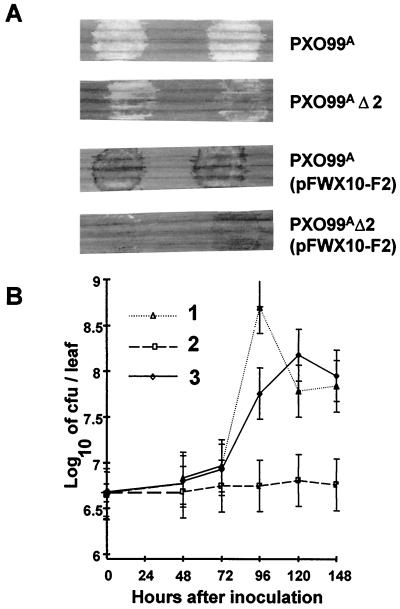
Cosmids p23-14, p23-44, and p2-2 were recovered from a genomic library of strain PXO86 by using p83-15, which contained a portion of the X. campestris pv. vesicatoria hrp region (10), as a probe. The maps of the clones were characterized by endonuclease restriction digests, Southern blotting, and DNA sequence analysis and shown to cover the regions corresponding to hrpA, hrpB, hrpC, and hrpD of X. campestris pv. vesicatoria (Fig. 1). Thirty-five strains with Tn5-gusA1 insertions in the region covered by p23-44 were generated from strain PXO99A of X. oryzae pv. oryzae. Eleven strains, with insertions in the 4.8- and 7.5-kb EcoRI fragments, had unaltered hrp gene function. Twenty-four strains, with insertions in the 8.3-, 1.4-, and 5.1-kb EcoRI fragments, lost the ability to elicit disease on rice and an HR on tomato (Fig. 1). The mapping data indicated that the insertions in hrp loci were located in the hrpB, hrpC, and hrpD operons. None of the Tn5-gusA1 insertions appeared to have inserted into the hrpA operon. Therefore, two insertions in hrpA were generated in pK4.0 by using pGPS-1 and recombined into the hrp region (Fig. 1, insertions 3230 and 2785). Both mutations resulted in hrp mutant phenotypes. The phenotypes of a representative hrp mutant after inoculation on rice and tomato are shown in Fig. 2A. Introduction of p23-44 into selected hrp mutants restored the ability to elicit disease on rice and an HR on tomato (Fig. 2A). In planta growth of a representative hrp mutant was reduced compared to that of the wild-type strain, while the levels of in planta growth of the mutant strains carrying p23-44 were similar to that of the parent strain PXO99A (Fig. 2B).
FIG. 1.
Restriction fragment map of the hrp region in X. oryzae pv. oryzae. The name of each fragment also indicates the size in kilobases. Vertical lines above the map indicate positions of Tn5-gusA1 insertion. Lines with filled circles indicate that the insertion abolished hrp function; lines without circles indicate insertions that did not cause loss of hrp activity. Arrows indicate positions and orientations of hrp operons. E, EcoRI; K, KpnI.
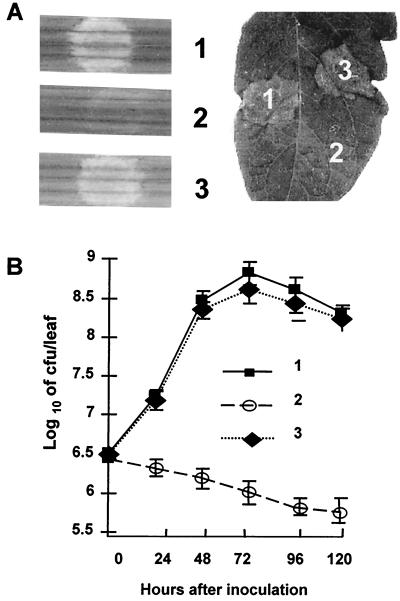
FIG. 2.
Effects of mutations in the hrp genes of X. oryzae pv. oryzae. (A) Phenotype of rice (cultivar IR24) and tomato leaf (VFN8) reactions to the following strains: 1, PXO99A; 2, PXO99A9mx (hrpC); 3, PXO99A9mx (p23-44). Water soaking and disease symptoms were evidenced by discoloration at the inoculation site on rice leaves (left). Inoculation in the hrp mutants resulted in no change in leaf coloration (leaf 2). HR on tomato leaf (right) is indicated by light gray patches (leaves 1 and 3). (B) Effect of hrp mutation on growth of bacteria in rice leaves. Numbering as for panel A.
X. oryzae pv. oryzae interacts with rice plants in a race-specific manner. PXO99A with avrXa10 or avrXa7 elicits an HR on rice cultivars with the corresponding resistance gene Xa10 or Xa7 (24). Three hrp mutants of X. oryzae pv. oryzae, PXO99A8mx, PXO99A9mx, and PXO99A56mx, whose insertions could be mapped in the hrpB, hrpC, and hrpD operons, respectively, were unable to elicit a race-specific HR when carrying avrXa10 (Table 2). Identical results were obtained with avrXa7 (data not shown).
TABLE 2.
Phenotypes of interaction between X. oryzae pv. oryzae strains and plants
| Strain | Phenotypea on:
|
|
|---|---|---|
| Rice (IRBB10b) | Tomato | |
| PXO99A | WS | HR |
| PXO99A8mx | NR | NR |
| PXO99A9mx | NR | NR |
| PXO99A56mx | NR | NR |
| PXO99A8mx(p23-44) | WS | HR |
| PXO99A9mx(p23-44) | WS | HR |
| PXO99A56mx(p23-44) | WS | HR |
| PXO99A(pFWX10-F2) | HR | HR |
| PXO99A8mx(pFWX10-F2) | NR | NR |
| PXO99A9mx(pFWX10-F2) | NR | NR |
| PXO99A56mx(pFWX10-F2) | NR | NR |
WS, water soaking (pathogenesis reaction); NR, no reaction.
IRBB10 is rice cultivar containing resistance gene Xa10.
Two novel hrp-associated loci are located in the left end of the hrp cluster.
The DNA sequence was determined for 12,165 bp from the BglI site in the 7.5-kb EcoRI fragment (E7.5) and extending 713 bp into the 1.4-kb EcoRI fragment of p23-44 (Fig. 3). The sequence data were organized into two portions. The first portion, starting at the second SalI site in E7.5, contained 10,096 bp and included transcription units A and B and two additional open reading frames, tentatively termed hpa1 and hpa2 (Fig. 3). Table 3 gives the positions and properties of the noted features in the first portion. The second portion, containing 2,075 bp and the IS element IS1114, extended upstream from the SalI site to a BglII site in the E7.5 fragment (Fig. 3).
FIG. 3.
Region of sequence analysis of the left hrp region of PXO86. Transcriptional units and open reading frames of the hrp genes are indicated by lines and open arrows, respectively. The direction of transcription and translation is indicated by direction of the arrow. Filled bars indicate sequenced regions. E, EcoRI; K, KpnI.
TABLE 3.
Summary of features of the sequence of the left hrp region of X. oryzae pv. oryzae
| Sequence positiona | Feature | Properties (aa, MW)b | Relatednessd
|
Hrc nomenclaturee | Flagellar homologf | |
|---|---|---|---|---|---|---|
| Xcv | Rs | |||||
| 569–129 | Hpa2 | 146c, 16.5 | ||||
| 884–908 | PIP-1 | |||||
| 976–1000 | PIP-2 | |||||
| 1136–1567 | Hpa1 | 143, 14.0 | ||||
| 4123–2306 | HrpA | 605, 64.0 | 96/99 | HrpA, 48/54 | HrcC | |
| 5036–4208 | HrpB8 | 236c, 24.9 | 96/99 | HrpC, 46/81 | HrcT | FliR |
| 5542–5033 | HrpB7 | 169, 18.8 | 95/100 | HrpD, 25/60 | ||
| 6863–5535 | HrpB6 | 422, 47.2 | 98/99 | HrpE, 59/89 | HrcN | FliI |
| 7554–6853 | HrpB5 | 233, 25.3 | 100/100 | HrpF, 25/54 | HrcL | FliHg |
| 8168–7539 | HrpB4 | 209, 22.2 | 97/100 | HrpH, 27/60 | ||
| 8937–8176 | HrpB3 | 232c, 24.9 | 94/100 | HrpI, 56/78 | HrcJ | FliF |
| 9331–8939 | HrpB2 | 110c, 11.8 | 98/100 | HrpJ, 31/73 | ||
| 9820–9365 | HrpB1 | 151, 15.9 | 96/99 | HrpK, 32/80 | ||
| 9901–9926 | PIP-3 | |||||
| 9941–9965 | PIP-4 | |||||
| 10035 | HrpC1 | NDh | ||||
Nucleotide position according to GenBank accession no. AF232057.
aa, number of amino acid residues; MW, molecular mass in kilodaltons.
Protein size after cleavage of putative signal peptide.
Relatedness to proteins of the same name from X. campestris pv. vesicatoria (Xcv) (17, 18) or to proteins from the hrp region of R. solanacearum (Rs) (21). The first number indicates the percentage of identical amino acid matches. The second number indicates the percentage of similar amino acid matches.
Classification within the highly conserved members of the Hrp pathway proteins according to Bogdanove et al. (8).
Related component of flagellar biosynthetic pathway (8).
ND, not determined.
The hrpA operon contained a single coding sequence for HrpA (HrcC, using the unified nomenclature [8]), which started at position 4123 and continued to position 2306. The sequence predicted a protein of 605 amino acids with 96% similarity to HrpA1 of X. campestris pv. vesicatoria (63). HrpA from X. oryzae pv. oryzae lacked two alanine residues corresponding to positions 295 and 296 in HrpA1 from X. campestris pv. vesicatoria (63) and was, therefore, two amino acids shorter than HrpA1 from X. campestris pv. vesicatoria. The hrpB locus of X. oryzae pv. oryzae contained eight coding regions extending from position 9820, which is the start of HrpB1, to the end of HrpB8 at 4211. The putative GTG start codon for HrpB1 was based on the alignment to HrpB1 of X. campestris pv. vesicatoria, which has the usual ATG start codon (18). A PIP box (PIP-3), which consists of two direct repeats (TTCGC) separated by 15 nucleotides, was located 81 bp upstream of the putative HrpB1-coding sequence and was similar to the PIP box found in X. campestris pv. vesicatoria (18) and R. solanacearum (21). PIP-3 was separated by 14 nucleotides from another PIP box (PIP-4) in the opposite direction. PIP-4 was located 155 nucleotides away from the start codon of the putative HrpC1-coding sequence. (Complete sequence analysis of the hrpC operon will be presented later.)
Sequence analysis at the left end of the 8.3-kb EcoRI fragment revealed an open reading frame for a protein of 143 amino acid residues, starting at position 1136, that had not previously been described for Xanthomonas and was tentatively named hpa1 (Fig. 4). A perfect consensus PIP box was located at position 975 and 135 bp upstream of hpa1. The putative protein encoded by hpa1 is glycine rich (26% glycine), particularly in the middle and C-terminal portions, and has no high degree of sequence similarity to proteins in the databases.
FIG. 4.
DNA sequence of hpa1. Sequences for a PIP box, restriction sites, and a ribosome binding site (SD) are underlined. The sequence of the deduced translation product is given in the single-letter code below the DNA sequence. The glycine-rich regions are double underlined. Asterisks indicate a potential transmembrane motif.
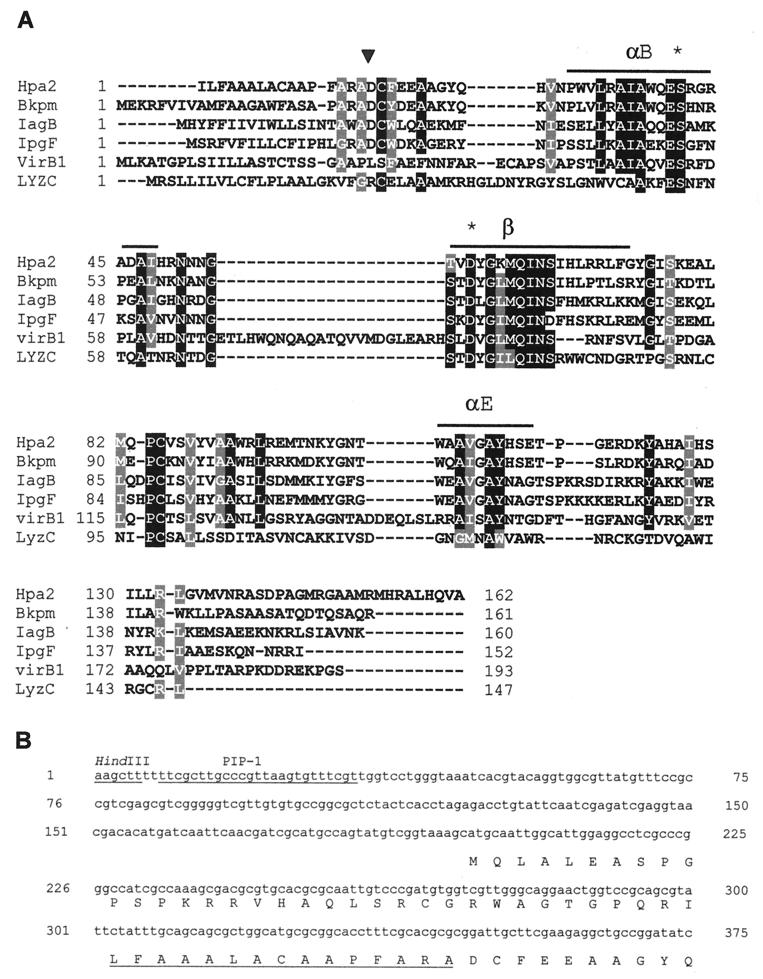
The hpa2 open reading frame was oriented in the opposite direction to hpa1. The putative protein product has a high degree of similarity at the amino acid level to a group of lysozyme-like proteins starting at position 569 (Fig. 5A). An imperfect PIP box (with C replaced by a T in the second TTCGC consensus repeat) was located upstream of hpa2 at position 884 and 165 bp upstream from the first ATG (Fig. 5B). The putative start codon for Hpa2 was unclear. The first upstream ATG was 150 bp from the region of similarity to lysozyme-like proteins (Fig. 5B). A consensus secretion signal sequence was identified within 54 bp upstream from the start of the sequence similarity to lysozyme-like proteins (Fig. 5B). The putative cleavage site was predicted to occur immediately before the start of the sequence similarity.
FIG. 5.
Sequence analysis of hpa2. (A) Sequence alignment of Hpa2 and selected proteins. Bkpm, Burkholderia pseudomallei (GenBank accession no. AAD05172); IagB, Salmonella enterica serovar Typhimurium (39); IpgF, Shigella flexneri (3); VirB1, Agrobacterium tumefaciens (40); LyzC, chicken (28). The conserved A and E α-helices (αB and αE) and β-sheet (β) in the structure of lysozyme are overlined. Asterisks indicate conserved asparagine and aspartate residues in the catalytic region of lysozyme. Gaps in the alignment are represented by dashes. Hpa2 is shown as starting from the isoleucine that is 16 residues upstream of the putative cleavage site. The triangle indicates the putative cleavage site. (B) Promoter region of hpa2. A HindIII site and imperfect PIP box (PIP-1) are underlined. Only amino acids starting from the last methionine before the conserved region are indicated. The predicted signal peptide in the protein product is underlined.
An IS element is located adjacent to hpa2 in PXO86.
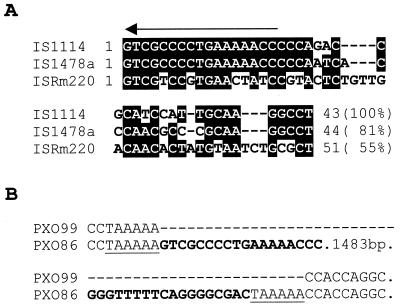
An IS element was identified 254 bp from the end of hpa2. The element is 1,519 bp long and bounded by an 18-bp perfect inverted repeat. IS1114 was similar in sequence to two recently described insertion elements from Rhizobium and X. campestris pv. campestris (Fig. 6A) (13, 50). The repeat was located within a 6-bp direct repeat (TAAAA) that may have been generated by the insertion process (Fig. 6B). Strain PXO99A did not contain IS1114 adjacent to hpa2, and the sequence analysis of the same region revealed only the TAAAA sequence with no duplication (Fig. 6B). The facts that the TAAAA sequence, which is duplicated at the ends of IS1114 in PXO86, was not duplicated in PXO99A and that no remnant of IS1114 was found at the corresponding region of PXO99A suggest that the insertion is unique to the PXO86 lineage. The sequence from the BglII site to the end of hpa2 without the IS element did not match any entries in the GenBank database. Therefore, the element also does not appear to have inserted into an identifiable genetic locus. Genetic loci that are involved in pathogenicity have been associated with transmissible genetic elements (30). These so-called pathogenicity islands may also have G+C compositions that differ from the G+C content of the bulk of the chromosomal DNA, reflecting the transfer among different species of bacteria (reviewed in reference 22). However, no evidence for a dramatic shift in the G+C content at the element's boundary with the hrp region was observed. We therefore could not find that the element had any relevance to hrp function. We believe that IS1114 is present near the hrp region in some lineages and probably inserted into the region by chance.
FIG. 6.
Sequence analysis of IS1114. (A) Sequence similarity of IS1114 to insertion elements IS1478a (13) and ISRm220-13-5 (50). The alignment shows only 43 to 51 bases of sequence from one end, with the overall sequence identity of the entire element to IS1114 given in parentheses. The arrow indicates an 18-bp repeat. Identities in two of the three elements are shaded. (B) Sequence alignment of genomic DNA from strain PXO99A with the region of insertion in strain PXO86. Only the 18-bp direct repeats are shown (boldface). Dashes indicate spaces introduced for optimal alignment. A direct 6-bp repeat at the site of insertion in PXO86 is underlined.
Deletion of the left end affects virulence.
A deletion mutant (PXO99AΔ2) covering the hpa1-hpa2 region was constructed by replacing the region between the SalI and XhoI sites of pK6.0B with the gene for resistance to kanamycin and subsequent recombination into the chromosome of PXO99A to give strain PXO99AΔ2 (58). PXO99AΔ2 was therefore missing hpa2 entirely, and hpa1 was truncated. Upon inoculation to rice, PXO99AΔ2 was found to cause reduced disease symptoms and, when harboring avrXa10, elicited a weak HR on rice plants with resistance gene Xa10 in terms of the intensity of browning (Fig. 7A). The reduced disease symptoms were accompanied by reduced bacterial populations in the leaf tissue (Fig. 7B). Similarly, the HR of the mutant on tomato was delayed and weaker in terms of the area that collapsed after inoculation (not shown). The reduced pathogenicity of the mutant could be complemented by reintroduction of p23-44 (Fig. 7B).
FIG. 7.
Effect of an hpa1-hpa2 deletion on virulence and avrXa10 activity. (A) Phenotypes of PXO99AΔ2 and PXO99AΔ2(pFWX10-F2), which contains the avirulence gene avrXa10, on susceptible (IR24) and resistant (BB10) rice cultivars. Susceptible leaves (upper two leaves) and resistant leaves (lower two leaves) were photographed 3 days after inoculation with the strain indicated at the right. (B) Growth of PXO99AΔ2 in susceptible rice cultivar IR24. 1, PXO99A; 2, PXO99AΔ2; 3, PXO99AΔ2(p23-44).
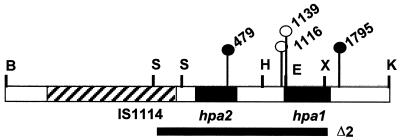
Insertions and one deletion were generated in pK6.0B, which by itself restored full pathogenicity to PXO99AΔ2, and the mutants were tested for the ability to restore pathogenicity (Fig. 8). The insertions in hpa2 (pK6.0B-479) and immediately downstream from hpa1 (pK6.0B-1795) did not affect the ability of pK6.0B to restore water soaking to PXO99AΔ2 (Table 4). Plasmids pK6.0B-1116 and pK6.0B-1139, on the other hand, failed to restore water soaking (Table 4). The insertion in pK6.0B-1116 was located 20 bp upstream of the hpa1 start codon and interrupted the presumed promoter elements from the coding sequence of hpa1. The plasmid pK6.0B-1139 had an insertion at the EcoRI site in the coding sequence of hpa1. Thus, plasmids with hpa1 mutations were unable to restore water soaking to PXO99AΔ2, indicating that the loss of hpa1 was the principal cause of the reduced virulence of PXO99AΔ2.
FIG. 8.
Map of single-gene mutations in hpa1 and hpa2. Insertion sites are indicated by lines with circles at the top. Filled circles indicate no loss of virulence; open circles indicate insertions that reduced virulence. The filled bar indicates the region missing in deletion Δ2. IS1114 is indicated by the hatched bar. B, BglII; E, EcoRI; H, HindIII; K, KpnI; S, SalI; X, XhoI.
TABLE 4.
Effects of mutations in the left end of the hrp region of X. oryzae pv. oryzae
| Strain | Reactiona |
|---|---|
| PXO99A(pHM1) | + |
| PXO99A9mx(pHM1) | − |
| PXO99AΔ2(pHM1) | ± |
| PXO99AΔ2(p23-44) | + |
| PXO99A(pFWX10-F2) | HR |
| PXO99AΔ2(pFWX10-F2) | WHR |
| PXO99AΔ2(pK6.0B) | + |
| PXO99AΔ2(pK6.0B-479) | + |
| PXO99AΔ2(pK6.0B-1116) | ± |
| PXO99AΔ2(pK6.0B-1139) | ± |
| PXO99AΔ2(pK6.0B-1795) | + |
+, water-soaking reaction on rice comparable to wild type; −, no water soaking; ±, weak or delayed water-soaking symptoms; HR, HR on rice with Xa10; WHR, weak HR.
Distribution of hpa1, hpa2, and IS1114 in species of Xanthomonas.
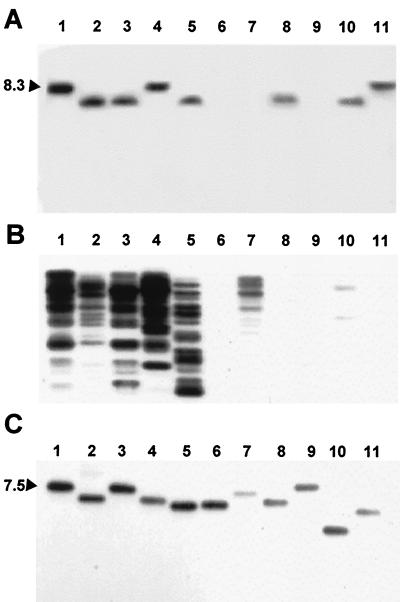
To determine the prevalence of the newly identified elements in a variety of other strains of X. oryzae pv. oryzae and X. campestris, gene-specific and element-specific probes were generated from hpa1, hpa2, and IS1114 by PCR or gel purification of an internal restriction fragment. The probes were then used in Southern analyses of genomic DNA. Both hpa1 and IS1114 hybridized to DNAs from all of the strains of X. oryzae pv. oryzae and the one strain of X. oryzae pv. oryzicola that were tested (Fig. 9A and B, lanes 1 to 5). Signal for hpa1 was detected in DNA from X. campestris pv. alfalfae KX-1, X. campestris pv. malvacearum H, and X. campestris pv. phaesoli SC-4A and was not detected in X. campestris pv. vesicatoria 81-23, X. campestris pv. campestris KXXC-1, or X. campestris pv. holcicola (Fig. 9A, lanes 6 to 11). Outside X. oryzae, IS1114 was detected only in X. campestris pv. campestris KXXC-1 and X. campestris pv. malvacearum H (Fig. 9B, lanes 7 and 10). In contrast to hpa1 and IS1114, hpa2 was detected in DNAs from all of the tested strains (Fig. 9C).
FIG. 9.
Southern analysis of hpa1, hpa2, and IS1114. (A) DNA probed with the EcoRI/XhoI fragment of hpa1. (B) DNA probed with the PCR fragment from IS1114. (C) DNA probed with the PCR fragment from hpa2. Genomic DNA was extracted from 11 strains of Xanthomonas spp. and digested with EcoRI (A and B) or SalI (C). Lanes 1, X. oryzae pv. oryzae PXO86; lanes 2, X. oryzae pv. oryzae PXO99A; lanes 3; X. oryzae pv. oryzae PXO177; lanes 4, X. oryzae pv. oryzae C191; lanes 5, X. oryzae pv. oryzicola BLS 303; lanes 6, X. campestris pv. vesicatoria 81-23; lanes 7, X. campestris pv. campestris Kxxc1; lanes 8, X. campestris pv. alfalfae KX-1; lanes 9, X. campestris pv. holcicola; lanes 10, X. campestris pv. malvacearum H; lanes 11, X. campestris pv. phaesoli SC-4A. See Materials and Methods for PCR primers and conditions. Numbers at the left indicate kilobase pairs.
DISCUSSION
The results presented here demonstrate that a type III secretory system, also known as the hrp system, has a critical role for pathogenicity of X. oryzae pv. oryzae on rice. Type III secretory systems play central roles in the ability of many gram-negative bacteria to colonize plant and animal hosts. In general, the systems are envisaged to direct the assembly of a supramolecular secretory apparatus similar in structure to the flagellar apparatus, which is also the product of a type III secretory system. Some components of the type III systems are conserved due to the structural requirements, while other components can be expected to reflect the adaption of the system to the particular niche of the bacterium (26). The hrpA and hrpB operons represent part of the conserved core of genes necessary for the assembly of the hrp system. The predicted proteins of the hrpA and hrpB operons had 94% or higher amino acid residue identity with the proteins from X. campestris pv. vesicatoria (10, 57). HrpA is a member of the HrcC class of hrp proteins and is localized to the outer membrane, where it is thought to function the transporter past the outer membrane layer (63). As a class, HrpA proteins have similarity to a variety of proteins from other type III systems, and in fact, HrpA ancestry can be traced to other secretory systems (63).
HrpB3, HrpB5, HrpB6, and HrpB8 of the hrpB operon are related to components of other type III systems, including the flagellar assembly pathway (8). HrpB8 and HrpB3, for example, are similar in amino acid sequence to FliR and FliF, which have been determined to be components of the inner membrane basal body and M-ring portion of the flagellar secretory apparatus, respectively (16, 27). HrpB1, HrpB2, HrpB4, and HrpB7 are proteins that, on the basis of sequence similarity, are unique to Xanthomonas and R. solanacearum. These proteins either have diverged from the ancestral secretion system or represent unique adaptations of the type III system in Xanthomonas and R. solanacearum. Divergence between the latter four proteins in X. oryzae pv. oryzae and X. campestris pv. vesicatoria was no greater than that for proteins with recognizable counterparts in other type III systems, and they therefore appear not to have diverged along species lines as a possible consequence of adaptation to the particular host plants.
The hrp mutations in X. oryzae pv. oryzae resulted in the loss of pathogenicity and prevented the elicitation of the nonhost HR on tomato and the race-specific HR on incompatible rice plants due to the avirulence genes avrXa10 and avrXa7, which are homologs of avrBss3 from X. campestris pv. vesicatoria (24). Activity due to avrBs3 was also lost when X. campestris pv. vesicatoria was hrp deficient (32), and dependence on hrp function for avirulence activity could be bypassed when avrBs3 and other members of the family from X. campestris pv. malvacearum were expressed in the plant cells (15, 56). In a separate study, we have observed a 75% reduction in the number of transformation foci after particle bombardment of resistant rice leaves with a plant-expressed copy of avrXa10 (66). Therefore, the protein products of avrXa10 and avrXa7 along with possible virulence factors are likely to be secreted by a hrp-encoded type III secretory apparatus into the cells of the rice plant.
Despite the high degree of similarity, the analysis of the left end of the hrp region of X. oryzae pv. oryzae revealed two genes, named hpa1 and hpa2, that were not found in X. campestris pv. vesicatoria. The putative hpa1 product has an amino acid composition similar to the compositions of the harpin proteins of P. syringae pathovars and Erwinia species and the harpin-like PopA protein of R. solanacearum (5, 20, 23, 61). These proteins share regions of high glycine content and are secreted via the type III pathway. Whether Hpa1 is secreted is unknown at present. Conditions for hrp-dependent secretion by X. campestris pv. vesicatoria have recently been determined (48). Thus, it may be possible to adapt the conditions to X. oryzae pv. oryzae and determine if the hpa1 product is secreted in a hrp-dependent manner. Like popA, hpa1 has a PIP box immediately upstream of the coding sequence and is likely to be regulated by the hrpXo gene product, which is a member of the AraC class of transcription factors (29, 44, 62).
Harpins gained attention by the fact that some harpins and PopA can elicit hypersensitive reactions on certain plants simply by injection of the proteins into the leaf tissue (5, 23, 46, 61). The protein product of hrpW has similarity to both harpin and pectate lyase (12, 20, 31). However, the elicitor properties are not shared by all harpins, and in the case of harpin from P. syringae pv. tomato, the protein elicited an HR on the host plant (46). Mutations in hrpZ, hrpW, and popA have no observable effect on the pathogenicity (1, 5, 12). Therefore, the biological relevance of the latter genes or the elicitor activities of their products is unknown. On the other hand, mutations in hrpN, from which harpins were originally named, abolished pathogenicity of Erwinia amylovora (61). Mutations in hpa1 reduced pathogenicity due to X. oryzae pv. oryzae. hpa1 is the only gene from Xanthomonas with a harpin-like product. However, sequence similarity with the hrpW gene from P. syringae pv. tomato in the DNAs from several Xanthomonas species was recently reported (12). Thus, Xanthomonas, like P. syringae and Erwinia species, may produce a variety of harpin-like proteins depending on the species or strain.
The role of the hpa2 locus, which encodes a lysozyme-like protein, is unclear. However, an argument can be made that the locus is another core component of the type III system. The locus appears to be present in all of the species of Xanthomonas that were examined, and related genes can be found in association with a variety of secretory systems, including type III systems of animal pathogens. On the one hand, the mutation of hpa2 had no apparent effect on pathogenicity under the conditions of the assay. Indeed, whether this protein is produced in vivo remains in question. The lack of a phenotype with the hpa2 mutants may reflect the lack of a requirement for this gene under the conditions of testing or environment, yet the locus may be required under natural infection conditions. None of the lysozyme-related proteins appear to be essential for the related systems under the conditions of testing. Mutations in virB1 severely reduced but did not eliminate tumor formation (40). Similarly, mutations in gene 19, which encodes a lysozyme-like protein in the R1-16 plasmid conjugation pathway, reduced but did not eliminate conjugative transfer of R1-16 (7). A mutation in ipgF, which is a locus in a type III pathway of Shigella flexneri, was reported to have no effect on pathogenicity (3). One obvious possibility for the function of the lysozyme-like proteins would be in the degradation of the peptidoglycan layer of the bacterial cell wall to accommodate the respective secretory apparatuses or associated pili (19, 33, 47). This possibility remains to be tested.
ACKNOWLEDGMENTS
We thank Diana Pavlisko for assistance in the preparation of the manuscript and Bing Yang and Nick Wills for excellent technical assistance.
This work was supported by grants 94-37303-0548 and 98-35303-6446 from the National Competitive Research Initiative of the U.S. Department of Agriculture. W.Z. was support in part by a grant from the Rockefeller Foundation.
Footnotes
Contribution number 99-500-J from the Kansas Agriculture Experiment Station.
REFERENCES
- 1.Alfano J R, Bauer D W, Milos T M, Collmer A. Analysis of the role of the Pseudomonas syringae pv. syringae HrpZ harpin in elicitation of the hypersensitive response in tobacco using functionally non-polar hrpZ deletion mutations, truncated HrpZ fragments, and hrmA mutations. Mol Microbiol. 1996;19:715–728. doi: 10.1046/j.1365-2958.1996.415946.x. [DOI] [PubMed] [Google Scholar]
- 2.Alfano J R, Collmer A. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J Bacteriol. 1997;179:5655–5662. doi: 10.1128/jb.179.18.5655-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaoui A, Menard R, Sansonetti P J, Parsot C. Characterization of the Shigella flexneri ipgD and ipgF genes, which are located in the proximal part of the mxi locus. Infect Immun. 1993;61:1707–1714. doi: 10.1128/iai.61.5.1707-1714.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Arlat M, Van Gijsegem F, Huet J C, Pernollet J C, Boucher C A. PopA1, a protein which induces a hypersensitive-like response on specific Petunia genotypes, is secreted via the Hrp pathway of Pseudomonas solanacearum. EMBO J. 1994;13:543–553. doi: 10.1002/j.1460-2075.1994.tb06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates-Wiley Interscience; 1991. [Google Scholar]
- 7.Bayer M, Eferl R, Zellnig G, Teferle K, Dijkstra A J, Kordyum E, Koraimann G, Hogenauer G. Gene 19 of Plasmid R1 is required for both efficient conjugative DNA transfer and bacteriophage R17 infection. J Bacteriol. 1995;177:4279–4288. doi: 10.1128/jb.177.15.4279-4288.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanove A J, Beer S V, Bonas U, Boucher C A, Collmer A, Coplin D L, Cornelis G R, Huang H-C, Hutcheson S W, Panopoulos N J, Van Gijsegem F. Unified nomenclature for broadly conserved hrp genes of phytopathogenic bacteria. Mol Microbiol. 1996;20:681–683. doi: 10.1046/j.1365-2958.1996.5731077.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonas U. hrp genes of phytopathogenic bacteria. In: Dangl J L, editor. Bacterial pathogenesis of plants and animals. Berlin, Germany: Springer-Verlag; 1994. pp. 79–98. [DOI] [PubMed] [Google Scholar]
- 10.Bonas U, Schulte R, Fenselau S, Minsavage G V, Staskawicz B J, Stall R E. Isolation of a gene cluster from Xanthomonas campestris pv. vesicatoria that determines pathogenicity and the hypersensitive response on pepper and tomato. Mol Plant-Microbe Interact. 1991;4:81–88. [Google Scholar]
- 11.Bonas U, Van den Ackerveken G. Recognition of bacterial avirulence proteins occurs inside the plant cell: a general phenomenon in resistance to bacterial diseases? Plant J. 1997;12:1–7. doi: 10.1046/j.1365-313x.1997.12010001.x. [DOI] [PubMed] [Google Scholar]
- 12.Charkowski A O, Alfano J R, Preston G, Yuan J, He S Y, Collmer A. The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J Bacteriol. 1998;180:5211–5217. doi: 10.1128/jb.180.19.5211-5217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J H, Hsieh Y Y, Hsiau S L, Lo T C, Shau C C. Characterization of insertions of IS476 and two newly identified insertion sequences, IS1478 and IS1479, in Xanthomonas campestris pv. campestris. J Bacteriol. 1999;181:1220–1228. doi: 10.1128/jb.181.4.1220-1228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig N L. Transposon Tn7. Curr Top Microbiol Immunol. 1996;204:27–48. doi: 10.1007/978-3-642-79795-8_2. [DOI] [PubMed] [Google Scholar]
- 15.de Feyter R, McFadden H, Dennis L. Five avirulence genes from Xanthomonas campestris pv. malvacearum cause genotype-specific cell death when expressed transiently in cotton. Mol Plant-Microbe Interact. 1998;11:698–701. [Google Scholar]
- 16.Fan F, Ohnishi K, Francis N R, Macnab R M. The FliP and FliR proteins of Salmonella typhimurium, putative components of the type III flagellar export apparatus, are located in the flagellar basal body. Mol Microbiol. 1997;26:1035–1046. doi: 10.1046/j.1365-2958.1997.6412010.x. [DOI] [PubMed] [Google Scholar]
- 17.Fenselau S, Balbo I, Bonas U. Determinants of pathogenicity in Xanthomonas campestris pv. vesicatoria are related to proteins involved in secretion in bacterial pathogens of animals. Mol Plant-Microbe Interact. 1992;5:390–396. doi: 10.1094/mpmi-5-390. [DOI] [PubMed] [Google Scholar]
- 18.Fenselau S, Bonas U. Sequence and expression analysis of the hrpB pathogenicity operon of Xanthomonas campestris pv. vesicatoria which encodes eight proteins with similarity to components of the Hrp, Ysc, Spa, and Fli secretion systems. Mol Plant-Microbe Interact. 1995;8:845–854. doi: 10.1094/mpmi-8-0845. [DOI] [PubMed] [Google Scholar]
- 19.Fullner K J, Lara J C, Nester E W. Pilus assembly by Agrobacterium T-DNA transfer genes. Science. 1996;273:1107–1109. doi: 10.1126/science.273.5278.1107. [DOI] [PubMed] [Google Scholar]
- 20.Gaudriault S, Brisset M N, Barny M A. HrpW of Erwinia amylovora, a new Hrp-secreted protein. FEBS Lett. 1998;428:224–228. doi: 10.1016/s0014-5793(98)00534-1. [DOI] [PubMed] [Google Scholar]
- 21.Gijsegem F, Gough C, Zischek C, Niqueux E, Arlat M, Genin S, Barberis P, German S, Castello P, Boucher C. The hrp gene locus of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol Microbiol. 1995;15:1095–1114. doi: 10.1111/j.1365-2958.1995.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 22.Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 23.He S Y, Huang H-C, Collmer A. Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the hrp pathway and elicits the hypersensitive response in plants. Cell. 1993;73:1255–1266. doi: 10.1016/0092-8674(93)90354-s. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins C M, White F F, Choi S H, Guo A, Leach J E. A family of avirulence genes from Xanthomonas oryzae pv. oryzae. Mol Plant-Microbe Interact. 1992;5:451–459. doi: 10.1094/mpmi-5-451. [DOI] [PubMed] [Google Scholar]
- 25.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huguet E, Bonas U. hrpf of Xanthomonas campestris pv. vesicatoria encodes an 87-kDa protein with homology to NoIX of Rhizobium fredii. Mol Plant-Microbe Interact. 1997;10:488–498. doi: 10.1094/MPMI.1997.10.4.488. [DOI] [PubMed] [Google Scholar]
- 27.Jones C J, Homma M, Macnab R M. L-, P-, and M-ring proteins of the flagellar basal body of Salmonella typhimurium: gene sequences and deduced protein sequences. J Bacteriol. 1989;171:3890–3900. doi: 10.1128/jb.171.7.3890-3900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung A, Sippel A E, Grez M, Schutz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci USA. 1980;77:5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamdar H V, Kamoun S, Kado C I. Restoration of pathogenicity of avirulent Xanthomonas oryzae pv. oryzae and X. campestris pathovars by reciprocal complementation with the hrpXo and hrpXc genes and identification of HrpX function by sequence analyses. J Bacteriol. 1993;175:2017–2025. doi: 10.1128/jb.175.7.2017-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearney B, Ronald P C, Dahlbeck D, Staskawicz B J. Molecular basis for evasion of plant host defense in bacterial spot disease of pepper. Nature. 1988;332:541–543. [Google Scholar]
- 31.Kim J F, Beer S V. HrpW of Erwinia amylovora, a new harpin that contains a domain homologous to pectate lyases of a distinct class. J Bacteriol. 1998;180:5203–5210. doi: 10.1128/jb.180.19.5203-5210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knoop V, Staskawicz B, Bonas U. Expression of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria is not under the control of hrp genes and is independent of plant factors. J Bacteriol. 1991;173:7142–7150. doi: 10.1128/jb.173.22.7142-7150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galan J E, Aizawa S-I. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 34.Leach J E, Rhoads M L, Vera Cruz C M, White F F, Mew T W, Leung H. Assessment of genetic diversity and population structure of Xanthomonas oryzae pv. oryzae with a repetitive DNA element. Appl Environ Microbiol. 1992;58:2188–2195. doi: 10.1128/aem.58.7.2188-2195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leong S A, Ditta G S, Helinski D R. Heme biosynthesis in Rhizobium. J Biol Chem. 1982;257:8724–8730. [PubMed] [Google Scholar]
- 36.Leyns F, De Cleene M, Swings J G, De Ley J. The host range of the genus Xanthomonas. Bot Rev. 1984;50:308–356. [Google Scholar]
- 37.Lindgren P B. The role of hrp genes during plant-bacterial interactions. Annu Rev Phytopathol. 1997;35:129–152. doi: 10.1146/annurev.phyto.35.1.129. [DOI] [PubMed] [Google Scholar]
- 38.Lindgren P B, Peet R C, Panopoulos N J. Gene cluster of Pseudomonas syringae pv. “phaseolicola” controls pathogenicity on bean plants and hypersensitivity on nonhost plants. J Bacteriol. 1986;168:512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miras I, Hermant D. Nucleotide sequence of iagA and iagB genes involved in invasion of HeLa cells by Salmonella enterica subsp. enterica ser. Typhi. Res Microbiol. 1995;146:17–20. doi: 10.1016/0923-2508(96)80267-1. [DOI] [PubMed] [Google Scholar]
- 40.Mushegian A R, Fullner K J, Koonin E V, Nester E W. A family of lysozyme-like virulence factors in bacterial pathogens of plants and animals. Proc Natl Acad Sci USA. 1996;93:7321–7326. doi: 10.1073/pnas.93.14.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakai K. Predicting various targeting signals in amino acid sequences. Bull Inst Chem Res Kyoto Univ. 1991;69:269–291. [Google Scholar]
- 42.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in Gram-negative bacteria. Proteins Struct Funct Genet. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 43.Ogiwara A, Uchiyama I, Takagi T, Kanehisa M. Construction and analysis of a profile library characterizing groups of structurally known proteins. Protein Sci. 1996;5:1991–1999. doi: 10.1002/pro.5560051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oku T, Alvarez A M, Kado C I. Conservation of the hypersensitivity-pathogenicity regulatory gene hrpX of Xanthomonas campestris and X. oryzae. DNA Sequencing. 1995;5:245–249. doi: 10.3109/10425179509030974. [DOI] [PubMed] [Google Scholar]
- 45.Pearson W R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- 46.Preston G, Huang H-C, He S Y, Collmer A. The HrpZ proteins of Pseudomonas syringae pvs. syringae, glycinea, and tomato are encoded by an operon containing Yersinia ysc homologs and elicit the hypersensitive response in tomato but not soybean. Mol Plant-Microbe Interact. 1995;8:717–732. doi: 10.1094/mpmi-8-0717. [DOI] [PubMed] [Google Scholar]
- 47.Roine E, Wei W, Yuan J, Nurmiaho-Lassila E-L, Kalkkinen N, Romantschuk M, He S Y. Hrp pilus: an hrp-dependent bacterial surface appendage produced by Pseudomonas syringae pv. tomato DC3000. Proc Natl Acad Sci USA. 1997;94:3459–3464. doi: 10.1073/pnas.94.7.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossier O, Wengelnik K, Hahn K, Bonas U. The Xanthomonas Hrp type III system secretes proteins from plant and mammalian bacterial pathogens. Proc Natl Acad Sci USA. 1999;96:9368–9373. doi: 10.1073/pnas.96.16.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 50.Selbitschka W, Zekri S, Schroeder G, Puehler A, Toro N. The Sinorhizobium meliloti insertion sequence (IS) elements ISRm102F34-1/ISRm7 and ISRm220-13-5 belong to a new family of insertion sequence elements. Fed Eur Microbiol Soc Microbiol Lett. 1999;172:1–7. doi: 10.1111/j.1574-6968.1999.tb13441.x. [DOI] [PubMed] [Google Scholar]
- 51.Sharma S B, Signer E R. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in planta revealed by transposon Tn5-gusA. Genes Dev. 1990;4:344–356. doi: 10.1101/gad.4.3.344. [DOI] [PubMed] [Google Scholar]
- 52.Shuman S. Novel approach to molecular cloning and polynucleotide synthesis using vaccinia DNA topoisomerase. J Biol Chem. 1994;269:32678–32684. [PubMed] [Google Scholar]
- 53.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology. 1983;1:784–791. [Google Scholar]
- 54.Swings J, Van den Mooter M, Vauterin L, Hoste B, Gillis M, Mew T W, Kersters K. Reclassification of the causal agents of bacterial blight (Xanthomonas campestris pv. oryzae) and bacterial leaf streak (Xanthomonas campestris pv. oryzicola) of rice as pathovars of Xanthomonas oryzae (ex Ishiyama 1922) sp. nov., nom. rev. Int J Syst Bacteriol. 1990;40:309–311. [Google Scholar]
- 55.Thompson J D, Higginsand D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van den Ackerveken G, Marois E, Bonas U. Recognition of the bacterial avirulence protein AvrBs3 occurs inside the host plant cell. Cell. 1996;87:1307–1316. doi: 10.1016/s0092-8674(00)81825-5. [DOI] [PubMed] [Google Scholar]
- 57.Van Gijsegem F, Gough C, Zischek C, Niqueux E, Arlat M, Genin S, Barberis P, German S, Castello P, Boucher C. The hrp gene locus of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol Microbiol. 1995;15:1095–1114. doi: 10.1111/j.1365-2958.1995.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 58.Vieira J, Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]
- 59.Vogler A P, Homma M, Irikura V M, Macnab R M. Salmonella typhimurium mutants defective in flagellar filament regrowth and sequence similarity of FliI to F0F1, vacuolar, and archaebacterial ATPase subunits. J Bacteriol. 1991;173:3564–3572. doi: 10.1128/jb.173.11.3564-3572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Heijne G. Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992;225:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- 61.Wei Z-M, Laby R J, Zumoff C H, Bauer D W, He S Y, Collmer A, Beer S V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992;257:85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]
- 62.Wengelnik K, Bonas U. HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J Bacteriol. 1996;178:3462–3469. doi: 10.1128/jb.178.12.3462-3469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wengelnik K, Marie C, Russel M, Bonas U. Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol. 1996;178:1061–1069. doi: 10.1128/jb.178.4.1061-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuk M H, Harvill E T, Miller J F. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]
- 65.Zhu W, Yang B, Chittoor J M, Johnson L B, White F F. AvrXA10 contains an acidic transcriptional activation domain in the functionally conserved C terminus. Mol Plant-Microbe Interact. 1998;11:824–832. doi: 10.1094/MPMI.1998.11.8.824. [DOI] [PubMed] [Google Scholar]
- 66.Zhu W, Yang B, Wills N, Johnson L B, White F F. The C terminus of AvrXa10 can be replaced by the transcriptional activation domain of VP16 from the herpes simplex virus. Plant Cell. 1999;11:1665–1674. doi: 10.1105/tpc.11.9.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]