Abstract
The behavior of gut microbiota is closely involved in sustaining balanced immune and metabolic homeostasis, and the dysbiosis of gut microbiota can lead to severe disease. Foods and dietary patterns are the primary drivers in shaping/designing gut microbiota compositions and their metabolites across the lifetime. This indicates the importance of functional molecules present in the food matrix in the life of gut microbiota and their influence on the host’s biological system. In this contribution, the effects of different dietary choices and bioactive compounds (i.e., phenolics, vitamins, carotenoids) on gut microbiome compositions and their metabolites are comprehensively discussed by focusing on neurotransmitters. This study may provide useful information that fills a gap in understanding the role of the gut microbiota and its alterations as affected by foods and food-derived bioactives.
Keywords: Gut microbiota, Gut-Brain-Axis, Bioactive compound, Gut microbiota-derived metabolite, Neurotransmitter
Gut microbiota
The collection of microorganisms including bacteria, archaea and eukaryotes inhabiting the gut is referred to as the ‘gut microbiota’ (Foster and Neufeld, 2013). The gut microbiota is engaged in sustaining balanced metabolism by contributing to the successful degradation of macromolecules ingested such as carbohydrates, proteins, and lipids (Chait et al., 2020). Recent findings show that gut microbiota plays a crucial role in the functions of the central nervous system (CNS) (Heijtz et al., 2011). Residential and commensal bacteria colonize the gut of human beings after birth and throughout life; 1014–1015 of bacteria along with 1000 distinct bacterial species live in the gut, contributing to the immune function, nutrient processing, and other aspects of the host’s physiology (Foster and Neufeld, 2013; Macpherson and Uhr, 2004; Tlaskalová-Hogenová et al., 2004).
Recent advanced techniques such as molecular and metagenomic approaches have enabled the characterization of the composition of the gut microbiota, revealing that the diverse microbiomes in the gut form a complex ecology and community (Foster and Neufeld, 2013). There are two predominant bacterial phyla in the gut: Firmicutes and Bacteroides, which account for more than 70% of the microbiome in the gut (Eckburg et al., 2005; Lay et al., 2005). Moreover, Proteobacteria, Fusobacteria, Actinobacteria, and Verrucomicrobia also reside in the gut in a lower number compared to Firmicutes and Bacteroides. A variety of environmental factors (i.e., genetics, diet, age, metabolism, geography, stress, and antibiotic treatment) affect the gut microbiota composition and behavior, which in turn influence its metabolic functions. In this review, we mainly focus on how food-derived bioactives affect gut microbiota profiles and their metabolites.
Diseases related to the dysbiosis of gut microbiota
The human gut possesses trillions of symbiotic microorganisms (bacteria, archaea, fungi, etc.) that play a crucial role in regulating the host’s unique physiology in health and disease (Foster and Neufeld, 2013). Gut dysbiosis is referred to as a disruption of gut microbiota homeostasis due to an imbalance in microflora, alterations in their functional profiles and metabolism, or changes in their distribution (Moos et al., 2016). A range of factors such as diet, age, genetics, and medications are responsible for gut dysbiosis (Chait et al., 2020; Heiman and Greenway, 2016; Maier et al., 2018; Odamaki et al., 2016).
Recent findings reported that the dysbiosis of gut microbiota is strongly associated with the occurrence of many severe diseases such as inflammatory bowel disease, type 2 diabetes, Crohn's disease, Parkinson’s disease, Alzheimer's disease, schizophrenia, and autism as well as mental illnesses such as major depressive disorder and anxiety (Carding et al., 2015; Clarke et al., 2013; Diaz et al., 2011; Gabbay et al., 2017; Nedic et al., 2021), indicating the importance of maintaining a well-balanced gut microbiota.
Gut dysbiosis by psychotropics
Psychotropic medications, such as antidepressants, mood stabilizers, antipsychotics, and anxiolytics, are widely applied in the treatment of many psychiatric disorders in which they exert their bioactivity by contributing to CNS reactions (Kaye et al., 2018). According to recent research, the alteration of gut microbiota diversity can be caused by psychotropic medications (Chait et al., 2020). Indeed, much attention has been paid to the alteration of gut microbiota by psychotropic administration in both in vitro and in vivo models. Chait et al. (2020) examined the antimicrobial activity of psychotropics in which the abundance of Akkermansia muciniphila, Bifidobacterium animalis and Bacteroides fragilis were remarkably changed by antidepressant drugs such as phenelzine, desipramine, venlafaxine, bupropion, (S)-citalopram, and aripiprazole. Moreover, olanzapine administration increased the abundance of Firmicutes and Erysipelotrichi, while it decreased the level of Bacteroidetes, Proteobacteria, and Actinobacteria in a rat model (Davey et al., 2012; Morgan et al., 2014). In addition to the above evidence, a variety of studies have been carried out to screen the alteration of the gut microbiota profile upon psychotropic administration (Cussotto et al., 2019; Lyte et al., 2019). However, research on how this alteration affects gut microbiota-derived metabolites is limited.
The importance of gut microbiota-derived metabolites
Metabolites secreted by gut microbiota have a significant function in the gut microbiota-brain axis; for instance, gut microbiota secrete diverse metabolites including neurotransmitters and their precursors, and these molecules influence the interaction between the gut and brain via the endocrine, immune, and neurotransmitter systems (Chen et al., 2021). For instance, gut bacteria secrete lipopolysaccharide and other endotoxins responsible for the activation of the peripheral immune system through immune cell activation and cytokine release, leading to the initiation of CNS inflammation (Caspani and Swann, 2019). Moreover, the gut microbiota produces metabolites such as gamma-aminobutyric acid (GABA), acetylcholine, short-chain fatty acid (SCFA), norepinephrine, and dopamine, and these molecules influence brain functions and conditions (Cox and Weiner, 2018; Cryan et al., 2020). Thus, gut microbiota-derived metabolites significantly affect the host’s body and brain health, emphasizing the importance of the alteration of gut microbiota diversity upon exposure to psychotropic administration. Until now, despite tremendous scientific efforts to understand the alteration/dysregulation of gut microbiota as affected by psychotropics, critical limitations remain in understanding changes in the metabolites of gut microbiota upon exposure to psychotropics and following the alteration of gut microbiota composition. Today, much progress has been made in mass spectrometry-based metabolomics in the identification and quantification of metabolites in a wide spectrum of biological samples (i.e., plant, microbial and mammalian samples), demonstrating that mass spectrometry-based is a rapid and high throughput approach in the determination of metabolite profiles in target samples. (Fernie et al., 2004; Gieger et al., 2008; Rinschen et al., 2019).
Gut-brain-axis
The gut-brain axis, which refers to the two-way communication between the gut microbiota and the brain, has a crucial role in neuronal development, cognitive regulation, and brain function (Cryan et al., 2020) (Fig. 1). There are two main pathways in transmitting information between two areas: “top-down” and “bottom-up”. The top-down pathway is regulated by the hypothalamus–pituitary–adrenal axis in which the cortisol and cytokine secreted by immune cells significantly influence the gut microbiota community locally and systemically, influencing the diversity of gut microbiota and gut permeability (Cryan and Dinan, 2012). Conversely, the gut microbiota can influence alterations in the levels of circulating cytokines, affecting brain functions, and is referred to as the “bottom-up” pathway. In this way, the level of tryptophan and its interaction with the vagus and enteric nerve act as key functions in the gut-microbiota-brain axis. In addition, recent findings indicate that the gut microbiota produces a wide spectrum of metabolites including neurotransmitters and their precursors, which then take part in the bottom-up pathway. For example, spore-forming bacteria produce metabolites that can improve the biosynthesis of serotonin in enterochromaffin cells (Chen et al., 2021). In addition, certain neurotransmitters and their precursors released by the gut microbiota and enteroendocrine cells enter into the blood circulation and eventually reach the brain. This demonstrates the significance of gut microbiota and their metabolites in the communication between the gut and brain, in particular in the “bottom-up” pathway.
Fig. 1.
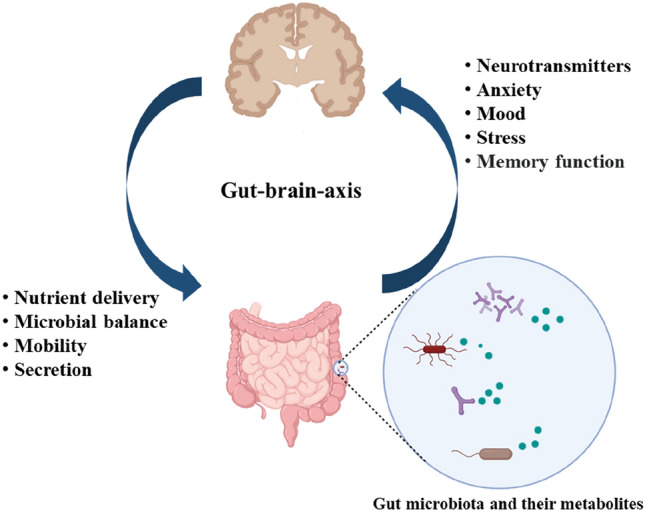
Gut-brain-axis: The importance of bidirectional communication between the brain and gut microbiota and their metabolite in controlling mental disorders
Gut microbiota-derived metabolites with a focus on neurotransmitters
Many recent studies have investigated the biosynthesis of neurotransmitters (GABA, SCFA, dopamine, acetylcholine, norepinephrine, etc.) by gut microbiota as their metabolites as well as their influence on the brain's functions (Cox and Weiner, 2018; Cryan et al., 2020). For example, some research groups reported that interference in producing monoamine due to gut dysbiosis resulted in depressive disorder in the animal system, indicating a strong influence of gut microbiota on the occurrence of mental disorders (Clarke et al., 2013; Diaz et al., 2011; Neufeld et al., 2011). Moreover, patients with depression showed a notable alteration in the level of Firmicutes, Actinobacteria, and Bacteroidetes compared to a control group (Zheng et al., 2016). Minato et al. (2017) reported that a significantly reduced level of Prevotellaceae was found in patients with progressive Parkinson’s disease, which led them to suggest that the abundance of Prevotellaceae is a biomarker for Parkinson’s disease. Moreover, Li et al. (2017) stated that the level of Faecalibacterium is strongly related to the development and neuropathology of Parkinson’s disease. The above evidence clearly shows the crucial roles of the diversity of gut microbiota and their metabolite profiles in sustaining a healthy brain.
Synthesis and production of neurotransmitters by gut microbiota
Dopamine is a major catecholaminergic neurotransmitter and plays a crucial role in many functions of the brain such as emotion, memory, attention, motivation, and reward (Klein et al., 2019; Kleinridders and Pothos, 2019). Increasing evidence has demonstrated that dysregulation in the production of dopamine is closely related to mental disorders, including depression and anxiety (Belujon and Grace, 2017; Camardese et al., 2014; Carpenter et al., 2012; Gonzalez-Arancibia et al., 2019; Moraga-Amaro et al., 2014). Some Bacillus species such as B. cereus, B. subtilis, and B. mycoides can synthesize dopamine (Tsavkelova et al., 2000). In addition, Hafnia alvei (NCIMB, 11,999), (NCIMB, 10,466), Klebsiella pneumoniae (NCIMB, 673), and morganii enable the production of dopamine in the gut (Özoğul, 2004; Shishov et al., 2009). Cryan and Dinan (2012) also demonstrated the production and secretion of dopamine by Bacillus and Escherichia species. However, the mechanism of dopamine synthesis in the gut microbiota has not yet been fully investigated.
Serotonin is engaged in diverse brain functions including mood, modulating reward, memory, cognition, learning, and other physiological processes (i.e., vasoconstriction and vomiting). Changes in the expression and production of serotonin in the brain lead to the pathogenesis of mental illnesses, including depressive disorders and anxiety (Helton and Lohoff, 2015). The main pathway for the biosynthesis of serotonin is through enterochromaffin cells in the gut, thereby tryptophan hydroxylase 1 (Tph1) is involved in the reaction as the rate-limiting enzyme (Kwon et al., 2019). The synthesis rate of serotonin by enterochromaffin cells is largely affected by the available concentration of tryptophan required for the reaction; therefore, sustaining an adequate amount of tryptophan in the gastrointestinal tract is important to maintaining an adequate level of serotonin.
Many studies have found serotonin-producing bacteria in the gut such as E. coli (K-12), Lactococcus lactis subsp. cremoris (MG 1363), Lactobacillus plantarum (FI8595), Candida spp., Streptococcus spp., Streptococcus thermophilus (NCFB2392), Escherichia spp., and Enterococcus spp. (Cryan and Dinan, 2012; Özoğul et al., 2012; Shishov et al., 2009). Gut microbiota also indirectly engage in the synthesis of serotonin; for example, enterochromaffin cells synthesize serotonin when they receive signals via gut microbiota-derived metabolites, which increase the expression of the gene TPH1. Moreover, SCFAs produced by gut microbiota elevate the production of serotonin in the enterochromaffin cells (Reigstad et al., 2015). However, additional research should be carried out to understand the direct pathways for the synthesis of serotonin in gut microbiota.
Transportation of gut microbiota-derived neurotransmitters to the brain
Gut microbiota-derived GABA is transferred to the brain via different pathways. Much attention has been paid to exploring the transportation mechanisms of GABA to the brain produced by the gut microbiota. GABA absorption in the intestinal system takes place via the transcellular pathway with the assistance of carrier proteins. GABA in plasma can enter the blood–brain barrier (BBB) via GABA transporters, including GABA transporter type 1, 2, 3, and 4, which are also localized in other organs including the kidneys and liver. The plasma membrane GABA transporters in the brain have a significant role in sustaining an adequate level of extracellular GABA around the synapses (Zhou and Danbolt, 2013). The GABA transporters are the active voltage-dependent system through which the inward electrochemical gradient of Na+ considerably influences the action of GABA transporters (Scimemi, 2014). In addition, the GABA transporter displays a weak micromolecular affinity to GABA, and it demands Cl− in the extracellular matrix (Scimemi, 2014). Therefore, the mechanism of GABA transportation from the intestinal system to the brain is well understood.
SCFAs produced by the fermentation of fiber by the gut microbiota are absorbed via colonocytes through sodium-coupled monocarboxylate transporters (SMCTs) and monocarboxylate transporters (MCTs), referred to as active transport (Vijay and Morris, 2014). The transportation of SCFAs occurs through MCT1 transporters in an H+-dependent, while they can also be transported via sodium-dependent and electrogenic SMCTs, which is referred to as SCFA anion transport (Stumpff, 2018). SCFAs present in colonocytes are metabolized through the citric acid cycle in the mitochondria to generate ATP and energy (Schönfeld and Wojtczak, 2016). However, certain portions of SCFAs in colonocytes are not metabolized, leading them to enter circulation for consumption as an essential energy source in hepatocytes, except for acetic acids, which are not metabolized in the liver (Schönfeld and Wojtczak, 2016). This implies that only a certain range of colon-derived SCFAs enter the systemic circulation; for instance, 36%, 9%, and 2% of gut microbiota-derived acetate, propionate, and butyrate, respectively, enter the blood plasma and tissue (Boets et al., 2015). Bloemen et al. (2009) demonstrated that the usual levels of acetate, propionate, and butyrate in portal blood were 260 μM, 30 μM, and 30 μM, respectively. However, the penetration abilities of SCFAs to the BBB have been rarely investigated; thus, further studies are needed for a better understanding of the effects of gut-derived neurotransmitters on the roles of the brain. Meanwhile, the majority of neurotransmitters, including norepinephrine, dopamine, and acetylcholine, in blood circulation cannot get through the BBB due to the absence of proper transporters (Chen et al., 2021). However, the precursor molecules of these neurotransmitters, including tryptophan and tyrosine, can penetrate the BBB. Thus, they can be localized in the relevant cells and utilized for the biosynthesis of neurotransmitters in the brain.
Alteration of gut microbiomes by bioactive compounds
Increasing findings show the remarkable influence of bioactive compounds in shaping the compositional and functional patterns of the gut microbiota. Many studies have shown that bioactive compounds derived from diverse food sources cause substantial alterations in the composition of the gut microbiota (Wen and Duffy 2017; Wu et al. 2011). The alterations of gut microbiota profiles as affected by food bioactives are summarized in Table 1.
Table 1.
Alteration in gut microbiota profiles by bioactive compounds
| Bioactive compound | Change in the composition of gut microbiome | Reference |
|---|---|---|
| Gallic acid | Reducing the counts of Bacteroides spp. and enhancement in the abundance of Atopobium spp. | Hidalgo et al. (2012) |
| Catechin | Simulate the growth of the C. coccoides–E. rectale group, Bifidobacterium spp. and E. coli, and repress the level of C. histolyticum group in in vitro batch-culture fermentation system | Tzounis et al. (2011) |
| Epicatechin | Increase in the counts of the C. coccoides–E. rectale group in in vitro batch-culture fermentation system | Tzounis et al. (2011) |
| Isoflavones | Enhancement in the abundance of C. coccoides-E. rectale cluster, F. prausnitzii subgroup, L.-Enterococcus group, and Bifidobacterium spp. | Clavel et al. (2005) |
| Quercetin | Increase in the abundance of Bacteroides, Bifidobacterium, Lactobacillus, and Clostridia and a decrease in Fusobacterium and Enterococcus in mice | Lin et al. (2019) |
| Stilbene (resveratrol) | Increase in the counts of Bifidobacterium and Lactobacillus during 20 days in the rats in vivo dietary intervention tests | Larrosa et al. (2009) |
| Malvidin-3-glucoside | Increase in the level of beneficial bacteria Bifidobacterium spp. and Lactobacillus spp. | Hidalgo et al. (2012) |
| Phloridzin | Elevate the adhesion of L. rhamnosus to Caco-2 cells | Parkar et al. (2008) |
| Astaxanthin |
Increase in the abundance of Proteobacteria and Bacteroides in six-week-old male and female BCO2 knockout mice Increase in the abundance of Actinobacteria and Bifidobacterium in male genetic background C57BL/6 J mice |
Lyu et al. (2018) |
| Eugenol | Increase in the population of Clostridales in mice | Wlodarska et al. (2015) |
| Curcumin | Increase in the population of Clostridium spp., Bacteroides spp., Citrobacter spp., Cronobacter spp., Enterobacter spp., Enterococcus spp., Klebsiella spp., Parabacteroides spp., and Pseudomonas spp. in human study | Peterson et al. (2018) |
Polyphenols also have regulatory effects on gut microbiota composition. Gallic acid significantly reduced the counts of Bacteroides spp. and increased the abundance of Atopobium spp. (Hidalgo et al., 2012). Catechin improved the growth of the Clostridium coccoides–Eubacterium rectale group, E. coli, and Bifidobacterium spp. and repressed the level of the Clostridium histolyticum group in in vitro batch-culture fermentation systems. The exposure of epicatechin to the gut microbiota increased the abundance of the C. coccoides–E. rectale group (Tzounis et al., 2011). Clavel et al. (2005) found that the administration of isoflavones at a dose of 100 mg/day for two months in human tests caused an enhancement in the level of the C. coccoides–E. rectale cluster, Faecalibacterium prausnitzii subgroup, Lactobacillus-Enterococcus group, and B. spp. The administration of stilbene (resveratrol) dramatically improved the counts of Bifidobacterium and Lactobacillus during 20 days in rats in in vivo dietary intervention tests (Larrosa et al., 2009). Quercetin elevated the level of Bacteroides, Bifidobacterium, Lactobacillus, and Clostridia and reduced those of Fusobacterium and Enterococcus in a mouse model (Lin et al., 2019).
Research has also been carried out regarding the influence of proanthocyanidin on gut microbiota composition. For instance, Hidalgo et al. (2012) tested the effects of malvidin-3-glucoside on the alteration of gut microbiota in batch-culture fermentation with human fecal bacteria. Malvidin-3-glucoside remarkably improved the level of the beneficial bacteria B. spp. and Lactobacillus spp. In addition to the above findings, many studies have reported on the alteration of gut microbiota as affected by diverse phenolic compounds. Phloridzin improved the adhesion of Lactobacillus rhamnosus to Caco-2 cells (Parkar et al., 2008).
Meanwhile, carotenoids also showed a remarkable influence on the alteration of gut microbiota profiles. For instance, β-carotene remarkably reduced the level of Bacteroidetes and the genus Prevotella and Blautia, while it led to an increase in the abundance of phyla Firmicutes, genera p-75-a5, and Parabacteroides (Li et al., 2021). Moreover, a β-carotene treatment significantly enhanced the level of Faecalibacterium in a rat model system (Zhu et al., 2021).
Recently, accumulating evidence has shown that vitamins from the plant- and animal-based foods lead to alterations in the microbiome profiles in the gut. The changes in gut microbiome composition can result from exposure to certain food-derived bioactives or indirectly due to changes in the physiology of gut and intestinal lumen conditions (Pham et al., 2021). Vitamins have been proven to be microbiome-modulators through several pathways. Some vitamins, including vitamins A, B6, C, and E, cause direct alterations in gut microbiome profiles (Castillo et al., 2016; Miki et al., 2017; Vergalito et al., 2018;). In this section, a wide spectrum of examples regarding the effect of vitamins on changes in gut microbiota composition are summarized.
Liu et al. (2017) reported that giving 200,000 IU of a vitamin A supplement to 64 young children suffering from autism disorders significantly increased the abundance of Bacteroidetes, while it reduced the abundance of Proteobacteria, Actinobacteria, Enterobacter, Escherichia-Shigella, and Clostridium. Lv et al. (2016) studied the alteration of gut microbiota of infants with persistent diarrhea profiles through a vitamin A supplement in which the results showed that the levels of Enterococcus, Enterococcaceae, and Lactobacillales were remarkably increased, while the abundance of Escherichia-Shigella was decreased. Meanwhile, another study showed that the administration of vitamin A to 16 adult patients with cystic fibrosis enhanced the level of Clostridium and Gemellales and significantly decreased Bacteroidetes/Bacteroidia/Bacteroidales (Li et al., 2017). Thus, the above examples provide crucial information regarding discrepancies in the alteration of gut microbiota profiles due to the supplementation of vitamin A depending on the health status of patients, the dose, and other environmental differences. Other bioactive molecules such as astaxanthin, eugenol, and curcumin also remarkably affect alterations of the composition of gut microbiota.
Changes in gut microbiota-derived metabolites by bioactives and diets
Recently, increasing research has focused on the influences of food-derived bioactives on alterations in gut microbiota composition and their metabolites, in particular in neurotransmitters. Changes in gut microbiota-derived metabolites as affected by bioactives are summarized in Table 2.
Table 2.
Changes in gut microbiota-derived metabolites by bioactives
| Diet/food ingredient | Alteration in gut microbiome-derived metabolite | Reference |
|---|---|---|
| Polyphenols in the water-insoluble cocoa fraction | Increase in the level of butyrate | Fogliano et al. (2011) |
| Apple juice extracts and red beet juice extracts | Increase in the contents of acetate and total short-chain fatty acids (propionic acid and butyric acid) in rats | Sembries et al (2006) |
| Anthocyanin-rich blueberry extract | Increase in the production of kynurenic acid by altering tryptophan metabolism | Marques et al. (2018) |
| Berberine | Promotion in the production of butyrate in rats | Wang et al. (2018) |
| Dietary fiber (resistant starch from potatoes) | Increase in the production of short-chain fatty acids including butyrate | Baxter et al. (2019) |
| Western-style diet (high in fat) |
Enhancing the level of Firmicutes/Bacteroidetes ratio and the abundance of Proteobacteria and Spirochaetes in mice model Reducing the level of SCFAs including acetic, propionic, and butyric acids in cecal contents along with altered anxiety-like behavior |
Ohland et al. (2013) |
| Carbohydrates (fiber) | A decrease in carbohydrates intake (including fiber) significantly reduced the richness of fiber-degrading bacteria, whereas the abundance of Eggerrthella, Lactococcus, and Streptococcus was increased, leading to a decrease in the production of SCFAs | Mardinoglu et al. (2018) |
Fogliano et al. (2011) investigated the influence of polyphenols in the water-insoluble cocoa fraction. They found an increase in the abundance of Bifidobacteria and Lactobacilli followed by the elevation of butyrate production, indicating that bioactives from diets can modulate gut microbiota composition and their metabolites. Sembries et al. (2006) demonstrated that apple juice extracts and red beet juice extracts enhanced the level of neurotransmitters such as acetate, propionic acid, and butyric acid in a rat model. Moreover, Marques et al. (2018) found an increase in the production of kynurenic acid by altering tryptophan metabolism in a rat model. Other studies also demonstrate improvements in the level of SCFAs in rat models upon exposure to food sources; namely, berberine promoted the production of butyrate in rats, and dietary fiber (resistant starch from potatoes) increased the production of SCFAs including butyrate (Baxter et al., 2019; Wang et al., 2018).
In addition, recent research has indicated a strong association between gut microbiota and mental health, and given that diets affect the diversity of gut microbiota to a large extent, the composition of a host’s diet may play an important role in their mental health. For example, a western-style diet elevated the Firmicutes/Bacteroidetes ratio and the abundance of Proteobacteria and Spirochaetes in a rat model. This led to a decrease in the level of SCFAs such as acetic and propionic in cecal contents and anxiety-like behavior (Ohland et al., 2013). In a recent human cohort study, a reduced level of carbohydrate intake resulted in a rapid rearrangement in the composition of human gut microbiota within 24 h; for instance, a reduction in carbohydrate intake significantly decreased the abundance of fiber-degrading bacteria, while the level of Eggerrthella, Streptococcus, and Lactococcus was enhanced followed by a decrease in the secretion of SCFAs (Mardinoglu et al., 2018).
In summary, although many studies have been conducted regarding alterations in the diversity of gut microbiota as affected by food ingredients, research on how food ingredients influence the alteration of gut microbiota-derived neurotransmitters is limited. However, based on the above investigations, it is expected that individual food ingredients/components play a significant role in modulating gut microbiota composition and the following changes in their secretion of neurotransmitters.
This review provides comprehensive, in-depth knowledge of the significant functions of gut microbiota in maintaining metabolism and homeostasis as well as the alteration of their profiles as affected by food intake and the consumption of food-derived bioactive compounds. Moreover, this contribution summarizes the significance of food sources and dietary choices in designing gut microbiota profiles and their metabolites. Food sources and bioactive compounds remarkably affect the composition of gut microbiomes and their metabolites, indicating their importance in sustaining balanced-immune and metabolic homeostasis. Despite the increasing research on the influence of food bioactives on gut microbiota-derived metabolites, there is still a lack of information on related fields. Nevertheless, this review provides a valuable summary for understanding the role of foods and food-derived bioactives in controlling the composition of gut microbiota and their metabolites.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio. 2019;10:e0256618. doi: 10.1128/mBio.02566-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. The International Journal of Neuropsychopharmacology. 2017;20:1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clinical Nutrition. 2009;28:657–661. doi: 10.1016/j.clnu.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Boets E, Deroover L, Houben E, Vermeulen K, Gomand SV, Delcour JA, Verbeke K. Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients. 2015;7:8916–8929. doi: 10.3390/nu7115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camardese G, Di Giuda D, Di Nicola M, Cocciolillo F, Giordano A, Janiri L, Guglielmo R. Imaging studies on dopamine transporter and depression: A review of literature and suggestions for future research. Journal of Psychiatric Research. 2014;51:7–18. doi: 10.1016/j.jpsychires.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microbial Ecology in Health and Disease. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AC, Saborido TP, Stanwood GD. Development of hyperactivity and anxiety responses in dopamine transporter-deficient mice. Developmental Neuroscience. 2012;34:250–257. doi: 10.1159/000336824. [DOI] [PubMed] [Google Scholar]
- Caspani G, Swann J. Small talk: Microbial metabolites involved in the signaling from microbiota to brain. Current Opinion in Pharmacology. 2019;48:99–106. doi: 10.1016/j.coph.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Castillo Y, Suzuki J, Watanabe K, Shimizu T, Watarai M. Effect of vitamin A on Listeria monocytogenes infection in a Silkworm model. Public Library of Science (PLoS) ONE. 2016;11:e0163747. doi: 10.1371/journal.pone.0163747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait YA, Mottawea W, Tompkins TA, Hammami R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Scientific Reports. 2020;10:17878. doi: 10.1038/s41598-020-74934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021;13:2099. doi: 10.3390/nu13062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Clavel T, Fallani M, Lepage P, Levenez F, Mathey J, Rochet V, Sérézat M, Sutren M, Henderson G, Bennetau-Pelissero C, Tondu F, Blaut M, Doré J, Coxam V. Isoflavones and functional foods alter the dominant intestinal microbiota in postmenopausal women. Journal of Nutrition. 2005;135:2786–2792. doi: 10.1093/jn/135.12.2786. [DOI] [PubMed] [Google Scholar]
- Cox LM, Weiner HL. Microbiota signaling pathways that influence neurologic disease. Neurotherapeutics. 2018;15:135–145. doi: 10.1007/s13311-017-0598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurology. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, Stanton C, Dinan TG, Cryan JF. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology. 2019;236:1671–1685. doi: 10.1007/s00213-018-5006-5. [DOI] [PubMed] [Google Scholar]
- Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, Dinan TG, Cryan JF. Gender-dependent consequences of chronic olanzapine in the rat: Effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. 2012;221:155–169. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Trethewey RN, Krotzky AJ, Willmitzer L. Metabolite profiling: From diagnostics to systems biology. Nature Reviews Molecular Cell Biology. 2004;5:763–769. doi: 10.1038/nrm1451. [DOI] [PubMed] [Google Scholar]
- Fogliano V, Corollaro ML, Vitaglione P, Napolitano A, Ferracane R, Travaglia F, Arlorio M, Costabile A, Klinder A, Gibson G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Molecular Nutrition and Food Research. 2011;55:44–55. doi: 10.1002/mnfr.201000360. [DOI] [PubMed] [Google Scholar]
- Foster JA, Neufeld KM. Gut–brain axis: How the microbiome influences anxiety and depression. Trends in Neurosciences. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Bradley KA, Mao X, Ostrover R, Kang G, Shungu DC. Anterior cingulate cortex gamma-aminobutyric acid deficits in youth with depression. Translational Psychiatry. 2017;7:e1216. doi: 10.1038/tp.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieger C, Geistlinger L, Altmaier E, Hrabé de Angelis M, Kronenberg F, Meitinger T, Mewes WH, Wichmann HE, Weinberger KM, Adamski J, Illig T, Suhre K. Genetics meets metabolomics: A genome-wide association study of metabolite profiles in human serum. Public Library of Science (PLoS) Genetics. 2008;4:e100282. doi: 10.1371/journal.pgen.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton SG, Lohoff FW. Serotonin pathway polymorphisms and the treatment of major depressive disorder and anxiety disorders. Pharmacogenomics. 2015;16:541–553. doi: 10.2217/pgs.15.15. [DOI] [PubMed] [Google Scholar]
- Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman ML, Greenway FL. A healthy gastrointestinal microbiome is dependent on dietary diversity. Molecular Metabolism. 2016;5:317–320. doi: 10.1016/j.molmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M, Oruna-Concha MJ, Kolida S, Walton GE, Kallithraka S, Spencer JP, de Pascual-Teresa S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. Journal of Agricultural and Food Chemistry. 2012;60:3882–3890. doi: 10.1021/jf3002153. [DOI] [PubMed] [Google Scholar]
- Kaye AD, Kline RJ, Thompson ER, Kaye AJ, Terracciano JA, Siddaiah HB, Urman RD, Cornett EM. Perioperative implications of common and newer psychotropic medications used in clinical practice. Best Practice & Research Clinical Anaesthesiology. 2018;32:187–202. doi: 10.1016/j.bpa.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. Dopamine: Functions, signaling, and association with neurological diseases. Cellular and Molecular Neurobiology. 2019;39:31–59. doi: 10.1007/s10571-018-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Pothos EN. Impact of brain insulin signaling on dopamine function, food intake, reward, and emotional behavior. Current Nutrition Reports. 2019;8:83–91. doi: 10.1007/s13668-019-0276-z. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Wang H, Denou E, Ghia JE, Rossi L, Fontes ME, Bernier SP, Shajib MDS, Banskota S, Collins SM, Surette MG, Khan WI. Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cellular and Molecular Gastroenterology and Hepatology. 2019;7:709–728. doi: 10.1016/j.jcmgh.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrosa M, Yañéz-Gascón MJ, Selma MV, González-Sarrías A, Toti S, Cerón JJ, Tomás-Barberán F, Dolara P, Espín JC. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. Journal of Agricultural and Food Chemistry. 2009;57:2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- Lay C, Sutren M, Rochet V, Saunier K, Doré J, Rigottier-Gois L. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environmental Microbiology. 2005;7:933–946. doi: 10.1111/j.1462-2920.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- Li L, Krause L, Somerset S. Associations between micronutrient intakes and gut microbiota in a group of adults with cystic fibrosis. Clinical Nutrition. 2017;36:1097–1104. doi: 10.1016/j.clnu.2016.06.029. [DOI] [PubMed] [Google Scholar]
- Li R, Li L, Hong P, Lang W, Hui J, Yang Y, Zheng X. β-Carotene prevents weaning-induced intestinal inflammation by modulating gut microbiota in piglets. Animal Bioscience. 2021;34:1221–1234. doi: 10.5713/ajas.19.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Piao M, Song Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in Citrobacter rodentium-infected mice. Frontiers in Microbiology. 2019;10:1092. doi: 10.3389/fmicb.2019.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu X, Xiong XQ, Yang T, Cui T, Hou NL, Lai X, Liu S, Guo M, Liang XH, Cheng Q, Chen J, Li TY. Effect of vitamin A supplementation on gut microbiota in children with autism spectrum disorders: A pilot study. BioMed Central (BMC) Microbiology. 2017;17:204. doi: 10.1186/s12866-017-1096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Wang Y, Yang T, Zhan X, Li Z, Hu H, Li T, Chen J. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. Journal of Clinical Biochemistry and Nutrition. 2016;59:113–121. doi: 10.3164/jcbn.15-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, Daniels KM, Schmitz-Esser S. Fluoxetine-induced alteration of murine gut microbial community structure: Evidence for a microbial endocrinology-based mechanism of action responsible for fluoxetine-induced side effects. Journal of Life & Environmental Science (PeerJ) 2019;7:e6199. doi: 10.7717/peerj.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y, Wu L, Wang F, Shen X, Lin D. Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Experimental Biology and Medicine. 2018;243:613–620. doi: 10.1177/1535370218763760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensmal intestinal bacteria. Annals of the New York Academy of Sciences. 2004;1029:36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, Räsänen SM, Lee S, Mancina RM, Bergentall M, Pietiläinen KH, Söderlund S, Matikainen N, Ståhlman M, Bergh PO, Adiels M, Piening BD, Granér M, Lundbom N, Williams KJ, Romeo S, Nielsen J, Snyder M, Uhlén M, Bergström G, Perkins R, Marschall HU, Bäckhed F, Taskinen MR, Borén J. An integrated understanding of the rapid metabolic benefits of a carbohydrate-restricted diet on hepatic steatosis in humans. Cell Metabolism. 2018;27:559–571.e5. doi: 10.1016/j.cmet.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques C, Fernandes I, Meireles M, Faria A, Spencer JPE, Mateus N, Calhau C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Scientific Reports. 2018;8:11341. doi: 10.1038/s41598-018-29744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Goto R, Fujimoto M, Okada N, Hardt WD. The bactericidal lectin RegIIIbeta prolongs gut colonization and enteropathy in the streptomycin mouse model for Salmonella diarrhea. Cell Host and Microbe. 2017;21:195–207. doi: 10.1016/j.chom.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K, Hirayama M. Progression of Parkinson's disease is associated with gut dysbiosis: Two-year follow-up study. Public Library of Science (PLoS) ONE. 2017;12:e0187307. doi: 10.1371/journal.pone.0187307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos WH, Faller DV, Harpp DN, Kanara I, Pernokas J, Powers WR, Steliou K. Microbiota and neurological disorders: A gut feeling. BioResearch Open Access. 2016;5:137–145. doi: 10.1089/biores.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga-Amaro R, Gonzalez H, Pacheco R, Stehberg J. Dopamine receptor D3 deficiency results in chronic depression and anxiety. Behavioural Brain Research. 2014;274:186–193. doi: 10.1016/j.bbr.2014.07.055. [DOI] [PubMed] [Google Scholar]
- Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, Bogue MA, Paredes SH, Yourstone S, Carroll IM, Kawula TH, Bower MA, Sartor RB, Sullivan PF. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. Public Library of Science (PLoS) One. 2014;9:e115225. doi: 10.1371/journal.pone.0115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Communicative & Integrative Biology. 2011;4:492–494. doi: 10.4161/cib.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BioMed Central (BMC) Microbiology. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E, Madsen KL. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013;38:1738–1747. doi: 10.1016/j.psyneuen.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Özoğul F. Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method. European Food Research and Technology. 2004;219:465–469. doi: 10.1007/s00217-004-0988-0. [DOI] [Google Scholar]
- Özoğul F, Kuley E, Özoğul Y, Özoğul I. The function of lactic acid bacteria on biogenic amines production by food-borne pathogens in arginine decarboxylase broth. Food Science and Technology Research. 2012;18:795–804. doi: 10.3136/fstr.18.795. [DOI] [Google Scholar]
- Parkar SG, Stevenson DE, Skinner MA. The potential influence of fruit polyphenols on colonic microflora and human gut health. International Journal of Food Microbiology. 2008;124:295–298. doi: 10.1016/j.ijfoodmicro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Pham VT, Dold S, Rehman A, Bird JK, Steinert RE. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutrition Research. 2021;95:35–53. doi: 10.1016/j.nutres.2021.09.001. [DOI] [PubMed] [Google Scholar]
- Rinschen MM, Ivanisevic J, Giera M, Siuzdak G. Identification of bioactive metabolites using activity metabolomics. Nature Reviews Molecular Cell Biology. 2019;20:353–367. doi: 10.1038/s41580-019-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. The Federation of American Societies of Experimental Biology (FASEB) Journal. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. Journal of Lipid Research. 2016;57:943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A. Structure, function, and plasticity of GABA transporters. Frontiers in Cellular Neuroscience. 2014;8:161. doi: 10.3389/fncel.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sembries S, Dongowski G, Mehrländer K, Will F, Dietrich H. Physiological effects of extraction juices from apple, grape, and red beet pomaces in rats. Journal of Agricultural and Food Chemistry. 2006;54:10269–10280. doi: 10.1021/jf0618168. [DOI] [PubMed] [Google Scholar]
- Shishov VAKT, Kudrin VS, Oleskin AV. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Prikladnaia Biokhimiia i Mikrobiologiia. 2009;45:550–554. [PubMed] [Google Scholar]
- Stumpff F. A look at the smelly side of physiology: Transport of short chain fatty acids. European Journal of Physiology. 2018;470:571–598. doi: 10.1007/s00424-017-2105-9. [DOI] [PubMed] [Google Scholar]
- Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, Kozáková H, Rossmann P, Bártová J, Sokol D, Funda DP, Borovská D, Reháková Z, Sinkora J, Hofman J, Drastich P, Kokesová A. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology Letters. 93: 97-108 (2004) [DOI] [PubMed]
- Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. The American Journal of Clinical Nutrition. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- Vergalito F, Pietrangelo L, Petronio Petronio G, Colitto F, Alfio Cutuli M, Magnifico I, Venditti N, Guerra G, Di Marco R. Vitamin E for prevention of biofilm-caused healthcare-associated infections. Open Medicine. 2018;15:14–21. doi: 10.1515/med-2020-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Current Pharmaceutical Design. 2014;20:1487–1498. doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Guan L, Li J, Lai M, Wen X. The effects of berberine on the gut microbiota in apc min/+ mice fed with a high fat diet. Molecules. 2018;23:2298. doi: 10.3390/molecules23092298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Duffy A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. The Journal of Nutrition. 2017;147:1468S–1475S. doi: 10.3945/jn.116.240754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska M, Willing BP, Bravo DM, Finlay BB. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Scientific Reports. 2015;5:9253. doi: 10.1038/srep09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Molecular Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC. GABA and glutamate transporters in brain. Frontiers in Endocrinology. 2013;4:165. doi: 10.3389/fendo.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Song Y, Liu H, Wu M, Gong H, Lan H, Zheng X. Gut microbiota regulation and anti-inflammatory effect of β-carotene in dextran sulfate sodium-stimulated ulcerative colitis in rats. Journal of Food Science. 2021;86:2118–2130. doi: 10.1111/1750-3841.15684. [DOI] [PubMed] [Google Scholar]


