Abstract
Dopamine-related abnormalities in the basal ganglia have been implicated in attention-deficit/hyperactivity disorder (ADHD). Iron plays a critical role in supporting dopaminergic function, and reduced brain iron and serum ferritin levels have been linked to ADHD symptom severity in children. Furthermore, the basal ganglia is a central brain region implicated in ADHD psychopathology and involved in motor and reward functions as well as emotional responding. The present study repurposed diffusion tensor imaging (DTI) to examine effects of an ADHD diagnosis and sex on iron deposition within the basal ganglia in children ages 8–12 years. We further explored associations between brain iron levels and ADHD symptom severity and affective symptoms. We observed reduced iron levels in children with ADHD in the bilateral limbic region of the striatum, as well as reduced levels of iron-deposition in males in the sensorimotor striatal subregion, regardless of diagnosis. Across the whole sample, iron-deposition increased with age in all regions. Brain-behavior analyses revealed that, across diagnostic groups, lower tissue-iron levels in bilateral limbic striatum correlated with greater ADHD symptom severity, whereas lower tissue-iron levels in the left limbic striatum only correlated with anxious, depressive and affective symptom severity. This study sheds light on the neurobiological underpinnings of ADHD, specifically highlighting the localization of tissue-iron deficiency in limbic regions, and providing support for repurposing DTI for brain iron analyses. Our findings highlight the need for further investigation of iron as a biomarker in the diagnosis and treatment of ADHD and sex differences.
Keywords: ADHD, Dopamine, Striatum, Limbic circuitry, Diffusion tensor imaging (DTI), Emotional dysregulation
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a highly prevalent neurodevelopmental disorder diagnosed in childhood and is characterized by developmentally inappropriate levels of inattentive, hyperactive and impulsive behavior. In the U.S., 8.4% of children 2–17 years of age have a diagnosis of ADHD (Danielson et al. 2018). The etiology of ADHD is complex and there is currently no consensus on a single neurobiological model that explains its pathophysiology (Curatolo et al. 2010). One predominant etiological theory of ADHD, derived from neuroimaging and molecular genetics, explains the disorder through deficits in synaptic dopamine resulting from abnormal functioning of the dopaminergic system (Swanson et al. 2007; Tripp and Wickens 2009). The striatum (including the caudate and putamen) and globus pallidus, comprising the basal ganglia, have been implicated in ADHD as they are central components in motor and reward functions as well as emotional responding (Tewari et al. 2016; Badgaiyan et al. 2010). These subcortical regions are also particularly sensitive to changes in dopaminergic function because of their high concentration of dopamine receptors, which are targeted by dopamine from the midbrain dopaminergic nuclei (Rommelfanger and Wichmann 2010; Sobel et al. 2010). The success of stimulant medication such as methylphenidate, which acts as a dopamine agonist and blocks the reuptake of dopamine into the presynaptic neuron, has supported the theory of dopaminergic dysfunction in ADHD (Cortese 2012). Moreover, reduced levels of the D2 and D3 subtypes of the dopamine receptor have been found to be significantly correlated with inattentive behavior in adults with ADHD (Prince 2008).
Brain imaging over the years has demonstrated multiple lines of evidence that point to structural, functional and dopamine-related abnormalities in the basal ganglia in individuals with ADHD. Structural neuroimaging studies report that in comparison to typically developing (TD) children, subcortical regions dense in dopamine receptors, namely the caudate and globus pallidus, are significantly smaller in size among children with ADHD (Qiu et al. 2009; Rosch et al. 2018; Swanson et al. 2007). Additionally, alterations in multifocal shape of the basal ganglia have been observed in males with ADHD, compared to typically developing males (Qiu et al. 2009; Seymour et al. 2017). Functional MRI imaging has also consistently demonstrated hypoactivation of the fronto-striatal circuitry during reward anticipation, cognitive control, and response inhibition in children with ADHD (see reviews by Cubillo et al. 2012; Faraone et al. 2015; Plichta and Scheres 2014), and children with ADHD show decreased blood flow to frontal lobes and the basal ganglia via single photon emission computed tomography (Lou et al. 1990). These findings are consistent with models of ADHD that postulate a hypoactive dopaminergic system contributing to atypical reward processing and higher impulsivity levels in those with ADHD (Plichta and Scheres, 2014) as well as greater emotional lability which is increasingly emphasized in models of ADHD (Shaw et al. 2014).
Iron is a key component in supporting the synthesis of dopamine and is, therefore, a critical biological factor to consider in the study of ADHD. Acting as a cofactor for tyrosine hydroxylase, an enzyme that catalyzes the conversion of tyrosine to dopamine (Dichtl et al. 2018), iron plays a critical role in supporting the dopaminergic system, as well as the development of oligodendrocytes and the production of myelin (Todorich et al. 2009). Iron deficiency has been suggested as an environmental risk factor linked to reduced behavioral and cognitive function in children (Pollit and Leibel 1976). Some studies suggest an association between increased ADHD symptom severity and reduced serum ferritin levels, supporting links of iron content and resulting dopaminergic system dysfunction in children with ADHD (Konofal et al. 2004; Sever et al. 1997; Tseng et al. 2018). Despite this, there have been only three prior investigations of basal ganglia iron concentration in children (ages 8–18) with ADHD and the sample sizes have been smaller than the present study. In line with the dopamine-deficit theory, studies revealed that medication-naive ADHD patients show lower indices of striatal and thalamic brain iron compared to healthy controls (Cortese et al. 2012) as well as psychostimulant-treated ADHD patients (Adisetiyo et al. 2014). In a follow-up study, one of these investigator teams showed that brain iron levels in the basal ganglia normalize with long-term psychostimulant treatment (Adisetiyo et al. 2019).
Given that ADHD is a disorder of brain development with evidence of delayed maturation and other age-related effects on brain structure and function (Nikolaidis et al. 2022; Shaw et al. 2006, 2012; Slobodin et al. 2018), it is important to consider developmental effects on brain tissue-iron concentration. Studies reveal a rapid increase in brain tissue-iron concentration in the first two decades of life, with stabilization in early adulthood (Aquino et al. 2009; Hallgren and Souranger 1958). A recent study found that the putamen and globus pallidus show markedly different developmental trajectories relative to other structures with prolonged periods of rapid iron accumulation that continue into the mid-20 s (Larsen et al. 2020a, b). An earlier plateau of tissue-iron accumulation in the globus pallidus is observed in adolescent females, compared to a prolonged increase through early adulthood in males (Larsen et al. 2020a, b). These developmental findings provide a basis for exploring whether insufficient brain iron accumulation in ADHD may reflect a lag in maturation and whether it is associated with other atypical patterns of brain development in ADHD (Shaw et al. 2006, 2012; Slobodin et al. 2018). Consideration of brain iron as a biomarker for ADHD could further our understanding of brain mechanisms underlying ADHD with the potential to support extant findings suggesting clinical prognosis and treatment.
In addition to impacts of age on brain and behavioral phenotypes of ADHD, recent research has increasingly revealed ADHD-based sex differences across brain and behavioral studies (Dirlikov et al. 2014; Patros et al. 2018; Rosch et al. 2016, 2018; Seymour et al. 2017). Findings from our group reveal that males tend to show a greater tendency for abnormalities in motor/premotor circuitry implicated in motor response control while females show greater tendency for abnormalities in prefrontal circuitry associated with top-down control of higher order behavioral and emotional responses (Jacobson et al. 2015). Furthermore, preschool and school-aged children show sex-related differences in basal ganglia morphology (Wang et al. 2018) with larger reductions of volume among 8–12 year old males, compared to females in the same age group (Qiu et al. 2009; Rosch et al. 2018; Seymour et al. 2017). Sex-related variations in subcortical brain iron indicate adult females show reduced levels of iron in the substantia nigra than do adult males (Persson et al. 2015) and that adult females have significantly lower ferritin iron than men in the caudate, thalamus, frontal lobe, genu and splenium of the corpus callosum (Bartzokis et al. 2007) however, few studies have examined sex differences in tissue-iron in children and adolescents and there have been no studies examining impacts of sex on brain tissue-iron in ADHD.
The striatum has been shown to be spatially heterogeneous in terms of function, connectivity (Cohen et al. 2009; Martinez et al. 2003; Middleton and Strick 2000; Postuma and Dagher 2006) and other relevant neurobiological factors, such as tissue-iron concentration (Deistung et al. 2013; Larsen and Luna 2015). The basal ganglia works largely in tandem with the frontal cortex via corticostriatal networks to plan and carry out complex functions that rely on processes rooted in emotion, cognition and motivation (Haber 2016). The ventral portion of the striatum receives input from limbic areas central in emotional responding (Catani et al. 2013), while the dorsal striatum receives cortical input from sensory-motor and executive areas (Haber 2016). Existing literature on ADHD shows impairments in all three functions, namely in executive functioning (Barkley 1997; Pennington and Ozonoff 1996) motor processes (Chen et al. 2021; Neidermeyer and Naidou 1997; Sweeney et al. 2018) and internalizing and externalizing psychopathology (which correspond to mood and anxiety disorders and disruptive behaviors, respectively) in children with ADHD (Biederman et al. 1991; Jarrett and Ollendick 2008; Jensen et al 1993; Seymour et al. 2017; Shaw et al. 2014). As such, examining brain iron through limbic/executive/sensorimotor striatal subregions allows for a more fine-grained approach in probing these three functions that are central to ADHD symptomatology.
Among multiple MRI techniques for imaging brain iron (Haacke et al. 2005; Langkammer et al. 2010; Ropele and Langkammer 2017), recently developed quantitative susceptibility mapping (QSM) (Deistung et al. 2017; Liu et al. 2015; Wang and Liu 2015; Wang et al. 2017) is recognized as the most advanced tool for measuring brain iron levels in gray matter (Langkammer et al. 2012) and for detecting brain iron changes during pathological conditions such as neurodegeneration (Ayton et al. 2017; Eskreis-Winkler et al. 2017; He et al. 2015) (van Bergen et al. 2016). However, a recent study has shown that brain iron estimates in deep nuclei including the basal ganglia generated from diffusion tensor imaging (DTI) are highly correlated with the gold standard estimates of QSM in these regions (Peterson et al. 2019). The paramagnetic properties of tissue-iron have a strong effect on T2-weighted MRI signal intensity (Langkammer et al. 2010), therefore allowing research to repurpose DTI as a proxy for measuring brain iron deposition.
In this study, we used DTI to evaluate brain iron in limbic, executive and sensorimotor subregions of the striatum and the globus pallidus. We assessed effects of diagnosis, sex and their interaction on iron deposition within the basal ganglia, and examined the relationship between brain iron and age. Further, we explored associations between brain iron deposition and measures of ADHD symptom severity, as well as measures of internalizing and externalizing behaviors. We hypothesized that children with ADHD would show reduced levels of iron in the striatal subregions and globus pallidus and that further, this reduction would be associated with greater ADHD symptom severity and higher incidence of internalizing and externalizing behaviors. We further hypothesized that brain iron deposition would increase with age and the iron accumulation trajectory may differ among children with and without ADHD. Given recent evidence of sex differences in brain structure and function in children with ADHD, we also explored whether diagnostic effects were similar in females and males.
Methods
Participants
Participants were mainly recruited through local public schools, but also through community-wide advertisements, pediatricians, and word-of-mouth. Children were screened for eligibility via parent telephone interviews. Those with histories of traumatic brain injury, neurological conditions, seizures, prenatal exposure to teratogens, genetic disorders, intellectual disability or other neurodevelopmental disorders were excluded from the current study. Eligible participants completed two laboratory sessions within a period of six months in order to maintain validity of data collected at each session. All participants met a Full Scale Intelligence Quotient (FSIQ) and General Ability Index (GAI) of at least 80 on The Weschler’s Intelligence Scales for Children (WISC; Fourth (n = 216) or Fifth Edition(n = 63)). All studies were approved by the Johns Hopkins Institutional Review Board and a parent or guardian of the child signed informed consent with children providing verbal assent.
Diffusion tensor imaging (DTI) data was collected for 537 children ages 8–12 years old who participated in at least one of several studies that were active between 2007 and 2019. For those with multiple visits (n = 14), a single visit per participant was selected based on availability of behavioral and imaging data to represent each person in the dataset once, and siblings were accounted for to include one child from each sibling pair (n = 23). Additionally, participants were excluded based on the following criteria: 1) low image quality (n = 131, 2) presence of familial history of ADHD in typically developing (TD) children (n = 66, 3) comorbid anxiety or depressive disorder (n = 11), and 4) usage of selective serotonin reuptake inhibitors or psychotropics at the time of the study (n = 13). Participants taking stimulant medication were asked to withhold their medication the day prior to and day of testing so as to eliminate effects of stimulants on measures of cognition, behavior and motor skills. One participant was taking a non-stimulant medication, atomoxetine, at the time of the study. The final analytic sample (see Table 1) included 279 participants, 107 of which were children with ADHD.
Table 1.
Demographic data of study participants
| ADHD (n = 107) |
TD (n = 172) |
Group Comparisons | Effects of Dx within sex |
||||
|---|---|---|---|---|---|---|---|
| Males (n = 76) | Females (n = 31) | Males (n = 119) | Females (n = 53) | p values |
d
|
||
| ADHD vs. TD | Males | Females | |||||
| Age (years) | 10.4 (1.4) | 10.0 (1.1) | 10.4 (1.2) | 10.1 (1.0) | 0.732 | 0.03 | 0.09 |
| Framewise Displacement | 0.28 (0.10) | 0.28 (0.15) | 0.25 (0.11) | 0.26 (0.12) | 0.083 | 0.29 | 0.15 |
| Socioeconomic Status | 52.7 (9.5) | 52.9 (9.7) | 53.5 (10.1) | 53.1 (8.8) | 0.584 | 0.09 | 0.03 |
| WISC GAI | 112.6 (12.2) | 110.2 (12.4) | 116.8 (12.1) | 111.4 (11.3) | 0.045 | 0.34* | 0.10 |
| Conners IA T | 70.4 (8.6) | 80.9 (9.7) | 45.0 (6.2) | 45.9 (6.1) | < 0.001 | 3.40** | 4.32** |
| Conners HI T | 70.4 (13.3) | 73.2 (14.8) | 46.7 (5.5) | 46.9 (5.8) | < 0.001 | 2.33** | 2.34** |
| CBCL Externalizing T | 54.6 (10.1) | 57.8 (8.3) | 42.9 (7.7) | 41.0 (7.1) | < 0.001 | 1.29** | 2.18** |
| CBCL Internalizing T | 53.9 (9.4) | 53.2 (9.8) | 45.6 (9.0) | 44.5 (9.2) | < 0.001 | 0.91** | 0.92** |
| CBCL Anx/Dep T | 55.4 (6.2) | 56.1 (5.8) | 51.5 (3.0) | 51.9 (3.5) | < 0.001 | 0.81** | 0.87** |
| CBCL Aff Problems T | 58.5 (6.1) | 58.3 (7.4) | 51.4 (2.6) | 50.7 (1.6) | < 0.001 | 1.53** | 1.42** |
| Globus Pallidus Volume | 2500 (259) | 2312 (179) | 2562 (241) | 2327 (188) | < 0.001 | 0.25 | 0.08 |
| Striatal Volume | 18,303 (1818) | 17,169 (1179) | 18,697 (1785) | 17,232 (1557) | < 0.001 ADHD males vs. females |
0.22 | 0.05 |
| ODD (n, %) | 27 (36%) | 12 (39%) | 0 | 0 | 0.756 | – | – |
| Stimulant Med (n, %) | 44 (58%) | 17 (55%) | 0 | 0 | 0.722 | – | – |
Values represent means and (standard deviations) unless otherwise noted. Framewise displacement refers to head movement in the MRI scanner and socioeconomic status is conceptualized based on household income, family education and family occupational status.
WISC GAI = Wechsler Intelligence Scale for Children General Ability Index; Conners IA T = Conners Parent Rating Scale DSM Inattention; Conners HI T = Conners Parent Rating Scale DSM Hyperactivity/Impulsivity; CBCL Anx/Dep T = Child Behavior Checklist Anxiety/Depression; CBCL Aff Problems T = Child Behavior Checklist Affective Problems; T = T-score adjusted for age and sex d = Cohen’s d value
p < 0.05
p < 0.01
Diagnostic procedures
A diagnosis of ADHD was determined using criteria from the DSM-IV or DSM-5 and confirmed through a structured or semi-structured parent interview, using either the Diagnostic Interview for Children and Adolescents (DICA-IV; n = 210) (Reich 2000) or the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; n = 69) (Kaufman et al. 2016). The Conners Parent Rating Scale-Revised or -Version 3 (Conners 1997, 2008) was used to provide a dimensional measure of ADHD symptom severity reflected in the DSM Inattention and Hyperactivity/Impulsivity scales (n = 271). The Child Behavior Checklist (CBCL), a questionnaire completed by parents to detect emotional and behavioral problems in children and adolescents.
(Achenbach and Ruffle 2000), was also completed (n = 229) providing dimensional scores regarding symptoms of internalizing and externalizing behaviors. Master’s level clinicians conducted all diagnostic interviews and diagnosis was verified by licensed doctoral level clinical psychologists highly experienced in the clinical assessment of ADHD and related childhood disorders.
Children with ADHD were excluded from the study if they met criteria on the DICA-IV or the K-SADS for psychosis, major depression, bipolar disorder, conduct disorder or anxiety disorders, including generalized anxiety disorder, social phobias and obsessive–compulsive disorder. Children with ADHD and a comorbid diagnosis of oppositional defiant disorder (ODD) were not excluded.
Participants were included in the TD group only if they did not meet diagnostic criteria for any psychiatric disorders based on information obtained from the diagnostic interview and the parent and teacher ADHD rating scales.
Neuroimaging
MRI Acquisition and preprocessing
Prior to scanning, participants experienced a mock scanning session inside an inactive MRI scanner to simulate the scanning experience and acclimate them to the space and sounds of a real MRI environment. A research assistant provided verbal feedback on the subject’s movement throughout the mock scan in order to help minimize motion during the real scan and obtain better quality data and ensure participant comfort.
DTI images were acquired on Philips 3 T MRI scanners (Philips Healthcare, Best, The Netherlands) using a single-shot echo-planar sequence with sensitivity encoding using either an 8- or 32-channel head coil. (TR = 6.356 s, TE = 75 ms, sensitivity encoding acceleration factor 2.5, 2.2 mm axial slices, acquisition matrix of 96 × 96 [FOV = 212 mm], anterior–posterior phase-encoding direction). Two scans were collected for each participant, with 32 non-collinear diffusion encoding directions (b value 700 s/mm3) and one b0 for each run. A double echo T2 image was collected to quantify magnetic field inhomogeneity and the second echo was used (TR = 4164 ms, TE = 12/80 ms, Flip Angle = 90°, Slice thickness = 2.2 mm; Slice number: 70; FOV = 212; Reconstructed matrix: 256 × 256. Duration = 4 min). A 3D T1-weighted MPRAGE image was also collected (TR/TE = 8.2/3.7 ms, flip angle = 8°; slice thickness = 1 mm; slice number = 150; FOV = 212 mm; reconstructed matrix: 240 × 240. Duration = 4.5 min) and used for spatial registration with an MNI T1 template. All data were processed with the FSL Diffusion Toolbox. The two DTI acquisitions were concatenated to improve signal-to-noise ratio. The FSL’s EDDY utility (Andersson et al. 2016a, 2016b) was then used to correct eddy current-induced distortions, between-volume head motion, and for detection and replacement of outliers. Susceptibility distortion artifacts were corrected using FSL’s tool TOPUP (Andersson et al. 2003; Smith et al. 2004) using the first b0 image and a structural T2-weighted image with the same geometry as the diffusion data but different phase-encoding direction (right-left). Registration to a non-distorted structural T2-weighted image with similar contrast has been shown to effectively correct susceptibility induced geometric distortions for better anatomical alignment compared to field-map correction (Tao et al. 2009; Wu et al. 2008). A diffusion tensor model was fit at each voxel using DTIFIT and BEDPOSTX (Behrens et al. 2003, 2007) was employed to model crossing fibers using a ball-and-stick diffusion model.
Quality control of diffusion data
The diffusion data were visually inspected and binned into one of three categories using a method outlined in Roalf et al (2016). In brief, every volume of the raw, uncorrected data was visually inspected for contamination caused by motion or gradient failure, and scans were assigned a rating based on overall presence of artifact. Only clear distortions and instances of dropout in the images counted toward this rating—volumes where head motion was observed but the signal appeared to remain intact were not counted. Scans with zero volumes affected by signal loss were assigned a rating of “Excellent,” scans with 1–14 volumes affected were rated “Good,” and scans with more than 14 poor quality volumes were rated “Poor”. Only scans with a rating of “Excellent” or “Good” were used in this analysis.
Intensity normalization and calculation of the proxy iron index from diffusion data
The methods described in Larsen and Luna (2015) were closely followed to process the T2-weighted diffusion data. Each diffusion-weighted volume of the eddy-corrected image was normalized to its corresponding volume mean. More specifically, each volume was divided by the average signal across all slices of the volume contained within the brain mask using FSLmaths. All normalized volumes were then averaged to generate a single intensity-normalized, eddy-corrected T2-weighted image for each participant (see Fig. 1). The mean signal as a proxy iron index and the volume of each selected region of interest per participant were then calculated. Note the mean T2-weighted (mT2w) signal is expected to be inversely associated with tissue-iron concentration, i.e. higher mT2w signal indicates lower iron concentration. As such, each plot displays a measure of tissue-iron index (1/mT2w signal) for better and more intuitive visualization, but mT2w signal was used for all statistical analysis and is included in all results and tables.
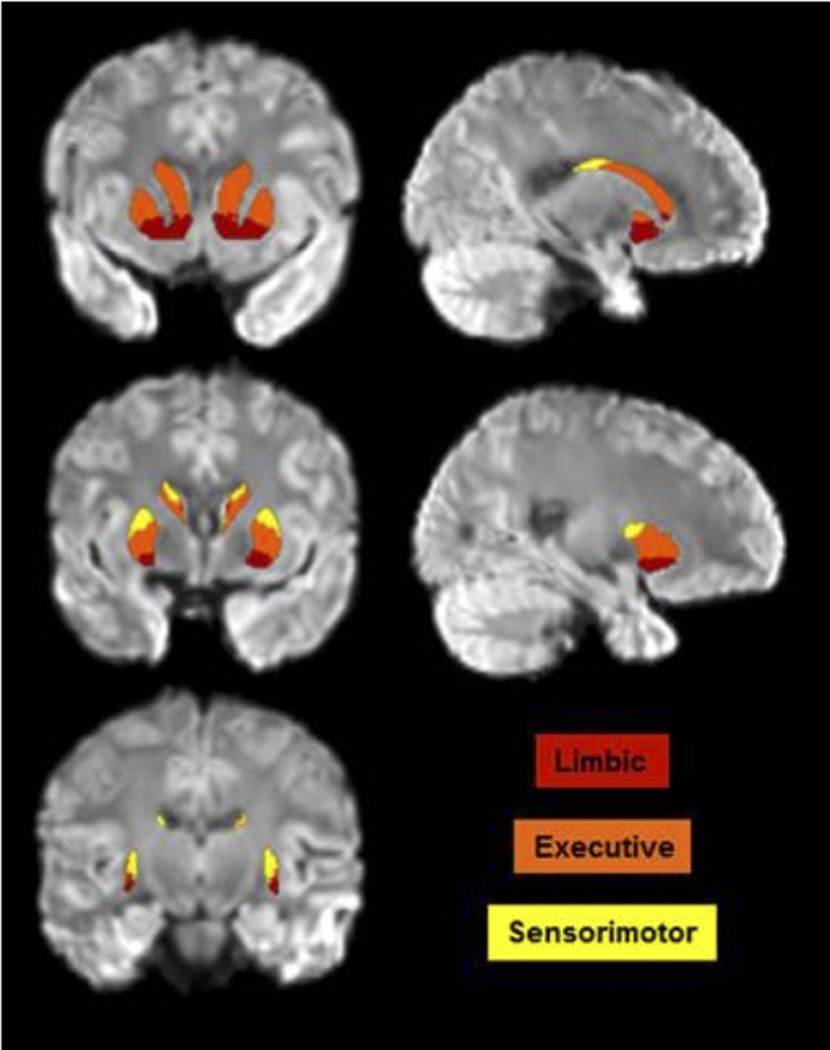
Fig. 1.
Intensity-normalized, eddy-corrected T2-weighted image with Oxford-GSK-Imanova Striatal Connectivity atlas labels overlaid
Regions of interest (ROIs)
Striatal subdivisions (limbic, executive and sensorimotor portions of the striatum) were labeled using the Oxford-GSK-Imanova Striatal Connectivity atlas (see atlas labels in Fig. 1) which uses information from diffusion-weighted imaging data and probabilistic tractography to segment the human striatum into connectivity-based subdivisions according to cortical-striatal anatomical connections (Tziortzi et al. 2014). The globus pallidus was labeled according to the Human Brain White Matter Atlas (Mori et al. 2008). Each participant’s T1-weighted MPRAGE was non-linearly registered to standard MNI (Montreal Neurological Institute) space in which both atlases are defined using FSL FNIRT. Both atlases were then brought to the space of each participant’s MPRAGE. Next, each MPRAGE image was linearly registered to participants’ eddy-corrected diffusion data using FSL’s FLIRT function. The transformation matrix of the linear registration from MPRAGE to diffusion was then applied to bring both atlases to diffusion space for further analyses to take place in native space.
Statistical analysis
Outliers were identified as any value that was outside the range of three standard deviations from the mean within each diagnostic group. Outlier analyses were performed for regional mT2w signal, framewise displacement (FD), regional brain volume, and scores on each symptom severity measure. No more than 3 outliers were removed for each measure, with the exception of the CBCL Anxiety/Depression (n = 5) and CBCL Affective Problems scales (n = 8).
A multivariate general linear model was used to examine main effects of diagnosis, sex and their interaction on regional mT2w signal with age, FD, and regional volume included as covariates. Covariates were selected based on observed group differences in the sample (see Table 1), as well as evidence from prior literature, showing age-related changes in brain iron accumulation from childhood to early adulthood (Aquino et al. 2009; Hallgren and Souranger 1958; Larsen et al. 2020a, b), reduced basal ganglia volumes in children with ADHD (Qiu et al. 2009; Rosch et al. 2018; Seymour et al. 2017), and increased sensitivity of diffusion-weighted imaging to head motion resulting in exacerbated signal attenuation confounding the underlying T2 signal (Gumus et al. 2014; Kaso and Ernst 2021) and downstream diffusion derived metrics (Roalf et al. 2016). Because the volume of the whole striatum and globus pallidus was included as one of three covariates, separate models were conducted for the striatal regions and globus pallidus. Significant multivariate effects were further probed with post hoc examination of univariate tests on individual ROIs without correction for multiple comparisons to determine which regions contributed to the significant multivariate effect. Cohen’s d was calculated as a measure of effect size for diagnostic group differences in tissue-iron. Secondary analyses conducted within the ADHD group examined the effects of stimulant use on regional brain iron using multivariate general linear models covarying for age, FD and regional volume. Finally, sensitivity analyses were conducted without any covariates and all significant results remained with no additional significant results.
Additionally, associations between mT2w signal and age were explored through Pearson’s partial correlations with FD and volume as covariates, and moderation analyses were performed to examine whether this relationship was moderated by diagnosis or a diagnosis-by-sex interaction. Similarly, Pearson’s partial correlations were examined between mT2w signal and questionnaire measures of ADHD and affective symptoms across the whole sample with FD and volume included as covariates (age was not included since the questionnaire measures are age-adjusted). A hierarchical approach was employed such that these relationships were only examined for regions where effects of diagnosis were observed on mT2w signal and questionnaire measures. A false discovery rate (FDR) correction of 0.05 was applied to all correlational tests (i.e., correcting for six comparisons for the associations with age and four comparisons for the brain-behavior correlations). Finally, moderation models were conducted to examine whether the relationship between mT2w signal and ADHD/affective symptoms was moderated by a diagnosis-by-sex interaction.
Results
Effects of diagnosis and sex on the mT2w signal in ROIs
Striatal subregions
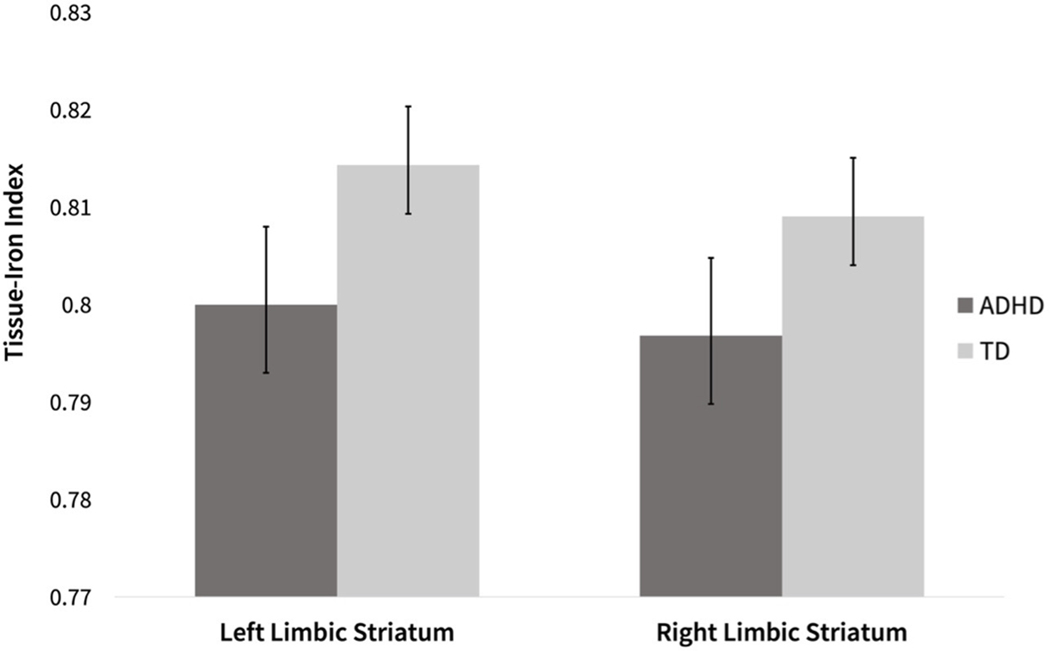
There were significant multivariate main effects of diagnosis (Dx) and sex on mT2w signal across all striatal regions (Dx: F(6, 261) = 2.4; p = 0.030, sex: F(6, 261) = 2.6; p = 0.020), with no multivariate Dx*sex interaction (p = 0.590). Between-subjects effects for individual ROIs (see Table 2) revealed a significant effect of diagnosis on mT2w signal in the left and right limbic striatal regions (left: F(1, 273) = 5.0; p = 0.026; right: F(1, 273) = 4.9; p = 0.028), such that children with ADHD showed higher mT2w signal and lower tissue-iron index (1/mT2w signal) in both regions indicating lower concentrations of tissue-iron (see Table 2 and Fig. 2). The diagnostic effect was similar among males (d = 0.326) and females (d = 0.267) for the left limbic striatum; for the right limbic striatum the effect size was particularly strong in females (females: d = 0.410) as compared to males: d = 0.204). There were no significant effects of diagnosis on the executive and sensorimotor subregions of the striatum.
Table 2.
(a) Mean and (SD) of regional mT2w signal for females and males with ADHD and TD controls. (b) Effects of diagnosis, sex and their interactions. (c) Effects of diagnosis within males and females, including Cohen’s d values
| a. | ADHD |
TD |
b. Main effects and interactions |
c. Effects of Diagnosis |
|||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Males (n = 76) | Females (n = 31) | Males (n = 119) | Females (n = 53) | Dx | Sex | Dx*Sex | Males | Females |
| p | p | p | p (d) | p (d) | |||||
| L Limbic | 1.25 (0.08) | 1.25 (0.06) | 1.23 (0.08) | 1.23 (0.07) | 0.026* | 0.656 | 0.615 | 0.013** (0.326) | 0.299 (0.267) |
| R Limbic | 1.25 (0.07) | 1.26 (0.06) | 1.24 (0.07) | 1.23 (0.06) | 0.028* | 0.627 | 0.591 | 0.127 (0.204) | 0.100 (0.410) |
| L Exec | 1.25 (0.06) | 1.24 (0.05) | 1.24 (0.06) | 1.24 (0.06) | 0.360 | 0.802 | 0.842 | 0.309 (0.139) | 0.667 (0.100) |
| R Exec | 1.23 (0.05) | 1.23 (0.05) | 1.23 (0.05) | 1.22 (0.06) | 0.403 | 0.319 | 0.424 | 0.971 (0.218) | 0.328 (0.016) |
| L Sm | 1.15 (0.06) | 1.15 (0.05) | 1.15 (0.06) | 1.15 (0.05) | 0.785 | 0.323 | 0.926 | 0.739 (0.047) | 0.914 (0.030) |
| R Sm | 1.20 (0.05) | 1.19 (0.05) | 1.20 (0.05) | 1.18 (0.05) | 0.854 | 0.004** | 0.796 | 0.687 (0.030) | 0.965 (0.031) |
| L GP | 0.83 (0.06) | 0.84 (0.05) | 0.83 (0.07) | 0.83 (0.06) | 0.731 | 0.895 | 0.584 | 0.856 (0.023) | 0.593 (0.162) |
| R GP | 0.87 (0.07) | 0.87 (0.05) | 0.87 (0.07) | 0.86 (0.07) | 0.903 | 0.375 | 0.358 | 0.473 (0.076) | 0.533 (0.188) |
L Limbic = left limbic striatum; R Limbic = right limbic striatum; L Exec = left executive striatum; R Exec = right executive striatum; L Sm = left sensorimotor striatum; R Sm = right sensorimotor striatum; L GP = left globus pallidus; R GP = right globus pallidus
p < 0.05
p < 0.01
Fig. 2.
Distribution of the estimated marginal tissue-iron index (1/mT2w signal) in the left limbic striatum and right limbic striatum in children with ADHD and TD controls
A significant effect of sex (across diagnosis) was observed on the mT2w signal in the right sensorimotor striatal subregion (F(1, 273) = 8.3, p = 0.028), such that males showed higher mT2w signal in this region, and consequently lower levels of brain iron (see Table 2). No significant effects of sex were observed on the limbic, executive, or left sensorimotor portions of the striatum, and there were no significant diagnosis-by-sex interaction effects for any of the striatal subregions.
Globus pallidus
There were no statistically significant findings for effects of diagnosis, sex, or their interaction on mT2w signal in the globus pallidus.
Effects of psychostimulant use on mT2w signal in ROIs
Among children with ADHD, there were no significant multivariate main effects of stimulant use on mT2w signal, or brain iron, (striatal regions: F(6, 200) = 1.0; p = 0.801; globus pallidus: F(2, 207) = 0.3; p = 0.450). There were also no significant between-subjects effects for individual ROIs (ps > 0.05).
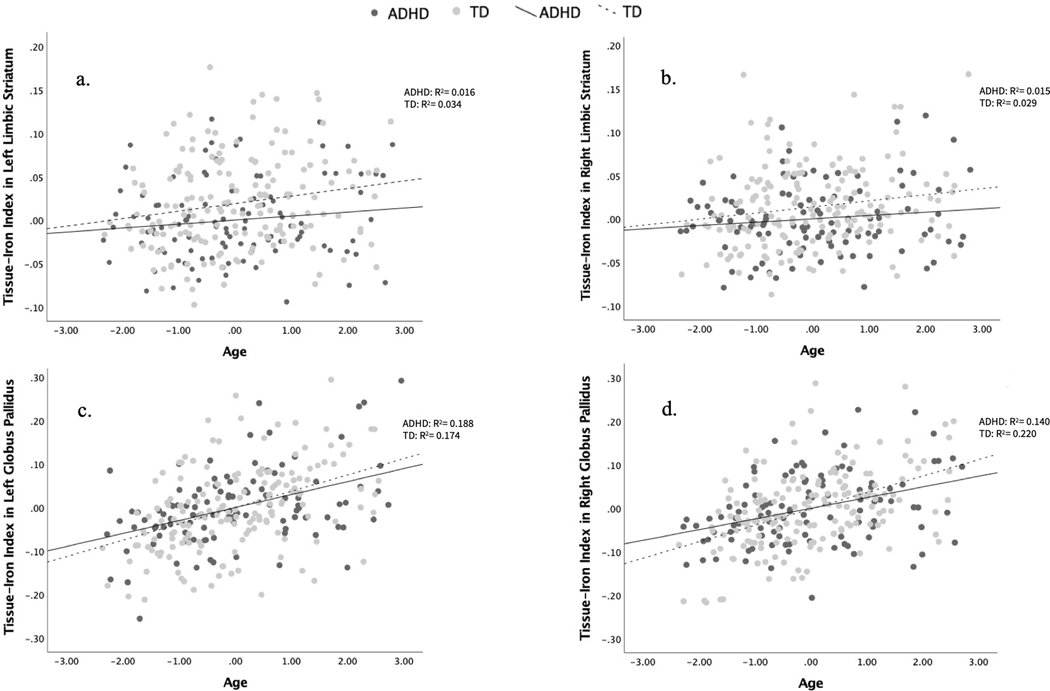
Associations between age and mT2w signal
In the overall sample, there was a negative correlation between mT2w signal and age across all regions, such that mT2w signal decreases and tissue-iron concentration increases linearly with age (Table 3). This relationship was significant and survived an FDR correction for the left and right limbic striatal region (left: p = 0.002; right: p = 0.001), the left executive striatal region (p = 0.028), the left sensorimotor striatal region (p = 0.033) and the left and right globus pallidus (left: p < 0.001; right: p < 0.001; Fig. 3a-d). Curve-estimation tests revealed that exponential curves do not fit the data differently than do linear curves. Moderation analyses revealed that diagnosis and the diagnosis-by-sex interaction did not significantly moderate the relationship between age and mean T2 (moderation test results reported in Table 3).
Table 3.
Pearson partial correlations between regional mT2w signal and age across the whole group (with FD and volume as covariates) and regression analyses testing whether an ADHD diagnosis (Dx*Age) or diagnosis-by-sex interaction (Dx*Sex*Age) moderated the relationship between age and mT2w signal
| ROI | Hemisphere | Dx*Age |
Dx*Sex*Age |
||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | β | SE | p | β | SE | p | ||
| Limbic Striatum | Left | − 0.190** | 0.002 | 0.015 | 0.017 | 0.387 | − 0.009 | 0.019 | 0.634 |
| Right | − 0.196** | 0.001 | − 0.001 | 0.015 | 0.945 | 0.011 | 0.017 | 0.494 | |
| Executive Striatum | Left | − 0.134* | 0.028 | 0.013 | 0.014 | 0.347 | − 0.017 | 0.016 | 0.261 |
| Right | − 0.076 | 0.210 | 0.004 | 0.012 | 0.737 | − 0.003 | 0.014 | 0.804 | |
| Sensorimotor Striatum | Left | − 0.130* | 0.033 | 0.005 | 0.013 | 0.703 | − 0.001 | 0.014 | 0.969 |
| Right | − 0.106 | 0.080 | − 0.007 | 0.011 | 0.528 | − 0.018 | 0.013 | 0.151 | |
| Globus Pallidus | Left | − 0.437** | < 0.001 | 0.019 | 0.013 | 0.147 | − 0.017 | 0.015 | 0.245 |
| Right | − 0.437** | < 0.001 | 0.010 | 0.013 | 0.442 | 0.001 | 0.015 | 0.935 | |
p < 0.05
p < 0.01
Fig. 3.
Correlation plots of age and tissue-iron index (1/mT2w signal) in the a left limbic striatum, b right limbic striatum, c left globus pallidus, d right globus pallidus
Correlations between mT2w signal and ADHD/affective symptom severity measures
Striatal subregions
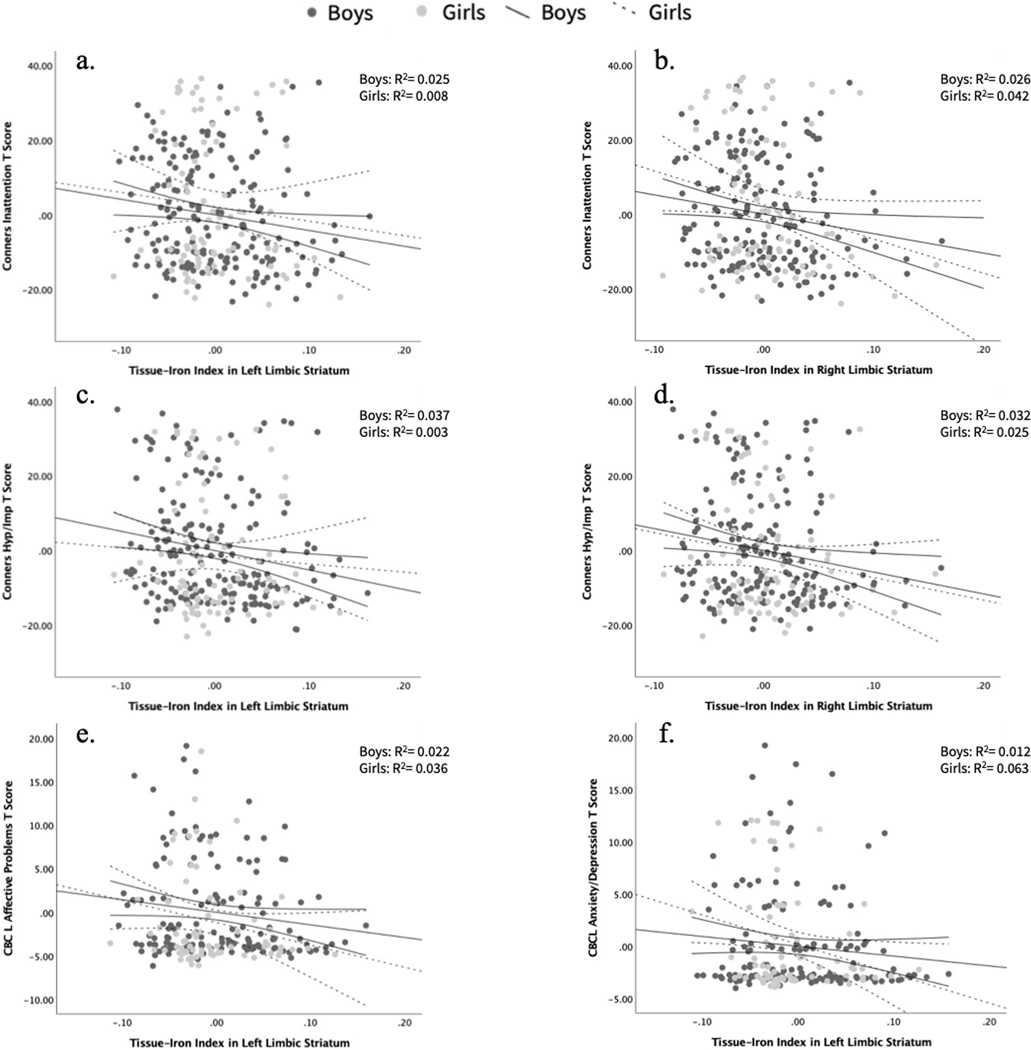
Across the whole sample (ADHD and TD groups), there were significant positive Pearson partial correlations between mT2w signal in the left and right limbic striatal regions and the Conners ADHD symptom severity scales (Table 4). Specifically, higher mT2w signal and thus, lower tissue-iron in these regions corresponded with higher scores and thus greater symptom severity on the Conners Inattentive scale (left: p = 0.040; right: p = 0.008) and Conners Hyperactivity/Impulsivity scale (left: p = 0.012; right: p = 0.005) (Fig. 4a–d).
Table 4.
Pearson partial correlations between regional mT2w signal and measures of ADHD symptom severity and emotional functioning
| Left Limbic Striatum |
Right Limbic Striatum |
|||||
|---|---|---|---|---|---|---|
| Whole Group |
Whole Group |
|||||
| r | p | df | r | p | df | |
| Conners Inattentive T | 0.126* | 0.040 | 265 | 0.163** | 0.008 | 265 |
| Conners Hyperactivity/Impulsivity T | 0.152* | 0.012 | 266 | 0.170** | 0.005 | 266 |
| CBCL Externalizing T | 0.080 | 0.232 | 224 | 0.078 | 0.246 | 224 |
| CBCL Internalizing T | 0.117 | 0.080 | 224 | 0.082 | 0.221 | 224 |
| CBCL Anxiety/Depression T | 0.135* | 0.046 | 219 | 0.057 | 0.402 | 219 |
| CBCL Affective Problems T | 0.142* | 0.036 | 216 | 0.120 | 0.077 | 216 |
Conners IA T = Conners Parent Rating Scale DSM Inattention T; Conners HI T = Conners Parent Rating Scale DSM Hyperactivity/Impulsivity T; CBCL = Child Behavior Checklist, T = T-score adjusted for age and sex
p < 0.05
p < 0.01
Fig. 4.
Brain-behavior correlation plots across diagnosis with 95% confidence intervals for a Conners Inattentive measure and tissue-iron index (1/mT2w signal) in the left limbic striatum and b right limbic striatum, c Conners Hyperactivity/Impulsivity measure and tissue-iron index in the left limbic striatum and d right limbic striatum, e CBCL Affective Problems measure and tissue-iron index in the left limbic striatum, f CBCL Anxiety/Depression measure and tissue-iron index in the left limbic striatum
Additionally, mT2w signal in the left limbic striatum was found to be positively correlated with the CBCL Anxiety and Depression Scale (p = 0.046) and the CBCL Affective Problems Questionnaire (p = 0.036), such that higher mT2w signal (i.e., lower tissue-iron) in the left limbic striatum was associated with parent reports of higher anxious and depressive symptoms as well as affective issues (Fig. 4e, f).
After FDR correction, only the right limbic striatum association with the Conners Inattentive and Hyperactivity/Impulsivity scales remained significant.
The relationship between mT2w signal in the left/right limbic striatum and symptom severity measures was not moderated by a diagnosis-by-sex interaction (See Supplementary Materials Table S1).
Discussion
The present study repurposed DTI as a proxy for measuring brain iron deposition to investigate ADHD diagnosis- and sex-related differences in mT2w signal within the basal ganglia. We further explored relationships between mT2w signal and measures of ADHD symptom severity and externalizing and internalizing symptoms. Note that even though the mT2w signal derived from DTI data and T2 relaxation rate in general is known to be affected by factors other than tissue iron, e.g. water content and myelin (see further discussion in 4.5), in the striatum and pallidum of the studied age group, tissue iron is believed to play a major role in determining the T2 values and the mT2w signal in these regions when considering their high iron and low myelin contents. Similar T2 relaxation-based measures in the basal ganglia have been demonstrated to associate with more accurate and specific tissue iron measures (Langkammer et al. 2010; Peterson et al. 2019). Therefore, in the present study, we mainly attribute observed differences in mT2w signal to differences in tissue iron levels. We observed increased mT2w signal (indicating reduced striatal iron-deposition) in children with ADHD, specifically in the bilateral limbic striatal regions. We also observed increased mT2w signal (indicating reduced levels of iron-deposition) in males, regardless of diagnosis, in the sensorimotor striatal subregion. Across the whole sample, we observed reduced mT2w signal, indicating increased iron-deposition, with age in all regions that did not differ as a function of diagnosis or sex, with the exception of the right sensorimotor striatum and right executive striatum wherein we saw the same directional association with age, albeit not significant. Brain-behavior analyses revealed that, across the whole sample, higher mT2w signal, indicating lower tissue-iron, in the bilateral limbic striatal region was correlated with greater ADHD symptom severity. Similarly, higher mT2w signal in the left limbic striatal region corresponded to higher parent-rated anxious, depressive and affective symptoms across children with and without ADHD with trend-level significance. These brain-behavior associations were similar across diagnostic groups and sex.
Iron and ADHD
Consistent with our a priori hypotheses, children with ADHD showed significantly increased levels of mT2w signal in the left and right limbic striatal subregions, indicating lower levels of tissue-iron compared to TD children. This is in line with the dopamine-deficit theory and prior literature which reports lower iron concentrations in the caudate, putamen and thalamus in children with ADHD compared to healthy controls (Cortese et al. 2012), as well as lower iron concentrations in the caudate and putamen of ADHD-nonmedicated children and adolescents compared to ADHD-medicated subgroups, and in ADHD-nonmedicated subgroups compared to controls (Adisetiyo et al. 2014). We explored whether there was an effect of current psychostimulant use on regional brain iron and found that children with ADHD taking stimulants did not differ in brain iron deposition from medication-naïve children with ADHD.
Of note, the specificity of ADHD-associated findings expand on prior studies reporting lower iron concentration in the striatum (caudate and putamen) in ADHD (Adisetiyo et al. (2014) and Cortese et al. (2012)) to show that this may be specific to the limbic subregion of the striatum and was not observed in the executive or sensorimotor subregions. However, it is important to note that their samples also included a broader age range including adolescents (ages 8–18 and 8–14, respectively). As discussed below, we observed age-related increases in iron concentration in the striatum, and prior studies have shown that there is a rapid accumulation of brain iron in early adolescence (Larsen et al. 2020a, b). Prominent models of adolescent brain development also suggest differential development of neural circuitry governing response to reward and cognitive control (Casey et al. 2016). Therefore, the specific pattern of brain anomalies observed in individuals with ADHD may vary across development relative to typical brain development, particularly during adolescence as a critical developmental period. Interestingly, Larsen et al. (2020a, b) recently found the strongest association between brain iron and presynaptic dopamine availability localized to the nucleus accumbens, a region of the striatum roughly corresponding to our designated limbic striatum. This supports our finding of reduced iron levels in the limbic striatum being linked to lower levels of presynaptic dopamine in children with ADHD, which is also in line with the dopaminergic deficit theory of ADHD (Luman et al. 2010; Sagvolden et al. 2005). Thus, our specific finding localized to the limbic striatum may be related to the higher concentration of dopaminergic receptors in this area or a function of the age range we examined speaking to the importance of longitudinal studies focused on these functional subdivisions of the striatum.
Moving beyond extant literature, by applying the Oxford-GSK-Imanova Striatal Connectivity atlas, (Tziortzi et al. 2013) we were able to examine specific functional domains of the striatum, including limbic striatum, an area that contributes extensively to emotional processing in circuit with orbitofrontal and other cortical regions (Catani et al. 2013; Rajmohan & Mohandas, 2017). Our findings revealing reduced tissue-iron specifically within the limbic striatal subdivision in children with ADHD highlight the importance of examining cortico-striatal limbic circuitry and emotional/affective function in the context of ADHD. Emotion dysregulation (Seymour et al. 2017; Shaw et al. 2014) and affective problems (Biederman et al. 1991; Jarrett and Ollendick 2008) are commonly observed in children with ADHD. Approximately 24% of children with ADHD demonstrate emotional difficulties (Sjöwall et al. 2013) and the presence of childhood emotional dysregulation is associated with increased rates of anxiety disorders, mood disorders, disruptive behavior disorders, and drug abuse in adulthood (Althoff et al. 2010). Furthermore, studies have shown that dopaminergic deficits, which contribute to an imbalance in limbic control systems (Sonuga-Barke 2002), are associated with increased impulsivity, a defining feature of ADHD (Swanson et al. 2007), as well as depressive symptoms such as anhedonia (Klimek et al. 2002).
ADHD has been associated with other abnormalities in limbic circuitry. The extant neuroimaging literature involving studies of children with ADHD compared to controls generally shows structural and functional abnormalities in frontal-striatal circuitry (see reviews by Cubillo et al. 2012; Faraone et al. 2015; Plichta and Scheres 2014). Specifically, studies have consistently shown reduced volume of the striatum, reduced white matter structural connectivity in fronto-striatal tracts, and underactivation of the caudate, and specifically the ventral striatum, during cognitive control and reward anticipation task-based fMRI. In addition, recent findings have suggested increased structural connectivity in individuals with ADHD compared to TD peers within subcortical limbic circuitry including the substantia nigra/ventral tegmental area-striatum tracts and greater connectivity strength was associated with more impulsive behavior (Elliot et al. 2021). Furthermore, a study of limbic white matter microstructure revealed that children with ADHD were found to show lower mean fractional anisotropy in the right cingulum, a major white matter tract associated with the limbic system, compared to a group of healthy controls (Stephens et al. 2021). We have also published findings of atypical fronto-subcortical intrinsic functional connectivity during resting-state fMRI among children with ADHD compared to TD controls (Rosch et al. 2018) and recently expanded upon these findings to demonstrate that this atypical functional connectivity was specific to the ventral striatum (Nikolaidis et al. in press). When combined with this extant literature, our findings of diminished levels of iron in the limbic striatum suggest that limbic substrates and pathways are altered both structurally and functionally in children with ADHD and that dopaminergic deficits in the limbic system may contribute to ADHD-related symptoms and related difficulties with emotion dysregulation as discussed below.
Prior studies have identified tissue-iron concentration as a measure of dopamine neurobiology (Adisetiyo et al. 2014; Larsen et al. 2020a, b). Given that dopaminergic dysfunction is a critical mechanism thought to underlie the presentation of ADHD (Cortese 2012), and that iron plays an integral role in supporting healthy dopaminergic function, the finding of reduced iron levels in children with ADHD provides support for a dopaminergic deficit model of ADHD (Luman et al. 2010; Sagvolden et al. 2005). The specific localization of diminished iron concentration in the limbic striatum, an area rich in dopamine receptors which are targeted by dopamine from the midbrain dopaminergic nuclei (Rommelfanger and Wichmann 2010; Sobel et al. 2010), hints more broadly to system-wide dopaminergic abnormalities. Moreover, diminished tissue-iron concentration affects normal cellular respiration and myelination, which is critical to neuronal development, synapse formation and plasticity, and network connectivity related to cognition, attention, emotion regulation, and motor control (Vértes and Bullmore 2015), domains that are impaired in ADHD.
Iron and sex
When examining sex effects on iron deposition in the striatum and globus pallidus, we found that males showed increased mT2w signal, or reduced iron in the left sensorimotor striatum compared to females. Surprisingly, very little is known regarding sex-related differences in brain iron. Several studies have reported that adult females show lower levels of iron in subcortical structures, including the caudate and thalamus (Bartzokis et al. 2007) and the substantia nigra (Persson et al. 2015). These findings are specific to adult females, and it is believed that estrogen levels mediate this decrease in iron (Grubić Kezele and Ćurko-Cofek 2020), which limits our ability to relate these findings to our own in pre-pubertal children. Larsen et al. (2020a, b) observed an earlier developmental plateau in tissue-iron in the globus pallidus in females at mid-20 s, compared to a continued increase throughout the ages of 8–27 in males. This sex difference in iron accumulation could partially explain reduced iron in the right sensorimotor region in males. Unfortunately, there is a lack of research on sex-related differences in brain iron in pediatric populations, thus we explored this question in the present study as understanding sex differences in relation to typical development is important for contextualizing ADHD-related sex differences.
Examination of diagnosis within sex revealed significant effects of diagnosis on mT2w signal in the left limbic striatum only in males. However, effect sizes were similar for both males and females and were moderately strong, with males showing only a slightly stronger effect. On the contrary, examining effect size in the right limbic striatum revealed a stronger effect size in females, despite neither sex group showing significant effects of diagnosis. These findings are preliminary and future study with larger sample sizes, particularly with more females with ADHD, would help to resolve whether sex has any significant impact on findings of ADHD-associated reductions in limbic striatal iron content.
Iron and age
Our findings show that across the whole sample, mT2w signal decreased indicating an increase in iron levels with age in the bilateral limbic striatum, the left executive striatum, the left sensorimotor striatum and most strongly in the bilateral globus pallidus. This is consistent with prior literature that describes a rapid increase in brain iron concentration in the first twenty years of life and maximum iron concentration in the globus pallidus (Aquino et al. 2009; Hallgren and Souranger 1958). These associations between iron and age are consistent with the extant literature and further corroborate the method of DTI for iron analyses. There was no evidence that the relationship between age and iron was moderated by, or differed as a function of diagnosis or a diagnosis-by-sex interaction. However, it will be important to examine differential change with age in children with ADHD in samples spanning a larger age range than the current study (ages 8–12) as this may depend on the developmental period.
Interestingly, the strength of the relationship of age with iron concentrations was strongest in the limbic striatum and globus pallidus, suggesting that the functional subdivisions of the striatum show differences in age-related brain iron accumulation and that the effect may not be uniform across all regions. This is in keeping with long-standing evidence that sensorimotor systems mature substantially earlier in child development than do executive/cognitive and limbic systems (Gilmore et al. 2018). This is the first paper reporting on age-related changes within distinct functional subdivisions (motor, executive, limbic) of the striatum. Overall, our age-related findings, while cross-sectional, provide foundation for pursuing future research with longitudinal cohorts, which will help to confirm and clarify localized patterns of age-related changes in iron concentration within the striatum.
Associations between Iron and ADHD, externalizing, and internalizing symptoms
Across our entire cohort of children with ADHD and TD controls, we found that increased mT2w signal, indicative of reduced levels of iron, in the right limbic striatum correlated significantly with higher parent-reported measures of ADHD symptom severity on the Conners Inattentive and Hyperactivity/Impulsivity scales, with trend-level significance in the left limbic striatum. These dimensional symptom associations are consistent with diagnostic group comparisons reported above. In 2010, Buckholtz et al. proposed a mechanistic theory stating that diminished midbrain D2 autoreceptor availability in healthy adults is implicated in impulsivity. This idea that dysregulation within ascending dopaminergic projection pathways produces deficits in impulse control relates to existing knowledge regarding iron’s role in supporting the dopaminergic system. Thus, it is not surprising to see that reduced levels of iron in both children with ADHD and TD controls is correlated with inattention, hyperactivity, and impulsivity. These associations, however, were not observed within the ADHD group itself, suggesting that the associations with symptom severity were principally driven by the group differences in limbic striatal iron concentration.
Associations across the whole group also revealed a positive trend-level association between mT2w signal and parent-rated affective symptoms on the CBCL. Specifically, higher mT2w signal (indicative of lower iron content) in the left limbic striatum was associated with greater anxious/depressive and affective symptoms as reported by parents. This relationship did not vary as a function of diagnosis or sex, with similar associations among females and males with and without ADHD. A recent study conducted in adolescent females supports this finding as it was revealed that those with lower serum ferritin (sF) concentrations (sF < 15 ng/mL) exhibited more severe depressive and anxiety symptoms compared to those with higher sF (sF < 20 ng/mL) concentrations and that lower sF concentration was associated with increased left caudate and bilateral putamen volume (Abbas et al. 2021). Collectively, these findings provide support for the role of iron in the limbic striatum as a neural correlate of childhood symptoms of ADHD and affective problems.
Limitations and future directions
There were some limitations to this study. Regarding our measurement approach, while the current gold standard for tissue-iron analyses uses quantitative susceptibility mapping (QSM), we chose to instead apply DTI, which is more commonly available, including in large-scale open access datasets. It is important to note that while tissue-iron content is certainly captured by T2-weighted signal, other factors such as tissue water content and myelin also contribute to T2 relaxation and are thus also being captured in the reported mT2w estimates, though to a lesser degree than tissue-iron in the studied ROIs. These effects lead to the limited sensitivity and specificity of T2 measures on tissue-iron, especially when compared to measures derived from T2* weighted sequences such as quantitative R2* and QSM (Haacke et al. 2005; Langkammer et al. 2010; Peterson et al. 2019; Schenker et al. 1993). Additionally, our mT2w signal is a normalized relative measure of the T2 relaxation obtained at a certain echo time specific to the scan protocol, which may hinder its direct quantitative comparison with other studies. However, DTI-derived mT2w signal measure can still serve as a useful, highly generalizable, proxy measure of tissue-iron concentration, particularly for iron-rich regions such as the basal ganglia (and deep cerebellar nuclei as one other example). Our findings using this approach, including hypothesized reductions in mT2w signal (increases in iron content) with age, provide additional construct validity for repurposing existing DTI datasets for conducting iron related analyses in large cohorts. In addition, the brain atlases used for striatum and globus pallidus labels were generated using adult subjects, and given the population for this study, replication of the analyses with a pediatric brain atlas would be more fitting.
Regarding characteristics of our sample, the sample sizes of the subgroups of females and males with ADHD differed substantially, with the fewest number of females with ADHD and the largest number of TD males. This imbalance did not provide enough statistical power to meaningfully conduct and interpret brain-behavior analyses within each subgroup. Similarly, the small sample of females made it difficult to examine effects of sex and to generalize findings to females with ADHD. Future analyses should replicate these analyses in a larger sample with better representation of females to increase statistical power for exploring sex effects. Another factor to possibly consider in future analyses is duration of psychostimulant use, which has been found to have an effect on brain iron (Adisetiyo et al. 2019). This was another limitation in the present study as we do not collect information regarding length of psychostimulant use. Furthermore, our sample was restricted to the age range of 8–12, providing a narrow window for examining developmental changes. Future studies with participants spanning early childhood through adolescence will be important. Finally, a follow-up study should collect QSM data to examine whether these findings remain consistent when performing analyses with what is considered the “gold-standard” approach.
Apart from brain iron investigations, prior studies have also examined serum ferritin levels and found reduced system iron levels in children with ADHD (Konofal et al. 2004; Lahat et al. 2011). Sever et al. (1997) found that treatment with Ferrocal resulted in increased mean serum ferritin levels and decreased parents’ score on the Conners Rating Scale. A recent meta-analysis from Degremont and colleagues (2021) revealed brain iron concentration to be more robustly associated with ADHD diagnosis than are peripheral measures of iron. Future studies of iron supplementation in ADHD would thereby benefit from examining changes in brain iron concentrations.
Conclusion
The present study has added to knowledge of the neurobiological underpinnings of ADHD, supporting previous findings of ADHD-associated reductions in striatal iron content with sample sizes much larger than prior studies and through use of widely available diffusion imaging data to estimate tissue-iron, which supports the repurposing of existing DTI datasets for iron content analyses. Crucially, our findings further suggest that these ADHD-associated reductions in iron content are localized to the limbic striatum, with these reductions related to ADHD symptom severity and affective symptoms. The findings provide preliminary support for examining iron content in ADHD and for further investigation of iron content as a biomarker relevant to ADHD diagnosis and treatment.
Supplementary Material
Acknowledgements
The authors wish to thank Joshua Robinson, B.S.a for his contributions to pre-processing and quality control of diffusion data.
Funding
This research was supported by the National Institutes of Health (R01MH085328; R01MH078160; K23MH101322; K23MH107734; P50HD103538). The MRI Equipment in this study was funded by NIH grant 1S10OD021648.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00221-022-06484-7.
Data availability The dataset analyzed for the current study is available in Open Science Framework, https://osf.io/mrqu8/?view_only=6e07a8b4211440b485da5ded155d46f4
Declarations
Ethics approval and consent to participate This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. After providing a complete study description to the participants, oral informed consent was obtained from a parent/guardian prior to the initial phone screening; written informed consent and assent were obtained from the parent/guardian and the child upon arrival at the initial laboratory visit.
Consent for publication Not applicable.
Conflict of interest The authors report no conflicts of interest.
References
- Abbas M, Gandy K, Salas R, Devaraj S, Calarge CA (2021) Iron deficiency and internalizing symptom severity in unmedicated adolescents: a pilot study Psychological medicine, 1–11. Adv Online Pub. 10.1017/S0033291721004098 [DOI] [PubMed]
- Achenbach TM, Ruffle TM (2000) The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 21(8):265–271. 10.1542/pir.21-8-265 [DOI] [PubMed] [Google Scholar]
- Adisetiyo V, Gray KM, Jensen JH, Helpern JA (2019) Brain iron levels in attention-deficit/hyperactivity disorder normalize as a function of psychostimulant treatment duration. NeuroImage Clinical 24:101993. 10.1016/j.nicl.2019.101993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adisetiyo V, Jensen JH, Tabesh A, Deardorff RL, Fieremans E, Di Martino A, Gray KM, Castellanos FX, Helpern JA (2014) Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: a noninvasive biomarker that responds to psychostimulant treatment? Radiology 272(2):524–532. 10.1148/radiol.14140047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoff RR, Verhulst FC, Rettew DC, Hudziak JJ, van der Ende J (2010) Adult outcomes of childhood dysregulation: a 14-year follow-up study. J Am Acad Child Adolesc Psychiatry 49(11):1105–1116. 10.1016/j.jaac.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Graham MS, Zsoldos E, Sotiropoulos SN (2016) Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 141:556–572. 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20(2):870–888. 10.1016/S1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- Andersson J, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M, Chiapparini L (2009) Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology 252(1):165–172. 10.1148/radiol.2522081399 [DOI] [PubMed] [Google Scholar]
- Ayton S, Fazlollahi A, Bourgeat P, Raniga P, Ng A, Lim YY, Diouf I, Farquharson S, Fripp J, Ames D, Doecke J, Desmond P, Ordidge R, Masters CL, Rowe CC, Maruff P, Villemagne VL, Salvado O, Bush AI (2017) Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain J Neurol. 140(8):2112–2119. 10.1093/brain/awx137 [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD (2010) Dopamine is released in the striatum during human emotional processing. NeuroReport 21(18):1172–1176. 10.1097/WNR.0b013e3283410955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121(1):65–94. 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J (2007) Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging 28(3):414–423. 10.1016/j.neurobiolaging.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34(1):144–155. 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003) Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50(5):1077–1088. 10.1002/mrm.10609 [DOI] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S (1991) Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry 148(5):564–577. 10.1176/ajp.148.5.564 [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH (2010) Dopaminergic network differences in human impulsivity. Science 329(5991):532. 10.1126/science.1185778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galván A, Somerville LH (2016) Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Dev Cogn Neurosci 17:128–130. 10.1016/j.dcn.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell’acqua F, Thiebaut de Schotten M (2013) A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev 37(8):1724–1737. 10.1016/j.neubiorev.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Chen C, Lidstone D, Crocetti D, Mostofsky SH, Nebel MB (2021) Increased interhemispheric somatomotor functional connectivity and mirror overflow in ADHD. NeuroImage Clinical 31:102759. 10.1016/j.nicl.2021.102759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Schoene-Bake JC, Elger CE, Weber B (2009) Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci 12(1):32–34. 10.1038/nn.2228 [DOI] [PubMed] [Google Scholar]
- Cortese S (2012) The neurobiology and genetics of attention-deficit/hyperactivity disorder (ADHD): what every clinician should know. Europ J Paediatric Neurol EJPN off J Europ Paediatric Neurol Soc 16(5):422–433. 10.1016/j.ejpn.2012.01.009 [DOI] [PubMed] [Google Scholar]
- Cortese S, Azoulay R, Castellanos FX, Chalard F, Lecendreux M, Chechin D, Delorme R, Sebag G, Sbarbati A, Mouren MC, Bernardina BD, Konofal E (2012) Brain iron levels in attention-deficit/hyperactivity disorder: a pilot MRI study. World J Biologic Psychiatry off J World Federation Soc Biologic Psychiatry 13(3):223–231. 10.3109/15622975.2011.570376 [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales—Revised. Published online 1997. Conners CK. Conners 3. Published online 2008.
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K (2012) A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex J Dev Study Nervous Sys Behav 48(2):194–215. 10.1016/j.cortex.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Curatolo P, D’Agati E, Moavero R (2010) The neurobiological basis of ADHD. Ital J Pediatr 36(1):79. 10.1186/1824-7288-36-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ (2018) Prevalence of parent-reported adhd diagnosis and associated treatment among U.S. children and adolescents. J Clin Child Adolescent Psychol off J Soc Clin Child Adolescent Psychol, Am Psychol Assoc Div. 47(2):199–212. 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degremont A, Jain R, Philippou E, Latunde-Dada GO (2021) Brain iron concentrations in the pathophysiology of children with attention deficit/hyperactivity disorder: a systematic review. Nutr Rev 79(5):615–626. 10.1093/nutrit/nuaa065 [DOI] [PubMed] [Google Scholar]
- Deistung A, Schäfer A, Schweser F, Biedermann U, Turner R, Reichenbach JR (2013) Toward in vivo histology: a comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. Neuroimage 65:299–314. 10.1016/j.neuroimage.2012.09.055 [DOI] [PubMed] [Google Scholar]
- Deistung A, Schweser F, Reichenbach JR (2017) Overview of quantitative susceptibility mapping. NMR Biomed. 10.1002/nbm.3569 [DOI] [PubMed]
- Dichtl S, Haschka D, Nairz M, Seifert M, Volani C, Lutz O, Weiss G (2018) Dopamine promotes cellular iron accumulation and oxidative stress responses in macrophages. Biochem Pharmacol 148:193–201. 10.1016/j.bcp.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Dirlikov B, Shiels Rosch K, Crocetti D, Denckla MB, Mahone EM, Mostofsky SH (2014) Distinct frontal lobe morphology in girls and boys with ADHD. NeuroImage Clinical 7:222–229. 10.1016/j.nicl.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H (2006) Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiat 60(10):1062–1070. 10.1016/j.biopsych.2005.12.020 [DOI] [PubMed] [Google Scholar]
- Elliott BL, D’Ardenne K, Mukherjee P, Schweitzer JB, McClure SM (2021) Limbic and executive meso- and nigrostriatal tracts predict impulsivity differences in attention-deficit/hyperactivity disorder. Cognitive neuroscience and neuroimaging, Biological psychiatry. 10.1016/j.bpsc.2021.05.002 [DOI] [PMC free article] [PubMed]
- Eskreis-Winkler S, Zhang Y, Zhang J, Liu Z, Dimov A, Gupta A, Wang Y (2017) The clinical utility of QSM: disease diagnosis, medical management, and surgical planning. NMR Biomed. 10.1002/nbm.3668 [DOI] [PubMed]
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJ, Tannock R, Franke B (2015) Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 1:15020. 10.1038/nrdp.2015.20 [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W (2018) Imaging structural and functional brain development in early childhood. Nat Rev Neurosci 19(3):123–137. 10.1038/nrn.2018.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubić Kezele T, Ćurko-Cofek B (2020) Age-related changes and sex-related differences in brain iron metabolism. Nutrients 12(9):2601. 10.3390/nu12092601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumus K, Keating B, Poser BA, Armstrong B, Chang L, Maclaren J, Prieto T, Speck O, Zaitsev M, Ernst T (2014) Prevention of motion-induced signal loss in diffusion-weighted echo-planar imaging by dynamic restoration of gradient moments. Magn Reson Med 71(6):2006–2013. 10.1002/mrm.24857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23(1):1–25. 10.1016/j.mri.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Haber SN (2016) Corticostriatal circuitry. Dialogues Clin Neurosci 18(1):7–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3(1):41–51. 10.1111/j.1471-4159.1958.tb12607.x [DOI] [PubMed] [Google Scholar]
- He N, Ling H, Ding B, Huang J, Zhang Y, Zhang Z, Liu C, Chen K, Yan F (2015) Region-specific disturbed iron distribution in early idiopathic Parkinson’s disease measured by quantitative susceptibility mapping. Hum Brain Mapp 36(11):4407–4420. 10.1002/hbm.22928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LA, Peterson DJ, Rosch KS, Crocetti D, Mori S, Mostofsky SH (2015) Sex-based dissociation of white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 54(11):938–946. 10.1016/j.jaac.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett MA, Ollendick TH (2008) A conceptual review of the comorbidity of attention-deficit/hyperactivity disorder and anxiety: implications for future research and practice. Clin Psychol Rev 28(7):1266–1280. 10.1016/j.cpr.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Jensen PS, Shervette RE 3rd, Xenakis SN, Richters J (1993) Anxiety and depressive disorders in attention deficit disorder with hyperactivity: new findings. Am J Psychiatry 150(8):1203–1209. 10.1176/ajp.150.8.1203 [DOI] [PubMed] [Google Scholar]
- Kaso A, Ernst T (2021) Motion-insensitive diffusion imaging of the brain using optical tracking and dynamic sequence updates. Magn Reson Med 86(2):926–934. 10.1002/mrm.28747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N (2016) Kiddie schedule for affective disorders and schizophrenia for school-aged children-present and lifetime version (Kiddie-SADS-PL 2016). Western Psychiatric Institute and Clinic and Yale University, Pittsburgh Pennsylvania [Google Scholar]
- Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA (2002) Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiat 52(7):740–748. 10.1016/s0006-3223(02)01383-5 [DOI] [PubMed] [Google Scholar]
- Konofal E, Lecendreux M, Arnulf I, Mouren MC (2004) Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 158(12):1113–1115. 10.1001/archpedi.158.12.1113 [DOI] [PubMed] [Google Scholar]
- Lahat E, Heyman E, Livne A, Goldman M, Berkovitch M, Zachor D (2011) Iron deficiency in children with attention deficit hyperactivity disorder. Israel Med Assoc J IMAJ 13(9):530–533 [PubMed] [Google Scholar]
- Larsen B, Bourque J, Moore TM, Adebimpe A, Calkins ME, Elliott MA, Gur RC, Gur RE, Moberg PJ, Roalf DR, Ruparel K, Turetsky BI, Vandekar SN, Wolf DH, Shinohara RT, Satterthwaite TD (2020a) Longitudinal development of brain iron is linked to cognition in youth. J Neurosci off J Soc Neurosci 40(9):1810–1818. 10.1523/JNEUROSCI.2434-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Luna B (2015) In vivo evidence of neurophysiological maturation of the human adolescent striatum. Dev Cogn Neurosci 12:74–85. 10.1016/j.dcn.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Olafsson V, Calabro F, Laymon C, Tervo-Clemmens B, Campbell E, Minhas D, Montez D, Price J, Luna B (2020b) Maturation of the human striatal dopamine system revealed by PET and quantitative MRI. Nat Commun 11(1):846. 10.1038/s41467-020-14693-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkammer C, Krebs N, Goessler W, Scheurer E, Ebner F, Yen K, Fazekas F, Ropele S (2010) Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 257(2):455–462. 10.1148/radiol.10100495 [DOI] [PubMed] [Google Scholar]
- Langkammer C, Schweser F, Krebs N, Deistung A, Goessler W, Scheurer E, Sommer K, Reishofer G, Yen K, Fazekas F, Ropele S, Reichenbach JR (2012) Quantitative susceptibility mapping (QSM) as a means to measure brain iron? Post Mortem Validation Study Neuroimage 62(3):1593–1599. 10.1016/j.neuroimage.2012.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li W, Tong KA, Yeom KW, Kuzminski S (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magnetic Res Imag JMRI 42(1):23–41. 10.1002/jmri.24768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Henriksen L, Bruhn P (1990) Focal cerebral dysfunction in developmental learning disabilities. Lancet (london, England) 335(8680):8–11. 10.1016/0140-6736(90)90136-s [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A (2010) Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobehav Rev 34(5):744–754. 10.1016/j.neubiorev.2009.11.021 [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M (2003) Imaging human mesolimbic dopamine transmission with positron emission. Tomography Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cerebral Blood Flow Metabolism off J Intern Soc Cerebral Blood Flow Metabolism. 23(3):285–300. 10.1097/01.WCB.0000048520.34839.1A [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2000) Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 31(2–3):236–250. 10.1016/s0165-0173(99)00040-5 [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40(2):570–582. 10.1016/j.neuroimage.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E, Naidu SB (1997) Attention-deficit hyperactivity disorder (ADHD) and frontal-motor cortex disconnection. Clinical EEG (electroencephalography) 28(3):130–136. 10.1177/155005949702800303 [DOI] [PubMed] [Google Scholar]
- Nikolaidis A, He X, Pekar J, Rosch K, Mostofsky SH (2022) Frontal corticostriatal functional connectivity reveals task positive and negative network dysregulation in relation to ADHD, sex, and inhibitory control. Develop Cognitive Neurosci. 54:101101. 10.1016/j.dcn.2022.101101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, Akhter K, Hua K, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Zhang J, Huang H, Miller MI, van Zijl PC, Mazziotta J, Mori S (2008) Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43(3):447–457. 10.1016/j.neuroimage.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patros C, Sweeney LK, Mahone EM, Mostofsky SH, & Rosch KS (2018) Greater delay discounting among girls, but not boys, with ADHD correlates with cognitive control. Child Neuropsychol J Normal Abnormal Develop Childhood Adolescence 24(8):1026–1046. 10.1080/09297049.2017.1359525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S (1996) Executive functions and developmental psychopathology. J Child Psychol Psychiatry 37(1):51–87. 10.1111/j.1469-7610.1996.tb01380.x [DOI] [PubMed] [Google Scholar]
- Persson N, Wu J, Zhang Q, Liu T, Shen J, Bao R, Ni M, Liu T, Wang Y, Spincemaille P (2015) Age and sex related differences in subcortical brain iron concentrations among healthy adults. Neuroimage 122:385–398. 10.1016/j.neuroimage.2015.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson ET, Kwon D, Luna B, Larsen B, Prouty D, De Bellis MD, Voyvodic J, Liu C, Li W, Pohl KM, Sullivan EV, Pfefferbaum A (2019) Distribution of brain iron accrual in adolescence: evidence from cross-sectional and longitudinal analysis. Hum Brain Mapp 40(5):1480–1495. 10.1002/hbm.24461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Scheres A (2014) Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev 38:125–134. 10.1016/j.neubiorev.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt E, Leibel RL (1976) Iron deficiency and behavior. J Pediatr 88(3):372–381. 10.1016/s0022-3476(76)80250-8 [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A (2006) Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 16(10):1508–1521. 10.1093/cercor/bhj088 [DOI] [PubMed] [Google Scholar]
- Prince J (2008) Catecholamine dysfunction in attention-deficit/hyperactivity disorder: an update. J Clin Psychopharmacol 28(3 Suppl 2):S39–S45. 10.1097/JCP.0b013e318174f92a [DOI] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, Mostofsky SH (2009) Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry 166(1):74–82. 10.1176/appi.ajp.2008.08030426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajmohan V, Mohandas E (2007) The limbic system. Indian J Psychiatry 49(2):132–139. 10.4103/0019-5545.33264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W (2000) Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry 39(1):59–66. 10.1097/00004583-200001000-00017 [DOI] [PubMed] [Google Scholar]
- Roalf DR, Quarmley M, Elliott MA, Satterthwaite TD, Vandekar SN, Ruparel K, Gennatas ED, Calkins ME, Moore TM, Hopson R, Prabhakaran K, Jackson CT, Verma R, Hakonarson H, Gur RC, Gur RE (2016) The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage 125:903–919. 10.1016/j.neuroimage.2015.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, Wichmann T (2010) Extrastriatal dopaminergic circuits of the Basal Ganglia. Front Neuroanat 4:139. 10.3389/fnana.2010.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropele S, Langkammer C (2017) Iron quantification with susceptibility. NMR Biomed. 10.1002/nbm.3534.10.1002/nbm.3534 [DOI] [PubMed]
- Rosch KS, Crocetti D, Hirabayashi K, Denckla MB, Mostofsky SH, Mahone EM (2018) Reduced subcortical volumes among preschool-age girls and boys with ADHD. Psychiatry Res Neuroimaging 271:67–74. 10.1016/j.pscychresns.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch KS, Mostofsky SH (2016) Increased delay discounting on a novel real-time task among girls, but not boys, with ADHD. J Intern Neuropsychol Soc JINS 22(1):12–23. 10.1017/S1355617715001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA (2005) A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci 28(3):397–468. 10.1017/S0140525X05000075 [DOI] [PubMed] [Google Scholar]
- Schenker C, Meier D, Wichmann W, Boesiger P, Valavanis A (1993) Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology 35(2):119–124. 10.1007/BF00593967 [DOI] [PubMed] [Google Scholar]
- Sever Y, Ashkenazi A, Tyano S, Weizman A (1997) Iron treatment in children with attention deficit hyperactivity disorder. Preliminary Rep Neuropsychobiol 35(4):178–180. 10.1159/000119341 [DOI] [PubMed] [Google Scholar]
- Seymour KE, Tang X, Crocetti D, Mostofsky SH, Miller MI, Rosch KS (2017) Anomalous subcortical morphology in boys, but not girls, with ADHD compared to typically developing controls and correlates with emotion dysregulation. Psychiatry Res Neuroimaging 261:20–28. 10.1016/j.pscychresns.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J (2006) Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 63(5):540–549. 10.1001/archpsyc.63.5.540 [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D (2012) Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiat 72(3):191–197. 10.1016/j.biopsych.2012.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, Leibenluft E (2014) Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry 171(3):276–293. 10.1176/appi.ajp.2013.13070966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöwall D, Roth L, Lindqvist S, Thorell LB (2013) Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. J Child Psychol Psychiatry 54(6):619–627. 10.1111/jcpp.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodin O, Cassuto H, Berger I (2018) Age-related changes in distractibility: developmental trajectory of sustained attention in ADHD. J Atten Disord 22(14):1333–1343. 10.1177/1087054715575066 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Sobel LJ, Bansal R, Maia TV, Sanchez J, Mazzone L, Durkin K, Liu J, Hao X, Ivanov I, Miller A, Greenhill LL, Peterson BS (2010) Basal ganglia surface morphology and the effects of stimulant medications in youth with attention deficit hyperactivity disorder. Am J Psychiatry 167(8):977–986. 10.1176/appi.ajp.2010.09091259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ (2002) Psychological heterogeneity in AD/HD–a dual pathway model of behaviour and cognition. Behav Brain Res 130(1–2):29–36. 10.1016/s0166-4328(01)00432-6 [DOI] [PubMed] [Google Scholar]
- Stephens K, Silk TJ, Anderson V, Hazell P, Enticott PG, Sciberras E (2021) Associations between limbic system white matter structure and socio-emotional functioning in children with ADHD + ASD. J Autism Dev Disord 51(8):2663–2672. 10.1007/s10803-020-04738-3 [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD (2007) Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev 17(1):39–59. 10.1007/s11065-007-9019-9 [DOI] [PubMed] [Google Scholar]
- Sweeney KL, Ryan M, Schneider H, Ferenc L, Denckla MB, Mahone EM (2018) Developmental trajectory of motor deficits in preschool children with ADHD. Dev Neuropsychol 43(5):419–429. 10.1080/87565641.2018.1466888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Fletcher PT., Gerber S, Whitaker RT (2009) A variational image-based approach to the correction of susceptibility artifacts in the alignment of diffusion weighted and structural MRI. Information processing in medical imaging: proceedings of the conference. 21:664–675. 10.1007/978-3-642-02498-6_55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari A, Jog R, Jog MS (2016) The striatum and subthalamic nucleus as independent and collaborative structures in motor control. Front Syst Neurosci 10:17. 10.3389/fnsys.2016.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR (2009) Oligodendrocytes and myelination: the role of iron. Glia 57(5):467–478. 10.1002/glia.20784 [DOI] [PubMed] [Google Scholar]
- Tripp G, Wickens JR (2009) Neurobiology of ADHD. Neuropharmacology 57(7–8):579–589. 10.1016/j.neuropharm.2009.07.026 [DOI] [PubMed] [Google Scholar]
- Tseng PT, Cheng YS, Yen CF, Chen YW, Stubbs B, Whiteley P, Carvalho AF, Li DJ, Chen TY, Yang WC, Tang CH, Chu CS, Yang WC, Liang HY, Wu CK, Lin PY (2018) Peripheral iron levels in children with attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Scientific Rep 8(1):788. 10.1038/s41598-017-19096-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Haber SN, Searle GE, Tsoumpas C, Long CJ, Shotbolt P, Douaud G, Jbabdi S, Behrens TE, Rabiner EA, Jenkinson M, Gunn RN (2014) Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb Cortex 24(5):1165–1177. 10.1093/cercor/bhs397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD (1998) Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 95(24):14494–14499. 10.1073/pnas.95.24.14494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen JM, Hua J, Unschuld PG, Lim IA, Jones CK, Margolis RL, Ross CA, van Zijl PC, Li X (2016) Quantitative susceptibility mapping suggests altered brain iron in premanifest huntington disease. AJNR Am J Neuroradiol 37(5):789–796. 10.3174/ajnr.A4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vértes PE, Bullmore ET (2015) Annual research review: growth connectomics–the organization and reorganization of brain networks during normal and abnormal development. J Child Psychol Psychiatry 56(3):299–320. 10.1111/jcpp.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu T (2015) Quantitative susceptibility mapping (QSM): decoding MRI data for a tissue magnetic biomarker. Magn Reson Med 73(1):82–101. 10.1002/mrm.25358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Spincemaille P, Liu Z, Dimov A, Deh K, Li J, Zhang Y, Yao Y, Gillen KM, Wilman AH, Gupta A, Tsiouris AJ, Kovanlikaya I, Chiang GC, Weinsaft JW, Tanenbaum L, Chen W, Zhu W, Chang S, Lou M, Prince MR (2017) Clinical quantitative susceptibility mapping (QSM): biometal imaging and its emerging roles in patient care. J Magnetic Res Imag JMRI 46(4):951–971. 10.1002/jmri.25693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu Q, Li S, Li G, Zuo C, Liao S, Long Y, Li S, Joshi RM (2018) Gender differences in anomalous subcortical morphology for children with ADHD. Neurosci Lett 665:176–181. 10.1016/j.neulet.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Welkie J, Babinski DE, Neely KA (2021) Sex and emotion regulation difficulties contribute to depression in young adults with attention-deficit/hyperactivity disorder. Psychol Rep 124(2):596–610. 10.1177/0033294120918803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Chang LC, Walker L, Lemaitre H, Barnett AS, Marenco S, Pierpaoli C. (2008) Comparison of EPI distortion correction methods in diffusion tensor MRI using a novel framework. Medical image computing and computer-assisted intervention: MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention. 11(2):321–329. 10.1007/978-3-540-85990-1_39 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.