Abstract
Cerebral blood flow (CBF) decreases across the adult lifespan; however, more studies are needed to understand the underlying mechanisms. This study measured CBF and cerebrovascular resistance (CVR) using a multimodality approach in 185 healthy adults (21–80 years). Color-coded duplex ultrasonography and phase-contrast MRI were used to measure CBF, CBF velocity, and vessel diameters of the internal carotid (ICA) and vertebral arteries (VA). MRI arterial spin labeling was used to measure brain perfusion. Transcranial Doppler was used to measure CBF velocity at the middle cerebral artery. Structural MRI was used to measure brain volume. CBF was presented as total blood flow (mL/min) and normalized CBF (nCBF, mL/100g/min). Mean arterial pressure was measured to calculate CVR. Age was associated with decreased CBF by ∼3.5 mL/min/year and nCBF by ∼0.19 mL/100g/min/year across the methods. CVR increased by ∼0.011 mmHg/mL/100g/min/year. Blood flow velocities in ICA and VA decreased with age ranging from 0.07–0.15 cm/s/year, while the vessel diameters remained similar among age groups. These findings suggest that age-related decreases in CBF can be attributed mainly to decreases in blood flow velocity in the large cerebral arteries and that increased CVR likely reflects the presence of cerebral vasoconstrictions in the small cerebral arterioles and/or capillaries.
Keywords: Age, cerebral blood flow, cerebrovascular resistance, magnetic resonance imaging, ultrasonography
Introduction
A sufficient and continuous blood supply of oxygen, nutrients, and energy substrate (i.e., glucose) to the brain is necessary to maintain normal neuronal function.1–4 In this regard, brain hypoperfusion has been increasingly recognized as a risk factor for age-related cognitive decline and Alzheimer’s disease and related dementias (ADRD).1–5 Indeed, a prospective, population-based study with a mean follow-up of 6.9 years reported that lower cerebral blood flow (CBF) at baseline was related to higher risk of developing dementia. 6 Similar findings were also reported in other studies.2,3 Therefore, a better understanding of age-related CBF changes and the underlying regulatory mechanisms in the context of ADRD prevention is essential for preserving brain health in old age.1,3,7,8
Age-related reduction of CBF has been reported extensively in previous studies, which may reflect either a reduction of cerebral metabolism,9–11 cerebrovascular dysfunction, or both.12–15 One hypothesis in support of cerebrovascular dysfunction is that age-related central arterial stiffening associated with increases in arterial pulsation (i.e., arterial pressure and blood flow pulsatility) 16 may expose cerebral arterioles and capillaries to augmented mechanical stress, thus leading to cerebral endothelial dysfunction, vasoconstriction, increases in cerebrovascular resistance (CVR), and decreases in CBF in older adults. 17
Of note, a longitudinal study in older adults with and without clinical diagnosis of Alzheimer’s disease (AD) showed that increases in CVR preceded reductions in CBF, and that the increases in CVR were able to predict the onset of clinical AD independent of alterations of cerebral metabolism. 18 This study highlights the importance of the calculation of CVR based on the measurement of blood pressure (BP) and CBF in the assessment of cerebrovascular function since CBF is influenced by BP despite the presence of cerebral autoregulation.1,19 At present, little information is available on CVR across the healthy adult lifespan12,13,15,20
CBF and CBF velocity have been measured using various non-invasive imaging modalities. 19 For example, phase-contrast magnetic resonance imaging (PC-MRI) and color-coded duplex ultrasonography (CDUS) have been used to measure both volumetric blood flow and blood flow velocity at the brain-feeding extracranial arteries [i.e., the internal carotid (ICA) and vertebral (VA) arteries].21–23 Furthermore, transcranial Doppler (TCD) has been used widely to measure blood flow velocity at the intracranial arteries [e.g., the middle cerebral artery (MCA)] to reflect changes in CBF24. Finally, MRI arterial spin labeling (ASL) has been used to measure both global and regional CBF. 25 Previous studies of age-related CBF reduction using these modalities have reported inconsistent findings, which may be ascribed to either the differences in the study populations or differences in the methodology itself or both.21,26–28 In most of the previous studies, age-related changes or differences in CBF were often measured by using only one of the aforementioned modalities,10,21,23,27–31 and it is difficult to compare these studies directly because of the different methods used for CBF measurement.13,32 Thus, measurement of CBF using a multimodality approach in the same cohort of subjects would provide significant insights into the differences or consistency of the methodologies used in the study of age effects on CBF and CBF velocity.
Furthermore, simultaneous measurements of CBF velocity and vessel diameters of the ICA and VA make it possible to determine whether age-related differences in CBF are determined mainly by alterations in blood flow velocity or the vessel diameter or both. Since large cerebral arteries such as the ICA and VA contribute importantly to the overall CVR, these measurements will also provide insight into whether age-related differences in CVR are related mainly to the vasoconstriction of the downstream small cerebral arterioles and/or capillaries.1,33
Therefore, the purpose of the present study was to characterize age-related differences in CBF, CBF velocity, CVR (based on simultaneous measurement of CBF and BP), and the ICA and VA diameter using a multimodality approach including 2D ultrasonography, PC-MRI, TCD, and ASL across the adult lifespan in healthy subjects aged between 21 and 80 years. We hypothesized that advanced age is associated with decreased CBF, CBF velocity, and increased CVR, and that different modalities used would show a similar magnitude of decreases in CBF and CBF velocity with age.13,32
Materials and methods
Participants
One hundred ninety-three healthy participants aged between 21 and 80 years were recruited through flyers and newspaper advertisements from the Dallas-Fort Worth metropolitan areas. Exclusion criteria were the presence of ischemic or structural heart disease screened by 12-lead electrocardiogram (ECG) and echocardiography, office BP >140/90 mmHg confirmed by ambulatory BP monitoring, carotid artery atherosclerotic plaque or stenosis with >50% occlusion imaged by ultrasonography, diabetes mellitus screened by the presence of symptoms, use of antidiabetic drugs, or fasting blood glucose >126 mg/dL, body mass index (BMI) >35 kg/m2, current cigarette smoking or history of cigarette smoking within the last two years, 34 excessive alcohol use or drug abuse, 35 history of brain trauma, the presence or history of cerebrovascular (e.g., stroke), neurological, psychiatric, or inflammatory disease, pregnant or breastfeeding women, and subjects with metal devices, plates or pins in their bodies that preclude them from MRI or having claustrophobia. To minimize the confounding effects of aerobic exercise training on CBF, 36 individuals who participate in structured aerobic exercise training programs (i.e. moderate-intensity, aerobic exercise training programs over 90 minutes per week over the past two years) were also excluded.
This study was approved by the Institutional Review Board (IRB) of the University of Texas Southwestern (UTSW) Medical Center and Texas Health Presbyterian Hospital Dallas and was performed in accordance with the guidelines of the Declaration of Helsinki and Belmont Reports. All subjects provided informed written consent prior to participation.
Data availability
The data from this study are available upon request from qualified investigators to the research team according to the data sharing policy and the UTSW IRB regulations.
Study design
Data collection of MRI and ultrasonography were performed on two separate days of visits within a time interval of 2–3 months to accommodate participant’s personal need while making the time interval between the study visits as short as possible. Participants were instructed to abstain from caffeinated beverages, alcohol, and vigorous exercise for >24 hours before the visits.
MRI data collection and analysis
MRI data were collected by a 3T MRI system (Philips Medical Systems, Best, the Netherlands) using an 8-channel transmit/receive head coil. Four imaging sequences were performed. First, 3D magnetization-prepared rapid acquisition gradient echo (MPRAGE) images were acquired with the following parameters: TR = 8.1 ms, TE = 3.7 ms, FA = 12°, FOV = 256 ×256 mm, number of slices = 160 (no gap), resolution = 1 × 1 × 1 mm3, SENSE factor = 2, and scan duration = 4 minutes. Seconds, a 3D time-of-flight (TOF) MR angiographic image of neck vessels was collected with the following parameters: repetition time (TR) = 23 ms, echo time (TE) = 3.5 ms, flip angle (FA) = 18°, field of view (FOV) = 160 × 160 mm, number of slices = 47, resolution = 0.3 × 0.3 × 1.5 mm3, a venous saturation slab placed above the imaging slab, and scan duration = 1 minute 15 seconds. An imaging plane for 2D PC-MRI was determined using the coronal and sagittal maximum-intensity-projections of the TOF MR angiographic image. Care was taken to select the imaging plane at the straight and perpendicular portion of vessels above the bifurcations of ICA and external carotid artery and below the bends of VA. Third, non-ECG gated PC-MRI was collected four times at different stages of the cardiac cycle (time interval = 6.5 seconds) and averaged to generate a single image. 32 The acquisition parameters of PC-MRI were as follows: TR = 20 ms, TE = 6.9 ms, FA = 15°, FOV =230 × 230 mm, resolution = 0.45 × 0.45 mm2, slice thickness = 5 mm, maximum velocity encoding (VENC) = 80 cm/s, and scan duration = 30 seconds. PC-MRI data were acquired after 10 minutes in a resting stage in the supine position. Fourth, pseudo-continuous ASL (pCASL) data were acquired with multi-slice single-shot 2-D echoplanar imaging (EPI) sequence (label duration = 1,650 ms, post labeling delay (PLD) = 1,525 ms, time of repetition = 4,260 ms, echo time = 14 ms, EPI factor = 35 ms, voxel size = 3 × 3 × 5 mm, field of view = 240 × 240 × 145 mm, slice number = 29). A total of 40 pairs of control and labeled images were acquired to average and increase the signal-to-noise ratio.
PC-MRI data were analyzed using the region of interest (ROI) method described in detail elsewhere. 37 Briefly, ROIs were manually drawn on the bilateral ICAs and VAs using the magnitude images. Vessel masks generated from the magnitude image were then applied to the phase image to obtain mean blood flow velocity in each of the arteries. CBF was calculated by multiplying mean blood flow velocity by the area of the corresponding vessels and added together to obtain total CBF. Total CBF was corrected for individual differences in brain size and expressed as blood flow (mL/min) per 100 grams of brain tissue. Brain volume was converted into brain mass by assuming a density of 1.06 g/mL. 38 Total brain volume was calculated by adding the volumes of the cerebrum, cerebellum, and brain stem (FreeSurfer, https://surfer.nmr.mgh.harvard.edu/fswiki). Individual total brain volume and gray matter (GM) and white matter (WM) volumes were divided by intracranial volume to obtain normalized values. All data analyses were performed blind to the clinical and personal information of participants.
The pCASL was processed by the FMRIB Software Library (FSL version 5.0, Oxford University, UK; https://www.fmrib.ox.ac.uk/fsl). Cerebral perfusion was calculated according to the recommended procedure of pCASL processing. 39 After pre-processing steps, individual perfusion maps were registered to their native MPRAGE space, and then cerebral perfusion data were extracted from 1) the cerebral cortex and 2) the global brain, including cortical and subcortical GM and WM by the FreeSurfer’s cross-sectional analysis pipeline (i.e., recon-all).
Ultrasonography data collection and analysis
All data were collected in an environmental control laboratory with an ambient temperature of ∼22°C. After participants rested in the supine position for at least 15 minutes, images and hemodynamics data were acquired. Brachial cuff BP and beat-by-beat arterial pressure waveforms from the right brachial artery were measured intermittently at least three times using an ECG-gated electro-sphygmomanometer (Suntech, Morrisville, NC, USA) and applanation tonometry (SphygmoCor 8.0; AtCor Medical, West Ryde, NSW, Australia). The beat-by-beat brachial arterial pressure waveform was calibrated to brachial systolic BP (SBP) and diastolic BP (DBP), and then mean arterial pressure (MAP) was calculated from the area under the curve of the brachial arterial pressure waveforms. Heart rate (HR) was monitored using a 3-lead ECG (Hewlett-Packard, Palo Alto, CA, USA). CBF velocity was obtained from the right MCA using TCD (Multi-Dop X2: Compumedics/DWL, Singen, Germany). A 2-MHz TCD probe was securely attached to the temporal bone acoustic window by using either an individually created mold to fit the facial bone structure or a probe holder (Spencer Technologies, Seattle, WA, USA) to keep the position and angle of the probe unchanged during the study. The insonation depth and angle were adjusted to optimize the signal quality according to a standard procedure. 24 End-tidal CO2 (EtCO2) was recorded using capnography (Capnograd; Novamatrix, Wallingford, CT, USA). During data collection, subjects were instructed to breathe normally through the nose for EtCO2 monitoring. To ensure stable hemodynamics throughout data collection, continuous BP monitoring was performed using a finger photoplethysmogram (Finapres; Ohmeda, Boulder, CO, USA). All the physiological variables were continuously recorded with data acquisition software (Acknowledge, BIOPAC Systems, Goleta, CA, USA).
Blood flow velocity and diameter of the ICA and VA were measured using a 3–12 MHz linear array transducer with an ultrasonography system (CX50, Philips Ultrasound, Bothell, WA, USA). ICA and VA images were obtained from both sides. At least 3 clips of duplex ultrasonography images were obtained to measure time-averaged mean velocity (TAMV) of the ≥5 complete and consecutive cardiac cycles from both the ICA and VA to calculate CBF. CBF pulsatility indexes (PI) at the MCA (via TCD), ICA, and VA (via CDUS) were calculated as systolic minus diastolic CBF velocity divided by mean CBF velocity. 40 Total CBF was calculated as a sum of blood flow measured from the bilateral ICAs and VAs using ultrasonography.22,32 Volumetric blood flow in each artery was calculated by the following equation: TAMV ×(Diameter2/2) × π × 60. TAMV from ≥15 complete cardiac cycles were extracted from duplex ultrasonographic images. The time-averaged arterial diameter was obtained from ultrasonographic images with a vessel length of ≥5 mm (Vascular Tool 5; Medical Imaging Applications, Coralville, IA) at the same location where averaged CBF velocity were measured. The procedures used to obtain the time-averaged CBF velocity and vessel diameter for total CBF calculation were taken to reduce the intrinsic CBF variability associated with respiration or other low-frequency physiological oscillations. 41 Normalized CBF (nCBF) was calculated by the following equation: nCBF = total CBF (mL/min)/brain tissue mass (100 g). Mean CBF velocity at MCA was averaged for at least 5 minutes. CVR was calculated by MAP divided by total CBF or nCBF, and CVR index (CVRi) was calculated by MAP divided by CBF velocity at the MCA. CVR was not calculated from PC-MRI data because BPs were not collected during the MRI scan. Recently, we have used this method to measure CBF in the ICAs and VAs and observed a coefficient of variation of ∼4.7% between the repeated measurements. 22 Representation of images of measurement and analysis of CBF using PC-MRI, ASL, CDUS, and TCD are shown in Figure S1.
Statistical analysis
We first conducted age groups [young (range 21–45 years), middle-aged (range 46–65 years), and old (range 66–80 years)] by sex (men or women) data analysis. Chi-square test was used to examine the age group differences in the categorical variables. Two-way analysis of variance (ANOVA) was used to test the main effects of age and sex, as well as the interaction effect of age and sex on demographic and physiological variables. After observing no notable interaction effect on hemodynamic variables, we used one-way ANOVA to focus upon age group differences. The Bonferroni method was used to correct for multiple pairwise comparisons. One-way analysis of covariance (ANCOVA) was used to examine the effects of interaction of age and sex on the age-related differences (slope) in CBF, CBF velocity, CBF pulsatility, and CVR. Next, Pearson product-moment correlation coefficient analysis was used to examine simple correlations among age and different modalities of CBF measurements. Comparisons between PC-MRI and CDUS measurements in the ICA and VA and between the left- and right-side measurements from the same vessels using the same methods were performed using paired t-test. Bland-Altman plots were used to examine the agreement of volumetric blood flow in the VA and ICA assessed by PC-MRI and CDUS. 42 Data normality was checked by the Shapiro-Wilk test and the visual inspection of histogram and Q-Q plots. A two-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, IL, USA).
Results
Data from 8 subjects were excluded from the analysis of MRI because of the low quality of images. Furthermore, 24 out of 193 subjects (12%) could not obtain TCD signals. This resulted in the final sample of 185 subjects in MRI and CDUS analysis and 169 subjects in TCD analysis. The average time between the study visits was 25 ± 22 days.
Participant characteristics
Participants’ demographic characteristics grouped in age and sex are presented in Table 1. The distributions of men and women, years of education, and MMSE score were similar across the age groups. Use of BP and cholesterol medications increased with age. SBP and MAP increased with age, whereas DBP peaked in middle age, and then decreased in the old group. Women had lower BPs compared with men. The absolute value of intracranial volume, total brain volume and GM and WM volumes were smaller in women than those in men, but these were reversed after normalized by intracranial volume. Total brain volume, GM, and WM volumes were decreased with age.
Table 1.
Participants' characteristics, systemic hemodynamics, and brain volume.
| Young |
Middle age |
Old |
p-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Men | Women | Men | Women | Men | Women | Age | Sex | Age × Sex |
| n | 21 | 31 | 26 | 42 | 21 | 44 | 0.634 | ||
| Age (years) | 35 ± 8 | 33 ± 7 | 56 ± 6 | 59 ± 5 | 71 ± 4 | 71 ± 5 | <0.001 | 0.566 | 0.026 |
| Height (cm) | 177 ± 7 | 163 ± 6 | 176 ± 8 | 163 ± 9 | 174 ± 8 | 162 ± 6 | 0.353 | <0.001 | 0.704 |
| Body mass (kg) | 81 ± 10 | 61 ± 11 | 88 ± 14 | 70 ± 11* | 83 ± 13 | 69 ± 11 | 0.001 | <0.001 | 0.337 |
| BMI (kg/m2) | 26 ± 3 | 23 ± 3 | 28 ± 4 | 26 ± 5* | 27 ± 4 | 26 ± 4* | <0.001 | 0.002 | 0.422 |
| Education (years) | 16 ± 2 | 16 ± 2 | 16 ± 2 | 16 ± 2 | 17 ± 2 | 16 ± 2 | 0.538 | 0.097 | 0.737 |
| MMSE (score) | 29 ± 1 | 29 ± 1 | 29 ± 1 | 29 ± 1 | 29 ± 1 | 29 ± 1 | 0.949 | 0.211 | 0.777 |
| Medication use | |||||||||
| Calcium-channel blockers | 0 (0%) | 0 (0%) | 5 (8%) | 0.008 | |||||
| Beta blocker | 0 (0%) | 0 (0%) | 0 (0%) | – | |||||
| ARBs | 0 (0%) | 1 (1%) | 5 (8%) | 0.037 | |||||
| ACE inhibitors | 0 (0%) | 7 (10%) | 12 (18%) | 0.004 | |||||
| Diuretics | 0 (0%) | 1 (1%) | 7 (11%) | 0.006 | |||||
| Statins | 2 (4%) | 11 (16%) | 22 (34%) | <0.001 | |||||
| Race (W/B/A) | (37/8/7) | (59/6/3) | (63/1/1) | 0.002 | |||||
| Systemic hemodynamics | |||||||||
| Heart rate (bpm) | 64 ± 8 | 67 ± 10 | 64 ± 9 | 63 ± 8 | 64 ± 7 | 64 ± 7 | 0.464 | 0.574 | 0.366 |
| SBP (mmHg) | 113 ± 8 | 105 ± 9 | 116 ± 12 | 112 ± 10* | 122 ± 12 | 117 ± 12*† | <0.001 | 0.001 | 0.509 |
| MAP (mmHg) | 86 ± 7 | 80 ± 7 | 90 ± 9 | 88 ± 8* | 92 ± 9 | 87 ± 10* | <0.001 | <0.001 | 0.507 |
| DBP (mmHg) | 72 ± 8 | 64 ± 6 | 74 ± 8 | 72 ± 7* | 74 ± 9 | 67 ± 8 | 0.004 | <0.001 | 0.185 |
| Brain volume | |||||||||
| ICV (mL) | 1660 ± 144 | 1464 ± 139 | 1679 ± 131 | 1419 ± 122 | 1738 ± 182 | 1457 ± 125 | 0.216 | <0.001 | 0.217 |
| Total volume (mL) | 1380 ± 101 | 1275 ± 101 | 1357 ± 115 | 1187 ± 83* | 1301 ± 100 | 1169 ± 73* | <0.001 | <0.001 | 0.167 |
| (%ICV) | 83 ± 3 | 87 ± 4 | 81 ± 3 | 84 ± 4* | 75 ± 6 | 81 ± 6*† | <0.001 | <0.001 | 0.256 |
| Gray matter (mL) | 812 ± 64 | 744 ± 56 | 780 ± 60 | 678 ± 47* | 739 ± 54 | 673 ± 45*† | <0.001 | <0.001 | 0.108 |
| (%ICV) | 49 ± 2 | 51 ± 2 | 46 ± 2 | 48 ± 3* | 43 ± 4 | 46 ± 4*† | <0.001 | <0.001 | 0.163 |
| White matter (mL) | 531 ± 59 | 483 ± 50 | 538 ± 62 | 465 ± 49 | 527 ± 56 | 454 ± 41 | 0.245 | <0.001 | 0.349 |
| (%ICV) | 32 ± 2 | 33 ± 2 | 32 ± 2 | 33 ± 2* | 30 ± 2 | 31 ± 2*† | <0.001 | 0.006 | 0.942 |
BMI: body mass index; MMSE: Mini-Mental State Examination; ARBs: angiotension receptor blockers; ACE: angiotension converting enzyme; W: white; B: black; A: Asian; bpm: beats per minute; BP: blood pressure; SBP: systolic BP; MAP: mean arterial pressure; DBP: diastolic BP, ICV: intracranial volume. The volume of total brain, gray matter, and white matter were normalized to the ICV (ICV%). Data are mean ± standard deviation. Bold values represent p < 0.05. Results from one-way analysis of variance p < 0.05 * vs young; p < 0.05 † vs. middle age.
Age- and sex-related differences in cerebral hemodynamics
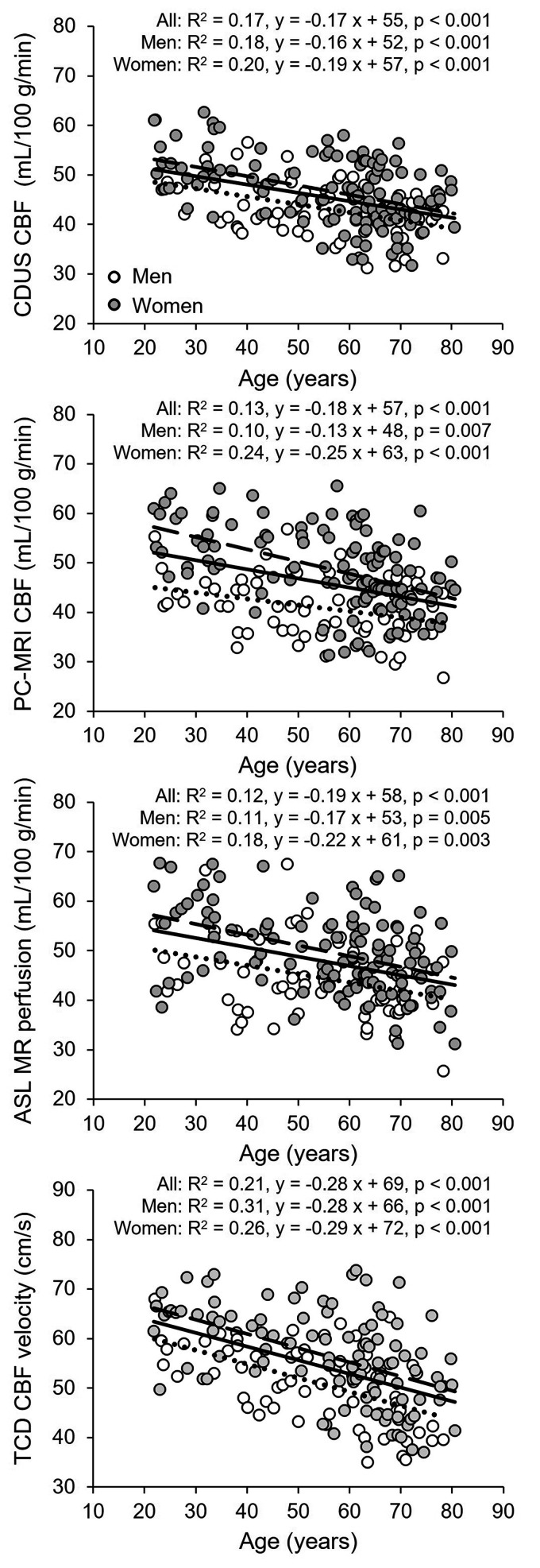
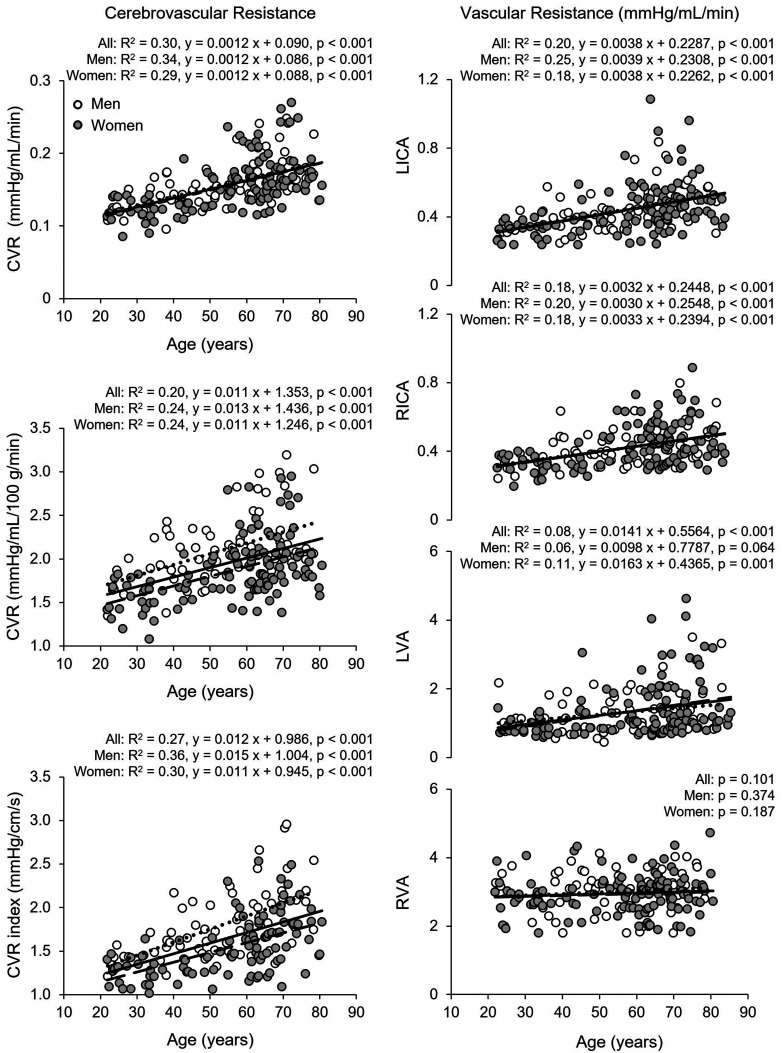
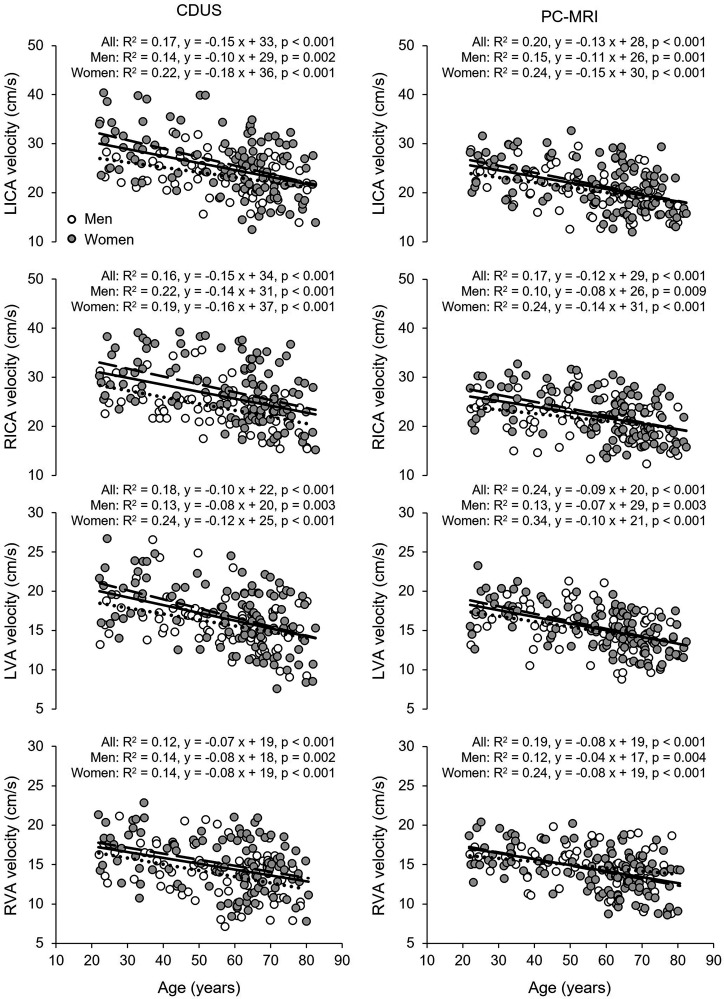
Total CBF and nCBF were lower and CBF pulsatility and CVR was higher in both the middle age and old groups than in the young group (Table 2 and Table S1). The old group also showed lower EtCO2 relative to the young group. Women had higher nCBF and CBF velocity measured at the MCA and lower CVR and CVRi than men (Table 2). Total CBF decreased linearly with age at a rate of 3.6 mL/min per year measured with CDUS and 3.4 mL/min per year with PC-MRI (Figure S2). nCBF also decreased linearly with age at a rate of 0.17 mL/100 g/min per year measured with CDUS, 0.18 mL/100 g/min per year with PC-MRI, and 0.19 mL/100 g/min per year with ASL associated with a reduction in CBF velocity of 0.28 cm/s per year measured at the MCA (Figure 1). CVR estimated from MAP and nCBF increased linearly with age at a rate of 0.011 mmHg/mL/100 g/min per year associated with an increase in CVRi of 0.012 mmHg/cm/s per year measured at the MCA (Figure 2). Vascular resistance of both the ICA and left VA also increased linearly with age (Figure 2). Corresponding to the inceases in CVR, CBF pulsatility in the MCA, ICA, and VA all increased linearly with age (Figure S3). Volumetric blood flow (Table S2) and blood flow velocity in the ICAs and VAs were lower in the middle age and old groups than those in the young group, whereas the vessel diameters were similar across the age groups (Table 3). Blood flow velocity in the VA and ICA measured by CDUS and in the ICA measured by PC-MRI was higher in women than men (Table 3). Blood flow velocity decreased linearly with age ranging from 0.07 to 0.15 cm/s per year (Figure 3). Of note, there are no interactions of age and sex in the results of one-way ANCOVA, suggesting that the rates of decreases in CBF and blood flow velocity and increases in CBF pulsatility and CVR with age were similar among women and men (Figures 1 to 3, Figure S2 and S3).
Table 2.
Cerebral hemodynamics measurements using CDUS, MRI, and TCD.
| Young |
Middle age |
Old |
p-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Men | Women | Men | Women | Men | Women | Age | Sex | Age × Sex |
| Color-coded duplex ultrasonography: CDUS | |||||||||
| Total CBF (mL/min) | 633 ± 76 | 654 ± 81 | 583 ± 82 | 544 ± 85* | 533 ± 73 | 516 ± 76*† | <0.001 | 0.316 | 0.125 |
| ICA (mL/min) | 482 ± 80 | 494 ± 73 | 441 ± 72 | 406 ± 70* | 396 ± 66 | 385 ± 73*† | <0.001 | 0.301 | 0.223 |
| VA (mL/min) | 151 ± 32 | 160 ± 31 | 143 ± 39 | 137 ± 46 | 137 ± 40 | 131 ± 38* | 0.018 | 0.885 | 0.542 |
| nCBF (mL/100 g/min) | 46 ± 6 | 51 ± 6 | 43 ± 6 | 46 ± 7* | 41 ± 5 | 44 ± 6* | <0.001 | <0.001 | 0.479 |
| CVR (mmHg/mL/min) | 0.14 ± 0.02 | 0.12 ± 0.02 | 0.16 ± 0.03 | 0.17 ± 0.03* | 0.18 ± 0.03 | 0.17 ± 0.04* | <0.001 | 0.520 | 0.165 |
| nCVR (mmHg/mL/100 g/min) | 1.90 ± 0.32 | 1.57 ± 0.21 | 2.13 ± 0.36 | 1.95 ± 0.33* | 2.28 ± 0.40 | 2.02 ± 0.37* | <0.001 | <0.001 | 0.479 |
| ICA (mmHg/mL/min) | 0.18 ± 0.04 | 0.16 ± 0.03 | 0.21 ± 0.04 | 0.22 ± 0.05* | 0.24 ± 0.05 | 0.24 ± 0.06*† | <0.001 | 0.707 | 0.183 |
| VA (mmHg/mL/min) | 0.60 ± 0.17 | 0.52 ± 0.12 | 0.69 ± 0.25 | 0.74 ± 0.34* | 0.76 ± 0.30 | 0.74 ± 0.28* | <0.001 | 0.598 | 0.443 |
| Phase contrast magnetic resonance imaging: PC-MRI | |||||||||
| Total CBF (mL/min) | 603 ± 73 | 692 ± 97 | 548 ± 89 | 578 ± 112* | 513 ± 80 | 521 ± 72*† | <0.001 | 0.003 | 0.063 |
| ICA (mL/min) | 461 ± 67 | 513 ± 74 | 411 ± 71 | 440 ± 82* | 389 ± 73 | 400 ± 65* | <0.001 | 0.008 | 0.365 |
| VA (mL/min) | 142 ± 36 | 179 ± 37 | 137 ± 40 | 138 ± 53* | 125 ± 37 | 121 ± 39* | <0.001 | 0.080 | 0.024 |
| nCBF (mL/100 g/min) | 44 ± 5 | 54 ± 7 | 41 ± 7 | 49 ± 9* | 39 ± 6 | 45 ± 6* | <0.001 | <0.001 | 0.133 |
| Arterial spin labeling: ASL (mL/100 g/min) | |||||||||
| Cortex perfusion | 47 ± 9 | 55 ± 8 | 46 ± 8 | 49 ± 8 | 41 ± 10 | 49 ± 10* | 0.002 | <0.001 | 0.212 |
| Global brain perfusion | 43 ± 8 | 52 ± 7 | 43 ± 7 | 47 ± 8 | 39 ± 9 | 46 ± 9* | 0.003 | <0.001 | 0.242 |
| Transcranial Doppler: TCD | |||||||||
| CBF velocity (cm/s) | 55 ± 6.2 | 62 ± 6.3 | 52 ± 7.4 | 57 ± 9.0* | 46 ± 8.5 | 51 ± 8.7*† | <0.001 | <0.001 | 0.692 |
| CVRi (mmHg/cm/s) | 1.59 ± 0.26 | 1.29 ± 0.16 | 1.78 ± 0.34 | 1.57 ± 0.32* | 2.07 ± 0.39 | 1.74 ± 0.32*† | <0.001 | <0.001 | 0.516 |
| PI (a.u) | 0.79 ± 010 | 0.80 ± 0.12 | 0.87 ± 0.12 | 0.90 ± 0.09* | 1.00 ± 0.10 | 1.03 ± 0.11*† | <0.001 | 0.241 | 0.786 |
| EtCO2 (mmHg) | 39 ± 3 | 38 ± 4 | 39 ± 3 | 38 ± 3 | 36 ± 4 | 37 ± 5* | 0.007 | 0.367 | 0.677 |
CBF: cerebral blood flow; ICA: internal carotid artery; VA: vertebral artery; nCBF: normalized total CBF by total brain mass. CVR: cerebrovascular resistance; nCVR, normalized CVR. GM: gray matter; WM: white matter; CVRi: CVR index; PI: pulsatility index; EtCO2: end-tidal CO2. Data are mean ± standard deviation. Bold values represent p < 0.05. Results from one-way analysis of variance p < 0.05 * vs young; p < 0.05 † vs. middle age. p < 0.05 * vs young; p < 0.05 † vs. middle age. Global brain perfusion was calculated by gray and white matter perfusion. No calculation of CVR for PC-MRI and ASL due to no blood pressure assessment on the visit or during MRI.
Figure 1.
Association of age with cerebral blood flow (CBF) measured by color-coded duplex ultrasonography (CDUS), Continued.phase-contrast MRI (PC-MRI), arterial spin labeling (ASL), and CBF velocity measured by transcranial Doppler (TCD) at the middle cerebral artery. Solid line, dotted line, and dashed lines represent the regression equations obtained for all subjects, men, and women, respectively. The rates of decreases in CBF and blood flow velocity were similar between women and men (p-values of the interaction of age and sex in one-way analysis of covariance: CDUS CBF, p = 0.085; PC-MRI CBF, p = 0.635; ASL, p = 0.970; TCD CBF velocity, p = 0.933).
Figure 2.
Association of age with cerebrovascular resistance (CVR) measured by color-coded duplex ultrasonography (CDUS) (left upper and middle panels), CVR index measured by transcranial Doppler (TCD) at the middle cerebral artery (left lower panel), and Continued.vascular resistance in the left (L) and right (R) internal carotid (ICA) and vertebral arteries (VA) (right panels). CVR was calculated as mean arterial pressure divided by total CBF (upper panel) and normalized CBF (middle panel). Solid line, dotted line, dashed lines represent the regression equations obtained for all subjects, men, and women, respectively. The rates of increases in CVR and CVR index were similar between women and men (p-values of the interaction of age and sex in one-way analysis of covariance: CVR (left upper panel) p = 0.678; CVR (left middle panel) p = 0.868; CVR index (left lower panel) p = 0.206; vascular resistance LICA p = 0.986; RICA p = 0.789; LVA p = 0.261; RVA p = 0.893).
Table 3.
Mean blood flow velocity and vessel diameter using CDUS and PC-MRI.
| Young |
Middle age |
Old |
p-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Men | Women | Men | Women | Men | Women | Age | Sex | Age×Sex |
| Color-coded duplex ultrasonography (CDUS) | |||||||||
| Velocity (cm/s) | |||||||||
| Left-ICA | 26 ± 4 | 30 ± 6 | 23 ± 4 | 26 ± 6* | 22 ± 5 | 23 ± 5* | <0.001 | 0.001 | 0.180 |
| Right-ICA | 26 ± 4 | 31 ± 5 | 25 ± 5 | 27 ± 6* | 21 ± 5 | 24 ± 5*† | <0.001 | <0.001 | 0.289 |
| Left-VA | 18 ± 3 | 20 ± 3 | 16 ± 3 | 17 ± 3* | 15 ± 4 | 15 ± 4*† | <0.001 | 0.023 | 0.430 |
| Right-VA | 15 ± 3 | 17 ± 3 | 13 ± 4 | 15 ± 4* | 13 ± 3 | 14 ± 3* | <0.001 | 0.008 | 0.949 |
| Diameter (mm) | |||||||||
| Left-ICA | 4.5 ± 0.4 | 4.2 ± 0.5 | 4.4 ± 0.5 | 4.1 ± 0.5 | 4.3 ± 0.4 | 4.2 ± 0.4 | 0.529 | <0.001 | 0.505 |
| Right-ICA | 4.4 ± 0.4 | 4.1 ± 0.3 | 4.4 ± 0.4 | 4.0 ± 0.6 | 4.6 ± 0.5 | 4.2 ± 0.6 | 0.117 | <0.001 | 0.582 |
| Left-VA | 3.1 ± 0.5 | 3.1 ± 0.4 | 3.3 ± 0.7 | 3.1 ± 0.5 | 3.2 ± 0.7 | 3.0 ± 0.7 | 0.683 | 0.096 | 0.561 |
| Right-VA | 3.0 ± 0.6 | 2.9 ± 0.6 | 2.9 ± 0.6 | 2.9 ± 0.5 | 3.2 ± 0.6 | 3.0 ± 0.6 | 0.054 | 0.301 | 0.697 |
| Phase contrast magnetic resonance imaging (PC-MRI) | |||||||||
| Velocity (cm/s) | |||||||||
| Left-ICA | 23 ± 4 | 25 ± 4 | 20 ± 4 | 22 ± 5* | 19 ± 4 | 19 ± 4* | <0.001 | 0.050 | 0.322 |
| Right-ICA | 23 ± 4 | 26 ± 4 | 22 ± 4 | 23 ± 5* | 20 ± 4 | 20 ± 4*† | <0.001 | 0.036 | 0.096 |
| Left-VA | 16 ± 2 | 18 ± 2 | 16 ± 4 | 16 ± 2* | 14 ± 2 | 14 ± 2*† | <0.001 | 0.146 | 0.096 |
| Right-VA | 16 ± 2 | 16 ± 2 | 15 ± 2 | 14 ± 3* | 14 ± 3 | 13 ± 3* | <0.001 | 0.360 | 0.320 |
| Diameter (mm) | |||||||||
| Left-ICA | 4.7 ± 0.5 | 4.7 ± 0.4 | 4.7 ± 0.5 | 4.7 ± 0.6 | 4.7 ± 0.5 | 4.7 ± 0.4 | 0.966 | 0.811 | 0.919 |
| Right-ICA | 4.7 ± 0.4 | 4.6 ± 0.4 | 4.5 ± 0.4 | 4.5 ± 0.5 | 4.5 ± 0.5 | 4.7 ± 0.5 | 0.316 | 0.549 | 0.678 |
| Left-VA | 3.1 ± 0.5 | 3.4 ± 0.4 | 3.2 ± 0.7 | 3.2 ± 0.5 | 3.1 ± 0.6 | 3.0 ± 0.6 | 0.136 | 0.462 | 0.112 |
| Right-VA | 2.9 ± 0.6 | 3.2 ± 0.5 | 2.8 ± 0.5 | 2.9 ± 0.6 | 2.9 ± 0.6 | 2.9 ± 0.6 | 0.113 | 0.117 | 0.534 |
ICA: internal carotid artery; VA: vertebral artery. Data are mean ± standard deviation. Bold values represent p < 0.05. Results from one-way analysis of variance p < 0.05 * vs young; p < 0.05 † vs. middle age. p < 0.05 * vs young; p < 0.05 † vs. middle age.
Figure 3.
Association of age with blood flow velocity measured by color-coded duplex ultrasonography (CDUS) (left panels) and phase-contrast MRI (PC-MRI) (right panels) in the left (L) and right (R) internal carotid (ICA) and the vertebral (VA) arteries. Solid line, dotted line, and dashed lines represent the regression equations obtained for all subjects, men, and women, respectively. The rates of decreases in blood flow velocity in the ICA and VA were similar between women and men (p-values of the interaction of age and sex in one-way analysis of covariance: CDUS (left panels) LICA, p = 0.129; RICA, p = 0.622; LVA, p = 0.224; RVA, p = 0.933; PC-MRI (right panels) LICA, p = 0.296; RICA, p = 0.121; LVA, p = 0.222; RVA, p = 0.262).
Correlations among CBF measurements
The correlations of CBF measured by CDUS, MRI, and TCD are presented in Table 4. Total CBF measured by CDUS and PC-MRI showed higher correlation than that assessed by ASL. The correlations of nCBF measurements were similar among these three methods. CBF velocity measured at the MCA by TCD showed low to moderate correlations with total CBF and nCBF measured by CDUS, PC-MRI, and ASL (Table 4). CBF velocity measured at the right MCA by TCD was correlated with the right ICA blood flow velocity (R2 = 0.22, p < 0.001), and volumetric blood flow (R2 = 0.24, p < 0.001) measured by CDUS. CBF PI at the right MCA by TCD was correlated with PI at right ICA measured by CDUS (R2 = 0.38. p < 0.001).
Table 4.
The correlations between CBF measurements by CDUS, MRI, and TCD.
| CDUS |
PC-MRI |
ASL |
TCD |
||||
|---|---|---|---|---|---|---|---|
| Total CBF | nCBF | Total CBF | nCBF | Cortex | Global brain | CBF velocity | |
| CDUS | |||||||
| Total CBF | 0.819 | 0.629 | 0.400 | 0.300 | 0.271 | 0.461 | |
| (mL/min) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | |
| nCBF | 0.497 | 0.565 | 0.423 | 0.427 | 0.503 | ||
| (mL/100 g/min) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | ||
| PC-MRI | |||||||
| Total CBF | 0.872 | 0.536 | 0.520 | 0.515 | |||
| (mL/min) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | |||
| nCBF | 0.624 | 0.637 | 0.515 | ||||
| (mL/100 g/min) | (<0.001) | (<0.001) | (<0.001) | ||||
| ASL | |||||||
| Cortex | 0.990 | 0.318 | |||||
| (mL/100 g/min) | (<0.001) | (<0.001) | |||||
| Global brain | 0.311 | ||||||
| (mL/100 g/min) | (<0.001) | ||||||
Data are Pearson’s product-moment correlation coefficients and (P-values). CDUS: color-coded duplex ultrasonography; CBF: cerebral blood flow; PC-MRI: phase contrast magnetic resonance imaging; ASL: arterial spin labeling; TCD: transcranial Doppler. Global brain perfusion was calculated by gray matter and white matter perfusion.
When the measurements from all age groups were pooled together, blood flow velocities in both the ICAs and the left VA measured with PC-MRI were significantly lower relative to those measured with CDUS (Table S3). Measurements of the ICA diameter with PC-MRI were significantly larger compared to those with CDUS, whereas no difference was observed in the VA (Table S3). No differences in volumetric blood flow were observed in either the ICA or VA between methods (Table S3). Blood flow measurements in all four arteries were correlated between methods (Figure S4). Blood flow measurements were lower by ∼18% in the right VA than that in the left VA regardless of the methods used (Table S3). Finally, Bland-Altman plots show that differences in blood flow measurements between PC-MRI and CDUS were not systemically biased (Figure S5). However, the wide dispersion of mean differences ( ± 1.96 SD) showed the presence of large individual variability of CBF measurements between the methods.
Discussion
This study investigated the age-related differences in CBF and CVR using MRI, ultrasonography, and TCD across the healthy adult lifespan. The main findings from the present study are as follows. First, advanced age was associated linearly with decreased CBF and increased CBF pulatility and CVR from the age of 20’s to 80s’, and the rates of decreases in CBF and increases in CBF pulatility and CVR were similar between women and men. Second, blood flow velocities measured at the ICAs, VAs, and MCA also decreased linearly with age, while the vessel diameters of the ICAs and VAs remained similar among the age groups. Of note, women had higher CBF and blood flow velocity and lower CVR than men. Third, measurements of total CBF and nCBF were correlated among CDUS, PC-MRI, and ASL, and measurements of blood flow velocity at the ICA, VA, and MCA were correlated between CDUS, PC-MRI, and TCD despite the presence of large individual differences.
Collectively, these findings, using a multimodality approach, suggested the presence of a decline of global brain perfusion with age, which can be attributed mainly to the decreases in blood flow velocity in the large cerebral arteries. Furthermore, increases in CBF pulatility and CVR with age suggest the presence of cerebral vasoconstriction, which likely occurs in the small cerebral arterioles and capillaries but not in the large cerebral arteries.
Age-related differences in cerebral hemodynamics
Age-related CBF alterations have been investigated extensively using various non-invasive imaging modalities. There is a general agreement that CBF declines linearly with age from early adulthood. 43 For instance, nCBF measured by PC-MRI decreased by 0.08 mL/100 g/min per year, 27 and cerebral cortical perfusion measured by ASL decreased by 0.11-0.36 mL/100g/min per year23,29,30 in healthy adults. Further, Buijs et al. reported that total CBF measured by PC-MRI as a sum of blood flow from the ICAs and the basilar artery decreased by 4.6 mL/min per year in those with no indication of cerebrovascular disease, 21 and Scheel et al. reported total CBF measured by CDUS from the bilateral ICAs and VAs decreased by 2.9 mL/min per year in healthy adults. 28 Our results are consistent with these previous studies, as we observed nCBF decreased by ∼0.18 mL/100 g/min and total CBF decreased by ∼3.5 mL/min per year in the healthy adult lifespan between 21 and 80 years (averaged rates across the measurement methods used).
The mechanisms of age-related reductions in CBF are not fully understood, and are likely to be multifactorial. 1 The potential factors include decreased cerebral metabolic rate associated with brain atrophy, thus a decreased demand of CBF with age9–11 or cerebrovascular dysfunction,12,13,15 or both. Advanced age decreases cerebral metabolic rate for oxygen consumption (CMRO2) and glucose utilization by ∼0.6% per year from early adulthood, and these reductions were associated with decreases in CBF.9,11 However, age-related increases in CMRO2 were also reported in previous studies.8,27 These discrepancies are likely related to the differences in the meausement methods (positron emission tomography scan vs. MRI) or the study population, or both.8,9,11,27
On the other hand, vascular aging per se may contribute to decreased CBF17. In this regard, central arterial stiffening associated with aging increases SBP and MAP, while DBP peaks around middle age and decreases in older age. 16 As a consequence, cerebral blood vessels, particularly the arterioles and capillary vascular beds, are exposed to augmented arterial pulsations 17 which may lead to cerebral endothelial dysfunction and increased vascular tone and vasoconstriction, thereby increasing CVR and decreasing CBF. 17 The elevated CBF pulsatility in the ICA, VA, and MCA with age observed in this study support this hypothesis. Alternatively, even though brain metabolic rate for oxygen or glucose utilization would remain unchanged, the presence of neurovascular decoupling may lead to vasoconstriction and inceases in CVR. 27
In this context, according to Poiseuille’s law, vascular resistance is inversely proportional to the fourth power of its radius. Thus, the diameter of a small cerebral arterioles and capillaries decreases (vasoconstriction) would lead to a significant increase in vascular resistance and reductions in blood flow. In this study, we observed that global CVR calculated from simultaneous measurments of BP and CBF using 2D ultrosonography increased by ∼0.011 mmHg/mL/100 g/min per year associated with significant reductions in blood flow at the ICAs and VAs and reductions of CBF velocity at the MCA. Interestingly, the vessel diameters measured at the ICA and VA remain similar among the age groups consistent with previous studies.28,31 These findings suggest that even though large cerebral arteries including the ICA and VA contribute importantly to the global CVR, 33 CBF decreases with age observed in this study are likely due to the vasoconstriction/reductions of the vessel diameters of small cerebral arterioles and capillary beds rather than the diamter changes of the large cerebral arteries.4,33 Furthermore, the observed reductions of blood flow velocity at the ICAs, VAs, and MCA with age suggest that the possibility of reduction of downstream blood flow velocity in the cerebral arterioles and capillary beds, which in turn may lead to reduced shear stress on endothelial cells and impaired flow-mediated vasodilation. 1 These vascular changes then would increase CVR and reduce CBF, formulating a vicious circle. 1
The lower resting EtCO2 we observed in older adults is consistent with previous findings.44,45 In a meta-analysis of age and blood acid-base equilibrium, arterial partial pressure CO2 (PaCO2), which is reflected by EtCO2, decreased by ∼3 mmHg in healthy subjects aged between 20 and 80 years. 45 Another study also demonstrated that EtCO2 reduced by ∼3 mmHg in older adults compared with young individuals. 44 It is not known whether and to what extent age-related reduction of EtCO2 or PaCO2 may contribute to increases in CVR and reduction in CBF.
Sex-related differences in cerebral hemodynamics
Women tend to have higher CBF levels than men,23,27,29,30 while some studies reported no sex-related differences.21,28 This discrepancy may be related to the methodological differences used to quantify CBF. For example, in a few, but not all of previous CBF studies with PC-MRI or CDUS, total CBF (mL/min) calculated as a sum of volumetric blood flow from the brain feeding arteries (i.e. the ICAs and VAs) showed no sex-related differences.21,28 However, normalization of total CBF by brain tissue mass (mL/100 g/min) have consistently shown higher CBF levels in women than men23,27,29,30 consistent with the sex-related differences in total CBF and nCBF observed in the present study. Our study extended these previous observations by showing that women also had lower CVR than men (Table 2). Interestingly, women also had higher blood flow velocity in the ICA and MCA than men, which may have contributed to the observed higher nCBF. However, it is also likely that these higher blood flow velocities were related to the smaller ICA diameters observed in women (Tables 2 and 3).
The biological mechanisms for the sex-related differences in CBF and CVR are unclear. Sex-related differences in cerebral metabolic rate, hematocrit, and sex hormones all may play a role. Women have higher levels of cerebral metabolic rate for glucose 46 and oxygen 23 and lower hematocrit 47 than men. All these factors can result in increases in CBF for the following reasons. First, higher CBF is needed to meet the higher glucose or oxygen utilization demand in women than men. Second, reduced oxygen-carrying capacity (due to lower hematocrit) would result in higher CBF to supply the brain with necessary oxygen. Third, lower hematocrit is associated with reduced blood viscosity which by itself may lead to higher blood flow velocity, higher CBF, or both. Further, differences in sex hormones may also contribute to the differences in CBF and CVR observed between men and women.48,49 Briefly, it has been shown that elevated blood estrogen level increased CBF by enhancing endothelial-derived nitric oxide and prostacyclin pathways leading to reduced cerebrovascular tone and vasodilation, while testosterone increases cerebrovascular tone. 48 The extent to which each of these factors may have contributed to the observed higher nCBF and lower CVR in women needs to be determined in future studies.
Methodological considerations
Understanding the agreement or differences among the modalities used to measure CBF is essential for data interpretation of our comparisons between different studies. Previous studies reported weak to moderate correlations between CBF measurement methods. For instance, Dolui et al. compared CBF measured by PC-MRI and ASL in 436 healthy adults and found on average about 34% of the ASL variance could be explained by PC-MRI. 50 Ambarki et al. reported 53% ASL variance could be explained by PC-MRI, but there was a significant difference in the CBF values between the two methods. 51 Tarumi et al. showed ∼31% of the mean CBF variability measured at the MCA using TCD could be explained by nCBF measured by PC-MRI13. In the present study, weak to moderate correlations among the modalities were also observed (Table 4). About 10–40% of one modality variance could be explained by other modalities, but a large amount of residual variability (60–90%) remains which could reflect either the methodological differences in CBF measurement or the influenence of spotaneous dynamic CBF oscillations presented at the different measurement times or both.19,41 Taken together, despite the consistency among the modalities used in the present study for revealing the age and sex-related differences in CBF and CBF velocity in the same subjects, caution is warranted when interpreting and comparing the results of CBF from the studies using different modalities in different study populations.
The comparison of CDUS with PC-MRI and ASL for CBF measurement is important because ultrasonography can be used under less demanding clinical conditions than MRI and other imaging modalities. 19 In this aspect, we previously reported the agreement between PC-MRI and CDUS among 31 healthy subjects. 32 Consistent with our previous study, we observed measurements of volumetric blood flow in the ICA and VA with CDUS are comparable to and correlated with PC-MRI. We also observed differences in blood flow velocity and vessel diameter measurement between the modalities in the previous study, which are confirmed in the present study (Table S3). These findings may relate to the differences in the imaging planes used for PC-MRI and CDUS. Indeed, the opposite differences between blood flow velocity and vessel diameter measurements were observed in the ICA but not in the VA (Table S3). This finding also suggests that VA morphology is likely to be more uniform along the cervical spine, whereas ICA is not. Notably, the observed lower blood flow in the right VA than the left side, regardless of the methods used, is also consistent with previous findings (Table S3).32
Clinical perspective
There is no cure for ADRD at present; 52 therefore, early prediction and prevention of ADRD is critical. Emerging evidence shows links between cerebrovascular dysfunction and the progression of ADRD.1,3,5 For example, brain hypoperfusion may be an early and strong pathophysiologic factor associated with ADRD development followed by cerebral metabolism, brain atrophy, and functional impairment. 53 A prospective, population-based study with a mean follow-up of 6.9 years found that lower CBF at baseline was related to higher risk for future onset of AD6. In addition, a 2-year follow-up study found that higher CVR at baseline accelerated cognitive declines. 18 In this regard, a better understanding of age-related CBF and CVR changes is essential for differentiating normal from pathophysiologic alterations in brain perfusion, structure, and function. 54
Strengths and limitations
There are several strengths in our study. First, CDUS and TCD ultrasonography used to measure CBF and CBF velocity are noninvasive and readily applicable in primary clinical settings to assess age, sex, or disease-related alternation of CBF or CBF velocity. Second, measurement of volumetric CBF in the ICA and VA using ultrasonography with simultaneous measurements of MAP allowed us to estimate CVR accurately, which may be difficult to implement using technologies such as MRI. Third, our study sample was vigorously screened to exclude major cerebral and cardiovascular disease to minimize the potential effects of other confounding factors on CBF and CVR, such as stroke, uncontrolled hypertension, history of cigarette smoking, and diabetes. 1 The major limitation was the cross-sectional nature of the present study; the age-related differences in CBF and CVR observed in this study only suggest but cannot imply its causal relation. A longitudinal study, even if it can be performed only in a short time priod of several years is likely to be critical to examine the role of increases in CVR and CBF pulsatility in age-related reduction of CBF. In this study, we used a fast (<2 min) and non-gated PC-MRI to measure mean CBF because of its potential advantage for clinical use. 8 However, this method cannot reveal pulsatile changes in blood flow velocity. In this aspect, time-resolved CINE PC-MRI may provide further information on arterial pulsation in both extracranial and intracranial arteries, which is a potential risk factor for cerebrovascular dysfunction. 55 In addition, we used a single PLD of 1,525 ms for pCASL data acquisition which may not be long enough to obtain reliable meaurement of white matter perfusion. 56 Recent development in the pCASL technology may improve measurement of white matter perfusion in future studies. 56 Nonetheless, the observed age differeneces of whole brain perfusion measured with pCASL were consistent with those of 2D ultrasonography and PC-MRI suggesting the relaibility of using pCASL to measure whole brain perufsion in this study. 39 Finally, similar to other studies of age-related differences or effects on CBF, we cannot dissect the direct effects of antihypertensive and cholesterol medication use on CBF (Table 1). However, recent systematic review and meta-analyses suggest that potential effects of these medications on CBF in otherwise healthy older adults are likely to be minimal.57–59
Conclusion
This study demonstrated that advanced age is associated with decreased CBF and increased CVR and CBF pulsatility across the healthy adult lifespan. Further, women had higher CBF and blood flow velocity and lower CVR than men. In addition, blood flow velocity at the ICA, VA, and MCA all were decreased with age, whereas the diameter of the ICA and VA remained similar among the age groups. We also observed that the age- and sex-related differences and the magnitude of CBF decline were similar among the measurements using CDUS, PC-MRI, and ASL and that the measurements of CBF using these modalities had weak to moderate correlations despite the presence of large individual differences. Collectively, these findings suggest that advanced age is associated with decreases in CBF which can be attributed mainly to the decreases of blood flow velocity in the large cerebral arteries. Furthermore, advanced age is associated with increases in CVR, which may reflect the presence of cerebral vasoconstriction in the small cerebral arterioles and/or capillaries but not in the large cerebral arteries.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231153741 for Cerebral blood flow and cerebrovascular resistance across the adult lifespan: A multimodality approach by Tsubasa Tomoto, Marilyn Lu, Ayaz M Khan, Jie Liu, Evan P Pasha, Takashi Tarumi and Rong Zhang in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors thank all our study participants for their willingness, time, and effort devoted to this study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Institute of Health (R01AG033106 and R01HL102457).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: T Tomoto wrote the manuscript; ML AMK, EPP, T Tarumi and RZ edited the manuscript. RZ designed research; AMK, JL, T Tarumi, and RZ performed or directed data collection; T Tomoto, ML, AK, JL, and T Tarumi analyzed data. All the authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
ORCID iD: Tsubasa Tomoto https://orcid.org/0000-0001-5936-0332
Supplementary material: Supplemental material for this article is available online.
References
- 1.Claassen J, Thijssen DHJ, Panerai RB, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev 2021; 101: 1487–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan W, Sehrawat P, Balachandrasekaran A, et al. Cerebral Blood flow is associated with diagnostic class and cognitive decline in Alzheimer's disease. J Alzheimers Dis 2020; 76: 1103–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hays CC, Zlatar ZZ, Wierenga CE.The utility of cerebral blood flow as a biomarker of preclinical Alzheimer's disease. Cell Mol Neurobiol 2016; 36: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C.Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 5.Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 2017; 18: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolters FJ, Zonneveld HI, Hofman A, et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation 2017; 136: 719–728. [DOI] [PubMed] [Google Scholar]
- 7.Nagata K, Yamazaki T, Takano D, et al. Cerebral circulation in aging. Ageing Res Rev 2016; 30: 49–60. [DOI] [PubMed] [Google Scholar]
- 8.Peng SL, Su P, Wang FN, et al. Optimization of phase-contrast MRI for the quantification of whole-brain cerebral blood flow. J Magn Reson Imaging 2015; 42: 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990; 113: 27–47. [DOI] [PubMed] [Google Scholar]
- 10.Marchal G, Rioux P, Petit-Taboue MC, et al. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol 1992; 49: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 11.Petit-Taboue MC, Landeau B, Desson JF, et al. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage 1998; 7: 176–184. [DOI] [PubMed] [Google Scholar]
- 12.Sugawara J, Tarumi T, Xing C, et al. Older age and male sex are associated with higher cerebrovascular impedance. J Appl Physiol (1985) 2021; 130: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarumi T, Ayaz Khan M, Liu J, et al. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab 2014; 34: 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas BP, Liu P, Park DC, et al. Cerebrovascular reactivity in the brain white matter: magnitude, temporal characteristics, and age effects. J Cereb Blood Flow Metab 2014; 34: 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomoto T, Riley J, Turner M, et al. Cerebral vasomotor reactivity during hypo- and hypercapnia across the adult lifespan. J Cereb Blood Flow Metab 2020; 40: 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell GF, Wang N, Palmisano JN, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation 2010; 122: 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorin-Trescases N, de Montgolfier O, Pincon A, et al. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol 2018; 314: H1214–H1224. H1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yew B, Nation DA. and Alzheimer’s Disease Neuroimaging Initiative. Cerebrovascular resistance: effects on cognitive decline, cortical atrophy, and progression to dementia. Brain 2017; 140: 1987–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantini S, Sassaroli A, Tgavalekos KT, et al. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016; 3: 031411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dastur DK.Cerebral blood flow and metabolism in normal human aging, pathological aging, and senile dementia. J Cereb Blood Flow Metab 1985; 5: 1–9. [DOI] [PubMed] [Google Scholar]
- 21.Buijs PC, Krabbe-Hartkamp MJ, Bakker CJ, et al. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology 1998; 209: 667–674. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Zhu YS, Khan MA, et al. Global brain hypoperfusion and oxygenation in amnestic mild cognitive impairment. Alzheimers Dement 2014; 10: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Zhu X, Feinberg D, et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med 2012; 68: 912–922. [DOI] [PubMed] [Google Scholar]
- 24.Aaslid R, Markwalder TM, Nornes H.Noninvasive transcranial doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982; 57: 769–774. [DOI] [PubMed] [Google Scholar]
- 25.Roberts DA, Detre JA, Bolinger L, et al. Quantitative magnetic resonance imaging of human brain perfusion at 1.5 T using steady-state inversion of arterial water. Proc Natl Acad Sci U S A 1994; 91: 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aanerud J, Borghammer P, Chakravarty MM, et al. Brain energy metabolism and blood flow differences in healthy aging. J Cereb Blood Flow Metab 2012; 32: 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 2011; 21: 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheel P, Ruge C, Petruch UR, et al. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke 2000; 31: 147–150. [DOI] [PubMed] [Google Scholar]
- 29.Alisch JSR, Khattar N, Kim RW, et al. Sex and age-related differences in cerebral blood flow investigated using pseudo-continuous arterial spin labeling magnetic resonance imaging. Aging (Albany NY) 2021; 13: 4911–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkes LM, Rashid W, Chard DT, et al. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 2004; 51: 736–743. [DOI] [PubMed] [Google Scholar]
- 31.Scheel P, Ruge C, Schoning M.Flow velocity and flow volume measurements in the extracranial carotid and vertebral arteries in healthy adults: reference data and the effects of age. Ultrasound Med Biol 2000; 26: 1261–1266. [DOI] [PubMed] [Google Scholar]
- 32.Khan MA, Liu J, Tarumi T, et al. Measurement of cerebral blood flow using phase contrast magnetic resonance imaging and duplex ultrasonography. J Cereb Blood Flow Metab 2017; 37: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faraci FM, Heistad DD.Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res 1990; 66: 8–17. [DOI] [PubMed] [Google Scholar]
- 34.Johnson HM, Gossett LK, Piper ME, et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol 2010; 55: 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Chronic Disease Prevention and Health Promotion Excessive Alcohol Use, www.cdc.gov/chronicdisease/resources/publications/factsheets/alcohol.htm (2022, accessed 30 November 2022).
- 36.Tarumi T, Zhang R.Cerebral hemodynamics of the aging brain: risk of Alzheimer disease and benefit of aerobic exercise. Front Physiol 2014; 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu F, Ge Y, Lu H.Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med 2009; 62: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herscovitch P, Raichle ME.What is the correct value for the brain–blood partition coefficient for water? J Cereb Blood Flow Metab 1985; 5: 65–69. [DOI] [PubMed] [Google Scholar]
- 39.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European Consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim MH, Cho YI, Jeong SK.Homocysteine and pulsatility index of cerebral arteries. Stroke 2009; 40: 3216–3220. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Zuckerman JH, Levine BD.Spontaneous fluctuations in cerebral blood flow: insights from extended-duration recordings in humans. Am J Physiol Heart Circ Physiol 2000; 278: H1848–55. [DOI] [PubMed] [Google Scholar]
- 42.Giavarina D.Understanding Bland Altman analysis. Biochem Med (Zagreb) 2015; 25: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lassen NA.Normal average value of cerebral blood flow in younger adults is 50 ml/100 g/min. J Cereb Blood Flow Metab 1985; 5: 347–349. [DOI] [PubMed] [Google Scholar]
- 44.Dhokalia A, Parsons DJ, Anderson DE.Resting end-tidal CO2 association with age, gender, and personality. Psychosom Med 1998; 60: 33–37. [DOI] [PubMed] [Google Scholar]
- 45.Frassetto L, Sebastian A.Age and systemic acid-base equilibrium: analysis of published data. J Gerontol A Biol Sci Med Sci 1996; 51: B91–9. [DOI] [PubMed] [Google Scholar]
- 46.Willis MW, Ketter TA, Kimbrell TA, et al. Age, sex and laterality effects on cerebral glucose metabolism in healthy adults. Psychiatry Res 2002; 114: 23–37. [DOI] [PubMed] [Google Scholar]
- 47.Smith LA, Melbourne A, Owen D, et al. Cortical cerebral blood flow in ageing: effects of haematocrit, sex, ethnicity and diabetes. Eur Radiol 2019; 29: 5549–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krause DN, Duckles SP, Pelligrino DA.Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol (1985) 2006; 101: 1252–1261. [DOI] [PubMed] [Google Scholar]
- 49.Orshal JM, Khalil RA.Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 2004; 286: R233–R249. [DOI] [PubMed] [Google Scholar]
- 50.Dolui S, Wang Z, Wang DJJ, et al. Comparison of non-invasive MRI measurements of cerebral blood flow in a large multisite cohort. J Cereb Blood Flow Metab 2016; 36: 1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ambarki K, Wahlin A, Zarrinkoob L, et al. Accuracy of parenchymal cerebral blood flow measurements using pseudocontinuous arterial spin-labeling in healthy volunteers. AJNR Am J Neuroradiol 2015; 36: 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.2021 Alzheimer's disease facts and figures. Alzheimers Dement 2021; 17: 327–406. [DOI] [PubMed] [Google Scholar]
- 53.Iturria-Medina Y, Sotero RC, Toussaint PJ, et al. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun 2016; 7: 11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw TG, Mortel KF, Meyer JS, et al. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology 1984; 34: 855–862. [DOI] [PubMed] [Google Scholar]
- 55.Harloff A, Albrecht F, Spreer J, et al. 3D blood flow characteristics in the carotid artery bifurcation assessed by flow-sensitive 4D MRI at 3T. Magn Reson Med 2009; 61: 65–74. [DOI] [PubMed] [Google Scholar]
- 56.Dolui S, Fan AP, Zhao MY, et al. Reliability of arterial spin labeling derived cerebral blood flow in periventricular white matter. Neuroimage Rep 2021; 1: 100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giannopoulos S, Katsanos AH, Tsivgoulis G, et al. Statins and cerebral hemodynamics. J Cereb Blood Flow Metab 2012; 32: 1973–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Rijssel AE, Stins BC, Beishon LC, et al. Effect of antihypertensive treatment on cerebral blood flow in older adults: a systematic review and meta-analysis. Hypertension 2022; 79: 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christie IN, Windsor R, Mutsaerts HJ, et al. Cerebral perfusion in untreated, controlled, and uncontrolled hypertension. J Cereb Blood Flow Metab 2022; 42: 2188–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231153741 for Cerebral blood flow and cerebrovascular resistance across the adult lifespan: A multimodality approach by Tsubasa Tomoto, Marilyn Lu, Ayaz M Khan, Jie Liu, Evan P Pasha, Takashi Tarumi and Rong Zhang in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
The data from this study are available upon request from qualified investigators to the research team according to the data sharing policy and the UTSW IRB regulations.