Supplemental Digital Content is available in the text.
Keywords: endoscopy, fecal calprotectin, histology, inflammatory bowel disease, pediatrics
Objectives:
Fecal calprotectin (FC) is a noninvasive marker of intestinal inflammation used for screening and ongoing monitoring of inflammatory bowel disease (IBD); it is unclear the association of specific FC values with disease activity. The aim of our study was to examine the association of FC values with endoscopic and histologic severity.
Methods:
We performed a retrospective chart review of patients who had FC done between 30 days and 1 day before colonoscopy at our institution. IBD patients were graded using the simple endoscopic score for Crohn’s disease or Mayo endoscopic score for ulcerative colitis. Histologic slides were graded using the Geboes method.
Results:
Three-hundred thirty-one patients were included in the study and 107 had IBD. For endoscopy, median FC was lowest for all IBD patients with no disease (181 μg/g) and highest in severe disease (921 μg/g), with significant difference between no disease and moderate and severe disease (P = 0.019, 0.003), and between mild and severe disease (P = 0.012). For histology, median FC was lowest with no disease (328 μg/g) and highest in severe disease (895 μg/g), with significant difference between no disease and moderate and severe disease (P = 0.021, 0.018). The control population had a significantly lower median FC than the IBD population in endoscopic remission (35.5 versus 181 μg/g; P = 0.018).
Conclusions:
There was a linear increase in FC values associated with increasing disease severity in the undifferentiated IBD cohort. Values for IBD patients in endoscopic remission were significantly different from our control population. FC may be a useful noninvasive marker to assess disease severity.
INTRODUCTION
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammatory disease of the gastrointestinal tract with a relapsing and remitting course (1,2). Treatment goals include mitigating associated complications arising from the natural course of the disease, such as fibrosis, strictures, and colorectal neoplasms (3). In the past, clinical remission was the treatment target; however, this does not significantly alter the natural course of the disease or decrease rate of hospitalizations, flares, and complications (4).
Furthermore, remission measured with clinical indices does not correlate with endoscopic remission in adults or children (5,6).
Therapeutic strategies have transitioned to targeting endoscopic remission and away from using clinical symptoms, as this has been shown to alter the natural history of disease, decrease hospitalizations, surgeries, and relapses (7,8). In more recent years, published data demonstrate that histologic inflammation can persist in the presence of endoscopic remission and, conversely, achievement of histologic remission may serve as a more reliable predictor of sustained remission (9,10). Assessing for endoscopic remission requires direct visualization with endoscopy and remains a costly, invasive, and uncomfortable procedure; thus, accurate noninvasive testing to assess for endoscopic or histologic remission would be useful for the patient and physician alike.
Fecal calprotectin (FC) is a cytosolic protein released by neutrophils into the lumen when inflammation is present in the gastrointestinal tract and acts as a surrogate marker of neutrophils in the intestinal mucosa (11,12). FC as a biomarker has shown promise in assessing intestinal inflammation in IBD in a noninvasive modality and has been shown to be more accurate than serologic markers for inflammation, such as C-reactive protein and erythrocyte sedimentation rate (13–15). Furthermore, FC correlates with endoscopic activity in IBD patients, although specific cutoff levels for disease severity have varied widely among multiple studies and may be affected by location (isolated small bowel versus colonic) and type of disease (CD versus UC) (16–18). Similarly, some adult studies show that FC correlates with inflammation on histology; however, those cutoff values differ from values associated with endoscopic remission (19,20).
The primary aim of our study was to examine the association of quantitative FC levels with endoscopic and histologic severity in pediatric patients with IBD. In addition, we aimed to determine if there were differences in FC levels in IBD patients with endoscopic remission compared to a cohort without underlying IBD.
MATERIALS AND METHODS
We conducted a retrospective chart review of patients who had FC done between 30 days and 1 day prior to a colonoscopy at a tertiary academic center between January 1, 2014, and May 30, 2018. Patients were excluded from analysis if there was documentation of infection or polyp.
IBD patients were identified by utilization of a locally maintained database for pediatric patients with IBD. Controls were defined as cases whose colonoscopy did not reveal a diagnosis of IBD and were used to compare FC values to IBD patients with endoscopic remission. For the FC test, all stool samples were refrigerated and analyzed by the same quantitative enzyme-linked immunosorbent assay from ARUP laboratories (Salt Lake City, UT). The assay used did not change throughout the study period. Demographics, laboratory values, pathology, and colonoscopy results were extracted from the medical record. This study was approved by the Institutional Review Board at University Hospitals Cleveland Medical Center, Cleveland, OH (STUDY20181164).
Endoscopic Scoring
A single pediatric gastroenterologist (J.M.) using the simple endoscopic score for CD (SES-CD) and Mayo endoscopic score for UC patients scored disease activity in all IBD patients and was blinded to all clinical information related to each case other than their diagnosis. SES-CD consists of 4 components, each with 0–3 points allocated, which composite the score, including presence and size of ulcers, extent of ulcerated surface, extent of affected surface and the presence and type of narrowing (21). All areas of the ileum and colon were examined, and areas with inflammation were used for grading. The Mayo endoscopic score for UC patients reflects intestinal inflammation, and the score is based on degree of erythema, friability, visible vascular pattern, presence of ulcerations, and spontaneous bleeding (22). Only colonoscopies (sigmoidoscopies excluded) were assessed and graded based on the area with the highest level of inflammation. Endoscopic assessment was done using video recordings of the colonoscopies or representative images when video was not available. When still images were used, we verified representative images from each section of the colon and small bowel to score the SES-CD. The raw score obtained from SES-CD was further stratified into the following categories: quiescent (0–2), mild (3–6), moderate (7–15), and severe (>15) (23). The Mayo endoscopic score ranged from 0 to 3, indicating varying levels of inflammation in UC patients, from no disease (0), mild disease (1), moderate disease to severe disease (2–3) (22).
Histologic Scoring
Disease activity in IBD patients was scored histologically using the Geboes method, a widely used scoring index that has shown good reproducibility (24). The Geboes score consists of 6 grades (0: architectural changes, 1: chronic inflammatory infiltrate, 2A: eosinophils in the lamina propria, 2B: neutrophils in the lamina propria, 3: neutrophils in the epithelium, 4: crypt destruction, 5: erosion and ulcers) that are then each further subdivided. The original hematoxylin and eosin-stained slides were reviewed by both a pathologist-in-training (C.G.) and a board-certified pediatric pathologist (S.S.) who primarily reads histologic slides of pediatric gastrointestinal pathology. They were blinded to the clinical and endoscopic details of each patient. The pathology resident (C.G.) scored each patient and the senior pathologist (S.S.) confirmed the score. Any discrepancy in score defaulted to the senior pathologist. The raw scores obtained from the Geboes method were then stratified into categories of no disease (0–1), mild disease (2), moderate disease (3–4), and severe disease (5), based on the experience of the senior pathologist (S.S.).
Statistics
For the descriptive statistics, patient and clinical characteristics were described using frequency and percentages for categorical variables. Categorical variables were analyzed with the chi-square test. Boxplots with multiple pairwise comparisons (testing the association of FC with disease severity) with the significance level were generated using ggpubr package with t test method and compare means function. Sensitivity and analysis were performed using “OptimalCutpoints” package in R software.
Non-IBD patients were matched to the IBD patients by age and gender using nearest method with the ratio 2:1 to generate the similar pairwise comparison. In this manner, a larger number of controls were collected to ensure appropriate matching of age and gender to the IBD cohort. P values of less than 0.05 were considered statistically significant for the analyses. All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC) and R software, version 3.5.3.
RESULTS
Patient Characteristics
Three-hundred thirty-one patients were included in the study, with 107 IBD patients and 224 controls. Of the IBD cohort, 63 patients had a diagnosis of CD and 55% percent were male, with a median age at endoscopy of 14 years (interquartile range [IQR], 11–16 years; range, 0–21 years). Gender and age distribution between CD and UC patients did not differ (Table 1). Of the IBD cohort, 45.8% were new diagnoses at time of endoscopy, and 61.7% (n = 66) had FC levels obtained within 14 days of colonoscopy. The majority of CD patients had ileocolonic disease (54%) and the majority of UC patients had pancolitis (65%) at initial diagnosis. Only a small percentage of established IBD patients had disease progression from time of initial diagnosis to time of assessment for this study.
TABLE 1.
Patient characteristics
| Characteristic | All IBD (n = 107) | Crohn’s disease (n = 63) | Ulcerative colitis (n = 44) | P |
|---|---|---|---|---|
| Age, y ± SD | 13.66 ± 3.4 | 13.27 ± 3.5 | 14.22 ± 3.1 | 0.1480 |
| Male, n (%) | 59 (55.1) | 36 (57.1) | 23 (52.3) | 0.6942 |
| Endoscopic disease severity, n (%) | 0.0110 | |||
| None | 13 (12.2) | 11 (17.5) | 2 (4.6) | |
| Mild | 20 (18.7) | 9 (14.3) | 11 (25.0) | |
| Moderate | 48 (44.9) | 23 (36.5) | 25 (56.8) | |
| Severe | 26 (24.3) | 20 (31.8) | 6 (13.6) | |
| Histologic disease severity, n (%) | 0.0481 | |||
| None | 20 (18.7) | 15 (23.8) | 5 (11.4) | |
| Mild | 17 (15.9) | 10 (15.9) | 7 (15.9) | |
| Moderate | 51 (47.7) | 23 (36.5) | 28 (63.6) | |
| Severe | 12 (11.2) | 10 (15.9) | 2 (4.6) | |
| Disease phenotype of Crohn’s disease at diagnosis | ||||
| Paris classification | Crohn’s disease | |||
| Age of patients at diagnosis, y, n (%) | ||||
| 0–10 | 14 (22.2) | |||
| 11–17 | 43 (68.3) | |||
| >17 | 6 (9.5) | |||
| Location of disease, n (%) | ||||
| Ileal | 24 (38.1) | |||
| Colonic | 5 (7.9) | |||
| Ileocolonic | 34 (54.0) | |||
| Penetrating characteristic, n (%) | ||||
| Nonstricturing nonpenetrating | 55 (87.3) | |||
| Stricturing | 4 (6.4) | |||
| Penetrating | 4 (6.4) | |||
| Growth delay, n (%) | ||||
| Absent | 41 (65.1) | |||
| Present | 22 (34.9) | |||
| Perianal findings, n (%) | ||||
| Absent | 51 (80.1) | |||
| Present | 12 (19.1) | |||
| Disease phenotype of ulcerative colitis at diagnosis | ||||
| Montreal classification | Ulcerative colitis | |||
| Extent of disease, n (%) | ||||
| Ulcerative proctitis | 6 (13.6) | |||
| Left-sided UC (to splenic flexure) | 6 (13.6) | |||
| Extensive (to hepatic flexure) | 3 (6.8) | |||
| Pancolitis (proximal to hepatic flexure) | 29 (65.9) | |||
| Severity of disease, n (%) | ||||
| Never severe | 22 (50.0) | |||
| Ever severe (required hospital admission) | 22 (50.0) | |||
IBD = inflammatory bowel disease; UC = ulcerative colitis.
The median age of the control population was 12.5 years (IQR, 7–16 years; range, 0–25 years), 52.2% were female, and gender distribution was no different between the IBD and control populations. The control population consisted of patients with noninflammatory diagnoses, such as irritable bowel syndrome, constipation, and functional abdominal pain, while 6% had a diagnosis of celiac disease or eosinophilic esophagitis.
Endoscopic Activity
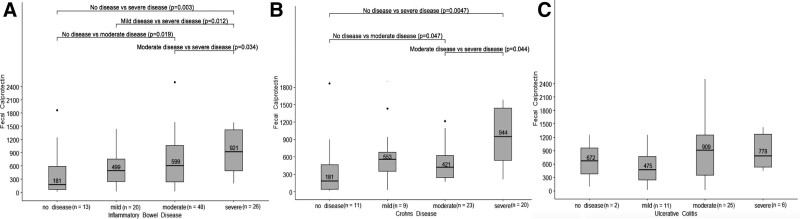
Videos for assessment of endoscopic disease were available for 48 (45%) patients in the IBD cohort, comprising 31 (49%) of the CD patients and 17 (39%) of the UC patients. When comparing endoscopic scoring and FC in the overall IBD cohort, the median FC was lowest in those with no disease (181 μg/g; IQR, 445–749 μg/g), followed by those with mild (499 μg/g; IQR, 42–779 μg/g) and moderate disease (599 μg/g; IQR, 244–1071 μg/g) and highest in those with severe disease (921 μg/g; IQR, 462–1422 μg/g) (Fig. 1A). Comparison of patients with no disease to moderate and severe disease (P = 0.019, 0.003 respectively) and between mild disease and severe disease (P = 0.012) revealed no significant differences.
FIGURE 1.
Fecal calprotectin association with endoscopic disease severity of IBD (A), Crohn's disease (B) and ulcerative colitis (C). IBD = inflammatory bowel disease.
In the CD patients, the median FC was lowest in those with no disease (181 μg/g; IQR, 21–595 μg/g) and highest in those with severe disease (944 μg/g; IQR, 448–1457 μg/g); there was significant difference in FC levels between no disease and moderate and severe disease (P = 0.047, 0.0047, respectively), and between moderate and severe disease (P = 0.044) (Fig. 1B). In the UC patients, a clear linear increase of median FC as disease severity increased was not found (Fig. 1C).
Histologic Activity
When examining histologic scoring to FC in IBD patients, the median FC level was lowest in IBD patients with no disease (328 μg/g; IQR, 116–833 μg/g), followed by those with mild (399 μg/g; IQR, 235–1064 μg/g) and moderate disease (674 μg/g; IQR, 325–1250 μg/g) and highest in those with severe disease (895 μg/g; IQR, 504–1434 μg/g) (Fig. 2A). There was significant difference when comparing no disease to moderate and severe disease (P = 0.021, 0.018, respectively).
FIGURE 2.
Fecal calprotectin association with histologic disease severity of IBD (A), Crohn's disease (B) and ulcerative colitis (C). IBD = inflammatory bowel disease.
In CD patients, median FC was lowest in those with mild disease (371 μg/g; IQR, 182–1010 μg/g), highest median FC 895 μg/g in those with severe activity (IQR, 397–1429), and significant differences were not found between severity groups (Fig. 2B). In UC patients, the median FC was lowest in those with no disease (94 μg/g; IQR, 30–654 μg/g) and highest in those with severe disease (1206 μg/g; IQR, 816–1597 μg/g), and FC levels between no disease and moderate disease differed significantly (P = 0.023) (Fig. 2C).
Analysis of Fecal Calprotectin and Endoscopic Activity
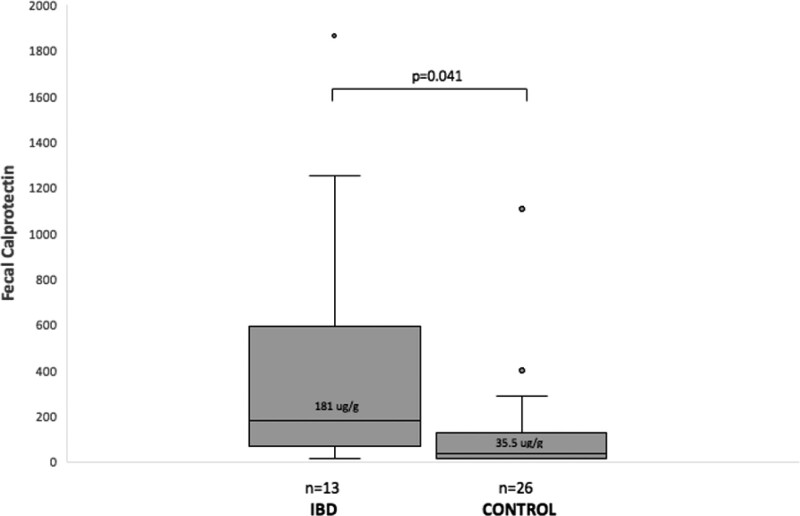
Sensitivity analysis of FC predicting endoscopic inflammation in the IBD cohort revealed that FC of >50 μg/g and >100 μg/g yielded sensitivity of 95% and 95% and specificity of 23% and 38% in predicting the presence of any inflammation, respectively. When matching the FC of endoscopically normal patients by age and gender, the control group (n = 26) had a significantly lower median FC than the IBD population (n = 13) with endoscopic remission (35.5 versus 181 μg/g; P = 0.041; Fig. 3).
FIGURE 3.
Comparison of median fecal calprotectin levels in IBD patients in endoscopic remission versus matched controls. IBD = inflammatory bowel disease.
DISCUSSION
This is one of the first pediatric studies comparing FC with endoscopic and histologic activity in an IBD cohort. We demonstrated significant differences in median FC when comparing them to endoscopic and histologic disease severity. We found a linear increase in FC levels as disease severity increases on both the macroscopic and microscopic level in our overall IBD cohort. On subanalysis of CD and UC, we found a less clear linear progression of median FC coinciding to disease severity. Comparison of the IBD cohort in endoscopic remission with the control population revealed a significant difference in FC levels (181 versus 35.5 μg/g), suggesting the normative FC values for pediatric patients with IBD in endoscopic remission may be higher than expected.
Published data on the applicability of FC as a noninvasive marker of inflammation in IBD patients have largely focused on the adult population and tends to assess the ability of FC to predict the presence of any histologic and endoscopic activity. Several adult studies have shown that IBD patients without endoscopic and/or histologic activity have lower FC levels compared with those with any activity (16, 19, 20, 25). FC levels correlate significantly with endoscopic disease severity in adult CD patients, however, SES-CD was stratified into 3 categories (no disease, mild, moderate/severe), potentially owing to more statistical significance in this category compared with our study (16). Relatively, fewer pediatric studies on the topic have been published. A pediatric study of CD patients revealed lower FC values in patients with mucosal and deep healing compared with those without healing (17). Our results reflect a unique level of granularity in pediatric data given we demonstrate differences in median FC values between disease severity and between activity versus remission on a macroscopic and microscopic scale.
FC has been shown to be a highly sensitive screening tool for IBD in the evaluation of undifferentiated pediatric patients with abdominal pain or diarrhea, when cutoff values for normal were set at 50–100 μg/g, with sensitivity up to 95% (26). We found similar results for our FC testing in our control population for 50 and 100 μg/g, with a sensitivity of 95% for both cutoff values. Larger studies are needed to more precisely determine cutoff values that predict not only IBD in undifferentiated patients but also presence of ulcers in known IBD patients. D’Haens et al (18) found the cutoff of 250 μg/g to be the most accurate predictor of the presence of ulcerations on endoscopy in adult CD patients. As the use of FC to assess and predict the need for additional endoscopic procedures and/or medication changes becomes more integrated into clinical practice, having a standard cutoff that reliably predicts endoscopic remission in pediatric IBD patients is an urgent need.
It is interesting to note that the median FC of those in histologic remission was comparatively higher than those in endoscopic remission when looking at our IBD cohort as a whole (328 versus 181 μg/g). Adult studies have shown lack of correlation between endoscopic lesions and histology and that histologic inflammation persists despite endoscopic remission, suggesting FC might be lower in those with histologic remission compared with endoscopic remission (10, 27). A large range of those in histologic remission (16–1865 μg/g; IQR, 116–833 μg/g) with presence of outliers may explain our findings. Further chart review of the outliers noted several patients with FC greater than 1000 μg/g whose total clinical picture was not suggestive of ongoing inflammation. Although it is generally accepted that FC has a stronger correlation with colonic disease, some studies do show correlation with small bowel disease (28). Due to the retrospective nature of this study, temporal small bowel imaging was not consistently available for review in the outliers, and it is possible that active small bowel disease could have contributed to some of these data points despite chart review suggesting clinical remission (ie, review of clinical notes with symptom description, laboratory values). Another consideration includes sampling variability, noting that biopsy samples are relatively small and may not always be taken precisely at the area of greatest inflammation. Undocumented or unidentified infection and unidentified polyps could conceivably contribute here.
Strengths of our study include assessing FC levels in an exclusively pediatric IBD cohort, using both endoscopic and histologic indices of disease severity. Disease activity assessments were made by a pediatric gastroenterologist with prior experience in SES-CD scoring and a pediatric pathologist specializing in gastrointestinal pathology.
Our study has several limitations. Up to 45% of the IBD cohort had videos available for endoscopic scoring, and we did not use a central reading process. While greater inter-rater reliability of endoscopic scoring systems has been shown using video recordings compared with photographs (29–31), other published reports suggest variability, and 1 study suggests at least moderate inter-rater reliability when using picture representation for the Mayo UC score (32). The variation in media in our study is a reflection of its retrospective nature. Similarly, there was no central reading process for the histologic grading, although the Geboes method has been shown to have good reproducibility (24). Sample size needs to be acknowledged as a significant limitation in the study, as evidenced by comparatively less significant findings on subanalysis of CD and UC. While a good linear progression of median FC was evident as disease severity increased endoscopically and histologically in the entire IBD cohort, subanalysis showed less progression, which limited our ability to accurately assess these subcohorts. Furthermore, sample size limited assessment of FC and disease activity by phenotype of UC and CD patients, which may have provided further insight into lack of correlation in the subanalyses, given it has been shown that FC correlates both with severity and location of disease (33). Outliers seemed to have impacted the relatively small sample size as noted by the range and IQR of each disease severity (See Supplementary Table 1a, b, http://links.lww.com/PG9/A61). FC levels also vary over the course of a day in active UC; additionally, we were unable to reliably document use of proton pump inhibitors or nonsteroidal anti-inflammatory medications, which may lead to elevated FC levels (34,35).
In conclusion, we demonstrate FC association with disease severity on endoscopic and histologic assessment in a pediatric IBD cohort. Complementing the published literature on this subject thus far, FC may be useful in predicting disease severity; however, larger multicenter, prospective studies are required to confirm these findings and allow for more extensive analysis based individual diagnoses and degree of severity.
Additionally, what constitutes a “normal” FC value for pediatric patients with IBD in endoscopic remission needs to be evaluated further with larger cohorts, as this may not be similar to otherwise healthy pediatric patients without IBD. While endoscopic surveillance and biopsy are still the gold standard for diagnosis and assessing disease severity in IBD patients, the use of FC as a noninvasive, cost-effective tool for serial assessment of IBD activity.
ACKNOWLEDGMENTS
We would like to thank Dr Sandra Kim for her generous and insightful review of the article.
Supplementary Material
Footnotes
Dr Sferra is a member of a scientific advisory committee for a research study sponsored by Merck & Co., Inc. Dr. Moses is on the speaker’s bureau for AbbVie, Inc. The remaining authors report no conflicts of interest.
Supplemental digital content is available for this article.
REFERENCES
- 1.Langholz E, Munkholm P, Davidson M, Binder V. Course of ulcerative coilitis: analysis of changes in disease activity over years. Gastroenterology. 2007;132:763–786. [DOI] [PubMed] [Google Scholar]
- 2.Magro F, Rodrigues A, Vieira AI, et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012;18:573–583. [DOI] [PubMed] [Google Scholar]
- 3.Torres J, Billioud V, Sachar DB, et al. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18:1356–1363. [DOI] [PubMed] [Google Scholar]
- 4.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baars JE, Nuij VJ, Oldenburg B, et al. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis. 2012;18:1634–1640. [DOI] [PubMed] [Google Scholar]
- 6.Turner D, Levine A, Walters TD, et al. Which PCDAI version best reflects intestinal inflammation in pediatric crohn disease? J Pediatr Gastroenterol Nutr. 2017;64:254–260. [DOI] [PubMed] [Google Scholar]
- 7.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. [DOI] [PubMed] [Google Scholar]
- 8.Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. [DOI] [PubMed] [Google Scholar]
- 9.Christensen B, Hanauer SB, Erlich J, et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol. 2017;15:1557–1564. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessissow T, Lemmens B, Ferrante M, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107:1684–1692. [DOI] [PubMed] [Google Scholar]
- 11.Kopylov U, Rosenfeld G, Bressler B, et al. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:742–756. [DOI] [PubMed] [Google Scholar]
- 12.Chang MH, Chou JW, Chen SM, et al. Faecal calprotectin as a novel biomarker for differentiating between inflammatory bowel disease and irritable bowel syndrome. Mol Med Rep. 2014;10:522–526. [DOI] [PubMed] [Google Scholar]
- 13.Gisbert JP, González-Lama Y, Maté J. [Role of biological markers in inflammatory bowel disease]. Gastroenterol Hepatol. 2007;30:117–129. [DOI] [PubMed] [Google Scholar]
- 14.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:524–534. [DOI] [PubMed] [Google Scholar]
- 16.Jusué V, Chaparro M, Gisbert JP. Accuracy of fecal calprotectin for the prediction of endoscopic activity in patients with inflammatory bowel disease. Dig Liver Dis. 2018;50:353–359. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein-Nakar I, Focht G, Church P, et al. ; ImageKids study group. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn’s disease. Clin Gastroenterol Hepatol. 2018;16:1089–1097. e4. [DOI] [PubMed] [Google Scholar]
- 18.D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. [DOI] [PubMed] [Google Scholar]
- 19.Guardiola J, Lobatón T, Rodríguez-Alonso L, et al. Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol. 2014;12:1865–1870. [DOI] [PubMed] [Google Scholar]
- 20.Mak WY, Buisson A, Andersen MJ, Jr, et al. Fecal calprotectin in assessing endoscopic and histological remission in patients with ulcerative colitis. Dig Dis Sci. 2018;63:1294–1301. [DOI] [PubMed] [Google Scholar]
- 21.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. [DOI] [PubMed] [Google Scholar]
- 23.Moskovitz DN, Daperno M, Van Assche G. Defining and validating cut-offs for the simple endoscopic score for Crohn’s disease. Gastrol. 2007;132:A173. [Google Scholar]
- 24.Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YW, Lee KM, Lee JM, et al. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J Intern Med. 2019;34:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:637–645. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg L, Nanda KS, Zenlea T, et al. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin Gastroenterol Hepatol. 2013;11:991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai T, Takeuchi K, Miyamura M, et al. Level of fecal calprotectin correlates with severity of small bowel Crohn’s disease, measured by balloon-assisted enteroscopy and computed tomography enterography. Clin Gastroenterol Hepatol. 2017;15:56–62. [DOI] [PubMed] [Google Scholar]
- 29.Khanna R, Zou G, D’Haens G, et al. Reliability among central readers in the evaluation of endoscopic findings from patients with Crohn’s disease. Gut. 2016;65:1119–1125. [DOI] [PubMed] [Google Scholar]
- 30.Dubcenco E, Zou G, Stitt L, et al. Effect of standardised scoring conventions on inter-rater reliability in the endoscopic evaluation of Crohn’s disease. J Crohns Colitis. 2016;10:1006–1014. [DOI] [PubMed] [Google Scholar]
- 31.Hart L, Chavannes M, Lakatos PL, et al. Do you see what i see? An assessment of endoscopic lesions recognition and description by gastroenterology trainees and staff physicians. Can J Gastroenterol Hepatol. 2019;20:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerur B, Litman HJ, Stern JB, et al. Correlation of endoscopic disease severity with pediatric ulcerative colitis activity index score in children and young adults with ulcerative colitis. World J Gastroenterol. 2017;23:3322–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawashima K, Ishihara S, Yuki T, et al. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calafat M, Cabré E, Mañosa M, et al. High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis. 2015;21:1072–1076. [DOI] [PubMed] [Google Scholar]
- 35.Maiden L, Thjodleifsson B, Theodors A, et al. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–1178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.