Abstract
Background & Aims:
Sarcopenic obesity is associated with higher rates of morbidity and mortality than seen with either sarcopenia or obesity alone. We aimed to define sarcopenic visceral obesity (SVO) using computed tomography (CT)-quantified skeletal muscle index (SMI) and visceral-to-subcutaneous adipose tissue ratio (VSR) and to examine its association with waitlist mortality in patients with cirrhosis.
Approach & Results:
Included were 326 adults with cirrhosis awaiting liver transplantation in the ambulatory setting with available abdominal CT within 6 months from enrollment between 2/2015–1/2018. SVO was defined as patients with sarcopenia (SMI <50 cm2/m2 in men and <39 cm2/m2 in women) and visceral obesity (VSR ≥1.21 in men and ≥0.48 in women). The percentage who met criteria for sarcopenia, visceral obesity, and SVO were 44%, 29%, and 13%, respectively. Cumulative incidence of waitlist mortality was higher in patients with SVO compared to patients with sarcopenia without visceral obesity or visceral obesity without sarcopenia at 12 months (40% vs 21% vs 12%) [overall logrank p=0.003]. In univariable Cox regression, SVO was associated with waitlist mortality (HR 3.42, 95% CI 1.58–7.39), which remained significant after adjusting for age, sex, diabetes, ascites, encephalopathy, MELDNa, liver frailty index, and different body compositions (HR 2.64, 95% CI 1.11–6.30).
Conclusions:
SVO was associated with increase waitlist mortality in patients with cirrhosis in the ambulatory setting awaiting liver transplantation. Concurrent loss of skeletal muscle and gain of adipose tissue seen in SVO quantified by CT may be a useful and objective measurement to identify patients at risk for suboptimal pre-transplant outcomes.
Keywords: body composition, visceral adipose tissue, visceral-to-subcutaneous adipose tissue ratio, subcutaneous adipose tissue, skeletal muscle mass
INTRODUCTION
Approximately 20–35% of patients with cirrhosis develop sarcopenic obesity as a result of concurrent loss of skeletal muscle mass and gain of adipose tissue. (1–3) These rates will likely continue to increase in light of the obesity epidemic and the aging of patients with cirrhosis. (4–6) The concordance of these two conditions is associated with higher rates of pre/post-transplant morbidity and mortality than seen with either sarcopenia or obesity alone. (1, 3, 7–9) However, there continues to be no unified consensus for defining sarcopenic obesity in patients with cirrhosis, largely due to the variability in diagnostic modalities and varying cutoffs to define sarcopenia and obesity. (1, 3, 7, 10)
Although obesity is generally defined by cutoffs of body mass index (BMI), it is considered to be an imperfect metric in patients with cirrhosis due to volume overload and inability to account for differences in fat distribution, which was the defining criteria of obesity (either BMI ≥25 or ≥30 kg/m2) used in prior studies assessing its effects in the presence of sarcopenia. (1, 3, 8, 11) Presently, cross-sectional imaging such as computed tomography (CT) is commonly used to provide direct measurements of body composition such as skeletal muscle mass, visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT), allowing for objective assessment of a patient’s nutritional and metabolic status. As such, prior studies have used CT-quantified VAT area of ≥100 cm2 to define visceral obesity in sarcopenic patients with cirrhosis, though this has primarily been in Asian populations without adjusting for sex and/or stature. (7–9, 12) A standardized criteria to define sarcopenic obesity while encompassing various aspects of body composition is needed to establish clinically relevant cutoffs that can be used for clinical decision-making.
It is well recognized that the accumulation of VAT is responsible for the majority of liver- and obesity-related complications in comparison to SAT, which is due to its different metabolic and inflammatory effects. (13–15) Considering that obesity is a heterogeneous condition with regional difference in adipose tissue depot, we hypothesized that the relative distribution of visceral adiposity in the form of visceral-to-subcutaneous adipose tissue ratio (VSR) rather than the absolute area of its different depots may serve as a more objective measure of visceral obesity. Specifically, we aimed to better define sarcopenic visceral obesity (SVO) by using CT-quantified VSR as a marker of visceral obesity, and to examine its association with waitlist mortality in patients with cirrhosis awaiting liver transplantation.
MATERIALS AND METHODS
Study Design and Patient Selection
For this cohort study, we prospectively enrolled adult patients with cirrhosis awaiting liver transplantation in the ambulatory setting at a single transplant center between February 1, 2015 and January 31, 2018 as part of the Functional Assessment in Liver Transplantation (FrAILT) cohort study, and had available abdominal CT scan within 6 months from enrollment. Patients were excluded if they had inadequate body composition measurements on CT (Supplementary Figure 1, http://links.lww.com/XCL/A0); furthermore, baseline demographic and laboratory characteristics of patients included and excluded in the study cohort is available in Supplementary Table 1, http://links.lww.com/XCL/A3. Baseline was defined as date of study enrollment. Demographic and clinical data, including primary liver disease etiology, comorbid conditions, and laboratory data were manually chart reviewed and abstracted from the electronic health records by trained study personnel who were blinded to all body composition data. Clinical evidence of ascites and hepatic encephalopathy were ascertained through a manual chart review and determined based on recorded clinical physical examination and/or management plan/note at the time of study enrollment. The degree/severity of ascites was considered mild/moderate if it was controlled with an oral diuretic regimen and severe/refractory if it required frequent large volume paracentesis equal or more than one time per month and/or underwent transjugular intrahepatic portosystemic shunt (TIPS) placement. Patients’ outcomes were obtained prospectively, which included death, delisting due to being too sick for liver transplantation, underwent living or deceased donor liver transplantation, deferred/deactivated due to social reasons, and still waiting. The primary outcome was waitlist mortality, defined as the combined outcome of death or delisting for being too sick for liver transplantation.
All research was conducted in accordance with both the Declarations of Helsinki and Istanbul. Written consent was provided by all subjects. This study was approved by the Institutional Review Board at the University of California, San Francisco (San Francisco, CA, USA).
Measurement of Body Compositions
Body composition was assessed using secondary analysis of abdominal CT scans as part of the liver transplant evaluation. CT-based measures of skeletal muscle (psoas, erector spinae, multifidus, quadratus lumborum, rectus abdominis, transverse abdominis, and internal/external oblique), VAT and SAT, were quantified (cm2) at the lumbar (L3) vertebral level using a post-processing workstation (General Electric Advanced Workstation 4.6, Volume Viewer software, GE Healthcare, Waukesha, WI, USA), which enabled specific tissue demarcation using standard Hounsfield Unit thresholds of −29 to 150 for skeletal muscle (16), −150 to −50 for VAT (17), and −190 to −30 for SAT (18). As reported in prior studies using these specific Hounsfield Unit thresholds, tissue areas were outlined on an individual CT section/slice resulting in a semiautomatic computed total cross-sectional area (cm2) by summing tissue pixels and multiplying by pixel surface area. (19) All CT images were analyzed by a trained radiologist who was blinded to all clinical and outcome data. All values were normalized by height (m2), resulting in a skeletal muscle index (SMI, cm2/m2), visceral adipose tissue index (VATI, cm2/m2), and subcutaneous adipose tissue index (SATI, cm2/m2). VSR was calculated by dividing VATI and SATI. Sarcopenia was defined by previously established cutpoints of SMI <50 cm2/m2 for men and <39 cm2/m2 for women which have been shown to be associated with pre-transplant mortality independent of age and MELD score. (20, 21)
Statistical Analysis
Data were summarized using numbers and percentages (%) for categorical variables or medians and interquartile ranges (IQR) for continuous variables. Variables were compared between groups using Wilcoxon rank-sum and Pearson’s chi-square tests, as appropriate. Waitlist survival time was defined as the time from study enrollment to death due to any causes or delisting due to being too sick for transplant. Patients were censored at date of transplant if they underwent a living or deceased donor liver transplant, date of waitlist removal for reasons other than being too sick (e.g., psychosocial reasons), or last known date of clinical follow-up. The survival outcome was summarized using the Kaplan-Meier method and compared between groups using the logrank test. Time-dependent receiver operating characteristic (ROC) method was used to identify optimal VSR cutoffs to predict waitlist mortality, where the concordance probability function (defined as the product of sensitivity and specificity) at month 12 is maximized to determine the cutoffs. (22, 23) The effect of SVO on waitlist mortality was assessed by univariate and multivariate Cox regression analyses. Established clinical prognostic factors of mortality and candidate predictors with a p-value <0.10 in the univariable analysis were evaluated for inclusion in the final multivariable model. We used Cox regression as our primary analytic technique – rather than competing risks regression, which is often the favored statistical method to treat liver transplant waitlist data (24) – to allow for more natural causal inference between difference in body composition and waitlist mortality. A two-sided p-value <0.05 was considered statistically significant. Analyses were performed using STATA, version 15.1 (StataCorp, College Station, TX, USA).
RESULTS
Baseline Patient Characteristics
A total of 326 patients with cirrhosis were included. Baseline characteristics of the cohort are shown in Table 1. The majority were male (69%) and non-Hispanic white (54%), with a median age of 61 years and BMI of 27.4 kg/m2. The primary etiology of cirrhosis was chronic hepatitis C (48%) and alcohol-related liver disease (19%), followed by nonalcoholic fatty liver disease or non-alcoholic steatohepatitis (14%), chronic hepatitis B (8%), and autoimmune or cholestatic liver disease (6%). Hepatocellular carcinoma was present in 62% of patients. Rates of hypertension were 44% and diabetes were 30%. The median (IQR) Model for End-Stage Liver Disease sodium (MELDNa) was 13 (10–17) and median (IQR) albumin was 3.3 (2.8–3.7) g/dL. Hepatic encephalopathy was present in 48% of patients. Ascites was present in 54% of patients, with 40% of patients categorized as mild/moderate and 14% as severe/refractory. In patients with severe ascites, 14 or 32% underwent TIPS placement.
Table 1.
Baseline demographic and laboratory characteristics in patients with and without sarcopenic visceral obesity
| Overall n=326 |
Sarcopenic visceral obesity n=41 |
Without sarcopenic visceral obesity n=285 |
p-value | |
|---|---|---|---|---|
| Age (years) | 61 (56–65) | 65 (61–67) | 60 (54–64) | <0.001 |
| Sex (male) | 225 (69) | 26 (63) | 199 (70) | 0.41 |
| Race | 0.18 | |||
| Non-Hispanic white | 177 (54) | 27 (66) | 150 (53) | |
| Hispanic white | 83 (25) | 5 (12) | 78 (27) | |
| Asian | 42 (13) | 8 (20) | 34 (12) | |
| Black | 19 (6) | 1 (2) | 18 (6) | |
| Other | 5 (1) | 0 (0) | 5 (2) | |
| Body mass index (kg/m2) | 27.4 (24.8–30.8) | 25.2 (22.8–27.8) | 27.7 (25.1–31.3) | <0.001 |
| Weight (kg) | 83.0 (70.8–93.9) | 75.8 (63.0–84.8) | 83.0 (71.2–93.0) | 0.003 |
| Etiology | 0.81 | |||
| Hepatitis C infection | 157 (48) | 19 (46) | 138 (48) | |
| Hepatitis B infection | 27 (8) | 4 (10) | 23 (8) | |
| Alcohol-related | 61 (19) | 5 (12) | 56 (20) | |
| NAFLD/NASH | 45 (14) | 7 (17) | 38 (13) | |
| AIH/PBC/PSC | 20 (6) | 3 (7) | 17 (6) | |
| Other | 16 (5) | 3 (7) | 13 (5) | |
| Hypertension | 143 (44) | 19 (46) | 124 (44) | 0.73 |
| Diabetes mellitus | 99 (30) | 14 (34) | 85 (30) | 0.57 |
| Coronary artery disease | 19 (6) | 3 (7) | 16 (6) | 0.66 |
| Ascites | 0.001 | |||
| Mild/moderate | 132 (40) | 13 (32) | 119 (42) | |
| Severe/refractory | 44 (14) | 13 (32) | 31 (11) | |
| Hepatic encephalopathy | 157 (48) | 23 (56) | 134 (47) | 0.28 |
| Hepatocellular carcinoma | 203 (62) | 26 (63) | 177 (62) | 0.87 |
| MELDNa | 13 (10–17) | 12 (9–16) | 13 (10–17) | 0.58 |
| Body composition | ||||
| SMI (cm2/m2) | 47 (41–53) | 39 (36–45) | 48 (43–54) | <0.001 |
| VATI (cm2/m2) | 42 (26–63) | 53 (33–75) | 41 (24–60) | 0.004 |
| SATI (cm2/m2) | 62 (44–88) | 44 (40–62) | 65 (47–89) | <0.001 |
| VSR | 0.62 (0.39–0.97) | 1.42 (0.64–1.81) | 0.57 (0.38–0.88) | <0.001 |
| Liver frailty index (LFI) | 3.7 (3.2–4.2) | 3.8 (3.6–4.7) | 3.6 (3.1–4.1) | 0.003 |
| Frailty (LFI >4.4) | 51 (16) | 39 (14) | 12 (29) | 0.01 |
| Outcomes | 0.02 | |||
| Death/Delisted | 79 (24) | 17 (41) | 62 (22) | |
| Transplant | 177 (54) | 20 (49) | 157 (55) | |
| Waiting | 13 (4) | 0 (0) | 13 (5) | |
| Deferred | 57 (17) | 4 (10) | 53 (19) | |
| Follow-up duration (month) | 10 (5–17) | 8 (3–13) | 10 (6–18) | 0.01 |
Values reported in median (IQR) or number (percentage). Abbreviations: AIH/PBC/PSC, autoimmune hepatitis/primary biliary cholangitis/primary sclerosing cholangitis; INR, international normalized ratio; MELDNa, model for end-stage liver disease-sodium; NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis; SATI, subcutaneous adipose tissue index; SMI, skeletal mass index; VATI, visceral adipose tissue index; VSR, visceral-to-subcutaneous adipose tissue index
The median (IQR) time between CT imaging and baseline was 1 (0–2) month and the median (IQR) time from CT imaging to clinical outcome was 12 (6–19) months. Overall median VATI and SATI were 42 and 62 cm2/m2, respectively, with a median VSR of 0.62 (Table 1). In men, overall median VATI and SATI were 47 and 59 cm2/m2, respectively, with a median VSR of 0.80. In women, overall median VATI and SATI were 30 and 73 cm2/m2, respectively, with a median VSR of 0.39. Although both VATI and SATI appeared to have a slight positive linear relationship with SMI, there was no significant direct linear association between VSR and SMI among both men (Supplementary Figure 2, http://links.lww.com/XCL/A1) and women (Supplementary Figure 3, http://links.lww.com/XCL/A2). A higher proportion of men (47%) met criterion for sarcopenia with a median SMI of 51 cm2/m2 compared to women (39%) with a median SMI of 41 cm2/m2. Baseline clinical characteristics according to sex are shown in Supplementary Table 1, http://links.lww.com/XCL/A3.
By the end of follow-up with a median duration of 10 (IQR 5–17) months, 79 (24%) had the primary outcome of death or delisting due to being too sick for liver transplantation, 177 (54%) underwent liver transplantation, 57 (17%) were deferred/delisted for psychosocial reasons, and 13 (4%) were still waiting. The number of patients who experienced waitlist mortality was 24 (7%) at 6 months and 48 (15%) at 12 months.
Identifying Optimal Cutoff Values for Visceral Obesity Associated with Waitlist Mortality
To identify an optimal cutoff of VSR to define visceral obesity, we first assessed the relationship between VSR and waitlist mortality stratified by sex based on the different visceral and subcutaneous adiposity observed in our cohort among men and women. Using the time-dependent ROC method to identify a cutoff that could be used in clinical practice, the optimal (highest concordance probability function at month 12) VSR cutoff of ≥1.21 for men (sensitivity 43% and specificity 88%) and ≥0.48 for women (sensitivity 41% and specificity 67%) were used to define visceral obesity in our cohort.
Association of Sarcopenic Visceral Obesity and Waitlist Mortality
We then defined SVO as the combination of sarcopenia (SMI <50 cm2/m2 for men and <39 cm2/m2 for women) and visceral obesity (VSR ≥1.21 for men and ≥0.48 for women). Distributions of various body compositions including SMI, VATI, SATI, and VSR among men and women, with and without SVO are shown Supplementary Table 2, http://links.lww.com/XCL/A3. Among the 326 patients in our cohort, 128 (39%) were categorized as non-sarcopenia and non-visceral obesity, 53 (16%) were categorized as non-sarcopenia and visceral obesity, 104 (32%) were categorized as sarcopenia and non-visceral obesity, and 41 (13%) were categorized as sarcopenia and visceral obesity. Baseline clinical characteristics in patients with and without SVO are shown in Table 1. Compared to patients without SVO, patients with SVO were significantly older (median age of 65 vs 60 years), had lower median BMI (25.2 vs 27.7 kg/m2), and lower median weight (75.8 vs 83.0 kg). Prevalence of ascites was slightly higher in patients with SVO (63% vs 53%, p=0.20) with a significantly higher proportion characterized as severe/refractory (32% vs 11%) compared to patients without SVO. Among patients with severe/refractory ascites, 14 patients underwent TIPS placement with no significant difference between patients with and without SVO (35% vs 23%, p=0.42). Both patients with and without SVO had similar rates of hepatocellular carcinoma (63% vs 62%, respectively), comorbidities with diabetes (34% vs 30%) and hypertension (46% vs 44%), and median MELDNa (12 vs 13). There were no differences in underlying etiology among patients with and without SVO.
Patients with SVO had a significantly higher cumulative incidence of waitlist mortality at 6 months (19% vs 6%, overall logrank p=0.003) and 12 months (40% vs 16%, overall logrank p<0.001) compared to patients without SVO. Among various body composition groups, the cumulative incidence of waitlist mortality was higher in patients with both sarcopenia and visceral obesity compared to patients with sarcopenia without visceral obesity and visceral obesity without sarcopenia at 6 months (19% vs 10% vs 2%, overall logrank p=0.009) and 12 months (40% vs 21% vs 12%, overall logrank p=0.003), as shown in Figure 1.The presence of SVO remained significantly associated with cumulative incidence of waitlist mortality at 12 months compared to those without even when stratified by sex: 48% vs 19% (overall logrank p=0.002) in women and 36% vs 15% (overall logrank p=0.05) in men.
Figure 1.
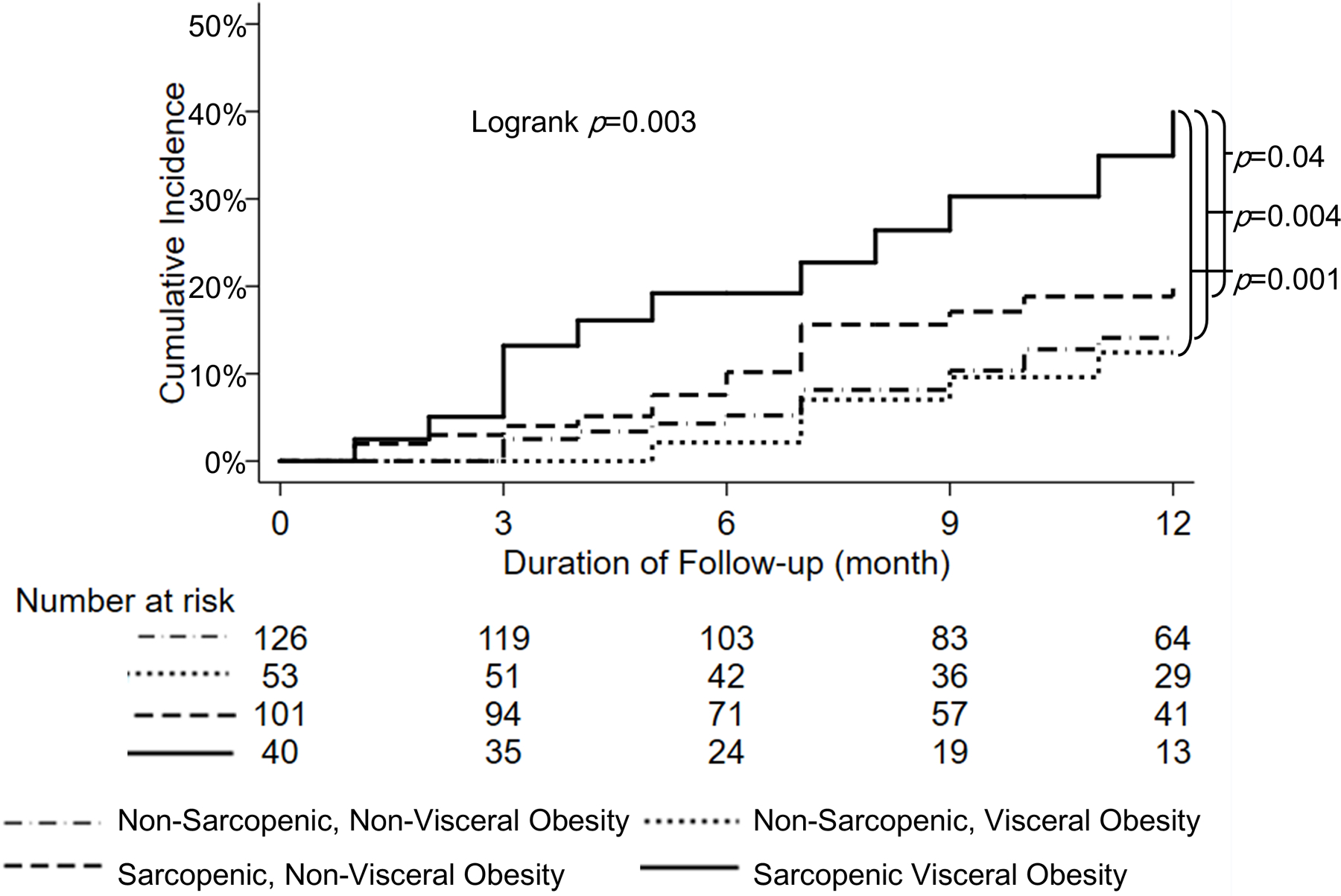
Cumulative incidence of waitlist mortality according to different subgroups of sarcopenia and visceral obesity through 12 months
In univariable Cox regression, SVO was associated with waitlist mortality (HR 3.42, 95% CI 1.58–7.39, p=0.002), which remained significant after adjusting for age, sex, diabetes, ascites, encephalopathy, MELDNa, liver frailty index, and different body compositions (HR 2.64, 95% CI 1.11–6.30, p=0.03) (Table 2).
Table 2.
Univariable and multivariable Cox regression for predictors of death or delisted for being too sick for liver transplantation at 12 months
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (year) | 1.01 | 0.97–1.04 | 0.70 | 1.01 | 0.97–1.06 | 0.58 |
| Sex (male) | 0.74 | 0.41–1.35 | 0.33 | 0.92 | 0.48–1.75 | 0.79 |
| Diabetes | 2.22 | 1.26–3.94 | 0.01 | 1.68 | 0.89–3.19 | 0.11 |
| Ascites | ||||||
| None | Ref | - | - | Ref | - | - |
| Mild/moderate | 3.11 | 1.54–6.30 | 0.002 | 2.23 | 1.03–4.81 | 0.04 |
| Severe/refractory | 4.21 | 1.78–9.92 | 0.001 | 1.58 | 0.54–4.63 | 0.40 |
| Hepatic encephalopathy | 2.68 | 1.43–5.01 | 0.002 | 1.34 | 0.66–2.74 | 0.42 |
| MELDNa (per 1 point) | 1.08 | 1.04–1.14 | 0.001 | 1.04 | 0.98–1.10 | 0.19 |
| Liver frailty index (per 0.1 point) | 2.13 | 1.54–2.95 | <0.001 | 1.41 | 0.94–2.09 | 0.09 |
| Body composition | ||||||
| Non-sarcopenia, non-visceral obesity | Ref | - | - | Ref | - | - |
| Non-sarcopenia, visceral obesity | 0.83 | 0.30–2.31 | 0.72 | 0.94 | 0.33–2.71 | 0.91 |
| Sarcopenia, non-visceral obesity | 1.60 | 0.78–3.39 | 0.20 | 1.70 | 0.82–3.52 | 0.15 |
| Sarcopenic visceral obesity | 3.42 | 1.58–7.39 | 0.002 | 2.64 | 1.11–6.30 | 0.03 |
Abbreviations: MELDNa, model for end-stage liver disease-sodium.
Comparison of Different Visceral Obesity Criteria: VSR, VAT area, and BMI
We then evaluated the degree of improvement in predicting waitlist mortality when VSR was used to define visceral obesity compared to other criteria used in prior studies: BMI ≥25.0 kg/m2 (3, 8, 11) or VAT area ≥100 cm2 (7–9). Similar to the above multivariable Cox regression model, after adjusting for age, sex, diabetes, ascites, encephalopathy, MELDNa, liver frailty index, and different body compositions, there was no longer any statistically significant association with waitlist mortality when SVO was defined by either VAT area ≥100 cm2 (HR 3.31, 95% CI 0.68–16.00, p=0.15) or BMI ≥25.0 kg/m2 (HR 1.08, 95% CI 0.34–3.45, p=0.90).
DISCUSSION
In this prospective cohort study of patients with cirrhosis awaiting liver transplantation in an ambulatory setting, we observed that sarcopenia and visceral obesity when defined by CT-quantified body compositions, were present in nearly one-half and one-third of patients, respectively. Over one-tenth of patients were categorized as having SVO based on the presence of both sarcopenia and visceral obesity, which were similar to rates reported in previous studies that included a heterogeneous cohort of patients with cirrhosis awaiting liver transplantation. (3, 15, 21) Presence of both sarcopenia and visceral obesity was independently associated with higher risk of waitlist mortality, resulting in 6- and 12-month waitlist mortality rate of 19% and 40%, respectively, compared to patients with either sarcopenia without visceral obesity or visceral obesity without sarcopenia.
Although body composition is commonly measured using BMI in clinical practice due to its ease of measurement, studies that evaluate the association between obesity – as measured by BMI – and outcomes in patients with cirrhosis are conflicting with some demonstrating that obesity is a risk factor for pre/post-transplant mortality (25–28) and others demonstrating no association (6, 29). This may be due to dynamic shifts in volume retention (e.g., ascites and peripheral edema), sex and racial differences of body composition (e.g., visceral vs subcutaneous fat, and skeletal muscle), and differences in criteria from prior studies in classifying obesity [e.g., BMI ≥25 kg/m2 (3, 8) or ≥30 kg/m2 (1), and/or VAT area ≥100 cm2 (7–9)]. Given these limitations, cross-sectional imaging with CT is emerging as a gold standard in non-invasive clinical assessment of body compositions allowing for objective evaluation of nutritional and metabolic status using readily available quantitative morphomics software with standardized Hounsfield Unit for specific tissue demarcation/quantification. However, some limitations to this method include the limited availability of CT scan performed outside of other clinical use such as hepatocellular surveillance/management and/or surgical planning and its limited potential applicability as the primary method of detecting sarcopenia and visceral obesity due to its cost and radiation exposure. Nonetheless, when available, this metric combining both muscle and adipose tissue quantification advances our understanding of pragmatic risk assessments. Although prior studies have used CT-quantified VAT area of ≥100 cm2 to define visceral obesity in sarcopenic patients with cirrhosis, it did not adjust for sex and/or stature (7–9). Additionally, body fat distribution may be more important than total body adiposity given the direct relationship of VAT and inverse relationship of SAT with clinical outcomes in patients with cirrhosis. (13, 30–34) As such, the relative distribution of adipose tissue with CT-quantified VSR rather than the absolute value of its different depots or anthropometric measurements may more comprehensively and objectively define visceral obesity.
In the present study, interestingly, BMI and weight were significantly lower in patients with SVO compared to patients without SVO (25.2 vs 27.7 kg/m2 and 75.8 vs 83.0 kg, respectively). This further highlights the unreliability of BMI as a marker of obesity, but more importantly, its ability to account for weight loss due to loss of muscle mass in sarcopenia as it is likely concealed by fluid retention with ascites, which was present in the majority of patients (63%) with SVO. Additionally, severe/refractory ascites requiring large volume paracentesis was observed in a higher proportion of patients with SVO (32% vs 11%) compared to those without SVO, and may be a predictor of frailty, the functional construct of sarcopenia. (35) In this study, presence of ascites in general was associated with increased waitlist mortality rather than the severe degree of ascites. Within the construct of SVO, encompassing both aspect of visceral adiposity (e.g., VATI and SATI in the form of VSR) along with sarcopenia (e.g., SMI) had a greater ability to predict waitlist mortality compared to solely using total VAT area or BMI cutoffs to define visceral obesity. Furthermore, only SVO defined by VSR was significantly associated with cumulative incidence of waitlist mortality through 12 months and was independently associated with waitlist mortality even after adjusting for age, sex, diabetes, ascites, encephalopathy, MELDNa, and different body compositions, which was neither observed when SVO was defined by VAT area ≥100 cm2 or BMI ≥25.0 kg/m2. Thus, the ability to identify different clinical phenotypes of patients with cirrhosis based on their body compositions (e.g., sarcopenic visceral obesity) may allow for better stratification of patients at higher risk for disease progression for more individualized management strategies with weight loss while preserving skeletal muscle mass and/or function.
It is important to note the differences in VSR cutoffs used to define SVO in this current study compared to our previous study, which included acutely ill patients with cirrhosis undergoing urgent evaluation and liver transplantation. (36) The primary reason for different VSR cutoffs was due to differences in quantified SATI. Although the role of SAT remains to be fully elucidated, limited prior studies have shown an inverse association with liver-related mortality due to its potential metabolic protective properties through its different inflammatory and adipocytokine profile. (13, 32) Furthermore, both skeletal muscle wasting and loss of adipose tissue has been shown to occur in the setting of critical illness. As such, it is not unexpected that there was a lower median SATI (32 vs 62 cm2/m2) in our more critically ill cohort as evidence by higher proportion of ascites (80% vs 54%), hepatic encephalopathy (63% vs 48%), and higher median MELDNa (33 vs 13) compared to this current cohort. We then conducted sensitivity analyses to categorize patients in this study with our previous VSR cutoffs (>1.54 in men and >1.37 in women) to define SVO. We observed that SVO remained significantly associated with waitlist mortality in univariable Cox regression (HR 3.02, 95% CI 1.12–8.15) though did not reach statistical significance in multivariable Cox regression (HR 2.14, 95% CI 0.73–6.28) after adjusting for the same cofactors (age, sex, diabetes, ascites, encephalopathy, MELDNa, liver frailty index, and different body compositions). Furthermore, we observed similar increased cumulative incidence rate of waitlist mortality through 12 months in patients with both sarcopenia and visceral obesity compared to patients with sarcopenia without visceral obesity and visceral obesity without sarcopenia at 6 months (18% vs 12% vs 5%) and 12 months (35% vs 25% vs 14%) (overall logrank p=0.03). Despite the differences in these studies, SVO remained associated with worse waitlist and post-transplant mortality in patients with cirrhosis, further supporting that the concordance of sarcopenic and visceral obesity is associated with higher rates of morbidity and mortality than seen with either alone.
We acknowledge several limitations to our study. We assessed and prospectively enrolled patients with cirrhosis awaiting liver transplantation in the ambulatory setting at a single tertiary-care liver transplant center; as such, our data may not be as generalizable to the general population of patients with cirrhosis, especially those who are acutely ill. However, the median MELDNa score of 13 in our patients is similar to the proportion of initial MELDNa score of <15 seen in the majority of patients awaiting liver transplantation in the United States. (37) Additionally, the prevalence of sarcopenia, visceral obesity, and SVO in our population were similar to that reported in prior studies of patients with cirrhosis awaiting liver transplantation using similar CT-quantification of body composition (3, 15, 21), further improving the generalizability of our findings. Body composition measurements were ascertained at the time of enrollment, which could have occurred at any time while the patient was on the waitlist and not necessarily at listing, though the median duration from CT imaging to enrollment was only 1 month. Lastly, potential VSR cutoffs using time-dependent ROC method was limited to 12 months to maximize the number of primary outcome/events (i.e., waitlist mortality) and number of patients with available follow-up. VSR cutoffs at shorter study duration (e.g., 30-day, 90-day, 180-day) were limited due to fewer number of primary outcome/events while cutoffs at longer study duration (e.g., 24-month, 36-month) were limited due to fewer number of patients with extended follow-up past 12 months among the various body composition groups. Larger studies including a heterogenous cohort of cirrhosis patients with longer follow-up duration are needed to explore different cutoffs at various timepoints and to validate our proposed VSR cutoffs in defining visceral obesity and its association along with sarcopenia resulting in suboptimal clinical outcomes.
In conclusion, we demonstrate that the concordance of sarcopenia and visceral obesity in patients with cirrhosis awaiting liver transplantation was associated with a higher risk of waitlist mortality than seen with ether condition alone. We propose using the relative distribution of adipose tissue with CT-quantified VSR rather than the absolute value of its different depots or anthropometric measurements as a better method to comprehensively define visceral obesity, and objectively identify patients at risk for suboptimal waitlist outcomes.
Supplementary Material
Supplementary Figure 1. Study flow chart
Supplementary Figure 2. Two-way scatter plots of (A) visceral adipose tissue index (cm2/m2), (B) subcutaneous adipose tissue index (cm2/m2), and (C) visceral-to-subcutaneous adipose tissue ratio according to skeletal muscle index (cm2/m2) among men
Supplementary Figure 3. Two-way scatter plots of (A) visceral adipose tissue index (cm2/m2), (B) subcutaneous adipose tissue index (cm2/m2), and (C) visceral-to-subcutaneous adipose tissue ratio according to skeletal muscle index (cm2/m2) among women
Financial Support:
This study was funded by NIH R01AG059183 (Lai), NIH P30DK026743 (Huang, Lai, Shui), NIH R21AG067554 (Lai), and NIH 5T32DK060414-18 (Ha). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
List of Abbreviations:
- BMI
body mass index
- CT
computed tomography
- IQR
interquartile range
- MELDNa
model for end-stage liver disease sodium
- SMI
skeletal muscle index
- SAT
subcutaneous adipose tissue
- SATI
subcutaneous adipose tissue index
- VAT
visceral adipose tissue
- VATI
visceral adipose tissue index
- VSR
visceral-to-subcutaneous adipose tissue ratio
Footnotes
COI Jennifer C. Lai advises Novo Nordisk, consults for Genfit and has received grants from Nestle Nutrition Institute.
REFERENCES
- 1.Carias S, Castellanos AL, Vilchez V, Nair R, Dela Cruz AC, Watkins J, Barrett T, et al. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol 2016;31:628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int 2018;38:1706–1717. [DOI] [PubMed] [Google Scholar]
- 3.Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandman D Obesity Management of Liver Transplant Waitlist Candidates and Recipients. Clin Liver Dis 2021;25:1–18. [DOI] [PubMed] [Google Scholar]
- 5.Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol 2015;31:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spengler EK, O’Leary JG, Te HS, Rogal S, Pillai AA, Al-Osaimi A, Desai A, et al. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation 2017;101:2288–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara N, Iwasa M, Sugimoto R, Mifuji-Moroka R, Yoshikawa K, Terasaka E, Hattori A, et al. Sarcopenia and Sarcopenic Obesity Are Prognostic Factors for Overall Survival in Patients with Cirrhosis. Intern Med 2016;55:863–870. [DOI] [PubMed] [Google Scholar]
- 8.Kamo N, Kaido T, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, Yao S, et al. Impact of sarcopenic obesity on outcomes in patients undergoing living donor liver transplantation. Clin Nutr 2019;38:2202–2209. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, Kamo N, et al. Impact of Sarcopenic Obesity on Outcomes in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Ann Surg 2019;269:924–931. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131–140. [DOI] [PubMed] [Google Scholar]
- 11.Hammad A, Kaido T, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, Kamo N, et al. Impact of sarcopenic overweight on the outcomes after living donor liver transplantation. Hepatobiliary Surg Nutr 2017;6:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Examination Committee of Criteria for ‘Obesity Disease’ in J, Japan Society for the Study of O. New criteria for ‘obesity disease’ in Japan. Circ J 2002;66:987–992. [DOI] [PubMed] [Google Scholar]
- 13.Ebadi M, Tandon P, Moctezuma-Velazquez C, Ghosh S, Baracos VE, Mazurak VC, Montano-Loza AJ. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol 2018;69:608–616. [DOI] [PubMed] [Google Scholar]
- 14.Kimura N, Tsuchiya A, Oda C, Kimura A, Hosaka K, Tominaga K, Hayashi K, et al. Visceral adipose tissue index and hepatocellular carcinoma are independent predictors of outcome in patients with cirrhosis having endoscopic treatment for esophageal varices. Dig Dis 2020. [DOI] [PubMed]
- 15.Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, Beaumont C, et al. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol 2015;6:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. [DOI] [PubMed] [Google Scholar]
- 17.Vehmas T, Kairemo KJ, Taavitsainen MJ. Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord 1996;20:570–573. [PubMed] [Google Scholar]
- 18.Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes 1986;10:53–67. [PubMed] [Google Scholar]
- 19.Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861–870. [DOI] [PubMed] [Google Scholar]
- 20.Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, Dunn MA, et al. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology 2019;70:1816–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano-Loza AJ, Dunn MA, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cattaneo M, Malighetti P, Spinelli D. Estimating receiver operative characteristic curves for time-dependent outcomes: The stroccurve package. The Stata Journal 2017;17:1015–1023. [Google Scholar]
- 23.Liu X Classification accuracy and cut point selection. Stat Med 2012;31:2676–2686. [DOI] [PubMed] [Google Scholar]
- 24.Kim WR, Therneau TM, Benson JT, Kremers WK, Rosen CB, Gores GJ, Dickson ER. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology 2006;43:345–351. [DOI] [PubMed] [Google Scholar]
- 25.Hakeem AR, Cockbain AJ, Raza SS, Pollard SG, Toogood GJ, Attia MA, Ahmad N, et al. Increased morbidity in overweight and obese liver transplant recipients: a single-center experience of 1325 patients from the United Kingdom. Liver Transpl 2013;19:551–562. [DOI] [PubMed] [Google Scholar]
- 26.Kardashian AA, Dodge JL, Roberts J, Brandman D. Weighing the risks: Morbid obesity and diabetes are associated with increased risk of death on the liver transplant waiting list. Liver Int 2018;38:553–563. [DOI] [PubMed] [Google Scholar]
- 27.LaMattina JC, Foley DP, Fernandez LA, Pirsch JD, Musat AI, D’Alessandro AM, Mezrich JD. Complications associated with liver transplantation in the obese recipient. Clin Transplant 2012;26:910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singhal A, Wilson GC, Wima K, Quillin RC, Cuffy M, Anwar N, Kaiser TE, et al. Impact of recipient morbid obesity on outcomes after liver transplantation. Transpl Int 2015;28:148–155. [DOI] [PubMed] [Google Scholar]
- 29.Fujikawa T, Fujita S, Mizuno S, Shenkman E, Vogel B, Lipori P, Hemming AW, et al. Clinical and financial impact of obesity on the outcome of liver transplantation. Transplant Proc 2006;38:3612–3614. [DOI] [PubMed] [Google Scholar]
- 30.Montano-Loza AJ, Mazurak VC, Ebadi M, Meza-Junco J, Sawyer MB, Baracos VE, Kneteman N. Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatology 2018;67:914–923. [DOI] [PubMed] [Google Scholar]
- 31.Ohki T, Tateishi R, Shiina S, Goto E, Sato T, Nakagawa H, Masuzaki R, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut 2009;58:839–844. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues SG, Brabandt B, Stirnimann G, Maurer MH, Berzigotti A. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int 2019;39:1672–1681. [DOI] [PubMed] [Google Scholar]
- 33.Terjimanian MN, Harbaugh CM, Hussain A, Olugbade KO, Jr., Waits SA, Wang SC, Sonnenday CJ, et al. Abdominal adiposity, body composition and survival after liver transplantation. Clin Transplant 2016;30:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, London R, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008;48:449–457. [DOI] [PubMed] [Google Scholar]
- 35.Lin FP, Visina JM, Bloomer PM, Dunn MA, Josbeno DA, Zhang X, Clemente-Sanchez A, et al. Prehabilitation-Driven Changes in Frailty Metrics Predict Mortality in Patients With Advanced Liver Disease. Am J Gastroenterol 2021;116:2105–2117. [DOI] [PubMed] [Google Scholar]
- 36.Ha NB, Montano-Loza AJ, Carey EJ, Lin S, Shui AM, Huang CY, Dunn MA, et al. Sarcopenic visceral obesity is associated with increased post-liver transplant mortality in acutely ill patients with cirrhosis. Am J Transplant 2022;22:2195–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong AJ, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, et al. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant 2021;21 Suppl 2:208–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Study flow chart
Supplementary Figure 2. Two-way scatter plots of (A) visceral adipose tissue index (cm2/m2), (B) subcutaneous adipose tissue index (cm2/m2), and (C) visceral-to-subcutaneous adipose tissue ratio according to skeletal muscle index (cm2/m2) among men
Supplementary Figure 3. Two-way scatter plots of (A) visceral adipose tissue index (cm2/m2), (B) subcutaneous adipose tissue index (cm2/m2), and (C) visceral-to-subcutaneous adipose tissue ratio according to skeletal muscle index (cm2/m2) among women


