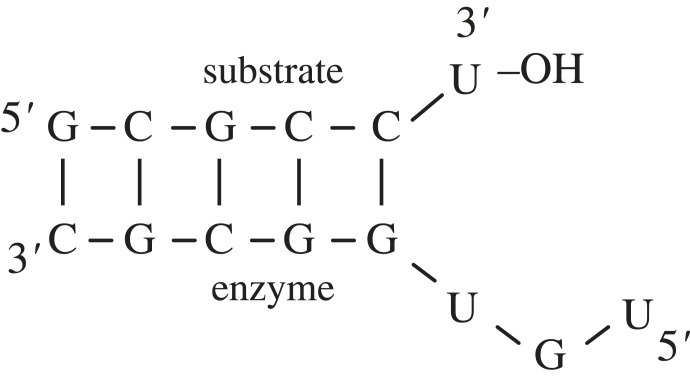Abstract
We present a scenario for the origin of biological coding, a semiotic relationship between chemical information stored in one location that links to chemical information stored in a separate location. Coding originated from cooperation between two, originally separate, collectively autocatalytic sets (CASs), one for nucleic acids and one for peptides. Upon interaction, a series of RNA folding-directed processes led to their joint cooperativity. The aminoacyl adenylate was the first covalent association made by these two CASs and solidified their interdependence, and is a palimpsest of this era, a relic of the original semiotic relationship between RNA and proteins. Coding was driven by selection pressure to eliminate waste in CASs. Eventually a 1 : 1 relationship between single amino acids and short RNA pieces was established, i.e. the ‘genetic code’. The two classes of aaRS enzymes are remnants of the complementary information in two RNA strands, as postulated by Rodin and Ohno. Every stage in the evolution of coding was driven by the downward selection on the components of a system to satisfy the Kantian whole. Coding was engendered because there were two chemically distinct classes of polymers needed for open-ended evolution; systems with only one polymer cannot exhibit this characteristic. Coding is thus synonymous with life as we know it.
Keywords: origins of life, coding, RNA, peptides, autocatalytic sets, mixed anhydrides
1. Introduction
The evolution of the genetic code is a massive mystery; there have been thousands of papers theorizing its origin and evolution (cf. [1,2]). But it is important to realize that the origins of biological coding as a phenomenon and the origins of the genetic code per se were fundamentally different events. The former preceded the latter, and it is possible that the two events were causally related.
The relationship between coding and the genetic code has been queried, mainly from the viewpoint of information theory. Gatlin [3] and later Yockey [4] focused on the parallels between computer coding and the biological genetic code. These authors emphasized the mapping aspect of the genetic code, and the types of functions that could map a set of nucleotides onto a set of amino acids. Yockey [4] framed the problem in terms of set theory and reiterated a definition of a code from that perspective as, ‘a unique mapping of the letters of alphabet A on to the letters of alphabet B’, following Perlwitz et al. [5].
No matter how it is perceived, coding is a situation in which information about an object or event is stored somewhere else. This is, at heart, an issue of semiotics. Because biology is a subset of chemistry having unique processes, we therefore need to consider semiotics from a chemical point of view. One informational chemical must point to another, as a sign, a signal or an icon. In the realm of nascent life, we must figure out why, and how, coding arose, and then deduce its downstream influences on the biotic world. Davies noted that ‘real’ life required coding; you can envisage trivial replicators but until you have coding, they do not advance [6].
Today, there is a distinct co-linearity between information stored in nucleotides (RNA) and information stored in amino acids (polypeptides). We perceive the information thus as ‘coded’ such that there must be a decoding apparatus that is responsible for chemically reconfiguring (translating) the information stored in RNA into polypeptides. This decoding is carried out by the joint action of aminoacyl tRNA (aaRS) enzymes, tRNAs and the ribosome. Humans can refer to the ‘genetic code’ as a look-up table to see the mapping of RNA information in codon (or anticodon) triplets and the amino acids that they specify. The question of why certain codons specify their cognate amino acids is a long-standing one in biology and has been the subject of 60 years of (mostly theoretical) inquiry. But the more fundamental question of why a code exists in the first place, and how it came to be—chemically and evolutionarily—remains open and understudied in origins research. The simple answer that information storage is best in nucleic acids and information manifestation is best in proteins, and therefore there must be some code, is not sufficient. At first glance, the chemistry of nucleotides and the chemistry of amino acids are so different that it is not apparent how a code could have arisen de novo. We need a theory of coding origins that takes into account information theoretic, chemical and evolutionary considerations to reconstruct a reasonable history of how RNA/peptide co-linearity gained a foothold in biology without a prior existence of complex molecular machinery. We also need to construct an origin of coding that is fully consistent with a continual reinforcement of the whole through the benefits imparted by the parts; this feature of life is a requisite to permit open-ended evolution.
The most thorough and thoughtful analyses of the origins of coding have been performed by Carter & Wills [7–10]. Their work focused on the origin, evolution and contemporary (and historical) substrate specificity of aaRS enzymes. A major takeaway from their analyses is that coding did not originate with catalytic RNAs (ribozymes), but instead was first established in archaic polypeptides, a conclusion reached by considering the reflexivity possible in aaRS and protein synthesis functions (cf. [8]). They derive insight from the observation that there are two distinct classes of aaRS, and that one can deduce an ancestral ‘gene’ on which these two classes are encoded on opposite, and thus interdependent, nucleic acid strands [11,12]. Class I enzymes, which tend to deal with the larger amino acids, have a catalytic core (HIGH/KMSKS) that involves amino acids that must be charged by class II enzymes (i.e. H, G, K and S). The converse is true, suggesting an ‘ancient hypercycle-like interdependence’ of the two enzyme classes [8]. This leads to a model of feedback loops that argue for a self-supporting (autocatalytic) protein world but not a self-supporting ribozymal world. Importantly, Carter & Wills downplay the primordial role of the ribosome in coding origins and instead posit that coding predated the earliest encoded peptides, or at least the two events were contemporaneous [7,9].
An alternative view can be derived from a careful analysis of the ribosome, and this has led to an inquiry into the relationship between the peptidyl-transferase centre and the coding phenomenon [13–16]. This view is that in the milieu of the proto-ribosome a symbiotic relationship between proteins and nucleic acids was birthed [16]. The proto-ribosome would have been a loose collection of RNA stem loops and divalent cations (first Fe2+, later Mg2+) having rudimentary catalytic activity. Although the origins of the original aaRS activity are not explicit in this model, one can infer that either aaRS specificity was broad at the root of the ribosomal tree, and/or that the elements of the ribosome itself originally provided a rudimentary mapping of amino acids onto nucleotide sequences [16,17].
Both the aaRS-centric view and the ribosome-centric view provide valuable insights into the history of protein synthesis and frame the macromolecular events that surrounded the advent of the coding phenomenon. However, neither adequately explains how coding itself came to be in the first place, nor what the ultimate chemical innovation was that cleared the path for the origins of life. In this paper, we propose that the mixed anhydride bond was just this innovation, and that its inclusion into autocatalytic sets was the spark of life.
2. Results
2.1. Chemical semiotics
To understand how one chemical acts as a sign (etc.) for another, we must consider how the earliest group interactions played out during the origins of life. Carter & Wills begin to address this semiotic issue. They state that, ‘The earliest genetic coding paradigm therefore required simultaneously solving three different recognition problems—ATP, amino acid, and tRNA—and finding two related catalytic mechanisms, each in two different ways (for class I and class II aaRS) in order to implement all events necessary to accomplish the symbolic conversion’ [7]. But the question remains: where do the aaRS (and their embodiment in a double-stranded RNA (dsRNA)) come from?
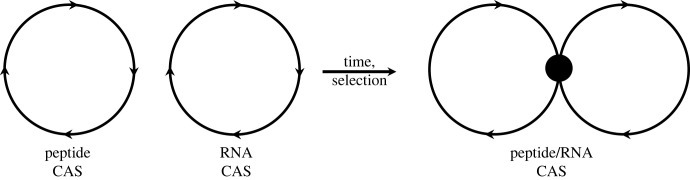
We have argued, implicitly, that the answer to this lies in a more fundamental––and pervasive—phenomenon, that of the collectively autocatalytic set (CAS) [18–21]. A CAS is a collection of molecules that spontaneously forms a network of interdependent catalytic connections to ensure self-propagation of the whole set. It is a chemical reaction network in which the molecules mutually catalyse each other's formation from a basic food set [20,21]. We would describe the aaRS as an entity that resulted from the intersection between two (previously independent) CASs: the RNA CAS and the peptide CAS. This intersection would be the first creation of a covalent bond between a component of the first (i.e. a nucleotide) and a component of the second (i.e. an amino acid). We represent this covalency as a black dot in figure 1. Such a hybrid molecule must originally have had some role in benefiting both CASs, and its subsequent transfer to a tRNA must have been a much later invention/requirement.
Figure 1.
The merging of two separate autocatalytic sets (CASs) to form a more complex one in which coding exists.
2.2. The mixed anhydride model of the origins of life
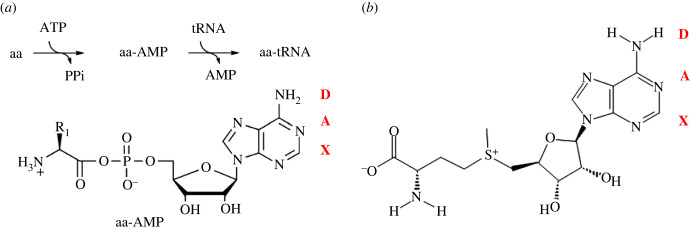
There is one obvious molecule that fits the description above perfectly: the aminoacyl adenylates. These are single amino acids that have been ‘energized’ with the formation of a covalent bond to an adenosine nucleotide. In reactions catalysed today by the aaRS, an ATP molecule is bound to an amino acid forming a mixed anhydride bond: a physical link between a nucleic acid precursor and a polypeptide precursor (figure 2a). Once formed, they serve as the building blocks for ribosomal-directed protein synthesis.
Figure 2.
(a) The formation of an aminoacyl-AMP via the creation of a mixed anhydride bond. These two reactions are performed today by the action of aaRS enzymes, whose substrate specificity manifests the genetic code. The hydrogen bonding donor (D) and acceptor (A) pattern, which mediates information transfer in nucleic acid interactions, is shown in red. (b) Another nucleotide–amino acid chimera, SAM.
One problem to solve here is, why ATP (as compared to GTP, UTP or CTP)? The answer to this question would address a long-standing enigma in biology. It could be as simple as the aqueous concentration of adenine was higher than that of any other nucleobase. Adenine was shown decades ago by Oró to be produced in roughly 1% yield as hydrogen cyanide (thought to be prebiotically abundant on the Earth) is heated at 60°C [22]. Or it could have been that the formation of the adenosine nucleotide (e.g. AMP, ADP, ATP or AppA) is/was thermodynamically more accessible than that of any other nucleotide.
Yet a more relevant possible explanation for the primacy of ATP and its utilization in energy transfer, including amino acid charging, is that the semiotic nature of the Watson–Crick surface of ATP was either the simplest of them all, or the easiest from which expansion into a larger code could take place. Reading from the most distal moiety from the glycosidic bond down the W–C face of a nucleobase, adenine reads (hydrogen bond) donor–acceptor–none (DAX; red letters in figure 2). Uracil would read ADA, being complementary to adenine (with a lower A that is used in wobble pairing), in the canonical pairing orientation. For completeness, guanine would read ADD and cytidine would read DAA.
From a binary coding perspective, the simplest possible way that four states can be encoded elsewhere is through two positions of a 0/1 code (Code I in table 1). However, if one allows for additional positions for either redundancy or punctuation, one can have three or more positions (e.g. Codes II and III in table 1). There is an analogy between Code I below and purine nucleobase information (ignoring wobble) and Code II below and pyrimidine nucleobase information. If the original coding only involved A–U, then it could follow Code I: DAX–ADX. But adding both pyrimidines would require Code II (DAD–ADA, DDA–AAD, etc.). Clearly then, a proto-biological system based on adenosine (and a complement, possibly uracil) would require the least complexity.
Table 1.
Three possible binary codes for four-letter alphabet.
| letter | code I | code II | code III |
|---|---|---|---|
| A | 0 0 | 0 0 0 | 0 0 0 0 0 |
| B | 0 1 | 0 1 1 | 0 0 1 1 1 |
| C | 1 0 | 1 0 1 | 1 1 0 0 1 |
| D | 1 1 | 1 1 0 | 1 1 1 1 0 |
Note that in the mixed anhydride, the P–O–C bond is formed very far from the W–C surface, especially when the nucleotide is in the anti-configuration. Consequently, the information is linked from one physical location (the W–C surface), through the ribose foundation, to another, the distal end of the molecule where the amino acid identity (R1) lies. The W–C surface thus acts as a sign (sensu C.S. Peirce) to point to a relatively distant object. Though today all mixed anhydrides used by aaRS enzymes are adenylates, at the time of the first intersection between RNA and peptide CASs, there may have been many covalent associations between nucleotides and amino acids. S-adenosylmethionine (SAM) is an example chimera with a different covalent linkage (figure 2b). As a third example of a covalent amino acid–nucleotide configuration, recently a novel hypothesis for the origins and role of the nucleotide–RNA interaction has been discovered in a synthetic reaction in which an amino acid is covalently attached to the Hoogsteen surface of nucleotides, aiding in amino acid condensation [23].
The covalent bond provided the permanent link between the sign and the object. This solidified coding. Prior to this, hydrogen bonds could presage coding, but their transient nature could not be used effectively in evolutionary selective processes. In contemporary translation, the sign has become covalently disambiguated with the object, giving rise to the genetic code (see below) and only aminoacyl-AMP remains as a palimpsest of that era.
2.3. A scenario of how RNA and proteins first interacted in a coding fashion
Two contemporaneous CASs, one of nucleotides/RNA and one of amino acids/peptides, could have existed, and their cooperation could have benefited both [19]. Laboratory research has shown that RNAs alone [24] or peptides alone [25] can form self-reproducing networks. Peptides can also self-condense and recombine through wet–dry cycles, aided by thioester chemistry [26,27]. Simple amino acids such as glycine and alanine can moreover be driven by carbonyl sulfide gas into ordered amyloid fibres that in turn drive the formation of more complex structures [28]. As such, these two types of polymers have the capacity to attain catalytic, constraint and task closure, and do thermodynamic work to construct themselves [21,29]. However, the growth potential of these is limited; only sub-exponential reproduction can be achieved. Without exponential reproduction, molecular networks cannot escape competing selfish parasites, and cannot evolve complexity [30–33].
Thus, the critical mutual benefit that each CAS would offer each other is the ability to achieve exponential reproduction. From a nucleic acid standpoint, one significant barrier to sustained reproduction is strand melting. This problem manifests itself as the ‘strand displacement problem’ and has been a long-standing obstacle to the synthetic creation of RNA autoreplicase ribozymes [34–36]. It is also the reason why its solution in the context of the PCR reaction revolutionized biological study and clinical practice: high temperatures induce strand separation and, along with thermostable polymerase enzymes, allow for exponential reproduction (replication in this case). From a protein standpoint, the opposite problem, of sorts, exists. Polypeptides do not have a reliable pattern of hybridization; one sequence cannot template another with a high degree of certainty and under a wide variety of environmental conditions. An enormous and phase transitionary advantage would result if certain amino acids or short peptides could have facilitated the melting of double-stranded RNAs, while certain nucleotides or proto-anticodons could have facilitated the annealing and/or ligation of amino acids.
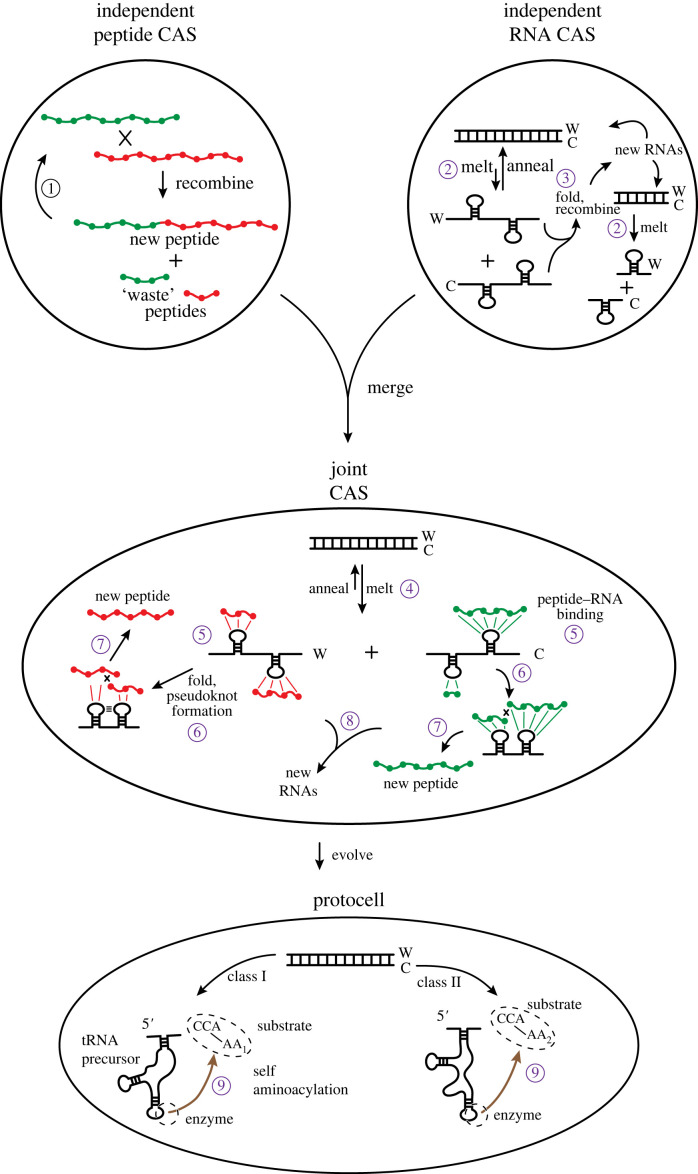
A scenario of mutualism therefore presents itself (figure 3). Originally there are two autocatalytic sets, one for peptides and one for RNAs. They operate independently, having been spawned from a prebiotic soup of small organics that included amino acids and nucleotides. One feature of these autocatalytic sets in our scenario is that they both run on recombination reactions primarily: trans-peptidation in the case of the peptide CAS and trans-esterification in the case of the RNA CAS. Other models of polymer CAS have been based mainly on cleavage/ligation reactions, and we do not preclude this mechanism although it requires a higher degree of chemical activation and is thus deemed less likely.
Figure 3.
Scenario for the origins of coding. Originally there are two independent CASs, a peptide CAS and an RNA CAS. In each, reproduction is possible on its own. In the peptide CAS, two peptides, green and red, if of sufficient length have the capacity to recombine to make new peptides. Small peptides of length, say, five amino acids or fewer do not possess sufficient complexity to recombine (or ligate, following [25]). They are ‘waste’ peptides, having low affinity for other short peptides. ① Longer products of reactions interact with other long products to create reaction cycles. Similar processes occur in the RNA CAS [24]. ② However, in the RNA CAS, there are two complementary strands of RNA upon annealing, a ‘Watson’ strand (W) and a ‘Crick’ strand (C). Annealing is favoured when two such strands interact; dsRNA also helps protect the RNA information from spontaneous hydrolysis. ③ If RNAs do melt, longer strands can adopt secondary structures including stem loops that engender catalytic activity. Shorter RNAs melt more easily, but have less tendency to possess catalytic activity. In both the independent CASs, reproduction is slow, sub-exponential, because of the structural, kinetic and thermodynamic barriers to proper catalytic events. Nevertheless, each CAS can operate without regard for the other. If, however, the two CASs find themselves in a situation in which they can interact productively, then the stage is set for coding, and life. In a joint CAS, each polymer provides assistance to the other, enhancing the other's reproductive rate. ④ Interaction with peptides shifts the equilibrium of the dsRNA to the melted, and potentially catalytically active, forms: ⑤ peptides, long or short, can bind to the loop regions of melted RNA strands. ⑥ The folding of single-stranded RNA into more complex stem loops and pseudoknots helps bring the peptides, including the shorter ones, into close contact so that ⑦ they can catalytically recombine/ligate to form larger peptides. One subset of peptides develops a tendency to bind the W strand of RNA, while another subset develops a tendency to bind the C strand. The advent of exactly two distinct subsets is the result of there being exactly two strands of RNA that form dsRNA [12]; dsRNA is the most thermodynamically stable nucleic acid complex. ⑧ Recombination/ligation of the RNAs proceeds as in the independent CAS case, although now its rate is augmented, eventually (through group selection) becoming exponential. Precursors to tRNAs could have arisen by the recombination of two similar stem loops [37,38]. Likewise, peptide reproduction is augmented because of the ability of smaller peptides to participate in the catalytic cycle. In the bottom, protocell stage, evolution drives the formation of the precursors to modern-day RNAs and proteins. Shorter and shorter peptides are selected for RNA loop binding, as the march to a 1 : 1 specificity between amino acids and nucleotide (triplets) is favoured by further reduction of waste (see text). ⑨ The RNAs are selected to covalently attach the single amino acids to their ends, forming the aminoacyl adenylates that are now palimpsests of this era. Coding arises in full when a unique associate between specific amino acids and specific RNA sequences becomes established; life is a consequence of the duality of polymer types and their association through this coding. Self-aminoacylation is the first aaRS function, shared with tRNA function in this model; the set of reproducing RNAs includes the precursors to tRNA, rRNA and aaRS activities, which later diverge and/or become taken over by peptides. The W and C strands of RNA each drive the evolution of the class I and II aaRS enzymes [12]. At each stage in this scenario, selection for the reproduction of the whole drives the evolutionary relationships of the parts (i.e. a Kantian whole).
Suppose that the ‘Watson’ (W) strand and the ‘Crick’ (C) strand of a dsRNA can each form one stem loop, but only when denatured from each other. There may be two, three, or many more such RNAs in a CAS, and a reproduction cycle is maintained via recombination (or perhaps template-directed replication). Yet because of slow melting, this reproduction is sub-exponential. The binding of an amino acid to the nascent loop of the stem loops would shift the equilibrium from dsRNA (W–C) to separate stem loops (W and C), each with an amino acid bound to the nucleotides in the loop region. This would be the equivalent of the modern-day anticodon, although at this point it would not yet be functioning in the same role. Nevertheless, by pure physical–chemical properties, each short contiguous set of nucleotides (e.g. three, although this need not be so, nor a fixed value) would bind a ‘cognate’ amino acid with some degree of specificity. This hypothesis, that trinucleotides bound amino acids prior to the full development of the (genetic) code, has often been proposed in various forms (e.g. [39]), but most noticeably as the stereochemical theory. Yarus et al. [40] have demonstrated that there is a statistically significant correlation between the binding constants in solution for amino acids and their cognate anticodon triplets. Rodin et al. [41] similarly have shown that amino acids bind preferentially to their modern-day anticodons, at least to the second and third positions. In our scenario, the W or C strands, when dissociated from each other, bind single amino acids or short peptides.
At first, this binding is rather weak and non-specific. These short peptides are too short to participate actively in the CAS; only peptides of length, say, eight amino acids or more possess enough structural complexity to be trans-peptidation catalysts. The smaller peptides are essentially non-productive members of their CAS; they are akin to waste products. Yet upon binding to the loops in an RNA, they become positioned, and ordered, in a way such that their recombination or ligation to make longer oligomers becomes enhanced.
At the same time, the binding has a twofold positive effect on the catalytic potential of the RNA. First, these short peptides help to melt the dsRNA, acting either similarly to the ligands seen in riboswitches [42] or as crude helicases [43]. In the former case, it is known that RNAs can bind amino acids and, as a result, have their secondary structures altered; glycine and SAM riboswitches are well characterized [44]. In fact, there are many different SAM riboswitches, and it is perhaps not a coincidence that SAM is also a covalent amino acid–nucleotide chimera, akin to aminoacyl-AMP, but with the amino acid moiety essentially inverted (figure 2b). Second, this binding also serves to stabilize the secondary structures of the nascent stem loops [42].
There is ample evidence that non-covalent binding between amino acids and RNA was an ancient event and one of important regulatory function [45]. Yarus [46] detected a specific and reversible binding site in the catalytic core of the Tetrahymena ribozyme for L-arginine. This amino acid, much more so than any of the other biological 19, or even the D-stereoisomer, binds to a triplet sequence of nucleotides at the guanosine binding site of the ribozyme that is critically involved in is catalytic (self-splicing) function and is actually a competitive inhibitor [47]. Since this discovery, hundreds of specific interactions between short (e.g. tri-) nucleotide sequences and certain amino acids (such as glycine and lysine) have been revealed, such as those in natural riboswitches and artificial aptamers [48]. In fact, the idea that a variety of random and (initially) chaotic peptide–RNA interactions formed and set the state for chemical evolution has been proposed before. The hybridization-dependent peptides proposed by Kunnev & Gospodinov [49] are a good example; any peptide–RNA-world concept would rely on such interaction.
2.3.1. Benefits to the RNA
The strand separation would benefit the RNA CAS. When the W and C strands are apart, they are free to form more complex secondary structures, interacting with other regions of the same strand as well as distant regions of the opposite strand. This would facilitate the formation of catalytic structures, which, in RNA, are often made possible by pseudoknotted configurations. A pseudoknot is a specific non-symmetrical pattern of nucleotide pairing which allows for catalytic nucleotides to be positioned at the active site, and/or by placing strain on particular phosphodiester bonds. These configurations are seen in ligase, replicase and HDV ribozymes, for example.
With conformational freedom and the catalytic capabilities that ensue, an RNA population would be more able to access the numbers and types of catalytic events (e.g. recombination reactions) that would permit exponential growth [19].
2.3.2. Benefits to the peptides
The binding to stem loops would benefit the peptide CAS. As free peptides, their ability to associate with each other in orientations that are productive (for trans-peptidation/ligation) is limited. In the well-studied case of template-directed ligation in peptide networks, the reproduction efficiency depends not only on the kinetic order of the reaction, but also on the ratio of reactions that are template-assisted to those that are template-free [50]. Peptide ligation reactions that contain a certain degree of templating can self-organize into small cross-catalytic networks, while those that do not can only form random, disorganized collections with a low rate of reproduction. Binding to an RNA scaffold affords peptides the ability to use templates that are not part of their own network. Experimental evidence for this exists in that RNAs can direct amino acid coupling in abiotic scenarios [23,51].
Two amino acids or peptides that are bound to the loop regions of two RNA stem loops can be positioned for recombination or ligation. One way to envision this is that one RNA strand, say the W strand, once free of its complementary C strand, forms two stem loops, which then position near each other in space through tertiary interactions. This type of conformation is seen in many ribozymes, such as the hammerhead [52]. Upon joining to form longer peptides, these molecules can better participate in the reactions of the peptide CAS. Short fragments are no longer waste; they become incorporated into the self-reproduction network.
2.3.3. Strand specificity sets the stage for coding
If two loops from the same strand of RNA form and bring their bound amino acids/peptides together to allow for recombination/ligation, a polarity develops that leads to a coding situation. Amino acids that bind to the loops formed from the W strand will preferentially be joined to one another, while amino acids that bind to the loops formed from the C strand. The reason for this is that loops on the same strand can interact through intramolecular rearrangement (i.e. folding) far more readily than they can with loops on (now) dissociated strands through intermolecular interactions. RNA folding drives peptide elongation.
Returning to the model presented in figure 3, there exists empirical support for the folding processes that bring the two amino acids (or short peptides) together upon rearrangement such that they can be recombined to form longer peptides. Consider that it has been shown that one ribozyme can have two distinct folds, each with its own unique catalytic activity. A fold with ligase activity or a fold with HDV self-cleavage activity is both accessible by single RNA sequences of length 90 nucleotides [53]. Moreover, HDV genomic/antigenomic sequences can be templates for each other's replication [54].
The intramolecular reinforcement leads to two subsets of amino acids, those affiliated with the W strand and those affiliated with the C strand. Because, by definition, the W and C strands are complementary, the minimal energy state for the entire system would be a symmetric one in which half of the participating amino acids at least transiently associate in one sub-network, while the other half associate in another sub-network. It is clear that this situation presages that of the aaRS enzymes, for which today there are 10 in each of two classes, and it is in agreement with the observation of Carter & Wills of reciprocity between two 10-member aaRS collections [8].
At this point, there would emerge a strong selection pressure for class uniformity and specificity. That is, peptides composed of pure class I (say) amino acids would become associated with the W (say) RNA strand, while peptides composed of class II amino acids would become associated with the C strand. This would not yet be coding per se, merely the advent of two clouds of informational-rich polymers.
Selection would strengthen their self-reinforcement. Let us explain why. If there are, say, 20 amino acids involved in the peptide CAS, then there are 8000 possible tripeptides. Let the set of N W stem loops bind ‘overlapping’ subsets of the 8000 tripeptides that have higher affinity for the W stem loops. Similarly, let this set of N C stem loops bind overlapping subsets of the 8000 tripeptides having higher affinity for C stem loops. Now consider the manner in which the peptides help the RNA CAS: to bind the W stem loops on the W strand, and bind the C stem loops on the C strand and help melt the dsRNA so that they can reproduce in the RNA CASs. Consider two extreme cases:
Case 1: Let there be a long pure W strand and its complementary long pure C strand. Let each strand have a modest number of stem loops, say 4–11. The N W stems bind W peptides (e.g. class I) and recombine or ligate them to create longer pure W polypeptides. The N C stem loops bind C peptides (e.g. class II) and recombine or ligate them to create longer pure C polypeptides. This is self-consistent: long W RNA strands have denatured to create W stem loops, the complementary long C RNA strands have denatured to create C stem loops. When these longer complementary RNA strands replicate, the W strand and the C strand can be pulled apart (melted) by binding, respectively W polypeptides and C polypeptides, and can reproduce exponentially.
Case 2: By contrast, consider that the two complementary long RNA strands each have both W stem loops and C stem loops in more or less random order along the RNA strands. These stem loops create polypeptides that are likewise random sequences of W and C amino acids. When the longer W–C and C–W RNA strands reproduce and W–C polypeptides try to bind W and bind C stem loops they will, but in general be out of sequential register. Because of this, the W–C polypeptides will not bind the two W–C strands efficiently, so will not effectively melt the two strands apart.
Thus, pure W and pure C RNA strands and pure W polypeptides and pure C polypeptides will form sets that reproduce more effectively. This sets up the scenario that further engrains coding. There will be a selection pressure towards longer RNA complementary strands, one pure W and one pure C. The pure W strands will interact with pure W peptides on nearby regions of the strand that are become proximal as a consequence of folding. The W strands drive the recombination of these peptides to elongate them. The analogous processes are occurring on the C strands with C peptides.
2.3.4. True coding arises upon a covalent interaction between RNA and peptides
At this point in our scenario so far, the stem loops of RNA are not conveying any information about the peptides to any other location. There is simply a selected tendency for W stem loops to interact with W peptides, and for C stem loops to interact with C peptides. The contemporary tRNA-aminoacyl synthetase activities are not yet fully integrated into nascent life.
However, the key event that, in one stroke, led to coding and the origins of life was the formation of a covalent bond between the amino acid and the RNA strand to which it is bound. This could only occur at a free end of the RNA. And although the chemical states of the two ends of the RNAs would have been quite variable in a chemical soup, containing a wide mixture of alkylation (methylation), amidation, hydroxylation and phosphorylation states, the last two such termini would be the most activated and thus amenable for bond formation. These would have been numerous to allow for RNA recombination/ligation anyway.
Again, the extant evidence for a mechanism of amino acid–RNA covalent attachment is plentiful. Turk et al. [55] demonstrated that a host of small and simple RNA motifs have the catalytic capacity to transfer an amino acid and covalently attach it to their 2′-hydroxyl groups on their 3′-terminal nucleotide (usually a uridine). In the most extreme case, the enzyme portion of the RNA could be as short as 5–8 nucleotides in length (figure 4). The substrate uridine was found to exist most often on the 3′ end of the sequence CCU, which mimics the CCA terminus of contemporary tRNAs (the attachment site of amino acids).
Figure 4.
An example of a small self-aminoacylating ribozyme discovered by Turk et al. [55]. The top strand is the substrate, while the bottom strand is the enzyme (8 nt in this case).
The amino acid source for transfer for these mini-trans-aminoacylation ribozymes is already ‘activated’ as an aminoacyl-AMP such as PheAMP (for phenylalanine) or MetAMP (for methionine). Therefore, the mixed anhydride bond must be formed prior to the activity of these mini ribozymes. Nevertheless, their existence and versatility portend two things. First, they suggest that RNAs could have performed at least one of the contemporary functions of aaRS enzymes, that of the charging of tRNA precursors. And second, they hint that the more rudimentary formation of the mixed anhydride bond, admittedly a greater thermodynamic challenge than aminoacylation, could have been a part of the small molecule CAS milieu prior to coding and life.
A progressive enhancement of the informational relationship between RNA sequences and amino acid identity solidified the coding. When, say, a stretch of W nucleotides was correlated with a five amino acid peptide, then the RNA is informationally linked to n5 possible peptides. If n were 20, and in contemporary biology, then this would mean 3.2 × 106 possible combinations could be associated with a particular RNA sequence, and the vast majority of these would be non-functional for the peptide–RNA CAS. This in turn would lead to a tremendous amount of waste in the peptide CAS. Evolutionary pressure would then be strong to tighten the relationship, by shortening the peptide length. If the stretch of W nucleotides were correlated to only tripeptides, then the associated products would number about 8000, an efficiency improvement (in terms of waste in the peptide CAS) of many orders of magnitude.
As the length of peptides, n, progressively shortens from 5 to 4 to 3 to 2 to 1, the waste decreases. On the other hand, as the length of peptides drops, the chance that those peptides synthesized can also play a role in the peptide CAS decreases. Thus, as the length n decreases towards 1, reproduction of the system must shift from the peptide CAS to reproduction by encoded peptides. In sum, there is strong selection pressure to minimize waste, and the highest informational efficiency would be achieved if there were a 1 : 1 correspondence between an RNA stretch and a single amino acid. At this point, there would be true coding, and a covalent bond between RNA and a single peptide, as in the aminoacyl adenylate, would manifest this code. The length of the RNA codon stretch today is known to be three, as first discussed by Gamow in that three nucleotides is the minimum binary number capable of encoding a set of amino acids that exceeds 16 (i.e. 20 or so).
Ultimately, each W and C RNA strands could evolve into the Rodin & Ohno duplex [12] as envisaged by Carter & Wills [8], and accordingly, W-encoded and C-encoded peptides could evolve into the Ur-aaRS enzymes as envisaged by Carter [56]. Of course, at some point during the honing of the peptide–RNA relationship towards 1 : 1, there was a transition to template-directed replication, both for peptides and for RNA. This contemporary form of reproduction post-dated coding. As hinted at by Carter & Wills though not fully articulated, symbolic coding eventually emulated hydrogen bonding [8], but the first key step was the formation of a covalent bond, now seen only in the mixed anhydride.
3. Discussion
We have presented a model of early polymeric molecular evolution that includes the origin and early development of coding. The relic of coding is the covalent bond found in the mixed anhydride molecule, central to contemporary translation. Coding was a direct consequence of the fact that two, chemically distinct polymers were needed for the genotype–phenotype duality that allows for full evolutionary freedom to explore fitness landscapes. An implication of this realization is that life as we know it could not have been possible without a code; simpler pre-life systems can undergo change but cannot encapsulate the characteristics of their environment into their ‘genomes’, and thus tend to end up in uninteresting and/or closed-ended patterns such as stable limit cycles or parasitic dualities (cf. [6]). In fact, we can make the claim that coding is life, or at least that coding is a necessary condition for life. As stated eloquently by Davies: ‘life = matter + information’. We have added coding: life = matter + information + coding [6].
Coding arises from the existence of two distinct biopolymers. We have discussed polypeptides and polynucleotides. However, we do not want to rule out the possibility that other polymers, particularly lipids, played key informational roles in the instigation of coding. Damer & Deamer [57], for example, proposed an attractive model of life's origins that involves as many as six polymeric types that interact to cooperatively sustain information. Yet from an Occam's Razor point of view, two cooperating systems would be the most accessible to any complex system; the critical point is simply that the number must be greater than one.
In our scenario, progress towards the level of complexity characteristic of life requires the interaction of CASs. Consequently, a requisite for life is the continual functioning of the parts to sustain the whole. Life is distinct from non-life in large part because it is a Kantian whole. There is downward selection from the whole to the parts; without the former, the latter are insignificant. Selection at every step of the process solidifies the whole (the CAS or, later, the joint CASs) and directs the chemical interactions of the parts. This is a continuously self-reinforcing phenomenon.
Importantly though, a tremendously significant phase transition occurs when both CASs reinforce each other. Their physical entanglement via the covalent bond of the mixed anhydride became manifest as coding. This may have been one of the most important phase transitions in the history of the universe in that it led to life. In the realm of particle physics, an entangled system is defined to be one whose quantum state cannot be factored as a product of states of its local constituents; that is to say, they are not individual particles but are an inseparable whole. A parallel process occurred at the macromolecular level and became life.
Previously we have noted that constraint closure drove the major transitions in the origins of life [21], and this scenario we present here is no exception. Work, the constrained release of energy into just a few degrees of freedom, was required to create the living situation. Having more than one biopolymer is itself a constraint. Another is that, in a CAS, there are more transitions than there are molecules.
Our scenario is just a working hypothesis. Yet it is amenable to possible experimental approaches for support or refutation. For example, one could put two CASs (peptides and RNAs) together and observe if their intersection leads to more than doubling in reproductive rates. A more restricted experiment would be to test if two RNA stem loops can spur the recombination or ligation of two peptides. Conversely, it should be relatively easy to test whether short peptides, through binding, help two regions of RNA that are otherwise fairly thermodynamically stable, melt.
4. Conclusion
Life as we know it today is dependent on the interaction among polymers. The canonical NASA definition that life ‘is a self-sustaining chemical system capable of Darwinian evolution’ requires that this inter-polymer relationship be quantized, and this in turn mandates coding. And perhaps more relevant are newer definitions of life that focus on the information transfer process more explicitly. Adami, for example, considers life to be a persisting state in which entropy is reduced compared to an abiotic maximum value [58]. In this view, the replication of information, rather than of organisms per se, is required. We agree with this concept, and stress that only a code (but not necessarily the ‘genetic code’) could provide the iterative feedback that is characteristic of evolution that pushes a system away from maximum chaos in a non-transient manner.
In our view, a code is any means by which information in one (polymer) molecule can direct the information in another. In the origins of life, this was less precise, but the honing of precision was essentially the ‘breath’ of life. Later, and especially today, this has become not only exquisitely precise but multi-faceted, encompassing epigenetic factors, reverse transcription, reverse translation and any other chemical means by which the state of one polymer can influence others.
Thinking about life and coding as mutually dependent phenomena, we may be able to address questions that could not have been answered before. Many phenomena have been discussed for 60 years regarding the code and its origins. We need to emphasize that life required at least two polymers. Polymers alone can perform combinatorics. Our point is that life, or Von Neuman's universal constructor, required two distinct chemistries working together. One would not be enough; any single-polymer CAS would not have access to the range of benefits needed to achieve constraint closure [21], informational organization [6] and complexity [17] needed for the persistence of life, as opposed to the transient existence of replicators. This relationship between the two required a code.
Today, the two-polymer relationship has evolved to a clear division of labour where nucleic acids store the bulk of the information (i.e. genotype) while peptides store the bulk of the functional capacity (i.e. phenotype). Few would disagree with this viewpoint. We feel that a stronger statement can be made, that coding was a requisite for life itself. This additional layer of organization may partly explain the apparent rarity of life in the universe.
Acknowledgements
We thank G. Kretovic for help with figure preparation.
Data accessibility
This article has no additional data.
Authors' contributions
S.A.K.: conceptualization, methodology, supervision and writing—review and editing; N.L.: formal analysis, investigation, methodology, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This project received no funding.
References
- 1.Koonin EV, Novozhilov AS. 2017. Origin and evolution of the universal genetic code. Annu. Rev. Genet. 51, 45-62. ( 10.1146/annurev-genet-120116-024713) [DOI] [PubMed] [Google Scholar]
- 2.Freeland SJ, Hurst LD. 1998. The genetic code is one in a million. J. Mol. Evol. 47, 238-248. ( 10.1007/PL00006381) [DOI] [PubMed] [Google Scholar]
- 3.Gatlin L. 1972. Information theory and the living system. New York, NY: Columbia University Press. [Google Scholar]
- 4.Yockey H. 2005. Information theory, evolution, and the origin of life. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Perlwitz MD, Burks C, Waterman MS. 1998. Pattern analysis of the genetic code. Adv. Appl. Math. 9, 7-21. ( 10.1016/0196-8858(88)90003-6) [DOI] [Google Scholar]
- 6.Davies PCW. 2020. The demon in the machine: how hidden webs of information are solving the mystery of life. Baltimore, MD: Penguin Books. [Google Scholar]
- 7.Carter CW, Wills PR. 2019. Experimental solutions to problems defining the origin of codon-directed protein synthesis. Biosystems 183, 103979. ( 10.1016/j.biosystems.2019.103979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter CW, Wills PR. 2018. Interdependence, reflexivity, fidelity, impedance matching, and the evolution of genetic coding. Mol. Biol. Evol. 35, 269-286. ( 10.1093/molbev/msx265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter CW, Wills PR. 2019. Class I and II aminoacyl-tRNA synthetase tRNA groove discrimination created the first synthetase–tRNA cognate pairs and was therefore essential to the origin of genetic coding. IUBMB Life 71, 1088-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter CW, Wills PR. 2021. Reciprocally-coupled gating: strange loops in bioenergetics, genetics, and catalysis. Biomolecules 11, 265. ( 10.3390/biom11020265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Giulio M. 1992. On the origin of the transfer RNA molecule. J. Theor. Biol. 159, 199-214. ( 10.1016/S0022-5193(05)80702-7) [DOI] [PubMed] [Google Scholar]
- 12.Rodin SN, Ohno S. 1995. Two types of aminoacyl-tRNA synthetases could be originally encoded by complementary strands of the same nucleic ACID. Orig. Life Evol. Biosph. 25, 565-589. ( 10.1007/BF01582025) [DOI] [PubMed] [Google Scholar]
- 13.Agmon I, Bashan A, Yonath A. 2006. On ribosome conservation and evolution. Isr. J. Ecol. Evol. 52, 359-374. ( 10.1560/IJEE_52_3-4_359) [DOI] [Google Scholar]
- 14.Davidovich C, et al. 2010. The proto-ribosome: an ancient nano-machine for peptide bond formation. Isr. J. Chem. 50, 29-35. ( 10.1002/ijch.201000012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao C, et al. 2013. Molecular paleontology: a biochemical model of the ancestral ribosome. Nucleic Acids Res. 41, 3373-3385. ( 10.1093/nar/gkt023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrov AS, et al. 2015. History of the ribosome and the origin of translation. Proc. Natl Acad. Sci. USA 112, 15 396-15 401. ( 10.1073/pnas.1509761112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowman JC, Petrov AS, Frenkel-Pinter M, Penev PI, Williams LD. 2020. Root of the tree: the significance, evolution, and origins of the ribosome. Chem. Rev. 120, 4848-4878. ( 10.1021/acs.chemrev.9b00742) [DOI] [PubMed] [Google Scholar]
- 18.Kauffman SA. 1971. Cellular homeostasis, epigenesis and replication in randomly aggregated macromolecular systems. J. Cybern. 1, 71-96. ( 10.1080/01969727108545830) [DOI] [Google Scholar]
- 19.Kauffman SA. 1993. The origins of order. Oxford, UK: Oxford University Press. [Google Scholar]
- 20.Hordijk W, Steel M. 2004. Detecting autocatalytic, self-sustaining sets in chemical reaction systems. J. Theor. Biol. 227, 451-461. ( 10.1016/j.jtbi.2003.11.020) [DOI] [PubMed] [Google Scholar]
- 21.Lehman NE, Kauffman SA. 2021. Constraint closure drove major transitions in the origins of life. Entropy 23, 105. ( 10.3390/e23010105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oró J. 1961. Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive Earth conditions. Nature 191, 1193-1194. ( 10.1038/1911193a0) [DOI] [PubMed] [Google Scholar]
- 23.Müller F, et al. 2022. A prebiotically plausible scenario of an RNA–peptide world. Nature 605, 279-284. ( 10.1038/s41586-022-04676-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaidya N, Manapat ML, Chen IA, Xulvi-Brunet R, Hayden EJ, Lehman N. 2012. Spontaneous network formation among cooperative RNA replicators. Nature 491, 72-77. ( 10.1038/nature11549) [DOI] [PubMed] [Google Scholar]
- 25.Ashkenasy G, Jagasia R, Yadav M, Ghadiri MR. 2004. Design of a directed molecular network. Proc. Natl Acad. Sci. USA 101, 10 872-10 877. ( 10.1073/pnas.0402674101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frenkel-Pinter M, et al. 2022. Differential oligomerization of alpha versus beta amino acids and hydroxy acids in abiotic proto-peptide synthesis reactions. Life 12, 265. ( 10.3390/life12020265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross DS, Deamer D. 2016. Dry/wet cycling and the thermodynamics and kinetics of prebiotic polymer synthesis. Life 6, 28. ( 10.3390/life6030028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwald J, Friedmann MP, Riek R. 2016. Amyloid aggregates arise from amino acid condensations under prebiotic conditions. Angew. Chem. Int. Ed. 55, 11 609-11 613. ( 10.1002/anie.201605321) [DOI] [PubMed] [Google Scholar]
- 29.Kauffman SA. 2019. A world beyond physics: the emergence and evolution of life. Oxford, UK: Oxford University Press. [Google Scholar]
- 30.von Kiedrowski G. 1993. Minimal replicator theory. I: parabolic versus exponential growth. In Bioorganic chemistry frontiers (eds Dugas H, Schmidtchen FP), pp. 113-146. Berlin, Germany: Springer. [Google Scholar]
- 31.Boerlijst MC, Hogeweg P. 1991. Spiral wave structure in pre-biotic evolution: hypercycles stable against parasites. Phys. Nonlinear Phenom. 48, 17-28. ( 10.1016/0167-2789(91)90049-F) [DOI] [Google Scholar]
- 32.Bansho Y, Furubayashi T, Ichihashi N, Yomo T. 2016. Host–parasite oscillation dynamics and evolution in a compartmentalized RNA replication system. Proc. Natl Acad. Sci. USA 113, 4045-4050. ( 10.1073/pnas.1524404113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuuchi R, Furubayashi T, Ichihashi N. 2022. Evolutionary transition from a single RNA replicator to a multiple replicator network. Nat. Commun. 13, 1460. ( 10.1038/s41467-022-29113-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston WK, Unrau PJ, Lawrence MS, Glasner ME, Bartel DP. 2001. RNA-catalyzed RNA polymerization: accurate and general RNA-templated primer extension. Science 292, 1319-1325. ( 10.1126/science.1060786) [DOI] [PubMed] [Google Scholar]
- 35.Cheng LKL, Unrau PJ. 2010. Closing the circle: replicating RNA with RNA. Cold Spring Harb. Perspect. Biol. 2, a002204. ( 10.1101/cshperspect.a002204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutschler H, Wochner A, Holliger P. 2015. Freeze–thaw cycles as drivers of complex ribozyme assembly. Nat. Chem. 7, 502-508. ( 10.1038/nchem.2251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton ZF. 2020. The 3-minihelix tRNA evolution theorem. J. Mol. Evol. 88, 234-242. ( 10.1007/s00239-020-09928-2) [DOI] [PubMed] [Google Scholar]
- 38.Lei L, Burton ZF. 2020. Evolution of life on Earth: tRNA, aminoacyl-tRNA synthetases and the genetic code. Life 10, 21. ( 10.3390/life10030021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter CW, Kraut J. 1974. A proposed model for interaction of polypeptides with RNA. Proc. Natl Acad. Sci. USA 71, 283-287. ( 10.1073/pnas.71.2.283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarus M, Widmann JJ, Knight R. 2009. RNA–amino acid binding: a stereochemical era for the genetic code. J. Mol. Evol. 69, 406-429. ( 10.1007/s00239-009-9270-1) [DOI] [PubMed] [Google Scholar]
- 41.Rodin AS, Szathmáry E, Rodin SN. 2011. On origin of genetic code and tRNA before translation. Biol. Direct 6, 14. ( 10.1186/1745-6150-6-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandal M, Breaker RR. 2004. Gene regulation by riboswitches. Nat. Rev. Mol. Cell Biol. 5, 451-463. ( 10.1038/nrm1403) [DOI] [PubMed] [Google Scholar]
- 43.Vyas P, Trofimyuk O, Longo LM, Deshmukh FK, Sharon M, Tawfik DS. 2021. Helicase-like functions in phosphate loop containing beta-alpha polypeptides. Proc. Natl Acad. Sci. USA 118, e2016131118. ( 10.1073/pnas.2016131118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breaker RR. 2012. Riboswitches and the RNA World. Cold Spring Harb. Perspect. Biol. 4, a003566. ( 10.1101/cshperspect.a003566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frenkel-Pinter M, et al. 2020. Mutually stabilizing interactions between proto-peptides and RNA. Nat. Commun. 11, 3137. ( 10.1038/s41467-020-16891-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yarus M. 1988. A specific amino acid binding site composed of RNA. Science 240, 1751-1758. ( 10.1126/science.3381099) [DOI] [PubMed] [Google Scholar]
- 47.Yarus M. 1991. An RNA-amino acid complex and the origin of the genetic code. New Biol. 3, 183-189. [PubMed] [Google Scholar]
- 48.Sudarsan N, Wickiser JK, Nakamura S, Ebert MS, Breaker RR. 2003. An mRNA structure in bacteria that controls gene expression by binding lysine. Genes Dev. 17, 2688-2697. ( 10.1101/gad.1140003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunnev D, Gospodinov A. 2018. Possible emergence of sequence specific RNA aminoacylation via peptide intermediary to initiate Darwinian evolution and code through origin of life. Life 8, 44. ( 10.3390/life8040044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dadon Z, Wagner N, Ashkenasy G. 2008. The road to non-enzymatic molecular networks. Angew. Chem. Int. Ed. 47, 6128-6136. ( 10.1002/anie.200702552) [DOI] [PubMed] [Google Scholar]
- 51.Harada K, et al. 2014. RNA-directed amino acid coupling as a model reaction for primitive coded translation. ChemBioChem 15, 794-798. ( 10.1002/cbic.201400029) [DOI] [PubMed] [Google Scholar]
- 52.Martick M, Scott WG. 2006. Tertiary contacts distant from the active site prime a ribozyme for catalysis. Cell 126, 309-320. ( 10.1016/j.cell.2006.06.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schultes EA, Bartel DP. 2000. One sequence, two ribozymes: implications for the emergence of new ribozyme folds. Science 289, 448-452. ( 10.1126/science.289.5478.448) [DOI] [PubMed] [Google Scholar]
- 54.Webb CHT, Lupták A. 2011. HDV-like self-cleaving ribozymes. RNA Biol. 8, 719-727. ( 10.4161/rna.8.5.16226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turk RM, Chumachenko NV, Yarus M. 2010. Multiple translational products from a five-nucleotide ribozyme. Proc. Natl Acad. Sci. USA 107, 4585-4589. ( 10.1073/pnas.0912895107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carter CW. 2014. Urzymology: experimental access to a key transition in the appearance of enzymes. J. Biol. Chem. 289, 30 213-30 220. ( 10.1074/jbc.R114.567495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damer B, Deamer D. 2015. Coupled phases and combinatorial selection in fluctuating hydrothermal pools: a scenario to guide experimental approaches to the origin of cellular life. Life 5, 872-887. ( 10.3390/life5010872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adami C. 2015. Information-theoretic considerations concerning the origin of life. Orig. Life Evol. Biospheres 45, 309-317. ( 10.1007/s11084-015-9439-0) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.