Abstract
To present a comprehensive synthesis of the effect of soluble fiber supplementation on blood lipid parameters in adults, a systematic search was undertaken in PubMed, Scopus, and ISI Web of Science of relevant articles published before November 2021. Randomized controlled trials (RCTs) evaluating the effects of soluble fibers on blood lipids in adults were included. We estimated the change in blood lipids for each 5 g/d increment in soluble fiber supplementation in each trial and then calculated the mean difference (MD) and 95% CI using a random-effects model. We estimated dose-dependent effects using a dose-response meta-analysis of differences in means. The risk of bias and certainty of the evidence was evaluated using the Cochrane risk of bias tool and the Grading Recommendations Assessment, Development, and Evaluation methodology, respectively. A total of 181 RCTs with 220 treatment arms (14,505 participants: 7348 cases and 7157 controls) were included. There was a significant reduction in LDL cholesterol (MD: −8.28 mg/dL, 95% CI: −11.38, −5.18), total cholesterol (TC) (MD: −10.82 mg/dL, 95% CI: −12.98, −8.67), TGs (MD: −5.55 mg/dL, 95% CI: −10.31, −0.79), and apolipoprotein B (Apo-B) (MD: −44.99 mg/L, 95% CI: −62.87, −27.12) after soluble fiber supplementation in the overall analysis. Each 5 g/d increase in soluble fiber supplementation had a significant reduction in TC (MD: −6.11 mg/dL, 95% CI: −7.61, −4.61) and LDL cholesterol (MD: −5.57 mg/dl, 95% CI: −7.44, −3.69). In a large meta-analysis of RCTs, results suggest that soluble fiber supplementation could contribute to the management of dyslipidemia and the reduction of cardiovascular disease risk.
Keywords: blood lipid, cardiovascular, meta-analysis, soluble fiber
Introduction
Despite sustained progress in the treatment and prevention of cardiovascular disease, it remains one of the leading causes of death worldwide [1]. According to the World Health Organization, 17.9 million individuals died from cardiovascular disease in 2019, accounting for 32% of all global deaths [2]. Cardiovascular disease also accounts for a substantial proportion of morbidity and places considerable economic burden on the individual and health care system [3]. Dyslipidemia, present in >50% of adults, is considered an important risk factor for cardiovascular disease incidence, characterized by elevated circulating concentrations of blood lipids such as cholesterol and TGs [4,5].
Dietary fiber, particularly water-soluble fibers, has demonstrated efficacy and tolerability in serum lipid management [6]. Dietary fibers as edible carbohydrate polymers are not hydrolyzed by endogenous enzymes and, hence, are not digested or absorbed in the human body [7,8]. Dietary fibers are categorized based on several features, such as insoluble and soluble forms. Insoluble fiber has a fecal bulking effect and passes through the digestive tract intact. Meanwhile, soluble fiber is the edible part of the plant that is resistant to digestion and do not contribute to fecal bulking, but could be fermented by colonic bacteria to short-chain fatty acids [7,8]. A body of knowledge suggested that the soluble fiber, which find in vegetables, certain fruits, beans, and oat products can be improved cardiovascular health status through lowering blood pressure, inflammatory markers, blood glucose, and lipids levels [9]. The main mechanisms of the lipid-lowering properties of dietary fiber are not yet completely understood; however, previous studies suggest a range of potential mechanisms including the capacity of soluble dietary fiber to form viscous solutions that delay gastric emptying, increase bile acid excretion, modulate the gut microbiome, and may decrease lipid uptake from the intestinal tract [7,8]. Despite the beneficial properties of dietary fiber reported in the literature, <5% of general population meet the recommended daily intake [9]. Therefore, a feasible strategy for improving intake may be through the use of isolated or synthetic viscous fiber supplements.
To date, a large number of clinical trial studies have investigated the effects of soluble fiber supplementation on blood lipids but the results are often varied and inconsistent. Given the contradictory outcomes on the effectiveness of soluble fiber on blood lipids, the objective of the current comprehensive systematic review and the dose-response meta-analysis was to synthesis data from individual investigations and to determine the overall treatment effect of soluble fiber on serum blood lipids.
Methods
The current dose-response meta-analysis has been conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines [10]. The study protocol was submitted and approved in the international prospective register of systematic reviews database, under the registration number: CRD42022334419.
Literature search and selection
A systematic search was conducted using the following electronic databases: ISI Web of Science, PubMed/MEDLINE, and Scopus, from inception up to November 2021 without any language or publication data restrictions. Electronic searches were complemented by hand searches of the reference lists of eligible articles. Search terms were a combination of keywords relevant to soluble fibers and study design to identify related publications. Further details about the search strategy are provided in Supplemental Table 1. Two reviewers (H.B. and E.N.E.) independently screened titles and abstracts according to the PICOS criteria (Population: adults; Intervention: use of soluble fiber; Comparator: placebo/no treatment group; Outcomes: total cholesterol (TC), TGs, LDL cholesterol; HDL cholesterol, all subtypes of apolipoprotein A (Apo-a) and apolipoprotein B (Apo-B); study design: RCTs and the below predefined inclusion and exclusion criteria to identify potential eligible trials.
Inclusion criteria
All human RCTs (either parallel or cross-over designs) that examined the effects of soluble fiber supplementation on adults aged 18 y or older, regardless of health status; evaluated the effect of soluble fiber (mixed soluble fiber or 1 of the soluble fiber subtypes) on at least 1 of the blood lipids parameters; compared the effect of different doses (g/d) of a specific soluble fiber on blood lipids across >1 study arm or compared the effect of the specific amount of soluble fiber (g/d) against a soluble fiber-free group; considered the change in blood lipid parameters as the primary or 1 of the secondary outcomes; provided mean, SD, and the number of participants in each study of change in blood lipids parameters across study arms or reported sufficient information to estimate those values were included.
Exclusion criteria
Studies were excluded if they were nonrandomized or quasi-experimental studies; conducted in participants under the age of 18 y (children and adolescents) or in pregnant or lactating women; did not have placebo or untreated control groups; used supplements as placebo; supplemented soluble fiber in combination with any other drugs, minerals, or botanicals (unless a separate arm controlled for the effect of the mixed substance); publications with duplicate data; follow-up duration <4 wk; reviews, conference letters, notes, reports, short surveys, and case reports. Discrepancies were resolved by discussion with the corresponding author (G.A.).
Data extraction
Two reviewers (A.G. and R.Z.) extracted the following information from eligible studies: first author’s name, year of publication, sex of participants, BMI and age of participants, study location, study design, follow-up duration and blinding, type of fiber based on viscosity and fermentability, dose of soluble fiber supplementation, form of administration, comparator, and background diet. For trials that evaluated multiple doses of soluble fiber supplementation, we included the highest dose in the analysis. When the data were reported at multiple measurements, only the outcomes at the end of the intervention were included in the analysis. In the case of multiple publications with duplicate and/or overlapped data for the same trial, the publication with most comprehensive and complete data was selected. Data were cross-checked to minimize potential errors, and disagreements were resolved through discussion with the corresponding author (G.A.).
Risk of bias
Two authors (E.N.E. and A.G.) independently assessed the risk of bias of each included trial. The Cochrane risk of bias tool for RCTs was used to assess each study as having a low, high, or unclear risk of bias on the following criteria: selection bias (sequence generation and allocation concealment), performance bias (blinding participants and personnel), detection bias (blinding outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and bias because of problems not covered elsewhere [11]. The overall quality of studies was graded as good if there were low risk of bias for >2 items, fair if there were low risk of bias for 2 items and poor if there was a low risk of bias for <2 items.
Statistical analyses
We examined the effect of soluble fiber supplementation on change in the following outcomes: TC (mg/dL), TGs (mg/dL), LDL cholesterol (mg/dL), HDL cholesterol (mg/dL), Apo-A (mg/L), and Apo-B (mg/L). The mean difference (MD) in blood lipids between the intervention (soluble fiber supplementation) and placebo or control groups and their 95% CIs were used as the effect sizes in the meta-analysis. A random-effects model was used to calculate the pooled effect size. If the studies did not report mean values and SDs of changes, we calculated these values by using data from measures before and after the intervention and the following formula: SD2 = [(SD baseline2 + SD final2) − (2 × R × SD baseline × SD final)], considering the correlation coefficient (R) of 0.5 [12]. All analyses were repeated using correlation coefficients of 0.2 and 0.8 to demonstrate that our results were not sensitive to the chosen correlation coefficient (R = 0.5). Furthermore, SD was computed by relevant formulas in studies that presented SEs, 95% CIs, and IQRs [13]. Heterogeneity across the studies was explored by the I2 statistic, described as I2 values > 50% or P < 0.05 [14]. To detect probable sources of heterogeneity, a series of predefined subgroup analyses based on health status (healthy, hypercholesterolemia, hyperlipidemia, diabetes, overweight-obese, hypertension, metabolic syndrome (MetS), kidney disease), gender (male, female or mixed), age (<50 y old vs. ≥50), BMI (<30 kg/m2 vs. ≥30), dose of soluble fiber (<10 g/d vs. ≥ 10 g), follow-up duration (< 8 wk vs. ≥8), fermentability (fermented vs. nonfermented), and viscosity (viscous vs. nonviscous) [[15], [16], [17]]. Influence analysis was conducted to test the potential impact of each trial on the pooled effect size. The potential for publication bias was tested using Egger’s test, Begg’s test, and by inspection of funnel plots.
Based on Crippa and Orsini's method, the mean and corresponding SD of change in blood lipids parameters, and the number of participants in each study arm, was used to conduct a random-effects model for each 5 g/d increase in soluble fiber supplementation in the intervention group on changes in blood lipid levels [18]. Additionally, we conducted a dose-response meta-analysis to clarify the shape of the effect of different doses of soluble fiber on blood lipids [19]. STATA software version 17.1 was used to conduct statistical analyses. A 2-tailed P value of <0.05 was considered significant.
Certainty of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the quality of evidence for each outcome based on the following domains: assessed risk of bias, publication bias, imprecision of results, heterogeneity, and indirectness of evidence [20]. The quality of evidence was categorized as high, moderate, low, and very low.
Results
Literature search
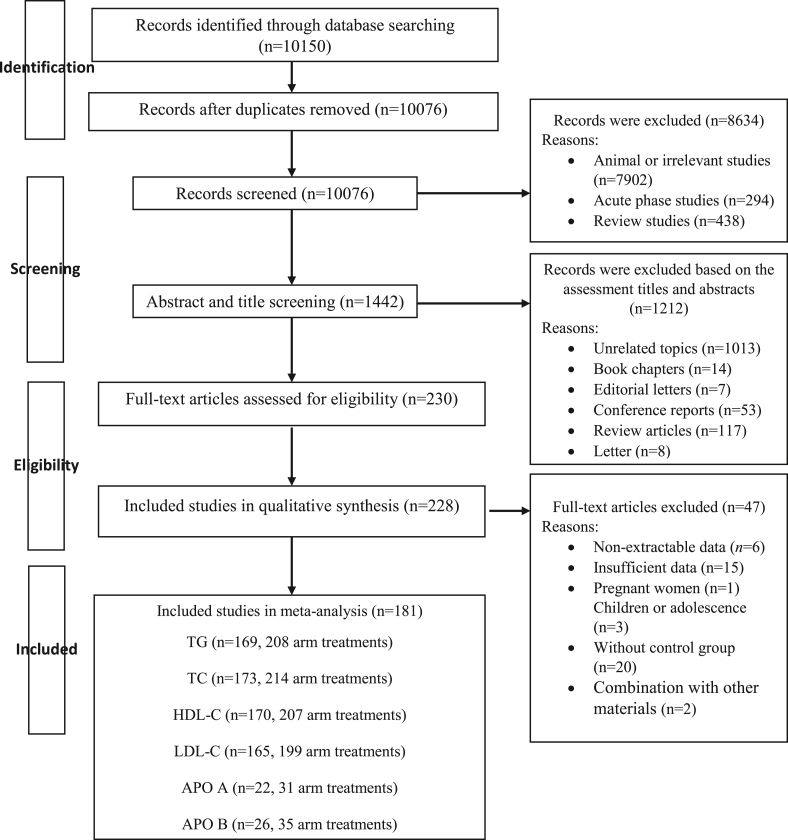
In total, 181 studies with 220 treatment arms, including 14,505 participants (7348 cases and 7157 controls), met our inclusion criteria and were included in our meta-analysis. Except for 1 study [21] with 3 treatment arms, all studies were included in the dose-response meta-analysis Supplemental Table 2. Detailed screening and data extraction process are depicted in Figure 1, and the list of excluded studies are presented in Supplemental Table 3.
FIGURE 1.
Flow chart of the process of the study selection.
Characteristics of included studies
The general characteristics of the included studies are shown in Supplemental Table 2. A total of 181 RCTs including 71% parallel and 29% cross-over design were included. Among the 181 RCTs, 47 studies included participants with hyperlipidemia (26%), 38 studies (20%) included participants with type 2 diabetes mellitus (T2DM), 28 studies (15%) included participants who were obese or overweight, 24 studies (13%) included healthy participants, 10 studies (5.5%) included participants with MetS, and the remaining included other diseases. The mean age of participants in RCTs ranged from 26 to 67 y and had a baseline BMI ranging from 19.2 to 32.7 kg/m2. Furthermore, the intervention duration of the included studies ranged from 4 to 52 wk. Twenty-two studies were conducted in men only (12%), 17 studies included only women (9%), and the remaining included both male and female participants. RCTs included for analysis were published between 1980 and 2021 and included the following countries of origin: 66 studies (36%) were conducted in Europe, 48 studies (26%) were conducted in North America, 25 studies (13%) were conducted in Asia, eleven studies (6%) were conducted in South America, and remaining were conducted in other of countries. Moreover, among all studies, 122 studies (68%) and 158 studies (87%) administrated viscous fiber and fermentable fiber, respectively. the intervention dose of soluble fiber varied from 0.03 to 45 g/d depending on the prescribed form of soluble fiber.
Meta-analysis results
TGs
The effect of the soluble fiber supplementation on TG concentration was examined in 169 studies with 208 treatment arms containing 12,445 subjects (6341 cases and 6104 controls). The pooled analysis demonstrated that soluble fiber supplementation significantly reduced TG compared with the placebo group (MD: −5.55 mg/dL, 95% CI: −10.31 to −0.79, P = 0.022). Significant between-study heterogeneity was present (I2 = 94.3%, P < 0.001) (Table 1).
TABLE 1.
The effect of soluble fiber supplements on blood lipids
| Variables | Pairwise meta-analysis |
Dose-response meta-analysis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, n | MD (95% CI) | P value | I2, % | Pheterogeneity | Dose, g/d | Studies, n | WMD (95% CI) | P value | I2, % | Pheterogeneity | |
| TG (mg/dL) | 208 | −5.55 (−10.31, −0.79) | 0.022 | 94.3 | <0.001 | 5 | 205 | −2.64 (−5.31, 0.03) | 0.052 | 94.8 | <0.001 |
| TC (mg/dL) | 214 | −10.82 (−12.98, −8.67) | <0.001 | 93.1 | <0.001 | 5 | 211 | −6.11 (−7.61, −4.60) | <0.001 | 94.7 | <0.001 |
| LDL cholesterol (mg/dL) | 199 | −8.28 (−11.38, −5.18) | <0.001 | 98.5 | <0.001 | 5 | 196 | −5.57 (−7.44, −3.69) | <0.001 | 98.4 | <0.001 |
| HDL cholesterol (mg/dL) | 207 | −0.03 (−0.45, 0.38) | 0.868 | 67.9 | <0.001 | 5 | 204 | 0.04 (−0.17, 0.26) | 0.693 | 65.6 | <0.001 |
| Apo-A (mg/L) | 31 | −10.47 (−31.16, 10.21) | 0.321 | 0.0 | 0.793 | 5 | 31 | −4.09 (−10.07, 1.89) | 0.180 | 0.0 | 0.830 |
| Apo-B (mg/L) | 35 | −44.99 (−62.87, −27.12) | <0.001 | 0.0 | 0.617 | 5 | 35 | −16.37 (−40.18, 7.42) | 0.177 | 86.8 | <0.001 |
Apo-A, apolipoprotein A; Apo-B, apolipoprotein B; MD, mean difference; TC; total cholesterol.
Additional analysis showed a greater reduction in TG in trials with a longer duration (≥8 wk) (MD: −7.29 mg/dL, 95% CI: −12.99 to −1.59) compared with the short duration (<8 wk), studies that supplemented soluble fiber with fermentable characteristics (MD: −5.04 mg/dL, 95% CI: −9.87 to −0.19) had a greater reduction in compared with other type of fiber, and when conducted in people with hyperlipidemia (MD: −22.23 mg/dL, 95% CI: −34.7 to −9.77) (Supplemental Table 4).
Sensitivity analysis suggested that no individual trial had a substantial impact on the overall pooled effect size. Statistically significant publication bias was found (P < 0.001, Begg’s test and P = 0.037, Egger’s test). Trim-and-fill method to detect sources of bias also suggested evidence of publication bias.
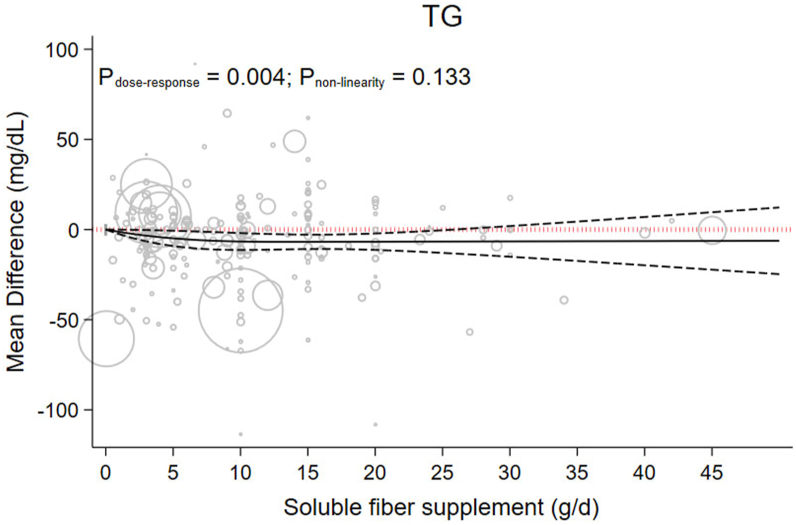
There was nonsignificant evidence suggesting that each 5 g/d increment in soluble fiber might reduce TG by −3.64 (−5.31 to 0.03), P = 0.052; (I2 = 94.8%, P < 0.001) (Table 1). Moreover, dose-dependent analysis showed a significant proportional decrease in TG with the increase in soluble fiber consumption Pdose-response = 0.004 in a linear manner Pnonlinearity =0.133. Results of the dose-response analysis suggest that 15 g/d of soluble provided the greatest decrease in TG (MD15g/d: −6.81, 95% CI: −10.81, −2.82) (Figure 2 and Table 2).
FIGURE 2.
The effects of different doses of soluble fiber supplementation on TG from the nonlinear dose-response meta-analysis.
TABLE 2.
The effects of different doses of soluble fiber supplements on blood lipids form the nonlinear dose-response meta-analysis (mean difference and 95% CI)
| Soluble fiber supplements (g/d) | 0 (Reference) | 5 | 8 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TG (mg/dL) | 0 | −4.89 (−9.14, −0.63) | −6.21 (−11.04, −1.39) | −6.63 (−11.33, −1.94) | −6.81 (−10.81, −2.82) | −6.73 (−11.30, −2.16) | −6.65 (−12.94, −0.35) | −6.56 (−15.07, 1.94) | −6.48 (−17.38, 4.42) | −6.40 (−19.79, 6.99) | −6.32 (−22.24, 9.61) |
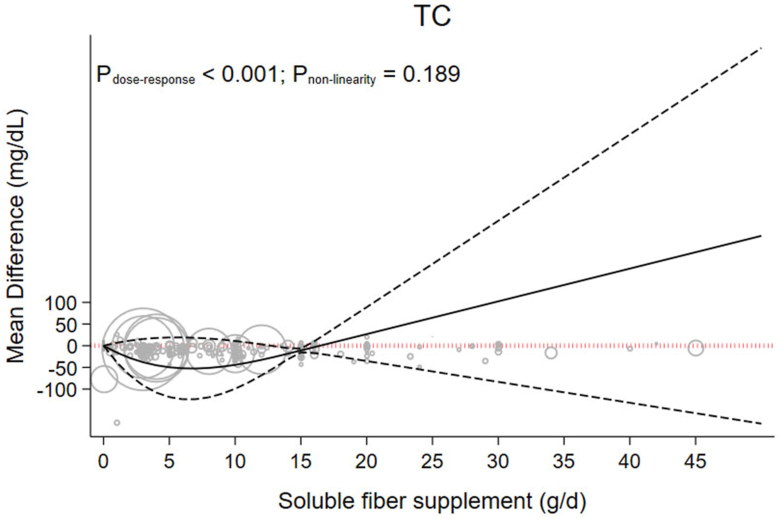
| TC (mg/dL) | 0 | −49.71 (−118.49, 19.08) | −51.24 (−119.06, 16.59) | −44.14 (−99.23, 10.95) | −10.94 (−15.36, −6.53) | 26.82 (−35.31, 88.95) | 64.61 (−59.34, 188.55) | 102.39 (−83.41, 288.19) | 140.17 (−107.5, 387.83) | 177.94 (−131.59, 487.47) | 215.71 (−155.69, 587.11) |
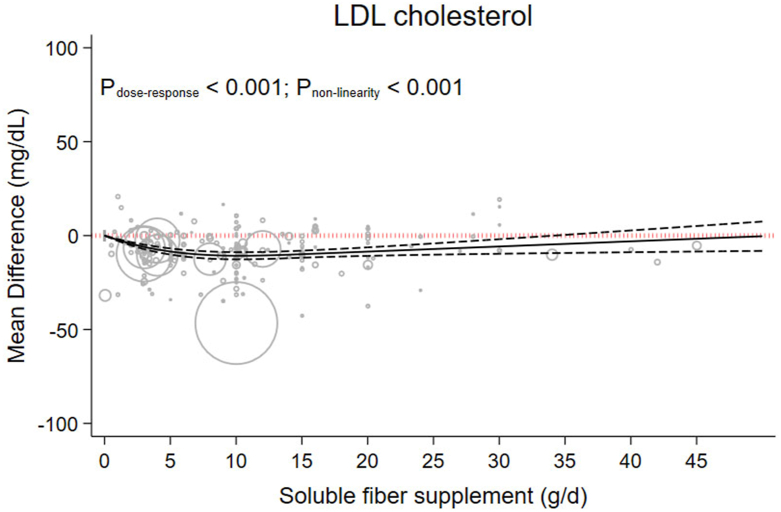
| LDL−C (mg/dL) | 0 | −8.49 (−10.12, −6.85) | −10.39 (−12.29, −8.49) | −10.75 (−12.66, −8.83) | −9.89 (−11.77, −8.02) | −8.53 (−10.80, −6.26) | −7.16 (−10.16, −4.16) | −5.79 (−9.66, −1.92) | −4.42 (−9.24, 0.39) | −3.06 (−8.85, 2.74) | −1.69 (−8.49, 5.11) |
| HDL−C (mg/dL) | 0 | −0.09 (−0.73, 0.54) | −0.06 (−0.80, 0.69) | −0.00 (−0.75, 0.74) | 0.18 (−0.53, 0.89) | 0.38 (−0.42, 1.17) | 0.58 (−0.43, 1.59) | 0.78 (−0.51, 2.07) | 0.98 (−0.62, 2.58) | 1.18 (−0.75, 3.11) | 1.38 (−0.89, 3.65) |
| Apo−A mg/L | 0 | −4.25 (−24.52, 16.02) | −6.74 (−31.99, 18.51) | −8.38 (−34.82, 18.07) | −12.42 (−37.96, 13.12) | −16.43 (−41.93, 9.06) | −20.44 (−50.39, 9.50) | −24.45 (−61.93, 13.02) | −28.46 (−75.08, 18.15) | −32.47 (−89.06, 24.11) | −34.081 (−94.79, 26.64) |
| Apo−B mg/L | 0 | −49.37 (−75.91, −22.84) | −51.49 (−78.19, −24.76) | −44.93 (−72.34, −17.52) | −13.31 (−65.79, 39.18) | 22.85 (−65.94, 111.02) | 59.01 (−76.21, 194.22) | 95.16 (−83.31, 273.64) | 131.32 (−90.71, 353.34) | 167.47 (−98.25, 433.19) | 181.941 (−101.29, 465.16) |
Apo-A, apolipoprotein A; Apo-B, apolipoprotein B; TC; total cholesterol.
42 g of soluble fiber supplement.
TC
Overall, 173 eligible studies with 214 treatment arms, including a total of 13,871 participants (7020 cases and 6851 controls) examined the effect of soluble fiber supplementation on TC. TC was significantly reduced after soluble fiber supplementation compared with the control group (MD: −10.82 mg/dL, 95% CI: −12.98 to −8.67, P < 0.001), with a significant between-study heterogeneity (I2 = 93.1%, P < 0.001) (Table 1).
Subgroup analysis based on viscosity and study population could explain this heterogeneity (I2 <50%, P < 0.05). Subgroup analysis also revealed a greater reduction in people with T2DM (MD: −17.32 mg/dL, 95% CI: −27.08 to −7.56) and MetS (MD: −14.99 mg/dL, 95% CI: −20.06 to −9.93) compared with other subgroups. Furthermore, there was an indication of a larger effect size in trials that implemented viscous fiber supplementation (MD: −12.99 mg/dL, 95% CI: −15.57 to −10.41) (Supplemental Table 4).
Sensitivity analysis suggested that no individual trial had a substantial impact on the overall pooled effect size. Furthermore, there was no indication of publication bias using the Egger’s test (P = 0.244) or Begg’s test (P = 0.267).
A linear dose-response meta-analyses indicated that each 5 g/d increment in soluble fiber supplementation reduced TC by −6.11 mg/dL (95% CI: −7.61, −4.60, P < 0.001; I2 = 94.7%, P < 0.001) (Table 1). Soluble fiber had a significant dose-dependent effect on TC (Pdose-response < 0.001, Pnonlinearity = 0.189). Similar to the results of the TG analysis, results of the dose-response analysis suggest that 15 g/d of soluble fiber provided the greatest decrease in TC (MD 15g/d: −10.94, 95% CI: −15.36, −6.53) (Figure 3 and Table 2).
FIGURE 3.
The effects of different doses of soluble fiber supplementation on TC from the nonlinear dose-response meta-analysis.
Low-density lipoprotein
The effect of soluble fiber supplementation on LDL cholesterol was investigated in 165 RCTs including 199 treatment arms, with 12,773 participants (6462 cases and 6311 controls). Pooled results indicated that soluble fiber supplementation significantly reduced LDL cholesterol compared with placebo (MD: −8.28 mg/dL, −11.38 to −5.18, P < 0.001). Significant heterogeneity was observed across studies (I2 = 98.5 %, P < 0.001) (Table 1). The treatment dose, gender, and study population were sources of heterogeneity (I2 < 50%, P < 0.05) (Supplemental Table 4).
Subgroup analysis determined that soluble fiber had a greater effect among participants with hyperlipidemia (MD: −17.85 mg/dL, −30.74 to −4.98), when using a viscous fiber type (MD: −9.78 mg/dL, −13.46 to −6.11), and in male participants (MD: −10.71 mg/dL, −14.48 to −9.95) in compared with another subset (Supplemental Table 4).
Sensitivity analysis suggested that no individual trial had a substantial impact on the overall pooled effect size. Statistically significant publication bias was found (P < 0.001, Begg’s test and P < 0.001, Egger’s test). Trim-and-fill analysis demonstrated that, with the publication of 247 studies, evidence of publication bias is reduced but the results remain stable.
Each 5 g/d increment in soluble fiber supplementation reduced LDL cholesterol by −5.57 mg/dL (−7.44 to −3.69, P < 0.001; I2 = 98.4%, P < 0.001) (Table 1). Dose-response analysis showed a nonlinear decrease in LDL cholesterol to 10 g/d (MD 10g/d: −10.75, 95% CI: −12.66, −8.83) (Figure 4 and Table 2).
FIGURE 4.
The effects of different doses of soluble fiber supplementation on LDL cholesterol from the nonlinear dose-response meta-analysis.
High-density lipoprotein
The pooled MD of 170 studies (207 treatment arms) involving 12,563 participants (6400 cases and 6163 controls) suggested that soluble fiber supplementation had no statistically significant effect in HDL cholesterol compared with placebo (MD: −0.03 mg/dL; 95% CI: −0.45 to 0.38, P = 0.868). However, significant heterogeneity was detected between the studies (I2 = 67.9%, P < 0.001) (Table 1).
Subgroup analysis based on study population and fermentability of the soluble fiber supplement were sources of heterogeneity (I2 < 50%, P < 0.05) (Supplemental Table 4). Soluble fiber supplementation had a nonsignificant beneficial effect on HDL cholesterol in studies that used a longer duration (MD: 0.18 mg/dL; 95% CI: −0.57 to 0.94) in compared with short duration, that included people with T2DM (MD: 2.32 mg/dl; 95% CI: 1.01 to 3.63) and hyperlipidemia (MD: 0.46 mg/dL; 95% CI: −0.50 to 1.43), and when doses more and equal than 10 g/d were investigated (MD: 0.13 mg/dL; 95% CI: −0.82 to 1.08) (Supplemental Table 4).
Sensitivity analysis suggested that overall estimates for HDL cholesterol concentration were not affected by the removal of any individual study. Statistically significant publication bias was not found (P = 0.098, Begg’s test and P = 0.172, Egger’s test).
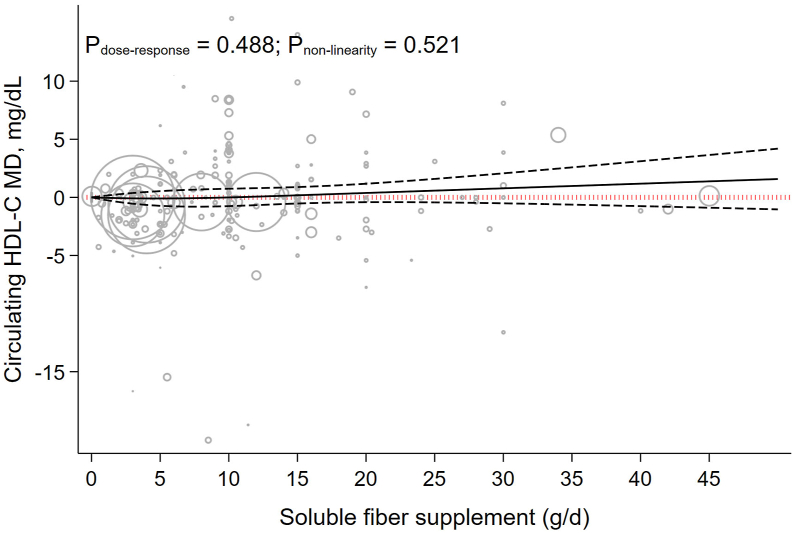
Each 5 g/d increment in soluble fiber supplementation increase HDL cholesterol by 0.04 mg/dL (−0.17 to 0.26, P = 0.693; I2 = 65.6%, P < 0.001) (Table 1). HDL cholesterol concentration had a nonsignificant association with soluble fiber supplementation dosage (Pnonlinearity = 0.521, Pdose-response = 0.488). In addition, dose-response analysis showed beneficial effect of soluble fiber on HDL cholesterol started from a dose 15 g/d (MD 15g/d: 0.18, 95% CI: −0.53, 0.89) (Table 2 and Figure 5).
FIGURE 5.
The effects of different doses of soluble fiber supplementation on HDL cholesterol from the nonlinear dose-response meta-analysis.
Apolipoprotein A
Thirty-two articles with 31 treatment arms, including a total of 2491 subjects (1300 intervention, 1191 placebo), reported the effect of soluble fiber consumption on Apo-A. Soluble fiber supplementation had no significant effect on Apo-A (MD: −10.47 mg/L, 95% CI: −31.16, 10.21; P = 0.321) and between-study heterogeneity was low (I2 = 0.0%, P = 0.793) (Table 1).
Findings from the sensitivity analysis revealed that the exclusion of any single study from the analysis did not alter the overall effect. Begg’s (P = 0.475) and Egger’s test (P = 0.440) suggested no evidence of publication bias.
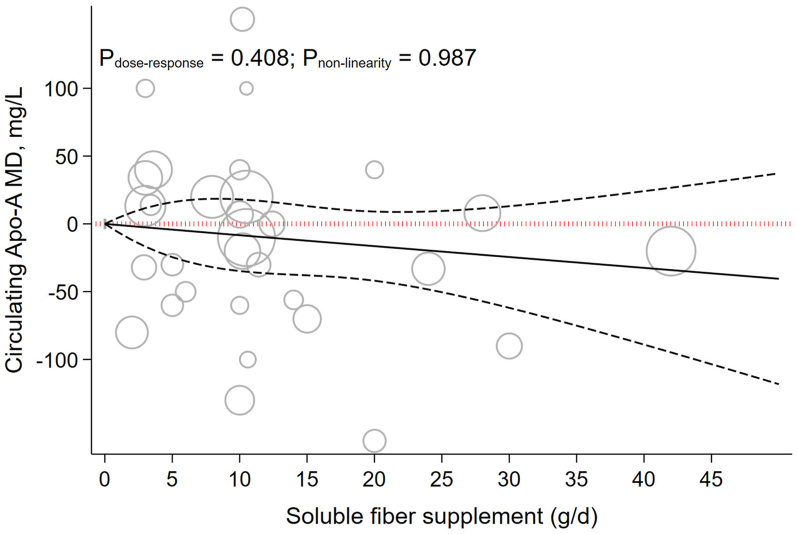
Each 5 g/d increment in soluble fiber supplementation reduced Apo-A by −4.09 mg/L (−10.07 to 1.89, P = 0.180; I2 = 0.0%, P = 0.830) (Table 1). Dose-dependent effects showed a nonsignificant reduction on Apo-A (Pnonlinearity = 0.987, Pdose-response = 0.408) (Figure 6).
FIGURE 6.
The effects of different doses of soluble fiber supplementation on Apo-A from the nonlinear dose-response meta-analysis.
Apolipoprotein B
Using data from 26 studies with 35 treatment arms containing 2644 participants (1377 cases and 1267 controls), meta-analysis suggested that soluble fiber significantly reduced Apo-B (MD: −44.99 mg/L, 95% CI: −62.87 to −27.12, P < 0.001) and that between-study heterogeneity was low (I2 = 0.0%, P = 0.617) (Table 1).
Subgroup analysis suggested that the effect size was greater in studies that had a >8 wk duration (MD: −32.53 mg/L, 95% CI: −59.64 to −5.42) used a dose of soluble fiber >10 g/d (MD: −62.60 mg/L, 95% CI: −94.00 to −31.21), and in people with MetS as (MD: −77.91 mg/L, 95% CI: −141.53 to −14.29) in compared with other subsets (Supplemental Table 4).
Sensitivity analyses revealed that the exclusion of any single study from the analysis did not alter the overall effect. Begg’s (P = 0.109) and Egger’s test (P = 0.015) suggested evidence of publication bias. The trim-and-fill analysis demonstrated that the results were similar to the original.
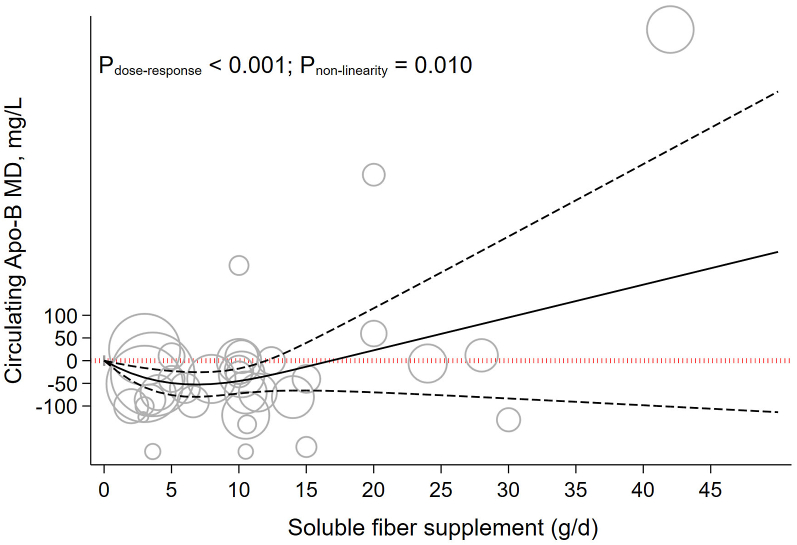
Each 5 g/d increment in soluble fiber supplementation reduced Apo-B by −16.37 mg/L (−40.18 to 7.42, P = 0.177; I2 = 86.8%, P < 0.001) (Table 1). Dose-response analysis suggests that soluble fiber supplementation had a significant reduction on Apo-B up to 8 g/d (MD8g/d: −51.49, 95% CI: −78.19, −24.79) (Pnonlinearity = 0.010, Pdose-response < 0.001) (Table 2 and Figure 7).
FIGURE 7.
The effects of different doses of soluble fiber supplementation on Apo-B from the nonlinear dose-response meta-analysis.
Risk of bias and certainty of evidence
Based on the Cochrane risk of bias tool, 138 studies (76.2%) were rated as having a good overall quality, 38 studies (20.9%) had fair overall quality, and 5 studies (2%) were rated as having poor overall quality. Supplemental Table 5 presents the quality of the studies. According to the GRADE approach, the certainty of the evidence was rated as very low for TG, LDL cholesterol, and HDL cholesterol and low for TC, Apo-A, and Apo-B outcomes. Supplemental Table 6 illustrates the details of the GRADE assessment.
Discussion
In this comprehensive dose-response meta-analysis, a total of 181 studies with 220 treatment arms, containing 14,505 participants were included. Soluble fiber supplementation improved serum TG, TC, LDL cholesterol, and Apo-B concentrations; however, it did not alter serum HDL cholesterol and Apo-A levels. In addition, nonlinear dose-response meta-analysis showed a significant effect of soluble fiber supplementation on serum TG, TC, and HDL cholesterol in 15 g/d and LDL cholesterol in 10 g/d.
The degree of improvement in lipid markers reported in this meta-analysis suggest that soluble fiber supplementation might be of great clinical importance. For example, we report the soluble fiber reduced LDL cholesterol concentrations by −8.28 mg/dL (−11.38 to −5.18). Previous research suggests that each 1-mmol/L (38.6 mg/dL) reduction in LDL cholesterol has can confer an average of 23% relative reduction in the risk of major vascular events including acute MI or other acute coronary syndrome, coronary revascularization, or stroke [22]. Subgroup analyses suggest that participants with T2DM, MetS, and hyperlipidemia experienced greater reductions in blood lipid parameters following soluble fiber supplementation, independent of dose, and duration. Considering the fermentability of soluble fibers, serum TG level was only reduced following fermentable fiber supplementation. Since most fermentable fibers are also viscous, we investigated whether fermentable viscous fiber supplementation induced greater reduction in lipid outcomes compared with other soluble fiber types. The effect of fermentable viscous, only viscous or only fermentable fiber supplementation on TC and LDL cholesterol levels was more pronounced that nonfermentable nonviscous fibers. Subgroup analyses of intervention duration and dosage revealed a greater reduction in serum TG levels in studies with a longer duration (≥8 wk) and supplementation dosage ≤ 10 g/d, respectively.
Findings in relation to other studies
To our knowledge, the present systematic review and meta-analysis of RCTs are the first to update the clinical evidence of the effect of soluble fibers on blood lipid parameters since 1998. Brown et al. [23] conducted a meta-analysis of 67 trials that investigated the cholesterol-lowering effects of various soluble fibers including pectin, oat bran, guar gum, and psyllium. They found significant reductions of both TC and LDL cholesterol by −1.74 mg/dL (95% CI: −2.08, −1.35) and −2.2 mg/dL (−2.7, −1.7), respectively, without a significant reduction in TG levels. Unlike our study, type of soluble fiber was not found to be a significant predictor of lipid changes. The dose of soluble fiber was also restricted, with only studies using 2–10 g/d of fiber being included. We included all eligible studies, without restricting dosage, which might explain greater reductions in TG, TC, and LDL cholesterol levels in our study. We also investigated the effects of different doses of fiber supplementation on each lipid outcome using a dose-response analyses.
Other existing systematic reviews and meta-analyses have mainly focused on individual soluble fiber types. Similar to our findings, a meta-analysis of 8 RCTs, on the effect of psyllium consumption on anthropometric and metabolic parameters in people with T2DM reported significant reductions in TG and LDL cholesterol [24]. Another meta-analysis showed that konjac glucomannan decreased LDL cholesterol and non-HDL cholesterol with no significant effect on Apo-B concentration [25]. A guar gum-focused meta-analysis with 17 RCTs observed significant reductions in TC [26]. Consumption of 3 g/d of oat or barley β -glucan induced significant reductions in TC and LDL cholesterol concentrations in another meta-analysis with 13 RCTs [27]. The results from 2 other meta-analyses of RCTs regarding the effects of individual soluble fibers on blood lipid profile were also in accordance with ours, demonstrating improvements in LDL cholesterol and TC, without changing TG and HDL cholesterol concentrations [28,29].
There was no significant effect of soluble fiber supplementation on serum HDL cholesterol levels; a finding that is consistent with several previous studies [24,26,28]. A review study of the meta-analyses conducted on the effects of glycemic index and dietary fibers on the serum HDL cholesterol levels concluded that dietary fibers had no significant effect on HDL cholesterol concentrations [30]. The impact of dietary alterations on circulating concentrations of HDL cholesterol is less clear than LDL cholesterol and seems to be sex-specific and with interracial differences [31].
Based on the findings from nonlinear dose-response meta-analysis, we proposed a supplementation dosage of 15 g/d for achieving better results of lipid outcomes; as higher doses might be associated with some undesirable effects such as gastrointestinal discomfort [32].
Proposed mechanisms of action
Several physiological mechanisms have been proposed to explain the lipid-lowering effect of soluble fibers. Soluble fibers, especially high viscous fibers, decrease gastric emptying time and delay the absorption of dietary cholesterol [33,34]. Delayed gastric emptying restricts postprandial glucose response and improves insulin resistance [33]. Insulin resistance and compensatory hyperinsulinemia are associated with increased cholesterol synthesis [35]. We found that the cholesterol-lowering effect of soluble fibers was greater in participants with T2DM and MetS, which might suggest that soluble fibers decrease cholesterol levels by insulin resistance-related mechanisms. Soluble fibers may also lower calorie intake via satiety-linked mechanisms and induce a modest effect on weight, thus reducing blood lipid levels indirectly [36]. Soluble fibers, mainly viscous fibers, increase ileum viscosity and inhibit micelle formation, and thus entrap bile acids, increasing fecal excretion of bile acids [26] and increasing LDL cholesterol receptor upregulation in the liver. Furthermore, soluble fermentable fibers are fermented in the colon by intestinal bacteria to produce short-chain fatty acids (SCFA) [37]. Two of these bacterial products, propionate, and acetate, have been proposed to influence several lipids metabolic pathways, including cholesterol synthesis, fatty acid synthesis, and fatty acid oxidation [38]. In addition, SCFAs may be involved in weight control by increasing the secretion of satiety-induced hormones such as glucagon-like peptide 1 and peptide YY (PYY) [39].
Heterogeneity
Significant heterogeneity was found in the analysis of all lipid outcomes except Apo-A and Apo-B levels, which might be because of the large variability in the target population and study characteristics across studies. Furthermore, although we performed several prespecified subgroup analyses to detect sources of heterogeneity, residual heterogeneity might persist within some subgroups. The results of subgroup analyses suggested that gender, health status of study population, study duration, viscosity and fermentability of soluble fiber, and dose of soluble fiber might influence the magnitude of effect.
Strengths and limitations of this study
The present study has several strengths. The beneficial effects of soluble fibers on blood lipid concentrations have long been proposed; however, this meta-analysis provides an updated and thorough assessment of all clinical trials conducted in this field. We performed a comprehensive assessment of study quality and biases to minimize the presence of confounding variables. Many of the studies included in this analysis were considered to be of high quality based on Cochrane risk of bias tool. Since trials included participants that were both healthy and nonhealthy, including type 2 diabetic, hyperlipidemic, and obese, our findings are highly generalizable.
Several potential limitations of this study should be noted. First, substantial publication bias was detected in Apo-B, HDL cholesterol, LDL cholesterol, and TG analyses, which might reduce confidence in the estimates. Although trim-and-fill computation modified the estimate of these outcomes, our findings could have been overestimated, and thus should be interpreted with this in mind. In addition, we only considered supplemental soluble fiber in our analyses, thus, the differential fiber content of diet between intervention groups could have affected our estimates.
Conclusion and Future Research
Our comprehensive meta-analysis demonstrates the significant beneficial effect of isolated soluble fiber supplementation on serum TG, TC, LDL cholesterol, and Apo-B levels. Based on our findings, increasing fiber intake using soluble fiber supplementation could be an effective intervention in the prevention and management of dyslipidemia, and consequently may contribute to the risk reduction of cardiovascular diseases. However, because of the high between-study heterogeneity and publication bias, our findings should be interpreted cautiously. Future RCTs with protocol-based strategy and consistent methodology considering the fiber content of diet or comparing it between intervention groups are warranted to establish clear recommendations in this regard.
Author disclosures
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare support from the Isfahan University of Medical Sciences, Isfahan, Iran, for the submitted work; no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
GA and AG designed research. HM, HB, ENE, RZ, HG, and AG conducted research. ST and AG analyzed the data. SM, AG, and RZ wrote the paper, which was critically revised for important intellectual content by all authors. WM edited the manuscript. GA had primary responsibility for final content. GA affirms that the manuscript is an honest, accurate, and transparent account of the study being reported. No important aspects of the study have been omitted and any discrepancies from the study as planned have been disclosed. All authors have read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.01.005.
Funding
The authors reported no funding received for this study.
Data Availability
No additional data are available.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . World Health Organization; 2014. Global status report on noncommunicable diseases 2014. [Google Scholar]
- 2.Abas R., Othman F., Thent Z.C.J.E.j. Effect of Momordica charantia fruit extract on vascular complication in type 1 diabetic rats. EXCLI. 2015;179:J14. doi: 10.17179/excli2014-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas H., Diamond J., Vieco A., Chaudhuri S., Shinnar E., Cromer S., et al. Global atlas of cardiovascular disease 2000–2016: the Path to Prevention and Control. Glob. Heart. 2018;13(3):143–163. doi: 10.1016/j.gheart.2018.09.511. [DOI] [PubMed] [Google Scholar]
- 4.Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020;41(24):2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedayatnia M., Asadi Z., Zare-Feyzabadi R., Yaghooti-Khorasani M., Ghazizadeh H., Ghaffarian-Zirak R., et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids. Health. Dis. 2020;19(1):42. doi: 10.1186/s12944-020-01204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haskell W.L., Spiller G.A., Jensen C.D., Ellis B.K., Gates J.E. Role of water-soluble dietary fiber in the management of elevated plasma cholesterol in healthy subjects. Am. J. Cardiol. 1992;69(5):433–439. doi: 10.1016/0002-9149(92)90980-d. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins D.J., Kendall C.W., Axelsen M., Augustin L.S., Vuksan V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr. Opin. Lipidol. 2000;11(1):49–56. doi: 10.1097/00041433-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gee J.M., Blackburn N.A., Johnson I.T. The influence of guar gum on intestinal cholesterol transport in the rat. Br. J. Nutr. 1983;50(2):215–224. doi: 10.1079/bjn19830091. [DOI] [PubMed] [Google Scholar]
- 9.Mobley A.R., Jones J.M., Rodriguez J., Slavin J., Zelman K.M. proceedings from the food & fiber summit. Multidisciplinary Digital Publishing Institute; 2014. Identifying practical solutions to meet America’s fiber needs. A1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [A2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al. John Wiley & Sons; 2019. Cochrane handbook for systematic reviews of interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J., Tompson S., Deeks J., Altman D. A meta-analysis on the effectiveness of smart-learning. BMJ. 2003;327(1):557–660. [Google Scholar]
- 15.Yuan F., Dong H., Gong J., Wang D., Hu M., Huang W., et al. A systematic review and meta-analysis of randomized controlled trials on the effects of turmeric and curcuminoids on blood lipids in adults with metabolic diseases. Adv. Nutr. 2019;10(5):791–802. doi: 10.1093/advances/nmz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney A.C., Rumble C.E., Billings L.M., George E.S.J. Effect of dietary and supplemental lycopene on cardiovascular risk factors: a systematic review and meta-analysis. Adv. Nutr. 2020;11(6):1453–1488. doi: 10.1093/advances/nmaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawla R., Patil G.R. Soluble dietary fiber. Compr. Rev. Food Sci. Food Saf. 2010;9(2):178–196. [Google Scholar]
- 18.Crippa A., Orsini N. Dose–response meta-analysis of differences in means. BMC Med. Res. Methodol. 2016;16(1):91. doi: 10.1186/s12874-016-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bretz F., Pinheiro J.C., Branson M. Combining multiple comparisons and modeling techniques in dose-response studies. Biometrics. 2005;61(3):738–748. doi: 10.1111/j.1541-0420.2005.00344.x. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichenametla S.N., Weidauer L.A., Wey H.E., Beare T.M., Specker B.L., Dey M. Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double blind controlled cross-over intervention. Mol. Nutr. Food Res. 2014;58(6):1365–1369. doi: 10.1002/mnfr.201300829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverman M.G., Ference B.A., Im K., Wiviott S.D., Giugliano R.P., Grundy S.M., et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 23.Brown L., Rosner B., Willett W.W., Sacks F.M. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am. J. Clin. Nutr. 1999;69(1):30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 24.Xiao Z., Chen H., Zhang Y., Deng H., Wang K., Bhagavathula A.S., et al. The effect of psyllium consumption on weight, body mass index, lipid profile, and glucose metabolism in diabetic patients: a systematic review and dose–response meta-analysis of randomized controlled trials. Phytother. Res. 2020;34(6):1237–1247. doi: 10.1002/ptr.6609. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.H., Jeong Y.S., Song J., Hwang K.-A., Noh G.M., Hwang I.G.J. Phenolic acid, carotenoid composition, and antioxidant activity of bitter melon (Momordica charantia L.) at different maturation stages. International Journal of Food Properties. 2017;20(suppl 3):S3078–S3087. sup3. [Google Scholar]
- 26.Wang N., Pan D., Guo Z., Xiang X., Wang S., Zhu J., Sun G. Effects of guar gum on blood lipid levels: a systematic review and meta-analysis on randomized clinical trials. J. Funct. Foods. 2021;85 [Google Scholar]
- 27.Tiwari U., Cummins E.J.N. Meta-analysis of the effect of β-glucan intake on blood cholesterol and glucose levels. Nutrition. 2011;27(10):1008–1016. doi: 10.1016/j.nut.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead A., Beck E.J., Tosh S., Wolever T.M.J.T. Cholesterol-lowering effects of oat β-glucan: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014;100(6):1413–1421. doi: 10.3945/ajcn.114.086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sood N., Baker W.L., Coleman C.I. Effect of glucomannan on plasma lipid and glucose concentrations, body weight, and blood pressure: systematic review and meta-analysis. Am. J. Clin. Nutr. 2008;88(4):1167–1175. doi: 10.1093/ajcn/88.4.1167. [DOI] [PubMed] [Google Scholar]
- 30.Yanai H., Tada N. Effects of glycemic index and intake of dietary fiber on serum HDL-cholesterol levels. J. Endocrinol. Metab. 2018;8(4):57–61. [Google Scholar]
- 31.Vincent M.J., Allen B., Palacios O.M., Haber L.T., Maki K.C. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am. J. Clin. Nutr. 2019;109(1):7–16. doi: 10.1093/ajcn/nqy273. [DOI] [PubMed] [Google Scholar]
- 32.Mysonhimer A.R., Holscher H.D. Gastrointestinal effects and tolerance of nondigestible carbohydrate consumption. Adv. Nutr. 2022;13(6):2237–2276. doi: 10.1093/advances/nmac094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolever T.M.S., Tosh S.M., Spruill S.E., Jenkins A.L., Ezatagha A., Duss R., et al. Increasing oat β-glucan viscosity in a breakfast meal slows gastric emptying and reduces glycemic and insulinemic responses but has no effect on appetite, food intake, or plasma ghrelin and PYY responses in healthy humans: a randomized, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2020;111(2):319–328. doi: 10.1093/ajcn/nqz285. [DOI] [PubMed] [Google Scholar]
- 34.Cohn J.S., Kamili A., Wat E., Chung R.W., Tandy S. Reduction in intestinal cholesterol absorption by various food components: mechanisms and implications. Atheroscl. 2010;1(suppl 11):45–48. doi: 10.1016/j.atherosclerosissup.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Hoenig M.R., Sellke F.W. Insulin resistance is associated with increased cholesterol synthesis, decreased cholesterol absorption and enhanced lipid response to statin therapy. Atherosclerosis. 2010;211(1):260–265. doi: 10.1016/j.atherosclerosis.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 36.Surampudi P., Enkhmaa B., Anuurad E., Berglund L. Lipid lowering with soluble dietary fiber. Current Atheroscl. Rep. 2016;18(12):75. doi: 10.1007/s11883-016-0624-z. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto J., Watanabe K., Taira S., Kasubuchi M., Li X., Irie J., et al. Barley β-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLOS ONE. 2018;13(4) doi: 10.1371/journal.pone.0196579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara H., Haga S., Aoyama Y., Kiriyama S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 1999;129(5):942–948. doi: 10.1093/jn/129.5.942. [DOI] [PubMed] [Google Scholar]
- 39.Hosseini E., Grootaert C., Verstraete W., Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011;69(5):245–258. doi: 10.1111/j.1753-4887.2011.00388.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data are available.