Significance
Across the globe, bird morphology is changing rapidly. Although trajectories of change are frequently consistent across studies, rates of change among species vary in magnitude—a phenomenon that remains unexplained. By analyzing two independently collected datasets demonstrating consistent changes in morphology in 129 species, we show that rates of phenotypic change are negatively correlated with body size—that is, smaller birds are changing faster. Why smaller-bodied species are changing faster is unknown, but we found little support for the hypothesis that generation length explains variation in the rates of change. Rather, body size appears to be a primary mediator of species’ phenotypic responses to contemporary climatic change and should be considered when testing hypotheses about the drivers of change.
Keywords: morphological change, generation length, body size, birds, evolutionary rates
Abstract
Variation in evolutionary rates among species is a defining characteristic of the tree of life and may be an important predictor of species’ capacities to adapt to rapid environmental change. It is broadly assumed that generation length is an important determinant of microevolutionary rates, and body size is often used as a proxy for generation length. However, body size has myriad biological correlates that could affect evolutionary rates independently from generation length. We leverage two large, independently collected datasets on recent morphological change in birds (52 migratory species breeding in North America and 77 South American resident species) to test how body size and generation length are related to the rates of contemporary morphological change. Both datasets show that birds have declined in body size and increased in wing length over the past 40 y. We found, in both systems, a consistent pattern wherein smaller species declined proportionally faster in body size and increased proportionally faster in wing length. By contrast, generation length explained less variation in evolutionary rates than did body size. Although the mechanisms warrant further investigation, our study demonstrates that body size is an important predictor of contemporary variation in morphological rates of change. Given the correlations between body size and a breadth of morphological, physiological, and ecological traits predicted to mediate phenotypic responses to environmental change, the relationship between body size and rates of phenotypic change should be considered when testing hypotheses about variation in adaptive responses to climate change.
Generation length is a fundamental unit of evolutionary biology and is the basis for many quantitative models of evolution (1–5). Scaling the amount of change over time in a trait by generation length makes evolutionary rates more comparable and biologically meaningful, especially when the life histories of focal taxa differ (6–8). Generation length is also essential to our understanding of evolutionary resiliency to contemporary environmental change (9–11). When compared on a given interval of time (e.g., years), species with shorter generation times are predicted to evolve faster because they experience more opportunities for shifts in gene frequencies (6, 7, 12). For example, long-lived organisms are expected to respond more slowly to environmental change than their short-lived counterparts, which in general have been shown to evolve remarkably rapidly (13, 14). However, owing to a paucity of studies examining rates of phenotypic change across many codistributed taxa experiencing similar selection pressures, it remains unclear whether generation length is an important general mediator of responses to rapid environmental change within and across taxonomic groups. Further, despite its hypothesized importance and broad application in efforts to understand variation in the rates of microevolutionary processes, much of the empirical evidence linking generation length and rates of phenotypic change has been at macroevolutionary scales (15, 16).
Characterizing generation length in a consistent manner across species is challenging, requiring estimates of multiple vital rates such as the age at first reproduction, longevity, and annual survival (17). Because these data are not available for the majority of species, body size is often used as a proxy for generation length, based on a strong correlation between body size and generation length across a wide range of taxa (18–20). Negative relationships between body size and rates of evolutionary processes have been demonstrated on macroevolutionary scales (21–25) and in studies of molecular evolution (22, 26–29). This influence of body size on evolutionary rates is routinely attributed to the correlation between body size and generation length. It has been hypothesized, however, that body size may influence evolutionary rates through mechanisms independent of generation length, such as metabolic rate, population size and density, or differences in the chance of DNA mutations (23, 27, 30, 31). Additionally, although body size and generation length tend to be correlated when taxa of highly disparate sizes are compared (e.g., mice versus elephants; 17), it is less clear whether linkages between size and life history persist in closely related species of more similar body size. Thus, comparing rates of evolutionary change among closely related species affords an opportunity to assess the relative effects of generation length and body size on rates of evolution. This can improve understanding of the factors that regulate the pace of evolution, which has been of persistent interest in evolutionary biology (32). Furthermore, identifying the biological determinants of variation in rates of contemporary evolution is critical for improving our ability to predict biotic responses to rapid global environmental change (11, 33).
Here, we leverage two datasets encompassing four decades of avian morphological change (34, 35) to test the importance of generation length and body size in mediating rates of contemporary phenotypic change. First, we use a long-term dataset of 52 species of mostly passerine migratory birds breeding in temperate and boreal North America based on morphological measurements of 70,716 specimens from bird-building collisions collected in Chicago, Illinois, United States, from 1978 to 2016. This dataset demonstrates near-universal declines in body size, with concurrent increases in wing length (34) in these species. Second, we use a dataset of 15,415 individuals from 77 nonmigratory Amazonian species collected over the same period through a long-term mist-netting effort (35). Remarkably, although the two datasets are nonoverlapping in species composition, geography, and life history, and data were collected independently using different methods, the Amazonian species have also experienced similarly widespread declines in body size with concurrent increases in wing length (35). That is, the species within and across both studies exhibit striking similarity in the directionality of morphological change (shrinking bodies and lengthening wings). Yet, in each study system, there is unexplained variation in the rates of morphological change among species over this 40-y period (35, 36), providing the opportunity for phylogenetic comparative analyses of the factors that mediate morphological rates.
For the North American species, we take advantage of the availability of demography-based estimates of generation length (37) to test the hypotheses that 1) species with shorter generation lengths experienced greater rates of morphological change over the past four decades, and 2) rates of morphological change covary with body size independently of generation length, given the evidence of direct links between body size and other evolutionary processes (22–24, 27, 31). Although species-specific empirical data on generation length are not available for the Amazonian species, we test the relationship between body size and rates of morphological change across both systems.
Both Weeks et al. (34) and Jirinec et al. (35) demonstrated a quantitative link between reductions in body size and increases in summer temperature in the North American and Amazonian datasets, respectively. Similar associations between warmer temperatures and declining body size in many of the same North American species have been demonstrated in separate studies of bird banding records (38, 39), as well as an independent reanalysis of the Chicago dataset (40). Weeks et al. (34) and Jirinec et al. (35) hypothesized that their observed increases in wing length represent adaptive compensatory responses to shrinking bodies that allow more efficient flight. It is not known whether the observed morphological changes in these systems are due to evolution, plasticity, or both (34), or whether additional ecological dynamics beyond temperature may be influencing morphological shifts. Regardless of the mechanistic underpinnings of the observed morphological changes described by (34, 35), the consistent direction of morphological shifts observed across species provides a unique opportunity to test whether fundamental organismal traits hypothesized to influence phenotypic rates of change—body size and generation length—mediate variation in species’ phenotypic responses to rapid environmental change.
Methods
Data Collection.
Our data on North American species come from a specimen-based dataset described in the study by Weeks et al. (34) that includes 70,716 individuals collected from 1978 to 2016, representing 52 species of North American migratory birds (SI Appendix, Table S1). The dataset is derived from individual birds that were retrieved by Field Museum staff and volunteers after they died following collisions with buildings in Chicago, IL, United States, during their spring or fall migrations. Body mass was measured using a digital scale, tarsus length and bill length were measured using digital calipers, and the length of the relaxed wing was measured using a wing rule. Individuals were sexed based on gonadal inspection and aged based on skull ossification to Hatch Year (fall birds hatched that summer) and After Hatch Year (all spring birds and all fall birds at least 1 y old). All measurements were taken by a single person (D.E.W.) prior to preparation of the individuals as museum specimens. The species span 11 families and 30 genera. Fifty of the 52 species are passerines, and the remaining two are a rail (Porzana carolina; Rallidae) and a woodpecker (Sphyrapicus varius; Picidae). While all species are migratory, they nest in a variety of habitats in temperate and boreal North America and winter in a range of environments throughout eastern North America and the Neotropics (34).
As explained by Weeks et al. (34), body mass data from migratory birds collected as collision specimens are likely unreliable for quantifying subtle intraspecific shifts in body size, owing to the large variation in body condition and fat content of birds collected on migration and uncertainties regarding the standardization of fat quantification in museum specimens over the years. Therefore, for quantifying intraspecific rates of morphological change in the Chicago dataset, we focus on tarsus, bill, and wing length.
The Amazonian data, described in the study by Jirinec et al. (35), are morphological measurements for 15,415 individuals of nonmigratory species from primary terra firme forest in the Brazilian Amazon that were captured, measured, and released in a 43 km2 study area as part of a long-term monitoring program (41) from 1979 to 2019 (SI Appendix, Table S2). The individuals are from 77 species spanning 65 genera, 21 families, and 5 orders and are broadly representative of the understory forest avifauna catchable with 2.5 m tall mist nets [~19% of the species found in the region; (42)]. As described in the study by Jirinec et al. (35), data were filtered to only include individuals captured in the dry season. Two measurements were consistently recorded throughout the entire study period—mass and wing length—and we thus limit our analyses to these two metrics when considering Amazonian species. As in the Chicago dataset, a wing rule was used to measure the relaxed wing length from the carpal joint to the tip of the longest primary. In the early years of data collection, mass was measured using analog spring scales (Pesola; Feusisberg, Switzerland), but around the year 2000, measurers transitioned to electronic balances. As discussed previously, this shift in tools was unlikely to bias mass measurements (35). In contrast to migratory species examined during migration in Chicago, tropical resident species do not experience wide fluctuation in mass and generally carry little fat (43), making mass a useful metric of intraspecific changes in body size when examining rates of morphological change for these species. Although age and sex were noted where possible, this information is less reliable when assessed based on external aspects of the phenotype such as plumage or bare parts coloration, in contrast to the measurement of gonads and subcutaneous inspection of skull ossification in the Chicago specimen dataset. Further, robust criteria for aging and sexing many tropical species based on external phenotype are still being developed and were not available in the earlier years of the study (43). Therefore, we do not consider age and sex in our analyses of the Amazonian species.
Statistical Analysis.
We tested for effects of generation length and body size on the rates of morphological change over the past four decades using two independent analytical frameworks: 1) a two-step approach in which we first estimated species-specific rates of morphological change in each trait using mixed-effects models and then modeled the resulting estimated rates of change as a function of mean body size and generation length using phylogenetic generalized least squares (PGLS); and 2) models that simultaneously estimate species’ rates of morphological change and the effects of body size and generation length on these rates in a hierarchical Bayesian framework.
Approach 1: Two-step analysis of rates of change in a PGLS framework.
Step 1: Estimating rates of morphological change.
We quantified species-specific rates of morphological change across the study period using linear mixed-effects models for the Chicago and Amazonian datasets separately. For the Chicago dataset, we used three separate models to estimate changes in each of the three trait variables (tarsus length, bill length, and wing length) as a function of year, sex, and age as fixed effects, with random intercepts and slopes for the effect of year for each species using the lme4 package (44) in R (45). We did not control for phylogeny when estimating species-specific slopes; rather, we control for phylogeny when analyzing those slopes as outcome variables in the downstream PGLS analysis (Step 2: PGLS models). Year was transformed to start at zero to make intercepts more interpretable. For the Amazonian dataset, we constructed similar models for the two available traits (mass and wing length) but, for the reasons discussed above, did not include age or sex as fixed effects.
Prior to analysis, all morphological measurements were log-transformed to allow for comparisons of proportional rates of change in morphological traits between differently sized species (46). The resulting random slopes for the year covariate in each model thus represent species-specific rates of percent change per year (SI Appendix, Methods S1) in tarsus length (Chicago only), bill length (Chicago only), wing length (Chicago and Amazon), and mass (Amazon only). Log-transforming the data resulted in models with normally distributed residual variance (SI Appendix, Figs. S1 and S2).
Step 2: PGLS models.
We next tested whether body size and generation length predict the rates of morphological change. Species’ mean body sizes were estimated directly from our measurement data as the arithmetic mean of body mass for all individuals of each species collected during the four-decade study period. The decline in mass observed over the study period in both datasets is negligible compared to interspecific differences in size, making this an appropriate estimate of species’ body size for use in comparative models (SI Appendix, Table S3).
For the species in the Chicago dataset, we quantified generation length following equation 1 in the study by Bird et al. (37) using demographic data on age of first reproduction (F), maximum longevity (L), and annual adult survival (S). These generation length estimates can be interpreted as the mean age at which a cohort of individuals produces offspring. We used the empirical F, L, and S values that were compiled from the literature in the study by Bird et al. (37); these data were available for 46 of the 52 species in the Chicago dataset but were not available for any species in the Amazonian dataset. Although Bird et al. (37) also provided model-based estimates of generation length for all species of birds in the world, their estimates of generation length for the Amazonian species are wholly based on interpolation of values measured in other species in the same genus or family owing to the paucity of data on population demography in many tropical species. While these model-based estimates are informative at a broader scale, for our purposes, using the interpolated estimates of generation length could preclude confident isolation of the effects of body size and generation length in our hypothesis-testing framework because body mass was one of the covariates used to produce the model-based estimates of generation length. Therefore, we limit our hypothesis testing of generation length to the 46 species in the Chicago dataset for which empirically derived estimates of generation length are available.
We modeled the species-specific rates of change in each trait (estimated in Step 1) as a function of species’ mean body size. We modeled each morphological trait from each dataset separately (tarsus length, bill length, and wing length from Chicago, and body mass and wing length from Amazonia). We also performed a combined analysis of wing length from both datasets. For the models with only Chicago species, we also included generation length as a predictor variable in the models. We used the logarithms of species’ mean body size and generation length to account for the nonlinear relationship between body size, generation length, and mensural traits (SI Appendix, Figs. S3 and S4). While generation length covaries with body size across taxa, the log-log correlation was not exceptionally high in our species (Pearson’s correlation coefficient r = 0.54, SI Appendix, Fig. S4), and thus it is reasonable to include both body size and generation length as predictors. We also assessed multicollinearity between body size and generation length using variance inflation factors (VIFs). Multicollinearity was low in models that included both body size and generation length (VIF < 2), indicating it is appropriate to assess the effects of these two predictors in the same model (47).
We performed PGLS analyses using simultaneous estimation of Pagels’ λ (48) for all models using the ape package (49) in R (45). The phylogenetic covariance matrix was based on 1,000 trees from the posterior distribution of a global phylogeny of birds (50) using the backbone phylogeny of Hackett et al. (51); the posterior distribution was used to generate a consensus tree with DendroPy (52), following (53). To test for the impacts of phylogenetic uncertainty on our results, we also repeated all PGLS analyses using 100 randomly selected trees from the posterior distribution of the global phylogeny of birds (50) and assessed the influence on parameter values.
For each trait in the Chicago dataset, we also used an information theoretic approach to compare PGLS models (using the consensus tree) that include the possible different combinations of generation length and species’ mean body size as predictors of rate of change. All models were compared using AIC (54).
Approach 2: Bayesian hierarchical multispecies models.
As a complementary analysis to jointly estimate the effects of body size and generation length on rates of morphological change and better account for error in estimated rates of change, we used Bayesian hierarchical multispecies models to simultaneously estimate species’ rates of morphological change and the effects of body size and generation length on these rates. In each model, we modeled the morphological measurement (M) of each individual (i) of each species (s) as a function of covariates (Cov) as:
The regression coefficients (β) were modeled as a function of species traits (Trait, indicating species’ mean body size and generation length) as
where the slope parameter γ quantifies the effects of the traits.
As in Approach 1, we fit hierarchical multispecies models to predict log-transformed tarsus and bill lengths (Chicago only), body mass (Amazon only), and wing length (Chicago and Amazon). Each model included year as a covariate and species' log-transformed mean body size as a trait. All Chicago models also included sex and age of each individual as covariates and species' log-transformed generation length as a trait. All covariates and species responses were scaled to a mean of zero and a SD of one to be compatible with the default prior distributions. Additionally, we fit a hierarchical multispecies model on the combined Chicago and Amazon datasets to model rates of change in wing length as a function of year, with species' log-transformed mean body size as a trait. All of the hierarchical multispecies models also included the appropriate consensus phylogeny (constructed as outlined above) to test for phylogenetic structure in the residual variation in species rates of change after accounting for traits.
We used the HMSC R-package (55, 56) to fit each model, with default diffuse prior distributions, using four Markov Chain Monte Carlo (MCMC) chains to sample the posterior distributions of all parameters. We ran each chain for 85,000 iterations and discarded the first 10,000 as burn-in. We thinned the remaining iterations by 50, producing 1,500 samples per chain (6,000 total). We evaluated potential scale reduction factors to ensure model convergence and effective sample size to ensure adequate independence of samples.
Results
Species’ mean body size varied from 5.47 (Regulus satrapa) to 107.90 g (Quiscalus quiscula) across the Chicago species (SI Appendix, Table S1) and 4.08 (Thalurania furcata) to 131.00 g (Momotus momota) across the Amazon species (SI Appendix, Table S2). Generation length in the Chicago dataset varied from 1.20 (Regulus satrapa) to 2.54 (Toxostoma rufum) years (SI Appendix, Table S1).
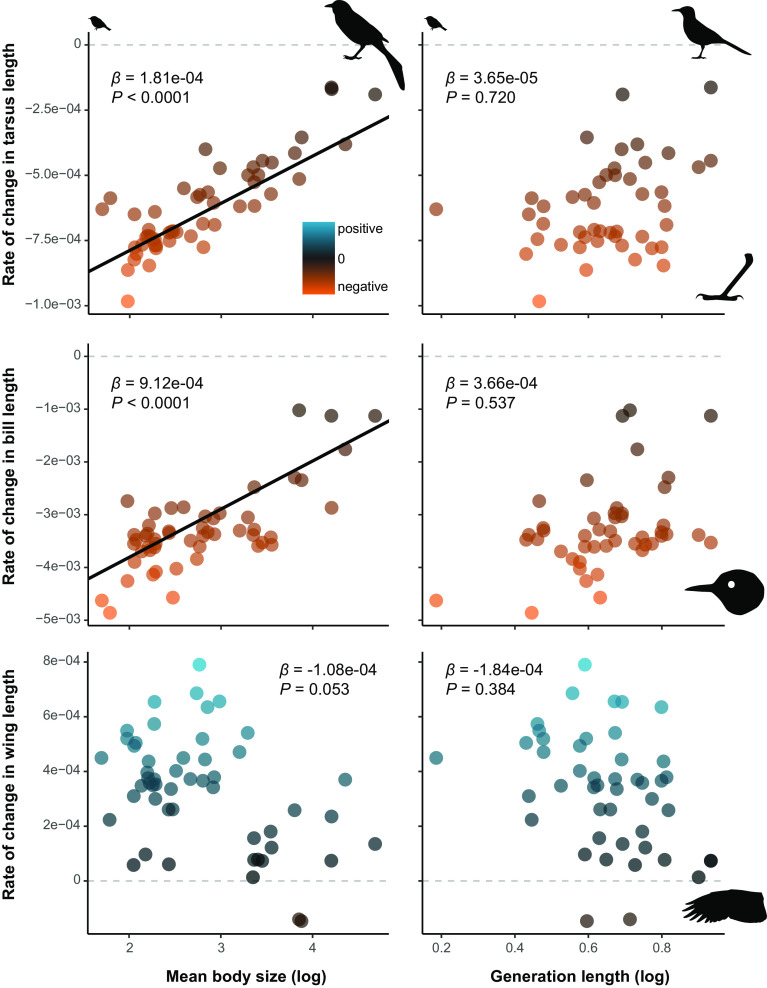
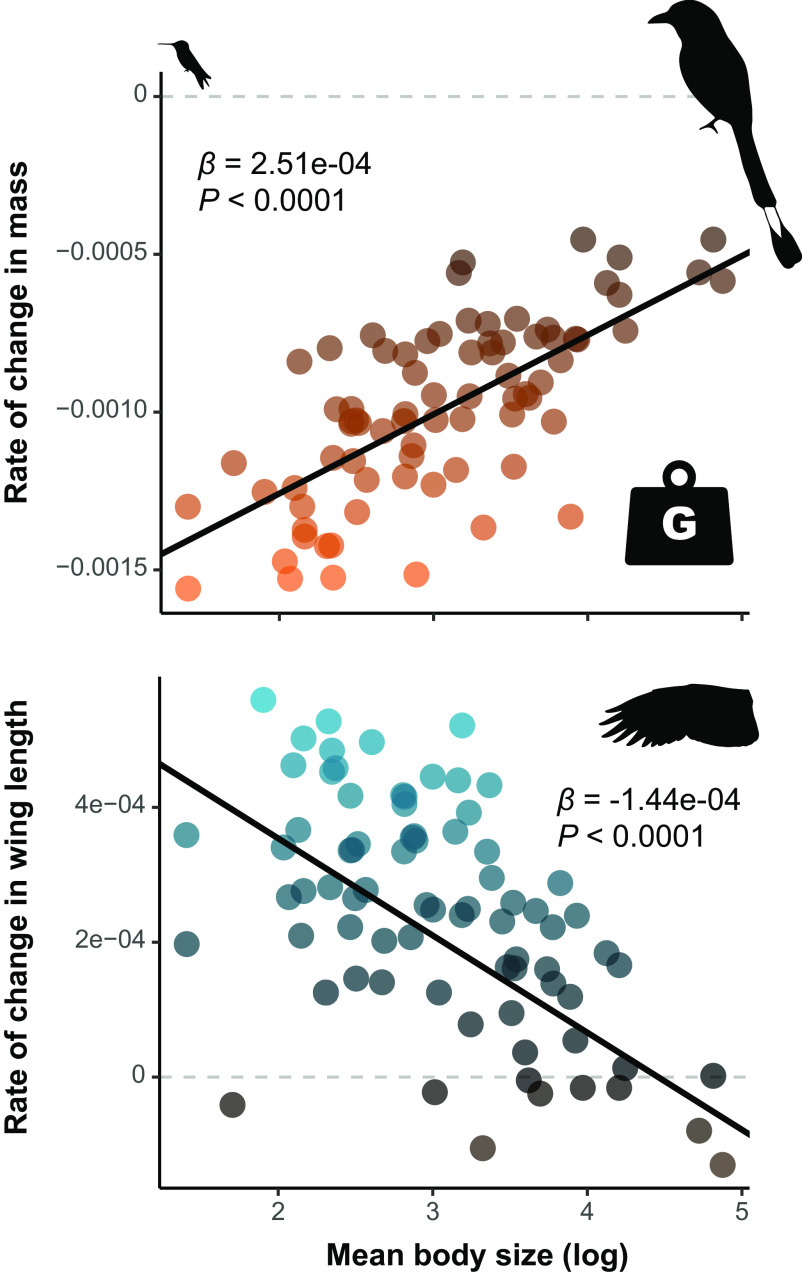
As previously reported by Weeks et al. (34) and Jirinec et al. (35), our mixed-effects models showed that most species declined in body mass (Amazonia), and tarsus and bill lengths (Chicago), and increased in wing length (both datasets), across the 40-y study period (Fig. 1). Species’ mean body size was significantly associated with rates of change in these traits in both the Chicago and Amazonian species (Figs. 1–3 and SI Appendix, Table S4), although this relationship was marginally nonsignificant for wing length when only the Chicago species were modeled. Specifically, smaller species exhibited greater rates of morphological change than larger species, such that smaller species declined proportionately more in tarsus length, bill length, and body mass, and increased proportionately more in wing length, than larger species (Fig. 4). For the Chicago models, generation length was not significantly associated with any rates of change when species mean body size was included in the model (Fig. 1, SI Appendix, Table S4).
Fig. 1.
Species’ mean body size predicts more variation in rates of phenotypic change than generation length, with smaller Chicago species changing in bill, tarsus, and wing lengths faster than larger species. Points show the rates of change for the 46 species that have empirically derived generation length estimates available. All rates of morphological change were derived from our mixed-effects models, with positive rates in blue and negative rates in orange. Black lines show the partial model coefficients where body size or generation length predicted rates of morphological change (i.e., P ≤ 0.05), with slope estimates and P-values derived from our PGLS analyses given in top corners. Bird silhouettes depict the species at the extremes of body mass (Regulus satrapa, Quiscalus quiscula) and generation length (R. satrapa, Toxostoma rufum).
Fig. 2.
Species’ mean body size predicts rates of phenotypic change, with smaller Amazonian species decreasing in body mass and increasing in wing length faster than larger species. Points show the rates of change for the 77 species derived from our mixed-effects models, with positive rates in blue and negative rates in orange. Black lines show the partial model coefficients where body size predicted rates of morphological change (i.e., P ≤ 0.05), with slope estimates and P-values derived from our PGLS analyses given in top corners. Bird silhouettes depict the species at the extremes of body mass (Thalurania furcata, Momotus momota).
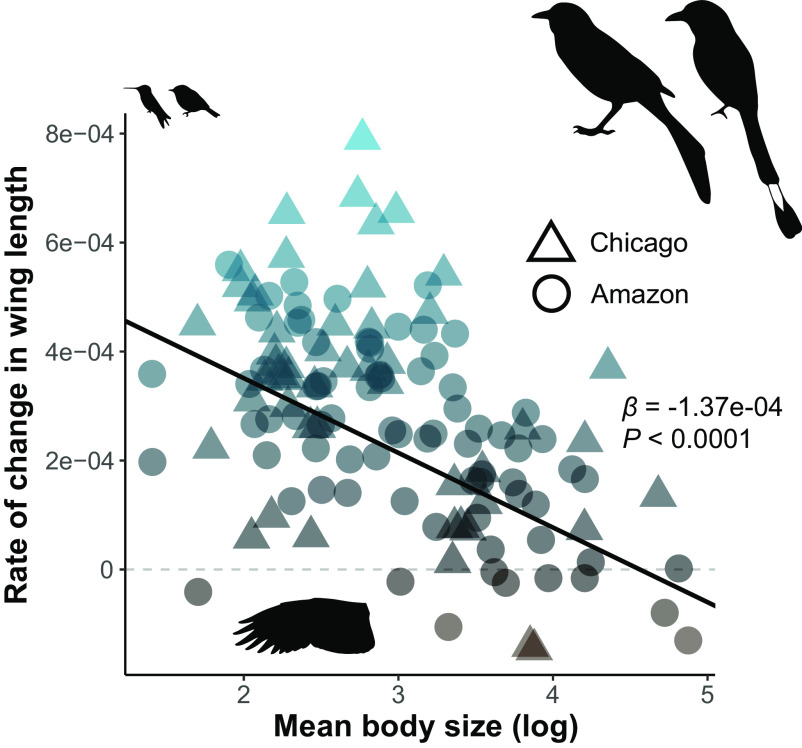
Fig. 3.
Rates of wing length change in the Chicago and Amazonian species were similar and were significantly associated with species’ mean body size. Points show the rates of change in wing length for each of the 52 Chicago and 77 Amazonian species derived from our mixed-effects models. The black line shows the partial model coefficient where body size predicted rate of change (i.e., P ≤ 0.05) with the slope estimate and p-value derived from our PGLS analysis given on the right. Bird silhouettes depict the species at the extremes of body mass for Amazonian (Thalurania furcata, Momotus momota) and Chicago (Regulus satrapa, Quiscalus quiscula) datasets.
Fig. 4.
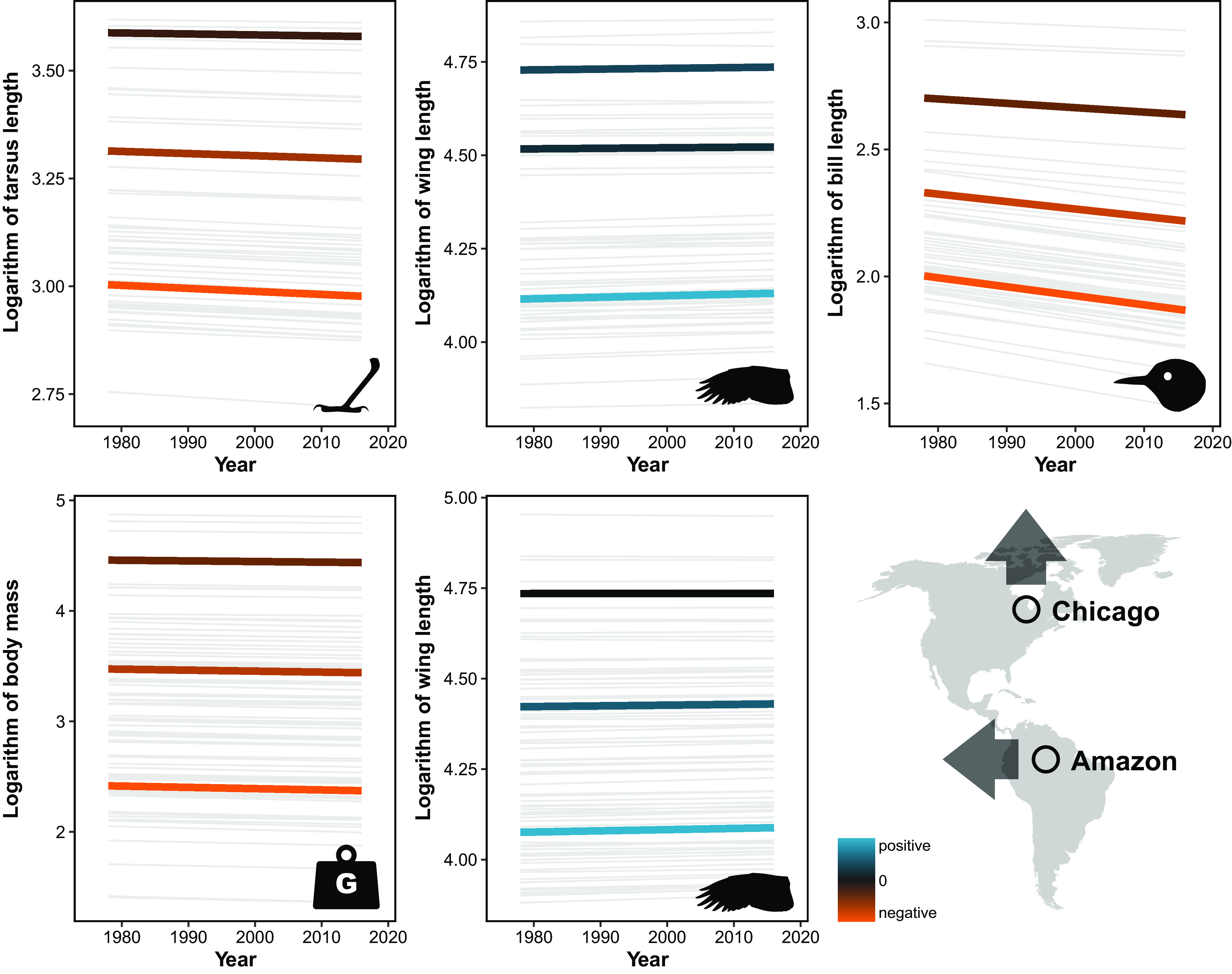
Temporal change in morphological traits over time for small-, medium-, and large-sized Chicago species (Top) and Amazonian species (Bottom), showing that smaller species have greater proportional change. The gray lines represent temporal trends for each of the species derived from our mixed-effects models. The three bold lines in each facet are the mean intercepts and slopes for birds with species’ mean body size of <20 g (small size, bottom lines), 20 to 60 g (medium size, middle lines), and >60 g (large size, top lines).
When model selection was used to determine the most appropriate model, models that included both generation length and species’ mean body size received similar support to those that included only species’ mean body size (i.e., ΔAIC ≤ 2, SI Appendix, Table S5), whereas models that only included generation length were not supported (ΔAIC > 2, SI Appendix, Table S5).
The hierarchical multispecies models provided similar evidence that smaller species experienced greater rates of change in morphological traits, and that generation length explained less variation in these rates than body size (Table 1, SI Appendix, Figs. S5-S7). The only relationship that differed across the two analytical approaches was the effect of species’ mean body size on rates of change in body mass in the Amazonian species, with lower support for this relationship in the hierarchical multispecies model (Table 1) than in the two-step PGLS model (SI Appendix, Table S4).
Table 1.
Species’ mean body size (Size) is consistently a better predictor of rate of change in morphological traits (tarsus, bill, and wing lengths) than generation length (GL)
| Dataset | Morphological trait | Predictor | Mean estimate | Posterior support (%) |
|---|---|---|---|---|
| Chicago | Tarsus length | Size | 6.19e-03 | 96.2 |
| GL | 6.32e-03 | 72.7 | ||
| Chicago | Bill length | Size | 9.97e-03 | 99.4 |
| GL | 6.42e-03 | 70.3 | ||
| Chicago | Wing length | Size | −3.15e-03 | 84.3 |
| GL | −6.82e-03 | 75.4 | ||
| Amazon | Body mass | Size | 5.00e-04 | 59.5 |
| Amazon | Wing length | Size | −4.03e-03 | 96.1 |
| Chicago & Amazon | Wing length | Size | −2.99e-03 | 96.4 |
Results are based on Bayesian hierarchical multispecies models that include all trait observations for Chicago, Amazon, and both datasets. Posterior support indicates % one-sided support.
Lastly, we performed several post-hoc supplementary analyses to test the robustness of our results to potential confounders or artifacts. First, we show that the relationship between body size and rates of morphological change is not an artifact of allometric scaling (SI Appendix, Methods S2). That is, the relationship between observed rates of morphological change through time and body size is not an epiphenomenal outcome of how mensural traits scale allometrically with body mass. Second, we tested whether per-species sample size is related to body size and whether it influences our ability to recover rates of change. We found species’ body size and sample sizes to be poorly correlated in either dataset (Pearson’s correlation coefficients r = -0.03 for the Chicago and r = -0.24 for the Amazon dataset), and sample size had no effect on our results (SI Appendix, Table S6). Third, we tested whether the phenology of migration within a species in the Chicago dataset (that is, the date when an individual collided with a building in Chicago) confounded the relationship between body size and rates of morphological change across years. We found no relationship with phenology (SI Appendix, Table S7), consistent with a previous assessment of the relationship between phenological patterns and rates of change in the same dataset (36). Finally, phylogenetic uncertainty had negligible effects on our results, with minimal differences between coefficients generated from PGLS models that were using a consensus tree versus randomly selected phylogenetic trees (50) (SI Appendix, Table S8).
Discussion
Over the past 40 y, smaller body size has been associated with greater rates of morphological change across 129 species of birds. Specifically, smaller species exhibited proportionately greater declines in mass, and tarsus and bill lengths, while also experiencing greater proportional increases in wing length (Figs. 1–3 and Table 1). For example, in the Chicago dataset, a species weighing less than 20 g has a tarsus length that is about 2.68% shorter at the end (2016) than at the beginning of the study (1978), whereas a species weighing more than 60 g shows only a 0.85% decline in tarsus length (Figs. 1 and 4). The relationships between body size and rates of change are remarkably consistent across systems. For the measurement that was taken in both systems (wing length), rates of change are broadly overlapping, indicating a similar magnitude of change as well as relationship between body size and rates of change across systems (Fig. 3).
After controlling for body size, we find no relationship between generation length and rates of morphological change in the North American species (Table 1 and SI Appendix, Tables S4 and S5). Although it is difficult to conclusively rule out some influence of generation length (see below), our results suggest that other biological factors associated with body size may be more important for determining contemporary rates of change than generation length per se.
Potential Mechanisms Connecting Size to Morphological Rates.
The biological mechanism underlying the observed link between body size and rates of morphological change requires further investigation. Contemporary declines in animal body size are typically ascribed to global warming (38, 57–62) and the declines in body size in our study systems have been shown to be associated with warming temperature (34, 35, 38). The available data do not allow us to test whether the morphological shifts described here are microevolutionary changes in response to natural selection. However, all the examined traits have medium to high heritability in wild birds (with a typical h2 = 0.4 to 0.6, (63)) and have been documented to evolve on contemporary timescales (64–67). Thus, if selection plays a role in the patterns described here, our results suggest that smaller bird species might be evolving faster because they experience stronger natural selection, are more responsive to selection, or both. Likewise, larger species may either be slower to adapt to changing conditions, or there may be behavioral or ecological correlates of larger body sizes that buffer larger species from the negative effects of warming temperatures. However, we can think of no clear ecological covariate of size that singularly explains the observed rate variation. The consistency in the results presented herein—both within and across different systems—indicates that a geographically, ecologically, and phylogenetically widespread mechanism is affecting birds.
Population genetic and molecular evolutionary theories provide two potential nonexclusive explanations for the observed patterns. First, smaller species tend to have larger effective population sizes (Ne) and higher genetic variance (68–70), theoretically allowing them to respond more rapidly to selection because beneficial mutations will be lost less frequently to drift and because slightly deleterious mutations are less likely to be fixed by drift (31, 71, 72). In our data, we found no effects of census population size, often treated as a proxy for Ne, on rates of morphological change (SI Appendix, Table S9). We also found poor correlations between species’ mean body size and variance in morphological traits (SI Appendix, Table S10). However, future research should explore the relationship between morphological rates of change and direct estimates of genetic variance and Ne. A second genetic explanation for the patterns we have recovered here is that smaller species have higher rates of molecular evolution, a pattern that itself remains difficult to explain but has been attributed to allometric relationships with factors such as basal metabolic rate, mutation risk and repair, and fecundity (27, 31, 73). These dynamics could allow small species to generate higher rates of potentially beneficial mutations and a higher probability that beneficial mutations increase in frequency in the population. Distinguishing the influence of body size per se versus generation length on molecular evolution is an ongoing challenge (31), and further study is required to understand whether hypothesized links between body size, mutation rate, and substitution rate (71) could explain the observed trends in morphological shifts.
Developmental plasticity might also play a role in the observed morphological shifts. In birds, warmer ambient temperature during development leads to faster metabolic rates (74), more rapid development (75–77), and smaller adult body size (78–81). This suggests that phenotypic plasticity might contribute to the observed morphological shifts. However, the role of developmental plasticity in contributing to body size declines was not supported in a recent study of a temperate passerine (82). More generally, it is not obvious why developmental plasticity should vary with body size such that smaller species would mount a stronger plastic response than larger species. Further, as plasticity is thought to provide phenotypic variation upon which selection may act when populations are confronted with novel environments (83, 84), the factors that mediate plastic responses are still of key importance for understanding the pace of adaptive evolution. Future studies using predictive modeling of size-driven variation in evolutionary rates will benefit from a better understanding of the degree to which the observed changes are genetically based versus mediated by phenotypic plasticity.
Implications for Testing the Influence of Other Traits Correlated with Body Size.
We have shown here that species’ mean body size is negatively correlated with rates of contemporary morphological change. This relationship has broad implications for understanding the role of other traits that are correlated with body size (e.g., brain size, geographic range, metabolism, and genome size) in determining rates of phenotypic evolution. In the Chicago data, for example, absolute brain mass is highly correlated with body size [Pearson’s correlation coefficient = 0.94; (40)] and species with smaller relative brain sizes have experienced much greater morphological change (40). This pattern was interpreted as evidence that cognitive ability buffers species from the effects of climate change (40). However, when absolute body size is correlated with an outcome variable of interest—in this case, rates of phenotypic change—it can be challenging to distinguish the effect of a trait that is first “corrected” for body size by regression (e.g., relative brain size), from the effects of body size itself (85). Given that our results show that body size is a major axis of variation of avian morphological responses to recent climate change, the specific influence of any particular trait that is highly correlated with body size may be difficult to disentangle from other physiological, ecological, developmental, and life history factors that correlate with body size.
Implications for Population Trajectories.
Our results provide insights into how birds from diverse biota are responding to global change. If the morphological changes we observe have an adaptive component, then we predict that the lower rates of change in larger species may exacerbate their extinction risks. Body size is highly correlated with extinction threat, such that larger species have an increased risk of extinction (86–88). This relationship is often hypothesized to be due to a correlation between body size and several life history and ecological traits that make large species more vulnerable to extinction threats including small population size, lower fecundity, and increased range requirements (87, 89–91), or due to their reduced potential to evolve rapidly, presumably due to long generation lengths (92). Our results suggest that large body size could further exacerbate extinction risk by limiting the potential to adapt to rapid, ongoing anthropogenic change. In contrast, the body size effect on evolutionary rates might increase persistence of small taxa if their rapidly changing morphology reflects a faster adaptive response to changing conditions. Yet, the degree to which the rapid changes in morphology observed in smaller species are truly adaptive remains unclear and shrinking body size may also have detrimental effects on populations. This is especially relevant for birds under increasing global temperatures, as smaller birds may have disproportionately high risk of mortality due to direct effects of climate change, for example acute dehydration (58, 93). Integrating body size and its relationship with adaptive potential into models seeking to understand extinction risk may improve our ability to predict biotic responses to environmental change.
The Potential Impacts of Variation in Generation Length and Morphological Change Estimates.
Our measurements of species’ mean body sizes are precise, as they are calculated from samples of hundreds and sometimes thousands of birds (SI Appendix, Table S1). By contrast, a limitation of our study is that the estimates of generation length, taken from ref. 37, are derived from potentially more variable samples and each component (age at first reproduction, longevity, and annual survival) has measurement error that is difficult to assess. Therefore, although our results do not support generation length as a strong predictor of rates of morphological change, it remains possible that improvements in our ability to estimate generation length from demographic data could yield stronger support for a role of generation length in driving these rates. To explore the potential impacts of generation length uncertainty on our results, we simulated a generation length variable that predicted rates of morphological change as well as does body size (SI Appendix, Methods S3). We then introduced random error to the estimates of generation length until the relationship between generation length and morphological change matched the empirical relationship we document. We found that a magnitude of error of approximately 10% may be sufficient to challenge our conclusion that body size is a better predictor of evolutionary change in our dataset. Future data-driven improvements of generation length estimates from demographic studies are necessary to test these relationships empirically.
While the data explored here on size change in Amazonian birds (35) are the only published data on these species, other studies have explored size change in some of the same North American species. For example, Youngflesh et al. (38) and Van Buskirk et al. (39) both used bird banding data to show that North American birds have been shrinking in mass. Interestingly, these studies did not find evidence for increasing absolute wing length as demonstrated by Weeks et al. (34), although Youngflesh et al. (38) found that wing length relative to body size has been increasing through time. Variation in sampling strategy and analytical approaches used to estimate morphological trends, and differences in reported results, complicate post-hoc comparison of the relationship between size and rates of morphological change across these and other studies. Our discovery that body size predicts rates of morphological change within the two datasets we analyzed suggests that broader exploration of body size as a determinant of evolutionary rates across systems is warranted.
Conclusions
In two independent datasets, body size predicts rates of morphological change in migratory and nonmigratory birds over the past 40 y, with smaller birds undergoing greater rates of decline in body mass, tarsus length, and bill length, and greater rates of increase in wing length (Figs. 1–4 and Table 1). The explanatory power of generation length is minimal when body size is controlled for in the models (Fig. 1, Table 1, and SI Appendix, Tables S4 and S5). Our results suggest that body size has a relationship with evolutionary rates independent of generation length, and we expect that multiple dynamics associated with body size may collectively drive the observed patterns. That is, although generation length may be one additive component of the relationship between body size and evolutionary rates, our findings question the long-held assumption that the association between body size and rates of evolution is driven principally by generation length. Future studies of diverse taxa across a wider range of body sizes and generation lengths, and continued improvement of the estimation of generation length from demographic data, are important for further testing the patterns revealed in our study. Developing a better understanding of the mechanistic links between size and rates of evolution is an important avenue of future research, especially because species persistence in a rapidly changing environment may require the capacity for rapid evolution. Body size may be a valuable predictor of adaptive capacity and the extent to which contemporary evolution may reduce the risk of extinction among species.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank the staff, curators, and volunteers of the Field Museum, and the Chicago Bird Collision Monitors, for their assistance in salvaging birds, as well as the many field assistants who collected data in Brazil. For helpful discussion and comments on the manuscript, we thank Jacob Berv, Philip Gingerich, Teresa Pegan, and Mark Urban. We also thank Rob Martin for help with calculating generation lengths using observed vital rates. For advice on statistical analyses, we thank the University of Michigan’s Center for Statistical Computing and Research and Jarrod Hadfield. M.Z. was supported by the Institute for Global Change Biology at the University of Michigan. S.T.G. was supported by an Eberly Research Fellowship of the Eberly College of Science at The Pennsylvania State University. This is publication no. 859 of the Biological Dynamics of Forest Fragments Project (BDFFP) Technical Series and no. 72 of the Amazonian Ornithology Technical Series of the Instituto Nacional de Pesquisas da Amazônia (INPA) Collections Program. USGS's contribution to the Work to be published constitutes a “work of the United States government” and therefore there is no domestic copyright protection and USGS's contribution is considered to be in the public domain. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. government.
Author contributions
M.Z., B.C.W., and B.M.W. designed research; M.Z., B.C.W., D.E.W., S.T.G., V.J., R.C.B., and B.M.W. performed research; M.Z., B.C.W., R.C.B., and B.M.W. analyzed data; and M.Z., B.C.W., D.E.W., S.T.G., V.J., R.C.B., and B.M.W. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. W.J. is a guest editor invited by the Editorial Board.
Contributor Information
Brian C. Weeks, Email: bcweeks@umich.edu.
Benjamin M. Winger, Email: wingerb@umich.edu.
Data, Materials, and Software Availability
All data and code are publicly available in the Dryad Digital Repository and Zenodo and can be accessed via: https://doi.org/10.5061/dryad.8pk0p2nhw (94), https://doi.org/10.5061/dryad.fqz612jsp (95), and https://doi.org/10.5061/dryad.rjdfn2zh2 (96).
Supporting Information
References
- 1.Lynch M., Lande R., “Evolution and extinction in response to environmental change” in Biotic Interactions and Global Change, Kareiva P., Kingsolver J. G., Huey R.-B., Eds. (Sinauer Associates, 1993), pp. 234–250. [Google Scholar]
- 2.Chevin L.-M., Lande R., Mace G. M., Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biol. 8, e1000357 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomulkiewicz R., Houle D., Demographic and genetic constraints on evolution. Am. Nat. 174, 218–229 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Falconer D. S., Introduction to Quantitative Genetics (Longman Scientific & Technical, ed. 3, 1989). [Google Scholar]
- 5.Gomulkiewicz R., Holt R. D., When does evolution by natural selection prevent extinction? Evolution 49, 201 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Haldane J. B. S., Suggestions as to quantitative measurement of rates of evolution. Evolution 3, 51–56 (1949). [DOI] [PubMed] [Google Scholar]
- 7.Gingerich P. D., Quantification and comparison of evolutionary rates. Am. J. Sci. 293 A, 453–478 (1993). [Google Scholar]
- 8.Gingerich P. D., Rates of evolution on the time scale of the evolutionary process. Genetica 112, 127–144 (2001). [PubMed] [Google Scholar]
- 9.Petit R. J., Hampe A., Some evolutionary consequences of being a tree. Annu. Rev. Ecol. Evol. Syst. 37, 187–214 (2006). [Google Scholar]
- 10.Jump A. S., Peñuelas J., Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann A. A., Sgrò C. M., Climate change and evolutionary adaptation. Nature 470, 479–485 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Simpson G. G., The Major Features of Evolution (Columbia University Press, 1953). [Google Scholar]
- 13.Lenski R. E., Rose M. R., Simpson S. C., Tadler S. C., Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during. Gener. J. Am. Soc. Aging 138, 1315–1341 (1991). [Google Scholar]
- 14.Bell G., Gonzalez A., Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 12, 942–948 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Marzluff J. M., Dial K. P., Life history correlates of taxonomic diversity. Ecology 72, 428–439 (1991). [Google Scholar]
- 16.Isaac N. J. B., Jones K. E., Gittleman J. L., Purvis A., Correlates of species richness in mammals: Body size, life history, and ecology. Am. Nat. 165, 600–607 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Charlesworth B., Evolution in Age-Structured Populations (Cambridge University Press, Cambridge, 1994). [Google Scholar]
- 18.Calder W., Size, Function, and Life History (Harvard University Press, Cambridge, MA, 1984). [Google Scholar]
- 19.Blueweiss L., et al. , Relationships between body size and some life history parameters. Oecologia 37, 257–272 (1978). [DOI] [PubMed] [Google Scholar]
- 20.Peters R. H., The Ecological Implications of Body Size (Cambridge University Press, Cambridge, UK, 1983). [Google Scholar]
- 21.Etienne R. S., et al. , Can clade age alone explain the relationship between body size and diversity? Interface Focus 2, 170–179 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wollenberg K. C., Vieites D. R., Glaw F., Vences M., Speciation in little: The role of range and body size in the diversification of Malagasy mantellid frogs. BMC Evol. Biol. 11, 217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper N., Purvis A., What factors shape rates of phenotypic evolution? A comparative study of cranial morphology of four mammalian clades. J. Evol. Biol. 22, 1024–1035 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Friedman S. T., Martinez C. M., Price S. A., Wainwright P. C., The influence of size on body shape diversification across Indo-Pacific shore fishes. Evolution 73, 1873–1884 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Cardini A., Polly P. D., Larger mammals have longer faces because of size-related constraints on skull form. Nat. Commun. 4, 2458 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Fontanillas E., Welch J. J., Thomas J. A., Bromham L., The influence of body size and net diversification rate on molecular evolution during the radiation of animal phyla. BMC Evol. Biol. 7, 1–12 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin A. P., Palumbi S. R., Body size, metabolic rate, generation time, and the molecular clock. Proc. Natl. Acad. Sci. U.S.A. 90, 4087–4091 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bromham L., Rambaut A., Harvey P. H., Determinants of rate variation in mammalian DNA sequence evolution. J. Mol. Evol. 43, 610–621 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Berv J. S., Field D. J., Genomic signature of an avian lilliput effect across the K-Pg extinction. Syst. Biol. 67, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunn G. B., Stanley S. E., Body size effects and rates of cytochrome b evolution in tube-nosed seabirds. Mol. Biol. Evol. 15, 1360–1371 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Bromham L., “Causes of variation in the rate of molecular evolution” in The Molecular Evolutionary Clock, Ho S. Y. W., Ed. (Springer, Cham, 2020), pp. 45–64. [Google Scholar]
- 32.Kinnison M. T., Hendry A. P., The pace of modern life II: From rates of contemporary microevolution to pattern and process. Genetica 112–113, 145–164 (2001). [PubMed] [Google Scholar]
- 33.Urban M. C., et al. , Improving the forecast for biodiversity under climate change. Science 353, aad8466 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Weeks B. C., et al. , Shared morphological consequences of global warming in North American migratory birds. Ecol. Lett. 23, 316–325 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Jirinec V., et al. , Morphological consequences of climate change for resident birds in intact Amazonian rainforest. Sci. Adv. 7, 1743 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimova M., Willard D. E., Winger B. M., Weeks B. C., Widespread shifts in bird migration phenology are decoupled from parallel shifts in morphology. J. Anim. Ecol. 90, 2348–2361 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Bird J. P., et al. , Generation lengths of the world’s birds and their implications for extinction risk. Conserv. Biol. 10.1111/cobi.13486 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Youngflesh C., Saracco J. F., Siegel R. B., Tingley M. W., Abiotic conditions shape spatial and temporal morphological variation in North American birds. Nat. Ecol. Evol. 2022, 1–11 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Van Buskirk J., Mulvihill R. S., Leberman R. C., Declining body sizes in North American birds associated with climate change. Oikos 119, 1047–1055 (2010). [Google Scholar]
- 40.Baldwin J. W., Garcia-Porta J., Botero C. A., Phenotypic responses to climate change are significantly dampened in big-brained birds. Ecol. Lett. 25, 939–947 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Stouffer P. C., Birds in fragmented Amazonian rainforest: Lessons from 40 years at the Biological Dynamics of Forest Fragments Project. Condor 122 (2020). [Google Scholar]
- 42.Rutt C. L., et al. , Twenty years later: An update to the birds of the biological dynamics of forest fragments project, Amazonas. Brazil. Rev. Bras. Ornitol. 25, 277–296 (2017). [Google Scholar]
- 43.Johnson E. I., Wolfe J. D., Molt in Neotropical Birds: Life History and Aging Criteria (CRC Press, Boca Raton, ed. 1, 2017). [Google Scholar]
- 44.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 48 (2015). [Google Scholar]
- 45.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2018). [Google Scholar]
- 46.Gingerich P. D., Rates of Evolution: A Quantitative Synthesis (Cambridge University Press, 2019). [Google Scholar]
- 47.O’Brien R. M., A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 41, 673–690 (2007). [Google Scholar]
- 48.Pagel M., Inferring the historical patterns of biological evolution. Nature 401, 877–884 (1999). [DOI] [PubMed] [Google Scholar]
- 49.Paradis E., Claude J., Strimmer K., APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Jetz W., Thomas G. H., Joy J. B., Hartmann K., Mooers A. O., The global diversity of birds in space and time. Nature 491, 444–448 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Hackett S. J., et al. , A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Sukumaran J., Holder M. T., DendroPy: A Python library for phylogenetic computing. Bioinformatics 26, 1569–1571 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Rubolini D., Liker A., Garamszegi L. Z., Møller A. P., Saino N., Using the birdtree.org website to obtain robust phylogenies for avian comparative studies: A primer. Curr. Zool. 61, 959–965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burnham K. P., Anderson D. R., Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2002). [Google Scholar]
- 55.Ovaskainen N., O., & Abrego, Joint Species Distribution Modelling: With Applications in R (Cambridge University Press, 2020). [Google Scholar]
- 56.Ovaskainen O., et al. , How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol. Lett. 20, 561–576 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Daufresne M., Lengfellner K., Sommer U., Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12788–12793 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardner J. L., Peters A., Kearney M. R., Joseph L., Heinsohn R., Declining body size: A third universal response to warming? Trends Ecol. Evol. 26, 285–291 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Sheridan J. A., Bickford D., Shrinking body size as an ecological response to climate change. Nat. Clim. Chang. 1, 401–406 (2011). [Google Scholar]
- 60.Forster J., Hirst A. G., Atkinson D., Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc. Natl. Acad. Sci. U.S.A. 109, 19310–19314 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yom-Tov Y., Geffen E., Recent spatial and temporal changes in body size of terrestrial vertebrates: Probable causes and pitfalls. Biol. Rev. 86, 531–541 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Dubiner S., Meiri S., Widespread recent changes in morphology of Old World birds, global warming the immediate suspect. Glob. Ecol Biogeogr 31, 791–801 (2022). [Google Scholar]
- 63.Merilä J., Sheldon B. C., “Avian Quantitative Genetics” in Current Ornithology (Springer, US, 2001), vol. 16, pp. 179–255. [Google Scholar]
- 64.Grant P. R., Grant B. R., Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296, 707–711 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Rolshausen G., Segelbacher G., Hobson K. A., Schaefer H. M., Contemporary evolution of reproductive isolation and phenotypic divergence in sympatry along a migratory divide. Curr. Biol. 19, 2097–2101 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Mathys B. A., Lockwood J. L., Contemporary morphological diversification of passerine birds introduced to the Hawaiian archipelago. Proc. R. Soc. B Biol. Sci. 278, 2392–2400 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garant D., Kruuk L. E. B., McCleery R. H., Sheldon B. C., Evolution in a changing environment: A case study with great tit fledging mass. Am. Nat. 164, 115–129 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Eo S. H., Doyle J. M., DeWoody J. A., Genetic diversity in birds is associated with body mass and habitat type. J. Zool. 283, 220–226 (2011). [Google Scholar]
- 69.Wooten M. C., Smith M. H., Large mammals are genetically less variable? Evolution 39, 210–212 (1985). [DOI] [PubMed] [Google Scholar]
- 70.Frankham R., Relationship of genetic variation to population size in wildlife. Conserv. Biol. 10, 1500–1508 (1996). [Google Scholar]
- 71.Lanfear R., Kokko H., Eyre-Walker A., Population size and the rate of evolution. Trends Ecol. Evol. 29, 33–41 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Botero-Castro F., Figuet E., Tilak M. K., Nabholz B., Galtier N., Avian genomes revisited: Hidden genes uncovered and the rates versus traits paradox in birds. Mol. Biol. Evol. 34, 3123–3131 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Gillooly J. F., Allen A. P., West G. B., Brown J. H., The rate of DNA evolution: Effects of body size and temperature on the molecular clock. Proc. Natl. Acad. Sci. U.S.A. 102, 140–145 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheldon E. L., McCowan L. S. C., McDiarmid C. S., Griffith S. C., Measuring the embryonic heart rate of wild birds: An opportunity to take the pulse on early development. Auk 135, 71–82 (2018). [Google Scholar]
- 75.Mertens J. A. L., Thermal conditions for successful breeding in Great Tits (Parus major L.) - I. Relation of growth and development of temperature regulation in nestling great tits. Oecologia 28, 1–29 (1977). [DOI] [PubMed] [Google Scholar]
- 76.Ospina E. A., Merrill L., Benson T. J., Incubation temperature impacts nestling growth and survival in an open-cup nesting passerine. Ecol. Evol. 8, 3270–3279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olson J. M., Growth, the development of endothermy, and the allocation of energy in red-winged blackbirds (Agelaius phoeniceus) during the nestling period. Physiol. Zool. 65, 124–152 (1992). [Google Scholar]
- 78.Andrew S. C., Hurley L. L., Mariette M. M., Griffith S. C., Higher temperatures during development reduce body size in the zebra finch in the laboratory and in the wild. J. Evol. Biol. 30, 2156–2164 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Wada H., et al. , Transient and permanent effects of suboptimal incubation temperatures on growth, metabolic rate, immune function and adrenocortical responses in zebra finches. J. Exp. Biol. 218, 2847–2855 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Cunningham S. J., Martin R. O., Hojem C. L., Hockey P. A. R., Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly-warming arid savanna: A study of common fiscals. PLoS One 8, e74613 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weeks B. C., et al. , Temperature, size and developmental plasticity in birds. Biol. Lett. 18, 20220357 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shipley J. R., Twining C. W., Taff C. C., Vitousek M. N., Winkler D. W., Selection counteracts developmental plasticity in body-size responses to climate change. Nat. Clim. Chang. 12, 863–868 (2022). [Google Scholar]
- 83.Price T. D., Qvarnstrom A., Irwin D. E., The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. B-Biological Sci. 270, 1433–1440 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.West-Eberhard M. J., Developmental Plasticity and Evolution (Oxford University Press, 2003). [Google Scholar]
- 85.Rogell B., Dowling D. K., Husby A., Controlling for body size leads to inferential biases in the biological sciences. Evol. Lett. 4, 73–82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cardillo M., et al. , Evolution: Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Gaston K. J., Blackburn T. M., Birds, body size and the threat of extinction. Philos. Trans. R. Soc. B Biol. Sci. 347, 205–212 (1995). [Google Scholar]
- 88.Olden J. D., Hogan Z. S., Vander Zanden M. J., Small fish, big fish, red fish, blue fish: Size-biased extinction risk of the world’s freshwater and marine fishes. Glob. Ecol. Biogeogr. 16, 694–701 (2007). [Google Scholar]
- 89.Bennett P. M., Owens I. P. F., Variation in extinction risk among birds: Chance or evolutionary predisposition? Proc. R. Soc. B Biol. Sci. 264, 401–408 (1997). [Google Scholar]
- 90.Pimm S. L., Jones H. L., Diamond J., On the risks of extinction. Am. Nat. 132, 757–785 (1988). [Google Scholar]
- 91.Hutchings J. A., Myers R. A., García V. B., Lucifora L. O., Kuparinen A., Life-history correlates of extinction risk and recovery potential. Ecol. Appl. 22, 1061–1067 (2012). [DOI] [PubMed] [Google Scholar]
- 92.Foden W. B., et al. , Identifying the world’s most climate change vulnerable species: A systematic trait-based assessment of all birds, amphibians and corals. PLoS One 8, e65427. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McKechnie A. E., Wolf B. O., Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weeks B. C., et al. , Shared morphological consequences of global warming in North American migratory birds. Dryad. 10.5061/dryad.8pk0p2nhw. Deposited 29 June 2021. [DOI] [PubMed]
- 95.Jirinec V., Stouffer P., Morphological consequences of climate change for resident birds in intact Amazonian rainforest. Dryad. 10.5061/dryad.fqz612jsp. Deposited 29 September 2021. [DOI] [PMC free article] [PubMed]
- 96.Zimova M., et al. Body size predicts the rate of contemporary morphological change in birds. Dryad. 10.5061/dryad.rjdfn2zh2. Deposited 19 April 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All data and code are publicly available in the Dryad Digital Repository and Zenodo and can be accessed via: https://doi.org/10.5061/dryad.8pk0p2nhw (94), https://doi.org/10.5061/dryad.fqz612jsp (95), and https://doi.org/10.5061/dryad.rjdfn2zh2 (96).