Abstract
G protein-coupled receptors (GPCRs), comprising the largest superfamily of cell surface receptors, serve as fundamental modulators of cardiac health and disease owing to their key roles in the regulation of heart rate, contractile dynamics, and cardiac function. Accordingly, GPCRs are heavily pursued as drug targets for a wide variety of cardiovascular diseases ranging from heart failure, cardiomyopathy, and arrhythmia to hypertension and coronary artery disease. Recent advancements in understanding the signalling mechanisms, regulation, and pharmacological properties of GPCRs have provided valuable insights that will guide the development of novel therapeutics. Herein, we review the cellular signalling mechanisms, pathophysiological roles, and pharmacological developments of the major GPCRs in the heart, highlighting the β-adrenergic, muscarinic, and angiotensin receptors as exemplar subfamilies.
Keywords: G protein-coupled receptors, Heart failure, Biased signalling, Allosteric modulators
Graphical Abstract
Graphical Abstract.
1. Introduction
G protein-coupled receptors (GPCRs), also known as 7 transmembrane domain receptors (7TMRs), encompass the largest and most extensively studied superfamily of cell surface receptors.1 GPCRs are activated by a diverse array of ligands including hormones, peptides, and neurotransmitters, and serve as key regulators of a variety of cellular responses. Given their involvement in many different physiological processes, GPCRs are highly pursued pharmacologically and represent the primary targets of ∼35% of all small molecule drugs currently approved by the Food and Drug Administration (FDA).2 Of the nearly 800 different human GPCR genes,3 more than 200 are expressed in the heart alone,4 underscoring their prominent role in regulating cardiac function and highlighting their potential as therapeutic targets in heart disease.
1.1. GPCR signalling mechanisms
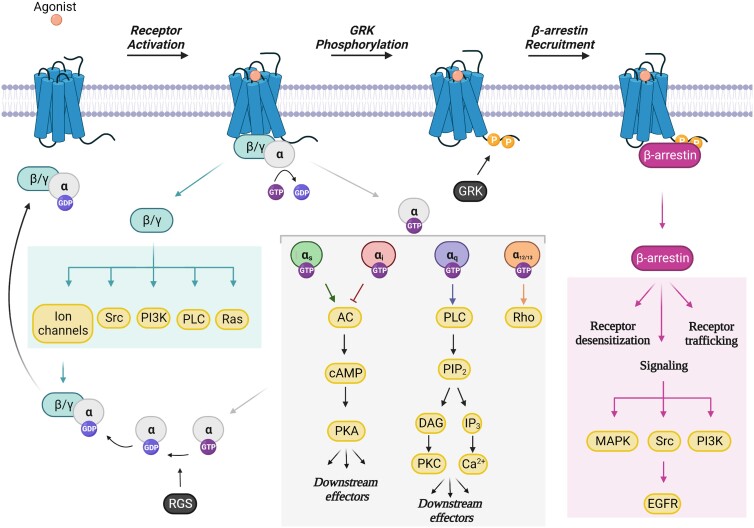
Canonically, GPCRs are activated via the binding of an agonist to the orthosteric ligand binding site on the extracellular surface (Figure 1). Agonist-bound GPCRs subsequently recruit heterotrimeric G proteins, consisting of Gα, Gβ, and Gγ subunits, and induce the exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) on Gα. This exchange stimulates the dissociation of Gα from Gβγ, generating two activated G protein units that independently transduce signals to downstream effectors.5
Figure 1.
GPCR activation and downstream signalling. Following agonist binding, GPCRs recruit heterotrimeric G proteins (Gα, β, γ) and induce the exchange of GDP for GTP on Gα. The Gα and Gβγ subunits subsequently dissociate forming two activated G protein units that independently transduce signals to downstream effectors. The four families of Gα (Gαs, Gαi, Gαq, and Gα12/13) activate distinct signalling cascades, thereby generating unique cellular responses. RGS proteins accelerate the GTPase activity of Gα, thereby leading to the inactivation of G protein signalling. GRK-mediated phosphorylation of the COOH-terminus of the receptor facilitates the recruitment of β-arrestin, which in turn, promotes receptor desensitization, receptor trafficking/recycling, and β-arrestin-dependent signalling mechanisms. GDP, guanosine diphosphate; GTP, guanosine triphosphate; PI3K, phosphoinositide 3-kinase; PLC, phospholipase C; AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacylglycerol; PKC, protein kinase C; IP3, inositol trisphosphate; MAPK, mitogen-activated protein kinase; EGFR, epidermal growth factor receptor; GRK, GPCR kinase; RGS, regulators of G protein signalling. Figure generated with Biorender.com.
The Gα subunit is divided into four major classes including Gαs, Gαi, Gαq/11, and Gα12/13, which each generate their own unique cellular responses (Figure 1).5 The Gαs subunit, a stimulatory Gα protein, activates adenylyl cyclase (AC) to generate second messenger cyclic adenosine monophosphate (cAMP) which results in the activation of protein kinase A (PKA). In the heart, PKA phosphorylates sarcomeric proteins, calcium regulators, and ion channels resulting in enhanced contractile and calcium cycling dynamics thereby mediating positive inotropic and chronotropic responses.6 In contrast, receptors that are coupled to Gαi inhibit AC, which leads to decreased production of cAMP and reduced PKA activity. Gαq-coupled receptors promote the activation of phospholipase C (PLC), which generates the second messengers, namely diacylglycerol (DAG) and inositol triphosphate (IP3) that stimulate protein kinase C (PKC) and calcium influx, respectively. Finally, Gα12/13 activates the small GTPase Rho.
To mediate signal termination, regulators of G protein signalling (RGS) and GPCR kinases (GRKs) are recruited to GTP-bound Gα subunits or agonist-activated GPCRs, respectively. RGS proteins serve to accelerate Gα GTP hydrolysis activity, causing the reassociation of Gα with Gβγ and the inactivation of downstream G protein signalling (Figure 1).7 In contrast, GRKs are recruited to agonist-activated GPCRs to phosphorylate the COOH-terminal tail of the receptor (Figure 1).8 This in turn, facilitates the recruitment of β-arrestin, a multifunctional adaptor protein, which promotes receptor desensitization via sterically uncoupling G proteins from activated GPCRs.5 β-arrestins also serve as scaffolds for cyclic nucleotide phosphodiesterases (PDEs) and diacylglycerol kinases (DGKs) that degrade second messengers cAMP and DAG, respectively, providing another mechanism to dampen signalling.9,10 In addition to their desensitization function, β-arrestins facilitate receptor internalization and trafficking to endosomes via binding clathrin and its adaptor protein AP2.11 Importantly, β-arrestins function as signal transducers via scaffolding major signalling complexes such as mitogen-activated protein kinases (MAPKs), Src, and phosphoinositide 3-kinase (PI3K).12
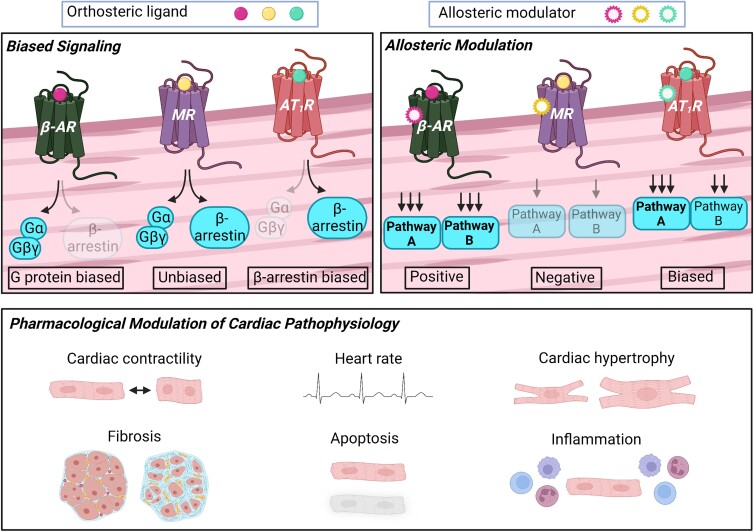
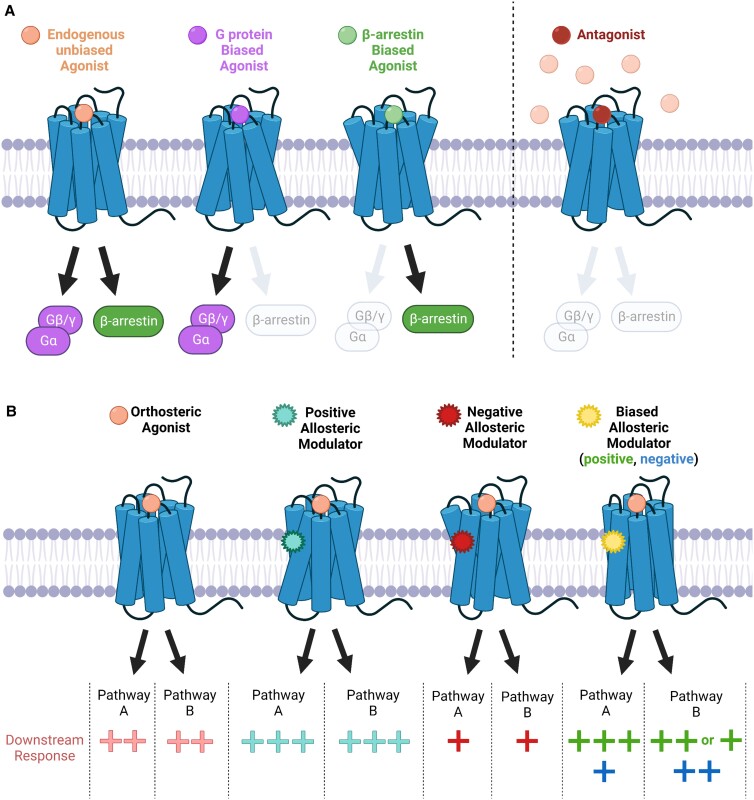
The discovery that β-arrestins serve as functional signal mediators has led to the emergence of a fundamental concept in GPCR biology termed biased agonism. Compared to the cognate endogenous ligands for each GPCR which activate multiple signalling pathways in an unbiased fashion, biased ligands stabilize distinct receptor conformations to preferentially activate either G protein or β-arrestin pathways (Figure 2A).13 The concept of biased agonism provides important implications in the development of therapeutics since drugs can be developed to target specific signalling pathways that will generate more beneficial physiological outcomes and/or reduce adverse side effects.
Figure 2.
Biased agonism and allosteric modulation of GPCR function. (A) Unbiased orthosteric agonists non-selectively activate G protein and β-arrestin signalling pathways, while biased orthosteric ligands preferentially activate one pathway over the other. Conversely, antagonists competitively inhibit agonist binding and thereby inactivate both G protein and β-arrestin signalling in a balanced fashion. (B) Allosteric modulators bind to sites that are topographically distinct from the orthosteric binding pocket of the receptor. Functionally, positive allosteric modulators enhance (+++) the activity of an orthosteric agonist, while negative allosteric modulators reduce (+) orthosteric activity. Biased allosteric modulators can preferentially enhance (positive; +++) or reduce (negative; +) the activity of a particular pathway (i.e. pathway A) while having no effect (++) and/or antagonizing (+) an alternative pathway (i.e. pathway B) in the presence of an orthosteric agonist. Figure generated with Biorender.com.
1.2. Allosteric modulation of GPCR function
Aside from the orthosteric binding region, ligands can target allosteric sites of the receptor (i.e. regions that are topographically distinct from the orthosteric binding pocket). These ligands, termed allosteric modulators, most often do not possess intrinsic activity on their own, but can enhance (positive allosteric modulator) or reduce (negative allosteric modulator) the activity of receptors stimulated by orthosteric ligands (Figure 2B).14 The identification of allosteric modulators that possess intrinsic activity in the absence of an orthosteric ligand has additionally established ‘ago-allosteric modulators’ as an entity.15 Importantly, both allosteric and ago-allosteric modulators can potentially act in a biased fashion by enhancing the activation of a particular signalling pathway while either having no effect or antagonizing alternative pathways (Figure 2B).16 Given that the orthosteric binding pocket is typically highly conserved among GPCR subtypes, while allosteric regions exhibit greater sequence and structural diversity, allosteric modulators have a higher potential for being subtype-specific and/or generating biased cellular effects.14
Herein, we review the cellular signalling mechanisms, physiological functions, clinical implications, and current pharmacological developments of the major subtypes of GPCRs that regulate cardiac physiology. Given the large number of different GPCRs expressed in the heart,4 we chose to focus on the receptors that have shown the greatest pharmacological potential and/or highest efficacy in the treatment of heart disease, including the β-adrenergic, muscarinic, and angiotensin receptor families.
2. β-Adrenergic receptors
Adrenergic receptors were initially subclassified into two distinct receptor families (α and β) by Ahlquist in 1948.17 Within the β-adrenergic (βAR) family, there are three major subtypes that include the β1AR, β2AR, and β3AR, which are encoded by the genes ADRB1, ADRB2, and ADRB3, respectively. All three βARs are endogenously activated by the catecholamine hormones epinephrine and norepinephrine (Figure 3). For the β1AR, norepinephrine has a slightly higher potency than epinephrine, while the agonist potency is reversed for the β2AR.18
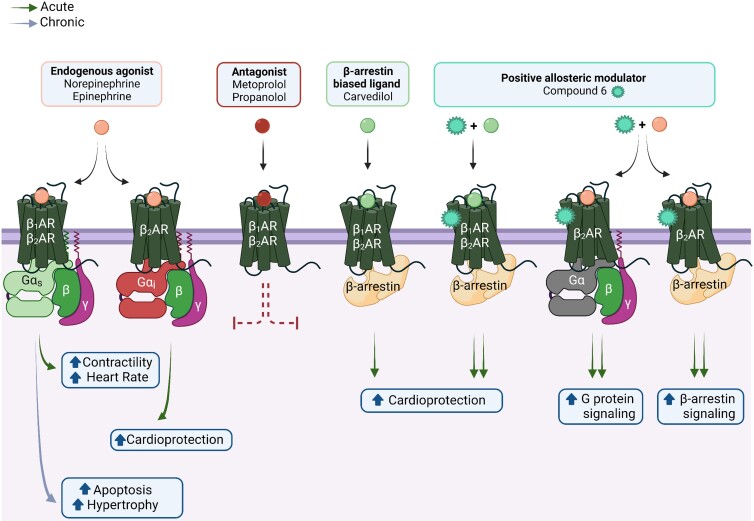
Figure 3.
Physiological and pathological effects of cardiac βAR signalling. Norepinephrine and epinephrine, the endogenous βAR agonists, activate both β1AR and β2AR subtypes, albeit with different potencies. In general, β1AR is coupled to Gαs and β2AR is coupled to both Gαs and Gαi. In the short term, the activation of β1AR/β2AR through Gαs generates positive chronotropic and inotropic responses; however, chronic activation through Gαs can induce cardiomyocyte apoptosis and hypertrophy. In contrast, Gαi-mediated signalling via β2AR is thought to be cardioprotective due to its anti-apoptotic and anti-fibrotic effects. Furthermore, both receptor subtypes can couple to β-arrestin, which also induces cardioprotective signalling cascades in the heart. Carvedilol, a clinically utilized β-arrestin-biased ligand, preferentially stimulates β-arrestin signalling while antagonizing G protein signalling. Notably, carvedilol-mediated β-arrestin-dependent cardioprotection is potentiated by Compound 6, a positive allosteric modulator of the β1AR and β2AR. Additionally, in response to agonist, Compound 6 potentiates G protein and β-arrestin signalling mediated by β2AR, but not β1AR. As expected, traditional antagonists such as metoprolol or propranolol, block βAR signalling via both G protein and β-arrestin. Figure generated with biorender.com.
2.1. Tissue expression and localization
The β1AR is the most common subtype in the heart, as it comprises about 80% of total βARs while the remaining 20% is mainly the β2AR.19 The β3AR, in contrast, is the least characterized to date and only constitutes about 3% of all cardiac βARs.20 Consistent with its increased abundance, the β1AR subtype displays a more widespread cellular distribution as it localizes to both the outer plasma membrane and T-tubule membranes of cardiomyocytes,21 whereas β2AR and β3AR are confined to the T-tubular network.21,22 In contrast to the healthy heart, failing human hearts exhibit a substantial reduction in βAR receptor density due to selective downregulation of β1AR.19 As a consequence, this shifts the β1AR:β2AR ratio from 80:20 to 60:40.19
2.2. Cellular signalling mechanisms
Under normal physiological conditions, the β1AR couples to Gαs as the signalling transducer, while the β2AR couples to both Gαs and Gαi, and the β3AR predominantly signals via Gαi.23,24 In addition to PKA activation via Gαs, stimulation of β1ARs increases calcium/calmodulin-dependent protein kinase II (CaMKII) activity in a β-arrestin-dependent manner.25 β2AR coupling to Gαi in the heart can activate cell survival signalling via Gβγ-mediated activation of PI3K and Akt.23 Furthermore, the dissociated Gβγ subunit from Gαi can also activate the Src-family tyrosine kinases and G protein Ras.26,27
Although β3ARs canonically signal via Gαi functioning to inhibit activation of AC and PKA, administration of the β3AR-selective agonist, L755-507, generated positive inotropic responses in transgenic mice overexpressing human β3AR.28 Moreover, stimulation with L755-507 resulted in increased AC activity in a pertussis-toxin insensitive fashion, indicating overexpressed β3ARs couple to Gαs.28 Unlike β1ARs and β2ARs that have rich serine/threonine residues that can be phosphorylated by GRKs, β3ARs lack these residues and therefore are more resistant to agonist-induced desensitization.24
Aside from their desensitization and internalization functions, β-arrestins also serve as signal transducers following βAR activation. Specifically, in response to an agonist, β-arrestin recruits c-Src to βAR, which subsequently induces the activation of extracellular signal-regulated kinases (ERK).29 Furthermore, β-arrestin-mediated c-Src stimulation leads to the activation of matrix metalloproteases (MMPs), which in turn, initiate epidermal growth factor receptor (EGFR) transactivation.30 Importantly, in vivo, βAR-mediated EGFR transactivation confers cardioprotection by preventing the development of ventricular dilation and contractile impairment under conditions of chronic catecholamine stimulation.30
2.3. Physiological functions and contribution to disease
Increased sympathetic nervous system activation and the adrenergic response are essential in maintaining circulatory support during acute heart failure, yet are associated with worse survival.31 Indeed, prolonged activation of catecholamine signalling is detrimental to the heart since this causes βAR desensitization, excessive CaMKII activation, and cardiomyocyte hypertrophy and apoptosis.25,32–34 Consistent with this, transgenic mice overexpressing β1AR exhibit depressed cardiac function, progressive hypertrophy, and fibrosis.35,36
Similarly, mice with cardiac-specific overexpression of β2AR display severe left ventricular dysfunction following aortic stenosis,37 and spontaneous tachyarrhythmia related to increased interstitial fibrosis.38 Furthermore, β2AR overexpressing mice are more susceptible to ischaemic injury than their wild-type counterparts, which is further exacerbated by pertussis-toxin treatment suggesting that the pathological manifestations of β2AR overexpression are mediated primarily by Gαs rather than Gαi.39 Indeed, β2AR activation is considered anti-apoptotic through its ability to couple to Gαi.40,41 Therefore, β2AR signalling may be either protective or deleterious in the heart depending on transducer coupling.
In addition to the evaluation of βAR overexpression, the generation of different βAR knockout mouse models revealed that the absence of βAR is also pathogenic. Particularly, β1AR knockout mice (β1AR−/−) die prenatally, and those that do survive lack chronotropic or inotropic responses after administration of isoproterenol.42 Although endogenous β2ARs are present in β1AR−/− hearts, they do not functionally compensate for the absence of β1ARs, suggesting that the β1AR subtype predominantly mediates the chronotropic/inotropic response in the heart.42 As further evidence for this, while β1AR/β2AR double knockout and β1AR−/− mice lack heart rate and contractile responses to catecholamine infusion,43,44 adult β2AR−/− mice respond normally.43,45 Moreover, progressive cardiac dysfunction following myocardial infarction is attenuated in β1AR−/− and β1AR/β2AR double knockout mice, but not β2AR−/− mice, demonstrating that regulation of cardiac function in normal hearts and following injury is predominantly mediated through β1ARs.43
On the other hand, the role of β3AR in cardiac function is relatively unclear. Several studies have reported that pharmacological activation and/or overexpression of β3ARs reduced contractility in healthy hearts,46–48 while others have suggested that β3AR overexpression will augment contractility.28 Moreover, overexpression or selective stimulation of β3AR by BRL 37 344 is protective against cardiac remodelling and dysfunction in response to pressure overload, myocardial infarction, or neurohormonal stimulation through a nitric oxide synthase (NOS)-mediated pathway.49–51 However, other studies have revealed that β3AR antagonism via administration of L-748 337 improved the cardiac function and exercise performance in a canine model of pacing-induced heart failure.52 Therefore, additional studies are needed to further interrogate the potential protective or detrimental impacts of β3AR in the heart.
2.4. Pharmacological perspectives
β-Blockers competitively antagonize the binding of endogenous catecholamines to the orthosteric site of βARs and are a mainstay in the treatment of chronic heart failure. Among the many different clinically utilized β-blockers,53 carvedilol possesses unique properties by virtue of its ability to recruit and activate cardioprotective β-arrestin signalling at both the β1AR and β2AR.54–57 Specifically, carvedilol stabilizes βARs in a particular conformation that initiates β-arrestin-mediated signalling while remaining uncoupled from Gαs.54 While the evidence that carvedilol is more effective as a therapeutic agent comes exclusively from experimental studies, a clinical trial revealed that carvedilol was superior to metoprolol in reducing all-cause mortality in patients with heart failure.58
Although β-blockers effectively reduce mortality, patients often experience side effects including fatigue and reduced functional capacity, which may limit their maximal effectiveness.59 This has led to an effort to identify new βAR ligands, particularly β-arrestin-biased ligands60 or allosteric modulators, aimed at preferentially enhancing the beneficial effects and minimizing the adverse effects of overall β-blockade.61 Recently, a positive allosteric modulator, Compound 6, was identified through a DNA-encoded small molecule library screen against the β2AR.62 Strikingly, Compound 6 increases the binding affinity of carvedilol for β1ARs57 and β2ARs,63 potentiates carvedilol-stimulated β-arrestin-dependent signalling,57,63 and provides enhanced cardioprotection to ischaemic injury.57
3. Muscarinic receptors
Muscarinic receptors (MRs) comprise a family of GPCRs that are activated by the principal neurotransmitter of the parasympathetic nervous system, acetylcholine (Figure 4). The five major subtypes, M1R through M5R, encoded by genes CHRM-1 to CHRM-5, respectively, range in size from ∼460–590 amino acids in length and exhibit significant sequence homology to each other.64,65 Given their ubiquitous expression across all organs,66 they are implicated in a wide array of physiological processes ranging from the regulation of cognitive, behavioural, sensory, motor, and autonomic functions mediated by MRs in the central nervous system (CNS), to the regulation of heart rate, smooth-muscle contraction, and glandular secretion mediated by MRs in peripheral tissues.67,68
Figure 4.
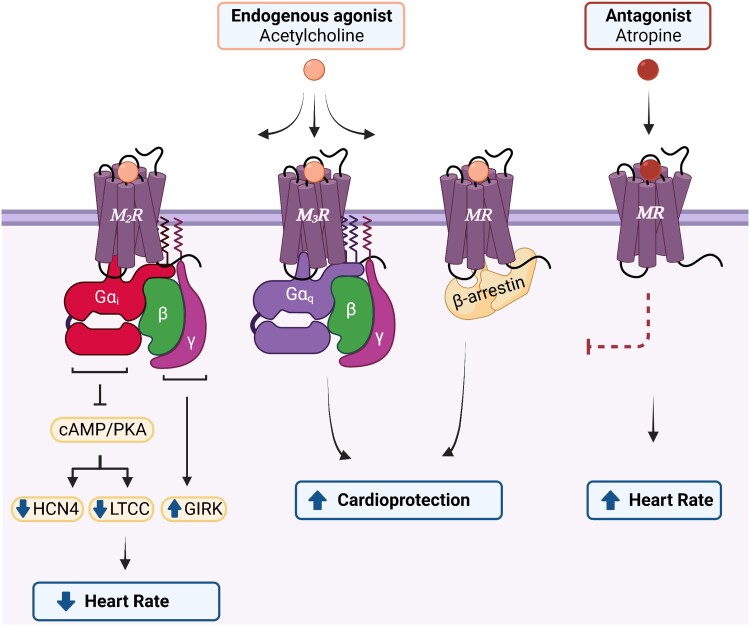
Physiological effects of cardiac MR signalling. The M2R and M3R, depicted herein as representative MR subtypes given that their physiological roles in the heart are the most extensively characterized, signal via distinct G protein classes. The M2R, coupled to Gαi, is the most abundant cardiac subtype and negatively regulates heart rate in response to the endogenous agonist, acetylcholine. In contrast, activation of M3R, coupled with Gαq, is considered cardioprotective due to its anti-arrhythmic/anti-fibrotic properties and protection against ischaemic injury. All MR subtypes couple to β-arrestin following agonist binding which initiates cardioprotective signalling cascades in the heart. In response to an antagonist, such as atropine, MR signalling is blocked and heart rate is increased. HCN4, hyperpolarization-activated cyclic nucleotide-gated 4 channel; LTCC, L-type Ca2+ channel; PKA, protein kinase A; GIRK, G protein-coupled inward rectifying K+ channel. Figure generated with biorender.com.
3.1. Tissue expression and localization
The M2R is the predominant subtype in the heart66 where its expression is almost 2.5-fold higher in the human atrium than in ventricles.69 The other subtypes are present in the heart as well, although to a lesser extent than M2R. While mRNA encoding all five subtypes has been reported in both atrial and ventricular human myocardium, immunoblotting experiments have only confirmed the expression of M1R, M2R, M3R, and M5R, but not M4R, at the protein level.70 Consistently, antibodies to all subtypes besides M4R positively stain the sarcolemma of human ventricular cardiomyocytes, with M3R and M5R additionally exhibiting strong localization to the intercalated disc.70 Although M4R protein expression has not been reported in the human heart, both functional and binding assays have revealed its presence in atrial myocardium from canines.71 This suggests that all five subtypes might be physiologically relevant in the heart but emphasizes that their functional significance may be species-dependent.
Evaluation of MR abundance in human patients with heart disease, on the other hand, has generated conflicting results. Early studies found a moderate, but not statistically significant, upregulation of M2R in the left ventricles of patients with end-stage heart failure.72 Corroborating this, positron emission tomography with radiolabeled 11C-MQNB, a muscarinic antagonist, revealed elevated MR levels in patients with idiopathic dilated cardiomyopathy perhaps as a compensatory mechanism to β-adrenergic signalling.73 However, recent studies measuring the levels of MRs in atrial tissues from patients with chronic atrial fibrillation reported no change in M2R expression, but an upregulation of the M1R subtype.74 This suggests that different cardiac disease states, chamber-specific differences, and/or receptor subtype-specific differences are important factors to consider when evaluating the impact of MRs in cardiac disease.
3.2. Cellular signalling mechanisms
Belonging to the Class A (rhodopsin-like) subtype of GPCRs, MRs undergo a stereotypical activation mechanism wherein agonist binding induces an outward movement in transmembrane helix 6 (TM6) that exposes a G protein binding cavity on the cytoplasmic surface.75 Molecular dynamics simulations of different agonist-occupied receptor conformations76 and cryo-EM structures of either M1R or M2R in complex with distinct heterotrimeric G protein families77 further revealed that conformational rearrangements in TM5, 6, and 7 correlate with the selective coupling of MRs to different G protein classes. Specifically, M2R and M4R, mediate signalling via Gαi, whereas M1R, M3R, and M5R predominantly couple to Gαq.66
The M2R and M4R receptors are coupled to the Gαi signal to inhibit AC leading to reduced PKA activity, reduced L-type calcium channel current, and suppression of the hyperpolarization-activated cyclic nucleotide-gated 4 (HCN4) channel. The HCN4 channel is a non-selective cation channel permeable to Na+/K+ that mediates the pacemaker or ‘funny’ current (If) in nodal cells and conduction tissues.78 Together, these mechanisms collectively counteract the positive chronotropic effects mediated by β-adrenergic-Gαs signalling, thereby modulating the heart rate response. In contrast, Gαq-coupled receptors M1R, M3R, and M5R promote the activation of PKC and stimulate calcium influx via second messengers DAG and IP3, respectively.
In addition to the signalling mechanisms mediated by Gα, the Gβγ subunit of the Gαi complex directly couples and activates the G protein-coupled inward rectifying K+ (GIRK) channel leading to K+ influx, hyperpolarization, and slowed heart rate in response to MR activation.79 Using atrial-specific or ventricle-specific GIRK knockout mice, Lee and colleagues demonstrated that MR-dependent regulation of heart rate is primarily mediated via atrial GIRK activation, rather than GIRK channels expressed in the ventricle.80 Of note, this mechanism can be activated by the Gβγ subunits originating from Gαq-coupled M1R stimulation as well.74 Finally, like other GPCRs, MRs are phosphorylated by GRKs in an agonist-dependent fashion resulting in the recruitment of β-arrestins which facilitate receptor desensitization, internalization, and downstream signalling.81
3.3. Physiological functions and contribution to disease
The lack of subtype-specific MR ligands and their heterogenous distribution across tissues has complicated the investigation of the individual roles of each member of the MR family. In order to study the unique function of each receptor subtype, mice constitutively deficient in each of the MRs have been generated via gene targeting.67 In addition to a wide array of cognitive and behavioural phenotypes mainly mediated through the loss of MRs in the CNS,67 mice deficient in MRs also exhibit profound abnormalities in cardiac autonomic regulation. In particular, while the application of carbachol, a muscarinic agonist, induced bradycardia in isolated atrial cells from wild-type mice, atrial cells from CHRM2−/− (M2R knockout) mice remained unaffected.82 In contrast, cardiomyocytes from M4R knockout mice, CHRM4−/−, show a very modest decrease in carbachol-induced bradycardia, suggesting that the M4R could instead participate in maximizing the bradycardic effects of carbachol stimulation, at least in the species where it is expressed.82 Although all other subtypes are detected to some level in the heart, the lack of compensatory effects mediated by the remaining subtypes in CHRM2−/− mice suggests that the M2R subtype predominantly mediates the negative chronotropic response in the heart.67,82
A significant functional role has been established for the Gαq-coupled M3R in the heart.83–88 In particular, pharmacological activation and/or overexpression of the M3R have been associated with reduced cardiac hypertrophy and myocardial injury induced by angiotensin II,83 reduced frequency of ventricular arrhythmias, cardiac fibrosis and electrical remodelling following chronic cardiac pressure overload,84–87 and is protective against cardiac damage following ischaemic injury.88 In contrast, the physiological roles of the remaining Gαq-coupled receptors in the muscarinic family, M1R and M5R, remain largely unstudied in the heart.
Notably, several recent studies have investigated the protective potential of cardiac MR stimulation and downstream signalling. For example, modulating MR activity could be clinically useful in the treatment of doxorubicin-induced cardiotoxicity. Cancer patients being treated with anthracycline-based chemotherapies (such as doxorubicin) are susceptible to developing left ventricular systolic dysfunction and arrhythmia, likely arising from imbalanced cardiac autonomic signalling.89,90 Notably, treatment with muscarinic agonist bethanechol improved overall cardiac function in a rat model of doxorubicin-induced cardiotoxicity by enhancing mitochondrial function, and reducing mitochondrial oxidative damage, cardiomyocyte apoptosis, and myocardial inflammation.89 In addition, recent studies conducted in zebrafish embryos demonstrated that the MR antagonist, tolterodine, initiates transcriptional changes in the heart that promote pacemaker cell fate. These findings proposed a new role for MRs in cardiac development and underscore the potential utility of MR antagonists as a therapy for developmentally related cardiac conduction system disorders.91
3.4. Pharmacological perspectives
Excessive sympathetic signalling and attenuated parasympathetic activity manifest early in the development of cardiac disease. In fact, depressed parasympathetic activity can occur as early as three days after the development of cardiac dysfunction and precedes the upregulation of sympathetic activity.92 Most current pharmacological interventions aim to suppress the over-active sympathetic nervous system (i.e. beta blockers). However, targeted modulation of parasympathetic pathways (i.e. via MR ligands) remains relatively under-exploited.
Aside from atropine, a muscarinic antagonist that is clinically utilized to acutely treat marked symptomatic bradycardia,93 the majority of muscarinic agonists or antagonists characterized to date have been mainly developed for a variety of non-cardiac conditions such as glaucoma, asthma, smooth-muscle disorders, chronic obstructive pulmonary disease (COPD), peptic ulcer, Sjorgren’s syndrome, cancer, and various neurological disorders.68 Due to the lack of subtype-selective MR ligands, and the ubiquitous expression of MR subtypes across different tissues, the clinical utility of the current therapies is limited due to the presence of non-selective side effects.68 For instance, the use of tiotropium, one of the six licensed MR antagonists used as a bronchodilator agent for the treatment of COPD,94 has the potential for cardiovascular risk. As evidence for this notion, recent studies have revealed that perfusion of tiotropium in the rat heart leads to cardiomyocyte cell damage via pathological calcium signalling.95
A major barrier in the development of subtype-selective MR drugs is the high degree of conservation of the amino acids forming the orthosteric binding pocket across the different subtypes.64 In particular, the five subtypes share 64–82% sequence identity overall and 82–92% identity within the transmembrane region which harbours the orthosteric binding pocket.64,77 Importantly, the crystal structure of the M3R revealed the presence of a relatively large cavity separate from the orthosteric binding pocket.96 Although the structural feature is generally conserved among all subtypes, the amino acids surrounding this region are more diverse, which potentially makes it a target for subtype-selective allosteric modulators.97 In line with this, allosteric modulators for the M1R, M4R, and M5R receptors have been developed that are predicted to bind to this region.98 To date, many subtype-specific allosteric modulators have been identified for all five MRs, however, they have predominantly been pursued for the treatment of CNS disorders.99 This warrants the further discovery and characterization of subtype-selective muscarinic allosteric modulators that might be clinically useful in the treatment of cardiovascular disease as well.
4. Angiotensin II receptors
Angiotensin II type 1 (AT1R) and Type 2 (AT2R) receptors, endogenously activated by the peptide hormone, Angiotensin II (Ang II), are additional members of the GPCR superfamily that are fundamental for the regulation of cardiovascular physiology (Figure 5). Although the role of AT1R is well established in the heart, the significance of AT2R remains highly controversial since altering the expression of AT2R does not affect cardiac function100,101 and findings regarding the role of AT2R in cardiac pathology appear contradictory. Therefore, we mainly focus on the AT1R while providing a summary of the most relevant aspects of the AT2R in cardiac pathophysiology.
Figure 5.
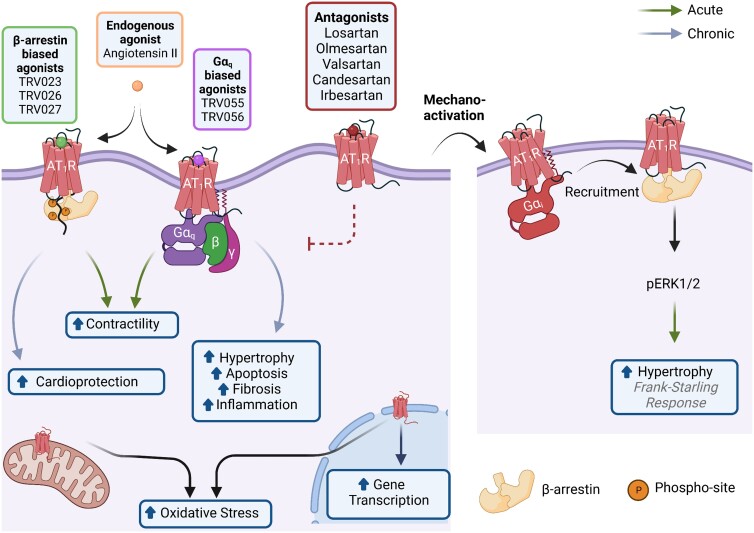
Physiological and pathological effects of cardiac AT1R signalling. The AT1R is a pleiotropic receptor that localizes to the cellular, mitochondrial, and nuclear membranes of cells. Ligands that preferentially stabilize the AT1R/β-arrestin complex (TRV023, TRV026, TRV027) increase the contractility of cardiomyocytes and have cardioprotective effects under long-term use. Likewise, ligands that preferentially stabilize the AT1R/Gαq complex (TRV055 and TRV056) increase the contractility of cardiomyocytes. However, chronic activation of this signalling pathway promotes cardiac hypertrophy, apoptosis, fibrosis, and inflammation. The endogenous ligand for the AT1R, Ang II, is unbiased towards both signalling pathways. AT1R antagonists such as Losartan, Olmesartan, and Valsartan, among others, stabilize the receptor in an inactive conformation thereby blocking the activation of any signalling cascade. Another activation mechanism of the AT1R is via membrane stretch. In turn, β-arrestin is recruited in a process mediated by Gαi and activates ERK1/2 phosphorylation regulating the Frank–Starling mechanism and inducing hypertrophy. Finally, the activation of the AT1R at mitochondrial or nuclear membranes induces oxidative stress and activates gene transcription. Figure generated with Biorender.com.
The translation of human AT1R comes from a single gene, AGTR1, which shares ∼95% homology to bovine and rat AT1Rs.102 Comparatively, two separate AT1R genes are found in mice and rats, Agtr1a and Agtr1b, that encode for the AT1AR and AT1BR, respectively. Both murine receptors share ∼95% homology and are functionally identical, yet are expressed in a tissue-specific manner.103 The AT1AR is the predominant isoform in rodent hearts and is therefore the main subtype addressed in this review when discussing AT1R in rodent models. Multiple reviews with a specific focus on the structure, trafficking, function, and/or pathophysiology of the AT1R are also available for complementary information.4,104–106
4.1. Tissue expression and localization
The AT1R is expressed in all cell types of the heart including the endothelial cells,107 vascular smooth-muscle cells,108 fibroblasts,109 myocardial cells,110 and immune cells.111 The AT1R represents 59% of the total AT receptors in human ventricles, compared to its homologue subtype, the AT2R, which comprises the remaining 41%.112 Conversely, the human atrium shows an inverted relationship where the AT1R:AT2R ratio is 30:70.113 Acute heart injury such as myocardial infarction upregulates AT1R expression, whereas chronic cardiac damage such as dilated cardiomyopathy downregulates its expression.112 Thus, the temporal nature of the cardiac disease is a key regulator of AT1R levels. Beyond the cellular membrane, AT1R further localizes to nuclear and mitochondrial membranes in the heart where it activates gene transcription and induces oxidative stress, respectively.114,115 This suggests that the localization within the cell is also an important factor for the signal transduction and function of AT1R.
4.2. Cellular signalling mechanisms
The ubiquitous expression of AT1Rs along with the endocrine, paracrine, autocrine, and/or intracrine effects of its endogenous ligand, Ang II, are main contributors to the multiple cellular effects (i.e. growth, contractility, inflammation, fibrosis, and apoptosis, among others) regulating the cardiovascular system.104 Independent from Ang II, AT1Rs can also be activated by the mechanical forces exerted within the cardiovascular system.116,117 Moreover, the AT1R can couple to multiple intracellular transducers including several G protein subtypes (i.e. Gαq/11, Gαs, Gαi/o, and Gα12/13) and β arrestins to initiate different cellular responses.118,119 The best-characterized signalling pathways for the AT1R in physiological conditions are the canonical Gαq pathway and the β arrestin pathway.
Like other GPCRs, the dynamic structure of the AT1R permits multiple active conformations that preferentially interact with distinct transducers and can be further stabilized by biased ligands.120,121 Importantly, the development of biased ligands for the AT1R has allowed for a more in-depth exploration of the signalling pathways downstream of the AT1R and their biological consequences. This is clearly illustrated in a recent proteomics study utilizing proximity ligation assay to identify more than a thousand functional and structural proteins proximal to the AT1R after activation with unbiased or biased ligands for the Gαq or the β-arrestin pathway.122 Understanding the effects of these ligands in the heart will enable more precise modulation of AT1R activity and will allow for the development of novel strategies to preferentially target beneficial signalling cascades in disease states.
4.3. Physiological functions and contributions to disease
Upon activation, Ang II binds to the AT1R and increases cardiomyocyte contractility by coupling to the canonical Gαq pathway as well as β-arrestin.123 While the latter observation was first shown in vitro using [Sar1, Ile4, Ile8]-Angiotensin II (SII), the first β-arrestin-biased ligand available for the AT1R,123 this concept was later demonstrated in vivo using the β-arrestin-biased AT1R ligands TRV023 and TRV027.124,125 While the regulation of calcium is central to the contractile response induced by both Ang II and β-arrestin-biased ligands (TRV023 and TRV 027), the mechanisms by which they regulate intracellular calcium are distinct. Specifically, stimulation with Ang II leads to increased intracellular calcium concentration via enhanced calcium release through the AT1R-Gαq-IP3/PKC signalling axis.126 In contrast, β-arrestin-biased activation of the AT1R increases myofilament calcium sensitivity without altering the global intracellular calcium transient, in part through modifying the phosphorylation status of myofilament proteins.127–129
In contrast to acute activation, chronic AT1R stimulation by direct stimulation with Ang II,130,131 overexpression of AT1R, or expression of constitutively active mutants of the AT1R132,133 induces cardiac hypertrophy. Similarly, increasing the activity of the local renin angiotensin system (RAS) of the heart by angiotensinogen overexpression, the main precursor of Ang II, also induces cardiac hypertrophy.134 The hypertrophic response induced by chronic AT1R stimulation is largely mediated by Gαq, since in vivo overexpression of Gαq or in vitro transfection of a constitutively activated Gαq mutant induces hypertrophy and apoptosis in cardiomyocytes.135 Moreover, overexpression of an inhibitor of Gαq blocks the development of cardiac hypertrophy induced by pressure overload.136 The alternative β-arrestin pathway might also contribute to this effect since cardiac-specific overexpression of the AT1R mutant lacking Gαq/Gαi coupling also induces hypertrophy.131,137 Nevertheless, numerous studies have demonstrated that β-arrestin-biased activation of AT1R is cardioprotective via promoting cardiomyocyte cell survival.116,125,138
Although the hypertrophic response mediated by chronic AT1R stimulation is well established, it has not been consistently observed. Recent studies demonstrate that mice overexpressing AT1R or a constitutively active AT1R mutant do not undergo hypertrophy despite developing fibrosis and/or ventricular dysfunction.139,140 Moreover, AT1R knockout mice develop cardiac hypertrophy following pressure overload or myocardial infarction, suggesting that the AT1R may not be critical for the development of hypertrophy in heart failure.141,142 Accordingly, chronic stimulation with Ang II in mice lacking AT1R in the kidney, yet expressing physiological levels of AT1R in the heart, does not develop cardiac hypertrophy.143 In line with this, selective expression of the AT1R in the kidney or in resistance vessels mimics the cardiac hypertrophy induced by chronic Ang II treatment observed in wild-type mice.143,144 Taken together, the development of cardiac hypertrophy following the manipulation of AT1R could be secondary to the increased peripheral resistance that induces pressure overload in the heart.
Other paracrine, stretch, and transactivation mechanisms of the AT1R are also contributors to cardiomyocyte growth. Indeed, Ang II-stimulated cardiac fibroblasts secrete exosomes and multiple cytokines such as transforming growth factor (TGF-1β) and interleukin-6 that stimulate cardiomyocytes to increase local Ang II production, AT1R expression, and induce hypertrophy.145–148 In addition, AT1R can be activated by mechanical stretch independent from Ang II, leading to hypertrophic growth.117 This mechano-activation mechanism appears to be dependent on Gαi and β-arrestin pathways.116,149,150 Mechanical stretch also increases the expression of the AT1R and other components of the RAS, while Ang II decreases AT1R expression in the heart.151 Therefore, mechano-activation of the AT1R behaves as a positive feedback loop for the cardiac RAS. Notably, this mechanism underlies the Frank–Starling response which describes the length-dependent activation of contractility wherein increased cardiac filling, and thus increased sarcomere length, enhances the force of contraction.127 Finally, AT1R stimulation transactivates EGFR through multiple intracellular mechanisms including β-arrestin recruitment and direct association/dimerization between both receptors ultimately inducing hypertrophy.152 Aside from cardiomyocyte growth, AT1R activation also leads to apoptosis, hyperplasia, fibrosis, and oxidative stress, which further contribute to disease.130,132,133,139
4.4. Pharmacological perspectives
Eight AT1R blockers (ARBs), which stabilize the receptor in an inactive state, have been approved by the FDA and are clinically utilized for the treatment of multiple cardiovascular diseases including heart failure.153,154 The AT1R β-arrestin-biased ligand TRV027 was also recently proposed as pharmacotherapy for acute heart failure. However, TRV027 failed to improve the clinical status of patients with this condition in clinical trials.155 This unexpected outcome might be related to the short duration of the treatment (1 month). Accordingly, Ryba and colleagues showed that a 3-month treatment with the β-arrestin-biased ligand TRV067 improves the cardiac function of mice with dilated cardiomyopathy.156 Alternatively, the developmental stage might also modify the efficacy of the treatment, since activation of the AT1R-β-arrestin2 pathway in neonatal or immature cardiomyocytes induces sustained cardiac contractility157 whereas this effect is short lasting in adult hearts of mice or rats.124,158 Thus, refocusing these novel compounds to treat heart failure for a longer period or in the paediatric population might prove therapeutic.
4.5. Angiotensin II Type 2 receptor
The AT2R subtype, only ∼34% identical to the AT1R,159 exhibits unique transducer coupling properties and complex pathophysiological effects. The first crystal structure available of the AT2R showed a non-canonical position of helix 8 that blocked the binding sites of G proteins and β-arrestins.160 This finding is consistent with multiple studies that reported a lack of signalling through G proteins or internalization.161 The recently crystalized AT2R bound to Ang II shows a rather canonical outward position of helix 8, raising the possibility of conformational selection by different ligands to induce multiple responses.162 Besides Ang II, new endogenous ligands derived from Ang II, such as Ang 1–7 and Ang 1–9, have been described as agonists for the AT2R that may be beneficial in the heart.163,164
In the human heart, AT2R has been detected in cardiomyocytes,165 fibroblasts,112 and coronary arteries.166 During heart failure or dilated cardiomyopathy, AT2R levels are downregulated or upregulated, respectively, suggesting that the regulation of the AT2R depends on the aetiology of the disease, or perhaps reflects alterations in the relative proportions of different cell types during the pathological process of adverse cardiac remodelling.112,165 Moreover, studies evaluating the contribution of AT2R to cardiac disease show conflicting results. Some claim that AT2R activation is associated with decreased hypertrophy and fibrosis.167–169 Conversely, others report that AT2R overexpression results in constitutive hypertrophy in neonatal rat cardiomyocytes, fibrosis, and heart failure,170,171 whereas deletion of the AT2R decreases hypertrophy following chronic Ang II administration or myocardial infarction.172,173 These discrepancies might be explained by the expression levels of the AT2R during the cardiac insult.174 Thus, pharmacological activation of AT2R during disease states when there are high expression levels of AT2R might prove therapeutic. Indeed, multiple recent studies using AT2R agonists in models of heart disease have shown cardioprotective effects in rodents.164,175,176 In summary, the AT2R is a potential therapeutic target in cardiac disease that needs further examination to better understand its pathophysiological significance.
5. Conclusion
GPCRs serve as excellent therapeutic targets for the identification of novel heart failure treatments given their diverse and essential roles in cardiac health and disease. The β-adrenergic, muscarinic, and angiotensin receptor families exemplify only 3 of the >200 types of GPCRs in the heart that fundamentally regulate cardiac function and demonstrate great pharmacological potential. Importantly, recent advancements in understanding the mechanisms of biased signalling coupled with the identification of novel allosteric modulators have enabled the discovery of more selective ligands possessing enhanced cardioprotective effects with reduced unwanted side effects. Nonetheless, the heterogeneous distribution of GPCRs across multiple cell types in the heart,4,177 combined with their overlapping signalling networks, and localization to cellular compartments aside from the cell surface,177–180 remain essential elements to consider in evaluating the physiological effects of these novel drugs. Further investigation of the biased signalling pathways, physiological functions, disease mechanisms, and pharmacological features of cardiac GPCRs will therefore prove impactful in designing novel therapeutics.
Contributor Information
Alyssa Grogan, Department of Medicine, Duke University Medical Center, DUMC 3104, 226 CARL Building, Durham, NC 27710, USA.
Emilio Y Lucero, Department of Medicine, Duke University Medical Center, DUMC 3104, 226 CARL Building, Durham, NC 27710, USA.
Haoran Jiang, Department of Medicine, Duke University Medical Center, DUMC 3104, 226 CARL Building, Durham, NC 27710, USA.
Howard A Rockman, Department of Medicine, Duke University Medical Center, DUMC 3104, 226 CARL Building, Durham, NC 27710, USA; Cell Biology, Duke University Medical Center, DUMC 3104, 226 CARL Building, 12 Durham, NC 27710, USA.
Funding
This work was supported by the National Institutes of Health Grant HL056687 to H.A.R.
References
- 1. Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 2002;3:639–650. [DOI] [PubMed] [Google Scholar]
- 2. Sriram K, Insel PA. G protein-coupled receptors as targets for approved drugs: how many targets and how many drugs? Mol Pharmacol 2018;93:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schioth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 2006;88:263–273. [DOI] [PubMed] [Google Scholar]
- 4. Wang J, Gareri C, Rockman HA. G-Protein-Coupled receptors in heart disease. Circ Res 2018;123:716–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang H, Galtes D, Wang J, Rockman HA. G protein-coupled receptor signaling: transducers and effectors. Am J Physiol Cell Physiol 2022;323:C731–C748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Chen J, Fontes SK, Bautista EN, Cheng Z. Physiological and pathological roles of protein kinase a in the heart. Cardiovasc Res 2022;118:386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang P, Mende U. Regulators of G-protein signaling in the heart and their potential as therapeutic targets. Circ Res 2011;109:320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem 1998;67:653–692. [DOI] [PubMed] [Google Scholar]
- 9. Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 2002;298:834–836. [DOI] [PubMed] [Google Scholar]
- 10. Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. Targeting of diacylglycerol degradation to M1 muscarinic receptors by beta-arrestins. Science 2007;315:663–666. [DOI] [PubMed] [Google Scholar]
- 11. Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci U S A 1999;96:3712–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jean-Charles PY, Kaur S, Shenoy SK. G protein-coupled receptor signaling through beta-arrestin-dependent mechanisms. J Cardiovasc Pharmacol 2017;70:142–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith JS, Lefkowitz RJ, Rajagopal S. Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov 2018;17:243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov 2002;1:198–210. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol Sci 2007;28:366–373. [DOI] [PubMed] [Google Scholar]
- 16. Slosky LM, Caron MG, Barak LS. Biased allosteric modulators: new frontiers in GPCR drug discovery. Trends Pharmacol Sci 2021;42:283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol 1948;153:586–600. [DOI] [PubMed] [Google Scholar]
- 18. Lefkowitz RJ. Editorial: selectivity in beta-adrenergic responses: clinical implications. Circulation 1974;49:783–786. [DOI] [PubMed] [Google Scholar]
- 19. Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res 1986;59:297–309. [DOI] [PubMed] [Google Scholar]
- 20. Michel LYM, Farah C, Balligand JL. The Beta3 adrenergic receptor in healthy and pathological cardiovascular tissues. Cells 2020;9:2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bathe-Peters M, Gmach P, Boltz HH, Einsiedel J, Gotthardt M, Hubner H, Gmeiner P, Lohse MJ, Annibale P. Visualization of beta-adrenergic receptor dynamics and differential localization in cardiomyocytes. Proc Natl Acad Sci U S A 2021;118:e2101119118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schobesberger S, Wright PT, Poulet C, Sanchez Alonso Mardones JL, Mansfield C, Friebe A, Harding SE, Balligand JL, Nikolaev VO, Gorelik J. beta3-Adrenoceptor redistribution impairs NO/cGMP/PDE2 signalling in failing cardiomyocytes. Elife 2020;9:e52221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiao RP. Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Sci STKE 2001;2001:re15. [DOI] [PubMed] [Google Scholar]
- 24. Cannavo A, Koch WJ. Targeting beta3-adrenergic receptors in the heart: selective agonism and beta-blockade. J Cardiovasc Pharmacol 2017;69:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mangmool S, Shukla AK, Rockman HA. Beta-Arrestin-dependent activation of ca(2+)/calmodulin kinase II after beta(1)-adrenergic receptor stimulation. J Cell Biol 2010;189:573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-src tyrosine kinase in G protein-coupled receptor- and gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem 1996;271:19443–19450. [DOI] [PubMed] [Google Scholar]
- 27. Hordijk PL, Verlaan I, van Corven EJ, Moolenaar WH. Protein tyrosine phosphorylation induced by lysophosphatidic acid in rat-1 fibroblasts. Evidence that phosphorylation of map kinase is mediated by the gi-p21ras pathway. J Biol Chem 1994;269:645–651. [PubMed] [Google Scholar]
- 28. Kohout TA, Takaoka H, McDonald PH, Perry SJ, Mao L, Lefkowitz RJ, Rockman HA. Augmentation of cardiac contractility mediated by the human beta(3)-adrenergic receptor overexpressed in the hearts of transgenic mice. Circulation 2001;104:2485–2491. [DOI] [PubMed] [Google Scholar]
- 29. Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-src protein kinase complexes. Science 1999;283:655–661. [DOI] [PubMed] [Google Scholar]
- 30. Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest 2007;117:2445–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984;311:819–823. [DOI] [PubMed] [Google Scholar]
- 32. Bristow MR, Hershberger RE, Port JD, Minobe W, Rasmussen R. Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol Pharmacol 1989;35:295–303. [PubMed] [Google Scholar]
- 33. Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 1993;87:454–463. [DOI] [PubMed] [Google Scholar]
- 34. Scheuer J. Catecholamines in cardiac hypertrophy. Am J Cardiol 1999;83:70H–74H. [DOI] [PubMed] [Google Scholar]
- 35. Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A 1999;96:7059–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, Roden R, Asano K, Blaxall BC, Wu SC, Communal C, Singh K, Colucci W, Bristow MR, Port DJ. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol 2000;32:817–830. [DOI] [PubMed] [Google Scholar]
- 37. Du XJ, Autelitano DJ, Dilley RJ, Wang B, Dart AM, Woodcock EA. Beta(2)-adrenergic receptor overexpression exacerbates development of heart failure after aortic stenosis. Circulation 2000;101:71–77. [DOI] [PubMed] [Google Scholar]
- 38. Nguyen MN, Kiriazis H, Ruggiero D, Gao XM, Su Y, Jian A, Han LP, McMullen JR, Du XJ. Spontaneous ventricular tachyarrhythmias in beta2-adrenoceptor transgenic mice in relation to cardiac interstitial fibrosis. Am J Physiol Heart Circ Physiol 2015;309:H946–H957. [DOI] [PubMed] [Google Scholar]
- 39. Cross HR, Steenbergen C, Lefkowitz RJ, Koch WJ, Murphy E. Overexpression of the cardiac beta(2)-adrenergic receptor and expression of a beta-adrenergic receptor kinase-1 (betaARK1) inhibitor both increase myocardial contractility but have differential effects on susceptibility to ischemic injury. Circ Res 1999;85:1077–1084. [DOI] [PubMed] [Google Scholar]
- 40. Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3'-kinase. Circ Res 2000;87:1172–1179. [DOI] [PubMed] [Google Scholar]
- 41. Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A 2001;98:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP Jr, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci U S A 1996;93:7375–7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoo B, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. Beta1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol 2009;297:H1377–H1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2-adrenergic receptors. J Biol Chem 1999;274:16701–16708. [DOI] [PubMed] [Google Scholar]
- 45. Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem 1999;274:16694–16700. [DOI] [PubMed] [Google Scholar]
- 46. Tavernier G, Toumaniantz G, Erfanian M, Heymann MF, Laurent K, Langin D, Gauthier C. Beta3-Adrenergic stimulation produces a decrease of cardiac contractility ex vivo in mice overexpressing the human beta3-adrenergic receptor. Cardiovasc Res 2003;59:288–296. [DOI] [PubMed] [Google Scholar]
- 47. Gauthier C, Leblais V, Kobzik L, Trochu JN, Khandoudi N, Bril A, Balligand JL, Le Marec H. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 1998;102:1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varghese P, Harrison RW, Lofthouse RA, Georgakopoulos D, Berkowitz DE, Hare JM. Beta(3)-adrenoceptor deficiency blocks nitric oxide-dependent inhibition of myocardial contractility. J Clin Invest 2000;106:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Belge C, Hammond J, Dubois-Deruy E, Manoury B, Hamelet J, Beauloye C, Markl A, Pouleur AC, Bertrand L, Esfahani H, Jnaoui K, Gotz KR, Nikolaev VO, Vanderper A, Herijgers P, Lobysheva I, Iaccarino G, Hilfiker-Kleiner D, Tavernier G, Langin D, Dessy C, Balligand JL. Enhanced expression of beta3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation 2014;129:451–462. [DOI] [PubMed] [Google Scholar]
- 50. Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, Ellis CL, Miller KL, Vandegaer K, Bedja D, Gabrielson KL, Paolocci N, Kass DA, Barouch LA. Cardioprotective effect of beta-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol 2012;59:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Niu X, Zhao L, Li X, Xue Y, Wang B, Lv Z, Chen J, Sun D, Zheng Q. Beta3-Adrenoreceptor stimulation protects against myocardial infarction injury via eNOS and nNOS activation. PLoS One 2014;9:e98713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Masutani S, Cheng HJ, Morimoto A, Hasegawa H, Han QH, Little WC, Cheng CP. Beta3-Adrenergic receptor antagonist improves exercise performance in pacing-induced heart failure. Am J Physiol Heart Circ Physiol 2013;305:H923–H930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kotecha D, Flather MD, Altman DG, Holmes J, Rosano G, Wikstrand J, Packer M, Coats AJS, Manzano L, Bohm M, van Veldhuisen DJ, Andersson B, Wedel H, von Lueder TG, Rigby AS, Hjalmarson A, Kjekshus J, Cleland JGF; Beta-Blockers in Heart Failure Collaborative Group . Heart rate and rhythm and the benefit of Beta-blockers in patients with heart failure. J Am Coll Cardiol 2017;69:2885–2896. [DOI] [PubMed] [Google Scholar]
- 54. Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci U S A 2007;104:16657–16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci U S A 2008;105:14555–14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim IM, Wang Y, Park KM, Tang Y, Teoh JP, Vinson J, Traynham CJ, Pironti G, Mao L, Su H, Johnson JA, Koch WJ, Rockman HA. Beta-arrestin1-biased beta1-adrenergic receptor signaling regulates microRNA processing. Circ Res 2014;114:833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang J, Pani B, Gokhan I, Xiong X, Kahsai AW, Jiang H, Ahn S, Lefkowitz RJ, Rockman HA. Beta-Arrestin-Biased allosteric modulator potentiates carvedilol-stimulated beta adrenergic receptor cardioprotection. Mol Pharmacol 2021;100:568–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A; Carvedilol Or Metoprolol European Trial Investigators . Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol European trial (COMET): randomised controlled trial. Lancet 2003;362:7–13. [DOI] [PubMed] [Google Scholar]
- 59. Bhatt AS, DeVore AD, DeWald TA, Swedberg K, Mentz RJ. Achieving a maximally tolerated beta-blocker dose in heart failure patients: is there room for improvement? J Am Coll Cardiol 2017;69:2542–2550. [DOI] [PubMed] [Google Scholar]
- 60. Wang J, Hanada K, Staus DP, Makara MA, Dahal GR, Chen Q, Ahles A, Engelhardt S, Rockman HA. Galphai is required for carvedilol-induced beta1 adrenergic receptor beta-arrestin biased signaling. Nat Commun 2017;8:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wisler JW, Rockman HA, Lefkowitz RJ. Biased G protein-coupled receptor signaling: changing the paradigm of drug discovery. Circulation 2018;137:2315–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahn S, Pani B, Kahsai AW, Olsen EK, Husemoen G, Vestergaard M, Jin L, Zhao S, Wingler LM, Rambarat PK, Simhal RK, Xu TT, Sun LD, Shim PJ, Staus DP, Huang LY, Franch T, Chen X, Lefkowitz RJ. Small-Molecule positive allosteric modulators of the beta2-adrenoceptor isolated from DNA-encoded libraries. Mol Pharmacol 2018;94:850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pani B, Ahn S, Rambarat PK, Vege S, Kahsai AW, Liu A, Valan BN, Staus DP, Costa T, Lefkowitz RJ. Unique positive cooperativity between the beta-arrestin-biased beta-blocker carvedilol and a small molecule positive allosteric modulator of the beta2-adrenergic receptor. Mol Pharmacol 2021;100:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peralta EG, Ashkenazi A, Winslow JW, Smith DH, Ramachandran J, Capon DJ. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J 1987;6:3923–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bonner TI, Young AC, Brann MR, Buckley NJ. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron 1988;1:403–410. [DOI] [PubMed] [Google Scholar]
- 66. Saternos HC, Almarghalani DA, Gibson HM, Meqdad MA, Antypas RB, Lingireddy A, AbouAlaiwi WA. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics 2018;50:1–9. [DOI] [PubMed] [Google Scholar]
- 67. Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol 2004;44:423–450. [DOI] [PubMed] [Google Scholar]
- 68. Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 2007;6:721–733. [DOI] [PubMed] [Google Scholar]
- 69. Deighton NM, Motomura S, Borquez D, Zerkowski HR, Doetsch N, Brodde OE. Muscarinic cholinoceptors in the human heart: demonstration, subclassification, and distribution. Naunyn Schmiedebergs Arch Pharmacol 1990;341:14–21. [DOI] [PubMed] [Google Scholar]
- 70. Wang H, Han H, Zhang L, Shi H, Schram G, Nattel S, Wang Z. Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol Pharmacol 2001;59:1029–1036. [DOI] [PubMed] [Google Scholar]
- 71. Shi H, Wang H, Wang Z. Identification and characterization of multiple subtypes of muscarinic acetylcholine receptors and their physiological functions in canine hearts. Mol Pharmacol 1999;55:497–507. [PubMed] [Google Scholar]
- 72. Le Guludec D, Cohen-Solal A, Delforge J, Delahaye N, Syrota A, Merlet P. Increased myocardial muscarinic receptor density in idiopathic dilated cardiomyopathy: an in vivo PET study. Circulation 1997;96:3416–3422. [DOI] [PubMed] [Google Scholar]
- 73. Giessler C, Dhein S, Ponicke K, Brodde OE. Muscarinic receptors in the failing human heart. Eur J Pharmacol 1999;375:197–202. [DOI] [PubMed] [Google Scholar]
- 74. Heijman J, Kirchner D, Kunze F, Chretien EM, Michel-Reher MB, Voigt N, Knaut M, Michel MC, Ravens U, Dobrev D. Muscarinic type-1 receptors contribute to IK, ACh in human atrial cardiomyocytes and are upregulated in patients with chronic atrial fibrillation. Int J Cardiol 2018;255:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou Q, Yang D, Wu M, Guo Y, Guo W, Zhong L, Cai X, Dai A, Jang W, Shakhnovich EI, Liu ZJ, Stevens RC, Lambert NA, Babu MM, Wang MW, Zhao S. Common activation mechanism of class A GPCRs. Elife 2019;8:e50279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Randakova A, Nelic D, Dolezal V, El-Fakahany EE, Boulos J, Jakubik J. Agonist-Specific conformations of the M2 muscarinic acetylcholine receptor assessed by molecular dynamics. J Chem Inf Model 2020;60:2325–2338. [DOI] [PubMed] [Google Scholar]
- 77. Maeda S, Qu Q, Robertson MJ, Skiniotis G, Kobilka BK. Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 2019;364:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zaza A, Robinson RB, DiFrancesco D. Basal responses of the L-type Ca2+ and hyperpolarization-activated currents to autonomic agonists in the rabbit sino-atrial node. J Physiol 1996;491:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Touhara KK, MacKinnon R. Molecular basis of signaling specificity between GIRK channels and GPCRs. Elife 2018;7:e42908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee SW, Anderson A, Guzman PA, Nakano A, Tolkacheva EG, Wickman K. Atrial GIRK channels mediate the effects of Vagus nerve stimulation on heart rate dynamics and arrhythmogenesis. Front Physiol 2018;9:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. van Koppen CJ, Kaiser B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacol Ther 2003;98:197–220. [DOI] [PubMed] [Google Scholar]
- 82. Stengel PW, Gomeza J, Wess J, Cohen ML. M(2) and M(4) receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther 2000;292:877–885. [PubMed] [Google Scholar]
- 83. Liu Y, Wang S, Wang C, Song H, Han H, Hang P, Jiang Y, Wei L, Huo R, Sun L, Gao X, Lu Y, Du Z. Upregulation of M(3) muscarinic receptor inhibits cardiac hypertrophy induced by angiotensin II. J Transl Med 2013;11:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu Y, Sun L, Pan Z, Bai Y, Wang N, Zhao J, Xu C, Li Z, Li B, Du Z, Lu Y, Gao X, Yang B. Overexpression of M(3) muscarinic receptor is a novel strategy for preventing sudden cardiac death in transgenic mice. Mol Med 2011;17:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu Y, Sun HL, Li DL, Wang LY, Gao Y, Wang YP, Du ZM, Lu YJ, Yang BF. Choline produces antiarrhythmic actions in animal models by cardiac M3 receptors: improvement of intracellular Ca2+handling as a common mechanism. Can J Physiol Pharmacol 2008;86:860–865. [DOI] [PubMed] [Google Scholar]
- 86. Zhao L, Chen T, Hang P, Li W, Guo J, Pan Y, Du J, Zheng Y, Du Z. Choline attenuates cardiac fibrosis by inhibiting p38MAPK signaling possibly by acting on M3 muscarinic acetylcholine receptor. Front Pharmacol 2019;10:1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen X, Bai Y, Sun H, Su Z, Guo J, Sun C, Du Z. Overexpression of M3 muscarinic receptor suppressed adverse electrical remodeling in hypertrophic myocardium via increasing repolarizing K+ currents. Cell Physiol Biochem 2017;43:915–925. [DOI] [PubMed] [Google Scholar]
- 88. Intachai K, Chattipakorn SC, Chattipakorn N, Shinlapawittayatorn K. Revisiting the cardioprotective effects of acetylcholine receptor activation against myocardial ischemia/reperfusion injury. Int J Mol Sci 2018;19:2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Prathumsap N, Ongnok B, Khuanjing T, Arinno A, Maneechote C, Apaijai N, Chunchai T, Arunsak B, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N. Acetylcholine receptor agonists provide cardioprotection in doxorubicin-induced cardiotoxicity via modulating muscarinic M2 and alpha7 nicotinic receptor expression. Transl Res 2022;243:33–51. [DOI] [PubMed] [Google Scholar]
- 90. Caru M, Corbin D, Perie D, Lemay V, Delfrate J, Drouin S, Bertout L, Krajinovic M, Laverdiere C, Andelfinger G, Sinnett D, Curnier D. Doxorubicin treatments induce significant changes on the cardiac autonomic nervous system in childhood acute lymphoblastic leukemia long-term survivors. Clin Res Cardiol 2019;108:1000–1008. [DOI] [PubMed] [Google Scholar]
- 91. Burczyk MS, Burkhalter MD, Tena TC, Grisanti LA, Kauk M, Matysik S, Donow C, Kustermann M, Rothe M, Cui Y, Raad F, Laue S, Moretti A, Zimmermann WH, Wess J, Kuhl M, Hoffmann C, Tilley DG, Philipp M. Muscarinic receptors promote pacemaker fate at the expense of secondary conduction system tissue in zebrafish. JCI Insight 2019;4:e121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ishise H, Asanoi H, Ishizaka S, Joho S, Kameyama T, Umeno K, Inoue H. Time course of sympathovagal imbalance and left ventricular dysfunction in conscious dogs with heart failure. J Appl Physiol (1985) 1998;84:1234–1241. [DOI] [PubMed] [Google Scholar]
- 93. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Circulation 2019;140:e382–e482. [DOI] [PubMed] [Google Scholar]
- 94. Matera MG, Cazzola M. Muscarinic receptor antagonists. Handb Exp Pharmacol 2017;237:41–62. [DOI] [PubMed] [Google Scholar]
- 95. Cassambai S, Mee CJ, Renshaw D, Hussain A. Tiotropium bromide, a long acting muscarinic receptor antagonist triggers intracellular calcium signalling in the heart. Toxicol Appl Pharmacol 2019;384:114778. [DOI] [PubMed] [Google Scholar]
- 96. Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO, Shaw DE, Weis WI, Wess J, Kobilka BK. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 2012;482:552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kruse AC, Li J, Hu J, Kobilka BK, Wess J. Novel insights into M3 muscarinic acetylcholine receptor physiology and structure. J Mol Neurosci 2014;53:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Digby GJ, Shirey JK, Conn PJ. Allosteric activators of muscarinic receptors as novel approaches for treatment of CNS disorders. Mol Biosyst 2010;6:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bock A, Schrage R, Mohr K. Allosteric modulators targeting CNS muscarinic receptors. Neuropharmacology 2018;136:427–437. [DOI] [PubMed] [Google Scholar]
- 100. Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature 1995;377:744–747. [DOI] [PubMed] [Google Scholar]
- 101. Sugino H, Ozono R, Kurisu S, Matsuura H, Ishida M, Oshima T, Kambe M, Teranishi Y, Masaki H, Matsubara H. Apoptosis is not increased in myocardium overexpressing type 2 angiotensin II receptor in transgenic mice. Hypertension 2001;37:1394–1398. [DOI] [PubMed] [Google Scholar]
- 102. Curnow KM, Pascoe L, White PC. Genetic analysis of the human type-1 angiotensin II receptor. Mol Endocrinol 1992;6:1113–1118. [DOI] [PubMed] [Google Scholar]
- 103. de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 2000;52:415–472. [PubMed] [Google Scholar]
- 104. Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 2018;98:1627–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG. International union of basic and clinical pharmacology. XCIX. Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli [corrected]. Pharmacol Rev 2015;67:754–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhang H, Luginina A, Mishin A, Baidya M, Shukla AK, Cherezov V. Structural insights into ligand recognition and activation of angiotensin receptors. Trends Pharmacol Sci 2021;42:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jacques D, Abdel Malak NA, Sader S, Perreault C. Angiotensin II and its receptors in human endocardial endothelial cells: role in modulating intracellular calcium. Can J Physiol Pharmacol 2003;81:259–266. [DOI] [PubMed] [Google Scholar]
- 108. Bkaily G, Sleiman S, Stephan J, Asselin C, Choufani S, Kamal M, Jacques D, Gobeil F Jr, D'Orleans-Juste P. Angiotensin II AT1 receptor internalization, translocation and de novo synthesis modulate cytosolic and nuclear calcium in human vascular smooth muscle cells. Can J Physiol Pharmacol 2003;81:274–287. [DOI] [PubMed] [Google Scholar]
- 109. Kawano H, Do YS, Kawano Y, Starnes V, Barr M, Law RE, Hsueh WA. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation 2000;101:1130–1137. [DOI] [PubMed] [Google Scholar]
- 110. Wharton J, Morgan K, Rutherford RA, Catravas JD, Chester A, Whitehead BF, De Leval MR, Yacoub MH, Polak JM. Differential distribution of angiotensin AT2 receptors in the Normal and failing human heart. J Pharmacol Exp Ther 1998;284:323–336. [PubMed] [Google Scholar]
- 111. Nataraj C, Oliverio MI, Mannon RB, Mannon PJ, Audoly LP, Amuchastegui CS, Ruiz P, Smithies O, Coffman TM. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest 1999;104:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tsutsumi Y, Matsubara H, Ohkubo N, Mori Y, Nozawa Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Moriguchi Y, Shibasaki Y, Kamihata H, Inada M, Iwasaka T. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ Res 1998;83:1035–1046. [DOI] [PubMed] [Google Scholar]
- 113. Regitz-Zagrosek V, Friedel N, Heymann A, Bauer P, Neuss M, Rolfs A, Steffen C, Hildebrandt A, Hetzer R, Fleck E. Regulation, chamber localization, and subtype distribution of angiotensin II receptors in human hearts. Circulation 1995;91:1461–1471. [DOI] [PubMed] [Google Scholar]
- 114. Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A 2011;108:14849–14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem 2010;285:22338–22349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. Beta-arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal 2010;3:ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 2004;6:499–506. [DOI] [PubMed] [Google Scholar]
- 118. Sauliere A, Bellot M, Paris H, Denis C, Finana F, Hansen JT, Altie MF, Seguelas MH, Pathak A, Hansen JL, Senard JM, Gales C. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat Chem Biol 2012;8:622–630. [DOI] [PubMed] [Google Scholar]
- 119. Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci U S A 2003;100:10782–10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wingler LM, Elgeti M, Hilger D, Latorraca NR, Lerch MT, Staus DP, Dror RO, Kobilka BK, Hubbell WL, Lefkowitz RJ. Angiotensin analogs with divergent bias stabilize distinct receptor conformations. Cell 2019;176:468–478 e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wingler LM, Skiba MA, McMahon C, Staus DP, Kleinhenz ALW, Suomivuori CM, Latorraca NR, Dror RO, Lefkowitz RJ, Kruse AC. Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR. Science 2020;367:888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pfeiffer CT, Wang J, Paulo JA, Jiang X, Gygi SP, Rockman HA. Mapping angiotensin II type 1 receptor-biased signaling using proximity labeling and proteomics identifies diverse actions of biased agonists. J Proteome Res 2021;20:3256–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci U S A 2006;103:16284–16289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. Selectively engaging beta-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther 2010;335:572–579. [DOI] [PubMed] [Google Scholar]
- 125. Kim KS, Abraham D, Williams B, Violin JD, Mao L, Rockman HA. Beta-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol 2012;303:H1001–H1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Freer RJ, Pappano AJ, Peach MJ, Bing KT, McLean MJ, Vogel S, Sperelakis N. Mechanism for the postive inotropic effect of angiotensin II on isolated cardiac muscle. Circ Res 1976;39:178–183. [DOI] [PubMed] [Google Scholar]
- 127. Abraham DM, Davis RT III, Warren CM, Mao L, Wolska BM, Solaro RJ, Rockman HA. Beta-Arrestin mediates the frank-starling mechanism of cardiac contractility. Proc Natl Acad Sci U S A 2016;113:14426–14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Monasky MM, Taglieri DM, Henze M, Warren CM, Utter MS, Soergel DG, Violin JD, Solaro RJ. The beta-arrestin-biased ligand TRV120023 inhibits angiotensin II-induced cardiac hypertrophy while preserving enhanced myofilament response to calcium. Am J Physiol Heart Circ Physiol 2013;305:H856–H866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tarigopula M, Davis RT III, Mungai PT, Ryba DM, Wieczorek DF, Cowan CL, Violin JD, Wolska BM, Solaro RJ. Cardiac myosin light chain phosphorylation and inotropic effects of a biased ligand, TRV120023, in a dilated cardiomyopathy model. Cardiovasc Res 2015;107:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sadoshima J, Izumo S. Molecular characterization of angiotensin II–induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res 1993;73:413–423. [DOI] [PubMed] [Google Scholar]
- 131. Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, Irie K, Holle E, Yu X, Kupershmidt S, Roden DM, Wagner T, Yatani A, Vatner DE, Vatner SF, Sadoshima J. Cardiac-specific overexpression of AT1 receptor mutant lacking G alpha q/G alpha i coupling causes hypertrophy and bradycardia in transgenic mice. J Clin Invest 2005;115:3045–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ainscough JF, Drinkhill MJ, Sedo A, Turner NA, Brooke DA, Balmforth AJ, Ball SG. Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovasc Res 2009;81:592–600. [DOI] [PubMed] [Google Scholar]
- 133. Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci U S A 2000;97:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mazzolai L, Nussberger J, Aubert JF, Brunner DB, Gabbiani G, Brunner HR, Pedrazzini T. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension 1998;31:1324–1330. [DOI] [PubMed] [Google Scholar]
- 135. Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW II. Enhanced galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci U S A 1998;95:10140–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science 1998;280:574–577. [DOI] [PubMed] [Google Scholar]
- 137. Rajagopal K, Lefkowitz RJ, Rockman HA. When 7 transmembrane receptors are not G protein-coupled receptors. J Clin Invest 2005;115:2971–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem 2004;279:24578–24584. [DOI] [PubMed] [Google Scholar]
- 139. Billet S, Bardin S, Verp S, Baudrie V, Michaud A, Conchon S, Muffat-Joly M, Escoubet B, Souil E, Hamard G, Bernstein KE, Gasc JM, Elghozi JL, Corvol P, Clauser E. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest 2007;117:1914–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rivard K, Grandy SA, Douillette A, Paradis P, Nemer M, Allen BG, Fiset C. Overexpression of type 1 angiotensin II receptors impairs excitation-contraction coupling in the mouse heart. Am J Physiol Heart Circ Physiol 2011;301:H2018–H2027. [DOI] [PubMed] [Google Scholar]
- 141. Bridgman P, Aronovitz MA, Kakkar R, Oliverio MI, Coffman TM, Rand WM, Konstam MA, Mendelsohn ME, Patten RD. Gender-specific patterns of left ventricular and myocyte remodeling following myocardial infarction in mice deficient in the angiotensin II type 1a receptor. Am J Physiol Heart Circ Physiol 2005;289:H586–H592. [DOI] [PubMed] [Google Scholar]
- 142. Harada K, Komuro I, Shiojima I, Hayashi D, Kudoh S, Mizuno T, Kijima K, Matsubara H, Sugaya T, Murakami K, Yazaki Y. Pressure overload induces cardiac hypertrophy in angiotensin II type 1A receptor knockout mice. Circulation 1998;97:1952–1959. [DOI] [PubMed] [Google Scholar]
- 143. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 2006;103:17985–17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sparks MA, Rianto F, Diaz E, Revoori R, Hoang T, Bouknight L, Stegbauer J, Vivekanandan-Giri A, Ruiz P, Pennathur S, Abraham DM, Gurley SB, Crowley SD, Coffman TM. Direct actions of AT1 (type 1 angiotensin) receptors in cardiomyocytes do not contribute to cardiac hypertrophy. Hypertension 2021;77:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol 1997;29:1947–1958. [DOI] [PubMed] [Google Scholar]
- 146. Fredj S, Bescond J, Louault C, Delwail A, Lecron JC, Potreau D. Role of interleukin-6 in cardiomyocyte/cardiac fibroblast interactions during myocyte hypertrophy and fibroblast proliferation. J Cell Physiol 2005;204:428–436. [DOI] [PubMed] [Google Scholar]
- 147. Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol 2015;89:268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest 2002;109:787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang J, Hanada K, Gareri C, Rockman HA. Mechanoactivation of the angiotensin II type 1 receptor induces beta-arrestin-biased signaling through galphai coupling. J Cell Biochem 2018;119:3586–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tang W, Strachan RT, Lefkowitz RJ, Rockman HA. Allosteric modulation of beta-arrestin-biased angiotensin II type 1 receptor signaling by membrane stretch. J Biol Chem 2014;289:28271–28283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Malhotra R, Sadoshima J, Brosius FC III, Izumo S. Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes in vitro. Circ Res 1999;85:137–146. [DOI] [PubMed] [Google Scholar]
- 152. Kagiyama S, Eguchi S, Frank GD, Inagami T, Zhang YC, Phillips MI. Angiotensin II-induced cardiac hypertrophy and hypertension are attenuated by epidermal growth factor receptor antisense. Circulation 2002;106:909–912. [DOI] [PubMed] [Google Scholar]
- 153. Brunner HR, Gavras H, Laragh JH. Angiotensin-II blockade in man by sar1-ala8-angiotensin II for understanding and treatment of high blood-pressure. Lancet 1973;2:1045–1048. [DOI] [PubMed] [Google Scholar]
- 154. Singh KD, Karnik SS. Angiotensin type 1 receptor blockers in heart failure. Curr Drug Targets 2020;21:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Pang PS, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA, Filippatos G, Levy PD, Metra M, Ponikowski P, Teerlink JR, Voors AA, Bharucha D, Goin K, Soergel DG, Felker GM. Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF). Eur Heart J 2017;38:2364–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ryba DM, Li J, Cowan CL, Russell B, Wolska BM, Solaro RJ. Long-Term biased beta-arrestin signaling improves cardiac structure and function in dilated cardiomyopathy. Circulation 2017;135:1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kashihara T, Kawagishi H, Nakada T, Numaga-Tomita T, Kadota S, Wolf EE, Du CK, Shiba Y, Morimoto S, Yamada M. Beta-Arrestin-Biased AT1 agonist TRV027 causes a neonatal-specific sustained positive inotropic effect without increasing heart rate. JACC Basic Transl Sci 2020;5:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kashihara T, Nakada T, Kojima K, Takeshita T, Yamada M. Angiotensin II activates CaV 1.2 ca(2+) channels through beta-arrestin2 and casein kinase 2 in mouse immature cardiomyocytes. J Physiol 2017;595:4207–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Nakajima M, Mukoyama M, Pratt RE, Horiuchi M, Dzau VJ. Cloning of cDNA and analysis of the gene for mouse angiotensin II type 2 receptor. Biochem Biophys Res Commun 1993;197:393–399. [DOI] [PubMed] [Google Scholar]
- 160. Zhang H, Han GW, Batyuk A, Ishchenko A, White KL, Patel N, Sadybekov A, Zamlynny B, Rudd MT, Hollenstein K, Tolstikova A, White TA, Hunter MS, Weierstall U, Liu W, Babaoglu K, Moore EL, Katz RD, Shipman JM, Garcia-Calvo M, Sharma S, Sheth P, Soisson SM, Stevens RC, Katritch V, Cherezov V. Structural basis for selectivity and diversity in angiotensin II receptors. Nature 2017;544:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Porrello ER, Delbridge LM, Thomas WG. The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front Biosci (Landmark Ed) 2009;14:958–972. [DOI] [PubMed] [Google Scholar]
- 162. Asada H, Inoue A, Ngako Kadji FM, Hirata K, Shiimura Y, Im D, Shimamura T, Nomura N, Iwanari H, Hamakubo T, Kusano-Arai O, Hisano H, Uemura T, Suno C, Aoki J, Iwata S. The crystal structure of angiotensin II type 2 receptor with endogenous peptide hormone. Structure 2020;28:418–425 e414. [DOI] [PubMed] [Google Scholar]
- 163. Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 2011;121:297–303. [DOI] [PubMed] [Google Scholar]
- 164. Mendoza-Torres E, Riquelme JA, Vielma A, Sagredo AR, Gabrielli L, Bravo-Sagua R, Jalil JE, Rothermel BA, Sanchez G, Ocaranza MP, Lavandero S. Protection of the myocardium against ischemia/reperfusion injury by angiotensin-(1–9) through an AT2R and akt-dependent mechanism. Pharmacol Res 2018;135:112–121. [DOI] [PubMed] [Google Scholar]
- 165. Matsumoto T, Ozono R, Oshima T, Matsuura H, Sueda T, Kajiyama G, Kambe M. Type 2 angiotensin II receptor is downregulated in cardiomyocytes of patients with heart failure. Cardiovasc Res 2000;46:73–81. [DOI] [PubMed] [Google Scholar]
- 166. Toedebusch R, Belenchia A, Pulakat L. Cell-Specific protective signaling induced by the novel AT2R-agonist NP-6A4 on human endothelial and smooth muscle cells. Front Pharmacol 2018;9:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Booz GW, Baker KM. Role of type 1 and type 2 angiotensin receptors in angiotensin II-induced cardiomyocyte hypertrophy. Hypertension 1996;28:635–640. [DOI] [PubMed] [Google Scholar]
- 168. Falcon BL, Stewart JM, Bourassa E, Katovich MJ, Walter G, Speth RC, Sumners C, Raizada MK. Angiotensin II type 2 receptor gene transfer elicits cardioprotective effects in an angiotensin II infusion rat model of hypertension. Physiol Genomics 2004;19:255–261. [DOI] [PubMed] [Google Scholar]
- 169. Wu L, Iwai M, Nakagami H, Chen R, Suzuki J, Akishita M, de Gasparo M, Horiuchi M. Effect of angiotensin II type 1 receptor blockade on cardiac remodeling in angiotensin II type 2 receptor null mice. Arterioscler Thromb Vasc Biol 2002;22:49–54. [DOI] [PubMed] [Google Scholar]
- 170. D'Amore A, Black MJ, Thomas WG. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension 2005;46:1347–1354. [DOI] [PubMed] [Google Scholar]
- 171. Yan X, Price RL, Nakayama M, Ito K, Schuldt AJ, Manning WJ, Sanbe A, Borg TK, Robbins J, Lorell BH. Ventricular-specific expression of angiotensin II type 2 receptors causes dilated cardiomyopathy and heart failure in transgenic mice. Am J Physiol Heart Circ Physiol 2003;285:H2179–H2187. [DOI] [PubMed] [Google Scholar]
- 172. Brede M, Roell W, Ritter O, Wiesmann F, Jahns R, Haase A, Fleischmann BK, Hein L. Cardiac hypertrophy is associated with decreased eNOS expression in angiotensin AT2 receptor-deficient mice. Hypertension 2003;42:1177–1182. [DOI] [PubMed] [Google Scholar]
- 173. Ichihara S, Senbonmatsu T, Price E Jr, Ichiki T, Gaffney FA, Inagami T. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation 2001;104:346–351. [DOI] [PubMed] [Google Scholar]
- 174. Xu J, Sun Y, Carretero OA, Zhu L, Harding P, Shesely EG, Dai X, Rhaleb NE, Peterson E, Yang XP. Effects of cardiac overexpression of the angiotensin II type 2 receptor on remodeling and dysfunction in mice post-myocardial infarction. Hypertension 2014;63:1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Lauer D, Slavic S, Sommerfeld M, Thone-Reineke C, Sharkovska Y, Hallberg A, Dahlof B, Kintscher U, Unger T, Steckelings UM, Kaschina E. Angiotensin type 2 receptor stimulation ameliorates left ventricular fibrosis and dysfunction via regulation of tissue inhibitor of matrix metalloproteinase 1/matrix metalloproteinase 9 axis and transforming growth factor beta1 in the rat heart. Hypertension 2014;63:e60–e67. [DOI] [PubMed] [Google Scholar]
- 176. Ocaranza M P, Riquelme JA, Garcia L, Jalil JE, Chiong M, Santos RAS, Lavandero S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol 2020;17:116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mazarura GR, Dallagnol JCC, Chatenet D, Allen BG, Hebert TE. The complicated lives of GPCRs in cardiac fibroblasts. Am J Physiol Cell Physiol 2022;323:C813–C822. [DOI] [PubMed] [Google Scholar]
- 178. Irannejad R, Pessino V, Mika D, Huang B, Wedegaertner PB, Conti M, von Zastrow M. Functional selectivity of GPCR-directed drug action through location bias. Nat Chem Biol 2017;13:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Nguyen AH, Thomsen ARB, Cahill TJ III, Huang R, Huang LY, Marcink T, Clarke OB, Heissel S, Masoudi A, Ben-Hail D, Samaan F, Dandey VP, Tan YZ, Hong C, Mahoney JP, Triest S, Little J IV, Chen X, Sunahara R, Steyaert J, Molina H, Yu Z, des Georges A, Lefkowitz RJ. Structure of an endosomal signaling GPCR-G protein-beta-arrestin megacomplex. Nat Struct Mol Biol 2019;26:1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Thomsen ARB, Plouffe B, Cahill TJIII, Shukla AK, Tarrasch JT, Dosey AM, Kahsai AW, Strachan RT, Pani B, Mahoney JP, Huang L, Breton B, Heydenreich FM, Sunahara RK, Skiniotis G, Bouvier M, Lefkowitz RJ. GPCR-G Protein-beta-Arrestin super-Complex mediates sustained G protein signaling. Cell 2016;166:907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]