Abstract
Humic compounds and related factors are the main constraints for the development of zooplankton in humic lakes, leading to low transfer efficiency in food webs. The results of this study indicated that some zooplankton species could have an advantage under these conditions. We found that the mass development of omnivorous Asplanchna priodonta in temperate humic lakes could be caused by the domination of high nutritional algae such as Gonyostomum semen and Botryococcus braunii. These algae are too large for most zooplankton to ingest, but A. priodonta can feed on a wide range of particles and benefit from this high-nutritional food. Small cladocerans (Ceriodaphnia, Bosmina) might be favored when picoplankton and small algae-dominate humic lakes. Therefore, some zooplankton species could have an advantage and control the development of phytoplankton, leading to the effective transfer of matter and energy in the planktonic food web in humic lakes.
Subject terms: Ecosystem ecology, Freshwater ecology
Introduction
Dystrophic (humic) lakes, according to the commonly accepted definition (after Thienemann 19251), are acid lakes poor in nutrients and organisms, with brown-colored water and organic matter originating mainly from peat bogs and forests surrounding the lake2. Humic substances dissolved in waters acidify them and can form complexes of phosphorus, ammonia, and metal ions3,4; therefore, the water contains small concentrations of dissolved mineral substances. A large content of humic substances gives the brown color of the water, which increases solar energy adsorption and increases the warming of the epilimnion thus increasing thermal stratification5–7. As a result of sharp thermal stratification, even lakes only a few meters deep can be dimictic or meromictic with a large deficiency of oxygen below the thermocline8.
Although humic lakes are usually considered as unproductive9,10, they may contain several groups of producers. Dystrophic lakes have large numbers of bacteria that can use carbon from humic substances11,12. Thus, the microbiological loop seems to play a more important role than the classic trophic pyramid in harmonious (clear water) lakes13. The important components of primary producers in humic lakes are photoautotrophic picoplankton and mixotrophic phytoplankton. Photoautotrophic picoplankton could be favored by intensive nutrient recycling within the microbial loop14, while mixotrophs and osmotrophs could take advantage of the hypolimnetic pool of ammonium, high bacterial production, and low light conditions15. Particularly, the large flagellate Gonyostomum semen forms dense blooms in humic lakes at boreal and temperate latitudes16–18. This may explain literature reports based on studies of more than 600 freshwater lakes, showing that primary production in humic lakes may be higher than that in clear lakes19.
On the other hand, the high biomass of phytoplankton contrasts with the low biomass of zooplankton in dystrophic lakes, which results in low efficiency of the transfer of matter and energy in planktonic food webs20–22. Furthermore, dystrophic lakes are unproductive from a fishery point of view, with low fish densities or an absence of fish23–25. Thus, a large amount of food and low fish pressure in temperate humic lakes usually do not lead to the mass development of large-bodied zooplankton, and the limiting factors for zooplankton growth are unclear. Some authors have indicated that humic stress related to the dystrophication process is responsible for limiting zooplankton development4,26, and others have suggested low food quality20. In less productive lakes, a low diversity of available food may also intensify competition in zooplankton27. Our previous research suggested that a combination of factors related to the dystrophication process constrained zooplankton development. On the one hand, it could be oxygen stress, as anoxic conditions can be found 3–4 m deep, and on the other hand, high ultraviolet radiation in surface waters limits optimal conditions for zooplankton to a very narrow water layer8.
Zooplankton communities in temperate dystrophic lakes are generally characterized by low species richness of crustaceans and rotifers and are often dominated by one or two species (Asplanchna priodonta, Ceriodaphnia quadrangula, and Eudiaptomus gracilis)25,28,29. Moreover, very similar zooplankton communities exist in different dystrophic lakes30. Zooplankton biomass in open water is generally low; however, mass development of one species has been observed in humic lakes29,31.
During this study, we observed high zooplankton biomass in some temperate dystrophic lakes. Therefore, the main aim of this paper was to determine the combination of factors that favor the mass development of zooplankton in humic lakes. The other purpose of the study was to analyze top-down (zooplankton grazing) and bottom-up (hydrochemistry) factors that affect phytoplankton communities. Finally, we estimated the transfer of matter in planktonic food webs by comparing the phytoplankton and zooplankton biomasses.
Results
Environmental conditions
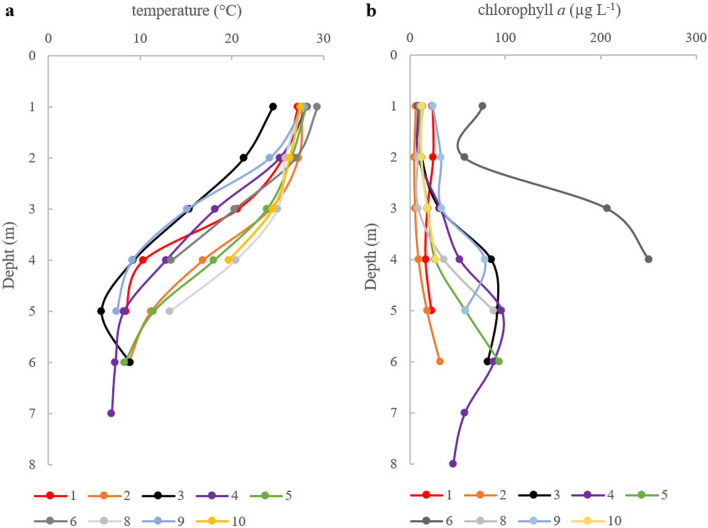
The visibility of the Secchi disk ranged from 1.0 to 3.0 m, with the lowest values in lakes no. 6, 8, and 9 and the highest values in lakes no. 2, and 3 (Table 1). The pH ranged from 5.1 to 6.5, with an average of 5.9 ± 0.5 (Table 1). The water was characterized by a low electrical conductivity (33.3 ± 19.9 µS cm−1) (Table 1) and high DOC concentration (8.7 ± 2.6 mg L−1) (Table S1). The hydrochemical dystrophy indices (HDI) ranged from 55.5 to 74.3, with an average of 64.6 ± 5.7 (Table 1), which indicated highly dystrophic conditions. The concentration of total nitrogen was 1.30 ± 0.75 mg L−1, and total phosphorus was 0.46 ± 0.13 mg L−1 (Table S1). However, the concentrations of dissolved nutrient forms were low. The average concentration of orthophosphates was 0.64 ± 0.74 µg L−1, except for the epilimnion of lake no. 1, where a very high concentration of orthophosphates (162 µg L−1) was noticed. The average concentrations of NH4+ and NO3- were 70 ± 15 µg L−1 and 30 ± 30 µg L−1, respectively (Table S1). We also found large differences in the concentrations of NH4+ and NO3− in the vertical profiles and between lakes. The temperature of surface waters was high and reached up to 29.9 °C, and there were sharp temperature gradients from the surface (Fig. 1a). This resulted in very shallow epilimnion zones where the thermocline began at a depth of 1–2 m, and the temperature at 4 m was approximately 10 °C (Fig. 1a). Thermal stratification was followed by oxygen stratification. The average concentrations of oxygen in the epilimnion, metalimnion, and hypolimnion were 8.2 ± 0.6 mg L−1, 6.5 ± 3.2 mg L−1, and 1.2 ± 1.0 mg L−1, respectively (Table S1). There were no significant differences in nutrient forms and DOC concentrations in the vertical profiles, except for NH4+ where the highest concentrations were found in hypolimnion zones (Table S1).
Table 1.
Characteristics of the studied dystrophic lakes in NE Poland.
| Lake no | Lake name | Latitude (n) | Longitude (e) | Area (ha) | Max depth (m) | SDV (m) | pH | EC (μS cm−1) | HDI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Borkowskie | 53°43′16ʺ | 21°32′57ʺ | 2.9 | 5.0 | 2.0 | 5.7 | 32.4 | 63.8 |
| 2 | Tobolinka | 55°01′36ʺ | 23°24′23ʺ | 2.6 | 7.5 | 3.0 | 6.5 | 74.2 | 55.5 |
| 3 | Rosochaty Róg | 53°45′45ʺ | 21°28′42ʺ | 2.2 | 7.5 | 2.7 | 6.1 | 13.3 | 69.6 |
| 4 | Kruczek Duży | 53°39′41ʺ | 21°24′21ʺ | 4.2 | 8.0 | 2.4 | 5.9 | 47.9 | 58.2 |
| 5 | Suchar Dembowskich | 54°02′18ʺ | 23°03′33ʺ | 3.1 | 5.5 | 1.8 | 6.4 | 11.7 | 69.1 |
| 6 | Ślepe | 53°53′33ʺ | 23°01′46ʺ | 4.8 | 6.5 | 1.0 | 5.3 | 18.1 | 74.3 |
| 7 | Martwe | 53°39′20ʺ | 21°34′07ʺ | 1.4 | 5.0 | 2.2 | 6.4 | 13.5 | 67.4 |
| 8 | Sęczek | 53°43′41ʺ | 21°32′41ʺ | 3.7 | 6.8 | 1.3 | 5.6 | 38.2 | 64.2 |
| 9 | Konopniak | 53°35′07ʺ | 21°33′09ʺ | 1.7 | 5.0 | 1.1 | 5.1 | 45.0 | 60.9 |
| 10 | Suchar Wielki | 54°01′40ʺ | 23°03′20ʺ | 11.0 | 8.0 | 2.1 | 5.7 | 38.6 | 62.7 |
SDV Secchi disk visibility, EC electrical conductivity, HDI Hydrochemical Dystrophy Index4.
Figure 1.
Vertical gradients of temperature (a) and chlorophyll a (b) in studied lakes (lake no. 7—no data).
Phytoplankton communities
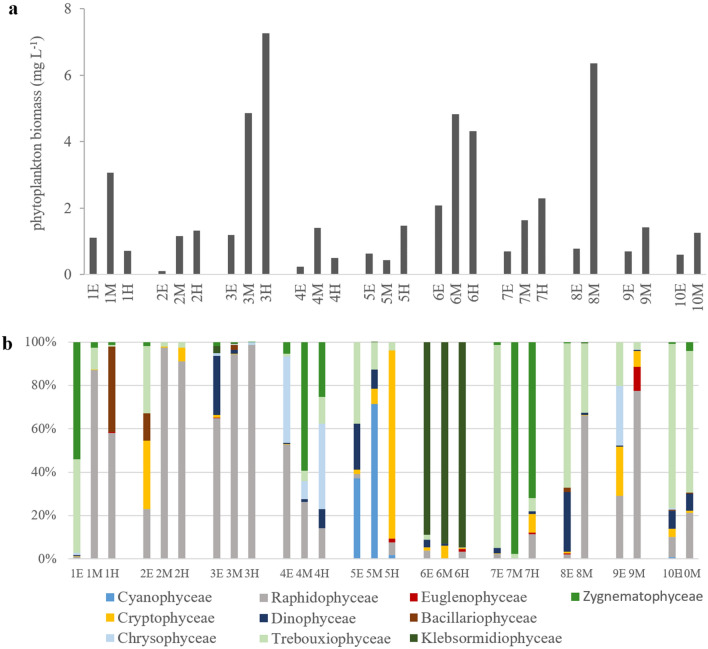
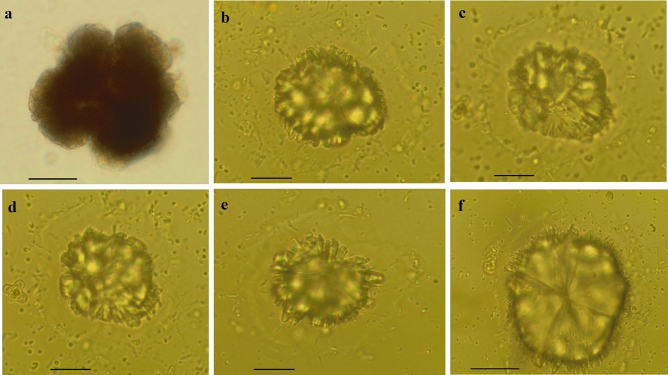
Phytoplankton were represented by 10 classes: Cyanophyceae, Dinophyceae, Cryptophyceae, Raphidophyceae, Chrysophyceae, Bacillariophyceae, Trebouxiophyceae, Zygnematophyceae, Euglenophyceae, and Klebsormidiophyceae (Fig. 2b). The flagellates were the most numerous group. Among them, the highest abundance reached Cryptomonas spp. (4661 ind. mL−1 in lake no. 5) (Cryptophyceae), Mallomonas sp. (1905 ind. mL−1 in lake no. 4) and D. pediforme (1029 ind. mL−1 in lake no. 4) (Chrysophyceae), and Euglena sp. (872 ind. mL−1 in lake no. 9) (Euglenophyceae). Cyanophyceae were determined only in four lakes no. 4, 5, 6 and 10. The most numerous Cyanophyceae was Merismopedia tenuissima (max. 15,805 ind. mL−1 in lake no. 5). Bacillariophyceae were represented only by Asterionella formosa in the hypolimnion of lake no. 1 (847 ind. mL−1). Spondylosium papillosum and Cosmarium sp. from Zygnematophyceae reached maximum abundance in the metalimnion of lake 4 (2135 ind. mL−1) and lake no. 7 (16,090 ind. mL−1), respectively. Among Trebouxiophyceae with small size the most numerous were Stichococcus sp. (149,760 ind. mL−1 lake no. 6), Oocystis spp. (4224 ind. mL−1 lake no. 9), unicellular Chlorococcales (lake no. 2, 4, 5, 9, 10—maximum 3859 ind. mL−1), Crucigeniella rectangularis (2850 ind. mL−1 in lake no. 10), Gloeotila cf. turfosa (2575 ind. mL−1 in lake no. 8), Ankistrodesmus falcatus (1548 ind. mL−1 in lake no. 7), and Monoraphidium komarkovae (737 ind. mL−1 in lake no. 5). Large colonies of Botryococcus braunii were found in most of the studied lakes, with the exception of lakes no. 4 and 9. In the epilimnion and metalimnion of lake no. 1, apart from a small number of typical colonies of B. braunii (epilimnion—24 colonies mL−1, metalimnion—12 colonies mL−1), huge numbers of ‘digested’ colonies (epilimnion—2214 colonies mL−1, metalimnion—1238 colonies mL−1) were noted (Fig. 3). Klebsormidiophyceae represented only one genus Elakatothrix (E. genevensis and E. spirostoma in lakes no. 1, 3, 5, 6, 8).
Figure 2.
Phytoplankton biomasses (a), and percentage share of main phytoplankton groups (b) in epilimnion (E), metalimnion (M), and hypolimnion (H) of studied lakes.
Figure 3.
Botryococcus braunii from lake no. 1 (Borkowskie). (a) normal colony; (b–f) ‘digested’ colonies. Scale bars are 20 µm. Images of colonies fixed with Lugol's solution were taken with an inverted microscope from the Utermöhl chamber.
The total phytoplankton biomass ranged from 0.11 mg L−1 (epilimnion of lake no. 2) to 7.26 mg L−1 (hypolimnion of lake no. 3) (Fig. 2a). Gonyostomum semen, the only representative of the Raphidophyceae, was present in each of the lakes but strongly dominated (> 50% total phytoplankton biomass) in only 6 of them in at least one of the layers (Fig. 2b, Table S2). In lake no. 3, this species dominated all three layers, reaching from 64.9% of the total phytoplankton biomass in the epilimnion to 98.2% in the hypolimnion. In the epilimnion of lake no. 4, it constituted 52.6% of the total phytoplankton biomass; in lake no. 1 it constituted 87.1% in the metalimnion and 95.6% in the hypolimnion; and in the metalimnion of lake no. 8 and 9 it constituted 66.0% and 77.6% of the total phytoplankton biomass, respectively. Apart from Raphidophyceae, more than 50% of the total phytoplankton biomass was found among Cyanophyceae (Merismopedia tenuissima in the metalimnion of lake no. 5), Cryptophyceae (Cryptomonas spp. in the hypolimnion of lake no. 5), Trebouxiophyceae (Stichococcus sp. in all layers of lake no. 6, Gloeotila cf. turfosa in the epilimnion of lake no. 8, unicellular Chloroococcales in all layers of lake no. 10), Zygnemathophyceae (Spondylosium papillosum in the metalimnion of lake no. 4 and Cosmarium sp. in the epilimnion and hypolimnion of lake no. 7 (Table S2).The chlorophyll a concentrations ranged from 5.2 to 250 µg L−1, with an average of 49.8 ± 59.2 µg L−1. The highest chl a concentrations were found in lake no. 6, while in other lakes, they did not exceed 100 µg L−1 (Fig. 1b). The lower water layers were characterized by higher chl a concentrations (Fig. 1b) and higher biomasses of phytoplankton (Fig. 2a).
Zooplankton communities
We identified 18 microcrustacean species (11 Cladocera, 5 Cyclopoida, and 2 Calanoida) (Table S3) and 29 Rotifera species (Table S4). The number of microcrustacean species in one lake ranged from 2 to 8 (Table S3), with an average of 5.2 ± 1.7 species. The number of Rotifera species in one lake ranged from 3 to 11 (Table S4), with an average of 7.4 ± 2.7 species.
Among Cladocera, Ceriodaphnia quadrangula was the most frequent species (all lakes except no. 6), often reaching high biomasses. Bosmina longirostris was found in six lakes and was the dominant component of zooplankton in lake no. 7 (Table S3). Diaphanosoma brachyurum was found in six lakes, reaching high biomass in lakes no. 2 and no. 3 (Table S3). Rare Holopedium gibberum was found in two lakes (no. 4 and no. 9), where it reached high biomasses. Scapholeberis mucronata and Daphnia cucullata were found in two lakes, while Alonella nana, Eurycercus lamellatus, Chydorus sphaericus, Daphnia longispina, and Polyphemus pediculus were found in single records (Table S3). The proportion of Cyclopoida in zooplankton was low in the studied lakes (Fig. 4a). The most common species were Mesocyclops leuckarti, found in four lakes, and Thermocyclops crassus, found in two lakes (Table S3). Diacyclops bicuspidatus, Eucyclops sp., and Cyclops sp. were single records (Table S3). Calanoida had a higher share than Cyclopoida, and they were the dominant component of zooplankton in lake no. 8 (Fig. 4a). The most common calanoid was Eudiaptomus gracilis (9 lakes), while Eudiaptomus graciloides was found only in lake no. 10 (Table S3).
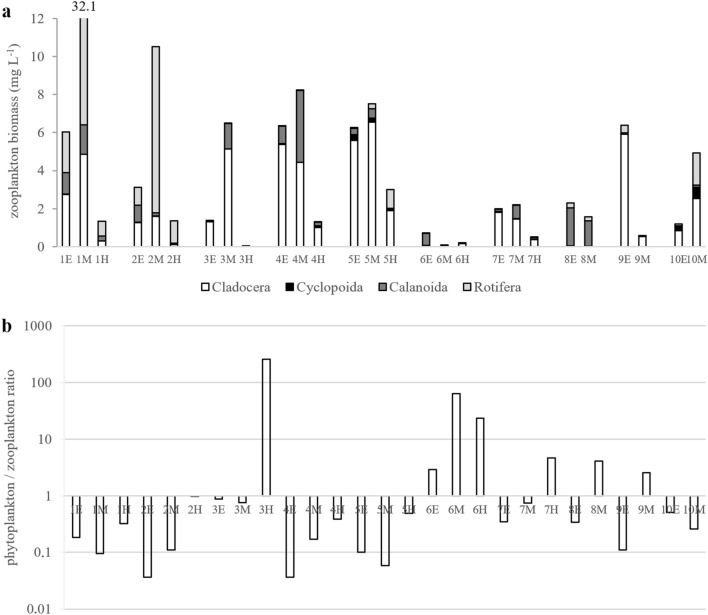
Figure 4.
Zooplankton biomasses with a share of Cladocera, Cyclopoida, Calanoida, and Rotifera (a), and the ratio of phytoplankton/zooplankton (b) biomasses in epilimnion (E), metalimnion (M), and hypolimnion (H) of studied lakes.
The most frequent rotifer species were Asplanchna priodonta and Trichocerca simoneae, which were found in 8 lakes. Asplanchna priodonta achieved very high densities in the metalimnion of lake no. 1 and 2 (1204 and 916 ind. L−1, respectively) and dominated in three other lakes. T. simoneae, on the contrary, although very frequent, had much lower densities, usually lower than 10 ind. L−1 and up to 156 ind. L−1 in lake no. 9. Present in at least half of the studied lakes were Conochiloides dossuarius, and two species of Polyarthra (P. remata and P. vulgaris) (Table S4).
Zooplankton biomass ranged from 0.03 to 32.1 mg L−1. Higher zooplankton biomass was found in lakes no. 1, 2, 4, and 5, and the lowest biomass was found in lakes no. 6, 7, and 8 (Fig. 4a). The very high biomass of zooplankton in lakes no. 2 and no. 1 was caused by the mass development of Asplanchna priodonta, even to 8.7 and 25.5 mg L−1, respectively. Due to its large size, the presence of this species had a strong impact on the biomass of Rotifera communities in the other six lakes, while higher biomass in lakes no. 4 and no. 5 was related to the large development of Ceriodaphnia quadrangula, even to 4.94 and 6.39 mg L−1, respectively. In the vertical profiles, the highest zooplankton biomasses were found in metalimnion zones, but in some lakes, high biomasses were also observed in epilimnion zones (Fig. 4a).
Phytoplankton–environmental relationship (bottom-up)
The ANOVA results indicated a weak relationship between chl a concentrations and all analyzed environmental parameters (F = 1.49; p = 0.24); however, temperature could be negatively related to chl a (F = 4.24; p = 0.057). The dominant species, Gonyostomum semen could be favored by higher DOC concentrations, lower temperatures and lower nutrient concentrations (Fig. 5). Euglenophyta was strongly related to DOC (Fig. 5), while Trebouxiophyceae and Cyanophyceae were favored by higher temperatures and nutrient concentrations (Fig. 5).
Figure 5.
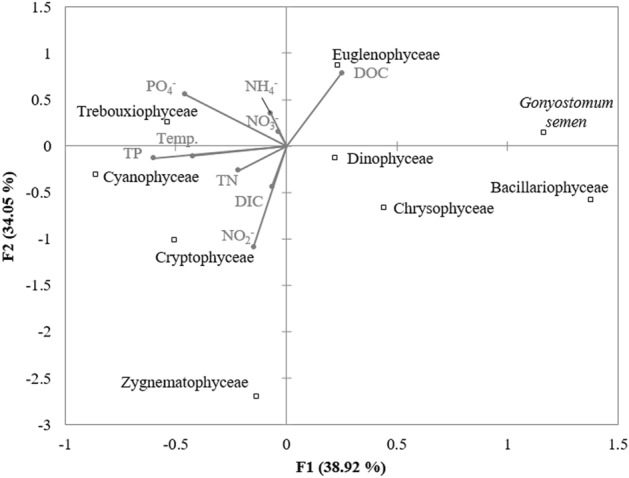
Relations between phytoplankton and physicochemical parameters of water (bottom-up) visualized by the Canonical Correspondence Analysis map.
Phytoplankton–zooplankton relationship (top-down)
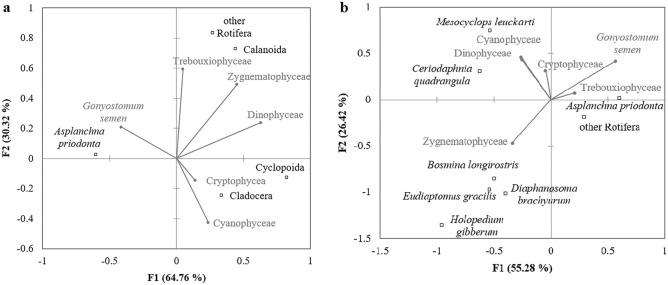
The results of our study suggest that the high biomass of zooplankton in lakes 1, 2, 4, 5 and 10 could control phytoplankton growth (Fig. 4b). We suppose that the extremely high biomass of Asplanchna priodonta in lake no. 1 could be caused by the development of Botryococcus braunii. We found that the number of ‘digested’ colonies of B. braunii (Fig. 3) was over 5 times higher than that of normal colonies (‘digested’ colonies were not counted) in lake no. 1, which may suggest that they were ingested by A. priodonta. In lake no. 2, the biomass of phytoplankton also could be controlled by a large number of A. priodonta. Additionally, in both lakes (no. 1 and 2), one of the highest biomasses of G. semen seems to support the development of A. priodonta (Fig. 6a,b). In lakes no. 4, 5, and 10, Cladocera dominated (Fig. 4a), which could control the development of small algae (i.e., Merismopedia tenuissima, Stichococcus sp., and unicellular Chlorococcales).
Figure 6.
Relations between phytoplankton and zooplankton (top-down) visualized by the Canonical Correspondence Analysis map. (a) all lakes; (b) lakes with ‘effective transfer’ (higher biomass of zooplankton than phytoplankton).
The biomass of the dominant phytoplankton species, Gonyostomum semen, could also be related to A. priodonta (Fig. 6a). The biomass of other species of Rotifera and Calanoida could be related to Trebouxiophyceae and Zygnematophyceae (Fig. 6a). Cladocera and Cyclopoida seem to be related to Cryptophyceae and Cyanophyceae (Fig. 6a). We obtained similar results when analyzing lakes with efficient transfer of matter (higher biomass of zooplankton than phytoplankton) in planktonic food webs (lakes no. 1, 2, 4, 5 and 10). Asplanchna priodonta with other rotifers was related to G. semen but also Trebouxiophyceae (Fig. 6b). Ceriodaphnia quadrangula and Mesocyclops leuckarti were related to Cyanophyceae and Dinophyceae (Fig. 6b). Bosmina longirostris, Diaphanosoma brachyurum, Holopedium gibberum, and Eudiaptomus gracilis were related to Zygnematophyceae (Fig. 6b).
Discussion
Humic lakes are traditionally viewed as unproductive lakes9,10 with a low transfer of energy and matter along the food chain20,21. Eutrophication and dystrophication similarly decrease the trophic transfer efficiency of essential substances between phytoplankton and zooplankton20,21. The development of large-bodied zooplankton in humic lakes is limited despite the low fish pressure and high availability of food resources (phytoplankton and bacterioplankton). The main limiting factors for zooplankton include (i) humic stress related to low pH and high dissolved organic carbon and humic substances26; (ii) sharp thermal and oxygen stratification and anoxic conditions from 3–4 m deep8; and (iii) high UV radiation in surface waters32. The results of this study indicated that some species may have an advantage under these specific conditions. The biomass of Asplanchna priodonta was positively related to the dominant algae Gonyostomum semen. During the last few decades, G. semen, has increased in abundance and distribution in brown water lakes of boreal and temperate regions and it is often the dominant component of phytoplankton in these lakes18. This species could be considered a valuable food source due to its high concentration of polyunsaturated fatty acids (especially EPA and C18 PUFAs), but it is too large for most zooplankton to ingest33. However, some species such as Asplanchna priodonta, Eudiaptomus gracilis and Holopedium gibberum can feed on G. semen at high rates17,34. The results of our study suggest that A. priodonta could have an advantage over other zooplankton species during the domination of G. semen in phytoplankton, while E. gracilis and H. gibberum were negatively related to G. semen biomass.
We found extremely high biomass of Asplanchna in lake no. 1, which could be the result of favorable food conditions. In addition to the high biomass of G. semen in this lake, we found that Asplanchna may selectively feed on Botryococcus braunii. The number of ‘digested’ colonies of B. braunii (Fig. 3) was over five times higher than that of normal colonies, which may suggest that they were ingested by Asplanchna. In this lake, we observed a very high concentration of orthophosphates in the epilimnion, which was 100 times higher than that in other lakes. Asplanchna may spit out the rest of digested food items again35, which was confirmed by our observations. The results of Kappes et al. show a strong preference of Asplanchna for Botryococcus terribilis, which was up to 99% of the food36. Other results also confirm that Asplanchna may change dietary preferences depending on the availability of food36–38. Botryococcus seems to be a ‘superenergetic’ food due to its considerable production of lipids, notably hydrocarbons39. Botryococcus braunii contains up to 61% of hydrocarbon dry weight, while other microalgae generally contain less than 5% of hydrocarbon dry weight40. Most of the hydrocarbons (approximately 95%) are located outside the cells in the colony matrix of Botryococcus, while other algae do not accumulate lipids and hydrocarbons extracellularly but rather as part of the cell membrane systems and as oil droplets within the cytoplasm39,41. The excellent food conditions may explain the very high biomass of Asplanchna observed in lake no. 1 and the approximately tenfold prevalence of dimictic female A. priodonta producing resting eggs. The production of these eggs requires much energy because the females hatching from them have much more lipid droplets than those hatched from subitaneous eggs42.
This study indicated that in some cases, dystrophic lakes could create favorable conditions for the intense development of zooplankton; however, it is mostly of one species. Therefore, the efficiency of the transfer of matter in the planktonic food webs of humic lakes could switch from being inefficient to efficient. Previous studies indicated rather low transfer efficiency of essential substances in food webs of humic lakes12,20,21. The results of our study suggest that the mass development of highly nutritional algae such as G. semen and B. braunii could favor the development of omnivorous A. priodonta, which could feed on a wide range of particles. Furthermore, the mass development of A. priodonta was previously observed in dystrophic lakes29,37.
Zooplankton in temperate dystrophic lakes have a specific and distinctive community structure. Among the most distinguishing features are the low diversity and species richness of crustaceans and rotifers, and community structures are dominated by one or two species29,30,43. Humic stress and related factors cause zooplankton communities to be composed mostly of cosmopolitan and eurytopic species. Among Cladocera the most common are Ceriodaphnia quadrangula, Bosmina longirostris, and Diaphanosoma brachyurum in temperate humic lakes29,44, while in boreal lakes, Holopedium gibberum and Daphnia longispina are often dominant31,43. H. gibberum is rare in temperate regions, where it is restricted mostly to relict lakes with soft water that are poor in dissolved salts of mainly calcium and magnesium45,46, but we found this rare species in two studied humic lakes. Another characteristic feature of humic lakes in Central Europe is the domination of Calanoida over Cyclopoida29,43. The results of our study confirm the relevant share of Calanoida in zooplankton biomass, with the presence of Eudiaptomus gracilis and E. graciloides.
Ejsmont-Karabin hypothesized that poor-in-species, pelagic communities in humic lakes are unsaturated with species; thus, biotic interactions are too weak to prevent colonization of their pelagial with new species47. Trichocerca simoneae is a new species to Poland. It colonizes most humic lakes and often dominates. In our study, it had a high frequency, but low density. A probable reason for its low density may be the presence of relatively high densities of rotifers and crustaceans as T. simoneae density was negatively connected with densities of rotifers and crustaceans in studies by Ejsmont-Karabin47. On the other hand, the species is probably dependent on a high abundance of Dinobryon sp., which was scarce in the studied lakes (JEK unpublished visual observations).
In summary, humic stress and related factors are the main constraints on the development of zooplankton in dystrophic lakes, which leads to low transfer efficiency in food webs. However, the results of our study indicated that some zooplankton species could have an advantage in these conditions. We found that the mass development of high nutritional algae such as Gonyostomum semen and Botryococcus braunii could favor the intense development of omnivorous Asplanchna priodonta, which could feed on a wide range of particles and benefit from these high nutritional food resources. Under these conditions, zooplankton successfully controlled phytoplankton development, which led to the effective transfer of matter and energy in the planktonic food web.
Methods
We analyzed 10 dystrophic (humic) lakes in NE Poland (Table 1). Five lakes were located in the Masurian Lakeland (no. 1, 3, 4, 7, 8), and five lakes were located in the Suwalki Lakeland (no. 2, 5, 6, 9, 10) among which three lakes were located in the Wigry National Park (no. 5, 9, 10). These lakes are mid-forest, usually oval, and without any outlets. All the studied lakes are surrounded by peat mosses that extend into the lakes to a considerable extent. The color of the water was brown-yellow and sometimes green. The lakes are small, and their areas range from 1.4 to 11 ha (Table 1). The maximum depth of the lakes ranged from 3.5 to 8 m (Table 1). The biomass and the number of fish species were very low in the studied lakes25,48. Dystrophic conditions were evaluated by the hydrochemical dystrophy index (HDI), which uses data on surface water pH, electric conductivity, and DIC/DOC ratio4.
The sampling was conducted in the middle of the summer stagnation (07–14.07.2021). Sampling stations were located close to the deepest point of each lake. Water samples for chemical analyses and zooplankton samples were taken from the epilimnion, metalimnion, and hypolimnion (if possible) with a 5 L Limnos sampler. For zooplankton samples, ten liters of water were filtered through a plankton net with a 50 µm mesh size and fixed with 4% formalin. Water samples for phytoplankton analysis were fixed with Lugol solution. The field measurements included the Secchi disc visibility (SDV), conductivity (EC), pH, and dissolved oxygen by the HQ40D Multi Meter (Hach-Lange GmbH, Germany). Chlorophyll a (chl a) concentrations and temperature were measured in situ by a submersible spectrofluorometer (FluoroProbe, bbe Moldaenke, Germany). Temperature measurements taken every few centimeters allowed us to determine the water layers for sampling49. Chemical analyses of the water samples were conducted in the laboratory immediately after collection. The concentrations of ions (PO43−, NH4+, NO3−, NO2−) were determined using a Dionex ICS 1100 ion chromatograph. The analyses of total phosphorus (TP) were conducted in the laboratory according to the conventional photocolorimetric method50. The concentrations of total nitrogen (TN), dissolved organic carbon (DOC) and dissolved inorganic carbon (DIC) were analyzed via high-temperature catalytic combustion using a TOC-L Series (Shimadzu, Japan).
Rotifers and crustaceans were determined to the species level and counted in the whole samples. Additionally, 10 length measurements for each species were made. Mean values of the animal length were used to estimate the wet weight of planktonic crustaceans by applying the equations from51 and for rotifers from52. Phytoplankton abundance was determined according to the Utermöhl method53. Cells, colonies, and filaments were counted. The biomass of the phytoplankton species was determined based on cell sizes and their approximations to simple geometric shapes54.
The relationship between zooplankton biomass and phytoplankton biomass can provide insight into the structure and function of lake biological communities55,56. Therefore, we used the ratio of phytoplankton biomass to zooplankton biomass to estimate the transfer of matter in planktonic food webs. Lakes with a higher biomass of zooplankton than phytoplankton in the whole profile were considered lakes with effective transfer of matter in planktonic food webs22,55. One-way analysis of variance (ANOVA) with type III sums of squares was used to test all pairwise differences between means. Canonical correspondence analysis (CCA) was performed to analyze the summarized effect of environmental factors on plankton communities (including vertical variability: epilimnion, metalimnion, and hypolimnion). Statistical analyses were performed with XLSTAT Ecology (Addinsoft).
Supplementary Information
Acknowledgements
This study was performed as a part of the project entitled: “Monitoring of habitat types with particular regard to the special areas of conservation of the Natura 2000 network in 2021” executed at the order of the Chief Inspectorate for Environmental Protection in Poland and financed by the National Fund for Environment Protection and Water Management.
Author contributions
M.K. conceptualization of research; M.K., M.G., and A.O. conducted the fieldwork; M.K. and J.E.-K. conducted zooplankton analysis; M.G. conducted phytoplankton analysis and wrote phytoplankton methods and results; M.K. statistical analysis and visualization; M.K. wrote the manuscript. All authors reviewed the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files (species list and raw data).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-35039-1.
References
- 1.Thienemann A. Die Binnengewässer Mitteleuropas. E. Schweizerbart'sche Verlagsbuchhandlung; 1925. [Google Scholar]
- 2.Wetzel R. Limnology: Lake and River Ecosystems. 3. Academic Press; 2001. [Google Scholar]
- 3.Górniak A, Jekatierynczuk-Rudczyk E, Dobrzyń P. Hydrochemistry of three dystrophic lakes in North eastern Poland. Acta Hydrochim. Hydrobiol. 1999;27:12–18. doi: 10.1002/(SICI)1521-401X(199901)27:1<12::AID-AHEH12>3.0.CO;2-X. [DOI] [Google Scholar]
- 4.Górniak A. A new version of the hydrochemical dystrophy index to evaluate dystrophy in lakes. Ecol. Ind. 2017;78:566–573. doi: 10.1016/j.ecolind.2017.03.030. [DOI] [Google Scholar]
- 5.Jones RI, Arvola L. Light penetration and some related characteristics ins mall forest lakes in Southern Finland. Verh. Int. Ver. Limnol. 1984;22:811–816. doi: 10.1080/03680770.1983.11897390. [DOI] [Google Scholar]
- 6.Snucin E, Gunn J. Interannual variation in the thermal structure of clear and colored lakes. Limnol. Oceanogr. 2000;45:1639–1644. doi: 10.4319/lo.2000.45.7.1639. [DOI] [Google Scholar]
- 7.Hakala A. Meromixis as a part of lake evolution: Observations and a revised classification of true meromictic lakes in Finland. Boreal Environ. Res. 2004;9:37–53. [Google Scholar]
- 8.Karpowicz M, Ejsmont-Karabin J. Influence of environmental factors on vertical distribution of zooplankton communities in humic lakes. Ann. Limnol. Int. J. Lim. 2018;54:17. doi: 10.1051/limn/2018004. [DOI] [Google Scholar]
- 9.Carpenter SR, Pace ML. Dystrophy and eutrophy in lake ecosystems: implications of fluctuating inputs. Oikos. 1997;78:3–14. doi: 10.2307/3545794. [DOI] [Google Scholar]
- 10.Del Giorgio PA, Cole JJ, Cimberlis A. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature. 1997;385:148–151. doi: 10.1038/385148a0. [DOI] [Google Scholar]
- 11.Karlsson J, Jansson M, Jonsson A. Similar relationships between pelagic primary and bacterial production in clearwater and humic lakes. Ecology. 2002;83:2902–2910. doi: 10.2307/3072025. [DOI] [Google Scholar]
- 12.Jackson TA, Hecky RE. Depression of primary productivity by humic matter in lake and reservoir waters of the boreal forest zone. Can. J. Fish. Aquat. Sci. 1980;37:2300–2317. doi: 10.1139/f80-277. [DOI] [Google Scholar]
- 13.Klavins M, Rodinov V, Druvieties I. Aquatic chemistry and humic substances in bog lakes in Latvia. Boreal Environ. Res. 2003;8:113–123. [Google Scholar]
- 14.Jasser I. The dynamics and importance of picoplankton in shallow, dystrophic lake in comparison with surface waters of two deep lakes with contrasting trophic status. Hydrobiologia. 1997;342(343):87–93. doi: 10.1023/A:1017057005313. [DOI] [Google Scholar]
- 15.Grabowska M, Hindák F, Hindáková A. Phototrophic microflora of dystrophic Lake Sęczek, Masuria, Poland. Oceanol. Hydrobiol. Stud. 2014;43:337–345. doi: 10.2478/s13545-014-0149-4. [DOI] [Google Scholar]
- 16.Karosienė J, Kasperovičienė J, Koreivienė J, Savadova K, Vitonytė I. Factors promoting persistence of the bloom-forming Gonyostomum semen in temperate lakes. Limnologica. 2016;60:51–58. doi: 10.1016/j.limno.2016.05.009. [DOI] [Google Scholar]
- 17.Pęczuła W, Grabowska M, Zieliński P, Karpowicz M, Danilczyk M. Vertical distribution of expansive, bloom-forming algae Gonyostomum semen vs. plankton community and water chemistry in four small humic lakes. Knowl. Manag. Aquat. Ecosyst. 2018;419:28. doi: 10.1051/kmae/2018017. [DOI] [Google Scholar]
- 18.Gollnisch RT, et al. Calcium and pH interaction limits bloom formation and expansion of a nuisance microalga. Limnol. Oceanogr. 2021;66:3523–3534. doi: 10.1002/lno.11896. [DOI] [Google Scholar]
- 19.Nürnberg GK, Shaw M. Productivity of clear and humic lakes: Nutrients, phytoplankton, bacteria. Hydrobiologia. 1998;382:97–112. doi: 10.1023/A:1003445406964. [DOI] [Google Scholar]
- 20.Taipale SJ, Vuorioc K, Strandberg U. Lake eutrophication and brownification downgrade availability and transfer of essential fatty acids for human consumption. Environ. Int. 2016;96:156–166. doi: 10.1016/j.envint.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Karpowicz M, et al. Effect of eutrophication and humification on nutrient cycles and transfer efficiency of matter in the freshwater food web. Hydrobiologia. 2020;847:2521–2540. doi: 10.1007/s10750-020-04271-5. [DOI] [Google Scholar]
- 22.Karpowicz M, et al. Transfer efficiency of carbon, nutrients, and polyunsaturated fatty acids in planktonic food webs under different environmental conditions. Ecol. Evol. 2021;11:8201–8214. doi: 10.1002/ece3.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rask M, Mannio J, Forsius M, Posch M, Vuorinen PJ. How many fish populations in Finland are affected by acid precipitation? Environ. Biol. Fish. 1995;42:51–63. doi: 10.1007/BF00002351. [DOI] [Google Scholar]
- 24.Finstad AG, Helland IP, Ugedal O, Hesthagen T, Hessen DO. Unimodal response of fish yield to dissolved organic carbon. Ecol. Lett. 2014;17:36–43. doi: 10.1111/ele.12201. [DOI] [PubMed] [Google Scholar]
- 25.Kalinowska K, et al. Under-ice environmental conditions, planktonic communities and ichthyofauna in dystrophic lakes. Eur. Zool. J. 2021;88:340–351. doi: 10.1080/24750263.2021.1889054. [DOI] [Google Scholar]
- 26.Robidoux M, del Giorgio P, Derry A. Effects of humic stress on the zooplankton from clear and DOC-rich lakes. Fresh. Biol. 2015;60:1263–1278. doi: 10.1111/fwb.12560. [DOI] [Google Scholar]
- 27.Karabin A, Ejsmont-Karabin J. An evidence for vertical migrations of small rotifers: A case of rotifer community in a dystrophic lake. Hydrobiologia. 2005;546:381–386. doi: 10.1007/s10750-005-4280-5. [DOI] [Google Scholar]
- 28.Arvola L, Metsälä TR, Similä A, Rask M. Phyto- and zooplankton in relation to water pH and humic content in small lakes in southern Finland. Verh. Int. Ver. Limnol. 1990;24:688–692. doi: 10.1080/03680770.1989.11898828. [DOI] [Google Scholar]
- 29.Karpowicz M, Ejsmont-Karabin J. Diversity and structure of pelagic zooplankton (Crustacea, Rotifera) in NE Poland. Water. 2021;13:456. doi: 10.3390/w13040456. [DOI] [Google Scholar]
- 30.Ejsmont-Karabin J, Kalinowska K, Karpowicz M. Structure of ciliate, rotifer, and crustacean communities in lake systems of Northeastern Poland. Polish River Basins Lakes II. 2020;87:77–101. doi: 10.1007/978-3-030-12139-6_4. [DOI] [Google Scholar]
- 31.Ojala A, Salonen K. Productivity of Daphnia longispina in a highly humic boreal lake. J. Plankton Res. 2001;23:1207–1216. doi: 10.1093/plankt/23.11.1207. [DOI] [Google Scholar]
- 32.Rautio M, Korhola A. Effects of ultraviolet radiation and dissolved organic carbon on the survival of subarctic zooplankton. Polar Biol. 2002;25:460–468. doi: 10.1007/s00300-002-0366-y. [DOI] [Google Scholar]
- 33.Strandberg U, et al. Increasing concentration of polyunsaturated fatty acids in browning boreal lakes is driven by nuisance alga Gonyostomum. Ecosphere. 2020;11:e03189. doi: 10.1002/ecs2.3189. [DOI] [Google Scholar]
- 34.Johansson KSL, Vrede T, Lebret K, Johnson RK. Zooplankton feeding on the nuisance flagellate Gonyostomum semen. PLoS ONE. 2013;8:e62557. doi: 10.1371/journal.pone.0062557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wegleńska T, Ejsmont-Karabin J, Rybak JI. Biotic interactions of the zooplankton community of a shallow, humic lake. Hydrobiologia. 1997;342:185–195. doi: 10.1023/A:1017062602104. [DOI] [Google Scholar]
- 36.Kappes H, Mechenich C, Sinsch U. Long-term dynamics of Asplanchna priodonta in Lake Windsborn with comments on the diet. Hydrobiologia. 2000;432:91–100. doi: 10.1023/A:1004022020346. [DOI] [Google Scholar]
- 37.Ejsmont-Karabin J. Studies on the feeding of planktonic polyphage Asplanchna priodonta Gosse (Rotatoria) Ekol. Pol. 1974;22:311–317. [Google Scholar]
- 38.Pociecha A, Wilk-Woźniak E. Comments on the diet of Asplanchna priodonta (Gosse, 1850) in the Dobczycki dam reservoir on the basis of field sample observations. Ocean. Hydrobiol. Stud. 2008;37:63–69. doi: 10.2478/v10009-008-0004-2. [DOI] [Google Scholar]
- 39.Metzger P, Largeau C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2005;66:486–496. doi: 10.1007/s00253-004-1779-z. [DOI] [PubMed] [Google Scholar]
- 40.Metzger P, Berkaloff C, Couté A, Casadevall E. Alkadieneand botryococcene-producing races of wild strains of Botryococcus braunii. Phytochem. 1985;24:2305–2312. doi: 10.1016/S0031-9422(00)83032-0. [DOI] [Google Scholar]
- 41.Suzuki R, et al. Transformation of lipid bodies related to hydrocarbon accumulation in a green alga, Botryococcus braunii (Race B) PLoS ONE. 2013;8:e81626. doi: 10.1371/journal.pone.0081626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbert JJ. Females from resting eggs and parthenogenetic eggs in the rotifer Brachionus calyciflorus: Lipid droplets, starvation resistance and reproduction. Freshw. Biol. 2004;49:1505–1515. doi: 10.1111/j.1365-2427.2004.01282.x. [DOI] [Google Scholar]
- 43.Hessen DO, Faafeng BA, Andersen T. Competition or niche segregation between Holopedium and Daphnia; empirical light on abiotic key parameters. Hydrobiologia. 1995;307:253–261. doi: 10.1007/BF00032019. [DOI] [Google Scholar]
- 44.Druvietis I, Springe G, Urtane L, Klavins M. Evaluation of plankton communities in small highly humic bog lakes in Latvia. Environ. Int. 1998;24:595–602. doi: 10.1016/S0160-4120(98)00038-5. [DOI] [Google Scholar]
- 45.Podshivalina VN, Sheveleva NG, Bayanov N. Biology and ecology of Holopedium gibberum (Branchiopoda: Cladocera: Ctenopoda) in the palearctic. Hydrobiol. J. 2012;48:28–36. doi: 10.1615/HydrobJ.v48.i6.20. [DOI] [Google Scholar]
- 46.Kuczyńska-Kippen N, Klimaszyk P, Piotrowicz R. Zooplankton communities in three adjacent softwater lobelia lakes of slightly differentiated morphology and trophic state. Limnol. Rev. 2017;17:207–214. doi: 10.1515/limre-2017-0019. [DOI] [Google Scholar]
- 47.Ejsmont-Karabin J. Rotifer invasion? On appearance and abundance of tropical species in lakes of North-Eastern Poland. Pol. J. Ecol. 2014;62:727–733. [Google Scholar]
- 48.Białokoz, W. & Krzywosz, T. Struktura ichtiofauny w jeziorach Wigierskiego Parku Narodowego. [Fish communities in lakes of the Wigry National Park]. In Lakes of Wigry National Park: Trophic Status and Protection Directions Zdanowski, B. (ed.). PAN. Kom. Naukowy “Człowiek i środowisko” Zeszyty Naukowe3, 153–162 (1992) (in Polish).
- 49.Karpowicz M, Ejsmont-Karabin J. Effect of metalimnetic gradient on phytoplankton and zooplankton (Rotifera, Crustacea) communities in different trophic conditions. Environ. Monit. Assess. 2017;189:367. doi: 10.1007/s10661-017-6055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neal C, Neal M, Wickham H. Phosphate measurement in natural waters: Two examples of analytical problems associated with silica interference using phosphomolybdic acid methodologies. Sci. Total Environ. 2000;251–252:513–542. doi: 10.1016/S0048-9697(00)00402-2. [DOI] [PubMed] [Google Scholar]
- 51.Błędzki LA, Rybak JI. Freshwater Crustacean Zooplankton of Europe. Springer; 2016. pp. 1–918. [Google Scholar]
- 52.Ejsmont-Karabin J. Empirical equations for biomass calculation of planktonic rotifers. Pol. Arch. Hydrobiol. 1998;45:513–522. [Google Scholar]
- 53.Utermöhl H. Zur vervollkommnung der quantitativen phytoplankton-methodik. Verhandl. Inte. Ver. Theor. Angew. Limnol. 1958;9:1–39. [Google Scholar]
- 54.Hillebrand H, Dürselen C-D, Kirschtel D, Pollingher U, Zohary T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999;35:403–424. doi: 10.1046/j.1529-8817.1999.3520403.x. [DOI] [Google Scholar]
- 55.Yuan LL, Pollard AI. Changes in the relationship between zooplankton and phytoplankton biomasses across a eutrophication gradient. Limnol. Oceanogr. 2018;63:2493–2507. doi: 10.1002/lno.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salonen K, et al. Planktonic food chains of a highly humic lake. Hydrobiologia. 1992;229:143–157. doi: 10.1007/BF00006997. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files (species list and raw data).