Abstract
This article describes the complex interactions occurring between diet, the gut microbiome, and bile acids in the etiology of fatty liver disease. Perhaps 25% of the world’s population may have nonalcoholic fatty liver disease (NAFLD) and a significant percentage (∼20%) of these individuals will progress to nonalcoholic steatohepatitis (NASH). Currently, the only recommended treatment for NAFLD and NASH is a change in diet and exercise. A Western-type diet containing high fructose corn syrup, fats, and cholesterol creates gut dysbiosis, increases intestinal permeability and uptake of LPS causing low-grade chronic inflammation in the body. Fructose is a “lipogenic” sugar that induces long-chain fatty acid (LCFA) synthesis in the liver. Inflammation decreases the oxidation of LCFA, allowing fat accumulation in hepatocytes. Hepatic bile acid transporters are downregulated by inflammation slowing their enterohepatic circulation and allowing conjugated bile acids (CBA) to increase in the serum and liver of NASH patients. High levels of CBA in the liver are hypothesized to activate sphingosine-1-phosphate receptor 2 (S1PR2), activating pro-inflammatory and fibrosis pathways enhancing NASH progression. Because inflammation appears to be a major physiological driving force in NAFLD/NASH, new drugs and treatment protocols may require the use of anti-inflammatory compounds, such as berberine, in combination with bile acid receptor agonists or antagonists. Emerging new molecular technologies may provide guidance in unraveling the complex physiological pathways driving fatty liver disease and better approaches to prevention and treatment.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is becoming a global epidemiological problem, which affects about 25% of the adult population (58). Progression of NAFLD to nonalcoholic steatohepatitis (NASH) is among the top etiologies for cirrhosis and hepatocellular carcinoma (HCC) (89). The imbalance between lipid uptake or de novo synthesis and lipid secretion results in excessive lipid accumulation in hepatocytes. It has been well recognized that inflammation is the key driving force of NAFLD to NASH progression (4). NAFLD/NASH is often associated with obesity, insulin resistance, type 2 diabetes (T2DM), and metabolic syndrome (90). However, its pathogenesis remains incompletely understood.
In the last few decades, scientists have recognized the human body as a complex ecosystem of interacting prokaryotic and eukaryotic cells. The adult human body consists of approximately 1013 mammalian cells and 2 to 5×1013 prokaryotic cells that colonize the human body at different sites (77). Moreover, the microbial metagenome is much greater than the human genome as the microbiome represents approximately 99% of the functional genes in the body (68). Elucidating the host-microbe interactions is very important in developing strategies to prevent and treat very common diseases of the liver and gastrointestinal tract (GI). Quantitatively, most of the bacteria associated with the human body are found in the GI tract, especially the colon that contains one of the most densely populated natural ecosystems known. In recent years, using the 16S rRNA gene, the most invariant bacterial gene, and high-throughput sequencing, scientists have a much better understanding of the diversity of the human gut microbiome. It has been estimated that the human colon contains at least 150 to 200 “phylotypes” at any given time. Bacterial 16S rRNA genes sharing >97% to 99% identity are generally referred to as a “phylotype” or operational taxonomic unit (OTU) (44). There have been identified six divisions/phyla of bacteria in the human colon. Two major divisions, the Bacteroidetes (Gram-negative anaerobes) and Firmicutes (Gram-positive anaerobes) represent greater than 90% of the total species of gut microbiota. Moreover, bacterial diversity in the colon ecosystem is almost entirely due to changes at lower taxonomic levels, that is, genus, species, and strains (24, 68).
The Role of Diet in Regulating Gut Microbiota and Inflammation
The composition of the human gut microbiota can be altered by diet, bile acids, liver diseases, antibiotics, gender, age, intestinal transit time, and numerous other factors. Dysbiosis of the gut microbiota can enhance the pathophysiology of several chronic diseases, including liver and GI diseases (71). The gut microbiota can utilize both endogenous and exogenous substrates for growth. Endogenous substrates include greater than 100g wet weight of sloughed intestinal cells per day as well as bile components. Host antigens may select for gut bacteria that are capable of degrading these as a source of carbon and energy. In this regard, Hoskins and Boulding (34) reported that fecal samples from blood group B individuals had 5×104 higher levels of bacteria capable of degrading B antigens compared to individuals with A or O blood groups. Exogenous or dietary substrates can be quite variable depending upon the type of diet consumed, which include Western, Mediterranean, Paleolithic, Vegetarian, Ketogenic as well as hybrid forms of each diet (27). Alteration of the structure of the human gut microbiome by changes to either plant-based or animal-based diets can occur within 24 to 48h postconsumption (17). A study by O’Keefe et al. (64) showed that switching the diets of African Americans (Western diet) with rural South Africans (plant-based diet high in fiber and resistant starch) for 2 weeks resulted in a rapid change in the gut microbiota for each group. African Americans on a Western diet are at high risk for colon cancer, while rural South Africans have a very low risk for colon cancer. The results showed that Ki67, a mucosal cell proliferative marker, and the fecal metabolome markedly changed in each group. African Americans showed a decrease in Ki67 in intestinal cells, secondary bile acids, deoxycholic acid (DCA), and lithocholic acid (LCA), and an increase in fecal butyric acid on the rural South African diet. The rural South Africans showed the opposite effects on the Western diet. Western-type diets containing high fructose corn syrup (HFCS), saturated fats, and cholesterol and low in complex carbohydrates also rapidly altered the gut microbiota composition, intestinal barrier function, and immune system (22, 74). Western-type diets have been reported to increase intestinal permeability with increased absorption of bacterial components, such as lipopolysaccharides (LPS) and lipoteichoic acids, which enhance the synthesis of pro-inflammatory cytokines and cyclooxygenase-2 (COX2) induction through toll-like receptor-4 (TLR4) and toll-like receptor-2 (TLR2), respectively, in the intestines and liver (51). Western diets create a low-grade chronic inflammation in the intestine and liver and systemically drive the pathophysiology of numerous chronic diseases (22, 74).
Western Diets and Hepatic Sugar Metabolism
During the last 40 years, there have been major changes in the dietary habits of individuals in the United States and worldwide, with associated increases in the numbers of overweight and obese individuals. One of the significant dietary changes was the wide-scale introduction of HFCS into diets in the early 1980s and beyond as well as increased long-chain saturated fatty acids (LCSF). Farmers have known for centuries that feeding corn to animals resulted in weight gain. HFCS is a liquid mixture of fructose (50%–65%) and the remainder glucose (65). Fructose is a “lipogenic” sugar in humans and other animals, and its transport and metabolism in the liver are mediated by different pathways compared to glucose (32). Fructose metabolism in the liver stimulates the synthesis of long-chain fatty acids (LCFA) via induction of sterol regulatory element-binding protein 1C (SREBP-1c), which induces genes encoding enzymes in the fatty acid biosynthesis pathway. The pathway of fructose metabolism in the human liver is mostly unregulated as compared to glucose metabolism. Once in the liver, fructose is phosphorylated by fructokinase C at carbon 1 producing fructose-1-phosphate, which is then cleaved to dihydroxyacetone phosphate and glyceraldehyde by aldolase B. Because fructose is rapidly metabolized with consumption of ATP, excess uric acid is formed through activation of AMP deaminase and is further catabolized to uric acid (19). The generation of uric acid may further enhance inflammation and fat accumulation in the liver. The combination of increased LCSF synthesis in the liver and decreased oxidation due to increased inflammation contributes to fatty liver disease.
LCSF, such as palmitate, has been reported to activate cellular stress pathways and the induction of JNK-dependent hepatocyte apoptosis (55). Moreover, LCSF stimulates the release of extracellular vesicles from hepatocytes containing tumor necrosis factor-related apoptosis-inducing ligand that induces the expression of pro-inflammatory cytokines IL1-β and IL-6 in macrophages (33). Inflammatory cytokines, such as TNF-α, can quench insulin signaling by activating the JNK signaling cascade by phosphorylating insulin receptor substrate 1 (2). The accumulation of fat in the liver, with enhanced inflammation, over time can lead to NAFLD and up to 20% of these individuals go on to develop NASH (39).
Enterohepatic Circulation of Bile Acids
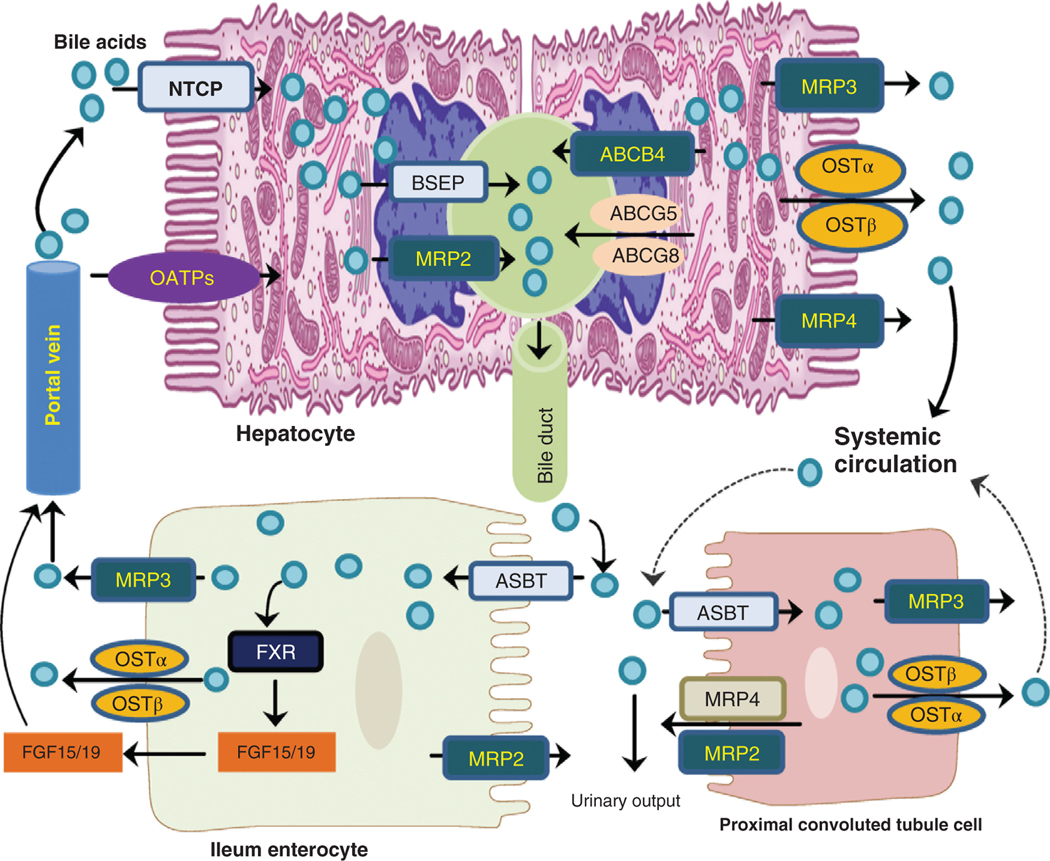
Bile acids are synthesized from free cholesterol in liver hepatocytes. The major bile acids biosynthetic pathway, termed the classical pathway, starts with the 7α-hydroxylation of cholesterol catalyzed by cholesterol 7α-hydroxylase (CYP7A1), located in the smooth ER (36, 93). In humans, this pathway leads to the synthesis of cholic acid (CA, 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid) and chenodeoxycholic acid (CDCA, 3α,7α-dihydroxy-5β-cholan-24-oic acid) via a multistep pathway (36). The alternative pathway of bile acid synthesis begins in the mitochondria with the 27-hydroxylation of cholesterol catalyzed by cholesterol 27-hydroxylase (CYP27A1). This pathway is believed to form mostly CDCA and may generate important regulatory oxysterols (70). Bile acids are conjugated at the 24-carboxyl group to either glycine or taurine before active transport from the hepatocyte primarily by the canalicular bile salt export protein [BSEP, ATP-binding cassette subfamily B, member 11 (ABCB11)] along with cholesterol (ABCG5/G8, ATP-binding cassette subfamily G, member 5/8), phosphatidylcholine (ABCB4, ATP-binding cassette subfamily B, member 4), conjugated bilirubin (multidrug resistance protein 2, MRP2), and other metabolites (93). Biliary bile components are stored in the gallbladder and released into the small bowel following a meal. Conjugated bile acids (CBA) function in the small bowel to promote the absorption of cholesterol, LCFA, as well as fat-soluble vitamins A, D, E, and K by forming mixed micelles that promote uptake by enterocytes. Bile acids move down the GI via gut peristalsis and are actively transported by ileal enterocytes by the apical sodium-dependent bile acid transporter (ASBT) (18, 93). Once inside ileal enterocytes, bile acids activate the farnesoid X receptor (FXR), upregulating the gene encoding fibroblast growth factor 15 (FGF-15) in mice, fibroblast growth factor 19 (FGF-19) in humans (76). Intracellular bile acids are transported into the portal blood by the heterodimeric organic solute transporter (OST), OSTα-OSTβ, a facilitated diffusion transporter on the basolateral membranes of ileal enterocytes. Bile acids and FGF-15/19 are transported to the liver via the portal vein. Bile acids are taken up by hepatocytes, primarily by the Na+-taurocholate co-transporting polypeptide (NTCP). In addition, the basolateral multidrug resistance-associated proteins (MRP3 or ABCC3 and MRP4 or ABCC4) and OSTα-OSTβ are involved in ATP-dependent bile acid export from hepatocytes to systemic circulation (42). FGF-15/19 binding to fibroblast growth factor receptor 4 (FGFR4) on hepatocytes activates the extracellular signal-regulated kinases (ERK) signaling cascade and downregulates CYP7A1, the rate-limiting enzyme in the classical bile acid synthesis pathway (Figure 1). In this manner, the enterohepatic circulation of bile acids helps to maintain homeostatic bile acid synthesis rates, glucose, lipid, energy metabolism as well as the immune system (13).
Figure 1. Bile acid transporters in the liver hepatocyte, ileal enterocyte, and proximal convoluted tubule in the kidney.
Bile acids are actively transported from the hepatocytes by the bile salt export protein (BSEP) or multidrug resistance protein 2 (MPR2) into bile duct. After secretion into the intestine, they are efficiently recovered by leal enterocytes using the apical sodium-dependent bile acid transporter (ASBT) and into the portal vein via the heterodimeric organic solute transporter (OSTα/β) on the basolateral membrane. Bile acids may also undergo hepatic-renal cycling, especially during cholestasis. Bile acids secreted by the kidney are usually modified by the sulfation of hydroxyl groups. The multidrug resistance-associated proteins (MRP3 or ABCC3 and MRP4 or ABCC4) and OSTα-OSTβ on the basolateral membrane are involved in ATP-dependent bile acid export from hepatocytes to systemic circulation.
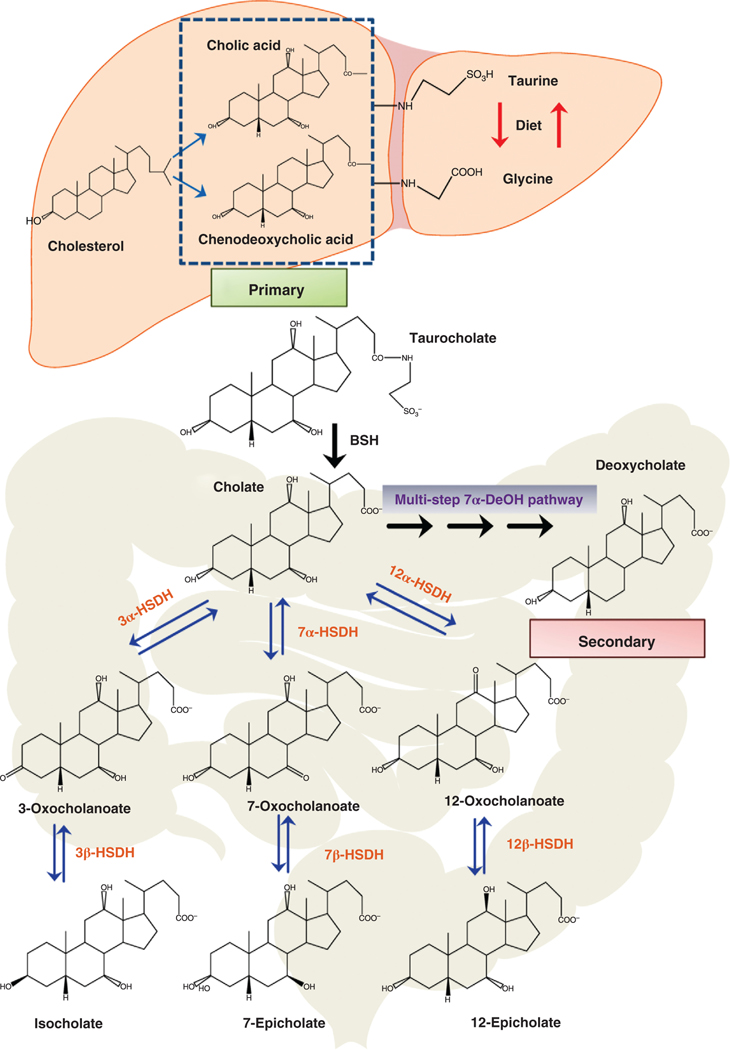
During the enterohepatic circulation of bile acids, several hundred milligrams of bile acid enter the colon, where gut bacteria can biotransform bile acids into a variety of metabolites (72, 73). However, the two most important “gateway” biotransformations are catalyzed by bile salt hydrolases (BSH) and the multistep bile acid 7α-dehydroxylation pathway (7α-DeOH) (Figure 2) (72). Hydrophobic secondary bile acids can be absorbed from the large bowel via passive diffusion and transported to the liver via the portal vein. The levels of DCA in biliary bile can be quite high (>50%) in some individuals as the human liver is not capable of the 7α-hydroxylation of DCA reforming CA, as is the case with rodents (72).
Figure 2. Synthesis of the primary bile acids cholic acid and chenodeoxycholic acid from cholesterol in liver hepatocytes and metabolism by gut bacteria.
The primary bile acids, cholic acid, and chenodeoxycholic acid are synthesized in the hepatocytes from cholesterol and conjugated with taurine or glycine. Taurocholate is biotransformed by gut bacteria expressing bile salt hydrolases (BSH) to cholic acid and taurine. Gut bacteria can oxidize hydroxyl groups at the 3α, 7α, and 12α position on the steroid ring by 3α-hydroxysteroid dehydrogenases (3α-HSDH), 7α-hydroxysteroid dehydrogenases (7α-HSDH), and 12α-hydroxysteroid dehydrogenases (12α-HSDH), respectively. Oxo-bile acids may be further metabolized at the 3β, 7β, or 12β position by 3β-hydroxysteroid dehydrogenase (3β-HSDH), 7β-hydroxysteroid dehydrogenase (7β-HSDH) and 12β-hydroxysteroid dehydrogenase (12β-HSDH), respectively, producing iso and epi bile acids. Primary bile acids can be biotransformed to secondary bile acids by removing the 7α-hydroxyl group via a multistep 7α-dehydroxylation (7α-DeOH) biochemical pathway found in some species of the genus Clostridium.
Bile acids are major regulators of the structure of the gut microbiome. Resistance to bile acids is a major selective pressure regulating the gut microbiome structure. Bile acids may alter the structure of the human gut microbiome in a variety of ways: (i) deconjugation of bile salts increases the hydrophobicity and alters the chemical properties of individual bile acids. Unconjugated bile acids are generally more toxic to gut bacteria than CBA (72). A small population of gut bacteria belonging to the genus Clostridium is able to convert the unconjugated primary bile acids, CA and CDCA, into DCA and LCA, respectively. DCA is reported to be up to 10-time more toxic to some gut bacteria than CA, probably because it disrupts bacterial cytoplasmic membrane structure and function (15). The epimerization of the 3α>3β-hydroxyl group of DCA by gut bacteria decreases the toxicity of this bile acid by decreasing hydrophobicity (21) (Figure 2). (ii) Bile acids have been reported to inhibit the growth and translocation of bacteria in the small bowel via an FXR-dependent mechanism involving the secretion of antibacterial peptides (37). This probably has a stronger effect on the composition of mucosal-associated gut microbiota than those found in the lumen of the intestine. (iii) Certain gut bacteria including, Clostridium scindens and other species of 7α-DeOH gut bacteria, secrete antibiotics or antibacterial compounds (40). These antibacterial compounds may be important regulators of the gut microbiome structure along with secondary bile acids. In this regard, feeding CA to rats shifted the gut microbiome from a approximately 1:1 ratio of Firmicutes/Bacteroidetes to >98% Firmicutes (38), and feeding CA to mice increased levels of bile acid 7α-DeOH bacteria approximately 1000-fold (72). Moreover, feeding a high-fat diet (HFD) containing fructose and cholesterol increases the Firmicutes/Bacteriodetes ratio, possibly due to increased bile acid loss into the colon.
Effects of Fatty Liver Disease on Hepatic Bile Acid Synthesis, Transporters, and Serum Bile Acid Levels
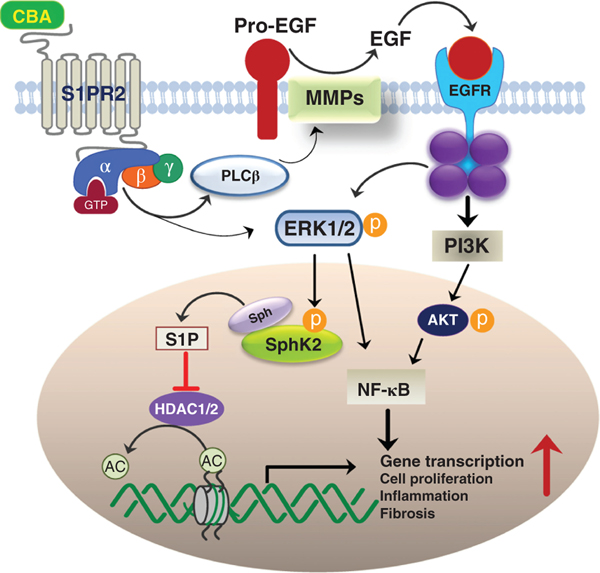
Changes in rates of primary bile acid synthesis in the liver, formation of secondary bile acids by gut bacteria, induction of FGF-15/19 in the intestines, and increase of the serum bile acid levels, may be important indicators of pathophysiological processes occurring in the liver-gut-microbiome axis due to fatty liver disease. The downregulation of hepatic NTPC by LPS was reported more than 20 years ago (81). Moreover, in a rat liver model of obstructive cholestasis, there was a downregulation of BSEP and NTCP in periportal hepatocytes due to induction of TNFα and IL-1β (23). The downregulation of BSEP is hypothesized to be primarily due to the negative effects of TNF-α and IL-1β on the interaction of FXR:RXR (retinoid x receptor) heterodimer that activates the BSEP promoter (26). The OSTα/β is a heterodimeric solute transport protein located in the basolateral membranes of liver and intestinal epithelial cells (8). OSTα/β was found to be significantly upregulated in liver tissues from NASH and primary biliary cholangitis (PBC) patients. Upregulation of OSTα/β is considered a physiological marker for cholestatic liver disease (56). However, levels of OSTα/β mRNA or protein have not been measured in NAFLD patients. Downregulation of NTCP and other hepatocyte uptake transporters by inflammation may increase the level of serum bile acids. In this regard, Puri et al. (67) identified and quantitated serum bile acids in biopsy-proven NAFLD and NASH patients and compared them to healthy controls. The results showed a significant increase in total conjugated primary bile acids and decreased secondary bile acids in NASH patients compared to NAFLD patients and controls. There was a stepwise increase in total serum bile acids from controls to NAFLD to NASH patients. The increase in serum CBA was associated with higher grades of steatosis, lobular and portal inflammation, and hepatocyte ballooning, correlating a role of increased serum bile acids with pathophysiological effects in the liver. Additional studies by Lake et al. (43) suggest that in NASH patients, there is a shift to more bile acid synthesis via the alternative pathway with upregulation of oxysterol 7α-hydroxylase (CYP7B1) and increased taurine conjugated primary bile acid. Studies by Mouzaki et al. (60) showed that NASH patients had significantly higher levels of total fecal unconjugated primary bile acids as compared to controls. Moreover, levels of the bile acid biosynthesis serum marker, 7α-hydroxy-4-cholesten-3-one (C4) was significantly upregulated in NASH patients; however, FGF-19 levels were not significantly different. Mouse models of NASH also show increases in hepatic inflammation markers, downregulation of bile acid transporters, except OSTβ, key genes involved in bile acid synthesis, and an increase in serum total conjugated primary bile acids, especially taurocholate (TCA) (80, 83) (Figure 3).
Figure 3. Activation of the ERK1/2 and AKT signaling pathways by conjugated bile acids (CBA) via S1PR2.
High levels of CBA activate S1PR2 in liver cells, enhancing the upregulation of genes encoding pro-inflammatory and fibrosis mediators. Phosphorylated ERK1/2 is translocated into the nucleus, where it activates sphingosine kinase 2 (SphK2) via phosphorylation. SphK2 produces sphingosine-1-phosphate (S1P) that is a potent inhibitor of histone deacetylase 1 and 2 (HDAC1/2), allowing the epigenetic upregulation of gene transcription.
Possible Role of Sphingosine1-phosphate Receptor 2 in NASH Progression
Studer et al. (79) first reported that CBA activate ERK1/2 and protein kinase B (AKT) signaling pathways through sphingosine-1-phosphate receptor 2 (S1PR2) in rodent hepatocytes. The activation of ERK1/2 and AKT by TCA in primary rat hepatocytes or in the chronic bile fistula rat was inhibited by JET-013, a chemical S1PR2 antagonist. Moreover, S1PR2 shRNA markedly inhibited ERK1/2 and AKT activation by TCA in primary rat hepatocytes. Additional studies discovered that S1PR2 activated nuclear sphingosine kinase 2 (SphK2) in mouse hepatocytes and the chronic bile fistula rat (61). Sphingosine-1-phosphate (S1P) has been shown to be an endogenous inhibitor of histone deacetylases 1 and 2 that regulate levels of histone acetylation and gene expression (30). SphK2 has been reported to be a key regulator of genes involved in LCFA metabolism, and S1PR2−/− and SphK2−/− mice rapidly developed fatty livers on (61, 62). SphK2 is significantly downregulated in a mouse model of NASH on an HFD (83). S1PR2 appears to be an important bile acid sensor during the feeding and fasting cycle and is activated by CBA returning from the intestines following a meal. CBAs secreted from the liver are stored in the gallbladder during fasting. The decreased levels of hepatic CBA cause the inactivation of S1PR2. In contrast, there is evidence that constant activation of S1PR2 results in the induction of pro-inflammatory and proliferative signaling pathways in cells in the liver that may occur during cholestasis. In this regard, TCA promoted cholangiocarcinoma cell growth and cyclooxygenase 2 expression via S1PR2 [Figure 3, (35, 49, 50)]. Our recent studies also showed that the long noncoding RNA H19 activates pro-inflammatory and fibrotic markers in cirrhotic liver and bile duct ligated mice. In the multidrug resistance 2 knockout (Mdr2−/−) mouse that serves as a model of cholestatic cholangiopathies, TCA induced expression of H19 and fibrotic genes via S1PR2 (46). The increased serum levels of CBA, especially TCA, have been reported in NASH patients and may activate S1PR2 in cholangiocytes and other liver cells to activate pro-inflammatory pathways and fibrotic gene expression, accelerating fatty liver pathogenesis. Finally, other studies reported that CBAs promote the growth of esophageal adenocarcinoma cells, activation of YAP and β-catenin signaling pathways via S1PR2 (48). It is unknown if S1PR2 and CBAs might play a role in HCC development.
Liver Disease and Gut Dysbiosis
A comparison of the gut microbiota in individuals with a healthy liver, NAFLD, NASH, and cirrhosis shows an increase in gut dysbiosis with advancing disease (7). Most studies show a decrease in gut microbiota diversity, an increase in bacteria containing LPS, and a decrease in bacteria producing short-chain fatty acids (SCFA), especially butyrate (57). Bile acids, SCFA, and LPS activate different biosynthetic, metabolic, pro-, and anti-inflammatory signaling pathways in host cells. In this regard, bile acids activate specific nuclear receptors (FXR, Vitamin D, PXR) and G-protein coupled receptors (GPR) (TGR5, S1PR2, M2,3-muscarinic) while SCFA activates GPR (41 and 43) and propionate and butyrate activate the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) (3, 75). LPS can bind to TLR-4 to activate pro-inflammatory signaling pathways (14). Western diets are associated with an increase in the risk of obesity, T2DM, NAFLD, and NASH and chronic low-grade inflammation (12). It has been observed that enhanced inflammation associated with HFD is due to the absorption of LPS and stimulation of the synthesis of pro-inflammatory cytokines through activating TLR-4. Higher levels of LPS have been reported in obese individuals compared to healthy controls, possibly due to higher levels of Gram-negative gut bacteria, especially members of the family Enterobacteriaceae, and increased intestinal barrier permeability (12, 59). Humans are one of the most sensitive animal species to the pro-inflammatory effects of LPS. Cani et al. (10) have reported that HFD can alter the composition of the gut microbiota, decreasing tight junction proteins, zonula occludens (ZO1) and occludin, allowing increase absorption of LPS. HFD may also increase absorption of LPS via the lymphatics by incorporation into chylomicrons in the small bowel. Germ-free animals and those treated with antibiotics are highly resistant to developing fatty liver disease implicating the gut microbiota as an important factor in inducing fatty liver disease (9).
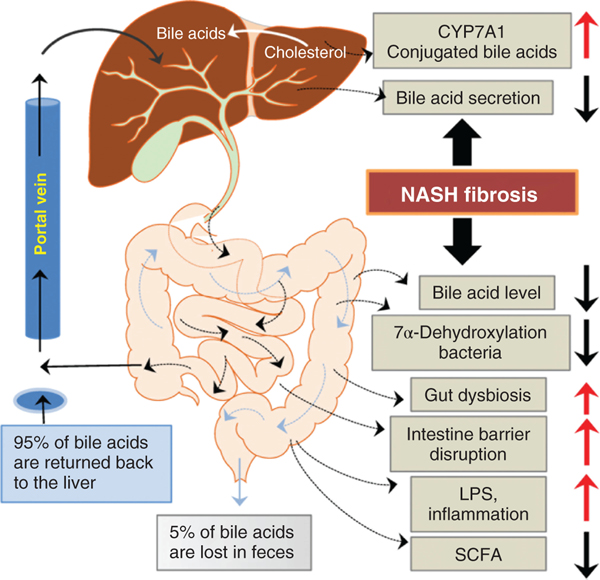
Slowing of the enterohepatic circulation brought on by increased systemic inflammation, downregulating hepatic bile acid transporters may decrease levels of bile acids in the intestine. This may have the effect of altering the gut microbiota as intestinal bile acids are important regulators of the structure of the gut microbiome. Moreover, bile acids are signaling molecules activating anti-inflammatory pathways through activation of FXR and TGR5, as well as increasing the secretion of gut antibacterial peptides (25). Moreover, intestinal bile acids regulate bile acid synthesis in the liver by stimulating the synthesis of FGF-19 in ileal enterocytes via activation of FXR (Figure 4) (41).
Figure 4. Alteration of the enterohepatic circulation of bile acids by NASH.
Liver diseases interrupt the enterohepatic circulation of bile acids, enhancing gut dysbiosis. This has the downstream effect of increasing gut permeability and absorption of pro-inflammatory mediators such as LPS. In NASH, there is an increase in the serum CBA that may activate the S1PR2 in hepatic cells, enhancing inflammation and activating pro-fibrotic gene expression in stellate cells.
Studies by Bajaj et al. (7) shows major changes in the stool microbiota composition comparing healthy controls to decompensated cirrhotic patients where there were significant decreases in potentially beneficial autochthonous bacteria including members of families Lachnospiraceae, Ruminococcaceae, and Clostridiales XIV and increases in potential pathogenic families including Staphyloccaeae, Enterobacteriaceae, and Enterococcaceae. Qin et al. (68) also observed major changes in cirrhotic patients compared to controls, and gene analysis suggested that many of the gut bacteria were from the oral cavity. Moreover, changes in both oral and stool microbiome in cirrhotic patients were also reported by Bajaj et al. (1, 6). It was discovered that in the salvia of cirrhotic patients, the levels of autochthonous bacterial families decreased and potential pathogenic families Enterobacteriaceae and Enterococcaceae increased. These results might represent a system-wide change in immunity to host microbiota in cirrhotic patients. In patients with cirrhosis, which have a smaller bile acid pool than control patients, there is a loss of bile acid 7α-DeOH gut bacteria and a shift to a more “toxic” Gram-negative gut microbiota (55). When patients with cirrhosis are transplanted with a new liver, there is an increase in bile acid secretion, an increase in fecal secondary bile acid synthesis, and a return to a more “normal” and diverse gut microbiota with less systemic inflammation (33). These results show the intimate connection between bile acids and the liver-gut axis (Figure 5).
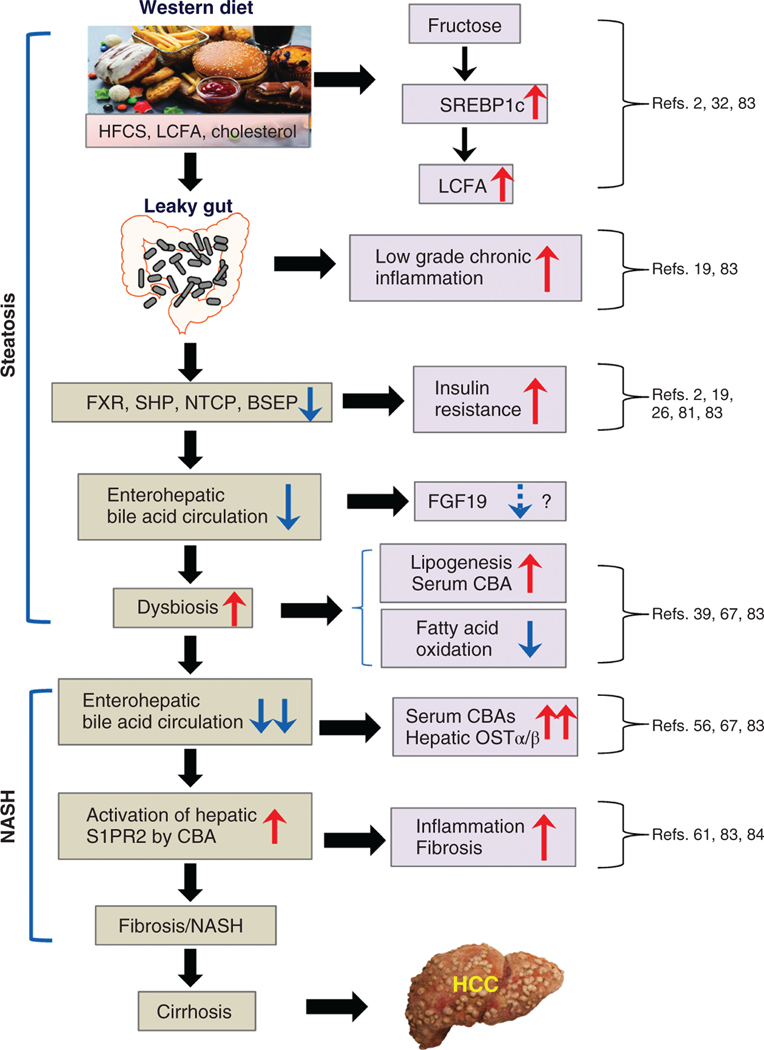
Figure 5. The possible role of diet, gut dysbiosis and bile acids in the development of steatosis and NASH.
The road to Steatosis and NASH begins by consuming a Western-type diet containing large amounts of HFCS and fats, resulting in constant low-grade systemic inflammation due to gut dysbiosis and absorption of pro-inflammatory bacterial molecules. Under these dietary conditions, there is an upregulation of long-chain fatty acid synthesis in the liver and decreased oxidation and secretion of fats. Inflammation also downregulates bile acid transporters in the liver, slowing their enterohepatic circulation and allowing an increase in CBA in the liver, which activates S1PR2 stimulating hepatic inflammation and fibrosis pathways and promoting the development of cirrhosis and hepatocellular carcinoma (HCC).
Possible Role of Berberine and Other Anti-inflammatory Compounds in Treating Fatty Liver Disease
Berberine is a natural pentacyclic isoquinoline alkaloid present in many plants used in ancient medicine, such as Berberis vulgaris (barberry fruit), goldenseal, Orgon grapes, Coptis chinensis, and has been used in Asia for thousands of years as a folk remedy for various digestive disorders, especially for diarrhea and infectious diseases (86). During the last two decades, berberine has been extensively studied for its beneficial effects on metabolic diseases, including NAFLD and diabetes (6). Numerous studies have reported that berberine has various biological activities, such as anti-inflammatory, lipid-lowering, and an antidiabetic effect (63). Our previous studies in rodent NAFLD models showed the beneficial effects of berberine on preventing NAFLD disease progression is mainly through modulating bile acid metabolism in the gut-liver axis (28). Although the bioavailability of intragastrically administered berberine was much lower than that of intraperitoneally administered berberine, it had a stronger lipid-lowing effect, indicating that the GI is the major functional site of berberine (28). A number of mechanisms have been identified underlying berberine’s beneficial effects, such as activating AMP-activated protein kinase (AMPK), inhibiting Nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation, promoting glucagon-like peptide-1 (GLP-1) secretion, attenuating ER stress and oxidative stress, regulating microRNAs (miRs) (20, 45, 52, 54, 69, 84). Our recent study using the best available diet-induced NASH mouse model showed that berberine significantly prevented NASH disease progression by targeting multiple pathways (5, 83). Bile acid analysis further showed that berberine had a significant impact on bile acid composition and fatty acid metabolism. Since berberine is mainly accumulated in the intestinal tract, our studies suggest that gut microbiota may be the primary target of berberine in modulating metabolic processes. A growing number of studies showed that berberine modulates not only the structure but also the number of gut microbiota (16, 29, 91, 92). A recent study reported that the combination of berberine, tocotrienols and coffee extracts improved metabolic profile and hepatic lipid accumulation in the HFD-feeding mouse NAFLD model via modulating gut microbiota and hepatic miR-122 and miR-34a (16).
Licorice is another ancient medicinal plant with a long history as a remedy for inflammatory diseases and metabolic disorders (31, 53, 87). Our previous study showed that 18β-glycyrrhetinic acid, the major component of licorice root extract, prevented free fatty acid-induced hepatic lipotoxicity via modulating lysosomal and mitochondrial functions (85). It also has been reported that glycyrrhetinic acid alleviated hepatic inflammation injury in viral hepatitis disease via the high mobility group box protein 1 (HMGB1)-TLR4 signaling pathway (78). A recent study showed that diammonium glycyrrhizinate exerted its protective effect against HFD-induced NAFLD via modulating gut microbiota and preventing HFD-induced disruption of intestinal barrier functions. Diammonium glycyrrhizinate reduced the ratio of Firmicutes-to-Bacteroidetes and increased the levels of SCFA-producing bacteria (47). There is increasing evidence indicating the beneficial effects of polyphenols, alkaloids, and terpenoids from herbal medicine, vegetables, and fruits in regulating lipid, glucose, and energy metabolism via different signaling pathways in the liver and modulating gut microbiota (66, 82, 88, 95).
Future Directions
The physiological interactions between diet, gut microbiome, and bile acids in regulating normal physiological and biochemical pathways in the body are just beginning to be elucidated. Dysregulation of these pathways increases the risk of chronic diseases, including fatty liver disease. These are important medical issues as there are no currently accepted medical treatments for fatty liver disease, such as NASH, other than dietary changes and exercise. In this regard, it is currently believed that 25% of the world’s population may have NAFLD, of which up to 20% may progress to NASH and perhaps 5% of these individuals may end up with cirrhosis and/or HCC (89). Because dietary habits affect so many physiological and biochemical pathways in the body, treatment protocols for NASH may require multiple approaches and drug combinations (94). For example, the FXR agonist (obeticholic acid) has been used to treat NASH patients but with limited success. However, in the background of enhanced inflammation, cellular levels of FXR may be downregulated. Moreover, phosphorylation of FXR by protein kinase C-zeta (PKC-zeta) is required for optimal activation but may be inhibited by inflammation (11). Therefore, chronic inflammation may alter drug effectiveness requiring an anti-inflammatory compound, such as berberine and other complementary medicines, for optimal treatment results. Fatty liver diseases appear to have complex etiologies, but with the emerging new molecular technologies, there is an opportunity to gain a better understanding of these and improved treatment protocols in the future.
Didactic Synopsis.
Major teaching points
Western-type diets containing high fructose corn syrup, lipids, and cholesterol create chronic inflammation in the body.
Bile acids are important signaling molecules regulating glucose, lipid, energy metabolism as well as the structure of the gut microbiome.
Inflammation appears to slow the enterohepatic circulation of bile acids allowing them to increase in serum, enhancing inflammation and fibrotic pathways in the liver.
Naturally occurring compounds, such as berberine, may be useful in treating fatty liver disease by inhibiting pro-inflammatory pathways arising in the gut.
Acknowledgements
This study was supported by VA Merit Awards (I01BX004033 to HZ; 1BX001328 to PBH; 2I0CX001076 to BJ); Research Career Scientist Award (IK6BX004477 to HZ); ShEEP grant (1IS1 BX004777-01 to HZ); National Institutes of Health Grants (R01 DK104893 and R01DK-057543 to HZ and PBH; 1 R21 AA026629-01 to HZ; R21TR003095 and RO1HS025412 to BJ). We thank Mrs. Elaine Kennedy for proofreading and English editing.
List of Abbreviations and Acronyms
- ABCB11
ATP-binding cassette subfamily B, member 11
- ABCG5/G8
ATP-binding cassette subfamily G, member 5/8
- AKT
protein kinase B
- AMPK
AMP-activated protein kinase
- ASBT
apical sodium-dependent bile acid transporter
- BSEP
bile salt export protein
- BSH
bile salt hydrolases
- CA
cholic acid
- CBA
conjugated bile acids
- CDCA
chenodeoxycholic acid
- COX2
cyclooxygenase-2
- CYP27A1
cholesterol 27-hydroxylase
- CYP7A1
cholesterol 7α-hydroxylase
- CYP7B1
oxysterol 7α-hydroxylase
- DCA
deoxycholic acid
- ERK
extracellular signal-regulated kinases
- FGF-15
fibroblast growth factor 15
- FGF-19
fibroblast growth factor 19
- FGFR4
fibroblast growth factor receptor 4
- FXR
farnesoid X receptor
- GI
gastrointestinal tract
- GLP-1
glucagon-like peptide-1
- GPR
G-protein coupled receptors
- HCC
hepatocellular carcinoma
- HFCS
high fructose corn syrup
- HFD
high-fat diet
- LCA
lithocholic acid
- LCFA
long-chain fatty acids
- LCSF
long-chain saturated fatty acids
- LPS
lipopolysaccharides
- miRs
microRNAs
- MPR2
multidrug resistance protein 2
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NLRP3
nod-like receptor family pyrin domain containing 3
- NTCP
Na+-taurocholate co-transporting polypeptide
- OST
organic solute transporter
- OTU
operational taxonomic unit
- PBC
primary biliary cholangitis
- PKC-zeta
protein kinase C-zeta
- PPARγ
peroxisome proliferator-activated receptor γ
- RXR
retinoid x receptor
- S1P
sphingosine-1-phosphate
- S1PR2
sphingosine-1-phosphate receptor 2
- SCFA
short-chain fatty acids
- SphK2
sphingosine kinase 2
- SREBP-1c
sterol regulatory element-binding protein 1c
- T2DM
type 2 diabetes
- TCA
taurocholate
- TLR2
toll-like receptor-2
- TLR4
toll-like receptor-4
- ZO1
zonula occludens
References
- 1.Acharya C, Sahingur SE, Bajaj JS. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight 2: e94416, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem 119: 105–110, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Ang Z, Ding JL. GPR41 and GPR43 in obesity and inflammation–Protective or causative? Front Immunol 7: 28, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci 61: 1294–1303, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min H-K, Mirshahi F, Bedossa P, Sun X, Hoshida Y, Koduru SV, Contaifer D, Warncke UO, Wijesinghe DS, Sanyal AJ. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol 65: 579–588, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, Thacker LR, Sanyal AJ, Kang DJ, Sikaroodi M, Gillevet PM. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 62: 1260–1271, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 60: 940–947, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaudoin JJ, Brouwer KLR, Malinen MM. Novel insights into the organic solute transporter alpha/beta, OSTalpha/beta: From the bench to the bedside. Pharmacol Ther 211: 107542, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boursier J, Diehl AM. Implication of gut microbiota in nonalcoholic fatty liver disease. PLoS Pathog 11: e1004559, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Cao R, Cronk ZX, Zha W, Sun L, Wang X, Fang Y, Studer E, Zhou H, Pandak WM, Dent P, Gil G, Hylemon PB. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(alpha)i-protein-coupled receptors and the AKT pathway. J Lipid Res 51: 2234–2244, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol 6: 110–119, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang JYL, Ferrell JM. Bile acid biology, pathophysiology, and therapeutics. Clin Liver Dis (Hoboken) 15: 91–94, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 78: 1233–1261, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman R, Lowe PJ, Billington D. Membrane lipid composition and susceptibility to bile salt damage. Biochim Biophys Acta 599: 294–300, 1980. [DOI] [PubMed] [Google Scholar]
- 16.Cossiga V, Lembo V, Nigro C, Mirra P, Miele C, D’Argenio V, Leone A, Mazzone G, Veneruso I, Guido M, Beguinot F, Caporaso N, Morisco F. The combination of berberine, tocotrienols and coffee extracts improves metabolic profile and liver steatosis by the modulation of gut microbiota and hepatic miR-122 and miR-34a expression in mice. Nutrients 13: 1281, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson PA. Roles of ileal ASBT and OSTalpha-OSTbeta in regulating bile acid signaling. Dig Dis 35: 261–266, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab 299: E685–E694, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Deng Y, Tang K, Chen R, Nie H, Liang S, Zhang J, Zhang Y, Yang Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp Ther Med 17: 2091–2098, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devlin AS, Fischbach MA. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat Chem Biol 11: 685–690, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do MH, Lee E, Oh MJ, Kim Y, Park HY. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 10: 571731, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donner MG, Schumacher S, Warskulat U, Heinemann J, Haussinger D. Obstructive cholestasis induces TNF-alpha- and IL-1-mediated periportal downregulation of Bsep and zonal regulation of Ntcp, Oatp1a4, and Oatp1b2. Am J Physiol Gastrointest Liver Physiol 293: G1134-G1146, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science 308: 1635–1638, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorucci S, Biagioli M, Zampella A, Distrutti E. Bile acids activated receptors regulate innate immunity. Front Immunol 9: 1853, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geier A, Zollner G, Dietrich CG, Wagner M, Fickert P, Denk H, van Rooijen N, Matern S, Gartung C, Trauner M. Cytokine-independent repression of rodent Ntcp in obstructive cholestasis. Hepatology 41: 470–477, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science 362: 776–780, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Gu S, Cao B, Sun R, Tang Y, Paletta JL, Wu X-L, Liu L, Zha W, Zhao C, Li Y, Radlon JM, Hylemon PB, Zhou H, Aa J, Wang G. A metabolomic and pharmacokinetic study on the mechanism underlying the lipid-lowering effect of orally administered berberine. Mol Biosyst 11: 463–474, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habtemariam S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol Res 155: 104722, 2020. [DOI] [PubMed] [Google Scholar]
- 30.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325: 1254–1257, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajiaghamohammadi AA, Ziaee A, Samimi R. The efficacy of licorice root extract in decreasing transaminase activities in non-alcoholic fatty liver disease: A randomized controlled clinical trial. Phytother Res 26: 1381–1384, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Hannou SA, Haslam DE, McKeown NM, Herman MA. Fructose metabolism and metabolic disease. J Clin Invest 128: 545–555, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology 150: 956–967, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoskins LC, Boulding ET. Degradation of blood group antigens in human colon ecosystems. II. A gene interaction in man that affects the fecal population density of certain enteric bacteria. J Clin Invest 57: 74–82, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hylemon PB, Takabe K, Dozmorov M, Nagahashi M, Zhou H. Bile acids as global regulators of hepatic nutrient metabolism. Liver Res 1: 10–16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res 50: 1509–1520, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A 103: 3920–3925, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141: 1773–1781, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DH, Tolan DR, Sanchez-Lozada LG, Rosen HR, Lanaspa MA, Diehl AM, Johnson RJ. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol 68: 1063–1075, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang JD, Myers CJ, Harris SC, Kakiyama G, Lee IK, Yun BS, Matsuzaki K, Furukawa M, Min HK, Bajaj JS, Zhou H, Hylemon PB. Bile acid 7alpha-dehydroxylating gut bacteria secrete antibiotics that inhibit clostridium difficile: Role of secondary bile acids. Cell Chem Biol 26: 27–34 e24, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: The FXR-FGF15/19 pathway. Dig Dis 33: 327–331, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Köck K, Ferslew BC, Netterberg I, Yang K, Urban TJ, Swaan PW, Stewart PW, Brouwer KLR. Risk factors for development of cholestatic drug-induced liver injury: Inhibition of hepatic basolateral bile acid transporters multidrug resistance-associated proteins 3 and 4. Drug Metab Dispos 42: 665–674, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lake AD, Novak P, Shipkova P, Aranibar N, Robertson D, Reily MD, Lu Z, Lehman-McKeeman LD, Cherrington NJ. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol Appl Pharmacol 268: 132–140, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124: 837–848, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Li CH, Tang SC, Wong CH, Wang Y, Jiang JD, Chen Y. Berberine induces miR-373 expression in hepatocytes to inactivate hepatic steatosis associated AKT-S6 kinase pathway. Eur J Pharmacol 825: 107–118, 2018. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Liu R, Yang J, Sun L, Zhang L, Jiang Z, Puri P, Gurley EC, Lai G, Tang Y, Huang Z, Pandak WM, Hylemon PB, Zhou H. The role of long noncoding RNA H19 in gender disparity of cholestatic liver injury in multidrug resistance 2 gene knockout mice. Hepatology 66: 869–884, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Liu T, Yan C, Xie R, Guo Z, Wang S, Zhang Y, Li Z, Wang B, Cao H. Diammonium glycyrrhizinate protects against nonalcoholic fatty liver disease in mice through modulation of gut microbiota and restoration of intestinal barrier. Mol Pharm 15: 3860–3870, 2018. [DOI] [PubMed] [Google Scholar]
- 48.Liu R, Li X, Hylemon PB, Zhou H. Conjugated bile acids promote invasive growth of esophageal adenocarcinoma cells and cancer stem cell expansion via sphingosine 1-phosphate receptor 2-mediated yes-associated protein activation. Am J Pathol 188: 2042–2058, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu R, Li X, Qiang X, Luo L, Hylemon PB, Jiang Z, Zhang L, Zhou H. Taurocholate induces cyclooxygenase-2 expression via the sphingosine 1-phosphate receptor 2 in a human cholangiocarcinoma cell line. J Biol Chem 290: 30988–31002, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, Zhang X, Zhang L, Shi R, Wang G, Pandak WM, Sirica AE, Hylemon PB, Zhou H. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology 60: 908–918, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov 7: 522–538, 2017. [DOI] [PubMed] [Google Scholar]
- 52.Lu SS, Yu YL, Zhu HJ, Liu XD, Liu L, Liu YW, Wang P, Xie L, Wang GJ. Berberine promotes glucagon-like peptide-1 (7–36) amide secretion in streptozotocin-induced diabetic rats. JEndocrinol 200: 159–165, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Madak-Erdogan Z, Gong P, Zhao YC, Xu L, Wrobel KU, Hartman JA, Wang M, Cam A, Iwaniec UT, Turner RT, Twaddle NC, Doerge DR, Khan IA, Katzenellenbogen JA, Katzenellenbogen BS, Helferich WG. Dietary licorice root supplementation reduces diet-induced weight gain, lipid deposition, and hepatic steatosis in ovariectomized mice without stimulating reproductive tissues and mammary gland. Mol Nutr FoodRes 60: 369–380, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mai W, Xu Y,Xu J,Zhao D,Ye L,Yu G,Wang Z,Lu Q,Lin J,Yang T,Gu C, Liu S, Zhong Y, Yang H. Berberine inhibits nod-like receptor family pyrin domain containing 3 inflammasome activation and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP axis. Front Pharmacol 11: 185, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malhi H, Kropp EM, Clavo VF, Kobrossi CR, Han J, Mauer AS, Yong J, Kaufman RJ. C/EBP homologousprotein-induced macrophage apoptosis protects mice from steatohepatitis. JBiolChem 288: 18624–18642, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malinen MM, Ali I, Bezencon J, Beaudoin JJ, Brouwer KLR. Organic solutetransporterOSTalpha/betaisoverexpressedinnonalcoholicsteatohepatitis and modulated by drugs associated with liver injury. Am J Physiol Gastrointest Liver Physiol 314: G597–G609, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7: 189–200, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moszak M, Szulińska M, Walczak-Gałęzewska M, Bogdański P. Nutritional approach targeting gut microbiota in NAFLD—To date. Int J Environ Res Public Health 18: 1616, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol 71: 1216–1228, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, Fung S, Fischer SE, McGilvray IG, Allard JP. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One 11: e0151829, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagahashi M, Takabe K, Liu R, Peng K, Wang X, Wang Y, Hait NC, Wang X, Allegood JC, Yamada A, Aoyagi T, Liang J, Pandak WM, Spiegel S, Hylemon PB, Zhou H. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology 61: 1216–1226, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagahashi M, Yuza K, Hirose Y, Nakajima M, Ramanathan R, Hait NC, Hylemon PB, Zhou H, Takabe K, Wakai T. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. J Lipid Res 57: 1636–1643, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Och A, Podgórski R, Nowak R. Biological activity of berberine—A summary update. Toxins 12: 713, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, Vipperla K, Naidoo V, Mtshali L, Tims S, Puylaert PG, DeLany J, Krasinskas A, Benefiel AC, Kaseb HO, Newton K, Nicholson JK, de Vos WM, Gaskins HR, Zoetendal EG. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun 6: 6342, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker K, Salas M, Nwosu VC. High fructose corn syrup: Production, uses and public health concerns. Biotechnol Mol Biol Rev 5: 9, 2010. [Google Scholar]
- 66.Perez-Burillo S, Hinojosa-Nogueira D, Pastoriza S, Rufian-Henares JA. Plant extracts as natural modulators of gut microbiota community structure and functionality. Heliyon 6: e05474, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min HK, Kumar DP, Kohli R, Zhou H, Hylemon PB, Contos MJ, Idowu M, Sanyal AJ. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 67: 534–548, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Meta HITC, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin S, Tang H, Li W, Gong Y, Li S, Huang J, Fang Y, Yuan W, Liu Y, Wang S, Guo Y, Guo Y, Xu Z. AMPK and its activator berberine in the treatment of neurodegenerative diseases. Curr Pharm Des 26: 5054–5066, 2020. [DOI] [PubMed] [Google Scholar]
- 70.Ren S, Hylemon P, Zhang ZP, Rodriguez-Agudo D, Marques D, Li X, Zhou H, Gil G, Pandak WM. Identification of a novel sulfonated oxysterol, 5-cholesten-3beta,25-diol 3-sulfonate, in hepatocyte nuclei and mitochondria. J Lipid Res 47: 1081–1090, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: Unraveling a complex relationship. Gut Microbes 4: 382–387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7: 22–39, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients 11: 2393, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord 20: 461–472, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schumacher JD, Guo GL. Pharmacologic modulation of bile acid-FXR-FGF15/FGF19 pathway for the treatment of nonalcoholic steatohepatitis. Handb Exp Pharmacol 256: 325–357, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14: e1002533, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi X, Yu L, Zhang Y, Liu Z, Zhang H, Zhang Y, Liu P, Du P. Glycyrrhetinic acid alleviates hepatic inflammation injury in viral hepatitis disease via a HMGB1-TLR4 signaling pathway. Int Immunopharmacol 84: 106578, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, Pandak WM, Dent P, Spiegel S, Shi R, Xu W, Liu X, Bohdan P, Zhang L, Zhou H, Hylemon PB. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 55: 267–276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suga T, Yamaguchi H, Ogura J, Shoji S, Maekawa M, Mano N. Altered bile acid composition and disposition in a mouse model of non-alcoholic steatohepatitis. Toxicol Appl Pharmacol 379: 114664, 2019. [DOI] [PubMed] [Google Scholar]
- 81.Trauner M, Arrese M, Lee H, Boyer JL, Karpen SJ. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J Clin Invest 101: 2092–2100, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang HN, Xiang JZ, Qi Z, Du M. Plant extracts in prevention of obesity. Crit Rev Food Sci Nutr 15: 1–14, 2020. DOI: 10.1080/10408398.2020.1852171. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Tai YL, Zhao D, Zhang Y, Yan J, Kakiyama G, Wang X, Gurley EC, Liu J, Liu J, Liu J, Lai G, Hylemon PB, Pandak WM, Chen W, Zhou H. Berberine prevents disease progression of nonalcoholic steatohepatitis through modulating multiple pathways. Cells 10: 210, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Zhou X, Zhao D, Wang X, Gurley EC, Liu R, Li X, Hylemon PB, Chen W, Zhou H. Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes. PLoS One 15: e0232630, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu X, Zhang L, Gurley E, Studer E, Shang J, Wang T, Wang C, Yan M, Jiang Z, Hylemon PB, Sanyal AJ, Pandak WM, Zhou H. Prevention of free fatty acid-induced hepatic lipotoxicity by 18β-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology 47: 1905–1915, 2008. [DOI] [PubMed] [Google Scholar]
- 86.Xu X, Yi H, Wu J, Kuang T, Zhang J, Li Q, Du H, Xu T, Jiang G, Fan G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed Pharmacother 133: 110984, 2021. [DOI] [PubMed] [Google Scholar]
- 87.Yang L, Jiang Y, Zhang Z, Hou J, Tian S, Liu Y. The anti-diabetic activity of licorice, a widely used Chinese herb. J Ethnopharmacol 263: 113216, 2020. [DOI] [PubMed] [Google Scholar]
- 88.Yi H, Xu D, Wu X, Xu F, Lin L, Zhou H. Isosteviol protects free fatty acid- and high fat diet-induced hepatic injury via modulating PKC-beta/p66Shc/ROS and endoplasmic reticulum stress pathways. Antioxid Redox Signal 30: 1949–1968, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-SunWong V, Yilmaz Y, George J, Fan J, Vos MB. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 69: 2672–2682, 2019. [DOI] [PubMed] [Google Scholar]
- 90.Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Chalasani NP, Anstee QM, Kowdley KV, George J, Goodman ZD, Lindor K. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 68: 361–371, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang L, Wu X, Yang R, Chen F, Liao Y, Zhu Z, Wu Z, Sun X, Wang L. Effects of berberine on the gastrointestinal microbiota. Front Cell Infect Microbiol 10: 588517, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, Ma J, Gu X, Xue Y, Huang S, Yang J, Chen L, Chen G, Qu S, Liang J, Qin L, Huang Q, Peng Y, Li Q, Wang X, Kong P, Hou G, Gao M, Shi Z, Li X, Qiu Y, Zou Y, Yang H, Wang J, Xu G, Lai S, Li J, Ning G, Wang W. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun 11: 5015, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids 86: 62–68, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou J, Cui S, He Q, Guo Y, Pan X, Zhang P, Huang N, Ge C, Wang G, Gonzalez FJ, Wang H, Hao H. SUMOylation inhibitors synergize with FXR agonists in combating liver fibrosis. Nat Commun 11: 240, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu JZ, Yi HW, Huang W, Pang T, Zhou HP, Wu XD. Fatty liver diseases, mechanisms, and potential therapeutic plant medicines. ChinJNat Med 18: 161–168, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]