Key Points
Question
How do heart failure etiology, treatment, and outcomes differ between groups of countries at different levels of economic development?
Findings
Ischemic heart disease and hypertension were the most common causes of heart failure. Half of patients with reduced ejection fraction received combined guideline-directed medications, with the lowest use in lower–middle-income and low-income countries. Mortality rates were more than 2-fold higher in lower–middle-income and low-income countries compared with high-income countries. In low-income countries, deaths were more frequent than hospitalizations, and the short-term risk of death associated with a hospitalization was 3- to 5-fold higher in lower–middle-income and low-income countries compared with high-income countries.
Meaning
These data may be useful for planning approaches to improve heart failure management globally.
Abstract
Importance
Most epidemiological studies of heart failure (HF) have been conducted in high-income countries with limited comparable data from middle- or low-income countries.
Objective
To examine differences in HF etiology, treatment, and outcomes between groups of countries at different levels of economic development.
Design, Setting, and Participants
Multinational HF registry of 23 341 participants in 40 high-income, upper–middle-income, lower–middle-income, and low-income countries, followed up for a median period of 2.0 years.
Main Outcomes and Measures
HF cause, HF medication use, hospitalization, and death.
Results
Mean (SD) age of participants was 63.1 (14.9) years, and 9119 (39.1%) were female. The most common cause of HF was ischemic heart disease (38.1%) followed by hypertension (20.2%). The proportion of participants with HF with reduced ejection fraction taking the combination of a β-blocker, renin-angiotensin system inhibitor, and mineralocorticoid receptor antagonist was highest in upper–middle-income (61.9%) and high-income countries (51.1%), and it was lowest in low-income (45.7%) and lower–middle-income countries (39.5%) (P < .001). The age- and sex- standardized mortality rate per 100 person-years was lowest in high-income countries (7.8 [95% CI, 7.5-8.2]), 9.3 (95% CI, 8.8-9.9) in upper–middle-income countries, 15.7 (95% CI, 15.0-16.4) in lower–middle-income countries, and it was highest in low-income countries (19.1 [95% CI, 17.6-20.7]). Hospitalization rates were more frequent than death rates in high-income countries (ratio = 3.8) and in upper–middle-income countries (ratio = 2.4), similar in lower–middle-income countries (ratio = 1.1), and less frequent in low-income countries (ratio = 0.6). The 30-day case-fatality rate after first hospital admission was lowest in high-income countries (6.7%), followed by upper–middle-income countries (9.7%), then lower–middle-income countries (21.1%), and highest in low-income countries (31.6%). The proportional risk of death within 30 days of a first hospital admission was 3- to 5-fold higher in lower–middle-income countries and low-income countries compared with high-income countries after adjusting for patient characteristics and use of long-term HF therapies.
Conclusions and Relevance
This study of HF patients from 40 different countries and derived from 4 different economic levels demonstrated differences in HF etiologies, management, and outcomes. These data may be useful in planning approaches to improve HF prevention and treatment globally.
This study uses data from the Global Congestive Heart Failure Registry, sorted by country and by country income level, to evaluate trends in heart failure etiologies, management, and outcomes.
Introduction
Approximately 64 million people are estimated to have heart failure (HF) globally, and its prevalence is increasing in many middle- and low-income countries.1 Much of the current understanding of HF epidemiology is based on studies conducted in high-income countries.2,3,4,5,6 While some evidence suggests that HF outcomes are worse in middle- and low-income countries, few studies have directly compared how the clinical course of HF differs between countries at different levels of socioeconomic development. Most data from middle- or low-income countries have been collected in clinical trials enrolling selected HF populations or from different registries that are not readily comparable.7,8,9,10,11 There is also evidence from one registry of hospitalized HF patients conducted in 44 countries that observed worse survival after hospital discharge in upper–middle-income countries and lower–middle-income countries compared with high-income countries, but there were no data reported strictly from low-income countries.12
To better inform how HF management can be improved globally, additional data are needed to understand how the condition, its treatment, and its clinical course differ between countries at different levels of socioeconomic development. The Global Congestive Heart Failure (G-CHF) registry was used for this study because the registry was designed to document whether the clinical profiles of HF, its management, and outcomes vary in different parts of the world.13 In this analysis of the G-CHF registry, we evaluated differences in the common causes of HF, the use of guideline-directed treatments, the risk of death, and the relationship between deaths and hospitalizations between high-income countries, upper–middle-income countries, lower–middle-income countries, and low-income countries.
Methods
Design and Participants
The design of the G-CHF registry has been previously published.13 It is a multinational registry of 23 341 patients with a diagnosis of HF and with prospective long-term clinical follow-up. Recruitment occurred between 2016 and 2020 from 257 centers in 40 countries across 5 continents. Seventeen of these countries were high-income countries, 10 were upper–middle-income countries, 9 were lower–middle-income countries, and 4 were low-income countries based on their World Bank classification at study initiation (eTable 1 in Supplement 1; Figure 1). Sites were chosen based on prior experience collaborating with the study’s central coordinating center or with a national coordinating center. Participants aged 18 years and older with a clinical diagnosis of HF were recruited from outpatient and hospital inpatient settings. While the diagnosis of HF was based on local investigator judgement, prior analyses have demonstrated that the study cohort is consistent with a HF population in terms of the prevalence of structural heart disease features consistent with HF, Framingham HF criteria in participants enrolled from the inpatient setting, and N-terminal pro-B type natriuretic peptide (NT-pro BNP) levels.13 The study was approved by the local ethics committees, and all participants provided written informed consent.
Figure 1. Countries Included in the Registry.
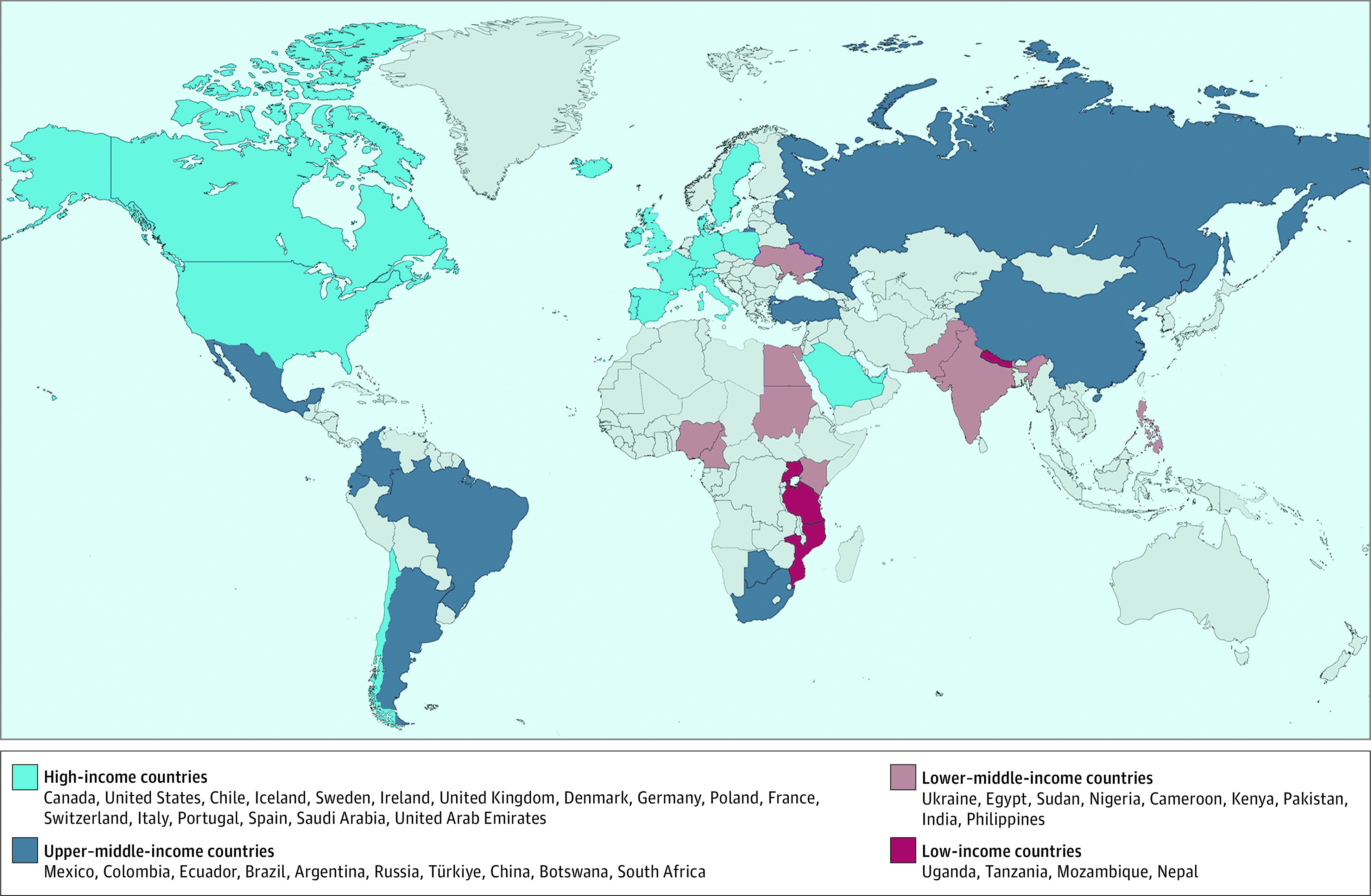
The countries are sorted by income level then listed by continent from west to east, north to south. Türkiye is the new spelling, approved by the United Nations in June 2022 and also by the US Board on Geographic Names, for the country formerly known as Turkey. The basis for this figure was created with MapChart.14
Data Collection
Data were collected on patient demographics, physical and laboratory measures, medical history, education level, medications, and cardiac device use. The following variables collected at baseline were used in this analysis: demographics (age, sex, and education); clinical characteristics and health behaviors (systolic blood pressure, left ventricular ejection fraction [LVEF], body mass index [BMI], kidney dysfunction, smoking, alcohol, diabetes, chronic obstructive pulmonary disease, duration of HF, HF etiology), New York Heart Association (NYHA) functional class, and use of HF therapies (medications, implantable cardioverter-defibrillator [ICD]). The clinical characteristics included for this analysis were chosen to be consistent with those in the Meta-Analysis Global Group in Chronic HF Risk Score, which represents a parsimonious set of variables that have been shown to predict mortality in HF patients in both the clinical trial and registry settings and regardless of ejection fraction.15,16 HF etiology was classified as due to ischemic heart disease, hypertension, rheumatic valve disease, nonrheumatic valve disease, idiopathic, or other causes based on the judgement of the local investigator. LVEF was recorded from echocardiography if performed within the year before enrollment, and using these data, participants were classified as having HF with reduced ejection fraction (HFrEF, LVEF≤40%), mildly reduced ejection fraction (HFmrEF, LVEF 41%-49%), or preserved ejection fraction (HFpEF, LVEF ≥50%).
Follow-up
Clinical events recorded before August 10, 2022, at a median follow-up period of 2.0 (IQR, 2.0-3.2) years, were included in this analysis. Data on clinical events were collected at 6-month intervals until the 2-year follow-up visit and then yearly thereafter. Clinical events were identified and categorized through participant self-report and review of available records. Additional information could be collected from a participant’s physician or next of kin if consent was obtained (see eAppendix 1 in Supplement 1 for additional description of participant follow-up). Causes of death and of hospitalizations were determined by the local investigators based on their review of the available clinical data. The primary outcome of this analysis was death from any cause.
Statistical Analysis
Baseline categorical variables were summarized as proportions, and continuous variables were reported as mean (SD) or median (IQR). The proportion of participants in each etiologic category of HF was summarized by country income level, and differences were compared using the χ2 statistic. At baseline, we calculated the proportions of participants with documented HFrEF who received any dose of a β-blocker, a renin-angiotensin-system (RAS) inhibitor (angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor neprilysin inhibitor), a mineralocorticoid receptor antagonist (MRA), and all 3 classes of drugs. Baseline ICD use was examined in participants with an ejection fraction of 35% or less. Differences in baseline treatment by country income level were compared using the χ2 statistic.
Hospitalization rates were based on the first new hospitalization event that occurred during the follow-up period. For patients who were enrolled as inpatients, the index hospitalization at the time of enrollment was not included in the calculation of hospitalization rates. Age- and sex-standardized rates of first hospitalization for any cause and of death from any cause were summarized by patient-years for each country income level. To determine the extent to which patient-level factors accounted for differences in the risk of death among countries grouped by income level, hazard ratios (HRs) with 95% CIs were compared using stepwise Cox regression models first adjusted for age and sex, then mutually adjusted for patient characteristics and use of chronic HF treatments. All potential confounding variables were measured and included in each Cox regression model as participant-level variables.
To examine the relationship between access to hospital care and mortality, we compared the rates of death, the first hospitalization, and the ratio of these outcomes by country income level. We also examined mortality during the first hospitalization period, which was defined as a death occurring within 30 days of the first hospital admission that occurred during follow-up. Hospitalization-related deaths were first examined by comparing the crude 30-day case fatality by country income level; then the proportional risk of death over this same period was examined by Cox regression. All statistical models were performed in participants with complete data, and we did not consider imputation methods for missing data. For all analyses, a P value of less than .05 was considered statistically significant. Analyses were executed using SAS and R statistical software.
Results
Clinical Characteristics
A total of 23 341 participants were included in this analysis. The proportion of missing data for variables in our analysis are reported in eTable 2 in Supplement 1. LVEF was collected if participants had echocardiography performed within the previous year. In 3661 (15.7%) participants, echocardiography was not performed within the prior year, and so LVEF was not collected. In an additional 493 (2.1%) participants, echocardiography was performed, but the LVEF value was missing. Data on BMI were missing in 572 (2.4%) participants. Other variables each had less than 1% missing data.
Mean (SD) age of the study cohort was 63.1 (14.9) years, and 14 222 (60.9%) were men (Table). Overall, 7371 (31.6%) participants were recruited as inpatients. Regarding participants’ level of education, 10 267 (44.2%) either had no formal or a primary level education. A total of 19 237 (82.6%) participants had an ejection fraction documented within the year prior to enrollment; of these 11 642 (60.5%) had an LVEF of less than or equal to 40%, 2909 (15.1%) had an ejection fraction between 41% and 49%, and 4686 (24.4%) had an ejection fraction of 50% or greater. A total of 14 005 (60.0%) participants were NYHA functional class I or II at the time of enrollment.
Table. Baseline Characteristics of the Study Populationa.
| Overall | Low-income countries | Lower–middle-income countries | Upper–middle-income countries | High-income countries | |
|---|---|---|---|---|---|
| Total No. | 23 341 | 1910 | 6947 | 5793 | 8691 |
| Age, median (IQR), y | 65 (54-74) | 59 (45-70) | 60 (48-69) | 67 (57-75) | 69 (59-77) |
| Age, mean (SD), y | 63.1 (14.9) | 57.1 (17.1) | 57.8 (15.4) | 65.3 (13.9) | 67.2 (12.9) |
| Female sex | 9119 (39.1) | 1027 (53.7) | 3094 (54.5) | 2232 (38.5) | 2766 (31.8) |
| Male sex | 14 222 (60.9) | 883 (46.3) | 3853 (55.5) | 3561 (61.5) | 5925 (68.2) |
| Education level | |||||
| No. | 23 248 | 1910 | 6947 | 5791 | 8600 |
| ≤Primary | 10 267 (44.2) | 1256 (65.8) | 3411 (49.1) | 2771 (47.9) | 2829 (32.9) |
| Secondary | 7749 (33.3) | 370 (19.4) | 2190 (31.5) | 1943 (33.6) | 3246 (37.7) |
| Postsecondary | 5232 (22.5) | 284 (14.9) | 1346 (19.4) | 1077 (18.6) | 2525 (29.4) |
| Enrollment from the inpatient hospitalization setting | 7371 (31.6) | 600 (31.4) | 2917 (42.0) | 1733 (29.9) | 2121 (24.4) |
| Clinical characteristics and health behaviors | |||||
| Body mass index, median (IQR)b | 26.5 (23.1-30.8) | 24.2 (21.2-28.3) | 24.8 (21.8-28.7) | 26.2 (23.2-30.1) | 28.4 (25.1-32.9) |
| Chronic obstructive pulmonary diseasec | 2452 (10.5) | 73 (3.8) | 421 (6.1) | 736 (12.7) | 1222 (14.1) |
| Current alcohol use | 5232 (22.5) | 137 (7.2) | 788 (11.3) | 757 (13.1) | 3550 (41.1) |
| Current tobacco use | 1967 (8.4) | 76 (4.0) | 436 (6.3) | 501 (8.6) | 954 (11.0) |
| Diabetesc | 7209 (30.9) | 253 (13.2) | 1832 (26.4) | 1704 (29.4) | 3420 (39.4) |
| Heart failure >12-mo duration | 16 071 (75.3) | 940 (49.2) | 3951 (56.9) | 4346 (75.1) | 6834 (78.6) |
| Hypertensionc | 15 337 (65.7) | 1120 (58.6) | 4002 (57.6) | 4021 (69.4) | 6194 (71.3) |
| Systolic blood pressure, median (IQR), mm Hg | 121 (109-136) | 120 (105-141) | 120 (109-135) | 122 (110-135) | 121 (109-135) |
| Left ventricular ejection fractiond | |||||
| No. | 19 237 | 1853 | 5896 | 4818 | 6670 |
| ≤40 | 11 642 (60.5) | 1140 (61.5) | 3685 (62.5) | 2554 (53.0) | 4263 (63.9) |
| 41-49 | 2909 (15.1) | 273 (14.7) | 710 (12.0) | 969 (20.1) | 957 (14.3) |
| ≥50 | 4686 (24.4) | 440 (23.7) | 1501 (25.5) | 1295 (26.9) | 1450 (21.7) |
| Stage 4-5 kidney dysfunctione | 963 (4.1) | 74 (3.9) | 151 (2.2) | 192 (3.3) | 546 (6.3) |
| New York Heart Association functional classf | |||||
| No. | 23 227 | 1910 | 6946 | 5786 | 8585 |
| I | 2658 (11.4) | 261 (13.7) | 497 (7.2) | 690 (11.9) | 1210 (14.1) |
| II | 11 347 (48.6) | 508 (26.6) | 3325 (47.9) | 2875 (49.7) | 4639 (54.0) |
| III | 7395 (31.8) | 670 (35.1) | 2504 (36.0) | 1743 (30.1) | 2478 (28.9) |
| IV | 1827 (7.9) | 471 (24.7) | 620 (8.9) | 478 (8.3) | 258 (3.0) |
Values are reported as No. (%) unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters squared.
Based on participant self-report.
Indicates participants who had an ejection fraction documented within 1 year prior to study enrollment.
Investigator reported stage 4 or 5 kidney dysfunction defined as an estimated glomerular filtration rate of less than 30 mL per minute based on local blood work.
Range, I to IV (higher values indicate greater heart failure symptom severity).
Compared with participants in high-income or upper–middle-income countries, those in lower–middle-income or low-income countries were younger at the time of enrollment, and a higher proportion were females. A higher proportion of participants from lower–middle-income or low-income countries were enrolled in the hospital inpatient setting and had a shorter known duration of HF prior to enrollment. Related medical conditions such as hypertension, diabetes, and chronic obstructive pulmonary disease were reported more commonly in participants from high-income and upper–middle-income countries compared with lower–middle-income or low-income countries. The type of HF based on LVEF was comparable across different country income levels, but a higher proportion of participants in lower–middle-income countries or low-income countries were enrolled with more advanced HF symptoms based on NYHA functional class.
HF Etiology
The most common cause of HF was ischemic heart disease (8899 [38.2%] participants), followed by hypertension (4709 [20.2%] participants), and idiopathic dilated cardiomyopathy (3590 [15.4%] participants) (panel A in Figure 2; eTable 3 in Supplement 1). Rheumatic valve disease accounted for 4.9% of cases (1133 participants), nonrheumatic valve disease accounted for 4.1% (944 participants), and 17.2% (4024 participants) were due to other etiologies. In high-income, upper–middle-income, and lower–middle-income countries, ischemic heart disease was the most common cause of HF; while in low-income countries, hypertension was the most common cause. The proportion of HF due to valve disease was highest in low-income countries, followed by lower–middle-income, upper–middle-income, and high-income countries. The higher proportion of HF due to valvular disease in low-income and lower–middle-income countries was due to a higher proportion of rheumatic heart disease (panel A in Figure 2; eTable 3 in Supplement 1).
Figure 2. Variation in the Causes of Heart Failure and the Use of Treatments for Heart Failure With Reduced Ejection Fraction by Country Income Level in a Registry Study of 40 Countries.
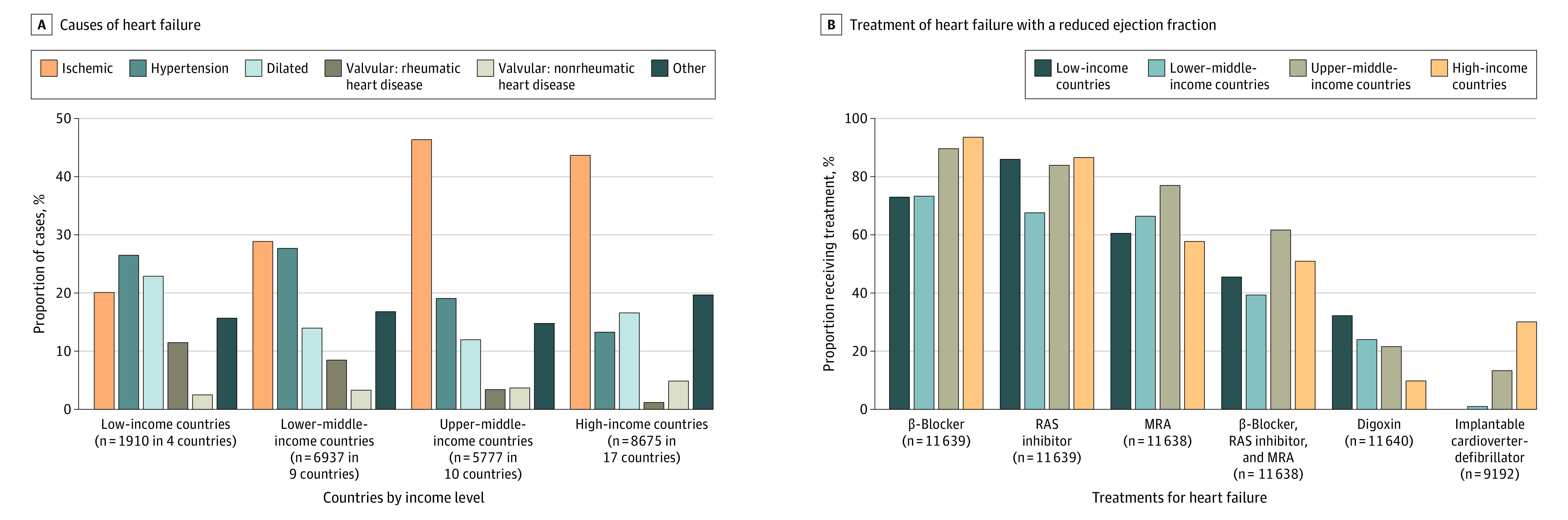
A, See eTable 3 in Supplement 1 for an expanded list of heart failure causes by income level.
B, See eTable 4 in Supplement 1 for an expanded list of heart failure treatments by country income level. Data report on the proportion of participants with an left ventricular ejection fraction of less than or equal to 40% taking a specific class of medication or combination of medications. Implantable cardioverter-defibrillator use is reported in participants with a left ventricular ejection fraction of less than or equal to 35%. RAS inhibitor refers to angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or an angiotensin receptor–neprilysin inhibitor.
MRA indicates mineralocorticoid receptor antagonist; RAS, renin-angiotensin system.
Treatment of HF With Reduced Ejection Fraction
In participants with a LVEF less than or equal to 40%, 9833 (84.5%) were prescribed a β-blocker for treatment, 9329 (80.1%) a RAS inhibitor, and 7587 (65.2%) an MRA. All 3 classes of medications were taken in 5733 (49.3%) participants. In high-income, upper–middle-income, and lower–middle-income countries, β-blockers were the most commonly used medication; while RAS inhibitors were the most commonly used in low-income countries (panel B in Figure 2; eTable 4 in Supplement 1). MRAs were the least commonly prescribed of the 3 medication classes in all groups of countries. Use of all 3 medication classes varied by country income level, with the highest use in upper–middle-income countries (61.9%) and high-income countries (51.1%), and the lowest use in low-income countries (45.7%) and lower–middle-income countries (39.5%) (P < .001). In participants with an LVEF of 35% or less, 1329 (14.5%) had an ICD, with the highest proportion in high-income countries (30.3%), and lowest in low-income countries (0.3%) (P < .001).
Death
Among the 23 341 enrolled participants, 23 277 (99.7%) had completed at least 1 follow-up visit and were included in the analysis of clinical events. Data on clinical events were obtained in 99.7% of participants at 6 months, 99.0% at 1 year, and 97.2% at 2 years. At a median follow-up of 2.0 years, 6035 deaths were recorded, and 4452 (73.7%) deaths were due to a cardiovascular cause. The proportion of deaths due to a cardiovascular cause varied between high-income countries (1131/1926 [58.7%]), upper–middle-income countries (858/1188 [72.2%]), lower–middle-income countries (1859/2205 [84.3%]), and low-income countries (604/716 [84.3%]). The age- and sex-standardized death rate was lowest in high-income countries (7.8 per 100 person-years [95% CI, 7.5-8.2]), but the death rate increased as country income level decreased (upper–middle-income countries, 9.3 [95% CI, 8.8-9.9]; lower–middle-income countries, 15.7 [95% CI, 15.0-16.4]; and low-income countries, 19.1 [95% CI, 17.6-20.7]) (Figure 3; eFigure 1 in Supplement 1). After adjusting for baseline patient characteristics and use of chronic HF treatments, the risk of death remained significantly higher in lower–middle-income countries (HR, 1.48 [95% CI, 1.37-1.60]) and in low-income countries (HR, 2.10 [95% CI, 1.90-2.33]) compared with high-income countries. Since chronic HF treatments have a larger impact on survival in HFrEF, we also examined the risk of death between groups of countries at different income levels in participants with an LVEF of 40% or less, and we adjusted for patient characteristics and the use of chronic HF medication therapies. These results were consistent with our primary findings (eTable 5 in Supplement 1). Results were also examined in groups stratified by HF duration and by enrollment as an inpatient or outpatient (eTable 6 in Supplement 1). While the risk of death by country income level differed between these stratified groups, in each group, the risk of death remained consistently higher in lower–middle-income countries and low-income countries compared with high-income countries.
Figure 3. Rate of Hospitalization, Rate of Death, and Proportional Risk of Death From Any Cause by Country Income Level.
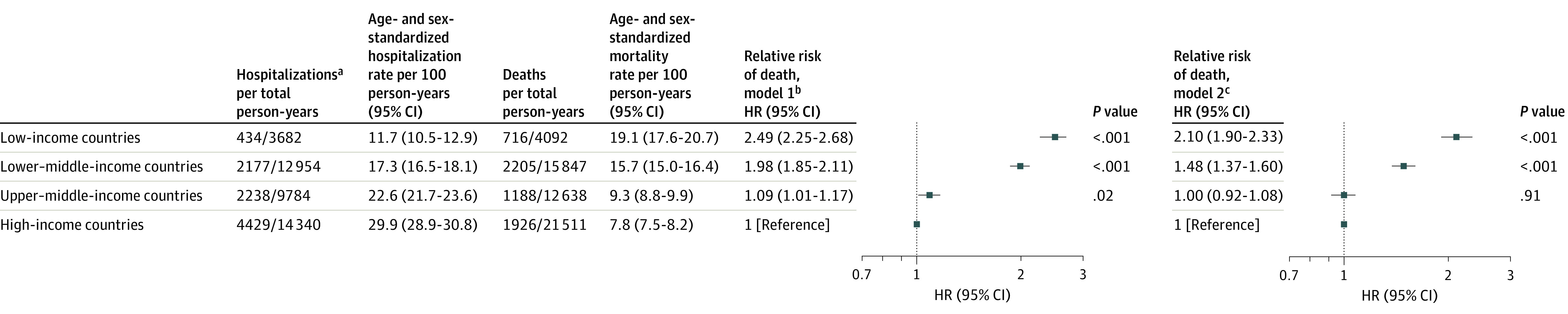
Events were captured at a median follow-up period of 2.0 years. HR indicates hazard ratio.
aData reflect the first hospitalization after enrollment. Observation hospitalizations are common in the US but uncommon in many of the other countries participating in the registry; therefore, observation vs inpatient hospitalizations were not specifically delineated in the registry.
bModel was adjusted for age and sex.
cModel was adjusted for variables in model 1 plus clinical characteristics (body mass index, systolic blood pressure, diabetes, chronic obstructive pulmonary disease, current tobacco use, stage 4-5 kidney dysfunction, left ventricular ejection fraction [>50%, 41%-49%, ≤40%], having no echocardiography performed within the previous year, New York Heart Association functional class, heart failure duration >12 months), heart failure etiology (ischemic, hypertension, dilated, rheumatic valvular, nonrheumatic valvular, other), inpatient recruitment, patient education level, chronic heart failure treatments (β-blocker, renin-angiotensin system inhibitor, mineralocorticoid receptor antagonist, implantable cardioverter-defibrillator).
Hospitalizations
During follow-up, 9278 participants were hospitalized. The rate of the first hospitalization that occurred during follow-up was highest in high-income countries (29.9 per 100 person-years [95% CI, 28.9-30.8]), but the rate decreased as country income level decreased (upper–middle-income countries, 22.6 [95% CI, 21.7-23.6]; lower–middle-income countries, 17.3 [95% CI, 16.5-18.1]; and low-income countries, 11.7 [95% CI, 10.5-12.9]) (Figure 3; eFigure 1 in Supplement 1). The rate of first hospitalization for HF was 8.0 per 100 person-years (95% CI, 7.6-8.4) in high-income countries, 10.6 per 100 person-years (95% CI, 10.0-11.3) in upper–middle-income countries, 10.0 per 100 person-years (95% CI, 9.4-10.6) in lower–middle-income countries, and 6.0 per 100 person-years (95% CI, 5.1-6.8) in low-income countries.
Relationship Between Hospitalizations and Deaths
Ratio of the Rate of First Hospitalization to the Rate of Death by Country Income Level
In high-income countries, the rate of a first hospitalization was higher than the rate of death (hospitalization to death ratio = 3.8). This ratio was lower in upper–middle-income countries (2.4) and in lower–middle-income countries (1.1). In low-income countries, the rate of death exceeded the rate of hospitalization (0.6) (Figure 3; eFigure 1 in Supplement 1). Results were similar in patients enrolled as inpatients and as outpatients (eFigure 1 in Supplement 1). Analyses at the country level also demonstrated that a lower gross national income correlated with a higher mortality rate and a lower hospitalization rate (eFigure 2 in Supplement 1).
Thirty-Day Case-Fatality Rate After the First Hospitalization Occurring in Follow-up
Unadjusted 30-day case fatality after the first hospital admission date was lowest in high-income countries (6.7%), but case fatality increased as country income level increased (upper–middle-income countries [9.7%], lower–middle-income countries [21.1%], and low-income countries [31.6%]), with similar trends whether or not the first hospitalization was due to HF (Figure 4; eFigure 3 in Supplement 1). Compared with high-income countries, after adjusting for patient characteristics and use of chronic HF treatments, the risk of death within 30 days after the first hospital admission remained higher in upper–middle-income countries (HR, 1.50 [95% CI, 1.24-1.82]), and it remained substantially higher in lower–middle-income countries (HR, 3.18 [95% CI, 2.64-3.83]) and in low-income countries (HR, 5.33 [95% CI, 4.20-6.77]) (Figure 4). This was consistent regardless of whether the hospitalization was due to HF or another cause (Figure 4).
Figure 4. Risk of Death Within 30 Days of the First Hospital Admission by Country Income Level.
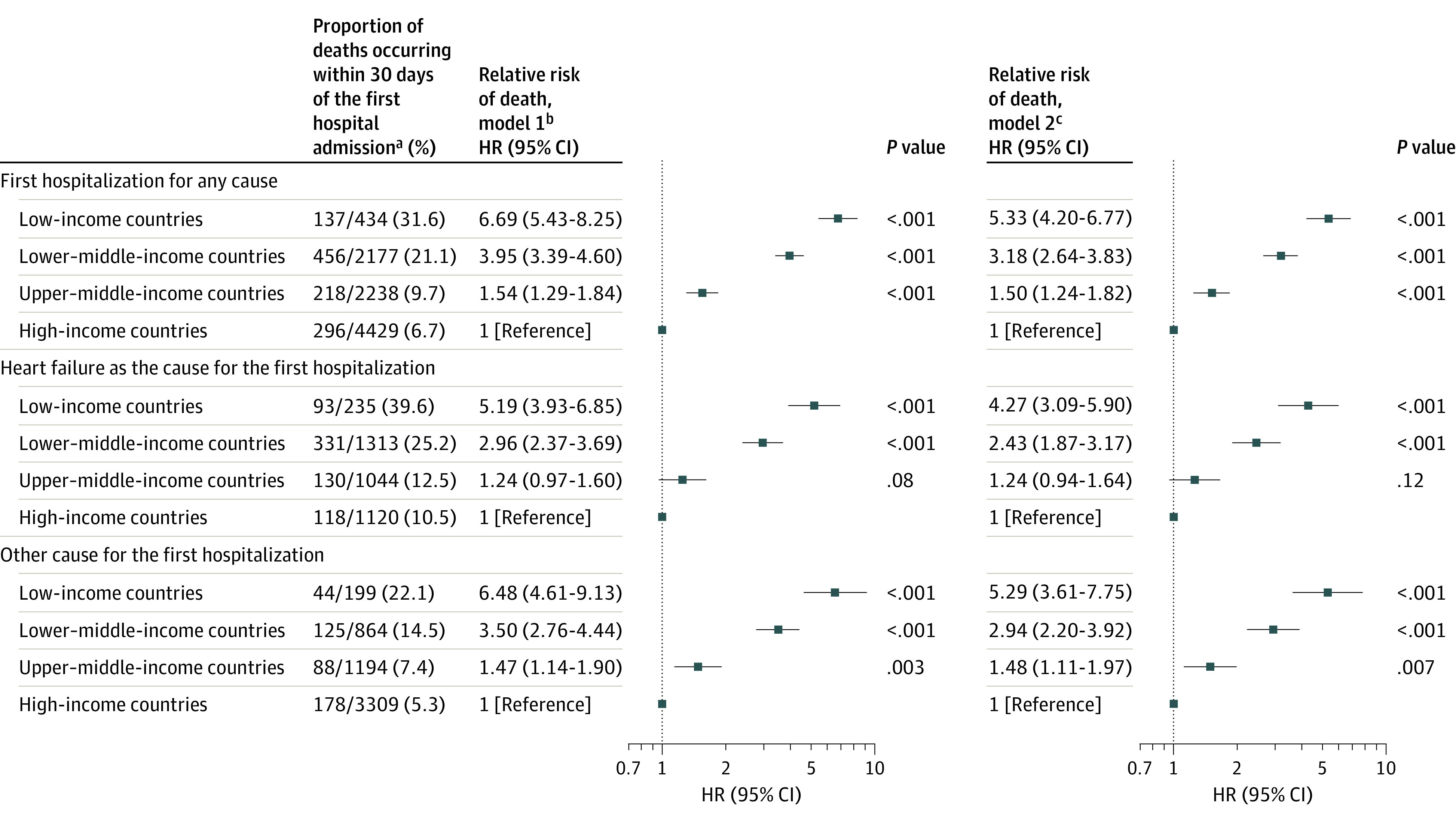
HR indicates hazard ratio.
aData reflect the first hospitalization after enrollment. Observation hospitalizations are common in the US but uncommon in many of the other countries participating in the registry; therefore, observation vs inpatient hospitalizations were not specifically delineated in the registry.
bModel was adjusted for age and sex.
cModel was adjusted for variables in model 1 plus clinical characteristics (body mass index, systolic blood pressure, diabetes, chronic obstructive pulmonary disease, current tobacco use, stage 4-5 kidney dysfunction, left ventricular ejection fraction [>50%, 41%-49%, ≤40%], having no echocardiography performed within the previous year, New York Heart Association functional class, heart failure duration >12 months), heart failure etiology (ischemic, hypertension, dilated, rheumatic valvular, nonrheumatic valvular, other), inpatient recruitment, patient education level, chronic heart failure treatments (β-blocker, renin-angiotensin system inhibitor, mineralocorticoid receptor antagonist, implantable cardioverter-defibrillator).
Discussion
To our knowledge, this is the largest prospective multinational registry of HF patients to study global variations in HF characteristics, management, and outcomes. There were several key findings from this analysis. First, while ischemic heart disease and hypertension were the most common causes of HF globally, the frequency of common etiologies varied considerably among countries at different income levels. Second, there was underutilization of chronic therapies that improve survival in HF in countries at all income levels, with the lowest use of combined medications and of ICDs in lower–middle-income and low-income countries. Third, HF patients in lower–middle-income and low-income countries were at a higher risk of death compared with those in high-income countries, which was only partly explained by differences in patient characteristics and the use of chronic HF therapies. Fourth, despite HF mortality being highest in lower–middle-income and low-income countries, use of acute hospital care was lowest, and when hospitalized, HF patients had a substantially higher mortality.
Our observation that the etiology of HF varies between countries at different income levels has important implications for the prevention and management of HF. There are large gaps in the management of ischemic heart disease and hypertension in many parts of the world, with less than half of individuals with ischemic heart disease in middle-income and low-income countries receiving secondary prevention medications, and only 12% of individuals with hypertension from low-income countries achieving adequate blood pressure control.17,18 As the burden of HF is expected to rise in many middle-income and low-income countries, it is important to highlight that a large proportion of cases could potentially be avoided by greater use of relatively low-cost medications for the management of hypertension and ischemic heart disease. The proportion of HF cases due to valvular disease was highest in low-income and lower–middle-income countries, and this was due to a higher proportion of rheumatic heart disease. In many countries where rheumatic fever is common, the management of HF due to rheumatic heart disease is limited by a lack of availability of valve surgery or balloon mitral valvuloplasty. For example, in Sub-Saharan Africa, it is estimated that 300 cardiac surgery cases are required per 1 million individuals; but there is only 1 cardiac surgeon per 3.3 million persons and approximately 22 centers that can provide cardiac surgical care to this population. Therefore, a lack of experienced personnel and infrastructure limits access to these procedures.19 In response to this need, formal strategies are being developed to expand local capacity, expertise, and training, and to reorganize current systems of care to improve the availability of these procedures in Africa.20,21 While up-front costs are high, developing an integrated strategy of secondary care for rheumatic heart disease (including secondary prophylaxis, stroke prevention, medical HF management, and surgical care) in regions with poor treatment access is still estimated to save overall costs by reducing stroke, HF morbidity, and mortality.22
Only about half of patients with HFrEF received the combination of a β-blocker, a RAS inhibitor, and an MRA, with the lowest rates of their combined use in low-income and lower–middle-income countries. Similar low rates of medication use have been observed in other contemporary HF registries and collectively suggest that essential medications for HFrEF management are underutilized in much of the world, particularly in low-income and lower–middle-income countries.23,24,25 We also observed different patterns in the use of individual medication classes in different groups of countries. In high-income countries, a high proportion of HF patients were taking β-blockers and RAS inhibitors, and the primary treatment gap in prescribing guideline-directed medications was related to a lower use of MRAs. By contrast, the higher use of MRAs in upper–middle-income countries contributed to the highest proportion of participants using all 3 medication classes in these countries. Lower–middle-income countries exhibited the lowest rates of use of all 3 medication classes; while in low-income countries, β-blockers and MRAs were underutilized in contrast with RAS inhibitors. There was even greater underutilization of ICDs in our cohort, especially in lower–middle-income and low-income countries. This is likely related to the complex range of system-, provider- and patient-level barriers that need to be overcome to support the use of ICDs, including the high cost of devices, inadequate health insurance coverage, limited infrastructure to support device implantation, and limited clinician procedural expertise.26,27
Patients in lower–middle-income and low-income countries had a substantially lower survival with HF compared with high-income countries. These findings are consistent with observations from several multinational clinical trials, indirect comparisons of regional HF registries, and the multinational REPORT-HF study, which focused on hospitalized HF patients.7,8,9,10,11,12 In lower–middle-income and low-income countries, participants were more commonly enrolled as inpatients with more advanced HF symptoms and less guideline-directed therapy, all of which could be associated with a higher risk of subsequent mortality. However, we also found that the higher risk of death in lower–middle-income and low-income countries persisted after accounting for patient-level characteristics and use of chronic HF therapies, suggesting that factors beyond these contribute to the higher risk of death observed in these countries. Since the clinical course of HF is characterized by acute episodes of decompensation where the risk of death abruptly increases, hospitalizations are an important opportunity to modify a patient´s risk through acute interventions that potentially prevent death.28,29 In this context, we observed that the lowest rates of hospitalization occurred in low-income and lower–middle-income countries despite these countries having the highest mortality rates for HF patients, suggesting that HF patients in these countries are limited in their access to acute hospital care. Moreover, when HF patients in low-income and lower–middle-income countries underwent hospitalization, their short-term risk of death was 3- to 5-fold higher than in high-income countries. Case fatality when the first hospitalization was due to HF was higher than from other causes, but the excess risk of death in lower–middle-income and low-income countries occurred with both types of hospitalizations.
Limitations
Our study also has some limitations. First, participants were recruited simultaneously from both inpatient and outpatient settings, and we cannot ensure that HF patients were consecutively enrolled across both enrollment pathways at all sites.
Second, the cause of HF was based on local investigator assessment according to locally available data, which could have resulted in some misclassification of HF etiology in countries with less available cardiac diagnostic resources.
Third, nonfatal events in our registry were primarily identified by participant self-report. While this could result in recall bias, our main nonfatal outcome of interest was hospitalization, which has very good agreement when ascertained through self-report compared with other routine surveillance methods.30,31
Fourth, some variables in our analysis had missing data. For most variables, the proportion of missing data was less than 1%, but we did not collect LVEF history or it was not recorded in 17.8% of participants. This may introduce bias if participants with missing data on LVEF differed in their baseline characteristics or treatment compared with participants without missing data.
Fifth, sodium-glucose cotransporter-2 inhibitor use was not routinely documented at baseline as these medications were not implemented into standard HF care at the time of the study’s initiation. We are now collecting data on their use during follow-up, and this will be reported in future research articles.
Sixth, estimates of medication use and survival tend to be higher in HF registries compared with the general community setting when examined in high-income countries.32 Such comparative data are not readily available in middle-income or low-income countries, but if consistent, would suggest that HF management and outcomes at the community level in lower–middle-income and low-income countries may be even worse than reported in our registry.
Conclusion
In conclusion, this study of HF patients from 40 different countries derived from 4 different income groups demonstrated differences in HF etiologies, management, and outcomes. These data may be useful in planning approaches to improve HF prevention and treatment globally.
eTable 1. Summary of Countries by Region and Countries Grouped by Income Status
eTable 2. Proportion of Missing Data for Variables Collected at Baseline for This Analysis
eTable 3. Causes of Heart Failure by Country Income Level
eTable 4. Use of Heart Failure Therapies in HFrEF by Country Income Level
eTable 5. Hazard Ratios for Death by Country Income Level After Adjusting for Patient Level Risk Factors in Participants With a Documented LVEF <40%
eTable 6. Analysis of Risk of Death in Participants Stratified by i) Duration on Known Heart Failure Prior to Enrollment and ii) Inpatient Versus Outpatient Enrollment
eFigure 1. Age- and Sex-Standardized Rates of Death and First Hospitalization for Any Cause by Countries Grouped by Income Level
eFigure 2. Country-Level Correlations of Gross National Income With the Rate of Death and the Rate of First Hospitalization
eFigure 3. Crude 30-Day Case Fatality From the Time of the First Hospital Admission, Which Was Due to A) Any Cause, B) Heart Failure, and C) Cause Other Than Heart Failure
eAppendix 1. Description of Study Follow-up
eAppendix 2. National Leaders (NLs), Principal Investigators (PIs), Co-Investigators (Co-Is), and Sub-Investigators (Sub-Is)
Co-Investigators and Study Staff
Data Sharing Statement
References
- 1.Bragazzi NL, Zhong W, Shu J, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28(15):1682-1690. doi: 10.1093/eurjpc/zwaa147 [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148(1):43-51. doi: 10.1016/j.ahj.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 3.Jonsson A, Edner M, Alehagen U, Dahlström U. Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail. 2010;12(1):25-31. doi: 10.1093/eurjhf/hfp175 [DOI] [PubMed] [Google Scholar]
- 4.Maggioni AP, Dahlström U, Filippatos G, et al. ; Heart Failure Association of the European Society of Cardiology (HFA) . EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808-817. doi: 10.1093/eurjhf/hft050 [DOI] [PubMed] [Google Scholar]
- 5.Adams KF Jr, Fonarow GC, Emerman CL, et al. ; ADHERE Scientific Advisory Committee and Investigators . Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209-216. doi: 10.1016/j.ahj.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 6.Savarese G, Vasko P, Jonsson Å, Edner M, Dahlström U, Lund LH. The Swedish Heart Failure Registry: a living, ongoing quality assurance and research in heart failure. Ups J Med Sci. 2019;124(1):65-69. doi: 10.1080/03009734.2018.1490831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristensen SL, Martinez F, Jhund PS, et al. Geographic variations in the PARADIGM-HF heart failure trial. Eur Heart J. 2016;37(41):3167-3174. doi: 10.1093/eurheartj/ehw226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair JEA, Zannad F, Konstam MA, et al. ; EVEREST Investigators . Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol. 2008;52(20):1640-1648. doi: 10.1016/j.jacc.2008.07.056 [DOI] [PubMed] [Google Scholar]
- 9.Dokainish H, Teo K, Zhu J, et al. ; INTER-CHF Investigators . Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health. 2017;5(7):e665-e672. doi: 10.1016/S2214-109X(17)30196-1 [DOI] [PubMed] [Google Scholar]
- 10.Sliwa K, Davison BA, Mayosi BM, et al. Readmission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry. Eur Heart J. 2013;34(40):3151-3159. doi: 10.1093/eurheartj/eht393 [DOI] [PubMed] [Google Scholar]
- 11.MacDonald MR, Tay WT, Teng T-HK, et al. ; ASIAN-F investigators . Regional variation of mortality in heart failure with reduced and preserved ejection fraction across Asia: outcomes in the ASIAN-HF Registry. J Am Heart Assoc. 2020;9(1):e012199. doi: 10.1161/JAHA.119.012199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tromp J, Bamadhaj S, Cleland JGF, et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Health. 2020;8(3):e411-e422. doi: 10.1016/S2214-109X(20)30004-8 [DOI] [PubMed] [Google Scholar]
- 13.Joseph P, Dokainish H, McCready T, et al. ; G-CHF Investigators . A multinational registry to study the characteristics and outcomes of heart failure patients: The global congestive heart failure (G-CHF) registry. Am Heart J. 2020;227:56-63. doi: 10.1016/j.ahj.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 14.MapChart . Create your own custom map. Created maps are licensed under a Creative Commons Attribution–ShareAlike 4.0 International License. Accessed April 20, 2023. https://www.mapchart.net
- 15.Pocock SJ, Ariti CA, McMurray JJV, et al. ; Meta-Analysis Global Group in Chronic Heart Failure . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34(19):1404-1413. doi: 10.1093/eurheartj/ehs337 [DOI] [PubMed] [Google Scholar]
- 16.Sartipy U, Dahlström U, Edner M, Lund LH. Predicting survival in heart failure: validation of the MAGGIC heart failure risk score in 51 043 patients from the Swedish Heart Failure Registry. Eur J Heart Fail. 2014;16(2):173-179. doi: 10.1111/ejhf.32 [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Islam S, Chow CK, et al. ; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378(9798):1231-1243. doi: 10.1016/S0140-6736(11)61215-4 [DOI] [PubMed] [Google Scholar]
- 18.Chow CK, Teo KK, Rangarajan S, et al. ; PURE (Prospective Urban Rural Epidemiology) Study investigators . Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310(9):959-968. doi: 10.1001/jama.2013.184182 [DOI] [PubMed] [Google Scholar]
- 19.Yankah C, Fynn-Thompson F, Antunes M, et al. Cardiac surgery capacity in sub-saharan Africa: quo vadis? Thorac Cardiovasc Surg. 2014;62(5):393-401. doi: 10.1055/s-0034-1383723 [DOI] [PubMed] [Google Scholar]
- 20.Zilla P, Bolman RM, Yacoub MH, et al. The Cape Town Declaration on Access to Cardiac Surgery in the Developing World. Cardiovasc J Afr. 2018;29(4):256-259. doi: 10.5830/CVJA-2018-046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miccio R, Quattrociocchi M, Valgoi L, et al. Treating children with advanced rheumatic heart disease in Sub-Saharan Africa: the NGO EMERGENCY’s project at the Salam Centre for Cardiac Surgery in Sudan. Front Pediatr. 2021;9:704729. doi: 10.3389/fped.2021.704729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coates MM, Sliwa K, Watkins DA, et al. An investment case for the prevention and management of rheumatic heart disease in the African Union 2021-30: a modelling study. Lancet Glob Health. 2021;9(7):e957-e966. doi: 10.1016/S2214-109X(21)00199-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damasceno A, Mayosi BM, Sani M, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012;172(18):1386-1394. doi: 10.1001/archinternmed.2012.3310 [DOI] [PubMed] [Google Scholar]
- 24.Teng TK, Tromp J, Tay WT, et al. ; ASIAN-HF investigators . Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Health. 2018;6(9):e1008-e1018. doi: 10.1016/S2214-109X(18)30306-1 [DOI] [PubMed] [Google Scholar]
- 25.Dokainish H, Teo K, Zhu J, et al. ; INTER-CHF Investigators . Heart failure in Africa, Asia, the Middle East and South America: the INTER-CHF study. Int J Cardiol. 2016;204:133-141. doi: 10.1016/j.ijcard.2015.11.183 [DOI] [PubMed] [Google Scholar]
- 26.Chia YMF, Teng TK, Tan ESJ, et al. Disparity between indications for and utilization of implantable cardioverter defibrillators in Asian patients with heart failure. Circ Cardiovasc Qual Outcomes. 2017;10(11):e003651. doi: 10.1161/CIRCOUTCOMES.116.003651 [DOI] [PubMed] [Google Scholar]
- 27.Kirkpatrick JN, Papini C, Baman TS, et al. Reuse of pacemakers and defibrillators in developing countries: logistical, legal, and ethical barriers and solutions. Heart Rhythm. 2010;7(11):1623-1627. doi: 10.1016/j.hrthm.2010.04.027 [DOI] [PubMed] [Google Scholar]
- 28.Abraham WT, Fonarow GC, Albert NM, et al. ; OPTIMIZE-HF Investigators and Coordinators . Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol. 2008;52(5):347-356. doi: 10.1016/j.jacc.2008.04.028 [DOI] [PubMed] [Google Scholar]
- 29.Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155(2):200-207. doi: 10.1016/j.ahj.2006.10.043 [DOI] [PubMed] [Google Scholar]
- 30.Short ME, Goetzel RZ, Pei X, et al. How accurate are self-reports? analysis of self-reported health care utilization and absence when compared with administrative data. J Occup Environ Med. 2009;51(7):786-796. doi: 10.1097/JOM.0b013e3181a86671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raina P, Torrance-Rynard V, Wong M, Woodward C. Agreement between self-reported and routinely collected health-care utilization data among seniors. Health Serv Res. 2002;37(3):751-774. doi: 10.1111/1475-6773.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund LH, Carrero J-J, Farahmand B, et al. Association between enrolment in a heart failure quality registry and subsequent mortality-a nationwide cohort study. Eur J Heart Fail. 2017;19(9):1107-1116. doi: 10.1002/ejhf.762 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary of Countries by Region and Countries Grouped by Income Status
eTable 2. Proportion of Missing Data for Variables Collected at Baseline for This Analysis
eTable 3. Causes of Heart Failure by Country Income Level
eTable 4. Use of Heart Failure Therapies in HFrEF by Country Income Level
eTable 5. Hazard Ratios for Death by Country Income Level After Adjusting for Patient Level Risk Factors in Participants With a Documented LVEF <40%
eTable 6. Analysis of Risk of Death in Participants Stratified by i) Duration on Known Heart Failure Prior to Enrollment and ii) Inpatient Versus Outpatient Enrollment
eFigure 1. Age- and Sex-Standardized Rates of Death and First Hospitalization for Any Cause by Countries Grouped by Income Level
eFigure 2. Country-Level Correlations of Gross National Income With the Rate of Death and the Rate of First Hospitalization
eFigure 3. Crude 30-Day Case Fatality From the Time of the First Hospital Admission, Which Was Due to A) Any Cause, B) Heart Failure, and C) Cause Other Than Heart Failure
eAppendix 1. Description of Study Follow-up
eAppendix 2. National Leaders (NLs), Principal Investigators (PIs), Co-Investigators (Co-Is), and Sub-Investigators (Sub-Is)
Co-Investigators and Study Staff
Data Sharing Statement


