Abstract
Objective:
The objective of this clinical practice guideline is to provide updated and new evidence-based recommendations for the comprehensive care of persons with diabetes mellitus to clinicians, diabetes-care teams, other health care professionals and stakeholders, and individuals with diabetes and their caregivers.
Methods:
The American Association of Clinical Endocrinology selected a task force of medical experts and staff who updated and assessed clinical questions and recommendations from the prior 2015 version of this guideline and conducted literature searches for relevant scientific papers published from January 1, 2015, through May 15, 2022. Selected studies from results of literature searches composed the evidence base to update 2015 recommendations as well as to develop new recommendations based on review of clinical evidence, current practice, expertise, and consensus, according to established American Association of Clinical Endocrinology protocol for guideline development.
Results:
This guideline includes 170 updated and new evidence-based clinical practice recommendations for the comprehensive care of persons with diabetes. Recommendations are divided into four sections: (1) screening, diagnosis, glycemic targets, and glycemic monitoring; (2) comorbidities and complications, including obesity and management with lifestyle, nutrition, and bariatric surgery, hypertension, dyslipidemia, retinopathy, neuropathy, diabetic kidney disease, and cardiovascular disease; (3) management of prediabetes, type 2 diabetes with antihyperglycemic pharmacotherapy and glycemic targets, type 1 diabetes with insulin therapy, hypoglycemia, hospitalized persons, and women with diabetes in pregnancy; (4) education and new topics regarding diabetes and infertility, nutritional supplements, secondary diabetes, social determinants of health, and virtual care, as well as updated recommendations on cancer risk, nonpharmacologic components of pediatric care plans, depression, education and team approach, occupational risk, role of sleep medicine, and vaccinations in persons with diabetes.
Conclusions:
This updated clinical practice guideline provides evidence-based recommendations to assist with person-centered, team-based clinical decision-making to improve the care of persons with diabetes mellitus.
Keywords: antihyperglycemic medications; atherosclerotic cardiovascular disease; cardiovascular diseases; diabetes; gestational; diabetes mellitus; diabetes mellitus, type 1; diabetes mellitus, type 2; diabetic nephropathies; diabetic neuropathies; diabetic retinopathy; dyslipidemias; guideline; hospitalization; hypertension; hypoglycemia; infertility; interdisciplinary communication; metabolic syndrome; obesity; occupations; prediabetic state; pregnancy; secondary diabetes; sleep apnea syndromes; telemedicine; vaccination
Lay Abstract
Advances in medications and tools to monitor blood sugar are helping persons with diabetes greatly improve control of their blood sugar levels, excess weight, high blood pressure, and quality of life. This American Association of Clinical Endocrinology guideline provides recommendations for the diagnosis and treatment of persons with prediabetes and diabetes and its prevention.
Care of persons with prediabetes and diabetes includes change in lifestyle with a focus on sleep, healthy eating, and exercise. Reaching goals for blood sugar, blood pressure, fats like cholesterol, and weight can prevent harm from diabetes to eyes, kidneys, heart, and nervous system. Many newer, safer drugs control blood sugar and reduce risk of heart and kidney disease. Some drugs also lower cholesterol and weight. Ways to check blood sugar levels with fingersticks or sensors placed under the skin (continuous glucose monitors) have improved, making it easier and safer for persons with diabetes to avoid both low and high blood sugars.
A team approach helps people best manage diabetes. The individual with diabetes is the center of the team and should help make decisions together with their doctors. In addition to doctors, the team may include educators, nurses, dietitians, pharmacists, foot doctors, psychologists, and other specialists.
This guideline addresses other topics of interest to those living with or at risk for diabetes such as health care visits by computer or phone, access to care, management of diabetes at work, sleep disorders, depression, infertility, risk of cancer, safety of nutritional supplements, and benefits of vaccines. Also included are specific care and treatment needs of pregnant women and those who are hospitalized.
The American Association of Clinical Endocrinology hopes that this guideline will improve the management of diabetes and benefit all who live with prediabetes or diabetes and their caregivers.
Introduction
This 2022 update of the American Association of Clinical Endocrinology (AACE) Clinical Practice Guideline: Developing a Comprehensive Diabetes Mellitus Care Plan includes revised and new recommendations for clinical practice based on evidence published since the previous edition of this clinical practice guideline (CPG) in 2015.1 This updated CPG provides evidence-based guidance to assist clinicians, diabetes-care teams, investigators, educators, and other health care professionals and stakeholders with decision-making in practice to improve prevention, diagnosis, and treatment of persons with diabetes mellitus (DM). Unless otherwise specified, persons with DM applies to adults.
The task force evaluated a vast pool of literature to revise, update, and create recommendations based on relevant new evidence of the highest quality that reflects advances in the diagnosis and management of DM with available new monitoring methods and therapies. Evidence from recent cardiovascular (CV) outcome trials (CVOTs); diabetic kidney disease (DKD), chronic kidney disease (CKD), and heart failure (HF) trials; and studies of antihyperglycemic therapy, diabetes technology, management of hypertension, neuropathy, hypoglycemia, obesity, obesity medications, and antihyperglycemic medications that also can produce significant weight reduction for the majority of those with DM who also are overweight have informed this guideline. Goals for treatment emphasize individualized targets for weight loss, glucose, lipids, and blood pressure (BP). In addition, this guideline promotes personalized management of DM with a focus on safety and advocates for a comprehensive approach to management of DM based on current evidence. Although glycemic control parameters such as hemoglobin A1c (A1C), postprandial glucose (PPG) excursions, fasting plasma glucose (FPG), continuous glucose monitoring (CGM) readings of time in/below/above range, and glycemic variability have an impact on risk of microvascular complications and CV disease (CVD), mortality, quality of life (QoL), and other factors also affect clinical outcomes in persons with DM. Therefore, in addition to glycemic control, recommendations consider micro- and macrovascular risk, including CV risk factors such as dyslipidemia, hypertension, and obesity.
Methods
The AACE CPG Oversight Committee confirmed the extent of new literature and the AACE Board of Directors approved development of this update of the 2015 AACE CPG to develop a comprehensive plan for the care of persons with DM in adherence to the 2017 AACE Protocol for Standardized Production of Clinical Practice Guidelines (Supplementary Tables 1–4).1,2 AACE followed a rigorous developmental process based on strict methodology to systematically collect, objectively evaluate, and clearly summarize available scientific literature to develop trustworthy recommendations for clinical practice regarding care of persons with DM.
A methodologist conducted comprehensive literature searches in PubMed using medical subject headings, field descriptions, and free-text terms to identify all possible studies that included human participants and were published in English between January 1, 2015, and May 15, 2022. Bibliographies of select articles were also reviewed to ensure inclusion of all possibly relevant studies. The literature searches, examination of reference lists from primary and review articles, and identification of online sources yielded an evidence pool of 11,606 discrete potential references, of which 1871 citations—1840 articles (including late-breaking/supplementary articles) and 31 web links—were included to support this guideline’s recommendations and background information.
At least 2 task force authors screened titles and abstracts of broad pools of evidence found in literature searches for each topic and submitted decisions to include or exclude each article along with rationale for exclusion. Disagreements about inclusion among reviewers were resolved by consensus with task force chairs and team leaders. Through this process, authors conducted a thorough appraisal of evidence based on the full scope of available literature to determine studies that best support each recommendation.
AACE methodologist and staff assigned evidence levels (ELs) 1 to 4 and study types to included studies according to established AACE evidence ratings (Supplementary Table 1). The task force considered the quality of each article in addition to ELs and study types to inform assigned grades for recommendations, which reflect the confidence and strength of evidence in aggregate (Supplementary Table 2 and 3). Recommendation qualifiers and subjective factors also informed the overall grade assigned to each recommendation (Supplementary Table 4). For some issues related to clinical practice and the care of persons with DM, there is little evidence of high quality available. Where the task force determined guidance to be necessary despite a lack of available supporting literature, a recommendation was developed based on expert opinion and consensus of task force authors’ collective experience, knowledge, and judgment. Therefore, although randomized controlled trials (RCTs) and meta-analyses of these trials (rated the highest EL 1) support many recommendations, derivative EL 4 publications that include other primary evidence (rated EL 1, EL 2, and EL 3) are sometimes also cited. This CPG is intended to complement other previously published AACE DM-related guidelines and consensus statements as well as other organizations’ DM-related guidance.
Questions related to clinical practice provide the framework for this guideline with answers in the form of recommendations. Task force authors revised prior questions where necessary and submitted contributions for new questions, which were integrated into the final document. This CPG includes 31 questions that cover the spectrum of DM management and 170 actionable clinical practice recommendations that provide brief, evidence-based answers to each question. Evidence bases summarize clinical context with a brief discussion of the best available scientific literature to support recommendations that answer corresponding questions. Although recommendations are concise and actionable, the evidence base for each specific topic provides additional information that explains the guidance for best clinical practice.
Table 1 lists all revised and new questions. Table 2 provides a summary of all questions and recommendations. Table 3 lists all tables, figures, and supplementary tables.
Table 1.
Summary of Questions
| Q = Question | |
|---|---|
| Section 1 | Screening, diagnosis, glycemic targets, glycemic monitoring |
| Q1 | How is the diagnosis of DM made and what is the current screening protocol for prediabetes and diabetes? |
| Q2 | What are the glycemic treatment goals for persons with DM? |
| Q3 | When and how should glucose monitoring be used? |
| Section 2 | Comorbidities and complications |
| Q4 | How should hypertension be managed in persons with DM? |
| Q5 | How should dyslipidemia be managed in persons with DM? |
| Q6 | How should DKD or CKD in DM be managed? |
| Q7 | How should retinopathy be managed in persons with DM? |
| Q8 | How should neuropathy be diagnosed and managed in persons with DM? |
| Q9 | How should antihyperglycemic agents be prioritized in persons with T2D at high risk for/or with established CVD? |
| Q10 | How should obesity be managed in persons with DM? |
| Section 3 | Management |
| Q11 | How should prediabetes be managed? |
| Q12 | How can glycemic targets be achieved in persons with T2D? |
| Q13 | How should insulin therapy be used for management of persons with T1D? |
| Q14 | How should hypoglycemia be managed? |
| Q15 | How should DM be managed in the hospital? |
| Q16 | How should DM in pregnancy be managed? |
| Section 4 | Education and other topics |
| Q17 | What education interventions have been shown to be most effective in management of persons with DM? |
| Q18 | What are the key nonpharmacological components of a comprehensive diabetes care plan in children and adolescents? |
| Q19.1 | Should persons with infertility be screened for DM? |
| Q19.2 | How should persons with preexisting diabetes mellitus and infertility be evaluated? |
| Q19.3 | Should men with DM and cardiometabolic disorders be assessed for hypogonadism? |
| Q20.1 | How should persons at risk for secondary diabetes be assessed? |
| Q20.2 | What are the best treatment strategies for management of secondary diabetes, such as posttransplant diabetes, cystic fibrosis‒related diabetes, and other forms of secondary diabetes? |
| Q21 | What is the role of sleep medicine in the care of persons with DM? |
| Q22 | Should screening for depression be a routine component of clinical assessment in persons with DM? |
| Q23 | Is the evaluation of SDOH in persons predisposed to or with DM useful in improving health outcomes? |
| Q24 | Is telehealth/virtual care an effective care-delivery model for the management of persons with DM? |
| Q25 | Which occupations have specific public safetyerelated diabetes management considerations? |
| Q26 | Is there a role for nutritional supplements in the management of DM and what might be the associated risks? |
| Q27 | How should potential increased cancer risk be managed in persons with obesity/T2D? |
| Q28 | Which vaccinations should be given to adults with DM? |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; DKD, diabetic kidney disease; DM, diabetes mellitus; SDOH, social determinants of health; T1D, type 1 diabetes, T2D, type 2 diabetes.
Table 2.
Summary of Recommendations
| Section 1. Screening, Diagnosis, Glycemic Targets, Glycemic Monitoring | |
|---|---|
|
| |
| Q 1: How is the diagnosis of diabetes mellitus made and what is the current screening protocol for prediabetes and diabetes? | |
| R 1.1 | The diagnosis of diabetes mellitus (DM) is based on the following criteria (Table 4): • Fasting plasma glucose (FPG) concentration ≥126 mg/dL (after ≥8 h of an overnight fast), or • Plasma glucose (PG) concentration ≥200 mg/dL 2 h after ingesting a 75-g oral glucose load after an overnight fast of at least 8 h, or • Symptoms of hyperglycemia (eg, polyuria, polydipsia, polyphagia) and a random (nonfasting) PG concentration ≥200 mg/dL, or • Hemoglobin A1c (A1C) level ≥6.5% Diagnosis of DM requires 2 abnormal test results, either from the same sample or two abnormal results on samples drawn on different days. However, a glucose level ≥200 mg/dL in the presence of symptoms for DM confirms the diagnosis of DM. Grade A; BEL 2 and expert opinion of task force |
| R 1.2 | Prediabetes is identified by the presence of impaired fasting glucose (IFG) (100 to 125 mg/dL), impaired glucose tolerance (IGT), which is a PG value of 140 to 199 mg/dL 2 h after ingesting 75 g of glucose, and/or A1C value between 5.7% and 6.4% (Table 4). A1C should be used only for screening for prediabetes. The diagnosis of prediabetes, which may manifest as either IFG or IGT, should be confirmed with glucose testing. Grade B; BEL 2 |
| R 1.3 | Type 1 diabetes (T1D) is characterized by marked insulin deficiency in the presence of hyperglycemia and positive autoantibody tests to glutamic acid decarboxylase (GAD65), pancreatic islet b cells (tyrosine phosphatase IA-2), and IA-2b zinc transporter (ZnT8), and/or insulin. The presence of immune markers and clinical presentation are needed to establish the correct diagnosis and to distinguish between T1D and type 2 diabetes (T2D) in children or adults, as well as to determine appropriate treatment. Grade A; BEL 2 |
| R 1.4 | T2D is characterized by progressive loss of b-cell insulin secretion and variable defects in insulin sensitivity. T2D is often asymptomatic and can remain undiagnosed for many years; therefore, all adults ≥35 y of age with risk factors should be screened for DM (Table 5). Grade A; BEL 1 |
| R 1.5 | Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance that begins or is first recognized during pregnancy and resolves postpartum. Pregnant women with risk factors for DM should be screened at the first prenatal visit for undiagnosed T2D using standard criteria (Table 4). Grade B; BEL 1 |
| R 1.6 | Screen all pregnant women for GDM at 24 to 28 weeks' gestation. Diagnose GDM with either the one-step or the two-step approach. • The one-step approach uses a 2-h 75-g oral glucose tolerance test (OGTT) after ≥8 h of fasting with diagnostic cutoffs of one or more FPG ≥92 mg/dL, 1-h PG ≥180 mg/dL, or 2-h PG ≥153 mg/dL. • The two-step approach uses a nonfasting 1-h 50-g glucose challenge test with 1-h PG screening threshold of 130 or 140 mg/dL. For women with a positive screening test, the 3-h 100-g OGTT is used for diagnosis with 2 or more PG tests that meet the following thresholds: FPG ≥95 mg/dL, 1-h ≥180 mg/dL, 2-h ≥155 mg/dL, 3-h ≥140 mg/dL. Grade A; BEL 1 |
| R 1.7 | Clinicians should consider evaluation for monogenic DM in any child or young adult with an atypical presentation, clinical course, or response to therapy. Monogenic DM includes neonatal diabetes and nonautoimmune diabetes of multiple genetic causes, also known as maturity-onset diabetes of the young. Most children with DM occurring under age 6 mo of age have a monogenic cause as autoimmune T1D rarely occurs before 6 mo of age. Other monogenic forms of diabetes are characterized by mutation of genes of transcription factors, genes regulating pancreatic development or atrophy, abnormal insulin genes, genes related to endoplasmic reticulum stress that impair insulin secretion or abnormal glucokinase genes that cause impaired insulin signaling. Grade B; BEL 2 |
|
Q 2: What are the glycemic treatment goals for persons with diabetes mellitus? | |
|
2.1 Outpatient Glucose Targets for Nonpregnant Adults
| |
| R 2.1.1 | An A1C level of ≤6.5% is recommended for most nonpregnant adults, if it can be achieved safely. To achieve this target A1C level, FPG may need to be <110 mg/dL, and the 2-h postprandial glucose (PPG) may need to be <140 mg/dL (Table 6). Glucose targets should be individualized with consideration for life expectancy, disease duration, presence or absence of micro- and macrovascular complications, cardiovascular disease (CVD) risk factors, comorbid conditions, and risk for hypoglycemia, as well as a person’s cognitive and psychological status. Grade A; BEL 1 |
| R 2.1.2 | Adopt less stringent glycemic goals (A1C 7% to 8%) in persons with a history of severe hypoglycemia, hypoglycemia unawareness, limited life expectancy, advanced renal disease, extensive comorbid conditions, or long-standing DM in which the A1C goal has been difficult to attain despite intensive efforts, so long as the person remains free of hyperglycemia-associated symptoms. Grade A; BEL 1 |
|
2.2 Inpatient Glucose Targets for Nonpregnant Adults | |
| R 2.2 | For most hospitalized persons with hyperglycemia in both the intensive care unit (ICU) and non-ICU settings, a glucose range of 140 to 180 mg/dL is recommended, provided this target can be safely achieved (Table 6). Grade A; BEL 1 |
|
2.3 Outpatient Glucose Targets for Pregnant Women | |
| R 2.3 | In women with GDM, the following glucose goals are recommended: fasting and preprandial glucose concentration ≤95 mg/dL and either a 1-h postmeal glucose value ≤140 mg/dL or a 2-h postmeal glucose value ≤120 mg/dL. In women with preexisting T1D or T2D who become pregnant, it is recommended that glucose be controlled to meet the following goals, but only if the goals can be safely achieved: premeal, bedtime, and overnight glucose values between 60 and 95 mg/dL; a 1-h PPG value between 110 and 140 mg/dL; a 2-h glucose 100 to 120 mg/dL. A secondary target would be an A1C level of <6% if it can be accomplished without significant hypoglycemia. Grade A; BEL 1 |
|
Q 3: When and how should glucose monitoring be used? | |
| R 3.1 | A1C should be measured at least semiannually in all persons with DM and at least quarterly in persons not at their glycemic target. Grade B; BEL 2 |
| R 3.2 | All persons who use insulin should use continuous glucose monitoring (CGM) or perform blood glucose monitoring (BGM) a minimum of twice daily and ideally before any insulin injection. More frequent BGM may be needed by persons who are taking multiple daily injections (MDI) injections, persons not at A1C targets, or those with history of hypoglycemia. Persons who do not require insulin or insulin secretagogue therapy may often benefit from BGM, especially to provide feedback about the effects of their lifestyle choices (diet and physical activity), and to assess response to pharmacologic therapy. Grade A; BEL 1 |
| R 3.3 | Real-time continuous glucose monitoring (rtCGM) or intermittently scanned continuous glucose monitoring (isCGM) is recommended for all persons with T1D, regardless of insulin delivery system, to improve A1C levels and to reduce the risk for hypoglycemia and DKA (see Fig. 6). Grade A; BEL 1 |
| R 3.4 | rtCGM or isCGM is recommended for persons with T2D who are treated with insulin therapy, or who have high risk for hypoglycemia and/or with hypoglycemia unawareness (see Figure 6). Grade A; BEL 1 |
|
Section 2. Comorbidities and Complications | |
|
Q 4: How should hypertension be managed in persons with diabetes mellitus?
| |
| R 4.1 | The recommended blood pressure (BP) goal for most persons with T1D, T2D, or prediabetes is <130/80 mm Hg (Table 7). Grade A; BEL 1 |
| R 4.2 | Therapeutic lifestyle interventions in persons with hypertension are recommended to include consultation with a registered dietitian for education about an overall healthy diet (such as the Mediterranean diet), weight management, reduced sodium intake (such as the Dietary Approaches to Stop Hypertension [DASH] diet), daily physical activity and regular exercise (several times a week), and as-needed consultation with a psychologist or certified diabetes care and education specialist (CDCES) to support long-term behavior change. (See also R 11.2 to R 11.4 and R 12.1.1 to R 12.1.5 on nutrition and lifestyle.) Grade A; BEL 1 |
| R 4.3 | If BP goals are unattained with therapeutic lifestyle changes, use antihypertensive pharmacotherapy to achieve individual BP treatment goals. Grade A; BEL 1 |
| R 4.4 | Select antihypertensive agents based on their ability to reduce BP to goal and prevent or slow the progression of micro- and macrovascular disease. Use either an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) for BP control and to delay the progression of DKD or chronic kidney disease (CKD) in DM (see also R 6.1 to R 6.6 on DKD or CKD in DM). Grade A; BEL 1 |
| R 4.5 | Intensify pharmacotherapy as needed to achieve BP goals. Antihypertensive therapy may include combinations of either an ACE inhibitor or an ARB plus any of the following agents: diuretics, calcium channel antagonists, combined alpha-beta blockers, and newer-generation beta blockers. Consider a mineralocorticoid receptor antagonist for resistant hypertension. Grade A; BEL 1 |
|
Q 5: How should dyslipidemia be managed in persons with diabetes mellitus? | |
| R 5.1 | All persons with prediabetes, T1D over the age of 40, or T2D should have a lipid panel (fasting or nonfasting) checked at diagnosis and annually to assess cardiovascular (CV) and metabolic disease risks, and at additional intervals as needed to monitor treatment to achieve lipid goals. Grade B; BEL 2 |
| R 5.2 | Therapeutic lifestyle interventions for dyslipidemia are recommended for all persons with prediabetes, T1D over the age of 40, or T2D, to include education with a registered dietitian about a healthy diet with emphasis on weight management, daily physical activity, and regular exercise (several times a week). Consultation with a psychologist or CDCES is recommended to support long-term behavior change. Grade A; BEL 1 |
| R 5.3 | Persons with prediabetes or T2D without atherosclerotic cardiovascular disease (ASCVD) and with less than 2 traditional risk factors should be assessed with the aid of ASCVD risk calculators to determine initiation and intensity of lipid-lowering therapy (Fig. 1 and Table 8). Grade A; BEL 1 |
| R 5.4 | Assess nontraditional ASCVD risk factors (Fig. 1) beyond a lipid panel to guide management when the initial shared decision is not self-evident. Grade B; BEL 2 |
| R 5.5 | Manage persons with prediabetes and persons with T1D over the age of 40 in the same manner as those with T2D. Grade A; BEL 1 |
| R 5.6 | In persons with high ASCVD risk, use a moderate-intensity statin regardless of DM type or status. In persons with very high ASCVD risk (T2D with 2 or more additional traditional ASCVD risk factors such as advancing age, hypertension, chronic kidney disease (CKD) stage 3a, cigarette smoking, family history of premature ASCVD in men <55 y and women <65 y, low high-density lipoprotein cholesterol (HDL-C), or high non-HDL-C), use a high-intensity statin regardless of baseline low-density lipoprotein cholesterol (LDL-C) level. For persons at extreme risk of ASCVD event (current ASCVD or target organ damage), use a high-intensity statin plus other therapies as needed to achieve lipid targets (Fig. 1 and Table 10). Grade A; BEL 1 |
| R 5.7 | Treatment targets for persons in a high ASCVD risk category are LDL-C <100 mg/dL, apolipoprotein B (apo B) <90 mg/dL, and non-HDL-C <130 mg/dL. Treatment targets for persons in a very high risk ASCVD category are LDL-C <70 mg/dL, apo B <80 mg/dL, and non-HDL-C <100 mg/dL. Treatment targets for persons with extreme risk of ASCVD include LDL-C <55 mg/dL, apo B <70 mg/dL, and non-HDL-C <90 mg/dL (Table 9 and Fig. 1). Grade A; BEL 1 |
| R 5.8 | Statins are recommended for the initial treatment of hypercholesterolemia. Monitor efficacy every 6 to 12 wk and increase the dose or intensity of statin as needed and tolerated to achieve LDL-C, apo B, and/or non-HDL-C goals based on individual ASCVD risk. Once lipid targets are achieved, lipid panel or apo B can be monitored less often (Fig. 1). Grade A; BEL 1 |
| R 5.9 | Combine the cholesterol absorption inhibitor ezetimibe with statin therapy when the desired lipid targets are not achieved with a maximally tolerated statin dose. If lipid targets are not achieved on this combination, add or substitute a proprotein convertase subtilisin/kexin type 9-lowering agent. Alternatively, add bempedoic acid to the maximally tolerated statin or consider adding icosapent ethyl (in persons with triglycerides 135 to 499 mg/dL) for ASCVD risk reduction. Grade A; BEL 1 |
| R 5.10 | Management of hypertriglyceridemia in persons with high ASCVD risk or very high ASCVD risk should begin with intensive lifestyle modification and statin therapy. In persons treated with a maximally tolerated statin who have triglyceride concentrations ≥200 mg/dL and HDL-C <40 mg/dL, add a fibrate or high-dose omega-3 fatty acid to achieve the desired apo B or non-HDL-C goal. Icosapent ethyl can be considered in persons with high or very high ASCVD risk (Fig. 2). Grade A; BEL 1 |
|
Q 6: How should DKD or CKD in DM be managed? | |
| R 6.1 | Annual assessment of serum creatinine to determine the estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio is recommended to identify, stage, and monitor progression of DKD, also referred to as CKD in DM. Begin annual DKD assessment 5 y after diagnosis in persons with T1D or at diagnosis in persons with T2D. Grade B; BEL 2 |
| R 6.2 | Advise persons with CKD in DM about optimal glycemic control, BP control, lipid control, and smoking cessation to reduce risks of development and progression of CKD and CVD. (See also R 4.1 to R 4.5 on BP control, R 5.1 to R 5.10 on lipid management, and R 12.1.1 to R 12.2.19 on glycemic control.) Grade A; BEL 1 |
| R 6.3 | Renin-angiotensin-aldosterone system blockade with an ARB or an ACE inhibitor is recommended for persons with albuminuria (T1D or T2D) to reduce risk of DKD or CKD in DM progression (see Fig. 3 for category definitions). Grade A; BEL 1 |
| R 6.4 | A sodium glucose cotransporter 2 inhibitor (SGLT2i) with proven benefit is recommended as foundational therapy for persons with T2D and CKD with eGFR ≥20 mL/min/1.73 m2 to reduce progression of CKD and risk of CVD. Grade A; BEL 1 |
| R 6.5 | A glucagon-like peptide-1 receptor agonist (GLP-1 RA) with proven benefit is recommended for persons with T2D and DKD or CKD in DM with eGFR ≥15 mL/min/1.73 m2 for glycemic control and to reduce risk of ASCVD and progression of albuminuria. Grade A; BEL 1 |
| R 6.6 | A non-steroidal mineralocorticoid receptor antagonist (finerenone) with proven kidney and CVD benefit is recommended for persons with T2D, an eGFR ≥25 mL/min/1.73 m2, normal serum potassium concentration, and albuminuria (ACR ≥30 mg/g) despite a maximum tolerated dose of a renin-angiotensin-system inhibitor. Grade A; BEL 1 |
|
Q 7: How should retinopathy be managed in persons with diabetes mellitus? | |
| R 7.1 | It is recommended that persons with T2D or adult-onset T1D should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist at the time of diagnosis or shortly after diagnosis. Individualized subsequent screening can be based on type and duration of DM, A1C or mean blood glucose (BG), BP, and the presence and grade of retinopathy. Grade A; BEL 2 and expert opinion of task force |
| R 7.2 | In persons with T1D, an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist should be performed within 5 y of diagnosis in children and adolescents. Grade B; BEL 4 and expert opinion of task force |
| R 7.3 | Women who are pregnant and have preexisting T1D or T2D should be monitored with eye examinations every trimester during pregnancy and in the postpartum period as determined by the severity of retinopathy during pregnancy. Grade B; BEL 2 |
| R 7.4 | Persons with greater than mild nonproliferative retinopathy should have examinations at least once a year and more frequently as advised by their eyecare specialist. Grade B; BEL 4 and expert opinion of task force |
| R 7.5 | Follow-up with eyecare specialists typically should occur on an annual basis, but persons with T1D or T2D who have had a normal ocular examination may be screened every 2 to 3 y. Grade B; BEL 2 and expert opinion of task force |
| R 7.6 | Optimal glucose, BP, weight, and lipid control should be implemented to slow the progression of retinopathy. Grade B; BEL 1 |
| R 7.7 | Artificial intelligence systems, authorized by the US Food and Drug Administration (FDA) for detecting greater than mild diabetic retinopathy, can be used as an alternative to traditional screening approaches. These systems can facilitate diagnosis of vision-threatening retinopathy and identification of persons who require ophthalmologic visits for treatment. Grade B; BEL 1 |
|
Q 8: How should neuropathy be diagnosed and managed in persons with diabetes mellitus? | |
| R 8.1 | Diabetic peripheral neuropathy (DPN) is a clinical diagnosis. A comprehensive differential diagnosis should be considered to rule out nondiabetic neuropathies. Grade B; BEL 2 |
| R 8.2 | Screening for DPN should be done at diagnosis of T2D, within 5 y of the diagnosis of T1D, and subsequently annually or whenever symptoms occur, by performing a clinical history and physical exam. Grade B; BEL 2 |
| R 8.3 | Assessments for DPN should include a careful history to assess target symptoms, and a combination of at least two of the following: vibration sensation using a 128-Hz tuning fork, pinprick sensation, temperature discrimination, 10-g monofilament testing on the dorsal aspect of the great toe bilaterally, and ankle reflexes. All these assessments should follow the typical DPN pattern, starting distally (the dorsal aspect of the hallux) on both sides and move proximally until a sensory threshold is identified. Grade A; BEL 2, upgraded by expert opinion of task force |
| R 8.4 | Screening for cardiovascular autonomic neuropathy (CAN) should be considered at diagnosis of T2D and at 5 y after the diagnosis of T1D, including youth. Screening for CAN should also be considered in the presence of DPN, DKD, 2 or more CV risk factors, hypoglycemia unawareness, high glucose variability, in persons with heart failure (HF), perioperatively, or in individuals presenting with autonomic symptoms. A careful differential to exclude other comorbidities or drug effects/interactions that could mimic CAN should be performed. Grade B; BEL 2 |
| R 8.5 | CV reflex tests (deep breathing, Valsalva, supine to standing) remain the gold standard and are recommended for assessment of CAN. Indices of heart rate variability derived from electrocardiogram recordings could also be used as an easier alternative for screening for CAN. Grade A; BEL 2, upgraded by expert opinion of task force |
| R 8.6 | Diabetic foot exams should be performed at every visit (in person or virtual) to identify deformities and to identify those at risk for late complications such as ulcerations and amputations. Grade A; BEL 1 |
| R 8.7 | Intensive glucose control applied as early as possible is recommended to prevent the onset of DPN and CAN in T1D. Achieving optimal control of glucose, BP, and lipid levels along with lifestyle interventions, including weight loss and exercise, are recommended to prevent DPN and CAN in T2D. Lifestyle interventions are effective for DPN and CAN prevention in persons with prediabetes/metabolic syndrome. Grade A; BEL 2, upgraded by expert opinion of task force |
| R 8.8 | Pregabalin, duloxetine, and capsaicin 8% patch are recommended for the treatment of neuropathic pain due to DM and have received regulatory approval in the United States. Current evidence shows that these agents are effective in reaching 30% to 50% reduction in pain in many individuals (Grade A; BEL 1). However, gabapentin and some tricyclic antidepressants may be as effective to achieve a clinically meaningful reduction in diabetic neuropathic pain (Grade B; BEL 1). Combining two or more agents from different classes may have enhanced benefits with lower adverse effects and risks than maximizing the dose of one medication or using opioids. The use of opioids, including tapentadol or tramadol, is NOT RECOMMENDED due to high risk of addiction and other complications. Grade A; BEL 1 |
| R 8.9 | Lifestyle interventions including a combination of regular aerobic, strengthening, and balance exercises, reduction of sedentary behavior, and dietary modification aimed at reducing calorie intake and increasing plant-based and polyunsaturated fats are recommended. Neuromodulatory techniques such as high-frequency spinal cord stimulation and combining pharmacological with nonpharmacological approaches should be considered in those with refractory painful DPN. Grade B; BEL 1 |
|
Q 9: How should antihyperglycemic agents be prioritized in persons with type 2 diabetes at high risk for or with established cardiovascular disease? | |
| R 9.1 | In persons with T2D and established ASCVD or at high risk for ASCVD, use GLP-1 RAs with proven CV benefits to reduce the risk of myocardial infarction, stroke, or CV death regardless of other glucose-lowering or CV therapies and independent of A1C. Grade A; BEL 1 |
| R 9.2 | In persons with T2D and established ASCVD or very high ASCVD risk, use SGLT2is with proven CV benefits to reduce the risk of hospitalization for HF, major adverse CV events, or CV death regardless of background glucose-lowering therapy, cardiovascular therapy, or A1C. Grade A; BEL 1 |
| R 9.3 | In persons with T2D and established HF (regardless of ejection fraction, background glucose-lowering or HF therapies, or A1C), use SGLT2is with proven HF benefits to reduce the risk of hospitalization for HF or CV death, and to improve HFerelated symptoms. Grade A; BEL 1 |
| R 9.4 | In persons with T2D and ASCVD or at high risk for ASCVD, use GLP-1 RAs with proven benefit for reduction in the risk of stroke. In persons with insulin resistance, prediabetes, or T2D and a prior transient ischemic attack or stroke, pioglitazone should be considered to reduce the risk of recurrent stroke. Grade A; BEL 1 |
|
Q 10: How should obesity be managed in persons with diabetes mellitus? | |
| R 10.1 | Persons with prediabetes, T1D or T2D, and obesity/adiposity-based chronic disease (ABCD) have 2 diseases, and each should be treated effectively with the goal of optimizing their respective outcomes. Grade B; BEL 2 and expert opinion of task force |
| R 10.2 | The diagnosis and evaluation of ABCD in persons with prediabetes, T1D, or T2D should include both anthropometric and clinical components. The anthropometric evaluation should include body mass index (BMI), confirmed by physical examination that excludes excess muscle mass, edema, or sarcopenia. Waist circumference (WC) should be measured as a marker of cardiometabolic disease (CMD) risk. Grade B; BEL 2 and expert opinion of task force |
| R 10.3 | For most adults, BMI values that indicate excess body weight are 25 to 29.9 kg/m2 for overweight and ≥30 kg/m2 for obesity, and WC threshold values ≥102 cm for men and ≥88 cm for women. Grade B; BEL 4 and expert opinion of task force |
| R 10.4 | The clinical evaluation of persons with both prediabetes, T1D, or T2D and ABCD should assess the presence and severity of weight-related complications including cardiometabolic complications such as dyslipidemia, hypertension, nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH), CVD, HF, and CKD; biomechanical complications such as obstructive sleep apnea (OSA), osteoarthritis, gastroesophageal reflux disease, and urinary incontinence; abnormalities involving sex steroids, such as infertility, polycystic ovary syndrome, and hypogonadism; as well as impact on psychological disorders and quality of life (QoL). Grade B; BEL 2 and expert opinion of task force |
| R 10.5 | Persons with T2D and ABCD should be treated with weight-loss interventions which will both improve glycemic control and prevent or treat ABCD complications. The target for weight loss should be >5% to ≥10% of baseline body weight. Grade A; BEL 1 |
| R 10.6 | Persons with T2D and ABCD should be instructed and supported in therapeutic lifestyle interventions that include a reduced-calorie healthy diet generally designed to produce a ≥500 kilocalorie daily energy deficit, daily physical activity, regular exercise (several times a week), and behavioral health practices. Grade A; BEL 1 |
| R 10.7 | The Mediterranean, low-fat, low-carbohydrate, very lowecarbohydrate, vegetarian, vegan, and DASH diets are recommended, safe, and effective for short-term (1–2 y) weight loss, though evidence of long-term risk reduction for CVD events and mortality exists only for the Mediterranean diet. Grade A; BEL 1 |
| R 10.8 | Persons with T2D and obesity/ABCD with BMI ≥27 kg/m2 should be treated with DM medications associated with weight loss (GLP-1 RAs, SGLT2is). In addition, for persons with prediabetes, T1D, or T2D who have obesity/ABCD, consider FDAeapproved weight-loss medications as an adjunct to lifestyle intervention to achieve lowering of A1C, reduction of CVD risk factors, treatment or prevention of other ABCD complications, and improvement in QoL. Grade A; BEL 1 |
| R 10.9 | Persons with a BMI ≥35 kg/m2 and one or more severe obesity-related complications remediable by weight loss, including T2D, high risk for T2D (insulin resistance, prediabetes, and/or metabolic syndrome), poorly controlled hypertension, NAFLD/NASH, OSA, osteoarthritis of the knee or hip, and urinary stress incontinence, should be considered for a bariatric procedure (668). Grade C; BEL 3 |
| R 10.10 | Persons with BMI 30 to 34.9 kg/m2 and T2D with inadequate glycemic control despite optimal lifestyle and medical therapy should be considered for a bariatric procedure (668). Grade B; BEL 2 |
|
Section 3. Management | |
|
Q 11: How should prediabetes be managed?
| |
| R 11.1 | Prediabetes is a metabolic and vascular disorder, and clinicians should actively treat people with prediabetes in order to prevent or at least delay progression to T2D and development of CVD complications. Grade A; BEL 1 |
| R 11.2 | In persons with prediabetes and/or metabolic syndrome or identified to be at high risk of T2D based on validated risk-staging instruments, the prevention of T2D can be addressed by lifestyle modifications that include a healthy meal plan, regular physical activity, and behavioral health practices and weight loss in persons with ABCD. The Mediterranean diet should be considered to reduce progression to T2D and risk of CVD. Low-fat, vegetarian, and DASH meal patterns can also be considered for prevention of T2D. Grade A, BEL 1 |
| R 11.3 | Clinicians should manage and monitor CVD risk factors in prediabetes and metabolic syndrome, including elevated BP, dyslipidemia, and excessive weight, with the same targets as for a person with T2D. Grade B; BEL 2 |
| R 11.4 | Lifestyle intervention should include aerobic and resistance physical activity in all persons with prediabetes and/or metabolic syndrome. The initial aerobic prescription may require a progressive increase in the volume and intensity of exercise, and the ultimate goal should be ≥150 min/week of moderate exercise performed during 3 to 5 sessions per week (Grade A; BEL 1). Resistance exercise should consist of single-set exercises that use the major muscle groups 2 to 3 times per week (Grade A; BEL 1). An increase in nonexercise and active leisure activity should be encouraged to reduce sedentary behavior (Grade B; BEL 2). |
| R 11.5 | Obesity medications, namely phentermine/topiramate ER, liraglutide 3 mg, or weekly semaglutide 2.4 mg, in conjunction with lifestyle therapy should be considered in persons with prediabetes and/or metabolic syndrome with ABCD, whether overweight (BMI 27 to 29.9 kg/m2) or with obesity (BMI ≥30 kg/m2), when needed to achieve and sustain 7% to 10% weight loss for prevention of T2D. Grade A; BEL 1 |
| R 11.6 | Although no medications have been approved for the treatment of prediabetes, diabetes medications including metformin, acarbose, pioglitazone, or GLP-1 RA can be considered in persons with prediabetes or in persons who also have ABCD and remain glucose-intolerant following weight loss using lifestyle and/or weight-loss medications. Grade A; BEL 1 |
|
Q 12: How can glycemic targets be achieved in persons with type 2 diabetes? | |
|
12.1 Therapeutic Lifestyle Changes
| |
| R 12.1.1 | All persons with prediabetes or DM should be prescribed, instructed, and supported in lifestyle interventions that include a healthy meal plan, regular physical activity, and healthful behavior practices. Individualized medical nutrition therapy (MNT) should be provided at the time of diagnosis (with intermittent re-education as needed during continued care) via evaluation and counseling by a trained registered dietitian, certified nutritionist, or a clinician knowledgeable in nutrition. Grade A, BEL 1 |
| R 12.1.2 | MNT should consider the overall treatment plan including medications, DM complications, physical activity, body weight goals, and avoidance of hypoglycemia, as well as personal and cultural preferences, health literacy and numeracy, psychological factors, readiness for change, social determinants of health (SDOH), and support systems. For people on insulin therapy, insulin dosage adjustments should match carbohydrate intake (eg, with use of carbohydrate counting). Grade A; BEL 1 |
| R 12.1.3 | The meal plan should contribute to therapeutic goals for control of glycemia, BP, lipids, CVD risk factors, and the prevention of DM complications. In selecting optimal meal patterns, certain Mediterranean diets should be considered which, over the long term, can protect against CVD events and premature mortality. Although there is a lack of long-term studies addressing CVD outcomes, multiple other meal plans have been shown to be safe and can achieve short-term benefits (1–2 y) regarding glycemia, BP, lipids, and CVD risk factors. These meal plans include low-fat, low-carbohydrate, very low-carbohydrate, vegetarian, vegan, and DASH diets. Grade A, BEL 1 |
| R 12.1.4 | Given the variety of meal plans demonstrated to be beneficial in management of DM, nutritional recommendations should consider personal and cultural dietary preferences. Until there is conclusive evidence comparing the benefits of different meal patterns and the availability of long-term safety data, health care professionals should emphasize foods and nutrients that contribute to high “diet quality” scores as assessed by the Healthy Eating Index (HEI); high HEI is associated with reduced risks of DM, CVD, and mortality and includes fruits, nonstarchy vegetables, whole grains, nuts, legumes, and fish, with limited consumption of added sugars, refined grains, red meat, and processed meats. Grade B; BEL 1 |
| R 12.1.5 | Lifestyle intervention in persons with DM should include an individualized prescription for physical activity involving aerobic and resistance exercise and reduction in sedentary behavior. The initial prescription for aerobic physical activity may require a progressive increase in the volume and intensity of exercise, and the ultimate goal should be ≥150 min/week of moderate exercise performed during 3 to 5 sessions per week. (Grade A; BEL 1). Moderate exercise is considered to be activity that achieves a heart rate that is 50% to 60% higher than one’s basal heart rate. The physical activity prescription also should include resistance exercise that use the major muscle groups 2 to 3 times per week (Grade A; BEL 1). Individuals should also incorporate flexibility and range-of-motion training. An increase in nonexercise and/or active leisure activity should be encouraged to reduce sedentary behavior (Grade A; BEL 1). |
|
12.2 Antihyperglycemic Pharmacotherapy for Persons with Type 2 Diabetes | |
| R 12.2.1 | Individualized pharmacotherapy for persons with T2D should be prescribed based on evidence for benefit that includes glucose lowering, avoidance of hypoglycemia and weight gain, and reduction of cardio-renal risk. Grade A; BEL 1 |
| R 12.2.2 | Persons with T2D and their health care professionals should use patient-centered shared decision-making to agree on therapy targets and treatments as well as a regimen for glucose monitoring (i.e., BGM, structured BGM, or CGM). Grade B; BEL 2 |
| R 12.2.3 | Glycemic targets include A1C, BGM, and, for those using CGM, achievement of CGM targets such as time in range (TIR), percentage in low and very low range, time above range, and glycemic variability (Table 6). Nonglycemic targets include avoidance of hypoglycemia, control of BP, lipids, other CVD risk factors, and achieving and maintaining a healthy body weight. Grade B; BEL 4 |
| R 12.2.4 | Independent of glycemic control, targets, or treatment, if there is established or high risk for ASCVD, HF, and/or CKD, clinicians should prescribe a GLP-1 RA or an SGLT2i with proven efficacy for the specific condition(s) of the person with T2D being treated (see also R 6.1 to R 6.6 on DKD or CKD in DM and R 9.1 to R 9.4 on ASCVD and HF). Grade A; BEL 1 |
| R 12.2.5 | DM therapy should be individualized based on level of glycemia and the presence of comorbidities, complications, and access. Metformin is often the preferred initial therapy. Other agents may be appropriate as first line or in addition to metformin to reduce BG and/or to address specific comorbidities (such as ASCVD, HF, CKD, obesity, NAFLD), independent of glucose-lowering effects. Grade A; BEL 1 |
| R 12.2.6 | For some recently diagnosed individuals with T2D and more severe hyperglycemia (A1C ≥7.5%), unlikely to attain the A1C target with a single agent, early combination pharmacotherapy should be considered, usually to include metformin plus another agent that does not cause hypoglycemia, especially a GLP-1 RA, SGLT2i, or dipeptidyl peptidase 4 (DPP-4) inhibitor. Grade A; BEL 1 |
| R 12.2.7 | For newly diagnosed persons with T2D and an entry A1C >9.0% and/or ≥1.5% above target, one should initiate, along with lifestyle modifications, dual- or possibly triple-combination pharmacotherapy usually including metformin. Basal insulin along with noninsulin therapy is recommended if there are significant signs or symptoms of hyperglycemia, especially including catabolism (eg, weight loss) or a very high A1C >10% (86 mmol/mol) or BG levels (≥300 mg/dL [16.7 mmol/L]). Grade A; BEL 1 |
| R 12.2.8 | Clinicians should discuss with persons with T2D the likelihood that most persons with T2D ultimately require a combination of multiple complementary antihyperglycemic agents, in addition to lifestyle interventions, to attain and maintain optimal glycemic control. Grade B; BEL 2 |
| R 12.2.9 | The DM care team should assess medication adherence and safety and glycemic control in persons with T2D quarterly or more frequently as needed. Subsequent visits will depend upon the metabolic targets achieved and the stability of metabolic control. Grade D; BEL 4 |
| R 12.2.10 | Persons with T2D who start on metformin should continue it unless intolerance or contraindications occur. When intensification of antihyperglycemic treatment is needed, other agents should be added to metformin. Grade B; BEL 2 |
| R 12.2.11 | Most persons with T2D who require intensification of antihyperglycemic therapy with a GLP-1 RA or insulin should initially be prescribed a GLP-1 RA. If further intensification is required, one should prescribe a basal insulin or a switch to a fixed-ratio combination of a basal insulin and a GLP-1 RA (insulin glargine U100 + lixisenatide [GlarLixi] or insulin degludec + liraglutide [IdegLira]). Grade A BEL 1 |
| R 12.2.12 | Insulin should be prescribed for persons with T2D when noninsulin antihyperglycemic therapy fails to achieve target glycemic control or when a person has symptomatic hyperglycemia. Grade A; BEL 1 |
| R 12.2.13 | Long-acting basal insulin analogs are the recommended initial choice of insulin therapy for persons with T2D. The insulin analogs glargine (U100 or U300), degludec (U100 or U200), or detemir are preferred over intermediate-acting Neutral Protamine Hagedorn (NPH) insulin because analog insulins have demonstrated less hypoglycemia in some studies. Glargine U300 and degludec can be associated with less hypoglycemia than glargine U100 or detemir. Grade A; BEL 1 |
| R 12.2.14 | Many persons with T2D receiving basal insulin and not at goal A1C can have significantly improved glycemia by the addition of a GLP-1 RA or being switched to a fixed-ratio combination basal insulineGLP-1 RA (GlarLixi or IdegLira). One of these changes should be considered before adding a meal-time insulin for postprandial glycemic control. Grade A; BEL 1 |
| R 12.2.15 | When control of postprandial hyperglycemia is needed and a basal insulin and a GLP-1 RA are already being used, preference should be given to rapid-acting insulins (the analogs lispro, aspart, and glulisine or the rapid-acting inhaled human insulin powder) over regular human insulin (see Table 18). The former have a more consistent and a more rapid onset and offset of action with less risk of hypoglycemia. Grade A; BEL 1 |
| R 12.2.16 | Ultra-rapid-acting insulins (faster-acting insulin aspart, lispro aabc, and [human insulin] inhalation powder) may allow a decrease in the time between insulin administration and food intake and reduce the postprandial peak of PG as compared with rapid-acting insulins. The significance of this on long-term complications is unknown. Grade A; BEL 1 |
| R 12.2.17 | Basal-bolus insulin regimens or continuous subcutaneous insulin infusion (CSII) (ie, insulin pump) allow for adjustment of insulin doses according to carbohydrate intake and activity levels and are recommended for intensive insulin therapy in persons with T2D. Grade C; BEL 1 |
| R 12.2.18 | Premixed insulin formulations (fixed combinations of shorter- and longer-acting components) of human or analog insulin may be considered for persons with T2D who have consistent dietary and exercise patterns and in whom adherence to more intensive insulin regimens is problematic. However, these preparations have reduced dosage flexibility and may increase the risk of hypoglycemia compared with basal insulin or basal-bolus regimens. Grade A; BEL 1 |
| R 12.2.19 | In persons with T2D who are treated with basal-bolus insulin therapy, adding a GLP-1 RA, or switching to a fixed-ratio combination of a GLP-1 RA and a basal insulin, or adding an SGLT2i or pramlintide (less commonly used) may be able to reduce postprandial hyperglycemia, A1C, and weight. GLP-1 RAs may also allow reduction or discontinuation of bolus insulin in some individuals. Grade A; BEL 1 |
|
Q 13: How should insulin therapy be used for management of persons with type 1 diabetes? | |
| R 13.1 | Insulin must be used to treat all persons with T1D. Grade A; BEL 1 |
| R 13.2 | Physiologic insulin replacement regimens, which provide both basal and prandial (meal-related or bolus) insulin, are recommended for most persons with T1D. Grade A; BEL 1 |
| R 13.3 | Achievement of glucose targets using either MDI of insulin or CSII, is needed to prevent development of life-threatening crises, such as acute hyperglycemic crises (DKA and hyperglycemic hyperosmolar state) and catabolic state. Grade A; BEL 1 |
| R 13.4 | A multi-component self-management DM education program is recommended for persons with T1D. Ideally, this is provided by a professional with expertise (ie, CDCES) in the topics of healthy lifestyle, insulin technique including prandial insulin dosing guided by carbohydrate counting and diet adjustments for special situations, such as physical activity and prolonged fasting. Instruction is also needed in how to deal with sick days and prevention of DKA and hypoglycemia, and other relevant issues. Due to changes in DM self-management practices and each individual’s medical history, personal and cultural background, and educational needs, specific education topics may need to be repeated at regular intervals. Grade A; BEL 1 |
| R 13.5 | The ideal insulin regimen should be personalized to an individual’s needs and glycemic targets, attempting to better emulate physiological insulin replacement to maintain near normoglycemia, to prevent the development and progression of DM complications, while minimizing hypoglycemia and providing flexibility for specific daily life situations/scenarios such as: exercise, sleep, acute illness, psychological stress, etc. Grade A; BEL 1 |
| R 13.6 | Insulin regimens usually should involve the use of insulin analogs for most persons with T1D and include the following approaches: a. MDI, which usually involve 1 to 2 subcutaneous injections daily of basal insulin to suppress ketogenesis and gluconeogenesis and to control glycemia between meals and overnight, and subcutaneous injections of prandial insulin or use of inhaled insulin before each meal to control meal-related glycemic excursions. CGM is the preferred method of glucose monitoring for all individuals with T1D. Grade A; BEL 1 b. Insulin pump therapy (CSII) provides constant/continuous infusion of fast-acting insulin driven by mechanical force and delivered via a cannula inserted under the skin. CSII can improve (or enhance) glycemic control and should be an option for insulin delivery for appropriate persons with DM. Ideally, these individuals should also use CGM as stated in R13.6.a. Grade B; BEL 1 c. Automated insulin delivery systems (AIDs), which include an insulin pump, an integrated CGM, and computer software algorithm, aim to better emulate physiological insulin replacement and achieve glycemic targets. This technology is recommended for many persons with T1D since its use has been shown to increase TIR while often reducing hypoglycemia or at least without causing increased hypoglycemia. Grade A; BEL 1 d. Open-loop (use of a pump and sensor which do not communicate) and sensor-augmented pump (SAP) systems: (CGM communicates with pump facilitating needed adjustments to basal rate; temporary interruption of insulin delivery when glucose levels are low or forecast to be low within 30 min). Insulin pump with a CGM or an SAP is recommended to manage persons with DM treated with intensive insulin management who prefer not to use AIDs or have no access to them. Grade D; BEL 4 |
|
Q 14: How should hypoglycemia be managed? | |
| R 14.1 | Oral intake of rapidly absorbed glucose (eg, glucose tablets or dietary sugar like fruit juice) followed by a snack or meal containing both protein and carbohydrates (eg, cheese and crackers or a peanut butter sandwich) should be used to treat hypoglycemia (measured glucose <70 mg/dL [3.9 mmol/L]) if a person is able to safely swallow. Grade A; BEL 1 |
| R 14.2 | Glucagon, in one of the currently available forms: intranasal, prefilled liquid stable nonaqueous formulation, prefilled aqueous liquid stable glucagon analogue or with reconstitution from powder, should be used to correct hypoglycemia if individuals are unable or unwilling to ingest carbohydrates orally. If there is no response after 15 min, an additional same dose may be administered. As soon as the individual is awake and able to swallow, they should receivea rapidly absorbed source of carbohydrate. Grade A; BEL 1 |
| R 14.3 | Persons with severe hypoglycemia with altered mental status or with prolonged hypoglycemia need to be hospitalized. If an individual has hypoglycemic unawareness and hypoglycemia-associated autonomic failure, several weeks of hypoglycemia avoidance may at least partially reverse hypoglycemia unawareness and may reduce the risk or prevent recurrence of severe hypoglycemia. Adjustment of an individual's long-term antihyperglycemic regimen may be necessary to further avoid recurrence of hypoglycemia. Grade B; BEL 1 |
| R 14.4 | In persons with T2D who develop hypoglycemia and are being treated with alpha-glucosidase inhibitors or with pancreatic diabetes, oral glucose or lactose-containing foods (dairy products) must be given because alpha-glucosidase inhibitors inhibit the breakdown and absorption of complex carbohydrates and disaccharides (eg, table sugars or starches). Grade A; BEL 1 |
| R 14.5 | Persons at risk for hypoglycemia should perform frequent BGM or preferably use CGM devices (see R 3.1 to R 3.4 on monitoring). Grade B; BEL 4 and expert opinion of task force |
|
Q 15: How should diabetes mellitus be managed in the hospital? | |
| R 15.1 | All hospitalized persons should have laboratory glucose testing on admission. Persons with DM or with admission hyperglycemia >140 mg/dL should have glucose monitoring during hospitalization. Grade B; BEL 1 |
| R 15.2 | To guide inpatient therapy and inform discharge planning, clinicians should measure A1C in all persons with DM, unless their A1C is known and was tested within the previous 3 mo. Grade B; BEL 2 |
| R 15.3 | Hospitalized persons with hyperglycemia but without known DM should have A1C measured to identify preexisting DM and inform discharge planning. Grade B; BEL 2 |
| R 15.4 | Initiate bedside point-of-care (POC) capillary glucose monitoring at an appropriately chosen schedule to guide therapy for hyperglycemia during hospitalization in all persons with DM, persons without prior DM who have hyperglycemia, and persons receiving therapies with a high risk of hyperglycemia, such as corticosteroids and enteral or parenteral nutrition. Grade A; BEL 1 |
| R 15.5 | For hospitalized persons with DM eating on a regular schedule, check POC BG before each meal and at bedtime, if clinically indicated. In hospitalized persons who are not eating (eg, NPO [nothing by mouth] or continuous feeding), initially check POC BG at least every 4 to 6 h. Additional checks may be warranted for those at higher risk of hypoglycemia. For those on intravenous (IV) insulin, POC BG should be obtained from every 30 min to every 2 h. Grade A; BEL 1 |
| R 15.6 | Although inpatient CGM has not received regulatory approval, CGM may be useful in inpatient settings, while complying with institutional policies and safety precautions. CGM may improve detection of severe hypoglycemic and hyperglycemic events, identify glucose trends and patterns, and improve satisfaction in persons with DM. Grade C; BEL 2 |
| R 15.7 | CGM may be considered under special regulatory allowance during the time of coronavirus disease 2019 (COVID-19) to reduce staff exposure and use of personal protective equipment and assist with glycemic monitoring of persons in the hospital setting. Grade C; BEL 2 |
| R 15.8 | Specialized inpatient DM teams and/or CDCES, if available, should be used to improve outcomes in hospitalized persons with DM or hyperglycemia. The use of virtual consults may be considered an alternative to support hospitals lacking these services. Grade B; BEL 1 |
| R 15.9 | For critically ill persons, IV insulin infusion is recommended to treat persistent hyperglycemia in the ICU using validated protocols that allow adjustment of insulin dose for glycemic excursions based on prespecified glucose targets. For those receiving IV insulin, POC testing should be performed every 30 to 120 min. Grade A; BEL 1 |
| R 15.10 | A glucose target of 140 to 180 mg/dL is recommended for most critically ill persons in the hospital setting. More intensive targets between 110 to 140 mg/dL may be appropriate in select populations, particularly critically ill persons postcardiothoracic or other surgeries, while minimizing the risk of hypoglycemia. Grade A; BEL 1 |
| R 15.11 | For most noncritically ill persons in the hospital setting, a glucose target of 140 to 180 mg/dL is recommended. For hospitalized persons who are able to achieve and maintain glycemic control without hypoglycemia, a lower target range (100 to 140 mg/dL) may be reasonable. For persons in a hospital setting with high clinical complexity, terminal illness, limited life expectancy, or high risk for hypoglycemia, less stringent targets are appropriate. Grade B; BEL 1 |
| R 15.12 | Insulin therapy following approved protocols is recommended as the preferred therapy for managing hyperglycemia in the hospital. For noncritically ill hospitalized persons with T2D, an individualized approach is recommended for consideration of noninsulin agents alone or in combination with insulin (see also R 15.16). Grade A; BEL 1 |
| R 15.13 | The insulin regimen for hospitalized persons with satisfactory meal intake should include basal, prandial, and correction doses. For those without adequate food intake, a regimen of basal, prandial, and correction doses should be used as necessary for glycemic control. Exclusive use of “sliding-scale” insulin should only be used for those whose glucoses are in the target range most of the time, and only occasionally exceed it. Grade A; BEL 1 |
| R 15.14 | The management of hyperglycemic emergencies, including DKA and hyperosmolar state, should include fully adequate fluid resuscitation to correct fluid deficits, electrolyte replacement (potassium), and insulin therapy. Simultaneous continued infusion of insulin and dextrose solutions after correction of hyperglycemia is often required until DKA resolves to avoid hypoglycemia. Grade A; BEL 1 |
| R 15.15 | Transition from IV insulin in the ICU to a subcutaneous insulin regimen is typically required when acidosis is resolved, and a person is no longer critically ill. A proactive regimen with scheduled subcutaneous insulin therapy, with basal, nutritional/prandial, and/or correctional doses, is recommended for most persons. Grade A; BEL 1 |
| R 15.16 | For hospitalized persons with T2D and mild admission hyperglycemia (glucose <180 mg/dL), a personalized approach is recommended for the use of noninsulin agents alone or in combination with basal insulin, aiming for the most efficacious regimen with the lowest hypoglycemic risk. For some hospitalized persons with T2D, DPP-4 inhibitors plus correction doses with rapid-acting insulin, or basal insulin plus DPP-4 inhibitors may be sufficient. Grade A; BEL 1 |
| R 15.17 | A hospital-wide standardized plan should be in place to prevent hypoglycemia. Each hypoglycemic episode should be documented, and appropriate adjustments should be made to prevent recurrence. Grade B; BEL 2 |
| R 15.18 | It is recommended to start discharge planning soon after hospital admission and to provide and document appropriate individualized plans for transition to an ambulatory setting and follow-up care at discharge for all persons with DM or newly diagnosed hyperglycemia. Grade A; BEL 1 |
|
Q 16: How should diabetes mellitus in pregnancy be managed? | |
| R 16.1 | For women with GDM, the following treatment goals are recommended: preprandial glucose concentration ≤95 mg/dL and either a 1-h postmeal glucose ≤140 mg/dL or a 2-h postmeal glucose ≤120 mg/dL to decrease adverse fetal outcomes. Grade C; BEL 4 and expert opinion of task force |
| R 16.2 | All women with preexisting DM (T1D, T2D, or previous GDM) need access to preconception care and counseling to ensure adequate nutrition, healthy weight, and glucose control before conception, during pregnancy, and in the postpartum period. Grade B; BEL 2 |
| R 16.3 | Rapid-acting insulin analogs (insulin-lispro, insulin-aspart) should be used to treat postprandial hyperglycemia in pregnant women. Grade B; BEL 1 |
| R 16.4 | Options for basal insulin include long-acting insulin (eg, NPH, detemir, or glargine) or rapid-acting insulin via a CSII. Regular insulin, although not recommended as first-line therapy, is acceptable to use in managing pregnant women with DM when rapid-acting insulin analogs are not available. Grade B; BEL 1 |
| R 16.5 | Insulin is the preferred therapeutic choice for pregnant women with GDM or T2D, but metformin has been given a category B for pregnancy with accumulating clinical evidence of metformin’s safety during the first trimester and beyond. Metformin has been shown to improve pregnancy and fetal outcomes except for increased rates of infants with SGA and later onset of obesity. The prescriber should discuss the potential risks and benefits of oral agent therapy during pregnancy as well as the need for longer-term outcome studies. Grade B; BEL 1 |
|
Section 4. Education and Other Topics | |
|
Q 17: What education interventions have been shown to be most effective in management of persons with diabetes mellitus?
| |
| R 17 | Comprehensive individualized DSMES is recommended at the time of DM diagnosis and subsequently as appropriate. Therapeutic lifestyle management must be discussed with all persons with DM or prediabetes at the time of diagnosis and throughout their lifetime. This includes MNT (with reduction and modification of caloric and fat intake to achieve weight loss in those who are overweight or obese), appropriately prescribed physical activity, avoidance of tobacco products, and adequate sleep quantity and quality. Additional topics commonly taught in DSMES programs outline principles of glycemia treatment options; BGM; insulin dosage adjustments; acute complications of DM; and prevention, recognition, and treatment of hypoglycemia. Grade A; BEL 1 |
|
Q 18: What are the key nonpharmacological components of a comprehensive diabetes care plan for children and adolescents? | |
| R 18.1 | T1D and T2D in children and adolescents should be managed in close consultation with the patient and their family members, involving school and daycare personnel whenever possible. Grade B; BEL 2 |
| R 18.2 | It is recommended that all children and adolescents with DM should be given age and culturally appropriate education and guidance for physical activity and lifestyle modification. Grade A; BEL 1 |
| R 18.3 | Interventions by family and/or community are recommended to improve dietary behavior and increase physical activity in efforts to prevent childhood obesity and T2D (Grade A). Game-based interventions also can be incorporated to enhance healthy lifestyle habits (Grade B). BEL 1 |
| R 18.4 | Routine psychological assessment with consideration of family stressors and psychosocial factors that may impact glycemic control is recommended for all youth with DM. Grade A; BEL 1 |
| R 18.5 | With the risk of glycemic control worsening during adolescence, coordinated, individualized, planned transition from pediatric to adult DM care is recommended for all adolescents. Grade A; BEL 1 |
|
Q 19.1: Should persons with infertility be screened for diabetes mellitus? | |
| R 19.1 | Men and women undergoing investigation for infertility and preparation for infertility interventions, including in vitro fertilization, should be screened for DM. Grade B; BEL 2 |
|
Q 19.2: How should persons with preexisting diabetes mellitus and infertility be evaluated? | |
| R 19.2 | For all persons with DM and possible infertility, in addition to routine endocrine evaluation, further collaborative consultation with a reproductive specialist should be considered. For women with T2D and infertility, or those with T1D who desire to preserve or estimate their fertility, anti-Müllerian hormone and midluteal progesterone levels may be assessed and screened for ovulatory dysfunction including anovulation. For men with DM and infertility, a standard semen analysis may be assessed, and an endocrine evaluation be initiated. Grade B; BEL 2 |
|
Q 19.3: Should men with diabetes mellitus and cardiometabolic disorders be assessed for hypogonadism? | |
| R 19.3 | All men with CMD including prediabetes, metabolic syndrome, obesity, and T2D should be assessed for hypogonadism by history and physical examination; test for testosterone deficiency in persons with loss of libido and/or loss of muscle strength or mass, erectile dysfunction, osteopenia, or infertility. Grade B; BEL 1 |
|
Q 20.1: How should persons at risk for secondary diabetes be assessed? | |
| R 20.1 | Persons with risk factors for developing secondary DM, such as postorgan transplantation, cystic fibrosis, chronic pancreatitis/postpartial pancreatectomy, or on medication associated with hyperglycemia, should be monitored routinely for IFG, IGT, and/or overt DM. Grade A; BEL 1 |
|
Q 20.2: What are the best treatment strategies for management of secondary diabetes, such as posttransplant diabetes, cystic fibrosis‒related diabetes, and other forms of secondary diabetes? | |
| R 20.2.1 | Select treatment for secondary DM based on the underlying pathophysiology. Insulin therapy is safe and effective, but alternative glucose-lowering agents may be considered in specific patient populations. Grade A; BEL 1 |
| R 20.2.2 | DPP-4 inhibitors can be safely used to improve glycemic control for posttransplant diabetes. Grade A; BEL 1 |
|
Q 21: What is the role of sleep medicine in the care of persons with diabetes? | |
| R 21.1 | Health care professionals should assess persons with T2D for symptoms and signs of OSA, especially in the presence of obesity or suggestive clinical features of OSA. Grade B; BEL 2 |
| R 21.2 | Based on resources available locally, persons suspected to have OSA should be referred to an appropriate center for diagnosis and management of OSA. Grade B; BEL 4 and Expert Opinion of Task Force |
| R 21.3 | Weight loss is recommended as the predominant intervention to improve both OSA and insulin sensitivity. In addition, devices that provide positive airway pressure as prescribed by a sleep specialist are effective. Grade A; BEL 1 |
|
Q 22: Should screening for depression be a routine component of clinical assessment in persons with diabetes mellitus? | |
| R 22 | Routine screening of adults with DM for depression and DM distress is recommended during each clinic encounter, if appropriate. Referral to mental health professionals should be made as soon as possible once depression is suspected or diagnosed. Grade A; BEL 1 |
|
Q 23: Is the evaluation of social determinants of health in persons predisposed to or with diabetes mellitus useful in improving health outcomes? | |
| R 23 | Clinicians should assess the SDOH in persons with DM to better guide them to the most appropriate resources. Interventional trials addressing SDOH and health inequities in DM are needed to evaluate reversibility of their impact. Grade B; BEL 1 |
|
Q 24: Is telehealth/virtual care an effective care-delivery model for the management of persons with diabetes mellitus? | |
| R 24 | Offer telehealth, if available and appropriate, to persons with DM as part of their wholistic health care. Grade A; BEL 1 |
|
Q 25: Which occupations have specific public safetyerelated diabetes management considerations? | |
| R 25 | Persons with DM who are engaged in occupations with public safety implications, such as commercial drivers and pilots, have special management requirements for certification. CGM to predict hypoglycemia in real time and pharmacotherapy that minimizes hypoglycemia are recommended as effective strategies for persons with DM who work in these occupations. Grade A; BEL 1 and expert opinion of task force |
|
Q 26: Is there a role for nutritional supplements in the management of diabetes and what might be the associated risks? | |
| R 26 | Nutritional supplements (ie, noncaloric oral supplements) have modest or neutral effects on glycemic control, lipids, and BP. Until proven scientifically, these supplements should not be used for managing DM or related CV risk factors among persons with DM. In view of potential harm, we recommend that persons with DM use caution and discuss with their physicians the use of unregulated nutritional supplements. Grade A; BEL 1 |
|
Q 27: How should potential increased cancer risk be managed in persons with obesity/type 2 diabetes? | |
| R 27.1 | Clinicians should recommend age, sex, and risk-appropriate screening for common cancers, especially those associated with obesity and DM. Grade B; BEL 2 |
| R 27.2 | With the increased risk of certain cancers in persons with obesity or DM, clinicians should educate persons regarding cancer risk and encourage a healthy lifestyle, including weight reduction. Grade A; BEL 1 |
|
Q 28: Which vaccinations should be given to persons with diabetes mellitus? | |
| R 28.1 | AACE supports the recommendations of the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) that all persons with DM receive age-appropriate vaccinations according to the CDC/ACIP schedule (7). Immunization recommendations for adults with DM are summarized in Table 21. Grade A; BEL 4 and expert opinion of task force |
| R 28.2 | An annual influenza vaccine is recommended for those with DM who are ≥6 mo old. Grade A; BEL 1 |
| R 28.3 | The 15- or 20-valent pneumococcal conjugate vaccine (PCV15 or PCV20) should be administered to all adults aged 19 to 64 y who have DM. When PCV15 is used, PPSV23 should be administered at least 12 mo following the dose of PCV15. A minimum interval of 8 wk may be used for adults with immunocompromising conditions. Grade B; BEL 3 |
| R 28.4 | For adults over 65 who have not previously received PCV or whose vaccination history is unknown, PCV15 or PCV20 should be administered. When PCV15 is used, it should be followed by a dose of PPSV23. Grade B; BEL 3 |
| R 28.5 | It is recommended to administer hepatitis B vaccinations to all individuals as soon after diagnosis of DM as possible up to age 59 y. Grade A; BEL 1 |
| R 28.6 | Consider hepatitis B vaccination of adults ≥60 y based on assessment of risk and likelihood of an adequate immune response. Grade C; BEL 4 |
| R 28.7 | Tetanus-diphtheria-pertussis (Tdap) vaccine is typically included with routine childhood vaccinations. However, all adults with DM should receive a tetanus-diphtheria (Td) booster every 10 y. Grade C; BEL 4 |
| R 28.8 | Health care professionals may consider recommending vaccines for the following diseases for persons with T2D based on individual needs: Tdap - tetanus, diphtheria, and pertussis (whooping cough); measles/mumps/rubella; varicella (chicken pox); and polio. In addition, persons traveling to other countries may require vaccines for endemic diseases. Grade D; BEL 4, expert opinion of task force |
| R 28.9 | Due to the increased risk for serious complications of COVID-19, persons with DM should be vaccinated against COVID-19 according to current guidelines. Grade B; BEL 2 |
| R 28.10 | Recombinant zoster vaccine is recommended for adults ≥50 y for protection against shingles according to the CDC/ACIP vaccination schedule. Grade A; BEL 1 |
| R 28.11 | Health care professionals should utilize interventions with demonstrated effectiveness in increasing vaccination rates to improve uptake of vaccination among persons with DM. Grade B; BEL 2 |
Table 3.
Summary of Tables and Figures
| Title of Table/Figure | |
|---|---|
| Overview | |
|
| |
| Table 1 | Summary of Questions |
| Table 2 | Summary of Recommendations |
| Table 3 | Summary of Tables and Figures |
|
| |
| Section 1 | Screening, Diagnosis, Glycemic Targets, Glycemic Monitoring |
|
| |
| Table 4 | Glucose Testing and Hemoglobin A1c Interpretation |
| Table 5 | Risk Factors for Prediabetes and Type 2 Diabetes: Criteria for Testing for Diabetes in Asymptomatic Adults |
| Table 6 | Glycemic Targets for Persons with Diabetes Mellitus |
|
| |
| Section 2 | Comorbidities and Complications |
|
| |
| Table 7 | Individualized Blood Pressure Goals for Persons with Type 1 or Type 2 Diabetes |
| Table 8 | Atherosclerotic Cardiovascular Disease Risk Calculators |
| Table 9 | Atherosclerotic Cardiovascular Disease Risk Categories, Characteristics, Lipid Targets, and Therapy |
| Table 10 | Drug-Effectiveness for Low-Density Lipoprotein Cholesterol-Lowering Therapy |
| Figure 1 | Decision Tree for Treating Hypercholesterolemia in Persons with Diabetes Mellitus |
| Figure 2 | Decision Tree for Hypertriglyceridemia in Persons with Diabetes Mellitus |
| Table 11 | Relationship among Categories for Albuminuria and Proteinuria |
| Figure 3 | Guide to Frequency of Monitoring (Number of Times per Year) by Glomerular Filtration Rate and Albuminuria Category |
| Table 12 | Mitigation of Side Effects for Newer Agents to Treat Diabetic Kidney Disease |
| Table 13 | Clinical Symptoms and Signs of Diabetic Peripheral Neuropathy |
| Figure 4 | Algorithm for Treatment of Diabetic Peripheral Neuropathy |
| Figure 5 | Antihyperglycemic Therapy for Persons with Type 2 Diabetes and Atherosclerotic Cardiovascular Disease (ASCVD), Very High Risk for ASCVD, Heart Failure, Cerebral Vascular Disease, or Chronic Kidney Disease |
| Table 14 | Food and Drug Administrationdapproved Pharmacotherapy for Weight Loss in Persons with Adiposity-based Chronic Disease |
|
| |
| Section 3 | Management |
|
| |
| Table 15 | Recommended Meal Patterns for Persons with Diabetes Mellitus |
| Table 16 | Profiles of Antihyperglycemic Medications |
| Table 17 | Recommended Steps for the Addition of Insulin to Antihyperglycemic Therapy |
| Table 18 | Recommended Steps for the Intensification of Insulin Therapy When Prandial Control Is Needed |
| Table 19 | Types of Insulin |
| Figure 6 | Matching Glucose Monitoring Option to Complexity of Antihyperglycemic Regimens |
| Table 20 | Glucagon Preparations for Treatment of Severe Hypoglycemia |
|
| |
| Section 4 | Education and Other Topics |
|
| |
| Table 21 | Vaccine Recommendations for Adults with Diabetes Mellitus |
|
| |
| Supplementary | Titles of Tables from AACE Protocol for Standardized Production of Clinical Practice Guidelines, Algorithms, and Checklists e 2017 Update Tables |
|
| |
| Supplementary Table 1 | Table 5: Revised Logical Ranking of Scientific Methodologies (Step I: Evidence Rating) |
| Supplementary Table 2 | Table 6: Revised Evaluation of Studies (Step II: Scientific Analysis and Subjective Factors) |
| Supplementary Table 3 | Table 7: Revised Evaluation of Recommendations (Step III: Recommendation Qualifiers) |
| Supplementary Table 4 | Table 8: Revised and Detailed Mapping Protocol (Step IV: Creating Initial Recommendation Grades) |
Task force authors discussed each recommendation in this updated CPG to achieve consensus regarding actionable language and grades of recommendations. Task force chairs and team leaders provided oversight throughout the entire development process. Semantic descriptors of “must,” “should,” and “may” generally but not strictly correlate with Grade A (strong), Grade B (intermediate), and Grade C (weak) recommendations, respectively; each semantic descriptor can be used with Grade D (no conclusive evidence and/or expert opinion) recommendations.2 Deviations from this mapping take into consideration further decision-making based on clinical expertise. Thus, the process leading to a final recommendation and grade was not rigid and incorporated expert discussion of objective and subjective factors that reflect optimal real-life clinical decision-making to enhance care of persons with DM whose individual circumstances and presentations differ. Ultimate clinical management is based on a combination of the best interest and input of each person with DM and reasonable clinical judgment of clinicians and diabetes care teams.
Limitations of the Literature
In the continually expanding and rapidly evolving literature on DM, there is significant heterogeneity among studies, including but not limited to differences in study design, comparators, and outcomes, as well as age, duration of DM, and other characteristics of participants. Many RCTs have employed an open-label design with potential bias or implemented a crossover design with an inherent limitation because the order of treatments may affect outcomes. Many well-designed studies are sponsored to some degree by industry, posing another challenge to interpretation, even though coordinating academic centers may have collected and analyzed data independently of sponsors. Recognizing these limitations, grading of the evidence base was informed by trial design, potential generalizability, risks, harms, and benefits.
Key Updates
Section 1, Screening, diagnosis, glycemic targets, and glycemic monitoring: Screening criteria for the diagnosis of DM along with glycemic targets have been refreshed for 2022. Incorporation of advances in CGM has been strongly recommended in insulin-treated persons with type 1 diabetes (T1D) and type 2 diabetes (T2D).
Section 2, Management of comorbidities, including obesity and its management with lifestyle, nutrition, and bariatric surgery; hypertension; dyslipidemia; and complications: retinopathy, neuropathy, DKD or CKD in DM, and CVD: Recognizing the importance of an individualized approach, person-centric recommendations for management of hypertension and dyslipidemia in those with DM have been updated. The importance of weight management throughout the natural history of DM has been stressed throughout the document. Recommendations on the prevention and management of retinopathy, neuropathy, and DKD or CKD in DM have been refreshed with consideration of the most recent advances. For those with DM and comorbidity of CVD or at high risk for CVD, the focus has shifted to the utility of antihyperglycemic agents and their impact on improving CV outcomes in those with atherosclerotic CV disease (ASCVD), HF, and/or with cerebrovascular disease.
Section 3, Management of prediabetes, T2D, and T1D with selection of glycemic targets, lifestyle interventions, antihyperglycemic pharmacotherapy (insulin therapy for all with T1D and select individuals with T2D); prevention, identification, and treatment of hypoglycemia; treatment of hospitalized persons with DM or those with hyperglycemia without diagnosis of DM; and women with GDM: Recommendations on identifying persons with prediabetes and incorporating validated approaches to prevention of DM and CV complications are included. In addition to a person’s social and medical scenarios, other factors suchas hypoglycemia, weight gain/loss, CVoutcomes, kidney outcomes, and adverse events have been considered in the most appropriate therapeutic choices. When appropriate and safe, emphasis on early combination therapy and early titration to combination of complementary pharmacotherapies is encouraged.
Section 4, Education and new topics regarding DM and infertility, nutritional supplements, posttransplantation, secondary diabetes, social determinants of health (SDOH), and virtual care, as well as updated recommendations on cancer risk, nonpharmacologic components of pediatric plans, depression, education and team approach, occupational risk, role of sleep medicine, and vaccinations in persons with DM: Common questions confronting clinicians in caring for those with DM are addressed in this section. Educational approaches and delivery of telehealth/virtual care, with recent studies of new platforms, are reviewed. Akin to pharmacologic therapies, no one approach is ideal for every individual with DM. Evidence-based recommendations are provided to approach male and female infertility, posttransplant diabetes, and secondary diabetes. Recommendations on topics that impact QoL such as sleep hygiene, depression, SDOH, and type of occupation are also included. During their care, persons with DM often ask about nutritional supplements and risk of cancer due to their condition or to their antihyperglycemic medications, which led to inclusion of several pragmatic safety-oriented recommendations. Finally, the use of vaccinations is recommended to maintain public health as well as to mitigate specific higher risks among those with DM.
Recommendations and Evidence Bases
Section 1: Screening, Diagnosis, Glycemic Targets, and Glycemic Monitoring
Question 1. How is the diagnosis of DM made and what is the current screening protocol for prediabetes and diabetes?
Recommendation 1.1
The diagnosis of DM is based on the following criteria (Table 4):
Table 4.
Glucose Testing and Hemoglobin A1C Interpretation
| Normal | Prediabetes | Diabetes |
|---|---|---|
| FPG <100 mg/dL | IFG FPG ≥100 to 125 mg/dL |
FPG ≥126 mg/dL |
| 2-h PG <140 mg/dL | IGT 2-h PG ≥140 to 199 mg/dL |
2-h PG ≥200 mg/dL Random PG ≥200 mg/dL + symptoms |
| A1C <5.5% | 5.7% to 6.4% For screening of prediabetesa |
≥6.5% Secondaryb |
Abbreviations: A1C = hemoglobin A1c; FPG = fasting plasma glucose; h = hour; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; PG = plasma glucose
A1C should be used only for screening prediabetes. The diagnosis of prediabetes, which may manifest as either IFG or IGT, should be con rmed with glucose testing.
Glucose criteria (ie, FPG or 2-h glucose after a 75-g oral glucose load) are preferred for the diagnosis of diabetes mellitus (DM). The same testdPG or A1C measurementd—should be repeated on a different day to confirm the diagnosis of DM. Two abnormal test results from the same sample confirm the diagnosis of DM. A glucose level ≥200 mg/dL in the presence of DM symptoms does not need to be confirmed.
FPG concentration 126 mg/dL (after 8 hours of an overnight fast), or
Plasma glucose (PG) concentration 200 mg/dL 2 hours after ingesting a 75-g oral glucose load after an overnight fast of at least 8 hours, or
Symptoms of hyperglycemia (eg, polyuria, polydipsia, polyphagia) and a random (nonfasting) PG concentration ≥200 mg/dL, or
A1C level ≥6.5%
Diagnosis of DM requires 2 abnormal test results, either from the same sample or 2 abnormal results on samples drawn on different days. However, a glucose level ≥200 mg/dL in the presence of symptoms for DM confirms the diagnosis of DM.
Grade A; BEL 2 and expert opinion of task force
Recommendation 1.2
Prediabetes is identified by the presence of impaired fasting glucose (IFG) (100 to 125 mg/dL), impaired glucose tolerance (IGT), which is a PG value of 140 to 199 mg/dL 2 hours after ingesting 75 g of glucose, and/or A1C value between 5.7% and 6.4% (Table 4). A1C should be used only for screening for prediabetes. The diagnosis of prediabetes, which may manifest as either IFG or IGT, should be confirmed with glucose testing.
Grade B; BEL 2
Recommendation 1.3
T1D is characterized by marked insulin deficiency in the presence of hyperglycemia and positive autoantibody tests to glutamic acid decarboxylase (GAD65), pancreatic islet b cells (tyrosine phosphatase IA-2), and IA-2b zinc transporter (ZnT8), and/or insulin. The presence of immune markers and clinical presentation are needed to establish the correct diagnosis and to distinguish between T1D and T2D in children or adults, as well as to determine appropriate treatment.
Grade A; BEL 2
Recommendation 1.4
T2D is characterized by progressive loss of b-cell insulin secretion and variable defects in insulin sensitivity. T2D is often asymptomatic and can remain undiagnosed for many years; therefore, all adults 35 years of age with risk factors should be screened for DM (Table 5).
Table 5.
Risk Factors for Prediabetes and Type 2 Diabetes: Criteria for Testing for Diabetes Mellitus in Asymptomatic Adultsa
| Age ≥35 ywithout other risk factors First-degree relative with diabetes History of CVD Overweight or obeseb Sedentary lifestyle Member of an at-risk racial or ethnic group: Asian, African American, Hispanic, Native American (Alaska Natives and American Indians), or Pacific Islander HDL-C <35 mg/dL (0.90 mmol/L) and/or a triglyceride level >250 mg/dL (2.82 mmol/L) IGT, IFG, and/or metabolic syndrome PCOS Acanthosis nigricans NAFLD Hypertension (BP >140/90 mm Hg) or on therapy for hypertension History of gestational diabetes mellitus or delivery of a baby weighing more than 4 kg (9 lb) Antipsychotic therapy for schizophrenia and/or severe bipolar disease Sleep disorders including OSA, chronic sleep deprivation, and night-shift occupation |
Abbreviations: A1C = hemoglobin A1c; BP = blood pressure; CVD = cardiovascular disease; HDL-C = high-density lipoprotein cholesterol; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; NAFLD = nonalcoholic fatty liver disease; OSA = obstructive sleep apnea; PCOS = polycystic ovary syndrome
Source: US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: Us preventive services task force recommendation statement. Jama. 2021;326(8):736–743. https://doi.org/10.1001/jama.2021.12531 [EL 4; NE].
Testing should be considered in all adults who are obese (body mass index [BMI] ≥30 kg/m2), and those who are overweight (BMI 25 to <30 kg/m2 or >23 kg/m2 in Asian Americans) and have additional risk factors.
Grade A; BEL 1
Recommendation 1.5
GDM is defined as carbohydrate intolerance that begins or is first recognized during pregnancy and resolves postpartum. Pregnant women with risk factors for DM should be screened at the first prenatal visit for undiagnosed T2D using standard criteria (Table 4).
Grade B; BEL 1
Recommendation 1.6
Screen all pregnant women for GDM at 24 to 28 weeks’ gestation. Diagnose GDM with either the one-step or the two-step approach.
The one-step approach uses a 2-hour 75-g oral glucose tolerance test (OGTT) after ≥8 hours of fasting with diagnostic cutoffs of one or more FPG ≥92 mg/dL, 1-hour PG ≥180 mg/dL, or 2-hour PG ≥153 mg/dL.
The two-step approach uses a nonfasting 1-hour 50-g glucose challenge test with 1-hour PG screening threshold of 130 or 140 mg/dL. For women with a positive screening test, the 3-hour 100-g OGTT is used for diagnosis with 2 or more PG tests that meet the following thresholds: FPG ≥95 mg/dL, 1-hour ≥180 mg/dL, 2-hour ≥155 mg/dL, 3-hour ≥140 mg/dL.
Grade A; BEL 1
Recommendation 1.7
Clinicians should consider evaluation for monogenic DM in any child or young adult with an atypical presentation, clinical course, or response to therapy. Monogenic DM includes neonatal diabetes and nonautoimmune diabetes of multiple genetic causes, also known as maturity-onset diabetes of the young (MODY). Most children with DM occurring under age 6 months of age have a monogenic cause as autoimmune T1D rarely occurs before 6 months of age. Other monogenic forms of diabetes are characterized by mutation of genes of transcription factors, genes regulating pancreatic development or atrophy, abnormal insulin genes, genes related to endoplasmic reticulum stress that impair insulin secretion, or abnormal glucokinase genes that cause impaired insulin signaling.
Grade B; BEL 2
Evidence Base 1: How is the diagnosis of DM made and what is the current screening protocol for prediabetes and DM?
Diagnosis of DM
DM refers to a group of metabolic disorders that result in hyperglycemia, regardless of the underlying etiology. DM is diagnosed by using any of 3 established criteria for elevated blood glucose (BG) concentrations (Table 4). FPG ≥126 mg/dL, 2-hour PG ≥ 200 mg/dL during 75-g OGTT, and A1C ≥ 6.5% are equally appropriate for diagnostic screening (Table 4). The concordance between FPG, 2-hour PG, and A1C is not perfect; thus, the diagnosis of DM requires 2 different (fasting glucose and A1C) abnormal test results, either from the same sample or 2 abnormal results on samples drawn on different days.3-5 A glucose level ≥200 mg/dL in the presence of hyperglycemia symptoms such as polyuria and polydipsia confirm the diagnosis of DM.6 In individuals with discordant results from 2 different tests, the test result that is above the diagnostic cut point should be repeated on a different day.4
The A1C captures chronic hyperglycemia and is the gold standard for assessment of long-term glycemic control and risk of chronic micro- and macrovascular complications; however, analyses of the fidelity of DM diagnosis using A1C have reported a lower sensitivity than FPG or 2-hour OGTT.4,5,7-10 A1C is known to be affected by nonglycemic factors such as changes in red blood cell maturity and survival and impaired renal function. A1C levels may be 0.4% to 0.6% higher in Blacks and Hispanics compared with Whites, despite equivalent levels of hyperglycemia in those with T2D.11-17 In view of physiological changes in pregnancy that could affect glycated hemoglobin levels, A1C should not be used for GDM screening or diagnosis of DM.18,19
Classification of DM
DM is classified as T1D, T2D, GDM, monogenic DM, and other less common conditions, such as diabetes related to pancreatic disease, drug-induced, or rare insulin resistance and mitochondrial syndromes.9,20,21 T1D accounts for 5% to 10% of all DM cases and occurs more commonly in children and young adults but can occur at any age. It is also more common in persons of European ancestry and is caused by absolute insulin deficiency that usually results from an immune-mediated destruction of the pancreatic b cells. The presence or absence of autoimmune markers (autoantibodies to glutamic acid decarboxylase [GAD65], pancreatic islet b cells [tyrosine phosphatase IA-2], and IA-2b zinc transporter [ZnT8], and/or insulin) in addition to the clinical presentation may help establish the correct diagnosis to distinguish between T1D and T2D in children or adults.22–27 Over 90% of newly diagnosed persons with T1D have 1 or more antibodies. The presence of >2 antibodies in a relative without diabetes of a person with T1D is highly predictive of developing T1D within 5 years. However, some forms of T1D have no evidence of autoimmunity and have been termed idiopathic. The clinical presentation and rate of b-cell destruction progression is variable, with higher rates of ketosis in children and slower progression in older adults. In some individuals with T1D in adulthood, the clinical presentation may follow a more indolent course (termed latent autoimmune diabetes in adults) with slower decline in b-cell insulin secretion. Many of these individuals are initially misdiagnosed as having T2D until the progression of insulin deficiency leads to insulin dependence.
Severe insulinopenia in T1D predisposes persons to diabetic ketoacidosis (DKA). However, DKA can also occur in persons with T2D.28–31 Worldwide epidemiological studies have reported that between 13% and 80% of individuals with T1D present with DKA. The percentage of adults with T2D who present with DKA at diagnosis is unknown; however, the number of people with ketosis-prone or atypical T2D has increased. Ketosis-prone T2D most frequently occurs among Blacks of African ancestry or persons who identify as Afro-Caribbean or Hispanic. In contrast to the long-term insulin requirement of autoimmune T1D, many persons with ketosis-prone T2D can discontinue insulin after a few months of therapy and maintain acceptable glycemic control for many years on either diet or noninsulin therapies. At presentation, persons with ketosis-prone T2D have significant impairment of both insulin secretion and insulin action; however, at the time of near-normoglycemia remission, insulin secretion and action improve to levels similar to hyperglycemic persons with ketosis-resistant T2D.
T2D is considered a polygenic condition with considerable heterogeneity in degrees of insulin deficiency and resistance and accounts for >90% of all cases of DM.32 Most persons with T2D are overweight or obese and have multiple risk factors for DM (Table 5). Insulin resistance and concurrent relative insulin deficiency and glucagon dysregulation underlie T2D pathophysiology.33–35 The Centers for Disease Control and Prevention (CDC) has reported a higher prevalence of diagnosed DM in African Americans, Hispanic Americans, and other persons of non-European origin compared with European Americans.36,37 T2D remains undiagnosed for years in many affected persons because they are frequently asymptomatic; therefore, screening individuals with multiple risk factors is needed to reduce the risk of long-term complications.38,39 Up to 25% of persons with T2D have already developed at least 1 microvascular complication by the time of diagnosis.40,41
Monogenic diabetes accounts for 1% to 3% of DM diagnosed under 30 years of age (~0.4% of all DM) and frequently occurs in pubertal children or young adults <35 years of age. Many, but not all, will have a family history over 3 generations.42–47 Monogenic diabetes includes neonatal diabetes, MODY, and diabetes associated with a variety of syndromes, including mitochondrial disorders, lipodystrophy syndromes, Wolfram syndrome, and many others. Because most types of MODY are autosomal dominant disorders, affected people have a 50% chance of passing along the gene mutation to their children. There are 14 genes that have been implicated as causes of MODY accounting for 11 different types of MODY.48,49 Establishing the correct diagnosis is important as appropriate treatment varies with the type of gene defect. MODY 2 can usually be managed with diet alone, while sulfonylurea (SU) therapy may be effective in MODY 1, 3, and 4.
Other causes of DM include diseases of the exocrine pancreas including (but not limited to) pancreatitis, trauma, cystic fibrosis, neoplastic disease, posttransplant diabetes, and iron deposition in hemochromatosis. Endocrine disorders including Cushing syndrome, acromegaly, glucagonoma, and pheochromocytoma can induce insulin resistance and T2D. Hyperglycemia may also be associated with the use of certain medications. Beyond the well-known agents (glucocorticoids, nicotinic acid, thiazides, interferon gamma, high-dose statins), agents such as atypical antipsychotics, immune checkpoint inhibitors, PI3 kinase inhibitors, tacrolimus, and octreotide have been shown to induce DM. Pathogenesis can be multifactorial with management depending on the severity of hyperglycemia. Careful follow-up for progression or regression of DM is necessary.
Screening and Diagnosis of GDM
All pregnant women should be screened for GDM at 24 to 28 weeks’ gestation. Universal screening is recommended, as selective screening (only in women with risk factors) would miss a significant number of women with GDM and universal screening has been shown to be cost-effective compared with selective screening.9,50–55 Children born to women with GDM have increased incidence of childhood adiposity and development of IGT in children aged 10 to 14 years compared with children born to mothers without GDM.56,57
GDM can be diagnosed with either the one-step or the two-step approach using the OGTT.58 The one-step approach consists of the 2-hour 75-g load oral glucose load after an overnight fast to diagnose GDM as recommended by the International Association for Diabetes and Pregnancy Study Groups criteria.9,52,54,55,59–63 The criteria for GDM are fasting glucose level ≥92 mg/dL, 1-hour postglucose challenge value ≥180 mg/dL, or 2-hour value ≥153 mg/dL. These criteria are based on trials such as the Hyperglycemia and Pregnancy Outcomes (HAPO) study.59,64 The International Association for Diabetes and Pregnancy Study Groups criteria result in overall higher percentages of women diagnosed with GDM. Until recently, there were no large RCTs comparing the effect of one-step and two-step approaches on maternal and neonatal outcomes. An RCT performed at the Kaiser Permanente health systems in California and Hawaii examined the one-step (75 g) and the two-step (50 g) sequential approach on neonatal and gestational outcomes.65 The study randomized 23,792 women to either the one-step or two-step approach. The diagnosis of GDM was twice as high in the one-step group compared with the two-step group (16.5% vs 8.5%). However, there were no differences in neonatal or maternal outcomes between the 2 approaches.65 The higher diagnosis of GDM in the one-step approach can lead to more resource utilization for subsequent maternal treatment and fetal monitoring.55,59,66 It should be noted that only 66% of the women randomized to the one-step approach adhered to the screening, whereas 92% of the women randomized to the two-step approach adhered to the assigned screening process. With the two-step sequential screening approach using a nonfasting 1-hour 50-g glucose challenge test between 24- and 28-weeks’ gestation, screening cutoffs are 130 mg/dL (90% sensitivity) or 140 mg/dL (80% sensitivity).67 For women with a positive screening test, the 3-hour 100-g OGTT following 8 or more hours of no caloric intake, GDM is diagnosed if 2 or more PG values meet or exceed the following thresholds: fasting level of 95 mg/dL, 1-hour level of ≥180 mg/dL, 2-hour level of ≥155 mg/dL, or 3-hour level of ≥140 mg/dL.68
Question 2. What are the glycemic treatment goals for persons with DM?
2.1. Outpatient Glucose Targets for Nonpregnant Adults
Recommendation 2.1.1
An A1C level of ≤6.5% is recommended for most nonpregnant adults, if it can be achieved safely. To achieve this target A1C level, FPG may need to be <110 mg/dL, and the 2-hour PPG may need to be <140 mg/dL (Table 6). Glucose targets should be individualized with consideration for life expectancy, disease duration, presence or absence of micro- and macrovascular complications, CVD risk factors, comorbid conditions, and risk for hypoglycemia, as well as a person’s cognitive and psychological status.
Table 6.
Glycemic Targets for Persons with Diabetes Mellitus
| Parameter | Treatment goal | |
|---|---|---|
| Glucose | ||
| A1C, % | Individualize on the basis of age, comorbid conditions, duration of disease; in general, ≤6.5 for most; closer to normal for healthy; less stringent for those at greater risk for hypoglycemia and/or adverse consequences from hypoglycemia; longer duration of diabetes; shorter life expectancy; comorbidities especially established vascular complications | |
| FPG, mg/dL | <110 | |
| 2-h PPG, mg/dL | <140 | |
| Inpatient hyperglycemia: glucose, mg/dL | 140 to 180 | |
| Weight | ||
| Weight loss | Reduce weight by >5% to ≥10%; avoid weight gain | |
| Diabetes type | Glucose range a,179 | Recommendations (% of readings) |
| T1D and T2D | <54 mg/dL (<3.0 mmol/L) | <1% |
| <70 mg/dL (<3.9 mmol/L) | <4% | |
| 70 to 180 mg/dL (3.9 to 10.0 mmol/L) | >70% | |
| >180 mg/dL (>10.0 mmol/L) | <25% | |
| >250 mg/dL (>13.9 mmol/L) | <5% | |
| Pregnancy with T1D | <54 mg/dL (<3.0 mmol/L) | <1% |
| <63 mg/dL (<3.5 mmol/L) | <4% | |
| 63 to 140 mg/dL (3.5 to 7.8 mmol/L) | >70% | |
| >140 mg/dL (>7.8 mmol/L) | <25% | |
| Pregnancy with gestational or T2D | 63 to 140 mg/dL (3.5 to 7.8 mmol/L) | >90% |
Abbreviations: A1C = hemoglobin A1c; FPG = fasting plasma glucose; PPG = postprandial glucose; T1D = type 1 diabetes; T2D = type 2 diabetes
Downloaded from CGM preferably, or other devices if CGM not available.
Grade A; BEL 1
Recommendation 2.1.2
Adopt less stringent glycemic goals (A1C 7% to 8%) in persons with a history of severe hypoglycemia, hypoglycemia unawareness, limited life expectancy, advanced renal disease, extensive comorbid conditions, or long-standing DM in which the A1C goal has been difficult to attain despite intensive efforts, so long as the person remains free of hyperglycemia-associated symptoms.
Grade A; BEL 1
2.2. Inpatient Glucose Targets for Nonpregnant Adults
Recommendation 2.2
For most hospitalized persons with hyperglycemia in both the intensive care unit (ICU) and non-ICU settings, a glucose range of 140 to 180 mg/dL is recommended, provided this target can be safely achieved (Table 6).
Grade A; BEL 1
2.3. Outpatient Glucose Targets for Pregnant Women
Recommendation 2.3
In women with GDM, the following glucose goals are recommended: fasting and PPG concentration ≤95 mg/dL and either a 1-hour postmeal glucose value ≤140 mg/dL or a 2-hour postmeal glucose value ≤120 mg/dL.
In women with preexisting T1D or T2D who become pregnant, it is recommended that glucose be controlled to meet the following goals, but only if the goals can be safely achieved: premeal, bedtime, and overnight glucose values between 60 and 95 mg/dL; a 1-hour PPG value between 110 and 140 mg/dL; a 2-hour glucose 100 to 120 mg/dL. A secondary target would be an A1C level of <6% if it can be accomplished without significant hypoglycemia.
Grade A; BEL 1
Evidence Base 2.1: Outpatient Glucose Targets for Nonpregnant Adults
There is no dispute that elevated glucose levels are associated with micro- and macrovascular complications of DM. Similarly, it has been accepted that strategies aimed at lowering glucose concentrations can lead to lower rates of microvascular and perhaps macroangiopathic complications.69 Significant reductions in the risk or progression of nephropathy were seen in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, which targeted an A1C <6.5% in the intensive therapy group vs standard approaches.70 Landmark trials near the turn of the 21st century confirmed that intensive approach to control was not necessarily associated with reduced CVD complication rates.71 Duration of DM and preexisting ASCVD appeared to negate the benefit of improved glycemic control. This data derived from older clinical trials, relying on therapies associated with hypoglycemia, must be interpreted in the context of recent positive CVOTs with 3 new classes of medications not associated with hypoglycemia. The newer classes of antihyperglycemic agents appear to be either neutral (dipeptidyl peptidase 4 [DPP-4] inhibitors) or successful (glucagon-like peptide-1 receptor agonist [GLP-1 RA] or a sodium glucose cotransporter 2 inhibitor [SGLT2i]) in lowering the risk of CVD complications in those with T2D.
Epidemiologic evidence shows a continuous relationship between A1C and CVD and all-cause mortality.72
No RCTs have yet established optimal glycemic targets in persons with T2D. Professional organizations have relied on results from existing intervention trials achieving improved A1C levels and epidemiologic analyses of various studies to arrive at consensus statements or expert opinions regarding targets. Thus, some have recommended a general target A1C level 6.5%, while others have recommended a general target of <7%.73–75 The potential risks of intensive glycemic control may be obviated by incorporating agents that are not associated with hypoglycemia, especially in persons with frequent severe hypoglycemia, hypoglycemia unawareness, or a very long duration of DM, and particularly in the presence of established and advanced atherosclerosis, advanced age, and terminal illness.69,70,76–81
Moreover, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, mortality increased with increasing A1C among intensively treated persons, with excess mortality only affecting persons whose A1C remained >7%.82 Similar U-shaped curves were found in a 7-year observational study of persons with T1D and a 22-year observational study of >20,000 persons with T2D.83,84 A corollary of this issue is the safety of those therapies in view of the demonstrated increase of frequency of severe hypoglycemia during attempts at intensive glycemic control.70,76,80,85,86 As discussed in Q14. How should hypoglycemia be managed?, much of the mortality in the ACCORD trial may have been related to hypoglycemia, and the hazard ratio (HR) for hypoglycemia-associated deaths was actually higher in the standard treatment than the intensive therapy groups.87
As discussed in Q12. How can glycemic targets be achieved in persons with T2D? as well as in the AACE Comprehensive Type 2 Diabetes Management Algorithm,73 some newer therapies carry a lower risk of hypoglycemia, which may enable more persons to safely achieve individualized target A1C levels.69,88–90 In addition, for persons with established ASCVD or multiple ASCVD risk factors, HF, or CKD, a GLP-1 RA or an SGLT2i with demonstrated CVD benefit is recommended as part of a glucose-lowering regimen independent of A1C. In such persons, GLP-1 RA and SGLT2is have been shown to have beneficial CVD benefits as well as benefits on indices of CKD (see Q12. How can glycemic targets be achieved in persons with T2D?).
Evidence Base 2.2: Inpatient Glucose Targets for Nonpregnant Adults
Inpatient hyperglycemia is associated with increased complications including surgical site infections, mortality, and increased length of hospital stay.91–93 The level of hyperglycemia at which complications occur is debated. In the surgical ICU, earlier studies have indicated that intensive glucose control (80 to 110 mg/dL) inconsistently resulted in decreased length of stay.94–96 The Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation study showed that more aggressive glucose targets of 81 to 108 mg/dL compared with a conventional glucose target (<180 mg/dL) in the ICU did not change length of stay, number of days needing mechanical ventilation, or need for renal replacement therapy.95 However, the intensively controlled group had increased mortality and higher rates of hypoglycemia.95,97 Lower levels of glycosylated hemoglobin in patients undergoing cardiac surgery are associated with a lower risk of early and late mortality, as well as in the incidence of postoperative acute kidney injury (AKI), neurologic complications, and wound infections.98,99
Further, the history of DM makes a difference as to whether postoperative complications occur. Those without preexisting DM or stress hyperglycemia have a higher incidence of more postheart surgery complications.91,100–103 Intensive glycemic control post-operatively, compared to less intensive targets, has resulted in equivocal or mixed results; however, other studies have shown no benefits.94,100,101,104 Intensive targets result in hypoglycemia, which, in turn, results in higher complications.105,106 Therefore, we recommend a glucose target of 140 to 180 mg/dL in the ICU setting. More intensive targets can be used (110 to 140 mg/dL) if they can be achieved without hypoglycemia.
It is unclear whether the targets for the ICU should be the same in the non-ICU setting. Overall, studies have shown that treatment including a basal insulin regimen resulted in better glycemic control compared with sliding-scale insulin alone.92,107–109 The better glycemic control resulted in improvements in rates of postsurgical complications.92 Most studies of inpatient DM have treated to goal glucose levels 110 to 140 or 140 to 180 mg/dL. Large multicenter studies are needed to assess which glycemic targets predict complications while avoiding hypoglycemia. With CGM being used more frequently in the inpatient setting, lower targets may be achieved while avoiding hypoglycemia.
Evidence Base 2.3: Outpatient Glucose Targets for Pregnant Women
Elevated BG levels at conception and during the early first trimester are associated with increased rates of congenital malformations, spontaneous abortion, intrauterine fetal demise, pre-eclampsia, preterm delivery, and perinatal mortality.110–114 Therefore, preconception counseling is essential for women with T1D and T2D to minimize pregnancy risks. The goals of preconception care should be tight glycemic control with an A1C <6.5%, without significant hypoglycemia, which will lower risks of congenital malformations, preeclampsia, and perinatal mortality.113
The HAPO study showed that increasing glycemia is associated with increased neonatal adverse outcomes such as macrosomia, neonatal hypoglycemia, and cesarean delivery.64,115–119
The targets for glycemic control during pregnancy for women with preexisting DM and GDM are based on the physiology of nondiabetic pregnancies. The A1C target of <6% is recommended as A1C ≥6% in the second and third trimesters was associated with macrosomia in persons with T1D in the Diabetes and Pre-Eclampsia Intervention Trial.120 Further, during pregnancy, A1C is reduced in women without GDM compared with nonpregnant women.121 One observational study in Australia in women with GDM examined the impact of reference glucose control (fasting glucose <98 mg/dL [5.5 mmol/L], 2-hour postprandial <126 mg/dL [7 mmol/L]) or tight glucose control (fasting <90 mg/dL [5 mmol/L], 2-hour PPG <120 mg/dL [6.7 mmol/L]) on neonatal outcomes.115 The study showed no difference in birthweights with the different glycemic targets. However, women with tighter glycemic control had higher adverse maternal outcomes such as increased cesarean section rates. There is an ongoing RCT (TARGET) that will assess the effect of tight glycemic control on both perinatal and maternal outcomes.116 In women with preexisting DM and GDM, postprandial rather than preprandial glucose levels were associated with better neonatal and maternal outcomes.117,122 Therefore, we suggest checking PPG levels in addition to fasting glucose and to target 2-hour BG of <120 mg/dL and a 1-hour BG <140 mg/dL, as there are no RCTs at the moment to inform us as to the best glycemic target.
CGM may help women achieve glucose goals and reduce hypoglycemia during pregnancy. Use of CGM can accurately identify glycemic patterns among pregnant women with DM. Data from RCTs on the effects of CGM use on maternal and fetal outcomes are limited and results are inconsistent. The CONCEPTT study’s participants were randomly assigned to either CGM in addition to capillary glucose monitoring or capillary glucose monitoring alone and showed a modest reduction in A1C levels, increased time in target, reduced hyperglycemia, and less glycemic variability.123 There was also lower incidence of large for gestational age (odds ratio [OR], 0.51; 95% CI, 0$28–0.90; P = .0210), fewer neonatal intensive care admissions lasting more than 24 hours (0.48; 95% CI, 0.26–0.86; P = .0157), fewer incidences of neonatal hypoglycemia (0.45; 95% CI, 0.22–0.89; P = .0250), and 1-day shorter length of hospital stay (P = .0091) in women with T1D on multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII). Improved glycemic control may result in improved neonatal health outcomes attributed to reduced exposure to maternal hyperglycemia. Some studies of CGM have included women with T1D and T2D and were performed with intermittently scanned CGM (isCGM) or blinded CGM, which did not show differences in neonatal outcomes.124,125
The use of CGM in women with GDM is limited. Some observational cohort studies that reported that the use of CGM resulted in lower mean glucose, lower standard deviation, and a higher percentage of time in target range were associated with lower risk of large-for-gestational-age births and other adverse neonatal outcomes.126,127 Although the available evidence is not strong to support use of CGM in pregnant women with T2D and GDM for maternal or neonatal benefits, it may be used in select persons who are at risk for hypoglycemia, especially those treated with insulin. With improving CGM technology, increased acceptability by pregnant women with DM is anticipated.
Question 3: When and how should glucose monitoring be used?
Recommendation 3.1
A1C should be measured at least semiannually in all persons with DM and at least quarterly in persons not at their glycemic target.
Grade B; BEL 2
Recommendation 3.2
All persons who use insulin should use CGM or perform BG monitoring (BGM) a minimum of twice daily and ideally before any insulin injection. More frequent BGM may be needed by persons who are taking multiple insulin injections, persons not at A1C targets, or those with history of hypoglycemia. Persons who do not require insulin or insulin secretagogue therapy may often benefit from BGM, especially to provide feedback about the effects of their lifestyle choices (diet and physical activity), and to assess response to pharmacologic therapy.
Grade A; BEL 1
Recommendation 3.3
Real-time CGM (rtCGM) or isCGM is recommended for all persons with T1D, regardless of insulin delivery system, to improve A1C levels and to reduce the risk for hypoglycemia and DKA.
Grade A; BEL 1
Recommendation 3.4
rtCGM or isCGM is recommended for persons with T2D who are treated with insulin therapy or who have high risk for hypoglycemia and/or with hypoglycemia unawareness.
Grade A; BEL 1
Evidence Base 3: When and how should glucose monitoring be used?
Current glucose monitoring strategies can be classified into 2 categories: patient self-monitoring, which would allow individuals to change behavior (diet and/or exercise) or medication dose (most often insulin), and long-term assessment, which allows both the person with DM and the clinician to evaluate overall glucose control and risk for complications over weeks or months. Current forms of self-monitoring include BGM and CGM, whereas long-term assessment is most often by A1C. A1C is considered the current gold standard for monitoring chronic hyperglycemia and provides an indication of the average of BG levels over the previous 3 months. It is associated with the risk for the development of long-term complications. However, A1C does not inform individuals about BG values on a daily basis; therefore, frequent measurements of BG levels are necessary for the day-to-day management of DM.
Glycated hemoglobin is quantified most commonly with methods that distinguish it from nonglycated hemoglobin on the basis of either charge (cation-exchange chromatography, electrophoresis, isoelectric focusing) or structural characteristics (affinity chromatography, immunoassays). A1C reflects average glycemia over the lifespan of the red blood cell (100 to 120 days), but 50% of A1C is determined by glycemia during the month preceding measurement. A1C is the metric used in clinical trials to assess the benefits of improved glycemic control.128 The frequency of A1C testing should depend on the clinical situation and treatment regimen. A1C should be measured at least twice yearly in all persons with DM and at least quarterly in persons not at target.129
Currently, 99% of laboratories in the United States use a standardized and certified assay traced to the Diabetes Control and Complications Trial.4,130 More recently, using CGM, each level of A1C was measured as “estimated average glucose.”131 There are numerous populations in which A1C may not reflect average glucose.132,133 These reasons can include changes in erythrocyte survival time (eg, hemolysis, splenomegaly, or use of epoetin alfa), alterations in the hemoglobin molecule (hemoglobinopathies), iron status, or recent blood transfusion.4,14,134 Renal failure also results in a different A1C level than would be seen in those with normal kidney function.135 In numerous cohorts and in national data, it has been shown that Blacks have higher A1C values than Whites in both the presence and absence of DM.12,13,133,136 Hispanic Americans have values of A1C that are intermediate between Blacks and Whites.136
Current glucose meters perform rapid tests with small blood volumes and are easily operated by laypersons with DM in the outpatient setting.137 They are equipped with a variety of features, ranging from storing results of glucose tests performed to simple pattern analysis, audible reporting of results, and wireless connectivity to smartphones. The Institutional Organization for Standardization specifies requirements for in vitro glucose monitoring systems that measure capillary BG for specific design verification procedures and for the validation of self-measurement performance by laypersons with DM.138–140 The 2013 Institutional Organization for Standardization 15 197 standard for glucose meter accuracy is stricter than the 2003 version. The standard requires that 95% of values fall within ±15 mg/dL for glucose <100 mg/dL and within ±15% at ≥100 mg/dL.140,141 In addition, at least 99% of pooled results shall fall within zones A and B of the consensus error grid.140,141
Frequency of BGM (in a retrospective analysis) has been shown to be predictive of A1C levels.142–146 In persons who are not using insulin, regular BGM did not result in significant differences in glycemic control.147–150 Use of structured BGM data to adjust medications is associated with greater A1C decreases than unstructured BGM.151,152
CGM has emerged as a standard of care for persons with DM who are treated with intensive insulin therapy. The indication for using new technologies and CGM for the management of DM was reported in the 2021 AACE Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons with Diabetes Mellitus.153 Based on the results of multiple studies reporting a strong linear relationship between percent time in range (TIR) (70–180 mg/dL) and A1C in persons with T1D and T2D, AACE recommends 2 metrics, percent TIR (>70%) and percent time below range (<70 mg/dL [<4%] and <54 mg/dL [<1%]), to be used as a starting point for the assessment of quality of glycemic control and as the basis for therapy adjustment.154,155 The primary goal for effective and safe glucose management is to reduce the percent time below range while increasing the percent TIR. These recommendations align with recent reports from other national and international organizations.129
The clinical efficacy of CGM has been demonstrated in numerous retrospective studies and RCTs of individuals with T1D regardless of insulin delivery method.156–161 Benefits of CGM in this population include reductions in A1C, fewer severe hypoglycemia events in children and adults, increased TIR, as well as significant reductions in hospitalizations for severe hypoglycemia and DKA.156,162–168 There is emerging evidence that CGM is efficacious in reducing hyperglycemia and A1C levels in insulin-treated persons with T2D, including those taking 1 or 2 doses of basal insulin.169–172
CGM has also been shown to be effective in reducing incidence of severe hypoglycemia in individuals with T1D and T2D who are treated with intensive insulin therapy.173 In addition, CGM use has been reported to reduce fear of hypoglycemia and increase confidence in avoiding/treating hypoglycemia.156,174,175 Adherence to monitoring and treatment is the greatest predictor of glycemic control in persons with DM.159,176–178
Recent recommendations in CGM technology that provide guidance for clinicians, researchers, and individuals with DM to utilize are shown in Table 6.179
Section 2: Comorbidities and Complications
Question 4: How should hypertension be managed in persons with DM?
Recommendation 4.1
The recommended BP goal for most persons with T1D, T2D, or prediabetes is <130/80 mm Hg (Table 7).
Table 7.
Individualized Blood Pressure Goals for Persons with Type 1 or Type 2 Diabetes
| Blood pressure (BP) <130/80 mm Hg is the recommended goal for persons with diabetes - BP control has a significant impact on morbidity and mortality. - BP goal may be set higher in persons with autonomic neuropathy, orthostatic hypotension, acute coronary syndrome, frailty, and medication intolerance. BP <120/70 mm Hg may be considered to limit progression of micro- and macrovascular disease in persons with the following: - Micro- or macroalbuminuria - Documented coronary heart disease (CHD) - Moderate-to-high risk for CHD - Peripheral vascular disease - Retinopathy |
Grade A; BEL 1
Recommendation 4.2
Therapeutic lifestyle interventions in persons with hypertension are recommended to include consultation with a registered dietitian for education about an overall healthy diet (such as the Mediterranean diet), weight management, reduced sodium intake (such as the Dietary Approaches to Stop Hypertension [DASH] diet), daily physical activity and regular exercise (several times a week), and as-needed consultation with a psychologist or certified diabetes care and education specialist (CDCES) to support long-term behavior change. (See also R 11.2 to 11.4 and R 12.1.1 to 12.1.5 on nutrition and lifestyle.)
Grade A; BEL 1
Recommendation 4.3
If BP goals are unattained with therapeutic lifestyle changes, use antihypertensive pharmacotherapy to achieve individual BP treatment goals.
Grade A; BEL 1
Recommendation 4.4
Select antihypertensive agents based on their ability to reduce BP to goal and prevent or slow the progression of micro- and macrovascular disease. Use either an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) for BP control and to delay the progression of DKD or CKD in DM (see also R 6.1 to R 6.6 on DKD or CKD in DM).
Grade A; BEL 1
Recommendation 4.5
Intensify pharmacotherapy as needed to achieve BP goals. Antihypertensive therapy may include combinations of either an ACE inhibitor or an ARB plus any of the following agents: diuretics, calcium channel antagonists, combined alpha-beta blockers, and newer-generation beta blockers. Consider a mineralocorticoid receptor antagonist for resistant hypertension.
Grade A; BEL 1
Evidence Base 4: How should hypertension be managed in persons with DM?
The majority of individuals with DM either have elevated BP or are receiving treatment for hypertension.180 Hypertension is not only more prevalent in persons with T2D than in the general population, but hypertension also predicts progression to T2D. Once diagnosed with hypertension, an individual is 2.5 times more likely to be diagnosed with T2D within the next 5 years.181,182 The combination of hypertension and T2D magnifies the risk of DM-related complications.183,184 The UK Prospective Diabetes Study (UKPDS) demonstrated that hypertension treatment decreased both micro- and macrovascular complications of T2D.185 This study showed that each 10 mm Hg decrease in systolic BP (achieved with either an ACE inhibitor [captopril] or a beta blocker [atenolol]) was associated with a 15% reduction in DM-related mortality, an 11% reduction in myocardial infarction (MI), and a 13% reduction in the microvascular complications of retinopathy or DKD.186
Subsequent trials that included large numbers of persons with T2D, including the Hypertension Optimal Treatment study, the Heart Outcomes Prevention Evaluation study, the Losartan Intervention for Endpoint Reduction in Hypertension study, the Anti-hypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, and the ADVANCE trial, have demonstrated that BP control improves CV outcomes when more intensive BP targets are set.187–191 Numerous other studies have also demonstrated decreased DKD/CKD and retinopathy progression.192–194 Based on these data, the Eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, AACE, American Diabetes Association (ADA), National Kidney Foundation-Kidney Disease Outcomes Quality Initiative, and American Heart Association (AHA) have recommended that BP in persons with T2D be controlled to <130/80 mm Hg.195–199 However, the preferred goal for BP lowering remains controversial as clinical trial data to support the level of <130/80 mm Hg are limited. Epidemiologic data suggest no evidence of a threshold for adverse outcomes with a BP level <115/75 mm Hg.200 Clinical trial data show that intensifying therapy with BP-lowering medications slows the progression of DKD and retinopathy, though vigilance in monitoring renal function during intensive BP therapy is recommended.185,186,201–203 Neither the ACCORD BP trial nor subanalyses of other large BP trials have shown that targeting a systolic BP <120 mm Hg (compared with <140 mm Hg) reduces risk of the composite outcome of fatal and nonfatal major CV events in persons with T2D, although stroke was significantly reduced (HR, 0.59; 95% CI, 0.39–0.89; P = .01).204–206 Thus, data from prospective RCTs have not found an overall positive effect of BP targets <120/70 mm Hg on CV or renal outcomes in persons with T2D.207,208 Various guidelines from different professional organizations have generally recommended a BP goal for persons with T2D of <130/80 mm Hg, with an option to individualize to the lower target of <120/70 mm Hg based on extrapolation from the Systolic Blood Pressure Intervention Trial in persons with hypertension without T2D.199,209–213
Once the diagnosis of hypertension is established, lowering BP decreases the risk of both micro- and macrovascular complications associated with T2D. Therapeutic lifestyle goals in persons with hypertension and T2D should include education about a healthy diet (such as the Mediterranean diet) with emphasis on weight management and reduced salt intake (such as the DASH diet), daily physical activity and regular exercise (several times a week) (also see the section on weight-loss therapy and lifestyle in Evidence Base 10).214–220 Individuals should be referred to a registered dietitian for diet education and as needed to a psychologist or CDCES to support long-term behavior change. Cognitive behavioral therapy can be used to support adherence to medications.221–225
Analysis of the UKPDS data suggests that BP lowering should be a priority in managing a person presenting with newly diagnosed hypertension and DM. Although glucose and lipid management remain important, BP lowering may have an additive and significant impact on morbidity and mortality, particularly in persons with standard vs intensive glycemic control.185,201,202,226,227
Accurate measurement of BP is fundamental to diagnosis and effective management of hypertension.210,211,228 The equipment, which can be aneroid, mercury, or electronic, should be inspected and validated on a regular maintenance schedule. Initial training and regularly scheduled retraining in the standardized technique for BP measurement provides consistency and reliability of BP readings. Individuals must be properly prepared and positioned to obtain an accurate BP; serial BP readings are recommended to be measured after being seated quietly for at least 5 minutes in a chair (rather than on an exam table) with feet on the floor and arm supported at heart level. Caffeine, exercise, and smoking should be avoided for at least 30 minutes prior to measurement. Measurement of BP in the standing position is also indicated in persons suspected to have postural hypotension. An appropriately sized cuff (ie, cuff bladder encircling at least 80% of the arm) should be used to ensure accuracy. At least 2, and preferably 3, serial measurements should be obtained, and the average BP recorded.
The use of 24-hour ambulatory BP monitoring (ABPM) is not currently included as part of the diagnostic criteria for hypertension, though it is an important tool for guiding management.211,228 Persons whose 24-hour ABPM mean BP exceeds 135/85 mm Hg are nearly twice as likely to have a CV event as those with values that remain <135/85 mm Hg, irrespective of the level of the office BP.229 Routine use of ABPM may be considered for the evaluation of white-coat hypertension, masked hypertension, and nighttime nondipping status, all of which are associated with increased long-term morbidity and mortality.230 ABPM provides a longer assessment of a person’s BP variability and may be utilized to guide BP management to facilitate medication adjustments and to avoid overtreatment.
BP goals are based upon evidence from clinical trials and should be individualized for persons with consideration of their anticipated lifespan and risk factors for heart disease, stroke, and DKD. The recommended BP goal for persons with T2D is <130/80 mm Hg based upon best available evidence-based data.183,226,227,231–235 In the presence of multiple CVD risk factors, consideration may be given for a more intensive BP goal of <120/70 mm Hg, provided it can be attained safely. A less intense individualized goal >130/80 mm Hg may be considered in persons who are older and frail, or who have complicated comorbidities of T2D to include autonomic neuropathy and orthostatic hypotension, acute coronary syndromes (acute MI or hospitalization with unstable angina), or medication intolerance.236,237 Frequent reassessment is needed to ensure that the BP goal is maintained without adverse effects of pharmacotherapy. If unacceptable side effects develop, consideration should be given to reducing dosage and/or changing the class of medication while intensifying therapeutic lifestyle changes.215,216,219 If such changes do not alleviate symptoms, then consideration should be given to relaxing the target to a higher BP goal based upon individual characteristics, preferences, and priorities (Table 7).
The UKPDS group performed a 10-year posttrial monitoring observational study that demonstrated a loss of benefit within 2 years if tight BP control was not maintained.201 These data reinforce the imperative to initiate BP-lowering therapy at diagnosis, to intensify treatment as needed to reach and maintain BP goal, and to monitor treatment safety and tolerance for enhanced compliance. The introduction of fixed-dose combination tablets combining 2 or 3 agents in 1 pill can facilitate compliance and adherence. The use of multiple fixed-dose combination pills can provide multiple-drug regimens with a reduced number of tablets and may help optimize adherence to reach BP goal.238
The selection of medications to lower BP in persons with T2D should be guided by individual-specific considerations and may include nontraditional BP-lowering agents such as SGLT2is and GLP-1 RAs, though these drugs alone may be inadequate to control BP.239–244 Clinical trials with diuretics, ACE inhibitors, ARBs, alpha-adrenergic blockers, and calcium channel antagonists have a demonstrated benefit in the treatment of hypertension in both T1D and T2D.187–189,210,245,246 The choice of pharmacologic agents is guided by additional considerations such as the presence of CKD, CVD, HF, or post-MI status; possible metabolic side effects; number of pills per day; and cost. During the course of T2D, an early primary goal is BP control to reduce risk of onset and progression of micro- and macrovascular disease, to include DKD and retinopathy.191
There appears to be inertia to treat residual hypertension in persons with T2D, and pharmacotherapy should be intensified when needed to achieve BP goals.247–251 ACE inhibitors or ARBs are indicated as pharmacotherapy for BP control and to delay the progression of CKD.252–254 They also have reported advantage over other treatments in reducing the risk of new-onset T2D in elderly persons.255 Thiazide diuretics can effectively lower BP in persons aged 65 years and older, though low-dose hydrochlorothiazide can negatively impact fasting BG, A1C, and high-density lipoprotein cholesterol (HDL-C).250,256,257 ACE inhibitors and ARBs should not be used together and should be used with caution with spironolactone to avoid hyperkalemia or AKI.258,259
BP control reduces the risk of stroke and major CVD events in persons with T2D.208,227 As heart disease develops, consideration of CV benefits factor into the choice of agents to lower BP. Given that diastolic heart disease develops early in T2D and the renin-angiotensin-aldosterone system (RAAS) plays a critical role in the pathophysiology of HF, the use of an ACE inhibitor or ARBs could be considered early in the treatment of hypertension, before the diagnosis of HF.260,261 However, the combination of multiple RAAS blockers (ie, ACE inhibitor, ARB, and/or renin inhibitor) should be avoided due to risk of hyperkalemia and AKI.262,263
Resistant hypertension is defined as BP ≥140/90 mm Hg in the presence of ≥3 antihypertensive agents at maximum tolerated doses, one of which is a diuretic.264 The initial assessment should include adherence to lifestyle recommendations, the antihypertensive drug regimen, and assessment for white-coat hypertension. Secondary causes of hypertension should be ruled out when suspected. The addition of a mineralocorticoid receptor antagonist should be considered for management of resistant hypertension in persons with T2D; however, monitoring for hyperkalemia and kidney function is necessary in those taking an ACE inhibitor or ARB.265
Question 5: How should dyslipidemia be managed in persons with DM?
Recommendation 5.1
All persons with prediabetes, T1D over the age of 40, or T2D should have a lipid panel (fasting or nonfasting) checked at diagnosis and annually to assess CV and metabolic disease risks and at additional intervals as needed to monitor treatment to achieve lipid goals.
Grade B; BEL 2
Recommendation 5.2
Therapeutic lifestyle interventions for dyslipidemia are recommended for all persons with prediabetes, T1D over the age of 40, or T2D, to include education with a registered dietitian about a healthy diet with emphasis on weight management, daily physical activity, and regular exercise (several times a week). Consultation with a psychologist or CDCES is recommended to support long-term behavior change.
Grade A; BEL 1
Recommendation 5.3
Persons with prediabetes or T2D without ASCVD and with less than 2 traditional risk factors should be assessed with the aid of ASCVD risk calculators to determine initiation and intensity of lipid-lowering therapy (Fig. 1 and Table 8).
Figure 1.

Decision Tree for Treating Hypercholesterolemia in Persons with Diabetes Mellitus. Copyright © 2022 AACE. May not be reproduced in any form without express written permission from Elsevier on behalf of AACE. Visit https://doi.org/10.1016/j.eprac.2022.08.002 to request copyright permission.
Table 8.
Atherosclerotic Cardiovascular Disease Risk Calculators
| ASCVD risk calculators (date) | Atherosclerotic cardiovascular disease risk factors |
ASCVD end points* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Family history | Current tobacco use | T2D | SBP |
Lipid profile (mg/dL) |
Other CVD RFs | |||||
| mmHg | Rx | TC | HDL-C | Rxa | ||||||||
| Reynolds CVD Risk Score (2007–2008) 290,291 | X | X | X | X | Xb | X | X | X | hs-CRP | 1,2,5,7 | ||
| Framingham CVD Risk Score (2008–2009) 292,293 | X | X | X | X | X | X | X | X | 1,2,3,4,6,7,8,9,10 | |||
| ACC/AHA Pooled Cohort CVD Risk Calculator (2013) 294,295 | Xc | X | X | X | X | X | X | X | 1,2,7,8 | |||
| MESA Risk Score (2015) 296 | Xd | X | Xe | X | X | X | X | X | X | X | CAC score | 1,2,5f |
Abbreviations: ACC/AHA = American College of Cardiology/American Heart Association; ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcification; CHD = coronary heart disease; CVD = cardiovascular disease; hs-CRP = high-sensitivity C-reactive protein; HDL-C = high-density lipoprotein cholesterol; mm Hg = millimeters mercury; MI = myocardial infarction; RF = risk factor; Rx = treatment; SBP = systolic blood pressure; T2D = type 2 diabetes; TC = total cholesterol
Endpoints: (1) CHD death, (2) nonfatal MI, (3) unstable angina, (4) stable angina, (5) coronary revascularization, (6) heart failure, (7) nonfatal stroke, (8) fatal stroke, (9) transient ischemic attack, (10) claudication
Use of lipid-lowering therapy.
Assessed by A1C value for women only, not for men.
Validated for adults 40–79 years of age.
Included non-Hispanic White, Hispanic, African American, and Chinese American ethnic groups.
Family history of MI at any age.
Also included resuscitated cardiac arrest.
Grade A; BEL 1
Recommendation 5.4
Assess nontraditional ASCVD risk factors (Fig. 1) beyond a lipid panel to guide management when the initial shared decision is not self-evident.
Grade B; BEL 2
Recommendation 5.5
Manage persons with prediabetes and persons with T1D over the age of 40 in the same manner as those with T2D.
Grade A; BEL 1
Recommendation 5.6
In persons with high ASCVD risk, use a moderate-intensity statin regardless of DM type or status. In persons with very high ASCVD risk (T2D with 2 or more additional traditional ASCVD risk factors such as advancing age, hypertension, CKD stage 3a, cigarette smoking, family history of premature ASCVD in men <55 years and women <65 years, low HDL-C, or high non-HDL-C), use a high-intensity statin regardless of baseline low-density lipoprotein cholesterol (LDL-C) level. For persons at extreme risk of ASCVD event (current ASCVD or target organ damage), use a high-intensity statin plus other therapies as needed to achieve lipid targets (Fig. 1 and Table 10).
Table 10.
Drug-Effectiveness for Low-Density Lipoprotein CholesteroleLowering Therapy
| Drug | Dose | % LDL-C lowering 318,324,* | Mechanism | Potential adverse effects | ||
|---|---|---|---|---|---|---|
| Statins | Dose intensity (mg/d; po) | |||||
|
| ||||||
| Low | Mod | High | Typical LDL-C decline basedon statin and dose intensity: Low <30% Mod 30% to 45% High ≥50% |
Inhibits HMG-CoA reductase, alters intracellular cholesterol metabolism resulting in LDL-R upregulation | Myalgias, fatigue, diabetogenic effect for both new onset T2D and increase in A1C,327,328 rare rhabdomyolysis (1–4/10,000 per year) | |
|
| ||||||
| Simvastatin | 10 | 20 to 40 | ||||
| Pravastatin | 10 to 20 | 40 to 80 | ||||
| Lovastatin | 20 | 40 | ||||
| Fluvastatin | 20 to 40 | 80b | ||||
| Pitavastatin | 2 to 4 mg | |||||
| Atorvastatin | 10 to 20 | 40 to 80 | ||||
| Rosuvastatin | 5 to 10 | 20 to 40 | ||||
|
| ||||||
| Cholesterol absorption inhibitor | ||||||
| Ezetimibe329,330 | 10 mg orally every day | 12% to 25% as mono-Rx(329–332); 25% when added to statin330,331 | Inhibits intestinal and biliary cholesterol absorption, decreasing hepatic stores and increasing LDL-R upregulation | Myalgias, fatigue, URI symptoms, GI symptoms | ||
|
| ||||||
| Bile acid sequestrants | ||||||
| Colesevelam | 625 mg/tab; 3 tabs bid | 8% to 16% as mono-Rx | Efficient binding of bile acids, lowering hepatic cholesterol, promoting LDL-R upregulation | GI symptoms, constipation, can bind other drugs; avoid if TG >300mg | ||
| Colestipol | 1 g/tab; 2 to 6 g/d | |||||
| Cholestyramine | 4 g/packet; 8 to 16 g/d | |||||
|
| ||||||
| PCSK9i | Initial dose | Max dose | ||||
| Alirocumab | 75 mg sc every 2 weeks | 300 mg sc every 4 weeks | 48% to 58%c | Decreases PCSK9 levels, leading to reduced hepatic LDL-R degradation and increased expression | ||
| Evolocumab | 140 mg sc every 2 weeks | 420 mg sc every 4 weeks | 63% to 71%c | |||
|
| ||||||
| ACL inhibitor | ||||||
| Bempedoic acida | 180 mg orally every day | 17% to 18% as mono-Rx. Further lowering when added to statin (+22%)(333) or ezetimibe (+13%)(329) | Inhibits ACLY, an upstream enzyme of HMG-CoA reductase | Myalgias, fatigue, URI symptoms, uric acid increase | ||
|
| ||||||
| PCSK9 siRNA | ||||||
| Inclisirana,334 | 284 mg every 6 months sc X2, then 284 mg every 6 months sc | 38% to 52% | small interfering | RNA directs breakdown of PCSK9 mRNA | ||
Abbreviations: A1C = hemoglobin A1c; ACLY = adenosine triphosphate (ATP) citrate lyase; bid = twice a day; d = day; g = grams; GI = gastrointestinal; HMG-CoA = 3-hydroxy 3-methylglutaryl coenzyme A; IV = intravenous; LDL-C = low-density lipoprotein cholesterol; LDL-R = LDL receptor; Max = maximal; mg = milligrams; Mod = moderate; mono-Rx = monotherapy; mRNA = messenger RNA; PCSK9i = proprotein convertase subtilisin/kexin type 9 inhibitor; RNA = ribonucleic acid; sc = subcutaneous; siRNA = small interfering RNA; T2D = type 2 diabetes; TG = triglyceride; tab = tablet; URI = upper respiratory infection
Additional sources: Casula M, Mozzanica F, Scotti L, et al. Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2017;27(5):396–406. doi: 10.1016/j.numecd.2017.03.001 [EL 2; MNRCT]; Mansi IA, Chansard M, Lingvay I, Zhang S, Halm EA, Alvarez CA. Association of statin therapy initiation with diabetes progression: A retrospective matched-cohort study. JAMA Intern Med. 2021;181(12):1562–1574. doi: 10.1001/jamainternmed.2021.5714 [EL 2; CS]; Ballantyne CM, Laufs U, Ray KK, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27(6):593–603. doi: 10.1177/2047487319864671 [EL 1; RCT]; Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489 [EL 1; RCT]; Wu NQ, Guo YL, Zhu CG, et al. Comparison of statin plus ezetimibe with double-dose statin on lipid profiles and inflammation markers. Lipids Health Dis. 2018;17(1):265. doi: 10.1186/s12944–018-0909-z [EL 1; RCT]; Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (ewtopia 75): A randomized, controlled trial. Circulation. 2019;140(12):992–1003. doi: 10.1161/circulationaha.118.039415 [EL 1; RCT]; Lalwani ND, Hanselman JC, MacDougall DE, Sterling LR, Cramer CT. Complementary low-density lipoprotein-cholesterol lowering and pharmacokinetics of adding bempedoic acid (ETC-1002) to high-dose atorvastatin background therapy in hypercholesterolemic patients: A randomized placebo-controlled trial. J Clin Lipidol. 2019;13(4):568–579. doi: 10.1016/j.jacl.2019.05.003 [EL 1; RCT]; US Food & Drug Administration (FDA). Inclisiran prescribing information/package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214012lbl.pdf Accessed February 19, 2022.[EL 4; NE].
Food and Drug Administration-approved for use in persons with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease who are taking maximally tolerated statin dose and require additional LDL-C lowering.
40 mg bid or XL-80 mg
In combination with statin therapy
Grade A; BEL 1
Recommendation 5.7
Treatment targets for persons in a high ASCVD risk category are LDL-C <100 mg/dL, apolipoprotein B (apo B) <90 mg/dL, and non-HDL-C <130 mg/dL. Treatment targets for persons in a very high risk ASCVD category are LDL-C <70 mg/dL, apo B <80 mg/dL, and non-HDL-C <100 mg/dL. Treatment targets for persons with extreme risk of ASCVD include LDL-C <55 mg/dL, apo B <70 mg/dL, and non-HDL-C <90 mg/dL (Table 9 and Fig. 1).
Table 9.
Atherosclerotic Cardiovascular Disease Risk Categories, Characteristics, Lipid Targets, and Therapya
| Risk categories | Risk characteristics | Approximate 10-y risk | Lipid targets | Therapy |
|---|---|---|---|---|
| High risk | T2D duration <10 y, T1D duration <20 y with <2 additional ASCVD risk factors; no TOD | <10% | LDL-C <100 mg/dL; apo B <90 mg/dL; non-HDL-C <130 mg/dL | Moderate-intensity statin to start, intensify as needed |
| Very high risk | T2D duration >10 y or T1D >20 y and age >40 y without ASCVD or severe TOD; ≥2 additional traditional ASCVD risk factors | 10% to 20% | LDL-C <70 mg/dL; apo B <80 mg/dL; non-HDL-C <100 mg/dL | High-intensity statin, addition of ezetimibe or bempedoic acid to reach lipid targets |
| Extreme risk | T2D or T1D with established ASCVD or severe TOD: eGFR <45 mL/min/1.73 m2; UACR >300 mg/g; ABI <0.9; left ventricular systolic or diastolic dysfunction | >20% | LDL-C <55 mg/dL; apo B <70 mg/dL; non-HDL-C <90 mg/dL | High-intensity statin, addition of ezetimibe, bempedoic acid, and/or PCSK9 agent to reach lipid targets |
Abbreviations: ABI = ankle-brachial index; apo B = apolipoprotein B-100; ASCVD = atherosclerotic cardiovascular disease; eGFR = estimated glomerular filtration rate; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9; T1D = type 1 diabetes; T2D = type 2 diabetes; TOD = target organ damage (left ventricular systolic or diastolic dysfunction, eGFR <45 mL/min/1.73 m2, and abnormal ankle-brachial index); UACR = urine albumin-to-creatinine ratio
Task force expert opinion
Grade A; BEL 1
Recommendation 5.8
Statins are recommended for the initial treatment of hyper-cholesterolemia. Monitor efficacy every 6 to 12 weeks and increase the dose or intensity of statin as needed and tolerated to achieve LDL-C, apo B, and/or non-HDL-C goals based on individual ASCVD risk. Once lipid targets are achieved, lipid panel or apo B can be monitored less often (Fig. 1).
Grade A; BEL 1
Recommendation 5.9
Combine the cholesterol absorption inhibitor ezetimibe with statin therapy when the desired lipid targets are not achieved with a maximally tolerated statin dose. If lipid targets are not achieved on this combination, add or substitute a proprotein convertase subtilisin/kexin type 9 (PCSK9)-lowering agent. Alternatively, add bempedoic acid to the maximally tolerated statin or consider adding icosapent ethyl (in persons with triglycerides 135 to 499 mg/dL) for ASCVD risk reduction.
Grade A; BEL 1
Recommendation 5.10
Management of hypertriglyceridemia in persons with high ASCVD risk or very high ASCVD risk should begin with intensive lifestyle modification and statin therapy. In persons treated with a maximally tolerated statin who have triglyceride concentrations ≥200 mg/dL and HDL-C <40 mg/dL, add a fibrate or high-dose omega-3 fatty acid to achieve the desired apo B or non-HDL-C goal. Icosapent ethyl can be considered in persons with high or very high ASCVD risk (Fig. 2).
Figure 2.
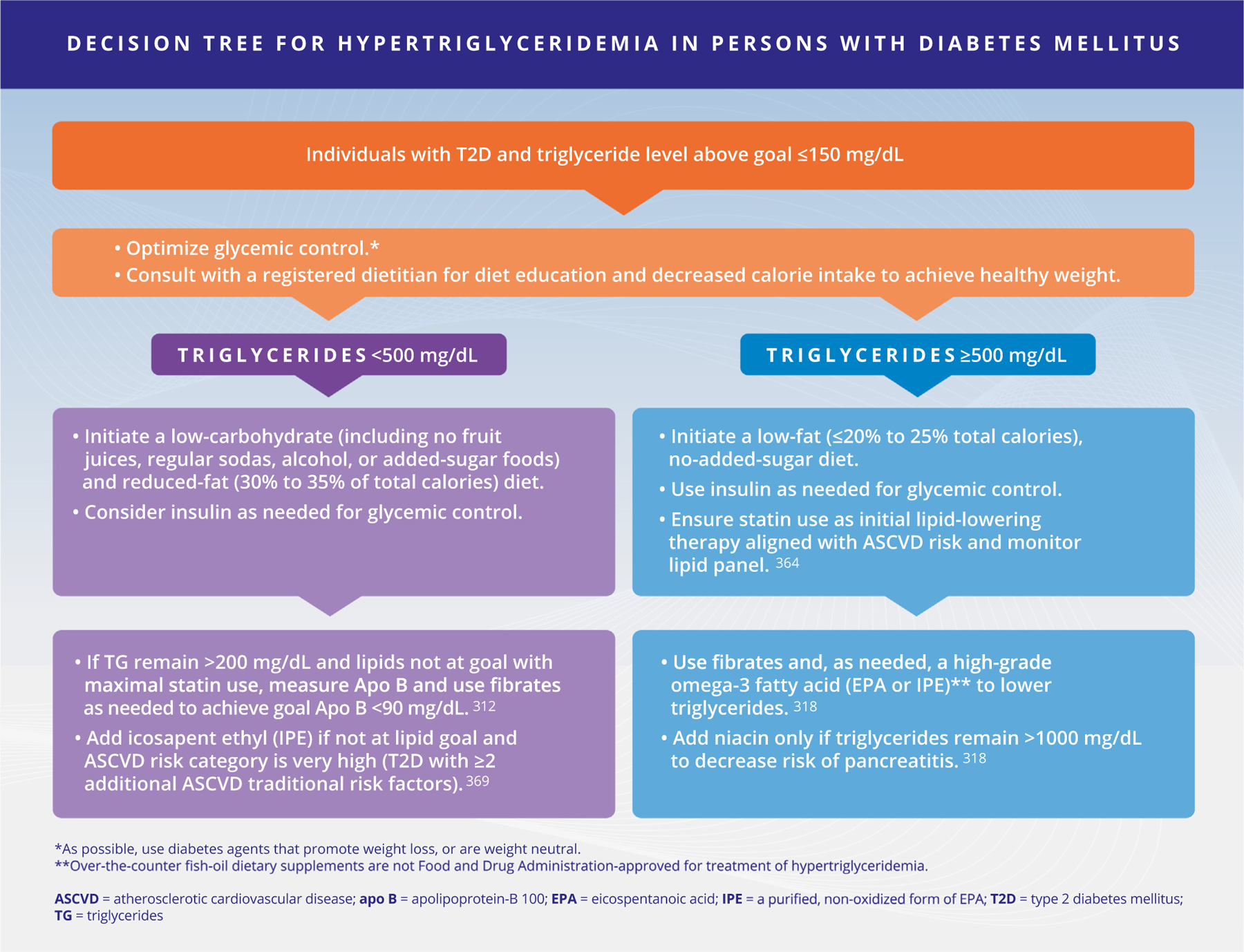
Decision Tree for Hypertriglyceridemia in Persons with Diabetes Mellitus. Copyright © 2022 AACE. May not be reproduced in any form without express written permission from Elsevier on behalf of AACE. Visit https://doi.org/10.1016/j.eprac.2022.08.002 to request copyright permission.
Grade A; BEL 1
Evidence Base 5: How should dyslipidemia be managed in persons with DM?
Dyslipidemia Screening
All persons should receive information about the benefits of enduring lifestyle changes, including daily physical activity, regular exercise (several times a week), and nutritional guidance designed to improve glucose, lipid, and BP profiles and maintain a healthy weight.266–274 Adherence to healthy lifestyle behaviors should be assessed frequently, with educational support from a registered dietitian, a CDCES, and/or a behavioral psychologist as needed to intensify therapeutic lifestyle change.
All persons with prediabetes, T1D over the age of 40 years, or T2D should be screened at diagnosis and monitored yearly with a lipid panel to include total cholesterol, triglycerides, HDL-C, and LDL-C. Fasting lipid panels, though helpful, are not necessary for therapeutic decisions, and nonfasting lipid panels may aid compliance with timely blood draws.275
Additional biomarkers, including apo B, lipoprotein(a), high-sensitivity C-reactive protein (CRP), coronary artery calcification score, and ankle-brachial index, are independent risk factors associated with increased risk of ASCVD events and may be helpful when the lipid management goal is unclear.276–285 These biomarkers may enhance understanding of an individual’s risk and inform decisions of initiating or intensifying pharmacotherapy.286 However, sequential monitoring of some of these biomarkers is not recommended at this time; high-sensitivity CRP is not mechanistically linked to the pathophysiology of atherosclerosis, coronary artery calcium changes are unlikely to be reversed, and lipoprotein(a) as a targetable marker requires validation by future outcome trials.287–289 In addition, repeat measures of these biomarkers add to costs and may result in unproven therapeutic strategies.
Where the decision for need to intervene with pharmacotherapy based upon the above risk factor assessments remains unclear, we recommend the use of a risk calculator290–296 (Table 8). In persons younger than 40 years, initiation of statin therapy for primary prevention of CVD in both men and women needs to be individualized, based on other risk factors and comorbidities.273 The use of various 10-year or lifetime risk calculators is an option to decide the intensity of treatment (Table 8). By definition, these calculators are based on observational data for risk prediction but have been verified for prediction accuracy using large databases. The use of a statin choice decision aid also may assist in shared decision-making between clinicians and persons considering statin treatment choices.297–299
Persons who have T1D with persistent proteinuria are at increased risk of premature atherosclerosis.300 In addition, the rising prevalence of overweight and obesity may contribute to increased rates of abnormal lipoprotein patterns related to insulin resistance among persons with T1D.301,302 Despite limited observational and RCT data, we recommend treating persons with T1D and proteinuria and those over the age of 40 in a similar fashion to those with T2D.
Very low-density lipoprotein (VLDL) is secreted by the liver and is strongly influenced by insulin and carbohydrate intake, whereas chylomicrons are derived from the intestine and are secreted in response to dietary fat intake. VLDL remnants (apo B-100) are atherogenic and the primary particles accumulating when triglycerides are between 250 to 500 mg/dL. VLDL and chylomicrons may coexist in hypertriglyceridemia >500 mg/dL, though chylomicron remnants (apo B-48) predominate with significantly increasing triglyceride levels >500 mg/dL.
Multiple disturbances in lipoprotein metabolism in individuals with prediabetes and T2D result from the combined effects of insulin deficiency, insulin resistance, and hyperglycemia.303–308 T2D dyslipidemia is characterized by increased levels of triglyceriderich lipoproteins (VLDL), intermediate-density lipoprotein, and remnant particlesdall apo Becontaining particlesd which metabolically lead to low levels of HDL-C and increased levels of small, dense LDL-C.278,309–311 Hypertriglyceridemia is indirectly linked with changes in HDL-C and LDL-C composition that are conducive to accelerated atherogenesis.312,313 Accumulating evidence favors the need to assess apo Becontaining lipoproteins to account for remnant lipoproteins and small dense lipoproteins responsible for residual ASCVD risk not seen with a standard lipid panel of total cholesterol, LDL-C, and HDL-C.314–316
Lipid Targets
Treatment targets for dyslipidemia in persons with DM or prediabetes and without ASCVD or target organ damage are based on the duration of DM and the presence of traditional ASCVD risk factors including advancing age, hypertension, CKD stage 3a, cigarette smoking, family history of premature ASCVD in men <55 years and women <65 years, low HDL-C, or high non-HDL-C (Fig. 1 and Table 9). T2D carries a high lifetime risk for developing ASCVD.317 In individuals with T2D, ASCVD risk should be assessed and stratified as high (persons with T1D <40 years of age or T2D duration <10 years and less than 2 additional ASCVD risk factors), very high (persons with T2D >10 years or T1D >20 years and age >40 plus 2 or more traditional ASCVD risk factors), or extreme risk (DM or prediabetes plus established ASCVD or target organ damage, including left ventricular systolic or diastolic dysfunction, estimated glomerular filtration rate [eGFR] <45 mL/min/1.73 m2, and ankle-brachial index <0.9) to help define lipid treatment targets and direct appropriate lipid-lowering therapy.318 Risk stratification in this manner can guide management strategies as well as laboratory testing to ensure efficacy of therapy. We recommend the use of apo B measurements as this is more accurate than non-HDL-C and predicts ASCVD risk more accurately than LDL-C.319 In persons at high risk, the lipid targets should be LDL-C <100 mg/dL, apo B <90 mg/dL, and non-HDL-C <130 mg/dL.314,315,320–325 In persons at very high risk for ASCVD, the lipid targets should be LDL-C <70 mg/dL, apo B <80 mg/dL, and non-HDL-C <100 mg/dL.306 In persons with DM at extreme risk of ASCVD events, the lipid targets should be LDL-C <55 mg/dL, apo B <70 mg/dL, and non-HDL-C <90 mg/dL.322–324,326 Note that the risk categories vary in name and definition in the references cited. The choice of statin and other lipid-lowering therapies prescribed should be based upon their relative intensity in lowering LDL-C required for lowering risk of ASCVD (Table 9 and Table 10).
Dyslipidemia Therapeutic Recommendations
To date, no RCT dedicated solely to lipid lowering in persons with T2D has examined secondary CVD prevention. However, several statin trials with large T2D subpopulations, including the GREACE (GREek Atorvastatin and Coronary-Heart-Disease Evaluation) study, TNT (Treating to New Targets) study, and PROVE IT (Pravastatin or Atorvastatin Evaluation and Infection Therapy) trial, have shown significant reductions in mortality and CVD events.327–330 It should be noted that the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus of 2410 participants with T2D randomized to 10 mg atorvastatin or placebo did not show any reduction in the composite CV endpoint for primary prevention.331 In low-risk persons with DM, whether DM per se leads to elevation of CV risk has been questioned.332 In very high-risk persons with T2D who have had a prior ASCVD event or those who have T2D plus 2 or more additional major ASCVD risk factors (advancing age, hypertension, CKD stage 3a, cigarette smoking, family history of premature ASCVD in men <55 years and women <65 years, low HDL-C, or high non-HDL-C), a high-intensity statin (Table 10) should be started along with therapeutic lifestyle changes regardless of baseline LDL-C level.333–335 Lipids should be rechecked within 12 weeks of initiating therapy, and the primary target is to attain apo B or non-HDL-C in goal.314,315,336–338 If the LDL-C or non-HDL-C concentration remains >70 mg/dL or >100 mg/dL, respectively, the statin dose should be increased with the goal of lowering LDL-C to <70 mg/dL and non-HDL-C to <100 mg/dL (Fig. 2 and Table 10). If these targets cannot be achieved with maximally tolerated statin therapy, then treatment considerations should include a more potent statin or the addition of ezetimibe.286,324–326,339–341 Where treatment goals are not met despite these strategies, bempedoic acid, or pharmacotherapy targeting PCSK9 should be considered.340,342
The high-risk ASCVD category (noted as moderate risk in some guidelines) describes persons with DM without known ASCVD and <2 major CV risk factors (advancing age, hypertension, CKD ≥ stage 3a, cigarette smoking, family history of premature ASCVD in men <55 years and women <65 years, low HDL-C, or high non-HDL-C). In such persons, treatmentshould beginwith therapeuticlifestylechanges for an initial 6- to 12-week trial. Goals for the primary targets LDL-C and non-HDL-C are <100 mg/dL and <130 mg/dL, respectively.245,343–346 The additional primary target of apo B lowering (<90 mg/dL) may also be considered for judgingtherapeutic efficacy.314,319,326 When the recommended goals are not being achieved after lifestyle interventions, statin therapy should be initiated, starting with a moderate-intensity statin. For persons older than 40 years without diagnosed ASCVD, but who have 2 or more additional major ASCVD risk factors (very high ASCVD risk), statin therapy may be considered even if the LDL-C concentration is <100 mg/dL.245,343–345 In persons with statin intolerance or unacceptable adverse drug effects, a bile acid sequestrant should be considered alone or in combination with statin tolerated at a lower dose 347,348 or a cholesterol absorption inhibitor.349–355 No study has yet been designed to investigate the CV outcomes benefit of adding bile acid sequestrants or cholesterol absorption inhibitor to statins in persons whose atherogenic markers (LDL-C, non-HDL-C, and apo B) are not already at target levels. Addition of PCSK9 inhibitors has been shown to be highly effective for lowering LDL-C and apo B, and for lowering CV event rates, but to date have not translated to overall mortality benefits.354
In persons with end-stage kidney disease to include hemodialysis treatment and in those with advanced HF, there is no clear evidence that LDL-Celowering therapy provides ASCVD benefit.356 Persons with eGFR <45 mL/min/1.73 m2 who are not dialysis-dependent are at very high to extremely high risk for ASCVD events and should be treated to achieve LDL-C, non-HDL-C, and apo B goals with a statin and ezetimibe,357 because higher doses of statins alone have not been proven to be safe in the setting of CKD. Such persons should be monitored closely to determine whether statin dose adjustment is necessary depending on comorbidities, drug interactions, and renal status.356 Increasing evidence now shows that older persons gain significant benefits from lipid lowering for primary prevention, and these benefits are even more impactful in persons with DM.358–361
In persons at LDL-C goal and who have a fasting triglyceride level ≥150 mg/dL or HDL-C level ≤35 mg/dL, glycemic control and lifestyle changes to maintain a healthy weight are recommended.324,343 In persons with fasting triglycerides of 200 to 499 mg/dL, despite tight adherence to a healthy diet (to include reduced intake of simple carbohydrates and avoidance of fruit juices and alcohol) and optimized glycemia control, prescribe statin therapy to the maximum tolerated dose to achieve goals for non-HDL-C or apo B, since all apo B–containing particles are potentially atherogenic.312,313,324,362,363 Nonstatin therapies in combination with statins are often required in these settings.364
In persons with persistently elevated fasting triglycerides >200 mg/dL who are receiving maximally tolerated LDL-Celowering statin therapy, adding triglyceride-lowering drugs such as a fibrate or high-dose omega-3 fatty acid may be helpful to further reduce non-HDL-C.365–368 The Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial used icosapent ethyl added to statin-treated persons and reported a significant beneficial CV outcome in participants with hypertriglyceridemia, though this effect was independent of triglyceride lowering.369 To date, aside from icosapent ethyl, fish oil therapy has not been shown to prevent CV adverse events.313,370,371
If the fasting triglyceride concentration is ≥500 mg/dL (ie, severe hypertriglyceridemia), begin treatment with a very low-fat diet and initiate a fibrate or high-dose omega-3-fatty acid treatment. Dietary therapy should be strongly emphasized as it remains the most poorly adherent triglyceride-lowering intervention, and all these therapies may be required in combination to control severe hypertriglyceridemia.372 Niacin use is not encouraged as it leads to dysglycemia but may be considered in refractory cases. Observational data and retrospective analyses support triglyceride-lowering therapy for prophylaxis against acute pancreatitis.373 Rule out other secondary causes and reassess lipid status when the triglyceride concentration is <500 mg/dL. Additional statin therapy and possibly other agents are usually required to achieve the primary LDL-C, apo B, and non-HDL-C goals.374–376 No RCT has yet been reported to investigate the benefit of reducing severe (>500 mg/dL) or moderate (>200 mg/dL) hypertriglyceridemia to prevent CVD.313,370,371
Modification of triglycerides with the proliferator activated receptor-alpha agonist fenofibrate failed to reduce ASCVD events in 2 separate trials in persons with T2D: (Fenofibrate Intervention and Event Lowering in Diabetes [FIELD]377 and ACCORD-Lipid),367 although the mean baseline triglyceride levels were 153 mg/dL in FIELD377 and 162 mg/dL in ACCORD-Lipid.367 Post hoc and prespecified subgroup analyses and meta-analyses of 5 major fibrate trialsdHelsinki Heart Study, Veterans Affairs HDL Intervention Trial, Bezafibrate Infarction Project, FIELD, and ACCORD-Lipiddhave shown a CV benefit in persons with moderate dyslipidemia (triglycerides >200 mg/dL and HDL-C <40 mg/dL, either isolated or together) but not in persons without dyslipidemia.286,378–381
Two separate RCTs tested the HDL-C–raising hypothesis inpersons with coronary artery disease optimally treated with statins with or without ezetimibe. In the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes trial, the atherogenic markers LDL-C, non-HDL-C, and apo B were 74, 108, and 81 mg/dL, respectively, prior to randomization.382 Before randomization in Heart Protection Study 2dTreatmentof HDL to Reduce the Incidence of Vascular Events, LDL-C, non-HDL-C, and apo B were 63, 84, and 68 mg/dL, respectively, and triglyceride and HDL-C levels were 120 mg/dL and 44 mg/dL, respectively.383 In each of these trials, the addition of niacin resulted in small improvements in lipids, but these changes were not accompanied by any significant reduction in ASCVD events.382,383 Thus, niacin cannot be recommended as adjunctive therapy if LDL-C, non-HDL-C, and apo B goals are already met. Niacin may have a role when optimized therapy fails to control triglycerides >1000 mg/dL.
Managing Dyslipidemia
If lipid goals are not achieved after initiating treatment, lipid-lowering therapy should be intensified, and apo B determination may also be useful to confirm goal attainment.73,286,314 LDL-C, calculated non-HDL-C (total cholesterol – HDL-C), and apo B are the primary targets of therapy, with respective goals set according to ASCVD risk stratification (Fig. 1). If LDL-C is at goal but non-HDL-C or apo B remain above goal after maximally tolerated statin therapy, consider additional apo B or triglyceride-lowering therapies, such as ezetimibe, a cholesterol absorption inhibitor, or PCSK9 inhibitor or PCSK9-interfering therapy.73,286,342
Lipid Management in Prediabetes
The principles and goals of lipid management in individuals with prediabetes are the same as those with DM described previously (Fig. 1). No randomized intervention trials dedicated to persons with prediabetes use ASCVD events as outcome measures. Lifestyle change for a healthy diet, daily activity and regular weekly exercise, and healthy weight maintenance should be emphasized for all persons with prediabetes.
Moderate-potency or high-potency statins, possibly combined with cholesterol absorption inhibitors or bile acid sequestrants, are effective for achieving LDL-C, non-HDL-C, and apo B goals in persons with prediabetes.324 Low HDL-C is also common in prediabetes, and low HDL-C and high triglycerides are both associated with increased atherogenic lipoprotein particles. Niacin is effective in raising HDL-C, but it also increases insulin resistance and may accelerate the appearance of overt DM. Fibrates may be considered, but the use of gemfibrozil is discouraged owing to its interaction with statin clearance and the risk for severe rhabdomyolysis.
Meta-analyses of statin RCTs indicate that statin use is associated with significant increases in the risk of progression to T2D among persons with prediabetes: a 9% increase with moderate statin dosing and 12% increase with high statin dosing.384,385 Persons with prediabetes should be warned of the potential added risk of conversion to DM with statin use. The net comparison of benefit vs risk is >4 ASCVD events prevented for one conversion from prediabetes to DM.386 This risk-benefit analysis, considering the individual risk of converting to DM vs prevention of ASCVD, should be discussed when initiating statin therapy.
Question 6: How should DKD or CKD in DM be managed?
DKD refers to kidney disease attributable to DM. DKD replaced the older term, “diabetic nephropathy,” which referred to specific glomerular lesions of nodular glomerulosclerosis and glomerular basement membrane thickening, because of emerging evidence for a variety of other types of structural kidney injury caused by DM.197
DM and CKD or CKD in DM refer to DM accompanied by low eGFR and/or albuminuria/proteinuria without specification for cause. This is the inclusion criteria used for most of the large outcomes trials for kidney disease in DM. Therefore, though many study participants may have had DKD, others could have had CKD from another cause in the setting of DM.
Recommendation 6.1
Annual assessment of serum creatinine to determine the eGFR and urine albumin-to-creatinine ratio (UACR) is recommended to identify, stage, and monitor progression of DKD, also referred to as CKD in DM. Begin annual DKD assessment 5 years after diagnosis in persons with T1D or at diagnosis in persons with T2D.
Grade B; BEL 2
Recommendation 6.2
Advise persons with CKD in DM about optimal glycemic control, BP control, lipid control, and smoking cessation to reduce risks of development and progression of CKD and CVD. (See also R 4.1 to R 4.5 on BP control, R 5.1 to R 5.10 on lipid management, and R 12.1.1 to R 12.2.19 on glycemic control.)
Grade A; BEL 1
|
Practice Points In moderate-to-severe CKD (stages 3 to 5), check UACR and eGFR more frequently (eg, every 3 to 6 months), depending on rate of progression and comorbidities. Measure UACR and eGFR after medication additions or adjustments (eg, ACE inhibitors, ARBs, SGLT2is, finerenone, nonsteroidal anti-inflammatory drugs, proton pump inhibitors) or change in clinical status that may affect kidney function (eg, iodinated contrast administration, acute illness). Assess for complications of CKD, including anemia and bone and mineral metabolism disorders, in severe CKD (stages 4 to 5). Referral to a nephrologist is recommended by CKD stage 4 or earlier if there are concerns about kidney disease diagnosis, rapid progression, complications, or management. |
Recommendation 6.3
RAAS blockade with an ARB or an ACE inhibitor is recommended for persons with albuminuria (T1D or T2D) to reduce risk of DKD or CKD in DM progression (see Fig. 3 for category definitions).
Figure 3.
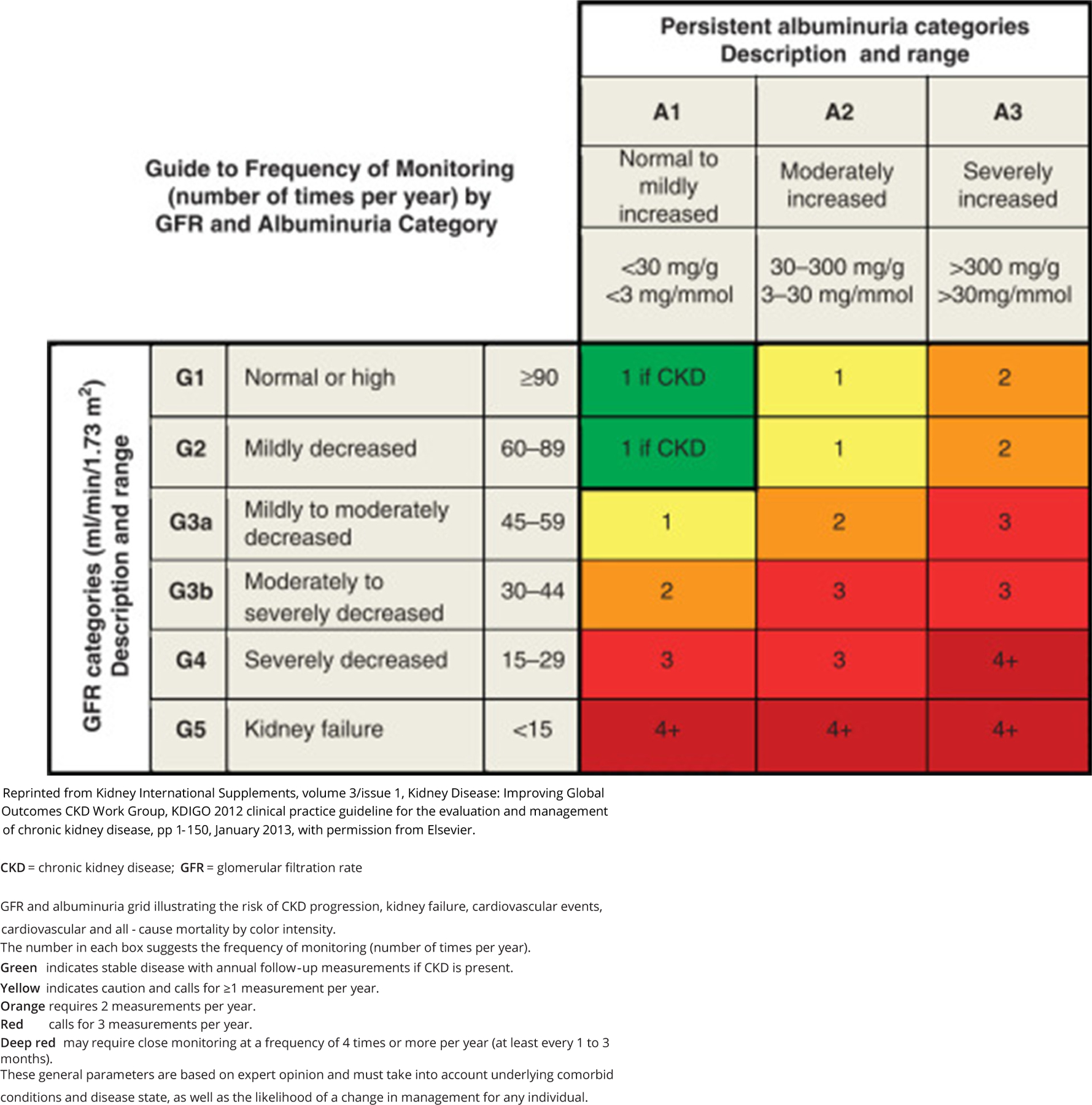
Guide to Frequency of Monitoring (Number of Times per Year) by Glomerular Filtration Rate and Albuminuria Category
Reprinted from Kidney International Supplements, volume 3/issue 1, Kidney Disease: Improving Global Outcomes CKD Work Group, KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease, pp 1– 150, January 2013, with permission from Elsevier.
CKD = chronic kidney disease; GFR = glomerular filtration rate
GFR and albuminuria grid illustrating the risk of CKD progression, kidney failure, cardiovascular events, cardiovascular and all - cause mortality by color intensity.
The number in each box suggests the frequency of monitoring (number of times per year).
Green indicates stable disease with annual follow-up measurements if CKD is present.
Yellow indicates caution and calls for ≥1 measurement per year.
Orange requires 2 measurements per year.
Red calls for 3 measurements per year.
Deep red may require close monitoring at a frequency of 4 times or more per year (at least every 1 to 3 months).
These general parameters are based on expert opinion and must take into account underlying comorbid conditions and disease state, as well as the likelihood of a change in management for any individual.
Grade A; BEL 1
Recommendation 6.4
An SGLT2i with proven benefit is recommended as foundational therapy for persons with T2D and CKD with eGFR ≥20 mL/min/1.73 m2 to reduce progression of CKD and risk of CVD.
Grade A; BEL 1
Recommendation 6.5
A GLP-1 RA with proven benefit is recommended for persons with T2D and DKD or CKD in DM with eGFR ≥15 mL/min/1.73 m2 for glycemic control and to reduce risk of ASCVD and progression of albuminuria.
Grade A; BEL 1
Recommendation 6.6
A nonsteroidal mineralocorticoid receptor antagonist (finerenone) with proven kidney and CVD benefit is recommended for persons with T2D, an eGFR ≥25 mL/min/1.73 m2, normal serum potassium concentration, and albuminuria (UACR 30 mg/g) despite a maximum tolerated dose of a renin-angiotensin system inhibitor.
Grade A; BEL 1
|
Practice Points Serum potassium levels and eGFR should be monitored within 2 to 4 weeks after initiating an ACE inhibitor, an ARB, an SGLT2i, finerenone, or with changes in these medications. Finerenone can be used for persistent albuminuria in addition to an ACE inhibitor or an ARB and SGLT2i, or in people with CKD in DM who cannot take an SGLT2i. In the absence of albuminuria and with normal BP, ACE inhibitors or ARBs do not prevent DKD onset. ACE inhibitors and ARBs should not be used together due to increased risks of adverse effects, particularly hyperkalemia and AKI. ACE inhibitors and ARBs are not safe for use in pregnant women. |
Evidence Base 6: How should DKD or CKD in DM be managed?
DKD or CKD in DM accounts for nearly half of all cases of kidney failure that require kidney replacement therapy (dialysis or transplant) in the United States and occurs in about 40% of persons with T2D and 30% of those with T1D, increasing with duration of DM.387–389 Classical diabetic nephropathy is represented histologically by the presence of basement membrane thickening, mesangial expansion, podocyte loss, and nodular or diffuse glomerulosclerosis.197,390 Many other pathological changes (tubulointerstitial inflammation and fibrosis, arteriolar hyalinosis, mesangiolysis, glomerular capillary aneurysms) may also occur as a consequence of DM.391 The pathologic changes may be present prior to development of albuminuria or low eGFR.390,392 Consequently, the term DKD is now preferred to diabetic nephropathy. Prevention of microvascular complications including DKD should be a managementgoal as earlyas the time of diagnosis of DM. In general, AACE concurs with guidelines by the Kidney Disease: Improving Global Outcomes (KDIGO) working group393 and the Kidney Disease Outcomes Quality Initiative Committee394 for the diagnosis and management of CKD in persons with DM, also known as DKD.
The KDIGO guidelines recommend phasing out the term micro-albuminuria and replacing it with the term albuminuria. Testing for the presence of albuminuria can be done using a spot urine sample or a timed collection, although the former is now preferred for reliability and simplicity. UACR levels >30 mg/g indicate kidney damage and are also a marker of CV risk.393,394 Increased urinary albumin may be seen in the setting of urinary tract or systemic infection, after exercise, or in the presence of hematuria, so confirmation is necessary to establish the diagnosis of DKD or CKD in DM. A UACR of >300 mg/g indicates greater damage and greater risk for progression to kidney failure and development of CKD complications such as anemia, CVD, and infections. Sudden onset or rapidly increasing albuminuria should prompt additional tests to assess for other types of kidney disease. Table 11 lists correlations between albuminuria, urine dipstick, and tests of total protein excretion.
Table 11.
| Categories | |||
|---|---|---|---|
|
| |||
| Measure | Normal to mildly increased (A1) | Moderately increased (A2) | Severely increased (A3) |
| AER (mg/24 h) | <30 | 30–300 | >300 |
| PER (mg/24 h) | <150 | 150–500 | >500 |
| ACR (mg/mmol) (mg/g) | <3 <30 |
3–30 30–300 |
>30 >300 |
| PCR (mg/mmol) (mg/g) | <15 <150 |
15–50 150–500 |
>50 >500 |
| Protein reagent strip | Negative to trace | Trace to + | + or greater |
Abbreviations: ACR = albumin-to-creatinine ratio; AER = albumin excretion rate; PCR = protein-to-creatinine ratio; PER = protein excretion rate.
Reprinted with permission from Macmillan Publishers Ltd: Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [EL 4; NE]
Albuminuria and proteinuria can be measured using excretion rates in timed urine collections, ratio of concentrations to creatinine concentration in spot urine samples and using reagent strips in spot urine samples. Relationships among measurement methods within a category are not exact. For example, the relationships between AER and ACR and between PER and PCR are based on the assumption that average creatinine excretion rate is approximately 1.0 g/d or 10 mmol/d. The conversions are rounded for pragmatic reasons. (For an exact conversion from mg/g of creatinine to mg/mmol of creatinine, multiply by 0.113.) Creatinine excretion varies with age, sex, race, and diet; therefore, the relationship among these categories is approximate only. ACR <10 mg/g (<1 mg/mmol) is considered “normal”; ACR 10 to 30 mg/g (1 to 3 mg/mmol) is considered “high normal.” ACR >2200 mg/g (>220 mg/mmol) is considered “nephrotic range.” The relationship between urine reagent strip results and other measures depends on urine concentration.
eGFR should be calculated from the serum creatinine by the Chronic Kidney Disease Epidemiology (CKD-EPI). The CKD-EPI equation is more accurate for calculation of eGFR above 60 mL/min/1.73 m2 than the prior Modification of Diet in Renal Disease equation, and the CKD-EPI equation is currently preferred.393 Importantly, the CKD-EPI equation has been updated to a new version that is race agnostic and termed CKD-EPI 2021. The American Society of Nephrology and the National Kidney Foundation have recommended immediate implementation for CKD-EPI 2021 as an important strategy to advance CKD care and reduce health disparities by racial identification.395–397 Figure 3 depicts the classification system for CKD that incorporates both eGFR and albuminuria in the risk assessment. Note that in Figure 3, stage 3 CKD has been divided into 2 categories: G3a for eGFR 45 to 60 mL/min/1.73 m2 and G3b for eGFR 30 to 45 mL/min/1.73 m2. The terminology used to describe CKD provides a composite picture by integrating the cause, eGFR, and UACR. For example, a person with DM, an eGFR of 40 mL/min/1.73 m2, and an UACR of 250 mg/g creatinine would be categorized as “diabetes/G3b/A2.” The “heat grid” shown in Figure 3 indicates the terminology, the level of risk for CVD events and progression of kidney disease by color intensity, and the recommended frequency for monitoring UACR and eGFR.393,398,399 Progression of CKD is considered rapid if the decline in eGFR is ≥5 mL/min/1.73 m2 per year or if the person has a rapid increase in albuminuria.
High levels, as well as variability, of both BG and BP are important risk factors for DKD. 89,184,400–403 Prevention of the development of DKD includes optimal control of glycemia and BP with RAAS inhibition as first-line therapy.394,404,405 Intensive glucose control (A1C levels <7% in T2D and <7.5% in T1D) in several clinical trials was found to reduce the risk of incident albuminuria (A2) and DKD onset.70,78,90,406–410 However, intensive glucose control has not been shown to diminish DKD or CKD in DM progression and may increase risk of CVD mortality in persons with established DKD or CKD in DM. Moreover, glycemic targets need to be individualized due to increased risk of hypoglycemia in persons with DKD or CKD in DM.
The KDIGO guidelines recommend that persons with CKD in general be treated to a BP <120/70 mm Hg, but <130/80 mm Hg may be appropriate in people who have DM or DKD or CKD in DM.209 Although care must be taken to avoid orthostasis and drug side effects, AACE recommends individualized BP targets, with a goal of <130/80 mm Hg for most persons (see Q4. How should hypertension be managed in persons with DM?).
Smoking cessation and lipid lowering are also important interventions for prevention of CVD complications of DM, which are increased at every level of CKD.398 Therapy with statins reduces the relative risk of major vascular events in persons with DM by 17% for every 39 mg/dL decrease in LDL-C.333 Persons with DM and CKD up to stage 4, including those who have had kidney transplants, receive CVD benefit from lipid-lowering with statins. However, the beneficial effect of statins is not apparent in persons who require dialysis.333,357,411–413
Slowing the progression of DKD or CKD in DM is critical for reducing risks of kidney failure and CVD, including HF, atherosclerotic events,414–422 and related causes of death. Therapies shown to positively affect albuminuria and declining eGFR include ACE inhibitors, ARBs, SGLT2is, GLP-1 RAs, and the nonsteroidal mineralocorticoid antagonist finerenone.197,209,239,242,393,423–435 Persons with albuminuria and T1D or T2D should be treated with an ACE inhibitor or ARB at the highest tolerated dose based on the drug label for approval.188,197,430,436–439 Data are lacking on the effectiveness of ACE inhibitors and ARBs in persons with DM and reduced eGFR who do not have albuminuria. However, AACE recommends RAAS blockade in all persons with DM who have CKD categories G2, G3a, G3b, and G4. The RAAS-blocking drugs may potentiate hyperkalemia and AKI when used with nonsteroidal anti-inflammatory drugs. Risk of AKI is also increased in persons with volume depletion or bilateral renal artery stenosis who use ACE inhibitors or ARBs. RAAS-blocking drugs are not safe for use in pregnancy. Combination therapy with an ACE inhibitor and an ARB or with a renin inhibitor added to another RAAS-blocking agent does not prolong survival or prevent progression of CKD.263,440,441 In persons with advanced CKD (G3b and higher), combination therapy increases the risk of hyperkalemia and AKI and is therefore not recommended.263,441,442
On top of the prevailing standard of care with an ACE inhibitor or an ARB, there has been a major upsurge in new highly effective therapies for people with T2D to reduce risks of DKD or CKD in DM progression, kidney failure, HF, ASCVD, and death for some agents. An SGLT2i with proven kidney protection is recommended for people with T2D who have an eGFR >25 mL/min/1.73 m2 irrespective of albuminuria to reduce risks of DKD or CKD in DM and CVD.425,443–469 In those with HF, one agent in the class, empagliflozin, can be used with an eGFR as low as 20 mL/min/1.73 m2.470 If the eGFR drops below this level on treatment, the SGLT2i does not have to be stopped unless the person proceeds to kidney failure requiring kidney replacement therapy by dialysis or kidney transplant. The recognized side effects of SGLT2 inhibition, including genital mycotic infections, volume depletion, DKA, or hypoglycemia when used with insulin or insulin secretagogues, are not greater in persons with lower levels of eGFR.471 Initial reports of higher rates of lower extremity amputation with canagliflozin have not been substantiated in subsequent studies of this agent or other SGLT2is. However, people with DKD or CKD in DM are at higher risk of lower extremity amputations in general, making good diabetic foot care essential (see R 8.6 on diabetic foot exams). Risk mitigation strategies for SGLT2is are the same as for other individuals with DM (Table 12). SGLT2is also tend to lower serum potassium, which may mitigate risks of hyperkalemia and allow greater use of other kidney and heart protective agents such as ACE inhibitors, ARBs, and mineralocorticoid antagonists. Additionally, the risk of AKI is actually reduced by 25% with SGLT2i use in persons with T2D.461
Table 12.
Mitigation of Side Effects for Newer Agents to Treat Diabetic Kidney Disease
| Side effects | Mitigation strategies |
|---|---|
| SGLT2 inhibitors | |
| Genital mycotic infections Volume depletion | ○ Hygiene, topical antifungals ○ Proactive dose reduction of diuretics in persons at risk for hypovolemia ○ Hold SGLT2is during GI illness (nausea, vomiting, diarrhea) ○ Improve glucose control to reduce glucosuria |
| Ketoacidosis | ○ Educate persons with DM on early recognition ○ “STOP DKA” protocol (stop SGLT2i, test for ketones, maintain fluid and carbohydrate intake, use maintenance and supplemental insulin) |
| Hypoglycemia | ○ Adjustment of background antihyperglycemic agents |
| GLP-1 receptor agonists | |
| Nausea/vomiting/diarrhea | ○ Patient education on tolerability and symptom recognition ○ Start at lowest dose and titrate slowly |
| Hypoglycemia | ○ Adjustment of background antihyperglycemic agents |
| Finerenone | |
| Hyperkalemia | ○ Dietary restriction of potassium ○ Thiazide or loop diuretics ○ SGLT2i ○ Potassium-binding agents (patiromer or sodium zirconium cyclosilicate) |
Abbreviations: DM = diabetes mellitus; GI = gastrointestinal; GLP-1 = glucagon-like peptide 1; SGLT2i = sodium-glucose cotransporter 2 inhibitor
A GLP-1 RA is recommended as another option to reduce risks of ASCVD, macroalbuminuria, and eGFR decline in T2D.429,472–479 In advanced CKD (eGFR <30 mL/min/1.73 m2), GLP-1 RAs retain glycemic efficacy without increased risk of hypoglycemia and can be used to control BG with an eGFR as low as 15 mL/min/1.73 m2, depending on the agent.429 Similar to the SGLT2is, side effects of GLP-1 RAs are not different in persons with lower levels of eGFR (Table 12). Because gastrointestinal (GI) symptoms such as nausea, vomiting, and diarrhea may occur more frequently when kidney function is reduced, consider slowing uptitration of drug doses. As for other persons with DM, adjustment of other glucose-lowering agents may be needed to prevent hypoglycemia.
The nonsteroidal mineralocorticoid antagonist finerenone is also recommended for kidney and heart protection in T2D with eGFR >25 mL/min/1.73 m2, normal serum potassium concentration, and albuminuria (UACR ≥ 30 mg/g) despite a maximally tolerated dose of a renin-angiotensin system inhibitor, because it reduced risks of substantial GFR decline, kidney failure, HF and ASCVD events, and related deaths in a broad T2D population ranging from those with microalbuminuria to advanced CKD. 434,435,480–482 The main side effect of finerenone is hyperkalemia, which usually can be managed with dietary restriction and concurrent use of diuretics or SGLT2is (Table 12). Potassium-binding agents such as partiromer or sodium zirconium cyclosilicate are also a consideration for control of hyperkalemia.
Dietary protein management may add benefit to risk factor control and drug therapies for CKD. KDIGO recommends limiting protein intake to 0.8 g/kg per day (the recommended daily allowance in the United States) in persons with DKD or eGFR <30 mL/min/1.73 m2.430 As described above, dietary approaches also help to control high levels of potassium as well as phosphorus. Sodium intake should be limited to 2 g per day in persons with DM who require antihypertensive medications. With obesity being a risk factor for hypertension and incident CKD, weight management including diet, physical activity, and other weight-loss strategies (eg, US Food and Drug Administration [FDA]-approved pharmacotherapy, bariatric surgery, GLP-1 RAs) may be considered for persons with DM.
Persons with CKD or CKD in DM are at risk for various types of drug toxicity and AKI. Glucose-lowering therapies may need to be modified to reduce hypoglycemia.483 Many other drugs should be avoided or used with caution in persons with low eGFR. Individuals should be informed of their CKD diagnosis and should avoid dehydration and imaging that requires gadolinium, high phosphate-containing bowel preparations, or high doses of iodinated contrast agents.
Persons with CKD in DM should undergo annual or more frequent assessment of electrolytes to assess potassium and acid-base status; blood counts to assess anemia status; and calcium, phosphorus, 25(OH) vitamin D, and parathyroid hormone (PTH) measurements to assess mineral metabolism.209 Hyperkalemia may be managed by dietary restriction, potassium binding agents, and adjustment of antihypertensive medications. For those with a serum bicarbonate level <22 mEq/L, the addition of oral sodium bicarbonate is recommended to correct the serum bicarbonate level. Anemia, defined as hemoglobin <13 g/dL in men and <12 g/dL in women, should be further investigated with iron, transferrin saturation, ferritin, vitamin B12, and folate levels.484 Deficiencies should be replaced and a transferrin saturation target of ≥30% achieved, regardless of ferritin level484. Iron given intravenously may produce better results than oral replacement. AACE recommends adequate calcium intake and achievement of 25(OH) vitamin D levels of >30 ng/dL in all persons. Supplementing vitamin D2 or D3 may reduce PTH in persons with CKD and secondary hyperparathyroidism.484,485 Active vitamin D preparations are usually necessary to keep the PTH level from increasing as eGFR declines. Hyperphosphatemia should be corrected into the normal range with dietary modification and use of phosphate binders.
Referral to a nephrologist is appropriate when the presentation is atypical, progression of albuminuria or decline in eGFR is rapid, or when secondary manifestations of CKD require expert advice. Referral of persons with stage 4 CKD to a nephrologist allows time for sufficient planning to accommodate individual personal needs.486 Kidney transplantation is the preferred kidney replacement therapy for persons with DM because long-term outcomes are superior to those achieved with dialysis. For persons with T1D, the possibility of combined kidney-pancreas transplantation delivers considerably better outcomes.487
Question 7: How should retinopathy be managed in persons with DM?
Recommendation 7.1
It is recommended that persons with T2D or adult-onset T1D should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist at the time of diagnosis or shortly after diagnosis. Individualized subsequent screening can be based on type and duration of DM, A1C or mean BG, BP, and the presence and grade of retinopathy.
Grade A; BEL 2 and expert opinion of task force
Recommendation 7.2
In persons with T1D, an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist should be performed within 5 years of diagnosis in children and adolescents.
Grade B; BEL 4 and expert opinion of task force
Recommendation 7.3
Women who are pregnant and have preexisting T1D or T2D should be monitored with eye examinations every trimester during pregnancy and in the postpartum period as determined by the severity of retinopathy during pregnancy.
Grade B; BEL 2
Recommendation 7.4
Persons with greater than mild nonproliferative retinopathy should have examinations at least once a year and more frequently as advised by their eyecare specialist.
Grade B; BEL 4 and expert opinion of task force
Recommendation 7.5
Follow-up with eyecare specialists typically should occur on an annual basis, but persons with T1D or T2D who have had a normal ocular examination may be screened every 2 to 3 years.
Grade B; BEL 2 and expert opinion of task force
Recommendation 7.6
Optimal glucose, BP, weight, and lipid control should be implemented to slow the progression of retinopathy.
Grade B; BEL 1
Recommendation 7.7
Artificial intelligence systems, authorized by the FDA for detecting greater than mild diabetic retinopathy, can be used as an alternative to traditional screening approaches. These systems can facilitate diagnosis of vision-threatening retinopathy and identification of persons who require ophthalmologic visits for treatment.
Grade B; BEL 1
Evidence Base 7: How should retinopathy be managed in persons with DM?
Diabetic retinopathy is the leading cause of blindness in adults. The stages of diabetic retinopathy include nonproliferative retinopathy, preproliferative retinopathy, and proliferative retinopathy, with macular edema occurring at any stage. The prognosis for retaining vision has improved dramatically over the past 40 years owing to improved metabolic and BP control.78,432,488–490 Diabetic retinopathy is present in 25% to 45% of persons with T2D, and between 2% and 8% of persons with T2D have proliferative retinopathy and/or macular edema.491 Diabetic retinopathy is present in approximately 20%, 40%, and 70% of persons with T2D after <10, 10 to 20, and >20 years of the disease, respectively, with prevalence rates of proliferative retinopathy and/or macular edema around 2%, 10%, and 25% at the respective durations.492 A 2020 meta-analysis of data from Europe revealed that any retinopathy was present in 25.7% of persons and diabetic macular edema occurred in 3.75%.493 The prevalence of diabetic retinopathy is predicted to continue to increase through the next 2 decades.494
Moreover, teenagers and young adults with T1D and T2D diagnosed during childhood (mean 7.8 years duration) have a prevalence of retinopathy of 9.1% and 5.6%, respectively.417,495 Higher levels of glycemia and BP, as well as the presence of nerve and renal diabetic complications, are associated with a greater likelihood of developing retinopathy.496,497 Nonhealing ulcers and bacterial infections increase the rate of progression of retinopathy,496,498 so persons with infections may merit close ophthalmologic care during this period; African American and Hispanic populations have increased likelihood of diabetic macular edema compared with non-Hispanic Whites.499
Like other complications, it is important to detect greater than mild nonproliferative retinopathy before vision is threatened. Ophthalmoscopy without pupil dilation is suboptimal so referral to an experienced ophthalmologist or optometrist for an annual dilated eye examination is recommended.500 Annual examinations have long been recommended as the standard approach, but data suggest that individualized risk assessment and persons with well-controlled DM and no retinopathy at baseline can be safely examined at 2- to 3-year intervals.501,502 The ophthalmologic examination can also detect other common conditions such as cataracts, glaucoma, and macular degeneration. The use of nonmydriatic fundus cameras equipped with digital transmission technology enables large-scale point-of-care (POC) screening for retinopathy.503 Screening programs have been most successful in defined populations with government-organized health care systems, such as in the United Kingdom and Scandinavia and in American Indian tribes rather than in the general population of the United States.503–506 Persons with abnormal retinal photographs are referred to an ophthalmologist for a complete eye examination. This two-step approach can be an effective strategy for retinopathy screening at the population level, particularly in remote areas.507
Artificial intelligence approaches to screening for diabetic retinopathy have progressed to the point of utility and cost-effectiveness.508–513 Given the relatively low prevalence of proliferative retinopathy and/or macular edema in persons with T2D during the first decade after diagnosis, however, the suggestion is now being made that persons with T2D who have had a negative ophthalmologic examination may safely have the screening interval increased to 2 or 3 years.514–517 Retinopathy develops over a period of 5 or more years from initial hyperglycemia, so screening should be initiated within 5 years of diagnosis in persons with T1D.518 Pregnancy is a risk factor for progression of retinopathy, and ophthalmologic examinations should be performed repeatedly during pregnancy and for 1 year postpartum.519 Persons with active lesions may be followed more frequently, whereas those who have had repeatedly normal eye findings can be seen less frequently. Elevated prepregnancy A1C and duration of T1D >10 years predict progression.520 Teenage girls may have more difficulty controlling T1D than do teenage boys and thus risk more complications.521 Thus, multiple systemic factors influence the risk of the development and progression of diabetic retinopathy.
Management of retinopathy requires attention to multiple systemic and ocular factors. Optimization of glucose and BP are the proven strategies for primary prevention of diabetic retinopathy and for slowing the progression of preexisting nonproliferative retinopathy.78,185,186,193,522,523
Other options that can stabilize retinopathy include lifestyle intervention or bariatric surgery in persons with T2D or physical activity in persons with T1D.524–527 One study suggested that dietary marine omega-3 fatty acids may slow sight-threatening retinopathy, but further investigation is needed.528
In addition, pharmacologic treatment approaches may have specific benefit in diabetic retinopathy, including ACE inhibitors, ARBs,529,530 and fibrate lipid-lowering agents.531–533 Research into other novel pharmacologic agents with potential benefits may lead to additional medical treatments.534
The ophthalmic treatment of proliferative retinopathy has evolved in the past decade. Panretinal laser photocoagulation has been the primary treatment for decades because it provides enduring effects. Anti-vascular endothelial growth factor (VEGF) antibodies also inhibit neovascularization.535 However, after 5 years of follow-up, the rate of visual field sensitivity is equivalent in the drug- and laser-treated groups.536 Moreover, persons who fail to return for continued anti-VEGF treatments are at risk of losing vision.537,538 Therefore, treatment should be individualized, in some cases combining panretinal photocoagulation and anti-VEGF therapy.539 Vitrectomy effectively restores vision for many persons with persistent vitreous hemorrhage, vitreous scarring, and detachment.540 Diabetic macular edema in the absence of proliferative retinopathy is most commonly treated with repeated anti-VEGF injections.541
Question 8: How should neuropathy be diagnosed and managed in persons with DM?
Recommendation 8.1
Diabetic peripheral neuropathy (DPN) is a clinical diagnosis. A comprehensive differential diagnosis should be considered to rule out nondiabetic neuropathies.
Grade B; BEL 2
Recommendation 8.2
Screening for DPN should be done at diagnosis of T2D, within 5 years of the diagnosis of T1D, and subsequently annually or whenever symptoms occur, by performing a clinical history and physical exam.
Grade B; BEL 2
Recommendation 8.3
Assessments for DPN should include a careful history to assess target symptoms and a combination of at least two of the following: vibration sensation using a 128-Hz tuning fork, pinprick sensation, temperature discrimination, 10-g monofilament testing on the dorsal aspect of the great toe bilaterally, and ankle reflexes. All these assessments should follow the typical DPN pattern, starting distally (the dorsal aspect of the hallux) on both sides and move proximally until a sensory threshold is identified.
Grade A; BEL 2, upgraded by expert opinion of task force
Recommendation 8.4
Screening for CV autonomic neuropathy (CAN) should be considered at diagnosis of T2D and at 5 years after the diagnosis of T1D, including youth. Screening for CAN should also be considered in the presence of DPN, DKD, 2 or more CV risk factors, hypoglycemia unawareness, high glucose variability, in persons with HF, peri-operatively, or in individuals presenting with autonomic symptoms. A careful differential to exclude other comorbidities or drug effects/interactions that could mimic CAN should be performed.
Grade B; BEL 2
Recommendation 8.5
CV reflex tests (deep breathing, Valsalva, supine to standing) remain the gold standard and are recommended for assessment of CAN. Indices of heart rate variability (HRV) derived from electro-cardiogram recordings could also be used as an easier alternative for screening for CAN.
Grade A; BEL 2, upgraded by expert opinion of task force
Recommendation 8.6
Diabetic foot exams should be performed at every visit (in person or virtual) to identify deformities and to identify those at risk for late complications such as ulcerations and amputations.
Grade A; BEL 1
Recommendation 8.7
Intensive glucose control applied as early as possible is recommended to prevent the onset of DPN and CAN in T1D. Achieving optimal control of glucose, BP, and lipid levels along with lifestyle interventions, including weight loss and exercise, are recommended to prevent DPN and CAN in T2D. Lifestyle interventions are effective for DPN and CAN prevention in persons with prediabetes/metabolic syndrome.
Grade A; BEL 2, upgraded by expert opinion of task force
Recommendation 8.8
Pregabalin, duloxetine, and capsaicin 8% patch are recommended for the treatment of neuropathic pain due to DM and have received regulatory approval in the United States. Current evidence shows that these agents are effective in reaching 30% to 50% reduction in pain in many individuals (Grade A; BEL 1). However, gabapentin and some tricyclic antidepressants may be as effective to achieve a clinically meaningful reduction in diabetic neuropathic pain (Grade B; BEL 1). Combining two or more agents from different classes may have enhanced benefits with lower adverse effects and risks than maximizing the dose of one medication or using opioids. The use of opioids, including tapentadol or tramadol, is NOT RECOMMENDED due to high risk of addiction and other complications.
Grade A; BEL 1
Recommendation 8.9
Lifestyle interventions including a combination of regular aerobic, strengthening, and balance exercises, reduction of sedentary behavior, and dietary modification aimed at reducing calorie intake and increasing plant-based and polyunsaturated fats are recommended. Neuromodulatory techniques such as high-frequency spinal cord stimulation and combining pharmacological with nonpharmacological approaches should be considered in those with refractory painful DPN.
Grade B; BEL 1
Evidence Base 8: How should neuropathy be diagnosed and managed in persons with DM?
Diabetic neuropathy affects about half of all persons with DM, contributing to substantial morbidity and mortality and resulting in a huge economic burden for DM care due to the increased risk of associated complications such as pain, sleep disturbances, falls and fractures, reduced QoL, polypharmacy, and socioeconomic consequences.417,418,542–552 Persons with prediabetes and/or obesity may also develop DPN.547,548,553
Consensus in the field is that diabetic neuropathies are defined by the presence of symptoms and/or signs of peripheral and autonomic nerve dysfunction in people with DM after the exclusion of other causes confirmed through clinical examination and appropriate differential tests. Among the various forms of diabetic neuropathy, distal symmetric polyneuropathy and diabetic autonomic neuropathies, particularly CAN, are by far the most studied.547 There are several atypical forms of diabetic neuropathy as well. A comprehensive classification of diabetic neuropathies was updated by the ADA’s 2017 position statement on diabetic neuropathy.547
Symptoms of DPN vary depending on the class of sensory nerve fibers involved. The earliest affected nerve fibers are usually the small myelinated and unmyelinated fibers; thus, the most common early symptoms are pain and dysesthesias (unpleasant sensations of burning).547,554,555 Neuropathic pain may be the first symptom that prompts persons to seek medical care and is present in up to 25% to 30% of individuals with DPN.547,555–559 Characteristically, the pain is burning, lancinating, tingling, or shooting (electric shock-like), with paresthesia, occurring in varying combinations, and is typically worse at night547 Neuropathic pain may be accompanied by an exaggerated response to painful stimuli (hyperalgesia) and pain evoked by contact, with socks, shoes, and bedclothes (allodynia) for example.547,556,557,560,561 Neuropathic pain can lead to interference with daily activities, disability, psychosocial impairment, and reduced health-related QoL.547,552,562,563 The direct and indirect economic burden associated with neuropathic pain is substantial.
The involvement of large fibers may cause numbness, tingling without pain, and loss of protective sensation.547 Persons can also initially present with a completely insensate, numb foot, stating their feet feel like they are wrapped in wool or that they are walking in thick socks.
There are several established clinical tests that may be easily used to assess small- and large-fiber function. In clinical care, assessment of pinprick and temperature (mostly cold) sensation provide reliable information on small-fiber function, whereas assessment of vibration perception (with a 128-Hz tuning fork), proprioception, light touch to 10-g monofilament, and ankle reflexes allow assessments of large-fiber function (Table 13) all in a “stocking and glove” distribution. Assessment of light touch perception using a 10-g monofilament should be performed on the dorsal aspect of the great toe bilaterally as recommended.547 However, it is important to consider that the 10-g monofilament is a useful clinical tool mainly for detecting advanced neuropathy and identifying persons at increased risk of ulceration and amputation.547,564 Loss of ankle reflexes occurs early, and later weakness of small foot muscles and dorsiflexors are also observed.547
Table 13.
Clinical Symptoms and Signs of Diabetic Peripheral Neuropathy
| Large myelinated nerve fibers | Small nerve fibers | |
|---|---|---|
| Symptoms | Numbness Tingling Poor balance |
Pain: Burning Electric shocks Stabbing pain Hyperalgesia Allodynia |
| Function | Pressure, balance | Nociception; protective sensation |
| Examination (clinically diagnostic)a | Ankle reflexes Reduced Abolished Vibration perceptiona Reduced Absent 10-g monofilamenta Reduced Absent Proprioception impaired |
Thermal (cold/hot) discriminationa Reduced Absent Pinprick sensationa Reduced Absent |
Document impairment/loss in symmetrical, distal to proximal pattern
The diagnosis of DPN is principally a clinical one. A combination of typical symptomatology and symmetrical distal sensory loss, or typical signs in the absence of symptoms, in a person with DM is highly suggestive of DPN and may not require additional evaluations or referral.547,565–567 Tests for small fibers such as pinprick and temperature discrimination are recommended for diagnosis to document the deficits in the same DPN specific pattern.547,556 Electrophysiological testing or referral to a neurologist is rarely needed for diagnosis, except in situations where the clinical features are atypical, the diagnosis is unclear, and a different etiology is suspected.547,554,565 However, the presence of atypical features including motor deficits greater than sensory, asymmetry of symptoms and signs, and rapid progression warrant a timely referral.547 Skin biopsy and/or standardized quantitative sensory testing are sensitive tests for small-fiber neuropathy and should be considered if the clinical features are atypical and a different etiology is suspected.
It is also important to remember that several peripheral neuropathies due to causes other than DM may coexist in persons with DM, mimic diabetic neuropathy, and may be treatable.547 Thus, undertaking a thorough family and medication history and performing relevant investigations are helpful to assess other potential etiologies, such as alcohol abuse, genetic neuropathies, neoplasia, toxic exposure, and amyloidosis. Laboratory screening includes vitamin B12 levels to test for B12 deficiency (particularly in persons treated with metformin), thyroid function tests, complete blood count, metabolic panel, and serum immunoelectrophoresis with immunofixation to test for a monoclonal gammopathy.547
As recommended by the Toronto Consensus on Diabetic Neuropathy,568 for research purposes, a diagnosis of confirmed diabetic neuropathy requires a combination of symptoms, signs, and abnormality of objective tests such as changes in nerve conduction studies. The symptoms and signs of DPN have been broadly covered above in the clinical section. However, for research, a range of assessments, including more objective measures and importantly, person-reported outcomes, has been developed over time and validated. The use of validated clinical instruments such as the Michigan Neuropathy Screening Instrument (most widely used in large cohorts of persons with T1D and T2D),69,417,546,550,569–571 the modified Toronto Clinical Neuropathy Scale,572 the Utah Early Neuropathy Scale,573 or the Neuropathy Disability Score574 are feasible and can be done in a standardized fashion in large cohorts of persons with DM. Instruments for painful DPN also have been validated.556,558
In addition to screening for DPN, a comprehensive foot examination at all outpatient office visits is necessary to identify foot ulcerations, infections, vascular insufficiency, and deformities that could lead to limb loss or mortality.575 The global burden of foot complications, including ulcerations, infections, and ischemia that may lead to amputation, is between 2.2% to 6.3% of persons with DM per year, and the lifetime risk of any foot complication is up to 34% in persons with DM.576,577 Recurrence of foot ulcerations after healing is common, up to 50% at 3 years.576 Most foot ulcerations have a neuropathic component577 and are preventable by foot care that includes daily inspection, nail and skin care, correction of foot deformities, and/or provision of appropriate footwear to accommodate structural changes.578 In-office examination should include inspection for vascular insufficiency, musculoskeletal deformities, skin breakdown, or abnormal skin callus formation in addition to the DPN examination.579 Referral to podiatry or others on a multispecialty team may prevent infections and ulcerations from progressing to limb loss.580 Risk assessment for diabetic foot problems is integral to comprehensive care of persons with DM.575
Prevention of Diabetic Peripheral Neuropathy
Prevention of diabetic neuropathies focuses on glucose control and lifestyle modifications. Enhanced glucose control in people with T1D dramatically reduces the incidence of DPN (78% relative risk reduction),78,542,547,549 and glucose control remains the strongest risk factor for DPN in T1D even in contemporary cohorts.417,542–544,547 Despite socioeconomic barriers being associated with DPN, one should aim to achieve near-normal glycemia in persons with T1D at risk of DPN.544 In contrast, enhanced glucose control in people with T2D reduces the risk of developing DPN more modestly.547,557 The ACCORD trial in individuals with T2D reported a modest but significant DPN risk reduction with intensive glycemia intervention after 5 years of follow-up,69 but other trials reported inconclusive effects.547 Specific glucose-lowering strategies may contribute to the discrepancy. For example, participants, particularly men, in the BARI 2D trial treated with insulin sensitizers had a lower incidence of DPN over 4 years than those treated with insulin/SUs.570 There is also emerging evidence that lifestyle modifications either as exercise alone (supervised aerobic with or without resistance training), combined dietary modification and exercise, or other behavioral interventions may have beneficial effects on preventing DPN in some persons with T2D or prediabetes.547,526,582,583,581–586 Emerging evidence shows a potential benefit of bariatric surgery on reducing risk of DPN.587
Management of Diabetic Peripheral Neuropathy
Despite major advances in elucidating the pathogenesis of diabetic neuropathy, there remains a lack of disease-modifying treatment options in individuals with DM; hence, there is an urgent need for more targeted research.
Currently, there is no convincing evidence supporting glucose control or lifestyle management as therapies for neuropathic pain in DM or prediabetes. At present, among pharmacological options, pregabalin588–598 and duloxetine594,595,599,600 are oral agents that have received regulatory approval for the treatment of neuropathic pain associated with DPN by the FDA and are effective for DPN pain reduction when using patient-reported outcomes.547 In addition, based on evidence from 2 large 12-week randomized multicenter trials in 2020, the FDA approved the cutaneous concentrated capsaicin 8% patch that works by desensitizing and interfering with the function of the transient receptor potential vanilloid 1 receptor, a protein involved in pain signaling. However, the patch needs to be applied for ~30 minutes and can be done only in the office with a physician present.601,602 The opioid, tapentadol, has received regulatory approval in the United States and Canada, but evidence for its use is, at best, inconclusive,603,604 and the ADA and other organizations strongly recommend against using any opioids for management of DPN pain.547 Reviewing evidence for the variety of agents that can modify DPN pain, one should use a stepwise approach and consider an individual’s comorbidities, socioeconomic status, and potential drug interactions.547,605 Nonsteroidal anti-inflammatory agents should be avoided for chronic pain management in persons with DM due to adverse kidney effects. Given the high risk of addiction, abuse, sedation, and other complications and psychosocial issues, even with short-term opioid use, opioids are not recommended in the treatment of painful DPN.547 High frequency (eg,10 kHz) spinal cord stimulation is a nonpharmacological approach that may be effective in persons with painful DPN that failed at least one medication, as suggested by a recent large RCT, leading to FDA approval in 2021 (Fig. 4).606,607
Figure 4.

Algorithm for Treatment of Diabetic Peripheral Neuropathy
Regular aerobic, strengthening, and balance exercise, alone or in combination; reduction of sedentary behavior; and dietary modification aimed at reducing calorie intake and increasing plant-based foods and polyunsaturated fats have all demonstrated positive outcomes for individuals with DPN, including for neuropathic pain reduction.581–583,586,608–610 Small-fiber neuropathies should be managed with foot protection (eg, padded socks); supportive shoes with orthotics, if necessary; regular foot and shoe inspection; prevention of heat injury; and use of emollient creams.547 Regular foot and nail care by a trained professional is recommended. Advanced stages of large-fiber neuropathies may require a multidisciplinary approach to include strategies to enhance muscle strength, gait, and balance training; titrate any pain or other medications that could promote dizziness and other side effects affecting gait and balance; orthotics to treat and prevent foot deformities; tendon lengthening for pes equinus from Achilles tendon shortening; and/or surgical reconstruction in case of deformities.547,609
Autonomic Neuropathies
Autonomic neuropathies affect the autonomic neurons (para-sympathetic, sympathetic, or both) and are associated with a variety of condition-specific symptoms and signs that should be evaluated during the medical history and physical examination of all individuals with DM.547 CAN is the most studied and clinically relevant of the diabetic autonomic neuropathies, whereas GI, genitourinary, and sudomotor dysfunction may also develop during the course of DM and impact a person’s QoL and optimal management.547
CV Autonomic Neuropathy
Prevalence rates for CAN vary from ~3% to 5% in early T1D, to 44% over 23 years of mean follow-up, even with the current standard of care.417,542 Prevalence rates as high as 60% have been reported in earlier cohorts with long-standing T2D or more advanced disease.547,611 Furthermore, even in more contemporary cohorts with recent-onset T2D prevalence rates of up to 25% have been observed,545,546 including in youth417,612–615 or at different degrees of glucose intolerance.616
Timely detection of CAN may help implementation of tailored interventions to prevent its progression and mitigate the risk of associated complications, including CVD, cardiac arrhythmias, myocardial dysfunction leading to congestive heart failure (CHF), CKD, and all-cause mortality.547,614,617–625 Unfortunately, CAN is frequently overlooked in clinical practice due to its characteristic subtle presentation earlier in disease.547,614
Screening and Diagnosis of CV Autonomic Neuropathy in Clinical Care
It could be quite challenging to detect CAN in its early stages in routine clinical care, as persons may be completely asymptomatic (subclinical CAN) and may only present with decreased HRV.547 The most common symptoms of CAN occur upon standing and include lightheadedness, weakness, palpitations, faintness, and syncope.547,614 Targeted questions to unveil these symptoms should be included with a medical history. The specificity of these symptoms for CAN is quite low as they may occur in many other endocrine disorders, CVD, or with use of various medications, requiring an appropriate differential.547 In addition, these symptoms occur late in the disease course. Clinicians should consider screening persons at risk for developing CAN for hypoglycemia unawareness and vice versa, as this may be associated with CAN.
Clinical Signs of CV Autonomic Neuropathy
Persons with subclinical CAN present with a decrease in HRV, usually with deep breathing or change in posture, considered the earliest clinical indicator of CAN.547,614 Although HRV testing is largely confined to the research setting, the CV reflex tests that assess changes in HRV during standardized clinical challenges such as deep breathing, Valsalva maneuver and supine-to-standing position remain the gold standard tests and are available for clinical care as well.547,614,626 However, even these relatively simple methods could be challenging in some clinical settings, highlighting the need for easily accessible diagnostic tools and/or biomarkers for screening and diagnosis of CAN for general clinical care.547,627 Indices of HRV derived from standard short electrocardiogram recordings have been recently validated as an alternative approach.627 Other evaluations requiring a variety of expensive devices have a low sensitivity and specificity for CAN and are not recommended for use in clinical care.614
As CAN progresses, persons may present with resting tachycardia with fixed heart rate (>100 bpm), exercise intolerance, nondipping BP and reverse dipping BP, and in most advanced cases with orthostatic hypotension (a fall in systolic or diastolic BP by >20 mm Hg or >10 mm Hg, respectively, upon standing without an appropriate increase in heart rate).547 Orthostatic hypotension is usually easy to document in the office by measuring the BP supine and after standing. However, as with symptoms, the specificity of the signs for CAN is low, and thorough differential is required.547 In a symptomatic person with a history of poor glucose control presenting with resting tachycardia or postural hypotension, clinicians may not need to perform additional CAN tests given costs and burden after excluding other potential causes.547
Management of CV Autonomic Neuropathy
Intensive glucose control is most effective to prevent CAN in T1D as documented by a 45% reduction in risk with intensive glucose control in the Diabetes Control and Complications Trial, a benefit that persisted during the Epidemiology of Diabetes Interventions and Complications study with a 30% reduction in incident CAN over an additional 14 years of follow-up.542,628,629 Glucose control as part of a multifactorial intervention that also targeted hypertension, dyslipidemia, and lifestyle demonstrated a 63% reduction in the rate of progression to CAN in a small T2D cohort participating in the Steno-2 trial.630 Analyses from the ACCORD trial reported that, after adjusting for multiple other risk factors, intensive glucose treatment significantly reduced CAN risk by 16% compared to standard intervention in a large cohort of more than 8 000 participants with T2D in the ACCORD trial over a mean 5-year follow-up.611 As for DPN, lifestyle modifications with diet and exercise have shown benefit in CAN prevention.631
Management of orthostatic hypotension involves both behavioral and pharmacological interventions. Behavioral supportive measures include: (1) avoiding abrupt changes in body position; (2) avoiding actions that elevate intraabdominal and intrathoracic pressures; (3) avoiding medications that would exacerbate hypotension such as tricyclic antidepressants, phenothiazines, and diuretics; (4) raising the head of the bed during sleep; (5) following a schedule of small and frequent meals to minimize postprandial hypotension; (6) considering physical counterpressure maneuvers such as leg crossing and squatting; and (7) hydrating with fluids and salt, if not contra-indicated.547 Pharmacological therapy includes midodrine and the more recent droxidopa, both of which are FDA approved for the management of orthostatic hypotension and may be considered in persons who fail nonpharmacological interventions. However, it is recommended to proceed with a very slow titration and use the minimally effective dose to avoid undesirable side effects. In selected severe cases, low-dose fludrocortisone may be also an option.547
Gastrointestinal Autonomic Neuropathy
GI neuropathies may involve any portion of the GI tract with manifestations including esophageal dysmotility, gastroparesis (delayed gastric emptying), constipation, diarrhea, and fecal incontinence.547 Among these, gastroparesis may the most common condition providers may consider in clinical practice.
Earlier prevalence data on gastroparesis are limited and inconsistent. However, more recent data from larger community-based studies reported that cumulative incidence of gastroparesis over 10 years was 5% in T1D compared with T2D (1%) and controls (1%). The prevalence of GI symptoms that could mimic gastroparesis increased substantially in recent years in the United States and other countries, although these are nonspecific.
Screening and Diagnosis in Clinical Care
A careful history may unveil symptoms such as early satiety, fullness, bloating, nausea, vomiting, dyspepsia, and abdominal pain. However, all these symptoms are nonspecific and resemble many other conditions, do not correspond with the severity of gastroparesis, and are poorly associated with abnormal gastric emptying.547 Thus, gastroparesis may be clinically silentin the majority of cases and symptoms and many persons may only present with unexplained early postprandial hypoglycemia followed by later hyperglycemia.547
Importantly, besides organic causes such as gastric outlet obstruction or peptic ulcer disease, hyperglycemia, hypoglycemia, and acute changes in BG are well documented to alter gastric emptying.547 A critical consideration is that several medications widely used in persons with DM, especially opioids, that have been unfortunately prescribed extensively for pain management, and GLP-1 RAs, directly affect gastric emptying and could mimic gastroparesis or cause iatrogenic gastroparesis.547 Therefore, a thorough differential to exclude all these factors known to affect gastric emptying (including esophagogastroduodenoscopy and/or a barium study of the esophagus, stomach, and upper GI tract), careful documentation of medication intake, and performing CGM if available should always be considered before conducting more specialized testing for gastroparesis and a firm diagnosis is established.
The diagnostic gold standard is the measurement of gastric emptying with scintigraphy of digestible solids at 15-minute intervals for 4 hours after food intake. Optimization of glucose levels prior to scanning is needed to avoid false-positive results. However, this test is burdensome, time consuming, not readily available, and costly. Recently, the use of 13C-octanoic acid breath test has been FDA approved and emerged as a much easier to use alternative.547
Other Forms of Diabetic Neuropathies
Other forms of diabetic neuropathies are mononeuritis such as cranial nerve palsies or entrapment neuropathies (eg, carpal tunnel syndrome, ulnar entrapment, and peroneal entrapment, among others).632–635 There may also be atypical variants of diabetic neuropathy such as small-fiber neuropathies, which present predominantly with pain and autonomic features.636,637 Risk factors include metabolic syndrome, IFG, and IGT.638–640
Question 9: How should antihyperglycemic agents be prioritized in persons with T2D at high risk for or with established CVD?
Recommendation 9.1
In persons with T2D and established ASCVD or at high risk for ASCVD, use GLP-1 RAs with proven CV benefits to reduce the risk of myocardial infarction, stroke, or CV death regardless of other glucose-lowering or CV therapies and independent of A1C.
Grade A; BEL 1
Recommendation 9.2
In persons with T2D and established ASCVD or very high ASCVD risk, use SGLT2is with proven CV benefits to reduce the risk of hospitalization for HF, major adverse CV events (MACE), or CV death regardless of background glucose-lowering therapy, CV therapy, or A1C.
Grade A; BEL 1
Recommendation 9.3
In persons with T2D and established HF (regardless of ejection fraction, background glucose-lowering or HF therapies, or A1C), use SGLT2is with proven HF benefits to reduce the risk of hospitalization for HF or CV death, and to improve HFerelated symptoms.
Grade A; BEL 1
Recommendation 9.4
In persons with T2D and ASCVD or at high risk for ASCVD, use GLP-1 RAs with proven benefit for reduction in the risk of stroke. In persons with insulin resistance, prediabetes, or T2D and a prior transient ischemic attack or stroke, pioglitazone should be considered to reduce the risk of recurrent stroke.
Grade A; BEL 1
Evidence Base 9: How should antihyperglycemic agents be prioritized inpersons with T2D athighriskforor withestablished CVD?
Evidence Base 9.1: DM and ASCVD
ASCVD remains the leading cause of morbidity and mortality in persons with T2D; therefore, prevention of ASCVD events is a key clinical priority. Multiple CVOTs have demonstrated that the use of GLP-1 RAs significantly lowers the risk of MACEdtypically defined as a composite of nonfatal MI, nonfatal stroke, or CV deathdin persons with T2D and either established ASCVD or at high risk for ASCVD. The category of “high risk” varies between studies, but in general includes persons with T2D, target organ damage, and/or risk factors for ASCVD. In specific, large trials of injectable GLP-1 RAs, once-daily liraglutide, and once-weekly albiglutide, semaglutide, dulaglutide, and efpeglenatide have all shown significant reduction in the risk of MACE.242,427,472,641–643 The trial of once-weekly exenatide demonstrated a directionally favorable effect on MACE but narrowly missed statistical significance.644 The outcome trial of once-daily lixisenatide was neutral.645 Initial CVOT of oral semaglutide demonstrated CV safety but was not powered for superiority.646
A 2021 comprehensive meta-analysis of 8 major GLP-1 RA CVOTs, comprising more than 60 000 participants, demonstrated that these agents reduce the risk of MACE by 14% (HR, 0.86; 95% CI, 0.80–0.93; P < .0001) as well as individual components of MACE, including MI, stroke, and CV death.643 In addition, GLP-1 RAs also reduced the risk of death from any cause by 12% (HR, 0.88; 95% CI, 0.82–0.94; P = .0001) and produced a modest but significant decrease in hospitalization for HF (HR, 0.89; 95% CI, 0.82–0.98; P = .013).643 Importantly, these benefits were consistent in persons with or without established ASCVD and did not differ based on GLP-1 RA structural homology (human vs exendin based), baseline A1C, or background antihyperglycemic therapy (Fig. 5).
Figure 5.

Antihyperglycemic Therapy for Persons with Type 2 Diabetes and Atherosclerotic Cardiovascular Disease (ASCVD), Very High Risk for ASCVD, Heart Failure, Cerebral Vascular Disease, or Chronic Kidney Disease. Copyright © 2022 AACE. May not be reproduced in any form without express written permission from Elsevier on behalf of AACE. Visit https://doi.org/10.1016/j.eprac.2022.08.002 to request copyright permission.
Evidence Base 9.2: DM and HF
The risk of HF is 2- to 4-fold higher in persons with DM compared with those without DM.647 Thus, prevention of HF is critically important. Multiple large CVOTs and kidney outcome trials of SGLT2is have demonstrated robust and consistent reductions in the risk of hospitalization for HF in persons with T2D.239,423,425,426,648 These trials tested different agents in the class (including empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin), and included both persons with and without established ASCVD, HF, and kidney disease.
A comprehensive meta-analysis of 6 major SGLT2i trials in individuals with T2D, comprising nearly 50,000 participants showed a 32% reduction in the risk of hospitalization for HF (HR, 0.68; 95% CI, 0.61–0.76), a 22% reduction in the composite of hypertensive HF and CV death (HR, 0.78; 95% CI, 0.73–0.84), and a 15% reduction in CV death (HR, 0.85; 95% CI, 0.78–0.93) with SGLT2is vs placebo.649 In addition, there was also a modest but significant 10% reduction in the risk of MACE (HR, 0.90; 95% CI, 0.85–0.95).649 All of these benefits were consistent regardless of presence or absence of ASCVD. Secondary analyses across several of these trials also showed that HF prevention benefits of SGLT2is are present regardless of baseline CV or antihyperglycemic therapies (including metformin) (Fig. 5).649
Evidence Base 9.3: DM, ASCVD, and HF
Persons with HF (regardless of left ventricular ejection fraction) have a high risk of death and hospitalizations and experience a high burden of debilitating symptoms, physical limitations, and a poor QoL. The prognosis is particularly unfavorable in persons with HF who have concomitant T2D. Several large RCTs, which in combination enrolled over 8000 persons, have demonstrated that SGLT2is significantly reduce the risk of CV death or worsening HF and improve symptoms, physical limitations, and QoL in persons with heart failure with reduced ejection fraction (HFrEF). In the DAPA-HF (Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure) trial, dapagliflozin reduced the primary endpoint of CV death or worsening HF by 26%, whereas in EMPEROR-REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), empagliflozin significantly reduced the risk of CV death or hospitalization for HF by 25%.457,470 Both of these studies, as well as a smaller RCT of dapagliflozin (DEFINE-HF [Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction]) also demonstrated significant improvement in HF-related symptoms with SGLT2is.650,651 The benefits were highly consistent in persons both with and without T2D; however, because persons with T2D had higher absolute risk, they experienced a greater absolute benefit with SGLT2is vs placebo.
More recently, empagliflozin also was shown to significantly reduce the risk of CV death or hospitalization for HF in nearly 5000 persons with HF and heart failure with preserved ejection fraction (HFpEF) by 21%.443 Furthermore, the PRESERVED-HF trial of 324 persons with HFpEF demonstrated a large, clinically meaningful, and significant improvement in symptoms, physical limitations, and exercise function with dapagliflozin, as compared with placebo.652 Similar to HFrEF trials of SGLT2is, in both HFpEF trials the benefits were consistent in persons with and without T2D.
In the SOLOIST-WHF (Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure) trial, which recruited ~1200 persons who were either hospitalized with HF or were recently discharged following hospitalization for HF, and all of whom had T2D, a mixed SGLT1/2 inhibitor sotagliflozin also significantly reduced the primary endpoint of total HF hospitalizations or CV death but is not yet FDA approved.653
Collectively, these data indicate that SGLT2is address all key goals of care in HF: reducing death and hospitalizations and improving symptoms, physical limitations, and QoL across the broad range of ejection fraction, and that persons with T2D derive a greater absolute benefit from these agents due to their higher baseline absolute risk (Fig. 5).
Evidence Base 9.4: DM and Stroke
Stroke is a devastating CV event, leading to disability, cognitive and physical dysfunction, recurrent strokes, and death. Persons with DM have a significantly higher risk of stroke, which is particularly pronounced in the elderly. In a cross-sectional study of 4346 persons aged ≥60 years using the National Health and Nutrition Examination Survey 2013–2018 dataset, the presence of DM increased the risk of stroke (OR, 28.019; 95% CI, 19.139–41.020) dramatically.654 Additionally, the risk of death from stroke in persons with DM is 1.6 to 1.9 times the death rate for persons without DM.655 The National Health Interview Survey conducted from 2000–2009 included participants with DM (8.2%) and showed that death attributable to cerebrovascular disease was significantly higher (HR, 1.48; 95% CI, 1.18–1.85) among those with DM compared to those without DM.656 In the large Trial Evaluating Cardiovascular Outcomes with Sitagliptin study population, 1084 deaths were adjudicated from the study population of 14,671 persons with DM and ASCVD.657 Death due to stroke (n = 65) was more common than death due to MI (n = 48) but less common than sudden death (n = 145).657
After the initial cerebrovascular accident (CVA), whether transient ischemic attack or stroke, the risk of recurrence and mortality increases. In an analysis of repeat hospitalization after first-ever lifetime stroke, DM was noted to increase the risk of repeat hospitalization for all causes.658 Among persons aged <65 years who were stroke survivors and admitted to a comprehensive stroke center in Ontario, Canada (N 8293), preexisting DM was associated with increased risk of in-hospital death (adjusted OR, 1.46; 95% CI, 1.14–1.87) or direct discharge to long-term care (adjusted OR, 1.65; 95% CI, 1.07–2.54).659 Among those discharged (N 7847), preexisting DM was associated with increased rate of death (adjusted hazards ratio [aHR], 1.68; 95% CI, 1.50–1.88), admission to long-term care (aHR, 1.37; 95% CI, 0.21–1.54]), and incident dementia (aHR, 1.44; 95% CI, 1.17–1.77).659 Clearly, prevention of incident and recurrent stroke is a high priority in the care of persons with DM. Comprehensive risk factor management for reduction of ASCVD including stroke is discussed in R 9.1 and R 9.4 of this document.
Glucagon-like Peptide-1 Receptor Agonists and Stroke
Recent randomized, placebo-controlled clinical trials with adjudicated CV outcomes have been informative regarding the impact of GLP-1 RAs on the risk of fatal and nonfatal stroke. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial randomized 9340 persons with T2D with CVD or at high risk for CVD and demonstrated lower risk for the composite outcome (MI, stroke, CV death) (HR, 0.86; 95% CI, 0.78–0.97; P < .001 for noninferiority, P = 0.01 for superiority).427 The HR for nonfatal stroke (HR, 0.89; 95% CI, 0.72–1.11; P = .30) provided the first indication that GLP-1 RAs could have a positive effect on stroke.427 SUSTAIN-6, the semaglutide CVOT, tested once-weekly injectable semaglutide and found a more pronounced effect on stroke (HR, 0.61; 95% CI, 0.38–0.99; P = .04).242 PIONEER-6, the CVOT for oral semaglutide, showed noninferiority for MACE and numerically fewer nonfatal strokes.646 Dulaglutide was tested in 9901 participants with DM and prior ASCVD or at high risk for ASCVD and showed reduction in MACE (HR, 0.88; 95% CI, 0.79–0.99; P = .026) and reduction in nonfatal stroke (HR, 0.76; 95% CI, 0.61–0.95; P = .017).642 In the CVOTs Harmony Outcomes (albiglutide vs placebo), EXSCEL (exenatide LA vs placebo), and AMPLITUDE-O (efpeglenatide vs placebo), there were numerically fewer strokes, but HRs were not significant.472,641,644 ELIXA (lixisenatide vs placebo after acute coronary syndrome) showed noninferiority for MACE but no effect on fatal or nonfatal stroke.645 Two meta-analyses of CVOTs of GLP-1 RAs vs placebo reported MACE outcomes, including stroke.643,660 A 2021 meta-analysis analyzed 7 RCTs (N 56,005 participants) with 174,163 patient years of follow-up and found that GLP-1 RAs reduced nonfatal stroke (RR, 0.85; 95% CI, 0.77–0.95) without statistically significant heterogeneity among the trials.660 A comprehensive meta-analysis of the 8 completed CVOTs of GLP-1 RAs vs placebo in persons with DM and either prior ASCVD or at high risk for ASCVD reported risk reduction for fatal or nonfatal stroke (HR, 0.83; 95% CI, 0.76–0.92; P = .0002).643 The number needed to treat, calculated over a weighted average median follow-up of 3.0 years to prevent one fatal or nonfatal stroke, was reported as 241 (120–1694).643 Another meta-analysis evaluated the efficacy and safety of GLP-1 RAs for stroke prevention in 8 RCTs comprising 56,251 participants and found that compared with placebo, GLP-1 RAs reduced nonfatal strokes (OR, 0.84; 95% CI, 0.76–0.94; P = .002) and all strokes (OR, 0.84; 95% CI, 0.75–0.93; P = .001).661
Currently, FDAeapproved indications for the GLP-1 RAs exenatide QW, lixisenatide, and oral semaglutide are to improve glycemic control in adults with T2D. Dulaglutide has an additional indication to reduce MACE for people with T2D with and without established CVD. Liraglutide and semaglutide SC are approved to reduce the risk of MI, CVA, or CV death in adults with T2D and CVD.662 No other antihyperglycemic agents have an FDAeapproved indication for CVA or stroke prevention (Fig. 5).
Pioglitazone and Stroke
Pioglitazone was arguably the first glucose-lowering medication to be tested in a placebo-controlled, dedicated CVOT. In the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive Study), 5238 persons with T2D and prior ASCVD were randomized to receive pioglitazone or placebo with follow-up of an average of 34.5 months.663 Although the primary composite endpoint was not significant, the main secondary endpoint consisting of all-cause mortality, nonfatal MI, and stroke was positive with an HR of 0.84 (95% CI, 0.72–0.98; P = .027).663 The HR for stroke was 0.81 (95% CI, 0.61–1.07), suggesting but not proving benefit.663 PROactive was followed by the IRIS trial (Pioglitazone after Ischemic Stroke or Transient Ischemic Attack), which randomized 3876 persons with insulin resistance but not DM to pioglitazone or placebo, with a follow-up of 4.8 years.664 The primary outcome of fatal or nonfatal stroke or MI was reduced (HR, 0.76; 95% CI, 0.62–0.93; P = .007).664 A post hoc analysis of the IRIS trial in the subset of participants with prediabetes and good adherence (A1C 5.7%-6.4%, N 1454) showed that pioglitazone reduced the outcomes of stoke (HR, 0.64; 95% CI 0.42–0.99) and stroke/MI (HR, 0.57; 95% CI, 0.39–0.84).665 Adverse events of HF, edema, and bone fracture were increased with pioglitazone.664 A meta-analysis of these trials plus others of varying size and duration reported that the use of pioglitazone reduced the risks of MACE and MI significantly, whereas there was a trend toward reducing recurrent stroke (RR, 0.81; 95% CI, 0.65–1.01) in persons without DM, similar to the result in persons with DM (RR, 0.78; 95% CI, 0.62–1.02).666 Another meta-analysis of the risk of recurrent stroke in persons with prior transient ischemic attack or stroke (N 4980 with insulin resistance, prediabetes, or DM) found that pioglitazone reduced the risk of recurrent stroke (HR, 0.68; 95% CI, 0.50–0.92; P = .01) and future major vascular events (HR, 0.75; 95% CI, 0.64–0.87; P = .0001) without heterogeneity across clinical trials (Fig. 5).667
Clinicians should discuss the risks and benefits of GLP-1 RAs with proven ASCVD risk reduction in persons with DM who are at high risk for stroke or who have had a prior stroke. This recommendation is concordant with evidence reviews and recommendations of other national and international bodies.199,431,662 Alternatively, consider pioglitazone for stoke prevention after the risks and benefits of this therapy have been evaluated clearly and presented to persons with DM so that adverse effects can be avoided. Additional study of these antihyperglycemic agents in persons at risk for stroke is clearly warranted.
Question 10: How should obesity be managed in persons with DM?
Recommendation 10.1
Persons with prediabetes, T1D or T2D, and obesity/adiposity-based chronic disease (ABCD) have 2 diseases, and each should be treated effectively with the goal of optimizing their respective outcomes.
Grade B; BEL 2 and expert opinion of task force
Recommendation 10.2
The diagnosis and evaluation of ABCD in persons with prediabetes, T1D, or T2D should include both anthropometric and clinical components. The anthropometric evaluation should include body mass index (BMI), confirmed by physical examination that excludes excess muscle mass, edema, or sarcopenia. Waist circumference (WC) should be measured as a marker of cardiometabolic disease (CMD) risk.
Grade B; BEL 2 and expert opinion of task force
Recommendation 10.3
For most adults, BMI values that indicate excess body weight are 25 to 29.9 kg/m2 for overweight and ≥30 kg/m2 for obesity, and WC threshold values ≥102 cm for men and ≥88 cm for women.
Grade B; BEL 4 and expert opinion of task force
Recommendation 10.4
The clinical evaluation of persons with both prediabetes, T1D, or T2D and ABCD should assess the presence and severity of weight-related complications including cardiometabolic complications such as dyslipidemia, hypertension, nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH), CVD, HF, and CKD; biomechanical complications such as obstructive sleep apnea (OSA), osteoarthritis, gastroesophageal reflux disease, and urinary incontinence; abnormalities involving sex steroids, such as infertility, polycystic ovary syndrome, and hypogonadism; as well as impact on psychological disorders and QoL.
Grade B; BEL 2 and expert opinion of task force
Recommendation 10.5
Persons with T2D and ABCD should be treated with weight-loss interventions which will both improve glycemic control and prevent or treat ABCD complications. The target for weight loss should be >5% to ≥10% of baseline body weight.
Grade A; BEL 1
Recommendation 10.6
Persons with T2D and ABCD should be instructed and supported in therapeutic lifestyle interventions that include a reduced-calorie healthy diet generally designed to produce a ≥500 kilocalorie daily energy deficit, daily physical activity, regular exercise (several times a week), and behavioral health practices.
Grade A; BEL 1
Recommendation 10.7
The Mediterranean, low-fat, low-carbohydrate, very lowecarbohydrate, vegetarian, vegan, and DASH diets are recommended, safe, and effective for short-term (1–2 years) weight loss, though evidence of long-term risk reduction for CVD events and mortality exists only for the Mediterranean diet.
Grade A; BEL 1
Recommendation 10.8
Persons with T2D and obesity/ABCD with BMI ≥27 kg/m2 should be treated with DM medications associated with weight loss (GLP-1 RAs, SGLT2is). In addition, for persons with prediabetes, T1D, or T2D who have obesity/ABCD, consider FDAeapproved weight-loss medications as an adjunct to lifestyle intervention to achieve lowering of A1C, reduction of CVD risk factors, treatment, or prevention of other ABCD complications, and improvement in QoL.
Grade A; BEL 1
Recommendation 10.9
Persons with a BMI ≥35 kg/m2 and one or more severe obesity-related complications remediable by weight loss, including T2D, high risk for T2D (insulin resistance, prediabetes, and/or metabolic syndrome), poorly controlled hypertension, NAFLD/NASH, OSA, osteoarthritis of the knee or hip, and urinary stress incontinence, should be considered for a bariatric procedure.668
Grade C; BEL 3
Recommendation 10.10
Persons with BMI 30 to 34.9 kg/m2 and T2D with inadequate glycemic control despite optimal lifestyle and medical therapy should be considered for a bariatric procedure.668
Grade B; BEL 2
Evidence Base 10: How should obesity be managed in persons with DM?
Diagnosis of Obesity
Increased adiposity occurs as a positive energy imbalance driven by dysregulated interactions involving central nervous system satiety factors, resulting in increased caloric intake and excess adipose tissue mass.669 Although lean individuals can have insulin resistance and CMD, weight gain together with CMD exacerbates insulin resistance and leads to greater storage of fat in the intra-abdominal depot, ectopic accumulation of fat in liver and muscle cells, and heightened dysregulation of adipocytokines and systemic inflammation.670–673 Weight gain increases risk of overt T2D by increasing insulin resistance, thereby placing greater metabolic stress on the pancreas b cells in individuals predisposed to b-cell fatigue. BMI is currently used as a general screening tool to diagnose overweight (BMI 25 to 29.9 kg/m2) and obesity (BMI ≥30 kg/m2), but these BMI cutoffs do not capture risk of adiposity among varied ethnic groups, nor do they identify individual CMD risk. ABCD is a medical diagnostic term that recognizes that excessive weight gain engenders abnormalities in the mass, distribution, and function of adipose tissue and leads to chronic complications that confer morbidity and mortality.672,673 Thus, persons with T2D and ABCD have 2 diseases that interact to worsen outcomes, and each requires effective therapy. Weight loss not only addresses glycemic control but also addresses both the prevention and treatment of other cardiometabolic and biomechanical complications of obesity.
The comprehensive diagnosis of ABCD requires both anthropometric and clinical components674,675 The anthropometric component is largely satisfied by BMI, which is used to diagnose individuals as lean (BMI 18.5 to 24.9 kg/m2), overweight (BMI 25 to 29.9 kg/m2), or obese (BMI ≥30 kg/m2). BMI can be further categorized as obese class I (BMI 30 to 34.9 kg/m2), obese class II (BMI 35 to 39.9 kg/m2), and obese class III (BMI ≥40 kg/m2). In South Asian, East Asian, and Southeast Asian populations, health is adversely affected at lower levels of BMI, and alternate criteria have been advocated, with BMI 18.5 to 22.9 kg/m2 indicative of normal weight, 23 to 24.9 kg/m2 overweight, and ≥25 kg/m2 obese.674 Clinical correlation is required because BMI may not reflect adipose tissue mass in individuals with increased muscle mass, sarcopenic obesity, paraplegia, frailty, edema, or other conditions that affect body composition. WC thresholds indicate increased risk of CMD and exhibit regional and ethnic variations.676 Distribution of fat to the abdominal/visceral compartment can be assessed by WC, using cutoff points of ≥102 cm for men and ≥88 cm for women in the United States and Canada and ≥94 cm in men and ≥80 cm in women in many other populations. In South Asian, Southeast Asian, and East Asian adults, WC ≥85 cm in men and ≥74 cm in women indicate excess abdominal fat.676,677
The limitation of BMI as a diagnosis of obesity is that BMI does not indicate the impact of excess adiposity on overall health.675 The health effects of excess adiposity are manifest by the development of adverse weight-related complications that are the cause of morbidity and mortality.672,673 Complications or relevant risk factors determine disease staging and indicate the need for more aggressive therapy to improve individual health. In persons with ABCD and T2D, this involves an assessment of the severity of T2D as well as other cardiometabolic and biomechanical complications because all individuals with T2D would be designated stage 2 (at least one severe complication) ABCD.
Weight-loss Therapy and Lifestyle
Weight loss of >5% to ≥10% or more is needed to achieve progressive and optimal improvements in A1C, BP, and lipids in persons with T2D and ABCD.678,679 Weight loss of 10% or more is required to remedy other common complications of ABCD, such as OSA 680–682 and NASH.683–685 The treat-to-target objectives for the degree of weight loss should be individualized both for improvement of glycemia and for improvements in ABCD complications, to include BP, lipids, osteoarthritis, urinary stress incontinence, NAFLD/NASH,686 and OSA. The reader is referred to the 2016 AACE guideline for care of persons with obesity for evidence attesting to the amount of weight loss needed and efficacy of weight-loss therapy for addressing ABCD complications.674
All persons with T2D and ABCD complications should be instructed and supported in therapeutic lifestyle interventions that include a healthy diet with emphasis on weight management, daily physical activity, and regular exercise (several times a week). Consultation with a psychologist or CDCES is recommended as needed to support long-term behavior change. The most important feature for diet modification is a reduced-calorie meal plan, which is essential for effective weight loss.687–690 The initial dietary prescription should generally be designed to produce a 500 kilocalories daily energy deficit. Very lowecalorie diets and meal substitutes can be considered and have the potential for T2D remission.689,691,692 The following reduced-calorie meal plans have been shown to be safe and effective for weight loss in the short term (1–2 years) in persons with T2D and ABCD: Mediterranean,693–700 low fat,689,690,701–704 low carbohydrate, very low carbohydrate,694,702–715 vegetarian and vegan,716–720 and DASH diets.721–723 However, evidence of long-term protection against CVD events and mortality exists only for the Mediterranean diet.698,724–726 Minimal differences in weight loss between reduced-calorie diets with different macronutrient composition allow health care professionals to personalize recommendations for foods and macronutrients on the basis of individual medical conditions, cultural and personal preferences, lifestyle, and behaviors.679
A second lifestyle modification may pertain to the potential challenges and barriers for increased physical activity and exercise that may exist in persons with T2D and high BMI. Physical activity and aerobic exercise guidelines are similar in persons with T2D independent of lean vs overweight/obese BMI.431,674,727,728 It is important to evaluate persons with T2D for contraindications, disabilities, and/or other physical limitations that may accompany increased adiposity. A physical activity and exercise program should be individualized for each person according to their personal preferences, goals, and physical limitations.727 Importantly, increased physical activity is a main component in any lifestyle program for achieving and maintaining weight loss. In the Look AHEAD (Action for Health in Diabetes) trial, 1-year results revealed a significant association between increased minutes of physical activity and degree of weight loss.689
A systematic review and meta-analysis of 10 RCTs assessing lifestyle interventions vs standard care in persons with T2D found a pooled effect of 3.33 kg average weight loss associated with a 0.29% decline in A1C.687 Another meta-analysis of 11 RCTs with 6754 participants employing lifestyle intervention (calorie restriction, regular physical activity/exercise and frequent contact support from health care professionals) duration of at least 1 year, showed significantly better effects on A1C, lipids, and BP in those with >5% weight loss compared to those with <5% weight loss.688
The benefits of an intensive lifestyle and behavioral weight-loss intervention in persons with T2D was rigorously examined in the Look AHEAD trial.678,689,690,729 This study randomized 5145 persons to an intensive lifestyle intervention or standard DM support and education. The intensive lifestyle group were placed on a low-fat and reduced-calorie diet ranging from 1200 to 1800 kilocalories per day based on initial weight. Liquid meal replacements were made available to participants who found this helpful for portion control to enhance adherence to the caloric goal. The physical activity goal was at least 175 minutes per week consisting of activities similar in intensity to brisk walking. The participants had frequent group and solo visits with support staff, and behavioral strategies were stressed, such as self-monitoring, goal setting, and problem-solving. Intensive lifestyle intervention resulted in 1-year weight loss of 8.6%, and 4-year weight loss of 4.7% compared with 1.1% in the standard group, and was accompanied by lower A1C with less need for DM medications, DM remission in ~10% of persons, lower diastolic and systolic BP, improved lipids (higher HDL-C, lower triglycerides), improvements in OSA as reflected by lower apnea hypopnea index scores, increased mobility, slower progression of nephropathy, and improved QoL.678,680,690,729–733 The magnitude of weight loss after 1 year in Look AHEAD was related to the frequency of using meal replacements, amount of physical activity performed, and attendance at behavioral support sessions.689 The principal outcome measure in Look AHEAD was CVD events, and the study was discontinued prematurely when an interim analysis showed no difference between treatment groups after 9.6 years median follow-up. Even so, in subanalyses, persons losing more than 10% of weight at 1 year experienced a 21% reduction in the composite CVD outcome.734
The Primary Care-Led Weight Management for Remission of Type 2 Diabetes (DIRECT) trial reported on persons with T2D and ABCD followedinprimary-care clinics inthe United Kingdomrandomized to a very lowecalorie diet vs standard care.691,692 The very lowecalorie diet group lost 10 kg after 1 year and experienced a DM remission rate of 46% compared to 1 kg weight loss and 4% remission rate in the control group. Remission of T2D was directly related to the amount of weight lost with the remission rate rising to 86% inpersons losing ≥15 kg at 1 year.691 After 2 years, 36% of persons with T2D remained in remission compared to 3% who received standard care.692
The Look AHEAD and DiRECT studies attest to the powerful benefits of lifestyle interventions and weight loss in persons with T2D and ABCD. These composite data indicate that weight loss should be a primary treatment modality in persons with T2D and ABCD.
Obesity and Diabetes Medications
Weight loss >5% to ≥10% is required for optimal treatment of most persons with T2D to optimally improve glycemia and address the risk, presence, and severity of ABCD cardiometabolic and biomechanical complications.678–683 Since this degree of sustained weight loss is not commonly achieved by lifestyle interventions alone,687,688 weight loss medications should be routinely considered as an adjunct to lifestyle inpersons with T2D and ABCD. These medications are approved when used in conjunction with a reduced-calorie diet plan for any person with BMI 27 to 29.9 kg/m2 who have at least one ABCD weight-related complication, to include T2D, or for any person with BMI ≥30 kg/m2 regardless of ABCD complications.
The addition of weight-loss medications has been shown to achieve significantly more weight loss than lifestyle interventions alone and produce greater A1C lowering and improvements in cardiometabolic risk factors. The mechanism of action for all weight loss medications (except orlistat) is to blunt appetite at the level of the central nervous system hypothalamic satiety centers, which thereby helps individuals adhere to a reduced-calorie diet. All FDAdapproved medications for chronic weight management have also been demonstrated to be effective and safe in both RCTs and a meta-analysis involving persons with T2D.735–742 The design of these studies was consistent in that all persons with T2D were treated with lifestyle intervention and then randomized to placebo vs weight-loss medication. The study’s weight-loss medication arms consistently resulted in: (1) greater weight loss than lifestyle alone, (2) lower A1C values despite less need for DM medications, (3) reductions in BP, (4) lower triglycerides and higher HDL-C, (5) decreased levels of hepatic transaminases, and (6) improvements in inflammatory and other biomarkers such as CRP, fibrinogen, and adiponectin, when compared to the control arms treated with lifestyle intervention plus placebo.
Medications used for weight loss include several sympathomimetic amines (phentermine, benzphetamine, and phendimetrazine) approved for short-term use (≤12 weeks), which makes these drugs ineffectual for treatment of ABCD as a chronic disease. There is a lack of rigorous long-term safety data available for the sympathomimetic amines because this criterion was not required at the time of their approval. Placebo-subtracted weight loss approximates 5.1% in individuals without T2D,743 and although longer-term cohort studies have been reported,744 there is a lack of clinical trial data assessing efficacy and safety in persons with T2D.
Orlistat at a dose of 120 mg 3 times per day taken with meals produced placebo-subtracted weight loss of ~4% after 1 year in persons without T2D745 and has been shown to be effective in those with T2D.735–737,739 Weight loss produced by orlistat led to A1C reductions of 0.75% after 1 year (baseline value 8.9%) in persons with T2D who were overweight or obese. In a meta-analysis of 7 RCTs involving 1 249 persons with overweight/obesity and T2D treated with orlistat, 23% of persons lost ≥5% weight and exhibited pooled mean weight loss of 8.6 kg with decrements of 1.16% in A1C, 5.3% in total cholesterol, and 5.2 mm Hg in systolic BP.739 A CVOT has not been performed for orlistat.
Phentermine/topiramate-extended release (ER) resulted in placebo-subtracted weight loss of ~8% to 9% in phase 3 RCTs that enrolled participants without T2D.746–748 In persons with T2D, phentermine/topiramate-ER administration led to placebo-subtracted weight loss of 9% to 10% at 1 year, and reduced A1C by 0.4% in persons with a baseline mean A1C of 7.0% and by 1.6% in those with a baseline mean A1C of 8.6% who had long-standing T2D treated with multiple medications.740,749 Weight loss was accompanied by improvements in lipids, BP, and CVD risk biomarkers. Importantly, these improvements were significantly greater than the lifestyle intervention alone and occurred despite greater reductions in the need for conventional DM drugs. A CVOT has not been performed for phentermine/topiramate-ER.
Naltrexone/bupropion-ER produced placebo-subtracted weight loss of ~4–5% in persons without T2D750–752 and in those with T2D led to a reduction in A1C of 0.6% vs 0.1% compared with placebo, with improvements in triglycerides and HDL-C.738 There was no weight loss benefit for BP and the drug is contraindicated in individuals with uncontrolled hypertension. A CVOT for naltrexone/bupropion-ER was terminated early, and there was insufficient data to assess CV safety.753
Liraglutide is an acylated human GLP-1 RA that is injected subcutaneously once per day. Liraglutide doses up to 1.8 mg/day are approved for glycemic control and to reduce the risk of major adverse CVD events in adults with T2D. The liraglutide dose-response for weight loss is greater than that for glycemic control, and 3 mg per day is approved for chronic weight management. In 3 studies of 56-week duration involving persons with obesity and dyslipidemia or hypertension, weight loss ranged from 6.2% to 8.0% with 3 mg liraglutide vs 0.2% to 2.9% with placebo.754–756 In persons with T2D, liraglutide 3 mg significantly reduced weight over 56 weeks by 6.0% and to a greater extent than liraglutide 1.8 mg (4.7%) and placebo (2.0 %).741 Reductions in A1C were also greater with liraglutide 3 mg (-1.3%) compared to liraglutide 1.8 mg (-1.1%) and placebo (0.3%). These differences in A1C were achieved while actively treating to an A1C target for liraglutide, with a greater number of persons requiring fewer DM medications or less need to increase DM medications with liraglutide 3 mg compared to liraglutide 1.8 mg. Liraglutide 3 mg, but not liraglutide 1.8 mg, significantly improved levels of total cholesterol, VLDL-cholesterol HDL-C, triglycerides, plasminogen activator inhibitor 1, and UACR compared with placebo.741 In clinical trials of liraglutide 3 mg, the incidence of cholelithiasis was greater than placebo.741,754
The GLP-1 RA semaglutide is acylated for binding to albumin and has an amino acid substitution to prevent degradation by DPP-4 that prolongs its half-life to allow once weekly subcutaneous injection. It is approved at doses of 0.5 mg and 1.0 mg per week for glycemic control in adults with T2D and to reduce the risk of major CVD events. Oral semaglutide 7 mg and 14 mg is approved for glycemic control but is not currently approved for chronic weight management, though a higher dose formulation is under development for obesity.
Subcutaneous semaglutide at the higher dose of 2.4 mg/week has been approved for chronic weight management based on results from 4 pivotal Semaglutide Treatment Effect in People with Obesity (STEP) trials.742,757–759 The STEP 1 Trial enrolled persons without T2D and demonstrated placebo-subtracted weight loss was 16.9% with on-treatment analysis (analogous to completers) and 14.9% weight loss with in-trial analysis (analogous to last observation carried forward with imputation).757 The STEP 1, 3, and 4 trials used semaglutide in conjunction with a lifestyle program and resulted in 16.9% to 18.2% weight-loss (using a completers-type analysis), which is superior to phase 3 trial results for other weight-loss medications, though without head-to-head drug comparison. A phase 2 study in persons with biopsy-proven NASH using a daily injection equivalent to semaglutide 2.4 g/week demonstrated 13% weight loss and improvement in hepatic fibrosis stage in 43% of participants compared to 1% weight loss and 33% fibrosis score improvement with placebo.760
The STEP 2 trial enrolled persons with T2D and ABCD and included 3 randomization groups, treatment with semaglutide at the dose approved for obesity (2.4 mg/week), semaglutide at the dose approved for T2D (1.0 mg/week), and placebo (Table 14).742 Placebo-subtracted weight loss was greater in persons taking semaglutide 2.4 mg (6.2%) compared to semaglutide 1.0 mg (3.6%), and semaglutide 2.4 mg in conjunction with lifestyle changes led to 10.6% weight loss in a completers-type analysis. In STEP 2, A1C lowering was relatively similar in both groups, though persons treated with semaglutide 2.4 mg achieved improvements in cardiometabolic risk factors, including WC, A1C, spontaneous bacterial peritonitis, lipids, UACR, CRP, and liver parameters.742
Table 14.
Food and Drug Administrationeapproved Pharmacotherapy for Weight Loss in Persons with Adiposity-based Chronic Disease
| Weight-loss medication | Dose; escalate as tolerated | Mechanism | Potential side effects | Warnings and contraindicationsa |
|---|---|---|---|---|
| Approved for short-term therapy (≤3 mo) | ||||
| Phentermine | Low-dose 15 mg every day; maximum dose 37.5 mg every day (by mouth)b | Sympathomimetic amine (decreases appetite); stimulates CNS activity | Restlessness, insomnia, headache, dry mouth, tachycardia, BP elevation | Pregnancy, active coronary artery disease, uncontrolled hypertension, hyperthyroidism, agitated states |
| Approved for chronic management of obesity | ||||
| Orlistat | Treatment dose 120 mg three times a day (by mouth with meals) | Gastrointestinal lipase inhibitor (decreased fat absorption) | Fat malabsorption, flatulence, fecal urgency, oily stools | Pregnancy, fat-soluble vitamin and drug malabsorption (do not use in organ transplant), renal oxalate stones, cholestasis |
| Phentermine/Topiramate-ER | Starting dose 3.75 mg/23 mg every day; treatment dose 7.5 mg/46 mg every day; maximum dose 15 mg/92 mg every day (by mouth) | Sympathomimetic amine (decreases appetite)/anticonvulsant, carbonic anhydrase inhibitor, gabaminergic (increases satiety) | Restlessness, insomnia, headache, dry mouth, tachycardia, BP elevation, paresthesia, dysgeusia, mood changes, mental clouding, blurred vision | Pregnancy, glaucoma, hyperthyroidism, metabolic acidosis, urolithiasis |
| Naltrexone-ER/Bupropion-ER | 8 mg/90 mg tablets; starting dose one tablet every day; treatment dose 2 tablets twice a day (by mouth) | Opioid receptor antagonist (decreases cravings)/dopamine-norepinephrine reuptake inhibitor (decreases appetite) | Nausea, vomiting, diarrhea, constipation, headache, fatigue, insomnia, agitation, mood changes, dry mouth, blurred vison | Pregnancy, seizure risk, uncontrolled hypertension, chronic opioid use |
| Liraglutide 3 mg | Starting dose 0.6 mg/d; maximum dose 3 mg/d (subcutaneous injection) | Glucagon-like peptide-1 receptor agonist (decreases appetite and delays gastric emptying) | Nausea, vomiting, diarrhea, constipation, headache, fatigue | Pregnancy, medullary thyroid cancer, MEN type 2, tachycardia, acute pancreatitis, acute gallbladder disease |
| Semaglutide 2.4 mg | Starting dose 0.25 mg/wk; maximum dose 2.4 mg/wk (subcutaneous injection) | Glucagon-like peptide-1 receptor agonist (decreases appetite and delays gastric emptying) | Nausea, vomiting, diarrhea, constipation, headache, fatigue | Pregnancy, medullary thyroid cancer, MEN type 2, tachycardia, acute pancreatitis, acute gallbladder disease, diabetic retinopathy |
Abbreviations: BP = blood pressure; CNS = central nervous system; ER = extended release; MEN = multiple endocrine neoplasia.
Weight-loss drugs should not be used during pregnancy, if planning to become pregnant, and during breastfeeding.
15 mg / 30 mg / 37.5 mg phentermine hydrochloride = 12 mg / 24 mg / 30 mg phentermine resin, respectively.
Although efficacy has been documented for weight-loss medications compared to placebo-subtracted weight loss, it is important to consider there is a wide range of weight loss reported in studies among these medications. Moreover, the 1-year efficacy can be predicted based on early response to weight loss.761 If certain thresholds for early weight loss are not met, the FDA prescribing recommendation is to either stop the medication, continue the medication and intensify lifestyle behaviors for diet and exercise, or switch to a different medication. Phentermine/topiramate-ER and naltrexone/bupropion-ER have 2- and 4-week time periods of dose uptitration, respectively, with a weight cut-off to stop the drug if <5% weight loss occurs at 12 weeks. Liraglutide 3 mg has a 4-week dose uptitration period and based on clinical trial data, the weight cutoff for stopping the drug is <4% weight loss at 16 weeks. The FDA prescribing information for orlistat and semaglutide 2.4 mg does not contain weight cutoff rules. Weight-loss medications should be considered and available to prescribe for any individual unless contraindicated to enhance the likelihood that a drug will be found effective for successful weight loss. Semaglutide 2.4 mg has the greatest placebo-subtracted weight loss in clinical trials, with only 14% of persons losing only <5% body weight.757,758 Persons with T2D are reported to have less weight loss in clinical trials than individuals without T2D, and in persons with T2D treated with semaglutide 2.4 mg, up to 27% have lost <5% weight.742
Benefits of phentermine/topiramate-ER in persons with T2D include lower A1C and less need for DM medication compared to placebo, as reported in the OB-202/DM-230 study in persons with T2D duration of 8 to 9 years, baseline A1C 8.7%, and an average 1.6 DM medications per person, and as reported in the CONQUER study in a subset of persons with T2D duration <5 years and baseline A1C 6.8%.740
FDA-approved weight-loss medications should be used with caution and monitored closely in adults aged ≥65 years with T2D and ABCD due to a relative lack of data addressing safety concerns. Additionally, persons aged ≥65 years with T2D and ABCD who are being considered for medical or surgical weight-loss therapy should be evaluated for bone loss (osteopenia/osteoporosis) and sarcopenia.
Despite the clinical benefits realized with weight-loss therapy in persons with T2D, there is more difficulty achieving and maintaining weight loss than in individuals without T2D.762 It is important to be aware that several medications used to treat DM result in weight gain.73,763,764 For achieving glycemic targets in individuals with T2D and ABCD, DM medications associated with weight loss (eg, GLP-1 RA, SGLT2is), or those associated with weight neutrality or minimal weight loss (metformin, DPP-4 inhibitors), should be considered over medications associated with weight gain (eg, insulin, SUs, meglitinides, thiazolidinediones [TZDs]) when possible, to the extent they are needed to achieve A1C targets.
Current DM medications associated with weight loss (eg, SGLT2is and GLP-1 RAs at doses approved for T2D) often do not usually produce sufficient weight loss for optimal treatment of ABCD. None of the current DM medications associated with weight loss, including GLP-1 RAs and SGLT2is, have resulted in more than 5.6% weight loss in clinical trials, and this includes dulaglutide,765 exenatide,766 exenatide-ER,767 liraglutide 1.8 mg,768 lixisenatide,769 semaglutide 1.0 mg/week,770,771 semaglutide 1.0 mg/week added to SGLT2i,772 oral semaglutide 14 mg,773 canagliflozin,774 dapagliflozin,775 and empagliflozin.776 These DM drugs do not achieve adequate weight loss for optimal treatment of ABCD in the majority of persons. In persons with T2D and ABCD, improvements in A1C, BP, and lipids require >5% weight loss and are progressive up to and exceeding 15% weight loss,678,679 whereas other common complications of ABCD, such as OSA and NASH, may require >10% weight loss for clinical benefits.680–685 Health care professionals who treat persons with T2D with GLP-1 RAs and SGLT2is without further consideration of other ABCD complications may not be effectively treating that person’s composite CMD risk.
Metabolic (Bariatric) Surgery and Endoscopic Devices
Bariatric surgery and endoscopic procedures are important therapeutic options in persons with T2D and ABCD.777–781 In clinical trials comparing bariatric surgery vs medical treatment in persons with T2D, bariatric surgery results in greater short-term and long-term lowering of A1C, including remission of T2D in some persons.777–783 Persons with T2D and ABCD who undergo bariatric surgical procedures must have careful evaluation pre- and peri-operatively due to anesthesia and surgical risks, and post-operatively because of risks of micronutrient deficiencies and hypoglycemia, particularly following malabsorptive procedures such as Roux-en-Y gastric bypass or biliopancreatic diversion.
While recommendations are adopted from the 2019 AACE/TOS/ASMBS bariatric surgery guideline,668 several key studies involving persons with T2D warrant mention. The STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial showed that metabolic surgery, when compared with intensive medical therapy (lifestyle counseling, weight management, self-monitoring of glucose, drug therapy), significantly improved outcomes for weight loss, DM remission, glycemic control, need for DM medications, lipid and BP medications, and QoL777 Five-year outcomes from a 2020 RCT reported that Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding achieved remission of T2D in 30% and 19% of persons, respectively, compared with 0% of controls undergoing intensive lifestyle weight intervention.784 Ten-year data from a single-center RCT in Italy showed that 37.5% participants randomized to a surgical intervention maintained DM remission (25% for Roux-en-Y gastric bypass and 50% for biliopancreatic diversion) compared with 5.5% of participants treated with medical therapy.783 In the prospective SOS (Swedish Obese Subjects) cohort study, bariatric surgery produced DM remission rates of 72% and 30% after 2 and 15 years, respectively, and was associated with a reduction in micro- and macrovascular DM complications, including risk of CV death.779,780 At a median follow-up of 20 years, the HR was 0.77 (P < .001) for death, with reduced death from CVD and cancer compared with the control cohort in the SOS cohort study.785 Thus, there are ample data to support bariatric surgery as an effective therapeutic approach in persons with T2D, obesity, and uncontrolled DM refractory to lifestyle and pharmacotherapy.
In addition to carrying over 3 recommendations from the 2019 bariatric surgery guideline,668 a new recommendation is added unique to the current guideline regarding endoscopic and orally ingested devices for weight loss in persons with T2D. The 2019 AACE/TOS/ASMBS bariatric surgery guideline reviewed evidence regarding endoscopic devices for treating obesity but did not make any recommendations for use due to lack of an adequate evidence base as of 2019. Various bariatric devices function by: (1) reducing the stomach’s capacity via space-occupying devices, such as intragastric balloons or orally ingested hydrogels, (2) inhibiting gastric emptying via a transpyloric shuttle, (3) evacuation of stomach contents following meals (aspiration therapy), or (4) preventing nutrient absorption across the duodenal mucosal surface.668 Some endoscopic and orally delivered devices have been approved by the FDA for treatment of obesity, including hydrogel capsules,786 intragastric balloon systems,787–788 a transpyloric shuttle that blocks gastric emptying,789 and gastric aspiration therapy that evacuates partial gastric contents following meals via a variation of a percutaneous endoscopic gastrostomy tube.790
None of these devices have been approved to treat T2D, though some trials included some persons with T2D. The hydrogel capsules are well tolerated and in the Gelesis Loss of Weight (GLOW) study produced 2% placebo-subtracted weight loss.786 However, 59% of participants treated with these hydrogel capsules achieved non-eplacebo-subtracted weight loss ≥5%, and persons with prediabetes or drug-naïve T2D were more likely to achieve a favorable weight-loss response.786 The intragastric balloon and transpyloric shuttle trials included some persons with T2D, but numbers were inadequate to assess safety and efficacy in those with T2D.787–789 Duodenal mucosal resurfacing involves a catheter-based hydrothermal ablation of the duodenal mucosa followed by subsequent regeneration of healthy new mucosa with therapeutic effects lasting up to 1 year. In April 2021, the duodenal mucosal resurfacing approach was given an FDA Breakthrough Device Designation for treatment in persons with T2D, which should accelerate its development and review.791 Clinical trials have demonstrated A1C lowering of 0.9% to 1.2% over 6 to 12 months irrespective of weight loss.792–794 Problems of duodenal stenosis treatable by endoscopic balloon dilation have been reduced via changes in catheter design. There is potential for duodenal mucosal resurfacing to be an option for therapy and an adjunct to oral medications in persons with T2D.
Persons Aged ≥65 Years with Type 2 Diabetes and Obesity
It is important to mention T2D and ABCD in persons aged ≥65 years due to the increasing number of persons in this category. Because relatively low numbers of elderly individuals have been included in clinical trials, there is a lack of rigorous efficacy and safety data, particularly regarding weight-loss medications. Weight-loss therapy should be used cautiously and monitored frequently in the elderly674,795 with clear health-related goals in mind, including glycemic control in T2D, prevention of T2D in persons with prediabetes, BP lowering, and improvements in osteoarthritis, mobility, and physical function, because available evidence supports weight-loss therapy in these conditions. As reviewed in the 2016 AACE guideline for care of persons with obesity,674 persons aged ≥65 years being considered for weight-loss therapy or bariatric surgery should be screened for sarcopenia by examining muscle strength and performing a review of systems assessing functionality. Endurance and resistance exercise becomes a valuable addition to lifestyle intervention because it preserves lean muscle mass during weight loss. Elderly individuals should receive adequate calcium and vitamin D for skeletal health, especially after bariatric surgery796 and should be screened for bone loss per usual guidelines797 because weight loss results in loss of bone mass798 and may increase risk for fracture.799,800 Weight-reduction interventions in elderly persons with ABCD and prediabetes or T2D should consider their nutritional status, eating habits, food availability, social support systems, risk of hypoglycemia, and cognitive abilities.
Section 3: Management of Prediabetes, T2D, and T1D With Selection of Glycemic Targets, Lifestyle Interventions, and Antihyperglycemic Pharmacotherapy (Insulin Therapy for all With T1D and Select Individuals With T2D); Prevention, Identification, and Treatment of Hypoglycemia; Treatment of Hospitalized Persons With DM or Those With Hyperglycemia Without Diagnosis of DM; and Women With GDM
Question 11: How should prediabetes be managed?
Recommendation 11.1
Prediabetes is a metabolic and vascular disorder, and clinicians should actively treat people with prediabetes in order to prevent or at least delay progression to T2D and development of CVD complications.
Grade A; BEL 1
Recommendation 11.2
In persons with prediabetes and/or metabolic syndrome or identified to be at high risk of T2D based on validated risk-staging instruments, the prevention of T2D can be addressed by lifestyle modifications that include a healthy meal plan, regular physical activity, and behavioral health practices and weight loss in persons with ABCD. The Mediterranean diet should be considered to reduce progression to T2D and risk of CVD. Low-fat, vegetarian, and DASH meal patterns can also be considered for prevention of T2D.
Grade A, BEL 1
Recommendation 11.3
Clinicians should manage and monitor CVD risk factors in prediabetes and metabolic syndrome, including elevated BP, dyslipidemia, and excessive weight, with the same targets as for a person with T2D.
Grade B; BEL 2
Recommendation 11.4
Lifestyle intervention should include aerobic and resistance physical activity in all persons with prediabetes and/or metabolic syndrome. The initial aerobic prescription may require a progressive increase in the volume and intensity of exercise, and the ultimate goal should be ≥150 minutes/week of moderate exercise performed during 3 to 5 sessions per week (Grade A; BEL 1). Resistance exercise should consist of single-set exercises that use the major muscle groups 2 to 3 times per week (Grade A; BEL 1). An increase in nonexercise and active leisure activity should be encouraged to reduce sedentary behavior (Grade B; BEL 2).
Recommendation 11.5
Obesity medications, namely phentermine/topiramate ER, liraglutide 3 mg, or weekly semaglutide 2.4 mg, in conjunction with lifestyle therapy, should be considered in persons with prediabetes and/or metabolic syndrome with ABCD, whether overweight (BMI 27 to 29.9 kg/m2) or with obesity (BMI ≥30 kg/m2), when needed to achieve and sustain 7% to 10% weight loss for prevention of T2D.
Grade A; BEL 1
Recommendation 11.6
Although no medications have been approved for the treatment of prediabetes, diabetes medications including metformin, acarbose, pioglitazone, or GLP-1 RA can be considered in persons with prediabetes or in persons who also have ABCD and remain glucose-intolerant following weight loss using lifestyle and/or weight-loss medications.
Grade A; BEL 1
Evidence Base 11: How should prediabetes be managed?
Prediabetes can be identified by the presence of IFG (FPG value of 100 to 125 mg/dL), or IGT (OGTT result of 140 to 199 mg/dL 2 hours after ingesting 75 g of glucose), or an A1C value of 5.7% to 6.4%.9 Metabolic syndrome, based on National Cholesterol Education Program IV Adult Treatment Panel III (NCEP ATP III) criteria, may be considered a prediabetes equivalent.801 Both prediabetes and metabolic syndrome confer increased risk of T2D and CVD.802–804 The risk of progressing from prediabetes to overt T2D are greatest for those persons with a history of GDM, strong family history of T2D, progressive increments in glycemia within the prediabetes range, and who meet criteria for a combination of IFG, IGT, or metabolic syndrome (any 2 out of 3).9,805,806
|
Goals of therapy in persons with prediabetes and metabolic syndrome ✓ Prevent progression to T2D ✓ Prevent progression to NASH ✓ Improve CVD risk factors via aggressive control of: • elevated BP • LDL-C • dyslipidemia ✓ Treat obesity or prevent excessive weight gain ✓ Improve functionality and ✓ QoL |
In treating prediabetes and metabolic syndrome, it is important to consider that these clinical states are integral to a chronic progressive pathophysiological process termed CMD, which, as the term implies, gives rise to both metabolic and vascular disease end-stage manifestations.670 At the core of CMD is the insulin-resistant state characterized by a glucoregulatory defect (ie, normal or elevated glycemia in the face of hyperinsulinemia) accompanied by multiple biochemical abnormalities involving molecular signaling, gene expression, oxidative stress, mitochondrial dysfunction, and accumulation of inflammatory macrophages in adipose tissue that alters release of adipocytokines into the circulation.671,807,808 These molecular processes have systemic consequences producing abnormal glucose tolerance, ectopic lipid accumulation within muscle and liver cells, systemic inflammation, dyslipidemia, vascular stiffness, elevated BP, and accelerated atherogenesis. Early in the course of CMD progression, the insulin-resistant state is largely subclinical. However, over time, disease progression gives rise to clinically identifiable states, namely prediabetes and metabolic syndrome, which indicate the presence of CMD and mark individuals at high risk of future T2D, NASH, hypertension, myocardial dysfunction, CVD events, and CKD. Furthermore, with the development of T2D, there is further amplification of vascular disease progression and risk of CVD events.670
Obesity plays a key role in CMD because it can exacerbate insulin resistance and impel this disease progression. AACE672 and the European Association for the Study of Obesity673 have advocated for the use of ABCD as a medical diagnostic term for obesity. The disease is adiposity based because it involves abnormalities in the mass, distribution, and function of adipose tissue, and is a chronic disease that gives rise to complications, both biomechanical and cardiometabolic, which confer morbidity and mortality. Therefore, ABCD indicates what we are treating and why we are treating it and underscores a complications-centric approach to treatment consistent with the 2016 AACE guideline for care of persons with obesity.674 Thus, ABCD is clinically meaningful in contradistinction to the BMI-based diagnosis that provides no indication of the impact on health675 and avoids multiple meanings and stigmatization associated with the term obesity.672 In this context, treatment of ABCD employing weight-loss therapy is highly effective for treating persons with prediabetes, metabolic syndrome, T2D, and CVD risk factors who also have overweight or obesity.809
The natural history of CMD has important implications regarding the treatment of prediabetes and metabolic syndrome. Aggressive preventive interventions are required to halt progression toward all end-stage manifestations of the disease.809 Thus, comprehensive risk factor management is required for the treatment and prevention of both metabolic and vascular outcomes. With this in mind, the goals of treatment in persons with prediabetes and/or metabolic syndrome are shown in the text box.75,805 Optimal management of lipids and BP in prediabetes equates with the recommendations for T2D itself (ie, a DM equivalent), as described in the 2017 AACE Guidelines for the Management of Dyslipidemia and Prevention of CVD286 and 2020 AACE Algorithm for Management of Dyslipidemia,318 because accelerated atherosclerosis predates the development of overt hyperglycemia and diagnosis of T2D.810
In all persons with prediabetes and/or metabolic syndrome, whether lean or with ABCD, dietary and physical activity aspects of lifestyle therapy are cornerstones of risk management in preventing progression to T2D.811–813 The most robust research available regarding eating/meal patterns for T2D prevention in prediabetes pertains to Mediterranean-style diets.698,724–726,814–816 In a subgroup analysis of the PREDIMED trial, nondiabetic persons with metabolic syndrome traits who were randomized to Mediterranean diets enriched with olive oil without restrictions on energy intake experienced a significant reduction in the progression to DM compared with standard dietary advice to avoid fats (HR [0.60; 95% CI, 0.43–0.85]).814,815 In addition, the PREDIMED trial showed that a Mediterranean-style eating pattern intervention enriched with olive oil or nuts over 4.8 years reduced the composite primary endpoint of MI, stroke, or CV death compared with a low-fat diet in individuals at risk for CVD with or without DM.724,725 The Lyon Diet Heart Study assessed the efficacy of Mediterranean diets for the secondary prevention of CVD events.726 Persons who had a previous MI were randomized to a Mediterranean diet or a diet typically consumed in northern European countries, and after 4 years, the Mediterranean diet group had reduced rates of reinfarction and mortality. Adherence to this eating pattern is associated with decreased risk for metabolic syndrome, reduced inflammation, hepatic steatosis, and improved renal function. Mediterranean diets have also been shown to reduce rates of progression to T2D independent of weight loss, and, therefore, can be recommended in lean persons with metabolic syndrome or prediabetes.814 An umbrella evaluation of meta-analyses affirmed that a higher adherence to a Mediterranean eating pattern was associated with lower incidence of mortality from T2D and CVD.698 Thus, Mediterranean diets are a highly rational choice as the dietary component of long-term lifestyle therapy in persons with cardiometabolic risk.
With respect to other meal patterns, there are limited RCT data available that address prevention of CMD outcomes in persons with prediabetes or metabolic syndrome. The DASH diet has been shown to reduce BP and is particularly effective in individuals who were hypertensive at baseline and/or self-identified as African American.817,818 Adherence to a DASH diet is also associated with a low prevalence of DM.819–821 Low-fat meal plans in the context of a comprehensive lifestyle intervention have been shown to promote weight loss, improve glucose tolerance, and prevent DM in large RCTs enrolling persons with obesity and IGT.811,822–828 A large number of cohort studies and epidemiological data demonstrate that vegetarian and vegan diets confer metabolic benefits and are associated with a lower risk of developing T2D.820,829–833 In a meta-analysis of 11 cohort studies,715 a low-carbohydrate diet was no different than a high-carbohydrate diet regarding incidence of DM715,834 and could be harmful if fat sources are derived from red meat.835 Given the limited evidence, it is unclear which meal pattern is optimal. There is a large body of data indicating that isocaloric substitution of specific macronutrients can improve insulin sensitivity assessed by clamp studies and CVD risk factors.836 These data would generally support macronutrient intake as follows: (1) limitations on fat intake, (2) emphasis on poly/mono-unsaturated fats over saturated fats, (3) no trans fats,835 (4) complex over simple carbohydrates, (5) whole grains over refined grains934, (6) fruits and vegetables,837 (7) dietary fiber,838 and (8) reduced consumption of processed food.839 Dietary enrichment of these macronutrients can enhance insulin sensitivity,836 aligning with the Mediterranean diet and other meal patterns that are epidemiologically associated with reduced prevalence of T2D,839 and predictably would be beneficial based on the role of insulin resistance in the pathophysiology of CMD. Indeed, these foods and macronutrients coincide with favorable scores on the Healthy Eating Index-2010 (HEI-2010), the Alternative HEI-2010 (AHEI-2010), the Alternative Mediterranean Diet Score, and the DASH scores, which are associated epidemiologically with lower prevalence of DM.723,840
There is a plethora of evidence in persons with prediabetes and metabolic syndrome that regular exercise can lower glycemia, improve CVD risk factors, and prevent or delay progression to DM, either in the form of an exercise program per se or as part of a comprehensive lifestyle plan.841–851 As is the case for persons with T2D, studies have demonstrated beneficial effects of both aerobic and resistance exercise and additive benefits when both forms of exercise are combined.727,847–852 The physical activity program optimally includes aerobic exercise, which should begin at a low level to allow a person to increase the intensity and duration of the exercise over time. Various guidelines have recommended that, ideally, a person should achieve at least 150 minutes per week of moderately intense aerobic exercise accomplished in 3 to 5 sessions.674,852,853 High-intensity interval training can be used to achieve comparable metabolic benefits of moderate aerobic exercise with less of a time commitment.854 A resistance exercise program should be added and should consist of single-set repetitions targeting the major muscle groups 2 to 3 times per week.674,852,853 Despite these recommendations, less intense exercise and walking programs can reduce risk of DM.846,855 A final component of a physical activity program is to reduce sedentary behavior and increase active leisure activity.856–859 The physical activity prescription should be compatible with individual preferences and take into account any health-related or physical limitations.674
In persons with ABCD (both overweight or with obesity) and prediabetes and/or metabolic syndrome, weight loss is a highly effective way to prevent progression to T2D.674 In addition, weight reduction prevents or treats multiple CVD risk factors and additional complications of ABCD.674 Whether due to lifestyle therapy, obesity medications, or bariatric surgery, weight loss has been shown to (1) enhance insulin sensitivity; (2) prevent or delay progression to T2D particularly in high-risk persons with prediabetes or metabolic syndrome; (3) improve hepatic steatosis; (4) lower BP; (5) improve dyslipidemia; and (6) ameliorate biomarkers of CVD risk, including CRP, interleukin 6 and other markers of inflammation, fibrinogen levels, and serum adiponectin concentrations.674 Thus, weight loss is perhaps the most effective therapeutic approach for preventing the progression of CMD to T2D and/or CVD events.
In persons with ABCD, the principles of lifestyle therapy are the same as those that generally apply in prediabetes and metabolic syndrome except that the meal plan is presented in a reduced-calorie format to achieve weight loss. Any one of the reviewed meal plans (Mediterranean, low-fat, low-carbohydrate, vegetarian, vegan, and DASH diets) can be used as diets that feature a healthy composition of foods and macronutrients that promote insulin sensitivity836 and are associated with improvements in CMD outcomes as defined by the HEI723,840 and other epidemiological data. Although any of these healthy eating patterns can safely be used in the short term for weight reduction and improvements in CVD risk factors, only the Mediterranean diet has been shownto be cardioprotective in the long term. To accomplish weight loss, the incorporation of very lowecalorie diets and meal substitutes into an overall dietary plan has been shownto be effective in achieving greater degrees of weight loss.689,860
Regarding lifestyle interventions in persons who have ABCD and prediabetes, 3 major RCTs, the Diabetes Prevention Program, the Finnish Diabetes Study, and the Da Qing Study, all demonstrated that lifestyle/behavioral therapy featuring a reduced-calorie diet (eg, caloric deficits of 500 to 1000 calories/day) and physical activity are highly effective in preventing T2D.811,822–828 These lifestyle interventions also improved other aspects of CMD including improvements in insulin sensitivity and CVD risk factors, such as BP, lipids, and markers of inflammation. In addition, long-term follow-up of participants in the Da Qing Study revealed that CVD events and mortality were reduced when comparing the combined subgroups treated with diet and exercise with the controls.828 The Diabetes Prevention Program study randomized persons with IGT to ordinary care, metformin, and lifestyle intervention subgroups, and after 4 years, lifestyle modification reduced progression to T2D by 58% and metformin by 31%, compared with placebo.811 Participants achieved approximately 6% mean weight loss at 2 years and 4% weight loss at 4 years in the lifestyle intervention arm, and there was a progressive 16% reduction in T2D risk with every kilogram of weight loss.822 With observational follow-up after termination of the study, there was still a significant reduction in the cumulative incidence of T2D in the lifestyle treatment group at 10 years, despite the fact that BMI levels had equalized among the 3 treatment arms.823,824 The Diabetes Prevention Program was a resource-intensive efficacy trial and was not designed to be directly deliverable in real-world settings. The translation of the structured lifestyle intervention used in the Diabetes Prevention Program to community-based programs, commercial programs, and programs using remote technologies have achieved less weight loss than observed in the Diabetes Prevention Program trial itself.861–869 Despite limited weight loss, some efforts have produced modest improvements in metabolic parameters and, when measured, reductions in incidence of DM. A meta-analysis of 44 Diabetes Prevention Program translation studies reported an average 9.3-month weight loss of 3.77 kg from participants’ baseline weight and a decrease in fasting glucose of 2.4 mg/dL.865 Another meta-analysis of 63 real-world DM prevention efforts demonstrated a weight loss of 2.2 kg in participants and 0.8 kg in controls, but still led to a reduction in incident DM by 25%.866 Since the degree of DM prevention is proportional to the degree of weight loss,822 these efforts at translation would predictably be less effective regarding prevention of DM.
It is important to consider the degree of weight loss that is optimal for DM prevention. In the Diabetes Prevention Program, maximal prevention of DM over 4 years was observed at about 7% to 10% weight loss.811,822 This is consistent with the study employing phentermine/topiramate-ER where weight loss of 10% reduced incident DM by 79% over 2 years, and any further weight loss to ≥15% did not lead to additional prevention.870 Bariatric surgery produces greater weight loss than observed following lifestyle and pharmacotherapy interventions, yet, in 2 studies, there was a maximum of 76% to 80% reduction in DM rates,871,872 similar to that observed with phentermine/topiramate-ER870 and liraglutide 3 mg873 despite lesser weight loss than achieved following bariatric surgery. These combined data suggest that 7% to 10% weight loss will reduce the risk of future T2D by ~80% and represents a threshold above which further weight loss may not result in additional preventive benefits. For this reason, 7% to 10% weight loss is the appropriate goal in preventing progression to T2D in persons with ABCD and prediabetes and/or metabolic syndrome,674,679 whether as a component of a structured lifestyle intervention program or in conjunction with obesity medications.
The addition of obesity medications to lifestyle interventions produces more weight loss than attributable to lifestyle intervention alone and leads to greater reductions in incident T2D and improvements in CVD risk factors.674 Currently approved obesity medications are shown in Table 14 and include phentermine for short-term therapy (≤3 months) and 5 medications approved for chronic obesity management. Orlistat diminishes intestinal fat absorption via lipase inhibition, but the remaining medications act centrally to suppress appetite. When used in combination with lifestyle therapy, orlistat produced greater weight loss compared with lifestyle changes plus placebo and reduced rates of DM by up to 52% among persons with IGT at baseline.874 Naltrexone-ER/bupropion-ER reduced body weight and A1C in persons with T2D738 but had minimal effects on fasting glucose in persons without DM;875 a DM prevention study has not been performed for naltrexone-ER/bupropion-ER. Greater degrees of weight loss in RCTs involving phentermine/topiramate-ER870 and liraglutide 3 mg873 were associated with larger reductions in rate of DM and improvement of CVD risk factors. Phentermine/topiramate-ER in persons with prediabetes or metabolic syndrome reduced the annualized incidence rates of T2D by 70.5% and 78.7% among persons receiving the 7.5/46 mg and 15/92 mg daily doses, respectively, over 2 years.870 These reductions were related to the degree of weight loss (10.9% and 12.1% in the low- and high-dose groups, respectively, vs 2.5% in the placebo group; P < .0001) and were accompanied by significant improvements in cardiometabolic parameters.870 High-dose liraglutide (3 mg/day) in persons with prediabetes reduced weight by 6.1% from baseline over 160 weeks compared with 1.9% in those randomized to placebo, and the cumulative progression to DM was reduced by 72.7%.873,876 In RCT phase 3 trials (STEP 1, 3, 4), semaglutide 2.4 mg once weekly has produced weight loss of 14.9% to 17.4% from baseline in persons with overweight or obesity compared with 2.4% to 5.7% in placebo.757–759 In the STEP 1 trial, 45% of persons randomized to semaglutide 2.4 mg/week had prediabetes at baseline and treatment converted many to normoglycemia, reducing the percent with prediabetes to 8.3% by the end of study with improvements in CVD risk factors, compared to 40% and 26% with prediabetes, respectively, on placebo.757 In addition, a greater number of persons progressed to overt T2D on placebo in the STEP 1 trial compared with semaglutide 2.4 mg,757 although a study powered to assess DM prevention has not yet been conducted using semaglutide 2.4 mg. Given these high rates of DM prevention, persons with prediabetes and/or metabolic syndrome with ABCD (BMI ≥27 kg/m2) should be considered for weight-loss therapy involving obesity medications. In addition, weight regain is frequently observed after lifestyle interventions accompanied by worsening of glucose tolerance and CVD risk factors.811,877 Obesity medications used together with lifestyle changes can be used to sustain a greater degree of weight loss over time to preserve CMD benefits.674,759
The at-risk pool of persons at risk of T2D is large,878,879 and it is not feasible or safe to treat all persons aggressively using all the tools of obesity medicine. However, the risk for developing T2D and CVD varies greatly among persons with ABCD. This presents opportunities for identifying and targeting persons at higher risk for more aggressive interventions.290,728,806,880–885 For DM risk, clinicians can use the Framingham Risk Score,880 the ADA Diabetes Risk Calculator,9,881 and Cardiometabolic Disease Staging.806,882,883 Cardiometabolic Disease Staging is based on the number and severity of metabolic syndrome traits and employs two models: (1) a validated categorical approach indicating that persons who meet criteria for a combination of IFG, IGT, or metabolic syndrome (any 2 of the 3) are at greatest risk of both T2D and CVD,806 and (2) a logistic regression equation providing a quantitative 10-year risk assessment, which has superior accuracy compared with ADA or Framingham risk scores.882 Additional tools for predicting CVD risk in persons with CMD include the American College of Cardiology (ACC)/AHA Omnibus Risk Estimator,728 Framingham Coronary Heart Disease Risk Score,886 and the Reynolds Risk Score.290 Given the rising personal and social cost of DM, clinicians and health care systems can use these strategies to identify persons at high risk for DM and employ more aggressive interventions in those persons who will most benefit. For example, the number-needed-to-treat to prevent one case of T2D using phentermine/topiramate-ER is markedly reduced among high-risk persons compared with low-risk using Cardiometabolic Disease Staging.887
There is strong evidence that oral glucose-lowering medications approved for DM reduce the progression of prediabetes to DM.888–896 Even so, no medications sanctioned for use in DM or obesity are approved by the FDA solely for the management of prediabetes and/or the prevention of T2D. The Diabetes Prevention Program randomized persons with IGT to placebo, a structured lifestyle intervention, or metformin, and assessed progression to T2D with average follow-up of 2.8 years.811 Metformin was effective as evidenced by a 31% decrease in progression to DM but was inferior to lifestyle that reduced DM incidence by 58% compared with placebo.811 Metformin was particularly effective in persons with A1C 6.1% to 6.4%, BMI ≥30 kg/m2, those aged <60 years, and women with prior GDM.897 Metformin can also be combined with linagliptin to decrease DM incidence over that observed with metformin alone.890 Additionally, acarbose may be associated with reduced risk of DM891–894 as well as coronary heart disease as shown in the STOP-NIDDM trial.892 More recent study did not show coronary benefit with acarbose but did show decreased progression to T2D.1058 There is also robust RCT evidence demonstrating that TZDs decrease the likelihood of progression from prediabetes to DM in studies employing rosiglitazone898 and ACT-NOW for pioglitazone.895,896
TZDs are the only medications that approach the effectiveness of weight-loss medications, such as phentermine/topiramate-ER870 and liraglutide 3 mg,873 to prevent DM in persons with prediabetes and obesity. Therefore, with respect to DM medications, metformin, acarbose, or TZDs can be used to prevent progression to T2D.75,805 It is important for clinicians to consider side effects and CVD benefits in the choice of these DM medications.664,892 A meta-analysis of 10 RCTs encompassing 20,872 participants, including both weight-loss/lifestyle and pharmacologic interventions, found that lifestyle approaches were superior to DM drug-based approaches in DM prevention and improved CVD risk factors.812 Thus, DM drugs should be reserved for the higher-risk populations who remain glucose intolerant following failed weight-loss interventions involving structured lifestyle interventions and obesity medications, or in lean persons with CMD.805 The preference for weight-loss therapy in persons with obesity is due to the high efficacy of weight loss or DM prevention, and this ameliorates the broad range of other obesity complications.
Question 12: How can glycemic targets be achieved in persons with T2D?
12.1. Therapeutic Lifestyle Changes
Recommendation 12.1.1
All persons with prediabetes or DM should be prescribed, instructed, and supported in lifestyle interventions that include a healthy meal plan, regular physical activity, and healthful behavior practices. Individualized medical nutrition therapy (MNT) should be provided at the time of diagnosis (with intermittent reeducation as needed during continued care) via evaluation and counseling by a trained registered dietitian, certified nutritionist, or a clinician knowledgeable in nutrition.
Grade A, BEL 1
Recommendation 12.1.2
MNT should consider the overall treatment plan including medications, DM complications, physical activity, body weight goals, and avoidance of hypoglycemia, as well as personal and cultural preferences, health literacy and numeracy, psychological factors, readiness for change, SDOH, and support systems. For people on insulin therapy, insulin dosage adjustments should match carbohydrate intake (eg, with use of carbohydrate counting).
Grade A; BEL 1
Recommendation 12.1.3
The meal plan should contribute to therapeutic goals for control of glycemia, BP, lipids, CVD risk factors, and the prevention of DM complications. In selecting optimal meal patterns, certain Mediterranean diets should be considered which, over the long term, can protect against CVD events and premature mortality. Although there is a lack of long-term studies addressing CVD outcomes, multiple other meal plans have been shown to be safe and can achieve short-term benefits (1–2 years) regarding glycemia, BP, lipids, and CVD risk factors. These meal plans include low-fat, low-carbohydrate, very-lowecarbohydrate, vegetarian, vegan, and DASH diets.
Grade A, BEL 1
Recommendation 12.1.4
Given the variety of meal plans demonstrated to be beneficial in management of DM, nutritional recommendations should consider personal and cultural dietary preferences. Until there is conclusive evidence comparing the benefits of different meal patterns and the availability of long-term safety data, health care professionals should emphasize foods and nutrients that contribute to high “diet quality” scores as assessed by the HEI; high HEI is associated with reduced risks of DM, CVD, and mortality and includes fruits, non-starchy vegetables, whole grains, nuts, legumes, and fish, with limited consumption of added sugars, refined grains, red meat, and processed meats.
Grade B; BEL 1
Recommendation 12.1.5
Lifestyle intervention in persons with DM should include an individualized prescription for physical activity involving aerobic and resistance exercise and reduction in sedentary behavior. The initial prescription for aerobic physical activity may require a progressive increase in the volume and intensity of exercise, and the ultimate goal should be ≥150 min/week of moderate exercise performed during 3 to 5 sessions per week. (Grade A; BEL 1). Moderate exercise is considered to be activity that achieves a heart rate that is 50% to 60% higher than one’s basal heart rate. The physical activity prescription also should include resistance exercise that use the major muscle groups 2 to 3 times per week (Grade A; BEL 1). Individuals should also incorporate flexibility and range-of-motion training. An increase in nonexercise and/or active leisure activity should be encouraged to reduce sedentary behavior (Grade A; BEL 1).
Evidence Base 12: How can glycemic targets be achieved in persons with T2D?
Evidence Base 12.1: Therapeutic Lifestyle Changes
MNT encompasses the delivery of evidence-based nutrition care for persons with DM in a manner that supports healthy eating behaviors, optimizing glycemic control, achieving and sustaining body weight goals, and reducing the risks of DM complications.899–901 MNT has several essential components including assessment, nutrition diagnosis, interventions (eg, education and counseling), and monitoring with the provision of long-term follow-up, adjusting meal patterns as needed to accommodate changes in medications and the clinical course of the disease.899,901 A registered dietitian nutritionist (RDN) is the ideal member of the health care team to provide MNT based on training and expertise,679,899,901–905 and MNT constitutes the regulatory definition of nutrition counseling for DM by an RDN in the United States.900,901 In T1D, T2D, and GDM, key objectives are to provide consistency in day-to-day carbohydrate intake, adjusting insulin doses to match carbohydrate intake (eg, use of carbohydrate counting), limitations in consumption of sucrose-containing or high-glycemic index foods, adequate protein intake, healthy meal patterns, weight management, regular physical activity, and adequate glucose monitoring.679 MNT is individualized to accommodate differences in nutritional needs, medications and A1C goals, personal and cultural preferences, access to healthful foods and other SDOH, health literacy and numeracy, readiness for change and other psychological factors, family and community support systems, and existing barriers to change.679,906–908
Data support the effectiveness of MNT delivered by RDNs for improving A1C, with absolute decreases of 0.3% to 2.0% in T2D and of 1.0% to 1.9% in T1D at 3 to 6 months.901 Ongoing MNT support is helpful in maintaining glycemic improvements679,901,902,909–912 accompanied by cost savings in a person’s care.913–915 MNT is a covered Medicare benefit and should also be adequately reimbursed by insurance and health care systems or bundled in value-based care models.
T2D is an end-stage development of CMD, and, in this context, persons with T2D are also at risk of other sequelae of CMD including hypertension, dyslipidemia, NAFLD/NASH, CVD (coronary artery disease, stroke, nontraumatic amputation), CHF (both HFrEF and HFpEF), and CKD.670 Therefore, the clinician should assess persons with T2D for the risk, presence, and severity of these disease manifestations and engage in comprehensive and aggressive prevention and treatment strategies. Furthermore, obesity can exacerbate insulin resistance and accelerate progression of CMD toward these end-stage developments, and weight loss is an effective intervention in preventing and treating T2D, as well as hypertension, dyslipidemia, NAFLD/NASH, CVD risk factors, and CKD.674 The role of obesity to worsen CMD is mediated by abnormalities in the mass, function, and distribution of adipose tissue (adiposity-based) causing progression to chronic end-stage complications (chronic disease).901 For this reason, we will use the term ABCD, as recommended by AACE672 and the EASO,673 as the medical diagnostic term for obesity to indicate what is being treated and why it is being treated.
Lifestyle therapy is a foundational aspect of treatment in persons with DM who also may have or are at risk of other CMD outcomes. All persons with DM should be instructed and supported in lifestyle interventions centered around MNT.679,899–901 The components of therapeutic lifestyle changes include healthful eating, regular physical activity, weight management in persons who have ABCD, sufficient sleep, avoidance of tobacco products, limited alcohol consumption, and stress reduction.
Successful lifestyle interventions also feature a package of behavioral interventions that are designed to promote adherence with the meal plan and physical activity prescriptions. Clinical trials have demonstrated the efficacy of lifestyle programs that include behavioral interventions and have underscored particular practices that are most likely to be associated with success.690,811 For example, persons who self-monitor and record weight, food intake, or physical activity are more likely to achieve weight management goals. Patient education is also advantageous and can be delivered face-to-face, in group meetings, or using remote technologies (telephone, texting, and Internet). The program should also be able to provide for clear and reasonable goal setting, strategies for stimulus control, and systematic approaches for problem-solving and stress reduction. Other components can include cognitive restructuring (ie, cognitive behavioral therapy), motivational interviewing, behavioral contracting, and mobilization of social support structures. DM can often be associated with depression, disordered eating (eg, binge-eating disorder), anxiety, and other psychiatric disorders, which can impair the effectiveness of lifestyle interventions. For this reason, psychological counseling and psychiatric care may be necessary. The behavior intervention package is effectively accomplished by a multidisciplinary team that can include combinations of dietitians, nurses, health educators, physical activity trainers or coaches, and clinical psychologists. As with the meal plan and physical activity components, behavioral lifestyle intervention should be tailored to a person’s ethnic, cultural, socioeconomic, and educational background.
Meal plans for persons with DM should be designed to assure adequate intake of all nutrients, optimize glycemic control, achieve and sustain body weight goals, reduce the risks of DM complications, and improve CVD risk factors.679,899–901 There should be consistency in day-to-day carbohydrate intake for persons on fixed medical regimens, or adjustments of insulin doses to meals that vary in carbohydrate content (eg, use of carbohydrate counting). The timing of meals and distribution of ingested calories through the day should be individualized with reference to medical therapy and physical activity and to avoid hypoglycemia. A physician and/or an RDN should discuss meal plan recommendations in plain language with persons at the initial visit after DM diagnosis and then periodically during follow-up outpatient visits679,899–901 and should include information on specific foods and meal planning, grocery shopping, and dining-out strategies. MNT and diabetes self-management education and support (DSMES) should assure an understanding of differences between protein, fat (saturated and unsaturated), and carbohydrates (sugars, starch, and fiber), and their effects on health and glucose excursions following meals.916–918 Persons with DM should also understand nutrition facts label information.916 MNT can address the metabolic needs of persons in more detailed discussions in terms of calories, grams, and other metrics, but should be individualized to accommodate differences in health literacy and numeracy, personal and cultural preferences, access to healthful foods, support systems, and other SDOH.
To achieve dietary goals in DM, studies have demonstrated that there is no ideal mix of macronutrients that can be broadly prescribed, and that current evidence has not established an ideal percentage of calories from carbohydrate, protein, and fat.679,901,919 Regarding whole foods, multiple meal patterns have been shown to be advantageous for the management of DM, which promote reductions in glycemia, BP, and CVD risk factors.679,901,920–924 These include the Mediterranean-style,694,695,697–700,724–726,814,815,821,925,926 low-fat,678,690,701–704,811,827,927,928 low-carbohydrate,704,707–714,926,929,930 vegetarian and vegan,716–720,831,833,931,932 and DASH721–723 diets, as shown in Table 15. Thus, while current evidence has identified meal patterns that are clinically advantageous in DM, studies addressing the comparative benefits have not identified a superior mealpattern for control of glycemiathat can be universally applied to all persons with DM. However, the long-term safety data demonstrating protection against CVD events, CVD mortality, and all-cause mortality is only available for Mediterranean-style diets.724,726 Thus, all meal plans in Table 15 can be used safely in the short term (1–2 years) to facilitate glucose control, lower BP, and improve lipids; however, long-term maintenance on a Mediterranean-style diet should be considered. In any event, meal plans and macronutrient distribution should be based on an individualized assessment of current eating patterns, personal preferences including health beliefs, economics and food access, cultural preferences (eg, tradition, culture, religion), as well as metabolic and clinical goals.679,899–901 In an RCT comparing the Atkins, Ornish, Weight Watchers, and Zone diets, weight change did not differ between diets, and adherence to the diet was the single most important criterion of successful weight loss.933 The key to adherence, then, is to individualize the dietary recommendation consistent with personal and cultural preferences, lifestyle, and behaviors.
Table 15.
Recommended Meal Patterns for Persons with Diabetes Mellitus
| Meal pattern | Macronutrient characteristics | Outcome evidence in diabetes (see text) | Comments |
|---|---|---|---|
| Mediterranean 694,695,697–700,724–726,814,815,821,925,926 | Uses olive oil as the principal source of dietary fat; fish and other seafood; vegetables, nuts, fruits, beans; whole grains; moderate dairy products; red meat on occasion; wine with meals; limited sweets | Reduces risk of DM; lowers A1C, BP, and triglycerides; improves hepatic steatosis; primary and secondary prevention of major CVD events and mortality | Only meal pattern with RCTs showing long-term benefits regarding CVD events and mortality |
| Low fat 678,690,701–704,827,927,928 | Emphasizes vegetables, fruits, starches (eg, breads, pasta, whole grains, starchy vegetables), lean protein sources, and low-fat dairy products. Defined here as total fat intake ≤30% of total calories and saturated fat intake ≤10% | As part of a structured lifestyle intervention, reduces risk of DM and reduces A1C, BP, triglycerides in T2D | No long-term safety data |
| Low carbohydrate 702–704,707–714,926,929,930 | Often defined as a reduction in carbohydrates to 26% to 45% of total calories. Emphasizes (i) vegetables low in carbohydrate content, (ii) meat, poultry, fish, shellfish, eggs, cheese, nuts, (iii) oils, butter, and avocado. Avoids foods high in starch and sugars such as pasta, rice, potatoes, bread, and some fruits | Reduces A1C, body weight, BP, and triglycerides, and increases HDL-C in T2D | No long-term safety data. When compared with low-fat diet, there are greater benefits early (3–6 mo) followed by equilibration at 1–2 y. |
| Very low carbohydrate 705,706 | Often defined as limiting nonfiber carbohydrate to 20 to 50 grams/d in order to induce ketosis, resulting in > 50% of calories from fat. Otherwise, similar to low carbohydrate | ||
| Vegetarian/vegan 722–726,837,839,937,938 | Vegetarian: plant-based diets devoid of all flesh foods but including egg (ovo) and/or dairy (lactose) products. Vegan: eliminates all flesh foods and animal-derived products. | Reduces risk of DM; lowers A1C; weight loss; lowers LDL-C and non–HDL-C | No long-term safety data; may require supplementation of vitamins and minerals |
| Dietary Approaches to Stop Hypertension (DASH) 727–729 | Limitations in sodium and adequate potassium; whole grains, vegetables, fruits; low-fat dairy products; poultry, fish; limits on saturated fat, red meat; limit sweets, and sugar-containing beverages. | Reduces risk of DM; reduces glycemia, BP, and lipids in DM | No long-term safety data |
Abbreviations: A1C = hemoglobin A1c; BP = blood pressure; CVD = cardiovascular disease; DM = diabetes mellitus; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; RCT = randomized controlled trial; T2D = type 2 diabetes
Mediterranean.
In addition to the prevention of DM,814,815,821 RCTs and cohort studies that included persons with T2D have demonstrated that Mediterranean-style diets lower A1C, body weight, and improve CVD risk factors.694,695,697–700,724–726,821,925,926 In addition, RCTs have demonstrated primary724 and secondary726 protection against CVD events, CVD mortality, and all-cause mortality in study populations comprised of ~50% with DM.724 In particular, the PREDIMED trial showed that a Mediterranean-style eating pattern intervention enriched with olive oil or nuts over 4.8 years reduced the composite primary end point of MI, stroke, or CV death compared with a low-fat diet in individuals at risk for CVD with or without DM.724
Low Fat.
Structured lifestyle interventions that include reduced-calorie low-fat diets in persons with overweight or obesity have been shown to prevent progression from prediabetes to DM,690,811,827 and to lower A1C, BP, and triglycerides in persons with T2D.678,690,702–704,927,928 In both prediabetes and T2D, most of these benefits are attributable to weight loss. Although diets that emphasize low glycemic index foods may not affect A1C compared with high glycemic index foods,701,901 the quantity of carbohydrate and anticipated glycemic response should be taken into account in adjusting rapid-acting insulin doses for any given meal.679,899–901
Low Carbohydrate.
Low-carbohydrate diets that reduce carbohydrates to 26% to 46% of daily calories and very lowecarbohydrate diets that restrict carbohydrates to 20 to 50 grams per day sufficient to induce ketosis are both safe for persons with T2D.707–714,929,930 Several systematic reviews agree that reduced-carbohydrate diets can produce greater reductions in A1C and body weight compared with low-fat diets in the short term (~3 to 6 months); however, benefits equilibrate at 1 to 2 years when persons on both diets achieve similar A1C, BP, and lipid levels.704,707–709 Even so, persons on low-carbohydrate diets may chronically experience the need for reductions in doses of DM medications.711,712
Vegetarian/Vegan.
Vegetarian and vegan diets are associated with lower risk of DM, and, in persons with DM, these diets have been shown to lower glycemia and improve CVD risk factors.831,833,931,716–720,833,932 Low-fat vegetarian or vegan diets may be associated with additional improvements in metabolic parameters.718,932
DASH.
The DASH can also be used safely in persons with DM and can produce improvements in glycemia, BP, and lipids.721–723
Until there is more conclusive evidence regarding comparative benefits of different eating patterns in individuals, health care professionals should at least emphasize foods or nutrients that are common among these meal patterns demonstrated to be beneficial in persons with DM. These foods and macronutrients include: (1) limiting consumption of added sugars and refined grains, (2) emphasizing nonstarchy vegetables,837 (3) intake of whole foods over highly processed foods, (4) increased fiber consumption,838,934 and (5) avoidance of trans fats835 and excess saturated fats with emphasis on mono- or polyunsaturated fats.679,839 The meal plans in Table 15 share an emphasis on these foods and macronutrients. In addition, in isocaloric substitution experiments, these macronutrients have been shown to increase insulin sensitivity in studies employing glucose clamps.836 Furthermore, a systemic review and meta-analysis assessed the association between diet quality as measured by the HEI, AHEI, and DASH score and multiple health outcomes.723 In general, these diets also emphasize fruits, vegetables, whole grains, nuts, legumes, and fish, moderate dairy (<1000 grams/day), and limits on red meats and processed meats.921 The meta-analysis found that diets scoring highly on the HEI, AHEI, and DASH were associated with significant reductions in the risk of all-cause mortality, CVD, cancer, T2D, and neurodegenerative disease by 22%, 22%, 16%, 18%, and 15%, respectively.723 Thus, health care professionals should provide individualized meal plans in which the foods and macronutrients described above are emphasized, consistent with the meal patterns in Table 15.
In the absence of underlying insufficiency, routine supplementation of vitamins and minerals is not necessary; a healthful eating meal plan can generally provide sufficient micronutrients.679,935 Specifically, chromium; vanadium; magnesium; vitamins A, C, and E; CoQ10; and herbal supplements including cinnamon, curcumin, or aloe vera for improving glycemia in persons with DM are not supported by evidence, or the data are conflicting, and, therefore, are not recommended.679,935
Metformin administration can cause vitamin B12 deficiency, perhaps due to impaired absorption.936 Clinicians should be wary of vitamin B12 deficiency, particularly in persons on metformin who develop peripheral neuropathy or anemia.937 Supplementation doses of 1000 mg orally per day can be effective.938
Lifestyle Therapy: Physical Activity
Increased physical activity is an important component of lifestyle therapy.674,727 Regular physical activity improves glucose control in persons with DM,939–943 even in the absence of weight loss.944–946 In addition to improving BG control, exercise has been shown to reduce CV risk factors, contribute to weight loss, and improve sense of well-being.943,946–948 Moderate to high volumes of aerobic activity are associated with substantially lower CV and overall mortality risks in both T1D and T2D.948,949 Structured exercise improves insulin sensitivity, cardiorespiratory fitness,950,951 muscle strength, and mobility.731 Physical activity is also an important component in weight loss and weight-loss maintenance. Individuals must be evaluated initially for contraindications and/or limitations to physical activity, and the physical activity prescription should be compatible with any health-related or physical limitations and consider patient preferences.
Studies have reported beneficial effects of both aerobic and resistance exercise, and additive benefits when both forms of exercise are combined on a regular basis.853,945,947,952–954 For cardiometabolic conditioning, the guidelines proposed by the ACC/AHA,728,852 AACE,286,674 ADA,199,679 European Society of Cardiology/European Association for the Study of Diabetes,431 and the American College of Sports Medicine727,853 are well aligned. The recommendations include 30 minutes of moderate intensity exercise 5 days per week for a total of 150 minutes/week, or 20 to 25 minutes of intense exercise 3 days per week for a total of 60 to 75 minutes/week, combined with resistance training involving each major muscle group 2 to 3 days per week.955 Persons with T2D and ABCD can also benefit from high-intensity interval training involving shorter durations of time engaged in exercise.854,956 Regular exercise not allowing more than 2–3 days to elapse between exercise sessions is recommended to maintain improvements in insulin sensitivity.956 Persons with DM tend to have lower VO2 max measurements, and the exercise prescription should initiate activities at a lower level as tolerated followed by a slow progression in the intensity, frequency, and duration of exercise.
The recommended targets for physical activity cannot always be achieved and individuals should be encouraged to engage in physical activity even if suboptimal. For example, studies have consistently shown that a walking program is associated with reductions in DM incidence,855 and low-intensity exercise can improve glycemic control in T2D.939,940
People with and without DM should be encouraged to reduce the amount of time spent being sedentary (eg, working at a computer, watching television) with durations of sedentary periods lasting less than 90 minutes and interrupted by >30-minute periods of activity such as standing, walking, or performing other light physical activities.728,855,957–965 Participating in leisure-time activity and avoiding extended sedentary periods may help prevent T2D for those at risk959,960 and may also aid in glycemic control for those with DM.961–964 Persons with DM should be recommended to engage in flexibility and range of motion training, which can have significant impacts on A1C, flexibility, muscle strength, and balance, especially in older adults with DM.943,962–964,947
As individuals intensify their exercise program, medical monitoring may be indicated to ensure safety and evaluate the effects on glycemic management. Health care professionals and persons with DM should together establish a physical activity prescription with the goal of long-term adherence. Specific recommendations and precautions will vary by the type of diabetes, age, type of activity, and presence of DM-related health complications. Clinicians should assess individuals for disabilities and other conditions that might preclude certain types of exercise or predispose to injury, such as advanced age, limited exercise tolerance, uncontrolled hypertension, claudication, untreated proliferative retinopathy, autonomic neuropathy, diabetic foot disease, and Charcot foot. Recommendations should be tailored to meet the specific needs and capabilities of each individual,947 and an incremental exercise prescription should be developed for each person according to both goals and limitations. Although routine testing for coronary artery disease may not be necessary,41 health care professionals should perform a careful history, assess CV risk factors, and be aware of the atypical or silent ischemia. Screening for coronary artery disease should be performed in persons at risk.
12.2. Antihyperglycemic Pharmacotherapy for Persons with Type 2 Diabetes
Recommendation 12.2.1
Individualized pharmacotherapy for persons with T2D should be prescribed based on evidence for benefit that includes glucose lowering, avoidance of hypoglycemia and weight gain, and reduction of cardio-renal risk.
Grade A; BEL 1
Recommendation 12.2.2
Persons with T2D and their health care professionals should use patient-centered shared decision-making to agree on therapy targets and treatments as well as a regimen for glucose monitoring (ie, BGM, structured BGM, or CGM).
Grade B; BEL 2
Recommendation 12.2.3
Glycemic targets include A1C, BGM, and, for those using CGM, achievement of CGM targets such as TIR, percentage in low and very low range, time above range, and glycemic variability (Table 6). Nonglycemic targets include avoidance of hypoglycemia, control of BP, lipids, other CVD risk factors, and achieving and maintaining a healthy body weight.
Grade B; BEL 4
Recommendation 12.2.4
Independent of glycemic control, targets, or treatment, if there is established or high risk for ASCVD, HF, and/or CKD, clinicians should prescribe a GLP-1 RA or an SGLT2i with proven efficacy for the specific condition(s) of the person with T2D being treated (see also R 6.1 to R 6.6 on DKD or CKD in DM and R 9.1 to R 9.4 on ASCVD and HF).
Grade A; BEL 1
Recommendation 12.2.5
DM therapy should be individualized based on level of glycemia and the presence of comorbidities, complications, and access. Metformin is often the preferred initial therapy. Other agents may be appropriate as first line or in addition to metformin to reduce BG and/or to address specific comorbidities (such as ASCVD, HF, CKD, obesity, NAFLD), independent of glucose-lowering effects.
Grade A; BEL 1
Recommendation 12.2.6
For some recently diagnosed individuals with T2D and more severe hyperglycemia (A1C ≥7.5%), unlikely to attain the A1C target with a single agent, early combination pharmacotherapy should be considered, usually to include metformin plus another agent that does not cause hypoglycemia, especially a GLP-1 RA, SGLT2i, or DPP-4 inhibitor.
Grade A; BEL 1
Recommendation 12.2.7
For newly diagnosed persons with T2D and an entry A1C >9.0% and/or ≥1.5% above target, one should initiate, along with lifestyle modifications, dual- or possibly triple-combination pharmacotherapy usually including metformin. Basal insulin along with noninsulin therapy is recommended if there are significant signs or symptoms of hyperglycemia, especially including catabolism (eg, weight loss) or a very high A1C >10% (86 mmol/mol) or BG levels (≥300 mg/dL [16.7 mmol/L]).
Grade A; BEL 1
Recommendation 12.2.8
Clinicians should discuss with persons with T2D the likelihood that most persons with T2D ultimately require a combination of multiple complementary antihyperglycemic agents, in addition to lifestyle interventions, to attain and maintain optimal glycemic control.
Grade B; BEL 2
Recommendation 12.2.9
The diabetes care team should assess medication adherence and safety and glycemic control in persons with T2D quarterly or more frequently as needed. Subsequent visits will depend upon the metabolic targets achieved and the stability of metabolic control.
Grade D; BEL 4
Recommendation 12.2.10
Persons with T2D who start on metformin should continue it unless intolerance or contraindications occur. When intensification of antihyperglycemic treatment is needed, other agents should be added to metformin.
Grade B; BEL 2
Recommendation 12.2.11
Most persons with T2D who require intensification of antihyperglycemic therapy with a GLP-1 RA or insulin should initially be prescribed a GLP-1 RA. If further intensification is required, one should prescribe a basal insulin or a switch to a fixed-ratio combination of a basal insulin and a GLP-1 RA (insulin glargine U100 + lixisenatide [GlarLixi] or insulin degludec + liraglutide [IdegLira]).
Grade A; BEL 1
Recommendation 12.2.12
Insulin should be prescribed for persons with T2D when non-insulin antihyperglycemic therapy fails to achieve target glycemic control or when a person has symptomatic hyperglycemia.
Grade A; BEL 1
Recommendation 12.2.13
Long-acting basal insulin analogs are the recommended initial choice of insulin therapy for persons with T2D. The insulin analogs glargine (U100 or U300), degludec (U100 or U200), or detemir are preferred over intermediate-acting Neutral Protamine Hagedorn (NPH) insulin because analog insulins have demonstrated less hypoglycemia in some studies. Glargine U300 and degludec can be associated with less hypoglycemia than glargine U100 or detemir.
Grade A; BEL 1
Recommendation 12.2.14
Many persons with T2D receiving basal insulin and not at goal A1C can have significantly improved glycemia by the addition of a GLP-1 RA or being switched to a fixed-ratio combination basal insulineGLP-1 RA (GlarLixi or IdegLira). One of these changes should be considered before adding a meal-time insulin for postprandial glycemic control.
Grade A; BEL 1
Recommendation 12.2.15
When control of postprandial hyperglycemia is needed and a basal insulin and a GLP-1 RA are already being used, preference should be given to rapid-acting insulins (the analogs lispro, aspart, and glulisine or the rapid-acting inhaled human insulin powder) over regular human insulin (see Table 18). The former have a more consistent and a more rapid onset and offset of action with less risk of hypoglycemia.
Table 18.
Recommended Steps for the Intensification of Insulin Therapy When Prandial Control Is Needed
| Therapeutic option | Insulin dose | Notes/caveats |
|---|---|---|
| Step 1. Add prandial therapy: Begin with Step 1A for T2D and Step 1B for T1D | ||
| Step 1A: GLP-1 RA, SGLT2 inhibitor, or DPP-4 inhibitor | If glucose goals remain unmet, add prandial insulin | |
| Step 1B: Prandial insulin | TDD 0.3 to 0.5 units/kg (50% basal; 50% prandial) | Basal + prandial insulin analogs preferred over (NPH + regular insulin) or premixed insulin |
| Step 2. Monitor for hyperglycemia; Titrate insulin every 2–3 days to reach glycemic goalsa | ||
| Fixed regimen | Increase TDD by 2 units/d | |
| Adjustable regimen | ||
| Elevated fasting BG | Increase HS basal doses | Increase dose by 10% to 20% depending on severity of BG elevation |
| Elevated premidday meal BG | Increase breakfast prandial insulin doses | |
| Elevated pre-evening meal BG | Increase midday prandial insulin dose | |
| Elevated bedtime BG | Increase dinner prandial insulin dose | |
| Premixed insulin | ||
| FBG/premeal BG >180 mg/dL | Increase AM or PM dose depending on times of BG elevation | Increase dose by 10% to 20% depending on severity of BG elevation |
| Step 3. Monitor for hypoglycemia | ||
| Adjustable regimen | ||
| Low fasting BG | Reduce HS basal dose | Decrease dose by 10% to 20% depending on severity of hypoglycemia |
| Low premidday meal BG | Reduce breakfast prandial dose | |
| Low pre-evening meal BG | Reduce midday prandial dose | |
| Low bedtime BG | Reduce evening prandial dose | |
| Premixed insulin | ||
| Low BGs in AM or PM | Reduce AM or PM dose depending on times of BG elevation | Reduce dose by 10% to 20% depending on severity of BG elevation |
Abbreviations: A1C = hemoglobin A1c; AM = morning; BG = blood glucose; DPP-4 = dipeptidyl peptidase 4; FBG = fasting blood glucose; GLP-1 RA = glucagon-like peptide 1 receptor agonist; HS = at bedtime; NPH = Neutral Protamine Hagedorn; PM = evening; PPG = postprandial glucose; SGLT2 = sodium glucose cotransporter 2; T1D = type 1 diabetes; T2D = type 2 diabetes; TDD = total daily dose
For most persons with T2D taking insulin, glucose goals are A1C <7% and fasting and premeal blood glucose <110 mg/dL in the absence of hypoglycemia. A1C and FBG targets may be adjusted based on a person’s age, duration of diabetes, presence of comorbidities, diabetic complications, and hypoglycemia risk.
Grade A; BEL 1
Recommendation 12.2.16
Ultra-rapid-acting insulins (faster-acting insulin aspart, lispro aabc, and [human insulin] inhalation powder) may allow a decrease in the time between insulin administration and food intake and reduce the postprandial peak of PG as compared with rapid-acting insulins. The significance of this on long-term complications is unknown.
Grade A; BEL 1
Recommendation 12.2.17
Basal-bolus insulin regimens or CSII (ie, insulin pump) allow for adjustment of insulin doses according to carbohydrate intake and activity levels and are recommended for intensive insulin therapy in persons with T2D.
Grade B; BEL 1
Recommendation 12.2.18
Premixed insulin formulations (fixed combinations of shorter- and longer-acting components) of human or analog insulin may be considered for persons with T2D who have consistent dietary and exercise patterns and in whom adherence to more intensive insulin regimens is problematic. However, these preparations have reduced dosage flexibility and may increase the risk of hypoglycemia compared with basal insulin or basal-bolus regimens.
Grade A; BEL 1
Recommendation 12.2.19
In persons with T2D who are treated with basal-bolus insulin therapy, adding a GLP-1 RA, or switching to a fixed-ratio combination of a GLP-1 RA and a basal insulin, or adding an SGLT2i or pramlintide (less commonly used) may be able to reduce postprandial hyperglycemia, A1C, and weight. GLP-1 RAs may also allow reduction or discontinuation of bolus insulin in some individuals.
Grade A; BEL 1
Evidence Base 12: How can glycemic targets be achieved in persons with T2D?
Evidence Base 12.2: Antihyperglycemic Pharmacotherapy
The goal of antihyperglycemic treatment in persons with T2D is to achieve clinical and laboratory targets (eg, glycemic, BMI, BP, plasma lipids, eGFR) with as few adverse consequences as possible and reduce the risk of DM-related complications. As shown in Table 16, antihyperglycemic agents vary in their impact on A1C, FPG, PPG, insulin secretion, insulin sensitivity, weight, BP as well as the potential for hypoglycemia and other adverse effects. There are also differences in demonstrated evidence for CV and renal benefits among individual antihyperglycemic agents even within the same class. The choice of specific antihyperglycemic agents for those with T2D should be personalized and guided by each individual's medical needs, shared decision-making with their clinicians, treatment goals, weight, comorbidities, presence of or estimated risk for chronic complications, A1C, glycemic profile obtained by either BGM or CGM, and history of or risk for hypoglycemia or increased risk for adverse consequences from hypoglycemia.966–968 These patient characteristics can be matched with an agent’s antihyperglycemic efficacy, tolerability, side-effect profile, ease of administration, convenience, cost-effectiveness, and extraglycemic effects.966–970 Minimizing the risks of hypoglycemia and weight gain and maximizing CV and renal benefits should be priorities. Affordability of and access to the prescribed medications also need to be considered.
Table 16.
Profiles of Antihyperglycemic Medications
| Antihyperglycemic efficacy (as monotherapy)c | Hypoglycemia (as monotherapy)e | Weight | ASCVD events | HF | Effect on CKD worsening/other issues in presence of CKD | GI S/S | |
|---|---|---|---|---|---|---|---|
| METFORMIN | ++ | Low risk | Neutral/slight loss | Neutral/slight benefit | Neutral | Neutral/contraindicated if eGFR <30 mL/min/ 1.73 m2; should not be initiated if eGFR <45 mL/min/1.73 m2. But once started, can continue to be used if stable eGFR >30 mL/min/1.73 m2, although reduction in dose is prudent if eGFR between 30 and 45 mL/min/1.73 m2. | Moderate |
|
| |||||||
| GLP-1 RA | +++ | Low risk | Loss | Demonstrated benefit reducing risk of MACE (dulaglutide; liraglutide; semaglutide SQ); Demonstrated reduced risk for stroke with semaglutide and dulaglutide | Neutral | Renal benefit demonstrated in CVOTs (dulaglutide, liraglutide, SQ semaglutide) largely due to decreased albuminuria; Worsening kidney function or AKI can occur in presence of volume depletion due to severe adverse GI S/S. Exenatide not recommended if eGFR below 45 mL/min/1.73 m2 or ESKD or if CrCl <30 mL/min | Moderate |
|
| |||||||
| DUAL GIP/GLP-1 RA | +++ | Low risk | Loss | CVOT being conducted | Neutral | One exploratory analysis showed slowing of eGFR decline in those with T2D and increased CV risk; Worsening kidney function or AKI can occur in presence of volume depletion due to severe adverse GI S/S | Moderate |
|
| |||||||
| SGLT2i | ++ | Low risk | Loss | Demonstrated benefit reducing risk of MACE (empagliflozin; canagliflozin); empagliflozin demonstrated benefit reducing risk of CV death and all-cause mortality | Demonstrated benefit reducing risk of HHF (see legenda) | Demonstrated benefit reducing risk for CKD progression (see legendb) | Neutral |
|
| |||||||
| DPP-4i | + | Low risk | Neutral | Noninferior to placebo | CVOT showed increased risk for HHF with saxagliptin; alogliptin should be used with caution in patients with CHF of NYHA functional classes III and IV. | Neutral/all but linagliptin require dose adjustment if decreased kidney function. | Neutral |
|
| |||||||
| AGI | + | Low risk | Neutral | Neutral | Neutral | Neutral/not recommended if serum creatinine >2.0 mg/dL | Moderate |
|
| |||||||
| TZD | ++ | Low risk | Gain | Potential reduced risk of MACE/stroke (pioglitazone) | Increased risk secondary to fluid retentiond | Neutral/potential for increased fluid accumulation | Neutral |
|
| |||||||
| SU/GLINIDE | ++/+ | Moderate-to-severe/mild-to-moderate increased risk for both with CKD | Gain | Neutral | Neutral | Neutral/increased risk of hypoglycemia | Neutral |
|
| |||||||
| COLSVL | + | Low risk | Neutral | Lowers LDL-C; Can increase TG levels; Contraindicated if serum TG >500 mg/dL or if history of hypertriglyceridemia-induced pancreatitis | Neutral | Neutral | Mild to Moderate |
|
| |||||||
| BCR-QR | + | Low risk | Neutral | No increased risk | Neutral | Neutral | Moderate |
|
| |||||||
| INSULIN (basal/basal bolus) | +++/++++ | Moderate-to-severe increased risk with CKD | Gain | Neutral | Monitor for fluid retention | Neutral/increased risk of hypoglycemia | Neutral |
|
| |||||||
| PRAMLINTIDE | + | Increased risk because indicated in those with T1D and T2D using mealtime insulin | Modest loss | Neutral | Neutral | Neutral | Moderate |
Abbreviations: AGI = alpha-glucosidase inhibitors; AKI = acute kidney injury; ASCVD = atherosclerotic cardiovascular disease; BCR-QR = bromocriptine quick release; CHF = congestive heart failure; CKD = chronic kidney disease; COLSVL = colesevelam; CrCl = creatinine clearance; CV = cardiovascular; CVD = CV disease; CVOT = CV outcome trial; DPP-4i = dipeptidyl peptidase 4 inhibitor; eGFR = estimated glomerular filtration rate; ESKD = end-stage kidney disease; GFR = glomerular filtration rate; GI = gastrointestinal; GIP = glucose-dependent insulinotropic polypeptides; GLP-1 RA = glucagon-like peptide-1 receptor agonist; HDL-C = high-density lipoprotein cholesterol; HF = heart failure; HHF = hospitalization for heart failure; HFrEF = heart failure with reduced ejection fraction; LDL-C = low-density lipoprotein cholesterol; MACE = major adverse cardiovascular event; S/S = signs & symptoms; SGLT2i = sodium glucose cotransporter 2 inhibitor; SU = sulfonylurea; T1D = type 1 diabetes; T2D = type 2 diabetes; TG = triglyceride; TZD = thiazolidinedione Disclaimer: The designated row of a medication class does not imply or indicate any preference or hierarchy. In addition, prescribers should always refer to the most recent published prescribing information for medications as well as consideration of local resources and individual patient circumstances. The evidence base content in the guideline has much more comprehensive information about antihyperglycemic medications including potential adverse events and how to reduce their risk and/or treat them.
Decreased HHF was seen in CVOTs with canagliflozin, empagliflozin, dapagliflozin, and ertugliflozin. Some subsequent studies had HF as primary outcomes and led to dapagliflozin receiving an indication to reduce risk of HHF in adults with T2D and either established CVD or multiple CV risk factors AND to reduce risk of CV death and HHF in adults (with or without T2D) with HFrEF (NYHA classes II-IV). Empagliflozin has indication to reduce the risk of CV death in adult patients with T2D and established CV disease AND to reduce the risk of CV death and HHF in adults (with or without T2D) with HF (not limited to HFrEF). Because of recent publication of the Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure (DELIVER) trial,1054a it is likely that the dapagliflozin HF indication will lose the limitation to HFrEF.
Canagliflozin has indication to reduce risk of ESKD, doubling of serum creatinine, CV death, in adults with T2D and diabetic nephropathy with albuminuria; HHF in those with a history of HF. Dapagliflozin has indication to reduce the risk of sustained eGFR decline, ESKD, CV death, in adults with CKD at risk of progression and HHF in those with history of HF. The EMPA-KIDNEY trial has been stopped early due to evidence of efficacy.
Efficacy dependent on baseline A1C and duration of diabetes.
TZDs are contraindicated in persons with NYHA Class III/IV CHF.
Agents with “low risk” for hypoglycemia may have that risk increased when combined with antihyperglycemic agents that themselves can cause hypoglycemia. The latter agents may need to have a lower dose in order to reduce hypoglycemia risk.
As monotherapy, most noninsulin antihyperglycemic agents reduce A1C by 0.5% to 2.0%. Larger decrements are seen in persons with more marked A1C elevations, likely explaining the apparent greater efficacy of some older agents in their clinical trials vs newer ones.73 Several GLP-1 RAs lower glucose more than other noninsulin antihyperglycemic agents.971 The various classes of glucose-lowering agents differ widely in nonantihyperglycemic respects (Table 16).
Detailed descriptions of available antihyperglycemic agents, their mechanisms of action, glycemic efficacy, extraglycemic effects, and rationale for use in different clinical situations can be found in the AACE Comprehensive T2D Management Algorithm73 and Table 16 as well as the 2022 ADA Standards of Care chapter on pharmacologic approaches to glycemic treatment972 and the 2018 ADA/EASD consensus report967 and its 2020 update.973,974 In addition to lowering glucose, a priority in DM management is to avoid or minimize the risks for hypoglycemia. Choosing agents that are associated with weight loss or minimal weight gain is also desirable. AACE preferentially recommends agents that can achieve these goals.
Metformin is often the preferred initial therapy for most persons with new-onset T2D. Once initiated, metformin should be continued as long as it is tolerated and not contraindicated. Metformin carries a low risk of hypoglycemia, is weight neutral, produces durable antihyperglycemic effects, and some studies suggest CV benefit. It is equally efficacious across all weight categories (normal, overweight, and obese) in T2D.975 However, it should not be used in persons with advanced renal impairment in which situation it can pose a risk of lactic acidosis.407,976–978 Metformin should not be used in persons with eGFR <30 mL/min/1.73 m2, and it should not be initiated in persons with an eGFR <45 mL/min/1.73 m2.73,979 However, once started, it can continue to be used in persons with stable eGFR >30 mL/min/1.73 m2 although reduction in total daily dose (TDD) is prudent in persons with eGFR between 30 and 45 mL/min/1.73 m2.
Metformin is sometimes associated with anorexia and weight loss and may cause GI adverse effects (eg, nausea, vomiting, dyspepsia, or diarrhea). Longer-term use of metformin may be associated with the development of vitamin B12 deficiency,980 and B12 levels should be monitored periodically. When metformin is contraindicated or not tolerated, acceptable alternatives include GLP-1 RAs, SGLT2is, DPP-4 inhibitors, and alpha-glucosidase inhibitors. TZDs, SUs, and glinides may also be used, although caution should be exercised owing to the potential for weight gain, hypoglycemia (not with TZDs), or other risks. Metformin can be used in combination with virtually all other antihyperglycemic agents, including insulin, in persons who do not reach their glycemic target on monotherapy. There are single pill combinations with many other oral antihyperglycemic agents including SUs, DPP-4 inhibitors, SGLT2is, TZDs and glinides.
SUs increase insulin secretion in a glucose level-independent fashion. Appropriate candidates for treatment with SUs are persons with T2D whose duration of DM is <5 years and who do not have end-organ complications (eg, CKD), for whom cost of antihyperglycemic agents is a major concern, and those who are willing to follow a healthy diet and exercise plan and perform BGM or CGM to reduce the likelihood or identify the occurrence of hypoglycemia.981 The use of pharmacoeconomic analyses of medication utilization should help inform prescribers and health systems of the cost-effectiveness of a particular medication. For unknown reasons, not all persons with T2D respond to SUs (primary failure), and antihyperglycemic effectiveness declines after several years of treatment in many persons (secondary failure).982,983 SU therapy may be associated with weight gain, but the main SU adverse event of concern is hypoglycemia, which can be more prolonged than that produced by insulin, particularly when longer-acting formulations (eg, glyburide) are used in older adults.984 Decreased kidney function also increases the risk of SU-associated hypoglycemia. Glinides’ mode of action and other properties are very similar to those of SUs, but the efficacy is less, and hypoglycemia potential is also less than with SUs.985
TZDs improve insulin sensitivity and can preserve or improve b cell secretory function in persons with T2D. In addition to their glycemic effects, these agents also improve a wide range of CV risk markers986,987 and may help prevent central nervous system insulin resistanceerelated cognitive dysfunction.988 Clinical studies and meta-analyses of RCTs reported that treatment with pioglitazone results in a statistically significant reduction in the composite outcome of nonfatal acute MI, stroke, and all-cause mortality (MACE).663,989,990 TZDs have been shown to have benefit in some persons with NASH686,991,992; however, TZDs lead to weight gain comparable to that with SU and insulin therapy.993 TZDs may also cause fluid retention (particularly in persons with cardiac or renal disease), which may contribute to TZD-associated weight gain and peripheral edema. The risk for both might be decreased by using lower doses of pioglitazone and avoiding the highest dose and/or perhaps use in combination with an SGLT2i and/or a GLP-1 RA.994 TZDs are not recommended in persons with symptomatic HF and are contraindicated in persons with New York Heart Association (NYHA) class III or class IV CHF. TZDs can also reduce bone mineral density and are associated with increased risk for bone fractures, especially in women, with the majority of fractures in the distal upper limb or distal lower limbs.995,996 The TZD rosiglitazone has been withdrawn from use in Europe and was severely restricted in the United States because of concerns over a possible increase in CVD risk.997 However, the FDA later lifted this restriction because additional data, including one large RCT, showed it was not associated with an increased risk.998,999 According to the FDA, pioglitazone, but not rosiglitazone, may be associated with increased rates of bladder cancer, although there is not enough evidence to support a clear association.1000,1001 A cumulative exposure analysis involving data from 1.01 million persons from multiple countries over 5.9 million person-years found no association between exposure to pioglitazone and bladder cancers.1002
GLP-1 RAs and DPP-4 inhibitors increase insulin and decrease glucagon secretion in a glycemic level-dependent manner. In addition to glucose lowering, the GLP-1 RAs may slow gastric emptying, promote early satiety, reduce food intake, and frequently are associated with weight loss. GLP-1 RAs are also associated with a decrease in BP accompanied by a small increase in pulse rate. There also can be improvements in lipid levels.1003,1004
Currently approved GLP-1 RAs include dulaglutide, exenatide, exenatide-ER, liraglutide, lixisenatide, and semaglutide, which are administered by injection on a twice daily, daily, or once weekly basis. There is also a form of semaglutide that is orally administered. These agents are often used as add-on therapies for persons with inadequately controlled DM despite oral therapy.242,642,1005-1020 Several clinical trials have compared the effects of adding a GLP-1 RA to insulin (glargine insulin or premixed insulin) in persons with inadequately controlled T2D on oral agents.1021-1030 All of the studies show equivalent or slightly better A1C lowering by GLP-1 RA with the advantages of a 2- to 3-kg weight loss and little or no additional hypoglycemia. Additionally, liraglutide, semaglutide, and dulaglutide have demonstrated reduction in MACE in CVOTs.242,642,1031 As a result, guidelines recommend use of GLP-1 RAs before initiation of insulin for most individuals with T2D (see Evidence Base 9: How should antihyperglycemic agents be prioritized in persons with T2D at high risk for or with established CVD?).
The most frequently experienced adverse effects with GLP-1 RAs are nausea, vomiting, and diarrhea, which may lead to discontinuation of the GLP-1 RA in 5% to 10% of persons, but usually these adverse symptoms diminish over time.1032
Although medullary thyroid carcinoma has not been shown to be caused by GLP-1 RAs in humans, all GLP-1 RAs except twice-daily exenatide and lixisenatide are contraindicated in persons with a personal or family history of medullary thyroid carcinoma and in persons with multiple endocrine neoplasia syndrome type 2. The FDA has stated that persons taking a GLP-1 RA do not need to be monitored for medullary thyroid carcinoma (eg, with calcitonin levels)1033 (also see discussion of pharmacologic therapies for DM and cancer risk or prognosis under Q27. How should potential increased cancer risk be managed in persons with obesity/T2D?).
Pancreatitis appears to be a rare association with use of GLP-1 RAs and DPP-4 inhibitors.1034,1035 Prescribing information for GLP-1 RAs and DPP-4 inhibitors generally states that these agents have not been studied in persons with a history of pancreatitis. Consider other antihyperglycemic therapies in persons with a history of pancreatitis.
Tirzepatide is a dual glucose-dependent insulinotropic peptide and GLP-1 RA recently approved by the FDA for improvement of glycemic control in persons with T2D. Individual trials have assessed the clinical profile of tirzepatide vs different comparators. A systematic analysis of seven completed trials with a total of 6609 participants1036 confirmed a dose-dependent (5, 10, or 15 mg weekly subcutaneous administration) superiority on glycemic efficacy, and reduction in body weight was evident with tirzepatide vs placebo, GLP-1 RAs, and basal insulin. Tirzepatide was associated with increased incidence of GI adverse events but no increase in risk of hypoglycemia. Tirzepatide appears to be useful for those already on metformin therapy. Based on some early promising data and ongoing trials, including a CVOT, clinical indications for weight loss and/or CV risk reduction may be sought.1037
DPP-4 inhibitors do not cause weight gain; linagliptin can be administered in persons with CKD at full dosage since it is not cleared by the kidneys. Sitagliptin, saxagliptin and alogliptin are renally cleared and require appropriate dose adjustment in the presence of decreased eGFR. DPP-4 inhibitors do not have significant GI adverse effects and may be used in early combination with metformin.1038–1043 CVOTs with DPP-4 inhibitors achieved non-inferiority compared with placebo for the occurrence of MACE.1044–1046 The trial comparing saxagliptin with placebo showed an increased likelihood of hospitalization for CHF1045 without increase in mortality. (Prescribing information states: Consider the risks and benefits of saxagliptin in patients who have known risk factors for HF. Monitor patients for signs and symptoms.) The FDA also noted a trend toward increased hospitalization for CHF without increase in mortality with alogliptin and stated “There is limited experience with alogliptin therapy in patients with CHF of New York Heart Association (NYHA) functional classes III and IV. Alogliptin should therefore, be used with caution in these patients,”1044,1047 Despite no evidence of increased risk in their CVOTs, the prescribing information for sitagliptin and linagliptin say because HF has been observed with other members of the DPP-4 inhibitor class, consider risks and benefits in patients who have known risk factors for HF. Monitor patients for signs and symptoms. The main adverse effects noted with DPP-4 inhibitors are a small increase in upper respiratory tract viral infections (rates of nasopharyngitis were 6.4% with a DPP-4 inhibitor vs 6.1% with comparators; risk ratio, 1.2; 95% CI, 1.0–1.4) and a rare hypersensitivity reaction.1032 Severe and disabling arthralgia has been reported in individuals taking DPP-4 inhibitors.1032
SGLT2is are the newest class of oral antihyperglycemic agents approved for treatment of individuals with T2D. The glucosuric effect of these agents reduces both glycemia and weight in most persons. Most also experience decreases in systolic BP. Dehydration due to increased diuresis could lead to hypotension.1048 Clinicians and persons with DM should be alert for the potential of postural hypotension, especially in older adults on loop diuretics. Although the antihyperglycemic effect can be diminished with decreasing eGFR, studies have shown that SGLT2is continue to exert their renal protective benefit for those with low eGFRs (eg, <45 mL/min/1.73 m2).423
By increasing glycosuria, SGLT2is may increase the risk of fungal genital tract infection and much less frequently urinary tract infection. Risk of DKA is increased in persons using SGLT2is, especially in those being treated with insulin (especially if there has been a recent reduction in their insulin dose) and/or those with acute illnesses and prolonged fasting.1049,1050 Small increases in LDL-C levels (4 to 8 mg/dL) occurred with canagliflozin, dapagliflozin, and empagliflozin in pivotal trials. Bone fracture has been described in post-marketing safety reporting.1051 Multiple studies have shown renal and CV benefits of SGLT2is239,424–426,444,1052–1054 (see also Evidence Base 9: How should antihyperglycemic agents be prioritized in persons with T2D at high risk for or with established CVD?). Empagliflozin and canagliflozin demonstrated reduction in MACE in CVOTs; empagliflozin also demonstrated decreased CV death and all-cause mortality. Empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin have shown a decrease in hospitalization for HF in their CVOTs. Dapagliflozin also has shown in people with HFrEF reduced risk of worsening HF or death from CV causes regardless of the presence or absence of DM.457 Empagliflozin has also demonstrated reduction in composite of CV death or hospitalization for worsening HF in those with HFrEF with or without T2D.470 More recently, empagliflozin was demonstrated to reduce the combined risk of CV death or hospitalization for HF in persons with HFpEF, regardless of the presence or absence of DM.239,425,426,443 Dapagliflozin has now been shown to reduce the combined risk of worsening HF or CV death in patients with HF and a mildly reduced or preserved ejection fraction.1054a Dapagliflozin received an FDA indication to reduce the risk of CV death and hospitalization for HF in adults with and without DM with HFrEF (NYHA Class II-IV). Empagliflozin received an indication for those with and without DM to reduce the risk of CV death and hospitalization for HF in adults with HF (HFrEF or HFpEF).
Based on the CREDENCE trial,423 the FDA has given canagliflozin an indication to reduce the risk of end-stage kidney disease, doubling of serum creatinine, CV death, and hospitalization for HF in adults with T2D and diabetic nephropathy with albuminuria. Dapagliflozin based on the DAPA-CKD RCT424 has received an FDA indication to reduce the risk of sustained eGFR decline, end-stage kidney disease, CV death, and hospitalization for HF in adults with CKD at risk of progression.
As a result of GLP-1 RA and SGLT2i studies above, independent of glycemic control or targets, individuals with T2D at significant risk for or with established ASCVD, HF, and/or CKD should be treated with a GLP-1 RA or SGLT2i with proven benefit for the individual’s specific conditions.
Colesevelam, alpha-glucosidase inhibitors, and bromocriptine primarily affect PPG levels and are worth consideration in selected persons. Colesevelam carries a low risk of hypoglycemia and also reduces LDL-C, for which it was originally developed. It also modestly increases triglyceride levels, and its main adverse effect is constipation, but it is not systemically absorbed and therefore is not likely to have systemic adverse effects.1055
Alpha-glucosidase inhibitors also have a low risk for hypoglycemia, although persons may not tolerate the GI side effects (eg, bloating, flatulence, and diarrhea). These may be reduced by starting with a low dose and slowly titrating the dose as needed. Acarbose has been shown to lower A1C and cause weight loss.1056,1057 Some clinical trials have suggested some CV benefit in persons with IGT or DM. However, in a large RCT1058 of Chinese participants with coronary heart disease and IGT, acarbose did not reduce the risk of MACE, but did reduce the incidence of DM.891,892
The dopamine receptor agonist bromocriptine does not cause hypoglycemia. It can cause nausea and orthostasis and should not be used in persons taking antipsychotic drugs. Bromocriptine in one study with a small number of events was associated with reduced CV event rates.1059
Because many persons do not achieve adequate glycemic control with monotherapy or are at risk for early loss of efficacy with metformin or another monotherapy, combining antihyperglycemic agents is often appropriate.1060 For some recently diagnosed individuals with T2D and more severe hyperglycemia, early combination pharmacotherapy should be considered, usually to include metformin plus another first-line agent that does not cause hypoglycemia, especially a GLP-1 RA or an SGLT2i, or DPP-4 inhibitor (see R 12.2.6 and R 12.2.7 and R 9.1 to R 9.4 on CVD). In the VERIFY trial,1040 initial combination therapy of metformin and the DPP-4 inhibitor vildagliptin was superior to sequential addition of medications in prolonging the occurrence of primary and secondary failure. For newly diagnosed persons with T2D and an entry A1C >9.0% and/or ≥1.5% above target, one should initiate, along with lifestyle modifications, dual or possibly triple combination pharmacotherapy usually including metformin and considering basal insulin. If there are significant symptoms of hyperglycemia, especially including catabolism (eg, weight loss) or a very high A1C >10% (86 mmol/mol) or BG levels (300 mg/dL [16.7 mmol/L]), insulin is recommended. The Efficacy and Durability of Initial Combination Therapy study compared efficacy of initial metformin/pioglitazone/exenatide in new-onset T2D vs sequential addition of metformin followed by glipizide and insulin. The decrease in A1C from triple therapy was greater at 6 months than that of conventional therapy and the superiority was maintained at 3 years.1061
Metformin is quite effective when administered in combination with other agents, as long as one avoids its use in persons with CKD (eGFR <30 mL/min/1.73 m2)73 or GI intolerance. SUs, in contrast, may be problematic when used in combinations because they can cause hypoglycemia and may reduce, eliminate, or minimize the weight-loss benefit of drugs such as metformin, GLP-1 RAs, and SGLT2is.993 See R 12.2.4 and Evidence Base 9: How should antihyperglycemic agents be prioritized in persons with T2D at high risk for or with established CVD? for those with established or high risk for ASCVD, HF, and/or CKD for recommendations about antihyperglycemic medications that should be used often in combination with metformin. Even for those without these conditions who require intensification of therapy, one should consider adding a GLP-1 RA and/or an SGLT2i, which would provide good glycemic lowering (especially with GLP-1 RA), reduction in weight and BP, and a low risk for hypoglycemia. Other medications that have a low risk for hypoglycemia are DPP-4 inhibitors and TZDs. Medications that tend to be less expensive than others are SUs and TZDs.
Insulin Use in T2D
Insulin is usually initiated in those with T2D when combination therapy with other agents fails to attain or maintain glycemic goals, or when an individual, whether drug naïve or on a treatment regimen, presents with an A1C level >9.0% and symptomatic hyperglycemia.73,1062 Once insulin is initiated, its beneficial A1C effect is stable for 2 to 4 years in the majority of persons.1063 Most persons with T2D who require intensification of antihyperglycemic therapy with a GLP-1 RA or insulin should initially be prescribed a GLP-1 RA. Insulin could then be added if further intensification is required. Several RCTs show that GLP-1 RAs vs basal insulin have equal or better glucose lowering, low risk for hypoglycemia, and weight reduction vs weight gain.1021,1064
Insulin therapy may be initiated as a basal, basal-bolus, prandial, or premixed regimen, although for most persons with T2D, starting with a basal insulin analog added to the existing antihyperglycemic regimen is preferred1065 (Tables 17 and 18). The combination of insulin with any antihyperglycemic agent raises the potential for hypoglycemia. Persons should be closely monitored, and those on SUs or glinides may require dosage reductions or discontinuation of the oral agent. TZDs can be associated with weight gain, edema, and increased risk of CHF in combination with insulin. Basal insulin analogs are preferred over NPH insulin because of a reduced risk of hypoglycemia.1066–1071 Newer “ultra-long-acting” basal insulin analogues, insulin glargine U300, and insulin degludec have been shown to be associated with less hypoglycemia than insulin glargine U100.1072,1073 The insulin regimen to be prescribed and the exact treatment goals should be discussed with the person with DM.
Table 17.
Recommended Steps for the Addition of Insulin to Antihyperglycemic Therapy
| Glucose value | Total daily dose | Notes/caveats |
|---|---|---|
| Step 1. Start basal (long-acting insulin) | ||
| A1C <8% | 0.1 to 0.2 units/kg | Consider discontinuing SU therapy; basal analogs preferred over NPH; bedtime dose preferred |
| A1C >8% | 0.2 to 0.3 units/kg | |
| Step 2. Titrate basal insulin every 2–3 d to reach glycemic goals a | ||
| Fixed regimen | Increase by 2 units/d | |
| Adjustable regimen | ||
| FBG >180 mg/dL | Add 4 units | |
| FBG 140 to 180 mg/dL | Add 2 units | |
| FBG 110 to 139 mg/dL | Add 1 unit | |
| Step 3. Monitor for hypoglycemia | ||
| BG <70 mg/dL | Reduce by 10% to 20% | |
| BG <40 mg/dL | Reduce by 20% to 40% | |
Abbreviations: A1C = hemoglobin A1c; BG = blood glucose; d = day; FBG = fasting blood glucose; NPH = Neutral Protamine Hagedorn; SU = sulfonylurea.
For most persons with T2D taking insulin, glucose goals are A1C <7% and fasting and premeal blood glucose <110 mg/dL in the absence of hypoglycemia. A1C and FBG targets may be adjusted based on a person’s age, duration of diabetes, presence of comorbidities, diabetic complications, and hypoglycemia risk.
Insulin-treated persons should be instructed in performance of BGM. Most insulin-treated persons with T2D should conduct BGM ≥2 times daily and ideally at least before each injection of insulin. The frequency and timing should be dictated by the particular needs and goals of the individual, as well as hypoglycemia risk. Emerging evidence suggests benefits of CGM use in insulin-treated persons with T2D170,1074 (see Q3. When and how should glucose monitoring be used?).
Premixed insulins are popular with some persons, but they provide less dosing flexibility and have been associated with a higher frequency of hypoglycemia compared to basal and basal-bolus regimens in many, but not all studies.1075–1079 Nevertheless, there are some persons for whom a simpler regimen is a reasonable compromise, and for this population, analog premixed insulins will provide better glycemic control with less hypoglycemia than the traditional, more affordable premixed NPH regimens.1080 The analog premixed insulin insulin degludec/insulin aspart may provide reductions in nocturnal hypoglycemia compared to glargine U100,1081 but a basal bolus regimen with an ultra-long-acting basal insulin analog and a rapid-acting prandial insulin analog may achieve more effective control with less hypoglycemia.1082 Different concentrations of the rapid-acting analogue may be beneficial in some populations.1083 With the BIAsp 30 preparation (premixed insulin analogue containing 30% soluble, rapid-acting insulin aspart and 70% intermediate-acting protamine-bound aspart in each injection), for higher A1C levels, a third injection prior to lunch may be preferable.1084
When mealtime glucose control is needed or when glycemic goals are not met on a basal insulin regimen plus oral agents or a GLP-1 RA, insulin therapy intensification to a basal-bolus regimen (using a rapid-acting insulin analog or inhaled insulin) should be considered (Table 19). Ultra-rapid acting insulins can reduce postprandial glycemic peak, but this effect on long-term complications is unknown.1085,1086 In addition, insulin human inhalation powder, a rapid-acting inhaled insulin, is effective at reducing postprandial peak, and studies in persons with T1D demonstrated that hypoglycemia was reduced with use of this inhaled insulin relative to insulin aspart,1087 but overall glycemic efficacy measured by A1C may not be as great as subcutaneous insulin.1088,1089
Table 19.
Types of Insulin
| Onset | Peak | Duration | |
|---|---|---|---|
| Basal insulins | |||
| Intermediate-acting human (cloudy) | |||
| Neutral Protamine Hagedorn (human) | 1–3 h | 5–8 h | Up to 18 h |
| Long-acting (clear) analogs | |||
| Detemir | 1.5 h | near peakless | 16–24 h |
| Glargine U100a | 1.5–2 h | near peakless | 24 h |
| Glargine U300 | 6 h | peakless | >30 h |
| Degludec U100 | 1 h | peakless | 42 h |
| Degludec U200 | 1 h | peakless | 42 h |
| Prandial insulins | |||
| Short-acting human | |||
| Regular | 30–60 min | 2–4 h | 5–8 h |
| Rapid-acting analogs | |||
| Aspart | 15 min | 1–1.5 h | 3–5 h |
| Glulisine | 12–30 min | 1–1.5 h | 3.5–5 h |
| Lisprob (U100 and U200) | 15–30 min | 1–2 h | 3–4.75 h |
| Faster-acting analogs | |||
| Faster aspart | 4 min | 0.5–1.5 h | 3–5 h |
| Lispro aabc | 15–17 min | ≈ 2 h | 4.6–7.3 h |
| Inhaled technosphere insulin | ≈ 12 min | 0.5–1 h | 1.5–3 h |
| Premixed Insulins (cloudy) | |||
| 70/30 NPH/Regular | These insulins contain a fixed ratio of intermediate-acting insulin and short- or rapid-acting insulin. These suspensions must be resuspended uniformly for more consistent glucose lowering. The timing and adjustment of these insulins depend on glucose levels and the individual kinetics of the insulin components. | ||
| 70/30, 60/40, 50/50 N/R | |||
| 70/30 aspart protamine/aspart | |||
| 75/25 lispro protamine/lispro | |||
| 50/50 lispro protamine/lispro | |||
Biosimilars are follow-on biologics which have been approved via the Public Health Service Act. Biosimilar designation allows the drugs to be interchangeable with the reference drug and are approved by the US Food and Drug Administration (FDA) to allow pharmacists to substitute without the need for an authorized prescription.
Clinicians should refer to the FDAdapproved prescribing information for the most current official product information on any of the insulins.
Degludec
Flexible dosing with at least 8 to 40 hours between injections was not associated with increased hypo- or hyperglycemia. Source: Mathieu C, Hollander P, Miranda-Palma B, et al. NN1250–3770 (BEGIN: Flex T1) Trial Investigators. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98(3):1154–1162 [EL 1; RCT].
Similar glycemic variability parameters, but better continuous glucose monitoring metrics (time in range, time above range, time below range) compared with glargine U300.1148
Detemir
Up to 40% to 50% of persons may require twice daily dosing (with 12 hours after the morning dose). Sources: Dornhorst A, Lüddeke HJ, Sreenan S, Koenen C, Hansen JB, Tsur A, Landstedt-Hallin L, et al. Safety and efficacy of insulin detemir in clinical practice: 14-week follow-up data from type 1 and type 2 diabetes patients in the PREDICTIVE European cohort. Int J Clin Pract. 2007;61(3):523–528 [EL 2; PCS]; and Heller S, Koenen C, Bode B, et al. Comparison of insulin detemir and insulin glargine in a basal-bolus regimen, with insulin aspart as the mealtime insulin, in patients with type 1 diabetes: a 52-week, multinational, randomized, open-label, parallel-group, treat-to-target noninferiority trial. Clin Ther. 2009;31(10):2086–2097 [EL 1; RCT].
Glargine U300
Persons with type 1 diabetes may require ~15% to 30% higher dose compared with glargine U100. Source: Porcellati F, Bolli GB, Fanelli CG. Pharmacokinetics and pharmacodynamics of basal insulins. Diabetes Technol Ther. 2011;13 Suppl 1:S15–S24 [EL 4; NE].
Prandial insulins
Based on onset of action, insulin should be taken at appropriate time to match the postprandial glucose absorption. All can be used in insulin pumps with preference given to rapid-acting insulins. Source: Bode B, Weinstein R, Bell D, McGill J, Nadeau D, Raskin P, Davidson J, Henry R, Huang WC, Reinhardt RR. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: A randomized study in type 1 diabetes. Diabetes Care. 2002;25(3):439–444 [EL 1; RCT].
Glargine available as branded, U100 biosimilar (2 preparations) or U100 follow-on biologic (single preparation)
Lispro available as branded, or a follow-on biologic (U-100)
CSII or insulin pumps are options for persons with T2D taking basal and prandial injections (MDI) of insulin.153,1090 Persons with T2D may also benefit from the use of a wearable device that delivers for basal insulin a continuous subcutaneous infusion of rapid-acting insulin and also allows 2-unit boluses of insulin when the wearer depresses a button.1091
Many people with T2D treated with MDI and CSII should also be using CGM, and a significant number of those treated using the wearable, patch-like device described above or receiving injections of basal insulin only would benefit greatly by use of CGM. More information about insulin pumps, CGM (including differences between rtCGM, isCGM), and open-loop and hybrid closed-loop (HCL) use of both insulin pumps and CGM can be found elsewhere in this guideline and in the 2021 AACE Advanced Diabetes Technology guideline.153
Use of the amylin analog pramlintide in conjunction with bolus insulin improves both glycemia and weight in persons with T2D.1092,1093 GLP-1 RAs and DPP-4 inhibitors have properties similar to those of pramlintide and also increase endogenous in- sulin secretion. The combination of basal insulin and incretin therapy decreases basal glucose and PPG and may minimize weight gain and the risk of hypoglycemia compared with basal-bolus insulin regimens. Pharmacokinetic and pharmacodynamic studies of combination GLP-1 RAs and basal insulin analogs have shown an additive effect on BG decreases.1021,1094–1097 Similarly, in persons with T2D who are treated with basal-bolus insulin therapy, adding a GLP-1 RA or switching to a fixed-ratio combination of a GLP-1 RA and a basal insulin or adding an SGLT2i1098 or pramlintide can serve as adjuncts to prandial insulin therapy to reduce postprandial hyperglycemia, A1C, and weight. GLP-1 RAs may also allow reduction or discontinuation of bolus insulin in some individuals. Long-acting GLP-1 RAs also reduce fasting glucose.1099
The combined use of DPP-4 inhibitors or SGLT2is with insulin can also help improve glycemic control with a relatively low risk of hypoglycemia, although the glycemic lowering is likely to be less than with GLP-1 RAs.1100,1101
U500 regular insulin (contains 500 units/mL of regular insulin) may improve glycemia in the therapy of persons with DM who have severe insulin resistance (eg, require >200 total units of insulin/day).1102–1105 The pharmacokinetics of U500 insulin is more like NPH than regular insulin but is variable and can pose a hypoglycemia risk and be associated with weight gain. U500 insulin should only be administered with a U500 insulin syringe or a U500 insulin pen device.
Hypoglycemia and weight gain are the most common adverse effects of insulin therapy.1106,1107 Rates and the clinical impact of hypoglycemia are frequently underestimated,1108 but about 7% to 15% of insulin-treated persons with T2D experience at least 1 episode of hypoglycemia per year,1109 and 1% to 2% have severe hypoglycemia.1106,1108 The frequency of hypoglycemia increases with intensive insulin targets, use of SUs, decreased caloric intake, delayed meals, exercise, alcohol consumption, CKD, T2D duration, and cognitive impairment.1108 Large, randomized trials have shown that participants with established T2D and a history of 1 or more severe hypoglycemic events had an approximately 2- to 4-fold higher rate of mortality for reasons that remain unknown.87,1110 It has been proposed that hypoglycemia may be a marker for persons at higher risk of death rather than being its proximate cause1108; nevertheless, avoidance of hypoglycemia by appropriately reducing insulin dosages seems prudent. Basal inulin analogs are associated with less hypoglycemia than human basal insulin such as NPH. U300 glargine and insulin degludec have a lower risk for hypoglycemia than U100 insulin glargine1031,1111 or insulin detemir.
Question 13: How should insulin therapy be used for management of persons with T1D?
Recommendation 13.1
Insulin must be used to treat all persons with T1D.
Grade A; BEL 1
Recommendation 13.2
Physiologic insulin replacement regimens, which provide both basal and prandial (meal-related or bolus) insulin, are recommended for most persons with T1D.
Grade A; BEL 1
Recommendation 13.3
Achievement of glucose targets using either MDI of insulin or CSII, is needed to prevent development of life-threatening crises, such as acute hyperglycemic crises (DKA and hyperglycemic hyperosmolar state) and catabolic state.
Grade A; BEL 1
Recommendation 13.4
A multi-component self-management diabetes education program is recommended for persons with T1D. Ideally, this is provided by a professional with expertise (ie, CDCES) in the topics of healthy lifestyle, insulin technique including prandial insulin dosing guided by carbohydrate counting, and diet adjustments for special situations, such as physical activity and prolonged fasting. Instruction is also needed in how to deal with sick days and prevention of DKA and hypoglycemia, and other relevant issues. Due to changes in DM self-management practices and each individual’s medical history, personal and cultural background, and educational needs, specific education topics may need to be repeated at regular intervals.
Grade A; BEL 1
Recommendation 13.5
The ideal insulin regimen should be personalized to an individual’s needs and glycemic targets, attempting to better emulate physiological insulin replacement to maintain near normoglycemia, to prevent the development and progression of DM complications, while minimizing hypoglycemia and providing flexibility for specific daily life situations/scenarios such as: exercise, sleep, acute illness, psychological stress, etc.
Grade A; BEL 1
Recommendation 13.6
Insulin regimens usually should involve the use of insulin analogs for most persons with T1D and include the following approaches:
-
MDI, which usually involve 1 to 2 subcutaneous injections daily of basal insulin to suppress ketogenesis and gluconeogenesis and to control glycemia between meals and overnight, and subcutaneous injections of prandial insulin or use of inhaled insulin before each meal to control meal-related glycemic excursions. CGM is the preferred method of glucose monitoring for all individuals with T1D.
Grade A; BEL 1
-
Insulin pump therapy (CSII) provides constant/continuous infusion of fast-acting insulin driven by mechanical force and delivered via a cannula inserted under the skin. CSII can improve (or enhance) glycemic control and should be an option for insulin delivery for appropriate persons with DM. Ideally, these individuals should also use CGM as stated in R13.6.a.
Grade B; BEL 1
-
Automated insulin delivery (AID) systems, which include an insulinpump, an integrated CGM, and computer software algorithm, aim to better emulate physiological insulin replacement and achieve glycemic targets. This technology is recommended for many persons with T1D since its use has been shown to increase TIR while often reducing hypoglycemia or at least without causing increased hypoglycemia.
Grade A; BEL 1
-
Open-loop (use of a pump and sensor which do not communicate) and sensor-augmented pump (SAP) systems: (CGM communicates with pump facilitating needed adjustments to basal rate; temporary interruption of insulin delivery when glucose levels are low or forecast to be low within 30 minutes). Insulin pump with a CGM or an SAP is recommended to manage persons with DM treated with intensive insulin management who prefer not to use AID systems or have no access to them.
Grade D; BEL 4
Evidence Base 13: How should insulin therapy be used for management of T1D?
Based on the World Health Organization’s Classification of Diabetes 2021,1112 T1D is defined as b-cell destruction and absolute insulin deficiency. Hence, insulin therapy is necessary for life in all persons with T1D (“all or nothing”).1113 Absolute (or near-absolute) insulin deficiency can result in acute hyperglycemic complications, including DKA, hyperosmolar hyperglycemic state, hypertriglyceridemia, and hypercatabolic state, which can be life-threatening.1114–1120 All persons with T1D should receive adequate basal insulin replacement, either via frequent injections or CSII, every day to prevent development of life-threating acute hyperglycemic complications.1117,1121 Inadequate (incomplete) insulin replacement, beyond the use of basal insulin, results in chronic hyperglycemia which is a driver for micro- and macrovascular DM-related complications in T1D. Intensive glycemic control with insulin therapy reduces the risk of these complications.200,1122,1123
Since publication of the prior CPG in 2015, there has been extensive development on insulin formulations and delivery, particularly in persons with T1D. Several advantages have been published on newer insulin analogs for both basal and prandial insulin; CSII development with adjunctive use of CGM; and the latest development in the use of nonadjunctive CGM with CSII, also called AID systems, HCL systems, or artificial pancreas device systems (discussed below, in Figure 6, and in 2021 AACE Advanced Diabetes Technology guideline).153
Figure 6.
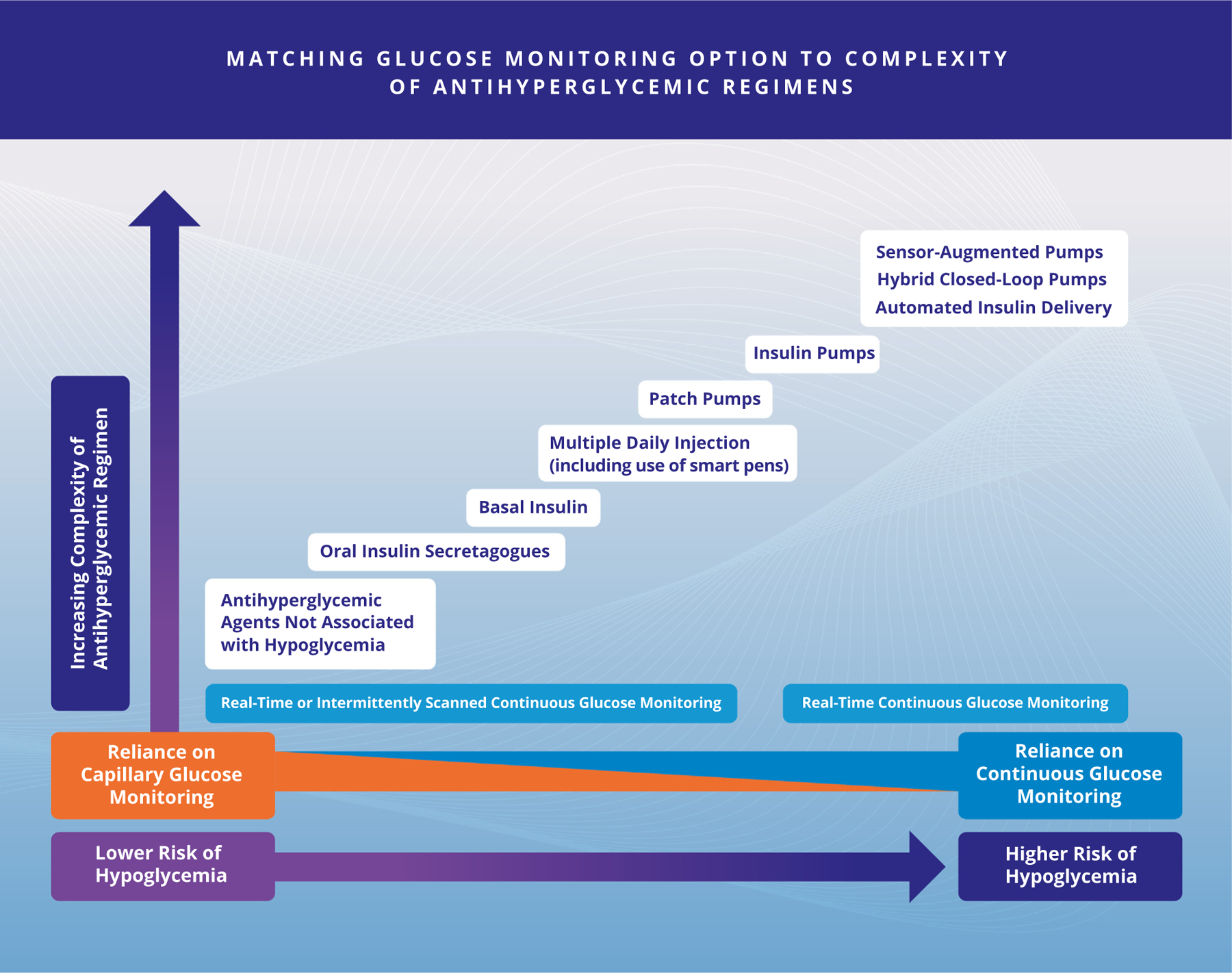
Matching Glucose Monitoring Option to Complexity of Antihyperglycemic Regimens. Copyright © 2022 AACE. May not be reproduced in any form without express written permission from Elsevier on behalf of AACE. Visit https://doi.org/10.1016/j.eprac.2022.08.002 to request copyright permission.
Physiologic insulin regimens including both basal and prandial insulin, provided by either MDI or CSII, have not been formally tested in RCTs against nonphysiologic insulin regimens (once or twice daily insulin). Rather, physiologic insulin regimens have been formally studied as one component of a comprehensive treatment strategy for persons with T1D.1106,1124,1118 In comparisons of regimens of MDI with BGM (without CGM) vs CSII for T1D, there have been small improvements in A1C, but substantial reductions in severe and nocturnal hypoglycemia.1113,1125–1127 However, in the REPOSE RCT, where all participants received a structured diabetes education program and adjusted insulin based on SBMG, treatment with CSII vs MDI resulted in reduction in severe hypoglycemia after 2 years in both groups, with a nonstatistically significant A1C benefit toward CSII, and better treatment satisfaction and QoL among CSII users.1128 Regardless of the insulin delivery method (MDI vs CSII), glycemic control metrics, including A1C and CGM TIR, hypoglycemic events, QoL, and patient satisfaction are substantially improved when MDI or CSII is augmented with CGM, with better results when CSII is combined with CGM or integrated into AID systems, compared with using CGM.158,160,1129–1131
Basic Principles of Insulin Therapy in Type 1 Diabetes
Several trials have demonstrated that physiologic regimens using basal insulin analogs may reduce hypoglycemia, and bolus insulin analogs may result in better control of PPG excursions for most persons with T1D.1132–1137 Hence, insulin analogs should be considered first-line choice for most persons with T1D (Table 19).
These effects were demonstrated initially when comparing first-generation insulin analogs to NPH insulin.1132,1134,1138,1139 Further improvements were confirmed when comparing second generation of ultra-long-acting basal to first-generation insulin analogs,1135,1137,1140–1145 which may translate into potential cost savings in real-world settings from avoiding severe hypoglycemic and hyperglycemic crises.1146 There are limited data comparing the latest basal insulin analogs degludec and glargine U300.1147,1148 The InRange RCT assessed the noninferiority of both basal insulins, as measured by CGM metrics.1149 Furthermore, a novel ultra-long-acting weekly insulin icodec is under development for persons with T1D.1150
Similarly, the use of rapid-acting analogs has resulted in less hypoglycemia, with small reductions in A1C compared to using regular human insulin, including MDI using NPH as basal insulin.1151–1153 The development of ultrarapid insulin analogs has resulted in better coverage of PPG excursions, compared with rapid-acting insulin analogs, as measured by BGM and CGM, but not all studies have resulted in A1C improvements or hypoglycemia reductions.1154–1157 Inhaled insulin with a faster peak of action and shorter duration is also available as prandial insulin, with the requirement of using repeated inhaled correctional insulin doses after 1 to 2 hours postmeal.1158,1159
The starting dose of insulin is usually estimated based on weight, with doses ranging from 0.4 to 0.5 units/kg/day of total insulin, with higher amounts required for persons who are obese (increasingly common in T1D) or have a sedentary lifestyle, as well as during puberty, pregnancy, and acute medical conditions. Conversely, lower starting insulin doses (0.2 to 0.3 units/kg/day) are recommended for older adults, those with renal failure, malnutrition, or low BMI.1124,1136
In general, basal insulin requirements are usually 40% to 50% of TDD insulin. Basal insulin doses are titrated to personalized target fasting glucose. In the ideal prandial/bolus regimen, the dose of prandial insulin is usually determined by estimating the carbohydrate content of the meal. Persons with DM should have formal training on carbohydrate counting as part of a multicomponent DSMES program, provided by professionals (CDCES) if available.1160–1164 Most would start with 1:15 insulin to carbohydrate (IC) ratio (1 unit for 15 g of consistent carbohydrates) or 450/TDD insulin if using regular insulin; 500/TDD if using rapid-acting IC ratio (eg, for someone using 50 units of insulin/day: 500/50 units = 1:10 IC ratio) as a starting point. The IC ratios can be adjusted based on an individual’s response to the calculated boluses of insulin. Insulin sensitivity factor (ISF) approximates the glucose-lowering effect of 1 unit of insulin in a particular individual (1500/TDD units for regular insulin vs 1800/TDD rapid acting insulin). These formulas are just starting points and need to be modified empirically for each person. Numerous mobile applications are also being used to assist with IC ratios and ISF.
IC ratios usually range from 1:20 for the very insulin-sensitive to 1:5 for insulin-resistant persons. Similarly, correction dose insulin for premeal or between-meal hyperglycemia is based on the ISF, also called insulin correctional factor, which is based on the overall insulin sensitivity of a person, loosely estimated by the individual’s TDD insulin. Although various formulas have been used to estimate the appropriate ISF, this parameter should only be viewed as an estimation due to numerous factors that can alter BG. The most commonly used formula is 1800/TDD insulin = number of mg/dL of glucose that will be reduced by 1 unit of insulin. Another key factor that should be appreciated is insulin action time. For most subcutaneous injections, this ranges from 4 to 6 hours. Ultrarapid insulins (ie, faster aspart, lispro aabc) have demonstrated earlier time of onset and action but similar duration of action.1165
With the knowledge of the IC ratio, ISF (insulin correctional factor), and insulin action time, persons with DM on MDI or CSII can calculate the appropriate correction dose of insulin. This is significantly simpler with CSII, as most pumps include bolus calculators to perform the calculations by pressing a few buttons. Most persons using MDI, however, will need to estimate the remaining “insulin on board” from the last injection of prandial insulin based on standard curves that can be provided to them.1124,1136
For persons using MDI or intensive insulin therapy, there are a variety of smart phone apps available that can assist persons with insulin dosing and calculations.1166 Similarly, several smart insulin pens have been developed, including devices that are specific to one insulin brand and others that can be used with different formulations. RCTs have validated their use in persons with T1D, providing benefits for avoidance of extreme glycemic events.1167–1169 This topic is reviewed in the 2021 AACE Advanced Diabetes Technology guideline.153
CSII should only be used by persons who are motivated and knowledgeable in DM self-care, including insulin adjustment. To ensure the safety of persons with DM, prescribing physicians must have expertise in CSII therapy, and CSII users must be thoroughly educated and periodically reevaluated. In 2018, an RCT using an open-loop CSII system found no glycemic benefit compared to MDI, although QoL scores were improved with pump therapy.1170 Training should be provided by personnel with expertise, particularly a CDCES or registered dietitian. Refer to R 17 on DSMES in Section 4 of this guideline.
Adjunctive Medications for Type 1 Diabetes
The amylin analog pramlintide, the only other medication approved for the treatment of T1D, is administered with prandial insulin. A1C reductions are consistently modest, and mild weight loss is common. Nausea is a common adverse effect. There is a potential risk of severe hypoglycemia if persons with DM do not appropriately reduce the prandial insulin dosage.1171–1174 Tachyphylaxis is often seen after several years of therapy.
There has been much interest in the use of metformin as an adjunctive therapy for T1D. A meta-analysis of 13 randomized placebo-controlled trials with 1183 participants with T1D reported small reductions in BMI, insulin requirements, total and LDL-C, but no differences in A1C, HDL-C, or triglyceride levels.1175 Given the lack of glycemic benefit and minimum other benefits on top of frequent GI side effects and a small risk of lactic acidosis, a recommendation for metformin use in T1D cannot be made at this time.
Another unapproved agent for T1D is the GLP-1 RAs, which have been studied for T1D for 2 indications. The first is b-cell preservation in newly diagnosed T1D. In conjunction with anti-IL-21 antibody, liraglutide was shown to provide small improvements of endogenous insulin secretion.1176 Secondly, similar to pramlintide, these agents inhibit glucagon secretion, delay gastric emptying, and promote satiety and weight loss. The largest trial with this class in T1D was with liraglutide, which showed a reduction of A1C of 0.4% with the highest 1.8 mg dose in addition to a 5 kg weight loss and insulin dose reductions.1177 Although this agent was more effective with those participants still producing endogenous insulin, the added hypoglycemia and ketosis noted has resulted in no attempt for FDA approval in T1D.
Of all of the unapproved adjunctive agents for T1D, there has been the most interest in the SGLT2i or SGLT1/2is. By inducing glycosuria, less hyperglycemic spikes, lower A1C levels, and weight loss could be expected. In one meta-analysis of 14 studies and with 4591 participants, A1C was reduced by 0.4% with a 2.7 kg weight loss and reductions in BP and insulin dosing.1178 A 3.38-fold increased risk of DKA (often euglycemic, now generally defined as a BG less than 250 mg/dL)1179 has resulted in no approval in the United States, although the European Medicine Agency has approved dapagliflozin (5 mg) and sotagliflozin (200 mg) for those with T1D and a BMI above 27 kg/m2.1180 Although there have been attempts to reduce risks,1181 at the present time, no recommendation for use of these agents to manage T1D can be made until the high risk of DKA can be safely mitigated.
AID Systems
The integration of glucose monitoring with insulin pump therapy has been an important goal in diabetes technology. Although connectivity of glucose meters to insulin pumps were initially found to be convenient to assist in calculating the bolus dose delivery, the evolution of CGM with sophisticated computerized algorithms has resulted in SAPs and more recently AID systems.
CGM used completely independently of insulin pump therapy (“open-loop”) or in conjunction with an SAP system (where insulin can be interrupted before or when glucose drops below a hypoglycemic threshold) has been shown to benefit glycemic control for all age groups with T1D.153,1182–1184 The use of SAP therapy has been shown to improve not just A1C or hypoglycemia but also glycemic variability and albuminuria1185–1187 when compared with MDI. Furthermore, scores for QoL and treatment satisfaction are also superior with SAP systems compared with MDI and BGM.1188–1191 This is important because not all areas of the world have access to AID systems.
HCL systems and AID systems: AID systems are recommended for all persons with T1D, since their use has been shown to increase TIR, especially in the overnight period, without causing an increased risk of hypoglycemia. For persons with DM with suboptimal glycemia, significant glycemic variability, impaired hypoglycemia awareness, or who allow for permissive hyperglycemia due to the fear of hypoglycemia, such AID systems should be considered.153,1131,1182–1184,1192,1193
Pivotal trials for 3 HCL systems have shown success in improving TIR and reducing hypoglycemia both in the pediatric and adult age group.1182–1184 In terms of insulin algorithms, target glucose choices and clinicians’ understanding of the impact of basal rates, insulin action time, and insulin sensitivity is paramount. Some systems use automated basal only, while others also use automated bolus for sustained hyperglycemia. Exercise is also addressed differently in each system. A meta-analysis of both approved and unapproved systems have shown improvements in glycemic control with closed-loop systems.1194 It is therefore important for clinicians to be familiar with each device. Do-it-yourself HCLs have also gained in popularity due to excellent glycemic results,1195 but as of this writing are not approved by the FDA.
Question 14: How should hypoglycemia be managed?
Recommendation 14.1
Oral intake of rapidly absorbed glucose (eg, glucose tablets or dietary sugar like fruit juice) followed by a snack or meal containing both protein and carbohydrates (eg, cheese and crackers or a peanut butter sandwich) should be used to treat hypoglycemia (measured glucose <70 mg/dL [3.9 mmol/L]) if a person is able to safely swallow.
Grade A; BEL 1
Recommendation 14.2
Glucagon, in one of the currently available forms: intranasal, prefilled liquid stable nonaqueous formulation, prefilled aqueous liquid stable glucagon analogue or with reconstitution from powder, should be used to correct hypoglycemia if individuals are unable or unwilling to ingest carbohydrates orally. If there is no response after 15 minutes, an additional same dose may be administered. As soon as the individual is awake and able to swallow, they should receive a rapidly absorbed source of carbohydrate.
Grade A; BEL 1
Recommendation 14.3
Persons with severe hypoglycemia with altered mental status or with prolonged hypoglycemia need to be hospitalized. If an individual has hypoglycemic unawareness and hypoglycemia-associated autonomic failure, several weeks of hypoglycemia avoidance may at least partially reverse hypoglycemia unawareness and may reduce the risk or prevent recurrence of severe hypoglycemia. Adjustment of an individual’s long-term antihyperglycemic regimen may be necessary to further avoid recurrence of hypoglycemia.
Grade B; BEL 1
Recommendation 14.4
In persons with T2D who develop hypoglycemia and are being treated with alpha-glucosidase inhibitors or with pancreatic diabetes, oral glucose or lactose-containing foods (dairy products) must be given because alpha-glucosidase inhibitors inhibit the breakdownand absorption of complex carbohydrates and disaccharides (eg, table sugars or starches).
Grade A; BEL 1
Recommendation 14.5
Persons at risk for hypoglycemia should perform frequent BGM or preferably use CGM devices (see R 3.1-R 3.4 on monitoring).
Grade B; BEL 4 and expert opinion of task force
Evidence Base 14: How should hypoglycemia be managed?
Definition of Hypoglycemia
The classical definition of hypoglycemia is a low BG level accompanied by symptoms of hypoglycemia (eg, palpitations, diaphoresis, hunger) that are relieved by the ingestion of glucose (ie, the Whipple triad).1196 A glucose of <70 mg/dL (3.9 mmol/L) is the classic threshold for hypoglycemia based on physiologic glucose regulation and neuroendocrine response in persons without DM.1197 Hypoglycemia may be asymptomatic, and any BG <70 mg/dL is generally considered hypoglycemia.1198 In persons with DM, hypoglycemia is separated into 3 levels. Level 1, a measurable glucose <70 mg/dL (3.9 mmol/L), but ≥54 mg/dL(3.0 mmol/L), can and should alert a person to act. Level 2 is a measurable glucose <54 mg/dL (3.0 mmol/L) that needs immediate action, as neurogenic and neuroglycopenic hypoglycemic symptoms begin to occur below this level. Level 3 is defined as a severe event characterized byaltered mental status and/or physical status requiring assistance.1198,1199 In addition, hypoglycemia symptoms can occur in the normal glucose range in a person with very high glucose levels that drop quickly. BGM and CGM can be helpful but are not necessarily diagnostic because of possible instances of glucose meter and sensor inaccuracy.
Symptoms of Hypoglycemia
Hypoglycemia manifests as neurogenic and/or neuroglycopenic symptoms that range in severity from mild to life-threatening and include anxiety, palpitations, tremor, sweating, hunger, paresthesia, behavioral changes, cognitive dysfunction, seizures, and coma. Certain hypoglycemia-related responses (psychomotor function) are altered in older adults compared with younger persons. Although severe hypoglycemia generally results in recognizable symptoms, mild-to-moderate hypoglycemia may remain asymptomatic and unreported in persons with DM. Even severe hypoglycemia is often unrecognized in those with hypoglycemia unawareness.1198
Etiology of Hypoglycemia
In persons with DM, iatrogenic hypoglycemia stems from an imbalance among insulin and/or insulinogenic (eg, SUs, glinides) therapy and food intake, physical activity, organ function (gluconeogenesis), and counter-regulation with glucagon and/or epinephrine (hypoglycemia-associated autonomic failure). Hyperinsulinemia, increased alcohol intake, starvation, and organ failure may be aggravating factors.1108,1200,1201 Noniatrogenic hypoglycemia (ie, insulinoma) is beyond the scope of this guideline.
Risks of Hypoglycemia
The primary cause of hypoglycemia is intensification of antihyperglycemic therapy (almost always using SUs [or to a lesser extent glinides] and/or insulin) aimed at achieving lower A1C targets, as demonstrated by intensive therapy trials.1202,1203 Over 3.5 years in the ACCORD study, severe hypoglycemia occurred at an annualized rate of 3.1% of persons in the intensive therapygroup (mean end point A1C 6.4%; target <6.0%) vs 1.0% per year in the standard therapy group (mean end point A1C 7.5%).76 During the ADVANCE trial, in which the goal A1C of 6.5% was met in the intensive group, 0.7% of intensively treated persons experienced severe hypoglycemia on an annual basis compared with 0.4% of persons per year in the standard care group.70 Finally, in the UKPDS, wherein intensive treatment led to a mean end point A1C of 7.0%, hypoglycemia occurred in 1.8% of insulin-treated persons per year in the intensive group vs 0.7% of conventionally treated persons per year.407 The risk of hypoglycemia is greater in older adults and those with longer DM duration, kidney failure, or lesser insulin reserve. Dementia is another important risk factor for hypoglycemia, and recurrent hypoglycemia appears to increase the risk of dementia.1204–1206 The failure to recognize symptoms of hypoglycemia can increase the risk of subsequent hypoglycemia by causing autonomic failure, leading to a cycle of recurrent hypoglycemia and hypoglycemia unawareness.1200
Sequelae of Hypoglycemia
Studies have suggested an association of hypoglycemia with adverse CV events. In the ADVANCE trial, severe hypoglycemia was associated with significant risk increases for CV events including death.1110 In ACCORD, hypoglycemia was considered a suspect behind the increased mortality observed in the intensive-treatment arm. However, glucose levels at time of death were unknown, and the hypothesis remains unproven.82,87 Moreover, the HR for hypoglycemia-related mortality was even higher in the standard therapy arm of that study (aHR in intensive treatment arm: 1.41, 95% CI, 1.03–1.93; in standard therapy arm: 2.30, 95% CI, 1.46–3.65).87 A meta-analysis of prospective and retrospective clinical trials demonstrated that severe hypoglycemia doubled the risk of CV events, whereas an observational trial showed that, over a period of 5 years, mortality was 3.4 times higher among persons who reported severe hypoglycemia at baseline.1207,1208 The proposed mechanism for these effects posits that hypoglycemia reduces baroreceptor sensitivity and increases sympathoadrenal system activity, which can trigger a fatal ventricular arrhythmia in the setting of reduced baroreflex sensitivity.1209 Other short- and long-term consequences of severe hypoglycemia include neurologic conditions ranging from temporary cognitive impairment to dementia as well as major vascular events such as stroke, MI, acute cardiac failure, ventricular arrhythmias, and sudden death.1108,1200,1210 The complications of hypoglycemia are also associated with short-term disability and higher health care costs.1211–1214
Management of Hypoglycemia
Oral administration of rapidly absorbed glucose (15 g) should be used to treat hypoglycemia (measured glucose <70 mg/dL (3.9 mmol/L) if a person is able to safely swallow.1215–1217 Subsequent confirmation of return of glucose levels to greater than normal range is recommended.
In persons with T2D being treated with alpha-glucosidase inhibitors, who develop hypoglycemia due to use of hypoglycemic agents, oral glucose or lactose-containing foods (dairy products) must be given because alpha-glucosidase inhibitors inhibit the breakdown and absorption of complex carbohydrates and disaccharides.1218–1225
If a person is unable to swallow or is unresponsive, subcutaneous, intramuscular, or intranasal glucagon or IV glucose should be given by a trained family member or medical personnel. There are at least 3 FDA-approved formulations of standard glucagon for reconstitution and injection. These formulations are supplied as lyophilized white powder requiring reconstitution using the liquid in an included prefilled syringe prior to injection as 1 mg per vial. The adult dose is 1 mg. For children weighing less than 44 lbs (20 kg), the dose is 0.5 mg.1226–1229
New, more stable formulations of glucagon have recently become available for clinical use: intranasal glucagon, dasiglucagon, and nonaqueous soluble glucagon. These new FDAeapproved formulations have demonstrated glycemic responses similar to standard glucagon formulations for the treatment of hypoglycemia but without the need of reconstitution.1230 Three mg of intranasal glucagon (1 mg glucagon per 10 mg dry powder) appears to have maximal effect.1231–1235 Nonaqueous glucagon and dasiglucagon can be administered via a prefilled syringe or auto-injector, reducing the steps to prepare and administer glucagon in the event of hypoglycemia.1236,1237 Dasiglucagon is a novel stable peptide analog of human glucagon consisting of 29 amino acids with 7 amino acid substitutions relative to native glucagon. In clinical trials, the time taken to increase glucose concentration to above 70 mg/dL was 6 minutes with doses of 0.3 mg and 0.6 mg of dasiglucagon, which is comparable to standard glucagon at doses of 0.5 mg and 1 mg.1237,1238 For all these forms of glucagon rescue, if there is no response after 15 minutes, an additional same dose may be administered subcutaneously while waiting for emergency assistance, which should be called for immediately after administering the first dose (Table 20).
Table 20.
Glucagon Preparations for Treatment of Severe Hypoglycemia
| Ready to use | Nasal | Injection | Stability | |
|---|---|---|---|---|
| Glucagon nasal powder | ✓ | ✓ | Can be stored at temperature up to 86 °F | |
| Dasiglucagon | ✓ | ✓ | 0.6 mg e shelf life is 12 mo at room temperature from date of removal from refrigeration* | |
| Glucagon prefilled syringe (Pen) | ✓ | ✓ | 1 mg** e 30 mo at room temperature 20–25 °C | |
| Glucagon emergency kit | Reconstitution required | ✓ 4 manufacturers | Can be stored at room temperature from 18 to 36 mo, depending on manufacturer |
The most common side effects of glucagon administration are nausea; vomiting; headache; runny nose; discomfort in nose; stuffy nose; redness in eyes; itchy nose, throat, and eyes; watery eyes
Refer to the full prescribing information for any prescribed glucagon formulation for the most current, US Food and Drug Administrationeapproved information.
Do not return product to refrigerator after storing at room temperature.
Dose for pediatric patients aged 2 to under 12 years of age who weigh <45 kg is 0.5 mg.
As soon as the individual is awake and able to swallow, they should receive a rapidly absorbed source of carbohydrate (eg, glucose tablets or dietary sugar like fruit juice) followed by a snack or meal containing both protein and carbohydrates (eg, cheese and crackers or a peanut butter sandwich).1216,1217,1233,1239–1241
Hypoglycemia is the primary limiting factor in the treatment of both T1D and T2D. It remains a significant barrier in terms of treatment adherence and achievement of glycemic goals.1108 Long-term management of hypoglycemia depends on appropriate adjustment of therapy to prevent hypoglycemia or reduce its frequency and severity in persons prone to hypoglycemia (eg, the elderly and persons with T1D). In T2D, hypoglycemia typically occurs in association with use of exogenous insulin, SUs (especially glyburide),1242 and glinides; symptoms may be mild, moderate, or severe. The risk of hypoglycemia may be further increased by the addition of other antihyperglycemic agents to SUs or insulin. Therefore, in adults with T2D, treatment strategies should emphasize the increased number of antihyperglycemic medication classes that are not associated with severe hypoglycemia (Table 16). Also, the role of hypoglycemia must be considered in determining ideal A1C goals for each patient.
BGM and especially CGM are important tactics to help persons prevent, identify, and document hypoglycemia, although it is essential that the glucose meter and CGM meet accuracy standards and that users are provided with education and support. CGM use is particularly important in persons with recurrent asymptomatic hypoglycemia (hypoglycemia unawareness, hypoglycemia-associated autonomic failure), recurrent hypoglycemia, and persons on regimens placing them at risk for hypoglycemia.153,1198
Persons with hypoglycemic unawareness are particularly susceptible to marked variations in glucose levels. Therapeutic approaches can minimize glycemic excursions and prevent hypoglycemia.1243–1247 Also, CGM, especially when connected to insulin pumps and HCL devices, can reduce occurrence of hypoglycemia.1248
Question 15: How should DM be managed in the hospital?
Recommendation 15.1
All hospitalized persons should have laboratory glucose testing on admission. Persons with DM or with admission hyperglycemia >140 mg/dL should have glucose monitoring during hospitalization.
Grade B; BEL 1
Recommendation 15.2
To guide inpatient therapy and inform discharge planning, clinicians should measure A1C in all persons with DM, unless their A1C is known and was tested within the previous 3 months.
Grade B; BEL 2
Recommendation 15.3
Hospitalized persons with hyperglycemia but without known DM should have A1C measured to identify preexisting DM and inform discharge planning.
Grade B; BEL 2
Recommendation 15.4
Initiate bedside POC capillary glucose monitoring at an appropriately chosen schedule to guide therapy for hyperglycemia during hospitalization in all persons with DM, persons without prior DM who have hyperglycemia, and persons receiving therapies with a high risk of hyperglycemia, such as corticosteroids and enteral or parenteral nutrition.
Grade A; BEL 1
Recommendation 15.5
For hospitalized persons with DM eating on a regular schedule, check POC BG before each meal and at bedtime, if clinically indicated. In hospitalized persons who are not eating (eg, NPO [nothing by mouth] or continuous feeding), initially check POC BG at least every 4 to 6 hours. Additional checks may be warranted for those at higher risk of hypoglycemia. For those on IV insulin, POC BG should be obtained from every 30 minutes to every 2 hours.
Grade A; BEL 1
Recommendation 15.6
Although inpatient CGM has not received regulatory approval, CGM may be useful in inpatient settings, while complying with institutional policies and safety precautions. CGM may improve detection of severe hypoglycemic and hyperglycemic events, identify glucose trends and patterns, and improve satisfaction in persons with DM.
Grade C; BEL 2
Recommendation 15.7
CGM may be considered under special regulatory allowance during the time of COVID-19 to reduce staff exposure and use of personal protective equipment and assist with glycemic monitoring of persons in the hospital setting.
Grade C; BEL 2
Recommendation 15.8
Specialized inpatient DM teams and/or CDCES, if available, should be used to improve outcomes in hospitalized persons with DM or hyperglycemia. The use of virtual consults may be considered an alternative to support hospitals lacking these services.
Grade B; BEL 1
Recommendation 15.9
For critically ill persons, IV insulin infusion is recommended to treat persistent hyperglycemia in the ICU using validated protocols that allow adjustment of insulin dose for glycemic excursions based on prespecified glucose targets. For those receiving IV insulin, POC testing should be performed every 30 to 120 minutes.
Grade A; BEL 1
Recommendation 15.10
A glucose target of 140 to 180 mg/dL is recommended for most critically ill persons in the hospital setting. More intensive targets between 110 to 140 mg/dL may be appropriate in select populations, particularly critically ill persons postcardiothoracic or other surgeries, while minimizing the risk of hypoglycemia.
Grade A; BEL 1
Recommendation 15.11
For most noncritically ill persons in the hospital setting, a glucose target of 140 to 180 mg/dL is recommended. For hospitalized persons who are able to achieve and maintain glycemic control without hypoglycemia, a lower target range (100 to 140 mg/dL) may be reasonable. For persons in a hospital setting with high clinical complexity, terminal illness, limited life expectancy, or high risk for hypoglycemia, less stringent targets are appropriate.
Grade B; BEL 1
Recommendation 15.12
Insulin therapy following approved protocols is recommended as the preferred therapy for managing hyperglycemia in the hospital. For noncritically ill hospitalized persons with T2D, an individualized approach is recommended for consideration of noninsulin agents alone or in combination with insulin (see also R 15.16).
Grade A; BEL 1
Recommendation 15.13
The insulin regimen for hospitalized persons with satisfactory meal intake should include basal, prandial, and correction doses. For those without adequate food intake, a regimen of basal, prandial, and correction doses should be used as necessary for glycemic control. Exclusive use of “sliding-scale” insulin should only be used for those whose glucoses are in the target range most of the time, and only occasionally exceed it.
Grade A; BEL 1
Recommendation 15.14
The management of hyperglycemic emergencies, including DKA and hyperosmolar state, should include fully adequate fluid resuscitation to correct fluid deficits, electrolyte replacement (potassium), and insulin therapy. Simultaneous continued infusion of insulin and dextrose solutions after correction of hyperglycemia is often required until DKA resolves to avoid hypoglycemia.
Grade A; BEL 1
Recommendation 15.15
Transition from IV insulin in the ICU to a subcutaneous insulin regimen is typically required when acidosis is resolved and a person is no longer critically ill. A proactive regimen with scheduled subcutaneous insulin therapy, with basal, nutritional/prandial, and/or correctional doses, is recommended for most persons.
Grade A; BEL 1
Recommendation 15.16
For hospitalized persons with T2D and mild admission hyperglycemia (glucose <180 mg/dL), a personalized approach is recommended for the use of noninsulin agents alone or in combination with basal insulin, aiming for the most efficacious regimen with the lowest hypoglycemic risk. For some hospitalized persons with T2D, DPP-4 inhibitors plus correction doses with rapid-acting insulin, or basal insulin plus DPP-4 inhibitors may be sufficient.
Grade A; BEL 1
Recommendation 15.17
A hospital-wide standardized plan should be in place to prevent hypoglycemia. Each hypoglycemic episode should be documented, and appropriate adjustments should be made to prevent recurrence.
Grade B; BEL 2
Recommendation 15.18
It is recommended to start discharge planning soon after hospital admission and to provide and document appropriate individualized plans for transition to an ambulatory setting and follow-up care at discharge for all persons with DM or newly diagnosed hyperglycemia.
Grade A; BEL 1
Evidence Base 15: How should DM be managed in the hospital?
DM affects up to ~10% of the US population36 and is even more common among hospitalized persons, present in up to 20% to 40% of admissions and has been particularly high during the COVID-19 pandemic.1249,1250 The association between inpatient hyperglycemia and increased risk for complications and mortality is well established in persons with and without previously diagnosed DM.1251–1254 Hyperglycemia is associated with prolonged hospital stay, increased incidence of infections, greater disability after hospital discharge, and death.91,1255,1256
Substantial evidence indicates that correction of hyperglycemia with insulin administration reduces hospital complications and mortality in critically ill persons, as well as those who receive care for general medicine and surgery.92,1257,1258 Several RCTs, including the real-world Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation study95,96,104 and meta-analyses1257,1259,1260 reported higher rates of severe hypoglycemia and increased morbidity and mortality with intensive insulin therapy (glycemic targets of 80 to 110 mg/dL) compared with more relaxed glycemic targets, demonstrating that intensive glycemic control (80 to 110 mg/dL) in critically ill persons may be difficult to achieve, with no consistent mortality benefits in all studies, and increased risk of complications in those treated intensively, compared to moderate glycemic targets.95,96,104 However, personalized glycemic targets between 110 to 140 mg/dL may improve outcomes in selected populations, particularly critically ill persons postcardiothoracic surgery, in hospital units that have shown low rates of hypoglycemia. In addition, minimizing glycemic variability, independent of glucose levels, could result in lower rates of complications and CV mortality in critically ill persons1261–1263 and in reduced hospital stays and mortality in non-ICU settings.1264 Thus, glucose targets <110 mg/dL are no longer universally recommended, and the AACE/ADA consensus statement on inpatient glycemic control favors more relaxed glycemic targets in the ICU, as high as 140 to 180 mg/dL.105
Treatment of Hyperglycemia in Persons in the Hospitalized Setting
Persons with DM have a 3-fold greater chance of hospitalization compared to those without DM, with 30% to 40% requiring 2 or more hospitalizations in any given year.1264–1266 It is well established that hyperglycemia in persons with or without a prior diagnosis of DM increases both mortality and disease-specific morbidity in hospitalized persons,91,105,1251,1267 and that goal-directed insulin therapy can improve outcomes.92,94,101 This topic has been extensively reviewed in the AACE/ADA consensus statement on inpatient hyperglycemia,105 2021 ADA Standards of Medical Care in DM,1268 and 2022 Endocrine Society clinical practice guideline titled Management of Hyperglycemia in Hospitalized Adult Patients in Noncritical Care Settings.99
The management of hyperglycemia in the hospital setting presents multiple challenges including variable nutritional status and altered levels of consciousness, as well as resource limitations for monitoring glycemia during these changes. Given the paramount importance of patient safety, reasonable glucose targets in the hospital setting should be set at modestly higher levels than targets for outpatients with DM. For noncritically ill persons, a premeal glucose target of <140 mg/dL and a random BG of <180 mg/dL are recommended, while avoiding hypoglycemia (BG <70 mg/dL). Additionally, glycemic targets should be modified according to clinical status. For persons who are able to achieve and maintain glycemic control without hypoglycemia, a lower target range may be reasonable. For persons with terminal illness, limited life expectancy, or high risk for hypoglycemia, higher target ranges may be reasonable.100,1259,1269–1274 Refer to Section 1 on Screening, Diagnosis, Glycemic Targets, and Glycemic Monitoring for additional guidance.
We recommend to check A1C for all persons with known DM, unless the A1C level is available and had been checked within the prior 3 months. A1C levels provide an overview of prior glycemic control, can predict response to therapy in the hospital, and guide discharge therapy.103,1275–1279
Some studies have demonstrated that the use of specialized DM management teams can result in better hyperglycemia correction, and avoidance of hypoglycemia, with positive impacts on readmissions and costs.1280–1283 The use of e-consults or virtual visits may be an alternative for hospitals lacking these services,1284 or for postdischarge DM follow-up.1285–1287
Management of Adults with Inpatient Hyperglycemia in the ICU
Insulin therapy is the preferred method of glycemic control in most hospitalized persons. IV infusion of insulin is the preferred route of administration for persons in the ICU. In the critical care setting, a variety of CSII protocols have been shown to be effective in achieving glycemic control with a low rate of hypoglycemic events and also to improve hospital outcomes.94,101,1288–1290
For ICU settings, most hospitals use institutional-based, nurse-driven protocols, with several validated protocols published.1291–1293 Automated, computerized, IV insulin protocols, including commercially available or institutional-based protocols, have improved glycemic control, with good acceptance by nursing personnel.1294–1307 The preference will depend on local needs, support, and cost to the institution. Preference should be given to use of regular insulin for IV administration,1308,1309 given lower cost and wide availability, and short-acting insulin analogs have shown effective glycemic control.1308
The management of hyperglycemic emergencies (including DKA and hyperosmolar state) should follow standardized protocols with aggressive fluid resuscitation, electrolytes replacement, and also insulin therapy.28,1310,1311 Persons with severe DKA should typically receive IV insulin therapy, whereas mild-to-moderate crises may be managed with frequent subcutaneous insulin administration and glucose monitoring protocols.28,1121,1310,1312 Caution is recommended in persons with advanced renal failure because standard IV fluid and insulin replacement may result in increased volume overload and hypoglycemia.1313,1314 Prevention and correction of hypokalemia and hypoglycemia should be proactively part of any treatment protocol. Severe hypokalemia (K ≤2.5 mEq/L) and severe hypoglycemia (BG <40 mg/dL) have been associated with increased mortality.1315
The addition of noninsulin agents, such as DPP-4 inhibitors or GLP-1 RAs, before admission or during the perioperative period, has not reduced rates of stress hyperglycemia and may increase nausea and vomiting rates1316–1324 among critically ill persons. While the use of these agents is safe and may result in lower glucose levels and insulin doses, we do not recommend addition of a DPP-4 inhibitor or GLP-1 RA to IV insulin therapy until efficacy is demonstrated.
Most persons with T2D and all persons with T1D in the ICU receiving IV insulin infusion will require transition to a subcutaneous insulin regimen.105 Those who are suitable for this transition ideally have a stable infusion rate and BG levels in the target range. Several studies1266,1325–1329 recommend starting at a daily insulin dose ~80% of the IV insulin used in the preceding 12 to 24 hours and splitting it into basal and bolus insulin.105 Persons without DM but with stress or newly diagnosed hyperglycemia who have required an insulin rate less than 1 to 2 units/hour at the time of transition may not require a scheduled subcutaneous insulin regimen.1330 Many of these individuals can be treated with correction insulin to determine if they will require scheduled subcutaneous insulin.
Management of Hospitalized Adults with Inpatient Hyperglycemia in the Non-ICU Setting
In the noncritically ill setting, scheduled subcutaneous insulin regimens with a combination of basal, nutritional, and correctional components is recommended. Prolonged use of “sliding scale” insulin as the sole method of glucose control is strongly discouraged. Clinicians should only consider using “sliding scale” insulin alone in persons whose glucoses are in the target range most of the time, and only occasionally exceed it (ie, with stress hyperglycemia or well-controlled DM).1331,1332
RCTs have shown that treatment with a basal prandial regimen using insulin analogs improved glycemic control with fewer hospital complications in general medical and surgical persons with T2D compared with sliding-scale regular insulin alone.92,107,108,1333–1336 Persons with T1D should be treated with basal-prandial insulin regimens to avoid severe hyperglycemia and DKA. In insulin-naïve persons with T2D, a starting insulin TDD between 0.3 and 0.5 units/kg/day is effective and safe in thosewho receive care for general medicine and surgery. Persons with T2D receiving insulin therapy before admission are at risk for severe hyperglycemia in the hospital if insulin therapy is discontinued. Assessment of the need for modification of the home insulinregimenis important as requirements varyaccordingto clinical stressors and altered caloric intake.105,1337 Lower starting insulin TDD of 0.20 to 0.25 units/kg are recommended in persons with impaired kidney function1338,1339 in the elderly, and in those with poor caloric intake.1339,1340 In addition, for persons whose glucose is controlled with insulinprior to admission, reducingtheinsulin TDD by 20% to 25% is recommended for those with poor caloric intake to avoid hypoglycemia in in the hospital setting,1340 though persons with uncontrolled DM may actually require higher doses. In a single-center RCT, the use of correctional insulin sliding scales for bedtime hyperglycemia did not improve glycemic control in persons with T2D.1341,1342
Several studies have compared different basal insulin formulations, including glargine U100, insulin detemir U100, glargine U300, and degludec U100, with similar glycemic control results.92,107,1343–1346 Although some RCTs have shown similar glycemic control and lower hypoglycemia with the use of insulin analogs1308 in low-resource settings, the use of NPH may result in similar glycemic control, with minimally increased hypoglycemia,1347,1348 albeit with higher insulin dose and injections per day. However, the use of pre-mixed insulin formulations (ie, 70/30) have resulted in significantly higher rates of hypoglycemia during hospitalization and are not recommended.1349
Though effective, the basal-bolus regimen is labor intensive, requires several injections per day, and is associated with risk of hypoglycemia, affecting up to 10% to 30% of noncritically ill persons.102,1333 Several studies have been published on the use of alternative approaches, aiming for a more personalized approach. Evidence suggests that the best regimen should be individualized to achieve glycemic targets with the lowest risk of hypoglycemia. Clinical judgment will guide the best plan, incorporating a person’s comorbidities, severity and complexity of disease, life expectancy, severity of acute hyperglycemia (ie, admission glucose), prior glycemic control (ie, A1C levels), and prior antihyperglycemic regimen (insulin-naïve vs insulin-treated persons).102,103,1266,1268
For noncritically ill persons with T2D and mild-to-moderate hyperglycemia (ie, admission glycemia below 180 to 200 mg/dL),1266 the use of basal insulin plus correctional insulin (basal-plus regimen),108 or basal insulin with DPP-4 inhibitors, or DPP-4 inhibitors plus correctional insulin doses with rapid-acting insulin may provide equal glycemic control to basal-bolus insulin.1350–1352 The basal-plus approach in an RCT resulted in similar glycemic control compared with a standard basal-bolus regime108 and can be an effective alternative with low insulin requirements, decreased oral intake, or when undergoing surgery.1266,1353
Studies have assessed inpatient uses of noninsulin agents with low hypoglycemic risk (DPP-4 inhibitors or GLP-1 RAs).1351,1352,1354–1358 These newer agents are not expected to increase risk of lactic acidosis in ill persons (ie, unlike metformin) nor enhance risk of hypoglycemia (ie, unlike SUs and similar secretagogues), or cause edema or CHF (ie, unlike TZDs). Oral DPP-4 inhibitors have been tested in RCTs in medical and surgical noncritically ill persons with mild hyperglycemia (admission glucose <180 mg/dL).1351,1354–1357 DPP-4 inhibitors combined with low-dose basal insulin have been similarly effective to basal-bolus insulin and associated with less hypoglycemia and less treatment burden.1351,1354–1357,1359 The use of SGLT2is have not been evaluated in hospitalized persons. In ambulatory studies, these agents increased the risk of infections (urinary, perineal), euglycemia DKA, acute renal failure, and hyperkalemia.974 The FDA recommends withholding SGLT2is 3 to 4 days before surgery.1360 We do not recommend SGLT2is in the hospital until further studies prove efficacy and safety, with the hypotheses derived from ambulatory studies where SGLT2is decreased cardio-renal and HF outcomes in persons with and without DM.974 Hence, SGLT2is added at discharge to appropriately chosen persons who are stable may decrease clinical inertia and improve long-term outcomes.
Hypoglycemia and Hospital Outcomes
Meta-analyses of RCTs have reported increased risk ratio of 6 to 7.7 times for occurrence of hypoglycemia with intensive insulin therapy vs conventional glycemic control in critically ill persons,1259,1361 with some studies showing a risk ratio >10.1259 Inpatient hypoglycemia has been associated with higher rates of hospital complications, longer hospital stays, higher health care resource utilization, and increased hospital mortality, creating a J-shaped relationship between glucose levels and death rates.1362,1363 BG <50 mg/dL was associated with 22.2% mortality compared with 2.3% without hypoglycemia.1364 Hypoglycemia is associated with adverse CV outcomes such as prolonged QT intervals, ischemic electrocardiogram changes, angina, arrhythmias, and death.1365 Despite these epidemiologic associations between hypoglycemia and poor clinical outcomes, data demonstrating that insulin-induced hypoglycemia is the direct cause of harm in hospitalized persons are sparse. The severity of hypoglycemia and not insulin therapy, per se, is associated with increased risk of mortality in critically ill persons.1363 Hypoglycemia resulting from severe systemic illness (spontaneous hypoglycemia), rather than insulin-induced hypoglycemia, is associated with increased risk of inpatient mortality and complications.1366–1368 Hospitals and hospital systems should implement nurse-driven protocols for the management of hypoglycemia.1369–1372 These protocols should include specific treatment options for different levels of hypoglycemia, with indications to repeat treatment options within 15 minutes until resolution.
SUs may be associated with prolonged hypoglycemia, especially in persons with impaired renal function. In some instances, individuals will present with hypoglycemia on admission or mild hyperglycemia that rapidly results in hypoglycemia upon initiation of insulin therapy in the hospital. With increased use of antihyperglycemic agents with prolonged half-life and duration of action up to 5 to 7 days (glargine U300, degludec, dulaglutide, semaglutide, long-acting exenatide), clinicians should be aware of potential prolonged glucose-lowering effects of these agents, particularly in the setting of decreased oral intake, prolonged fasting episodes, decreased renal function, or liver failure during hospitalization (expert recommendations). Detailed medication reconciliation should occur upon admission to avoid these situations and assist early discharge planning.
Some retrospective studies have demonstrated severe hypoglycemia inpersons with and without DMtreated with IV bolus of insulin for hyperkalemia in the emergency room or while hospitalized.1373,1374 The majority of these cases occur in persons with advanced CKD, often requiring dialysis treatment. Rates of severe hypoglycemia events in ambulatory persons with end-stage kidney disease on dialysis are 10-fold higher than among other nondialysis persons with CKD and often associated with prolonged hypoglycemic episodes and poor response to regular hypoglycemia treatment.1375 The most effective treatment is prevention with modified hyperkalemia treatment protocols, using lower insulin doses (5 units vs 10 units) and coadministration of dextrose (25 to 50 g IV).1376,1377 However, this approach has not resulted in reduced rates of hypoglycemia in all studies, concluding that frequent glucose monitoring for up to 6 hours is recommended after using IV insulin to correct hyperkalemia1376,1377 especially in persons at higher risk: without DM, with previous use of insulin or glucose-lowering agents, with pretreatment normoglycemia or mild hyperglycemia, or undergoing hemodialysis.1373,1374
Recommendations after Hospital Discharge
Persons with stress or hospital-related hyperglycemia, defined as any BG concentration >140 mg/dL without evidence of previous DM, should undergo A1C testing during admission or hospital stay.102,1276–1278 Measurement of A1C may differentiate persons with stress hyperglycemia from those with previously undiagnosed DM, as well as identifying persons with known DM who will benefit from intensified glycemic management. In the presence of hyperglycemia, an A1C >6.5% suggests the diagnosis of DM. Because up to 40% to 50% of persons admitted with stress-related hyperglycemia have confirmed DM at 1 year they should be closely monitored after discharge.1378,1379 A systematic review of 18 studies (N = 111,078 participants) found that the prevalence of DM after discharge was 4%, 12%, and 28% for persons with inpatient normoglycemia (fasting <100 mg/dL and random <140 mg/dL), mild hyperglycemia (fasting <126 mg/dL and random <200 mg/dL), and severe hyperglycemia (fasting >126 mg/dL and random >200 mg/dL, respectively).1279
The transition of persons from the hospital to ambulatory or subacute settings is a high-risk period for medication errors, but also an opportunity to improve glycemic control, avoiding what is often termed clinical inertia.102,1285,1286,1380 Persons should have careful review of all prescribed medications to be taken post discharge and ideally have any needed prescriptions filled prior to discharge. Discharge algorithms based on A1C levels provide discharge guidance for hospitalized persons.1275 For persons with A1C <7% and no hypoglycemia, it is recommended to restart prior ambulatory antihyperglycemic regimen, unless a new clinical indication or new contraindications require other adjustment. It is recommended to decrease (50% reduction) or stop SUs, aiming to reduce the risk of hypoglycemia with a low admission A1C. For persons with admission A1C between 7% and 9%, modification or intensification of therapy is recommended, including adding basal insulin, if clinically indicated. Addition of 50% of hospital dose of basal insulin is suggested. In persons with A1C >9%, the addition of basal insulin at 80% of hospital dose is suggested.1275 Recent evidence suggests that adding GLP-1 RAs at discharge to select persons with uncontrolled T2D may result in better glycemic control, less hypoglycemia, and weight loss compared with adding basal insulin.1381
With several newer antihyperglycemic agents showing benefits beyond glucose reduction such as prevention of CV events or kidney disease progression, hospital discharge may be an important opportunity to revise prior antihyperglycemic therapy. For instance, many persons with T2D and underlying CVD admitted with CV event, HF, or kidney failure may have indications to use SGLT2 inhibitors or GLP-1 RAs. However, the evidence is very limited in the setting of acute illness. Trials of SGLT2 in the hospital and at discharge are ongoing.1382,1383 Starting SGLT2is for hospitalized persons with HF is not recommended at this time. There are limited studies assessing its efficacy or safety in hospitalized persons.1384 If persons are stable and ready for discharge, starting these agents at discharge and communicating with the patient’s primary clinician may represent a good opportunity to avoid clinical inertia, with preliminary studies showing no adverse safety signals.1384
Glucose Monitoring in the Hospital
Bedside capillary POC testing is the preferred method for guiding ongoing glycemic management of hospitalized persons.99 Some glucose meters received approval for hospital use among persons in the ICU and non-ICU setting.1385 It is recommended that all hospitals develop procedures for maintaining and calibrating glucose meters in use.
POC testing is usually performed 4 times a day: before meals and at bedtime for persons who are eating. For those who are not able to eat, have orders for holding food before procedures or as part of therapy, or receiving continuous enteral nutrition; POC testing is recommended every 4 to 6 hours. More frequent glucose monitoring is indicated in persons treated with CSII or after a medication change that could alter glycemic control, such as corticosteroid use, abrupt discontinuation of enteral or parenteral nutrition, or frequent episodes of hypoglycemia. For inpatients with steroid-induced hyperglycemia or with posttransplant diabetes receiving daily steroids, CGM studies have demonstrated that while fasting glucose could be within range, hyperglycemia is mostly detected in the afternoon and evening.1386
Prospective observational studies, using the current standard of care of checking capillary glucose before meals and at bedtime, have shown that about 45% of persons experience asymptomatic hypoglycemia events.1387,1388 Hence, current methods for monitoring hypoglycemia in hospitals often fail to detect most hypoglycemic episodes, particularly asymptomatic or nocturnal hypoglycemia, which may be the most dangerous episodes.1389 While still investigational, consensus guidelines and experts agree that CGM may better detect and prevent hypoglycemic episodes.1390,1391 Innovative methods, such as a “glucose telemetry system”1392–1394 or other approaches using CGM may provide better glycemic monitoring, including predictive tools or alarms before hypoglycemia occurs enabling prevention. CGM in the hospital is not currently approved by regulatory agencies, with ongoing validation studies.1395 Research on implementing CGM targeting approval of some devices for hospital use has been focused mostly on the ICU. Earlier devices were invasive and required capillary glucose calibration. Hence, use was not widely adapted by clinicians and hospitals, leading to lack of availability of these sensors at this time.1390
During the COVID-19 pandemic, hospitals faced a critical need to minimize personnel exposure and save personal protective equipment. Several hospitals implemented emergency use, particularly of newer factory-calibrated CGM not requiring capillary glucose calibrations, for persons in ICU and non-ICU medical and surgical settings.1266,1389,1390,1396,1397 Some studies have shown potential improvements in detection of glycemic excursions and prevention of hypoglycemia, specifically with the use of glucose telemetry systems.1392–1394 However, there are no intervention studies showing benefits of CGM to adjust therapy. While expert consensus expects CGM in hospitalized persons to improve detection of glycemic excursion and overall glycemic control,153,1390 CGM remains investigational in the hospital.1398,1399
With proper protocols in place, persons previously using CGM can be allowed to continue using their devices during hospitalization, unless clinically not appropriate.153,1390 Given limited approval from regulatory agencies of CGM devices, adjustment of antihyperglycemic therapy should be performed with the use of hospital-calibrated glucose meters, per local hospital policies. CGM values outside the desired range, specifically hypoglycemia (BG <70 mg/dL or <54 mg/dL), should prompt nursing notification by the patient to be confirmed with hospital-calibrated glucose meters. The implications of these hospital policies are unknown when applied to SAP therapy or AID systems (eg, HCL systems, artificial pancreas devices), since the insulin pump uses glucose information received from the CGM without information from POC testing or direct interaction with a practitioner.153
MNT for Persons in the Hospital Setting
MNT is an essential component of inpatient glycemic management in persons with DM and hyperglycemia. The goals of inpatient MNT for persons with DM are to help optimize glycemic control, provide adequate calories to meet metabolic demands, address individual needs based on personal food preferences, and provide a discharge plan for follow-up care. Most hospitalized persons require 25 to 35 calories/kg/day; critically ill persons require between 15 and 25 calories/kg/day.1400–1405 This translates to a diet containing approximately 1800 to 2000 calories/day or about 200 g of carbohydrate per day divided between meals. Care must be taken not to overfeed hospitalized persons because this may exacerbate hyperglycemia. No single meal-planning system is ideal for hospitalized persons. However, hospitals should provide a consistent carbohydrate DM meal-planning system.1400,1402–1405 The carbohydrate components of breakfast, lunch, dinner, and snacks may vary, but the day-to-day carbohydrate content of specific meals and snacks should be kept constant. Persons requiring clear or full liquid diets should receive about 200 g of carbohydrate per day in equally divided amounts at meal and snack times. Persons on liquid diets, in particular during the perioperative period, do not meet these nutritional needs. Increasing evidence indicates that a person’s food intake should be initiated as quickly as possible with progression from clear liquids to full liquids to solid foods as rapidly as tolerated postsurgery.1402–1406 Early enteral feeding is safe and well tolerated and is associated with reduced wound morbidity, improved wound healing, fewer septic complications, diminished weight loss, and improved protein kinetics.1406 Among persons in the ICU, the use diabetes-specific formulas improved glycemic control, decreased insulin requirements, and risk of infections relative to the standard formulas.1407
Question 16: How should DM in pregnancy be managed?
Recommendation 16.1
For women with GDM, the following treatment goals are recommended: preprandial glucose concentration ≤95 mg/dL and either a 1-hour postmeal glucose ≤140 mg/dL or a 2-hour postmeal glucose ≤120 mg/dL to decrease adverse fetal outcomes.
Grade C; BEL 4 and expert opinion of task force
Recommendation 16.2
All women with preexisting DM (T1D, T2D, or previous GDM) need access to preconception care and counseling to ensure adequate nutrition, healthy weight, and glucose control before conception, during pregnancy, and in the postpartum period.
Grade B; BEL 2
Recommendation 16.3
Rapid-acting insulin analogs (insulin-lispro, insulin-aspart) should be used to treat postprandial hyperglycemia in pregnant women.
Grade B; BEL 1
Recommendation 16.4
Options for basal insulin include long-acting insulin (eg, NPH, detemir, or glargine) or rapid-acting insulin via a CSII. Regular insulin, although not recommended as first-line therapy, is acceptable to use in managing pregnant women with DM when rapid-acting insulin analogs are not available.
Grade B; BEL 1
Recommendation 16.5
Insulin is the preferred therapeutic choice for pregnant women with GDM or T2D, but metformin has been given a category B for pregnancy with accumulating clinical evidence of metformin's safety during the first trimester and beyond. Metformin has been shown to improve pregnancy and fetal outcomes except for increased rates of infants with SGA and later onset of obesity. The prescriber should discuss the potential risks and benefits of oral agent therapy during pregnancy as well as the need for longer-term outcome studies.
Grade B; BEL 1
Evidence Base 16: How should DM in pregnancy be managed?
Abnormal glucose tolerance develops at higher rates and at younger ages among offspring of women with DM. A 2021 study examining the trends in GDM from 2011–2019 suggested that the rates of GDM have increased across all racial and ethnic subgroups.1408 Maternal DM is one of the strongest risk factors for the development of T2D among children.1409–1411 By the time these offspring reach childbearing age, they are very likely to be obese and have DM, thereby perpetuating a vicious cycle with significant implications for public health and health care costs.1411 That this is not simply a genetic predisposition is inferred from the finding of lower rates of DM in offspring of women who were born before their mothers developed DM1412; this is true among sibling pairs whose birth dates straddle the onset of their mother’s DM.1409 Thus, all women with DM in childbearing years should have preconception care and guidance to target an A1C level of <6.5%.75,1413–1417
The HAPO study confirmed findings in Pima Indians1409 that, even among offspring of women without GDM as it is currently defined, there is a linear association between maternal glucose concentration during pregnancy and newborn weight, rates of large-for-gestational-age, and cesarean delivery.64,1418–1421 DM during pregnancy and even maternal obesity itself1418 set the stage for a vicious cycle with offspring of mothers with DM during pregnancy being more likely to become obese and to develop DM at younger ages.1420 Maternal DM and obesity, although major risk factors for the metabolic health of the offspring, are not the only factors at play in the early stages of childhood that can have lasting adverse effects on offspring. Both low and high birth weight are associated with higher rates of DM.1421 Abnormal birth weight directly affects the offspring and leads to higher rates of GDM eventually in the offspring, thereby compounding the vicious cycle. Early diagnosis and treatment of DM, careful preconception care and guidance for women with DM or at risk for GDM, and meticulous control of glucose abnormalities throughout pregnancy are currently our best hope to break this perpetuating cycle.67,1422,1423
Thus, women with risk factors for DM (Table 5) should be screened at the first prenatal visit for undiagnosed T2D using standard criteria (Table 4), and all pregnant women without a prior diagnosis of DM should be screened for GDM with a 2-hour OGTT using a 75-g glucose load or a 2-step 1-hour/3-hour OGTT at 24 to 28 weeks’ gestation.63,1424 Glucose criteria diagnostic for GDM are an FPG >92 mg/dL, 1-hour postglucose challenge value ≥180 mg/dL, or 2-hour value ≥153 mg/dL.59
For women with GDM, glucose should be managed with the following treatment goals: preprandial glucose concentration ≤95 mg/dL and either a 1-hour postmeal glucose ≤140 mg/dL or a 2-hour postmeal glucose ≤120 mg/dL to decrease fetal macrosomia. However, no controlled trials have been performed to identify ideal glycemic target beyond the outcome of macrosomia, and the majority of the studies are extrapolated from T2D in pregnancy vs GDM data.122,1425,1426
Maternal diet modification and control is the initial intervention for a new diagnosis of GDM. Referral for nutrition counseling or meeting with a certified diabetes educator to discuss label reading, carbohydrate counting, and meal splitting is recommended. While initial treatment of GDM involves modifying maternal diet, if medication is needed beyond diet control, split dosing with long-acting and rapid-acting insulin is recommended. First-line therapy for GDM involves using NPH, detemir, or glargine; regular insulin is considered for treatment if long-acting insulin analogs are not available.1427–1429 Furthermore, while insulin is the recommended therapy, if not available or if unable to safely initiate, oral agents such as metformin or glyburide have not shown increased adverse pregnancy outcomes and therefore can be considered.1430–1435 If concerns for postprandial hyperglycemia occur, rapid-acting insulin and analogs are recommended.75,1436–1438
In T1D, optimal care may necessitate CGM and CSII utilization (often already in use given the longevity of the disease). Rapid-acting insulin analogs for pump therapy that have been studied in pregnancy include lispro and aspart.1439–1442 Data that detemir is safe in pregnancy are convincing.1443–1446 Glargine is widely used; however, there are still no conclusive reports on its safety as performing an RCT in pregnant persons with T1D has its own challenges. Discussion for women with glargine should include considering maternal risks vs fetal benefits and understanding that drug studies can clearly demonstrate concern when abnormal fetal outcomes are above the baseline population risk (1% to 2%). When that is not present, it is unclear whether documented fetal abnormalities are due to the drug or baseline population rate. Therefore, if glargine has been successful in maintaining a person with T1D in a euglycemic state, potentially changing medication in pregnancy and altering maternal blood sugar levels is riskier than fetal risk.
Insulin is the preferred treatment for women with pregnancy and DM. For those with GDM or pregestational T2D, and insulin is inaccessible, metformin and glyburide may be alternative options. The prescriber should discuss the potential risks and benefits of oral agent therapy during pregnancy as well as the need for longer-term outcome studies.1447,1448
Finally, women with GDM specifically need to have appropriate postpartum follow-up with a 2-hour OGTT within 6 weeks of delivery and referral to primary care for appropriate monitoring for T2D development.
Section 4: Select Additional Topics on Education, Nonpharmacologic Components of a Care Plan for Children and Adolescents, Male and Female Infertility, Secondary Diabetes, Posttransplant Diabetes, Sleep Medicine, Depression, SDOH, Virtual Health, Occupational Safety, Nutritional Supplements, Cancer Risk, and Vaccinations
Question 17: What education interventions have been shown to be most effective in management of persons with DM?
Recommendation 17
Comprehensive individualized DSMES is recommended at the time of DM diagnosis and subsequently as appropriate. Therapeutic lifestyle management must be discussed with all persons with DM or prediabetes at the time of diagnosis and throughout their lifetime. This includes MNT (with reduction and modification of caloric and fat intake to achieve weight loss in those who are overweight or obese), appropriately prescribed physical activity, avoidance of tobacco products, and adequate sleep quantity and quality. Additional topics commonly taught in DSMES programs outline principles of glycemia treatment options; BGM; insulin dosage adjustments; acute complications of DM; and prevention, recognition, and treatment of hypoglycemia.
Grade A; BEL 1
Evidence Base 17: What education interventions have been shown to be most effective in management of persons with DM?
DSMES is an ongoing educational program that imparts skills and knowledge needed for DM self-care throughout the life span.907 In a disease that is largely self-managed, DSMES includes nutrition, physical activity, and an understanding of treatment relevant to glycemic control, knowledge regarding the natural history of DM, and measures to prevent cardiometabolic and microvascular disease outcomes. Evidence indicates that DSMES imparts enhanced knowledge of DM and self-care practices resulting in improved A1C values,909,912,1449–1451 modest reductions in weight, better QoL,912,1451,1452 and lower mortality risk,1453 while at the same time leading to a reduction in health care costs.1454,1455 In a systematic review, patient education for those with DM has been suggested to be both cost-effective1456,1457 as well as to reduce complications and overall mortality.1458,1453
A personalized educational approach to persons with DM, leveraging members of a multidisciplinary team, will lead to greater self-management skills.1459–1463 When designing a patient education program, one should consider individual preferences and needs, social-educational status, health literacy, and learning barriers along with the types of locally available resources. The frequency and intensity of education are based on the natural history of the DM and particular situations such as pregnancy, CVD, initiation of insulin, or intensive insulin therapy incorporating pump or sensor technology. The National Institutes of Health1464 and AACE disease state resources provide useful online resources and education.
Patient education has proven to be effective for persons with T1D and T2D909,1465 across a variety of ages,1466 in rural1467,1468 and urban settings,1469 individually or in groups,1470 as well as across cultures and ethnicities,225,1471–1473 particularly if cultural and psychosocial issues are addressed.906,1474,1475
Patient education can be successful in many formats including individual sessions, group sessions,1476 telephone or video,1456,1469,1477,1478 and computer-based programs.1479,1480 Internet-based DSMES services and telemedicine approaches for DM prevention and the management of T2D management have also been shown to be effective.1481–1484 Digital enhancement of patient education curriculum via online formats, social media, and gamification has the potential to increase access to and engagement with DSMES.1485–1487
Members of the DM educational team can include nurses,1488,1489 dietitians, and pharmacists.1490,1491 In addition, nonmedical colleagues such as patient peers1492 or community health workers1493–1495 may also contribute to self-efficacy of a person with DM. A CDCES, after fulfilling eligibility criteria and passing the certification exam,1496 can be a critical member of a DM education program with their educational expertise as well as real-world knowledge of DM management. The goal is to improve a person’s knowledge of DM as well as their competency in DM self-management. Efforts to continually engage persons with DM may help sustain the outcomes achieved by the initial structured educational program.1497
Question 18: What are the key nonpharmacological components of a comprehensive diabetes care plan for children and adolescents?
Recommendation 18.1
T1D and T2D in children and adolescents should be managed in close consultation with the patient and their family members, involving school and daycare personnel whenever possible.
Grade B; BEL 2
Recommendation 18.2
It is recommended that all children and adolescents with DM should be given age and culturally appropriate education and guidance for physical activity and lifestyle modification.
Grade A; BEL 1
Recommendation 18.3
Interventions by family and/or community are recommended to improve dietary behavior and increase physical activity in efforts to prevent childhood obesity and T2D (Grade A). Game-based interventions also can be incorporated to enhance healthy lifestyle habits (Grade B).
BEL 1
Recommendation 18.4
Routine psychological assessment with consideration of family stressors and psychosocial factors that may impact glycemic control is recommended for all youth with DM.
Grade A; BEL 1
Recommendation 18.5
With the risk of glycemic control worsening during adolescence, coordinated, individualized, planned transition from pediatric to adult DM care is recommended for all adolescents.
Grade A; BEL 1
Evidence Base 18: What are the key nonpharmacological components of a comprehensive diabetes care plan for children and adolescents?
Parental involvement in DM care and monitoring has been shown to improve adherencetotreatmentplans as wellasglycemiccontrol in childhood,1498–1500 and parental support through adolescence has been shown to improve DM outcomes.1501 Similarly, because many children spend large portions of their day at school or in daycare, it is essential to communicate and coordinate with school personnel and other childcare providers to optimize glycemic control.1501,1502
Improvement in fitness and/or diet correlate with improvement in glycemic control in youth with T2D. The largest study of youth with T2D, TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) clinical trial, demonstrated that achieving a healthy lifestyle in this age group is challenging,1503 but when successful, it results in decreased A1C and homeostatic model assessment for insulin resistance.1504 More data are needed to determine optimal interventions for these positive lifestyle changes. In T1D, though physical activity may not improve glycemic control, it is important for preventing obesity and building healthy habits for adulthood and is an integral part of a diabetes care plan when coupled with hypoglycemia avoidance strategies.1502
DM care providers for children and young adults must recognize the prevalence of mental health disorders in this population as well as the impact of psychological concerns on glycemic control.1505 It is important that care providers understand normal cognitive and psychological development in youth as well as signs of mental illnesses such as depression/anxiety, eating disorders, and substance use disorders in children and young adults. Targeted depression-prevention programs with DM specific content have been shown to reduce DM distress and depression among youth with T1D compared with advanced DM education alone.1506 Mental health professionals should be included in the care team when needed for appropriate support.1498,1507,1508
Childhood obesity is a primary contributor to the development of T2D in children and young adults. Family-based interventions such as nutrition counseling with psychological support and more frequent family meals have been shown to be effective in obesity prevention.1509–1513 Inadequate sleep duration is an important contributor to childhood obesity.1514
Physical activity improves BMI in overweight and obese children.1515,1516 Games used to increase physical activity and increase nutritional knowledge have been effective.1517 Exergaming interventions at home and at school have been shown to increase physical activity in children and to improve BMI z-score and cardiometabolic parameters.1518–1520 There is evidence that community programs involving cross-age peers can be effective at delivering nutritional interventions1521 and in reducing BMI,1522 particularly in low-income or minority populations.
Although before and after-school interventions to increase physical activity in school-age children and adolescents have been effective in improving BMI and preventing obesity,1523,1524 results from in-school nutrition and lifestyle education programs have been inconsistent.1525–1531 Such interventions may be more effective when combined with family and community involvement.1525,1532,1533
There has been considerable interest in transitional care from pediatrics to adult care for persons with DM. Glycemic control has been shown to worsen during transition from pediatric to adult care for persons with T1D and T2D.1534,1535 There is strong consensus that an organized, planned process is necessary to appropriately transition persons from pediatric to adult DM care. However, due to limited studies there is insufficient evidence to support a particular transitional care model.1536–1541 Transitional care models may vary with respect to type of multidisciplinary staffing, separate vs joint clinics with pediatrician and adult physician, individual vs group education approaches and many other variables. Since no particular approach to transition has been found to be superior, more studies are needed to identify the most effective transitional care model.
Question 19.1: Should persons with infertility be screened for DM?
Recommendation 19.1
Men and women undergoing investigation for infertility and preparation for infertility interventions, including in vitro fertilization, should be screened for DM.
Grade B; BEL 2
Question 19.2: How should persons with preexisting DM and infertility be evaluated?
Recommendation 19.2
For all persons with DM and possible infertility, in addition to routine endocrine evaluation, further collaborative consultation with a reproductive specialist should be considered. For women with T2D and infertility, or those with T1D who desire to preserve or estimate their fertility, anti-Müllerian hormone and midluteal progesterone levels may be assessed and screened for ovulatory dysfunction including anovulation. For men with DM and infertility, a standard semen analysis may be assessed, and an endocrine evaluation be initiated.
Grade B; BEL 2
Question 19.3: Should men with DM and cardiometabolic disorders be assessed for hypogonadism?
Recommendation 19.3
All men with CMD including prediabetes, metabolic syndrome, obesity, and T2D should be assessed for hypogonadism by history and physical examination; test for testosterone deficiency in persons with loss of libido and/or loss of muscle strength or mass, erectile dysfunction, osteopenia, or infertility.
Grade B; BEL 1
Evidence Base 19:
19.1 Should persons with infertility be screened for DM?
19.2 How should persons with preexisting DM and infertility be evaluated?
19.3 Should men with DM and cardiometabolic disorders be assessed for hypogonadism?
Many persons with infertility have not had appropriate evaluations for underlying medical causes. Men and women with undiagnosed, untreated, or undertreated DM may have higher rates of infertility. For those diagnosed with DM, it also is important to emphasize that appropriate glycemic control is the best way to safeguard current and future fertility, and preconception care is essential for improved pregnancy outcomes.1542,1543 Desired outcomes of fertility treatments may be reduced in persons with undiagnosed controlled DM. Since infertility treatments are also resource intensive, it may be prudent to screen for DM universally in persons seeking evaluation for infertility.1544
Men with DM (T1D or T2D) have higher rates of infertility and a reduced number of offspring (T1D). 1545–1548 IGT also is present in a significant proportion of men undergoing investigation for primary infertility.1549 The presence of metabolic syndrome components has been associated with poor sperm morphology and erectile dysfunction.1550 Hyperglycemia has been shown to impair gamete number and competency oligoasthenospermia) as well as erectile and ejaculatory function.1551 DM is associated with worsened sperm quality including decreased concentration, progressive motility, and sperm morphology.1546 The molecular mechanisms that underlie these findings are not fully understood. There may be impaired sperm mitochondrial function in men with T1D and epidydimal dysfunction.1546,1552 For men with T2D, semen analysis can display findings of increased oxidative stress.1546 Men with DM also may have lower testosterone levels due to decreased hypothalamic gonadotropin-releasing hormone drive and/or damage to the testes. Initial investigation can include semen analysis and an endocrine evaluation for secondary hypogonadism and/or secondary hypothyroidism.1547,1553–1556
There appears to be a higher likelihood for undiagnosed hyperglycemia in those seeking fertility.1557 In preclinical or animal models, hyperglycemia can impact oocyte competence by known and unknown mechanisms.1558,1559 In addition, women with DM may have higher rates of ovulatory dysfunction including hypothalamic hypogonadism.1560 Womenwith autoimmune DM may be especially prone to accelerated oocyte atresia and early menopause,1561 which may be one explanation for decreased numbers of offspring of women with T1D compared with controls or unaffected siblings,1548 especially for those with earlier childhood onset. Women with T1D with hyperglycemia may also have hypothalamic-pituitary-gonadal axis dysfunction, which can contribute to oligo- or amenorrhea.1562 Initial investigations include measurement of anti-Müllerian hormone and midluteal progesterone levels.1563,1564 Collaboration with a reproductive specialist is recommended to discern risk factors for infertility.
There is a significant knowledge gap regarding the impact of pharmacologic treatments for DM on gamete health, fertility, and pregnancy as well as for reproductive technologies.1565 Current data do not allow us to determine which treatments for DM could preserve or compromise fertility.
An additional consideration is that men with CMD, whether characterized by prediabetes, metabolic syndrome, obesity, or T2D, are at increased risk of hypogonadism and testosterone deficiency.1566–1569 Testosterone may impair gonadotropin secretion and result in impaired spermatogenesis.1570 Furthermore, testosterone replacement improves glycemia, dyslipidemia, hepatic steatosis, body composition, and CVD risk and also prevents progression from prediabetes to T2D.1571–1574 Therefore, it is important to screen for and treat hypogonadism and testosterone deficiency in men with CMD and ABCD.
Question 20.1: How should persons at risk for secondary diabetes be assessed?
Recommendation 20.1
Persons withrisk factors for developing secondary diabetes, such as postorgan transplantation, cystic fibrosis, chronic pancreatitis/postpartial pancreatectomy, or on medication associated with hyperglycemia, should be monitored routinely for IFG, IGT, and/or overt DM.
Grade A; BEL 1
Evidence Base 20.1: How should persons at risk for secondary diabetes be assessed?
Common forms of secondary diabetes include posttransplant diabetes (PTDM), cystic fibrosiserelated diabetes (CFRD), pancreatogenic diabetes (type 3c), and diabetes associated with certain medications such as corticosteroids, protease inhibitors, and, most recently, immune checkpoint inhibitors. These conditions have unique pathophysiologic mechanisms with associated risk factors such as genetic (human leukocyte antigen typing, family history), clinical phenotypes (overweight/obese), or medication-related disruption of normal glucose metabolism. Others are related to underlying primary disease process, such as cystic fibrosis, resulting in a loss of pancreatic function. Similarly, diabetes related to chronic pancreatitis or postpartial pancreatectomy is a common complication, with one meta-analysis demonstrating an incidence of up to 77% after distal pancreatectomy.1575
The risk factors for PTDM are similar to those for T2D, particularly with demographics, and obesity1576–1578 with the additive effects of immunosuppressive medications resulting in insulin deficiency as well as insulin resistance. Other more unique risk factors in this population include genetic polymorphisms, polycystic kidney disease, hepatitis C, and cytomegalovirus status.1579–1581 Of the various immunosuppressive agents, belatacept1582,1583 appears to be the least diabetogenic and tacrolimus having the highest risk for PTDM.1584–1590 Electrolyte abnormalities such as hypomagnesemia may also add to the risk.1586 It is still unclear if steroid-sparing regimens decrease the risk of PTDM with studies showing mixed results.1591–1595 Other immunosuppressive therapy associated with a lower risk of PTDM include antithymocyte globulin and Interleukin 2 receptor antagonists.1583,1589,1596,1597
Early screening and diagnosis are important as PTDM is associated with increased adverse outcomes in persons with transplant.1598,1599
An increasing body of new literature points to the association of immune checkpoint inhibitors with new-onset hyperglycemia and DM. At this time, most of the literature is in the form of case reports demonstrating hyperglycemia, new-onset diabetes, and DKA, either with or without autoimmune markers of T1D.1579,1600–1605 Presentation may range from mild hyperglycemia to frank DM, ranging from 1% to 6%.1606,1607 Therefore, persons on immune checkpoint inhibitor therapy should be monitored closely for early detection and therapy for hyperglycemia and the long-term development of DM.
Corticosteroids and protease inhibitors (anti-retroviral agents) also cause insulin resistance and IGT and are strongly associated with secondary DM.1608–1611 With all these secondary causes of hyperglycemia, a high index of suspicion is necessary to screen for both fasting and postprandial hyperglycemia to diagnose new onset of DM.
Question 20.2: What are the best treatment strategies for management of secondary diabetes, such as posttransplant diabetes, cystic fibrosis‒related diabetes, and other forms of secondary diabetes?
Recommendation 20.2.1
Select treatment for secondary DM based on the underlying pathophysiology. Insulin therapy is safe and effective, but alternative glucose-lowering agents may be considered in specific patient populations.
Grade A; BEL 1
Recommendation 20.2.2
DPP-4 inhibitors can be safely used to improve glycemic control for posttransplant diabetes.
Grade A; BEL 1
Evidence Base 20.2: What are the best treatment strategies for management of secondary diabetes, such as posttransplant diabetes, cystic fibrosis‒related diabetes, and other forms of secondary diabetes?
Treatment strategies vary based on the etiology of the secondary DM. Other than PTDM, clinical trials for specific therapies for the various forms of secondary DM have been limited in size and scope. In any secondary DM with obvious insulin deficiency or diabetic emergencies such as hyperosmolar hyperglycemic syndrome or DKA, insulin is the primary therapy. Insulin therapy is efficacious in all forms of secondary DM.
There is limited evidence to support any particular non-insulin therapy in secondary DM. There are several studies on PTDM and oral antihyperglycemia agents, including repaglinide, DPP-4 inhibitors, and pioglitazone.1612 In particular, DPP-4 inhibitors have been studied the most in several RCTs of persons with kidney transplant and have been shown to be safe and efficacious.1612–1614 The choice to use immunosuppressive agents is also a consideration in both prevention and management of PTDM. Use of antithymocyte globulin, belatacept, and minimization or elimination of tacrolimus in the immunosuppressive regimen has been shown to reduce hyperglycemia and new onset PTDM.1590,1615,1616 SGLT2is are emerging as a potential option for PTDM and have demonstrated safety and efficacy.1612,1617–1619
Studies on intensive lifestyle intervention for secondary DM are limited. However, one prospective study demonstrated a beneficial role of lifestyle intervention in persons with transplant.1620
Studies on CFRD are small and limited. In a 24-month RCT of newly diagnosed CFRD, repaglinide was demonstrated to be equivalent to insulin.1621 However, insulin remains the most common therapy for CFRD, particularly when corticosteroid therapy is also used for CF management.
Question 21: What is the role of sleep medicine in the care of persons with DM?
Recommendation 21.1
Health care professionals should assess persons with T2D for symptoms and signs of OSA, especially in the presence of obesity or suggestive clinical features of OSA.
Grade B; BEL 2
Recommendation 21.2
Based on resources available locally, persons suspected to have OSA should be referred to an appropriate center for diagnosis and management of OSA.
Grade B; BEL 4 and Expert Opinion of Task Force
Recommendation 21.3
Weight loss is recommended as the predominant intervention to improve both OSA and insulin sensitivity. In addition, devices that provide positive airway pressure as prescribed by a sleep specialist are effective.
Grade A; BEL 1
Evidence Base 21: What is the role of sleep medicine in the care of persons with DM?
The National Sleep Foundation1622 recommends an average of at least 7 hours of sleep for adults ≥18 years of age. Chronic inefficient sleep duration has been reported to be associated adversely with obesity, DM,1623 hypertension, CVD, and increased mortality. OSA is prevalent in persons with T2D (58%-77%) and even higher (86%) in those with both T2D and obesity.1624
The American Academy of Sleep Medicine recently emphasized the detrimental effects of sleep disorders and the importance of sleep education and routine screening for sleep disorders by health care professionals as strategies for optimizing healthy sleep.1625 Screening in the office should assess for symptoms associated with OSA such as snoring, apnea or choking during sleep, unrefreshed sleep, excessive daytime sleepiness, or fatigue, especially in overweight or obese individuals with DM. Some clinics have supplemented clinical screening with the use of home oximetry. Clinical screening with a tool such as the STOP-Bang questionnaire can be augmented with home sleep apnea testing depending on available resources.1626,1627
Treatment of OSA in persons with prediabetes and DM with continuous positive airway pressure (CPAP) improves OSA symptoms.1623,1628–1631 Weight loss whether via lifestyle intervention or pharmacologic approaches also has been shown to result in improvements of OSA.1628–1630,1632–1636 From Sleep AHEAD study data, intensive lifestyle intervention resulting in weight loss had a greater impact on the apnea-hypopnea index and OSA remission than standard DMsupport and education, and this wassustained at 10 years.1635
The effects on A1C are more variable with one study suggesting that despite improvements in insulin resistance, CPAP treatment of OSA does not necessarily improve A1C.1637 However, a 2021 meta-analysis found that in adults with T2D and OSA, treatment with CPAP resulted in significant improvement in A1C.1638 More consistently, weight loss was independently reported to improve both sleep apnea and A1C in those with T2D.1634
Question 22: Should screening for depression be a routine component of clinical assessment in persons with DM?
Recommendation 22
Routine screening of adults with DM for depression and DM distress is recommended during each clinic encounter, if appropriate. Referral to mental health professionals should be made as soon as possible once depression is suspected or diagnosed.
Grade A; BEL 1
Evidence Base 22: Should screening for depression be a routine component of clinical assessment in persons with DM?
Depression is highly prevalent in those with T2D and if untreated, can be associated with poor adherence to lifestyle and medical regimens and potentially lead to more CVD and other DM-related complications.1639,1640 DM-related distress, anxiety, subthreshold depression, having more than 3 chronic diseases and having stressful life events can predict depression and should trigger screening with effective tools like the WHO Wellbeing Index (WHO-5), the Patient Health Questionaire-9, or the Beck Depression Inventory II.1641–1644 For DM distress, The Problem Areas in Diabetes Scale and the Diabetes Distress Scale are available.1643 Screening for and treatment of depression among persons with DM has been associated with reduction in DM comorbidities and better outcomes for glycemic control and DM complications.1645–1648
Cognitive behavioral therapy delivered by trained mental health personnel, either face to face or through virtual web-based media, is effective in reducing depression and improving self-care and DM outcomes.1645–1647,1649,1650 Although chronic use of antidepressant medication has been associated with a modestly increased relative risk of T2D, this may reflect the association of DM with depression rather than an adverse effect of these agents.1651,1652 Selective serotonin reuptake inhibitors (SSRIs) appear to improve glycemic outcomes independent of their effect on depression or weight.1653 Treatment of depression with SSRIs among persons with DM has been associated with weight reduction in some reports.1654,1655 These interventions for depression were most effective when combined with exercise and DM education in several RCTs.1647,1656,1657
Question 23: Is the evaluation of SDOH in persons predisposed to or with DM useful in improving health outcomes?
Recommendation 23
Clinicians should assess SDOH in persons with DM to better guide them to the most appropriate resources. Interventional trials addressing SDOH and health inequities in DM are needed to evaluate reversibility of their impact.
Grade B; BEL 1
Evidence Base 23: Is the evaluation of SDOH in persons predisposed to or with DM useful in improving health outcomes?
Considering only biologic variables of DM may result in partial understanding of the etiology of DM outcomes. In addition to assessing biologic variables of a disease, evaluation of SDOH will lead to a greater contextual understanding of the natural history of disease. SDOH are the conditions in which people are born, grow, live, work, and age. These circumstances are shaped by the distribution of money, power, and resources at global, national, and local levels. The SDOH are mostly responsible for health inequitiesdthe unfair and avoidable differences in health status seen within and between countries.1658 The World Health Organization lists the following examples of SDOH:
Income and social protection
Level of education
Unemployment and job insecurity
Working life conditions
Food insecurity
Housing, basic amenities, and the environment
Early childhood development
Social inclusion and nondiscrimination
Structural conflict
Access to affordable health services of decent quality
Socioeconomic factors have been associated with poor lifestyle practices like physical inactivity and smoking.1659 Research shows that social determinants can be more important than health care or lifestyle choices by themselves in influencing health outcomes. For example, numerous studies suggest that SDOH account for between 30% to 55% of health outcomes.1660 Those persons with lower socioeconomic status have a higher risk for developing T2D, worse DM control, and more DM-related complications.1661–1663 For example, lower socioeconomic status has been associated with increased risk of developing DKA in T1D1664 and diabetic retinopathy in T2D.1665 In addition, estimates show that the contribution of sectors outside health to population health outcomes exceeds the contribution from the health sector.1666 A study looking at food insecurity among Latinos with T2D reported decreased sleep quality attributed to anxiety, depression, and DM distress from food insecurity.1667 Job-related insecurity was associated with diabetic retinopathy in T2D.1665 The increased prevalence of DM among Native Americans is well known and attention must be paid not only to biologic causes and medical management but also to sociocultural and environmental factors.1668
Various interventions with specific strategies targeting the underlying disparities resulting from social factors have been shown to be effective in reducing the burden posed by SDOH.1669,1670 Recognition of SDOH and engaging community stakeholders and resources may result in lower-cost programs to improve metabolic health. Once disparities and adverse SDOH are apparent, connecting impacted persons with DM to appropriate community resources that address housing, nutrition, and health care access should be helpful. This should result in a public health societal approach in addition to medical-biological approaches.1669
There are inadequate equity-related considerations in DM trials limiting the relevance and applicability of their data to disadvantaged populations.1671 Targeted recruitment and explicit focus on SDOH and health inequities are recommended to close this gap.1672
Question 24: Is telehealth/virtual care an effective care-delivery model for the management of persons with DM?
Recommendation 24
Offer telehealth, if available and appropriate, to persons with DM as part of their wholistic health care.
Grade A; BEL 1
Evidence Base 24: Is telehealth/virtual care an effective care-delivery model for the management of persons with DM?
With globally expanding access to virtual platforms, telehealth is becoming a mainstream component of health care delivery. Mobile applications, web-based interventions, virtual coaching, and other electronic tools have been shown to improve DM self-management.153 While DM consists of multiple complex factors, each of which could be amenable to different technological approaches, the focus of this discussion is consideration of the outcome of improved glycemic control.
With respect to glycemic control, telehealth appears noninferior (as good or superior) to traditional health care delivery for persons with T1D or T2D, particularly in those who require more interactions, are newly diagnosed, have higher A1C levels, are diagnosed with GDM, or have other comorbid conditions.1673–1681 Telehealth may reduce the incidence of hypoglycemia.1682 The virtual care approach appears to be effective across age or racial lines.1683
Telehealth also allows for integration of a multidisciplinary team, which could include nurses, pharmacists, dieticians, and other health care professionals, for an individual’s health care.1684–1686
Telehealth appears to be cost-effective and is likely to be even more economical in the future. Incorporation of automated recommendations regarding dosing of one’s insulin or antihyperglycemic medications will further enhance economies of scale.1687–1691
Future research is needed to determine the impact of the interplay between an individual’s preference of platform and type of health care delivery (telehealth vs hybrid vs traditional models). Beyond improvement in glycemic control, there may be additional benefits of improved adherence, improvement in comorbid conditions, and reduced cost of health care per person.
For information on incorporation of DM technology into one’s practice, please refer to the 2021 AACE Advanced Diabetes Technology Guideline.153
Question 25: Which occupations have specific public safetyerelated diabetes management considerations?
Recommendation 25
Persons with DM who are engaged in occupations with public safety implications, such as commercial drivers and pilots, have special management requirements for certification. CGM to predict hypoglycemia in real time and pharmacotherapy that minimizes hypoglycemia are recommended as effective strategies for persons with DM who work in these occupations.
Grade A; BEL 1 and expert opinion of task force
Evidence Base 25: Which occupations have specific public safetyerelated diabetes management considerations?
It is important to note that existing evidence for the association of commercial vehicle accidents and DM does not consider recent advances in nonhypoglycemic therapies and in glucose monitoring technology, both of which have potential to reduce the risk of hypoglycemia-related accidents. More studies are needed on the impact of these advances on vehicular safety. Nevertheless, the licensing and certification of two occupations in particular, commercial vehicle drivers and airline pilots, have become more favorable in recent years. In 2018, the Department of Transportation Federal Motor Carrier Safety Administration (FMCSA) reversed the previous blanket exclusion against insulin use with a new rule for interstate commercial drivers with DM. This new rule was the first update on DM since 1970 and allows medical certification by obtaining an assessment from a treating clinician that the applicant has “properly controlled insulin-treated diabetes” and is on a “stable insulin regimen.” The treating clinician determines whether a particular individual meets these criteria, which do not include a threshold A1C value. Proliferative diabetic retinopathy is a permanently disqualifying complication; treating clinicians can assess other complications on an individual basis to determine if they impair one’s ability to operate a commercial vehicle driver safely. A medical examiner then determines if the individual meets the FMCSA’s physical qualification standards. These individuals must also consult their state licensing agencies for their laws to reflect this new federal rule.1692,1693
Similarly, in 2019, the Federal Aviation Administration outlined considerations for insulin-treated T1D or T2D with a CGM option. This policy permits special issuance of medical certification to some applicants who provide medical documentation of their history of treatment, accidents, and current medical status by an endocrinologist. For first- and second-class airman certification, CGM data is a requirement, whereas third-class airmen may use non-CGM protocol.1694
Risk of Accidents and Potential Treatment-Associated Hypoglycemia
An area of great concern has been whether DM might lead operators of commercial vehicles (eg, bus, truck, taxi, ferry, or airplane) to lose control and have an accident, putting themselves or others at risk of injury. Eye disease associated with DM, including various forms of retinopathy and cataracts, is of course a potential cause of impaired driving ability. There is general consensus that ascertainment of the visual acuity of commercial motor vehicle drivers or airline pilots is a reasonable measure for such risk. Similarly, coronary artery disease, CVD, musculoskeletal conditions, and diabetic neuropathy might in various ways impair safe driving or piloting ability.
Hypoglycemia may impair judgment and motor ability, which could increase the likelihood of an accident during operation of a motor vehicle or airplane. The Federal Motor Carrier Safety Administration Evidence Report on Diabetes and Commercial Motor Vehicle Driver Safety addresses some key aspects of these hypoglycemia-related issues.1695 Taken as a whole, individuals with DM do not have a significantly increased risk of motor vehicle accidents compared with drivers without DM. However, a separate analysis of studies conducted within the United States showed a 25% increase in risk of accidents, whereas studies conducted outside the United States showed no increased risk. This was particularly true when non-US and US cohorts of insulin-treated persons were compared. An analysis of 2 available US studies showed a 2.75-fold greater risk of motor vehicle accidents when insulin-treated persons were compared with individuals without DM (P = .001), while studies from outside the United States demonstrated no significant difference in accident risk.1695
A meta-analysis restricted to US studies of persons with DM not using pharmacologic treatment or using oral antihyperglycemic agents did not show a significant increase in risk of accidents. Among individual studies included in the analysis, use of SU did not significantly increase the risk of accidents.1696–1698 However, SU treatment is associated with a greater likelihood of hypoglycemia than all other noninsulin antihyperglycemic agents (metformin, TZDs, alpha-glucosidase inhibitors, DPP-4 inhibitors, and GLP-1 RAs) and carries a nearly a 2-fold greater likelihood of hypoglycemia than basal insulin.1699 Studies of insulin users involved mostly persons with T1D, but the use of a basal insulin analog as the sole administered insulin for T2D is associated with considerably lower hypoglycemia rates than older insulin preparations or the use of basal-bolus treatment.1065
With respect to pilots with DM, recent experience in Europe reported no episodes of pilot incapacitation nor worsening of glycemic control with insulin-treated DM.1700
Unfortunately, reliable large population studies of motor vehicle accidents involving persons with T2D treated with current approaches are not available (studies of oral antihyperglycemic agents included in the meta-analysis examined data from the late 1980s to early 1990s). Although in a post hoc analysis, one study demonstrated the potential role of CGM in predicting hypoglycemia more consistently than intermittent BGM.1701 Advances in vehicle technology combined with reliable rtCGM should result in safer driving or flying.1702
Although, the diagnosis of DM has not been shown to be directly associated with increased collision risk, persons with older age and on insulin therapy tend to have a higher risk.1703 A validated patient questionnaire may be a useful tool for clinicians to predict and reduce driving mishaps among persons with DM.1704 Treatment efforts should focus on agents with reduced likelihood of causing hypoglycemia.
Although commercial drivers and pilots with DM are highly scrutinized, those with shift work or extended periods of work should also have customized regimens of therapeutic dosing and scheduling, nutrition variability, and glycemic monitoring. The goal is to support an individual’s productivity and safety.
Question 26: Is there a role for nutritional supplements in the management of DM and what might be the associated risks?
Recommendation 26
Nutritional supplements (ie, noncaloric oral supplements) have modest or neutral effects on glycemic control, lipids, and BP. Until proven scientifically, these supplements should not be used for managing DM or related CV risk factors among persons with DM. In view of potential harm, we recommend that persons with DM use caution and discuss with their physicians the use of unregulated nutritional supplements.
Grade A; BEL 1
Evidence Base 26: Is there a role for nutritional supplements in the management of DM and what might be the associated risks?
Nutritional supplements are a heterogenous group of substances marketed without prescription with varying effects on glycemic control and CV risk factors such as hypertension and dyslipidemia in persons with DM. They include vitamins, minerals, herbs or botanical products, and probiotics. These supplements generally are not regulated by governmental approval agencies and have inconsistent composition and quality.
Probiotics are among the most studied nutritional supplements. Several RCTs and systematic reviews/meta-analyses have noted a positive effect of probiotics on glucose, A1C, lipids, and BP.1705–1714 The main limitation of these studies is the wide variation in methodologies including differences in the type, formulation, concentration, and duration of exposure to probiotics.
A meta-analysis reported that psyllium when taken before meals led to significant improvement in fastin BG (−37.0 mg/dL; P < .001) and A1C (−0.97%; P = .048) among persons with T2D.1715 Zinc supplementation at 20 mg daily in persons with prediabetes in an RCT resulted in a reduction in BG, decreased insulin resistance, improved β-cell function, and reduced progression to DM compared with controls.1716
Other nutritional supplements, specifically resveratrol, selenium, and vitamin D, have mixed effects on glycemic control and other CV risk factors.1705,1717–1724 Some reports suggest potential harm with the use of these agents. One systematic review found potential increase in risk for developing DM with selenium supplementation, but an RCT found no harm to b cells or insulin sensitivity.1720,1721
Question 27: How should potential increased cancer risk be managed in persons with obesity/T2D?
Recommendation 27.1
Clinicians should recommend age, sex, and risk-appropriate screening for common cancers, especially those associated with obesity and DM.
Grade B; BEL 2
Recommendation 27.2
With the increased risk of certain cancers in persons with obesity or DM, clinicians should educate persons regarding cancer risk and encourage a healthy lifestyle, including weight reduction.
Grade A; BEL 1
Evidence Base 27: How should potentially increased cancer risk be managed in persons with obesity/T2D?
Epidemiologic evidence suggests increased risks of cancer and cancer mortality in persons with obesity and/or DM.1725–1730 There also may be an additive interaction of overweight or obesity with DM, further increasing cancer risk and mortality.1731,1732
Persons who are overweight (>25 kg/m2) or obese (>30 kg/m2) may have an increased risk of a variety of cancers, although risk may be modified by age, sex, race, menopausal status, duration of obesity, anthropomorphic distribution of adiposity, and the presence of additional metabolic syndrome components.1730,1732–1738 Variably increased risk has been reported for cancers of the breast (postmenopausal) endometrium ovary gall bladder, stomach and esophagus, kidney, thyroid (papillary, follicular, and anaplastic but not medullary), colon, bladder, and pancreas.1733,1734,1739–1752 There also is increased risk of hematologic malignancies with leukemia (acute myeloid leukemia), malignant and multiple myeloma, and non-Hodgkin and Hodgkin lymphoma.1753,1754 Increased BMI may, however, be protective for lung cancer (in never smokers),1755 although a large meta-analysis found increased lung cancer risk among current smokers, past smokers, and never smokers with increased abdominal obesity measured by WC.1756 An inverse relationship between BMI and prostate cancer has been reported,1757 but this may be dependent on racial background with increased risk in African American men.1735 There is evidence of an association of obesity and high-grade aggressive prostate cancers.1758 In premenopausal women, increased BMI may be protective overall for breast cancer1759 but not with hormone receptor negative breast cancer.1760 Although the pathophysiologic mechanisms that drive an increase in cancer risk with obesity have not been clearly elucidated, higher BMI is associated with increased systemic levels of endogenous insulin, insulin-like growth factors, adipokines, inflammatory cytokines, and angiogenic factors that have potential procancerous effects. The local interaction of adipose tissue and tumor cell microenvironments may also be important in the promotion of cancer.1761,1762
DM also is reported to be associated with the risk of specific cancers, although it is challenging to isolate DM-associated risk from that of comorbid obesity.1732 Most of the available evidence is generated from T2D cohorts, possibly because it is the most prevalent DM, although increased risk of certain cancers also has been reported for persons with T1D.1763 There also may be cancer detection bias with increased screening after diagnosis of DM,1732 although there also is evidence that persons with DM may be underscreened for certain cancers compared to those without DM.1764 T2D has been shown to be associated with increased risk for hepatic,1765 bladder,1766 pancreatic,1767,1768 and colorectal cancers.1769 For womenwith DM, increased risk has been reported for endometrial1770 and breast cancer.1771 Sex differences also are reported with women at higher risk than men for oral, gastric, colorectal, and kidney cancers as well as leukemia, but with decreased risk for liver cancer.1772,1773 Bladder cancer risk may be higher in men.1766 There is an inverse association between the risk of prostate cancer and DM,1774 although the use of antihyperglycemic agents may have diminished the apparent DM-associated risk in epidemiologic studies.1775 Importantly, elevated BG and DM are associated with increased prostate cancer mortality.1775–1777 In addition to the obesity-related mechanisms for cancer risk that are discussed above, hyperinsulinemia and hyperglycemia may promote a microenvironment amenable to cancer cell proliferation with activation of mitogenic signaling.1778
With the understanding of the increased cancer risk, up-to-date age- and sex-appropriate cancer screening is imperative in persons with obesity and/or DM but is not always met.1764 Education regarding cancer risk for persons with overweight/obesity and/or DM also may encourage adherence to lifestyle modifications and weight loss,1779 but a major knowledge gap is how such interventions impact risk of cancer in the long term. There are data to support a modest reduction of obesity-related cancers with weight loss (HR, 0.84; 95% CI, 0.68–1.04) in persons with DM but without a significant impact on total cancer incidence or mortality.1780
Pharmacologic Therapies for DM and Cancer Risk or Prognosis
To date, no definitive relationship has been established between specific antihyperglycemic agents and an increased risk of cancer or cancer-related mortality. The evidence for the effects of specific antihyperglycemic agents on cancer risk is confounded by factors such as obesity, hyperinsulinemia, glycemic control, and combination pharmacotherapy in DM.
Metformin
Metformin mayeither be neutral or modestly protective regarding cancer incidence and mortality; however, most of the available evidence was gathered from observational studies with varied designs and risk for bias.1781–1783 A decreased risk of colorectal adenomas and colorectal carcinoma is a consistent finding with a potential for a survival benefit for colorectal carcinoma.1784–1790 Modest survival benefits also have been reported for breast, ovarian, endometrial, prostate, lung, kidney, liver, and earlier stage pancreatic cancers.1791–1800 There is no reported effect of metformin on the incidence or overall survival for bladder cancer.1801 The effect of metformin on cancer outcomes (prostate, breast, lung, colorectal, pancreas) is currently being explored in multiple prospective trials, including with metformin as an adjuvant to chemotherapy. Although definitive statements regarding the benefits of metformin and cancer cannot be made, the above findings could inform the decision to initiate metformin as a treatment in persons with DM and specific cancers.
Thiazolidinediones
Large population-based cohort studies have found that pioglitazone is associated with a modestly increased risk of bladder cancer when compared with other oral DM therapies including the TZD rosiglitazone as the comparator.1802,1803 However, evidence from a large study of over 1 million persons from several international cohorts did not find an association with cumulative exposure to pioglitazone and bladder cancer1002 and 10 years of observation of participants fromthe PROACTIVE did not find increased risk of bladder cancer (0.8% pioglitazone vs 1.2% placebo).1804 TZD therapy in general is not associated with other cancers and a modest reduction in overall cancer risk has been reported for pioglitazone.1781
Incretin Therapies
A concern about increased risk of pancreatic cancer with incretin therapies wasraised bya studyofhumanpancreata from persons with DM on incretin therapy compared to controls, which reported exocrine dysplasia and alpha-cell hyperplasia.1805 However, a thorough review of available data conducted by the FDA and the European Medicines Agency did not uncover evidence to support a causal association.1806 Retrospective and meta-analysis of data from large placebo-controlled clinical trials with GLP-1 RAs and DPP-4 inhibitors have not found an increased risk of pancreatic cancer.1034,1807,1808
An increase in thyroid C-cell hyperplasia, adenomas, and medullary thyroid carcinomas was observed in preclinical rodent studies of liraglutide, while exenatide has been shown to cause nodular C-cell lesions without medullary thyroid carcinoma, 1809,1810 leading to concerns regarding the potential for development of medullary thyroid carcinoma in persons with DM on GLP-1 RAs. From placebo-controlled clinical trial data, there is no evidence of increased calcitonin or C-cell neoplasia in humans,1811 and meta-analyses have not uncovered increased risk for thyroid cancer with GLP-1 RAs or DPP-4 inhibitors.1808,1812,1813 Human calcitonin-producing C-cells do not express GLP-1 RAs as do rodent C-cells. Overall, there is no evidence that incretin-based therapies increase risk for medullary thyroid carcinoma in humans.1808,1812,1813 Nonetheless, GLP-1 RAs should not be used in individuals with a personal or family history of medullary thyroid carcinoma or in persons with multiple endocrine neoplasia syndrome type 2.
SGLT2 Inhibitors
Among the SGLT2is, more cases of bladder cancer occurred among dapagliflozin-treated than control-treated persons in clinical trials, and the product labeling indicates that this agent should not be used inpersons with active bladder cancer and should be used with caution in persons with a history of bladder cancer.1814 Warnings regarding bladder cancer are not included in the canagliflozin or empagliflozin prescribing information.1815,1816 There was no increased bladder cancer risk in a large meta-analysis of multiple SGLT2is,1817 although a separate meta-analysis suggested that there could be some increased risk, but a causal relationship was inconclusive.1818 Overall risk for all cancers is not increased with SGLT2is.1817,1818
Sulfonylureas
There is no evidence for increased cancer risk for SUs compared to controls in RCTs, although cancer risk may be higher compared to a metformin comparator in cohort studies.1819
Insulin
Contrary to preliminary cohort-level evidence suggesting that exogenous insulin may be associated with an increased cancer risk,1820 particularly glargine, the large-scale ORIGIN (Outcome Reduction with an Initial Glargine Intervention) trial did not substantiate this risk.1821 In ORIGIN, >6000 participants received insulin glargine over a median trial duration of 6 years with no associated increased risk of any cancer (HR, 1.0; 95% CI, 0.88–1.13) or cancer death (HR, 0.94; 95% CI, 0.77–1.15), including breast, lung, colon, and prostate cancers.1821 A meta-analysis of data from 10 cohort studies examined insulin use and overall cancer risk found an increased risk of 28% for persons with DM using insulin compared with non-users.1822 Given that endogenous hyperinsulinemia is one of the proposed factors for the link between cancer and obesity and DM, an improved understanding of the impact of insulin therapy on cancer risk and progression is imperative.
Question 28: Which vaccinations should be given to persons with DM?
Recommendation 28.1
AACE supports the recommendations of the CDC Advisory Committee on Immunization Practices (ACIP) that all persons with DM receive age-appropriate vaccinations according to the CDC/ACIP schedule.1823 Immunization recommendations for adults with DM are summarized in Table 21.
Table 21.
Vaccine Recommendations for Adults with Diabetes Mellitusa
| Vaccine | Recommendation | Grade and best evidence level |
|---|---|---|
| Age-appropriate vaccines | All persons should receive according to the CDC/ACIP immunization schedules: https://www.cdc.gov/vaccines/schedules/index.html. | A 4 |
|
Influenza IIV4 or RIV4 or LAIV |
Annually | A 1 |
|
Pneumococcal PCV15 and PCV20 Age, 19–64 y |
PCV15 or PCV20 for all adults aged 19 to 64 y who have underlying medical conditions, including DM. When PCV15 is used, PPSV23 should be administered at least 12 months following the dose of PCV15. A minimum interval of 8 weeks may be used for adults with immunocompromising conditions. | B 3 |
|
Pneumococcal PCV15 and PCV20 Ages ≥65 y |
For adults over age 65 y who have not previously received PCV or whose vaccination history is unknown, PCV15 or PCV20 should be administered. When PCV15 is used, it should be followed by a dose of PPSV23. | B 3 |
| Hepatitis B | All adults aged ≤59 y | A 1 |
| HepB | Based on risk and quality of immune response for adults aged ≥60 y | C4 |
|
Tetanus, diphtheria, acellular pertussis Tdap |
Every 10 y following completion of the primary series | C 4 |
| COVID-19 | All persons per FDA approval or emergency use authorization | B 2 |
|
Varicella RZV |
All adults aged ≥50 y | A 1 |
Abbreviations: ACIP = Advisory Committee on Immunization Practices; CDC = Centers for Disease Control and Prevention; COVID-19 = coronavirus disease 2019; DM = diabetes mellitus; FDA = Food and Drug Administration; IIV4 = quadrivalent inactivated influenza vaccine; LAIV = live, attenuated influenza vaccine; PCV15 and PCV20 = pneumococcal conjugate vaccines; PPSV23 = pneumococcal polysaccharide vaccine; RIV4 = quadrivalent recombinant influenza vaccine; RZV = recombinant zoster vaccine.
For child/adolescent specic immunization recommendations, refer to the CDC Immunization Schedules: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
Grade A; BEL 4 and expert opinion of task force
Recommendation 28.2
An annual influenza vaccine is recommended for those with DM who are ≥6 months old.
Grade A; BEL 1
Recommendation 28.3
The 15- or 20-valent pneumococcal conjugate vaccine (PCV15 or PCV20) should be administered to all adults aged 19 to 64 years who have DM. When PCV15 is used, PPSV23 should be administered at least 12 months following the dose of PCV15. A minimum interval of 8 weeks may beused for adults withimmunocompromising conditions.
Grade B; BEL 3
Recommendation 28.4
For adults over 65 who have not previously received PCV or whose vaccination history is unknown, PCV15 or PCV20 should be administered. When PCV15 is used, it should be followed by a dose of PPSV23.
Grade B; BEL 3
Recommendation 28.5
It is recommended to administer hepatitis B vaccinations to all individuals as soon after diagnosis of DM as possible up to age 59 years.
Grade A; BEL 1
Recommendation 28.6
Consider hepatitis B vaccination of adults ≥60 years based on assessment of risk and likelihood of an adequate immune response.
Grade C; BEL 4
Recommendation 28.7
Tetanus-diphtheria-pertussis (Tdap) vaccine is typically included with routine childhood vaccinations. However, all adults with DM should receive a tetanus-diphtheria (Td) booster every 10 years.
Grade C; BEL 4
Recommendation 28.8
Health care professionals may consider recommending vaccines for the following diseases for persons with T2D based on individual needs: Tdap - tetanus, diphtheria, and pertussis (whooping cough); measles/mumps/rubella; varicella (chicken pox); and polio. In addition, persons traveling to other countries may require vaccines for endemic diseases.
Grade D; BEL 4, expert opinion of task force
Recommendation 28.9
Due to the increased risk for serious complications of COVID-19, persons with DM should be vaccinated against COVID-19 according to current guidelines.
Grade B; BEL 2
Recommendation 28.10
Recombinant zoster vaccine (RZV) is recommended for adults aged ≥50 years for protection against shingles according to the CDC/ACIP vaccination schedule.
Grade A; BEL 1
Recommendation 28.11
Health care professionals should utilize interventions with demonstrated effectiveness in increasing vaccination rates to improve uptake of vaccination among persons with DM.
Grade B; BEL 2
Evidence Base 28: Which vaccinations should be given to persons with DM?
Bacterial and viral infections cause significant morbidity and mortality in persons with DM.1824 A cohort study of adults <65 years of age with DM showed that DM increased the risk of influenza-associated hospitalizations by 6% (risk ratio, 1.06; 95% CI, 1.02–1.10; absolute risk difference 6 per 1000 adults per year), even though the rates of influenza and pneumonia were similar between diabetic and nondiabetic populations (P = .11).1825 Both community-acquired and nosocomial infections with pneumococcal bacteria may also be higher among persons with DM, who may also be at greater risk of death from these diseases.1826-1828 However, vaccines can safely and effectively reduce serious complications from influenza. A systematic review found reduction in all-cause mortality ranging between 33% and 68% among persons older than 65 years with DM and with seasonal influenza vaccination.1829 Other systematic reviews have demonstrated effective immunogenicity of influenza vaccine with decreased risk for hospitalization and mortality among persons with DM (especially those aged >65 years) compared with healthy individuals.1830,1831 An RCT evaluating the safety of the inactivated influenza vaccine in persons with DM compared with controls found that the vaccine was tolerated with mild-to-moderate adverse effects and with similar immune response among persons with DM compared to those without DM.1832 CDC/ACIP recommends a yearly influenza vaccine for all individuals with DM, although live attenuated influenza vaccine should be used with caution because its safety in persons with DM has not been established.1823 Inactivated influenza vaccine may be considered for persons with DM.1833 The CDC also provides references and resources related to influenza.1834
The CDC/ACIP also recommends a single dose of PCV15 or PCV20 for adults with DM who have not previously received PCV or whose previous vaccination history is unknown). When PCV15 is used, it should be followed by a dose of PPSV23.1835 The updated CDC recommendations are based on several trials demonstrating safety and immunogenicity of the new conjugate vaccines, PCV15 and PCV20, which were comparable to PCV13. No studies of clinical efficacy studies were included.
Hepatitis B vaccination is recommended for all persons with DM aged 59 years or younger and should be considered for persons 60 years or older with shared clinical decision-making based on risk assessment and likelihood of an adequate immune response. A prospective, multicenter RCT found that seroprotection following hepatitis B vaccination was lower among persons with DM compared with non-DM individuals and tended to wane with older age.1836 A 2-dose hepatitis B vaccine, HBsAg-1018, had greater seroprotection rates (90%) among persons with DM compared with the 3-dose hepatitis B vaccine (65.1%) 28 weeks after vaccination.1837 A similar finding was reported by another RCT involving persons with DM and CKD.1838
Individuals with DM, when infected with COVID-19, are more likely to be hospitalized, need higher levels of care, and have higher mortality.1839,1840 COVID-19 vaccination has demonstrated efficacy in reducing these adverse outcomes. Hence, individuals with DM should be vaccinated once eligible according to current recommendations.1841,1842
The CDC recommends the tetanus, diphtheria, and acellular pertussis (Tdap) vaccine as part of the child/adolescent immunization schedule and for adults every 10 years due to waning immunity.1843 Persons with DM may be more susceptible to respiratory infections and tetanus. A meta-analysis of observational studies found an increased risk of respiratory infections in persons with DM (odds ratio, 1.35; 95% CI, 1.28–1.43).1844 Although tetanus infections overall are rare, a surveillance study reported an increased risk of mortality in persons over age 65 (relative risk, 5.1; 95% CI, 2.1–12.2) and in persons with DM (relative risk, 2.4; 95% CI, 1.2–4.8).1845 Tdap is also recommended for tetanus prophylaxis in wound management, which could be important for persons with DM who have foot ulcers.1846
The recombinant zoster vaccine (RZV) is recommended for adults 50 years of age and older according to the CDC/ACIP schedule.1823 A large meta-analysis found that older adults who received RZV had a lower incidence of herpes zoster (relative risk, 0.08, 95% CI, 0.03–0.23) after over 3 years of follow-up compared with placebo. Following vaccination, a 2019 meta-analysis found that persons who received RZV had a mild-to-moderate systemic or injection site reaction, but no serious adverse effects or death compared with placebo.1847 An RCT reported a 100% vaccine response rate following RZV injection given either via subcutaneous or intramuscular route.1848 Coadministration of RZV with PPSV23 in adults aged ≥50 years in a 2018 RCT resulted in no immunologic interference or safety concerns between the 2 vaccines.1849 The live attenuated zoster vaccine, which was found to be safe and effective in reducing herpes zoster among adults ≥50 years,1847,1850 is no longer available for use in the United States.
Interventions with demonstrated effectiveness to increase vaccination rates include strategies that involve convenience of vaccinations, better communication with persons, enhanced vaccination systems with motivation by designated vaccination champions and the coadministration of compatible vaccines while avoiding vaccine coadministration that may decrease immune response to individual vaccines.1851-1855 These strategies should be utilized to improve vaccination rates in the DM population. The CDC Standards for Immunization Practice support clinicians in addressing low vaccination rates (https://www.cdc.gov/vaccines/hcp/adults/for-practice/standards/index.html). The practice standards include the following key points:
- Assess immunization status of all persons with DM at every encounter.
- Stay up to date on the latest recommendations from the CDC (https://www.cdc.gov/vaccines/schedules/index.html). Vaccination recommendations change frequently, so clinicians should always find the most recent recommendations before advising their patients.
- Implement policies or workflow changes to facilitate review of immunizations by care team staff and to provide patient reminders.
- Strongly recommend any vaccines a person may need (see Table 21 for vaccine recommendations for adults with DM).
- Provide a nonjudgmental environment to address any questions or concerns.
- A strong recommendation from a clinician is the best predictor for a person choosing to get immunized.
Administer vaccines, if stocked in practice, or refer persons with DM to local vaccine providers, which may include primary care clinicians, pharmacies, or public health offices.
Document administered or received vaccines in the electronic health record and the state immunization registry.
Future Directions
DM is a paradoxical condition in which most clinicians are familiar with its prevalence and impact on health, but many are not fully knowledgeable about all the nuances of optimal management. Simultaneously, the advance of big data, outcome studies, and therapeutic and monitoring capabilities have shifted the paradigm of DM management.
Reflecting the evolving natural history of T2D in the United States, screening for DM should start at 35 years of age. Although the glycemic criteria for the official diagnosis of DM have not changed, it has been increasingly apparent that those with prediabetes need to have their CVD risk factors managed as aggressively as those with DM. The impact of health disparities and adverse SDOH in both developed and developing nations have been shown to affect the QoL as well as metabolic control in those with DM. However, there is need for interventional studies in these areas to demonstrate improvement in DM outcomes. Access to proper nutrition and effective medicines will continue to be a difficult challenge.
For those with prediabetes/DM and obesity, it is paramount that achieving and maintaining effective weight loss is the key to improving glycemic control as well as management of CVD risk, neuropathy, OSA, and other complications of DM. Lifestyle optimization remain the cornerstone treatment for those with DM. The arrival of new peptide therapies may approach weight loss observed with bariatric procedures should revolutionize obesity management. Avoidance of weight gain or weight loss have become key differentiators of therapeutic choices.
We have primarily classified DM into 2 types based on insulin deficiency vs insulin resistance and the presence of autoimmune destruction of the endocrine pancreas. As DM management moves to personalized approaches in the era of precision medicine, there have been early efforts to subcategorize T2D based on phenotype and metabolic characteristics. Pharmacogenomic strategies related to prediction of drug efficacy and/or safety will be incorporated into clinical practice. Some of the subtypes of DM include monogenic forms, and genetic screening will likely become more cost-effective to inform clinicians about the most appropriate treatments and genetic counseling. Secondary forms of DM, such as posttransplant diabetes and CFRD should be screened in order to begin earlier and timely pathophysiologic-focused treatment.
Adverse outcomes that can occur in the inpatient setting or during pregnancy need to be further studied with the use of CGM technology. CGM and its metrics provide a deeper description of glycemia that should further complement the use of A1C and lead to improved clinical insights into both hyperglycemia and hypoglycemia. The safety net provided by CGM cannot be discounted, for CGM studies may provide more information about the frequency and impact of level one hypoglycemia. Select workers such as commercial drivers and pilots have been highly regulated with respect to concurrent DM. Recently, these regulations have been updated allowing persons with DM to preserve their occupation. There will be more progress toward developing an “artificial pancreas” with closed-loop insulin delivery and/or faster-acting prandial and longer-lasting basal insulins and/or delivery systems providing both insulin and glucagon as well as the potential for smart insulins and insulin delivery into the portal system.
The default aggressive management of hypertension and dyslipidemia in those with DM is universally accepted. For those with established ASCVD, options such as PCSK9 targeted therapies allow even greater reduction in LDL-C levels. The pathogenic involvement of apo B-100 in atherosclerosis is well understood; more CVOTs examining apo B-100 as a metabolic target may inform revisions of future recommendations.
The SGLT2i and the GLP-1 RA classes have CV benefit independent of glucose-lowering mechanisms. There likely will be greater utilization of these agents in persons without DM by our primary care and cardiology colleagues as well as endocrinologists.
DKD, retinopathy, and neuropathy remain prominent microvascular complications. Use of retinal photographs evaluated by artificial intelligence programs promise increased and potentially more accurate retinopathy screening. In addition, newer therapies, such as anti-VEGF intravitreal injections with or without concomitant laser therapy have revolutionized the management of macular edema and vision-threatening retinopathy while reducing adverse effects of treatment. Diabetic neuropathy, including peripheral sensorimotor loss, autonomic dysfunction, and cardiac autonomic neuropathy are not uncommon in persons with DM; earlier recognition and new, more efficacious therapies are needed.
This task force has incorporated the latest landmark CVOTs and other RCTs examining the effect of antihyperglycemic agents on CVD, HF, and CKD outcomes and has made strong recommendations for appropriate use of SGLT-2is and GLP-1 RAs in ASCVD, HF, and cerebrovascular disease. These recommendations along with development of new therapies and further ASCVD, HF, and CKD outcome studies are likely to enhance the ability to care for those with these disorders, including some without DM.
Glycemic management will be more oriented to use of agents that do not cause hypoglycemia, that promote weight loss and those that reduce risk of cardio-renal disease. Both long-acting and rapid-acting insulin analogs have expanded options for persons who require insulin. CGM and insulin pumps with or without AID allow persons to achieve glycemic goals more safely.
Avoidance of hypoglycemia is key to achieving euglycemia safely, which is possible with newer antihyperglycemic therapies and the use of CGM. Additionally, treatment of severe hypoglycemia will be greatly improved with the advent of newer formulations of and delivery methods for glucagon and glucagon analogs. More work is needed to discover methods to restore hypoglycemic awareness.
To paraphrase Dr. Eliot Joslin, “The person with diabetes who knows the most, lives the longest.” In the 21st century, multidisciplinary education can be personalized with respect to age group, type of diabetes, language, and location. The task force reviewed hundreds of articles uncovering many innovative approaches to delivery of diabetes education. There is no single platform or approach that will benefit every individual. The key will be to find the right approach for the right patient.
Mental health is often suboptimally managed in those with DM, often due to time and resource constraints. There is need for more professional mental health expertise to address this burgeoning need. Sleep apnea ishighly prevalent buta neglectedcomorbidity inpersons with T2D; early diagnosis and future advances in management may contribute to improved DM control. Since persons with DM are more susceptible to more severe infections, validated proven vaccinations, as recommended by public health agencies, should be more consistently administered to those who would benefit from them.
We address virtual telemedicine, virtual/digital medicine, which has risen to prominence during the COVID-19 pandemic. Early evidence suggests that virtual care can be a satisfying experience for the clinician and the patient and lead to comparable outcomes. In the near future, telemedicine will become seamlessly integrated into traditional care programs, improving access to care. Patient-generated data will be incorporated into one’s medical record allowing more informed medical decisions. Artificial intelligence and machine-learning applications will lead to unexpected clinical insights.
Conclusions
A number of newer antihyperglycemic therapies have enhanced safety with reduced or very low risk for hypoglycemia, and at least two classes, SGLT2is and GLP-1 RAs, have been found to reduce the risk of CVD, HF, and/or CKD, independent of glycemic control. Medical management of obesity continues to advance with significant improvements in weight loss. Insulin formulations are available to address more individual lifestyles and medical profiles. Insulin delivery platforms and CGM technologies are improving rapidly and converging toward a closed-loop system.
Future improvements in the organization of health care delivery are critical for overall management of DM and will require coordination and cooperation from a multidisciplinary team that is patient centered and uses shared decision-making.
Despite the optimism from recent developments, access remains a significant challenge and will require, in future, greater cooperation of public and private sectors.
Supplementary Material
Acknowledgments
We thank the CPG Oversight Committee for their thoughtful reviews and insightful comments on this guideline update. We also thank Stephanie Adams, PhD, for assistance in assigning evidence levels, study types, and references; Melanie Bird, PhD, MSAM, for assistance with evidence review, document review, and references; Kendall Alexander, MPH, for assistance with document review; and Amy Ogunsulade, BS, for assistance with references.
Funding
This CPG on developing a comprehensive plan for the care of persons with diabetes mellitus was developed with financial support from the American Association of Clinical Endocrinology (AACE). All members who served on this AACE task force completed work on the manuscript electronically and met via video conferences. AACE received no outside funding for the development of this guideline. Volunteer authors on this task force received no remuneration for their participation in development of this guideline.
Abbreviations
- AACE
American Association of Clinical Endocrinology
- ABCD
adiposity-based chronic disease
- ABPM
ambulatory BP monitoring
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ACE
angiotensin-converting enzyme
- ACIP
CDC Advisory Committee on Immunization Practices
- ADA
American Diabetes Association
- ADVANCE
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- AHA
American Heart Association
- AHEI
Alternative Healthy Eating Index
- aHR
adjusted hazard ratio
- AID
automated insulin delivery
- AKI
acute kidney injury
- apo B
apolipoprotein B
- ARB
angiotensin II receptor blocker
- ASCVD
atherosclerotic cardiovascular disease
- A1C
hemoglobin A1c
- BG
blood glucose
- BGM
blood glucose monitoring
- BMI
body mass index
- BP
blood pressure
- CAN
cardiovascular autonomic neuropathy
- CDC
Centers for Disease Control and Prevention
- CDCES
certified diabetes care and education specialist
- CFRD
cystic fibrosiserelated diabetes
- CGM
continuous glucose monitoring
- CHF
congestive heart failure
- CKD
chronic kidney disease
- CKD-EPI
Chronic Kidney Disease Epidemiology
- CMD
cardiometabolic disease
- CPAP
continuous positive airway pressure
- CPG
clinical practice guideline
- CRP
C-reactive protein
- CSII
continuous subcutaneous insulin infusion
- CV
cardiovascular
- CVA
cerebrovascular accident
- CVD
cardiovascular disease
- CVOT
cardiovascular outcome trial
- DASH
Dietary Approaches to Stop Hypertension
- DKA
diabetic ketoacidosis
- DKD
diabetic kidney disease
- DM
diabetes mellitus
- DPN
diabetic peripheral neuropathy
- DPP-4
dipeptidyl peptidase 4
- DSMES
diabetes self-management education and support
- eGFR
estimated glomerular filtration rate
- EL
evidence level
- FDA
Food and Drug Administration
- FIELD
Fenofibrate Intervention and Event Lowering in Diabetes
- FPG
fasting plasma glucose
- GDM
gestational diabetes mellitus
- GFR
glomerular filtration rate
- GI
gastrointestinal
- GLP-1 RA
glucagon-like peptide-1 receptor agonist
- HAPO
Hyperglycemia and Pregnancy Outcomes
- HCL
hybrid closed-loop
- HDL-C
high-density lipoprotein cholesterol
- HEI
Healthy Eating Index
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HR
hazard ratio
- HRV
heart rate variability
- IC
insulin to carbohydrate
- ICU
intensive care unit
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- isCGM
intermittently scanned CGM
- ISF
insulin sensitivity factor
- IV
intravenous
- KDIGO
Kidney Disease: Improving Global Outcomes
- LDL-C
low-density lipoprotein cholesterol
- MACE
major adverse cardiovascular event
- MDI
multiple daily injections
- MI
myocardial infarction
- MNT
medical nutrition therapy
- MODY
maturity-onset diabetes of the young
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NPH
Neutral Protamine Hagedorn
- OGTT
oral glucose tolerance test
- OR
odds ratio
- OSA
obstructive sleep apnea
- PCSK9
proprotein convertase subtilisin/kexin type 9
- PCV
pneumococcal conjugate vaccine
- PG
plasma glucose
- POC
point-of-care
- PPG
postprandial glucose
- PTDM
posttransplant diabetes
- PTH
parathyroid hormone
- QoL
quality of life
- RAAS
renin-angiotensin-aldosterone system
- RCT
randomized controlled trial
- RDN
registered dietitian nutritionist
- rtCGM
real-time CGM
- RZV
recombinant zoster vaccine
- SAP
sensor-augmented pump
- SDOH
social determinants of health
- SGLT2i
sodiumglucose cotransporter 2 inhibitor
- SU
sulfonylurea
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- Tdap
tetanus-diphtheria-pertussis
- TDD
total daily dose
- TIR
time in range
- TZD
thiazolidinedione
- UACR
urine albumin-to-creatinine ratio
- UKPDS
UK Prospective Diabetes Study
- US
United States
- VEGF
vascular endothelial growth factor
- VLDL
very low-density lipoprotein
- WC
waist circumference
Footnotes
Disclaimer: American Association of Clinical Endocrinology clinical practice guidelines include systematically developed recommendations to assist health care professionals in medical decision-making for specific clinical conditions. Most of the content herein is based on scientific evidence. In areas of uncertainty, or when clarification is required, expert opinion and professional judgment were applied.
This guideline is a working document that reflects the state of the field at the time of publication. Since rapid changes in this area are expected, periodic revisions are inevitable. We encourage health care professionals to use this information in conjunction with their best clinical judgment. The presented recommendations may not be appropriate in all situations. Any decision(s) by health care professionals to apply the recommendations provided in this guideline, including prescribing of any medications, must be made in consideration of the recommendations presented, the most recently published prescribing information for medications, local resources, and individual patient circumstances.
Disclosures and Conflicts of Interest Policy
The Task Force was empaneled in accordance with AACE’s Conflict of Interest (COI) Policy and approved by the AACE COI Subcommittee. All members of the expert Task Force completed AACE’s disclosure form regarding any multiplicities of interests related to commercial and direct financial relationships within the preceding 12 months with companies that develop products connected with endocrine disorders. Categories for disclosure include employment, stock or other ownership, direct financial relationships (eg, speaker or consultant), research funding, authorship or panel involvement on a guideline related to an overlapping topic, or other situations related to a perceived COI. The AACE COI Subcommittee reviewed these disclosures against an AACE-approved list of affected companies for this guideline and reached consensus regarding members who could serve on the Task Force in the nonconflicted majority, those who could serve in the conflicted minority with management strategy, and those who were disqualified from serving on the Task Force. The AACE CPG Oversight Committee reviewed and approved the AACE COI Subcommittee’s decisions regarding manageable COI and empanelment. Members of this Task Force were reminded to update potential disclosures if new potential conflicts arose during their appointments and to verifycurrency of disclosures. AACE made every effort to minimize the potential for conflicts of interest that could influence the recommendations of this CPG.
Disclosures
Chair
Lawrence Blonde, MD, FACP, MACE, Endocrinology Department, Ochsner Health, New Orleans, Louisiana
consultant: Merck; consultant, payment to institution: Corcept Therapeutics, Janssen Pharmaceuticals, Lyndra Therapeutics Inc., Novo Nordisk, Salix Pharmaceuticals; consultant/speaker, payment to institution: Sanofi
Vice Chair
Guillermo E. Umpierrez, MD, CDCES, MACP, FACE, Emory University, Atlanta, Georgia
research support and national or overall principal investigator: AstraZeneca, Dexcom, Novo Nordisk; President, Medicine & Science: American Diabetes Association; guideline/white paper development: Endocrine Society e Hospital Diabetes Guidelines, Society of Critical Care e ICU Diabetes Guidelines
Team Leaders
S. Sethu Reddy, MD, MBA, FACP, MACE, Central Michigan University, Mount Pleasant, Michigan
reports no potential conflicts of interest
Janet B. McGill, MD, FACP, FACE, Washington University in St. Louis, St. Louis, Missouri
consultant: Bayer, Boehringer Ingelheim, Eli Lilly and Company, Salix; research support and national or overall principal investigator: Beta Bionics, Dexcom, Medtronic, Novo Nordisk
Task Force Members
Sarah L. Berga, MD, University at Buffalo, Buffalo, New York
consultant: Ava AG; Ferring Pharmaceuticals SW Reproductive Medicine and Maternal Health Advisory Board; ClearBlue Medical Advisory Board; NovoNordisk; Amazon Affiliates; editorial boards and positions (gratis): American Journal of Obstetrics and Gynecology Advisory Board, Human Reproduction Update Associate Editor, International Society for Gynecological Endocrinology Executive Committee Member, Journal of Obstetrics and Gynecology Canada International Editorial Board, Mayo Clinic Proceedings Editorial Board, Menopause Editorial Board, UpToDate Peer Review Board; service: Salem Academy and College Board of Trustees Member
Michael Bush, MD, FACE, Cedars-Sinai, Beverly Hills, California
consultant: Eli Lilly and Company
Suchitra Chandrasekaran, MD, MSCE, FACOG, Emory University, Atlanta, Georgia
reports no potential conflicts of interest
Ralph A. DeFronzo, MD, University of Texas, San Antonio, Texas
advisory board: AstraZeneca, Boehringer Ingelheim, Intarcia, Novo Nordisk; research support: AstraZeneca, Boehringer Ingel-heim, Merck; speakers’ bureau: AstraZeneca
Daniel Einhorn, MD, FACP, FACE, Scripps Whittier Diabetes Institute, La Jolla, California
consultant/speakers’ bureau: Abbott, Boehringer Ingelheim, Corcept Therapeutics, Eli Lilly and Company, Epitracker, GlySens Incorporated, Intuity Medical, Janssen Pharmaceuticals, Novo Nordisk; research support: AstraZeneca, Eli Lilly and Company, Mylan, Novo Nordisk
Rodolfo J. Galindo, MD, FACE, Emory University, Atlanta, Georgia
consultant: Boehringer Ingelheim, Eli Lilly and Company, Sanofi, Weight Watchers; research support to Emory University for investigator-initiated studies and national or overall principal investigator: Dexcom, Eli Lilly and Company, Novo Nordisk; partially supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under Award Numbers P30DK111024, K23DK123384
Thomas W. Gardner, MD, MS, University of Michigan, Ann Arbor, Michigan
research funding: American Diabetes Association, JDRF, National Institutes of Health, Novo Nordisk Fond, Taubman Medical Research Institute and Coulter Translational Research Program at the University of Michigan; prior industry funding: Zebra Biologics
Rajesh Garg, MD, Lundquist Institute/Harbor-UCLA Medical Center, Torrance, California
research support and national or overall principal investigator: Duke Clinical Research Institute, NIH/NIDDK, Quest Diagnostics
W. Timothy Garvey, MD, FACE, University of Alabama at Birmingham, Birmingham, Alabama
volunteer advisory board member without receipt of financial compensation: Boehringer Ingelheim, JAZZ Pharmaceuticals, Novo Nordisk, and Pfizer; site principal investigator for multi-centered clinical trials sponsored/funded through university: Eli Lilly, Epitomee, Novo Nordisk, and Pfizer
Irl B. Hirsch, MD, University of Washington, Seattle, Washington
advisory board: Abbott, Bigfoot, GWave, Roche; research support: Beta Bionics, Insulet, Medtronic Diabetes Care; guideline/white paper development: American Diabetes Association/European Association for the Study of Diabetes e Management of Type 1 Diabetes consensus report, Endocrine Society e Guidelines for Inpatient Diabetes Management
Daniel L. Hurley, MD, FACE, Mayo Clinic, Rochester, Minnesota
reports no potential conflicts of interest
Kenneth Izuora, MD, MBA, FACE, University of NevadaeLas Vegas, Las Vegas, Nevada
research support: Novo Nordisk
Mikhail Kosiborod, MD, FACC, FAHA, Saint Luke’s Mid America Heart Institute, Kansas City, Missouri
consultant/advisory board: Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly and Company, Esperion Therapeutics, Janssen, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Vifor Pharma; research support and national or overall principal investigator: Astra Zeneca, Boehringer Ingelheim; national or overall principal investigator: Novo Nordisk; guideline/white paper development: American College of Cardiology review of American Diabetes Association’s Standards of Care
Darin Olson, MD, Colorado Mountain Medical, LLC, Avon, Colorado
research support and site investigator: AbbVie, Kowa
Shailendra B. Patel, MD, PhD, University of Cincinnati Medical Center, Cincinnati, Ohio
speaker: Rhythm Pharmaceuticals Inc.
Rodica Pop-Busui, MD, PhD, University of Michigan, Ann Arbor, Michigan
consultant: Averitas Pharma, Bayer, Boehringer Ingelheim, Nevro, Regenacy; research support: AstraZeneca; steering committee: Novo Nordisk (SOUL CVOT)
Archana R. Sadhu, MD, Houston Methodist; Weill Cornell Medicine; Texas A&M College of Medicine; Houston, Texas
advisory board: Abbott
Susan L. Samson, MD, PhD, FRCPC, FACE, Mayo Clinic, Jacksonville, Florida
research support, steering committee, and national or overall principal investigator: Chiasma, Novartis
Carla Stec, MA, American Association of Clinical Endocrinology, Jacksonville, FL
reports no potential conflicts of interest
William V. Tamborlane Jr, MD, Yale School of Medicine, New Haven, Connecticut
research support: AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Takeda
Katherine R. Tuttle, MD, FASN, FACP, FNKF, University of Washington and Providence Health Care, Seattle and Spokane, Washington
consultant: AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly and Company, Gilead, Goldfinch Bio, Novo Nordisk; research support and national or overall principal investigator: Bayer, Goldfinch Bio; research funding: Novo Nordisk; guideline/white paper development: Kidney Disease: Improving Global Outcomes & American Society of Nephrology DKD Collaborative e Clinical Practice Guidelines and white paper on diabetes and CKD
Christine Twining, MD, FACE, Maine Medical Center, Scarborough, Maine
reports no potential conflicts of interest
Adrian Vella, MD, Mayo Clinic, Rochester, Minnesota
consultant: Crinetics Pharmaceuticals, Rezolute, vTv Therapeutics, Zeeland Pharmaceuticals; research support and national or overall principal investigator: Novo Nordisk; Editor in Chief: Metabolic Syndrome & Related Disorders; Associate Editor: Diabetes Care
Priyathama Vellanki, MD, Emory University, Atlanta, Georgia
consultant: Takeda Pharmaceutical Company; research support and national or overall principal investigator: NIH K23 DK 11324–01A1
Sandra L. Weber, MD, FACP, FACE, University of South Carolina School of Medicine-Greenville, Prisma Health System, Greenville, South Carolina
research support and national or overall principal investigator: Novo Nordisk
Panel Composition
The Task Force was empaneled in accordance with AACE’s COI Policy. This evidence-based CPG was developed by a group of credentialed medical professionals in the fields of endocrinology, cardiology, neurology, nephrology, obstetrics and gynecology, ophthalmology, and pediatrics, and a methodologic specialist.
Review Process
Drafts of this guideline were reviewed and approved by all task force members, the AACE CPG Oversight Committee, the AACE Board of Directors, and peer reviewers for Endocrine Practice.
Updating Policy
AACE reviews and updates or retires its evidence-based guidelines every 3 to 5 years or after significant scientific developments or change in public policy as determined by the AACE executive leadership, AACE CPG Oversight Committee, and relevant AACE Disease-State Network.
Document Expiration Date: October 2025
References are followed by an evidence level [EL] rating of 1, 2, 3, or 4. See Supplementary Table 1 for additional information about evidence level ratings. References with an “a” suffix denote late-breaking or supplementary references.
References
- 1.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract 2015;21(suppl 1):1–87. 10.4158/EP15672. GL [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mechanick JI, Pessah-Pollack R, Camacho P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology protocol for standardized production of clinical practice guidelines, algorithms, and checklists - 2017 update. Endocr Pract 2017;23(8):1006–1021. 10.4158/EP171866. GL [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Wang D, Matsushita K, Grams ME, Coresh J. Prognostic implications of single-sample confirmatory testing for undiagnosed diabetes: A prospective cohort study. Ann Intern Med 2018;169(3):156–164. 10.7326/M18-0091 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacks DB. A1c vs glucose testing: A comparison. Diabetes Care 2011;34(2): 518–523. 10.2337/dc10-1546 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karnchanasorn R, Huang J, Ou HY, et al. Comparison of the current diagnostic criterion of HbA1c with fasting and 2-hour plasma glucose concentration. J Diabetes Res 2016;2016:6195494. 10.1155/2016/6195494 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broome DT, Pantalone KM, Kashyap SR, Philipson LH. Approach to the patient with mody-monogenic diabetes. J Clin Endocrinol Metab 2021;106(1): 237–250. 10.1210/clinem/dgaa710 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1C as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysis. BMJ Open 2017;7(7):e015949. 10.1136/bmjopen-2017-015949 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 2010;33(3):562–568. 10.2337/dc09-1524 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S17–S38. 10.2337/dc22-S002 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 10.Gillett MJ. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes: Diabetes care. Clin Biochem Rev 2009;30(4): 197–200, 32(7):1327–1334. [EL 4; NE]. [PMC free article] [PubMed] [Google Scholar]
- 11.Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL. Effect of ethnicity on HbA1c levels in individuals without diabetes: systematic review and meta-analysis. PLOS ONE 2017;12(2):e0171315. 10.1371/journal.-pone.0171315 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F, Moellering DR, Garvey WT. Use of HbA1c for diagnoses of diabetes and prediabetes: comparison with diagnoses based on fasting and 2-hr glucose values and effects of gender, race, and age. Metab Syndr Relat Disord 2014;12(5):258–268. 10.1089/met.2013.0128 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman WH. Are there clinical implications of racial differences in HbA1c? Yes, to not consider can do great harm. Diabetes Care 2016;39(8):1458–1461. 10.2337/dc15-2686 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacy ME, Wellenius GA, Sumner AE, et al. Association of sickle cell trait with hemoglobin A1c in African Americans. JAMA 2017;317(5):507–515. 10.1001/jama.2016.21035 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson AD. HbA1c for type 2 diabetes diagnosis in Africans and African Americans: personalized medicine now. PLOS Med 2017;14(9):e1002384. 10.1371/journal.pmed.1002384 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler E, Leong A, Liu CT, et al. Impact of common genetic determinants of hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis. PLOS Med 2017;14(9): e1002383. 10.1371/journal.pmed.1002383 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1C levels. Ann Intern Med 2017;167(2):95–102. 10.7326/M16-2596 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 18.Versantvoort AR, van Roosmalen J, Radder JK. Course of HbA1c in non-diabetic pregnancy related to birth weight. Neth J Med 2013;71(1):22–25 [EL 3; CCS]. [PubMed] [Google Scholar]
- 19.Khalafallah A, Phuah E, Al-Barazan AM, et al. Glycosylated haemoglobin for screening and diagnosis of gestational diabetes mellitus. BMJ Open 2016;6(4): e011059. 10.1136/bmjopen-2016-011059 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26(11):3160–3167. 10.2337/diacare.26.11.3160 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 21.Orchard TJ, Temprosa M, Barrett-Connor E, et al. Long-term effects of the diabetes prevention program interventions on cardiovascular risk factors: A report from the DPP Outcomes Study. Diabet Med J Br Diabet Assoc 2013;30(1):46–55. 10.1111/j.1464-5491.2012.03750.x [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buzzetti R, Zampetti S, Maddaloni E. Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol 2017;13(11):674–686. 10.1038/nrendo.2017.99 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 23.. Evans-Molina C, Sims EK, DiMeglio LA, et al. В cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight 2018;3(15):e120877. 10.1172/jci.insight.120877 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: A scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38(10):1964–1974. 10.2337/dc15-1419 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.. Oikawa Y, Tanaka H, Uchida J, et al. Slowly progressive insulin-dependent (type 1) diabetes positive for anti-GAD antibody ELISA test may be strongly associated with a future insulin-dependent state. Endocr J 2017;64(2): 163–170. 10.1507/endocrj.EJ16-0328 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 26.Sosenko JM, Skyler JS, DiMeglio LA, et al. A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 2015;38(2):271–276. 10.2337/dc14-1813 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steck AK, Vehik K, Bonifacio E, et al. Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: the environmental determinants of diabetes in the young (TEDDY). Diabetes Care 2015;38(5):808–813. 10.2337/dc14-2426 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32(7):1335–1343. 10.2337/dc09-9032 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerji MA, Chaiken RL, Huey H, et al. GAD antibody negative NIDDM in adult Black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4. Flatbush diabetes. Diabetes 1994;43(6): 741–745. 10.2337/diab.43.6.741 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 30.Vellanki P, Umpierrez GE. Diabetic ketoacidosis: A common debut of diabetes among African Americans with type 2 diabetes. Endocr Pract 2017;23(8): 971–978. 10.4158/EP161679. RA [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vellanki P, Smiley DD, Stefanovski D, et al. Randomized controlled study of metformin and sitagliptin on long-term normoglycemia remission in African American patients with hyperglycemic crises. Diabetes Care 2016;39(11): 1948–1955. 10.2337/dc16-0406 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci 2020;21(17). 10.3390/ijms21176275 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: A pathophysiologic and therapeutic makeover. J Clin Invest 2012;122(1):4–12. 10.1172/JCI60016 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104(6):787–794. 10.1172/JCI7231 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of pima indians. N Engl J Med 1993;329(27):1988–1992. 10.1056/NEJM199312303292703 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed January 6, 2021, 2020.
- 37.Lebovitz HE, Banerji MA. Ketosis-prone diabetes (Flatbush diabetes): an emerging worldwide clinically important entity. Curr Diabetes Rep 2018;18(11):120. 10.1007/s11892-018-1075-4 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razavi LN, Ebenibo S, Edeoga C, Wan J, Dagogo-Jack S. Five-year glycemic trajectories among healthy African-American and European-American offspring of parents with type 2 diabetes. Am J Med Sci 2020;359(5): 266–270. 10.1016/j.amjms.2020.03.005 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James D, Umekwe N, Edeoga C, Nyenwe E, Dagogo-Jack S. Multi-year reproducibility of hyperinsulinemic euglycemic clamp-derived insulin sensitivity in free-living adults: association with incident prediabetes in the pop-abc study. Metabolism 2020;109:154263. 10.1016/j.metabol.2020.154263 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia 1991;34(12):877–890 [EL 1; RCT]. [PubMed] [Google Scholar]
- 41.Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med 2008;359(15):1618–1620. 10.1056/NEJMe0807625 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 42.Naylor RN, Philipson LH. Diagnosis and clinical management of monogenic diabetes. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext South. MDText.com, Inc. 2020. MA: Dartmouth; 2021. [EL 4; NE]. [Google Scholar]
- 43.Barbetti F, D'Annunzio G. Genetic causes and treatment of neonatal diabetes and early childhood diabetes. Best Pract Res Clin Endocrinol Metab 2018;32(4): 575–591. 10.1016/j.beem.2018.06.008 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 44.Bennett JT, Vasta V, Zhang M, et al. Molecular genetic testing of patients with monogenic diabetes and hyperinsulinism. Mol Genet Metab 2015;114(3): 451–458. 10.1016/j.ymgme.2014.12.304 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chambers C, Fouts A, Dong F, et al. Characteristics of maturity onset diabetes of the young in a large diabetes center. Pediatr Diabetes 2016;17(5):360–367. 10.1111/pedi.12289 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Franco E, Flanagan SE, Houghton JA, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet 2015;386(9997):957–963. 10.1016/S0140-6736(15)60098-8 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson SR, Ellis JJ, Leo PJ, et al. Comprehensive genetic screening: the prevalence of maturity-onset diabetes of the young gene variants in a population-based childhood diabetes cohort. Pediatr Diabetes 2019;20(1): 57–64. 10.1111/pedi.12766 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd MH, Shields BM, Hudson M, et al. A UK nationwide prospective study of treatment change in mody: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin. Diabetologia 2018;61(12):2520–2527. 10.1007/s00125-018-4728-6 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warncke K, Kummer S, Raile K, et al. Frequency and characteristics of MODY 1 (HNF4A mutation) and MODY 5 (HNF1B mutation): analysis from the dpv database. J Clin Endocrinol Metab 2019;104(3):845–855. 10.1210/jc.2018-01696 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 50.Nankervis A, McIntyre HD, Moses R, et al. ADIPS consensus guidelines for the testing and diagnosis of hyperglycaemia in pregnancy in Australia and New Zealand (modified 2014) https://www.adips.org/downloads/2014ADIPSGDMGuidelinesV18.11.2014_000.pdf. Accessed January 14, 2022. [EL 4; NE].
- 51.Zhu WW, Yang HX, Wei YM, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care 2013;36(3):586–590. 10.2337/dc12-1157 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benhalima K, Mathieu C, Van Assche A, et al. Survey by the European Board and College of Obstetrics and Gynaecology on screening for gestational diabetes in Europe. Eur J Obstet Gynecol Reprod Biol 2016;201:197–202. 10.1016/j.ejogrb.2016.04.003 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 53.Miailhe G, Kayem G, Girard G, Legardeur H, Mandelbrot L. Selective rather than universal screening for gestational diabetes mellitus? Eur J Obstet Gynecol Reprod Biol 2015;191:95–100. 10.1016/j.ejogrb.2015.05.003 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization guideline. Diabetes Res Clin Pract 2014;103(3):341–363. 10.1016/j.diabres.2013.10.012 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 55.Hod M, Kapur A, Sacks DA, et al. The Interntational Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet 2015;S173–211(suppl 3):131. 10.1016/s0020-7292(15)30033-3 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 56.Lowe WL Jr, Scholtens DM, Kuang A, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 2019;42(3): 372–380. 10.2337/dc18-1646 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lowe WL Jr, Lowe LP, Kuang A, et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia 2019;62(4):598–610. 10.1007/s00125-018-4809-6 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964;13:278–285 [EL 4; NE]. [PubMed] [Google Scholar]
- 59.International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676–682. 10.2337/dc09-1848 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 190: Gestational diabetes mellitus ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol 2018;131(2):e49–e64. 10.1097/AOG.0000000000002501 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 61.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144(7):768–773. 10.1016/0002-9378(82)90349-0 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 62.Brown FM, Wyckoff J. Application of one-step IADPSG vs two-step diagnostic criteria for gestational diabetes in the real world: impact on health services, clinical care, and outcomes. Curr Diabetes Rep 2017;17(10):85. 10.1007/s11892-017-0922-z [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farrar D, Duley L, Dowswell T, Lawlor DA. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev 2017;8(8):Cd007122. 10.1002/14651858.CD007122.pub4 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358(19): 1991–2002. 10.1056/NEJMoa0707943 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 65.Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med 2021;384(10):895–904. 10.1056/NEJMoa2026028 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sacks DA, Hadden DR, Maresh M, et al. Frequency of gestational diabetes mellitus at collaborating centers based on iadpsg consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Diabetes Care 2012;35(3):526–528. 10.2337/dc11-1641 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on gestational diabetes mellitus. Diabetes Care 2007;30(suppl 2):S251–S260. 10.2337/dc07-s225 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 68.Egan AM, Dow ML, Vella A. A review of the pathophysiology and management of diabetes in pregnancy. Mayo Clin Proc 2020;95(12):2734–2746. 10.1016/j.mayocp.2020.02.019 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 69.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376(9739):419–430. 10.1016/S0140-6736(10)60576-4 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.ADVANCE Collaborative Group, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358(24):2560–2572. 10.1056/NEJMoa0802987 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez-Gutierrez R, Gonzalez-Gonzalez JG, Zuñiga-Hernandez JA, McCoy RG. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ 2019;367:l5887. 10.1136/bmj.l5887 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 72.Khaw KT, Wareham N, Bingham S, et al. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004;141(6):413–420. 10.7326/0003-4819-141-6-200409210-00006 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 73.Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract 2020;26(1):107–139. 10.4158/CS-2019-0472 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 74.National Institute for Health and Care Excellence (NICE). Type 1 diabetes in adults: diagnosis and management: NICE guideline [NG17] https://www.nice.org.uk/guidance/ng17. Accessed January 14, 2022. [EL 4; NE]. [PubMed]
- 75.American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 3. Prevention or delay of type 2 diabetes and associated comorbidities: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022;45(Suppl 1):S39–S45. 10.2337/dc22-S003 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 76.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358(24):2545–2559. 10.1056/NEJMoa0802743 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63(1):225–232. 10.1046/j.1523-1755.2003.00712.x [EL 3; DS]. [DOI] [PubMed] [Google Scholar]
- 78.Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329(14):977–986. 10.1056/NEJM199309303291401 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 79.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, Lachin JM, Genuth S, et al. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342(6):381–389. 10.1056/NEJM200002103420603 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360(2): 129–139. 10.1056/NEJMoa0808431 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 81.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359(15): 1577–1589. 10.1056/NEJMoa0806470 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 82.Riddle MC, Ambrosius WT, Brillon DJ, et al. Epidemiologic relationships between A1c and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010;33(5):983–990. 10.2337/dc09-1278 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schoenaker DA, Simon D, Chaturvedi N. Glycemic control and all-cause mortality risk in type 1 diabetes patients: the EURODIAB Prospective Complications Study. J Clin Endocrinol Metab 2014;99(3):800–807. 10.1210/jc.2013-2824 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 84.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: A retrospective cohort study. Lancet 2010;375(9713): 481–489. 10.1016/S0140-6736(09)61969-3 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 85.ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364(9):818–828. 10.1056/NEJMoa1006524 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zoungas S, Chalmers J, Ninomiya T, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 2012;55(3):636–643. 10.1007/s00125-011-2404-1 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 87.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ (Clin Res Ed) 2010;340: b4909. 10.1136/bmj.b4909 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glucose tolerance and mortality: comparison of who and American Diabetes Association diagnostic criteria. The decode study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999;354(9179):617–621 [EL 2; PCS]. [PubMed] [Google Scholar]
- 89.Lind M, Pivodic A, Svensson AM, et al. HbA(1c) level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ (Clin Res Ed) 2019;366:l4894. 10.1136/bmj.l4894 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28(2):103–117. 10.1016/0168-8227(95)01064-k [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 91.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87(3):978–982. 10.1210/jcem.87.3.8341 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 92.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011;34(2):256–261. 10.2337/dc10-1407 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Vught LA, Wiewel MA, Klein Klouwenberg PM, et al. Admission hyperglycemia in critically ill sepsis patients: Association with outcome and host response. Crit Care Med 2016;44(7):1338–1346. 10.1097/CCM.0000000000001650 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 94.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345(19):1359–1367. 10.1056/NEJMoa011300 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 95.NICE-SUGAR Study Investigators, Finfer S, Chittock DR, et al. Intensive vs conventional glucose control in critically ill patients. N Engl J Med 2009;360(13):1283–1297. 10.1056/NEJMoa0810625 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 96.Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multicentre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009;35(10):1738–1748. 10.1007/s00134-009-1585-2 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 97.NICE-SUGAR Study Investigators, Finfer S, Liu B, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012;367(12):1108–1118. 10.1056/NEJMoa1204942 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 98.Corazzari C, Matteucci M, Kołodziejczak M, et al. Impact of preoperative glycometabolic status on outcomes in cardiac surgery: systematic review and meta-analysis. J Thorac Cardiovasc Surg 2021. 10.1016/j.jtcvs.2021.05.035 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 99.Korytkowski MT, Muniyappa R, Antinori-Lent K, et al. Management of hyperglycemia in hospitalized adult patients in non-critical care settings: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2022;107(8):2101–2128. 10.1210/clinem/dgac278 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive vs conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care 2015;38(9): 1665–1672. 10.2337/dc15-0303 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003;125(5):1007–1021. 10.1067/mtc.2003.181 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 102.Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97(1):16–38. 10.1210/jc.2011-2098 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 103.Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diabetes Care 2015;38(12):e202–e203. 10.2337/dc15-1835 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358(2):125–139. 10.1056/NEJMoa070716 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 105.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32(6): 1119–1131. 10.2337/dc09-9029 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maynard G, Berg K, Kulasa K, O’Malley C, Rogers KM, eds. The Glycemic Control Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes 2nd ed. Society of Hospital Medicine; 2015. [EL 4; NE]. [Google Scholar]
- 107.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007;30(9):2181–2186. 10.2337/dc07-0295 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 108.Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care 2013;36(8):2169–2174. 10.2337/dc12-1988 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phillips VL, Byrd AL, Adeel S, et al. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus vs sliding scale insulin regimens. Pharmacoecon Open 2017;1(2):109–115. 10.1007/s41669-017-0020-9 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guerin A, Nisenbaum R, Ray JG. Use of maternal GHb concentration to estimate the risk of congenital anomalies in the offspring of women with prepregnancy diabetes. Diabetes Care 2007;30(7):1920–1925. 10.2337/dc07-0278 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 111.Jensen DM, Damm P, Moelsted-Pedersen L, et al. Outcomes in type 1 diabetic pregnancies: A nationwide, population-based study. Diabetes Care 2004;27(12): 2819–2823. 10.2337/diacare.27.12.2819 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 112.Wahabi HA, Alzeidan RA, Bawazeer GA, Alansari LA. Esmaeil SA Preconception care for diabetic women for improving maternal and fetal outcomes: A systematic review and meta-analysis. BMC Preg Childbirth 2010;10:63. 10.1186/1471-2393-10-63 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feldman AZ, Brown FM. Management of type 1 diabetes in pregnancy. Curr Diabetes Rep 2016;16(8):76. 10.1007/s11892-016-0765-z [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wahabi HA, Alzeidan RA. Esmaeil SA Pre-pregnancy care for women with pregestational diabetes mellitus: A systematic review and meta-analysis. BMC Public Health 2012;12:792. 10.1186/1471-2458-12-792 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abell SK, Boyle JA, Earnest A, et al. Impact of different glycaemic treatment targets on pregnancy outcomes in gestational diabetes. Diabet Med 2019;36(2):177–183. 10.1111/dme.13799 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 116.Crowther CA, Alsweiler JM, Hughes R, Brown J. TARGET Study Group Tight or less tight glycaemic targets for women with gestational diabetes mellitus for reducing maternal and perinatal morbidity? (TARGET): study protocol for a stepped wedge randomised trial. BMC Preg Childbirth 2018;18(1):425. 10.1186/s12884-018-2060-2 [EL 1; RCT protocol]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jovanovic-Peterson L, Peterson CM, Reed GF, et al. Maternal postprandial glucose levels and infant birth weight: the diabetes in early pregnancy study. Am J Obstet Gynecol 1991;164(1 Pt 1):103–111. 10.1016/0002-9378(91)90637-7 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 118.Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med 2019;32(17):2905–2914. 10.1080/14767058.2018.1449203 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 119.Cho HY, Jung I, Kim SJ. The association between maternal hyperglycemia and perinatal outcomes in gestational diabetes mellitus patients: A retrospective cohort study. Medicine 2016;95(36):e4712. 10.1097/MD.0000000000004712 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maresh MJ, Holmes VA, Patterson CC, et al. Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care 2015;38(1):34–42. 10.2337/dc14-1755 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 121.Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 2004;27(5):1200–1201. 10.2337/diacare.27.5.1200 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 122.de Veciana M, Major CA, Morgan MA, et al. Postprandial vs preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med 1995;333(19):1237–1241. 10.1056/NEJM199511093331901 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 123.Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): A multicentre international randomised controlled trial. Lancet 2017;390(10110):2347–2359. 10.1016/S0140-6736(17)32400-5 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Voormolen DN, DeVries JH, Sanson RME, et al. Continuous glucose monitoring during diabetic pregnancy (GlucoMOMS): A multicentre randomized controlled trial. Diabetes Obes Metab 2018;20(8):1894–1902. 10.1111/dom.13310 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 125.Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: A randomized controlled trial. Diabetes Care 2013;36(7):1877–1883. 10.2337/dc12-2360 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kristensen K, Ögge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia 2019;62(7):1143–1153. 10.1007/s00125-019-4850-0 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Márquez-Pardo R, Torres-Barea I, Córdoba-Doña JA, et al. Continuous glucose monitoring and glycemic patterns in pregnant women with gestational diabetes mellitus. Diabetes Technol Ther 2020;22(4):271–277. 10.1089/dia.2019.0319 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 128.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321(7258):405–412. 10.1136/bmj.321.7258.405 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 7. Diabetes technology: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S97–S112. 10.2337/dc22-S007 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 130.Little RR, Rohlfing CL, Sacks DB. National Glycohemoglobin Standardization Program (NGSP) Steering Committee Status of hemoglobin A1c measurement and goals for improvement: From chaos toorder for improving diabetes care. Clin Chem 2011;57(2):205–214. 10.1373/clinchem.2010.148841 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 131.Nathan DM, Kuenen J, Borg R, et al. Translating the A1c assay into estimated average glucose values. Diabetes Care 2008;31(8):1473–1478. 10.2337/dc08-0545 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dagogo-Jack S Pitfalls in the use of HbA₁(c) as a diagnostic test: the ethnic conundrum. Nat Rev Endocrinol 2010;6(10):589–593. 10.1038/nrendo.2010.126 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 133.Selvin E Are there clinical implications of racial differences in HbA1c? A difference, to be a difference, must make a difference. Diabetes Care 2016;39(8):1462–1467. 10.2337/dc16-0042 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rohlfing C, Hanson S, Little RR. Measurement of hemoglobin A1c in patients with sickle cell trait. JAMA 2017;317(21):2237. 10.1001/jama.2017.4643 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 135.Little RR, Rohlfing CL, Tennill AL, et al. Measurement of HbA(1c) in patients with chronic renal failure. Clin Chim Acta Int J Clin Chem 2013;418:73–76. 10.1016/j.cca.2012.12.022 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Herman WH Do race and ethnicity impact hemoglobin A1c independent of glycemia? J Diabetes Sci Technol 2009;3(4):656–660. 10.1177/193229680900300406 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Malanda UL, Welschen LM, Riphagen II, et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev 2012;1:CD005060. 10.1002/14651858.CD005060. pub3 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Freckmann G, Schmid C, Baumstark A, et al. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy: relevant differences among ISO 15197:2003, ISO 15197: 2013, and current FDA recommendations. J Diabetes Sci Technol 2015;9(4): 885–894. 10.1177/1932296815580160 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eerdekens GJ, Rex S, Mesotten D. Accuracy of blood glucose measurement and blood glucose targets. J Diabetes Sci Technol 2020;14(3):553–559. 10.1177/1932296820905581 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freckmann G, Pleus S, Grady M, Setford S. Levy B Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J Diabetes Sci Technol 2019;13(3):575–583. 10.1177/1932296818812062 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.International Organization for Standardization (ISO) ISO 15197:2013(en) in vitro diagnostic test systemsdrequirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus https://www.iso.org/obp/ui/#iso:std:iso:15197:ed-2:v1:en. Accessed January 11, 2022.
- 142.Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes 2011;12(1):11–17. 10.1111/j.1399-5448.2010.00650.x [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 143.Davidson PC, Bode BW, Steed RD. Hebblewhite HR A cause-and-effect-based mathematical curvilinear model that predicts the effects of self-monitoring of blood glucose frequency on hemoglobin A1c and is suitable for statistical correlations. J Diabetes Sci Technol 2007;1(6):850–856. 10.1177/193229680700100608 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care 2013;36(7): 2009–2014. 10.2337/dc12-1770 [EL 2; RCCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Minder AE, Albrecht D, Sch€afer J, Zulewski H. Frequency of blood glucose testing in well educated patients with diabetes mellitus type 1: how often is enough? Diabetes Res Clin Pract 2013;101(1):57–61. 10.1016/j.diabres.2012.12.024 [EL 3; CCS]. [DOI] [PubMed] [Google Scholar]
- 146.Elgart JF, González L, Prestes M, Rucci E. Gagliardino JJ Frequency of self-monitoring blood glucose and attainment of HbA1c target values. Acta diabetol 2016;53(1):57–62. 10.1007/s00592-015-0745-9 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 147.O'Kane MJ, Bunting B, Copeland M, Coates VE. ESMON study group Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ (Clin Res Ed) 2008;336(7654):1174–1177. 10.1136/bmj.39534.571644. BE [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Willett LR Acp journal club. Meta-analysis: self-monitoring in non-insulin-treated type 2 diabetes improved HbA1c by 0.25. Ann Intern Med 2012;156(12): JC6–JC12. 10.7326/0003-4819-156-12-201206190-02012 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 149.Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ 2007;335(7611):132. 10.1136/bmj.39247.447431. BE [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hortensius J, Kleefstra N, Landman GWD, et al. Effects of three frequencies of self-monitored blood glucose on HbA1c and quality of life in patients with type 2 diabetes with once daily insulin and stable control: A randomized trial. BMC Res Notes 2018;11(1):26. 10.1186/s13104-018-3138-7 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mannucci E, Antenore A, Giorgino F. Scavini M Effects of structured vs unstructured self-monitoring of blood glucose on glucose control in patients with non-insulin-treated type 2 diabetes: A meta-analysis of randomized controlled trials. J Diabetes Sci Technol 2018;12(1):183–189. 10.1177/1932296817719290 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Di Molfetta S, Bosi E, Ceriello A, et al. Structured self-monitoring of blood glucose is associated with more appropriate therapeutic interventions than unstructured self-monitoring: A novel analysis of data from the prisma trial. Diabetes Res Clin Pract 2021;181:109070. 10.1016/j.dia-bres.2021.109070 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 153.Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology clinical practice guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract 2021;27(6): 505–537. 10.1016/j.eprac.2021.04.008 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 154.Vigersky RA, McMahon C. The relationship of hemoglobin A1c to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21(2):81–85. 10.1089/dia.2018.0310 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 155.Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019;13(4): 614–626. 10.1177/1932296818822496 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the gold randomized clinical trial. JAMA 2017;317(4):379–387. 10.1001/jama.2016.19976 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 157.Aleppo G, Ruedy KJ, Riddlesworth TD, et al. REPLACE-BG: A randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care 2017;40(4):538–545. 10.2337/dc16-2482 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317(4): 371–378. 10.1001/jama.2016.19975 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 159.Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring vs usual care in patients with type 2 diabetes receiving multiple daily insulin injections: A randomized trial. Ann Intern Med 2017;167(6):365–374. 10.7326/M16-2855 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 160.Šoupal J, Petruželková L, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care 2020;43(1):37–43. 10.2337/dc19-0888 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 161.Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: A randomized clinical trial. JAMA 2020;323(23):2388–2396. 10.1001/jama.2020.6940 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paris I, Henry C, Pirard F, Gérard AC, Colin IM. The new FreeStyle libre flash glucose monitoring system improves the glycaemic control in a cohort of people with type 1 diabetes followed in real-life conditions over a period of one year. Endocrinol Diabetes Metab 2018;1(3):e00023. 10.1002/edm2.23 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther 2020;11(1): 279–291. 10.1007/s13300-019-00741-9 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: A multicentre, non-masked, randomised controlled trial. Lancet 2016;388(10057): 2254–2263. 10.1016/S0140-6736(16)31535-5 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 165.van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): A randomised, open-label, crossover trial. Lancet Diabetes Endocrinol 2016;4(11):893–902. 10.1016/S2213-8587(16)30193-0 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 166.Charleer S, De Block C, Van Huffel L, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): A prospective observational real-world cohort study. Diabetes Care 2020;43(2): 389–397. 10.2337/dc19-1610 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 167.Fokkert M, van Dijk P, Edens M, et al. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care 2019;7(1):e000809. 10.1136/bmjdrc-2019-000809 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Charleer S, Mathieu C, Nobels F, et al. Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: A real-world study. J Clin Endocrinol Metab 2018;103(3):1224–1232. 10.1210/jc.2017-02498 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 169.Manski-Nankervis J, Yates CJ, Blackberry I, et al. Impact of insulin initiation on glycaemic variability and glucose profiles in a primary healthcare type 2 diabetes cohort: analysis of continuous glucose monitoring data from the INITIATION study. Diabet Med 2016;33(6):803–811. 10.1111/dme.12979 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 170.Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: A randomized clinical trial. JAMA 2021;325(22):2262–2272. 10.1001/jama.2021.7444 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: A multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8(1):55–73. 10.1007/s13300-016-0223-6 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Haak T, Hanaire H, Ajjan R, et al. Use of flash glucose-sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther 2017;8(3):573–586. 10.1007/s13300-017-0255-6 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: A randomized clinical trial. JAMA 2020;323(23):2397–2406. 10.1001/jama.2020.6928 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Haugstvedt A, Wentzel-Larsen T, Graue M, Søvik O. Rokne B Fear of hypoglycaemia in mothers and fathers of children with type 1 diabetes is associated with poor glycaemic control and parental emotional distress: A population-based study. Diabet Med J Br Diabet Assoc 2010;27(1):72–78. 10.1111/j.1464-5491.2009.02867.x [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 175.Davis TME, Dwyer P, England M, Fegan PG. Davis WA Efficacy of intermittently scanned continuous glucose monitoring in the prevention of recurrent severe hypoglycemia. Diabetes Technol Ther 2020;22(5):367–373. 10.1089/dia.2019.0331 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 176.Davis SN, Horton ES, Battelino T, et al. STAR 3 Randomized controlled trial to compare sensor-augmented insulin pump therapy with multiple daily injections in the treatment of type 1 diabetes: research design, methods, and baseline characteristics of enrolled subjects. Star. Diabetes Technol Ther 2010;12(4): 249–255. 10.1089/dia.2009.0145 [EL 1; RCT protocol]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pearce KL, Noakes M, Keogh J. Clifton PM Effect of carbohydrate distribution on postprandial glucose peaks with the use of continuous glucose monitoring in type 2 diabetes. Am J Clin Nutr 2008;87(3):638–644. 10.1093/ajcn/87.3.638 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 178.Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ. Vigersky RA The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol 2011;5(3):668–675. 10.1177/193229681100500320 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42(8):1593–1603. 10.2337/dci19-0028 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Suh DC, Kim CM, Choi IS, Plauschinat CA, Barone JA. Trends in blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988–2004. J Hypertens 2009;27(9):1908–1916. 10.1097/HJH.0b013e32832d4aee [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 181.Gress TW, Nieto FJ, Shahar E, Wofford MR. Brancati FL Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis risk in communities study. N Engl J Med 2000;342(13): 905–912. 10.1056/NEJM200003303421301 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 182.Sowers JR, Williams M, Epstein M, Bakris G. Hypertension in patients with diabetes. Strategies for drug therapy to reduce complications. Postgrad Med 2000;107(4):47–54, 60. 10.3810/pgm.2000.04.990 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 183.Brunström M Carlberg B Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ (Clin Res Ed) 2016;352:i717. 10.1136/bmj.i717 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Leehey DJ, Zhang JH, Emanuele NV, et al. BP and renal outcomes in diabetic kidney disease: the Veterans Affairs Nephropathy in Diabetes trial. Clin J Am Soc Nephrol CJASN 2015;10(12):2159–2169. 10.2215/CJN.02850315 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ (Clin Res Ed) 1998;317(7160): 703–713 [EL 1; RCT]. [PMC free article] [PubMed] [Google Scholar]
- 186.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ (Clin Res Ed) 2000;321(7258): 412–419. 10.1136/bmj.321.7258.412 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomised trial. Lancet 1998;351(9118):1755–1762. 10.1016/S0140-6736(98)04311-6 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 188.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000;355(9200):253–259 [EL 1; RCT]. [PubMed] [Google Scholar]
- 189.Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002;359(9311): 995–1003. 10.1016/S0140-6736(02)08089-3 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 190.Whelton PK, Barzilay J, Cushman WC, et al. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). Arch Intern Med 2005;165(12):1401–1409. 10.1001/archinte.165.12.1401 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 191.Rahman F, McEvoy JW, Ohkuma T, et al. Effects of blood pressure lowering on clinical outcomes according to baseline blood pressure and cardiovascular risk in patients with type 2 diabetes mellitus. Hypertens (Dallas. TX 2019;73(6): 1291–1299. 10.1161/HYPERTENSIONAHA.118.12414 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yusufu M, Zhang X, Sun X, Raat H, Wang N. How to perform better intervention to prevent and control diabetic retinopathy among patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2019;156:107834. 10.1016/j.diabres.2019.107834 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 193.Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev 2015;1:Cd006127. 10.1002/14651858.CD006127. pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kato S, Maruyama S, Makino H, et al. Anti-albuminuric effects of spironolactone in patients with type 2 diabetic nephropathy: A multicenter, randomized clinical trial. Clin Exp Nephrol 2015;19(6):1098–1106. 10.1007/s10157-015-1106-2 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Armstrong C Joint National Committee JNC8 guidelines for the management of hypertension in adults. Am Fam Physician 2014;90(7):503–504 [EL 4; NE]. [PubMed] [Google Scholar]
- 196.Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertens (Dallas. TX 1979;75(6): 1334–1357. 10.1161/HYPERTENSIONAHA.120.15026 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 197.National Kidney Foundation. KDOQI™ clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis Off J Natl Kidney Found 2007;49(suppl 2):S12–S154. 10.1053/j.ajkd.2006.12.005 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 198.Torre JJ, Bloomgarden ZT, Dickey RA, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of hypertension. Endocr Pract 2006;12(2):193–222 [EL 4; NE]. [PubMed] [Google Scholar]
- 199.American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S144–S174. 10.2337/dc22-S010 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 200.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287(19):2563–2569. 10.1001/jama.287.19.2563 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359(15):1565–1576. 10.1056/NEJMoa0806359 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 202.Beddhu S, Chertow GM, Greene T, et al. Effects of intensive systolic blood pressure lowering on cardiovascular events and mortality in patients with type 2 diabetes mellitus on standard glycemic control and in those without diabetes mellitus: reconciling results from ACCORD BP and SPRINT. J Am Heart Assoc 2018;7(18):e009326. 10.1161/JAHA.118.009326 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ohkuma T, Jun M, Rodgers A, et al. Acute increases in serum creatinine after starting angiotensin-converting enzyme inhibitor-based therapy and effects of its continuation on major clinical outcomes in type 2 diabetes mellitus. Hypertension TX. 2019:84–91. 10.1161/HYPERTENSIONAHA.118.12060 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 204.ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362(17):1575–1585. 10.1056/NEJMoa1001286 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tsujimoto T, Kajio H. Benefits of intensive blood pressure treatment in patients with type 2 diabetes mellitus receiving standard but not intensive glycemic control. Hypertension. TX 2018:323–330. 10.1161/HYPERTENSIONAHA.118.11408 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 206.Xie XX, Liu P, Wan FY, et al. Blood pressure lowering and stroke events in type 2 diabetes: A network meta-analysis of randomized controlled trials. Int J Cardiol 2016;208:141–146. 10.1016/j.ijcard.2016.01.197 [EL 2; NMA]. [DOI] [PubMed] [Google Scholar]
- 207.Beddhu S, Greene T, Boucher R, et al. Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: secondary analyses of two randomised controlled trials. Lancet Diabetes Endocrinol 2018;6(7):555–563. 10.1016/S2213-8587(18)30099-8 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang J, Chen Y, Xu W, et al. Effects of intensive blood pressure lowering on mortality and cardiovascular and renal outcomes in type 2 diabetic patients: A meta-analysis. PLOS ONE 2019;14(4):e0215362. 10.1371/journal.pone.0215362 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group Group KDIGOKBPW. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021;99(3s):S1–S87. 10.1016/j.kint.2020.11.003 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 210.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA 2014;311(5):507–520. 10.1001/jama.2013.284427 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 211.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018;36(10):1953–2041. 10.1097/HJH.0000000000001940 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 212.Go AS, Bauman MA, Coleman King SM, et al. An effective approach to high blood pressure control: A science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension. TX 2014:878–885. 10.1161/HYP.0000000000000003 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: A statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich Conn) 2014;16(1):14–26. 10.1111/jch.12237 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.. Davis CR, Hodgson JM, Woodman R, et al. A Mediterranean diet lowers blood pressure and improves endothelial function: results from the MedLey randomized intervention trial. Am J Clin Nutr 2017;105(6):1305–1313. 10.3945/ajcn.116.146803 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 215.Filippou CD, Tsioufis CP, Thomopoulos CG, et al. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: A systematic review and meta-analysis of randomized controlled trials. Adv Nutr 2020;11(5):1150–1160. 10.1093/advances/nmaa041 [El 1: MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Banerjee T, Crews DC, Tuot DS, et al. Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int 2019;95(6):1433–1442. 10.1016/j.kint.2018.12.027 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Alvarez C, Ramirez-Campillo R, Martinez-Salazar C, et al. Low-volume high-intensity interval training as a therapy for type 2 diabetes. Int J Sports Med 2016;37(9):723–729. 10.1055/s-0042-104935 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 218.Gorostegi-Anduaga I, Maldonado-Martín S, MartinezAguirre-Betolaza A, et al. Effects on cardiovascular risk scores and vascular age after aerobic exercise and nutritional intervention in sedentary and overweight/obese adults with primary hypertension: the EXERDIET-HTA randomized trial study. High Blood Press Cardiovasc Prev 2018;25(4):361–368. 10.1007/s40292-018-0281-0 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 219.Haywood CJ, Prendergast LA, Lim R, et al. Obesity in older adults: effect of degree of weight loss on cardiovascular markers and medications. Clin Obes 2019;9(4):e12316. 10.1111/cob.12316 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 220.Lee JY, Ryu S, Sung KC. Association of baseline level of physical activity and its temporal changes with incident hypertension and diabetes mellitus. Eur J Prev Cardiol 2018;25(10):1065–1073. 10.1177/2047487318774419 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 221.Gilardini L, Redaelli G, Croci M, et al. Effect of a modest weight loss in normalizing blood pressure in obese subjects on antihypertensive drugs. Obes Facts 2016;9(4):251–258. 10.1159/000445504 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Abughosh S, Wang X, Serna O, et al. A motivational interviewing intervention by pharmacy students to improve medication adherence. J Manag Care Spec Pharm 2017;23(5):549–560. 10.18553/jmcp.2017.23.5.549 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schoenthaler AM, Lancaster KJ, Chaplin W, et al. Cluster randomized clinical trial of FAITH (Faith-Based Approaches in the Treatment of Hypertension) in blacks. Circ Cardiovasc Qual Outcomes 2018;11(10):e004691. 10.1161/CIRCOUTCOMES.118.004691 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 224.Grant AB, Seixas A, Frederickson K, et al. Effect of expectation of care on adherence to antihypertensive medications among hypertensive blacks: analysis of the counseling African Americans to control hypertension (CAATCH) trial. J Clin Hypertens (Greenwich Conn) 2016;18(7):690–696. 10.1111/jch.12736 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fitzpatrick SL, Golden SH, Stewart K, et al. Effect of DECIDE (Decision-Making Education for Choices In Diabetes Everyday) program delivery modalities on clinical and behavioral outcomes in urban African Americans with type 2 diabetes: A randomized trial. Diabetes Care 2016;39(12):2149–2157. 10.2337/dc16-0941 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Aggarwal R, Petrie B, Bala W, Chiu N. Mortality outcomes with intensive blood pressure targets in chronic kidney disease patients. Hypertens (Dallas. TX 2019;73(6):1275–1282. 10.1161/HYPERTENSIONAHA.119.12697 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 227.Aggarwal R, Steinkamp J, Chiu N, Petrie B, Mirzan H. Intensive blood pressure targets for diabetic and other high-risk populations: A pooled individual patient data analysis. Hypertension. TX 2018;71(5):833–839. 10.1161/HYPERTENSIONAHA.117.10713 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 228.Cheung AK, Chang TI, Cushman WC, et al. Executive summary of the KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021;99(3):559–569. 10.1016/j.kint.2020.10.026 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 229.Verdecchia P Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertens (Dallas. TX 2000;35(3):844–851. 10.1161/01.hyp.35.3.844 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 230.Shen J, Li ZM, He LZ, et al. Comparison of ambulatory blood pressure and clinic blood pressure in relation to cardiovascular diseases in diabetic patients. Medicine 2017;96(33):e7807. 10.1097/MD.0000000000007807 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Buckley LF, Dixon DL, Wohlford GFt, Wijesinghe DS, Baker WL, Van Tassell BW. Effect of intensive blood pressure control in patients with type 2 diabetes mellitus over 9 years of follow-up: A subgroup analysis of high-risk ACCORDION trial participants. Diabetes Obes Metab 2018;20(6):1499–1502. 10.1111/dom.13248 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 232.Atkins ER. Rodgers A More vs less blood pressure lowering: an update. Clin Ther 2016;38(10):2135–2141. 10.1016/j.clinthera.2016.08.007 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 233.Brouwer TF, Vehmeijer JT, Kalkman DN, et al. Intensive blood pressure lowering in patients with and patients without type 2 diabetes: A pooled analysis from two randomized trials. Diabetes Care 2018;41(6):1142–1148. 10.2337/dc17-1722 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 234.Böhm M, Schumacher H, Teo KK, et al. Cardiovascular outcomes and achieved blood pressure in patients with and without diabetes at high cardiovascular risk. Eur Heart J 2019;40(25):2032–2043. 10.1093/eurheartj/ehz149 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 235.Ó Hartaigh B, Szymonifka J. Okin PM Achieving target SBP for lowering the risk of major adverse cardiovascular events in persons with diabetes mellitus. J Hypertens 2018;36(1):101–109. 10.1097/HJH.0000000000001515 [EL 1, RCT]. [DOI] [PubMed] [Google Scholar]
- 236.Fleg JL, Evans GW, Margolis KL, et al. Orthostatic hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertens (Dallas. TX 2016;68(4):888–895. 10.1161/HYPERTENSIONAHA.116.07474 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.White WB, Jalil F, Cushman WC, et al. Average clinician-measured blood pressures and cardiovascular outcomes in patients with type 2 diabetes mellitus and ischemic heart disease in the EXAMIN trial. J Am Heart Assoc 2018;7(20):e009114. 10.1161/JAHA.118.009114 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Simonyi G Benefits of fixed dose combination of ramipril/amlodipine in hypertensive diabetic patients: A subgroup analysis of RAMONA trial. Chin Med J (Engl) 2016;129(10):1224–1228. 10.4103/0366-6999.181959 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373(22):2117–2128. 10.1056/NEJMoa1504720 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 240.Ferdinand KC, Izzo JL, Lee J, et al. Antihyperglycemic and blood pressure effects of empagliflozin in black patients with type 2 diabetes mellitus and hypertension. Circulation 2019;139(18):2098–2109. 10.1161/CIRCULATIONAHA.118.036568 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 241.Weber MA, Mansfield TA, Alessi F, et al. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin-angiotensin system blockade. Blood Press 2016;25(2):93–103. 10.3109/08037051.2015.1116258 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 242.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375(19):1834–1844. 10.1056/NEJMoa1607141 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 243.Leiter LA, Bain SC, Bhatt DL, et al. The effect of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across baseline blood pressure categories: analysis of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab 2020;22(9):1690–1695. 10.1111/dom.14079 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mancia G, Cannon CP, Tikkanen I, et al. Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertens (Dallas. TX 2016;68(6): 1355–1364. 10.1161/HYPERTENSIONAHA.116.07703 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 245.Rahman M, Pressel S, Davis BR, et al. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: A report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 2005;165(8):936–946. 10.1001/archinte.165.8.936 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 246.Kunimura A, Himuro N, Fujiyoshi A, et al. The effects of renin-angiotensin system inhibitors on mortality, cardiovascular events, and renal events in hypertensive patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Hypertens Res Off J Jpn Soc Hypertens 2019;42(5):669–680. 10.1038/s41440-019-0234-6 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 247.Perez A, Levin A. Alam N A comparison of the use of clinical-guideline-recommended antihypertensive regimens in Mexican American, non-Hispanic black, and non-Hispanic white adults with type 2 diabetes and hypertension in the United States: Nhanes 2003–2012. Diabetes Educ 2016;42(6):739–747. 10.1177/0145721716666680 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 248.Petrie JR, Marso SP, Bain SC, et al. LEADER-4: blood pressure control in patients with type 2 diabetes and high cardiovascular risk: baseline data from the LEADER randomized trial. J Hypertens 2016;34(6):1140–1150. 10.1097/HJH.0000000000000890 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Navar AM, Gallup DS, Lokhnygina Y, et al. Hypertension control in adults with diabetes mellitus and recurrent cardiovascular events: global results from the trial evaluating cardiovascular outcomes with sitagliptin. Hypertens (Dallas. TX 2017;70(5):907–914. 10.1161/HYPERTENSIONAHA.117.09482 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Karashima S, Yoneda T, Kometani M, et al. Angiotensin II receptor blocker combined with eplerenone or hydrochlorothiazide for hypertensive patients with diabetes mellitus. Clin Exp Hypertens. New York, NY 2016;38(7): 565–570. 10.3109/10641963.2016.1151526 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 251.Imaizumi S, Shiga Y, Ogawa M, et al. Randomized trial of an increased dose of calcium channel blocker or angiotensin II type 1 receptor blocker as an add-on intensive depressor therapy in type 2 diabetes mellitus patients with uncontrolled essential hypertension: the academie study. Heart Vessels 2019;34(4):698–710. 10.1007/s00380-018-1286-2 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 252.Uzu T, Araki SI, Kashiwagi A, et al. Comparative effects of direct renin inhibitor and angiotensin receptor blocker on albuminuria in hypertensive patients with type 2 diabetes. A randomized controlled trial. PLOS ONE 2016;11(12): e0164936. 10.1371/journal.pone.0164936 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Saglimbene V, Palmer SC, Ruospo M, et al. The long-term impact of renin-angiotensin system (RAS) inhibition on cardiorenal outcomes (LIRICO): A randomized, controlled trial. J Am Soc Nephrol 2018;29(12):2890–2899. 10.1681/ASN.2018040443 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Persson F, Lindhardt M, Rossing P. Parving HH Prevention of microalbuminuria using early intervention with renin-angiotensin system inhibitors in patients with type 2 diabetes: A systematic review. J Renin Angiotensin Aldosterone Syst 2016;17(3):1470320316652047. 10.1177/1470320316652047 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang J, Tong A, Dai Y, et al. Comparative risk of new-onset diabetes mellitus for antihypertensive drugs in elderly: A bayesian network meta-analysis. J Clin Hypertens (Greenwich Conn) 2019;21(8):1082–1090. 10.1111/jch.13598 [EL 2; NMA]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hanon O, Caillard L, Chaussade E, Hernandorena I. Boully C Blood pressure-lowering efficacy of indapamide sr/amlodipine combination in older patients with hypertension: A post hoc analysis of the NESTOR trial (Natrilix sr vs Enalapril in Hypertensive Type 2 Diabetics with Microalbuminuria). J Clin Hypertens (Greenwich Conn) 2017;19(10):965–972. 10.1111/jch.13053 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lin JJ, Chang HC, Ku CT. Chen HY Hydrochlorothiazide hypertension treatment induced metabolic effects in type 2 diabetes: A meta-analysis of parallel-design RCTs. Eur Rev Med Pharmacol Sci 2016;20(13):2926–2934 [EL 1; MRCT]. [PubMed] [Google Scholar]
- 258.Feng Y, Huang R, Kavanagh J, et al. Efficacy and safety of dual blockade of the renin-angiotensin-aldosterone system in diabetic kidney disease: A meta-analysis. Am J Cardiovasc Drugs Drugs Devices Other Interv 2019;19(3): 259–286. 10.1007/s40256-018-00321-5 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 259.Chen Y, Liu P, Chen X, et al. Effects of different doses of irbesartan combined with spironolactone on urinary albumin excretion rate in elderly patients with early type 2 diabetic nephropathy. Am J Med Sci 2018;355(5):418–424. 10.1016/j.amjms.2018.01.017 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 260.Soufi Taleb Bendiab N, Meziane-Tani A, Ouabdesselam S, et al. Factors associated with global longitudinal strain decline in hypertensive patients with normal left ventricular ejection fraction. Eur J Prev Cardiol 2017;24(14): 1463–1472. 10.1177/2047487317721644 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 261.Soufi Taleb Bendiab N, Ouabdesselam S, Henaoui L, et al. Impact of diabetes on cardiac function in patients with high blood pressure. Int J Environ Res Public Health 2021;18(12):6553. 10.3390/ijerph18126553 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367(23):2204–2213. 10.1056/NEJMoa1208799 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 263.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369(20):1892–1903. 10.1056/NEJMoa1303154 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 264.Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: A scientific statement from the American Heart Association. Hypertens (Dallas. TX 2018;72(5):e53–e90. 10.1161/HYP.0000000000000084 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Williams B, MacDonald TM, Morant S, et al. Spironolactone vs placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, cross-over trial. Lancet 2015;386(10008):2059–2068. 10.1016/S0140-6736(15)00257-3 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gadowski AM, Nanayakkara N, Heritier S, et al. Association between dietary intake and lipid-lowering therapy: prospective analysis of data from Australian diabetes, obesity, and lifestyle study (AUSDIAB) using a quantile regression approach. Nutrients 2019;11(8):1858. 10.3390/nu11081858 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ (Clin Res Ed) 2015;351:h3978. 10.1136/bmj.h3978 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wing RR, Espeland MA, Clark JM, et al. Association of weight loss maintenance and weight regain on 4-year changes in cvd risk factors: the action for health in diabetes (Look AHEAD) clinical trial. Diabetes Care 2016;39(8):1345–1355. 10.2337/dc16-0509 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sharma AE, Willard-Grace R, Hessler D, Bodenheimer T, Thom DH. What happens after health coaching? Observational study 1 year following a randomized controlled trial. Ann Fam Med 2016;14(3):200–207. 10.1370/afm.1924 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pedersen LR, Olsen RH, Anholm C, et al. Effects of 1 year of exercise training vs combined exercise training and weight loss on body composition, low-grade inflammation and lipids in overweight patients with coronary artery disease: A randomized trial. Cardiovasc Diabetol 2019;18(1):127. 10.1186/s12933-019-0934-x [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kim Y, Keogh JB. Clifton PM Differential effects of red meat/refined grain diet and dairy/chicken/nuts/whole grain diet on glucose, insulin and triglyceride in a randomized crossover study. Nutrients 2016;8(11):687. 10.3390/nu8110687 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gomez-Marin B, Gomez-Delgado F, Lopez-Moreno J, et al. Long-term consumption of a Mediterranean diet improves postprandial lipemia in patients with type 2 diabetes: the Cordioprev randomized trial. Am J Clin Nutr 2018;108(5):963–970. 10.1093/ajcn/nqy144 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 273.Safai N, Carstensen B, Vestergaard H. Ridderstråle M Impact of a multifactorial treatment programme on clinical outcomes and cardiovascular risk estimates: A retrospective cohort study from a specialised diabetes centre in Denmark. BMJ Open 2018;8(3):e019214. 10.1136/bmjopen-2017-019214 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Amadid H, Johansen NB, Bjerregaard AL, et al. The role of physical activity in the development of first cardiovascular disease event: A tree-structured survival analysis of the Danish ADDITION-PRO cohort. Cardiovasc Diabetol 2018;17(1):126. 10.1186/s12933-018-0769-x [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mora S, Chang CL, Moorthy MV, Sever PS. Association of nonfasting vs fasting lipid levels with risk of major coronary events in the Anglo-Scandinavian Cardiac Outcomes Trial-lipid lowering arm. JAMA Intern Med 2019;179(7):898–905. 10.1001/jamainternmed.2019.0392 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sathiyakumar V, Park J, Quispe R, et al. Impact of novel low-density lipoprotein-cholesterol assessment on the utility of secondary non-high-density lipoprotein-c and apolipoprotein B targets in selected worldwide dyslipidemia guidelines. Circulation 2018;138(3):244–254. 10.1161/CIRCULATIONAHA.117.032463 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 277.Sniderman AD, St-Pierre AC, Cantin B, et al. Concordance/discordance between plasma apolipoprotein B levels and the cholesterol indexes of atherosclerotic risk. Am J Cardiol 2003;91(10):1173–1177. 10.1016/s0002-9149(03)00262-5 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 278.Konishi H, Miyauchi K, Shitara J, et al. Impact of lipoprotein(a) on long-term outcomes in patients with diabetes mellitus who underwent percutaneous coronary intervention. Am J Cardiol 2016;118(12):1781–1785. 10.1016/j.amjcard.2016.08.067 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 279.Al Rifai M, McEvoy JW, Nasir K, et al. Traditional cardiovascular disease risk factors associated with one-year all-cause mortality among those with coronary artery calcium scores ≥400. Atherosclerosis 2015;241(2):495–497. 10.1016/j.atherosclerosis.2015.06.002 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 280.Lee JH, Han D. B Warranty period of zero coronary artery calcium score for predicting all-cause mortality according to cardiac risk burden in asymptomatic Korean adults. Circ J Off J Jpn Circ Soc 2016;80(11):2356–2361. 10.1253/circj.CJ-16-0731 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 281.Park GM, Lee JH, Lee SW, et al. Comparison of coronary computed tomographic angiographic findings in asymptomatic subjects with vs without diabetes mellitus. Am J Cardiol 2015;116(3):372–378. 10.1016/j.amjcard.2015.04.046 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 282.Pereira AC, Gomez LM, Bittencourt MS, et al. Age, gender, and race-based coronary artery calcium score percentiles in the Brazilian longitudinal study of adult health (ELSA-Brasil). Clin Cardiol 2016;39(6):352–359. 10.1002/clc.22539 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tomizawa N, Nojo T, Inoh S, Nakamura S. Difference of coronary artery disease severity, extent and plaque characteristics between patients with hypertension, diabetes mellitus or dyslipidemia. Int J Cardiovasc Imaging 2015;31(1):205–212. 10.1007/s10554-014-0542-5 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 284.Lucaroni F, Cicciarella Modica D, Macino M, et al. Can risk be predicted? An umbrella systematic review of current risk prediction models for cardiovascular diseases, diabetes and hypertension. BMJ Open 2019;9(12):e030234. 10.1136/bmjopen-2019-030234 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Nie F, He J, Cao H, Hu X Predictive value of abnormal ankle-brachial index in patients with diabetes: A meta-analysis. Diabetes Res Clin Pract 2021;174: 108723. 10.1016/j.diabres.2021.108723 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 286.Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017;23(Suppl 2):1–87. 10.4158/EP171764.APPGL [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 287.Zacho J, Tybjaerg-Hansen A, Jensen JS, et al. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008;359(18): 1897–1908. 10.1056/NEJMoa0707402 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 288.Lai R, Ju J, Lin Q, Xu H. Coronary artery calcification under statin therapy and its effect on cardiovascular outcomes: A systematic review and meta-analysis. Front Cardiovasc Med 2020;7:600497. 10.3389/fcvm.2020.600497 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388(10057): 2239–2253. 10.1016/S0140-6736(16)31009-1 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 290.Ridker PM, Buring JE, Rifai N. Cook NR Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 2007;297(6):611–619. 10.1001/jama.297.6.611 [EL 3; DS]. [DOI] [PubMed] [Google Scholar]
- 291.Ridker PM, Paynter NP, Rifai N, Gaziano JM. Cook NR C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation 2008;118(22):2243–2251, 2244p following 2251. 10.1161/CIRCULATIONAHA.108.814251 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.D'Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008;117(6): 743–753. 10.1161/CIRCULATIONAHA.107.699579 [EL 3; DS]. [DOI] [PubMed] [Google Scholar]
- 293.Pencina MJ, D'Agostino RB Sr, Larson MG, Massaro JM. Vasan RS Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation 2009;119(24):3078–3084. 10.1161/CIRCULATIONAHA.108.816694 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 suppl 2):S49–S73. 10.1161/01.cir.0000437741.48606.98 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 295.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 B):2935–2959. 10.1016/j.jacc.2013.11.005 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.McClelland RL, Jorgensen NW, Budoff M, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66(15):1643–1653. 10.1016/j.jacc.2015.08.035 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mayo Clinic. Statin Choice Decision Aid [EL 4; NE] https://statindecisionaid.mayoclinic.org/. Accessed January 13, 2022.
- 298.Mann DM, Ponieman D, Montori VM, Arciniega J, McGinn T. The statin choice decision aid in primary care: A randomized trial. Patient Educ Couns. patient ed 2010;80(1):138–140. 10.1016/j.pec.2009.10.008 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 299.Ye S, Leppin AL, Chan AY, et al. An informatics approach to implement support for shared decision making for primary prevention statin therapy. MDM Policy Pract 2018;3(1):2381468318777752. 10.1177/2381468318777752 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Bakris GL. Molitch M Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care 2014;37(3):867–875. 10.2337/dc13-1870 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 301.Purnell JQ, Zinman B, Brunzell JD. DCCT/EDIC Research Group The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) study. Circulation 2013;127(2):180–187. 10.1161/CIRCULATIONAHA.111.077487 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Edqvist J, Rawshani A, Adiels M, et al. BMI, mortality, and cardiovascular outcomes in type 1 diabetes: findings against an obesity paradox. Diabetes Care 2019;42(7):1297–1304. 10.2337/dc18-1446 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 303.Flores-Le Roux JA, Comin J, Pedro-Botet J, et al. Seven-year mortality in heart failure patients with undiagnosed diabetes: an observational study. Cardiovasc Diabetol 2011;10:39. 10.1186/1475-2840-10-39 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Giraldez RR, Clare RM, Lopes RD, et al. Prevalence and clinical outcomes of undiagnosed diabetes mellitus and prediabetes among patients with high-risk non-ST-segment elevation acute coronary syndrome. Am Heart J 2013;165(6):918–925.e2. 10.1016/j.ahj.2013.01.005 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 305.Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet 2002;359(9324):2140–2144. 10.1016/S0140-6736(02)09089-X [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 306.Wang N, Fulcher J, Abeysuriya N, et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: A systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol 2020;8(1):36–49. 10.1016/S2213-8587(19)30388-2 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 307.de Ritter R, Sep SJS, van der Kallen CJH, et al. Adverse differences in cardiometabolic risk factor levels between individuals with prediabetes and normal glucose metabolism are more pronounced in women than in men: the Maastricht study. BMJ Open Diabetes Res Care 2019;7(1):e000787. 10.1136/bmjdrc-2019-000787 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Hashemi Madani N, Ismail-Beigi F, Poustchi H, et al. Impaired fasting glucose and major adverse cardiovascular events by hypertension and dyslipidemia status: the Golestan cohort study. BMC Cardiovasc Disord 2020;20(1):113. 10.1186/s12872-020-01390-8 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Taskinen MR Diabetic dyslipidaemia: From basic research to clinical practice. Diabetologia 2003;46(6):733–749. 10.1007/s00125-003-1111-y [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 310.Halcox JP, Banegas JR, Roy C, et al. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc Disord 2017;17(1):160. 10.1186/s12872-017-0591-5 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Jin JL, Zhang HW, Cao YX, et al. Association of small dense low-density lipoprotein with cardiovascular outcome in patients with coronary artery disease and diabetes: A prospective, observational cohort study. Cardiovasc Diabetol 2020;19(1):45. 10.1186/s12933-020-01015-6 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ference BA, Kastelein JJP, Ray KK, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA 2019;321(4):364–373. 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ye X, Kong W, Zafar MI, Chen LL. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Cardiovasc Diabetol 2019;18(1):48. 10.1186/s12933-019-0851-z [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Johannesen CDL, Mortensen MB, Langsted A. Nordestgaard BG Apolipoprotein B and non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J Am Coll Cardiol 2021;77(11):1439–1450. 10.1016/j.jacc.2021.01.027 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 315.Khan SU, Khan MU, Valavoor S, et al. Association of lowering apolipoprotein B with cardiovascular outcomes across various lipid-lowering therapies: systematic review and meta-analysis of trials. Eur J Prev Cardiol 2020;27(12): 1255–1268. 10.1177/2047487319871733 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Richardson TG, Sanderson E, Palmer TM, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLOS Med 2020;17(3):e1003062. 10.1371/journal.pmed.1003062 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018;379(7):633–644. 10.1056/NEJMoa1800256 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 318.Handelsman Y, Jellinger PS, Guerin CK, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm - 2020 executive summary. Endocr Pract 2020;26(10):1196–1224. 10.4158/CS-2020-0490 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 319.Sniderman AD, Thanassoulis G, Glavinovic T, et al. Apolipoprotein B particles and cardiovascular disease: A narrative review. JAMA Cardiol 2019;4(12): 1287–1295. 10.1001/jamacardio.2019.3780 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes 2011;4(3):337–345. 10.1161/CIRCOUTCOMES.110.959247 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 321.Robinson JG, Wang S. Jacobson TA Meta-analysis of comparison of effectiveness of lowering apolipoprotein B vs low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol 2012;110(10):1468–1476. 10.1016/j.amjcard.2012.07.007. EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 322.AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices, Cole TG, Contois JH, et al. Association of apolipoprotein B and nuclear magnetic resonance spectroscopy-derived LDL particle number with outcomes in 25 clinical studies: assessment by the AACC Lipoprotein and Vascular Diseases Division Working Group on Best Practices. Clin Chem 2013;59(5):752–770. 10.1373/clinchem.2012.196733 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 323.Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract 2015;21(4):438–447. 10.4158/EP15693.CS. [DOI] [PubMed] [Google Scholar]
- 324.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139(25):e1082–e1143. 10.1161/CIR.0000000000000625 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Authors/Task Force Members. ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019;290:140–205. 10.1016/j.atherosclerosis.2019.08.014 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 326.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41(1):111–188. 10.1093/eurheartj/ehz455 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 327.Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care 2006;29(6):1220–1226. 10.2337/dc05-2465 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 328.Athyros VG, Papageorgiou AA, Symeonidis AN, et al. Early benefit from structured care with atorvastatin in patients with coronary heart disease and diabetes mellitus. Angiology 2003;54(6):679–690. 10.1177/000331970305400607 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 329.Ahmed S, Cannon CP, Murphy SA. Braunwald E Acute coronary syndromes and diabetes: is intensive lipid lowering beneficial? Results of the PROVE-IT-TIMI 22 trial. Eur Heart J 2006;27(19):2323–2329. 10.1093/eurheartj/ehl220 [EL 1; RCT, subgroup]. [DOI] [PubMed] [Google Scholar]
- 330.Eeg-Olofsson K, Zethelius B, Gudbjörnsdottir S, et al. Considerably decreased risk of cardiovascular disease with combined reductions in HbA1c, blood pressure and blood lipids in type 2 diabetes: report from the Swedish National Diabetes Register. Diab Vasc Dis Res 2016;13(4):268–277. 10.1177/1479164116637311 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 331.Knopp RH, d'Emden M, Smilde JG. Pocock SJ Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care 2006;29(7):1478–1485. 10.2337/dc05-2415 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 332.Lee CD, Folsom AR, Pankow JS, Brancati FL. Atherosclerosis Risk in Communities (ARIC) Study Investigators Cardiovascular events in diabetic and nondiabetic adults with or without history of myocardial infarction. Circulation 2004;109(7): 855–860. 10.1161/01.CIR.0000116389.61864. DE [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 333.Kearney PM, Blackwell L, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 2008;371(9607):117–125. 10.1016/S0140-6736(08)60104-X [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 334.Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376(9753):1670–1681. 10.1016/S0140-6736(10)61350-5 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Liu Z, Xu Y, Hao H, et al. Efficacy of high intensity atorvastatin vs moderate intensity atorvastatin for acute coronary syndrome patients with diabetes mellitus. Int J Cardiol 2016;222:22–26. 10.1016/j.ijcard.2016.07.140 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 336.Bruno G, Merletti F, Biggeri A, et al. Effect of age on the association of non-high-density-lipoprotein cholesterol and apolipoprotein B with cardiovascular mortality in a Mediterranean population with type 2 diabetes: the Casale Monferrato study. Diabetologia 2006;49(5):937–944. 10.1007/s00125-006-0195-6 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 337.Chien KL, Hsu HC, Su TC, et al. Apolipoprotein B and non-high density lipoprotein cholesterol and the risk of coronary heart disease in Chinese. J Lipid Res 2007;48(11):2499–2505. 10.1194/jlr.M700213-JLR200 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 338.Pischon T, Girman CJ, Sacks FM, et al. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation 2005;112(22):3375–3383. 10.1161/CIRCULATIONAHA.104.532499 [EL 2; NCCS]. [DOI] [PubMed] [Google Scholar]
- 339.Le NA, Tomassini JE, Tershakovec AM, Neff DR, Wilson PW. Effect of switching from statin monotherapy to ezetimibe/simvastatin combination therapy compared with other intensified lipid-lowering strategies on lipoprotein subclasses in diabetic patients with symptomatic cardiovascular disease. J Am Heart Assoc 2015;4(10):e001675. 10.1161/JAHA.114.001675 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73(24):3210–3227. 10.1016/j.jacc.2018.11.004 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 341.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372(25):2387–2397. 10.1056/NEJMoa1410489 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 342.Taskinen MR, Del Prato S, Bujas-Bobanovic M, et al. Efficacy and safety of alirocumab in individuals with type 2 diabetes mellitus with or without mixed dyslipidaemia: analysis of the ODYSSEY long term trial. Atherosclerosis 2018;276:124–130. 10.1016/j.atherosclerosis.2018.07.017 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 343.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359(21):2195–2207. 10.1056/NEJMoa0807646 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 344.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364(9435):685–696. 10.1016/S0140-6736(04)16895-5 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 345.Collins R, Armitage J, Parish S, et al. MRC/BHF heart protection study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet 2003;361(9374):2005–2016. 10.1016/s0140-6736(03)13636-7 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 346.Kaasenbrood L, Poulter NR, Sever PS, et al. Development and validation of a model to predict absolute vascular risk reduction by moderate-intensity statin therapy in individual patients with type 2 diabetes mellitus: the Anglo Scandinavian Cardiac Outcomes Trial, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, and Collaborative Atorvastatin Diabetes Study. Circ Cardiovasc Qual Outcomes 2016;9(3):213–221. 10.1161/CIRCOUTCOMES.115.001980 [EL 3; DS]. [DOI] [PubMed] [Google Scholar]
- 347.The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251(3):365–374 [EL 1; RCT]. [PubMed] [Google Scholar]
- 348.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta-analysis. JAMA 2016;316(12):1289–1297. 10.1001/jama.2016.13985 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 349.Amarenco P, Kim JS, Labreuche J, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med 2020;382(1):9. 10.1056/NEJMoa1910355 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 350.Bohula EA, Wiviott SD, Giugliano RP, et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE-IT (Improved Reduction of outcomes: Vytorin Efficacy International Trial). Circulation 2017;136(25):2440–2450. 10.1161/CIRCULATIONAHA.117.029095 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 351.Ji MS, Jeong MH, Ahn YK, et al. Clinical outcome of statin plus ezetimibe vs high-intensity statin therapy in patients with acute myocardial infarction propensity-score matching analysis. Int J Cardiol 2016;225:50–59. 10.1016/j.ijcard.2016.09.082 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 352.Lotta LA, Sharp SJ, Burgess S, et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: A meta-analysis. JAMA 2016;316(13):1383–1391. 10.1001/jama.2016.14568 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Nußbaumer B, Glechner A, Kaminski-Hartenthaler A, Mahlknecht P, Gartlehner G. Ezetimibe-statin combination therapy. Dtsch arztebl int 2016;113(26):445–453. 10.3238/arztebl.2016.0445 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Schmidt AF, Pearce LS, Wilkins JT, et al. Pcsk9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;4(4):Cd011748. 10.1002/14651858.CD011748. pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Zieve F, Wenger NK, Ben-Yehuda O, et al. Safety and efficacy of ezetimibe added to atorvastatin vs up titration of atorvastatin to 40 mg in patients > or = 65 years of age (from the ZETia in the ELDerly [ZETELD] study). Am J Cardiol 2010;105(5):656–663. 10.1016/j.amjcard.2009.10.029 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 356.Tonelli M, Wanner C, Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. Lipid management in chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2013 clinical practice guideline. Ann Intern Med 2014;160(3):182. 10.7326/M13-2453 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 357.Baigent C, Landray MJ, Reith C, et al. The effects of lowering ldl cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): A randomised placebo-controlled trial. Lancet 2011;377(9784):2181–2192. 10.1016/S0140-6736(11)60739-3 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ramos R, Comas-Cufí M, Martí-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ (Clin Res Ed) 2018;362:k3359. 10.1136/bmj.k3359 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Ponce OJ, Larrea-Mantilla L, Hemmingsen B, et al. Lipid-lowering agents in older individuals: A systematic review and meta-analysis of randomized clinical trials. J Clin Endocrinol Metab 2019;104(5):1585–1594. 10.1210/jc.2019-00195 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 360.Lind L, Sundström J, Ärnlöv J, Lampa E. Impact of aging on the strength of cardiovascular risk factors: A longitudinal study over 40 years. J Am Heart Assoc 2018;7(1):e007061. 10.1161/JAHA.117.007061 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Yourman LC, Cenzer IS, Boscardin WJ, et al. Evaluation of time to benefit of statins for the primary prevention of cardiovascular events in adults aged 50 to 75 years: A meta-analysis. JAMA Intern Med 2021;181(2):179–185. 10.1001/jamainternmed.2020.6084 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Liu J, Sempos CT, Donahue RP, et al. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am J Cardiol 2006;98(10):1363–1368. 10.1016/j.amjcard.2006.06.032 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 363.Lu W, Resnick HE, Jablonski KA, et al. Non-hdl cholesterol as a predictor of cardiovascular disease in type 2 diabetes: the strong heart study. Diabetes Care 2003;26(1):16–23. 10.2337/diacare.26.1.16 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 364.Diabetes Atorvastin Lipid Intervention (DALI) Study Group The effect of aggressive vs standard lipid lowering by atorvastatin on diabetic dyslipidemia: the dali study: A double-blind, randomized, placebo-controlled trial in patients with type 2 diabetes and diabetic dyslipidemia. Diabetes Care 2001;24(8): 1335–1341. 10.2337/diacare.24.8.1335 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 365.Marston NA, Giugliano RP, Im K, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: A systematic review and meta-regression analysis of randomized controlled trials. Circulation 2019;140(16):1308–1317. 10.1161/CIRCULATIONAHA.119.041998 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Scott R, O'Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care 2009;32(3): 493–498. 10.2337/dc08-1543 [EL 2; NRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.ACCORD Study Group, Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362(17): 1563–1574. 10.1056/NEJMoa1001282 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302(18): 1993–2000. 10.1001/jama.2009.1619 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380(1):11–22. 10.1056/NEJMoa1812792 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 370.Bowman L, Mafham M, Wallendszus K, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379(16):1540–1550. 10.1056/NEJMoa1804989 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 371.Brinton EA, Ballantyne CM, Guyton JR, et al. Lipid effects of icosapent ethyl in women with diabetes mellitus and persistent high triglycerides on statin treatment: ANCHOR trial subanalysis. J Womens Health (Larchmt) 2018;27(9): 1170–1176. 10.1089/jwh.2017.6757 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97(9):2969–2989. 10.1210/jc.2011-3213 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yang AL. McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology 2020;20(5):795–800. 10.1016/j.pan.2020.06.005 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 374.Brunzell JD. Clinical practice. Hypertriglyceridemia. N Engl J Med 2007;357(10):1009–1017. 10.1056/NEJMcp070061 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 375.Hegele RA, Ginsberg HN, Chapman MJ, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014;2(8):655–666. 10.1016/S2213-8587(13)70191-8 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Christian JB, Arondekar B, Buysman EK, et al. Determining triglyceride reductions needed for clinical impact in severe hypertriglyceridemia. Am J Med 2014;127(1):36–44.e31. 10.1016/j.amjmed.2013.09.018 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 377.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366(9500):1849–1861. 10.1016/S0140-6736(05)67667-2 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 378.Rosenblit PD. Do persons with diabetes benefit from combination statin and fibrate therapy? Curr Cardiol Rep 2012;14(1):112–124. 10.1007/s11886-011-0237-7 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 379.Lee M, Saver JL, Towfighi A, Chow J, Ovbiagele B. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: A meta-analysis. Atherosclerosis 2011;217(2):492–498. 10.1016/j.atherosclerosis.2011.04.020 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 380.Bruckert E, Labreuche J, Deplanque D, Touboul PJ, Amarenco P. Fibrates effect on cardiovascular risk is greater in patients with high triglyceride levels or atherogenic dyslipidemia profile: A systematic review and meta-analysis. J Cardiovasc Pharmacol 2011;57(2):267–272. 10.1097/FJC.0b013e318202709f [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 381.Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med 2010;363(7):692–694; author reply 694 [EL 4; NE]. 10.1056/NEJMc1006407. [DOI] [PubMed] [Google Scholar]
- 382.Investigators AIM-HIGH, Boden WE Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365(24):2255–2267. 10.1056/NEJMoa1107579 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 383.HPS-2 THRIVE Collaboration Group. HPS-2 THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013;34(17):1279–1291. 10.1093/eurheartj/eht055 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA 2011;305(24):2556–2564. 10.1001/jama.2011.860 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 385.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010;375(9716): 735–742. 10.1016/S0140-6736(09)61965-6 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 386.Maki KC, Ridker PM, Brown WV. An assessment by the Statin Diabetes Safety Task Force: 2014 update. J Clin Lipidol 2014;8(3 suppl):S17–S29. 10.1016/j.jacl.2014.02.012 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 387.de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305(24):2532–2539. 10.1001/jama.2011.861 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Metsärinne K, Bröijersen A, Kantola I, et al. High prevalence of chronic kidney disease in Finnish patients with type 2 diabetes treated in primary care. Prim Care Diabetes 2015;9(1):31–38. 10.1016/j.pcd.2014.06.001 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 389.Schroeder EB, Powers JD, O'Connor PJ, et al. Prevalence of chronic kidney disease among individuals with diabetes in the SUPREME-DM project, 2005–2011. J Diabetes Its Complications 2015;29(5):637–643. 10.1016/j.jdiacomp.2015.04.007 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 390.Fioretto P Mauer M Histopathology of diabetic nephropathy. Semin Nephrol 2007;27(2):195–207. 10.1016/j.semnephrol.2007.01.012 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol CJASN 2017;12(12):2032–2045. 10.2215/CJN.11491116 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Alicic RZ, Johnson EJ, Tuttle KR. Inflammatory mechanisms as new biomarkers and therapeutic targets for diabetic kidney disease. Adv Chronic Kidney Dis 2018;25(2):181–191. 10.1053/j.ackd.2017.12.002 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 393.Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 394.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis Off J Natl Kidney Found 2012;60(5): 850–886. 10.1053/j.ajkd.2012.07.005 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 395.Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: Recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol 2021;32(12):2994–3015. 10.1681/ASN.2021070988 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385(19): 1737–1749. 10.1056/NEJMoa2102953 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Williams WW, Hogan JW, Ingelfinger JR. Time to eliminate health care disparities in the estimation of kidney function. N Engl J Med 2021;385(19): 1804–1806. 10.1056/NEJMe2114918 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 398.Newman DJ, Mattock MB, Dawnay AB, et al. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess (Winchester, England) 2005;9(30):iii–vi:xiii-163. 10.3310/hta9300 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 399.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO controversies conference report. Kidney Int 2011;80(1):17–28. 10.1038/ki.2010.483 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 400.Nordwall M, Abrahamsson M, Dhir M, et al. Impact of HbA1c, followed from onset of type 1 diabetes, on the development of severe retinopathy and nephropathy: the VISS study (Vascular Diabetic Complications in Southeast Sweden). Diabetes Care 2015;38(2):308–315. 10.2337/dc14-1203 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 401.Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol 2018;17(1):33. 10.1186/s12933-018-0677-0 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ku E, McCulloch CE, Mauer M, et al. Association between blood pressure and adverse renal events in type 1 diabetes. Diabetes Care 2016;39(12): 2218–2224. 10.2337/dc16-0857 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Sohn MW, Epstein N, Huang ES, et al. Visit-to-visit systolic blood pressure variability and microvascular complications among patients with diabetes. J Diabetes Its Complications 2017;31(1):195–201. 10.1016/j.jdiacomp.2016.09.003 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Gæde P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the STENO-2 randomised trial. Diabetologia 2016;59(11):2298–2307. 10.1007/s00125-016-4065-6 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Griffin SJ, Rutten GEHM, Khunti K, et al. Long-term effects of intensive multifactorial therapy in individuals with screen-detected type 2 diabetes in primary care: 10-year follow-up of the ADDITION-Europe cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7(12):925–937. 10.1016/S2213-8587(19)30349-3 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 406.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):854–865 [EL 2; PHAS]. [PubMed] [Google Scholar]
- 407.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):837–853 [EL 1; RCT]. [PubMed] [Google Scholar]
- 408.Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: A meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 2017;5(6):431–437. 10.1016/S2213-8587(17)30104-3 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 409.Papademetriou V, Lovato L, Doumas M, et al. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int 2015;87(3):649–659. 10.1038/ki.2014.296 [EL 1; PHAS]. [DOI] [PubMed] [Google Scholar]
- 410.Kuo IC, Lin HY, Niu SW, et al. Glycated hemoglobin and outcomes in patients with advanced diabetic chronic kidney disease. Sci Rep 2016;6:20028. 10.1038/srep20028 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353(3):238–248. 10.1056/NEJMoa043545 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 412.Palmer SC, Craig JC, Navaneethan SD, et al. Benefits and harms of statin therapy for persons with chronic kidney disease: A systematic review and meta-analysis. Ann Intern Med 2012;157(4):263–275. 10.7326/0003-4819-157-4-201208210-00007 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Slinin Y, Ishani A, Rector T, et al. Management of hyperglycemia, dyslipidemia, and albuminuria in patients with diabetes and CKD: A systematic review for a KDOQI clinical practice guideline. Am J Kidney Dis Off J Natl Kidney Found 2012;60(5):747–769. 10.1053/j.ajkd.2012.07.017 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 414.Bjerg L, Hulman A, Carstensen B, et al. Effect of duration and burden of microvascular complications on mortality rate in type 1 diabetes: an observational clinical cohort study. Diabetologia 2019;62(4):633–643. 10.1007/s00125-019-4812-6 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 415.Bramlage P, Lanzinger S, van Mark G, et al. Patient and disease characteristics of type-2 diabetes patients with or without chronic kidney disease: an analysis of the German DPV and DIVE databases. Cardiovasc Diabetol 2019;18(1):33. 10.1186/s12933-019-0837-x [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Chen PM, Wada T, Chiang CK. Prognostic value of proteinuria and glomerular filtration rate on Taiwanese patients with diabetes mellitus and advanced chronic kidney disease: A single center experience. Clin Exp Nephrol 2017;21(2):307–315. 10.1007/s10157-016-1290-8 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 417.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317(8): 825–835. 10.1001/jama.2017.0686 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Garofolo M, Gualdani E, Giannarelli R, et al. Microvascular complications burden (nephropathy, retinopathy and peripheral polyneuropathy) affects risk of major vascular events and all-cause mortality in type 1 diabetes: A 10-year follow-up study. Cardiovasc Diabetol 2019;18(1):159. 10.1186/s12933-019-0961-7 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Huang CY, Ting WH, Lo FS, et al. Factors associated with diabetic nephropathy in children, adolescents, and adults with type 1 diabetes. J Formos Med Assoc Taiwan Yi Zhi 2017;116(12):924–932. 10.1016/j.jfma.2017.09.015 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 420.Koye DN, Magliano DJ, Reid CM, et al. Risk of progression of nonalbuminuric CKD to end-stage kidney disease in people with diabetes: the CRIC (Chronic Renal Insufficiency Cohort) study. Am J Kidney Dis 2018;72(5):653–661. 10.1053/j.ajkd.2018.02.364 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 421.Tanaka N, Babazono T, Takagi M, et al. Albuminuria and reduced glomerular filtration rate for predicting the renal outcomes in type 2 diabetic patients. Nephrology (Carlton) 2015;20(8):531–538. 10.1111/nep.12446 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 422.Thorn LM, Gordin D, Harjutsalo V, et al. The presence and consequence of nonalbuminuric chronic kidney disease in patients with type 1 diabetes. Diabetes Care 2015;38(11):2128–2133. 10.2337/dc15-0641 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 423.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380(24):2295–2306. 10.1056/NEJMoa1811744 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 424.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383(15):1436–1446. 10.1056/NEJMoa2024816 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 425.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377(7):644–657. 10.1056/NEJMoa1611925 w. [DOI] [PubMed] [Google Scholar]
- 426.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380(4):347–357. 10.1056/NEJMoa1812389 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 427.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375(4):311–322. 10.1056/NEJMoa1603827 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: Randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Pioneer. Diabetes Care 2019;42(9):1724–1732. 10.2337/dc19-0749 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 429.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide vs insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018;6(8):605–617. 10.1016/S2213-8587(18)30104-9 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 430.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020;98(4S):S1–S115. 10.1016/j.kint.2020.06.019 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 431.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41(2):255–323. 10.1093/eurheartj/ehz486 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 432.American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care 2021;44(Suppl 1):S151–S167. 10.2337/dc21-S011 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 433.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertens (Dallas. TX 2018;71(6):e13–e115. 10.1161/HYP.0000000000000065 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 434.Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383(23):2219–2229. 10.1056/NEJMoa2025845 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 435.Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385(24):2252–2263. 10.1056/NEJMoa2110956 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 436.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993;329(20):1456–1462. 10.1056/NEJM199311113292004 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 437.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345(12):861–869. 10.1056/NEJMoa011161 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 438.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345(12):851–860. 10.1056/NEJMoa011303 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 439.Bangalore S, Fakheri R, Toklu B, Messerli FH. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ (Clin Res Ed) 2016;352:i438. 10.1136/bmj.i438 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Parving HH, Persson F, Lewis JB. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008;358(23):2433–2446. 10.1056/NEJMoa0708379 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 441.Investigators ONTARGET, Yusuf S Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358(15): 1547–1559. 10.1056/NEJMoa0801317 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 442.Halimi JM, Asmar R, Ribstein J. Optimal nephroprotection: use, misuse and misconceptions about blockade of the renin-angiotensin system. Lessons from the ONTARGET and other recent trials. Diabetes Metab 2009;35(6): 425–430. 10.1016/j.diabet.2009.05.003 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 443.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385(16):1451–1461. 10.1056/NEJMoa2107038 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 444.Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-REDUCED trial. Circulation 2021;143(4):337–349. 10.1161/CIRCULATIONAHA.120.051824 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Cannon CP, Perkovic V, Agarwal R, et al. Evaluating the effects of canagliflozin on cardiovascular and renal events in patients with type 2 diabetes mellitus and chronic kidney disease according to baseline HbA1c, including those with HbA1c <7%: results from the CREDENCE trial. Circulation 2020;141(5): 407–410. 10.1161/CIRCULATIONAHA.119.044359 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 446.Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG outcome randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5(8):610–621. 10.1016/S2213-8587(17)30182-1 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 447.Dekkers CCJ, Petrykiv S, Laverman GD, et al. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab 2018;20(8):1988–1993. 10.1111/dom.13301 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Heerspink HJL, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019;62(7): 1154–1166. 10.1007/s00125-019-4859-4 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Heerspink HJL, Sjöström CD, Inzucchi SE, et al. Reduction in albuminuria with dapagliflozin cannot be predicted by baseline clinical characteristics or changes in most other risk markers. Diabetes Obes Metab 2019;21(3): 720–725. 10.1111/dom.13579 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Heerspink HJL, Sjöström CD, Jongs N, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: A pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J 2021;42(13): 1216–1227. 10.1093/eurheartj/ehab094 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Inzucchi SE, Kosiborod M, Fitchett D, et al. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation 2018;138(17):1904–1907. 10.1161/CIRCULATIONAHA.118.035759 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 452.Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation 2021;143(4):298–309. 10.1161/CIRCULATIONAHA.120.050391 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors vs other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation 2017;136(3):249–259. 10.1161/CIRCULATIONAHA.117.029190 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation 2019;140(9): 739–750. 10.1161/CIRCULATIONAHA.119.042007 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018;137(4): 323–334. 10.1161/CIRCULATIONAHA.117.032038 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Mayer GJ, Wanner C, Weir MR, et al. Analysis from the EMPA-REG OUTCOME(®) trial indicates empagliflozin may assist in preventing the progression of chronic kidney disease in patients with type 2 diabetes irrespective of medications that alter intrarenal hemodynamics. Kidney Int 2019;96(2):489–504. 10.1016/j.kint.2019.02.033 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 457.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381(21): 1995–2008. 10.1056/NEJMoa1911303 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 458.McMurray JJV, Wheeler DC, Stefánsson BV, et al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation 2021;143(5):438–448. 10.1161/CIRCULATIONAHA.120.051675 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 459.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019;7(8):606–617. 10.1016/S2213-8587(19)30180-9 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 460.Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation 2018;138(15): 1537–1550. 10.1161/CIRCULATIONAHA.118.035901 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7(11):845–854. 10.1016/S2213-8587(19)30256-6 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 462.Persson F, Rossing P, Vart P, et al. Efficacy and safety of dapagliflozin by baseline glycemic status: A prespecified analysis from the DAPA-CKD trial. Diabetes Care 2021;44(8):1894–1897. 10.2337/dc21-0300 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 2017;60(2):215–225. 10.1007/s00125-016-4157-3 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol 2020;16(6):317–336. 10.1038/s41581-020-0256-y [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375(4):323–334. 10.1056/NEJMoa1515920 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 466.Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018;137(2):119–129. 10.1161/CIRCULATIONAHA.117.028268 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 467.Wheeler DC, Stefánsson BV, Jongs N, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021;9(1):22–31. 10.1016/S2213-8587(20)30369-7 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 468.Zannad F, Ferreira JP, Pocock SJ, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-REDUCED. Circulation 2021;143(4):310–321. 10.1161/CIRCULATIONAHA.120.051685 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Kawai Y, Uneda K, Yamada T, et al. Comparison of effects of SGLT-2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in type 2 diabetes mellitus patients with/without albuminuria: A systematic review and network meta-analysis. Diabetes Res Clin Pract 2022;183:109146. 10.1016/j.diabres.2021.109146 [EL 2: NMA]. [DOI] [PubMed] [Google Scholar]
- 470.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383(15):1413–1424. 10.1056/NEJMoa2022190 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 471.Tuttle KR, Levin A, Nangaku M, et al. Safety of empagliflozin in patients with type 2 diabetes and chronic kidney disease: pooled analysis of placebo-controlled clinical trials. Diabetes Care 2022;45(6):1445–1452. 10.2337/dc21-2034 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med 2021;385(10):896–907. 10.1056/NEJMoa2108269 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 473.Mann JFE, Buse JB, Idorn T, et al. Potential kidney protection with liraglutide and semaglutide: exploratory mediation analysis. Diabetes Obes Metab 2021;23(9):2058–2066. 10.1111/dom.14443 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Mann JFE, Fonseca V, Mosenzon O, et al. Effects of liraglutide vs placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation 2018;138(25):2908–2918. 10.1161/CIRCULATIONAHA.118.036418 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Mann JFE, Fonseca VA, Poulter NR, et al. Safety of liraglutide in type 2 diabetes and chronic kidney disease. Clin J Am Soc Nephrol 2020;15(4):465–473. 10.2215/CJN.11881019 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Mann JFE, Hansen T, Idorn T, et al. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: A post-hoc analysis of the SUSTAIN 1–7 randomised controlled trials. Lancet Diabetes Endocrinol 2020;8(11):880–893. 10.1016/S2213-8587(20)30313-2 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 477.Mann JFE, Ørsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017;377(9):839–848. 10.1056/NEJMoa1616011 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 478.Mosenzon O, Bain SC, Heerspink HJL, et al. Cardiovascular and renal outcomes by baseline albuminuria status and renal function: results from the LEADER randomized trial. Diabetes Obes Metab 2020;22(11):2077–2088. 10.1111/dom.14126 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Persson F, Bain SC, Mosenzon O, et al. Changes in albuminuria predict cardiovascular and renal outcomes in type 2 diabetes: A post hoc analysis of the LEADER trial. Diabetes Care 2021;44(4):1020–1026. 10.2337/dc20-1622 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Filippatos G, Anker SD, Agarwal R, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation 2021;143(6):540–552. 10.1161/CIRCULATIONAHA.120.051898 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Filippatos G, Bakris GL, Pitt B, et al. Finerenone reduces new-onset atrial fibrillation in patients with chronic kidney disease and type 2 diabetes. J Am Coll Cardiol 2021;78(2):142–152. 10.1016/j.jacc.2021.04.079 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 482.Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022;43(6):474–484. 10.1093/eurheartj/ehab777 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Koro CE, Lee BH. Bowlin SJ Antidiabetic medication use and prevalence of chronic kidney disease among patients with type 2 diabetes mellitus in the United States. Clin Ther 2009;31(11):2608–2617. 10.1016/j.clinthera.2009.10.020 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 484.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012;2(4):279–335 [EL 4; NE]. [Google Scholar]
- 485.Al-Aly Z, Qazi RA, González EA, Zeringue A, Martin KJ. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis Off J Natl Kidney Found 2007;50(1):59–68. 10.1053/j.ajkd.2007.04.010 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 486.Levinsky NG Specialist evaluation in chronic kidney disease: too little, too late. Ann Intern Med 2002;137(6):542–543. 10.7326/0003-4819-137-6-200209170-00016 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 487.Becker BN, Brazy PC, Becker YT, et al. Simultaneous pancreas-kidney transplantation reduces excess mortality in type 1 diabetic patients with end-stage renal disease. Kidney Int 2000;57(5):2129–2135. 10.1046/j.1523-1755.2000.00064.x [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 488.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: Risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 2001;44(2):156–163. 10.1007/s001250051594 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 489.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med 2012;366(13):1227–1239. 10.1056/NEJMra1005073 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 490.Fang M, Selvin E. Thirty-year trends in complications in U.S. Adults with newly diagnosed type 2 diabetes. Diabetes Care 2021;44(3):699–706. 10.2337/dc20-2304 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Ruta LM, Magliano DJ, Lemesurier R, et al. Prevalence of diabetic retinopathy in type 2 diabetes in developing and developed countries. Diabet Med 2013;30(4):387–398. 10.1111/dme.12119 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 492.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35(3):556–564. 10.2337/dc11-1909 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Li JQ, Welchowski T, Schmid M, et al. Prevalence, incidence and future projection of diabetic eye disease in Europe: A systematic review and meta-analysis. Eur J Epidemiol 2020;35(1):11–23. 10.1007/s10654-019-00560-z [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 494.Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 2021;128(11):1580–1591. 10.1016/j.ophtha.2021.04.027 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 495.Wang SY, Andrews CA, Herman WH, Gardner TW, Stein JD. Incidence and risk factors for developing diabetic retinopathy among youths with type 1 or type 2 diabetes throughout the United States. Ophthalmology 2017;124(4): 424–430. 10.1016/j.ophtha.2016.10.031 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Harris Nwanyanwu K, Talwar N, Gardner TW, et al. Predicting development of proliferative diabetic retinopathy. Diabetes Care 2013;36(6):1562–1568. 10.2337/dc12-0790 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Hainsworth DP, Bebu I, Aiello LP, et al. Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC study. Diabetes Care 2019;42(5):875–882. 10.2337/dc18-2308 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Simonsen JR, Järvinen A, Hietala K, et al. Bacterial infections as novel risk factors of severe diabetic retinopathy in individuals with type 1 diabetes. Br J Ophthalmol 2021;105(8):1104–1110. 10.1136/bjophthalmol-2020-316202 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol 2014;132(11): 1334–1340. 10.1001/jamaophthalmol.2014.2854 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Williams R, Airey M, Baxter H, et al. Epidemiology of diabetic retinopathy and macular oedema: A systematic review. Eye (Lond Engl) 2004;18(10): 963–983. 10.1038/sj.eye.6701476 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 501.Lund SH, Aspelund T, Kirby P, et al. Individualised risk assessment for diabetic retinopathy and optimisation of screening intervals: A scientific approach to reducing healthcare costs. Br J Ophthalmol 2016;100(5):683–687. 10.1136/bjophthalmol-2015-307341 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.DCCT/EDIC Research Group, Nathan DM, Bebu I, et al. Frequency of evidence-based screening for retinopathy in type 1 diabetes. N Engl J Med 2017;376(16):1507–1516. 10.1056/NEJMoa1612836 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Hansen AB, Hartvig NV, Jensen MS, et al. Diabetic retinopathy screening using digital non-mydriatic fundus photography and automated image analysis. Acta ophthalmol Scand 2004;82(6):666–672. 10.1111/j.1600-0420.2004.00350.x [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 504.Scanlon PH. The English National Screening Programme for diabetic retinopathy 2003–2016. Acta diabetol 2017;54(6):515–525. 10.1007/s00592-017-0974-1 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Bursell SE, Fonda SJ, Lewis DG, Horton MB. Prevalence of diabetic retinopathy and diabetic macular edema in a primary care-based teleophthalmology program for American Indians and Alaskan natives. PLOS ONE 2018;13(6): e0198551. 10.1371/journal.pone.0198551 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Mansberger SL, Sheppler C, Barker G, et al. Long-term comparative effectiveness of telemedicine in providing diabetic retinopathy screening examinations: A randomized clinical trial. JAMA Ophthalmol 2015;133(5):518–525. 10.1001/jamaophthalmol.2015.1 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Ahmed J, Ward TP, Bursell SE, et al. The sensitivity and specificity of non-mydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care 2006;29(10):2205–2209. 10.2337/dc06-0295 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 508.Wolf RM, Liu TYA, Thomas C, et al. The SEE Study: safety, efficacy, and equity of implementing autonomous artificial intelligence for diagnosing diabetic retinopathy in youth. Diabetes Care 2021;44(3):781–787. 10.2337/dc20-1671 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 509.Channa R, Wolf R, Abramoff MD. Autonomous artificial intelligence in diabetic retinopathy: From algorithm to clinical application. J Diabetes Sci Technol 2021;15(3):695–698. 10.1177/1932296820909900 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Ting DSW, Cheung CY, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017;318(22): 2211–2223. 10.1001/jama.2017.18152 [EL 3; DS]]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Bellemo V, Lim G, Rim TH, et al. Artificial intelligence screening for diabetic retinopathy: the real-world emerging application. Curr Diabetes Rep 2019;19(9):72. 10.1007/s11892-019-1189-3 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 512.Maa AY, Medert CM, Lu X, et al. Diagnostic accuracy of technology-based eye care services: the Technology-based Eye Care Services Compare Trial Part I. Ophthalmology 2020;127(1):38–44. 10.1016/j.ophtha.2019.07.026 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 513.Conlin PR, Asefzadeh B, Pasquale LR, et al. Accuracy of a technology-assisted eye exam in evaluation of referable diabetic retinopathy and concomitant ocular diseases. Br J Ophthalmol 2015;99(12):1622–1627. 10.1136/bjophthalmol-2014-306536 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 514.Chalk D, Pitt M, Vaidya B, Stein K. Can the retinal screening interval be safely increased to 2 years for type 2 diabetic patients without retinopathy? Diabetes Care 2012;35(8):1663–1668. 10.2337/dc11-2282 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Looker HC, Nyangoma SO, Cromie DT, et al. Predicted impact of extending the screening interval for diabetic retinopathy: the Scottish Diabetic Retinopathy Screening Programme. Diabetologia 2013;56(8):1716–1725. 10.1007/s00125-013-2928-7 [EL 2; RCCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Leese GP, Stratton IM, Land M, et al. Progression of diabetes retinal status within community screening programs and potential implications for screening intervals. Diabetes Care 2015;38(3):488–494. 10.2337/dc14-1778 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 517.Agardh E, Tababat-Khani P. Adopting 3-year screening intervals for sight-threatening retinal vascular lesions in type 2 diabetic subjects without retinopathy. Diabetes Care 2011;34(6):1318–1319. 10.2337/dc10-2308 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care 2017;40(3):412–418. 10.2337/dc16-2641 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Control Diabetes and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care 2000;23(8):1084–1091. 10.2337/diacare.23.8.1084 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Bourry J, Courteville H, Ramdane N, et al. Progression of diabetic retinopathy and predictors of its development and progression during pregnancy in patients with type 1 diabetes: A report of 499 pregnancies. Diabetes Care 2021;44(1):181–187. 10.2337/dc20-0904 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 521.Samuelsson U, Anderzén J, Gudbjörnsdottir S, et al. Teenage girls with type 1 diabetes have poorer metabolic control than boys and face more complications in early adulthood. J Diabetes Its Complications 2016;30(5):917–922. 10.1016/j.jdiacomp.2016.02.007 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 522.Barr CC. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive insulin therapy, by the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N. Engl. J. Med 342:381–9. Surv Ophthalmol. 2001 2000;45(5):459–460. 10.1016/s0039-6257(01)00187-4 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 523.Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group and the Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the action to control cardiovascular risk in diabetes (ACCORD) follow-on study. Diabetes Care 2016;39(7): 1089–1100. 10.2337/dc16-0024 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Aro A, Kauppinen A, Kivinen N, et al. Life style intervention improves retinopathy status-the Finnish Diabetes Prevention Study. Nutrients 2019;11(7): 1691. 10.3390/nu11071691 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Koh ES, Han KD, Kim MK, et al. Weight change and microvascular outcomes in patients with new-onset diabetes: A nationwide cohort study. Korean J Intern Med 2021;36(4):932–941. 10.3904/kjim.2020.121 [EL 2; CS]]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.O'Brien R, Johnson E, Haneuse S, et al. Microvascular outcomes in patients with diabetes after bariatric surgery vs usual care: A matched cohort study. Ann Intern Med 2018;169(5):300–310. 10.7326/M17-2383 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Tikkanen-Dolenc H, Wadén J, Forsblom C, et al. Frequent physical activity is associated with reduced risk of severe diabetic retinopathy in type 1 diabetes. Acta diabetol 2020;57(5):527–534. 10.1007/s00592-019-01454-y [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Sala-Vila A, Díaz-López A, Valls-Pedret C, et al. Dietary marine u-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: prospective investigation from the predimed trial. JAMA Ophthalmol 2016;134(10):1142–1149. 10.1001/jamaophthalmol.2016.2906 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 529.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361(1):40–51. 10.1056/NEJMoa0808400 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Sjølie AK, Klein R, Porta M, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-PROTECT 2): A randomised placebo-controlled trial. Lancet 2008;372(9647):1385–1393. 10.1016/S0140-6736(08)61411-7 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 531.ACCORD Study Group, ACCORD Eye Study Group, Chew EY, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363(3):233–244. 10.1056/NEJMoa1001288 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007;370(9600):1687–1697. 10.1016/S0140-6736(07)61607-9 [EL 1; RCT; substudy]. [DOI] [PubMed] [Google Scholar]
- 533.Morgan CL, Owens DR, Aubonnet P, et al. Primary prevention of diabetic retinopathy with fibrates: A retrospective, matched cohort study. BMJ Open 2013;3(12):e004025. 10.1136/bmjopen-2013-004025 [EL 2; RCCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Scott IU, Jackson GR, Quillen DA, et al. Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in patients with severe nonproliferative or non-high-risk proliferative diabetic retinopathy: A randomized clinical trial. JAMA Ophthalmol 2014;132(5):535–543. 10.1001/jamaophthalmol.2014.93 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 535.Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA Ophthalmol 2018;136(10): 1138–1148. 10.1001/jamaophthalmol.2018.3255 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Maguire MG, Liu D, Glassman AR, et al. Visual field changes over 5 years in patients treated with panretinal photocoagulation or ranibizumab for proliferative diabetic retinopathy. JAMA Ophthalmol 2020;138(3):285–293. 10.1001/jamaophthalmol.2019.5939 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Obeid A, Su D, Patel SN, et al. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation vs intravitreal anti-vascular endothelial growth factor. Ophthalmology 2019;126(3):407–413. 10.1016/j.ophtha.2018.07.027 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 538.Weiss M, Sim DA, Herold T, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti-vasular endothelial growth factor therapy in daily practice. Retina 2018;38(12):2293–2300. 10.1097/IAE.0000000000001892 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 539.Arevalo JF, Beatson B, Pan-American Collaborative Retina Study Group (PACORES). Lessons learned from pacores in proliferative diabetic retinopathy management, data from Latin America and Spain: the Asbury Lecture 2020. Retina 2022;42(1):4–10. 10.1097/IAE.0000000000003226 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 540.Smiddy WE, Flynn HW Jr. Vitrectomy in the management of diabetic retinopathy. Surv Ophthalmol 1999;43(6):491–507. 10.1016/s0039-6257(99)00036-3 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 541.Bressler SB, Glassman AR, Almukhtar T, et al. Five-year outcomes of ranibizumab with prompt or deferred laser vs laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol 2016;164:57–68. 10.1016/j.ajo.2015.12.025 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Braffett BH, Gubitosi-Klug RA, Albers JW, et al. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2020;69(5): 1000–1010. 10.2337/db19-1046 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Mizokami-Stout KR, Li Z, Foster NC, et al. The contemporary prevalence of diabetic neuropathy in type 1 diabetes: findings from the T1D Exchange. Diabetes Care 2020;43(4):806–812. 10.2337/dc19-1583 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Jeyam A, McGurnaghan SJ, Blackbourn LAK, et al. Diabetic neuropathy is a substantial burden in people with type 1 diabetes and is strongly associated with socioeconomic disadvantage: A population-representative study from Scotland. Diabetes Care 2020;43(4):734–742. 10.2337/dc19-1582 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 545.Andersen ST, Witte DR, Fleischer J, et al. Risk factors for the presence and progression of cardiovascular autonomic neuropathy in type 2 diabetes: ADDITION-Denmark. Diabetes Care 2018;41(12):2586–2594. 10.2337/dc18-1411 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 546.Mather KJ, Bebu I, Baker C, et al. Prevalence of microvascular and macrovascular disease in the glycemia reduction approaches in diabetes - a comparative effectiveness (GRADE) study cohort. Diabetes Res Clin Pract 2020;165:108235. 10.1016/j.diabres.2020.108235 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care 2017;40(1): 136–154. 10.2337/dc16-2042 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Ziegler D, Strom A, Lobmann R, et al. High prevalence of diagnosed and undiagnosed polyneuropathy in subjects with and without diabetes participating in a nationwide educational initiative (PROTECT study). J Diabetes Its Complications 2015;29(8):998–1002. 10.1016/j.jdiacomp.2015.09.008 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 549.Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications study. Diabetologia 1996;39(11): 1377–1384. 10.1007/s001250050586 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 550.Bongaerts BW, Ziegler D, Shaw JE, et al. A clinical screening score for diabetic polyneuropathy: KORA F4 and AUDDIAB studies. J Diabetes Its Complications 2015;29(1):44–49. 10.1016/j.jdiacomp.2014.09.014 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 551.Andersen ST, Witte DR, Dalsgaard EM, et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care 2018;41(5):1068–1075. 10.2337/dc17-2062 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 552.D'Amato C, Morganti R, Greco C, et al. Diabetic peripheral neuropathic pain is a stronger predictor of depression than other diabetic complications and comorbidities. Diabetes Vasc Dis Res 2016;13(6):418–428. 10.1177/1479164116653240 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 553.Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol 2018;5(4):397–405. 10.1002/acn3.531 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Khoshnoodi MA, Truelove S, Burakgazi A, et al. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol 2016;73(6):684–690. 10.1001/jama-neurol.2016.0057 [EL 2; CCS]. [DOI] [PubMed] [Google Scholar]
- 555.Pop-Busui R, Boulton AJM, Sosenko JM. Peripheral and autonomic neuropathy in diabetes. In: Cowie CC, Casagrande SS, Menke A, et al. , eds. Diabetes in America Bethesda (MD) Interest. US: National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [EL 4; NE]. [PubMed] [Google Scholar]
- 556.Truini A, Spallone V, Morganti R, et al. A cross-sectional study investigating frequency and features of definitely diagnosed diabetic painful polyneuropathy. Pain 2018;159(12):2658–2666. 10.1097/j.pain.0000000000001378 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 557.Ang L, Cowdin N, Mizokami-Stout K. Pop-Busui R Update on the management of diabetic neuropathy. Diabetes Spectr 2018;31(3):224–233. 10.2337/ds18-0036 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Alam U, Sloan G, Tesfaye S. Treating pain in diabetic neuropathy: current and developmental drugs. Drugs 2020;80(4):363–384. 10.1007/s40265-020-01259-2 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 559.Gylfadottir SS, Christensen DH, Nicolaisen SK, et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: A cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. Pain 2020;161(3):574–583. 10.1097/j.pain.0000000000001744 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Ziegler D, Rathmann W, Dickhaus T, et al. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg surveys S2 and S3. Pain Med 2009;10(2):393–400. 10.1111/j.1526-4637.2008.00555.x [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 561.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 2014;13(9):924–935. 10.1016/S1474-4422(14)70102-4 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 562.O'Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 2009;27(2):95–112. 10.2165/00019053-200927020-00002 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 563.Sadosky A, Hopper J, Parsons B. Painful diabetic peripheral neuropathy: results of a survey characterizing the perspectives and misperceptions of patients and healthcare practitioners. Patient 2014;7(1):107–114. 10.1007/s40271-013-0038-8 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Wang F, Zhang J, Yu J, et al. Diagnostic accuracy of monofilament tests for detecting diabetic peripheral neuropathy: A systematic review and meta-analysis. J Diabetes Res 2017;2017:8787261. 10.1155/2017/8787261 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Callaghan BC, Kerber KA, Lisabeth LL, et al. Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol 2014;71(9):1143–1149. 10.1001/jamaneurol.2014.1279 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Jensen TS, Karlsson P, Gylfadottir SS, et al. Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain 2021;144(6):1632–1645. 10.1093/brain/awab079 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Rajan S, Campagnolo M, Callaghan B, Gibbons CH. Sudomotor function testing by electrochemical skin conductance: does it really measure sudomotor function? Clin Auton Res 2019;29(1):31–39. 10.1007/s10286-018-0540-0 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 568.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33(10):2285–2293. 10.2337/dc10-1303 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Martin CL, Albers JW, Pop-Busui R, DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37(1):31–38. 10.2337/dc13-2114 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Pop-Busui R, Lu J, Brooks MM, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the bypass angioplasty revascularization investigation 2 diabetes (BARI 2D) cohort. Diabetes Care 2013;36(10):3208–3215. 10.2337/dc13-0012 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Zilliox LA, Ruby SK, Singh S, Zhan M, Russell JW. Clinical neuropathy scales in neuropathy associated with impaired glucose tolerance. J Diabetes Its Complications 2015;29(3):372–377. 10.1016/j.jdiacomp.2015.01.011 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 2002;25(11):2048–2052. 10.2337/diacare.25.11.2048 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 573.Singleton JR, Bixby B, Russell JW, et al. The Utah Early Neuropathy Scale: A sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst JPNS 2008;13(3):218–227. 10.1111/j.1529-8027.2008.00180.x [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 574.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36(2):150–154. 10.1007/BF00400697 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 575.Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31(8): 1679–1685. 10.2337/dc08-9021 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366(9498):1719–1724. 10.1016/S0140-6736(05)67698-2 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 577.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376(24):2367–2375. 10.1056/NEJMra1615439 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 578.Crawford F, Cezard G, Chappell FM, et al. A systematic review and individual patient data meta-analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS). Health Technol Assess (Winchester, England) 2015;19(57):1–210. 10.3310/hta19570 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Miller JD, Carter E, Shih J, et al. Erratum. J Fam Pract 2015;64(8):452 [EL 4; NE]. [PubMed] [Google Scholar]
- 580.Schmidt BM, Holmes CM, Ye W, Pop-Busui R. A tale of two eras: mining big data from electronic health records to determine limb salvage rates with podiatry. Curr Diabetes Rev 2019;15(6):497–502. 10.2174/1573399814666181017104818 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Its Complications 2006;20(4):216–223. 10.1016/j.jdiacomp.2005.07.005 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 582.Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Its Complications 2012;26(5):424–429. 10.1016/j.jdiacomp.2012.05.007 [EL 2; CS; pilot]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Streckmann F, Zopf EM, Lehmann HC, et al. Exercise intervention studies in patients with peripheral neuropathy: A systematic review. Sports Med (Auckland NZ) 2014;44(9):1289–1304. 10.1007/s40279-014-0207-5 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 584.Singleton JR, Marcus RL, Jackson JE, et al. Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann Clin Transl Neurol 2014;1(10):844–849. 10.1002/acn3.125 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 2006;29(6):1294–1299. 10.2337/dc06-0224 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 586.Otis JD, Sanderson K, Hardway C, et al. A randomized controlled pilot study of a cognitive-behavioral therapy approach for painful diabetic peripheral neuropathy. J Pain 2013;14(5):475–482. 10.1016/j.jpain.2012.12.013 [EL 1; RCT; pilot]. [DOI] [PubMed] [Google Scholar]
- 587.Aghili R, Malek M, Tanha K, Mottaghi A. The effect of bariatric surgery on peripheral polyneuropathy: A systematic review and meta-analysis. Obes Surg 2019;29(9):3010–3020. 10.1007/s11695-019-04004-1 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 588.Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011;76(20):1758–1765. 10.1212/WNL.0b013e3182166ebe [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Derry S, Bell RF, Straube S, et al. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev 2019;1(1):CD007076. 10.1002/14651858.CD007076. pub3 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Huffman C, Stacey BR, Tuchman M, et al. Efficacy and safety of pregabalin in the treatment of patients with painful diabetic peripheral neuropathy and pain on walking. Clin J Pain 2015;31(11):946–958. 10.1097/AJP.0000000000000198 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 591.Mimenza Alvarado A, Aguilar Navarro S. Clinical trial assessing the efficacy of gabapentin plus B complex (b1/b12) vs pregabalin for treating painful diabetic neuropathy. J Diabetes Res 2016;2016:4078695. 10.1155/2016/4078695 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Parsons B, Li C. The efficacy of pregabalin in patients with moderate and severe pain due to diabetic peripheral neuropathy. Curr Med Res Opin 2016;32(5):929–937. 10.1185/03007995.2016.1151776 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 593.Raskin P, Huffman C, Yurkewicz L, et al. Pregabalin in patients with painful diabetic peripheral neuropathy using an NSAID for other pain conditions: A double-blind crossover study. Clin J Pain 2016;32(3):203–210. 10.1097/AJP.0000000000000254 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 594.Roy MK, Kuriakose AS, Varma SK, Jacob LA, Beegum NJ. A study on comparative efficacy and cost effectiveness of pregabalin and duloxetine used in diabetic neuropathic pain. Diabetes Metab Syndr 2017;11(1):31–35. 10.1016/j.dsx.2016.07.003 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 595.Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”ea multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain 2013;154(12):2616–2625. 10.1016/j.pain.2013.05.043 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 596.Arnold LM, McCarberg BH, Clair AG, et al. Dose-response of pregabalin for diabetic peripheral neuropathy, postherpetic neuralgia, and fibromyalgia. Postgrad Med 2017;129(8):921–933. 10.1080/00325481.2017.1384691 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 597.Parsons B, Emir B, Knapp L. Examining the time to improvement of sleep interference with pregabalin in patients with painful diabetic peripheral neuropathy and postherpetic neuralgia. Am J Ther 2015;22(4):257–268. 10.1097/MJT.0000000000000100 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 598.Pérez C, Latymer M, Almas M, et al. Does duration of neuropathic pain impact the effectiveness of pregabalin? Pain Pract Off J World Inst Pain 2017;17(4): 470–479. 10.1111/papr.12469 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 599.Yasuda H, Hotta N, Kasuga M, et al. Efficacy and safety of 40 mg or 60 mg duloxetine in Japanese adults with diabetic neuropathic pain: results from a randomized, 52-week, open-label study. J Diabetes Investig 2016;7(1): 100–108. 10.1111/jdi.12361 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Majdinasab N, Kaveyani H, Azizi M. A comparative double-blind randomized study on the effectiveness of duloxetine and gabapentin on painful diabetic peripheral polyneuropathy. Drug Des Dev Ther 2019;13:1985–1992. 10.2147/DDDT.S185995 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: A randomized, double-blind, placebo-controlled study. J Pain 2017;18(1):42–53. 10.1016/j.jpain.2016.09.008 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 602.Vinik AI, Perrot S, Vinik EJ, et al. Capsaicin 8% patch repeat treatment plus standard of care (SOC) vs SOC alone in painful diabetic peripheral neuropathy: A randomised, 52-week, open-label, safety study. BMC Neurol 2016;16(1): 251. 10.1186/s12883-016-0752-7 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin 2011;27(1):151–162. 10.1185/03007995.2010.537589 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 604.Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care 2014;37(8):2302–2309. 10.2337/dc13-2291 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 605.Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2017;6(6):Cd007938. 10.1002/14651858.CD007938. pub4 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: A randomized clinical trial. JAMA Neurol 2021;78(6):687–698. 10.1001/jamaneurol.2021.0538 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Petersen EA, Stauss TG, Scowcroft JA, et al. Durability of high-frequency 10-kHz spinal cord stimulation for patients with painful diabetic neuropathy refractory to conventional treatments: 12-month results from a randomized controlled trial. Diabetes Care 2022;45(1):e3–e6. 10.2337/dc21-1813 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Khan KS, Andersen H. The impact of diabetic neuropathy on activities of daily living, postural balance and risk of falls - a systematic review. J Diabetes Sci Technol 2022;1932296821997921:289–294. 10.1177/1932296821997921 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Khan KS, Christensen DH, Nicolaisen SK, et al. Falls and fractures associated with type 2 diabetic polyneuropathy: A cross-sectional nationwide questionnaire study. J Diabetes Investig 2021;12(10):1827–1834. 10.1111/jdi.13542 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Venkataraman K, Tai BC, Khoo EYH, et al. Short-term strength and balance training does not improve quality of life but improves functional status in individuals with diabetic peripheral neuropathy: A randomised controlled trial. Diabetologia 2019;62(12):2200–2210. 10.1007/s00125-019-04979-7 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Tang Y, Shah H, Bueno Junior CR, et al. Intensive risk factor management and cardiovascular autonomic neuropathy in type 2 diabetes: the ACCORD trial. Diabetes Care 2021;44(1):164–173. 10.2337/dc20-1842 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Zoppini G, Cacciatori V, Raimondo D, et al. Prevalence of cardiovascular autonomic neuropathy in a cohort of patients with newly diagnosed type 2 diabetes: the Verona Newly Diagnosed type 2 Diabetes Study (VNDS). Diabetes Care 2015;38(8):1487–1493. 10.2337/dc15-0081 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 613.Cseh D, Climie RE, Offredo L, et al. Type 2 diabetes mellitus is independently associated with decreased neural baroreflex sensitivity: the Paris Prospective Study III. Arterioscler Thromb Vasc Biol 2020;40(5):1420–1428. 10.1161/ATVBAHA.120.314102 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 614.Ang L, Dillon B, Mizokami-Stout K, Pop-Busui R. Cardiovascular autonomic neuropathy: A silent killer with long reach. Auton Neurosci Basic Clin 2020;225:102646. 10.1016/j.autneu.2020.102646 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 615.Jaiswal M, Divers J, Urbina EM, et al. Cardiovascular autonomic neuropathy in adolescents and young adults with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth cohort study. Pediatr Diabetes 2018;19(4):680–689. 10.1111/pedi.12633 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Ziegler D, Voss A, Rathmann W, et al. Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: the KORA S4 survey. Diabetologia 2015;58(5):1118–1128. 10.1007/s00125-015-3534-7 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 617.Pop-Busui R, Evans GW, Gerstein HC, et al. Effects of cardiac autonomic dysfunction on mortality risk in the action to control cardiovascular risk in diabetes (ACCORD) trial. Diabetes Care 2010;33(7):1578–1584. 10.2337/dc10-0125 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Pop-Busui R, Cleary PA, Braffett BH, et al. Association between cardiovascular autonomic neuropathy and left ventricular dysfunction: DCCT/EDIC study (diabetes control and complications trial/epidemiology of diabetes interventions and complications). J Am Coll Cardiol 2013;61(4):447–454. 10.1016/j.jacc.2012.10.028 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Pop-Busui R, Kirkwood I, Schmid H, et al. Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol 2004;44(12):2368–2374. 10.1016/j.jacc.2004.09.033 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 620.Pop-Busui R, Braffett BH, Zinman B, et al. Cardiovascular autonomic neuropathy and cardiovascular outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 2017;40(1):94–100. 10.2337/dc16-1397 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Sacre JW, Franjic B, Jellis CL, et al. Association of cardiac autonomic neuropathy with subclinical myocardial dysfunction in type 2 diabetes. JACC Cardiovasc Imaging 2010;3(12):1207–1215. 10.1016/j.jcmg.2010.09.014 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 622.Wheelock KM, Jaiswal M, Martin CL, et al. Cardiovascular autonomic neuropathy associates with nephropathy lesions in American Indians with type 2 diabetes. J Diabetes Its Complications 2016;30(5):873–879. 10.1016/j.jdiacomp.2016.03.008 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Nattero-Chávez L, Redondo López S, Alonso Díaz S, et al. Association of cardiovascular autonomic dysfunction with peripheral arterial stiffness in patients with type 1 diabetes. J Clin Endocrinol Metab 2019;104(7):2675–2684. 10.1210/jc.2018-02729 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 624.Matsutani D, Sakamoto M, Iuchi H, et al. Glycemic variability in continuous glucose monitoring is inversely associated with baroreflex sensitivity in type 2 diabetes: A preliminary report. Cardiovasc Diabetol 2018;17(1):36. 10.1186/s12933-018-0683-2 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Orlov S, Cherney DZ, Pop-Busui R, et al. Cardiac autonomic neuropathy and early progressive renal decline in patients with nonmacroalbuminuric type 1 diabetes. Clin J Am Soc Nephrol CJASN 2015;10(7):1136–1144. 10.2215/CJN.11441114 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011;27(7):639–653. 10.1002/dmrr.1239 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 627.Pop-Busui R, Backlund JC, Bebu I, et al. Utility of using electrocardiogram measures of heart rate variability as a measure of cardiovascular autonomic neuropathy in type 1 diabetes patients. J Diabetes Investig 2022;13(1): 125–133. 10.1111/jdi.13635 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Pop-Busui R, Low PA, Waberski BH, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009; 119(22):2886–2893. 10.1161/CIRCULATIONAHA.108.837369 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Lachin JM, Bebu I, Bergenstal RM, et al. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the Diabetes Control and Complications Trial. Diabetes Care 2017;40(6):777–783. 10.2337/dc16-2426 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348(5): 383–393. 10.1056/NEJMoa021778 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 631.Carnethon MR, Prineas RJ, Temprosa M, et al. The association among autonomic nervous system function, incident diabetes, and intervention arm in the diabetes prevention program. Diabetes Care 2006;29(4):914–919. 10.2337/diacare.29.04.06.dc05-1729 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care 2005;28(4):956–962. 10.2337/diacare.28.4.956 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 633.England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: A definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64(2):199–207. 10.1212/01.WNL.0000149522, 32823.EA [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 634.Dyck PJ, Davies JL, Clark VM, et al. Modeling chronic glycemic exposure variables as correlates and predictors of microvascular complications of diabetes. Diabetes Care 2006;29(10):2282–2288. 10.2337/dc06-0525 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 635.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352(4):341–350. 10.1056/NEJMoa032782 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 636.Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: A reversible painful autonomic neuropathy. Ann Neurol 2010;67(4):534–541. 10.1002/ana.21952 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Tesfaye S, Vileikyte L, Rayman G, et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev 2011;27(7):629–638. 10.1002/dmrr.1225 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 638.Pittenger GL, Mehrabyan A, Simmons K, et al. Small fiber neuropathy is associated with the metabolic syndrome. Metab Syndr Relat Disord 2005;3(2): 113–121. 10.1089/met.2005.3.113 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 639.Singleton JR, Smith AG. Bromberg MB Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001;24(8):1448–1453. 10.2337/diacare.24.8.1448 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 640.Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve 2001;24(9): 1225–1228. 10.1002/mus.1136 [EL 3; CCS]. [DOI] [PubMed] [Google Scholar]
- 641.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018;392(10157):1519–1529. 10.1016/S0140-6736(18)32261-X [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 642.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019;394(10193):121–130. 10.1016/S0140-6736(19)31149-3 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 643.Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9(10):653–662. 10.1016/S2213-8587(21)00203-5 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 644.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377(13): 1228–1239. 10.1056/NEJMoa1612917 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373(23): 2247–2257. 10.1056/NEJMoa1509225 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 646.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381(9):841–851. 10.1056/NEJMoa1901118 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 647.Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes mellitus and heart failure, a scientific statement from the American Heart Association and Heart Failure Society of America. J Card Fail 2019;25(8):584–619. 10.1016/j.cardfail.2019.05.007 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 648.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383(15):1425–1435. 10.1056/NEJMoa2004967 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 649.McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A meta-analysis. JAMA Cardiol 2021;6(2):148–158. 10.1001/jama-cardio.2020.4511 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation 2019;140(18):1463–1476. 10.1161/CIRCULATIONAHA.119.042929 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 651.Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396(10254):819–829. 10.1016/S0140-6736(20)31824-9 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 652.Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat Med 2021;27(11):1954–1960. 10.1038/s41591-021-01536-x [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384(2):117–128. 10.1056/NEJMoa2030183 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 654.Ren Z, Fu X. Stroke risk factors in United States: an analysis of the 2013–2018 National Health and Nutrition Examination Survey. Int J Gen Med 2021;14: 6135–6147. 10.2147/IJGM.S327075 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 2018;391(10138):2430–2440. 10.1016/S0140-6736(18)30314-3 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 656.Liu L, Simon B, Shi J, Mallhi AK, Eisen HJ. Impact of diabetes mellitus on risk of cardiovascular disease and all-cause mortality: evidence on health outcomes and antidiabetic treatment in United States adults. World J Diabetes 2016;7(18):449–461. 10.4239/wjd.v7.i18.449 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Sharma A, Green JB, Dunning A, et al. Causes of death in a contemporary cohort of patients with type 2 diabetes and atherosclerotic cardiovascular disease: insights from the tecos trial. Diabetes Care 2017;40(12):1763–1770. 10.2337/dc17-1091 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 658.Abreu P, Magalh~aes R, Baptista D, Azevedo E, Correia M. Admission and readmission/death patterns in hospitalized and non-hospitalized first-ever-in-a-lifetime stroke patients during the first year: A population-based incidence study. Front Neurol 2021;12:685821. 10.3389/fneur.2021.685821 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.MacIntosh BJ, Cohen E, Colby-Milley J, et al. Diabetes mellitus is associated with poor in-hospital and long-term outcomes in young and midlife stroke survivors. J Am Heart Assoc 2021;10(14):e019991. 10.1161/JAHA.120.019991 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Grewal S, Zaman N, Borgatta L, et al. Usefulness of glucagon-like peptide-1 receptor agonists to reduce adverse cardiovascular disease events in patients with type 2 diabetes mellitus. Am J Cardiol 2021;154:48–53. 10.1016/j.amjcard.2021.05.043 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 661.Malhotra K, Katsanos AH, Lambadiari V, et al. GLP-1 receptor agonists in diabetes for stroke prevention: A systematic review and meta-analysis. J Neurol 2020;267(7):2117–2122. 10.1007/s00415-020-09813-4 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 662.Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76(9):1117–1145. 10.1016/j.jacc.2020.05.037 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROACTIVE study (Prospective Pioglitazone Clinical Trial in Macrovascular Events): A randomised controlled trial. Lancet 2005;366(9493):1279–1289. 10.1016/S0140-6736(05)67528-9 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 664.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374(14):1321–1331. 10.1056/NEJMoa1506930 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Spence JD, Viscoli CM, Inzucchi SE, et al. Pioglitazone therapy in patients with stroke and prediabetes: A post hoc analysis of the IRIS randomized clinical trial. JAMA Neurol 2019;76(5):526–535. 10.1001/jama-neurol.2019.0079 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Liao HW, Saver JL, Wu YL, et al. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, prediabetes and type 2 diabetes: A systematic review and meta-analysis. BMJ Open 2017;7(1):e013927. 10.1136/bmjopen-2016-013927 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Lee M, Saver JL, Liao HW, Lin CH, Ovbiagele B. Pioglitazone for secondary stroke prevention: A systematic review and meta-analysis. Stroke 2017;48(2):388–393. 10.1161/STROKEAHA.116.013977 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 668.Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists - executive summary. Endocr Pract 2019;25(12):1346–1359. 10.4158/GL-2019-0406 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 669.Sumithran P Proietto J The defence of body weight: A physiological basis for weight regain after weight loss. Clin Sci 2013;124(4):231–241. 10.1042/CS20120223 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 670.Mechanick JI, Farkouh ME, Newman JD. Garvey WT Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers: JACC state-of-the-art review. J Am Coll Cardiol 2020;75(5):525–538. 10.1016/j.jacc.2019.11.044 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Reaven G Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol 2012;32(8):1754–1759. 10.1161/ATVBAHA.111.241885 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 672.Mechanick JI, Hurley DL. Garvey WT Adiposity-based chronic disease as a new diagnostic term: the American Association of Clinical Endocrinologists and American College of Endocrinology position statement. Endocr Pract 2017;23(3):372–378. 10.4158/EP161688.PS [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 673.Frühbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts 2019;12(2):131–136. 10.1159/000497124 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016;22(suppl 3):1–203. 10.4158/EP161365. GL [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 675.Garvey WT, Garber AJ, Mechanick JI, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the 2014 advanced framework for a new diagnosis of obesity as a chronic disease. Endocr Pract 2014;20(9):977–989. 10.4158/EP14280.PS [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640–1645. 10.1161/CIRCULATIONAHA.109.192644 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 677.Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adultsestudy on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002;15(1):83–96 [EL 2; MNRCT]. [PubMed] [Google Scholar]
- 678.Look AHEAD Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369(2): 145–154. 10.1056/NEJMoa1212914 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019;42(5): 731–754. 10.2337/dci19-0014 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009;169(17):1619–1626. 10.1001/archinternmed.2009.266 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Kuna ST, Reboussin DM, Borradaile KE, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep 2013;36(5):641–649A. 10.5665/sleep.2618 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep 2012;35(11):1529–1539. 10.5665/sleep.2204 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51(1):121–129. 10.1002/hep.23276 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Liu X, Lazenby AJ, Clements RH, Jhala N, Abrams GA. Resolution of nonalcoholic steatohepatits after gastric bypass surgery. Obes Surg 2007;17(4): 486–492. 10.1007/s11695-007-9086-2 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 685.Barker KB, Palekar NA, Bowers SP, et al. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol 2006;101(2): 368–373. 10.1111/j.1572-0241.2006.00419.x [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 686.Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract 2022;28(5):528–562. 10.1016/j.eprac.2022.03.010 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 687.Terranova CO, Brakenridge CL, Lawler SP, Eakin EG, Reeves MM. Effectiveness of lifestyle-based weight loss interventions for adults with type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab 2015;17(4): 371–378. 10.1111/dom.12430 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 688.Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet 2015;115(9):1447–1463. 10.1016/j.jand.2015.02.031 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 689.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17(4):713–722. 10.1038/oby.2008.637 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.. Look AHEAD Research Group, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the look ahead trial. Arch Intern Med 2010;170(17):1566–1575. 10.1001/archinternmed.2010.334 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DIRECT): an open-label, cluster-randomised trial. Lancet 2018;391(10120):541–551. 10.1016/S0140-6736(17)33102-1 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 692.Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DIRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7(5): 344–355. 10.1016/S2213-8587(19)30068-3 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 693.Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: A systematic review. Diabetes Res Clin Pract 2010;89(2):97–102. 10.1016/j.diabres.2010.04.019 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 694.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359(3):229–241. 10.1056/NEJMoa0708681 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 695.Esposito K, Maiorino MI, Ciotola M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: A randomized trial. Ann Intern Med 2009;151(5): 306–314. 10.7326/0003-4819-151-5-200909010-00004 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 696.Esposito K, Maiorino MI, Di Palo C, Giugliano D, Campanian Postprandial Hyperglycemia Study Group. Adherence to a Mediterranean diet and glycaemic control in type 2 diabetes mellitus. Diabet Med 2009;26(9):900–907. 10.1111/j.1464-5491.2009.02798.x [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 697.Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: A 1-year prospective randomized intervention study. Diabetes Obes Metab 2010;12(3): 204–209. 10.1111/j.1463-1326.2009.01151.x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 698.Galbete C, Schwingshackl L, Schwedhelm C, Boeing H, Schulze MB. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: an umbrella review of meta-analyses. Eur J Epidemiol 2018;33(10):909–931. 10.1007/s10654-018-0427-3 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: A meta-analysis. Eur J Clin Nutr 2015;69(11):1200–1208. 10.1038/ejcn.2014.243 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 700.Itsiopoulos C, Brazionis L, Kaimakamis M, et al. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr Metab Cardiovasc Dis NMCD 2011;21(9):740–747. 10.1016/j.numecd.2010.03.005 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 701.Vega-López S, Venn BJ, Slavin JL. Relevance of the glycemic index and glycemic load for body weight, diabetes, and cardiovascular disease. Nutrients 2018;10(10):1361. 10.3390/nu10101361 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Brehm BJ, Lattin BL, Summer SS, et al. One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care 2009;32(2):215–220. 10.2337/dc08-0687 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Davis NJ, Tomuta N, Schechter C, et al. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet vs a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care 2009;32(7): 1147–1152. 10.2337/dc08-2108 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Gardner CD, Trepanowrski JF, Del Gobbo LC, et al. Effect of low-fat vs low-carbohydrate diet on 12-month rweight loss in overweight adults and the association with genotype pattern or insulin secretion: the dietfits randomized clinical trial. JAMA 2018;319(7):667–679. 10.1001/jama.2018.0245 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Saslow LR, Daubenmier JJ, Moskowitz JT, et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate vs very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr Diabetes 2017;7(12):304. 10.1038/s41387-017-0006-9 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ (Clin Res Ed) 2021;372:m4743. 10.1136/bmj.m4743 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Sainsbury E, Kizirian NV, Partridge SR, et al. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract 2018;139:239–252. 10.1016/j.diabres.2018.02.026 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 708.van Zuuren EJ, Fedorowicz Z, Kuijpers T, Pijl H. Effects of low-carbohydrate-compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: A systematic review including grade assessments. Am J Clin Nutr 2018;108(2):300–331. 10.1093/ajcn/nqy096 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 709.Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care 2017;5(1):e000354. 10.1136/bmjdrc-2016-000354 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Guldbrand H, Dizdar B, Bunjaku B, et al. In type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss. Diabetologia 2012;55(8):2118–2127. 10.1007/s00125-012-2567-4 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Tay J, Thompson CH, Luscombe-Marsh ND, et al. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet vs a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes Metab 2018;20(4):858–871. 10.1111/dom.13164 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 712.Mayer SB, Jeffreys AS, Olsen MK, et al. Two diets with different haemoglobin A1c and antiglycaemic medication effects despite similar weight loss in type 2 diabetes. Diabetes Obes Metab 2014;16(1):90–93. 10.1111/dom.12191 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Wheatley SD, Deakin TA, Arjomandkhah NC, Hollinrake PB, Reeves TE. Low carbohydrate dietary approaches for people with type 2 diabetes-a narrative review. Front Nutr 2021;8:687658. 10.3389/fnut.2021.687658 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 2015;31(1):1–13. 10.1016/j.nut.2014.06.011 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 715.Noto H, Goto A, Tsujimoto T, Noda M. Long-term low-carbohydrate diets and type 2 diabetes risk: A systematic review and meta-analysis of observational studies. J Gen Fam Med 2016;17(1):60–70. 10.14442/jgfm.17.1_60 [EL 2; MNRCT]. [DOI] [Google Scholar]
- 716.Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89(5):1588se1596s. 10.3945/ajcn.2009.26736H [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Barnard ND, Katcher HI, Jenkins DJ, Cohen J, Turner-McGrievy G. Vegetarian and vegan diets in type 2 diabetes management. Nutr Rev 2009;67(5): 255–263. 10.1111/j.1753-4887.2009.00198.x [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 718.Nicholson AS, Sklar M, Barnard ND, et al. Toward improved management of NIDDM: A randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med 1999;29(2):87–91. 10.1006/pmed.1999.0529 [EL 1; RCT pilot]. [DOI] [PubMed] [Google Scholar]
- 719.Rinaldi S, Campbell EE, Fournier J, O'Connor C, Madill J. A comprehensive review of the literature supporting recommendations from the Canadian Diabetes Association for the use of a plant-based diet for management of type 2 diabetes. Can J Diabetes 2016;40(5):471–477. 10.1016/j.jcjd.2016.02.011 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 720.Pawlak R Vegetarian diets in the prevention and management of diabetes and its complications. Diabetes Spectr 2017;30(2):82–88. 10.2337/ds16-0057 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Azadbakht L, Fard NR, Karimi M, et al. Effects of the dietary approaches to stop hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: A randomized crossover clinical trial. Diabetes Care 2011;34(1):55–57. 10.2337/dc10-0676 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Paula TP, Viana LV, Neto AT, et al. Effects of the DASH diet and walking on blood pressure in patients with type 2 diabetes and uncontrolled hypertension: A randomized controlled trial. J Clin Hypertens (Greenwich Conn) 2015;17(11):895–901. 10.1111/jch.12597 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the healthy eating index, alternate healthy eating index, Dietary Approaches to Stop Hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2018;118(1): 74–100.e111. 10.1016/j.jand.2017.08.024 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 724.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378(25):e34. 10.1056/NEJMoa1800389 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 725.Salas-Salvadó J, Díaz-López A, Ruiz-Canela M, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the predimed-plus trial. Diabetes Care 2019;42(5):777–788. 10.2337/dc18-0836 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 726.de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999;99(6): 779–785. 10.1161/01.cir.99.6.779 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 727.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes. Diabetes Care 2010;33(12):e147–e167. 10.2337/dc10-9990 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Arnett DK, Blumenthal RS, Albert MAMA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74(10):e177–e232. 10.1016/j.jacc.2019.03.010 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34(7):1481–1486. 10.2337/dc10-2415 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Gregg EW, Chen H, Wagenknecht LE, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012;308(23): 2489–2496. 10.1001/jama.2012.67929 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med 2012;366(13):1209–1217. 10.1056/NEJMoa1110294 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Rubin RR, Wadden TA, Bahnson JL, et al. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: the Look AHEAD trial. Diabetes Care 2014;37(6):1544–1553. 10.2337/dc13-1928 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Look AHEAD Research Group. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: A secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2014;2(10):801–809. 10.1016/S2213-8587(14)70156-1 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Look AHEAD Research Group, Gregg EW, Jakicic JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4(11):913–921. 10.1016/S2213-8587(16)30162-0 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Miles JM, Leiter L, Hollander P, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care 2002;25(7):1123–1128. 10.2337/diacare.25.7.1123 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 736.Hanefeld M, Sachse G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: A randomized, placebo-controlled trial. Diabetes Obes Metab 2002;4(6):415–423. 10.1046/j.1463-1326.2002.00237.x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 737.Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care 1998;21(8):1288–1294. 10.2337/diacare.21.8.1288 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 738.Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013;36(12):4022–4029. 10.2337/dc13-0234 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Ruof J, Golay A, Berne C, et al. Orlistat in responding obese type 2 diabetic patients: meta-analysis findings and cost-effectiveness as rationales for reimbursement in Sweden and Switzerland. Int J Obes (Lond) 2005;29(5): 517–523. 10.1038/sj.ijo.0802925 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 740.Garvey WT, Ryan DH, Bohannon NJ, et al. Weight-loss therapy in type 2 diabetes: effects of phentermine and topiramate extended release. Diabetes Care 2014;37(12):3309–3316. 10.2337/dc14-0930 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 741.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes Randomized Clinical Trial. JAMA 2015;314(7):687–699. 10.1001/jama.2015.9676 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 742.Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2$4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021;397(10278):971–984. 10.1016/S0140-6736(21)00213-0 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 743.Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate vs phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21(11):2163–2171. 10.1002/oby.20584 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 744.Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity (Silver Spring) 2011;19(12): 2351–2360. 10.1038/oby.2011.94 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 745.O'Meara S, Riemsma R, Shirran L, Mather L, ter Riet G. A systematic review of the clinical effectiveness of orlistat used for the management of obesity. Obes Rev 2004;5(1):51–68. 10.1111/j.1467-789x.2004.00125.x [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 746.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012;95(2):297–308. 10.3945/ajcn.111.024927 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: A randomized controlled trial (EQUIP). Obesity (Silver Spring) 2012;20(2):330–342. 10.1038/oby.2011.330 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): A randomised, placebo-controlled, phase 3 trial. Lancet 2011;377(9774):1341–1352. 10.1016/S0140-6736(11)60205-5 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 749.Henry RR, Chilton R, Garvey WT. New options for the treatment of obesity and type 2 diabetes mellitus (narrative review). J Diabetes Its Complications 2013;27(5):508–518. 10.1016/j.jdiacomp.2013.04.011 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone sr/bupropion sr on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring) 2013;21(5):935–943. 10.1002/oby.20309 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone sr/bupropion sr combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19(1):110–120. 10.1038/oby.2010.147 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010;376(9741):595–605. 10.1016/S0140-6736(10)60888-4 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 753.Nissen SE, Wolski KE, Prcela L, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: A randomized clinical trial. JAMA 2016;315(10): 990–1004. 10.1001/jama.2016.1558 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 754.Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373(1):11–22. 10.1056/NEJMoa1411892 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 755.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009;374(9701):1606–1616. 10.1016/S0140-6736(09)61375-1 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 756.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance Randomized Study. Int J Obes (Lond) 2013;37(11): 1443–1451. 10.1038/ijo.2013.120 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 757.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384(11):989–1002. 10.1056/NEJMoa2032183 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 758.Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA 2021;325(14):1403–1413. 10.1001/jama.2021.1831 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA 2021;325(14):1414–1425. 10.1001/jama.2021.3224 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384(12):1113–1124. 10.1056/NEJMoa2028395 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 761.Fujioka K, O'Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring) 2016;24(11):2278–2288. 10.1002/oby.21629 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Wing RR, Marcus MD, Epstein LH, Salata R. Type ii diabetic subjects lose less weight than their overweight nondiabetic spouses. Diabetes Care 1987;10(5): 563–566. 10.2337/diacare.10.5.563 [EL 2; NRCT]. [DOI] [PubMed] [Google Scholar]
- 763.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100(2):342–362. 10.1210/jc.2014-3415 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 764.Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: A systematic review. QJM Mon J Assoc Phys 2007;100(7):395–404. 10.1093/qjmed/hcm044 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 765.Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy vs metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 2014;37(8): 2168–2176. 10.2337/dc13-2759 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 766.Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008;30(8):1448–1460. 10.1016/j.clinthera.2008.08.006 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 767.Grimm M, Han J, Weaver C, et al. Efficacy, safety, and tolerability of exenatide once weekly in patients with type 2 diabetes mellitus: an integrated analysis of the duration trials. Postgrad Med 2013;125(3):47–57. 10.3810/pgm.2013.05.2660 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 768.Garber A, Henry R, Ratner R, et al. Liraglutide vs glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373(9662):473–481. 10.1016/S0140-6736(08)61246-5 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 769.Fonseca VA, Alvarado-Ruiz R, Raccah D, et al. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: A randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GET-GOAL-MONO). Diabetes Care 2012;35(6):1225–1231. 10.2337/dc11-1935 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide vs exenatide er in subjects with type 2 diabetes (SUSTAIN 3): A 56-week, open-label, randomized clinical trial. Diabetes Care 2018;41(2):258–266. 10.2337/dc17-0417 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 771.Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy vs placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017;5(4):251–260. 10.1016/S2213-8587(17)30013-X [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 772.Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): A randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2019;7(5):356–367. 10.1016/S2213-8587(19)30066-X [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 773.Pratley R, Amod A, Hoff ST, et al. Oral semaglutide vs subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): A randomised, double-blind, phase 3a trial. Lancet 2019;394(10192):39–50. 10.1016/S0140-6736(19)31271-1 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 774.Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013;15(4):372–382. 10.1111/dom.12054 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010;33(10):2217–2224. 10.2337/dc10-0612 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013;1(3):208–219. 10.1016/S2213-8587(13)70084-6 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 777.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery vs intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376(7):641–651. 10.1056/NEJMoa1600869. EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351(26):2683–2693. 10.1056/NEJMoa035622 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 779.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307(1):56–65. 10.1001/jama.2011.1914 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 780.. Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311(22):2297–2304. 10.1001/jama.2014.5988 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 781.Horwitz D, Saunders JK, Ude-Welcome A, et al. Three-year follow-up comparing metabolic surgery vs medical weight management in patients with type 2 diabetes and bmi 30–35. The role of sRAGE biomarker as predictor of satisfactory outcomes. Surg Obes Relat Dis 2016;12(7):1337–1341. 10.1016/j.soard.2016.01.016 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 782.Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA 2018;319(3):291–301. 10.1001/jama.2017.21055 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery vs conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2021;397(10271): 293–304. 10.1016/S0140-6736(20)32649-0 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 784.Courcoulas AP, Gallagher JW, Neiberg RH, et al. Bariatric surgery vs lifestyle intervention for diabetes treatment: 5-year outcomes from a randomized trial. J Clin Endocrinol Metab 2020;105(3):866–876. 10.1210/clinem/dgaa006 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish obese subjects study. N Engl J Med 2020;383(16): 1535–1543. 10.1056/NEJMoa2002449 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Greenway FL, Aronne LJ, Raben A, et al. A randomized, double-blind, placebo-controlled study of gelesis100: A novel nonsystemic oral hydrogel for weight loss. Obesity (Silver Spring) 2019;27(2):205–216. 10.1002/oby.22347 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Genco A, Bruni T, Doldi SB, et al. Bioenterics intragastric balloon: the Italian experience with 2,515 patients. Obes Surg 2005;15(8):1161–1164. 10.1381/0960892055002202 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 788.Ponce J, Woodman G, Swain J, et al. The REDUCE pivotal trial: A prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 2015;11(4): 874–881. 10.1016/j.soard.2014.12.006 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 789.Marinos G, Eliades C, Raman Muthusamy V, Greenway F. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: clinical results from a 3- and 6-month study. Surg Obes Relat Dis 2014;10(5): 929–934. 10.1016/j.soard.2014.03.005 [EL 3; PRECLIN]. [DOI] [PubMed] [Google Scholar]
- 790.Nyström M, Machytka E, Norén E, et al. Aspiration therapy as a tool to treat obesity: 1- to 4-year results in a 201-patient multi-center post-market European Registry Study. Obes Surg 2018;28(7):1860–1868. 10.1007/s11695-017-3096-5 [EL 3; PRECLIN]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791.Fracytl health. Fractyl announces FDA breakthrough designation for Revita DMR in insulin-treated type 2 diabetes https://www.fractyl.com/fractyl-announces-fda-breakthrough-device-designation-for-revita-dmr-in-insulin-treated-type-2-diabetes/.
- 792.Rajagopalan H, Cherrington AD, Thompson CC, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes: 6-month interim analysis from the first-in-human proof-of-concept study. Diabetes Care 2016;39(12):2254–2261. 10.2337/dc16-0383 [EL 3; PRECLIN]. [DOI] [PubMed] [Google Scholar]
- 793.van Baar ACG, Holleman F, Crenier L, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut 2020;69(2): 295–303. 10.1136/gutjnl-2019-318349 [EL 3; PRECLIN]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Hadefi A, Huberty V, Lemmers A, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes. Dig Dis (Basel, Switzerland) 2018;36(4):322–324. 10.1159/000487078 [EL 3; SCRI]. [DOI] [PubMed] [Google Scholar]
- 795.Haywood C, Sumithran P. Treatment of obesity in older persons-a systematic review. Obes Rev Off J Int Assoc Study Obes 2019;20(4):588–598. 10.1111/obr.12815 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 796.Crawford MR, Pham N, Khan L, et al. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery vs medical therapy at 5 years. Endocr Pract 2018;24(3):256–264. 10.4158/EP-2017-0072 [EL 1; RCT, ancillary]. [DOI] [PubMed] [Google Scholar]
- 797.Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract 2020;26(Suppl 1):1–46. 10.4158/GL-2020-0524SUPPL. [El 4; NE]. [DOI] [PubMed] [Google Scholar]
- 798.Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab 2008;93(10):3735–3740. 10.1210/jc.2008-0481 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: A population-based study. Osteoporos Int J Established Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 2014;25(1): 151–158. 10.1007/s00198-013-2463-x [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ (Clin Res Ed) 2012;345:e5085. 10.1136/bmj.e5085 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Bethel MA, Hyland KA, Chacra AR, et al. Updated risk factors should be used to predict development of diabetes. J Diabetes Its Complications 2017;31(5): 859–863. 10.1016/j.jdiacomp.2017.02.012 [EL 3; DS]. [DOI] [PubMed] [Google Scholar]
- 802.de Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn Study. JAMA 2001;285(16):2109–2113. 10.1001/jama.285.16.2109 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 803.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288(21): 2709–2716. 10.1001/jama.288.21.2709 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 804.Laaksonen DE, Lakka HM, Niskanen LK, et al. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol 2002;156(11):1070–1077. 10.1093/aje/kwf145 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 805.Garber AJ, Handelsman Y, Einhorn D, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract 2008;14(7):933–946. 10.4158/EP.14.7.933 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 806.Guo F, Moellering DR, Garvey WT. The progression of cardiometabolic disease: validation of a new cardiometabolic disease staging system applicable to obesity. Obesity (Silver Spring) 2014;22(1):110–118. 10.1002/oby.20585 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010;72:219–246. 10.1146/annurev-physiol-021909-135846 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 808.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLOS ONE 2012;7(12):e52036. 10.1371/journal.pone.0052036 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic-based chronic disease, addressing knowledge and clinical practice gaps: JACC state-of-the-art review. J Am Coll Cardiol 2020;75(5):539–555. 10.1016/j.jacc.2019.11.046 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 1990;263(21): 2893–2898. 10.1001/jama.263.21.2893 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 811.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403. 10.1056/NEJMoa012512 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta-analysis of randomised controlled clinical trials. Eur J Cardiovasc Prev Rehabil 2011;18(6):813–823. 10.1177/1741826711421687 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 813.Yamaoka K, Nemoto A, Tango T. Comparison of the effectiveness of lifestyle modification with other treatments on the incidence of type 2 diabetes in people at high risk: A network meta-analysis. Nutrients 2019;11(6):1373. 10.3390/nu11061373 [EL 2; NMA]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 814.Salas-Salvadó J, Bulló M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the predimed-reus nutrition intervention randomized trial. Diabetes Care 2011;34(1):14–19. 10.2337/dc10-1288 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Salas-Salvadó J, Bulló M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann Intern Med 2014;160(1):1–10. 10.7326/M13-1725 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 816.Pérez-Ferre N, Del Valle L, Torrejón MJ, et al. Diabetes mellitus and abnormal glucose tolerance development after gestational diabetes: A three-year, prospective, randomized, clinical-based, Mediterranean lifestyle interventional study with parallel groups. Clin Nutr 2015;34(4):579–585. 10.1016/j.clnu.2014.09.005 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 817.Svetkey LP, Simons-Morton D, Vollmer WM, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the dietary approaches to stop hypertension (DASH) randomized clinical trial. Arch Intern Med 1999;159(3): 285–293. 10.1001/archinte.159.3.285 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 818.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. Dash-Sodium Collaborative Research Group. N Engl J Med 2001;344(1):3–10. 10.1056/NEJM200101043440101 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 819.Liese AD, Nichols M, Sun X, D'Agostino RB Jr, Haffner SM. Adherence to the DASH diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care 2009;32(8):1434–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820.Malik VS, Li Y, Tobias DK, Pan A, Hu FB. Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol 2016;183(8):715–728. 10.1093/aje/kwv268 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821.Esposito K, Chiodini P, Maiorino MI, et al. Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine 2014;47(1): 107–116. 10.1007/s12020-014-0264-4 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 822.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29(9):2102–2107. 10.2337/dc06-0560 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823.. Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study Diabetes prevention program research. Lancet 2009;374(9702):1677–1686. 10.1016/s0140-6736(09)61457-4 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Diabetes Prevention Program Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3(11):866–875. 10.1016/S2213-8587(15)00291-0 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Laaksonen DE, Lindström J, Lakka TA, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes 2005;54(1):158–165. 10.2337/diabetes.54.1.158 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 826.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish diabetes prevention study. Lancet 2006;368(9548):1673–1679. 10.1016/S0140-6736(06)69701-8 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 827.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes study. Diabetes Care 1997;20(4):537–544. 10.2337/diacare.20.4.537 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 828.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention study: A 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2(6):474–480. 10.1016/S2213-8587(14)70057-9 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 829.Chiu THT, Pan WH, Lin MN, Lin CL. Vegetarian diet, change in dietary patterns, and diabetes risk: A prospective study. Nutr Diabetes 2018;8(1):12. 10.1038/s41387-018-0022-4 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Becerra-Tomás N, Díaz-López A, Rosique-Esteban N, et al. Legume consumption is inversely associated with type 2 diabetes incidence in adults: A prospective assessment from the PREDIMED study. Clin Nutr 2018;37(3): 906–913. 10.1016/j.clnu.2017.03.015 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 831.Lee Y, Park K. Adherence to a vegetarian diet and diabetes risk: A systematic review and meta-analysis of observational studies. Nutrients 2017;9(6):603. 10.3390/nu9060603 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association between plant-based dietary patterns and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA Intern Med 2019;179(10):1335–1344. 10.1001/jamainternmed.2019.2195 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Pollakova D, Andreadi A, Pacifici F, et al. The impact of vegan diet in the prevention and treatment of type 2 diabetes: A systematic review. Nutrients 2021;13(6):2123. 10.3390/nu13062123 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.de Koning L, Fung TT, Liao X, et al. Low-carbohydrate diet scores and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93(4):844–850. 10.3945/ajcn.110.004333 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Bendsen NT, Christensen R, Bartels EM, Astrup A. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: A systematic review and meta-analysis of cohort studies. Eur J Clin Nutr 2011;65(7):773–783. 10.1038/ejcn.2011.34 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 836.Lara-Castro C, Diet Garvey WT. insulin resistance, and obesity: zoning in on data for Atkins dieters living in South Beach. J Clin Endocrinol Metab 2004;89(9):4197–4205. 10.1210/jc.2004-0683 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 837.Jia X, Zhong L, Song Y, et al. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Prim Care Diabetes 2016;10(4):272–280. 10.1016/j.pcd.2015.12.004 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 838.Honsek C, Kabisch S, Kemper M, et al. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: the randomised controlled Optimal Fibre Trial (OPTIFIT). Diabetologia 2018;61(6):1295–1305. 10.1007/s00125-018-4582-6 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 839.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014; 383(9933):1999–2007. 10.1016/S0140-6736(14)60613-9 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Jacobs S, Harmon BE, Boushey CJ, et al. A priori-defined diet quality indexes and risk of type 2 diabetes: the multiethnic cohort. Diabetologia 2015;58(1): 98–112. 10.1007/s00125-014-3404-8 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.Hrubeniuk TJ, Bouchard DR, Goulet EDB, Gurd B, Sénéchal M. The ability of exercise to meaningfully improve glucose tolerance in people living with prediabetes: A meta-analysis. Scand J Med Sci Sports 2020;30(2):209–216. 10.1111/sms.13567 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 842.Jadhav RA, Hazari A, Monterio A, Kumar S, Maiya AG. Effect of physical activity intervention in prediabetes: A systematic review with meta-analysis. J Phys Act Health 2017;14(9):745–755. 10.1123/jpah.2016-0632 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 843.Slentz CA, Bateman LA, Willis LH, et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: A randomised controlled trial. Diabetologia 2016;59(10):2088–2098. 10.1007/s00125-016-4051-z [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Kerrison G, Gillis RB, Jiwani SI, et al. The effectiveness of lifestyle adaptation for the prevention of prediabetes in adults: A systematic review. J Diabetes Res 2017;2017:8493145. 10.1155/2017/8493145 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845.Hemmingsen B, Gimenez-Perez G, Mauricio D, et al. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev 2017;12(12):Cd003054. 10.1002/14651858.CD003054. pub4 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: A systematic review. Diabetes Care 2007;30(3):744–752. 10.2337/dc06-1842 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 847.Dai X, Zhai L, Chen Q, et al. Two-year-supervised resistance training prevented diabetes incidence in people with prediabetes: A randomised control trial. Diabetes Metab Res Rev 2019;35(5):e3143. 10.1002/dmrr.3143 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 848.Davy BM, Winett RA, Savla J, et al. Resist diabetes: A randomized clinical trial for resistance training maintenance in adults with prediabetes. PLOS ONE 2017;12(2):e0172610. 10.1371/journal.pone.0172610 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 849.Strasser B, Siebert U, Schobersberger W. Resistance training in the treatment of the metabolic syndrome: A systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Med (Auckland NZ) 2010;40(5):397–415. 10.2165/11531380-000000000-00000 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 850.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann Intern Med 2007;147(6):357–369. 10.7326/0003-4819-147-6-200709180-00005 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 851.Aguiar EJ, Morgan PJ, Collins CE, Plotnikoff RC, Callister R. Efficacy of interventions that include diet, aerobic and resistance training components for type 2 diabetes prevention: A systematic review with meta-analysis. Int J Behav Nutr Phys Act 2014;11. 10.1186/1479-5868-11-2 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25B):2960–2984. 10.1016/j.jacc.2013.11.003 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 853.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care 2010;33(12): 2692–2696. 10.2337/dc10-1548 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.De Nardi AT, Tolves T, Lenzi TL, Signori LU, Silva AMVD. High-intensity interval training vs continuous training on physiological and metabolic variables in prediabetes and type 2 diabetes: A meta-analysis. Diabetes Res Clin Pract 2018;137:149–159. 10.1016/j.diabres.2017.12.017 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 855.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: A prospective study. JAMA 1999;282(15):1433–1439. 10.1001/jama.282.15.1433 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 856.Van Der Berg JD, Van Der Veld JHPM, De Waard EAC, et al. Replacement effects of sedentary time on metabolic outcomes: The Maastricht study. Med Sci Sports Exerc 2017;49(7):1351–1358. 10.1249/mss.0000000000001248 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 857.Loh R, Stamatakis E, Folkerts D, Allgrove JE, Moir HJ. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: A systematic review and meta-analysis. Sports Med 2020;50(2):295–330. 10.1007/s40279-019-01183-w [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Edwardson CL, Henson J, Bodicoat DH, et al. Associations of reallocating sitting time into standing or stepping with glucose, insulin and insulin sensitivity: A cross-sectional analysis of adults at risk of type 2 diabetes. BMJ Open 2017;7(1): e014267. 10.1136/bmjopen-2016-014267 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 859.McCarthy M, Edwardson CL, Davies MJ, et al. Change in sedentary time, physical activity, bodyweight, and HbA1c in high-risk adults. Med Sci Sports Exerc 2017;49(6):1120–1125. 10.1249/MSS.0000000000001218 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Noronha JC, Nishi SK, Braunstein CR, et al. The effect of liquid meal replacements on cardiometabolic risk factors in overweight/obese individuals with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2019;42(5):767–776. 10.2337/dc18-2270 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 861.Hesselink AE, Rutten GE, Slootmaker SM, et al. Effects of a lifestyle program in subjects with impaired fasting glucose, a pragmatic cluster-randomized controlled trial. BMC Fam Pract 2015;16:183. 10.1186/s12875-015-0394-7 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Bian RR, Piatt GA, Sen A, et al. The effect of technology-mediated diabetes prevention interventions on weight: A meta-analysis. J Med Internet Res 2017;19(3):e76. 10.2196/jmir.4709 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile Phone: A randomized controlled trial among persons with prediabetes. J Med Internet Res 2015;17(10):e240. 10.2196/jmir.4897 [[EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864.Vitolins MZ, Blackwell CS, Katula JA, Isom SP, Case LD. Long-term weight loss maintenance in the continuation of a randomized diabetes prevention translational study: the healthy living partnerships to prevent diabetes (HELP PD) continuation trial. Diabetes Care 2019;42(9):1653–1660. 10.2337/dc19-0295 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Mudaliar U, Zabetian A, Goodman M, et al. Cardiometabolic risk factor changes observed in diabetes prevention programs in US settings: A systematic review and meta-analysis. PLOS Med 2016;13(7):e1002095. 10.1371/journal.pmed.1002095 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Galaviz KI, Weber MB, Straus A, et al. Global diabetes prevention interventions: A systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care 2018;41(7): 1526–1534. 10.2337/dc17-2222 [EL 2; NMA]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867.Baetge C, Earnest CP, Lockard B, et al. Efficacy of a randomized trial examining commercial weight loss programs and exercise on metabolic syndrome in overweight and obese women. Appl Physiol Nutr Metab 2017;42(2):216–227. 10.1139/apnm-2016-0456 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 868.Ford CN, Weber MB, Staimez LR, et al. Dietary changes in a diabetes prevention intervention among people with prediabetes: the Diabetes Community Lifestyle Improvement Program trial. Acta diabetol 2019;56(2):197–209. 10.1007/s00592-018-1249-1 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 869.Kramer MK, Vanderwood KK, Arena VC, et al. Evaluation of a diabetes prevention program lifestyle intervention in older adults: A randomized controlled study in three senior/community centers of varying socioeconomic status. Diabetes Educ 2018;44(2):118–129. 10.1177/0145721718759982 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 870.Garvey WT, Ryan DH, Henry R, et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care 2014;37(4):912–921. 10.2337/dc13-1518 [EL 1; RCT, subanalysis]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367(8): 695–704. 10.1056/NEJMoa1112082 [EL 2; NRCT]. [DOI] [PubMed] [Google Scholar]
- 872.Booth H, Khan O, Prevost T, et al. Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. Lancet Diabetes Endocrinol 2014;2(12):963–968. 10.1016/S2213-8587(14)70214-1 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 873.le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide vs placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017; 389(10077):1399–1409. 10.1016/S0140-6736(17)30069-7 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 874.Torgerson JS, Hauptman J, Boldrin MN. Sjöström L Xenical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27(1):155–161. 10.2337/diacare.27.1.155 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 875.Oh TJ. The role of anti-obesity medication in prevention of diabetes and its complications. J Obes Metab Syndr 2019;28(3):158–166. 10.7570/jomes.2019.28.3.158 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876.Ard J, Cannon A, Lewis CE, et al. Efficacy and safety of liraglutide 3.0 mg for weight management are similar across races: subgroup analysis across the scale and phase II randomized trials. Diabetes Obes Metab 2016;18(4): 430–435. 10.1111/dom.12632 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 877.Beavers KM, Case LD, Blackwell CS, et al. Effects of weight regain following intentional weight loss on glucoregulatory function in overweight and obese adults with prediabetes. Obes Res Clin Pract 2015;9(3):266–273. 10.1016/j.orcp.2014.09.003 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 878.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020;360(360):1–8 [EL 4; NE]. [PubMed] [Google Scholar]
- 879.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015;313(19):1973–1974. 10.1001/jama.2015.4260 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 880.Wilson PW, Meigs JB, Sullivan L, et al. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham offspring study. Arch Intern Med 2007;167(10):1068–1074. 10.1001/archinte.167.10.1068 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 881.Bang H, Edwards AM, Bomback AS, et al. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med 2009;151(11): 775–783. 10.7326/0003-4819-151-11-200912010-00005 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882.Wilkinson L, Yi N, Mehta T, Judd S, Garvey WT. Development and validation of a model for predicting incident type 2 diabetes using quantitative clinical data and a Bayesian logistic model: A nationwide cohort and modeling study. PLOS Med 2020;17(8):e1003232. 10.1371/journal.pmed.1003232 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Guo F, Garvey WT. Development of a weighted cardiometabolic disease staging (CMDS) system for the prediction of future diabetes. J Clin Endocrinol Metab 2015;100(10):3871–3877. 10.1210/jc.2015-2691 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884.DeBoer MD, Filipp SL, Gurka MJ. Use of a metabolic syndrome severity Z score to track risk during treatment of prediabetes: an analysis of the diabetes prevention program. Diabetes Care 2018;41(11):2421–2430. 10.2337/dc18-1079 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Giráldez-García C, Sangrós FJ, Díaz-Redondo A, et al. Cardiometabolic risk profiles in patients with impaired fasting glucose and/or hemoglobin A1c 5.7% to 6.4%: evidence for a gradient according to diagnostic criteria: the predaps study. Medicine 2015;94(44):e1935. 10.1097/MD.0000000000001935 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 886.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham study. Am J Cardiol 1976;38(1):46–51. 10.1016/0002-9149(76)90061-8 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 887.Guo F, Garvey WT. Cardiometabolic disease staging predicts effectiveness of weight-loss therapy to prevent type 2 diabetes: pooled results from phase III clinical trials assessing phentermine/topiramate extended release. Diabetes Care 2017;40(7):856–862. 10.2337/dc17-0088 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Stevens JW, Khunti K, Harvey R, et al. Preventing the progression to type 2 diabetes mellitus in adults at high risk: A systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res Clin Pract 2015;107(3):320–331. 10.1016/j.diabres.2015.01.027 [EL 2; NMA]. [DOI] [PubMed] [Google Scholar]
- 889.Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2019;42(4):601–608. 10.2337/dc18-1970 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Guardado-Mendoza R, Salazar-López SS, álvarez-Canales M, et al. The combination of linagliptin, metformin and lifestyle modification to prevent type 2 diabetes (PRELLIM). A randomized clinical trial. Metabolism 2020;104: 154054. 10.1016/j.metabol.2019.154054 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 891.Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359(9323): 2072–2077. 10.1016/S0140-6736(02)08905-5 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 892.Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the stop-niddm trial. JAMA 2003;290(4):486–494. 10.1001/jama.290.4.486 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 893.Hu R, Li Y, Lv Q, Wu T, Tong N. Acarbose monotherapy and type 2 diabetes prevention in eastern and western prediabetes: an ethnicity-specific meta-analysis. Clin Ther 2015;37(8):1798–1812. 10.1016/j.clinthera.2015.05.504 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 894.Moelands SV, Lucassen PL, Akkermans RP, De Grauw WJ, Van de Laar FA. Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev 2018;12(12):Cd005061. 10.1002/14651858.CD005061. pub3 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364(12): 1104–1115. 10.1056/NEJMoa1010949 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 896.Ipsen EØ, Madsen KS, Chi Y, et al. Pioglitazone for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus. Cochrane Database Syst Rev 2020;11(11):Cd013516. 10.1002/14651858.CD013516. pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897.Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2019;42(4):601–608. 10.2337/dc18-1970 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898.Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): A double-blind randomised controlled study. Lancet 2010;376(9735): 103–111. 10.1016/S0140-6736(10)60746-5 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 899.Powers MA, Bardsley J, Cypress M, et al. Diabetes self-management education and support in type 2 diabetes: A joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Care 2015;38(7):1372–1382. 10.2337/dc15-0730 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 900.Davidson P, Ross T, Castor C. Academy of Nutrition and Dietetics: Revised 2017 standards of practice and standards of professional performance for registered dietitian nutritionists (competent, proficient, and expert) in diabetes care. J Acad Nutr Diet 2018;118(5):932–946.e948. 10.1016/j.jand.2018.03.007 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 901.Franz MJ, MacLeod J, Evert A, et al. Academy of Nutrition and Dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: systematic review of evidence for medical nutrition therapy effectiveness and recommendations for integration into the nutrition care process. J Acad Nutr Diet 2017;117(10): 1659–1679. 10.1016/j.jand.2017.03.022 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 902.Møller G, Andersen HK, Snorgaard O. A systematic review and meta-analysis of nutrition therapy compared with dietary advice in patients with type 2 diabetes. Am J Clin Nutr 2017;106(6):1394–1400. 10.3945/ajcn.116.139626 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 903.Parker AR, Byham-Gray L, Denmark R, Winkle PJ. The effect of medical nutrition therapy by a registered dietitian nutritionist in patients with prediabetes participating in a randomized controlled clinical research trial. J Acad Nutr Diet 2014;114(11):1739–1748. 10.1016/j.jand.2014.07.020 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 904.Battista MC, Labonté M, Ménard J, et al. Dietitian-coached management in combination with annual endocrinologist follow up improves global metabolic and cardiovascular health in diabetic participants after 24 months. Appl Physiol Nutr Metab 2012;37(4):610–620. 10.1139/h2012-025 [EL 2; NRCT]. [DOI] [PubMed] [Google Scholar]
- 905.Briggs Early K, Stanley K. Position of the academy of nutrition and dietetics: the role of medical nutrition therapy and registered dietitian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J Acad Nutr Diet 2018;118(2):343–353. 10.1016/j.jand.2017.11.021 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 906.Lynch EB, Liebman R, Ventrelle J, Avery EF, Richardson D. A self-management intervention for African Americans with comorbid diabetes and hypertension: A pilot randomized controlled trial. Prev Chronic Dis 2014;11:E90. 10.5888/pcd11.130349 [EL 1; RCT pilot]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 907.Beck J, Greenwood DA, Blanton L, et al. 2017 national standards for diabetes self-management education and support. Diabetes Care 2017;40(10): 1409–1419. 10.2337/dci17-0025 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 908.Ku GM, Kegels G. Effects of the first line diabetes care (FiLDCare) self-management education and support project on knowledge, attitudes, perceptions, self-management practices and glycaemic control: A quasi-experimental study conducted in the Northern Philippines. BMJ Open 2014;4(8):e005317. 10.1136/bmjopen-2014-005317 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909.Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: A systematic review of the effect on glycemic control. Patient Educ Couns. patient ed 2016;99(6):926–943. 10.1016/j.pec.2015.11.003 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 910.. Razaz JM, Rahmani J, Varkaneh HK, et al. The health effects of medical nutrition therapy by dietitians in patients with diabetes: A systematic review and meta-analysis: nutrition therapy and diabetes. Prim Care Diabetes 2019;13(5):399–408. 10.1016/j.pcd.2019.05.001 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 911.Valkama A, Koivusalo S, Lindström J, et al. The effect of dietary counselling on food intakes in pregnant women at risk for gestational diabetes: A secondary analysis of a randomised controlled trial RADIEL. Eur J Clin Nutr 2016;70(8): 912–917. 10.1038/ejcn.2015.205 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 912.Ferguson S, Swan M, Smaldone A. Does diabetes self-management education in conjunction with primary care improve glycemic control in Hispanic patients? A systematic review and meta-analysis. Diabetes Educ 2015;41(4):472–484. 10.1177/0145721715584404 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 913.Franz MJ, Splett PL, Monk A, et al. Cost-effectiveness of medical nutrition therapy provided by dietitians for persons with non-insulin-dependent diabetes mellitus. J Am Diet Assoc 1995;95(9):1018–1024. 10.1016/S0002-8223(95)00277-4 [EL 3; ECON]. [DOI] [PubMed] [Google Scholar]
- 914.Wolf AM, Siadaty M, Yaeger B, et al. Effects of lifestyle intervention on health care costs: improving control with activity and nutrition (ICAN). J Am Diet Assoc 2007;107(8):1365–1373. 10.1016/j.jada.2007.05.015 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 915.Pritchard DA, Hyndman J, Taba F. Nutritional counselling in general practice: A cost effective analysis. J Epidemiol Community Health 1999;53(5):311–316. 10.1136/jech.53.5.311 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916.Lewis JE, Arheart KL, LeBlanc WG, et al. Food label use and awareness of nutritional information and recommendations among persons with chronic disease. Am J Clin Nutr 2009;90(5):1351–1357. 10.3945/ajcn.2009.27684 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917.Wheeler ML, Pi-Sunyer FX. Carbohydrate issues: type and amount. J Am Diet Assoc 2008;108(4 suppl 1):S34–S39. 10.1016/j.jada.2008.01.024 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 918.Palou A, Bonet ML, Picó C. On the role and fate of sugars in human nutrition and health. Introduction. Obes Rev Off J Int Assoc Study Obes 2009;10(suppl 1): 1–8. 10.1111/j.1467-789X.2008.00560.x [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 919.Wheeler ML, Dunbar SA, Jaacks LM, et al. Macronutrients, food groups, and eating patterns in the management of diabetes: A systematic review of the literature, 2010. Diabetes Care 2012;35(2):434–445. 10.2337/dc11-2216 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920.Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol 2018;33(2):157–170. 10.1007/s10654-017-0352-x [EL 2; NMA]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 921.Schwingshackl L, Schwedhelm C, Hoffmann G, et al. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2017;105(6):1462–1473. 10.3945/ajcn.117.153148 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 922.Benson G, Hayes J. An update on the Mediterranean, vegetarian, and DASH eating patterns in people with type 2 diabetes. Diabetes Spectr 2020;33(2): 125–132. 10.2337/ds19-0073 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923.de Carvalho GB, Dias-Vasconcelos NL, Santos RKF, et al. Effect of different dietary patterns on glycemic control in individuals with type 2 diabetes mellitus: A systematic review. Crit Rev Food Sci Nutr 2020;60(12):1999–2010. 10.1080/10408398.2019.1624498 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 924.Papamichou D, Panagiotakos DB, Itsiopoulos C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr Metab Cardiovasc Dis NMCD 2019;29(6):531–543. 10.1016/j.numecd.2019.02.004 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 925.Toobert DJ, Glasgow RE, Strycker LA, et al. Biologic and quality-of-life outcomes from the Mediterranean lifestyle program: A randomized clinical trial. Diabetes Care 2003;26(8):2288–2293. 10.2337/diacare.26.8.2288 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 926.Rodríguez-Villar C, Pérez-Heras A, Mercadé I, Casals E, Ros E. Comparison of a high-carbohydrate and a high-monounsaturated fat, olive oil-rich diet on the susceptibility of LDL to oxidative modification in subjects with type 2 diabetes mellitus. Diabet Med 2004;21(2):142–149. 10.1046/j.1464-5491.2003.01086.x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 927.Look AHEAD Research Group, Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the Look AHEAD trial. Diabetes Care 2007;30(6): 1374–1383. 10.2337/dc07-0048 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.Barnard RJ, Massey MR, Cherny S, O'Brien LT. Pritikin N Long-term use of a high-complex-carbohydrate, high-fiber, low-fat diet and exercise in the treatment of niddm patients. Diabetes Care 1983;6(3):268–273. 10.2337/diacare.6.3.268 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 929.Micha R, Wallace SK. Mozaffarian D Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010;121(21):2271–2283. 10.1161/CIRCULATIONAHA.109.924977 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 930.Markovic TP, Muirhead R, Overs S, et al. Randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in women at high risk of gestational diabetes mellitus: the GI Baby 3 study. Diabetes Care 2016;39(1):31–38. 10.2337/dc15-0572 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 931.Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009;32(5):791–796. 10.2337/dc08-1886 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 932.Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006;29(8):1777–1783. 10.2337/dc06-0606 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 933.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005;293(1):43–53. 10.1001/jama.293.1.43 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 934.Reynolds AN, Akerman AP. Mann J Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLOS Med 2020;17(3): e1003053. 10.1371/journal.pmed.1003053 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Mechanick JI, Brett EM. Chausmer AB American Association of Clinical Endocrinologists medical guidelines for the clinical use of dietary supplements and nutraceuticals. Endocr Pract 2003;9(5):417–470. 10.4158/EP.9.5.417 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 936.Aroda VR, Edelstein SL, Goldberg RB, et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab 2016;101(4):1754–1761. 10.1210/jc.2015-3754 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937.Yang W, Cai X, Wu H, Ji L. Associations between metformin use and vitamin B(12) levels, anemia, and neuropathy in patients with diabetes: A meta-analysis. J Diabetes 2019;11(9):729–743. 10.1111/1753-0407.12900 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 938.Butler CC, Vidal-Alaball J, Cannings-John R, et al. Oral vitamin B12 vs intramuscular vitamin B12 for vitamin B12 deficiency: A systematic review of randomized controlled trials. Fam Pract 2006;23(3):279–285. 10.1093/fampra/cml008 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 939.Manders RJ, Van Dijk JW, van Loon LJ. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc 2010;42(2):219–225. 10.1249/MSS.0b013e3181b3b16d [EL 1; RCT; small sample size]. [DOI] [PubMed] [Google Scholar]
- 940.Hansen D, Dendale P, Jonkers RA, et al. Continuous low- to moderate-intensity exercise training is as effective as moderate-to high-intensity exercise training at lowering blood HbA1c in obese type 2 diabetes patients. Diabetologia 2009;52(9):1789–1797. 10.1007/s00125-009-1354-3 [EL 2; NRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 941.Praet SF, Manders RJ, Lieverse AG, et al. Influence of acute exercise on hyperglycemia in insulin-treated type 2 diabetes. Med Sci Sports Exerc 2006;38(12):2037–2044. 10.1249/01.mss.0000235352.09061, 1d [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 942.De Feyter HM, Praet SF, van den Broek NM, et al. Exercise training improves glycemic control in long-standing insulin-treated type 2 diabetic patients. Diabetes Care 2007;30(10):2511–2513. 10.2337/dc07-0183 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 943.2018 Physical Activity Guidelines Advisory Committee. 2018 physical activity guidelines advisory committee scientific report In: Serv US, (Doha)H, ed. Washington, DC: [EL 4; NE]. [Google Scholar]
- 944.Boulé NG, Haddad E, Kenny GP, Wells GA. Sigal RJ Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA 2001;286(10):1218–1227. 10.1001/jama.286.10.1218 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 945.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: A randomized controlled trial. JAMA 2010;304(20):2253–2262. 10.1001/jama.2010.1710 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946.Balducci S, Zanuso S, Nicolucci A, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: A randomized controlled trial: the Italian Diabetes and Exercise study (IDES). Arch Intern Med 2010;170(20):1794–1803. 10.1001/archinternmed.2010.380 [EL 1: RCT]. [DOI] [PubMed] [Google Scholar]
- 947.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016;39(11):2065–2079. 10.2337/dc16-1728 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 948.Sluik D, Buijsse B, Muckelbauer R, et al. Physical activity and mortality in individuals with diabetes mellitus: A prospective study and meta-analysis. Arch Intern Med 2012;172(17):1285–1295. 10.1001/archinternmed.2012.3130 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 949.Tikkanen-Dolenc H, Wadén J, Forsblom C, et al. Physical activity reduces risk of premature mortality in patients with type 1 diabetes with and without kidney disease. Diabetes Care 2017;40(12):1727–1732. 10.2337/dc17-0615 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 950.Boulé NG, Kenny GP, Haddad E, Wells GA. Sigal RJ Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia 2003;46(8):1071–1081. 10.1007/s00125-003-1160-2 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 951.Pandey A, Patel KV, Bahnson JL, et al. Association of intensive lifestyle intervention, fitness, and body mass index with risk of heart failure in overweight or obese adults with type 2 diabetes mellitus: an analysis from the Look AHEAD trial. Circulation 2020;141(16):1295–1306. 10.1161/CIRCULATIONAHA.119.044865 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 952.Johannsen NM, Swift DL, Lavie CJ, et al. Categorical analysis of the impact of aerobic and resistance exercise training, alone and in combination, on cardiorespiratory fitness levels in patients with type 2 diabetes: results from the HART-D study. Diabetes Care 2013;36(10):3305–3312. 10.2337/dc12-2194 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 953.Snowling NJ. Hopkins WG Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: A meta-analysis. Diabetes Care 2006;29(11):2518–2527. 10.2337/dc06-1317 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 954.Willey KA. Singh MA Battling insulin resistance in elderly obese people with type 2 diabetes: bring on the heavy weights. Diabetes Care 2003;26(5): 1580–1588. 10.2337/diacare.26.5.1580 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 955.Health HTHCSoP. Obesity prevention source: examples of moderate and vigorous physical activity https://www.hsph.harvard.edu/obesity-prevention-source/moderate-and-vigorous-physical-activity/. Accessed February 23, 2022.
- 956.. Jelleyman C, Yates T, O'Donovan G, et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes Rev 2015;16(11):942–961. 10.1111/obr.12317 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 957.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 2009;41(5):998–1005. 10.1249/MSS.0b013e3181930355 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 958.Dempsey PC, Larsen RN, Sethi P, et al. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care 2016;39(6):964–972. 10.2337/dc15-2336 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 959.Schellenberg ES, Dryden DM, Vandermeer B, Ha C. Korownyk C Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med 2013;159(8):543–551. 10.7326/0003-4819-159-8-201310150-00007 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 960.Wang Y, Lee DC, Brellenthin AG, et al. Leisure-time running reduces the risk of incident type 2 diabetes. Am J Med 2019;132(10):1225–1232. 10.1016/j.amjmed.2019.04.035 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 961.Pai LW, Li TC, Hwu YJ, et al. The effectiveness of regular leisure-time physical activities on long-term glycemic control in people with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract 2016;113: 77–85. 10.1016/j.diabres.2016.01.011 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 962.Cui J, Yan JH, Yan LM, et al. Effects of yoga in adults with type 2 diabetes mellitus: A meta-analysis. J Diabetes Investig 2017;8(2):201–209. 10.1111/jdi.12548 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963.Lee MS, Jun JH, Lim HJ, Lim HS. A systematic review and meta-analysis of tai chi for treating type 2 diabetes. Maturitas 2015;80(1):14–23. 10.1016/j.maturitas.2014.09.008 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 964.Rees JL, Johnson ST. Boulé NG Aquatic exercise for adults with type 2 diabetes: A meta-analysis. Acta diabetol 2017;54(10):895–904. 10.1007/s00592-017-1023-9 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 965.Bax JJ, Young LH, Frye RL, et al. Screening for coronary artery disease in patients with diabetes. Diabetes Care 2007;30(10):2729–2736. 10.2337/dc07-9927 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 966.Rodriguez-Gutierrez R, Gionfriddo MR, Ospina NS, et al. Shared decision making in endocrinology: Present and future directions. Lancet Diabetes Endocrinol 2016;4(8):706–716. 10.1016/S2213-8587(15)00468-4 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 967.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41(12):2669–2701. 10.2337/dci18-0033 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 968.Powell RE, Zaccardi F, Beebe C, et al. Strategies for overcoming therapeutic inertia in type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab 2021;23(9):2137–2154. 10.1111/dom.14455 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 969.Boels AM, Hart HE, Rutten GE. Vos RC Personalised treatment targets in type 2 diabetes patients: the Dutch approach. Prim Care Diabetes 2017;11(1):71–77. 10.1016/j.pcd.2016.08.001 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 970.Heller SR, Pratley RE, Sinclair A, et al. Glycaemic outcomes of an individualized treatment approach for older vulnerable patients: A randomized, controlled study in type 2 diabetes mellitus (IMPERIUM). Diabetes Obes Metab 2018;20(1):148–156. 10.1111/dom.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971.Goldenberg RM, Steen O. Semaglutide: review and place in therapy for adults with type 2 diabetes. Can J Diabetes 2019;43(2):136–145. 10.1016/j.jcjd.2018.05.008 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 972.American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1): S125–S143. 10.2337/dc22-S009 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 973.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43(2):487–493. 10.2337/dci19-0066 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 974.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020;63(2):221–228. 10.1007/s00125-019-05039-w [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 975.Boussageon R, Supper I, Bejan-Angoulvant T, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: A meta-analysis of randomised controlled trials. PLOS Med 2012;9(4):e1001204. 10.1371/journal.pmed.1001204 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 976.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355(23): 2427–2443. 10.1056/NEJMoa066224 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 977.Bailey CJ, Turner RC. Metformin. N Engl J Med 1996;334(9):574–579. 10.1056/NEJM199602293340906 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 978.Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: A cohort study. Ann Intern Med 2012;157(9):601–610. 10.7326/0003-4819-157-9-201211060-00003 [EL 2; RCCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care 2011;34(6):1431–1437. 10.2337/dc10-2361 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: Randomised placebo controlled trial. BMJ (Clin Res Ed) 2010;340:c2181. 10.1136/bmj.c2181 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Martono DP, Lub R, Lambers Heerspink HJ, et al. Predictors of response in initial users of metformin and sulphonylurea derivatives: A systematic review. Diabet Med J Br Diabet Assoc 2015;32(7):853–864. 10.1111/dme.12688 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 982.Wright A, Burden AC, Paisey RB. Sulfonylurea inadequacy: Efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002;25(2):330–336. 10.2337/diacare.25.2.330 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 983.Kahn SE, Lachin JM, Zinman B, et al. Effects of rosiglitazone, glyburide, and metformin on b-cell function and insulin sensitivity in ADOPT. Diabetes 2011;60(5):1552–1560. 10.2337/db10-1392 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984.Thulé PM, Sulfonylureas Umpierrez G. A new look at old therapy. Curr Diabetes Rep 2014;14(4):473. 10.1007/s11892-014-0473-5 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 985.Omori K, Nomoto H, Nakamura A, et al. Reduction in glucose fluctuations in elderly patients with type 2 diabetes using repaglinide: A randomized controlled trial of repaglinide vs sulfonylurea. J Diabetes Investig 2019;10(2): 367–374. 10.1111/jdi.12889 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Gerstein HC, Ratner RE, Cannon CP, et al. Effect of rosiglitazone on progression of coronary atherosclerosis in patients with type 2 diabetes mellitus and coronary artery disease: the assessment on the prevention of progression by rosiglitazone on atherosclerosis in diabetes patients with cardiovascular history trial. Circulation 2010;121(10):1176–1187. 10.1161/CIRCULATIONAHA.109.881003 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 987.Riche DM, Valderrama R. Henyan NN Thiazolidinediones and risk of repeat target vessel revascularization following percutaneous coronary intervention: A meta-analysis. Diabetes Care 2007;30(2):384–388. 10.2337/dc06-1854 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 988.Abbatecola AM, Lattanzio F, Spazzafumo L, et al. Adiposity predicts cognitive decline in older persons with diabetes: A 2-year follow-up. PLOS ONE 2010;5(4):e10333. 10.1371/journal.pone.0010333 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 989.Lincoff AM, Wolski K, Nicholls SJ. Nissen SE Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA 2007;298(10):1180–1188. 10.1001/jama.298.10.1180 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 990.Zhou Y, Huang Y, Ji X, et al. Pioglitazone for the primary and secondary prevention of cardiovascular and renal outcomes in patients with or at high risk of type 2 diabetes mellitus: A meta-analysis. J Clin Endocrinol Metab 2020;105(5):dgz252. 10.1210/clinem/dgz252 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 991.Stein LL, Dong MH. Loomba R Insulin sensitizers in nonalcoholic fatty liver disease and steatohepatitis: current status. Adv Ther 2009;26(10):893–907. 10.1007/s12325-009-0072-z [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 992.Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial. Ann Intern Med 2016;165(5):305–315. 10.7326/M15-1774 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 993.Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147(6):386–399. 10.7326/0003-4819-147-6-200709180-00178 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 994.Spence JD, Viscoli C, Kernan WN, et al. Efficacy of lower doses of pioglitazone after stroke or transient ischaemic attack in patients with insulin resistance. Diabetes Obes Metab 2022;24(6):1150–1158. 10.1111/dom.14687 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 995.Kahn SE, Zinman B, Lachin JM, et al. Rosiglitazone-associated fractures in type 2 diabetes: an analysis from a diabetes outcome progression trial (adopt). Diabetes Care 2008;31(5):845–851. 10.2337/dc07-2270 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 996.Schwartz AV, Sellmeyer DE, Vittinghoff E, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab 2006;91(9): 3349–3354. 10.1210/jc.2005-2226 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 997.Woodcock J, Sharfstein JM. Hamburg M Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med 2010;363(16): 1489–1491. 10.1056/NEJMp1010788 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 998.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): A multicentre, randomised, open-label trial. record. Lancet 2009;373(9681):2125–2135. 10.1016/S0140-6736(09)60953-3 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 999.Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs–in-sights from the rosiglitazone experience. N Engl J Med 2013;369(14): 1285–1287. 10.1056/NEJMp1309610 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1000.Wang T, Ning G, Bloomgarden Z. Diabetes and cancer relationships. J Diabetes 2013;5(4):378–390. 10.1111/1753-0407.12057 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1001.Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015;314(3):265–277. 10.1001/jama.2015.7996 [EL 2; NCCS]. [DOI] [PubMed] [Google Scholar]
- 1002.. Levin D, Bell S, Sund R, et al. Pioglitazone and bladder cancer risk: A multipopulation pooled, cumulative exposure analysis. Diabetologia 2015;58(3): 493–504. 10.1007/s00125-014-3456-9 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1003.. Duan XY, Liu SY, Yin DG. Comparative efficacy of 5 sodium glucose cotransporter 2 inhibitor and 7 glucagon-like peptide 1 receptor agonists interventions on cardiorenal outcomes in type 2 diabetes patients: A network meta-analysis based on cardiovascular or renal outcome trials. Medicine 2021;100(30): e26431. 10.1097/md.0000000000026431 [EL 2; NMA]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1004.. Tran S, Retnakaran R, Zinman B, Kramer CK. Efficacy of glucagon-like peptide-1 receptor agonists compared to dipeptidyl peptidase-4 inhibitors for the management of type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes Metab 2018;20(Suppl 1):68–76. 10.1111/dom.13137 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1005.. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: A proof-of-concept study. Diabetes Care 2010;33(7):1509–1515. 10.2337/dc09-2191 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006.. Riddle MC, Henry RR, Poon TH, et al. Exenatide elicits sustained glycaemic control and progressive reduction of body weight in patients with type 2 diabetes inadequately controlled by sulphonylureas with or without metformin. Diabetes Metab Res Rev 2006;22(6):483–491. 10.1002/dmrr.646 [EL 2; OLES]. [DOI] [PubMed] [Google Scholar]
- 1007.. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28(5): 1092–1100. 10.2337/diacare.28.5.1092 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1008.. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28(5):1083–1091. 10.2337/diacare.28.5.1083 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1009.. Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: A randomized trial. Ann Intern Med 2007;146(7):477–485. 10.7326/0003-4819-146-7-200704030-00003 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1010.. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD). Diabetes Care 2009;32(7):1224–1230. 10.2337/dc08-2124 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1011.. Nauck M, Marre M. Adding liraglutide to oral antidiabetic drug monotherapy: Efficacy and weight benefits. Postgrad Med 2009;121(3):5–15. 10.3810/pgm.2009.05.1997 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1012.. Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human glp-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabetic Med 2009;26(3): 268–278. 10.1111/j.1464-5491.2009.02666.x [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1013.. Li M, Yang Y, Jiang D, Ying M, Wang Y, Zhao R. Efficacy and safety of liraglutide versus sitagliptin both in combination with metformin in patients with type 2 diabetes: A systematic review and meta-analysis. Medicine 2017;96(39):e8161. 10.1097/md.0000000000008161 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014.. Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab 2017;19(4):524–536. 10.1111/dom.12849 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1015.. Nauck MA, Stewart MW, Perkins C, et al. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia 2016;59(2):266–274. 10.1007/s00125-015-3795-1 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1016.. Odawara M, Miyagawa J, Iwamoto N, Takita Y, Imaoka T, Takamura T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study. Diabetes Obes Metab 2016;18(3):249–257. 10.1111/dom.12602 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1017.. Aroda VR, Bailey TS, Cariou B, et al. Effect of adding insulin degludec to treatment in patients with type 2 diabetes inadequately controlled with metformin and liraglutide: A double-blind randomized controlled trial (BEGIN: ADD TO GLP-1 study). Diabetes Obes Metab 2016;18(7):663–670. 10.1111/dom.12661 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1018.. Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: A meta-analysis. Lancet Diabetes Endocrinol 2018;6(2):105–113. 10.1016/s2213-8587(17)30412-6 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1019.. Martinez L, Penfornis A, Gautier JF, et al. Effectiveness and persistence of liraglutide treatment among patients with type 2 diabetes treated in primary care and specialist settings: A subgroup analysis from the evidence study, a prospective, 2-year follow-up, observational, post-marketing study. Adv Ther 2017;34(3):674–685. 10.1007/s12325-017-0476-0 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1020.. Shomali ME, Ørsted DD, Cannon AJ. Efficacy and safety of liraglutide, a once-daily human glucagon-like peptide-1 receptor agonist, in African-American people with type 2 diabetes: A meta-analysis of sub-population data from seven phase III trials. Diabetic Med 2017;34(2):197–203. 10.1111/dme.13185 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1021.. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): A randomised controlled trial. Diabetologia 2009;52(10):2046–2055. 10.1007/s00125-009-1472-y [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1022.. Bergenstal R, Lewin A, Bailey T, Chang D, Gylvin T, Roberts V. Efficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylurea. Curr Med Res Opin 2009;25(1):65–75. 10.1185/03007990802597951 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1023.. Blevins T, Han J, Nicewarner D, Chen S, Oliveira JH, Aronoff S. Exenatide is non-inferior to insulin in reducing HbA1c: An integrated analysis of 1423 patients with type 2 diabetes. Postgrad Med 2010;122(3):118–128. 10.3810/pgm.2010.05.2149 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1024.. Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: The DUAL VII randomized clinical trial. Diabetes Care 2018;41(5):1009–1016. 10.2337/dc17-1114 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1025.. Dailey GE, Dex TA, Roberts M, Liu M, Meneilly GS. Efficacy and safety of lixisenatide as add-on therapy to basal insulin in older adults with type 2 diabetes in the GETGOAL-O study. J Diabetes 2019;11(12):971–981. 10.1111/1753-0407.12952 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1026.. Kaneko S, Nishijima K, Bosch-Traberg H, Kaku K, Seino Y. Efficacy and safety of adding liraglutide to existing insulin regimens in Japanese patients with type 2 diabetes mellitus: A post-hoc analysis of a phase 3 randomized clinical trial. J Diabetes Investig 2018;9(4):840–849. 10.1111/jdi.12793 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1027.. Lind M, Hirsch IB, Tuomilehto J, et al. Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: Randomised clinical trial (MDI Liraglutide trial). BMJ 2015;351:h5364. 10.1136/bmj.h5364 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1028.. Pantalone KM, Patel H, Yu M, Fernández Landó L. Dulaglutide 1.5 mg as an add-on option for patients uncontrolled on insulin: Subgroup analysis by age, duration of diabetes and baseline glycated haemoglobin concentration. Diabetes Obes Metab 2018;20(6):1461–1469. 10.1111/dom.13252 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029.. Rodbard HW, Buse JB, Woo V, et al. Benefits of combination of insulin degludec and liraglutide are independent of baseline glycated haemoglobin level and duration of type 2 diabetes. Diabetes Obes Metab 2016;18(1): 40–48. 10.1111/dom.12574 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.. Leiter LA, Gross JL, Chow F, Miller D, Johnson S, Ahrén B. Once weekly glucagon-like peptide-1 receptor agonist albiglutide vs. prandial insulin added to basal insulin in patients with type 2 diabetes mellitus: Results over 52 weeks. J Diabetes Complications 2017;31(8):1283–1285. 10.1016/j.jdiacomp.2017.05.010 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1031.. Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 2017;377(8):723–732. 10.1056/NEJMoa1615692 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1032.. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA 2007;298(2): 194–206. 10.1001/jama.298.2.194 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1033.. Parks M, Rosebraugh C. Weighing risks and benefits of liraglutideethe FDA's review of a new antidiabetic therapy. N Engl J Med 2010;362(9):774–777. 10.1056/NEJMp1001578 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1034.Buse JB, Bethel MA, Green JB, et al. Pancreatic safety of sitagliptin in the TECOS study. Diabetes Care 2017;40(2):164–170. 10.2337/dc15-2780 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1035.. DeVries JH, Rosenstock J. DPP-4 inhibitor-related pancreatitis: Rare but real. Diabetes Care 2017;40(2):161–163. 10.2337/dci16-0035 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1036.. Karagiannis T, Avgerinos I, Liakos A, et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: A systematic review and meta-analysis. Diabetologia 2022:1–11. 10.1007/s00125-022-05715-4 [EL1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1037.. Sattar N, McGuire DK, Pavo I, et al. Tirzepatide cardiovascular event risk assessment: A pre-specified meta-analysis. Nat Med 2022;28(3):591–598. 10.1038/s41591-022-01707-4 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1038.. Amblee A, Lious D, Fogelfeld L. Combination of saxagliptin and metformin is effective as initial therapy in new-onset type 2 diabetes mellitus with severe hyperglycemia. J Clin Endocrinol Metab 2016;101(6):2528–2535. 10.1210/jc.2015-4097 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1039.. Bajaj HS, Ye C, Jain E, Venn K, Stein E, Aronson R. Glycemic improvement with a fixed-dose combination of DPP-4 inhibitor + metformin in patients with type 2 diabetes (GIFT study). Diabetes Obes Metab 2018;20(1): 195–199. 10.1111/dom.13040 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1040.. Matthews DR, Paldánius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): A 5-year, multicentre, randomised, double-blind trial. Lancet 2019;394(10208):1519–1529. 10.1016/s0140-6736(19)32131-2 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1041.. Ross SA, Caballero AE, Del Prato S, et al. Linagliptin plus metformin in patients with newly diagnosed type 2 diabetes and marked hyperglycemia. Postgrad Med 2016;128(8):747–754. 10.1080/00325481.2016.1238280 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 1042.. Wang W, Yang J, Yang G, et al. Efficacy and safety of linagliptin in Asian patients with type 2 diabetes mellitus inadequately controlled by metformin: A multinational 24-week, randomized clinical trial. J of diabetes 2016;8(2):229–237. 10.1111/1753-0407.12284 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1043.. Bloomgarden Z, Drexler A. What role will 'gliptins' play in glycemic control? Cleveland Clinic J of medicine 2008;75(4):305–310. 10.3949/ccjm.75.4.305 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1044.. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369(14): 1327–1335. 10.1056/NEJMoa1305889 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1045.. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369(14): 1317–1326. 10.1056/NEJMoa1307684 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1046.. Ning G, Bandgar T, Hehnke U, Lee J, Chan JCN. Efficacy and safety of linagliptin in 2681 Asian patients stratified by age, obesity, and renal function: A pooled analysis of randomized clinical trials. Adv Ther 2017;34(9): 2150–2162. 10.1007/s12325-017-0595-7 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1047.. Nesina (alogliptin [as alogliptin benzoate]) product monograph, page 4. In: Inc. TC, ed.2021. [Google Scholar]
- 1048.. Bloomgarden Z Sodium glucose transporter 2 inhibition: A new approach to diabetes treatment. J Diabetes 2013;5(3):225–227. 10.1111/1753-0407.12065 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1049.. Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodiumglucose cotransporter 2 inhibition. Diabetes Care 2015;38(9):1687–1693. 10.2337/dc15-0843 [EL 3; CCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050.. Goldenberg RM, Berard LD, Cheng AYY, et al. SGLT-2 inhibitor-associated diabetic ketoacidosis: Clinical review and recommendations for prevention and diagnosis. Clin Ther 2016;38(12):2654–2664.e2651. 10.1016/j.clinthera.2016.11.002 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1051.. Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. Clin Endocrinol Metab 2016;101(1):157–166. 10.1210/jc.2015-3167 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1052.. Piperidou A, Sarafidis P, Boutou A, et al. The effect of SGLT-2 inhibitors on albuminuria and proteinuria in diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J Hypertens 2019;37(7): 1334–1343. 10.1097/hjh.0000000000002050 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1053.. Sinclair AJ, Bode B, Harris S, et al. Efficacy and safety of canagliflozin in individuals aged 75 and older with type 2 diabetes mellitus: A pooled analysis. J Am Geriatric Soc 2016;64(3):543–552. 10.1111/jgs.14028 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1054.. Persson F, Nyström T, Jørgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study. Diabetes Obes Metab 2018;20(2):344–351. 10.1111/dom.13077 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]; 1054a. Solomon SD, McMurray JJV, Claggett B, et al. DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022. 10.1056/NEJMoa2206286. Epub ahead of print. [DOI] [Google Scholar]
- 1055.. Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: The clinical evidence. Diabetes Obes Metab 2010;12(5):384–392. 10.1111/j.1463-1326.2009.01181.x [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1056.. Yang HK, Lee SH, Shin J, et al. Acarbose add-on therapy in patients with type 2 diabetes mellitus with metformin and sitagliptin failure: A multicenter, randomized, double-blind, placebo-controlled study. Diabetes Metab J 2019;43(3):287–301. 10.4093/dmj.2018.0054 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1057.. Schnell O, Weng J, Sheu WH, et al. Acarbose reduces body weight irrespective of glycemic control in patients with diabetes: Results of a worldwide, non-interventional, observational study data pool. J Diabetes Complications 2016;30(4):628–637. 10.1016/j.jdiacomp.2016.01.023 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1058.. Holman RR, Coleman RL, Chan JCN, et al. Effects of acarose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5(11):877–886. 10.1016/s2213-8587(17)30309-1 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1059.. Gaziano JM, Cincotta AH, O'Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010;33(7):1503–1508. 10.2337/dc09-2009 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1060.. Weiss T, Iglay K, Gulati T, Rajpathak S, Yang L, Blonde L. Secondary metformin monotherapy failure in individuals with type 2 diabetes mellitus. Open Diabetes Res Care 2021;9(1):e002127. 10.1136/bmjdrc-2021-002127 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1061.. Abdul-Ghani M, Puckett C, Adams J, et al. Durability of triple combination therapy versus stepwise addition therapy in patients with new-onset T2DM: 3-year follow-up of EDICT. Diabetes Care 2021;44(2):433–439. 10.2337/dc20-0978 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1062.. Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: Systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab 2012;14(3):228–233. 10.1111/j.1463-1326.2011.01512.x [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1063.. Sidorenkov G, van Boven JFM, Hoekstra T, Nijpels G, Hoogenberg K, Denig P. HbA1c response after insulin initiation in patients with type 2 diabetes mellitus in real life practice: Identifying distinct subgroups. Diabetes Obes Metab 2018;20(8):1957–1964. 10.1111/dom.13332 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1064.. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): A randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017;5(5):355–366. 10.1016/s2213-8587(17)30085-2 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1065.. Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361(18):1736–1747. 10.1056/NEJMoa0905479 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1066.. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29(6): 1269–1274. 10.2337/dc05-1365 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1067.. Home PD, Fritsche A, Schinzel S, Massi-Benedetti M. Meta-analysis of individual patient data to assess the risk of hypoglycaemia in people with type 2 diabetes using NPH insulin or insulin glargine. Diabetes Obes Metab 2010;12(9):772–779. 10.1111/j.1463-1326.2010.01232.x [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1068.. Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: A meta-analysis. Diabetes Res Clin Pract 2008;81(2):184–189. 10.1016/j.diabres.2008.04.007 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1069.. Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26(11):3080–3086. 10.2337/diacare.26.11.3080 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1070.. Ji L, Zhang P, Zhu D, et al. Comparative effectiveness and safety of different basal insulins in a real-world setting. Diabetes Obes Metab 2017;19(8): 1116–1126. 10.1111/dom.12920 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1071.. Lipska KJ, Parker MM, Moffet HH, Huang ES, Karter AJ. Association of initiation of basal insulin analogs vs Neutral Protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA 2018;320(1):53–62. 10.1001/jama.2018.7993 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1072.. Heller SR, DeVries JH, Wysham C, Hansen CT, Hansen MV, Frier BM. Lower rates of hypoglycaemia in older individuals with type 2 diabetes using insulin degludec versus insulin glargine u100: Results from switch 2. Diabetes Obes Metab 2019;21(7):1634–1641. 10.1111/dom.13708 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1073.. Roussel R, d'Emden MC, Fisher M, et al. Glycaemic control and hypoglycaemia in people with type 2 diabetes switching from twice-daily basal insulin to once-daily insulin glargine 300 u/ml or insulin glargine 100 u/ml (EDITION 1 and EDITION 2 subgroup analysis). Diabetes Obes Metab 2018;20(2):448–452. 10.1111/dom.13071 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1074.. Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA 2021;325(22): 2273–2284. 10.1001/jama.2021.6530 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1075.. Janka HU, Plewe G, Riddle MC, Kliebe-Frisch C, Schweitzer MA, Yki-Järvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care 2005;28(2):254–259. 10.2337/diacare.28.2.254 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1076.. Tunis SL, Sauriol L, Minshall ME. Cost effectiveness of insulin glargine plus oral antidiabetes drugs compared with premixed insulin alone in patients with type 2 diabetes mellitus in Canada. Appl Health Econ Health Policy 2010;8(4):267–280. 10.2165/11535380-000000000-00000 [EL 3; ECON]. [DOI] [PubMed] [Google Scholar]
- 1077.. Yki-Jarvinen H, Kauppila M, Kujansuu E, et al. Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1992;327(20):1426–1433. 10.1056/nejm199211123272005 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1078.. Jin SM, Kim JH, Min KW, et al. Basal-prandial versus premixed insulin in patients with type 2 diabetes requiring insulin intensification after basal insulin optimization: A 24-week randomized non-inferiority trial. J Diabetes 2016;8(3):405–413. 10.1111/1753-0407.12312 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1079.. Vora J, Cohen N, Evans M, Hockey A, Speight J, Whately-Smith C. Intensifying insulin regimen after basal insulin optimization in adults with type 2 diabetes: A 24-week, randomized, open-label trial comparing insulin glargine plus insulin glulisine with biphasic insulin aspart (LanScape). Diabetes Obes Metab 2015;17(12):1133–1141. 10.1111/dom.12528 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1080.. Franek E, Haluzík M, Canecki Varžić S, et al. Twice-daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin-naïve adults with type 2 diabetes. Diabetic Med 2016;33(4): 497–505. 10.1111/dme.12982 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1081.. Kumar A, Franek E, Wise J, Niemeyer M, Mersebach H, Simó R. Efficacy and safety of once-daily insulin degludec/insulin aspart versus insulin glargine (u100) for 52 weeks in insulin-naïve patients with type 2 diabetes: A randomized controlled trial. PloS One 2016;11(10):e0163350. 10.1371/journal.pone.0163350 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1082.. Kawaguchi Y, Sawa J, Hamai C, Nishimura Y, Kumeda Y. Comparison of the efficacy and safety of insulin degludec/aspart (twice-daily injections), insulin glargine 300 u/ml, and insulin glulisine (basal-bolus therapy). J Diabetes Investig 2019;10(6):1527–1536. 10.1111/jdi.13038 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1083.. Watada H, Imori M, Li P, Iwamoto N. Insulin lispro 25/75 and insulin lispro 50/50 as starter insulin in japanese patients with type 2 diabetes: Subanalysis of the classify randomized trial. Endocrine J 2017;64(7):705–717. 10.1507/endocrj.EJ17-0020 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1084.. Yang W, Ersoy C, Wang G, et al. Efficacy and safety of three-times-daily versus twice-daily biphasic insulin aspart 30 in patients with type 2 diabetes mellitus inadequately controlled with basal insulin combined with oral antidiabetic drugs. Diabetes Res Clin Pract 2019;150:158–166. 10.1016/j.diabres.2019.02.023 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1085.. Blevins T, Zhang Q, Frias JP, Jinnouchi H, Chang AM. Randomized double-blind clinical trial comparing ultra rapid lispro with lispro in a basal-bolus regimen in patients with type 2 diabetes: PRONTO-T2D. Diabetes Care 2020;43(12):2991–2998. 10.2337/dc19-2550 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1086.. Bowering K, Harvey J, Kolaczynski JW, Snyder JW, Bode BW. Mealtime fast-acting insulin aspart versus insulin aspart for controlling postprandial hyperglycaemia in people with insulin-resistant type 2 diabetes. Diabetic Med 2019;36(6):771–775. 10.1111/dme.13866 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1087.. Seaquist ER, Blonde L, McGill JB, et al. Hypoglycaemia is reduced with use of inhaled Technosphere(®) Insulin relative to insulin aspart in type 1 diabetes mellitus. Diabetic Med 2020;37(5):752–759. 10.1111/dme.14202 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1088.. Rosenstock J, Franco D, Korpachev V, et al. Inhaled Technosphere Insulin versus inhaled technosphere placebo in insulin-naïve subjects with type 2 diabetes inadequately controlled on oral antidiabetes agents. Diabetes Care 2015;38(12):2274–2281. 10.2337/dc15-0629 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1089.. Pittas AG, Westcott GP, Balk EM. Efficacy, safety, and patient acceptability of Technosphere inhaled insulin for people with diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015;3(11):886–894. 10.1016/s2213-8587(15)00280-6 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1090.. Reznik Y, Cohen O, Aronson R, et al. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): A randomised open-label controlled trial. Lancet 2014;384(9950):1265–1272. 10.1016/s0140-6736(14)61037-0 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1091.. Grunberger G, Rosenfeld CR, Bode BW, et al. Effectiveness of V-Go(®) for patients with type 2 diabetes in a real-world setting: A prospective observational study. Drugs Real World Outcomes 2020;7(1):31–40. 10.1007/s40801-019-00173-8 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1092.. Peyrot M, Rubin RR, Polonsky WH, Best JH. Patient reported outcomes in adults with type 2 diabetes on basal insulin randomized to addition of mealtime pramlintide or rapid-acting insulin analogs. Curr Med Res Opin 2010;26(5):1047–1054. 10.1185/03007991003634759 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1093.. Riddle M, Pencek R, Charenkavanich S, Lutz K, Wilhelm K, Porter L. Randomized comparison of pramlintide or mealtime insulin added to basal insulin treatment for patients with type 2 diabetes. Diabetes Care 2009;32(9): 1577–1582. 10.2337/dc09-0395 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1094.. Bell DS, Dharmalingam M, Kumar S, Sawakhande RB. Triple oral fixed-dose diabetes polypill versus insulin plus metformin efficacy demonstration study in the treatment of advanced type 2 diabetes (TrIED study III). Diabetes Obes Metab 2011;13(9):800–805. 10.1111/j.1463-1326.2011.01408.x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1095.. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: A review of their efficacy and tolerability. Diabetes Care 2011;34(Suppl 2): S279–S284. 10.2337/dc11-s231 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1096.. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: A randomized, controlled trial. Ann Intern Med 2011;154(2):103–112. 10.7326/0003-4819-154-2-201101180-00300 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1097.. DeVries JH, Bain SC, Rodbard HW, et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1c targets. Diabetes Care 2012;35(7): 1446–1454. 10.2337/dc11-1928 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1098.. Giugliano D, Longo M, Caruso P, et al. Feasibility of simplification from a basal-bolus insulin regimen to a fixed-ratio formulation of basal insulin plus a glp-1ra or to basal insulin plus an SGLT2 inhibitor: Beyond, a randomized, pragmatic trial. Diabetes Care 2021;44(6):1353–1360. 10.2337/dc20-2623 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1099.. Rosenstock J, Emral R, Sauque-Reyna L, et al. Advancing therapy in suboptimally controlled basal insulin-treated type 2 diabetes: Clinical outcomes with iGlarLixi versus premix BIAsp 30 in the SoliMix randomized controlled trial. Diabetes Care 2021;44(10):2361–2370. 10.2337/dc21-0393 [EL 1; RCT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1100.. Vilsbøll T, Rosenstock J, Yki-Järvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010;12(2):167–177. 10.1111/j.1463-1326.2009.01173.x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1101.Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin 2012;28(4):513–523. 10.1185/03007995.2012.665046 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1102.Hood RC, Borra S, Fan L, Pollom RD, Huang A, Chen J. Treatment patterns and outcomes, before and after humulin R U-500 initiation, among high-dose type 2 diabetes mellitus patients in the united states. Endocr Pract 2021;27(8):798–806. 10.1016/j.eprac.2021.05.006 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1103.Reutrakul S, Wroblewski K, Brown RL. Clinical use of U-500 regular insulin: Review and meta-analysis. J Diabetes Sci Technol 2012;6(2):412–420. 10.1177/193229681200600229 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1104.Umpierrez GE, Holt EH, Einhorn D, McGill JB. Concentrated insulins: Clinical update of therapeutic options. Endocr Pract 2020;26(Suppl 3):1–12. 10.4158/ep-2019-0607 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1105.Hood RC, Arakaki RF, Wysham C, Li YG, Settles JA, Jackson JA. Two treatment approaches for human regular U-500 insulin in patients with type 2 diabetes not achieving adequate glycemic control on high-dose U-100 insulin therapy with or without oral agents: A randomized, titration-to-target clinical trial. Endocrine Pract 2015;21(7):782–793. 10.4158/ep15612.Or [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1106.DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: Scientific review. JAMA 2003;289(17):2254–2264. 10.1001/jama.289.17.2254 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1107.Balkau B, Home PD, Vincent M, Marre M, Freemantle N. Factors associated with weight gain in people with type 2 diabetes starting on insulin. Diabetes Care 2014;37(8):2108–2113. 10.2337/dc13-3010 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1108.Moghissi E, Ismail-Beigi F, Devine RC. Hypoglycemia: Minimizing its impact in type 2 diabetes. Endocr Pract 2013;19(3):526–535. 10.4158/ep13005. Ra [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1109.UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: Effects of treatment modalities and their duration. Diabetologia 2007;50(6):1140–1147. 10.1007/s00125-007-0599-y [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1110.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363(15):1410–1418. 10.1056/NEJMoa1003795 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1111.Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/ml versus glargine 100 units/ml in people with type 2 diabetes using oral agents and basal insulin: Glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37(12):3235–3243. 10.2337/dc14-0990 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1112.Adler A, Bennett P, Colagiuri Chair S, et al. Reprint of: Classification of diabetes mellitus. Diabetes Res Clin Pract 2021. 10.1016/j.diabres.2021.108972, 108972 epub ahead of print. [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1113.Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: A systematic review and meta-analysis. Ann Intern Med 2012;157(5): 336–347. 10.7326/0003-4819-157-5-201209040-00508 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1114.Umpierrez G, Korytkowski M. Diabetic emergencies - ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nature Rev Endocrinol. 2 016;12(4):222–232. 10.1038/nrendo.2016.15 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1115.Foster DW, McGarry JD. The metabolic derangements and treatment of diabetic ketoacidosis. N Engl J Med 1983;309(3):159–169. 10.1056/nejm198307213090307 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1116.Miles JM, Rizza RA, Haymond MW, Gerich JE. Effects of acute insulin deficiency on glucose and ketone body turnover in man: Evidence for the primacy of overproduction of glucose and ketone bodies in the genesis of diabetic ketoacidosis. Diabetes 1980;29(11):926–930. 10.2337/diab.29.11.926 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1117.Voss TS, Vendelbo MH, Kampmann U, et al. Substrate metabolism, hormone and cytokine levels and adipose tissue signalling in individuals with type 1 diabetes after insulin withdrawal and subsequent insulin therapy to model the initiating steps of ketoacidosis. Diabetologia 2019;62(3):494–503. 10.1007/s00125-018-4785-x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1118.Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2021;44(11):2589–2625. 10.2337/dci21-0043 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1119.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004;53(8):2079–2086. 10.2337/diabetes.53.8.2079 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1120.Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care 2004;27(8):1873–1878. 10.2337/diacare.27.8.1873 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1121.Umpierrez GE, Latif K, Stoever J, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Am J Med 2004;117(5):291–296. 10.1016/j.amjmed.2004.05.010 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1122.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353(25):2643–2653. 10.1056/NEJMoa052187 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1123.Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev 2014;2014(2):Cd009122. 10.1002/14651858.CD009122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1124.Mathieu C, Gillard P, Benhalima K. Insulin analogues in type 1 diabetes mellitus: Getting better all the time. Nat Rev Endocrinol 2017;13(7): 385–399. 10.1038/nrendo.2017.39 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1125.Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev 2010;1:Cd005103. 10.1002/14651858.CD005103. pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1126.Monami M, Lamanna C, Marchionni N, Mannucci E. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 2 diabetes: A meta-analysis. Exp Clin Endocrinol Diabetes 2009;117(5):220–222. 10.1055/s-0028-1119405 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1127.Benkhadra K, Alahdab F, Tamhane SU, McCoy RG, Prokop LJ, Murad MH. Continuous subcutaneous insulin infusion versus multiple daily injections in individuals with type 1 diabetes: A systematic review and meta-analysis. Endocrine 2017;55(1):77–84. 10.1007/s12020-016-1039-x [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1128.Heller S, White D, Lee E, et al. A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: The repose trial. Health Technol Assess 2017;21(20):1–278. 10.3310/hta21200 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1129.Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: Results from the diamond trial. J Diabetes Sci Technol 2017;11(6):1138–1146. 10.1177/1932296817704445 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130.Cardona-Hernandez R, Schwandt A, Alkandari H, et al. Glycemic outcome associated with insulin pump and glucose sensor use in children and adolescents with type 1 diabetes. Data from the international pediatric registry SWEET. Diabetes Care 2021;44(5):1176–1184. 10.2337/dc20-1674 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1131.Beck RW, Riddlesworth TD, Ruedy KJ, et al. Effect of initiating use of an insulin pump in adults with type 1 diabetes using multiple daily insulin injections and continuous glucose monitoring (DIAMOND): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5(9):700–708. 10.1016/s2213-8587(17)30217-6 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1132.Tricco AC, Ashoor HM, Antony J, et al. Safety, effectiveness, and cost effectiveness of long acting versus intermediate acting insulin for patients with type 1 diabetes: Systematic review and network meta-analysis. BMJ 2014;349:g5459. 10.1136/bmj.g5459 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1133.Hemmingsen B, Metzendorf MI, Richter B. (ultra-)long-acting insulin analogues for people with type 1 diabetes mellitus. Cochrane Database Syst Rev 2021;3(3):Cd013498. 10.1002/14651858.CD013498.pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1134.Laranjeira FO, de Andrade KRC, Figueiredo A, Silva EN, Pereira MG. Long-acting insulin analogues for type 1 diabetes: An overview of systematic reviews and meta-analysis of randomized controlled trials. PloS One 2018;13(4):e0194801. 10.1371/journal.pone.0194801 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1135.Lane W, Bailey TS, Gerety G, et al. Effect of insulin degludec vs insulin glargine u100 on hypoglycemia in patients with type 1 diabetes: The SWITCH 1 randomized clinical trial. JAMA 2017;318(1):33–44. 10.1001/jama.2017.7115 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1136.Hirsch IB, Juneja R, Beals JM, Antalis CJ, Wright EE. The evolution of insulin and how it informs therapy and treatment choices. Endocr Rev 2020;41(5): 733–755. 10.1210/endrev/bnaa015 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1137.Thalange N, Deeb L, Iotova V, et al. Insulin degludec in combination with bolus insulin aspart is safe and effective in children and adolescents with type 1 diabetes. Pediatr Diabetes 2015;16(3):164–176. 10.1111/pedi.12263 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1138.Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study group of insulin glargine in type 1 diabetes. Diabetes Care 2000;23(5):639–643. 10.2337/diacare.23.5.639 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1139.Raskin P, Klaff L, Bergenstal R, Hallé JP, Donley D, Mecca T. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with Insulin Glargine in Type 1 Diabetes. Diabetes Care 2000;23(11):1666–1671. 10.2337/diacare.23.11.1666 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1140.Thalange N, Gundgaard J, Parekh W, Tutkunkardas D. Cost analysis of insulin degludec in comparison with insulin detemir in treatment of children and adolescents with type 1 diabetes in the uk. BMJ Open Diabetes Res Care 2019;7(1):e000664. 10.1136/bmjdrc-2019-000664 [EL 3; ECON]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1141.Fadini GP, Feher M, Hansen TK, et al. Switching to degludec from other basal insulins is associated with reduced hypoglycemia rates: A prospective study. J Clin Endocrinol Metab 2019;104(12):5977–5990. 10.1210/jc.2019-01021 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1142.Danne T, Matsuhisa M, Sussebach C, et al. Lower risk of severe hypoglycaemia with insulin glargine 300 u/ml versus glargine 100 u/ml in participants with type 1 diabetes: A meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab 2020;22(10):1880–1885. 10.1111/dom.14109 [EL1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1143.Laviola L, Porcellati F, Bruttomesso D, Larosa M, Rossi MC, Nicolucci A. Comparative effectiveness of switching from first-generation basal insulin to glargine 300 u/ml or degludec 100 u/ml in type 1 diabetes: The RESTORE-1 study. Diabetes Ther 2021;12(2):509–525. 10.1007/s13300-020-00982-z [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1144.Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/ml versus glargine 100 units/ml in people with type 1 diabetes: A randomized, phase 3a, open-label clinical trial (edition 4). Diabetes Care 2015;38(12): 2217–2225. 10.2337/dc15-0249 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1145.Russell-Jones D, Gall MA, Niemeyer M, Diamant M, Del Prato S. Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. Insulin glargine: A meta-analysis of seven clinical trials. Nutr Metab Cardiovasc Dis 2015;25(10):898–905. 10.1016/j.numecd.2015.06.005 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1146.Lee TY, Kuo S, Yang CY, Ou HT. Cost-effectiveness of long-acting insulin analogues vs intermediate/long-acting human insulin for type 1 diabetes: A population-based cohort followed over 10 years. Br J Clin Pharmacol 2020;86(5):852–860. 10.1111/bcp.14188 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1147.Battelino T, Edelman SV, Nishimura R, Bergenstal RM. Comparison of second-generation basal insulin analogs: A review of the evidence from continuous glucose monitoring. Diabetes Technol Ther 2021;23(1):20–30. 10.1089/dia.2020.0180 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1148.Miura H, Sakaguchi K, Otowa-Suematsu N, et al. Effects of insulin degludec and insulin glargine u300 on glycaemic stability in individuals with type 1 diabetes: A multicentre, randomized controlled crossover study. Diabetes Obes Metab 2020;22(12):2356–2363. 10.1111/dom.14161 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1149.Battelino T, Bosnyak Z, Danne T, et al. InRange: Comparison of the second-generation basal insulin analogues glargine 300 u/ml and degludec 100 u/ml in persons with type 1 diabetes using continuous glucose monitoring-study design. Diabetes Ther 2020;11(4): 1017–1027. 10.1007/s13300-020-00781-6 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1150.A research study to compare a new weekly insulin, insulin icodec, and an available daily insulin, insulin degludec, both in combination with mealtime insulin in people with type 1 diabetes (ONWARDS 6) Clinicaltrials.Gov identifier: NCT04848480. Available at: https://clinicaltrials.gov/ct2/show/NCT04848480. Accessed February 21, 2022.
- 1151.Brunelle BL, Llewelyn J, Anderson JH Jr, Gale EA, Koivisto VA. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. ? 1998;21(10):1726–1731. 10.2337/diacare.21.10.1726 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1152.Fullerton B, Siebenhofer A, Jeitler K, et al. Short-acting insulin analogues versus regular human insulin for adults with type 1 diabetes mellitus. Cochrane Database Syst Rev 2016;2016(6):Cd012161. 10.1002/14651858.Cd012161 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1153.Melo KFS, Bahia LR, Pasinato B, et al. Short-acting insulin analogues versus regular human insulin on postprandial glucose and hypoglycemia in type 1 diabetes mellitus: A systematic review and meta-analysis. Diabetol Metab Syndr 2019;11(2). 10.1186/s13098-018-0397-3 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1154.Russell-Jones D, Bode BW, De Block C, et al. Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: Results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (ONSET 1). Diabetes Care 2017;40(7):943–950. 10.2337/dc16-1771 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1155.Klaff L, Cao D, Dellva MA, et al. Ultra rapid lispro improves postprandial glucose control compared with lispro in patients with type 1 diabetes: Results from the 26-week PRONTO-T1D study. Diabetes Obes Metab 2020;22(10):1799–1807. 10.1111/dom.14100 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1156.Klonoff DC, Evans ML, Lane W, et al. A randomized, multicentre trial evaluating the efficacy and safety of fast-acting insulin aspart in continuous subcutaneous insulin infusion in adults with type 1 diabetes (onset 5). Diabetes Obes Metab 2019;21(4):961–967. 10.1111/dom.13610 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1157.Mathieu C, Bode BW, Franek E, et al. Efficacy and safety of fast-acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): A 52-week, randomized, treat-to-target, phase III trial. Diabetes Obes Metab 2018;20(5):1148–1155. 10.1111/dom.13205 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1158.Akturk HK, Snell-Bergeon JK, Rewers A, et al. Improved postprandial glucose with inhaled technosphere insulin compared with insulin aspart in patients with type 1 diabetes on multiple daily injections: The STAT study. Diabetes Technol Ther 2018;20(10):639–647. 10.1089/dia.2018.0200 [EL 1; RCT, pilot]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1159.Bode BW, McGill JB, Lorber DL, Gross JL, Chang PC, Bregman DB. Inhaled Technosphere insulin compared with injected prandial insulin in type 1 diabetes: A randomized 24-week trial. Diabetes Care 2015;38(12): 2266–2273. 10.2337/dc15-0075 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1160.Hood KK, Rohan JM, Peterson CM, Drotar D. Interventions with adherence-promoting components in pediatric type 1 diabetes: Meta-analysis of their impact on glycemic control. Diabetes Care 2010;33(7):1658–1664. 10.2337/dc09-2268 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1161.Sterner Isaksson S, Bensow Bacos M, Eliasson B, et al. Effects of nutrition education using a food-based approach, carbohydrate counting or routine care in type 1 diabetes: 12 months prospective randomized trial. BMJ Open Diabetes Res Care 2021;9(1):e001971. 10.1136/bmjdrc-2020-001971 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1162.Bell KJ, Barclay AW, Petocz P, Colagiuri S, Brand-Miller JC. Efficacy of carbohydrate counting in type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 2014;2(2):133–140. 10.1016/s2213-8587(13)70144-x [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1163.Vaz EC, Porfírio GJM, Nunes HRC, Nunes-Nogueira VDS. Effectiveness and safety of carbohydrate counting in the management of adult patients with type 1 diabetes mellitus: A systematic review and meta-analysis. Arch Endocrinol Metab 2018;62(3):337–345. 10.20945/2359-3997000000045 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1164.DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: Dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325(7367): 746. 10.1136/bmj.325.7367.746 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1165.Bowering K, Case C, Harvey J, et al. Faster aspart versus insulin aspart as part of a basal-bolus regimen in inadequately controlled type 2 diabetes: The ONSET 2 trial. Diabetes Care 2017;40(7):951–957. 10.2337/dc16-1770 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1166.Sun C, Malcolm JC, Wong B, Shorr R, Doyle MA. Improving glycemic control in adults and children with type 1 diabetes with the use of smartphone-based mobile applications: A systematic review. Can J Diabetes 2019;43(1):51–58.e53. 10.1016/j.jcjd.2018.03.010 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1167.Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, et al. Efficacy of Insulclock in patients with poorly controlled type 1 diabetes mellitus: A pilot, randomized clinical trial. Diabetes Technol Ther 2020;22(9):686–690. 10.1089/dia.2019.0427 [EL 1; RCT, pilot]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1168.Adolfsson P, Hartvig NV, Kaas A, Møller JB, Hellman J. Increased time in range and fewer missed bolus injections after introduction of a smart connected insulin pen. Diabetes Technol Ther 2020;22(10):709–718. 10.1089/dia.2019.0411 [EL 2; PCS proof-of-concept]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1169.Munshi MN, Slyne C, Greenberg JM, et al. Nonadherence to insulin therapy detected by bluetooth-enabled pen cap is associated with poor glycemic control. Diabetes Care 2019;42(6):1129–1131. 10.2337/dc18-1631 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1170.Blair J, McKay A, Ridyard C, et al. Continuous subcutaneous insulin infusion versus multiple daily injections in children and young people at diagnosis of type 1 diabetes: The scipi rct. Health Technol Assess 2018;22(42):1–112. 10.3310/hta22420 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1171.Edelman S, Garg S, Frias J, et al. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care 2006;29(10):2189–2195. 10.2337/dc06-0042 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1172.Ratner RE, Dickey R, Fineman M, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: A 1-year, randomized controlled trial. Diabetic Med 2004;21(11):1204–1212. 10.1111/j.1464-5491.2004.01319.x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1173.Whitehouse F, Kruger DF, Fineman M, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 2002;25(4): 724–730. 10.2337/diacare.25.4.724 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1174.Lee NJ, Norris SL, Thakurta S. Efficacy and harms of the hypoglycemic agent pramlintide in diabetes mellitus. Ann Fam Med 2010;8(6):542–549. 10.1370/afm.1174 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1175.Meng H, Zhang A, Liang Y, Hao J, Zhang X, Lu J. Effect of metformin on glycaemic control in patients with type 1 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metab Res Rev 2018;34(4):e2983. 10.1002/dmrr.2983 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1176.von Herrath M, Bain SC, Bode B, et al. Anti-interleukin-21 antibody and liraglutide for the preservation of b-cell function in adults with recent-onset type 1 diabetes: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 2021;9(4):212–224. 10.1016/s2213-8587(21)00019-x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1177.Ahrén B, Hirsch IB, Pieber TR, et al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: The adjunct two randomized trial. Diabetes Care 2016;39(10):1693–1701. 10.2337/dc16-0690 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1178.Yamada T, Shojima N, Noma H, Yamauchi T, Kadowaki T. Sodium-glucose co-transporter-2 inhibitors as add-on therapy to insulin for type 1 diabetes mellitus: Systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab 2018;20(7):1755–1761. 10.1111/dom.13260 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1179.Rawla P, Vellipuram AR, Bandaru SS, Pradeep Raj J. Euglycemic diabetic ketoacidosis: A diagnostic and therapeutic dilemma. Endocrinol Diabetes Metab Case Rep 2017;2017:17–0081. 10.1530/edm-17-0081 [EL 3; CCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1180.Taylor SI, Blau JE, Rother KI, Beitelshees AL. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: Balancing benefits and risks. Lancet Diabetes Endocrinol 2019;7(12):949–958. 10.1016/s2213-8587(19)30154-8 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1181.Garg SK, Peters AL, Buse JB, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: A STICH protocol. Diabetes Technol Ther 2018;20(9):571–575. 10.1089/dia.2018.0246 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1182.Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44(7):1630–1640. 10.2337/dc21-0172 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1183.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381(18):1707–1717. 10.1056/NEJMoa1907863 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1184.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19(3):155–163. 10.1089/dia.2016.0421 [EL 2; NRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1185.Rosenlund S, Hansen TW, Andersen S, Rossing P. Effect of 4 years subcutaneous insulin infusion treatment on albuminuria, kidney function and HbA1c compared with multiple daily injections: A longitudinal follow-up study. Diabetic Med 2015;32(11):1445–1452. 10.1111/dme.12950 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1186.. Rosenlund S, Hansen TW, Rossing P, Andersen S. Effect of sensor-augmented pump treatment versus multiple daily injections on albuminuria: A 1-year randomized study. J Clin Endocrinol Metab 2015;100(11):4181–4188. 10.1210/jc.2015-2839 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1187.Buse JB, Kudva YC, Battelino T, Davis SN, Shin J, Welsh JB. Effects of sensor-augmented pump therapy on glycemic variability in well-controlled type 1 diabetes in the STAR 3 study. Diabetes Technol Ther 2012;14(7):644–647. 10.1089/dia.2011.0294 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1188.Beato-Víbora PI, Quirós-López C, Lázaro-Martín L, et al. Impact of sensor-augmented pump therapy with predictive low-glucose suspend function on glycemic control and patient satisfaction in adults and children with type 1 diabetes. Diabetes Technol Ther 2018;20(11):738–743. 10.1089/dia.2018.0199 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1189.Bomba F, Müller-Godeffroy E, von Sengbusch S. Experiences in sensor-augmented pump therapy in families with two children with type 1 diabetes: A qualitative study. Exp Clin Endocrinol Diabetes 2018;126(3): 162–167. 10.1055/s-0043-110479 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1190.Šoupal J, Petruželková L, Flekač M, et al. Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52 weeks of follow-up: A comisair study. Diabetes Technol Ther 2016;18(9):532–538. 10.1089/dia.2016.0171 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1191.Pala L, Dicembrini I, Mannucci E. Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: An updated meta-analysis of randomized clinical trials. Acta Diabetologica 2019;56(9): 973–980. 10.1007/s00592-019-01326-5 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1192.Fang Z, Liu M, Tao J, Li C, Zou F, Zhang W. Efficacy and safety of closed-loop insulin delivery versus sensor-augmented pump in the treatment of adults with type 1 diabetes: A systematic review and meta-analysis of randomized-controlled trials. J Endocrinol Invest 2021. 10.1007/s40618-021-01674-6 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1193.Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: A multicentre, 12-week randomised trial. Lancet 2018;392(10155):1321–1329. 10.1016/s0140-6736(18)31947-0 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1194.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: Systematic review and meta-analysis. BMJ 2018;361(k1310). 10.1136/bmj.k1310 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1195.Lum JW, Bailey RJ, Barnes-Lomen V, et al. A real-world prospective study of the safety and effectiveness of the loop open source automated insulin delivery system. Diabetes Technol Ther 2021;23(5):367–375. 10.1089/dia.2020.0535 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1196.Whipple AO. The surgical therapy of hyperinsulinism. J Int Chir 1938;3: 237–276 [EL 4; NE]. [Google Scholar]
- 1197.Cryer PE. In: Jefferson L, Cherrington A, eds. Handbook of physiology: A critical, comprehensive presentation of physiological knowledge and concepts New York: Oxford University Press, 2001: 1057–1092. [EL 4; NE] [Google Scholar]
- 1198.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36(5):1384–1395. 10.2337/dc12-2480 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1199.Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA(1c) for type 1 diabetes: A consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF international, the Leona M. And Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40(12):1622–1630. 10.2337/dc17-1624 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1200.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2013;369(4):362–372. 10.1056/NEJMra1215228 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1201.. Tetzschner R, Nørgaard K, Ranjan A. Effects of alcohol on plasma glucose and prevention of alcohol-induced hypoglycemia in type 1 diabetes-a systematic review with GRADE. Diabetes Metab Res Rev 2018;34(3). 10.1002/dmrr.2965 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1202.Gubitosi-Klug RA, Braffett BH, White NH, et al. Risk of severe hypoglycemia in type 1 diabetes over 30 years of follow-up in the DCCT/EDIC study. Diabetes Care 2017;40(8):1010–1016. 10.2337/dc16-2723 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1203.Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes 1997;46(2): 271–286 [EL 1; RCT]. [PubMed] [Google Scholar]
- 1204.Kostev K, Dippel FW, Rathmann W. Predictors of hypoglycaemia in insulin-treated type 2 diabetes patients in primary care: A retrospective database analysis. Prim Care Diabetes 2014;8(2):127–131. 10.1016/j.pcd.2013.10.001 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1205.Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UKea nested case-control analysis. Diabetes Obes Metab 2014;16(9):801–811. 10.1111/dom.12282 [EL 2; NCCS]. [DOI] [PubMed] [Google Scholar]
- 1206.Feinkohl I, Aung PP, Keller M, et al. Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: The Edinburgh type 2 diabetes study. Diabetes Care 2014;37(2):507–515. 10.2337/dc13-1384 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1207.Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: Systematic review and meta-analysis with bias analysis. BMJ 2013;347:f4533. 10.1136/bmj.f4533 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1208.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35(9):1897–1901. 10.2337/dc11-2054 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1209.Cryer PE. Death during intensive glycemic therapy of diabetes: Mechanisms and implications. Am J Med 2011;124(11):993–996. 10.1016/j.amjmed.2011.08.008 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1210.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26(6):1902–1912. 10.2337/diacare.26.6.1902 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1211.Liu S, Zhao Y, Hempe JM, Fonseca V, Shi L. Economic burden of hypoglycemia in patients with type 2 diabetes. Expert Rev Pharmacoecon Outcomes Res 2012;12(1):47–51. 10.1586/erp.11.87 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1212.Bajpai S, Wong-Jacobson S, Liu D, et al. Health care resource utilization and cost of severe hypoglycemia treatment in insulin-treated patients with diabetes in the united states. Manag Care Spec Pharm 2021;27(3):385–391. 10.18553/jmcp.2021.27.3.385 [EL 3; ECON]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1213.Strizek A, Chang CJ, Furnback W, Wang B, Lebrec J, Lew T. The cost of hypoglycemia associated with type 2 diabetes mellitus in taiwan. Value Health Reg Issues 2019;18:84–90. 10.1016/j.vhri.2019.01.002 [EL 3; ECON]. [DOI] [PubMed] [Google Scholar]
- 1214.Wong CKH, Tong T, Cheng GHL, et al. Direct medical costs in the preceding, event and subsequent years of a first severe hypoglycaemia episode requiring hospitalization: A population-based cohort study. Diabetes Obes Metab 2019;21(6):1330–1339. 10.1111/dom.13657 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1215.Carlson JN, Schunder-Tatzber S, Neilson CJ, Hood N. Dietary sugars versus glucose tablets for first-aid treatment of symptomatic hypoglycaemia in awake patients with diabetes: A systematic review and meta-analysis. Emerg Med J 2017;34(2):100–106. 10.1136/emermed-2015-205637 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1216.Krebs JD, Weatherall M, Corley B, Wiltshire E, McTavish L. Optimizing the management of hypoglycaemia in individuals with type 2 diabetes: A randomized crossover comparison of a weight-based protocol compared with two fixed-dose glucose regimens. Diabetes Obes Metab 2018;20(5): 1256–1261. 10.1111/dom.13231 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1217.McTavish L, Corley B, Weatherall M, Wiltshire E, Krebs JD. Weight-based carbohydrate treatment of hypoglycaemia in people with type 1 diabetes using insulin pump therapy: A randomized crossover clinical trial. Diabetic Med 2018;35(3):339–346. 10.1111/dme.13576 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1218.Kiyosue A, Seino Y, Nishijima K, Bosch-Traberg H, Kaku K. Safety and efficacy of the combination of the glucagon-like peptide-1 receptor agonist liraglutide with an oral antidiabetic drug in japanese patients with type 2 diabetes: Post-hoc analysis of a randomized, 52-week, open-label, parallel-group trial. J Diabetes Investig 2018;9(4):831–839. 10.1111/jdi.12759 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1219.Talaviya PA, Saboo BD, Dodiya HG, et al. Retrospective comparison of voglibose or acarbose as an add-on therapy to sulfonylureas in Western Indian patients with uncontrolled overweight/obese type 2 diabetes. Diabetes Metab Syndr 2016;10(2):88–91. 10.1016/j.dsx.2015.09.021 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1220.Tschöpe D, Bramlage P, Schneider S, Gitt AK. Incidence, characteristics and impact of hypoglycaemia in patients receiving intensified treatment for inadequately controlled type 2 diabetes mellitus. Diabetes Vasc Dis Res 2016;13(1):2–12. 10.1177/1479164115610470 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1221.Wang JS, Lee IT, Lee WJ, et al. Glycemic excursions are positively associated with changes in duration of asymptomatic hypoglycemia after treatment intensification in patients with type 2 diabetes. Diabetes Res Clin Pract 2016;113:108–115. 10.1016/j.diabres.2015.12.010 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1222.Wang RR, Lv ZM, Dan YP, Chen KY, Zhang C. Effects of acarbose and siglitine on blood glucose fluctuation and islet b-cell function in patients with type 2 diabetes mellitus. J Biol Regul Homeost Agents 2019;33(2):365–374 [EL 1; RCT]. [PubMed] [Google Scholar]
- 1223.Wang W, Ning G, Ma J, et al. A randomized clinical trial of the safety and efficacy of sitagliptin in patients with type 2 diabetes mellitus inadequately controlled by acarbose alone. Curr Med Res Opin 2017;33(4):693–699. 10.1080/03007995.2016.1277200 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1224.Wu H, Liu J, Lou Q, et al. Comparative assessment of the efficacy and safety of acarbose and metformin combined with premixed insulin in patients with type 2 diabetes mellitus. Medicine 2017;96(35):e7533. 10.1097/md.0000000000007533 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1225.Zhu D, Gan S, Liu Y, et al. Dorzagliatin monotherapy in chinese patients with type 2 diabetes: A dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. The Lancet Diabetes Endocrinol 2018;6(8): 627–636. 10.1016/s2213-8587(18)30105-0 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1226.Guzman CB, Dulude H, Piché C, et al. Effects of common cold and concomitant administration of nasal decongestant on the pharmacokinetics and pharmacodynamics of nasal glucagon in otherwise healthy participants: A randomized clinical trial. Diabetes Obes Metab 2018;20(3):646–653. 10.1111/dom.13134 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1227.Haymond MW, DuBose SN, Rickels MR, et al. Efficacy and safety of mini-dose glucagon for treatment of nonsevere hypoglycemia in adults with type 1 diabetes. J Clin Endocrinol Metab 2017;102(8):2994–3001. 10.1210/jc.2017-00591 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1228.Ranjan A, Schmidt S, Damm-Frydenberg C, et al. Low-carbohydrate diet impairs the effect of glucagon in the treatment of insulin-induced mild hypoglycemia: A randomized crossover study. Diabetes Care 2017;40(1): 132–135. 10.2337/dc16-1472 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1229.Ranjan A, Schmidt S, Madsbad S, Holst JJ, Nørgaard K. Effects of subcutaneous, low-dose glucagon on insulin-induced mild hypoglycaemia in patients with insulin pump treated type 1 diabetes. Diabetes Obes Metab 2016;18(4):410–418. 10.1111/dom.12627 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1230.Hawkes CP, De Leon DD, Rickels MR. Novel preparations of glucagon for the prevention and treatment of hypoglycemia. Curr Diab Rep 2019;19(10):97. 10.1007/s11892-019-1216-4 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1231.Christiansen MP, Cummins M, Prestrelski S, Close NC, Nguyen A, Junaidi K. Comparison of a ready-to-use liquid glucagon injection administered by autoinjector to glucagon emergency kit for the symptomatic relief of severe hypoglycemia: Two randomized crossover non-inferiority studies. BMJ Open Diabetes Res Care 2021;9(1):e002137. 10.1136/bmjdrc-2021-002137 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1232.Pontiroli AE. Intranasal glucagon: A promising approach for treatment of severe hypoglycemia. J Diabetes Sci Technol 2015;9(1):38–43. 10.1177/1932296814557518 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1233.Sherr JL, Ruedy KJ, Foster NC, et al. Glucagon nasal powder: A promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care 2016;39(4):555–562. 10.2337/dc15-1606 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1234.Rickels MR, Ruedy KJ, Foster NC, et al. Intranasal glucagon for treatment of insulin-induced hypoglycemia in adults with type 1 diabetes: A randomized crossover noninferiority study. Diabetes Care 2016;39(2):264–270. 10.2337/dc15-1498 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1235.US Food & Drug Administration (FDA). Baqsimi (glucagon) nasal powder prescribing information Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2019/210134s000lbl.pdf [EL 4; NE]. [Google Scholar]
- 1236.Newswanger B, Ammons S, Phadnis N, et al. Development of a highly stable, nonaqueous glucagon formulation for delivery via infusion pump systems. J Diabetes Sci Technol 2015;9(1):24–33. 10.1177/1932296814565131 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1237.Hövelmann U, Bysted BV, Mouritzen U, et al. Pharmacokinetic and pharmacodynamic characteristics of dasiglucagon, a novel soluble and stable glucagon analog. Diabetes Care 2018;41(3):531–537. 10.2337/dc17-1402 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1238.Hövelmann U, Olsen MB, Mouritzen U, Lamers D, Kronshage B, Heise T. Low doses of dasiglucagon consistently increase plasma glucose levels from hypoglycaemia and euglycaemia in people with type 1 diabetes mellitus. Diabetes Obes Metab 2019;21(3):601–610. 10.1111/dom.13562 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1239.De Buck E, Borra V, Carlson JN, Zideman DA, Singletary EM, Djärv T. First aid glucose administration routes for symptomatic hypoglycaemia. Cochrane Database Syst Rev 2019;4(4):Cd013283. 10.1002/14651858.CD013283.pub2 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1240.Hochuli M, Christ E, Meienberg F, Lehmann R, Krützfeldt J, Baumgartner MR. Alternative nighttime nutrition regimens in glycogen storage disease type i: A controlled crossover study. J Inherit Metab Dis 2015;38(6):1093–1098. 10.1007/s10545-015-9864-2 [EL2; NRCT]. [DOI] [PubMed] [Google Scholar]
- 1241.Rickels MR, DuBose SN, Toschi E, et al. Mini-dose glucagon as a novel approach to prevent exercise-induced hypoglycemia in type 1 diabetes. Diabetes Care 2018;41(9):1909–1916. 10.2337/dc18-0051 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1242.Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: A comparison of glyburide with other secretagogues and with insulin. Diabetes Care 2007;30(2):389–394. 10.2337/dc06-1789 [EL 1: MRCT]. [DOI] [PubMed] [Google Scholar]
- 1243.Fritsche A, Stefan N, Häring H, Gerich J, Stumvoll M. Avoidance of hypoglycemia restores hypoglycemia awareness by increasing beta-adrenergic sensitivity in type 1 diabetes. Ann Intern Med 2001;134(9 Pt 1):729–736. 10.7326/0003-4819-134-9_part_1-200105010-00009 [EL 2; NRCT]. [DOI] [PubMed] [Google Scholar]
- 1244.Fritsche A, Stumvoll M, Häring HU, Gerich JE. Reversal of hypoglycemia unawareness in a long-term type 1 diabetic patient by improvement of beta-adrenergic sensitivity after prevention of hypoglycemia. J Clin Endocrinol Metab 2000;85(2):523–525. 10.1210/jcem.85.2.6353 [EL 3; SCR]. [DOI] [PubMed] [Google Scholar]
- 1245.Johansen NJ, Christensen MB. A systematic review on insulin overdose cases: Clinical course, complications and treatment options. Basic Clin Pharmacol Toxicol 2018;122(6):650–659. 10.1111/bcpt.12957 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1246.Pathak RD, Schroeder EB, Seaquist ER, et al. Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in U.S. integrated health care delivery systems: 2005–2011. Diabetes Care 2016;39(3):363–370. 10.2337/dc15-0858 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1247.Shah BR, Walji S, Kiss A, James JE, Lowe JM. Derivation and validation of a risk-prediction tool for hypoglycemia in hospitalized adults with diabetes: The hypoglycemia during hospitalization (HyDHo) score. Can J Diabetes 2019;43(4):278–282.e271. 10.1016/j.jcjd.2018.08.061 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1248.Pinsker JE, Leas S, Müller L, Habif S. Real-world improvements in hypoglycemia in an insulin-dependent cohort with diabetes mellitus pre/post tandem basal-iq technology remote software update. Endocr Pract 2020;26(7): 714–721. 10.4158/ep-2019-0554 [EL 3; DS]. [DOI] [PubMed] [Google Scholar]
- 1249.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323(20):2052–2059. 10.1001/jama.2020.6775 [EL 3; CCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1250.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, march 1–30, 2020. MMWR Morbidity and mortality weekly report 2020;69(15):458–464. 10.15585/mmwr.mm6915e3 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1251.Falciglia M, Freyberg RW, Almenoff PL, D'Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Critical Care Med 2009;37(12):3001–3009. 10.1097/CCM.0b013e3181b083f7 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1252.Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010;33(8):1783–1788. 10.2337/dc10-0304 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1253.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet 2000;355(9206):773–778. 10.1016/s0140-6736(99)08415-9 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1254.Davis G, Fayfman M, Reyes-Umpierrez D, et al. Stress hyperglycemia in general surgery: Why should we care? J Diabetes Complications 2018;32(3):305–309. 10.1016/j.jdiacomp.2017.11.010 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1255.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: A report from the surgical care and outcomes assessment program. Ann Surg 2013;257(1): 8–14. 10.1097/SLA.0b013e31827b6bbc [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1256.Kutz A, Struja T, Hausfater P, et al. The association of admission hyperglycaemia and adverse clinical outcome in medical emergencies: The multinational, prospective, observational triage study. Diabetic Med 2017;34(7):973–982. 10.1111/dme.13325 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1257.Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: A systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97(1):49–58. 10.1210/jc.2011-2100 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1258.Negreiros PH, Bau A, Nadruz W, et al. Intensive treatment of hyperglycemia in the acute phase of myocardial infarction: The tenuous balance between effectiveness and safety - a systematic review and meta-analysis of randomized clinical trials. Rev Assoc Med Bras 2019;65(1):24–32. 10.1590/1806-9282.65.1.24 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1259.Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: A meta-analysis including NICE-SUGAR study data. CMAJ 2009;180(8):821–827. 10.1503/cmaj.090206 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1260.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA 2008;300(8):933–944. 10.1001/jama.300.8.933 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1261.Lipska KJ, Venkitachalam L, Gosch K, et al. Glucose variability and mortality in patients hospitalized with acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2012;5(4):550–557. 10.1161/circoutcomes.111.963298 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1262.Farrokhi F, Chandra P, Smiley D, et al. Glucose variability is an independent predictor of mortality in hospitalized patients treated with total parenteral nutrition. Endocr Pract 2014;20(1):41–45. 10.4158/ep13131.Or [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1263.Krinsley JS. Glycemic variability: A strong independent predictor of mortality in critically ill patients. Critical Care Med 2008;36(11):3008–3013. 10.1097/CCM.0b013e31818b38d2 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1264.Mendez CE, Mok KT, Ata A, Tanenberg RJ, Calles-Escandon J, Umpierrez GE. Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. Diabetes Care 2013;36(12):4091–4097. 10.2337/dc12-2430 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1265.Donnan PT, Leese GP, Morris AD. Hospitalizations for people withtype 1 and type 2 diabetes compared with the nondiabetic population of Tayside, Scotland: A retrospective cohort study of resource use. Diabetes Care 2000;23(12): 1774–1779. 10.2337/diacare.23.12.1774 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1266.Pasquel FJ, Lansang MC, Dhatariya K, Umpierrez GE. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol 2021;9(3):174–188. 10.1016/s2213-8587(20)30381-8 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1267.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: Implications for patients with and without recognized diabetes. Circulation 2005;111(23): 3078–3086. 10.1161/circulationaha.104.517839 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1268.American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 16. Diabetes care in the hospital: Standards of medical care in diabetes-2022. Diabetes Care 2022;45(Supplement_1):S244–S253. 10.2337/dc22-S016 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1269.Cardona S, Pasquel FJ, Fayfman M, et al. Hospitalization costs and clinical outcomes in cabg patients treated with intensive insulin therapy. J Diabetes Complications 2017;31(4):742–747. 10.1016/j.jdiacomp.2017.01.003 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 1270.Song F, Zhong LJ, Han L, et al. Intensive insulin therapy for septic patients: A meta-analysis of randomized controlled trials. Biomed Res Int 2014;2014: 698265. 10.1155/2014/698265 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1271.Kansagara D, Fu R, Freeman M, Wolf F, Helfand M. Intensive insulin therapy in hospitalized patients: A systematic review. Ann Intern Med 2011;154(4): 268–282. 10.7326/0003-4819-154-4-201102150-00008 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1272.Pittas AG, Siegel RD, Lau J. Insulin therapy and in-hospital mortality in critically ill patients: Systematic review and meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr 2006;30(2):164–172. 10.1177/0148607106030002164 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1273.Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: A meta-analysis of randomized controlled trials. Arch Intern Med 2004;164(18):2005–2011. 10.1001/archinte.164.18.2005 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1274.Yamada T, Shojima N, Noma H, Yamauchi T, Kadowaki T. Glycemic control, mortality, and hypoglycemia in critically ill patients: A systematic review and network meta-analysis of randomized controlled trials. Intensive Care Med 2017;43(1):1–15. 10.1007/s00134-016-4523-0 [EL 2; NMA]. [DOI] [PubMed] [Google Scholar]
- 1275.Umpierrez GE, Reyes D, Smiley D, et al. Hospital discharge algorithm based on admission HbA1c for the management of patients with type 2 diabetes. Diabetes Care 2014;37(11):2934–2939. 10.2337/dc14-0479 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1276.Nanayakkara N, Nguyen H, Churilov L, et al. Inpatient HbA1c testing: A prospective observational study. BMJ Open Diabetes Res Care 2015;3(1): e000113. 10.1136/bmjdrc-2015-000113 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1277.Ekinci EI, Kong A, Churilov L, et al. Using automated HbA1c testing to detect diabetes mellitus in orthopedic inpatients and its effect on outcomes. PloS One 2017;12(1):e0168471. 10.1371/journal.pone.0168471 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1278.Gomez-Peralta F, Abreu C, Andreu-Urioste L, et al. Point-of-care capillary hba1c measurement in the emergency department: A useful tool to detect unrecognized and uncontrolled diabetes. Int J Emerg Med 2016;9(1):7. 10.1186/s12245-016-0107-6 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1279.Jivanji CJ, Asrani VM, Windsor JA, Petrov MS. New-onset diabetes after acute and critical illness: A systematic review. Mayo Clin Proc 2017;92(5): 762–773. 10.1016/j.mayocp.2016.12.020 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1280.Bansal V, Mottalib A, Pawar TK, et al. Inpatient diabetes management by specialized diabetes team versus primary service team in non-critical care units: Impact on 30-day readmission rate and hospital cost. BMJ Open Diabetes Res Care 2018;6(1):e000460. 10.1136/bmjdrc-2017-000460 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1281.Davies M, Dixon S, Currie CJ, Davis RE, Peters JR. Evaluation of a hospital diabetes specialist nursing service: A randomized controlled trial. Diabetic Med 2001;18(4):301–307. 10.1046/j.1464-5491.2001.00470.x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1282.Haque WZ, Demidowich AP, Sidhaye A, Golden SH, Zilbermint M. The financial impact of an inpatient diabetes management service. Curr Diab Rep 2021;21(2):5. 10.1007/s11892-020-01374-0 [EL 3; ECON]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1283.Mandel SR, Langan S, Mathioudakis NN, et al. Retrospective study of inpatient diabetes management service, length of stay and 30-day readmission rate of patients with diabetes at a community hospital. J Community Hosp Intern Med Perspect 2019;9(2):64–73. 10.1080/20009666.2019.1593782 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1284.Rushakoff RJ, Sullivan MM, MacMaster HW, et al. Association between a virtual glucose management service and glycemic control in hospitalized adult patients: An observational study. Ann Intern Med 2017;166(9): 621–627. 10.7326/m16-1413 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1285.Garg R, Hurwitz S, Rein R, Schuman B, Underwood P, Bhandari S. Effect of follow-up by a hospital diabetes care team on diabetes control at one year after discharge from the hospital. Diabetes Res Clin Pract 2017;133:78–84. 10.1016/j.diabres.2017.08.014 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1286.Magee MF, Nassar CM, Mete M, White K, Youssef GA, Dubin JS. The synergy to enable glycemic control following emergency department discharge program for adults with type 2 diabetes: Step-diabetes. Endocr Pract 2015;21(11):1227–1239. 10.4158/ep15655.Or [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1287.Ullal J, Dignan C, Cwik R, McFarland R, Gaines M, Aloi JA. Utility of computer-guided decision support system in discharge insulin dosing and diabetes-related readmissions. J Diabetes Sci Technol 2021;15(2):523–524. 10.1177/1932296820949282 [EL 3; PRECLIN]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1288.Goldberg PA, Siegel MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004;27(2):461–467. 10.2337/diacare.27.2.461 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1289.Brown G, Dodek P. Intravenous insulin nomogram improves blood glucose control in the critically ill. Critical Care Med 2001;29(9):1714–1719. 10.1097/00003246-200109000-00010 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1290.Higgs M, Fernandez R. The effect of insulin therapy algorithms on blood glucose levels in patients following cardiac surgery: A systematic review. JBI Database System Rev Implement Rep 2015;13(5):205–243. 10.11124/jbisrir-2015-1911 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1291.Eslami S, Abu-Hanna A, de Jonge E, de Keizer NF. Tight glycemic control and computerized decision-support systems: A systematic review. Intensive Care Med 2009;35(9):1505–1517. 10.1007/s00134-009-1542-0 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1292.Davis GM, Galindo RJ, Migdal AL, Umpierrez GE. Diabetes technology in the inpatient setting for management of hyperglycemia. Endocrinol Metab Clin North Am 2020;49(1):79–93. 10.1016/j.ecl.2019.11.002 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1293.Galindo RJ, Fayfman M, Umpierrez GE. Perioperative management of hyperglycemia and diabetes in cardiac surgery patients. Endocrinol Metab Clin North Am 2018;47(1):203–222. 10.1016/j.ecl.2017.10.005 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1294.Juneja R, Roudebush C, Kumar N, et al. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes Technol Ther 2007;9(3):232–240. 10.1089/dia.2006.0015 [EL 3; PRECLIN]. [DOI] [PubMed] [Google Scholar]
- 1295.Davidson PC, Steed RD, Bode BW. Glucommander: A computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care 2005;28(10):2418–2423. 10.2337/diacare.28.10.2418 [EL 3; PRECLIN]. [DOI] [PubMed] [Google Scholar]
- 1296.Boord JB, Sharifi M, Greevy RA, et al. Computer-based insulin infusion protocol improves glycemia control over manual protocol. J Am Med Inform Assoc 2007;14(3):278–287. 10.1197/jamia.M2292 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1297.Dortch MJ, Mowery NT, Ozdas A, et al. A computerized insulin infusion titration protocol improves glucose control with less hypoglycemia compared to a manual titration protocol in a trauma intensive care unit. JPEN J Parenter Enteral Nutr 2008;32(1):18–27. 10.1177/014860710803200118 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1298.Pachler C, Plank J, Weinhandl H, et al. Tight glycaemic control by an automated algorithm with time-variant sampling in medical icu patients. Intensive Care Med 2008;34(7):1224–1230. 10.1007/s00134-008-1033-8 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1299.Ullal J, Aloi JA, Reyes-Umpierrez D, et al. Comparison of computer-guided versus standard insulin infusion regimens in patients with diabetic ketoacidosis. J Diabetes Sci Technol 2018;12(1):39–46. 10.1177/1932296817750899 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1300.John SM, Waters KL, Jivani K. Evaluating the implementation of the EndoTool glycemic control software system. Diabetes Spectr 2018;31(1):26–30. 10.2337/ds16-0061 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1301.Tanenberg RJ, Hardee S, Rothermel C, Drake AJ 3rd. Use of a computer-guided glucose management system to improve glycemic control and address national quality measures: A 7-year, retrospective observation study at a tertiary care teaching hospital. Endocr Pract 2017;23(3):331–341. 10.4158/ep161402.Or [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1302.Newton CA, Smiley D, Bode BW, et al. A comparison study of continuous insulin infusion protocols in the medical intensive care unit: Computer-guided vs. Standard column-based algorithms. Journal Hosp Med 2010;5(8):432–437. 10.1002/jhm.816 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1303.Juneja R, Roudebush CP, Nasraway SA, et al. Computerized intensive insulin dosing can mitigate hypoglycemia and achieve tight glycemic control when glucose measurement is performed frequently and on time. Crit Care 2009;13(5):R163. 10.1186/cc8129 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1304.Olinghouse C Development of a computerized intravenous insulin application (AutoCal) at Kaiser Permanente Northwest, integrated into Kaiser Permanente HealthConnect: Impact on safety and nursing workload. Perm J 2012;16(3):67–70 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1305.Saur NM, Kongable GL, Holewinski S, O'Brien K, Nasraway SA Jr. Software-guided insulin dosing: Tight glycemic control and decreased glycemic derangements in critically ill patients. Mayo Clinic Proc 2013;88(9):920–929. 10.1016/j.mayocp.2013.07.003 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1306.Hermayer KL, Neal DE, Hushion TV, et al. Outcomes of a cardiothoracic intensive care web-based online intravenous insulin infusion calculator study at a medical university hospital. Diabetes Technol Ther 2007;9(6): 523–534. 10.1089/dia.2007.0225 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1307.Aloi J, Bode BW, Ullal J, et al. Comparison of an electronic glycemic management system versus provider-managed subcutaneous basal bolus insulin therapy in the hospital setting. J Diabetes Sci Technol 2017;11(1):12–16. 10.1177/1932296816664746 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1308.Umpierrez GE, Jones S, Smiley D, et al. Insulin analogs versus human insulin in the treatment of patients with diabetic ketoacidosis: A randomized controlled trial. Diabetes Care 2009;32(7):1164–1169. 10.2337/dc09-0169 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1309.Tran TTT, Pease A, Wood AJ, et al. Review of evidence for adult diabetic ketoacidosis management protocols. Front Endocrinol 2017;8:106. 10.3389/fendo.2017.00106 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1310.Scott AR. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabetic Med 2015;32(6):714–724. 10.1111/dme.12757 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1311.Joint British Diabetes Societies for Inpatient Care. The management of diabetic ketoacidosis in adults Revised june 2021. Available at: https://abcd.care/resource/jbds-02-management-diabetic-ketoacidosis-adults. Accessed February 21, 2022 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1312.Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers 2020;6(1):40. 10.1038/s41572-020-0165-1 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1313.Galindo RJ, Pasquel FJ, Vellanki P, et al. Biochemical parameters of diabetes ketoacidosis in patients with end-stage kidney disease and preserved renal function. J Clin Endocrinol Metab 2021;106(7):e2673–e2679. 10.1210/clinem/dgab126 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1314.Galindo RJ, Pasquel FJ, Fayfman M, et al. Clinical characteristics and outcomes of patients with end-stage renal disease hospitalized with diabetes ketoacidosis. BMJ Open Diabetes Res Care 2020;8(1):e000763. 10.1136/bmjdrc-2019-000763 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1315.Pasquel FJ, Tsegka K, Wang H, et al. Clinical outcomes in patients with isolated or combined diabetic ketoacidosis and hyperosmolar hyperglycemic state: A retrospective, hospital-based cohort study. Diabetes Care 2020;43(2):349–357. 10.2337/dc19-1168 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1316.Fayfman M, Davis G, Duggan EW, et al. Sitagliptin for prevention of stress hyperglycemia in patients without diabetes undergoing general surgery: A pilot randomized study. J Diabetes Complications 2018;32(12):1091–1096. 10.1016/j.jdiacomp.2018.08.014 [EL 1; RCT, pilot]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1317.Cardona S, Tsegka K, Pasquel FJ, et al. Sitagliptin for the prevention of stress hyperglycemia in patients without diabetes undergoing coronary artery bypass graft (CABG) surgery. BMJ Open Diabetes Res Care 2019;7(1): e000703. 10.1136/bmjdrc-2019-000703 [EL 1; RCT, pilot]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1318.Cardona S, Tsegka K, Pasquel FJ, et al. Sitagliptin for the prevention and treatment of perioperative hyperglycaemia in patients with type 2 diabetes undergoing cardiac surgery: A randomized controlled trial. Diabetes Obes Metab 2021;23(2):480–488. 10.1111/dom.14241 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1319.Abuannadi M, Kosiborod M, Riggs L, et al. Management of hyperglycemia with the administration of intravenous exenatide to patients in the cardiac intensive care unit. Endocr Pract 2013;19(1):81–90. 10.4158/ep12196.Or [EL 2; NRCT]. [DOI] [PubMed] [Google Scholar]
- 1320.Kohl BA, Hammond MS, Cucchiara AJ, Ochroch EA. Intravenous GLP-1 (7–36) amide for prevention of hyperglycemia during cardiac surgery: A randomized, double-blind, placebo-controlled study. J Cardiothorac Vasc Anesth 2014;28(3):618–625. 10.1053/j.jvca.2013.06.021 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1321.Besch G, Perrotti A, Mauny F, et al. Clinical effectiveness of intravenous exenatide infusion in perioperative glycemic control after coronary artery bypass graft surgery: A phase II/III randomized trial. Anesthesiology 2017;127(5):775–787. 10.1097/aln.0000000000001838 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1322.Lipš M, Mráz M, Kloučková J, et al. Effect of continuous exenatide infusion on cardiac function and peri-operative glucose control in patients undergoing cardiac surgery: A single-blind, randomized controlled trial. Diabetes Obes Metab 2017;19(12):1818–1822. 10.1111/dom.13029 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1323.Kaneko S, Ueda Y, Tahara Y. GLP1 receptor agonist liraglutide is an effective therapeutic option for perioperative glycemic control in type 2 diabetes within enhanced recovery after surgery (eras) protocols. Eur Surg Res 2018;59(5–6):349–360. 10.1159/000494768 [EL 1; RCT; safety]. [DOI] [PubMed] [Google Scholar]
- 1324.Hulst AH, Visscher MJ, Godfried MB, et al. Liraglutide for perioperative management of hyperglycaemia in cardiac surgery patients: A multicentre randomized superiority trial. Diabetes Obes Metab 2020;22(4):557–565. 10.1111/dom.13927 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1325.Doolin MK, Walroth TA, Harris SA, Whitten JA, Fritschle-Hilliard AC. Transition from intravenous to subcutaneous insulin in critically ill adults. J Diabetes Sci Technol 2016;10(4):932–938. 10.1177/1932296816629985 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1326.Shomali ME, Herr DL, Hill PC, Pehlivanova M, Sharretts JM, Magee MF. Conversion from intravenous insulin to subcutaneous insulin after cardiovascular surgery: Transition to target study. Diabetes Technol Ther 2011;13(2):121–126. 10.1089/dia.2010.0124 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1327.Lansang MC, Zhou K, Korytkowski MT. Inpatient hyperglycemia and transitions of care: A systematic review. Endocr Pract 2021;27(4):370–377. 10.1016/j.eprac.2021.01.016 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1328.Kreider KE, Lien LF. Transitioning safely from intravenous to subcutaneous insulin. Curr Diab Rep 2015;15(5):23. 10.1007/s11892-015-0595-4 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1329.Pichardo-Lowden A, Umpierrez G, Lehman EB, et al. Clinical decision support to improve management of diabetes and dysglycemia in the hospital: A path to optimizing practice and outcomes. BMJ Open Diabetes Res Care 2021;9(1). 10.1136/bmjdrc-2020-001557 [EL 2; CS pilot]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1330.Kavanagh BP, McCowen KC. Clinical practice. Glycemic control in the ICU. N Engl J Med 2010;363(26):2540–2546. 10.1056/NEJMcp1001115 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1331.Migdal AL, Fortin-Leung C, Pasquel F, Wang H, Peng L, Umpierrez GE. Inpatient glycemic control with sliding scale insulin in noncritical patients with type 2 diabetes: Who can slide? J Hosp Med 2021;16(8):462–468. 10.12788/jhm.3654 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1332.Sadhu AR, Serrano IA, Xu J, et al. Continuous glucose monitoring in critically ill patients with COVID-19: Results of an emergent pilot study. J Diabetes Sci Technol 2020;14(6):1065–1073. 10.1177/1932296820964264 [EL 3; DS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1333.Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev 2017;33(5). 10.1002/dmrr.2885 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1334.Colunga-Lozano LE, Gonzalez Torres FJ, Delgado-Figueroa N, et al. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst Rev 2018;11(11):Cd011296. 10.1002/14651858.CD011296.pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1335.Lee YY, Lin YM, Leu WJ, et al. Sliding-scale insulin used for blood glucose control: A meta-analysis of randomized controlled trials. Metabolism 2015;64(9):1183–1192. 10.1016/j.metabol.2015.05.011 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1336.Kyi M, Colman PG, Wraight PR, et al. Early intervention for diabetes in medical and surgical inpatients decreases hyperglycemia and hospital-acquired infections: A cluster randomized trial. Diabetes Care 2019;42(5): 832–840. 10.2337/dc18-2342 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1337.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med 2006;355(18):1903–1911. 10.1056/NEJMcp060094 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1338.Baldwin D, Zander J, Munoz C, et al. A randomized trial of two weight-based doses of insulin glargine and glulisine in hospitalized subjects with type 2 diabetes and renal insufficiency. Diabetes Care 2012;35(10):1970–1974. 10.2337/dc12-0578 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1339.Rubin DJ, Rybin D, Doros G, McDonnell ME. Weight-based, insulin dose-related hypoglycemia in hospitalized patients with diabetes. Diabetes Care 2011;34(8):1723–1728. 10.2337/dc10-2434 [EL 2; RCCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1340.Farrokhi F, Klindukhova O, Chandra P, et al. Risk factors for inpatient hypoglycemia during subcutaneous insulin therapy in non-critically ill patients with type 2 diabetes. J Diabetes Sci Technol 2012;6(5):1022–1029. 10.1177/193229681200600505 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1341.Vellanki P, Bean R, Oyedokun FA, et al. Randomized controlled trial of insulin supplementation for correction of bedtime hyperglycemia in hospitalized patients with type 2 diabetes. Diabetes Care 2015;38(4):568–574. 10.2337/dc14-1796 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1342.Vellanki P, Cardona S, Galindo RJ, et al. Efficacy and Safety of Intensive Versus Nonintensive supplemental insulin with a basal-Bolus insulin regimen in hospitalized patients with type 2 diabetes: A Randomized Clinical Study. Diabetes Care 2022:dc211606. 10.2337/dc21-1606. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1343.Pasquel FJ, Lansang MC, Khowaja A, et al. A randomized controlled trial comparing glargine U300 and glargine U100 for the inpatient management of medicine and surgery patients with type 2 diabetes: Glargine U300 hospital trial. Diabetes Care 2020;43(6):1242–1248. 10.2337/dc19-1940 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1344.Galindo RJ, Davis GM, Fayfman M, et al. Comparison of efficacy and safety of glargine and detemir insulin in the management of inpatient hyperglycemia and diabetes. Endocr Pract 2017;23(9):1059–1066. 10.4158/ep171804.Or [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1345.. Perez A, Carrasco-Sánchez FJ, González C, et al. Efficacy and safety of insulin glargine 300 U/ml (Gla-300) during hospitalization and therapy intensification at discharge in patients with insufficiently controlled type 2 diabetes: Results of the phase iv COBALTA trial. BMJ Open Diabetes Res Care 2020;8(1):e001518. 10.1136/bmjdrc-2020-001518 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1346.Galindo RJ, Pasquel FJ, Vellanki P, et al. Degludec hospital trial: A randomized controlled trial comparing insulin degludec U100 and glargine U100 for the inpatient management of patients with type 2 diabetes. Diabetes Obes Metab 2022;24(1):42–49. 10.1111/dom.14544 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1347.Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94(2):564–569. 10.1210/jc.2008-1441 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1348.Schroeder JE, Liebergall M, Raz I, et al. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: A randomized prospective study. Diabetes Metab Res Rev 2012;28(1):71–75. 10.1002/dmrr.1217 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1349.Bellido V, Suarez L, Rodriguez MG, et al. Comparison of basal-bolus and premixed insulin regimens in hospitalized patients with type 2 diabetes. Diabetes Care 2015;38(12):2211–2216. 10.2337/dc15-0160 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1350.Pérez-Belmonte LM, Osuna-Sanchez J, Millán-Gómez M, et al. Glycaemic efficacy and safety of linagliptin for the management of non-cardiac surgery patients with type 2 diabetes in a real-world setting: Linasurg study. Ann Med 2019;51(3–4):252–261. 10.1080/07853890.2019.1613672 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1351.Fayfman M, Galindo RJ, Rubin DJ, et al. A randomized controlled trial on the safety and efficacy of exenatide therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes. Diabetes Care 2019;42(3):450–456. 10.2337/dc18-1760 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1352.Fushimi N, Shibuya T, Yoshida Y, Ito S, Hachiya H, Mori A. Dulaglutide-combined basal plus correction insulin therapy contributes to ideal glycemic control in non-critical hospitalized patients. J Diabetes Investig 2020;11(1): 125–131. 10.1111/jdi.13093 [EL 1; RCT, pilot]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1353.Amir M, Sinha V, Kistangari G, Lansang MC. Clinical characteristics of patients with type 2 diabetes mellitus continued on oral antidiabetes medications in the hospital. Endocr Pract 2020;26(2):167–173. 10.4158/ep-2018-0524 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1354.Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: A pilot, randomized, controlled study. Diabetes Care 2013;36(11):3430–3435. 10.2337/dc13-0277 [EL 1; RCT, pilot]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1355.Vellanki P, Rasouli N, Baldwin D, et al. Glycaemic efficacy and safety of linagliptin compared to a basal-bolus insulin regimen in patients with type 2 diabetes undergoing non-cardiac surgery: A multicentre randomized clinical trial. Diabetes Obes Metab 2019;21(4):837–843. 10.1111/dom.13587 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1356.Garg R, Schuman B, Hurwitz S, Metzger C, Bhandari S. Safety and efficacy of saxagliptin for glycemic control in non-critically ill hospitalized patients. BMJ Open Diabetes Res Care 2017;5(1):e000394. 10.1136/bmjdrc-2017-000394 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1357.Pasquel FJ, Gianchandani R, Rubin DJ, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): A multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol 2017;5(2):125–133. 10.1016/s2213-8587(16)30402-8 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1358.Rabizadeh S, Tavakoli Ardakani MA, Mouodi M, et al. Dpp4 inhibitors in the management of hospitalized patients with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. Adv Ther 2020;37(9): 3660–3675. 10.1007/s12325-020-01434-7 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1359.Pasquel FJ, Umpierrez GE. Annals for hospitalists inpatient notes - how we treat hyperglycemia in the hospital. Ann Intern Med 2021;174(8):Ho2–Ho4. 10.7326/m21-2789 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1360.US Food & Drug Administration (FDA). FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious. Accessed February 20, 2022 [EL 4; NE]. [Google Scholar]
- 1361.Marik PE, Preiser JC. Toward understanding tight glycemic control in the icu: A systematic review and metaanalysis. Chest 2010;137(3):544–551. 10.1378/chest.09-1737 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1362.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: Defining the optimal outcomes-based measure of risk. Circulation 2008;117(8):1018–1027. 10.1161/circulationaha.107.740498 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1363.Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clinic proceedings 2010;85(3):217–224. 10.4065/mcp.2009.0394 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1364.Stagnaro-Green A, Barton MK, Linekin PL, Corkery E, deBeer K, Roman SH. Mortality in hospitalized patients with hypoglycemia and severe hyperglycemia. Mt Sinai J Med 1995;62(6):422–426 [EL 2; PCS]. [PubMed] [Google Scholar]
- 1365.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: A study based on continuous monitoring. Diabetes Care 2003;26(5):1485–1489. 10.2337/diacare.26.5.1485 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1366.Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med 2011;124(11): 1028–1035. 10.1016/j.amjmed.2011.07.011 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1367.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 2009;301(15):1556–1564. 10.1001/jama.2009.496 [EL 2; RCCS]. [DOI] [PubMed] [Google Scholar]
- 1368.Gamble JM, Eurich DT, Marrie TJ, Majumdar SR. Admission hypoglycemia and increased mortality in patients hospitalized with pneumonia. The Am J Med 2010;123(6). 10.1016/j.amjmed.2009.11.021, 556.e511–556. [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1369.Lake A, Arthur A, Byrne C, Davenport K, Yamamoto JM, Murphy HR. The effect of hypoglycaemia during hospital admission on health-related outcomes for people with diabetes: A systematic review and meta-analysis. Diabetic Med 2019;36(11):1349–1359. 10.1111/dme.14115 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1370.Mathioudakis NN, Abusamaan MS, Shakarchi AF, et al. Development and validation of a machine learning model to predict near-term risk of iatrogenic hypoglycemia in hospitalized patients. JAMA Netw Open 2021;4(1): e2030913. 10.1001/jamanetworkopen.2020.30913 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1371.Maynard G, Kulasa K, Ramos P, et al. Impact of a hypoglycemia reduction bundle and a systems approach to inpatient glycemic management. Endocr Pract 2015;21(4):355–367. 10.4158/ep14367.Or [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1372.Shelton C, Demidowich AP, Motevalli M, et al. Retrospective quality improvement study of insulin-induced hypoglycemia and implementation of hospital-wide initiatives. J Diabetes Sci Technol 2021;15(4):733–740. 10.1177/19322968211008513 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1373.Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med 2012;7(3):239–242. 10.1002/jhm.977 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1374.Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J 2014;7(3):248–250. 10.1093/ckj/sfu026 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1375.Galindo RJ, Ali MK, Funni SA, et al. Hypoglycemic and hyperglycemic crises among u.S. Adults with diabetes and end-stage kidney disease: Population-based study, 2013–2017. Diabetes Care 2022;45(1):100–107. 10.2337/dc21-1579 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1376.Pierce DA, Russell G, Pirkle JL Jr. Incidence of hypoglycemia in patients with low egfr treated with insulin and dextrose for hyperkalemia. Ann Pharmacother 2015;49(12):1322–1326. 10.1177/1060028015607559 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1377.Farina N, Anderson C. Impact of dextrose dose on hypoglycemia development following treatment of hyperkalemia. Ther Adv Drug Saf 2018;9(6): 323–329. 10.1177/2042098618768725 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1378.Greci LS, Kailasam M, Malkani S, et al. Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care 2003;26(4): 1064–1068. 10.2337/diacare.26.4.1064 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1379.Ali Abdelhamid Y, Kar P, Finnis ME, et al. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: A systematic review and meta-analysis. Crit Care 2016;20(1):301. 10.1186/s13054-016-1471-6 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1380.Driver BE, Klein LR, Cole JB, Prekker ME, Fagerstrom ET, Miner JR. Comparison of two glycemic discharge goals in ED patients with hyperglycemia, a randomized trial. Am J Emerg Med 2019;37(7):1295–1300. 10.1016/j.ajem.2018.09.053 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1381.Pasquel FJ, Urrutia MA, Cardona S, et al. Liraglutide hospital discharge trial: A randomized controlled trial comparing the safety and efficacy of liraglutide versus insulin glargine for the management of patients with type 2 diabetes after hospital discharge. Diabetes Obes Metab 2021;23(6):1351–1360. 10.1111/dom.14347 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1382.A study to test the effect of empagliflozin in patients who are in hospital for acute heart failure Clinicaltrials.Gov identifier: NCT04157751. Available at: https://clinicaltrials.gov/ct2/show/NCT04157751. Accessed February 20, 2022.
- 1383.Dapagliflozin heart failure readmission Clinicaltrials.Gov identifier: NCT04249778. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04249778. Accessed February 20, 2022.
- 1384.Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2021;9(9):586–594. 10.1016/s2213-8587(21)00180-7 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1385.Klonoff DC, Umpierrez GE, Rice MJ. A milestone in point of care capillary blood glucose monitoring of critically ill hospitalized patients. J Diabetes Sci Technol 2018;12(6):1095–1100. 10.1177/1932296818801607 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1386.Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab 2011;96(6):1789–1796. 10.1210/jc.2010-2729 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1387.Cardona S, Gomez PC, Vellanki P, et al. Clinical characteristics and outcomes of symptomatic and asymptomatic hypoglycemia in hospitalized patients with diabetes. BMJ Open Diabetes Res Care 2018;6(1):e000607. 10.1136/bmjdrc-2018-000607 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1388.Criner KE, Kim HN, Ali H, et al. Hypoglycemia symptoms are reduced in hospitalized patients with diabetes. J Diabetes Complications 2021;35(10): 107976. 10.1016/j.jdiacomp.2021.107976 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1389.Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the FreeStyle Libre Pro flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care 2020;43(11):2730–2735. 10.2337/dc19-2073 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1390.Galindo RJ, Umpierrez GE, Rushakoff RJ, et al. Continuous glucose monitors and automated insulin dosing systems in the hospital consensus guideline. J Diabetes Sci Technol 2020;14(6):1035–1064. 10.1177/1932296820954163 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1391.Galindo RJ, Aleppo G, Klonoff DC, et al. Implementation of continuous glucose monitoring in the hospital: Emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol 2020;14(4):822–832. 10.1177/1932296820932903 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1392.Singh LG, Satyarengga M, Marcano I, et al. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: The glucose telemetry system, a randomized clinical trial. Diabetes Care 2020;43(11):2736–2743. 10.2337/dc20-0840 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1393.Spanakis EK, Levitt DL, Siddiqui T, et al. The effect of continuous glucose monitoring in preventing inpatient hypoglycemia in general wards: The glucose telemetry system. J Diabetes Sci Technol 2018;12(1):20–25. 10.1177/1932296817748964 [EL 3; PRECLIN]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1394.Spanakis EK, Singh LG, Siddiqui T, et al. Association of glucose variability at the last day of hospitalization with 30-day readmission in adults with diabetes. BMJ Open Diabetes Res Care 2020;8(1):e000990. 10.1136/bmjdrc-2019-000990 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1395.Dexcom g6 intervention study. Clinicaltrials.Gov identifier: NCT03877068. Available at: https://clinicaltrials.gov/ct2/show/NCT03877068. Accessed February 20, 2022.
- 1396.Davis GM, Spanakis EK, Migdal AL, et al. Accuracy of Dexcom G6 continuous glucose monitoring in non-critically ill hospitalized patients with diabetes. Diabetes Care 2021;44(7):1641–1646. 10.2337/dc20-2856 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1397.Agarwal S, Mathew J, Davis GM, et al. Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care 2021;44(3):847–849. 10.2337/dc20-2219 [EL 3; PRECLIN]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1398.Davis GM, Faulds E, Walker T, et al. Remote continuous glucose monitoring with a computerized insulin infusion protocol for critically ill patients in a COVID-19 medical ICU: Proof of concept. Diabetes Care 2021;44(4): 1055–1058. 10.2337/dc20-2085 [EL 3; PRECLIN]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1399.Perez-Guzman MC, Duggan E, Gibanica S, et al. Continuous glucose monitoring in the operating room and cardiac intensive care unit. Diabetes Care 2021;44(3):e50–e52. 10.2337/dc20-2386 [EL 3; PRECLIN]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1400.Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetese2006: A position statement of the American Diabetes Association. Diabetes Care 2006;29(9):2140–2157. 10.2337/dc06-9914 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1401.McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33(3): 277–316. 10.1177/0148607109335234 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1402.Drincic AT, Knezevich JT, Akkireddy P. Nutrition and hyperglycemia management in the inpatient setting (meals on demand, parenteral, or enteral nutrition). Curr Diab Rep 2017;17(8):59. 10.1007/s11892-017-0882-3 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1403.Vennard KC, Selen DJ, Gilbert MP. The management of hyperglycemia in noncritically ill hospitalized patients treated with continuous enteral or parenteral nutrition. Endocr Pract 2018;24(10):900–906. 10.4158/ep-2018-0150 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1404.Laesser CI, Cumming P, Reber E, Stanga Z, Muka T, Bally L. Management of glucose control in noncritically ill, hospitalized patients receiving parenteral and/or enteral nutrition: A systematic review. J Clin Med 2019;8(7):935. 10.3390/jcm8070935 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1405.Korytkowski MT, Salata RJ, Koerbel GL, et al. Insulin therapy and glycemic control in hospitalized patients with diabetes during enteral nutrition therapy: A randomized controlled clinical trial. Diabetes Care 2009;32(4): 594–596. 10.2337/dc08-1436 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1406.Warren J, Bhalla V, Cresci G. Postoperative diet advancement: Surgical dogma vs evidence-based medicine. Nutr Clin Pract 2011;26(2):115–125. 10.1177/0884533611400231 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1407.Mesejo A, Montejo-González JC, Vaquerizo-Alonso C, et al. Diabetes-specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: A prospective, open-label, blind-randomized, multicenter study. Crit Care 2015;19:390. 10.1186/s13054-015-1108-1 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1408.Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the us, 2011–2019. JAMA 2021;326(7): 660–669. 10.1001/jama.2021.7217 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1409.Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med 1983;308(5):242–245. 10.1056/nejm198302033080502 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1410.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: A study of discordant sibships. Diabetes 2000;49(12):2208–2211. 10.2337/diabetes.49.12.2208 [EL 2; CCS]. [DOI] [PubMed] [Google Scholar]
- 1411.Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: Incidence, risk factors and pathogenesis. Diabetes Metab Rev 1990;6(1):1–27. 10.1002/dmr.5610060101 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1412.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes 1988;37(5):622–628. 10.2337/diab.37.5.622 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1413.Jovanovic L, Savas H, Mehta M, Trujillo A, Pettitt DJ. Frequent monitoring of A1c during pregnancy as a treatment tool to guide therapy. Diabetes Care 2011;34(1):53–54. 10.2337/dc10-1455 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1414.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA 2005;294(21):2751–2757. 10.1001/jama.294.21.2751 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1415.Buchanan TA, Xiang AH. Gestational diabetes mellitus. The Journal of clinical investigation 2005;115(3):485–491. 10.1172/jci24531 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1416.Chodick G, Elchalal U, Sella T, et al. The risk of overt diabetes mellitus among women with gestational diabetes: A population-based study. Diabetic Med 2010;27(7):779–785. 10.1111/j.1464-5491.2010.02995.x [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1417.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009;373(9677):1773–1779. 10.1016/s0140-6736(09)60731-5 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1418.Classification of diabetes mellitus Geneva: World Health Organization; 2019. [EL 4; NE]. [Google Scholar]
- 1419.Pettitt DJ, Jovanovic L. Low birth weight as a risk factor for gestational diabetes, diabetes, and impaired glucose tolerance during pregnancy. Diabetes Care 2007;30(Suppl 2):S147–S149. 10.2337/dc07-s207 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1420.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32(6): 1076–1080. 10.2337/dc08-2077 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1421.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991;303(6809):1019–1022. 10.1136/bmj.303.6809.1019 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1422.American Diabetes Association. Medical management of pregnancy complicated by diabetes fourth edition Jovanovic L, ed. Alexandria, VA: American Diabetes Association, 2009. [Google Scholar]
- 1423.Russell MA, Phipps MG, Olson CL, Welch HG, Carpenter MW. Rates ofpostpartum glucose testing after gestational diabetes mellitus. Obstet Gynecol 2006;108(6): 1456–1462. 10.1097/01.Aog.0000245446.85868.73 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1424.Vandorsten JP, Dodson WC, Espeland MA, et al. Nih consensus development conference: Diagnosing gestational diabetes mellitus. NIH consensus and state-of-the-science statements 2013;29(1):1–31 [EL 4; NE]. [PubMed] [Google Scholar]
- 1425.Durnwald CP, Mele L, Spong CY, et al. Glycemic characteristics and neonatal outcomes of women treated for mild gestational diabetes. Obstet Gynecol 2011;117(4):819–827. 10.1097/AOG.0b013e31820fc6cf [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1426.Weisz B, Shrim A, Homko CJ, Schiff E, Epstein GS, Sivan E. One hour versus two hours postprandial glucose measurement in gestational diabetes: A prospective study. J Perinatol 2005;25(4):241–244. 10.1038/sj.jp.7211243 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1427.Herrera KM, Rosenn BM, Foroutan J, et al. Randomized controlled trial of insulin detemir versus nph for the treatment of pregnant women with diabetes. Am J Obstet Gynecol 2015;213(3). 10.1016/j.ajog.2015.06.010, 426.e421–427. [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1428.Koren R, Toledano Y, Hod M. The use of insulin detemir during pregnancy: A safety evaluation. Expert Opin Drug Safety 2015;14(4):593–599. 10.1517/14740338.2015.1013533 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1429.Lv S, Wang J, Xu Y. Safety of insulin analogs during pregnancy: A meta-analysis. Arch Gynecol Obstet 2015;292(4):749–756. 10.1007/s00404-015-3692-3 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1430.Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Trends in glyburide compared with insulin use for gestational diabetes treatment in the united states, 2000–2011. Obstet Gynecol 2014;123(6): 1177–1184. 10.1097/aog.0000000000000285 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1431.Eyal S, Easterling TR, Carr D, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos 2010;38(5):833–840. 10.1124/dmd.109.031245 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1432.Farrar D, Simmonds M, Bryant M, et al. Treatments for gestational diabetes: A systematic review and meta-analysis. BMJ Open 2017;7(6):e015557. 10.1136/bmjopen-2016-015557 [EL 2; NMA]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1433.Poolsup N, Suksomboon N, Amin M. Efficacy and safety of oral antidiabetic drugs in comparison to insulin in treating gestational diabetes mellitus: A meta-analysis. PloS One 2014;9(10):e109985. 10.1371/journal.pone.0109985 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1434.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 2008;358(19): 2003–2015. 10.1056/NEJMoa0707193 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1435.Spaulonci CP, Bernardes LS, Trindade TC, Zugaib M, Francisco RP. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol 2013;209(1):34.e31–34.e37. 10.1016/j.ajog.2013.03.022 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1436.Anderson JH Jr, Brunelle RL, Koivisto VA, et al. Reduction of postprandial hyperglycemia and frequency of hypoglycemia in IDDM patients on insulin-analog treatment. Multicenter Insulin Lispro Study Group. Diabetes 1997;46(2):265–270. 10.2337/diab.46.2.265 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1437.Fishel Bartal M, Ward C, Refuerzo JS, et al. Basal insulin analogs versus Neutral Protamine Hagedorn for type 2 diabetics. Am J Perinatol 2020;37(1): 30–36. 10.1055/s-0039-1694733 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1438.O'Neill SM, Kenny LC, Khashan AS, West HM, Smyth RM, Kearney PM. Different insulin types and regimens for pregnant women with pre-existing diabetes. Cochrane Database Syst Rev 2017;2(2):Cd011880. 10.1002/14651858.CD011880.pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1439.Cypryk K, Sobczak M, Pertynška-Marczewska M, et al. Pregnancy complications and perinatal outcome in diabetic women treated with humalog (insulin lispro) or regular human insulin during pregnancy. Med Sci Monit 2004;10(2):Pi29–Pi32 [EL 2; NRCT]. [PubMed] [Google Scholar]
- 1440.Lapolla A, Dalfrá MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: An italian experience. Acta Diabetol 2008;45(1):61–66. 10.1007/s00592-008-0024-0 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1441.Masson EA, Patmore JE, Brash PD, et al. Pregnancy outcome in type 1 diabetes mellitus treated with insulin lispro (humalog) ? 2003;20(1):46–50. 10.1046/j.1464-5491.2003.00840.x [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1442.Mathiesen ER, Kinsley B, Amiel SA, et al. Maternal glycemic control and hypoglycemia in type 1 diabetic pregnancy: A randomized trial of insulin aspart versus human insulin in 322 pregnant women. Diabetes Care 2007;30(4):771–776. 10.2337/dc06-1887 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1443.Sciacca L, Marotta V, Insalaco F, et al. Use of insulin detemir during pregnancy. Nutr Metab Cardiovasc Dis 2010;20(4):e15–e16. 10.1016/j.numecd.2009.12.010 [EL 3; SCR]. [DOI] [PubMed] [Google Scholar]
- 1444.Lapolla A, Di Cianni G, Bruttomesso D, et al. Use of insulin detemir in pregnancy: A report on 10 type 1 diabetic women. Diabetic Med 2009;26(11):1181–1182. 10.1111/j.1464-5491.2009.02852.x [EL 3; RCSS]. [DOI] [PubMed] [Google Scholar]
- 1445.Mathiesen ER, Damm P, Jovanovic L, et al. Basal insulin analogues in diabetic pregnancy: A literature review and baseline results of a randomised, controlled trial in type 1 diabetes. Diabetes Metab Res Rev 2011;27(6): 543–551. 10.1002/dmrr.1213 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1446.Mathiesen ER, Hod M, Ivanisevic M, et al. Maternal efficacy and safety outcomes in a randomized, controlled trial comparing insulin detemir with NPH insulin in 310 pregnant women with type 1 diabetes. Diabetes Care 2012;35(10):2012–2017. 10.2337/dc11-2264 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1447.Fornes R, Simin J, Nguyen MH, et al. Pregnancy, perinatal and childhood outcomes in women with and without polycystic ovary syndrome and metformin during pregnancy: A nationwide population-based study. Reprod Biol Endocrinol 2022;20(1):30. 10.1186/s12958-022-00905-6 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1448.Newman C, Dunne FP. Metformin for pregnancy and beyond: The pros and cons. Diabetic Med 2022;39(3):e14700. 10.1111/dme.14700 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1449.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: A meta-analysis of the effect on glycemic control. Diabetes Care 2002;25(7):1159–1171. 10.2337/diacare.25.7.1159 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1450.Marincic PZ, Salazar MV, Hardin A, et al. Diabetes self-management education and medical nutrition therapy: A multisite study documenting the efficacy of registered dietitian nutritionist interventions in the management of glycemic control and diabetic dyslipidemia through retrospective chart review. J Acad Nutr Dietetics 2019;119(3):449–463. 10.1016/j.jand.2018.06.303 [EL 3; CCS]. [DOI] [PubMed] [Google Scholar]
- 1451.Cooke D, Bond R, Lawton J, et al. Structured type 1 diabetes education delivered within routine care: Impact on glycemic control and diabetes-specific quality of life. Diabetes Care 2013;36(2):270–272. 10.2337/dc12-0080 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1452.Cochran J, Conn VS. Meta-analysis of quality of life outcomes following diabetes self-management training. Diabetes Educ 2008;34(5):815–823. 10.1177/0145721708323640 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1453.He X, Li J, Wang B, et al. Diabetes self-management education reduces risk of all-cause mortality in type 2 diabetes patients: A systematic review and meta-analysis. Endocrine 2017;55(3):712–731. 10.1007/s12020-016-1168-2 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1454.Robbins JM, Thatcher GE, Webb DA, Valdmanis VG. Nutritionist visits, diabetes classes, and hospitalization rates and charges: The urban diabetes study. Diabetes Care 2008;31(4):655–660. 10.2337/dc07-1871 [EL 3; ECON]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1455.Strawbridge LM, Lloyd JT, Meadow A, Riley GF, Howell BL. One-year outcomes of diabetes self-management training among medicare beneficiaries newly diagnosed with diabetes. Medical care 2017;55(4):391–397. 10.1097/mlr.0000000000000653 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1456.Schechter CB, Walker EA, Ortega FM, Chamany S, Silver LD. Costs and effects of a telephonic diabetes self-management support intervention using health educators. J Diabetes Complications 2016;30(2):300–305. 10.1016/j.jdiacomp.2015.11.017 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1457.Teljeur C, Moran PS, Walshe S, et al. Economic evaluation of chronic disease self-management for people with diabetes: A systematic review. Diabetic Med 2017;34(8):1040–1049. 10.1111/dme.13281 [EL 2; MNRCT; EL 3; ECON]. [DOI] [PubMed] [Google Scholar]
- 1458.Musuuza J, Sutherland BL, Kurter S, Balasubramanian P, Bartels CM, Brennan MB. A systematic review of multidisciplinary teams to reduce major amputations for patients with diabetic foot ulcers. J Vasc Surg 2020;71(4): 1433–1446.e1433. 10.1016/j.jvs.2019.08.244 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1459.Siaw MYL, Lee JY. Multidisciplinary collaborative care in the management of patients with uncontrolled diabetes: A systematic review and meta-analysis. Int J Clin Pract 2019;73(2):e13288. 10.1111/ijcp.13288 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1460.Fazel MT, Bagalagel A, Lee JK, Martin JR, Slack MK. Impact of diabetes care by pharmacists as part of health care team in ambulatory settings: A systematic review and meta-analysis. Ann Pharmacother 2017;51(10):890–907. 10.1177/1060028017711454 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1461.McGill M, Blonde L, Chan JCN, Khunti K, Lavalle FJ, Bailey CJ. The interdisciplinary team in type 2 diabetes management: Challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol 2017;7:21–27. 10.1016/j.jcte.2016.12.001 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1462.Miller-Rosales C, Rodriguez HP. Interdisciplinary primary care team expertise and diabetes care management. J Am Board Fam Med 2021;34(1): 151–161. 10.3122/jabfm.2021.01.200187 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1463.Diabetes Canada Clinical Practice Guidelines Expert Committee, Clement M, Filteau P, et al. Organization of diabetes care. Can J Diabetes 2018;42(Suppl 1):S27–S35. 10.1016/j.jcjd.2017.10.005 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1464.National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes overview Available at: https://www.niddk.nih.gov/health-information/diabetes/overview. Accessed January 14, 2022.
- 1465.Cunningham AT, Crittendon DR, White N, Mills GD, Diaz V, LaNoue MD. The effect of diabetes self-management education on hba1c and quality of life in african-americans: A systematic review and meta-analysis. BMC Health Services Res 2018;18(1):367. 10.1186/s12913-018-3186-7 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1466.Sherifali D, Bai JW, Kenny M, Warren R, Ali MU. Diabetes self-management programmes in older adults: A systematic review and meta-analysis. Diabetic Med 2015;32(11):1404–1414. 10.1111/dme.12780 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1467.Lepard MG, Joseph AL, Agne AA, Cherrington AL. Diabetes self-management interventions for adults with type 2 diabetes living in rural areas: A systematic literature review. Curr Diab Rep 2015;15(6):608. 10.1007/s11892-015-0608-3 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1468.Paz-Pacheco E, Sandoval MA, Ardena GJ, et al. Effectiveness of a community-based diabetes self-management education (dsme) program in a rural agricultural setting. Prim Health Care Res Dev 2017;18(1):35–49. 10.1017/s1463423616000335 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1469.Heitkemper EM, Mamykina L, Travers J, Smaldone A. Do health information technology self-management interventions improve glycemic control in medically underserved adults with diabetes? A systematic review and meta-analysis. J Am Med Inform Assoc 2017;24(5):1024–1035. 10.1093/jamia/ocx025 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1470.Odgers-Jewell K, Ball LE, Kelly JT, Isenring EA, Reidlinger DP, Thomas R. Effectiveness of group-based self-management education for individuals with type 2 diabetes: A systematic review with meta-analyses and meta-regression. Diabetic Med 2017;34(8):1027–1039. 10.1111/dme.13340 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1471.Gaillard T, Amponsah G, Osei K. Patient-centered community diabetes education program improves glycemic control in African-American patients with poorly controlled type 2 diabetes: Importance of point of care metabolic measurements. J Natl Black Nurses Assoc 2015;26(1):50–57 [EL 1; RCT]. [PubMed] [Google Scholar]
- 1472.Navodia N, Wahoush O, Tang T, Yost J, Ibrahim S, Sherifali D. Culturally tailored self-management interventions for South Asians with type 2 diabetes: A systematic review. Can J Diabetes 2019;43(6):445–452. 10.1016/j.jcjd.2019.04.010 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1473.Creamer J, Attridge M, Ramsden M, Cannings-John R, Hawthorne K. Culturally appropriate health education for type 2 diabetes in ethnic minority groups: An updated cochrane review of randomized controlled trials. Diabetic Med 2016;33(2):169–183. 10.1111/dme.12865 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1474.Glazier RH, Bajcar J, Kennie NR, Willson K. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care 2006;29(7):1675–1688. 10.2337/dc05-1942 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1475.Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: Randomized clinical trial. Arch Intern Med 2011;171(5):453–459. 10.1001/archinternmed.2011.70 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1476.do Rosário Pinto M, Parreira P, Basto ML, Dos Santos Mendes Mónico L. Impact of a structured multicomponent educational intervention program on metabolic control of patients with type 2 diabetes. BMC Endocrine Disord 2017;17(1):77. 10.1186/s12902-017-0222-2 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1477.Egede LE, Williams JS, Voronca DC, Gebregziabher M, Lynch CP. Telephone-delivered behavioral skills intervention for African American adults with type 2 diabetes: A randomized controlled trial. J General Intern Med 2017;32(7):775–782. 10.1007/s11606-017-4023-0 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1478.Rush KL, Hatt L, Janke R, Burton L, Ferrier M, Tetrault M. The efficacy of telehealth delivered educational approaches for patients with chronic diseases: A systematic review. Patient Educ Couns 2018;101(8):1310–1321. 10.1016/j.pec.2018.02.006 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1479.Bailey RA, Pfeifer M, Shillington AC, et al. Effect of a patient decision aid (pda) for type 2 diabetes on knowledge, decisional self-efficacy, and decisional conflict. BMC Health Services Res 2016;16:10. 10.1186/s12913-016-1262-4 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1480.Booth AO, Lowis C, Hunter SJ, Dean M, Cardwell CR, McKinley MC. Development and evaluation of a computer-based, self-management tool for people recently diagnosed with type 2 diabetes. J Diabetes Res 2016;2016: 3192673. 10.1155/2016/3192673 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1481.Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: A 2-year non-randomized clinical trial. Front Endocrinol 2019;10:348. 10.3389/fendo.2019.00348 [EL 2; NRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1482.Sepah SC, Jiang L, Peters AL. Long-term outcomes of a web-based diabetes prevention program: 2-year results of a single-arm longitudinal study. J Med Internet Res 2015;17(4):e92. 10.2196/jmir.4052 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1483.Greenwood DA, Gee PM, Fatkin KJ, Peeples M. A systematic review of reviews evaluating technology-enabled diabetes self-management education and support. J Diabetes Sci Technol 2017;11(5):1015–1027. 10.1177/1932296817713506 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1484.Benson GA, Sidebottom A, Hayes J, et al. Impact of ENHANCED (diEtitiaNs Helping pAtieNts CarE for Diabetes) Telemedicine Randomized Controlled Trial on Diabetes Optimal Care Outcomes in Patients with Type 2 Diabetes. J Acad Nutr Dietetics 2019;119(4):585–598. 10.1016/j.jand.2018.11.013 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1485.Diehl LA, Souza RM, Gordan PA, Esteves RZ, Coelho IC. Insuonline, an electronic game for medical education on insulin therapy: A randomized controlled trial with primary care physicians. J Med Internet Res 2017;19(3): e72. 10.2196/jmir.6944 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1486.Dong Y, Wang P, Dai Z, et al. Increased self-care activities and glycemic control rate in relation to health education via wechat among diabetes patients: A randomized clinical trial. Medicine 2018;97(50):e13632. 10.1097/md.0000000000013632 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1487.Yang Q, Fang P. Impact of the conversation map tools in patients with type 2 diabetes mellitus: A PRISMA-compliant meta-analysis of randomized controlled trials. Medicine 2016;95(40):e4664. 10.1097/md.0000000000004664 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1488.Azami G, Soh KL, Sazlina SG, et al. Effect of a nurse-led diabetes self-management education program on glycosylated hemoglobin among adults with type 2 diabetes. J Diabetes Res 2018;2018:4930157. 10.1155/2018/4930157 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1489.Odnoletkova I, Goderis G, Nobels F, et al. Optimizing diabetes control in people with type 2 diabetes through nurse-led telecoaching. Diabetic Med 2016;33(6):777–785. 10.1111/dme.13092 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1490.Cani CG, Lopes Lda S, Queiroz M, Nery M. Improvement in medication adherence and self-management of diabetes with a clinical pharmacy program: A randomized controlled trial in patients with type 2 diabetes undergoing insulin therapy at a teaching hospital. Clinics 2015;70(2): 102–106. 10.6061/clinics/2015(02)06 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1491.Aguiar PM, da Silva CHP, Chiann C, Dórea EL, Lyra DP Jr, Storpirtis S. Pharmacist-physician collaborative care model for patients with uncontrolled type 2 diabetes in Brazil: Results from a randomized controlled trial. J Eval Clin Pract 2018;24(1):22–30. 10.1111/jep.12606 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1492.Gatlin TK, Serafica R, Johnson M. Systematic review of peer education intervention programmes among individuals with type 2 diabetes. J Clin Nursing 2017;26(23–24):4212–4222. 10.1111/jocn.13991 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1493.Aponte J, Jackson TD, Wyka K, Ikechi C. Healtheffectiveness of community health workers as a diabetes self-management intervention. Diabetes Vasc Dis Res 2017;14(4):316–326. 10.1177/1479164117696229 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1494.Piatt GA, Rodgers EA, Xue L, Zgibor JC. Integration and utilization of peer leaders for diabetes self-management support: Results from project SEED (Support, Education, and Evaluation in Diabetes). Diabetes Educ 2018;44(4): 373–382. 10.1177/0145721718777855 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1495.Rodriguez HP, Friedberg MW, Vargas-Bustamante A, Chen X, Martinez AE, Roby DH. The impact of integrating medical assistants and community health workers on diabetes care management in community health centers. BMC Health Services Res 2018;18(1):875. 10.1186/s12913-018-3710-9 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1496.Certification Board for Diabetes Care and Education. Thinking about earning the CDCES? Available at: https://www.cbdce.org/. Accessed July 22, 2021.
- 1497.Campbell F, Lawton J, Rankin D, et al. Follow-up support for effective type 1 diabetes self-management (the FUSED model): A systematic review and meta-ethnography of the barriers, facilitators and recommendations for sustaining self-management skills after attending a structured education programme. BMC Health Services Res 2018;18(1):898. 10.1186/s12913-018-3655-z [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1498.Carcone AI, Ellis DA, Chen X, Naar S, Cunningham PB, Moltz K. Multisystemic therapy improves the patient-provider relationship in families of adolescents with poorly controlled insulin dependent diabetes. J Clin Psychol Med Settings 2015;22(2–3):169–178. 10.1007/s10880-015-9422-y [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1499.Robinson EM, Weaver P, Chen R, Streisand R, Holmes CS. A model of parental distress and factors that mediate its link with parental monitoring of youth diabetes care, adherence, and glycemic control. Health Psychol 2016;35(12): 1373–1382. 10.1037/hea0000406 [EL 2; PHAS/CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1500.AlHaidar AM, AlShehri NA, AlHussaini MA. Family support and its association with glycemic control in adolescents with type 1 diabetes mellitus in Riyadh, Saudi Arabia. J Diabetes Res 2020;2020:5151604. 10.1155/2020/5151604 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1501.Cameron FJ, Garvey K, Hood KK, Acerini CL, Codner E. ISPAD clinical practice consensus guidelines 2018: Diabetes in adolescence. Pediatr diabetes 2018;19(Suppl 27):250–261. 10.1111/pedi.12702 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1502.American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 14. Children and adolescents: Standards of medical care in diabetes-2022. Diabetes Care 2022;45(Supplement_1):S208–S231. 10.2337/dc22-S014 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1503.Kriska A, El Ghormli L, Copeland KC, et al. Impact of lifestyle behavior change on glycemic control in youth with type 2 diabetes. Pediatr Diabetes 2018;19(1):36–44. 10.1111/pedi.12526 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1504.Marson EC, Delevatti RS, Prado AK, Netto N, Kruel LF. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: A systematic review and meta-analysis. Prev Med 2016;93:211–218. 10.1016/j.ypmed.2016.10.020 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1505.Delamater AM, de Wit M, McDarby V, et al. ISPAD clinical practice consensus guidelines 2018: Psychological care of children and adolescents with type 1 diabetes. Pediatr diabetes 2018;19(Suppl 27):237–249. 10.1111/pedi.12736 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1506.Weissberg-Benchell J, Shapiro JB, Bryant FB, Hood KK. Supporting teen problem-solving (STEPS) 3 year outcomes: Preventing diabetes-specific emotional distress and depressive symptoms in adolescents with type 1 diabetes. J Consult Clin Psychol 2020;88(11):1019–1031. 10.1037/ccp0000608 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1507.Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroendocrinology 2016;70:70–84. 10.1016/j.psyneuen.2016.04.019 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1508.Martinez K, Frazer SF, Dempster M, Hamill A, Fleming H, McCorry NK. Psychological factors associated with diabetes self-management among adolescents with type 1 diabetes: A systematic review. J Health Psychol 2018;23(13): 1749–1765. 10.1177/1359105316669580 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1509.Yackobovitch-Gavan M, Wolf Linhard D, Nagelberg N, et al. Intervention for childhood obesity based on parents only or parents and child compared with follow-up alone. Pediatr Obes 2018;13(11):647–655. 10.1111/ijpo.12263 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1510.Horning ML, Schow R, Friend SE, Loth K, Neumark-Sztainer D, Fulkerson JA. Family dinner frequency interacts with dinnertime context in associations with child and parent BMI outcomes. J Fam Psychol 2017;31(7):945–951. 10.1037/fam0000330 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1511.Potter C, Ferriday D, Griggs RL, Hamilton-Shield JP, Rogers PJ, Brunstrom JM. Parental beliefs about portion size, not children's own beliefs, predict child BMI. Pediatr Obes 2018;13(4):232–238. 10.1111/ijpo.12218 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1512.French SA, Sherwood NE, Veblen-Mortenson S, et al. Multicomponent obesity prevention intervention in low-income preschoolers: Primary and subgroup analyses of the net-works randomized clinical trial, 2012–2017. Am J Public Health 2018;108(12):1695–1706. 10.2105/ajph.2018.304696 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1513.Soltero EG, Olson ML, Williams AN, et al. Effects of a community-based diabetes prevention program for latino youth with obesity: A randomized controlled trial. Obesity 2018;26(12):1856–1865. 10.1002/oby.22300 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1514.Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: A systematic review and bias-adjusted meta-analysis. Obes Rev 2015;16(2):137–149. 10.1111/obr.12245 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1515.Kelley GA, Kelley KS, Pate RR. Exercise and bmi in overweight and obese children and adolescents: A systematic review and trial sequential meta-analysis. BioMed Res Int 2015;2015:704539. 10.1155/2015/704539 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1516.Miguel-Berges ML, Reilly JJ, Moreno Aznar LA, Jiménez-Pavón D. Associations between pedometer-determined physical activity and adiposity in children and adolescents: Systematic review. Clin J Sport Med 2018;28(1): 64–75. 10.1097/jsm.0000000000000419 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1517.Viggiano E, Viggiano A, Di Costanzo A, et al. Healthy lifestyle promotion in primary schools through the board game kaledo: A pilot cluster randomized trial. Eur J Pediatr 2018;177(9):1371–1375. 10.1007/s00431-018-3091-4 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1518.Staiano AE, Beyl RA, Guan W, Hendrick CA, Hsia DS, Newton RL Jr. Home-based exergaming among children with overweight and obesity: A randomized clinical trial. Pediatr Obes 2018;13(11):724–733. 10.1111/ijpo.12438 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1519.Trost SG, Sundal D, Foster GD, Lent MR, Vojta D. Effects of a pediatric weight management program with and without active video games a randomized trial. JAMA Pediatr 2014;168(5):407–413. 10.1001/jamapediatrics.2013.3436 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1520.Staiano AE, Marker AM, Beyl RA, Hsia DS, Katzmarzyk PT, Newton RL. A randomized controlled trial of dance exergaming for exercise training in overweight and obese adolescent girls. Pediatr Obes 2017;12(2):120–128. 10.1111/ijpo.12117 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1521.Sato PM, Steeves EA, Carnell S, et al. A youth mentor-led nutritional intervention in urban recreation centers: A promising strategy for childhood obesity prevention in low-income neighborhoods. Health Educ Res 2016;31(2):195–206. 10.1093/her/cyw011 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1522.Arlinghaus KR, Moreno JP, Reesor L, Hernandez DC, Johnston CA. Compañeros: High school students mentor middle school students to address obesity among Hispanic adolescents. Prev Chronic Dis 2017;14:E92. 10.5888/pcd14.170130 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1523.Llauradó E, Tarro L, Moriña D, Aceves-Martins M, Giralt M, SoláR. Follow-up of a healthy lifestyle education program (the edal study): Four years after cessation of randomized controlled trial intervention. BMC Public Health 2018;18(1):104. 10.1186/s12889-017-5006-0 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1524.Whooten RC, Perkins ME, Gerber MW, Taveras EM. Effects of before-school physical activity on obesity prevention and wellness. Am J Prevent Med 2018;54(4):510–518. 10.1016/j.amepre.2018.01.017 [EL 2; NRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1525.Adab P, Pallan MJ, Lancashire ER, et al. Effectiveness of a childhood obesity prevention programme delivered through schools, targeting 6 and 7 year olds: Cluster randomised controlled trial (WAVES study). BMJ 2018;360: k211. 10.1136/bmj.k211 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1526.Heo M, Jimenez CC, Lim J, et al. Effective nationwide school-based participatory extramural program on adolescent body mass index, health knowledge and behaviors. BMC Pediatr 2018;18(1):7. 10.1186/s12887-017-0975-9 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1527.Hung LS, Tidwell DK, Hall ME, Lee ML, Briley CA, Hunt BP. A meta-analysis of school-based obesity prevention programs demonstrates limited efficacy of decreasing childhood obesity. Nutr Res 2015;35(3):229–240. 10.1016/j.nutres.2015.01.002 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1528.Oosterhoff M, Joore M, Ferreira I. The effects of school-based lifestyle interventions on body mass index and blood pressure: A multivariate multilevel meta-analysis of randomized controlled trials. Obes Rev 2016;17(11): 1131–1153. 10.1111/obr.12446 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1529.Pablos A, Nebot V, Vañó-Vicent V, Ceca D, Elvira L. Effectiveness of a school-based program focusing on diet and health habits taught through physical exercise. Appl Physiol Nutr Metab 2018;43(4):331–337. 10.1139/apnm-2017-0348 [EL 2; NRCT]. [DOI] [PubMed] [Google Scholar]
- 1530.Price C, Cohen D, Pribis P, Cerami J. Nutrition education and body mass index in grades k-12: A systematic review. J Sch Health 2017;87(9):715–720. 10.1111/josh.12544 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1531.Scherr RE, Linnell JD, Dharmar M, et al. A multicomponent, school-based intervention, the shaping healthy choices program, improves nutrition-related outcomes. J Nutr Educ Behav 2017;49(5):368–379.e361. 10.1016/j.jneb.2016.12.007 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1532.Verjans-Janssen SRB, van de Kolk I, Van Kann DHH, Kremers SPJ, Gerards S. Effectiveness of school-based physical activity and nutrition interventions with direct parental involvement on children's BMI and energy balance-related behaviors - a systematic review. PloS One 2018;13(9):e0204560. 10.1371/journal.pone.0204560 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1533.Weihrauch-Blüher S, Kromeyer-Hauschild K, Graf C, et al. Current guidelines for obesity prevention in childhood and adolescence. Obes Facts 2018;11(3): 263–276. 10.1159/000486512 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1534.Agarwal S, Raymond JK, Isom S, et al. Transfer from paediatric to adult care for young adults with type 2 diabetes: The search for diabetes in youth study. Diabetic Med 2018;35(4):504–512. 10.1111/dme.13589 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1535.Shulman R, Shah BR, Fu L, Chafe R, Guttmann A. Diabetes transition care and adverse events: A population-based cohort study in Ontario, Canada. Diabetic Med 2018;35(11):1515–1522. 10.1111/dme.13782 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1536.Campbell F, Biggs K, Aldiss SK, et al. Transition of care for adolescents from paediatric services to adult health services. Cochrane Database Syst Rev 2016;4:Cd009794. 10.1002/14651858.CD009794. pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1537.Chu PY, Maslow GR, von Isenburg M, Chung RJ. Systematic review of the impact of transition interventions for adolescents with chronic illness on transfer from pediatric to adult healthcare. J Pediatr Nurs 2015;30(5): e19–e27. 10.1016/j.pedn.2015.05.022 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1538.Wong CA, Miller VA, Murphy K, et al. Effect of financial incentives on glucose monitoring adherence and glycemic control among adolescents and young adults with type 1 diabetes: A randomized clinical trial. JAMA Pediatr 2017;171(12):1176–1183. 10.1001/jamapediatrics.2017.3233 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1539.Floyd BD, Block JM, Buckingham BB, et al. Stabilization of glycemic control and improved quality of life using a shared medical appointment model in adolescents with type 1 diabetes in suboptimal control. Pediatr diabetes 2017;18(3):204–212. 10.1111/pedi.12373 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1540.Schultz AT, Smaldone A. Components of interventions that improve transitions to adult care for adolescents with type 1 diabetes. J Adolesc Health 2017;60(2):133–146. 10.1016/j.jadohealth.2016.10.002 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1541.Spaic T, Robinson T, Goldbloom E, et al. Closing the gap: Results of the multicenter Canadian randomized controlled trial of structured transition in young adults with type 1 diabetes. Diabetes Care 2019;42(6):1018–1026. 10.2337/dc18-2187 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1542.Wahabi HA, Fayed A, Esmaeil S, et al. Systematic review and meta-analysis of the effectiveness of pre-pregnancy care for women with diabetes for improving maternal and perinatal outcomes. PloS one 2020;15(8): e0237571. 10.1371/journal.pone.0237571 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1543.Callec R, Perdriolle-Galet E, Sery GA, Morel O. Type 2 diabetes in pregnancy: Rates of fetal malformations and level of preconception care. J Obstet Gynaecol 2014;34(7):648–649. 10.3109/01443615.2014.925856 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1544.Goldwire Tutt T Healthcare policy: Federally mandated insurance coverage for infertility treatment Available at: https://journals.library.columbia.edu/index.php/cswr/article/view/7587. Accessed January 10, 2022. [Google Scholar]
- 1545.Raheem OA, Hehemann MC, Rogers MJ, Fustok JN, Hirsch IB, Walsh TJ. Does type 1 diabetes affect male infertility: Type 1 diabetes exchange registry-based analysis. Soc Int Urol J 2021;2(3):139–143 [EL 2; ES]. [Google Scholar]
- 1546.Condorelli RA, La Vignera S, Mongioì LM, Alamo A, Calogero AE. Diabetes mellitus and infertility: Different pathophysiological effects in type 1 and type 2 on sperm function. Front Endocrinol 2018;9:268. 10.3389/fendo.2018.00268 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1547.Maresch CC, Stute DC, Alves MG, Oliveira PF, de Kretser DM, Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum Reprod Update 2018;24(1):86–105. 10.1093/humupd/dmx033 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1548.Wiebe JC, Santana A, Medina-Rodríguez N, et al. Fertility is reduced in women and in men with type 1 diabetes: Results from the Type 1 Diabetes Genetics Consortium (T1DGC). Diabetologia 2014;57(12):2501–2504. 10.1007/s00125-014-3376-8 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1549.Boeri L, Capogrosso P, Ventimiglia E, et al. Undiagnosed prediabetes is highly prevalent in primary infertile men - results from a cross-sectional study. BJU international 2019;123(6):1070–1077. 10.1111/bju.14558 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1550.Lotti F, Corona G, Degli Innocenti S, et al. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology 2013;1(2):229–239. 10.1111/j.2047-2927.2012.00031.x [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1551.Pergialiotis V, Prodromidou A, Frountzas M, Korou LM, Vlachos GD, Perrea D. Diabetes mellitus and functional sperm characteristics: A meta-analysis of observational studies. Diabetes Complications 2016;30(6):1167–1176. 10.1016/j.jdiacomp.2016.04.002 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1552.La Vignera S, Condorelli RA, Di Mauro M, et al. Reproductive function in male patients with type 1 diabetes mellitus. Andrology 2015;3(6):1082–1087. 10.1111/andr.12097 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1553.Barratt CLR, Björndahl L, De Jonge CJ, et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Human reproduction update 2017;23(6):660–680. 10.1093/humupd/dmx021 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1554.Louters M, Pearlman M, Solsrud E, Pearlman A. Functional hypogonadism among patients with obesity, diabetes, and metabolic syndrome. Int J Impotence Research 2021. 10.1038/s41443-021-00496-7 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1555.Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: A longitudinal study. Diabetes Care 2003;26(4): 1181–1185. 10.2337/diacare.26.4.1181 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1556.Dwyer AA, Chavan NR, Lewkowitz-Shpuntoff H, et al. Functional hypogonadotropic hypogonadism in men: Underlying neuroendocrine mechanisms and natural history. J Clin Endocrinol Metab 2019;104(8):3403–3414. 10.1210/jc.2018-02697 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1557.Thong EP, Codner E, Laven JSE, Teede H. Diabetes: A metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol 2020;8(2):134–149. 10.1016/s2213-8587(19)30345-6 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1558.Wang Q, Moley KH. Maternal diabetes and oocyte quality. Mitochondrion 2010;10(5):403–410. 10.1016/j.mito.2010.03.002 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1559.Frank LA, Sutton-McDowall ML, Gilchrist RB, Thompson JG. The effect of peri-conception hyperglycaemia and the involvement of the hexosamine biosynthesis pathway in mediating oocyte and embryo developmental competence. Mol Reprod Dev 2014;81(5):391–408. 10.1002/mrd.22299 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1560.Wellons MF, Matthews JJ, Kim C. Ovarian aging in women with diabetes: An overview. Maturitas 2017;96:109–113. 10.1016/j.maturitas.2016.11.019 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1561.Dorman JS, Steenkiste AR, Foley TP, et al. Menopause in type 1 diabetic women: Is it premature? Diabetes 2001;50(8):1857–1862. 10.2337/diabetes.50.8.1857 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1562.Codner E, Merino PM, Tena-Sempere M. Female reproduction and type 1 diabetes: From mechanisms to clinical findings. Hum Reprod Update 2012;18(5):568–585. 10.1093/humupd/dms024 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1563.Moolhuijsen LME, Visser JA. Anti-müllerian hormone and ovarian reserve: Update on assessing ovarian function. J Clin Endocrinol Metab 2020;105(11): 3361–3373. 10.1210/clinem/dgaa513 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1564.Hansen KR, Eisenberg E, Baker V, et al. Midluteal progesterone: A marker of treatment outcomes in couples with unexplained infertility. J Clin Endocrinol Metab 2018;103(7):2743–2751. 10.1210/jc.2018-00642 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1565.Tso LO, Costello MF, Albuquerque LET, Andriolo RB, Macedo CR. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2020;12(12):Cd006105. 10.1002/14651858.CD006105.pub4 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1566.Corona G, Rastrelli G, Balercia G, et al. Hormonal association and sexual dysfunction in patients with impaired fasting glucose: A cross-sectional and longitudinal study. J Sex Med 2012;9(6):1669–1680. 10.1111/j.1743-6109.2012.02717.x [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1567.Rabijewski M, Papierska L, Piątkiewicz P. The prevalence of prediabetes in population of polish men with late-onset hypogonadism. Aging Male 2014;17(3): 141–146. 10.3109/13685538.2014.936000 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1568.Ho CH, Yu HJ, Wang CY, et al. Prediabetes is associated with an increased risk of testosterone deficiency, independent of obesity and metabolic syndrome. PloS One 2013;8(9):e74173. 10.1371/journal.pone.0074173 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1569.Rastrelli G, Filippi S, Sforza A, Maggi M, Corona G. Metabolic syndrome in male hypogonadism. Front Horm Res 2018;49:131–155. 10.1159/000485999 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1570.Song SH, Sung S, Her YS, et al. Misuse of testosterone replacement therapy in men in infertile couples and its influence on infertility treatment. Clin Exp Reprod Med 2019;46(4):173–177. 10.5653/cerm.2019.00290 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1571.Yassin A, Haider A, Haider KS, et al. Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: Eight-year data from a registry study. Diabetes Care 2019;42(6):1104–1111. 10.2337/dc18-2388 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1572.Traish AM, Haider A, Haider KS, Doros G, Saad F. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: A real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther 2017;22(5):414–433. 10.1177/1074248417691136 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1573.Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: Systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab 2013;27(4):557–579. 10.1016/j.beem.2013.05.002. EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1574.Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: Results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010;7(10):3495–3503. 10.1111/j.1743-6109.2010.01931.x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1575.De Bruijn KM, van Eijck CH. New-onset diabetes after distal pancreatectomy: A systematic review. Ann Surg 2015;261(5):854–861. 10.1097/sla.0000000000000819 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1576.Chang S, Jiang J. Association of body mass index and the risk of new-onset diabetes after kidney transplantation: A meta-analysis. Transplant Proc 2018;50(5):1316–1325. 10.1016/j.transproceed.2018.02.075 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1577.Gaynor JJ, Ciancio G, Guerra G, et al. Multivariable risk of developing new onset diabetes after transplant-results from a single-center study of 481 adult, primary kidney transplant recipients. Clin Transplant 2015;29(4): 301–310. 10.1111/ctr.12510 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1578.Beckmann S, Drent G, Ruppar T, Nikolić N, De Geest S. Body weight parameters are related to morbidity and mortality after liver transplantation: A systematic review and meta-analysis. Transplantation 2019;103(11): 2287–2303. 10.1097/tp.0000000000002811 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1579.Magis Q, Gaudy-Marqueste C, Basire A, et al. Diabetes and blood glucose disorders under anti-PD1. J Immunother 2018;41(5):232–240. 10.1097/cji.0000000000000218 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1580.Chen T, Jia H, Li J, Chen X, Zhou H, Tian H. New onset diabetes mellitus after liver transplantation and hepatitis c virus infection: Meta-analysis of clinical studies. Transpl Int 2009;22(4):408–415. 10.1111/j.1432-2277.2008.00804.x [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1581.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and hcv seropositive status after renal transplantation: Meta-analysis of clinical studies. Am J Transplant 2005;5(10): 2433–2440. 10.1111/j.1600-6143.2005.01040.x [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1582.Schaefer HM, Kizilisik AT, Feurer I, et al. Short-term results under three different immunosuppressive regimens at one center. Transplantation Proc 2006;38(10):3466–3467. 10.1016/j.transproceed.2006.10.098 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1583.Vanrenterghem Y, Bresnahan B, Campistol J, et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies). Transplantation 2011;91(9):976–983. 10.1097/TP.0b013e31820c10eb [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 1584.Aasebø W, Midtvedt K, Valderhaug TG, et al. Impaired glucose homeostasis in renal transplant recipients receiving basiliximab. Nephrol Dial Transplant 2010;25(4):1289–1293. 10.1093/ndt/gfp617 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1585.Borda B, Lengyel C, Várkonyi T, et al. Side effects of the calcineurin inhibitor, such as new-onset diabetes after kidney transplantation. Acta Physiol Hung 2014;101(3):388–394. 10.1556/APhysiol.101.2014.3.13 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1586.Cheungpasitporn W, Thongprayoon C, Harindhanavudhi T, Edmonds PJ, Erickson SB. Hypomagnesemia linked to new-onset diabetes mellitus after kidney transplantation: A systematic review and meta-analysis. Endocr Res 2016;41(2):142–147. 10.3109/07435800.2015.1094088 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1587.Cheungpasitporn W, Thongprayoon C, Vijayvargiya P, Anthanont P, Erickson SB. The risk for new-onset diabetes mellitus after kidney transplantation in patients with autosomal dominant polycystic kidney disease: A systematic review and meta-analysis. Can J Diabetes 2016;40(6):521–528. 10.1016/j.jcjd.2016.03.001 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1588.Claes K, Meier-Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H. Effect of different immunosuppressive regimens on the evolution of distinct metabolic parameters: Evidence from the symphony study. Nephrol Dial Transplant 2012;27(2):850–857. 10.1093/ndt/gfr238 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1589.Ducloux D, Courivaud C, Bamoulid J, et al. Immune phenotype predicts new onset diabetes after kidney transplantation. Hum Immunol 2019;80(11): 937–942. 10.1016/j.humimm.2019.08.006 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1590.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: A systematic review and meta-analysis. Am J Transplant 2004;4(4):583–595. 10.1046/j.1600-6143.2003.00372.x [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1591.Gheith OA, Nematalla AH, Bakr MA, Refaie A, Shokeir AA, Ghoneim MA. Steroid avoidance reduce the cost of morbidities after live-donor renal allotransplants: A prospective, randomized, controlled study. Exp Clin Transplant 2011;9(2):121–127 [EL 1; RCT]. [PubMed] [Google Scholar]
- 1592.Hill P, Cross NB, Barnett AN, Palmer SC, Webster AC. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev 2017;1(1):Cd004759. 10.1002/14651858.CD004759.pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1593.Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation 2010;89(1):1–14. 10.1097/TP.0b013e3181c518cc [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1594.Kato T, Gaynor JJ, Yoshida H, et al. Randomized trial of steroid-free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis c virus: Impact on hepatic fibrosis progression at one year. Transplantation 2007;84(7):829–835. 10.1097/01.tp.0000282914.20578.7b [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1595.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 2008;248(4):564–577. 10.1097/SLA.0b013e318187d1da [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1596.Talawila N, Pengel LH. Does belatacept improve outcomes for kidney transplant recipients? A systematic review. Transpl Int 2015;28(11): 1251–1264. 10.1111/tri.12605 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1597.Goralczyk AD, Hauke N, Bari N, Tsui TY, Lorf T, Obed A. Interleukin 2 receptor antagonists for liver transplant recipients: A systematic review and meta-analysis of controlled studies. Hepatology 2011;54(2):541–554. 10.1002/hep.24385 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1598.Thiruvengadam S, Hutchison B, Lim W, et al. Intensive monitoring for post-transplant diabetes mellitus and treatment with dipeptidyl peptidase-4 inhibitor therapy. Diabetes Metab Syndr 2019;13(3):1857–1863. 10.1016/j.dsx.2019.04.020 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1599.Kim HJ, Jung SH, Kim JJ, et al. New-onset diabetes mellitus after heart transplantation - incidence, risk factors and impact on clinical outcome. Circ J 2017;81(6):806–814. 10.1253/circj.CJ-16-0963 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1600.Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: An emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care 2019;7(1):e000591. 10.1136/bmjdrc-2018-000591 [EL 3; CCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1601.Gauci ML, Boudou P, Squara PA, et al. Checkpoint inhibitor treatment induces an increase in HbA1c in nondiabetic patients. Melanoma Res 2019;29(3): 328–332. 10.1097/cmr.0000000000000585 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1602.Leiter A, Carroll E, Brooks D, et al. Characterization of hyperglycemia in patients receiving immune checkpoint inhibitors: Beyond autoimmune insulin-dependent diabetes. Diabetes Res Clin Pract 2021;172(108633). 10.1016/j.diabres.2020.108633 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1603.Monami M, Naletto L, Nreu B, Dicembrini I, Sesti G, Mannucci E. Immune checkpoints inhibitors and hyperglycemia: A meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2020;162:108115. 10.1016/j.diabres.2020.108115 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1604.Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. A systematic review and meta-analysis on the regulation of programmed cell death-1 on t-cells in type 2 diabetes. Medicine (Baltimore) 2021;100(15):e25488. 10.1097/md.0000000000025488 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1605.Zhao Z, Wang X, Bao XQ, Ning J, Shang M, Zhang D. Autoimmune polyendocrine syndrome induced by immune checkpoint inhibitors: A systematic review. Cancer Immunol Immunother 2021;70(6):1527–1540. 10.1007/s00262-020-02699-1 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1606.Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur J Endocrinol 2018;178(2):173–180. 10.1530/eje-17-0810 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1607.Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: Insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018;67(8):1471–1480. 10.2337/dbi18-0002 [EL 3; CCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1608.van Raalte DH, Brands M, van der Zijl NJ, et al. Low-dose glucocorticoid treatment affects multiple aspects of intermediary metabolism in healthy humans: A randomised controlled trial. Diabetologia 2011;54(8): 2103–2112. 10.1007/s00125-011-2174-9 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1609.van Raalte DH, Diamant M, Ouwens DM, et al. Glucocorticoid treatment impairs microvascular function in healthy men in association with its adverse effects on glucose metabolism and blood pressure: A randomised controlled trial. Diabetologia 2013;56(11):2383–2391. 10.1007/s00125-013-3016-8 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1610.den Uyl D, van Raalte DH, Nurmohamed MT, et al. Metabolic effects of high-dose prednisolone treatment in early rheumatoid arthritis: Balance between diabetogenic effects and inflammation reduction. Arthritis Rheum 2012;64(3):639–646. 10.1002/art.33378 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1611.Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in hiv-1-infected patients. AIDS 1998;12(15):F167–F173. 10.1097/00002030-199815000-00001 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1612.Lo C, Toyama T, Oshima M, et al. Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients. Cochrane Database Syst Rev 2020;8:Cd009966. 10.1002/14651858.CD009966.pub3 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1613.Haidinger M, Werzowa J, Hecking M, et al. Efficacy and safety of vildagliptin in new-onset diabetes after kidney transplantationea randomized, double-blind, placebo-controlled trial. Am J Transplant 2014;14(1):115–123. 10.1111/ajt.12518 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1614.Strøm Halden TA, Åsberg A, Vik K, Hartmann A, Jenssen T. Short-term efficacy and safety of sitagliptin treatment in long-term stable renal recipients with new-onset diabetes after transplantation. Nephrol Dial Transplant 2014;29(4):926–933. 10.1093/ndt/gft536 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1615.Oberholzer J, Thielke J, Hatipoglu B, Testa G, Sankary HN, Benedetti E. Immediate conversion from tacrolimus to cyclosporine in the treatment of posttransplantation diabetes mellitus. Transplantation Proc 2005;37(2): 999–1000. 10.1016/j.transproceed.2004.12.085 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1616.Wissing KM, Abramowicz D, Weekers L, et al. Prospective randomized study of conversion from tacrolimus to cyclosporine a to improve glucose metabolism in patients with posttransplant diabetes mellitus after renal transplantation. J Transplant 2018;18(7):1726–1734. 10.1111/ajt.14665 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1617.Werzowa J, Hecking M, Haidinger M, et al. Vildagliptin and pioglitazone in patients with impaired glucose tolerance after kidney transplantation: A randomized, placebo-controlled clinical trial. Transplantation 2013;95(3): 456–462. 10.1097/TP.0b013e318276a20e [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1618.Halden TAS, Kvitne KE, Midtvedt K, et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care 2019;42(6):1067–1074. 10.2337/dc19-0093 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1619.Ramos-Cebrián M, Torregrosa JV, Gutiérrez-Dalmau A, Oppenheimer F, Campistol JM. Conversion from tacrolimus to cyclosporine could improve control of posttransplant diabetes mellitus after renal transplantation. Transplantation Proc 2007;39(7):2251–2253. 10.1016/j.transproceed.2007.06.035 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1620.Sharif A, Moore R, Baboolal K. Influence of lifestyle modification in renal transplant recipients with postprandial hyperglycemia. Transplantation 2008;85(3):353–358. 10.1097/TP.0b013e3181605ebf [EL 2; NRCT]. [DOI] [PubMed] [Google Scholar]
- 1621.Ballmann M, Hubert D, Assael BM, et al. Repaglinide versus insulin for newly diagnosed diabetes in patients with cystic fibrosis: A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018;6(2):114–121. 10.1016/s2213-8587(17)30400-x [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1622.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's updated sleep duration recommendations: Final report. Sleep Health 2015;1(4):233–243. 10.1016/j.sleh.2015.10.004 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1623.Chen L, Kuang J, Pei JH, et al. Continuous positive airway pressure and diabetes risk in sleep apnea patients: A systemic review and meta-analysis. Eur J Intern Med 2017;39:39–50. 10.1016/j.ejim.2016.11.010 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1624.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 2010;181(5):507–513. 10.1164/rccm.200909-1423OC [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1625.Ramar K, Malhotra RK, Carden KA, et al. Sleep is essential to health: An american academy of sleep medicine position statement. American Academy of Sleep Medicine position statement. J Clin Sleep Med 2021;17(10): 2115–2119. 10.5664/jcsm.9476 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1626.Pivetta B, Chen L, Nagappa M, et al. Use and performance of the STOP-bang questionnaire for obstructive sleep apnea screening across geographic regions: A systematic review and meta-analysis. JAMA Netw Open. J Clin Sleep Med 2021;4(3):e211009. 10.1001/jamanetworkopen.2021.1009 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1627.Westlake K, Dostalova V, Plihalova A, Pretl M, Polak J. The clinical impact of systematic screening for obstructive sleep apnea in a type 2 diabetes population-adherence to the screening-diagnostic process and the acceptance and adherence to the CPAP therapy compared to regular sleep clinic patients. Front Endocrinol 2018;9:714. 10.3389/fendo.2018.00714 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1628.Shaw JE, Punjabi NM, Naughton MT, et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. American Academy of Sleep Medicine position statement. J Clin Sleep Med 2016;194(4): 486–492. 10.1164/rccm.201511-2260OC [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1629.Martínez-Cerón E, Barquiel B, Bezos AM, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med 2016;194(4):476–485. 10.1164/rccm.201510-1942OC [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1630.Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: A systematic review and meta-analysis. NPJ Prim Care Respir Med 2015;25:15005. 10.1038/npjpcrm.2015.5 [EL2; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1631.Abud R, Salgueiro M, Drake L, Reyes T, Jorquera J, Labarca G. Efficacy of continuous positive airway pressure (CPAP) preventing type 2 diabetes mellitus in patients with obstructive sleep apnea hypopnea syndrome (OSAHS) and insulin resistance: A systematic review and meta-analysis. Sleep Med 2019;62:14–21. 10.1016/j.sleep.2018.12.017 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1632.Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: The SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond) 2016;40(8): 1310–1319. 10.1038/ijo.2016.52 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1633.Kline CE, Reboussin DM, Foster GD, et al. The effect of changes in cardiorespiratory fitness and weight on obstructive sleep apnea severity in overweight adults with type 2 diabetes. Sleep 2016;39(2):317–325. 10.5665/sleep.5436 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1634.Shechter A, Foster GD, Lang W, et al. Effects of a lifestyle intervention on REM sleep-related OSA severity in obese individuals with type 2 diabetes. J Sleep Res 2017;26(6):747–755. 10.1111/jsr.12559 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1635.Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the sleep AHEAD study. Am J Respir Crit Care Med 2021;203(2):221–229. 10.1164/rccm.201912-2511OC [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1636.Lam JCM, Lai AYK, Tam TCC, Yuen MMA, Lam KSL, Ip MSM. Cpap therapy for patients with sleep apnea and type 2 diabetes mellitus improves control of blood pressure. Sleep Breath 2017;21(2):377–386. 10.1007/s11325-016-1428-7 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1637.Loffler KA, Heeley E, Freed R, et al. Continuous positive airway pressure treatment, glycemia, and diabetes risk in obstructive sleep apnea and comorbid cardiovascular disease. Diabetes Care 2020;43(8):1859–1867. 10.2337/dc19-2006 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1638.Shang W, Zhang Y, Wang G, Han D. Benefits of continuous positive airway pressure on glycaemic control and insulin resistance in patients with type 2 diabetes and obstructive sleep apnoea: A meta-analysis. Diabetes Obes Metab 2021;23(2):540–548. 10.1111/dom.14247 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1639.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care 2000;23(7):934–942. 10.2337/diacare.23.7.934 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1640.Pan A, Lucas M, Sun Q, et al. Increased mortality risk in women with depression and diabetes mellitus. Arch Gen Psychiatry 2011;68(1):42–50. 10.1001/archgenpsychiatry.2010.176 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1641.Reimer A, Schmitt A, Ehrmann D, Kulzer B, Hermanns N. Reduction of diabetes-related distress predicts improved depressive symptoms: A secondary analysis of the DIAMOS study. PloS One 2017;12(7):e0181218. 10.1371/journal.pone.0181218 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1642.Pols AD, Adriaanse MC, van Tulder MW, et al. Two-year effectiveness of a stepped-care depression prevention intervention and predictors of incident depression in primary care patients with diabetes type 2 and/or coronary heart disease and subthreshold depression: Data from the Step-Dep cluster randomised controlled trial. BMJ Open 2018;8(10):e020412. 10.1136/bmjopen-2017-020412 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1643.Owens-Gary MD, Zhang X, Jawanda S, Bullard KM, Allweiss P, Smith BD. The importance of addressing depression and diabetes distress in adults with type 2 diabetes. J General Intern Med 2019;34(2):320–324. 10.1007/s11606-018-4705-2 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1644.Rauwerda NL, Tovote KA, Peeters A, et al. WHO-5 and BDI-II are acceptable screening instruments for depression in people with diabet. Diabet. Med 2018;35(12):1678–1685. 10.1111/dme.13779 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1645.Wroe AL, Rennie EW, Sollesse S, Chapman J, Hassy A. Is cognitive behavioural therapy focusing on depression and anxiety effective for people with long-term physical health conditions? A controlled trial in the context of type 2 diabetes mellitus. Behav Cogn Psychother 2018;46(2):129–147. 10.1017/s1352465817000492 [EL 2; NRCT]. [DOI] [PubMed] [Google Scholar]
- 1646.Kanapathy J, Bogle V. The effectiveness of cognitive behavioural therapy for depressed patients with diabetes: A systematic review. J Health Psychol 2019;24(1):137–149. 10.1177/1359105317713360 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1647.de Groot M, Shubrook JH, Hornsby WG Jr, et al. Program ACTIVE II: Outcomes from a randomized, multistate community-based depression treatment for rural and urban adults with type 2 diabetes. Diabetes Care 2019;42(7): 1185–1193. 10.2337/dc18-2400 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1648.Ell K, Aranda MP, Wu S, Oh H, Lee PJ, Guterman J. Promotora assisted depression and self-care management among predominantly Latinos with concurrent chronic illness: Safety net care system clinical trial results. Contemp Clin Trials 2017;61:1–9. 10.1016/j.cct.2017.07.001 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1649.Clarke J, Sanatkar S, Baldwin PA, et al. A web-based cognitive behavior therapy intervention to improve social and occupational functioning in adults with type 2 diabetes (the SpringBoarD trial): Randomized controlled trial. J Med Internet Res 2019;21(5):e12246. 10.2196/12246 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1650.Mehta S, Peynenburg VA, Hadjistavropoulos HD. Internet-delivered cognitive behaviour therapy for chronic health conditions: A systematic review and meta-analysis. J Behav Med 2019;42(2):169–187. 10.1007/s10865-018-9984-x [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1651.Heiskanen TH, Koivumaa-Honkanen HT, Niskanen LK, et al. Depression and major weight gain: A 6-year prospective follow-up of outpatients. Compr Psychiatry 2013;54(6):599–604. 10.1016/j.comppsych.2013.02.001 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1652.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363(27):2611–2620. 10.1056/NEJMoa1003955 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1653.Radojkovic J, Sikanic N, Bukumiric Z, Tadic M, Kostic N, Babic R. Improvement of glycemic control in insulin-dependent diabetics with depression by concomitant treatment with antidepressants. Med Sci Monit 2016;22: 2133–2143. 10.12659/msm.899571 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1654.Tharmaraja T, Stahl D, Hopkins CWP, et al. The association between selective serotonin reuptake inhibitors and glycemia: A systematic review and meta-analysis of randomized controlled trials. Psychosom Med 2019;81(7): 570–583. 10.1097/psy.0000000000000707 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1655.Rachdi C, Damak R, Fekih Romdhane F, Ouertani H, Cheour M. Impact of sertraline on weight, waist circumference and glycemic control: A prospective clinical trial on depressive diabetic type 2 patients. Prim Care Diabetes 2019;13(1):57–62. 10.1016/j.pcd.2018.09.003 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1656.Inouye J, Li D, Davis J, Arakaki R. Psychosocial and clinical outcomes of a cognitive behavioral therapy for Asians and Pacific Islanders with type 2 diabetes: A randomized clinical trial. Hawaii J Med Public Health 2015;74(11):360–368 [EL 1; RCT]. [PMC free article] [PubMed] [Google Scholar]
- 1657.Wagner JA, Bermudez-Millan A, Damio G, et al. A randomized, controlled trial of a stress management intervention for latinos with type 2 diabetes delivered by community health workers: Outcomes for psychological well-being, glycemic control, and cortisol. Diabetes Res Clin Pract 2016;120: 162–170. 10.1016/j.diabres.2016.07.022 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1658.World Health Organization. About social determinants of health, 2020. Available at: https://www.who.int/social_determinants/sdh_definition/. Accessed January 26, 2022. [EL 4; NE]. [Google Scholar]
- 1659.Andersen MB, Bjørkman AD, Pedersen M, Ekholm O, Molsted S. Social inequality in lifestyle, motivation to change lifestyle and received health advice in individuals with diabetes: A nationwide study. Scand J Public Health 2020;48(8): 847–854. 10.1177/1403494819885727 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1660.Marmot M, Allen JJ. Social determinants of health equity. Am J Public Health 2014;104(Suppl 4):S517–S519. 10.2105/ajph.2014.302200 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1661.Asadi-Lari M, Khosravi A, Nedjat S, et al. Socioeconomic status and prevalence of self-reported diabetes among adults in tehran: Results from a large population-based cross-sectional study (Urban Heart-2). J Endocrinol Invest 2016;39(5):515–522. 10.1007/s40618-015-0384-6 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1662.Bijlsma-Rutte A, Rutters F, Elders PJM, Bot SDM, Nijpels G. Socio-economic status and hbA(1c) in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev 2018;34(6):e3008. 10.1002/dmrr.3008 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1663.Tan ML, Manski-Nankervis JA, Thuraisingam S, Jenkins A, O'Neal D, Furler J. Socioeconomic status and time in glucose target range in people with type 2 diabetes: A baseline analysis of the GP-OSMOTIC study. BMC Endocr Disord 2018;18(1):47. 10.1186/s12902-018-0279-6 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1664.Lindner LME, Rathmann W, Rosenbauer J. Inequalities in glycaemic control, hypoglycaemia and diabetic ketoacidosis according to socio-economic status and area-level deprivation in type 1 diabetes mellitus: A systematic review. Diabet. Med 2018;35(1):12–32. 10.1111/dme.13519 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1665.Silverberg EL, Sterling TW, Williams TH, Castro G, Rodriguez de la Vega P, Barengo NC. The association between social determinants of health and self-reported diabetic retinopathy: An exploratory analysis. Int J Environ Res Public Health 2021;18(2). 10.3390/ijerph18020792 [EL 2; CSS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1666.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: A scientific review. Diabetes Care 2020;44(1):258–279. 10.2337/dci20-0053 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1667.Bermúdez-Millán A, Pérez-Escamilla R, Segura-Pérez S, et al. Psychological distress mediates the association between food insecurity and suboptimal sleep quality in Latinos with type 2 diabetes mellitus. J Nutr 2016;146(10): 2051–2057. 10.3945/jn.116.231365 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1668.Scarton LJ, de Groot M. Emotional and behavioral aspects of diabetes in american indians/alaska natives. American Indians/Alaska Natives. Health Educ Behav 2017;44(1):70–82. 10.1177/1090198116639289 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1669.Mayer VL, Vangeepuram N, Fei K, et al. Outcomes of a weight loss intervention to prevent diabetes among low-income residents of East Harlem, New York. Health Educ Behav 2019;46(6):1073–1082. 10.1177/1090198119868232 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1670.Meader N, King K, Wright K, et al. Multiple risk behavior interventions: Meta-analyses of RCTs. Am J Prevent Med 2017;53(1):e19–e30. 10.1016/j.amepre.2017.01.032 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1671.Lu JB, Danko KJ, Elfassy MD, Welch V, Grimshaw JM, Ivers NM. Do quality improvement initiatives for diabetes care address social inequities? Secondary analysis of a systematic review. BMJ Open 2018;8(2):e018826. 10.1136/bmjopen-2017-018826 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1672.Morone J Systematic review of sociodemographic representation and cultural responsiveness in psychosocial and behavioral interventions with adolescents with type 1 diabetes. J Diabetes 2019;11(7):582–592. 10.1111/1753-0407.12889 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1673.Sood A, Watts SA, Johnson JK, Hirth S, Aron DC. Telemedicine consultation for patients with diabetes mellitus: A cluster randomised controlled trial. J Telemed Telecare 2018;24(6):385–391. 10.1177/1357633x17704346 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1674.Bertuzzi F, Stefani I, Rivolta B, et al. Teleconsultation in type 1 diabetes mellitus (TELEDIABE). Acta Diabetol 2018;55(2):185–192. 10.1007/s00592-017-1084-9 [EL 1; RCT, feasibility]. [DOI] [PubMed] [Google Scholar]
- 1675.Yaron M, Sher B, Sorek D, et al. A randomized controlled trial comparing a telemedicine therapeutic intervention with routine care in adults with type 1 diabetes mellitus treated by insulin pumps. Acta Diabetol 2019;56(6): 667–673. 10.1007/s00592-019-01300-1 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1676.Ruiz de Adana MS, Alhambra-Expósito MR, Muñoz-Garach A, et al. Randomized study to evaluate the impact of telemedicine care in patients with type 1 diabetes with multiple doses of insulin and suboptimal HbA(1c) in Andalusia (Spain): PLATEDIAN study. Diabetes Care 2020;43(2):337–342. 10.2337/dc19-0739 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1677.Lee SWH, Chan CKY, Chua SS, Chaiyakunapruk N. Comparative effectiveness of telemedicine strategies on type 2 diabetes management: A systematic review and network meta-analysis. Sci Rep 2017;7(1):12680. 10.1038/s41598-017-12987-z [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1678.Rasmussen OW, Lauszus FF, Loekke M. Telemedicine compared with standard care in type 2 diabetes mellitus: A randomized trial in an outpatient clinic. J Telemed Telecare 2016;22(6):363–368. 10.1177/1357633x15608984 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1679.Jeong JY, Jeon JH, Bae KH, et al. Smart care based on telemonitoring and telemedicine for type 2 diabetes care: Multi-center randomized controlled trial. Telemed J E Health 2018;24(8):604–613. 10.1089/tmj.2017.0203 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1680.Xie W, Dai P, Qin Y, Wu M, Yang B, Yu X. Effectiveness of telemedicine for pregnant women with gestational diabetes mellitus: An updated meta-analysis of 32 randomized controlled trials with trial sequential analysis. BMC Pregnancy Childbirth 2020;20(1):198. 10.1186/s12884-020-02892-1 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1681.Cai X, Qiu S, Luo D, Wang L, Lu Y, Li M. Mobile application interventions and weight loss in type 2 diabetes: A meta-analysis. Obesity (Silver Spring) 2020;28(3):502–509. 10.1002/oby.22715 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1682.Hu Y, Wen X, Wang F, et al. Effect of telemedicine intervention on hypoglycaemia in diabetes patients: A systematic review and meta-analysis of randomised controlled trials. Telemed Telecare 2019;25(7):402–413. 10.1177/1357633x18776823 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1683.Kobe EA, Diamantidis CJ, Bosworth HB, et al. Racial differences in the effectiveness of a multifactorial telehealth intervention to slow diabetic kidney disease. Med Care 2020;58(11):968–973. 10.1097/mlr.0000000000001387 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1684.Cohen LB, Taveira TH, Wu WC, Pirraglia PA. Pharmacist-led telehealth disease management program for patients with diabetes and depression. J Telemed Telecare 2020;26(5):294–302. 10.1177/1357633x18822575 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1685.Carter BL, Levy B, Gryzlak B, et al. Cluster-randomized trial to evaluate a centralized clinical pharmacy service in private family medicine offices. Circ Cardiovasc Qual Outcomes 2018;11(6):e004188. 10.1161/circoutcomes.117.004188 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1686.Kempf K, Altpeter B, Berger J, et al. Efficacy of the telemedical lifestyle intervention program TeLiPro in advanced stages of type 2 diabetes: A randomized controlled trial. Diabetes Care 2017;40(7):863–871. 10.2337/dc17-0303 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1687.Rinaldi G, Hijazi A, Haghparast-Bidgoli H. Cost and cost-effectiveness of mHealth interventions for the prevention and control of type 2 diabetes mellitus: A systematic review. Diabetes Res Clin Pract 2020:162 10.1016/j.diabres.2020.108084, 108084. [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1688.Timpel P, Oswald S, Schwarz PEH, Harst L. Mapping the evidence on the effectiveness of telemedicine interventions in diabetes, dyslipidemia, and hypertension: An umbrella review of systematic reviews and meta-analyses. J Med Internet Res 2020;22(3):e16791. 10.2196/16791 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1689.Cho JH, Kim HS, Yoo SH, et al. An internet-based health gateway device for interactive communication and automatic data uploading: Clinical efficacy for type 2 diabetes in a multi-centre trial. J Telemed Telecare 2017;23(6): 595–604. 10.1177/1357633x16657500 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1690.Agarwal P, Mukerji G, Desveaux L, et al. Mobile app for improved self-management of type 2 diabetes: Multicenter pragmatic randomized controlled trial. JMIR Mhealth Uhealth 2019;7(1):e10321. 10.2196/10321 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1691.Warren R, Carlisle K, Mihala G, Scuffham PA. Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: A randomised controlled trial. J Telemed Telecare 2018;24(9):586–595. 10.1177/1357633x17723943 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1692.American Diabetes Association. Federal motor carrier safety administration (FMCSA) final new rule on insulin-treated diabetes: Frequently asked questions Available at: https://www.diabetes.org/sites/default/files/2019-08/FMCSA%20Final%20Rule%20FAQ.pdf. Accessed September 27, 2021. [Google Scholar]
- 1693.Federal Motor Carrier Safety Administration. Qualifications of drivers; diabetes standard Available at: https://www.fmcsa.dot.gov/regulations/rulemaking/2018-20161. Accessed September 27, 2021. [Google Scholar]
- 1694.Aviation Administration Federal. Guide for aviation medical examiners. Decision considerations disease protocols - diabetes mellitus type i or type ii - insulin treated - CGM option Available at: https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/dec_cons/disease_prot/itdm/. Accessed January 10, 2022. [Google Scholar]
- 1695.Federal Motor Carrier Safety Administration. Diabetes and commercial motor vehicle driver safety: Updated evidence report presentation Available at: https://www.fmcsa.dot.gov/diabetes-and-commercial-motor-vehicle-driver-safety-updated-evidence-report-presentation. Accessed September 27, 2021.
- 1696.Hemmelgarn B, Lévesque LE, Suissa S. Anti-diabetic drug use and the risk of motor vehicle crash in the elderly. Can J Clin Pharmacol 2006;13(1): e112–e120 [EL 2; RCCS]. [PubMed] [Google Scholar]
- 1697.McGwin G Jr, Sims RV, Pulley L, Roseman JM. Diabetes and automobile crashes in the elderly. A population-based case-control study. Diabetes Care 1999;22(2):220–227. 10.2337/diacare.22.2.220 [EL 2; RCCS]. [DOI] [PubMed] [Google Scholar]
- 1698.Koepsell TD, Wolf ME, McCloskey L, et al. Medical conditions and motor vehicle collision injuries in older adults. J Am Geriatr Soc 1994;42(7):695–700. 10.1111/j.1532-5415.1994.tb06526.x [EL 2; RCCS]. [DOI] [PubMed] [Google Scholar]
- 1699.Liu SC, Tu YK, Chien MN, Chien KL. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: A network meta-analysis. Diabetes Obes Metab 2012;14(9):810–820. 10.1111/j.1463-1326.2012.01606.x [EL 2; NMA]. [DOI] [PubMed] [Google Scholar]
- 1700.Garden GL, Hine JL, Mitchell SJ, et al. An evaluation of the safety of pilots with insulin-treated diabetes in Europe flying commercial and noncommercial aircraft. Diabetes Care 2020;43(12):2923–2929. 10.2337/dc20-0277 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1701.Rayman G, Kröger J, Bolinder J. Could Freestyle Libre(™) sensor glucose data support decisions for safe driving? Diabetic Med 2018;35(4):491–494. 10.1111/dme.13515 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1702.Drincic A, Rizzo M, Desouza C, Merickel J. Chapter 16 - digital health technologies, diabetes, and driving (meet your new backseat driver). Diabetes Digital Health Elsevier; 2020:219–230 [EL 4; NE]. [Google Scholar]
- 1703.Hostiuc S, Negoi I, Hostiuc M. Diabetes and collision risk. A meta-analysis and meta-regression. Int J Clin Pract 2016;70(7):554–568. 10.1111/ijcp.12832 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1704.Cox DJ, Gonder-Frederick LA, Singh H, et al. Predicting and reducing driving mishaps among drivers with type 1 diabetes. Diabetes Care 2017;40(6): 742–750. 10.2337/dc16-0995 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1705.Akbari M, Tamtaji OR, Lankarani KB, et al. The effects of resveratrol supplementation on endothelial function and blood pressures among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc Prev 2019;26(4):305–319. 10.1007/s40292-019-00324-6 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1706.Akbari M, Tamtaji OR, Lankarani KB, et al. The effects of resveratrol on lipid profiles and liver enzymes in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis 2020;19(1):25. 10.1186/s12944-020-1198-x [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1707.Ardeshirlarijani E, Tabatabaei-Malazy O, Mohseni S, Qorbani M, Larijani B, Baradar Jalili R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. Daru 2019;27(2):827–837. 10.1007/s40199-019-00302-2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1708.He J, Zhang F, Han Y. Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: A meta-analysis of RCTS. Medicine 2017;96(51):e9166. 10.1097/md.0000000000009166 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1709.Hu YM, Zhou F, Yuan Y, Xu YC. Effects of probiotics supplement in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. Med Clin (Barc) 2017;148(8):362–370. 10.1016/j.medcli.2016.11.036 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1710.Samah S, Ramasamy K, Lim SM, Neoh CF. Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract 2016;118:172–182. 10.1016/j.diabres.2016.06.014 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1711.Sun J, Buys NJ. Glucose- and glycaemic factor-lowering effects of probiotics on diabetes: A meta-analysis of randomised placebo-controlled trials. Br J Nutr 2016;115(7):1167–1177. 10.1017/s0007114516000076 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1712.Wang X, Juan QF, He YW, Zhuang L, Fang YY, Wang YH. Multiple effects of probiotics on different types of diabetes: A systematic review and meta-analysis of randomized, placebo-controlled trials. J Pediatr Endocrinol Metab 2017;30(6):611–622. 10.1515/jpem-2016-0230 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1713.Yao K, Zeng L, He Q, Wang W, Lei J, Zou X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: A meta-analysis of 12 randomized controlled trials. Med Sci Monit 2017;23:3044–3053. 10.12659/msm.902600 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1714.Zhang Q, Wu Y, Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina (Kaunas) 2016;52(1):28–34. 10.1016/j.medici.2015.11.008 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1715.Gibb RD, McRorie JW Jr, Russell DA, Hasselblad V, D'Alessio DA. Psyllium fiber improves glycemic control proportional to loss of glycemic control: A meta-analysis of data in euglycemic subjects, patients at risk of type 2 diabetes mellitus, and patients being treated for type 2 diabetes mellitus. Am J Clin Nutr 2015;102(6):1604–1614. 10.3945/ajcn.115.106989 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1716.Ranasinghe P, Wathurapatha WS, Galappatthy P, Katulanda P, Jayawardena R, Constantine GR. Zinc supplementation in prediabetes: A randomized double-blind placebo-controlled clinical trial. J Diabetes 2018;10(5):386–397. 10.1111/1753-0407.12621 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1717.Bo S, Ponzo V, Ciccone G, et al. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol Res 2016;111:896–905. 10.1016/j.phrs.2016.08.010 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1718.Jeyaraman MM, Al-Yousif NSH, Singh Mann A, et al. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2020;1(1): Cd011919. 10.1002/14651858.CD011919.pub2 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1719.Abdelhaleem IA, Brakat AM, Adayel HM, Asla MM, Rizk MA, Aboalfetoh AY. The effects of resveratrol on glycemic control and cardiometabolic parameters in patients with T2DM: A systematic review and meta-analysis. Med Clin (Barc) 2021. 10.1016/j.medcli.2021.06.028. S0025–7753(0021)00472–00473 online ahead of print. [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1720.Vinceti M, Filippini T, Rothman KJ. Selenium exposure and the risk of type 2 diabetes: A systematic review and meta-analysis. Eur J Epidemiol 2018;33(9):789–810. 10.1007/s10654-018-0422-8 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1721.Jacobs ET, Lance P, Mandarino LJ, et al. Selenium supplementation and insulin resistance in a randomized, clinical trial. BMJ Open Diabetes Res Care 2019;7(1):e000613. 10.1136/bmjdrc-2018-000613 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1722.Lerchbaum E, Trummer C, Theiler-Schwetz V, et al. Effects of vitamin D supplementation on body composition and metabolic risk factors in men: A randomized controlled trial. Nutrients 2019;11(8):1894. 10.3390/nu11081894 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1723.Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Nutrients 2018;10(3):375. 10.3390/nu10030375 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1724.Raygan F, Ostadmohammadi V, Bahmani F, Asemi Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 2018;84(Pt A):50–55. 10.1016/j.pnpbp.2018.02.007 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1725.Liu X, Ji J, Sundquist K, Sundquist J, Hemminki K. The impact of type 2 diabetes mellitus on cancer-specific survival: A follow-up study in sweden. Cancer 2012;118(5):1353–1361. 10.1002/cncr.26420 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1726.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. Adults. N Engl J Med 2003;348(17):1625–1638. 10.1056/NEJMoa021423 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1727.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. Adults. Diabetes Care 2012;35(9):1835–1844. 10.2337/dc12-0002 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1728.Petrelli F, Cortellini A, Indini A, et al. Association of obesity with survival outcomes in patients with cancer: A systematic review and meta-analysis. JAMA Netw Open 2021;4(3):e213520. 10.1001/jamanetworkopen.2021.3520 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1729.Freisling H, Arnold M, Soerjomataram I, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: Meta-analysis of individual participant data of seven prospective cohorts in europe. Br J Cancer 2017;116(11):1486–1497. 10.1038/bjc.2017.106 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1730.Xue K, Li FF, Chen YW, Zhou YH, He J. Body mass index and the risk of cancer in women compared with men: A meta-analysis of prospective cohort studies. Eur J Cancer Prev 2017;26(1):94–105. 10.1097/cej.0000000000000231 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1731.Soltani S, Abdollahi S, Aune D, Jayedi A. Body mass index and cancer risk in patients with type 2 diabetes: A dose-response meta-analysis of cohort studies. Sci Rep 2021;11(1):2479. 10.1038/s41598-021-81671-0 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1732.Drake I, Gullberg B, Sonestedt E, et al. Type 2 diabetes, adiposity and cancer morbidity and mortality risk taking into account competing risk of non-cancer deaths in a prospective cohort setting. Int J Cancer 2017;141(6): 1170–1180. 10.1002/ijc.30824 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1733.Kabat GC, Kim MY, Lee JS, et al. Metabolic obesity phenotypes and risk of breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2017;26(12):1730–1735. 10.1158/1055-9965.Epi-17-0495 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1734.Schlesinger S, Lieb W, Koch M, et al. Body weight gain and risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Obes Rev 2015;16(7):607–619. 10.1111/obr.12286 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1735.Barrington WE, Schenk JM, Etzioni R, et al. Difference in association of obesity with prostate cancer risk between us african american and non-hispanic white men in the selenium and vitamin e cancer prevention trial (SELECT). JAMA Oncol 2015;1(3):342–349. 10.1001/jamaoncol.2015.0513 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1736.Agnoli C, Grioni S, Sieri S, et al. Metabolic syndrome and breast cancer risk: A case-cohort study nested in a multicentre italian cohort. PloS One 2015;10(6):e0128891. 10.1371/journal.pone.0128891 [EL 2; NCCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1737.De Ridder J, Julian-Almárcegui C, Mullee A, et al. Comparison of anthropometric measurements of adiposity in relation to cancer risk: A systematic review of prospective studies. Cancer Causes Control 2016;27(3):291–300. 10.1007/s10552-015-0709-y [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1738.Arnold M, Freisling H, Stolzenberg-Solomon R, et al. Overweight duration in older adults and cancer risk: A study of cohorts in europe and the united states. Eur J Epidemiol 2016;31(9):893–904. 10.1007/s10654-016-0169-z [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1739.Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the women's health initiative randomized clinical trials. JAMA Oncol 2015;1(5): 611–621. 10.1001/jamaoncol.2015.1546 [EL 2; PHAS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1740.Aune D, Navarro Rosenblatt DA, Chan DS, et al. Anthropometric factors and endometrial cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Ann Oncol 2015;26(8):1635–1648. 10.1093/annonc/mdv142 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1741.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: A systematic review and meta-analysis. Eur J Cancer 2007;43(4):690–709. 10.1016/j.ejca.2006.11.010 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1742.Aune D, Navarro Rosenblatt DA, Chan DS, et al. Anthropometric factors and ovarian cancer risk: A systematic review and nonlinear dose-response meta-analysis of prospective studies. Int J Cancer 2015;136(8):1888–1898. 10.1002/ijc.29207 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1743.Foong KW, Bolton H. Obesity and ovarian cancer risk: A systematic review. Post reproductive health 2017;23(4):183–198. 10.1177/2053369117709225 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1744.Li ZM, Wu ZX, Han B, et al. The association between BMI and gallbladder cancer risk: A meta-analysis. Oncotarget 2016;7(28):43669–43679. 10.18632/oncotarget.9664 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1745.Du X, Hidayat K, Shi BM. Abdominal obesity and gastroesophageal cancer risk: Systematic review and meta-analysis of prospective studies. Biosci Rep 2017;37(3). 10.1042/bsr20160474 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1746.Gelfond J, Al-Bayati O, Kabra A, Iffrig K, Kaushik D, Liss MA. Modifiable risk factors to reduce renal cell carcinoma incidence: Insight from the PLCO trial. Urol Oncol 2018;36(7):340.e341–340.e346. 10.1016/j.urolonc.2018.04.011 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1747.Liu X, Sun Q, Hou H, et al. The association between BMI and kidney cancer risk: An updated dose-response meta-analysis in accordance with PRISMA guideline. Medicine (Baltimore) 2018;97(44):e12860. 10.1097/md.0000000000012860 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1748.Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: A systematic review and meta-analysis. Obes Rev 2015;16(12): 1042–1054. 10.1111/obr.12321 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1749.Hidayat K, Yang CM, Shi BM. Body fatness at an early age and risk of colorectal cancer. International journal of cancer 2018;142(4):729–740. 10.1002/ijc.31100 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1750.Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB. Obesity and risk of bladder cancer: A dose-response meta-analysis of 15 cohort studies. PloS One 2015;10(3):e0119313. 10.1371/journal.pone.0119313 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1751.Zhao L, Tian X, Duan X, Ye Y, Sun M, Huang J. Association of body mass index with bladder cancer risk: A dose-response meta-analysis of prospective cohort studies. Oncotarget 2017;8(20):33990–34000. 10.18632/oncotarget.16722 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1752.Genkinger JM, Kitahara CM, Bernstein L, et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann Oncol 2015;26(11):2257–2266. 10.1093/annonc/mdv355 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1753.Li S, Chen L, Jin W, et al. Influence of body mass index on incidence and prognosis of acute myeloid leukemia and acute promyelocytic leukemia: A meta-analysis. Sci Rep 2017;7(1):17998. 10.1038/s41598-017-18278-x [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1754.Psaltopoulou T, Sergentanis TN, Ntanasis-Stathopoulos I, Tzanninis IG, Riza E, Dimopoulos MA. Anthropometric characteristics, physical activity and risk of hematological malignancies: A systematic review and meta-analysis of cohort studies. Int J Cancer 2019;145(2):347–359. 10.1002/ijc.32109 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1755.Zhu H, Zhang S. Body mass index and lung cancer risk in never smokers: A meta-analysis. BMC cancer 2018;18(1):635. 10.1186/s12885-018-4543-y [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1756.Hidayat K, Du X, Chen G, Shi M, Shi B. Abdominal obesity and lung cancer risk: Systematic review and meta-analysis of prospective studies. Nutrients 2016;8(12):810. 10.3390/nu8120810 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1757.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst 2003;95(16): 1240–1244. 10.1093/jnci/djg009 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1758.Zhang X, Zhou G, Sun B, et al. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncol Lett 2015;9(3):1307–1312. 10.3892/ol.2014.2841 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1759.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008;371(9612):569–578. 10.1016/s0140-6736(08)60269-x [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1760.Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the breast cancer association consortium studies. J Natl Cancer Inst 2011;103(3):250–263. 10.1093/jnci/djq526 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1761.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol 2019;15(3): 139–154. 10.1038/s41574-018-0126-x [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1762.Cozzo AJ, Fuller AM, Makowski L. Contribution of adipose tissue to development of cancer. Compr Physiol 2017;8(1):237–282. 10.1002/cphy.c170008 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1763.Carstensen B, Read SH, Friis S, et al. Cancer incidence in persons with type 1 diabetes: A five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia 2016;59(5):980–988. 10.1007/s00125-016-3884-9 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1764.Bhatia D, Lega IC, Wu W, Lipscombe LL. Breast, cervical and colorectal cancer screening in adults with diabetes: A systematic review and meta-analysis. Diabetologia 2020;63(1):34–48. 10.1007/s00125-019-04995-7 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1765.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006;4(3):369–380. 10.1016/j.cgh.2005.12.007 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1766.Xu Y, Huo R, Chen X, Yu X. Diabetes mellitus and the risk of bladder cancer: A PRISMA-compliant meta-analysis of cohort studies. Medicine 2017;96(46):e8588. 10.1097/md.0000000000008588 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1767.Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer 2005;92(11):2076–2083. 10.1038/sj.bjc.6602619 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1768.Bruenderman EH, Martin RC 2nd. High-risk population in sporadic pancreatic adenocarcinoma: Guidelines for screening. J Surg Res 2015;194(1): 212–219. 10.1016/j.jss.2014.06.046 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1769.Shi J, Xiong L, Li J, et al. A linear dose-response relationship between fasting plasma glucose and colorectal cancer risk: Systematic review and meta-analysis. Sci Rep 2015;5:17591. 10.1038/srep17591 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1770.Saed L, Varse F, Baradaran HR, et al. The effect of diabetes on the risk of endometrial cancer: An updated a systematic review and meta-analysis. BMC Cancer 2019;19(1):527. 10.1186/s12885-019-5748-4 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1771.Boyle P, Boniol M, Koechlin A, et al. Diabetes and breast cancer risk: A meta-analysis. Br J Cancer 2012;107(9):1608–1617. 10.1038/bjc.2012.414 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1772.Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: A systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia 2018;61(10):2140–2154. 10.1007/s00125-018-4664-5 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1773.Guraya SY. Association of type 2 diabetes mellitus and the risk of colorectal cancer: A meta-analysis and systematic review. World J Gastroenterol 2015;21(19):6026–6031. 10.3748/wjg.v21.i19.6026 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1774.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006;15(11):2056–2062. 10.1158/1055-9965.Epi-06-0410 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1775.Murtola TJ, Vihervuori VJ, Lahtela J, et al. Fasting blood glucose, glycaemic control and prostate cancer risk in the Finnish randomized study of screening for prostate cancer. Br J Cancer 2018;118(9):1248–1254. 10.1038/s41416-018-0055-4 [EL 2; ES]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1776.Murtola TJ, S€alli SM, Talala K, Taari K, Tammela TLJ, Auvinen A. Blood glucose, glucose balance, and disease-specific survival after prostate cancer diagnosis in the Finnish randomized study of screening for prostate cancer. Prostate Cancer Prostatic Dis 2019;22(3):453–460. 10.1038/s41391-018-0123-0 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1777.Cai H, Xu Z, Xu T, Yu B, Zou Q. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: A meta-analysis of 11 cohort studies. Diabetes Metab Res Rev 2015;31(4):336–343. 10.1002/dmrr.2582 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1778.Wang M, Yang Y, Liao Z. Diabetes and cancer: Epidemiological and biological links. World Jo Diabetes 2020;11(6):227–238. 10.4239/wjd.v11.i6.227 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1779.Harvie M, Pegington M, French D, et al. Breast cancer risk status influences uptake, retention and efficacy of a weight loss programme amongst breast cancer screening attendees: Two randomised controlled feasibility trials. BMC Cancer 2019;19(1):1089. 10.1186/s12885-019-6279-8 [EL 1; RCT feasibility]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1780.Look AHEAD Research Group, Yeh HC, Bantle JP, et al. Intensive weight loss intervention and cancer risk in adults with type 2 diabetes: Analysis of the look ahead randomized clinical trial. Obesity (Silver Spring) 2020;28(9): 1678–1686. 10.1002/oby.22936 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1781.Wu L, Zhu J, Prokop LJ, Murad MH. Pharmacologic therapy of diabetes and overall cancer risk and mortality: A meta-analysis of 265 studies. Sci Rep 2015;5:10147. 10.1038/srep10147 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1782.Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res Rev 2017;40:31–44. 10.1016/j.arr.2017.08.003 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1783.Farmer RE, Ford D, Forbes HJ, et al. Metformin and cancer in type 2 diabetes: A systematic review and comprehensive bias evaluation. Int J Epidemiol 2017;46(2):728–744. 10.1093/ije/dyw275 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1784.Hou YC, Hu Q, Huang J, Fang JY, Xiong H. Metformin therapy and the risk of colorectal adenoma in patients with type 2 diabetes: A meta-analysis. Oncotarget 2017;8(5):8843–8853. 10.18632/oncotarget.13633 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1785.Jung YS, Park CH, Eun CS, Park DI, Han DS. Metformin use and the risk of colorectal adenoma: A systematic review and meta-analysis. J Gastroenterol Hepatol 2017;32(5):957–965. 10.1111/jgh.13639 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1786.Liu F, Yan L, Wang Z, et al. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Oncotarget 2017;8(9):16017–16026. 10.18632/oncotarget.13762 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1787.Rokkas T, Portincasa P. Colon neoplasia in patients with type 2 diabetes on metformin: A meta-analysis. Eur J Intern Med 2016;33:60–66. 10.1016/j.ejim.2016.05.027 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1788.Meng F, Song L, Wang W. Metformin improves overall survival of colorectal cancer patients with diabetes: A meta-analysis. J Diabetes Res 2017;2017: 5063239. 10.1155/2017/5063239 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1789.He XK, Su TT, Si JM, Sun LM. Metformin is associated with slightly reduced risk of colorectal cancer and moderate survival benefits in diabetes mellitus: A meta-analysis. Medicine 2016;95(7):e2749. 10.1097/md.0000000000002749 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1790.Du L, Wang M, Kang Y, et al. Prognostic role of metformin intake in diabetic patients with colorectal cancer: An updated qualitative evidence of cohort studies. Oncotarget 2017;8(16):26448–26459. 10.18632/oncotarget.14688 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1791.Tang GH, Satkunam M, Pond GR, et al. Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: A grade-assessed systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2018;27(6):627–635. 10.1158/1055-9965.Epi-17-0936 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1792.Shi J, Liu B, Wang H, Zhang T, Yang L. Association of metformin use with ovarian cancer incidence and prognosis: A systematic review and meta-analysis. Int Journal Gynecol Cancer 2019;29(1):140–146. 10.1136/ijgc-2018-000060 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1793.Perez-Lopez FR, Pasupuleti V, Gianuzzi X, Palma-Ardiles G, Hernandez-Fernandez W, Hernandez AV. Systematic review and meta-analysis of the effect of metformin treatment on overall mortality rates in women with endometrial cancer and type 2 diabetes mellitus. Maturitas 2017;101:6–11. 10.1016/j.maturitas.2017.04.001 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1794.Chu D, Wu J, Wang K, et al. Effect of metformin use on the risk and prognosis of endometrial cancer: A systematic review and meta-analysis. BMC Cancer 2018;18(1):438. 10.1186/s12885-018-4334-5 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1795.Stopsack KH, Ziehr DR, Rider JR, Giovannucci EL. Metformin and prostate cancer mortality: A meta-analysis. Cancer Causes Control 2016;27(1): 105–113. 10.1007/s10552-015-0687-0 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1796.Xin WX, Fang L, Fang QL, Zheng XW, Ding HY, Huang P. Effect of hypoglycemic agents on survival outcomes of lung cancer patients with diabetes mellitus: A meta-analysis. Medicine 2018;97(9):e0035. 10.1097/md.0000000000010035 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1797.Li Y, Hu L, Xia Q, Yuan Y, Mi Y. The impact of metformin use on survival in kidney cancer patients with diabetes: A meta-analysis. Int Urol Nephrol 2017;49(6): 975–981. 10.1007/s11255-017-1548-4 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1798.Ma S, Zheng Y, Xiao Y, Zhou P, Tan H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine 2017;96(19):e6888. 10.1097/md.0000000000006888 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1799.Shi YQ, Zhou XC, Du P, et al. Relationships are between metformin use and survival in pancreatic cancer patients concurrent with diabetes: A systematic review and meta-analysis. Medicine 2020;99(37):e21687. 10.1097/md.0000000000021687 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1800.Wan G, Sun X, Li F, et al. Survival benefit of metformin adjuvant treatment for pancreatic cancer patients: A systematic review and aeta-analysis. Int J Exp Cell Physiol Biochem Pharmacol 2018;49(3):837–847. 10.1159/000493214 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1801.Hu J, Chen JB, Cui Y, et al. Association of metformin intake with bladder cancer risk and oncologic outcomes in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Medicine 2018;97(30):e11596. 10.1097/md.0000000000011596 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1802.Tuccori M, Filion KB, Yin H, Yu OH, Platt RW, Azoulay L. Pioglitazone use and risk of bladder cancer: Population based cohort study. BMJ 2016;352:i1541. 10.1136/bmj.i1541 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1803.Garry EM, Buse JB, Lund JL, Pate V, Stürmer T. Comparative safety of pioglitazone versus clinically meaningful treatment alternatives concerning the risk of bladder cancer in older US adults with type 2 diabetes. Diabetes Obes Metab 2018;20(1):129–140. 10.1111/dom.13049 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1804.Erdmann E, Harding S, Lam H, Perez A. Ten-year observational follow-up of proactive: A randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes Metab 2016;18(3):266–273. 10.1111/dom.12608 [EL 2; CS]. [DOI] [PubMed] [Google Scholar]
- 1805.Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62(7):2595–2604. 10.2337/db12-1686 [EL 3; BR]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1806.Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugseFDA and EMA assessment. N Engl J Med 2014;370(9):794–797. 10.1056/NEJMp1314078 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1807.Cao C, Yang S, Zhou Z. GLP-1 receptor agonists and pancreatic safety concerns in type 2 diabetic patients: Data from cardiovascular outcome trials. Endocrine 2020;68(3):518–525. 10.1007/s12020-020-02223-6 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1808.Abd El Aziz M, Cahyadi O, Meier JJ, Schmidt WE, Nauck MA. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: A meta-analysis based on cardiovascular outcomes trials. Diabetes Obes Metab 2020;22(4):699–704. 10.1111/dom.13924 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1809.US Food & Drug Administration. Victoza (liraglutide injection) prescribing information/package insert Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022341s031lbl.pdf [EL 4; NE]. [Google Scholar]
- 1810.Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid c-cells causing calcitonin release and c-cell proliferation. Endocrinology 2010;151(4):1473–1486. 10.1210/en.2009-1272 [EL 3; DS]. [DOI] [PubMed] [Google Scholar]
- 1811.Hegedüs L, Sherman SI, Tuttle RM, et al. No evidence of increase in calcitonin concentrations or development of C-cell malignancy in response to liraglutide for up to 5 years in the LEADER trial. Diabetes Care 2018;41(3): 620–622. 10.2337/dc17-1956 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1812.Cao C, Yang S, Zhou Z. GLP-1 receptor agonists and risk of cancer in type 2 diabetes: An updated meta-analysis of randomized controlled trials. Endocrine 2019;66(2):157–165. 10.1007/s12020-019-02055-z [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1813.Overbeek JA, Bakker M, van der Heijden A, van Herk-Sukel MPP, Herings RMC, Nijpels G. Risk of dipeptidyl peptidase-4 (DPP-4) inhibitors on site-specific cancer: A systematic review and meta-analysis. Diabetes Metab Res Rev 2018;34(5):e3004. 10.1002/dmrr.3004 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1814.US Food & Drug Administration. Farxiga (dapagliflozin) prescribing information/package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202293s020lbl.pdf [EL 4; NE]. [Google Scholar]
- 1815.US Food & Drug Administration. Invokana (canagliflozin) prescribing information/package insert Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204042s034lbl.pdf [EL 4; NE]. [Google Scholar]
- 1816.US Food & Drug Administration. Jardiance (empagliflozin) prescribing information/package insert Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/204629s026lbl.pdf [EL 4; NE]. [Google Scholar]
- 1817.Dicembrini I, Nreu B, Mannucci E, Monami M. Sodium-glucose co-transporter-2 (SGLT-2) inhibitors and cancer: A meta-analysis of randomized controlled trials. Diabetes Obes Metab 2019;21(8):1871–1877. 10.1111/dom.13745 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1818.Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Diabetologia 2017;60(10):1862–1872. 10.1007/s00125-017-4370-8 [EL 1; MRCT]. [DOI] [PubMed] [Google Scholar]
- 1819.Chen Y, Du L, Li L, et al. Cancer risk of sulfonylureas in patients with type 2 diabetes mellitus: A systematic review. J Diabetes 2017;9(5):482–494. 10.1111/1753-0407.12435 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1820.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: A cohort study. Diabetologia 2009;52(9):1732–1744. 10.1007/s00125-009-1418-4 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1821.ORIGIN Trial Investigators, Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367(4):319–328. 10.1056/NEJMoa1203858 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1822.Janghorbani M, Dehghani M, Salehi-Marzijarani M. Systematic review and meta-analysis of insulin therapy and risk of cancer. Hormones Cancer 2012;3(4): 137–146. 10.1007/s12672-012-0112-z [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1823.Centers for Disease Control and Prevention. Immunization schedules Available at: https://www.cdc.gov/vaccines/schedules/. Accessed January 6, 2022.
- 1824.Smith SA, Poland GA. Use of influenza and pneumococcal vaccines in people with diabetes. Diabetes Care 2000;23(1):95–108. 10.2337/diacare.23.1.95 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1825.Lau D, Eurich DT, Majumdar SR, Katz A, Johnson JA. Working-age adults with diabetes experience greater susceptibility to seasonal influenza: A population-based cohort study. Diabetologia 2014;57(4):690–698. 10.1007/s00125-013-3158-8 [EL 2; ES]. [DOI] [PubMed] [Google Scholar]
- 1826.McKane CK, Marmarelis M, Mendu ML, Moromizato T, Gibbons FK, Christopher KB. Diabetes mellitus and community-acquired bloodstream infections in the critically ill. J Crit Care 2014;29(1):70–76. 10.1016/j.jcrc.2013.08.019 [EL 2; CSS]. [DOI] [PubMed] [Google Scholar]
- 1827.Tsakiridou E, Makris D, Chatzipantazi V, et al. Diabetes and hemoglobin a1c as risk factors for nosocomial infections in critically ill patients. Crit Care Res Pract 2013;2013:279479. 10.1155/2013/279479 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1828.Adamuz J, Viasus D, Jiménez-Martínez E, et al. Incidence, timing and risk factors associated with 1-year mortality after hospitalization for community-acquired pneumonia. J Infection 2014;68(6):534–541. 10.1016/j.jinf.2014.02.006 [EL 2; PCS]. [DOI] [PubMed] [Google Scholar]
- 1829.Casanova L, Gobin N, Villani P, Verger P. Bias in the measure of the effectiveness of seasonal influenza vaccination among diabetics. Primary Care Diabetes 2016;10(6):398–406. 10.1016/j.pcd.2016.05.005 [EL 2; MNRCT]. [DOI] [PubMed] [Google Scholar]
- 1830.Dos Santos G, Tahrat H, Bekkat-Berkani R. Immunogenicity, safety, and effectiveness of seasonal influenza vaccination in patients with diabetes mellitus: A systematic review. Human Vaccines Immunother 2018;14(8):1853–1866. 10.1080/21645515.2018.1446719 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1831.Remschmidt C, Wichmann O, Harder T. Vaccines for the prevention of seasonal influenza in patients with diabetes: Systematic review and meta-analysis. BMC Med 2015;13:53. 10.1186/s12916-015-0295-6 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1832.Seo YB, Baek JH, Lee J, et al. Long-term immunogenicity and safety of a conventional influenza vaccine in patients with type 2 diabetes. Clin Vaccine Immunol 2015;22(11):1160–1165. 10.1128/cvi.00288-15 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1833.Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2020–21 influenza season. MMWR Recomm Rep 2020;69(8):1–24. 10.15585/mmwr.rr6908a1 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1834.Centers for Disease Control and Prevention. Influenza Flu Available at: https://www.cdc.gov/flu/. Accessed February 22, 2022.
- 1835.Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. Adults: Updated recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71(4):109–117. 10.15585/mmwr.mm7104a1 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1836.Van Der Meeren O, Peterson JT, Dionne M, et al. Prospective clinical trial of hepatitis b vaccination in adults with and without type-2 diabetes mellitus. Human Vaccines Immunother 2016;12(8):2197–2203. 10.1080/21645515.2016.1164362 [EL 2; NRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1837.Jackson S, Lentino J, Kopp J, et al. Immunogenicity of a two-dose investigational hepatitis b vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine 2018;36(5): 668–674. 10.1016/j.vaccine.2017.12.038 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1838.Janssen JM, Heyward WL, Martin JT, Janssen RS. Immunogenicity and safety of an investigational hepatitis b vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis b vaccine in patients with chronic kidney disease and type 2 diabetes mellitus. Vaccine 2015;33(7):833–837. 10.1016/j.vaccine.2014.12.060 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1839.Gregory JM, Slaughter JC, Duffus SH, et al. HBsAg-19 severity is tripled in the diabetes community: A prospective analysis of the pandemic's impact in type 1 and type 2 diabetes. Diabetes Care 2021;44(2):526–532. 10.2337/dc20-2260 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1840.Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the united states. J Diabetes Sci Technol 2020;14(4):813–821. 10.1177/1932296820924469 [EL 2; CS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1841.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed January 6, 2022.
- 1842.Ferretti F, Cannatelli R, Benucci M, et al. How to manage COVID-19 vaccination in immune-mediated inflammatory diseases: An expert opinion by IMIDS study group. Front Immunol 2021;12:656362. 10.3389/fimmu.2021.656362 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1843.Havers FP, Moro PL, Hunter P, Hariri S, Bernstein H. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: Updated recommendations of the Advisory Committee on Immunization Practices - United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69(3):77–83. 10.15585/mmwr.mm6903a5 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1844.Abu-Ashour W, Twells L, Valcour J, et al. The association between diabetes mellitus and incident infections: A systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res Care 2017;5(1):e000336. 10.1136/bmjdrc-2016-000336 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1845.Tetanus surveillance d United States, 2001–2008. MMWR Morb Mortal Wkly Rep 2011;60(12):365–369 [EL 2; CSS]. [PubMed] [Google Scholar]
- 1846.Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Reports 2018;67(2):1–44. 10.15585/mmwr.rr6702a1 [EL 4; NE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1847.Gagliardi AM, Andriolo BN, Torloni MR, et al. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst Rev 2019;2019(11): CD008858. 10.1002/14651858.CD008858.pub4 [EL 1; MRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1848.Vink P, Shiramoto M, Ogawa M, et al. Safety and immunogenicity of a herpes zoster subunit vaccine in Japanese population aged ≥50 years when administered subcutaneously vs. intramuscularly. Hum Vaccin Immunother 2017;13(3):574–578. 10.1080/21645515.2016.1232787 [EL 1; RCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1849.Maréchal C, Lal H, Poder A, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine co-administered with the 23-valent pneumococcal polysaccharide vaccine in adults ≥50 years of age: A randomized trial. Vaccine 2018;36(29):4278–4286. 10.1016/j.vaccine.2018.05.110 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1850.Ohfuji S, Ito K, Inoue M, et al. Safety of live attenuated varicella-zoster vaccine in patients with underlying illnesses compared with healthy adults: A prospective cohort study. BMC Infect Dis 2019;19(1):95. 10.1186/s12879-019-3719-7 [EL 2; PCS]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1851.Nowalk MP, Moehling KK, Zhang S, Raviotta JM, Zimmerman RK, Lin CJ. Using the 4 pillars to increase vaccination among high-risk adults: Who benefits? Am J Manag Care 2017;23(11):651–655 [EL 2; PHAS]. [PMC free article] [PubMed] [Google Scholar]
- 1852.Ofori-Anyinam O, Leroux-Roels G, Drame M, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine co-administered with a 23-valent pneumococcal polysaccharide vaccine versus separate administration, in adults ≥50years of age: Results from a phase III, randomized, non-inferiority trial. Vaccine 2017;35(46):6321–6328. 10.1016/j.vaccine.2017.09.012 [EL 1; RCT]. [DOI] [PubMed] [Google Scholar]
- 1853.Hata A, Ishioka T, Oishi K, Katayama T, Ohkubo T. Altered immunogenicity of 23-valent pneumococcal polysaccharide vaccine in elderly patients with diabetes who revealed lower responses to concomitant administration of BIKEN varicella zoster vaccine: Results of post hoc analysis of a randomized double-blind trial. J Diabetes Complicat 2019;33(3):243–248. 10.1016/j.jdiacomp.2018.11.003 [EL 2; PHAS]. [DOI] [PubMed] [Google Scholar]
- 1854.Ridda I, Chamberlain R, Haber R, Rashid H. Letter to the editor to: Verger P and Dubé E Restoring confidence in vaccines in the COVID-19 era, expert review of vaccines, 2020; 19(11):991–3. Exp Rev Vaccines 2021;20(4): 479–481. 10.1080/14760584.2021.1903880 [EL 4; NE]. [DOI] [PubMed] [Google Scholar]
- 1855.Jacobson Vann JC, Jacobson RM, Coyne-Beasley T, Asafu-Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev 2018;1(1):Cd003941. 10.1002/14651858.CD003941. pub3 [EL 2; MNRCT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


