Abstract
Background:
Alcohol use disorder (AUD) and post-traumatic stress disorder (PTSD) are highly comorbid, yet there is a lack of preclinical research investigating how prior ethanol (EtOH) dependence influences the development of a PTSD-like phenotype. Furthermore, the neuroimmune system has been implicated in the development of both AUD and PTSD, but the extent of glial involvement in this context remains unclear. A rodent model was developed to address this gap in the literature.
Methods:
We used a 15-day exposure to the 5% w/v EtOH low-fat Lieber-DeCarli liquid diet in combination with the stress-enhanced fear learning (SEFL) paradigm to investigate the effects of chronic EtOH consumption on the development of a PTSD-like phenotype. Next, we used a reverse transcription quantitative real-time polymerase chain reaction to quantify mRNA expression of glial cell markers GFAP (astrocytes) and CD68 (microglia) following severe footshock stress in EtOH-withdrawn rats. Finally, we tested the functional contribution of dorsal hippocampal (DH) astrocytes in the development of SEFL in EtOH-dependent rats using astrocyte-specific Gi designer receptors exclusively activated by designer drugs (Gi-DREADD).
Results:
Results demonstrate that chronic EtOH consumption and withdrawal exacerbate future SEFL. Additionally, we found significantly increased GFAP mRNA expression in the dorsal and ventral hippocampus and amygdalar complex following the severe stressor in EtOH-withdrawn animals. Finally, the stimulation of the astroglial Gi-DREADD during EtOH withdrawal prevented the EtOH-induced enhancement of SEFL.
Conclusions:
Collectively, results indicate that prior EtOH dependence and withdrawal combined with a severe stressor potentiate future enhanced fear learning. Furthermore, DH astrocytes significantly contribute to this change in behavior. Overall, these studies provide insight into the comorbidity of AUD and PTSD and the potential neurobiological mechanisms behind increased susceptibility to a PTSD-like phenotype in individuals with AUD.
Keywords: astrocytes, dorsal hippocampus, enhanced fear learning, ethanol, PTSD
INTRODUCTION
Alcohol use disorder (AUD) and post-traumatic stress disorder (PTSD) are highly prevalent in society, with the rates of AUD and PTSD alone at 29.1% and 7.8%, respectively (Back & Jones, 2018). These disorders are often comorbid, and alcohol is one of the most abused drugs among patients with a PTSD diagnosis (Debell et al., 2014; Gilpin & Weiner, 2017; Jacobsen et al., 2001). Furthermore, clinical research suggests that alcohol misuse preceding severe stress increases vulnerability to PTSD. For example, soldiers with a prior history of AUD have an increased likelihood of developing PTSD (Stein et al., 2017), and extent of alcohol use predicted PTSD symptom severity (McFarlane et al., 2009). These studies support a broader susceptibility hypothesis that states drug use may increase vulnerability to the development of PTSD following a traumatic event (Chilcoat & Breslau, 1998). Unfortunately, current treatments for comorbid AUD and PTSD do not successfully address the symptoms of both disorders (Norman & Hamblen, 2017; Petrakis & Simpson, 2017). Thus, understanding the neurobiological mechanisms by which prior AUD promotes susceptibility to a PTSD-like phenotype is essential for the development of effective treatment strategies.
Despite evidence raised by clinical research, preclinical data primarily focuses on prior stressors impacting future ethanol (EtOH) drinking. For example, stressors like social defeat (Norman et al., 2015), exposure to predator odor (Edwards et al., 2013), and forced swim (Anderson et al., 2016) increase EtOH intake in rodents. Moreover, completion of the stress-enhanced fear learning paradigm (SEFL), a rodent model of PTSD-like behavior, significantly increased EtOH consumption (Gonzalez et al., 2021; Meyer et al., 2013), further demonstrating that prior stress can exacerbate EtOH drinking behavior. While some work has exposed animals to EtOH prior to PTSD-like stressors, these experiments assess changes in EtOH drinking following stress and do not examine EtOH-induced changes in PTSD-like behavior itself. For example, previous exposure to EtOH increases EtOH preference and consumption following exposure to PTSD-like stressors (Piggott et al., 2020). Likewise, Zoladz et al. (2018) confirmed that PTSD-like stress increases EtOH intake in EtOH-experienced animals, but also demonstrated that stress leads to decreased EtOH intake in EtOH-naïve animals. Collectively, the current literature illustrates how aspects of EtOH drinking and dependence change with the introduction of PTSD-like stressors, but there is a lack of research investigating how prior EtOH consumption impacts the development of a PTSD-like phenotype.
To address this gap in knowledge, we combined two existing animal models to examine the effects of EtOH dependence on the development of a PTSD-like phenotype. Adult rats were given the low-fat Lieber-DeCarli liquid diet, a nutritionally complete food source containing 5% w/v EtOH that has been used to model chronic EtOH consumption (Guo et al., 2018; Lieber et al., 1989; Lieber & DeCarli, 1982, 1989). The Lieber-DeCarli liquid diet reliably (1) produces behaviors related to EtOH withdrawal, (2) results in the consumption of pharmacologically relevant doses of EtOH, and (3) produces chronic EtOH-induced changes in various brain regions and in behavior (Chen et al., 2019; Pandey et al., 1999; Piano et al., 2005). As 15 days of exposure to an EtOH liquid diet is sufficient to produce EtOH dependence and withdrawal behaviors (Chen et al., 2019; Wills et al., 2008, 2009), we used that timeline here. Following the establishment of EtOH dependence, we utilized the SEFL paradigm. SEFL is a rodent model of fear conditioning designed to mimic aspects of PTSD, specifically the hallmark symptom of enhanced reactivity to future stressors (Rau et al., 2005). This behavioral model has been described at length previously (Rau et al., 2005) and reliably produces PTSD-like behavior in our laboratory (Jones et al., 2015; Jones, Lebonville, et al., 2018). To examine the effects of chronic EtOH consumption and withdrawal on future PTSD-like behavior, animals were exposed to 15 days of the low-fat Lieber-DeCarli diet. Then, at 24 h into EtOH withdrawal, animals experienced the severe stressor of SEFL, followed by the observation of freezing behavior to assess learned fear.
To examine the neurobiological mechanisms underlying the effects of chronic EtOH consumption and withdrawal on SEFL, we focused on glial cells, as recent evidence suggests that glial- and neuroimmune-related mechanisms contribute to both AUD and PTSD (Erickson et al., 2019; Jones, Paniccia, et al., 2018; Li et al., 2022). However, the literature reveals inconsistent changes in glial cells caused by EtOH dependence and severe stress. Firstly, astrocytes have been investigated in both behaviors by observation of changes in glial fibrillary acidic protein (GFAP), a cytoskeletal protein expressed in the major branches of astroglia (Eng et al., 2000) that has historically been quantified as a marker for astrocyte reactivity (Hol & Pekny, 2015). Increased GFAP has been observed following several methodologies of EtOH exposure, including 10-day intragastric administration (Qin & Crews, 2012) and various lengths of chronic EtOH consumption (Alfonso-Loeches et al., 2010; Evrard et al., 2006). However, the literature indicates inconsistent changes in GFAP expression following the development of a PTSD-like phenotype. For instance, fear conditioning has been shown to decrease GFAP expression in the hippocampus 1 h following stress exposure (Choi et al., 2016). Conversely, our own laboratory found no change in GFAP expression 48 h following the severe stressor in the SEFL paradigm (Jones, Lebonville, et al., 2018). Interestingly, we observed a decrease in the microglial marker ionized calcium binding adaptor molecule (Iba-1) at this 48 h timepoint, indicating a potential role for hippocampal microglia in SEFL. However, others have found contrasting evidence, with increased microglial activation and number following footshock stress (Li et al., 2021; Smith et al., 2019). Similarly, chronic EtOH consumption and repeated intragastric administration of EtOH increase microglial activation, as evidenced by morphological changes and increased expression of proinflammatory cytokines (Cruz et al., 2017; Qin & Crews, 2012). Based on existing evidence that variations in glial cell reactivity and structure occur following both chronic EtOH and severe stress exposure, we quantified gene expression for markers associated with astroglial and microglial activation, GFAP and CD68, respectively, using reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR). We focused our investigation 24 h after severe stress exposure in animals with a history of chronic EtOH consumption compared with pair-fed controls. We examined GFAP and CD68 in the dorsal hippocampus (DH), ventral hippocampus (VH), and amygdala, as neuroinflammation in these regions has been associated with anxiety-related disorders (Won & Kim, 2020), and these regions are implicated in both AUD and PTSD (Gilpin & Weiner, 2017; Suh & Ressler, 2018).
To causally demonstrate that glial cells contribute to increased vulnerability to PTSD-like behavior following chronic EtOH intake, we selectively expressed Gi designer receptors exclusively activated by designer drugs (Gi-DREADD) in DH astrocytes using an adeno-associated viral construct. Using this technique, we have previously shown that Gi-DREADD expression is restricted to DH astrocytes (Jones, Paniccia, et al., 2018; Paniccia et al., 2018) and that stimulation of astroglial Gi-DREADD following Context A prevents the development of enhanced fear learning (Jones, Paniccia, et al., 2018). Since EtOH withdrawal occurs over a length of time, we administered CNO at two timepoints–once at 4 h into withdrawal, when blood EtOH concentration (BEC) levels drop and near zero mg/dl (Wills et al., 2008, 2009), and again at 12 h into withdrawal, when observable withdrawal behaviors begin to peak (Macey et al., 1996). We hypothesized that stimulation of hippocampal astroglial Gi-DREADD would prevent the development of a PTSD-like phenotype following chronic EtOH consumption and withdrawal.
Developing a rodent model of combined chronic EtOH consumption and withdrawal and SEFL enabled us to effectively test how prior EtOH dependence influences subsequent PTSD-like behavior. Experiments 1 and 2 characterized this newly developed model, and we hypothesized that chronic EtOH dependence and withdrawal would exacerbate future SEFL. Next, Experiment 3 aimed to uncover neurobiological changes following severe stress exposure in EtOH-withdrawn animals. Finally, Experiment 4 causally tested the role of hippocampal astroglial activity in EtOH- and stress-induced enhanced fear learning. Collectively, these studies are the first to test the effect of prior chronic EtOH dependence on future SEFL, and the neuroimmune mechanisms responsible for AUD-induced vulnerability to PTSD-like behavior.
MATERIALS AND METHODS
Subjects
Adult male Sprague Dawley rats (200–225 g, Charles River Laboratories) were individually housed under a reversed 12 h light–dark cycle. Animals had ad libitum access to water and were handled regularly throughout experiments. Animals were provided the Control Diet formula of the low-fat Lieber-DeCarli '83, '89, Shake and Pour Rodent Liquid Diet (Bio-Serv) ad libitum (150 ml provided) upon arrival to the vivarium, and at all times with the exception of the chronic EtOH consumption paradigm. Details regarding diet during this paradigm are described below. All procedures were conducted with approval from the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Drug administration
Clozapine-N-oxide (CNO; Sigma) was dissolved in a vehicle of 0.9% sterile saline with 0.5% dimethyl sulfoxide (DMSO). CNO (3 mg/kg) or vehicle was administered subcutaneously at 4 and 12 h into EtOH withdrawal in Experiment 4.
Surgical procedures
Stereotaxic surgery was performed as previously described (Jones, Paniccia, et al., 2018; Paniccia et al., 2018). In Experiment 4, rats were anesthetized with a 1 ml/kg intraperitoneal injection of 9:1 (vol/vol) ketamine hydrochloride (100 mg/ml) mixed with xylazine (100 mg/ml). AAV8-GFAP-hM4D(Gi)-mCherry (9.8 × 1012 particles/ml, UNC Vector Core) was infused bilaterally in the DH (AP −3.4 mm, ML ±3.1 mm, DV −3.2 mm relative to bregma, 15° angle) at a rate of 0.05 to 1 μl/min, at a volume of 0.7 μl per hemisphere. The DREADD construct was packaged in AAV8 by the Vector Core at the University of North Carolina at Chapel Hill. Injectors were left in place for 10 min following infusion, so virus could diffuse away from the injection site. Following surgery, animals remained in the home cage for 1 week before EtOH consumption began, culminating in a total of 3 weeks from the completion of surgeries to allow for viral expression prior to CNO injections.
Chronic EtOH consumption liquid diet model
The Low-Fat Lieber-DeCarli '83, '89, Shake and Pour Rodent Liquid Diet (Bio-Serv) was prepared and stored according to manufacturer instructions, with the addition of using a hand immersion blender to achieve a smooth consistency (as recommended by Guo etal., 2018). A 150 ml glass liquid diet feeding tube (Bio-Serv) was provided in the home cage, and consumption and body weight were measured daily. Animals were provided the Control Diet formula ad libitum (150 ml provided) upon arrival to the vivarium and had ad libitum access for an additional 24 h to habituate to the new food source. In Experiment 4, animals received ad libitum access to food pellets throughout surgery and recovery before beginning 48 h of Control Diet habituation. Following habituation, animals were randomly assigned to two groups, with half receiving a 5% w/v EtOH Diet formula while the other half continued to receive Control Diet. EtOH and control groups were pair-fed to equate caloric intake, such that the control group was provided the average millilitres of diet consumed by the EtOH group on the prior day (Knapp et al., 2016; Overstreet et al., 2002). Animals were exposed to EtOH Diet (or pair-fed Control Diet) for 15 days. On the following day, the EtOH group's diet was replaced with Control Diet, marking the loss of access to EtOH, and beginning of withdrawal. Following the 15-day EtOH exposure, all animals were provided Control Diet for the entirety of the experiment.
Withdrawal behavior analysis
To assess whether animals were EtOH-dependent following 15 days of EtOH Diet exposure, we examined withdrawal behaviors 12 and 24 h following the replacement of EtOH Diet with Control Diet. Animals (n = 3 to 4 per group) were placed in open-field Plexiglas boxes (42 cm × 42 cm × 30 cm). Withdrawal behaviors (rearing, tail rigidity, and abnormal gait) were video recorded for 30 min. Experimenters scored withdrawal behaviors blind to the experiment condition. Rearing was scored by counting the number of times the behavior occurred during the 30 min session. Tail rigidity and abnormal gait scores were determined by the presence (one point) or absence (0 points) of the behavior in each 5 min time bin of the 30 min session. Presence of tail rigidity was defined as the tail being rigid and lifted entirely above the ground, extended in a straight line from the animal's rear. Presence of abnormal gait was defined as a stumble or broad-based gait.
Tail blood collection and BEC analysis
To investigate the BEC of animals (n = 9) throughout the chronic EtOH consumption paradigm, tail blood samples were collected during the last hour of the dark cycle on days 1, 5, 10, and 15 of EtOH Diet consumption, similar to previously published methods (Overstreet et al., 2002; Wills et al., 2008). Blood was additionally collected when access to EtOH Diet was removed (0 h into withdrawal) and 24 h into withdrawal. Animals were restrained in a Broome-style restrainer (Plas-Labs) and blood was obtained via tail clip. Samples were centrifuged at 4°C, 10,000 g for 15 min immediately following collection and plasma was extracted, then frozen at −20°C until BEC analysis. BECs were obtained using an AM1 Alcohol Analyzer (Analox Instruments). Each plasma sample was analyzed twice, and the average value was used for subsequent analysis.
SEFL
SEFL was performed after the completion of the chronic EtOH consumption paradigm in Experiment 2 (n = 24). This protocol has been previously described at length (Jones et al., 2015; Jones, Lebonville, et al., 2018; Jones, Paniccia, et al., 2018; Parekh et al., 2020, 2021; Rau et al., 2005). Briefly, 24 h following the replacement of EtOH Diet with Control Diet, animals were placed into Context A (BRS/LVE, Laurel, MD; H 26.7 cm × D 24.8 cm × 30.7 cm) for 90 min, an environment with distinct olfactory, auditory, and tactile characteristics from the home cage. Animals were randomly assigned to experience either 15 scrambled footshocks (2 mA, 1 s) on a 6 min variable time schedule or no shock in Context A. Animals were returned to the home cage for 5 days, after which they were placed in Context B (Med Associates), an environment with distinct olfactory, auditory, and tactile characteristics from the home cage and from Context A, for 15 min of habituation. On the following day, animals were returned to Context B and all received a single scrambled footshock (1 mA, 1 s), and then were placed back into Context B 1, 2, 7, and 14 days later to measure freezing behavior, a measure of learned fear defined as the lack of all movement except that required for breathing. Context B behavior sessions were video recorded. Freezing behavior was analyzed using EthoVision XT video tracking software (Noldus Information Technology, Inc.). The activity analysis feature (Activity Threshold = 10) was used to calculate the percentage of time spent inactive by each animal during each Context B session. Statistical outliers in freezing behavior were determined using the Grubbs' test and dropped from analysis (four animals were excluded for high levels of freezing in both Experiments 2 and 4).
This paradigm was modified during Experiments 3 and 4. In Experiment 3 (n = 36), animals were sacrificed 24 h after exposure to Context A to capture changes in gene expression occurring after both chronic EtOH consumption/withdrawal and severe stress. During Experiment 4 (n = 40), CNO injections were administered at 4 and 12 h into EtOH withdrawal, followed by Context A exposure at 24 h into withdrawal, and the SEFL paradigm proceeded as described above.
Tissue collection and histology
In Experiment 3, animals were sacrificed via cervical dislocation. Brains were removed, flash-frozen in isopentane (Fisher Scientific), and stored at −80°C. Brain regions of interest (DH, VH, and amygdalar complex) were dissected via a sterile disposable biopsy punch (1.5 mm for amygdala and VH, 2 mm for DH; Braintree Scientific, Inc.). Dissected tissue was immediately placed in 500μl ice-cold TriReagent (Molecular Research Center) inside 2 ml homogenizing bead tubes and frozen at −80°C until RNA extraction.
To verify DREADD expression was restricted to DH astrocytes following Experiment 4, animals were euthanized and tissue was prepared as described previously (Paniccia et al., 2018; Parekh et al., 2020, 2021). Animals were anesthetized with 9:1 (vol/vol) ketamine hydrochloride (100 mg/ml) mixed with xylazine (100 mg/ml) and transcardially perfused with ice-cold phosphate buffer (PB; 0.1 M, pH = 7.4) followed by 4% paraformaldehyde in 0.1 M PB. Brains were removed and postfixed in 4% paraformaldehyde for 6 h at 4°C, and then transferred to 30% sucrose with 0.1% sodium azide at 4°C. Following sucrose saturation of the tissue, 40 μm coronal slices were obtained using a cryostat (Leica CM 3050 S, Leica Microsystems). Slices were stored in ethylene glycol and polyvinylpyrrolidone in 0.1 M PB solution at −20°C.
RNA extraction, cDNA synthesis, and RT-qPCR
Tissue for RT-qPCR was prepared as described previously (Lebonville et al., 2020; Paniccia et al., 2018, 2021), with modifications made in the RNA extraction step. Briefly, brain tissue punches were homogenized (Precellys Instruments) and BCP (1-Bromo-3-Chloropropane; Molecular Research Center) was added to homogenate for phase separation. Then, the RNEasy Lipid Tissue Mini Kit (Qiagen) was used to purify RNA samples according to manufacturer instructions.
A spectrophotometer (Epoch™, BioTek Instruments Inc.) was used to verify the purity and concentration of RNA in each sample. RNA concentrations were read using the Take3 Application and Gen5 Software for Nucleic Acid Quantification (BioTek Instruments Inc.), and A260/280 ratios were assessed to ensure purity. Sample mRNA input concentration was equalized using PCR-grade water, then cDNA was synthesized using the Advantage for RT-PCR Kit (ClonTech) following manufacturer directions and using the Veriti 96 Well Fast Thermal Cycler (Applied Biosystems, ThermoFisher Scientific).
RT-qPCR was performed using the TaqMan Fast Advanced Master Mix kit (Applied Biosystems, ThermoFisher Scientific) following the manufacturer's instructions. Each reaction contained 1.5 μl of cDNA sample, and reactions were completed in triplicate on a 384-well plate. A no-template control was added to verify purity. Gene expression was analyzed using TaqMan Gene Expression Assays (FAM-MGB; ThermoFisher Scientific): GFAP (Assay ID: Rn00566603_m1), CD68 (Assay ID: Rn01495634_g1), and the reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Assay ID: Rn01775763_g1). 384-well plates were run in the QuantStudio™ 6 Flex RealTime PCR System (Applied Biosystems, ThermoFisher Scientific). Data were collected using the QuantStudio™ RealTime PCR Software with the following run method: 50°C for 2 min for PCR product contamination degradation, hold at 95°C for 20 s for polymerase activation, then 45 PCR cycles at 95°C for 1 s and 60°C for 20 s with data collection at the end of each cycle. Data were analyzed using the comparative CT (ΔΔCT) method. Data for genes of interest were normalized to the reference gene (GAPDH) and then normalized to the average of reference normalized values.
Immunohistochemistry and microscopy
Fluorescent immunohistochemistry was used to verify the specificity of Gi-DREADD expression in DH astrocytes in Experiment 4. The immunohistochemistry protocol used here has been described previously (Jones, Lebonville, et al., 2018; Jones, Paniccia, et al., 2018; Paniccia et al., 2018; Parekh et al., 2020,2021). Tissue was incubated with the following primary antibodies: rabbit anti-GFAP (1:1000, Agilent Technologies, Cat# Z0334), mouse anti-NeuN (1:1000, Millipore-Sigma, Cat # MAB377), and the corresponding secondary antibodies: goat anti-rabbit Alexa Fluor-405 (1:1000, ThermoFisher Scientific, Cat #A11008), goat anti-mouse Alexa Fluor-488 (1:1000, ThermoFisher Scientific, Cat #35501BID). Sections were mounted onto SuperFrost Plus slides (Fisher Scientific) using Vectashield hardset mounting medium (Vector Laboratories).
Confocal microscopy (Zeiss LSM800) was used to verify the specificity of DREADD to DH astrocytes by examining mCherry signal. Representative images were acquired with the following settings: 1024 × 1024 frame size, 16-bit resolution, and frame average of 4. Images were deconvolved using Bitplane AutoQuant X3 and exported to Bitplane Imaris software. mCherry signal was expected to be bilateral, restricted to the DH, and specifically in astrocytes. Animals that did not meet these criteria were excluded from analysis.
Statistical analysis
Statistics were analyzed using SPSS Statistics software (IBM). All data were tested for assumptions of parametric tests, including normality and equal variances among groups. If assumptions were not met, nonparametric statistical tests were used. To analyze BEC and EtOH consumption in Experiment 1A, within-group analyses were conducted via a paired-samples t-test to compare two timepoints or a one-way repeated measures (RM) ANOVA for three timepoints. In Experiment 1B, there were unequal variances among groups, so nonparametric Mann–Whitney U tests were used to examine between-group differences in EtOH withdrawal behaviors of the two diet groups (Control and EtOH). In Experiments 2 and 4, behavioral measures were not normally distributed, and so, a nonparametric Kruskal–Wallis test was used to analyze between-group differences among the four experimental groups in baseline freezing. Then, nonparametric Mann–Whitney U tests were used to investigate a priori hypotheses of differences in freezing behavior between two experimental groups on each test day. Finally, Experiment 3 used 2 (diet type) × 2 (shock) factorial ANOVAs to analyze between-group differences in mRNA expression for each gene of interest within each brain region of interest.
RESULTS
Experiment 1: Liquid diet chronic EtOH consumption model
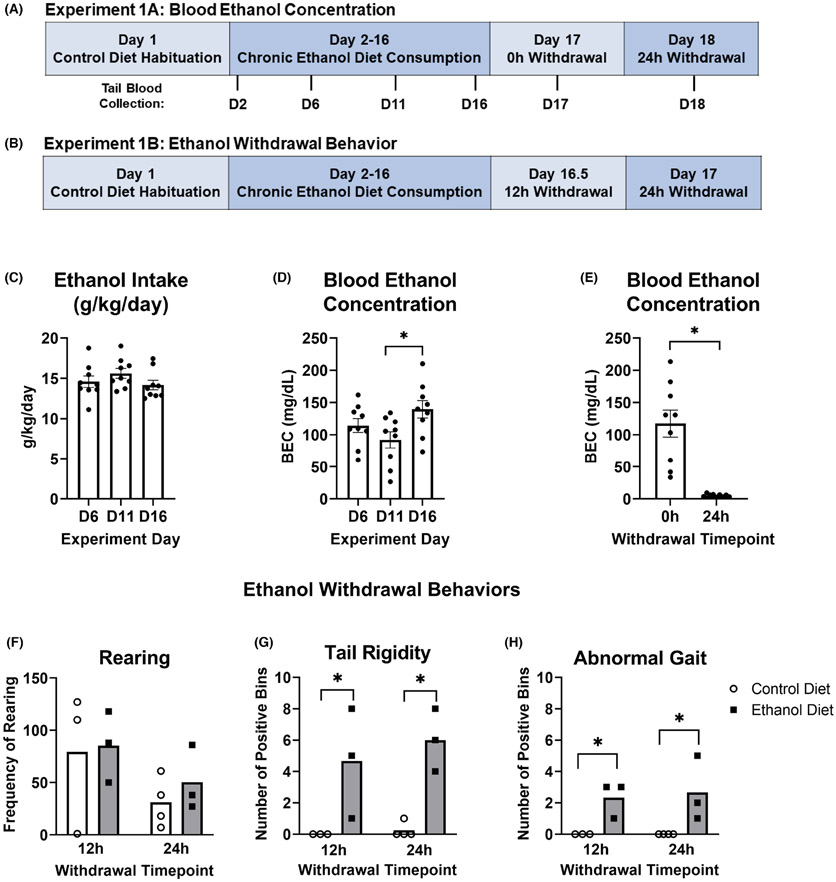
For Experiment 1A (Figure 1A), a one-way RM ANOVA revealed no significant differences in EtOH consumption (g/kg/day) across the 3 days where tail blood was collected after 24 h access to EtOH Diet, F (2, 16) = 1.869, p = 0.186 (Figure 1C). A second one-way RM ANOVA revealed an overall difference in BEC across the same three test days, F (2, 16) = 8.386, p = 0.003 (Figure 1D). Bonferroni post hoc analyses revealed a significant increase in BEC between D11 and D16 (p = 0.008), but no difference between any other test day (D6 vs. D11: p = 0.293; D6 vs. D16: p = 0.193). Finally, a paired-samples t-test revealed a significant decrease in BEC between 0 and 24 h withdrawal (t[8] = 5.253, p = 0.001; Figure 1E).
FIGURE 1.
Chronic consumption of the low-fat Lieber-DeCarli liquid diet produces EtOH dependence. Experiment timelines for experiment 1A (A) and experiment 1B (B) are shown. (C) Average EtOH intake (g/kg/day) on days where tail blood was sampled following 24 h access to EtOH increased from D6 to D11 but was otherwise consistent. (D) Animals showed average BECs greater than 80 mg/dl on all days where tail blood was sampled after 24 h access to EtOH Diet. Average BECs were consistent, with the exception of a significant increase in BEC on D16 compared with D11. (E) There was a significant decrease in average BEC between 0 h (D17) and 24 h (D18) EtOH withdrawal. (F) There was no significant difference in rearing behavior between EtOH Diet and Control Diet groups at either 12 h or 24 h withdrawal. EtOH Diet animals showed significantly more EtOH withdrawal behaviors, tail rigidity (G), and abnormal gait (H), at both 12 and 24 h withdrawal. *p < 0.05
In Experiment 1B (Figure 1B), nonparametric Mann–Whitney U tests were used to analyze group differences for rearing, tail rigidity, and abnormal gait withdrawal behaviors. There were no significant differences between Control Diet and EtOH Diet animals in rearing at either 12 h (z = −0.218, p = 1.000) or 24 h (z = −0.892, p = 0.400) withdrawal (Figure 1F). EtOH Diet animals displayed significantly more tail rigidity than Control Diet animals both at 12 h (z = 2.087, p = 0.037) and 24 h (z = 2.201, p = 0.028; Figure 1G). Likewise, EtOH Diet animals showed significantly more abnormal gait than Control Diet animals at both 12 h (z = 2.121, p = 0.034) and 24 h (z = 2.341, p = 0.019; Figure 1H).
Experiment 2: Effect of chronic EtOH consumption and withdrawal on SEFL
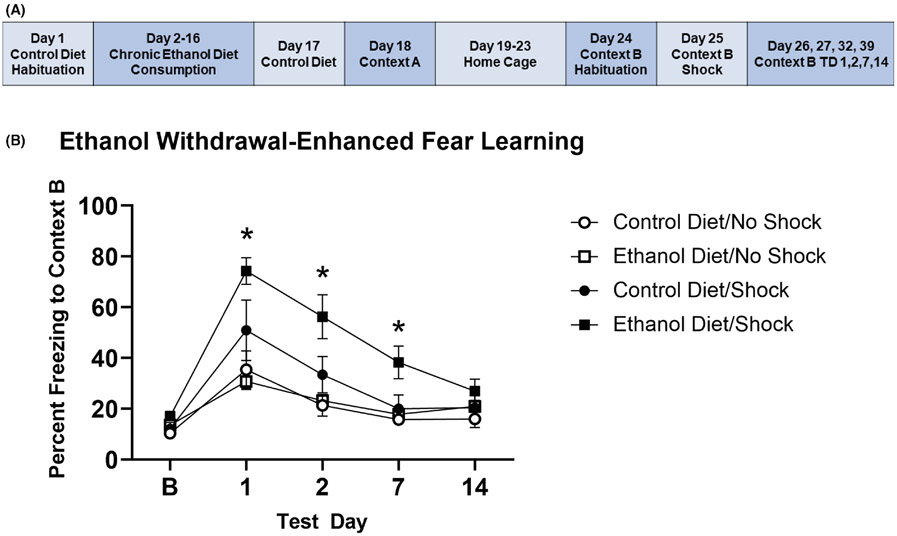
In Experiment 2 (Figure 2A), there was no effect of EtOH or footshock in Context A on baseline freezing in Context B, indicating a lack of generalization of fear to the new context (Kruskal–Wallis H = 5.272; p = 0.153). Given our hypothesis that there would be significant group differences in freezing for the Control Diet/Shock and EtOH Diet/Shock groups relative to their respective No Shock control groups, we first analyzed whether the No Shock groups were different. There was no effect of diet type on freezing in Context B following the absence of footshock in Context A, given the lack of significant Mann–Whitney U tests between the Control Diet/No Shock and EtOH Diet/No Shock groups at baseline and on all test days: Baseline (z = −1.056, p = 0.343), TD1 (z = −0.081, p = 1.000), TD2 (z = −0.893, p = 0.432), TD7 (z = −1.056, p = 0.343), and TD14 (z = −1.868, p = 0.073). Given this lack of difference in freezing behavior, we combined these two groups into a single No Shock control group. Mann–Whitney U tests revealed no significant effect of footshock in Context A on freezing, as the Control Diet/Shock group was not significantly different from the No Shock control group on any test day: TD1 (z = −1.183, p = 0.261), TD2 (z = −1.352, p = 0.196), TD7 (z = −0.719, p = 0.482), TD14 (z = −1.183, p = 0.261). Interestingly, the EtOH Diet/Shock group showed significantly enhanced freezing compared with the No Shock group on TD1 (z = −3.091, p = 0.001), TD2 (z = −3.091, p = 0.001), and TD7 (z = −3.372, p < 0.001). By TD14, the EtOH Diet/Shock group no longer showed freezing significantly different from the No Shock controls (z = −1.686, p = 0.102; Figure 2B).
FIGURE 2.
Chronic EtOH consumption and 24 h withdrawal exacerbate SEFL. (A) Experiment timeline for experiment 2. (B) The EtOH Diet/Shock group spent significantly more time freezing in context B on TD1, TD2, and TD7 than No Shock group. There were no significant differences between the Control Diet/Shock group and No Shock groups. * difference between EtOH Diet/Shock and No Shock groups with p < 0.05; error bars represent standard error. TD = context B test day
Experiment 3: Effect of chronic EtOH consumption and withdrawal and severe footshock stress on markers of glial activation
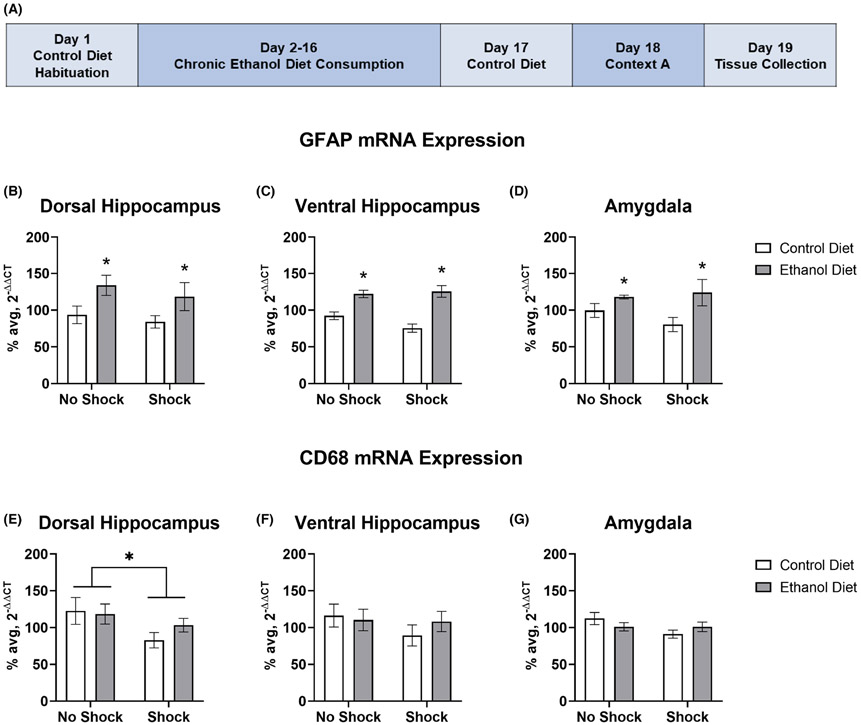
In Experiment 3 (Figure 3A), 2 × 2 ANOVAs revealed a significant main effect of diet type on GFAP mRNA expression in the DH, F (1, 30) = 7.253, p = 0.011 (Figure 3B), VH, F (1, 29) = 37.632, p < 0.001 (Figure 3C), and amygdala complex, F (1, 29) = 9.519, p = 0.004 (Figure 3D) with EtOH Diet groups showing significantly more GFAP mRNA expression than Control Diet groups in all three brain regions of interest. However, there was no significant main effect of shock on GFAP mRNA expression in the DH, F (1, 30) = 0.966, p = 0.334, VH, F (1, 29) = 2.211, p = 0.148, or amygdala, F (1, 29) = 1.277, p = 0.268. Likewise, there was no interaction of diet type and shock on GFAP mRNA expression in the DH, F (1, 30) = 0.091, p = 0.765, VH, F (1, 29) = 2.94, p = 0.097, or amygdala, F (1, 29) = 1.092, p = 0.305.
FIGURE 3.
Chronic EtOH consumption and 24 h withdrawal enhance GFAP mRNA expression across multiple brain regions, while severe footshock stress decreases CD68 mRNA expression in the DH. (A) Experiment timeline for experiment 3. GFAP mRNA expression was significantly increased in EtOH Diet groups compared with Control Diet groups in the DH (B), VH (C), and the amygdala (D) 48 h following the start of EtOH withdrawal. Groups that experienced severe footshock stress showed significantly decreased mRNA expression of CD68 in the DH (E), while no changes in CD68 mRNA expression were found in the VH (F) or amygdala (G). *p < 0.05; error bars represent standard error
In the DH, a 2 × 2 ANOVA revealed a significant main effect of shock on CD68 mRNA expression, F (1, 30) = 4.842, p = 0.036 (Figure 3E), with decreased expression in Shock groups. However, there were no changes to CD68 mRNA expression due to shock in either the VH, F (1, 30) = 1.803, p = 0.189 (Figure 3F) or amygdala, F (1, 28) = 2.498, p = 0.125 (Figure 3G). Additionally, there was no significant main effect of diet type on CD68 mRNA expression in the DH, F(1, 30) = 0.973, p = 0.332, VH, F (1, 30) = 0.711, p = 0.406, or amygdala, F (1, 28) = 0.003, p = 0.956. Likewise, there was no interaction of diet type and shock on CD68 mRNA expression in the DH, F (1, 30) = 1.311, p = 0.261, VH, F (1, 30) = 0.621, p = 0.437, or amygdala, F (1, 28) = 2.439, p = 0.130.
Experiment 4: Effect of astroglial Gi-DREADD stimulation on EtOH-induced increases in SEFL
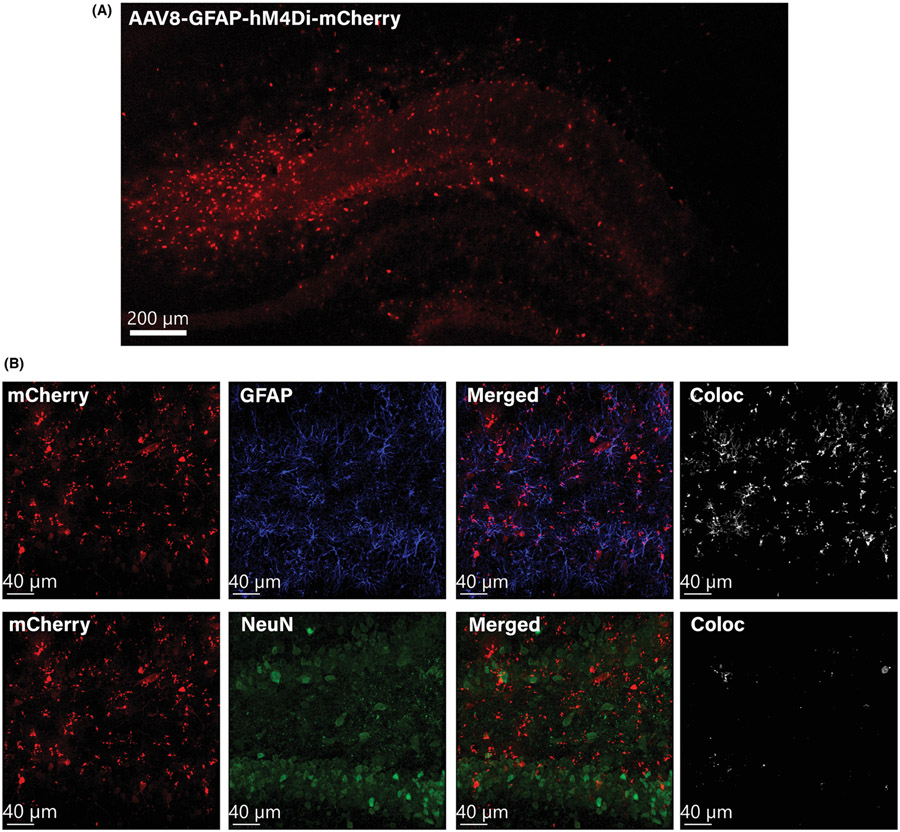
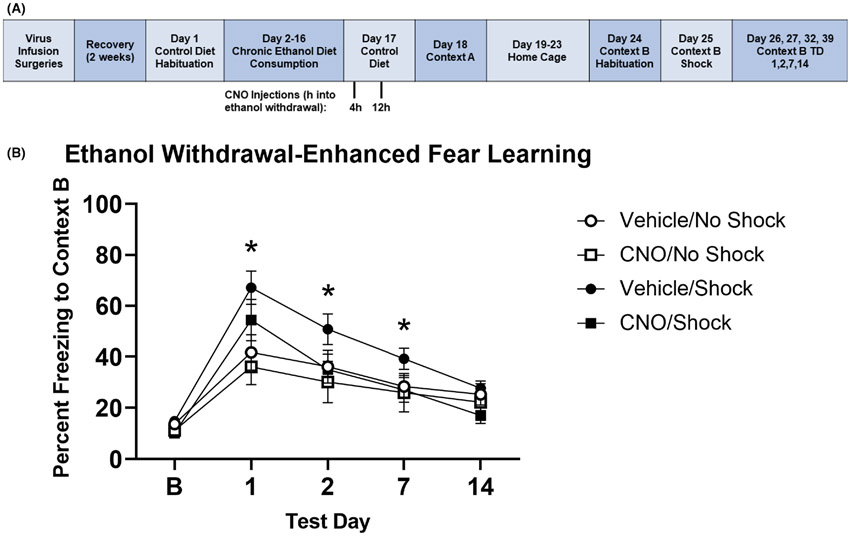
Representative images of DH astroglial-specific DREADD expression are shown in Figure 4. In Experiment 4 (Figure 5A), there was no significant effect of CNO administration or footshock in Context A on baseline freezing in Context B (Kruskal–Wallis H = 5.681, p = 0.128), demonstrating a lack of generalization of fear to the new context. Similar to the approach for Experiment 2, we first analyzed group differences between the CNO/No Shock and Vehicle/No Shock groups, and determined there were no significant differences in freezing on any test day: Baseline (z = −1.073, p = 0.295), TD1 (z = −0.714, p = 0.534), TD2 (z = −0.857, p = 0.445), TD7 (z = −0.857, p = 0.445), TD14 (z = −0.857, p = 0.445). Given the lack of differences between the two No Shock groups, they were combined into a single No Shock control group for subsequent analysis. There was a significant effect of shock on freezing in Context B, with the Vehicle/Shock group freezing significantly more than the No Shock control group on TD1 (z = −2.905, p = 0.003), TD2 (z = −2.170, p = 0.030), and TD7 (z = −2.104, p = 0.036), but no difference on TD14 (z = −1.235, p = 0.235). Mann–Whitney U tests revealed no significant differences between the CNO/Shock group and the No Shock control group on TD1 (z = −1.503, p = 0.144), TD2 (z = −0.167, p = 0.896), and TD7 (z = −0.167, p = 0.896), though there was a trending difference on TD14 (z = −1.970, p = 0.051) with the CNO/Shock group freezing less than the No Shock control group (Figure 5B).
FIGURE 4.
AAV8-GFAP-hM4Di-mCherry is expressed in dorsal hippocampal astrocytes. (A) A representative 10× confocal tile image shows mCherry expression throughout the DH. (B) Representative confocal images at 20× show that the DREADD construct (mCherry) is primarily colocalized with the astrocyte marker GFAP (Alexa Fluor 405) and has minimal localization (<5%) with the neuronal marker NeuN (Alexa Fluor 488)
FIGURE 5.
Stimulation of astroglial Gi-DREADD in the DH reduces EtOH-exacerbated SEFL. (A) Experiment timeline for experiment 4. (B) EtOH Diet animals again showed an enhancement of SEFL, as the Vehicle/Shock group froze significantly more than the No Shock groups on TD1, TD2, and TD7. There was no difference between the CNO/Shock group and No Shock groups, indicating that CNO injections during EtOH withdrawal decreased the EtOH and shock-induced increase in freezing. * difference between Vehicle/Shock and No Shock control group with p < 0.05; error bars represent standard error. TD = context B test day
DISCUSSION
This study is the first to demonstrate that prior chronic EtOH consumption and withdrawal exacerbates future SEFL and that DH astrocytes are critically involved in this change in behavior. Animals exposed to chronic EtOH consumption, EtOH withdrawal, and severe footshock exhibited significantly enhanced freezing behavior in response to a future mild stressor. Additionally, chronic EtOH consumption and withdrawal resulted in increased GFAP mRNA expression in all brain regions examined. Given the importance of the DH in the development of a PTSD-like phenotype and the observed EtOH-induced increases in DH GFAP expression, we tested the involvement of DH astroglia in mediating the EtOH-induced exacerbation of SEFL. We demonstrate that stimulation of astroglial Gi-DREADD in the DH prevented the enhanced freezing behavior displayed by EtOH- and shock-exposed animals. Overall, these findings provide critical evidence that prior EtOH dependence increases susceptibility to a PTSD-like phenotype and that DH astrocytes are involved in mediating this effect.
In Experiment 1, we establish that 15-day consumption of the 5% w/v EtOH low-fat Lieber-DeCarli liquid diet results in the consumption of clinically relevant doses of EtOH, with average BECs greater than 80 mg/dl, the “binge-level” equivalent, on all days where tail blood was measured. BECs and EtOH intake measured in g/kg/day were consistent with previous reports using a liquid diet model (Piano et al., 2005; Wills et al., 2008). Differences in BECs likely reflect slight changes in day-to-day drinking patterns, as animals exposed to liquid diet may consume EtOH throughout the day instead of at consistent times of peak consumption, as seen with other EtOH models (Hiller-Sturmhöfel & Kulkosky, 2001). Further, we show that the 15-day exposure to the low-fat Lieber-DeCarli liquid diet produces EtOH dependence, as EtOH Diet animals showed significantly more tail rigidity and abnormal gait at both 12 and 24 h withdrawal. These behaviors are specific to EtOH withdrawal and present at similar timepoints after EtOH cessation (Fleming et al., 2019; Macey et al., 1996; Uzbay & Kayaalp, 1995). Overall, the results of Experiment 1 established that the low-fat Lieber-DeCarli liquid diet produces EtOH dependence and results in EtOH consumption similar to that which has been previously established.
Using this EtOH consumption and withdrawal model, we are the first to show that chronic EtOH consumption exacerbates the development of a PTSD-like phenotype. EtOH-dependent rats exposed to the severe stressor (Context A) displayed significantly enhanced freezing behavior relative to nonshocked groups. These findings suggest that EtOH dependence and severe stress during withdrawal increase vulnerability to the development of a PTSD-like phenotype. These behavioral results support the susceptibility hypothesis and mirror clinical findings that individuals with AUD have a higher risk of developing PTSD (e.g., Stein et al., 2017). Interestingly, animals that consumed EtOH and experienced withdrawal but did not experience severe stress did not display any change in freezing behavior compared with nonshocked Control Diet animals. This suggests that EtOH dependence and 24 h withdrawal alone are not sufficient to produce enhanced fear learning.
Differences between the Control Diet/Shock and nonshocked groups did not achieve significance. Given that the SEFL model reliably produces enhanced fear learning (Jones et al., 2015; Jones, Lebonville, et al., 2018; Jones, Paniccia, et al., 2018), it is surprising that an effect was not seen in this group. In addition to the potential of within-group variability to obscure group differences, the weight of liquid diet animals compared with animals on a typical pellet food diet could have differed and affected sensitivity to shock. It is common for animals on the Lieber-DeCarli diet to slow their growth trajectories for the first 2 to 3 weeks of consuming this diet (Guo et al., 2018), and we did observe less weight gain in these animals. Evidence also shows that meal-feeding rats can alter the circadian rhythms of corticosterone (Moberg et al., 1975). While animals have 24 h access to the liquid diet during this paradigm, a fresh diet is replaced at the same time each day. Thus, if changes in eating patterns differed between groups such that Control Diet animals consumed food in a meal-like pattern, this could potentially affect basal patterns of corticosterone levels, which in turn might alter the reaction to stress. Despite these potential group differences, if the footshock in Context A was an insufficient stressor, the EtOH Diet/Shock group would likely not have shown enhanced freezing, as EtOH Diet alone did not produce enhanced freezing. Remarkably, this combined effect of chronic EtOH consumption/withdrawal and severe stress exposure produces a robust conditioned fear response that was replicated in Experiment 4. While these data support a growing body of literature suggesting that previous experience with EtOH enhances vulnerability to the development of PTSD-like behavior, we sought to examine the neurobiological mechanisms responsible for EtOH-induced susceptibility to PTSD.
Our data demonstrate that chronic EtOH consumption and withdrawal result in increased GFAP mRNA expression in the DH, VH, and amygdalar complex. This EtOH withdrawal-induced increase in GFAP expression is consistent with previous research (Alfonso-Loeches et al., 2010; Evrard et al., 2006). While GFAP is a traditional marker for astrocyte reactivity (Hol & Pekny, 2015), GFAP may not be expressed in every astrocyte, and changes in GFAP alone are not sufficient to determine astrocyte reactivity (Escartin et al., 2021; Guttenplan & Liddelow, 2019; Khakh &Sofroniew, 2015). Thus, these data are but one indication that EtOH consumption and withdrawal may enhance astrocyte reactivity, but the further assessment of additional astrocytic markers is needed to make this determination. However, the literature supports that astrocyte function is altered following EtOH exposure, typically in a proinflammatory direction. For instance, EtOH exposure upregulates signal transducer and activator of transcription 3 (STAT3), which controls the transcription of immune-related genes (Chen et al., 2021). STAT3 was found to be frequently bound to GFAP and related to EtOH withdrawal-induced depressive-like behaviors (Chen et al., 2021). Additionally, EtOH has been shown to activate both toll-like receptor 4 (TLR4) and interleukin-1 receptor 1 (IL-1R1) in astrocyte culture (Blanco et al., 2005), and genetic knockdown of TLR4 decreased astrocyte production of proinflammatory cytokines in vivo (Alfonso-Loeches et al., 2010). Given this literature, it is possible that chronic EtOH exposure and withdrawal affected astrocyte function in the current study through the upregulation of proinflammatory signaling. Supporting this, our laboratory has previously demonstrated that withdrawal from opioids induces increased expression of DH GFAP and IL-1 protein (Parekh et al., 2020). Future studies should assess astrocyte-specific gene and protein profiles throughout EtOH withdrawal, as this would provide insight into the specific signaling molecules involved in potential EtOH-induced increases of astrocyte reactivity.
In addition to changes in hippocampal and amygdalar GFAP mRNA expression, severe footshock stress selectively decreased CD68 mRNA expression in the DH 24 h following severe stress exposure. There were no alterations in CD68 gene expression within the VH or amygdalar complex. Importantly, these results replicate our previous findings showing that Iba-1 immunoreactivity in the DH is decreased 48 h after the severe footshock stressor in Context A (Jones, Lebonville, et al., 2018). Collectively, data from our laboratory suggest that DH microglia are altered as a result of severe stress exposure, suggesting a potential role in the development of a PTSD-like phenotype, independent of substance use disorders. Future experiments could examine microglial activation profiles and morphology to gain a more in-depth view of the impact of severe stress on DH microglia. However, we did not observe changes in DH CD68 expression due to chronic EtOH consumption, and as such, follow-up experiments focused on the contribution of astrocytes in the increased susceptibility to a PTSD-like phenotype following EtOH dependence.
Given the marked increase in GFAP gene expression following chronic EtOH consumption and withdrawal in the DH, an area in which astrocytes have been shown to be functionally relevant to SEFL (Jones, Paniccia, et al., 2018), the current study examined how in vivo manipulation of astrocyte activity during withdrawal altered future fear learning behavior. Excitingly, we replicated results from Experiment 2, in which EtOH-dependent animals exposed to severe stress displayed significantly enhanced fear learning. There were also no significant differences between the No Shock/CNO and No Shock/Vehicle groups, suggesting no effect of CNO alone on freezing. Remarkably, stimulation of astroglial Gi-DREADD during EtOH withdrawal decreased the combined effect of chronic EtOH consumption/withdrawal and severe stress on enhanced fear learning. In other words, when EtOH Diet/Shock subjects experienced CNO administration during EtOH withdrawal, they no longer exhibited enhanced freezing behavior in Context B. Given that stimulation of astrocyte, Gi signaling is known to decrease LPS-induced increases in GFAP positive cell counts and immunoreactivity and decrease proinflammatory cytokines (Kim et al., 2021), our causal DREADD manipulation likely decreased DH astroglial GFAP expression and/or proinflammatory cytokine signaling. As we have previously demonstrated the involvement of astrocyte-dependent cytokine signaling in the development of SEFL (Jones, Lebonville, et al., 2018), as well as in opioid withdrawal-induced enhanced fear learning (Parekh et al., 2020), we hypothesize that astrocyte-dependent neuroimmune signaling is responsible for the development of an exacerbated PTSD-like phenotype in EtOH-dependent animals.
It is likely that EtOH consumption and withdrawal change hippocampal astrocytes, such that these EtOH-induced adaptations result in altered astroglial expression profiles, structural changes, and signaling mechanisms. These hypothesized changes in astrocyte function may subsequently affect how animals learn about the Context A severe stress, ultimately leading to the exacerbation of SEFL seen in Experiments 2 and 4 caused by this impaired learning. For instance, the increase in GFAP expression observed in Experiment 3 may be one indication of increased astrocyte reactivity following EtOH consumption, a result that has been well-documented following both short- and long-term EtOH consumption and withdrawal (Hayes et al., 2013; Miguel-Hidalgo, 2006; Vallés et al., 2004). These changes in astrocyte function may also be related to an increase in proinflammatory signaling, as discussed previously (Alfonso-Loeches et al., 2010; Blanco et al., 2005; Chen et al., 2021). Increased levels of proinflammatory cytokines, such as interleukin-1β and tumor necrosis factor α, impair learning and memory (Goshen et al., 2007; Habbas et al., 2015). Therefore, we believe it is possible that chronic EtOH consumption and withdrawal induces increases in DH astrocyte proinflammatory signaling, which lead to future impairments in learning about the severe stressor in Context A. While EtOH exposure increases GFAP mRNA expression, it appears that this alone does not enhance future fear learning—the combination of EtOH exposure and severe footshock stress is necessary to see this behavioral effect. Thus, EtOH exposure may broadly drive increases in proinflammatory cytokines from DH astrocytes, but these neuroimmune changes only cause enhanced impairment of learning and memory following the addition of severe stress exposure. To investigate whether this proposed mechanism is true, future studies should investigate DH astrocyte-specific neuroimmune gene and protein profiles, and examine proinflammatory cytokine expression and signaling following stimulation of the astroglial Gi-DREADD, an inhibitor of the exacerbated PTSD-like phenotype.
Collectively, the current study indicates that EtOH dependence exacerbates future SEFL and that this change in behavior is, at least in part, mediated by DH astrocytes. These results support clinical literature suggesting AUD may increase susceptibility to future PTSD. Additionally, these results add to the growing literature suggesting astrocytes, and the neuroimmune system more broadly, can act as novel targets for treatments addressing comorbid AUD and PTSD.
ACKNOWLEDGMENTS
The authors would like to thank Rhiannon Thomas, Lydia Adams, and Thery Sanon for their technical support and assistance in data collection.
Funding information
National Institute on Alcohol Abuse and Alcoholism, Grant/Award Number: AA013573, AA022048, AA025809 and AA027463; National Institute on Drug Abuse, Grant/Award Number: DA007244, DA034721, DA047054, DA048241 and DA053781. JEP was supported by DA047054.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I & Guerri C (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. The Journal of Neuroscience, 30, 8285–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF & Becker HC (2016) Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology, 233, 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE & Jones JL (2018) Alcohol use disorder and posttraumatic stress disorder: an introduction. Alcoholism, Clinical and Experimental Research, 42, 836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco AM, Vallés SL, Pascual M & Guerri C (2005) Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. Journal of Immunology, 175, 6893–6899. [DOI] [PubMed] [Google Scholar]
- Chen W-Y, Chen H, Hamada K, Gatta E, Chen Y, Zhang H et al. (2021) Transcriptomics identifies STAT3 as a key regulator of hippocampal gene expression and anhedonia during withdrawal from chronic alcohol exposure. Translational Psychiatry, 11, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-Y, Zhang H, Gatta E, Glover EJ, Pandey SC & Lasek AW (2019) The histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) alleviates depression-like behavior and normalizes epigenetic changes in the hippocampus during ethanol withdrawal. Alcohol, 78, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD & Breslau N (1998) Investigations of causal pathways between PTSD and drug use disorders. Addictive Behaviors, 23, 827–840. [DOI] [PubMed] [Google Scholar]
- Choi M, Ahn S, Yang E-J, Kim H, Chong YH & Kim H-S (2016) Hippocampus-based contextual memory alters the morphological characteristics of astrocytes in the dentate gyrus. Molecular Brain, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz C, Meireles M & Silva SM (2017) Chronic ethanol intake induces partial microglial activation that is not reversed by long-term ethanol withdrawal in the rat hippocampal formation. Neurotoxicology, 60, 107–115. [DOI] [PubMed] [Google Scholar]
- Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S et al. (2014) A systematic review of the comorbidity between PTSD and alcohol misuse. Social Psychiatry and Psychiatric Epidemiology, 49, 1401–1425. [DOI] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF et al. (2013) Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Translational Psychiatry, 3, e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS & Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochemical Research, 25, 1439–1451. [DOI] [PubMed] [Google Scholar]
- Erickson EK, Grantham EK, Warden AS & Harris RA (2019) Neuroimmune signaling in alcohol use disorder. Pharmacology, Biochemistry, and Behavior, 177, 34–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Galea E, Lakatos A, O'Callaghan JP, Petzold GC, Serrano-Pozo A et al. (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience, 24, 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard SG, Duhalde-Vega M, Tagliaferro P, Mirochnic S, Caltana LR & Brusco A (2006) A low chronic ethanol exposure induces morphological changes in the adolescent rat brain that are not fully recovered even after a long abstinence: an immunohistochemical study. Experimental Neurology, 200, 438–459. [DOI] [PubMed] [Google Scholar]
- Fleming W, Jones Q, Chandra U, Saini A, Walker D, Francis R et al. (2019) Withdrawal from brief repeated alcohol treatment in adolescent and adult male and female rats. Alcoholism, Clinical and Experimental Research, 43, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW & Weiner JL (2017) Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder. Genes, Brain, and Behavior, 16, 15–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez ST, Marty V, Spigelman I, Reise SP & Fanselow MS (2021) Impact of stress resilience and susceptibility on fear learning, anxiety, and alcohol intake. Neurobiology of Stress, 15, 100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T et al. (2007) A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology, 32, 1106–1115. [DOI] [PubMed] [Google Scholar]
- Guo F, Zheng K, Benedé-Ubieto R, Cubero FJ & Nevzorova YA (2018) The Lieber-DeCarli diet-a flagship model for experimental alcoholic liver disease. Alcoholism, Clinical and Experimental Research, 42, 1828–1840. [DOI] [PubMed] [Google Scholar]
- Guttenplan KA & Liddelow SA (2019) Astrocytes and microglia: models and tools. The Journal of Experimental Medicine, 216, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N et al. (2015) Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell, 163, 1730–1741. [DOI] [PubMed] [Google Scholar]
- Hayes DM, Deeny MA, Shaner CA & Nixon K (2013) Determining the threshold for alcohol-induced brain damage: new evidence with gliosis markers. Alcoholism, Clinical and Experimental Research, 37, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller-Sturmhöfel S & Kulkosky P (2001) Chronobiological regulation of alcohol intake. Alcohol Research & Health, 25, 141–148. [PMC free article] [PubMed] [Google Scholar]
- Hol EM & Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Current Opinion in Cell Biology, 32, 121–130. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM & Kosten TR (2001) Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. The American Journal of Psychiatry, 158, 1184–1190. [DOI] [PubMed] [Google Scholar]
- Jones ME, Lebonville CL, Barrus D & Lysle DT (2015) The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology, 40, 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Lebonville CL, Paniccia JE, Balentine ME, Reissner KJ & Lysle DT (2018) Hippocampal interleukin-1 mediates stress-enhanced fear learning: a potential role for astrocyte-derived interleukin-1β. Brain, Behavior, and Immunity, 67, 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Paniccia JE, Lebonville CL, Reissner KJ & Lysle DT (2018) Chemogenetic manipulation of dorsal hippocampal astrocytes protects against the development of stress-enhanced fear learning. Neuroscience, 388, 45–56. [DOI] [PubMed] [Google Scholar]
- Khakh BS&Sofroniew MV(2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nature Neuroscience, 18, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-H, Rahman MH, Lee WH & Suk K (2021) Chemogenetic stimulation of the Gi pathway in astrocytes suppresses neuroinflammation. Pharmacology Research & Perspectives, 9, e00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Harper KM, Whitman BA, Zimomra Z & Breese GR (2016) Stress and withdrawal from chronic ethanol induce selective changes in neuroimmune mRNAs in differing brain sites. Brain Sciences, 6, E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebonville CL, Paniccia JE, Parekh SV, Wangler LM, Jones ME, Fuchs RA et al. (2020) Expression of a heroin contextually conditioned immune effect in male rats requires CAMKIIα-expressing neurons in dorsal, but not ventral, subiculum and hippocampal CA1. Brain, Behavior, and Immunity, 89, 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhang D & Verkhratsky A (2022) Astrocytes in post-traumatic stress disorder. Neuroscience Bulletin, 38(8), 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liao Y, Dong Y, Li X, Li J, Cheng Y et al. (2021) Microglial deletion and inhibition alleviate behavior of post-traumatic stress disorder in mice. Journal of Neuroinflammation, 18, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS & DeCarli LM (1982) The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcoholism, Clinical and Experimental Research, 6, 523–531. [DOI] [PubMed] [Google Scholar]
- Lieber CS & DeCarli LM (1989) Liquid diet technique of ethanol administration: 1989 update. Alcohol and Alcoholism, 24, 197–211. [PubMed] [Google Scholar]
- Lieber CS, Decarli LM & Sorrell MF (1989) Experimental methods of ethanol administration. Hepatology, 10, 501–510. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC & Koob GF (1996) Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol, 13, 163–170. [DOI] [PubMed] [Google Scholar]
- McFarlane AC, Browne D, Bryant RA, O'Donnell M, Silove D, Creamer M et al. (2009) A longitudinal analysis of alcohol consumption and the risk of posttraumatic symptoms. Journal of Affective Disorders, 118, 166–172. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS & Spigelman I (2013) Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcoholism, Clinical and Experimental Research, 37, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ (2006) Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. A/cohoi and Alcoholism, 41, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg GP, Bellinger LL & Mendel VE (1975) Effect of meal feeding on daily rhythms of plasma corticosterone and growth hormone in the rat. NEN, 19, 160–169. [DOI] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF et al. (2015) Social stress and escalated drug self-administration in mice I. alcohol and corticosterone. Psychopharmacology, 232, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SB & Hamblen JL (2017) Promising directions for treating comorbid PTSD and substance use disorder. Alcoholism, Clinical and Experimental Research, 41, 708–710. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ & Breese GR (2002) Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcoholism, Clinical and Experimental Research, 26, 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang D, Mittal N & Nayyar D (1999) Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. The Journal of Pharmacology and Experimental Therapeutics, 288, 866–878. [PubMed] [Google Scholar]
- Paniccia JE, Lebonville CL, Jones ME, Parekh SV, Fuchs RA & Lysle DT (2018) Dorsal hippocampal neural immune signaling regulates heroin-conditioned immunomodulation but not heroin-conditioned place preference. Brain, Behavior, and Immunity, 73, 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniccia JE, Weckstein TN, Lebonville CL & Lysle DT (2021) Female rats express heroin-induced and -conditioned suppression of peripheral nitric oxide production in response to endotoxin challenge. Brain, Behavior, and Immunity, 91, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh SV, Paniccia JE, Adams LO & Lysle DT (2021) Hippocampal TNF-α signaling mediates heroin withdrawal-enhanced fear learning and withdrawal-induced weight loss. Molecular Neurobiology, 58, 2963–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh SV, Paniccia JE, Lebonville CL & Lysle DT (2020) Dorsal hippocampal interleukin-1 signaling mediates heroin withdrawal-enhanced fear learning. Psychopharmacology, 237, 3653–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL & Simpson TL (2017) Posttraumatic stress disorder and alcohol use disorder: a critical review of pharmacologic treatments. Alcoholism, Clinical and Experimental Research, 41, 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano MR, Carrigan TM & Schwertz DW (2005) Sex differences in ethanol liquid diet consumption in Sprague-Dawley rats. Alcohol, 35, 113–118. [DOI] [PubMed] [Google Scholar]
- Piggott VM, Lloyd SC, Perrine SA & Conti AC (2020) Chronic intermittent ethanol exposure increases ethanol consumption following traumatic stress exposure in mice. Frontiers in Behavioral Neuroscience, 14, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L &Crews FT (2012) NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. Journal of Neuroinflammation, 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP & Fanselow MS (2005) Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews, 29, 1207–1223. [DOI] [PubMed] [Google Scholar]
- Smith KL, Kassem MS, Clarke DJ, Kuligowski MP, Bedoya-Pérez MA, Todd SM et al. (2019) Microglial cell hyper-ramification and neuronal dendritic spine loss in the hippocampus and medial prefrontal cortex in a mouse model of PTSD. Brain, Behavior, and Immunity, 80, 889–899. [DOI] [PubMed] [Google Scholar]
- Stein MB, Campbell-Sills L, Gelernter J, He F, Heeringa SG, Nock MJ et al. (2017) Alcohol misuse and co-occurring mental disorders among new soldiers in the U.S. Army. Alcoholism: Clinical and Experimental Research, 41, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J Ressler KJ (2018) Common biological mechanisms of alcohol use disorder and post-traumatic stress disorder. Alcohol Research: Current Reviews, 39, 131–145. [PMC free article] [PubMed] [Google Scholar]
- Uzbay IT & Kayaalp SO (1995) A modified liquid diet of chronic ethanol administration: validation by ethanol withdrawal syndrome in rats. Pharmacological Research, 31, 37–42. [DOI] [PubMed] [Google Scholar]
- Vallés SL, Blanco AM, Pascual M Guerri C (2004) Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathology, 14, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH & Breese GR (2008) Differential dietary ethanol intake and blood ethanol levels in and adult rats: effects on anxiety-like behavior and seizure thresholds. Alcoholism, Clinical and Experimental Research, 32, 1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH & Breese GR (2009) Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcoholism, Clinical and Experimental Research, 33, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won E & Kim Y-K (2020) Neuroinflammation-associated alterations of the brain as potential neural biomarkers in anxiety disorders. International Journal of Molecular Sciences, 21, 6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Eisenmann ED, Rose RM, Kohls BA, Johnson BL, Robinson KL et al. (2018) Predator-based psychosocial stress model of PTSD differentially influences voluntary ethanol consumption depending on methodology. Alcohol, 70, 33–41. [DOI] [PubMed] [Google Scholar]