Abstract
Adenosarcoma can mimic high-grade endometrial stromal sarcoma with ZC3H7B-BCOR fusion that may show entrapped glands and often exhibits diffuse BCOR expression. We encountered diffuse BCOR expression in rare adenosarcomas and sought to define its frequency among a larger cohort of these tumors. BCOR immunohistochemistry was performed on archival formalin-fixed paraffin-embedded tumor tissue in 13 of 14 adenosarcomas with and without stromal overgrowth arising in the uterus or ovary. Staining intensity and percentage of positive tumor nuclei in the mesenchymal component were evaluated. Eleven cases with sufficient tumoral tissue were subjected to fluorescence in situ hybridization for the detection of BCOR, BCORL1, NUTM1, ZC3H7B, and JAZF1 rearrangement. Three cases were subjected to targeted RNA sequencing. BCOR was expressed in 9 of 13 (70%) tumors, including 6 with and 3 without stromal overgrowth. Moderate to strong staining in >70% of cells was seen throughout one low- and six high-grade tumors, five of which had stromal overgrowth. No staining was seen in three low- and 1 high-grade tumor with stromal overgrowth. One tumor demonstrating extensive sex cord-like differentiation and diffuse BCOR expression harbored JAZF1 and BCORL1 rearrangements. No BCOR or BCORL1 rearrangement was identified in the remaining tumors. BCOR expression is seen in most adenosarcomas with and without stromal overgrowth. BCORL1 rearrangement is seen in rare tumors with diffuse BCOR expression. Assessment of BCOR or BCORL1 rearrangement status is required in adenosarcomas demonstrating BCOR expression.
Keywords: BCOR, BCORL1, JAZF1, adenosarcoma, endometrial stromal sarcoma
INTRODUCTION
Mullerian adenosarcoma is a rare biphasic tumor that occurs in all age groups but most frequently affects postmenopausal women and arises most commonly in the uterine corpus followed by the uterine cervix, ovary, fallopian tube, or vagina (1). Most lesions are easily recognizable by their characteristic phyllodes-like architecture, periglandular stromal condensation and low-grade cytology (2). However, high-grade cytologic features, heterologous elements, and stromal overgrowth may occur and cause diagnostic confusion when architectural features characteristic of adenosarcoma are obscured (3). There is particularly substantial morphologic overlap between adenosarcoma with homologous stromal overgrowth and high-grade endometrial stromal sarcoma harboring ZC3H7B-BCOR gene fusion, especially when entrapped endometrioid glands may be found in the latter (4, 5). In practice, we have also anecdotally encountered variable BCOR expression, a diagnostic marker helpful in the evaluation of high-grade endometrial stromal sarcomas, among adenosarcomas (6).
While BCOR genetic abnormalities play important roles in the development of high-grade endometrial stromal sarcoma in the form of gene rearrangement or internal tandem duplication, they so far do not appear to contribute to the highly heterogeneous genetic landscape of adenosarcomas. MDM2/CDK4 gene amplifications are the most common genetic abnormality and have been detected in 16–28% of lesions (7–9). Additional alterations with variable detection rates across studies include TERT and MYBL1 amplifications; FGFR2, KMT2C, DICER1, ATRX, and TP53 mutations; and ESR1-NCOA2 and ESR1-NCOA3 fusions (7–9). Some of these genetic aberrations have also been found in other uterine tumor types, such as DICER1 mutations in cervical rhabdomyosarcoma (10, 11), and ESR1-NCOA2 and ESR1-NCOA3 fusions in uterine tumor resembling ovarian sex cord tumor (12–14).
To date, BCOR expression appears to be a robust diagnostic marker of high-grade endometrial stromal sarcomas harboring YWHAE rearrangement and BCOR genetic abnormalities (6). However, BCOR staining patterns have not been evaluated in adenosarcomas, which may be confused with high-grade endometrial stromal sarcoma in the setting of stromal overgrowth (4). In this study, we sought to define the frequency of BCOR expression among adenosarcomas with and without stromal overgrowth and correlate BCOR staining with BCOR and BCORL1 gene rearrangement status by fluorescence in situ hybridization (FISH).
MATERIALS AND METHODS
Case selection
All available hematoxylin-and-eosin stained slides and pathology reports of adenosarcomas diagnosed between 2013 and 2019 were collected from the pathology archives of Memorial Sloan Kettering Cancer Center, New York, NY, USA and University of Medicine, Pharmacy, Sciences and Technology of Targu Mures, Targu Mures, Romania using the search term “adenosarcoma.” The slides were reviewed by a gynecologic pathologist for confirmation of diagnosis based on the presence of the following features: (1) biphasic morphology with benign epithelial and malignant mesenchymal components, (2) phyllodes-like architecture, and (3) periglandular stromal condensation. Tumor size, FIGO stage, histologic grade, presence of heterologous elements, and presence of stromal overgrowth were recorded. Tumors were assigned low-grade if the mesenchymal component showed only mild to moderate cytologic atypia and low mitotic activity (index of <10 mitotic figures per 10 high power fields), resembling a low-grade endometrial stromal sarcoma or low-grade fibrosarcoma. Tumors were considered high-grade if the mesenchymal component exhibited severe cytologic atypia and brisk mitotic activity (index of ≥10 mitotic figures per 10 high power fields). Stromal overgrowth was recorded when it comprised at least 25% of the overall tumor area. Clinical features, including patient age, disease presentation, treatment, and outcome, were also extracted from available medical records.
Immunohistochemistry
Immunohistochemical staining for BCOR was performed on all tumors as previously described (6). Briefly, five μm whole tumoral sections mounted on charged glass slides were stained with a commercially available monoclonal antibody, clone C-10 (sc-514576; Santa Cruz, Dallas, TX, USA) at 1:150 dilutions (1.7 μg/ml) generated against the N-terminus (1–300 amino acid) of BCOR. Staining was performed on the Leica Bond-3 autostainer (Leica, Buffalo Grove, IL). Immunohistochemical stains were evaluated by two pathologists (SC, VM). Intensity of staining (negative, weak, moderate, strong) and estimated percentage of positive tumor nuclei in the mesenchymal component of all tumors tested were recorded.
FISH
Eleven tumors with sufficient material were subjected to break-apart FISH analysis for BCOR and BCORL1 gene rearrangement (15). For tumors with confirmed BCOR or BCORL1 rearrangement, break-apart FISH for possible fusion partners, NUTM1, ZC3H7B, and JAZF1 gene rearrangements was also undertaken. FISH was performed on formalin-fixed paraffin-embedded whole tumoral sections mounted on charged glass slides using custom bacterial artificial chromosomes (BAC) probes flanking BCOR (RP11–21D3, RP11–1105N2, RP11–37K20, RP11–973F20) BCORL1 (RP11– 671B10, RP11–246J10, RP11–460L15, RP11–383B16), NUTM1 (RP11–1141P10, RP11–477L8, RP11–1084A12), ZC3H7B (RP11–1078O11, RP11–110H11), and JAZF1 genes, obtained from BAC/PAC sources of Children’s Hospital of Oakland Research Institute (CHORI, http://bacpac.chori.org ) (Oakland, CA, USA). Briefly, slides were pre-treated, de-paraffinized and hybridized with denatured BAC probes. Slides underwent post-hybridization incubation, washing, and counterstaining with DAPI. Two hundred tumor nuclei were examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems). Gene rearrangement was confirmed when at least 20% of tumor nuclei showed a break-apart signal.
Targeted RNA sequencing
Three tumors were subjected to the Archer FusionPlex Custom Solid Panel (ArcherDC Inc., Boulder, CO), a next-generation targeted RNA sequencing assay utilizing the Anchored Multiplex PCR technology that detects gene fusions and oncogenic isoforms in selected protein-coding exons of 62 genes (16). Tumor RNA was extracted from five μm, formalin-fixed, paraffin-embedded tumoral sections followed by cDNA synthesis and library preparation. Final targeted amplicons were sequenced on an Illumina MiSeq. Data were analyzed using the Archer Software (version 4.0.10; ArcherDX Inc.).
RESULTS
Clinicopathologic features
Fourteen adenosarcomas, including twelve uterine and two ovarian, with available tumoral material were identified (Table 1). Median patient age was 64 (range, 23 to 77) years. Patients with uterine tumors presented with vaginal bleeding or a uterine mass, while patients with ovarian primaries presented with a pelvic mass. All were treated surgically with three and one patient receiving chemotherapy and chemoradiation, respectively. Nine and three patients presented with FIGO stage IA and IB uterine disease. Five patients recurred at 6 to 21 months, and all were alive with disease at last follow up. Six patients had no evidence of disease at 7 to 72 months after initial diagnosis. Patients with ovarian primaries presented with FIGO stage IC and IIB disease, the latter who recurred two months after initial presentation.
Table 1.
Clinical features
| Case | Age, y | Signs and symptoms | Site | FIGO stage | Treatment | Recurrence, mo | Last follow-up, mo |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 35 | Vaginal bleeding | Uterus | IA | Surgery, chemotherapy | 6 | Alive with disease, 24 |
| 2 | 77 | Vaginal bleeding | Uterus | IA | Surgery, chemoradiation | 11 | Alive with disease, 38 |
| 3 | 32 | Vaginal bleeding | Uterus | IA | Surgery | No evidence of disease, 30 | |
| 4 | 56 | Vaginal bleeding | Uterus | IA | Surgery, chemotherapy | 11 | Alive with disease, 18 |
| 5 | 57 | Vaginal bleeding | Uterus | IA | Surgery | No evidence of disease, 26 | |
| 6 | 77 | Vaginal bleeding | Uterus | IA | Surgery | 20 | Alive with disease, 24 |
| 7 | 56 | Vaginal bleeding | Uterus | IB | Surgery | 21 | Alive with disease, 21 |
| 8 | 70 | Pelvic mass | Ovary | IIB | Surgery | 2 | Alive with disease, 2 |
| 9 | 23 | Vaginal bleeding | Uterus | IB | Surgery, chemotherapy | No evidence of disease, 19 | |
| 10 | 69 | Vaginal bleeding | Uterus | IA | Surgery | No evidence of disease, 7 | |
| 11 | 70 | Vaginal bleeding | Uterus | IB | Surgery | No evidence of disease, 72 | |
| 12 | 69 | Pelvic mass | Ovary | IC | Surgery | Lost to follow up | |
| 13 | 75 | Uterine mass | Uterus | IA | Surgery | 6 | Alive with disease, 12 |
| 14 | 58 | Uterine mass | Uterus | IA | Surgery | No evidence of disease, 35 | |
Median tumor size was 6.3 (range, 1.7 to 13.0) cm (Table 2). Five tumors were low-grade, and nine were high-grade (Figure 1A–C). Stromal overgrowth was seen in eight high-grade tumors and not in any of the low-grade tumors. Variant features, defined as sex cord-like differentiation and heterologous elements, were present in eight tumors. Sex cord-like differentiation was seen in four tumors, two of which were high-grade (Figure 2). Heterologous rhabdomyosarcomatous elements were seen in three high-grade tumors with stromal overgrowth, including one also demonstrating rare teratoid elements. Ossification and lipomatous differentiation were seen in two low-grade tumors.
Table 2.
Pathologic features
| Case | Size, cm | Grade | Stromal Overgrowth | Variant features | BCOR expression | Fusion status |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 1.7 | Low | No | Strong, >95% | Negative | |
| 2 | 3.2 | Low | No | Lipomatous and sex cord differentiation | Negative | Negative |
| 3 | 5.7 | Low | No | Negative | Negative | |
| 4 | 5.6 | High | Yes | Negative | Negative | |
| 5 | 3.1 | High | Yes | Moderate, 70% | Negative | |
| 6 | 13.0 | High | Yes | Weak, 20% | Negative | |
| 7 | 7.8 | Low | No | Ossification | Weak, 20% | Negative |
| 8 | 10.6 | High | Yes | Rhabdomyosarcoma | Strong, >95% | Negative |
| 9 | 6.8 | High | Yes | Rhabdomyosarcoma, teratoid elements | Moderate, >95% | Negative |
| 10 | 7.3 | High | No | Sex cord differentiation | Strong, >95% | JAZF1-BCORL1 |
| 11 | 3.5 | Low | No | Sex cord differentiation | Negative | Negative |
| 12 | 14.9 | High | Yes | Sex cord differentiation | Not performed | Negative |
| 13 | 13.0 | High | Yes | Rhabdomyosarcoma | Strong, >95% | Negative |
| 14 | 3.8 | High | Yes | Strong, >95% | Negative | |
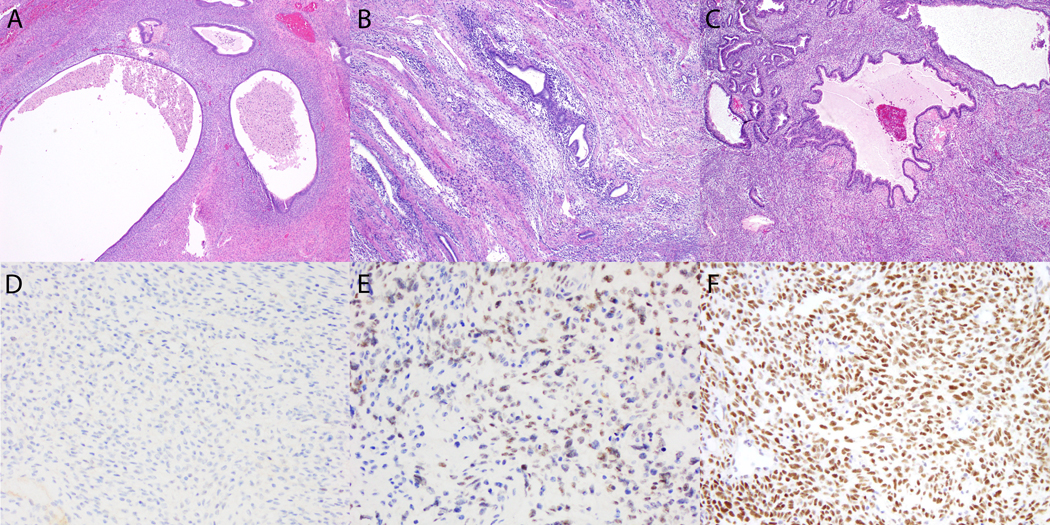
Figure 1.
BCOR expression in adenosarcoma. (A-C) Periglandular stromal condensation is seen in (A) low-grade and (B, C) high-grade tumors, one which exhibited stromal overgrowth (not pictured). (D) BCOR expression is absent in the low-grade tumor. (E, F) Strong BCOR expression is seen in the mesenchymal component of both high-grade tumors.
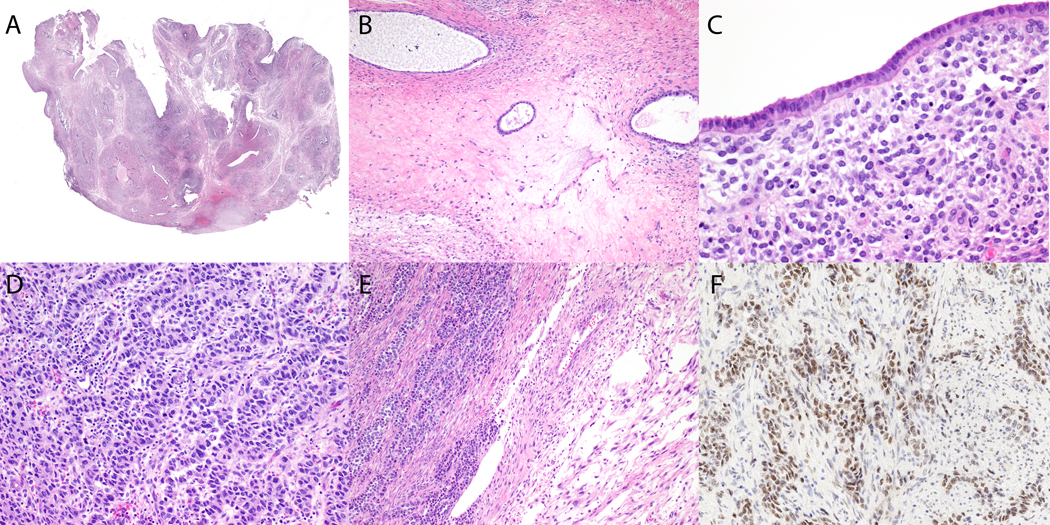
Figure 2.
High-grade adenosarcoma with JAZF1-BCORL1 fusion. (A) Phyllodes growth pattern and periglandular stromal condensation is visible on low magnification. (B) Periglandular stromal condensation surrounds cystically dilated glands and is associated with fibrotic stroma. (C) Stromal cells adjacent to the cytologically bland epithelium have mild to moderate nuclear atypia. (D, E) Cells with marked cytologic atypia form cords and trabeculae adjacent to (E) a fibromyxoid spindle cell component. (F) Strong BCOR expression is seen in the sex cord-like foci.
Immunohistochemical findings
Nuclear BCOR staining was present in the mesenchymal component of 9 of 13 (70%) tumors, including 6 high-grade tumors with stromal overgrowth as well as 1 high- and 2 low-grade tumors without stromal overgrowth (Table 2). Moderate to strong BCOR expression in at least 70% of cells was seen in the mesenchymal component of one low- and six high-grade tumors; three in the latter group demonstrated stromal overgrowth (Figure 1D–F). Weak staining in 20% of cells was seen in the mesenchymal component of one low- and one high-grade tumor, the latter exhibiting stromal overgrowth. No staining was seen in three low- and one high-grade tumor with stromal overgrowth.
Among seven patients with high-grade uterine adenosarcomas with BCOR expression, two with FIGO stage IA disease recurred at 6 and 20 months after initial presentation, while the remaining five were alive with no evidence of disease at 7 to 35 months follow-up. The patient with a FIGO stage IIB ovarian high-grade adenosarcoma demonstrating BCOR immunoreactivity recurred two months after initial presentation. Of the two patients with BCOR-positive uterine low-grade adenosarcoma, both recurred 6 and 21 months after presentation.
FISH findings
Among 11 tumors subjected to BCOR and BCORL1 FISH, only one tumor (case 10) with strong BCOR expression in ≥95% of tumor cells harbored a BCORL1 gene rearrangement in the mesenchymal component only. Additional FISH analysis identified JAZF1 as the fusion partner in the mesenchymal component only, and no rearrangements of BCOR, NUTM1, or ZC3H7B. No BCOR or BCORL1 rearrangement was detected in the remaining 10 tumors subjected to FISH (Table 2).
Next generation sequencing findings
No gene fusions were identified in the three tumors that were subjected to targeted RNA sequencing (Table 2).
Genotype-phenotype correlation
The gross and histologic features of case 10 were re-reviewed after FISH confirmation of a JAZF1-BCORL1 fusion. A 7.3 cm tan polypoid mass involved the endometrium with no gross involvement of the underlying myometrium. The tumor was biphasic with an atypical spindle cell proliferation associated with bland often cystic endometrioid glands, some of which imparting a phyllodes-like appearance (Figure 2A). Periglandular stromal condensation was readily seen on low magnification, separated by scattered foci of edematous and fibrotic stroma (Figure 2B). Most of the periglandular spindle cells had intermediate-size round to oval nuclei with clumped chromatin, small nucleoli, and scant eosinophilic cytoplasm (Figure 2C). Mitotic index was 12 mitotic figures per 10 high power fields in this region. In rare scattered foci, the periglandular spindle cells showed enlarged pleomorphic nuclei with clumped chromatin, prominent nucleoli, occasional intranuclear inclusions, and a moderate amount of eosinophilic to foamy cytoplasm (Figure 2D). These cells also formed sheets, nests, cords, trabeculae, and rare tubules devoid of an epithelial component (Figure 2E). Mitotic index in these areas was 30 mitotic figures per 10 high power fields. One area also showed fascicular growth of a fibromyxoid spindle cell component (Figure 2E). BCOR expression was strong and diffuse throughout the tumor, including sex cord-like foci (Figure 2F) with confirmation of BCORL1 rearrangement by FISH (Figure 3).
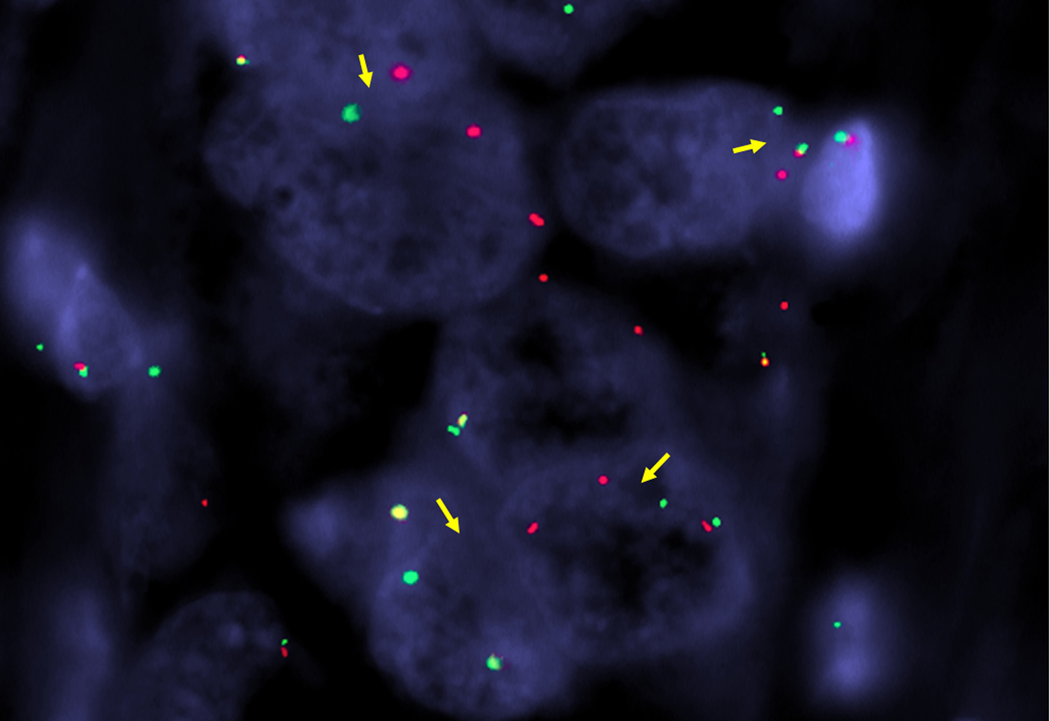
Figure 3.
BCORL1 rearrangement in adenosarcoma. Separation of green telomeric and red centromeric signals (arrows) flanking the gene confirm BCORL1 rearrangement by fluorescence in situ hybridization.
DISCUSSION
Nuclear BCOR expression is frequently observed in the mesenchymal component of Mullerian adenosarcomas, including both low- and high-grade lesions with and without stromal overgrowth. Moderate to strong staining in at least 70% of tumor cells in the sarcomatous component is present in 45% of tumors and does not appear associated with grade, stromal overgrowth, or clinical outcomes. This extent of BCOR expression does not correspond with the presence of BCOR rearrangement in adenosarcomas, suggesting that using BCOR expression alone as a surrogate marker of BCOR fusion-positive high-grade endometrial stromal sarcoma may represent a potential diagnostic pitfall. Rare adenosarcomas with BCOR overexpression may, however, harbor BCORL1 gene fusion. These findings suggest that molecular assessment of BCOR and BCORL1 rearrangement status may be helpful in the diagnostic evaluation of any uterine sarcoma demonstrating BCOR overexpression.
We recently reported the clinical utility of BCOR expression in the diagnostic evaluation of high-grade endometrial stromal sarcomas and undifferentiated uterine sarcomas (6, 15). BCOR expression has a sensitivity of 86% (95% CI, 70–95%) and specificity of 100% (95% CI, 97–100%) in the detection of high-grade endometrial stromal sarcomas with YWHAE-NUTM2 fusion, ZC3H7B-BCOR fusion, and BCOR internal tandem duplication, with positive and negative predictive values of 100% and 97%, respectively (6, 15). Diffuse moderate to strong BCOR expression has not been seen in endometrial stromal nodules, low-grade endometrial stromal sarcomas, leiomyomas, and most leiomyosarcomas (6). Our new findings, however, demonstrate that BCOR overexpression may also be seen in a large subset of Mullerian adenosarcomas lacking BCOR rearrangements. This is particularly problematic in the diagnostic evaluation of any uterine spindle cell sarcoma with fibromyxoid features in which adenosarcoma with stromal overgrowth and ZC3H7B-BCOR fusion-positive high-grade endometrial stromal sarcoma are the main differential diagnoses. In this setting, BCOR overexpression may not be used to support a provisional diagnosis of high-grade endometrial stromal sarcoma with probable ZC3H7B-BCOR fusion. The mechanism of BCOR expression in adenosarcoma is unclear and requires further investigation.
One of our cases demonstrating BCOR overexpression, however, harbored a JAZF1-BCORL1 fusion which has not been previously described in adenosarcomas. This tumor exhibited features diagnostic of adenosarcoma and was biphasic with both periglandular stromal condensation and phyllodes-like architecture. While sex cord-like differentiation is common among adenosarcomas (17), this tumor was unusual by the degree of cytologic atypia and mitotic activity observed in the sex cord-like foci. JAZF1-BCORL1 fusion with rearrangement between JAZF1 exons 1–3 and exons 5–12 of BCORL1 has been recently reported in a tumor classified as recurrent low-grade endometrial stromal sarcoma (18). However, published histologic images demonstrate sheets of spindle cells with nuclear enlargement, prominent nucleoli, and ample eosinophilic cytoplasm without perivascular whorling and associated with myxoid matrix. These findings are somewhat unusual for low-grade endometrial stromal sarcoma, and histologic review of the primary uterine tumor to assess features of adenosarcoma or high-grade endometrial stromal sarcoma may be of interest. It is feasible that our case represents a high-grade endometrial stromal sarcoma recapitulating adenosarcoma-like architecture. It is also possible that identical gene fusions are observed across various uterine mesenchymal tumor types, i.e. ESR1 rearrangement with NCOA2 or NCOA3 fusion partners in uterine tumors resembling ovarian sex cord tumor (12–14) and adenosarcomas (7, 9).
BCORL1 (BCL6 corepressor-like 1) encodes a transcriptional corepressor homolog to BCOR and results in transcriptional repression by interacting with class II histone deacetylase and C-terminal-binding protein (19). Amino acid sequence homology between BCORL1 and BCOR likely accounts the observed BCOR overexpression. BCORL1 rearrangements have been previously described as rare events in ossifying fibromyxoid tumors (CREBBP-BCORL1 fusion) (20) and hepatocellular carcinoma (BCORL1-ELF4 fusion) (21). Inactivating mutations of BCORL1 have also been reported in subsets of acute myeloid leukemia (22, 23), myelodysplastic syndrome (24), chronic myelomonocytic leukemia (25), aplastic anemia (26), and intracranial germ cell tumors (27).
JAZF1 (JAZF zinc finger 1) encodes a nuclear factor that represses transcription by interacting with nuclear orphan receptor TR4 (28). Mutations have only been reported in endometrial stromal tumors in which JAZF1 rearrangement is found most frequently with SUZ12 followed by PHF1 in endometrial stromal nodules and low-grade endometrial stromal sarcomas (29). The JAZF1-SUZ12 fusion product appears to disrupt the polycomb repressive complex 2 and decreases H3K27me3, thereby resulting in an oncogenic-like protein involved in endometrial stromal sarcoma tumorigenesis (30–32).
In summary, we demonstrate BCOR expression in a large proportion of Mullerian adenosarcomas of any grade and with or without stromal overgrowth. Lesions with BCOR overexpression lack BCOR gene rearrangement, suggesting that the use of BCOR overexpression in isolation may be misleading. Rare lesions with BCOR overexpression may harbor JAZF1-BCORL1 fusion, expanding the genetic spectrum of adenosarcomas. Molecular assessment of BCOR and BCORL1 rearrangement status is recommended in the diagnostic evaluation of any uterine sarcoma demonstrating BCOR overexpression to confirm BCOR fusion-positive high-grade endometrial stromal sarcoma and the rare adenosarcoma harboring BCORL1 fusion.
References
- 1.Huang PS, Chang WC, Huang SC. Mullerian adenosarcoma: a review of cases and literature. Eur J Gynaecol Oncol. 2014;35:617–620. [PubMed] [Google Scholar]
- 2.McCluggage WG. Mullerian adenosarcoma of the female genital tract. Adv Anat Pathol. 2010;17:122–129. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson A, Amemiya Y, Seth A, et al. High-grade mullerian adenosarcoma: genomic and clinicopathologic characterization of a distinct neoplasm with prevalent TP53 pathway alterations and aggressive behavior. Am J Surg Pathol. 2017;41:1513–1522. [DOI] [PubMed] [Google Scholar]
- 4.Lewis N, Soslow RA, Delair DF, et al. ZC3H7B-BCOR high-grade endometrial stromal sarcomas: a report of 17 cases of a newly defined entity. Mod Pathol. 2018;31:674–684. [DOI] [PubMed] [Google Scholar]
- 5.Hoang LN, Aneja A, Conlon N, et al. Novel high-grade endometrial stromal sarcoma: a morphologic mimicker of myxoid leiomyosarcoma. Am J Surg Pathol. 2017;41:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang S, Lee CH, Stewart CJR, et al. BCOR is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod Pathol. 2017;30:1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piscuoglio S, Burke KA, Ng CK, et al. Uterine adenosarcomas are mesenchymal neoplasms. J Pathol. 2016;238:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howitt BE, Sholl LM, Dal Cin P, et al. Targeted genomic analysis of Mullerian adenosarcoma. J Pathol. 2015;235:37–49. [DOI] [PubMed] [Google Scholar]
- 9.Bean GR, Anderson J, Sangoi AR, et al. DICER1 mutations are frequent in mullerian adenosarcomas and are independent of rhabdomyosarcomatous differentiation. Mod Pathol. 2019;32:280–289. [DOI] [PubMed] [Google Scholar]
- 10.Foulkes WD, Bahubeshi A, Hamel N, et al. Extending the phenotypes associated with DICER1 mutations. Hum Mutat. 2011;32:1381–1384. [DOI] [PubMed] [Google Scholar]
- 11.de Kock L, Rivera B, Revil T, et al. Sequencing of DICER1 in sarcomas identifies biallelic somatic DICER1 mutations in an adult-onset embryonal rhabdomyosarcoma. Br J Cancer. 2017;116:1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goebel EA, Hernandez Bonilla S, Dong F, et al. Uterine tumor resembling ovarian sex cord tumor (UTROSCT): a morphologic and molecular study of 26 cases confirms recurrent NCOA1–3 rearrangement. Am J Surg Pathol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CH, Kao YC, Lee WR, et al. Clinicopathologic characterization of GREB1-rearranged uterine sarcomas with variable sex-cord differentiation. Am J Surg Pathol. 2019;43:928–942. [DOI] [PubMed] [Google Scholar]
- 14.Dickson BC, Childs TJ, Colgan TJ, et al. Uterine tumor resembling ovarian sex cord tumor: a distinct entity characterized by recurrent NCOA2/3 gene fusions. Am J Surg Pathol. 2019;43:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotzia P, Benayed R, Mullaney K, et al. Undifferentiated uterine sarcomas represent under-recognized high-grade endometrial stromal sarcomas. Am J Surg Pathol. 2019;43:662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–1484. [DOI] [PubMed] [Google Scholar]
- 17.Stolnicu S, Molnar C, Barsan I, et al. The Impact on survival of an extensive sex cord-like component in mullerian adenosarcomas: a study comprising 6 cases. Int J Gynecol Pathol. 2016;35:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen AJ, Ali SM, Gowen K, et al. A recurrent endometrial stromal sarcoma harbors the novel fusion JAZF1-BCORL1. Gynecol Oncol Rep. 2017;20:51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagan JK, Arnold J, Hanchard KJ, et al. A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J Biol Chem. 2007;282:15248–15257. [DOI] [PubMed] [Google Scholar]
- 20.Kao YC, Sung YS, Zhang L, et al. Expanding the molecular signature of ossifying fibromyxoid tumors with two novel gene fusions: CREBBP-BCORL1 and KDM2A-WWTR1. Genes Chromosomes Cancer. 2017;56:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Totoki Y, Tatsuno K, Yamamoto S, et al. High-resolution characterization of a hepatocellular carcinoma genome. Nat Genet. 2011;43:464–469. [DOI] [PubMed] [Google Scholar]
- 22.de Rooij JD, van den Heuvel-Eibrink MM, Hermkens MC, et al. BCOR and BCORL1 mutations in pediatric acute myeloid leukemia. Haematologica. 2015;100:e194–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiacci E, Grossmann V, Martelli MP, et al. The corepressors BCOR and BCORL1: two novel players in acute myeloid leukemia. Haematologica. 2012;97:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damm F, Chesnais V, Nagata Y, et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood. 2013;122:3169–3177. [DOI] [PubMed] [Google Scholar]
- 25.Rotunno G, Guglielmelli P, Biamonte F, et al. Mutational analysis of BCORL1 in the leukemic transformation of chronic myeloproliferative neoplasms. Ann Hematol. 2014;93:523–524. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Yamaguchi S, Burstein MD, et al. Novel somatic and germline mutations in intracranial germ cell tumours. Nature. 2014;511:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima T, Fujino S, Nakanishi G, et al. TIP27: a novel repressor of the nuclear orphan receptor TAK1/TR4. Nucleic Acids Res. 2004;32:4194–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoang L, Chiang S, Lee CH. Endometrial stromal sarcomas and related neoplasms: new developments and diagnostic considerations. Pathology. 2018;50:162–177. [DOI] [PubMed] [Google Scholar]
- 30.Ma X, Wang J, Wang J, et al. The JAZF1-SUZ12 fusion protein disrupts PRC2 complexes and impairs chromatin repression during human endometrial stromal tumorogenesis. Oncotarget. 2017;8:4062–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Ma X, Wang J, et al. Effects of rearrangement and allelic exclusion of JJAZ1/SUZ12 on cell proliferation and survival. Proc Natl Acad Sci U S A. 2007;104:20001–20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koontz JI, Soreng AL, Nucci M, et al. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci U S A. 2001;98:6348–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]