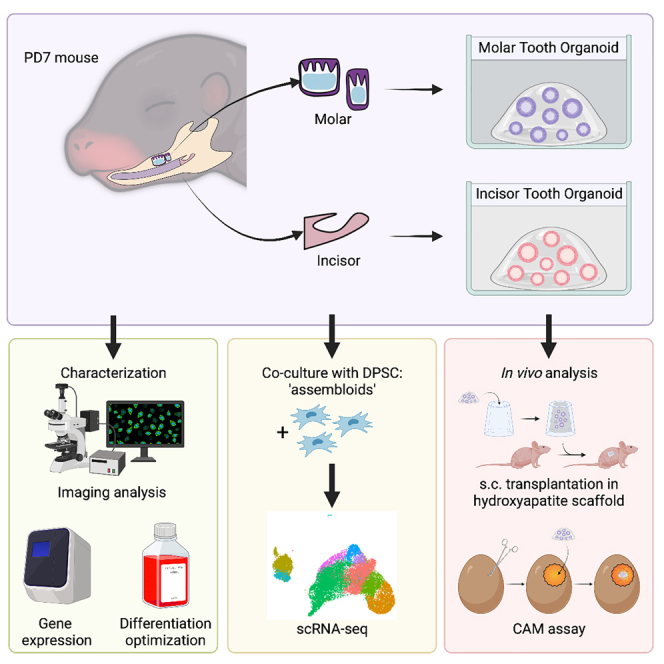
Summary
Organoid models provide powerful tools to study tissue biology and development in a dish. Presently, organoids have not yet been developed from mouse tooth. Here, we established tooth organoids (TOs) from early-postnatal mouse molar and incisor, which are long-term expandable, express dental epithelium stem cell (DESC) markers, and recapitulate key properties of the dental epithelium in a tooth-type-specific manner. TOs display in vitro differentiation capacity toward ameloblast-resembling cells, even more pronounced in assembloids in which dental mesenchymal (pulp) stem cells are combined with the organoid DESCs. Single-cell transcriptomics supports this developmental potential and reveals co-differentiation into junctional epithelium- and odontoblast-/cementoblast-like cells in the assembloids. Finally, TOs survive and show ameloblast-resembling differentiation also in vivo. The developed organoid models provide new tools to study mouse tooth-type-specific biology and development and gain deeper molecular and functional insights that may eventually help to achieve future human biological tooth repair and replacement.
Keywords: organoids, tooth development, stem cells, incisors, molars, dental epithelium, ameloblasts, assembloids, single-cell RNA-sequencing
Graphical abstract
Highlights
-
•
Tooth organoids (TOs) are established from early-postnatal mouse molar and incisor
-
•
TOs recapitulate both dental epithelium (DE) and tooth-type-specific characteristics
-
•
TOs mirror DE differentiation properties in vitro and in vivo
-
•
scRNA-seq inquiry supports differentiation to ameloblasts and junctional epithelium
Hermans et al. developed long-term expandable 3D tooth organoids from mouse molar and incisor. The organoids mirror the dental epithelium in a tooth-type-specific manner and show differentiation potential toward enamel-producing cells and tooth-protecting epithelium. These properties advance mouse tooth organoids as exciting tools to unravel tooth biology and development and promote tooth engineering and repair.
Introduction
In the last decade, organoid technology has proven to be a powerful tool to explore tissue biology and development (Clevers, 2016; Boretto et al., 2017; Artegiani and Clevers, 2018; Cox et al., 2019; Hemeryck et al., 2022). Tissue-derived organoids develop when tissue (stem) cells or fragments are embedded in a 3D extracellular matrix scaffold (typically Matrigel) and exposed to a defined growth factor cocktail that replicates key tissue stem cell niche and developmental signaling factors. These organoid models closely recapitulate phenotypical and functional characteristics of the tissue of origin, much better than traditional 2D cell cultures, and they are highly and long-term expandable with preservation of their characteristics (Clevers, 2016; Hemeryck et al., 2022). Importantly, these stem cell organoids are able to differentiate into specific tissue cell types following exposure to differentiation factors or co-culture with other cell types (Artegiani and Clevers, 2018).
Although in vivo experiments using genetically modified mice have provided important insights into mouse tooth development and biology, deep knowledge is still missing, particularly regarding overlap or distinctions between molars and incisors, largely due to a lack of reliable and tractable in vitro models. Previous cell culture models of mouse dental epithelium (DE) cells, including 2D immortalized, non-physiological cell lines, 2D pluripotent stem cell-derived DE-like cells, and 3D (incisor) spheroids or tooth germ aggregates, lack the important assets of tissue-derived organoids such as faithful recapitulation of tissue-specific phenotype and function and robust long-term expandability (Nakao et al., 2007; Sarkar et al., 2014; Binder et al., 2020; Miao et al., 2022). Moreover, many of the previous models were derived from only one tooth type, thus not allowing decipherment of molar- and incisor-specific biology and development.
In this study, we report the establishment of organoid models starting from both mouse molar and incisor. The obtained epithelial organoids are long-term expandable, recapitulate tooth-specific characteristics, and show dental epithelium stem cell (DESC) differentiation properties, both in vivo and in vitro, the latter further reinforced by the presence of dental mesenchymal (pulp) stem cells, thereby mirroring the important epithelial-mesenchymal crosstalk as occurring during tooth development.
Taken together, our study provides a new powerful tool to explore and contrast mouse molar and incisor biology and development. Together with our recently developed human tooth-derived organoid model (Hemeryck et al., 2022), the here established mouse tooth organoids (TOs) form a highly valuable arsenal of in vitro research tools to decipher tooth biology and development and to open translational perspectives toward tooth repair and replacement, envisioned to eventually be instrumental to counter and cure the highly prevalent and burdening tooth pathologies.
Results
Establishing organoids from mouse molar and incisor
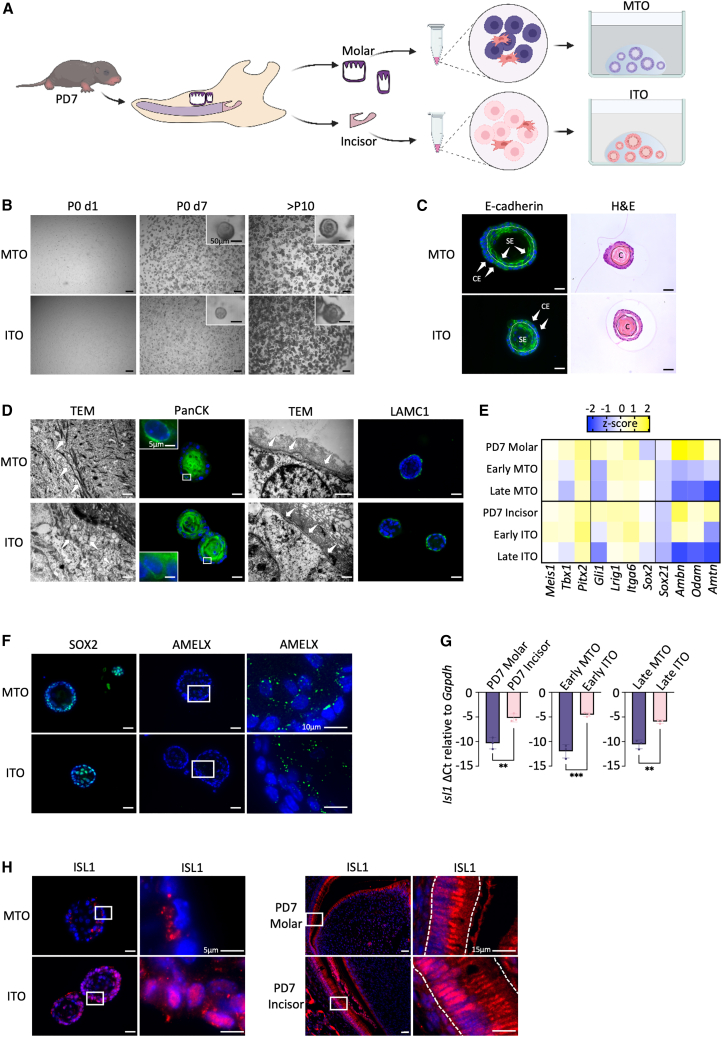
To establish mouse epithelial TOs, we dissected developing (unerupted) teeth from early-postnatal (i.e., postnatal day 7, PD7) mice and isolated the dental epithelium and attached dental follicle from molars and the DESC-containing apical ends from incisors (Figure 1A). Following enzymatic and mechanical trituration, the cell mixture was seeded in Matrigel droplets and cultured in a precisely defined medium, designated as “tooth organoid medium” (TOM), encompassing key stem cell niche factors previously identified to enable development and growth of human tooth organoids (Hemeryck et al., 2022). In particular, canonical organoid (stem cell) growth and differentiation-inhibiting factors such as wingless-type MMTV integration site (WNT) activators (R-spondin 1 [RSPO1] and WNT3A), bone morphogenetic protein (BMP) inhibitor (Noggin), p38 mitogen-activate protein kinase (MAPK) inhibitor (SB202190), and transforming growth factor β (TGFβ) inhibitor (A83-01) and more specific (dental) stem cell niche regulatory growth factors such as sonic hedgehog (SHH), fibroblast growth factors (FGF), and insulin-like growth factor-1 (IGF1) are included in the TOM (for exact composition, see Tables 1 and S1). Notably, our previously defined TOM did not need epidermal growth factor (EGF) (Hemeryck et al., 2022), which is peculiar since it is a prototypical component in nearly all organoid media.
Figure 1.
Development and characterization of organoids from mouse molar and incisor
(A) Schematic of experimental setup for derivation of TOs from early-postnatal (PD7) mouse molar and incisor.
(B) Progressive development (bright-field pictures) of TOs after initial seeding (passage 0 (P0) day 1 (d1)), 7 days after initial seeding (P0 d7), and after long-term culture for more than 10 passages (>P10). Magnified view is in boxes.
(C) Immunofluorescence (IF) and histological (H&E) analysis of TO phenotype and morphology. Regarding E-cadherin IF (green), arrows indicate outer layer cuboidal epithelium (CE) or inner layer stratified epithelium (SE). Nuclei are counterstained with Hoechst33342 (blue; for all IF images). Delineation between layers is indicated by white dotted line. Black dotted line delineates the centrally localized core region in H&E.
(D) Ultrastructural (TEM) and IF (of indicated markers) characterization of TO. Arrows indicate keratin filaments (left TEM images) or outer basement membrane (right TEM images). Boxed areas are magnified.
(E) Heatmap of gene expression of DE TFs, proposed DESC markers, and EMPs in primary molar and incisor tissue (PD7) and early- and late-passage TOs, as quantified by qRT-PCR analysis. Data are presented as relative expression to Gapdh (ΔCt) and Z score normalized. Colors range from blue (low expression) to yellow (high expression).
(F) IF analysis of SOX2 and AMELX in TOs (green). Boxed areas are magnified.
(G) Bar graph (mean ± standard error of the mean [SEM]) showing expression of Isl1 relative to Gapdh (ΔCt) for primary molar and incisor tissue (left), early-passage TOs (middle), and late-passage TOs (right). Data points represent biological replicates from independently obtained primary tissue and independently established organoid lines; unpaired t test.
(H) IF analysis of ISL1 in TOs and primary molar and incisor. Boxed areas are magnified.
Scale bars: 250 μm for bright-field images, 25 μm for IF and H&E images, and 1 μm for TEM images, unless indicated otherwise. ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S1.
Table 1.
Tooth organoid medium (TOM) composition
| Product | Concentration | Supplier | Catalog number |
|---|---|---|---|
| Serum-free defined medium (SFDM) | For composition, see Table S1 | ||
| A83-01 | 0.5 μM | Sigma-Aldrich | SML0788 |
| B27 (without vitamin A) | 1X | Gibco | 12587-010 |
| Cholera toxin | 100 ng/mL | Sigma-Aldrich | C8052-.5mg |
| FGF2 (=basic FGF) | 20 ng/mL | R&D Systems | 234-FSE |
| FGF8 | 200 ng/mL | Peprotech | AF-100-25 |
| FGF10 | 100 ng/mL | Peprotech | 100-26 |
| L-glutamine | 2 mM | Gibco | 25030081 |
| IGF1 | 100 ng/mL | Peprotech | 100-11 |
| N2 | 1X | Gibco | 17502-048 |
| N-acetyl L-cysteine | 1.25 mM | Sigma-Aldrich | A7250 |
| Nicotinamide | 10 mM | Sigma-Aldrich | N0636 |
| Noggin | 100 ng/mL | Peprotech | 120-10C |
| RSPO1 | 200 ng/mL | Peprotech | 120-38 |
| SB202190 | 10 μM | Biotechne (Tocris) | 1264 |
| SHH | 100 ng/mL | R&D Systems | 464-SH |
| WNT3A | 200 ng/mL | R&D Systems | 5036-WN |
| EGFa | 20 ng/ML | R&D Systems | 236-EG |
For all results from Figure 3 onward, TOM was by default supplemented with EGF.
Organoids swiftly develop from both molar and incisor tissue (referred to as molar TO [MTO] and incisor TO [ITO]) and can be stably expanded and long-term passaged, at present for more than 10 passages (i.e., longer than 3 months; Figure 1B). Both MTOs and ITOs are epithelial in nature (as shown by E-cadherin expression) and display a dense morphology with an outer layer of cuboidal epithelium, an intermediate layer of stratified epithelium, and a dense inner core (Figure 1C). Moreover, the TOs are characterized by an abundance of cytokeratin (CK) filaments, especially in the core region with lower level in the periphery, as shown by transmission electron microscopy (TEM) and CK immunoreactivity (including CK5, CK14, and CK8/18; Figures 1D and S1A). Desmosomes, which play an important role in anchoring CK filaments and mediating cell-cell adhesion, are also abundantly detected in the TO, especially toward the CK-rich core region (TEM and desmoglein 1 [DSG1] immunoreactivity; Figure S1B). Both organoid types are boarded by laminin-containing basement membrane (TEM and laminin subunit gamma 1 [LAMC1] immunoreactivity; Figure 1D), indicating organoid polarity.
Interestingly, both MTOs and ITOs express key transcription factors (TFs) involved in the development of the DE (e.g., Meis1, Tbx1, and Pitx2), as well as proposed DESC markers (e.g., Gli1, Lrig1, Itga6, and the well-established Sox2/SOX2) (Figures 1E and 1F), all as found in the primary tissue (Figure 1E) (Catón et al., 2009; Sanz-Navarro et al., 2019; Hermans et al., 2021). Overall, these markers remain expressed in late-passage (P6) TOs (Figure 1E). However, lower levels of ameloblast (AB) markers such as the TF Sox21 and the enamel matrix proteins (EMPs) Ambn, Odam, and Amtn are observed when compared with early-passage TOs (i.e., immediately after seeding; P0) (Figure 1E), which is most likely due to the disappearance of certain seeded cell types (such as AB) at further passaging in typical organoid culture conditions (Fujii and Sato, 2020; Saito et al., 2020; Hemeryck et al., 2022). Moreover, as also found before in human TOs and present in developing DESCs (Hemeryck et al., 2022), the EMP AMELX was detected in the mouse TOs in its typical punctuated pattern (Figure 1F).
Of note, ISL LIM homeobox 1 (ISL1) is known as a key TF involved in development of the incisor DE (Naveau et al., 2017). Accordingly, we find higher gene expression in PD7 incisor than molar. Interestingly, this expression difference is faithfully recapitulated in the organoids (early and late passage; Figure 1G). This observation was further validated by immunofluorescence analysis (Figure 1H) (Naveau et al., 2017). In ITOs, ISL1 is found in the majority of cells where it is localized in both nucleus and cytoplasm, whereas in MTOs, ISL1 is detected in only a few cells, being localized in the cytoplasm. Interestingly, these findings match the in vivo situation; in incisors, ISL1 is abundantly observed in the nuclei (and cytoplasm) of the majority of ABs, whereas in molars, it is detected only in the cytoplasm of the densely packed molar ABs. Our findings show that MTOs and ITOs retain tooth-specific transcriptional programs. In further support, principal component analysis (PCA) of bulk RNA-sequencing (RNA-seq) data generated from both TO types reveals clustering of the organoids based on their tooth of origin (Figure S1C). Moreover, this RNA-seq analysis confirmed incisor-specific expression of Isl1 in ITOs and identified molar-specific expression of Irx1 and Irx2 in MTOs, and both findings were further validated in primary mouse tooth tissue by applying our recently published single-cell RNA-seq (scRNA-seq) atlas (Figures S1D and S1E) (Hermans et al., 2022).
Taken together, we successfully established epithelial organoids from early-postnatal mouse molar and incisor that recapitulate key (tooth-specific) phenotypic DE features.
Differential response of MTOs and ITOs to exogenous EGF exposure, recapitulating in vivo behavior
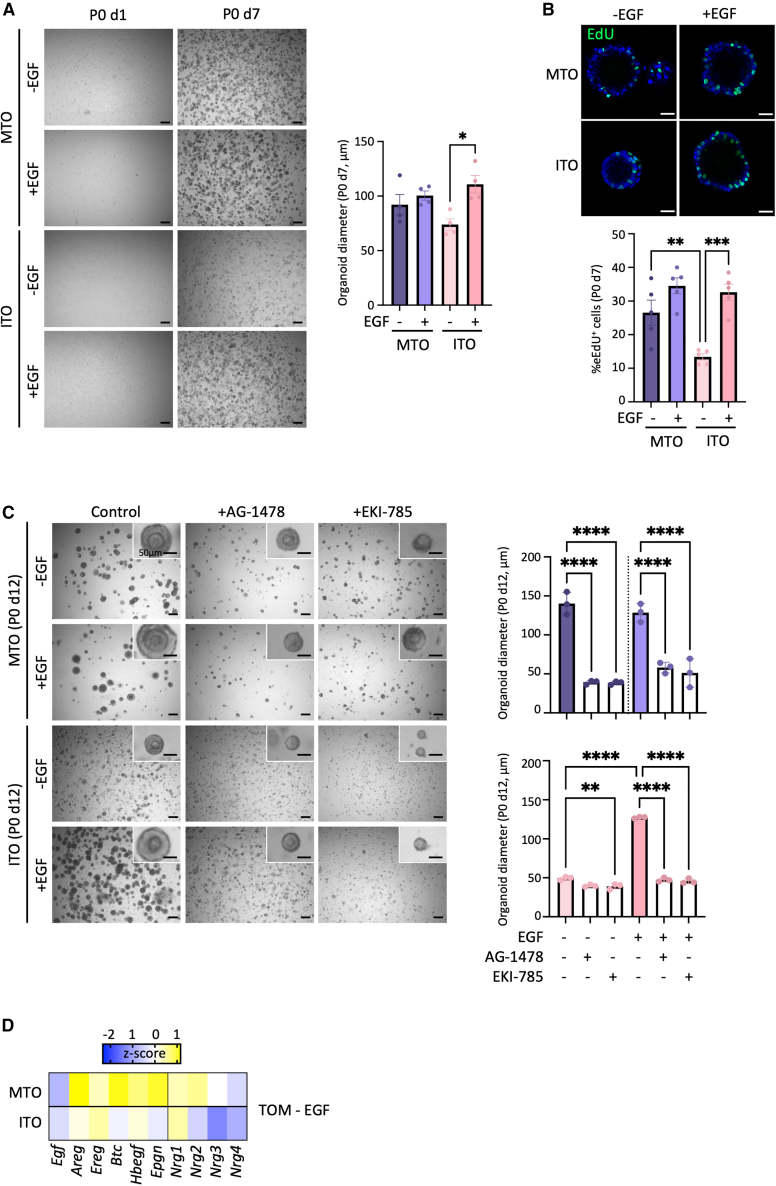
Intriguingly, EGF, typically needed to establish organoids from tissues, is not essential for mouse TO development and passaging, in line with our recent observation in organoid development from human tooth (Hemeryck et al., 2022). However, when EGF is supplemented to the established TOM, the size of ITOs increases, although MTO diameter is not affected (Figure 2A). In accordance, proliferative activity (as analyzed by EdU incorporation) is significantly augmented in ITOs when exposed to EGF but not in MTOs (Figure 2B). Of note, basal proliferation of MTOs (i.e., in TOM without EGF) is higher than of ITOs (Figure 2B). Both organoid types can be maintained long-term in the presence of EGF, comparable to culturing without EGF, and retain similar morphological and phenotypical characteristics, including expression of E-cadherin, SOX2, and AMELX, and reduced Isl1 expression in MTOs compared with ITOs (Figures S2A and S2B). Finally, we observed that the maintenance of ITO culture is facilitated with EGF, epitomized by the significantly reduced time between passaging (Figure S2C).
Figure 2.
Differential response of TOs to exogenous EGF depending on tooth of origin
(A) Left: bright-field images of P0 d1 and d7 TOs grown in TOM without exogenous EGF (–EGF) or with EGF (+EGF). Right: bar graph (mean ± SEM) showing organoid diameter on d7. Data points represent biological replicates from independently established organoid lines; one-way analysis of variance (ANOVA) with Šídák’s multiple comparisons test.
(B) Top: proliferative activity of TOs grown with or without EGF as assessed by EdU incorporation (green). Nuclei are counterstained with Hoechst33342 (blue). Bottom: bar graph showing the proportion of EdU+ cells in TOs (mean ± SEM). Data points represent biological replicates from independently established organoid lines; one-way ANOVA with Šídák’s multiple comparisons test.
(C) Left: bright-field images of P0 d12 TOs grown in TOM–EGF or TOM+EGF and treated with EGFR inhibitors AG-1478 or EKI-785. Right: bar graphs (mean ± SEM) showing TO diameter on d12. Data points represent biological replicates from independently established organoid lines; one-way ANOVA with Šídák’s multiple comparisons test.
(D) Heatmap of gene expression of EGF pathway ligands in TOs (grown without EGF) as quantified by qRT-PCR analysis. Data are presented as relative expression to Gapdh (ΔCt) and Z score normalized. Colors range from blue (low expression) to yellow (high expression).
Scale bars: 250 μm for bright-field images and 25 μm for IF images, unless indicated otherwise. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S2.
Differential sensitivity of MTOs and ITOs to exogenous EGF may have to do with the presence of, and differences in, endogenous EGF activity. Blocking the EGF receptor (EGFR) with a reversible (AG-1478) or irreversible (EKI-785) inhibitor in TOM (i.e., without EGF supplementation) results in a prominent reduction of MTO growth (diameter), while only marginally affecting ITO size (only with EKI-785; Figure 2C). The specificity of the inhibitors on EGFR signaling was confirmed by their inhibitory effect on organoids grown in the presence of exogenous EGF (Figure 2C). When EGF is added to ITOs that are first grown in TOM without EGF for 5 days, organoid growth (diameter) is increased (versus growth without EGF for the full period) (Figure S2D). When EGF is removed after the first 5 days in the presence of EGF, ITO growth is decreased (versus growth with EGF for the full period). In contrast, molar-derived organoids are insensitive to analogous removal or addition of exogenous EGF (Figure S2D). Thus, MTOs appear to have sufficient endogenous EGF(R) activity for optimal growth, whereas ITOs show better growth when the system is exogenously stimulated. In accordance, expression of several EGF family ligands that signal through the EGF receptor family (via homo- or heterodimerization of EGFR, ErbB2, ErbB3, and ErbB4), including Areg, Btc, Hbegf, Epgn, Nrg2, Nrg3, and Nrg4) (Wee and Wang, 2017), is higher in MTOs than ITOs (Figure 2D).
Taken together, MTOs and ITOs represent interesting tools to in vitro study tooth-specific molecular signaling and development. Due to better growth characteristics of ITOs in the presence of EGF (Figures 2A–2C, S2C, and S2D), we supplemented TOM with EGF for all further experiments in which MTOs and ITOs were compared.
MTOs and ITOs are amenable to AB-resembling differentiation
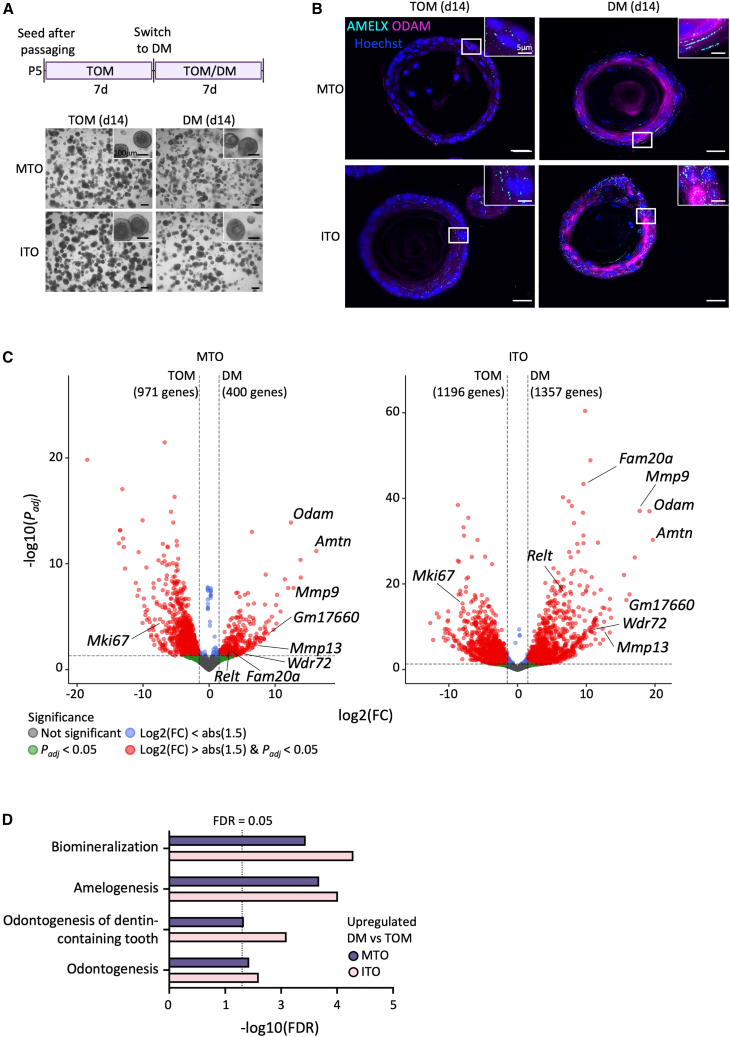
During tooth development, DESCs differentiate into ABs, which form the tooth enamel (Hermans et al., 2021) by first depositing EMP, to subsequently enable mineralization and formation of hydroxyapatite crystals using the deposited protein matrix as a guide (Bai et al., 2020; Welborn, 2020). During this process, differentiating DESCs first acquire a secretory-stage phenotype (sABs, which produce the EMP scaffold) and then a maturation-stage nature (mABs, which drive mineralization and degrade the EMP template), each characterized by distinct EMP profiles (AMELX/AMBN and ODAM/AMTN expression, respectively) (Ganss and Abbarin, 2014; Welborn, 2020). Here, we investigated whether the DESC organoids are able to differentiate toward ABs. Therefore, we removed stemness- and proliferation-promoting growth factors from TOM and added BMP2, BMP4, and TGFβ1 (Table S2; further referred to as differentiation medium [DM]), signaling factors that have been shown to be important for in vivo AB development (Gao et al., 2009; Xie et al., 2016).
Organoids (P5), expanded in TOM for 7 days, were switched to DM for an additional 7 days, causing no overt morphological changes (Figure 3A). However, interestingly, a prominent increase in EMP expression at protein (AMELX, ODAM) and/or gene (Ambn, Amtn, Odam) level was observed (Figures 3B and S3A). RNA-seq analysis revealed clear shifts in PCA pattern upon culture in DM, overall showing clustering of samples according to tooth type and culture condition (Figure S3B and supplemental information). Differentially expressed gene (DEG) analysis uncovered 400 upregulated genes in MTO+DM compared with MTO+TOM, and 1,357 upregulated genes in ITO+DM compared with ITO+TOM, of which 233 are shared between both organoid types (Figures 3C and S3C). Numerous amelogenesis-associated genes are found enriched in DM-exposed MTOs and ITOs versus TOM-grown controls, such as Fam20a, Relt, and Wdr72; the EMPs Amtn and Odam; and the matrix metallopeptidases Mmp9 and Mmp13 (Figure 3C) (El-Sayed et al., 2009; O’Sullivan et al., 2011; Feng et al., 2012; Kim et al., 2019; Vasconcelos et al., 2019). In addition, organoids exposed to DM express Gm17660, belonging to the same gene family and genomic locus as Amtn and Odam, and associated with mABs and the tooth epithelium-derived and enamel-bound junctional epithelium (JE) (Ganss and Abbarin, 2014; Moffatt et al., 2014; Yajima-Himuro et al., 2014). Gene ontology (GO) analysis further supported the acquisition of an AB-resembling fate in DM- versus TOM-grown organoids, identifying biological processes such as “amelogenesis” and “odontogenesis” (i.e., tooth development) as significantly enriched upon DM culture (Figure 3D). Simultaneously, GO analysis revealed upregulation of apoptosis processes in DM-grown TOs (Figure S3D). Apoptosis is a natural step in the AB life cycle, with ABs undergoing apoptosis during the transition from sABs to mABs and in early mAB stage (Abramyan et al., 2021). Quantification of cleaved caspase-3 (CC3) immunofluorescence shows an increased number of apoptotic cells upon DM culture in both MTOs and ITOs (Figure S3E). Their proportion (20%–30%) is very similar to the reported proportion observed in vivo (25%) during both transition from sABs to mABs as well as in early mAB stage (Smith and Warshawsky, 1977). Simultaneously, the number of proliferating (Ki67+) cells goes down (Figure S3F), further corroborated by decreased Mki67 expression (Figure 3C) and downregulated “cell cycle” processes in DM- versus TOM-cultured TOs (Figure S3G).
Figure 3.
In vitro differentiation of TOs toward AB-resembling cells
(A) Top: timeline of experimental setup. Bottom: bright-field images of P5 d14 TOs grown in TOM or switched to DM after 7 days. Magnified view is in boxes.
(B) IF analysis of AMELX (cyan) and ODAM (magenta) in TOs. Nuclei are counterstained with Hoechst33342 (blue). Boxed areas are magnified.
(C) Volcano plot with log2(fold change [FC]) versus –log10(Padj) value) of RNA-seq data from MTOs (left panel) and ITOs (right panel). Statistically upregulated genes between TOM- and DM-grown TOs (left for TOM and right for DM) are indicated in red, as determined by a combination of log2(FC) > the absolute (abs) value of ±1.5 and Padj < 0.05.
(D) Significant (FDR ≤ 0.05, indicated by dotted line) DEG-based GO terms enriched in DM-grown TOs compared with TOM-cultured controls.
Scale bars: 250 μm for bright-field images and 25 μm for IF images, unless indicated otherwise. See also Figure S3.
Together, our findings indicate that our organoid models, both from molar and incisor, are amenable to differentiation toward AB-resembling cells in vitro. From the data obtained, it appears that the acquired phenotype more resembles the mAB than the preceding sAB stage, as supported by predominant increase of mAB markers and noticeable apoptosis.
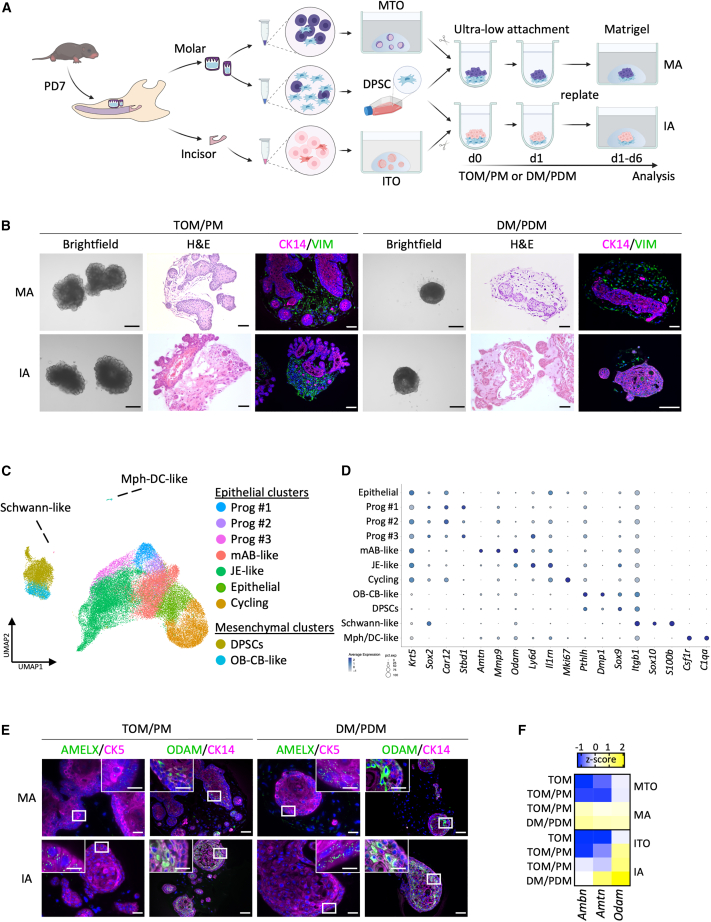
Assembloids combining organoid DESCs with mesenchymal dental pulp stem cells recapitulate developmental epithelial-mesenchymal interactions and co-differentiation
During tooth development, the DE generates ABs while the dental mesenchyme contributes to the dentin-producing odontoblasts (OBs) and root cement-fabricating cementoblasts (CBs) (Hermans et al., 2021). Throughout the various stages of tooth development, epithelial-mesenchymal interactions play a crucial role, driving odontogenesis and differentiation of the mature cell types (Hermans et al., 2021). To mimic these developmental interactions, we established a co-culture model by combining TO DESCs with mesenchymal dental pulp stem cells (DPSCs), following a similar approach as we previously described for human tooth (Figure 4A; see experimental procedures) (Hemeryck et al., 2022). These tooth “assembloids” (referring to self-organizing 3D cellular constructs resulting from the combination of epithelial organoids with other cell types such as mesenchymal cells; Rawlings et al., 2021; Kanton and Paşca, 2022) were established and cultured either in a 1:1 ratio of TOM and pulp medium (PM) or a 1:1 ratio of DM and pulp differentiation medium (PDM) (Figure 4A; Tables S3 and S4). The molar and incisor assembloids (referred to as MAs and IAs, respectively) show similar morphology that changes according to culture medium (Figure 4B). TOM/PM-grown assembloids mainly display a bubbled outline composed of small organoid units, whereas DM/PDM-grown assembloids largely show a smooth contour. In all conditions, the assembloids contain distinct epithelial (CK14+) and mesenchymal (vimentin, VIM+) domains (Figure 4B).
Figure 4.
Establishment and characterization of tooth assembloids combining organoid DESCs with mesenchymal DPSCs
(A) Schematic of experimental setup for the development of tooth assembloids by combining TO cells with mesenchymal DPSCs.
(B) Bright-field, H&E, and IF (of indicated markers) images of MAs and IAs grown in TOM/PM or DM/PDM. Nuclei of IF images are counterstained with Hoechst33342.
(C) Annotated UMAP plot of integrated assembloid scRNA-seq datasets, i.e., from MA+TOM/PM, MA+DM/PDM, IA+TOM/PM, and IA+DM/PDM (n = 2 biological replicates of each experimental condition).
(D) Dotplot displaying the percentage of cells (dot size) expressing key marker genes of the different annotated cell clusters (average expression levels indicated by color intensity; see scale).
(E) IF analysis of AMELX and ODAM (green) and indicated cytokeratins (CK; magenta) in tooth assembloids. Nuclei are counterstained with Hoechst33342 (blue). Boxed areas are magnified.
(F) Heatmap of gene expression of AB-resembling differentiation in tooth assembloids as quantified by qRT-PCR analysis. Data are presented as relative expression to Gapdh (ΔCt) and Z score normalized. Colors range from blue (low expression) to yellow (high expression).
Scale bars: 250 μm for bright-field images, 50 μm for H&E, CK14/VIM, and ODAM/CK14 (20 μm for close-ups) IF images, 25 μm for AMELX/CK5 (10 μm for close-ups) IF images. See also Figure S4.
To more granularly characterize the tooth assembloids, we applied scRNA-seq analysis on MAs and IAs grown in TOM/PM and DM/PDM conditions (Table S5). Following quality control, data processing, and integration, 43,891 cells were retained (Figures S4A and S4B). Eleven distinct clusters were discerned and annotated, based on marker expression (Figures 4C, 4D, S4C, and S4D) (Hermans et al., 2022). As expected, two large “superclusters” of epithelial or mesenchymal cells were identified. Within the epithelial supercluster, several clusters were classified including “cycling” cells, non-cycling non-differentiated “epithelial” cells, progenitor cells (“Prog” #1, #2, and #3), and two distinct, more differentiated cell populations, i.e., a “mAB-like” (Amtn+/Odam+/Mmp9+/Gm17660+) and “JE-like” (Ly6D+/Il1rn+/BC037156+ [also known as Fdcsp]) cell cluster, both more abundant in DM/PDM-grown assembloids (Figures 4C, 4D, S4C, and S4D) (Feng et al., 2012; Ganss and Abbarin, 2014; Hermans et al., 2022). Comparison of the top 20 DEGs between mAB- and JE-like clusters further confirmed specific expression of amelogenesis-associated genes (Amtn, Odam, Mmp9, Gm17660, Lamc2, Lama3, Lamb3, and Tmsb4x) or JE-linked genes (Ly6d, Il1rn, BC037156 [Fdcsp], and Anxa1) in the respective clusters (Figure S4D) (Hayashi et al., 2010; Feng et al., 2012; Kim et al., 2013; Ganss and Abbarin, 2014; Kiyoshima et al., 2014; Moffatt et al., 2014; Gostyńska et al., 2016; Wazen et al., 2016; Hermans et al., 2022). Both groups of genes were elevated in DM/PDM-cultured compared with TOM/PM-grown assembloids, indicating enhanced differentiation and maturation when exposed to differentiation media (Figure S4E). Expression of JE-related genes is also detected in the monocultured MTOs and ITOs and found upregulated following culture in DM (Figures S4F and S4G), indicating that mesenchymal interaction is not essentially needed for the acquisition of a JE-like cell fate. On the other hand, assembloids show enhanced differentiation toward AB-resembling cells compared with organoid monocultures, which is still further promoted by exposure to DM/PDM (Figures 4E, 4F, S4C–S4E). Indeed, whereas TOM-cultured TOs lack ODAM expression and TOM/PM-cultured organoids show no or only little ODAM protein signal (Figure S4H), the presence of DPSCs is sufficient to lead to prominent ODAM signal in the assembloid epithelium, which is visibly further enhanced by exposure to DM/PDM (Figure 4E). Similarly, gene expression of Ambn, Amtn, and Odam is augmented in assembloids compared with TOs alone, and further elevated when cultured in DM/PDM (Figure 4F).
Regarding the mesenchymal supercluster, both DPSCs and OB-CB-like cells (Dmp1+/Pthlh+) are discerned (Figures 4C and 4D). Gene expression analysis of known OBs (Dspp, Dmp1), CBs (Pthlh), and mineralization markers (Ibsp, Spp1, and Col1a1) shows upregulation in DM/PDM culture, thereby supporting their further differentiation (Figures 4C, 4D, and S4I). Unexpectedly, a small cluster of “Schwann-like” cells (Sox10+/S100b+) is identified in DM/PDM-grown assembloids (Figures 4C, 4D, and S4C). In previous work, we have shown that human DPSCs can differentiate into Schwann cells in vitro (Martens et al., 2014), although not yet demonstrated for mouse DPSCs. Finally, a small cluster of macrophage/dendritic cell-like (“Mph-DC-like”) cells (Csf1r+/C1qa+) of unknown origin is detected in the assembloids, more clearly after culture in DM/PDM (Figures 4C, 4D, and S4C).
Taken together, combining organoid DESCs with mesenchymal DPSCs empowers the differentiation toward (m)AB-resembling as well as OB-CB-like cells, thereby mimicking the key outcome of developmental epithelial-mesenchymal interactions as occurring in vivo. Interestingly, acquisition of a JE-like fate was also observed. During tooth development, the JE is derived from the DE, likely from both the “reduced enamel epithelium” (REE; i.e., the layer of mAB and outer enamel epithelium covering the deposited enamel prior to eruption) and the DESC-containing epithelial cell rests of Malassez (ERM) (Kato et al., 2019; Hermans et al., 2022). Together, our findings propose that the organoids/assembloids acquire a late-stage DE phenotype, encompassing both mAB and JE phases.
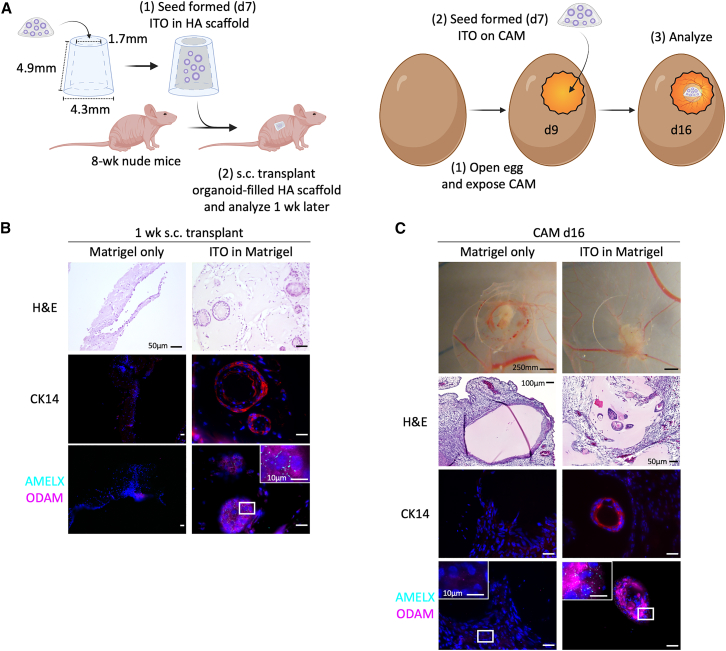
In vivo survival and differentiation potential of mouse TOs
To evaluate whether mouse TOs are able to survive and differentiate in vivo—a requirement for future tooth regeneration/replacement endeavors—we applied TOM-grown ITOs in two complementary in vivo transplantation assays, i.e., subcutaneous (s.c.) implantation into immunodeficient (nude) mouse of a 3D printed hydroxyapatite scaffold seeded with Matrigel and organoids (Hemeryck et al., 2022) or grafting onto chicken chorioallantoic membrane (CAM) (Bronckaers et al., 2013, 2021) (Figure 5A). In both models, ITOs are able to survive, retain epithelial nature (CK14+), and produce both AMELX and ODAM (Figures 5B and 5C) (the latter not present before transplantation, see, e.g., Figure 3B). Together, these proof-of-principle experiments show that mouse TOs are able to survive and spontaneously differentiate toward AB-like cells in vivo.
Figure 5.
In vivo survival and differentiation potential of TOs
(A) Schematic overview of in vivo transplantation approaches. Left: s.c. transplantation of ITOs seeded in 3D-printed hydroxyapatite (HA) construct. Right: grafting onto chicken CAM.
(B) H&E and IF (of indicated markers) images of s.c. transplanted ITOs or negative controls (Matrigel only) after 1 week (wk).
(C) CAM, H&E, or IF (of indicated markers) images of grafted ITOs or negative controls (Matrigel only) at d10. Boxed areas are magnified.
Scalebars: 50 μm for H&E, 25 μm for IF, and 250 mm for CAM images, unless otherwise indicated.
Discussion
In this study, we report the development of long-term expandable TOs from mouse molar and incisor. The established TOs recapitulate key properties of mouse DE. In contrast to many other available methods to study DE in vitro, our TO model provides more accurate mirroring of tooth-specific biology in vitro. Among others, our observations support a previous study in which ISL1 was abundantly detected in developing mouse incisors but only barely in emerging molars (Naveau et al., 2017). Moreover, genetic deletion of Isl1 in the DE resulted in incisor but not molar enamel defects (Naveau et al., 2017). In addition to displaying tooth-specific expression profiles, both MTOs and ITOs show tooth-specific responses to exogenous growth factor signaling as shown by differential response to EGF supplementation, which, importantly, recapitulates in vivo observations. Indeed, perinatal injection of EGF in rodents results in precocious eruption of incisors but not rodents (Rhodes et al., 1987; Cielinski et al., 1995; Naveau et al., 2017). Current models are either developed from one tooth type (i.e., immortalized cell lines derived from molar enamel organ) or are not specified (i.e., differentiation of AB/DE-like cells from 2D pluripotent stem cells), whereas other methods are not expandable and start from embryonic material (Nakao et al., 2007; Sarkar et al., 2014; Miao et al., 2021). The ability to mimic tooth-type-specific paradigms and development in vitro will be invaluable to further elucidate key factors and pathways driving differential development of both tooth types.
In addition, we show that both MTOs and ITOs are amenable to in vitro differentiate toward AB-like cells, either alone or when activated by co-culture with DPSCs, mirroring signaling interactions naturally occurring in vivo. Notably, our data suggest that AB differentiation appears predisposed toward a more mature phenotype, representing the mAB stage, which in vivo plays an important role in the final mineralization and maturation of the tooth enamel. Firstly, DM-grown TOs and assembloids predominantly express the mAB EMP markers Amtn and Odam/ODAM, as well as other mAB markers such as Gm17660 (Ganss and Abbarin, 2014). Secondly, differentiation of TOs is accompanied by a loss of proliferation and increased apoptosis. The latter phenomenon is strongly associated with the transition from sAB to the mAB stage, as well as during the early mAB stage of the AB life cycle (Smith and Warshawsky, 1977). Interestingly, the proportion of apoptotic cells in DM-grown organoids was similar to the proportion identified in vivo (Smith and Warshawsky, 1977).
At the same time, our data suggest the acquisition of a JE-like cell fate in DM-grown TOs and in assembloids. In vivo, JE plays an important role forming the bridge between the enamel surface and oral gingival epithelium, and it functions as an important immunological barrier to oral micro-organisms (Fischer and Aparicio, 2022). Previously, use of bioengineered tooth germs revealed that the JE is derived from the odontogenic DE, which also gives rise to the AB lineage (Yajima-Himuro et al., 2014). Although JE is traditionally thought to develop from the REE (and thus from mABs and outer enamel epithelium), there is previously reported support that ERM may also contribute to JE (Kato et al., 2019; Hermans et al., 2022). Further analysis of TOs and assembloids (including our developed ERM-derived human tooth models; Hemeryck et al., 2022) during differentiation (i.e., at different timepoints) may provide further insight into these hypotheses. Together, the presence of both mAB- and JE-like cells suggests that our currently developed differentiation protocols drive TOs toward a more mature, late-stage DE phenotype. Further development and optimization of differentiation protocols to specifically enrich for one specific cell type (i.e., mAB- or JE-like cells) or to instead acquire sAB nature will provide a tunable and flexible model for future research.
Importantly, organoids are highly adaptable tools for disease modeling and regenerative medicine or tooth bioengineering endeavors. TOs can be derived from transgenic mouse models of tooth disease (e.g., mice mimicking amelogenesis imperfecta), thereby having the ethical advantage of reducing the number of animals used, as organoids are highly amendable (Gibson et al., 2001; Paine et al., 2003; Pugach and Gibson, 2014). Another application may be co-culture with oral micro-organisms to study the immunological barrier function of JE. In addition, due to their high, long-term expandability in vitro and ability to survive and differentiate in vitro and in vivo (as demonstrated in this study), TOs are an ideal cellular source of DE cells for tooth tissue bioengineering. Finally, through enabling epithelial-mesenchymal reciprocal signaling, epithelial TOs may be the missing link needed to stimulate in vitro differentiation of OBs. Considerable research has established that DPSCs can differentiate into mineralizing cells resembling OBs; however, an accurate representation of OBs is still lacking (Tsutsui, 2020). Data from our developed tooth assembloids indicate that DPSCs may acquire an OB-CB-like phenotype. Further research is now needed to optimize the differentiation parameters for development of authentic OBs in vitro.
In summary, this novel mouse TO model provides a valuable tool to study mouse tooth DE/DESCs, dental epithelial-mesenchymal interactions, and AB/JE differentiation while allowing further elucidation of tooth-type-specific features. As such, TOs, both from mouse molar and incisor as developed here and from human ERM obtained from extracted third molars as previously described (Hemeryck et al., 2022), have great potential to further unravel tooth biology and repair and may be an alluring tool to eventually enable tooth bioengineering strategies.
Experimental procedures
Detailed methods are provided in the supplemental information.
Resource availability
Corresponding author
Further information and requests for resources and reagents should be directed to and will be fulfilled upon reasonable request by the corresponding authors, Annelies Bronckaers (annelies.bronckaers@uhasselt.be) and Hugo Vankelecom (hugo.vankelecom@kuleuven.be).
Materials availability
The study did not generate new unique reagents.
Establishing organoid cultures from mouse molar and incisor
Whole (unerupted) molars, including surrounding dental follicle and attached epithelium, as well as apical ends of (unerupted) incisors were carefully isolated from PD7 mice (ethical approval P056/2022, KU Leuven). Dissociated molar and incisor DE cell material was plated in serum-free defined medium (SFDM; Table S1) and growth factor-reduced Matrigel at a 30:70 ratio and cultured in defined TOM (Table 1). TOs were passaged every 7–10 days.
Establishment of mouse tooth assembloids
Mouse tooth assembloids were established using a similar protocol as we previously described for human tooth assembloids (Hemeryck et al., 2022). Briefly, single-cell dissociated TOs and DPSCs were combined in round-bottom low-attachment plates using a layered approach and cultured in 10% growth factor reduced Matrigel with 90% of either a 1:1 mixture of TOM/PM or DM/PDM. After 24 h, the formed structures were plated in 70% Matrigel to generate tooth assembloids that were maintained in the respective 1:1 media mixtures.
Histochemical, immunostaining, EdU incorporation, and TEM analysis
Samples were fixed in paraformaldehyde and paraffin embedded. Derived sections were subjected to hematoxylin and eosin (H&E) or immunofluorescence staining (for antibodies, see Table S6). EdU labeling in TOs was performed using the Click-iT EdU Alexa Fluor 488 kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. TO samples were prepared for TEM as previously described in detail (Lambrichts et al., 1993; Cox et al., 2019). For all analyses, representative images are shown.
Gene expression analysis by qRT-PCR
Total RNA from dissociated molar and incisor tissue, TOs, and tooth assembloids was subjected to quantitative reverse transcription (real-time) PCR (qRT-PCR) using specific forward and reverse primers (Table S7), all as described before (Cox et al., 2019). Gene expression levels were calculated as ΔCt values relative to Gapdh (Cttarget – Cthousekeeping), and Z score normalization was performed.
RNA-seq analysis
For bulk RNA-seq analysis, RNA was isolated from P0 MTOs and ITOs grown in TOM or from P5 MTOs and ITOs grown in TOM+EGF and exposed to DM or not. For scRNA-seq analysis, MAs and IAs, exposed to DM/PDM or not, were dissociated into single cells.
Subcutaneous transplantation of ITOs
Matrigel with d7 ITOs (P5) was pipetted into hydroxyapatite scaffolds that were s.c. grown in immunodeficient mice for 1 week, as in detail described elsewhere (Bronckaers et al., 2021; Hemeryck et al., 2022) (ethical approval protocol 202138, UHasselt).
Chicken CAM assay
CAM assay was performed as previously in detail described (Bronckaers et al., 2013). In short, pre-solidified Matrigel droplets with d7 ITOs (P5) were applied onto the CAM of fertilized eggs, and the graft was removed 1 week later. Droplets of Matrigel alone served as negative controls.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (v9.3.1) for macOS and are specified in the figure legends. All experiments were performed with ≥3 (unless otherwise indicated) independent biological experiments (i.e., organoid lines established from independent mouse litters).
Author contributions
F.H. designed the concepts and experiments, performed the experiments and the data analysis, interpreted the results, and wrote the manuscript. L.H. co-designed the concepts and provided technical and conceptual input. C.B. contributed to organoid differentiation experiments. M.T.P. contributed to assembloid experiments. S.H. contributed to TEM analysis. H.K. performed and co-interpreted TEM analysis. D.L. co-supervised the scRNA-seq experiments. I.L. co-supervised the project, co-designed concepts and experiments, and co-interpreted results. A.B. co-supervised the project, co-designed concepts and experiments, performed TEM and in vivo experiments, co-interpreted the results and co-wrote the manuscript. H.V. supervised the entire project, co-designed the organoid concepts and experiments, co-interpreted the results, and vastly amended the manuscript. All co-authors critically read and approved the manuscript.
Acknowledgments
We are grateful to Evelyne Van Kerckhove (UHasselt), Marc Jans (UHasselt), Jeanine Santermans (UHasselt), and Veerle Vanslembrouck (KU Leuven) for valuable technical help. We thank Dr. Diether Lambrechts’ group (VIB, KU Leuven) for technical assistance in 10× Genomics. Computational resources for transcriptome analyses were provided by the “Vlaams Supercomputer Centrum” (VSC), managed by the Fund for Scientific Research (FWO), Flanders (Belgium). We are also grateful to the Imaging Core (VIB, KU Leuven) and the CIC (KU Leuven) for use of microscopes and the Center for Brain & Disease Research (CBD) Histology unit (VIB, KU Leuven) for use of histology equipment. We acknowledge the use of the TEM platforms at VIB-KU Leuven, UHasselt, and Tohoku University. The authors also thank Dr. Adrian Ranga (KU Leuven), Dr. Ronald Driesen (UHasselt), and other non-coauthor members of the Laboratory of Tissue Plasticity in Health and Disease (KU Leuven) for their input. Certain figures were created using BioRender.com or GraphPad Prism (v9.3.1) for macOS.
This work was supported by grants from KU Leuven (Research Fund) and Fund for Scientific Research (FWO) Flanders. L.H. was an FWO PhD Fellow (1S84718N). C.B. is an FWO PhD Fellow (1129323N), while F.H. was supported by an FWO project grant (G061819FWO). Use of the Zeiss LSM 780 – SP Mai Tai HP DS is supported by Hercules AKUL/11/37 and FWO G.0929.15 funding to Dr. Pieter Vanden Berghe (CIC, KU Leuven), while the application of the JEOL FLASH 1400 TEM was supported by Hercules FWO funding I000220N to I.L.
Conflict of interests
The authors declare no competing interests.
Published: April 20, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.03.011.
Contributor Information
Annelies Bronckaers, Email: annelies.bronckaers@uhasselt.be.
Hugo Vankelecom, Email: hugo.vankelecom@kuleuven.be.
Supplemental information
Data availability
RNA-seq and scRNA-seq data have been deposited to the ArrayExpress database (accession numbers E-MTAB-12557 [https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-12557?accession=E-MTAB-12557] and E-MTAB-12544 [https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-12544?accession=E-MTAB-12544]).
References
- Abramyan J., Geetha-Loganathan P., Šulcová M., Buchtová M. Role of cell death in cellular processes during odontogenesis. Front. Cell Dev. Biol. 2021;9:671475. doi: 10.3389/fcell.2021.671475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani B., Clevers H. Use and application of 3D-organoid technology. Hum. Mol. Genet. 2018;27:R99–R107. doi: 10.3389/fcell.2021.671475. [DOI] [PubMed] [Google Scholar]
- Bai Y., Yu Z., Ackerman L., Zhang Y., Bonde J., Li W., Cheng Y., Habelitz S. Protein nanoribbons template enamel mineralization. Proc. Natl. Acad. Sci. USA. 2020;117:19201–19208. doi: 10.1073/pnas.2007838117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M., Biggs L.C., Kronenberg M.S., Schneider P., Thesleff I., Balic A. Novel strategies for expansion of tooth epithelial stem cells and ameloblast generation. Sci. Rep. 2020;10:4963. doi: 10.1038/s41598-020-60708-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boretto M., Cox B., Noben M., Hendriks N., Fassbender A., Roose H., Amant F., Timmerman D., Tomassetti C., Vanhie A., et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144:1775–1786. doi: 10.1242/dev.148478. [DOI] [PubMed] [Google Scholar]
- Bronckaers A., Hilkens P., Fanton Y., Struys T., Gervois P., Politis C., Martens W., Lambrichts I. Angiogenic properties of human dental pulp stem cells. PLoS One. 2013;8:e71104. doi: 10.1371/journal.pone.0071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronckaers A., Hilkens P., Wolfs E., Lambrichts I. In: Vascular Morphogenesis. Methods in Molecular Biology. Ribatti D., editor. Humana Press Inc.; 2021. By the skin of your teeth: a subcutaneous mouse model to study pulp regeneration; pp. 223–232. [DOI] [PubMed] [Google Scholar]
- Catón J., Luder H.U., Zoupa M., Bradman M., Bluteau G., Tucker A.S., Klein O., Mitsiadis T.A. Enamel-free teeth: Tbx1 deletion affects amelogenesis in rodent incisors. Dev. Biol. 2009;328:493–505. doi: 10.1016/j.ydbio.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cielinski M.J., Jolie M., Wise G.E., Marks S.C. The contrasting effects of colony-stimulating factor-1 and epidermal growth factor on tooth eruption in the rat. Connect. Tissue Res. 1995;32:165–169. doi: 10.3109/03008209509013720. [DOI] [PubMed] [Google Scholar]
- Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- Cox B., Laporte E., Vennekens A., Kobayashi H., Nys C., Van Zundert I., Uji-I H., Vercauteren Drubbel A., Beck B., Roose H., et al. Organoids from pituitary as a novel research model toward pituitary stem cell exploration. J. Endocrinol. 2019;240:287–308. doi: 10.1530/JOE-18-0462. [DOI] [PubMed] [Google Scholar]
- El-Sayed W., Parry D.A., Shore R.C., Ahmed M., Jafri H., Rashid Y., Al-Bahlani S., Al Harasi S., Kirkham J., Inglehearn C.F., Mighell A.J. Mutations in the beta propeller WDR72 cause autosomal-recessive hypomaturation amelogenesis imperfecta. Am. J. Hum. Genet. 2009;85:699–705. doi: 10.1016/j.ajhg.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., McDaniel J.S., Chuang H.H., Huang O., Rakian A., Xu X., Steffensen B., Donly K.J., MacDougall M., Chen S. Binding of amelogenin to MMP-9 and their co-expression in developing mouse teeth. J. Mol. Histol. 2012;43:473–485. doi: 10.1007/s10735-012-9423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N.G., Aparicio C. Junctional epithelium and hemidesmosomes: tape and rivets for solving the “percutaneous device dilemma” in dental and other permanent implants. Bioact. Mater. 2022;18:178–198. doi: 10.1016/j.bioactmat.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M., Sato T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat. Mater. 2020;20:156–169. doi: 10.1038/s41563-020-0754-0. [DOI] [PubMed] [Google Scholar]
- Ganss B., Abbarin N. Maturation and beyond: proteins in the developmental continuum from enamel epithelium to junctional epithelium. Front. Physiol. 2014;5:371. doi: 10.3389/fphys.2014.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Li D., Han T., Sun Y., Zhang J. TGF-beta1 and TGFBR1 are expressed in ameloblasts and promote MMP20 expression. Anat. Rec. 2009;292:885–890. doi: 10.1002/ar.20901. [DOI] [PubMed] [Google Scholar]
- Gibson C.W., Yuan Z.A., Hall B., Longenecker G., Chen E., Thyagarajan T., Sreenath T., Wright J.T., Decker S., Piddington R., et al. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J. Biol. Chem. 2001;276:31871–31875. doi: 10.1074/jbc.M104624200. [DOI] [PubMed] [Google Scholar]
- Gostyńska K.B., Yan Yuen W., Pasmooij A.M.G., Stellingsma C., Pas H.H., Lemmink H., Jonkman M.F. Carriers with functional null mutations in LAMA3 have localized enamel abnormalities due to haploinsufficiency. Eur. J. Hum. Genet. 2016;25:94–99. doi: 10.1038/ejhg.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Matsunaga T., Yamamoto G., Nishii K., Usui M., Yamamoto M., Tachikawa T. Comprehensive analysis of gene expression in the junctional epithelium by laser microdissection and microarray analysis. J. Periodontal. Res. 2010;45:618–625. doi: 10.1111/j.1600-0765.2010.01276.x. [DOI] [PubMed] [Google Scholar]
- Hemeryck L., Hermans F., Chappell J., Kobayashi H., Lambrechts D., Lambrichts I., Bronckaers A., Vankelecom H. Organoids from human tooth showing epithelial stemness phenotype and differentiation potential. Cell. Mol. Life Sci. 2022;79:153. doi: 10.1007/s00018-022-04183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans F., Bueds C., Hemeryck L., Lambrichts I., Bronckaers A., Vankelecom H. Establishment of inclusive single-cell transcriptome atlases from mouse and human tooth as powerful resource for dental research. Front. Cell Dev. Biol. 2022;10:1021459. doi: 10.3389/fcell.2022.1021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans F., Hemeryck L., Lambrichts I., Bronckaers A., Vankelecom H. Intertwined signaling pathways governing tooth development: a give-and-take between canonical wnt and shh. Front. Cell Dev. Biol. 2021;9:758203. doi: 10.3389/fcell.2021.758203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanton S., Paşca S.P. Human assembloids. Development. 2022;149:dev201120. doi: 10.1242/dev.201120. [DOI] [PubMed] [Google Scholar]
- Kato M., Tanaka J., Aizawa R., Yajima-Himuro S., Seki T., Tanaka K., Yamada A., Ogawa M., Kamijo R., Tsuji T., et al. Visualization of junctional epithelial cell replacement by oral gingival epithelial cells over a life time and after gingivectomy. Sci. Rep. 2019;9:7640. doi: 10.1038/s41598-019-44065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Seymen F., Lee K.E., Ko J., Yildirim M., Tuna E.B., Gencay K., Shin T.J., Kyun H.K., Simmer J.P., Hu J.C.C. LAMB3 mutations causing autosomal-dominant amelogenesis imperfecta. J. Dent. Res. 2013;92:899–904. doi: 10.1177/0022034513502054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Zhang H., Seymen F., Koruyucu M., Hu Y., Kang J., Kim Y.J., Ikeda A., Kasimoglu Y., Bayram M., et al. Mutations in RELT cause autosomal recessive amelogenesis imperfecta. Clin. Genet. 2019;95:375–383. doi: 10.1111/cge.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyoshima T., Fujiwara H., Nagata K., Wada H., Ookuma Y.F., Shiotsuka M., Kihara M., Hasegawa K., Someya H., Sakai H. Induction of dental epithelial cell differentiation marker gene expression in non-odontogenic human keratinocytes by transfection with thymosin beta 4. Stem Cell Res. 2014;12:309–322. doi: 10.1016/j.scr.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Lambrichts I., Creemers J., Van Steenberghe D. Periodontal neural endings intimately relate to epithelial rests of Malassez in humans. A light and electron microscope study. J. Anat. 1993;182:153–162. [PMC free article] [PubMed] [Google Scholar]
- Martens W., Sanen K., Georgiou M., Struys T., Bronckaers A., Ameloot M., Phillips J., Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. Faseb. J. 2014;28:1634–1643. doi: 10.1096/fj.13-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao X., Niibe K., Fu Y., Zhang M., Nattasit P., Ohori-Morita Y., Nakamura T., Jiang X., Egusa H. Epiprofin transcriptional activation promotes ameloblast induction from mouse induced pluripotent stem cells via the BMP-smad signaling Axis. Front. Bioeng. Biotechnol. 2022;10:890882. doi: 10.3389/fbioe.2022.890882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao X., Niibe K., Zhang M., Liu Z., Nattasit P., Ohori-Morita Y., Nakamura T., Jiang X., Egusa H. Stage-specific role of amelx activation in stepwise ameloblast induction from mouse induced pluripotent stem cells. Int. J. Mol. Sci. 2021;22:7195. doi: 10.3390/ijms22137195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt P., Wazen R.M., Dos Santos Neves J., Nanci A. Characterisation of secretory calcium-binding phosphoprotein-proline-glutamine-rich 1: a novel basal lamina component expressed at cell-tooth interfaces. Cell Tissue Res. 2014;358:843–855. doi: 10.1007/s00441-014-1989-3. [DOI] [PubMed] [Google Scholar]
- Nakao K., Morita R., Saji Y., Ishida K., Tomita Y., Ogawa M., Saitoh M., Tomooka Y., Tsuji T. The development of a bioengineered organ germ method. Nat. Methods. 2007;4:227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- Naveau A., Zhang B., Meng B., Sutherland M.T., Prochazkova M., Wen T., Marangoni P., Jones K.B., Cox T.C., Ganss B., et al. Isl1 controls patterning and mineralization of enamel in the continuously renewing mouse incisor. J. Bone Miner. Res. 2017;32:2219–2231. doi: 10.1002/jbmr.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan J., Bitu C.C., Daly S.B., Urquhart J.E., Barron M.J., Bhaskar S.S., Martelli-Júnior H., dos Santos Neto P.E., Mansilla M.A., Murray J.C., et al. Whole-Exome sequencing identifies FAM20A mutations as a cause of amelogenesis imperfecta and gingival hyperplasia syndrome. Am. J. Hum. Genet. 2011;88:616–620. doi: 10.1016/j.ajhg.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine M.L., Wang H.J., Luo W., Krebsbach P.H., Snead M.L. A transgenic animal model resembling amelogenesis imperfecta related to ameloblastin overexpression. J. Biol. Chem. 2003;278:19447–19452. doi: 10.1074/jbc.M300445200. [DOI] [PubMed] [Google Scholar]
- Pugach M.K., Gibson C.W. Analysis of enamel development using murine model systems: approaches and limitations. Front. Physiol. 2014;5:313. doi: 10.3389/fphys.2014.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings T.M., Makwana K., Taylor D.M., Molè M.A., Fishwick K.J., Tryfonos M., Odendaal J., Hawkes A., Zernicka-Goetz M., Hartshorne G.M., et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. Elife. 2021;10:e69603. doi: 10.7554/eLife.69603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J.A., Fitzgibbon D.H., Macchiarulo P.A., Murphy R.A. Epidermal growth factor-induced precocious incisor eruption is associated with decreased tooth size. Dev. Biol. 1987;121:247–252. doi: 10.1016/0012-1606(87)90156-4. [DOI] [PubMed] [Google Scholar]
- Saito K., Michon F., Yamada A., Inuzuka H., Yamaguchi S., Fukumoto E., Yoshizaki K., Nakamura T., Arakaki M., Chiba Y., et al. Sox21 regulates Anapc10 expression and determines the fate of ectodermal organ. iScience. 2020;23:101329. doi: 10.1016/j.isci.2020.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Navarro M., Delgado I., Torres M., Mustonen T., Michon F., Rice D.P. Dental epithelial stem cells express the development regulator Meis1. Front. Physiol. 2019;10:249. doi: 10.3389/fphys.2019.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J., Simanian E.J., Tuggy S.Y., Bartlett J.D., Snead M.L., Sugiyama T., Paine M.L. Comparison of two mouse ameloblast-like cell lines for enamel-specific gene expression. Front. Physiol. 2014;5:277. doi: 10.3389/fphys.2014.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.E., Warshawsky H. Quantitative analysis of cell turnover in the enamel organ of the rat incisor. Evidence for ameloblast death immediately after enamel matrix secretion. Anat. Rec. 1977;187:63–98. doi: 10.1002/ar.1091870106. [DOI] [PubMed] [Google Scholar]
- Tsutsui T.W. Dental pulp stem cells: advances to applications. Stem Cells Cloning. 2020;13:33–42. doi: 10.2147/SCCAA.S166759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos K.R., Arid J., Evangelista S., Oliveira S., Dutra A.L., Silva L.A.B., Segato R.A.B., Vieira A.R., Nelson-Filho P., Küchler E.C. MMP13 contributes to dental caries associated with developmental defects of enamel. Caries Res. 2019;53:441–446. doi: 10.1159/000496372. [DOI] [PubMed] [Google Scholar]
- Wazen R.M., Viegas-Costa L.C., Fouillen A., Moffatt P., Adair-Kirk T.L., Senior R.M., Nanci A. Laminin γ2 knockout mice rescued with the human protein exhibit enamel maturation defects. Matrix Biol. 2016;52–54:207–218. doi: 10.1016/j.matbio.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Wee P., Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers. 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welborn V.V. Enamel synthesis explained. Proc. Natl. Acad. Sci. USA. 2020;117:21847–21848. doi: 10.1073/pnas.2014394117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Liu C., Zhang H., Jani P.H., Lu Y., Wang X., Zhang B., Qin C. Abrogation of epithelial BMP2 and BMP4 causes Amelogenesis Imperfecta by reducing MMP20 and KLK4 expression. Sci. Rep. 2016;6:25364. doi: 10.1038/srep25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima-Himuro S., Oshima M., Yamamoto G., Ogawa M., Furuya M., Tanaka J., Nishii K., Mishima K., Tachikawa T., Tsuji T., Yamamoto M. The junctional epithelium originates from the odontogenic epithelium of an erupted tooth. Sci. Rep. 2014;4:4867. doi: 10.1038/srep04867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq and scRNA-seq data have been deposited to the ArrayExpress database (accession numbers E-MTAB-12557 [https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-12557?accession=E-MTAB-12557] and E-MTAB-12544 [https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-12544?accession=E-MTAB-12544]).