Abstract
We cloned the rpoN (ntrA and glnF) gene encoding ς54 from the phytopathogen Pseudomonas syringae pv. maculicola strain ES4326. The P. syringae ES4326 rpoN gene complemented Pseudomonas aeruginosa, Escherichia coli, and Klebsiella aerogenes rpoN mutants for a variety of rpoN mutant phenotypes, including the inability to utilize nitrate as sole nitrogen source. DNA sequence analysis of the P. syringae ES4326 rpoN gene revealed that the deduced amino acid sequence was most similar (86% identity; 95% similarity) to the ς54 protein encoded by the Pseudomonas putida rpoN gene. A marker exchange protocol was used to construct an ES4326 rpoN insertional mutation, rpoN::Kmr. In contrast to wild-type ES4326, ES4326 rpoN::Kmr was nonmotile and could not utilize nitrate, urea, C4-dicarboxylic acids, several amino acids, or concentrations of ammonia below 2 mM as nitrogen sources. rpoN was essential for production of the phytotoxin coronatine and for expression of the structural genes encoding coronamic acid. In addition, ES4326 rpoN::Kmr did not multiply or elicit disease symptoms when infiltrated into Arabidopsis thaliana leaves, did not elicit the accumulation of several Arabidopsis defense-related mRNAs, and did not elicit a hypersensitive response (HR) when infiltrated into tobacco (Nicotiana tabacum) leaves. Furthermore, whereas P. syringae ES4326 carrying the avirulence gene avrRpt2 elicited an HR when infiltrated into Arabidopsis ecotype Columbia leaves, ES4326 rpoN::Kmr carrying avrRpt2 elicited no response. Constitutive expression of ES4326 hrpL in ES4326 rpoN::Kmr partially restored defense-related mRNA accumulation, showing a direct role for the hrp cluster in host defense gene induction in a compatible host-pathogen interaction. However, constitutive expression of hrpL in ES4326 rpoN::Kmr did not restore coronatine production, showing that coronatine biosynthesis requires factors other than hrpL.
The rpoN gene encodes the alternate sigma factor ς54, which works in conjunction with the NtrC class of transcriptional activators to control the expression of many genes in response to nutritional and environmental conditions (2, 54). For example, genes involved in nitrogen, hydrogen, and catabolite utilization are frequently regulated by ς54 (6, 35, 48, 95). In the case of pathogenic bacteria, rpoN mediates expression of virulence-related factors such as pilin in Pseudomonas aeruginosa and flagellin in Vibrio anguillarum (24, 61, 86).
For some phytopathogenic bacteria, rpoN has been implicated indirectly as a regulator of pathogenicity-related genes known as the hrp gene cluster (17, 18). Pseudomonas syringae pv. syringae strain 61, for example, contains a 25-kb hrp cluster consisting of several complementation groups comprising at least 27 genes (8, 26). Several hrp genes encode proteins that have a high degree of homology to components of the type III secretory pathway of Yersinia species which are responsible for translocating Yersinia outer membrane proteins into mammalian cells (13, 15, 55, 83). By analogy, it is proposed that hrp-encoded proteins in phytopathogenic bacteria are involved in the transport of pathogenicity-related factors into plant cells.
The acronym hrp stands for hypersensitive response and pathogenicity. hrp genes are required not only for pathogenicity of a virulent pathogen but also for the elicitation of the hypersensitive response (HR) which occurs on some hosts (44, 45). The HR involves rapid, but localized, programmed plant cell death and is believed to restrict pathogen spread (1, 37). There is mounting evidence that the elicitation of an HR is mediated by the specific interaction between the products of a plant resistance gene (R gene) and a pathogen avirulence (avr) gene (43, 80, 85). It appears likely that at least some avr genes encode pathogenicity-related factors (34, 47, 71, 84) that are transported into plant cells via the hrp-encoded transport machinery (58, 59, 90). In the absence of a corresponding R gene product, the avr product enhances virulence; however, in hosts which have the corresponding R gene, recognition of the avr gene product enhances host resistance. Interestingly, most avr genes are also coordinately regulated with genes in the hrp cluster (27, 29, 46, 67, 71, 77, 97).
The HR is accompanied by the induction of defense-related genes (7, 91) that are differentially expressed depending on the particular pair of avr and R gene products eliciting the HR (70, 72). Defense gene induction also occurs in the absence of the HR during compatible pathogen-host interactions, although usually later and at lower levels than those occurring during an HR (11, 32, 66). Furthermore, hrp mutations that presumably block the export of avr gene products have been found to reduce, but not eliminate, defense gene induction (60). Collectively, these results suggest that there are a variety of signaling pathways that activate host responses.
In P. syringae the circuitry of hrp regulation appears to involve a transcriptional activation cascade. At the top of the cascade are two regulatory genes, hrpR and hrpS, which are required for expression of the remaining hrp genes in the cluster (12, 18). Both hrpR and hrpS encode proteins consist almost exclusively of the domain conserved among transcriptional activators such as NtrC, DctD, and NifA that work in concert with ς54 (21, 22; reviewed in references 2 and 54). The hrpR product activates hrpS expression, and the hrpS product activates hrpL transcription (17, 18). HrpL is an alternate sigma factor homologous to AlgU of P. aeruginosa and is thought to activate transcription of the remaining genes in the hrp cluster (96, 97). The factor(s) involved in the regulation of hrpRS remains obscure. Nevertheless, the central role of hrpS in this cascade and the HrpS-NtrC homology predicts that rpoN would be required for activation of the hrp gene cluster in P. syringae.
Despite the highly conserved and clustered nature of hrp genes among phytopathogenic bacteria, transcriptional regulation of the hrp genes is achieved by different mechanisms in different species. In Ralstonia solanacearum, HrpB, an AraC-like transcriptional activator, controls hrp gene expression (14). Similarly, in Xanthomonas campestris pv. vesicatoria, hrp gene expression is regulated by an OmpR homolog, HrpG, which in turn activates HrpX, another AraC-like activator that activates the remaining hrp genes (79, 92). This latter regulatory cascade is consistent with the fact that rpoN is not required for hrp expression or pathogenicity in X. campestris pv. vesicatoria (25).
The experiments described here utilize a pathogenicity system that involves the infection of Arabidopsis thaliana with P. syringae pv. maculicola strain ES4326. Strain ES4326 belongs to the leaf spotting class of phytopathogenic pseudomonads (78), proliferates extensively in Arabidopsis ecotype Columbia leaves, and causes the development of water-soaked disease lesions (9, 11). In contrast, ES4326 carrying the avirulence gene avrRpt2 elicits a visible HR about 16 h after infiltration and proliferates 50- to 100-fold less than the wild-type strain ES4326 (11). Using this system, we describe experiments that examine the role of ς54 in the pathogenicity of P. syringae ES4326. Our results indicate that ς54 is an important virulence factor for P. syringae and is required for the elicitation of an HR by P. syringae in both host and nonhost plants.
MATERIALS AND METHODS
Bacterial strains and media.
Bacterial strains and plasmids used in this work are listed in Table 1. P. syringae pv. maculicola strain ES4326 and its derivatives were grown at 28°C in L broth (50), minimal M9 salts media (50), or King's B medium (36). Escherichia coli, Klebsiella aerogenes, and P. aeruginosa strains were grown at 37°C in L broth or M9 minimal salts medium. For clarity, ES4326 carrying plasmid pLH12 (which carries the avirulence gene avrRpt2) is referred to as ES4326 (avrRpt2). Carbon and nitrogen source utilization tests for ES4326 rpoN mutants were performed in M9 salts minimal medium by providing a carbon source at 10 mM and by replacing ammonium chloride with an alternative nitrogen source at 5 mM when required. Bacterial motility was tested on “swarm plates” consisting of 0.3% agar, 0.5% NaCl and 0.5% tryptone (38). Antibiotic concentrations for E. coli and P. syringae strains were as follows: streptomycin, 150 μg/ml; kanamycin, 25 μg/ml; tetracycline, 12 μg/ml; gentamicin, 20 μg/ml; and spectinomycin, 20 μg/ml. Interspecies complementation tests of the E. coli rpoN mutant by ES4326 rpoN were carried out on M9 minimal salts agar supplemented with 0.2% glutamine and 20 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml (Sigma).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F−lacZΔM15 endA1 recA1 hsdR17 supE44 thi-1 gyrA relA1 λ− | Bethesda Research Laboratories (20) |
| MM294 | G. Walker (4) | |
| Th1 λgln101 | lacΔ rpoNΔ glnA-lacZ | D. Ow (62) |
| K. aerogenes HG63 rpoN | rpoN | D. Ow (62) |
| P. aeruginosa PAK N1 | Gmr cassette in rpoN | S. Lory (31) |
| P. syringae pv. maculicola | ||
| ES4326 | Wild-type | K. Davis (9) |
| ES4326 rpoN::Kmr | Kmr cassette in rpoN | This study |
| P. syringae pv. tomato | ||
| DC3000 | Wild type | D. Cuppels (57) |
| DC3661 | COR-defective mutant | D. Cuppels (57) |
| P. syringae pv. glycinea | ||
| PG4180.N9 | COR-producing strain | C. Bender (88) |
| PG4180.P2 | Gmr cassette in corR | C. Bender (65) |
| Plasmids | ||
| pRK2013 | Kmr Tra+, mating helper | G. Ditta (10) |
| pPH1 | Gmr Spr, chasing plasmid | |
| pLAFR3 | Tcr cosmid cloning vector | B. Staskawicz (81) |
| pBluescript SK(+) | Apr, cloning vector | Stratagene, Inc., La Jolla, Calif. |
| pGEM-7Zf(+) | Apr, cloning vector | Promega Inc., Madison, Wis. |
| pUC4K | Source of Kmr cassette from Tn903 | Pharmacia, Inc., Bridgewater, N.J. |
| pJSR1 | Apr, cosmid cloning vector | J. Shao (68) |
| pKI11 | Source of P. aeruginosa rpoN gene | S. Lory (31) |
| pLH12 | 1.4-kb SalI fragment containing avrRpt2 in pLAFR3 | R. Innes (93) |
| pPG101 | 17.2-kb insert of ES4326 DNA carrying rpoN in pLAFR3 | This study |
| pPG102 | 4.5-kb BamHI-EcoRI fragment containing ES4326 rpoN in pLAFR3 | This study |
| pLAFR-RK | pLAFR3 containing rpoN::Kmr | This study |
| pRN5; pNR9 | pBluescript SK(+) carrying 4.5-kb HindIII fragment containing ES4326 rpoN gene in opposite orientations | This study |
| pRG960sd | Smr Spr, contains promoterless uidA with start codon and Shine-Dalgarno sequence | C. Bender (89) |
| pRGMU7 | Smr Spr, contains 1.5-kb PstI-AatI fragment containing cmaABT promoter inserted into pRG960sd | C. Bender (87) |
| pHRPLC | lacZ-hrpL transcriptional fusion in pLAFR3 | E. Hendrickson (23) |
Bacterial genetics.
pLAFR3 derivatives were introduced into Pseudomonas strains via triparental matings with MM294(pRK2013) (10). Aspartate-utilizing pseudorevertants of strain ES4326 rpoN::Kmr were obtained by plating approximately 109 CFU on M9 agar plates containing succinate and aspartate as carbon sources at 10 mM and the appropriate antibiotics (streptomycin and kanamycin).
Plant pathogenicity assays.
Arabidopsis ecotype Columbia was germinated, grown, and inoculated with ES4326 strains. Bacterial strains were grown overnight in King's B, subcultured and grown to mid-log phase, resuspended in 10 mM MgSO4, and inoculated into the underside of the leaf at a titer of 104 CFU/cm2 using a disposable syringe. Growth of P. syringae strains in leaves was measured by individually grinding four to six 0.2-cm2 leaf punches (excised with a no. 2 cork borer) in 10 mM MgSO4, plating appropriate dilutions on King's B medium containing the appropriate antibiotics, and counting the CFU. For RNA blot analysis, entire Arabidopsis leaves infiltrated with ES4326 bacterial suspensions were harvested, frozen in liquid nitrogen at the indicated times, and stored at −80°C until needed (see below). Nicotiana tabacum (tobacco) cultivar Xanthi was grown under greenhouse conditions and inoculated with ES4326 strains and assayed for the HR as previously described (82).
Cosmid library constructions.
Total bacterial genomic DNA was prepared from strain ES4326 as described previously (3), partially digested with Sau3A, and size fractionated on a 14-ml sucrose gradient (50). DNA fragments of approximately 20 kb were purified and ligated with linearized pLAFR3 that had been prepared to promote the formation of concatemers (50). Packaging, infection, and plating of the cosmid clones were performed using the Giga Gold packaging kit according to the manufacturer's specifications (Stratagene, La Jolla, Calif.).
Nucleic acid manipulations.
Routine manipulations such as DNA blots and plasmid DNA isolation were performed as described earlier (3). Restriction enzymes, T4 DNA ligase, and calf intestine phosphatase were purchased from Boehringer Mannheim and New England BioLabs and used according to the manufacturer's specifications. Deletions in plasmids were created using the Erase-a-Base kit (Promega, Madison, Wis.). Isolation of Arabidopsis mRNA and RNA blot analysis was carried out as described previously (11). Hybridizations were performed at stringent conditions (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 65°C) as described earlier (50). 32P-labeled DNA probes for use in hybridizations were prepared as described previously for the Pal1, PR1, BGL2 (PR2), PR5, and GST1 pathogen-induced genes (16, 73).
Cloning and sequencing the ES4326 rpoN gene.
DNA blot analysis data indicated that ES4326 contained a single 4.5-kb HindIII fragment that hybridized to a 4.2-kb XhoI fragment in plasmid pKI11 containing the P. aeruginosa strain PAK rpoN gene (31). Approximately 1,400 clones from a cosmid library of strain ES4326 DNA were screened by colony hybridization using the rpoN probe derived from P. aeruginosa. A hybridizing clone, pPG101, carrying a 17.2-kb insert was identified and shown to contain the 4.5-kb HindIII fragment previously detected by Southern blot analysis (data not shown).
E. coli strain TH1 λgln101, which contains deletions in rpoN and lacZ and a glnA-lacZ reporter construct, was used as an assay for functional ES4326 rpoN clones by plating subclones of pPG101 onto M9 medium containing 0.2% glutamine and 20 μg of X-Gal per ml. The 4.5-kb HindIII fragment from pPG101 contained a functional rpoN gene that complemented the E. coli rpoN mutation in TH1 λgln101. The rpoN gene in this construct presumably contained its own promoter since this fragment activated the glnA-lacZ fusion in TH1 λgln101 when cloned in the HindIII site of pBluescript SK(+) in either orientation (plasmids pRN5 and pRN9). For subsequent use in Pseudomonas spp., the 4.5-kb fragment containing ES4326 rpoN was subcloned into cosmid pLAFR3. A 4.5-kb HindIII fragment from pRN5 was subcloned into pGem7Zf. Using the pGem7Zf polylinker sites, the rpoN gene was recloned as a 4.5-kb EcoRI-BamHI fragment in pLAFR3 to produce pPG102. Plasmids pRN5 and pRN9 were used to derive a series of nested deletions starting from either end of the 4.5 HindIII fragment. This analysis showed that the ES4326 rpoN gene was located near the left end of the 4.5-kb fragment (data not shown).
DNA sequence analysis was initiated at the middle XhoI site in Fig. 1 and continued in both directions using synthetic oligonucleotides for a total of approximately 1,900 bp. A single large open reading frame that encodes a protein that is highly homologous to ς54 in other bacterial species was identified in the 1,900-bp region.
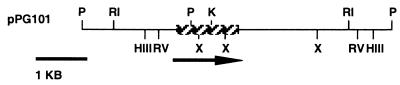
FIG. 1.
Restriction map of the ES4326 rpoN region. The stippled box indicates the region sequenced. The horizontal arrow indicates the direction of transcription. Restriction enzyme sites: HIII, HindIII; RI, EcoRI; RV, EcoRV; P, PstI; K, KpnI; X, XhoI.
Insertional mutagenesis of the ES4326 rpoN gene.
pGem7Zf containing the 4.5-kb rpoN fragment was digested with PstI, and a 1.24-kb fragment encoding the aminoglycoside 3′-phosphotransferase activity of Tn903 (Pharmacia) from pUC4K was ligated to the PstI-digested ends. A 5.8-kb EcoRI-BamHI fragment from this plasmid was then subcloned into pLAFR3. The resulting plasmid (pLAFR-RK) was used to recombine the mutated rpoN gene (referred to as rpoN::Kmr) into strain ES4326 by first mobilizing pLAFR-RK into ES4326 and then introducing plasmid pPH1, which confers gentamicin (Gm) resistance. Cultures were grown under selection for Kmr and Gmr, and individual colonies were screened for tetracycline sensitivity (76). Southern blot analysis of chromosomal DNA prepared from a putative rpoN mutant confirmed the insertion of Kmr into the rpoN gene (data not shown).
COR preparation and assay.
Coronatine (COR) synthesis by strain ES4326 was assayed using two approaches. In the first procedure (74), a 5-ml culture of ES4326 grown overnight in King's B medium was used to inoculate 50 ml of Woolley's liquid medium (94), where potassium nitrate was replaced with 5 mM arginine to facilitate rapid growth of the ES4326 rpoN-Kmr mutant. Cultures (50 ml) were shaken at 20°C for 6 days, at which point the optical density at 600 nm (OD600) was measured, and the cells were pelleted and weighed. The supernatants were acidified to pH 2.0 with HCl and extracted with 50 ml of ethyl acetate. The organic phase was lyophilized to dryness, and the residue was resuspended in 2.0 ml of H2O/g of wet bacterial pellet. Then, 10-μl droplets containing dilutions of either purified COR or the COR preparation described above were inoculated into Arabidopsis and tomato leaves. Elicitation of red anthocyanin pigments on Arabidopsis leaves and chlorosis on tomato leaves was assayed 3 to 7 days later.
In the second method, P. syringae strains were grown at 18°C in Hoitink-Sinden medium optimized for COR production (HSC) (63), and the supernatants were analyzed for COR production by high-pressure liquid chromatography (HPLC) 5 days after inoculation (63). Each strain was inoculated into three replicate aliquots (10 ml) of HSC medium for evaluation of COR production, and each experiment was repeated.
Nucleotide sequence accession number.
The rpoN sequence from P. syringae pv. maculicola has been assigned accession number AF199600 in the GenBank database.
RESULTS
Cloning the strain ES4326 rpoN gene.
An interspecies hybridization strategy was used to isolate pPG101, a cosmid clone that carried a presumptive P. syringae ES4326 rpoN gene. Plasmid pPG101 complemented the inability of E. coli, K. aerogenes, and P. aeruginosa rpoN mutants (strains TH1 λgln101, HG63 rpoN, and PAKN1, respectively) to utilize 10 mM nitrate and 10 mM ammonia as sole nitrogen sources; the inability of the E. coli rpoN mutant to utilize histidine, arginine, or proline as sole nitrogen sources; and the lack of motility of the P. aeruginosa rpoN mutant. As described in Materials and Methods and as illustrated in Fig. 1, the ES4326 rpoN gene on pPG101 was mapped to a 4.5-kb-HindIII fragment, and a 1,900-bp region containing the presumptive rpoN gene was sequenced. DNA sequence analysis (Fig. 2) revealed that the presumptive ES4326 rpoN gene encodes a protein with 86% identity and 95% similarity to the rpoN gene of P. putida.
FIG. 2.
Amino acid alignment of the ES4326 protein with ς54 from diverse bacterial species. The sequences are listed in decreasing order of conservation with ς54 from P. syringae pv. maculicola. (P_Syr). Abbreviations and references: P_Put, Pseudomonas putida (30); P_Aer, Pseudomonas aeruginosa (33); A_Vin, Azotobacter vinelandii (53); S_Typ, Salmonella enterica serovar Typhimurium (66); E_Col, Escherichia coli (28). Shading: black, conserved amino acids; gray, conservative substitutions.
Construction and metabolic phenotypes of a strain ES4326 rpoN insertional mutant.
An ES4326 rpoN mutant was constructed by subcloning rpoN from pPG101, inserting a DNA cassette conferring kanamycin resistance into the PstI site (located at codon 162) of rpoN, transferring the mutated rpoN gene to pLAFR3, and marker exchanging the mutant gene into the ES4326 genome (see Materials and Methods). ES4326 rpoN::Kmr exhibited an array of phenotypes typical of rpoN mutants, including the inability to grow on nitrate and urea as sole nitrogen sources, lack of motility, and inability to grow on a variety of C4-dicarboxylic acids as sole carbon sources, including aspartate, succinate, and fumarate, as well as the tricarboxylic acid intermediate α-ketoglutarate (data not shown). Unlike rpoN mutants of enteric bacteria (49, 86), however, ES4326 rpoN::Kmr was able to grow on glucose and ammonia as the sole carbon and nitrogen sources if ammonia was present at concentrations higher than 2 mM.
The ability of ES4326 rpoN::Kmr to utilize a variety of amino acids as nitrogen sources was tested on minimal M9 solid medium. The rpoN mutant failed to utilize aspartate, proline, histidine, and methionine as nitrogen sources, which supported growth of large colonies of wild-type ES4326. Both wild-type and the rpoN mutant formed large colonies on arginine, lysine, asparagine, glutamine, and glutamate, although the rpoN mutant grew somewhat slower than the wild type (5 days to form a large colony compared to 3 days for the wild type). The wild-type ES4326 formed small colonies, and the rpoN mutant formed even smaller colonies on serine, leucine, threonine, isoleucine, or alanine. Phenylalanine and cysteine were not utilized by the wild type or the rpoN mutant. As in the case of P. putida rpoN mutants, all amino acids that served as a sole nitrogen source for the ES4326 rpoN::Kmr mutant also served as sole carbon source with the exception of lysine, which served only as a nitrogen source. The best growth rate for ES4326 rpoN::Kmr was observed when the medium was supplemented with glutamate, where the growth was equivalent to the wild-type strain.
Strain ES4326 rpoN is nonpathogenic on Arabidopsis and cannot elicit an HR.
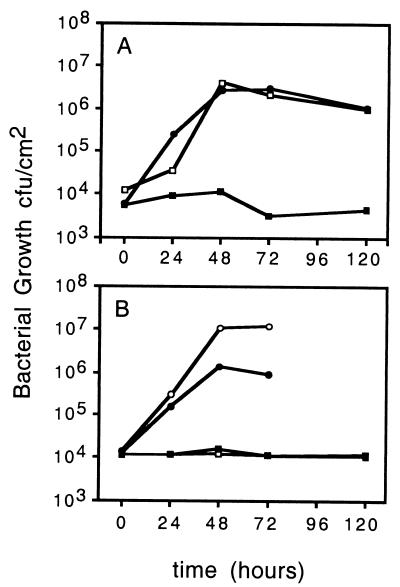
As described previously (11), infiltration of Arabidopsis leaves with ES4326 at a titer of 103 to 104 CFU/cm2 of leaf area resulted in the development of characteristic disease symptoms, including spreading chlorosis, water-soaked lesions, and growth of the infiltrated bacteria to a titer of approximately 107 CFU/cm2 (Fig. 3A). In contrast, Arabidopsis leaves infiltrated with ES4326 rpoN::Kmr exhibited no symptoms, even when inoculated with 108 CFU/cm2. Furthermore, the titer of ES4326 rpoN::Kmr in Arabidopsis leaves remained consistently low for the duration of the experiment (Fig. 3A). ES4326 rpoN::Kmr carrying pPG102, which carries wild-type rpoN, exhibited the same pathogenic phenotype as ES4326, and its growth in Arabidopsis leaves was indistinguishable from that of the wild type (Fig. 3A). This result indicated that the nonpathogenic phenotype of ES4326 rpoN::Kmr was due to the disruption of the rpoN gene. However, because the complementing plasmid contains about 2,000 bp downstream of rpoN, these data do not rule out the possibility that the insertion in rpoN exerts polarity on a downstream gene and that this downstream gene is required for pathogenicity.
FIG. 3.
Growth of ES4326 rpoN::Kmr in Arabidopsis leaves. Six-week-old Arabidopsis (Columbia) seedlings were infiltrated with bacterial suspensions at a titer of 104 CFU/cm2 of leaf area, and bacterial populations were determined as described in Materials and Methods. Each value represents the average of at least four leaf discs. (A) Symbols: □, ES4326(pLAFR3); ■, ES4326 rpoN::Kmr (pLAFR3); ●, ES4326 rpoN::Kmr (pPG102) (rpoN+). The experiment was repeated with similar results. (B) Symbols: ○, ES4326(pLAFR3); ●, ES4326 (avrRpt2); ■, ES4326 rpoN::Kmr (pLAFR3); □, ES4326 rpoN::Kmr (avrRpt2). The experiment was repeated with similar results.
ES4326 carrying avrRpt2 on plasmid pLH12 [ES4326 (avrRpt2)] elicits an HR on Arabidopsis ecotype Columbia instead of disease symptoms, and the HR is accompanied by a 10- to 100-fold reduction in bacterial growth compared to ES4326 in infiltrated leaves (Fig. 3B). In contrast, ES4326 rpoN::Kmr (avrRpt2) failed to elicit an HR or any other visible symptom, even when the inoculum was 20 times higher than ES4326 (avrRpt2) (data not shown). As shown in Fig. 3B, ES4326 rpoN::Kmr (avrRpt2), like ES4326 (avrRpt2), failed to multiply in Arabidopsis leaves. Infiltration of ES4326 into tobacco leaves also results in the elicitation of an HR (not shown). In confirmation of the results obtained in Arabidopsis, infiltration of tobacco leaves with ES4326 rpoN::Kmr did not result in the appearance of any visible symptoms (not shown).
The pathogenicity defect of ES4326 rpoN::Kmr is not solely due to the inability to assimilate aspartate.
Because genes encoding dicarboxylic acid permease are regulated by DctD, an NtrC homolog, rpoN mutants in a variety of species cannot utilize dicarboxylic acids as carbon or nitrogen sources. R. meliloti mutants defective in aspartate aminotransferase cannot utilize aspartate and are defective in symbiosis, suggesting that aspartate may be a major carbon source for symbiotic bacterial cells (69). Therefore, we tested whether ES4326 rpoN::Kmr is nonpathogenic solely because it cannot assimilate aspartate. We selected a ES4326 rpoN::Kmr pseudorevertant, as described in Materials and Methods, that was able to utilize aspartate as a carbon source. This revertant still had an RpoN− phenotype with respect to its inability to use nitrate and succinate and its lack of motility. The Asp+ revertant failed to grow in A. thaliana or to elicit an HR in tobacco or Arabidopsis (data not shown).
Strain ES4326 rpoN::Kmr fails to synthesize the phytotoxin coronatine.
One possible explanation for the reduced virulence of ES4326 rpoN::Kmr is the inability to produce virulence factors such as toxins. Many P. syringae pathovars, including ES4326, produce a chlorosis-inducing phytotoxin, coronatine, which is composed of an ethyl cyclopropyl amino acid linked to a polyketide moiety (19). COR production is regulated by a modified two-component regulatory system that controls the expression of essential COR biosynthetic genes. The regulators CorR and CorP are related to response regulators of the ROIII group, while CorS is similar to the corresponding histidine protein kinase sensors. To determine whether COR biosynthesis requires rpoN, crude COR was extracted from strains ES4326, ES4326 rpoN::Kmr, the COR-producing strain P. syringae pv. tomato DC3000, and DC3661, a COR− mutant of DC3000. The COR extracted from DC3000 and ES4326 elicited typical COR-induced symptoms, chlorosis and anthocyanin accumulation, respectively, on tomato and Arabidopsis leaves. These symptoms were not detected with organic acids extracted from ES4326 rpoN::Kmr or DC3661. Further characterization was carried out by quantitatively analyzing COR production using HPLC. As shown in Table 2, ES4326 produced 51 mg of COR/g of protein, a level comparable to that produced by P. syringae pv. glycinea strain PG4180.N9, a high-yielding COR producer which has been used in many genetic investigations (65, 88). However, ES4326 rpoN::Kmr produced only 0.3 mg of COR, a level comparable to PG4180.P2, a corR mutant of PG4180.N9 which is considered completely defective in COR production (65).
TABLE 2.
COR production and cor gene transcriptional activity in selected strains of P. syringaea
| Strain | COR production (mg/g of protein) | Transcriptional activity (U of GUS/mg of protein)b |
|---|---|---|
| P. syringae pv. maculicola | ||
| ES4326 | 51.0 | 347.0 |
| ES4326 rpoN | 0.3 | 8.5 |
| ES4326 rpoN + pHRPLC | 0.0 | ND |
| P. syringae pv. glycinea | ||
| PG4180.N9 | 43.4 | 390.0 |
| PG4180.P2 | 0.3 | 12.6 |
Values for COR and glucuronidase (GUS) activity represent the average of two experiments with three replicates each. Means within each column were analyzed using Duncan's multiple-range test. The protein content in the cell lysates was determined with the Bio-Rad protein assay kit as recommended by the manufacturer.
Transcriptional activity in the COR biosynthetic gene cluster was evaluated by introducing pRGMU7 into each strain and measuring the glucuronidase activity in the resulting transconjugants. ND, not determined.
The structural genes encoding COR production belong primarily to two distinct transcriptional units: the cmaABT transcript which is essential for production of coronamic acid and the cfl-CFA transcript which encodes coronafacic acid (5). Coronamic acid and coronafacic acid function as the two key intermediates in the biosynthetic pathway to coronatine (5). The effect of the rpoN mutation on transcriptional activity in the COR biosynthetic gene cluster was investigated by measuring β-glucuronidase activity from pRGMU7, a construct containing the cmaABT promoter fused to uidA (87). As shown in Table 2, when pRGMU7 was introduced into the two COR-producing strains, ES4326 and PG4180.N9, transcriptional activity from the cmaABT promoter was comparable (347 and 390 U GUS, respectively). However, β-glucuronidase activity in the rpoN mutant containing pRGMU7 was extremely low and was comparable to the low level of expression in PG4180.P2(pRGMU7). It is important to note that PG4180.P2 is defective in corR, a gene which encodes a positive transcriptional activator of the cmaABT transcript (64). The present data suggest that a functional rpoN is required for expression of the cmaABT transcript in P. syringae pv. maculicola.
In the accompanying study (23), we demonstrate that rpoN in ES4326 is required for the expression of hrpL, which encodes an alternative sigma factor and is required for expression of the ES4326 hrp genes and avrRpt2 (12, 17, 29, 97). We also show that constitutive expression of hrpL on plasmid pHRPLC restores the ability of ES4326 rpoN::Kmr to elicit disease symptoms in A. thaliana and an HR in tobacco. However, pHRPLC did not restore COR production to ES4326 rpoN::Kmr (Table 2), indicating that COR biosynthesis is not dependent on hrpL in ES4326 but on a separate regulatory pathway that also requires rpoN.
Strain ES4326 rpoN::Kmr fails to activate high-level expression of Arabidopsis defense-related genes.
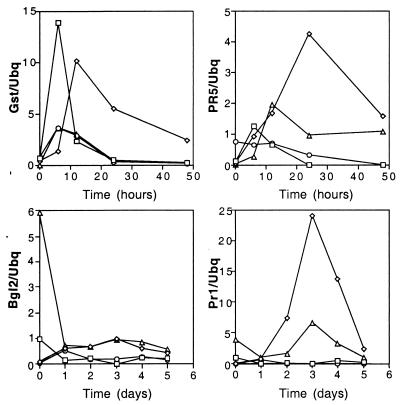
The infiltration of Arabidopsis leaves with ES4326 normally leads to the accumulation of mRNAs corresponding to a variety of Arabidopsis defense-related genes, including PR2 (BGL2), GST1, PR5 and PR1, which encode β-1,3-glucanase, glutathione S-transferase, a thaumatin-like protein, and a protein with unknown activity, respectively (42, 51, 52, 91). Each of these genes shows a different induction pattern. In general, GST1 and PR5 are induced within several hours after infection, whereas PR1 and BGL2 are induced later than 24 h postinfection. In contrast to ES4326, very little accumulation of the BGL2, GST1, PR1, and PR5 transcripts was seen following infiltration with ES4326 rpoN::Kmr (Fig. 4), which gave results similar to those for the MgSO4 control. This demonstrated that a factor under rpoN control is necessary for defense gene induction.
FIG. 4.
Induction of Arabidopsis defense-related mRNAs following infiltration of ES4326 rpoN::Kmr. Leaves were infiltrated with bacterial suspensions normalized to 0.1 OD600 as described in Materials and Methods. Filters containing 5 μg of total RNA per lane were probed with radiolabeled DNA fragments encoding the Arabidopsis GST1, PR5, BGL2, and PR1 genes. The filters were also probed with a radiolabeled probe corresponding to the Arabidopsis ubiquitin gene UBQ. Phosphorimager data are expressed as a ratio of defense gene to UBQ gene induction. Symbols: □, 10 mM MgSO4; ◊, ES4326; ○, ES4326 rpoN::Kmr; ▵, ES4326 rpoN::Kmr (pHRPLC).
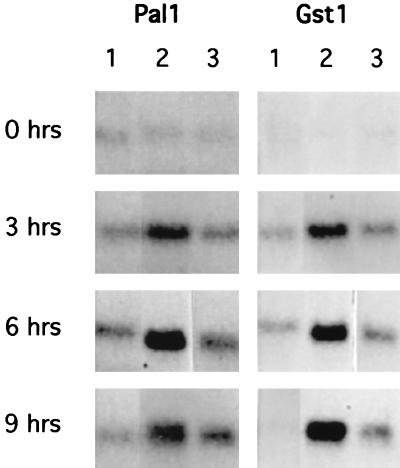
Even more rapid induction of defense-related genes is seen accompanying the HR in incompatible interactions (11, 32, 66). Two such genes, Pal1 and Gst1, which encode phenylalanine ammonia lysase and glutathione S-transferase, respectively, show early, high-level accumulation of mRNAs in response to the avirulence gene avrRpt2 (11, 16). ES4326 rpoN::Kmr carrying the avrRpt2 gene induces little accumulation of Pal1 or Gst1 mRNA compared to ES4326 (avrRpt2) (Fig. 5). Again, the rpoN mutant gave results similar to those for the MgSO4 control.
FIG. 5.
Induction of Arabidopsis defense-related mRNAs following infiltration of ES4326 rpoN::Kmr carrying the avirulence gene avrRpt2. Leaves were infiltrated with bacterial suspensions normalized to 0.1 OD600 as described in Materials and Methods. Filters containing 5 μg of total RNA per lane were probed with radiolabeled DNA fragments encoding the Arabidopsis Pal1 gene or the Arabidopsis GST1 gene. Lanes: 1, 10 mM MgSO4; 2, ES4326 (avrRpt2); 3, ES4326 rpoN::Kmr (avrRpt2).
In the accompanying study we report that constitutive expression of the sigma factor HrpL restores disease and HR phenotypes to ES4326 rpoN::Kmr (23). Curiously, however, constitutive expression of hrpL did not restore in planta growth to ES4326 rpoN::Kmr. As seen in Fig. 4, constitutive expression of hrpL in ES4326 rpoN::Kmr restored partial activation of the PR1, PR2 (BGL2), and PR5 genes in infiltrated plants, suggesting that defense gene activation and the elicitation of disease symptoms are a consequence of HrpL-dependent bacterial factors rather than growth of the pathogen per se. One difficulty encountered in this experiment was the instability of pHRPLC in wild-type ES4326 in planta (23). Thus, it was not possible to examine PR gene expression in response to ES4326 (pHRPLC).
DISCUSSION
ES4326 rpoN gene.
The amino acid sequence of the ES4326 ς54 protein is most closely related to the P. putida ς54 protein, and the phenotype of the ES4326 rpoN mutant resembles the phenotype of P. putida rpoN mutants (38). Both P. syringae and P. putida rpoN mutants grow more slowly than their wild-type counterparts in all media tested and were unable to utilize several uncharged amino acids as substrates. Neither is a glutamine auxotroph, and both can utilize NH4 as a sole nitrogen source. They can also utilize lysine as a nitrogen source though not as a carbon source. In P. putida, the inability of the rpoN mutant to grow on lysine is probably due to the fact that lysine decarboxylase is under ς54 control (38). The major phenotypic difference that we observed between the P. putida and the P. syringae rpoN mutants was that the P. syringae rpoN mutant could not utilize proline and histidine as nitrogen sources, whereas the P. putida rpoN mutant could.
Lack of growth of strain ES4326 rpoN on low concentrations of ammonia and other nitrogen sources.
The inability of ES4326 rpoN::Kmr to grow on low concentrations of ammonia (<1 mM) and various amino acids is presumably caused by the lack of glnA expression (which encodes glutamine synthetase), which is under ς54 control in several other bacterial species (54, 86). On the other hand, the ES4326 rpoN mutant is not a glutamine auxotroph, since it grew well when high concentrations of ammonia (>20 mM) or several amino acids other than glutamine were supplied as the sole nitrogen source. One explanation for these results is that P. syringae, like Rhizobium and Bradyrhizobium species, has more than one gene encoding glutamine synthetase, one that is expressed at high levels under ς54 control and a second copy that is expressed at low levels and is ς54 independent (41). Alternatively, P. syringae could have a single gene encoding glutamine synthetase, which requires ς54 for the high-level expression needed for ammonia assimilation but which has sufficient basal expression to prevent auxotrophy. rpoN mutants of most soil bacteria, including Azotobacter vinelandii, P. putida, and Agrobacterium tumefaciens, are not glutamine auxotrophs (39, 40, 53, 74, 75).
Growth rate of strain ES4326 rpoN.
The ES4326 rpoN mutant displayed slower growth rates than the wild type in each medium examined, including M9 supplemented with 0.2 mM glutamine. Thus, it appears unlikely that the slower growth of the rpoN mutant can be explained solely on the basis of decreased levels of glnA expression. While it is possible that the growth deficit is due to a secondary mutation, plasmid pPG102, which carries the wild-type rpoN gene, fully complemented every rpoN-related phenotype tested, including growth and pathogenesis in Arabidopsis leaves.
Nonpathogenic phenotype of strain ES4326 rpoN.
Given the pleiotropic phenotype of rpoN mutants, it is not possible to state precisely why the ES4326 rpoN mutant failed to elicit disease symptoms and to grow in Arabidopsis leaves or to elicit an HR. In the accompanying study we show that the absence of a functional ς54 in ES4326 blocks the transcription of hrp genes downstream of hrpRS (23), which would account for the nonpathogenic and HR-deficient phenotypes. However, we also report that the constitutive expression of hrpL in ES4326 rpoN::Kmr restored the elicitation of disease symptoms but failed to restore growth of ES4326 rpoN::Kmr in planta, implying that the absence of hrp functions is not the sole reason for the nonpathogenic phenotype of ES4326 rpoN::Kmr.
Our experiments using a pseudorevertant of the ES4326 rpoN mutant that was able to utilize aspartate eliminated the possibility that the rpoN mutant is nonpathogenic solely due to its inability to utilize this amino acid. However, it is possible that ES4326 rpoN::Kmr has another metabolic defect that contributes to the nonpathogenic phenotype. The rpoN mutant is also unable to utilize proline, histidine, and methionine, and if ammonia serves as the main nitrogen source during infection then leaf concentrations of less than 1 to 2 mM would likely stop the growth of ES4326 rpoN::Kmr. Finally, as discussed in the next section, rpoN is involved in the production of at least one known toxin, COR, which could help explain the reduced virulence.
rpoN-mediated regulation of COR synthesis.
The data in Table 2 demonstrate that ES4326 rpoN::Kmr does not produce COR, which contributes to lesion expansion, chlorosis, and bacterial multiplication in Arabidopsis (56). Although a COR− mutant is not available for P. syringae pv. maculicola, pHRPLC, which expresses hrpL constitutively, restored some disease symptoms but not COR production to ES4326 rpoN::Kmr. However, because pHRPLC failed to restore in planta growth to the mutant (23), it remains possible that some of the growth defect in ES4326 rpoN::Kmr could be caused by loss of COR production.
The data in Table 2 show that rpoN is also required for the expression of the cmaABT transcript, which encodes proteins that produce coronamic acid, an intermediate in the COR pathway (5). This was surprising since a conserved −24(GG)/−12(GC) motif is lacking upstream of the cmaA transcriptional start site (87). Thus, ς54 control of cmaABT expression is probably mediated indirectly through another regulatory gene whose expression is directly controlled by ς54. Possible candidates for ς54 control inside the COR gene cluster include corP and corR, which encode response regulators with uncharacterized upstream sequences. Alternatively, ς54 might control the expression of regulatory genes unlinked to the COR biosynthetic gene cluster.
Reduced induction of the host defense response by ES4326 rpoN.
ES4326 rpoN::Kmr failed to induce defense gene induction in Arabidopsis during both compatible and incompatible interactions. These results contrast with those reported previously for hrp mutants. A nonpathogenic hrp deletion mutant of a compatible X. campestris pv. campestris strain elicited the expression of a variety of defense genes in the turnip to approximately 50% of their normal expression levels (60). Similarly, an incompatible X. campestris pv. armoraciae strain with an hrp deletion did not induce an HR in the turnip but still induced defense gene expression (60). In the P. syringae pv. phaseolicola-bean interaction, approximately the same level of defense gene induction occurred with incompatible wild-type and hrp deletion strains (32). One way to explain the discrepancy observed in defense gene induction by rpoN and hrp mutants is that important factors for defense gene induction may lie outside of the hrp pathway but under rpoN control. While there are reasons to believe that phytotoxins and avr genes may contribute to defense gene induction, they seem unlikely explanations for this phenomenon (5, 34, 47, 71). Although production of the phytotoxin COR is rpoN dependent, in ES4326, COR− mutants of DC3000 elicited more defense gene induction than the wild-type strain (56). Similarly, avr gene products are thought to require a functioning hrp cluster for activity and are therefore unlikely candidates for hrp-independent defense induction factors (34, 47, 71, 84). Finally, the fact that X. campestris pv. vesicatoria rpoN mutants are fully pathogenic (25) indicates that, at least in the case of this species, rpoN does not regulate any essential pathogenicity factors.
Our experiments also indicate an important role for hrp-dependent factors in defense gene induction. When hrpL was constitutively expressed in ES4326 rpoN::Kmr, both hrp gene expression (23) and defense gene induction (Fig. 4) were restored. This result indicates that at least some inducing factors require genes downstream of hrpL for expression, function, or both. As mentioned above, avr genes are a likely source of hrp-dependent defense-inducing factors. We also report that the partial restoration of defense gene induction by hrpL is accompanied by restoration of disease symptoms and host cell death (23). This restored host cell death may also play a role in the activation of host defense responses. P. syringae products secreted by the Hrp system could result in necrotic or programmed cell death which in turn activates defense gene induction in neighboring cells.
ACKNOWLEDGMENTS
Erik L. Hendrickson and Pablo Guevara contributed equally to this work.
This work was supported by NIH grant GM48707 awarded to F.M.A. and NSF grant MCB-9603618 awarded to C.L.B.
REFERENCES
- 1.Agrios G N. Plant pathology. 4th ed. San Diego, Calif: Academic Press, Inc.; 1997. [Google Scholar]
- 2.Albright L M, Huala E, Ausubel F M. Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet. 1989;23:311–336. doi: 10.1146/annurev.ge.23.120189.001523. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1996. [Google Scholar]
- 4.Backman K, Ptashne M. Maximizing gene expression on a plasmid using recombination in vitro. Cell. 1978;13:65–71. doi: 10.1016/0092-8674(78)90138-1. [DOI] [PubMed] [Google Scholar]
- 5.Bender C, Alarcon-Chaidez F, Gross D. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev. 1999;63:266–292. doi: 10.1128/mmbr.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black L K, Maier R J. IHF- and RpoN-dependent regulation of hydrogenase expression in Bradyrhizobium japonicum. Mol Microbiol. 1995;16:405–413. doi: 10.1111/j.1365-2958.1995.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 7.Carr J P, Dixon D C, Nikolau B J, Voelkerding K V, Klessig D F. Synthesis and localization of pathogenesis-related proteins in tobacco. Mol Cell Biol. 1987;7:1580–1583. doi: 10.1128/mcb.7.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charkowski A, Huang H, Collmer A. Altered localization of HrpZ in Pseudomonas syringae pv. syringae hrp mutants suggests that different components of the type III secretion pathway control protein translocation across the inner and outer membranes of gram-negative bacteria. J Bacteriol. 1997;179:3966–3974. doi: 10.1128/jb.179.12.3866-3874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis K R, Schott E, Ausubel F M. Virulence of selected phytopathogenic pseudomonads in Arabidopsis thaliana. Mol Plant-Microbe Interact. 1991;4:477–488. [Google Scholar]
- 10.Ditta G, Stanfield S, Corbin D, Helinski D R. Broad-host-range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA. 1980;27:7347–7451. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong X, Mindrinos M, Davis K R, Ausubel F M. Induction of Arabidopsis thaliana defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell. 1991;3:61–72. doi: 10.1105/tpc.3.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fellay R, Rahme L G, Mindrinos M N, Frederick R D, Pisi A, Panopoulos N J. Genes and signals controlling the Pseudomonas syringae pv. phaseolicola-plant interaction. In: Hennecke H, Verma D P S, editors. Molecular genetics of plant-microbe interactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 45–52. [Google Scholar]
- 13.Fenselau S, Balbo I, Bonas U. Determinants of pathogenicity in Xanthomonas campestris pv. vesicatoria are related to proteins involved in secretion in bacterial pathogens of animals. Mol Plant-Microbe Interact. 1992;5:390–396. doi: 10.1094/mpmi-5-390. [DOI] [PubMed] [Google Scholar]
- 14.Genin S, Gough C L, Zischek C, Boucher C A. Evidence that the hrpB gene encodes a positive regulator of pathogenicity genes from Pseudomonas solanacearum. Mol Microbiol. 1992;6:3065–3076. doi: 10.1111/j.1365-2958.1992.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 15.Gough C L, Genin S, Zischek C, Boucher C A. hrp genes of Pseudomonas solanacearum are homologous to pathogenicity determinants of animal pathogenic bacteria and are conserved among plant pathogenic bacteria. Mol Plant-Microbe Interact. 1992;5:384–389. doi: 10.1094/mpmi-5-384. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg J T, Guo A, Klessig D F, Ausubel F M. Programmed cell death in plants: a pathogen-triggered response activated coordinately with multiple defense functions. Cell. 1994;77:551–563. doi: 10.1016/0092-8674(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 17.Grimm C, Aufsatz W, Panopoulos N J. The hrpRS locus of Pseudomonas syringae pv. phaseolicola constitutes of complex regulatory unit. Mol Microbiol. 1995;15:155–165. doi: 10.1111/j.1365-2958.1995.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 18.Grimm C G, Panopoulos N J. The predicted protein product of a pathogenicity locus from Pseudomonas syringae pv. phaseolicola is homologous to a highly conserved domain of several prokaryotic regulatory proteins. J Bacteriol. 1989;171:5031–5038. doi: 10.1128/jb.171.9.5031-5038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross D C. Molecular and genetic analysis of toxin production by pathovars of Pseudomonas syringae. Annu Rev Phytopathol. 1991;29:247–278. [Google Scholar]
- 20.Hanahan D, Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- 21.Haula E, Ausubel F M. The central domain of Rhizobium meliloti NifA is sufficient to activate transcription from the R. meliloti nifH promoter. J Bacteriol. 1989;171:3354–3365. doi: 10.1128/jb.171.6.3354-3365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haula E, Moon A L, Ausubel F M. The central domain of Rhizobium leguminosarum DctD functions independently to activate transcription. J Bacteriol. 1992;174:1428–1431. doi: 10.1128/jb.174.4.1428-1431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrickson E, Guevara P, Ausubel F M. The alternative sigma factor RpoN is required for hrp activity in Pseudomonas syringae pv. maculicola and acts at the level of hrpL transcription. J Bacteriol. 2000;182:3508–3516. doi: 10.1128/jb.182.12.3508-3516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbs M, Mattick J S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 25.Horns T, Bonas U. The rpoN gene of Xanthomonas campestris pv. vesicatoria is not required for pathogenicity. Mol Plant-Microbe Interact. 1996;9:856–859. doi: 10.1094/mpmi-9-0856. [DOI] [PubMed] [Google Scholar]
- 26.Huang H-C, Lin R-H, Chang C-J, Collmer A, Deng W-L. The complete hrp gene cluster of Pseudomonas syringae pv. syringae 61 includes two blocks of genes required for harpin Pss secretion that are arranged colinearaly with Yersinia ysc homologs. Mol Plant-Microbe Interact. 1995;8:733–746. doi: 10.1094/mpmi-8-0733. [DOI] [PubMed] [Google Scholar]
- 27.Huynh T V, Dahlbeck D, Staskawicz B J. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science. 1989;245:1374–1377. doi: 10.1126/science.2781284. [DOI] [PubMed] [Google Scholar]
- 28.Imaishi H, Gomada M, Inouye S, Nakazawa A. Physical map location of the rpoN gene of Escherichia coli. J Bacteriol. 1993;175:1550–1551. doi: 10.1128/jb.175.5.1550-1551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Innes R, Bent A, Kunkel B, Bisgrove S, Staskawicz B. Molecular analysis of avirulence gene avrRpt2 and identification of a putative regulatory sequence common to all known Pseudomonas syringae avirulence genes. J Bacteriol. 1993;175:4859–4869. doi: 10.1128/jb.175.15.4859-4869.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inouye S, Yamada M, Nakazawa T. Cloning and sequence analysis of the ntrA (rpoN) gene of Pseudomonas putida. Gene. 1989;85:154–152. doi: 10.1016/0378-1119(89)90474-5. [DOI] [PubMed] [Google Scholar]
- 31.Ishimoto K S, Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci USA. 1989;86:1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakobek J L, Lindgren P B. Generalized induction of defense responses in bean is not correlated with the induction of the hypersensitive reaction. Plant Cell. 1993;5:49–56. doi: 10.1105/tpc.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin S, Ishimoto K, Lory S. Nucleotide sequence of the rpoN gene and characterization of two downstream open reading frames in Pseudomonas aeruginosa. J Bacteriol. 1994;176:1316–1322. doi: 10.1128/jb.176.5.1316-1322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kearney B, Staskawicz B J. Widespread distribution and fitness contribution of Xanthomonas campestris avirulence gene avrBs2. Nature. 1990;346:385–386. doi: 10.1038/346385a0. [DOI] [PubMed] [Google Scholar]
- 35.Kessler B, Marqués S, Köhler T, Ramos J L, Timmis K N, de Lorenzo V. Cross talk between catabolic pathways in Pseudomonas putida: XylS-dependent and-independent activation of the TOL meta operon requires the same cis-acting sequences within the Pm promoter. J Bacteriol. 1994;176:5578–5582. doi: 10.1128/jb.176.17.5578-5582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King E O, Ward M K, Raney D E. Two simple media for the demonstration of phycocyanin and fluorescin. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 37.Klement Z. Hypersensitivity. In: Mount M S, Lacy G H, editors. Phytopathogenic procaryotes. Vol. 2. New York, N.Y: Academic Press, Inc.; 1982. pp. 149–177. [Google Scholar]
- 38.Köhler T, Harayama S, Ramos J-L, Timmis K N. Involvement of Pseudomonas putida Rpo ς factor in regulation of various metabolic functions. J Bacteriol. 1989;171:4326–4333. doi: 10.1128/jb.171.8.4326-4333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kullik I, Fritsche S, Knobel H, Sanjuan J, Hennecke H, Fischer H-M. Bradyrhizobium japonicum has two differentially regulated, functional homologs of the ς54 gene (rpoN) J Bacteriol. 1991;173:1125–1138. doi: 10.1128/jb.173.3.1125-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kustu S, Santero E, Keener J, Popham D, Weiss D. Expression of ς-54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kustu S, Santero E, Keener J, Popham D, Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamb C J. Plant disease resistance genes in signal perception and transduction. Cell. 1994;76:419–422. doi: 10.1016/0092-8674(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 43.Leister R T, Ausubel F M, Katagiri F. Molecular recognition of pathogen attack occurs inside of plant cells in plant disease resistance specified by the Arabidopsis genes RPS2 and RPM1. Proc Natl Acad Sci USA. 1996;93:15497–15502. doi: 10.1073/pnas.93.26.15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindgren P B, Panopoulos N J, Staskawicz B J, Dahlbeck D. Genes required for pathogenicity and hypersensitivity are conserved and interchangeable among pathovars of Pseudomonas syringae. Mol Gen Genet. 1988;211:499–506. [Google Scholar]
- 45.Lindgren P B, Peet R C, Panopoulos N J. Gene cluster of Pseudomonas syringae pv. “phaseolicola” controls pathogenicity on bean and hypersensitivity on non-host plants. J Bacteriol. 1986;168:512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lorang J M, Keen N T. Characterization of avrE from Pseudomonas syringae pv. tomato: a hrp-linked avirulence locus consisting of at least two transcriptional units. Mol Plant-Microbe Interact. 1995;8:49–57. doi: 10.1094/mpmi-8-0049. [DOI] [PubMed] [Google Scholar]
- 47.Lorang J M, Shen H, Kobayashi D, Cooksey D, Keen N T. avrA and avrE in Pseudomonas syringae pv. tomato PT2 play a role in virulence on tomato plants. Mol Plant-Microbe Interact. 1994;7:508–515. [Google Scholar]
- 48.Macaluso A, Best E A, Bender R A. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J Bacteriol. 1990;172:7249–7255. doi: 10.1128/jb.172.12.7249-7255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magasanik B. Regulation of nitrogen utilization. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1344–1356. [Google Scholar]
- 50.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 51.Mauch F, Hadwiger L A, Boller T. Antifungal hydrolases in pea tissue. I. Plant Physiol. 1988;87:325–333. doi: 10.1104/pp.87.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mauch F, Mauch-Mani B, Boller T. Antifungal hydrolases in pea tissue. II Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol. 1988;88:936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merrick M, Gibbons J, Toukdarian A. The nucleotide sequence of the sigma factor gene ntrA (rpoN) of Azotobacter vinellandii: analysis of conserved sequences in NtrA proteins. Mol Gen Genet. 1987;210:323–330. doi: 10.1007/BF00325701. [DOI] [PubMed] [Google Scholar]
- 54.Merrick M J. In a class of its own—the RNA polymerase sigma factor ς54 (ςN) Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 55.Miller W, Mindrinos M N, Rahme L G, Frederick R D, Grimm C, Gressman R, Kyriakides X, Kokkinidis M, Panopoulos N J. Pseudomonas syringae pv. phaseolicola-plant interactions: host-pathogen signalling through cascade control of hrp gene expression. In: Nester E W, Verma D P S, editors. Advances in molecular genetics of plant-microbe interactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 267–274. [Google Scholar]
- 56.Mittal S, Davis K R. Role of the phytotoxin coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Mol Plant-Microbe Interact. 1995;8:165–171. doi: 10.1094/mpmi-8-0165. [DOI] [PubMed] [Google Scholar]
- 57.Moore R A, Starratt A N, Ma S-W, Morris V L, Cuppels D A. Identification of a chromosomal region required for biosynthesis of the phytotoxin coronatine by Pseudomonas syringae pv. tomato. Can J Microbiol. 1989;35:910–917. [Google Scholar]
- 58.Mudgett M, Staskawicz B. Characterization of the Pseudomonas syringae pv. tomato AvrRpt2 protein: demonstration of secretion and processing during bacterial pathogenesis. Mol Microbiol. 1999;32:927–941. doi: 10.1046/j.1365-2958.1999.01403.x. [DOI] [PubMed] [Google Scholar]
- 59.Mudgett M, Staskawicz B. Protein signaling via type III secretion pathways in phytopathogenic bacteria. Curr Opin Microbiol. 1998;1:109–114. doi: 10.1016/s1369-5274(98)80150-1. [DOI] [PubMed] [Google Scholar]
- 60.Newman M-A, Conrads-Strauch J, Scofield G, Daniels M J, Dow J M. Defense-related gene induction in Brassica campestris in response to defined mutants of Xanthomonas campestris with altered pathogenicity. Mol Plant-Microbe Interact. 1994;7:553–563. doi: 10.1094/mpmi-7-0553. [DOI] [PubMed] [Google Scholar]
- 61.O'Toole R, Milton D L, Wolf Watz H. Chemotactic motility is required for invasion of the fish pathogen Vibrio anguillarum. Mol Microbiol. 1996;19:625–637. doi: 10.1046/j.1365-2958.1996.412927.x. [DOI] [PubMed] [Google Scholar]
- 62.Ow D W, Ausubel F M. Regulation of nitrogen metabolism genes by nifA gene product in Klebsiella pneumoniae. Nature. 1983;301:307–313. doi: 10.1038/301307a0. [DOI] [PubMed] [Google Scholar]
- 63.Palmer D A, Bender C L. Effects of environmental and nutritional factors on production of the polyketide phytotoxin coronatine by Pseudomonas syringae pv. glycinea. Appl Environ Microbiol. 1993;59:1619–1626. doi: 10.1128/aem.59.5.1619-1626.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peñaloza-Vàzquez A, Bender C. Characterization of CorR, a transcriptional activator which is required for biosynthesis of the phytotoxin coronatine. J Bacteriol. 1998;180:6252–6259. doi: 10.1128/jb.180.23.6252-6259.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peñaloza-Vàzquez A, Rangaswamy V, Ullrich M, Bailey A, Bende C L. Use of translational fusions to the maltose binding protein to produce and purify proteins in Pseudomonas syringae and assess their activity in vivo. Mol Plant-Microbe Interact. 1996;9:637–641. doi: 10.1094/mpmi-9-0637. [DOI] [PubMed] [Google Scholar]
- 66.Pontier D, Godiard L, Marco Y, Roby D. hsr203J, a tobacco gene whose activation is rapid, highly localized, and specific for incompatible plant/pathogen interactions. Plant J. 1994;5:507–521. doi: 10.1046/j.1365-313x.1994.5040507.x. [DOI] [PubMed] [Google Scholar]
- 67.Puri N, Jenner C, Bennett M, Stewart R, Mansfield J, Lyons N, Taylor J. Expression of avrPphB, an avirulence gene from Pseudomonas syringae pv. phaseolicola, and the delivery of signals causing the hypersensitive reaction in bean. Mol Plant-Microbe Interact. 1997;10:247–256. doi: 10.1094/MPMI.1997.10.2.247. [DOI] [PubMed] [Google Scholar]
- 68.Rahme L G, Stevens E J, Wolfort S F, Shao J, Tompkins R G, Ausubel F M. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 69.Rastogi V K, Watson R J. Aspartate aminotransferase activity is required for aspartate catabolism and symbiotic nitrogen fixation in Rhizobium meliloti. J Bacteriol. 1991;173:2879–2887. doi: 10.1128/jb.173.9.2879-2887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reuber T L, Ausubel F M. Isolation of Arabidopsis genes that differentiate between disease resistance responses mediated by RPS2 and RPM1 disease resistance genes. Plant Cell. 1996;8:241–249. doi: 10.1105/tpc.8.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ritter C, Dangl J L. The avrRpm1 gene of Pseudomonas syringae pv. maculicola is required for virulence on Arabidopsis. Mol Plant-Microbe Interact. 1995;8:444–453. doi: 10.1094/mpmi-8-0444. [DOI] [PubMed] [Google Scholar]
- 72.Ritter C, Dangl J L. Interference between two specific pathogen recognition events mediated by distinct plant disease resistance genes. Plant Cell. 1996;8:251–257. doi: 10.1105/tpc.8.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogers E E, Ausubel F M. Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell. 1997;9:305–316. doi: 10.1105/tpc.9.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Römermann D, Warrelmann J, Bender R A, Friedrich B. An rpoN-like gene of Alcaligenes eutrophus and Pseudomonas facilis controls expression of diverse metabolic pathways, including hydrogen oxidation. J Bacteriol. 1989;171:1093–1099. doi: 10.1128/jb.171.2.1093-1099.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ronson C W, Nixon B T, Albright L M, Ausubel F M. Rhizobium meliloti ntrA (rpoN) gene is required for diverse metabolic functions. J Bacteriol. 1987;169:2424–2431. doi: 10.1128/jb.169.6.2424-2431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruvkun G B, Ausubel F M. A general method for site-directed mutagenesis in prokaryotes: construction of mutations in symbiotic nitrogen fixation genes of Rhizobium meliloti. Nature. 1981;289:85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- 77.Salmeron J M, Staskawicz B J. Molecular characterization and hrp dependence of the avirulence gene avrPto from Pseudomonas syringae pv. tomato. Mol Gen Genet. 1993;239:6–16. doi: 10.1007/BF00281595. [DOI] [PubMed] [Google Scholar]
- 78.Schroth M N, Hildebrand D C, Panopoulos N. Phytopathogenic pseudomonads and related plant-associated pseudomonads. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. Vol. 3. New York, N.Y: Springer-Verlag; 1992. pp. 3104–3131. [Google Scholar]
- 79.Schulte R, Bonas U. Expression of the Xanthomonas campestris pv. vesicatoria hrp gene cluster, which determines pathogenicity and hypersensitivity in pepper and tomato, is plant inducible. J Bacteriol. 1992;174:815–823. doi: 10.1128/jb.174.3.815-823.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scofield S R, Tobias C M, Rathjen J P, Chang J H, Lavelle D T, Michelmore R W, Staskawicz B J. Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science. 1996;274:2063–2065. doi: 10.1126/science.274.5295.2063. [DOI] [PubMed] [Google Scholar]
- 81.Staskawicz B J, Dahlbeck D, Keen N T. Cloned avirulence gene of Pseudomonas syringae pv. glycinea determines race-specific incompatibility on Glycine max (L.) Merr. Proc Natl Acad Sci USA. 1984;81:6024–6028. doi: 10.1073/pnas.81.19.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Staskawicz B J, Dahlbeck D, Keen N T, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 84.Swarup S, Yang Y, Kingsley M T, Gabriel D W. An Xanthomonas citri pathogenicity gene, pthA, pleiotropically encodes gratuitous avirulence on nonhosts. Mol Plant-Microbe Interact. 1992;5:204–213. doi: 10.1094/mpmi-5-204. [DOI] [PubMed] [Google Scholar]
- 85.Tang X, Frederick R D, Zhou J, Halterman D A, Jia Y, Martin G B. Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science. 1996;274:2060–2063. doi: 10.1126/science.274.5295.2060. [DOI] [PubMed] [Google Scholar]
- 86.Totten P A, Lara J C, Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990;172:389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ullrich M, Bender C L. The biosynthetic gene cluster for coronamic acid, an ethylcylopropyl amino acid, contains genes homologous to amino acid activating enzymes and thioesters. J Bacteriol. 1994;176:7574–7586. doi: 10.1128/jb.176.24.7574-7586.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ullrich M, Guenzi A C, Mitchell R E, Bender C L. Cloning and expression of genes required for coronamic acid (2-ethyl-1-aminocyclopropane 1-carboxylic acid), and intermediate in the biosynthesis of the phytotoxin coronatine. Appl Environ Microbiol. 1994;60:2890–2897. doi: 10.1128/aem.60.8.2890-2897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van den Eede G, Deblaere R, Goethals K, Montagu M V, Holsters M. Broad host range and promoter selection vectors for bacteria that interact with plants. Mol Plant-Microbe Interact. 1992;5:228–234. doi: 10.1094/mpmi-5-228. [DOI] [PubMed] [Google Scholar]
- 90.van Dijk K, Fouts D E, Rehm A H, Hill A R, Collmer A, Alfano J R. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type III) protein secretion system in a temperature- and pH-sensitive manner. J Bacteriol. 1999;181:4790–4797. doi: 10.1128/jb.181.16.4790-4797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Loon L C. Pathogenesis-related proteins. Plant Mol Biol. 1985;4:111–116. doi: 10.1007/BF02418757. [DOI] [PubMed] [Google Scholar]
- 92.Wengelnik K, Marie C, Russel M, Bonas U. Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol. 1996;178:1061–1069. doi: 10.1128/jb.178.4.1061-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whalen M C, Innes R W, Bent A F, Staskawicz B J. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woolley D P, Pringle R B, Braun A C. Isolation of the phytopathogenic toxin of Pseudomonas tabaci, an antagonist of methionine. J Biol Chem. 1952;197:407–417. [PubMed] [Google Scholar]
- 95.Wu Z-L, Charles T C, Wang H, Nester E W. The ntrA gene of Agrobacterium tumefaciens: identification, cloning, and phenotype of a site-directed mutant. J Bacteriol. 1992;174:2720–2723. doi: 10.1128/jb.174.8.2720-2723.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao Y, Heu S, Yi J, Lu Y, Hutcheson S W. Identification of a putative alternate sigma factor and characterization of a multicomponent regulatory cascade controlling the expression of Pseudomonas syringae pv. syringae Pss61 hrp and hrmA genes. J Bacteriol. 1994;176:1025–1036. doi: 10.1128/jb.176.4.1025-1036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao Y, Hutcheson S W. A single promoter sequence recognized by a newly identified alternate sigma factor directs expression of pathogenicity and host range determinants in Pseudomonas syringae. J Bacteriol. 1994;176:3089–3091. doi: 10.1128/jb.176.10.3089-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]