Background:
Evidence suggests that selenium supplementation could be useful in the treatment of Hashimoto thyroiditis (HT), but the available trials are heterogeneous. This study investigates clinically relevant effects of selenium supplementation in patients with HT.
Methods:
A systematic search was performed in PubMed, Web of Science, EMBASE, Scopus, and the Cochrane Library. The latest update was performed on December 3, 2022. We investigated the changes in thyroid peroxidase antibodies (TPOAb) and thyroglobulin antibodies (TgAb) after selenium supplementation. The effect sizes were expressed as weighted mean difference (WMD) with 95% confidence intervals (CIs).
Results:
After screening and full-text assessment, 7 controlled trials comprising 342 patients were included in the systematic review. The results showed that there was no significant change in TPOAb levels (WMD = −124.28 [95% CI: −631.08 to 382.52], P = .631, I2 = 94.5%) after 3 months of treatment. But there was a significant decrease in TPOAb levels (WMD = −284.00 [95% CI: −553.41 to −14.60], P < .05, I2 = 93.9%) and TgAb levels (WMD = −159.86 [95% CI: −293.48 to −26.24], P < .05, I2 = 85.3%) after 6 months of treatment.
Conclusions:
Selenium supplementation reduces serum TPOAb and TgAb levels after 6 months of treatment in patients with HT, but future studies are warranted to evaluate health-related quality or disease progression.
Keywords: Hashimoto thyroiditis, selenium supplementation, thyroglobulin antibodies, thyroid peroxidase antibodies
1. Introduction
Hashimoto thyroiditis (HT) is an autoimmune thyroid disorder and affects approximately 1 to 2% of the general population, especially children and women.[1,2] HT is characterized by lymphocyte infiltration, elevated plasma thyroid peroxidase antibodies (TPOAb), thyroglobulin antibodies (TgAb), and reduced echogenicity of thyroid parenchyma.[3] The slowly developing chronic autoimmune damage of the thyroid follicular cells frequently leads to hypothyroidism.[4]
In recent years, selenium supplementation is proposed for the treatment of HT patients. The thyroid gland has the highest selenium concentrations per unit weight among all tissues.[5,6] Selenium is considered to have substantial structural and functional roles in thyroid cell physiology and thyroid hormone metabolism.[7] Its deficiency could induce thyroid cell damage and autoimmune destruction of the gland.[8,9]
However, in human experiments, the efficacy of selenium supplementation in HT patients remains controversial. The majority of these studies have focused on serum TPOAb and TgAb autoantibody levels.[10–16] Some studies showed that selenium supplementation has a beneficial effect on thyroid autoimmunity in patients with HT.[10–14] But, others showed that selenium supplementation has no significant effect on serum thyroid autoantibody levels.[15,16] Therefore, the objective of this systematic review and meta-analysis was to assess the available evidence of the effect of selenium supplementation on thyroid autoantibody levels in patients with HT.
2. Material and methods
2.1. Search strategy
Using the search terms “selenium” AND “Hashimoto,” “selenium” AND “thyroiditis,” “selenomethionine” AND “Hashimoto” and “selenomethionine” AND “thyroiditis,” we searched the following databases: PubMed, Web of Science, EMBASE, Scopus, and the Cochrane Library. The latest update was performed on December 3, 2022. We also screened the reference lists of all retrieved articles to identify other potentially relevant literature. This meta-analysis was performed in accordance with the 2009 Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols.[17]
2.2. Inclusion and exclusion criteria
Studies were included if they met all the following criteria: patients with HT (positive TPOAb, reduced echogenicity of thyroid parenchyma); underwent selenium supplementation therapy; the data of TPOAb or TgAb were available.
Studies were excluded if they met any of the following characteristics: nonhuman studies; duplicate studies; lack the data of TPOAb and TgAb. Two investigators (Xiao-Qing Quan and Gui-Ying Qiu) read the literature independently of each other. Disagreements were solved by discussion with other investigators.
2.3. Data extraction and quality assessment
The following data were extracted: the first author, publication year, country of patients, sample size (the number of patients and the ratio of men to women), mean age, study design, outcomes, duration, baseline thyrotropin levels, the data of TPOAb and TgAb (mean with standard deviations). The Newcastle–Ottawa Scale was used to assess the quality of each included study. One star was assigned to each quality feature and denoted a score of 1. The total scores were calculated for each study. A study with Scores 4 and 5 was considered low quality, Scores 6 and 7 considered intermediate quality, and Scores 8 and 9 considered high quality.
2.4. Ethics
In this study, ethical approval was not necessary because the included data was based on previously published articles, and no original clinical data was collected or utilized.
2.5. Statistical analysis
All statistical analyses in the present study were conducted with STATA statistical software (version 13.1). If the data were presented as mean with interquartile range, the standard deviation was estimated using the method as interquartile range/1.35. This meta-analysis investigated the changes in antibodies after 3-month and 6-month selenium supplementation. We used weighted mean difference (WMD) and its 95% confidence intervals (CIs) to express the effect sizes. A 2-sided P value < 0.05 was considered statistically significant.
Meta-regression analyses were used to assess the correlation between sample size, percentage of females, mean age, baseline antibody (TPOAb and TgAb) levels, baseline thyrotropin levels, and WMD. Subgroup analyses were performed based on baseline antibody (TPOAb and TgAb) levels. Cochrane Q and I2 texts were used to assess between-study heterogeneity. I2 < 25% was regarded as low amounts of heterogeneity. An I2 value of 25 to 50% was regarded as moderate amounts of heterogeneity. I2 > 50% was regarded as a high amount of heterogeneity. A fixed-effects model was applied in the absence of significant heterogeneity (I2 ≤ 50%), or the random-effect model was applied (I2 > 50%).
In order to evaluate the stability of the overall effect size, we conducted a sensitivity analysis using the 1-study remove approach. Potential publication bias was explored using Egger test. A P value > 0.05 was considered that there was no publication bias.
3. Results
3.1. Study identification and selection
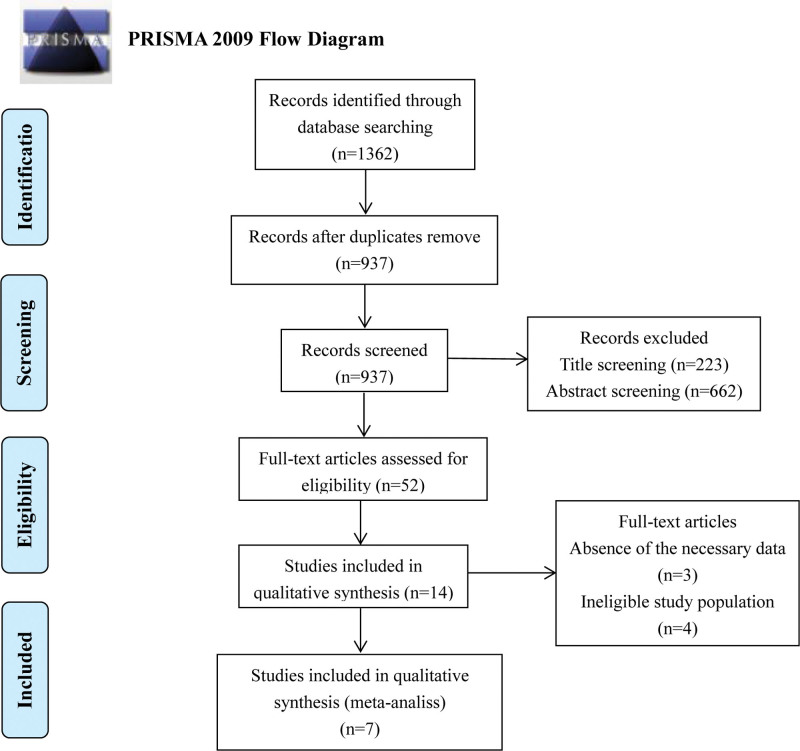
A flowchart of our search strategy and results was shown in Figure 1. Initially, a total of 1362 studies were included after the primary search from multiple databases. Search results were shown in Supplemental Table 1, Supplemental Digital Content, http://links.lww.com/MD/J2. After removing duplicate articles, screening titles, and abstracts, a total of 14 papers remained. Three of the remaining studies lacked the data of TPOAb and TgAb, and 4 studies had an ineligible study population (not HT patients). Eventually, 7 studies published from 2008 to 2019 were selected for our meta-analysis.[10–16] The quality assessment of inclusion studies based on the Newcastle–Ottawa Scale was shown in Supplemental Table 2, Supplemental Digital Content, http://links.lww.com/MD/J3.
Figure 1.
Flow diagram of literature search and study selection.
The main characteristics of the included literature were summarized in Table 1. A total of 342 subjects were included. Three studies were conducted in Poland by Krysiak et al (named Krysiak A, Krysiak B, Krysiak C),[10,11,14] Krysiak A enrolled only adult male patients,[10] Krysiak B enrolled only female patients who were recruited between 2016 and 2017[11] and Krysiak C enrolled female patients who were recruited before 2012.[14] Two studies were conducted in Italy by Nordio et al (Named Nordio A, Nordio B),[12,13] patients in Nordio A received only selenomethionine treatment[12] and in Nordio B received only myo-inositol plus selenomethionine treatment.[13] One study was conducted in Turkey[15] and one in Austria.[16] Patients of 4 studies received the treatment of selenomethionine 200 μg/day.[10,11,14,16] The remaining patients of 3 studies separately received selenomethionine 50 μg/day,[15] selenium 83μg/day[12] and myo-inositol 600mg/day plus selenium 83 μg/day.[13] Five studies compared the antibody (TPOAb and TgAb) levels before and after treatment,[10–13,15] 2 studies compared the antibody levels between experimental and placebo groups.[14,16]
Table 1.
Characteristics of included studies.
| Study | Country | Sample size (male/female) | Mean age (yr) | Baseline TPOAb levels (mean ± SD) | Baseline TgAb levels (mean ± SD) | Study design | Outcomes | Duration (mo) |
|---|---|---|---|---|---|---|---|---|
| Krysiak A 2019[10] | Poland | 17 (17/0) | 34 | 878 ± 286 | 783 ± 312 | Selenomethionine (200 μg/d) vs pretreatment | TPOAb, TgAb, TSH, fT4, fT3 | 6 |
| Krysiak B 2018[11] | Poland | 22 (0/22) | 48 | 892 ± 247 | 810 ± 301 | Selenomethionine (200 μg/d) vs pretreatment |
TPOAb, TgAb, TSH, ft3 | 6 |
| Nordio A 2017[12] | Italy | 84 (10/74) | 40.9 | 820.13 ± 513.99 | 415.43 ± 275.89 | l-selenomethionine (selenium 83μg/d) vs pretreatment | TPOAb, TgAb, TSH, fT4, fT3 | 6 |
| Nordio B 2017[13] | Italy | 87 (8/79) | 42.3 | 720.67 ± 52.39 | 344.96 ± 23.77 | Myo-inositol 600mg/d plus selenium 83 μg/d Vs pretreatment | TPOAb, TgAb, TSH, fT4, fT3 | 6 |
| Krysiak C 2012[14] | Poland | 73 (0/73) | 40.5 | 1682 ± 395 | 1720 ± 498 | selenomethionine (200 μg/d) vs placebo |
TPOAb, TgAb, TSH, fT4, fT3 | 3, 6 |
| Onal 2012[15] | Turkey | 23 (7/16) | 12.3 | 210 ± 349 | 272 ± 712 | 50 μg l-selenomethionine vs pretreatment |
TPOAb, TgAb, TSH, fT4 | 3 |
| Karanikas 2008[16] | Austria | 36 (0/36) | 47 | 524 ± 452 | NA | Selenomethionine (200 μg/d) vs placebo |
TPOAb, TSH, fT4, Se | 3 |
fT3 = triiodothyronine, fT4 = thyroxine, NA = not available, SD = standard deviations, TgAb = thyroglobulin antibodies, TPOAb = thyroid peroxidase antibodies, TSH = thyrotropin.
3.2. Change in TPOAb levels and TgAb levels
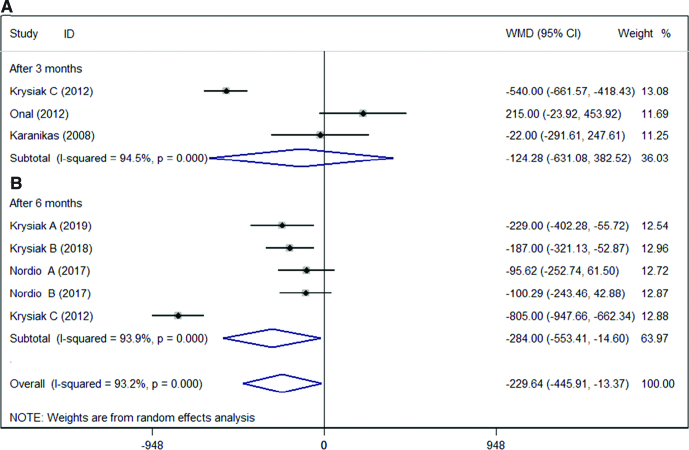
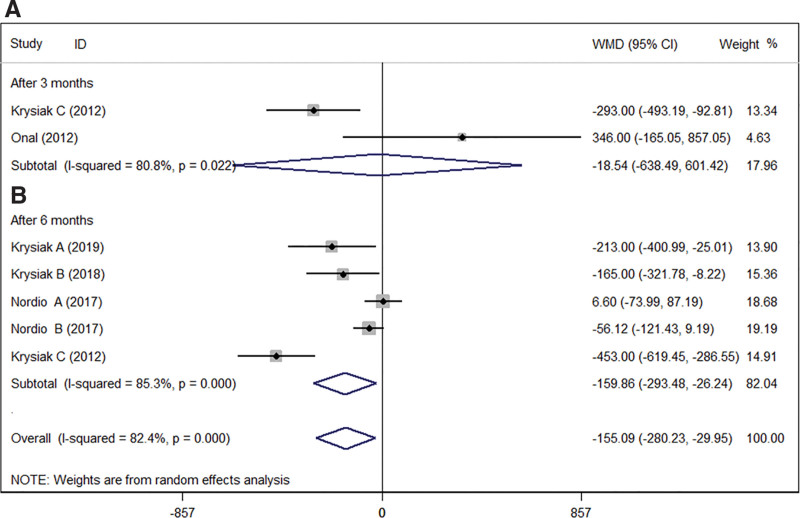
We compared the antibody (TPOAb and TgAb) levels after selenium supplementation. The results showed in Figures 2 and 3. The results indicated that the antibody levels (TPOAb levels and TgAb levels) were significantly decreased in HT patients after selenium supplementation. Interestingly, there was no significant change in TPOAb levels (WMD = −124.28; 95% CI: −631.08 to 382.52], P = .631, I2 = 94.5%, Fig. 2A)[14–16] after 3 months treatment. Only 2 studies counted TgAb levels after 3 months of treatment, and the results indicated that selenium supplementation had no significant effect on TgAb levels in HT patients (WMD = −18.54 [95% CI: −638.49 to 601.42], P = .953, I2 = 80.8%, Fig. 3A).[14,15] This result might be related to the sample size. But there was a significant decrease in TPOAb levels (WMD = −284.00 [95% CI: −553.41 to −14.60], P < .05, I2 = 93.9%, Fig. 2B)[10–14] and TgAb levels (WMD = −159.86 [95% CI: −293.48 to −26.24], P < .05, I2 = 85.3%, Fig. 3B)[10–14] after 6 months of treatment.
Figure 2.
Association between admission antibody TPOAb levels and selenium supplementation after (A) 3 months or (B) 6 months. CI = confidence interval, TPOAb = thyroid peroxidase antibodies, WMD = weighted mean difference.
Figure 3.
Association between admission antibody TgAb levels and selenium supplementation after 3 months (A) or 6 months (B). CI = confidence interval, TgAb = thyroglobulin antibodies, WMD = weighted mean difference.
3.3. Meta-regression and subgroup analyses
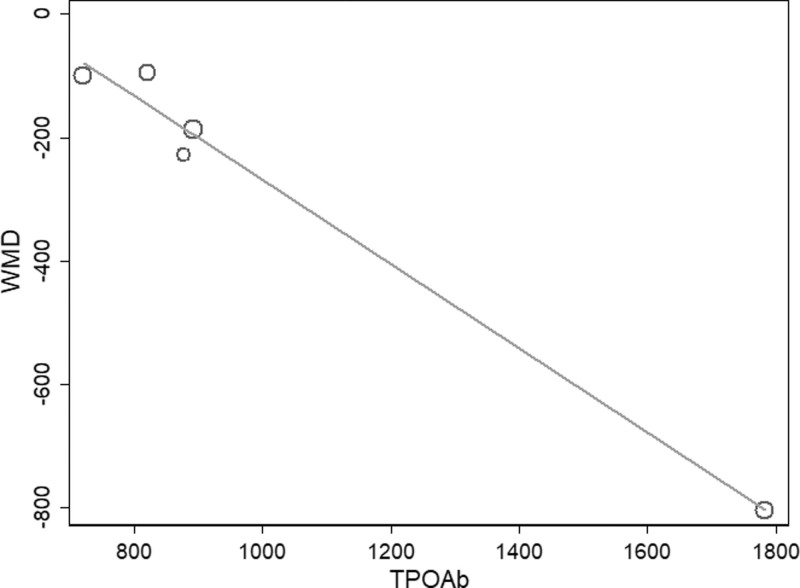
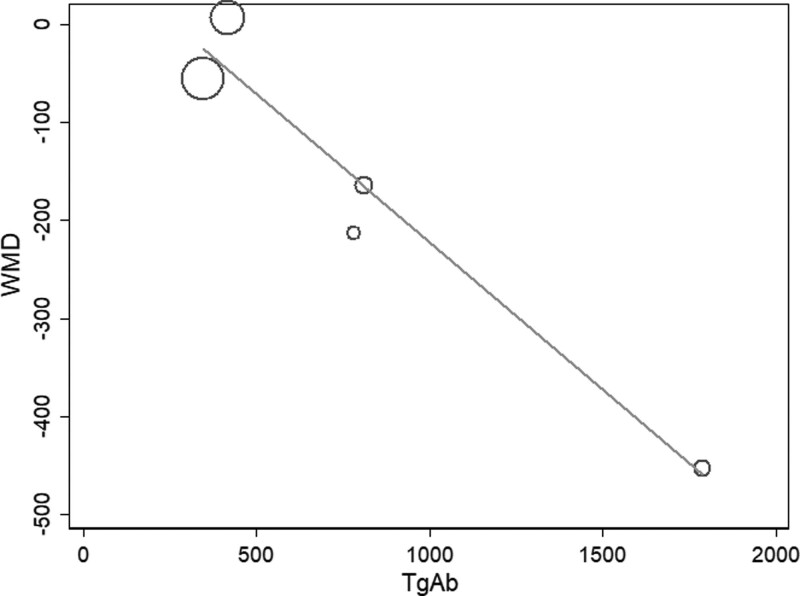
Because of the high amounts of heterogeneity between studies, we conducted meta-regression and subgroup analyses. In our meta-regression analyses, we investigated the correlation between sample size, percentage of females, mean age, baseline antibody (TPOAb and TgAb) levels, baseline thyrotropin levels, and WMD. The results showed that only baseline antibody (TPOAb and TgAb) levels were correlated with WMD at 6 months of studies (TPOAb, P = .004, Fig. 4; TgAb, P = .02, Fig. 5).
Figure 4.
Meta-regression analysis assessing the impact TPOAb on WMD. TPOAb = thyroid peroxidase antibodies, WMD = weighted mean difference.
Figure 5.
Meta-regression analysis assessing the impact TgAb on WMD. TgAb = thyroglobulin antibodies, WMD = weighted mean difference.
According to the baseline antibody (TPOAb and TgAb) levels, we conducted subgroup analyses. The results were shown in Table 2. In TPOAb or TgAb > 1000 IU/mL subgroup, there was a significant decrease in antibody (TPOAb and TgAb) levels after 3 months or 6 months of selenium supplementation therapy. In TPOAb < 1000 IU/mL subgroup, there was a significant decrease only in TPOAb levels after 6 months of treatment (WMD = −150.33 [CI: −225.25 to −75.42], P < .05, I2 = 0%, Table 2).
Table 2.
Subgroup analyses based on baseline antibody (TPOAb and TgAb) levels.
| Subgroup | Number of studies | WMD | 95% CI | I 2 | P (I2) | P value for overall effect |
|---|---|---|---|---|---|---|
| Baseline TPOAb levels | ||||||
| After 3 mo | ||||||
| >1000 | 1[14] | −540.00 | −661.57 to −418.43 | NA | NA | <.05 |
| <1000 | 2[15,16] | 105.07 | −126.57 to 336.72 | 39.9% | .197 | .374 |
| After 6 mo | ||||||
| >1000 | 1[14] | −805.00 | −947.66 to −662.34 | NA | NA | <.05 |
| <1000 | 4[10–13] | −150.33 | −225.25 to −75.42 | 0% | .569 | <.05 |
| Baseline TgAb levels | ||||||
| After 3 mo | ||||||
| >1000 | 1[14] | −293.00 | −493.19 to −92.81 | NA | NA | <.05 |
| <1000 | 1[15] | 346.00 | −165.05 to 857.05 | NA | NA | .185 |
| After 6 mo | ||||||
| >1000 | 1[14] | −453.00 | −619.45 to −286.55 | NA | NA | <.05 |
| <1000 | 4[10–13] | −75.53 | −156.80 to 5.75 | 56.2% | .077 | .069 |
CI = confidence interval, NA = not available, TgAb = thyroglobulin antibodies, TPOAb = thyroid peroxidase antibodies, WMD = weighted mean difference.
3.4. Sensitivity analysis
In our sensitivity analysis, we used the leave-one-out strategy to assess the stability of overall results. Serum TPOAb levels still had a significant decrease after 6 months of treatment. However, as Krysiak C (study of patients with the highest serum antibody levels at baseline)[14] left out, serum TgAb levels no longer showed a significant decrease after 6 months of treatment (WMD = −75.53 [CI: −156.80 to 5.57], P = .069, I2 = 56.2%).
3.5. Publication bias
Because there was heterogeneity between studies, all outcomes were downgraded for inconsistency. Publication bias was assessed using Egger test. The results showed that there was no evidence of publication bias.
4. Discussion
HT is the commonest cause of spontaneous hypothyroidism in children and adolescents and is mainly prevalent in women. HT is characterized by the presence of complement-fixing autoantibodies to TPOAb and TgAb, which are closely associated with autoimmune destruction of the thyroid and overt thyroid dysfunction.[1,18] Selenium may catalyze the extra thyroid production of T3 from T4, and a selenium deficiency can increase thyroid necrosis and reduce compensatory epithelial regeneration.[19,20]
Previous studies have reported that selenium is important for antioxidant defense and adjuvant supplementation decreased TPOAb and TgAb.[21,22] We conducted this meta-analysis to assess the effect of selenium supplementation on thyroid autoantibody levels in patients with HT. The present study showed that selenium supplementation for 3 months did not reduce serum TPOAb and TgAb levels, but selenium supplementation for 6 months might be an effective method to reduce serum antibody levels.
Selenium is an essential trace element that is required in small amounts for the correct functioning of the immune system and the thyroid gland.[23,24] One of the organs with the highest selenium concentration is the thyroid gland. Enhance, the content of selenium is essential for normal thyroid function and thyroid hormone homeostasis. Selenium can be incorporated into selenoproteins by synthesizing tRNA of selenocysteine-bound.[25] These selenoproteins are produced in the liver and exert actions in the selenium and other tissues, mainly having a role in antioxidant functions. Selenoproteins are involved in thyroid hormone metabolism and regulation of the redox state and reduce the inflammatory status in patients with HT.[12,26] Selenium deficiency contributes to decreased activity of glutathione peroxidases and deiodinases, which can lead to oxidative damage and the synthesis of triiodothyronine.[7,27]
Due to the high degree of heterogeneity between studies, we conducted meta-regression and subgroup analyses based on sample size, percentage of females, mean age, baseline antibody (TPOAb and TgAb) levels, and baseline thyrotropin levels. The results showed that baseline antibody (TPOAb and TgAb) levels might be the source of heterogeneity. Baseline antibody (TPOAb and TgAb) levels were a negative correlation with WMD at 6 months of selenium supplementation. In other words, the higher the baseline serum antibody (TPOAb and TgAb) levels, the lower of WMD after 6 months of selenium supplementation. We hypothesized that patients with high serum antibody levels might benefit from selenium supplementation. After sensitivity analysis, the results of TPOAb were still robust and indicated that selenium supplementation was effective. But we need to be cautious about the results of selenium supplementation to reduce TgAb levels.
There are some limitations of our meta-analysis. Firstly, we hypothesized that selenium supplementation might have a beneficial effect in patients with high serum antibody levels, but we were unclear of the exact mechanism based on the available evidence. Secondly, because few studies investigated selenium supplementation at 3 months in patients with HT, we were not sure whether there was also a negative correlation between baseline serum antibody (TPOAb and TgAb) levels and WMD. Thirdly, the sample size included in our meta-analysis was small, so the results would have more bias.
Based on the current evidence, selenium supplementation therapy might reduce serum TPOAb and TgAb levels after 6 months of treatment in patients with HT. Meanwhile, it was reasonable to speculate that patients with high serum antibody levels would benefit from selenium supplementation. The above results did not mean that selenium supplementation must be routinely used in clinical treatment, because the reduction of antibodies did not mean the improvement of thyroid function. Future prospective cohort studies are warranted to provide more evidence.
Acknowledgments
The authors thank all participants who contributed to this meta-analysis.
Author contributions
Conceptualization: Xiang-Qi Kong, Xiao-Qing Quan.
Data curation: Xiang-Qi Kong, Gui-Ying Qiu, Xiao-Qing Quan.
Formal analysis: Zhong-Bin Yang.
Investigation: Gui-Ying Qiu, Zhi-Xiong Tan.
Methodology: Gui-Ying Qiu, Zhong-Bin Yang.
Software: Zhong-Bin Yang, Zhi-Xiong Tan.
Supervision: Gui-Ying Qiu, Zhong-Bin Yang.Validation: Gui-Ying Qiu, Zhi-Xiong Tan.
Visualization: Zhong-Bin Yang.
Writing – original draft: Xiang-Qi Kong, Xiao-Qing Quan.
Writing – review & editing: Xiang-Qi Kong, Xiao-Qing Quan.
Supplementary Material
Abbreviations:
- CI
- confidence interval
- HT
- Hashimoto thyroiditis
- TgAb
- thyroglobulin antibodies
- TPOAb
- thyroid peroxidase antibodies
- WMD
- weighted mean difference
X-QK and G-YQ contributed equally to this work.
This work is supported by the Guangdong Basic and Applied Basic Research Foundation (2021A1515220123).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
How to cite this article: Kong X-Q, Qiu G-Y, Yang Z-B, Tan Z-X, Quan X-Q. Clinical efficacy of selenium supplementation in patients with Hashimoto thyroiditis: A systematic review and meta-analysis. Medicine 2023;102:20(e33791).
Contributor Information
Xiang-Qi Kong, Email: 1246813570@qq.com.
Gui-Ying Qiu, Email: 1193112321@qq.com.
Zhong-Bin Yang, Email: 2591784066@qq.com.
Zhi-Xiong Tan, Email: 2511604035@qq.com.
References
- [1].Ralli M, Angeletti D, Fiore M, et al. Hashimoto’s thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. 2020;19:102649. [DOI] [PubMed] [Google Scholar]
- [2].Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol. 2014;170:R241–52. [DOI] [PubMed] [Google Scholar]
- [3].Guan H, de Morais NS, Stuart J, et al. Discordance of serological and sonographic markers for Hashimoto’s thyroiditis with gold standard histopathology. Eur J Endocrinol. 2019;181:539–44. [DOI] [PubMed] [Google Scholar]
- [4].Ucan B, Sahin M, Sayki Arslan M, et al. Vitamin D treatment in patients with hashimoto’s thyroiditis may decrease the development of hypothyroidism. international journal for vitamin and nutrition research. internationale zeitschrift fur vitamin- und ernahrungsforschung. J Int de Vitaminologie et de Nutr. 2016;86:9–17. [DOI] [PubMed] [Google Scholar]
- [5].Duntas LH. The role of iodine and selenium in autoimmune thyroiditis. Horm Metab Res. 2015;47:721–6. [DOI] [PubMed] [Google Scholar]
- [6].Valea A, Georgescu CE. Selenoproteins in human body: focus on thyroid pathophysiology. Hormones (Athens). 2018. [DOI] [PubMed] [Google Scholar]
- [7].Gorini F, Sabatino L. Selenium: an element of life essential for thyroid function. 2021;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Di Bari F, Granese R, Le Donne M, et al. Autoimmune abnormalities of postpartum thyroid diseases. Front Endocrinol (Lausanne). 2017;8:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gianopoulou E, Margaritis K, Karampasi E, et al. Selenium supplementation and Hashimoto’s thyroiditis: an update. Clin Nutr ESPEN. 2018;24:177. [Google Scholar]
- [10].Krysiak R, Szkrobka W, Okopien B. The effect of vitamin D and selenomethionine on thyroid antibody titers, hypothalamic-pituitary-thyroid axis activity and thyroid function tests in men with Hashimoto’s thyroiditis: a pilot study. Pharmacol Rep. 2019;71:243–7. [DOI] [PubMed] [Google Scholar]
- [11].Krysiak R, Szkrobka W, Okopien B. Atorvastatin potentiates the effect of selenomethionine on thyroid autoimmunity in euthyroid women with Hashimoto’s thyroiditis. Curr Med Res Opin. 2019;35:675–81. [DOI] [PubMed] [Google Scholar]
- [12].Nordio M, Basciani S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto’s patients with subclinical hypothyroidism. Eur Rev Med Pharmacol Sci. 2017;21(2 Suppl):51–9. [PubMed] [Google Scholar]
- [13].Nordio M, Basciani S. Treatment with myo-inositol and selenium ensures euthyroidism in patients with autoimmune thyroiditis. Int J Endocrinol. 2017;2017:2549491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krysiak R, Okopien B. Haemostatic effects of levothyroxine and selenomethionine in euthyroid patients with Hashimoto’s thyroiditis. Thromb Haemost. 2012;108:973–80. [DOI] [PubMed] [Google Scholar]
- [15].Onal H, Keskindemirci G, Adal E, et al. Effects of selenium supplementation in the early stage of autoimmune thyroiditis in childhood: an open-label pilot study. J Pediatr Endocrinol Metab. 2012;25:639–44. [DOI] [PubMed] [Google Scholar]
- [16].Karanikas G, Schuetz M, Kontur S, et al. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid. 2008;18:7–12. [DOI] [PubMed] [Google Scholar]
- [17].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed.). 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kyritsi EM, Kanaka-Gantenbein C. Autoimmune thyroid disease in specific genetic syndromes in childhood and adolescence. Front Endocrinol (Lausanne). 2020;11:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34–44. [DOI] [PubMed] [Google Scholar]
- [20].Winther KH, Rayman MP. Selenium in thyroid disorders - essential knowledge for clinicians. 2020;16:165–76. [DOI] [PubMed] [Google Scholar]
- [21].Negro R, Greco G, Mangieri T, et al. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab. 2007;92:1263–8. [DOI] [PubMed] [Google Scholar]
- [22].Schmidt M, Voell M, Rahlff I, et al. Long-term follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (Hashimoto’s thyroiditis) treated with levothyroxine. Thyroid. 2008;18:755–60. [DOI] [PubMed] [Google Scholar]
- [23].Gheorghiu ML, Badiu C. Selenium involvement in mitochondrial function in thyroid disorders. 2020;19:25–30. [DOI] [PubMed] [Google Scholar]
- [24].Wang X, Zhang Y, Fan T, et al. Effective mobilization with etoposide and cyclophosphamide for collection of peripheral blood stem cell in patients with multiple myeloma. Clin Invest Med. Medecine clinique et experimentale. 2020;43:E27–32. [DOI] [PubMed] [Google Scholar]
- [25].Wallenberg M, Misra S, Björnstedt M. Selenium cytotoxicity in cancer. Basic Clin Pharmacol Toxicol. 2014;114:377–86. [DOI] [PubMed] [Google Scholar]
- [26].Malik AE, Issekutz TB, Derfalvi B. The role of type III interferons in human disease. Clin Invest Medi. Medecine clinique et experimentale. 2021;44:E5–18. [DOI] [PubMed] [Google Scholar]
- [27].Jablonska E, Vinceti M. Selenium and human health: witnessing a copernican revolution? J Environ Sci Health. 2015;33:328–68. [DOI] [PubMed] [Google Scholar]