OBJECTIVES:
There is a crucial unmet need for biomarker-guided diagnostic and prognostic enrichment in clinical trials evaluating immune modulating therapies in critically ill patients. Low monocyte expression of human leukocyte antigen-DR (mHLA-DR), considered as a reference surrogate to identify immunosuppressed patients, has been proposed for patient stratification in immunostimulation approaches. However, its widespread use in clinic has been somewhat hampered by technical constraints inherent to flow cytometry technology. The objective of the present study was to evaluate the ability of a prototype multiplex polymerase chain reaction tool (immune profiling panel [IPP]) to identify immunosuppressed ICU patients characterized by a low mHLA-DR expression.
DESIGN:
Retrospective observational cohort study.
SETTING:
Adult ICU in a University Hospital, Lyon, France.
PATIENTS:
Critically ill patients with various etiologies enrolled in the REAnimation Low Immune Status Marker study (NCT02638779).
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
mHLA-DR and IPP data were obtained from 1,731 blood samples collected from critically ill patients with various etiologies and healthy volunteers. A partial least square regression model combining the expression levels of IPP markers was trained and used for the identification of samples from patients presenting with evidence of immunosuppression, defined here as mHLADR less than 8,000 antibodies bound per cell (AB/C). The IPP gene set had an area under the receiver operating characteristic curve (AUC) of 0.86 (95% CI 0.83–0.89) for the identification of immunosuppressed patients. In addition, when applied to the 123 patients still in the ICU at days 5–7 after admission, IPP similarly enriched the number of patients with ICU-acquired infections in the immunosuppressed group (26%), in comparison with low mHLA-DR (22%).
CONCLUSIONS:
This study reports on the potential of the IPP gene set to identify ICU patients presenting with mHLA-DR less than 8,000 AB/C. Upon further optimization and validation, this molecular tool may help in the stratification of patients that could benefit from immunostimulation in the context of personalized medicine.
Keywords: gene expression, human leukocyte antigen-DR, immunologic monitoring, intensive care units, monocytes
KEY POINTS.
Question: Can a prototype multiplex polymerase chain reaction tool (immune profiling panel, IPP) identify ICU patients with evidence of immunosuppression, defined as monocyte expression of human leukocyte antigen-DR expression less than 8,000 antibodies bound per cell?
Findings: In this retrospective observational cohort study, IPP had an area under the receiver operating characteristic curve of 0.86 for the identification of samples indicative of immunosuppression in ICU patients. When applied to patients still in the ICU at days 5–7, IPP enriched the number of patients with ICU-acquired infections in the immunosuppressed group.
Meaning: This study reports on the potential of an IPP prototype to identify immunosuppressed ICU patients with an automated system. This may help in guiding future personalized immunomodulating strategies.
BACKGROUND
Syndrome heterogeneity has been suggested as the main reason to explain the failure of decades of anti-inflammatory strategies for the treatment of sepsis (1). This highlights the crucial need for patient stratification to enable a more individualized approach. Immunoinflammatory response following sepsis is complex as it rapidly evolves over time and varies according to location and cell type, in a compartmentalized manner (2). Briefly, at the systemic level, sepsis pathophysiology is characterized by a tremendous inflammatory response that is accompanied by concomitant anti-inflammatory feedback acting as a compensatory mechanism. As a result, immunosuppression temporarily occurs but may persist in subgroups of patients. This immunosuppressive phase has been repeatedly found to induce detrimental consequences on patients’ outcomes (3, 4). A similar mechanism is involved after noninfectious insults such as trauma or major surgery (5). Consequently, adjunctive immunostimulation is now being explored as a means to counterbalance this immunosuppressive response and restore patients’ immune functions. However, in order not to reproduce errors from the past in anti-inflammatory strategies, this novel approach would benefit from identifying groups of patients presenting with a high level of immunosuppression. Since there is no clinical sign of immunosuppression, such stratification should be based on immune biomarkers. Consensus now exists for considering low monocyte expression of human leukocyte antigen-DR expression (mHLA-DR) as the reference surrogate to diagnose sepsis-induced immunosuppression (3). Thanks to standardized measurement, a clinical decision-making threshold has been proposed, that is, mHLA-DR less than 8,000 antibodies bound per cell (AB/C), and used to stratify sepsis patients in a successful pioneering immunostimulation study (6). Several ongoing clinical trials also based patient stratification on the same threshold (NCT02361528, NCT04990232).
To date, mHLA-DR expression is assessed by flow cytometry, a technology that presents barriers to clinical deployment, including preanalytical constraints, the need for skilled technicians, and a lack of round-the-clock access.
Recently, various transcriptomic approaches have been proposed to better characterize septic patients. Among them is the immune profiling panel (IPP), a prototype multiplex polymerase chain reaction (PCR) tool that enables assessment of a set of messenger RNA (mRNA) markers involved in the main immune functions dysregulated in ICU patients (7). Most importantly, this overview of patients’ immune status can be obtained in whole blood in less than an hour using the fully automated BioFire FilmArray system (BioFire Diagnostics, LLC, Salt Lake City, UT).
The objective of the present study was to evaluate the ability of the IPP gene set to identify patients presenting with mHLA-DR expression less than 8,000 AB/C, a threshold indicative of immunosuppression (6). For this purpose, we took advantage of the REAnimation Low Immune Status Marker (REALISM) study (5) for which more than 1,700 samples with known mHLA-DR values were available.
METHODS
Patients and Samples
Patients and samples are from the REALISM study, in which 353 critically ill patients with different etiologies (sepsis, trauma, elective surgery) were enrolled, as well as 175 healthy volunteers. Peripheral whole blood was collected in EDTA and in PAXgene Blood RNA tubes (PreAnalytiX GmbH, Hombrechtikon, Switzerland) at different time-points from study inclusion to day 60, with up to seven samples per patient (5, 8). Healthy volunteers were sampled once. Written informed consent was obtained from each healthy volunteer and patient upon inclusion. The study protocol was approved on December 3, 2015, by the Institutional Review Board (Comité de Protection des Personnes Sud-Est II) under number 2015-42-2 and is in accordance with the Helsinki Declaration of 1975. It was also registered at ClinicalTrials.gov (NCT02638779).
IPP Measurement
PAXgene samples were stabilized for at least 2 hours after collection at room temperature and then frozen at –80°C following the manufacturer’s recommendations. RNA was then isolated using Maxwell HT simplyRNA Kit (AX2420; Promega Corporation, Madison, WI). RNA concentration was determined using QuantiFluor RNA system (E3310; Promega Corporation) on the GloMax Discover Microplate Reader (Promega). RNA integrity was assessed using the RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA) on the Agilent 2100 Bioanalyzer (Agilent Technologies).
The determination of the mRNA expression level of host response markers was performed on the FilmArray Torch Instrument (BioFire), as previously described (7, 9), by injecting 200 ng of isolated RNA in the IPP prototype (not submitted for regulatory review at the time of writing). Briefly, all freeze dried reagents are enclosed in a disposable pouch. After hydration and sample injection, the pouch is loaded in the FilmArray Torch Instrument (BioFire), in which sample preparation, reverse transcription, and nested PCR are performed. Among the 26 mRNAs available on IPP, we selected 11 markers on the basis of the literature (10–15) and preliminary analysis from the REALISM study (5) (Table 1). Normalized expression values were computed for each marker and used for the analyses.
TABLE 1.
Prototype Immune Profiling Panel Gene Set
| Genes | Name |
|---|---|
| C3AR1 | Complement C3a receptor 1 |
| CD177 | CD177 molecule |
| CD3D | CD3d molecule |
| CD74 | CD74 molecule |
| CIITA | Class II major histocompatibility complex transactivator |
| CTLA4 | Cytotoxic T-lymphocyte associated protein 4 |
| CX3CR1 | C-X3-C motif chemokine receptor 1 |
| IFNG | Interferon gamma |
| IL1R2 | Interleukin 1 receptor 2 |
| S100A9 | S100 calcium binding protein A9 |
| TAP2 | Transporter 2, adenosine triphosphate binding cassette subfamily B member |
mHLA-DR Measurement
The determination of the number of human leukocyte antigen-DR (HLA-DR) molecules per monocyte using the BD Quantibrite standardized method (HLA-DR: 340827; Quantibrite: 340495; Becton Dickenson, Franklin Lakes, NJ) was performed on fresh EDTA blood samples, within 3 hours after collection, as previously described (16). Samples were considered as immunosuppressed when mHLA-DR less than 8,000 AB/C and as immunocompetent otherwise.
Statistical Analysis
IPP Model for the Identification of Samples With mHLA-DR Less Than 8,000 AB/C
All samples for which both mHLA-DR and IPP measurements were available were used in the analysis.
A machine learning approach was implemented to predict immune status (i.e., immunosuppressed or immunocompetent), based on a linear regression that used a space dimension reduction: the partial least square-discriminant analysis (PLS-DA) regression. This classification method can simultaneously manage a large number of predictors, even if they are highly intercorrelated, which would hinder the identification of the features primarily associated with the outcome. Data were divided into a train set containing 1,221 samples (70%) to build the model and a test set containing the 510 remaining samples (30%) to assess the performance. The separation between the train and test set was balanced on mHLA-DR classes, sampling time-point, and patient etiology. The PLS-DA regression model combining the centered and scaled expression levels of the 11 IPP markers was trained using repeated cross-validation 20X-5-fold for the prediction of mHLA-DR below 8,000 AB/C. Synthetic minority oversampling technique oversampling was used to balance the training set between categories. Fine-tuning (number of components) was performed manually from one to the maximum number of components, with mean area under the receiver operating characteristic curve (AUC) across repetitions used to select the best number of components. The final PLS-DA model was built on the entire training set. Model performance was then evaluated on the test set by calculating AUC and its CI (bootstrap method, 2,000 replicates), accuracy, sensitivity, specificity, positive predictive value, and negative predictive value.
IPP Performance in the Group of Patients Still in the ICU at Days 5–7
The IPP model was applied to all the patients who were still in the ICU at days 5–7 and who had not developed any ICU-acquired infection at this time. This group of patients, still at risk of poor outcomes, may constitute a target for patient stratification before immunotherapy administration. A descriptive analysis of patient outcomes according to immune status defined by mHLA-DR or predicted by the IPP model was performed, using appropriate statistical tests.
Statistical analyses were performed using R software Version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria), with caret v6.0-91 and pls v2.8-0 packages for machine learning.
RESULTS
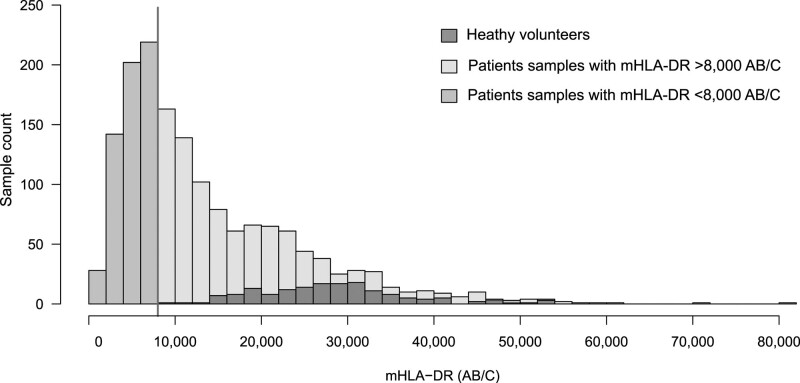
mHLA-DR and IPP measurements were available for 1,731 samples (163 from healthy volunteers and 1,568 from 351 patients) (detailed description in [5]). Overall, 34% of mHLA-DR values were below 8,000 AB/C (Fig. 1).
Figure 1.
Distribution of monocyte expression of human leukocyte antigen-DR (mHLA-DR) levels. mHLA-DR measurement was available in 1,731 samples distributed as follows: 163 samples from healthy volunteers (HVs), and 1,568 samples from 106 sepsis, 136 trauma, 109 surgery patients. mHLA-DR values ranged from 439 to 80,066 AB/C. The vertical line corresponds to the value of 8,000 AB/C. mHLA-DR was below 8,000 antibodies bound per cell (AB/C) in 591 patients samples (34%) and above 8,000 AB/C in 1,140 samples (66%), of which 977 samples were from patients, and 163 from HV.
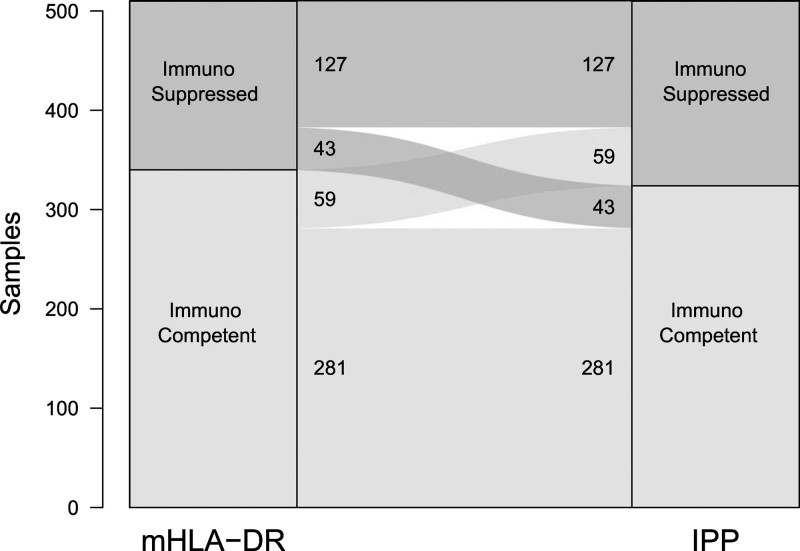
CD3D, CD74, CIITA, CTLA4, CX3CR1, IFNg, and TAP2 were down-regulated in the immunosuppressed group, whereas C3AR1, CD177, IL1R2, and S100A9 were up-regulated (eFig. 1, http://links.lww.com/CCM/H303). The final trained model used the combined expression levels of these 11 markers to predict the mHLA-DR class, defined by the 8,000 AB/C threshold. It exhibited good performance, with an AUC of 0.86 (95% CI 0.83–0.89) on the test set, and a concordance of 80% with true mHLA-DR classes (Fig. 2). A sensitivity analysis performed without healthy volunteers showed a concordance of 78% (Table 2).
Figure 2.
Concordance between monocyte expression of human leukocyte antigen-DR (mHLA-DR) and immune profiling panel (IPP) classification in the test set, including healthy volunteers (n = 510). Groups of samples based on mHLA-DR value (< 8,000 antibodies bound per cell [AB/C] for immunosuppressed and ≥ 8,000 AB/C for immunocompetent status) are represented on the left of each alluvial plot, and prediction using IPP gene set is represented on the right. Concordance is 80% on the test set.
TABLE 2.
Performance of Immune Profiling Panel Prototype for the Identification of Immunosuppressed Status on Train and Test Sets, With and Without Samples From Healthy Volunteers
| Parameter | Train Set (n = 1,221) | Test Set (n = 510) | Test Set Without Healthy Volunteers (n = 462) |
|---|---|---|---|
| Area under the receiver operating characteristic curve (95% CI) | 0.87 (0.85–0.89) | 0.86 (0.83–0.89) | 0.84 (0.81–0.88) |
| Sensitivity, % | 71 | 75 | 75 |
| Specificity, % | 83 | 83 | 80 |
| Negative predictive value, % | 85 | 87 | 84 |
| Positive predictive value, % | 69 | 68 | 69 |
The immunosuppressed status is defined by monocyte expression of human leukocyte antigen-DR < 8,000 antibodies bound per cell.
For comparison, a logistic regression model using only CD74 mRNA expression as a single surrogate of mHLA-DR (17) had lower performance (AUC 0.77 [95% CI 0.73–0.81]), illustrating the significant contribution of the IPP gene set.
We also evaluated the proportions of immunosuppressed patients in the different etiologies (i.e., sepsis, trauma, and surgery patients) as well as their evolution over time. As depicted in eFigure 2 (http://links.lww.com/CCM/H303), we observed that patients were identified as immunosuppressed in all etiologies and that the evolution was comparable between the subgroups, with a decreasing proportion of immunosuppressed patients over time.
To further assess the clinical potential of the IPP gene set, we considered all the patients still in the ICU at days 5–7 after admission, who may constitute a target for stratification before administration of immune therapy. For this subgroup of 123 patients, the concordance in immune status defined by mHLA-DR and by IPP gene set was 76%. Clinical outcomes were found to be similar between those identified as immunosuppressed by either mHLADR or IPP (Table 3). Of note, in the group of immunosuppressed patients identified by IPP, 26% developed ICU-acquired infections (IAIs), as compared to 22% in the low mHLA-DR group (no significant difference).
Table 3.
Clinical Description and Outcomes of Patients Still in the ICU at Days 5–7 After Admission
| Variables | Overall, N = 123 | Immune Status Defined by Monocyte Expression of Human Leukocyte Antigen-DR | Immune Status Defined by Immune Profiling Panel | ||||
|---|---|---|---|---|---|---|---|
| Immunocompetent, N = 52 (42%) | Immunosuppressed, N = 71 (58%) | p | Immunocompetent, N = 55 (44%) | Immunosuppressed, N = 68 (56%) | p | ||
| Mechanical ventilation D30 FD |
n = 80 26 (20–29) |
n = 26 28 (26–29) |
n = 54 25 (8–28) |
0.004 |
n = 27 28 (26–29) |
n = 53 25 (12–28) |
0.005 |
| ICU D28 FD | 18 (12–20) | 20 (17–21) | 14 (6–19) | < 0.001 | 20 (18–21) | 14 (4–19) | < 0.001 |
| Hospital D28 FD | 0 (0–9) | 7 (0–14) | 0 (0–4) | < 0.001 | 8 (0–13) | 0 (0–3) | < 0.001 |
| D28 death | 8 (6.5) | 0 (0) | 8 (11) | 0.020 | 1 (1.8) | 7 (10) | 0.074 |
| D30 ICU-acquired infection | 21 (17) | 5 (9.6) | 16 (22) | 0.088 | 3 (5.4) | 18 (26) | 0.003 |
FD = free day.
Variables are described in all patients (overall) and in immune status groups defined by monocyte expression of human leukocyte antigen-DR (mHLA-DR) or by immune profiling panel (IPP). Categorical variables are expressed as n (%) and continuous variables as median (interquartile range). Comparisons between immune status groups defined by mHLA-DR or by IPP were performed with χ2 or Fisher exact tests for qualitative variables and Mann-Whitney tests for quantitative variables, as appropriate. Values in bold indicate significance at p < 0.05. mHLA-DR and IPP measurements were censored at the occurrence of ICU-acquired infection.
DISCUSSION
This study reports that assessing the expression level of a set of mRNA markers involved in various aspects of the immune response using an integrated PCR platform is a promising tool to classify patients according to their immune status as defined by their level of mHLA-DR. These findings may facilitate the identification of ICU patients with persistent immunosuppression that may be reversed by immune stimulation.
In the last decades, many anti-inflammatory strategies in critically ill septic patients have failed to show a benefit on clinical outcomes. It has been hypothesized that this was probably not related to the evaluated drugs per se, but rather to the underlying multifactorial heterogeneity of enrolled patients, which may impact not only the patients’ immune status but also their response to treatment (1). This has prompted investigators to search for predictive enrichment strategies in order to restrict treatment to the patients who would most likely benefit. So far, the putative favorable impact of a biomarker-based patient stratification in early anti-inflammatory trials has mainly been shown through post hoc analyses (18). On the immunostimulation side, some preliminary studies using mHLA-DR as a stratification marker showed a beneficial effect on immune function and/or clinical outcomes in patients treated by interferon-γ or GM-CSF (3). However, the flow cytometry constraints have likely restricted the use of mHLA-DR in routine clinical practice so far (19), even if recent improvements in sample stabilization could facilitate measurements once validated in large clinical studies (20).
We observed in the present study that IPP identified most of the immunosuppressed patients characterized by a low mHLA-DR. Most importantly, IPP may offer several advantages. The preanalytical process is facilitated by the stabilization of whole blood RNA within the PAXgene tube, without sample preparation. With the current prototype IPP tool, results are obtained within an hour, using the fully automated and easy to use BioFire FilmArray system.
Previous studies have reported good correlation between mHLA-DR and mRNA levels of different molecules contributing to HLA complexes, for example, CD74, HLA-DRA, and CIITA (14, 17), but, to the best of our knowledge, this is the first study comparing the classification of patients obtained with a transcriptomic approach to the classification obtained with mHLA-DR assessed by flow cytometry as a reference method. Furthermore, another asset of IPP is that it relies on genes selected from previous works on sepsis-induced immunosuppression and accumulated knowledge on sepsis immunology. Thus, results directly provide information on immune dysfunctions of concern.
Despite a good concordance between mHLA-DR and IPP, a few samples were misclassified. This could be due to the intrinsic variability of both techniques. Another explanation lies in the differential regulation of the expression level of a protein (mHLA-DR) and several mRNA markers. In addition, whereas HLA-DR is measured at the cell level solely in monocytes, IPP measures mRNA in whole blood, including monocytes but also other cells expressing HLA-DR (e.g., B lymphocytes) and captures other immune alterations, not necessarily linked to mHLA-DR regulation. The latter point could be illustrated by the fact that IPP similarly identified patients who went on to develop IAI. This observation also highlights the complexity and heterogeneity of immune alterations, which may not be captured by a single marker. In line with this, as IPP could give a rapid view of immune functions (i.e., beyond antigen presentation), one may envision other applications and targeted therapies (i.e., lymphocyte alteration, inflammation) that, nevertheless, need to be further carefully investigated.
This work acknowledges several limitations. First, in this study, an immunosuppressed status was defined by a mHLA-DR expression below 8,000 AB/C. Although immunosuppression is a continuum, rather than a dichotomized state, a threshold was needed to support our analytical approach. For this purpose, we used the threshold (i.e., < 8,000 AB/C) that has been previously used in different clinical studies for treating patients with the most severe immunosuppression (6). In addition, this threshold is currently used in ongoing trials (NCT02361528 and NCT04990232). Notably, recent observational studies (21, 22) also reported on this threshold to define groups of patients at increased risk of deleterious outcomes and similarly used 8,000 AB/C as a threshold for identifying immunosuppression. That said, next prospective investigations may focus on a more continuous adjustment of the IPP algorithm according to various levels of immunosuppression. A second limitation is that the present study is monocentric and retrospective. Results remain to be confirmed in an independent dataset, encompassing data from different centers. Furthermore, the data were not fully independent, since samples were collected at multiple time-points for some individuals. However, results obtained from 123 patients sampled once at days 5–7 provided similar concordance value as that obtained from all samples. Finally, the current version of IPP is a preliminary prototype that deserves further optimization of analytical aspects, as well as optimization of the decision threshold of the algorithm.
CONCLUSIONS
This study illustrates the potential of IPP to identify ICU patients presenting with mHLA-DR less than 8,000 AB/C. These patients are believed to be at increased risk of developing deleterious events and thus could be selected to benefit from targeted immunostimulating treatment. Upon confirmation and validation, IPP could contribute to the pragmatic generalization of immunomonitoring of ICU patients and the improved efficiency of patient enrolment in clinical trials, thus paving the way for a personalized medicine approach in critical care patients. Most importantly, IPP may fill in the gap between the ambitions of omics-based medicine and the challenges of clinical practice.
ACKNOWLEDGMENTS
We thank Katia Imhoff for her support for statistical analysis, as well as Maxime Bodinier, Claire Tardiveau, and Marie-Angélique Cazalis for sharing their expertise during data analysis and interpretation. REAnimation Low Immune Status Marker (REALISM) study group members are as follows: Hospices Civils de Lyon: Sophie Arnal, Caroline Augris-Mathieu, Frederique Bayle, Liana Caruso, Charles-Eric Ber, Asma Ben-Amor, Anne-Sophie Bellocq, Farida Benatir, Anne Bertin-Maghit, Marc Bertin-Maghit, Andre Boibieux, Yves Bouffard, Jean-Christophe Cejka, Valerie Cerro, Jullien Crozon-Clauzel, Julien Davidson, Sophie Debord-Peguet, Benjamin Delwarde, Robert Deleat-Besson, Claire Delsuc, Bertrand Devigne, Laure Fayolle-Pivot, Alexandre Faure, Bernard Floccard, Julie Gatel, Charline Genin, Thibaut Girardot, Arnaud Gregoire, Baptiste Hengy, Laetitia Huriaux, Catherine Jadaud, Alain Lepape, Veronique Leray, Anne-Claire Lukaszewicz, Guillaume Marcotte, Olivier Martin, Marie Matray, Delphine Maucort-Boulch, Pascal Meuret, Celine Monard, Florent Moriceau, Guillaume Monneret, Nathalie Panel, Najia Rahali, Thomas Rimmele, Cyrille Truc, Thomas Uberti, Helene Vallin, Fabienne Venet, Sylvie Tissot, and Abbes Zadam. bioMérieux: Sophie Blein, Karen Brengel-Pesce, Elisabeth Cerrato, Valerie Cheynet, Emmanuelle Gallet-Gorius, Audrey Guichard, Camille Jourdan, Natacha Koenig, Francois Mallet, Boris Meunier, Virginie Moucadel, Marine Mommert, Guy Oriol, Alexandre Pachot, Estelle Peronnet, Claire Schrevel, Olivier Tabone, Julien Textoris, and Javier Yugueros Marcos. BIOASTER: Jeremie Becker, Frederic Bequet, Yacine Bounab, Florian Brajon, Bertrand Canard, Muriel Collus, Nathalie Garcon, Irene Gorse, Cyril Guyard, Fabien Lavocat, Philippe Leissner, Karen Louis, Maxime Mistretta, Jeanne Moriniere, Yoann Mouscaz, Laura Noailles, Magali Perret, Frederic Reynier, Cindy Riffaud, Mary-Luz Rol, Nicolas Sapay, Trang Tran, and Christophe Vedrine. SANOFI: Christophe Carre, Pierre Cortez, Aymeric de Monfort, Karine Florin, Laurent Fraisse, Isabelle Fugier, Sandrine Payrard, Annick Peleraux, and Laurence Quemeneur. École supérieure de physique et de chimie industrielles: Andrew Griffiths and Stephanie Toetsch. GlaxoSmithKline: Teri Ashton, Peter J. Gough, Scott B. Berger, David Gardiner, Iain Gillespie, Aidan Macnamara, Aparna Raychaudhuri, Rob Smylie, Lionel Tan, and Craig Tipple.
Supplementary Material
Footnotes
Drs. Brengel-Pesce and Monneret contributed equally. Drs. Peronnet, Blein, Venet, Brengel-Pesce, and Monneret contributed to conceptualization. Dr. Blein contributed to data curation. Drs. Blein and Terraz contributed to formal analysis. Drs. Peronnet, Blein, Cerrato, Fleurie, Llitjos, Kreitmann, Terraz, Conti, Gossez, Rimmelé, Textoris, Lukaszewicz, Brengel-Pesce, and Monneret contributed to investigation. Drs. Rimmelé, Textoris, and Lukaszewicz contributed to resources. Drs. Fleurie, Brengel-Pesce, and Monneret contributed to supervision. Drs. Peronnet and Terraz contributed to visualization. Drs. Peronnet, Brengel-Pesce, and Monneret contributed to writing—original draft. Drs. Blein, Venet, Cerrato, Fleurie, Llitjos, Kreitmann, Terraz, Conti, Gossez, Rimmelé, Textoris, Lukaszewicz, Brengel-Pesce, and Monneret contributed to writing—review and editing.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The REAnimation Low Immune Status Marker study received funding from the Agence Nationale de la Recherche through a grant awarded to BIOASTER (grant number ANR-10-AIRT-03) and from bioMérieux, Sanofi, and GlaxoSmithKline. The Agence Nationale de la Recherche was not involved in the study design, collection, analysis, interpretation of data, and in writing the article. All partners involved in the study agreed on the study design, collection, analysis, interpretation of data, and in writing the report.
Drs. Peronnet, Blein, Cerrato, Fleurie, Llitjos, Textoris, and Brengel-Pesce are employees of bioMérieux. Drs. Peronnet, Cerrato, Fleurie, Llitjos, Kreitmann, Terraz, Conti, Rimmelé, Lukaszewicz, Brengel-Pesce, and Monneret work in a joint research unit, cofunded by the Hospices Civils de Lyon and bioMérieux. Drs. Peronnet, Venet, and Monneret are coinventors in patent applications covering the following markers: CX3CR1 and S100A9. Drs. Peronnet, Venet, Rimmelé, Textoris, and Monneret are coinventors in patent applications covering the following markers: CX3CR1, IL1R2, C3AR1, CD177, CIITA, and TAP2. BioFire—a bioMérieux company—holds patents on the technology. This does not alter the authors’ adherence to all the policies on sharing data and materials. Drs. Peronnet’s, Blein’s, Cerrato’s, Fleurie’s, Llitjos’, Terraz’s, and Lukaszewicz’s institutions received funding from the Agence Nationale de la Recherche; they received support for article research from bioMérieux, Sanofi, and GlaxoSmithKline. Drs. Peronnet, Blein, Cerrato, Fleurie, Kreitmann, Terraz, Textoris, and Brengel-Pesce received funding from bioMérieux. Drs. Peronnet, Cerrato, and Lukaszewicz disclosed that they are coinventors on patent applications. Dr. Peronnet disclosed that her partner is employed by bioMérieux. The remaining authors have disclosed that they do not have any potential conflict of interest.
The REAnimation Low Immune Status Marker (REALISM) study group members are listed in the Acknowledgements.
Contributor Information
Collaborators: Sophie Arnal, Caroline Augris-Mathieu, Frederique Bayle, Liana Caruso, Charles-Eric Ber, Asma Ben-Amor, Anne-Sophie Bellocq, Farida Benatir, Anne Bertin-Maghit, Marc Bertin-Maghit, Andre Boibieux, Yves Bouffard, Jean-Christophe Cejka, Valerie Cerro, Jullien Crozon-Clauzel, Julien Davidson, Sophie Debord-Peguet, Benjamin Delwarde, Robert Deleat-Besson, Claire Delsuc, Bertrand Devigne, Laure Fayolle-Pivot, Alexandre Faure, Bernard Floccard, Julie Gatel, Charline Genin, Thibaut Girardot, Arnaud Gregoire, Baptiste Hengy, Laetitia Huriaux, Catherine Jadaud, Alain Lepape, Veronique Leray, Anne-Claire Lukaszewicz, Guillaume Marcotte, Olivier Martin, Marie Matray, Delphine Maucort-Boulch, Pascal Meuret, Celine Monard, Florent Moriceau, Guillaume Monneret, Nathalie Panel, Najia Rahali, Thomas Rimmele, Cyrille Truc, Thomas Uberti, Helene Vallin, Fabienne Venet, Sylvie Tissot, Abbes Zadam, Sophie Blein, Karen Brengel-Pesce, Elisabeth Cerrato, Valerie Cheynet, Emmanuelle Gallet-Gorius, Audrey Guichard, Camille Jourdan, Natacha Koenig, Francois Mallet, Boris Meunier, Virginie Moucade, Marine Mommert, Guy Oriol, Alexandre Pachot, Estelle Peronnet, Claire Schrevel, Olivier Tabone, Julien Textoris, Javier Yugueros Marcos, Jeremie Becker, Frederic Bequet, Yacine Bounab, Florian Brajon, Bertrand Canard, Muriel Collus, Nathalie Garcon, Irene Gorse, Cyril Guyard, Fabien Lavocat, Philippe Leissner, Karen Louis, Maxime Mistretta, Jeanne Moriniere, Yoann Mouscaz, Laura Noailles, Magali Perret, Frederic Reynier, Cindy Riffaud, Mary-Luz Rol, Nicolas Sapay, Trang Tran, Christophe Vedrine, Christophe Carre, Pierre Cortez, Aymeric de Monfort, Karine Florin, Laurent Fraisse, Isabelle Fugier, Sandrine Payrard, Annick Peleraux, Laurence Quemeneur, Andrew Griffiths, Stephanie Toetsch, Teri Ashton, Peter J. Gough, Scott B. Berger, David Gardiner, Iain Gillespie, Aidan Macnamara, Aparna Raychaudhuri, Rob Smylie, Lionel Tan, and Craig Tipple
REFERENCES
- 1.Marshall JC: Why have clinical trials in sepsis failed? Trends Mol Med 2014; 20:195–203 [DOI] [PubMed] [Google Scholar]
- 2.Cavaillon JM, Annane D: Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res 2006; 12:151–170 [DOI] [PubMed] [Google Scholar]
- 3.Torres LK, Pickkers P, van der Poll T: Sepsis-induced immunosuppression. Annu Rev Physiol 2022; 84:157–181 [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Monneret G, Payen D: Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13:862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venet F, Textoris J, Blein S, et al. : Immune profiling demonstrates a common immune signature of delayed acquired immunodeficiency in patients with various etiologies of severe injury. Crit Care Med 2021; 50:565–575 [DOI] [PubMed] [Google Scholar]
- 6.Meisel C, Schefold JC, Pschowski R, et al. : Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: A double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 2009; 180:640–648 [DOI] [PubMed] [Google Scholar]
- 7.Tawfik DM, Vachot L, Bocquet A, et al. : Immune profiling panel: A proof-of-concept study of a new multiplex molecular tool to assess the immune status of critically ill patients. J Infect Dis 2020; 222(Supplement_2):S84–S95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rol ML, Venet F, Rimmele T, et al. ; REALISM Study Group: The reanimation low immune status markers (REALISM) project: A protocol for broad characterisation and follow-up of injury-induced immunosuppression in intensive care unit (ICU) critically ill patients. BMJ Open 2017; 7:e015734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poritz MA, Blaschke AJ, Byington CL, et al. : FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: Development and application to respiratory tract infection. PLoS One 2011; 6:e26047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demaret J, Venet F, Plassais J, et al. : Identification of CD177 as the most dysregulated parameter in a microarray study of purified neutrophils from septic shock patients. Immunol Lett 2016; 178:122–130 [DOI] [PubMed] [Google Scholar]
- 11.Scicluna BP, van Vught LA, Zwinderman AH, et al. : Classification of patients with sepsis according to blood genomic endotype: A prospective cohort study. Lancet Respir Med 2017; 5:816–826 [DOI] [PubMed] [Google Scholar]
- 12.Fontaine M, Planel S, Peronnet E, et al. : S100A8/A9 mRNA induction in an ex vivo model of endotoxin tolerance: Roles of IL-10 and IFNγ. PLoS One 2014; 9:e100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friggeri A, Cazalis M-A, Pachot A, et al. : Decreased CX3CR1 messenger RNA expression is an independent molecular biomarker of early and late mortality in critically ill patients. Critical Care 2016; 20:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peronnet E, Venet F, Maucort-Boulch D, et al. ; MIP Rea Study Group: Association between mRNA expression of CD74 and IL10 and risk of ICU-acquired infections: A multicenter cohort study. Intensive Care Med 2017; 43:1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peronnet E, Nguyen K, Cerrato E, et al. : Evaluation of mRNA biomarkers to identify risk of hospital acquired infections in children admitted to paediatric intensive care unit. PLoS One 2016; 11:e0152388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Döcke W-D, Höflich C, Davis KA, et al. : Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR Expression: A multicenter standardized study. Clin Chem 2005; 51:2341–2347 [DOI] [PubMed] [Google Scholar]
- 17.Cazalis M-A, Friggeri A, Cavé L, et al. : Decreased HLA-DR antigen-associated invariant chain (CD74) mRNA expression predicts mortality after septic shock. Crit Care 2013; 17:R287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shakoory B, Carcillo JA, Chatham WW, et al. : Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 2016; 44:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demaret J, Walencik A, Jacob M-C: Inter-laboratory assessment of flow cytometric monocyte HLA-DR expression in clinical samples. Cytometry Part B 2013; 84B:59–62 [DOI] [PubMed] [Google Scholar]
- 20.Hamada S, Jeannet R, Gossez M, et al. : Bicentric evaluation of stabilizing sampling tubes for assessment of monocyte HLA-DR expression in clinical samples. Cytometry B Clin Cytom 2022; 102:384–389 [DOI] [PubMed] [Google Scholar]
- 21.de Roquetaillade C, Dupuis C, Faivre V, et al. : Monitoring of circulating monocyte HLA-DR expression in a large cohort of intensive care patients: Relation with secondary infections. Ann Intensive Care 2022; 12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremblay JA, Peron F, Kreitmann L, et al. ; REALISM Study Group: A stratification strategy to predict secondary infection in critical illness-induced immune dysfunction: The REALIST score. Ann Intensive Care 2022; 12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]