Abstract
A 10‐year‐old mixed breed male cat presented with clinical signs related to chronic orthopaedic pain. Upon physical examination, pain was noted, based on the feline Musculoskeletal Pain Index (FMPI). An analgesic treatment with a full spectrum cannabis oil (1.8% CBD and 0.8% THC) was proposed for 30 days (0,5 mg/kg based on CBD). The FMPI scale score decreased more than 50%. This case reported a satisfactory outcome for the patient and the owner, although this medication could increase ALT. Given the paucity of literature published to date on the treatment of veterinary species with cannabis‐based medications, further clinical and pharmacokinetic studies are necessary to study the safety and efficacy of its use.
Keywords: chronic pain, cannabidiol, cannabis, osteoarthritis, tetrahydrocannabinol
The analgesic properties of cannabis‐based pharmaceutical formulations have been proved in chronic pain in humans, but there is lack of literature in veterinary medicine. To our knowledge, this is the first reported case about the use of full spectrum cannabis sativa oil (with CBD and THC) in a cat with osteoarthritic pain. The aim of this report is to reinforce the importance of cannabis‐based products as analgesics.
![]()
1. CASE REPORT
A 10‐year‐old mixed breed male neutered cat presented with clinical signs related to chronic orthopaedic pain. The cat´s owner reported a decrease in its usual activity level, difficulty jumping and vocalisation during defecation, amongst others. Upon physical examination, pain was noted, based on the feline Musculoskeletal Pain Index (FMPI). An analgesic treatment with a full spectrum cannabis oil (1.8% CBD and 0.8% THC) was proposed. The treatment was initiated with CBD 0.5 mg/kg q12h PO. However after 2 days of treatment sedation was observed, thus the dose was decreased to 0.25 mg/kg PO q12h. The alanine aminotransferase (ALT) showed an increase of approximately 3.2 times (from 51 IU/L to 168 IU/L) with 30‐day treatment. Pain score decreased treatment from 13 to 5 points on the FMPI.
Full spectrum Cannabis sativa oil containing CBD and THC exhibited an analgesic effect in a cat with chronic pain. The owner stated that the improvement in the patient's quality of life outweighed the few adverse effects presented.
Cannabis‐based pharmaceutical formulations with analgesic properties have been proved in to be useful humans with chronic pain, but there is lack of literature in veterinary medicine. To our knowledge, this is the first reported case about the use of full spectrum cannabis sativa oil in a cat with osteoarthritic pain. The aim of this report is to highlight the usefulness of cannabis‐based products as analgesics in cats.
2. CLINICAL FEATURES
A 10‐year‐old mixed breed male spayed cat was referred to a teaching hospital for evaluation due to behavioural changes and deterioration in activity level. Major complaints included a decrease in socialisation, reluctance to jump onto surfaces, difficulty jumping in the litter box, vocalisation when defecating, excessive grooming and self‐mutilation, and sporadic muscle spasms and intolerance to soft touch at the lumbosacral region. The patient's history included onychectomy and severe trauma, consisting of falling from a fifth floor when he was 2 years old. At the time, analgesics were administered for a few days, but no further studies were performed.
The clinical signs reported developed approximately one year before presentation. The patient was taken to a private veterinary clinic; the differential diagnoses included lymphoma and inflammatory bowel disease. Treatment with prednisolone was initiated, starting with 0.5 mg/kg q24h PO, lowering the dose every 3 days until reaching a dose of 0.05 mg/kg q48h PO. In addition, a liver protectant was administered for 2 months: nicotinamide 16 mg and coline 31 mg q12h PO (Proteliv; Holliday‐Scott S.A.).
Although there were not any signs of colon inflammation in the recheck abdominal ultrasound exam 5 months later, the cat continued receiving prednisolone at the minimum effective dose. Nevertheless, behavioural changes and reluctance to touch persisted until the owner decided to seek a second opinion.
Upon presentation to the Veterinary Hospital, a physical examination was performed and vital parameters were within normal limits for the species. Mild dehydration was observed, which was assumed to be associated with the patient's difficulty ambulating, making the patient reluctant to walk to the water bowl as needed. Subsequently, an orthopaedic examination was performed revealing marked discomfort on lumbosacral palpation. Thermography images were obtained (FLIR E8) from the forelimbs, hindlimbs and dorsum, and the lumbar temperature was higher than other parts of the spinal column. Lumbosacral and coxofemoral radiographic examination showed lumbosacral stenosis (Figure 1), bilateral acetabular sclerosis and periosteitis (Figure 2). These signs were consistent with lumbosacral instability and coxofermoral degenerative joint disease.
FIGURE 1.
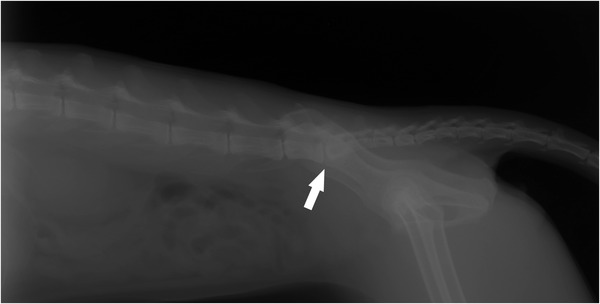
Left lateral view. Slight decrease in the lumbosacral joint space with facet joint sclerosis, which could suggest lumbosacral instability (white arrow).
FIGURE 2.
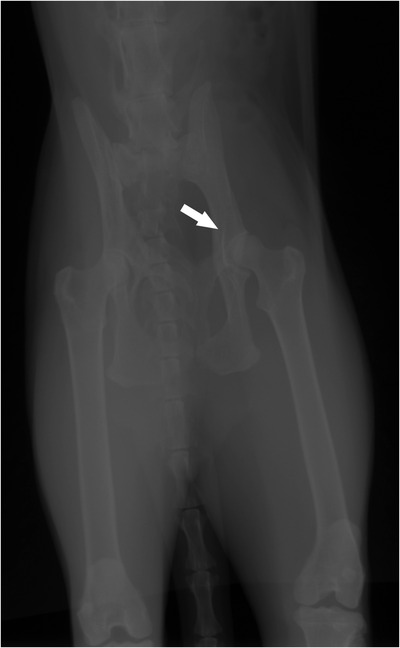
Left hip joint. Acetabular sclerosis and mild periosteal reaction on the cranial edge of the acetabulum, which could suggest incipient degenerative (white arrow).
Following radiographic examination, blood samples were obtained and submitted for CBC and biochemical analysis. The results showed an elevated alkaline phosphatase (ALP) of 107 IU/L. The remaining values were within normal limits for the species (Table 1). Due to haemolysis in the EDTA tube, it was not possible to perform a CBC on day 0. However, no abnormalities were noted on the CBC results corresponding to the last day of treatment (day 30).
TABLE 1.
Biochemistry panel from a 10‐year‐old mixed breed male cat with osteoarthritis, before and after 30 days of analgesic treatment with full spectrum Cannabis sativa oil
| Parameters | Before treatment D 0 | After treatment D 30 | RI |
|---|---|---|---|
| Urea (mg/dl) | 46.54 | 43.97 | 21.42–74.99 |
| Creatinine (mg/dl) | 1.4 | 0.9 | 1.0–2.0 |
| Albumin (g/dl) | 3.3 | 3.1 | 2.6–4.0 |
| Globulins (g/l) | 3.61 | 3.64 | 2.6–5.1 |
| Total protein (g/dl) | 6.9 | 6.8 | 6.0–8.1 |
| AST (IU/l) | 35 | 30 | 14–51 |
| ALT (U/l) | 51 | 168† | 5–65 |
| GGT (IU/l) | 1 | 3 | 1–8 |
| ALP (U/l) | 107† | 93† | 10–70 |
| Cholesterol (mg/dl) | 178 | 156 | 66–317 |
| Total bilirubin (mg/dl) | 0 | 0 | 0.0–0.5 |
RI = reference interval; AST = aspartate aminotransferase; ALT = alanine aminotransferase; GGT = gamma‐glutamyl transferase; ALP = alkaline phosphatase.
†Above reference interval.
Lastly, the Feline Musculoskeletal Pain Index (FMPI) was implemented, which is a validated tool to measure pain resulting from chronic musculoskeletal disorders such as osteoarthritis. It consists of 17 questions about the cat's general activities and mobility, rating it from normal/not painful to abnormal behaviour/worst pain (0–4), and should be answered by the same owner at each visit. The results may be compared during the follow up consults to assess the efficacy of the analgesic treatment implemented (Benito et al., 2013). Three hypothetical questions did not apply to this case; therefore, they were excluded from the questionnaire, resulting in 14 questions answered. The total score on day 0 (before treatment) was 13 points, on a scale where 56 total points would be the worst achievable assessment.
A treatment with Cannabis sativa oil was recommended. The medication was manufactured at the Faculty of Chemistry of Universidad de la Republica, from plan material authorized by the regulatory authority of Uruguay (IRCCA), which ensures plant quality regarding chemical contaminants and presence of pathogens. The plant material was characterized by determining the content of main cannabinoids by liquid chromathography (HPLC‐DAD) and the terpene profile by gas chromatography with flame ionization detection (G‐C‐FID). Plant material was heated at 120°C for 1 h, to achieve the decarboxylation of the acid cannabinoids. An ethanolic extract was made with the decarboxylated material. The extract was filtered and the solvent was removed in a rotary evaporator. The medicinal oil was prepared from the dry extract using olive oil as a vehicle. Finally, the THC and CBD content int the oil was determined by G‐C‐FID, obtaining a concentration of 18 mg/mL of CBD and 8 mg/mL ofTHC.
The medication was initiated at 0.5 mg/kg PO q12h; however, the patient exhibited moderate sedation and ataxia during the first 2 days of treatment. Consequently, the dose was reduced to 0.25 mg/kg, and the adverse effects resolved. In addition, the patient was continued on his previous treatment with prednisolone at 0.05 mg/kg q48h PO for possible inflammatory bowel disease. The patient was closely monitored during the treatment period, to detect any possible adverse effects or signs of excessive pain, but rescue analgesia was not required.
To assess the patient's pain, the owner was asked to complete the FMPI every 3 days, for a total of 30 days (Table 2). The score obtained on the last day of treatment (day 30) was 5 from a total achievable 56 points.
TABLE 2.
Pain assessment with Feline Musculoskeletal Pain Index (FMPI) during 30‐day analgesic treatment with a full spectrum Cannabis sativa oil
| Day | D0 | D3 | D6 | D9 | D12 | D15 | D18 | D21 | D24 | D27 | D30 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | 13 | 13 | 8 | 7 | 7 | 4 | 6 | 6 | 6 | 6 | 5 |
On the last day of treatment, the patient was scheduled for a follow‐up consult. Blood samples were obtained and submitted for CBC and biochemical analysis. The ALT showed an increase of approximately 3.2 times compared to day 0 (from 51 IU/L to 168 IU/L) (Table 1). Upon physical examination, the patient was less reactive to palpation in lumbosacral area.
The patient returned to the primary care veterinarian for follow up, and continued on treatment with another manufactured cannabis oil (50/50 THC/CBD formula), and 6 months later still appears comfortable with a better quality of life.
3. DISCUSSION
Chronic pain is commonly described as pain that persists beyond the normal healing period; therefore, it should be addressed through management of the clinical signs (Epstein et al., 2015). Diagnosis and treatment of chronic pain in cats is suboptimal (Lascelles et al., 2010) and typically does not have an easily identifiable start, as the behavioural changes develop slowly and are often subtle. It has been reported that up to 90% of cats have radiographic evidence of degenerative joint disease (Lascelles, 2010) and about 50% of these cats have clinical signs of impairment due to osteoarthritic pain, which affects mobility, gait, ability to jump and climb stairs (Klinck et al., 2012). The treatment of chronic pain with classic analgesic drugs, such as opioids and anti‐inflammatories, presents limitations due to their low bioavailability, gastrointestinal, hepatic or renal adverse effects, and restricted access to certain drugs by veterinarians (Rychel, 2010). A medicinal analgesic approach with less side effects and low cost would be very beneficial.
THC has agonist actions at CB1 and CB2 receptors, exerting anti‐inflammatory, antineoplastic, analgesic, muscle relaxant, and anti‐spasmodic effects; it also increases appetite and inhibits nausea. It is also the component of the plant with psychoactive properties. CBD has anti‐inflammatory, anxiolytic, antipsychotic and immunomodulatory properties, and is a weak CB1 and CB2 receptor ligand. These molecules also act on other targets, such G‐protein‐coupled receptors (GPR55, GPR18, GPR119). CBD is a reverse agonist at the transient receptor potential of vanilloid‐type 1 channel (TRPV1) which plays important role in pain signaling, and activates the peroxisome proliferator‐activated nuclear receptors (PPARs), profoundly involved in the transcriptional regulation of several cytokines on the immune system. CBD also inhibits the transient receptor potential melastatin type‐8 (TRPM8), and the fatty acid amide hydrolase (FAAH), responsible for anandamide catabolism (Di Marzo & Piscitelli, 2015). These therapeutic effects of THC and CBD have been described to have synergetic effects with other phytochemicals of the plant as Terpenes and other cannabinoids themselves (Nixon et al., 2021).
Infrared thermography is a method for recognising painful areas, as a change in superficial temperature may be an indicator of pain (Vainionpää et al., 2012). The increase in temperature in this case could be the result of a local inflammatory process, which has a correlation with clinical metrology instruments scores in cats (Vainionpää et al., 2012).
In this case, the cat showed a decrease of 38% in the pain score in a 30‐day treatment. The owner reported an improvement in the patient's quality of life, including the use of the litter box without difficulties, interaction with other cats and increased activity level such as jumping onto higher surfaces, behaviour that it had not shown for the past few years.
Even though cats appear to have lower absorption or more rapid elimination of CBD after oral administration than dogs, they also a higher tolerance to THCs ‘intoxicating effects’ (Deabold et al., 2019). It has been reported that CBD and THC doses up to 30 mg/kg and 41.5 mg/kg respectively are well tolerated in healthy cats. Mild, transient and self‐limiting adverse effects, such as lethargy, hypothermia and ataxia, were mainly observed in animals receiving oils that contained THC (Kulpa et al., 2021). The cat from this report showed mild ataxia in the first 2 days, but it was rapidly reversed with a lower dose.
In this case report, we observed that the ALT showed an increase of approximately 3.2 times on day 30 compared to day 0. In scientific studies that have used cannabis in cats, no significant increases in ALT have been observed. However, ALT is frequently noted to increase in dogs but normalises following discontinuation of CBD (Deabold et al., 2019; Kulpa et al., 2021).
4. CONCLUSION
This case reported a satisfactory outcome for the patient and the owner, suggesting that cannabis‐based medications could be a therapeutic alternative for cats with osteoarthritis.
Veterinarians still present doubts and concerns about using Cannabis sativa formulations on their patients; consequently, further clinical and pharmacokinetic studies are necessary to study the safety and efficacy of its use.
AUTHOR CONTRIBUTIONS
Erica Matias Gutierre: investigation, writing – original draft; Nadia Crosignani: supervision, writing – review & editing; Carlos Garcia Carnelli: investigation, methodology, writing – review & editing; Andrea di Mateo: investigation, writing – review & editing; Lucciana Recchi: writing – original draft.
FUNDING INFORMATION
The authors declare no sources of funding for the work presented here.
CONFLICT OF INTEREST
The authors declare no conflicts of interest for the work presented here.
PEER REVIEW
Yes, I would like my name to appear with my report on Publons: https://publons.com/publon/10.1002/vms3.1057.
ACKNOWLEDGMENTS
None.
Gutierre, E. , Crosignani, N. , García‐Carnelli, C. , di Mateo, A. , & Recchi, L. (2023). A case report of CBD and THC as analgesic therapy in a cat with chronic osteoarthritic pain. Veterinary Medicine and Science, 9, 1021–1025. 10.1002/vms3.1057
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Benito, J. , Hansen, B. , DePuy, V. , Davidson, G. S. , Thomson, A. , Simpson, W. , Roe, S. , Hardie, E. , & Lascelles, B. D. X. (2013). Feline musculoskeletal pain index: Responsiveness and testing of criterion validity. Journal of Veterinary Internal Medicine, 27(3), 474–482. [DOI] [PubMed] [Google Scholar]
- Deabold, K. A. , Schwark, W. S. , Wolf, L. , & Wakshlag, J. J. (2019). Single‐dose pharmacokinetics and preliminary safety assessment with use of CBD‐rich hemp nutraceutical in healthy dogs and cats. Animals, 9(10), 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo, V. , & Piscitelli, F. (2015). The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics, 12(4), 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, M. , Rodan, I. , Griffenhagen, G. , Kadrlik, J. , Petty, M. C. , Robertson, S. A. , Simpson, W. , AHAA , & AAFP . (2015). AAHA/AAFP pain management guidelines for dogs and cats. Journal of the American Animal Hospital Association, 51(2), 67–84. [DOI] [PubMed] [Google Scholar]
- Klinck, M. P. , Frank, D. , Guillot, M. , & Troncy, E. (2012). Owner‐perceived signs and veterinary diagnosis in 50 cases of feline osteoarthritis. The Canadian Veterinary Journal, 53(11), 1181. [PMC free article] [PubMed] [Google Scholar]
- Kulpa, J. E. , Paulionis, L. J. , Eglit, G. M. , & Vaughn, D. M. (2021). Safety and tolerability of escalating cannabinoid doses in healthy cats. Journal of Feline Medicine and Surgery, 23(12), 1162–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles, B. , Henry, I. I. I. J. , Brown, J. , Robertson, I. , Sumrell, A. T. , Simpson, W. , Wheeler, S. , Hansen, B. D. , Zamprogno, H. , Freire, M. , & Pease, A. (2010). Cross‐sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Veterinary Surgery, 39(5), 535–544. [DOI] [PubMed] [Google Scholar]
- Lascelles, B. (2010). Feline degenerative joint disease. Veterinary Surgery, 39(1), 2–13. [DOI] [PubMed] [Google Scholar]
- Nixon, J. , Abramovici, H. , Cabecinha, A. , Martinez‐Farina, C. , Hui, J. , & Ellis, L. (2021). Assessing the bioactivity of cannabis extracts in larval zebrafish. Journal of Cannabis Research, 3(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychel, J. K. (2010). Diagnosis and treatment of osteoarthritis. Topics in Companion Animal Medicine, 25(1), 20–25. [DOI] [PubMed] [Google Scholar]
- Vainionpää, M. H. , Raekallio, M. R. , Junnila, J. J. T. , Hielm‐Björkman, A. K. , Snellman, M. P. M. , & Vainio, O. M. (2012). A comparison of thermographic imaging, physical examination and modified questionnaire as an instrument to assess painful conditions in cats. Journal of Feline Medicine and Surgery, 15(2), 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


