Abstract
Despite higher bone mineral density (BMD), women with obesity are at an increased risk of fracture compared to normal-weight women. Optimal adolescent bone accrual is critical for normal peak bone mass acquisition and future bone health. Whereas several studies have examined the impact of low body weight on bone accrual in youth, data are lacking regarding the impact of obesity on bone accrual. We examined bone accrual over one year in young women with moderate to severe obesity (OB) (n=21) versus normal-weight controls (NWC) (n=50). Participants were 13–25 years old. We used dual-energy X-ray absorptiometry to assess areal BMD (aBMD) and high resolution peripheral quantitative computed tomography (distal radius and tibia) to assess volumetric BMD (vBMD), bone geometry, and microarchitecture. Analyses were controlled for age and race. The mean age was 18.7 ± 2.7 years. OB and NWC were similar for age, race, height, and physical activity. OB had a higher BMI (p<0.0001) and younger menarchal age (p=0.022) than NWC. Over one year, OB did not demonstrate the increase in total hip BMD observed in NWC (p=0.03). Increases in percent cortical area and cortical thickness, and cortical and total vBMD at the radius were lower in OB than in NWC (p≤0.037). Groups did not differ for tibial bone accrual. We demonstrate that longitudinal bone accrual is impaired at the total hip and radial cortex in young women with obesity, raising concerns regarding their future bone health.
Keywords: Obesity, Overweight, Adolescents, Bone accrual, Bone density, Bone geometry, Bone microarchitecture
1. Introduction
The adolescent and young adult years are crucial periods for bone accrual. Optimal bone accrual during this time lays the foundation for optimal peak bone mass, and hence for current and future bone health and fracture risk1. Many factors affect bone accrual, and the extremes of weight are one such modifiable factor. Children with obesity have a 25% higher risk of extremity fractures compared to their normal-weight peers2,3. Cross-sectional studies have revealed both positive and negative effects of obesity on areal and volumetric bone mineral density (BMD), bone geometry, and microarchitecture - essential determinants of bone strength4,5. Early life cohort studies suggest that obesity during childhood is associated with higher areal BMD at the hip and total body during adolescence as measured by dual-energy X-ray absorptiometry (DXA)6. However, our cross-sectional study using high-resolution peripheral quantitative computed tomography (HRpQCT) demonstrated deficits in the cortical bone at the radius in adolescents with obesity versus their normal-weight counterparts7. Further, the effects of body composition (both lean and fat mass) have been examined, with studies suggesting a positive effect of lean mass and a detrimental effect of fat mass on volumetric BMD in girls with obesity8,9.
Data from longitudinal studies evaluating bone accrual in youth with obesity are limited. One study assessing the impact of exercise on bone accrual reported smaller gains in those with obesity compared to normal-weight preadolescents10. Prospective data regarding the impact of obesity on volumetric BMD, bone geometry, and microarchitecture are currently lacking. Therefore, in this study, we examined changes in areal and volumetric BMD, bone geometry, and microarchitecture (using DXA and HRpQCT), and in estimates of bone strength (using micro finite element analysis) over one year in young females with moderate to severe obesity compared to their normal-weight counterparts. We hypothesized that females with obesity would demonstrate lesser increments in bone accrual, especially at the non-weight bearing radius, compared to normal-weight females.
2. Participants and Methods
2.1. Participant Selection:
Study participants included 71 adolescent and young adult women, 13–25 years of age. Of these, 21 had moderate to severe obesity (OB), with body mass index (BMI) ≥ 35 kg/m2 or ≥ 120% of the 95th percentile of BMI for age and sex with at least one obesity-related co-morbidity or a BMI ≥40 kg/m2 or ≥ 140% of the 95th percentile of BMI and were recruited between 2015 and 2021 in a prospective observational study (NCT02557438) from several tertiary care obesity treatment centers. 50 participants were normal weight controls (NWC) with a BMI between 18.5 and 25 kg/m2, and were recruited through advertisements on institutional recruitment forums and college job boards between 2010 to 2018 in two separate studies (NCT00946192 and NCT01301183)). Exclusion criteria included the use of medications that may affect bone metabolism (except calcium, vitamin D, or hormonal contraception) in the eight weeks prior to the baseline visit, or health conditions that impact bone except those directly related to obesity such as polycystic ovarian syndrome (PCOS) or diabetes. We did not exclude participants on combined oral contraceptive pills (OCPs) to ensure a representative population given that many adolescents and young adult females take these medications for contraception or management of PCOS. We did exclude those on depot medroxyprogesterone, given its known deleterious effects on bone but did not exclude young women using the progestin-releasing intrauterine device or progestin implants, given their limited systemic and bone effects11. We obtained informed consent from participants at least 18 years old or parents of participants < 18 years, and informed assent from participants < 18 years. The study was reviewed and approved by the Mass General Brigham Institutional Review Board. It is Health Insurance Portability and Accountability Act (HIPAA) compliant.
2.2. Methods:
A screening visit was performed to confirm eligibility, followed by study visits at baseline (BL) and at one year. A complete history was obtained, and physical examination was performed at each visit. Subjects self-reported race and ethnicity. Height was measured as the mean of three measurements using a wall-mounted stadiometer, and weight to the nearest 0.1 kilograms (kg) with an electronic scale. We calculated BMI as weight in kg/(height in meters)2, and BMI z-score using CDC tables12. Physical activity was recorded as the average hours of exercise per week reported by the participant. All participants were offered daily calcium and vitamin D supplements based on their baseline levels to optimize calcium intake and absorption. Blood samples were obtained for serum calcium, and 25 hydroxyvitamin D (25OHD) levels. Participants underwent DXA scans at the lumbar spine, total hip, femoral neck, and whole body, and HRpQCT scans at the distal radius and tibia. Participants having either DXA or HRpQCT scans at one-year follow-up were included in the analysis. Bone age for participants <18 years old was calculated by comparing left wrist radiographs of subjects with the nearest matching reference radiographs using the atlas of Greulich and Pyle13. We did not assess bone ages for those 18 years and older due to expected maturity of their bones by this age.
Areal Bone Mineral Density (aBMD) and Body Composition Assessment:
DXA (Hologic QDR 4500; Hologic Inc, Waltham, MA) was used to determine aBMD of the lumbar spine, total hip, femoral neck, and whole body, as well as for body composition (fat and lean mass) assessment. All participants were scanned using the same machine for baseline and follow-up visits Coefficients of variation for aBMD, fat mass, and lean mass at our institution are between 0.8% to 2.1%. We used the Hologic pediatric database to calculate aBMD Z-scores for participants <20.5 years of age at follow-up and the adult database for participants ≥20.5 years of age at follow-up (to avoid having to transition from the pediatric to the adult database over the course of the study for any participant). We did not use the Longitudinal Bone Mineral Density in Childhood study database to calculate Z-scores or height Z-score adjusted Z-scores are this database only goes up to 20 years, and our study included participants older than 20 years. We report whole body (and not whole body less head) Z-scores based on data available to us.
Volumetric Bone Mineral Density (vBMD) and Strength Estimates Assessment:
We used HRpQCT (XtremeCT; Scanco Medical AG®, Bassersdorf, Switzerland) to determine vBMD at the ultra-distal radius (9.5mm from a reference line manually placed at the endplate) and distal tibia (22.5 mm from a reference line manually placed at the endplate). Scans used 60 kVp effective energy and 100 ms integration time, obtaining 110 CT slices (9.02 mm) with an isotropic voxel of 82 μm3. We chose fixed sites because participants had mostly attained adult height with minimal or no changes in height anticipated given their baseline bone age. All measurements were performed at the non-dominant wrist and leg unless there was a history of fracture at that site in the last six months, in which case the non-fractured side was assessed. All participants were scanned on the same HRpQCT instrument. The scans were matched for region of interest at baseline and one year. We used 2D registration of the outer bone contours to match the follow-up scans to the baseline images, using software provided by Scanco Medical AG. Image acquisition and analysis followed published guidelines14. Automated analysis was used for measures of (i) bone geometry (cross-sectional area of cortical and trabecular bone compartments and cortical thickness), (ii) trabecular microarchitecture, including trabecular number and thickness, and (iii) vBMD (for total, cortical, and trabecular bone). Extended cortical analysis assessed cortical porosity, which contributes to fracture risk independent of other measures15. Failure load, a strength estimate, was calculated using micro finite element analysis (μFEA) via mathematical modeling of the effects of simulated mechanical load on bone and scaling the resultant load from a 1% apparent compressive strain until 2% of all elements reached an effective strain > 7000 μ. Same-day reproducibility for repeated measurements is 0.2 to 1.4% for density values, 0.3 to 8.6% for trabecular parameters, 0.6 to 2.4% for cortical parameters, 7.3 to 20.2% for cortical porosity measurements, and 2.1 to 3.0% for FEA measures. Failure load estimated from FEA correlates strongly (r2 = 0.75) with experimentally measured failure loads producing radii fractures in human cadavers16.
Biochemical Analysis:
Calcium levels were measured using a colorimetric assay (sensitivity 0.8 mg/dL, intra-assay CV 0.9–3.0% for calcium; LabCorp Esoteric Testing, Burlington, NC). Serum 25OHD was assessed by immunochemiluminometric assay (sensitivity 4.0 ng/mL, intra-assay CV 4.8–7.7%; LabCorp Esoteric Testing, Burlington, NC), and PTH by an electrochemiluminescent immunoassay (sensitivity 1 pg/mL, intra-assay CV 1.6–2.6%; Beckman Coulter, Fullerton, CA).
2.3. Statistical Analysis:
Data were analyzed using JMP software (SAS Institute Inc., Cary, NC), Version 16.0. We first assessed data for normality using the Shapiro-Wilk test and report values as mean ± SEM (for parametric data) or median and interquartile range (for nonparametric data). A p-value of < 0.05 was used to denote statistical significance. All reported bone outcome analyses were controlled for age and race. For baseline comparisons, we used the Student t-test or the Wilcoxon rank sum test to compare differences across groups depending on whether or not data were normally distributed. For categorical variables, the chi-square test was used for between-group comparisons. For within-group comparisons over one year, the paired t-test or Wilcoxon sign-rank test was used depending on data distribution. To control for the effect of other confounders such as menarchal age, change in lean mass or use of oral contraceptives on bone parameters after adjusting for race and age, we performed multivariable analyses. In order to determine the impact of one-year change in BMI on bone parameters, we added this variable to the regression model. The stability of the model was managed by adjusting for < N/10 variables in the regression model as per the one-in-ten rule of thumb. We additionally checked for the variance inflation factor in the multivariable analysis, and a value of ≥2 was used as a measure of collinearity between the variables at which point, they were excluded from the analysis.
3. Results
3.1. Anthropometric measures and clinical characteristics at baseline (Table 1):
Table 1:
Baseline characteristics for subjects with obesity (OB) and normal-weight controls (NWC)
| Baseline Characteristics | OB | NWC | p-value |
|---|---|---|---|
| n=21 | n=50 | ||
| Age, years | 17.82 ± 0.64 | 19.04 ± 0.36 | 0.107 |
| Race (white/black/Asian/others) | 15/2/1/3 | 31/4/9/6 | 0.337 |
| Height, cm | 164.23 ± 1.43 | 163.50 ± 0.89 | 0.666 |
| Height z-score for those under 20 years | 0.42 ± 0.30 | 0.16 ± 0.17 | 0.471 |
| Weight, kg | 115.67 ± 3.85 | 57.02 ± 0.79 | <0.0001 |
| Weight z-score for those under 20 years | 2.57 ± 0.07 | 0.10 ± 0.11 | <0.0001 |
| BMI, kg/m 2 | 42.76 ± 1.10 | 21.33 ± 0.25 | <0.0001 |
| BMI z-score | 2.38 ± 0.04 | 0.32 ± 0.10 | <0.0001 |
| Physical activity, hours/week | 4.75 ± 0.72 | 6.69 ± 0.89 | 0.0927 |
| Age at menarche, years | 11.00 (10.88,13) | 12.75 (12,13) | 0.022 |
| Bone age for those under 18 years, years | 17.00 (16.50, 17.00) | 16.00 (14.63, 16.38) | 0.011 |
| Calcium, mg/dL | 9.18 ± 0.07 | 9.06 ± 0.11 | 0.321 |
| 25OHD, ng/mL | 22.5 (18.73, 28.75) | 24 (19.7, 32.15) | 0.388 |
| Breast Tanner stage (1/2/3/4/5) | 0/1/0/1/17 | 0/0/3/5/42 | 0.243 |
Data presented as mean ± SEM or median (interquartile range)
Significant p values of <0.05 are bolded
BMI: Body Mass Index; 25OHD: 25-hydroxycholecalciferol
The mean age for the cohort was 18.7 ± 2.7 years. OB vs. NWC groups were similar for age, race, height, and physical activity at baseline. OB had higher mean weight, BMI, and BMI z-scores and younger mean menarchal age compared to NWC. Bone age for participants <18 years was higher in OB (n=10) vs. NWC (n=12). 52.4% of the OB and 76% of the NWCs had a mature bone age. There was no difference between the groups in baseline serum calcium and 25OHD levels. Five OB participants were on metformin at their baseline visit and continued this during the study, while none had diabetes or prediabetes. Ten out of twenty-one participants in the OB group had a history of non-stress fractures, while ten out of fifty participants in the NWC group had a history of fractures (stress=1, non-stress=8, both=1) (p=0.079). Nine OB participants were on OCPs at the baseline visit and continued it during the study, and three had intrauterine progesterone-releasing devices, while none of the NWCs were on hormonal contraceptives.
3.2. Body composition at baseline and changes over one year in anthropometric measures and body composition (Table 2):
Table 2:
Baseline characteristics and changes over a year in DXA variables including body composition and bone parameters for subjects with obesity (OB) and normal-weight controls (NWC)
| Anthropometric Measures | BL OB | BL NWC | p value controlling for age and race | Changes over one year in OB | Changes over one year in NWC | p-value controlling for age and race | p-value controlling for age, race and change in BMI |
|---|---|---|---|---|---|---|---|
| Height, cm | 164.2 (160.7, 168.4) | 163.7 (158.4, 168.6) | 0.959 | 0.4 (−0.2, 0.7) | 0.3 (−0.2, 0.6) | 0.542 | - |
| Weight, kg | 116.1 (98.8, 128.7) | 56.1 (53.7, 60.1) | <0.0001 | 1.3 (−2.3, 4.9) | 0.5 (−0.5, 2.1)# | 0.551 | - |
| BMI, kg/m2 | 41.6 (38.1, 46.7) | 21.2 (20.1, 22.6) | <0.0001 | 0.0 (−0.7, 1.7) | 0.1 (−0.2, 0.7) | 0.526 | - |
| DXA Body Composition | |||||||
| Total Lean Mass, kg | 57.92 ± 1.57 | 40.11 ± 0.56 | <0.0001 | 1.16 ± 0.48# | −0.08 ± 0.17 | 0.030 | - |
| Total Fat Mass, kg | 58.04 (46.59, 63.72) | 15.98 (14.42, 17.37) | <0.0001 | 0.04 (−2.83, 3.11) | −0.16 (−0.74, 1.20) | 0.042 | - |
| Percent Fat Mass, % | 48.80 (45.09, 51.22) | 28.21 (25.58, 31.13) | <0.0001 | −0.68 (−2.33, 0.82) | −0.19 (−1.09, 1.19) | 0.182 | - |
| DXA Bone Variables | |||||||
| Lumbar Spine BMD, g/cm2 | 1.08 ± 0.02 | 0.96 ± 0.01 | <0.0001 | 0.016 ± 0.009 | 0.014 ± 0.004# | 0.925 | 0.912 |
| Lumbar Spine BMD Z scores | 0.80 (0, 1.55) | −0.5 (−1.2, 0) | <0.0001 | 0.00 (−0.25, 0.30) | 0.00 (−0.10, 0.20) | 0.907 | 0.987 |
| Total Hip BMD, g/cm2 | 1.15 ± 0.03 | 0.98 ± 0.01 | <0.0001 | −0.001 ± 0.008 | 0.009 ± 0.003# | 0.030 | 0.076 |
| Total Hip BMD Z scores | 1.70 (0.80, 2.55) | 0.20 (−0.59, 0.88) | <0.0001 | −0.10 (−0.30, 0.15) | 0.03 (−0.10, 0.20)# | 0.018 | 0.046 |
| Femoral Neck BMD, g/cm2 | 1.04 ± 0.03 | 0.85 ± 0.01 | <0.0001 | −0.002 ± 0.012 | 0.006 ± 0.004 | 0.557 | 0.964 |
| Femoral Neck BMD Z scores | 1.65 ± 0.26 | −0.17 ± 0.12 | <0.0001 | −0.10 ± 0.12 | 0.06 ± 0.05 | 0.385 | 0.694 |
| Whole Body BMD, g/cm2 | 1.07 (1.00, 1.13) | 1.04 (1.00, 1.12) | 0.883 | 0.017 (−0.018, 0.035) | 0.001 (−0.012, 0.022) | 0.094 | 0.145 |
| Whole Body BMD Z scores | −0.05 ± 0.26 | −0.42 ± 0.14 | 0.660 | 0.03 ± 0.14 | −0.10 ± 0.05# | 0.091 | 0.139 |
Data presented as mean ± SEM or median (interquartile range)
Significant p values of <0.05 are bolded
Signifies reversal in the directionality of the relationship between the groups after adjusted analyses
Signifies significant within-group change over one year
BMD: Bone Mineral Density. BMI: Body Mass Index
Over one year, there was no difference between groups for change in height, weight, and BMI, although a within-group increase in weight was observed in NWC (p=0.030). At baseline, lean and fat mass were higher in OB than in NWC. Over one year, the OB group had increases in the total lean mass, not seen in NWC.
3.3. DXA bone variables at baseline and changes over one year (Table 2):
Areal BMD and BMD Z scores at the lumbar spine, total hip, and femoral neck were higher in OB vs NWC at baseline after controlling for baseline age and race. There was a within-group increase in lumbar spine BMD, total hip BMD, total hip BMD Z-scores and a decrease in whole body BMD Z-scores in NWCs over one year (p=0.0006, 0.0008, 0018, 0.047 respectively). These changes were not observed in the OB group. The groups differed significantly for changes over a year in total hip BMD and BMD-Z scores (after controlling for baseline age and race). Our findings held for differences between groups for changes in total hip BMD Z-scores over a year even after controlling for changes in BMI over the year. Figure 1 shows percent changes in BMD over one year.
Figure 1:
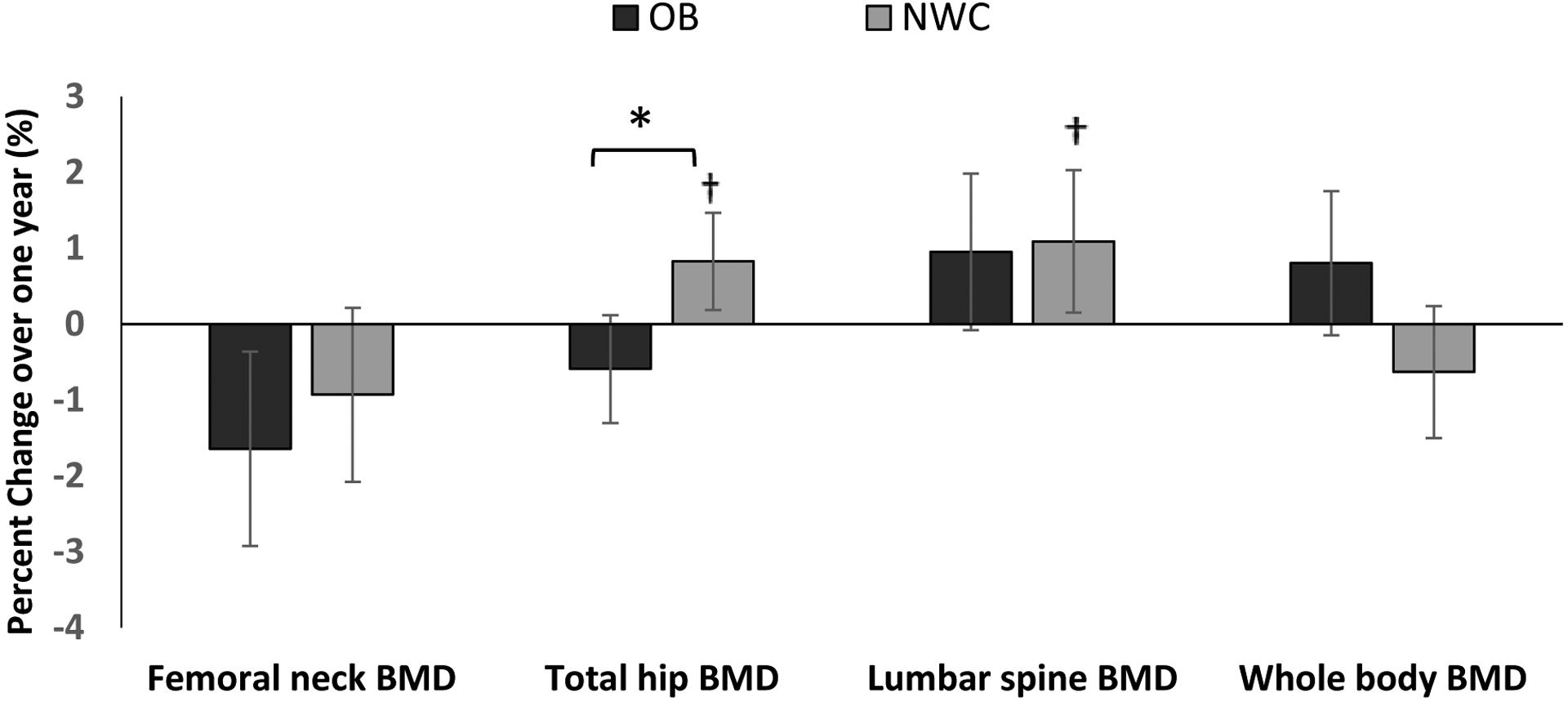
Percent change in absolute BMD values (as assessed by DXA) in subjects with obesity (OB) and normal-weight controls (NWC) over one year, after controlling for age and race *p < 0.05 for between group comparison, † p<0.05 for within group change
3.4. HRpQCT bone variables at baseline and changes over one year (Table 3):
Table 3:
Baseline characteristics and changes over one year in HRpQCT variables at the radius and tibia for subjects with obesity (OB) and normal-weight controls (NWC) groups
| HRpQCT Variables | BL OB | BL NWC | p value controlling for age and race | Changes over one year in OB | Changes over one year in NWC | p-value controlling for age and race | p-value controlling for age, race and change in BMI |
|---|---|---|---|---|---|---|---|
| Radius | |||||||
| Percent Cortical Area | 24.00 (17.79, 25.87) | 20.37 (16.02, 26.01) | 0.102 | −0.33 (−1.45, 1.22) | 0.57 (0.00, 1.21)# | 0.006 | 0.008 |
| Cortical Thickness, mm | 0.877 ± 0.047 | 0.780 ± 0.034 | 0.020 | −0.015 (−0.055, 0.050) | 0.02 (−0.003, 0.045)# | 0.012 | 0.016 |
| Cortical Porosity, % | 1.19 (0.88, 1.37) | 0.79 (0.46, 1.34) | 0.546 | 0.025 (−0.235, 0.285) | −0.010 (−0.188, 0.355) | 0.739 | 0.733 |
| Percent Trabecular Area | 77.42 ± 1.33 | 79.15 ± 1.09 | 0.102 | 0.33 (−1.22, 1.46) | −0.57 (−1.21, −0.00)# | 0.006 | 0.008 |
| Trabecular Thickness, mm | 0.080 (0.066, 0.090) | 0.072 (0.064, 0.078) | 0.139 | −0.001 (−0.004, 0.009) | 0.002 (−0.002, 0.004) | 0.422 | 0.408 |
| Trabecular Number, 1/mm | 2.02 (1.97, 2.23) | 1.95 (1.85, 2.15) | 0.059 | 0.01 (−0.15, 0.18) | −0.03 (−0.09, 0.10) | 0.614 | 0.600 |
| Trabecular Separation, mm | 0.397 (0.385, 0.432) | 0.436 (0.394, 0.474) | 0.031 | 0.000 (−0.034, 0.029) | 0.008 (−0.022, 0.024) | 0.611 | 0.594 |
| Cortical vBMD, mgHA/cm3 | 840.2 (809.8, 879.8) | 846.1 (801.1, 881.9) | 0.067 | 9.0 (−5.1,18.1) | 14.0 (4.6, 22.1)# | 0.034 | 0.036 |
| Trabecular vBMD, mgHA/cm3 | 197 (169.2, 220.8) | 172.1 (145.7, 194.1) | 0.029 | 1.6 (0.1, 3.5)# | 1.6 (−2.7, 4.4) | 0.631 | 0.601 |
| Total vBMD, mgHA/cm3 | 343.3 (321.6, 392.4) | 325.7 (267.3, 362.1) | 0.030 | 2.6 (−9.3, 14.8) | 5.2 (0.8, 13.0)# | 0.037 | 0.046 |
| Stiffness, kN/mm | 94.258 (75.693, 110.371) | 74.930 (68.724, 87.537) | 0.003 | 0.805 (−6.662, 3.254) | 0.353 (−1.627, 4.767) | 0.161 | 0.191 |
| Tibia | |||||||
| Percent Cortical Area | 20.42 (16.97, 23.40) | 18.40 (15.16, 22.21) | 0.021 | 0.35 (0.17, 0.70)# | 0.20 (0.07, 0.38)# | 0.517 | 0.443 |
| Cortical Thickness, mm | 1.367 ± 0.061 | 1.223 ± 1.035 | 0.005 | 0.02 (0.01, 0.06)# | 0.01 (0.00, 0.03)# | 0.466 | 0.402 |
| Cortical Porosity, % | 3.65 (2.44, 4.78) | 1.49 (0.98, 4.78) | 0.002 | −0.070 (−0.335, 0.795) | 0.430 (−0.145, 1.320)# | 0.292 | 0.134 |
| Percent Trabecular Area | 79.58 (76.60, 83.03) | 81.25 (77.62, 84.23) | 0.021 | −0.35 (−0.70, −0.17)# | −0.20 (−0.38, −0.07)# | 0.517 | 0.443 |
| Trabecular Thickness, mm | 0.076 (0.068, 0.089) | 0.083 (0.074, 0.095) | 0.085 | 0.002 (0.000, 0.006)# | 0.001 (−0.003, 0.006) | 0.384 | 0.467 |
| Trabecular Number, 1/mm | 2.500 (2.185, 2.635) | 1.920 (1.780, 2.130) | <0.0001 | −0.080 (−0.155, 0.045) | −0.010 (−0.150, 0.060) | 0.606 | 0.743 |
| Trabecular Separation, mm | 0.323 (0.301, 0.370) | 0.431 (0.390, 0.470) | <0.0001 | 0.010 (−0.007, 0.022) | 0.003 (−0.018, 0.029) | 0.871 | 0.934 |
| Cortical vBMD, mgHA/cm3 | 868.9 (854.25, 894.1) | 880.8 (850.5, 912.0) | 0.676 | 5.8 (1.4, 10.2)# | 6.7 (0.5, 18.5)# | 0.352 | 0.337 |
| Trabecular vBMD, mgHA/cm3 | 229.5 (199.2, 249.7) | 199.4 (170.8, 221.1) | 0.003 | 2.7 (−0.4, 3.7)# | 1.6 (−0.2, 3.3)# | 0.625 | 0.663 |
| Total vBMD, mgHA/cm3 | 359.4 (334.5, 387.3) | 332.4 (290.6, 361.1) | 0.005 | 4.1 (2.3, 7.5)# | 3.3 (1.4, 7.5)# | 0.613 | 0.581 |
| Stiffness, kN/mm | 250.915 (218.948, 277.303) | 220.837 (205.147, 243.585) | 0.013 | 4.502 (0.044, 10.897)# | 2.984 (−0.276, 7.340)# | 0.561 | 0.636 |
Data presented as mean ± SEM or median (interquartile range)
Significant p values of <0.05 are bolded
Signifies reversal of the relationship between the groups after adjusted analyses
Signifies significant within-group change over one year
vBMD: Volumetric Bone Mineral Density
Radius:
At baseline, after controlling for age and race, OB compared to NWC had higher cortical thickness, lower trabecular separation, higher total and trabecular vBMD, and stiffness.
Within the OB group there was an increase in trabecular vBMD (p= 0.031) over one year, whereas within the NWC group there were increases in percent cortical area, cortical thickness, cortical vBMD, total vBMD, and a decrease in percent trabecular area (p≤0.009 for all). For between-group comparisons, the NWC group had greater increases in percent cortical area, cortical thickness, cortical and total vBMD, and greater decreases in percent trabecular area over a year than the OB group after controlling for baseline age and race. These findings held after also controlling for one-year change in BMI. Figure 2 shows percent changes in these parameters over one year (which were similar to results for absolute changes).
Figure 2:
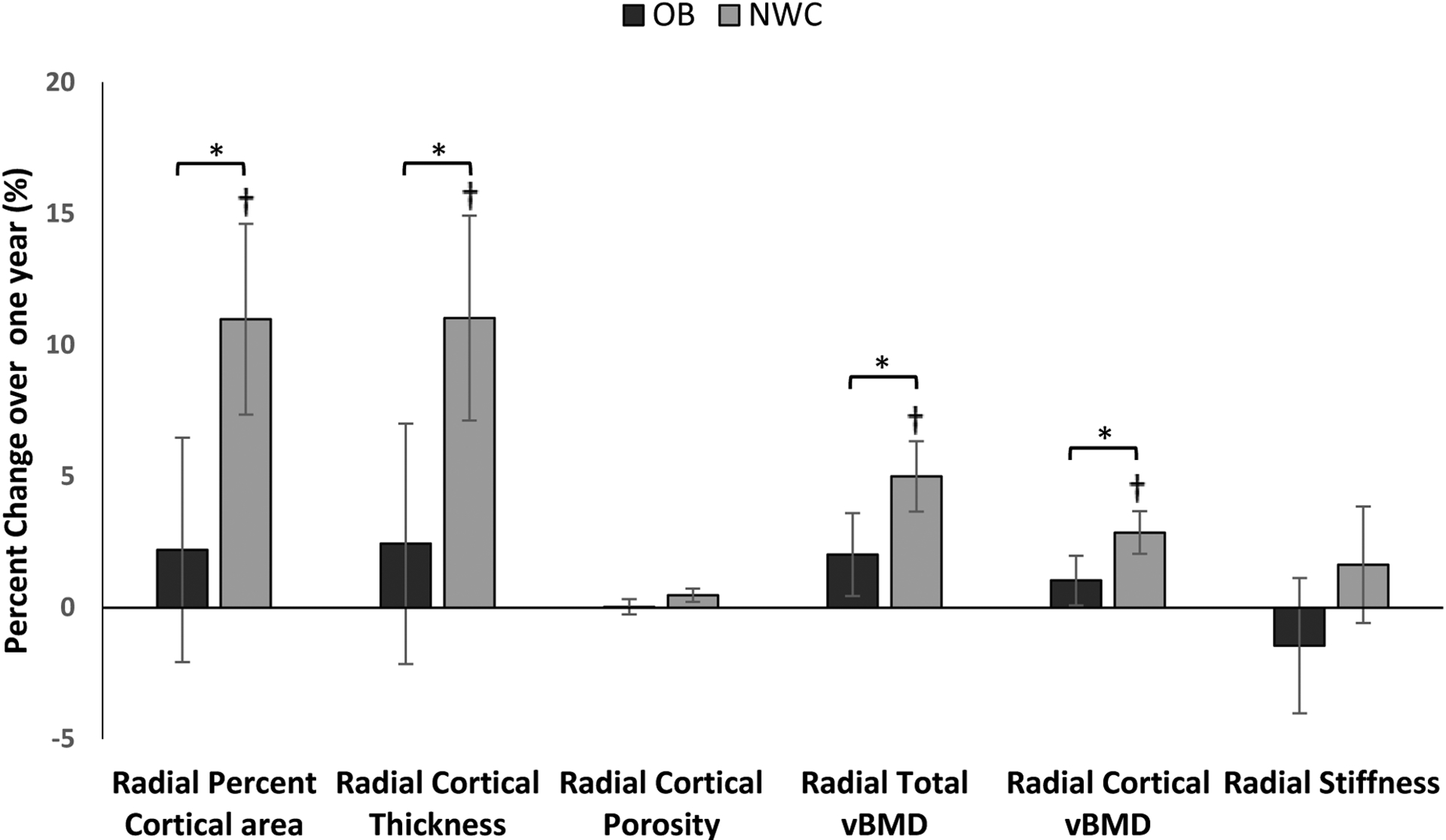
Percent change in absolute values of radial HRpQCT variables between subjects with obesity (OB) and normal-weight controls (NWC) over one year, after controlling for age and race *p < 0.05 for between group comparison, † p<0.05 for within group change
Tibia:
At baseline, OB vs. NWC had higher percent cortical area, cortical thickness and porosity, higher trabecular number and lower percent trabecular area and trabecular separation, and higher total and trabecular vBMD and stiffness.
Over one year, OB demonstrated a within-group increase in percent cortical area (p= <0.002), cortical thickness (p=0.004), trabecular thickness (p=0.026), cortical vBMD (p=0.018), trabecular vBMD (p=0.029), total vBMD (p=0.0002), and stiffness (p=0.002) with a decrease in percent trabecular area (p=0.002). NWC showed similar within-group changes, with increases in percent cortical area (p<0.0001), cortical thickness (p<0.0001), cortical porosity (p=0.001), cortical vBMD (p<0.0001), trabecular vBMD (p=0.006), total vBMD (p<0.0001), and stiffness (p=0.0008) and decreases in percent trabecular area (p=<0.0001). There were no differences between the two groups in bone accrual over one year in tibial cortical or trabecular bone. Percent changes in these parameters over one year showed similar results (data not shown).
3.5. Controlling for Other Possible Confounders:
When we controlled for baseline lean mass, in addition to controlling for baseline age and race, the groups did not differ for baseline bone measures (data not shown). When we controlled for one-year change in lean mass (rather than one-year change in BMI), in addition to controlling for age and race, our results for between group differences were essentially unchanged (data not shown). On adding menarchal age to the regression model that included baseline age and race, differences between groups for bone accrual over one year did not change, except for change in radial cortical vBMD which became a trend (p=0.067).
On controlling for use of oral contraceptives (in addition to baseline age and race), differences in bone accrual over one year between the groups were overall similar to results without controlling for oral contraceptive use other than as indicated below. This analysis demonstrated greater increases in lumbar BMD in OB vs NWC (0.03 ± 0.01 vs. 0.01 ± 0.01; p=0.04) and greater decreases in radial stiffness in OB vs NWC (−5626.60 ± 2017.17 vs. −27.04 ± 1446.60; p=0.008) over a year. Further, there was a trend for greater increases in lumbar BMD Z-scores in OB vs NWC (0.14 ± 0.09 vs. −0.01 ± 0.07; p= 0.078) and lesser increases in tibial cortical porosity in OB vs NWC (0.22 ± 0.35 vs. 1.00 ± 0.23; p=0.056).
On excluding participants on metformin from the analysis, differences between groups for one-year change in total hip BMD (p=0.035), total hip BMD Z-scores (p=0.020), radial cortical area (p=0.019) and radial cortical thickness (p=0.031) remained significant while those for radial total vBMD (p=0.058) and radial cortical vBMD (p=0.073) become a trend; differences between groups for changes in whole body BMD (p=0.009) and whole body BMD Z-scores (p=0.007) became significant.
4. Discussion:
Despite a greater representation of adolescents with obesity in those with extremity fractures, data suggest that areal and volumetric bone density are higher in adolescents and young adults with obesity compared to normal-weight individuals2,17. However, we and others have reported suboptimal bone adaptation to body weight in those with obesity, which likely explains their higher fracture risk despite higher BMD3,7,9,18. We have previously reported cross-sectional differences in bone parameters between adolescent girls with obesity and normal-weight adolescents, which are replicated in this cohort as well7. We have now examined longitudinal changes in areal and volumetric BMD, bone geometry, microarchitecture, and strength estimates over one year in those with obesity compared with normal-weight controls.
We found that adolescent and young women with obesity did not show the increase in total hip BMD that was observed in young women with normal weight. In contrast to our findings, studies in peri- and postmenopausal women have reported similar changes in hip BMD over time in participants with and without obesity19,20. Recent data on increased risk of early hip fracture in women with obesity further elaborates the detrimental effect of obesity on bone health21. We noted no differences between the normal weight group and the group with obesity for bone accrual at the lumbar spine or total body. These results differ from those reported by Mengel et al, who found a lesser increment in lumbar spine BMD in young adolescent Estonian boys who had larger BMI gains in the three-year duration of the study as compared with those who had smaller BMI increases22. The difference in sex, age and follow-up duration between our and the Estonian cohort may explain the difference in findings as both sex and age can impact bone accrual23–25. Further, literature also supports greater increases in total body BMD in normal-weight adolescents as compared to those with obesity after physical activity intervention suggesting that higher adiposity has an adverse effect on bone accrual.10 Thus, studies with longer follow-up duration and those evaluating the factors affecting the bone accrual in young women with obesity are needed.
We found that increases in cortical area and thickness at the non-weight bearing radius, observed in normal-weight young women over one year were not evident in those with obesity. The lower accrual rates at the radial cortex seen in our study may explain the low radial cortical vBMD reported previously in cross-sectional studies of adolescents and young adults with obesity7,18. Wey et al have previously demonstrated a negative association of fat mass with longitudinal changes in radial cortical vBMD, which supports our findings of greater increases in both total and cortical vBMD in normal-weight young women who have lower total fat mass than those with obesity18. The greater risk of wrist fracture in adolescents with obesity, as reported in the literature, may be explained, partly, by the suboptimal accrual demonstrated at the radial cortex in our study3,26.
We found no differences in OB vs. NWC groups for changes in bone parameters at the weight-bearing tibia over 12 months, Wetzsteon et al have previously reported a greater increase in absolute bone strength at the tibia over 16 months in overweight vs. healthy-weight children between the ages of 9–11 years, although these changes were suboptimal when controlled for fat mass, showing that bone strength did not adapt to excess body fat27. In contrast to our results at the tibia, in a study of young girls aged 8–13 years, Laddu et al reported that a two-year longitudinal increase in tibial bone strength index as assessed by peripheral quantitative CT (pQCT), was positively associated with baseline fat mass. However, changes in tibial cortical vBMD were higher for girls in the middle versus the highest tertile of baseline android fat mass suggesting that higher levels of fat mass may affect cortical vBMD29. The lack of differences in our cohort may stem from the older age of our participants, specifically because bone accrual slows down during the later adolescent years23,24.
Since our adjusted analysis for differences in bone accrual between the OB vs. NWC after controlling for menarchal age and changes in BMI showed similar results to the unadjusted analysis, there is a need to explore further contributing factors. Differences in changes in bone accrual at the radius vs. the tibia between OB and NWC groups are likely because of more pronounced effects of mechanical loading in OB at weight-bearing sites, such as the tibia, as opposed to non-weight bearing sites, such as the radius29. Greater mechanical loading at the weight-bearing tibia in OB appears to be protective, mitigating the negative systemic effects of obesity.
Limitations of our study include a relatively small sample size and that the study participants were followed for a short duration. Since our study only had female participants, future studies with both male and female participants will be important to investigate the patterns of bone accrual over a longer duration of time. The impact of puberty and growth on bone likely differs in males versus females with obesity due to the differential effects of obesity on these physiological processes; further exploration of bone outcomes in males with obesity is thus important. Adjusting for menarchal age statistically might not entirely capture the contribution of puberty; however, this mitigates the impact of longer estrogen exposure in girls with obesity due to lower menarchal age. We did not assess differences in hormonal parameters that may impact bone accrual in the two groups, and physical activity was also not addressed in detail. Further, contraceptive use was very different between the two groups, making the effect of hormones on the changes in bone outcomes a potential contributory factor. However, we also ran our analyses after controlling for use of oral contraceptives.
In addition, we had access to whole body BMD Z-scores, but not whole body less head BMD Z-scores for this cohort, and the ISCD guidelines prefer the latter. We could not use the Longitudinal Bone Mineral Density in Childhood Study database to calculate whole body less head BMD Z-scores as this database does not go past 20 years, and several of our participants were older than 20 years. We used a fixed scan site for the HRpQCT evaluations vs. the relative site that is sometimes used in growing children. However, it is unlikely that this impacted our results as our cohort had attained near adult height at study onset and had minimal height increments over the duration of the study. A significant strength of our study includes the use of HRpQCT to assess volumetric BMD, bone geometry, and microarchitecture, especially because DXA results can be impacted by body composition differences between groups. Ours is one of the very few studies assessing longitudinal bone accrual for adolescents and young adults with obesity in comparison to normal-weight controls using such techniques.
In conclusion, our study demonstrates that longitudinal bone accrual over one year is impaired at the total hip and radius in adolescents and young adult females with obesity compared to normal-weight controls, while no differences are observed in tibial bone accrual.
Highlights:
Bone accrual is impaired at the hip and radial cortex in women with obesity (OB)
The increase in hip bone mineral density (BMD) seen in controls is lacking in OB
Increases in radial cortical area, thickness and vBMD are lower in OB than controls
Funding:
This work was supported by NIH grants R01DK103946 (MM, MAB), R01DK062249 (MM), R01HD060827 (MM), K24HD071843 (MM), K23DK110419-01(VS), P30DK040561 (VS), K24DK109940 (MAB), P30DK057521 (VS), 1UL1TR001102 (MM), 1UL1TR002541-01, 1S10RR023405-01
Abbreviations:
- NWC
Normal-weight controls
- OB
Subjects with obesity
- BMD
Bone mineral density
- aBMD
Areal bone mineral density
- DXA
Dual-energy X-ray absorptiometry
- vBMD
Volumetric bone mineral density
- HRpQCT
High resolution peripheral quantitative computed tomography
- μFEA
Microfinite element analysis
- BMI
Body mass index
- PCOS
Polycystic ovarian syndrome
- OCPs
Combined oral contraceptive pills
- 25OHD
Serum 25 hydroxy vitamin D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration: NCT02557438, NCT00946192, NCT01301183
CRediT Author Statement
Vibha Singhal – Conceptualization, Final data analysis and writing the first draft
Snimarjot Kaur – Data curation, analysis and writing the manuscript
Lea Abou Haidar – Data analysis and manuscript editing
Hang Lee – Data analysis and Manuscript editing
Miriam A. Bredella –Supervision, Funding acquisition, and Manuscript editing
Madhusmita Misra- Conceptualization, Supervision, Funding acquisition, Manuscript editing
References:
- (1).Boot AM; de Ridder MAJ; van der Sluis IM; van Slobbe I; Krenning EP; Keizer-Schrama S. M. P. F. de M. Peak Bone Mineral Density, Lean Body Mass and Fractures. Bone 2010, 46 (2), 336–341. 10.1016/j.bone.2009.10.003. [DOI] [PubMed] [Google Scholar]
- (2).Fintini D; Cianfarani S; Cofini M; Andreoletti A; Ubertini GM; Cappa M; Manco M The Bones of Children With Obesity. Front. Endocrinol 2020, 11, 200. 10.3389/fendo.2020.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Goulding A; Jones IE; Taylor RW; Manning PJ; Williams SM More Broken Bones: A 4-Year Double Cohort Study of Young Girls with and without Distal Forearm Fractures. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 2000, 15 (10), 2011–2018. 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- (4).Brandi ML Microarchitecture, the Key to Bone Quality. Rheumatology 2009, 48 (suppl 4), iv3–iv8. 10.1093/rheumatology/kep273. [DOI] [PubMed] [Google Scholar]
- (5).Leonard MB; Shults J; Wilson BA; Tershakovec AM; Zemel BS Obesity during Childhood and Adolescence Augments Bone Mass and Bone Dimensions. Am. J. Clin. Nutr 2004, 80 (2), 514–523. 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- (6).Evensen E; Skeie G; Wilsgaard T; Christoffersen T; Dennison E; Furberg A; Grimnes G; Winther A; Emaus N How Is Adolescent Bone Mass and Density Influenced by Early Life Body Size and Growth? The Tromsø Study: Fit Futures—A Longitudinal Cohort Study From Norway. JBMR Plus 2018, 2 (5), 268–280. 10.1002/jbm4.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Singhal V; Sanchita S; Malhotra S; Bose A; Flores LPT; Valera R; Stanford FC; Slattery M; Rosenblum J; Goldstein MA; Schorr M; Ackerman KE; Miller KK; Klibanski A; Bredella MA; Misra M Suboptimal Bone Microarchitecure in Adolescent Girls with Obesity Compared to Normal-Weight Controls and Girls with Anorexia Nervosa. Bone 2019, 122, 246–253. 10.1016/j.bone.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Farr JN; Chen Z; Lisse JR; Lohman TG; Going SB Relationship of Total Body Fat Mass to Weight-Bearing Bone Volumetric Density, Geometry, and Strength in Young Girls. Bone 2010, 46 (4), 977–984. 10.1016/j.bone.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Dimitri P; Wales JK; Bishop N Fat and bone in children: Differential effects of obesity on bone size and mass according to fracture history. J. Bone Miner. Res 2010, 25 (3), 527–536. 10.1359/jbmr.090823. [DOI] [PubMed] [Google Scholar]
- (10).Kondiboyina V; Raine LB; Kramer AF; Khan NA; Hillman CH; Shefelbine SJ Skeletal Effects of Nine Months of Physical Activity in Obese and Healthy Weight Children. Med. Sci. Sports Exerc 2020, 52 (2), 434–440. 10.1249/MSS.0000000000002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hadji P; Colli E; Regidor P-A Bone Health in Estrogen-Free Contraception. Osteoporos. Int 2019, 30 (12), 2391–2400. 10.1007/s00198-019-05103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ogden CL; Kuczmarski RJ; Flegal KM; Mei Z; Guo S; Wei R; Grummer-Strawn LM; Curtin LR; Roche AF; Johnson CL Centers for Disease Control and Prevention 2000 Growth Charts for the United States: Improvements to the 1977 National Center for Health Statistics Version. Pediatrics 2002, 109 (1), 45–60. 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- (13).Pyle SI; Greulich WW Radiographic Atlas of Skeletal Development of the Hand and Wrist; Stanford University Press: Stanford, 1959. [Google Scholar]
- (14).Whittier DE, Boyd SK, Burghardt AJ, Paccou J, Ghasem-Zadeh A, Chapurlat R, Engelke K, Bouxsein ML Guidelines for the assessment of bone density and microarchitecture in vivo using high-resolution peripheral quantitative computed tomography. Osteoporosis International. 2020, 31(9), 1607–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Seeman E Growth and Age-Related Abnormalities in Cortical Structure and Fracture Risk. Endocrinol. Metab 2015, 30 (4), 419–428. 10.3803/EnM.2015.30.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pistoia W; van Rietbergen B; Lochmüller E-M; Lill CA; Eckstein F; Rüegsegger P Estimation of Distal Radius Failure Load with Micro-Finite Element Analysis Models Based on Three-Dimensional Peripheral Quantitative Computed Tomography Images. Bone 2002, 30 (6), 842–848. 10.1016/s8756-3282(02)00736-6. [DOI] [PubMed] [Google Scholar]
- (17).Farr JN; Dimitri P The Impact of Fat and Obesity on Bone Microarchitecture and Strength in Children. Calcif. Tissue Int 2017, 100 (5), 500–513. 10.1007/s00223-016-0218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Wey HE; Binkley TL; Beare TM; Wey CL; Specker BL Cross-Sectional versus Longitudinal Associations of Lean and Fat Mass with PQCT Bone Outcomes in Children. J. Clin. Endocrinol. Metab 2011, 96 (1), 106–114. 10.1210/jc.2010-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lloyd JT; Alley DE; Hochberg MC; Waldstein SR; Harris TB; Kritchevsky SB; Schwartz AV; Strotmeyer ES; Womack C; Orwig DL CHANGES IN BONE MINERAL DENSITY OVER TIME BY BODY MASS INDEX IN THE HEALTH ABC STUDY. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2016, 27 (6), 2109–2116. 10.1007/s00198-016-3506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Holecki M; Chudek J; Titz-Bober M; Więcek A; Zahorska-Markiewicz B; Duława J Changes of Bone Mineral Density in Obese Perimenopausal Women during 5-Year Follow-Up. Pol. Arch. Med. Wewn 2012, 122 (4), 139–147. 10.20452/pamw.1175. [DOI] [PubMed] [Google Scholar]
- (21).Rikkonen T; Sund R; Sirola J; Honkanen R; Poole KES; Kröger H Obesity Is Associated with Early Hip Fracture Risk in Postmenopausal Women: A 25-Year Follow-Up. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2021, 32 (4), 769–777. 10.1007/s00198-020-05665-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Mengel E; Tillmann V; Remmel L; Kool P; Purge P; Lätt E; Jürimäe J Extensive BMI Gain in Puberty Is Associated with Lower Increments in Bone Mineral Density in Estonian Boys with Overweight and Obesity: A 3-Year Longitudinal Study. Calcif. Tissue Int 2017, 101 (2), 174–181. 10.1007/s00223-017-0273-4. [DOI] [PubMed] [Google Scholar]
- (23).Bachrach LK; Hastie T; Wang MC; Narasimhan B; Marcus R Bone Mineral Acquisition in Healthy Asian, Hispanic, Black, and Caucasian Youth: A Longitudinal Study. J. Clin. Endocrinol. Metab 1999, 84 (12), 4702–4712. 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- (24).Nilsen OA; Ahmed LA; Winther A; Christoffersen T; Furberg A-S; Grimnes G; Dennison E; Emaus N Changes and Tracking of Bone Mineral Density in Late Adolescence: The Tromsø Study, Fit Futures. Arch. Osteoporos 2017, 12 (1), 37. 10.1007/s11657-017-0328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Sayers A; Tobias JH Fat Mass Exerts a Greater Effect on Cortical Bone Mass in Girls than Boys. J. Clin. Endocrinol. Metab 2010, 95 (2), 699–706. 10.1210/jc.2009-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Singhal V; Huynh C; Nimmala S; Mitchell DM; Pedreira CC; Bader A; Flanders K; Zheng J; Bouxsein ML; Misra M; Bredella MA Load-to-Strength Ratio at the Radius Is Higher in Adolescent and Young Adult Females with Obesity Compared to Normal-Weight Controls. Bone 2022, 164, 116515. 10.1016/j.bone.2022.116515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wetzsteon RJ; Petit MA; Macdonald HM; Hughes JM; Beck TJ; McKay HA Bone Structure and Volumetric BMD in Overweight Children: A Longitudinal Study. J. Bone Miner. Res 2008, 23 (12), 1946–1953. 10.1359/jbmr.080810. [DOI] [PubMed] [Google Scholar]
- (28).Laddu DR; Farr JN; Laudermilk MJ; Lee VR; Blew RM; Stump C; Houtkooper L; Lohman TG; Going SB Longitudinal Relationships between Whole Body and Central Adiposity on Weight-Bearing Bone Geometry, Density, and Bone Strength: A PQCT Study in Young Girls. Arch. Osteoporos 2013, 8 (0), 156. 10.1007/s11657-013-0156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Leonard MB; Zemel BS; Wrotniak BH; Klieger SB; Shults J; Stallings VA; Stettler N Tibia and Radius Bone Geometry and Volumetric Density in Obese Compared to Non-Obese Adolescents. Bone 2015, 73, 69–76. 10.1016/j.bone.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


