Abstract
Introduction
Immune checkpoint inhibitors (ICIs) can cause inflammatory and immune-related adverse events (irAEs) that might worsen the course of COVID-19. We conducted a systematic review (PROSPERO ID: CRD42022307545) to evaluate the clinical course and complications of COVID-19 in patients with cancer receiving ICI.
Methods
We searched Medline and Embase through January 5, 2022. We included studies evaluating patients with cancer who received ICI and developed COVID-19. Outcomes included mortality, severe COVID-19, intensive care unit (ICU) and hospital admissions, irAEs, and serious adverse events. We pooled data with random effects meta-analysis.
Results
Twenty-five studies met study eligibility (n = 36,532 patients: 15,497 had COVID-19 and 3220 received ICI). Most studies (71.4%) had a high risk of comparability bias. There were no significant differences in mortality (relative risk [RR] 1.29; 95% CI 0.62-2.69), ICU admission (RR 1.20; 95% CI 0.71-2.00), and hospital admission (RR 0.91; 95% CI 0.79-1.06) when comparing patients treated with ICI with patients without cancer treatment. When pooling adjusted odds ratios (ORs), no statistically significant differences were observed in mortality (OR 0.95; 95% CI 0.57-1.60), severe COVID-19 (OR 1.05; 95% CI 0.45-2.46), or hospital admission (OR 2.02; 95% CI 0.96-4.27), when comparing patients treated with ICIs versus patients with cancer without ICI therapy. No significant differences were observed when comparing clinical outcomes in patients receiving ICIs versus patients receiving any of the other anticancer therapies.
Conclusion
Although current evidence is limited, COVID-19 clinical outcomes of patients with cancer receiving ICI therapy appear to be similar to those not receiving oncologic treatment or other cancer therapies.
Keywords: SARS-CoV-2, COVID-19, immune checkpoint inhibitors, immune-related adverse events, immunotherapy
INTRODUCTION
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified in December 2019 and disseminated rapidly across the globe, resulting in a pandemia. Infection with this coronavirus can cause coronavirus disease 2019 (COVID-19), with symptoms that range from mild (most commonly) to severe or critical disease with lung involvement, and with a case fatality rate of approximately 2-3%.[1,2]
Patients with cancer and COVID-19 have a higher risk of developing severe disease and death.[3] In a recent observational study including over 500,000 patients with COVID-19, those with cancer who received cancer treatment within 3 months before COVID-19 diagnosis had an increased risk of death, hospitalization, and intensive care unit (ICU) admission.[4] Yet, the type of cancer treatment received might influence the outcomes of patients with COVID-19. In a large national cohort including 398,579 patients with cancer, recent cytotoxic therapy was associated with an increased risk of mortality, whereas immunotherapy or targeted therapy were not.[5]
Immune checkpoint inhibitors (ICIs) enhance tumor immunity, but their effects are indiscriminate and can cause inflammatory and immune-related adverse events (irAEs) in nontarget organs.[6] Because some of the critical complications of COVID-19 are thought to be caused by an exaggerated immune response resulting in massive release of cytokines, it is conceivable that patients with cancer receiving ICI therapy who acquire COVID-19 might be at risk of developing such complications. However, the results of previous studies are inconsistent, with some studies reporting no increased risk in adverse outcomes, whereas others report a higher risk compared with other therapies.[5,7] Because of these discrepancies, we conducted a systematic review of existing literature to evaluate the clinical course and potential complications of COVID-19 in patients with cancer receiving ICI therapy.
METHODS
This review was registered in PROSPERO (registration no.: CRD42022307545) and is reported in accordance with the PRISMA (Preferred Reporting Items of Systematic Reviews and Meta-Analysis) guidelines.
Eligibility Criteria
We included studies (prospective and retrospective) with at least five patients treated with ICIs and with a comparison group. For inclusion studies, we considered where the cancer treatment was administered within 90 days prior to COVID-19 diagnosis. We also included case series and reports to identify potential unusual adverse events. Studies were considered if they included adults ≥ 18 years of age with any type of advanced cancer who were diagnosed with COVID-19. We included any ICI therapy, i.e., programmed cell death protein 1s (PD-1s; pembrolizumab, nivolumab, cemiplimab), programmed death-ligand 1s (PD-L1s; atezolizumab, avelumab, durvalumab), and cytotoxic T lymphocyte-associated protein 4 (CTLA-4; ipilimumab). We considered type of comparison group (e.g., chemotherapy, chemotherapy plus immunotherapy, hormone therapy, targeted therapy, no treatment). We excluded studies if insufficient information for analysis was provided, if the type of immunotherapy received by the participants was not specified, or if the studies were on pediatric populations. We also excluded case reports of pneumonitis (and its complications) because this outcome could not be considered an unusual adverse event in the context of SARS-CoV-2 infection.
Finally, we also included studies of patients receiving ICIs, comparing those who developed COVID-19 with those who did not, in order to compare the incidence of irAEs or other serious adverse events in both groups.
Information Sources and Search Strategy
An expert librarian searched two electronic databases Medline and Embase (through Ovid) from December 1, 2019, to January 5, 2022. We also checked the reference lists of other systematic reviews and retrieved those studies that were considered appropriate.
Terms were searched using subject headings and keywords as needed. The search terms were combined using Boolean operators AND/OR. Search strategies are as shown in Supplemental Table S1 (available online). The search was limited to English language articles. We used EndNote X9 (Clarivate) to manage references.
Two reviewers (JIR and MLO) independently screened the citations retrieved by the search and selected the studies of interest. Disagreements were resolved by discussion and consensus. Data were extracted by one reviewer (JIR) using the web-based software platform Covidence (Veritas Health Innovation). The following information was extracted: (1) general study information (i.e., year of publication, country, study design); (2) population characteristics (i.e., age, gender, number of patients); (3) intervention characteristics (i.e., number of patients under ICI treatment, number of patients in comparison group, interval between ICI treatment and COVID-19 diagnosis, types of ICI, types of comparison [chemotherapy, chemotherapy + ICI, targeted therapy]); (4) outcomes (primary outcomes: mortality, ICU admission, rate of irAEs and type of irAE, hospital admission; and secondary outcomes: systemic complications [venous thrombosis/pulmonary embolism (VTE), ischemic stroke], severe disease [according to the author's definition], mechanical ventilation, pneumonitis). We collected information on COVID-19-related mortality, where the numerator is the number of COVID-19-related deaths and the denominator is the number of patients with COVID-19 infection.
Assessment of Studies and Analysis
The risk of bias was assessed by one reviewer (JIR). We used the Newcastle-Ottawa Scale (NOS) to assess the methodological quality of observational studies. This scale consists of three components: patient selection, study comparability, and outcome assessment. It ranges from 0, indicating highest risk of bias, to 9, indicating lowest risk of bias. We planned a priori to assess and quantify publication bias using funnel plots and Egger's test if more than 10 studies reported on the primary outcome. However, data were insufficient to perform this analysis.
We performed the statistical analysis using Review Manager version 5.3 (RevMan). We presented the measure of association as RRs and their corresponding 95% CI. If the data were not suitable for pooling, we synthesized the results narratively. Synthesis was performed according to comparison groups and primary outcomes.
We calculated the RRs when studies provided raw data from the frequency of events and sample sizes. We used the Mantel-Haenszel method for meta-analysis of dichotomous raw data. We converted reported ORs into log ORs to quantify the variable's effect in studies reporting ORs, using generic inverse-variance method with random effects model to pool them (when needed, 1 OR used for consistency of the referent group in pooled estimates). Adjusted estimates were used for primary analyses where possible to decrease potential confounder bias. Data were pooled using random effects models. For meta-analysis with only two studies, we also used fixed effects models following the recommendation of Chen et al.[8]
We assessed heterogeneity using I2 statistics. We considered that heterogeneity was present when the I2 was higher than 40%. We grouped studies by type of effect estimate used (unadjusted versus adjusted) and type of design (prospective versus retrospective) to determine the potential effect on the results.
Sensitivity analysis was performed for the main comparisons (i.e., ICI versus no cancer treatment and ICI versus chemotherapy), excluding studies with high risk of bias (NOS score lower than 6) for the outcome “mortality” to determine differential effects of the quality of the primary studies. To evaluate the occurrence of unusual adverse events of ICIs in patients with COVID-19 diagnosis, we identified and summarized data from case reports and case series that reported adverse events.
We evaluated the quality of the evidence for each outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework, which considers risk of bias, indirectness, inconsistency, imprecision, and publication bias.[9] We created summary of findings tables for the comparison of ICI versus no ICI using the adjusted ORs and rating the certainty of the evidence as high (indicating that further research is very unlikely to change our confidence in the estimate of effect), moderate (indicating that further research is likely to have a significant effect on our confidence in the estimate of effect and may change the estimate), low (indicating that further research is very likely to have a significant effect on our confidence in the estimate of effect and is likely to change the estimate), or very low (indicating that any estimate of effect is very uncertain).
RESULTS
The search strategy identified 621 unique citations (Fig. 1). After review, 25 studies were included for analysis.[7,10-33] Overall 36,532 patients were included: 15,497 subjects had COVID-19 and 3220 received ICI.
Figure 1.
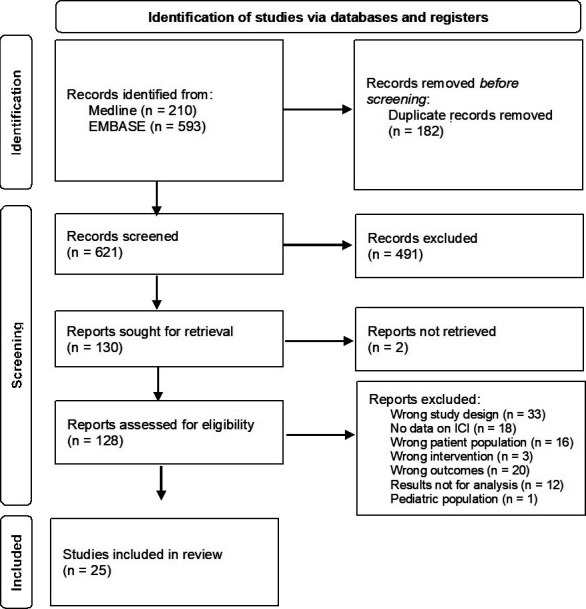
PRISMA flow diagram of study selection.
Cohort studies' characteristics are described in Table 1. There were 21 cohort studies (three prospective and 18 retrospective), one case series, and three case reports. With regard to the outcomes reported, 16 studies reported mortality; seven reported hospital admission; four reported severe COVID-19 infection; one reported clinical worsening; one reported mechanical ventilation; one reported venous thromboembolism; one reported the composite outcome respiratory failure or death; one reported the composite outcome of ICU admission, intubation, or do not intubate (DNI) order; two reported irAEs; and one study reported serious adverse events. ICI therapy was compared with chemotherapy in 13 studies;[10,11,13-15,17,18,20-23,26,33] targeted therapy in 11 studies;[10,13-17,20,22-24,33] hormone therapy in six studies;[10,15,16,20,26,33] patients with cancer without therapy in six studies;[11,14,15,17,19,24] chemotherapy + immunotherapy in three studies;[13,14,22] and healthy subjects in one study.[13] Additional characteristics are shown in Supplemental Table S2 (available online). The median age of participants ranged between 61 and 80 years old.
Table 1.
Characteristics of cohort studies comparing ICIs with other treatments or no treatment in patients with cancer and COVID-19
|
Study
|
Total No. of Patients
|
Patients on ICI,
n
(%) |
Comparison Group
|
Outcomes
|
| Retrospective cohorts | ||||
| Albiges 2020[10] | 178 | 19 (10.7) | Chemotherapy, targeted therapy, hormone therapy | Hospital admission, clinical worsening, ICU admission |
| Assaad 2020[11] | 55 | 3 (5.5) | Chemotherapy, no treatment | Mortality |
| Dai 2020[13] | 105 | 6 (5.7) | Chemotherapy, targeted therapy, chemotherapy + immunotherapy, healthy controls | Mortality |
| Garassino 2020[14] | 200 | 24 (12) | Chemotherapy, targeted therapy, chemotherapy + immunotherapy, no treatment | Hospital admission |
| Gonzalez-Cao 2020[24] | 50 | 22 (44) | Targeted therapy, no treatment | Mortality |
| Grivas 2021[15]a | 4966 | 238 (4.8) | Chemotherapy, targeted therapy, no treatment, hormone therapy | Mortality, ICU admission, hospital admission, mechanical ventilation |
| Gulati 2021[16] | 4217 | 199 (4.7) | Targeted therapy, hormone therapy | Thrombotic complications, ICU admission, mortality |
| Hwang 2021[17]b | 1267 | 12 (1) | Chemotherapy, targeted therapy, no treatment | Mortality |
| Jee 2021[18] | 820 | 51 (6.2) | Chemotherapy | Mortality, respiratory failure |
| Klebanov 2021[19]c | 21,693 | 1545 (7.1) | No treatment | COVID-19, mortality |
| Lara 2020[20] | 121 | 8 (6.6) | Chemotherapy, targeted therapy, hormone therapy | Mortality, severe COVID-19 |
| Lin 2021[21] | 106 | 10 (10) | Chemotherapy | COVID-19, hospital admission |
| Luo 2020[22]d | 102 | 26 (25) | Chemotherapy, chemotherapy + immunotherapy, targeted therapy | Hospital admission, ICU/intubation/DNI, mortality |
| Moritz 2021[35] | 13 | 13 (100) | NA | Mortality, irAEs |
| Pinato 2020[27]e | 890 | 38 (4.3) | No ICI | Mortality |
| Robilotti 2020[7]f | 423 | 31 (7.3) | No ICI | Hospital admission, mortality |
| Trojaniello 2021[36] | 343 | 343 (100) | NA | COVID-19, irAEs |
| Tyan 2020[32]g | 1222 | 611 (50) | No ICI | Mortality |
| Prospective cohorts | ||||
| Mandala 2021[23] | 293 | 52 (17.7) | Chemotherapy, targeted therapy | COVID-19, serious adverse events, adverse events, mortality |
| Nichetti 2020[26] | 11 | 4 (36.4) | Chemotherapy, hormone therapy | Mortality |
| Yarza 2020[33]h | 63 | 8 (12.7) | Chemotherapy, targeted therapy, hormone therapy | Respiratory failure |
Adjusted analysis: aage, sex, race, comorbidities, cancer status, cancer type, timing of anticancer therapy, anti-COVID-19; bage, sex, race, income, comorbidities, malignancy, cancer status, cancer treatment; cage, sex, race and ethnicity, median income, local infection rate, Charlson comorbidity index; dsex, smoking status, esex, age, comorbidities, tumor stage, tumor status, fage, lung cancer, hematologic malignancy, metastatic disease, gage, sex, anticancer therapy, hage, sex, ECOG, metastasis, chronic obstructive pulmonary disease, previous venous thromboembolism .
COPD: chronic obstructive pulmonary disease; DNI: do not intubate; ECOG: Eastern Cooperative Oncology Group performance status; ICI: immune checkpoint inhibitor; ICU: intensive care unit; irAEs: immune-related adverse events; NA: not applicable; VTE: previous venous thromboembolism.
Most of the studies had high risk of bias in several domains (Supplemental Table S3, available online); 71% of the studies (n = 15) had high risk of confounding bias due to no adjustment for confounding factors.
Results of Cohort Studies
The reported rates of people worsening, deaths, hospital and ICU admissions, and other outcomes per intervention group are shown in Supplemental Table S4 (available online).
ICI Therapy Versus No Cancer Therapy
Outcomes in patients with cancer treated with ICIs versus no cancer therapy are presented in Supplemental Fig. S1.
Mortality was reported in five studies including 308 patients on ICI therapy and 4069 controls.[11,14,15,17,24] No statistically significant differences were observed in the pooled estimate (RR 1.29; 95% CI 0.62-2.69; I2 = 79%). Certainty of evidence was very low (rated down for imprecision, inconsistency, risk of bias). In the sensitivity analysis (Supplemental Fig. S7) including only studies with low risk of bias, no statistically significant differences were observed in the pooled estimate (RR 1.59; 95% CI 0.73-3.48; I2 = 81%).
Hospital admission rates were reported in two studies including 282 patients on ICI therapy and 2859 controls. No statistically significant differences were observed in the pooled estimate[14,15] (RR 0.91; 95% CI 0.79-1.06; I2 = 0%). Certainty of evidence was very low (rated down for imprecision, risk of bias).
ICU admission rates were reported in one study including 248 patients on ICI therapy and 2807 controls.[15] No statistically significant differences were observed (RR 1.20; 95% CI 0.71-2.00). Certainty of evidence was very low (rated down for imprecision, risk of bias).
ICI Therapy Versus Chemotherapy
Outcomes in patients with cancer treated with ICIs versus chemotherapy are presented in Supplemental Fig. S2.
Mortality was reported in nine studies including 393 patients on ICIs and 1025 controls.[10,11,13-15,17,22,23,26] No statistically significant differences were observed (OR 0.84; 95% CI 0.65-1.07; I2 = 1%). Certainty of evidence was low (rated down for imprecision, risk of bias). In the sensitivity analysis (Supplemental Fig. S8) including only studies with low risk of bias, no statistically significant differences were observed in the pooled estimate (RR 0.87 95%, CI 0.66-1.14; I2 = 5%).
Hospital admission rates were reported in five studies including 370 patients on ICIs and 922 controls.[14,15,21-23] No statistically significant differences were observed (RR 0.93; 95% CI 0.81-1.08; I2 = 0%). Certainty of evidence was low (rated down for imprecision, risk of bias).
ICU admission rates were reported in two studies including 274 patients on ICI and 828 controls.[15,22] No statistically significant differences were observed (RR 1.06; 95% CI 0.41-2.75; I2 = 73%). Certainty of evidence was very low (rated down for inconsistency, imprecision, risk of bias).
Severe COVID-19 was reported in three studies including 35 patients on ICIs and 137 controls.[10,20,33] No statistically significant differences were observed (RR 0.93; 95% CI 0.38-2.24; I2 = 58%). Certainty of evidence was low (rated down for imprecision, risk of bias).
Respiratory failure or death was reported in one study, including 51 patients on ICIs and 38 controls,[18] combined both outcomes. No statistically significant differences were observed (RR 0.75; 95% CI 0.40-1.37). Certainty of evidence was very low (rated down for imprecision, risk of bias).
ICI Therapy Versus Targeted Therapy
Outcomes in patients with cancer treated with ICIs versus targeted therapy are presented in Supplemental Fig. S3.
Mortality was reported in eight studies including 364 patients on ICIs and 851 controls.[10,13-15,17,22,24,33] No statistically significant differences were observed (RR 1.28; 95% CI 0.93-1.75; I2 = 6%). Certainty of evidence was very low (rated down for imprecision, risk of bias).
Hospital admission rates were reported in three studies including 308 patients on ICIs and 731 controls.[14,15,22] No statistically significant differences were observed (RR 1.00; 95% CI 0.77-1.28; I2 = 43%). Certainty of evidence was very low (rated down for imprecision, risk of bias).
Severe COVID-19 was reported in three studies including 35 patients on ICIs and 50 controls.[10,20,33] No statistically significant differences were observed in the pooled estimate (RR 1.29; 95% CI 0.59-2.82; I2 = 0%). Certainty of evidence was very low (rated down for imprecision, risk of bias).
Venous thromboembolism was reported in one study including 139 patients on ICIs and 675 controls.[16] No statistically significant differences were observed in the pool estimate (RR 1.26; 95% CI 0.80-1.98). Certainty of evidence was very low (rated down for imprecision, risk of bias).
ICI Therapy Versus No ICI Therapy
Outcomes in patients with cancer treated with ICIs versus no ICIs are presented in Supplemental Fig. S4.
Mortality was reported in two studies including 47 patients on ICI therapy and 238 controls.[19,32] No statistically significant differences were observed in the pooled estimate (RR 1.14; 95% CI 0.64-2.04; I2 = 33%). Certainty of evidence was very low (rated down for inconsistency, imprecision, risk of bias).
Five studies reported adjusted ORs for this outcome.[15,19,22,32,33] No statistically significant differences were observed in the adjusted analysis (OR 0.95; 95% CI 0.57-1.60; I2 = 25%). Figure 2 shows the OR for all studies adjusting for covariates (mortality or severe COVID-19). The studies adjusted for the following covariates: age,[7,15,17,19,22,27,32,33] sex,[15,17,19,22,27,32,33] race,[15,17,19] comorbidities,[15,17,19,27] cancer status,[15,17,27] cancer type,[15] timing of anticancer therapy,[15] anti-COVID-19 therapy,[15] income,[17,19] ethnicity,[19] local infection rate,[19] smoking status,[22] tumor stage,[27] metastatic disease,[7,33] lung cancer,[7] hematologic malignancy,[7] anticancer therapy,[17,32] Eastern Cooperative Oncology Group (ECOG) performance status scale,[33] chronic obstructive pulmonary disease,[33] and previous venous thromboembolism.[33] One of the studies did not report the interval between the cancer treatment and the COVID-19 diagnosis[34]; therefore, we performed a subgroup analysis with this study separated from the others (Supplemental Fig. S4). Certainty of evidence was very low (rated down for imprecision, risk of bias). See Table 2.
Figure 2.
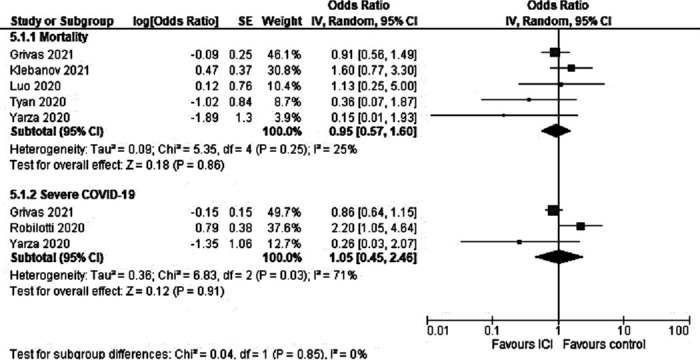
Forest plot comparing patients with cancer treated with ICIs versus those not treated with ICI. Adjusted ORs. ICI: immune checkpoint inhibitor; OR: odds ratio.
Table 2.
COVID-19 outcomes in patients with cancer receiving ICIs compared to those not receiving ICI Therapy
|
Outcomes
|
Anticipated Absolute Effects
|
Relative Effect, OR (95% CI)
|
No. of Studies
|
Certainty of the Evidence (GRADE)
|
Comments
|
|
|
Risk With No ICI
|
Risk With ICI (95% CI)
|
|||||
| Mortality | 100 per 1000 | 95 per 1000 (60–151) | 0.95 (0.57–1.60)a | 5 | Very lowb,d | There is uncertainty based on the quality of evidence if the risk of COVID-19 mortality is higher in patients with cancer exposed to ICI compared with those not exposed to ICI. |
| Hospital admission | 300 per 1000 | 464 per 1000 (291–647) | 2.02 (0.96– 4.27)a | 2 | Very lowd | There is uncertainty based on the quality of evidence if the risk of COVID-19 hospital admission is higher in patients with cancer exposed to ICI compared with those not exposed to ICI. |
| Severe COVID-19 | 120 per 1000 | 125 per 1000 (58–251) | 1.05 (0.45–2.46)a | 3 | Very lowc,d | There is uncertainty based on the quality of evidence if the risk of severe COVID-19 is higher in patients with cancer exposed to ICI compared with those not exposed to ICI. |
Adjusted OR.
There is high risk of bias assessed with the Newcastle-Ottawa Scale specifically in the selection and outcome domains.
There is heterogeneity not explained due to chance.
The true effect can benefit either the experimental or the control group.
CI: confidence interval; GRADE: Grading of Recommendations, Assessment, Development and Evaluations; ICI: immune checkpoint inhibitor; OR: odds ratio.
Hospital admission rates were reported in one study including 25 on ICIs and 25 controls.[32] No statistically significant differences were observed (RR 0.79; 95% CI 0.63-1.00). Certainty of evidence was very low (rated down for imprecision, risk of bias).
Two studies reported adjusted ORs for this outcome.[7,22] The pool estimate favored the control group, although the lower limit of the CI crossed the line of null effect (OR 2.02; 95% CI 0.96-4.27; I2 = 0%). Certainty of evidence was very low (rated down for imprecision, risk of bias).
ICU admission rates were reported in one study including 25 patients on ICIs and 25 controls.[32] No statistically significant differences were observed (RR 0.50; 95% CI 0.20-1.25). Certainty of evidence was very low (rated down for imprecision, risk of bias).
Severe COVID-19 was reported in three studies reported adjusted ORs for this outcome.[7,15,33] No statistically significant differences were observed (OR 1.05; 95% CI 0.45-2.46; I2 = 71%) (Fig. 2). Certainty of evidence was very low (rated down for inconsistency, imprecision, risk of bias).
ICI Therapy Versus Hormone Therapy
Outcomes in patients with cancer treated with ICIs versus hormone therapy are presented in Supplemental Fig. S5.
Mortality was reported in two studies including 252 patients on ICIs and 485 controls.[15,26] No statistically significant differences were observed in the pooled estimate (RR 1.10; 95% CI 0.42-2.90) in the random effects model analysis. In the fixed effects model, the RR was 1.53 (95% CI 1.05-2.22), favoring the control group. Certainty of evidence was very low (rated down for inconsistency, imprecision, risk of bias).
Severe COVID-19 was reported in two studies including 16 patients on ICIs and 16 controls.[20,33] No statistically significant differences were observed in the random effects model analysis (RR 1.65; 95% CI 0.08-33.66) and in the fixed effects model (RR 1.08; 95% CI 0.52-2.25). Certainty of evidence was very low (rated down for inconsistency, imprecision, risk of bias).
ICI Therapy Alone Versus ICIs Plus Chemotherapy
Outcomes in patients with cancer treated with ICI therapy versus ICI and chemotherapy are presented in Supplemental Fig. S6.
Mortality was reported in two studies including 32 patients on ICIs and 17 controls.[13,22] No statistically significant differences were observed (RR 0.74; 95% CI 0.28-2.00 [random effects model], RR 0.74; 95% CI 0.28-1.98 [fixed effects model]). Certainty of evidence was very low (rated down for imprecision, risk of bias).
Hospital admission rates were reported in two studies including 60 patients on ICIs and 34 controls.[14,22] No statistically significant differences were observed (RR 0.89; 95% CI 0.72-1.11 [random effects model and fixed effects model]). Certainty of evidence was very low (rated down for imprecision, risk of bias).
ICI Therapy and COVID-19
Serious adverse events were reported in one study including patients on ICIs, including 52 SARS-CoV-2 positive, and 107 SARS-CoV-2 negative.[23] Patients with cancer who received ICI therapy and had COVID-19 had a higher risk of serious adverse events than those who did not have COVID-19 (RR 4.63; 95% CI 1.50-14.34), with pneumonitis being the most common serious adverse event. Serious adverse events were related to COVID-19. Certainty of evidence was very low (rated down for imprecision, risk of bias).
Two studies including 30 patients reported the rate of irAEs in patients treated with ICI and diagnosed with COVID-19.[35,36] No irAEs were observed in these studies.
Results of Case Reports
Three case reports[12,28,30] and one case series[29] reported the characteristics of five patients with cancer receiving ICIs who developed an unusual adverse event after having COVID-19, and it was hypothesized that the infection triggered the event in these patients. Reported unusual adverse events after havingCOVID-19 in these patients were (1) acute tubulointerstitial nephritis, (2) hemophagocytic lymphohistiocytosis, (3) digital ischemia, (4) urticarial popular lesions, and (5) erythema multiforme. A more detailed description of the case reports is provided in the Supplemental Appendix.
DISCUSSION
In this systematic review, patients with cancer receiving ICI therapy who acquired COVID-19 did not have statistically significantly worse clinical outcomes compared with those not receiving ICI therapy. We examined several COVID-19 clinical outcomes including mortality, ICU and hospital admissions, and severe COVID-19. No statistically significant differences were observed when comparing patients receiving ICI with patients receiving no treatment or other oncologic treatments.
A few prior systematic reviews have also examined COVID-19 outcomes in patients with cancer receiving ICI therapy; however, our systematic review adds to the literature with a more recent search through January 2022, including cohort studies and case reports. We included several clinical outcomes that were not identified previously, including irAEs. Our results are consistent with other studies. The most recent systematic review was that published by Liu et al.,[37] with a search performed through May 2021 reporting incidence and mortality. In this study, compared with other cancer treatments, ICI treatment neither increased the incidence of COVID-19 (OR 0.84; 95% CI 0.60-1.18) nor the mortality (OR 1.22; 95% CI 0.91-1.62).37 Other previous systematic reviews showed similar results.[38-40] Lazarus et al.[38] searched through February 2021 and evaluated other clinical outcomes besides mortality; however, they included 11 studies for analysis, compared with 21 included in our review. Finally, the other two systematic reviews (Park et al. and Qian et al.)[39,40] searched through January 2021 and October 2020, respectively.
Unlike other reviews, we also identified irAEs or other serious adverse events. Two studies evaluated irAEs, and no events were reported.[35,36] Another study reported a higher risk of serious adverse events in patients receiving ICIs who developed COVID-19 compared with those patients receiving ICI without COVID-19. They considered serious adverse events to be death, life-threating toxicity, hospitalization (initial or prolonged), disability, and permanent organ damage. Although more patients with COVID-19 had pneumonitis compared to those without COVID-19, there were no differences in irAEs between the two groups in other organs.
We also examined case series and reports to identify potential unusual adverse events that occurred in patients receiving ICIs who developed COVID-19. The adverse events reported were acute tubulointerstitial nephritis, hemophagocytic lymphohistiocytosis, digital ischemia, urticarial popular lesions, and erythema multiforme. While attribution is not possible, small case series and reports can alert researchers to the occurrence of serious events that can then be more systematically evaluated.
The quality of the evidence for many outcomes was rated as low or very low, largely attributable to all studies being observational. More than half of the primary studies had a high risk of bias, and most of the studies did not provide an adjusted analysis. Therefore, most of our meta-analyses were unadjusted. Another limitation is that several studies did not provide specific information about the type of immunotherapy given to patients.
CONCLUSION
This systematic review provides an updated synthesis of evidence about the prognosis of COVID-19 in patients with cancer receiving ICI therapy. Overall, ICI therapy was not significantly associated with worse COVID-19 outcomes compared with other cancer therapies or no therapy. No increase in irAEs was reported, but most studies did not evaluate immune toxicity secondary to ICI treatment.
Supplemental Material
Supplemental materials are available online with the article.
Supplementary Material
Funding Statement
Source of support: This study was supported by the National Cancer Institute (K08 grant CA237619, Maria A. Lopez-Olivo, MD, PhD, Principal Investigator), and the Cancer Survivorship Research Grant at the University of Texas MD Anderson Cancer Center (FP00015598). Partial support was provided through MD Anderson's Cancer Center Support Grant (P30 CA016672). The study sponsors did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: Dr. Suarez-Almazor has received consultant fees in the past 12 months from Pfizer, Eli Lilly, and Bristol Myers Squibb/Celgene, unrelated to this study.
References
- 1.Gosain R, Abdou Y, Singh A, et al. COVID-19 and cancer: a comprehensive review. Curr Oncol Rep . 2020;22(5):53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA . 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Lunski MJ, Burton J, Tawagi K, et al. Multivariate mortality analyses in COVID-19: comparing patients with cancer and patients without cancer in Louisiana. Cancer . 2021;127:266–274. doi: 10.1002/cncr.33243. [DOI] [PubMed] [Google Scholar]
- 4.Chavez-MacGregor M, Lei X, Zhao H, et al. Evaluation of COVID-19 mortality and adverse outcomes in US patients with or without cancer. JAMA Oncol . 2021. [DOI] [PMC free article] [PubMed]
- 5.Sharafeldin N, Bates B, Song Q, et al. Outcomes of COVID-19 in patients with cancer: report from the National COVID Cohort Collaborative (N3C) J Clin Oncol . 2021;39:2232–2246. doi: 10.1200/JCO.21.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med . 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med . 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D-G, Fang D, Wilson JR. Meta-analysis of two studies with random effects. J Minim Invasive Gynecol . 2017;24:689–690. doi: 10.1016/j.jmig.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ . 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albiges L, Foulon S, Bayle A, et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. Nat Cancer . 2020;1:965–975. doi: 10.1038/s43018-020-00120-5. [DOI] [PubMed] [Google Scholar]
- 11.Assaad S, Avrillon V, Fournier ML, et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer . 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buyansky D, Fallaha C, Gougeon F, et al. Acute tubulointerstitial nephritis in a patient on anti-programmed death-ligand 1 triggered by COVID-19: a case report. Can J Kidney Health Dis . 2021;8:20543581211014745. doi: 10.1177/20543581211014745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov . 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol . 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol . 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulati S, Shah S, Kulkarni A, et al. Thrombotic complications with SARS-CoV-2 infection in patients with cancer on high-risk therapies: data from the COVID-19 and Cancer Consortium (CCC19) J Clin Oncol . 2021;39:e18788–e. [Google Scholar]
- 17.Hwang C, Izano MA, Thompson MA, et al. Rapid real-world data analysis of patients with cancer, with and without COVID-19, across distinct health systems. Cancer Rep (Hoboken) . 2021;4:e1388. doi: 10.1002/cnr2.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jee J, Stonestrom AJ, Devlin S, et al. Oncologic immunomodulatory agents in patients with cancer and COVID-19. Sci Rep . 2021;11:4814. doi: 10.1038/s41598-021-84137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klebanov N, Pahalyants V, Murphy WS, et al. Risk of COVID-19 in patients with cancer receiving immune checkpoint inhibitors. Oncologist . 2021;26:e898–e901. doi: 10.1002/onco.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lara OD, O'Cearbhaill RE, Smith MJ, et al. COVID-19 outcomes of patients with gynecologic cancer in New York City. Cancer . 2020;126:4294–4303. doi: 10.1002/cncr.33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin DD, Wu Y, Toom S, et al. Clinical determinants differentiating the severity of SARS-CoV-2 infection in cancer patients: hospital care or home recovery. Front Med (Lausanne) . 2021;8:604221. doi: 10.3389/fmed.2021.604221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo J, Rizvi H, Preeshagul IR, et al. COVID-19 in patients with lung cancer. Ann Oncol . 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandala M, Lorigan P, De Luca M, et al. SARS-CoV-2 infection and adverse events in patients with cancer receiving immune checkpoint inhibitors: an observational prospective study. J Immunother Cancer . 2021;9:e001694. doi: 10.1136/jitc-2020-001694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Cao M, Basa MA, Puertolas T, et al. Cancer immunotherapy does not increase the risk of death by COVID-19 in melanoma patients. medRxiv . 2020. [DOI]
- 25.Moritz RKC, Gutzmer R, Zimmer L, et al. SARS-CoV-2 infections in melanoma patients treated with PD-1 inhibitors: a survey of the German ADOREG melanoma registry. Eur J Cancer . 2021;144:382–385. doi: 10.1016/j.ejca.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichetti F, Bini M, Ambrosini M, et al. COVID-19 risk for patients undergoing anticancer treatment at the outpatient clinic of the National Cancer Institute of Milan: the COVINT study. ESMO Open . 5e000883. 2020. [DOI] [PMC free article] [PubMed]
- 27.Pinato DJ, Zambelli A, Aguilar-Company J, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov . 2020;10:1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos-Ruperto L, Busca-Arenzana C, Valdivieso J, et al. COVID-19 and pembrolizumab-induced secondary hemophagocytic lymphohistiocytosis: a case report. SN Compr Clin Med . 2021;3:1412–1415. doi: 10.1007/s42399-021-00882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolfo C, Cardona AF, Ruiz-Patiño A, et al. Atypical skin manifestations during immune checkpoint blockage in coronavirus disease 2019-infected patients with lung cancer. J Thorac Oncol . 2020;15:1767–1772. doi: 10.1016/j.jtho.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serra-García L, Bosch-Amate X, Alamon-Reig F, et al. Digital ischemia triggered by coronavirus disease 2019 in a patient under cemiplimab treatment. Int J Dermatol . 2021;60:e30–30e2. doi: 10.1111/ijd.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trojaniello C, Vitale MG, Ascierto PA. Checkpoint inhibitor therapy for skin cancer may be safe in patients with asymptomatic COVID-19. Ann Oncol . 2021;32:674–676. doi: 10.1016/j.annonc.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyan K, Bui A-T, Giobbie-Hurder A, et al. Impact of COVID-19 on cancer patients receiving immune checkpoint inhibitors. J Immunother Cancer . 2020;8:A296–A298. doi: 10.36401/JIPO-20-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarza R, Bover M, Paredes D, et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur J Cancer . 2020;135:242–250. doi: 10.1016/j.ejca.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klebanov N, Pahalyants V, Murphy WS, et al. Risk of COVID-19 in patients with cancer receiving immune checkpoint inhibitors. Oncologist . 2021;26:e898–e901. doi: 10.1002/onco.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moritz RKC, Gutzmer R, Zimmer L, et al. SARS-CoV-2 infections in melanoma patients treated with PD-1 inhibitors: a survey of the German ADOREG melanoma registry. Eur J Cancer . 2021;144:382–385. doi: 10.1016/j.ejca.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trojaniello C, Vitale MG, Ascierto PA. Checkpoint inhibitor therapy for skin cancer may be safe in patients with asymptomatic COVID-19. Ann Oncol . 2021;32:674–676. doi: 10.1016/j.annonc.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Liu S, Qin Y, et al. Does prior exposure to immune checkpoint inhibitors treatment affect incidence and mortality of COVID-19 among the cancer patients: the systematic review and meta-analysis. Int Immunopharmacol . 2021;101:108242. doi: 10.1016/j.intimp.2021.108242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazarus G, Budiman RA, Rinaldi I. Does immune checkpoint inhibitor increase the risks of poor outcomes in COVID-19-infected cancer patients? A systematic review and meta-analysis. Cancer Immunol Immunother . 2021;26:26. doi: 10.1007/s00262-021-02990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park R, Lee SA, Kim SY, et al. Association of active oncologic treatment and risk of death in cancer patients with COVID-19: a systematic review and meta-analysis of patient data. Acta Oncol . 2021;60:13–19. doi: 10.1080/0284186X.2020.1837946. [DOI] [PubMed] [Google Scholar]
- 40.Qian W, Ye Y, Zuo L, et al. Immune checkpoint inhibitors use and effects on prognosis of COVID-19 infection: a systematic review and meta-analysis. Immunotherapy . 2021;13:1271–1282. doi: 10.2217/imt-2021-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


