Abstract
Purpose:
MiR-146a upregulated in limbus vs. central cornea and in diabetic vs. non-diabetic limbus has emerged as an important immune and inflammatory signaling mediator in corneal epithelial wound healing. Our aim was to investigate the potential inflammation-related miR-146a target genes and their roles in normal and impaired diabetic corneal epithelial wound healing.
Methods:
Our previous data from RNA-seq combined with quantitative proteomics of limbal epithelial cells (LECs) transfected with miR-146a mimic vs. mimic control were analyzed. Western blot and immunostaining were used to confirm the expression of miR-146a inflammatory target proteins in LECs and organ-cultured corneas. Luminex assay was performed on conditioned media at 6- and 20-hours post-wounding in miR-146a Mimic/Inhibitor transfected normal and diabetic cultured LECs.
Results:
Overexpression of miR-146a decreased the expression of pro-inflammatory TRAF6 and IRAK1 and downstream target NF-κB after challenge with lipopolysaccharide (LPS) or wounding. Additionally, miR-146a overexpression suppressed the production of downstream inflammatory mediators including secreted cytokines IL-1α, IL-1β, IL-6 and IL-8, and chemokines CXCL1, CXCL2 and CXCL5. These cytokines and chemokines were upregulated in normal but not in diabetic LEC during wounding. Furthermore, we achieved normalized levels of altered secreted cytokines and chemokines in diabetic wounded LEC via specific inhibition of miR-146a.
Conclusion:
Our study documented significant impact of miR-146a on the expression of inflammatory mediators at the mRNA and protein levels during acute inflammatory responses and wound healing, providing insights into the regulatory role of miR-146a in corneal epithelial homeostasis in normal and diabetic conditions.
Keywords: chemokines, cytokines, diabetic cornea, limbal stem cells, microRNA, miR-146a, NF-κB inflammatory pathway, wound healing
1. Introduction
The corneal epithelium is the outermost layer of the cornea, constantly exposed to the environment and at significant risk of injury and infection. As part of corneal homeostasis, the epithelium is renewed and/or healed by limbal epithelial stem cells (LESC) residing at the corneoscleral junction [1, 2]. Proper maintenance of the corneal epithelium is necessary to preserve vision and corneal health. Pathological conditions such as diabetes mellitus (DM), can impair corneal homeostasis, resulting in impaired epithelial renewal and wound healing, a common complication of diabetes [3].
Previously, we found altered expression in diabetic corneal epithelium of wound healing mediators such as HGF/c-met [4], MMP-10, cathepsin F [5] and phosphorylated EGFR (p-EGFR), a direct target of upregulated microRNA 146a (miR-146a) [6]. It is now well documented that miRNAs have an important role in regulating numerous vital cellular functions [7, 8]. MiRNAs are non-coding, single strand RNA molecules, 19–23 nucleotides in length that act on the post-transcriptional level to block translation or target mRNAs for degradation [9, 10]. The specific roles of miRNAs in different cell types and pathological conditions are an area of active research.
We documented increased miR-146a expression in diabetic relative to healthy corneal epithelium [6]. In both groups, miR-146a was enriched in the limbus vs. the central cornea. Normal corneas overexpressing miR-146a as well as diabetic corneas had delayed epithelial wound healing and cell migration, whereas inhibition of miR-146a in diabetic corneas significantly enhanced wound healing [11]. Transfection with miR-146a mimic resulted in decreased levels of epithelial wound healing mediators phosphorylated p38 (p-p38) and p-EGFR [11]. Our previous RNA-seq transcriptomic analysis was combined with quantitative proteomics of miR-146a transfected limbal epithelial cells (LECs). It has revealed that miR-146a regulates the expression of a large number of genes and corresponding proteins including Notch signaling and inflammatory mediators, such as IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6), and downstream targets in inflammatory pathways [12]. IRAK1 and TRAF6 are important adaptor molecules acting upstream of NF-κB. MiR-146a has emerged as an important immune and inflammatory signaling regulator in many tissues often via its target genes IRAK1 and TRAF6 [13–16], which are induced following treatment with bacterial endotoxin, lipopolysaccharide (LPS) [17–20].
Numerous studies have shown the role of inflammation-related NF-κB, cytokines and chemokines [21–23] in the healing of diabetic corneal wounds [24]. In addition to our transcriptomics and proteomics analyses, previous studies in other cell types revealed inflammatory signaling molecules as potential targets of miR-146a [12, 13, 25, 26]. This distinct pathway is known to regulate corneal epithelial homeostasis during wound healing.
In the present study, we examined the potential inflammation-related miR-146a target genes and their roles in normal and impaired diabetic corneal epithelial wound healing using LECs transfected with miR-146a mimic or inhibitor. We found that overexpression of miR-146a decreased TRAF6 and IRAK1 after LPS challenge as a model of induced inflammation [27,28] or during wound healing. Furthermore, we showed a significant surge in the expression of secreted inflammatory cytokines IL-1α and IL-1β and modest increase of IL-6 and IL-8 at 6 hours post-wounding in normal but not in DM LECs vs. their respective non-wounded controls. We also restored non-diabetic levels of altered secreted cytokines and chemokines in diabetic wounded LEC via inhibition of miR-146a. As a result, miR-146a overexpression significantly suppressed the production of inflammatory mediators in the NF-κB pathway. Additionally, knockdown of TRAF6 and IRAK1 expression using corresponding siRNAs in primary LECs showed downregulation of p-NF-κB and of the secretion of proinflammatory cytokines and chemokines. Our study documented the large impact of miR-146a on the expression of inflammatory mediators via targeting TRAF6 and IRAK1, providing insights into its regulatory role in corneal epithelial homeostasis and wound healing.
2. Materials and Methods
2.1. Human Corneas
Age-matched human autopsy normal and diabetic cadaver corneas (Table 1) were received in Optisol medium (Chiron Vision, Claremont, CA) from the National Disease Research Interchange (NDRI, Philadelphia, PA). NDRI uses a human tissue collection protocol approved by a managerial committee and subject to National Institutes of Health oversight. Samples were received within 24–48 hours of procurement. This work was covered by approved IRB protocol Pro00019393 from Cedars-Sinai Medical Center and adhered to ARVO guidelines.
Table 1.
Donor characteristics
| Case number | Age | Gender | Cause of death | History of eye diseases |
|---|---|---|---|---|
| N16–13 | 87 | M | Ruptured abdominal aortic aneurysm | IOL |
| N16–14 | 76 | F | Cardiac pulmonary failure | -- |
| N17–06 | 93 | F | Myocardial infarction | IOL |
| N17–07 | 73 | M | Cardiac arrest | IOL, Cataract surgery |
| N17–10 | 80 | F | Cerebrovascular/Stroke | IOL, Cataract surgery |
| N17–11 | 52 | M | Hypertension | IOL |
| N17–14 | 51 | F | Intracerebral hemorrhage | Cataract |
| N17–24 | 75 | M | Cardiac arrest | -- |
| N18–19 | 73 | M | Multi-system organ failure | -- |
| N18–23 | 57 | F | Cerebrovascular accident | -- |
| N18–36 | 74 | M | Cardiopulmonary arrest | -- |
| N19–04 | 71 | M | Cardiopulmonary arrest | IOL, Cataract surgery |
| N19–13 | 66 | M | Cardiopulmonary arrest | LASIK surgery |
| N19–32 | 70 | M | Respiratory failure | IOL |
| N19–35 | 35 | M | KCL overdose | -- |
| N19–38 | 63 | M | Cardiac arrest | |
| N20–03 | 64 | F | Cardiac arrest | -- |
| N20–05 | 73 | M | Shortness of breath | -- |
| DM15–04 | 81 | M | Congestive heart failure | T2D |
| DM18–12 | 76 | M | Respiratory failure | T2D |
| DM18–14 | 63 | M | Respiratory failure | T2D |
| DM18–22 | 73 | F | Cardiopulmonary arrest | T2D, Cataracts |
| DM18–34 | 75 | M | Respiratory failure | DM |
| DM19–43 | 75 | F | Cardiac arrest | T2D |
N, normal; DM, diabetic mellitus; M, male; F, female; IOL, intraocular lens; IOL, intraocular lens; T2D, type 2 diabetes.
2.2. Primary Limbal Epithelial Cell Isolation and cell culture maintenance
Primary Limbal Epithelial Cells (LECs) were isolated from human corneas as previously described [11]. Briefly, epithelial cells were removed from corneoscleral rims following Dispase/Trypsin digestion. Cells were placed in plates coated with a mixture of fibronectin, collagen IV, and limbal-expressed laminin-521 [11]. Cells were cultured and maintained in EpiLife media containing Human Keratinocyte Growth Supplement (HKGS), N-2 supplement, B27 supplement, 1X antibiotic/antimycotic mixture, with added 10 ng/ml epidermal growth factor (EGF) (Thermo Fisher Scientific, Waltham, MA, USA) [11]. Cells were used up to passage 5.
2.3. miRNA/siRNA Transfection of Primary LECs and Organ-Cultured Corneas
As described previously [12], primary human LECs were transfected with 50 nM has-miR-146a-5p pre-miR miRNA precursor or anti-miR inhibitor with respective negative controls for 48 hours using Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific). In some experiments, cells were treated for 24 hours with 10 ng/mL LPS prior to transfection. For siRNA experiments, 60–80% confluent primary human LECs were transfected with 50–100 nM of TRAF6 or IRAK1 siRNA along with control siRNA (Horizon Discovery, Lafayette, CO) for 24 hours using DharmaFECT transfection reagent (Horizon Discovery) and used for further analysis 72 hours after transfection. For in vitro wound healing assay, transfected cells were scratch wounded with a 200-μl pipet tip at confluency, and then incubated in new media for 6 or 20 hours before conditioned media collection for Luminex assay. Corneal organ cultures were established as described previously [11] and were maintained in Dulbecco’s Modified Eagle’s Medium with 1X insulin-transferrin-selenite (Sigma-Aldrich, St Louis, MO, USA) and 1X antibiotic/antimycotic mix (Thermo Fisher Scientific). Each cornea in a pair was transfected with 50 nM has-miR-146a-5p mimic or inhibitor with their respective negative controls for 48 hours using Lipofectamine RNAiMAX transfection reagent. After additional incubation period of 3–5 days, the transfected corneas were processed for western blot. To determine the fraction of LECs transfected with miRs, cells were transfected with Cy3-labeled scrambled-sequence miRNA using Lipofectamine RNAiMAX transfection reagent.
2.4. Western Blot Analysis
Western blotting was performed as described previously [6]. Briefly, 8% to 16% gradient Tris-glycine SDS polyacrylamide gels were used (Thermo Fisher Scientific). Protein was transferred to nitrocellulose membranes and blocked using Blotting Grade Blocker (BioRad, Hercules, CA, USA) before being probed with primary antibodies (Table 2). IRDye 800 CW or 680 RD goat anti-mouse or anti-rabbit secondary antibodies (Li-Cor Biosciences, Lincoln, NE, USA) were used. Blots were imaged and quantified using Odyssey CLX imaging system (Li-Cor Biosciences).
Table 2.
Primary antibody list
| Antigen | Antibody | Source | MW (kDa) | Dilution |
|---|---|---|---|---|
| TRAF6 | Rabbit mAb 8028S | Cell Signaling | 60 | 1:500 |
| IRAK1 | Rabbit mAb 4504S | Cell Signaling | 78 | 1:500 |
| NF-κB | Rabbit mAb 8242S | Cell Signaling | 65 | 1:1000 |
| p-NF-κB | Rabbit mAb 3033S | Cell Signaling | 65 | 1:400 |
| IκB | Rabbit mAb 4812 | Cell Signaling | 39 | 1:250 |
| p-IκB | Mouse mAb 9246 | Cell Signaling | 39 | 1:250 |
| β-actin | Mouse mAb 3700 | Cell Signaling | 45 | 1:1000 |
mAb, monoclonal antibody.
2.5. Luminex Assay of LEC Supernatant
Quantitative determination of select cytokines and chemokines was performed by the UCLA Immune Assessment Core using a customized panel of Human Magnetic Luminex Assay kit (R&D Systems, Minneapolis, MN, USA) per the manufacturer’s instructions. Briefly, 50 μl undiluted cell culture supernatant was mixed with 50 μl magnetic microparticle bead cocktail and allowed to incubate for 2 hours at room temperature while shaking. After washing the plates three times with wash buffer in a Biotek ELx405 washer, 50 μl of diluted biotin-antibody cocktail was added and incubated for 1 hour at room temperature (RT). 50 μl streptavidin-phycoerythrin conjugate was then added to the reaction mixture and incubated for another 30 minutes at RT. Following two washes, beads were resuspended in sheath fluid, and fluorescence was quantified using a Luminex 200 instrument (R&D Systems). Data were analyzed using MILLIPLEX Analyst 5.1 software.
2.6. Immunostaining
Primary cultured LEC were fixed in 10% formalin, permeabilized and immunostained as previously described [11]. Cultured cells were incubated with primary antibodies (Table 2) in blocking solution overnight at 4 °C, followed the next day by 1 h incubation of cross-species adsorbed secondary antibodies conjugated with either Alexa Fluor 488 or Alexa Fluor 594 (Abcam, Waltham, MA, USA) at RT. Slides were mounted with ProLong Gold Antifade Mounting with DAPI (Thermo Fisher Scientific). For each marker the same exposure time was used when photographing slides. Immunofluorescent staining was analyzed for a number of positive cells using ImageJ software. Negative controls without a primary antibody were included in each experiment.
2.7. Statistical Analysis
Western blot densitometry was analyzed by Student’s t-test for two groups, with p < 0.05 considered significant.
3. Results
3.1. Transcriptomic and proteomic analyses of miR-146a inflammatory targets in primary LECs
Previously, our combined transcriptomic and proteomic analysis showed that overexpression of miR-146a in primary LECs significantly decreased mRNA and protein levels of its known targets such as EGFR, Notch-2 and Numb [12]. Ingenuity pathway analysis of differentially expressed mRNAs in miR-146a mimic vs. mimic control showed significant differences in inflammatory signaling pathways such as STAT3, NF-κB, cytokines mediating inflammatory pathways, IL-17 signaling, CXC chemokine receptors and TLR signaling [12] (Figure 1A). We also found a decrease in the mRNA expression level of known regulators of the NF-κB inflammatory pathway, IRAK1, TRAF6, cytokines (IL1A, and IL1B) and CXC family chemokines (CXCL1, CXCL2, CXCL3, CXCL5, and CXCL8), with similar decrease of TRAF6, IL-1α, and IL-1β protein levels by proteomics analysis as shown in Figure 1B [12].
Figure 1.
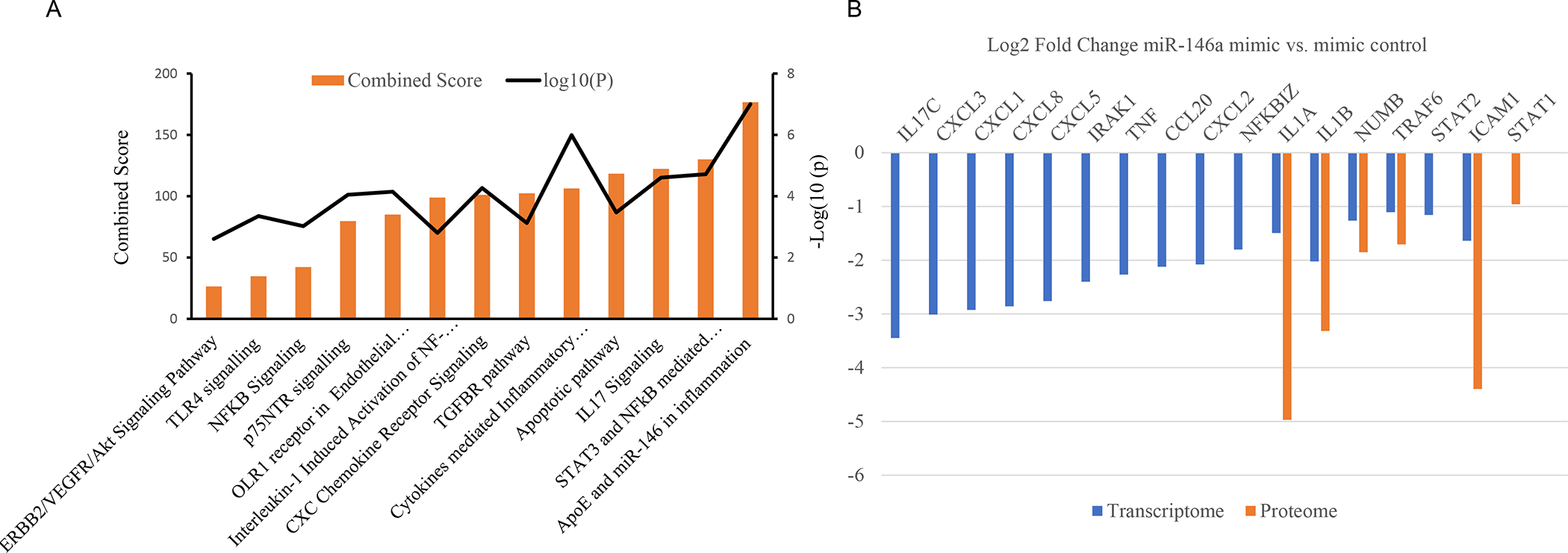
Inflammatory Target genes identified in human primary LECs from individual donors transfected with miR-146a mimic vs. mimic control in transcriptome (n = 8) and in proteome (n=4) using quantitative differential expression analysis. A. Pathway analysis indicates inflammatory responses are the significantly altered pathways in transcriptome analysis. B. Transfection with miR-146a mimic using RNAiMax decreased mRNA and protein expression levels of some miR-146a predicted targets such as TRAF6, IL-1α, IL-1β.
3.2. Effect of miR-146a on NF-κB Inflammatory Pathway Proteins in Human Normal LECs and Organ-Cultured Corneas by targeting TRAF6 and IRAK1
Changes in inflammatory target proteins in miR146a mimic-transfected cells were further confirmed by western analysis and immunostaining. Upregulation and downregulation of miR-146a were confirmed previously by Quantitative Real-Time RT-PCR (QRT-PCR) in mimic- and inhibitor-transfected cells, respectively [12]. In addition, Cy3-labeled miRNA was shown by fluorescence microscopy to transfect about 80% to 90% of LEC (Supplementary Figure 1). Upregulation of miR-146a mimic in LPS-treated LECs significantly decreased protein expression of its targets, TRAF6 and IRAK1, relative to control-transfected cells, whereas the inhibitor had opposite effects (Figure 2A, B). Inhibition of miR-146a resulted in significant increase of TRAF6 expression and modest increase of IRAK1, which didn’t reach significance (Figure 2A, B). In line with this result, western analysis of LPS-treated organ-cultured corneas transfected with miR-146a mimic showed a significant decrease in the expression of both TRAF6 and IRAK1, compared to fellow corneas transfected with mimic control (Figure 2A, B). Accordingly, there was a concomitant significant decrease of p-NF-κB expression level in miR-146a mimic vs. mimic control and respective increase in miR-146a inhibitor vs. inhibitor control transfected LECs (Figure 2C).
Figure 2.
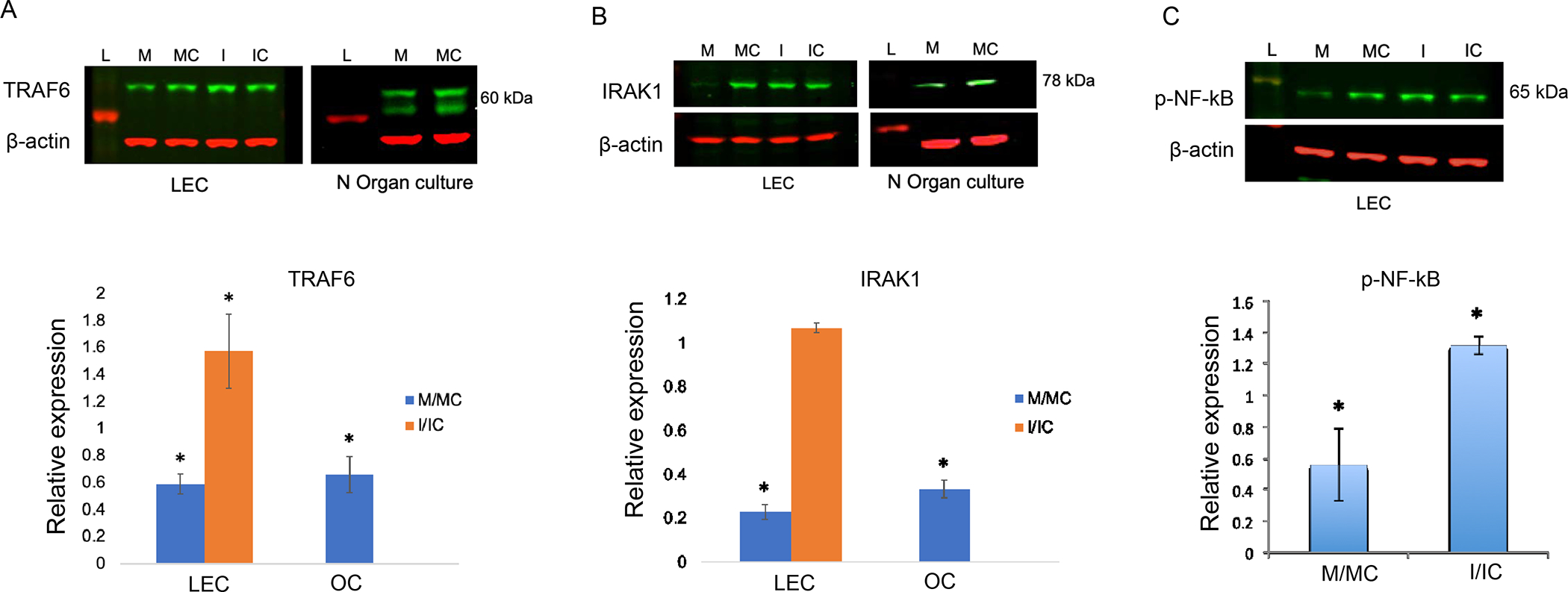
Differences in inflammatory regulators in miR-146a mimic/ inhibitor transfected LECs (n=4) and organ-cultured corneas (OC, n=3) by western blot analysis. Transfection with miR-146a mimic (M) vs. mimic control (MC) significantly decreased TRAF6 (A) and IRAK1 (B) expression levels in LPS treated normal primary LECs and organ-cultured corneas. Whereas the miR-146 inhibitor (I) vs. inhibitor control (IC) increased expression of TRAF6 (A) and IRAK1 (B) in LPS treated normal primary LECs. C. Transfection with mimic decreased, whereas the inhibitor increased p-NFkB expression in LPS treated normal primary LECs compared to their corresponding controls, MC and IC, respectively. Data are mean ± SEM, relative to β-actin. * p<.05, **p<.01 significant p values.
3.3. Effect of miR-146a on NF-κB Inflammatory Pathway Proteins in Human Diabetic LECs.
Diabetic primary LECs were challenged with LPS prior to miR-146a treatment. Transfection with miR-146a mimic decreased the expression of TRAF6 and IRAK1 in diabetic LECs compared to controls, whereas miR-146a inhibitor treatment increased both proteins (Figure 3). To mimic diabetic state, normal human LECs were transfected with mimic to upregulate miR-146a. Immunostaining of miR-146a mimic treated normal LECs showed a similar decrease in the number of NF-κB positive cells compared to cells transfected with mimic control, in line with the western blot, and similar to the level of expression observed in non-treated (NT) diabetic LECs (Figure 4A). Accordingly, immunostaining of diabetic LEC transfected with miR-146a inhibitor showed a similar increase in the number of NF-κB positive cells compared to cells transfected with inhibitor control, in line with western blot and similar to the level of expression observed in non-treated (NT) normal LECs (Figure 4B).
Figure 3.
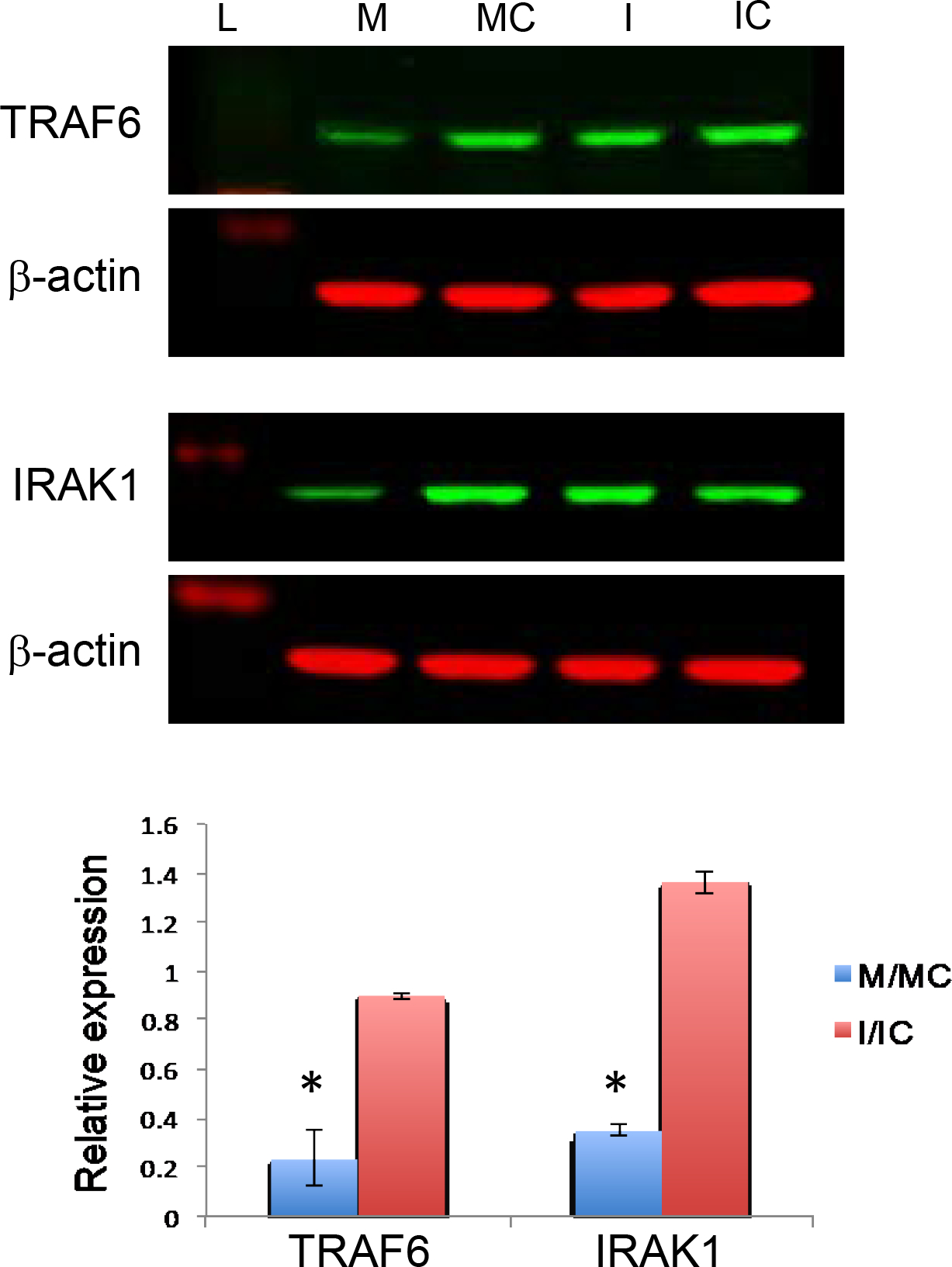
Differences in inflammatory regulators in miR-146a mimic/ inhibitor transfected diabetic LECs by western blot analysis. MiR-146a mimic (M) decreased, whereas the inhibitor (I) increased expression of TRAF6 and IRAK1 in LPS treated primary diabetic LECs compared to their corresponding controls, mimic control (MC) and inhibitor control (IC), respectively. Data are mean ± SEM, relative to β-actin * p<.05, **p<.01 significant p values.
Figure 4.
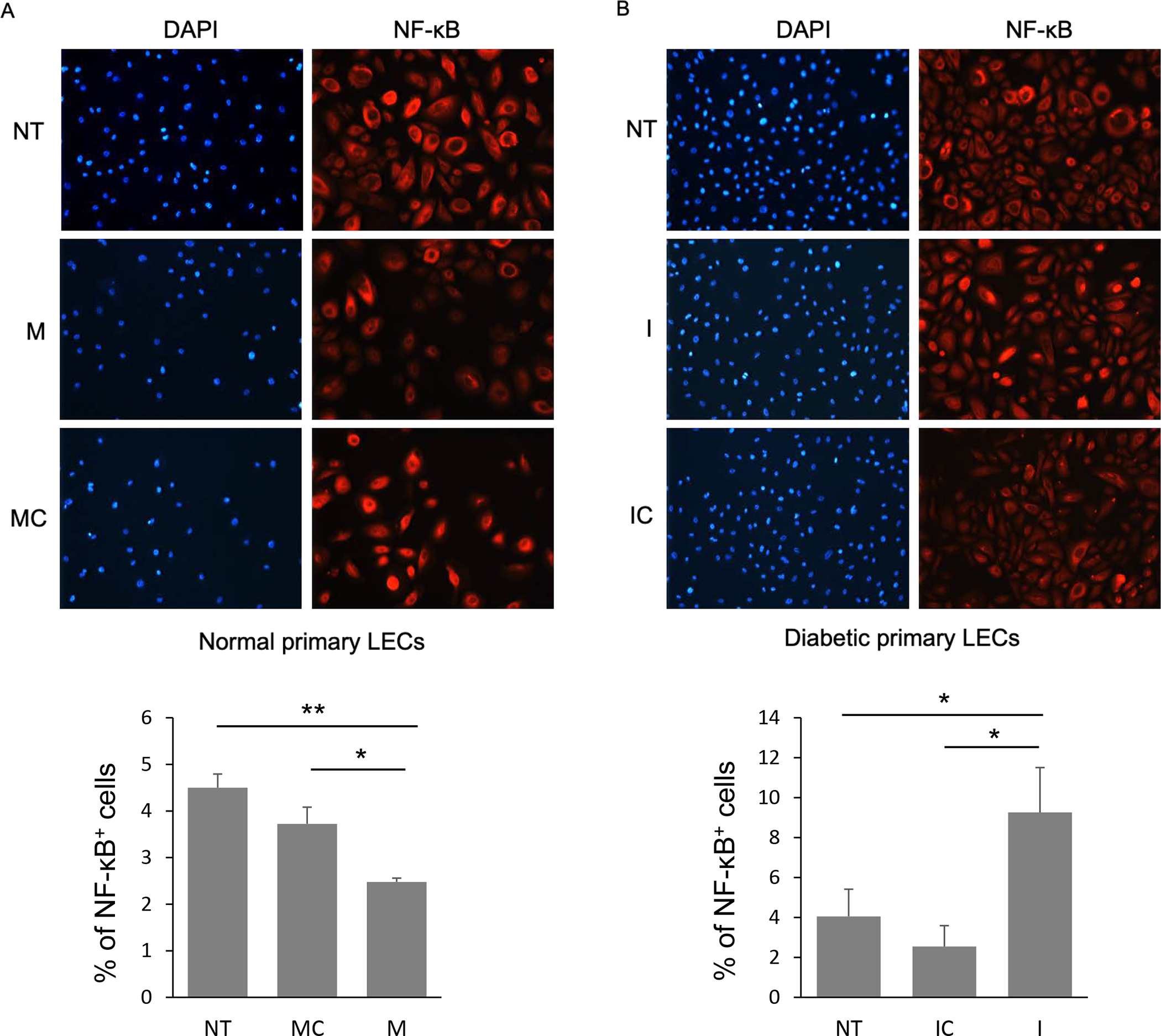
NF-κB expression level in miR-146a mimic (M) and inhibitor (I) transfected normal and diabetic LECs, respectively, using immunostaining with quantification of percent of NF-κB positive cells. Transfection with miR-146a mimic decreased NF-κB expression level in primary normal LECs, whereas the inhibitor increased the expression of NF-κB in primary diabetic LECs compared to their corresponding controls, mimic control (MC) and inhibitor control (IC), respectively. Nuclear staining using DAPI; NT, not treated.
3.4. Cytokine/Chemokine Expression in Wounded Normal and Diabetic Human LECs
The expression of secreted cytokines was examined by Luminex assay at two time points during the healing process of wounded normal and DM human LECs. Confluent normal and diabetic cultured cells were scratch wounded and conditioned media were collected at 6 and 20 hours post-wounding for Luminex assay; cytokine levels were compared to their unwounded controls. Luminex analysis showed significant downregulation of cytokine IL-1β, IL-6 and chemokines CXCL1, CXCL2 expressions in the conditioned media of DM vs. normal unwounded LECs (Figure 5, Supplementary Figure 2). By 6 hours post-wounding, there was a significant increase of inflammatory secreted cytokines, IL-1α and IL-1β, in normal LEC media, and modest increase of IL-6 and IL-8, which didn’t reach significance (Figure 5). 20 hours post-wounding, IL-1α, IL-1β and IL6 increase was still observed, but the cytokine expressions seemed to be returning to their background levels (Figure 5). Interestingly, no cytokine induction was observed in DM LECs media at both 6 and 20 hours post-wounding (Figure 5). In normal LECs media, the expression of chemokines CXCL1, CXCL2, and CXCL5 didn’t change at 6 hours post-wounding; however, there was a distinct but non-significant upregulation at 20 h post-wounding (Supplementary Figure 2). However, in non-wounded diabetic cell media CXCL1, CXCL2 levels were significantly lower than in normal LEC media (Supplementary Figure 2).
Figure 5.
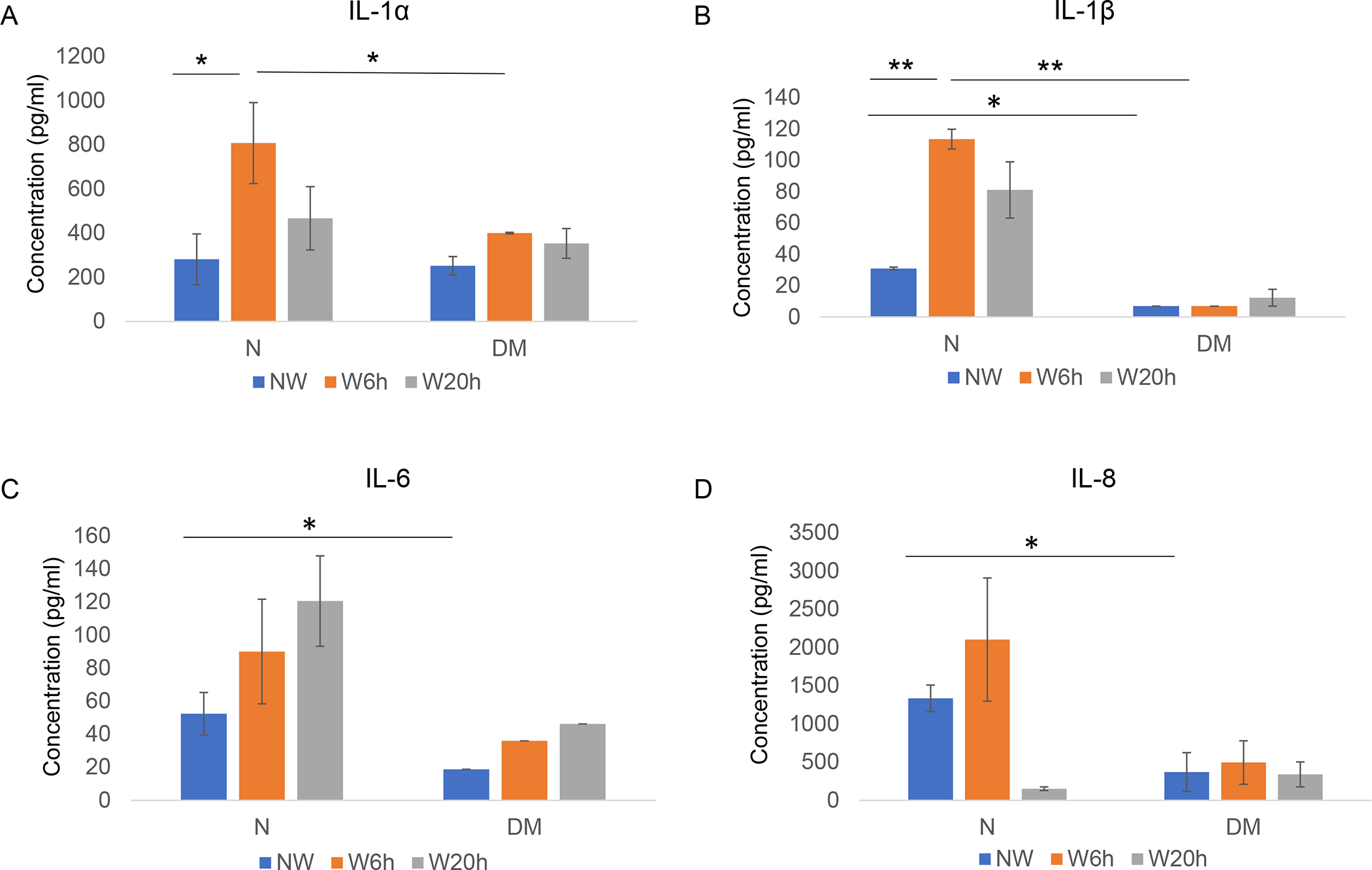
Luminex analysis of secretory cytokine levels at 6 and 20 h post-wounding in normal and diabetic primary LECs media. There was a significant surge in IL-1α (A) and IL-1β (B) expression levels at 6 h and modest increase at 20 h post-wounding in normal wounded LECs. IL-6 (C) and IL-8 (D) expression levels modestly increased at 6 h post-wounding in normal LECs that did not reach significance. No significant changes of any cytokines were observed at 6 and 20 h post-wounding in DM LECs. Data are mean ± SEM, * p<.05, **p<.01 significant p values.
3.5. Luminex Analysis of Cytokine/Chemokines Changes Due to miR-146a
Normal and DM LECs were transfected with miR-146a mimic and inhibitor, and their corresponding controls prior to growing to confluency and being wounded. Conditioned media were collected at 6 and 20 hours post-wounding for Luminex assay. Normal LECs treated with miR-146a mimic showed downregulation of secreted cytokines (Figure 6) and chemokines (Figure 7) post-wounding, similar to the level of expression observed in DM LECs. In line with these results, DM LECs treated with miR-146a inhibitor showed upregulation of secreted cytokines mostly at 6 h (Figure 6) and chemokines mostly at 20 h (Figure 7) post-wounding relative to their respective control treated cells, similar to the level of expression observed in normal LECs.
Figure 6.
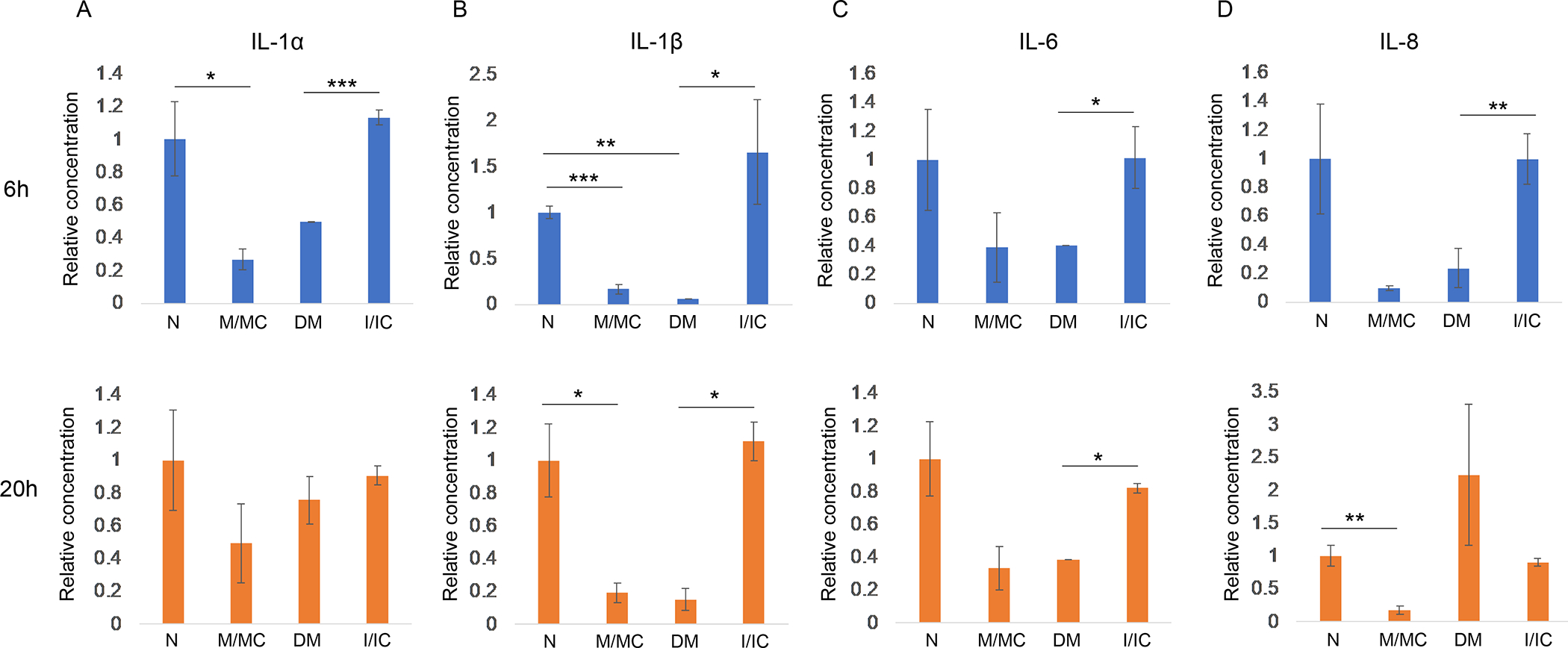
Secretory cytokine levels at 6 h and 20 h post-wounded normal primary LECs transfected with miR-146a mimic (M) vs. mimic control (MC), and diabetic primary LECs transfected with miR-146a inhibitor (I) vs. inhibitor control (IC). IL-1α (A) and IL-1β (B), IL6 (C) and IL8 (D) showed significant to modest decrease in M vs. MC transfected normal LECs at 6 h and 20 h post-wounding. While transfected DM LECs with inhibitor vs. IC showed significant to modest increase at 6 h and 20 h post-wounding similar to the level observed in non-treated normal LECs. Data are mean ± SEM, * p<.05, **p<.01, ***p<.005 significant p values.
Figure 7.
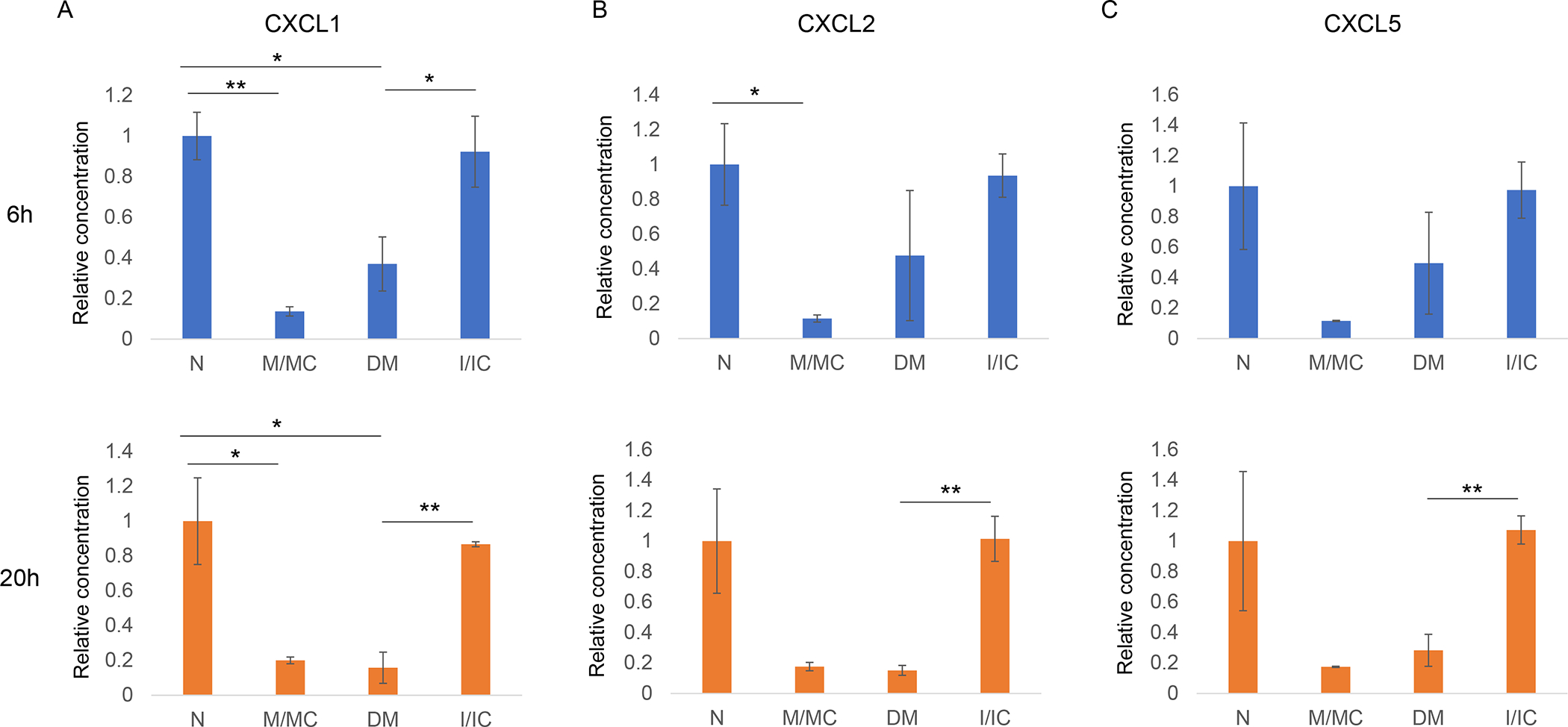
Secretory chemokine levels at 6 and 20 h post-wounding in normal primary LECs transfected with miR-146a mimic (M) and its control (MC), and diabetic primary LECs transfected with miR-146a inhibitor (I) and inhibitor control (IC). A. At both 6 and 20 h post-wounding, CXCL1 significantly decreased in M vs. MC treated normal LECs, whereas significantly increased in DM cells transfected with I vs. IC. B. CXCL2 significantly decreased at 6 h post-wounding in M vs. MC treated normal LECs and significantly increased at 20 h post wounded in I vs. IC treated DM LECs. C. CXCL5 significantly increased in I vs. IC transfected diabetic LECs at 20 h post-wounding. Data are mean ± SEM, * p<.05, **p<.01 significant p values.
3.6. Luminex Analysis of Cytokine/Chemokine Changes after Knockdown of TRAF6 and IRAK1.
Primary LECs were transfected with siTRAF6 or siIRAK1 or negative control (sicontrol) for 72 h prior to growing to confluency and being wounded. Western blot analysis showed significant downregulation of TRAF6 and IRAK1 protein expression levels after transfection compared to sicontrol (Supplementary Figure 3A and B). In addition, there was a significant decline in p-NF-κB expression level in siTRAF6 and siIRAK1 transfected LECs by immunostaining (Supplementary Figure 3C). Luminex analysis of conditioned media 6h post-wounding revealed significant decrease of secreted inflammatory cytokines, IL-6, IL8 and CXCL1, and downregulation of IL-1α, IL-1β, CXCL2 and CXCL5 which didn’t reach significance in primary LEC media after transfection with siTRAF6 or siIRAK1 (Supplementary Figure 4).
4. Discussion
As the cornea is continuously exposed to external injury, infection and diseases such as diabetes, it is important to understand the regulation of inflammatory responses and wound healing in corneal epithelium. Our previous studies suggested that upregulated miR-146a in the stem cell-enriched limbal region vs. central human cornea plays a regulatory role in corneal epithelial wound healing and limbal stem cell maintenance by direct targeting of EGFR and Notch signaling pathways, respectively [6, 11, 12]. Additionally, our data revealed abnormally increased expression of miR-146a in the diabetic cornea [11]. A hallmark of corneal diabetic complications is impaired wound healing, which was normalized by downregulating miR-146a expression using its inhibitor in human diabetic organ-cultured corneas [11].
Moreover, our integrated analysis of miRNA-mRNA and miRNA-protein expression of LECs transfected with miR-146a mimic has identified several miR-146a-targeted pathways important for corneal epithelial homeostasis, such as EGFR, Notch, anchoring junctions, adherens junctions, TGF-β and inflammation-related signaling [12].
In the present study, as a further step to investigate the regulatory role of miR-146a, we investigated the effects of miR-146a on the inflammatory responses during epithelial wound healing and acute inflammation in normal and diabetic corneas. The important regulatory role of miR-146a has been shown for many cellular functions and diseases due to targeting different genes in cell type specific manner [11, 29–34]. A number of studies in addition to our integrated transcriptomics and proteomics analysis revealed inflammatory signaling molecules as potential targets of miR-146a (Figure 1) [12–16, 35]. This signaling is known to regulate corneal epithelial homeostasis during inflammation and wound healing [21–23]. Therefore, we aimed for the first time to understand the functional importance of different miR-146a targeted pathways in diabetes-associated corneal changes.
Studies of miR-146a activity in inflammation indicated that it acts in a negative feedback loop to regulate NF-κB activity in different tissues [13]. Expression of miR-146a is induced by inflammatory stimuli of the NF-κB pathway, but then it acts to inhibit this pathway via targeting adaptor proteins such as CARD10, IRAK1, and TRAF6 [13, 25, 36]. Thus, it plays an important role in controlling the response to acute inflammatory challenges but also has definite functions in diseases with chronic inflammation such as diabetes. Due to its control of TLR and NF-κB response, miR-146a has also been linked to the pathogenesis of certain cancers [26, 37]. In a study of metabolic diseases in mice, knockout of miR-146a resulted in significant weight gain and increased activation of pro-inflammatory genes. MiR-146a was able to regulate inflammation in metabolic tissues and effected metabolic processes via direct targeting of TRAF6 [38]. Study of replicative senescence in the human trabecular meshwork cells found that miR-146a upregulation helped reduce the production of inflammatory mediators such as IL-6 and IL-8 as well as production of reactive oxygen species, helping mitigate possible harmful effects of senescent cells on their surrounding tissue [39]. An investigation of brain impairment in rats with chronic type 2 diabetes documented a negative correlation between miR-146a expression and inflammation and oxidative stress indicators [40]. Multiple studies have indicated an important role for miR-146a in models of diabetic retinopathy. As NF-κB plays an important function in pathogenesis of diabetic retinopathy, miR-146a was found to work as part of a negative feedback regulation by suppressing CARD10 to prevent thrombin-induced NF-κB activation in human retinal endothelial cells [25]. Intravitreal delivery of miR-146a in type 1 diabetic rats inhibited diabetes-induced upregulation of NF-κB, decreasing microvascular leakage and retinal functional defects [36]. Current evidence suggests that miR-146a has an important function in controlling both acute inflammation and inflammatory responses in chronic pathological conditions.
We demonstrated for the first time in limbal epithelium that miR-146a directly targets IRAK1 and TRAF6 in LPS-induced acute inflammatory responses. This leads to downregulation of NF-κB expression, in addition to downregulation of downstream inflammatory secreted cytokines including IL-1α/β, IL-6, IL-8 and chemokines including CXCL1, CXCL2, and CXCL5 (Figure 1B) that have major roles in wound healing in addition to inflammatory responses. Our study showed a significant surge in the expression of secreted IL-1α and IL-1β vs. control cell media, which was attenuated by 20 hours post-wounding (Figure 5). Conversely, in diabetic LECs, no increase in secreted inflammatory cytokine levels was observed at 6 or 20 hours post-wounding vs. control media. Although there was a modest increase in secreted chemokines CXCL1, CXCL2 and CXCL5 expression levels at 20 h post-wounding, more studies should be done to show if they have a role in wound healing process. However, there was a significant downregulation of CXCL1 and CXCL2 in non-wounded diabetic vs. normal LEC media (Supplementary Figure 2). Interestingly, the levels of altered secreted cytokines and chemokines were normalized in diabetic wounded LEC with miR-146a inhibition. Whereas upregulation of miR-146a in normal LECs led to altered levels of secreted cytokines and chemokines similar to the levels observed in untreated diabetic LECs (Figures 6 and 7).
The abovementioned data confirmed that the overexpression of miR-146 decreased NF-κB, its upstream regulators TRAF6 and IRAK1, and downstream secreted inflammatory cytokines in primary LECs. Further, knockdown of TRAF6 and IRAK1 expression using siRNAs in primary LECs led to downregulation of p-NF-κB (Supplementary Figure 3) and reduced secretion of proinflammatory cytokines and chemokines (Supplementary Figure 4). In silico analysis of miR-146a predicted targets with the presence of miR-146a binding site in TRAF6- and IRAK1– 3′-UTRs suggests that both TRAF6 and IRAK1 are targets of miR-146a [41]. These data confirmed the molecular mechanism by which miR-146a regulates NF-κB signaling pathways and its downstream secreted inflammatory cytokines via TRAF6 and IRAK1. Our results are consistent with previous studies showing that TRAF6 or IRAK1 knockdown inhibited pro-inflammatory cytokine secretion by downregulating p-NF-κB expression level [41,42].
Study of chemokine secretion in response to injury indicates that CXCL1, CXCL2, and CXCL5 may play a role in recruiting neutrophils to the site of injury [43–45]. Neutrophil recruitment is an important part of proper and timely wound healing. Neutrophil depletion resulted in substantially delayed corneal wound closure compared to WT; the majority of neutrophils in WT mice were adjacent to the wound edge [35, 46]. It is thus important to properly regulate these pro-inflammatory chemokines in order to maintain an effective healing environment [44].
Corneal epithelium is repaired by proliferation and migration of transit amplifying (TA) cells differentiated from LESCs. Proper corneal homeostasis is maintained by these small populations of stem cells. Inflammation in the limbus may have significant effect on stem cell niche maintenance. Dysregulation of the inflammatory response can cause alteration of the limbal niche, resulting in loss or dysfunction of limbal epithelial stem cells [47–49]. In addition, the release of epithelial inflammatory cytokines has significant effects on the underlying stromal layers of the cornea [50]. Failure to control the inflammatory response in the cornea can result in scarring and neovascularization, leading to loss of visual acuity [51]. Therefore, we speculate that the upregulation of miR-146a in diabetic cornea, especially in the limbus, could be a defense mechanism to prevent acute or diabetic chronic inflammation in stem cell enriched limbal region, however, leading to an expense of compromising wound healing in the diabetic corneal epithelium.
Our present study showed that the multiplicity of target genes enables miR-146a to regulate complex gene networks and influence their downstream interactions. Our data show that miR-146a acts as (1) a fine-tuning mechanism to prevent an overstimulation of acute inflammatory responses through its known targets, TRAF6 and IRAK1, upstream regulators of NF-κB, and (2) a regulator of epithelial wound healing by impacting downstream cytokine mediators. Therefore, its overexpression in the diabetic cornea may explain our novel finding of a blunted response of these corneas to inflammatory stimulators, including wounding, which may delay this process. Additionally, it may cause slow wound repair in diabetic corneas by cumulative effects of targeting EGFR and Notch signaling, by impairing migration and limbal stem cell differentiation, respectively [6, 12].
Since wound healing is a multistep process that involves consecutive and concerted changes in many genes, miR-based therapeutic approach with its advantage of targeting and normalizing several genes at once, may provide a more viable and effective strategy than individual gene- or protein-based therapeutics. In addition, their small size, ability to be easily modified with no immunogenicity effects make them a valid alternative to many other therapeutic approaches. However, the timing and duration of treatment should be considered to avoid off-targeting. Our previous translational therapeutic approach using miR-146a inhibitor restored normal-like wound healing and marker expressions in diabetic organ-cultured corneas apparently by lifting miR-146a-mediated cumulative inhibition of several genes such as EGFR and Notch, and inflammatory mediators controlling cytokine and chemokine release. Therefore, inhibition of miR-146a in diabetic cornea would normalize the expression of several diabetes-altered genes involved in the healing process.
5. Conclusion
In conclusion, our results provide insights into the regulatory network of miR-146a in control of multiple gene expression in different pathways to orchestrate and balance cellular functions and act in an immediate fine-tuning response to the external and/or internal stimuli. Such regulatory network may explain the impact of aberrant miR-146a expression in the diabetic cornea on wound healing and epithelial maintenance. Overall, for the first time, we have uncovered an important aspect in the process of healing and renewal of the corneal epithelium regulated by miR-146a and related to inflammation control.
Supplementary Material
Supplementary Figure 1. Fluorescence microscopy of Cy3-labeled scrambled miRNA transfected primary LECs. Primary LECs transfection with 50 nm Cy3-labeled scrambled miRNA using RNAiMax revealed that 80% to 90% of cells were transfected.
Supplementary Figure 2. Secretory chemokine levels at 6 and 20 h post-wounding in normal (N) and diabetic (DM) primary LECs. There were no significant changes of CXCL1, CXCL2, CXCL5 expression levels at 6 and 20 h post wounding in both N and DM LECs. However, the expression level of both CXCL1 and CXCL2 were significantly downregulated in DM vs. N LECs. Data are mean ± SEM, * p<.05, **p<.01 significant p values.
Supplementary Figure 3. Knockdown of TRAF6 and IRAK1 decreased p-NF-κB expression level. The primary LECs were transfected with siTRAF6, siIRAK1, or sicontrol for 72 h. The protein expression levels of TRAF6 (A) and IRAK1 (B) were significantly downregulated in comparison to sicontrol by western blot. (C) Transfection with siTRAF6 or siIRAK1 decreased p-NF-κB expression level compared to sicontrol in primary LECs by immunostaining. TRAF6, tumor necrosis factor receptor associated factor 6; IRAK1, interleukin-1-receptor-associated kinase 1; NF-κB, nuclear factor-κB.
Supplementary Figure 4. Luminex analysis of secretory cytokine/chemokine levels at 6h post-wounding in siTRAF6 or siIRAK1 transfected primary LECs media. There was a significant downregulation in IL-6 (C) and IL-8 (D) and CXCL1 (F) expression levels at 6 h post-wounding. There was also a downregulation of IL-1α (A), IL-1β (B), CXCL2 (F) and CXCL5 (G), which didn’t reach significance in primary LEC media after transfection with siTRAF6 or siIRAK1.
Acknowledgments
We would like to thank intern Steven T. Chun (UCLA) and research associate Cynthia Amador (Cedars-Sinai) for their technical assistance in fluorescent immunostaining and western blotting. Presented in part at the International Society for Eye Research (ISER) Congress, Belfast, Ireland, September 2018.
Funding:
This research was funded by the National Institute of Health (NIH), R01 grants EY025377, EY029829 (MS), EY013431, EY031377 (AVL), and grants from the Board of Governors Regenerative Medicine Institute.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, and Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010;363:147–55. [DOI] [PubMed] [Google Scholar]
- 2.Ordonez P and Di Girolamo N. Limbal epithelial stem cells: role of the niche microenvironment. Stem Cells 2012;30:100–7. [DOI] [PubMed] [Google Scholar]
- 3.Shah R, Amador C, Tormanen K, Ghiam S, Saghizadeh M, Arumugaswami V, et al. Systemic diseases and the cornea. Exp Eye Res 2021;204:108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saghizadeh M, Kramerov AA, Yu FS, Castro MG, and Ljubimov AV. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Invest Ophthalmol Vis Sci 2010;51:1970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saghizadeh M, Kramerov AA, Yaghoobzadeh Y, Hu J, Ljubimova JY, Black KL, et al. Adenovirus-driven overexpression of proteinases in organ-cultured normal human corneas leads to diabetic-like changes. Brain Res Bull 2010;81:262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funari VA, Winkler M, Brown J, Dimitrijevich SD, Ljubimov AV, and Saghizadeh M. Differentially expressed wound healing-related microRNAs in the human diabetic cornea. PLoS One 2013; 8:e84425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennecke J, Hipfner DR, Stark A, Russell RB, and Cohen SM. Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 2003;113:25–36. [DOI] [PubMed] [Google Scholar]
- 8.Decembrini S, Bressan D, Vignali R, Pitto L, Mariotti S, Rainaldi G, et al. MicroRNAs couple cell fate and developmental timing in retina. Proc Natl Acad Sci U S A 2009; 106:21179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys DT, Westman BJ, Martin DI, and Preiss T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc Natl Acad Sci U S A 2005;102:16961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu L, Fan J, and Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A 2006;103:4034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler MA, Dib C, Ljubimov AV, and Saghizadeh M. Targeting miR-146a to treat delayed wound healing in human diabetic organ-cultured corneas. PLoS One 2014;9: e114692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poe AJ, Kulkarni M, Leszczynska A, Tang J, Shah R, Jami-Alahmadi Y, et al. Integrated transcriptome and proteome analyses reveal the regulatory role of miR-146a in human limbal epithelium via Notch Signaling. Cells 2020;9:2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taganov KD, Boldin MP, Chang KJ, and Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006;103:12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie YF, Shu R, Jiang SY, Liu DL, Ni J, and Zhang XL. MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J Inflamm (Lond) 2013;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou J, Wang P, Lin L, Liu X, Ma F, An H, et al. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol 2009;183:2150–8. [DOI] [PubMed] [Google Scholar]
- 16.Gao M, Wang X, Zhang X, Ha T, Ma H, Liu L, et al. Attenuation of cardiac dysfunction in polymicrobial sepsis by microRNA-146a is mediated via targeting of IRAK1 and TRAF6 expression. J Immunol 2015;195:672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao L, Yan J, Feng D, Ye S, Yang T, Wei H, et al. Critical role of TLR4 on the microglia activation induced by maternal LPS exposure leading to ASD-like behavior of offspring. Front Cell Dev Biol 2021;9:634837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Z, Gong H, Li Y, Jie K, Ding C, Shao Q, et al. Upregulation of miR-146a contributes to the suppression of inflammatory responses in LPS-induced acute lung injury. Exp Lung Res 2013;39:275–82. [DOI] [PubMed] [Google Scholar]
- 19.Nahid MA, Satoh M, and Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol 2011;8:388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Zeng Z, Shen X, Wu Z, Dong Y, and Cheng JC. MicroRNA-146a-5p negatively regulates pro-Inflammatory cytokine secretion and cell activation in lipopolysaccharide stimulated human hepatic stellate cells through inhibition of Toll-like receptor 4 signaling pathways. Int J Mol Sci 2016;17:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim M, Goldstein MH, Tuli S, and Schultz GS. Growth factor, cytokine and protease interactions during corneal wound healing. Ocul Surf 2003;1:53–65. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SE, Mohan RR, Mohan RR, Ambrosio R Jr., Hong J, and Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res 2001;20:625–37. [DOI] [PubMed] [Google Scholar]
- 23.Bu Y, Shih KC, Kwok SS, Chan YK, Lo AC, Chan TCY, et al. Experimental modeling of cornea wound healing in diabetes: clinical applications and beyond. BMJ Open Diabetes Res Care 2019;7: e000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, and D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 2007;170:1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cowan C, Muraleedharan CK, O’Donnell JJ 3rd, Singh PK, Lum H, Kumar A, et al. MicroRNA-146 inhibits thrombin-induced NF-kappaB activation and subsequent inflammatory responses in human retinal endothelial cells. Invest Ophthalmol Vis Sci 2014;55:4944–51. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Li X, Li T, Wang L, Wu X, Liu J, et al. Multiple roles of microRNA-146a in immune responses and hepatocellular carcinoma. Oncol Lett 2019;18:5033–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seemann S, Zohles F, Lupp A. Comprehensive comparison of three different animal models for systemic inflammation. Journal of Biomedical Science 2017; 24:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catorce MV, Gevorkian G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Current Neuropharmacology 2016;14:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Liu Y, Li L, Su B, Yang L, Fan W, et al. Involvement of inflammation-related miR-155 and miR-146a in diabetic nephropathy: implications for glomerular endothelial injury. BMC Nephrol 2014;15:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang N, Chen FE, and Long ZW. Mechanism of microRNA-146a/Notch2 signaling regulating IL-6 in Graves Ophthalmopathy. Cell Physiol Biochem 2017;41:1285–1297. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Zhang W, Zhang L, Chen X, Liu F, Zhang J, et al. MiR-146a-5p mediates epithelial-mesenchymal transition of oesophageal squamous cell carcinoma via targeting Notch2. Br J Cancer 2016;115:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starczynowski DT, Kuchenbauer F, Wegrzyn J, Rouhi A, Petriv O, Hansen CL, et al. MicroRNA-146a disrupts hematopoietic differentiation and survival. Exp Hematol 2011; 39:167–178 e4. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Jiang Q, Jiang XQ, Li DQ, Jiang XC, Wu XB, et al. MiR-146a promoted breast cancer proliferation and invasion by regulating NM23-H1. J Biochem 2020;167:41–48. [DOI] [PubMed] [Google Scholar]
- 34.King JK, Ung NM, Paing MH, Contreras JR, Alberti MO, Fernando TR, et al. Regulation of marginal zone B-Cell differentiation by microRNA-146a. Front Immunol 2016;7: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavker RM, Kaplan N, McMahon KM, Calvert AE, Henrich SE, Onay UV, Lu KQ, Peng H, Thaxton CS. Synthetic high-density lipoprotein nanoparticles: Good things in small packages. Ocul Surf. 2021;21:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang P, Muraleedharan CK, and Xu S. Intraocular delivery of miR-146 inhibits diabetes-induced retinal functional defects in diabetic rat model. Invest Ophthalmol Vis Sci 2017;58:1646–1655. [DOI] [PubMed] [Google Scholar]
- 37.Shahriar A, Ghaleh-Aziz Shiva G, Ghader B, Farhad J, Hosein A, and Parsa H. The dual role of miR-146a in metastasis and disease progression. Biomed Pharmacother 2020; 126:110099. [DOI] [PubMed] [Google Scholar]
- 38.Runtsch MC, Nelson MC, Lee SH, Voth W, Alexander M, Hu R, et al. Anti-inflammatory microRNA-146a protects mice from diet-induced metabolic disease. PLoS Genet 2019; 15:e1007970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li G, Luna C, Qiu J, Epstein DL, and Gonzalez P. Modulation of inflammatory markers by miR-146a during replicative senescence in trabecular meshwork cells. Invest Ophthalmol Vis Sci 2010;51:2976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Y, Chu A, Feng Y, Chen L, Shao Y, Luo Q, et al. MicroRNA-146a: A Comprehensive Indicator of Inflammation and Oxidative Stress Status Induced in the Brain of Chronic T2DM Rats. Front Pharmacol 2018;9:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou J, Deng Q, Deng X, Zhong W, Liu S, Zhong Z. MicroRNA-146a-5p alleviates lipopolysaccharide-induced NLRP3 inflammasome injury and pro-inflammatory cytokine production via the regulation of TRAF6 and IRAK1 in human umbilical vein endothelial cells (HUVECs). Ann Transl Med 2021;9:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saba R, Sorensen DL, Booth SA. MicroRNA-146a: A Dominant, Negative Regulator of the Innate Immune Response. Front Immunol 2014;5:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spandau UH, Toksoy A, Verhaart S, Gillitzer R, and Kruse FE. High expression of chemokines Gro-alpha (CXCL-1), IL-8 (CXCL-8), and MCP-1 (CCL-2) in inflamed human corneas in vivo. Arch Ophthalmol 2003;121:825–31. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Magadi S, Li Z, Smith CW, and Burns AR. IL-20 promotes epithelial healing of the injured mouse cornea. Exp Eye Res 2017;154:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridiandries A, Tan JTM, and Bursill CA. The role of chemokines in wound healing. Int J Mol Sci 2018;19:3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrazzo G, Bellner L, Halilovic A, Li Volti G, Drago F, Dunn MW, et al. The role of neutrophils in corneal wound healing in HO-2 null mice. PLoS One 2011;6:e21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowell CS and Radtke F. Corneal epithelial stem cells and their niche at a glance. J Cell Sci 2017;130:1021–1025. [DOI] [PubMed] [Google Scholar]
- 48.Tseng SC, He H, Zhang S, and Chen SY. Niche regulation of limbal epithelial stem cells: Relationship between Inflammation and Regeneration. Ocul Surf 2016;14:100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Notara M, Lentzsch A, Coroneo M, and Cursiefen C. The Role of limbal epithelial stem cells in regulating corneal (lymph) angiogenic privilege and the micromilieu of the limbal niche following UV exposure. Stem Cells Int 2018;2018:8620172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nowell CS, Odermatt PD, Azzolin L, Hohnel S, Wagner EF, Fantner GE, et al. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat Cell Biol 2016;18:168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mobaraki M, Abbasi R, Omidian Vandchali S, Ghaffari M, Moztarzadeh F, and Mozafari M. Corneal repair and regeneration: Current concepts and future directions. Front Bioeng Biotechnol 2019;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Fluorescence microscopy of Cy3-labeled scrambled miRNA transfected primary LECs. Primary LECs transfection with 50 nm Cy3-labeled scrambled miRNA using RNAiMax revealed that 80% to 90% of cells were transfected.
Supplementary Figure 2. Secretory chemokine levels at 6 and 20 h post-wounding in normal (N) and diabetic (DM) primary LECs. There were no significant changes of CXCL1, CXCL2, CXCL5 expression levels at 6 and 20 h post wounding in both N and DM LECs. However, the expression level of both CXCL1 and CXCL2 were significantly downregulated in DM vs. N LECs. Data are mean ± SEM, * p<.05, **p<.01 significant p values.
Supplementary Figure 3. Knockdown of TRAF6 and IRAK1 decreased p-NF-κB expression level. The primary LECs were transfected with siTRAF6, siIRAK1, or sicontrol for 72 h. The protein expression levels of TRAF6 (A) and IRAK1 (B) were significantly downregulated in comparison to sicontrol by western blot. (C) Transfection with siTRAF6 or siIRAK1 decreased p-NF-κB expression level compared to sicontrol in primary LECs by immunostaining. TRAF6, tumor necrosis factor receptor associated factor 6; IRAK1, interleukin-1-receptor-associated kinase 1; NF-κB, nuclear factor-κB.
Supplementary Figure 4. Luminex analysis of secretory cytokine/chemokine levels at 6h post-wounding in siTRAF6 or siIRAK1 transfected primary LECs media. There was a significant downregulation in IL-6 (C) and IL-8 (D) and CXCL1 (F) expression levels at 6 h post-wounding. There was also a downregulation of IL-1α (A), IL-1β (B), CXCL2 (F) and CXCL5 (G), which didn’t reach significance in primary LEC media after transfection with siTRAF6 or siIRAK1.


