Abstract
Epidermal growth factor (EGF) can be efficiently used in wound healing process; but the main obstacle of its clinical use is its susceptibility to proteolysis and maintaining its effective concentration in the site of action. In this study, chitosan nanoparticles containing EGF is formulated using a simple method to increase its stability in physiological pH as well as protect its biological activity and effectiveness in wound healing process. Nanoparticles with different ratios of chitosan/EGF were prepared and evaluated in vitro and in vivo. Obtained results showed nanoparticles with 2:1 ratio of chitosan/EGF were able to release 80% of encapsulated protein after 12 h. Cell proliferation study demonstrated that prepared nanoparticles could protect EGF functionality in physiological pH. In vivo results showed that nanoparticles with 2:1 ratio of chitosan/EGF could significantly accelerate the wound closure‐rate, re‐epithelialisation and collagen deposition. In conclusion, the designed nanoparticles in optimal ratio can be considered as a potential vehicle for EGF delivery to wounds with the aim of improving healing process.
Keywords: nanoparticles, polymers, proteins, wounds
Formulated chitosan based nanoparticles containing EGF could efficiently protect biological activity of EGF and accelerated wound healing process by topical application.
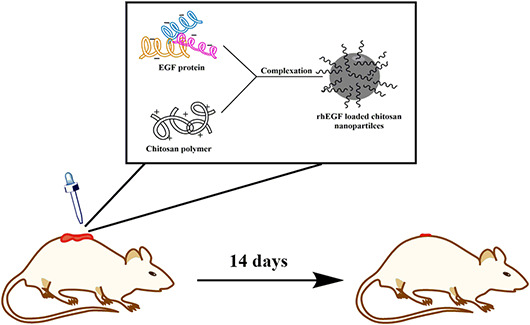
1. INTRODUCTION
Epidermal growth factor (EGF) is a single‐chain protein containing 53 amino acid residues (Molecular weight = 6045 Da) and three disulphide bridges, which show a potent regeneration activity [1]. This protein can accelerate wound healing process by stimulating the proliferation of epidermal cells as well as other cell types such as fibroblasts and endothelial cells [1, 2]. Epidermal growth factor protein acts by binding to the EGF receptor–tyrosine kinase, thereby initiating a series of events which regulate cell proliferation process [3]. Topical administration of EGF can accelerate dermal regeneration of partial thickness burns or split‐thickness incisions in vivo [4, 5]. According to these properties, EGF can be used as an attractive candidate for dermal regeneration in wound healing process. However, studies have shown that EGF is susceptible to proteolysis and has low stability in physiological condition. Furthermore, the main obstacle of its clinical usage is its short half‐life and difficulty to maintaining its effective concentration at the wound site [6, 7, 8].
Preparation of a robust EGF delivery system to improve its stability and bioavailability has been recently investigated in some studies [9]. In recent years, polymeric nanoparticles have been considered as a potential protein‐delivery systems [10]. Chitosan as a natural linear cationic polysaccharide could be a good candidate for bimolecular delivery [11, 12]. Studies have shown chitosan can regulate the inflammatory cells functions which leads to positive effect on wound healing process [13, 14]. Moreover, chitosan nanoparticles can enhance biomolecules stability and protect them from enzymatic degradations [15, 16]. For protein and peptide delivery, the use of biocompatible polymer along with anti‐inflammatory characteristics is advantageous [17, 18, 19, 20].
In the current study, we used simple complexation method for preparing EGF loaded nanoparticles. In this method, crosslinker agents was not be used in order to facilitate the scale up process of EGF loaded nanoparticles production. Furthermore, we stabilised EGF by encapsulation in chitosan nanoparticles and evaluated in vivo functionality of prepared nanoparticles in wound healing process.
2. MATERIALS AND METHODS
2.1. Materials
Chitosan (MW: 127kD, deacetylation degree: 97%) was purchased from Primex Co. (Island). Epidermal growth factor were purchased from Sigma Aldrich Co. (Germany). Human melanoma skin cancer (A375) cells were obtained from the cell bank of the Avicenna Research Institute (Iran). Dulbecco's Modified Eagles' Medium (DMEM) was purchased from Gibco Co.(USA). The rest of the materials used in the cell‐culture study, heat‐inactivated foetal bovine serum, trypsin, sodium2,3,‐bis(2‐methoxy‐4‐nitro‐5‐sulfophenyl)‐5‐[(phenylamino)‐carbonyl]‐2H‐tetrazolium inner salt (XTT) and powder PMS are from Sigma‐Aldrich Chemical Co. (Germany). All chemicals were of analytical grade.
2.2. Preparation of protein‐loaded chitosan nanoparticles
Chitosan nanoparticles were prepared using simple complexation technique. Briefly, chitosan solution was prepared at a concentration of 1 mg/ml in 0.25% acetic acid and placed on a stirrer at 25°C for 48 h. The pH of the chitosan solution was adjusted to 4.5 by NaOH 1 N. Nanoparticles with various ratios of chitosan/EGF (1:2, 1:1,2:1, 4:1, 6:1, and 8:1) were prepared using chitosan solution (1 mg/ml) and EGF solution (50 μg/ml in acetic acid 10 mM pH = 8) in different volumes. In all experiments, chitosan solution was stirred (500 rpm) at room temperature, and then EGF was added to it dropwise. Finally, prepared nanoparticles incubated 20 min at room temperature.
2.3. Size distribution and zeta potential
The mean particle size, polydispersity index, and zeta potential of different nanoparticles were determined by Nanozatasizer Malvern ZSP (England) at a wave length of 633 nm. The laser beam angle was 173 for measuring particle size at 25°C. Each sample was measured three times.
2.4. Morphological examination of nanoparticles
The morphology of protein‐loaded chitosan nanoparticles was evaluated using a Zeiss Transmission Electron Microscope (TEM) EM10C (at 80 kV). The Sample was placed on a slide and allowed to air dried at room temperature, then observed by TEM.
2.5. Protein loading efficiency
To evaluate the protein loading efficiency, EGF loaded chitosan nanoparticles with different ratios of chitosan/EGF (1:2, 1:1,2:1, 4:1, and 6:1) were centrifuged for 1 h at 5300 g and 4°C to precipitate nanoparticles. Unbound protein was collected from the supernatant. The concentration of free EGF was determined using an HPLC instrument (Knauer,Germany). The samples were analysed using a reversed‐phase C18 column (particle size 5 µm, 4.6 × 250 mm). The mobile phase was 100% water (A) and 100% acetonitrile (B). The gradient elution was as follows: 100% A to 100% B for 15 min at flow rate of 0.5 ml/min and 280 nm in the UV detector. A calibration curve of free EGF was used to calculate the percentage of loaded polypeptide according to the Equation (1):
| (1) |
All measurements were performed in triplicate.
2.6. In vitro protein release
In vitro release study of EGF loaded chitosan nanoparticles with different ratios of chitosan/EGF (2:1, 4:1 and 6:1) was carried out by dialysis sac. 1 ml of solution containing chitosan/EGF nanoparticles with a concentration of 50 µg/ml was placed in a dialysis sac (pore size of 12,000 Da) and the sac was immersed in a constantly stirred receiver vessel containing phosphate buffer (11 ml, pH = 7.4) at 32 ± 0.5°C. The release study was carried out for 24 h, and at pre‐decided time intervals 2 ml sample was withdrawn from the receiver vessel and replaced with fresh buffer. The samples were analysed using an HPLC according to the method described in session 2.6, and release profile was obtained. All measurements were performed in triplicate.
2.7. Cell proliferation assay
XTT method was used to evaluate the performance of EGF‐chitosan nanoparticles with different ratios of chitosan/EGF (2:1, 4:1 and 6:1) on cell proliferation using skin melanoma cell line (A375). For this purpose, 7 × 103 cells were seeded into 96‐well plates. After cells confluency about 60%, the samples or controls were added to the wells in triplicate. 50 μL of each sample (EGF loaded nanoparticles in different ratios, free EGF and free chitosan nanoparticles) was added to each well containing 100 μL DMEM (phenol red free) and incubated at 37°C with 5% CO2 for 24 h. Then 50 μL of XTT reagent (50 μg of XTT and 0.38 μg of PMS per well) was added to each well and incubated in 37°C, 5% CO2 for 4 h. After incubation, the absorbance of each well was measured at 450 nm with BioTekELISA reader. All measurements were performed in triplicate.
2.8. In vitro stability assay
The ability of chitosan nanoparticles to protect EGF functionality was investigated in vitro. Firstly, free EGF incubated at different pHs by using sodium citrate buffer (100 mM at pHs 3.5, 5.5), and tris buffer (50 mM at pHs 7.5, 9.5) at room temperature for 2 h. Cell proliferation assay was performed on incubated free EGF according to described XTT method. Due to instability of free EGF protein at pH 7.5, nanoparticles with different ratios of chitosan/EGF (2:1, 4:1 and 6:1) were incubated in pH 7.5 for 2 h then cell proliferation assay was performed based on described XTT method (the protein concentration was 50 μg/ml). Fresh and free EGF protein and unloaded chitosan nanoparticles were used as control and all measurements were performed in triplicate.
2.9. In vivo wound healing study
In the present study, 20 mature Sprague‐Dawley rats weighing 200–300 g were used. The rats were purchased from the Centre of Medicine at the Tehran University of Medical Science and treated according to the discipline of animal management and welfare of the University of Helsinki, Finland. Animals were placed in a standard cage with free access to tap water and a rodent's pellet chow diet. Then, the rats were divided into five experimental groups including control, free chitosan nanoparticles, free EGF, EGF loaded nanoparticles in chitosan/EGF ratio 2:1 and 6:1. (n = 5 in each group). The rats were anesthetised using a mixture of ketamine10% and sedaxyl 20 mg/ml in a ratio of 70 to 30. Excision wounds of approximately 1 cm2 were made via cutting out of a skin layer from the shaved area. Groups received different topical formulations on the wounds area (protein concentration was 50 μg/ml) in the first 3 days in a row and the rats receiving no treatment served as a control group. The percentage of wound healing was measured according to the following equation [21]:
2.10. Wound histological analysis
After 14 days, the animals were sacrificed by spinal cord injury under anaesthesia. Wound skin tissues (each in 3 × 2 cm) with full thickness were removed, and paraffin‐embedded sections were prepared. The sections (each with 2 mm thickness) were cut with a microtome, cutting perpendicular to the width of the skin surface. The sections were all stained with haematoxylin‐eosin are analysed by Masson's trichrome staining. The degrees of epithelialisation, fibrosis, inflammation, and granulation were assessed for all of the specimens. In order to calculate the wound healing process quantitatively, histopathological changes of skin tissue (the degrees of epithelialisation, fibrosis, inflammation, and granulation) were blindly scored by an expert clinical pathologist as mild (+1), moderate (+2) and severe (+3).
2.11. Statistical analysis
All data are reported as mean ± standard deviation. The results were analysed using t‐test and two ANOVA at the significance level of P < 0.05 by GraphPad Prism software (version 5.04 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com).
3. RESULTS AND DISCUSSION
3.1. Characterisation of nanoparticles
Size, zeta potential, and poly dispersity index of prepared nanoparticles were investigated. According to the obtained results (Supplementary material), by increasing the ratio of chitosan to EGF from 0.5 to 4 particle size of nanoparticles were decreased. However, at the ratios 6 and 8 the size of nanoparticles increases significantly. Furthermore, the nanoparticles with higher zeta potential were found at ratios of 6 and 8 which related to higher amount of chitosan in these ratios. In this study, using of organic solvents and high temperatures have been avoided. This approach is very important for safe preparation of protein loaded nanoparticles for clinical application. Furthermore, in current study we did not use crosslinker agents for preparation of nanoparticles which could facilitate the production of EGF loaded nanoparticles in scale up stage. Prepared nanoparticles with desire features revealed the size range within 63.5–127 nm which is in a range of optimum particle size for biological efficiency [22, 23]. The average zeta potential of chitosan nanoparticle containing EGF in this study was within +35 to +40 mV that provides good stability and reasonable dermal penetration. Zeta potential affects the efficiency of encapsulation, colloidal stability and the interaction of the particle with the cell. Nanoparticles with cationic charge in contrast to anionic and neutral charges could penetrate into the deeper layers of skin within 8 h [6]. Recently, Jayakumar et al. found that the penetration amounts of nanoparticles covered with cationic charges were 2–6 times higher than those with negative charges [6].
3.2. Morphological examination of chitosan/rhEGF nanoparticles
According to the image obtained by TEM (Supplementary material), the prepared nanoparticles were spherical, distinct and regular. It is noteworthy that the hydrodynamic diameter of the particles measured by light scattering was higher than the size estimated by microscopy. This can be explained by the dehydration of the CS nanoparticles during sample preparation for imaging. Furthermore, previous studies demonstrated that the shape and morphology of nanoparticles can influence on their ability to penetrate into the skin. Montiero et al. indicated spherical nanoparticles can penetrate deeply into the dermal layers, while egg shape nanoparticles accumulate only within the epidermis layer within 8 h [24, 25].
3.3. Protein loading efficiency
CS nanoparticles exhibited a high encapsulation efficiency of up to 90% in all ratios except at ratio of 1:2 (supplementary material). Ratios 4:1 and 6:1 revealed loading efficiency of 96.4% and 97.4% respectively. There was apparent correlation between the chitosan ratios and encapsulation efficiency of protein. The results in this study exhibited a relative high encapsulation efficiency more than 90% which was complied with other studies (31).
3.4. In vitro protein release
The results of in vitro release of EGF protein from chitosan nanoparticles at three different ratios of chitosan/EGF (2:1, 4:1 and 6:1) has been shown in Figure 1. In all formulations an initial burst release in 2 h has been observed which could be related to the EGF presents at the surface of nanoparticles [26]. After 12 h, the release rates reached a constant level. The rate of release in ratios 2:1 and 4:1 in the first 4 h was about 75%, and after 12 h reached to 81%. However, the release rate of 6:1 ratio was slower in compared with other ratios (59% and 65% of the protein was released after 4 and 12 h respectively).
FIGURE 1.
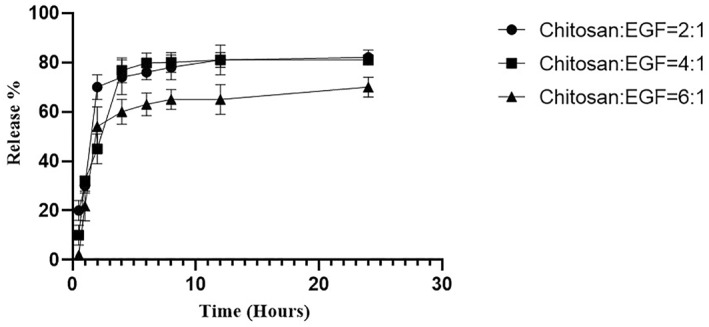
The release rate of epidermal growth factor (EGF) protein from chitosan nanoparticles in ratios 2: 1, 4: 1, and 6: 1. Data are represented as mean ± SD (n = 3).
3.5. Cell proliferation assay
In vitro proliferation of A375 cells induced by EGF loaded chitosan nanoparticles was evaluated to determine the biological activity of prepared nanoparticles with different ratios of chitosan/EGF (2:1, 4:1 and 6:1). According to Figure 2, cell proliferation induction by all three EGF loaded nanoparticles was significantly higher than free EGF. Prepared nanoparticles at ratios 2:1 and 4:1 induced cell proliferation more than 1.5 fold and nanoparticles with ratio 6:1 induced cell proliferation about 2 fold. Additionally, free chitosan nanoparticles also induced cell proliferation. Obtained results demonstrated chitosan nanoparticles have two effects; firstly, protection of EGF activity and secondly synergistic behaviour with EGF on cell growth.
FIGURE 2.
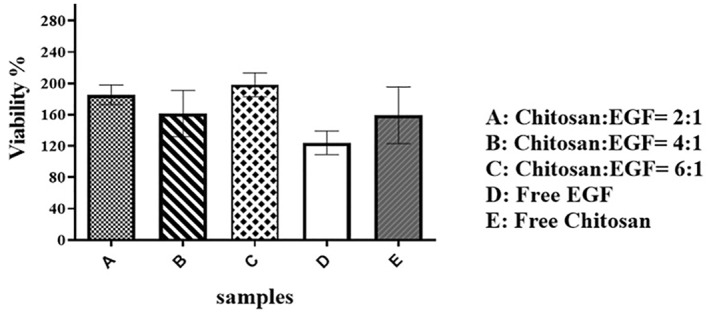
Cell proliferation assay of A375 cells after 24 h exposure to epidermal growth factor (EGF) loaded chitosan nanoparticles in different ratios. Data are represented as mean ± SD (n = 3).
3.6. In vitro stability evaluation
The ability of chitosan nanoparticles to protect EGF functionality was investigated in vitro. Firstly, according to Figure 3, cell proliferation after 2 h incubation of free EGF at pH = 7.5 was significantly lower than fresh EGF (p value = 0.0053). However, the biological activity of other samples was not changed significantly. Due to instability of free EGF protein at pH 7.5, the ability of nanoparticles to protect EGF functionality were evaluated in pH 7.5 for 2 h. The obtained results (Figure 4) showed that EGF functionality preserved by chitosan nanoparticles with different ratios of chitosan/EGF in compared with free EGF protein after 2 h incubation at pH 7.5.
FIGURE 3.
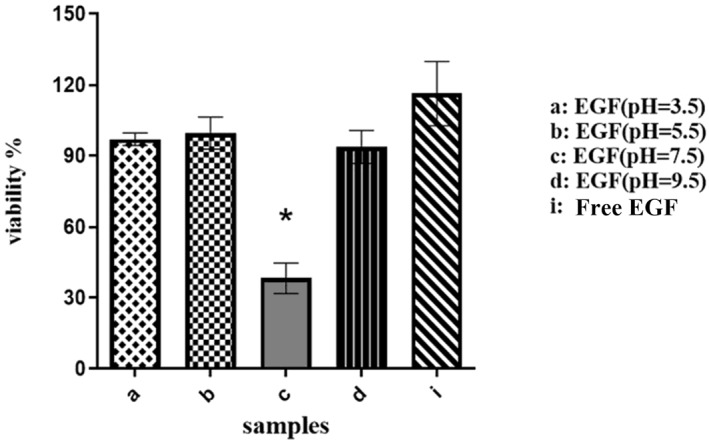
Biological activity of free epidermal growth factor (EGF) at different pHs for 2 h after incubation on A375 cells. Data are represented as mean ± SD (n = 3). *Denotes significant differences (P < 0.05).
FIGURE 4.
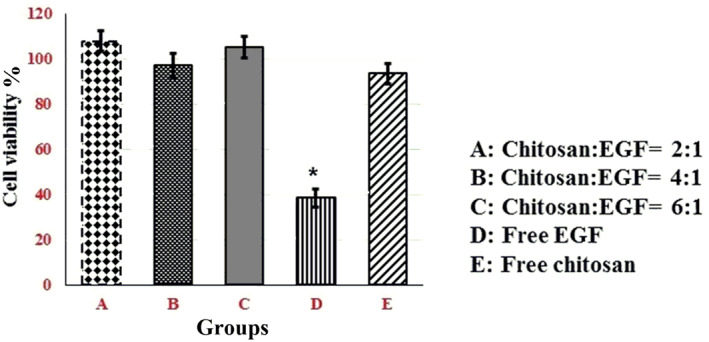
Cell proliferation assay of A375 cells after 2 h exposure to epidermal growth factor (EGF) loaded chitosan nanoparticles in different ratios at pH 7.5. Data are represented as mean ± SD (n = 3). *Denotes significant differences (P < 0.05).
This result can be related to isoelectric point of EGF, which is near 7 as estimated theoretically with https://web.expasy.org/cgi‐bin/protparam/protparam. Thus, EGF has lowest solubility at pH 7.5 and it becomes toxic for cells because of protein precipitation. Prepared chitosan nanoparticles with different ratios of chitosan/EGF could completely preserve biological activity of EGF in that condition. However, cell proliferation induction by chitosan/EGF in 4:1 ratio was slower in compared with 2:1 and 6:1 ratios. It could be related to release rate of protein within 2 h. According to the release profile, the 4:1 ratio after 2 h released only 45% of EGF but 2:1 and 6:1 ratios released 70 and 54% of active component respectively.
3.7. In vivo wound healing study
To evaluate the wound healing ability of prepared nanoparticles, the healing process of Rat skin wound was analysed macroscopically for 14 days. According to the obtained results (Figure 5), a typical pattern of healing has been observed in all five groups and the wounds of all groups remained open and similar after 4 days. The results obtained after 7 and 10 days showed that wound healing percentage was significantly higher in chitosan/EGF nanoparticles with ratio of 2:1. Also, the wound healing process was faster in ratio 2:1. This observation reveals that EGF stabilising by chitosan nanoparticles did not reduce its functionality and biological effect. Moreover, according to Figure 5 wound healing rate of chitosan: EGF (2:1) were significantly higher than other treatments. Because after 7 and 10 days only chitosan: EGF (2:1) group showed significant difference with control group regarding wound healing percentage.
FIGURE 5.
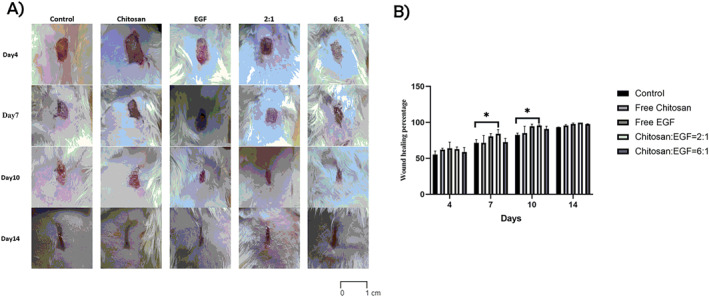
(a) Wound images of macroscopic pattern of wound healing for 14 days in different groups. (b) Wound healing percentage in treated animals at 4, 7, 10, and 14 days from wound creation. Data are represented as mean ± SD (n = 5). *Denotes significant differences (P < 0.05).
Previous studies have shown administration of chitosan nanoparticles containing EGF led to accelerate the wound healing process in compared with control and free EGF administration which can be related to EGF protection from degradation, sustained releasing and enhancing skin permeation. In this study induction of wound healing by chitosan/EGF nanoparticles with ratio 2:1 was significantly higher than control groups after 7 days (84% and 71% respectively). However, the wound healing process of chitosan/EGF nanoparticle with 6:1 ratio was slower than 2:1 ratio and free EGF which could be related to release rate of EGF. According to the obtained release profile, the 6:1 ratio after 12 h released only 65% of EGF but 2:1 ratio could release 81%. Ling et al, observed topical administration of chitosan nanoparticles containing five different bioactive agents including EGF, ascorbic acid, hydrocortisone, insulin and 1,25‐dihydroxy vitamin D3 could relatively complete wound healing process after 24 days in compared with untreated groups which was treated after 28 days [27]. In other study results showed that topical administration of EGF loaded carboxymethyl chitosan nanoparticles could significantly accelerated the wound healing process in treated group with 2:1 ratio (95% wound healing percentage after 10 days however control group reached to 93% after 14 days) that is in good agreement with our obtained results [26]. In their study, researchers used a chitosan derivative which can be more costly in scale up and commercialisation.
3.8. Wound histological analysis
The scores of epithelisation, inflammation, granulation, and fibrosis at the end of day 14 are summarised in Table 1. Results showed that the epithelisation processes were complete in all groups except the control. Additionally, the inflammation in the control and EGF groups was moderate in compare with other groups that mas mild after 14 days treatment. The granulation of skin tissue was severed in control group and chitosan groups showed less granulation. Interestingly, the degree of fibrosis was observed in all treatment groups but it was less in chitosan nanoparticles with different ratios of chitosan/EGF (2:1 and 6:1) (Figure 6). Furthermore, the obtained result showed the collagen deposition in the treatment groups by chitosan/EGF ratio 2:1 was significantly higher in compared with other groups after 14 days treatment (Figure 7).
TABLE 1.
Scores of skin histopathological changes in the animal model of wound healing after 14 days treatment. Data are represented as mean ± SD (n = 5)
| Treatments | Epithelialisation | Inflammation | Granulation | Fibrosis |
|---|---|---|---|---|
| Control | Incomplete | 2 | 3 | 2 |
| Free Chitosan | 2 | 1 | 1 | 2 |
| Free EGF | 3 | 2 | 2 | 2 |
| Chitosan: EGF = 2/1 | 3 | 1 | 2 | 1 |
| Chitosan: EGF = 6/1 | 2 | 1 | 2 | 1 |
FIGURE 6.
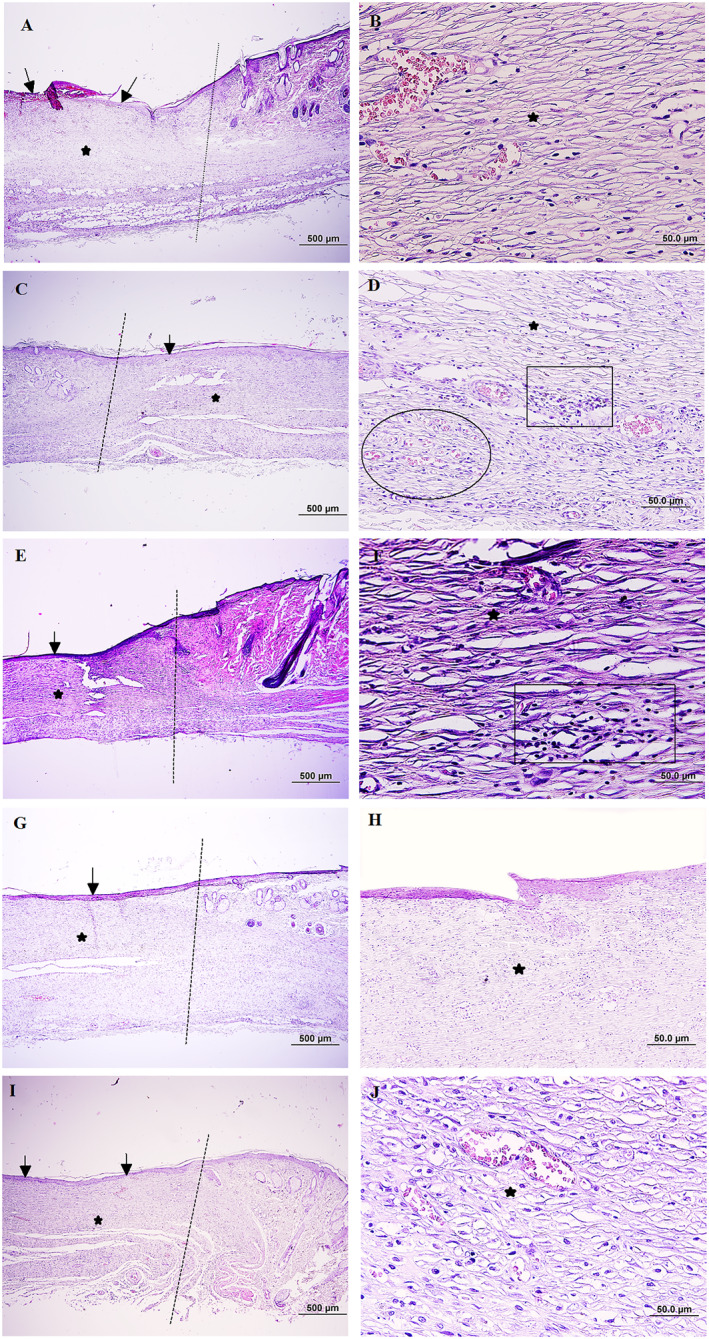
Microscopical evaluation of wound healing 14 days after wound induction, dot lines indicates the demarcation between the intact site and wound site (a): Control group, the partially re‐epithelisation is evident in wound bed (black arrow) and also immature granulation tissue in dermis is shown (black star), (b): Pervious slide with higher magnification, (c) Wound dressing with chitosan, note to appropriate re‐epithelisation (black arrow) and the newly formed dermis accumulated with hypercellular granulation tissue (star), (d): Higher magnification of pervious slide, neoangiogenesis (ellipsoid) and infiltration of inflammatory cells (rectangle) are evident, (e) Wound dressing with epidermal growth factor (EGF), the amount of epithelial regeneration seems appropriate (black arrow), and also note dermal reformation (star) (f) Pervious slide with higher magnification, presence of inflammatory cells in the granulation tissue (rectangle) is seen, (g) Wound dressing with chitosan/EGF nanoparticles ratio 2:1, note to proper re‐epithelisation (black arrow) and the dermis is almost filled with a mature granulation tissue (star), (h): Pervious side with higher magnification, (i): Wound dressing with chitosan/EGF nanoparticles ratio 6:1, the regeneration in epithelium (black arrow) and dermis (star) looks proper, (j) Pervious side with higher magnification, (H & E, Scale bar: A, C, E, G, and (i) 500 μm, B, D, E, H, and (j) 50.0 μm).
FIGURE 7.
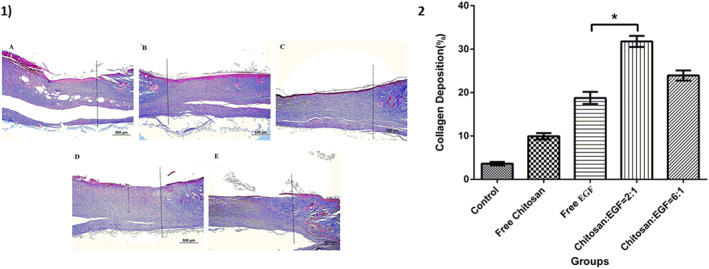
1) Masson's trichrome staining of healed, dot lines indicate the demarcation between the intact site and wound site. Blue staining represents deposition of collagen fibres. (a): Control group, (b): Wound dressing with chitosan, (c): Wound dressing with epidermal growth factor (EGF), (d): Wound dressing with chitosan/EGF nanoparticles ratio 2:1, (e): Wound dressing with chitosan/EGF nanoparticles ratio 6:1, (Masson's trichrome, Scale bar: A‐E: 500 μm). 2) Collagen deposition percentage in different groups after 14 days treatment. Data are represented as mean ± SD (n = 3). *Denotes significant differences (P < 0.0001).
Wound healing process involves three main phases including inflammation, proliferation and remodelling which required a coordinated response of different cells including fibroblasts, keratinocytes and vascular endothelial cells [28]. Our obtained results from histological studies demonstrated that treated groups by different nanoparticles had mild inflammation and fibrosis in compare with other groups. Presence of EGF in the wound area could promote wound healing process by accelerating angiogenesis, inducing the epithelial formation and collagen accumulation which was observed in previous studies [26, 29, 30].
4. CONCLUSION
In conclusion, this study exhibited prepared chitosan nanoparticles by simple complexation method in optimal chitosan to rhEGF ratio can be considered as a robust carrier for local delivery of EGF for wound healing process. The results were interesting since prepared nanoparticles could be able to preserve EGF bioactivity over time and accelerated wound healing in compared free EGF. Additionally, wound treatment by prepared nanoparticle showed less tissue fibrosis and high collagen deposition in wound tissue. The finding of our study suggested the prepared chitosan nanoparticles as a potential vehicle for safe delivery of EGF for wound healing applications.
AUTHOR CONTRIBUTIONS
Samaneh Montazeri: Formal analysis, Investigation, Writing – original draft. Ali Rastegari: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. Zohreh Mohammadi: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. Mahboobeh Nazari: Conceptualisation, Methodology, Validation. Maryam Yousefi: Conceptualisation, Methodology, Validation. Fatemeh Yazdi Samadi: Conceptualization, Methodology, Validation. Somayeh Najafzadeh: Conceptualization, Methodology. Mehdi Aghsami: Methodology, Validation.
CONFLICT OF INTEREST
None of the authors has not any conflict of interest. This study received no specific grant.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENT
This work would not have been possible without the invaluable excellent technical assistance of Dr Hanieh Jafari, Dr. Hananeh Golshahi Ms. Sanaz Rezaie and Mr. Ali Hamrahi.
Montazeri, S. , et al.: Chitosan nanoparticle loaded by epidermal growth factor as a potential protein carrier for wound healing: in vitro and in vivo studies. IET Nanobiotechnol. 17(3), 204–211 (2023). 10.1049/nbt2.12116
Contributor Information
Ali Rastegari, Email: mohammadi.z@iums.ac.ir.
Zohreh Mohammadi, Email: rastegari.a@iums.ac.ir.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Khanbanha, N. , et al.: Healing efficacy of an EGF impregnated triple gel based wound dressing: in vitro and in vivo studies. BioMed Res. Int. 2014, 1–10 (2014). 10.1155/2014/493732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson, N.R. , Wang, Y. : Controlled delivery of heparin‐binding EGF‐like growth factor yields fast and comprehensive wound healing. J. Contr. Release 166(2), 124–9 (2013). 10.1016/j.jconrel.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson, N.R. , Wang, Y. : Coacervate delivery of HB‐EGF accelerates healing of type 2 diabetic wounds. Wound Repair Regen. 23(4), 591–600 (2015). 10.1111/wrr.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chu, H. , et al.: Injectable fibroblast growth factor‐2 coacervate for persistent angiogenesis. Proc. Natl. Acad. Sci. USA 108(33), 13444–9 (2011). 10.1073/pnas.1110121108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Godwin, J.W. , Pinto, A.R. , Rosenthal, N.A. : Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 110(23), 9415–20 (2013). 10.1073/pnas.1300290110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jayakumar, R. , et al.: Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 29(3), 322–37 (2011). 10.1016/j.biotechadv.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 7. Stoscheck, C.M. , Carpenter, G. : Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J. Cell Biol. 98(3), 1048–53 (1984). 10.1083/jcb.98.3.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alvarez‐Lorenzo, C. , et al.: Crosslinked ionic polysaccharides for stimuli‐sensitive drug delivery. Adv. Drug Deliv. Rev. 65(9), 1148–71 (2013). 10.1016/j.addr.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 9. Dompé, M. , et al.: Thermoresponsive complex coacervate‐based underwater adhesive. Adv. Mater. 31(21), 1808179 (2019). 10.1002/adma.201808179 [DOI] [PubMed] [Google Scholar]
- 10. Peregrino, M. , Seabra, A. : Chitosan‐based nanomaterials for skin regeneration. Med. Sci. 4(3), 352–81 (2017). 10.3934/medsci.2017.3.352 [DOI] [Google Scholar]
- 11. Pfalzgraff, A. , Brandenburg, K. , Weindl, G. : Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 9, 281 (2018). 10.3389/fphar.2018.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dash, M. , et al.: Chitosan—a versatile semi‐synthetic polymer in biomedical applications. Prog. Polym. Sci. 36(8), 981–1014 (2011). 10.1016/j.progpolymsci.2011.02.001 [DOI] [Google Scholar]
- 13. Grolman, J.M. : Function Follows Form: Novel Structured Encapsulation Materials via Microfluidics. University of Illinois at Urbana‐Champaign; (2016) [Google Scholar]
- 14. Younes, I. , Rinaudo, M. : Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 13(3), 1133–74 (2015). 10.3390/md13031133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson, N.R. , Wang, Y. : Coacervate Delivery Systems for Proteins and Small Molecule Drugs. Taylor & Francis; (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loureiro, J.A. , et al.: Resveratrol and grape extract‐loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 22(2), 277 (2017). 10.3390/molecules22020277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamman, J.H. : Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 8(4), 1305–22 (2010). 10.3390/md8041305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhattarai, N. , Gunn, J. , Zhang, M. : Chitosan‐based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 62(1), 83–99 (2010). 10.1016/j.addr.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 19. Awada, H.K. , Johnson, N.R. , Wang, Y. : Dual delivery of vascular endothelial growth factor and hepatocyte growth factor coacervate displays strong angiogenic effects. Macromol. Biosci. 14(5), 679–86 (2014). 10.1002/mabi.201300486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Assa, F. , et al.: Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 37(4), 492–509 (2017). 10.1080/07388551.2016.1185389 [DOI] [PubMed] [Google Scholar]
- 21. Kahkeshani, N. , et al.: Antioxidant and burn healing potential of Galium odoratum extracts. Research in pharmaceutical sciences 8(3), 197 (2013) [PMC free article] [PubMed] [Google Scholar]
- 22. Serra, R. , et al.: Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti‐infect. Ther. 13(5), 605–13 (2015). 10.1586/14787210.2015.1023291 [DOI] [PubMed] [Google Scholar]
- 23. Siafaka, P.I. , et al.: Porous dressings of modified chitosan with poly (2‐hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr. Polym. 143, 90–9 (2016). 10.1016/j.carbpol.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 24. Black, K.A. , et al.: Protein encapsulation via polypeptide complex coacervation. ACS Macro Lett. 3(10), 1088–91 (2014). 10.1021/mz500529v [DOI] [PubMed] [Google Scholar]
- 25. Smijs, T.G. , Bouwstra, J.A. : Focus on skin as a possible port of entry for solid nanoparticles and the toxicological impact. J. Biomed. Nanotechnol. 6(5), 469–84 (2010). 10.1166/jbn.2010.1146 [DOI] [PubMed] [Google Scholar]
- 26. Zhang, P. , Liu, C. : Enhancement of skin wound healing by rhEGF‐loaded carboxymethyl chitosan nanoparticles. Polymers 12(7), 1612 (2020). 10.3390/polym12071612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li, L. , et al.: Fabrication of guanidinylated chitosan nanoparticles loaded with bioactive factors for facilitating wound healing. International Journal of Polymeric Materials and Polymeric Biomaterials 71(3), 173–9 (2022). 10.1080/00914037.2020.1817017 [DOI] [Google Scholar]
- 28. Chigurupati, S. , et al.: Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials 34(9), 2194–201 (2013). 10.1016/j.biomaterials.2012.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gainza, G. , et al.: A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF‐loaded lipid nanoparticles: in vitro bioactivity and in vivo effectiveness in healing‐impaired db/db mice. J. Contr. Release 185, 51–61 (2014). 10.1016/j.jconrel.2014.04.032 [DOI] [PubMed] [Google Scholar]
- 30. Hardwicke, J. , et al.: Bioresponsive dextrin− rhEGF conjugates: in vitro evaluation in models relevant to its proposed use as a treatment for chronic wounds. Mol. Pharm. 7(3), 699–707 (2010). 10.1021/mp9002656 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


