Abstract
A cold-sensitive secY mutant (secY125) with an amino acid substitution in the first periplasmic domain causes in vivo retardation of protein export. Inverted membrane vesicles prepared from this mutant were as active as the wild-type membrane vesicles in translocation of a minute amount of radioactive preprotein. The mutant membrane also allowed enhanced insertion of SecA, and this SecA insertion was dependent on the SecD and SecF functions. These and other observations suggested that the early events in translocation, such as SecA-dependent insertion of the signal sequence region, is actually enhanced by the SecY125 alteration. In contrast, since the mutant membrane vesicles had decreased capacity to translocate chemical quantity of pro-OmpA and since they were readily inactivated by pretreatment of the vesicles under the conditions in which a pro-OmpA translocation intermediate once accumulated, the late translocation functions appear to be impaired. We conclude that this periplasmic secY mutation causes unbalanced early and late functions in translocation, compromising the translocase's ability to catalyze multiple rounds of reactions.
A significant fraction of gene products encoded by the Escherichia coli genome are transported across the cytoplasmic membrane to the cell surface locations. Most of them are believed to use the Sec translocase for their delivery across the membrane. The Sec system is comprised of a cytosolic chaperone, SecB, the preprotein-driving ATPase, SecA, and integral membrane components, SecY, SecE, SecG, SecD, SecF, and YajC. In recent years, there has been remarkable progress in the elucidation of the modes of functions of some of the Sec factors (for reviews, see references 9 and 34).
The earliest process of protein translocation is the targeting of a preprotein to the translocase on the membrane. SecB possesses dual roles in the targeting step, prevention of the preprotein from unwanted folding and its presentation to the membrane-associated SecA protein. SecA then undergoes ATP- and preprotein-dependent insertion into the membrane, thereby initiating translocation by inserting the signal sequence and the early mature region of the preprotein (11). This process, referred to as the early translocation reaction, is followed by a mid translocation reaction, where translocation of the bulk of the mature sequence occurs. Finally, the translocated polypeptide is released into the milieu of the opposite periplasmic side (late translocation reaction). The signal peptide is cleaved off at certain point during this series of reactions.
Relatively little is understood about the mid to late reactions of translocation. The membrane inserted SecA releases the preprotein and deinserts itself from the membrane in a manner dependent on hydrolysis of ATP (11). While this ATPase can drive complete translocation by repeating the insertion/deinsertion cycles, the proton motive force (PMF) across the membrane can also facilitate translocation (8, 27). It should also be noted that the SecA actions as outlined above are executed in its close interaction with the SecYEG components in the membrane (12, 17).
Among the integral membrane Sec factors, the central subunits are SecY and SecE, which can be reconstituted into proteoliposomes having a basal activity of SecA-dependent translocation (1, 6). SecY and SecE may constitute an intramembranous channel for the translocation. SecG enhances the activity of the SecYE channel, probably by facilitating the SecA function through its topology inversion in the membrane (16, 22, 30). Roles assigned for SecD and SecF include facilitation of the release of a translocated polypeptide from the periplasmic membrane surface (19), stabilization of the inserted state of SecA (10, 12), and maintenance of PMF (4).
In this study, we characterized a secY mutation (secY125) with a Ser78-to-Phe amino acid alteration in the first periplasmic domain of this multi-membrane-spanning protein. This was one of the cold-sensitive mutants that we isolated earlier (32). While it exhibits a clear protein export defect at 20°C in vivo, inverted membrane vesicles (IMVs) prepared from this mutant were as active in vitro as the wild-type IMV. Evidence suggests that the early steps of translocation may be rather enhanced by this mutation whereas overall reaction capacity and a late step reaction are impaired. Thus, the mutation may provide a useful opportunity to study roles played by SecY in substeps of protein translocation reactions.
MATERIALS AND METHODS
E. coli strains.
A cold-sensitive secY125 mutant was isolated previously (32), and the mutation was introduced into appropriate strains using zhd-33::Tn10 (tetracycline resistance) or rpsE (spectinomycin resistance) as a selective marker in P1vir-mediated transduction (20). TY8 and TY0 were, respectively, secY125 zhd-33::Tn10 rpsE and secY+ zhd-33::Tn10 rpsE transductants of AD202, a derivative of MC4100 (28) carrying ompT::kan (2). GN08 and TW156 were respective Δ(uncB-uncC) (15) derivatives of the above two strains. THE562 (MC4100, secY125 rpsE ΔompT leu::Tn10 ara+ tgt::kan Pbad::yajC secDF) and its secY+ counterpart, THE575, carried the secD operon placed under the ara promoter and were constructed as follows. Derivatives carrying secY125-rpsE and secY+-rpsE were constructed from AD179 (MC4100, ΔompT [2]) by P1 transduction using rpsE as a selective marker. They were then transduced to tetracycline resistant and ara+ using an ara+ leu::Tn10 strain as a donor. Subsequently, these transductants were further transduced to kanamycin resistant (and arabinose requiring) using strain JP325 (MC4100, Δara714 araC+ tgt::kan Pbad::yajC secDF; donated by Joe Pogliano and Jon Beckwith) as a donor.
Materials.
IMVs were prepared from strains GN08 and TW156 (see above for strain descriptions) as described previously (35) and washed with 6 M urea (31). The wild-type SecA protein was overproduced and purified as described previously (17). SecB was purified as described elsewhere (35). The precursor form of OmpA (pro-OmpA) was purified as described previously (7, 26). [125I]NaI (17.5 Ci/mg of I; 100 mCi/ml) and [35S]methionine (1,175 Ci/mmol) were purchased from ICN and from American Radiolabeled Chemicals, Inc., respectively.
Media.
L medium contained 10 g of tryptone, 5 g of yeast extract, 5 g of NaCl, and 1.7 mmol of NaOH per liter. Minimal medium M9 was as described in reference 28.
In vitro protein translocation.
[35S]methionine-labeled pro-OmpA was synthesized in vitro and subjected to the in vitro translocation reaction as described previously (17). It was mixed with unlabeled and purified pro-OmpA, when indicated, for measuring chemical amounts of translocation. The reaction mixture contained IMV (500 μg of protein/ml), SecA (10 μg/ml), SecB (15 μg/ml), ATP (2 mM, unless otherwise indicated), an ATP regeneration system (5 mM phosphocreatine and 100 μg of creatine kinase per ml), 50 mM Tris-HCl buffer (pH 7.5), 5 mM MgSO4, and 500 μg of bovine serum albumin per ml. After incubation at 37 or 20°C for indicated lengths of time, samples were chilled on ice and treated with proteinase K (100 μg/ml) at 0°C for 20 min followed by trichloroacetic acid precipitation and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Translocated OmpA was quantified by a Fuji BAS2000 or BAS1800 phosphorimager.
Insertion of the 30-kDa segment of SecA into IMV.
SecA was covalently modified with 125I as described by Economou and Wickner (11), with minor modifications; 200 μl of solution containing 50 mM Tris-HCl (pH 8.0), SecA (200 μg), [125I]NaI (0.2 mCi), 50 mM KCl, 5 mM MgCl2, and 10% glycerol was added to an Iodogen (100 μg; obtained from Pierce)-coated tube and incubated for 20 min on ice. SecA insertion assay was essentially as described previously (11, 17), using IMV from an appropriate strain.
SecA binding assay.
Quantitative assay for SecA binding to IMV was carried out using different concentrations of SecA that contained a fixed amount of 125I-labeled SecA essentially as described by Hartl et al. (13). Data were analyzed by Scatchard analysis.
Pulse-chase study of in vivo protein export.
Cells were grown on M9-glycerol (0.4%) medium supplemented with 0.4% maltose, 5 mM cyclic AMP, and 18 amino acids (20 μg of each [excluding methionine and cysteine] per ml) and pulse-labeled with [35S]methionine (5 to 13 μCi/ml, 1,175 Ci/mmol; obtained from American Radiolabeled Chemicals) for indicated periods. Chase with unlabeled methionine and immunoprecipitation with anti-maltose-binding protein and anti-OmpA sera were as described previously (5), except that protein A-Sepharose was used as an immunoadsorbent. Immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis, and precursor and mature forms of maltose-binding protein and OmpA were visualized by exposure to a phosphorimager plate.
RESULTS
In vivo phenotypes of the secY125 mutant.
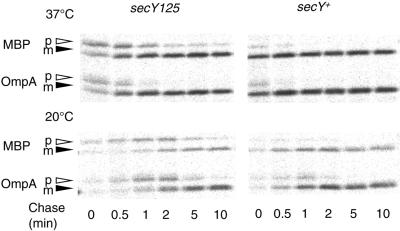
The secY125 mutant is unique in that it is the sole loss-of-function mutant so far identified as having an amino acid substitution in a periplasmic domain (32). It is a leaky cold-sensitive mutant, showing retarded growth at 20°C. When export of OmpA and maltose-binding protein was examined by pulse-chase experiments, this mutant showed an appreciable protein export defect even at 37°C (Fig. 1, upper panel); the defect became exaggerated within 20 min upon temperature down-shift to 20°C (Fig. 1, lower panel).
FIG. 1.
The secY125 mutant is defective in protein export. Cells of TY0 (secY+) and TY8 (secY125) were grown in M9-glycerol-maltose medium at 37°C (upper panel) and then shifted to 20°C for 20 min (lower panel). Cells were pulse-labeled for 0.5 min with [35S]methionine and chased for 0, 0.5, 1, 2, 5, and 10 min, as indicated. Whole cell proteins, precipitated by trichloroacetic acid, were dissolved in SDS-containing solution, diluted with Triton X-100-containing solution, and subjected to immunoprecipitation using antibodies against maltose-binding protein (MBP) and OmpA. After separation by SDS-polyacrylamide gel electrophoresis, precursor (p) and mature (m) forms of these proteins were detected by phosphorimager exposure.
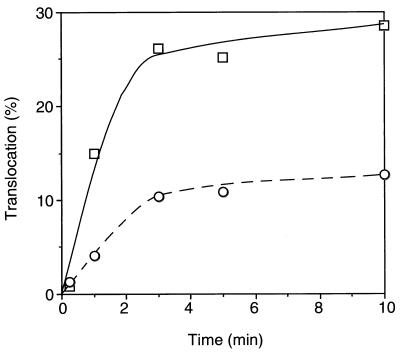
In vitro translocation of radiolabeled preprotein.
A standard in vitro assay of the E. coli protein translocation system uses IMV, SecA, and in vitro-translated and radiolabeled precursor protein, most often pro-OmpA. In this standard reaction, a number of cold-sensitive secY mutants showed severe defects when used as the sources of IMV (31). In many cases such a defect was observed even when the reaction was carried out at 37°C. When IMV prepared from the secY125 mutant was assayed, essentially the wild-type translocation activities were observed at both 37 and 20°C (Fig. 2). These results raised a question about the altered determinant in the mutant that causes impaired protein export in vivo but does not cause appreciable translocation defect in vitro. In the assay system used in the experiments shown in Fig. 2, translocation was defined as sequestration of precursor protein, which was minute in chemical amount, in a protease-inaccessible location. It is not established at what stage of the translocation process the substrate protein acquires the protease resistance, although it must be at a later stage. Also, it is likely that we assayed only a single round (or a few rounds) of reaction per a unit of the translocase machinery. In contrast, the Sec translocase in vivo must function repeatedly and must complete each reaction.
FIG. 2.
Translocation of in vitro-translated proOmpA. In vitro-translated and [35S]methionine-labeled pro-OmpA was precipitated with trichloroacetic acid, dissolved in urea solution, and subjected to translocation reaction in the presence of SecA and ATP into IMV prepared from secY+ (strain TW156; squares) and secY125 (strain GN08; circles) cells. Reactions were carried out at 37°C (solid symbols) or 20°C (open symbols), and translocated molecules that resisted proteinase K were quantitated after gel electrophoresis and phosphorimager exposure.
Enhanced SecA insertion into the secY125 IMV.
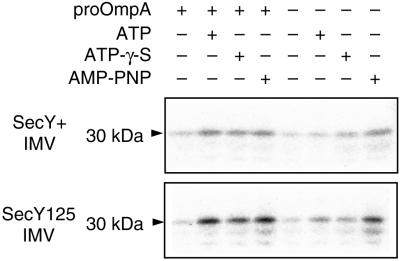
SecA insertion assays were carried out using a 125I-labeled SecA preparation and IMVs prepared from the wild-type and secY125 cells. The reaction mixtures were trypsinized to observe the 30-kDa inserted fragment of SecA (11). SecA insertion into the wild-type IMV was dependent on the addition of both ATP and pro-OmpA (Fig. 3, lane 2). This mode of insertion is referred to as productive insertion. In the presence of a nonhydrolyzable analog of ATP, SecA insertion was observed irrespective of the presence or absence of pro-OmpA (idling insertion; Fig. 3, lanes 3, 4, 7, and 8). SecA insertion reactions, both productive and idling, were apparently enhanced about two- to threefold when IMV from the secY125 mutant was used (Fig. 3, compare upper and lower panels).
FIG. 3.
Mutational enhancement of SecA insertion reaction. 125I-SecA (4 μg) and urea-treated IMV (35 μg of protein) from wild-type (upper panel) or secY125 (lower panel) cells were preincubated at 0°C (200 μl), and IMV-SecA complex was isolated by centrifugation and subjected to SecA insertion reaction (50 μl) containing the 125I-SecA-bound IMV (5 μg) in the presence or absence of pro-OmpA (3.3 μg), ATP (2 mM), ATPγS (2 mM), and adenosine 5′-(β,γ-imino)triphosphate (AMP-PNP) (2 mM), as indicated, at 37°C for 15 min. After trypsin digestion, the 30-kDa fragment (indicated by an arrowhead) was visualized by SDS-polyacrylamide gel electrophoresis and phosphorimager exposure.
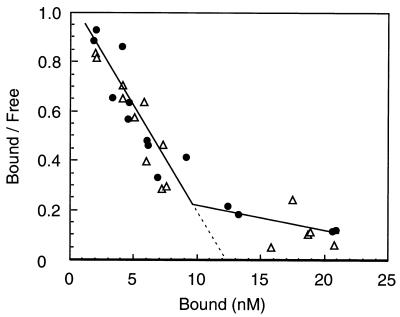
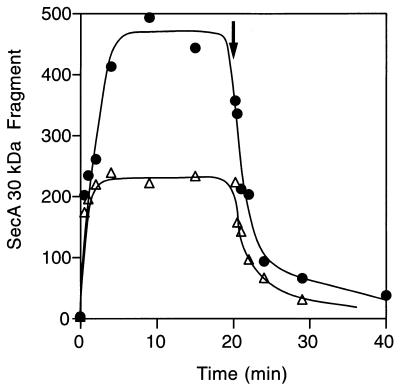
Scatchard analysis of SecA high-affinity binding (Fig. 4) indicated that wild-type and secY125 IMVs had similar affinities to SecA. The numbers of high-affinity binding sites in these membrane preparations were also similar (Fig. 4, abscissa intersection of the extrapolated first phase). Thus, the secY125 alteration does not affect the SecYEG binding steps of SecA. Since the assay for SecA insertion measures the steady state of the insertion/deinsertion equilibrium (11), enhanced SecA insertion can be ascribed either to a bona fide enhancement of insertion or an inhibition of deinsertion. Our previous measurements indicated that IMV prepared from the secY125 mutant can support essentially the normal level of SecA translocation ATPase activity (31; T. Yoshihisa, personal communication). Since deinsertion is coupled with ATP hydrolysis (11, 12), this result argues against the possibility that deinsertion was lowered by the mutation. We directly measured the kinetics of deinsertion (Fig. 5). To observe the deinsertion phase, unlabeled SecA was added in excess at 20 min after the onset of the insertion reaction at 37°C. Insertion reaction into the secY125 IMV continued until it reached a higher than wild-type steady state (Fig. 5). Upon addition of unlabeled SecA, the 125I-labeled SecA molecules were rapidly chased from both the wild-type and secY125 IMVs (Fig. 5). The rapidity of the chase in the secY125 reaction was no less than the wild-type reaction. Thus, we did not obtain any results that point to a decreased SecA deinsertion for the secY125 membrane vesicles. These results indicate that SecA can more readily insert into the mutant membrane than into the wild-type membrane.
FIG. 4.
Scatchard analysis of SecA binding. SecA (4 to 400 nM, of which 125I-SecA occupied 4 nM) was mixed with urea-washed IMV (100 μg/ml) in a 50-μl reaction at 0°C for 30 min, followed by isolation of IMV-SecA complex by centrifugation. Radioactivities of the bottom and supernatant were determined by an LKB γ-ray counter. Solid circles, wild-type IMV; open triangles, SecY125 IMV. Kd values estimated were 16 nM for both IMVs, and the number of high-affinity binding sites was estimated to be 3.3 × 1012 per μg (protein) of IMV.
FIG. 5.
Time courses of SecA insertion and deinsertion. 125I-SecA (8.2 μg) and urea-treated IMV (130 μg of protein) from wild-type (open triangles) or secY125 (solid circles) cells were preincubated at 0°C (200 μl). IMV-SecA complex was isolated by centrifugation and subjected to SecA insertion reaction (690 μl) in the presence of 1 mM ATP at 37°C. At 20 min (shown by arrow), unlabeled SecA (2.9 μM) was added to follow the deinsertion phase. Samples of 50 μl were withdrawn during the incubation, followed by trypsin digestion and subsequent gel electrophoresis and visualization of the 30-kDa fragment. Radioactivities were quantitated with a BAS2000 phosphorimager and reported as arbitrary units.
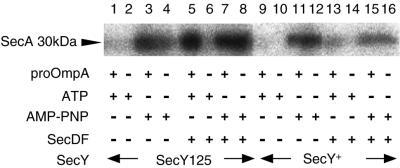
It was reported that SecD and SecF act to stabilize the inserted state of SecA (10, 12). We examined whether the enhanced insertion of SecA into the secY125 IMV was also dependent on the SecD and SecF proteins. We constructed secY+ and secY125 strains, in which the chromosomal secD operon was placed under the control of the ara promoter (24; J. Pogliano, personal communication). When cells were cultured in the presence of glucose but in the absence of arabinose for 1.5 h, the cellular content of SecD decreased to less than one-fifth of the normal value (data not shown). IMVs were prepared from these cells and assayed for SecA insertion. IMVs from both the secY+ and the secY125 strains supported SecA insertion in the presence of a nonhydrolyzable ATP analog (Fig. 6, lanes 3, 4, 11, and 12). In contrast, the pro-OmpA- and ATP-dependent insertion was not observed with these membrane vesicles of decreased SecD content (Fig. 6, lanes 1 and 9). Thus, the enhanced insertion of SecA observed with the mutant membrane vesicles requires the stabilization of the inserted state by the SecD and/or SecF proteins. The altered SecY125 protein allows an increased efficiency of SecA insertion, which is normal at least in its dependence on SecDF functions. The mutation does not create an entirely independent pathway of generating the trypsin-resistant SecA fragment.
FIG. 6.
SecA insertion into IMVs with decreased SecDF contents. Cells of strains THE562 (secY125 Pbad::yajC secDF; lanes 1 to 8) and THE575 (secY+ Pbad::yajC secDF; lanes 9 to 16) were grown in L medium supplemented with 0.2% arabinose and 0.1 mM cyclic AMP and sampled (lanes 5 to 8 and 13 to 16). To deplete SecD and SecF contents, cultures grown as above were centrifuged, and cells were washed twice with arabinose-free medium and grown for 90 min in the same medium supplemented further with 0.2% glucose (lanes 1 to 4 and 9 to 12). The SecD contents of the latter samples were shown to be decreased at least fivefold. IMVs were prepared from these samples, washed with urea, and subjected to the SecA insertion assay as described in the legend to Fig. 3. The 30-kDa inserted fragment of 125I-labeled SecA was visualized by phosphorimager.
Decreased translocation capacity and the late translocation functions in the secY125 IMV.
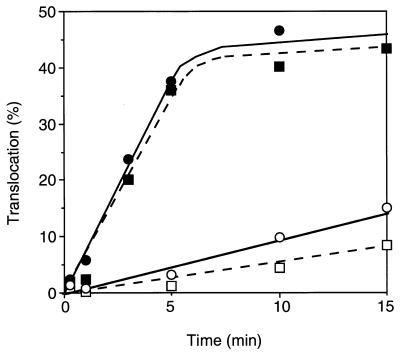
The results of the SecA insertion assay suggest that an early reaction in translocation is stimulated by the secY125 mutation. The overall translocation activity was not significantly affected when we carried out the translocation assays using radiochemical levels of substrate (Fig. 2). We then repeated the translocation assays using chemical amounts of pro-OmpA. In the experiment shown in Fig. 7, unlabeled and purified pro-OmpA was mixed with the 35S-labeled product, giving a substrate concentration of 25 μg/ml. Under these conditions, the secY125 IMV was only about 40% as active as the wild-type IMV (Fig. 7). It is thus suggested that the capacity of translocation is significantly decreased, raising a possibility that the altered translocation channel may be subject to blockage due to a defect in a later step of translocation.
FIG. 7.
In vitro translocation activities in the presence of excess pro-OmpA. A mixture of [35S]methionine-labeled pro-OmpA, as described for Fig. 2, and unlabeled pro-OmpA (25 μg/ml) was subjected to translocation reaction into the wild-type (squares) and secY125 (circles) IMVs. The reaction mixture was slightly modified; it contained 50 mM sodium phosphate buffer (pH 7.5) instead of Tris-HCl, 50 μg of SecA per ml, 1 mM ATP, 5 mM succinate, and no bovine serum albumin. Radioactive and translocated OmpA was quantitated after gel electrophoresis and phosphorimager exposure.
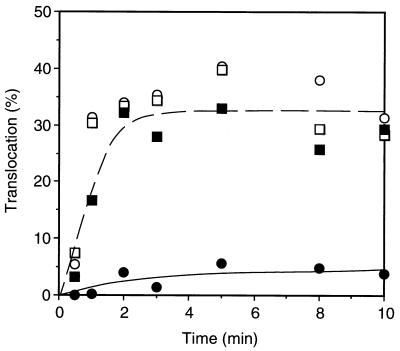
We confirmed that in vitro translocation reaction in the presence of a low concentration of ATP resulted in accumulation of a translocation intermediate of pro-OmpA, as reported by Schiebel et al. (27). This reaction occurred similarly with the wild-type and secY125 IMVs (data not shown). The mutant and the wild-type IMVs were subjected to the above reaction with 2 μM ATP and a chemical amount of pro-OmpA and then reisolated by centrifugation. The pro-OmpA intermediate-loaded IMVs were then incubated for 10 min with 2 mM ATP and 5 mM succinate to complete later stages of translocation. Subsequently, [35S]methionine-labeled pro-OmpA was added, and incubation continued for another 10 min. Control samples did not receive pro-OmpA in the preloading step. Figure 8 depicts time courses of translocation of [35S]methionine-labeled pro-OmoA. Translocation activity of the wild-type IMV remained unaltered or only slightly inhibited after it was subjected to the intermediate loading (in the presence of a low concentration of ATP) and the subsequent chase (in the presence of a high concentration of ATP); in contrast, the SecY125 IMV was markedly inactivated when it had been loaded with the pro-OmpA intermediate (Fig. 8). The fact that the inactivation was caused by the pretreatment of the mutant IMV with a chemical amount of pro-OmpA is consistent with the lowered translocation capacity of the SecY125 IMV. We suppose it likely that, whereas pro-OmpA is completely chased away from the wild-type IMV, it somehow remains SecY125 IMV associated even after the chase reaction in the presence of a concentration of high ATP. Thus, unlike the wild-type IMV, the mutant IMV may not be able to recycle efficiently for multiple rounds of reactions.
FIG. 8.
Inactivation of the SecY125 IMV by preloading of a translocation intermediate of pro-OmpA. IMVs prepared from strains TW156 (secY+; squares) and GN08 (secY125; circles), each 500 μg of protein/ml, were preincubated at 37°C for 5 min and then mixed with the reaction mixture for translocation with 2 μM ATP and with (solid symbols) or without (open symbols) 100 μg of pro-OmpA per ml, followed by incubation at 37°C for 10 min. IMVs were then isolated by centrifugation at 131,000 × g for 15 min at 4°C and resuspended in the same volume of reaction mixture containing 2 mM ATP and 5 mM potassium succinate. After incubation at 37°C for 10 min, a mixture of [35S]methionine-labeled pro-OmpA and unlabeled pro-OmpA (5 μg/ml) was added, and incubation continued for an additional 10 min. Samples were removed during the last incubation for measurement of translocated radioactive OmpA.
DISCUSSION
After being targeted to the membrane-bound translocase, a preprotein must initiate translocation by inserting its signal peptide region into the membrane. This is a distinct “early” reaction that requires ATP binding but not its hydrolysis (27, 33). This reaction may also be characterized by its incompatibility with the presence of positively charged residues at a segment (of about 30 amino acids) that is C-terminally adjacent to the signal peptide (3, 35). It is likely that the ATP-dependent SecA insertion is a key reaction for the initiation.
Here we presented evidence that SecA insertion reaction is enhanced by the SecY125 alteration (Ser78 to Phe) in the first periplasmic domain of SecY. This effect was not due to an increased SecA binding to the membrane, nor was it due to a decreased ATP hydrolysis-coupled deinsertion of SecA. The latter conclusion was supported by the following results. First, direct measurement of the deinsertion phase showed no appreciable retardation for the reaction involving SecY125 IMV. Second, SecY125 IMV supported normal ATPase activity of SecA (31; Yoshihisa, personal communication). Finally, SecY125-dependent enhancement was observed even when the insertion was driven by a nonhydrolyzable ATP analog (Fig. 3; Fig. 6, compare lanes 7 and 8 with lanes 15 and 16). The lowered SecDF content resulted in the abolishment of the ATP-dependent mode of insertion into both wild-type and SecY125 IMV. Thus, the mutational enhancement involves a normal mode of the reaction, at least with respect to its dependence on the SecDF functions. The fact that the ATP analog-dependent mode of insertion occurred even when the SecDF content was lowered is consistent with the notion that SecDF normally downregulates the ATP hydrolysis-coupled deinsertion. The insertion that is observed in response to a nonhydrolyzable analog of ATP still requires the integrity of the SecYE channel (18), although it is a distinct reaction in that it can be affected differentially by the secY205 mutation (17). Since the latter mode of insertion as well was stimulated by the SecY125 alteration, the secY125 mutation is likely to affect a step that is common between the productive and idling reactions (17) of SecA.
The mechanism by which a periplasmic alteration affects the SecA insertion process merits some discussion. Since some portions of SecA, when inserted, seem to become accessible from the periplasmic side (14, 25), an interaction between a periplasmic region of SecY and SecA is conceivable. In this case, the SecY125 alteration may stabilize such an interaction, resulting in the enhancement of insertion. It was indeed reported that an N-terminal portion of SecY, including the first periplasmic region, can be decorated with SecA in a ligand blotting assay after gel electrophoresis (29). It is also possible that the SecY125 alteration affects an interaction of SecY with the SecDF component, which stabilizes the inserted SecA. Still another possibility may be that the periplasmic alteration exerts an allosteric effect on a cytosolic domain of SecY such that the insertion reaction of SecA is enhanced.
We observed a decreased PMF dependence in the SecY125-mediated translocation of a pro-OmpF derivative protein, which normally shows a strong dependence on the PMF (H. Mori and K. Ito, unpublished data). Translocation of this protein into the wild-type and secY125 IMVs occurred at similar efficiencies in the presence of PMF. When the PMF was dissipated, the wild-type activity was lowered about fourfold, but more than 50% of the secY125 activity remained. Multiple roles have been assigned for PMF in translocation facilitation. PMF may facilitate translocation of preprotein that has just been released from SecA (8). It may also facilitate the SecA reaction cycles by enhancing the deinsertion process (21). PrlA mutant forms of SecY render the translocation less dependent on PMF, suggesting a role of PMF in an early step of translocation, in which signal sequence is inserted into the membrane (23). Consistent with this view, we observed a selective enhancement of the early translocation step by PMF, at least in a secY mutant membrane vesicles (Mori and Ito, unpublished). Thus, our observation that PMF dependence of the OmpF protein is alleviated by the secY125 mutation is consistent with this mutation's enhancing effect on the early translocation reaction. We also noted that the secY125 mutant exhibited a Prl phenotype, being able to suppress a signal sequence mutation in lamB (G. Matsumoto, T. Yoshihisa, and K. Ito, unpublished data).
While the early translocation reactions appear to be enhanced by the secY125 mutation, the defect of the mutant membrane vesicles became apparent when translocation of an excess amount of pro-OmpA was examined. Furthermore, the mutant membrane was much more easily inactivated when it had been treated with pro-OmpA and a low concentration of ATP, a condition in which a translocation intermediate accumulates. These results strongly suggest that the SecY125 translocase is defective in some aspect of the late step reactions, thereby being blocked with the pro-OmpA molecules that once accumulated in an intermediate state. Obviously, it is important to demonstrate the late function defects in direct biochemical assays. For instance, one could monitor the folding states of in vitro-translocated products. Thus, the substrate protein may have remained membrane bound and unfolded after it reacted with the SecY125 IMV, compared with the substrate that reacted with the wild-type IMV. Alternatively, cross-linking with a membrane-permeable cross-linker may also be feasible. In this case, the substrate protein is expected to be cross-linkable with SecY125 without imposition of an artificial condition that halts translocation. The periplasmic localization of the mutation site is consistent with the abnormal late functions of the mutationally altered translocase. The secY125 mutant may prove useful in the dissection of the mid to late processes, which are central to the translocation mechanisms but only insufficiently understood.
ACKNOWLEDGMENTS
This work was supported by grants from CREST, JST (Japan Science and Technology Corporation), and the Ministry of Education, Science and Culture, Japan. G.M. and T.H. were supported by JSPS Research Fellowships for Young Scientists.
We thank Tohru Yoshihisa for discussion and unpublished information, Yoshinori Akiyama and Ei-ichi Matsuo for discussion, Joe Pogliano for a bacterial strain, and Kiyoko Mochizuki, Yusuke Shimizu, and Toshiki Yabe for technical assistance.
REFERENCES
- 1.Akimaru J, Matsuyama S-I, Tokuda H, Mizushima S. Reconstitution of a protein translocation system containing purified SecY, SecE, and SecA from Escherichia coli. Proc Natl Acad Sci USA. 1991;88:6545–6549. doi: 10.1073/pnas.88.15.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama Y, Ito K. SecY protein, a membrane embedded secretion factor of E. coli, is cleaved by the OmpT protease in vitro. Biochem Biophys Res Commun. 1990;167:711–715. doi: 10.1016/0006-291x(90)92083-c. [DOI] [PubMed] [Google Scholar]
- 3.Andersson H, von Heijne G. A 30-residue-long “export initiation domain” adjacent to the signal sequence is critical for protein translocation across the inner membrane of Escherichia coli. Proc Natl Acad Sci USA. 1991;88:9751–9754. doi: 10.1073/pnas.88.21.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arkowitz R A, Wickner W. SecD and SecF are required for the proton electrochemical gradient stimulation of preprotein translocation. EMBO J. 1994;13:954–963. doi: 10.1002/j.1460-2075.1994.tb06340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba T, Jacq A, Brickman E, Beckwith J, Taura T, Ueguchi C, Akiyama Y, Ito K. Characterization of cold-sensitive secY mutants of Escherichia coli. J Bacteriol. 1990;172:7005–7010. doi: 10.1128/jb.172.12.7005-7010.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brundage L, Hendrick J P, Schiebel E, Driessen A J M, Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990;62:649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- 7.Crooke E, Brundage L, Rice M, Wickner W. ProOmpA spontaneously folds into a membrane assembly competent state which trigger factor stabilizes. EMBO J. 1988;7:1831–1835. doi: 10.1002/j.1460-2075.1988.tb03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driessen A J M. Precursor protein translocation by the Escherichia coli translocase is directed by the proton motive force. EMBO J. 1992;11:847–853. doi: 10.1002/j.1460-2075.1992.tb05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driessen A J M, Fekkes P, van der Wolk J P. The Sec system. Curr Opin Microbiol. 1998;1:216–222. doi: 10.1016/s1369-5274(98)80014-3. [DOI] [PubMed] [Google Scholar]
- 10.Duong F, Wickner W. The SecDFyajC domain of preprotein translocase controls preprotein movement by regulating SecA membrane cycling. EMBO J. 1997;16:4871–4879. doi: 10.1093/emboj/16.16.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 12.Economou A, Pogliano J A, Beckwith J, Oliver D B, Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell. 1995;83:1171–1181. doi: 10.1016/0092-8674(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 13.Hartl F-U, Lecker S, Schiebel E, Hendrick J P, Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y J, Rajapandi T, Oliver D. SecA protein is exposed to the periplasmic surface of the E. coli inner membrane in its active state. Cell. 1994;78:845–853. doi: 10.1016/s0092-8674(94)90602-5. [DOI] [PubMed] [Google Scholar]
- 15.Klionsky D J, Brusilow W S A, Simoni R D. In vivo evidence for the role of the ɛ subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol. 1984;160:1055–1060. doi: 10.1128/jb.160.3.1055-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto G, Mori H, Ito K. Roles of SecG in ATP- and SecA-dependent protein translocation. Proc Natl Acad Sci USA. 1998;95:13567–13572. doi: 10.1073/pnas.95.23.13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto G, Yoshihisa T, Ito K. SecY and SecA interact to allow SecA insertion and protein translocation across the Escherichia coli plasma membrane. EMBO J. 1997;16:6384–6393. doi: 10.1093/emboj/16.21.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo E, Mori H, Shimoike T, Ito K. Syd, a SecY-interacting protein, excludes SecA from the SecYE complex with altered SecY24 subunit. J Biol Chem. 1998;273:18835–18840. doi: 10.1074/jbc.273.30.18835. [DOI] [PubMed] [Google Scholar]
- 19.Matsuyama S-I, Fujita Y, Mizushima S. SecD is involved in the release of translocated secretory proteins from the cytoplasmic membrane of Escherichia coli. EMBO J. 1993;12:265–270. doi: 10.1002/j.1460-2075.1993.tb05652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 21.Nishiyama K, Fukuda A, Morita K, Tokuda H. Membrane deinsertion of SecA underlying proton motive force-dependent stimulation of protein translocation. EMBO J. 1999;18:1049–1058. doi: 10.1093/emboj/18.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishiyama K-I, Suzuki T, Tokuda H. Inversion of the membrane topology of SecG coupled with SecA-dependent protein translocation. Cell. 1996;85:71–81. doi: 10.1016/s0092-8674(00)81083-1. [DOI] [PubMed] [Google Scholar]
- 23.Nouwen N, de Kruijff B, Tommassen J. prlA suppressors in Escherichia coli relieve the proton electrochemical gradient dependency of translocation of wild-type precursors. Proc Natl Acad Sci USA. 1996;93:5953–5957. doi: 10.1073/pnas.93.12.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pogliano J A, Beckwith J. SecD and SecF facilitate protein export in Escherichia coli. EMBO J. 1994;13:554–561. doi: 10.1002/j.1460-2075.1994.tb06293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramamurthy V, Oliver D. Topology of the integral membrane form of Escherichia coli SecA protein reveals multiple periplasmically exposed regions and modulation by ATP binding. J Biol Chem. 1997;272:23239–23246. doi: 10.1074/jbc.272.37.23239. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Mori H, Yoshida M, Tagaya M, Mizushima S. In vitro analysis of the stop-transfer process during translocation across the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1997;272:20082–20087. doi: 10.1074/jbc.272.32.20082. [DOI] [PubMed] [Google Scholar]
- 27.Schiebel E, Driessen A J M, Hartl F-U, Wickner W. ΔμH+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991;64:927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- 28.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 29.Snyders S, Ramamurthy V, Oliver D. Identification of a region of interaction between Escherichia coli SecA and SecY proteins. J Biol Chem. 1997;272:11302–11306. doi: 10.1074/jbc.272.17.11302. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Nishiyama K-I, Tokuda H. Coupled structure change of SecA and SecG revealed by the synthetic lethality of the secAcsR11 and ΔsecG::kan double mutant. Mol Microbiol. 1998;29:331–342. doi: 10.1046/j.1365-2958.1998.00937.x. [DOI] [PubMed] [Google Scholar]
- 31.Taura T, Yoshihisa T, Ito K. Protein translocation functions of Escherichia coli SecY: in vitro characterization of cold-sensitive secY mutants. Biochimie. 1997;79:512–517. doi: 10.1016/s0300-9084(97)82744-7. [DOI] [PubMed] [Google Scholar]
- 32.Taura T, Akiyama Y, Ito K. Genetic analysis of SecY: additional export-defective mutations and factors affecting their phenotypes. Mol Gen Genet. 1994;243:261–269. doi: 10.1007/BF00301061. [DOI] [PubMed] [Google Scholar]
- 33.van der Wolk J P, de Wit J G, Driessen A J M. The catalytic cycle of the Escherichia coli SecA ATPase comprises two distinct preprotein translocation. EMBO J. 1997;17:3631–3639. doi: 10.1093/emboj/16.24.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickner W, Leonard M R. Escherichia coli preprotein translocase. J Biol Chem. 1996;271:29514–29516. doi: 10.1074/jbc.271.47.29514. [DOI] [PubMed] [Google Scholar]
- 35.Yoshihisa T, Ito K. Pro-OmpA derivatives with a his6 tag in their N-terminal “translocation initiation domains” are arrested by Ni2+ at an early post-targeting stage of translocation. J Biol Chem. 1996;271:9429–9436. doi: 10.1074/jbc.271.16.9429. [DOI] [PubMed] [Google Scholar]