Abstract
Sirtuins (SIRT1-SIRT7), a family of nicotinamide adenine dinucleotide (NAD+)-dependent enzymes, are key regulators of life span and metabolism. In addition to acting as deacetylates, some sirtuins have the properties of deacylase, decrotonylase, adenosine diphosphate (ADP)-ribosyltransferase, lipoamidase, desuccinylase, demalonylase, deglutarylase, and demyristolyase. Mitochondrial dysfunction occurs early on and acts causally in the pathogenesis of neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD). Sirtuins are implicated in the regulation of mitochondrial quality control, which is highly associated with the pathogenesis of neurodegenerative diseases. There is growing evidence indicating that sirtuins are promising and well-documented molecular targets for the treatment of mitochondrial dysfunction and neurodegenerative disorders by regulating mitochondrial quality control, including mitochondrial biogenesis, mitophagy, mitochondrial fission/fusion dynamics, and mitochondrial unfolded protein responses (mtUPR). Therefore, elucidation of the molecular etiology of sirtuin-mediated mitochondrial quality control points to new prospects for the treatment of neurodegenerative diseases. However, the mechanisms underlying sirtuin-mediated mitochondrial quality control remain obscure. In this review, we update and summarize the current understanding of the structure, function, and regulation of sirtuins with an emphasis on the cumulative and putative effects of sirtuins on mitochondrial biology and neurodegenerative diseases, particularly their roles in mitochondrial quality control. In addition, we outline the potential therapeutic applications for neurodegenerative diseases of targeting sirtuin-mediated mitochondrial quality control through exercise training, calorie restriction, and sirtuin modulators in neurodegenerative diseases.
Keywords: Sirtuins, structure, regulation, mitochondrial quality control, neurodegenerative disease, sirtuin modulators
1. Introduction
Neurodegenerative diseases, a heterogeneous class of diseases, are characterized by a slow, progressive loss of selective neuronal cell populations and neuronal dysfunction [1]. Aging is a primary risk factor for neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD). As the population ages, the incidence and prevalence of neurodegenerative diseases continue to rise. Global statistics suggest that AD is increasing steadily with age; it is estimated to affect up to 5.8 million Americans and more than 50 million individuals worldwide [2]. The prevalence of neurodegenerative diseases has grown enormously and poses an increasing financial burden and challenge to society, families, and individual patients [3]. Globally, PD is the second most common neurodegenerative disease (after AD), affecting more than 6 million individuals [4]. Approximately 10.6 to 13.7 people per 100,000 in Western populations suffer from HD [5]. Extensive studies have explored the pathogenesis of and interventions for neurodegenerative disorders. There are, however, not fully effective or definitive pharmacological treatments for these diseases.
Mitochondria are major contributors to energy metabolism, apoptosis, and cellular signaling. As highly metabolically active cells, neurons are particularly sensitive to alterations in mitochondrial function due to the high amount of energy required for neuronal activity [6]. It is remarkable that in all major examples of these diseases, mitochondrial dysfunction occurs early on and acts causally in the pathogenesis of the disease [7, 8]. Mitochondria isolated from the animal and postmortem human brain tissues exhibit increased functional heterogeneity with age, accompanied by increased oxidative damage, decreased electron transport chain function, disruption of membrane potential, impaired Ca2+ homeostasis, and/or the accumulation of dysfunctional mitochondria, all of which may contribute to the pathogenesis of neurodegenerative diseases [8]. Robust mitochondrial function is more critical for neurons than other cells. Mitochondrial quality control is key to maintaining the integrity and function of mitochondria [9]. Therefore, unraveling the molecular etiology of dysregulated mitochondrial quality control may provide new prospects for the treatment and prevention of neurodegenerative diseases.
Mammalian sirtuins are a class of highly conserved nicotinamide adenine dinucleotide (NAD+)-dependent enzymes [10]. Although their primary function is deacetylation, some sirtuins also have decrotonylase, adenosine diphosphate (ADP)-ribosyltransferase, lipoamidase, desuccinylase, demalonylase, deglutarylase, and demyristolyase [11] (Fig. 1). Mass spectrometry studies hint that a variety of mitochondrial proteins implicated in metabolism and energy production are acetylated, which suggests that sirtuins potentially regulate multiple mitochondrial biological processes [12]. Sirtuins have aroused widespread interest as crucial regulators of mitochondrial quality control and neurodegeneration, such as the changes seen in AD, PD, and HD [13]. Nevertheless, the underlying role of sirtuin-mediated mitochondrial quality control in the pathogenesis of neurodegenerative disorders is still not well elucidated. Therefore, the fundamental purpose of this review is to outline the current understanding of sirtuins and shed light on how various aspects of sirtuin-mediated mitochondrial quality control, including mitochondrial biogenesis, mitophagy, mitochondrial dynamics, and mitochondrial unfolded protein response (mtUPR), regulate the pathogenesis of neurodegenerative disease. In addition, we establish the efficacy of sirtuins as molecular therapeutic targets for restoring mitochondrial quality control in neurodegenerative diseases.
Figure 1.
Multiple enzymatic functions of sirtuins. Sirtuins are a family of NAD+-dependent enzymes. SIRT1, SIRT2, SIRT4, and SIRT6 display deacylase activity. SIRT3 exhibits decrotonylase activity. SIRT4 and SIRT6 exhibit ADP-ribosyl transferase activity. SIRT5 is a cofactor in the desuccinylation and demalonylation of target proteins. SIRT7 show desuccinylase activity.
2. What is it that causes the divergent biological functions of each isoform of the sirtuin family?
Sirtuins are the mammalian homologs of yeast silent information regulator 2 (Sir2), a key modulator of longevity and cell senescence in Saccharomyces cerevisiae [14]. To date, seven members of the sirtuin family have been identified: SIRT1-SIRT7. There was an overlap in enzymatic activity among different sirtuin isoforms. Besides, some sirtuins can act on the same targets, such as p53. Thus, what accounts for the divergent biological functions of each isoform in this family?
2.1 Structure, subcellular localization, and enzymatic activity
Sirtuins share a conserved catalytic core domain that comprises a highly conserved NAD+-binding domain that is primarily folded by Rossmann and a less conserved domain containing zinc finger structures (Fig. 2). The cracks between the NAD+-binding and structural zinc-binding domains provide substrate-binding sites. Although these members are relatively conserved, they contain diverse N- and C-terminal extensions [15]. Given their different expression and subcellular localization, unique binding substrates, and distinct enzymatic activities, each member of the sirtuin family exhibits varied physiological activities.
Figure 2.
Structure and subcellular localization of human sirtuins. The positions of the amino acid are indicated on each schematic. Domains are shown in different colors.
2.1.1 SIRT1
The human SIRT1 gene is located on chromosome 10q21.3, contains eight introns and nine exons, and encodes a protein that comprises 747 amino acids. SIRT1 is predominantly localized in the nucleus and has a broad range of roles in the regulation of multiple fundamental processes associated with aging, including metabolism, inflammation, stress, apoptosis, and DNA repair [16].
2.1.2 SIRT2
The SIRT2 gene is located on chromosome 19q13.2 and consists of 21,064 bases. The total length of SIRT2 contains 389 amino acids. It comprises a central catalytic domain of 232 amino acids that is flanked by N- and C-terminal helical extensions [17]. SIRT2 exists in two forms: a protein with a molecular weight of about 43 kDa and a short form with a molecular weight of about 39.5 kDa. SIRT2 is mainly located in the cytoplasm, but it is also present in the nucleus and mitochondria [18]. It is the most abundant member of the sirtuin family in the aging central nervous system [19]. In addition to deacetylation, SIRT2 also has defatty-acylase and deacylase activities [17].
2.1.3 SIRT3
SIRT3 is encoded by the nuclear genome locus on chromosome 11p15.5 and is about 21 kb in length. It is composed of a catalytic core of approximately 275 amino acid residues flanked by N- and C-terminal extensions. Two isomers of the SIRT3 protein are encoded: a full-length protein of about 44 kDa and a short form of approximately 28 kDa [20]. Macromolecule SIRT3 is localized in the mitochondria, nucleus, and cytoplasm, whereas the short-form SIRT3 exists exclusively in mitochondria [21]. SIRT3 regulates a wide spectrum of mitochondrial functions by catalyzing the deacetylation of mitochondrial proteins.
2.1.4 SIRT4
The human SIRT4 gene is located on q24.31 of chromosome 12 and encodes a protein of about 35.2 kDa. Sequence and structural analysis of SIRT4 indicated that it contains the requisite amino acids involved in the deacylase reactions. SIRT4 is composed of a homologous sirtuin deacylase domain, a conserved catalytic histidine (H161), and a NAD+-binding region [22]. It is not only present in the mitochondria but also in the nucleus and cytoplasm [23]. SIRT4 has specific deacetylase [24], lipoamidase [25], ADP-ribosyltransferase [26], and deacylase activities [22].
2.1.5 SIRT5
SIRT5 is located on chromosome 6p23. The SIRT5 gene encodes four SIRT5 protein isomers, of which SIRT5iso1 and SIRT5iso2 are the most studied. SIRT5iso1 contains 310 amino acid residues, whereas SIRT5iso2 comprises 299 amino acids. SIRT5 consists of a large domain and a small domain. The large domain is a NAD+-binding motif folded by Rossmann and the small domain is also called the zinc-binding domain [27]. SIRT5 is a mitochondrial protein with a variety of enzymatic functions, including deacetylation [28], desuccinylation [29], demalonylation [30], and deglutarylation [31].
2.1.6 SIRT6
SIRT6 is located at p13 of chromosome 19 (19p13.3). It has a molecular weight of about 39 kDa and encodes a protein composed of 355 amino acid residues. Two isomers of SIRT6 exist, encoded by seven and eight exons, respectively. SIRT6 lacks the NAD+-binding region and only contains one divergent zinc-binding domain, which is slightly different from other sirtuins [32]. Note that SIRT6 has a stable helical structure for NAD+ binding. It is found in the nucleus and has multiple enzymatic activities, including deacetylase [33], mono-ADP-ribosyltransferase [34], and defatty-acylase [35].
2.1.7 SIRT7
The human SIRT7 gene is located on chromosome 17q25.3 and comprises 9,385 bases. The protein encoded by the SIRT7 gene contains 400 amino acids with a molecular mass of about 44.9 kDa. Like other sirtuins, SIRT7 retains the central catalytic domain, whereas the flanking N- and C-terminal extensions are unique. SIRT7 is abundant in the nucleus and is highly enriched in the nucleolar compartment [36]. There are also small amounts of SIRT7 in the cytoplasm. SIRT7 has the properties of deacetylase [37], desuccinylase [38], mono-ADP-ribosyltransferase [39], defatty-acylase [40], decrotonylase [41], and deglutarylase [42].
2.2 Transcriptional regulation of sirtuins
Transcriptional regulation in eukaryotes occurs in the proximal region of the promoter. Sirtuins play a significant role in regulating cell metabolism and cellular functions such as inflammatory responses, oxidative stress, cell senescence, and apoptosis. Nevertheless, how they are transcriptionally regulated is still not well elucidated (Fig. 3).
Figure 3.
Mechanisms of action that regulate sirtuin expression. The regulatory mechanisms of sirtuin expression include transcriptional regulation and post-transcriptional regulation.
2.2.1 SIRT1
The transcription of SIRT1 is regulated by DNA methylation and several transcription factors. Under certain pathological conditions, DNA hypermethylation is associated with decreased SIRT1 expression, which can be enhanced through treatment with DNA methylation inhibitors [43]. Moreover, SIRT1 transcription is modulated by multiple negative feedback loops. The transcription factors p53 [44] and hypermethylated in cancer 1 (HIC1) [45] suppressed SIRT1 transcription, whereas E2F transcription factor-1 (E2F1) [46], forkhead box class O3a (FOXO3a), and c-Myc [47] promoted SIRT1 transcription. In turn, SIRT1 directly deacetylated p53, HIC1, E2F1, FOXO3a, and c-Myc. Additionally, peroxisome proliferator-activated receptor α (PPARα) [48], PPARβ/δ [49], and cAMP response element-binding (CREB) [50] upregulated SIRT1 transcription, whereas carbohydrate response element-binding protein (ChREBP) [50] and PPARγ [51] decreased SIRT1 levels.
2.2.2 SIRT2
SIRT2 transcriptional regulation can occur under different physiological and pathological conditions. Inoue et al. reported that histone deacetylation of the 5′ untranslated region (UTR) of SIRT2 was involved in the downregulation of SIRT2 in glioma cells [52]. Besides, the transcription factor NK2 homeobox 2.2 (Nkx2.2) and hypoxia-inducible factor 1α (HIF-1α) negatively regulated SIRT2 transcription. Nkx2.2 inhibited SIRT2 expression by interacting with the SIRT2 promoter via histone deacetylase 1 (HDAC-1) [53], whereas HIF-1α bound to the hypoxia response element in the SIRT2 promoter [54]. Li et al. discovered that suppression of the Wnt/β-catenin pathway elevated the promoter activity and mRNA expression of SIRT2. SIRT2 expression is regulated transcriptionally by β-catenin through interacting with its promoter [55]. Moreover, the hepatitis B viral protein HBx participated in the enhancement of the mRNA and protein levels of SIRT2 by binding to the SIRT2 promoter [56].
2.2.3 SIRT3
The transcription factor nuclear factor kappa B (NF-kB) [57], thyroid hormone receptor beta (TRbeta) [58], mechanistic target of rapamycin complex 1 (mTORC1) signal [59], and nuclear respiratory factor 2 (Nrf2) [60] promoted SIRT3 transcription by enhancing activation of the SIRT3 promoter. A surprising finding by Kanwal et al. showed that mitochondrial SIRT3 and nuclear SIRT6 regulated each other’s activity [61]. SIRT6 regulates SIRT3 levels by strengthening Nrf2-dependent SIRT3 gene transcription. Mechanistically, on the one hand, SIRT6 interacted with Nrf2 and antagonized its interaction with Kelch-like ECH-associated protein 1 (Keap1), a negative regulator of Nrf2. On the other hand, SIRT6 alleviated Keap1 expression. Conversely, androgen receptor (AR) and its coregulator steroid receptor coactivator-2 (SRC-2) inhibited SIRT3 transcription by recruiting histone deacetylase 2 to the SIRT3 promoter [62]. Proliferator-activated receptor γ coactivator 1α (PGC-1α) promoted SIRT3 gene transcription through activating estrogen-related receptor-α (ERR-α) and binding to the proximal promoter region of SIRT3 [63]. Moreover, Carnevale et al. reported that SIRT1 deacetylated the ZF5 region on the SIRT3 promoter, leading to SIRT3 transcription inhibition [64]. It is intriguing that a lack of CR6-interacting factor 1 (CRIF1) triggered ubiquitination-mediated degradation of PGC-1α and Nrf2, eventually reducing SIRT3 expression [65]. Furthermore, transforming growth factor-β1 (TGF-β1) repressed SIRT3 transcription and decreased its deacetylase activity in lung fibroblasts [66].
2.2.4 SIRT4
Transcription factors, such as c-Myb [67], FOXM1 [68], homeobox A5 (HOXA5), E2F1, CCAAT/enhancer-binding protein β (CEBPβ), and PGC-1α [69], transcriptionally activated SIRT4 expression, whereas Nrf1, ubiquitin-like with plant homeodomain and ring finger domains 1 (UHRF1) [70], CREB1, paired box 4 (PAX4), interferon regulatory factor 4 (IRF4) [71], mTORC1 [72], and C-terminal-binding protein (CtBP) [73] transcriptionally suppressed SIRT4 expression. Besides, transcriptional activation of SIRT4 can be mediated by the demethylation of the SIRT4 promoter [67].
2.2.5 SIRT5
At the transcriptional level, SIRT5 is under the control of AMP-activated kinase (AMPK) and PGC-1α, which have opposite effects on its mRNA levels. In one study, AMPK overexpression downregulated SIRT5 mRNA levels in mouse primary hepatocytes by 58%, whereas elevated PGC-1α upregulated SIRT5 mRNA and protein expression in an ERRα- and PPARα-dependent manner [74]. Moreover, during bovine adipocyte differentiation, SIRT5 transcription was negatively regulated by Kruppel-like factor 6 (KLF6) and CEBPβ and positively modulated by demethylation, E2F4, KLF2, Nrf1, MYOD, and PPARα [75, 76].
2.2.6 SIRT6
The expression of SIRT6 can be regulated at the transcriptional level. Transcription factors E2F1 [77] and Runt-related transcription factor 2 (RUNX2) [78] were involved in inhibiting SIRT6 transcription. In contrast, FOXO3a [79, 80], c-Fos, and Nrf1 [81] promoted SIRT6 transcription and subsequently repressed glycolysis. In addition, the expression of SIRT6 mRNA was decreased in p53-/- mice, indicating that p53 can interact with SIRT6 and activate its transcription [82].
2.2.7 SIRT7
There are many challenges and opportunities in the field of SIRT7 transcriptional regulation. The SMAD3/4 complex and its co-factor HDAC8 negatively regulated SIRT7 transcription via local chromatin remodeling [83], whereas CEBPα inhibited SIRT7 transcription through recruiting HDAC3 [84]. Moreover, the activation of SIRT7 transcription was linked to alterations in acetylation, which can be influenced by the ketone body β-hydroxybutyrate (β-OHB)/HDAC2 signaling pathway [85].
2.3 Post-transcriptional regulation of sirtuins
A plethora of proteins is involved in regulating mRNA stability and translation [86]. Moreover, non-coding RNAs (ncRNAs), especially microRNAs (miRNAs), are involved in the post-transcriptional regulation of sirtuins. Mechanistically, miRNAs bind to the 3′ UTR of target mRNA to promote mRNA degradation or repress mRNA translation [87]. As competitive endogenous RNAs, long ncRNAs (lncRNAs) and circular RNAs (circRNAs) regulate the post-transcription of SIRT1 by sponging miRNAs [88].
2.3.1 SIRT1
RNA binding protein human antigen R (HuR) was implicated in the stabilization of SIRT1 mRNA by binding to its 3′ UTR [86]. With cell senescence, reduced HuR expression leads to decreased SIRT1 expression levels. Alterations in miRNA patterns, such as miRNA-9, miRNA-9-5p, miRNA-23a, miRNA-34a, miRNA-132, miRNA-146a, miRNA-155, and miRNA-181, miRNA-200a-3p, miRNA-212, and miRNA-384-5p, seem to be responsible for the decreased expression of SIRT1 [89-97]. In addition, Li et al. pointed out that SIRT1 antisense lncRNA promoted SIRT1 stabilization and improved SIRT1 mRNA and protein levels through competing with miRNA-34a to bind to the SIRT1 3′ UTR [98, 99]. Besides, the post-transcription of SIRT1 was also regulated by the lncRNA MIAT/miRNA-132 and circHDAC9/miRNA-138 axes [100, 101].
2.3.2 SIRT2
By directly binding to the quaking response element (QRE) located in the 3′ UTR of SIRT2 mRNA, the RNA-binding protein quaking (QKI) upregulated the expression and stability of SIRT2 mRNA [102]. Additionally, several miRNAs and lncRNAs were implicated in the post-transcriptional regulation of SIRT2. MiRNAs, such as miRNA-7, miRNA-140-5p, miRNA-146a, and miRNA-486-3p negatively regulated SIRT2 expression levels [103-106]. LncRNA NEAT1 positively modulated the mRNA stability and expression of SIRT2 by sponging miRNA-221-3p [107].
2.3.3 SIRT3
It has been reported that many ncRNAs are associated with the post-transcription of SIRT3. Multiple miRNAs, such as miRNA-28-5p, miRNA-31, miRNA-195, miRNA-214, and miRNA-494-3p negatively regulated SIRT3 expression [108-112]. LncRNA FENDRR has inhibitory effects on the progression of gastric cancer via miRNA-421/SIRT3/Notch-1 pathway [113]. Furthermore, circFAM160A2 interacted with miRNA-505-3p and SIRT3 to promote mitochondrial stabilization [114]. CircCCDC66 promoted the progression of osteoarthritis by sponging miRNA-3622b-5p and upregulating SIRT3 expression [115].
2.3.4 SIRT4
NcRNAs participate in modulating SIRT4 expression. Several miRNAs can inhibit the expression of SIRT4. Exosomal miRNA-320a derived from human amniotic mesenchymal stem cells downregulated SIRT4 to suppress oxidative stress [116]. Moreover, miRNA-130b-5p and miRNA-424 play a role in the downregulation of SIRT4 [117, 118]. LncRNA MALAT1 attenuated cardiac hypertrophy via the miRNA-93-5p/SIRT4 axis [119], whereas circOMA1 aggravated breast cancer by regulating the miRNA-1276/SIRT4 axis [120].
2.3.5 SIRT5
MiRNAs such as miRNA-19b, miRNA-145-5p, and miRNA-299-3p downregulated SIRT5 expression [121-123], whereas lncRNA SNHG14 and linc01234 upregulated SIRT5 expression through sponging miRNA-656-3p and miRNA-27b-5p, respectively [124, 125]. Moreover, lncRNA APCDD1L-AS1 inhibited autophagic degradation of epidermal growth factor receptors via the miRNA-1322/miRNA-1972/miRNA-324-3p/SIRT5 pathway in lung adenocarcinoma [126]. CircKlhl8 promoted endothelial progenitor cell-mediated diabetic wound healing by sponging miRNA-212-3p and increasing SIRT5 levels [127].
2.3.6 SIRT6
A large amount of evidence indicates that ncRNAs are involved in modulating SIRT6. MiRNAs such as miRNA-34a, miRNA-34c-5p, miRNA-186, and miRNA-654-5p target SIRT6 for silencing [128-131]. Besides, lncRNA SNHG12 alleviated hypertensive vascular endothelial injury via the miRNA-25-3p/SIRT6 pathway [132]. CircITCH, a tumor-suppressive circRNA, attenuated doxorubicin-induced cardiotoxicity by the miRNA-330-5p/SIRT6 axis [133] and improved streptozotocin-induced diabetic renal fibrosis through the miRNA-33a-5p/SIRT6 axis [134].
2.3.7 SIRT7
NcRNAs regulate the expression of SIRT7. Massive studies have identified that miRNAs such as miRNA-125a-5p, miRNA-125b, miRNA-152-3p, and miRNA-770 serve as upstream regulators of SIRT7 by targeting its 3′ UTR [135-137]. Besides, lncRNA AC007207.2 drove the upregulation of SIRT7 by sponging miRNA-1306-5p in osteosarcoma cells [138], while lincRNA p21 elevated SIRT7 levels by sponging miRNA-17-5p in vascular smooth muscle cells. SIRT7 expression was regulated by circRNA ZNF609 and circRNA PVT1 via miRNA-138-5p [139] and miRNA-125b/miRNA-200a sponging [140], respectively.
2.4 Post-translational modification of sirtuins
In addition to being regulated at the transcriptional and post-transcriptional levels, sirtuins are also affected by post-translational modifications, including phosphorylation, methylation, acetylation, ubiquitination, SUMOylation, O-GlcNAcylation, S-nitrosylation, and S-glutathionylation (Fig.4).
Figure 4.
Post-translational modifications of sirtuins. Sirtuins can be affected by post-translational modifications, including phosphorylation, ubiquitination, SUMOylation, methylation, acetylation, O-GlcNAcylation, S-nitrosylation, and S-glutathionylation.
2.4.1 SIRT1
Post-translational modifications of SIRT1 include ubiquitination [141], SUMOylation [142], phosphorylation [143], methylation [144], O-GlcNAcylation [145], S-nitrosylation [146], and S-glutathionylation [147], which play important roles in regulating SIRT1 function. E3 ubiquitin ligase SMURF2 inhibited the proliferation of colorectal cancer cells and tumor growth through interacting with SIRT1 and mediating its ubiquitination and degradation [141]. SUMOylation of SIRT1 at lysine 734 enhanced its deacetylation ability. Conversely, SUMO-specific peptidase 1 (SENP1)-mediated SIRT1 de-SUMOylation downregulated its enzymatic activity [142]. SIRT1 phosphorylation is mediated by a variety of kinases and is crucial to SIRT1 enzymatic activity. Casein kinase 2 (CK2)-mediated SIRT1 phosphorylation on serine residues 154, 649, 651, and 683 [148] and c-Jun N-terminal kinase 1 (JNK1)-mediated phosphorylation on serine 27, serine 47, and threonine 530 [149] markedly promoted SIRT1 activity and function. In addition, cyclin-dependent kinase 1 (CDK1) phosphorylated SIRT1 on threonine 530 and serine 540 [143]. Wang et al. revealed that Janus kinase 1 (JAK1) catalyzed the phosphorylation of SIRT1 at tyrosine residues 280 and 301 without altering its deacetylase activity [150]. SIRT1 can be O-GlcNAcylated at serine 549 and has cytoprotective effects by increasing its deacetylase activity during stress [145]. Methyltransferase Set7/9 indirectly regulated p53 function by methylating SIRT1 at multiple lysine residues within its N-terminus [144]. Inducible nitric oxide synthase (iNOS) and inflammatory stimuli triggered the S-nitrosylation of SIRT1 and inhibited its enzymatic activity [146, 151]. Glutaredoxin 2 participated in vascular development by removing the S-glutathionylation of SIRT1 [152].
2.4.2 SIRT2
The function of SIRT2 is influenced by post-translational modifications. Cyclin-dependent kinases regulate SIRT2 phosphorylation. CDK-1 can phosphorylate SIRT2 at serine 368 [153], whereas CDK-5 induced SIRT2 phosphorylation at serine residues 331, 335, 368, and 372 [154, 155]. Mass spectrometry confirmed that in the cellular model of PD, SIRT2 was phosphorylated by GSK3β at serine residues 327, 331, and 335 [156]. In addition, AMPK phosphorylated SIRT2 at threonine 101 [157]. SRC proto-oncogene, nonreceptor tyrosine kinase (c-Src) reduced the SIRT2 protein levels via phosphorylation of SIRT2 at tyrosine 104 [158]. Moreover, p300 drove the acetylation of SIRT2 and then reduced the deacetylase activity of SIRT2 [159]. SIRT2-SUMOylation at lysine 183 and 340 plays a crucial role in cellular signal transduction and tumor suppression [160]. Casitas B-lineage lymphoma (Cbl) proteins Cbl-b and c-Cbl promoted the stability and protein expression of SIRT2 via ubiquitination [161]. E3 ubiquitin ligase (HRD1)-mediated SIRT2 ubiquitination and degradation in lung tumorigenesis [162].
2.4.3 SIRT3
At the post-translational level, SIRT3 is affected by phosphorylation, SUMOylation, acetylation, and ubiquitination. CDK1-induced SIRT3 phosphorylation at threonine 150 and serine 159 increased SIRT3 enzymatic activity [57]. SUMOylation negatively regulated SIRT3 enzymatic activity. SENP1-mediated SIRT3 de-SUMOylation at lysine 288 upregulated its deacetylase activity and participated in mitochondrial metabolism [163]. SIRT3 can also be modified by acetylation. In one study, Kwon et al. found that SIRT3 was acetylated at lysine 57 in aged and obese mice [164]. Moreover, berberine [165] and lncRNA DACH1 [166] promoted ubiquitination-mediated SIRT3 degradation by activating the AMPK pathway and increasing intracellular reactive oxygen species (ROS) levels.
2.4.4 SIRT4
Compared to other sirtuins, only a few publications focused on post-translational modifications of SIRT4. For instance, Li et al. found that P21-activated kinase 6 (PAK6) was implicated in mitochondrial apoptosis by promoting ubiquitination-mediated SIRT4 degradation [167]. More extensive studies are required to uncover the modifications underlying SIRT4.
2.4.5 SIRT5
Several researchers have proposed that SIRT5 is regulated by ubiquitination. Yao et al. indicated that tripartite motif-containing protein 21 (TRIM21) interacted with SIRT5 and catalyzed the ubiquitination and degradation of SIRT5. Besides, herpes virus-associated ubiquitin-specific protease (HAUSP) mediated SIRT5 de-ubiquitination and stabilization. In a feedback loop, ubiquitination-induced SIRT5 degradation sustained TRIM21 acetylation at lysine 351 [168]. Moreover, cyclin F-Skp1, CUL1, and the F-box protein family of E3 ubiquitin ligase complex regulated the ubiquitination, abundance, and stability of SIRT5 [169].
2.4.6 SIRT6
The enzymatic activity and protein stability of SIRT6 are modulated by post-translational modifications. As the SIRT6 N-terminus is responsible for its interaction with SIRT1, Meng et al. suggested that SIRT1 mediated the deacetylation of SIRT6 at lysine 33 [170]. Under stress conditions, SIRT6 was phosphorylated by JNK at serine 10. SIRT6 phosphorylation drove its mono-ADP-ribosyltransferase activity on poly(ADP-ribose) polymerase 1 (PARP1) and stimulated the repair of double-strand DNA breaks [171]. Ubiquitin ligase CHIP induced noncanonical ubiquitination of SIRT6 at lysine 170 [172]. Mammalian ubiquitin-specific peptidase 10 (USP10) [173] and USP48 [174] were SIRT6-specific de-ubiquitinases. USP48 was positively regulated by methyltransferase-like 14 (Mettl14)-induced m6A modification and promoted SIRT6 de-ubiquitination at lysine 33 and lysine 128. Moreover, SIRT6 ubiquitylation at lysine 160 was mediated by UBE3A [175]. NAD(P)H: quinone oxidoreductase 1 (NQO1) repressed proteasome-mediated degradation of SIRT6 [176]. In addition, SIRT6 can be modified by SUMOylation and decreased its deacetylase activity of histone H3 at lysine 56 [177].
2.4.7 SIRT7
The activity of SIRT7 is affected by methylation and ubiquitination. In vivo and in vitro studies have revealed that protein arginine methyltransferase 6 (PRMT6) directly interacted with and methylated SIRT7 at arginine 388, leading to the suppression of SIRT7 deacetylase activity [178]. Besides, USP7- [179] and USP17L2-mediated [180] SIRT7 de-ubiquitination alleviated its enzymatic activity, whereas Cullin 4 RING E3 ubiquitin ligase (CRL4)-DCAF1 E3 ligase triggered SIRT7 ubiquitination and degradation [181].
2.5 The NAD/NADH ratio
Because sirtuins are a family of NAD+-dependent enzymes, the intracellular level of NAD is key to their enzymatic activities. In addition to their roles in gene expression, NAD+ and NADH play significant roles in energy metabolism and mitochondrial function [182]. Using magnetic resonance-based NAD detection in vivo, Zhu et al. revealed age-dependent reductions in NAD+ levels, NAD+/NADH redox potential, total NAD content, and age-dependent increases in intracellular NADH in the brains of healthy subjects [183]. The level of NAD+ in the brain decreases with age and negatively affects the activity of sirtuins [184]. Many studies have confirmed that the NAD+/NADH ratio is highly related to the activity of sirtuins in diverse pathophysiological processes. Crucially, exogenously introduced NAD+ increased sirtuin activity [185]. Nicotinamide mononucleotide adenylyltransferase (Nmnat) is a crucial enzyme in NAD biosynthesis. Cai et al. revealed that Nmnat2 ameliorated angiotensin II-induced cardiac hypertrophy by maintaining NAD levels and activating SIRT6 [186].
A broad range of evidence has described the function and regulatory mechanisms of sirtuins under different physiological conditions. Indeed, understanding of the functions of sirtuins is improving.
3. Mitochondrial quality control in the pathogenesis of neurodegenerative diseases
Aging neurons frequently show evidence of mitochondrial dysfunction, which is associated with the pathogenesis of neurodegeneration [6]. Recently, the role of mitochondrial quality control in neurodegenerative diseases has received increased attention. Advances in mitochondrial quality control, including mitochondrial biogenesis, mitophagy, mitochondrial fusion and fission, and mtUPR, provide attractive approaches to the prevention and treatment of neurodegenerative diseases (Fig. 5).
Figure 5.
Mitochondrial quality control. (A) Mitochondrial biogenesis. Once activated by either deacetylation or phosphorylation, proliferator-activated receptor γ coactivator 1α (PGC-1α) triggers the activation of the nuclear respiratory factors (NRFs)/mitochondrial transcription factor A (TFAM) pathway, promoting mitochondrial biogenesis. (B) Mitophagy. The phosphatase and tensin homolog-induced putative protein kinase 1 (PINK1)/Parkin-mediated mitophagy pathway is involved in the formation of autophagosomes. (C) Mitochondrial dynamics. Mitochondrial fission is regulated by dynamin-related protein 1 (DRP1) and fission protein 1 (FIS1), whereas mitochondrial fusion is modulated by mitofusin (MFN) 1/2 and optic atrophy 1 (OPA1). (D) Mitochondrial unfolded protein response. Molecular chaperones and proteases mediate the degradation or refolding of misfolded/unfolded mitochondrial proteins.
3.1 Mitochondrial biogenesis in neurodegenerative diseases
Mitochondrial biogenesis, the process by which new mitochondria are generated from existing mitochondria, is mediated by PGC-1α [187]. PGC-1α, once activated by either deacetylation or phosphorylation, triggers the activation of the NRFs/mitochondrial transcription factor A (TFAM) signaling pathway, subsequently favoring mitochondrial biogenesis by the production of mitochondrial DNA (mtDNA) and proteins [188]. Mitochondrial biosynthesis is required to sustain mitochondrial respiration, maintain mitochondrial redox balance, and prevent mitochondrial apoptosis. Mitochondrial biogenesis is crucial to maintaining mitochondrial homeostasis.
Impaired mitochondrial biogenesis is associated with the development of AD. In one study, pyramidal neurons in the AD hippocampus showed a significant reduction in mtDNA copy numbers and disruption of mitochondrial biogenesis transcriptome signaling compared to the control hippocampus [189]. Besides, PGC-1α mRNA expression was remarkably decreased with the deterioration of dementia in the AD brain and was negatively correlated with amyloid beta (Aβ) and Tau pathology [190]. Notably, Sheng et al. demonstrated that the expression of PGC-1α, NRF1, NRF2, and TFAM in the AD hippocampus was decreased. In vitro studies have shown that restoration of PGC-1α expression has a salvage effect on mitochondrial and neuronal function in AD cellular models [191]. Moreover, PGC-1α modulates β-site amyloid precursor protein cleaving enzyme 1 (BACE1) and synergies with SIRT1-mediated PPARγ deacetylation constitute a key mechanism regulating Aβ production in AD [192].
Regulation of mitochondrial biogenesis is a promising therapeutic target for PD [193]. PGC-1α single nucleotide polymorphisms rs6821591 and rs2970848 were relative to the risk of onset of PD [194]. Besides, inhibition of PGC-1α activity is a primary cause of PINK1 and Parkin mutations-induced family PD [195]. PGC-1α was reduced in the leukocytes, substantia nigra, and brain. Another study revealed that mRNA levels of mitochondrial biogenesis activator genes such as PGC-1α and NRF1 and repressor genes such as ZFP746 and Mybbp1a were evidently decreased at all stages in MPTP-induced PD mouse models [196]. Ye et al. demonstrated that PGC-1α overexpression suppressed MPP+-induced mitochondrial dysfunction in SHSY5Y cells and showed a neuroprotective role in HD via the ERRα, PPARγ, and NRF1 pathways [197].
Defective mitochondrial biogenesis and impaired PGC-1α signaling pathway are associated with striatal vulnerability in HD [198]. The mRNA levels of PGC-1α in the striatum were decreased in early HD patients [199]. Mutant huntingtin (mTTT) inhibited PGC-1α gene transcription by interfering with the cAMP response element-binding (CREB)/TAF4-dependent transcriptional pathway. Overexpression of PGC-1α in the striatum partially reversed mTTT-induced neurotoxicity in the striatal neurons [199]. Additionally, the PGC-1α downstream transcription factors NRF1 and TFAM have been proposed as genetic modifiers of HD [200].
3.2 Mitophagy in neurodegenerative diseases
Mitophagy is a process of selective elimination of mitochondrial dysfunction through the autophagy-lysosomal pathway [201]. One important mitophagy pathway in mammals relies on phosphatase and tensin homolog-induced putative protein kinase 1 (PINK1) and the E3 ubiquitin ligase Parkin [202]. Impaired mitophagy leads to the accumulation of damaged mitochondria, which ultimately causes the progression of neurodegenerative diseases.
Affected neurons in AD suffer from mitochondrial dysfunction and early-onset bioenergetic defects, which contribute to Aβ and Tau pathology. Compelling evidence suggested that mitophagy was impaired in AD, resulting in the accumulation of dysfunctional mitochondria [203]. In cellular and animal AD models, energy deficiency and oxidative stress drove Aβ and Tau accumulation, leading to synaptic and cognitive dysfunction, which in turn impinged on mitophagy. On the one hand, overexpression of phosphorylated Tau alleviated mitophagy through increasing mitochondrial membrane potential and decreasing PINK1 and Parkin levels [204]. On the other hand, elevated mitophagy eliminated Tau hyperphosphorylation and Aβ pathology in neuronal cells and reversed memory impairment in animal models of AD [205, 206]. Therefore, stimulating or promoting mitophagy may block the pathological process of AD and be a potential strategy for AD treatment.
The critical role of mitophagy in the pathogenesis of PD highlights the potential therapeutic application of mitophagy modulation. Many PD-associated genes are related to mitophagy defects. Loss of PINK1 or Parkin function inhibited mitophagy, leading to mitochondrial dysfunction and loss of dopaminergic neurons [207]. In addition, LRRK2 mutant is the most common monogenic cause of PD. RAB10 is a substrate of LRRK2 kinase that accumulates on depolarized mitochondria in a PINK1- and Parkin-like manner. LRRK2 mutation disrupted depolarization-induced mitophagy through repressing mitochondrial accumulation of RAB10 [208].
HD is a common neurodegenerative disease associated with abnormal polyglutamine (PolyQ) expansion-induced misfolding proteins. Mitochondrial glyceraldehyde-3-phosphate dehydrogenase (GAPDH) interacted with expanded PolyQ tracts and disturbed GAPDH-mediated mitophagy, resulting in the accumulation of damaged mitochondria and cell death [209]. Of note, mHTT impairs mitophagy in HD [210]. Huntingtin protein acted as a scaffold protein of autophagy protein 11 (ATG11) and was implicated in selective autophagy [211]. Further studies showed that mitophagy was altered in the presence of mHTT through a reduction in the ability of mitochondria to target autophagosomes. Khalil et al. found that overexpression of PINK1 in HD striatal cells could partially restore mitophagy [212].
3.3 Mitochondrial dynamics in neurodegenerative diseases
Mitochondrial fusion and fission are crucial in mammals, and their impairment is strongly associated with a broad range of diseases, especially neurodegenerative diseases [213]. Mitochondrial fusion is a multistep process that includes outer and inner mitochondrial membrane fusion. Mechanistically, outer mitochondrial membrane fusion is mediated by mitochondria-related proteins mitofusin1 (MFN1) and MFN2, whereas inner mitochondrial membrane fusion is regulated by optic atrophy 1 (OPA1) [214]. Mitochondrial fission proteins include dynamin-related protein 1 (DRP1) and mitochondrial fission protein 1 (FIS1) [215]. In addition, mitochondrial fission is also mediated by other proteins, including mitochondrial fission factor (MFF), mitochondrial dynamic protein 49 (MiD49), and MiD51 [213].
Imbalance of mitochondrial fission and fusion is a key driver of mitochondrial dysfunction and neuronal injury in the AD brain [216]. Immunoblot analysis showed that the levels of DRP1, OPA1, MFN1, and MFN2 were dramatically decreased in AD, whereas the levels of FIS1 were vastly increased [216]. DRP1 interacted with Aβ and phosphorylated Tau, resulting in a series of neurotoxic events, including excessive mitochondrial fragmentation and ROS production, and mitochondrial bioenergetics and synaptic defects, ultimately causing neuronal and cognitive impairment [217]. Baek et al. revealed that DRP1 inhibition attenuated mitochondrial fragmentation, alleviated mitochondrial membrane potential loss, and ameliorated ROS generation in Aβ-treated neurons. Moreover, suppression of DRP1 rescued learning and memory deficits in AD mice and prevented mitochondrial fragmentation, BACE1 expression, and Aβ deposition in the AD brain [218]. Indeed, Aβ-induced nitric oxide generation was involved in mitochondrial fission, synaptic loss, and neuronal dysfunction partly through DRP1 S-nitrosylation, whereas DRP1 nitrosylation prevention eliminated these deleterious effects [219]. Furthermore, Aβ-induced DRP1 phosphorylation triggered excessive mitochondrial fission, ultimately leading to neuronal apoptosis [220]. Therefore, targeting mitochondrial fission may be a promising therapeutic method for AD.
DRP1-mediated aberrant mitochondrial fission contributes to PD [221]. Elevated DRP1 promoted mitochondrial fission and dopaminergic neuronal apoptosis, whereas suppression of DRP1 reversed aberrant mitochondrial fission and attenuated cell apoptosis [222]. Additionally, LRRK2 participated in regulating mitochondrial dynamics by enhancing and interacting with DRP1 [223]. PD-related genes such as PINK1 and Parkin were also found to exhibit vital roles in the modulation of mitochondrial dynamics [224]. Overexpression of α-synuclein contributes to mitochondrial fragmentation [225].
In HD patients, the mRNA and protein levels of DRP1 and FIS1 were increased, whereas the expression of MFN1 and MFN2 was decreased, which was consistent with the elevated mitochondrial fragmentation in cortical neurons [226]. Shirendeb et al. reported that mHTT interacted with DRP1 to enhance the enzymatic activity of GTPase DRP1 in postmortem HD brains and primary neurons from transgenic BACHD mice, worsening abnormal mitochondrial dynamics and ultimately resulting in defective axonal transport and synaptic degeneration in HD [227]. Moreover, mHTT promoted post-translational modifications and enhanced DRP1 activity and mitochondrial fission [228]. Inhibition of DRP1 overactivation downregulated neuropathological and behavioral deficits in zQ175 knock-in HD mouse models [229].
3.4 mtUPR in neurodegenerative diseases
mtUPR is a stress-protective response that ameliorates mitochondrial damage and maintains mitochondrial proteostasis by repairing or eliminating misfolded proteins [230]. Mitochondrial chaperones, such as heat shock protein 9 (HSP9), HSP10, HSP60, and HSP70, are implicated in helping restore misfolded proteins to normal conformations and improving the correct folding of newly synthesized proteins. Proteases, including CLP protease proteolytic (CLPP), YME1-like 1 ATPase (YME1L1), and Lon protease, are involved in degrading unwanted proteins [231].
AD is characterized by the accumulation of two insoluble protein aggregates, Aβ plaques and tau neurofibrillary tangles. AD is marked by an excess of faulty or unfolded proteins, which activates UPR [232]. In vivo and in vitro studies have shown that mtUPR was activated in the AD mice brain and Aβ25-35-treated SHSY5Y cells. Inhibition of mtUPR intensified Aβ25-35-induced cytotoxic effects on SHSY5Y cells, suggesting that mtUPR plays a protective role in the progression of AD [233]. Sorrentino et al. reported that mtUPR was essential for maintaining mitochondrial proteostasis. Moreover, increased mitochondrial proteostasis inhibited Aβ aggregation in cells, worms, and mouse models of AD [234]. The expression of mtUPR-related genes, including HSP60, CLPP, and YME1L1, was significantly increased by 40% to 60% in individuals with sporadic AD and by approximately 70% to 90% in familial AD subjects compared to cognitively intact controls [235]. Promoting the activation of mtUPR has emerged as an effective therapeutic modality for AD.
mtUPR is also implicated in PD. In C. elegans, mitochondrial damage causes the nuclear translocation of the transcription factor ATFS-1 to activate mtUPR [236]. PINK1 mutant activated ATFS-1-dependent mtUPR and promoted dopamine neuron survival in PD [237]. Furthermore, mtUPR overactivation increased α-synuclein-induced dopaminergic neurotoxicity, whereas ATFS-1 deficiency attenuated α-synuclein-related neurotoxicity [236]. In a nutshell, mtUPR activation suppressed PD by enhancing the survival of dopaminergic neurons, whereas excessive mtUPR activation was detrimental to PD.
Berendzen et al. found that polyQ-40 bound to mitochondria and elicited mtUPR in C. elegans neurons, hinting at an association between mtUPR and HD [238]. The mitochondrial transporter ABCB10 implicated in the mtUPR pathway was decreased in fibroblasts from HD patients and in striatal cells from HD mice. mHTT inhibited mtUPR by impairing the stability of ABCB10 mRNA [239]. Additionally, the levels of HSP60 and CLPP were significantly reduced in the ABCB10-deficient HD model. ABCB10 was involved in modulating CHOP, a transcription factor of HSP60 and CLPP. These findings suggest that moderate activation of mtUPR may have a therapeutic effect on HD.
4. Roles of sirtuins in mitochondrial quality control
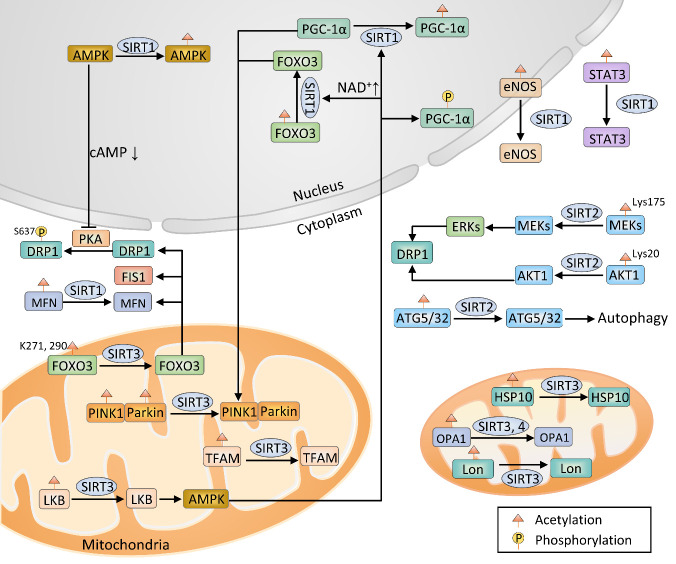
Sirtuins are involved in almost all aspects of mitochondrial metabolism and homeostasis, protecting mitochondria from various types of damage. With a deeper understanding of the molecular mechanisms of the acetylation of multiple mitochondrial proteins involved in stress and metabolism, sirtuins are implicated in regulating mitochondrial quality control, including mitochondrial biogenesis, mitophagy, mitochondrial fusion and fission, and mtUPR (Fig. 6).
Figure 6.
Sirtuin-mediated mitochondrial quality control in neurodegeneration. SIRT1 catalyzes the deacetylation of proliferator-activated receptor γ coactivator 1α (PGC-1α), AMP-activated protein kinase (AMPK), forkhead box class O3 (FOXO3), mitofusin (MFN), signal transducer and activator of transcription 3 (STAT3), and endothelial nitric oxide synthase (eNOS) and participates in mitochondrial quality control. PINK1/Parkin pathway-mediated mitophagy is activated by either the SIRT1/FOXO3 axis or SIRT3. Additionally, SIRT2 is involved in mitochondrial fission by regulating DRP1 via the SIRT2/mitogen-activated protein kinase kinase-1 (MEK1)/extracellular signal-regulated kinase (ERK)/dynamin-related protein 1 (DRP1) and SIRT2/serine/threonine-protein kinase AKT1/DRP1 pathways. Autophagy protein (ATG) 5/32-mediated mitophagy is regulated by SIRT2. SIRT3 deacetylates HSP10 and Lon proteases and regulates mitochondrial unfolded protein response (mtUPR). Liver kinase B (LKB), mitochondrial transcription factor A (TFAM), FOXO3, and optic atrophy type 1 (OPA1) have been identified as SIRT3 substrates. Additionally, SIRT4 modulates mitochondrial dynamics by regulating OPA1.
4.1 Sirtuins in mitochondrial biogenesis
There is converging evidence that sirtuins play a significant role in regulating mitochondrial biogenesis. SIRT1 is heavily involved in mitochondrial biogenesis and longevity. The SIRT1/PGC-1α signaling pathway showed an endogenous neuroprotective role in confronting epileptic seizure-induced neuronal cell damage [240]. In addition, SIRT1 indirectly regulated mitochondrial biogenesis by upregulating endothelial nitric oxide synthase or catalyzing signal transducer and activator of transcription 3 (STAT3) deacetylation [241]. SIRT2 plays various roles in cellular metabolism, especially during aging. Mitochondrial dysfunction and neurite outgrowth suppression were mediated by high d-glucose concentration-induced increases in polyol pathway activity and further depletion of SIRT2 [242]. Furthermore, SIRT2-knockout mice showed mitochondrial failure, redox imbalance, and energy depletion [243]. SIRT3 overexpression elicited activation of the AMPK signaling pathway via the deacetylation of liver kinase B1 (LKB1), promoting mitochondrial biogenesis and mitochondrial turnover [244]. Activated AMPK promoted SIRT1 activity by enhancing cellular NAD+ levels, leading to the deacetylation of SIRT1 downstream targets [245]. The PGC-1α/SIRT3/UCP2 axis was participated in improving mitochondrial biogenesis, dynamics, and function [246]. SIRT3 triggered the deacetylation and activation of FOXO3, subsequently upregulating the expression of PGC-1α and TFAM [247]. Moreover, SIRT3 promoted mitochondrial biogenesis through enhancing the content of mtDNA and the deacetylation of TFAM [248]. SIRT4 has been linked to mitochondria-derived signaling cascades by the regulation of AMPK and PGC-1α [249]. Moreover, PGC-1α overexpression and food withdrawal increased the levels of SIRT5 mRNA. Activated AMPK decreased the expression of SIRT5 mRNA. Overexpression of SIRT5 enhanced oxygen consumption and ATP generation but did not affect mitochondrial biogenesis [74]. Similarly, SIRT6 regulated mitochondrial biogenesis by increasing the activities of AMPK and PGC-1α or reducing the activity of Sox6 [250, 251]. Furthermore, arginine 388 methylation of SIRT7 modulated mitochondrial respiration and mitochondrial biogenesis [178]. Nevertheless, the molecular mechanisms of SIRT4, SIRT5, SIRT6, and SIRT7 underlying mitochondrial biogenesis in neurodegenerative diseases remain elusive.
4.2 Sirtuins in mitophagy
Sirtuins play a critical role in the regulation of mitophagy via acetylation. SIRT1 signaling cascade mediated mitophagy and PINK1/Parkin mitochondrial translocation in human neuroblastoma SHSY5Y cells through activation of the PGC-1α pathways [252]. In vitro studies have confirmed that the levels of autophagy markers light chain 3B (LC3B) and Beclin1 were significantly reduced and the expression of p62 was pronouncedly increased after pretreatment with SIRT1 inhibitor EX527, indicating that the inactivation of SIRT1 impeded mitophagy. SIRT1-mediated PINK1/Parkin-dependent mitophagy has neuroprotective effects [253]. Besides, activated SIRT1 promoted mitophagy and ameliorated mitochondrial dysfunction by activating the FOXO1/3/PINK1/parkin signaling pathway [254]. Chang et al. found the possible function of miRNA-302 in improving mitophagy and preserving mitochondrial function through activation of the SIRT1/AMPK/PGC-1α pathway [255]. SIRT2 appears to have unique mitochondrial targets and directs the relevant components of mitophagy. SIRT2 overactivation drove the dysregulation of autophagy and mitophagy. Elevated SIRT2 levels and decreased tubulin lysine 40 acetylation were found in the AD brain [256]. Intriguingly, microtubule assembly was not altered in MPP+-treated SIRT2-knockout mice neurons, which maintained normal autophagic flux. The inhibition of SIRT2 enhanced the acetylation of α-tubulin and accelerated the trafficking and clearance of misfolded protein [257]. During aging, increased mitophagy activity regulated by the SIRT2/ATG32 axis was a significant phenomenon associated with α-synuclein-induced toxicity [258]. Besides, mitophagy was impaired in SIRT2-deficient neurons due to the acetylation of ATG5, an E3 ubiquitin ligase [259]. SIRT3 facilitated mitophagy by directly deacetylating PINK1 and Parkin and indirectly regulating PINK1 via the SIRT3/FOXO3 pathway [260]. SIRT3 suppression reversed mitophagy induction through reducing the levels of LC3-II/LC3-I, p62, FOXO3α, and BINP3 [261]. One study confirmed that SIRT3-mediated deacetylation and activation of FOXO3 promoted the expression of LC3, Nix, and BNIP3. The expression of LC3, Nix, and BNIP3 was decreased in SIRT3-knockdown cells [247]. Furthermore, activated SIRT4 and SIRT5 alleviated mitophagy, whereas activated SIRT6 promoted mitophagy. Lang et al. reported that SIRT4 emerged as a stress-triggered factor and exhibited a role in reducing mitophagy, leading to mitochondrial dysfunction and mitochondrial quality control impairment [262]. Autophagy and mitophagy were enhanced in SIRT5-deficient cells and decreased in SIRT5-overexpressed cells [263]. During starvation, SIRT5 deletion enhanced mitophagy by attenuating mitochondrial elongation [264]. Conversely, SIRT1-mediated activation of the SIRT6/AMPK signaling pathway increased mitophagy and sustained mitochondrial function through intensifying PINK1/Parkin and the LC3II/LC3I ratio [265]. No relevant studies on the relationship between SIRT7 and mitophagy have been reported.
4.3 Sirtuins in mitochondrial dynamics
Increasing evidence has revealed the primary role of sirtuins in mitochondrial fusion and fission dynamics. SIRT1 was associated with mitochondrial fusion and fission. It promoted mitochondrial elongation through MFN1 deacetylation and accumulation [266]. Activated SIRT1 induced mitochondrial fission by the accumulation of FIS1 and activation of DRP1 via the SIRT1/AMPK/protein kinase A (PKA)/DRP1 pathway. Nicotinamide (NAM)-induced SIRT1 activation leads to mitochondrial fission, at least partly by inhibiting DRP1 phosphorylation to activate DRP1. Mechanistically, activated SIRT1 triggered AMPK activation, which subsequently reduced cellular cAMP levels and repressed the activity of PKA [267]. In addition, after inhibiting Ca2+ transfer to mitochondria, SIRT1 works collaboratively with AMPK to promote mitochondrial fragmentation through the deacetylation of cortactin and actin cytoskeleton [268]. SIRT2 inhibition enhanced mitochondrial fission through activating DRP1. SIRT2 modulated mitochondrial dynamics via two axes, SIRT2/mitogen-activated protein kinase kinase-1 (MEK1)/extracellular signal-regulated kinase (ERK)/DRP1 axis and SIRT2/serine/threonine-protein kinase AKT1/DRP1 axis [269]. SIRT3 affected mitochondrial dynamics by interacting with OPA1 and FOXO3. It induced the deacetylation of OPA1 and upregulated its GTPase activity, promoting the preservation of mitochondrial networking [270]. Likewise, SIRT3-mediated FOXO3 deacetylation at K271 and K290 enhanced the expression of MFN2, DRP1, and FIS1 [271]. The SIRT4-OPA1 signaling cascade was associated with mitochondrial quality control. Elevated SIRT4 interacted with and stabilized OPA1, promoting mitochondrial fusion in an enzyme-dependent manner and counteracting mitochondrial fission and mitophagy [262]. Moreover, SIRT5 regulated mitochondrial dynamics. Expression of MiD51 and FIS1 was increased in SIRT5-deletion mouse embryonic fibroblasts, resulting in mitochondrial fission [264]. However, the roles of SIRT6 and SIRT7 in mitochondrial fusion and fission remain obscure.
4.4 Sirtuins in mtUPR
The NAD+/sirtuin pathway regulates lifespan through activation of mtUPR and FOXO signaling [272]. In one study, pretreatment with the SIRT1 activator resveratrol improved postoperative learning and memory impairment in aged mice and significantly reduced the expression of UPR-related proteins and inflammatory molecules in the hippocampus [273]. Another study found that the expression and activity of SIRT3 in the hippocampus were markedly increased after treatment with honokiol, accompanied by activation of mtUPR [274]. Manganese superoxide dismutase (MnSOD), HSP10, and Lon protease have been identified as STRT3 substrates [275-277]. The relationship between sirtuins and mtUPR is still obscure.
These emerging data support the potential applications of sirtuins in regulating mitochondrial quality control and mitochondrial biology, hinting at the involvement of sirtuins in neurodegenerative diseases and providing promising therapeutic targets for neurodegenerative diseases. More extensive studies, however, are required to uncover the underlying mechanisms and roles of diverse sirtuins in mitochondrial dysfunction.
5. Roles of sirtuin-mediated mitochondrial quality control in neurodegenerative diseases
Mitochondrial dysfunction is a key and early contributor to neurodegenerative diseases [278]. Sirtuins have a highly regulatory effect on mitochondrial function, making them exciting targets for the treatment of neurodegenerative diseases. Emerging studies have shown the role of sirtuins in neurodegeneration.
5.1 AD
AD is the most common neurodegenerative disorder. The pathology of AD is characterized by synaptic loss and cognitive decline due to the accumulation of Aβ and p-Tau neurofibrillary tangles in the brain [279]. There is particularly strong data showing that Aβ was involved in regulating mitochondrial function and neuronal damage through sirtuins. Aβ reduced SIRT1 and its downstream signaling, leading to increased intracellular ROS accumulation and mitochondrial dysfunction [280].
Rashmita et al. found that serum levels of SIRT1, SIRT3, and SIRT6 were remarkably reduced in AD subjects compared with mild cognitive impairment subjects and geriatric controls. In contrast, there were no significant differences in the expression of SIRT2, SIRT4, SIRT5, and SIRT7 among the three groups [281]. Reciprocally, another study found that the expression of SIRT5 increased as AD progressed [282]. SIRT1 suppressed Aβ generation via biased amyloid precursor protein processing toward the non-amyloidogenic pathway. Decreased SIRT3 levels were highly correlated with cerebral cortical Aβ pathology in AD patients. By preserving mitochondrial function, SIRT3 enhanced the functionality of the GABAergic interneuron and ameliorated neuronal network hyperactivity through inhibiting Aβ-associated dysfunction and degeneration in AppPs1 AD mice [283]. Conversely, activated SIRT2 contributes to mitophagy dysfunction. Silva et al. observed elevated SIRT2 levels and decreased tubulin acetylation in cells containing AD patient mtDNA and AD brains. The lack of SIRT2 improved mitophagy and recovered microtubule stabilization, promoting Aβ elimination and neuronal cell survival [256]. Besides, SIRT5 inhibited neurotrophic pathways and Aβ accumulation in AD through promoting autophagy [284]. SIRT6 plays a role in maintaining genomic stability in the brain, and its loss could potentially cause toxic Tau stability and phosphorylation [285]. These findings indicate a close association between sirtuin-mediated mitochondrial quality control and AD.
5.2 PD
PD is the second most common neurodegenerative disease (after AD), with an average age-standardized annual incidence of 160 cases per 100,000 people age 65 or older in high-income countries [286]. Mitochondrial dysfunction contributes to the occurrence and development of sporadic and familial PD. Numerous lines of evidence indicated a bidirectional relationship between α-synuclein and mitochondrial dysfunction [287]. In addition, most PD-related genes, such as PINK1, SNCA, LRRK2, and CHCHD2, are implicated in the modulation of mitochondrial homeostasis [202].
Rita et al. found a decreased SIRT1 mRNA level, an increased SIRT6 mRNA level, and an unchanged SIRT2 mRNA level in PD patients in comparison to controls [288]. Emerging research supports the notion that upregulation of SIRT1, SIRT3, and SIRT5 confers neuroprotection against PD. SIRT6 was upregulated in the PD brain and exhibited a proinflammatory and pathogenic role in PD [289]. SIRT1 modulated α-synuclein clearance and phosphorylation in PD through the modulation of mitochondrial function, mitophagy, and heat shock factor 1 deacetylation, decreasing α-synuclein accumulation [290]. Additionally, α-synuclein induced mitochondrial dysfunction via a SIRT3-dependent pathway. α-synuclein interacted with mitochondria, suppressing mitochondrial biogenesis and reducing SIRT3 protein levels. A reduction in SIRT3 levels was accompanied by decreased phosphorylation of AMPK and CREB and increased phosphorylation of DRP1. Besides, treatment with the AMPK agonist 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside upregulated SIRT3 expression and reduced α-synuclein generation [291]. SIRT3 overexpression has shown beneficial effects on neuron-saving by stabilizing mitochondrial biogenetics in PD rat models. Pharmacological elevation of SIRT3 levels decreased α-synuclein oligomers and improved mitochondrial biogenesis against α-synuclein-induced mitochondrial dysfunction [291]. SIRT5 attenuated MPTP-induced nigrostriatal dopaminergic neuron degeneration through increasing SOD2 levels and mitochondrial antioxidant capacity [292]. Divertingly, there are conflicting reports on the role of SIRT2 in PD. In one study, SIRT2-knockout mice reduced α-synuclein toxicity and decreased MPTP-induced dopaminergic cell damage [293]. However, SIRT2 inhibition enhanced α-synuclein aggregation and promoted neural cell death [294]. In another study, SIRT6 overexpression developed more severe pathology, whereas SIRT6 knockout mice were protected from MPTP-induced PD [289]. These findings support the claim that sirtuin-mediated mitochondrial quality control is a key mechanism in PD.
5.3 HD
HD is an autosomal dominant, progressive neurodegenerative disease. Huntingtin, the mutant protein in HD, is strongly associated with cellular damage, including mitochondrial dysfunction, synaptic disruption, and decreased axonal transport rates [6]. mHTT is involved in regulating mitochondrial dysfunction. Additionally, mHTT can directly interact with mitochondrial proteins and act as a translocase of the inner membrane 23 (TIM23), disrupting mitochondrial proteostasis and promoting ROS production and HD progression [295].
Baldo et al. showed that SIRT1 was enhanced in the striatum and cerebral cortex, SIRT2 was only increased in the striatum, and SIRT3 was not changed in HD patients [296]. SIRT1 protected neurons from the toxicity of mHTT. Data on the role of SIRT2 in HD are mixed. The accumulation of mHTT increased sterols in neurons, whereas inhibition of SIRT2 decreased sterols through inhibiting the nuclear trafficking of SREBP-2 [297]. In contrast, Bobrowska et al. found that a reduction in SIRT2 did not affect tubulin acetylation in the brain or the development of HD [298]. In addition, the expression of mHTT inhibited SIRT3 deacetylase activity, further leading to a decrease in intracellular NAD+ levels and mitochondrial biogenesis [299]. SIRT3 provided neuroprotection in HD through modulating oxidative stress and mitochondrial dynamics. For example, ε-viniferin increased SIRT3-mediated FOXO3 deacetylation and alleviated rotenone-induced cell apoptosis by maintaining mitochondrial homeostasis and reducing oxidative stress [300]. Compelling evidence suggested the potential therapeutic implications of sirtuins for neurodegenerative disorders.
6. Pharmacological exploitation of sirtuins for therapeutic purposes
Given their important role in regulating mitochondrial quality control, it is not surprising that sirtuins are involved in the regulation of neurodegeneration. Therefore, sirtuins have been proposed as promising therapeutic targets for neurodegenerative diseases. Mitochondrial dysfunction occurring in neurodegenerative disorders can be partially reversed by exercise training, calorie restriction, and sirtuin modulators to regulate sirtuin activity.
6.1 Exercise training and calorie restriction
Exercise training and calorie restriction are beneficial for controlling disorders associated with aging, especially neurodegenerative diseases. Exercise can alleviate the progression of cognitive impairment in the elderly [301]. Physical exercise has a protective effect on the vulnerability of dopaminergic neurons and the progression of PD. An intriguing study found a reduction in mRNA levels of SIRT1 and SIRT3 in the nigral region of sedentary aged rats compared to young rats. In contrast, treadmill running significantly increased mRNA and protein levels of SIRT1 and SIRT3 in the nigral region of aged rats but not in young rats [302]. Elevated SIRT1 levels induced by treadmill exercise facilitated the non-amyloidogenic pathway and suppressed the amyloidogenic pathway through increasing ADAM-10 and upregulating PGC-1α, respectively [303]. Exercise inhibited α-synuclein levels in a PD mouse model via SIRT1 [304]. In addition, physical exercise efficiently protected against the development of AD by mitigating mitochondrial dysfunction via SIRT1/FOXO1/3/PINK1/parkin-related mitophagy [254]. Calorie restriction enhanced the life span and ameliorated brain health. Ma et al. reported that the neuroprotective role of calorie restriction was partially ascribable to the SIRT1/mTOR pathway and SIRT1-mediated prevention of AD-type Aβ neuropathology [305].
6.2 Drug discovery and the development of sirtuin modulators
In addition to physical exercise and calorie restriction, a great deal of research has shown the potential therapeutic applications of sirtuin modulators in the prevention and treatment of neurodegenerative diseases. Some of these compounds have a positive effect on improving mitochondrial quality control.
6.2.1 Cell and animal models
Natural phytochemical compounds modulate sirtuin activity. Resveratrol is the most well-known activator of SIRT1 [306]. It is beneficial to mitochondrial biogenesis and prevents metabolic decline. An intriguing study found enhanced mitochondrial function and biogenesis in mice treated with moderate doses of resveratrol, while SIRT1 knockout mice did not have these benefits [307]. Another study reported that resveratrol activated SIRT1-relevant mitochondrial biogenesis through the SIRT1/PGC-1α/NRF1/TFAM pathway, alleviating sodium fluoride-induced neurotoxicity [308]. Furthermore, sildenafil inhibited the expression of β-secretase 1 and the generation of Aβ via the CREB/PGC-1α and SIRT1/PGC-1α signaling pathways [309]. Rhein promoted mitochondrial biogenesis through upregulating protein expression of SIRT1 and its downstream PGC-1α and NRF1, thereby attenuating Aβ1-42 oligomer-induced neuronal apoptosis [310, 311]. In addition, Dezfouli et al. observed increased protein expression of SIRT1 and TFAM, along with elevated mtDNA copy numbers, in the hippocampus of aβ-injected rats treated with melatonin for 14 consecutive days. However, these effects were interdicted by the administration of EX527, highlighting that SIRT1 signaling was implicated in the neuroprotective role of melatonin [312]. Sesamol, a nutritional component of sesame, ameliorated neuron damage and cognitive deficits in mice fed a high-fat and high-fructose diet, partly by enhancing the mRNA levels of SIRT1 and PGC-1α [313]. Honokiol, a natural product extracted from the bark of Magnolia officinalis, dramatically attenuated cognitive and synaptic impairments through enhancing the protein expression and activity of SIRT3 and activating mitophagy and mtUPR in male APP/PS1 mice [274]. Fingolimod rescued spatial memory impairment in a rat model of chronic cerebral hypoperfusion, which is associated with the regulatory role of SIRT3 in mitochondrial dysfunction and neuroinflammation [314]. Moreover, administration of nicotinamide mononucleotide, a NAD+ precursor, enhanced mitochondrial NAD+ pools in the hippocampus and triggered a global reduction in SIRT3-mediated mitochondrial protein acetylation. Therefore, the interaction between phosphorylated DRP1 and mitochondria was decreased, resulting in less mitochondrial fragmentation [315].
Additionally, SIRT1-mediated mitochondrial biogenesis has shown neuroprotective effects on dopaminergic neurons in PD models [316]. Urolithin A, a gut metabolite produced from foods containing ellagic acid, like walnuts, berries, and pomegranates, had a protective role in PD by markedly strengthening mitochondrial biogenesis via the SIRT1/PGC-1α pathway and ameliorating 6-OHDA-induced mitochondrial dysfunction [317]. Embelin is a natural product similar in structure to ubiquinone. Rao et al. found that embelin treatment promoted mitochondrial biogenesis through upregulating the protein levels of pAMPK, SIRT1, and PGC-1α [318]. β-Lapachone, a compound extracted from Lapacho bark, upregulated SIRT1 expression, CREB phosphorylation, and PGC-1α deacetylation, inhibiting HD phenotype [319]. Ghrelin, a brain-gut peptide, attenuated MPTP/MPP+-induced neurotoxicity by activating the AMPK/SIRT1/PGC-1α signaling pathway and promoting PINK1/Parkin mitochondrial translocation [252].
Furthermore, a small molecule inhibitor DDQ-treated Tau (P301L) transgenic mouse model showed higher levels of mitochondrial biogenesis and fusion, as well as higher mRNA and protein levels of sirtuins compared to untreated Tau mice [320]. Moreover, synthetic compounds, such as AGK-2 and AK-7 (SIRT2 inhibitors), have neuroprotective effects. AGK-2 reduced astrocyte activation and proinflammatory molecule production in an in vitro model of AD [321]. Another study indicated that AGK-2 and AK-7 suppressed SIRT2 and ameliorated cognitive performance in AD mouse models [322]. All these findings broaden our current understanding that improved mitochondrial quality control is essential to prevent neurodegeneration.
6.2.2 Clinical trials
Most evidence supporting the neuroprotective effects of sirtuin-targeted drug interventions comes from cell or animal experiments. Human studies evaluating the long-term impacts are particularly few, even inconsistent. Several clinical trials have demonstrated the therapeutic efficacy of sirtuin modulators in neurodegenerative diseases. For example, Turner et al. examined the tolerability and safety of resveratrol in a 52-week, randomized, double-blind, placebo-controlled, multicenter phase 2 trial of 119 patients with mild to moderate AD. The findings provided Class II evidence that resveratrol is safe for AD patients and well tolerated [323]. Moreover, in a retrospective study of AD subjects (NCT01504854), resveratrol treatment markedly decreased the levels of metalloproteinase-9, fibroblast growth factor-2, macrophage-derived chemokines, and IL-4 at 52 weeks compared to placebo [324]. These compelling findings support the idea that sirtuins may be potential therapeutic targets for mitochondrial dysfunction and neurodegenerative disorders. However, future clinical trials with larger samples and longer durations are needed to explore the neuroprotective benefits of sirtuins for neurodegenerative diseases.
7. Conclusions and perspectives
Neurodegenerative diseases are characterized by a slow, progressive loss of selective neuronal cell populations and neuronal dysfunction. The prevalence and incidence of neurodegenerative disorders increase significantly with age. However, there are still not fully effective and definitive pharmacological treatments for neuro-degenerative diseases. There is strong evidence that mitochondrial dysfunction occurs early on and acts causally in the pathogenesis of all major neuro-degenerative diseases. Dysregulated sirtuin activity is strongly related to mitochondrial dysfunction, which provides an attractive direction for the intervention and treatment of neurodegenerative disorders. Sirtuins are promising therapeutic targets for neurodegenerative diseases. They are highly conserved NAD+-dependent enzymes that regulate longevity and multiple physiological processes, such as mitochondrial biogenesis, autophagy, mitochondrial dynamics, and mtUPR, through catalyzing a wide range of specific substrates. Given the interesting and encouraging results for regulating sirtuin activity through exercise training, calorie restriction, and the development of sirtuin modulators in cellular and animal models of neurodegenerative diseases, as well as the results of clinical trials, sirtuins emerge as potential therapeutic targets for mitochondrial quality control and neurodegenerative disorders. Compelling evidence for the protective effects on neurodegeneration, combined with continued research on the structure, transcriptional regulation, post-transcriptional regulation, and post-translational modifications of sirtuins, and further exploration of the detailed mechanisms of action of sirtuin-mediated mitochondrial quality control in neurodegenerative diseases, make the targeting of sirtuins a promising strategy for the treatment of neurodegenerative disorders. Future work is needed to elucidate the exact molecular mechanisms among sirtuins, mitochondrial function, and neurodegenerative diseases. Additionally, exploration of novel sirtuin modulators and the development of comparative studies of cells, animals, and clinical trials may facilitate the discovery and development of effective anti-neurodegeneration drugs.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82071593 and 82101663); National Key R&D (or Research and Development) Program of China (No. 2020YFC2009000 and 2020 YFC2009001).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors' contributions
XH wrote the manuscript and drew the figures. LYY and LLS collected literature and drew the figures. LYS conceived the idea and directed the writing. All authors have read and approved the article.
References
- [1].Baker-Nigh A, Vahedi S, Davis EG, Weintraub S, Bigio EH, Klein WL, et al. (2015). Neuronal amyloid-β accumulation within cholinergic basal forebrain in ageing and Alzheimer's disease. Brain, 138:1722-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Neff RA, Wang M, Vatansever S, Guo L, Ming C, Wang Q, et al. (2021). Molecular subtyping of Alzheimer's disease using RNA sequencing data reveals novel mechanisms and targets. Sci Adv, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vaquer-Alicea J, Diamond MI (2019). Propagation of Protein Aggregation in Neurodegenerative Diseases. Annu Rev Biochem, 88:785-810. [DOI] [PubMed] [Google Scholar]
- [4].Tolosa E, Garrido A, Scholz SW, Poewe W (2021). Challenges in the diagnosis of Parkinson's disease. Lancet Neurol, 20:385-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McColgan P, Tabrizi SJ (2018). Huntington's disease: a clinical review. Eur J Neurol, 25:24-34. [DOI] [PubMed] [Google Scholar]
- [6].Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. (2019). Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol, 15:565-581. [DOI] [PubMed] [Google Scholar]
- [7].Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, et al. (2010). PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol, 189:211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, et al. (2006). Thermoregulatory and metabolic defects in Huntington's disease transgenic mice implicate PGC-1alpha in Huntington's disease neurodegeneration. Cell Metab, 4:349-362. [DOI] [PubMed] [Google Scholar]
- [9].Palikaras K, Lionaki E, Tavernarakis N (2015). Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ, 22:1399-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ji Z, Liu GH, Qu J (2022). Mitochondrial sirtuins, metabolism, and aging. J Genet Genomics, 49:287-298. [DOI] [PubMed] [Google Scholar]
- [11].Houtkooper RH, Pirinen E, Auwerx J (2012). Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol, 13:225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. (2010). Regulation of cellular metabolism by protein lysine acetylation. Science, 327:1000-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Satoh A, Imai SI, Guarente L (2017). The brain, sirtuins, and ageing. Nat Rev Neurosci, 18:362-374. [DOI] [PubMed] [Google Scholar]
- [14].Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000). Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature, 403:795-800. [DOI] [PubMed] [Google Scholar]
- [15].Haigis MC, Guarente LP (2006). Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev, 20:2913-2921. [DOI] [PubMed] [Google Scholar]
- [16].Jiao F, Gong Z (2020). The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxid Med Cell Longev, 2020:6782872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang M, Zhang Y, Komaniecki GP, Lu X, Cao J, Zhang M, et al. (2022). Golgi stress induces SIRT2 to counteract Shigella infection via defatty-acylation. Nat Commun, 13:4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kida Y, Goligorsky MS (2016). Sirtuins, Cell Senescence, and Vascular Aging. Can J Cardiol, 32:634-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen X, Lu W, Wu D (2021). Sirtuin 2 (SIRT2): Confusing Roles in the Pathophysiology of Neurological Disorders. Front Neurosci, 15:614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schwer B, North BJ, Frye RA, Ott M, Verdin E (2002). The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol, 158:647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang T, Wang Y, Liu L, Jiang Z, Li X, Tong R, et al. (2020). Research progress on sirtuins family members and cell senescence. Eur J Med Chem, 193:112207. [DOI] [PubMed] [Google Scholar]
- [22].Anderson KA, Huynh FK, Fisher-Wellman K, Stuart JD, Peterson BS, Douros JD, et al. (2017). SIRT4 Is a Lysine Deacylase that Controls Leucine Metabolism and Insulin Secretion. Cell Metab, 25:838-855.e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ramadani-Muja J, Gottschalk B, Pfeil K, Burgstaller S, Rauter T, Bischof H, et al. (2019). Visualization of Sirtuin 4 Distribution between Mitochondria and the Nucleus, Based on Bimolecular Fluorescence Self-Complementation. Cells, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lei MZ, Li XX, Zhang Y, Li JT, Zhang F, Wang YP, et al. (2020). Acetylation promotes BCAT2 degradation to suppress BCAA catabolism and pancreatic cancer growth. Signal Transduct Target Ther, 5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mathias RA, Greco TM, Oberstein A, Budayeva HG, Chakrabarti R, Rowland EA, et al. (2014). Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell, 159:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Du J, Jiang H, Lin H (2009). Investigating the ADP-ribosyltransferase activity of sirtuins with NAD analogues and 32P-NAD. Biochemistry, 48:2878-2890. [DOI] [PubMed] [Google Scholar]
- [27].Wang Y, Chen H, Zha X (2022). Overview of SIRT5 as a potential therapeutic target: Structure, function and inhibitors. Eur J Med Chem, 236:114363. [DOI] [PubMed] [Google Scholar]
- [28].Ogura M, Nakamura Y, Tanaka D, Zhuang X, Fujita Y, Obara A, et al. (2010). Overexpression of SIRT5 confirms its involvement in deacetylation and activation of carbamoyl phosphate synthetase 1. Biochem Biophys Res Commun, 393:73-78. [DOI] [PubMed] [Google Scholar]
- [29].Yang X, Wang Z, Li X, Liu B, Liu M, Liu L, et al. (2018). SHMT2 Desuccinylation by SIRT5 Drives Cancer Cell Proliferation. Cancer Res, 78:372-386. [DOI] [PubMed] [Google Scholar]
- [30].He X, Li T, Qin K, Luo S, Li Z, Ji Q, et al. (2021). Demalonylation of DDX3 by Sirtuin 5 promotes antiviral innate immune responses. Theranostics, 11:7235-7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou L, Wang F, Sun R, Chen X, Zhang M, Xu Q, et al. (2016). SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep, 17:811-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu G, Chen H, Liu H, Zhang W, Zhou J (2021). Emerging roles of SIRT6 in human diseases and its modulators. Med Res Rev, 41:1089-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, et al. (2010). The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell, 140:280-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, et al. (2011). SIRT6 promotes DNA repair under stress by activating PARP1. Science, 332:1443-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, et al. (2013). SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature, 496:110-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kiran S, Chatterjee N, Singh S, Kaul SC, Wadhwa R, Ramakrishna G (2013). Intracellular distribution of human SIRT7 and mapping of the nuclear/nucleolar localization signal. Febs j, 280:3451-3466. [DOI] [PubMed] [Google Scholar]
- [37].Tang M, Li Z, Zhang C, Lu X, Tu B, Cao Z, et al. (2019). SIRT7-mediated ATM deacetylation is essential for its deactivation and DNA damage repair. Sci Adv, 5:eaav1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, et al. (2016). SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun, 7:12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Simonet NG, Thackray JK, Vazquez BN, Ianni A, Espinosa-Alcantud M, Morales-Sanfrutos J, et al. (2020). SirT7 auto-ADP-ribosylation regulates glucose starvation response through mH2A1. Sci Adv, 6:eaaz2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tong Z, Wang M, Wang Y, Kim DD, Grenier JK, Cao J, et al. (2017). SIRT7 Is an RNA-Activated Protein Lysine Deacylase. ACS Chem Biol, 12:300-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yu AQ, Wang J, Jiang ST, Yuan LQ, Ma HY, Hu YM, et al. (2021). SIRT7-Induced PHF5A Decrotonylation Regulates Aging Progress Through Alternative Splicing-Mediated Downregulation of CDK2. Front Cell Dev Biol, 9:710479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bao X, Liu Z, Zhang W, Gladysz K, Fung YME, Tian G, et al. (2019). Glutarylation of Histone H4 Lysine 91 Regulates Chromatin Dynamics. Mol Cell, 76:660-675.e669. [DOI] [PubMed] [Google Scholar]
- [43].Chen Z, Gong L, Zhang P, Li Y, Liu B, Zhang L, et al. (2019). Epigenetic Down-Regulation of Sirt 1 via DNA Methylation and Oxidative Stress Signaling Contributes to the Gestational Diabetes Mellitus-Induced Fetal Programming of Heart Ischemia-Sensitive Phenotype in Late Life. Int J Biol Sci, 15:1240-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nemoto S, Fergusson MM, Finkel T (2004). Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science, 306:2105-2108. [DOI] [PubMed] [Google Scholar]
- [45].Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB (2005). Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell, 123:437-448. [DOI] [PubMed] [Google Scholar]
- [46].Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, et al. (2006). Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol, 8:1025-1031. [DOI] [PubMed] [Google Scholar]
- [47].Yuan J, Minter-Dykhouse K, Lou Z (2009). A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol, 185:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hayashida S, Arimoto A, Kuramoto Y, Kozako T, Honda S, Shimeno H, et al. (2010). Fasting promotes the expression of SIRT1, an NAD+ -dependent protein deacetylase, via activation of PPARalpha in mice. Mol Cell Biochem, 339:285-292. [DOI] [PubMed] [Google Scholar]
- [49].Li Y, Cao R, Gu T, Cao C, Chen T, Guan Y, et al. (2022). PPARβ/δ Augments IL-1β-Induced COX-2 Expression and PGE2 Biosynthesis in Human Mesangial Cells via the Activation of SIRT1. Metabolites, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, et al. (2011). CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep, 12:1069-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Han L, Zhou R, Niu J, McNutt MA, Wang P, Tong T (2010). SIRT1 is regulated by a PPAR{γ}-SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res, 38:7458-7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Inoue T, Hiratsuka M, Osaki M, Yamada H, Kishimoto I, Yamaguchi S, et al. (2007). SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene, 26:945-957. [DOI] [PubMed] [Google Scholar]
- [53].Ji S, Doucette JR, Nazarali AJ (2011). Sirt2 is a novel in vivo downstream target of Nkx2.2 and enhances oligodendroglial cell differentiation. J Mol Cell Biol, 3:351-359. [DOI] [PubMed] [Google Scholar]
- [54].Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, et al. (2012). Dietary obesity-associated Hif1α activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev, 26:259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li C, Zhou Y, Kim JT, Sengoku T, Alstott MC, Weiss HL, et al. (2021). Regulation of SIRT2 by Wnt/β-catenin signaling pathway in colorectal cancer cells. Biochim Biophys Acta Mol Cell Res, 1868:118966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cheng ST, Ren JH, Cai XF, Jiang H, Chen J (2018). HBx-elevated SIRT2 promotes HBV replication and hepatocarcinogenesis. Biochem Biophys Res Commun, 496:904-910. [DOI] [PubMed] [Google Scholar]
- [57].Liu R, Fan M, Candas D, Qin L, Zhang X, Eldridge A, et al. (2015). CDK1-Mediated SIRT3 Activation Enhances Mitochondrial Function and Tumor Radioresistance. Mol Cancer Ther, 14:2090-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu M, Zhang X, Wang Y (2021). Curcumin Alleviates Aβ(42)-Induced Neuronal Metabolic Dysfunction via the Thrb/SIRT3 Axis and Improves Cognition in APP(TG) Mice. Neurochem Res, 46:3166-3178. [DOI] [PubMed] [Google Scholar]
- [59].Liu R, Zeng LW, Gong R, Yuan F, Shu HB, Li S (2021). mTORC1 activity regulates post-translational modifications of glycine decarboxylase to modulate glycine metabolism and tumorigenesis. Nat Commun, 12:4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Satterstrom FK, Swindell WR, Laurent G, Vyas S, Bulyk ML, Haigis MC (2015). Nuclear respiratory factor 2 induces SIRT3 expression. Aging Cell, 14:818-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kanwal A, Pillai VB, Samant S, Gupta M, Gupta MP (2019). The nuclear and mitochondrial sirtuins, Sirt6 and Sirt3, regulate each other's activity and protect the heart from developing obesity-mediated diabetic cardiomyopathy. Faseb j, 33:10872-10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sawant Dessai A, Dominguez MP, Chen UI, Hasper J, Prechtl C, Yu C, et al. (2021). Transcriptional Repression of SIRT3 Potentiates Mitochondrial Aconitase Activation to Drive Aggressive Prostate Cancer to the Bone. Cancer Res, 81:50-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Giralt A, Hondares E, Villena JA, Ribas F, Díaz-Delfín J, Giralt M, et al. (2011). Peroxisome proliferator-activated receptor-gamma coactivator-1alpha controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J Biol Chem, 286:16958-16966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Carnevale I, Pellegrini L, D'Aquila P, Saladini S, Lococo E, Polletta L, et al. (2017). SIRT1-SIRT3 Axis Regulates Cellular Response to Oxidative Stress and Etoposide. J Cell Physiol, 232:1835-1844. [DOI] [PubMed] [Google Scholar]
- [65].Kim S, Piao S, Lee I, Nagar H, Choi SJ, Shin N, et al. (2020). CR6 interacting factor 1 deficiency induces premature senescence via SIRT3 inhibition in endothelial cells. Free Radic Biol Med, 150:161-171. [DOI] [PubMed] [Google Scholar]
- [66].Rehan M, Kurundkar D, Kurundkar AR, Logsdon NJ, Smith SR, Chanda D, et al. (2021). Restoration of SIRT3 gene expression by airway delivery resolves age-associated persistent lung fibrosis in mice. Nat Aging, 1:205-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hong J, Wang X, Mei C, Wang H, Zan L (2019). DNA Methylation and Transcription Factors Competitively Regulate SIRT4 Promoter Activity in Bovine Adipocytes: Roles of NRF1 and CMYB. DNA Cell Biol, 38:63-75. [DOI] [PubMed] [Google Scholar]
- [68].Xu X, Zhang L, Hua F, Zhang C, Zhang C, Mi X, et al. (2021). FOXM1-activated SIRT4 inhibits NF-κB signaling and NLRP3 inflammasome to alleviate kidney injury and podocyte pyroptosis in diabetic nephropathy. Exp Cell Res, 408:112863. [DOI] [PubMed] [Google Scholar]
- [69].Amano H, Chaudhury A, Rodriguez-Aguayo C, Lu L, Akhanov V, Catic A, et al. (2019). Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell Metab, 29:1274-1290.e1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hu Q, Qin Y, Ji S, Xu W, Liu W, Sun Q, et al. (2019). UHRF1 promotes aerobic glycolysis and proliferation via suppression of SIRT4 in pancreatic cancer. Cancer Lett, 452:226-236. [DOI] [PubMed] [Google Scholar]
- [71].Hong J, Li S, Wang X, Mei C, Zan L (2018). Study of expression analysis of SIRT4 and the coordinate regulation of bovine adipocyte differentiation by SIRT4 and its transcription factors. Biosci Rep, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Csibi A, Fendt SM, Li C, Poulogiannis G, Choo AY, Chapski DJ, et al. (2013). The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell, 153:840-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang L, Zhou H, Wang Y, Cui G, Di LJ (2015). CtBP maintains cancer cell growth and metabolic homeostasis via regulating SIRT4. Cell Death Dis, 6:e1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Buler M, Aatsinki SM, Izzi V, Uusimaa J, Hakkola J (2014). SIRT5 is under the control of PGC-1α and AMPK and is involved in regulation of mitochondrial energy metabolism. Faseb j, 28:3225-3237. [DOI] [PubMed] [Google Scholar]
- [75].Hong J, Wang X, Mei C, Zan L (2019). Competitive regulation by transcription factors and DNA methylation in the bovine SIRT5 promoter: Roles of E2F4 and KLF6. Gene, 684:39-46. [DOI] [PubMed] [Google Scholar]
- [76].Hong J, Mei C, Wang X, Cheng G, Zan L (2018). Bioinformatics Analysis and Competitive Regulation by Transcription Factors of SIRT5 at the Core Promoter Region Using Bovine Adipocytes. DNA Cell Biol, 37:1003-1015. [DOI] [PubMed] [Google Scholar]
- [77].Wu M, Seto E, Zhang J (2015). E2F1 enhances glycolysis through suppressing Sirt6 transcription in cancer cells. Oncotarget, 6:11252-11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Choe M, Brusgard JL, Chumsri S, Bhandary L, Zhao XF, Lu S, et al. (2015). The RUNX2 Transcription Factor Negatively Regulates SIRT6 Expression to Alter Glucose Metabolism in Breast Cancer Cells. J Cell Biochem, 116:2210-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dong Z, Yang J, Li L, Tan L, Shi P, Zhang J, et al. (2020). FOXO3a-SIRT6 axis suppresses aerobic glycolysis in melanoma. Int J Oncol, 56:728-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Dong Z, Zhong X, Lei Q, Chen F, Cui H (2019). Transcriptional activation of SIRT6 via FKHRL1/FOXO3a inhibits the Warburg effect in glioblastoma cells. Cell Signal, 60:100-113. [DOI] [PubMed] [Google Scholar]
- [81].Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, et al. (2010). Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab, 12:224-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhou Y, Fan X, Jiao T, Li W, Chen P, Jiang Y, et al. (2021). SIRT6 as a key event linking P53 and NRF2 counteracts APAP-induced hepatotoxicity through inhibiting oxidative stress and promoting hepatocyte proliferation. Acta Pharm Sin B, 11:89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tang X, Li G, Su F, Cai Y, Shi L, Meng Y, et al. (2020). HDAC8 cooperates with SMAD3/4 complex to suppress SIRT7 and promote cell survival and migration. Nucleic Acids Res, 48:2912-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Liu GF, Lu JY, Zhang YJ, Zhang LX, Lu GD, Xie ZJ, et al. (2016). C/EBPα negatively regulates SIRT7 expression via recruiting HDAC3 to the upstream-promoter of hepatocellular carcinoma cells. Biochim Biophys Acta, 1859:348-354. [DOI] [PubMed] [Google Scholar]
- [85].Xu S, Tao H, Cao W, Cao L, Lin Y, Zhao SM, et al. (2021). Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct Target Ther, 6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Abdelmohsen K, Pullmann R, Jr., Lal A, Kim HH, Galban S, Yang X, et al. (2007). Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell, 25:543-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bartel DP (2009). MicroRNAs: target recognition and regulatory functions. Cell, 136:215-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ni YQ, Xu H, Liu YS (2022). Roles of Long Non-coding RNAs in the Development of Aging-Related Neurodegenerative Diseases. Front Mol Neurosci, 15:844193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Cieślik M, Czapski GA, Wójtowicz S, Wieczorek I, Wencel PL, Strosznajder RP, et al. (2020). Alterations of Transcription of Genes Coding Anti-oxidative and Mitochondria-Related Proteins in Amyloid β Toxicity: Relevance to Alzheimer's Disease. Mol Neurobiol, 57:1374-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hadar A, Milanesi E, Walczak M, Puzianowska-Kuźnicka M, Kuźnicki J, Squassina A, et al. (2018). SIRT1, miR-132 and miR-212 link human longevity to Alzheimer's Disease. Sci Rep, 8:8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hernandez-Rapp J, Rainone S, Goupil C, Dorval V, Smith PY, Saint-Pierre M, et al. (2016). microRNA-132/212 deficiency enhances Aβ production and senile plaque deposition in Alzheimer's disease triple transgenic mice. Sci Rep, 6:30953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Weinberg RB, Mufson EJ, Counts SE (2015). Evidence for a neuroprotective microRNA pathway in amnestic mild cognitive impairment. Front Neurosci, 9:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sarkar S, Engler-Chiurazzi EB, Cavendish JZ, Povroznik JM, Russell AE, Quintana DD, et al. (2019). Over-expression of miR-34a induces rapid cognitive impairment and Alzheimer's disease-like pathology. Brain Res, 1721:146327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rodriguez-Ortiz CJ, Baglietto-Vargas D, Martinez-Coria H, LaFerla FM, Kitazawa M (2014). Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J Alzheimers Dis, 42:1229-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang QS, Liu W, Lu GX (2017). miR-200a-3p promotes b-Amyloid-induced neuronal apoptosis through down-regulation of SIRT1 in Alzheimer's disease. J Biosci, 42:397-404. [DOI] [PubMed] [Google Scholar]
- [96].Tao H, Liu Y, Hou Y (2020). miRNA-384-5p regulates the progression of Parkinson's disease by targeting SIRT1 in mice and SH-SY5Y cell. Int J Mol Med, 45:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wang Z, Sun L, Jia K, Wang H, Wang X (2019). miR-9-5p modulates the progression of Parkinson's disease by targeting SIRT1. Neurosci Lett, 701:226-233. [DOI] [PubMed] [Google Scholar]
- [98].Li B, Hu Y, Li X, Jin G, Chen X, Chen G, et al. (2018). Sirt1 Antisense Long Noncoding RNA Promotes Cardiomyocyte Proliferation by Enhancing the Stability of Sirt1. J Am Heart Assoc, 7:e009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wang GQ, Wang Y, Xiong Y, Chen XC, Ma ML, Cai R, et al. (2016). Sirt1 AS lncRNA interacts with its mRNA to inhibit muscle formation by attenuating function of miR-34a. Sci Rep, 6:21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Xu X, Zhang Y, Kang Y, Liu S, Wang Y, Wang Y, et al. (2021). LncRNA MIAT Inhibits MPP(+)-Induced Neuronal Damage Through Regulating the miR-132/SIRT1 Axis in PC12 Cells. Neurochem Res, 46:3365-3374. [DOI] [PubMed] [Google Scholar]
- [101].Lu Y, Tan L, Wang X (2019). Circular HDAC9/microRNA-138/Sirtuin-1 Pathway Mediates Synaptic and Amyloid Precursor Protein Processing Deficits in Alzheimer's Disease. Neurosci Bull, 35:877-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Thangaraj MP, Furber KL, Gan JK, Ji S, Sobchishin L, Doucette JR, et al. (2017). RNA-binding Protein Quaking Stabilizes Sirt2 mRNA during Oligodendroglial Differentiation. J Biol Chem, 292:5166-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Li S, Lv X, Zhai K, Xu R, Zhang Y, Zhao S, et al. (2016). MicroRNA-7 inhibits neuronal apoptosis in a cellular Parkinson's disease model by targeting Bax and Sirt2. Am J Transl Res, 8:993-1004. [PMC free article] [PubMed] [Google Scholar]
- [104].Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L, et al. (2018). MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol, 15:284-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yu Y, An X, Fan D (2021). Histone Deacetylase Sirtuin 2 Enhances Viability of Trophoblasts Through p65-Mediated MicroRNA-146a/ACKR2 Axis. Reprod Sci, 28:1370-1381. [DOI] [PubMed] [Google Scholar]
- [106].Wang Y, Cai Y, Huang H, Chen X, Chen X, Chen X, et al. (2018). miR-486-3p Influences the Neurotoxicity of a-Synuclein by Targeting the SIRT2 Gene and the Polymorphisms at Target Sites Contributing to Parkinson's Disease. Cell Physiol Biochem, 51:2732-2745. [DOI] [PubMed] [Google Scholar]
- [107].Zhuang L, Xia W, Chen D, Ye Y, Hu T, Li S, et al. (2020). Exosomal LncRNA-NEAT1 derived from MIF-treated mesenchymal stem cells protected against doxorubicin-induced cardiac senescence through sponging miR-221-3p. J Nanobiotechnology, 18:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Poulsen RC, Knowles HJ, Carr AJ, Hulley PA (2014). Cell differentiation versus cell death: extracellular glucose is a key determinant of cell fate following oxidative stress exposure. Cell Death Dis, 5:e1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kao YY, Chou CH, Yeh LY, Chen YF, Chang KW, Liu CJ, et al. (2019). MicroRNA miR-31 targets SIRT3 to disrupt mitochondrial activity and increase oxidative stress in oral carcinoma. Cancer Lett, 456:40-48. [DOI] [PubMed] [Google Scholar]
- [110].Fan X, Xiao M, Zhang Q, Li N, Bu P (2019). miR-195-Sirt3 Axis Regulates Angiotensin II-Induced Hippocampal Apoptosis and Learning Impairment in Mice. Psychol Res Behav Manag, 12:1099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zhang Y, Sun M, Wang D, Hu Y, Wang R, Diao H, et al. (2022). FTZ protects against cardiac hypertrophy and oxidative injury via microRNA-214 / SIRT3 signaling pathway. Biomed Pharmacother, 148:112696. [DOI] [PubMed] [Google Scholar]
- [112].Geng L, Zhang T, Liu W, Chen Y (2018). miR-494-3p modulates the progression of in vitro and in vivo Parkinson's disease models by targeting SIRT3. Neurosci Lett, 675:23-30. [DOI] [PubMed] [Google Scholar]
- [113].Ma J, Zhao G, Du J, Li J, Lin G, Zhang J (2021). LncRNA FENDRR Inhibits Gastric Cancer Cell Proliferation and Invasion via the miR-421/SIRT3/Notch-1 Axis. Cancer Manag Res, 13:9175-9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bao J, Lin C, Zhou X, Ma D, Ge L, Xu K, et al. (2021). circFAM160A2 Promotes Mitochondrial Stabilization and Apoptosis Reduction in Osteoarthritis Chondrocytes by Targeting miR-505-3p and SIRT3. Oxid Med Cell Longev, 2021:5712280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhang C, Lu Y, Yuan F, Jiang S (2022). Circular RNA CCDC66 Regulates Osteoarthritis Progression by Targeting miR-3622b-5p. Gerontology, 68:431-441. [DOI] [PubMed] [Google Scholar]
- [116].Ding C, Qian C, Hou S, Lu J, Zou Q, Li H, et al. (2020). Exosomal miRNA-320a Is Released from hAMSCs and Regulates SIRT4 to Prevent Reactive Oxygen Species Generation in POI. Mol Ther Nucleic Acids, 21:37-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang H, Wang Z, Wang Y, Li X, Yang W, Wei S, et al. (2021). miRNA-130b-5p promotes hepatic stellate cell activation and the development of liver fibrosis by suppressing SIRT4 expression. J Cell Mol Med, 25:7381-7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Yu M, Ozaki T, Sun D, Xing H, Wei B, An J, et al. (2020). HIF-1α-dependent miR-424 induction confers cisplatin resistance on bladder cancer cells through down-regulation of pro-apoptotic UNC5B and SIRT4. J Exp Clin Cancer Res, 39:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ji H, Qu J, Peng W, Yang L (2022). Downregulation of lncRNA MALAT1 Inhibits Angiotensin II-induced Hypertrophic Effects of Cardiomyocytes by Regulating SIRT4 via miR-93-5p. Int Heart J, 63:602-611. [DOI] [PubMed] [Google Scholar]
- [120].Xu L, Xu K, Xiang L, Yan J (2021). Circular RNA OMA1 regulates the progression of breast cancer via modulation of the miR-1276/SIRT4 axis. Mol Med Rep, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sun RP, Xi QY, Sun JJ, Cheng X, Zhu YL, Ye DZ, et al. (2016). In low protein diets, microRNA-19b regulates urea synthesis by targeting SIRT5. Sci Rep, 6:33291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Li Y, Gao M, Yin LH, Xu LN, Qi Y, Sun P, et al. (2021). Dioscin ameliorates methotrexate-induced liver and kidney damages via adjusting miRNA-145-5p-mediated oxidative stress. Free Radic Biol Med, 169:99-109. [DOI] [PubMed] [Google Scholar]
- [123].Dang S, Zhou J, Wang Z, Wang K, Dai S, He S (2018). MiR-299-3p functions as a tumor suppressor via targeting Sirtuin 5 in hepatocellular carcinoma. Biomed Pharmacother, 106:966-975. [DOI] [PubMed] [Google Scholar]
- [124].Tang SJ, Yang JB (2020). LncRNA SNHG14 aggravates invasion and migration as ceRNA via regulating miR-656-3p/SIRT5 pathway in hepatocellular carcinoma. Mol Cell Biochem, 473:143-153. [DOI] [PubMed] [Google Scholar]
- [125].Ke F, Ren C, Zhai Z, Gao X, Wei J, Zhu Y, et al. (2022). LINC01234 regulates microRNA-27b-5p to induce the migration, invasion and self-renewal of ovarian cancer stem cells through targeting SIRT5. Cell Cycle, 21:1020-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wu J, Zheng C, Wang Y, Yang Z, Li C, Fang W, et al. (2021). LncRNA APCDD1L-AS1 induces icotinib resistance by inhibition of EGFR autophagic degradation via the miR-1322/miR-1972/miR-324-3p-SIRT5 axis in lung adenocarcinoma. Biomark Res, 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Shang B, Xu T, Hu N, Mao Y, Du X (2021). Circ-Klhl8 overexpression increased the therapeutic effect of EPCs in diabetic wound healing via the miR-212-3p/SIRT5 axis. J Diabetes Complications, 35:108020. [DOI] [PubMed] [Google Scholar]
- [128].Dotto GP, Karine L (2014). miR-34a/SIRT6 in squamous differentiation and cancer. Cell Cycle, 13:1055-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Li N, Mao D, Cao Y, Li H, Ren F, Li K (2018). Downregulation of SIRT6 by miR-34c-5p is associated with poor prognosis and promotes colon cancer proliferation through inhibiting apoptosis via the JAK2/STAT3 signaling pathway. Int J Oncol, 52:1515-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Xiao J, Qin S, Li W, Yao L, Huang P, Liao J, et al. (2020). Osteogenic differentiation of rat bone mesenchymal stem cells modulated by MiR-186 via SIRT6. Life Sci, 253:117660. [DOI] [PubMed] [Google Scholar]
- [131].Xu XZ, Song H, Zhao Y, Zhang L (2020). MiR-654-5p regulated cell progression and tumor growth through targeting SIRT6 in osteosarcoma. Eur Rev Med Pharmacol Sci, 24:3517-3525. [DOI] [PubMed] [Google Scholar]
- [132].Qian W, Zheng ZQ, Nie JG, Liu LJ, Meng XZ, Sun H, et al. (2021). LncRNA SNHG12 alleviates hypertensive vascular endothelial injury through miR-25-3p/SIRT6 pathway. J Leukoc Biol, 110:651-661. [DOI] [PubMed] [Google Scholar]
- [133].Han D, Wang Y, Wang Y, Dai X, Zhou T, Chen J, et al. (2020). The Tumor-Suppressive Human Circular RNA CircITCH Sponges miR-330-5p to Ameliorate Doxorubicin-Induced Cardiotoxicity Through Upregulating SIRT6, Survivin, and SERCA2a. Circ Res, 127:e108-e125. [DOI] [PubMed] [Google Scholar]
- [134].Liu J, Duan P, Xu C, Xu D, Liu Y, Jiang J (2021). CircRNA circ-ITCH improves renal inflammation and fibrosis in streptozotocin-induced diabetic mice by regulating the miR-33a-5p/SIRT6 axis. Inflamm Res, 70:835-846. [DOI] [PubMed] [Google Scholar]
- [135].Kim JK, Noh JH, Jung KH, Eun JW, Bae HJ, Kim MG, et al. (2013). Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology, 57:1055-1067. [DOI] [PubMed] [Google Scholar]
- [136].Wang Y, Wu XQ, Cai JR, Ji HX, Xu T (2022). SIRT7 silencing by miR-152-3p confers cell apoptosis and renal functional impairment induced by renal ischaemia/reperfusion injury. Int Urol Nephrol. [DOI] [PubMed] [Google Scholar]
- [137].Jia B, Zhang S, Wu S, Zhu Q, Li W (2021). MiR-770 promotes oral squamous cell carcinoma migration and invasion by regulating the Sirt7/Smad4 pathway. IUBMB Life, 73:264-272. [DOI] [PubMed] [Google Scholar]
- [138].Dang Y, Zhou Y, Ou X, Wang Q, Wei D, Xie F (2021). lncRNA AC007207.2 Promotes Malignant Properties of Osteosarcoma via the miR-1306-5p/SIRT7 Axis. Cancer Manag Res, 13:7277-7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Liu Q, Cui W, Yang C, Du LP (2021). Circular RNA ZNF609 drives tumor progression by regulating the miR-138-5p/SIRT7 axis in melanoma. Aging (Albany NY), 13:19822-19834. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [140].Luo C, Ling GX, Lei BF, Feng X, Xie XY, Fang C, et al. (2021). Circular RNA PVT1 silencing prevents ischemia-reperfusion injury in rat by targeting microRNA-125b and microRNA-200a. J Mol Cell Cardiol, 159:80-90. [DOI] [PubMed] [Google Scholar]
- [141].Yu L, Dong L, Li H, Liu Z, Luo Z, Duan G, et al. (2020). Ubiquitination-mediated degradation of SIRT1 by SMURF2 suppresses CRC cell proliferation and tumorigenesis. Oncogene, 39:4450-4464. [DOI] [PubMed] [Google Scholar]
- [142].Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, et al. (2007). SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol, 9:1253-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, et al. (2008). Phosphorylation regulates SIRT1 function. PLoS One, 3:e4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Liu X, Wang D, Zhao Y, Tu B, Zheng Z, Wang L, et al. (2011). Methyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1). Proc Natl Acad Sci U S A, 108:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Han C, Gu Y, Shan H, Mi W, Sun J, Shi M, et al. (2017). O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat Commun, 8:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Shinozaki S, Chang K, Sakai M, Shimizu N, Yamada M, Tanaka T, et al. (2014). Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci Signal, 7:ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Mustafa Rizvi SH, Shao D, Tsukahara Y, Pimentel DR, Weisbrod RM, Hamburg NM, et al. (2021). Oxidized GAPDH transfers S-glutathionylation to a nuclear protein Sirtuin-1 leading to apoptosis. Free Radic Biol Med, 174:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Kang H, Jung JW, Kim MK, Chung JH (2009). CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS One, 4:e6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, et al. (2009). JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One, 4:e8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wang W, Li F, Xu Y, Wei J, Zhang Y, Yang H, et al. (2018). JAK1-mediated Sirt1 phosphorylation functions as a negative feedback of the JAK1-STAT3 pathway. J Biol Chem, 293:11067-11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Nakazawa H, Chang K, Shinozaki S, Yasukawa T, Ishimaru K, Yasuhara S, et al. (2017). iNOS as a Driver of Inflammation and Apoptosis in Mouse Skeletal Muscle after Burn Injury: Possible Involvement of Sirt1 S-Nitrosylation-Mediated Acetylation of p65 NF-κB and p53. PLoS One, 12:e0170391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Bräutigam L, Jensen LD, Poschmann G, Nyström S, Bannenberg S, Dreij K, et al. (2013). Glutaredoxin regulates vascular development by reversible glutathionylation of sirtuin 1. Proc Natl Acad Sci U S A, 110:20057-20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].North BJ, Verdin E (2007). Mitotic regulation of SIRT2 by cyclin-dependent kinase 1-dependent phosphorylation. J Biol Chem, 282:19546-19555. [DOI] [PubMed] [Google Scholar]
- [154].Zhang Z, Zhang P, Qi GJ, Jiao FJ, Wang QZ, Yan JG, et al. (2018). CDK5-mediated phosphorylation of Sirt2 contributes to depressive-like behavior induced by social defeat stress. Biochim Biophys Acta Mol Basis Dis, 1864:533-541. [DOI] [PubMed] [Google Scholar]
- [155].Yan J, Zhang P, Tan J, Li M, Xu X, Shao X, et al. (2022). Cdk5 phosphorylation-induced SIRT2 nuclear translocation promotes the death of dopaminergic neurons in Parkinson's disease. NPJ Parkinsons Dis, 8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Liu S, Zhou Z, Zhang L, Meng S, Li S, Wang X (2019). Inhibition of SIRT2 by Targeting GSK3β-Mediated Phosphorylation Alleviates SIRT2 Toxicity in SH-SY5Y Cells. Front Cell Neurosci, 13:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ramakrishnan G, Davaakhuu G, Kaplun L, Chung WC, Rana A, Atfi A, et al. (2014). Sirt2 deacetylase is a novel AKT binding partner critical for AKT activation by insulin. J Biol Chem, 289:6054-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Choi YH, Kim H, Lee SH, Jin YH, Lee KY (2014). Src regulates the activity of SIRT2. Biochem Biophys Res Commun, 450:1120-1125. [DOI] [PubMed] [Google Scholar]
- [159].Han Y, Jin YH, Kim YJ, Kang BY, Choi HJ, Kim DW, et al. (2008). Acetylation of Sirt2 by p300 attenuates its deacetylase activity. Biochem Biophys Res Commun, 375:576-580. [DOI] [PubMed] [Google Scholar]
- [160].Lu W, Wang Q, Xu C, Yuan H, Fan Q, Chen B, et al. (2021). SUMOylation is essential for Sirt2 tumor-suppressor function in neuroblastoma. Neoplasia, 23:129-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Choi YH, Kim H, Han Y, Jin YH, Lee KY (2014). Cbl regulates the activity of SIRT2. Biochem Biophys Res Commun, 453:557-562. [DOI] [PubMed] [Google Scholar]
- [162].Liu L, Yu L, Zeng C, Long H, Duan G, Yin G, et al. (2020). E3 Ubiquitin Ligase HRD1 Promotes Lung Tumorigenesis by Promoting Sirtuin 2 Ubiquitination and Degradation. Mol Cell Biol, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Zhang Y, Shen Y, Wei W, Wang W, Jiang D, Ren Y, et al. (2022). Dysregulation of SIRT3 SUMOylation Confers AML Chemoresistance via Controlling HES1-Dependent Fatty Acid Oxidation. Int J Mol Sci, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Kwon S, Seok S, Yau P, Li X, Kemper B, Kemper JK (2017). Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J Biol Chem, 292:17312-17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Li W, Li D, Kuang H, Feng X, Ai W, Wang Y, et al. (2020). Berberine increases glucose uptake and intracellular ROS levels by promoting Sirtuin 3 ubiquitination. Biomed Pharmacother, 121:109563. [DOI] [PubMed] [Google Scholar]
- [166].Zhang Q, Li D, Dong X, Zhang X, Liu J, Peng L, et al. (2022). LncDACH1 promotes mitochondrial oxidative stress of cardiomyocytes by interacting with sirtuin3 and aggravates diabetic cardiomyopathy. Sci China Life Sci, 65:1198-1212. [DOI] [PubMed] [Google Scholar]
- [167].Li T, Li Y, Liu T, Hu B, Li J, Liu C, et al. (2020). Mitochondrial PAK6 inhibits prostate cancer cell apoptosis via the PAK6-SIRT4-ANT2 complex. Theranostics, 10:2571-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Yao P, Chen T, Jiang P, Li L, Du W (2022). Functional skewing of TRIM21-SIRT5 interplay dictates IL-1β production in DSS-induced colitis. EMBO Rep, 23:e54391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Mills CA, Wang X, Bhatt DP, Grimsrud PA, Matson JP, Lahiri D, et al. (2021). Sirtuin 5 Is Regulated by the SCF(Cyclin F) Ubiquitin Ligase and Is Involved in Cell Cycle Control. Mol Cell Biol, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Meng F, Qian M, Peng B, Peng L, Wang X, Zheng K, et al. (2020). Synergy between SIRT1 and SIRT6 helps recognize DNA breaks and potentiates the DNA damage response and repair in humans and mice. Elife, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Van Meter M, Simon M, Tombline G, May A, Morello TD, Hubbard BP, et al. (2016). JNK phosphorylates SIRT6 to stimulate DNA double-strand break repair in response to oxidative stress by recruiting PARP1 to DNA breaks. Cell reports, 16:2641-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ronnebaum SM, Wu Y, McDonough H, Patterson C (2013). The ubiquitin ligase CHIP prevents SirT6 degradation through noncanonical ubiquitination. Mol Cell Biol, 33:4461-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q, et al. (2013). USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep, 5:1639-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Du L, Li Y, Kang M, Feng M, Ren Y, Dai H, et al. (2021). USP48 Is Upregulated by Mettl14 to Attenuate Hepatocellular Carcinoma via Regulating SIRT6 Stabilization. Cancer Res, 81:3822-3834. [DOI] [PubMed] [Google Scholar]
- [175].Kohli S, Bhardwaj A, Kumari R, Das S (2018). SIRT6 Is a Target of Regulation by UBE3A That Contributes to Liver Tumorigenesis in an ANXA2-Dependent Manner. Cancer Res, 78:645-658. [DOI] [PubMed] [Google Scholar]
- [176].Zhou HZ, Zeng HQ, Yuan D, Ren JH, Cheng ST, Yu HB, et al. (2019). NQO1 potentiates apoptosis evasion and upregulates XIAP via inhibiting proteasome-mediated degradation SIRT6 in hepatocellular carcinoma. Cell Commun Signal, 17:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Cai J, Zuo Y, Wang T, Cao Y, Cai R, Chen FL, et al. (2016). A crucial role of SUMOylation in modulating Sirt6 deacetylation of H3 at lysine 56 and its tumor suppressive activity. Oncogene, 35:4949-4956. [DOI] [PubMed] [Google Scholar]
- [178].Yan WW, Liang YL, Zhang QX, Wang D, Lei MZ, Qu J, et al. (2018). Arginine methylation of SIRT7 couples glucose sensing with mitochondria biogenesis. EMBO Rep, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Jiang L, Xiong J, Zhan J, Yuan F, Tang M, Zhang C, et al. (2017). Ubiquitin-specific peptidase 7 (USP7)-mediated deubiquitination of the histone deacetylase SIRT7 regulates gluconeogenesis. J Biol Chem, 292:13296-13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Su Y, Wu C, Chang Y, Li L, Chen Y, Jia X, et al. (2022). USP17L2-SIRT7 axis regulates DNA damage repair and chemoresistance in breast cancer cells. Breast Cancer Res Treat. [DOI] [PubMed] [Google Scholar]
- [181].Zhou X, Monnie C, DeLucia M, Ahn J (2021). HIV-1 Vpr activates host CRL4-DCAF1 E3 ligase to degrade histone deacetylase SIRT7. Virol J, 18:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Ma Y, Nie H, Chen H, Li J, Hong Y, Wang B, et al. (2015). NAD/NADH metabolism and NAD-dependent enzymes in cell death and ischemic brain injury: current advances and therapeutic implications. Curr Med Chem, 22:1239-1247. [DOI] [PubMed] [Google Scholar]
- [183].Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W (2015). In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A, 112:2876-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R (2011). Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One, 6:e19194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [185].Jang SY, Kang HT, Hwang ES (2012). Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem, 287:19304-19314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Cai Y, Yu SS, He Y, Bi XY, Gao S, Yan TD, et al. (2021). EGCG inhibits pressure overload-induced cardiac hypertrophy via the PSMB5/Nmnat2/SIRT6-dependent signalling pathways. Acta Physiol (Oxf), 231:e13602. [DOI] [PubMed] [Google Scholar]
- [187].Cardanho-Ramos C, Morais VA (2021). Mitochondrial Biogenesis in Neurons: How and Where. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Li PA, Hou X, Hao S (2017). Mitochondrial biogenesis in neurodegeneration. J Neurosci Res, 95:2025-2029. [DOI] [PubMed] [Google Scholar]
- [189].Rice AC, Keeney PM, Algarzae NK, Ladd AC, Thomas RR, Bennett JP, Jr. (2014). Mitochondrial DNA copy numbers in pyramidal neurons are decreased and mitochondrial biogenesis transcriptome signaling is disrupted in Alzheimer's disease hippocampi. J Alzheimers Dis, 40:319-330. [DOI] [PubMed] [Google Scholar]
- [190].Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, et al. (2009). PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol, 66:352-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, et al. (2012). Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer's disease. J Neurochem, 120:419-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Wang R, Li JJ, Diao S, Kwak YD, Liu L, Zhi L, et al. (2013). Metabolic stress modulates Alzheimer's β-secretase gene transcription via SIRT1-PPARγ-PGC-1 in neurons. Cell Metab, 17:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, et al. (2010). PGC-1α, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med, 2:52ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Clark J, Reddy S, Zheng K, Betensky RA, Simon DK (2011). Association of PGC-1alpha polymorphisms with age of onset and risk of Parkinson's disease. BMC Med Genet, 12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Cheng Q, Chen J, Guo H, Lu JL, Zhou J, Guo XY, et al. (2021). Pyrroloquinoline quinone promotes mitochondrial biogenesis in rotenone-induced Parkinson's disease model via AMPK activation. Acta Pharmacol Sin, 42:665-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Rudenok MM, Alieva AK, Starovatykh JS, Nesterov MS, Stanishevskaya VA, Kolacheva AA, et al. (2020). Expression analysis of genes involved in mitochondrial biogenesis in mice with MPTP-induced model of Parkinson's disease. Mol Genet Metab Rep, 23:100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ye Q, Huang W, Li D, Si E, Wang J, Wang Y, et al. (2016). Overexpression of PGC-1α Influences Mitochondrial Signal Transduction of Dopaminergic Neurons. Mol Neurobiol, 53:3756-3770. [DOI] [PubMed] [Google Scholar]
- [198].Guedes-Dias P, Pinho BR, Soares TR, de Proença J, Duchen MR, Oliveira JM (2016). Mitochondrial dynamics and quality control in Huntington's disease. Neurobiol Dis, 90:51-57. [DOI] [PubMed] [Google Scholar]
- [199].Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D (2006). Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell, 127:59-69. [DOI] [PubMed] [Google Scholar]
- [200].Taherzadeh-Fard E, Saft C, Akkad DA, Wieczorek S, Haghikia A, Chan A, et al. (2011). PGC-1alpha downstream transcription factors NRF-1 and TFAM are genetic modifiers of Huntington disease. Mol Neurodegener, 6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K (2021). Molecular mechanisms and physiological functions of mitophagy. Embo j, 40:e104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Malpartida AB, Williamson M, Narendra DP, Wade-Martins R, Ryan BJ (2021). Mitochondrial Dysfunction and Mitophagy in Parkinson's Disease: From Mechanism to Therapy. Trends Biochem Sci, 46:329-343. [DOI] [PubMed] [Google Scholar]
- [203].Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, et al. (2017). Mitophagy and Alzheimer's Disease: Cellular and Molecular Mechanisms. Trends Neurosci, 40:151-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Hu Y, Li XC, Wang ZH, Luo Y, Zhang X, Liu XP, et al. (2016). Tau accumulation impairs mitophagy via increasing mitochondrial membrane potential and reducing mitochondrial Parkin. Oncotarget, 7:17356-17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, et al. (2019). Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat Neurosci, 22:401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Chen C, Yang C, Wang J, Huang X, Yu H, Li S, et al. (2021). Melatonin ameliorates cognitive deficits through improving mitophagy in a mouse model of Alzheimer's disease. J Pineal Res, 71:e12774. [DOI] [PubMed] [Google Scholar]
- [207].Liu J, Liu W, Li R, Yang H (2019). Mitophagy in Parkinson's Disease: From Pathogenesis to Treatment. Cells, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Wauters F, Cornelissen T, Imberechts D, Martin S, Koentjoro B, Sue C, et al. (2020). LRRK2 mutations impair depolarization-induced mitophagy through inhibition of mitochondrial accumulation of RAB10. Autophagy, 16:203-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Hwang S, Disatnik MH, Mochly-Rosen D (2015). Impaired GAPDH-induced mitophagy contributes to the pathology of Huntington's disease. EMBO Mol Med, 7:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Franco-Iborra S, Plaza-Zabala A, Montpeyo M, Sebastian D, Vila M, Martinez-Vicente M (2021). Mutant HTT (huntingtin) impairs mitophagy in a cellular model of Huntington disease. Autophagy, 17:672-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Ochaba J, Lukacsovich T, Csikos G, Zheng S, Margulis J, Salazar L, et al. (2014). Potential function for the Huntingtin protein as a scaffold for selective autophagy. Proc Natl Acad Sci U S A, 111:16889-16894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Khalil B, El Fissi N, Aouane A, Cabirol-Pol MJ, Rival T, Liévens JC (2015). PINK1-induced mitophagy promotes neuroprotection in Huntington's disease. Cell Death Dis, 6:e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Chan DC (2020). Mitochondrial Dynamics and Its Involvement in Disease. Annu Rev Pathol, 15:235-259. [DOI] [PubMed] [Google Scholar]
- [214].Sabouny R, Shutt TE (2020). Reciprocal Regulation of Mitochondrial Fission and Fusion. Trends Biochem Sci, 45:564-577. [DOI] [PubMed] [Google Scholar]
- [215].Meng H, Yan WY, Lei YH, Wan Z, Hou YY, Sun LK, et al. (2019). SIRT3 Regulation of Mitochondrial Quality Control in Neurodegenerative Diseases. Front Aging Neurosci, 11:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, et al. (2009). Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci, 29:9090-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Manczak M, Reddy PH (2012). Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer's disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet, 21:2538-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Baek SH, Park SJ, Jeong JI, Kim SH, Han J, Kyung JW, et al. (2017). Inhibition of Drp1 Ameliorates Synaptic Depression, Aβ Deposition, and Cognitive Impairment in an Alzheimer's Disease Model. J Neurosci, 37:5099-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, et al. (2009). S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science, 324:102-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Kim DI, Lee KH, Gabr AA, Choi GE, Kim JS, Ko SH, et al. (2016). Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochim Biophys Acta, 1863:2820-2834. [DOI] [PubMed] [Google Scholar]
- [221].Zhang Q, Hu C, Huang J, Liu W, Lai W, Leng F, et al. (2019). ROCK1 induces dopaminergic nerve cell apoptosis via the activation of Drp1-mediated aberrant mitochondrial fission in Parkinson's disease. Exp Mol Med, 51:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Wang X, Su B, Liu W, He X, Gao Y, Castellani RJ, et al. (2011). DLP1-dependent mitochondrial fragmentation mediates 1-methyl-4-phenylpyridinium toxicity in neurons: implications for Parkinson's disease. Aging Cell, 10:807-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, et al. (2012). LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet, 21:1931-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Bose A, Beal MF (2016). Mitochondrial dysfunction in Parkinson's disease. J Neurochem, 139 Suppl 1:216-231. [DOI] [PubMed] [Google Scholar]
- [225].Pozo Devoto VM, Falzone TL (2017). Mitochondrial dynamics in Parkinson's disease: a role for α-synuclein? Dis Model Mech, 10:1075-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, et al. (2011). Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: implications for selective neuronal damage. Hum Mol Genet, 20:1438-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, et al. (2012). Mutant huntingtin's interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington's disease. Hum Mol Genet, 21:406-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Chang CR, Blackstone C (2010). Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci, 1201:34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Zhao Y, Sun X, Qi X (2018). Inhibition of Drp1 hyperactivation reduces neuropathology and behavioral deficits in zQ175 knock-in mouse model of Huntington's disease. Biochem Biophys Res Commun, 507:319-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Zhu L, Luo X, Fu N, Chen L (2021). Mitochondrial unfolded protein response: A novel pathway in metabolism and immunity. Pharmacol Res, 168:105603. [DOI] [PubMed] [Google Scholar]
- [231].Zhu L, Zhou Q, He L, Chen L (2021). Mitochondrial unfolded protein response: An emerging pathway in human diseases. Free Radic Biol Med, 163:125-134. [DOI] [PubMed] [Google Scholar]
- [232].Poirier Y, Grimm A, Schmitt K, Eckert A (2019). Link between the unfolded protein response and dysregulation of mitochondrial bioenergetics in Alzheimer's disease. Cell Mol Life Sci, 76:1419-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Shen Y, Ding M, Xie Z, Liu X, Yang H, Jin S, et al. (2019). Activation of Mitochondrial Unfolded Protein Response in SHSY5Y Expressing APP Cells and APP/PS1 Mice. Front Cell Neurosci, 13:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D'Amico D, et al. (2017). Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature, 552:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Beck JS, Mufson EJ, Counts SE (2016). Evidence for Mitochondrial UPR Gene Activation in Familial and Sporadic Alzheimer's Disease. Curr Alzheimer Res, 13:610-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Martinez BA, Petersen DA, Gaeta AL, Stanley SP, Caldwell GA, Caldwell KA (2017). Dysregulation of the Mitochondrial Unfolded Protein Response Induces Non-Apoptotic Dopaminergic Neurodegeneration in C. elegans Models of Parkinson's Disease. J Neurosci, 37:11085-11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Cooper JF, Machiela E, Dues DJ, Spielbauer KK, Senchuk MM, Van Raamsdonk JM (2017). Activation of the mitochondrial unfolded protein response promotes longevity and dopamine neuron survival in Parkinson's disease models. Sci Rep, 7:16441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Berendzen KM, Durieux J, Shao LW, Tian Y, Kim HE, Wolff S, et al. (2016). Neuroendocrine Coordination of Mitochondrial Stress Signaling and Proteostasis. Cell, 166:1553-1563.e1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. (2004). Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science, 304:1158-1160. [DOI] [PubMed] [Google Scholar]
- [240].Chuang YC, Chen SD, Jou SB, Lin TK, Chen SF, Chen NC, et al. (2019). Sirtuin 1 Regulates Mitochondrial Biogenesis and Provides an Endogenous Neuroprotective Mechanism Against Seizure-Induced Neuronal Cell Death in the Hippocampus Following Status Epilepticus. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Bernier M, Paul RK, Martin-Montalvo A, Scheibye-Knudsen M, Song S, He HJ, et al. (2011). Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J Biol Chem, 286:19270-19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Schartner E, Sabbir MG, Saleh A, Silva RV, Roy Chowdhury S, Smith DR, et al. (2018). High glucose concentration suppresses a SIRT2 regulated pathway that enhances neurite outgrowth in cultured adult sensory neurons. Exp Neurol, 309:134-147. [DOI] [PubMed] [Google Scholar]
- [243].Fourcade S, Morató L, Parameswaran J, Ruiz M, Ruiz-Cortés T, Jové M, et al. (2017). Loss of SIRT2 leads to axonal degeneration and locomotor disability associated with redox and energy imbalance. Aging Cell, 16:1404-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Xin T, Lu C (2020). SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging (Albany NY), 12:16224-16237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature, 458:1056-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Hasan-Olive MM, Lauritzen KH, Ali M, Rasmussen LJ, Storm-Mathisen J, Bergersen LH (2019). A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem Res, 44:22-37. [DOI] [PubMed] [Google Scholar]
- [247].Tseng AH, Shieh SS, Wang DL (2013). SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med, 63:222-234. [DOI] [PubMed] [Google Scholar]
- [248].Sun Q, Kang RR, Chen KG, Liu K, Ma Z, Liu C, et al. (2021). Sirtuin 3 is required for the protective effect of Resveratrol on Manganese-induced disruption of mitochondrial biogenesis in primary cultured neurons. J Neurochem, 156:121-135. [DOI] [PubMed] [Google Scholar]
- [249].Ho L, Titus AS, Banerjee KK, George S, Lin W, Deota S, et al. (2013). SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK. Aging (Albany NY), 5:835-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Yu LM, Dong X, Xue XD, Xu S, Zhang X, Xu YL, et al. (2021). Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: Role of SIRT6. J Pineal Res, 70:e12698. [DOI] [PubMed] [Google Scholar]
- [251].Song MY, Han CY, Moon YJ, Lee JH, Bae EJ, Park BH (2022). Sirt6 reprograms myofibers to oxidative type through CREB-dependent Sox6 suppression. Nat Commun, 13:1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Wang H, Dou S, Zhu J, Shao Z, Wang C, Xu X, et al. (2021). Ghrelin protects against rotenone-induced cytotoxicity: Involvement of mitophagy and the AMPK/SIRT1/PGC1α pathway. Neuropeptides, 87:102134. [DOI] [PubMed] [Google Scholar]
- [253].Huang S, Hong Z, Zhang L, Guo J, Li Y, Li K (2022). CERKL alleviates ischemia reperfusion-induced nervous system injury through modulating the SIRT1/PINK1/Parkin pathway and mitophagy induction. Biol Chem. [DOI] [PubMed] [Google Scholar]
- [254].Zhao N, Xia J, Xu B (2021). Physical exercise may exert its therapeutic influence on Alzheimer's disease through the reversal of mitochondrial dysfunction via SIRT1-FOXO1/3-PINK1-Parkin-mediated mitophagy. J Sport Health Sci, 10:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Chang CC, Tsou SH, Chen WJ, Ho YJ, Hung HC, Liu GY, et al. (2021). miR-302 Attenuates Mutant Huntingtin-Induced Cytotoxicity through Restoration of Autophagy and Insulin Sensitivity. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Silva DF, Esteves AR, Oliveira CR, Cardoso SM (2017). Mitochondrial Metabolism Power SIRT2-Dependent Deficient Traffic Causing Alzheimer's-Disease Related Pathology. Mol Neurobiol, 54:4021-4040. [DOI] [PubMed] [Google Scholar]
- [257].Esteves AR, Arduíno DM, Silva DF, Viana SD, Pereira FC, Cardoso SM (2018). Mitochondrial Metabolism Regulates Microtubule Acetylome and Autophagy Trough Sirtuin-2: Impact for Parkinson's Disease. Mol Neurobiol, 55:1440-1462. [DOI] [PubMed] [Google Scholar]
- [258].Sampaio-Marques B, Felgueiras C, Silva A, Rodrigues M, Tenreiro S, Franssens V, et al. (2012). SNCA (α-synuclein)-induced toxicity in yeast cells is dependent on sirtuin 2 (Sir2)-mediated mitophagy. Autophagy, 8:1494-1509. [DOI] [PubMed] [Google Scholar]
- [259].Liu G, Park SH, Imbesi M, Nathan WJ, Zou X, Zhu Y, et al. (2017). Loss of NAD-Dependent Protein Deacetylase Sirtuin-2 Alters Mitochondrial Protein Acetylation and Dysregulates Mitophagy. Antioxid Redox Signal, 26:849-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Zhou ZD, Tan EK (2020). Oxidized nicotinamide adenine dinucleotide-dependent mitochondrial deacetylase sirtuin-3 as a potential therapeutic target of Parkinson's disease. Ageing Res Rev, 62:101107. [DOI] [PubMed] [Google Scholar]
- [261].Yu W, Lyu J, Jia L, Sheng M, Yu H, Du H (2020). Dexmedetomidine Ameliorates Hippocampus Injury and Cognitive Dysfunction Induced by Hepatic Ischemia/Reperfusion by Activating SIRT3-Mediated Mitophagy and Inhibiting Activation of the NLRP3 Inflammasome in Young Rats. Oxid Med Cell Longev, 2020:7385458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Lang A, Anand R, Altinoluk-Hambüchen S, Ezzahoini H, Stefanski A, Iram A, et al. (2017). SIRT4 interacts with OPA1 and regulates mitochondrial quality control and mitophagy. Aging (Albany NY), 9:2163-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Polletta L, Vernucci E, Carnevale I, Arcangeli T, Rotili D, Palmerio S, et al. (2015). SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy, 11:253-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Guedouari H, Daigle T, Scorrano L, Hebert-Chatelain E (2017). Sirtuin 5 protects mitochondria from fragmentation and degradation during starvation. Biochim Biophys Acta Mol Cell Res, 1864:169-176. [DOI] [PubMed] [Google Scholar]
- [265].Hong YX, Wu WY, Song F, Wu C, Li GR, Wang Y (2021). Cardiac senescence is alleviated by the natural flavone acacetin via enhancing mitophagy. Aging (Albany NY), 13:16381-16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Oanh NTK, Park YY, Cho H (2017). Mitochondria elongation is mediated through SIRT1-mediated MFN1 stabilization. Cell Signal, 38:67-75. [DOI] [PubMed] [Google Scholar]
- [267].Song SB, Park JS, Jang SY, Hwang ES (2021). Nicotinamide Treatment Facilitates Mitochondrial Fission through Drp1 Activation Mediated by SIRT1-Induced Changes in Cellular Levels of cAMP and Ca(2). Cells, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Lovy A, Ahumada-Castro U, Bustos G, Farias P, Gonzalez-Billault C, Molgó J, et al. (2020). Concerted Action of AMPK and Sirtuin-1 Induces Mitochondrial Fragmentation Upon Inhibition of Ca(2+) Transfer to Mitochondria. Front Cell Dev Biol, 8:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Cha Y, Kim T, Jeon J, Jang Y, Kim PB, Lopes C, et al. (2021). SIRT2 regulates mitochondrial dynamics and reprogramming via MEK1-ERK-DRP1 and AKT1-DRP1 axes. Cell Rep, 37:110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, et al. (2014). SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol, 34:807-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Tyagi A, Nguyen CU, Chong T, Michel CR, Fritz KS, Reisdorph N, et al. (2018). SIRT3 deficiency-induced mitochondrial dysfunction and inflammasome formation in the brain. Sci Rep, 8:17547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, et al. (2013). The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell, 154:430-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Wang B, Ge S, Xiong W, Xue Z (2018). Effects of resveratrol pretreatment on endoplasmic reticulum stress and cognitive function after surgery in aged mice. BMC Anesthesiol, 18:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Hou M, Bao W, Gao Y, Chen J, Song G (2022). Honokiol improves cognitive impairment in APP/PS1 mice through activating mitophagy and mitochondrial unfolded protein response. Chem Biol Interact, 351:109741. [DOI] [PubMed] [Google Scholar]
- [275].Shi H, Deng HX, Gius D, Schumacker PT, Surmeier DJ, Ma YC (2017). Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Hum Mol Genet, 26:1915-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Lu Z, Chen Y, Aponte AM, Battaglia V, Gucek M, Sack MN (2015). Prolonged fasting identifies heat shock protein 10 as a Sirtuin 3 substrate: elucidating a new mechanism linking mitochondrial protein acetylation to fatty acid oxidation enzyme folding and function. J Biol Chem, 290:2466-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Gibellini L, Pinti M, Beretti F, Pierri CL, Onofrio A, Riccio M, et al. (2014). Sirtuin 3 interacts with Lon protease and regulates its acetylation status. Mitochondrion, 18:76-81. [DOI] [PubMed] [Google Scholar]
- [278].Lin MT, Beal MF (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 443:787-795. [DOI] [PubMed] [Google Scholar]
- [279].Silva MVF, Loures CMG, Alves LCV, de Souza LC, Borges KBG, Carvalho MDG (2019). Alzheimer's disease: risk factors and potentially protective measures. J Biomed Sci, 26:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Lin CL, Cheng YS, Li HH, Chiu PY, Chang YT, Ho YJ, et al. (2016). Amyloid-β suppresses AMP-activated protein kinase (AMPK) signaling and contributes to α-synuclein-induced cytotoxicity. Exp Neurol, 275 Pt 1:84-98. [DOI] [PubMed] [Google Scholar]
- [281].Pradhan R, Singh AK, Kumar P, Bajpai S, Pathak M, Chatterjee P, et al. (2022). Blood Circulatory Level of Seven Sirtuins in Alzheimer's Disease: Potent Biomarker Based on Translational Research. Mol Neurobiol, 59:1440-1451. [DOI] [PubMed] [Google Scholar]
- [282].Lutz MI, Milenkovic I, Regelsberger G, Kovacs GG (2014). Distinct patterns of sirtuin expression during progression of Alzheimer's disease. Neuromolecular Med, 16:405-414. [DOI] [PubMed] [Google Scholar]
- [283].Cheng A, Wang J, Ghena N, Zhao Q, Perone I, King TM, et al. (2020). SIRT3 Haploinsufficiency Aggravates Loss of GABAergic Interneurons and Neuronal Network Hyperexcitability in an Alzheimer's Disease Model. J Neurosci, 40:694-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Wu S, Wei Y, Li J, Bai Y, Yin P, Wang S (2021). SIRT5 Represses Neurotrophic Pathways and Aβ Production in Alzheimer's Disease by Targeting Autophagy. ACS Chem Neurosci, 12:4428-4437. [DOI] [PubMed] [Google Scholar]
- [285].Kaluski S, Portillo M, Besnard A, Stein D, Einav M, Zhong L, et al. (2017). Neuroprotective Functions for the Histone Deacetylase SIRT6. Cell Rep, 18:3052-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Ascherio A, Schwarzschild MA (2016). The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol, 15:1257-1272. [DOI] [PubMed] [Google Scholar]
- [287].Rocha EM, De Miranda B, Sanders LH (2018). Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson's disease. Neurobiol Dis, 109:249-257. [DOI] [PubMed] [Google Scholar]
- [288].Maszlag-Török R, Boros FA, Vécsei L, Klivényi P (2021). Gene variants and expression changes of SIRT1 and SIRT6 in peripheral blood are associated with Parkinson's disease. Sci Rep, 11:10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Nicholatos JW, Francisco AB, Bender CA, Yeh T, Lugay FJ, Salazar JE, et al. (2018). Nicotine promotes neuron survival and partially protects from Parkinson's disease by suppressing SIRT6. Acta Neuropathol Commun, 6:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Li X, Feng Y, Wang XX, Truong D, Wu YC (2020). The Critical Role of SIRT1 in Parkinson's Disease: Mechanism and Therapeutic Considerations. Aging Dis, 11:1608-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Park JH, Burgess JD, Faroqi AH, DeMeo NN, Fiesel FC, Springer W, et al. (2020). Alpha-synuclein-induced mitochondrial dysfunction is mediated via a sirtuin 3-dependent pathway. Mol Neurodegener, 15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Liu L, Peritore C, Ginsberg J, Shih J, Arun S, Donmez G (2015). Protective role of SIRT5 against motor deficit and dopaminergic degeneration in MPTP-induced mice model of Parkinson's disease. Behav Brain Res, 281:215-221. [DOI] [PubMed] [Google Scholar]
- [293].Chopra V, Quinti L, Kim J, Vollor L, Narayanan KL, Edgerly C, et al. (2012). The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington's disease mouse models. Cell Rep, 2:1492-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Singh P, Hanson PS, Morris CM (2017). Sirtuin-2 Protects Neural Cells from Oxidative Stress and Is Elevated in Neurodegeneration. Parkinsons Dis, 2017:2643587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Fão L, Rego AC (2021). Mitochondrial and Redox-Based Therapeutic Strategies in Huntington's Disease. Antioxid Redox Signal, 34:650-673. [DOI] [PubMed] [Google Scholar]
- [296].Baldo B, Gabery S, Soylu-Kucharz R, Cheong RY, Henningsen JB, Englund E, et al. (2019). SIRT1 is increased in affected brain regions and hypothalamic metabolic pathways are altered in Huntington disease. Neuropathol Appl Neurobiol, 45:361-379. [DOI] [PubMed] [Google Scholar]
- [297].Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, et al. (2010). SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A, 107:7927-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Bobrowska A, Donmez G, Weiss A, Guarente L, Bates G (2012). SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington's disease phenotypes in vivo. PLoS One, 7:e34805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Fu J, Jin J, Cichewicz RH, Hageman SA, Ellis TK, Xiang L, et al. (2012). trans-(-)-ε-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J Biol Chem, 287:24460-24472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Zhang S, Ma Y, Feng J (2020). Neuroprotective mechanisms of ε-viniferin in a rotenone-induced cell model of Parkinson's disease: significance of SIRT3-mediated FOXO3 deacetylation. Neural Regen Res, 15:2143-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Cui MY, Lin Y, Sheng JY, Zhang X, Cui RJ (2018). Exercise Intervention Associated with Cognitive Improvement in Alzheimer's Disease. Neural Plast, 2018:9234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Muñoz A, Corrêa CL, Lopez-Lopez A, Costa-Besada MA, Diaz-Ruiz C, Labandeira-Garcia JL (2018). Physical Exercise Improves Aging-Related Changes in Angiotensin, IGF-1, SIRT1, SIRT3, and VEGF in the Substantia Nigra. J Gerontol A Biol Sci Med Sci, 73:1594-1601. [DOI] [PubMed] [Google Scholar]
- [303].Koo JH, Kang EB, Oh YS, Yang DS, Cho JY (2017). Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer's disease. Exp Neurol, 288:142-152. [DOI] [PubMed] [Google Scholar]
- [304].Koo JH, Cho JY (2017). Treadmill Exercise Attenuates α-Synuclein Levels by Promoting Mitochondrial Function and Autophagy Possibly via SIRT1 in the Chronic MPTP/P-Induced Mouse Model of Parkinson's Disease. Neurotox Res, 32:473-486. [DOI] [PubMed] [Google Scholar]
- [305].Ma L, Dong W, Wang R, Li Y, Xu B, Zhang J, et al. (2015). Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res Bull, 116:67-72. [DOI] [PubMed] [Google Scholar]
- [306].Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell, 127:1109-1122. [DOI] [PubMed] [Google Scholar]
- [307].Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. (2012). SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab, 15:675-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Zhao Q, Tian Z, Zhou G, Niu Q, Chen J, Li P, et al. (2020). SIRT1-dependent mitochondrial biogenesis supports therapeutic effects of resveratrol against neurodevelopment damage by fluoride. Theranostics, 10:4822-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Sanders O (2020). Sildenafil for the Treatment of Alzheimer's Disease: A Systematic Review. J Alzheimers Dis Rep, 4:91-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Yin Z, Geng X, Zhang Z, Wang Y, Gao X (2021). Rhein Relieves Oxidative Stress in an Aβ(1-42) Oligomer-Burdened Neuron Model by Activating the SIRT1/PGC-1α-Regulated Mitochondrial Biogenesis. Front Pharmacol, 12:746711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Yin Z, Gao D, Du K, Han C, Liu Y, Wang Y, et al. (2022). Rhein Ameliorates Cognitive Impairment in an APP/PS1 Transgenic Mouse Model of Alzheimer's Disease by Relieving Oxidative Stress through Activating the SIRT1/PGC-1α Pathway. Oxid Med Cell Longev, 2022:2524832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Ansari Dezfouli M, Zahmatkesh M, Farahmandfar M, Khodagholi F (2019). Melatonin protective effect against amyloid β-induced neurotoxicity mediated by mitochondrial biogenesis; involvement of hippocampal Sirtuin-1 signaling pathway. Physiol Behav, 204:65-75. [DOI] [PubMed] [Google Scholar]
- [313].Liu Z, Sun Y, Qiao Q, Zhao T, Zhang W, Ren B, et al. (2017). Sesamol ameliorates high-fat and high-fructose induced cognitive defects via improving insulin signaling disruption in the central nervous system. Food Funct, 8:710-719. [DOI] [PubMed] [Google Scholar]
- [314].Zhang M, Hu Y, Zhang J, Zhang J (2020). FTY720 Prevents Spatial Memory Impairment in a Rat Model of Chronic Cerebral Hypoperfusion via a SIRT3-Independent Pathway. Front Aging Neurosci, 12:593364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Klimova N, Long A, Kristian T (2019). Nicotinamide mononucleotide alters mitochondrial dynamics by SIRT3-dependent mechanism in male mice. J Neurosci Res, 97:975-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Chen Y, Jiang Y, Yang Y, Huang X, Sun C (2021). SIRT1 Protects Dopaminergic Neurons in Parkinson's Disease Models via PGC-1α-Mediated Mitochondrial Biogenesis. Neurotox Res, 39:1393-1404. [DOI] [PubMed] [Google Scholar]
- [317].Liu J, Jiang J, Qiu J, Wang L, Zhuo J, Wang B, et al. (2022). Urolithin A protects dopaminergic neurons in experimental models of Parkinson's disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1α signaling pathway. Food Funct, 13:375-385. [DOI] [PubMed] [Google Scholar]
- [318].Rao SP, Sharma N, Kalivendi SV (2020). Embelin averts MPTP-induced dysfunction in mitochondrial bioenergetics and biogenesis via activation of SIRT1. Biochim Biophys Acta Bioenerg, 1861:148157. [DOI] [PubMed] [Google Scholar]
- [319].Lee M, Ban JJ, Chung JY, Im W, Kim M (2018). Amelioration of Huntington's disease phenotypes by Beta-Lapachone is associated with increases in Sirt1 expression, CREB phosphorylation and PGC-1α deacetylation. PLoS One, 13:e0195968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Vijayan M, George M, Bunquin LE, Bose C, Reddy PH (2022). Protective effects of a small-molecule inhibitor DDQ against tau-induced toxicities in a transgenic tau mouse model of Alzheimer's disease. Hum Mol Genet, 31:1022-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Scuderi C, Stecca C, Bronzuoli MR, Rotili D, Valente S, Mai A, et al. (2014). Sirtuin modulators control reactive gliosis in an in vitro model of Alzheimer's disease. Front Pharmacol, 5:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Biella G, Fusco F, Nardo E, Bernocchi O, Colombo A, Lichtenthaler SF, et al. (2016). Sirtuin 2 Inhibition Improves Cognitive Performance and Acts on Amyloid-β Protein Precursor Processing in Two Alzheimer's Disease Mouse Models. J Alzheimers Dis, 53:1193-1207. [DOI] [PubMed] [Google Scholar]
- [323].Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. (2015). A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology, 85:1383-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Moussa C, Hebron M, Huang X, Ahn J, Rissman RA, Aisen PS, et al. (2017). Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer's disease. J Neuroinflammation, 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]