Abstract
Dopaminergic medication is widely used to alleviate motor symptoms of Parkinson’s disease, but these medications also impact cognition with significant variability across patients. It is hypothesized that dopaminergic medication impacts cognition and working memory in Parkinson’s disease by modulating frontoparietal-basal ganglia cognitive control circuits, but little is known about the underlying causal signalling mechanisms and their relation to individual differences in response to dopaminergic medication. Here we use a novel state-space computational model with ultra-fast (490 ms resolution) functional MRI to investigate dynamic causal signalling in frontoparietal-basal ganglia circuits associated with working memory in 44 Parkinson’s disease patients ON and OFF dopaminergic medication, as well as matched 36 healthy controls.
Our analysis revealed aberrant causal signalling in frontoparietal-basal ganglia circuits in Parkinson’s disease patients OFF medication. Importantly, aberrant signalling was normalized by dopaminergic medication and a novel quantitative distance measure predicted individual differences in cognitive change associated with medication in Parkinson’s disease patients. These findings were specific to causal signalling measures, as no such effects were detected with conventional non-causal connectivity measures. Our analysis also identified a specific frontoparietal causal signalling pathway from right middle frontal gyrus to right posterior parietal cortex that is impaired in Parkinson’s disease. Unlike in healthy controls, the strength of causal interactions in this pathway did not increase with working memory load and the strength of load-dependent causal weights was not related to individual differences in working memory task performance in Parkinson’s disease patients OFF medication. However, dopaminergic medication in Parkinson’s disease patients reinstated the relation with working memory performance.
Our findings provide new insights into aberrant causal brain circuit dynamics during working memory and identify mechanisms by which dopaminergic medication normalizes cognitive control circuits.
Keywords: cognitive control, dopamine, working memory, dynamical, state-space models
Cai et al. report that in patients with Parkinson’s disease, aberrant causal signalling in working memory circuits is normalized by dopaminergic medication and is predictive of cognitive response to medication. The findings reveal neurobiological mechanisms by which dopaminergic medication improves cognitive control.
Introduction
Cognitive impairment is a pervasive non-motor symptom of Parkinson’s disease with over 40% of all non-demented Parkinson’s disease patients meeting criteria for mild cognitive impairment.1 There are no effective treatments for cognitive deficits in Parkinson’s disease,2 which stands in stark contrast to an armamentarium of dopaminergic medications that provide effective relief for Parkinson’s disease motor symptoms.3 Almost all Parkinson’s disease patients take dopaminergic medications to improve motor symptoms associated with the disorder,4 and these pharmacological treatments have also been shown to impact working memory and executive functions,5–7 which are reliant on distributed frontoparietal-basal ganglia regions influenced by dopaminergic signalling.8–10 However, little is known about underlying causal signalling mechanisms and their relation to individual cognitive differences in dopaminergic medication. Here, we use novel computational methods and a system neuroscience approach to investigate aberrant dynamic causal circuits in Parkinson’s disease and to examine the effect of dopamine on brain circuit dynamics in individual patients ON and OFF dopaminergic medication.
Lewy body neuronal inclusion is the pathological hallmark of Parkinson’s disease, with subsequent degeneration of midbrain dopaminergic neurons and dopamine depletion in the basal ganglia.11 Optimal dopamine signalling is critical for normal functioning of frontoparietal-basal ganglia circuits involved in working memory.12–15 While some studies have suggested that dopaminergic mediation used to treat the motor symptoms of Parkinson’s disease has no beneficial or even have detrimental effect on cognitive functions,16–20 others point to improved treatment response in both motoric and cognitive domains.21–25 Such inconsistent medication effects have also been observed within the working memory domain.5–7,24 Notably studies have revealed dissociable effects of dopaminergic medication on different cognitive tasks in the same cohorts of Parkinson’s disease patients,26,27 suggesting that the cognitive effects of dopaminergic medication may depend on several factors including individual differences in functioning of cortical-subcortical circuits taxed by specific cognitive demands.13 We address this possibility here by using computational modelling of dynamic causal signalling in frontoparietal-basal ganglia systems important for working memory and examining how dopaminergic medication in Parkinson’s disease affects frontoparietal-basal ganglia dynamics and its relation to working memory performance and cognitive profiles.
Working memory, a component of executive function that refers to the ability to maintain and manipulate information in the absence of sensory input,28–30 is one of most prominent cognitive domains of impairment in Parkinson’s disease patients.31 Neuroimaging studies in Parkinson’s disease patients have reported contradictory findings regarding activation profiles associated with working memory with evidence for reduced activation in Parkinson’s disease32,33 or compensatory activation in frontal and parietal cortices as well as basal ganglia.34–36 Although dopaminergic medication in Parkinson’s disease has been shown to modulate activation in prefrontal cortex and striatum during working memory,37,38 a recent meta-analysis of 22 studies investigating working memory in Parkinson’s disease failed to pinpoint a specific neural correlate associated with executive or working memory dysfunction in Parkinson’s disease.39 The authors argued that the lack of findings might arise from methodological inconsistencies and a lack of quantitatively rigorous analysis of functional brain circuits. Crucially, as most neuroimaging studies of Parkinson’s disease to date have focused on identifying abnormal responses in regional brain activation, little is known about how aberrant context-dependent causal interactions in cognitive control circuits underlying working memory and executive function lead to cognitive dysfunction.40 We address this gap by examining how dopaminergic medication influences causal signalling in cognitive control circuits and determining whether global dopaminergic-induced changes in these circuits improves cognition.
Here, we investigate causal dynamic circuit mechanisms, involving frontoparietal-basal ganglia systems that are consistently implicated in working memory, in two groups of individuals: (i) Parkinson’s disease patients ON (PD-ON) and OFF (PD-OFF) dopaminergic medication; and (ii) age-, sex-, education- and head motion-matched healthy controls. We probed dynamic causal interactions between brain regions using multivariate dynamic state-space systems identification (MDSI).41–43 MDSI uses a state-space model for estimating context-dependent dynamic causal interactions in latent neuronal signals after taking into account inter-regional variations in haemodynamic response. A particular advantage of MDSI is that it does not require testing a large number of prespecified models, which is especially problematic as the number of the models to be tested increases exponentially with the number of nodes.43 This approach not only enabled us to probe large-scale causal circuits associated with working memory, but also allowed us to determine how Parkinson’s disease and dopaminergic medication asymmetrically affects causal circuits.
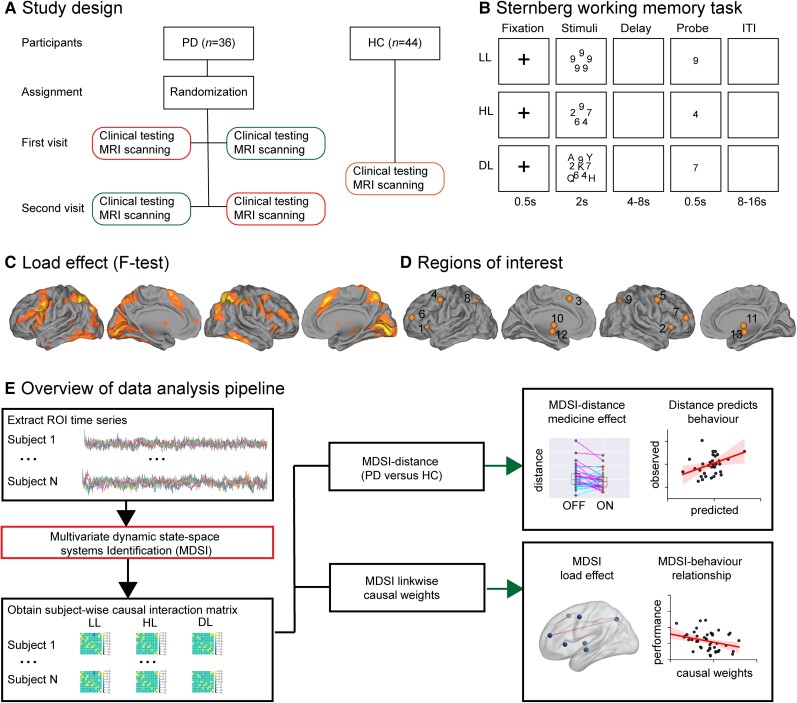
Eighty Parkinson’s disease patients and healthy control participants completed a Sternberg working memory task during functional MRI scanning. Each Parkinson’s disease participant completed cognitive testing and MRI scanning in both ON and OFF medication sessions in a within-subject design so that each acted as his/her own control (Fig. 1A). Participants viewed a set of stimuli for 2 s (Fig. 1B), and following a jittered delay period varying between 4 and 8 s, were presented with a probe to which they indicated whether the probe was part of the stimulus set they had viewed earlier. Working memory load was modulated across three levels: low load (LL), high load (HL) and distractor load (DL). In the low load condition, stimuli consisted of a set of five identical digits; in the high load condition, stimuli consisted of five different digits; and in the distractor load condition, stimuli included different digits and task-irrelevant letters. Each Parkinson’s disease participant also completed the Symbol Digit Modalities Test (SDMT), a brief test of working memory, attention switching, and processing speed that has been widely used to probe general cognitive functioning in Parkinson’s disease,45–47 in both the ON and OFF medication states. This design allowed us to probe dopaminergic effects of medication on causal brain circuit dynamics and their relation to both task performance and standardized measures of cognition.
Figure 1.
Task design and data analysis pipeline. (A) Within-subject study design. Each Parkinson’s disease (PD) participant has two visits, one while ON medication (PD-ON; green) and the other while OFF medication (PD-OFF; red). During each visit, Parkinson’s disease participants underwent clinical (MDS-UPDRS and SDMT) testing and MRI scanning with performance on an event-related Sternberg working memory functional MRI task. (B) Illustration of the low-load (LL), high-load (HL) and distractor-load (DL) Sternberg working memory task conditions. (C) ANOVA analysis amongst healthy controls (HC) and PD-OFF was used to uncover load effects (LL versus HL versus DL) and identify regions of interest (ROIs). Statistical map was thresholded at P < 0.001 FWE corrected. (D) Regions of interest used in the MDSI analysis: (1) lAI, (2) rAI, (3) DMPFC, (4) lPM, (5) rPM, (6) lMFG, (7) rMFG, (8) lPPC, (9) rPPC, (10) lPUT/GP and (11) rPUT/GP. (12) lSTN and (13) rSTN coordinates were determined from a previous study.44 (E) Overview of data analysis pipeline. We first extracted timeseries from each region of interest and applied MDSI to determine dynamic causal interactions from each participant in the LL, HL and DL task conditions. MDSI-derived causal influences were then used to investigate: (i) dissimilarity in network-level causal signalling between Parkinson’s disease and healthy controls, and whether this dissimilarity was reduced by dopaminergic medication; (ii) whether dopaminergic medication-related changes in network dissimilarity is related to individual differences in cognition; (iii) links that showed load-dependent effects on causal signalling; and (iv) the relationship between causal signalling and working memory task performance. AI = anterior insula; DMPFC = dorsomedial prefrontal cortex; MFG = middle frontal gyrus; l = left; PM = premotor cortex; PPC = posterior parietal cortex; PUT/GP = putamen/globus pallidus; r = right; STN = subthalamic nuclei.
Figure 1E provides an overview of our data analysis pipeline. We first identified frontoparietal-basal ganglia regions involved in the Sternberg working memory task (Fig. 1C and D) and used MDSI to compute directed causal interactions between these regions of interest. Second, we evaluated network-level causal signalling mechanisms, their modulation by dopaminergic medication in Parkinson’s disease participants, and relation to standardized measures of cognitive functioning. We computed a distance metric (Fig. 2A) to quantify the degree of similarity between healthy controls and each Parkinson’s disease participant ON and OFF dopaminergic medication, and tested the hypothesis that dopaminergic medication reduces dissimilarity in dynamic causal interactions within frontoparietal-basal ganglia circuits in Parkinson’s disease. We further hypothesized that changes in similarity of dynamic causal interactions would predict dopamine-related changes in general cognitive function. Finally, we identified specific causal links that showed load-dependent deficits in Parkinson’s disease compared to healthy control participants, and investigated their relation to dopaminergic medication and behavioural performance on the in-scanner Sternberg task. We hypothesized that frontoparietal and prefrontal-basal ganglia links would show impairments in Parkinson’s disease and that the degree of impairment would predict task deficits. Our findings described below demonstrate that dynamic causal interactions involving distributed brain regions important for working memory are aberrant in Parkinson’s disease and that dopaminergic medication restores the function of frontoparietal-basal ganglia circuitry in these patients.
Figure 2.
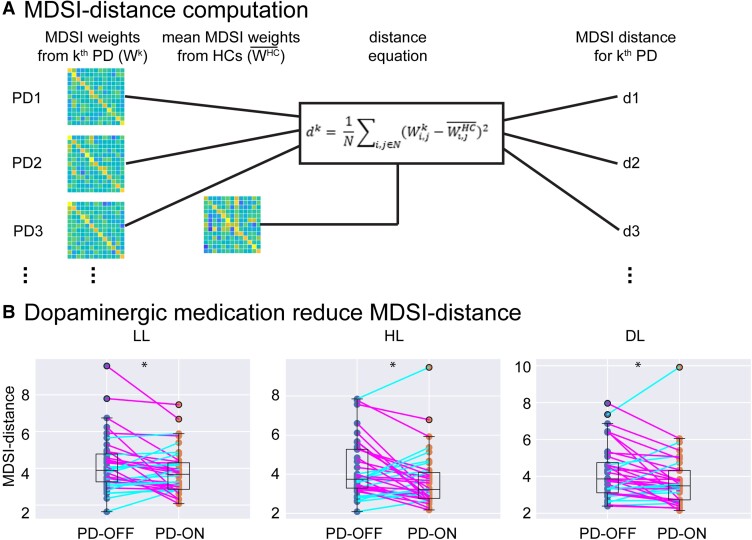
Dopaminergic medication reduces MDSI distance in Parkinson’s disease. (A) Schematic illustration of the algorithm used to compute MDSI-based dissimilarity measure (distance) in each Parkinson’s disease participant. The distance (d) is defined by the sum of the square of MDSI weight difference between each Parkinson’s disease participant and the mean of the healthy controls group. Note that dk is the distance metric for kth Parkinson’s disease participants; N is the total number of non-diagonal edge (i,j) in MDSI weight matrix per condition; is the MDSI weight on edge (i,j) for kth Parkinson’s disease participant; is the mean MDSI weight on edge (i,j) for all healthy controls. (B) Dopaminergic medication reduces MDSI-based distance, which quantifies the dissimilarity in network-level causal signalling between each Parkinson’s disease participant and the healthy controls group, in each task condition (i.e. LL, HL, DL) (all P-values < 0.05). *P < 0.05.
Materials and methods
Participants
All participants were enrolled in the Stanford Alzheimer’s Disease Research Center. Inclusion criteria for healthy controls included age ≥60 years; no neurological, psychiatric or medical conditions causing cognitive impairment determined through history and neurological examination; and cognitively normal as determined by clinical consensus after formal testing that included the National Alzheimer’s Coordinating Center Uniform Data Set (version 3) neuropsychological battery. Parkinson’s disease was determined by UK Brain Bank criteria48 after a comprehensive neurological screening exam and the Movement Disorders Society-Unified Parkinson’s disease Rating Scale motor assessment (MDS-UPDRS part III)49 both OFF and ON dopaminergic medications. Parkinson’s disease participants completed formal neuropsychological testing with the Uniform Data Set version 3 battery, which occurred within 6 months of the functional MRI session. A total of 48 healthy controls completed the Sternberg task in one functional MRI session and 39 Parkinson’s disease participants completed the Sternberg task in two separate ON and OFF functional MRI sessions in random order. Two participants (healthy controls) were excluded for excessive motion [i.e. mean motion >1.5 standard deviations (SD) above the interquartile range (IQR)], three participants (two healthy controls, one Parkinson’s disease) were excluded for poor task performance (i.e. <65% accuracy), and two participants (two Parkinson’s disease) were excluded because MDSI parameter estimation did not converge. Thus, a total of 44 healthy controls (71 ± 6 years old; 25 female/19 male; Table 1) and 36 Parkinson’s disease participants (69 ± 7 years old; 21 female/15 male; 29 Parkinson’s disease with no cognitive impairment, seven Parkinson’s disease with mild cognitive impairment) were included in the final analyses. The sample size was determined by power analysis (Supplementary material).
Table 1.
Demographic information and behavioural performance
| CTL | PD | CTL versus PD-OFF | PD-OFF versus PD-ON | ||||
|---|---|---|---|---|---|---|---|
| PD-OFF | PD-ON | t-/chi-stats | P-value | t-/chi-stats | P-value | ||
| Sample size | 44 | 36 | |||||
| Age (years) | 71 ± 6 | 69 ± 7 | 1.81 | 0.07 | |||
| Sex (f/m) | 25/19 | 21/15 | 0 | 1 | |||
| Education (years) | 17 ± 2 | 17 ± 2 | 0.66 | 0.51 | |||
| MDS-UPDRS III | 35 ± 11 | 22 ± 8 | 9.31 | 5.34 × 10−11 | |||
| SDMT | 51 ± 11 | 45 ± 11 | 46 ± 13 | 2.04 | 0.04 | 0.71 | 0.49 |
| Max Disp (mm) | 2.29 ± 1.29 | 2.39 ± 1.88 | 2.15 ± 0.90 | 0.27 | 0.79 | 0.82 | 0.42 |
| Max FD (mm) | 1.06 ± 1.06 | 1.06 ± 0.95 | 0.86 ± 0.42 | 0.002 | 0.99 | 1.41 | 0.17 |
| Stern LL ACC (%) | 97 ± 4 | 97 ± 4 | 97 ± 6 | 0.39 | 0.7 | 0.4 | 0.69 |
| Stern HL ACC (%) | 92 ± 5 | 94 ± 7 | 91 ± 9 | 2.21 | 0.03 | 2.08 | 0.04 |
| Stern DL ACC (%) | 93 ± 9 | 93 ± 8 | 93 ± 9 | 0.03 | 0.98 | 0.2 | 0.85 |
| Stern LL RT (ms) | 998 ± 229 | 1017 ± 219 | 1054 ± 272 | 0.38 | 0.71 | 1.21 | 0.23 |
| Stern HL RT (ms) | 1282 ± 243 | 1292 ± 280 | 1344 ± 327 | 0.17 | 0.87 | 1.41 | 0.17 |
| Stern DL RT (ms) | 1290 ± 253 | 1326 ± 312 | 1363 ± 352 | 0.55 | 0.58 | 0.93 | 0.36 |
ACC = anterior cingulate cortex; CTL = healthy controls; Disp = displacement; FD = frame-wise displacement; Max = maximum; MDS-UPDS = Movement Disorder Society–Unified Parkinson’s Disease Rating Scale; PD = Parkinson’s disease; Stern = Sternberg.
All participants provided written consent and the Stanford University Institutional Review Board approved all study protocols.
Symbol Digit Modality Test
The SDMT is a sensitive neuropsychological test that assesses working memory, attention switching and processing speed, and has been shown to be sensitive to cognitive changes in Parkinson’s disease.45–47 Each participant in the Parkinson’s disease group completed the written SDMT during the ON and OFF medication states.
Sternberg task
Participants performed a modified Sternberg working memory task during functional MRI (Fig. 1B). Each trial consisted of either low-load (LL), high-load (HL) or distractor-load (DL) working memory conditions. Accuracy and mean reaction time (RT) were recorded for each trial. Each scan included four runs, with each consisting of six LL, six HL and six DL working memory trials randomly intermixed. The stimulus presentations were implemented using E-Prime (v2.0; Psychology Software Tools, Pittsburgh, PA; 2002) and projected at the centre of the screen. Prior to each functional MRI session, participants completed a practice session of the task.
Data acquisition
The functional MRI images were collected using a 3 T scanner. A total of 790 functional images were acquired using multiband echo-planar imaging with the following parameters: 42 slices aligned with the anterior-posterior commissure line, with interleaved order, repetition time 490 ms, echo time = 30 ms, multi-band factor = 6, flip angle 45°, field of view = 222 × 222 mm, matrix = 74 × 74, 3 mm slice thickness and voxel size = 3 × 3 × 3 mm. The first 12 time points were removed to allow for signal equilibration, leaving 778 time points for each participant. Each participant’s T1-weighted anatomical scan had been acquired using a magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence (256 slices with a 176 × 256 matrix; voxel size 1.00 × 0.977 × 0.977smm3).
Functional MRI preprocessing
A standard preprocessing pipeline was implemented using SPM12 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), as well as in-house programs in MATLAB (MathWorks). Details described in the Supplementary material.
General linear model analysis
For each task and subject, a general linear model (GLM) was used, which included regressors of interest for LL, HL and DL conditions and six nuisance regressors for head motion. Both canonical haemodynamic response function (HRF) and its time derivative were used to convolve the stimulus function to form the regressors. The significant activation patterns were determined using a voxel-wise height threshold of P < 0.01 and an extent threshold of P < 0.01 with family-wise error (FWE) correction using a non-stationary suprathreshold cluster-size approach based on Monte Carlo simulations.50 Brain activation maps associated with specific task conditions and Parkinson’s disease-related differences are shown in Supplementary Fig. 3.
Working memory network
We built a network model involving frontoparietal and basal ganglia regions most consistently implicated in working memory, including middle frontal gyrus (MFG)/dorsolateral prefrontal cortex (DLPFC), posterior parietal cortex (PPC), premotor cortex (PMC), dorsomedial prefrontal cortex (DMPFC), bilateral anterior insula (AI) and putamen/globus pallidus (PUT/GP).40,51–53 The precise location of network nodes was based on task activation peaks that overlapped with these brain regions, as demarcated by the Brainnetome atlas.54 Peaks were determined using combined task-functional MRI data from both healthy controls and Parkinson’s disease groups, and a one-way ANOVA with a factor of task condition (LL, HL, DL) (Fig. 1C and D). Bilateral subthalamus nuclei (STN), whose coordinates were determined by a high resolution structural MRI study,44 were also included in the network given its critical role in cognitive impairment55–58 and stimulation-based treatment in Parkinson’s disease patients,59–62 as well as its role in dopaminergic modulation of brain circuits.63 Both factors are particularly relevant in the context of our investigation of the effects of dopamine modulation on causal signalling among brain areas involved in working memory. Moreover, our Sternberg working memory task included a distractor load condition that requires greater inhibitory control processes, which are known to engage the STN as demonstrated in animal64–67 and human studies.68–71 Finally, the STN is specifically involved in high-conflict decision-making, which is necessary for accurate performance on the Sternberg task, through its role in the hyperdirect pathway involving the prefrontal cortex.55–58
Mean time series were extracted from each of the resulting 13 working memory network nodes. A multiple linear regression approach with six realignment parameters (three translations and three rotations) was applied to reduce head motion-related artefacts, and the resulting time series were further linearly detrended, normalized and high-pass filtered (>0.008 Hz).
MDSI model for estimating causal interactions from functional MRI data
MDSI estimates context-dependent causal interactions between multiple brain regions in latent quasineuronal state while accounting for variations in haemodynamic responses in these regions.43 Analysis of MDSI, its modulation by task, group and medication, and relation to behaviour are described in the Supplementary material.
MDSI distance analysis
To evaluate the effect of dopamine treatment on global causal mechanisms in Parkinson’s disease, we developed a distance metric to quantify the dissimilarity in dynamic causal interactions between Parkinson’s disease ON and OFF medication condition in comparison to healthy controls. The distance (d) is defined by the sum of square of causal weight difference between each Parkinson’s disease participant and mean of the healthy control group. The absolute geometric distance allows us to quantify overall divergence of each Parkinson’s disease participant from healthy controls across all the network connections. The algorithm used to compute the MDSI distance for each Parkinson’s disease participant is illustrated in Fig. 2A. Paired t-tests were used to examine whether the distance of between PD-OFF and healthy controls is significantly different from the distance between PD-ON and healthy controls.
MDSI distance predicts cognitive function
To examine whether MDSI distance could account for individual differences in the effect of dopaminergic medication on cognition in Parkinson’s disease, we conducted multivariate regression analysis using linear support vector regression (SVR). The MDSI distances in each task condition, LL, HL and DL, were used as features to predict the difference in SDMT scores assessed during ON and OFF states. One Parkinson’s disease participant did not complete the SDMT test and two outliers were identified using a 2.5 SD of the group mean cut-off, leaving data from 33 Parkinson’s disease participants for this analysis. The model was evaluated using the leave-one-out cross validation. Each time, one data-point was selected as a test set and the rest of the data were used as a training set. The training set was then used to train a SVR model, which was then applied to the test set for classification. This procedure was repeated n times with each data-point used exactly once as a test set. Pearson’s correlations were used to evaluate prediction performance.
Psychophysiological analysis
We used general psychophysiological interaction (gPPI)72 to estimate non-causal task modulated connectivity, and details are described in the Supplementary material.
Data availability
All data used in this study will be shared upon request from qualified investigators.
Results
Cognitive impairments in Parkinson’s disease participants
Parkinson’s disease participants evaluated OFF dopaminergic medication had significantly worse SDMT scores than healthy controls [t(71) = 2.04, P = 0.04, Cohen’s d = 0.46, Table 1]. No significant difference was found between healthy controls and PD-ON groups (P = 0.11). We then examined the effect of dopaminergic medication on cognition (Table 1). There was no significant difference between PD-OFF and PD-ON in SDMT (P > 0.4).
Working memory performance in PD-OFF patients versus healthy controls
Both healthy control and Parkinson’s disease participants showed high performance on the Sternberg task, with average accuracies over 90% in all conditions (i.e. LL, HL, DL) (Table 1). A two-way analysis of variance (ANOVA) with factors group (healthy controls, PD-OFF) and condition (LL, HL and DL) revealed a significant main effect of condition [F(2,156) = 10.39, P = 5.78 × 10−05, Cohen’s f = 0.12] such that accuracies were significantly higher in LL compared to HL [t(79) = 4.97, P = 3.80 × 10−06, Cohen’s d = 0.56] and DL [t(79) = 3.45, P = 0.0008, Cohen’s d = 0.39] conditions (Table 1); accuracies in HL and DL conditions were equivalent [t (79) = 0.32, P = 0.75, Cohen’s d = 0.04]. There was no significant interaction between group and condition [F(2,156) = 1.74, P = 0.18, Cohen’s f = 0.02] and no significant main effect of group [F(1,78) = 1.38, P = 0.24, Cohen’s f = 0.02].
A similar ANOVA on reaction time (RT) on correct trials revealed a significant main effect of condition [F(2,156) = 179.85, P = 2 × 10−16, Cohen’s f = 1.52] such that responses were significantly faster in the LL in comparison to HL [t(79) = 14.66, P = 2.2 × 10−16, Cohen’s d = 1.64] and DL [t(79) = 14.53, P = 2.2 × 10−16, Cohen’s d = 1.62] conditions (Table 1), but there was no difference in reaction time between HL and DL conditions [t(79) = 1.73, P = 0.09, Cohen’s d = 0.19]. There was no significant interaction between group and condition [F(2,156) = 0.28, P = 0.76, Cohen’s f = 0.06] and no significant main effect of group [F(1,78) = 0.16, P = 0.69, Cohen’s f = 0.05].
Working memory performance in PD-OFF versus PD-ON participants
A two-way repeated measures ANOVA with factors Parkinson’s disease medication state (PD-OFF, PD-ON) and task condition (LL, HL and DL) revealed a significant main effect of task condition [F(2,70) = 11.09, P = 6.6 × 10−05, Cohen’s f = 0.56] such that accuracies were significantly higher in the LL compared to HL [t(71) = 4.61, P = 1.76 × 10−05, Cohen’s d = 0.54] and DL [t(71) = 3.75, P = 0.0004, Cohen’s d = 0.44] conditions; there was no significant difference in accuracy between HL and DL conditions [t(71) = 0.38, P = 0.70, Cohen’s d = 0.05]. There was no significant interaction between Parkinson’s disease medication state and task condition [F(2,70) = 2.60, P = 0.08, Cohen’s f = 0.17] and no significant main effect of medication state [F(1,35) = 1.48, P = 0.23, Cohen’s f = 0.16].
A similar repeated measures ANOVA was performed with reaction time on correct trials. This analysis revealed a significant main effect of task condition [F(2,70) = 79.4, P = 2 × 10−16, Cohen’s f = 1.51] such that responses were faster for LL compared to HL [t(71) = 11.82, P = 2.2 × 10−16, Cohen’s d = 1.39] and DL [t(71) = 12.37, P = 2.2 × 10−16, Cohen’s d = 1.46] conditions, as well as for HL compared to the DL condition [t(71) = 2.07, P = 0.04, Cohen’s d = 0.24]. There was no significant interaction between Parkinson’s disease medication state and task condition [F(2,70) = 0.24, P = 0.79, Cohen’s f = 0.08] and no significant main effect of medication state [F(1,35) = 1.67, P = 0.21, Cohen’s f = 0.22].
Dynamic causal interactions in the Sternberg working memory task
We first identified frontoparietal-basal ganglia regions of interest that showed task-load effects in a combined group of healthy controls and PD-OFF participants (Fig. 1C), all of which have been widely implicated in a range of working memory tasks (Fig. 1D and Table 2). To investigate condition-specific causal interactions between all nodes of the frontoparietal-basal ganglia circuit in each participant, we applied MDSI and the strength of causal interactions was estimated in the latent neuronal space across all nodes without having to test multiple models, allowing us to determine a directed asymmetric 13 × 13 connectivity matrix. Our analysis revealed multiple significant directed causal interactions between frontoparietal-basal ganglia network during LL, HL, DL conditions (P = 0.05, FDR corrected) in healthy controls, PD-OFF, and PD-ON groups (Supplementary Fig. 1).
Table 2.
Working memory regions of interest used in the MDSI analysis
| Index | Regions of interest | x | y | z |
|---|---|---|---|---|
| 1 | Right PM | 50 | −2 | 48 |
| 2 | Left PM | −50 | −2 | 48 |
| 3 | Right PPC | 26 | −64 | 44 |
| 4 | Left PPC | −26 | −64 | 44 |
| 5 | Midline DMPFC | −1 | 16 | 50 |
| 6 | Right MFG | 38 | 47 | 17 |
| 7 | Left MFG | −38 | 47 | 17 |
| 8 | Right AI | −32 | 22 | 2 |
| 9 | Left AI | 32 | 22 | 2 |
| 10 | Right PUT | 27 | −11 | 4 |
| 11 | Left PUT | −27 | −11 | 4 |
| 12 | Right STN | 11 | −12.5 | −7 |
| 13 | Left STN | −8 | −13.5 | −7 |
AI = anterior insula; DMPFC = dorsomedial prefrontal cortex; PM = premotor cortex; PUT = putamen.
Dopaminergic medication improves network level causal interactions in Parkinson’s disease
To examine whether dopaminergic medication improves causal signalling mechanisms in Parkinson’s disease at the network-level, we computed a distance measure to quantify the extent of dissimilarity in dynamic causal interactions among all the nodes in the frontoparietal-basal ganglia network in each Parkinson’s disease medication state (OFF or ON) relative to healthy controls. Briefly, distance metric was defined as the sum of square of differences in causal weights across all region of interest pairs between each Parkinson’s disease participant and the healthy control group (Fig. 2A). We conducted a two-way ANOVA with factors medication (OFF, ON) and task condition (LL, HL and DL). Although there was no significant interaction between medication state and task condition [F(2,70) = 0.33, P = 0.72, Cohen’s f = 0.10] and no significant main effect of task condition [F(2,70) = 0.09, P = 0.92, Cohen’s f = 0.05], there was a significant main effect of medication state [F(1,70) = 7.45, P < 0.01, Cohen’s f = 0.46]. Post hoc analysis revealed that distance between PD-OFF and healthy controls was significantly greater than that between PD-ON and healthy controls in LL (t(35) = 2.04, P = 0.04, Cohen’s d = 0.34], HL [t(35) = 2.44, P = 0.02, Cohen’s d = 0.41] and DL [t(35) = 2.26, P = 0.03, Cohen’s d = 0.38, Fig. 2B] conditions. These results demonstrate that dopaminergic medication improves network-level causal interactions in the Parkinson’s disease group.
To further test whether the dopaminergic medication effect on network-level signalling in the Parkinson’s disease group is specific to causal interactions, we conducted the same analysis in non-causal task-modulated connectivity estimated using gPPI. We used the same distance metric to estimate dissimilarity in network level gPPI weights between Parkinson’s disease and healthy controls in ON and OFF sessions. There was no significant difference in distance metric in any task condition between the ON and OFF sessions (P-values > 0.3), indicating that dopaminergic medication specifically improves casual interactions rather than connectivity in general.
Relation between changes in network level causal interactions and cognition with dopaminergic medication
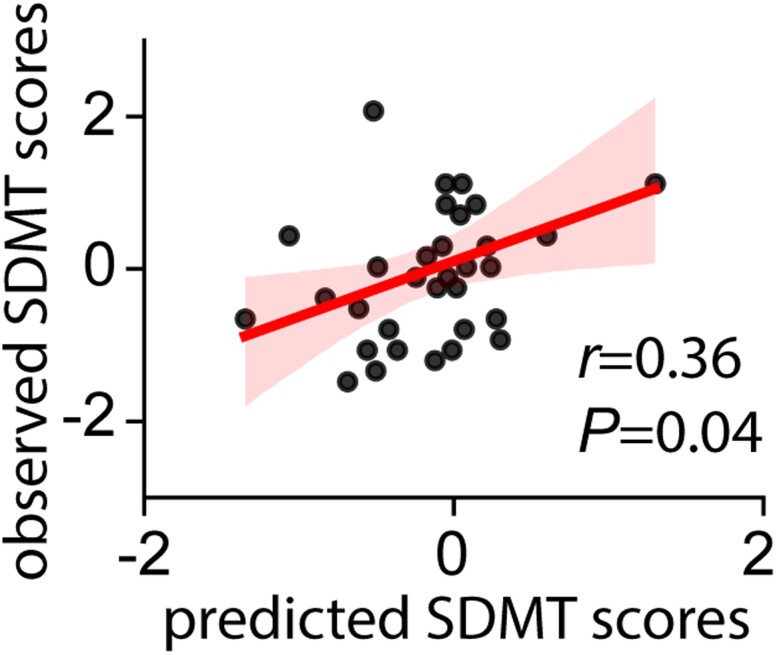
Next, we examined whether changes in network-level causal signalling is related to individual differences in cognitive function with dopaminergic medication. We trained a support vector regression model based on network distance between the PD-ON and PD-OFF states, to predict changes in SDMT scores between ON and OFF dopaminergic medication and evaluated performance of the model using leave-one-out cross validation. Network distance changes accurately predicted SDMT changes between ON and OFF states (r = 0.36, P = 0.04, Fig. 3). These results demonstrate that changes in causal signalling patterns within cognitive control circuitry contributes to cognitive changes in Parkinson’s disease.
Figure 3.
Medication effect on MDSI distance in relation to cognitive function. MDSI-based distance in causal signalling patterns predicted changes in SDMT scores between ON and OFF medication states in Parkinson’s disease participants (r = 0.36, P = 0.04). A support vector regression model based on network distance between the PD-ON and PD-OFF states was used to predict changes in SDMT scores between ON and OFF dopaminergic medication.
Working memory load-dependent modulation of dynamic causal interactions and relation to behaviour in healthy controls
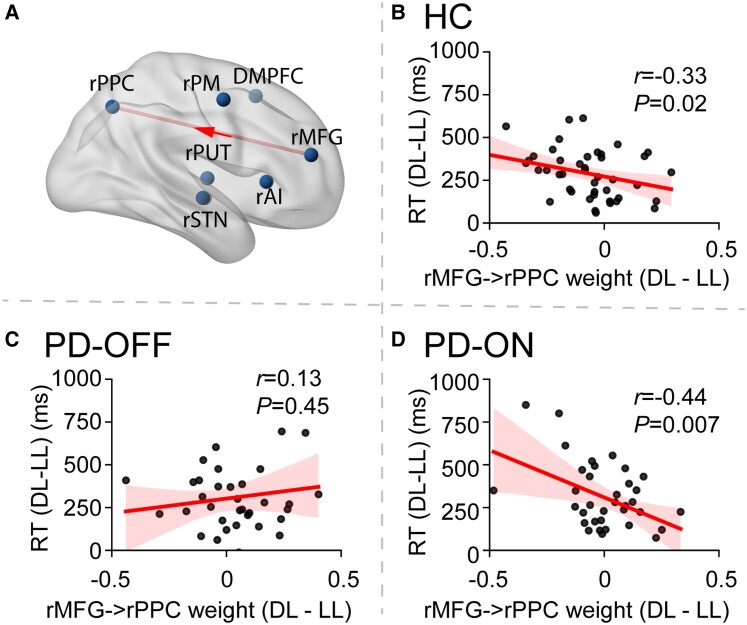
Next, we identified causal signalling pathways that showed consistent working memory load-dependent modulation in healthy controls and determined whether these pathways are associated with individual differences in healthy control working memory task performance. Paired t-tests revealed that in healthy controls, load-dependent modulation of the causal interaction from the rMFG to rPPC (rMFG → rPPC) was significant in the HL versus LL conditions (P < 0.05, FDR corrected, Fig. 4A) and in the DL versus LL conditions (P = 0.006), suggesting a consistent load effect in causal interaction of rMFG → rPPC across high load conditions (Supplementary Fig. 2). There was no significant difference between the DL versus HL conditions (P > 0.3). Notably, load-dependent modulation of this frontoparietal causal link was highly right lateralized (P < 0.01, Supplementary material).
Figure 4.
Causal signalling in frontoparietal network in relation to load effect. (A) MDSI analysis revealed a significant load-dependent casual influence from rMFG to rPPC in healthy controls (HC). (B–D) Relation between the strength of causal signalling from the rMFG to rPPC (rMFG → rPPC) and working memory performance is rescued by dopaminergic medication. (B) Healthy controls; (C) Parkinson’s disease participants OFF dopaminergic medication (PD-OFF); and (D) Parkinson’s disease participants ON medication (PD-ON). RT = reaction time.
Next, we sought to determine whether this causal link rMFG → rPPC was behaviourally relevant. Our analysis focused on working memory load effects in the relation to reaction time since accuracy was uniformly high. We found that the strength of dynamic casual interaction was not significantly related to Sternberg performance when the HL versus LL contrast was used (P > 0.45). However, the strength of the causal link rMFG → rPPC contrasting DL versus LL conditions was correlated with reaction time differences between these condition (r = –0.33, P = 0.02, Fig. 4B).
Dopaminergic modulation of the relation between rMFG → rPPC and behavioural performance in Parkinson’s disease
Having identified rMFG → rPPC as a causal signalling pathway that showed consistent working memory load-dependent modulation in healthy controls and a direct relation to working memory task performance, we then determined whether this link is impaired in PD-OFF. First, we conducted a two-way ANOVA with factors group (healthy controls, PD-OFF) and condition (LL, HL and DL) revealed a significant interaction between group and condition [F(2,156) = 8.56, P = 0.0003, Cohen’s f = 0.33] but no significant main effect of group [F(1,78) = 0.10, P = 0.75, Cohen’s f = 0.04] and condition [F(2,156) = 1.95, P = 0.15, Cohen’s f = 0.16]. Post hoc analysis found that the load-dependent strength of the causal link rMFG → rPPC was significantly weaker in PD-OFF compared to healthy controls in both the DL and HL relative to LL task conditions (P-values < 0.005, Supplementary Fig. 2). We then examined whether dopaminergic medication improves load-dependent modulation of the causal link rMFG → rPPC in Parkinson’s disease and did not find significant effect in either DL or HL relative to LL task conditions (P-values > 0.05).
Next, we examined whether dopaminergic medication restores the load-dependent relation between rMFG → rPPC causal interactions and behavioural performance assessed using RT, as was discovered in healthy controls above. We found that the strength of the causal link rMFG → rPPC was not significant in the PD-OFF group (r = 0.13, P = 0.45, Fig. 4C) but was significant in the PD-ON group (r = –0.44, P = 0.007, Fig. 4D). Comparison of the correlations confirmed that the correlation coefficient was significantly weaker in PD-OFF than healthy controls (P = 0.02, Fisher’s z-test) and significantly weaker in PD-OFF than PD-ON (P = 0.005, Dunn and Clark’s z-test). These results suggest that stronger load-dependent causal interaction in rMFG → rPPC is associated with better behavioural performance, and this relationship is impaired in Parkinson’s disease but restored by dopaminergic medication.
To further examine whether the relationship between the strength of the causal link rMFG → rPPC and behavioural performance was confounded by age, sex and head motion, we conducted multiple linear regression analyses. Our analyses confirmed that the causal strength of rMFG → rPPC was the only significant predictor of reaction times differences between DL and LL task conditions in both healthy control and PD-ON groups after controlling age, sex and frame-wise displacement (P-values < 0.05, Table 3). These results demonstrate that the robustness of the relationship between dynamic causal interaction of rMFG → rPPC and behavioural performance.
Table 3.
Relation between load-dependent modulation of the strength of causal signalling between the right middle frontal gyrus and posterior parietal cortex (rMFG → rPPC) and reaction time differences between DL and LL task conditions
| Beta | t-value | P-value | |
|---|---|---|---|
| Healthy controls | |||
| rMFG → rPPC | −0.26 | −2.24 | 0.03* |
| Age | 0.003 | 0.66 | 0.51 |
| Sex | −0.001 | −0.04 | 0.97 |
| Mean FD | −0.33 | −0.54 | 0.59 |
| PD-OFF | |||
| rMFG → rPPC | 0.18 | 0.76 | 0.45 |
| Age | 0.002 | 0.38 | 0.71 |
| Sex | 0.002 | 0.05 | 0.96 |
| Mean FD | 0.34 | 0.31 | 0.76 |
| PD-ON | |||
| rMFG → rPPC | −0.54 | −2.55 | 0.02* |
| Age | 0.001 | 0.17 | 0.86 |
| Sex | 0.006 | 0.17 | 0.86 |
| Mean FD | −0.19 | −0.25 | 0.81 |
The strength of the causal link rMFG → rPPC contrasting DL versus LL conditions was correlated with reaction time differences between these conditions in healthy controls and PD-ON, but not in the PD-OFF, groups. Results of multiple linear regression analyses controlling for age, gender, and head motion. FD = frame-wise displacement. *P < 0.05.
Finally, we determined whether the relation between the strength of the causal link rMFG → rPPC and behavioural performance could be uncovered by gPPI; no significant brain-behaviour relations were found with gPPI (P > 0.3). These results demonstrate the specificity of brain-behaviour relations estimated by MDSI (see Supplementary material for additional details).
Robustness of the main findings
We conducted additional analyses to determine the robustness of our findings. Specifically, we examined whether medication effect on network-level causal signalling and its relation to cognition are stable without inclusion of STN-related connections, as well as the impact of other medication factors. Results from these analyses, described in the Supplementary material, were equivalent to our main findings, highlighting the robustness of our findings.
Discussion
We used novel computational tools and state-space causal modelling to investigate dynamic causal circuits underlying working memory in Parkinson’s disease patients ON and OFF dopaminergic medication. Our study incorporated several innovations at the computational, methodological and design levels. First, rather than examining regional activation or static functional connectivity, we used high temporal functional MRI sampling of 490 ms to probe dynamic causal mechanisms in Parkinson’s disease patients ON and OFF dopaminergic medication. This allowed us to uncover dynamic processes that are not observable with conventional approaches, as demonstrated by our lack of findings using acausal functional connectivity techniques. Second, we used a novel state-space approach that allows testing of modulatory effects of working memory on all links. Our approach overcomes the limitation of having to test a limited set of models in a combinatorically large space of models that precludes testing of all possible models. Finally, we examined task-related casual circuits involved in cognitive control using a larger sample (Parkinson’s disease = 36, healthy controls = 44) than previous studies39 and incorporated a within-subject design to examine dopaminergic effects in Parkinson’s disease participants. Thus, we are able to examine how within-subject level changes due to dopaminergic medication relate to dynamic brain circuits and cognition.
Our analysis revealed aberrant causal signalling in frontoparietal-basal ganglia circuits in Parkinson’s disease patients OFF medication, which were normalized by dopaminergic medication. Quantitative distance measures predicted individual differences in cognitive change associated with medication in Parkinson’s disease. We also identified a specific frontoparietal causal signalling pathway that is impaired in Parkinson’s disease patients. More specifically, unlike in healthy controls, the causal interaction from rMFG to rPPC (rMFG → rPPC) was not modulated by working memory load and the strength of load-dependent causal weights was not related to individual differences in working memory performance in Parkinson’s disease participants OFF medication. However, dopaminergic medication reinstated the relation between load-dependent casual interactions from rMFG to rPPC and working memory task performance. Our findings provide novel insights into aberrant causal brain circuit dynamics during working memory and demonstrate that dopaminergic medication normalizes cognitive control circuits.
L-DOPA normalizes aberrant network-level causal signalling in frontoparietal-basal ganglia cognitive control circuits
Most investigations examining the neural correlates of working memory in Parkinson’s disease have focused on regional task activation.9,32,73–77 A recent meta-analysis of 13 studies examining working memory in Parkinson’s disease failed to identify consistent abnormal activation during working memory performance in Parkinson’s disease patients compared to healthy controls.39 Similarly, the few studies that have examined task-modulated connectivity have also yielded inconsistent findings.78–80 For example, one study showed a lack of attention-modulated connectivity between prefrontal regions and premotor cortex in Parkinson’s disease patients.80 Another study reported both increased and decreased cognitive control-modulated cortical-basal ganglia connectivity in Parkinson’s disease.79 Other studies have reported reduced corticocortical and cortical-subcortical connectivity in Parkinson’s disease patients following working memory training.81 Resting-state connectivity has been examined more extensively and a recent meta-analysis showed reduced default mode network connectivity in Parkinson’s disease patients with cognitive impairment.82 However, resting-state does not provide insight into brain activity during cognitive activity and indeed, one study showed that despite resting-state compromise in Parkinson’s disease, task-related connectivity can be adequately engaged to enable near normal task performance.79
We took a quantitatively rigorous circuit analysis approach to probe dynamic causal mechanisms in the human brain. MDSI simultaneously estimates the causality between regions under each task condition within the same modelling framework while accounting for variations in hemodynamic responses in these regions. Importantly, we used a multivariate circuit distance measure to determine the effect of dopaminergic medication on causal circuit dissimilarity between Parkinson’s disease and healthy controls. Our analysis revealed that dopaminergic medication reduced dissimilarity of causal circuit signalling between Parkinson’s disease and healthy controls in each of the three working memory load conditions. We suggest that multivariate circuit measures involving distributed cortico-cortico and cortico-basal ganglia circuits engaged in working memory and cognitive control40,69,83–85 may allow us to better capture the effects of dopaminergic medication. Such measures may also allow us to overcome limitations of previous approaches which have focused on individual brain regions or specific inter-regional links which have resulted in inconsistent findings.39 Crucially, the present study is the first to demonstrate normalization of network-level causal signalling through dopaminergic medications in Parkinson’s disease.
Network-level causal signalling predicts individual differences in dopaminergic treatment response
Levodopa and dopaminergic agonists are highly effective in alleviating motor symptoms associated with dopamine deficiency in Parkinson’s disease patients.4 However, their effects on cognition in Parkinson’s disease varies considerably across individuals and is likely influenced by factors such as differences in medication history, dosage, type of medication used, metabolism and dopamine regulation.86,87 Given the wide range of factors that can influence dopaminergic medication-related cognitive changes, examination of individual differences has the potential to provide better insight into mechanisms of treatment response. We therefore assessed the relation between medication-related changes in network similarity and changes in cognitive functioning using the SDMT, a brief cognitive test of working memory, attention switching, and processing speed.45–47 We found that dopaminergic modulation of network-level causal signalling predicted medication-related changes in cognitive functioning in Parkinson’s disease patients. Importantly, we tested this relationship using a cross-validation procedure such that a trained model is used to predict the medication effect on cognition based on the medication effect on global network-level signalling of unseen data. Results suggest that alterations in network-level dynamic causal interactions in the frontoparietal-basal ganglia cognitive control system are a mechanism by which dopaminergic medication affects cognitive functioning in Parkinson’s disease patients. Our results further suggest that medication-related changes in network-level signalling may be an objective biomarker of treatment response.
Dopamine reinstates relation between frontoparietal causal signalling and working memory performance in Parkinson’s disease
The MFG and PPC are key nodes of the frontoparietal working memory network.88–91 Their neuronal activity profiles are tightly linked to the ability to maintain and manipulate the content of working memory.30,92–96 Human neuroimaging studies have consistently reported activation of the MFG and PPC during working memory task performance83,97–101 and have furthermore highlighted consistent co-activation of the two regions across a wide range of working memory tasks.51–53,102 Consistent with these reports, we found robust co-activation of the rMFG and rPPC in healthy controls as well as Parkinson’s disease participants. Crucially, we observed significant load-dependent modulation in causal signalling from the rMFG to rPPC (rMFG → rPPC) in healthy controls. Notably, this brain-behaviour relationship was only observed in the more demanding DL condition which required participants to suppress attention to distractors while encoding task-relevant stimuli. This result is consistent with the hypothesis that the MFG plays a key role in top-down control of working memory, including selection of task-relevant information and suppression of task-irrelevant information,83,103 whereas the PPC plays an important role in temporal storage of information.104,105 Consistent with this hypothesis, a recent study has shown dissociable effects from stimulation of the two regions in a working memory task using transcranial magnetic stimulation (TMS).106 Specifically, theta-TMS (excitatory to neural activity) on MFG improves performance on trials with task-relevant cues whereas alpha-TMS (inhibitory to neural activity) on PPC has positive effect on trials with task-irrelevant cues.
The higher load-dependent modulation of dynamic causal interactions in the right frontoparietal circuit, compared to the left, may reflect right hemispheric dominance across a broad range of cognitive control tasks.107,108 Interestingly, a previous n-back working memory study in healthy controls, using visually displayed letters, has also found the right lateralized increase in dynamic causal interactions with cognitive load in connection between DLPFC and parietal cortex.109
More importantly, our analysis identified aberrancies in top-down causal signalling from the rMFG to the rPPC in Parkinson’s disease patients. In healthy controls, the strength of rMFG → rPPC load-dependent causal interactions predicted individual differences in reaction time, demonstrating the relevance of this causal pathway for efficient task performance during the presence of distractors. In Parkinson’s disease patients OFF dopaminergic medication, the rMFG → rPPC link was significantly less modulated during the presence of distractors, and there was no relation between the strength of interaction and working memory performance. Notably, like in healthy controls, the strength of causal rMFG → rPPC signalling was correlated with working memory performance in the Parkinson’s disease ON group. Thus, dopaminergic medication restores behaviourally relevant causal signalling from the rMFG to rPPC in Parkinson’s disease patients, likely allowing for more efficient manipulation of relevant versus irrelevant information, reduced interference and ultimately improved working memory performance. Our findings are consistent with reports that dopamine specifically reduces interference110 and that the ability to suppress distractions which is impaired in PD-OFF participants improves with dopaminergic medication.21 This aligns well with our finding that it was during the DL task, which specifically requires suppression of distractors, that dopaminergic medication reinstated the relationship between causal signalling from rMFG to rPPC and working memory performance. Our findings thus suggest that causal signalling from rMFG to rPPC may be an underlying mechanism of impaired gating of relevant versus irrelevant items and thereby increased interference in Parkinson’s disease.111
Limitations
We found that dopaminergic modulation of network-level causal signalling in Parkinson’s disease patients predicted medication-related changes in cognitive functioning as assessed using the SDMT, a standardized test of working memory, attention switching, and processing speed that has been widely used to probe general cognitive functioning in Parkinson’s disease.45–47 However, dopaminergic modulation on network-level causal signalling did not directly predict medication-related changes in performance on the Sternberg working memory functional MRI task, likely due to the high levels of task performance in Parkinson’s disease patients (>90% accuracy in all task conditions). While our findings highlight the role of causal interaction between MFG and PPC in the DL task, associated with selection of task-relevant information and suppression of task-irrelevant information, a limitation here is that our study could not dissociate specific working memory and cognitive control processes. Finally, due to computational limitations, we were not able to examine dynamic causal interactions across all task-related regions covering the entire brain. This does not mean that other left out brain regions, such as caudate and cerebellum, are not important for working memory, Parkinson’s disease, or dopaminergic modulation. Future work is needed using computational algorithms that can handle large network size, along with fast-sampling rate as used here, to probe the role of other brain areas implicated in working memory.
Conclusion
We used state-space modelling to uncover causal signalling mechanisms within a core frontoparietal-basal ganglia circuit implicated in working memory in Parkinson’s disease patients and further examined the effects of dopaminergic medication. Our analysis revealed that dopaminergic medication can normalize abnormality in network-level causal signalling in Parkinson’s disease and the extent of dopaminergic modulation on causal mechanism in frontoparietal-basal ganglia network can predict the effect of medication on cognition. More specifically, in comparison to healthy controls, Parkinson’s disease patients have weakened load-dependent modulation of frontal-parietal causal interaction. Dopaminergic medication can restore the association between causal strength of rMFG → rPPC and working memory performance in Parkinson’s disease patients, similar to what is observed in healthy controls, but this was diminished in Parkinson’s disease patients when they were OFF medication. Our findings highlight aberrant causal signalling between key working memory regions as an important neurobiological feature of Parkinson’s disease and provide novel evidence for supporting positive effects of dopaminergic medication in high-order cognitive system in Parkinson’s disease patients. The approach and methods developed here are useful for probing broad medication effects on cognitive systems in neurological and psychiatric disorders.
Supplementary Material
Abbreviations
- DL
distractor load
- HL
high load
- LL
low load
- MDSI
multivariate dynamic state-space systems identification
- MFG
middle frontal gyrus
- PPC
posterior parietal cortex
- SDMT
Symbol Digit Modalities Test
- STN
subthalamic nuclei
Contributor Information
Weidong Cai, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA; Wu Tsai Neurosciences Institute, Stanford University School of Medicine, Stanford, CA 94305, USA.
Christina B Young, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA.
Rui Yuan, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA.
Byeongwook Lee, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA.
Sephira Ryman, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA.
Jeehyun Kim, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA.
Laurice Yang, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA.
Victor W Henderson, Wu Tsai Neurosciences Institute, Stanford University School of Medicine, Stanford, CA 94305, USA; Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA; Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA 94305, USA.
Kathleen L Poston, Wu Tsai Neurosciences Institute, Stanford University School of Medicine, Stanford, CA 94305, USA; Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA; Department of Neurosurgery, Stanford University School of Medicine, Stanford, CA 94305, USA.
Vinod Menon, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA; Wu Tsai Neurosciences Institute, Stanford University School of Medicine, Stanford, CA 94305, USA; Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA.
Funding
This research was supported by grants from the National Institute of Health (P50 AG047366, P30 AG066515, P50 NS062684, NS08608505, MH121069).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Baiano C, Barone P, Trojano L, Santangelo G. camcPrevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov Disord. 2020;35(1):45–54. [DOI] [PubMed] [Google Scholar]
- 2. Hely MA, Reid WGJ, Adena MA, Halliday GA, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. [DOI] [PubMed] [Google Scholar]
- 3. Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson’s disease. Prog Neurobiol. 2007;81(1):29–44. [DOI] [PubMed] [Google Scholar]
- 4. Huse DM, Castelli-Haley J, Orsini LS, Lenhart G, Abdalla JA. Patterns of initial pharmacotherapy for Parkinson’s disease in the United States. J Geriatr Psychiatry Neurol. 2006;19(2):91–97. [DOI] [PubMed] [Google Scholar]
- 5. Skeel RL, Crosson B, Nadeau SE, Algina J, Bauer RM, Fennell EB. Basal ganglia dysfunction, working memory, and sentence comprehension in patients with Parkinson’s disease. Neuropsychologia. 2001;39(9):962–971. [DOI] [PubMed] [Google Scholar]
- 6. Cools R, Miyakawa A, Sheridan M, D’Esposito M. Enhanced frontal function in Parkinson’s disease. Brain. 2010;133:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moustafa AA, Sherman SJ, Frank MJ. A dopaminergic basis for working memory, learning and attentional shifting in Parkinsonism. Neuropsychologia. 2008;46(13):3144–3156. [DOI] [PubMed] [Google Scholar]
- 8. Lewis SJG, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia. 2003;41(6):645–654. [DOI] [PubMed] [Google Scholar]
- 9. Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125(Pt 3):584–594. [DOI] [PubMed] [Google Scholar]
- 10. Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–1302. [DOI] [PubMed] [Google Scholar]
- 11. Arnaoutoglou NA, O’Brien JT, Underwood BR. Dementia with Lewy bodies—from scientific knowledge to clinical insights. Nat Rev Neurol. 2019;15(2):103–112. [DOI] [PubMed] [Google Scholar]
- 12. Bell PT, Gilat M, O’Callaghan C, et al. Dopaminergic basis for impairments in functional connectivity across subdivisions of the striatum in Parkinson’s disease. Hum Brain Mapp. 2015;36(4):1278–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hazy TE, Frank MJ, O’Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc Lond B Biol Sci. 2007;362(1485):1601–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landau SM, Lal R, O’Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19(2):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang YT, Georgiev D, Foltynie T, Limousin P, Speekenbrink M, Jahanshahi M. Different effects of dopaminergic medication on perceptual decision-making in Parkinson’s disease as a function of task difficulty and speed-accuracy instructions. Neuropsychologia. 2015;75:577–587. [DOI] [PubMed] [Google Scholar]
- 17. Michely J, Barbe MT, Hoffstaedter F, et al. Differential effects of dopaminergic medication on basic motor performance and executive functions in Parkinson’s disease. Neuropsychologia. 2012;50(10):2506–2514. [DOI] [PubMed] [Google Scholar]
- 18. Osman M, Ryterska A, Karimi K, et al. The effects of dopaminergic medication on dynamic decision making in Parkinson’s disease. Neuropsychologia. 2014;53:157–164. [DOI] [PubMed] [Google Scholar]
- 19. Ruitenberg MFL, Abrahamse EL, Santens P, Notebaert W. The effect of dopaminergic medication on conflict adaptation in Parkinson’s disease. J Neuropsychol. 2019;13(1):121–135. [DOI] [PubMed] [Google Scholar]
- 20. Trempler I, Burkner PC, El-Sourani N, et al. Impaired context-sensitive adjustment of behaviour in Parkinson’s disease patients tested on and off medication: An fMRI study. Neuroimage. 2020;212:116674. [DOI] [PubMed] [Google Scholar]
- 21. Bayram E, Litvan I, Wright BA, Grembowski C, Shen Q, Harrington DL. Dopamine effects on memory load and distraction during visuospatial working memory in cognitively normal Parkinson’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2021:812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beigi M, Wilkinson L, Gobet F, Parton A, Jahanshahi M. Levodopa medication improves incidental sequence learning in Parkinson’s disease. Neuropsychologia. 2016;93(Pt A):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fournet N, Moreaud O, Roulin JL, Naegele B, Pellat J. Working memory functioning in medicated Parkinson’s disease patients and the effect of withdrawal of dopaminergic medication. Neuropsychology. 2000;14(2):247–253. [DOI] [PubMed] [Google Scholar]
- 24. Lewis SJG, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43(6):823–832. [DOI] [PubMed] [Google Scholar]
- 25. Williams IA, Obeso I, Jahanshahi M. Dopaminergic medication improves cognitive control under low cognitive demand in Parkinson’s disease. Neuropsychology. 2020;34(5):551–559. [DOI] [PubMed] [Google Scholar]
- 26. Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11(12):1136–1143. [DOI] [PubMed] [Google Scholar]
- 27. Slagter HA, van Wouwe NC, Kanoff K, et al. Dopamine and temporal attention: an attentional blink study in Parkinson’s disease patients on and off medication. Neuropsychologia. 2016;91:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baddeley AD. Working memory. Oxford psychology series. Clarendon Press; Oxford University Press; 1986:xi, 289 p [Google Scholar]
- 29. D’Esposito M, Postle BR. The cognitive neuroscience of working memory. Annual review of psychology. 2015;66:115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. [DOI] [PubMed] [Google Scholar]
- 31. Watson GS, Leverenz JB. Profile of cognitive impairment in Parkinson’s disease. Brain Pathol. 2010;20(3):640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ekman U, Eriksson J, Forsgren L, Mo SJ, Riklund K, Nyberg L. Functional brain activity and presynaptic dopamine uptake in patients with Parkinson’s disease and mild cognitive impairment: a cross-sectional study. Lancet Neurol. 2012;11(8):679–687. [DOI] [PubMed] [Google Scholar]
- 33. Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23(15):6351–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marklund P, Larsson A, Elgh E, et al. Temporal dynamics of basal ganglia under-recruitment in Parkinson’s disease: transient caudate abnormalities during updating of working memory. Brain. 2008;132(Pt 2):336–346. [DOI] [PubMed] [Google Scholar]
- 35. Poston KL, YorkWilliams S, Zhang K, et al. Compensatory neural mechanisms in cognitively unimpaired Parkinson disease. Ann Neurol. 2016;79(3):448–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trujillo JP, Gerrits NJ, Veltman DJ, Berendse HW, van der Werf YD, van den Heuvel OA. Reduced neural connectivity but increased task-related activity during working memory in de novo Parkinson patients. Hum Brain Mapp. 2015;36(4):1554–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aarts E, Nusselein AA, Smittenaar P, Helmich RC, Bloem BR, Cools R. Greater striatal responses to medication in Parkinson’s disease are associated with better task-switching but worse reward performance. Neuropsychologia. 2014;62:390–397. [DOI] [PubMed] [Google Scholar]
- 38. Nieuwhof F, Bloem BR, Reelick MF, et al. Impaired dual tasking in Parkinson’s disease is associated with reduced focusing of cortico-striatal activity. Brain. 2017;140(5):1384–1398. [DOI] [PubMed] [Google Scholar]
- 39. Giehl K, Tahmasian M, Eickhoff SB, van Eimeren T. Imaging executive functions in Parkinson’s disease: An activation likelihood estimation meta-analysis. Parkinsonism Relat Disord. 2019;63:137–142. [DOI] [PubMed] [Google Scholar]
- 40. Cai W, Ryali S, Pasumarthy R, Talasila V, Menon V. Dynamic causal brain circuits during working memory and their functional controllability. Nat Commun. 2021;12(1):3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ryali S, Chen TW, Supekar K, et al. Multivariate dynamical systems-based estimation of causal brain interactions in fMRI: group-level validation using benchmark data, neurophysiological models and human connectome project data. J Neurosci Meth. 2016;268:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryali S, Shih YYI, Chen TW, et al. Combining optogenetic stimulation and fMRI to validate a multivariate dynamical systems model for estimating causal brain interactions. Neuroimage. 2016;132:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ryali S, Supekar K, Chen T, Menon V. Multivariate dynamical systems models for estimating causal interactions in fMRI. Neuroimage. 2011;54(2):807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forstmann BU, Keuken MC, Jahfari S, et al. Cortico-subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. Neuroimage. 2012;60(1):370–375. [DOI] [PubMed] [Google Scholar]
- 45. Gabrieli JDE, Singh J, Stebbins GT, Goetz CG. Reduced working memory span in Parkinson’s disease: evidence for the role of frontostriatal system in working and strategic memory. Neuropsychology. 1996;10(3):322–332. [Google Scholar]
- 46. Pascoe M, Alamri Y, Dalrymple-Alford J, Anderson T, MacAskill M. The symbol-digit modalities test in mild cognitive impairment: evidence from Parkinson’s disease patients. Eur Neurol. 2018;79(3–4):206–210. [DOI] [PubMed] [Google Scholar]
- 47. Starkstein SE, Preziosi TJ, Berthier ML, Bolduc PL, Mayberg HS, Robinson RG. Depression and cognitive impairment in Parkinson’s disease. Brain. 1989;112:1141–1153. [DOI] [PubMed] [Google Scholar]
- 48. Litvan I, Bhatia KP, Burn DJ, et al. Movement disorders society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18(5):467–486. [DOI] [PubMed] [Google Scholar]
- 49. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 50. Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12(5):419–446. [DOI] [PubMed] [Google Scholar]
- 51. Nee DE, Brown JW, Askren MK, et al. A meta-analysis of executive components of working memory. Cereb Cortex. 2013;23(2):264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rottschy C, Langner R, Dogan I, et al. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60(1):830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fan L, Li H, Zhuo J, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26(8):3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cavanagh JF, Wiecki TV, Cohen MX, et al. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nature Neuroscience. 2011;14(11):1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Drummond NM, Chen R. Deep brain stimulation and recordings: insights into the contributions of subthalamic nucleus in cognition. Neuroimage. 2020;222:117300. [DOI] [PubMed] [Google Scholar]
- 57. Kelley R, Flouty O, Emmons EB, et al. A human prefrontal-subthalamic circuit for cognitive control. Brain. 2018;141(1):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weintraub DB, Zaghloul KA. The role of the subthalamic nucleus in cognition. Rev Neurosci. 2013;24(2):125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. New Engl J Med. 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 60. Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. New Engl J Med. 1998;339(16):1105–1111. [DOI] [PubMed] [Google Scholar]
- 61. Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord 2006;21(Suppl 14):S290–S304. [DOI] [PubMed] [Google Scholar]
- 62. Lozano AM, Lipsman N, Bergman H, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15(3):148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stefani A, Trendafilov V, Liguori C, Fedele E, Galati S. Subthalamic nucleus deep brain stimulation on motor-symptoms of Parkinson’s disease: focus on neurochemistry. Prog Neurobiol. 2017;151:157–174. [DOI] [PubMed] [Google Scholar]
- 64. Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex. 2008;18(1):178–188. [DOI] [PubMed] [Google Scholar]
- 65. Fife KH, Gutierrez-Reed NA, Zell V, et al. Causal role for the subthalamic nucleus in interrupting behavior. Elife. 2017;6:e27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pasquereau B, Turner RS. A selective role for ventromedial subthalamic nucleus in inhibitory control. Elife. 2017;6:e31627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schmidt R, Leventhal DK, Mallet N, Chen FJ, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci. 2013;16(8):1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26(9):2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cai W, Duberg K, Padmanabhan A, et al. Hyperdirect insula-basal-ganglia pathway and adult-like maturity of global brain responses predict inhibitory control in children. Nat Commun. 2019;10(1):4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Swann N, Poizner H, Houser M, et al. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson’s disease. J Neurosci. 2011;31(15):5721–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van den Wildenberg WPM, van Boxtel GJM, van der Molen MW, Bosch DA, Speelman JD, Brunia CHM. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cognitive Neurosci. 2006;18(4):626–636. [DOI] [PubMed] [Google Scholar]
- 72. McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Caminiti SP, Siri C, Guidi L, Antonini A, Perani D. The neural correlates of spatial and object working memory in elderly and Parkinson’s disease subjects. Behav Neurol. 2015;2015:123636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grossman M, Cooke A, DeVita C, et al. Grammatical and resource components of sentence processing in Parkinson’s disease: an fMRI study. Neurology. 2003;60(5):775–781. [DOI] [PubMed] [Google Scholar]
- 75. Marie RM, Lozza C, Chavoix C, Defer GL, Baron JC. Functional imaging of working memory in Parkinson’s disease: compensations and deficits. J Neuroimaging. 2007;17(4):277–285. [DOI] [PubMed] [Google Scholar]
- 76. Rottschy C, Kleiman A, Dogan I, et al. Diminished activation of motor working-memory networks in Parkinson’s disease. PLoS One. 2013;8(4):e61786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ventre-Dominey J, Bourret S, Mollion H, Broussolle E, Dominey PF. Dissociable dorsal and ventral frontostriatal working memory circuits: evidence from subthalamic stimulation in Parkinson’s disease. Hum Brain Mapp. 2014;35(2):552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Harrington DL, Castillo GN, Greenberg PA, et al. Neurobehavioral mechanisms of temporal processing deficits in Parkinson’s disease. PLoS One. 2011;6(2):e17461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Muller-Oehring EM, Sullivan EV, Pfefferbaum A, et al. Task-rest modulation of basal ganglia connectivity in mild to moderate Parkinson’s disease. Brain Imaging Behav. 2015;9(3):619–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rowe J, Stephan KE, Friston KJ, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson’s disease—impaired effective connectivity among frontal cortical regions. Brain. 2002;125:276–289. [DOI] [PubMed] [Google Scholar]
- 81. Giehl K, Ophey A, Hammes J, et al. Working memory training increases neural efficiency in Parkinson’s disease: a randomized controlled trial. Brain Commun. 2020;2(2):fcaa115. 10.1093/braincomms/fcaa115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wolters AF, van de Weijer SCF, Leentjens AFG, Duits AA, Jacobs HIL, Kuijf ML. Resting-state fMRI in Parkinson’s disease patients with cognitive impairment: a meta-analysis. Parkinsonism Relat Disord. 2019;62:16–27. [DOI] [PubMed] [Google Scholar]
- 83. McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11(1):103–107. [DOI] [PubMed] [Google Scholar]
- 84. O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18(2):283–328. [DOI] [PubMed] [Google Scholar]
- 85. Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proc Natl Acad Sci U S A. 2010;107(42):18167–18172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Poletti M, Bonuccelli U. Acute and chronic cognitive effects of levodopa and dopamine agonists on patients with Parkinson’s disease: a review. Ther Adv Psychopharmacol. 2013;3(2):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Roy MA, Doiron M, Talon-Croteau J, Dupre N, Simard M. Effects of antiparkinson medication on cognition in Parkinson’s disease: a systematic review. Can J Neurol Sci. 2018;45(4):375–404. [DOI] [PubMed] [Google Scholar]
- 88. Christophel TB, Klink PC, Spitzer B, Roelfsema PR, Haynes JD. The distributed nature of working memory. Trends Cogn Sci. 2017;21(2):111–124. [DOI] [PubMed] [Google Scholar]
- 89. Funahashi S. Working memory in the prefrontal cortex. Brain Sci. 2017;7(5):49. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lara AH, Wallis JD. The role of prefrontal cortex in working memory: a mini review. Front Syst Neurosci. 2015;9:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nee DE, D’Esposito M. The representational basis of working memory. Curr Top Behav Neurosci. 2018;37:213–230. [DOI] [PubMed] [Google Scholar]
- 92. Funahashi S, Bruce CJ, Goldmanrakic PS. Mnemonic coding of visual space in the monkeys dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–349. [DOI] [PubMed] [Google Scholar]
- 93. Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci. 1994;14(5 Pt 1):2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Goldmanrakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, ed. Handbook of physiology: the nervous system. American Physiological Society; 1987:373–417. [Google Scholar]
- 95. Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 1996;16(16):5154–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133(1):44–54. [DOI] [PubMed] [Google Scholar]
- 97. Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. [DOI] [PubMed] [Google Scholar]
- 98. D’Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37(11):1303–1315. [DOI] [PubMed] [Google Scholar]
- 99. Jonides J, Schumacher EH, Smith EE, et al. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18(13):5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci. 2002;14(4):659–671. [DOI] [PubMed] [Google Scholar]
- 101. Rowe JB, Toni I, Josephs O, Frackowiak RSJ, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288(5471):1656–1660. [DOI] [PubMed] [Google Scholar]
- 102. Daniel TA, Katz JS, Robinson JL. Delayed match-to-sample in working memory: a BrainMap meta-analysis. Biol Psychol. 2016;120:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Edin F, Klingberg T, Johansson P, McNab F, Tegner J, Compte A. Mechanism for top-down control of working memory capacity. Proc Natl Acad Sci U S A. 2009;106(16):6802–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428(6984):751–754. [DOI] [PubMed] [Google Scholar]
- 105. Christophel TB, Hebart MN, Haynes JD. Decoding the contents of visual short-term memory from human visual and parietal cortex. J Neurosci. 2012;32(38):12983–12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Riddle J, Scimeca JM, Cellier D, Dhanani S, D’Esposito M. Causal evidence for a role of theta and alpha oscillations in the control of working memory. Curr Biol. May. 2020;30(9):1748–1754.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cai W, Ryali S, Chen T, Li CS, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci. 2014;34(44):14652–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dima D, Jogia J, Frangou S. Dynamic causal modeling of load-dependent modulation of effective connectivity within the verbal working memory network. Hum Brain Mapp. 2014;35(7):3025–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fallon SJ, Mattiesing RM, Muhammed K, Manohar S, Husain M. Fractionating the neurocognitive mechanisms underlying working memory: independent wffects of dopamine and Parkinson’s disease. Cerebral Cortex. 2017;27(12):5727–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zokaei N, Husain M. Working memory in Alzheimer’s disease and Parkinson’s disease. Curr Top Behav Neurosci. 2019;41:325–344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study will be shared upon request from qualified investigators.