Abstract
The devH gene was identified in a screen for Anabaena sp. strain PCC 7120 sequences whose transcripts increase in abundance during a heterocyst development time course. The product of devH contains a helix-turn-helix motif similar to the DNA binding domain of members of the cyclic AMP receptor protein family, and the protein is most closely related to the cyanobacterial transcriptional activator NtcA. devH transcripts are barely detectable in vegetative cells and are induced approximately fivefold after nitrogen starvation. This induction is absent in the two developmental mutants hetR and ntcA. The gene is expressed as monocistronic transcripts with multiple 5′ termini, and the ∼500-bp region 5′ to devH was shown to have promoter activity in vivo. The devH gene was insertionally inactivated by the integration of plasmid sequences within the open reading frame. Nitrogen starvation of the devH mutant induces heterocysts of wild-type morphology, but the mutant is inviable in the absence of fixed nitrogen and unable to reduce acetylene aerobically.
The cyanobacteria are a diverse group of prokaryotes that perform oxygenic photosynthesis. A subset of the cyanobacteria are capable of nitrogen fixation, a process that is inhibited by oxygen. These diazotropic cyanobacteria have unique problems, as they not only live aerobically but produce oxygen as a byproduct of photosynthesis. The solution for some of the filamentous cyanobacteria such as Anabaena sp. strain PCC 7120 is the differentiation of the heterocyst, a cell designed for and devoted to nitrogen fixation.
Heterocyst development is induced by starvation for fixed nitrogen. Within about 12 h after nitrogen deprivation, approximately every 10th cell of the filament has become morphologically distinct and committed to the differentiation pathway. Heterocyst maturation is complete by 36 to 48 h after nitrogen step-down, and the cell is terminally differentiated and ceases cell division (reviewed in reference 26).
The development of a vegetative cell into a heterocyst includes a large number of morphological and biochemical alterations that facilitate nitrogen fixation (26). Although our knowledge of heterocyst differentiation has increased dramatically during the past decade, many gaps remain in our understanding of the developmental pathway. In particular, very little is known about how genes are regulated during this process. As an example, virtually nothing is known about how nitrogen fixation genes are activated within the heterocyst cell. Our laboratory is interested in how genes are regulated at the level of transcription during heterocyst differentiation. As a means to identify new genes important to development, as well as to expand our collection of genes for transcriptional studies, we conducted a screen for genomic sequences whose transcripts increase in abundance during differentiation (S. E. Curtis and P. B. Hebbar, unpublished data). One of the sequences identified in the screen, devH, may provide a good model for the study of gene induction during development and may improve our understanding of the regulatory cascade involved in heterocyst development. The predicted DevH amino acid sequence contains a helix-turn-helix domain like those of cyclic AMP receptor protein (CRP) family members (5) and is most similar to the cyanobacterial regulatory protein NtcA (9, 23, 25). In this report, we describe the characterization of the devH gene and show that it is required for heterocyst function.
MATERIALS AND METHODS
Strains and culture conditions.
Strains used in this study are listed in Table 1. Liquid cultures of cyanobacteria were grown in modified Kratz and Meyers (K&M) (12) or BG-11 (17) medium. For growth in fixed nitrogen (+N), K&M medium was supplemented with 2.5 mM (NH4)2SO4 and BG-11 was supplemented with 17.6 mM NaNO3. Agar (1.5%) was added to the medium prior to autoclaving for plate culture. BG-11 (−N) and K&M (−N) lack a source of combined nitrogen. Cultures were grown at 28°C under 80 to 120 microeinsteins of cool white fluorescent lighting per m2 per s. Liquid cultures were shaken and bubbled with air. Neomycin (40 μg/ml) was used for selective growth of strain A57, and spectinomycin and streptomycin (2 μg/ml each) were used for strain AMC236.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| Anabaena spp. | ||
| PCC 7120 | Wild type | R. Haselkorn |
| AMC236 | PCC 7120 ntcA::Ω Spr–Smr cassette | 25 |
| 216 | PCC 7120 hetR | 4 |
| A57 | PCC 7120 devH::pBN1-239B | This study |
| A58 | PCC 7120 703::pPL2-239 | This study |
| A59 | PCC 7120 703::pPL2Δ | This study |
| E. coli | ||
| LE392 | supE44 supF58 hsdR514 galK2 galT22 metB1 trpR55 lacY1 | 18 |
| DH5α | F′ recA φ80dlacZΔM15 | Bethesda Research Laboratories |
| Plasmids | ||
| pBluescript KS(+); pBS | Cloning vector, Apr | Stratagene |
| pBR322 | Cloning vector Apr Tetr | 3 |
| pIC20H | Cloning vector, Apr | 14 |
| pMC1871 | Contains lacZ gene for reporter gene constructs, Tetr | Pharmacia |
| pRL648 | Contains neomycin resistance cassette, Nmr | 8 |
| pRNAseP | 300-bp EcoRI genomic PCR fragment of rnpB in pBS, Apr | This study |
| pAD239 | 240-bp Sau3A1 fragment in pBS, Apr | This study |
| pAD239IP | 0.7-kb PstI-BamHI inverse PCR fragment in pBS, Apr | This study |
| pAD239-1 | 0.5-kb HindIII fragment containing devH in pBS, Apr | This study |
| pAD239-2 | 0.7-kb HindIII fragment containing the 3′ flanking region of devH in pBS, Apr | This study |
| pAD239-3 | 2.5-kb EcoRI fragment containing devH and part of the lambda vector sequence in pBs, Apr | This study |
| pAD239-4 | 1.4-kb HindIII fragment containing the 5′ flanking region of devH in pBS, Apr | This study |
| pPL1A | 0.7-kb HindIII/BamHI fragment of the 703 locus in pBR322, Apr | This study |
| pIC20H-lacZ | 2.9-kb PstI fragment from pMC1971 in pIC20H | This study |
| pPL2A | 2.9-kb HindIII fragment from pIC20H-lacZ in pPL1A, Nmr | This study |
| pPL2AΔ | pPL2A with region between BglII and SmaI sites deleted, Nmr | This study |
| pPL2-239 | 531-bp promoter fragment of devH in pPL2A, Nmr | This study |
| pBN1 | 1.1-kb EcoRI fragment containing the Nmr cassette from pRL648 in pBR322, Nmr | This study |
| pBN1-239B | 0.4-kb HincII fragment from pAD239IP in pBN1, Nmr | This study |
| Oligonucleotides | ||
| 239-1 | 5′ TGG GAT CCA CCA CTC TGC CAA CCA G 3′ | This study |
| 239-2 | 5′ CTG CTG CAG ACA AAT CAA GTT ATC CC 3′ | This study |
| 239-3 | 5′ GCT AGT CGA CAT TGC CCT TGG 3′ | This study |
| 239-B | 5′ TTT GTT ACC TAT GCA AT 3′ | This study |
| 239-C | 5′ TTC GGC TGA AGA CAA AG 3′ | This study |
| 239-E | 5′ GGT TTT TAT GAG GGG CT 3′ | This study |
| 239-R | 5′ GAG AAT TCG CCA GTC GCA GAG CTA 3′ | This study |
| 239-S | 5′ ATG CCC GGG AAC AGT CAT GAA GCT TTA 3′ | This study |
| 239-U | 5′ ATG AGA TCT TGC CAG TCT CAG AGC T 3′ | This study |
| 239-V | 5′ AAA ACC CGG GTG GAG ATT GCA TAG GTA 3′ | This study |
| 239-Y | 5′ CCC TAT GGC AGA TGA CA 3′ | This study |
| RNAP-1 | 5′ GTC AGA ATT CCC TCC CAT ATC CAT T 3′ | This study |
| RNAP-2 | 5′ AGA GAA TTC GTG GTA AGC CGG GTT C 3′ | This study |
For cyanobacterial developmental time courses, cells were grown essentially as previously described (10). A 500-ml culture of mid-log-phase vegetative cells (optical density at 750 nm [OD750] = 0.5) in K&M (+N) was collected by filtration, washed with 500 ml of K&M (−N) and resuspended in 500 ml of K&M (−N). Just before the removal of fixed nitrogen, and at 24 and 48 h after nitrogen step-down, cells from 200 ml of culture were collected by filtration and frozen at −80°C for RNA isolation. Heterocyst development was monitored by microscopy during induction. Proheterocysts and mature heterocysts were apparent by 24 and 48 h after nitrogen step-down, respectively.
In the cyanobacterial growth rate experiments, cultures were monitored spectrophotometrically as the OD750. Cultures of the wild type and strain A57 grown for 5 to 6 days were used as the inoculum for 500-ml cultures in BG-11 (+N) and BG-11 (−N). Strain A57 was grown in the presence and absence of neomycin selection. The BG-11 (+N) cultures were grown for 20 days and the BG-11 (−N) cultures were grown for 20 days. Samples of 3 ml of each culture were removed for optical density measurements at 24-h time intervals. At the end of the growth experiment, the cells were collected for genomic DNA isolation. Photomicrographs were taken with a Zeiss Axioplan microscope using differential interference contrast or bright-field optics. Filaments were stained with 0.015% alcian blue (Sigma, St. Louis, Mo.) for 10 min to stain heterocysts.
Escherichia coli strains DH5α (Bethesda Research Laboratories, Gaithersburg, Md.) and LE392 were the hosts for plasmids and phages, respectively, and were grown in 2YT broth (19) for liquid culture and 2YT solidified with 1.5% agar for plate cultures. Ampicillin and neomycin at 50 and 40 μg/ml, respectively, were used for selective growth.
Acetylene reduction assays.
Liquid cultures were grown in BG-11 and then transferred to BG-11 (−N) for 48 h. The cultures were harvested and suspended to an OD750 of 1.0. A 0.5-ml aliquot of each suspension was placed in a 20-ml stoppered tube and incubated in the light at 28°C in the presence of 10% (vol/vol) C2H2 in air. The accumulation of C2H4, the product of C2H2 reduction, was measured using analytical gas chromatography (Carle AGC 211 flame ionization detector; Shimadzu Corporation, Kyoto, Japan) after 6 h of incubation and according to the manufacturer's instructions. The gas chromatography column was packed with Porapak TR.
Isolation and analysis of nucleic acids.
Total cyanobacterial DNA was isolated as previously described (16), and phage DNA and plasmid DNA were isolated as previously described (18).
DNA sequences were determined by the chain termination method (19) using Sequenase (version 2.0; U.S. Biochemical, Cleveland, Ohio) according to the manufacturer's instructions. Double-stranded sequencing of the appropriate inserts in the clones was performed by using a series of complementary synthetic oligonucleotide primers. Sequence analysis and comparisons were performed using the Sequencher (version 3.0) software package (Gene Code Corporation, Ann Arbor, Mich.). The nucleotide and deduced amino acid sequences were used to search the GenBank database using the National Center for Biotechnology Information BLAST programs (1, 2). Codon usage was analyzed using CodonUse 3.5.3f software (unpublished program by Conrad Halling). Amino acid sequence comparisons were performed using the CLUSTALW program (22) and the BESTFIT program of the Wisconsin package (version 9.0; Genetics Computer Group, Inc.).
Total RNA was isolated from cyanobacterial filaments using the Rapid Total RNA Isolation kit (5 Prime→3 Prime Inc., Boulder, Colo.) according to the manufacturer's instructions. For Northern blots, 10 μg of total RNA was denatured with formaldehyde and formamide, fractionated on 1.5% agarose gel containing 2.2 M formaldehyde, and transferred to Nytran membranes (18).
In the reverse transcriptase PCR (RT-PCR) experiments, first-strand cDNA synthesis was performed using total RNA isolated from cells starved for fixed nitrogen for 24 h and the site-specific primer 239-S or 239-3. Primer sets 239-R–239-S and 239-E–239-S were used with first strand cDNA as the template to amplify PCR products (Table 1).
Primer extension assays were performed and analyzed according to the manufacturer's instructions using the Primer Extension Assay System (Promega Corporation, Madison, Wis.) with 10 μg of total RNA from cells starved for fixed nitrogen for 24 h. Primers used for this assay were 239-C and 239-Y.
Probe preparation, hybridization conditions, and quantitation of transcripts.
32P-labeled random primed probes were prepared using a Rediprime kit (Amersham Pharmacia Biotech, Piscataway, N.J.) according to the manufacturer's instructions. The probe fragments used were (i) for devH, a 0.5-kb HindIII fragment of pAD239-1 and (ii) for rnpB, a 0.3-kb EcoRI fragment of pRNAseP.
Blots were prehybridized in 6× SSC (1× SSC is 0.15 M NaCl and 15 mM sodium citrate [pH 7.0]), 5× Denhardt's reagent (1× Denhardt's reagent is 0.02% [wt/vol] Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% bovine serum albumin) and 0.5% sodium dodecyl sulfate (SDS) at 60°C for 30 min. Hybridizations were performed at 60°C for 12 to 16 h in 6× SSC, 1× Denhardt's reagent, and 0.5% SDS. Filters were washed three times for 20 min each at 60°C with 0.1× SSC–0.5% SDS. For reuse, blots were stripped by boiling in 0.1× SSC–0.5% SDS for 10 min.
Hybridization signals were detected by exposure to Kodak Biomax MR film (Eastman Kodak Co., Rochester, N.Y.) with an intensifying screen for 12 to 16 h at −80°C, or by exposure to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, Calif.) for 2 to 24 h. PhosphorImager screens were scanned on a Molecular Dynamics PhosphorImager, and signals were quantitated using the ImageQuant program (Molecular Dynamics). The rnpB signal was quantitated and used to normalize for loading differences on Northern blots. In the analysis of Northern blots, the normalized probe signal was quantitated and divided by the value before nitrogen step-down (0 h), which was set to 1.0.
Cloning of the devH region and rnpB gene.
Phages, plasmids, and oligonucleotides used in this study are listed in Table 1. The vector pBluescript KS(+) (Stratagene, La Jolla, Calif.) was used for plasmid cloning unless otherwise specified. Clone pAD239 has a 240 bp insert with a partial sequence of devH. Clone pAD239IP has a 0.7-kb PstI-BamHI insert, generated by performing inverse PCR using ligated HincII-digested Anabaena sp. strain PCC 7120 genomic DNA and primers 239-1 and 239-2. Genomic fragments that overlap the devH sequence were isolated from a recombinant phage library of Sau3A1 partial fragments of Anabaena sp. strain PCC 7120 genomic DNA in the lambda vector λ47.1 (10) and were subcloned from lambda clones λ4-1, λ5-1, and λ16-1. The inserts of clones pAD239-1 (0.5-kb HindIII fragment) and pAD239-2 (0.7-kb HindIII fragment) were subcloned from λ4-1, the pAD239-3 insert (2.5-kb EcoRI fragment with λ arm sequences at one end) was subcloned from λ5-1, and the pAD239-4 insert (1.4-kb HindIII fragment) was subcloned from λ 16-1.
The rnpB gene was cloned by PCR amplification of a 300-bp EcoRI fragment (24) from Anabaena sp. strain PCC 7120 genomic DNA using primers RNAP-1 and RNAP-2 (Table 1).
Inactivation of devH.
The devH gene was insertionally inactivated by the homologous recombination of plasmid sequences into the gene. A suicide vector, pBN1, was created by inserting a 1.1-kb EcoRI fragment containing the neomycin resistance cassette from pRL648 (8) into the EcoRI site of pBR322. A 0.4-kb HincII fragment from pAD239IP containing an internal part of the devH gene was inserted into the ScaI site of pBN1 to produce pBN1-239B.
The pBN1-239B plasmid was transferred into Anabaena sp. strain PCC 7120 via conjugation from E. coli using standard procedures (7). Exconjugants were selected and grown in BG-11 (+N) medium containing neomycin. Integration of plasmid sequences within the devH gene was confirmed by Southern blot analysis of genomic DNA.
Construction and assays of promoter fusions.
A plasmid vector designed to assay promoter activity in vivo was constructed by first cloning a 700-bp HindIII-BamHI fragment 3′ to the Anabaena sp. strain PCC 7120 atp1 operon (15), denoted the 703 locus, and a 1.1-kb npt gene from pRL648 into the plasmid pBR322 to yield pPL1A. The 703 region is a silent locus, as it does not contain any open reading frames (ORFs) of more than 50 amino acids, and does not hybridize to cellular transcripts before or after starvation for combined nitrogen (data not shown). A 2.9-kb PstI fragment from plasmid pMC1871 (Pharmacia LKB Biotechnology Inc., Piscataway, N.J.) containing a promoterless lacZ gene was cloned into the PstI site of pIC20H to yield pIC20H-lacZ. The HindIII fragment from pIC20H-lacZ containing the lacZ gene and a portion of the pIC20H multiple cloning site was cloned into the HindIII site of pPL1A to yield pPL2A. The pPL2A plasmid is a suicide vector, as it cannot replicate in Anabaena sp. strain PCC 7120. An additional construct, pPL2AΔ, was made by deleting the region between the BglII and SmaI sites of pPL2A. Constructs were integrated into the chromosome via homologous recombination at the 703 locus.
A fragment containing the first five codons of devH and the 516 bp upstream was generated by PCR amplification of Anabaena sp. strain PCC 7120 genomic DNA with primers 239-U and 239-V. This fragment was cloned into pPL2A which had been digested with BglII and SmaI to yield pPL2A-239. This construction has an in-frame fusion of the first few codons of devH with the lacZ gene, placing lacZ under the transcriptional and translational control signals of devH.
The pPL2A-239 and pPL2AΔ plasmids were transferred into Anabaena sp. strain PCC 7120 via conjugation from E. coli using standard procedures (7). Exconjugants were selected and grown in BG-11 (+N) medium containing neomycin. Intergration of plasmid sequences within the 703 locus was confirmed by Southern blot analysis of genomic DNA.
Strains with integrated pPL2A-239 (A58) and pPL2AΔ (A59) were assayed for β-galactosidase levels by enzyme-linked immunosorbent assay (ELISA) using a β-galactosidase ELISA kit (Bio-Rad Laboratories, Hercules, Calif.) according to the manufacturer's instructions.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the devH and 703 regions are AF242565 and AF24564, respectively.
RESULTS
devH encodes a putative DNA binding protein.
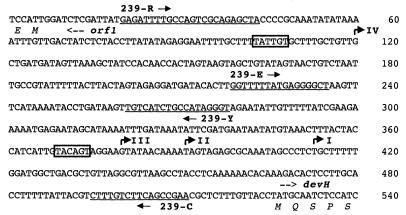
A screen for Anabaena sp. strain PCC 7120 genomic sequences up-regulated after nitrogen starvation identified a number of previously uncharacterized sequences (S. E. Curtis and P. B. Hebbar, unpublished data). One such clone identified in the screen, AD239, was chosen for further study because it hybridizes to transcripts that increase dramatically during development. A 2.5-kb region spanning the 240-bp fragment of AD239 was isolated and sequenced (Fig. 1). The original fragment contains part of a 700-bp ORF designated devH, as determined on the basis of characteristics described in sections below. A divergently oriented partial ORF designated ORF1 initiates ∼500 bp 5′ to devH. Both devH and ORF1 display excellent codon usage for Anabaena sp. strain PCC 7120 (data not shown). The ∼800-bp region 3′ to devH lacks ORFs of more than 50 codons with good codon usage for Anabaena sp. strain PCC 7120.
FIG. 1.
Restriction map of the devH locus and organization of the ORFs. (A) The ORFs and direction of transcription are indicated by the arrows. ORF1 is incomplete. The following restriction sites are indicated as italicized letters: EcoRI (E), HincII (Hc), HindIII (H), and Sau3AI (S). Not all Sau3AI sites are shown. The arrowheads represent primers used for inverse PCR (239-1 and 239-2) or transcript mapping (239-3, 239-B, 239-E, 239-R, and 239-S). (B) Fragments subcloned in pBluescript KS(+).
The sequence of the ORF1 translation product does not show similarity to database sequences. The devH translation product (DevH) has similarity to a family of transcriptional regulators of which the E. coli CRP is the prototype. DevH is most similar to several cyanobacterial proteins of the CRP family, with the closest fit to NtcA (Fig. 2A), a transcriptional activator that acts as a global nitrogen regulator in both heterocystous (9, 25) and nonheterocystous cyanobacteria (23). A devH homolog is absent in the completely sequenced genome of the unicellular, non-nitrogen-fixing cyanobacterium Synechocystis sp. strain PCC 6803 (11).
FIG. 2.
Amino acid sequence alignment using the CLUSTALW alignment program. (A) Amino acid sequence alignment of Anabaena sp. strain PCC 7120 DevH and NtcA proteins. Asterisks indicate identities and dots indicate similarities. (B) Amino acid sequence alignment of the DNA binding domain (helix-turn-helix motif) of DevH, Anabaena sp. strain PCC 7120 NtcA (9, 25), Synechocystis sp. strain PCC 6803 CysR homolog Q55854, Synechococcus sp. strain PCC 7942 CysR (13), Synechococcus sp. strain PCC 7942 transcriptional activator S51093, E. coli CRP (5), and E. coli Fnr (21). Asterisks indicate positions at which residues are completely conserved; plus signs indicate positions at which at least five of the seven residues are identical.
The strongest similarity with CRP family members is observed in the carboxyl-terminal region of the proteins, as shown in Fig. 2B. In particular, the helix-turn-helix domain implicated in DNA binding by CRP (20) is highly conserved between NtcA and DevH. However, there is little similarity between DevH and members of the CRP family outside the carboxyl region. The amino-terminal regions of the proteins in this family have five conserved glycine residues that are associated with a β-roll structure essential for the regulatory properties of CRP (5). DevH lacks these conserved glycine residues as well as CRP residues involved in cyclic AMP binding (5). The cysteine residues present in the amino terminus of E. coli FNR protein, presumably the binding site for metal ions that constitute the oxygen sensor (21), are also absent in DevH.
devH expression is up-regulated during development in the wild type but not in two developmental mutants.
To examine the expression of devH transcripts during development, cultures were starved for fixed nitrogen and samples were collected at 0, 24, and 48 h after nitrogen step-down. Such cultures contain filaments that are a mixture of vegetative cells and developing heterocyst cells. Total RNA samples from across the time course were analyzed with a probe internal to the devH gene. A major devH transcript of 1.0 kb and a less-abundant, 1.25-kb transcript were identified (Fig. 3A). Both transcripts had the same expression profile; each was barely detectable in vegetative cells and strongly induced at 24 and 48 h after nitrogen starvation. The major-to-minor transcript ratio was approximately 5:1. The increase in transcript abundance was apparent by 6 h after nitrogen step-down, the earliest time point examined (data not shown). Quantitation of the levels of the major transcript indicated an approximately fivefold increase in transcript abundance after 24 h of nitrogen step-down (Fig. 3C) and a fourfold increase at 48 h after step-down.
FIG. 3.
(A and B) Northern analysis of devH transcripts during a time course study of heterocyst development in wild-type cells, the ntcA mutant, and the hetR mutant. Samples of total RNA (10 μg) from cells starved for fixed nitrogen for 0, 24, and 48 h were fractionated, blotted, and hybridized with a devH-specific probe (A) and an rnpB-specific probe (B). The same blot was used for both hybridizations. (C) Quantitation of the ∼1.0-kb devH transcript. The transcript level was normalized using the rnpB signal, and then the transcript level was divided by the value for the wild type at 0 h of nitrogen step-down (arbitrarily set to 1.0). The values are the average of three independent experiments. Error bars indicate standard deviations.
devH expression was also analyzed in two developmental mutants, strains 216 (hetR) and AMC236 (ntcA). Both hetR and ntcA are required for heterocyst differentiation. hetR is a regulatory gene found in heterocystous cyanobacteria (4) which encodes a protease (27). ntcA is a universal cyanobacterial global nitrogen regulator which acts as a transcription factor (9, 23, 25). The expression of devH in the mutants before nitrogen step-down was similar to that in the wild type (Fig. 3C); however, the increase in devH transcript abundance after nitrogen starvation observed in the wild type was absent in both mutants (Fig. 3C).
The devH transcription unit is monocistronic.
The devH transcription unit was mapped using RT-PCR. First-strand cDNA was synthesized from total RNA isolated from cells 24 h after nitrogen starvation using primers 239-S and 239-3. Primer 239-S, which maps just 3′ of the devH ORF, yielded cDNA that could be amplified using upstream primers. However, attempts to generate cDNA with primer 239-3, which maps 320 bp 3′ to devH, were unsuccessful. The cDNA generated with primer 239-S was used in amplification reactions with upstream primers to roughly map the 5′ termini of devH transcripts. PCR products were generated from primer sets 239-E–239-S and 239-B–239-S but were not obtained with primer set 239-R–239-S. These results collectively suggest that devH transcripts initiate in the region delimited by primers 239-E and 239-R and terminate in the region delimited by primers 239-S and 239-3 (Fig. 1). Consistent with these data, a probe for the 700-bp HindIII fragment 3′ to devH (Fig. 1) did not detect devH transcripts (data not shown).
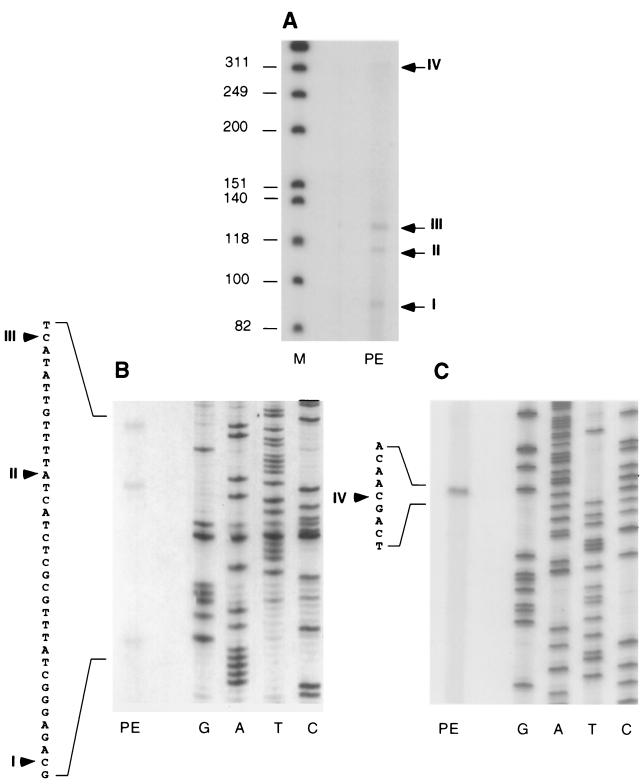
To map potential transcription initiation sites, primer extension analyses were performed with RNA isolated from cells 24 h after nitrogen step-down using two oligonucleotide primers (Fig. 4 and 5). Transcripts with four distinct 5′ termini, designated transcripts I to IV, were detected with primer 239-C (Fig. 4 and 5A and B). Three of the transcripts were of similar abundance, and their termini mapped relatively close together, at 112, 136, and 157 bp from the 5′ end of the devH ORF. The longest and least-abundant transcript was mapped to 406 bp from the 5′ end of devH using primer 239-Y (Fig. 4 and 5C). The 5′ termini of transcripts III and IV mapped 3′ to regions that conform to the −10 consensus sequences for ς70 promoters, but the corresponding −35 consensus sequences are absent (Fig. 4).
FIG. 4.
Nucleotide and amino acid sequence of the devH-ORF1 intergenic region. Primers used for RT-PCR and primer extension assays are underlined and labeled (239-R has a restriction site engineered at the 5′ end). The 5′ transcript termini of devH are shown as bent arrows and are labeled I to IV. Potential ς70 promoter −10 consensus sequences are boxed.
FIG. 5.
Identification of the 5′ termini of devH transcripts. Total RNA (10 μg) from cells grown for 24 h in the absence of fixed nitrogen was hybridized to 32P-end-labeled primers 239-C (A and B) and 239-Y (C). The primer-extended products (PE) were electrophoresed alongside labeled φX174/HincII (M) or a sequence ladder (GATC) generated with the same primer (B and C). The arrowheads indicate the positions where the primer-extended products map on the sequence.
To examine whether any of the devH transcripts identified show a differential pattern of expression across a developmental time course, the primer extension analysis was repeated using RNA samples from cells collected at 0, 12, 24, 36, and 48 h after nitrogen starvation. All four transcripts were identified at each time point in the same relative abundance (data not shown).
It was noted that the major and minor devH transcript species observed on Northern blots differed by ∼250 bp, similar to the distance between the 5′ map sites for transcript IV and transcripts I to III. To investigate this further, a probe was generated for the region between primers 239-R and 239-Y, which is contained only in transcript IV. This probe detected only the 1.25-kb transcript on the Northern blot used in Fig. 3 (data not shown), thus indicating that transcript IV corresponds to the 1.25-kb devH transcript. Consistent with this, the ratio of transcripts I through III to transcript IV was roughly 5:1, similar to the ratio of 1.0- to 1.25-kb transcripts.
devH promoter activity in vivo.
The plasmid pPL2A-239 was constructed to determine if the region 5′ to devH which contains the putative transcription start sites shows promoter activity in vivo. This vector contains a transcriptional fusion between the devH 5′ flanking region and a promoterless lacZ gene in the suicide vector pPL2A and a region of homology with a silent locus in the Anabaena sp. strain PCC 7120 genome which allows integration of promoter fusion constructs into a common chromosomal environment. Strains A58 and A59 with chromosomally integrated copies of pPL2-239 and pPL2AΔ (which lacks a promoter insert), respectively, were grown to mid-log phase and subjected to a nitrogen step-down time course. Protein extracts at each time point (0, 24, and 48 h) were assayed for β-galactosidase protein levels by ELISA. In a representative experiment, the levels of β-galactosidase were barely detectable in strain A59. Strain A58 showed a threefold increase in β-galactosidase levels at 24 and 48 h (approximately 135 ng/mg of total cellular protein) after nitrogen step-down relative to levels at 0 h (approximately 46.6 ng/mg of total cellular protein).
devH is required for heterocyst function.
To investigate the role of the devH gene in heterocyst development, the wild-type devH gene was insertionally inactivated by the integration of plasmid sequences within the gene. A suicide vector, pBN1-239B, which contains an internal fragment of the devH gene and a neomycin resistance marker (Fig. 6A), was transferred into Anabaena sp. strain PCC 7120, and neomycin-resistant colonies were selected. Genomic DNA analyzed from one of the neomycin-resistant strains, A57, contained restriction fragments of the sizes predicted from an insertion of the plasmid within the devH sequences via a single homologous recombination (Fig. 6B). In a HindIII digest of DNA from strain A57, a devH probe detected a 4.3-kb fragment in addition to the 700-, 500-, and 200-bp fragments seen in the wild type (Fig. 6B). This 4.3-kb fragment contains one copy of the inactivated devH gene and a portion of the pBN1-239B plasmid (Fig. 6A). In an EcoRI digest strain of A57 DNA, a 4.6-kb fragment is observed in addition to the >5.0-kb wild-type fragment. In a HincII digest, the 0.7-kb wild-type band is replaced by two fragments of 500 bp and 3.9 kb in strain A57. Anabaena sp. strain PCC 7120 cells contain multiple copies of a single chromosome. The results presented in Fig. 6 demonstrate that mutagenesis had gone to completion and that all chromosomal copies of devH were interrupted.
FIG. 6.
Insertional inactivation of the devH gene. (A) Map of the devH locus, pBN1-239C, and the site of insertion. Arrows indicate the orientation of gene transcription. (B) Southern analysis of the genomic DNA from the wild-type strain (lanes 1 to 3) and strain A57 (lanes 5 to 7). Samples digested with HindIII (H), EcoRI (E), and HincII (Hc) were hybridized to the 0.7-kb PstI-BamHI fragment from pAD239IP.
RNA was isolated from strain A57 after nitrogen starvation for 0, 24, and 48 h. No devH transcripts were detectable at any stage of the developmental time course, indicating that the gene interruption leads to a loss of devH transcript accumulation (data not shown). The same RNA samples were analyzed for expression of the ntcA and hetR transcripts. Each of these genes showed a wild-type expression profile during development in the mutant A57 (data not shown).
The growth characteristics of the devH disruption mutant were examined in medium with and without fixed nitrogen, in combinations with and without antibiotic selection. The devH mutant had growth characteristics similar to those of the wild type in the presence of fixed nitrogen, with or without antibiotic selection (Fig. 7A). However, when the devH mutant was starved for fixed nitrogen in medium containing neomycin, the filaments bleached and then died (Fig. 7B). Regularly spaced cells with the morphology of heterocysts were apparent by 24 h after nitrogen step-down (Fig. 8E). Like the wild type, these cells stain with alcian blue (Fig. 8F), a dye specific for the polysaccharride layer of the heterocyst envelope. Unlike the wild type, however, the A57 heterocysts did not appear to form the polar granules characteristic of mature heterocysts (Fig. 8C). An unusual feature observed in some of the A57 heterocysts is a structure that resembles the septum of a dividing cell (Fig. 8G and H).
FIG. 7.
Growth of mutant strain A57 and the wild type in BG11 (+N) (A) and BG11 (−N) (B). (C) Southern analysis of the genomic DNA from cells after the growth experiments. Samples were digested with HindIII and HincII and hybridized to the 0.7-kb PstI-BamHI fragment from pAD239IP. Asterisks indicate the 0.7-kb wild-type diagnostic fragment.
FIG. 8.
Light microscopy of Anabaena cultures. Wild type (WT) (A) and A57 (B) grown in the presence of fixed nitrogen. (C and D) WT cells grown in the absence of fixed nitrogen and stained with alcian blue. (E to H) A57 grown in the absence of fixed nitrogen and stained with alcian blue. (A to C and E) Differential interference contrast images; (D and F to H) bright-field images. Solid arrowheads indicate heterocysts in the WT and heterocyst-like cells in the A57 strain; open arrows indicate heterocyst-like structures in the A57 strain which appear to have septa.
The appearance of heterocysts in the devH mutant after nitrogen starvation suggested that the defect in the devH mutant might lie in the ability to fix nitrogen. To test the ability of the mutant to fix nitrogen aerobically, acetylene reduction assays were conducted with the wild type and with A57 after each had been starved for fixed nitrogen for 48 and 72 h. These studies showed that the mutant was unable to reduce acetylene to ethylene in the presence of air (less than 2% of the wild-type nitrogenase activity).
In contrast to the results obtained with A57 grown in the presence of antibiotic selection, when the devH mutant was starved for fixed nitrogen in the absence of neomycin, the cells initially grew slowly and then bleached (Fig. 7B). After about 12 days of nitrogen starvation, green cells appeared and cell division resumed (Fig. 7B). At the end of the growth experiment, cells with the morphology of mature heterocysts were observed at regular intervals along the filament (data not shown).
Genomic DNA was isolated from the devH mutant at the end of the growth experiments and analyzed with regard to the status of the devH gene interruption (Fig. 7C). The plasmid sequence that interrupts the devH gene appeared to be stably maintained throughout culturing in both the presence and absence of antibiotic when fixed nitrogen was contained in the medium (Fig. 7C). However, when the mutant was cultured on medium lacking fixed nitrogen and lacking neomycin, a mixture of wild-type devH and interrupted devH mutant DNA fragments was observed. This is most readily seen in the HincII digest of DNA from A57 grown in the absence of fixed nitrogen and neomycin (Fig. 7C), in which the 0.7-kb fragment diagnostic of the wild type is apparent. Thus, under the pressure of nitrogen starvation and in the absence of antibiotic selection, some of the chromosomes lose the plasmid interruption in devH and apparently restore wild-type activity.
DISCUSSION
The devH gene was identified in a screen for sequences whose transcripts increase in abundance during heterocyst development (S. E. Curtis and P. B. Hebbar, unpublished data). The DevH protein has a DNA binding motif characteristic of members of the CRP family and is most closely related to the NtcA proteins of cyanobacteria. NtcA has been shown to be a transcriptional activator and global nitrogen regulator in cyanobacteria (23) and is required for heterocyst development (9, 25). The helix-turn-helix domains near the C termini of CRP family members are involved in DNA binding. NtcA and DevH have very similar sequences in the helix-turn-helix domain, particularly in the second helix. In CRP, the second helix is the recognition helix and fits into the major groove of DNA at the binding site (5). Thus one might expect the DNA sequences of the sites to which NtcA and DevH bind to be very similar.
The devH transcripts are expressed at very low levels in vegetative cells and induced approximately fivefold following nitrogen step-down. There is excellent agreement between the devH transcript mapping data and Northern analyses. The data are consistent with two classes of devH monocistronic transcripts (transcripts I through III and transcript IV) of different abundances which terminate in the 220-bp region 3′ to devH and differ in length by approximately 250 bp at their 5′ termini. Primer extension analyses of transcripts across the developmental time course did not reveal the appearance of new transcripts after nitrogen starvation. Thus, the increase in transcript abundance during development likely does not derive from the use of new promoters, as has been shown for the glnA and gnd genes of Anabaena sp. strain PCC 7120 (6). The number of devH transcripts identified is not unusual, as many cyanobacterial genes have been shown to have transcripts with multiple 5′ termini (6). The ∼500-bp region 5′ to devH was shown to have promoter activity in vivo and to contain the elements necessary for induction after nitrogen starvation. Characterization of the devH promoter(s) and other cis elements involved in regulation will require additional promoter assays in vivo.
The devH expression pattern during a developmental time course study and the absence of a devH homolog in Synechocystis sp. strain PCC 6803 suggested that the devH gene product may be involved in heterocyst development or nitrogen fixation. A devH mutant produces heterocyst-like cells after nitrogen starvation but cannot reduce acetylene aerobically. The inability to fix nitrogen is correlated with the absence of expression of the nif structural genes (nifHDK operon) in the devH mutant after nitrogen step-down (data not shown). The devH gene is not induced in the ntcA and hetR mutant backgrounds, suggesting that it functions downstream of these genes in the genetic hierarchy. The effect of the ntcA mutation on devH may be indirect, as the ntcA mutation abolishes hetR expression (9). The devH gene was given its designation based on its product, expression pattern, and the phenotype of the mutant, which suggest that DevH is involved in some unknown development-specific function. Unlike an ntcA mutant (9, 25), the devH mutant produces heterocyst-like cells, and thus devH may not be involved in the structural development of heterocysts. The very low level of devH transcripts in vegetative cells and the abundance of these transcripts by 6 h after nitrogen starvation suggest that the gene acts relatively early in development in a heterocyst-specific manner. Given that DevH has characteristics of a DNA binding protein, it is likely that it controls the expression of the gene(s) required for heterocyst development and function. The identification of these putative targets and the role of DevH will be the focus of future experiments.
ACKNOWLEDGMENTS
We thank Pat Ligon and Dawn Chasse for technical assistance, Jim Mahaffey for performing the microscopy, and Royden Saah and Paul Bishop for providing equipment and advice for the acetylene reduction assays.
This work was supported by NSF grants DMB-9019039 and MCB-9507490. P.B.H. was supported in part by a Graduate Assistance in Areas of National Need (GAANN) Fellowship in Biotechnology from the Department of Education, administered by the N.C. State University Graduate School.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolivar F, Rodriguez R, Greene P, Betlach M, Heyneker H, Boyer H. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 4.Buikema W J, Haselkorn R. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 1991;5:321–330. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- 5.Crothers D M, Steitz T A. Transcriptional activation by Escherichia coli CAP protein. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 501–534. [Google Scholar]
- 6.Curtis S E, Martin J A. The transcription apparatus and the regulation of transcription initiation. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer; 1994. pp. 613–639. [Google Scholar]
- 7.Elhai J, Wolk C P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- 8.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the non viability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 9.Frias J E, Flores E, Herrero A. Requirement of the regulatory protein NtcA for the expression of nitrogen assimilation and heterocyst development genes in the cyanobacterium Anabaena sp. PCC 7120. Mol Microbiol. 1994;14:823–832. doi: 10.1111/j.1365-2958.1994.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 10.Golden J W, Robinson S J, Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985;314:419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko T, Tabata S. Complete genome structure of the unicellular cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 1997;38:1171–1176. doi: 10.1093/oxfordjournals.pcp.a029103. [DOI] [PubMed] [Google Scholar]
- 12.Kratz W A, Meyers J. Nutrition and growth of several blue-green algae. Am J Bot. 1955;42:282–287. [Google Scholar]
- 13.Laudenbach D E, Grossman A R. Characterization and mutagenesis of sulfur-regulated genes in a cyanobacterium: evidence for function in sulfate transport. J Bacteriol. 1991;173:2739–2750. doi: 10.1128/jb.173.9.2739-2750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh J, Enfle M, Wykes E. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984;32:481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 15.McCarn D F, Whitaker R A, Alam J, Vrba J M, Curtis S E. Genes encoding the alpha, gamma, delta, and four F0 subunits of ATP synthase constitute an operon in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1988;170:3448–3458. doi: 10.1128/jb.170.8.3448-3458.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice D, Mazur B J, Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982;257:13157–13163. [PubMed] [Google Scholar]
- 17.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Genetic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Sanger F, Air G M, Barrell B G, Brown N L, Coulson A R, Fiddes C A, Hutchison C A, Slocombe P M, Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977;265:687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- 20.Savery N J, Lloyd G S, Kainz M, Gaal T, Ross W, Ebright R H, Gourse R L, Busby S J. Transcription activation at class II CRP-dependent promoters: identification of determinants in the C-terminal domain of the RNA polymerase alpha subunit. EMBO J. 1998;17:3439–3447. doi: 10.1093/emboj/17.12.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiro S, Guest J R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990;6:399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- 22.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vega-Palas M, Flores E, Herrero A. NtcA, a global nitrogen regulator from the cyanobacterium Synechococcus that belongs to the Crp family of bacterial regulators. Mol Microbiol. 1992;6:1853–1859. doi: 10.1111/j.1365-2958.1992.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 24.Vioque A. Analysis of the gene encoding the RNA subunit of ribonuclease P from cyanobacteria. Nucleic Acids Res. 1992;20:6331–6337. doi: 10.1093/nar/20.23.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei T F, Ramasubramanian T S, Golden J W. Anabaena sp. strain PCC 7120 ntcA gene required for growth on nitrate and heterocyst development. J Bacteriol. 1994;176:4473–4482. doi: 10.1128/jb.176.15.4473-4482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolk C P. Heterocyst formation. Annu Rev Genet. 1996;30:59–78. doi: 10.1146/annurev.genet.30.1.59. [DOI] [PubMed] [Google Scholar]
- 27.Zhou R, Wei X, Jiang N, Li H, Zhao J. Evidence that HetR is an unusual serine-type protease. Proc Natl Acad Sci USA. 1998;95:4959–4963. doi: 10.1073/pnas.95.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]