Abstract
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 is an ongoing health concern. In addition to affecting the respiratory system, COVID-19 can potentially damage other systems in the body, leading to extra-pulmonary manifestations. Hepatic manifestations are among the common consequences of COVID-19. Although the precise mechanism of liver injury is still questionable, several mechanisms have been hypothesized, including direct viral effect, cytokine storm, hypoxic-ischemic injury, hypoxia-reperfusion injury, ferroptosis, and hepatotoxic medications. Risk factors of COVID-19-induced liver injury include severe COVID-19 infection, male gender, advanced age, obesity, and underlying diseases. The presentations of liver involvement comprise abnormalities in liver enzymes and radiologic findings, which can be utilized to predict the prognosis. Increased gamma-glutamyltransferase, aspartate aminotransferase, and alanine aminotransferase levels with hypoalbuminemia can indicate severe liver injury and anticipate the need for intensive care units’ hospitalization. In imaging, a lower liver-to-spleen ratio and liver computed tomography attenuation may indicate a more severe illness. Furthermore, chronic liver disease patients are at a higher risk for severe disease and death from COVID-19. Nonalcoholic fatty liver disease had the highest risk of advanced COVID-19 disease and death, followed by metabolic-associated fatty liver disease and cirrhosis. In addition to COVID-19-induced liver injury, the pandemic has also altered the epidemiology and pattern of some hepatic diseases, such as alcoholic liver disease and hepatitis B. Therefore, it warrants special vigilance and awareness by healthcare professionals to screen and treat COVID-19-associated liver injury accordingly.
Keywords: SARS-CoV-2, COVID-19, Liver injury, Chronic liver disease, Management, Liver transplant
Core Tip: Severe acute respiratory syndrome coronavirus-2 can involve the liver and cause damage through different mechanisms. Liver injury can be diagnosed based on alterations in the liver function tests, which can also predict the disease severity and fatality. In patients without underlying liver disease, liver injury is typically mild and can be treated with supportive care. However, it requires additional awareness and appropriate therapy in patients with chronic liver diseases, including autoimmune hepatitis, viral hepatitis, liver cirrhosis, liver transplantation, and nonalcoholic fatty liver disease, which we have discussed in detail.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has tremendously influenced global public health since its emergence in December 2019. Despite the effectiveness of worldwide vaccinations, the disease is still a substantial threat[1]. As of 6 January 2023, the World Health Organization (WHO) recorded over 657 million confirmed cases of COVID-19 and over 6.6 million deaths[2].
COVID-19 was first recognized as a respiratory disease with variable manifestations, from asymptomatic and mild symptoms to acute respiratory distress syndrome and death. However, further investigations determined that COVID-19 can cause various extra-pulmonary manifestations and result in multi-organ dysfunction[3]. Various theories have been suggested to explain how COVID-19 causes gastrointestinal (GI) system involvement. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) penetrates the host cells via angiotensin converting enzyme 2 (ACE2) receptors, which exist in different tissues, including the GI system. This may result in GI manifestations such as nausea, vomiting, diarrhea, anorexia, and hepatic manifestations[4]. After the lungs, the liver is the second most frequent organ impacted by COVID-19[5]. Liver involvement usually presents as mild to moderate elevations of liver enzymes. Although, in certain circumstances, including patients with severe COVID-19 infection and underlying comorbidities (i.e., diabetes and hypertension) significant elevations of liver enzymes and liver dysfunction are more probable to happen[6,7]. COVID-19-induced liver injury may develop in patients regardless of the presence of underlying liver diseases; however, the likelihood of poorer outcomes is higher in patients with underlying chronic liver disease (CLD)[5,8]. Chronic liver diseases, including viral hepatitis, autoimmune hepatitis, liver cirrhosis, and fatty liver disease constitute a significant worldwide health burden. About 3% of COVID-19 patients have CLD, and this group has a higher risk of developing severe disease and death[9]. Moreover, these individuals have impaired immune systems due to their use of immunosuppressive drugs. Therefore, it is essential to consider stringent monitoring and develop appropriate treatments for these patients[10].
In this review, we aimed to comprehensively investigate different related aspects of liver injury in COVID-19. We discuss the pathophysiology, epidemiology, clinical manifestations, management, and outcomes of COVID-19 patients with liver injury. We also covered important topics like the interaction between COVID-19 and various types of liver disease, as well as unique considerations for particular populations with CLD and liver transplant (LT) recipients.
MECHANISMS AND PATHOPHYSIOLOGY OF LIVER INJURY IN COVID-19 PATIENTS
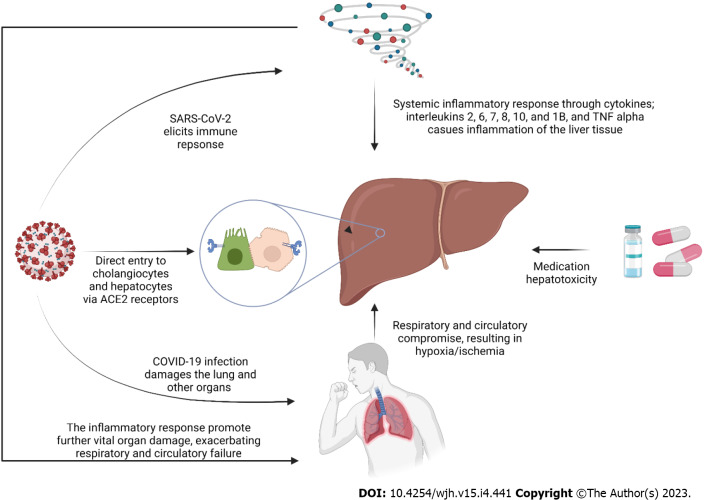
Although the exact mechanism of liver injury in COVID-19 is yet to be understood, several pathways have been proposed. The direct viral effect, drug hepatotoxicity, systematic inflammatory response (cytokine storm), decompensation of pre-existing liver disease, and hypoxic liver injury are among the suggested hypotheses[11]. Figure 1 demonstrates the proposed mechanisms of liver injury in COVID-19.
Figure 1.
Proposed mechanisms of liver injury in coronavirus disease 2019 patients. SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2; ACE2: Angiotensin converting enzyme 2; COVID-19: Coronavirus disease 2019; TNF alpha: Tumor necrosis factor alpha.
Direct viral effect
Although almost none of the current histopathological reports has shown a typical hepatitis picture[12], some evidence argues in favor of liver tropism and the direct effect of the virus on the liver. Firstly, the SARS-CoV-2 receptor, ACE2, is found on the surface of the hepatic Kupffer cells, hepatocytes, and cholangiocytes which helps the virus enter these cells and, as a result, makes the liver a potential target organ for the virus[13-15]. Secondly, Wang et al[16] discovered the SARS-CoV-2 spike structures in the cytoplasm of hepatocytes in two COVID-19 cases. The spatial presence of SARS-CoV-2 RNA and spike protein was also proved by previous studies in hepatic cells, which implies replication of the virus and direct infection of hepatic parenchyma[13,17]. Wanner et al[13] discovered a lower viral load in the liver than in the lungs of one patient, suggesting this may be the reason we do not typically observe cytopathic changes (i.e., hepatitis pattern) in the liver of COVID-19 patients.
Systemic inflammatory response (cytokine storm)
In response to COVID-19 and in order to hinder viral replication and evoke the adaptive immune response, the innate immune system is activated[18]. Severe cases of COVID-19 are associated with an exaggerated immune response indicated by high levels of C-reactive protein (CRP), pro-inflammatory T helper 17 cells, and cytokines[18,19]. Cytokines in severe cases of COVID-19 include interleukins (IL)-2, 6, 7, 8, 10, and 1B and Tumor necrosis factor alpha (TNF-α)[18-20]. Pathological studies have demonstrated non-specific inflammatory changes in the hepatocytes, including steatosis, lymphocyte infiltration, and Kupffer cell hyperplasia[19]. This immune system dysregulation can result in multiple organ involvement, including the liver. The cytokine storm not only affects the liver by triggering the inflammatory response and recruiting macrophages; but also plays a role in further indirect damage by promoting thrombotic events, circulatory changes, and failure of other organs[20].
Hypoxic-ischemic injury, hypoxia-reperfusion injury, and liver congestion
While shock as the result of the COVID-19 infection itself is not a common finding[21], it is a common entity in intensive care units (ICU)[22]. COVID-19 patients are susceptible to all types of shock (i.e., cardiogenic, septic, hypovolemic, and obstructive)[21,23,24]. While shock reduces liver perfusion, respiratory failure can also produce hypoxic harm to the liver, even in the absence of ischemia[22,25]. On the other hand, COVID-19 is known to induce microangiopathy and thromboembolism that further compromises the liver blood supply[26]. In the context of COVID-19, liver damage and coagulopathy are connected. Rise in transaminases have been shown to correlate with abnormal coagulopathy markers, such as prothrombin time, international normalized ratio, fibrinogen, D-dimer, fibrin/fibrinogen degradation products and platelet count[27]. Different organ involvements in COVID-19, including cardiac, pulmonary, and vascular involvement, paired with the high metabolic activity of the liver, makes it a highly susceptible organ to hypoxic-ischemic damage[28]. In the autopsy of COVID-19 patients, ischemic-type hepatic necrosis and lipid droplet accumulation (steatosis) have been found in histopathologic evaluations, both findings in favor of hypoxic-ischemic liver damage[12,29]. However, the hallmark of hypoxic hepatitis is a significant increase in liver enzymes, and the increase reported in the COVID-19 scenario is notably less[30]. Hence some have hypothesized that hypoxia-reperfusion is another explanatory mechanism[23]. Impairment of the venous drainage of the liver can contribute to blood stasis and hepatic congestion. Hepatic congestion is caused by the stasis of blood within the liver parenchyma due to the compromise of hepatic venous drainage[31]. Congestion of the hepatic sinusoids was estimated at 34.7% in a histopathologic study[32]. Cardiac events resulting in right-sided heart failure, decompensation of heart failure, and pulmonary thromboembolism can contribute to liver congestion in COVID-19 patients[23]. In a review by Kukla et al[33], hepatic dysfunction was associated with mechanical ventilation, especially with high rates of positive end-expiratory pressure (PEEP). They hypothesized that high PEEP (18-20 cm H2O) causes high right atrial pressure resulting in liver congestion in a mechanism similar to right-sided heart failure. In a hemodynamic study of COVID-19 patients, mechanical ventilation and PEEP were associated with left ventricular underfilling[25].
Ferroptosis
Ferroptosis is a novel form of cell death, which is characterized by iron accumulation and lipid peroxidation[34]. The oxidative stress caused by iron overload is an important trigger for liver injury as well as other vital organ damage[35]. COVID-19 is believed to impact iron metabolism[36]. In the condition of cytokine storm, IL-6 stimulates the production of hepcidin in the liver, which is an inhibitor of iron export and leads to iron sequestration inside cells[37]. It is hypothesized that SARS-CoV-2 triggers the recognition of blood iron-binding molecules by receptors in many organs, including the liver, thereby causing an influx of iron ions. The accumulated iron, along with agents like lipid and hydrogen peroxide, triggers the Fenton reaction, producing massive lipid reactive oxygen species that cause cell membrane damage[38]. In conclusion, ferroptosis is a potentially important mechanism of organ damage in COVID-19; however, the extent of its role and whether treatments that inhibit ferroptosis can be helpful in preventing organ damage in COVID-19 remains to be determined[37,39].
Medications
Medication hepatotoxicity has strong evidence as the cause of liver injury in the context of COVID-19[40]. Owing to the novel nature of the COVID-19 infection and the lack of evidence-based guidelines, various medications have been used for treatment, many of which have potential hepatotoxic properties. These include antivirals (including remdesivir, lopinavir/ritonavir, Favipiravir), hydroxychloroquine, antibiotics (e.g., azithromycin), corticosteroids, tocilizumab, and antipyretics, specifically acetaminophen[23,40,41]. Remdesivir has been associated with transient increases in liver enzymes in Gilead company trials in less than 5% of participants and was indicated to possibly be cytotoxic to human hepatocytes at clinically relevant exposures[42,43]. Consistently, liver injury in remdesivir-treated COVID-19 cases was mostly mild, according to published literature[44-46]. Wang et al[34] reported that 7% of a remdesivir-treated group developed grade 1-2 aspartate aminotransferase (AST) elevation compared to 12% in a placebo-controlled group, and 1% developed grade 1-2 alanine aminotransferase (ALT) elevation. Case reports have revealed that associate Favipiravir, another antiviral agent used to treat COVID-19, with liver injury[47,48]. However, a systematic review on the safety of Favipiravir in COVID-19 failed to prove the association between the drug and liver injury[49]. The use of lopinavir/ritonavir has been linked to liver toxicity in multiple studies of COVID-19 patients[40]. Serum aminotransferases increase in a large number of individuals using antiretroviral regimens containing ritonavir. Moderate to severe elevations in the serum aminotransferase levels are detected in up to 15% of patients treated with full dosages of ritonavir[50]. In the setting of COVID-19, Cai et al[51] also found lopinavir/ritonavir to be associated with increased odds of liver injury. Yip et al[52] showed that the use of lopinavir/ritonavir ± ribavirin + interferon beta was independently associated with elevated liver enzymes. The use of systemic corticosteroids is highly encouraged in COVID-19, especially in patients requiring oxygen supplement therapy[53]. A cohort study in Hong Kong reported the independent association of corticosteroids with elevated AST/ALT[52]. However, extensive data on the hepatotoxicity of corticosteroids is non-existent, and it’s usually confined to case reports[53]. Some have even proposed them to treat drug-induced liver injury[54]. One potential way of corticosteroid-induced liver toxicity may be its role in inducing non-alcoholic steatohepatitis[53]. Tocilizumab, a humanized recombinant monoclonal antibody, is used to combat systematic inflammatory response in COVID-19 because of its anti-IL-6 properties[55]. Hepatotoxicity with transient, mild to moderate transaminase increase is its well-known side effect, but severe drug-induced liver injury is a rare event[55,56]. Hydroxychloroquine and chloroquine are the standard medications to treat malaria and had been explored as treatment options for COVID-19 due to their in vitro anti-SARS-CoV-2 properties[57]. Liver injury associated with hydroxychloroquine is uncommon and limited to case reports[58-60]. Azithromycin, a macrolide antibiotic, was also advocated for the treatment of COVID-19 owing to its cytokine-suppressing features, potentially preserving the epithelial cells and inhibiting lung fibrosis[61]. Azithromycin is known to cause liver injury, with both hepatocellular and cholestatic patterns[62,63]. Ivermectin, an anti-parasite drug, was reported to be associated with one case of severe hepatitis; however, there is insufficient data to comment on the effects of ivermectin on the liver function of COVID-19 patients, and more research is needed to clarify this[40,64]. So far, there is no report in the literature about acetaminophen-associated hepatotoxicity in COVID-19 patients; this is likely because acetaminophen-induced hepatotoxicity is highly dose-dependent, and its safe dose is well-established[65]. Additionally, it should be highlighted that, despite dosages used in patients normally belonging within safe ranges, combination therapy with various hepatotoxic drugs may have a synergistic effect.
COVID-19 AND LIVER MANIFESTATIONS
COVID-19 is usually regarded as a respiratory disease; however, subsequent research revealed extra-pulmonary manifestations of COVID-19, including cardiovascular, gastrointestinal, renal, ocular, neurological, psychiatric, dermatological, and endocrinology symptoms[66,67]. GI manifestations of COVID-19 are among the most common extra-pulmonary manifestations, and they can emerge even before the appearance of respiratory symptoms. Also, they can be the sole manifestation in some COVID-19 patients[68]. It seems that patients with COVID-19 who experience GI symptoms have a higher risk of elevated liver enzymes and liver injury[18,69]. The prevalence of liver injury in COVID-19 patients ranges widely from 21.5% to 45.71%[70]. This wide range is because of the specific characteristics of different populations and cut-off level discrepancies[71]. Liver injury commonly manifests as abnormalities in liver enzymes in the absence of particular clinical symptoms. Hence, close monitoring of liver enzymes in COVID-19 patients is essential for timely diagnosis of liver injury. In a meta-analysis by Yadav et al[70], the prevalence of liver enzymes abnormalities among COVID-19 patients ranged from 37.2% to 76.3%. In another meta-analysis, an elevation in the liver enzymes was reported in about 25% of COVID-19 patients, and the prevalence of increased AST and ALT was 23.2% and 21.2%, respectively. Gamma-glutamyltransferase (GGT) was elevated in 15%, total bilirubin in 9.7%, and alkaline phosphatase (ALP) in 4% of the patients[69].
RISK FACTORS FOR LIVER INJURY AND PREDICTORS OF DISEASE SEVERITY
Certain risk factors can predispose patients to develop liver injury. Some laboratory findings, such as lymphopenia, elevated AST/ALT ratio, and higher erythrocyte sedimentation rate and CRP levels are linked to the development of liver injury in COVID-19 patients. It is hypothesized that a systemic inflammatory response and cytokine storm are the cause of liver damage[70,72]. In addition, male gender, obesity, and advanced age are the risk factors for liver injury in COVID-19 patients[51]. The higher odds of COVID-19 induced liver injury among males may be due to the higher prevalence of existing risk factors, such as nonalcoholic fatty liver disease (NAFLD) and alcohol intake[73,74]. Previous studies have linked abnormalities in liver enzymes and liver injury to disease severity and mortality[75]. The abnormalities in liver function tests (LFT) can be used to predict the outcome of the disease. In a study conducted by Cai et al[51], the patients who had hepatocellular and mixed patterns of liver involvement were at higher risk of developing severe disease. A hepatocellular pattern was determined as an increase in aminotransferases (ALT/AST) levels higher than three times the upper limit of normal (ULN), while a mixed pattern was determined as an increase in aminotransferases greater than three times the ULN and ALP/GGT greater than twice the ULN.
Weber et al[76] demonstrated the correspondence between abnormal baseline liver enzymes and disease progression in patients without underlying liver disease. Increased GGT, AST, and ALT levels with hypoalbuminemia upon admission predicted the severity of the illness and the requirement for ICU hospitalization. Other studies have confirmed the link between low albumin levels and the severity of COVID-19. Hypoalbuminemia in severely ill COVID-19 patients is likely due to a combination of impaired hepatic albumin production and dietary deficiencies[18,77]. Phipps et al[75] revealed that higher levels of peak ALT and markers of inflammation, such as ferritin and IL-6, were strongly linked to progression to severe liver injury and a poorer outcome.
RADIOLOGIC FINDINGS OF THE LIVER IN COVID-19 PATIENTS
Some radiologic manifestations of liver damage have been reported in COVID-19 patients. On abdominal computed tomography (CT) scans of COVID-19 patients, hepatic hypodensity and fat stranding around the gallbladder were observed[78]. Some findings, such as a decrease in liver-to-spleen attenuation ratio and liver CT attenuation, may be relevant to the severity of the disease[79,80]. Chen et al[72] demonstrated that hepatic steatosis on CT scan was observed in 11.3% of COVID-19 cases, which enhanced the risk of hepatic dysfunction. In addition, they mentioned that while a decrease in hepatic attenuation on a baseline CT scan is rampant, it is usually a temporary condition that improves on subsequent CT scans. The most prevalent findings of abdominal ultrasound of COVID-19 patients in ICU were hepatomegaly and biliary abnormalities, including common bile duct dilatation, gallbladder distention, and wall thickening[81]. Also, it was shown that in patients with mildly increased liver enzymes, hepatic ultrasonography was often not significant. However, in patients with a substantial rise in liver enzymes, hepatic ultrasound may detect vascular abnormalities or cholestatic changes, which is tied to higher fatality rates[82].
COVID-19 VACCINATION AND LIVER INJURY
With widespread vaccination against COVID-19, many reports of immune-mediated liver injury (ILI) associated with vaccination have emerged[83-87]. Most cases develop a hepatocellular pattern of injury[86]. The proposed hypothesis is that due to the similarity between S protein and liver specific proteins, the immune response elicited against the S protein, which is encoded by the vaccines, may cause autoimmune-like hepatitis[88]. Histopathological evaluation of the patients with ILI demonstrated portal lymphoplasmacytic infiltration and interface hepatitis[89]. The prognosis of immune-mediated vaccine-induced liver injury seems to be excellent with corticosteroid treatment, with only 4.3% death among patients who received steroids[89].
Roy et al[89] reviewed ILI following COVID-19 vaccinations. Most of the patients with ILI were female, with a mean age of 55.3 years. Moderna mRNA–1273 was the most common culprit for ILI following COVID-19 vaccinations, followed by Pfizer-BioNTech BNT162b2 mRNA and AstraZeneca ChAdOx1 nCoV-19 vaccine. Inactivated vaccines can also contribute to ILI; however, the reports are much more scarce than mRNA vaccines[90]. The most common presentation of ILI was jaundice, which was present in 78.3% of the patients, and bilirubin, ALT, and ALP levels were elevated. There is a dispute over the diagnosis of ILI following vaccination. Physicians should consider the clinical symptoms, liver function tests, histopathological findings, and chronological association between vaccine injection and presentations of the symptoms.
OUTCOME, MANAGEMENT, AND CONSIDERATIONS IN SPECIAL POPULATIONS
Liver involvement is a common finding in patients with COVID-19; however, it is frequently mild, transitory, and resolves without management[75,91]. Nevertheless, patients with underlying liver diseases, including hepatitis, cirrhosis, or LT recipients, are at higher risk of severe diseases. It is estimated that the probability of developing severe disease and death due to COVID-19 was 2.44 times greater in patients with CLD compared to those without underlying liver diseases [Odds ratio (OR) for severity = 2.44, 95%CI: 1.89-3.16; OR for mortality = 2.35, 95%CI: 1.84-3.00][92]. Among different underlying liver diseases, NAFLD was associated with the highest odds of severe disease and death due to COVID-19, followed by metabolic-associated fatty liver disease (MAFLD) and cirrhosis[92]. Severity and mortality rates after COVID-19 were not impacted by viral hepatitis significantly. Table 1 demonstrates the strength of the association between several major underlying liver diseases and the severity and mortality of COVID-19.
Table 1.
The association of different underlying liver diseases with clinical outcomes of coronavirus disease 2019
|
Underlying liver disease/condition
|
Findings
|
| Autoimmune hepatitis | AIH vs other CLDs: No significant differences in hospital admission (76% vs 85%; P = 0.06), ICU admission (29% vs 23%; P = 0.240), and mortality (23% vs 20%; P = 0.643) rates; AIH vs non-CLDs: Higher hospitalization rate, but similar rate for other outcomes; Severity of AIH associates with COVID-19 mortality, as follows: Age OR per 10 years: 2.01 (95%CI: 1.07-3.81); Child-Pugh B cirrhosis OR: 42.48 (95%CI: 4.41-409.53); Child-Pugh C cirrhosis OR: 69.30 (95%CI: 2.83-1694.50)[93] |
| Viral hepatitis | The risk of severe COVID-19 is higher (RR: 1.68, 95%CI: 1.26-2.22)[191]; however, another meta-analysis showed no association between viral hepatitis and poorer outcomes (pooled OR = 1.29, 95%CI: 0.36-4.63)[92] |
| Cirrhosis | Cirrhotic patients experienced more severe disease (pooled OR = 3.09, 95%CI, 1.95–4.89)[92]; Severity of cirrhosis associates with COVID-19 severity, as follows: Child-Pugh A cirrhosis OR: 1.90 (95%CI: 1.03-3.52); Child-Pugh B cirrhosis OR: 4.14 (95%CI: 2.4-7.65); Child-Pugh C cirrhosis OR: 9.32 (95%CI: 4.80-18.08)[111] |
| Liver transplant | No significant difference in mortality rates between LT and non-LT participants (OR: 0.8, 95%CI: 0.6-1.08); The time between the transplantation and COVID-19 did not affect the mortality rate (OR: 1.5, 95%CI: 0.63-3.56); Severe COVID-19 infection was observed in 23% of the participants with LT[129] |
| NAFLD | NAFLD was associated with more severe COVID-19 (AOR: 2.60, 95%CI: 2.24-3.02), more ICU admission (AOR: 1.66, 95%CI: 1.26-2.20), but not higher mortality rates (AOR: 1.01, 95%CI: 0.65-1.58)[148] |
| MAFLD | MAFLD increased the risk of severe COVID-19 (OR: 1.80, 95%CI: 1.53-2.13). No association was found between the presence of MAFLD and the occurrence of COVID-19 death[192] |
| Pregnancy | 29.7% of pregnant patients with COVID-19 had liver injury; Liver injury can predispose pregnant females to experience more severe COVID-19, however, their neonates do not have worsen prognosis[157] |
COVID-19: Coronavirus disease 2019; AIH: Autoimmune hepatitis; AOR: Adjusted odds ratio; CLD: Chronic liver disease; ICU: Intensive care unit; LT: Liver transplant; MAFLD: Metabolic associated fatty liver disease; MMF: Mycophenolate mofetil; NAFLD: Non-alcoholic fatty liver disease; OR: Odds ratio; RR: Relative risk.
Autoimmune hepatitis
The most common presentations of COVID-19 in patients with autoimmune hepatitis (AIH) were respiratory (74%) and GI (26%) manifestations[93]. However, AIH patients are more likely to present with GI manifestations than the general population (26% vs 14%). The mortality rate and cause of death of the AIH group were not significantly different from the non-AIH CLD group. Age (OR per 10 years: 2.01, 95%CI: 1.07-3.81), Child-Pugh B (OR: 42.48, 95%CI: 4.41-409.53), and Child-Pugh C (OR: 69.30, 95%CI: 2.83-1694.50) cirrhosis were the determinants of mortality within AIH patients[93].
AIH is an immune-mediated liver disease, which is mainly responsive to immunosuppressive therapy[94]. Single corticosteroid therapy or in combination with azathioprine are the standard immunosuppressive treatments for AIH[95]. Other drugs used to treat AIH include mycophenolate mofetil (MMF), calcineurin inhibitors, and TNF-α blockers[95]. The immunosuppression status following the use of these drugs enhances the risk of bacterial and viral infections in these patients[95] and might theoretically lead to a more severe COVID-19. Therefore, it is crucial to closely monitor the immunosuppressed AIH patients affected by COVID-19. In patients with simultaneous AIH and COVID-19, immunosuppressive therapy should be given after evaluating the risks and benefits. It is suggested that immunosuppressive therapy can be lifesaving in severe AIH patients[96]. Thus, it is not wise to discontinue the treatment as it might predispose the patients to a higher risk of relapse. In conclusion, empirical reduction in the doses of immunosuppressive drugs in these patients during the course of COVID-19 can be potentially harmful[97]; however, the immunosuppression therapy can be delayed until the COVID-19 polymerase chain reaction (PCR) test becomes negative in milder forms of AIH[96].
In addition to the impacts of COVID-19 on the management of AIH during the pandemic, SARS-CoV-2 has been proposed as a possible trigger for AIH. In a study by Kabaçam et al[96], COVID-19 patients were diagnosed with AIH after presenting with high serum aminotransferase and IgG levels. It should be noted that there is an AIH-like liver injury that might occur following COVID-19 vaccination. The laboratory findings are similar to AIH in these cases, and there is a good response to corticosteroids in these patients[98,99].
Viral hepatitis B
The prevalence of hepatitis B virus in patients with COVID-19 ranges from 0.1% in a study in the United States to 12.2% in China[100,101]. Interestingly, these prevalence rates were not significantly higher than the general population. Furthermore, there is no well-established association between HBV infection and severe COVID-19 disease[101-104]. Surprisingly, some studies reported a milder course of COVID-19 in patients with HBV[101,103,105,106]. One hypothesis is that patients with HBV might experience a state of “immune exhaustion”, which can be responsible for a lower chance of developing cytokine storm[107].
The treatment of COVID-19 with corticosteroids and other immunosuppressive drugs, including tocilizumab, may lead to the reactivation of HBV infection in these patients. Therefore, screening for HBV in COVID-19 patients with elevated liver enzymes in endemic HBV populations is highly recommended[104]. Several treatments were recommended for COVID-19 infection in HBV patients. A suggested method is drug repositioning, which means that the antiviral HBV drugs could also be used to treat COVID-19 infection[108,109]. In COVID-19 HBsAg-positive patients who are not receiving anti-HBV medication, continuous corticosteroid or immunosuppressive drugs consumption necessitates prophylaxis with tenofovir disoproxil fumarate, tenofovir alafenamide, and entecavir to reduce the likelihood of HBV reactivation and liver failure[104].
Liver cirrhosis
COVID-19 is one of the main contributors to the exacerbation of preexisting liver cirrhosis[110]. It is found that liver cirrhosis decompensation occurs in nearly half of the cirrhotic patients with COVID-19, one-fifth of whom might not develop any pulmonary manifestations[111]. Therefore, physicians should be advised to consider COVID-19 in patients with cirrhosis exacerbation, even in the absence of respiratory symptoms. Because of cirrhosis-associated immune dysfunction, patients with decompensated liver cirrhosis are more susceptible to COVID-19[112,113]. The mortality rate in COVID-19 patients with decompensated liver cirrhosis was shown to be about 50%[114]. The main predictor of mortality in cirrhotic patients with COVID-19 is the severity of underlying cirrhosis[111]. Child-Pugh C cirrhosis has the highest odds for mortality among patients with CLD (OR = 9.32, 95%CI: 4.80-18.08), while the odds ratio for Child-Pugh A was 1.90 (95%CI: 1.03-3.52). Other predictors of mortality were higher age and concurrent alcoholic liver disease. One study reported that mortality, ventilation support, hospitalization, and ICU admission rates were significantly higher in patients with higher model for end-stage liver disease (MELD) scores, especially a score above ten[115]. However, whether COVID-19 can worsen the outcomes in hospitalized patients with cirrhosis remains controversial. While some reports demonstrate that COVID-19 can elevate mortality rates in cirrhotic patients[110,116,117], other studies found that COVID-19 did not significantly increase mortality in these patients[118,119]. Monitoring liver function tests, early detection of liver complications caused by a coronavirus, and broad-spectrum antibiotic therapy in case of secondary bacterial infection are highly recommended for successful management[112].
The humoral response to COVID-19 vaccines is 94% in patients with CLD or cirrhosis. The mRNA vaccines are more efficacious than inactivated vaccines in these patients[120].
LT
The success rate of transplantation is associated closely with the postoperative immunosuppression treatment to prevent organ rejection[121]. Since the emergence of the pandemic, there has been a debate on the use and dosing of immunosuppressive drugs for these patients, as they act like a double-edged sword[122]. While they can profoundly decrease the chance of organ rejection, severe immunosuppression can theoretically lead to uncontrolled viral replication and severe COVID-19[123,124]. MMF and calcineurin inhibitors, including tacrolimus are among the main immunosuppressive drugs used in LT patients[125,126]. MMF inhibits the proliferation of lymphocytes and suppresses cell-mediated immunity and antibody formation[127]. LT patients who were previously treated with MMF have a higher risk of experiencing severe COVID-19 infection, especially those who have received doses more than 1000 mg/day[127]. They also suggested a reduction in the dose of MMF or temporary conversion to other immunosuppressive drugs, including tacrolimus in LT patients[127]. On the other hand, a meta-analysis found that the continued administration of certain immunosuppressive drugs during COVID-19 resulted in a lower probability of severe disease[128]. Among the immunosuppressive drugs, the mammalian target of rapamycin inhibitor (mTORi) was the most effective in preventing severe disease, followed by calcineurin inhibitors, steroids, and antimetabolites.
Further studies, including a meta-analysis, indicated that the rate of severe COVID-19 and death was not higher in LT patients[129]. Most deaths due to COVID-19 were associated with the cytokine storm and inflammatory process, not the result of direct viral replication[130]. Therefore, not only is immunosuppression not harmful, but also it could prevent cytokine storm and reduce mortality. Guarino et al[131] demonstrated that LT patients were more likely to become symptomatic; however, they did not exhibit more severe disease and death due to COVID-19. They did not reduce the dose of immunosuppressive drugs unless in severe and critical patients. This heterogeneity among the results can be attributed to the baseline characteristics of the patients, including the presence of other comorbidities, mean age, time from the transplant to COVID-19, and immunosuppressive drug. The strengths of the associations of these factors were as follows: (1) Age > 70 [Hazard ratio (HR): 4.16, 95%CI: 1.78-9.73]; (2) Tacrolimus use (HR: 0.55, 95%CI: 0.31-0.99); (3) Diabetes (HR: 1.95, 95%CI: 1.06-3.58); (4) Chronic kidney disease (HR: 1.97, 95%CI: 1.05-3.67); and (5) Time between transplantation and COVID-19 (HR: 0.55, 95%CI: 0.31-0.99)[132,133]. Therefore, we suggest that physicians assess the risk of severe disease in LT patients by considering age, comorbidities, and the time between the transplant and COVID-19. It is not necessary to modify the immunosuppressive drugs for all the patients; they can be closely monitored for the occurrence of risk factors and manifestations of severe disease and, if present, reducing the dose or changing the drugs can be applied. Besides, tacrolimus-based or mTORi-based immunosuppressive regimens have been proven to be safer with more favorable outcomes[128,134].
In addition, the response to COVID-19 vaccination is another important challenge in immunosuppressed patients. It is found that discontinuation or dose reduction of MMF optimizes the COVID-19 vaccination response in LT patients[135,136]. Moreover, it is necessary to consider booster shots in immunosuppressed patients who do not demonstrate the appropriate response to vaccinations[137]. On the other hand, pre-exposure prophylaxis with tixagevimab/cilgavima can be beneficial in LT patients with severe immunosuppression who did not respond adequately to COVID-19 vaccination or COVID-19 vaccine contraindication[138]. The humoral response to COVID-19 vaccines is 66% for patients with LT[120]. Luo et al[120] also revealed that the worse humoral response to COVID-19 vaccinations in LT patients was observed in patients with a history of using MMF (OR: 3.27, 95%CI: 1.45-7.41), diabetes (OR: 2.75, 95%CI: 1.48-5.09), and the use of > 2 immunosuppressive drugs (OR: 3.13, 95%CI: 1.22-7.99).
Transplant candidates waiting for a donor are also considered high-risk due to their underlying liver disease. COVID-19 can complicate the LT process as an affected patient cannot undergo transplant surgery until declared recovered from COVID-19. It is suggested that the transplant should be performed with one negative COVID-19 reverse transcription-PCR test 24 h prior to the transplantation[139]. It was found that COVID-19 infection before liver transplantation in patients with high MELD scores was not associated with a higher mortality rate[140]. As a result, postponing the surgery to a later time than what is suggested above is not recommended.
Nonalcoholic fatty liver disease
NAFLD is a liver manifestation of metabolic syndrome and is the most common CLD all over the world, affecting 30%-40% of the world population[141,142]. The spectrum of NAFLD ranges from hepatocellular steatosis to advanced liver cirrhosis[143]. There was a great heterogeneity across different studies which assessed the severity outcomes of COVID-19 in patients with NAFLD[144-147]. Two meta-analyses suggested a more severe disease course and higher rates of ICU admission in NAFLD patients[92,148].
MAFLD is a more recent term to describe fatty liver disease in the context of metabolic syndrome[142]. It is considered a better reflection of the disease pathogenesis and can contribute to a more appropriate disease classification and management. NAFLD patients were diagnosed and included in the studies mainly based on their imaging (computed tomography or sonography) findings; however, metabolic risk abnormalities should be considered beside the imaging findings of hepatic steatosis to label a patient as MAFLD[149]. The existence of MAFLD can lead to the release of more inflammatory cytokines and worsens the inflammatory process in COVID-19 infected patients[150]. Gao et al[151] found that the elevated level of IL-6 was associated with poorer COVID-19 outcomes in MAFLD patients. Leptin dysregulation in metabolic syndrome and obesity can be responsible for the more prominent cytokine storm observed in these conditions[152]. Therefore, it seems that metabolic syndrome features which are incorporated in the definition of MAFLD are the determinants of the disease severity in patients with fatty liver. This also clarifies the inconsistency of the results about disease severity in NAFLD patients with COVID-19, but a more consistent result for MAFLD that we described above.
Another predictor of disease severity is the degree of NAFLD or MAFLD and liver fibrosis. Targher et al[146] found that MAFLD patients with intermediate to high fibrosis-4 (FIB-4) scores had poorer COVID-19 outcomes (OR for intermediate FIB-4 = 4.32, 95%CI: 1.94-9.59; OR for high FIB-4 = 5.73, 95%CI: 1.84-17.9). Another study found that the increase in various liver fibrosis scores, including FIB-4, aspartate aminotransferase to platelet ratio index, NAFLD fibrosis score, and Forns scores was associated with worse COVID-19 outcomes and, as a result, can be used as prognostic factors[153]. However, their accuracy in identifying liver fibrosis is not acceptable in young individuals and both extremes of body mass index[154,155].
Pregnancy
The presence of liver involvement can partially impact COVID-19 prognosis in pregnant patients. It is shown that the duration of admission in COVID-19 pregnant females is more prolonged in those with liver involvement[156]. Moreover, pregnant females with liver injury are at higher risk of developing severe disease[157]. Nevertheless, their neonates do not have an excessive risk for worsening prognosis.
The prevalence of COVID-19-related liver injury in pregnant women is nearly 30%, which is relatively similar to the general population[157]. The first step in managing a pregnant patient who presents with COVID-19 and liver injury is to identify the cause of liver damage and exclude obstetric diagnoses. COVID-19-related liver involvement can be mistaken for preeclampsia[158], HELLP syndrome[159], acute fatty liver of pregnancy[158], and intrahepatic cholestasis[160]. All these conditions warrant early diagnosis and require certain urgent management. Therefore, it is highly recommended to monitor liver function tests in pregnant females[157].
Myalgia is one of the most common presentations of COVID-19, which is usually managed with acetaminophen plus non-steroidal anti-inflammatory drugs (NSAID)[161]. As the use of high-dose NSAID is not recommended during pregnancy, especially during the third trimester, higher doses of acetaminophen might be prescribed to control the pain, which might exacerbate the underlying liver disease. Therefore, physicians should calculate the safe dose of acetaminophen to prevent the exacerbation of underlying liver disease[162].
Infants and children
Severe COVID-19 disease in adults is linked to dysfunctional cellular immunity and unchecked inflammatory cytokines production[163]. The unaffected immunosuppressive and cellular elements of the immune system in children can result in a less aggressive course of COVID-19. This milder course of COVID-19 can explain the lower rate of elevated AST and ALT in children (6%-22%)[164,165], compared to adults (14%-53%)[166]. The most common hepatic presentation of COVID-19 is the elevation of liver enzymes. Elevated ALT is usually seen in older male children[167]. Supportive treatment is generally considered for managing COVID-19-affected children with liver involvement.
In addition to COVID-19-related liver injury, pediatricians should be aware of the management of COVID-19 in children with pre-existing liver disease. It was revealed that the severity of COVID-19 was not higher in children with CLD[168]. Therefore, children with stable CLD can be managed virtually with a well-organized telemedicine system. Similar to adults, in children with autoimmune liver disease or LT, who are receiving immunosuppressive drugs, it is not recommended to decrease the dose of drugs in mild cases, as it can increase the chance of organ rejection[163].
Neonates can be affected by COVID-19 via three routes: antenatal, peripartum, and postnatal. Liver involvement in neonates is mainly characterized by elevated liver enzymes. In these cases, the first step is to exclude other causes of abnormal liver function tests[169]. Remdesivir should be administered in neonates with COVID-19 with great caution and only in severe cases as it can worsen liver injury in COVID-19- infected neonates[170].
EPIDEMIOLOGICAL ASPECTS OF LIVER DISEASE DURING THE COVID-19 PANDEMIC
COVID-19 can cause liver injury via different mechanisms discussed above. In addition to these mechanisms, the COVID-19 pandemic contributed to some changes in the pattern and epidemiology of some of the hepatic diseases.
Alcoholic liver disease
The amount of alcohol consumption and prevalence of alcoholic liver disease have risen during the COVID-19 pandemic. Several reports from different countries worldwide demonstrated a significant rise in the amount of alcohol use during the pandemic, including the United States[171], China[172], and England[173]. It is postulated that this increase resulted from financial problems, job loss, and mental distress during the pandemic[174-176]. Besides, the psychological impacts of the loss of beloved ones, social isolation, lockdown, and other mitigation strategies that were implemented to control the disease have resulted in a greater level of mental distress and, as a result, aggravated alcohol use even more[177-179]. Therefore, it was expected to observe higher rates of alcoholic liver diseases and their burden. Numerous reports from across the globe provided data regarding the increased burden, hospitalization, and mortality due to excessive alcohol consumption during the pandemic. A study from Canada found that the rates of monthly hospitalization due to alcoholic hepatitis increased from 11.6 before March 2020 to 22.1 cases per 10000 amid the pandemic, which indicates a nearly two-fold increase in the number of cases[180]. A similar increase rate was also observed in another study from England[181]. Two reports from the United States suggested a nearly 50% increase in the case admission due to alcoholic hepatitis during the pandemic[182,183]. In concordance with the increase in case hospitalization, we also observed a 20% rise in mortality rates due to alcoholic hepatitis[184]. Females and younger individuals are more likely to experience these increasing trends in hospitalization and mortality rates due to alcoholic liver disease[183,184]. Several strategies can be implemented to mitigate the detrimental effects of the COVID-19 pandemic on patients with alcohol use disorders. Developing a well-organized telemedicine system can help better identification of high-risk persons and provide consultation, health-related suggestions, and surveillance[179]. The screening programs can also be used for earlier identification of at-risk groups for alcohol use disorder. Alcohol Use Disorders Identification Test – Consumption and Single Alcohol Screening Question are two recommended screening tools for assessing alcohol use disorder and are suggested to be used for all persons over 18 during primary care evaluation[185,186].
Hepatitis B virus
In addition to alcoholic liver disease, HBV infection is another hepatic disease which is affected by the COVID-19 pandemic. Implementation of the strategies to mitigate the spread of the pandemic, including the lockdown, resulted in more difficult access to the healthcare facilities and, thus, reduced the willingness of many patients to seek medical services[187]. As a result, the number of HBV tests was reduced, and HBV diagnosis and treatment were impacted negatively amid the pandemic[188]. In addition, there are reports which indicate that the failure of the screening programs lowers the number of HBV vaccination and aggravates the preexisting health inequalities during the pandemic[188,189]. All these can contribute to a notable rise in the number of HBV cases. Similar to what we proposed to tackle the burden associated with the alcoholic liver disease during the pandemic, telemedicine and remote screening programs can be useful to resolve the pandemic-related disruptions in HBV infection management. Also, integrating the messaging system and contact tracing into the screening programs is suggested to increase the efficacy of screening; however, their effectiveness is yet to be determined by future studies[190].
CONCLUSION
Liver injury is a relatively common manifestation of COVID-19, mostly presenting with elevated liver enzymes. Different mechanisms for COVID-19-induced liver injury, including direct viral effect, cytokine storm, hepatic congestion, hypoxia, and reperfusion injury are proposed. Most cases of liver injury are mild and only require supportive treatment; however, there are some underlying liver diseases which might require special considerations. NAFLD and MAFLD were associated with the highest OR for severe COVID-19. Also, more severe disease is expected in patients with liver cirrhosis, especially cases with more advanced liver fibrosis. Despite the immunocompromised status in LT and AIH patients, they are not at a significantly higher risk of severe disease, and there is no need to empirically reduce or change the immunosuppressive drugs.
ACKNOWLEDGEMENTS
The authors would like to thank the Research Consultation Center of Shiraz University of Medical Science for their assistance in revising the manuscript.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 28, 2022
First decision: January 3, 2023
Article in press: March 20, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Melo FF, Brazil; Kumar R, India S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
Contributor Information
Romina Roshanshad, Student Research Committee, School of Medicine, Shiraz University of Medical Sciences, Shiraz 7184731443, Iran.
Amirhossein Roshanshad, Department of MPH, Shiraz University of Medical Sciences, Shiraz 7184731443, Iran. aroshanshad@gmail.com.
Reza Fereidooni, Health Policy Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz 7134814336, Iran.
Mahnaz Hosseini-Bensenjan, Hematology Research Center, Shiraz University of Medical Sciences, Shiraz 7134814336, Iran.
References
- 1.Nka AD, Ka'e AC, Bouba Y, Ngoufack Jagni Semengue E, Tommo Tchouaket MC, Takou D, Pabo W, Fainguem N, Sosso SM, Colizzi V, Perno CF, Fokam J. Global burden of SARS-CoV-2 infection, hospitalization and case fatality rate among COVID-19 vaccinated individuals and its associated factors: A systematic review and meta-analysis protocol. PLoS One. 2022;17:e0272839. doi: 10.1371/journal.pone.0272839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World health organization. WHO Coronavirus (COVID-19) Dashboard, 2023. [cited 8 January 2023]. In: World health organization [internet]. Available from: https://covid19.who.int/
- 3.Thakur V, Ratho RK, Kumar P, Bhatia SK, Bora I, Mohi GK, Saxena SK, Devi M, Yadav D, Mehariya S. Multi-Organ Involvement in COVID-19: Beyond Pulmonary Manifestations. J Clin Med. 2021;10 doi: 10.3390/jcm10030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyk W, Janik MK, Portincasa P, Milkiewicz P, Lammert F, Krawczyk M. COVID-19: Focus on the lungs but do not forget the gastrointestinal tract. Eur J Clin Invest. 2020;50:e13276. doi: 10.1111/eci.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pazgan-Simon M, Serafińska S, Kukla M, Kucharska M, Zuwała-Jagiełło J, Buczyńska I, Zielińska K, Simon K. Liver Injury in Patients with COVID-19 without Underlying Liver Disease. J Clin Med. 2022;11 doi: 10.3390/jcm11020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aleem A, Shah H. Gastrointestinal And Hepatic Manifestations Of Coronavirus (COVID-19). 2022 May 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan- [PubMed] [Google Scholar]
- 8.Krishnan A, Prichett L, Liu Y, Ting PS, Alqahtani SA, Kim AK, Ma M, Hamilton JP, Woreta TA, Chen PH. Risk of Severe Illness and Risk Factors of Outcomes of COVID-19 in Hospitalized Patients with Chronic Liver Disease in a Major U. S. Hospital Network. Can J Gastroenterol Hepatol. 2022;2022:8407990. doi: 10.1155/2022/8407990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612–620. doi: 10.1007/s12072-020-10078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali N, Hossain K. Liver injury in severe COVID-19 infection: current insights and challenges. Expert Rev Gastroenterol Hepatol. 2020;14:879–884. doi: 10.1080/17474124.2020.1794812. [DOI] [PubMed] [Google Scholar]
- 12.Fassan M, Mescoli C, Sbaraglia M, Guzzardo V, Russo FP, Fabris R, Trevenzoli M, Pelizzaro F, Cattelan AM, Basso C, Navalesi P, Farinati F, Vettor R, Dei Tos AP. Liver histopathology in COVID-19 patients: A mono-Institutional series of liver biopsies and autopsy specimens. Pathol Res Pract. 2021;221:153451. doi: 10.1016/j.prp.2021.153451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanner N, Andrieux G, Badia-I-Mompel P, Edler C, Pfefferle S, Lindenmeyer MT, Schmidt-Lauber C, Czogalla J, Wong MN, Okabayashi Y, Braun F, Lütgehetmann M, Meister E, Lu S, Noriega MLM, Günther T, Grundhoff A, Fischer N, Bräuninger H, Lindner D, Westermann D, Haas F, Roedl K, Kluge S, Addo MM, Huber S, Lohse AW, Reiser J, Ondruschka B, Sperhake JP, Saez-Rodriguez J, Boerries M, Hayek SS, Aepfelbacher M, Scaturro P, Puelles VG, Huber TB. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4:310–319. doi: 10.1038/s42255-022-00552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitiello A, La Porta R, D'Aiuto V, Ferrara F. The risks of liver injury in COVID-19 patients and pharmacological management to reduce or prevent the damage induced. Egypt Liver J. 2021;11:11. doi: 10.1186/s43066-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes E. Infection of liver hepatocytes with SARS-CoV-2. Nat Metab. 2022;4:301–302. doi: 10.1038/s42255-022-00554-4. [DOI] [PubMed] [Google Scholar]
- 18.Sivandzadeh GR, Askari H, Safarpour AR, Ejtehadi F, Raeis-Abdollahi E, Vaez Lari A, Abazari MF, Tarkesh F, Bagheri Lankarani K. COVID-19 infection and liver injury: Clinical features, biomarkers, potential mechanisms, treatment, and management challenges. World J Clin Cases. 2021;9:6178–6200. doi: 10.12998/wjcc.v9.i22.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian D, Ye Q. Hepatic complications of COVID-19 and its treatment. J Med Virol. 2020;92:1818–1824. doi: 10.1002/jmv.26036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taneva G, Dimitrov D, Velikova T. Liver dysfunction as a cytokine storm manifestation and prognostic factor for severe COVID-19. World J Hepatol. 2021;13:2005–2012. doi: 10.4254/wjh.v13.i12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanidziar D, Bittner EA. Hypotension, Systemic Inflammatory Response Syndrome, and COVID-19: A Clinical Conundrum. Anesth Analg. 2020;131:e175–e176. doi: 10.1213/ANE.0000000000005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciobanu AO, Gherasim L. Ischemic Hepatitis - Intercorrelated Pathology. Maedica (Bucur) 2018;13:5–11. [PMC free article] [PubMed] [Google Scholar]
- 23.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olwal CO, Nganyewo NN, Tapela K, Djomkam Zune AL, Owoicho O, Bediako Y, Duodu S. Parallels in Sepsis and COVID-19 Conditions: Implications for Managing Severe COVID-19. Front Immunol. 2021;12:602848. doi: 10.3389/fimmu.2021.602848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollenberg SM, Safi L, Parrillo JE, Fata M, Klinkhammer B, Gayed N, Glotzer T, Go RC, Gourna-Paleoudis E, Landers D, Jamal S, Shah N, Shah R, Tancredi J, Turi ZG. Hemodynamic Profiles of Shock in Patients With COVID-19. Am J Cardiol. 2021;153:135–139. doi: 10.1016/j.amjcard.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Liu Y, Cheng Z, Yu X, Li Y. COVID-19-associated liver injury: Clinical characteristics, pathophysiological mechanisms and treatment management. Biomed Pharmacother. 2022;154:113568. doi: 10.1016/j.biopha.2022.113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Ardes D, Boccatonda A, Cocco G, Fabiani S, Rossi I, Bucci M, Guagnano MT, Schiavone C, Cipollone F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J Gastroenterol. 2022;28:1102–1112. doi: 10.3748/wjg.v28.i11.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao CL, Rapkiewicz A, Maghsoodi-Deerwester M, Gupta M, Cao W, Palaia T, Zhou J, Ram B, Vo D, Rafiee B, Hossein-Zadeh Z, Dabiri B, Hanna I. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19) Hum Pathol. 2021;109:59–68. doi: 10.1016/j.humpath.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol. 2016;4:263–268. doi: 10.14218/JCTH.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gore RM, Levine MS. Liver in Cardiac Disease. High-Yield Imaging: Gastrointestinal. Elsevier Health Sciences. 2010:668–670. [Google Scholar]
- 32.Díaz LA, Idalsoaga F, Cannistra M, Candia R, Cabrera D, Barrera F, Soza A, Graham R, Riquelme A, Arrese M, Leise MD, Arab JP. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: A systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693–7706. doi: 10.3748/wjg.v26.i48.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kukla M, Skonieczna-Żydecka K, Kotfis K, Maciejewska D, Łoniewski I, Lara LF, Pazgan-Simon M, Stachowska E, Kaczmarczyk M, Koulaouzidis A, Marlicz W. COVID-19, MERS and SARS with Concomitant Liver Injury-Systematic Review of the Existing Literature. J Clin Med. 2020;9 doi: 10.3390/jcm9051420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Wang Y, Jiang R, Xue R, Yin X, Wu M, Meng Q. Ferroptosis in liver disease: new insights into disease mechanisms. Cell Death Discov. 2021;7:276. doi: 10.1038/s41420-021-00660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Serum Iron Level as a Potential Predictor of Coronavirus Disease 2019 Severity and Mortality: A Retrospective Study. Open Forum Infect Dis. 2020;7:ofaa250. doi: 10.1093/ofid/ofaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fratta Pasini AM, Stranieri C, Girelli D, Busti F, Cominacini L. Is Ferroptosis a Key Component of the Process Leading to Multiorgan Damage in COVID-19? Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10111677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M, Lai CL. SARS-CoV-2 infection: can ferroptosis be a potential treatment target for multiple organ involvement? Cell Death Discov. 2020;6:130. doi: 10.1038/s41420-020-00369-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Xu Y, Zhang K, Shen L, Deng M. Ferroptosis in COVID-19-related liver injury: A potential mechanism and therapeutic target. Front Cell Infect Microbiol. 2022;12:922511. doi: 10.3389/fcimb.2022.922511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sodeifian F, Seyedalhosseini ZS, Kian N, Eftekhari M, Najari S, Mirsaeidi M, Farsi Y, Nasiri MJ. Drug-Induced Liver Injury in COVID-19 Patients: A Systematic Review. Front Med (Lausanne) 2021;8:731436. doi: 10.3389/fmed.2021.731436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roshanshad A, Kamalipour A, Ashraf MA, Roshanshad R, Jafari S, Nazemi P, Akbari M. The efficacy of remdesivir in coronavirus disease 2019 (COVID-19): a systematic review. Iran J Microbiol. 2020;12:376–387. doi: 10.18502/ijm.v12i5.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Medicines Agency. Summary on compassionate use: Remdesivir Gilead. 2020 Apr 3. [cited 3 Sep 2022]. In: European Medicines Agency [internet]. Available from: https://www.ema.europa.eu/en/news/ema-provides-recommendations-compassionate-use-remdesivir-covid-19 .
- 43.Rahimi MM, Jahantabi E, Lotfi B, Forouzesh M, Valizadeh R, Farshid S. Renal and liver injury following the treatment of COVID-19 by remdesivir. J Nephropathol. 2021;10:1–4. [Google Scholar]
- 44.Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, De Rosa R, Corcione A, Durante-Mangoni E. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14:881–883. doi: 10.1007/s12072-020-10077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Laar SA, de Boer MGJ, Gombert-Handoko KB, Guchelaar HJ, Zwaveling J LUMC-Covid-19 research group. Liver and kidney function in patients with Covid-19 treated with remdesivir. Br J Clin Pharmacol. 2021;87:4450–4454. doi: 10.1111/bcp.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aleem A, Mahadevaiah G, Shariff N, Kothadia JP. Hepatic manifestations of COVID-19 and effect of remdesivir on liver function in patients with COVID-19 illness. Proc (Bayl Univ Med Cent) 2021;34:473–477. doi: 10.1080/08998280.2021.1885289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar P, Kulkarni A, Sharma M, Rao PN, Reddy DN. Favipiravir-induced Liver Injury in Patients with Coronavirus Disease 2019. J Clin Transl Hepatol. 2021;9:276–278. doi: 10.14218/JCTH.2021.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamazaki S, Suzuki T, Sayama M, Nakada TA, Igari H, Ishii I. Suspected cholestatic liver injury induced by favipiravir in a patient with COVID-19. J Infect Chemother. 2021;27:390–392. doi: 10.1016/j.jiac.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prakash A, Singh H, Kaur H, Semwal A, Sarma P, Bhattacharyya A, Dhibar DP, Medhi B. Systematic review and meta-analysis of effectiveness and safety of favipiravir in the management of novel coronavirus (COVID-19) patients. Indian J Pharmacol. 2020;52:414–421. doi: 10.4103/ijp.ijp_998_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020;43:615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733–742. doi: 10.1136/gutjnl-2020-321726. [DOI] [PubMed] [Google Scholar]
- 53.Gabrielli M, Franza L, Esperide A, Gasparrini I, Gasbarrini A, Franceschi F, On Behalf Of Gemelli Against Covid. Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu PF, Xie WF. Corticosteroid therapy in drug-induced liver injury: Pros and cons. J Dig Dis. 2019;20:122–126. doi: 10.1111/1751-2980.12697. [DOI] [PubMed] [Google Scholar]
- 55.Muhović D, Bojović J, Bulatović A, Vukčević B, Ratković M, Lazović R, Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40:1901–1905. doi: 10.1111/liv.14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509–519. doi: 10.1177/2050640620924157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdel Galil SM. Hydroxychloroquine-induced toxic hepatitis in a patient with systemic lupus erythematosus: a case report. Lupus. 2015;24:638–640. doi: 10.1177/0961203314561667. [DOI] [PubMed] [Google Scholar]
- 59.Makin AJ, Wendon J, Fitt S, Portmann BC, Williams R. Fulminant hepatic failure secondary to hydroxychloroquine. Gut. 1994;35:569–570. doi: 10.1136/gut.35.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falcão MB, Pamplona de Góes Cavalcanti L, Filgueiras Filho NM, Antunes de Brito CA. Case Report: Hepatotoxicity Associated with the Use of Hydroxychloroquine in a Patient with COVID-19. Am J Trop Med Hyg. 2020;102:1214–1216. doi: 10.4269/ajtmh.20-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, Grau S. Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti Infect Ther. 2021;19:147–163. doi: 10.1080/14787210.2020.1813024. [DOI] [PubMed] [Google Scholar]
- 62.Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, Gu J, Hoofnagle JH, Chalasani N. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13:369–376.e3. doi: 10.1016/j.cgh.2014.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brito CA, Barros FM, Lopes EP. Mechanisms and consequences of COVID-19 associated liver injury: What can we affirm? World J Hepatol. 2020;12:413–422. doi: 10.4254/wjh.v12.i8.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veit O, Beck B, Steuerwald M, Hatz C. First case of ivermectin-induced severe hepatitis. Trans R Soc Trop Med Hyg. 2006;100:795–797. doi: 10.1016/j.trstmh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–548, vi. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Elrobaa IH, New KJ. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front Public Health. 2021;9:711616. doi: 10.3389/fpubh.2021.711616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roshanshad A, Ashraf MA, Roshanshad R, Kharmandar A, Zomorodian SA, Ashraf H. Ocular Manifestations of Patients with Coronavirus Disease 2019: A Comprehensive Review. J Ophthalmic Vis Res. 2021;16:234–247. doi: 10.18502/jovr.v16i2.9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc. 2020;83:521–523. doi: 10.1097/JCMA.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990–995. doi: 10.1097/MEG.0000000000001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, Zhiwei L, Adhikari VP, Liang T. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807–809. doi: 10.1136/gutjnl-2020-322072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai Q, Xu L, Chen J. Reply to: "Liver tests abnormalities in COVID-19: trick or treat? J Hepatol. 2020;73:1277–1278. doi: 10.1016/j.jhep.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen F, Chen W, Chen J, Xu D, Xie W, Wang X, Xie Y. Clinical features and risk factors of COVID-19-associated liver injury and function: A retrospective analysis of 830 cases. Ann Hepatol. 2021;21:100267. doi: 10.1016/j.aohep.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, Suzuki A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology. 2019;70:1457–1469. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kezer CA, Simonetto DA, Shah VH. Sex Differences in Alcohol Consumption and Alcohol-Associated Liver Disease. Mayo Clin Proc. 2021;96:1006–1016. doi: 10.1016/j.mayocp.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 75.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weber S, Hellmuth JC, Scherer C, Muenchhoff M, Mayerle J, Gerbes AL. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: a prospective cohort study. Gut. 2021;70:1925–1932. doi: 10.1136/gutjnl-2020-323800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soetedjo NNM, Iryaningrum MR, Damara FA, Permadhi I, Sutanto LB, Hartono H, Rasyid H. Prognostic properties of hypoalbuminemia in COVID-19 patients: A systematic review and diagnostic meta-analysis. Clin Nutr ESPEN. 2021;45:120–126. doi: 10.1016/j.clnesp.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lei P, Zhang L, Han P, Zheng C, Tong Q, Shang H, Yang F, Hu Y, Li X, Song Y. Liver injury in patients with COVID-19: clinical profiles, CT findings, the correlation of the severity with liver injury. Hepatol Int. 2020;14:733–742. doi: 10.1007/s12072-020-10087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gul Y, Kilicarslan G, Cilengir AH, Balaban M, Gul E. The Relationship of Liver and Pancreas Density With Chest Computed Tomography Score Progression and Laboratory Findings in Patients With COVID-19. J Comput Assist Tomogr. 2022;46:848–853. doi: 10.1097/RCT.0000000000001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uchida Y, Uemura H, Yamaba S, Hamada D, Tarumoto N, Maesaki S, Mochida S. Significance of liver dysfunction associated with decreased hepatic CT attenuation values in Japanese patients with severe COVID-19. J Gastroenterol. 2020;55:1098–1106. doi: 10.1007/s00535-020-01717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdelmohsen MA, Alkandari BM, Gupta VK, ElBeheiry AA. Diagnostic value of abdominal sonography in confirmed COVID-19 intensive care patients. Egypt J Radiol Nucl Med. 2020;51:198. [Google Scholar]
- 82.Spogis J, Hagen F, Thaiss WM, Hoffmann T, Malek N, Nikolaou K, Berg CP, Singer S, Bösmüller H, Kreth F, Kaufmann S. Sonographic findings in coronavirus disease-19 associated liver damage. PLoS One. 2021;16:e0244781. doi: 10.1371/journal.pone.0244781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong CKH, Mak LY, Au ICH, Lai FTT, Li X, Wan EYF, Chui CSL, Chan EWY, Cheng WY, Cheng FWT, Yuen MF, Wong ICK. Risk of acute liver injury following the mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines. J Hepatol. 2022;77:1339–1348. doi: 10.1016/j.jhep.2022.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mann R, Sekhon S. Drug-Induced Liver Injury After COVID-19 Vaccine. Cureus. 2021;13:e16491. doi: 10.7759/cureus.16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hines A, Shen JG, Olazagasti C, Shams S. Immune thrombocytopenic purpura and acute liver injury after COVID-19 vaccine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shroff H, Satapathy SK, Crawford JM, Todd NJ, VanWagner LB. Liver injury following SARS-CoV-2 vaccination: A multicenter case series. J Hepatol. 2022;76:211–214. doi: 10.1016/j.jhep.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lodato F, Larocca A, D'Errico A, Cennamo V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: Coincidence, autoimmunity or drug-related liver injury. J Hepatol. 2021;75:1254–1256. doi: 10.1016/j.jhep.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Efe C, Kulkarni AV, Terziroli Beretta-Piccoli B, Magro B, Stättermayer A, Cengiz M, Clayton-Chubb D, Lammert C, Bernsmeier C, Gül Ö, la Tijera FH, Anders M, Lytvyak E, Akın M, Purnak T, Liberal R, Peralta M, Ebik B, Duman S, Demir N, Balaban Y, Urzua Á, Contreras F, Venturelli MG, Bilgiç Y, Medina A, Girala M, Günşar F, Londoño MC, Androutsakos T, Kisch A, Yurci A, Güzelbulut F, Çağın YF, Avcı E, Akyıldız M, Dindar-Demiray EK, Harputluoğlu M, Kumar R, Satapathy SK, Mendizabal M, Silva M, Fagiuoli S, Roberts SK, Soylu NK, Idilman R, Yoshida EM, Montano-Loza AJ, Dalekos GN, Ridruejo E, Schiano TD, Wahlin S. Liver injury after SARS-CoV-2 vaccination: Features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022;76:1576–1586. doi: 10.1002/hep.32572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roy A, Verma N, Singh S, Pradhan P, Taneja S, Singh M. Immune-mediated liver injury following COVID-19 vaccination: A systematic review. Hepatol Commun. 2022;6:2513–2522. doi: 10.1002/hep4.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mekritthikrai K, Jaru-Ampornpan P, Komolmit P, Thanapirom K. Autoimmune Hepatitis Triggered by COVID-19 Vaccine: The First Case From Inactivated Vaccine. ACG Case Rep J. 2022;9:e00811. doi: 10.14309/crj.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS. COVID-19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta-Analysis. Prev Chronic Dis. 2022;19:E53. doi: 10.5888/pcd19.210228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, Baldelli L, Kennedy J, Mercer C, Ozga AK, Casar C, Schramm C contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis, Moon AM, Webb GJ, Lohse AW. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liberal R, Grant CR, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126–139. doi: 10.1016/j.jaut.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 95.Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 96.Kabaçam G, Wahlin S, Efe C. Autoimmune hepatitis triggered by COVID-19: A report of two cases. Liver Int. 2021;41:2527–2528. doi: 10.1111/liv.15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gerussi A, Rigamonti C, Elia C, Cazzagon N, Floreani A, Pozzi R, Pozzoni P, Claar E, Pasulo L, Fagiuoli S, Cristoferi L, Carbone M, Invernizzi P. Coronavirus Disease 2019 in Autoimmune Hepatitis: A Lesson From Immunosuppressed Patients. Hepatol Commun. 2020;4:1257–1262. doi: 10.1002/hep4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lleo A, Cazzagon N, Rigamonti C, Cabibbo G, Lai Q, Muratori L, Carbone M Italian Association for the Study of the Liver. Clinical update on risks and efficacy of anti-SARS-CoV-2 vaccines in patients with autoimmune hepatitis and summary of reports on post-vaccination liver injury. Dig Liver Dis. 2022;54:722–726. doi: 10.1016/j.dld.2022.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nur Dagli S, Efe C. Coronavirus disease 2019 (COVID-19) in autoimmune hepatitis. Hepatol Forum. 2022;3:68–70. doi: 10.14744/hf.2022.2022.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, Mo P, Yao L, Yang R, Gao S, Gui X, Hou W, Xiong Y, Li J, Zhang Y. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol Sin. 2020;35:842–845. doi: 10.1007/s12250-020-00276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Li C, Wang J, Zhu C, Zhu L, Ji F, Liu L, Xu T, Zhang B, Xue L, Yan X, Huang R, Wu C. A case series of COVID-19 patients with chronic hepatitis B virus infection. J Med Virol. 2020;92:2785–2791. doi: 10.1002/jmv.26201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alqahtani SA, Buti M. COVID-19 and hepatitis B infection. Antivir Ther. 2020;25:389–397. doi: 10.3851/IMP3382. [DOI] [PubMed] [Google Scholar]
- 105.Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, Cheng J. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504–1507. doi: 10.1111/jvh.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J, Wang T, Cai Q, Sun L, Huang D, Zhou G, He Q, Wang FS, Liu L, Chen J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol Res. 2020;50:1211–1221. doi: 10.1111/hepr.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choe JW, Jung YK, Yim HJ, Seo GH. Clinical Effect of Hepatitis B Virus on COVID-19 Infected Patients: A Nationwide Population-Based Study Using the Health Insurance Review & Assessment Service Database. J Korean Med Sci. 2022;37:e29. doi: 10.3346/jkms.2022.37.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xue H, Li J, Xie H, Wang Y. Review of Drug Repositioning Approaches and Resources. Int J Biol Sci. 2018;14:1232–1244. doi: 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beraldo RF, Marcondes MB, Dos Santos MNM, Grillo TG, Pires GBT, de Oliveira CV. COVID-19 in a Patient with Liver Cirrhosis. Am J Case Rep. 2021;22:e929948. doi: 10.12659/AJCR.929948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19:112–134. doi: 10.1038/s41575-021-00520-7. [DOI] [PubMed] [Google Scholar]
- 114.Belli LS, Duvoux C, Cortesi PA, Facchetti R, Iacob S, Perricone G, Radenne S, Conti S, Patrono D, Berlakovich G, Hann A, Pasulo L, Castells L, Faitot F, Detry O, Invernizzi F, Magini G, De Simone P, Kounis I, Morelli MC, Díaz Fontenla F, Ericzon BG, Loinaz C, Johnston C, Gheorghe L, Lesurtel M, Romagnoli R, Kollmann D, Perera MTP, Fagiuoli S, Mirza D, Coilly A, Toso C, Zieniewicz K, Elkrief L, Karam V, Adam R, den Hoed C, Merli M, Puoti M, De Carlis L, Oniscu GC, Piano S, Angeli P, Fondevila C, Polak WG for all the centres contributing to the ELITA-ELTR COVID-19 Registry. COVID-19 in liver transplant candidates: pretransplant and post-transplant outcomes - an ELITA/ELTR multicentre cohort study. Gut. 2021;70:1914–1924. doi: 10.1136/gutjnl-2021-324879. [DOI] [PubMed] [Google Scholar]
- 115.Kaya Y, Gülcü O, Aksakal E, Kalkan K, Aydın SŞ, Kaya A, Bostan S. A significant predictor of in-hospital and long-term mortality and progression in COVID-19 patients: The end-stage liver disease (MELD) score model. J Med Virol. 2023;95:e28109. doi: 10.1002/jmv.28109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ge J, Pletcher MJ, Lai JC N3C Consortium. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487–1501.e5. doi: 10.1053/j.gastro.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ioannou GN, Liang PS, Locke E, Green P, Berry K, O'Hare AM, Shah JA, Crothers K, Eastment MC, Fan VS, Dominitz JA. Cirrhosis and Severe Acute Respiratory Syndrome Coronavirus 2 Infection in US Veterans: Risk of Infection, Hospitalization, Ventilation, and Mortality. Hepatology. 2021;74:322–335. doi: 10.1002/hep.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jeon D, Son M, Choi J. Impact of liver cirrhosis on the clinical outcomes of patients with COVID-19: a nationwide cohort study of Korea. Korean J Intern Med. 2021;36:1092–1101. doi: 10.3904/kjim.2020.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luo D, Chen X, Du J, Mei B, Wang A, Kuang F, Fang C, Gan Y, Peng F, Yang X, Dahmen U, Li B, Song S. Immunogenicity of COVID-19 vaccines in chronic liver disease patients and liver transplant recipients: A systematic review and meta-analysis. Liver Int. 2023;43:34–48. doi: 10.1111/liv.15403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Claeys E, Vermeire K. Immunosuppressive drugs in organ transplantation to prevent allograft rejection: Mode of action and side effects. J Immunological Sci. 2019;3:14–21. [Google Scholar]
- 122.Fraser J, Mousley J, Testro A, Smibert OC, Koshy AN. Clinical Presentation, Treatment, and Mortality Rate in Liver Transplant Recipients With Coronavirus Disease 2019: A Systematic Review and Quantitative Analysis. Transplant Proc. 2020;52:2676–2683. doi: 10.1016/j.transproceed.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alfishawy M, Elbendary A, Mohamed M, Nassar M. COVID-19 Mortality in Transplant Recipients. Int J Organ Transplant Med. 2020;11:145–162. [PMC free article] [PubMed] [Google Scholar]
- 124.Becchetti C, Gschwend SG, Dufour JF, Banz V. COVID-19 in Liver Transplant Recipients: A Systematic Review. J Clin Med. 2021;10 doi: 10.3390/jcm10174015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kullar R, Patel AP, Saab S. COVID-19 in Liver Transplant Recipients. J Clin Transl Hepatol. 2021;9:545–550. doi: 10.14218/JCTH.2020.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anness A, Siddiqui F. COVID-19 complicated by hepatic dysfunction in a 28-week pregnant woman. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yadav DK, Adhikari VP, Ling Q, Liang T. Immunosuppressants in Liver Transplant Recipients With Coronavirus Disease 2019: Capability or Catastrophe? Front Med (Lausanne) 2021;8:756922. doi: 10.3389/fmed.2021.756922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kulkarni AV, Tevethia HV, Premkumar M, Arab JP, Candia R, Kumar K, Kumar P, Sharma M, Rao PN, Reddy DN. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guarino M, Cossiga V, Loperto I, Esposito I, Ortolani R, Fiorentino A, Pontillo G, De Coppi L, Cozza V, Galeota Lanza A, Di Costanzo GG, Picciotto FP, Morisco F. COVID-19 in liver transplant recipients: incidence, hospitalization and outcome in an Italian prospective double-centre study. Sci Rep. 2022;12:4831. doi: 10.1038/s41598-022-08947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, Coilly A, Ericzon BG, Loinaz C, Cuervas-Mons V, Zambelli M, Llado L, Diaz-Fontenla F, Invernizzi F, Patrono D, Faitot F, Bhooori S, Pirenne J, Perricone G, Magini G, Castells L, Detry O, Cruchaga PM, Colmenero J, Berrevoet F, Rodriguez G, Ysebaert D, Radenne S, Metselaar H, Morelli C, De Carlis LG, Polak WG, Duvoux C ELITA-ELTR COVID-19 Registry. Protective Role of Tacrolimus, Deleterious Role of Age and Comorbidities in Liver Transplant Recipients With Covid-19: Results From the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021;160:1151–1163.e3. doi: 10.1053/j.gastro.2020.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Choudhary NS, Dhampalwar S, Saraf N, Soin AS. Outcomes of COVID-19 in Patients with Cirrhosis or Liver Transplantation. J Clin Exp Hepatol. 2021;11:713–719. doi: 10.1016/j.jceh.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rodriguez-Peralvarez M, Salcedo M, Colmenero J, Pons JA. Modulating immunosuppression in liver transplant patients with COVID-19. Gut. 2021;70:1412–1414. doi: 10.1136/gutjnl-2020-322620. [DOI] [PubMed] [Google Scholar]
- 135.Meunier L, Malezieux E, Ursic Bedoya J, Faure S, Echenne M, Debourdeau A, Meszaros M, Pageaux GP. Mycophenolate mofetil discontinuation increases severe acute respiratory syndrome coronavirus 2 vaccine response in nonresponder liver transplantation recipients: A proof of concept. Liver Transpl. 2023;29:114–117. doi: 10.1002/lt.26569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zecca E, Rizzi M, Tonello S, Matino E, Costanzo M, Rizzi E, Casciaro GF, Manfredi GF, Acquaviva A, Gagliardi I, Calzaducca E, Mallela VR, D'Onghia D, Minisini R, Bellan M, Castello LM, Gavelli F, Avanzi GC, Patrucco F, Chiocchetti A, Pirisi M, Rigamonti C, Lilleri D, Sola D, Sainaghi PP. Ongoing Mycophenolate Treatment Impairs Anti-SARS-CoV-2 Vaccination Response in Patients Affected by Chronic Inflammatory Autoimmune Diseases or Liver Transplantation Recipients: Results of the RIVALSA Prospective Cohort. Viruses. 2022;14 doi: 10.3390/v14081766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sripongpun P, Pinpathomrat N, Bruminhent J, Kaewdech A. Coronavirus Disease 2019 Vaccinations in Patients With Chronic Liver Disease and Liver Transplant Recipients: An Update. Front Med (Lausanne) 2022;9:924454. doi: 10.3389/fmed.2022.924454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kleiboeker HL, Jorgenson MR, Smith JA. Myalgia in liver transplant recipients after receiving tixagevimab/cilgavimab for pre-exposure prophylaxis of COVID-19: A case series. Transpl Infect Dis. 2022;24:e13932. doi: 10.1111/tid.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Di Maira T, Berenguer M. COVID-19 and liver transplantation. Nat Rev Gastroenterol Hepatol. 2020;17:526–528. doi: 10.1038/s41575-020-0347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nacif LS, Fernandes MR, Waisberg DR, Pinheiro RS, Rocha-Santos V, Galvão F, Andraus W, Carneiro-D'Albuquerque L. Liver transplant after SARS-CoV-2 infection: A systematic review. Clinics (Sao Paulo) 2022;77:100042. doi: 10.1016/j.clinsp.2022.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nath P, Kumar R, Mallick B, Das S, Anand A, Panigrahi SC, Duseja A, Acharya SK, Chawla YK, Praharaj DL. Effect of Nonalcoholic Fatty Liver Disease (NAFLD) on COVID-19: A Single-Center Study of 3983 Patients With Review of Literature. Cureus. 2022;14:e26683. doi: 10.7759/cureus.26683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 143.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 144.Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2021;33:1578–1581. doi: 10.1097/MEG.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, Hosein Z, Padda I, Mangat J, Altaf M. Comorbidity and its Impact on Patients with COVID-19. SN Compr Clin Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 147.Madan K, Rastogi R, Bhargava R, Dagar V, Singla V, Sahu A, Singh P, Garg P, Aggarwal B, Singh RK. Is Fatty Liver Associated with Increased Mortality and Morbidity in Coronavirus Disease 2019 (COVID-19) Pneumonia? J Clin Exp Hepatol. 2022;12:1320–1327. doi: 10.1016/j.jceh.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Singh A, Hussain S, Antony B. Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: A comprehensive systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15:813–822. doi: 10.1016/j.dsx.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What's in a name? Liver Int. 2020;40:1254–1261. doi: 10.1111/liv.14478. [DOI] [PubMed] [Google Scholar]
- 150.Hayat U, Ashfaq MZ, Johnson L, Ford R, Wuthnow C, Kadado K, El Jurdi K, Okut H, Kilgore WR, Assi M, Siddiqui AA. The Association of Metabolic-Associated Fatty Liver Disease with Clinical Outcomes of COVID-19: A Systematic Review and Meta-Analysis. Kans J Med. 2022;15:241–246. doi: 10.17161/kjm.vol15.16522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gao F, Zheng KI, Yan HD, Sun QF, Pan KH, Wang TY, Chen YP, Targher G, Byrne CD, George J, Zheng MH. Association and Interaction Between Serum Interleukin-6 Levels and Metabolic Dysfunction-Associated Fatty Liver Disease in Patients With Severe Coronavirus Disease 2019. Front Endocrinol (Lausanne) 2021;12:604100. doi: 10.3389/fendo.2021.604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maurya R, Sebastian P, Namdeo M, Devender M, Gertler A. COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality. Front Immunol. 2021;12:649359. doi: 10.3389/fimmu.2021.649359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu M, Mei K, Tan Z, Huang S, Liu F, Deng C, Ma J, Yu P, Liu X. Liver Fibrosis Scores and Hospitalization, Mechanical Ventilation, Severity, and Death in Patients with COVID-19: A Systematic Review and Dose-Response Meta-Analysis. Can J Gastroenterol Hepatol. 2022;2022:7235860. doi: 10.1155/2022/7235860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eren F, Kaya E, Yilmaz Y. Accuracy of Fibrosis-4 index and non-alcoholic fatty liver disease fibrosis scores in metabolic (dysfunction) associated fatty liver disease according to body mass index: failure in the prediction of advanced fibrosis in lean and morbidly obese individuals. Eur J Gastroenterol Hepatol. 2022;34:98–103. doi: 10.1097/MEG.0000000000001946. [DOI] [PubMed] [Google Scholar]
- 155.McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Can E, Oğlak SC, Ölmez F. Abnormal liver function tests in pregnant patients with COVID-19 - a retrospective cohort study in a tertiary center. Ginekol Pol. 2022 doi: 10.5603/GP.a2021.0182. [DOI] [PubMed] [Google Scholar]
- 157.Deng G, Zeng F, Zhang L, Chen H, Chen X, Yin M. Characteristics of pregnant patients with COVID-19 and liver injury. J Hepatol. 2020;73:989–991. doi: 10.1016/j.jhep.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Choudhary A, Singh V, Bharadwaj M, Barik A. Pregnancy With SARS-CoV-2 Infection Complicated by Preeclampsia and Acute Fatty Liver of Pregnancy. Cureus. 2021;13:e15645. doi: 10.7759/cureus.15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ronnje L, Länsberg JK, Vikhareva O, Hansson SR, Herbst A, Zaigham M. Complicated COVID-19 in pregnancy: a case report with severe liver and coagulation dysfunction promptly improved by delivery. BMC Pregnancy Childbirth. 2020;20:511. doi: 10.1186/s12884-020-03172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Denızlı R, Sakcak B, Farisoğulları N, Peker MEM, Sınacı S, Kara Ö, Tanacan A, Tekın ÖM, Şahın D. The İmpact of Elevated Liver Enzymes and İntrahepatic Cholestasis of Pregnancy on the Course of COVID-19 in Pregnant Women. SN Compr Clin Med. 2022;4:184. doi: 10.1007/s42399-022-01267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang L, Yang N, Yang J, Zhao S, Su C. A Review: The Manifestations, Mechanisms, and Treatments of Musculoskeletal Pain in Patients With COVID-19. Front Pain Res (Lausanne) 2022;3:826160. doi: 10.3389/fpain.2022.826160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shah S, Banh ET, Koury K, Bhatia G, Nandi R, Gulur P. Pain Management in Pregnancy: Multimodal Approaches. Pain Res Treat. 2015;2015:987483. doi: 10.1155/2015/987483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Di Giorgio A, Hartleif S, Warner S, Kelly D. COVID-19 in Children With Liver Disease. Front Pediatr. 2021;9:616381. doi: 10.3389/fped.2021.616381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang D, Ju XL, Xie F, Lu Y, Li FY, Huang HH, Fang XL, Li YJ, Wang JY, Yi B, Yue JX, Wang J, Wang LX, Li B, Wang Y, Qiu BP, Zhou ZY, Li KL, Sun JH, Liu XG, Li GD, Wang YJ, Cao AH, Chen YN. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. 2020;58:269–274. doi: 10.3760/cma.j.cn112140-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 166.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 167.Perez A, Cantor A, Rudolph B, Miller J, Kogan-Liberman D, Gao Q, Da Silva B, Margolis KG, Ovchinsky N, Martinez M. Liver involvement in children with SARS-COV-2 infection: Two distinct clinical phenotypes caused by the same virus. Liver Int. 2021;41:2068–2075. doi: 10.1111/liv.14887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Di Giorgio A, Nicastro E, Arnaboldi S, Montini O, Di Stasio F, D'Antiga L, Gaio P, Fovino LN, Cananzi M, Pinon M, Calvo PL, Camelli V. "Health status of children with chronic liver disease during the SARS-CoV-2 outbreak: results from a multicentre study". Clin Res Hepatol Gastroenterol. 2021;45:101610. doi: 10.1016/j.clinre.2020.101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stolfi I, Conti MG, Marciano A, Dito L, Natale F, Bartolucci M, Cellitti R, Regoli D, Ticchiarelli A, Pangallo I, Pagano F, Ajassa C, Brunelli R, Terrin G. Liver Involvement in SARS-CoV-2 Vertically Infected Newborn: A Case Report. Front Pediatr. 2021;9:701722. doi: 10.3389/fped.2021.701722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kaur M, Tiwari D, Sidana V, Mukhopadhyay K. Remdesivir-Induced Liver Injury in a COVID-Positive Newborn. Indian J Pediatr. 2022;89:826. doi: 10.1007/s12098-022-04237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee BP, Dodge JL, Leventhal A, Terrault NA. Retail Alcohol and Tobacco Sales During COVID-19. Ann Intern Med. 2021;174:1027–1029. doi: 10.7326/M20-7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ahmed MZ, Ahmed O, Aibao Z, Hanbin S, Siyu L, Ahmad A. Epidemic of COVID-19 in China and associated Psychological Problems. Asian J Psychiatr. 2020;51:102092. doi: 10.1016/j.ajp.2020.102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jackson SE, Garnett C, Shahab L, Oldham M, Brown J. Association of the COVID-19 lockdown with smoking, drinking and attempts to quit in England: an analysis of 2019-20 data. Addiction. 2021;116:1233–1244. doi: 10.1111/add.15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pfefferbaum B, North CS. Mental Health and the Covid-19 Pandemic. N Engl J Med. 2020;383:510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 175.Keyes KM, Hatzenbuehler ML, Hasin DS. Stressful life experiences, alcohol consumption, and alcohol use disorders: the epidemiologic evidence for four main types of stressors. Psychopharmacology (Berl) 2011;218:1–17. doi: 10.1007/s00213-011-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.de Goeij MC, Suhrcke M, Toffolutti V, van de Mheen D, Schoenmakers TM, Kunst AE. How economic crises affect alcohol consumption and alcohol-related health problems: a realist systematic review. Soc Sci Med. 2015;131:131–146. doi: 10.1016/j.socscimed.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 177.Carson J, Gunda A, Qasim K, Allen R, Bradley M, Prescott J. Losing a Loved One During the Covid-19 Pandemic: An On-Line Survey Looking at the Effects on Traumatic Stress, Coping and Post-Traumatic Growth. Omega (Westport) 2021:302228211049683. doi: 10.1177/00302228211049683. [DOI] [PubMed] [Google Scholar]
- 178.Le TM, Wang W, Zhornitsky S, Dhingra I, Chen Y, Zhang S, Li CR. The Neural Processes Interlinking Social Isolation, Social Support, and Problem Alcohol Use. Int J Neuropsychopharmacol. 2021;24:333–343. doi: 10.1093/ijnp/pyaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deutsch-Link S, Curtis B, Singal AK. Covid-19 and alcohol associated liver disease. Dig Liver Dis. 2022;54:1459–1468. doi: 10.1016/j.dld.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shaheen AA, Kong K, Ma C, Doktorchik C, Coffin CS, Swain MG, Burak KW, Congly SE, Lee SS, Sadler M, Borman M, Abraldes JG. Impact of the COVID-19 Pandemic on Hospitalizations for Alcoholic Hepatitis or Cirrhosis in Alberta, Canada. Clin Gastroenterol Hepatol. 2022;20:e1170–e1179. doi: 10.1016/j.cgh.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cargill Z, Kattiparambil S, Hansi N, Barnabas A, Shawcross DL, Williams R, Agarwal K. Severe alcohol-related liver disease admissions post-COVID-19 lockdown: canary in the coal mine? Frontline Gastroenterol. 2021;12:354–355. doi: 10.1136/flgastro-2020-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gonzalez HC, Zhou Y, Nimri FM, Rupp LB, Trudeau S, Gordon SC. Alcohol-related hepatitis admissions increased 50% in the first months of the COVID-19 pandemic in the USA. Liver Int. 2022;42:762–764. doi: 10.1111/liv.15172. [DOI] [PubMed] [Google Scholar]
- 183.Sohal A, Khalid S, Green V, Gulati A, Roytman M. The Pandemic Within the Pandemic: Unprecedented Rise in Alcohol-related Hepatitis During the COVID-19 Pandemic. J Clin Gastroenterol. 2022;56:e171–e175. doi: 10.1097/MCG.0000000000001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Deutsch-Link S, Jiang Y, Peery AF, Barritt AS, Bataller R, Moon AM. Alcohol-Associated Liver Disease Mortality Increased From 2017 to 2020 and Accelerated During the COVID-19 Pandemic. Clin Gastroenterol Hepatol. 2022;20:2142–2144.e2. doi: 10.1016/j.cgh.2022.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vickers Smith R, Kranzler HR, Justice AC, Tate JP VA Million Veteran Program. Longitudinal Drinking Patterns and Their Clinical Correlates in Million Veteran Program Participants. Alcohol Clin Exp Res. 2019;43:465–472. doi: 10.1111/acer.13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899–1909. doi: 10.1001/jama.2018.16789. [DOI] [PubMed] [Google Scholar]
- 187.Sonneveld MJ, Veldhuijzen IK, van de Laar TJW, Op de Coul ELM, van der Meer AJ. Decrease in viral hepatitis diagnoses during the COVID-19 pandemic in the Netherlands. J Hepatol. 2022;77:896–897. doi: 10.1016/j.jhep.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pley CM, McNaughton AL, Matthews PC, Lourenço J. The global impact of the COVID-19 pandemic on the prevention, diagnosis and treatment of hepatitis B virus (HBV) infection. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2020-004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xiridou M, Adam P, Meiberg A, Visser M, Matser A, de Wit J, Op de Coul E. The impact of the COVID-19 pandemic on hepatitis B virus vaccination and transmission among men who have sex with men: A mathematical modelling study. Vaccine. 2022;40:4889–4896. doi: 10.1016/j.vaccine.2022.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ha YP, Sun Y, Wilkinson J, Wang S, Chien L, Wu M, Wang E, Freeland C. Implementation and outcomes of a remote hepatitis B screening program designed to overcome COVID-19 pandemic-related disruptions to community-based screenings for Asians in Greater Philadelphia: A descriptive study. Health Sci Rep. 2022;5:e761. doi: 10.1002/hsr2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hariyanto TI, Jodhinata C, Halim DA, Kurniawan A. Association between viral hepatitis and increased risk of severe coronavirus disease 2019 (COVID-19) outcome: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2022;15:9–14. [PMC free article] [PubMed] [Google Scholar]
- 192.Tao Z, Li Y, Cheng B, Zhou T, Gao Y. Risk of Severe COVID-19 Increased by Metabolic Dysfunction-associated Fatty Liver Disease: A Meta-analysis. J Clin Gastroenterol. 2021;55:830–835. doi: 10.1097/MCG.0000000000001605. [DOI] [PMC free article] [PubMed] [Google Scholar]