Abstract
Entomotherapy, the use of insects for medicinal purposes, has been practised for centuries in many countries around the world. More than 2100 edible insect species are eaten by humans, but little is known about the possibility of using these insects as a promising alternative to traditional pharmaceuticals for treating diseases. This review offers a fundamental understanding of the therapeutic applications of insects and how they might be used in medicine. In this review, 235 insect species from 15 orders are reported to be used as medicine. Hymenoptera contains the largest medicinal insect species, followed by Coleoptera, Orthoptera, Lepidoptera, and Blattodea. Scientists have examined and validated the potential uses of insects along with their products and by-products in treating various diseases, and records show that they are primarily used to treat digestive and skin disorders. Insects are known to be rich sources of bioactive compounds, explaining their therapeutic features such as anti-inflammatory, antimicrobial, antiviral, and so on. Challenges associated with the consumption of insects (entomophagy) and their therapeutic uses include regulation barriers and consumer acceptance. Moreover, the overexploitation of medicinal insects in their natural habitat has led to a population crisis, thus necessitating the investigation and development of their mass-rearing procedure. Lastly, this review suggests potential directions for developing insects used in medicine and offers advice for scientists interested in entomotherapy. In future, entomotherapy may become a sustainable and cost-effective solution for treating various ailments and has the potential to revolutionize modern medicine.
Keywords: Entomotherapy, Medicine, Diseases, Entomophagy, Consumer acceptance, Mass rearing, Edible insect
1. Introduction
Entomotherapy is another name for using insects and insect-derived products for therapeutic purposes [1,2]. Insects and their derived products contain natural compounds with a wide range of biological significance, including antibacterial, antifungal, antiviral, anticancer, antioxidant, anti-inflammatory, and immunomodulatory properties [[3], [4], [5], [6]]. Insects are used as live, cooked, ground, infusions, plasters, salves, ointments, and various other ways [6]. Due to these properties, many communities worldwide have used insects for treating illness. For instance, communities in countries like India, China, and Thailand use insects on the advice of local doctors and elders to treat ailments, such as kidney disease, swelling, intestinal disorders, fortified blood, postpartum hemorrhage, lung diseases like asthma and chronic cough, liver and stomach ailments, toothaches, rheumatism, and other conditions. Moreover, some tribes use bedbugs to treat pain and inflammation in the leg fingers caused by nail insertion or other injuries, while mud from the nest of termites is used to treat inflammation in the body. Several studies have also shown that honey, honeybee larvae and pupae are utilized for various health conditions, including gastrointestinal disorders, gastric problems, mental distress, treatment of external wounds, and maggot therapy [[7], [8], [9], [10]].
Entomotherapy has been practised in many countries around the world. According to Wigglesworth [11], many people in Europe throughout the seventeenth century believed that insects had some therapeutic value. These Europeans used insects to treat many health-related complications, such as epilepsy, earaches, scratches, rheumatism and anaemia [12]. Recent research into the antitumoral potential of the Chartergellus-CP1 peptide found in Chartergellus communis wasp venom in two different breast cancer cell lines (HR+ and triple-negative) showed encouraging results by killing just cancer cells while leaving healthy cells alone [13]. Blister beetles were used as an aphrodisiac throughout Europe, but recent advances show that they can also reduce pain from kidney stones, urinary tract infections and burns [14]. According to Verma and Prasad [15], these beetles contain cantharidin, which has a protein blocker that fights infections. These proteins can target only the infected cells, making them ideal for use in the immune system's fight against infections. Despite these therapeutic uses of insects and insect-based products, many studies have mainly focussed on their nutritional properties. In contrast to earlier studies, the information in our review offers a more fundamental understanding of the medicinal applications of insects and how they might be used in contemporary medicine. A thorough review of the literature is given, and the history, effects, and opportunities associated with the use of various insect species worldwide are discussed, focusing on papers highlighting the identification of insects to the lowest taxonomic rank possible and their publications in peer-reviewed journals.
In this review, we also discussed many insect species used for medicinal purposes, at which stages these species are utilized, and the impact of these insects on human health. We examine if entomotherapy is met with the same opposition as entomophagy. The earlier ideas of gathering insects, the requirement for industrial manufacturing to create significant amounts of insect-based medication, and what insect mass production would entail are discussed. Our review suggests potential directions for developing insects used in medicine and offers advice for scientists interested in entomotherapy.
2. History and evolution of the use of insects for medicinal purposes
Insects in medicine have a long history of application in many societies worldwide by different tribes. Medicinal uses of insects, such as silkworms (Bombyx mori L.), date back to at least 3000 years in China. At the same time, honeybees (Apis mellifera L.) were first recorded during the Xizho Dynasty (about 1100–771 B.C.). Tao Hongjing's “Mingyi Bielu” (Southern and Northern Dynasties, 420–589 A.D.) expanded “Shennong Bencaojing” to include information on nine additional species of medicinal insects. In his book “Compendium of Materia Medica” (1587), Li Shizhen listed seventy-three different insects used for medical purposes. As a result, 105 bug species were included in the supplementary volume to the “Compendium of Materia Medica” by Zhao Xuemin (Qing Dynasty, 1616–1911 A.D.). According to Robert James, who quoted the Dioscorides, “grasshoppers in a suffumigation relieve under a dysury (difficult micturition), especially as is incident to the female sex”. When insects are bruised and mixed with sugar, they are used to treat ulcers and also serve as dewormers [16]. In some parts of the world, earwigs were used to treat convulsion by first drying, powdering and mixing it with the urine of hare to treat ear complications [16]. Research has shown that the Maya employed maggots for therapeutic purposes 1000 years ago [17]. The lac bug (Kerria lacca Kerr.) has been used as medicines since the 3rd century [18].
In some parts of Brazil, ants mixed with sugar and added to coffee or juice was useful in treating diseases associated with vision [19]. The therapeutic uses of insects have been evolving since ancient times [20]. For instance, silkworm pupae were only used for one purpose, that was as feed for livestock [21]. However, they have been recently used in modern medicine [22]. Recent advances in entomotherapy include maggot therapy, which involves the selective removal of necrotic tissue from soft tissue wounds with the insertion of life, disinfected blowfly larvae [23]. There have been recent advancements in the use of bees in apitherapy. Melittin, a key peptide found in bee venom, has shown promise in treating inflammation associated with rheumatoid arthritis and multiple sclerosis. Melittin blocks the expression of genes for inflammation, thereby reducing pain. Apitherapy has also provided more insight into its application to treat diseases, like Parkinson's disease by analysing the effects of propolis and royal jelly on the disease [24].
At present, there are about 1000 insects that have been documented to have medicinal properties in different countries worldwide, and includes Africa, India, Japan, Korea, South America, Spain, Tibet and Turkey [25,26]. However, out of the 1000 insects in medicine, about half of them from 70 genera, 63 families and 14 orders have been reported from China. In the Tibetan region of China, eleven insects, including flies, ants, butterflies, cicadas, and four kinds of beetles, such as diving beetles and blister beetles, were identified as insects with medicinal properties [27]. Apart from China, at least 50 different human diseases and conditions had been linked to the use of 50 different insect species from 28 families and 11 orders, have been recorded from India [28].
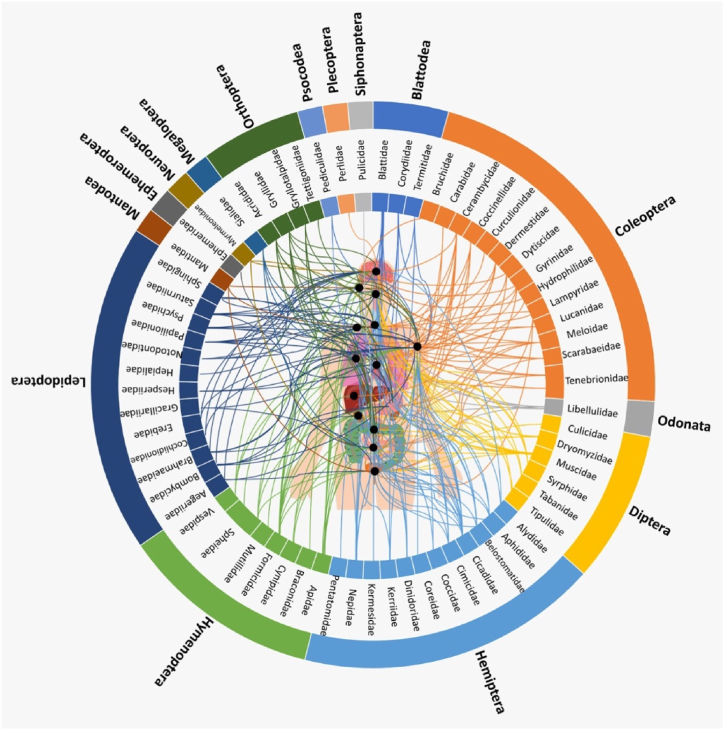
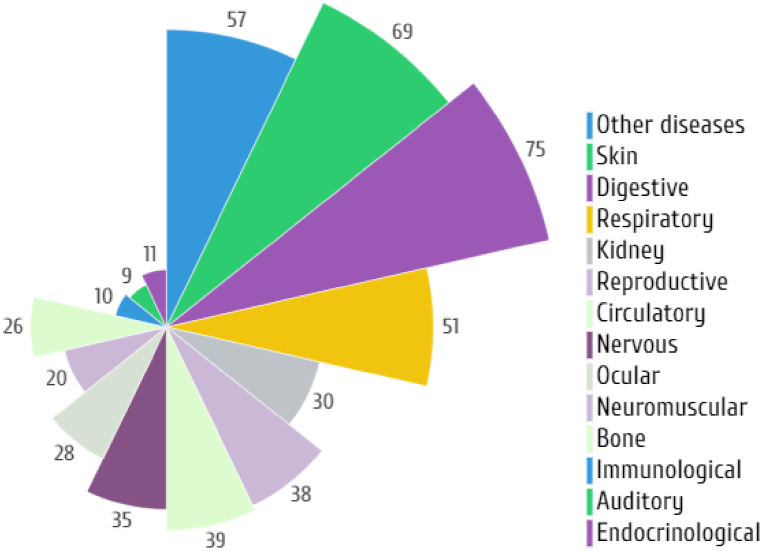
A large number of insect species belonging to different orders, such as Blattodea, Coleoptera, Diptera, Odonata, Hemiptera, Hymenoptera, Lepidoptera, Mantodea, Orthoptera, that have been useful in the treatment of various diseases are presented in Table 1 and illustrated in Fig. 1. It is worth mentioning that most of the identified medicinal insect were used to treat digestive and skin diseases. However, the records of application methods (e.g., oral, external applied) are limited, therefore, more detail in application description is needed in future studies on medicinal insect used by indigenous tribes across the globe. In more detail, the number of insect species used in the treatment of various diseases is shown in Fig. 2.
Table 1.
Various insect species and their records of medicinal uses.
| Order | Family | Scientific name | Therapeutic benefits | Ways of utilization | References |
|---|---|---|---|---|---|
| Blatodea | Blattidae | Periplaneta americana (Linnaeus) | Burning, gastroenteritis, earache, rectal prolapse, shingles, skin, stomach disorders, consitipation, heartburn, colic; whooping cough, boils, dropsy, wart, Bright's disease, ulcers, stimulate lactation, anti-tumor, whooping cough, difficulty urinating, renal colic, and asthma | Oral | [1,12,16,19,[29], [30], [31]] |
| Periplaneta fuliginosa (Serville) | Skin and stomach disorders | Non-specified | [12,16,19,26,31,32] | ||
| Blattella germanica (Linnaeus) | Skin and stomach disorders | Non-specified | [26] | ||
| Blatta orientalis (Linnaeus) | Skin, stomach disorders, tetanus and ear pain, anti-asthmatic, anti-anaphylactic properties dropsy, pleurisy, and pericarditis | Non-specified | [26] | ||
| Corydiidae | Eupolyphaga sinensis (Walker) | Ischemic heart disease, cardiac function; hepatic diseases, gynecopathy, and atherosclerosis and epilepsy | [12,16,19,31] | ||
| Polyphaga plancyi (Bolívar) | Menstrual problems, fracture, amenorrhea | Non-specified | [19] | ||
| Termitidae (termites) | Microcerotermes exiguus (Hagen) | Asthma, bronchitis, influenza, whooping cough, and flu | Non-specified | [[33], [34], [35]] | |
| Nasutitermes corniger (Motschulsky) | Asthma, cough, flu, and sore throat | Non-specified | [34] | ||
| Nasutitermes macrocephalus (Silvestri) | Asthma, Leakage, Bronchitis, ‘catarrh in the chest’ coughs, influenza, sore throat, sinusitis, tonsillitis, and hoarseness | Non-specified | [34] | ||
| Odontotermes feae (Wasmann) | Asthma | Oral | [36] | ||
| Macrotermes bellicosus (Smeathman) | Suture wounds | Non-specified | [37] | ||
| Odontotermes formosanus (Shiraki) | Ulcer, Better health, Body pain, Rheumatics, Anemia and Enhancement of lactation | Non-specified | [33] | ||
| Macrotermes sp. | Sexual impotence, inflammation, dislocation, congenita malformation, headache, vomiting, diarrhea, articular pain, bone pain, sprain, general fatigue, fracture, gonorrhea, and child malnutrition | Topical and Oral | [38,39] | ||
| Pseudacanthotermes spiniger (Sjoestedt) | Fungus and bacterial infection | Non-specified | [39] | ||
| Nasutitermes sp. | Inflammation | Topical | [39] | ||
| Microtermes obesi (Holmgren) | Liver disorder | Oral | [25] | ||
| Trinervitermes sp. | Mumps, burn, fracture, iron deficiency, dropsy, inflammation, edemas, wound and vomiting | Topical | [39] | ||
| Coleoptera | Bruchidae | Pachymerus nucleorum (Fabr.) | Earache | Non-specified | [40] |
| Cicindelidae | Cicindela chinensis (DeGeer) | Skin, tumours and gynaecological problems | Non-specified | [26] | |
| Pheropsophus spp. | Alcoholism | Oral | [29] | ||
| Cerambycidae | Apriona rugicollis (Chevrolat) | Lung problems, cramps, and palsy | Non-specified | [26] | |
| Batocera rubus (Linnaeus) | Analgesic and gastro-intestinal problems, treating malaria, typhoid and aphrodisiac | Oral | [28] | ||
| Batocera parryi (Hope) | Analgesic and gastro-intestinal problems, treating malaria, typhoid, and aphrodisiac | Oral | [28] | ||
| Batocera rufomaculata (De Geer) | Analgesic and gastro-intestinal problems, treating malaria, typhoid, and aphrodisiac | Oral | [28] | ||
| Chloridolum thaliodes (Bates) | Treating smallpox | Non-specified | [26] | ||
| Batocera lineolata (Chevrolat) | Mitigate cramps, cancer therapy and diphtheria, smallpox | Non-specified | [26] | ||
| Orthosoma brunneum (Forster) | Analgesic and gastro-intestinal problems, treating malaria, typhoid, and aphrodisiac | Oral | [28] | ||
| Aromia moschata (Linnaeus) | Vesicatory and acted like cantharides | Non-specified | [16] | ||
| Coccinellidae | Coccinella septempunctata (Linnaeus) | Wound | Non-specified | [39] | |
| Curculionidae | Larinus maculatus (Gyllenhal) | Respiratory organs | Non-specified | [16] | |
| Brachycerus ornatus (Drury) | Stomach pains | Non-specified | [8,41] | ||
| Dermestidae | Ips typographus (Linnaeus) | Vesicators and opening abscesses | Non-specified | [16] | |
| Dytiscidae | Cybister brevis (Aubé) | Asthma, respiratory and stomach problems | Non-specified | [26] | |
| Cybister chinensis (Motschulsky) | Asthma, respiratory and stomach problems | Non-specified | [26] | ||
| Cybister tripunctatus (Olivier) | Asthma, respiratory and stomach problems | Non-specified | [26] | ||
| Rhantus pulverosus (Stephens) | Skin disorders | Non-specified | [26] | ||
| Gyrinidae | Gyrinus curtus (Motschulsky) | Lung and stomach problems, fever, and cramps | Non-specified | [26] | |
| Gyrinus japonicus (Sharp) | Lung and stomach problems, fever, and cramps | Non-specified | [26] | ||
| Dineutus marginatus (Sharp) | Lung and stomach problems, fever, and cramps | Non-specified | [26] | ||
| Hydrophilidae | Sternolophus rufipes (Fabricius) | Skin disorders, cramps, and whooping cough | Non-specified | [26] | |
| Hydrophilus affinis (Thunberg) | Skin disorders, cramps, and whooping cough | Non-specified | [26] | ||
| Hydrophilus acuminatus (Motschulsky) | Skin disorders, cramps, and whooping cough | Non-specified | [26] | ||
| Lampyridae | Lampyridae spp. | Cancer | Oral | [29] | |
| Aquatica lateralis (Motschulsky) | Bleedings, tumours, whooping cough, haemorrhoids, and as hair tonic | Non-specified | [26] | ||
| Lucanidae | Lucanus macrifemoratus (Motschulsky) | Treatments of gynaecological problems | Non-specified | [26] | |
| Prosopocoilus inclinatus (Motschulsky) | Treatments of gynaecological problems | Non-specified | [26] | ||
| Meloidae | Epicauta gorhami (Marseul) | Treatments of hair, skin excretory (kidney) system, rabies and warts | Non-specified | [25] | |
| Mylabris pustulata (Latreille) | Dog bite and Hydrophobia | Non-specified | [26] | ||
| Mylabris sp. | Blisters and warts | Topical | [25,28] | ||
| Lytta vesicatoria (Linnaeus) | Urinary disorders and aphrodisiac | Non-specified | [8,42] | ||
| Berberomeloe majalis (Linnaeus) | Warts | Non-specified | [16] | ||
| Pseudomeloe andensis Guérin-Méneville | Warts | Non-specified | [16] | ||
| Palembus dermestoides (Farmaire) | Sexual impotence, ophthalmological problems, rheumatism, and weakness | Non-specified | [8,43] | ||
| Lytta sp. | Sickle cell anemia | Oral | [39] | ||
| Scarabaeidae | Melolontha vulgaris (Fabricius) | Scratches, anemia, and rheumatism | Non-specified | [8,12] | |
| Scarabaeus laticollis (Linnaeus) | Painful urination | Non-specified | [39] | ||
| Propomacrus sp. | Cough | Non-specified | [1] | ||
| Strategus aloeus (Linnaeus) | Aphrodisiac | Non-specified | [8] | ||
| Megasoma actaeon (Linnaeus) | Aphrodisiac | Non-specified | [8] | ||
| Tenebrionidae | Alphitobius diaperinus (Panzer) | Diabetes and obesity | Non-specified | [44,45] | |
| Palembus dermestoides (Farmaire) | Asthma, arthritis, tuberculosis and sexual impotence | Non-specified | [46] | ||
| Blaps sulcata (Laporte de Castelnau) | Scorpion bites | Non-specified | [16] | ||
| Tenebrionidae | Tenebrio molitor (Linnaeus) | Anti-inflammatory (stroke) | Non-specified | [47] | |
| Odonata | Libellulidae | Sympetrum darwinianum (Selys) | Throat aches, asthma, tumours, fever, and whooping cough | Non-specified | [26] |
| Sympetrum pedemontanum (Müller in Allioni) | Asthma | Non-specified | [26] | ||
| Sympetrum croceolum (Selys) | Asthma | Non-specified | [26] | ||
| Sympetrum frequens (Sélys) | Asthma | Non-specified | [26] | ||
| Orthetrum albistylum (Selys) | Asthma | Non-specified | [26] | ||
| Crocothemis servilia (Drury) | Ear, eye, throat and gut problems, fever, diphtheria, and cough | Non-specified | [26] | ||
| Diptera | Culicidae | Culex pipiens (Linnaeus) | Venereal diseases | Non-specified | [26] |
| Aedes japonicus (Theobald) | Venereal diseases | Non-specified | [26] | ||
| Aedes albopictus (Skuse) | Venereal diseases | Non-specified | [26] | ||
| Anopheles japonicus Coluzzi | Venereal diseases | Non-specified | [26] | ||
| Dryomyzidae | Dryomyza formosa Wiedemann | Fever, snake bite, gut and stomach problems and vision | Non-specified | [26] | |
| Muscidae | Fannia canicularis (Linnaeus) | Snake bites, fever, gut and stomach problems, vision, tooth ache, skin disorders and haemorrhoids | Non-specified | [26] | |
| Musca domestica (Linnaeus) | Sickle cell anaemia, male infertility, eye cysts, baldness, scorpion and snake bites, fever, gut and stomach problems, vision Fever, tooth ache, skin disorders and haemorrhoids | Oral | [26,39] | ||
| Muscina stabulans (Fallen) | Snake bites, fever, gut and stomach problems and vision tooth ache, skin disorders and haemorrhoids | Non-specified | [26] | ||
| Fannia canicularis (Linnaeus) | Snake bites, fever, gut and stomach problems and vision tooth ache, skin disorders and haemorrhoids | Non-specified | [16,26,39] | ||
| Calliphora lata (Coquillett) | Snake bites, fever, gut and stomach problems, vision, tooth ache and skin disorders and venereal diseases | Non-specified | [26] | ||
| Syrphidae | Eristalis tenax (Linnaeus) | Vision, tooth ache, fever, and cramps | Non-specified | [26] | |
| Tabanidae | Tabanus trigonus Coquillett | Vision and tumours | Non-specified | [26] | |
| Tabanus rufidens (Bigot) | Vision and tumours | Non-specified | [26] | ||
| Tabanus chrysurus (Loew) | Vision and tumours | Non-specified | [26] | ||
| Tabanus mandarinus (Schiner) | Vision and tumours | Non-specified | [26] | ||
| Tipulidae | Tipula oleracea (Linnaeus) | Analgesic and measles in children | Oral | [28] | |
| Hemiptera | Alydidae | Leptocorisa varicornis (Fabricius) | Fever | Oral | [29] |
| Aphididae | Schlechtendalia chinensis (Bell) | Eggs used in connection with bleedings, intestinal and uterine problems; adults in connection with cough, dysentery, and haemorrhoids | Non-specified | [26] | |
| Belostomatidae | Lethocerus deyrollei (Vuillefroy) | Eggs used in connection with bleedings, intestinal and uterine problems; adults in connection with cough, dysentery, and haemorrhoids | Non-specified | [26] | |
| Lethocerus indicus (Lepeletier and Serville) | Nocturnal emission, gastro-intestinal problems, rheumatoid arthritis, and wound healing | Oral | [1,28] | ||
| Cicadidae | Terpnosia vacua (Kato) | Anaemia; ear problems, tooth ache, fever as well as kidney problems, tumours, smallpox, coughs, and haemorrhoids; Migraine headache and ear infection | Non-specified | [26] | |
| Platypleura kaempferi (Fabricius) | Anaemia; ear problems, tooth ache, fever as well as kidney problems, tumours, smallpox, coughs, and haemorrhoids | Non-specified | [26] | ||
| Graptopsaltria nigrofuscata (Motschulsky) | Anaemia; ear problems, tooth ache, fever as well as kidney problems, tumours, smallpox, coughs, and haemorrhoids | Non-specified | [26] | ||
| Cryptotympana japonensis (Kato) | Anaemia; ear problems, tooth ache, fever as well as kidney problems, tumours, smallpox, coughs, and haemorrhoids | Non-specified | [26] | ||
| Huechys sanguinea (DeGeer) | Migraine headaches and ear infections | Non-specified | [8] | ||
| Tanna japonensis (Distant) | Anaemia; ear problems, tooth ache, fever as well as kidney problems, tumours, smallpox, coughs, and haemorrhoids | Non-specified | [26] | ||
| Hyalessa maculaticollis (Motschulsky) | Anaemia; ear problems, tooth ache, fever as well as kidney problems, tumours, smallpox, coughs, and haemorrhoids | Non-specified | [26] | ||
| Meimuna opalifera (Walker) | Anaemia; ear problems, tooth ache, fever as well as kidney problems, tumours, smallpox, coughs, and haemorrhoids | Non-specified | [26] | ||
| Cimicidae | Cimex lectularius Linnaeus | Venom of snakes, lethargy, urinary problems, eyes, ears, hysterical suffocation, worms, and epileptic attacks | Non-specified | [16] | |
| Coccidae | Ericerus pela (Chavannes) | Bleedings, lung and stomach problems and warts | Non-specified | [26] | |
| Coreidae | Thasus gigas (Klug) | Diabetes | Oral | [28,48] | |
| Dinidoridae | Coridius singhalanus (Distant) | Fever, treating jaundice, malaria and to increase milk production. | Non-specified | [45] | |
| Kerriidae | Kerria lacca (Kerr) | Diarrhea, indigestion, measles, macula, and scabies | Non-specified | [6,49] | |
| Kermesidae | Kermes ilicis (Linnaeus) | To prevent abortion from strain and injury, and menstrual problems | Non-specified | [16] | |
| Nepidae | Laccotrephes japonensis (Scott) | Eggs used in connection with bleedings, intestinal and uterine problems; adults in connection with cough, dysentery and haemorrhoids | Non-specified | [26,50] | |
| Ranatra chinensis (Mayr) | Eggs used in connection with bleedings, intestinal and uterine problems; adults in connection with cough, dysentery, and haemorrhoids | Non-specified | [26] | ||
| Ranatra unicolor (Scott) | Eggs used in connection with bleedings, intestinal and uterine problems; adults in connection with cough, dysentery, and haemorrhoids | Non-specified | [26] | ||
| Laccotrephes ruber (Linnaeus) | Cardiovascular (blood purification) | Oral | [28] | ||
| Pentatomidae | Udonga montana (Distant) | Pain | Oral | [28] | |
| Hymenoptera | Apidae | Apis cerana indica (Fabricius) | Cough, fever, cancer, cracks, diabetes and scars, cold, sore throat, burns, tongue ulcer, gastritis, and wart | Oral | [1,6,25,29,51,52] |
| Apis cerana japonica (Radoszkowski) | Skin, respiratory, urinary, and intestinal disorders, snake bite and rabies; skin and digestive problems and snake bite: Larvae and adults in connection with rheumatism, influenza, the common cold and whooping cough; wax for freckles and constipation | Oral | [26] | ||
| Apis dorsata Fabricius | Cracks and scars, skin, respiratory, urinary, and intestinal disorders, snake bite and rabies, skin and digestive problems, rheumatism, influenza, common cold, whooping cough; wax for freckles and constipation, cold, cough and sore throat, burns, tongue ulcer, gastritis, anti-inflammatory, anti-nociceptive, and anti-arthritic properties. | Oral | [6,25,26,51] | ||
| Apis florea (Fabricius) | Respiratory problems (coughs), cold, sore throat, burns, tongue ulcer, gastritis, and wart | on-specified | [28,51] | ||
| Apis laboriosa (Smith) | Respiratory problems (coughs) | on-specified | [28] | ||
| Apis mellifera (Linnaeus) | Throat pain, irregular menstruation, cough, cold, general fatigue, sickle cell anemia, and burns and cuts, menopausal problems, Intestinal, helminthiasis, strangulated hernia, sexual impotence, insomnia, memory losss, heart diseases, difficulty breathing, voice extinction, pneumonia, bladder lithiasis, diabetes, constipation, hemorrhage in women, nausea, burns, pyrosis, toxin, stomach aches, foot pain, gonorrhea, ulcer, itching, anal bleeding, amenorrhea and infertility | on-specified | [8,25,53,54] | ||
| Lepidotrigona arcifera (Cockerell) | Gynaecological/andrological problems, and venomous animal bites | on-specified | [28,51] | ||
| Lophotrigona canifrons (Smith) | Gynaecological/andrological problems, and venomous animal bites | on-specified | [28,51] | ||
| Melipona indecisa (Cockerell) | Sour throat | on-specified | [55] | ||
| Melipona mimetica (Cockerell) | Balm, blood kidney, eyes, inflammation and sour | on-specified | [55] | ||
| Melipona scutellaris (Latreille) | Cough | on-specified | [8] | ||
| Nannotrigona perilampoides (Cresson) | Eye | on-specified | [55] | ||
| Paratrigona eutaeniata Camargo et Moure | Eyes | on-specified | [55] | ||
| Scaptotrigona ederi Engel | Balm, kidney, eyes, inflammation sour throat, tumor, wound healing | on-specified | [55] | ||
| Trigona spinipes (Fabricius) | Cough | on-specified | [8] | ||
| Xylocopa appendiculata (Smith) | Fever, respiratory/lung ailments, and haemorrhoids | on-specified | [26] | ||
| Braconidae | Euurobracon penetrator (Smith) | Cases of cramp | on-specified | [26] | |
| Cynipidae | Diplolepis rosae (Linnaeus) | Diarrhea and dysentery, and for scurvy, stone and worms | on-specified | [16] | |
| Formicidae | Pogonomyrmex californicus (Buckley) | Panacea | Oral | [56] | |
| Tetraponera rufonigera (Jordan) | Body pain | Oral | [29] | ||
| Oecophylla smaragdina (Fabricius) | Coughs, fever, gastritis, malaria, typhoid, edema, sinus infections, analgesic, common cold, Jaundice, enteric problems, whooping hungriness, cancer and nose bleeding, malaria, throat pain, breathing problem, asthma, boils/pox, measles, for the treatment of detoxification blood, arresting hemorrhage during miscarriages, restoration of uterus, removal of any aftermath from the uterine canal after childbirth, stimulating pulse and heartbeat, and dizziness | Oral | [6,25,28,29,51,[57], [58], [59], [60]] | ||
| Myrmicaria brunnea (Saunders) | Body ache | Oral | [29] | ||
| Pseudoneoponera rufipes (Jerdon) | Scabies, toothache, wounds, high blood pressure and malaria | Non-specified | [25] | ||
| Polyrhachis dives Smith | Rheumatoid, osteoarthritis, inflammatory diseases, and central nervous system | Oral | [61] | ||
| Camponotus maculatus (Fabricius) | Azoospermia | Oral | [39] | ||
| Pseudoneoponera rufipes (Jerdon) | Toothaches and blood pressure | Non-specified | [6] | ||
| Tetramorium sp | Anti-bacterial properties, sprain, Inflammation, cyst, hip pain, headache, neurological problems, retention of acute urinary, gynecological problems, and chronic cough | Topical and Oral | [39] | ||
| Camponotus sp. | Foot pain and retention of acute urinary | Topical and Oral | [39] | ||
| Pachycondyla sp. | Knee pain, headache, stomach aches, neurological problems, retention of acute urinary and toxin | Topical and Oral | [39] | ||
| Mutillidae | Dasymutilla ocidentalis (Linnaeus) | Chickenpox | Non-specified | [62] | |
| Sphecidae | Sceliphron sp. | Inflammation, vomiting, allergy due to stings, sprain, hiccups, female infertility, lipoma, soa throat, hip pain, foot pain, mumps, cough, fontanel problem, vomiting and migraine | Non-specified | [25] | |
| Vespidae | Vespula vulgaris (Linnaeus) | Lipoma, heart diseases and whitlow | Topical and Oral | [39] | |
| Polistes carolina (Linnaeus) | Piles and general wound | Non-specified | [25] | ||
| Vespa affinis (Linnaeus) | Cancer | Oral | [29] | ||
| Vespa mandarinia (Smith) | Skin diseases, fever respiratory problems, whooping cough, ear, eye and dental problems, skin disorders and cramps | Non-specified | [26] | ||
| Vespa auraria (Smith) | Skin diseases, fever, respiratory problems, whooping cough, ear, eye and dental problems, cramps, and haemorrhoids | Non-specified | [26] | ||
| Lepidoptera | Aegeriidae | Paranthrene regalis (Butler) | Stomach upsets, cramps, gynaecological issues, and diphtheria | Non-specified | [26] |
| Bombycidae | Bombyx mori (Linnaeus) | Pneumonia, stopping bleedings, throat troubles, fever, and snake bite Pupae used in connection with throat problems, tuberculosis, kidney problems, bleedings, counter snake bite, vertigo and convulsions and fever |
Non-specified | [16,25,26] | |
| Brahmaeidae | Brahmaea japonica (Butler) | Cramps, respiratory, anemia and stomach troubles | Non-specified | [26] | |
| Cochlidionidae | Cnidocampa flavescens (Walker) | Cramps, vision | Non-specified | [26] | |
| Erebidae | Euproctis chrysorrhoea (Linnaeus) | homeopathic tinctures | Non-specified | [16] | |
| Spilosoma obliqua (Walker) | Dog bites | Non-specified | [6] | ||
| Gracillariidae | Stomphastis thraustica (Meyrick) | Fever and to increase milk flow in lactating women | Non-specified | [6,63] | |
| Hesperiidae | Erionota torus (Evans) | Sexual weakness and venomous animal bites | Oral | [28] | |
| Hepialidae | Endoclita excrescens (Butler) | Lung and stomach troubles and snake bite | Non-specified | [26] | |
| Notodontidae | Bombyx processionea (Linnaeus) | Homeopathic tinctures | Non-specified | [16] | |
| Papilionidae | Pachliopta aristolochiae (Fabricius) | Snake bite | Non-specified | [25] | |
| Holocerus vicarious Karsch | Fever and cramps | Non-specified | [26] | ||
| Papilio xuthus (Linnaeus) | Fever and cramps, skin disorders, lumps, and tumours | Non-specified | [26] | ||
| Papilio machaon (Linnaeus) | Fever and cramps, skin disorders, lumps, and tumours | Non-specified | [26] | ||
| Papilio protenor (Cramer) | Fever and cramps, skin disorders, lumps, and tumours | Non-specified | [26] | ||
| Papilio macilentus (Janson) | Fever and cramps, skin disorders, lumps, and tumours | Non-specified | [26] | ||
| Byasa alcinous (Klug) | Fever and cramps, skin disorders, lumps, and tumours | Non-specified | [26] | ||
| Graphium sarpedon nipponus (Fruhstorfer) | Fever and cramps, skin disorders, lumps, and tumours | Non-specified | [26] | ||
| Psychidae | Cryptothelea minuscula (Butler) | Toothache and respiratory problems | Non-specified | [26] | |
| Oiketicus kirbyi (Guilding) | Asthma, earache, and hemorrhage | Non-specified | [6] | ||
| Saturniidae | Antheraea yamamai (Guérin-Méneville) | Asthma, cramps, throat and skin troubles, lumps, and cramps | Non-specified | [26] | |
| Antheraea pernyi (Guérin-Méneville) | Tumor growths and lumps | Non-specified | [26] | ||
| Samia cynthia (Drury) | Analgesic, blood pressure and diabetes | Non-specified | [25] | ||
| Caligula japonica (Moore) | Skin problems | Non-specified | [26] | ||
| Cirina butyrospermi (Vuillet) | Asthma, arteria, hypertension, avitaminosis, abdominal bloating, diabetes, and tetanus | Oral | [39] | ||
| Rhodinia fugax (Butler) | Whooping cough | Non-specified | [26] | ||
| Sphingidae | Deilephila elpenor (Linnaeus) | Tuberculosis, stomach upsets, lumps, tumours and fever | Non-specified | [26] | |
| Agrius convolvuli (Linnaeus) | Tuberculosis, stomach upsets, lumps, tumours and fever | Non-specified | [26] | ||
| Psilogramma increta (Walker) | Tuberculosis, stomach upsets, lumps, tumours and fever | Non-specified | [26] | ||
| Theretra nessus (Drury) | Tuberculosis, stomach upsets, lumps, tumours and fever | Non-specified | [26] | ||
| Theretra oldenlandiae (Fabricius) | Tuberculosis, stomach upsets, umps, tumours and fever | Non-specified | [26] | ||
| Macroglossum stellatarum (Linnaeus) | Tuberculosis, stomach upsets, umps, tumours and fever | Non-specified | [26] | ||
| Mantodea | Mantidae | Hierodula coarctata (Saussure) | Urological problems (enuresis) | Oral | [28] |
| Mantis religiosa (Linnaeus) | Otorrhoea, fever, beriberi, tooth ache, fever, hair, and respiratory problems | Topical | [26,29] | ||
| Tenodera sinensis (Saussure) | Otorrhoea, fever, beriberi, tooth ache, warts, fever, hair and respiratory problems | Masticate on warts | [26,28] | ||
| Tenodera angustipennis (Saussure) | Otorrhoea, fever, beriberi, tooth ache, fever, hair, and respiratory problems | Non-specified | [26] | ||
| Statilia maculata (Thunberg) | Otorrhoea, fever, beriberi, tooth ache, fever, hair, and respiratory problems | Non-specified | [26] | ||
| Hierodula patellifera (Serville) | Otorrhoea, fever, beriberi, tooth ache, fever, hair, and respiratory problems | Non-specified | [26] | ||
| Ephemeroptera | Ephemeridae | Ephemera danica Müller | Stomach disturbance | Non-specified | [26] |
| Neuroptera | Myrmeleonidae | Hagenomyia micans (McLachlan) | Fever, migraine/headaches, beriberi, gonorrhea, and whooping cough | Non-specified | [26] |
| Megaloptera | Sialidae | Protohermes grandis (Thunberg) | Lung, stomach, and gut problems | Non-specified | [26] |
| Orthoptera | Acrididae | Oxya sp. | Nocturnal emission | Oral | [1] |
| Oxya velox (Fabricius) | Adults used in cases of fever, respiratory, skin, and gynaecological problems, effective in treating cancer, haemorrhoids and anaemia | Non-specified | [26] | ||
| Oxya vicina Wattenwyl. | Fever, respiratory, skin, gynaecological problems, cancer, haemorrhoids and anaemia | Non-specified | [26] | ||
| Acrida bicolor (Thunber) | Hypertention | Non-specified | [8,38] | ||
| Hieroglyphus banian (Fabricius) | Dog bite | Non-specified | [25] | ||
| Locusta migratoria (Linnaeus) | Effective antidote to scorpion bites, piles, and thirst | Non-specified | [16] | ||
| Schistocerca gregaria (Forsskål) | Wound | Topical | [39] | ||
| Melanoplus sp. | Gastrointestinal problems | Oral | [28] | ||
| Gryllidae | Tarbinskiellus portentosus (Lichtenstein) | Malaria, headaches, and gastro-intestinal problems | Oral | [28] | |
| Gryllus assimilis (Fabricius) | Urine retention | Non-specified | [8,62] | ||
| Acheta domesticus (Linnaeus) | Pain, deafness, eyesight, and pancreas health | Oral | [29,39] | ||
| Gryllotalpidae | Scapteriscus borellii (Giglio-Tos) | Intestinal worms | Oral | [29] | |
| Gryllotalpa africana (Palisot de Beauvois) | Fever, mitigate skin and kidney troubles, fight tumor growths and venereal disease | Non-specified | [26] | ||
| Tettigoniidae | Tettigonia verrucivora (Kirby) | Warts | Non-specified | [16] | |
| Psocodea | Pediculidae | Pediculus humanus (Linnaeus) | Jaundice, venereal diseases | Non-specified | [26,64] |
| Plecoptera | Perlidae | Perla tinctipennis (McLachlan) | Cramps | Non-specified | [28] |
| Perla tibialis (Pictet) | Cramps | Non-specified | [28] | ||
| Siphonaptera | Pulicidae | Pulex irritans (Linnaeus) | Venereal diseases | Non-specified | [26] |
| Ctenocephalides canis (Curtis, 1826) | Venereal diseases | Non-specified | [26] | ||
| Ctenocephalides felis (Bouché, 1835) | Venereal diseases | Non-specified | [26] |
Fig. 1.
The use of various types of insects for medicinal purposes.
Fig. 2.
Number of insect species to alleviate diseases.
3. Effects and consequences of using medicinal insects
3.1. Insect species used for medicinal purposes and their associated stage being used
In total, 235 valid species were documented in several literatures that summarized insects used in folk medicine, which include insects from China [65,66], India [28], Africa [39], and Latin America [63]. Table 2 listed all the 235 species from 15 different orders, within which Hymenoptera contains the largest medicinal insect species count (62 species), followed by Coleoptera (47), Orthoptera (28), Lepidoptera (23), and Blattodea (21). The other orders contain much less (e.g., ≤11) species, which sum up to 55. At the family level, Apidae (27) contains the largest medicinal insect species documented, followed by Vespidae (19), Formicidae (15), Gryllidae (11), Cerambycidae (10), Meloidae (9), Termitidae (9), Acrididae (8), Libellulidae (8), Cicadidae (8), and Mantidae (7), which sum up to 50% of the 235 species documented. Some genera contain more than one medicinal insect species. For example, seven species were reported in genus Melipona, seven species were reported in genus Vespa, and another seven species were reported in genus Apis.
Table 2.
Insect species recorded in folk medicine and the stage and ingredient used.
| Order | Family | Species | Stage or ingredient used | Reference |
|---|---|---|---|---|
| Blattodea | Blaberidae | Epilampra sp. | Nymph/Adults | [28] |
| Opisthoplatia orientalis (Burmeister) | Nymph/Adults | [66] | ||
| Rhyparobia maderae (Fabricius) | Non-specified | [63] | ||
| Blattidae | Blatta orientalis L. | Non-specified | [66] | |
| Blattella germanica L. | Nymphs/Adults | [66] | ||
| Eurycotis manni Rehn | Nymph | [63] | ||
| Periplaneta americana L. | Adults | [39,63,66] | ||
| Periplaneta australasiae (Fabricius) | Non-specified | [66] | ||
| Corydiidae | Eupolyphaga sinensis (Walker) | Nymph/Adults | [66] | |
| Eupolyphaga yunnanesis (Chopard) | Nymph/Adults | [66] | ||
| Rhinotermitidae | Coptotermes formosanus Shiraki | Adults | [66] | |
| Reticulitermes flaviceps Oshima | Adults | [66] | ||
| Termitidae | Macrotermes annandalei (Silvestri) | Larvae/Adults | [66] | |
| Macrotermes barneyi Light | Non-specified | [65,66] | ||
| Macrotermes sp. | Adults/Nest | [28,39] | ||
| Microcerotermes exiguus (Hagen) | Non-specified | [63] | ||
| Nasutitermes corniger (Motschulsky) | Non-specified | [63] | ||
| Nasutitermes macrocephalus (Silvestri) | Non-specified | [63] | ||
| Nasutitermes sp. | Nest | [39] | ||
| Odontotermes formosanus (Shiraki) | Non-specified | [66] | ||
| Trinervitermes sp. | Adults, Nest | [39] | ||
| Coleoptera | Buprestidae | Chalcophora japonica Gory | Adults | [65] |
| Carabidae | Pheropsophus jessoensis (A.Morawitz) | Non-specified | [65,66] | |
| Cerambycidae | Anoplophora chinensis (Forster) | Larvae/Adults | [66] | |
| Anoplophora glabripennis (Motschulsky) | Larvae | [65] | ||
| Apriona germari Hope | Larvae/Adults | [65,66] | ||
| Aromia bungii (Faldermann) | Larvae | [65] | ||
| Batocera horsfieldi (Hope) | Adults | [66] | ||
| Batocera parryi Hope | Larvae | [28] | ||
| Batocera rubus L. | Larvae | [28] | ||
| Batocera rufomaculata (De Geer) | Larvae | [28] | ||
| Macrodontia cervicornis (L.) | Non-specified | [63] | ||
| Orthosoma brunneum (Forster) | Larvae | [63] | ||
| Chrysomelidae | Coraliomela brunnea (Thunberg) | Non-specified | [63] | |
| Pachymerus nucleorum (Fabricius) | Non-specified | [63] | ||
| Coccinellidae | Coccinella septempunctata L. | Adults | [39] | |
| Curculionidae | Rhina barbirostris Champion | Non-specified | [63] | |
| Rhinostomus barbirostris (Fabricius) | Non-specified | [63] | ||
| Rhynchophorus palmarum L. | Non-specified | [63] | ||
| Dytiscidae | Cybister japonicus Sharp | Adults | [65] | |
| Cybister limbatus (Fabricius) | Adults | [28] | ||
| Cybister tripunctatus lateralis (Fabricius) | Adults | [28] | ||
| Elateridae | Pleonomus canaliculatus (Faldermann) | Non-specified | [66] | |
| Gyrinidae | Gyrinus curtus Motschulsky | Non-specified | [66] | |
| Hydrophilidae | Hydrophilus caschmirensis Redtenbacher | Adults | [28] | |
| Lampyridae | Luciola ficta Olivier | Larvae/Adults | [66] | |
| Meloidae | Epicauta hirticornis Haag-Rutenberg | Non-specified | [66] | |
| Lytta caraganae (Pallas) | Non-specified | [66] | ||
| Lytta sp. | Adults | [39] | ||
| Meloe coarctatus Motschulsky | Non-specified | [66] | ||
| Mylabris cichorii L. | Adults | [66] | ||
| Mylabris phalerata (Pallas) | Non-specified | [66] | ||
| Mylabris sp. | Adults | [28] | ||
| Palembus dermestoides (Fairmaire) | Non-specified | [63] | ||
| Pseudomeloe andensis (Guérin-Méneville) | Non-specified | [63] | ||
| Melolonthidae | Holotrichia diomphalia Bates | Larvae, Adults | [65,66] | |
| Holotrichia morosa Waterhouse | Larvae/Adults | [66] | ||
| Holotrichia oblita (Faldermann) | Larvae/Adults | [66] | ||
| Polyphylla gracilicornis (Blanchard) | Adults | [65] | ||
| Rutelidae | Anomala corpulenta Motschulsky | Larvae/Adults | [66] | |
| Scarabaeidae | Allomyrina dichotoma L. | Larvae | [65,66] | |
| Potosia (Liocola) brevitarsis (Lewis) | Larvae | [65] | ||
| Potosia (Liocola) brevitarsis (Lewis) | Larvae/Adults | [66] | ||
| Scarabaeus laticollis L. | Rolled dung | [39] | ||
| Geotrupes laevistriatus Motschulsky | Adults | [66] | ||
| Geotrupes substriatellus L. | Adults | [66] | ||
| Tenebrionidae | Martianus dermestoides (Chevrolat) | Non-specified | [66] | |
| Tenebrio molitor L. | Chitin | [65,66] | ||
| Dermaptera | Forficulidae | Forficula auricularia L. | Non-specified | [63] |
| Diptera | Calliphoridae | Chrysomyia megacephala (Fabricius) | Larvae | [66] |
| Muscidae | Musca domestica L. | Larvae/Adults | [39,63] | |
| Tabanidae | Tabanus mandarinus Schiner | Larvae/Adults | [66] | |
| Tachinidae | Musca domestica vicina L. | Larvae/Adults | [65] | |
| Tipulidae | Tipula sp. | Larvae | [28] | |
| Hemiptera | Aetalionidae | Darthula hardwickii (Gray) | Nymph | [28] |
| Belostomatidae | Lethocerus indicus Lepeletier and Serville | Adults | [28,66] | |
| Cicadidae | Cicada flammata Distant | Cicada periostracum (exuviae) | [66] | |
| Cryptotympana atrata (Fabricius) | Adults/Cicada periostracum (Exuviae) | [65,66] | ||
| Cryptotympana mandarina Distant | Cicada periostracum (exuviae) | [66] | ||
| Huechys philamata (Fabricius) | Non-specified | [66] | ||
| Huechys sanguinea (DeGeer) | Non-specified | [66] | ||
| Oncotympana maculaticollis (Motschulsky) | Non-specified | [65] | ||
| Oncotympana maculaticollis (Motschulsky) | Cicada periostracum (exuviae) | [66] | ||
| Platypleura kaempferi (Fabricius) | Non-specified | [65] | ||
| Ericerus pela (Chavannes) | Wax produced by male | [66] | ||
| Notobitus meleagris (Fabricius) | Adults | [28] | ||
| Dinidoridae | Aspongopus nepalensis Westwood | Adults | [28] | |
| Coridius singhalanus Distant | Non-specified | [28] | ||
| Cyclopelta parva Distant | Adults | [66] | ||
| Fulgoridae | Lycorma delicatula (White) | Adults | [65,66] | |
| Gerridae | Rhagadotarsus kraepelini Breddin | Non-specified | [66] | |
| Nepidae | Laccotrephes ruber L. | Adults | [28] | |
| Pentatomidae | Aspongopus chinensis Dallas | Adults | [65,66] | |
| Udonga montana Distant | Adults | [28] | ||
| Nezara viridula smaragdula (Fabricius) | Non-specified | [65] | ||
| Tessaratomidae | Tessaratoma papilllosa (Drury) | Adults | [66] | |
| Tessaratoma quadrata Distant | Adults | [66] | ||
| Hymenoptera | Apidae | Apis andreniformis Smith | Non-specified | [66] |
| Apis cerana Fabricius | Larvae/Bee venom/Bee wax/Honey/Royal jelly/Bee pollen | [65,66] | ||
| Apis cerana indica Fabricius | Larvae/Pupae/Cocoon/Adults/Bee comb/Bee wax/Honey | [28] | ||
| Apis dorsata Fabricius | Larvae/Pupae/Cocoon/Adults/Bee comb/Bee wax/Honey/Pollen | [28,66] | ||
| Apis florea Fabricius | Larvae/Pupae/Cocoon/Honey/Bee comb | [28,66] | ||
| Apis laboriosa Smith | Larvae/Pupae/Cocoon/Honey/Bee comb/Pollen | [28] | ||
| Apis mellifera L. | Larvae/Adults/Bee venom/Bee wax/Honey/Royal jelly/Bee pollen/Propolis | [39,63,65,66] | ||
| Cephalotrigona capitata (Smith) | Non-specified | [63] | ||
| Frieseomelitta silvestrii (Friese) | Non-specified | [63] | ||
| Frieseomelitta varia (Lepeletier) | Non-specified | [63] | ||
| Lepidotrigona arcifera (Cockerell) | Honey/Nest | [28] | ||
| Lestrimelitta limao (Smith) | Non-specified | [63] | ||
| Melipona asilvai Moure | Non-specified | [63] | ||
| Melipona compressipes (Fabricius) | Non-specified | [63] | ||
| Melipona mandacaia Smith | Non-specified | [63] | ||
| Melipona marginata Lepeletier | Non-specified | [63] | ||
| Melipona quadrifasciata Lepeletier | Non-specified | [63] | ||
| Melipona scutellaris Latreille | Non-specified | [63] | ||
| Melipona subnitida Ducke | Non-specified | [63] | ||
| Partamona Cupira (Smith) | Non-specified | [63] | ||
| Platynopoda magnifica Cockerell | Non-specified | [66] | ||
| Plebeia emerina (Friese) | Non-specified | [63] | ||
| Tetragonisca angustula (Latreille) | Non-specified | [63] | ||
| Trigona mosquito Smith | Non-specified | [63] | ||
| Trigona spinipes (Fabricius) | Non-specified | [63] | ||
| Xylocopa appendiculata Smith | Adults | [65,66] | ||
| Xylocopa sinensis Smith | Non-specified | [66] | ||
| Formicidae | Acromyrmex landolti (Forel) | Non-specified | [63] | |
| Atta cephalotes L. | Non-specified | [63] | ||
| Atta serdens L. | Non-specified | [63] | ||
| Camponotus japonicus Mayr | Non-specified | [66] | ||
| Camponotus maculatus (Fabricius) | Adults | [39] | ||
| Camponotus sp. | Adults/Nest | [39] | ||
| Campsomeris annulata Fabricius | Non-specified | [66] | ||
| Dinoponera quadriceps Kempf | Non-specified | [63] | ||
| Formica fusca L. | Non-specified | [66] | ||
| Oecophylla smaragdina (Fabricius) | Adults | [28,66] | ||
| Pachycondyla sp. | Nest | [39] | ||
| Polyrhachis dives Smith | Non-specified | [66] | ||
| Polyrhachis vicina Roger | Non-specified | [65,66] | ||
| Solenopsis saevissima (Smith) | Non-specified | [63] | ||
| Tetramorium sp. | Nest | [39] | ||
| Sphecidae | Sceliphron sp. | Pupae/Cocoon/Adults/Nest | [39] | |
| Vespidae | Apoica pallens (Fabricius) | Non-specified | [63] | |
| Brachygastra lecheguana (Latreille) | Non-specified | [63] | ||
| Parapolybia varia (Fabricius) | Adults, Bee comb | [66] | ||
| Polistes canadensis L. | Non-specified | [63] | ||
| Polistes chinensis (Fabricius) | Larvae/Adults/Bee comb | [66] | ||
| Polistes macaensis (Fabricius) | Adults | [65] | ||
| Polybia sericea (Olivier) | Non-specified | [63] | ||
| Protonectarina sylveirae (Saussure) | Non-specified | [63] | ||
| Protopolybia exigua (Saussure) | Non-specified | [63] | ||
| Provespa barthelemyi (Byusson) | Adults | [28] | ||
| Synoeca surinama (L.) | Non-specified | [63] | ||
| Vespa affinis (L.) | Bee comb | [66] | ||
| Vespa bicolor Fabricius | Adults | [65] | ||
| Vespa ducalis Smith | Adults | [65] | ||
| Vespa mandarinia Smith | Bee comb | [28,65,66] | ||
| Vespa nigrithorax Buusson | Bee comb | [66] | ||
| Vespa tropica L. | Larvae/Pupae/Cocoon/Adults/ | [28] | ||
| Vespa velutina auraria Smith | Bee comb | [65,66] | ||
| Vespula vulgaris L. | Nest | [39] | ||
| Lepidoptera | Bombycidae | Bombyx mori L. | Eggs/Larvae/Pupae/Cocoon/Male adults/Beauveria bassiana infected larvae/Pupae/Exuviae | [65,66] |
| Cossidae | Cossus sp. | Larvae, Adults | [28] | |
| Crambidae | Omphisa fuscidentalis Hampson | Larvae | [28] | |
| Erebidae | Arctia caja (L.) | Non-specified | [66] | |
| Hepialidae | Thitarodes armoricanus Oberthür | Cordyceps sp. Infected larvae | [65] | |
| Erionota torus Evans | Larvae | [28] | ||
| Lasiocampidae | Malacosoma sp. | Larvae | [28] | |
| Limacodidae | Cnidocampa flavescens (Walker) | Pupae/Cocoon | [66] | |
| Monema flavescens Walker | Pupae/cocoon | [65] | ||
| Thosea sinensis Walker | Pupae/Cocoon | [66] | ||
| Noctuidae | Agrotis ipsilon (Hufnagel) | Cordyceps hawkesii infected larvae | [66] | |
| Nymphalidae | Polygonia c-aureum L. | Adults | [65] | |
| Papilionidae | Papilio machaon L. | Larvae/Pupae/Cocoon | [66] | |
| Papilio xuthus L. | Larvae | [65,66] | ||
| Pieridae | Pieris rapae (L.) | Adults | [65,66] | |
| Psychidae | Oiketicus kirbyi Guilding | Non-specified | [63] | |
| Pyralidae | Aglossa dimidiatus Haworth | Frass | [66] | |
| Ostrinia nubilalis (Hübner) | Larvae | [65,66] | ||
| Proceras venosatum (Walker) | Larvae | [66] | ||
| Saturniidae | Antheraea pernyi (Guérin-Meneville) | Pupae/Cocoon | [65,66] | |
| Cirina butyrospermi Vuillot | Non-specified | [39] | ||
| Philosamia cynthia Grote | Larvae/Pupae/Cocoon | [65,66] | ||
| Samia cynthia ricini (Boisduval) | Larvae | [28] | ||
| Mantodea | Mantidae | Hierodula coarctata Saussure | Adults | [28] |
| Hierodula patellifera Serville | Eggs/Adults | [65,66] | ||
| Mantis religiosa Linnaeus | Eggs | [66] | ||
| Paratenodera sinensis (Saussure) | Eggs | [66] | ||
| Statilia maculata Thunberg | Eggs/Adults | [65,66] | ||
| Tenodera angustipennis (Saussure) | Eggs | [66] | ||
| Tenodera sinensis Saussure | Egg/Adults | [28,65] | ||
| Neuroptera | Myrmeleontidae | Euroleon sinicus (Navás) | Larvae | [66] |
| Myrmeleon sp. | Larvae | [28] | ||
| Odonata | Aeschnidae | Aeschna melanictera Selys | Adults | [66] |
| Anax parthenope julius Brauer | Adults | [66] | ||
| Gomphidae | Gomphidia confluens Selys | Non-specified | [65] | |
| Libellulidae | Crocothemis servilia Drury | Nymphs | [28,66] | |
| Diplacodes trivialis Rambur | Nymphs | [28] | ||
| Neurothemis fulvia Drury | Nymphs | [28] | ||
| Orthetrum pruinosum neglectum Rambur | Nymphs | [28] | ||
| Orthetrum sabina Drury | Nymphs | [28] | ||
| Orthetrum triangulare Selys | Nymphs | [28] | ||
| Pantala flavescens Fabricius | Nymphs | [28,65,66] | ||
| Potamarcha congener Rambur | Nymphs | [28] | ||
| Orthoptera | Acrididae | Acrida cinerea (Thunberg) | Adults | [66] |
| Acrida cinerea (Thunberg) | Adults | [65,66] | ||
| Ceracris kiangsu Tsai | Non-specified | [66] | ||
| Locusta migratoria (L.) | Non-specified | [65,66] | ||
| Melanoplus sp. | Adults | [28] | ||
| Oxya chinensis (Thunberg) | Adults | [65,66] | ||
| Patanga japonica (Bolívar) | Adults | [65,66] | ||
| Schistocerca gregaria (Forskål) | Adults | [39] | ||
| Gryllidae | Acheta domesticus (L.) | Non-specified | [39,63] | |
| Brachytrupes portentosus (Lichtenstein) | Non-specified | [66] | ||
| Gryllus assimilis (Fabricius) | Non-specified | [63] | ||
| Gryllus mitratus Burmeister | Adults | [66] | ||
| Gryllus sp. | Adults | [28] | ||
| Gryllus testaceus Wallker | Non-specified | [66] | ||
| Loxoblemmus doenitzi Stein | Nymph/Adults | [65,66] | ||
| Scapsipedus micado Saussure | Adults | [65] | ||
| Tarbinskiellus portentosus (Lichtenstein) | Adults | [28] | ||
| Teleogryllus emma (Ohmachi and Matsuura) | Adults | [65] | ||
| Velarifictorus aspersus (Walker) | Adults | [65] | ||
| Gryllotalpidae | Gryllotalpa orientalis Burmeister | Nymph/Adults | [65,66] | |
| Gryllotalpa unispina Saussure | Non-specified | [66] | ||
| Phalangopsidae | Paragryllus temulentus Saussure | Non-specified | [63] | |
| Tettigoniidae | Elimaea securigera Brunner von Wattenwyl | Adults | [28] | |
| Gampsocleis buergeri (De Haan) | Male | [66] | ||
| Gampsocleis gratiosa Brunner Von Wattenwyl | Adults | [65] | ||
| Gampsocleis sedakovii obscura (Walker) | Adults | [65] | ||
| Mecopoda elongata (L.) | Non-specified | [66] | ||
| Pseudophyllus titan White | Adults | [28] | ||
| Phasmatodea | Lonchodidae | Carausius sp. | Adults | [28] |
| Psocodea | Pediculidae | Pediculus humanus L. | Non-specified | [63] |
| Trichoptera | Phryganeidae | Phryganea japonica McLachlan | Larvae | [66] |
| Zygentoma | Lepismatidae | Lepisma saccharina L. | Non-specified | [66] |
| Lepisma villosa Fabricius | Non-specified | [66] |
Among those 235 species, 151 were documented with the specific stage or product (e.g., feces, nest, etc.) used. Adult stage (90 species) was the most documented stage, followed by larvae/nymphs (60), pupae/cocoon (13), and eggs (7). The usage of adults and larvae/nymph are distributed widely among different orders (e.g., 2/3 orders were documented). On the contrary, the usage of eggs (e.g., Lepidoptera and Mantodea) and pupae/cocoon (e.g., Hymenoptera and Lepidoptera) are limited in two orders, respectively. Besides, fungus infected larvae are only documented in three species in Lepidoptera, which are the Beauveria bassiana infected Bombyx mori (L.), Cordyceps sinensis infected Thitarodes armoricanus Oberthür, and Cordyceps hawkesii infected Agrotis ipsilon (Hufnagel).
Other than insects per se, byproducts from 31 species were documented. Byproducts from species in Hymenoptera are the most documented (e.g., 18 out of 31 species), for example, bee wax, honey, royal jelly, bee pollen, bee comb, and bee venom from the family Apidae, bee comb from the family Vespidae, and nest from the family Formicidae (Table 2).
3.2. Health effects of medicinal insects and their associated mechanisms
The international classification of diseases system ICD10 (Table 3) is used here to sort the health effects of insects mentioned in literature except wound healing, which cannot be sorted in a single group of disease. The ICD10 system was published by the World Health Organization (WHO) in 1994 (more details can be found in the ICD10 Interactive Self Learning Tool, https://apps.who.int/classifications/apps/icd/icd10training/). Modern research (i.e., 2012–2022) that studied medicinal functions with species family documented in the above five summarized literatures [28,39,63,65,66] were screened out on Web of Science™. We identified ∼300 articles, which cover 23 families.
Table 3.
ICD1O code and the associated diseases.
| ICD10 code | Disease classified |
|---|---|
| A00-B99 | Certain infectious and parasitic diseases |
| C00-D49 | Neoplasms |
| D50-D89 | Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism |
| E00-E89 | Endocrine, nutritional, and metabolic diseases |
| F01–F99 | Mental, Behavioral and Neurodevelopmental disorders |
| G00-G99 | Diseases of the nervous system |
| H00–H59 | Diseases of the eye and adnexa |
| H60–H95 | Diseases of the ear and mastoid process |
| I00–I99 | Diseases of the circulatory system |
| J00-J99 | Diseases of the respiratory system |
| K00–K95 | Diseases of the digestive system |
| L00-L99 | Diseases of the skin and subcutaneous tissue |
| M00-M99 | Diseases of the musculoskeletal system and connective tissue |
| N00–N99 | Diseases of the genitourinary system |
| O00–O99 | Pregnancy, childbirth, and the puerperium |
| P00–P96 | Certain conditions originating in the perinatal period |
| Q00-Q99 | Congenital malformations, deformations, and chromosomal abnormalities |
| R00-R99 | Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified |
| S00-T88 | Injury, poisoning and certain other consequences of external causes |
| U00–U85 | Codes for special purposes |
| V00–Y99 | External causes of morbidity |
| Z00-Z99 | Factors influencing health status and contact with health services |
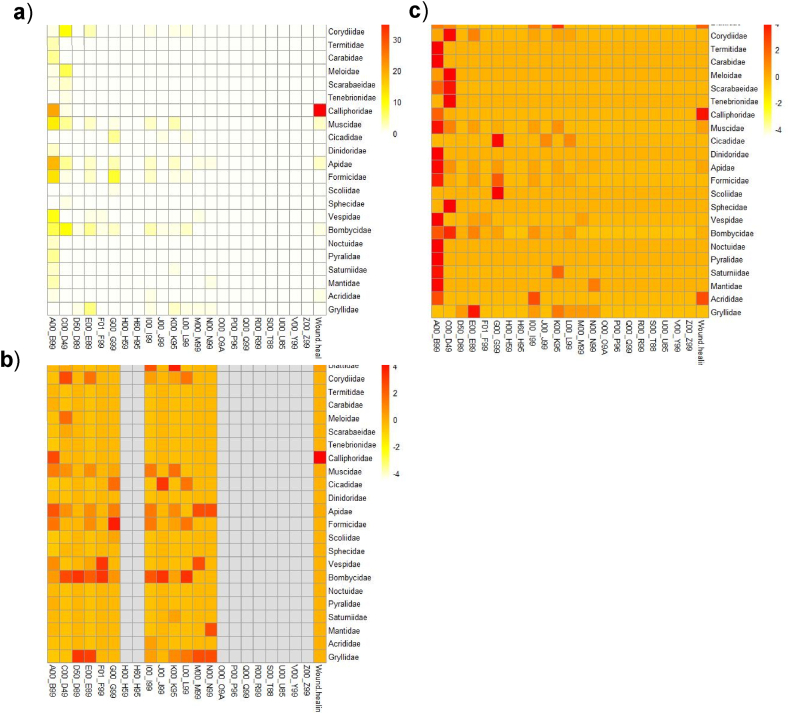
The focus on health effects of insects in modern medicine has changed significantly compared to folk medicine. Insects that used to fight against infectious and parasitic diseases (ICD A00-B99) counted ∼36% of the total research, followed by insects promote wound healing (counted ∼17%) and anti-neoplasms (ICD C00-D49, counted ∼15%). Heatmap analysis (Fig. 3a–c) showed the association between diseases and the insect families. Insect families used in different groups of diseases are diverse except wound healing, which was heavily focused on the use of Calliphoridae (Fig. 3b). For example, infection related diseases were frequently used with insects in Calliphoridae, Muscidae, Apidae, and Formicidae. Neoplasm studies frequently used insects in Corydiidae, Meloidae, and Bombycidae. Distributions of associated disease in each insect family vary (Fig. 3c). For example, Blattidae was mostly used in digestive system disease research. Cicadidae and Scollidae were mostly used in nervous system disease research.
Fig. 3.
Heatmap of associations between insect families and diseases. a) showed with numbers of papers; b) scaled by disease; and c) scaled by family of insect. International classification of diseases (ICD10) is used here to sort the diseases with code A to Z (https://www.icd10data.com/ICD10CM/Codes). Wound healing is added since it does not belong to any ICD10 code.
The choice of insects in disease research seems to be heavily impacted by the documentary of folk medicine and the market availability. Below is a detailed description of functions mentioned in modern research based on the ICD system with mechanisms (Table 4) and ingredients (Table 5) documented.
Table 4.
Health effects of medicinal insects and the associated mechanisms documented.
| ICD 10 | Effects | Mechanisms | Reference(s) |
|---|---|---|---|
| A00-B99 | Antibacteria | Peptide deformylase | [67] |
| Membrane permeability alteration and disruption | [68] | ||
| DNA formation inhibition and damage | [68] | ||
| Biofilm damage | [69] | ||
| Antifungus | Reduced bacterial adherence to human keratinocytes | [70] | |
| Membrane disruption | [71] | ||
| Antivirus | ROS production | [72] | |
| Parasite inhibition | Potentiating innate immunity function | [73] | |
| C00-D49 | Antitumor | Inhibit cell adhesion | [72] |
| Restrain cell migration and invasion | [72] | ||
| Antiproliferation | [74] | ||
| Apoptosis | [75] | ||
| Immunomodulatory | [74] | ||
| Anti-oxidation | [76] | ||
| Reduce inflammation | [76] | ||
| E00-E89 | Antihyperglycemic and antidiabetic | Advanced glycation end products (AGEs) inhibition | [77,78] |
| α-glucosidase inhibition | [79,80] | ||
| Beta cell function improvement | [81] | ||
| Reduce blood lipid and prevent obesity | Energy metabolism balance regulation | [82] | |
| AMPK/mTOR signaling pathway activation | [82] | ||
| Cholesterol metabolism-related biochemical parameters regulation | [83] | ||
| Combating malnutrition | Protein supplement | [84] | |
| F01–F99 | Anti-anxiety | may work with the B2-receptors and B1-receptors | [85] |
| Anti-depression | antioxidant and estrogenic properties | [86] | |
| G00-G99 | Neuroprotection | Anti-oxidation | [87] |
| Cell cycle inhibition | |||
| preventing cyclin D1 up-regulation | [87] | ||
| Reduce inflammation | |||
| Mitogen activated protein kinase (MAPK) inhibition | [87] | ||
| Prevent apoptosis | |||
| Bax inhibition | [88] | ||
| Caspase-3 inhibition | [88,89] | ||
| Nrf2/HO-1 pathway regulation | [90,91] | ||
| BDNF/TrkB pathway regulation | [90] | ||
| Nurr1 expression | [92] | ||
| Venom immunotherapy | Specific IgE reduction and IgG4 induction | [93] | |
| I00–I99 | Against thrombosis | FXa inhibition | [94] |
| Antiplatelet aggregation | [94,95] | ||
| Plasminogen activation and fibrin (ogen) hydrolyzation | [80] | ||
| Against hypertension | Regulating vascular tone | [96] | |
| Angiotensin-converting enzyme (ACE) inhibition | [96,97] | ||
| J00-J99 | Anti-tussive and anti-asthma | Cytokines and neuropeptides regulation | [98] |
| TRPA1/TRPV1/TRPV5 channels regulation | [98] | ||
| GATA-3/Th2 and IL-17/RORγt pathways regulation | [99] | ||
| K00–K95 | Hepatoprotection | Reduce inflammation | [100,101] |
| Relevant signaling pathways regulation | [101] | ||
| Anti-oxidation | [100] | ||
| Gastroprotection | Reduce inflammation | [102,103] | |
| NF-kappa B signaling pathway regulation | [104,105] | ||
| Anti-oxidation | [103] | ||
| Intestinal microbiota regulation | [[106], [107], [108], [109]] | ||
| Neovascularization | [102] | ||
| Growth factor expression enhancement | [102] | ||
| L00-L99 | Reduce melanogenesis | NA | [110] |
| Photoaging protection | Reduced UVB-induced skin winkles | [111] | |
| Anti-oxidation | [111,112] | ||
| Reduce inflammation | [111] | ||
| Alleviated the epidermal barrier dysfunction | [111] | ||
| Reduce collagen breakdown | [111] | ||
| Psoriasis | Reduced immune response | [113] | |
| Attenuated epidermal proliferation | [113] | ||
| Dermatitis | Reduce inflammation | [2] | |
| Wound healing | Wound healing | Biosurgical debridement | [114] |
| Disinfection | |||
| Anti-bacteria | [[115], [116], [117]] | ||
| Wound healing | |||
| Stimulated keratinocytes | [118,119] | ||
| Pro-fibrogenic and pro-angiogeneic effects | [120,121] | ||
| Blood coagulation | [114] | ||
| Cell proliferation, tissue reconstruction | [104,115,122] | ||
| Reduce inflammatory cytokines | [115,123,124] | ||
| Glycosidases (glycoside hydrolases) | [124] |
Table 5.
Medicinal insect species and their effective ingredients.
| ICD 10 | Order | Family | Species | Ingredients | Reference(s) |
|---|---|---|---|---|---|
| A00-B99 | Blattodea | Blattidae | Periplaneta americana L. | Unsaturated fatty acid | [125] |
| Gut microbiota | [126] | ||||
| Termitidae | Odontotermes formosanus (Shiraki) | Microbiota | |||
| Macrotermes sp. | Actinomycetes | [127] | |||
| Coleoptera | Meloidae | Meloidae sp. | Terpenoid - Cantharidin | [128] | |
| Scarabaeidae | Copris tripartitus Waterhouse | AMP - Coprisin | [129] | ||
| Diptera | Muscidae | Musca domestica L. | Proteins - Lectin | [130] | |
| AMP - Cecropin, attacin, lebocin | [131] | ||||
| Calliphoridae | Lucilia sericata (Meigen) | AMP - Lucifensin, lucimycin, attacins, cecropins, diptericins, proline-rich peptides, and sarcotoxins | [132] | ||
| Cochliomyia macellaria (Fabricius) | Excretions and secretions | [133] | |||
| Calliphora Vicina Robineau-Desvoidy | Excretions and secretions | [134] | |||
| Sarconesiopsis magellanica (Le Guillou) | Excretions and secretions | [135] | |||
| Drosophilidae | Drosophila melanogaster Meigen | AMP – Drosocin, Mtk-1, Mtk-2 | [136] | ||
| Hemiptera | Dinidoridae | Coridius chinensis (Dallas) | lysozyme - CcLys2 | [137] | |
| AMP - CcAMP1 | [138] | ||||
| Hymenoptera | Apidae | Melipona scutellaris Latreille | AMP - meliponamycin A, meliponamycin B | [139] | |
| Melipona orbignyi (Guérin-Méneville) | Geopropolis extract | [140] | |||
| Formicidae | Apterostigma dentigerum Wheeler | Microbiota – Pseudonocardia producing antibiotic (pseudonocardones) | [141] | ||
| Tetramorium bicarinatum (Nylander) | AMP - Bicarinalin | [142] | |||
| Hymenoptera | Vespidae | Agelaia pallipes (Olivier) | AMP - pronectin | [143] | |
| Polybia dimorpha Richards | AMP - Polydim-I | [144] | |||
| Polybia paulista Ihering | AMP - Polybia-CP | [72] | |||
| Vespa affinis L. | AMP - Mastoparan-AF | [145] | |||
| Lepidoptera | Bombycidae | Bombyx mori L. | AMP - Cecropin A, Cecropin B, moricin | [146] | |
| Microbiota - Yeast-melanin | [147] | ||||
| Noctuidae | Spodoptera litura (Fabricius) | AMP - Lebocin | [148] | ||
| Mantodea | Mantidae | Mantidis sp. | Ootheca lipid extract - Sesquiterpenoids, monoterpenes | [149] | |
| Sphodromantis viridis Forsskål | AMP - Mastoparan-S | [150] | |||
| C00-D49 | Blattodea | Corydiidae | Eupolyphaga sinensis (Walker) | Protein - EPS72 | [125] |
| Polysaccharide | [74] | ||||
| Coleoptera | Meloidae | Mylabris sp. | Norcantharidin | [81,151] | |
| Mylabris phalerata Pallas | Cantharidin | [15,152,153] | |||
| Scarabaeidae | Copris tripartitus Waterhouse | Coprisin - CopA3 | [154] | ||
| Scarabaeus sacer L. | Chitosan | [155] | |||
| Diptera | Calliphoridae | Chrysomya albiceps (Wiedemann) | Carboxymethyl derivative of chitosan | [156] | |
| Sarcophaga aegyptiaca (Salem) | Carboxymethyl derivative of chitosan | [156] | |||
| Muscidae | Musca domestica L. | Anti-tumor peptide | [157,158] | ||
| Microbiota - Bacillus subtilis - extracellular polymeric substance | [159] | ||||
| Lepidoptera | Bombycidae | Bombyx mori L. | Beauveria bassiana infected larvae | ||
| Cyclodepsipeptide - bassianolide | [160] | ||||
| Cordycepin | [161] | ||||
| Cecropin A | [146] | ||||
| Peptide - BmCecA and BmCecD | [162] | ||||
| Hymenoptera | Streptomycetaceae | Sceliphron madraspatanum (Fabricius) | Micorbiota - Streptomyces sp. - strepantibins A-C | [163] | |
| E00-E89 | Lepidoptera | Bombycidae | Bombyx mori L. | Flavonoids and free amino acids | [80] |
| Blattodea | Corydiidae | Eupolyphaga sinensis (Walker) | Peptide DP17 | [82] | |
| Peptide (AR-9) | [164] | ||||
| Diptera | Muscidae | Musca domestica L. | Extract | [79,83] | |
| Hymenoptera | Apidae | Apis mellifera L. | Propolis - epicatechin and p-coumaric | [165] | |
| Bombus ignitus (Smith) | Glycosaminoglycan | [166] | |||
| Vespidae | Vespa basalis Smith | Peptide- Mastoparan B | [167] | ||
| Orthoptera | Gryllidae | Gryllus bimaculatus De Geer | Glycosaminoglycan | [166,168] | |
| Ethanol extract | [169] | ||||
| Gryllus assimilis (Fabricius) | Protein hydrolysates | [77] | |||
| F01-f99 | Hymenoptera | Vespidae | Polybia paulista Ihering | venom | [85] |
| Lepidoptera | Bombycidae | Bombyx mori L. | Silk syrup | [86] | |
| G00-G99 | Diptera | Muscidae | Musca domestica L. | Larval meal | [87] |
| Hemiptera | Cicadidae | Cryptotympana pustulata Fabricius | Cicadidae periostracum - N-acetyldopamine dimers | [170] | |
| Cicadidae sp. | Cicadidae periostracum - cyclic peptide | [89] | |||
| Hymenoptera | Apidae | Apis mellifera L. | Venom - melittin | [88] | |
| Apidae sp. | Propolis - Caffeic acid phenethyl ester | [90] | |||
| Formicidae | Polyrhachis dives Smith | Dopamine derivatives | [61] | ||
| Myrmecia pilosula F. Smith | Venom | [171] | |||
| Dinoponera quadriceps Kempf | Venom | [[172], [173], [174]] | |||
| Scoliidae | Scolia decorata ventralis Smith | Venom - peptides | [175] | ||
| I00–I99 | Blattodea | Corydiidae | Eupolyphaga sinensis Walker | Serine proteases | [176] |
| Protein | [176] | ||||
| Blattidae | American cockroach L. | Xinmailong | [96] | ||
| Hymenoptera | Apidae | Apis mellifera L. | Polyphenol - epicatechin and p-coumaric | [165] | |
| Formicidae | Dinoponera quadriceps Kempf | Venom | [95] | ||
| Oecophylla smaragdina Fabricius | Proteins | [97] | |||
| Lepidoptera | Bombycidae | Bombyx mori L. | Protein - sericin | [177] | |
| Peptide | [178] | ||||
| Pupae oil | [179] | ||||
| Orthoptera | Acrididae | Oxya chinensis sinuosa Mistshenko | N-acetyldopamine dimers | [94] | |
| Gryllidae | Gryllus assimilis (Fabricius) | Protein hydrolysates | [77] | ||
| K00–K95 | Blattodea | Blattidae | Periplaneta americana L. | Extracts | [101,107] |
| Oligosaccharides | [106]) | ||||
| Ethanol extract - Kangfuxin | [102,105] | ||||
| Antimicrobial peptide (Periplanetasin-2) | [103] | ||||
| Corydiidae | Eupolyphaga sinensis (Walker) | Peptide | [109] | ||
| Diptera | Muscidae | Musca domestica L. | Low molecular weight peptides | [108,180] | |
| Stomoxys calcitrans L. | Metabolites | [181] | |||
| Hymenoptera | Apidae | Trigona sp. | Honey | [182] | |
| Formicidae | Oecophylla smaradina Fabricius | Ethanolic extract | [183] (2019) | ||
| Lepidoptera | Saturniidae | Antheraea pernyi (Guérin-Méneville) | Silk fibroin | [184] | |
| Bombycidae | Bombyx mori L. | Peptide - Gloverin A2 (BMGlvA2) | [185] | ||
| Orthoptera | Crididae | Oxya chinensis sinuosa (Mistshenko) | Extracts | [186] | |
| Gryllidae | Gryllus bimaculatus De Geer | Extracts | [100,111] | ||
| Protaetia brevitarsis (Lewis) | Extracts | [186] | |||
| L00-L99 | Blattodea | Corydiidae | Eupolyphaga sinensis (walker) | Polypeptides | [112] |
| Coleoptera | Scarabaeidae | Allomyrina dichotoma L. | Extract | [111] | |
| Protaetia brevitarsis seulensis (Kolbe) | Extract | [111] | |||
| Tenebrionidae | Tenebrio molitor L. | Extract | [111] | ||
| Hemiptera | Cicadidae | Cicadidae sp. | Cicadidae Periostracum | [2] | |
| Lepidoptera | Bombycidae | Bombyx mori (L.) | Freeze-dried mature silkworm powder | [110] | |
| Cocoon sericin | [187] | ||||
| Feces | [2] | ||||
| Orthoptera | Gryllidae | Gryllus bimaculatus De Geer | Extract | [111] | |
| Wound healing | Blattodea | Blattidae | Periplaneta americana (L.) | Extracts | [122] |
| Phenolic Derivatives | [188] | ||||
| Periplanpyrazine | [189] | ||||
| Kangfuxin liquid | [120] | ||||
| Diptera | Calliphoridae | Lucilia sericata (Meigen) | Excretions/secretions | [124,190,191] | |
| DNAse | [192] | ||||
| Angiopoietin-1 enzyme | [193] | ||||
| Allantoin | [194] | ||||
| Lysozymes | [195] | ||||
| Signal peptide protease | [196] | ||||
| Prenyl metalloproteinase | [196] | ||||
| Serine protease | [114,196,197] | ||||
| Chymotrypsin | [198] | ||||
| Sarconesiopsis magellanica (Le Guillou) | Excretions/secretions | [199] | |||
| AMP | [68,200] | ||||
| Proteases | [201] | ||||
| Fat body and hemolymph extract | [121] | ||||
| Hymenoptera | Apidae | Apis mellifera L. | Venom | [118] | |
| Lepidoptera | Bombycidae | Bombyx mori (L.) | Silk fibroin | [185] |
3.2.1. Health effects associated with infectious and parasitic diseases (A00-B99)
Most (e.g., ∼65%) of the research focused on antibacterial effects. At least 30 species of bacteria were determined to be inhibited by insect derivatives (Table 6), including but not limiting to bacteria associated with wound infection (e.g., Bacillus sp., Staphylococcus sp., and Proteus sp.), digestive system infection (e.g., Helicobacter pylori, Bacillus cereus, Citrobacter freundii, Escherichia coli, and Salmonella enterica), urinary tract infection (e.g., Enterobacter cloacae, Enterococcus faecalis, Acinetobacter baumannii, and Serratia marcescens), and other infections (e.g., Listeria monocytogenes and Haemophilus influenzae). Besides, at least 13 species of fungus (Aspergillus sp., Penicillium sp., Trichoderma sp., and Candida sp.) (Table 7), five viruses (e.g., Rift Valley fever virus, Coxsackie B4 virus, Hepatitis B virus, Hepatitis A virus, and Herpes simplex virus) (Table 8), and ten parasites (e.g., Trypanosoma cruzi, Leishmania sp. Plasmodium sp., and Haemonchus contortus) (Table 9) were determined can be inhibited by insect derivatives. Mechanisms include reducing bacterial adherence to human keratinocytes [70], biofilm interruption [69], membrane permeability alteration and disruption [68], peptide deformation [67], ROS production [72], and DNA formation inhibition and damage [68]. The active ingredients are mainly antimicrobe peptides (AMP). For example, coprisin [129], lebocin [148], drosocin [136], pronectin [143], cecropin [146], etc. Besides, certain unsaturated fatty acid [125], protein (e.g., lectin [130] and lysozyme [137], and terpenoid (e.g., cantharidin [128]) also showed antimicrobe/virus effects. The insect associated microbes contributed as well, for example the actinomycetes isolated from Termitidae sp [127]. and the melanin extracted from yeast in B. mori [147].
Table 6.
Bacteria inhibited by medicinal insects with insect species shown as examples.
| Bacteria Inhibited | Order | Family | Species | Stage or ingredients used | Reference(s) |
|---|---|---|---|---|---|
| Helicobacter pylori | Propolis from bees with no specific species mentioned | Propolis | [67] | ||
| Micrococcus flavus | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Micrococcus luteus | Lepidoptera | Bombycidae | Bombyx mori (L.) | Antimicrobial peptides | [203] |
| Micrococcus tetragenus | Blattodea | Termitidae | Odontotermes formosanus (Shiraki) | Associated microbiota | [127] |
| Mycobacterium abscessus subsp. Massiliense | Hymenoptera | Vespidae | Polybia dimorpha Richards | Venom | [144] |
| Bacillus pumilus | Lepidoptera | Saturniidae | Antheraea mylitta (L.) | Antimicrobial peptides | [204] |
| Bacillus subtilis | Lepidoptera | Bombycidae | Bombyx mori (L.) | Ethyl acetate extract | [203] |
| Bacillus cereus | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Listeria monocytogenes | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Methicillin-resistant Staphylococcus aureus | Blattodea | Blattidae | Periplaneta americana (L.) | Associated microbiota | [205] |
| Staphylococcus aureus | honeybee-specific lactic acid bacteria with no specifc species mentioned | Associated microbiota | [206] | ||
| Staphylococcus epidermidis | Diptera | Calliphoridae | Lucilia cuprina (Wiedemann) | Secretions | [207] |
| Staphylococcus xylosus | Hymenoptera | Formicidae | Tetramorium bicarinatum (Nylander) | Antimicrobial peptides | [142] |
| Streptococcus pyogenes | Hymenoptera | Apidae | Frieseomelitta nigra (Cresson) | Honey | [208] |
| Citrobacter freundii | honeys with no specific species mentioned | Honey | [209] | ||
| Enterobacter cloacae | Blattodea | Blattidae | Periplaneta americana (L.) | Associated microbiota | [126] |
| Enterococcus faecalis | Diptera | Calliphoridae | Lucilia sericata (Meigen) | Antimicrobial peptides | [132] |
| Escherichia coli | Hymenoptera | Apidae | Melipona orbignyi (Guérin-Méneville) | Geopropolis | [140] |
| Klebsiella pneumonia | honeys with no specific species mentioned | Honey | [209] | ||
| Proteus mirabilis | Diptera | Calliphoridae | Lucilia sericata (Meigen) | Secretions | [69] |
| Proteus vulgaris | Diptera | Calliphoridae | Lucilia sericata (Meigen) | Antimicrobial peptides | [132] |
| Salmonella enterica | Blattodea | Blattidae | Periplaneta americana (L.) | Associated microbiota | [126] |
| Salmonella infantis | honeys with no specific species mentioned | Honey | [209] | ||
| Salmonella typhimurium | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Legionella gormanii | Lepidoptera | Pyralidae | Galleria mellonella (L.) | Hemolymph polypeptides | [210] |
| Acinetobacter baumannii | honeys with no specific species mentioned | Honey | [209] | ||
| Haemophilus influenzae | Hymenoptera | Apidae | Frieseomelitta nigra (Cresson) | Honey | [208] |
| Pseudomonas aeruginosa | Diptera | Calliphoridae | Lucilia cuprina (Wiedemann) | Secretions | [207] |
| Pseudomonas fluorescens | Hymenoptera | Formicidae | Solenopsis invicta (Buren) | Venom | [211] |
| Serratia marcescens | Diptera | Calliphoridae | Chrysomya sp. | Secretions | [212] |
Table 7.
Fungus inhibited by medicinal insects with insect species shown as examples.
| Fungus inhibited | Order | Family | Species | Stage or ingredients used | References |
|---|---|---|---|---|---|
| Aspergillus flavus | Blattodea | Blattidae | Periplaneta americana (L.) | Associated microbiota | [126] |
| Aspergillus fumigatus | Blattodea | Blattidae | Periplaneta americana (L.) | Associated microbiota | [126] |
| Aspergillus niger | Blattodea | Blattidae | Periplaneta americana (L.) | Associated microbiota | [126] |
| Aspergillus ochraceus | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Aspergillus versicolor | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Aspergillus flavus | Blattodea | Blattidae | Periplaneta americana (L.) | Associated microbiota | [126] |
| Penicillium funiculosum | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Penicillium italicum | Blattodea | Blattidae | Periplaneta americana (L.) | Associated microbiota | [126] |
| Penicillium ochrochloron | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Penicillium verrucosum var. Cyclopium | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Trichoderma viride | Coleoptera | Carabidae | Calosoma sycophanta L. | Secretions | [202] |
| Trichophyton rubrum | Blattodea | Termitidae | Nasutitermes sp. | Associated microbiota | [213] |
| Candida albicans | Blattodea | Blattidae | Periplaneta americana (L.) | Associated microbiota | [126] |
Table 8.
Virus inhibited by medicinal insects with insect species shown as examples.
| Virus inhibited | Order | Family | Species | Stage or ingredients used | References |
|---|---|---|---|---|---|
| Rift Valley Fever virus | Diptera | Calliphoridae | Lucilia cuprina (Wiedemann) | Secretions | [214] |
| Coxsackie B4 virus | Diptera | Calliphoridae | Lucilia cuprina (Wiedemann) | Secretions | [214] |
| Hepatitis B virus | Blattodea | Corydiidae | Eupolyphaga sinensis (Walker) | Polysaccharide | [73] |
| Hepatitis A virus | Coleoptera | Curculionidae | Rhynchophorus ferrugineus (Olivier) | Larval extract | [215] |
| Herpes simplex virus | Coleoptera | Curculionidae | Rhynchophorus ferrugineus (Olivier) | Larval extract | [215] |
Table 9.
Parasites inhibited by medicinal insects with insect species shown as examples.
| Parasite inhibited | Order | Family | Species | Stage or ingredients used | References |
|---|---|---|---|---|---|
| Trypanosoma cruzi | Hymenoptera | Formicidae | Dinoponera quadriceps (Kempf) | Dinoponeratoxin peptides | [216] |
| Leishmania infantum | Hymenoptera | Apidae | Melipona scutellaris Latreille | Associated microbiota | [139] |
| Leishmania panamensis | Diptera | Calliphoridae | Lucilia sericata (Meigen) | Secretions | [135] |
| Leishmania major | Diptera | Calliphoridae | Lucilia sericata (Meigen) | Secretions | [217] |
| Leishmania amazonensis | Diptera | Muscidae | Musca domestica (L.) | Larvae | [218] |
| Leishmania tropica | Diptera | Calliphoridae | Lucilia sericata (Meigen) | Secretions | [219] |
| Leishmania donovani | Hymenoptera | Formicidae | Cyphomyrmex sp. | Associated microbiota | [220] |
| Plasmodium falciparum | Diptera | Drosophilidae | Drosophila melanogaster Meigen | Antimicrobial peptides | [136] |
| Plasmodium berghei | Hymenoptera | Formicidae | Apterostigma dentigerum Wheeler | Associated microbiota | [141] |
| Haemonchus contortus | Hymenoptera | Formicidae | Neoponera sp. | Venoms | [221] |
3.2.2. Health effects associated with neoplasms (C00-D49)
At least 15 types of anticancer activities were documented, including breast cancer, liver cancer, colorectal cancer, lung cancer, ovarian cancer, colon cancer, pancreatic cancer, esophageal cancer, cervical cancer, tongue cancer, bladder cancer, leukemia, murine melanoma, and two types of ascites cancer. Insect-derived ingredients can inhibit tumor cell adhesion [222], restrain cell migration and invasion [222], and induce cell antiproliferation [223] and apoptosis [146] by regulating different pathways, for example the Akt [75], Mapk [224], and PKC [91] pathways. In addition, the antioxidant [225], anti-inflammatory [76], and immunomodulatory [74] functions of insect-derived ingredients contribute to their antitumor effects. Cantharidin from blister beetles (Meloidae) [153], cordycepin [161], and cecropin [146] from silkworms (Bombycidae) have gain many attentions with their anti-tumor effects. Since the cantharidin has certain toxic side effects, a synthetic derivation of cantharidin named noncantharidin has been developed and widely used in modern anti-tumor medicine [81]. Eupolyphaga sinensis (Walker) (Blattodea: Corydiidae) is another well-known traditional Chinese anti-tumor medicine, from which the extracted polysaccharide [74] and a protein named EPS72 [125] have been determined as the active ingredients. Recently, chitosan derivations from scarab beetles (Scarabaeidae) [155] and blowflies (Calliphoridae) [156] were determined to be the effective ingredients as well.
3.2.3. Health effects associated with blood and blood-forming organs and certain disorders involving the immune mechanism (D50-D89)
Not much research can be classified in this group besides nutritional anemia. Insects are rich in nutrients and have been proved to be effective diet supplements [226]. For example, the consumption of cricket could help to prevent children nutritional anemia by providing sufficient energy, iron, and zinc [227].
3.2.4. Anti-hyperglycemia and anti-hyperlipidemia are the two major effects associated with endocrine, nutritional, and metabolic diseases (E00-E89)
The anti-hyperglycemic effect works through advanced glycation end products (AGEs) inhibition [78], α-glucosidase inhibition [176], and beta cell improvement [79]. Active ingredients, for example flavonoids and free amino acids from B. mori [80] and epicatechin and p-coumaric from A. mellifera propolis [165] have shown the ability to regulate blood sugar and prevent/treat diabetes. Anti-hyperlipidemic effects works through energy metabolism balancing [82], AMPK/mTOR pathway activation [82], and cholesterol metabolism-related biochemical parameters regulation [83], and therefore showed obesity prevention potentials [167]. Peptides, for example, DP17 [82] and AR-9 isolated from E. sinensis [164] and Mastoparan B isolated from Vespa basalis Smith (Hymenoptera: Vespidae) [167] and glycosaminoglycan from Gryllus bimaculatus De Geer (Orthoptera: Gryllidae) [166] and Bombus ignitus (Smith) (Hymenoptera: Apidae) [166] have been determined to be the effective ingredients.
3.2.5. Anti-anxiety and anti-depression were the documented effects that associated with mental, behavioral, and neurodevelopmental disorders (F01–F99)
The bradykinin-related peptide isolated from Polybia paulista Ihering (Hymenoptera: Vespidae) venom was determined to be the active ingredients again anxiety, which may work with the B2-receptors and B1-receptors [85]. The silk syrup produced from B. mori cocoon was determined to have anti-depression effect, which may be due to its antioxidant and estrogenic properties [86].
3.2.6. Neuroprotection and venom immunotherapy are the major functions associated with nervous system (G00-G99)
The mechanisms of insect neuroprotective effects include antioxidation [87], anti-inflammation [87], cell cycle inhibition [87], and apoptosis prevention [88], which can prevent neurodegenerative diseases (e.g., Alzheimer's disease) [87], Parkinson's disease [170], Amyotrophic lateral sclerosis [228], and epilepsy [174]. Venom immunotherapy is an effective treatment for systemic allergic reactions to Hymenoptera venom. The potential mechanisms (e.g., the initial desensitization of effector cells, the regulation of IgG and IgE level, and the associated inflammatory effects) were recently reviewed by Demšar Luzar et al. [229]. Hymenoptera venom was widely documented as traditional medicine or therapy targeting the nervous system and has been studied and used in the modern medicinal system. Peptide [175] and melittin [88] isolated from venom are documented as the effective ingredients.
3.2.7. Anti-thrombosis and anti-hypertension are the mostly documented effects associated with circulatory system (I00–I99)
Anti-thrombosis works through plasminogen activation and fibrinogen (a major determinant of plasma and blood viscosity) hydrolyzation [80], FXa inhibition, and antiplatelet aggregation [94]. Anti-hypertensive effect mainly works through angiotensin-converting enzyme (ACE) inhibition [178]. The identified effective ingredients are, for example, serine proteases from E. sinensis [176], sericin from B. mori [187], polyphenol (e.g., epicatechin and p-coumaric) from A. mellifera, and N-acetyldopamine dimers from Oxya chinensis sinuosa Mishchenko (Orthoptera: Acrididae) [94].
3.2.8. Anti-tussive and anti-asthmatic effects are health functions associated with respiratory system (J00-J99)
Bombyx batryticatus (i.e., the dried silkworm larvae after infected by fungi Beauveria bassiana) and cicada periostracum (i.e., the cast-off shell of the cicada Cryptotympana pustulata (Fabricius)) were well known against respiratory disease in traditional Chinese medicine and have been recommended as potential medicines fighting against SARS-CoV-2 [230]. They help against respiratory disease through cytokines and neuropeptides [98], TRPA1/TRPV1/TRPV5 channels [98], and GATA-3/Th2 and IL-17/RORγt pathways [99] regulations.
3.2.9. Hepatoprotection and gastroprotection are key effects associated with digestive system (K00–K95)
The inflammation reduction [101] and anti-oxidation [100] effects of insects play important role in against the digestive system disease such as diarrhea [181], gastric ulcer [102], and prevent liver damage after acute alcohol exposure [101]. Intestinal microbiota regulation is another key mechanism for gastroprotection [106,107]. Besides, neovascularization and growth factor expression enhancement were determined in preventing recurrence of gastric ulcer [102]. The gastroprotective effect of peptides isolated from B. mori [185], E. sinensis [109], Musca domestica L. (Diptera: Muscidae) [180], and P. americana [103] have been confirmed. Among the species tested, the P. americana gained a lot of attention in digestive system protection, from which the oligosaccharides [106] and an antimicrobial peptide (Periplanetasin-2) [103] have been identified as effective ingredients. The extract of P. americana has been developed into a commercial medicine named Kangfuxin solution in China [102].
3.2.10. Health effects associated with skin and subcutaneous tissue (L00-L99)
Besides the antibacterial function described in group A00-B99, the antioxidation and anti-inflammation effects of insects help to reduce psoriasis [177], dermatitis [2] and UVB-induced melanogenesis [110] and aging [112]. For example, silkworms B. mori have been used in skin protection for a long history. The cocoon sericin [187], freeze-dried silkworm powder [110], and even the feces [2] were determined contributed to skin protection.
3.2.11. Health effects associated with musculoskeletal system and connective tissue (M00-M99)
Insects used in rheumatism and arthritis are well-known in traditional medical systems. The anti-inflammation [231] and anti-oxidation [232] effects of insects confirmed in modern research revealed the mechanisms underline. Glycosaminoglycan extracted from G. bimaculatus was determined to be an effective ingredient, which produced a significant anti-edema effect [231].
3.2.12. Health effects associated with wound healing
Wound healing is one of the research hot spots in medicinal insects, which does not belong to any ICD 10 categories since many diseases can lead to wound formation. Wound healing can be separated into therapy with and without maggots. Debridement (i.e., the process of larval feeding on necrotic tissues), disinfection (i.e., anti-bacteria functions mentioned above) and wound healing (e.g., through keratinocytes stimulation [119], cell proliferation [122], blood coagulation [114], and pro-angiogenesis [122]) are the three main mechanisms. Lucilia sericata (Meigen) is the most used insect in maggot therapy. The excretions and secretions from maggot larvae have shown outstanding effects in wound healing, from which mainly proteins/enzymes (e.g., angiopoietin-1 enzyme [193] and serine protease [197] were determined effectively promote angiogenesis and cell proliferation. Besides, insect-derived products such as honey, bee venom, chitosan, and sericin have used in wound healing [233].
4. Medicinal uses of common edible insects
Most forms of traditional medicine rely on plants and plant-derived components [26]. Nevertheless, for centuries, animals are often used as part of folk pharmacopoeia [28,234,235]. Both domesticated and wild fauna resources are used in zootherapy, which involves the application of animals to treat diseases and include them in magic rituals and religious rites [236]. Medicines originating from animals are made either directly from the whole animal or its parts [235]. Insects in medicine fall under an umbrella terminology called “integrative medicine”, which refers to a medical practice that blends traditional treatment with complementary and alternative medicine techniques and, has been safe and effective through scientific research [[237], [238], [239], [240]]. Edible insects are rich in proteins, fats, fiber, vitamins, and minerals but also seem to contain large amounts of polyphenols able to have a key role in specific bioactivities as antioxidant functions. They also exert other activities, such as anti-inflammatory and anticancer activity, antityrosinase, antigenotoxic, and pancreatic lipase inhibitory activities.
Because of bee's medicinal and nutritional benefits, honey has been utilized for thousands of years [241]. In many societies, honey, a bee product, has long been regarded as a therapeutic remedy, and there are about 300 types of honey worldwide [242]. Honey, bee pollen, propolis, royal jelly, beeswax, and even bee venom are some honeybee products that have been used in folk medicine for millennia across the globe [243]. Anti-inflammatory, antimicrobial, antifungal, antiviral, and antioxidant properties have all been observed in these insect-derived products. These antioxidant, antimicrobial, and other medicinal properties are more effective than sucrose in treating diabetes [242]. Another important product from bees, bee venom, has been utilized as a treatment method in East Asia since the second century, making it one of the region's oldest medical practicesf [244]. The chemical structure of bee venom is intricate, involving many different enzymes, peptides, proteins, smaller molecules (amino acids, catecholamines, carbohydrates, and minerals), and lipids that make up honeybee venom [245]. It also contains Melittin, apamin, MCD peptide, histamine, hyaluronidase, and phospholipase-A2 for bee venom's primary components. However, melitin, a peptide obtained from the European honeybee Apis mellifera has been well-studied by several authors [246]. Due to its high cytolytic action, it has proven to be highly effective against tumours [247,248]. Bee venom is an allergen agent that causes Asthma, allergic rhinoconjunctivitis, and atopic eczema by stimulating the production of allergen specific CD4+ T cells in susceptible individuals [245]. Bees provide health benefits because they contain many different metabolites, such as folic acid, thiamine, biotin, niacin, tocopherol, polyphenols, phytosterols, and enzymes and coenzymes. The beneficial properties include antioxidant, antibacterial, antifungal, and hepatoprotective [241,249]. A recent study by Amr et al. [250] on female rats showed that boneybee products had the potential to reduce oxidative stress, increase cadmium (Cd) excretion via the kidneys, and modify intestinal absorption of the metal.
The larvae of the Australian Sawfly contain novel macrocarpa and grandinol. These chemical components were assessed against Bacillus subtilis and showed positive antimicrobial effects against the bacteria [6,251]. The larvae of Sawfly Tenthredo zonula Klug also contain phenolic compounds, such as flavonoid glycosides, flavonol oligoglycosides, and naphthodianthrones, and have been evaluated for their health properties [252].
The Chinese black ant (Polyrhachis dives) is an edible insect with kidney-detoxifying and antiinflammatory properties [253]. The ants contain several compounds essential for immunosuppressive, antinflammatory and renoprotective effects. Recently, several compounds were isolated from the species (Fig. 4).
Fig. 4.
Structure of thirteen nitrogen containing, non-peptide substances extracted and isolated from the edible Chinese black ants (Polyrhachis dives). a) 5-(3-Indolylmethyl)-nicotinsaureamide; b) β-Carboline-3-carboxamide; c) 4-Pyridin-3-yl-phenol; d) harman; e) β-carboline; f) S-1-(1′-hydroxyethyl)-β-carboline; g) 8-hydroxy-4-quinolone; h) 1,2,3,4-tetrahydroquinoline; i) niacinamide; j) 3-hydroxypyridine; k) 2,5-disubstituted oxazole; l) glutamine methyl ester; and m) cyclo-(L-Pro-L-Phe). Source: [253].
The Chinese medicinal Insect Blaps japanensis, has been used to treat many diseases, including fever, cough, rheumatism, cancer, and inflammatory disorders. The species contains blapsols (Fig. 5a) and dopamine dimers (Fig. 5b and 5c). These chemical components have been evaluated for their effectiveness against cyclooxygenase (COX) enzymes COX-1 and COX-2. The enzymes catalyze the conversion of arachidonic acid to prostaglandins, which is useful in pain, fever, and inflammation [6,254,255].
Fig. 5.
The blapsols a) and dopamine dimers (b and c) extracted from Blaps japanensis. Source [6].
The silkworm pupae are helpful to human health because of their high nutritional value and the many pharmacological effects they can have when consumed [73]. A vasorelaxant derived from the pupae of Bombyx mori, dimethyl adenosine, inhibits phosphodiesterase and stimulates nitric oxide production in endothelial cells, thus serving as a potential drug for treating vasculogenic impotence [22].
Chinese, Korean, and Japanese acupuncture and traditional medicine practitioners have used bee venom (B·V.) to treat inflammatory illnesses by administering a sterile bee sting or injecting a prepared B.V. solution [256,257]. The use of insects and insect-derived products for disease treatment is substantially lower than typically claimed in Asia, Europe and Africa [258]. A study conducted in Kadiogo and Houet showed that about 19 insects belonging to 6 orders were important in treating about 78 different diseases and conditions, such as vomiting, headaches, deafness, pain and Inflammation [39]. Many insects are also used to treat different kinds of diseases in India [28]. The insects and insect-derived products used as medicine in India and Burkina Faso are illustrated in Fig. 6, in which the Giant water bug Lethocerus indicus, dragonfly nymphs, large timber-boring larvae, freshly harvested Apis florea bee comb, nest entrances of stingless bees, Vespa mandarinia comb, blister beetle Mylabris sp., larvae of antlion and Myrmeleon sp., are recorded from Nagaland (Fig. 6 a-i) and larvae of Cossus sp., larvae of banana skipper Erionota torus, Epilambra sp. Cockroach, Periplaneta Americana, Macrotermes sp., Apis mellifera, Pachycondyla sp., Lytta sp., and Camponotus maculatus are reported from Kadiogo and Houët provinces (Fig. 6 j-r) [28,39]. Although the Angami people of India employ the larvae of the banana skipper Erionata torus to treat dangerous animal bites, the Lotha people utilise them as an aphrodisiac. Mylabris sp. Is used to treat blisters and warts in India and are also included in the traditional Chinese medical pharmacopoeia and Korean medical pharmacopoeia. A study by Ouango et al. [39] showed that insects had been used in Burkina Faso to treat many diseases. The traditional healers in Burkina Faso utilise the cockroach Periplaneta americana (Fig. 6), to relieve ear pain. Microtermi spp. Is also used to treat diarrhea and fractures in Burkina Faso. Asthma, rheumatological pathologies, bladder lithiasis, burns, constipation, difficulty breathing, general fatigue, gynaecological problems, heart diseases, hip pain, insomnia, intestinal helminthiasis, and voice extinction are just some of the many conditions that can be helped by medicines derived from Apis mellifera. The bees are also used to reat female infertility and male impotence. The blister beetle Lytta vesicatoria is urinary track infection. Insects and insect-based substances have a long history of usage as food and feed in many parts of the world [259]. In many regions of the world, entomotherapy is used by various segments of society. In Northeast India, locals have identified twelve insect species as having medicinal value. These insects are being employed by the tribes and used to cure a wide range of illnesses in humans and domestic animals [260]. Coughs, fevers, nighttime emetic production, burns, and gastrointestinal illness were all treated with one of nine species found in Bangladesh [1,28].
Fig. 6.
Certain medicinal insect and insect products of Nagaland (a–i) and Kadiogo and Houët provinces in Burkina Faso (j-r). a) Giant water bug Lethocerus indicus; b) dragonfly nymphs; c: large timber-boring larvae; d) freshly harvested Apis florea bee comb; e-f) nest entrances of stingless bees; g)Vespa mandarinia comb sold at local market, Kohima district; h) blister beetle Mylabris sp.; i) larvae of antlion Myrmeleon sp.; j) larvae of Cossus sp.; k) larvae of banana skipper Erionota torus; l)Epilambra sp. Cockroach, m)Periplaneta americana; n)Macrotermes sp.; o)Apis mellifera; p)Pachycondyla sp.; q)Lytta sp.; r)Camponotus maculatus. Source: [28,39].
4.1. Regulations of entomotherapy and entomophagy
Eating insects, or “entomophagy,” has evoked a wide range of feelings in people. Many psychological hurdles must be overcome before it can become mainstream because it is commonly held that neophobia and revulsion are the primary psychological factors that people use to reject entomophagy. With insects in medicine many illnesses have been treated with insects and insect extracts in folk medicine [261]. However, there are challenges associated with the consumption of insects and their therapeutic uses. Traditional medicine is still widely used in many parts of the world, including India, Korea, China, South America, and Africa. However, the practice of tradiational medicine has received little attention in Western culture and economically developed nations [6]. Zimmer [17] reported claimed that the Maya people have been utilizing maggots for therapeutic purposes for about 1000 years. These larvae consume decaying tissue which serves as a habitat for gangrene-causing bacteria that can cause health problems. In many African countries, there is limited access to modern medicine so traditional medicine, which frequently involves the use of insects, is nonetheless widely practiced in some parts of the continent [8,258].
4.2. Differences in entomotherapy between western countries and other regions in the world
Acceptance is still a barrier to the widespread use of insects as a medicinal resource for treating diseases and illnesses, especially in developed countries where most people view insects with distaste [262]. However, traditional medicine practices are widely accepted and documented in Chinese and Korean society, but less is known about similar traditions in Africa [263]. Traditional medicine, which sometimes includes insects, is nevertheless widely practised in parts of Africa where access to modern medicine is limited. This alternate medicine has largely received less attention since the advent of modern medicine, partly because of the baffling directions for various treatments [8,45]. As a result, entomotherapy, where insects are used to treat illness, is sometimes disregarded as superstition in many parts of the world. However, traditional medicine is still used in many parts of the world, such as India, Korea, China, South America, and Africa, despite being less popular in Western culture and economically developed nations [6,264]. It is believed that the general public in South America was more open to the concept of using insects as medicine because they were already using them as food in the past [8]. However, in Europe, therapeutic uses appear to have come before gastronomic ones [26,265]. Stick insects are used for treating calluses, warts and prickling spines in the Naga tribes, but in North Korea, they are considered to contain potent healing powers and are used to cleanse the body and remove stomach upset [266].
5. Production of the insects
5.1. Earlier principles of collecting the insects
A review of medical use of insects can at least date back to 2000 years ago when the book Sheng Nong's Herbal Classic described multiple medicinal applications of insects [267]. Till nowadays, only a few species are successfully mass produced [268]. More than 90% of the edible insects are collected from the field [[268], [269], [270]].
Insects for medicinal purposes differ from insects as food and feed for nutritional purposes in their exact medical effects requirements. Since the nutrients of insects vary even within species [271], principles of insect wide harvesting may include detail description of the stage, sex, time, location, and process to ensure their medical effects. For example, the B. mori adult used in medicine should be the newly emerged male with wings and legs removed [66]. Cicadidae periostracum should be collected in late summer and early autumn when cicadas newly emerged [66]. The best time to collect Mylabris sp. Beetles is the first month of autumn among the thorns of specific plants (e.g., Sophora sp.) [27]. Rhynchophorus palmarum (L.) (Coleoptera: Curculionidae) larvae collected from the native palm tree Mauritia flexuosa (L.f.) (Arecaceae: Arecaceae) is with the best healing properties [272].
Due to the uncertainty (e.g., quantity and quality across season and location) of wide harvesting, some insects have been domesticated for their commercial value. Insect domestication has at least 7000 years history [270], for example the honeybees and silkworms were domesticated during agricultural development [268]. Besides, crickets, mealworms, and the America cockroaches have been successively reared artificially. For example, a farm in Shandong, China, was reported to produce 20,000 kg of dry cockroaches annually [269]. However, not all the insects can be raised completely in artificial conditions. For example, locusts, wasps, and dragonflies are raised in a semi-domesticated way, which means part of the lifecycle is raised indoor or the nature habitat is manipulated to promote production [269]. Most of the rest of the medicinal insects are then still collected in the wide manually by local farmers.
Alternatively, if the effective ingredients have been identified, producing the specific ingredients rather than the insects would be a promising option. For example, the fermentation extract of mycelia from cultivated of C. sinensis is widely used in commercial sale with similar medical function, which has been an effective substitute of wide collection of infected ghost months, Hepialus armoricanus (Oberthür) (Lepidoptera: Hepialidae) [273].
5.2. Need for industrial production to produce large quantities of insect-based medicine
The need for insect-based medicine is increasing (Table 10). The emerged medical issues, for example, the increasing cases of cancer due to aging and antibiotic resistance problems leading to urgent requirements of novel drugs [274,275]. Therefore, medicinal insects, which can be potential sources for novel drug discovery, have gained increasing attention in the past decades due to their well-documented functions (e.g., anti-cancer and antimicrobe) [6]. Moreover, people are paying increasing attention to preventive medicine [276]. The willing of health and healing in daily life resulting in the debate of the concept of “food as medicine” [277]. To promote traditional medicine, World Health Organization (WHO) has established the global center for traditional medicine in India [278].
Table 10.
Factors pull/push towards medicinal insect mass production.
| The need for medicinal insect mass production | References |
|---|---|
| Factors pull towards medicinal insect mass production | |
| The need for novel drugs | [274,275] |
| The need for preventive medicines | [276] |
| The establishment of the WHO Global Center for Traditional Medicine | [278] |
| Factors push towards medicinal insect mass production | |
| Over-exploitation leads to species crisis and habitat destruction | [273] |
| Quantity variation | [279] |
| Quality variation | [76] |
| Safety concerns | [280] |
The increased need for medicinal insects has led to overexploitation resulting in severe insect population crisis and habitat damage. For example, the C. sinensis infected ghost moth larva is an important anti-cancer Chinese medicine resource. However, the geographic distribution is confined, which is only available in soil of Qinghai-Tibet Plateau with 3500–5000 altitudes [273]. Overexploitation has pushed the local ghost moth larva facing extinction [273]. Another example is from the bamboo caterpillar, Omphisa fuscidentalis Hampson (Lepidoptera: Crambidae). The traditional harvesting activity usually cut down the entire bamboo clumps which is destructive [281].
Besides, the variation across season is a major challenge in commercialization of wild harvested insects. For example, in Republic of Congo, the migratory locust, Locusta migratoria (L.) (Orthoptera: Acrididae) is only available in November and December while the Termite is only available from November to next April [279]. Referring to the quality variation, modern research has determined many biotic and abiotic factors associated with quality variation. For example, the antimicrobe effects of honey have been determined to vary by species [282], geographical locations [76], types of flowers [225], and the age of honey [208].
More importantly, wild harvested insects have huge safety concerns. Heavy metal is a big concern nowadays due to civilization pollution. For example, the copper level of the wide harvested Mylabris sp. (Coleoptera: Meloidae), which used as an anti-cancer resource, was once determined reaching ∼45 mg/kg resulting in carcinogenic risk [283]. Pathogen contamination is another health risk. For example, pathogens (e.g., Bacillus sp. and Staphylococcus sp.) associated with foodborne disease have been determined in raw edible grasshoppers, Ruspolia differens (Serville) (Orthoptera: Terrigoniidae), in Uganda [284]. Besides, agricultural residues, for example veterinary drugs, antibiotics, and mycotoxins, found in wide harvesting insects become major biohazards in medicinal insect market [280].
5.3. What would mass production of insects look like?
Medicinal insects are only small parts of the beneficial use of insects. Besides bees and silkworms, the mass production of sterile screwworm, Musca macellaria Fabricius (Diptera: Calliphoridae) [285] on artificial diet for bio-control purpose is a milestone in insect mass production [270]. The edible insect has gained significantly increasing attention in the past decade as a nutrient pack for food and feed. Insects in general have higher feed conversion efficiency and lower environmental impacts compared to traditional livestock, which are believed to be one of the key solutions against food crisis [270]. Accordingly, the amount of investment, research, and company work on insect mass production increased significantly [268] after the Food and Agriculture Organization of the United Nations (FAO) recommended insects as food and feed in 2014 [286].
Though the nutrient and environmental requirements differ by species. There are following issues need to be considered before setting up an insect farm (Table 11). To set up a mass production farm is not as easy as a small-scale farm because the high density of insect and the subsequent issues related (e.g., metabolic heat and disease).
Table 11.
Potential aspects to consider for medicinal insect mass production.
| Aspects | Examples | References |
|---|---|---|
| Breeding | Genetic diversity, inbreeding depression, etc. | [280] |
| Feed source | Stable quantity and quality, cost efficiency, nutrient requirements, physical form, etc. | [287,288] |
| Facility | Location, logistic, mass and energy/heat balance, process-type, abiotic factors, remote sensing monitoring system, etc. | [[289], [290], [291]] |
| Processing | Harvesting, killing methods, decontamination, end product form, etc. | [292] |
| Packaging and storage | Lipid oxidation, re-moisturization, etc. | [293] |
| Insect disease | Virus, bacteria, fungus, mites, etc. | [294] |
| Hygiene and sanitation | IPIFF Guide on Good Hygiene Practices | [295] |
| Regulations | Novel food regulation | [296,297] |
5.3.1. Breeding
Now most of the edible insects are obtained by trading and a few are wide-collected and reproduced indoor, which the generic diversity is generally uncleared while the concept of insect breeding for food and feed is new [280]. Domestication is a gene selection process. Attention should be paid to insect industrialization, especially medicinal insects, to avoid inbreeding depression, effective ingredient reduction, and increasing vulnerability to pests and diseases [298]. For example, selection for silkworm cocoon weight trait after four generation resulted in poor survival rates [298].
5.3.2. Feed source
The standard quality and continuous supply of feed is essential for insect mass rearing. Depending on the type of insect, the range of feed sources availability varies. For oligophagous like silkworms, the mass production of mulberries is required traditionally. In order to facilitate the sericulture, artificial diets for silkworms have been particularly developed [299]. Under the scope of edible insect production, omnivorous (e.g., crickets) insects are preferred and huge efforts have been put towards the organic waste stream exploitation and formulation [287,288] to meet the low-eco impact willing in insect farming. Besides feed source exploitation, the nutrient (species, stage, and age dependent) and physical form (mouthpart dependent) requirements [268] should be deeply studied to ensure a healthy and reproductive colony.
5.3.3. Facility
The location, mass and energy/heat balances [289], modelling and simulation [290], logistic [291], and the process-type (e.g., batch and continuous systems) should be carefully considered ahead to ensure environmental control system (e.g., temperature, humidity, and ventilation) meet the insect requirements and the workflow is optimized. Life cycle assessment (LCA) [300] and hazard analysis and critical control points system (HACCP) [301] plus a remote sensing monitoring system would be helpful in dealing with such complex system and towards precision agriculture [302].
5.3.4. Processing
Traditionally, most of the medicinal insects were sun dried and then boiled or fried before consumption [66]. Open and unhygienic drying conditions can cause microbes contamination [303]. Along with the development of edible insect industry, more processing methods were addressed, for example freeze drying, oven-frying, fluidized bed drying, microwave drying [292]. Further processing for protein, lipid, and chitin extraction can be achieved by pressing, ultrasound-assisted extraction, cold atmospheric pressure plasma, and dry fractionation [292]. As an alternative to drying, which is considered as an energy-consuming process, fermentation could be applied to raw insects [304]. While the above process would be enough for edible insects as food and feed, further processing (i.e., refining) may be required for medicinal insects processing to concentrate on the effective ingredients, for example the mass production of Xinmailong requires bioactive fraction extracted from P. americana [305].
5.3.5. Packaging and storage
Lipid oxidation can generate toxic products, which are correlated with inflammatory diseases, cancer, atherosclerosis, and aging [306]. Oxidation is common during processing and storage especially for lipid-rich products like insects [293]. Antioxidants and vacuum-filling nitrogen packaging were determined to be a good method to avoid storage-phase oxidation [293]. Proper packaging and storage environment can also help in remaining low moisture content to suppress microbe growth [307].
5.3.6. Insect disease
Mass production pushed insects to growth and develop at a high density, which provides optimal conditions for insect disease transmission [294]. For example, the A. domesticus densovirus (AdDNV) has caused mass mortality in cricket farms [308]. Hygiene is essential in insect farming to prevent disease transmission. However, once contaminated, shut down the production line and deeply clean the facility seems to be the only option in many cases [308]. Up to date, little is known about the insect pathogens, therefore a programmed called INSECT DOCTORS has been funded in Europe for insect disease specific research [309].
5.3.7. Other
While the above aspects are more related to technical issues, other challenges (e.g., trained technicians, labors, and regulations) need to be overcome by the whole community (e.g., academia, industry, and the consumer society). For example, to avoid food-borne disease contamination, insect farms should follow some hygiene and sanitation protocol. A detail guide on hygiene practices of insect farming can be found on the website of international platform of insects for food and feed [295]. The guide covers legislative requirements from feed stream preparation to harvesting and processing. In European Union (EU) market, insects are viewed as novel food which must be approved following the Novel Food Regulation (EU) 2015/2283. Regulations on insects as food and feed has been reviewed by Ref. [297]. Regulations [296] though ensure consumers receive a good quality product; it usually takes a long time to come out. Therefore, to mass produce medicinal insects that are documented with specific effects, to synthesize the effect compounds, or to discover medicinal effects among approved edible insects, the discussion remains.
6. Future perspectives and conclusions
Insects have been widely used as medicinal resources in many parts of the world since ancient times. Insects can be used alone or combination with medicinal plants in the treatment of diseases [39]. The promotion and application of medicinal insects play a key role in all existing disease treatments. Though insects form part of the human diet in many countries and regions of the world, their use for medicinal purposes is often not promoted, and Western practice of entomotherapy seems dominant. A wide variety of insect species from different orders, such as Blattodea, Coleoptera, Hemiptera Hymenoptera, Lepidoptera, Odonata, and Orthoptera, contribute to the treatment of diseases in humans. Nevertheless, clinical trials assessing diseases' treatment through entomotherapy have received little attention than insects and insect-derived products utilized as food and feed. In the form of eggs, larvae/nymphs, adults and their derived products, insects provide alternative medicinal properties to modern medicine, though a few studies have attempted to address this issue in some countries. Even countries that use insects and insect-derived products have focused on a few geographical locations. As a result, global records on insect and insect-derived products for disease control are poorly documented worldwide, especially in Africa. More than 2100 insect species are eaten by humans in a wide variety of regions and countries, but little is known about the possibility of using these edible insects in the study and development of new medications and vaccines to battle disease. As a result, medicinal uses of insects, including treating diseases induced by pollution, microorganisms, allergens, and other higher animals, such as snakes and scorpions, could provide insight into the benefits of insects in treating emerging diseases and illnesses. Understating sustainable methods of rearing insects in medicine is critical for biodiversity conservation and prevention. However, insect farming is a concern regarding environmental issues and safety.
In effect, captive farming of edible insects can offer feed and food for animals and humans, respectively, and provide resources for the pharmaceutical industry to discover drugs for various health-related complications. Specifically, among others, future research should provide proper identification of these medicinal insects using molecular tools and conduct further investigation to verify and assess the viability of utilizing insects in the drug discovery process. Limited information exists on the reservations about entomotherapy as there are about entomophagy. Moreover, a clear understanding of the therapeutic use of insects and insect-derived products between Western countries and other regions worldwide requires further investigations. There is also a need to assess consumer opinions/consumer science on entomotherapy. Progress has been towards using insects for medicinal purposes. However, knowledge about their side effects is generally lacking. The relationship between disease treatment using insects and insect-derived products and allergenic effects in patients should be considered in future studies. While a clinical evaluation of medicinal plants’ efficacy and safety have been considered by several researchers, such information on medicinal uses insects is poorly documented. Future works should also investigate knowledge, perception, and willingness to apply and pay for entomotherapy. In addition, implications of using insects and insect-derived products on insect biodiversity conservation, the use of insects for the treatment of animal diseases and the contribution of insects to drug discovery may offer a new direction and solution to emerging diseases. Furthermore, among insects used for therapeutic purposes, some are pests responsible for diseases in plants and humans and others play a role in biological control as predators and pollinators of crops. In view for environment and biodiversity conservation, there is a need to select few samples of these insects in investigations necessary to ecological balance.
Author contribution statement
S.A.S. – Conceptualization, Validation, Formal Analysis, Resources, Writing - Original Draft, Writing - Review and Editing, Visualization, Data Curation, Project administration, Supervision. C.L. – Writing - Original Draft. O.F.A. – Writing - Original Draft. I.F. – Conceptualization, Writing - Review and Editing. M.A.H. – Formal Analysis, Validation. J.A.M.P. - Review and Editing. A.B. - Data Curation. A.G. - Data Curation. J.S.C. - Review and Editing.
Funding
This research was funded by FCT-Fundação para a Ciência e a Tecnologia through the CQM Base Fund - UIDB/00674/2020, and Programmatic Fund - UIDP/00674/2020, by Interreg MAC 2014–2020 Cooperacion territorial through AD4MAC project (MAC2/1.1 b/350), and by ARDITI-Agência Regional para o Desenvolvimento da Investigação Tecnologia e Inovação, through the project M1420-01-0145-FEDER-000005 - Centro de Química da Madeira - CQM+ (Madeira 14–20 Program) and the Post-Doctoral fellowship given to JAMP (Project M1420–09–5369-FSE-000001). The authors also acknowledge FCT and Madeira 14–2020 program to the Portuguese Mass Spectrometry Network (RNEM) through PROEQUIPRAM program, M14-20 M1420-01-0145-FEDER-000008).
Conflict of interest
The authors declare no conflict of interest.
Data availability statement
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Shahida Anusha Siddiqui, Email: S.Siddiqui@dil-ev.de.
José S. Câmara, Email: jsc@staff.uma.pt.
References
- 1.Dev S., Hassan K., Claes J., Mozahid M.N., Khatun H., Mondal M.F. Practices of entomophagy and entomotherapy in Bangladesh. J. Insects as Food Feed. 2020;6:515–524. doi: 10.3920/JIFF2020.0038. [DOI] [Google Scholar]
- 2.Park G., Moon B.C., Lim H. Effects of 14 Chung-bu medicinal materials described in the dongui bogam on inflammatory cytokines production in HaCaT keratinocytes. J. Soc. Cosmet. Sci. Korea. 2020;46:195–204. [Google Scholar]
- 3.Guarnieri A., Triunfo M., Scieuzo C., Ianniciello D., Tafi E., Hahn T., Zibek S., Salvia R., De Bonis A., Falabella P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022;12:8084. doi: 10.1038/s41598-022-12150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khayrova A., Lopatin S., Shagdarova B., Sinitsyna O., Sinitsyn A., Varlamov V. Evaluation of antibacterial and antifungal properties of low molecular weight chitosan extracted from Hermetia illucens relative to crab chitosan. Molecules. 2022;27:577. doi: 10.3390/molecules27020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saadoun J.H., Sogari G., Bernini V., Camorali C., Rossi F., Neviani E., Lazzi C. A critical review of intrinsic and extrinsic antimicrobial properties of insects. Trends Food Sci. Technol. 2022;122:40–48. doi: 10.1016/j.tifs.2022.02.018. [DOI] [Google Scholar]
- 6.Seabrooks L., Hu L. Insects: an underrepresented resource for the discovery of biologically active natural products. Acta Pharm. Sin. B. 2017;7:409–426. doi: 10.1016/j.apsb.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hossain M.L., Lim L.Y., Hammer K., Hettiarachchi D., Locher C. Honey-based medicinal formulations: a critical review. Appl. Sci. 2021;11:5159. doi: 10.3390/app11115159. [DOI] [Google Scholar]
- 8.Costa-Neto E.M. Animal-based medicines: biological prospection and the sustainable use of zootherapeutic resources. An. Acad. Bras. Cienc. 2005;77:33–43. doi: 10.1590/S0001-37652005000100004. [DOI] [PubMed] [Google Scholar]
- 9.Al-Kafaween M.A., Alwahsh M., Mohd Hilmi A.B., Abulebdah D.H. Physicochemical characteristics and bioactive compounds of different types of honey and their biological and therapeutic properties: a comprehensive review. Antibiotics. 2023;12:337. doi: 10.3390/antibiotics12020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meda A., Lamien C.E., Millogo J., Romito M., Nacoulma O.G. Therapeutic uses of honey and honeybee larvae in central Burkina Faso. J. Ethnopharmacol. 2004;95:103–107. doi: 10.1016/j.jep.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Wigglesworth V.B. Springer US; Boston, MA: 1976. Insects and the Life of Man. [DOI] [Google Scholar]
- 12.Ratcliffe B.C. The significance of scarab beetles in the ethnoentomology of non-industrial, indigenous peoples. Proc. First Int. Congr. Ethnobiol. 1990;1:159–185. [Google Scholar]
- 13.Soares S., Lopes K.S., Mortari M., Oliveira H., Bastos V. Antitumoral potential of Chartergellus-CP1 peptide from Chartergellus communis wasp venom in two different breast cancer cell lines (HR+ and triple-negative) Toxicon. 2022;216:148–156. doi: 10.1016/j.toxicon.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Whitman Andrés, Martínez-Díaz Ibáñez-Escribano, Olmeda González-Coloma. Antiparasitic properties of cantharidin and the blister beetle Berberomeloe majalis (Coleoptera: Meloidae) Toxins. 2019;11:234. doi: 10.3390/toxins11040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma A.K., Prasad S.B. Bioactive component, cantharidin from Mylabris cichorii and its antitumor activity against ehrlich ascites carcinoma. Cell Biol. Toxicol. 2012;28:133–147. doi: 10.1007/s10565-011-9206-6. [DOI] [PubMed] [Google Scholar]
- 16.Weiss H.B. Entomological medicaments of the past. J. New York Entomol. Soc. 1947;55:155–168. [Google Scholar]
- 17.Zimmer C. The healing power of maggots. Discover. 1993;17 [Google Scholar]
- 18.Zimian D., Yonghua Z., Xiwu G. Medicinal insects in China, ecol. Food Nutr. 1997;36:209–220. doi: 10.1080/03670244.1997.9991516. [DOI] [Google Scholar]
- 19.Costa-Neto E.M. The use of insects in folk medicine in the state of Bahia, northeastern Brazil, with notes on insects reported elsewherein Brazilian folk medicine. Hum. Ecol. 2002;30:245–263. doi: 10.1023/A:1015696830997. [DOI] [Google Scholar]
- 20.Bairagi S.H. Insects with potential medicinal significance: a review. Biomed. J. Sci. Tech. Res. 2019;16 doi: 10.26717/BJSTR.2019.16.002849. [DOI] [Google Scholar]
- 21.Sheikh I.U., Banday I.M., Shaista Nissa I.S., Bushra Zaffer I., Bulbul I.K., Husbandry A., Nissa S.S., Zaffer B., Bulbul K. Utilization of silkworm pupae meal as an alternative source of protein in the diet of livestock and poultry: a review. J. Entomol. Zool. Stud. 2018;6:1010–1016. [Google Scholar]
- 22.Rather L.J., Ansari M.F., Li Q. In: Nat. Mater. Prod. From Insects Chem. Appl. Kumar D., Shahid M., editors. Springer International Publishing; Cham: 2020. Recent advances in the insect natural product chemistry: structural diversity and their applications; pp. 67–94. [DOI] [Google Scholar]
- 23.Harvey M.L., Dadour I.R., Gasz N.E. Maggot therapy in chronic wounds: new approaches to historical practices. Ann. Entomol. Soc. Am. 2021;114:415–424. doi: 10.1093/aesa/saab012. [DOI] [Google Scholar]
- 24.Ali A.M., Kunugi H. Apitherapy for Parkinson's disease: a focus on the effects of propolis and royal jelly. Oxid. Med. Cell. Longev. 2020;2020:1–18. doi: 10.1155/2020/1727142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhary P., Kumar Sharma A., Kumar Mishra Y., Nayak S. Entomotherapy medicinal significance of insects: a review. Pharm. Innov. 2022;11:25–29. http://www.thepharmajournal.com [Google Scholar]
- 26.Meyer-Rochow V.B. Therapeutic arthropods and other, largely terrestrial, folk-medicinally important invertebrates: a comparative survey and review. J. Ethnobiol. Ethnomed. 2017;13:9. doi: 10.1186/s13002-017-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czaja O. 2019. The use of insects in Tibetan medicine, Études Mongoles et Sibériennes, Centrasiatiques et Tibétaines. [DOI] [Google Scholar]
- 28.Mozhui L., Kakati L.N., Meyer-Rochow V.B. Entomotherapy: a study of medicinal insects of seven ethnic groups in Nagaland, North-East India. J. Ethnobiol. Ethnomed. 2021;17:17. doi: 10.1186/s13002-021-00444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borah M.P., Prasad S.B. Ethnozoological study of animals based medicine used by traditional healers and indigenous inhabitants in the adjoining areas of Gibbon Wildlife Sanctuary, Assam, India. J. Ethnobiol. Ethnomed. 2017;13:39. doi: 10.1186/s13002-017-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y., Yang A., Tu P., Hu Z. Anti-tumor effects of the American cockroach, Periplaneta americana. Chin. Med. 2017;12:26. doi: 10.1186/s13020-017-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin H.-Q., Wang B., Zhang J.-D., Lin H.-Q., Qiao Y., Wang R., Liu F.-Y. Effect of traditional Chinese medicine Shu-Mai-Tang on attenuating TNFα-induced myocardial fibrosis in myocardial ischemia rats. J. Ethnopharmacol. 2008;118:133–139. doi: 10.1016/j.jep.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Alonso-Castro A.J. Use of medicinal fauna in Mexican traditional medicine. J. Ethnopharmacol. 2014;152:53–70. doi: 10.1016/j.jep.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Albuquerque U.P., Melo J.G., Medeiros M.F., Menezes I.R., Moura G.J., Asfora El-Deir A.C., Nóbrega Alves R.R., de Medeiros P.M., de Sousa Araújo T.A., Alves Ramos M., Silva R.R., Almeida A.L., Almeida C. de F.C. Natural products from ethnodirected studies: revisiting the ethnobiology of the zombie poison, Evidence-Based Complement. Alternative Med. 2012;2012:1–19. doi: 10.1155/2012/202508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira F.S., Albuquerque U.P., Coutinho H.D.M., Almeida W. de O., Alves R.R. da N. The trade in medicinal animals in northeastern Brazil, Evidence-Based Complement. Alternative Med. 2013;22:1–20. doi: 10.1155/2012/126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Figueirêdo R.E.C.R., Vasconcellos A., Policarpo I.S., Alves R.R.N. Edible and medicinal termites: a global overview. J. Ethnobiol. Ethnomed. 2015;11:29. doi: 10.1186/s13002-015-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilsanand V. 2005. Utilization of Termite, Odontotermes Formosanus by Tribes of South India in Medicine and Food. [Google Scholar]
- 37.Marie R. Strasbourg University; 1955. Contribution a l’histoire des insectes en the'rapeutique. [Google Scholar]
- 38.Mbata K.J. Traditional uses of ar- thropods in Zambia: II. Medicinal and miscellaneous uses. Food Insects Newsl. 1991;12:1–7. [Google Scholar]
- 39.Ouango M., Romba R., Drabo S.F., Ouedraogo N., Gnankiné O. Indigenous knowledge system associated with the uses of insects for therapeutic or medicinal purposes in two main provinces of Burkina Faso, West Africa. J. Ethnobiol. Ethnomed. 2022;18:50. doi: 10.1186/s13002-022-00547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marques Jose G.W., Costa-Neto E.M. Insects as folk medicine in the state of alagoas, Brazil. Proc. 8th Int. Conf. Tradit. Med. Folk. 1994;4:115–119. [Google Scholar]
- 41.Green S.V. The bushman as an entomologist. ANTENNA. 1998;22:4–8. [Google Scholar]
- 42.Berenbaum M. Addison-Wesley; Reading: 1995. Bugs in the System: Insects and Their Impact on Human Affairs. [Google Scholar]
- 43.Costa-Neto E.M. Recursos animais utilizados na medicina tradicional dos índios Pankararé que habitam no nordeste do estado da Bahia, Brasil. Actual. Biol. 1999;21:69–79. [Google Scholar]
- 44.Lacroix I.M.E., Dávalos Terán I., Fogliano V., Wichers H.J. Investigation into the potential of commercially available lesser mealworm (A. diaperinus) protein to serve as sources of peptides with DPP‐IV inhibitory activity. Int. J. Food Sci. Technol. 2019;54:696–704. doi: 10.1111/ijfs.13982. [DOI] [Google Scholar]
- 45.Verheyen G.R., Pieters L., Maregesi S., Van Miert S. Insects as diet and therapy: perspectives on their use for combating diabetes mellitus in Tanzania. Pharmaceuticals. 2021;14:1273. doi: 10.3390/ph14121273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoenle P.O., Blüthgen N., Brückner A., Kronauer D.J.C., Fiala B., Donoso D.A., Smith M.A., Ospina Jara B., von Beeren C. Species-level predation network uncovers high prey specificity in a Neotropical army ant community. Mol. Ecol. 2019;28:2423–2440. doi: 10.1111/mec.15078. [DOI] [PubMed] [Google Scholar]
- 47.Zielińska E., Baraniak B., Karaś M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus , Tenebrio molitor , Schistocerca gragaria) Int. J. Food Sci. Technol. 2018;53:2542–2551. doi: 10.1111/ijfs.13848. [DOI] [Google Scholar]
- 48.Nallely M., Esmeralda V., Merari A., Gisela G., Josefina R., Minarda D., Carlos R. Is ingestion of Thasus gigas (Xamues) an alimentary culture or an auxiliary treatment for type II diabetes? Afr. J. Tradit., Complementary Altern. Med. 2014;11:131. doi: 10.4314/ajtcam.v11i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J., Wang H., Huang J., Li G., Wang Q., Xu W., Chen Y., Zhang K., Wang J. Sesquiterpene acids from Shellac and their bioactivities evaluation. Fitoterapia. 2014;97:64–70. doi: 10.1016/j.fitote.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Kritsky G. Take two cicadas and call me in the morning. Bull. Entomol. Soc. Am. 2014;33:139–141. doi: 10.1093/besa/33.3.139. [DOI] [Google Scholar]
- 51.Langthasa S., Teron R., Tamuli A.K. Cross-cultural entomotherapy in dima hasao district, Assam. Indian J. Entomol. 2019;81:526. doi: 10.5958/0974-8172.2019.00114.7. [DOI] [Google Scholar]
- 52.Prakash S., Bhargava H.R. Apis cerana bee venom: it is antidiabetic and anti-dandruff activity against Malassezia furfur. World Appl. Sci. J. 2014;32:343–348. doi: 10.5829/idosi.wasj.2014.32.03.947. [DOI] [Google Scholar]
- 53.Mousavi S.M., Imani S., Haghighi S., Mousavi S.E., Karimi A. Effect of Iranian honey bee (Apis mellifera) venom on blood glucose and insulin in diabetic rats. J. Arthropod. Borne. Dis. 2012;6:136–143. [PMC free article] [PubMed] [Google Scholar]
- 54.Chinlampianga M., Singh R.K., Shukla A.C. Ethnozoological diversity of Northeast India: empirical learning with traditional knowledge holders of Mizoram and Arunachal Pradesh. Indian J. Tradit. Knowl. 2013;12:18–30. [Google Scholar]
- 55.Vit P., Vargas O., Lpez T., Valle F. Meliponini biodiversity and medicinal uses of pot-honey from El Oro province in Ecuador. Emir. J. Food Agric. 2015;27:502–506. doi: 10.9755/ejfa.2015.04.079. [DOI] [Google Scholar]
- 56.Groark K.P. Taxonomic identity of “hallucinogenic” harvester ant (Pogonomyrmex californicus) confirmed. J. Ethnobiol. 2001;21:133–144. [Google Scholar]
- 57.Dutta L., Ghosh S.S., Deka P., Deka K. Terrestrial edible insects and their therapeutic value in Moridhal Panchayat of Dhemaji district , Assam. Int. J. Fauna Biol. Stud. 2016;3:11–14. [Google Scholar]
- 58.Jena S., Das S.S., Sahu H.K. Traditional value of red weaver ant (Oecophylla smaragdina) as food and medicine in Mayurbhanj District of Odisha, India. Int. J. Res. Appl. Sci. Eng. Technol. 2020;8:936–946. doi: 10.22214/ijraset.2020.5148. [DOI] [Google Scholar]
- 59.Rastogi N. Provisioning services from ants: food and pharmaceuticals. Asian Myrmecology. 2011;4:103–120. [Google Scholar]
- 60.Vidhu V.V., Evans D.A. Ethnoentomological values of Oecophylla smaragdina (Fabricius) Curr. Sci. 2015;109:572–579. [Google Scholar]
- 61.Tang J.-J., Zhang L., Jiang L.-P., Di L., Yan Y.-M., Tu Z.-C., Yang C.-P., Zuo Z.-L., Hou B., Xia H.-L., Chen Y.-B., Cheng Y.-X. Dopamine derivatives from the insect Polyrhachis dives as inhibitors of ROCK1/2 and stimulators of neural stem cell proliferation. Tetrahedron. 2014;70:8852–8857. doi: 10.1016/j.tet.2014.09.095. [DOI] [Google Scholar]
- 62.Maya E.M.A. Universidad Nacional Auto′noma de Me′xico, Iztacala; Hidalgo: 2000. Etnoentomologı'a de la comunidad Hñähñu, El Dexthi-San Juanico. [Google Scholar]
- 63.Alves R.R., Alves H.N. The faunal drugstore: animal-based remedies used in traditional medicines in Latin America. J. Ethnobiol. Ethnomed. 2011;7:9. doi: 10.1186/1746-4269-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vallejo J.R., González J.A. The use of the head louse as a remedy for jaundice in Spanish folk medicine: an overview. J. Ethnobiol. Ethnomed. 2013;9:52. doi: 10.1186/1746-4269-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Che J. Chemical Industry Press; 2019. A Guide to 100 Edible and Medicinal Insects. [Google Scholar]
- 66.Yang D. China Science and Technology Press; 2015. Important Chinese Medicinal Insects. [Google Scholar]
- 67.Cui K., Lu W., Zhu L., Shen X., Huang J. Caffeic acid phenethyl ester (CAPE), an active component of propolis, inhibits Helicobacter pylori peptide deformylase activity. Biochem. Biophys. Res. Commun. 2013;435:289–294. doi: 10.1016/j.bbrc.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 68.Díaz-Roa A., Espinoza-Culupú A., Torres-García O., Borges M.M., Avino I.N., Alves F.L., Miranda A., Patarroyo M.A., da Silva P.I., Bello F.J. Sarconesin II, a new antimicrobial peptide isolated from Sarconesiopsis magellanica excretions and secretions. Molecules. 2019;24:2077. doi: 10.3390/molecules24112077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bohova J., Majtan J., Majtan V., Takac P. Selective antibiofilm effects of Lucilia sericata larvae secretions/excretions against wound pathogens, Evidence-Based Complement. Alternative Med. 2014;2014:1–9. doi: 10.1155/2014/857360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.da Cunha M.G., de Cássia Orlandi Sardi J., Freires I.A., Franchin M., Rosalen P.L. Antimicrobial, anti-adherence and antibiofilm activity against Staphylococcus aureus of a 4-phenyl coumarin derivative isolated from Brazilian geopropolis. Microb. Pathog. 2020;139 doi: 10.1016/j.micpath.2019.103855. [DOI] [PubMed] [Google Scholar]
- 71.Dodou Lima H.V., de Paula Cavalcante C.S., Rádis-Baptista G. Antifungal in vitro activity of pilosulin- and ponericin-like peptides from the giant ant Dinoponera quadriceps and synergistic effects with antimycotic drugs. Antibiotics. 2020;9:354. doi: 10.3390/antibiotics9060354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang K., Jia F., Dang W., Zhao Y., Zhu R., Sun M., Qiu S., An X., Ma Z., Zhu Y., Yan J., Kong Z., Yan W., Wang R. Antifungal effect and action mechanism of antimicrobial peptide polybia-CP. J. Pept. Sci. 2016;22:28–35. doi: 10.1002/psc.2835. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X., Su H., Yu H., Ding J., Deng W., Qin B., Zhou C., Dou J., Guo M. A polysaccharide from Eupolyphaga sinensis Walker with Anti-HBV activities in vitro and in vivo. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.827128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie X., Shen W., Zhou Y., Ma L., Xu D., Ding J., He L., Shen B., Zhou C. Characterization of a polysaccharide from Eupolyphaga sinensis Walker and its effective antitumor activity via lymphocyte activation. Int. J. Biol. Macromol. 2020;162:31–42. doi: 10.1016/j.ijbiomac.2020.06.120. [DOI] [PubMed] [Google Scholar]
- 75.Ma H., Li X., Che J., Fan H., Liu Q., Xia H. The inhibitory effect of Periplaneta americana L. on hepatocellular carcinoma: explore the anti-hepatocellular carcinoma active site and its mechanism of action. J. Ethnopharmacol. 2022;291 doi: 10.1016/j.jep.2021.114884. [DOI] [PubMed] [Google Scholar]
- 76.Badrulhisham N.S.R., Ab Hamid S.N.P., Ismail M.A.H., Yong Y.K., Muhamad Zakuan N., Harith H.H., Saidi H.I., Nurdin A. Harvested locations influence the total phenolic content, antioxidant levels, cytotoxic, and anti-inflammatory activities of stingless bee honey. J. Asia Pac. Entomol. 2020;23:950–956. doi: 10.1016/j.aspen.2020.07.015. [DOI] [Google Scholar]
- 77.de Matos F.M., de Lacerda J.T.J.G., Zanetti G., de Castro R.J.S. Production of black cricket protein hydrolysates with α-amylase, α-glucosidase and angiotensin I-converting enzyme inhibitory activities using a mixture of proteases. Biocatal. Agric. Biotechnol. 2022;39 doi: 10.1016/j.bcab.2022.102276. [DOI] [Google Scholar]
- 78.Melo da Cunha J. da S., Alfredo T.M., dos Santos J.M., Alves Junior V.V., Rabelo L.A., Lima E.S., Boleti A.P. de A., Carollo C.A., dos Santos E.L., de Picoli Souza K. Antioxidant, antihyperglycemic, and antidiabetic activity of Apis mellifera bee tea. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mei H., Xu J., He Y., Yang X., Liu W., Tian W., Zeng Y., Zhu J. Protein-rich extract of Musca domestica larvae alleviated metabolic disorder in STZ-induced type 2 diabetic rat model via hepatoprotective and pancreatic β-cell protective activities. J. Biosci. 2018;43:969–983. doi: 10.1007/s12038-018-9804-z. [DOI] [PubMed] [Google Scholar]
- 80.Wang H.-Y., Wang Y.-J., Zhou L.-X., Zhu L., Zhang Y.-Q. Isolation and bioactivities of a non-sericin component from cocoon shell silk sericin of the silkworm Bombyx mori. Food Funct. 2012;3:150–158. doi: 10.1039/C1FO10148J. [DOI] [PubMed] [Google Scholar]
- 81.Liu M., Tu J., Feng Y., Zhang J., Wu J. Synergistic co-delivery of diacid metabolite of norcantharidin and ABT-737 based on folate-modified lipid bilayer-coated mesoporous silica nanoparticle against hepatic carcinoma. J. Nanobiotechnol. 2020;18:114. doi: 10.1186/s12951-020-00677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shan J., Ping-Ping D., Hao-Ran L.I., Jing X.U., Hua-Jian L.I., Ying-Ying Y.U., Long D., Peng G., Shao-Ping W., Jia-Yu Z. [Study on lipid-lowering mechanism of active peptide DP17 from Eupolyphaga steleophaga in hyperlipidemia rats] Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chinese Mater. medica. 2020;45:5265–5272. doi: 10.19540/j.cnki.cjcmm.20200709.403. [DOI] [PubMed] [Google Scholar]
- 83.Park B.-S., Park S.-O. Lowering lipid mechanism of the ethanol extracts from maggot of Musca domestica in rats fed a high-cholesterol diet. Int. J. Pharmacol. 2015;11:1–9. doi: 10.3923/ijp.2015.1.9. [DOI] [Google Scholar]
- 84.Bergmans R.S., Nikodemova M., Stull V.J., Rapp A., Malecki K.M.C. Comparison of cricket diet with peanut-based and milk-based diets in the recovery from protein malnutrition in mice and the impact on growth, metabolism and immune function. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.dos Anjos L.C., Gomes F.M.M., do Couto L.L., Mourão C.A., Moreira K.G., Silva L.P., Mortari M.R. Anxiolytic activity and evaluation of potentially adverse effects of a bradykinin-related peptide isolated from a social wasp venom. Life Sci. 2016;149:153–159. doi: 10.1016/j.lfs.2016.02.063. [DOI] [PubMed] [Google Scholar]
- 86.Zeinalpour Y., Kabiri M., Rahimi R., Karimi R. The effect of medicinal syrup made from silkworm cocoon on mixed anxiety-depression disorder: a triple-blind randomized clinical trial, Iran. Red Crescent Med. J. 2021;23 doi: 10.32592/ircmj.2021.23.12.1345. [DOI] [Google Scholar]
- 87.He Y., Yang X., Jiao M., Anoopkumar-Dukie S., Zeng Y., Mei H. Housefly (Musca domestica) larvae powder, preventing oxidative stress injury via regulation of UCP4 and CyclinD1 and modulation of JNK and P38 signaling in APP/PS1 mice. Food Funct. 2019;10:235–243. doi: 10.1039/C8FO02052C. [DOI] [PubMed] [Google Scholar]
- 88.Han S.M., Kim J.M., Park K.K., Chang Y.C., Pak S.C. Neuroprotective effects of melittin on hydrogen peroxide-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. BMC Compl. Alternative Med. 2014;14:286. doi: 10.1186/1472-6882-14-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thapa P., Katila N., Choi D.-Y., Choi H., Nam J.-W. Suntamide A, a neuroprotective cyclic peptide from Cicadidae Periostracum. Bioorg. Chem. 2021;106 doi: 10.1016/j.bioorg.2020.104493. [DOI] [PubMed] [Google Scholar]
- 90.Kurauchi Y., Hisatsune A., Isohama Y., Mishima S., Katsuki H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic factor. Br. J. Pharmacol. 2012;166:1151–1168. doi: 10.1111/j.1476-5381.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang N., Shu H.-Y., Huang T., Zhang Q.-L., Li D., Zhang G.-Q., Peng X.-Y., Liu C.-F., Luo W.-F., Hu L.-F. Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lim H.-S., Kim J.-S., Moon B.C., Choi G., Ryu S.M., Lee J., Ang M.J., Jeon M., Moon C., Park G. Cicadidae periostracum, the cast-off skin of cicada, protects dopaminergic neurons in a model of Parkinson's disease. Oxid. Med. Cell. Longev. 2019;2019:1–17. doi: 10.1155/2019/5797512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Albanesi M., Nico A., Sinisi A., Giliberti L., Rossi M.P., Rossini M., Kourtis G., Rucco A.S., Loconte F., Muolo L., Zurlo M., Di Bona D., Caiaffa M.F., Macchia L. A 13-year real-life study on efficacy, safety and biological effects of Vespula venom immunotherapy. Clin. Mol. Allergy. 2018;16:2. doi: 10.1186/s12948-017-0079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee W., Lee H., Kim M.-A., Choi J., Kim K.-M., Hwang J.S., Na M., Bae J.-S. Evaluation of novel factor Xa inhibitors from Oxya chinensis sinuosa with anti-platelet aggregation activity. Sci. Rep. 2017;7:7934. doi: 10.1038/s41598-017-08330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.da Costa Madeira J., Quinet Y.P., Nonato D.T.T., Sousa P.L., Chaves E.M.C., Júnior J.E.R.H., Pereira M.G., Assreuy A.M.S. Novel pharmacological properties of Dinoponera quadriceps giant ant venom. Nat. Prod. Commun. 2015;10 doi: 10.1177/1934578X1501000930. 1934578X1501000. [DOI] [PubMed] [Google Scholar]
- 96.Yang C., Zhang L., Dai R., Ji S., Li F., Shao W., Que Y., Hu L., Lin Q. Vasoconstrictive effect of Xinmailong injection in rat aorta. Afr. J. Tradit., Complementary Altern. Med. 2015;12:46. doi: 10.4314/ajtcam.v12i5.7. [DOI] [Google Scholar]
- 97.Pattarayingsakul W., Nilavongse A., Reamtong O., Chittavanich P., Mungsantisuk I., Mathong Y., Prasitwuttisak W., Panbangred W. Angiotensin-converting enzyme inhibitory and antioxidant peptides from digestion of larvae and pupae of Asian weaver ant, Oecophylla smaragdina , Fabricius. J. Sci. Food Agric. 2017;97:3133–3140. doi: 10.1002/jsfa.8155. [DOI] [PubMed] [Google Scholar]
- 98.Wang P., Shang E., Fan X. Effect of San’ao decoction with scorpio and Bombyx batryticatus on CVA mice model via airway inflammation and regulation of TRPA1/TRPV1/TRPV5 channels. J. Ethnopharmacol. 2021;264 doi: 10.1016/j.jep.2020.113342. [DOI] [PubMed] [Google Scholar]
- 99.Kim S.-H., Hong J.-H., Yang W.-K., Kim H.-J., An H.-J., Lee Y.-C. Cryptotympana pustulata extract and its main active component, oleic acid, inhibit ovalbumin-induced allergic airway inflammation through inhibition of Th2/GATA-3 and interleukin-17/RORγt signaling pathways in asthmatic mice. Molecules. 2021;26:1854. doi: 10.3390/molecules26071854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang B.B., Chang M.H., Lee J.H., Heo W., Kim J.K., Pan J.H., Kim Y.J., Kim J.H. The edible insect Gryllus bimaculatus protects against gut-derived inflammatory responses and liver damage in mice after acute alcohol exposure. Nutrients. 2019;11:857. doi: 10.3390/nu11040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li D., Li W., Chen Y., Liu L., Ma D., Wang H., Zhang L., Zhao S., Peng Q. Anti-fibrotic role and mechanism of Periplaneta americana extracts in CCl4-induced hepatic fibrosis in rats. Acta Biochim. Biophys. Sin. 2018;50:491–498. doi: 10.1093/abbs/gmy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tian M., Dong J., Wang Z., Lu S., Geng F. The effects and mechanism of Kangfuxin on improving healing quality and preventing recurrence of gastric ulcer. Biomed. Pharmacother. 2021;138 doi: 10.1016/j.biopha.2021.111513. [DOI] [PubMed] [Google Scholar]
- 103.Yoon I.N., Lu L.F., Hong J., Zhang P., Kim D.H., Kang J.K., Hwang J.S., Kim H. The American cockroach peptide periplanetasin-4 inhibits Clostridium difficile toxin A-induced cell toxicities and inflammatory responses in the mouse gut. J. Pept. Sci. 2017;23:833–839. doi: 10.1002/psc.3046. [DOI] [PubMed] [Google Scholar]
- 104.Lin Q., Fu Q., Su G., Chen D., Yu B., Luo Y., Zheng P., Mao X., Huang Z., Yu J., Luo J., Yan H., He J. Protective effect of Bombyx mori gloverin on intestinal epithelial cells exposure to enterotoxigenic E. coli. Braz. J. Microbiol. 2021;52:1235–1245. doi: 10.1007/s42770-021-00532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen Y., Sun J., Niu C., Yu D., Chen Z., Cong W., Geng F. Mechanistic evaluation of gastroprotective effects of Kangfuxin on ethanol-induced gastric ulcer in mice. Chem. Biol. Interact. 2017;273:115–124. doi: 10.1016/j.cbi.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 106.Lu K., Zhou J., Deng J., Li Y., Wu C., Bao J. Periplaneta americana oligosaccharides exert anti-inflammatory activity through immunoregulation and modulation of gut microbiota in acute colitis mice model. Molecules. 2021;26:1718. doi: 10.3390/molecules26061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma X., Hu Y., Li X., Zheng X., Wang Y., Zhang J., Fu C., Geng F. Periplaneta americana ameliorates dextran sulfate sodium-induced ulcerative colitis in rats by Keap1/Nrf-2 activation, intestinal barrier function, and gut microbiota regulation. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peng S., Ling X., Rui W., Jin X., Chu F. LMWP (S3-3) from the larvae of Musca domestica Alleviate D-IBS by adjusting the gut microbiota. Molecules. 2022;27:4517. doi: 10.3390/molecules27144517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang S.P. Effect of active peptides of Eupolyphaga on intestinal flora in rats with hyperlipemia. Chin. Pharmacol. Bull. 2020;621–626:621–626. [Google Scholar]
- 110.Kim H.-J., Kim K.-Y., Ji S.-D., Lee H.-T. Anti-melanogenic activity of steamed and freeze-dried mature silkworm powder. J. Asia Pac. Entomol. 2017;20:1001–1006. doi: 10.1016/j.aspen.2017.07.013. [DOI] [Google Scholar]
- 111.Im A.-R., Ji K.-Y., Park I., Lee J.Y., Kim K.M., Na M., Chae S. Anti-photoaging effects of four insect extracts by downregulating matrix metalloproteinase expression via mitogen-activated protein kinase-dependent signaling. Nutrients. 2019;11:1159. doi: 10.3390/nu11051159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang N., Zhao Y., Shi Y., Chen R., Fu X., Zhao Y. Polypeptides extracted from Eupolyphaga sinensis Walker via enzymic digestion alleviate UV radiation-induced skin photoaging. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108636. [DOI] [PubMed] [Google Scholar]
- 113.Rujimongkon K., Ampawong S., Reamtong O., Buaban T., Aramwit P. The therapeutic effects of Bombyx mori sericin on rat skin psoriasis through modulated epidermal immunity and attenuated cell proliferation. J. Tradit. Complement. Med. 2021;11:587–597. doi: 10.1016/j.jtcme.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kahl M., Gökçen A., Fischer S., Bäumer M., Wiesner J., Lochnit G., Wygrecka M., Vilcinskas A., Preissner K. Maggot excretion products from the blowfly Lucilia sericata contain contact phase/intrinsic pathway-like proteases with procoagulant functions. Thromb. Haemostasis. 2015;114:277–288. doi: 10.1160/TH14-06-0499. [DOI] [PubMed] [Google Scholar]
- 115.de Masiero F.S., Nassu M.P., Soares M.P., Thyssen P.J. Histological patterns in healing chronic wounds using Cochliomyia macellaria (Diptera: Calliphoridae) larvae and other therapeutic measures. Parasitol. Res. 2015;114:2865–2872. doi: 10.1007/s00436-015-4487-y. [DOI] [PubMed] [Google Scholar]
- 116.Borkataki S., Katoch R., Goswani P., Bhat A., Chakrabotty D. Acceleration of cutaneous wound healing by Lucilia sericata maggots in diabetic Wistar rats. Trop. Biomed. 2021;38:86–93. doi: 10.47665/tb.38.1.015. [DOI] [PubMed] [Google Scholar]
- 117.Szczepanowski Z., Grabarek B.O., Boroń D., Tukiendorf A., Kulik‐Parobczy I., Miszczyk L. Microbiological effects in patients with leg ulcers and diabetic foot treated with Lucilia sericata larvae. Int. Wound J. 2022;19:135–143. doi: 10.1111/iwj.13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Han S., Park K., Nicholls Y., Macfarlane N., Duncan G. Effects of honeybee (Apis mellifera) venom on keratinocyte migration in vitro. Phcog. Mag. 2013;9:220. doi: 10.4103/0973-1296.113271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martinotti S., Pellavio G., Laforenza U., Ranzato E. Propolis induces AQP3 expression: a possible way of action in wound healing. Molecules. 2019;24:1544. doi: 10.3390/molecules24081544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Z., Hu Y., Li J., Zhang C., Gao F., Ma X., Zhang J., Fu C., Geng F. A feasible biocompatible hydrogel film embedding Periplaneta americana extract for acute wound healing. Int. J. Pharm. 2019;571 doi: 10.1016/j.ijpharm.2019.118707. [DOI] [PubMed] [Google Scholar]
- 121.Góngora J., Díaz-Roa A., Ramírez-Hernández A., Cortés-Vecino J.A., Gaona M.A., Patarroyo M.A., Bello F. Evaluating the effect of Sarconesiopsis magellanica (Diptera: Calliphoridae) larvae-derived haemolymph and fat body extracts on chronic wounds in diabetic rabbits. J. Diabetes Res. 2015;2015:1–10. doi: 10.1155/2015/270253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zou Y., Zhang M., Zeng D., Ruan Y., Shen L., Mu Z., Zou J., Xie C., Yang Z., Qian Z., Xu R., Li S., Kang Q., Zou H., Zhao S., Liu L., Wang K., Wang X., Zhang X. Periplaneta americana extracts accelerate liver regeneration via a complex network of pathways. Front. Pharmacol. 2020;11:1–12. doi: 10.3389/fphar.2020.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li X., Liu N., Xia X., Zhang S., Bai J., Wang J. The effects of maggot secretions on the inflammatory cytokines in serum of traumatic rats. Afr. J. Tradit., Complementary Altern. Med. 2013;10 doi: 10.4314/ajtcam.v10i4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Telford G., Brown A.P., Rich A., English J.S.C., Pritchard D.I. Wound debridement potential of glycosidases of the wound-healing maggot, Lucilia sericata. Med. Vet. Entomol. 2012;26:291–299. doi: 10.1111/j.1365-2915.2011.01000.x. [DOI] [PubMed] [Google Scholar]
- 125.Kui W., Ying F., Long S.U.N., Zhao H.E., Zhi-yong C. Isolation of ethyl acetate extract from Periplaneta americana and its antimicrobial activity. For. Res. 2013;26:163–166. http://www.lykxyj.com//article/id/20130206 [Google Scholar]
- 126.Amer A., Hamdy B., Mahmoud D., Elanany M., Rady M., Alahmadi T., Alharbi S., AlAshaal S. Antagonistic activity of bacteria isolated from the Periplaneta americana L. gut against some multidrug-resistant human pathogens. Antibiotics. 2021;10:294. doi: 10.3390/antibiotics10030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Long Y., Zhang Y., Huang F., Liu S., Gao T., Zhang Y. Diversity and antimicrobial activities of culturable actinomycetes from Odontotermes formosanus (Blattaria: Termitidae) BMC Microbiol. 2022;22:80. doi: 10.1186/s12866-022-02501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maroufi Y., Ghaffarifar F., Dalimi A., Sharifi Z. Interferon-Gamma and Interlukin-4 patterns in BALB/c mice suffering from cutaneous leishmaniasis treated with cantharidin. Jundishapur J. Microbiol. 2014;7 doi: 10.5812/jjm.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee J., Lee D., Choi H., Kim H.H., Kim H., Hwang J.S., Lee D.G., Il Kim J. Synthesis and antimicrobial activity of cysteine-free coprisin nonapeptides. Biochem. Biophys. Res. Commun. 2014;443:483–488. doi: 10.1016/j.bbrc.2013.11.125. [DOI] [PubMed] [Google Scholar]
- 130.Xu C., Cao X., Wang Y., Wang Q., Sun R. Purification of a galactose-specific lectin with antibacterial and mitogenic activity from Musca domestica pupae. J. Pure Appl. Microbiol. 2013;7:494–503. [Google Scholar]
- 131.Zhou J., Fang N.-N., Zheng Y., Liu K.-Y., Mao B., Kong L.-N., Chen Y., Ai H. Identification and characterization of two novel C-type lectins from the larvae of housefly, Musca domestica L. Arch. Insect Biochem. Physiol. 2018;98 doi: 10.1002/arch.21467. [DOI] [PubMed] [Google Scholar]
- 132.Pöppel A.-K., Vogel H., Wiesner J., Vilcinskas A. Antimicrobial peptides expressed in medicinal maggots of the blow fly Lucilia sericata show combinatorial activity against bacteria. Antimicrob. Agents Chemother. 2015;59:2508–2514. doi: 10.1128/AAC.05180-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Masiero F.S., Aquino M.F.K., Nassu M.P., Pereira D.I.B., Leite D.S., Thyssen P.J. First record of larval secretions of Cochliomyia macellaria (Fabricius, 1775) (Diptera: Calliphoridae) inhibiting the growth of Staphylococcus aureus and Pseudomonas aeruginosa. Neotrop. Entomol. 2017;46:125–129. doi: 10.1007/s13744-016-0444-4. [DOI] [PubMed] [Google Scholar]
- 134.Dallavecchia D.L., Ricardo E., Silva A.S., Rodrigues A.G. Antibacterial and antifungal activity of excretions and secretions of Calliphora vicina. Med. Vet. Entomol. 2021;35:225–229. doi: 10.1111/mve.12486. [DOI] [PubMed] [Google Scholar]
- 135.Laverde-Paz M.J., Echeverry M.C., Patarroyo M.A., Bello F.J. Evaluating the anti-leishmania activity of Lucilia sericata and Sarconesiopsis magellanica blowfly larval excretions/secretions in an in vitro model. Acta Trop. 2018;177:44–50. doi: 10.1016/j.actatropica.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 136.Tonk M., Pierrot C., Cabezas-Cruz A., Rahnamaeian M., Khalife J., Vilcinskas A. The Drosophila melanogaster antimicrobial peptides Mtk-1 and Mtk-2 are active against the malarial parasite Plasmodium falciparum. Parasitol. Res. 2019;118:1993–1998. doi: 10.1007/s00436-019-06305-x. [DOI] [PubMed] [Google Scholar]
- 137.Huang H., Du J., Li S.-W., Gong T. Identification and functional analysis of a lysozyme gene from Coridius chinensis (Hemiptera: dinidoridae) Biology. 2021;10:330. doi: 10.3390/biology10040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Shang-Wei Du Juan Z.B.-S. Isolation, purification, and detection of the antimicrobial activity of the antimicrobial peptide CcAMP1 from Coridius chinensis (Hemiptera: dinidoridae) Acta Entomol. Sin. 2015;58:610–616. http://www.insect.org.cn [Google Scholar]
- 139.Menegatti C., Lourenzon V.B., Rodríguez-Hernández D., da Paixão Melo W.G., Ferreira L.L.G., Andricopulo A.D., do Nascimento F.S., Pupo M.T. Meliponamycins: antimicrobials from stingless bee-associated Streptomyces sp. J. Nat. Prod. 2020;83:610–616. doi: 10.1021/acs.jnatprod.9b01011. [DOI] [PubMed] [Google Scholar]
- 140.Santos H., Campos J., Santos C., Balestieri J., Silva D., Carollo C., de Picoli Souza K., Estevinho L., dos Santos E. Chemical profile and antioxidant, anti-inflammatory, antimutagenic and antimicrobial activities of geopropolis from the stingless bee Melipona orbignyi. Int. J. Mol. Sci. 2017;18:953. doi: 10.3390/ijms18050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Carr G., Derbyshire E.R., Caldera E., Currie C.R., Clardy J. Antibiotic and antimalarial quinones from fungus-growing ant-associated Pseudonocardia sp. J. Nat. Prod. 2012;75:1806–1809. doi: 10.1021/np300380t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rifflet A., Gavalda S., Téné N., Orivel J., Leprince J., Guilhaudis L., Génin E., Vétillard A., Treilhou M. Identification and characterization of a novel antimicrobial peptide from the venom of the ant Tetramorium bicarinatum. Peptides. 2012;38:363–370. doi: 10.1016/j.peptides.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 143.Meng Y.-C., Mo X.-G., He T.-T., Wen X.-X., Nieh J.-C., Yang X.-W., Tan K. New bioactive peptides from the venom gland of a social hornet Vespa velutina. Toxicon. 2021;199:94–100. doi: 10.1016/j.toxicon.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 144.das Neves R.C., Trentini M.M., de Castro e Silva J., Simon K.S., Bocca A.L., Silva L.P., Mortari M.R., Kipnis A., Junqueira-Kipnis A.P. Antimycobacterial activity of a new peptide Polydim-I isolated from neotropical social wasp Polybia dimorpha. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin C.-H., Hou R.F., Shyu C.-L., Shia W.-Y., Lin C.-F., Tu W.-C. In vitro activity of mastoparan-AF alone and in combination with clinically used antibiotics against multiple-antibiotic-resistant Escherichia coli isolates from animals. Peptides. 2012;36:114–120. doi: 10.1016/j.peptides.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 146.Ramos-Martín F., Herrera-León C., D'Amelio N. Molecular basis of the anticancer, apoptotic and antibacterial activities of Bombyx mori Cecropin A. Arch. Biochem. Biophys. 2022;715 doi: 10.1016/j.abb.2021.109095. [DOI] [PubMed] [Google Scholar]
- 147.Barretto D.A., Vootla S.K. Biological activities of melanin pigment extracted from Bombyx mori gut-associated yeast Cryptococcus rajasthanensis KY627764. World J. Microbiol. Biotechnol. 2020;36:159. doi: 10.1007/s11274-020-02924-0. [DOI] [PubMed] [Google Scholar]
- 148.Yang L., Zhan M., Zhuo Y., Dang X., Li M., Xu Y., Zhou X., Yu X., Rao X. Characterization of the active fragments of Spodoptera litura Lebocin‐1. Arch. Insect Biochem. Physiol. 2020;103 doi: 10.1002/arch.21626. [DOI] [PubMed] [Google Scholar]
- 149.Wang W., Zhang N., Chanda W., Liu M., ud Din S.R., Diao Y., Liu L., Cao J., Wang X., Li X., Ning A., Huang M., Zhong M. Antibacterial and anti-biofilm activity of the lipid extract from mantidis ootheca on Pseudomonas aeruginosa. J. Zhejiang Univ. B. 2018;19:364–371. doi: 10.1631/jzus.B1700356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zare-Zardini H., Taheri-Kafrani A., Ordooei M., Ebrahimi L., Tolueinia B., Soleimanizadeh M. Identification and biochemical characterization of a new antibacterial and antifungal peptide derived from the insect Sphodromantis viridis. Biochemist. 2015;80:433–440. doi: 10.1134/S0006297915040069. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Q.-Y., Yue X.-Q., Jiang Y.-P., Han T., Xin H.-L. FAM46C is critical for the anti-proliferation and pro-apoptotic effects of norcantharidin in hepatocellular carcinoma cells. Sci. Rep. 2017;7:396. doi: 10.1038/s41598-017-00313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chun J., Park M.K., Ko H., Lee K., Kim Y.S. Bioassay-guided isolation of cantharidin from blister beetles and its anticancer activity through inhibition of epidermal growth factor receptor-mediated STAT3 and Akt pathways. J. Nat. Med. 2018;72:937–945. doi: 10.1007/s11418-018-1226-6. [DOI] [PubMed] [Google Scholar]
- 153.Hsia T.-C., Yu C.-C., Hsiao Y.-T., Wu S.-H., Bau D.-T., Lu H.-F., Huang Y.-P., Lin J.-G., Chang S.-J., Chung J.-G. Cantharidin impairs cell migration and invasion of human lung cancer NCI-H460 cells via UPA and MAPK signaling pathways. Anticancer Res. 2016;36:5989–5998. doi: 10.21873/anticanres.11187. [DOI] [PubMed] [Google Scholar]
- 154.Kang B.-R., Kim H., Nam S.-H., Yun E.-Y., Kim S.-R., Ahn M.-Y., Chang J.-S., Hwang J.-S. CopA3 peptide from Copris tripartitus induces apoptosis in human leukemia cells via a caspase-independent pathway. BMB Rep. 2012;45:85–90. doi: 10.5483/BMBRep.2012.45.2.85. [DOI] [PubMed] [Google Scholar]
- 155.Abdel Wahid A., Maged M., Mohamed A. Evaluation of Scarabaeus sacer derived-chitosan, anti cancer potentials and related changes: in vitro study. J. Egypt. Soc. Parasitol. 2018;48:443–448. doi: 10.12816/0050452. [DOI] [Google Scholar]
- 156.Abdel Rahman R.M., Ghaffar H.A., Alkhuriji A.F., Khalil M.I., Amaly N., El-Kott A.F., Sultan A.S., Capasso R. Dipteran carboxymethyl chitosan as an inexhaustible derivative with a potential antiproliferative activity in hepatocellular carcinoma cells, Evidence-Based Complement. Alternative Med. 2020;2020:1–14. doi: 10.1155/2020/4396305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cheng D., Meng M., Zhang X., Wang C. An anti-tumor peptide from Musca domestica pupae (MATP) induces apoptosis in human liver cancer cells HepG2 cells through a ROS-JNK pathway. Int. J. Pept. Res. Therapeut. 2017;23:101–109. doi: 10.1007/s10989-016-9541-9. [DOI] [Google Scholar]
- 158.Zhang R., Cao X., Wang C., Hou L., Nie J., Zhou M., Feng Y. An antitumor peptide from Musca domestica pupae (MATP) induces apoptosis in HepG2 cells through a JNK-mediated and Akt-mediated NF-κB pathway. Anti Cancer Drugs. 2012;23:827–835. doi: 10.1097/CAD.0b013e32835455f1. [DOI] [PubMed] [Google Scholar]
- 159.Zhang L., Yi H. Potential antitumor and anti-inflammatory activities of an extracellular polymeric substance (EPS) from Bacillus subtilis isolated from a housefly. Sci. Rep. 2022;12:1383. doi: 10.1038/s41598-022-05143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee S., Lee S., Eom H., Kang H., Yu J., Lee T., Baek J., Lee D., Suh W., Kim K., (-)-Bassianolide A cyclodepsipeptide from Bombycis corpus: total synthesis and evaluation of its antitumor activity. Planta Med. 2016;81:S1–S381. doi: 10.1055/s-0036-1596774. [DOI] [Google Scholar]
- 161.Qiu W., Wu J., Choi J.-H., Hirai H., Nishida H., Kawagishi H. Cytotoxic compounds against cancer cells from Bombyx mori inoculated with Cordyceps militaris. Biosci. Biotechnol. Biochem. 2017;81:1224–1226. doi: 10.1080/09168451.2017.1289075. [DOI] [PubMed] [Google Scholar]
- 162.Xu P., Lv D., Wang X., Wang Y., Hou C., Gao K., Guo X. Inhibitory effects of Bombyx mori antimicrobial peptide cecropins on esophageal cancer cells. Eur. J. Pharmacol. 2020;887 doi: 10.1016/j.ejphar.2020.173434. [DOI] [PubMed] [Google Scholar]
- 163.Song Y.-J., Zheng H.-B., Peng A.-H., Ma J.-H., Lu D.-D., Li X., Zhang H.-Y., Xie W.-D., Strepantibins A.-C. Hexokinase II inhibitors from a mud dauber wasp associated Streptomyces sp. J. Nat. Prod. 2019;82:1114–1119. doi: 10.1021/acs.jnatprod.8b00821. [DOI] [PubMed] [Google Scholar]
- 164.Wang H., Dong P., Liu X., Zhang Z., Li H., Li Y., Zhang J., Dai L., Wang S. Active peptide AR-9 from Eupolyphaga sinensis reduces blood lipid and hepatic lipid accumulation by restoring gut flora and its metabolites in a high fat diet–induced hyperlipidemia rat. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.918505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cauich-kumul R., Sauri-duch E., Toledo-lópez V. Propolis of Apis mellifera from Yucatán , México : study of biological properties Propóleos de Apis mellifera de Yucatán. Mexico: Estudio de propiedades biológicas. 2020;7:1–11. doi: 10.19136/era.a7n3.2604. [DOI] [Google Scholar]
- 166.Ahn M., Kim B., Kim H., Yoon H., Jee S., Hwang J., Park K.-K. Anti-obesity effect of Bombus ignitus queen glycosaminoglycans in rats on a high-fat diet. Int. J. Mol. Sci. 2017;18:681. doi: 10.3390/ijms18030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim J.H., Jo M.J., Go H.J., Park N.G., Do Kim G. Anti-adipogenic effect of mastoparan B analogue peptide on 3T3-L1 preadipocytes, Bangladesh. J. Pharmacol. 2018;13:333–339. doi: 10.3329/bjp.v13i4.37351. [DOI] [Google Scholar]
- 168.Ahn M.Y., Kim B.J., Kim H.J., Jin J.M., Yoon H.J., Hwang J.S., Lee B.M. Anti-diabetic activity of field cricket glycosaminoglycan by ameliorating oxidative stress. BMC Complement. Med. Ther. 2020;20:232. doi: 10.1186/s12906-020-03027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ahn M.Y., Kim M.-J., Kwon R.H., Hwang J.S., Park K.-K. Gene expression profiling and inhibition of adipose tissue accumulation of G. bimaculatus extract in rats on high fat diet. Lipids Health Dis. 2015;14:116. doi: 10.1186/s12944-015-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Thapa P., Katila N., Choi H., Han A.R., Choi D.Y., Nam J.W. Neuroprotective effects of n-acetyldopamine dimers from cicadidae periostracum. Nat. Prod. Sci. 2021;27:161–168. doi: 10.20307/nps.2021.27.3.161. [DOI] [Google Scholar]
- 171.Wanandy T., Honda-Okubo Y., Davies N.W., Rose H.E., Heddle R.J., Brown S.G.A., Woodman R.J., Petrovsky N., Wiese M.D. Pharmaceutical and preclinical evaluation of Advax adjuvant as a dose-sparing strategy for ant venom immunotherapy. J. Pharm. Biomed. Anal. 2019;172:1–8. doi: 10.1016/j.jpba.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lopes K.S., Rios E.R.V., Lima C.N. de C., Linhares M.I., Torres A.F.C., Havt A., Quinet Y.P., Fonteles M.M. de F., Martins A.M.C. The effects of the Brazilian ant Dinoponera quadriceps venom on chemically induced seizure models. Neurochem. Int. 2013;63:141–145. doi: 10.1016/j.neuint.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 173.Nôga D.A.M.F., Cagni F.C., Santos J.R., Silva D., Azevedo D.L.O., Araújo A., Silva R.H., Ribeiro A.M. Pro- and anticonvulsant effects of the ant Dinoponera quadriceps (Kempf) venom in mice. Neotrop. Entomol. 2015;44:410–417. doi: 10.1007/s13744-015-0292-7. [DOI] [PubMed] [Google Scholar]
- 174.Nôga D., Brandão L., Cagni F., Silva D., de Azevedo D., Araújo A., dos Santos W., Miranda A., da Silva R., Ribeiro A. Anticonvulsant effects of fractions isolated from Dinoponera quadriceps (Kempt) ant venom (Formicidae: ponerinae) Toxins. 2016;9:5. doi: 10.3390/toxins9010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Alberto-Silva C., Portaro F.C.V., Kodama R.T., Pantaleão H.Q., Rangel M., Nihei K., Konno K. Novel neuroprotective peptides in the venom of the solitary scoliid wasp Scolia decorata ventralis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021;27 doi: 10.1590/1678-9199-jvatitd-2020-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Y., Yan H., Wang Y., Yang H., Wei L., Xiao Y., Ye H., Lai R., Liu R. Proteomics and transcriptome analysis coupled with pharmacological test reveals the diversity of anti-thrombosis proteins from the medicinal insect, Eupolyphaga sinensis. Insect Biochem. Mol. Biol. 2012;42:537–544. doi: 10.1016/j.ibmb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 177.Rujimongkon K., Ampawong S., Reamtong O., Buaban T., Aramwit P. The therapeutic effects of Bombyx mori sericin on rat skin psoriasis through modulated epidermal immunity and attenuated cell proliferation. J. Tradit. Complement. Med. 2022;11:587–597. doi: 10.1016/j.jtcme.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ko S.-C., Kang N., Kim E.-A., Kang M.C., Lee S.-H., Kang S.-M., Lee J.-B., Jeon B.-T., Kim S.-K., Park S.-J., Park P.-J., Jung W.-K., Kim D., Jeon Y.-J. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012;47:2005–2011. doi: 10.1016/j.procbio.2012.07.015. [DOI] [Google Scholar]
- 179.Kim Y.J., Lee K.P., Lee D.Y., Kim Y.T., Baek S., Yoon M.S. Inhibitory effect of modified silkworm pupae oil in PDGF-BB-induced proliferation and migration of vascular smooth muscle cells. Food Sci. Biotechnol. 2020;29:1091–1099. doi: 10.1007/s10068-020-00742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chu F., Jin X., Ma H. Anti-diarrhea effects and identification of Musca domestica larvae low molecular weight peptides (LMWP) J. Pharm. Biomed. Anal. 2019;173:162–168. doi: 10.1016/j.jpba.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 181.Jia Z., Wu A., He M., Zhang L., Wang C., Chen A. Metabolites of stable fly reduce diarrhea in mice by modulating the immune system, antioxidants, and composition of gut microbiota. Microb. Pathog. 2019;134 doi: 10.1016/j.micpath.2019.103557. [DOI] [PubMed] [Google Scholar]
- 182.Yazan L.S., Zainal N.A., Ali R.M., Muhamad Zali M.F.S., Sze O.Y., Sim T.Y., Gopalsamy B., Ling V.F., Sapuan S., Esa N., Haron A.S., Ansar F.H.Z., Mokhtar A.M.A., Alwi S.S.S. Antiulcer properties of kelulut honey against ethanol-induced gastric ulcer. Pertanika J. Sci. Technol. 2018;26:121–132. [Google Scholar]
- 183.Natarajan P., Singh R.S., Balamurugan K. Evaluation of anti-ulcer activity of ethanolic extract of Oecophylla smaradina in albino rats. Int. J. Pharma Sci. Res. 2019;10:3946–3950. doi: 10.13040/IJPSR.0975-8232.10(8).3946-50. [DOI] [Google Scholar]
- 184.Ma Y., Duan L., Sun J., Gou S., Chen F., Liang Y., Dai F., Xiao B. Oral nanotherapeutics based on Antheraea pernyi silk fibroin for synergistic treatment of ulcerative colitis. Biomaterials. 2022;282 doi: 10.1016/j.biomaterials.2022.121410. [DOI] [PubMed] [Google Scholar]
- 185.Lin M.-J., Lu M.-C., Chang H.-Y. Sustained release of insulin-like growth factor-1 from Bombyx mori L. silk fibroin delivery for diabetic wound therapy. Int. J. Mol. Sci. 2021;22:6267. doi: 10.3390/ijms22126267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Im A.-R., Yang W.-K., Park Y.-C., Kim S., Chae S. Hepatoprotective effects of insect extracts in an animal model of nonalcoholic fatty liver disease. Nutrients. 2018;10:735. doi: 10.3390/nu10060735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rujimongkon K., Ampawong S., Isarangkul D., Reamtong O., Aramwit P. Sericin-mediated improvement of dysmorphic cardiac mitochondria from hypercholesterolaemia is associated with maintaining mitochondrial dynamics, energy production, and mitochondrial structure. Pharm. Biol. 2022;60:708–721. doi: 10.1080/13880209.2022.2055088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yan Y.M., Zhu H.J., Xiang B., Qi J.J., Cheng Y.X. Phenolic derivatives from Periplaneta americana. Nat. Prod. Commun. 2017;12:1769–1772. doi: 10.1177/1934578x1701201130. [DOI] [Google Scholar]
- 189.Yan Y.-M., Xiang B., Zhu H.-J., Qi J.-J., Hou B., Geng F.-N., Cheng Y.-X. N -containing compounds from Periplaneta americana and their activities against wound healing. J. Asian Nat. Prod. Res. 2019;21:93–102. doi: 10.1080/10286020.2018.1450392. [DOI] [PubMed] [Google Scholar]
- 190.Díaz-Roa A., Gaona M.A., Segura N.A., Ramírez-Hernández A., Cortés-Vecino J.A., Patarroyo M.A., Bello F. Evaluating Sarconesiopsis magellanica blowfly-derived larval therapy and comparing it to Lucilia sericata-derived therapy in an animal model. Acta Trop. 2016;154:34–41. doi: 10.1016/j.actatropica.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 191.Tombulturk F.K., Soydas T., Sarac E.Y., Tuncdemir M., Coskunpinar E., Polat E., Sirekbasan S., Kanigur-Sultuybek G. Regulation of MMP 2 and MMP 9 expressions modulated by AP-1 (c-jun) in wound healing: improving role of Lucilia sericata in diabetic rats. Acta Diabetol. 2019;56:177–186. doi: 10.1007/s00592-018-1237-5. [DOI] [PubMed] [Google Scholar]
- 192.Brown A., Horobin A., Blount D.G., Hill P.J., English J., Rich A., Williams P.M., Pritchard D.I. Blow fly Lucilia sericata nuclease digests DNA associated with wound slough/eschar and with Pseudomonas aeruginosa biofilm. Med. Vet. Entomol. 2012;26:432–439. doi: 10.1111/j.1365-2915.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 193.Alipour H., Shahriari-Namadi M., Ebrahimi S., Moemenbellah-Fard M.D. Wound healing potential: evaluation of molecular profiling and amplification of Lucilia sericata angiopoietin-1 mRNA mid-part. BMC Res. Notes. 2020;13:308. doi: 10.1186/s13104-020-05141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Baumann A., Skaljac M., Lehmann R., Vilcinskas A., Franta Z. Urate Oxidase produced by Lucilia sericata medical maggots is localized in Malpighian tubes and facilitates allantoin production. Insect Biochem. Mol. Biol. 2017;83:44–53. doi: 10.1016/j.ibmb.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 195.Valachova I., Takac P., Majtan J. Midgut lysozymes of Lucilia sericata - new antimicrobials involved in maggot debridement therapy. Insect Mol. Biol. 2014;23:779–787. doi: 10.1111/imb.12122. [DOI] [PubMed] [Google Scholar]
- 196.Valachova I., Majtan T., Takac P., Majtan J. Identification and characterisation of different proteases in Lucilia sericata medicinal maggots involved in maggot debridement therapy. J. Appl. Biomed. 2014;12:171–177. doi: 10.1016/j.jab.2014.01.001. [DOI] [Google Scholar]
- 197.Pöppel A.-K., Kahl M., Baumann A., Wiesner J., Gökçen A., Beckert A., Preissner K.T., Vilcinskas A., Franta Z. A Jonah-like chymotrypsin from the therapeutic maggot Lucilia sericata plays a role in wound debridement and coagulation. Insect Biochem. Mol. Biol. 2016;70:138–147. doi: 10.1016/j.ibmb.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 198.Harris L.G., Nigam Y., Sawyer J., Mack D., Pritchard D.I. Lucilia sericata chymotrypsin disrupts [rotein adhesin-mediated staphylococcal biofilm formation. Appl. Environ. Microbiol. 2013;79:1393–1395. doi: 10.1128/AEM.03689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Díaz-Roa A., Gaona M.A., Segura N.A., Suárez D., Patarroyo M.A., Bello F.J. Sarconesiopsis magellanica (Diptera: Calliphoridae) excretions and secretions have potent antibacterial activity. Acta Trop. 2014;136:37–43. doi: 10.1016/j.actatropica.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 200.Díaz-Roa A., Patarroyo M.A., Bello F.J., Da Silva P.I. Sarconesin: Sarconesiopsis magellanica blowfly larval excretions and secretions with antibacterial properties. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pinilla Y.T., Patarroyo M.A., Velandia M.L., Segura N.A., Bello F.J. The effects of Sarconesiopsis magellanica larvae (Diptera: Calliphoridae) excretions and secretions on fibroblasts. Acta Trop. 2015;142:26–33. doi: 10.1016/j.actatropica.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 202.Nenadić M., Soković M., Glamočlija J., Ćirić A., Perić-Mataruga V., Ilijin L., Tešević V., Todosijević M., Vujisić L., Vesović N., Ćurčić S. The pygidial gland secretion of the forest caterpillar hunter, Calosoma (Calosoma) sycophanta: the antimicrobial properties against human pathogens. Appl. Microbiol. Biotechnol. 2017;101:977–985. doi: 10.1007/s00253-016-8082-7. [DOI] [PubMed] [Google Scholar]
- 203.Mastore M., Quadroni S., Caramella S., Brivio M.F. The silkworm as a source of natural antimicrobial preparations: efficacy on various bacterial strains. Antibiotics. 2021;10:1339. doi: 10.3390/antibiotics10111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dutta S.R., Gauri S.S., Mondal B., Vemula A., Halder S.K., Mondal K.C., Ghosh A.K. Screening of antimicrobial peptides from hemolymph extract of tasar silkworm Antheraea mylitta against urinary tract and wound infecting multidrug-resistant bacteria. Acta Biol. Szeged. 2016;60:49–55. [Google Scholar]
- 205.Chen Z., Ou P., Liu L., Jin X. Anti-MRSA activity of Actinomycin X2 and Collismycin A produced by Streptomyces globisporus WA5-2-37 from the intestinal tract of american cockroach (Periplaneta americana) Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Olofsson T.C., Butler É., Lindholm C., Nilson B., Michanek P., Vásquez A. Fighting off wound pathogens in horses with honeybee lactic acid bacteria. Curr. Microbiol. 2016;73:463–473. doi: 10.1007/s00284-016-1080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Teh C.H., Nazni W.A., Lee H.L., Fairuz A., Tan S.B., Sofian-Azirun M. In vitro antibacterial activity and physicochemical properties of a crude methanol extract of the larvae of the blow fly Lucilia cuprina. Med. Vet. Entomol. 2013;27:414–420. doi: 10.1111/mve.12012. [DOI] [PubMed] [Google Scholar]
- 208.Brown E., O'Brien M., Georges K., Suepaul S. Physical characteristics and antimicrobial properties of Apis mellifera, Frieseomelitta nigra and Melipona favosa bee honeys from apiaries in Trinidad and Tobago. BMC Complement. Med. Ther. 2020;20:85. doi: 10.1186/s12906-020-2829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tsavea E., Mossialos D. Antibacterial activity of honeys produced in Mount Olympus area against nosocomial and foodborne pathogens is mainly attributed to hydrogen peroxide and proteinaceous compounds. J. Apicult. Res. 2019;58:756–763. doi: 10.1080/00218839.2019.1649570. [DOI] [Google Scholar]
- 210.Chmiel E., Palusinska-Szysz M., Zdybicka-Barabas A., Cytryńska M., Mak P. The effect of Galleria mellonella hemolymph polypeptides on Legionella gormanii. Acta Biochim. Pol. 2014;61 doi: 10.18388/abp.2014_1933. [DOI] [PubMed] [Google Scholar]
- 211.de Carvalho D.B., Fox E.G.P., dos Santos D.G., de Sousa J.S., Freire D.M.G., Nogueira F.C.S., Domont G.B., de Castilho L.V.A., Machado E. de A. Fire ant venom alkaloids inhibit biofilm formation. Toxins. 2019;11:420. doi: 10.3390/toxins11070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ratcliffe N.A., Vieira C.S., Mendonça P.M., Caetano R.L., Queiroz M.M. de C., Garcia E.S., Mello C.B., Azambuja P. Detection and preliminary physico-chemical properties of antimicrobial components in the native excretions/secretions of three species of Chrysomya (Diptera, Calliphoridae) in Brazil. Acta Trop. 2015;147:6–11. doi: 10.1016/j.actatropica.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 213.Nirma C., Eparvier V., Stien D. Antifungal agents from Pseudallescheria boydii SNB-CN73 isolated from a Nasutitermes sp. termite. J. Nat. Prod. 2013;76:988–991. doi: 10.1021/np4001703. [DOI] [PubMed] [Google Scholar]
- 214.Abdel-Samad M.R.K. Antiviral and virucidal activities of Lucilia cuprina maggots' excretion/secretion (Diptera: Calliphoridae): first work. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hassan M., Shehata A., Farag M., Shehab A., Mansour M., Abdel-Aziz A. Antibacterial, antiviral and cytotoxic activities of Rhynchophorus ferrugineus (Coleoptera: dryophthoridae) and spodoptera littoralis (Lepidoptera: noctuidae) larval extracts. J. Egypt. Soc. Parasitol. 2018;48:289–299. doi: 10.12816/0050436. [DOI] [Google Scholar]
- 216.Lima D.B., Mello C.P., Bandeira I.C.J., Pessoa Bezerra de Menezes R.R.P., Sampaio T.L., Falcão C.B., Morlighem J.-É.R.L., Rádis-Baptista G., Martins A.M.C. The dinoponeratoxin peptides from the giant ant Dinoponera quadriceps display in vitro antitrypanosomal activity. Biol. Chem. 2018;399:187–196. doi: 10.1515/hsz-2017-0198. [DOI] [PubMed] [Google Scholar]
- 217.Sherafati J., Dayer M.S., Ghaffarifar F. Therapeutic effects of Lucilia sericata larval excretion/secretion products on Leishmania major under in vitro and in vivo conditions. Parasites Vectors. 2022;15:212. doi: 10.1186/s13071-022-05322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Reyes Parrado A.E., Arrivillaga-Henríquez J., Oviedo Araújo M.J., Scorza Dagert J.V., Ron Garrido L. Terapia larval con Musca domestica en el tratamiento de la úlcera leishmánicaen un modelo murino. Acta Biol. Colomb. 2020;25:82–95. doi: 10.15446/abc.v25n1.77177. [DOI] [Google Scholar]
- 219.Polat E., Cakan H., Aslan M., Sirekbasan S., Kutlubay Z., Ipek T., Ozbilgin A. Detection of anti-leishmanial effect of the Lucilia sericata larval secretions in vitro and in vivo on Leishmania tropica: first work. Exp. Parasitol. 2012;132:129–134. doi: 10.1016/j.exppara.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 220.Ortega H.E., Ferreira L.L.G., Melo W.G.P., Oliveira A.L.L., Ramos Alvarenga R.F., Lopes N.P., Bugni T.S., Andricopulo A.D., Pupo M.T. Antifungal compounds from Streptomyces associated with attine ants also inhibit Leishmania donovani. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nixon S.A., Robinson S.D., Agwa A.J., Walker A.A., Choudhary S., Touchard A., Undheim E.A.B., Robertson A., Vetter I., Schroeder C.I., Kotze A.C., Herzig V., King G.F. Multipurpose peptides: the venoms of Amazonian stinging ants contain anthelmintic ponericins with diverse predatory and defensive activities. Biochem. Pharmacol. 2021;192 doi: 10.1016/j.bcp.2021.114693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang F., Wu N., Wei J., Liu J., Zhao J., Ji A., Lin X. A novel protein from Eupolyphaga sinensis inhibits adhesion, migration, and invasion of human lung cancer A549 cells. Biochem. Cell. Biol. 2013;91:244–251. doi: 10.1139/bcb-2013-0002. [DOI] [PubMed] [Google Scholar]
- 223.Park H., Tuan N., Oh J., Son Y., Hamann M., Stone R., Kelly M., Oh S., Na M. Sesterterpenoid and steroid metabolites from a deep-water Alaska sponge inhibit Wnt/β-catenin signaling in colon cancer cells. Mar. Drugs. 2018;16:297. doi: 10.3390/md16090297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wu X., Sun Z., Shi B., Zhang X. Periplaneta americana extract inhibits proliferation and motility of human breast cancer cells through MAPK/ERK signaling pathway and promotes cell apoptosis. Curr. Top. Nutraceutical Res. 2021;19:194–198. doi: 10.37290/ctnr2641-452X.19:194-198. [DOI] [Google Scholar]
- 225.Zhang Y.-Z., Si J.-J., Li S.-S., Zhang G.-Z., Wang S., Zheng H.-Q., Hu F.-L. Chemical analyses and antimicrobial activity of nine kinds of unifloral Chinese honeys compared to Manuka honey (12+ and 20+) Molecules. 2021;26:2778. doi: 10.3390/molecules26092778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nowakowski A.C., Miller A.C., Miller M.E., Xiao H., Wu X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 2022;62:3499–3508. doi: 10.1080/10408398.2020.1867053. [DOI] [PubMed] [Google Scholar]
- 227.Menasria L., Blaney S., Main B., Vong L., Hun V., Raminashvili D., Chhea C., Chiasson L., Leblanc C. Mitigated impact of provision of local foods combined with nutrition education and counseling on young child nutritional status in Cambodia. Nutrients. 2018;10:1450. doi: 10.3390/nu10101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cai M., Choi S.-M., Yang E. The effects of bee venom acupuncture on the central nervous system and muscle in an animal hSOD1G93A mutant. Toxins. 2015;7:846–858. doi: 10.3390/toxins7030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Demšar Luzar A., Korošec P., Košnik M., Zidarn M., Rijavec M. Hymenoptera venom immunotherapy: immune mechanisms of induced protection and tolerance. Cells. 2021;10:1575. doi: 10.3390/cells10071575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang Z., Yang L. Chinese herbal medicine: fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 2021;270 doi: 10.1016/j.jep.2021.113869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ahn M.Y., Han J.W., Hwang J.S., Yun E.Y., Lee B.M. Anti-inflammatory effect of glycosaminoglycan derived from Gryllus bimaculatus (a type of cricket, insect) on adjuvant-treated chronic arthritis rat model. J. Toxicol. Environ. Health, Part A. 2014;77:1332–1345. doi: 10.1080/15287394.2014.951591. [DOI] [PubMed] [Google Scholar]
- 232.Dutta P., Dey T., Manna P., Kalita J. Antioxidant potential of Vespa affinis L., a traditional edible insect species of North East India. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.El-Ashram S., El-Samad L.M., Basha A.A., El Wakil A. Naturally-derived targeted therapy for wound healing: beyond classical strategies. Pharmacol. Res. 2021;170 doi: 10.1016/j.phrs.2021.105749. [DOI] [PubMed] [Google Scholar]
- 234.Chantawannakul P. From entomophagy to entomotherapy. Front. Biosci. 2020;25:4802. doi: 10.2741/4802. [DOI] [PubMed] [Google Scholar]
- 235.Loko L.E.Y., Medegan Fagla S., Orobiyi A., Glinma B., Toffa J., Koukoui O., Djogbenou L., Gbaguidi F. Traditional knowledge of invertebrates used for medicine and magical–religious purposes by traditional healers and indigenous populations in the Plateau Department, Republic of Benin. J. Ethnobiol. Ethnomed. 2019;15:66. doi: 10.1186/s13002-019-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Alves R.R.N., Rosa I.L., Léo Neto N.A., Voeks R. Animals for the gods: magical and religious faunal use and trade in Brazil. Hum. Ecol. 2012;40:751–780. doi: 10.1007/s10745-012-9516-1. [DOI] [Google Scholar]
- 237.El Hajj M., Holst L. Herbal medicine use during pregnancy: a review of the literature with a special focus on sub-saharan Africa. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.James P.B., Wardle J., Steel A., Adams J. Traditional, complementary and alternative medicine use in Sub-Saharan Africa: a systematic review. BMJ Glob. Health. 2018;3 doi: 10.1136/bmjgh-2018-000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mudonhi N., Nunu W.N. Traditional medicine utilisation among pregnant women in sub-saharan african countries: a systematic review of literature. Inq. J. Heal. Care Organ. Provision, Financ. 2022;59 doi: 10.1177/00469580221088618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mwaka A.D., Abbo C., Kinengyere A.A. Traditional and complementary medicine use among adult cancer patients undergoing conventional treatment in sub-saharan Africa: a scoping review on the use, safety and risks. Cancer Manag. Res. 2020;12:3699–3712. doi: 10.2147/CMAR.S251975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Meo S.A., Al-Asiri S.A., Mahesar A.L., Ansari M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017;24:975–978. doi: 10.1016/j.sjbs.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nikhat S., Fazil M. History, phytochemistry, experimental pharmacology and clinical uses of honey: a comprehensive review with special reference to Unani medicine. J. Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114614. [DOI] [PubMed] [Google Scholar]
- 243.Sforcin J.M., Bankova V., Kuropatnicki A.K. Medical benefits of honeybee products, Evidence-Based Complement. Alternative Med. 2017;2017:1–2. doi: 10.1155/2017/2702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zurier R.B., Mitnick H., Bloomgarden D., Weissmann G. Effect of bee venom on experimental arthritis. Ann. Rheum. Dis. 1973;32:466–470. doi: 10.1136/ard.32.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kolayli S., Keskin M. Natural bee products and their apitherapeutic applications. Bull. ESA. 2020;66:175–196. doi: 10.1016/B978-0-12-817907-9.00007-6. [DOI] [Google Scholar]
- 246.Donev R. Academic Press; 2015. Protein and Peptide Nanoparticles for Drug Delivery. [Google Scholar]
- 247.Gajski G., Garaj-Vrhovac V., Melittin A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013;36:697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 248.Jallouk A.P., Palekar R.U., Pan H., Schlesinger P.H., Wickline S.A. Modifications of natural peptides for nanoparticle and drug design. Adv. Protein Chem. Struct. Biol. 2015;98:57–91. doi: 10.1016/bs.apcsb.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Denisow B., Denisow-Pietrzyk M. Biological and therapeutic properties of bee pollen: a review. J. Sci. Food Agric. 2016;96:4303–4309. doi: 10.1002/jsfa.7729. [DOI] [PubMed] [Google Scholar]
- 250.Amr A., Abd El-Wahed A., El-Seedi H.R., Khalifa S.A.M., Augustyniak M., El-Samad L.M., Abdel Karim A.E., El Wakil A. UPLC-MS/MS analysis of naturally derived Apis mellifera products and their promising effects against cadmium-induced adverse effects in female rats. Nutrients. 2022;15:119. doi: 10.3390/nu15010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yin W., Zhao C., Liao C., Trowell S., Rickards R.W. Preparative isolation of novel antimicrobial compounds from Pergidae sp. by reversed-phase high-performance liquid chromatography. Chem. Nat. Compd. 2013;49:41–45. doi: 10.1007/s10600-013-0501-8. [DOI] [Google Scholar]
- 252.Crockett S.L., Boevé J.-L. Flavonoid glycosides and naphthodianthrones in the sawfly Tenthredo zonula and its host-plants. Hypericum perforatum and H. hirsutum, J. Chem. Ecol. 2011;37:943–952. doi: 10.1007/s10886-011-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tang J.-J., Fang P., Xia H.-L., Tu Z.-C., Hou B.-Y., Yan Y.-M., Di L., Zhang L., Cheng Y.-X. Constituents from the edible Chinese black ants (Polyrhachis dives) showing protective effect on rat mesangial cells and anti-inflammatory activity. Food Res. Int. 2015;67:163–168. doi: 10.1016/j.foodres.2014.11.022. [DOI] [Google Scholar]
- 254.Narsinghani T., Sharma R. Lead optimization on conventional non-steroidal anti-inflammatory drugs: an approach to reduce gastrointestinal toxicity. Chem. Biol. Drug Des. 2014;84:1–23. doi: 10.1111/cbdd.12292. [DOI] [PubMed] [Google Scholar]
- 255.Yan Y.-M., Li L.-J., Qin X.-C., Lu Q., Tu Z.-C., Cheng Y.-X. Compounds from the insect Blaps japanensis with COX-1 and COX-2 inhibitory activities. Bioorg. Med. Chem. Lett. 2015;25:2469–2472. doi: 10.1016/j.bmcl.2015.04.085. [DOI] [PubMed] [Google Scholar]
- 256.Abd El-Wahed A.A., Khalifa S.A.M., Sheikh B.Y., Farag M.A., Saeed A., Larik F.A., Koca-Caliskan U., AlAjmi M.F., Hassan M., Wahabi H.A., Hegazy M.-E.F., Algethami A.F., Büttner S., El-Seedi H.R. Bee venom composition: from chemistry to biological activity. 2019. 459–484. [DOI]
- 257.Munstedt K., Hackethal A., Schmidt K. Bee venom therapy, bee venom acupuncture or apipuncture--what is the evidence behind the various health claims? Am. Bee J. 2005;145:665–668. [Google Scholar]
- 258.Oyebode O., Kandala N.-B., Chilton P.J., Lilford R.J. Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Pol. Plann. 2016;31:984–991. doi: 10.1093/heapol/czw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Meyer-Rochow V.B., Chakravorty J. Notes on entomophagy and entomotherapy generally and information on the situation in India in particular. Appl. Entomol. Zool. 2013;48:105–112. doi: 10.1007/s13355-013-0171-9. [DOI] [Google Scholar]
- 260.Chakravorty J., Ghosh S., Meyer-Rochow V.B. Practices of entomophagy and entomotherapy by members of the Nyishi and Galo tribes, two ethnic groups of the state of Arunachal Pradesh (North-East India) J. Ethnobiol. Ethnomed. 2011;7:5. doi: 10.1186/1746-4269-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Devi W.D., Bonysana R., Kapesa K., Mukherjee P.K., Rajashekar Y. Edible insects: as traditional medicine for human wellness, Futur. Foods. 2023;7 doi: 10.1016/j.fufo.2023.100219. [DOI] [Google Scholar]
- 262.Lensvelt E.J.S., Steenbekkers L.P.A. Exploring consumer acceptance of entomophagy: a survey and experiment in Australia and The Netherlands, Ecol. Food Nutr. 2014;53:543–561. doi: 10.1080/03670244.2013.879865. [DOI] [PubMed] [Google Scholar]
- 263.Innocent E. Trends and challenges towards integration of traditional medicine in formal health care system: historical perspectives and an Appraisal of education curricula in Sub-Sahara Africa. J. Intercult. Ethnopharmacol. 2016;5:312. doi: 10.5455/jice.20160421125217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cherniack E.P. Bugs as drugs, Part 1: insects: the “new” alternative medicine for the 21st century? Alternative Med. Rev. 2010;15:124–135. [PubMed] [Google Scholar]
- 265.Shelomi M. Why we still don't eat insects: assessing entomophagy promotion through a diffusion of innovations framework. Trends Food Sci. Technol. 2015;45:311–318. doi: 10.1016/j.tifs.2015.06.008. [DOI] [Google Scholar]
- 266.Meyer-Rochow V.B. Ethno-entomological observations from North Korea (officially known as the “Democratic People's Republic of Korea”) J. Ethnobiol. Ethnomed. 2013;9:7. doi: 10.1186/1746-4269-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Feng Y., Zhao M., He Z., Chen Z., Sun L. Research and utilization of medicinal insects in China. Entomol. Res. 2009;39:313–316. doi: 10.1111/j.1748-5967.2009.00236.x. [DOI] [Google Scholar]
- 268.Cortes Ortiz J.A., Ruiz A.T., Morales-Ramos J.A., Thomas M., Rojas M.G., Tomberlin J.K., Yi L., Han R., Giroud L., Jullien R.L. In: Insects as Sustain. Food Ingredients. Dossey A.T., Morales-Ramos J.A., Rojas M.G., editors. Elsevier; 2016. Insect mass production technologies; pp. 153–201. [DOI] [Google Scholar]
- 269.Feng Y., Chen X.-M., Zhao M., He Z., Sun L., Wang C.-Y., Ding W.-F. Edible insects in China: utilization and prospects. Insect Sci. 2018;25:184–198. doi: 10.1111/1744-7917.12449. [DOI] [PubMed] [Google Scholar]
- 270.Rumpold B.A., Schlüter O.K. Potential and challenges of insects as an innovative source for food and feed production. Innovat. Food Sci. Emerg. Technol. 2013;17:1–11. doi: 10.1016/j.ifset.2012.11.005. [DOI] [Google Scholar]
- 271.Meyer-Rochow V.B., Gahukar R.T., Ghosh S., Jung C. Chemical composition, nutrient quality and acceptability of edible insects are affected by species, developmental stage, gender, diet, and processing method. Foods. 2021;10:1036. doi: 10.3390/foods10051036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Delgado C., Romero R., Vásquez Espinoza R., Trigozo M., Correa R. Rhynchophorus palmarum used in traditional medicine in the Peruvian Amazon. Ethnobiol. Lett. 2019;10:120–128. doi: 10.14237/ebl.10.1.2019.1271. [DOI] [Google Scholar]
- 273.Zhou Y., Wang M., Zhang H., Huang Z., Ma J. Comparative study of the composition of cultivated, naturally grown Cordyceps sinensis, and stiff worms across different sampling years. PLoS One. 2019;14:1–15. doi: 10.1371/journal.pone.0225750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chamberlin S.R., Blucher A., Wu G., Shinto L., Choonoo G., Kulesz-Martin M., McWeeney S. Natural product target network reveals potential for cancer combination therapies. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Huang X., Yang Z., Xie Q., Zhang Z., Zhang H., Ma J. Natural products for treating colorectal cancer: a mechanistic review. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109142. [DOI] [PubMed] [Google Scholar]
- 276.Razzak M.I., Imran M., Xu G. Big data analytics for preventive medicine. Neural Comput. Appl. 2020;32:4417–4451. doi: 10.1007/s00521-019-04095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Medical News Today . 2022. Can Food Be Medicine? Pros and Cons.https://www.medicalnewstoday.com/articles/can-food-be-medicine-pros-and-cons [Google Scholar]
- 278.World Health Organization . 2022. WHO Establishes the Global Centre for Traditional Medicine in India.https://www.who.int/news/item/25-03-2022-who-establishes-the-global-centre-for-traditional-medicine-in-india [Google Scholar]
- 279.Ishara J., Ayagirwe R., Karume K., Mushagalusa G.N., Bugeme D., Niassy S., Udomkun P., Kinyuru J. Inventory reveals wide biodiversity of edible insects in the Eastern Democratic Republic of Congo. Sci. Rep. 2022;12:1576. doi: 10.1038/s41598-022-05607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Skotnicka M., Karwowska K., Kłobukowski F., Borkowska A., Pieszko M. Possibilities of the development of edible insect-based foods in europe. Foods. 2021;10:766. doi: 10.3390/foods10040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hanboonsong Y., Jamjanya T., Durst P.B. RAP Publication; 2013. Six-legged Livestock: Edible Insect Farming, Collection and Marketing in Thailand. [Google Scholar]
- 282.Mercês M.D., Peralta E.D., Uetanabaro A.P.T., Lucchese A.M. Atividade antimicrobiana de méis de cinco espécies de abelhas brasileiras sem ferrão. Ciência Rural. 2013;43:672–675. doi: 10.1590/S0103-84782013005000016. [DOI] [Google Scholar]
- 283.Yang X., Tian Y., Liu H., Ren Y., Yang Z., Li X., Du C., Liu C., Wu F. Heavy metal pollution analysis and health risk assessment of two medicinal insects of Mylabris. Biol. Trace Elem. Res. 2022;200:1892–1901. doi: 10.1007/s12011-021-02775-2. [DOI] [PubMed] [Google Scholar]
- 284.Ssepuuya G., Wynants E., Verreth C., Crauwels S., Lievens B., Claes J., Nakimbugwe D., Van Campenhout L. Microbial characterisation of the edible grasshopper Ruspolia differens in raw condition after wild-harvesting in Uganda. Food Microbiol. 2019;77:106–117. doi: 10.1016/j.fm.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 285.Scott M.J., Concha C., Welch J.B., Phillips P.L., Skoda S.R. Review of research advances in the screwworm eradication program over the past 25 years. Entomol. Exp. Appl. 2017;164:226–236. doi: 10.1111/eea.12607. [DOI] [Google Scholar]
- 286.Van Huis A., Dicke M., Van Loon J.J. Wageningen Academic Publisher; 2015. Insects to Feed the World. [Google Scholar]
- 287.Varelas V. Food wastes as a potential new source for edible insect mass production for food and feed: a review. Fermentation. 2019;5:81. doi: 10.3390/fermentation5030081. [DOI] [Google Scholar]
- 288.Varelas V., Langton M. Forest biomass waste as a potential innovative source for rearing edible insects for food and feed – a review. Innovat. Food Sci. Emerg. Technol. 2017;41:193–205. doi: 10.1016/j.ifset.2017.03.007. [DOI] [Google Scholar]
- 289.Kok R. Preliminary project design for insect production: part 1 – overall mass and energy/heat balances. J. Insects as Food Feed. 2021;7:499–509. doi: 10.3920/JIFF2020.0055. [DOI] [Google Scholar]
- 290.Kok R. Preliminary project design for insect production: part 2 – organism kinetics, system dynamics and the role of modelling & simulation. J. Insects as Food Feed. 2021;7:511–523. doi: 10.3920/JIFF2020.0146. [DOI] [Google Scholar]
- 291.Kok R. Preliminary project design for insect production: part 4 – facility considerations. J. Insects as Food Feed. 2021;7:541–551. doi: 10.3920/JIFF2020.0164. [DOI] [Google Scholar]
- 292.Melgar‐Lalanne G., Hernández‐Álvarez A., Salinas‐Castro A. Edible insects processing: traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019;18:1166–1191. doi: 10.1111/1541-4337.12463. [DOI] [PubMed] [Google Scholar]
- 293.Gan J., Zhao M., He Z., Sun L., Li X., Feng Y. The effects of antioxidants and packaging methods on inhibiting lipid oxidation in deep fried crickets (Gryllus bimaculatus) during storage. Foods. 2022;11:326. doi: 10.3390/foods11030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Eilenberg J., Vlak J.M., Nielsen-LeRoux C., Cappellozza S., Jensen A.B. Diseases in insects produced for food and feed. J. Insects as Food Feed. 2015;1:87–102. doi: 10.3920/JIFF2014.0022. [DOI] [Google Scholar]
- 295.IPIFF . 2022. IPIFF Guide on Good Hygiene Practices for European Union (EU) Producers of Insects as Food and Feed.https://ipiff.org/good-hygiene-practices/ [Google Scholar]
- 296.Wilderspin D.E., Halloran A. Edible Insects Sustain. Food Syst. Springer International Publishing; Cham: 2018. The effects of regulation, legislation and policy on consumption of edible insects in the global south; pp. 443–455. [DOI] [Google Scholar]
- 297.Lähteenmäki-Uutela A., Marimuthu S., Meijer N. Regulations on insects as food and feed: a global comparison. J. Insects as Food Feed. 2021;7:849–856. doi: 10.3920/JIFF2020.0066. [DOI] [Google Scholar]
- 298.Bindroo B.B., Manthira Moorthy S. Genetic divergence, implication of diversity, and conservation of silkworm, Bombyx mori. Int. J. Biodivers. 2014;2014:1–15. doi: 10.1155/2014/564850. [DOI] [Google Scholar]
- 299.Bhattacharyya P., Jha S., Mandal P., Ghosh A. Artificial diet based silkworm rearing system-A review. Int. J. Pure Appl. Biosci. 2016;4:114–122. doi: 10.18782/2320-7051.2402. [DOI] [Google Scholar]
- 300.Smetana S., Spykman R., Heinz V. Environmental aspects of insect mass production. J. Insects as Food Feed. 2021;7:553–571. doi: 10.3920/JIFF2020.0116. [DOI] [Google Scholar]
- 301.Arévalo Arévalo H.A., Menjura Rojas E.M., Barragán Fonseca K.B., Vásquez Mejía S.M. Implementation of the HACCP system for production of Tenebrio molitor larvae meal. Food Control. 2022;138 doi: 10.1016/j.foodcont.2022.109030. [DOI] [Google Scholar]
- 302.Triantafyllou A., Sarigiannidis P., Bibi S. Precision agriculture: a remote sensing monitoring system architecture. Information. 2019;10:348. doi: 10.3390/info10110348. [DOI] [Google Scholar]
- 303.Lee J.H., Kim T.-K., Jeong C.H., Yong H.I., Cha J.Y., Kim B.-K., Choi Y.-S. Biological activity and processing technologies of edible insects: a review. Food Sci. Biotechnol. 2021;30:1003–1023. doi: 10.1007/s10068-021-00942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Castro-López C., Santiago-López L., Vallejo-Cordoba B., González-Córdova A.F., Liceaga A.M., García H.S., Hernández-Mendoza A. An insight to fermented edible insects: a global perspective and prospective. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109750. [DOI] [PubMed] [Google Scholar]
- 305.Peiyun Xiao Y.Y., Shi G., Li K., Yang M., Zhao Y. 2014. The Preparation of Blattaria Anti-hepatic Fibrosis Activity Extract and Detection Method. [Google Scholar]
- 306.Vieira S.A., Zhang G., Decker E.A. Biological implications of lipid oxidation products. J. Am. Oil Chem. Soc. 2017;94:339–351. doi: 10.1007/s11746-017-2958-2. [DOI] [Google Scholar]
- 307.Kamau E., Mutungi C., Kinyuru J., Imathiu S., Tanga C., Affognon H., Ekesi S., Nakimbugwe D., Fiaboe K.K.M. Moisture adsorption properties and shelf-life estimation of dried and pulverised edible house cricket Acheta domesticus (L.) and black soldier fly larvae Hermetia illucens (L.) Food Res. Int. 2018;106:420–427. doi: 10.1016/j.foodres.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 308.Van Huis A., Tomberlin J.K. Wageningen Academic Publisher; 2016. Insects as Food and Feed: from Production to Consumption. [Google Scholar]
- 309.Insect Doctor . 2020. Educating Insect Pathologists to Prevent Infectious Diseases in Mass-Reared Insects.https://www.insectdoctors.eu/en/insectdoctors/about.htm [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.