Abstract
This communication describes a variety of nucleophilic ring openings of N-arylated oxathiazinane heterocycles. We find that this reaction is compatible with phenoxides, naphthoxides, and thiolates and allows for the rapid assembly of N-aryl-amino ethers and N-aryl-amino thioethers. Fourteen examples are shown and a mechanistic pathway is hypothesized.
Keywords: Nucleophile, Sulfamate, Oxathiazinane, Aza-Wacker
Introduction
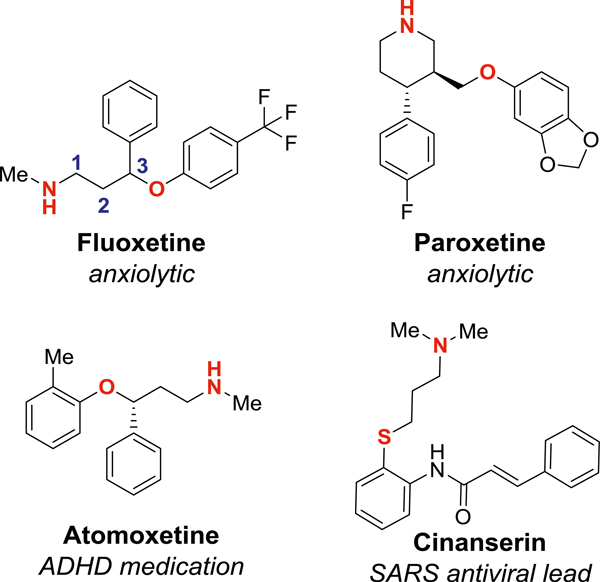
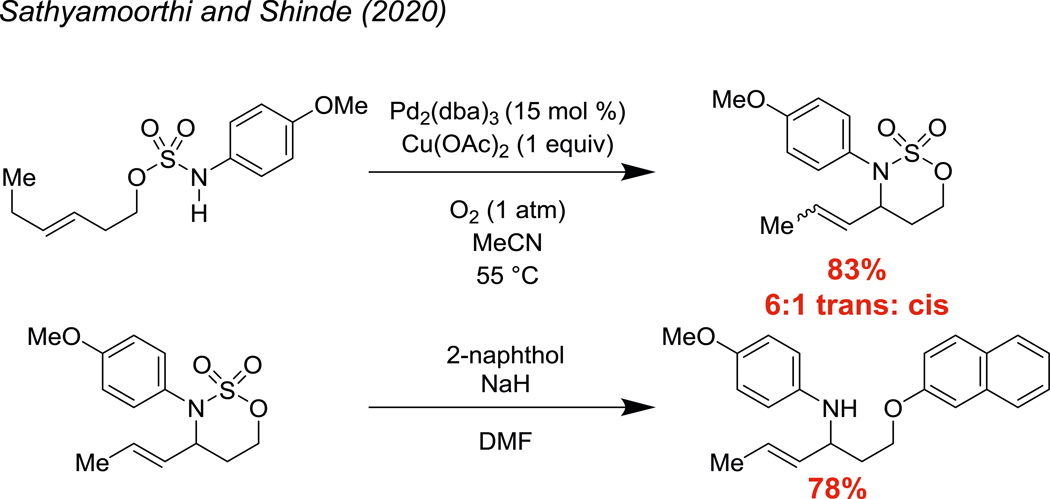
The 1,3-amino ether and 1,3-amino thioether motifs are common in biologically active compounds, including FDA approved pharmaceuticals (Fig. 1) (Andersen et al. 2009; Chen et al. 2005). Our laboratory recently disclosed an oxidative cyclization of sulfamate esters onto alkenes to yield a variety of 6-membered N-arylated oxathiazinane heterocycles with pendant unsaturation (Fig. 2) (Shinde and Sathyamoorthi 2020). In our previous work, we showed one example of ring opening with sodium 2-naphthoxide. While the nucleophilic ring opening of 5-membered cyclic sulfamates (oxathiazolidines) with phenoxide and thiophenoxide has been extensively explored (Malhotra et al. 2015; Baraznenok et al. 2005; Carreras et al. 2007; Bower et al. 2007; Okuda and Tomioka 1994), examples of the corresponding transformation with 6-membered cyclic sulfamates (oxathiazinanes) remain limited (Meléndez and Lubell 2003; Atfani et al. 2001). To our knowledge, apart from the lone example in our first disclosure, this reaction has not yet been explored with N-arylated sulfamates. We thus sought to explore the generality of this reaction and, in this communication, we are pleased to report that the nucleophilic ring opening of diverse oxathiazinanes is possible with a variety of alkoxides, naphthoxides, phenoxides, and thiolates.
Fig. 1.
1,3-amino ethers and 1,3-amino thioethers are vital motifs in clinically approved pharmaceutics
Fig. 2.
a An aza-Wacker cyclization of alkenyl sulfamate esters. b Lone example of naphthoxide opening of an N-aryl oxathiazinane heterocycle
Materials and methods
General considerations
All reagents were obtained commercially unless otherwise noted. Solvents purchased from Sigma Aldrich (HPLC Grade) were used directly and without purification. Infrared (IR) spectra were recorded on a Thermo Scientific™ Nicolet™ iS™5 FT-IR Spectrometer; data are reported in frequency of absorption (cm−1). NMR spectra were recorded on a Bruker Avance 400 operating at 400 and 100 MHz. 1H NMR spectra were recorded at 400 MHz. Data are recorded as: chemical shift in ppm referenced internally using residue solvent peaks, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, or overlap of nonequivalent resonances), integration, coupling constant (Hz). 13C NMR spectra were recorded at 100 MHz. Exact mass spectra were recorded using an electrospray ion source either in positive mode or negative mode and with a time-of-flight analyzer on a Waters LCT PremierTM mass spectrometer and are given in m/z. TLC was performed on pre-coated glass plates (Merck) and visualized either with a UV lamp (254 nm) or by dipping into a solution of KMnO4–K2CO3 in water followed by heating. Flash chromatography was performed on silica gel (230–400 mesh). Reversed phase HPLC was performed on a Hamilton PRP-1.7 μm, 21.2 × 250 mm, C18 column.
Representative experimental procedure for nucleophilic ring opening of N-arylatedoxathiazinane heterocycles
Reactions were performed on a 0.2 mmol scale, unless otherwise specified. A 25 ml round bottom flask was charged with phenol, naphthol, or thiol nucleophile (1.3 equivalents) and 2 ml of DMF. The flask was cooled to 0 °C, and dry NaH (1.3 equivalents) was added in portions. The resulting suspension was stirred for 15–20 min. Subsequently, a solution of the oxathiazinane precursor in 1 mL of DMF was added dropwise. The reaction mixture warmed to room temperature overnight. Following this time, the reaction was quenched with 1 M HCl (2 mL) and transferred to a separatory funnel with an additional 15 mL of deionized H2O. The aqueous mixture was subsequently basified with 1 M NaOH. Following this, the aqueous phase was extracted with three portions of ethyl acetate, the organic fractions were pooled, washed once with brine solution, and dried with Na2SO4. After removal of solvent under reduced pressure, the resulting residue was purified by chromatography on silica gel (gradient of 3–5% ethyl acetate in hexanes) to yield ring-opened N-aryl-amino ether or N-arylamino thioether product.
Characterization of products in Table 1 (starting materials have been fully characterized elsewhere (Shinde and Sathyamoorthi 2020))
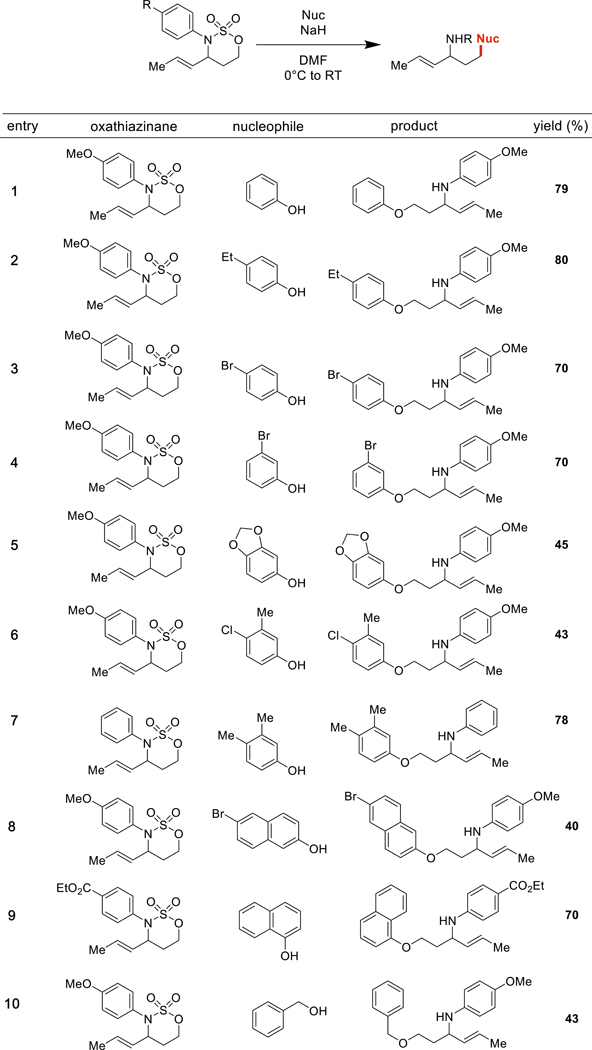
Table 1.
Scope of oxathiazinane heterocycle and nucleophile
|
|
(E)-4-Methoxy-N-(1-phenoxyhex-4-en-3-yl)aniline
(Table 1, Product Entry 1): Viscous light-yellow liquid (47 mg, 79%); 1H NMR (400 MHz, CDCl3) δ 1.7 (ddd, J = 6.5, 1.7, 0.8 Hz, 3H), 1.9–2.2 (m, 2H), 3.6 (s, 1H), 3.8 (s, 3H), 4.0–4.2 (m, 3H), 5.4 (ddq, J = 15.4, 6.8, 1.6 Hz, 1H), 5.7 (dqd, J = 15.4, 6.5, 1.1 Hz, 1H), 6.5–6.7 (m, 2H), 6.7–6.8 (m, 2H), 6.9–7.0 (m, 3H), 7.3–7.4 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 17.8, 35.5, 54.0, 55.8, 65.1, 114.6, 114.8, 115.0, 120.8, 126.8, 129.5, 132.7, 141.8, 152.1, 158.9; FT-IR 3347, 2937, 1510, 1031, 820 cm−1; HRMS calcd C19H24NO2 298.1807 found 298.1792 (MH+).
(E)-N-(1-(4-Ethylphenoxy)hex-4-en-3-yl)-4-methoxyaniline
(Table 1, Product Entry 2): Viscous light-yellow liquid (52 mg, 80%); 1H NMR (400 MHz, CDCl3) δ 1.1 (t, J = 7.6 Hz, 3H), 1.6 (ddd, J = 6.4, 1.7, 0.8 Hz, 3H), 1.8–2.0 (m, 2H), 2.5 (q, J = 7.6 Hz, 2H), 3.6 (s, 1H), 3.7 (s, 3H), 3.8–4.1 (m, 3H), 5.3 (ddq, J = 15.3, 6.7, 1.6 Hz, 1H), 5.5–5.7 (m, 1H), 6.4–6.6 (m, 2H), 6.6–6.7 (m, 2H), 6.7–6.8 (m, 2H), 7.0–7.1 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 15.9, 17.7, 28.0, 35.6, 54.1, 55.8, 65.3, 114.5, 114.8, 114.9, 126.7, 128.7, 132.7, 136.5, 141.9, 152.0, 156.9; FT-IR 3317, 2920, 1454, 1024, 764 cm−1; HRMS calcd C21H28NO2 326.2120 found 326.2127 (MH+).
(E)-N-(1-(4-Bromophenoxy)hex-4-en-3-yl)-4-methoxyaniline
(Table 1, Product Entry 3): Viscous light-yellow liquid (53mg, 70%); 1H NMR (400MHz, CDCl3) δ 1.7 (ddd, J=6.5, 1.7, 0.8 Hz, 3H), 1.9–2.2 (m, 2H), 3.4–3.7 (s, 1H), 3.8 (s, 3H), 3.9–4.2 (m, 3H), 5.4–5.5 (m, 1H), 5.7 (dtd, J=14.0, 6.5, 1.1 Hz, 1H), 6.6–6.7 (m, 2H), 6.8–6.9 (m, 4H), 7.4–7.5 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 17.8, 35.4, 53.9, 55.8, 65.5, 112.9, 114.9, 115.0, 116.4, 126.9, 132.3, 132.6, 141.7, 152.1, 158.0; FT-IR 3317, 2920, 1462, 1026, 750 cm−1; HRMS calcd C19H23NO2Br 376.0912 found 376.0959 (MH+).
(E)-N-(1-(3-Bromophenoxy)hex-4-en-3-yl)-4-methoxyaniline
(Table 1, Product Entry 4): Viscous light-yellow liquid (53 mg, 70%); 1H NMR (400 MHz, CDCl3) δ 1.7 (dt, J = 6.3, 1.4 Hz, 3H), 2.0 (dtdd, J = 20.6, 14.1, 6.7, 5.5 Hz, 2H), 3.6 (t, J = 18.1 Hz, 1H), 3.8 (s, 3H), 3.9–4.2 (m, 3H), 5.4 (ddq, J = 15.3, 6.8, 1.7 Hz, 1H), 5.7 (dqd, J = 15.3, 6.5, 1.1 Hz, 1H), 6.6–6.6 (m, 2H), 6.7–6.8 (m, 2H), 6.8–6.9 (m, 1H), 7.0–7.2 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 17.8, 35.4, 53.8, 55.8, 65.4, 113.6, 114.9, 115.0, 117.8, 122.8, 123.9, 127.0, 130.6, 132.5, 141.7, 152.1, 159.7; FT-IR 3396, 2920, 1511, 1035, 750 cm−1; HRMS calcd C19H23NO2Br 376.0912 found 376.0887 (MH+).
(E)-N-(1-(benzo[d][1,3]dioxol-5-yloxy)hex-4-en-3-yl)-4-methoxyaniline
(Table 1, Product Entry 5): Yellow oil (31 mg, 45%); 1H NMR (400 MHz, CDCl3) δ 6.76–6.66 (m, 2H), 6.62 (d, J = 8.5 Hz, 1H), 6.59–6.48 (m, 2H), 6.42 (d, J = 2.5 Hz, 1H), 6.24 (dd, J = 8.5, 2.5 Hz, 1H), 5.83 (s, 2H), 5.58 (dqd, J = 15.3, 6.5, 1.1 Hz, 1H), 5.31 (ddq, J = 15.3, 6.8, 1.6 Hz, 1H), 3.92 (dddd, J = 27.8, 9.6, 6.7, 5.5 Hz, 3H), 3.66 (s, 3H), 2.04–1.82 (m, 2H), 1.59 (ddd, J = 6.4, 1.6, 0.8 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 154.34, 152.36, 148.24, 141.68, 132.27, 127.16, 115.38, 114.79, 107.95, 105.77, 101.12, 98.12, 66.12, 55.79, 54.45, 35.32, 29.71, 17.77; FT-IR 3400, 2916, 1501, 1036, 749 cm−1; HRMS calcd C20H24NO4 342.1705 found 342.1687 (MH+).
(E)-N-(1-(4-chloro-3-methylphenoxy)hex-4-en-3-yl)-4-methoxyaniline
(Table 1, Product Entry 6): Viscous light-yellow liquid (26 mg, 43%); 1H NMR (400 MHz, CDCl3) 7.21 (d, J = 8.7 Hz, 1H), 6.78–6.76 (m, 2H), 6.75–6.73 (m, 1H), 6.67 (ddd, J = 8.8, 3.0, 0.6 Hz, 1H), 6.61–6.55 (m, 2H), 5.65 (dtd, J = 15.3, 6.5, 1.0 Hz, 1H), 5.43–5.32 (m, 1H), 4.10–3.93 (m, 3H), 3.74 (s, 3H), 2.33 (s, 3H), 2.07–1.95 (m, 2H), 1.67 (ddd, J = 6.5, 1.6, 0.8 Hz, 3H);13C NMR (101 MHz, CDCl3) δ 157.5, 152.2, 141.8, 137.1, 132.7, 129.7, 126.9, 126.0, 117.2, 115.1, 114.9, 113.3, 65.6, 55.9, 54.0, 35.6, 20.4, 17.9; FT-IR 3395, 2931, 1509, 1038, 818 cm−1; HRMS calcd C20H25ClNO2 346.1574 found 346.1565 (MH+).
(E)-N-(1-(3,4-dimethylphenoxy)hex-4-en-3-yl)aniline
(Table 1, Product Entry 7): Viscous light-yellow liquid; Yield: (45 mg, 78%); 1H NMR (400 MHz, CDCl3) δ 7.18–7.10 (m, 2H), 7.02 (d, J = 8.2 Hz, 1H), 6.72 (d, J = 2.8 Hz, 1H), 6.69–6.57 (m, 4H), 5.74–5.61 (m, 1H), 5.45–5.35 (m, 1H), 4.13–3.97 (m, 3H), 3.93 (s, 1H), 2.23 (s, 3H), 2.19 (s, 3H), 2.03 (dtd, J = 7.4, 6.6, 5.4 Hz, 2H), 1.67 (ddd, J = 6.4, 1.6, 0.7 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 157.1, 147.8, 137.8, 132.5, 130.4, 129.2, 128.9, 126.8, 117.2, 116.3, 113.6, 111.7, 65.3, 53.2, 35.7, 20.2, 18.9, 17.9; FT-IR 3403, 3050, 2917, 1501, 1049, 748 cm−1; HRMS calcd C20H26NO 296.2014 found 296.2006 (MH+).
(E)-N-(1-((6-bromonaphthalen-2-yl)oxy)hex-4-en-3-yl)-4-methoxyaniline
(Table 1, Product Entry 8): Light Brown Solid (34 mg, 40%); 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 1.9 Hz, 1H), 7.57 (d, J = 8.9 Hz, 1H), 7.48 (d, J = 8.7 Hz, 1H), 7.41 (dd, J = 8.7, 2.0 Hz, 1H), 7.10 (dd, J = 9.0, 2.5 Hz, 1H), 7.00 (d, J = 2.6 Hz, 1H), 6.70–6.63 (m, 2H), 6.62–6.39 (m, 2H), 5.60 (dqd, J = 15.4, 6.5, 1.1 Hz, 1H), 5.34 (ddq, J = 15.3, 6.8, 1.6 Hz, 1H), 4.23–3.85 (m, 3H), 3.65 (s, 3H), 2.15–1.89 (m, 2H), 1.60 (ddd, J = 6.5, 1.7, 0.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 157.12, 152.17, 141.61, 133.06, 132.53, 130.05, 129.66, 129.62, 128.52, 128.39, 126.95, 119.96, 117.06, 115.06, 114.84, 106.76, 65.28, 55.81, 54.01, 35.43, 17.78. FT-IR 3355, 2929, 1510, 1016, 817 cm−1; HRMS calcd C23H25BrNO2 426.1069 found 426.1059 (MH+).
Ethyl (E)-4-((1-(naphthalen-1-yloxy)hex-4-en-3-yl)amino) benzoate
(Table 1, Product Entry 9): Done on 0.167 mmol scale; Viscous light-yellow liquid (54.5 mg, 70%); 1H NMR (400 MHz, CDCl3) δ 1.4 (t, J = 7.1 Hz, 3H), 1.7 (ddd, J = 6.6, 1.7, 0.9 Hz, 3H), 2.3 (tdd, J = 6.4, 4.9, 3.7 Hz, 2H), 4.2–4.4 (m, 5H), 4.6 (d, J = 6.6 Hz, 1H), 5.5 (ddq, J = 15.3, 6.4, 1.6 Hz, 1H), 5.7–5.8 (m, 1H), 6.6 (dq, J = 9.8, 2.7, 2.3 Hz, 2H), 6.8 (dd, J = 7.6, 1.0 Hz, 1H), 7.3–7.4 (m, 1H), 7.5 (d, J = 8.2 Hz, 1H), 7.5–7.6 (m, 2H), 7.8–7.9 (m, 3H), 8.3 (ddd, J = 6.2, 3.4, 0.8 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 14.5, 17.7, 35.2, 52.9, 60.1, 65.1, 104.8, 112.1, 118.7, 120.6, 121.7, 125.4, 125.6, 125.8, 126.5, 127.6, 127.7, 131.1, 131.4, 134.6, 151.2, 154.3, 166.8; FT-IR 3374, 2933, 1694, 1510, 1036, 753 cm−1; HRMS calcd C25H28NO3 390.2069 found 390.2084 (MH+).
(E)-N-(1-(benzyloxy)hex-4-en-3-yl)-4-methoxyaniline
(Table 1, Product Entry 10): Yellow oil (25.8 mg, 43%); 1H NMR (400 MHz, CDCl3) δ 7.32–7.20 (m, 5H), 6.72–6.63 (m, 2H), 6.56–6.46 (m, 2H), 5.53 (dqd, J = 15.4, 6.4, 1.1 Hz, 1H), 5.28 (ddq, J = 15.3, 6.7, 1.6 Hz, 1H), 4.42 (d, J = 1.1 Hz, 2H), 3.86–3.73 (m, 1H), 3.66 (s, 3H), 3.53 (dddd, J = 28.3, 9.5, 6.6, 5.3 Hz, 2H), 1.80 (qd, J = 6.6, 5.1 Hz, 2H), 1.57 (ddd, J = 6.5, 1.7, 0.8 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 152.12, 141.47, 138.35, 132.61, 128.40, 127.70, 127.63, 126.59, 115.17, 114.74, 73.11, 67.68, 55.81, 54.90, 35.74, 17.73; FT-IR 3383, 2916, 1510, 1036, 748 cm−1; HRMS calcd for C10H26NO2 312.1964 found 312.1936 (MH+).
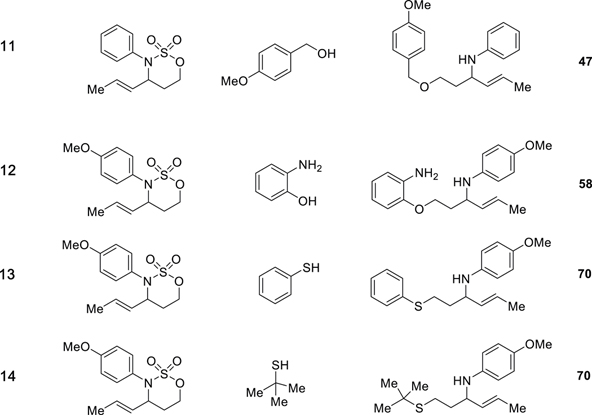
(E)-N-(1-((4-methoxybenzyl)oxy)hex-4-en-3-yl)aniline
(Table 1, Product Entry 11): Viscous light-yellow liquid (28 mg, 47%); 1H NMR (400 MHz, CDCl3) δ 7.26–7.24 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.85 (m, 2H), 6.69–6.64 (m, 1H), 6.60–6.56 (m, 2H), 5.63 (dqd, J = 15.3, 6.4, 1.1 Hz, 1H), 5.36 (ddq, J = 15.3, 6.3, 1.6 Hz, 1H), 4.43 (s, 2H), 4.11 (s, 1H), 3.97 (q, J = 6.8, 6.4 Hz, 1H), 3.82 (s, 3H), 3.59 (dddd, J = 28.3, 9.5, 6.6, 5.2 Hz, 2H), 1.87 (tdd, J = 6.6, 5.2, 4.0 Hz, 2H), 1.66 (ddd, J = 6.5, 1.7, 0.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.3, 148.0, 132.8, 130.6, 129.4, 129.2, 126.3, 116.9, 113.9, 113.4, 72.9, 67.5, 55.4, 53.6, 36.0, 17.8; FT-IR 3394, 2915, 1509, 1094, 748 cm−1; HRMS calcd for C20H26NO2 312.1964 found 312.1942 (MH+).
(E)-N-(1-(2-Aminophenoxy)hex-4-en-3-yl)-4-methoxyaniline
(Table 1, Product Entry 12): Viscous light-yellow liquid (36 mg, 58%); 1H NMR (400 MHz, CDCl3) δ 1.7 (ddd, J = 6.4, 1.7, 0.9 Hz, 3H), 2.0–2.2 (m, 2H), 3.7 (s, 5H), 4.0 (q, J = 6.6 Hz, 1H), 4.1–4.2 (m, 2H), 5.4 (ddq, J = 15.3, 6.7, 1.6 Hz, 1H), 5.7 (dqd, J = 15.4, 6.5, 1.1 Hz, 1H), 6.6–6.7 (m, 2H), 6.7–6.9 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 13 C NMR (101 MHz, CDCl3) δ 17.8, 35.6, 54.0, 55.8, 65.6, 112.0, 114.8, 115.0, 115.2, 118.5, 121.4, 126.8, 132.7, 136.4, 141.7, 146.5, 152.1; FT-IR 3383, 2938, 1509, 1038, 819 cm−1; HRMS calcd for C19H25N2O2 313.1916 found 313.1884 (MH+).
(E)-4-methoxy-N-(1-(phenylthio)hex-4-en-3-yl)aniline
(Table 1, Product Entry 13): Yellow oil (54 mg, 70%); 1H NMR (500 MHz, CDCl3) δ 7.25 (dt, J = 8.3, 1.1 Hz, 2H), 7.19 (d, J = 7.5 Hz, 2H), 7.09 (d, J = 6.7 Hz, 1H), 6.84–6.61 (m, 2H), 6.59–6.42 (m, 2H), 5.61–5.50 (m, 1H), 5.24 (ddt, J = 15.4, 7.0, 1.5 Hz, 1H), 3.80 (q, J = 6.8 Hz, 1H), 3.66 (s, 3H), 3.00–2.85 (m, 2H), 1.90–1.71 (m, 2H), 1.59 (dd, J = 6.5, 1.6 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 152.40, 140.91, 136.36, 132.08, 129.24, 128.92, 127.44, 125.98, 115.43, 114.80, 55.79, 35.08, 30.32, 29.72, 17.79; FT-IR 3372, 2917, 1510, 1038, 819 cm−1; HRMS calcd for C19H24NOS 314.1579 found 314.1580 (MH+).
(E)-N-(1-(tert-butylthio)hex-4-en-3-yl)-4-methoxyaniline
(Table 1, Product Entry 14): Viscous light-yellow liquid (36 mg, 70%); 1H NMR (400 MHz, CDCl3) δ 6.75 (d, J = 8.9 Hz, 2H), 6.58 (d, J = 9.0 Hz, 2H), 5.65 (dqd, J = 15.4, 6.5, 1.1 Hz, 1H), 5.32 (ddq, J = 15.3, 6.9, 1.6 Hz, 1H), 3.83 (q, J = 6.7 Hz, 1H), 3.74 (s, 3H), 3.36 (s, 1H), 2.61 (t, J = 7.4 Hz, 2H), 1.86–1.72 (m, 2H), 1.67 (ddd, J = 6.5, 1.7, 0.7 Hz, 3H), 1.31 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 152.2, 141.9, 132.8, 126.9, 115.1, 114.9, 56.0, 42.3, 36.1, 31.1, 25.1, 17.9; FT-IR 3364, 2957, 1510, 1039, 818 cm−1; HRMS calcd C17H28NOS 294.1892 found 294.1902 (MH+).
Results and discussion
We conducted a systematic exploration of oxathiazinane heterocycles with various O− and S− nucleophiles. We were pleased to find that ring opening with sodium phenoxide proceeded with high efficiency (Table 1, Entry 1); the reaction with sodium 4-ethylphenoxide (Table 1, Entry 2) was similarly successful. Opening with sodium 4-bromophenoxide provided product with a slightly lower yield (Table 1, Entry 3); we were somewhat surprised to observe that transposing the bromide to the meta position (Table 1, Entry 4) did not affect the reaction. With sesamol (Table 1, Entry 5) and 4-chloro-3-methylphenol (Table 1, Entry 6), the efficiency of the reaction dropped, but the amino ether product still formed in synthetically useful yields. We were not confined to ring opening of N-4-methoxyphenyl oxathiazinane heterocycles. Opening of an N-phenyl oxathiazinane (Table 1, Entries 7 and 11) as well as an N-4-ethoxycarbonyl oxathiazinane (Table 1, Entry 9) was also possible. Further, in addition to sodium aryloxides, sodium alkoxides (benzyloxide and 4-methoxybenzyloxide) were competent nucleophiles (Table 1, Entries 10 and 11). We envision that these benzyl groups may be facilely removed (Ghosh and Kulkarni 2020; Llàcer et al. 2006) to yield valuable N-aryl-amino alcohol molecules. With 2-aminophenol, we observed product solely from O− attack (Table 1, Entry 12). To our delight, ring opening with aryl and alkylthiols was both possible and efficient (Table 1, Entries 13 and 14).
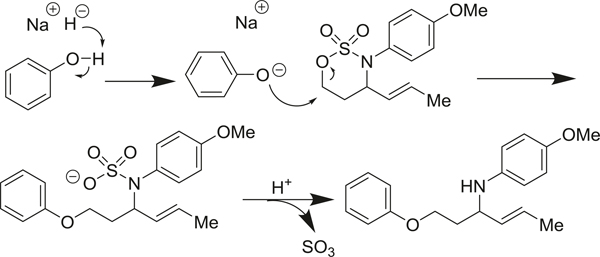
Our proposed mechanism (Fig. 3) takes inspiration from what has been postulated for analogous nucleophilic openings of oxathiazolidines (Meléndez and Lubell 2003). NaH deprotonation of phenol forms phenoxide which then selectively cleaves the C–O bond of the oxathiazinane heterocycle in a nucleophilic displacement. During acidic workup, SO3 is liberated and the resulting aryl nitrogen anion is quenched.
Fig. 3.
Proposed mechanism for nucleophilic ring opening of N-aryl-oxathiazinane heterocycles
Conclusion
We have presented our findings on the nucleophilic ring openings of N-arylated oxathiazinane heterocycles with phenoxides, naphthoxides, and thiolates. Based on our investigations, we postulate this to be a fairly general reaction for the rapid assembly of N-aryl-amino ethers and N-aryl-amino thioethers. We hope that this work contributes to the rising importance of the oxathiazinane heterocycle in synthetic organic chemistry.
Supplementary Material
Acknowledgements
This work was supported by start-up funding provided jointly by the University of Kansas Office of the Provost and the Department of Medicinal Chemistry as well as a grant from the COBRE Protein Structure and Function Small Grants Program. Support for the NMR instrumentation was provided by the NIH Shared Instrumentation Grant No. S10RR014767.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Supplementary information The online version of this article (https://doi.org/10.1007/s00044-020-02556-x) contains supplementary material, which is available to authorized users.
References
- Andersen J, Kristensen AS, Bang-Andersen B, Strømgaard K (2009) Recent advances in the understanding of the interaction of anti-depressant drugs with serotonin and norepinephrine transporters. Chem Commun 3677–3692 [DOI] [PubMed] [Google Scholar]
- Atfani M, Wei L, Lubell WD (2001) N-(9-(9-Phenylfluorenyl)) homoserine-derived cyclic sulfamidates: novel chiral educts for the synthesis of enantiopure γ-substituted α-amino acids. Org Lett 3:2965–2968 [DOI] [PubMed] [Google Scholar]
- Baraznenok IL, Jonsson E, Claesson A (2005) 3-(2,5-dihydro-1H-pyrrol-2-ylmethoxy)pyridines: synthesis and analgesic activity. Bioorg Med Chem Lett 15:1637–1640 [DOI] [PubMed] [Google Scholar]
- Bower JF, Szeto P, Gallagher T (2007) Enantiopure 1,4-benzoxazines via 1,2-cyclic sulfamidates. synthesis of levofloxacin. Org Lett 9:3283–3286 [DOI] [PubMed] [Google Scholar]
- Carreras J, Avenoza A, Busto JH, Peregrina JM (2007) Synthesis of azabicyclo[2.2.n]alkane systems as analogues of 3-[1-methyl-2-(S)-pyrrolidinyl-methoxy]pyridine (A-84543). J Org Chem 72:3112–3115 [DOI] [PubMed] [Google Scholar]
- Chen L, Gui C, Luo X, Yang Q, Günther S, Scandella E, Drosten C, Bai D, He X, Ludewig B, Chen J, Luo H, Yang Y, Yang Y, Zou J, Thiel V, Chen K, Shen J, Shen X, Jiang H (2005) Cinanserin is an inhibitor of the 3C-like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro. J Virol 79:7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B, Kulkarni SS (2020) Advances in protecting groups for oligosaccharide synthesis. Chem Asian J 15:450–462 [DOI] [PubMed] [Google Scholar]
- Llàcer E, Romea P, Urpí F (2006) Studies on the hydrogenolysis of benzyl ethers. Tetrahedron Lett 47:5815–5818 [Google Scholar]
- Malhotra R, Dey TK, Basu S, Hajra S (2015) Enantiopure synthesis of dihydrobenzo[1,4]-oxazine-3-carboxylic acids and a route to benzoxazinyl oxazolidinones. Org Biomol Chem 13:3211–3219 [DOI] [PubMed] [Google Scholar]
- Meléndez RE, Lubell WD (2003) Synthesis and reactivity of cyclic sulfamidites and sulfamidates. Tetrahedron 59:2581–2616 [Google Scholar]
- Okuda M, Tomioka K (1994) Stereo- and regiochemical aspects of the Mitsunobu reaction in synthesis of chiral amino ether ligands for asymmetric reactions. Tetrahedron Lett 35:4585–4586 [Google Scholar]
- Shinde AH, Sathyamoorthi S (2020) Oxidative cyclization of sulfamates onto pendant alkenes. Org Lett 22:896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.