Abstract
Objective:
To define a generic diet to protect human health and food system sustainability based on three dimensions: animal:plant ratio, degree of food processing and food diversity.
Design/setting:
The percentages of maximum animal and ultra-processed energy content were evaluated from scientific papers (Web of Science database) and reports from international scientific institutions. Then, a weekly French standard diet, including these percentages and food diversity (≥42 different foods), was designed to calculate adequacy to nutritional needs.
Results:
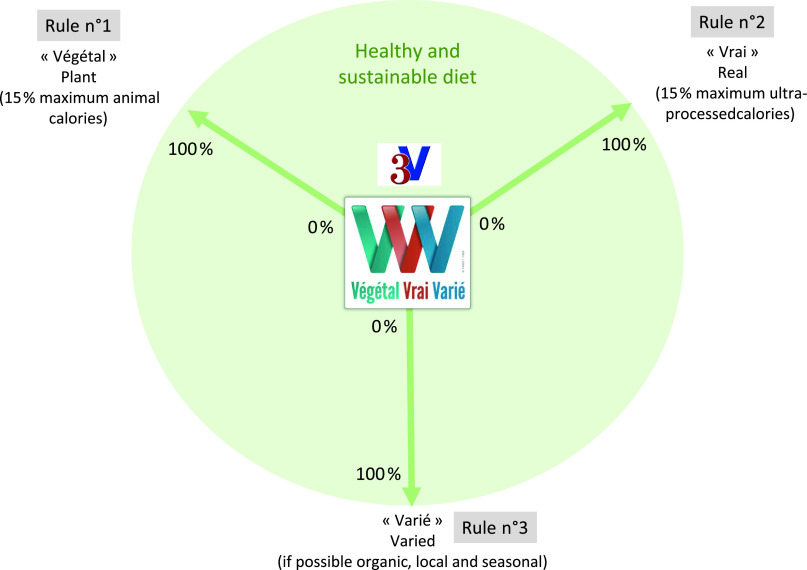
Based on traditional and scientifically based healthy diets, and on foresight scenarios for sustainable diets at horizon 2050, a median daily animal energy content intake of 15 % was found to be protective towards both human health and environment. Based on epidemiological studies associating ultra-processed energy consumption with increased overweight/obesity risk, a precautionary threshold of approximately 15 % ultra-processed energy content was observed. The French diet allows addressing all nutritional needs and other nutritional indicators such as maximum salt and simple sugar consumption, α-linolenic acid:linoleic acid ratio and essential amino acids. This diet was named the ‘3V rule’ for Végétal (plant), Vrai (real) and Varié (varied, if possible organic, local and seasonal). This generic diet can be adapted according to regional traditions and environmental characteristics. Excluding only one dimension of it would threaten both health and food system sustainability.
Conclusions:
Tending towards a 3V-based diet, while respecting local constraints, should allow preserving human health, environment (greenhouse gas emissions, pollution, deforestation, etc.), small farmers, animal welfare and biodiversity, culinary traditions and socioeconomics (including an alleviation of public health cost).
Keywords: 3V rule, Plant, Real, Varied, Health, Food systems, Sustainability
Today, recommended diets should not consider only consumers’ health but also food system sustainability(1). At first view, defining such a diet, covering all four securities at once (sanitary, health, nutritional and environmental), appears to be a tremendous task(2–4). In all cases, nutritional sciences should be included in a transdisciplinary approach to include all the four criteria. Therefore, adopting a more holistic perspective should be preferred over the present single-nutrient/food group reductionist approach(5) for which contradictory results have been obtained at present. Indeed, some studies showed that high nutritional quality, healthy diets and/or diets adhering to food-based dietary guidelines are not necessarily associated with lower greenhouse gas emissions (GHGE)(6–10), while other studies are more moderate(11–16). Moreover, GHGE is not the only issue to consider to define sustainable diet; there are also land use, water footprint, biodiversity, socioeconomic aspects and animal well-being. FAO of the United Nations defines sustainable diets as those that protect human health, the environment (pollution, deforestation, GHGE, etc.), small famers, culinary traditions and socioeconomics (healthy foods accessible to everyone, social life, fair trade, etc.)(17,18), to which animal biodiversity and welfare(19) can also be added. In addition, it is worth mentioning that the diets of each world region depend on different economic, pedo-climatic and agronomic conditions. Therefore, the design of a sustainable diet should be sufficiently generic and based on the specific local conditions of each country respecting culinary traditions first derived from local agricultural production.
Scientific evidence has shown that excess animal energy content, notably red and processed meats, is the main factor threatening both food system sustainability(20–23) and health(24–28), some studies investigating both at once(29–31). However, several recently published reports and papers demonstrated that a balanced consumption of meat is sustainable for both health and environment(32–36) ‘as they play a critical role in improving nutrition, reducing poverty, improving gender equity, improving livelihoods, increasing food security, and improving health’(36). Therefore, the issue is not to suppress animal foods but to achieve a win–win–win approach to synergistically protect human health, the environment and animal welfare by significantly reducing animal food production and consumption(37).
Similarly to animal energy content, those from ultra-processed foods (UPF) have been associated with an increased risk of several chronic diseases or conditions in over thirty epidemiological studies(38,39). These results, together with previous evidence about the influence of the degree of food processing on health (e.g., whole-grain v. refined cereals, raw fruits v. sweetened fruit juices, or red v. processed meats), showed that the degree of processing matters for defining food health potential(1,40) more than their nutrient contents alone(41). It has been also suggested that they are not associated with food system sustainability, notably due to increased plastic pollution, deforestation, intensive monocultures, energy-demanding technological processes, GHGE and excess water use(42).
Besides focusing on animal and ultra-processed foods, it is well admitted now that ‘eating varied’ ensures an optimum supply of synergistic bioactive protective compounds against chronic diseases(43,44) as well as increases and improves environmental biodiversity(1) contributing to its sustainability. Indeed, in some world regions, monotonous diets are shown to result in micronutrient deficiencies (mainly vitamin A, proteins, Fe, iodine and Zn)(45) in developing/emerging countries, or in developed countries when the western diet includes excess ‘empty’ ultra-processed energy content(46–52). Thus, dietary diversity in developing countries is shown to be positively associated with nutritional adequacy(53–55). On the contrary, dietary diversity in developed countries has not been necessarily associated with positive health outcomes in contrast with diet quality(56). The reason is that diversity in these countries is generally associated with consuming more diversified UPF, not fulfilling healthy eating criteria(38). Therefore, the well-known ‘eat varied’ applies to diverse raw agricultural commodities and mildly processed nutrient-dense foods.
From the above considerations, a generic and sustainable complex diet should be holistic and composed of diversified and high-quality foods containing a certain animal:plant ratio and a lower degree of food processing. However, healthy diets advocated today are either regional, for example, the Mediterranean or Nordic diet, or national (e.g., food pyramids or plates). The basis of these diets is essentially nutritional, and many failed to consider the growing rate of UPF share worldwide(38,57), threatening traditional diets in return.
The aim of the current study was to design a generic diet based on three dimensions at the same time, as identified previously(1). Qualitative, rather than quantitative, recommendations allow the generic diet to be easily extrapolated and adapted to regional socioeconomic, climatic and agronomic specificities. Using a data mining approach including both original papers and foresight scenarios from several international institutions, we determined daily maximum animal and ultra-processed energy percentages for a healthy and sustainable diet while tending towards diversity among both animal- and plant-based foods. The definition of such a generic diet first intends to be disease-preventive, that is, increasing people’s healthy life-years while preserving food system sustainability.
Methods
Determination of animal:plant energetic ratio
A literature search was carried out through mining of scientific literature from two main sources:
Studies of the association between traditional a priori (i.e., defined prior to the study) and a posteriori protective diets and health: The diets were selected based on meta-analyses, reviews and/or prospective cohort studies (when meta-analyses not available) of their associations with health outcomes, including risks of all-cause mortality, overweight/obesity, type 2 diabetes, cancers, CVD, mental illnesses (e.g., depression, Alzheimer’s disease, Parkinson’s disease, cognitive decline) and/or other chronic conditions (e.g., osteoporosis, hypertension or glycaemic control). Our search was carried out with the ISI Web of Science up to December 2019. Diets with no proven health benefits, or not sufficiently studied, were not selected.
Scientific reports defining sustainable diets at horizon 2050 as protective of both human health and different environmental outcomes (i.e., GHGE, land and pesticide use, water footprint, food waste, etc.): A search was carried out for foresight collective expertise from the websites of international organisations, government institutions, research institutes and private foundations up to December 2019. Only those reports showing the animal energetic share and those taking into consideration environmental outcomes were selected.
Our objective was not to review scientific papers and reports but extract data about daily animal energy shares to reach both human health and environmental sustainability. Otherwise, it should be remembered that the health protectiveness of diets, as defined by the current study, is mainly based on their associations or correlated relationships, not causality. However, accumulating scientific, as generally used for defining national dietary recommendations, was considered sufficient to select such diets as protective of human health.
For each selected diet, the recommended minimum and maximum number of servings per day was collected and converted into daily energy percentages. The energy conversion, as given by either the French Ciqual(58) or American USDA(59) databases, was based on recommended serving sizes(60) of generic foods representative of the animal product category, that is, white meat, red meat, milk, cheese, yogurt, eggs, seafood and fish (combining fatty and lean fishes). Overall, by averaging all generic animal products, one serving of animal product corresponded to approximately 7·5 % of daily energy content (Table 1). However, serving sizes may vary according to countries, and not normalised at the European level. Serving sizes chosen by the current study correspond to those recommended by dietitians in France(60). Finally, a median serving of animal products was calculated based on the reported daily minimum serving of each diet.
Table 1.
| Serving size (g) | kcal | |
|---|---|---|
| Butter | 10 | 74·4 |
| Cheese (n 12‡) | 38 | 132·2 |
| Milk | 250 | 115·5 |
| Egg | 60 | 85·2 |
| Yogurt | 125 | 79·9 |
| White meat (n 7‡) | 128 | 222·1 |
| Red meat (n 5‡) | 122 | 232·3 |
| White ham and bacon (n 3‡) | 60 | 68·3 |
| Sausages (n 4‡) | 75 | 236·9 |
| Mortadella | 26 | 78·3 |
| Salami | 50 | 228·5 |
| Fatty fishes (n 3‡) | 102 | 173·0 |
| Lean fishes (n 3‡) | 100 | 110·8 |
| Offals (n 6‡) | 163 | 297·6 |
| Seafoods (n 4‡) | 112 | 103·8 |
| Means | 95 | 149 |
| % daily kilocalories from one serving of animal food§ | 7·5 | |
Based on LaNutrition.fr website (https://www.lanutrition.fr/bien-dans-son-assiette/bien-manger/les-recommandations-de-lanutrition.fr/une-portion-cest-combien).
Based on French Ciqual database.
Number of different foods.
Based on a daily basis of 2000 kcal for an adult, that is, (149 × 100)/2000.
Determination of non-ultra-processed: ultra-processed energetic ratio
In brief, UPF were derived from the NOVA classification(61) and ‘are formulations of ingredients, mostly of exclusive industrial use, that result from a series of industrial processes (hence “ultra-processed”) … Processes include the fractioning of whole foods into substances, chemical modifications of these substances, assembly of unmodified and modified food substances, frequent use of cosmetic additives and sophisticated packaging’ (pp. 936 and 937)(62). For example, UPF may include, among others, carbonated soft drinks, sweet or savoury packaged snacks, chocolate, candies (confectionery), ice cream, mass-produced packaged breads and buns, margarines and other spreads, biscuits, pastries, cakes and cake mixes, breakfast ‘cereals’, burgers, hot dogs and other reconstituted meat products, etc.(62) To date, thirty-four epidemiological studies have reported about excess ultra-processed energy consumption and the risks of chronic diseases and/or metabolic dysregulations(38,39). The most studied chronic disease was overweight/obesity, with three ecological studies, five cross-sectional studies and four longitudinal prospective studies(39). Overweight/obesity was the most studied metabolic dysregulation, and is the first step to chronic diseases such as CVD and/or cancers(63). Therefore, obesity risk was the criterion used to select studies for the evaluation of two thresholds: (i) the median threshold of ultra-processed energetic intake at which the risk of overweight/obesity begins to significantly increase, and calculated from all selected studies; and (ii) the precautionary threshold, which corresponds to the minimum consumption of ultra-processed energy content at which the risk of overweight/obesity begins to significantly increase.
The 3V rule and nutritional needs
Based on the evaluated thresholds of animal and ultra-processed energetic percentages, we then tested the ability of a 3V-based diet to address nutritional needs by defining a weekly standard diet pattern based on common staple foods consumed in France, considered as an industrialised country (Table 2), from the French Ciqual(58) and American USDA(59) databases. This French generic diet also addressed food diversity, that is, at least two different food varieties were used among red meat, white meat, seafood, fish, eggs and dairy products and among cereals, nuts and seeds, legumes, fruits, vegetables and tubers. For example, for the cereal group, maize and wheat were used, not only wheat-based products; for fruits, more than one fruit type was used; for dairy products, cheese, yogurt and milk were used, and so on. From this theoretical and generic diet, the level of macronutrients, fibre and micronutrients (vitamins, minerals and trace elements) was calculated, and included at-risk nutrients when decreasing animal products or consuming too many UPF. These latter nutrients include essential amino acids (EAA, 13 g/d), SFA (12 % maximum of daily kilocalories), simple sugars from added sugars, fruit juices and honey as defined by the WHO (daily 10 % maximum energy content)(64), vitamin B12, vitamin D, vitamin A, Ca, iodine and salt, and linoleic acid (LA), α-linolenic acid (ALA), EPA, DHA, with a selected average conversion rates of ALA in EPA and DHA of 14 and 5 %, respectively(65).
Table 2.
Number of recommended animal servings and energy content for health-protective diets worldwide
| Protective diets | Min–max servings per day* | Average daily energy content (%)† | References for health outcomes |
|---|---|---|---|
| Thirty-seven countries | |||
| Actual | – | 26·7 | (37) |
| National recommendation | – | 21·9 | (37) |
| French consumption | |||
| Actual: INCA3 | Approximately 5–6 | 37–45 | (88) |
| Recommendation: PNNS4 | Approximately 3–4 | 23–30 | (89,127) |
| Traditional diets | |||
| Okinawan | Approximately 0–3(67) | 11·3 | Global health(66,67,128,129) |
| Palaeolithic | Approximately 6–7(68) | 48·8 | Weight, BMI and waist circumference(130); CVD risk factors(131) |
| Mediterranean | Approximately 2–4(70) | 22·5 | All-cause mortality(132–135); chronic diseases(69,136,137); bone mineral density and fractures(138,139); CVD(140–143); hypertension(144); cancers(145–149); mental health(143,150–152); type 2 diabetes(153–155); glycaemic control, body weight and CV risk factors(156,157) |
| Nordic/Baltic Sea | Approximately 2–3(158) | 18·8 | Global health(71); insulinaemia(159); cardiometabolic markers(160); cognitive functions(161) |
| Generic diets | |||
| Prudent | Approximately 1–4 | 18·8 | Cognitive decline(162); insulin resistance(163); global health(75) |
| Vegetarian | Approximately 1–3(164) | 15·0 | Cardiometabolic risk factors(165); coronary/ischemic heart disease mortality(166–168); type 2 diabetes(169); inflammatory biomarkers(170); cancers(167,168); body weight(171); blood pressure(172); glycaemic control(173); triglyceridaemia(174) |
| DASH | Approximately 2–4(77) | 22·5 | Colorectal cancer(175,176); metabolic syndrome(177); serum inflammatory markers(178); body weight and waist circumference(179,180); CV risk factors(181); CVD(182) blood pressure(179,183); glycaemic control(184) |
| Anti-inflammatory | Approximately 0–1 | 3·8 | All-cause mortality(185); CVD(186); depression(187); obesity(188) |
DASH, Dietary Approach to Stop Hypertension.
Number of minimum and maximum servings has been defined from data available in scientific papers and recommended food guide pyramids found on websites.
Two servings of animal-based foods daily is around 15 % energy content based on a weekly 3 V-based diet (see Table 1).
The calculations were based on a standard 2000 kcal/d for a healthy adult, including 1·5 l/d of mineral water. The weekly supply of nutrients was compared with the dietary reference intake (DRI) in percentages and other recommendations. Finally, based on the French food guide pyramid, the diet was also designed to balance – among plant-based foods – (i) whole-grain cereals, legumes, nuts and seeds, that is, approximately 30–40 % daily energy content, (ii) fruits and vegetables, that is, approximately 20–30 % daily energy content and (iii) added fats and sugars, that is, approximately 15 % daily energy content.
Results
Dimension 1: determination of a median optimal value of daily animal energy intake
The most studied diets were Okinawan, prudent, vegetarian, Mediterranean, Palaeolithic, Dietary Approach to Stop Hypertension (DASH), anti-inflammatory and Nordic diets (Table 2). Others such as Inuit, portfolio, flexitarian and ketogenic diets were very specific diets and/or not sufficiently studied. Scientific evidence towards their protective potentials was not enough relevant or consensual in order to include them in the current study. Notably, the ketogenic diet was tested in very specific pathological and clinical conditions, not as a preventive diet towards the abovementioned chronic conditions; the Inuit diet is confined to a very specific region; and there were no epidemiological studies on the portfolio diet.
We found four a priori traditional diets (i.e., Okinawan, Palaeolithic, Mediterranean and Nordic/Baltic Sea diets) and four a posteriori generic diets (i.e., prudent, vegetarian, anti-inflammatory and DASH diets) as being potentially protective against the development of chronic diseases (Table 2):
The traditional Okinawan diet is a low-energy, nutrient-dense, antioxidant-rich diet pattern(66,67). The number of servings of animal products is 0–3 per day, that is, approximately 11·3 % energy content(67).
The Palaeolithic diet is the richest in animal products (6–7 servings per day), allowing around equal proportions of animal/plant energy content daily(68).
The Mediterranean diet(69) provides an average of 2–4 servings daily corresponding to approximately 22·5 % energy content(70).
The Nordic/Baltic Sea diet is an umbrella term that encompasses any interpretation that combines food-based dietary guidelines with local Nordic foods(71,72), corresponding to approximately 22·5 % animal energy content, that is, 2–3 servings of animal products per day(73).
Based on a posteriori scientific evidence, four generic diets have been proposed to prevent chronic diseases (Table 2):
The prudent diet – in which saturated/trans-fat intake is <10 % of total energy content and cholesterol is <300 mg/d and/or fibre intake is ≥25 g/d for women and ≥35 g/d for men(74) – is typically high in total fat (35–45 %) but low in SFA (7–8 % of energy), with an average of 1–4 servings of animal products per day, that is, approximately 18·8 % energy content(75).
The vegetarian diet(76) includes approximately 1–3 servings per day of animal products, that is, an average of 15 % daily energy content.
DASH(77) indicates 2–4 servings of animal products per day(77), that is, an average of 22·5 % animal energy content.
The anti-inflammatory diet indicates fewer animal products with 0–1 daily serving, that is, approximately 9·4 % energy content.
Considering these eight traditional/a posteriori generic diets and the minimum number of recommended daily animal servings (Table 2), a median of 11·3 % animal energy content per day was reached, that is, 1·5 servings per day.
Several international agencies have proposed foresight scenarios to define sustainable diets to protect both human health and the environment at horizon 2050, mainly by reducing environmental impacts arising due to food systems (Table 3). Our search identified eight relevant scenarios that take into consideration such environmental impacts as climate change, land and water use and biodiversity, among others: Agrimonde-Terra(78), EAT-Lancet(79), WWF2050(80,81), Nordic Sufficiency(82), Swiss FeedNoFood2050(83), Afterres 2050(84), IDDRI(85) and Millennium Institute(86) (Table 3).
Table 3.
Number of animal daily energy content calculated from foresight diets for both health and sustainable food systems
| Foresight scenarios at horizon 2050 | Animal energy content (%) per day | Environmental outcomes | References |
|---|---|---|---|
| International/European scenarios | |||
| Millennium Institute: scenario 4 | Approximately 16·0 | Food loss and waste, crop production for animal feed | (86) |
| Agrimonde-Terra (INRA-Cirad): ‘healthy’ scenario | Approximately 16·7 | Climate change (GHGE), land use, urbanisation, cropping and livestock systems | (78) |
| EAT-Lancet | Approximately 13·6 | Land system change, biodiversity loss, freshwater use, climate change (GHGE), N and P cycling, food waste | (79) |
| IDDRI: TYFA scenario | Approximately 13·5 | Land use, natural prairies and biodiversity, symbiotic N, extensification of livestock and plant production, abandonment of pesticides, climate change | (85) |
| Regional scenarios | |||
| WWF2050: French scenario | Approximately 25·7 | Land and water use, climate change (GHGE), nitrogen balance, use of fertilisers and phytosanitary products | (80,81) |
| Afterres 2050: French scenario | Approximately 24·3 | Climate change (GHGE), land use, pesticide use, plant proteins, non-food valuations, intensive livestock | (84) |
| Nordic Council of Ministers: Nordic sufficiency scenario | Approximately 17·9 | Local resources, organic farming system, climate changes (GHGE), biodiversity, grazing, food waste, eutrophication, N balance, pesticides | (82) |
| Research Institute of Organic Agriculture FiBL: Swiss FeedNoFood2050 scenario | Approximately 14·7 | Environmental, economic and social performance | (83) |
GHGE, greenhouse gas emission; TYFA, Ten Years for Agroecology; FiBL, Swiss Research Institute of Organic Agriculture.
Concerning international foresight scenarios, Agrimonde-Terra, focusing on land use for a healthy diet, is based on 500 kcal of animal energy content among a total of 3000 kcal, that is, 16·7 % animal energy content(78). EAT-Lancet designed a ‘universal’ healthy diet for a sustainable food system with approximately 13·6 % animal energy content(79). The Millennium Institute, focusing on total food waste and losses and total harvested area, proposed four scenarios, among which scenario four takes the most environmental issues into consideration, with approximately 16·0 % daily animal energy content. At the European level, the IDDRI proposed the TYFA (Ten Years for Agroecology) scenario that corresponds to around 13·5 % daily animal energy content(85).
Concerning regional scenarios, the French scenarios proposed approximately 24·3 % animal energy content for Afterres 2050(87) and approximately 25·7 % animal energy content for WWF France(80,81). The Swiss Research Institute of Organic Agriculture (FiBL) proposed an optimised diet, called the FeedNoFood2050 scenario, that assumes an improved use of agricultural land by feeding only grass and by-products to livestock, which are not competing with direct human nutrition, that is, do not require arable land (neither in Switzerland nor abroad), thereby allowing good social, health, economic and environmental sustainability compared with their reference diet(83). It corresponds to approximately 14·7 % daily animal energy content (Table 4). Finally, the Nordic Council of Ministers proposed a generic sufficiency scenario for all Nordic countries, with approximately 17·9 % daily animal energy content(82).
Table 4.
Number of maximum ultra-processed daily energy content for increased risk of overweight/obesity (from epidemiological studies)
| Country | Type of study and population | Daily energy content from ultra-processed foods (%)* | Overweight/obesity prevalence | References |
|---|---|---|---|---|
| Brazil | Cross-sectional: 55 970 Brazilian households | 17·3 (Q2) v. 11·0 (Q1) | +2·1 % obesity prevalence (predictive value) | (91) |
| Brazil | Cross-sectional: 30 243 individuals aged ≥10 years | 14–22 (Q2) v. 0–13 (Q1) | +29 % obesity prevalence | (90) |
| Spanish | Longitudinal: 8451 middle-aged Spanish university graduates | 18·6 (Q2) v. 10·4† (Q1) | +15 % incident overweight/obesity | (95) |
| USA | Cross-sectional: 15 977 adults (20–64 years) | 36·6–49·9 (Q2) v. ≤36·5 (Q1); 50·0–60·9 (Q3) v. ≤36·5 (Q1) | +20 % overweight; +19 % obesity | (93) |
| Brazil | Longitudinal: 4525 civil servants aged 35–74 years at baseline | 23·9–30·8 (Q3) v. 0–17·7 (Q1); between 0 and 30 % (restricted cubic spline analyses) | +36 % incident overweight/obesity; linear increase in overweight/obesity risk up to approximately +59 %, then plateaued | (94) |
| Canada | Cross-sectional: 19 363 adults aged ≥18 years | 36·1 (Q2) v. 20·1 (Q1); for each +10 % | +8 % obesity risk; +3 % obesity risk | (92) |
| Median maximum UPF energetic threshold from which obesity risk significantly increases | 21·5 | |||
| Precautionary UPF energetic threshold | ≥14·0 % → +29 %; approximately 14·2 % → +29 % | Logistic regression models; cubic spline analyses | (90,94) | |
UPF, ultra-processed food.
As defined by NOVA classification.
Servings were converted into kilocalories based on the 3 V-based diet (Table 2), that is, one serving is approximately 6·9 % kcal daily.
Taking into account traditional/a posteriori generic diets (median 11·3 %) and foresight diet scenarios (median 16·4 %) together, a median value of approximately 15·0 % animal energy content was reached. In comparison, actual animal energy consumption in France is approximately 37–45 %(88) and that recommended by French PNNS is approximately 25–30 %(89) (Table 2). Beyond France, considering thirty-seven countries, the average recommendation of daily animal energy intake is 21·9 v. 26·7 % for actual consumption (Table 2)(37).
Dimension 2: determination of the maximum value of daily ultra-processed energy content
Six epidemiological studies – four cross-sectional(90–93) and two longitudinal(94,95) – investigated the association between increased risk of overweight/obesity and excess ultra-processed energetic consumption (Table 4). In the first Brazilian study involving 55 970 households, obesity risk increased by 2·1 % beyond 17·3 % ultra-processed energy content(91). In the second Brazilian study involving 30 243 individuals aged ≥10 years, obesity risk increased by 29 % with the share of ultra-processed energy content increasing from 14 to 22 %(90). In the third study conducted in the USA, an overweight risk of over 20 % was observed from 36·6 % UPF energy content(93). Finally, in a Canadian cross-sectional study involving 19 363 adults aged ≥18 years, obesity risk increased by 8 % from 36·1 % ultra-processed energy content, and by 3 % for each 10 % increase of ultra-processed energy content(92). Concerning longitudinal studies, the first Brazilian cohort comprised 4525 civil servants aged 35–74 years at baseline, and obesity risk increased by 36 % from 23·9 % ultra-processed energy content, with a linear increase between 0 and 30 % ultra-processed energy content up to approximately 59 % risk of overweight/obesity(94). The second longitudinal study was carried out involving in 8451 middle-aged Spanish university graduates, where overweight/obesity risk significantly increased from 2·7 servings per day of UPF, that is, approximately 18·6 % of energy content(95).
From these studies, the median daily ultra-processed energetic percentage at which obesity risk begins to significantly increase is approximately 21·3 % (Table 5). Taking the lowest precautionary threshold, we obtained 14·0 % at which obesity risks significantly increased by over 29 %(90). At 14 %, a cubic spline analysis in the longitudinal study by Canhada et al. (94) showed an increased overweight/obesity risk of approximately 29 % (Table 5).
Table 5.
A weekly standard French diet simulating the 3V rule*
| Day 1 | Serving size | kcal | Day 2 | Serving size | kcal | Day 3 | Serving size | kcal | Day 4 | Serving size | kcal | Day 5 | Serving size | kcal | Day 6 | Serving size | kcal | Day 7 | Serving size | kcal | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breakfast | Muesli | 50 | 212 | Muesli | 50 | 212 | Muesli | 50 | 212 | Muesli | 50 | 212 | Breakfast cereals | 30 | 117 | Muesli | 50 | 212 | Croissant | 90 | 338 |
| Milk | 150 | 69 | Milk | 150 | 69 | Milk | 150 | 69 | Milk | 150 | 69 | Milk | 150 | 69 | Yogurt | 175 | 112 | Coffee | 240 | 11 | |
| Sugar | 24 | 96 | Honey | 30 | 98 | Sugar | 24 | 96 | Sugar | 24 | 96 | Sugar | 24 | 96 | Sugar | 24 | 96 | Sugar | 24 | 96 | |
| Orange | 200 | 94 | Orange juice | 200 | 90 | Banana | 240 | 216 | Soya milk | 50 | 30 | Kiwi | 230 | 135 | Apple juice | 200 | 85 | ||||
| Coffee | 60 | 3 | Coffee | 60 | 3 | Coffee | 60 | 3 | Thé | 200 | 1 | Tea | 200 | 1 | Coffee | 60 | 3 | ||||
| Lunch | Beetroot salad | 100 | 47 | Avocado | 200 | 310 | Grapefruits | 120 | 54 | Hard boiled eggs | 50 | 67·0 | Salad | 50 | 7 | Carrot purée | 175 | 112 | |||
| Vinaigrette | 30 | 139 | Vinaigrette | 30 | 139 | Sugar | 12 | 48 | Mayonnaise | 15 | 104 | Vinaigrette | 30 | 139 | Vinaigrette | 30 | 139 | Industrial chips | 30 | 164 | |
| Fried chicken wing | 100 | 213 | Fried lean fish | 75 | 94 | Industrial beefsteak | 100 | 231 | Fried fatty fish | 85 | 194 | Veal liver | 100 | 137 | Pie pâté | 75 | 219 | Cheese | 25 | 87 | |
| Fried potatoes | 200 | 276 | Fish sauce with lemon | 15 | 19 | Boiled lentils with salt | 100 | 124 | Virgin rapeseed oil | 15 | 135 | Wholemeal pasta | 210 | 302 | Cola soda | 330 | 138 | Royal pizza | 100 | 226 | |
| Vanilla ice-cream | 50 | 101 | Brown rice | 150 | 237 | Virgin rapeseed oil | 15 | 135 | Cauliflower | 175 | 37 | Fruit yogurt | 125 | 80 | Strawberries | 90 | 31 | Chocolate fondant | 40 | 146 | |
| Whole-grain wheat bread | 75 | 192 | Virgin rapeseed oil | 15 | 135 | Apple sauce | 130 | 133 | Lemon pie | 75 | 287 | Multi-cereal bread | 75 | 179 | Multi-cereal bread | 75 | 179 | Whole-grain wheat bread | 50 | 128 | |
| Fruit salad | 130 | 65 | Whole-grain wheat bread | 75 | 192 | Whole-grain maize bread | 75 | 299 | |||||||||||||
| Whole-grain wheat bread | 75 | 192 | |||||||||||||||||||
| Collation | Dried fruits | 36 | 109 | Banana | 240 | 216 | Almonds | 35 | 222 | Walnut | 30 | 213 | Apple | 276 | 149 | Industrial biscuits | 50 | 180 | Whole-grain wheat bread | 50 | 128 |
| Butter | 10 | 73·0 | |||||||||||||||||||
| Chocolate | 20 | 110·6 | |||||||||||||||||||
| Diner | Vegetable soup | 234 | 92 | Salad of raw vegetables | 150 | 59 | Vegetable soup | 234 | 87 | Lentil salad | 100 | 124 | Bean soup | 250 | 333 | Salad of raw vegetables | 150 | 59 | Salad of raw vegetables | 75 | 14 |
| Pineapple | 80 | 43 | Vinaigrette | 30 | 139 | Plum | 100 | 49 | Vinaigrette | 30 | 139 | Apple | 276 | 149 | Vinaigrette | 30 | 139 | Vinaigrette | 30 | 139 | |
| Whole-grain wheat bread | 75 | 192 | Whole-grain wheat bread | 75 | 192 | French baguette | 50 | 141 | Grape | 100 | 128 | Multi-cereal bread | 50 | 119 | Fruit salad | 130 | 65 | Homemade fruit cake | 90 | 352 | |
| Virgin rapeseed oil | 15 | 135 | Dried seaweed (Nori) | 10 | 25·5 | Virgin rapeseed oil | 15 | 135 | Whole-grain maize bread | 50 | 199 | Virgin rapeseed oil | 15 | 135 | Multi-cereal bread | 75 | 179 | Industrial refined bread | 50 | 141 |
The diet comprises 14·1 and 15·0 % energy content from ultra-processed and animal foods, respectively.  , animal foods;
, animal foods;  , ultra-processed foods;
, ultra-processed foods;  , both ultra-processed and animal-based foods.
, both ultra-processed and animal-based foods.
Fifteen per cent maximum animal and ultra-processed energy content v. nutritional needs
Based on generic staple foods and including both maximum 15 % animal and ultra-processed energy content and a variety of food groups (≥42 different animal and plant-based foods), a standard French generic diet was designed (Table 5). This diet comprises 36·1, 51·6 and 12·4 % lipid, carbohydrate and protein energy content, respectively. Whole grains and nuts, fruits and vegetables and added plant oils and sugars account for 33·7, 20·2 and 14·5 % of total energy content, respectively.
With reference to a daily energy requirement of 2000 kcal for a healthy adult, this standard diet supplies 2054 daily energy content, including 14·5 and 15·4 % energy content provided by ultra-processed and/or animal foods, respectively, that is, some animal-based foods were also ultra-processed (Table 5). A further analysis of this diet proceeded to address the levels of nutrients according to dietary recommended intake (Table 6). Sparing a few, most of the nutrients were found to be close to DRI. Added sugars were slightly below the maximum 10 % recommended by the WHO, that is, 9·3 %. SFA contributed to 9·1 % compared with the maximum recommended intake of 12 % daily energy content. Essential amino acids were largely addressed, that is, >13 g/d. If EPA and DHA were below recommendations (45 and 94 %, respectively), the contribution from ALA converting into EPA (approximately 14 % of ALA) and DHA (approximately 5 % of ALA) allowed reaching the DRI with 163 and 136 %, respectively. In addition, salt consumption and the LA:ALA ratio were below the maximum recommended level of 5 g/d and < 5, respectively. Without the addition of dried seaweed (nori), iodine DRI was not reached, at only 88 %. If veal liver contributed greatly to the vitamin A supply (106·8 % of DRI without veal liver, instead of 306·8 %), then β-carotene remains a relevant supply of provitamin A. Finally, vitamin D was only 19·1 % of the DRI, without considering vitamin D synthesis through exposure to sun.
Table 6.
A weekly simulation of a 3 V-based French diet against dietary reference intakes (DRI)*
| Nutrients | DRI | % DRI |
|---|---|---|
| Energy content | 2000 kcal† | 101·3 |
| Proteins (11·6 %) | 58·1 g/d‡ § | 104·6 |
| Carbohydrates (49·1 %) | 238 g/d§ | 103·3 |
| Lipids (37·5 %) | 83·3 g/d§ | 94·7 |
| Essential amino acids | 13 g/d | 164·2 |
| Simple (added) sugars∥ | 10 % kcal maximum | 92·7 (9·3 % kcal) |
| SFA | 12 % of kcal maximum | 75·8 (9·1 % kcal) |
| Fibre | 30 g/d | 102·8 |
| LA (C18:2 n-6) | 8·9 g/d | 109·0 |
| ALA (C18:3 n-3) | 1·8 g/d | 116·9 |
| ALA/LA | 5 maximum | 4·6 |
| EPA (C20:5 n-3) | 250 mg/d | 44·7 (162·6 with ALA)¶ |
| DHA (C22:6 n-3) | 250 mg/d | 93·6 (135·6 with ALA)¶ |
| Vitamin B1 | 1·3 mg/d | 105·9 |
| Vitamin B2 | 1·8 mg/d | 111·4 |
| Vitamin B3 | 17·4 mg/d | 120·3 |
| Vitamin B5 | 5·8 mg/d | 111·7 |
| Vitamin B6 | 1·8 mg/d | 118·7 |
| Vitamin B9 | 330 μg/d | 144·7 |
| Vitamin B12 | 4 μg/d | 304·6 |
| Vitamin C | 110 mg/d | 111·6 |
| Vitamin A (retinol equivalent)** | 750 μg/d | 306·8 |
| Vitamin D | 15 μg/d | 19·1 |
| Vitamin E | 10·5 mg/d | 143·3 |
| Vitamin K | 70 μg/d | 147·9 |
| Ca | 900 mg/d | 94·3 |
| Cu | 1·3 mg/d | 342·0 |
| Fe | 11 mg/d | 119·1 |
| Zn | 7·5 mg/d | 108·7 |
| Mg | 420 mg/d | 109·6 |
| Mn | 2·8 mg/d | 190·5 |
| Se | 70 μg/d | 293·6 |
| P | 700 mg/d | 164·4 |
| Iodine | 150 μg/d | 136·2 |
| K | 2000 mg/d | 149·7 |
| Chloride | 2300 mg/d | 121·7 |
| Na | 1900 mg/d | 192·4 |
| Salt | 5·0 g/d maximum | 4·7 g/d |
LA, linoleic acid; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
See Table 5: 15 % ultra-processed foods represent 2·25 % of ultra-processed animal energy content among the 15 % (i.e., two servings per week) and 12·75 % of plant energy content among the 85 % (i.e., twelve servings per week); the diet comprises 12·1, 35·2 and 52·7 % of protein, lipid and carbohydrate energy content, respectively; 20·2, 33·7 and 14·5 % of fruits/vegetables, whole grains/legumes/nuts and added fat/sugar energy content, respectively, based on 1·5 l/d of mineral water.
For a healthy adult.
For an adult weighing 70 kg.
Daily intakes of macronutrients are only indicative because recommendations are 10–20 % for proteins, 40–55 % for carbohydrates and 35–40 % for lipids.
Simple sugars from added sugars, fruit juices and honey.
Conversion rates of ALA to EPA and DHA are on average 14 % (8–20) and 5 % (0·5–9), respectively(65).
Including β-carotene converted to vitamin A (where 12 mg β-carotene = 1 mg vitamin A equivalent).
Discussion
Our results showed that it is possible to design a complex diet compatible with both protection of human health and food system sustainability. Based on the ‘3V rule’ – for Végétal (plant), Vrai (real) and Varié (varied, if possible organic, local and seasonal), in French – these three dimensions are interconnected in the proposed diet. Therefore, a 3V-based diet may range between 15 and 30 % of both animal and/or ultra-processed energy content. For example, if all animal products are ultra-processed, the remaining 85 % energy content are all non-ultra-processed, plant-based products; and if all animal energy content are non-ultra-processed, then the remaining energy content are 15 % ultra-processed, plant-based energy content and 70 % are non-ultra-processed, plant-based energy content.
Otherwise, although tending towards a certain form of genericity and converging towards similar conclusions of EAT-Lancet (see key message no 4)(79), the current study had different purposes. While EAT-Lancet notably focused on the percentage shares of different food groups for reaching ‘universal’ sustainability, the current study aimed to extract the potential generic and fundamental dimensions that would help in defining a healthy and sustainable diet, and that can be adapted and also used as levers by different public health policies. Thus, none of the diets considered by the current study (to propose percentage shares of animal and ultra-processed energy content) have taken into consideration the three dimensions of the 3V rule. In addition, the degree of food processing, based on the UPF concept, was not directly considered in EAT-Lancet. Finally, the 3V rule appears more to be an ‘umbrella concept’ for a downstream (rather than upstream) appropriation by the society at large through dieticians, nutritionists, restaurateurs, consumers and encompassing the abovementioned studies and reports.
The 3V rule and nutritional needs
Our calculations were made within the framework of a French-style diet characteristic of developed countries. However, similar simulations could be done within other contexts where different types of animal- and plant-based products are consumed. Fifteen percentage of animal energy content may appear small at first view, but it is sufficient to supply all the essential macro- and micronutrients, provided the third rule (varied) is correctly applied (Tables 5 and 6). In addition, a reduction in ultra-processed energy content (‘empty’ energy content) will bring in new, minimally processed nutrient-dense foods rich in bioactive compounds that are protective against chronic diseases (i.e., more fibre, vitamins, minerals and antioxidants)(46–51,96,97). Notably, iodine and vitamin A DRI are easier to address when including a weekly serving of seaweeds/seafood (e.g., dried seaweeds) and offal/giblets (e.g., animal liver), respectively. Concerning EPA and DHA, a sufficient supply in ALA, notably through adequate use of plant oils, allows the DRI to be reached. Concerning vitamin D, the issue is different because many diets worldwide do not address its DRI, and many people in most countries are more or less vitamin D-deficient(98). Therefore, vitamin D deficiency is not due to the proposed 3V rule-based diet. Approximately one billion people worldwide have low levels of vitamin D in their blood(99). Notably, according to a 2011 study, 41·6 % of adults in the USA are deficient, although their omnivorous diet is still rather rich in animal energy content(100). Such deficiency may be attributable to a high consumption of ‘empty’ energy content, that is, a nutrient-poor diet(101), and low sun exposure. Normally, adequate exposure to sun (UV B radiation) should allow addressing the vitamin D DRI(98), with differences in the needed exposure time between summer (10–20 min) and winter (2 h) periods. Beyond the mere quality of the diet, the question of vitamin D supplementation deserves to be addressed. Another solution for people not getting enough exposure to sun would be to consume approximately 35 g of cod liver oil weekly to achieve 100 % of DRI(58).
Reducing animal energy content to 15 % daily
The first ‘Végétal’ rule is probably the most problematic and controversial, for which it is very difficult to reach consensus. Globally, a consensus over achieving the balance between animal- and plant-based food energy content to improve the healthiness of a diet is still lacking. A theoretical value of 15 % animal energy content was proposed based on literature and determining a median value from combined minimal values proposed within these supposedly healthy diets. First, the median animal energy intake of eight recognised protective diets is 13·1 %, but only 11·3 % when considering the minimum number of daily servings. The eight selected scenarios for reaching diet sustainability at horizon 2050 converge to a median value of 16·4 % daily animal energy content. Combining both observational and foresight diets, a level of approximately 15 % daily animal energy content appeared to be optimum, particularly when respecting the two other rules, that is, ‘Vrai’ and ‘Varié’. Therefore, including environmental outcomes does not substantially modify the animal energy share of a diet to be protective of human health.
In developed countries where animal foods take a high level of energy share, plant-based foods are reported to be a relevant alternative(11,12,15,31,37), even if some geographical regions, such as the Arctic, are more hostile for plant growth. In France where animal energy content are reaching approximately 37–45 % of energy share(88), at least 20 % can be replaced by under-consumed niche foods such as whole-grain cereals(102), nuts and seeds(88) and legumes(88), all currently consumed under 14 g/d. In addition, this could contribute to sustainable crop production, especially legumes(103). A diet based on a maximum of 15 % animal energy content is close to the recently emphasised healthy flexitarian diet(104), meaning that animal foods would become an exception, which is not commonly the case today in western countries. In addition, as recently pointed out, ‘an increase in the adoption of plant-based diets presents an opportunity for the world to re-evaluate how meat can be sustainably produced, with greater emphasis on animal welfare, nutritional value, product safety, better utilisation, and distribution channels’(105). Notably, Shepon et al. (106) demonstrated that by replacing all animal-based foods in the US diet with plant-based alternatives will add enough food to feed 350 million more people, while pointing out the importance of dietary shifts to improve food availability and security. This dietary shift may well correspond to the well-known flexitarian diet (for which, however, the dimension ‘real’ (‘Vrai’) is lacking in its accepted definition, notably through the level of UPF, and for which the dimension ‘varied’ is incomplete through the absence of ‘local’ and ‘seasonality’ criteria). Applying the 3V rule is also in agreement with the fact that a shift towards 85 % energy content being provided by plant-based foods should be very effective in reducing GHGE(107). Other researchers suggested that moving to a flexitarian diet will lead to reduced GHGE (−54 %), land use (−8 %) and water footprint (−11 %)(108). The impact of dietary change was reported to be the highest on GHGE by partially replacing meat with plant-based foods, with effects being reduced through a replacement with mixed foods or dairy(109).
However, the impact of dietary changes on sustainability is also driven by the national income levels(110). In high-income countries, the nationally recommended diets are associated with reductions in GHGE, eutrophication and land use from 13·0 to 24·8 %, 9·8 to 21·3 %, and 5·7 to 17·6 %, respectively, while in upper-middle-income nations, such a recommended diet is only associated with slight decreases in impacts of 0·8–12·2 %, 7·7–19·4 % and 7·2–18·6 %(110). The authors concluded that ‘reduced environmental impact in high-income countries is driven by reductions in energy content (≈54 % of effect) and a change in composition (≈46 %)’ while in low- and middle-income nations, the increased environmental impacts are associated with increased intake in animal products’(110). Therefore, the adoption of a 3 V-based diet would mean a drastic reduction in the use of animal products in developed countries, stabilisation in emerging countries and an increase in developing countries where the daily animal energy share is under 10 % most of the time.
An important but neglected issue when considering food system sustainability is animal welfare. Scherer et al.(19,37) were among the first to quantify the impacts of animal consumption on animal welfare through the development of an animal protection index. They found that animal welfare loss, associated with the current omnivorous average diet, is mostly driven by poultry and egg consumption (for animal life-years suffered and loss of morally adjusted animal lives) and by seafood consumption (for loss of animal lives)(19). The 3V rule, leading to the consumption of fewer animal products, but of higher quality animals raised in extensive conditions, is necessarily associated with improved animal wellbeing.
Limiting ultra-processed energy content to 15 % daily
The worldwide consumption of ultra-processed energy content is high, especially in western and Anglo-Saxon countries, often >40–50 % of daily energy content, and is growing rapidly in emerging countries(57,111). In France, it accounts for at least 36 % of daily energy content(112), even more when including the poorest who consume most of these products that are low in protective micronutrient density(113). Based on the NOVA classification(61), a decreased intake of energy content would imply more home cooking of bulk plant-based foods and returning to mildly/minimally processed foods such as pasta, plain yogurts, artisanal cheeses, breads and delicatessen, frozen or canned plant/animal-based products, dried fruits, nuts and seeds, whole fruits, muesli, butter, and so forth. As for animal energy content, a decreased intake of UPF for improving healthiness is recommended by international institutions based on evidence from prospective epidemiological studies(17,38). According to literature, a precautionary level of 15 % of daily energy content is proposed.
From epidemiological studies on obesity risk (Table 4), this 15 % threshold is only a proposition of a precautionary maximum threshold at which overweight/obesity risk was found to increase by approximately 29 % in two studies(90,94), awaiting other studies to be reported. Fifteen per cent of ultra-processed energy content correspond to approximately 1–2 servings per day; these foods might be generally more energy-dense than minimally processed foods due to added fats and sugars and lower water activity(96). Disparity among the selected studies was observed, wherein overweight/obesity risks began to significantly increase from the higher daily UPF energy shares in USA and Canada (≥36 %) than in Brazil and Spain (≥14 %). However, in the Canadian(92) and American(93) studies, the chosen reference quintiles were rather high for the energetic percentage of UPF (36·5 and 20·1 %), suggesting that significant effects may have been found with a lower reference quintile, as in Spain and Brazil where references were all <18 % daily energy content.
Besides obesity risk, other studies linked UPF with mortality risk(114–117). In a longitudinal study involving a restricted cubic spline analysis of the association between UPF and all-cause mortality, it was found that for each additional serving of UPF, all-cause mortality increased by 18 % (adjusted hazard ratio 1·18, 95 % CI 1·05, 1·33)(117). In a recent study employing restricted cubic spline analyses, a dose–response analysis showed that replacing only 10 % UPF energy content (isoenergetic substitution) with either processed or un-/minimally processed foods might significantly decrease the risk of mortality by approximately 20 and 56 %, respectively(115). For CVD risk, a similar dose–response analysis showed that an increase of 15 % by weight of UPF in the diet increased the risk by approximately 26 % (P-value for non-linearity = 0·39)(118). In a Brazilian modelling study, the authors reduced the intake of saturated fat, trans-fat, salt and added sugar in ultra-processed foods by 75 %, substituting this reduction with a 75 % increase in unprocessed or minimally processed foods(119). The results showed that such a scenario might lead to a reduction in CVD risk by 29 %.
The interconnectedness of the 3V rule
In the current study, three main dimensions were identified to holistically reach food system sustainability: Végétal (plant), Vrai (real) and Varié (varied), preferably organic, local and/or seasonal(1). These three rules are clearly interconnected (Fig. 1), meaning that no one dimension can be excluded without impairing both human health and food system sustainability:
First, except for niche populations (e.g., the Inuits), too many animal energy content would threaten both human health(120,121) and the environment(122), also impacting animal welfare.
Second, replacing real foods with too much of UPF has been consistently shown to increase the risk of several chronic diseases(38,39). Therefore, as suggested for CVD risk in the US adult population, it is not only sufficient to consume more plant-based foods; they must be minimally/normally processed(123). Actually, for each point increase in highly processed plant food serving, it has been shown that CHD risk similarly increased as for each point increase in animal food serving(123).
Finally, replacing the third rule ‘Varied’ by ‘Monotonous’ inevitably leads to micronutrient deficiencies, as observed in some developing countries, for example, sub-Saharan African and South Asian countries, where staple foods are generally not very varied, such as white glutinous rice in Asia or cereal variety in Africa, which may constitute up to >50 % of daily energy content(124). This rule also includes ‘local’ and ‘seasonal’ criteria to be conscious that eco-friendly, healthy foods produced in one country and transported into and consumed by another country may negatively impact environmental factors.
Fig. 1.
Interconnectedness of the 3V rule
Limitations of the current study
Calculations used in the current study were based on French standard serving sizes. Therefore, they are not representative of what can be found worldwide, depending on countries and their traditional habits. On average, a French standard animal serving size is 95 g, corresponding to around 7·5 % of daily energy requirement for an adult (Table 1). The converging figure of 15 % daily animal energy content is therefore only indicative, and allows for some variability around it.
Otherwise, the simulated 3V-based diet is based on French culinary uses, questioning its extrapolation to other culinary traditions. However, the results clearly emphasise the importance of consuming less of UPF with ‘empty energy’ and more of nutrient-dense whole-grain breads, virgin plant oils and various vegetables, legumes and fruits to adequately address all nutritional needs. Food diversity (i.e., varied) is certainly the key point of this 3V rule-based diet.
The median 15 % daily UPF energy threshold is based on six epidemiological studies only, with four cross-sectional studies being of lower quality compared with longitudinal ones. Therefore, 15 % is only indicative. However, a cubic spline analysis in Canhada et al. (94) showed a linear relationship between UPF energy consumption and overweight/obesity risk within a range 0·5–30 % energy content, and the risk begins to increase below 15 %.
Conclusions and perspectives
The ‘3V rule’ diet is a generic diet with three fundamental dimensions: animal:plant food ratio, degree of processing and food variety. In most of the healthy diets analysed here, the second rule – ‘Vrai’ (‘real’) – was neglected, probably because the degree of food processing only received attention recently. However, such a diet would probably not be applicable to niche populations represented by more traditional ethnicities such as Masai, Inuit, Aborigines and other hunter-gatherers whose diets (generally close to a keto-Palaeolithic diet) had long beed adapted to their environment(125,126). It can be noticed that these diets fit with at least ‘Vrai’ and – to a lesser extent – ‘Varié’ dimensions of the 3V rule, since their environment seems to be less favourable to developing food crops, while providing wild animals. Nevertheless, if these particular diets may have been conceived when the worldwide population was under one billion, they may no longer be sustainable today when applying such diets to nearly eight billion people by 2030, and probably 9–10 billion by 2050, many of whom may be living in very large cities. Additionally, the 3V diet is also compatible with a livestock polyculture system, implying animal and plant biodiversity and a circular bio-economy approach to improve the agricultural system.
As discussed previously(1), the 3V rule is sufficiently generic to be regionalised according to specific climatic, environmental, traditional and culinary uses worldwide and, finally, be part of a holistic territorial food plan, involving several stakeholders from society, science and politics. Therefore, a generic 3V-based diet is not in opposition to actual traditional worldwide protective diets. This generic diet does not intend to substitute regional diversity due to different agronomic, climatic, cultural, socioeconomic and religious beliefs, but, rather, to serve as the basic generic rules for improving their sustainability at horizon 2050 while considering regional specificities and growing population worldwide. Notably, the animal:plant energetic ratio and the food diversity may be adapted to different regions worldwide.
Otherwise, this qualitative diet appears to be easy to adopt without an extensive knowledge of nutrition. This diet does not refer to any nutrient while indirectly naturally offering all nutritional needs, allowing the majority of the population to appropriate a healthy diet. Difficulties will probably arise in replacing animal foods with more whole-grain cereals, legumes, nuts and seeds, while limiting highly palatable UPF. A vigilant point of this diet is to consume sufficient virgin plant oils and varied foods.
An interesting interventional study could consist to progressively moving a significant number of individuals consuming an omnivorous western diet (diet rich in animal and ultra-processed energy content) to a 3V rule-based diet and to measure short-term physiological/metabolic parameters with a follow-up of chronic disease prevalence over several years. Another perspective would be to use the lever of the 3V-based diet to develop healthy and sustainable regional diets and to adapt each rule to traditional, climatic, agronomic and socioeconomic specificities through modelling of three-dimensional abacuses.
Acknowledgements
Acknowledgements: None. Financial support: The current research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest: A.F. is a member of the Siga Society since 2017, which is a start-up dedicated to the development of a food scoring tool based on the degree of processing. Authorship: A.F. led the study and defined the 3V rule based on scientific literature. A.F. wrote the manuscript. E.R. contributed to the interpretation of results and to the final writing of the manuscript. Ethics of human subject participation: None.
References
- 1. Fardet A & Rock E (2018) Reductionist nutrition research has meaning only within the framework of holistic thinking. Adv Nutr 9, 655–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gazan R, Brouzes CMC, Vieux F et al. (2018) Mathematical optimization to explore tomorrow’s sustainable diets: a narrative review. Adv Nutr 9, 602–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rutten M, Achterbosch TJ, de Boer IJM et al. (2018) Metrics, models and foresight for European sustainable food and nutrition security: the vision of the SUSFANS project. Agric Sys 163, 45–57. [Google Scholar]
- 4. Ronzon T, Paillard S, Chemineau P et al. (2013) Elements for a foresight debate on food sustainability. In Food System Sustainability: Insights from duALIne, pp. 176–197 [Esnouf C, Russel M & Bricas N, editors]. Cambridge: Cambridge University Press. [Google Scholar]
- 5. Fardet A & Rock E (2014) Toward a new philosophy of preventive nutrition: from a reductionist to a holistic paradigm to improve nutritional recommendations. Adv Nutr 5, 430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vieux F, Soler L-G, Touazi D et al. (2013) High nutritional quality is not associated with low greenhouse gas emissions in self-selected diets of French adults. Am J Clin Nutr 97, 569–583. [DOI] [PubMed] [Google Scholar]
- 7. Payne CLR, Scarborough P & Cobiac L (2016) Do low-carbon-emission diets lead to higher nutritional quality and positive health outcomes? A systematic review of the literature. Public Health Nutr 19, 2654–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van de Kamp ME, van Doorenb C, Hollander A et al. (2018) Healthy diets with reduced environmental impact? The greenhouse gas emissions of various diets adhering to the Dutch food based dietary guidelines. Food Res Int 104, 14–24. [DOI] [PubMed] [Google Scholar]
- 9. Horgan GW, Perrin A, Whybrow S et al. (2016) Achieving dietary recommendations and reducing greenhouse gas emissions: modelling diets to minimise the change from current intakes. Int J Behav Nutr Phys Act 13, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hitaj C, Rehkamp S, Canning P et al. (2019) Greenhouse gas emissions in the United States food system: current and healthy diet scenarios. ES&T 53, 5493–5503. [DOI] [PubMed] [Google Scholar]
- 11. Sjors C, Hedenus F, Sjolander A et al. (2017) Adherence to dietary recommendations for Swedish adults across categories of greenhouse gas emissions from food. Public Health Nutr 20, 3381–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seconda L, Baudry J, Alles B et al. (2018) Comparing nutritional, economic, and environmental performances of diets according to their levels of greenhouse gas emissions. Clim Change 148, 155–172. [Google Scholar]
- 13. Balter K, Sjors C, Sjolander A et al. (2017) Is a diet low in greenhouse gas emissions a nutritious diet? Analyses of self-selected diets in the LifeGene study. Arch Public Health 75, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murakami K & Livingstone MBE (2018) Greenhouse gas emissions of self-selected diets in the UK and their association with diet quality: is energy under-reporting a problem? Nutr J 17, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macdiarmid JI, Kyle J, Horgan GW et al. (2012) Sustainable diets for the future: can we contribute to reducing greenhouse gas emissions by eating a healthy diet? Am J Clin Nutr 96, 632–639. [DOI] [PubMed] [Google Scholar]
- 16. Musicus AA, Moran AJ, Lawman HG et al. (2019) Online randomized controlled trials of restaurant sodium warning labels. Am J Prev Med 57, e181–e193. [DOI] [PubMed] [Google Scholar]
- 17. Food and Agriculture Organization & World Health Organization (2019). FAO-WHO Sustainable Healthy Diets: Guiding Principles. Rome, Italy: FAO & WHO. [Google Scholar]
- 18. Food and Agriculture Organization (2014) The Second International Conference on Nutrition (ICN2): Committing to a Future Free of Malnutrition, 19–21 November 2014 “Better Nutrition, Better Lives”. Rome, Italy: FAO.
- 19. Scherer L, Tomasik B, Rueda O et al. (2018) Framework for integrating animal welfare into life cycle sustainability assessment. Int J Life Cycle Assess 23, 1476–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caillavet F, Fadhuile A & Nichèle V (2016) Taxing animal-based foods for sustainability: environmental, nutritional and social perspectives in France. Eur Rev Agric Econ 43, 537–560. [Google Scholar]
- 21. Millward DJ & Garnett T (2010) Food and the planet: nutritional dilemmas of greenhouse gas emission reductions through reduced intakes of meat and dairy foods. Proc Nutr Soc 69, 103–118. [DOI] [PubMed] [Google Scholar]
- 22. Stoll-Kleemann S & Schmidt UJ (2016) Reducing meat consumption in developed and transition countries to counter climate change and biodiversity loss: a review of influence factors. Reg Environ Change 17, 1261–1277. [Google Scholar]
- 23. Llonch P, Haskell MJ, Dewhurst RJ et al. (2016) Current available strategies to mitigate greenhouse gas emissions in livestock systems: an animal welfare perspective. Animal 11, 274–284. [DOI] [PubMed] [Google Scholar]
- 24. Farvid MS, Stern MC, Norat T et al. (2018) Consumption of red and processed meat and breast cancer incidence: a systematic review and meta-analysis of prospective studies. Int J Cancer 143, 2787–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ranabhat CL, Park M-B & Kim C-B (2020) Influence of alcohol and red meat consumption on life expectancy: results of 164 countries from 1992 to 2013. Nutrients 12, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng Y, Li Y, Satija A et al. (2019) Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ 365, l2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Brandt PA (2019) Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in The Netherlands Cohort Study. Eur J Epidemio 34, 351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Händel MN, Cardoso I, Rasmussen KM et al. (2019) Processed meat intake and chronic disease morbidity and mortality: an overview of systematic reviews and meta-analyses. PLOS ONE 14, e0223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aston LM, Smith JN & Powles JW (2012) Impact of a reduced red and processed meat dietary pattern on disease risks and greenhouse gas emissions in the UK: a modelling study. BMJ Open 2, e001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. White RR & Hall MB (2017) Nutritional and greenhouse gas impacts of removing animals from US agriculture. PNAS 114, E10301–E10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scarborough P, Appleby PN, Mizdrak A et al. (2014) Dietary greenhouse gas emissions of meat-eaters, fish-eaters, vegetarians and vegans in the UK. Clim Change 125, 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernardi E, Capri E & Pulina G (2019) The Sustainability of Meat and Cured Meats In Italy: Nutritional Aspect, Food Safety, Environmental Impact, Animal Welfare, Circular Economy, Fight Against Waste. Milano: FrancoAngeli. [Google Scholar]
- 33. Food and Agriculture Organization, Harinder PSM & Philippe A (2014) Towards a concept of sustainable animal diets. Report Based on the Collated Results of a Survey of Stakeholder Views. FAO Animal Production and Health Report No. 7, Rome.
- 34. Hyland JJ, Henchion M, McCarthy M et al. (2017) The role of meat in strategies to achieve a sustainable diet lower in greenhouse gas emissions: a review. Meat Sci 132, 189–195. [DOI] [PubMed] [Google Scholar]
- 35. Rijsberman F (2017) The key role of the meat industry in transformation to a low-carbon, climate resilient, sustainable economy. Meat Sci 132, 2–5. [DOI] [PubMed] [Google Scholar]
- 36. Adesogan AT, Havelaar AH, McKune SL et al. (2019) Animal source foods: Sustainability problem or malnutrition and sustainability solution? Perspective matters. Glob Food Secur 25, 100325. [Google Scholar]
- 37. Scherer L, Behrens P & Tukker A (2019) Opportunity for a dietary win-win-win in nutrition, environment, and animal welfare. One Earth 1, 349–360. [Google Scholar]
- 38. Food and Agriculture Organization, Monteiro CA, Cannon G et al. (2019) Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System. Rome, Italy: FAO. [Google Scholar]
- 39. Fardet A & Rock E (2019) Ultra-processed foods: a new holistic paradigm? Trends Food Sci Technol 93, 174–184. [Google Scholar]
- 40. Fardet A (2018) Chapter 3: Characterization of the degree of food processing in relation with its health potential and effects. Adv Food Nutr Res 85, 79–121. [DOI] [PubMed] [Google Scholar]
- 41. Monteiro CA (2009) Nutrition and health: the issue is not food, nor nutrients, so much as processing. Public Health Nutr 12, 729–731. [DOI] [PubMed] [Google Scholar]
- 42. Ministry of Health of Brazil (2014) Dietary Guidelines for the Brazilian Population . São Paulo: Secretariat of Health Care, Primary Health Care Department.
- 43. Jacobs DR & Tapsell LC (2013) Food synergy: the key to a healthy diet. Proc Nutr Soc 72, 200–206. [DOI] [PubMed] [Google Scholar]
- 44. Jacobs DR & Steffen LM (2003) Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr 78, 508S–513S. [DOI] [PubMed] [Google Scholar]
- 45. Food and Agriculture Organization (2002) The State of Food Security and Nutrition in the World. Rome, Italy: FAO. [Google Scholar]
- 46. Marron-Ponce JA, Flores M, Cediel G et al. (2019) Associations between consumption of ultra-processed foods and intake of nutrients related to chronic non-communicable diseases in Mexico. J Acad Nutr Diet 119, 1852–1865. [DOI] [PubMed] [Google Scholar]
- 47. Machado PP, Steele EM, Levy RB et al. (2019) Ultra-processed foods and recommended intake levels of nutrients linked to non-communicable diseases in Australia: evidence from a nationally representative cross-sectional study. BMJ Open 9, e029544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gupta S, Hawk T, Aggarwal A et al. (2019) Characterizing ultra-processed foods by energy density, nutrient density and cost. Front Nutr 6, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rauber F, da Costa Louzada ML, Steele E et al. (2018) Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014). Nutrients 10, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cornwell B, Villamor E, Mora-Plazas M et al. (2018) Processed and ultra-processed foods are associated with lower-quality nutrient profiles in children from Colombia. Public Health Nutr 21, 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luiten CM, Steenhuis IH, Eyles H et al. (2016) Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets. Public Health Nutr 19, 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Louzada ML, Martins AP, Canella DS et al. (2015) Impact of ultra-processed foods on micronutrient content in the Brazilian diet. Rev Saude Publica 49, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rani V, Arends DE & Brouwer ID (2010) Dietary diversity as an indicator of micronutrient adequacy of the diet of five to eight year old Indian rural children. Nutr Food Sci 40, 466–476. [Google Scholar]
- 54. Steyn NP, Nel JH, Nantel G et al. (2006) Food variety and dietary diversity scores in children: are they good indicators of dietary adequacy? Public Health Nutr 9, 644–650. [DOI] [PubMed] [Google Scholar]
- 55. Nguyen PH, Huybregts L, Sanghvi TG et al. (2018) Dietary diversity predicts the adequacy of micronutrient intake in pregnant adolescent girls and women in Bangladesh, but use of the 5-group cutoff poorly identifies individuals with inadequate intake. J Nutr 148, 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Oliveira Otto MC, Padhye NS, Bertoni AG et al. (2015) Everything in moderation: dietary diversity and quality, central obesity and risk of diabetes. PLoS One 10, e0141341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. PAHO & World Health Organization (2019) Ultra-Processed Food and Drink Products in Latin America: Sales, Sources, Nutrient Profiles, and Policy Implications. Washington, DC, USA: Pan American Health Organization. [Google Scholar]
- 58. ANSES (2017) Table CIQUAL. Nutritional composition of foods. Maisons-Alfort, France: Agence nationale de sécurité sanitairede l’alimentation, de l’environnementet du travail; available at https://ciqual.anses.fr/ (accessed November 2019).
- 59. U.S. Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory (2005) USDA National Nutrient Database for Standard Reference, Release 18; available at https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site-pages/sr17-sr28/ (accessed November 2019).
- 60. LaNutrition.fr (2007) How much is one serving? Available at https://www.lanutrition.fr/bien-dans-son-assiette/bien-manger/les-recommandations-de-lanutrition.fr/une-portion-cest-combien- (accessed November 2019).
- 61. Moubarac J-C, Parra DC, Cannon G et al. (2014) Food classification systems based on food processing: significance and implications for policies and actions: a systematic literature review and assessment. Curr Obes Rep 3, 256–272. [DOI] [PubMed] [Google Scholar]
- 62. Monteiro CA, Cannon G, Levy RB et al. (2019) Ultra-processed foods: what they are and how to identify them? Public Health Nutr 22, 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fardet A & Boirie Y (2013) Associations between diet-related diseases and impaired physiological mechanisms: a holistic approach based on meta-analyses to identify targets for preventive nutrition. Nutr Rev 71, 643–656. [DOI] [PubMed] [Google Scholar]
- 64. World Health Organization (2015) Sugars Intake for Adults and Children: Guideline: Department of Nutrition for Health and Development. Switzerland: WHO. [Google Scholar]
- 65. Stark AH, Crawford MA & Reifen R (2008) Update on α-linolenic acid. Nutr Rev 66, 326–332. [DOI] [PubMed] [Google Scholar]
- 66. Willcox DC, Willcox BJ, Todoriki H et al. (2009) The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr 28, 500S–516S. [DOI] [PubMed] [Google Scholar]
- 67. Willcox DC, Scapagnini G & Willcox BJ (2014) Healthy aging diets other than the Mediterranean: a focus on the Okinawan diet. Mech Ageing Dev 136, 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cordain L (2002) The nutritional characteristics of a contemporary diet based upon Paleolithic food groups. JANA 5, 15–24. [Google Scholar]
- 69. Martinez-Lacoba R, Pardo-Garcia I, Amo-Saus E et al. (2018) Mediterranean diet and health outcomes: a systematic meta-review. Eur J Public Health 28, 955–961. [DOI] [PubMed] [Google Scholar]
- 70. D’Alessandro A, Lampignano L & De Pergola G (2019) Mediterranean diet pyramid: a proposal for Italian people: a systematic review of prospective studies to derive serving sizes. Nutrients 11, 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Meltzer HM, Brantsæter AL, Trolle E et al. (2019) Environmental sustainability perspectives of the Nordic diet. Nutrients 11, 2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nordic Council of Ministers (2014) Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity, 5th ed. Copenhagen, Denmark: Nordic Council of Ministers. [Google Scholar]
- 73. Mithril C, Dragsted LO, Meyer C et al. (2013) Dietary composition and nutrient content of the New Nordic diet. Public Health Nutr 16, 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lichtenstein AH, Appel LJ, Brands M et al. (2006) Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol 26, 2186–2191. [DOI] [PubMed] [Google Scholar]
- 75. Enas EA, Senthilkumar A, Chennikkara H et al. (2003) Prudent diet and preventive nutrition from pediatrics to geriatrics: current knowledge and practical recommendations. Indian Heart J 55, 310–338. [PubMed] [Google Scholar]
- 76. Parker HW & Vadiveloo MK (2019) Diet quality of vegetarian diets compared with nonvegetarian diets: a systematic review. Nutr Rev 77, 144–160. [DOI] [PubMed] [Google Scholar]
- 77. Rifai L & Silver MA (2016) A review of the DASH diet as an optimal dietary plan for symptomatic heart failure. Prog Cardiovasc Dis 58, 548–554. [DOI] [PubMed] [Google Scholar]
- 78. Le Mouël C, de Lattre-Gasquet M & Mora O (2018) Land Use and Food Security in 2050: A Narrow Road (Agrimonde-Terra). Paris: Quaé Publisher. [Google Scholar]
- 79. Willett W, Rockström J, Loken B et al. (2019) Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 393, 2–8. [DOI] [PubMed] [Google Scholar]
- 80. WWF France (2017) Eco2Initiative, Towards a low-carbon, healthy and affordable diet – Multidimensional comparative study of sustainable food baskets: carbon impact, nutritional quality and costs (part 1); available at https://www.wwf.fr/sites/default/files/doc-2017-11/171109_rapport_vers_une_alimentation_bas_carbone_saine_abordable_0.pdf (accessed March 2020).
- 81. WWF France (2018) Eco2Initiative, Towards a low-carbon, healthy and affordable diet – Multidimensional comparative study of sustainable food baskets: carbon impact, nutritional quality and costs (part 2); available at –https://www.wwf.fr/sites/default/files/doc-2018-10/20181015_etude_vers_alimentation_bas_carbone_saine_abordable_volet2-min.pdf (accessed March 2020).
- 82. Karlsson J, Röös E, Sjunnestrand T et al. (2017) Future Nordic Diets: Exploring Ways For Sustainably Feeding the Nordics. Copenhagen: Nordisk Ministerråd. [Google Scholar]
- 83. Stolze M, Schader C, Müller A et al. (2019) Sustainable and Healthy Diets: Trade-Offs and Synergies. Final Scientific Report: NRP 69 “Healthy Nutrition and Sustainable Food Production”. Switzerland: Research Institute of Organic Agriculture FiBL. [Google Scholar]
- 84. Solagro (2014) Afterres2050: A Sustainable Scenario for Agriculture and Land Use in France by 2050. Toulouse, France:Solagro. [Google Scholar]
- 85. Poux X & Aubert P-M (2018) An Agroecological Europe in 2050: Multifunctional Agriculture for Healthy Food – Lessons from a Modeling of the European Food System. Paris, France: IDDRI (Institut du développement durable et des relations internationales).
- 86. Millennium Institute (2013) Global Food and Nutrition Scenarios – Final Report. Washington, DC.
- 87. Solagro (2019) The Reverse of Our Plate – Changing Our Diet to Preserve Our Health and Our Environment. Toulouse, France: Solagro. [Google Scholar]
- 88. ANSES (2017) National Individual Study of Food Consumption n°3 (INCA 3). Maisons-Alfort, France.
- 89. Haut Conseil de la santé publique (2018) Opinion on Quantified Public Health Objectives for Public Health Nutrition Policy (PNNS) 2018–2022. Paris, France: Haut Conseil de la santé publique. [Google Scholar]
- 90. Louzada ML, Baraldi LG, Steele EM et al. (2015) Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev Med 81, 9–15. [DOI] [PubMed] [Google Scholar]
- 91. Canella DS, Levy RB, Martins APB et al. (2014) Ultra-processed food products and obesity in Brazilian households (2008–2009). Plos One 9, e92752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nardocci M, Leclerc B-S, Louzada M-L et al. (2019) Consumption of ultra-processed foods and obesity in Canada. Can J Public Health 110, 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Juul F, Martinez-Steele E, Parekh N et al. (2018) Ultra-processed food consumption and excess weight among US adults. Brit J Nutr 120, 90–100. [DOI] [PubMed] [Google Scholar]
- 94. Canhada S, Luft VC, Giatti L et al. (2020) Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr 23, 1076–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mendonca RD, Pimenta AM, Gea A et al. (2016) Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr 104, 1433–1440. [DOI] [PubMed] [Google Scholar]
- 96. Fardet A, Lakhssassi S & Briffaz A (2018) Beyond nutritional-based food indices: a data mining approach to search for a quantitative holistic index reflecting the degree of food processing and including physicochemical properties. Foods Funct 9, 561–572. [DOI] [PubMed] [Google Scholar]
- 97. Fardet A, Méjean C, Labouré H et al. (2017) The degree of processing of foods which are most widely consumed by the French elderly population is associated with satiety and glycemic potentials and nutrient profiles. Food Funct 8, 651–658. [DOI] [PubMed] [Google Scholar]
- 98. Palacios C & Gonzalez L (2014) Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 144, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sahota O (2014) Understanding vitamin D deficiency. Age Ageing 43, 589–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Forrest KY & Stuhldreher WL (2011) Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 31, 48–54. [DOI] [PubMed] [Google Scholar]
- 101. Slining MM & Popkin BM (2012) Trends in sources of empty calories for 2–18 olds in the US: 1977–2008. FASEB J 26, Meeting Abstract. [Google Scholar]
- 102. Bellisle F, Hébel P, Colin J et al. (2014) Consumption of whole grains in French children, adolescents and adults. Brit J Nutr 112, 1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Foyer CH, Lam H-M, Nguyen HT et al. (2016) Neglecting legumes has compromised human health and sustainable food production. Nat Plants 2, 16112. [DOI] [PubMed] [Google Scholar]
- 104. Derbyshire EJ (2017) Flexitarian diets and health: a review of the evidence-based-literature. Front Nutr 3, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hicks TM, Knowles SO & Farouk MM (2018) Global provisioning of red meat for Flexitarian diets. Front Nutr 3, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shepon A, Eshel G, Noor E et al. (2018) The opportunity cost of animal based diets exceeds all food losses. Proc Natl Acad Sci U S A 115, 3804–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Springmann M, Clark M, Mason-D’Croz D et al. (2018) Options for keeping the food system within environmental limits. Nature 562, 519–525. [DOI] [PubMed] [Google Scholar]
- 108. Batlle-Bayer L, Aldaco R, Bala A et al. (2019) Towards sustainable dietary patterns under a water-energy-food nexus life cycle thinking approach. Curr Opin Environ Sci Health 13, 61–67. [Google Scholar]
- 109. Hallström E, Carlsson-Kanyama A & Börjesson P (2015) Environmental impact of dietary change: a systematic review. J Cleaner Prod 91, 1–11. [Google Scholar]
- 110. Behrens P, Kiefte-de Jong JC, Bosker T et al. (2017) Evaluating the environmental impacts of dietary recommendations. Proc Natl Acad Sci U S A 114, 13412–13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Monteiro CA, Moubarac J-C, Levy RB et al. (2017) Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr 21, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Julia C, Martinez L, Alles B et al. (2018) Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Sante study. Public Health Nutr 21, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Maillot M, Darmon N, Vieux F et al. (2007) Low energy density and high nutritional quality are each associated with higher diet costs in French adults. Am J Clin Nutr 86, 690–696. [DOI] [PubMed] [Google Scholar]
- 114. Moreira PVL, Baraldi LG, Moubarac J-C et al. (2015) Comparing different policy scenarios to reduce the consumption of ultra-processed foods in UK: impact on cardiovascular disease mortality using a modelling approach. Plos One 10, e0118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Blanco-Rojo R, Sandoval-Insausti H, López-Garcia E et al. (2019) Consumption of ultra-processed foods and mortality: a national prospective cohort in Spain. Mayo Clin Proc 94, 2178–2188. [DOI] [PubMed] [Google Scholar]
- 116. Kim H, Hu EA & Rebholz CM (2019) Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr 22, 1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I et al. (2019) Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 365, l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Srour B, Fezeu LK, Kesse-Guyot E et al. (2019) Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ 365, l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Moreira PVL, Hyseni L, Moubarac J-C et al. (2018) Effects of reducing processed culinary ingredients and ultra-processed foods in the Brazilian diet: a cardiovascular modelling study. Public Health Nutr 21, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. World Health Organization (2015) ARC monographs evaluate consumption of red meat and processed meat; available at https://wwwiarcfr/en/media-centre/pr/2015/pdfs/pr240_Epdf (accessed November 2019).
- 121. Boutron-Ruault M-C, Mesrine S & Pierre F (2017) Chapter 12: Meat consumption and health outcomes. In Vegetarian and Plant-Based Diets in Health and Disease Prevention [Mariotti F, editor]. San Diego: Elsevier. [Google Scholar]
- 122. Tilman D & Clark M (2014) Global diets link environmental sustainability and human health. Nature 515, 518–522. [DOI] [PubMed] [Google Scholar]
- 123. Satija A, Bhupathiraju SN, Spiegelman D et al. (2017) Healthful and unhealthful plant-based diets and the risk of coronary heart disease in US adults. J Am Coll Cardiol 70, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Food and Agriculture Organization (2001) La nutrition dans les pays en développement [Nutrition in Developing Countries]. Rome, Italy: FAO. [Google Scholar]
- 125. Fumagalli M, Moltke I, Grarup N et al. (2015) Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science 349, 1343–1347. [DOI] [PubMed] [Google Scholar]
- 126. Hockett B & Haws J (2003) Nutritional ecology and diachronic trends in Paleolithic diet and health. Evol Anthropol 12, 211–216. [Google Scholar]
- 127. Ministère des Solidarités et de la Santé (2019) Programme National Nutrition Santé 2019–2023; available at https://solidarites-sante.gouv.fr/IMG/pdf/pnns4_2019-2023.pdf (accessed November 2019).
- 128. Salen P & de Lorgeril M (2011) The Okinawan diet: a modern view of an ancestral healthy lifestyle. In Healthy Agriculture, Healthy Nutrition, Healthy People. 102, pp. 114–123 [Simopoulos AP, editor]. Basel, Karger: World Review Nutrition Diet. [DOI] [PubMed] [Google Scholar]
- 129. Yamori Y, Miura A & Taira K (2001) Implications from and for food cultures for cardiovascular diseases: Japanese food, particularly Okinawan diets. Asia Pac J Clin Nutr 10, 144–145. [DOI] [PubMed] [Google Scholar]
- 130. de Menezes EVA, Sampaio HAD, Carioca AAF et al. (2019) Influence of Paleolithic diet on anthropometric markers in chronic diseases: systematic review and meta-analysis. Nut J 18, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ghaedi E, Mohammadi M, Mohammadi H et al. (2019) Effects of a Paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr 10, 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Soltani S, Jayedi A, Shab-Bidar S et al. (2019) Adherence to the Mediterranean diet in relation to all-cause mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Adv Nutr 10, 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Eleftheriou D, Benetou V, Trichopoulou A et al. (2018) Mediterranean diet and its components in relation to all-cause mortality: meta-analysis. Br J Nutr 120, 1081–1097. [DOI] [PubMed] [Google Scholar]
- 134. Bonaccio M, Di Castelnuovo A, Costanzo S et al. (2018) Mediterranean diet and mortality in the elderly: a prospective cohort study and a meta-analysis. Br J Nutr 120, 841–854. [DOI] [PubMed] [Google Scholar]
- 135. Sofi F, Macchi C, Abbate R et al. (2014) Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 17, 2769–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Galbete C, Schwingshackl L, Schwedhelm C et al. (2018) Evaluating Mediterranean diet and risk of chronic disease in cohort studies: an umbrella review of meta-analyses. Eur J Epidemiol 33, 909–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sofi F, Abbate R, Gensini GF et al. (2010) Accruing evidence on benefits of adherence to the Mediterranean diet on health an updated systematic review and meta-analysis. Am J Clin Nutr 92, 1189–1196. [DOI] [PubMed] [Google Scholar]
- 138. Malmir H, Saneei P, Larijani B et al. (2018) Adherence to Mediterranean diet in relation to bone mineral density and risk of fracture: a systematic review and meta-analysis of observational studies. Eur J Nutr 57, 2147–2160. [DOI] [PubMed] [Google Scholar]
- 139. Dinu M, Pagliai G, Casini A et al. (2018) Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr 72, 30–43. [DOI] [PubMed] [Google Scholar]
- 140. Rosato V, Temple NJ, La Vecchia C et al. (2019) Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur J Nutr 58, 173–191. [DOI] [PubMed] [Google Scholar]
- 141. Grosso G, Marventano S, Yang J et al. (2017) A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: are individual components equal? Crit Rev Food Sci Nutr 57, 3218–3232. [DOI] [PubMed] [Google Scholar]
- 142. Liyanage T, Ninomiya T, Wang A et al. (2016) Effects of the Mediterranean diet on cardiovascular outcomes: a systematic review and meta-analysis. Plos One 11, e0159252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Psaltopoulou T, Sergentanis TN, Panagiotakos DB et al. (2013) Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol 74, 580–591. [DOI] [PubMed] [Google Scholar]
- 144. Nissensohn M, Roman-Vinas B, Sanchez-Villegas A et al. (2016) The effect of the Mediterranean diet on hypertension: a systematic review and meta-analysis. J Nutr Educ Behav 48, 42–53. [DOI] [PubMed] [Google Scholar]
- 145. Li Y, Hu BQ, Wu XJ et al. (2018) Adherence to Mediterranean diet and the risk of breast cancer: a meta-analysis. Trans Cancer Res 7, 1290–1297. [Google Scholar]
- 146. van den Brandt PA & Schulpen M (2017) Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer 140, 2220–2231. [DOI] [PubMed] [Google Scholar]
- 147. Schwingshackl L, Schwedhelm C, Galbete C et al. (2017) Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients 9, 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Schwingshackl L & Hoffmann G (2015) Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med 4, 1933–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Schwingshackl L & Hoffmann G (2014) Adherence to Mediterranean diet and risk of cancer: A systematic review and meta-analysis of observational studies. Int J Cancer 135, 1884–1897. [DOI] [PubMed] [Google Scholar]
- 150. Wu L & Sun DL (2017) Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep 7, 41317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Loughrey DG, Lavecchia S, Brennan S et al. (2017) The impact of the Mediterranean diet on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Adv Nutr 8, 571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Singh B, Parsaik AK, Mielke MM et al. (2014) Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dis 39, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Schwingshackl L, Missbach B, Konig J et al. (2015) Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr 18, 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Panagiotakos D, Pitsavos C, Koloverou E et al. (2014) Mediterranean diet and diabetes development: a meta-analysis of 12 studies and 140,001 individuals. J Am Coll Cardiol 63, A1349–A1349. [Google Scholar]
- 155. Koloverou E, Esposito K, Giugliano D et al. (2014) The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 63, 903–911. [DOI] [PubMed] [Google Scholar]
- 156. Huo R, Du T, Xu Y et al. (2015) Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr 69, 1200–1208. [DOI] [PubMed] [Google Scholar]
- 157. Esposito K, Maiorino MI, Bellastella G et al. (2015) A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open 5, e008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Adamsson V, Reumark A, Cederholm T et al. (2012) What is a healthy Nordic diet? Foods and nutrients in the NORDIET study. Food Nutr Res 56, 18189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zimorovat A, Mohammadi M, Ramezani-Jolfaie N et al. (2020) The healthy Nordic diet for blood glucose control: a systematic review and meta-analysis of randomized controlled clinical trials. Acta Diabetol 57, 1–12. [DOI] [PubMed] [Google Scholar]
- 160. Ramezani-Jolfaie N, Mohammadi M & Salehi-Abargouei A (2019) The effect of healthy Nordic diet on cardio-metabolic markers: a systematic review and meta-analysis of randomized controlled clinical trials. Eur J Nutr 58, 2159–2174. [DOI] [PubMed] [Google Scholar]
- 161. Shakersain B, Rizzuto D, Larsson SC et al. (2018) The Nordic prudent diet reduces risk of cognitive decline in the Swedish older adults: a population-based cohort study. Nutrients 10, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shakersain B, Santoni G, Larsson SC et al. (2016) Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimers Dement 12, 100–109. [DOI] [PubMed] [Google Scholar]
- 163. Villegas R, Salim A, Flynn A et al. (2004) Prudent diet and the risk of insulin resistance. Nutr Metab Cardiovasc Dis 14, 334–343. [DOI] [PubMed] [Google Scholar]
- 164. Bradbury K, Tong T & Key T (2017) Dietary intake of high-protein foods and other major foods in meat-eaters, poultry-eaters, fish-eaters, vegetarians, and vegans in UK Biobank. Nutrients 9, 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Viguiliouk E, Kendall CWC, Kahleova H et al. (2019) Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr 38, 1133–1145. [DOI] [PubMed] [Google Scholar]
- 166. Glenn AJ, Viguiliouk E, Seider M et al. (2019) Relation of vegetarian dietary patterns with major cardiovascular outcomes: a systematic review and meta-analysis of prospective cohort studies. Front Nutr 6, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Dinu M, Abbate R, Gensini GF et al. (2017) Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr 57, 3640–3649. [DOI] [PubMed] [Google Scholar]
- 168. Huang T, Yang B, Zheng JS et al. (2012) Cardiovascular disease mortality and cancer incidence in vegetarians: a meta-Analysis and systematic review. Ann Nutr Metabol 60, 233–240. [DOI] [PubMed] [Google Scholar]
- 169. Lee Y & Park K (2017) Adherence to a vegetarian diet and diabetes risk: a systematic review and meta-analysis of observational studies. Nutrients 9, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Haghighatdoost F, Bellissimo N, de Zepetnek JOT et al. (2017) Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nutr 20, 2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Barnard ND, Levin SM & Yokoyama Y (2015) A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet 115, 954–969. [DOI] [PubMed] [Google Scholar]
- 172. Yokoyama Y, Nishimura K, Barnard ND et al. (2014) Vegetarian diets and blood pressure: A meta-analysis. JAMA Int Med 174, 577–587. [DOI] [PubMed] [Google Scholar]
- 173. Yokoyama Y, Barnard ND, Levin SM et al. (2014) Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther 4, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zhang Z, Ma G, Chen S et al. (2013) Comparison of plasma triacylglycerol levels in vegetarians and omnivores: a meta-analysis. Nutrition 29, 426–430. [DOI] [PubMed] [Google Scholar]
- 175. Mohsenpour MA, Fallah-Moshkani R, Ghiasvand R et al. (2019) Adherence to Dietary Approaches to Stop Hypertension (DASH)-style diet and the risk of cancer: a systematic review and meta-analysis of cohort studies. J Am Coll Nutr 38, 513–525. [DOI] [PubMed] [Google Scholar]
- 176. Mohseni R, Mohseni F, Alizadeh S et al. (2019) The association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies. Nutr Cancer 72, 778–790. [DOI] [PubMed] [Google Scholar]
- 177. Akhlaghi M (2019) Dietary Approaches to Stop Hypertension (DASH): potential mechanisms of action against risk factors of the metabolic syndrome. Nutr Res Rev 33, 1–18. [DOI] [PubMed] [Google Scholar]
- 178. Soltani S, Chitsazi MJ & Salehi-Abargouei A (2018) The effect of Dietary Approaches to Stop Hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr 37, 542–550. [DOI] [PubMed] [Google Scholar]
- 179. Bricarello LP, Poltronieri F, Fernandes R et al. (2018) Effects of the Dietary Approach to Stop Hypertension (DASH) diet on blood pressure, overweight and obesity in adolescents: a systematic review. Clin Nutr Espen 28, 1–11. [DOI] [PubMed] [Google Scholar]
- 180. Soltani S, Shirani F, Chitsazi MJ et al. (2016) The effect of Dietary Approaches to Stop Hypertension (DASH) diet on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Obes Rev 17, 442–454. [DOI] [PubMed] [Google Scholar]
- 181. Siervo M, Lara J, Chowdhury S et al. (2015) Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Brit J Nutr 113, 1–15. [DOI] [PubMed] [Google Scholar]
- 182. Salehi-Abargouei A, Maghsoudi Z, Shirani F et al. (2013) Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases-incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition 29, 611–618. [DOI] [PubMed] [Google Scholar]
- 183. Saneei P, Salehi-Abargouei A, Esmaillzadeh A et al. (2014) Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis 24, 1253–1261. [DOI] [PubMed] [Google Scholar]
- 184. Shirani F, Salehi-Abargouei A & Azadbakht L (2013) Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition 29, 939–947. [DOI] [PubMed] [Google Scholar]
- 185. Kaluza J, Hakansson N, Harris HR et al. (2019) Influence of anti-inflammatory diet and smoking on mortality and survival in men and women: two prospective cohort studies. J Intern Med 285, 75–91. [DOI] [PubMed] [Google Scholar]
- 186. Kaluza J, Stackelberg O, Harris HR et al. (2019) Anti-inflammatory diet and risk of abdominal aortic aneurysm in two Swedish cohorts. Heart 105, 1876–1883. [DOI] [PubMed] [Google Scholar]
- 187. Tolkien K, Bradburn S & Murgatroyd C (2019) An anti-inflammatory diet as a potential intervention for depressive disorders: a systematic review and meta-analysis. Clin Nutr 38, 2045–2052. [DOI] [PubMed] [Google Scholar]
- 188. Sears B & Ricordi C (2011) Anti-inflammatory nutrition as a pharmacological approach to treat obesity. J Obes 2011, 431985. [DOI] [PMC free article] [PubMed] [Google Scholar]