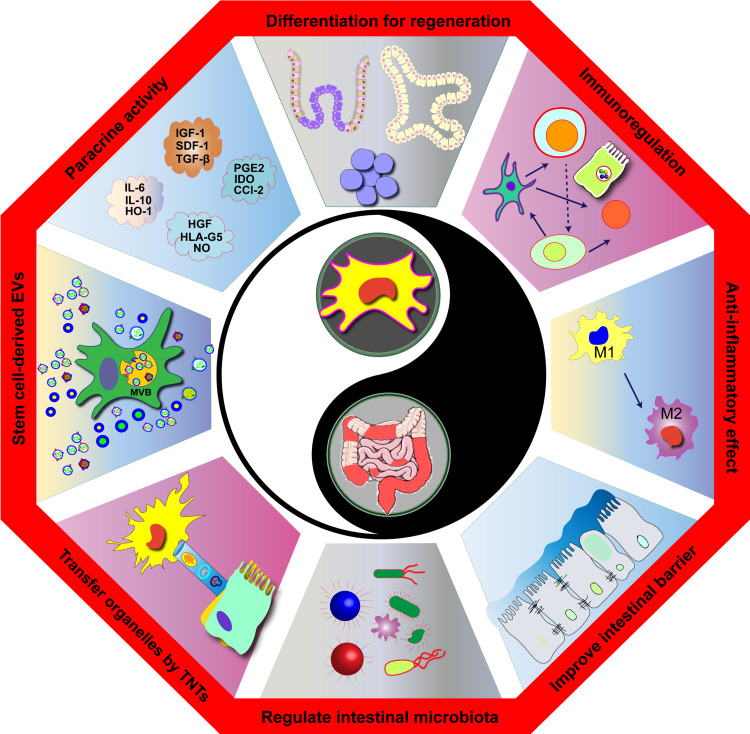
Abstract
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a group of chronic inflammatory diseases of the gastrointestinal tract. Repeated inflammation can lead to complications, such as intestinal fistula, obstruction, perforation, and bleeding. Unfortunately, achieving durable remission and mucosal healing (MH) with current treatments is difficult. Stem cells (SCs) have the potential to modulate immunity, suppress inflammation, and have anti-apoptotic and pro-angiogenic effects, making them an ideal therapeutic strategy to target chronic inflammation and intestinal damage in IBD. In recent years, hematopoietic stem cells (HSCs) and adult mesenchymal stem cells (MSCs) have shown efficacy in treating IBD. In addition, numerous clinical trials have evaluated the efficiency of MSCs in treating the disease. This review summarizes the current research progress on the safety and efficacy of SC-based therapy for IBD in both preclinical models and clinical trials. We discuss potential mechanisms of SC therapy, including tissue repair, paracrine effects, and the promotion of angiogenesis, immune regulation, and anti-inflammatory effects. We also summarize current SC engineering strategies aimed at enhancing the immunosuppressive and regenerative capabilities of SCs for treating intestinal diseases. Additionally, we highlight current limitations and future perspectives of SC-related therapy for IBD.
Keywords: stem cells, mesenchymal stromal cells, HSCs, immunosuppression, inflammatory bowel disease, ulcerative colitis, Crohn’s disease
Graphic Abstract
Introduction
Inflammatory Bowel Disease (IBD) has a multifaceted etiology, including genetic susceptibility, immune dysregulation, external intestinal flora, and environmental factors.1 Both internal and external causes can compromise the intestinal mucosal barrier, leading to chronic, non-specific inflammation, local structural changes, and intestinal dysfunction.2 However, the extent and nature of the lesions are not limited to the intestinal mucosa and inflammation.3 Repeated inflammation and microcirculation disorders can cause intestinal fistulae, stenosis, obstruction, perforation, gastrointestinal (GI) bleeding, sepsis, and other complications, increasing the risk of intestinal cell cancerization and death.1,4,5 Although the overall morbidity of IBD remains at 0.5% in North America and Europe, its incidence is increasing yearly in Asia, Africa, and South America, resulting in a growing number of patients.4–7
Drugs used to treat IBD primarily target the systemic immune system, whether through non-targeted immunosuppressive agents or biological agents targeting TNF-alpha, integrin-alpha 4 beta 7, interleukin-12 (IL-12), IL-23, and other factors. Their goal is to suppress excessive immune responses and active inflammatory responses.8,9 However, inhibiting the inflammatory response alone is insufficient to completely cure IBD and achieve long-term remission and mucosal healing (MH).10,11
Stem cells (SCs) can differentiate, proliferate, and regulate various tissue cells through secretory functions.12 Intestinal resident SCs, mainly intestinal stem cells (ISCs) and resident mesenchymal stem cells (MSCs), maintain the structure and function of intestinal structural and immune cells, preserving intestinal mucosal integrity and immune homeostasis.13 Absence or abnormal activation of these SCs may cause mucosal and immune homeostasis disorders, which are the primary pathogenesis of IBD.13 IBD is characterized by the loss of control of immune cells, damage to intestinal structural cells, and abnormalities of intestinal resident SCs.14,15 Transplantation of SCs can regulate or rebuild immune cells, repair or supplement structural cells, such as intestinal epithelial cells (IECs), and potentially lead to a complete cure of IBD.16,17 This review summarizes recent research on the use of SCs for treating IBD, providing targeted treatment recommendations and research strategies for IBD patients.
Research has shown that multiple types of SCs continuously generate and replace IECs, immune cells, and more, thereby maintaining the integrity of the intestinal epithelium and the homeostasis of intestinal immunity. The absence or abnormal activation of intestinal resident SCs can lead to intestinal epithelial dysfunction and immune dysfunction, which are key pathogenic factors of IBD. Transplantation of SCs into the intestinal lesions of patients may offer a potential cure for IBD by repairing intestinal epithelial dysfunction and reestablishing immune homeostasis, resulting in MH and long-term remission.
Commonly Used SC Types for IBD Treatment
Human cells can be classified into general cells and SCs based on their ability to proliferate and differentiate.18 General cells, also called terminal differentiated cells, have a limited lifespan and lack the capacity to proliferate and differentiate.19 In contrast, SCs have the potential for unlimited proliferation and differentiation and are regulated by their micro-environment, or niche, which allows them to differentiate into various cell types and maintain the structural and functional integrity of the organ.14 SCs are further classified into totipotent, pluripotent, and unipotent SCs based on their differentiation potential, with totipotent SCs capable of self-renewal and differentiation into any cell type, pluripotent SCs capable of differentiating into multiple cell types, and unipotent SCs able to differentiate into only one cell type. Additionally, SCs can regulate human cells, acting as cell producers and managers in the body.15,17 However, SCs in vivo are also affected by their local niche and can undergo structural changes, damage, depletion, cancerization, and even death,20 which can affect their differentiation and secretion functions. SC therapy involves transplanting exogenous SCs into patients with impaired SC structure and function, supplementing, resetting, or transforming their own SCs to repair or replace damaged cells or tissues and cure diseases.21 Various types of SCs, including hematopoietic stem cells (HSCs), MSCs, ISCs, and induced pluripotent stem cells (iPSCs), have been used for the treatment of IBD.12,22 Please refer to Table 1 for a list of commonly used SC types for IBD treatment.
Table 1.
For a List of Commonly Used SC Types for IBD Treatment
| Advantages | Disadvantages | |
|---|---|---|
| HSCs | ||
| HSCs have a high differentiation potential and can reconstruct the hematopoietic and immune systems. HSCT technology is mature and mainly used for the treatment of hematologic tumors, and has been found to be effective in IBD. |
HSCs are highly immunogenic. HSCT requires search for a match, marrow clearance therapy and immunosuppression. There are invasive operations, many adverse effects and high relapse rate. Not an option for the treatment of IBD alone. |
|
| MSCs | ||
| MSCs can be multidirectionally differentiated and have strong immunomodulatory and tissue repair capabilities. MSCs have low immunogenicity, MSCT does not require searching for a match and marrow clearance therapy, and there is no significant difference in efficacy and safety between allogeneic or autologous transplantation. The source is widely available, and can be derived from bone marrow, cord blood, peripheral blood or adipose tissue. Infusion is flexible, with the option of intravenous, intraperitoneal, subcutaneous, or local injection routes. |
MSCs are heterogeneous and can change with the microenvironment, lose their immunomodulatory capacity, or even become pro-inflammatory cells. MSCs remain weakly immunogenic, potentially tumorigenic or pro-tumorigenic risk. MSCs are injected intravenously and are mostly sequestered in organs such as liver and lung, with very little homing to the site of IBD lesions (< 1%). |
|
| BM-MSCs | Classical source, most studied, easy to extract and isolate. High differentiation potential and immunomodulatory ability. |
Bone marrow extraction is a highly invasive operation with a short in vitro survival time and proliferation and differentiation potential related to the age of the donor. |
| hUC-MSC UCBSCs | The MSCs of the umbilical cord are abundant, less immunogenic, and have greater differentiation potential and regulatory immunity. It can be expanded in vitro and frozen for a long time. | It can only be obtained during delivery, and there is a general lack of awareness of cord and cord blood preservation among the population. |
| PBSCs | Peripheral blood is easily accessible. | Low levels and lack of specific markers of MSCs in peripheral blood make them difficult to isolate and obtain. |
| ASCs | Adequate source, easy to obtain, easy to isolate and easy to cultivate. Strong immunomodulatory and tissue repair ability, stable biological properties. |
The ability to proliferate and differentiate is relatively weak. |
| ISCs | ||
| ISCs can differentiate into various types of IECs and resident immune cells, capable of repairing intestinal epithelium and regulating intestinal immune cells. ISCs are relatively new in research and have great potential to be cultivated into intestinal organoids, which are the best source of SCs for IBD treatment. | The extraction and culture of ISCs are relatively difficult. Relying on endoscopic manipulation and in vitro 3D cell culture techniques, the process is difficult and relatively complicated. | |
| iPSCs | ||
| It is easily accessible, malleable, and can be induced to form iISCs, iMSCs, and iESCs with similar functions as ISCs, MSCs, and ESCs. Preclinical studies clarify the therapeutic effects of iPSCs in IBD, and no clinical studies in IBD are available. | The preparation process is complex and requires precise control, with a high failure rate and uncertain safety. Induced SCs are not exactly the same as in vivo SCs in terms of gene expression, phenotypic characteristics, proliferation, and differentiation. |
|
| pESCs | ||
| pESCs are totipotent SCs that have been successfully induced into ISCs. pESCs are still in preclinical studies and there are no clinical studies on IBD yet. | The preparation process is complex and requires precise control, with high failure rates and uncertain safety. Faced with the problem of differences between induced SCs and in vivo SCs. |
|
Abbreviations: ASCs, adipose mesenchymal stem cells; BM-MSCs, Bone marrow mesenchymal stem cells; HSCs, hematopoietic stem cells; HSCT, hematopoietic stem cells transplantation; IBD, Inflammatory bowel disease; iESCs, Induced embryonic SCs; iISCs, Induced intestinal stem cells; iMSCs, MSCs derived from iPSCs; iPSCs, induced pluripotent stem cells; ISCs, intestinal stem cells; MSCs, mesenchymal stem cells; PBSCs, Peripheral blood stem cells; pESCs, Parthenogenetic embryonic stem cells; IECs, intestinal epithelial cells; UCBSCs, Umbilical Cord Blood stem cells; hUC-MSC, Umbilical cord mesenchymal stem cells.
HSCs
Human HSCs possess potent proliferation and differentiation capabilities, enabling them to differentiate into blood and immune cells, as well as migrate to damaged tissues and differentiate into various “structural cells”, such as epithelial cells, endothelial cells (ECs), and fibroblasts.23 Moreover, HSCs also have the ability to regulate blood, immune, and tissue cells.23 Hematopoietic stem cell transplantation (HSCT) can fully restore the hematopoietic and immune systems and prevent abnormal blood and immune cell differentiation.24 Although HSCT technology is well-established and has been used to treat hematological tumors, its application to IBD treatment is limited by its high immunogenicity, the need for a donor match, marrow clearance therapy, invasiveness, associated adverse effects, as well as a high recurrence rate. Additionally, immunosuppression is required, which further adds to its limitations.25–27
MSCs
MSCs are pluripotent SCs that are widely distributed throughout the human body, including the bone marrow, umbilical cord, placenta, amniotic fluid, fat, and other tissues.28 These cells have the ability to actively proliferate and differentiate in multiple directions,29 including osteoblasts, chondrocytes, adipocytes, and cardiomyocytes.30 Researchers have successfully induced bone marrow MSCs to differentiate into IECs by using fetal intestinal wall connective tissue.19 In addition to their differentiation potential, MSCs possess strong immune regulation and tissue repair abilities, as well as targeted and directional migration to injured tissue sites. They also have the ability to promote SC implantation and provide hematopoietic support.29,31
Studies have found that intestinal resident mesenchymal stem cells (MtSCs) in patients with IBD exhibit abnormal differentiation or depletion, making it difficult to regulate immune cells and repair IECs.13 Mesenchymal stem cell transplantation (MSCT), as a supplement or alternative to MtSCs, can regulate immune cells and repair IECs and has become the most widely used SC therapy for IBD.15 The use of MSCs for IBD treatment offers several advantages. Firstly, MSCs have low immunogenicity and do not generally require matching or myeloablative treatment, allowing for immunosuppressive therapy to be given as needed after transplantation.19 Secondly, MSCs can be easily obtained from multiple sources, such as umbilical cord blood, peripheral blood, or fat, and can be easily separated and cultured.14 Thirdly, MSCs can be administered through various methods, such as intravenous, intraperitoneal, subcutaneous, or local injection, allowing for the best method to be selected based on the type of lesion, with the transplantation process being similar to that of ordinary drug injection.17 Finally, both autologous and allogeneic MSCs can be used with no significant difference in efficacy and safety.17
However, it is worth noting that MSCs are heterogeneous and can change with the microenvironment, losing their immunomodulatory ability and even becoming pro-inflammatory cells.32 There are many differences between MSCs from different tissue sources in the treatment of IBD (Table 1). There are potential pathogenic risks associated with the colonization of allogeneic MSCs in vivo, such as potential immunogenicity and potential tumorigenicity.19 Attention should also be paid to the homing rate of MSCs, most of which stay in the liver, lung, and other organs after intravenous, intraperitoneal, and subcutaneous injection, with fewer than 1% homing to intestinal lesions. Therefore, MSCs mainly rely on paracrine signaling to regulate intestinal immunity and repair the intestinal mucosal barrier rather than through proliferation and differentiation.17
ISCs
IECs have a short lifespan of 3–5 days and rely on the continuous division and replenishment of ISCs located in the intestinal crypts to maintain the integrity of the intestinal epithelial barrier.33,34 ISCs possess pluripotency and can differentiate into various IECs and intestinal resident immune cells, such as T cells, B cells, and dendritic cells (DCs).35,36 In response to intestinal mucosal injury, ISCs can proliferate and differentiate to repair the injured mucosa and regulate resident immune cells in the intestine.37–39 Dysfunction of IECs is a crucial factor in the development of IBD, and studies have found abnormal differentiation or insufficient regeneration of ISCs in IBD patients.38 ISCs are an excellent source of SCs for the treatment of IBD due to their vital role in the maintenance of intestinal structure and function.40 However, the extraction and in vitro culture of ISCs are challenging.40 Recent advancements in 3D culture technology have led to the successful extraction, isolation, and in vitro culture of human ISCs by recreating the “niche” of ISCs in vitro, resulting in the development of “intestinal organoids” for in vitro cell research. The application of ISCs in animal models of IBD has shown initial success, providing hope for the application of ISCs in the treatment of IBD.41 The extraction and in vitro culture of intestinal organoids from ISCs require complex procedures, including endoscopic input, to ensure implantation at the site of intestinal lesions.42
iPSCs
Transcription factor genes, including Oct4, Sox, Myc, and Klf4, have been introduced into somatic cells, such as fibroblasts or blood cells, through retrovirus, leading to the generation of iPSCs.43 These cells possess similar morphology, function, and gene expression to embryonic stem cells (ESCs) and can be induced into various cell types, including iESCs, iMSCs, or iISCs, exhibiting similar functions to ESCs, MSCs, or ISCs, as confirmed in vitro and animal experiments. iPSCs offer several advantages over ESCs, including the avoidance of ethical issues and immune rejection.44–46 Spence et al have demonstrated the feasibility of using iPSCs to generate 3D intestinal tissues that closely resemble human intestinal tissues.47–49 After a series of induction and culture, iPSCs are differentiated into IECs, indicating their ability to produce tissue-specific cells. However, the preparation process of iPSCs is complex and requires precise control, often resulting in a high failure rate and uncertain safety. Moreover, although iPSCs can be induced into iESCs, iMSCs, or iISCs, their gene expression, phenotypic characteristics, proliferation, differentiation, apoptosis, and aging may not be identical.48
Parthenogenetic Embryonic Stem Cells (pESCs)
ESCs pose a high risk of cancer due to ethical restrictions, making it difficult to control and conduct clinical research on IBD. To overcome these issues, oocytes can be stimulated using physical and chemical methods to develop into parthenogenetic blastocysts, which can give rise to pluripotent stem cells known as pESCs.
These cells can be induced to differentiate into the three germ layers, and recent studies have shown successful induction of human pESCs into intestinal epithelial stem cells, bringing hope for the treatment of IBD.44,46 However, the preparation process is complex, requiring precise control, and has a high failure rate with uncertain security.46 Furthermore, ESCs or ISCs that have been differentiated in vitro may differ from their in vivo counterparts in terms of surface markers and functions. Therefore, their efficacy in clinical settings remains to be verified.50
The Mechanisms of SC Therapy in IBD
After implantation, SCs have been proven to persist in the body for an extended period and demonstrate a lasting effect.12,21 Each implanted SC represents a relatively independent life activity unit that can adapt well to the internal environment and serve as a producer and manager of human cells.22 SCs act on IBD lesions by regulating immune cells and repairing the intestinal mucosal barrier, which directly combats chronic intestinal inflammation.51 In addition to addressing inflammation, SCs can play a beneficial role in enhancing intestinal microecology, resolving microcirculation disorders, and preventing fibrosis and cancerization, among other benefits.15
Regulating the Immune System
Implanted SCs affect their own SCs and immune cells through differentiation and secretion.52 HSCT, following marrow clearing, replaces the body’s own SCs, while MSCT and iPSC transplantation (ISCT) primarily serve as supplements and regulators of the body’s own SCs. HSCT induces the differentiation of various types of immune cells, while MSCT and ISCT primarily regulate immune cells through the secretion of cytokines or vesicles.30,53 For patients with IBD, MSCs primarily regulate immune cells in the intestinal tract by secreting cytokines or vesicles. They regulate the polarization of macrophages to the M2 type, inhibit the proliferation of helper T cells Th1 and Th17, promote the differentiation of regulatory T cells, and inhibit the maturation and differentiation of DC, ultimately inhibiting inflammation. However, there is limited evidence supporting the homing of MSCs to the intestinal mucosa, and it remains unclear whether they can differentiate into immune cells.54–56
T Lymphocytes
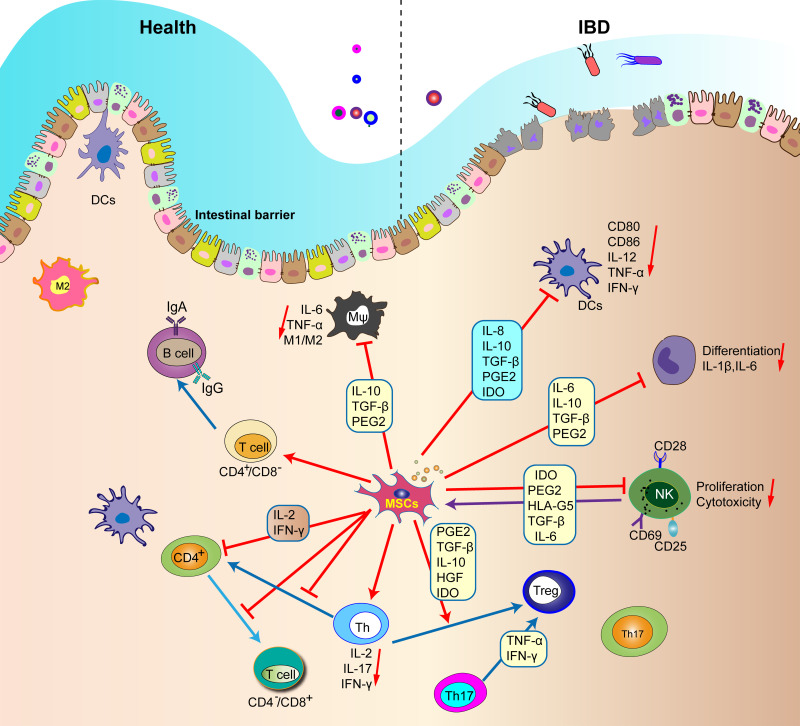
MSCs express indoleamine 2,3-dioxygenase (IDO), which can lead to tryptophan depletion and the production of tryptophan metabolites, promoting apoptosis of Th1 cells and inducing the differentiation and maturation of Th2 cells (Figure 1).57 Additionally, MSCs inhibit the expression of pro-inflammatory factors, such as TNF-α, IFN-γ, and IL-17, by Th1 cells and enhance the expression of anti-inflammatory factors, such as IL-10 and TGF-β, by Th2 cells. They also promote the differentiation of Treg cells in CD4+T cells and inhibit the differentiation of Th1 and Th17 cells, thereby exerting an immunomodulatory role.58,59 Moreover, MSCs can inhibit the signal transducer and activator of transcription 3 (STAT-3) pathway, reducing IL-17 expression and inhibiting the differentiation of T lymphocytes to Th17 cells while promoting differentiation to Treg cells by regulating the STAT3/STAT5 signaling pathway.60,61
Figure 1.
MSCs regulate the function of all immune cells in the intestinal tract.
Notes: MSCs inhibit inflammatory M1 macrophages and promote their transformation to M2 phenotype. MSCs inhibit the maturation of DC and the secretion of tumor necrosis factor-α, interferon-γ and IL-12 by DC. In addition, MSC can secrete PEG2, IL-10, TGF-β, inducible, IDO and IL-6 to inhibit the proliferation of T cells and NK cells, thus reduce the production of inflammatory cytokines in Th1 and Th17, and inhibit immune response and inflammation.
Abbreviations: IDO, indoleamine 2,3-dioxygenase; DCs, dendritic cells; MSCs, Mesenchymal stem cells.
Macrophages
Inflammation-stimulated MSCs have been shown to secrete prostaglandin E2 (PGE2) and tumor necrosis factor α stimulated gene-6 (TSG-6).62,63 TSG-6 has been found to inhibit neutrophil migration, signal transduction of tissue-resident immune cells, and polarize macrophages into the M2 subtype (Figure 1). PGE2 can also bind to macrophage receptors, leading to a shift from the M1 subtype to the M2 subtype and inhibiting inflammation.64
DCs
Research has indicated that MSCs can down-regulate the expression of CD80, CD86, and IL-12 in DCs, thereby inhibiting DC maturation and activation. In addition, MSCs can inhibit the migration of mature DCs and weaken the antigen presentation ability of DCs.65 Moreover, MSCs have the capacity to induce DCs to differentiate into tolerogenic dendritic cells (tDCs), which can effectively reduce inflammation by inhibiting effector T cells and promoting the activation of Treg cells (Figure 1).66
Repair of Intestinal Mucosal Barrier
Studies have demonstrated that MSCs repair the intestinal mucosal barrier mainly by secreting cytokines or vesicles. Firstly, MSCs regulate the growth microenvironment of ISCs and promote their differentiation into IECs by secreting certain factors.67 Secondly, MSCs secrete factors that promote the repair of IECs and reduce intestinal wall permeability.68 Additionally, vesicles secreted by MSCs have been shown to inhibit apoptosis of IECs by reducing the cleavage of caspase-3, caspase-8, and caspase-9 in animal models of IBD.67 Furthermore, MSCs can promote pro-angiogenesis through secretion by promoting lymphatic endothelial cell (LEC) proliferation, migration, and lymphangiogenesis. MSCs can also reduce oxidative stress damage in tissue cells by inhibiting the production of endogenous toxins, such as oxygen radicals.69–71
While MSCs can differentiate into IECs and myofibroblasts when homing to the intestinal mucosa, this method of repair is rare and not the primary repair mechanism.72,73 Local injection of MSC preparations has been found to significantly increase the homing rate in IBD lesions and produce good local effects, which is the basis for the clinical application of local injection in the treatment of perianal fistula Crohn’s disease (CD).12,19
Improving Intestinal Microecology
The intestinal microbiota and host cells are interdependent and together constitute the intestinal microecology.74 Their interaction results in a state of dynamic equilibrium, which is referred to as intestinal microecological homeostasis. It has become a consensus that the disorder of intestinal microecology is the primary pathogenesis of IBD. The maintenance of intestinal microecological homeostasis is heavily reliant on intestinal immunity and mucosal barrier function. MSCs have an indirect impact on improving intestinal microecology through their effects on intestinal immunity and mucosal barrier function.75 Furthermore, MSCs secrete various antimicrobial peptides, such as IL-10, PGE2, IDO, and IL-17, which have demonstrated antibacterial activity in multiple studies.76–78
Promoting Angiogenesis
Microvascular dysfunction and damage to the endothelial barrier occur in IBD, which can impact colon tissue perfusion and healing.79 MSCs secrete various angiogenic factors, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), and angiopoietin-1, which promote EC proliferation and the formation of new blood vessels.80,81 Furthermore, MSCs can differentiate into vascular ECs and promote angiogenesis.82 A study has also shown that MSCs can successfully differentiate into colon vascular ECs and promote angiogenesis.82
Anti Fibrosis
Intestinal fibrosis is a common complication of IBD that often leads to intestinal stenosis and obstruction in patients with CD.83 Research has identified the TGF-β signaling pathway as the core mechanism driving intestinal fibrosis.84 BM-MSCs have been shown to reverse the epithelial-mesenchymal transition (EMT) of TGF-β1-treated IEC-6 cells by carrying miR-200b, resulting in a significant reversal of intestinal fibrosis.85,86 Furthermore, studies on placental MSCs have revealed that inhibiting Rho/MRTF/SRF expression can downregulate TGF-β1-induced fibroblast activation, reduce collagen deposition in the intestinal wall, and thus inhibit intestinal fibrosis.87,88
SCS in Colorectal Cancer Cryotherapy
While the relationship between SCs and intestinal tumors is complex and controversial, many studies have shown that MSCs have certain anti-tumor properties.89,90 For example, MSCs can migrate to the intestine after intraperitoneal injection and prevent the occurrence of colitis-related colorectal cancer by inhibiting tumor cell proliferation and inducing tumor cell apoptosis.91,92 Additionally, some studies suggest that MSCs can modify the intestinal microbiome by increasing the number of anti-tumor bacteria, such as Parabacteroides and Staphylococcus, thus preventing tumor formation.93,94
Therapeutic Effects of SCs in IBD
Numerous studies have explored the potential of SCs in treating IBD. To conduct a thorough review, we searched the PubMed and Web of Science Library databases using keywords, such as “stem cell therapy”, “mesenchymal stromal cells”, “HSCs”, “immunosuppression”, “inflammatory bowel disease”, “ulcerative colitis”, “Crohn’s disease”, and “perianal fistula CD”. We also searched the NIH clinical trial database (https://ClinicalTrials.gov/) for clinical trials of MSCs in the treatment of IBD. So far, 34 clinical trials involving MSCs have been registered and implemented for treating IBD.12,95 From these studies, we carefully selected representative, complete, and high-quality studies and classified them into preclinical studies, completed clinical studies, and ongoing clinical studies. We summarized the operational process and research results, evaluated the feasibility, effectiveness, and safety, and objectively reflected on the research status.
Preclinical Use of SCs for IBD Treatment
Preclinical research has been conducted on the application of various types of SCs, including HSCs, MSCs, ISCs, iPSCs, and pESCs, for treating IBD. These studies have evaluated the efficacy, feasibility, and safety of SC therapy in animal models of IBD and have gradually revealed the internal mechanisms underlying the therapy. These findings have shown us the potential application prospects and limitations of SC therapy for IBD.16,96 However, further exploration and improvement of strategies are still necessary to lay the foundation for clinical research in this field.
HSCs
HSCT has been widely used clinically for the treatment of hematologic diseases. However, due to its complexity, there have been few preclinical studies conducted on HSCT, and almost no studies have been conducted on animal models of IBD.25
MSCs
A large number of preclinical studies have been conducted at the cellular level and on animal models of IBD regarding MSCs.19 Most of these studies have confirmed the efficacy of MSCs in treating IBD, with desirable feasibility and safety.15 Therefore, MSCs have become the primary choice for SC therapy for IBD. In several studies, MSCs from human umbilical cord blood, bone marrow, and adipose tissue are used to treat IBD animal models, achieving the goal of promoting symptom relief and pathological tissue repair.97–99
Qu et al have infused donor rats with BM-MSCs labeled with the fluorescent dye PKH26 into rats that have suffered indomethacin-induced GI tract injury. PKH26-positive BM-MSCs are subsequently found in the damaged mucosa, and the mucosal layer and crypt layer of the small intestine are thicker in BM-MSC-treated rats. This suggests that the therapeutic effect of MSCs is related to the regenerative repair of damaged tissues.100
Lian et al have established a mouse model of intestinal fibrosis by increasing the dose of TNBS by enema for 7 weeks, followed by introducing MSCs by enema for treatment. The results show that MSCs inhibit the expression of TGF-β and phosphorylation of Smad2 and Smad3 after TNBS induction, thereby inhibiting EMT and reversing intestinal fibrosis.85 Table 2 summarizes the preclinical studies on MSCs for the treatment of IBD completed in recent years.
Table 2.
Summarizes the Preclinical Studies on MSCs for the Treatment of IBD Completed in Recent Years
| Author/Time References | Animal Model | SCs Sources | SCs Route | SCs Dose (Cells) | Follow-Up Period | Primary Outcomes |
|---|---|---|---|---|---|---|
| Barnhoorn (2020)145 | DSS-induced colitis | BM-MSC | IP | 2 × 106 cells | 11 days | MSCs after in vivo aggregation show a favorable RNA expression profile for the treatment of colitis. MSCs spheroids showed high expression of Ki-67 and low levels of apoptotic marker cleaved caspase-3. Locally applied MSCs and MSCs spheroids are both able to ameliorate DSS-induced colitis and show similar clinical effects. |
| Barnhoorn (2020)145 | DSS-induced colitis | BM-MSC | Endoscopic | 2 × 106 cells | 4–6 days | Endoscopic injection can be a feasible and effective novel application route for MSCs therapy in patients with luminal IBD. |
| Chao (2016)146 | TNBS-induced colitis | UCBSC | IP | 10×106 cells | 14 days | The mortality in UCBSC-treated TNBS mice was 20% (55% in colitis model). IL-20 and TGF-Beta were significantly higher in UCBSC-treated mice (p = 0.04 and 0.02 respectively). |
| Cheng (2017)147 | DSS-induced colitis | BM-MSC | IV | 5×106 cells | 8 days | IL-25-MSCs treatment significantly attenuate the colon shortening (12 ± 0.62 cm); IL-25 could enhance immunomodulatory ability of MSCs via inhibiting Th17 immune response and promoting the regulation of Tregs cells. |
| de Aguiar (2018)148 | DSS-induced colitis | ASCs | IP | 10×106 cells | 7 days | ASCs-treated mice did not present severe reduction in colon length, and presented a reduced tissue damage score index (3). Significant reduction of IFN-gamma and TNF-Alpha, and reduction of IL-6 and MCP-1 protein levels. ASCs treatment reduced DCs and macrophages presence in the colon. |
| de Aguiar (2018)148 | DSS-induced colitis | ASCs | IP | 2 × 106 cells | 7 days | ASCs ameliorated the severity of DSS-induced colitis, reducing colitis pathological score and preventing colon shortening. |
| de la Portilla (2013)149 | TNBS-induced colitis | ASCs | Local | 60×106 cells | 24 weeks | First study which shows the homing migration of ASCs to areas of experimentally-induced colitis following rectal installation. |
| de la Portilla (2018)150 | TNBS-induced colitis | ASCs | Local | 2 × 106 cells | 10 days | There were no differences in component rectal wall thicknesses with a higher Hunter score in the treated group compared with the controls. |
| Fu (2017)151 | TNBS-induced colitis | ASCs | IP | 2 × 106 cells | 6 days | Decrease the weight loss and DAI score, MPO activity. Reduced levels of ROR and IL-17A; inhibited STAT3 phosphorylation, but increased STAT5 phosphorylation. |
| González-Rey (2009)152 | DSS-induced chronic colitis | ASCs | IP | 10×106 cells | 27 days | ASCs treatment protects against DSS-induced acute colitis as well as chronic severe colitis (p 0.01 and p 0.001 respectively); reduces colonic inflammatory responses in DSS-induced chronic colitis (p 0.001). |
| Gregoire (2018)153 | Fistula Crohn’s disease | ASCs | Local | (3−30) ×106 cells | 8 weeks | 6/8 fistulas healed, 2/8 improved. |
| Heidari (2021)56 | DSS-induced colitis | ASCs | IP | 10×106 cells | 34 days | There was no significant difference in the survival rate among the study groups; however, there was a significant increase in terms of the colon length (p 0.005). In the treated mice the level of mucosal damage was significantly lower (p 0.005). |
| Heidari (2021)56 | DSS-induced colitis | ASCs | IP | 2 × 106 cells | 34 days | The regulatory effects of ASCs and their CM in inflammatory conditions because of colitis. |
| In Kap (2010)137 | DSS-induced colitis | MSCs | IV | 1 × 106 cells | 7 days | Anti-addressin Ab coating on MSCs increased cell delivery to inflamed colon and increased the efficacy of MSCs treatment of IBD. |
| Jianxia Hu (2016)154 | Luminal Crohn’s disease | UCBSC | IV | 0.5×106 cells | 3 months | 30/36 patients showed good response and diffuse and deep ulcer formation and severe inflammatory mucosa were improved markedly. |
| Lee (2016)155 | DSS-induced colitis | BM-MSC | IV | 10×106 cells | 33 days | IL-10 production was upregulated by about 10-fold in BM-MSC-treated mice and showed a preventive effect on weight loss. |
| Lee (2016)155 | DSS-induced colitis | BM-MSC | IV | 30×106 cells | 33 days | Infusion of BM-MSC at the onset of disease exerted preventive and rapid recovery effects. |
| Lee (2018)156 | DSS-induced colitis | UCBSC | IP | 2×106 cells | 12 days | The survival rate was further increased by co-treatment compared to UCBSC or MIS416 single treatments; colon lengths were significantly increased in cotreatment; colonic inflammation was more effectively resolved by co-treatment with MIS416 and UCBSC, and only co-treatment markedly decreased fibrosis and enhanced tissue regeneration. |
| Legaki (2016)147 | DSS-induced colitis | ASCs | IP | 1.5×106 cells 200 µL /dose |
7 days | CM treatment significantly decreased the extension and severity of the inflammation in comparison to the DSS-treated mice; the relative expression levels of IL-10 mRNA were significantly increased, and TGFb1 was significantly higher (p 0.0001). |
| Mao (2017)157 | DSS-induced colitis | UCBSC | IV | 1.3×106 cells | 11 days | Exosomes from MSC have profound effects on alleviating DSS-induced IBD and may exert their impact through the modulation of IL-7 expression in macrophages. |
| Martín (2018)103 | TNBS-induced colitis | ASCs | Local | 10×106 cells | 11 days | Submucosal injection of human ASCs ameliorates the course of TNBS colitis in immunocompetent rats. |
| Martin Arranz (2018)158 | TNBS-induced colitis | ASCs | Endoscopic | 10× 06 cells | 11 days | The endoscopic score improved in the ASCs group by 47.1% ± 5.3% vs 21.8% ± 6.6% in the vehicle group. |
| Miyamoto (2017)159 | TNBS-induced colitis | ASCs | IV and Local | 1×106 cells IV and 400 µL Local |
7 days | ASCs transplantation significantly decreased the number of neutrophils, attenuated acute inflammation. In the TNBS-CM gel group ulcers were shallow and bleeding was not detected, therefore improved endoscopic score. In the gel group mRNA expression levels of TNF-Alpha, CXCL1, CCL2 and IL-6 were increased. |
| Molendijk (2015)160 | Fistula Crohn’s disease | BM-MSC | Local | 10,30, 90×106 cells | 6, 12, 24 weeks | At week twelve, 3 of 9 individual fistulas had healed in group 1 (33.3%), 6 of 7 had healed in group 2 (85.7%), 2 of 7 had healed in group 3 (28.6%), and 3 of 9 had healed in the placebo group (33.3%). |
| Pak (2018)161 | DSS-induced colitis | BM-MSC ASCs |
Endoscopic | 8×105, 1.1×106 cells | 1–3 days | The success rate was 37.60% for ASCs group and 35.20% for BM-MSC group. |
| Panés (2016)109 | Fistula Crohn’s disease | ASCs | Local | 120×106 cells | 24 weeks | Remission in the ITT (53 of 107 [50%] vs 36 of 105 [34%]; difference 15.2%, 97.5% CI 0.2–30.3; p = 0.024) C × 601 vs placebo. |
| Panés (2018)157 | Fistula Crohn’s disease | ASCs | Local | 120×106 cells | 52 weeks | C × 601 achieved combined remission (56.3%) vs controls (38.6%). |
| Park (2018)109 | DSS-induced colitis | ASCs | IP | 10×106 cells | 20 days | The results suggest that PGE2, produced by co-culture of ASCs and THP-1, reduces M1 population, decreased the frequency of macrophage transition. |
| Park (2018)162 | DSS-induced colitis | ASCs | IP | 2 × 106 cells | 20 days | ASCs can suppress the inflammatory response by controlling the macrophage population, and ASCs may be therapeutically useful for the treatment of IBD. |
| Pouya (2018)163 | DSS-induced colitis | MSCs | IP | 500 µL, ×3 | 10 days | After infusion, colon inflammation was reduced and histopathological analysis showed a decrease in mucosal degeneration. |
| Song (2017)164 | DSS-induced colitis | MSC-Exo UCBSC |
IP | 150µg 10×106 cells; |
36 days | MSC-Exo ameliorates the clinical parameters in DSS-induced colitis; the treated group showed significantly less MPO activity. The level of IL-17 was significantly decreased, whereas those of IL-10 and TGF-Beta1 were increased. |
| Song (2018)165 | DSS-induced colitis | Canine ASCs | IP | 2×106 + TSG-6 siRNA | 10 days | ASCs-secreted TSG-6 reduced inflammatory response and apoptosis in the colon; intraperitoneally infused ASCs did not migrate to the inflamed colon; increased M2 macrophages in the inflamed colon. |
| Soontararak (2018)48 | DSS-induced colitis | iMSCs ASCs |
IV | 3 × 106 cells | 19 days | Colonic tissues from mice treated with either iMSCs or ASCs exhibited an overall reduction in transmural inflammation, with significantly less infiltration of inflammatory. Cells in the lamina propria, diminished mucosal ulceration and decreased mucosal collapse and granulation tissue formation. |
| Tanaka (2008)166 | DSS-induced colitis | BM-MSC | IV | 5 × 106 cells | 7 days | In the rectum of treated rats the mRNA expression of TNF-alpha and IL-1Beta was markedly decreased to (43.7 ± 25.5% p 0.05 and 14.5 ± 12% p 0.01 respectively), as well as COX-2 16.5 ± 15.2% (p 0.01). |
| Tanaka (2008)166 | DSS-induced colitis | MSCs | IV | 5 × 106 cells | 7 days | Exogenous MSCs accumulated in inflamed tissues and ameliorated DSS-induced colitis via a local anti-inflammatory action. |
| Wang (2016)130 | DSS-induced colitis | BM-MSC | IP | 0.5×106 cells | 10 days | Intraperitoneal injection is the best delivery way for MSCs: showed better mucosa recovery and higher cell engraftment at inflamed colon. |
| Wu (2018)167 | DSS-induced colitis | UCBSC | IV | 400µg UC-MSC | 11 days | Exosomes from UCBSCs have profound effects on alleviating DSS-induced IBD and may exert their function by regulating the ubiquitin modification level. |
| Xu (2018)168 | DSS-induced colitis | ERC | IV | 3 × 106 cells | 10 days | ERC treatment significantly reduced the levels of TNF-Alpha, IL-1Beta and IL-6; ERC downregulated the expanded Th1 and Th17 cells in colitis, and elevated the proportion of Tregs in lymphocytes; ERC inhibited B-cell activation, differentiation and IgG production in colitis. |
| Yu (2017)169 | DSS-induced colitis | T-MSCs | IP | 20,40, × 106 cells | 30 days | Co-culture with T-MSCs clearly inhibited the PMA-stimulated proliferation of splenocytes by 60%; T-MSCs [×4] treated mice’s survival rate was improved to that of the normal. T-MSCs [×2] injection also significantly improved the survival rate to 89% of the control. T-MSCs [×4] treatment inhibits DSS-induced colon shortening. |
Abbreviations: AF-MSC, Amniotic fluid Mesenchymal stem cell; ASCs, adipose mesenchymal stem cells; BM-MSCs, Bone marrow mesenchymal stem cells; CCL2, Chemokine Ligand 2; CD, Crohn’s disease; CXCL1, Chemokine Ligand 1; DAI, Disease activity index; DCs, Dendritic cells; DSS, Dextran Sulfate Sodium Salt; ERC, Endometrial regenerative cells; IBD, Inflammatory bowel disease; IFN-γ, interferon-γ; IL-10, interleukin-10; IL-17A, interleukin-17A; IL-1β, interleukin-1β; IL-20, interleukin-20; IL-25, interleukin-25; IL-6, interleukin-6; IL-7, interleukin-7; iMSCs, mesenchymal stem cells derived from induced pluripotent stem cells; IP, intraperitoneal injection; iPSCs, induced pluripotent stem cells; IV, intravenous injection; MCP-1, Monocyte Chemotactic Protein-1; MPO, myeloperoxidase; MSC-Exo, Mesenchymal stem cell exosomes; MSCs, mesenchymal stem cells; MSCT, mesenchymal stem cells transplantation; PFCD, perianal fistula Crohn’s disease; PGE2, prostaglandin E2; PMA, THP-1 nuclear extract lysate; ROR, Retinoic acid related orphan receptor; SCs, stem cells; STAT3, signal transducer and activator of translation-3; TGF-β, transforming growth factor-β; THP-1, Tohoku Hospital Pediatrics-1; T-MSCs, Tonsil MSCs; TNBS, 2,4,6-trinitro-Benzenesulfonic acid; TNF-alpha, tumor necrosis factor alpha; TSG-6, tumor necrosis factor α stimulated gene-6; UCBSCs, Umbilical Cord Blood stem cells.
ISCs
ISCs have been identified as a promising therapy for SCs, with their feasibility being affirmed through breakthrough technology. The efficacy of ISCs in treating IBD has also been preliminarily confirmed in preclinical studies. In recent years, the establishment of an in vitro ISC culture system and advancements in 3D culture technology have brought about promising prospects for patients with IBD or other refractory GI diseases.101
Fukuda et al are the first to isolate intestinal crypts (containing ISCs) from EGFP transgenic mice and form colonic-like organs after 1 week of 3D culture. The culture medium containing colonic organoids is subsequently injected into an IBD mouse model induced by ethylene dinitril tetraacetic acid (EDTA) enema. One day after the injection, EGFP cells are found to be scattered on the surface of the recipient’s colon, partially retaining the organoid structure. Two weeks later, EGFP cells are found to display a complex structure with some downward invagination to form a crypt-like structure. At week 4, the EGFP epithelium is stably sunk into the recipient’s colon. This demonstrates that in vitro cultured mouse intestinal epithelial-like organs are able to reconstruct new epithelium in the recipient’s colon.102
Shaker et al have cultured single SCs from the colon of mice to produce tiny organs of intestinal epithelial tissue, which are then transplanted into the damaged intestine of mice through an enema. The tiny organs attach to the damaged area and repair the mucosal damage. After 25 weeks of tracking, the tiny organs still show self-renewal ability, and the weight of the treated mice increases significantly.103
Recently, Watanabe et al have formed “intestinal organoids” from human colon fragments and mouse colon segments by 3D in vitro culture, respectively. They are injected into the intestine of a DSS-induced IBD model mouse with a thin tube, and the anus of the mouse is subsequently sutured. The transplanted intestinal organoid is confirmed to be attached to the damaged area of the mouse intestine by fluorescence detection, preserving the intestinal epithelial structure. Subsequently, the attached intestinal organoid is found to be able to expand in the mucosa of the mouse intestinal colon and reconstruct new intestinal epithelial tissue. Finally, as observed from the mouse colon specimens and tissue examination, the transplantation of intestinal-like organs significantly reduces the inflammatory response in the colon, and the intestinal damage is repaired.104
iPSCs
Preclinical research on iPSCs has yielded promising results. In a mouse model of IBD, iMSCs are found to be as effective as adipose MSCs (ASCs), promoting intestinal angiogenesis and stimulating the proliferation of IECs and Lgr5+ ISCs.48 In another study, iPSCs are induced from mouse skin fibroblasts and injected into mice with DSS-induced colitis. The mice that receive the iPSCs demonstrate a significant improvement in clinical symptom scores after just 1 day. After 72 hours, the colon specimens from these mice are significantly longer and heavier, with reduced transmural inflammation, mucosal ulceration, and infiltration of inflammatory cells in the intestinal mucosa.48
Spence et al have successfully generated definitive endoderm (DE) from human iPSCs by inducing them with Activin A, a nodal-related TGF-β molecule. They then transfer the cells to a culture environment containing Wnt3a and FGF4 to create an “intestinal organoid” with a crypt structure of the intestinal epithelium containing various types of IECs and intestinal endocrine cells.49
Completed Clinical Studies of SCs for IBD Treatment
Clinical research on IBD has primarily focused on MSCs and HSCs, with little attention given to ISCs and iPSCs.
HSCT
The safety of HSCT for treating IBD has faced significant challenges due to its complex operation and many adverse reactions. As a result, HSCT is gradually being replaced by MSCT. While most completed clinical studies have achieved certain efficacy, some studies have shown no response to IBD treatment and have faced severe adverse reactions.26 Burt et al have reported on 24 patients with refractory CD who are treated with autologous nonmyeloablative HSCT and immunosuppression using cyclophosphamide (CTX) and horse anti-thymocyte globulin (ATG), with no transplant-related deaths. The recurrence-free survival rates are 91%, 63%, 57%, 39%, and 19% at 1–5 years after transplantation, respectively. Nine patients have survived for more than 5 years without recurrence.105 Cassinotti et al have performed autologous HSCT on four patients with refractory CD. In the third month, the primary endpoint of clinical remission is achieved in all patients. After a median follow-up of 16.5 months, all affected patients achieved complete fistula closure, and three out of four patients maintained both clinical and endoscopic remission even after the withdrawal of all drugs.106 The ASTIC study (autologous stem cell international Crohn’s disease trial) is a multicenter, randomized, Phase III intervention study. Twenty-three patients with refractory CD underwent autologous HSCT, while 22 received standard CD treatment (controls). The results show that only two out of 23 patients in the SC therapy group achieved complete clinical and endoscopic remission, whereas only one out of 22 patients in the control group achieved clinical and endoscopic remission, with no statistical difference. The study has also found that all patients who received SC therapy experienced different degrees of complications, and infection is the most common.107 However, when the evaluation criteria are lowered, the proportion of patients receiving HSCT treatment who stopped using immunosuppressive drugs is significantly higher than that of the control group, and the Crohn’s disease activity index (CDAI) is also significantly lower than that of the control group. The quality of life and the remission of intestinal lesions under endoscopy are better than those of the control group, indicating that HSCT treatment still has a positive effect on these patients.107
MSCs
Clinical studies on MSCs have primarily focused on patients with refractory perianal fistulous CD and refractory intestinal luminal IBD. MSCs can be sourced from various tissues, such as bone marrow, umbilical cord, and adipose tissue. Adipose tissue is a readily available and easy-to-culture source of MSCs that is increasingly used in clinical applications, and it now accounts for one-third of all MSC sources. The differentiation and secretory functions of MSCs are influenced by their growth environment, and by utilizing culture pretreatment and content modification, MSCs with specific functions can be generated. However, due to the heterogeneity of MSCs, there is still a lack of uniform research standards, leading to mixed results in clinical trials. The clinical applications of MSCs in IBD are broadly divided into two categories: local application in CD and intractable intraluminal IBD.29
Box 1 MSCs for Local Application of CD
Local application of MSCs has been found to be effective in treating refractory perianal fistula in Crohn’s disease (PFCD). The first clinical preparation of MSCs for PFCD, darvadstrocel/Cx601 (Takeda) (allogeneic adipose-derived stem cells), has already entered clinical treatment.108 More optimized MSC preparations and derivatives are currently in clinical trial stages. In a review of 32 Phase I–III clinical trials of HSCT for CD complicated by anal fistula, it is found that more than half of the patients achieved complete remission, at least 2/3 of the patients responded to treatment, and no serious adverse effects were reported.19 In a study conducted by Panés J, 212 patients with refractory, complex, and active perianal fistulas are included. Of these patients, 107 received allogeneic, expanded, and induced SCs (Cx601) treatment, while the remaining 105 were in the placebo group and received routine treatment. The intention-to-treat (ITT) analysis reveals that 53 individuals in the Cx601 group (50%) and 36 in the placebo group (34%) achieved combined remission. Among the patients who received Cx601 treatment, 18 (17%) of 103 experienced treatment-related adverse events compared to 30 (29%) of 103 in the placebo group. This study concludes that Cx601 is an effective and safe treatment for patients with refractory, complex, and active perianal fistulas.109 Another study has included 40 patients with PFCD. Of these, 25 patients are in the darvadstrocel treatment group (an expanded allogeneic adipose-derived stem cells), while the remaining 15 are in the control group. Thirty-seven patients completed the 104-week follow-up, and seven treatment-emergent serious adverse events were reported. At week 104, clinical remission is reported in 14/25 (56%) patients in the darvadstrocel group and 6/15 (40%) patients in the control group.110
Box 2 MSCs for Intractable Intraluminal IBD
For refractory intraluminal IBD, MSCs can be administered intravenously or intraperitoneally. This approach has shown promise in achieving relief of clinical symptoms and improvement of mucosal lesions while also being highly safe and cost-effective.15,19
In a recent randomized controlled trial (RCT), 82 patients with refractory intracavitary IBD were included, and 41 patients were randomly assigned to receive a total of four peripheral intravenous infusions of 1×106 UC MSCs/kg once a week. After 12 months of treatment, the CDAI decreased by 62.5±23.2 in the UC-MSC group compared to 23.6±12.4 in the control group. Additionally, the Harvey-Bradshaw index (HBI) decreased by 3.4±1.2 in the UC-MSC group and by 1.2±0.58 in the control group. The corticosteroid dosage also decreased by 4.2±0.84 mg/day in the UC-MSC group and by 1.2±0.35 mg/day in the control group. These results suggest that UC-MSCs are effective in treating refractory intracavitary IBD.111
In a Phase II clinical study, 16 patients with intractable intraluminal CD and a CDAI score >250 were included. Subjects received intravenous infusions of allogeneic MSCs (2 × 106 cells/kg body weight) weekly for 4 weeks. At day 42, among the 15 patients who completed the study, the mean CDAI score decreased from 370 to 203. Twelve patients had a clinical response, and seven patients had endoscopic improvement (47%) with a decrease in mean CD endoscopic index of severity (CDEIS) scores from 21.5 to 11.0.112
Knyazev et al have included 34 patients with refractory intracavitary CD, all of whom were treated with intravenous infusion of allogeneic BM-MSC preparations. Some patients (n=19) received azathioprine (AZA) combination therapy. The CDAI assessed before and 2, 6, and 12 months after transplantation was 337.6±17.1, 118.9±12.4, 110.3±11.1, and 99.9±10.8, respectively, with a significant decrease in CDAI after transplantation and comparable CDAI scores in both groups. Blood immunoassays show that the levels of pro-inflammatory factors, such as INF-γ, TNF-α, and IL-12-β, are significantly decreased and more pronounced in the combined ASA group.113 Table 3 summarizes the clinical studies of MSCs in treating IBD completed in recent years.
Table 3.
Summarizes the Clinical Studies of MSCs in Treating IBD Completed in Recent Years
| Author, Time and Country | Phase | Clinical Indication | Number | SC Sources Route | Adjuvant Therapy | Follow-Up Period | Primary Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|
| Oyama Y. 2005, USA | I | Refractory CD | 12 | Auto-HSCs IV | CTX and equine ATG | 7–37 months | 11 patients entered a sustained remission, only 1 patient has developed a recurrence of active CD, which occurred 15 months after HSCT. | [170] |
| Burt R. K. 2010, USA | I | Severe CD | 24 | Auto-HSCs IV | CTX and equine ATG | 1–5 years | Eighteen of 24 patients are 5 or more years after transplantation. | [105] |
| Clerici M. 2011, Italy | N/A | Active moderate-severe CD | 7 | Auto-HSCs IV | CTX and rabbit ATG | 1 year | Most of whom showed clinical and endoscopic complete remission and were maintained for one year. | [171] |
| Hasselblatt P. 2012, Germany | I/II | Refractory CD | 12 | Auto-PBSCs IV | High-dose CTX | 0.5–10.3 years | 5 patients achieved a clinical and endoscopic remission within 6 months after auto-PBSCT. 7/9 relapses patients reduced maintenance dose of conventional drugs. |
[172] |
| Hawkey C J. 2015, United Kingdom | III | Refractory CD | 45 | Auto-HSCs IV | High-dose CTX | 1 year | Compared with conventional therapy, did not result in a statistically significant improvement in sustained disease remission at 1 year. | [107] |
| Kotlarz D. 2012, Germany | II | IBD with IL-10 gene defect | 66 | Allo-HSCs IV | Lemtrada F-ara-A treosulfan | 2 years | Allo-HSCT was performed in 5 patients to induce sustained clinical remission. | [173] |
| Liang J. 2012, China | N/A | Refractory IBD 4 CD, 3 UC | 7 | Allo-MSCs IV | Steroids and/or immunosuppressants | Mean 19 months | Diarrhea frequency and abdominal pain/cramps gradually improved in all the seven patients, and the index score decreases. | [174] |
| Forbes G. M. 2013 Australia | II | Luminal CD | 16 | Allo-MSCs Local | Corticosteroid, AZA | 42 days | In a phase 2 study, administration of allogeneic MSCs reduced CDAI and CDEIS scores in patients with refractory luminal CD. | [112] |
| GarciaOlmo D. 2003, Spain | II | RFCD | 1 | ASCs Local | Olsalazine | 3 months | Since the surgical procedure 3 month ago the patient has not experienced vaginal flatus or fecal incontinence through her vagina. | [175] |
| GarciaOlmo D. 2009, Spain | II | Complex PFCD | 24 | ASCs Local | Fibrin glue | 1 year | Combination therapy appears to achieve higher rates of healing than fibrin glue alone. | [176] |
| Guadalajara H. 2011, Spain | II | PFCD | 49 | ASCs Local | Fibrin glue | 3 years | A low proportion of the stem cell-treated patients with closure after the procedure. Remained free of recurrence after more than 3 years of follow-up. | [177] |
| Herreros M. D. 2012, Spain | III | Complex Anal fistula | 200 | ASCs Local | Fibrin glue | 1 year | Achieving healing rates of approximately 40% at 6 months and of more than 50% at 1-year follow-up. | [178] |
| Dietz A. B. 2017 USA | I | Perianal CD | 12 | Auto-ASCs. Local | Nothing | <6 month | 10/12 (83%) achieved combined remission at 24 weeks by fistula exam and MRI. | [179] |
| Dige A. 2019 Denmark | I | Perianal CD | 21 | Auto-ASCs. Repeatable Local |
Nothing | <6 month | 12/21 (57%) achieved clinical remission 6 months after last injection. 8/9 patient who underwent MRI had complete resolution at 6 months. |
[180] |
| Lightner A. L. 2020 USA | I | Rectovaginal CD | 5 | Auto-ASCs. Local |
Nothing | <6 month | 3/5 (60%) with complete clinical response. 2/5 (40%) with partial clinical response. 0/5 patients with radiographic remission |
[181] |
| Nikolic M. 2021 Austria | I | Rectovaginal CD | 4 | Allo-ASCs. Local | Nothing | <6 month | Fistula closure and absence of drainage on exam at 6 months. 1/4 (25%) achieved clinical healing at 6 months. | [182] |
| Cho Y. B. 2015, Korea | II | Perianal CD | 41 | Auto-ASCs. Local | Nothing | >6 month | At 24 months, complete healing was observed in 21/26 (80.8%) patients in mPP group and 27/36 (75%) in mITT group. | [183] |
| Ciccocioppo R. 2015, Italy | I | Perianal CD | 10 | Auto-BM-MSCs. Local | Nothing | >6 month | Fistula relapse-free survival was 88% at 1 year, 50% at 2 years, and remaining at 37% for the remainder of the six-year follow-up. Mean CDAI score decreased from 300 to 150 at 6 years. |
[184] |
| Garcia-Olmo D. 2015, Spain | I | CD (3 with Perianal CD) | 10 | Auto-ASCs. Local / IV. | Nothing | >6 month | 2/3 (66%) of CD patients achieved complete healing (re-epithelialization and absence of suppuration at 1 year) at 1 year. | [185] |
| Park K. J. 2016, Korea | I | Perianal CD | 6 | Auto-ASCs. Local | Nothing | >6 month | 2/3 (66.7%) in group 1 and 1/3 (33.3%) in group 2 achieved clinical healing at 8 months. | [186] |
| García-Arranz M. 2016, Spain | I/II | Rectovaginal CD | 10 | Auto-ASCs. Local | Nothing | >6 month | Of 5 total patients who completed study, 3 achieved clinical healing (60%) (re-epithelialization of both vaginal and rectal sides and absence of drainage) at 52 weeks. | [187] |
| Panés J. 2018 Austria | III | Perianal CD | 212 | Auto-ASCs (107 Local) Placebo (105) |
Nothing | >6 month | Using mITT, 58/103 (56.3%) of patients in treatment arm achieved combined remission (absence of external openings on exam and absence of collections > 2 cm on MRI) vs 39/101 (38.6%) in control arm at week 52. | [157] |
| Wainstein C. 2018, Chile | I | Perianal CD | 9 | Auto-ASCS Local | Nothing | >6 month | 8/9 (88.9%) patients with complete healing (absence of suppuration from the external fistula opening and complete epithelialization), 1/9 (11.1%) patients with partial healing at median follow-up of 31 months. | [188] |
| Knyazev O. V. 2018 Russia | II | Perianal CD | 36 | Auto-BM-MSCs Local | Nothing | >6 month | At 3 and 6 months, healing of 66.6% (8/12) in group 1, 60% (6/10) in group 2, and 7.14% (1/14) in group 3. At 1-year, healing of 58.3% (7/12) in group 1, 60% (6/10) in group 2, and 14.3% (2/14) in group 3. |
[189] |
| Herreros M. D. 2019, Spain | I/II | CD (18 with perianal CD) | 45 | Auto-ASCs allo-ASCs Local / IV |
Nothing | >6 month | 55.5% of CD patients achieved healing (absence of suppuration). CD patients receiving SVF, auto-ASCs and allo-ASCs were cured by 40%, 66.6% and 55.5% respectively. |
[190] |
| Barnhoorn M. C. 2020, The Netherlands | I | Perianal CD | 21 | Auto-BM-MSCs Local | Nothing | >6 month | Group 1: 3/4 (75%) healing at 4 years. Group 2: 4/4 (100%) healing at 4 years. Group 3: 2/5 (20%) healing at 4 years. Group 4: 0/3 (0%) healing at 4 years. |
[145] |
| Zhou C. 2020, China | II | Perianal CD | 22 | Auto-ASCs Local | Nothing | >6 month | 3 months: 10/11 (91%) in treatment arm vs 5/11 (45.5%) in placebo arm. 6 months: 8/11 (72.7%) in treatment arm vs 6/11 (54.5%) in placebo arm. 12 months: 7/11 (63.6%) in treatment arm vs 6/11 (54.5%) in placebo arm. |
[191] |
| Laureti, S. 2020, Italy | II | Perianal CD | 15 | ASCs Lipogems® Local | Nothing | >6 month | 10/15 (66.7%) with combined remission (closure of all external openings on exam and absence of collections > 3mm on MRI) at 24 months. | [192] |
| Melmed G. Y. 2015, Italy | Ib/IIa | Luminal CD | 50 | PDA-001 1U, 4U IV |
Nothing | >6 month | Decrease in CDAI by ≥100 points and/or 25% from baseline at weeks 4 and 6. Phase I b: primary efficacy not reported Phase IIb: Placebo: 0/16 1U PDA-001: 5/15 (33%) 4U PDA-001: 5/13 (38.5%) |
[193] |
| Dhere T. 2016, USA | I | Luminal CD | 12 | Auto- BM-MSCs IV | N/A | >6 month | 5/11 (45.4%) achieved clinical response (decrease in CDAI by ≥ 100 points at 2 weeks). | [194] |
| Hu J. 2016, China | I/II | With UC | 70 | UCBSCs IV | N/A | >6 month | 29/34 (85.3%) with clinical response (decrease in total Mayo UC activity score of ≥3 and ≥30% from baseline) in group I vs 6/36 (16.7%) at 3 months. | [154] |
| Knyazev O. V. 2016 Russia | I | With UC | 22 | Auto-BM-MSCs IV | AZA | >6 month | Remission rate of 50% (6/12) in treatment group vs 10% (1/10) in control group at 3 years. Remission duration of 22 months in treatment group vs 20 months in control group at 3 years. |
[195] |
| Gregoire C. 2018 Belgium | I/II | Luminal CD | 13 | Auto-BM-MSCs IV. | N/A | >6 month | 2/13 (15.4%) with clinical response (decrease in CDAI by ≥100 points) at 8 weeks. | [153] |
| Zhang J. 2018, China | I | Luminal CD | 82 | UCBSCs IV. | Corticosteroid | >6 month | Decrease in CDAI, HBI, and corticosteroid usage. | [111] |
| Knyazev O V. 2018 Russia | I/II | Luminal CD | 34 | BM-MSCs IV. | AZA | >6 month | Clinical remission (CDAI < 150) at 12 months. | [113] |
Abbreviations: Allo, Allogeneic; ASCs, adipose mesenchymal stem cells; Auto, Autogenous; BM-MSCs, Bone marrow mesenchymal stem cells; CD, Crohn’s disease; CDAI, Crohn’s disease activity index; CDEIS, Crohn’s Disease Endoscopic Index of Severity; HBI, Harvey-Bradshaw index; HSCT, hematopoietic stem cell transplantation; IBD, Inflammatory bowel disease; IL-10, interleukin-10; IP, intraperitoneal injection; IV, intravenous injection; mPP, Massively Parallel Processing; MRI, Magnetic resonance imaging; MSCs, mesenchymal stem cells; MSCT, mesenchymal stem cells transplantation; PBSCT, Peripheral blood stem cell transplantation; PDA-001, Human placenta-derived cells; PFCD, perianal fistula Crohn’s disease; RFCD, rectovaginal fistula CD; SCs, stem cells; SVF, Stromal Vascular Fraction; UC, Ulcerative colitis; UCBSCs, Umbilical Cord Blood stem cells; CTX, Cyclophosphamide; ATG, antithymocyte globulin; F-ara-A, fludarabine; AZA, azathioprine; N/A, Not provided.
Ongoing Clinical Studies of SCs for IBD Treatment
While there have been some completed clinical trials investigating the use of MSCs in the treatment of IBD, there are a number of limitations to these studies. For instance, there are few double-blind RCTs with relatively small sample sizes, and the standards used across these trials have not been uniform, which makes it difficult to draw clear and convincing conclusions. Although local injection of MSCs has shown promise in treating PFCD, intravenous injection of MSCs for refractory intestinal IBD has not yet produced the desired effect, and the efficacy of some trials remains uncertain.19 In order to address these limitations, ongoing and future clinical trials should focus on improving treatment strategies and standardizing protocols. Key technologies, such as optimizing the extraction and culture process of SCs, will help to ensure the best possible SC preparation.114 It is important to clearly define the indications for SC therapy, determine the optimal route and dosage, and gradually expand the use of SC therapy for treating IBD. It is hoped that ongoing clinical trials investigating MSCs will provide clearer answers. The ongoing clinical trials investigating MSCs for IBD are summarized in Table 4.
Table 4.
The Ongoing Clinical Trials Investigating MSCs for IBD
| NCT Number | Sponsor Country | Phase | Clinical Indication | SC Sources | MSC Dose (Cells) | Administration Route | Status |
|---|---|---|---|---|---|---|---|
| 03299413 | Jordan | I/II | IBD | W. Jelly MSCs | 1.2×109 cells | iv. | Active |
| 03115749 | Montpellier | N/A | IBD | Intestinal MSCs | N/A | N/A | No recruiting |
| 01914887 | Spain | I/II | UC | Allogeneic ASCs | 6×107 cells | Colonoscope | Recruiting |
| 01874015 | Spain | I | CD | BM-MSCs | N/A | N/A | Recruiting |
| 01157650 | USA | I/ II | CD | Autologous MSCs | N/A | N/A | Completed |
| 00294112 | USA | II | CD | Adult human MSCs | 2, 8×106 cells | iv. | Completed |
| 02677350 | USA | I | CD | BM-MSCs | 2×107 cells | iv. | Withdrawn |
| 02445547 | China | I/II | CD | UCBSCs | 1×106 cell/kg | iv. | Completed |
| 00543374 | USA | III | CD | PROCHYMAL® | 0.6–1.2×109 cells | iv. | Completed |
| 00482092 | USA | III | CD | MSCs | 0.6–1.2×109 cells | iv. | Completed |
| 01540292 | Belgium | I/II | CD | MSCs | 1.5–2 ×106 cell/kg | iv. | Unknown status |
| 04519671 | USA | I/II | CD | BM-MSCs | 7.5×107 cells | iv. | Recruiting |
| 04519684 | USA | I/II | CD | BM-MSCs | 7.5×107 cells | iv. | Recruiting |
| 01144962 | USA | I/II | PFCD | BM-MSCs | 1–9×107 cells | Local injection | Completed |
| 04519697 | Nether-land | I/II | PFCD | MSCs | 7.5×107 cells | Local injection | Recruiting |
| 04073472 | USA | I | PFCD | BM-MSCs | 6×107 cells | Local injection | No recruiting |
| 04548583 | USA | I/II | CD | BM-MSCs | 1.5–3×108 cells | Endoscopic | Recruiting |
| 03183661 | China | I | CD | Allogenic-ASCs | 2, 8×106 cell/ kg | iv. | Enrolling |
| 01221428 | Austria | I/II | UC | UCBSCs | 2×107 cells | iv. | Unknown |
| 01541579 | Austria | III | PFCD | ASCs | 1.2×108 cells | Local injection | Completed |
| 04312113 | USA | I | UC | Autologous-ASCs | 1.5–3×107 cells | Intraarterial | Recruiting |
| 04543994 | USA | I/II | UC | BM-MSCs | 1.5, 3×108 cells | Endoscopic | Recruiting |
| 01233960 | USA | III | UC | MSCs | 2×108 cells | Intraarterial | Completed |
| 03609905 | China | I/II | UC | ASCs | 5×107 cells | Colonoscope | Recruiting |
| 03901235 | Belgium | I/II | N/A | MSCs | N/A | Local injection | Recruiting |
| 02442037 | China | I/II | UC | UCBSCs | 1×106 cell/kg | iv. | Recruiting |
| 02580617 | Korea | I | CD | ASCs | 0.15–1×108 cells | N/A | Recruiting |
| 01510431 | USA | N/A | CD | MSCs | 2×108 cells | N/A | Not available |
| 02403232 | Italy | II | CD | ASCs | N/A | N/A | Recruiting |
| 02403232 | Korea | I/II | CD | UCBSCs | 0.5, 1×108 cells | iv. | Unknown status |
| 02926300 | Korea | I/II | CD | SCs | Not provided. | N/A | Recruiting |
| 03220243 | USA | I | PFCD | MSCs | 2×107 cells | Local injection | Completed |
| 01915927 | USA | I | PFCD | MSCs | 2×107 cells | Local injection | Completed |
| 03449069 | USA | I | PFCD | MSCs | 2×107 cells | Local injection | Recruiting |
Abbreviations: ASCs, adipose mesenchymal stem cells; BM-MSCs, Bone marrow mesenchymal stem cells; CD, Crohn’s disease; IBD, Inflammatory bowel disease; iv., intravenous injection; MSCs, mesenchymal stem cells; PFCD, perianal fistula Crohn’s disease; PROCHYMAL®, Human Adult stem cells; SCs, stem cells; UC, Ulcerative colitis; UCBSCs, Umbilical Cord Blood stem cells; USA, United States, N/A, Not provided, W. Jelly, Wharton Jelly.
The emergence of ISCs and iPSCs represents a promising avenue for future research, and clinical trials will be conducted gradually.115,116 Recently, the research team at Tokyo Medical and Dental University has reported the successful transplantation of the world’s first “organoid” into the intestinal mucosa of a refractory UC patient, marking the beginning of the organoid SC era. The team collected normal intestinal mucosa (including ISCs) from the patient under endoscopy, cultured it in vitro for approximately 1 month, and constructed an “organoid” with a diameter of 0.1–0.2 mm. The “organoid” is then transplanted to the intestinal lesion site under endoscopy and fixed in place with a degradable film. According to reports, the patient’s intestinal symptoms have improved, and they are in good condition.
Improvement Strategies of SC-Based IBD Therapies
Clinical trials have provided valuable insights into the potential applications and efficacy of SC therapy for IBD. However, it is important to acknowledge the limitations of current SC therapy, including its efficacy, technology, and safety.117 When administered intravenously or intraperitoneally, only a small number of SCs home to intestinal lesions, with their effect primarily dependent on their secretory function. The secretion of SCs is also greatly influenced by their “niche” environment. Obtaining a large number of high-quality, homogeneous SC preparations remains technically challenging. Safety concerns associated with SC therapy include microcirculatory and coagulation dysfunction, immune rejection, and the risk of tumor formation.96,114
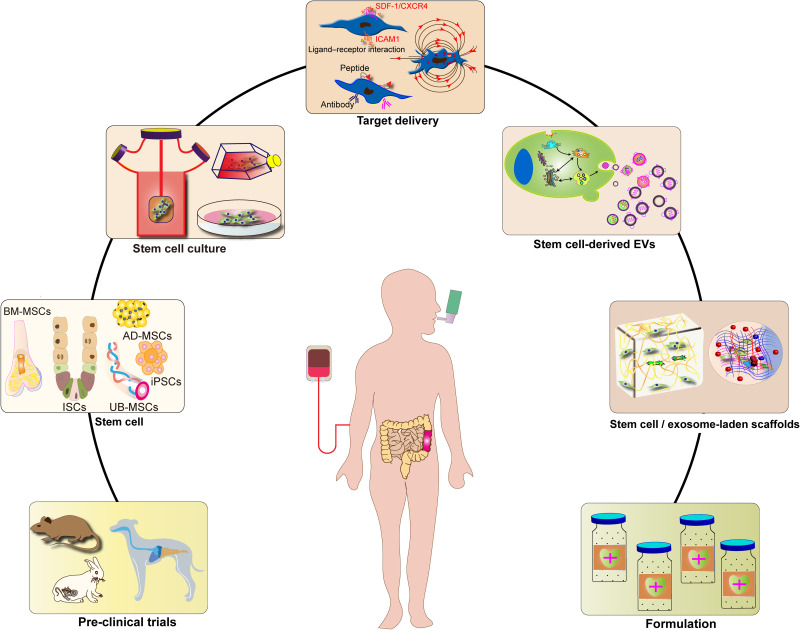
To enhance the use of SC therapy in treating IBD, several modifications have been proposed, such as changes to the culture conditions and cultivation methods for SCs, modification of SC contents and genetic genes, and extraction of SC derivatives. These strategies have the potential to optimize SC therapy and improve its efficacy and safety in treating IBD.114 Figure 2 illustrates some of these improvement strategies for SC-based IBD therapies.
Figure 2.
Stem cell-based therapy strategies for inflammatory bowel diseases.
Box 1 Changing SC Culture for IBD
3D Culture Method
Cells can migrate and grow in 3D culture vectors made from different materials, forming 3D cell-vector complexes.114,118 The 3D culture environment provides a better simulation of the in vivo SC growth environment and can more effectively regulate cell proliferation and differentiation. This allows for the cultivation of more cell types and the formation of tissue structures similar to those found in vivo.119 For instance, human ISCs can be cultured in 3D to generate intestinal epithelial organs, which can be used as IBD disease models. The introduction of human ISCs through the anus can also improve the targeting and colonization rate of SCs, further increasing the therapeutic potential.104
In another example, MSCs are cultured in 3D microsphere scaffolds constructed from poly(lactic-co-glycolic acid) (PLGA), resulting in improved viability of the MSCs. The addition of a broad-spectrum caspase inhibitor (IDN6556) to the 3D medium further increases the cell viability of MSCs, which is validated by intraperitoneal injection into an IBD mouse model.120
Changing Culture Conditions
The cultivation of SCs not only requires media but also an environment that mimics their in vivo growth. The proliferation and differentiation of SCs can be induced purposefully by adjusting the chemical, physical, or microbial environment of the culture medium, which enhances the therapeutic effects of SCs in IBD.90,121 For instance, in one study, various exosome-free fetal bovine sera (exosome-free FBS) were added to the medium of MSCs, resulting in enhanced immunomodulatory effects of MSCs in an animal model of IBD.122,123 The addition of drugs or biomolecules, such as aspirin, B fibroblast growth factor (FGF), all-trans retinoic acid (ATRA), activin A, and FGF2, to the culture medium has also been reported to enhance the beneficial effects of MSC treatment.124,125 Furthermore, the addition of pro-inflammatory cytokines, such as IFN-γ, TNF-α, and IL-1β, to the culture medium has been shown to increase the immunosuppressive function of MSCs.126,127
Box 2 Modification of SCs for IBD
Genetic Modification
To increase the function and efficacy of SCs, specific functional genes can be introduced into them through gene transfer viral vectors. For instance, IL-10 can be genetically delivered to MSCs, resulting in MSCs with IL-10 overexpression that enhance immunosuppression and tissue repair.128,129 Additionally, genes expressing IL-37b can be delivered to MSCs, and MSCs expressing IL-37b can inhibit the production of pro-inflammatory cytokines and chemokines, as well as suppress tissue migration and infiltration of neutrophils.130 In mouse models of IBD, both MSCs expressing IL-35 and MSCs expressing IL-25 have been shown to increase the therapeutic effect of MSC therapy.131,132 The therapeutic effect of MSCs can also be increased by gene transduction to produce miR-181a overexpressing MSCs, where the secreted miR-181a can regulate the development of T and B cells.133 Furthermore, there are increasing attempts to apply CRISPR-Cas9-mediated gene modifications to the therapeutic field of MSCs, which can enhance the ability of MSCs to regulate immunity.134,135
Adding Surface Markers
Surface markers play a crucial role in the recognition and targeting of SCs. By introducing cellular markers to SCs or exosomes, the recognition of IBD lesion sites can be enhanced, and the targeting of SCs can be improved, thereby increasing the homing ability of SCs or exosomes to specific sites. For instance, in one study, MSCs were incubated with antibodies directed against vascular cell adhesion molecules (VCAM1), which resulted in an increased therapeutic effect of VCAM1-treated MSCs as they were found to migrate more effectively to the injured colon.136,137 In another study, pretreating MSCs with stromal-derived factor 1 (SDF-1) induced an increased expression of CXCR4 chemokine receptors, allowing more MSCs to colonize the injured colon more rapidly, thereby enhancing their therapeutic effect.138 Additionally, the use of intercellular adhesion molecule 1 (ICAM1) overexpressing MSCs in an IBD mouse model is designed to direct infusion of ICAM1-MSCs to inflamed intestinal tissues and spleens, thereby increasing their therapeutic efficacy.139
Box 3 SCs Derivatives for IBD
The use of MSC-derived extracellular vesicles (EVs), particularly exosomes, as a substitute for MSC therapy has been validated in animal experiments, and clinical trials have reported comparable therapeutic outcomes.98,140 Mechanistic studies have revealed that MSCs act as immune modulators or repairers of the intestinal mucosal barrier by targeting immune cells or structural cells at sites of intestinal inflammation, mainly through paracrine EVs that carry a large number of bioactive molecules.141 The extraction of MSC-derived EVs is an attractive strategy for MSC-based therapy. EVs offer the advantages of cell-free therapy, reducing the potential immune rejection of MSCs and the risk of tumor formation. Furthermore, EVs have the ability to cross multiple physiological barriers in the body, act as carrier vesicles, and better preserve the formulation.142,143 The study conducted by Yang et al involved the intraperitoneal injection of exosome preparations derived from the supernatant of human UC-MSC cultures into IBD model mice. Through carrying TSG-6, MSC-derived EVs repair the mucosal barrier and regulate intestinal immunity, significantly improving IBD symptoms and reducing mortality.144
Conclusions and Future Perspectives
Although current treatments for IBD can provide temporary relief from clinical symptoms, achieving sustained remission and MH remains a challenge. SCs have the potential to directly improve chronic inflammation in the intestine by modulating immune cells and repairing the intestinal mucosal barrier. Furthermore, SCs can indirectly improve intestinal inflammatory damage and prevent IBD complications by positively impacting intestinal microecology, microcirculatory disorders, fibrosis, and carcinogenesis. The use of SCs in IBD treatment is expected to achieve sustained remission and MH. Preclinical studies of SCs in IBD models are increasing, and various SCs, such as MSCs, HSCs, ISCs, and iPSCs, have demonstrated positive efficacy and stable safety in IBD animal models, making them suitable for further clinical studies. Although HSCT can significantly improve IBD in clinical studies, its safety and cost-effectiveness are low, and it is not a viable option for the treatment of IBD alone. However, most clinical trials of MSCs applied to refractory IBD have reported positive efficacy and can achieve durable remission, especially in patients with refractory PFCD, confirming their safety and effectiveness. Additionally, clinical trials involving MSCs-derived EVs are underway. With the advancement of key technologies, SCs, such as ISCs and iPSCs, are gradually being applied in clinical studies for IBD treatment. Intestinal organoids derived from SCs in 3D culture are also being investigated in clinical research on IBD.104
However, significant progress is still needed to achieve safe, efficient, and standardized use of SC therapy for IBD. Firstly, achieving homogeneity in SCs is crucial. SCs are influenced by tissue sources and culture environments, leading to significant heterogeneity. Currently, there are no uniform standards and control comparisons in the tissue source and culture of SCs, making it challenging to achieve homogeneity in screening IBD patients. This heterogeneity affects the consistency of IBD clinical studies, leading to significant individual differences in study results and obstacles to sharing and applying results, ultimately limiting clinical translation. Secondly, the potential hazards of SC therapy must be considered. Compared to chemical drugs or biological agents, SCs are complex, and much is still unknown about their structure and function. Current observations of the effects of SCs in vivo are limited to particular diseases, and the full extent of their effects in vivo is not yet fully understood. These potential therapeutic risks limit clinical translation. Thirdly, standardization of SC therapy is necessary. Currently, there are no uniform standards regarding infusion mode, infusion dose, and time interval of SCs, and clear indications and contraindications have yet to be established.19
Currently, studies on SCs in IBD are primarily focused on the preclinical phase, with clinical applications limited to localized fistulas and refractory IBD. However, as the technology for SCs applications continues to mature, SCs are poised to become a routine “weapon” in clinical treatment and can be applied to general IBD patients in the future. Inflammation, injury, and neoplastic lesions of the digestive tract can all be targets for SC treatment.
Acknowledgments
The authors thank prof. Yu-Qiang Nie for editing the manuscript.
Funding Statement
This work was supported by Science and Technology Innovation Committee of Shenzhen (No. JCYJ20200109150700942, No. JCYJ20150403101028164, No. JCYC20170307100911479, No. JCYJ20190807145617113 and No. JCYJ20210324113802006), the Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515011581) and National Natural Science Foundation of China (No. 81800489).
Abbreviations
ASCs, adipose MSCs; ASTIC study, autologous stem cell international Crohn ‘s disease trial; ATRA, all-trans retinoic acid; BM-MSCs, Bone marrow MSCs; CD, Crohn’s disease; CDAI, Crohn’s disease activity index; CRISPR-Cas9, Clustered Regularly Interspersed Short Palindromic Repeats-Cas9; CXCR4, CXC chemokine receptor 4; DCs, Dendritic cells; ECs, endothelial cells; EMT, epithelial-mesenchymal transition; ESCs, embryonic SCs; EV, extracellular vesicle; FGF, fibroblast growth factor; HSCs, hematopoietic stem cells; HSCT, HSCs transplantation; IBD, Inflammatory bowel disease; ICAM1, intercellular adhesion molecule 1; ICAM1-MSCs, intercellular adhesion molecule 1-overexpressing MSCs; IDO, indoleamine 2,3-dioxygenase; IECs, intestinal epithelial cells; IFN- γ, interferon- γ; IL-10, interleukin-10; IL-12, interleukin-12; IL-17, interleukin-17; IL-1β, interleukin-1β; IL-23, interleukin-23; IL-37b, interleukin-37b; iMSCs, MSCs derived from iPSCs; iPSCs, IPSCs; ISCs, ISCs; LEC, lymphatic endothelial cell; Lgr5+, leucine rich repeat containing G protein coupled receptor 5; LIF, leukemia inhibitory factor; MNM, modified neuronal medium; MSCs, Mesenchymal SCs; MSCT, MSCs transplantation; MtSCs, intestinal resident MSCs; PBSCs, Peripheral blood SCs; PDGF, platelet-derived growth factor; pESCs, Parthenogenetic embryonic SCs; PFCD, perianal fistula Crohn’s disease; PGE2, prostaglandin E2; PLGA, poly lactic-co-glycolic acid; SCs, stem cells; SDF-1, stromal derived factor 1; STAT-3, signal transducer and activator of translation-3; tDC, tolerogenic dendritic cell; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; TSG-6, tumor necrosis factor α stimulated gene-6; UC, Ulcerative colitis; UCBSCs, Umbilical Cord Blood SCs; VCAM1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94(1):155–165. doi: 10.1016/j.mayocp.2018.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lightner AL. Stem cell therapy for inflammatory bowel disease. Clin Transl Gastroenterol. 2017;8(3):e82. doi: 10.1038/ctg.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019:7247238. doi: 10.1155/2019/7247238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56–66. doi: 10.1038/s41575-020-00360-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156(5):1345–1353.e1344. doi: 10.1053/j.gastro.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 6.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi: 10.1016/s0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 7.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150 [DOI] [PubMed] [Google Scholar]
- 8.Rogler G, Vavricka S, Schoepfer A, Lakatos PL. Mucosal healing and deep remission: what does it mean? World J Gastroenterol. 2013;19:7552–7560. doi: 10.3748/wjg.v19.i43.7552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hommes D, Colombel JF, Emery P, Greco M, Sandborn WJ. Changing Crohn’s disease management: need for new goals and indices to prevent disability and improve quality of life. J Crohns Colitis. 2012;6 Suppl 2:S224–S234. doi: 10.1016/s1873-9946(12)60502-9 [DOI] [PubMed] [Google Scholar]
- 10.Abraham BP, Ahmed T, Ali T. Inflammatory bowel disease: pathophysiology and current therapeutic approaches. Handb Exp Pharmacol. 2017;239:115–146. doi: 10.1007/164_2016_122 [DOI] [PubMed] [Google Scholar]
- 11.Wright EK, Ding NS, Niewiadomski O. Management of inflammatory bowel disease. Med J Aust. 2018;209(7):318–323. doi: 10.5694/mja17.01001 [DOI] [PubMed] [Google Scholar]
- 12.Zhang HM, Yuan S, Meng H, et al.. Stem cell-based therapies for inflammatory bowel disease. Int J Mol Sci. 2022;23. doi: 10.3390/ijms23158494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grim C, Noble R, Uribe G, et al. Impairment of tissue-resident mesenchymal stem cells in chronic ulcerative colitis and crohn’s disease. J Crohns Colitis. 2021;15(8):1362–1375. doi: 10.1093/ecco-jcc/jjab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Wu Q, Tam PKH. Immunomodulatory mechanisms of mesenchymal stem cells and their potential clinical applications. Int J Mol Sci. 2022;23. doi: 10.3390/ijms231710023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eiro N, Fraile M, González-Jubete A, González LO, Vizoso FJ. Mesenchymal (stem) stromal cells based as new therapeutic alternative in inflammatory bowel disease: basic mechanisms, experimental and clinical evidence, and challenges. Int J Mol Sci. 2022;23(16):8905. doi: 10.3390/ijms23168905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra R, Dhawan P, Srivastava AS, Singh AB. Inflammatory bowel disease: therapeutic limitations and prospective of the stem cell therapy. World J Stem Cells. 2020;12(10):1050–1066. doi: 10.4252/wjsc.v12.i10.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Che Z, Ye Z, Zhang X, et al. Mesenchymal stem/stromal cells in the pathogenesis and regenerative therapy of inflammatory bowel diseases. Front Immunol. 2022;13:952071. doi: 10.3389/fimmu.2022.952071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artinger KB, Watanabe M. Introduction to “stem cells” special issue. Birth Defects Res. 2022;114:921–925. doi: 10.1002/bdr2.2061 [DOI] [PubMed] [Google Scholar]
- 19.Ko JZ-H, Johnson S, Dave M. Efficacy and safety of mesenchymal stem/stromal cell therapy for inflammatory bowel diseases: an up-to-date systematic review. Biomolecules. 2021;11(1):82. doi: 10.3390/biom11010082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherman SE, Agudo J. Immune-mediated specific depletion of intestinal stem cells. Methods Mol Biol. 2020;2171:25–39. doi: 10.1007/978-1-0716-0747-3_2 [DOI] [PubMed] [Google Scholar]
- 21.Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85(1):3–10. doi: 10.1159/000345615 [DOI] [PubMed] [Google Scholar]
- 22.Biehl JK, Russell B. Introduction to stem cell therapy. J Cardiovasc Nurs. 2009;24(2):98–105. doi: 10.1097/JCN.0b013e318197a6a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(11):2204–2213. doi: 10.1242/jcs.02932 [DOI] [PubMed] [Google Scholar]
- 24.Goodell M. Introduction to a review series on hematopoietic stem cells. Blood. 2015;125(17):2587. doi: 10.1182/blood-2015-03-615005 [DOI] [PubMed] [Google Scholar]
- 25.DiNicola CA, Zand A, Hommes DW. Autologous hematopoietic stem cells for refractory Crohn’s disease. Expert Opin Biol Ther. 2017;17(5):555–564. doi: 10.1080/14712598.2017.1305355 [DOI] [PubMed] [Google Scholar]
- 26.Ruiz MA, Junior RLK, Piron-Ruiz L, et al. Medical, ethical, and legal aspects of hematopoietic stem cell transplantation for Crohn’s disease in Brazil. World J Stem Cells. 2020;12(10):1113–1123. doi: 10.4252/wjsc.v12.i10.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balassa K, Danby R, Rocha V. Haematopoietic stem cell transplants: principles and indications. Br J Hosp Med. 2019;80(1):33–39. doi: 10.12968/hmed.2019.80.1.33 [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, et al. Mesenchymal stem cells current clinical applications: a systematic review. Arch Med Res. 2021;52(1):93–101. doi: 10.1016/j.arcmed.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Wang S, Ding X, Guo J, Tian Z. Potential methods for improving the efficacy of mesenchymal stem cells in the treatment of inflammatory bowel diseases. Scand J Immunol. 2020;92(3):e12897. doi: 10.1111/sji.12897 [DOI] [PubMed] [Google Scholar]
- 30.Mao F, Wu Y, Tang X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017;2017:5356760. doi: 10.1155/2017/5356760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarsenova M, Kim Y, Raziyeva K, et al. Recent advances to enhance the immunomodulatory potential of mesenchymal stem cells. Front Immunol. 2022;13:1010399. doi: 10.3389/fimmu.2022.1010399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Wolf C, van de Bovenkamp M, Hoefnagel M. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy. 2017;19(7):784–797. doi: 10.1016/j.jcyt.2017.03.076 [DOI] [PubMed] [Google Scholar]
- 33.Umar S. Intestinal stem cells. Curr Gastroenterol Rep. 2010;12(5):340–348. doi: 10.1007/s11894-010-0130-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nalapareddy K, Zheng Y, Geiger H. Aging of intestinal stem cells. Stem Cell Rep. 2022;17(4):734–740. doi: 10.1016/j.stemcr.2022.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Walker NM, Cook MT, Ootani A, Clarke LL. Functional Cftr in crypt epithelium of organotypic enteroid cultures from murine small intestine. Am J Physiol Cell Physiol. 2012;302(10):C1492–C1503. doi: 10.1152/ajpcell.00392.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22(14):1856–1864. doi: 10.1101/gad.1674008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16(1):19–34. doi: 10.1038/s41575-018-0081-y [DOI] [PubMed] [Google Scholar]
- 38.Neal MD, Richardson WM, Sodhi CP, Russo A, Hackam DJ. Intestinal stem cells and their roles during mucosal injury and repair. J Surg Res. 2011;167(1):1–8. doi: 10.1016/j.jss.2010.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guiu J, Hannezo E, Yui S, et al. Tracing the origin of adult intestinal stem cells. Nature. 2019;570(7759):107–111. doi: 10.1038/s41586-019-1212-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan DW, Barker N. Intestinal stem cells and their defining niche. Curr Top Dev Biol. 2014;107:77–107. doi: 10.1016/b978-0-12-416022-4.00003-2 [DOI] [PubMed] [Google Scholar]
- 41.Jasper H. Intestinal stem cell aging: origins and interventions. Annu Rev Physiol. 2020;82(1):203–226. doi: 10.1146/annurev-physiol-021119-034359 [DOI] [PubMed] [Google Scholar]
- 42.Sugimoto S, Sato T. Establishment of 3D intestinal organoid cultures from intestinal stem cells. Methods Mol Biol. 2017;1612:97–105. doi: 10.1007/978-1-4939-7021-6_7 [DOI] [PubMed] [Google Scholar]
- 43.Liang R, Xiao X, Luo L, et al. Efficient definitive endoderm differentiation from human parthenogenetic embryonic stem cells induced by activin A and Wnt3a. Ann Clin Lab Sci. 2020;50(4):468–473. [PubMed] [Google Scholar]
- 44.Liang R, Wang Z, Kong X, et al. Differentiation of human parthenogenetic embryonic stem cells into functional hepatocyte-like cells. Organogenesis. 2020;16(4):137–148. doi: 10.1080/15476278.2020.1848237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez R, Garitaonandia I, Crain A, et al. Proof of concept studies exploring the safety and functional activity of human parthenogenetic-derived neural stem cells for the treatment of Parkinson’s disease. Cell Transplant. 2015;24(4):681–690. doi: 10.3727/096368915x687769 [DOI] [PubMed] [Google Scholar]
- 46.Schickl H, Braun M, Dabrock P. Ways out of the patenting prohibition? Human parthenogenetic and induced pluripotent stem cells. Bioethics. 2017;31(5):409–417. doi: 10.1111/bioe.12334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira AF, Calin GA, Picanço-Castro V, et al. Hematopoietic stem cells from induced pluripotent stem cells – considering the role of microRNA as a cell differentiation regulator. J Cell Sci. 2018;131(4). doi: 10.1242/jcs.203018 [DOI] [PubMed] [Google Scholar]
- 48.Soontararak S, Chow L, Johnson V, et al. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl Med. 2018;7(6):456–467. doi: 10.1002/sctm.17-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–109. doi: 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sagi I, Chia G, Golan-Lev T, et al. Derivation and differentiation of haploid human embryonic stem cells. Nature. 2016;532(7597):107–111. doi: 10.1038/nature17408 [DOI] [PubMed] [Google Scholar]
- 51.Lu Y, Xu Y, Zhang S, et al. Human gingiva-derived mesenchymal stem cells alleviate inflammatory bowel disease via IL-10 signalling-dependent modulation of immune cells. Scand J Immunol. 2019;90(3):e12751. doi: 10.1111/sji.12751 [DOI] [PubMed] [Google Scholar]
- 52.Otsuka R, Wada H, Murata T, Seino K-I. Immune reaction and regulation in transplantation based on pluripotent stem cell technology. Inflamm Regen. 2020;40(1):12. doi: 10.1186/s41232-020-00125-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao L, Xu H, Wang G, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int Immunopharmacol. 2019;72:264–274. doi: 10.1016/j.intimp.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Pei B, Yan J, et al. hucMSC-derived exosomes alleviate the deterioration of colitis via the miR-146a/SUMO1 axis. Mol Pharm. 2022;19(2):484–493. doi: 10.1021/acs.molpharmaceut.1c00450 [DOI] [PubMed] [Google Scholar]
- 55.Jiang -X-X, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586 [DOI] [PubMed] [Google Scholar]
- 56.Heidari N, Abbasi‐Kenarsari H, Namaki S, et al. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236(8):5906–5920. doi: 10.1002/jcp.30275 [DOI] [PubMed] [Google Scholar]
- 57.Mounayar M, Kefaloyianni E, Smith B, et al. PI3kα and STAT1 interplay regulates human mesenchymal stem cell immune polarization. Stem Cells. 2015;33(6):1892–1901. doi: 10.1002/stem.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin Z, Wang P-Y, Wan -J-J, et al. MicroRNA124-IL6R mediates the effect of nicotine in inflammatory bowel disease by shifting Th1/Th2 balance toward Th1. Front Immunol. 2020;11:235. doi: 10.3389/fimmu.2020.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terraza-Aguirre C, Campos-Mora M, Elizondo-Vega R, et al. Mechanisms behind the immunoregulatory dialogue between mesenchymal stem cells and Th17 cells. Cells. 2020;9(7):1660. doi: 10.3390/cells9071660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reis M, Mavin E, Nicholson L, et al. Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front Immunol. 2018;9:2538. doi: 10.3389/fimmu.2018.02538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian X, An N, Ren Y, et al. Immunosuppressive effects of mesenchymal stem cells-derived exosomes. Stem Cell Rev Rep. 2021;17(2):411–427. doi: 10.1007/s12015-020-10040-7 [DOI] [PubMed] [Google Scholar]
- 62.Song W-J, Li Q, Ryu M-O, et al. Canine adipose tissue-derived mesenchymal stem cells pre-treated with TNF-alpha enhance immunomodulatory effects in inflammatory bowel disease in mice. Res Vet Sci. 2019;125:176–184. doi: 10.1016/j.rvsc.2019.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Ma K, Zhang L, Xu H, Zhang N. Human umbilical cord blood derived-mesenchymal stem cells alleviate dextran sulfate sodium-induced colitis by increasing regulatory T cells in mice. Front Cell Dev Biol. 2020;8:604021. doi: 10.3389/fcell.2020.604021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao X, Duan L, Hou H, et al. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE 2 -mediated M2 macrophage polarization. Theranostics. 2020;10(17):7697–7709. doi: 10.7150/thno.45434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.García JR, Quirós M, Han WM, et al. IFN-γ-tethered hydrogels enhance mesenchymal stem cell-based immunomodulation and promote tissue repair. Biomaterials. 2019;220:119403. doi: 10.1016/j.biomaterials.2019.119403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. doi: 10.1038/ni.3002 [DOI] [PubMed] [Google Scholar]
- 67.Yang J, Liu XX, Fan H, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10(10):e0140551. doi: 10.3389/fimmu.2022.952071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. 2016;51(6):942–947. doi: 10.1016/j.jpedsurg.2016.02.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129(11):2182–2189. doi: 10.1242/jcs.170373 [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Wang H, Cao J, Ye C. Exosomes from adipose-derived stem cells promotes VEGF-C-dependent lymphangiogenesis by regulating miRNA-132/TGF-β pathway. Cell Physiol Biochem. 2018;49:160–171. doi: 10.1159/000492851 [DOI] [PubMed] [Google Scholar]
- 71.Guillén MI, Platas J, Pérez Del Caz MD, Mirabet V, Alcaraz MJ. Paracrine anti-inflammatory effects of adipose tissue-derived mesenchymal stem cells in human monocytes. Front Physiol. 2018;9:661. doi: 10.3389/fphys.2018.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Komori M, Tsuji S, Tsujii M, et al. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109–118. doi: 10.1111/j.1067-1927.2005.130114.x [DOI] [PubMed] [Google Scholar]
- 73.Hayashi Y, Tsuji S, Tsujii M, et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523–531. doi: 10.1124/jpet.108.137083 [DOI] [PubMed] [Google Scholar]
- 74.Ahlawat S, Kumar P, Mohan H, Goyal S, Sharma KK. Inflammatory bowel disease: tri-directional relationship between microbiota, immune system and intestinal epithelium. Crit Rev Microbiol. 2021;47:254–273. doi: 10.1080/1040841x.2021.1876631 [DOI] [PubMed] [Google Scholar]
- 75.Bailey CC, Zhong G, Huang IC, Farzan M. IFITM-family proteins: the cell’s first line of antiviral defense. Annu Rev Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yagi H, Chen AF, Hirsch D, et al. Antimicrobial activity of mesenchymal stem cells against Staphylococcus aureus. Stem Cell Res Ther. 2020;11:293. doi: 10.1186/s13287-020-01807-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alcayaga-Miranda F, Cuenca J, Martin A, et al. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther. 2015;6:199. doi: 10.1186/s13287-015-0192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang R, Liu Y, Kelk P, et al. A subset of IL-17(+) mesenchymal stem cells possesses anti-Candida albicans effect. Cell Res. 2013;23:107–121. doi: 10.1038/cr.2012.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Danese S, Sans M, de la Motte C, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130(7):2060–2073. doi: 10.1053/j.gastro.2006.03.054 [DOI] [PubMed] [Google Scholar]
- 80.Manieri NA, Mack MR, Himmelrich MD, et al. Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. J Clin Invest. 2015;125:3606–3618. doi: 10.1172/jci81423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tao H, Han Z, Han ZC, Li Z. Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int. 2016;2016:1314709. doi: 10.1155/2016/1314709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C, Li Y, Yang M, et al. Efficient differentiation of bone marrow mesenchymal stem cells into endothelial cells in vitro. Eur J Vasc Endovasc Surg. 2018;55(2):257–265. doi: 10.1016/j.ejvs.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Huang B, Jin T, et al. Intestinal fibrosis in inflammatory bowel disease and the prospects of mesenchymal stem cell therapy. Front Immunol. 2022;13:835005. doi: 10.3389/fimmu.2022.835005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lovisa S, Genovese G, Danese S. Role of epithelial-to-mesenchymal transition in inflammatory bowel disease. J Crohns Colitis. 2019;13(5):659–668. doi: 10.1093/ecco-jcc/jjy201 [DOI] [PubMed] [Google Scholar]
- 85.Lian L, Huang Q, Zhang L, et al. Anti-fibrogenic potential of mesenchymal stromal cells in treating fibrosis in crohn’s disease. Dig Dis Sci. 2018;63(7):1821–1834. doi: 10.1007/s10620-018-5082-8 [DOI] [PubMed] [Google Scholar]
- 86.Choi YJ, Koo JB, Kim HY, et al. Umbilical cord/placenta-derived mesenchymal stem cells inhibit fibrogenic activation in human intestinal myofibroblasts via inhibition of myocardin-related transcription factor A. Stem Cell Res Ther. 2019;10(1):291. doi: 10.1186/s13287-019-1385-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Srivastava AS, Feng Z, Mishra R, et al. Embryonic stem cells ameliorate piroxicam-induced colitis in IL10−/− KO mice. Biochem Biophys Res Commun. 2007;361(4):953–959. doi: 10.1016/j.bbrc.2007.07.139 [DOI] [PubMed] [Google Scholar]
- 88.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 89.Wang M, Cai J, Huang F, et al. Pre-treatment of human umbilical cord-derived mesenchymal stem cells with interleukin-6 abolishes their growth-promoting effect on gastric cancer cells. Int J Mol Med. 2015;35(2):367–375. doi: 10.3892/ijmm.2014.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng X-B, He X-W, Zhang L-J, et al. Bone marrow-derived CXCR4-overexpressing MSCs display increased homing to intestine and ameliorate colitis-associated tumorigenesis in mice. Gastroenterol Rep. 2019;7(2):127–138. doi: 10.1093/gastro/goy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He R, Han C, Li Y, Qian W, Hou X. Cancer-preventive role of bone marrow-derived mesenchymal stem cells on colitis-associated colorectal cancer: roles of gut microbiota involved. Front Cell Dev Biol. 2021;9:642948. doi: 10.3389/fcell.2021.642948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koh GY, Kane A, Lee K, et al. Parabacteroides distasonis attenuates toll-like receptor 4 signaling and Akt activation and blocks colon tumor formation in high-fat diet-fed azoxymethane-treated mice. Int J Cancer. 2018;143(7):1797–1805. doi: 10.1002/ijc.31559 [DOI] [PubMed] [Google Scholar]
- 93.Zhang X, Hu X, Rao X. Apoptosis induced by Staphylococcus aureus toxins. Microbiol Res. 2017;205:19–24. doi: 10.1016/j.micres.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 94.Nakatsuji T, Chen TH, Butcher AM, et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Science Adv. 2018;4(2):eaao4502. doi: 10.1126/sciadv.aao4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang LT, Liu KJ, Sytwu HK, Yen ML, Yen BL. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl Med. 2021;10:1288–1303. doi: 10.1002/sctm.21-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi MY, Liu L, Yang FY. Strategies to improve the effect of mesenchymal stem cell therapy on inflammatory bowel disease. World J Stem Cells. 2022;14:684–699. doi: 10.4252/wjsc.v14.i9.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Banerjee A, Bizzaro D, Burra P, et al. Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice. Stem Cell Res Ther. 2015;6(1):79. doi: 10.1186/s13287-015-0073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robinson AM, Miller S, Payne N, et al. Neuroprotective potential of mesenchymal stem cell-based therapy in acute stages of TNBS-induced colitis in guinea-pigs. PLoS One. 2015;10(9):e0139023. doi: 10.1371/journal.pone.0139023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie M, Qin H, Luo Q, et al. Comparison of adipose-derived and bone marrow mesenchymal stromal cells in a murine model of crohn’s disease. Dig Dis Sci. 2017;62(1):115–123. doi: 10.1007/s10620-016-4166-6 [DOI] [PubMed] [Google Scholar]
- 100.Qu B, Xin G-R, Zhao L-X, et al. Testing stem cell therapy in a rat model of inflammatory bowel disease: role of bone marrow stem cells and stem cell factor in mucosal regeneration. PLoS One. 2014;9(10):e107891. doi: 10.1371/journal.pone.0107891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watanabe M. Retraction: adult tissue stem cell therapy for gastrointestinal diseases by Mamoru Watanabe. J Gastroenterol Hepatol. 2018;33(1):329. doi: 10.1111/jgh.12555 [DOI] [PubMed] [Google Scholar]
- 102.Fukuda M, Mizutani T, Mochizuki W, et al. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28(16):1752–1757. doi: 10.1101/gad.245233.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaker A, Rubin DC. Stem cells: one step closer to gut repair. Nature. 2012;485(7397):181–182. doi: 10.1038/485181a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watanabe S, Kobayashi S, Ogasawara N, et al. Transplantation of intestinal organoids into a mouse model of colitis. Nat Protoc. 2022;17(3):649–671. doi: 10.1038/s41596-021-00658-3 [DOI] [PubMed] [Google Scholar]
- 105.Burt RK, Craig RM, Milanetti F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood. 2010;116(26):6123–6132. doi: 10.1182/blood-2010-06-292391 [DOI] [PubMed] [Google Scholar]
- 106.Cassinotti A, Annaloro C, Ardizzone S, et al. Autologous haematopoietic stem cell transplantation without CD34+ cell selection in refractory Crohn’s disease. Gut. 2008;57(2):211–217. doi: 10.1136/gut.2007.128694 [DOI] [PubMed] [Google Scholar]
- 107.Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn Disease: a randomized clinical trial. JAMA. 2015;314(23):2524–2534. doi: 10.1001/jama.2015.16700 [DOI] [PubMed] [Google Scholar]
- 108.Bislenghi G, Wolthuis A, Van Assche G, et al. Cx601 (darvadstrocel) for the treatment of perianal fistulizing Crohn’s disease. Expert Opin Biol Ther. 2019;19(7):607–616. doi: 10.1080/14712598.2019.1623876 [DOI] [PubMed] [Google Scholar]
- 109.Panes J, García-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a Phase 3 randomised, double-blind controlled trial. Lancet. 2016;388(10051):1281–1290. doi: 10.1016/S0140-6736(16)31203-X [DOI] [PubMed] [Google Scholar]
- 110.García-Olmo D, García-Arranz M, Herreros D, et al. A Phase I clinical trial of the treatment of Crohn’s Fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48(7):1416–1423. doi: 10.1007/s10350-005-0052-6 [DOI] [PubMed] [Google Scholar]
- 111.Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical cord mesenchymal stem cell treatment for Crohn’s disease: a randomized controlled clinical trial. Gut Liver. 2018;12(1):73–78. doi: 10.5009/gnl17035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Forbes GM, Sturm MJ, Leong RW, et al. A Phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12(1):64–71. doi: 10.1016/j.cgh.2013.06.021 [DOI] [PubMed] [Google Scholar]
- 113.Knyazev OV, Kagramanova AV, Fadeeva NA, et al. Mesenchymal stromal cells of bone marrow and azathioprine in Crohn’s disease therapy. Ter Arkh. 2018;90(2):47–52. doi: 10.26442/terarkh201890247-52 [DOI] [PubMed] [Google Scholar]
- 114.Seo Y, Kang M-J, Kim H-S. Strategies to potentiate paracrine therapeutic efficacy of mesenchymal stem cells in inflammatory diseases. Int J Mol Sci. 2021;22(7):3397. doi: 10.3390/ijms22073397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lucafò M, Muzzo A, Marcuzzi M, et al. Patient-derived organoids for therapy personalization in inflammatory bowel diseases. World J Gastroenterol. 2022;28(24):2636–2653. doi: 10.3748/wjg.v28.i24.2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arjmand B, Rabbani Z, Soveyzi F, et al.. Advancement of organoid technology in regenerative medicine. Regen Eng Transl Med. 2022:1–14. doi: 10.1007/s40883-022-00271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lopez-Santalla M, Garin MI. Improving the efficacy of mesenchymal stem/stromal-based therapy for treatment of inflammatory bowel diseases. Biomedicines. 2021;9(11):1507. doi: 10.3390/biomedicines9111507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Petrenko Y, Syková E, Kubinová Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res Ther. 2017;8(1):94. doi: 10.1186/s13287-017-0558-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y, Shu Z, Qian K, Wang J, Zhu H. Harnessing the properties of biomaterial to enhance the immunomodulation of mesenchymal stem cells. Tissue Eng Part B Rev. 2019;25(6):492–499. doi: 10.1089/ten.TEB.2019.0131 [DOI] [PubMed] [Google Scholar]
- 120.Hanson SE, King SN, Kim J, et al. The effect of mesenchymal stromal cell–hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue Eng Part A. 2011;17(19–20):2463–2471. doi: 10.1089/ten.TEA.2010.0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H, Shen S, Fu H, et al. Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells Int. 2019;2019:9671206. doi: 10.1155/2019/9671206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kang JY, Oh M-K, Joo H, et al. Xeno-free condition enhances therapeutic functions of human Wharton’s Jelly-derived mesenchymal stem cells against experimental colitis by upregulated indoleamine 2,3-dioxygenase activity. J Clin Med. 2020;9(9):2913. doi: 10.3390/jcm9092913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu X, Wu D, Mu Y, Zhao Y, Ma Z. Serum-free medium enhances the therapeutic effects of umbilical cord mesenchymal stromal cells on a murine model for acute colitis. Front Bioeng Biotechnol. 2020;8:586. doi: 10.3389/fbioe.2020.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Y, Xiao F, Hu X, et al. Recovery and maintenance of NESTIN expression in umbilical cord-MSC using a novel culture medium. AMB Express. 2020;10(1):132. doi: 10.1186/s13568-020-01067-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salmenkari H, Laitinen A, Forsgård RA, et al. The use of unlicensed bone marrow-derived platelet lysate-expanded mesenchymal stromal cells in colitis: a pre-clinical study. Cytotherapy. 2019;21(2):175–188. doi: 10.1016/j.jcyt.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 126.Shin T-H, Ahn J-S, Oh S-J, et al. TNF-α priming elicits robust immunomodulatory potential of human tonsil-derived mesenchymal stem cells to alleviate murine colitis. Biomedicines. 2020;8(12):561. doi: 10.3390/biomedicines8120561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu D, Zhao Y, Wang H, et al. IL-1β pre-stimulation enhances the therapeutic effects of endometrial regenerative cells on experimental colitis. Stem Cell Res Ther. 2021;12(1):324. doi: 10.1186/s13287-021-02392-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Payne NL, Dantanarayana A, Sun G, et al. Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination. Cell Adh Migr. 2012;6(3):179–189. doi: 10.4161/cam.20341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song SY, Hong J, Go S, et al. Interleukin-4 gene transfection and spheroid formation potentiate therapeutic efficacy of mesenchymal stem cells for osteoarthritis. Adv Healthcare Mater. 2020;9(5):e1901612. doi: 10.1002/adhm.201901612 [DOI] [PubMed] [Google Scholar]
- 130.Wang W-Q, Dong K, Zhou L, et al. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin. 2015;36(11):1377–1387. doi: 10.1038/aps.2015.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nan Z, Fan H, Tang Q, et al. Dual expression of CXCR4 and IL-35 enhances the therapeutic effects of BMSCs on TNBS-induced colitis in rats through expansion of tregs and suppression of Th17 cells. Biochem Biophys Res Commun. 2018;499(4):727–734. doi: 10.1016/j.bbrc.2018.03.043 [DOI] [PubMed] [Google Scholar]
- 132.Fu Y, Ni J, Chen J, et al. Dual-functionalized MSCs that express CX3CR1 and IL-25 exhibit enhanced therapeutic effects on inflammatory bowel disease. Mol Ther. 2020;28(4):1214–1228. doi: 10.1016/j.ymthe.2020.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu L, Wang Y, Fan H, et al. MicroRNA-181a regulates local immune balance by inhibiting proliferation and immunosuppressive properties of mesenchymal stem cells. Stem Cells. 2012;30(8):1756–1770. doi: 10.1002/stem.1156 [DOI] [PubMed] [Google Scholar]
- 134.Lu Q, Chen R, Du S, et al. Activation of the cGAS-STING pathway combined with CRISPR-Cas9 gene editing triggering long-term immunotherapy. Biomaterials. 2022;291:121871. doi: 10.1016/j.biomaterials.2022.121871 [DOI] [PubMed] [Google Scholar]
- 135.Crippa S, Conti A, Vavassori V, et al. Mesenchymal stromal cells improve the transplantation outcome of CRISPR-Cas9 gene-edited human HSPCs. Mol Ther. 2022;30(10):3333. doi: 10.1016/j.ymthe.2022.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen Q, Li Y, Chen Z, Du H, Wan J. Anti-VCAM 1 antibody-coated mesenchymal stromal cells attenuate experimental colitis via immunomodulation. Med Sci Monit. 2019;25:4457–4468. doi: 10.12659/msm.914238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ko IK, Kim B-G, Awadallah A, et al. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18(7):1365–1372. doi: 10.1038/mt.2010.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li X, Lan X, Zhao Y, et al. SDF-1/CXCR4 axis enhances the immunomodulation of human endometrial regenerative cells in alleviating experimental colitis. Stem Cell Res Ther. 2019;10(1):204. doi: 10.1186/s13287-019-1298-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li X, Wang Q, Ding L, et al. Intercellular adhesion molecule-1 enhances the therapeutic effects of MSCs in a dextran sulfate sodium-induced colitis models by promoting MSCs homing to murine colons and spleens. Stem Cell Res Ther. 2019;10(1):267. doi: 10.1186/s13287-019-1384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Robinson AM, Sakkal S, Park A, et al. Mesenchymal stem cells and conditioned medium avert enteric neuropathy and colon dysfunction in guinea pig TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2014;307(11):G1115–G1129. doi: 10.1152/ajpgi.00174.2014 [DOI] [PubMed] [Google Scholar]
- 141.Heidari M, Pouya S, Baghaei K, et al. The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J Cell Physiol. 2018;233:8754–8766. doi: 10.1002/jcp.26765 [DOI] [PubMed] [Google Scholar]
- 142.Lykov AP, Poveshchenko OV, Bondarenko NA, et al. Therapeutic potential of biomedical cell product in DSS-induced inflammation in the small intestine of C57Bl/6J mice. Bull Exp Biol Med. 2018;165:576–580. doi: 10.1007/s10517-018-4216-5 [DOI] [PubMed] [Google Scholar]
- 143.Lee KE, Jung S-A, Joo Y-H, et al. The efficacy of conditioned medium released by tonsil-derived mesenchymal stem cells in a chronic murine colitis model. PLoS One. 2019;14(12):e0225739. doi: 10.1371/journal.pone.0225739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang S, Liang X, Song J, et al. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12:315. doi: 10.1186/s13287-021-02404-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barnhoorn MC, Wasser MN, Roelofs H, et al. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for crohn’s disease perianal fistulas. J Crohns Colitis. 2020;14:64–70. doi: 10.1093/ecco-jcc/jjz116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chao K, Zhang S, Qiu Y, et al. Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5(+) B regulatory cells. Stem Cell Res Ther. 2016;7:109. doi: 10.1186/s13287-016-0376-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Legaki E, Roubelakis MG, Theodoropoulos GE, et al. Therapeutic potential of secreted molecules derived from human amniotic fluid mesenchymal stem/stroma cells in a mice model of colitis. Stem Cell Rev Rep. 2016;12(5):604–612. doi: 10.1007/s12015-016-9677-1 [DOI] [PubMed] [Google Scholar]
- 148.de Aguiar CF, Castoldi A, Andrade-Oliveira V, et al. Mesenchymal stromal cells modulate gut inflammation in experimental colitis. Inflammopharmacology. 2018;26(1):251–260. doi: 10.1007/s10787-017-0404-6 [DOI] [PubMed] [Google Scholar]
- 149.de la Portilla F, Alba F, García-Olmo D, et al. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323. doi: 10.1007/s00384-012-1581-9 [DOI] [PubMed] [Google Scholar]
- 150.de la Portilla F, Yuste Y, Pereira S, et al. Local mesenchymal stem cell therapy in experimentally induced colitis in the rat. Int J Stem Cell. 2018;11(1):39–47. doi: 10.15283/ijsc17074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fu ZW, Zhang ZY, Ge HY. Mesenteric injection of adipose-derived mesenchymal stem cells relieves experimentally-induced colitis in rats by regulating Th17/Treg cell balance. Am J Transl Res. 2018;10:54–66. [PMC free article] [PubMed] [Google Scholar]
- 152.Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58(7):929–939. doi: 10.1136/gut.2008.168534 [DOI] [PubMed] [Google Scholar]
- 153.Gregoire C, Briquet A, Pirenne C, et al. Allogeneic mesenchymal stromal cells for refractory luminal Crohn’s disease: a Phase I–II study. Dig Liver Dis. 2018;50(11):1251–1255. doi: 10.1016/j.dld.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 154.Hu J, Zhao G, Zhang L, et al. Safety and therapeutic effect of mesenchymal stem cell infusion on moderate to severe ulcerative colitis. Exp Ther Med. 2016;12(5):2983–2989. doi: 10.3892/etm.2016.3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee HJ, Oh S-H, Jang HW, et al. Long-term effects of bone marrow-derived mesenchymal stem cells in dextran sulfate sodium-induced murine chronic colitis. Gut Liver. 2016;10(3):412–419. doi: 10.5009/gnl15229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee B-C, Shin N, Lee JY, et al. MIS416 enhances therapeutic functions of human umbilical cord blood-derived mesenchymal stem cells against experimental colitis by modulating systemic immune milieu. Front Immunol. 2018;9:1078. doi: 10.3389/fimmu.2018.01078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Panés J, García-Olmo D, Van Assche G, et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2018;154(5):1334–1342.e1334. doi: 10.1053/j.gastro.2017.12.020 [DOI] [PubMed] [Google Scholar]
- 158.Martín Arranz E, Martín Arranz MD, Robredo T, et al. Endoscopic submucosal injection of adipose-derived mesenchymal stem cells ameliorates TNBS-induced colitis in rats and prevents stenosis. Stem Cell Res Ther. 2018;9(1):95. doi: 10.1186/s13287-018-0837-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Miyamoto S, Ohnishi S, Onishi R, et al. Therapeutic effects of human amnion-derived mesenchymal stem cell transplantation and conditioned medium enema in rats with trinitrobenzene sulfonic acid-induced colitis. Am J Transl Res. 2017;9(3):940–952. [PMC free article] [PubMed] [Google Scholar]
- 160.Molendijk I, Bonsing BA, Roelofs H, et al. Allogeneic bone marrow–derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2015;149(4):918–927.e916. doi: 10.1053/j.gastro.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 161.Pak S, Hwang SW, Shim IK, et al. Endoscopic transplantation of mesenchymal stem cell sheets in experimental colitis in rats. Sci Rep. 2018;8(1):11314. doi: 10.1038/s41598-018-29617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Park S-R, Kim J-W, Jun H-S, et al. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther. 2018;26(2):606–617. doi: 10.1016/j.ymthe.2017.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pouya S, Heidari M, Baghaei K, et al. Study the effects of mesenchymal stem cell conditioned medium injection in mouse model of acute colitis. Int Immunopharmacol. 2018;54:86–94. doi: 10.1016/j.intimp.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 164.Song J-Y, Kang HJ, Hong JS, et al. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci Rep. 2017;7(1):9412. doi: 10.1038/s41598-017-09827-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Song W-J, Li Q, Ryu M-O, et al. TSG-6 released from intraperitoneally injected canine adipose tissue-derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Res Ther. 2018;9(1):91. doi: 10.1186/s13287-018-0841-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tanaka F, Tominaga K, Ochi M, et al. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008;83(23–24):771–779. doi: 10.1016/j.lfs.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 167.Wu Y, Qiu W, Xu X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am J Transl Res. 2018;10(7):2026–2036. [PMC free article] [PubMed] [Google Scholar]
- 168.Xu X, Wang Y, Zhang B, et al. Treatment of experimental colitis by endometrial regenerative cells through regulation of B lymphocytes in mice. Stem Cell Res Ther. 2018;9(1):146. doi: 10.1186/s13287-018-0874-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yu Y, Song EM, Lee KE, et al. Therapeutic potential of tonsil-derived mesenchymal stem cells in dextran sulfate sodium-induced experimental murine colitis. PLoS One. 2017;12(8):e0183141. doi: 10.1371/journal.pone.0183141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005;128(3):552–563. doi: 10.1053/j.gastro.2004.11.051 [DOI] [PubMed] [Google Scholar]
- 171.Clerici M, Cassinotti A, Onida F, et al. Immunomodulatory effects of unselected haematopoietic stem cells autotransplantation in refractory Crohn’s disease. Dig Liver Dis. 2011;43(12):946–952. doi: 10.1016/j.dld.2011.07.021 [DOI] [PubMed] [Google Scholar]
- 172.Hasselblatt P, Drognitz K, Potthoff K, et al. Remission of refractory Crohn’s disease by high-dose cyclophosphamide and autologous peripheral blood stem cell transplantation. Aliment Pharmacol Ther. 2012;36(8):725–735. doi: 10.1111/apt.12032 [DOI] [PubMed] [Google Scholar]
- 173.Kotlarz D, Beier R, Murugan D, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143(2):347–355. doi: 10.1053/j.gastro.2012.04.045 [DOI] [PubMed] [Google Scholar]
- 174.Liang J, Zhang H, Wang D, et al. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012;61(3):468–469. doi: 10.1136/gutjnl-2011-300083 [DOI] [PubMed] [Google Scholar]
- 175.Garca-Olmo D, García-Arranz M, García LG, et al. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18(5):451–454. doi: 10.1007/s00384-003-0490-3 [DOI] [PubMed] [Google Scholar]
- 176.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52(1):79–86. doi: 10.1007/DCR.0b013e3181973487 [DOI] [PubMed] [Google Scholar]
- 177.Guadalajara H, Herreros D, De-La-Quintana P, et al. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27(5):595–600. doi: 10.1007/s00384-011-1350-1 [DOI] [PubMed] [Google Scholar]
- 178.Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula advanced therapy trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55(7):762–772. doi: 10.1097/DCR.0b013e318255364a [DOI] [PubMed] [Google Scholar]
- 179.Dietz AB, Dozois EJ, Fletcher JG, et al. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2017;153(1):59–62.e52. doi: 10.1053/j.gastro.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dige A, Hougaard HT, Agnholt J, et al. Efficacy of injection of freshly collected autologous adipose tissue into perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2019;156(8):2208–2216.e2201. doi: 10.1053/j.gastro.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 181.Lightner AL, Dozois EJ, Dietz AB, et al. Matrix-delivered autologous mesenchymal stem cell therapy for refractory rectovaginal Crohn’s fistulas. Inflamm Bowel Dis. 2020;26(5):670–677. doi: 10.1093/ibd/izz215 [DOI] [PubMed] [Google Scholar]
- 182.Nikolic M, Stift A, Reinisch W, et al. Allogeneic expanded adipose-derived stem cells in the treatment of rectovaginal fistulas in Crohn’s disease. Colorectal Dis. 2021;23(1):153–158. doi: 10.1111/codi.15324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cho YB, Park KJ, Yoon SN, et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med. 2015;4(5):532–537. doi: 10.5966/sctm.2014-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ciccocioppo R, Gallia A, Sgarella A, et al. Long-term follow-up of Crohn disease fistulas after local injections of bone marrow–derived mesenchymal stem cells. Mayo Clin Proc. 2015;90(6):747–755. doi: 10.1016/j.mayocp.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 185.Garcia-Olmo D, Guadalajara H, Rubio-Perez I, et al. Recurrent anal fistulae: limited surgery supported by stem cells. World J Gastroenterol. 2015;21(11):3330–3336. doi: 10.3748/wjg.v21.i11.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Park KJ, Ryoo S-B, Kim JS, et al. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: a pilot clinical trial. Colorectal Dis. 2016;18(5):468–476. doi: 10.1111/codi.13223 [DOI] [PubMed] [Google Scholar]
- 187.García-Arranz M, Herreros MD, González-Gómez C, et al. Treatment of Crohn’s-related rectovaginal fistula with allogeneic expanded-adipose derived stem cells: a phase I-IIa clinical trial. Stem Cells Transl Med. 2016;5(11):1441–1446. doi: 10.5966/sctm.2015-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wainstein C, Quera R, Fluxá D, et al. Stem cell therapy in refractory perineal Crohn’s disease: long-term follow-up. Colorectal Dis. 2018;20(3):O68–O75. doi: 10.1111/codi.14002 [DOI] [PubMed] [Google Scholar]
- 189.Knyazev OV, Fadeeva NA, Kagramanova AV, et al. Stem cell therapy for perianal Crohn’s disease. Ter Arkh. 2018;90(3):60–66. doi: 10.26442/terarkh201890360-66 [DOI] [PubMed] [Google Scholar]
- 190.Herreros MD, Garcia-Olmo D, Guadalajara H, et al. Stem cell therapy: a compassionate use program in perianal fistula. Stem Cells Int. 2019;2019:6132340. doi: 10.1155/2019/6132340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou C, Li M, Zhang Y, et al. Autologous adipose-derived stem cells for the treatment of Crohn’s fistula-in-ano: an open-label, controlled trial. Stem Cell Res Ther. 2020;11(1):124. doi: 10.1186/s13287-020-01636-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Laureti S, Gionchetti P, Cappelli A, et al. Refractory complex Crohn’s perianal fistulas: a role for autologous microfragmented adipose tissue injection. Inflamm Bowel Dis. 2020;26(2):321–330. doi: 10.1093/ibd/izz051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Melmed GY, Pandak WM, Casey K, et al. Human placenta-derived cells (PDA-001) for the treatment of moderate-to-severe Crohnʼs disease. Inflamm Bowel Dis. 2015;21(8):1809–1816. doi: 10.1097/mib.0000000000000441 [DOI] [PubMed] [Google Scholar]
- 194.Dhere T, Copland I, Garcia M, et al. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn’s disease - a Phase 1 trial with three doses. Aliment Pharmacol Ther. 2016;44(5):471–481. doi: 10.1111/apt.13717 [DOI] [PubMed] [Google Scholar]
- 195.Knyazev OV, Parfenov AI, Konoplyannikov AG, Boldyreva ON. Мезенхимальные стволовые клетки в комбинированном лечении язвенного колита [Use of mesenchymal stem cells in the combination therapy of ulcerative colitis]. Ter Arkh. 2016;88(2):44–48. Russian. doi: 10.17116/terarkh201688244-48 [DOI] [PubMed] [Google Scholar]