Abstract
Background
Heart failure (HF) is predominately a chronic disease. There are overlaps in HF and chronic disease research and care. Chronic disease and HF research are conducted with multiple goals. The overarching goal is “optimized patient outcomes at maximum cost-effectiveness”. However, observations on patients can come with many variables; thus, we see differences in clinical translation. This document discusses an argument for three important gaps common to HF and chronic disease, i.e., screening, self-management, and patient-reported outcomes (PRO), and provides a glance of how it could fit into the evidence tree. Pertinent arguments for a framework for health services and models of care are provided as a prelude to future consensus.
Methodology
1) A preliminary literature review to identify a taxonomy for cardiovascular research, and 2) a review of the published literature describing the translation of research studies into clinical practice for cardiovascular disorders. A spectrum from observational to large randomized controlled trials to post-marketing studies were identified.
Discussion
A brief discussion on traditional research and differences focusing on screening, mixed methods research concepts, and chronic diseases models of care. Six steps to facilitate this: 1) Research design; 2) Research application (translation) i. routine ii. challenges; 3. Transforming research to translational level; 4. Funding and infrastructure; 5. Clinical Centres of Research Excellence (CCRE) and collaboration; 6. Governance and cost-effectiveness.
Conclusion
Implementation research that aims to link research findings to improved patient outcomes in an efficient and effective way is a neglected area. Skills required to perform implementation research are complex. Ways to maximize translational impacts for chronic disease research to clinical practice are described in a HF context.
Keywords: Chronic disease, clinical translation, heart failure, health policy, screening, patient-reported outcomes
1. INTRODUCTION
The medical field is undergoing unprecedented exposure to a technologically advancing world and a profusion of information. The goal, however, is simple and clear: to provide medical care to improve patient’s quality of life and health outcomes journey within a framework steered by evidence that is concise and implementable from consensus within a cost structure provided by administrators, i.e., achieving optimal jurisdiction defined cost-effectiveness [1, 2]. In context to services, Krumholtz’s taxonomy of disease management [3] outlines eight domains to prioritise. Health clusters or jurisdictions have variable needs or capacities to transition services from ‘What actually works’ and what do we need to understand better?’.
Regards evidence, the early 1990’s evidence-based medicine (EBM) movement and offshoots like the Cochrane Collaboration reinvigorated objectivity as the core foundation of medical decision making and practice [4]. In time two issues became noticeable, firstly the process itself, with the focus of evidence weighted toward devices and pharmaceuticals, biases and conflicts of interest in the investigative process, and applicability and generalisability to populations beyond the trial cohort; secondly, medical practice, where medicine has an artistic side. Lay terms like “Pub Test” or “common sense” were features that included clinician's experience and reasoning to offer an authoritative opinion or clinical judgement to execute a solution. EBM itself sits in a silo that overlaps with clinical practice. For example, when individualising care, when are clinicians comfortable questioning “What constitutes evidence?”, “to whom does it apply?” and understanding the deductive hypothesis-generating or the reasoning processes health practitioners should undertake [3-6].
Before advancing, it is vital to acknowledge successes; firstly, RCT driven evidence has unequivocally increased population health by leaps and bounds; secondly, it is not possible to find evidence at an individual level, thus focus should be on understanding “what constitutes a significant difference in an observation, to warrant further attention to patients or groups?”. Thus, there is a link between evidence generation and research translation. “Evidence Translation”, hence, includes the evidence as definitive statistical proof of a hypothesis, and translation as extending validity to all populations needing such care. As finding both evidence and translational solutions is not always straightforward, we focus on three differences, an overview of the process and models to link them. Furthermore, guidelines are excellent references for current evidence, they are not performance measures in clinical practice [7-10]. Specifically we observe that chronic care models do not always implement RCT findings equitably. In this review, we explore:
1. Common research paradigms for heart failure (HF) and chronic disease,
2. Screening, Self-management and Mixed methods research to extract risk and patient-reported performance, and
3. Propose strategies to address these issues.
2. AIMS
This review sets out to provide a broad direction intended to continue this dialogue into the future. We acknowledge that ultimate decisions are devised on ‘acceptable or agreed jurisdiction defined cost-effectiveness’. The specific aims are to:
1. Perform a literature review on traditional research, governance, and translational research.
2. Describe screening methods to optimise patient-reported chronic disease risks.
3. Describes the various methods to obtain mixed methods qualitative and quantitative data to inform screening performance measures.
4. Describe a direction for HF within established chronic care models (CCM).
3. LITERATURE REVIEW
Chronic Disease and self-management programs (CDSMP) focused on Wagner’s model of care and Flinders Program of Chronic Disease Self-Management. For HF guidelines, we surveyed the three professional cardiac societies that influence the Australian health care system, The ACC/AHA, ESC and CSANZ. We reviewed the medical literature for relevant terminology in this area and research methods to attain them. For cardiovascular diseases (CVD), the American European systems published guidelines and focussed updates within a 3-5-year period. Committees, working groups or councils provide more focus on advanced subspecialty areas. In this vein, these societies have produced position papers in several areas of governance and translational research. The ACC and ESC have established and updated literature in the form of guidelines, expert consensus and position documents, competence and training statements, performance measures, appropriate use criteria, health policy statements, data standards, clinical alerts, survey and data reports, and other broader areas.
4. BUILDING A STEP-LOCK RESEARCH TRANSLATION AND COST-EFFECTIVENESS GOVERNANCE FRAMEWORK
“When the student is ready, the teacher will appear”. This proverb raises an important philosophical question for medical translation and implementation, i.e., “what are we needing to learn, and how do we gain relevant guidance?”. It is simple as merely to create a policy and implement a finding, as we may encounter resistance among practitioners who expressed their clinical judgment or experiences. Thus, we are both students and teachers in this. The unifying question is one of governance, how do we gain, interpret and act on evidence against existing consensus based framework (Figs. 1 and 2)?
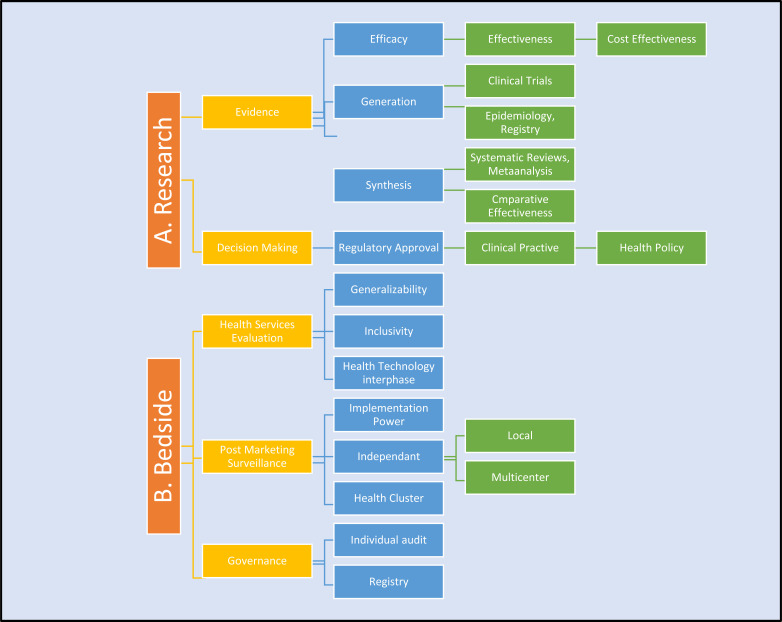
Fig. (1).
Research Structure and Implementation Pathways. A. Research studies answer local questions, broad global questions or the cost and outcome equation, through graded control of variables, limiting subconscious and other biases, and statistical support to calculate power and validity. Efficacy – how it works; Effectiveness – does it work?; Cost-effectiveness – is it worth it?. B. Governance – processes are not equally regulated at all levels to ensure all levels of health services can freely interpret evidence for individual patient needs. Translation of decision require 3 tiers, evidence, client, health services and political will. Generalizability, inclusivity of stakeholders can influence events post evidence generation [11].
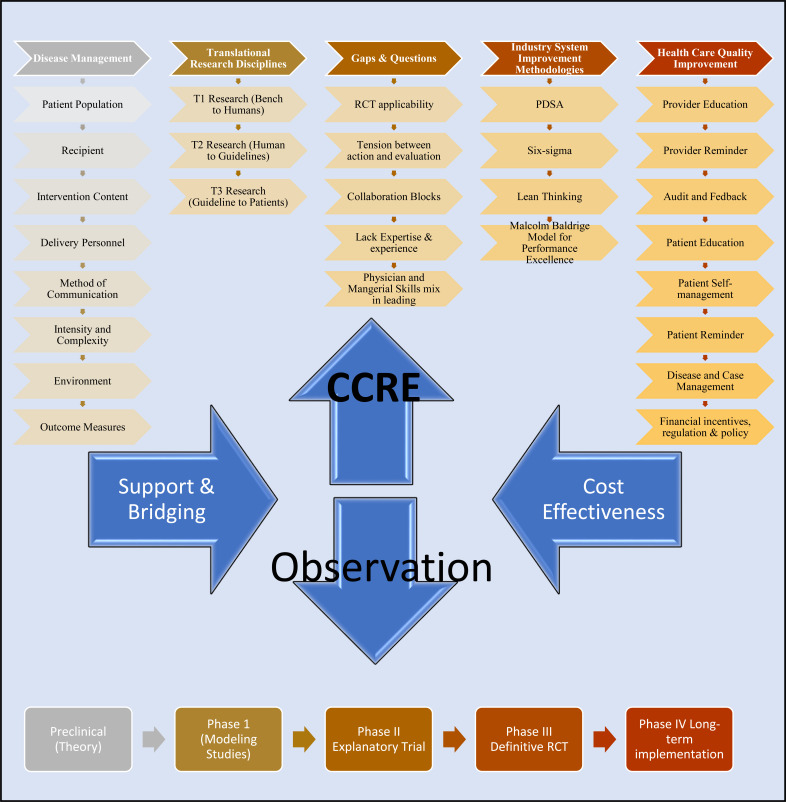
Fig. (2).
CCRE quality improvement and outcomes research bridge and support. The role for Clinical Centre For Excellence (CCRE) is outlined. Clinical care is shaped around standardised Disease Management e.g Krumholtz 8 domain program. Clinical Observation are at the heart of interactions. Important findings require the necessary support or bridging to achieve cost-effectiveness. Depending on the level of expertise and experience CCRE could help health clusters navigate question of research design, system gaps, and improvement methodology based on Industry or Health care concepts. It is vital that infrastructure within a cluster be used first and CCRE engaged when health clusters require assistance. Concepts modified from ref [3,10, 16]. Disease Management: 1. Patient Population - Risk Status, Comorbid Condition, Non-clinical features; 2. Recipient - Patient/ Caregiver; Case Provider; 3. Intervention Content - Patient/ Caregiver Education, Medical Management, Peer Support, Remote Monitoring; 4. Delivery Personnel – Nurse, Physician, Pharmacist, social workers, Dieticians, Physical therapists, Psychologists, Case managers, Care coordinators; 5. Method of Communication – Face-to-face individual, Face-to-face group, Telephone in person, Telephone Mechanised, Internet; 6. Intensity and Complexity – Duration, Frequency/ Periodicity, Complexity; 7. Environment – Hospital in-patient, Hospital out-patient, Home-based; 8. Outcome Measures – Clinical measures, Process Measures, Quality of life measures, Patient Satisfaction, Provider Satisfaction. Translational Research Disciplines: 1. T1 Bench to Human - Basic Science, Molecular Biology, Genetics, Technology Assessment, Animal Research, Phase I & II clinical trials; 2. T2 Human to Guideline Phase 3 clinical trials, observational studies, evidence synthesis and guidelines, clinical epidemiology, comparative effectiveness, policy and ethics; 3. T3 Guideline to Patient - Implementation and Dissemination, systems redesign, communication theory, behavioural and management science, organizational development, patient encounter research.
4.1. Traditional Research Framework and Paradigms
Western research is built on the paradigm of sequence (Fig. 1); for example, a taxonomy and populating those domains with variables that can be actioned and measured, such as performance indicators. This evidenced framework is overseen by a standardisation framework that delivers consensus on the parameters for the observations. Paradigms change when the parameters within any framework shift beyond the current boundaries forming a new framework. Thus, Cardiology research has two essential ingredients as its unchanging paradigms: observation described within consensus standardised measures and governance-on-governance or monitoring of all steps in all processes. In practice, each health professional is responsible for ensuring that the standard of care is chronologically present. It remains unclear what the expectations are when large grey areas are observed. Frameworks are set by National bodies such as Speciality Colleges and approved institutions like hospitals that enforce them [11].
Research starts with the intention of establishing an answer for a defined question through a systematic process [11]. Mandatory CMU Continuous Medical Education (CME) is welcomed and is mostly sufficient governance for health staff. All evidence has a shelf-life and applicability. Incidental observations scheduled governance audits to accumulate new data, some of which require action. Starting this process can occur via literature reviews, and when data is pooled, meta-analysis or systematic reviews can generate a new perspective. If novel data is required, observations, case and cohort studies are conducted. Experiments can also be designed on animals and humans. When an understanding takes shape, the findings can be consolidated by testing the hypotheses in controlled studies, to eliminate bias. This process can traditionally take years and large funds. It is largely assumed that a finding is translatable, however, phase-4 (post-trial) studies are not mandatory nor feasible across many health systems. Thus, it is important for us to consolidate the important achievements through the EBM process and equally continuously explore avenues to improve on this. For example, we can break down evidence-translation into two parts with three questions [12-20]:
What is the generalisability of the evidence in the community?
• What are the research aims?
• Is the research hypothesis addressing the question?
• Who or what does the finding apply to when translating to the real-world community?
What is the applicability of the evidence in individual clinical practice?
• Does the study findings apply to the Australian community at large?
• Are there examples in groups outside the enrolment cohort-criteria who have significantly abnormal health outcomes, and are their outcomes using the new finding below previously used best practice or trial treatment arm?
• Are there systems in place to prospectively audit this observation and, if required, translate a new solution?
4.2. Screening for Congestive Heart Failure Risk
There are 4 stages of Congestive Heart Failure (Fig. 3). Oxford textbook of medicine defines two components of screening, “Medical screening is the systematic application of a test or inquiry to identify individuals at sufficient risk of a specific disorder to benefit from further investigation or direct preventive action (these individuals not having sought medical attention on account of symptoms of that disorder). The key to this definition is that the early detection of disease is not an end in itself; bringing forward a diagnosis without altering the prognosis is useless and may be harmful....” [21, 22]. There has been ambiguity and standardisation in terminology through the years [21, 23]; for CHF, in this paper, we utilise the broader meaning of the identification of problems prior to onset.
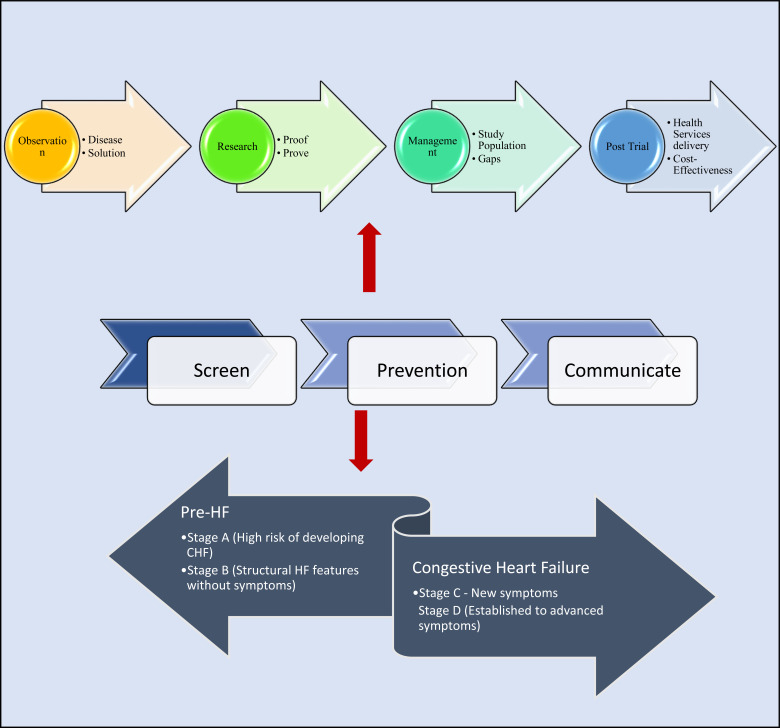
Fig. (3).
Screening and prevention clinical-research paradigm. Figure highlights the progression of medical practice. Clinical observation of diseases and discussed to gain directions for solutions. Research identifies early proofs and via clinical trials prove these early findings. Clinical management takes shape by having evidence for trial populations. Gaps will exist and this is examined as post trial or phase IV observations. The eventual goal of post-trial studies are linked between how to achieve cost-effectiveness or the most acceptable balance between service cost and achievable outcomes. Three essential determinants of cost effectiveness of any disease are public health issues. These have a chronological factor in common which is to be done early e.g. screening for diseases, preventing progression and communication between client and health systems.
There is no doubt that screening for key diseases in childhood, endemic regions and older populations followed by either preventive or prognostic management strategies have seen improvements in global health and life expectancies [24, 25]. However, the burden, management and cost-effectiveness of chronic diseases, e.g., CHF, must take a different approach, at least in developed nations, as there has been a plateau in mortality and readmissions and an escalation in cost and prevalence [26]. As there will be no cure, the disease trajectory will wax and wane and reach a terminal phase after many years; from a primary practice perspective, some legitimate questions for cost-effectiveness are:
a. What are primary care models for stages a to d?
b. What are the boundaries and overlaps to set a screening, prevention and monitoring program for stages a to d?
c. What are the rate-determining elements at each stage?
d. Are scoring tools limited to the risk of incipient disease sufficient, and are there overlaps in risk factors relevant across a patient's health journey?
e. At what level is sharing of information and resources beneficial?
Global risk assessment tools improve primary care provider's accuracy in predicting risk [23, 27]. This is important as absolute risk reduction can vary from 3-6% for an individual baseline risk of 10%, and a given treatment's relative risk reduction is 30% [28, 29]. Univariate clinical predictors such as angina for coronary artery disease are less pronounced for CHF, and imaging modalities like echocardiography are not feasible population screening tools [30]. Among the established CHF scoring tools, the majority have been formulated from population databases and provide risk from multiple variables [31]. These include Framingham Heart study, Health ABC, Atherosclerosis Risk in Communities Study, Multi-ethnic Study of Atherosclerosis and in-hospital score with GWTG-Heart Failure Risk Score [32-36]. There are overlaps in risk factors for CHF and other cardiac and metabolic syndromes, e.g., Diabetes and metabolic syndromes, hypertension, coronary artery disease, rheumatic fever, excess alcohol and drug use, family history, cancer and prescription drugs that can damage heart tissue; and emerging factors (atrial fibrillation and sleep apnoea). It is thus increasingly clear that individuals at risk of CHF are at risk of cardiac, circulatory, and metabolic diseases primarily or as future comorbidity. Secondarily, as the disease develops, inherent risks warrant screening measures equally. Risk scoring and screening should similarly go through stages.
4.3. Mixed Methods Research and Optimising Patient-Reported Outcomes
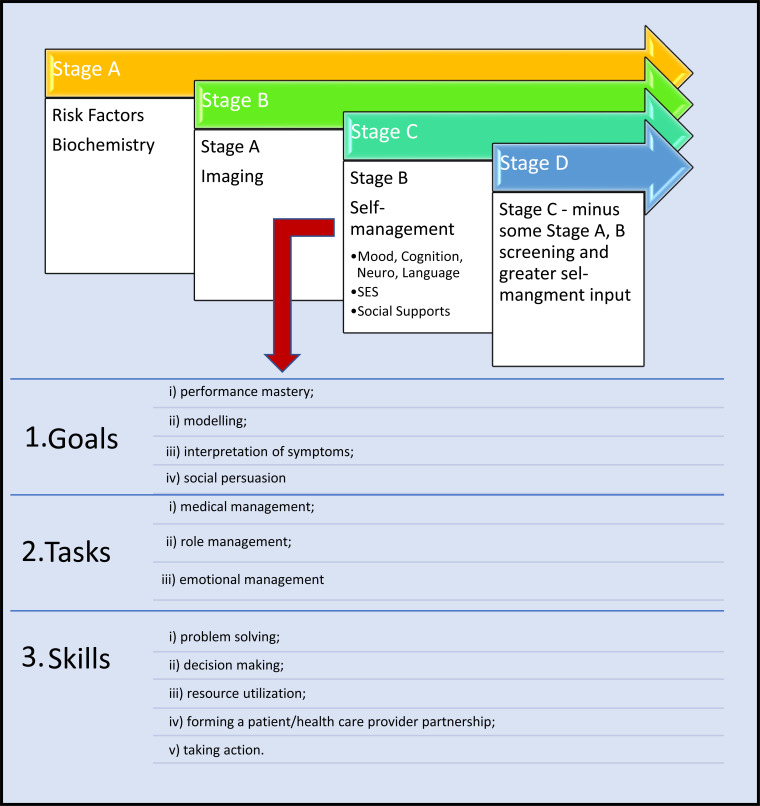
Risk scores rely on patient-reported information. As screening progresses to the various stages of diseases, the domains extracted increase in complexity (Fig. 3 and Table 1). There are three key components for chronic disease self-management programs (CDSMP) and mastery of each of these to become good self-managers is the ultimate goal [37, 38]. Poor self-managers are highlighted at the onset of the CDSM program teaching and patient interviews, predominately by limitations identified in the neurocognitive axis (comprehension, communication, physical limitations (language, dexterity, age, musculoskeletal system) and supports (geography, isolation, socioeconomics). When screening interrogates measures relevant for stages B and C, the complexity of questioning increases. Poor self-managers, in return, reduce attendance of follow-up or monitoring tests, medication compliance or seek medical assistance early. These practical and psychological factors are important barriers.
Table 1.
Heart failure stages and patient reported measures.
| Topic | Pro and Cons | Refs. |
|---|---|---|
| Disease Management, Guidelines (clinical trial consensus), post-trial strategies | P: Covers all HF C: Not sufficient to address Gaps |
[2, 7, 26, 44, 45] |
| Personalised Medicine | P: could be effective C: Costly |
Current review |
| Screening | P: Identify vulnerable cohort C: No effective proven tools |
Current review |
| Self-management and PRO | P: Cost effective C: Subjective. Translational Gaps |
Current review |
| Translational Models | P: Jurisdiction defined C: Requires a broad consensus |
[11] Current review |
| Health Technology and Logistics | P: Cost effective. Relevant to all patients and health services C: Large Translational Gaps remain |
[1, Current review] |
For example, Dickson et al. found that 79% of 114 patients reported 2 or more comorbidities that influenced self-care. Clark et al. reviewed 49 studies and noted that many patients were motivated and sought assistance to improve their adherence to care plans. However, deficiencies can exist in knowledge, training, and assessment. Mismatches between client, support group and health system beliefs, variable involvement of the family and, finally, failure to structure programs around patients’ normal routines. Marti et al. surveyed 308 CHF patients and showed that self-reported behavioural adherence can be low and selective in a variety of areas, including alcohol intake, smoking cessation and exercise. Patients also go through a process of developing skills and understanding where these new skills sit with their health-related beliefs. Changes in behaviour can occur with variable timeframes, in CHF disease. Depressive symptoms can manifest as physical or intellectual impediments, can be difficult to assess, are poorly detected and contribute to poor self-care. Other barriers include socioeconomic status, which can alter patient priorities, and communication barriers for which technology can be of benefit. Non-modifiable barriers in age, sex, culture or language, ethnicity, educational status and cognition are important. Published reports from the Middle East, Asia and Europe show that these factors can be addressed. In Australia, multicultural health care workers contributed to improvements observed in patient self-management skills with positive outcome trends. Multilingual patients with higher intelligence, Cognitive impairment predict good and poor self-care, respectively [37-41].
The points above suggest that to answer the CHF question, the study requires a hypothesis informing quantitative data, matched with hypothesis-generating qualitative data. In Mixed Methods Research (MMR), we integrate into a single study both qualitative and quantitative research methodologies. Quantitative CHF research methods are largely standardized [2, 42-44]. Quantitative research has several theoretical foundations, e.g., Phenomenology, grounded theory, ethnography, and historical and case studies, which allow the extraction of broad data from cohort perspectives. Focus groups are vital to standardize subjectivity within the theories and the questions generated in CHF study to data categories into subsequently clinical guidelines. Patient interviews highlight qualitative lens on complex patient behaviors, demography and multimorbidity [38]. What does MMR add? Outcomes research via quantitative data and deductive reasoning (starting with extant hypotheses and testing them with observations), will demonstrate existing and routine CHF care, including policies, on the observable outcomes during this period on individuals and cohorts. Outcomes research via qualitative data (inductive reasoning - starting with observations on a phenomenon and developing hypotheses) leans on hypothesis generation from complex health system's interactions with complex social behaviours, including patients and provider's personal interaction with health services (e.g., beliefs, values, and motivations), perceptions of quality of care, organisational and structure in implementing guideline-based care. This field uses principles across disciplines, fields, subject matter, and even approaches, that are exploratory, e.g., starts with observation and develop hypotheses and novel insights [39,40].
4.4. Directions for Developing a Framework for Clinical Translation
The lens that gauges health services is vital in interpreting epidemiology and outcomes data. We believe screening and PRO-self-management axis are relatively underdeveloped and has tremendous importance in translation and achieving population-level cost-effectiveness. Clinical translation and cost-effectiveness or pathways that determine treatments and its cost could be considered synonymous. Two areas stand out between trial evidence and clinical translation: firstly, ‘vulnerable patients’ who are unable to receive (including compliance issues) optimal medical therapies; secondly, ‘readmission’. Both contribute to preventable costs. Articles in this series have addressed vulnerable patients, risk scoring, health technologies, data management, logistics and health services research [45, 46] (Fig 4 and Table 2). In this section, we string together those concepts and the above points toward clinical translation:
Fig. (4).
Screening and self-management and patient reported outcomes link.
Table 2.
CHF management strategies and gaps.
| Stage | Risk | Factor | Risk Scoring Tool | Goal | Evidence |
|---|---|---|---|---|---|
| A | CCF development | Hypertension. Diabetes. Coronary artery disease. Metabolic syndrome. History alcohol abuse. Rheumatic fever. Family History Drugs e.g cancer drugs OSA AF |
Questionnaire 12-lead ECG |
Detect CHF at good cost-effectiveness | High |
| B-D | Management | Self-Management Cognition Frailty Nursing Home Complication screens |
Questionnaire | Exercise and activity Diet Smoking and exercise Medication + monitoring Device interrogation Decompensations |
High |
| Unmet Needs | Questionnaire | Variable Telehealth | Low | ||
| Telepharmacy | Questionnaire | Variable Teleleath | Low | ||
| Readmission | quality of medical management, early reassessment, health literacy, neuropsychological status, financial means functional status |
Note: The references provide a direction only and not intended to be a comprehensive list. Abbreviations: HF - heart failure; PRO - patient reported outcomes.
a. Understanding translational gaps and devising solutions:
These two quotes summarise the issues in this area:
• “Tightly controlled trials are poor predictors of real-world outcomes” William Aryitey
• “The biggest limitation of clinical trials is that they deal with rigidly-defined patient populations in a highly-controlled fashion. My colleague (and JACC Associate Editor) Barry Greenberg has likened trials to “Muzak” (bland background music sometimes referred to as “elevator” or “telephone on hold” music) as compared with clinical practice, which is analogous to Mozart. Clinical practice deals with broad patient populations from all strata of society, with diverse aetiologies, variable durations and degrees of disease, and frequent comorbidities. Like Mozart, it is heterogeneous and expansive. In contrast, RCTs usually deal with narrow populations with restricted aetiologies and severity of disease in whom most comorbidities have been excluded. Like Muzak, they tend to be homogenous and confined. It is not surprising, therefore, that the results of clinical trials are frequently not easily translated to individual patients [1].” Anthony N. DeMaria
b. Consolidating on what we have learned.
• What have we learned from RCT Registries? This most recognised observational tool is relatively simple, with strong governance and internal quality. Parameters or performance measures are extracted to inform questions. Today, data storage (small and big), health systems integration can see a largely retrospective tool superseded by continuous real-time performance measurement. Standardisation of real-world clinical data, to replicate trial-level standards, is probably the new area that will need exploration.
• What have we learned from large Randomized Controlled trials? An industry that provides gold-standard proof of hypotheses and the foundation of EBM. Evidence is gathered in exclusivity, and the implementation is generic. However, post RCT translational or phase-4 studies are relatively underdone11. Large studies from the author's jurisdiction include the ANBP 1 and 2, and the ASPREE trial recruiting in excess of 19000 patients with follow-up infrastructure beyond ten years. While racial heterogeneity was again low at 10%, the infrastructure to conduct such studies is vital [47,48].
• Interpreting RCT’s:
i. Not efficient at establishing effectiveness. Bayesian methods are needed in translation.
ii. As governance is greater, internal validity control of bias increases, but external validity (applicability and generalisability) diminishes.
iii. External validity is subject to individual judgement; excluded group solutions are lacking.
iv. Industry is vital in trial innovation and must be supported within a framework.
v. Greater emphasis on post-trial works to broaden the evidence base.
vi. Existing trial infrastructure could inform subsequent health economic evaluation.
• Existing research infrastructure: In the author's jurisdiction, larger funders include NHMRC, ARC, MRFF and are supported by smaller foundations that advocate research translation processes either through studies or collaborations. The MRFF has a 10-year plan and funds a number of portfolio initiates across the health continuum [49, 50]. The Australian Government, Department of Health oversees the initiative [51], and the National Health and Medical Research Council (NHMRC) handles the grants [52, 53]. The NHMRC is the leading advocate in this space with three initiatives, hosting The Annual NHMRC Research Translation Symposium since 2012, The Advanced Health Research and Translation Centres (AHRTCs) and Centres for Innovation in Regional Health (CIRH) initiatives and numerous funding schemes. The Australian Research Council (ARC) funding non-medical research has a slight overlap in this space. Industry links facilitated by academics and the Government have created translational bridges, with commercialization sectors, for basic sciences, including BioCurate [54]; and clinical sciences such as The Australian Centre For Health Services Innovation (AusHSI), a research, consultancy and training organization [55].
c. Directions for Developing a Framework for Clinical Translation and Implementation
The largest caveat in EBM is in phase-4. To advance this, first, understand the objectives; secondly, the framework and governance mechanisms:
4.4.1. Objectives
i. From a clinician's perspective, translation is to exercise clinical judgement or translate guideline-based evidence for any individual patient's needs. While cardiology specialty has the greatest representation of large RCT’s, this prolific accumulation of trial evidence has created a void in clinical judgement for routine scenarios, and doubts when new variables are introduced1; however, as Stone quotes, “A p-value is no substitute for a brain [9].”
Outside study population, study endpoints, particularly when using surrogate, composite endpoints, sub-group analyses or multiple testing, can lead to misjudgements in wider practice. Examples include: CAST (Cardiac Arrhythmia Suppression Trial), where suppressing ventricular ectopy increased mortality; torcetrapib increased mortality despite higher high-density lipoprotein; composite endpoints such as hospitalisation and death have hugely different significance; and finally, chance findings as with the ISIS (International Study of Infarct Survival) trial identifying aspirin benefits by astrological sign [1, 7, 9, 56-60]. Beyond pathophysiology compatibility, e.g., pill size, dosing intervals, combinations, broader effects on comorbidities and a rigid formulary, [61, 62] are not compatible with the evidence. In today's environment, stronger scientific foundations are more likely to influence rigid clinical practice [18].
Thus, in determining the objective, the most important factor is: firstly, the majority of trial evidence is generalisable, but in some settings, they are not universally generalisable; secondly, not all recommendations can come from RCT’s, if they have not been conducted or smaller trials provide clinically important findings from representative populations [1, 17, 18, 20, 56]; finally, how prevalent are the issues:
1. Common: Are guidelines flexible enough to accommodate heterogeneous needs, e.g., using agents of similar classes for extra class benefits (vasodilatory beta-blockers) or in the simplicity of dosing?
2. Low to Intermediate: Therapeutics may have multiple clinical effects, beneficial or detrimental (COX-II and cardiovascular risk), time effect (late-stent thrombosis) or efficacy (HF class beta-blockers).
3. Rare issue: Are there specific pathophysiological, phenotypic or genotypic factors that apply selectively to some groups, for example, antihypertensives and HF therapies such as lisinopril, losartan, and bucindolol in African Americans?
4. Negative trials – lower publication rates, hence data is missing from the evidence pool.
4.4.2. IMPLEMENT-IT
ii. Governance Structure and Framework
Translational ‘Framework’ pathways are not a mainstay across health systems. Health policy institutes are a good arbiter for this (Fig. 5). Some of the challenges include:
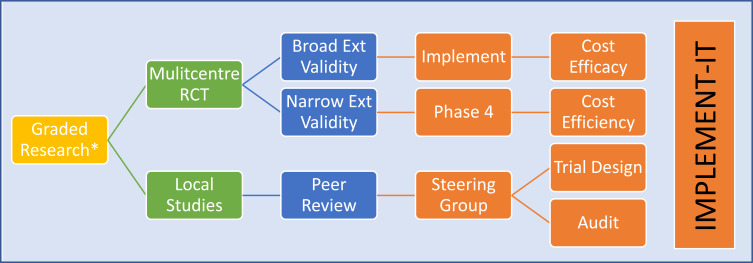
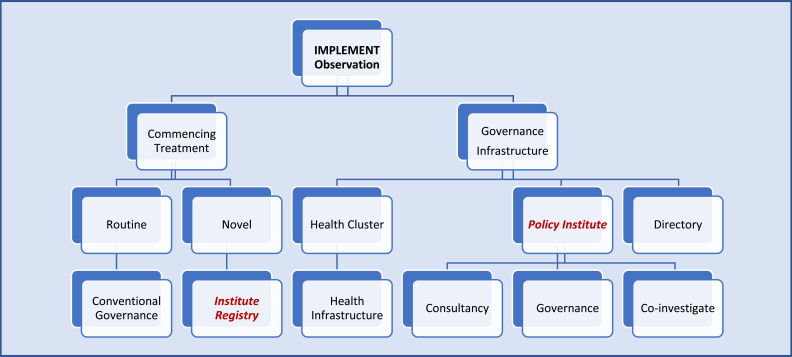
Fig. (5).
Pathways For health policy institutes to steer translational research. (a). Pathway for translation and cost-efficacy. The common final endpoint of all research is cost effectiveness. This is also the least actioned and most important arm of translational research. All health jurisdictions should have a mechanism to answer questions to ensure safe and robust health translation and act on variations. IMPLEMENT-IT is a concept for a Policy Institute to take up the role to create governance structures and provide consultancy to other Australian institutes to implement all levels of research that has relevance to individual patients. It is envisaged the Institute will assist in six subdomains: 1) Research design; 2) Research application (translation) i. routine ii. challenges; 3) Transforming research to translational level; 4) Funding and infrastructure; 5) Bridging and collaboration; 6) Governance and cost-efficacy.
1. Infrastructure exists for clinical pathways, administering newly approved therapies. Standard operating procedures and governance for the topics raised above lags and translation for individual patient needs remain varied.
2. Cost-effectiveness and “ cost-accountability*” – remain the principal goal. Importantly, no such entity exists with regulatory powers to attain this goal (Fig. 5a).
3. Developing Models of Care and Enforcing targets - Health System Governance Structure and Framework should build on the strength inherent in health systems (Fig. 5b). Enforcement appears to be an important grey area. Health funding is a complex nit of on the ground factors and the political environment. Services also benefit from the volume that improves skills and centralising and sharing of processes. This will naturally require service jurisdictions e.g., health clusters. From this balance of volume, skills and efficiency, the next step is exploring models to generate health industries with local economies and innovation (research and development) capabilities [16, 17, 21, 62-75].
(b). Governance framework for translational research. External validity of clinical trials are increasingly relevant for Australian populations. Observation may note marked regional variations. Guidelines do not always incorporate recommendations for the heterogeneity and demographic differences that may lead to the observed variations in outcomes. The process of accumulating powered, valid, generalisable evidence is rigorous and can be difficult. Smaller non-trial-based studies can be limited by power and hence translatability to the bedside to make it into guidelines. However, all evidence could have local or selective applications. In this regard investing in IMPLEMENT-IT, Policy Institute infrastructure to govern, consult and participate in the field could be advantageous to Australian Cardiovascular community. Directory is a hub role to link to relevant health or research organisations.
“IMPLEMENT-IT “ – In this hypothetical example (Fig. 5), the ‘Foundations and Jurisdiction for
Governance’ take three roles in evidence-translation. The boundaries are for a health cluster or defined geography with share health agenda. Local Health Policy Research Institutes act as think tanks providing white papers on potential links and the means to achieve them. Local health clinical services include primary care, tertiary specialists, and public or private medical and allied health specialists. The objective is cost-effectiveness and the differences discussed are CHF screening and optimising PRO interpretation in self-management efficacy. The additional advantage of centralising the policy aspect is that CHF is a chronic disease, translating this process to other diseases becomes much easier.
The options for treatments for chronic diseases such as CHF are advanced. There are, however, differences in translation, particularly at a community level, in readmissions and achieving optimal cost-effectiveness. The wider consideration may appear as a link between policy and clinical arms (e.g., IMPLEMENT-IT) and defining the boundaries to enforce (e.g., cost-accountability).
CONCLUSION: FUTURE OF TRANSLATION, GOVERNANCE IN CARDIOVASCULAR PRACTICE
“We have come to rely on trial data so much that trainees sometimes now feel paralyzed to make a decision in the absence of data from an RCT.” The data from RCTs represents the beginning of the decision-making process, not the end. The experience and wisdom of a thoughtful physician can make an important contribution to the application of the evidence base that is available [1].” Anthony N. DeMaria
Clinical medicine is essentially a collaborative effort. Paradigms continue to evolve and questions remain on our approach. If we look at medicine as one paradigm of health improvement and at an acceptable societal cost, then what remains is the framework for taxonomies, guidelines, and evidence translation as examples. Evidence-based medicine has successfully created a rigorous process from an observation to generate evidence. The consistent shortfalls in this process have been in implementation and cost-effectiveness. This review brings together broad pillars to create an impetus for future discussion on improving the implementation of chronic diseases with an HF focus.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- HF
Heart Failure
- PRO
Patient-Reported Outcomes
- CDSMP
Chronic Disease and Self-Management Programs
- CVD
Cardiovascular Diseases
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
All co-authors have won independent and governmental research funding. None pose a conflict of interest for this review.
NOTE
*Cost-accountability - we introduce this term to infer the enforcement of cost-effectiveness with a jurisdiction context for clinical practice. This term implies the clinical goal or targets that best reflect theoretical calculated cost-effectiveness. Regulatory bodies can use this as an enforcement benchmark.
REFERENCES
- 1.Smith S.C., Jr Risk-reduction therapy: The challenge to change. Presented at the 68th scientific sessions of the American Heart Association November 13, 1995 Anaheim, California. Circulation. 1996;93(12):2205–2211. doi: 10.1161/01.CIR.93.12.2205. [DOI] [PubMed] [Google Scholar]
- 2.Krumholz H.M., Currie P.M., Riegel B., et al. American Heart Association Disease Management Taxonomy Writing Group. A taxonomy for disease management: A scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006;114(13):1432–1445. doi: 10.1161/CIRCULATIONAHA.106.177322. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan D.J., Julian D.G. Achievements and limitations of evidence-based medicine. J. Am. Coll. Cardiol. 2016;68(2):204–213. doi: 10.1016/j.jacc.2016.03.600. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt G., Cairns J., Churchill D., et al. Evidence-based medicine: A new approach to teaching the practice of medicine. JAMA. 1992;268(17):2420–2425. doi: 10.1001/jama.1992.03490170092032. [DOI] [PubMed] [Google Scholar]
- 5.Loughlin M., Bluhm R., Buetow S., et al. Reason and value: Making reasoning fit for practice. J. Eval. Clin. Pract. 2012;18(5):929–937. doi: 10.1111/j.1365-2753.2012.01896.x. [DOI] [PubMed] [Google Scholar]
- 6.DeMaria A.N. Clinical trials and clinical judgment. J. Am. Coll. Cardiol. 2008;51(11):1120–1122. doi: 10.1016/j.jacc.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Ghali J.K., Massie B.M., Mann D.L., Rich M.W. Heart failure guidelines, performance measures, and the practice of medicine: Mind the gap. J. Am. Coll. Cardiol. 2010;56(25):2077–2080. doi: 10.1016/j.jacc.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Bonow R.O., Bennett S., Casey D.E., Ganiats T.G., Hlatky M.A., Konstam M.A. ACC/AHA clinical performance measures for adults with chronic heart failure. J. Am. Coll. Cardiol. 2005;46(6):1144–1178. doi: 10.1016/j.jacc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Stone G.W., Pocock S.J. Randomized trials, statistics, and clinical inference. J. Am. Coll. Cardiol. 2010;55(5):428–431. doi: 10.1016/j.jacc.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 10.Ting H.H., Shojania K.G., Montori V.M., Bradley E.H. Quality improvement: Science and action. Circulation. 2009;119(14):1962–1974. doi: 10.1161/CIRCULATIONAHA.108.768895. [DOI] [PubMed] [Google Scholar]
- 11.Iyngkaran P., Liew D., McDonald P., et al. Phase 4 studies in heart failure - what is done and what is needed? Curr. Cardiol. Rev. 2016;12(3):216–230. doi: 10.2174/1573403X12666160606121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford E.S., Ajani U.A., Croft J.B., et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N. Engl. J. Med. 2007;356(23):2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 13.Geboers H., van der Horst M., Mokkink H., et al. Setting up improvement projects in small scale primary care practices: Feasibility of a model for continuous quality improvement. Qual. Health Care. 1999;8(1):36–42. doi: 10.1136/qshc.8.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geboers H., Mokkink H., van Montfort P., van den Hoogen H., van den Bosch W., Grol R. Continuous quality improvement in small general medical practices: The attitudes of general practitioners and other practice staff. Int. J. Qual. Health Care. 2001;13(5):391–397. doi: 10.1093/intqhc/13.5.391. [DOI] [PubMed] [Google Scholar]
- 15.Shojania KG, McDonald KM, Wachter RM, Owens DK. Closing the quality gap: A critical analysis of quality improvement strategies. agency for healthcare research and quality publication No. 04–0051–1. 2004. Available from: http://www.ahrq.gov/downloads/pub/evidence/pdf/qualgap1/qualgap1.pdf. [PubMed]
- 16.Hlatky M.A., Douglas P.S., Cook N.L., et al. Future directions for cardiovascular disease comparative effectiveness research: Report of a workshop sponsored by the National Heart, Lung, and Blood Institute. J. Am. Coll. Cardiol. 2012;60(7):569–580. doi: 10.1016/j.jacc.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gitt A.K., Bueno H., Danchin N., et al. The role of cardiac registries in evidence-based medicine. Eur. Heart J. 2010;31(5):525–529. doi: 10.1093/eurheartj/ehp596. [DOI] [PubMed] [Google Scholar]
- 18.Grol R., Dalhuijsen J., Thomas S., Veld C., Rutten G., Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: Observational study. BMJ. 1998;317(7162):858–861. doi: 10.1136/bmj.317.7162.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAlister F.A., van Diepen S., Padwal R.S., Johnson J.A., Majumdar S.R. How evidence-based are the recommendations in evidence-based guidelines? PLoS Med. 2007;4(8):e250. doi: 10.1371/journal.pmed.0040250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warrell D.A., Cox T.M., Firth J.D., editors. 55. Wald N, Law M. Chapter: Medical screening.Oxford Textbook of Medicine. 5th ed. Oxford University Press; 2018. [Google Scholar]
- 22.Wald N.J. The definition of screening. J. Med. Screen. 2001;8(1):1. doi: 10.1136/jms.8.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Rossello X., Dorresteijn J.A., Janssen A., et al. Risk prediction tools in cardiovascular disease prevention. Eur. Heart J. Acute Cardiovasc. Care. 2020;9(5):522–532. doi: 10.1177/2048872619858285. [DOI] [PubMed] [Google Scholar]
- 24. Available from: https://www.who.int/healthinfo/global_burden_disease/en/ [Accessed on Apr 27, 2022].
- 25. The Global Health Observatory. Available from: https://www.who.int/data/gho [Accessed on Apr 27, 2022].
- 26.Iyngkaran P., Liew D., Neil C., Driscoll A., Marwick T.H., Hare D.L. Moving from heart failure guidelines to clinical practice: Gaps contributing to readmissions in patients with multiple comorbidities and older age. Clin. Med. Insights Cardiol. 2018;12:1179546818809358. doi: 10.1177/1179546818809358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matheny M, McPheeters M, Glasser A, et al. Systematic review of cardiovascular disease risk assessment tools. 2011. [PubMed]
- 28.Barratt A., Wyer P.C., Hatala R., et al. Evidence-Based Medicine Teaching Tips Working Group. Tips for learners of evidence-based medicine: 1. Relative risk reduction, absolute risk reduction and number needed to treat. CMAJ. 2004;171(4):353–358. doi: 10.1503/cmaj.1021197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace M.L., Ricco J.A., Barrett B. Screening strategies for cardiovascular disease in asymptomatic adults. Prim. Care. 2014;41(2):371–397. doi: 10.1016/j.pop.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyngkaran P., Chan W., Liew D., et al. Risk stratification for coronary artery disease in multi-ethnic populations: Are there broader considerations for cost efficiency? World J. Methodol. 2019;9(1):1–19. doi: 10.5662/wjm.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho J.E., Magnani J.W. The MESA heart failure risk score: Can’t we do more? Heart. 2015;101(1):7–9. doi: 10.1136/heartjnl-2014-306459. [DOI] [PubMed] [Google Scholar]
- 32.Kannel W.B., D’Agostino R.B., Silbershatz H., Belanger A.J., Wilson P.W., Levy D. Profile for estimating risk of heart failure. Arch. Intern. Med. 1999;159(11):1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 33.Butler J., Kalogeropoulos A., Georgiopoulou V., et al. Health ABC Study. Incident heart failure prediction in the elderly: The health ABC heart failure score. Circ. Heart Fail. 2008;1(2):125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal S.K., Chambless L.E., Ballantyne C.M., et al. Prediction of incident heart failure in general practice: The Atherosclerosis Risk in Communities (ARIC) Study. Circ. Heart Fail. 2012;5(4):422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chahal H., Bluemke D., Wu C., et al. Heart failure risk prediction in community dwelling adults: The Multi-Ethnic Study of Atherosclerosis Study. Heart. 2015;101:58–64. doi: 10.1136/heartjnl-2014-305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyle M., Wan S.H., Murphree D. Predictive value of the get with the guidelines heart failure risk score in unselected cardiac intensive care unit patients. J. Am. Heart Assoc. 2020;9(3) doi: 10.1161/JAHA.119.012439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan J.E., Osborne R.H. Chronic disease self-management education programs: Challenges ahead. Med. J. Aust. 2007;186(2):84–87. doi: 10.5694/j.1326-5377.2007.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 38.Iyngkaran P. Self-managing heart failure in remote australia - translating concepts into clinical practice. Curr. Cardiol. Rev. 2016;12(4):270–284. doi: 10.2174/1573403X12666160703183001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curry L.A., Nembhard I.M., Bradley E.H. Qualitative and mixed methods provide unique contributions to outcomes research. Circulation. 2009;119(10):1442–1452. doi: 10.1161/CIRCULATIONAHA.107.742775. [DOI] [PubMed] [Google Scholar]
- 40.Curry L.A., Krumholz H.M., O’Cathain A., Plano Clark V.L., Cherlin E., Bradley E.H. Mixed methods in biomedical and health services research. Circ. Cardiovasc. Qual. Outcomes. 2013;6(1):119–123. doi: 10.1161/CIRCOUTCOMES.112.967885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyngkaran P. Northern territory heart failure initiative – clinical audit (NTHFI – CA)- A prospective database on the quality of care and outcomes for acute decompensated heart failure admission in the northern territory - study design and rationale. BMJ Open. 2014;4(1):e004137. doi: 10.1136/bmjopen-2013-004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonow R.O., Ganiats T.G., Beam C.T., Blake K., Casey D.E., Jr, Goodlin S.J. etal. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure. Circulation. 2012;125:2382–2401. doi: 10.1161/CIR.0b013e3182507bec. [DOI] [PubMed] [Google Scholar]
- 43.Spertus J.A., Bonow R.O., Chan P., et al. ACCF/AHA Task Force on Performance Measures. ACCF/AHA new insights into the methodology of performance measurement: A report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures. Circulation. 2010;122(20):2091–2106. doi: 10.1161/CIR.0b013e3181f7d78c. [DOI] [PubMed] [Google Scholar]
- 44.Bonow R.O., et al. Clinical performance measures for adults with chronic heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures. Circulation. 2005;112:1853–1887. doi: 10.1161/CIRCULATIONAHA.105.170072. [DOI] [PubMed] [Google Scholar]
- 45.Iyngkaran P., Hare D.L. Heart failure paradigms in the developed world - a reflection - part 1. Curr. Cardiol. Rev. 2021;17(5):e221021197368. doi: 10.2174/1573403X1705211022154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seward J.B. Paradigm shift in medical data management: Big data and small data. JACC Cardiovasc. Imaging. 2017;10(11):1304–1306. doi: 10.1016/j.jcmg.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Ho C.L.B., Breslin M., Doust J., Reid C.M., Nelson M.R. Effectiveness of blood pressure-lowering drug treatment by levels of absolute risk: Post hoc analysis of the Australian National Blood Pressure Study. BMJ Open. 2018;8(3):e017723. doi: 10.1136/bmjopen-2017-017723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Available from: https://aspree.org/aus/
- 49.Grimshaw J.M., Eccles M.P., Lavis J.N., Hill S.J., Squires J.E. Knowledge translation of research findings. Implement. Sci. 2012;7:50. doi: 10.1186/1748-5908-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Available from: https://www.health.gov.au/initiatives-and-programs/medical-research-future-fund/mrff-research-themes/research-translation [Accessed on Apr 27, 2022].
- 51. Available from: https://www.health.gov.au/initiatives-and-programs/rapid-applied-research-translation-initiative [Accessed on Apr 27, 2022].
- 52. Available from: https://www.nhmrc.gov.au/research-policy/research-translation-and-impact [Accessed on Apr 27, 2022].
- 53. Available from: https://www.nhmrc.gov.au/research-policy/research-translation-and-impact/annual-nhmrc-research-translation-symposium [Accessed on Apr 27, 2022].
- 54. Available from: https://www.biocurate.com/ [Accessed on Apr 27, 2022].
- 55. Available from: https://www.aushsi.org.au [Accessed on Apr 27, 2022].
- 56.Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N. Engl. J. Med. 1989;321(6):406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 57.Barter P.J., Caulfield M., Eriksson M., et al. Effects of torceptrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007;357:2180–2183. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 58.ISIS 2 Collaborative Group. Randomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 59.Iyngkaran P., Majoni W., Cass A., et al. Northern Territory perspectives on heart failure with comorbidities – understanding trial validity and exploring collaborative opportunities to broaden the evidence base. Heart Lung Circ. 2015;24(6):536–543. doi: 10.1016/j.hlc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Iyngkaran P., Kangaharan N., Zimmet H., et al. Heart failure in minority populations - Impediments to optimal treatment in Australian aborigines. Curr. Cardiol. Rev. 2016;12(3):166–179. doi: 10.2174/1573403X12666160606115034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iyngkaran P., Thomas M.C., Johnson R., et al. Contextualizing genetics for regional heart failure care. Curr. Cardiol. Rev. 2016;12(3):231–242. doi: 10.2174/1573403X12666160606123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones D.W., Peterson E.D., Bonow R.O., et al. Translating research into practice for healthcare providers: The American Heart Association’s strategy for building healthier lives, free of cardiovascular diseases and stroke. Circulation. 2008;118(6):687–696. doi: 10.1161/CIRCULATIONAHA.108.189934. [DOI] [PubMed] [Google Scholar]
- 63.Drozda J.P., Bufalino V.J., Fasules J.W., et al. ACC 2009 Advocacy position statement: Principles for comparative effectiveness research. J. Am. Coll. Cardiol. 2009;54(18):1744–1746. doi: 10.1016/j.jacc.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Clark A.M., Savard L.A., Thompson D.R. What is the strength of evidence for heart failure disease-management programs? J. Am. Coll. Cardiol. 2009;54(5):397–401. doi: 10.1016/j.jacc.2009.04.051. [DOI] [PubMed] [Google Scholar]
- 65.Hachamovitch R., Di Carli M.F. Methods and limitations of assessing new noninvasive tests: Part II: Outcomes-based validation and reliability assessment of noninvasive testing. Circulation. 2008;117(21):2793–2801. doi: 10.1161/CIRCULATIONAHA.107.714006. [DOI] [PubMed] [Google Scholar]
- 66.Metra M., Dei Cas L., Massie B.M. Treatment of heart failure in the elderly: Never say it’s too late. Eur. Heart J. 2009;30(4):391–393. doi: 10.1093/eurheartj/ehp024. [DOI] [PubMed] [Google Scholar]
- 67.Ho P.M., Peterson P.N., Masoudi F.A. Evaluating the evidence: Is there a rigid hierarchy? Circulation. 2008;118(16):1675–1684. doi: 10.1161/CIRCULATIONAHA.107.721357. [DOI] [PubMed] [Google Scholar]
- 68.Krumholz H.M. Outcomes research: Generating evidence for best practice and policies. Circulation. 2008;118(3):309–318. doi: 10.1161/CIRCULATIONAHA.107.690917. [DOI] [PubMed] [Google Scholar]
- 69.Havranek E.P., Mujahid M.S., Barr D.A., et al. American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2015;132(9):873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 70.Stewart S., Riegel B., Boyd C., et al. Establishing a pragmatic framework to optimise health outcomes in heart failure and multimorbidity (ARISE-HF): A multidisciplinary position statement. Int. J. Cardiol. 2016;212:1–10. doi: 10.1016/j.ijcard.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riegel B., Lee C.S., Sochalski J. Developing an instrument to measure heart failure disease management program intensity and complexity. Circ. Cardiovasc. Qual. Outcomes. 2010;3(3):324–330. doi: 10.1161/CIRCOUTCOMES.109.877324. [DOI] [PubMed] [Google Scholar]
- 72.Zeitz C., Beltrame J. Case for Action proposal: Appropriateness and performance in the management of cardiovascular disease In Australian hospitals. Submitted by the NHMRC Research Translation Faculty Cardiovascular Health & Stroke Steering Group. 2014. Available from: www.nhmrc.gov.au/research/research-translation/research-translation-faculty/ideas-research-translation-faculty-cases.
- 73.Holmes D.R. Overcoming the challenges of conducting early feasibility studies of medical devices in the United States. J. Am. Coll. Cardiol. 2016;68:1908–1915. doi: 10.1016/j.jacc.2016.07.769. [DOI] [PubMed] [Google Scholar]
- 74.Gal D., Thijs B., Glänzel W., Sipido K.R. A changing landscape in cardiovascular research publication output: Bridging the translational gap. J. Am. Coll. Cardiol. 2018;71(14):1584–1589. doi: 10.1016/j.jacc.2018.01.073. [DOI] [PubMed] [Google Scholar]
- 75.McGlynn E.A., Asch S.M., Adams J., et al. The quality of health care delivered to adults in the United States. N. Engl. J. Med. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]