Abstract
Background
The role of thyroid hormones is crucial in the response to stress and critical illness, which has been reported to be closely associated with a poor prognosis in patients admitted to the intensive care unit (ICU). This study aimed to explore the relationship between thyroid hormone and prognosis in septic shock patients.
Methods
A total of 186 patients with septic shock were enrolled in the analytical study between December 2014 and September 2022. The baseline variables and thyroid hormone were collected. The patients were divided into survivor group and non-survivor group according to whether they died during the ICU hospitalization. Among 186 patients with septic shock, 123 (66.13%) were in the survivor group and 63 (33.87%) were in the non-survivor group.
Results
There were significant differences in the indictors of free triiodothyronine (FT3) (p = 0.000), triiodothyronine (T3) (p = 0.000), T3/FT3 (p = 0.000), acute physiology and chronic health evaluation II score (APACHE II) (p = 0.000), sequential organ failure assessment score (SOFA) (p = 0.000), pulse rate (p = 0.020), creatinine (p = 0.008), PaO2/FiO2 (p = 0.000), length of stay (p = 0.000) and hospitalization expenses (p = 0.000) in ICU between the two groups. FT3 [odds ratio (OR): 1.062, 95% confidence interval(CI): (0.021, 0.447), p = 0.003], T3 (OR: 0.291, 95% CI: 0.172-0.975, p = 0.037) and T3/FT3 (OR: 0.985, 95% CI:0.974-0.996, p = 0.006) were independent risk factors of the short-term prognosis of septic shock patients after adjustment. The areas under the receiver operating characteristic curves for T3 was associated with ICU mortality (AUC = 0.796, p < 0.05) and was higher than that for FT3 (AUC = 0.670, p < 0.05) and T3/FT3 (AUC = 0.712, p < 0.05). A Kaplan-Meier curve showed that patients with T3 greater than 0.48 nmol/L had a significantly higher survival rate than the patients with T3 less than 0.48 nmol/L.
Conclusions
The decrease in serum level of T3 in patients with septic shock is associated with ICU mortality. Early detection of serum T3 level could help clinicians to identify septic shock patients at high risk of clinical deterioration.
Keywords: Septic shock, Thyroid hormone, Prognosis, Mortality, Intensive care unit
Introduction
Sepsis refers to a life-threatening syndrome caused by a dysregulated host response to infection that may progress to fatal shock and contributes to 11.0 million deaths worldwide in 2017, representing the mortality closing to 20% worldwide (Rhodes et al., 2017; Singer et al., 2016; Rudd et al., 2020). Alarmingly, the prevalence and mortality rate is still continuing its upward trend (Suarez De La Rica, Gilsanz & Maseda, 2016). Septic patients frequently require expensive and intensive treatments, which estimated to be $27,461 per case, thus making sepsis a major public health concern (Fleischmann et al., 2016; Arefian et al., 2017; Dietz et al., 2017). Sepsis is associated with dysfunction of multiple organs and hypotension or shock tending to contribute to hypoxia, which would reduce the ability of cells to produce ATP. Thyroid hormones play a crucial role in the response to stress and critical illness. Therefore, the impact of severe systemic diseases on thyroid metabolism is increasingly emphasised by researchers. Decreased thyroid hormones level has often been called low T3 syndrome or euthyroid sick syndrome or nonthyroidal illness syndrome (NTIS) (Liu et al., 2016; Van den Berghe, 2014), which has been described as the key role of thyroid hormones in counteracting biochemical catabolism leading to sepsis or septic shock (Warner & Beckett, 2010).
The relationship between thyroid hormone and the severity of sepsis/septic shock is getting more and more attention (Langouche, Jacobs & Van den Berghe, 2019; Dietrich et al., 2008; Bertoli et al., 2017). A study from China showed that free riiodothyronine (FT3) could be used as a predictor of all-cause mortality in ICU patients more than a decade ago, and the predictive efficacy was further improved when combined with the APACHE II score (Wang et al., 2012). However, the study did not specifically observe the effect of thyroid hormones on outcomes in patients with septic shock. Subsequent research found that early detection of serum FT3 and FT4 levels could help clinicians to identify patients at high risk of clinical deterioration (Liu et al., 2021). Nevertheless, the study enrolled smaller numbers of critically ill patients and the origin of septic shock patients was limited to EICU, which could not be representive of most ICU septic shock patients in China and around the world. Considering the aforementioned condition, whether current thyroid hormone level could be used as a prognostic indicator of septic shock patients is still controversial and needed further study.
Therefore, further research is needed to clarify the association between NTIS and the prognosis of septic shock patients in the ICU. The aim of this study was to undertake a investigation of septic shock patients admitted to all ICU in our hospital to identify the prognostic value of thyroid hormones levels and provide reference for clinical research of thyroid hormone in the treatment of septic shock.
Patients and Methods
Participants
This single-center, retrospective study recruited patients admitted to the ICU of Nanjing Hospital Affiliated to Nanjing Medical University between December 2014 and September 2022. The septic shock was defined according to the diagnostic criteria of Sepsis and Septic Shock 3.0 published in 2016 (Rhodes et al., 2017). The exclusion criteria were as follows: (1) Patients with less than 24 h stay or more than 30 days in ICU, (2) patients with hypothalamic-pituitary disease, (3) patients administered with antithyroid drugs or other iodine containing drugs in one month, e.g., miodarone and glucocorticoid, (4) patients with severe immunodefciency and hypothyroid or hyperthyroid diagnosis history, (5) age <18 years. The enrolled patients were divided into survivor group and non-survivor group according to whether they died during the ICU hospitalization. The survivor group was defined as patients who improved after treatment and were discharged or transferred from ICU to the general ward. The non-survivor group was defined as patients who died after treatment during the ICU hospitalization.
Data collection
Datebase—Data for the study analysis were derived from the electronic medical records (EMR) which include demographic information, comorbid diseases such as coronary heart disease (60.22%, 112/186), hypertension (36.56%, 68/186), diabetes mellitus (26.88%, 50/186) and cerebrovascular disease (17.74%, 33/186). The infected site of septic shock leading to ICU admission such as respiratory infection (4.09%, 82/186), urinary tract infection (23.12%, 43/186), bloodstream infection (7.53%, 14/186), abdominal cavity infection (7.53%, 14/186) and others (4.30%, 8/186) were recorded.
Blood samples were taken from the patients on the first day of the ICU admission for detection of laboratory indicators and thyroid hormone, before the drug treatment started. Levels of FT3, FT4, T3, T4 and TSH were measured on the next morning after ICU admission by the chemiluminescent immunometric assay method using an Abbott ARCHITECT i2000 analyzer (Abbott, Chicago, IL, USA) in the Department of Nuclear Medicine of our hospital. In order to ensure the consistency of T3 and FT3 units, T3*1000/FT3 is taken as the ratio of T3/FT3. According to the manufacturer’s instructions, the normal reference intervals used were: 2.63−5.7 pmol/L for FT3, 0.89−2.44 nmol/L for T3, 9.0–19.0 pmol/L for FT4, 62.68–150.84 nmol/L for T4 and 0.35−4.94 mIU/L for TSH.
The septic shock patients’ vital signs and related laboratory indicators were recorded upon admission to the ICU, including systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), blood gas analysis, white blood cell (WBC), procalcitonin (PCT), interleukin-6 (IL-6), platelet (PLT) and creatinine. Blood gas analysis including the indicator of lactic acid were detected in the ICU using a multifunction blood gas analyzer (Rayto, USA). The other laboratory INDICATORS were detected by the Laboratory Department of Nanjing First Hospital.
The acute physiology and chronic health evaluation II score (APACHE II) and sequential organ failure assessment score (SOFA) were completed within 24 h of admission to the ICU for the enrolled patients. The duration of mechanical ventilation, length of stay in ICU, whether to use continuous renal replacement therapy (CRRT) and costs of hospitalization were recorded.
Research ethics
This study protocol was reviewed and approved by the Institutional Review Board of Nanjing Hospital Affiliated to Nanjing Medical University (No. KY20201102-03) and was conducted in accordance with the Declaration of Helsinki. The requirement of informed consent for the study was exempt due to restrained database access for analysis purposes only.
Statistical analyses
All data were analyzed using SPSS 20.0. Data were presented as median and interquartile ratio (IQR) or numbers, as appropriate. Continuous and categorical variables were compared using Mann–Whitney U test. Univariate and multivariable logistic regression analysis were conducted to identify the independent risk factors for ICU mortality. The receiver operating characteristic (ROC) curves for the ability of thyroid hormone to predict the ICU mortality were analyzed. Kaplan–Meier analysis was used to estimate the probability of survival and comparison was made by Log-rank test. A P value of <0.05 was considered statistically significant.
Results
Baseline of patient characteristics
There were two hundred and forty-seven patients who met the septic shock definition by Sepsis-3. Sixty-one patients were excluded based on exclusion criteria, resulting in a cohort of one hundred and eighty-six patients, including one hundred and twenty-three patients in the survivor group and sixty-three patients in the non-survivor group (Fig. 1). The median age in survivor group was 72.38 ± 9.64 years with 61.79% being men and the non-survivor group was 74.57 ± 8.57 years with 69.84% being men. The baseline and clinical characteristics of the study population upon shock recognition were collected, including demographic information, infection sites leading to ICU admission, past medical history, vital signs, APACHE II score at shock recognition and laboratory indicators. The non-survivor group had a higher APACHE II score, SOFA score and creatinine level (30.28 ± 6.67 vs 24.03 ± 5.90, p = 0.000; 12.14 ± 3.00 vs 8.67 ± 3.12, p = 0.000; 299.38 ± 125.19 vs 248.20 ± 123.59, p = 0.008), while the PaO2/FiO2 in the non-survivor group was significantly lower than that in the survivor group [165.43 (98.63, 200.39) vs 217.54 (198.63, 267.32)]. In addition, no significant difference was found in infection sites, past medical history, WBC, PCT, IL-6 and albumin and so on. Details of the two groups are shown in Table 1.
Figure 1. Flow chart of patients in the study cohort.
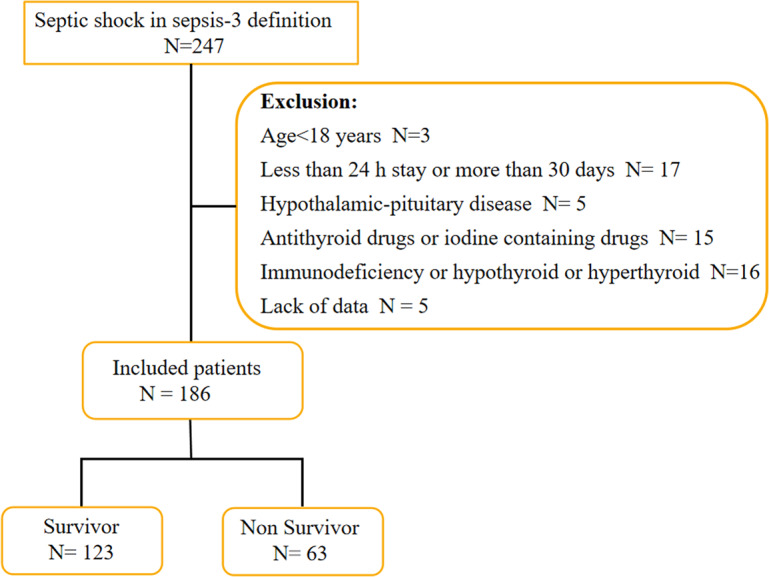
Table 1. Baseline and clinical characteristics of the study population.
| Patients characteristics | Survivors (n = 123) | Non-survivors (n = 63) | P |
|---|---|---|---|
| Male, n (%) | 76 | 44 | 0.277 |
| Age, mean ± SD | 72.38 ± 9.64 | 74.57 ± 8.57 | 0.199 |
| Infection sites leading to ICU admission, n(%) | 0.761 | ||
| Respiratory infection | 55 (44.72%) | 27 (42.85%) | |
| Urinary tract infection | 27 (21.95%) | 16 (25.40%) | |
| Bloodstream infections | 11 (8.94%) | 3 (4.76%) | |
| Abdominal cavity infection | 24 (19.51%) | 15 (23.81%) | |
| Others | 6 (4.88%) | 2 (3.17%) | |
| Past medical history, n (%) | 0.877 | ||
| Coronary heart disease | 65 (85.53%) | 47 (74.60%) | |
| Hypertension | 42 (55.26%) | 26 (41.27%) | |
| Diabetes mellitus | 32 (42.11%) | 18 (28.57%) | |
| Cerebrovascular disease | 19 (25%) | 14 (22.22%) | |
| APACHE II score, mean ± SD | 24.03 ± 5.90 | 30.28 ± 6.67 | 0.000 |
| SOFA score, mean ± SD | 8.67 ± 3.12 | 12.14 ± 3.00 | 0.000 |
| Vital signs on ICU admission | |||
| SBP, mmHg, median (IQR) | 82.00 (78.00, 87.00) | 85.00 (79.00, 90.00) | 0.146 |
| DBP, mmHg, median (IQR) | 50.00 (48.00, 60.00) | 50.00 (45.00, 60.00) | 0.273 |
| MAP, mmHg, median (IQR) | 61.67 (57.33. 68.33) | 61.67 (56.67, 70.00) | 0.915 |
| Pulse rate, beats/min,, median (IQR) | 123.00 (104.00, 139.00) | 147.00 (105.00, 153.00) | 0.020 |
| Body temperature, °C, mean ± SD | 38.42 ± 0.71 | 38.59 ± 0.71 | 0.137 |
| Respiratory rate, breaths/min, mean ± SD | 33.87 ± 9.77 | 35.02 ± 11.43 | 0.476 |
| Laboratory indicators | |||
| WBC, ×109/L, mean ± SD | 16.32 ± 8.18 | 14.40 ± 7.17 | 0.116 |
| PCT, ng/mL, median (IQR) | 16.27 (5.31, 35.10) | 16.87 (5.52, 35.29) | 0.368 |
| IL-6, pg/mL, median (IQR) | 84.27 (27.38, 243.00) | 129.91 (26.31, 1015,24) | 0.086 |
| Platelet, ×109 /L, median (IQR) | 90.00 (65.00, 190.00) | 140.00 (57.00, 210.00) | 0.390 |
| Albumin, g/L, median (IQR) | 30.00 (27.00, 43.00) | 31.00 (27.00, 40.00) | 0.539 |
| Creatinine, μmol/L, mean ± SD | 248.20 ± 123.59 | 299.38 ± 125.19 | 0.008 |
| PaO2/FiO2, mmHg, median (IQR) | 217.54 (198.63, 267.32) | 165.43 (98.63, 200.39) | 0.000 |
| Lactate, mmol/L, median (IQR) | 11.40 (8.80, 12.60) | 11.40 (9.40, 12.80) | 0.653 |
Notes.
- APACHE II score
- acute physiology and chronic health evaluation II score
- ICU
- intensive care unit
- WBC
- White blood cell
- PCT
- Procalcitonin
- SBP
- Systolic blood pressure
- DBP
- Diastolic blood pressure
- MAP
- Mean arterial pressure
- SOFA score
- Sequential Organ Failure Assessment score
- IL-6
- interleukin-6
Serum thyroid hormone levels
Blood samples were taken from 186 patients with septic shock on the first day of the ICU admission for thyroid hormone detection. No statistical difference of the serum TSH, FT4 and T4 seen between non-survivor and survivor groups [0.80 ± 0.79 vs 0.64 ± 0.61, p = 0.175; 12.19 (9.78,12.84) vs 12.32 (10.22,12.49), p = 0.575; 69.41 (63.02,75.30) vs 74.93 (55.75,83.03), p = 0.209]. While the levels of FT3, T3 and T3/FT3 in non-survivor group were significantly lower than that in survivor group (all p = 0.000; Fig. 2).
Figure 2. Serum level of FT3, FT4, TSH, T3,T4 and T3/FT3 in survivors and non-survivors.
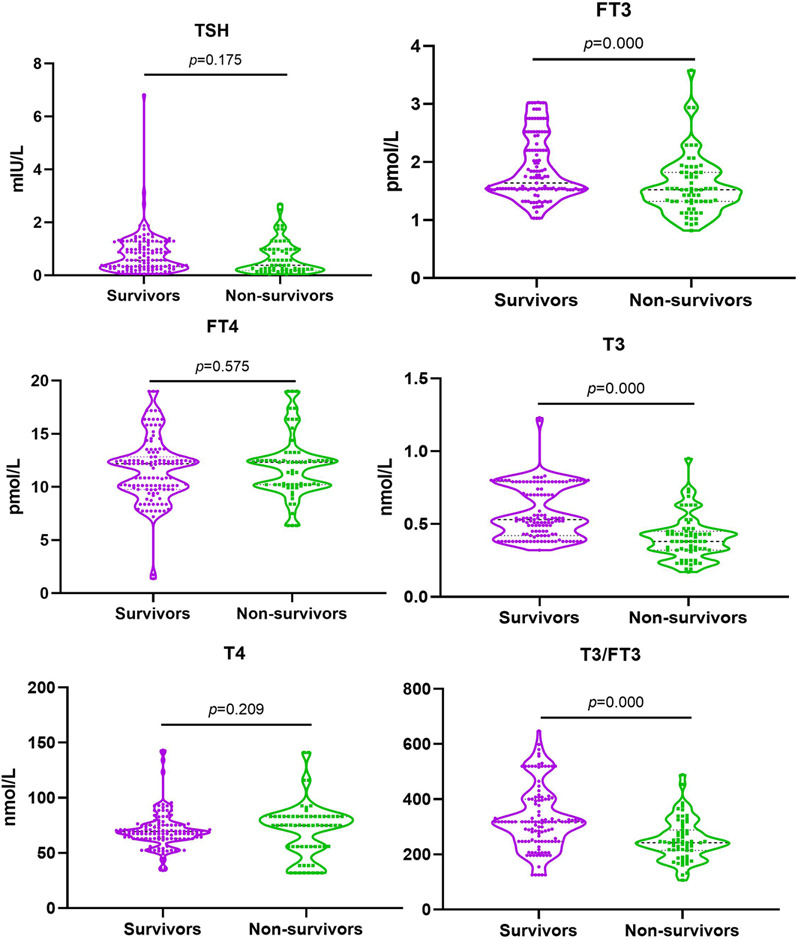
Abbreviations: FT3, free triiodothyronine; FT4, free thyroxine; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone. Comparison between the survivors and non-survivors groups using independent sample T test. P < 0.05.
Comparison of outcomes
The mechanical ventilation (MV) rate was higher in non-survivor group than that in the survivors group (χ2 = 12.148, p = 0.000). The length of ICU stay in the survivor group was longer and renal replacement therapy rate decreased significantly than that in the non-survivor group [7.00 (5.00,11.00) vs 5.00 (2.00, 9.00), p = 0.000; 6.50% vs 36.51%, χ2 = 11.600, p = 0.000]. It is worth noting that the costs of hospitalization in ICU were significantly higher in the non-survivor group than that in the survivor group (6.14 ± 4.67 vs 8.73 ± 8.23, p = 0.000). Details of the two groups are shown in Table 2.
Table 2. Outcomes of the two groups.
| Outcomes | Survivors (n = 123) | Non-survivors (n = 63) | P |
|---|---|---|---|
| MV rate,n (%) | 61 (49.59%) | 48 (76.19%) | 0.000 |
| Duration of MV (d) | 5.28 ± 3.77 | 5.94 ± 4.88 | 0.303 |
| Renal replacement therapy, n (%) | 18 (14.63%) | 23 (36.51%) | 0.001 |
| Length of ICU stay (d), median (IQR) | 7.00 (5.00,11.00) | 5.00 (2.00, 9.00) | 0.000 |
| Hospitalization expenses in ICU, mean ± SD, (×10,000 yuan) | 6.14 ± 4.67 | 8.73 ± 8.23 | 0.000 |
Notes.
- MV
- mechanical ventilation
- ICU
- intensive care unit
- IQR
- interquartile range
Factors associated with ICU mortality
The multivariable logistical regression analysis suggested that FT3, T3, T3/FT3, Creatinine and PaO2/FiO2 were independent factors for ICU mortality (FT3: OR = 0.097, 95%CI [0.021–0.447], p = 0.075; T3:OR =0.291, 95%CI [0.172–0.975], p = 0.037; T3/FT3: OR = 0.985, 95%CI [0.974–0.996], p = 0.006; Creatinine: OR =1.004, 95%CI [1.001–1.008], p = 0.019; PaO2/FiO2: OR = 0.986, 95%CI [0.979–0.993], p = 0.000), as shown in Table 3.
Table 3. The performance of FT3, T3 and T3/FT3 at admission for predicting ICU mortality in ICU septic shock patients.
| Indicators | β | S (S.E.) | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| APACHE II | 0.06 | 0.034 | 3.159 | 0.075 | 1.062 | (0.994, 1.135) |
| SOFA | 0.349 | 0.061 | 33.128 | 0.000 | 1.418 | (1.259, 1.597) |
| FT3 | −2.337 | 0.782 | 8.929 | 0.003 | 0.097 | (0.021, 0.447) |
| T3 | −0.534 | 2.986 | 0.006 | 0.037 | 0.291 | (0.172, 0.975) |
| T3/FT3 | 0.015 | 0.006 | 7.611 | 0.006 | 0.985 | (0.974, 0.996) |
| Creatinine | 0.004 | 0.002 | 5.468 | 0.019 | 1.004 | (1.001, 1.008) |
Notes.
Adjusted for age, gender, baseline APACHE II score and SOFA score.
- APACHE II score
- acute physiology and chronic health evaluation II score
- SOFA score
- sequential organ failure assessment score
- OR
- odds ratio
- CI
- confidence interval
Predictive value of thyroid hormones for ICU mortality
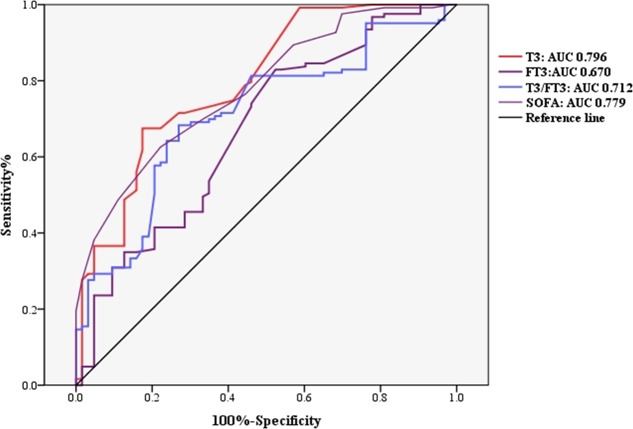
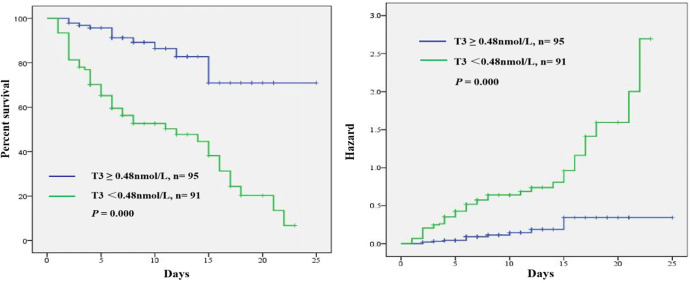
To investigate whether thyroid hormone is useful in predicting ICU mortality in septic shock patients, ROC analysis was conducted using clinical and laboratory indicators. Among the SOFA, serum FT3, T3 and T3/FT3, AUC for serum T3 (AUC 0.796,95% CI [0.727–0.864], p = 0.000) was higher than the AUC for FT3 (AUC 0.670, 95% CI [0.587–0.753], p = 0.000) and T3/FT3 (AUC 0.712, 95% CI [0.635–0.788], p = 0.000) and SOFA (AUC 0.779, 95% CI [0.712–0.845], p = 0.000), as shown in Fig. 3. With a cut-off value of 0.48 nmol/L of T3 determined on the ROC curve in the derivation cohort and Kaplan -Meier survival curves were established (Fig. 4). The septic shock patients’ survival rates were significantly different when stratified according to serum T3 level on day of ICU admission and the patients with higher serum T3 level had better survival than that with lower serum T3 level.
Figure 3. Receiver operating characteristic (ROC) curve analysis.
Abbreviations: FT3, Free triiodothyronine; T3, Triiodothyronine; SOFA score, Sequential organ failure assessment score. ROC curves for ICU mortality prediction of adult patients with septic shock by the serum FT3, T3 and T3/FT3 at admission. The area under the ROC curve (AUC) is shown.
Figure 4. Kaplan Meier survival curves of 186 adult patients with septic shock based on the T3 cut-off value 0.48 nmol/L on day of ICU admission.
A significant difference was measured between the two curves (p < 0.01, log-rank test).
Discussion
In this retrospective study, we sought the potential relationship between thyroid hormone levels measured in the first 24 h after ICU admission and prognosis in patients with septic shock admitted to the intensive care unit (ICU) . The results showed that the mortality of septic shock patients admitted to the ICU is 33.87% (63/186), which is consistent with the previous study (Chambers et al., 2018). In addition, there was a significant lower level of both T3 and FT3 in the non-survivors than that in the survivors. However, we did not find any significant differences in terms of serum TSH, FT4 and T4 levels at admission between the two groups. Although the topic in this study is similiar to the existed studies, some of the differences observed in the present study may be attributed to more patients and statistical indicators included and the difference in the term definition of “survivor and non survivor” (Liu et al., 2021 and Cornu et al., 2020). What is noteworthy is that the length of ICU stay were shorter in the non-survivors, although the ICU hospitalization expenses were higher, which may be due to non-survivors requiring longer periods of CRRT and mechanical ventilation, higher doses of vasoactive drugs besides more critical illness and poorer treatment response.
The univariable analyses suggested that the mortality of septic shock patients admitted to ICU was closely associated with APACHE II score, SOFA score, PaO2/FiO2, FT3, T3 and T3/FT3 levels. The ROC analysis showed that the thyroid hormone T3 has a higher specificity of 82.6% as a single indicator, which is of great prognostic value compared to FT3, T3/FT3 and SOFA score. Higher T3 specificity tends to indicate low false-positive rates. In clinical practice, T3 is a single laboratory test and might provide information about the prognosis of septic shock patients quickly and objectively, which of the predictive value is higher than the indicators of APACHE II, SOFA, FT3 and T3/FT3. Moreover, a cut-off value of 0.48nmol/L T3 indicated a higher risk of ICU mortality.
As we all know, APACHE II score is based on the worst data obtained within the first post-admission and is not recalculated during the patients’ stay. Therefore, the higher the scores reached, the higher the risk of critically ill patients in-hospital mortality. APACHE II score system is considered as ‘gold standards’ in prognostication among severely ill patients in individual ICUs worldwide, due to including lager numbers of indicators that offer significant advantages over a single indicator, and no single indicator has been reported to date to exceed the APACHE II score in terms of the prognostic value for mortality (Vincent & Moreno, 2010; Haq et al., 2014; Alizadeh et al., 2014; Lee et al., 2017; Ryan et al., 2016). In the present study, we found that the APACHE II score in the non-survivors group was significantly higher than that in the survival group,which is consistent with Lee and his research that APACHE II score is strongly associated with mortality in severely ill patients (Lee et al., 2017). However, the P value of APACHE II score was more than 0.05 in the multivariable logistic regression analysis for ICU mortality. Therefore, our team did not explain more about APACHE II score, sensitivity and specificity of APACHE II score compare others, nor did we conduct further studies on the prognosis of APACHE II in patients with septic shock.
The causes of poor prognosis of septic shock patients admitted to ICU due to decreased thyroid hormone levels may include immune regulation disorder, coagulation dysregulation and sepsis related cardiomyopathy (Soehnlein, 2019; Datta & Scalise, 2004; Perrotta et al., 2014; Montesinos & Pellizas, 2019). From an evolutionary point of view, the decreased thyroid hormone levels can be interpreted as an attempt to protect the organism by reducing energy consumption and catabolism and may be beneficial in the acute setting (Ingels, Gunst & Van den Berghe, 2018). However, several studies have reported that decreased thyroid hormone levels could also cause immune system dysfunction (Slag et al., 1981), coagulation system disorder (Luo, Yu & Li, 2017), septic cardiac dysfunction (Iervasi & Nicolini, 2013), and acute respiratory distress syndrome (Kim et al., 2018). Therefore, the question arises whether low thyroid hormone levels should be treated to improve organ function in septic shock. An early experimental study confirmed that thyroid hormone supplementation in septic rats had a beneficial effect and resulted in a lower rate of mortality (Inan et al., 2003). Although some clinical studies have also demonstrated that thyroid hormone supplementation may be helpful in critically ill patients (Kumar et al., 2018), well-conducted randomized controlled trials are needed to further evaluate the role of thyroid hormone supplementation in septic shock. Furthermore, it is worth noting that no significant difference was found in the duration of mechanical ventilation between the two groups in our study, although the non-survivors had a higher mechanical ventilation rate (48/63 vs 61/123, p = 0.000). The above phenomenon may be related to the inclusion of non-invasive mechanical ventilation into the category of mechanical ventilation.
However, we could not ignored that there were several limitations in the present study. First, the decrease in serum thyroid hormones levels of septic shock patients is a dynamic process developing over time (Boelen et al., 2004). But we only detected the thyroid hormoneone levle in the first 24 h of ICU admission, therefore, we could not observe the changs in thyroid hormone levels of septic shock patients during the ICU treatment. In addition, the result of endocrine-based testing may be related to the timing of sampling. It has been reported that serum thyroid hormone level may require 4 days to reach a nadir after the onset of critical illness (Woolf et al., 1988). Finally, dopamine, which can induce iatrogenic hypothyroidism in patients with critical illness (Van den Berghe, De Zegher & Lauwers, 1994a; Van den Berghe, De Zegher & Lauwers, 1994b; Schilling et al., 2004), was not excluded as a confounder in our study.
In conclusion, the data presented in this study pointed out a good performance of thyroid hormone indices in predicting the patients of septic shock, and septic shock patients with higher T3 were associated with good outcome, which can potentially be taken into consideration as new prognostic factors in sepsis shock patients. Of course, the present study has given some insight into the issue and further studies ideally multicentric and prospective with a larger cohort will be needed to corroborate these findings. On all accounts, we should pay more attention to the thyroid hormones of septic shock patients upon admission in ICU.
Supplemental Information
Funding Statement
This work was supported by the Nanjing medical science and technology development plan project of China (YKK22115, YKK20114). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Caizhi Sun conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Lei Bao conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Lei Guo performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Jingjing Wei performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Yang Song performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Hua Shen performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Haidong Qin analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study protocol was reviewed and approved by the Institutional Review Board of Nanjing Hospital Affiliated to Nanjing Medical University (No. KY20201102-03) and was conducted in accordance with the Declaration of Helsinki.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.
References
- Alizadeh et al. (2014).Alizadeh AM, Hassanian-Moghaddam H, Shadnia S, Zamani N, Mehrpour O. Simplified acute physiology score II/acute physiology and chronic health evaluation II and prediction of the mortality and later development of complications in poisoned patients admitted to intensive care unit. Basic & Clinical Pharmacology & Toxicology. 2014;115:297–300. doi: 10.1111/bcpt.12210. [DOI] [PubMed] [Google Scholar]
- Arefian et al. (2017).Arefian H, Heublein S, Scherag A, Brunkhorst FM, Younis MZ, Moerer O, Fischer D, Hartmann M. Hospital-related cost of sepsis: a systematic review. Journal of Infection. 2017;74:107–117. doi: 10.1016/j.jinf.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Van den Berghe (2014).Van den Berghe G. Non-thyroidal illness in the ICU: a syndrome with different faces. Thyroid. 2014;24:1456–1465. doi: 10.1089/thy.2014.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe, De Zegher & Lauwers (1994a).Van den Berghe G, De Zegher F, Lauwers P. Dopamine and the sick euthyroid syndrome in critical illness. Clinical Endocrinology. 1994a;41:731–737. doi: 10.1111/j.1365-2265.1994.tb02787.x. [DOI] [PubMed] [Google Scholar]
- Van den Berghe, De Zegher & Lauwers (1994b).Van den Berghe G, De Zegher F, Lauwers P. Dopamine suppresses pituitary function in infants and children. Critical Care Medicine. 1994b;22:1747–1753. doi: 10.1097/00003246-199422110-00008. [DOI] [PubMed] [Google Scholar]
- Bertoli et al. (2017).Bertoli A, Valentini A, Cianfarani MA, Gasbarra E, Tarantino U, Federici M. Low FT3: a possible marker of frailty in the elderly. Clinical Interventions in Aging. 2017;12:335–341. doi: 10.2147/CIA.S125934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelen et al. (2004).Boelen A, Kwakkel J, Thijssen-Timmer DC, Alkemade A, Fliers E, Wiersinga WM. Simultaneous changes in central and peripheral components of the hypothalamus-pituitary-thyroid axis in lipopolysaccharide-induced acute illness in mice. Journal of Endocrinology. 2004;182:315–323. doi: 10.1677/joe.0.1820315. [DOI] [PubMed] [Google Scholar]
- Chambers et al. (2018).Chambers KA, Park AY, Banuelos RC, Darger BF, Akkanti BH, Macaluso A, Thangam M, Doshi PB. Outcomes of severe sepsis and septic shock patients after stratification by initial lactate value. World Journal of Emergency Medicine. 2018;9:113–117. doi: 10.5847/wjem.j.1920-8642.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu et al. (2020).Cornu MG, Martinuzzi ALN, Roel P, Sanhueza L, Sepúlveda ME, Orozco MS, Sánchez CA, Gulino M. Incidence of low-triiodothyronine syndrome in patients with septic shock. Revista Brasileira de Terapia Intensiva. 2020;32:514–520. doi: 10.5935/0103-507X.20200088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta & Scalise (2004).Datta D, Scalise P. Hypothyroidism and failure to wean in patients receiving prolonged mechanical ventilation at a regional weaning center. Chest. 2004;126:1307–1312. doi: 10.1016/S0012-3692(15)31311-8. [DOI] [PubMed] [Google Scholar]
- Dietrich et al. (2008).Dietrich JW, Stachon A, Antic B, Klein HH, Hering S. The AQUA-FONTIS study: protocol of a multidisciplinary, cross-sectional and prospective longitudinal study for developing standardized diagnostics and classification of non-thyroidal illness syndrome. BMC Endocrine Disorders. 2008;8:13. doi: 10.1186/1472-6823-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz et al. (2017).Dietz BW, Jones TK, Small DS, Gaieski DF, Mikkelsen ME. The relationship between index hospitalizations, sepsis, and death or transition to hospice care during 30-day hospital readmissions. Medical Care. 2017;55:362–370. doi: 10.1097/MLR.0000000000000669. [DOI] [PubMed] [Google Scholar]
- Fleischmann et al. (2016).Fleischmann C, Thomas-Rueddel DO, Hartmann M, Hartog CS, Welte T, Heublein S, Dennler U, Reinhart K. Hospital incidence and mortality rates of sepsis. Deutsches Arzteblatt International. 2016;113:159–166. doi: 10.3238/arztebl.2016.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq et al. (2014).Haq A, Patil S, Parcells AL, Chamberlain RS. The simplified acute physiology score III is superior to the simplified acute physiology score II and acute physiology and chronic health evaluation II in predicting surgical and ICU mortality in the oldest old. Current Gerontology and Geriatrics Research. 2014;2014:934852. doi: 10.1155/2014/934852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iervasi & Nicolini (2013).Iervasi G, Nicolini G. Thyroid hormone and cardiovascular system: from basic concepts to clinical application. Internal and Emergency Medicine. 2013;8(Supp 1):S71–S74. doi: 10.1007/s11739-013-0911-4. [DOI] [PubMed] [Google Scholar]
- Inan et al. (2003).Inan M, Koyuncu A, Aydin C, Turan M, Gokgoz S, Sen M. Thyroid hormone supplementation in sepsis: an experimental study. Surgery Today. 2003;33:24–29. doi: 10.1007/s005950300004. [DOI] [PubMed] [Google Scholar]
- Ingels, Gunst & Van den Berghe (2018).Ingels C, Gunst J, Van den Berghe G. Endocrine and metabolic alterations in sepsis and implications for treatment. Critical Care Clinics. 2018;34:81–96. doi: 10.1016/j.ccc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2018).Kim JG, Shin H, Kim W, Lim TH, Jang B, Cho Y, Choi KS, Ahn C, Lee J, Na MK. The value of decreased thyroid hormone for predicting mortality in adult septic patients: a systematic review and meta-analysis. Scientific Reports. 2018;8:14137. doi: 10.1038/s41598-018-32543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. (2018).Kumar E, McCurdy MT, Koch CA, Hamadah A, Fülöp T, Gharaibeh KA. Impairment of thyroid function in critically ill patients in the intensive care units. The American Journal of the Medical Sciences. 2018;355:281–285. doi: 10.1016/j.amjms.2017.06.026. [DOI] [PubMed] [Google Scholar]
- Langouche, Jacobs & Van den Berghe (2019).Langouche L, Jacobs A, Van den Berghe G. Nonthyroidal illness syndrome across the ages. Journal of the Endocrine Society. 2019;3:2313–2325. doi: 10.1210/js.2019-00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2017).Lee JH, Hwang SY, Kim HR, Kim YW, Kang MJ, Cho KW, Lee DW, Kim YH. Effectiveness of the sequential organ failure assessment, acute physiology and chronic health evaluation II, and simplified acute physiology score II prognostic scoring systems in paraquat-poisoned patients in the intensive care unit. Human & Experimental Toxicology. 2017;36:431–437. doi: 10.1177/0960327116657602. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2016).Liu J, Wu X, Lu F, Zhao L, Shi L, Xu F. Low T3 syndrome is a strong predictor of poor outcomes in patients with community-acquired pneumonia. Scientific Reports. 2016;6:22271. doi: 10.1038/srep22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2021).Liu YC, Jiang TY, Chen ZS, Qi AL, Gao YL, Li SX, Yu MM, Chai YF, Shou ST. Thyroid hormone disorders: a predictor of mortality in patients with septic shock defined by Sepsis-3? Internal and Emergency Medicine. 2021;16:967–973. doi: 10.1007/s11739-020-02546-2. [DOI] [PubMed] [Google Scholar]
- Luo, Yu & Li (2017).Luo B, Yu Z, Li Y. Thyroid hormone disorders and sepsis. Bio-Medical Materials and Engineering. 2017;28:S237–S241. doi: 10.3233/BME-171646. [DOI] [PubMed] [Google Scholar]
- Montesinos & Pellizas (2019).Montesinos MDM, Pellizas CG. Thyroid hormone action on innate immunity. Frontiers in Endocrinology. 2019;10:350. doi: 10.3389/fendo.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta et al. (2014).Perrotta C, Buldorini M, Assi E, Cazzato D, Palma CDe, Clementi E, Cervia D. The thyroid hormone triiodothyronine controls macrophage maturation and functions: protective role during inflammation. The American Journal of Pathology. 2014;184:230–247. doi: 10.1016/j.ajpath.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Rhodes et al. (2017).Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Poll TVander, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Medicine. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- Rudd et al. (2020).Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan et al. (2016).Ryan HM, Sharma S, Magee LA, Ansermino JM, MacDonell K, Payne BA, Walley KR, Von Dadelszen P. The usefulness of the APACHE II score in obstetric critical care: a structured review. Journal of Obstetrics and Gynaecology Canada. 2016;38:909–918. doi: 10.1016/j.jogc.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Schilling et al. (2004).Schilling T, Gründling M, Strang CM, Möritz KU, Siegmund W, Hachenberg T. Effects of dopexamine, dobutamine or dopamine on prolactin and thyreotropin serum concentrations in high-risk surgical patients. Intensive Care Medicine. 2004;30:1127–113. doi: 10.1007/s00134-004-2279-4. [DOI] [PubMed] [Google Scholar]
- Singer et al. (2016).Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, Poll Tvander, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slag et al. (1981).Slag MF, Morley JE, Elson MK, Crowson TW, Nuttall FQ, Shafer RB. Hypothyroxinemia in critically ill patients as a predictor of high mortality. JAMA. 1981;245:43–45. doi: 10.1001/jama.1981.03310260021020. [DOI] [PubMed] [Google Scholar]
- Soehnlein (2019).Soehnlein O. Neutrophil research, Quo Vadis? Trends in Immunology. 2019;40:561–564. doi: 10.1016/j.it.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Suarez De La Rica, Gilsanz & Maseda (2016).Suarez De La Rica A, Gilsanz F, Maseda E. Epidemiologic trends of sepsis in western countries. Annals of Translational Medicine. 2016;4:325. doi: 10.21037/atm.2016.08.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent & Moreno (2010).Vincent JL, Moreno R. Clinical review: scoring systems in the critically ill. Critical Care. 2010;14:207. doi: 10.1186/cc8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2012).Wang F, Pan W, Wang H, Wang S, Pan S, Ge J. Relationship between thyroid function and ICU mortality: a prospective observation study. Critical Care. 2012;16(1):R11. doi: 10.1186/cc11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner & Beckett (2010).Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. Journal of Endocrinology. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- Woolf et al. (1988).Woolf PD, Lee LA, Hamill RW, McDonald JV. Thyroid test abnormalities in traumatic brain injury: correlation with neurologic impairment and sympathetic nervous system activation. The American Journal of Medicine. 1988;84:201–208. doi: 10.1016/0002-9343(88)90414-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.