Abstract
Ferroptosis is a form of regulated cell death triggered by the iron‐dependent peroxidation of phospholipids. Interactions of iron and lipid metabolism factors jointly promote ferroptosis. Ferroptosis has been demonstrated to be involved in the development of various diseases, such as tumors and degenerative diseases (e.g., aortic dissection), and targeting ferroptosis is expected to be an effective strategy for the treatment of these diseases. Recent studies have shown that the regulation of ferroptosis is affected by multiple mechanisms, including genetics, epigenetics, posttranscriptional modifications, and protein posttranslational modifications. Epigenetic changes have garnered considerable attention due to their importance in regulating biological processes and potential druggability. There have been many studies on the epigenetic regulation of ferroptosis, including histone modifications (e.g., histone acetylation and methylation), DNA methylation, and noncoding RNAs (e.g., miRNAs, circRNAs, and lncRNAs). In this review, we summarize recent advances in research on the epigenetic mechanisms involved in ferroptosis, with a description of RNA N6‐methyladenosine (m6A) methylation included, and the importance of epigenetic regulation in biological processes and ferroptosis‐related diseases, which provides reference for the clinical application of epigenetic regulators in the treatment of related diseases by targeting ferroptosis.
Keywords: DNA methylation, epigenetics, ferroptosis, histone modifications, noncoding RNA, RNA m6A methylation
Ferroptosis is a form of iron‐dependent regulated cell death driven by lipid peroxidation. Current studies have revealed that iron metabolism, lipid metabolism, and redox system jointly regulate ferroptosis. In this review, we highlight epigenetic mechanisms including histone modifications, DNA methylation, noncoding RNAs, and RNA m6A modifications in ferroptosis regulation, which provides potential therapeutic targets for ferroptosis‐related diseases.
1. INTRODUCTION
Ferroptosis is a type of programmed cell death that was discovered in 2012. 1 Different from apoptosis, necrosis, and autophagy, ferroptosis is driven by extensive iron‐dependent lipid peroxidation. Ferroptosis is closely related to cellular metabolism. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Disorders of iron metabolism and lipid metabolism can disrupt the balance between the production and degradation of intracellular reactive oxygen species (ROS), causing ROS accumulation and resulting in cell death. Additionally, studies have pointed out that the occurrence of tumors is often accompanied by the inhibition of ferroptosis, which suggests that multiple proto‐oncogene and tumor suppressor pathways interact to jointly regulate ferroptosis. 10 , 11 , 12 , 13 , 14
Epigenetic regulation refers to a model of regulation that can affect gene expression or activity but does not involve changes to the DNA sequence. 15 It is involved in the regulation of gene expression through the mediation of transcriptional and posttranscriptional processes. Histone modifications, DNA methylation, and certain noncoding RNAs (ncRNAs) are mainly involved in transcriptional regulation, while microRNAs (miRNAs) are mainly involved in the regulation of posttranscriptional processes. 15 During epigenetic regulation, four types of regulators “writers,” “erasers,” and “readers,” which are critical for the addition, removal and recognition of epigenetic marks, respectively, and “remodelers,” which moderate the chromatin state, make epigenetic modifications dynamic and reversible. 16 Increasing evidence suggests that the expression of ferroptosis‐related genes (FRGs) is regulated not only by canonical signaling pathways but also by epigenetic mechanisms. 17 This review summarizes the progress of ferroptosis study focusing on the epigenetic regulatory mechanisms such as histone modifications, DNA methylation, ncRNAs, and RNA m6A modification, highlighting the role of epigenetic regulators in biological processes and ferroptosis‐related diseases therapy (Figure 1).
FIGURE 1.
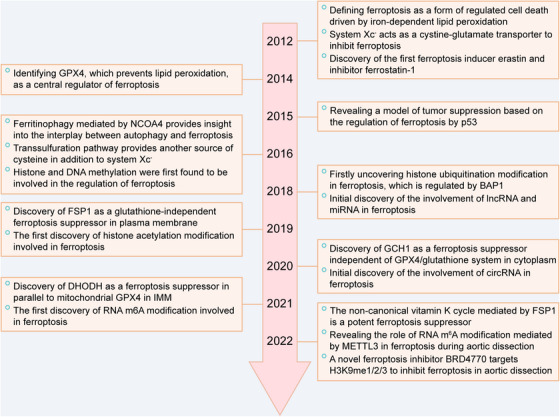
Key discoveries in ferroptosis‐related research. Key molecules related to ferroptosis discovered in different years and the first appearance of different epigenetic modifications targeting ferroptosis. BAP1, BRCA1‐associated protein 1; DHODH, dihydroorotate dehydrogenase; FSP1, ferroptosis suppressor protein 1; GCH1, GTP cyclohydrolase 1; GPX4, glutathione peroxidase 4; METTL3, methyltransferase 3; NCOA4, nuclear receptor coactivator 4; IMM, inner mitochondrial membrane.
2. THE BASIS OF FERROPTOSIS
As a form of novel programmed cell death, the execution of ferroptosis is driven by iron‐dependent lipid peroxidation. The core events leading to cell death in ferroptosis are considered to be the accumulation of lipid peroxides and iron‐dependent ROS. In this part, the focus is on cellular metabolism, including iron and lipid metabolism, as well as some classical signaling pathways related to ferroptosis.
2.1. Iron metabolism during ferroptosis
Ferroptosis is an iron‐dependent form of programmed cell death. Iron molecules with redox activity in cells constitute the “labile iron pool (LIP)” and is called “ferrous iron (Fe2+)”, 18 and their accumulation is a necessary condition of ferroptosis. Mechanistically, the LIP catalyzes the formation of hydroxyl radicals and hydroxides from hydrogen peroxide via the nonenzymatic Fenton reaction. Iron chelators such as dexrazoxane, deferoxamine, and ciclopirox inhibit ferroptosis by binding Fe2+. 19 , 20 In addition to the Fenton reaction, Fe2+ participates in the production of ROS, serving as the cofactor of iron‐dependent enzymes, which are required for the lipid peroxidation in ferroptosis. 3 Therefore, we deduce that the sources, transport mechanisms, storage modalities, and utilization of iron all affect the ferroptosis sensitivity of cells.
Iron is found as two ionic forms in cells: ferrous (Fe2+) and ferric (Fe3+). Endogenous iron is derived from hemoglobin iron (Fe2+) released by aging red blood cells. Heme oxygenase 1 (HMOX1) catalyzes the release of Fe2+ from heme, which provides a major intracellular source of iron, and maintains cellular iron homeostasis. 21 Overexpression of HMOX1 can induce ferroptosis by the accumulation of Fe2+ released from heme, 19 , 22 which indicates that there is a beneficial threshold of HMOX1 expression. Iron derived from food takes two forms: nonheme iron (Fe3+), which needs to be reduced to Fe2+ to be absorbed in the body, and heme iron (Fe2+), which can be directly absorbed by intestinal mucosal cells without being complexing cofactors. Ammonium ferric citrate and hemin promote erastin/FINO2‐induced ferroptosis by increasing the sources of iron. 22 , 23 Regarding the transport of iron, transferrin (TF) binds to Fe3+, which is in nontoxic form, and induces ferroptosis via its iron‐loading capacity. 4 Moreover, lactotransferrin (LTF), an iron‐binding transport protein, acts like TF to promote ferroptosis by directly increasing iron intake. 24 However, a recent cell experiment revealed that the lipogenic regulator SREBP2 inhibited ferroptosis by increasing TF levels in circulating melanoma cells. 25 A similar result of an animal experiment has also been reported. In Tf‐knockout mice, exogenous TF protected against ferroptosis induced by a high‐iron diet. 26 These paradoxical results indicated that the role of TF in ferroptosis might depend on the balance between TF‐bound iron and non‐TF‐bound iron. Ferritin is the main storage form of intracellular iron. Evidence shows that hypoxia increases ferritin‐based storage of Fe3+ and reduces the LIP, which leads to greater resistance to ferroptosis. 27 In contrast, cellular processes that reduce ferritins, such as autophagy, release Fe2+, which is the substrate of the Fenton reaction, increasing cell sensitivity to ferroptosis. 5 , 28 This selective form of autophagy targeting ferritin is named as ferritinophagy, which is mediated by nuclear receptor coactivator 4 (NCOA4). 29 In this process, NCOA4 acts as a selective cargo receptor to bind with ferritin and delivers it for lysosomal degradation, finally leading to ferroptosis by the increase of LIP. The novel ferroptosis inhibitor 9a exactly targets NCOA4 to disrupt the interaction between NCOA4 and ferritin, reducing intracellular Fe2+. 30 Additionally, the roles of TF and ferritin are not isolated. The link between TF and ferritin depends on a membrane protein named transferrin receptor 1 (TFR1). Extracellular iron binds with TF, and accumulate within cells in the form of ferritin via TFR1. 31 Obviously, regulation which targets TFR1 has been associated with ferroptosis. Specifically, TFR1 enhanced ferroptosis induced by ferritinophagy, and in erastin‐treated wild‐type fibroblasts, ferritinophagy led to enhanced TFR1 expression. 28 In addition, HUW1, a ubiquitin E3 ligase, has been confirmed to be a newly discovered inhibitor of ferroptosis by targeting the degradation of TFR1 (Figure 2). 32 As previously mentioned, iron is an important cofactor. In ferroptosis, iron is a cofactor of lipoxygenase (LOX) and cytochrome P450 oxidoreductase (POR), which have been identified as iron‐dependent enzymes that drive ferroptosis. 3 Notably, recent study suggested that POR is more likely than LOX to play a dominant role in ferroptosis. 2
FIGURE 2.
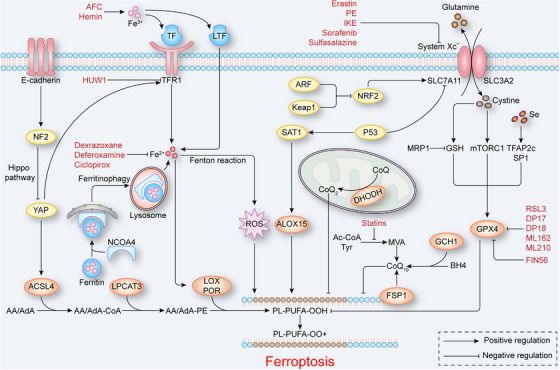
The core mechanisms of ferroptosis. TF, LTF, TFR1, and ferritin are critical for maintaining the balance of intracellular ferrous and regulating the production of ROS and the activity of LOX and POR. System Xc− mediates cysteine intake, GSH synthesis and GPX4 activation, inhibiting lipid peroxidation. Simultaneously, FSP1 and BH4 promote the production of CoQ10, and DHODH promotes the production of CoQ2, and they both protect cells from ferroptosis. P53 promotes and NRF2 inhibits ferroptosis by regulating the expression of SLC7A11, and E‐cadherin inhibits the expression of TFR1 and ACSL4 through the NF2‐YAP signaling pathway to inhibit ferroptosis. ACSL4 catalyzes AA/AdA to generate AA/AdA‐CoA. Subsequently, LPCAT3 catalyzes AA/AdA‐CoA to generate AA/AdA‐PE. Finally, LOX and POR catalyze AA/AdA PE to generate PL‐PUFA‐OOH and induce ferroptosis. The red text indicates ferroptosis regulators. AA/AdA, arachidonic acid/adrenic acid; ACSL4, acyl‐CoA synthetase long‐chain family member 4; BH4, tetrahydrobiopterin; CoA, coenzyme A; CoQ2, coenzyme Q2; CoQ10, coenzyme Q10; DHODH, dihydroorotate dehydrogenase; FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; GSH, glutathione; LOX, lipoxygenase; LPCAT3, lysophosphatidylcholine acyltransferase 3; LTF, lactotransferrin; NF2, neurofibromin 2; NRF2, nuclear factor erythroid 2‐related factor 2; PE, phosphatidylethanolamine; PL‐PUFA‐OOH, oxidized PUFA‐containing phospholipids; POR, cytochrome P450 oxidoreductase; ROS, reactive oxygen species; TF, transferrin; TFR1, transferrin receptor 1; YAP, yes‐associated protein 1.
In conclusion, the imbalance of cellular iron homeostasis, especially the accumulation of intracellular Fe2+, can induce ferroptosis. Therefore, reducing the concentration of intracellular Fe2+ can effectively inhibit ferroptosis, and maintaining iron homeostasis can be a potential therapeutic strategy to prevent or treat ferroptosis‐related diseases.
2.2. Lipid metabolism during ferroptosis
It has been reported that ferroptosis proceeds in two steps: initiation and amplification. Initiation, which involves the selection of substrates, involvement of enzymes and disruption of antioxidant systems, depends on the lipid peroxides produced due to LOX and POR action, whereas amplification, mediated by the LIP, depends on the spread of peroxides across the membrane. 33 Here we introduce lipid metabolism mainly in the context of ferroptosis initiation.
With respect to substrates, polyunsaturated fatty acids (PUFAs) have been identified as the lipids most prone to peroxidation during ferroptosis due to their bis‐allylic sites. 34 Moreover, monounsaturated fatty acids (MUFAs) do not readily undergo peroxidation due to the lack of bis‐allylic sites and have been demonstrated to be ferroptosis inhibitors. 34 Ultimately, acyl‐CoA synthetase long‐chain family member 3 (ACSL3) and stearoyl‐CoA desaturase 1 (SCD1) increase cellular resistance to ferroptosis in a MUFA‐dependent manner. 35 , 36 Further study has shown that phosphatidylethanolamine (PE) with arachidonic acid (AA) or adrenic acid (AdA) chains is the most critical substrate involved in ferroptosis. 37 In addition, oxidized PUFA‐containing phospholipids (PL‐PUFA‐OOH), not free oxidized PUFAs (PUFA‐OOH), cause ferroptosis, 37 which means that free PUFAs must be esterified to form membrane phospholipids. This process requires the participation of enzymes such as ACSL4 and lysophosphatidylcholine acyltransferase 3 (LPCAT3). 38 ACSL4 catalyzes the formation of AA/AdA‐CoA to drive free PUFAs into a readily oxidizable state, and LPCAT3 then catalyzes phospholipid remodeling to form AA/AdA‐PE. 39
The antioxidant systems are key to protect cells from ferroptosis, and its disruption leads to the accumulation of peroxidized phospholipids, which in turn causes ferroptosis. GPX4, a key antioxidant in ferroptosis, can reduce the formation of phospholipid hydroperoxides and thus has been identified as an inhibitor of ferroptosis. 6 Obviously, targeting GPX4 effectively regulates ferroptosis. Some small‐molecule compounds such as RSL3, DP17, DP18, ML162, and ML210, have been identified as ferroptosis inducers because they directly or indirectly inhibit the activity of GPX4. 40 GPX4 is essentially a selenoprotein whose molecular structure contains selenocysteine, in which the sulfur in cysteine is replaced with selenium. The utilization of selenocysteine by GPX4 increases its antiferroptic effect. 41 Thus, the regulation of ferroptosis by selenium is clear. Studies have shown that selenium supplementation can effectively increase ferroptosis resistance in cell and animal models. 41 , 42 At the transcription level, selenium may increase the expression of GPX4 in neurons by coactivating the transcription factors TFAP2c and SP1. 42 In addition to selenium, GSH, a substrate that provides reducing equivalents for GPX4, is integral to the antioxidant effect of GPX4. 43 Clearly, GSH deficiency increases ferroptosis susceptibility. A genome screen performed for identifying regulators of ferroptosis found that multidrug resistance protein 1 (MRP1), which mediates GSH efflux, increased the sensitivity of tumor cells to ferroptosis by pumping out GSH. 44 GSH is synthesized from cysteine, 45 which can be derived from glutamate‐cystine transport system Xc− or transsulfuration cysteine biosynthesis pathway. System Xc−, which is composed of a light chain subunit encoded by SLC7A11 and a heavy chain subunit encoded by SLC3A2, exchanges extracellular cystine with intracellular glutamate and cystine is reduced to cysteine to promote the synthesis of GSH. 46 Indeed, the first ferroptosis inducer to be identified, erastin, was found to mainly inhibit system Xc−, resulting in the depletion of GSH and a reduction in GPX4 activity. 1 Additional system Xc− inhibitors have subsequently discovered, such as piperazine erastin, imidazole ketone erastin, sorafenib, and sulfasalazine. 40 In addition to promoting GSH synthesis, system Xc−‐mediated cystine transport promotes GPX4 synthesis. Mechanistically, mechanistic target of rapamycin complex 1 (mTORC1) couples cystine uptake with GPX4 synthesis, and cystine may facilitate GPX4 synthesis partly through the Rag‐mTORC1‐4EBP signaling axis in a GSH‐independent way. 47 However, the mechanism through which cystine recognizes mTORC1 remains to be further investigated. As another source of cysteine, transsulfuration cysteine biosynthesis pathway is an alternative antioxidant process when system Xc− is inhibited. There are two key enzymes cystathionine β‐synthase (CBS) and cystathionine γ‐lyase (CTH) in the pathway. CBS catalyzes the synthesis of cystathionine with methionine cycle intermediate homocysteine as the substrate, and then cystathionine can be cleaved by CTH to release cysteine. 48 In prolonged erastin‐treated cells, transsulfuration cysteine biosynthesis pathway was found to be continuously activated since the upregulation of CBS, which confers erastin‐induced ferroptosis resistance. 49 Additionally, the knockdown of cysteinyl tRNA synthetase 1 (CARS), an enzyme that links cysteine with tRNAs for protein translation, inhibits erastin‐induced ferroptosis by the upregulation of transsulfuration genes including CBS, CTH, PSAT1, and PSPH. 50
In ferroptosis in addition to GPX4, nonmitochondrial coenzyme Q10 (CoQ10), a major antioxidant system inhibits lipid peroxidation by trapping radical intermediates. CoQ10 synthesized in vivo is derived from mevalonate (MVA) generated by tyrosine and acetyl‐CoA through a series of enzymatic reactions. Notably, the synthesis of MVA directly affects the antioxidant capacity of CoQ10. Statins, which reduce MVA production by inhibiting HMG‐CoA reductase, sensitizes cells to FIN56, a ferroptosis inducer that promotes GPX4 degradation. 7 In addition, a recent study has shown that inhibition of the MVA pathway by statins inactivated GPX4 to induce ferroptosis. 51 This evidence shows that the induction of ferroptosis by statins includes the involvement of both CoQ10 and GPX4; therefore, further exploration into whether their effects are mutual or independent is necessary. Studies have shown that ferroptosis suppressor protein 1 (FSP1), which is also called apoptosis‐inducing factor mitochondria‐associated 2, prevent GPX4‐deficient cells from ferroptosis by acting as an NADPH‐dependent CoQ oxidoreductase to regenerate reduced CoQ10, 8 , 9 which suggests that regulation of ferroptosis by CoQ10 is independent of GPX4. Therefore, the NADPH–FSP1–CoQ10 ferroptosis pathway was identified as a parallel system to the GSH–GPX4 pathway that regulates ferroptosis. Moreover, a recent study found that noncanonical vitamin K acts as a potential ferroptosis inhibitor through its reducing form hydroquinone (VKH2). 52 Since the similar structure properties between vitamin K and CoQ10, FSP1 was also found to act as a vitamin K reductase. However, in FSP1‐KO cells, high dose of phylloquinone (vitamin K1) and menaquinone‐4 (vitamin K3) can still prevent ferroptosis, which indicates the mechanism of vitamin K cycle preventing ferroptosis still needs further study. 52 In addition, a recent antagonistic ferroptosis gene screening revealed another independent ferroptosis inhibition pathway, the GCH1–BH4 pathway. GTP cyclohydrolase 1 (GCH1) is the rate‐limiting enzyme of tetrahydrobiopterin/dihydrobiopterin (BH4/BH2) production, and BH4 promotes the production of reduced CoQ10 and selectively inhibits lipid peroxidation. 53 Notably, as the first enzyme that catalyzes CoQ10 biosynthesis, ferroptosis induced by CoQ2 deletion was not reversed by FSP1, 9 but dihydroorotate dehydrogenase (DHODH) in the mitochondrial inner membrane inhibited ferroptosis through this pathway. Mechanistically, DHODH couples the oxidation of DHO with the reduction of CoQ and generates CoQ2 in the mitochondrial inner membrane 54 (Figure 2). Notably, it was found that mitochondrial plays a critical role in cysteine deprivation‐induced ferroptosis, but not in GPX4 inhibition‐induced ferroptosis, 55 which suggests that the cellular protective system against ferroptosis mediated by DHODH in the mitochondrial inner membrane is independent of GPX4.
In general, the antioxidant system involved in lipid metabolism can be categorized into at least six pathways, namely, the system Xc−–GPX4, MVA–CoQ10, FSP1‐reduced CoQ10, FSP1–VKH2, GCH1–BH4, and DHODH–CoQ2 pathways. Many epigenetic mechanisms regulating these pathways have been elucidated, 56 which are discussed in depth in subsequent subsections.
2.3. The signaling pathways in ferroptosis
Inhibiting tumor growth has been shown to be a physiological function of ferroptosis. Several oncogenic and tumor suppressor pathways are involved in ferroptosis. In these pathways, SLC7A11 is considered a central link. The expression of SLC7A11 is regulated by different upstream molecules, such as p53 and NRF2.
The tumor suppressor protein p53 represses the transcription of SLC7A11, 10 and this effect is affected by p53 protein acetylation modification. A study demonstrated that the acetylation‐defective mutant p533kR (K117R+K161R+K162R) retains the ability to regulate ferroptosis by downregulating SLC7A11 unless the fourth acetylation site K98R is mutated along with other lysine sites. 57 In addition to affecting GPX4 activity or synthesis through the p53–SLC7A11 axis, downregulation of SLC7A11 by p53 indirectly activates arachidonate 12‐lipooxygenase (ALOX12), which is an ACSL4‐independent pathway. 14 In addition to SLC7A11, p53 regulates other downstream targets. For example, p53 activates the spermidine/spermine N1‐acetyltransferase 1 (SAT1) gene, which induces lipid peroxidation to induce ferroptosis. In this process, the expression level of arachidonate 15‐lipoxygenase (ALOX15) is correlated with SAT1 induction. 58 Additionally, p53 promotes the expression of glutaminase 2 (GLS2) gene, which encodes a mitochondrial GLS to produce glutamate from glutamine. 59 With increasing production of glutamate, GLS2 leads to enhanced mitochondrial respiration and ATP generation by regulating energy metabolism. Ultimately, mitochondrial GLS2, but not cytosolic GLS1, has been confirmed to induce glutaminolysis‐associated ferroptosis. 4 , 60 The above evidence supports the promoting effect of p53 on ferroptosis. However, p53 can also inhibit ferroptosis in a transcription‐independent way. In colorectal cancer, depletion of p53 prevents nuclear accumulation of dipeptidyl‐peptidase‐4 (DPP4), which promotes DPP4‐dependent lipid peroxidation to result in ferroptosis. 61 The inhibiting effect of p53 on ferroptosis may also be attributed to the calcium‐independent phospholipase iPLA2β. As a direct target of p53, iPLA2β‐mediated lipid detoxification is critical for inhibiting ROS‐induced ferroptosis. Interestingly, iPLA2β is differentially regulated by p53. Under low levels of stress, p53 can active the expression of iPLA2β, but this activation is diminished under high levels of stress. 62 Overall, p53 plays a dual role in ferroptosis, which needs further study to find out whether cell environment or cell type determines the regulatory effect of p53 on ferroptosis.
In contrast to p53, nuclear factor erythroid 2‐related factor 2 (NRF2) promotes the expression of SLC7A11. 63 Two upstream molecules of NRF2, kelch‐like ECH‐associated protein 1 (KEAP1) and alternative reading‐frame protein (ARF), have also been found to be associated with ferroptosis. The tumor suppressor KEAP1 binds to NRF2 in the cytoplasm and degrades NRF2 via ubiquitination. Under oxidative stress, NRF2 dissociates from KEAP1, is translocated to the nucleus, where it binds to the antioxidant response element to activate the transcription of downstream genes, 11 , 64 such as SLC7A11. ARF regulates NRF2 in a KEAP1‐independent manner and inhibits CBP‐dependent NRF2 acetylation to inhibit NRF2 transcriptional activity, ultimately promoting ferroptosis. 12
Both p53 and NRF2 mediate intracellular signal transduction. Moreover, a ferroptosis‐regulating pathway that depends on intercellular signaling has been identified in epithelial cells. Intercellular communication is mediated by E‐cadherin, which activates the intracellular Hippo pathway to negatively regulate the proto‐oncogenic transcriptional coactivator YAP. The NF2‐YAP pathway ultimately downregulates the expression of ACSL4 and TFR to inhibit ferroptosis (Figure 2). 13 Mesenchymal or metastatic cancer cells have been shown to be sensitive to ferroptosis‐inducing agents, 65 possibly because of decreased cadherin activity during the epithelial–mesenchymal transition (EMT).
In conclusion, iron metabolism, lipid metabolism, and tumor‐related signaling pathways interact with each other during ferroptosis, and lipid metabolism can be regarded as the core link among these pathways. Perturbed iron metabolism disrupts the dynamic balance of ROS generation and elimination, which in turn causes lipid oxidative stress. Fe2+ participates in this stress‐including process as cofactors for enzymes such as LOX and POR. In addition to regulating antioxidant systems such as GPX4 and system Xc−, tumor‐related signaling pathways affect the activity of enzymes required for lipid peroxidation such as ALOX12. The formation of lipid peroxides on the membrane is a result of lipid metabolism in ferroptosis; however, the specific mechanism of ferroptosis induced by membrane lipid peroxides needs to be further studied.
3. EPIGENETIC REGULATION IN FERROPTOSIS
Epigenetics refers to heritable changes in gene function that ultimately lead to phenotypic changes without changes in the DNA sequence. Increasing evidence suggest that epigenetic regulation affects ferroptosis through gene transcription, posttranscription, or posttranslation processes, and targeting epigenetic mechanisms in ferroptosis is expected to provide a new direction for the treatment of ferroptosis‐related diseases. In this context, we will focus on the mechanisms of epigenetic regulation including histone modifications, DNA methylation, ncRNAs, and RNA modifications in ferroptosis.
3.1. Histone modifications in ferroptosis
Histone modifications regulate gene expression by altering the structural state of chromatin, and they include acetylation, methylation, phosphorylation, adenylation, and ubiquitination. Here, we mainly describe the regulatory mechanisms of acetylation, methylation and ubiquitination in ferroptosis.
3.1.1. Histone acetylation in ferroptosis
Histone acetylation neutralizes its positive charge and impairs the ability of histones to bind DNA, which leads to depolymerized nucleosomes and the activation of gene transcriptional. 66 , 67 Histone acetylation depends on the bromodomain‐containing protein (BRD) family, histone acetyltransferases (HATs), and histone deacetylases (HDACs). The BRD family recognizes acetylation marks. 68 In cancer cells, the BRD4 inhibitor JQ1 induces ferroptosis by enhancing the expression of an HDAC named SIRT1, which decreases the H3K27ac level at upstream of BRD4, ultimately affecting the recognition of acetylation sites on the histones at GPX4 and SLC7A11 genes. 69 Two competing enzymes, HATs and HDACs are critical for regulating histone acetylation. 70 Histone hyperacetylation by HATs is associated with transcriptional activation. For instance, NRF2 recruits P300 and CBP, and P300/CBP‐associated factor (PCAF) may increase H3K9ac level at NRF2 to regulate ferroptosis in renal tubulointerstitial fibrosis. 71 In contrast, ketamine, an inhibitor of lysine acetyltransferase 5 (KAT5), reduces H3K27ac levels at GPX4 promoter regions to promote ferroptosis in breast cancer (Figure 3A). 72 In addition, in liver cancer, two transcription factors, hepatocyte nuclear factor 4 alpha (HNF4A) and HIC ZBTB transcriptional repressor 1 (HIC1), competitively bind with KAT2B, playing opposite roles regulating GSH production; the former has been identified a ferroptosis inhibitor, and the latter has been identified a ferroptosis inducer. 73 Similar to HAT inhibitors, histone deacetylation mediated by HDACs also exhibits transcriptional repression. NAD+‐dependent HDACs such as SIRT1 and SIRT3 trigger ferroptosis induction by inhibiting EMT markers in cancer cells. 65 , 74 Of course, the effect of HDAC is reversed by HDAC inhibitors in the tumor microenvironment. HDAC inhibitors such as BEBT‐908 hyperacetylate p53 to promote ferroptosis signaling. 75 Nevertheless, the regulation of ferroptosis by BEBT‐908 may be affected by other signaling pathways such as the PI3K pathway. Other HDAC inhibitors, such as quisinostat and vorinostat, also induce ferroptosis by inhibiting GPX4 and system Xc− expression respectively; however, their targets are not clear. 76 , 77 Interestingly, the regulation of ferroptosis in different cells may be opposite even after treatment with the same HDAC inhibitors. A study revealed that in neurons and cancer cells with similar erastin‐induced ferroptosis mechanism, class I HDAC inhibitors enhanced ferroptosis in cancer cells, while protecting neurons from ferroptosis. The cell‐specific biological effects of HDAC inhibitors may result from the differential expression of distinct HDAC8 between cancer cells and primary neurons. 78 Therefore, it is necessary to further study the cell‐specific expression mechanism of HDAC, which will help us to understand how histone acetylation modifications precisely regulate ferroptosis.
FIGURE 3.
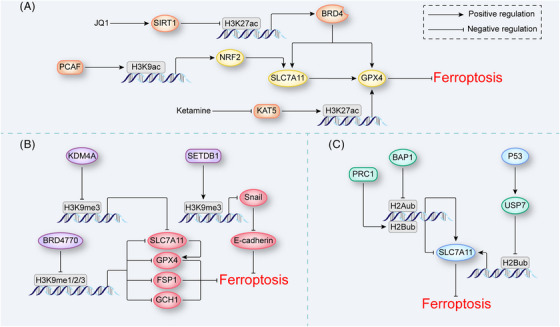
Histone modifications in ferroptosis. (A) Histone acetylation in ferroptosis. JQ1 and ketamine reduce H3K27ac abundance at BRD4 and GPX4 genes to induce ferroptosis, respectively. PCAF increases H3K27ac abundance at NRF2 gene to inhibit ferroptosis. (B) Histone methylation in ferroptosis. KDM4A reduces and SETDB1 increases H3K9me3 abundance at SLC7A11 and Snail genes, respectively, to regulate ferroptosis. BRD4770 reduces H3K9me1/2/3 abundance at SLC7A11, GPX4, FSP1, and GCH1 genes simultaneously to inhibit ferroptosis. (C) Histone ubiquitination in ferroptosis. PRC1 increases and BAP1 reduces the H2Aub abundance at SLC7A11 gene, both proteins induce ferroptosis. P53 increases the expression of USP7, which reduces H2Bub abundance at SLC7A11 gene to induce ferroptosis. BAP1, BRCA1‐associated protein 1; BRD4, bromodomain‐containing protein 4; FSP1, ferroptosis suppressor protein 1; GCH1, GTP cyclohydrolase 1; GPX4, glutathione peroxidase 4; KDM4A, lysine demethylase 4A; NRF2, nuclear factor erythroid 2‐related factor 2; PCAF, P300/CBP‐associated factor; PRC1, protein regulator of cytokinesis 1; SETDB1, SET domain bifurcated 1; USP7, ubiquitin‐specific peptidase 7.
3.1.2. Histone methylation in ferroptosis
Another important form of histone modification, histone methylation, mainly involves modification of an N‐terminal lysine (K) or arginine (R) residue in the H3 or H4 histone. 79 , 80 , 81 , 82 Generally, different methylation sites and the number of methyl groups on H3 and H4 indicate different biological functions. Among these modifications, H3K4me1/2/3, H3K36me1/2/3, and H3K79me1/2/3 usually mediate transcriptional activation, while H3K9me3, H3K27me3, and H4K20me2/3 usually repress transcription. 82 , 83
The histone methylation modifications related to ferroptosis mainly involve methylation of H3K4 and H3K9 catalyzed by histone methyltransferases (HMTs). 84 Regarding the methylation of H3K4, a study found that GPX4 was more highly expressed in tumor cells than in normal cells partially because of the increased abundance of H3K4me3 at the promoter of GPX4. 85 Additionally, in gastric cancer, methionine adenosyltransferase 2A (MAT2A) promoted the production of the methylation donor SAM, which upregulated ACSL3 by increasing H3K4me3 abundance at the promoter, sequentially inhibiting ferroptosis. 86 In addition, methylation of H3K4 indirectly regulates ferroptosis by affecting the recognition of histone acetylation sites. JQ1 inhibited the expression of an HMT named G9a, reduced H3K4me3 abundance at BRD4, and further induced ferroptosis in cancer cells. 69 These results suggested that the H3K4me3 modification inhibits ferroptosis in cancer cells. Reasonably, one can assume that the methylation of H3K9 induces ferroptosis, and this outcome has also been confirmed to some extent. In breast cancer, mucin1‐C (MUC1‐C), a transmembrane oncoprotein, bound with the CD44 variant to stabilize the SLC7A11 molecule. In turn, H3K9me2/3 at the MUC1‐C promoter inhibited MUC1‐C gene transcription, which influenced the ability of GPX4 to induce ferroptosis. 87 Similar results have been shown not only in tumors but also in progressive diseases. In a pulmonary fibrosis model, SET domain bifurcated 1 (SETDB1) indirectly induced E‐cadherin expression by increasing H3K9me3 abundance at the Snail promoter, which in turn enhanced TGF‐β‐induced ferroptosis. 88 Experiments related to histone demethylases, which are specifically critical for removing methyl groups from modified histones, have further proven this inference. For example, lysine demethylase 4A (KDM4A) protected against ferroptosis by decreasing H3K9me3 abundance at the SLC7A11 promoter to upregulate the expression of SLC7A11 in osteosarcoma. 89
These studies on histone methylation were conducted mostly in the context of cancer and degenerative diseases, which have been shown to be closely affected by ferroptosis. Interestingly, the findings of our group and others have suggested that multiple forms of regulated cell death are involved in the development of aortic dissection (AD), and histone methylation plays a critical role in these biological processes. 83 , 90 , 91 , 92 , 93 , 94 , 95 More importantly, our recently published results revealed that ferroptosis, in particular, is involved in the development of AD. 96 Furthermore, by screening multiple inhibitors of methyltransferases, we found that BRD4770 negatively regulated ferroptosis in VSMCs by inhibiting the H3K9me1/2/3 modifications. BRD4770 inhibited ferroptosis not only by inducing the expression of classical ferroptosis regulatory genes, such as FSP1, SLC7A11, GPX4, and GCH1 (Figure 3B), but also by inhibiting inflammation‐related genes activation. Moreover, BRD4770 protected against AD development by inhibiting ferroptosis. 96 In agreement with those of other studies, our results confirmed that H3K9me3 promotes ferroptosis. However, in clear cell renal cell carcinoma (ccRCC), suppressor of variegation 3−9 homolog 1 (SUV39H1), an HMT that deposits H3K9me3, has been found to protect cells against ferroptosis. Mechanistically, SUV39H1 targets the promoter of dipeptidlypeptidase‐4 (DDP4) and inhibits the expression of DPP4, which binds to NADPH oxidase 1 (NOX1) to induce lipid peroxidation. 97 It seems that the regulation effects of H3K9me3 on ferroptosis differ in different cells; therefore, it is necessary to explore the factors, such as cell microenvironment, which influence the biological effects of histone methylation modification in different cell lines.
3.1.3. Histone ubiquitination in ferroptosis
In cancer cells, current knowledge suggests that the regulation of ferroptosis by histone ubiquitination mainly involves histone 2A ubiquitination (H2Aub) and histone 2B ubiquitination (H2Bub), both of which have been associated with the expression of SLC7A11. 98 , 99 The level of the tumor suppressor BRCA1‐associated protein 1 (BAP1) decreases and the ubiquitin ligase of H2Aub named PRC1 increases H2Aub occupancy at the SLC7A11 promoter. In theory, in contrast to the regulation of H2Aub by BRP1, regulatory effects induced by PRC1 produces different phenotypes; however, both regulatory proteins repressed SLC7A11 expression. 98 , 100 This unexpected result suggested that H2Aub itself is not the sole mediator of these regulatory effects; in fact, the homeostasis of H2A ubiquitination and deubiquitination influences H2Aub‐mediated target gene expression. In addition, p53 promotes the recruitment of ubiquitin‐specific peptidase 7 (USP7) and leads to a decrease in H2Bub abundance at the SLC7A11 gene regulatory region, decreasing the expression of SLC7A11 in a p53 transcription factor‐independent manner (Figure 3C). 99
In general, histone modifications, such as acetylation, methylation, or ubiquitination, can modulate the susceptibility of cells to ferroptosis by affecting the expression of genes involved in ferroptosis‐related metabolism pathways. However, the effects of histone modifications in ferroptosis can be context dependent, and different patterns of modifications may have opposite or synergistic effects on ferroptotic cells. Further research is needed to fully understand the mechanisms underlying the regulation of ferroptosis by histone modifications.
3.2. DNA methylation in ferroptosis
DNA methylation is a common epigenetic modification in eukaryotic cells. In mammals, DNA methylation usually involves SAM as the methyl group donor and is mainly catalyzed by DNA methyltransferases (DNMTs), such as DNMT1, DNMT3A, and DNMT3B, and the DNA methylation level is always negatively correlated with its expression level. 101
Evidence suggests that DNA methylation regulates ferroptosis by participating in lipid metabolism. During the generation of substrates, a study revealed that overexpression of elongation of very long‐chain fatty acid protein 5 (ELOVL5) and fatty acid desaturases 1 (FADS1) in mesenchymal‐type gastric cancer cells (GCs), which promote the biosynthesis of PUFAs, was downregulated in intestinal‐type GCs due to the methylation of the ELOVL5 and FADS1 promotors. 102 In addition, in lung cancer, lymphoid‐specific helicase (LSH), a protein belonging to the sucrose nonfermenting 2 family of chromatin‐remodeling enzymes, directly modifies DNA methylation with WD repeat domain 76 (WDR76) to activate metabolic genes, including GLUT1, FADS2, and SCD1, inhibiting ferroptosis by decreasing lipid ROS levels. 103 The effect of LSH in this process was antagonized by DDB1‐ and CUL4‐associated factor 8 (DCAF8). 104 DNA methylation also directly regulates the expression of Ferroptosis‐related molecules such as GPX4 and SLC7A11. In rheumatoid arthritis, glycine enhanced SAM‐mediated Gpx4 promoter methylation catalyzed by DNMT1, DNMT3A, and DNMT3B to induce ferroptosis. 105 The DNMT1 inhibitor 6‐thioguanine has also been confirmed to be a ferroptosis inducer in gastric cancer, which may be related to its indirect inactivation of system Xc−. 106 In addition, DNA methylation is involved in the regulation of intercellular interactions in ferroptosis. The DNMT1 inhibitor 5‐aza‐CdR decreased the methylation level of cadherin‐1 (CDH1) in head and neck cancer, increasing E‐cadherin expression and decreasing ferroptosis sensitivity (Figure 4A). 65 These findings clearly demonstrated that DNA methylation actively regulates ferroptosis. Notably, oxidative stress and iron metabolism directly affect DNA methylation levels. 107 , 108 , 109 , 110 A recent study found that chronic iron exposure increased LIP levels in colon cells to promote ferroptosis while reactively triggering demethylation of NRF2 targets such as NOQ1 and GPX2, which are protective factors against ferroptosis and this epigenetic change was time‐dependent and reversible. 111 This finding suggests that DNA methylation and ferroptosis may be mutually causal, and the determination of whether this causal relationship is involved in other FRGs or epigenetic regulatory effects requires further study.
FIGURE 4.
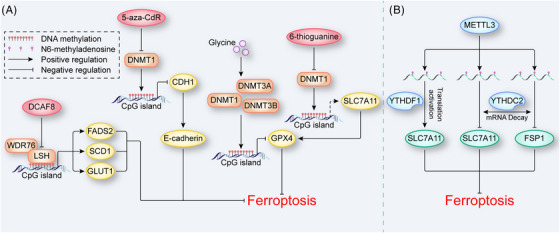
DNA and RNA methylation in ferroptosis. (A) DNA methylation in ferroptosis. LSH interacts with WDR76 to inhibit ferroptosis by regulating DNA methylation, and this effect is antagonized by DCAF8. DNMTs including DNMT1, DNMT3A, and DNMT3B increase the DNA methylation levels of target genes to regulate ferroptosis. In contrast, DNMTs inhibitors, such as 5‐aza‐CdR and 6‐thioguanine, antagonize the biological effects of DNMTs. (B) RNA m6A modification in ferroptosis. METTL3 promotes the m6A modification of SLC7A11 and FSP1 mRNA. YTHDF1 recognizes the m6A marks on SLC7A11 mRNA and increases its translation to inhibit ferroptosis. YTHDC2 recognizes the m6A marks on SLC7A11 and FSP1 mRNA, leading to mRNA decay to induce ferroptosis. The dotted line represents indirect regulation. DCAF8, DDB1‐ and CUL4‐associated factor 8; DNMT, DNA methyltransferase; FSP1, ferroptosis suppressor protein 1; LSH, lymphoid‐specific helicase; METTL3, methyltransferase 3; WDR76, WD repeat domain 76; YTHDC2, YTH domain containing 2; YTHDF1, YTH N6‐methyladenosine RNA binding protein 1.
In summary, DNA methylation plays a critical role in regulating ferroptosis through various mechanisms, including the regulation of lipid metabolism and the modulation of intercellular interactions. Deeply understanding the mechanisms of DNA methylation in ferroptosis is crucial for the development of effective therapies for ferroptosis‐related diseases.
3.3. Noncoding RNAs in ferroptosis
In recent years, increasing number of ncRNAs with biological functions have been discovered. NcRNAs can be classified into two main types: constitutive ncRNAs and regulatory ncRNAs. Constitutive ncRNAs act like housekeeping genes in translation and splicing, and they include ribosomal RNAs, transfer RNAs, and small nuclear RNAs. From an epigenetic perspective, regulatory ncRNAs are more interesting because they are involved in the modification of transcriptional and posttranscriptional processes. Here, we mainly describe the roles of certain regulatory ncRNAs in ferroptosis.
3.3.1. miRNAs in ferroptosis
MiRNAs constitute a class of noncoding single‐stranded RNA molecules approximately 22 nucleotides in length and encoded by endogenous genes. MiRNAs regulate gene expression at the mRNA level. Mechanistically, miRNAs participate in epigenetic regulation mainly by binding to the 3′UTR of target sequences, inhibiting mRNA translation or promoting mRNA degradation. The identification of FRGs in pulmonary arterial hypertension led to the construction of a network involving miRNA and transcription factors, 112 which showed that miRNAs are involved in the regulation of ferroptosis. This regulatory mechanism was confirmed by iron metabolism gene interference therapy based on miRNA for cancers. 113 Fortunately, a case has been described in which nanomedicine with a miRNA targeting ferroptosis was used to treat tumors in vivo. 114
The regulation of ferroptosis by miRNAs is not homogeneous but multifaceted and has been document not only in ferroptosis‐related disease models such as tumor, ischemic injury, and degenerative disease models 115 , 116 , 117 , 118 , 119 , 120 but also in normal tissues. 121 , 122 Multiple metabolic pathways have been identified as major links between miRNAs and ferroptosis. MiR‐302a‐3p and miR‐335 respectively target ferroportin and ferritin to promote ferroptosis by regulating iron metabolism. 115 , 117 Additionally, miR‐30e‐5p targets specificity protein 1 (SP1), which is an important molecule in energy metabolism, to inhibit the AMPK pathway and thus induce ferroptosis. 121 Regarding classical signaling pathways, miR‐5096, miR‐375, and miR‐378a‐3p downregulate the expression of SLC7A11 to induce ferroptosis, 116 , 118 , 123 in addition, miR‐15a‐5p, miR‐324‐3p, miR‐182‐5p, and miR‐541‐3p promote ferroptosis by inhibiting GPX4 expression. 118 , 119 , 124 , 125 Additionally, miR‐214‐3p promotes ferroptosis by targeting ATF4, 126 which is a critical mediator of endoplasmic reticulum (ER) stress. 127 Notably, ER stress plays a dual role in ferroptosis. In human glioma cells ATF4 acts as a negative ferroptosis regulator by upregulating SLC7A11, 128 which is consistent with the above result. However, in breast cancer, ATF4 upregulates the expression of ChaC glutathione specific gamma‐glutamylcyclotransferase 1 (CHAC1) to promote the cystine starvation‐induced ferroptosis. 129
In addition to inducing ferroptosis, certain miRNAs inhibit ferroptosis. During lipid metabolism, miR‐670‐3p and miR‐424‐5p suppress ferroptosis by inhibiting ACSL4 expression. 130 , 131 MiR‐16‐92 also causes ferroptosis resistance by inhibiting the expression of zinc lipoprotein A20, which is a molecule upstream of ACSL4. 122 Similarly, as the upstream molecules of SLC7A11, p53, and NRF2 are regulated by miRNAs. MiR‐122‐5p and miR‐130b‐3p inhibit ferroptosis by targeting p53 and DKK1, respectively, with the latter blocking the NRF2 signaling pathway. 132 , 133 In addition, miR‐545, miR‐137, miR‐190a‐5p, miR‐23a‐3p, miR‐212‐5p, and miR‐194 respectively target other metabolism‐related genes TF, SLC1A5, Gls2, Dmt1, Ptgs2, and Bach1, to inhibit ferroptosis. 120 , 134 , 135 , 136 , 137 , 138
Notably, a miRNA does not act on a single gene. For example, miR‐7‐5p inhibits ferroptosis by upregulating the expression of ferritin and simultaneously downregulating the expression of ALOX12 and decreasing the level of LiperFluo. 139 MiRNAs action is also coordinated with other epigenetic mechanisms to regulate ferroptosis. MiR‐34a‐5p downregulates the expression of SIRT1 to increase the sensitivity of cadmium‐induced ferroptosis, 140 and miR‐522 protects against ferroptosis by downregulating ALOX15 expression facilitated by USP7. 141 The latter process requires the assistance of heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1) (Figure 5).
FIGURE 5.
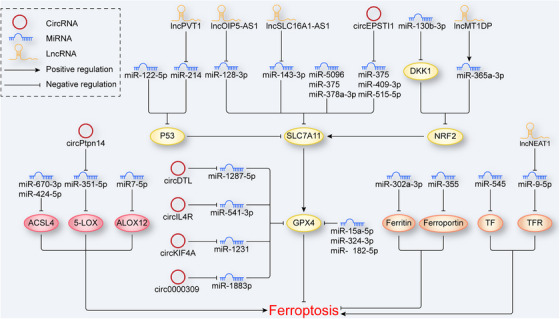
NcRNAs in ferroptosis. In ferroptosis, regulation by ncRNAs can be classified into three types. (1) NcRNAs in lipid metabolism. NcRNAs regulate ferroptosis by targeting lipid metabolism‐related molecules, such as ACSL4, 5‐LOX, and ALOX12. (2) NcRNAs in classical signaling pathways. NcRNAs regulate ferroptosis by targeting the p53/NRF2–SLC7A11–GPX4 axis. (3) NcRNAs in iron metabolism. NcRNAs regulate ferroptosis by targeting iron metabolism‐related molecules such as ferroportin, ferritin, TF, and TFR. 5‐LOX, 5‐lipoxygenase; ACSL4, acyl‐CoA synthetase long‐chain family member 4; ALOX12, arachidonate 12‐lipooxygenase; NcRNA, noncoding RNA; NRF2, nuclear factor erythroid 2‐related factor 2; TF, transferrin; TFR, transferrin receptor.
3.3.2. Circular RNAs in ferroptosis
A circular RNA (circRNA) is a special ncRNA molecule that is back‐spliced from pre‐mRNA, and it is a recently growing research hotspot in the field of ncRNAs. Similar to those on miRNA, studies on the regulation of ferroptosis by circRNAs have been focused mainly on tumors, diabetes and ischemic diseases. 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152
CircRNAs, which belong to competing endogenous RNAs (ceRNAs), directly sponge miRNA via their miRNA‐binding sites, thereby eliminating the inhibitory effect of miRNAs on their target genes. Depending on the miRNA targeted by a circRNA, the regulatory effect on ferroptosis differs. Some tumor‐related genes have been confirmed to be directly or indirectly associated with ferroptosis via circRNA‐miRNA‐target molecule regulatory networks. CircRHOT1, circ‐0008035, circRNA1615, and circPSEN1 protect against ferroptosis via the miR‐106a‐5/STAT3, miR‐599/EIF4A1, miR152/LPR6, and miR‐200b‐3p/cofilin‐2 axes, respectively. 142 , 143 , 150 , 152 Additionally, in lipid metabolism, circPtpn14 positively regulates ferroptosis by targeting miR‐351‐5p, inhibiting the expression of 5‐LOX, 153 while circDTL, circIL4R, circKIF4A, and cicr0000309 increase the expression of GPX4 by sponging miR‐1287‐5p, miR‐541‐3p, miR‐1231, and miR‐188‐3p respectively, inhibiting ferroptosis. 125 , 144 , 145 , 151 Notably, a single circRNA carries multiple miRNA‐binding sites. CircEPSTI1 inhibits ferroptosis by simultaneously sponging miR‐375, miR‐409‐3p, and miR‐515‐5p to upregulate SLC7A11 expression (Figure 5). 146
In addition, circRNAs interact with RNA‐binding proteins to regulate gene expression. Hsa‐circ‐0008367 binds with ALKBH5, a negative regulator of autophagy, to promote ferroptosis. 147 Notably, it has been proven that partial circRNAs show the potential to serve as a template for protein translation, 148 , 149 , 154 and certain encoded proteins can active ferroptosis‐related signaling pathway 149 ; however, whether circRNAs regulate ferroptosis through this mechanism remains to be confirmed.
3.3.3. Long ncRNAs in ferroptosis
Long noncoding RNAs (lncRNAs) constitute a class of ncRNAs greater than 200 nucleotides in length. Many studies have shown that the abnormal expression of lncRNAs is closely related to the pathogenesis of diseases such as tumors, degenerative diseases and ischemic injury, 155 , 156 , 157 , 158 which have been proven to be associated with ferroptosis. On this basis, an increasing number of ferroptosis‐related lncRNAs have been discovered. Additionally, in different tumors such as lung adenocarcinoma (LUAD), breast cancer, colon cancer, and bladder cancer, ferroptosis‐related lncRNA signatures related to prognosis have been established via Cox regression analysis. 159 , 160 , 161 , 162
Mechanistically, lncRNAs mainly regulate ferroptosis through posttranscriptional processes. First, some lncRNAs regulate ferroptosis through a ceRNA network, which is similar to that of circRNA. LncRNA PVT1 actives p53 gene expression through miR‐214 to induce ferroptosis. 158 As a molecule downstream of p53, SLC7A11 is also regulated via the ceRNA network. The lncRNA OIP5‐AS1 and lncRNA SLC16A1‐AS1 separately upregulate SLC7A11 expression by targeting miR‐128‐3p and inhibit ferroptosis. 163 , 164 In addition to system Xc−, the ceRNA network targets another amino acid transporter. LncRNA ZFAS1 negatively regulates the expression of miR‐150‐5p, which targets SLC38A1, a regulator of glutamine absorption and metabolism, to induce ferroptosis. 165 In addition, lncRNA participate in the regulation of the transsulfuration pathway, which is critical for the generation of cysteine. LncRNA LINC00336 interacts with ELAV‐like RNA‐binding protein 1 (ELAVL1) to inhibit miR6852, leading to cell resistance to ferroptosis by upregulating CBS expression. 166 These abovementioned results suggested that lncRNAs are extensively involved in lipid metabolism in ferroptosis. Additionally, studies have identified lncRNAs as key mediators regulating iron metabolism during ferroptosis. LncRNA NEAT1 increases while lncRNA PR11‐89 decreases the cellular iron concentration to regulate ferroptosis, in which the former sponges miR‐9‐5p to upregulate the expression of TFR and GOT1 and the latter sponges miR‐129‐5p to upregulate the expression of PROM2. 167 , 168 Interestingly, a recent study found diametrically opposing regulation to that mediated by the ceRNA network, where lncRNAs stabilize the structure of miRNAs. 169 This kind of regulation has also been found in relation to ferroptosis. For example, lncRNA MT1DP targets miR‐365a‐3p to downregulate the expression of NRF2, increasing cellular sensitivity to ferroptosis. 170 It is necessary to find more lncRNAs similar to lncRNA MT1DP to complement the lncRNA‐miRNA regulatory network. Second, lncRNAs bind with mRNAs to regulate the translation process. LncRNA GABPB1‐AS1 directly inhibits the translation of GABPB1 mRNA, which downregulates the expression of peroxiredoxin‐5 (PRDX5) to induce ferroptosis. 171 Additionally, certain lncRNAs regulate mRNA translation via lncRNA‐protein complexes. The lncRNA 00925 binds with the pumilio RNA‐binding family member 2 (Pum2) protein, leading to the degradation of Prdx6 mRNA, 172 while the lncRNA ASMTL‐AS1 recruits U2AF2, which stabilizes the structure of SAT1 mRNA to induce ferroptosis (Figure 5). 173
In addition to the posttranscriptional regulation, lncRNAs directly or indirectly regulate the transcription of genes. LncRNA Meg3, located both in the nucleus and cytoplasm, directly binds to the p53 gene and induces ferroptosis through the p53–GPX4 axis, 174 while the cytosolic lncRNA P53RRA necessarily cooperates with ras GTPase‐activating protein‐binding protein 1 (GABP1) to active the p53 gene. 175 The regulation of genes by lncRNA LINC00618 also requires the participation of proteins. The lncRNA LINC00618 promotes ferroptosis in an apoptosis‐dependent manner and inhibits the expression of SLC7A11 by attenuating LSH, inducing the transcription of SLC7A11 after recruitment to the SLC7A11 promoter. 176
Overall, ncRNAs can regulate ferroptosis in multiple ways, and the epigenetic mechanisms of ncRNA action are both interrelated and independent. Therefore, it is necessary to establish a complete lncRNA/circRNA–miRNA epigenetic regulatory network. In addition, many ncRNAs are located in knowledge blind spots, and how many of these ncRNAs are related to ferroptosis is unknown and deserves further exploration.
3.4. RNA modifications in ferroptosis
RNA methylation, which has become a hot topic in epigenetic research over the past decade, accounts for more than 60% of all RNA modifications, and among RNA methylation modifications, RNA m6A is the most prevalent posttranscription modification of mRNAs. 177 The biological function of the RNA m6A modification depends on “writers,” “erasers,” and “readers.” The RNA m6A writers mainly include METTL3, METTL14, WTAP, and KIAA1429, which mediate the methylation of RNA. ALKBH5 and FTO are erasers, that are critical for the demethylation of RNA m6A. Readers, RNA m6A‐binding proteins, recognize mRNA with the m6A mark; the readers mainly include the YTH domain protein family and HNRNP family. 178
Evidence suggests that the RNA m6A modification regulates ferroptosis. In non‐small cell lung carcinoma, FSP1, a glutathione‐independent ferroptosis inhibitor with an mRNA carrying five m6A sites, is upregulated by METTL3 sponging. 179 This result has been confirmed by the latest research performed by our group. In the aortas of type A AD patients, the protein level of METTL3 was negatively correlated with the expression of FSP1. We also found that METTL3 inhibited the expression of SLC7A11 to promote ferroptosis in human aortic smooth muscle cells. 180 However, in other diseases, such as hepatoblastoma and LUAD, METTL3 enhanced the stability of SLC7A11 mRNA to suppress ferroptosis. 181 , 182 These diametrically opposed effects may depend on the differential expression of the reader. Generally, YTHDF1 can promote the translation of its target mRNA. 183 , 184 , 185 For example, in hepatocellular carcinoma (HCC), YTHDF1 recognizes the m6A mark on SLC7A11 mRNA to enhance the inhibition of ferroptosis, 182 and in hepatic stellate cells, YTHDF1 also recognizes m6A mark on the BECN1 mRNA, enhancing ferritinophagy to induce ferroptosis. 186 However, another member of the YTH family, YTHDF2 exhibits biological effects opposite to YTHDF1 in that it causes mRNA's decay. 187 A study coupled YTHDF2 and METTL14 and demonstrated that the degradation of SLC7A11 mRNA by YTHDF2 requires the METTL14‐mediated RNA m6A modification in HCC. 188 Additionally, in LUAD, YTHDC2 targets SLC3A2 and SLC7A11 in an RNA m6A‐dependent manner to act as an endogenous ferroptosis inducer (Figure 4B). 189 Erasers are also involved in the regulation of ferroptosis. In hypopharyngeal squamous cell carcinoma, ALKBH5 targets RNA m6A residues in the 3′UTR of the NRF2 transcript and mediates transcriptional repression to promote ferroptosis. 190 In lung cells, black phosphorus quantum dots decreased the expression level of ALKBH5, which increased the global RNA m6A level, causing lipid peroxidation, iron overload and mitochondrial dysfunction. 187 As another eraser, FTO downregulated the expression of SLC7A11 to induce ferroptosis in thyroid cancer. 191 Moreover, the RNA m6A modification has also been linked to ncRNAs to regulate ferroptosis. In doxorubicin‐treated cardiomyocytes, METTL14 promoted the RNA m6A modification of the lncRNA KCNQ1OT1, which is a sponge of miR‐7‐5p. Deficiency in miR‐7‐5p induced ferroptosis by increasing the level of TFR. 192
Although studies on the regulation of ferroptosis mediated by the RNA m6A modification have been carried out only in recent years, clearly, the recent research on RNA m6A modification is focused mostly on writers and readers, with few studies on erasers. In addition, RNA modification is not limited to the m6A mark, and other common RNA modifications such as 2′‐O‐methylation (Nm) and pseudouracil (ψ) may be directions of further research.
4. THE ROLE OF EPIGENETIC REGULATORS IN FERROPTOSIS‐RELATED BIOLOGICAL PROCESSES AND DISEASES
4.1. Ferroptosis‐related biological processes
The role of ferroptosis in biological process has always been discussed. Indeed, ferroptosis has been implicated to cancer since its discovery, 16 and various tumor suppressors have been subsequently found to exhibit tumor inhibition effect through increasing cellular ferroptosis sensitivity. The role of tumor suppressor p53 in ferroptosis has been thoroughly investigated. Although there is ongoing debate regarding the precise role of p53 in regulating ferroptosis, it is widely believed that its primary role is to promote ferroptosis. As previously mentioned, p53 can suppress the occurrence of ferroptosis by regulating different targets including SLC7A11, ALOX12, SAT1, and GLS2. 10 , 14 , 58 , 59 Further research has indicated that p53‐mediated ferroptosis has been found to be sufficient for tumor suppression in animal model. 57 Therefore, we can reasonably assume that ferroptosis plays a critical role in tumor suppression process. Additionally, immune function may be another potential biological effect of ferroptosis. Evidence shows that ferroptosis‐related molecules is regulated by various cytokines. Interferon‐γ inhibits the expression of system Xc−, 193 while interleukins 4 and interleukins 13 upregulate the expression of ALOX15 with downregulating expression of GPX4. 194 Moreover, it has been found that ferroptosis may participate in physiological process such as erythropoiesis and aging with the generation of an antibody (HNEJ‐1), which is responsible for the reorganization of 4‐hydroxy‐2‐nonenal (HNE). 195
In conclusion, the tumor‐suppressing role of ferroptosis is well established, and the immunological functions of this process still require further confirmation. Furthermore, it remains to be determined whether ferroptosis is involved in other biological processes, and additional research is needed to fully address these questions.
4.2. Ferroptosis‐related diseases
Although the physiological function of ferroptosis has not been demonstrated clearly, numerous studies have shown the role of ferroptosis in a variety of diseases. With its physiological tumor suppression function, inhibition of ferroptosis can drive the development of cancer. A total analysis of pan‐cancer patients revealed higher expression level of GPX4 in tumor tissues than in normal tissues. 85 It was also reported that selenoprotein is upregulated in tumor, which suggests the enhanced expression level of GPX4 in the development of tumor. 196 These results demonstrated that inhibition of ferroptosis plays a critical role in tumorigenesis. Notably, the differential expression of GPX4 is not present in all types of tumors. In types including thymoma, bladder urothelial carcinoma, esophageal carcinoma, cervical/endocervical cancer, and skin cutaneous melanoma, GPX4 expression was not significantly higher than normal tissues. 85 This result indicates that the presence of other differentially expressed ferroptosis‐related molecules, which are valuable to be explored as specific tumor biomarkers. Interestingly, it has been reported that ferroptosis may have a tumor induction effect. In pancreatic ductal adenocarcinoma, cancer cells undergoing ferroptosis released a type of damage‐associated molecular pattern named KRAG12D to tumor microenvironment, which is subsequently internalized by macrophages via AGER pathway, inducing a shift in macrophages towards a pro‐tumor M2‐like phenotype and tumor growth. 197 Therefore, it is essential to investigate the possible influence of the cellular microenvironment on the impact of ferroptosis in tumors.
In addition to cancer, ferroptosis is involved in non‐neoplastic diseases. Evidence has indicated that ferroptosis is a major contributor to the pathogenesis of ischemia/reperfusion injuries (IRI) in different organs including the kidney, heart, and liver. In GPX4 knockdown mouse, induction of ferroptosis by depletion of GPX4 increased IRI‐mediated acute renal failure. 198 Similarly, overexpression of the ATP‐releasing pathway protein pannexin 1 induced ferroptosis via the MAPK/ERK signaling pathway in mouse subjected to renal IRI. 199 Conversely, inhibition of ferroptosis can alleviate organ injury caused by IRI. Ferroptosis inhibitors, such as iron chelators and glutaminolysis inhibitors, have been demonstrated to ameliorate IRI‐mediated cardiac injury in an ex vivo mouse heart model. 4 This result was further confirmed by another in vivo study, which indicated that iron chelators and ferrostatin‐1 can reduce acute or chronic IRI‐mediated heart failure. 19 Moreover, Liproxstatin‐1, a small molecule ferroptosis inhibitors, has been reported to be able to inhibit ferroptosis in IRI‐mediated hepatic damage in a preclinical model. 198 Additionally, ferroptosis has been linked to a range of other diseases, such as liver and lung fibrosis, autoimmune diseases, and neurodegeneration. 26 , 200 , 201 , 202 , 203 However, further research is necessary to confirm the causative role of ferroptosis in these conditions.
In general, the regulation of ferroptosis has the potential to provide substantial clinical advantages for relative biological disorders, and epigenetic modifications offer a promising approach in inducing or inhibiting ferroptosis.
4.3. The application of epigenetic regulators targeting ferroptosis in biological processes and diseases
Epigenetics play a role in biological processes related to ferroptosis, with tumor suppression being the main focus. One example of this involvement is the regulation of GPX4 gene expression. Specifically, increased levels of H3K4me3 and H3K27ac, as well as decreased levels of DNA methylation have been observed at the promoter region of GPX4 gene in various tumors. 85 This epigenetic regulation of GPX4 gene suggested promising potential for the use of epigenetic regulators in the treatment of ferroptosis‐related diseases. Indeed, many epigenetic drugs or compounds have shown excellent effects in the therapy of ferroptosis‐related diseases (Table 1).
TABLE 1.
Epigenetic drugs/compounds in ferroptosis‐related diseases therapy.
| Drug/compounds | Target | Molecular mechanism | Effects on ferroptosis | Diseases | Stage of research | References |
|---|---|---|---|---|---|---|
| JQ1 | BRD4 inhibitor | JQ1 induces the expression of SIRT1 and inhibits the expression of G9a to downregulate BRD4, decreasing the expression of GPX4 and SLC7A11 | Promotion | Breast cancer; lung adenocarcinoma | In vivo | 69 |
| Ketamine | KAT5 inhibitor | Ketamine inhibits the expression of KAT5 to downregulate the expression of GPX4 | Promotion | Breast cancer | In vitro | 72 |
| BEBT‐908 | HDACs inhibitor | BEBT‐908 hyperacetylates p53 to upregulate its expression | Promotion | Colon adenocarcinoma | In vivo | 75 |
| Quisinostat | HDACs inhibitor | Unknown | Promotion | Tongue squamous cell carcinoma | In vivo | 76 |
| Vorinostat | HDACs inhibitor | Unknown | Promotion | Lung adenocarcinoma | In vitro | 77 |
| 5‐aza‐CdR | DNMT1 inhibitor | 5‐aza‐CdR decreases the methylation level of CDH1 to upregulate the expression of E‐cadherin | Inhibition | Head and neck cancer | In vitro | 65 |
| BRD4770 | H3K9me 1/2/3 inhibitor | BRD4770 inhibits H3K9me1/2/3 abundance to upregulate the expression of FSP1, SLC7A11, GPX4, and GCH1 | Inhibition | Aortic dissection | In vivo | 96 |
Abbreviations: BRD4, bromodomain‐containing protein 4; CDH1, cadherin‐1; DNMT1, DNA methyltransferases 1; FSP1, ferroptosis suppressor protein 1; GCH1, GTP cyclohydrolase 1; GPX4, glutathione peroxidase 4; HDACs, histone deacetylases; KAT5, lysine acetyltransferase 5.
For different cancer, epigenetic regulators show potential of clinical application. In breast cancer, the BRD4 inhibitor JQ1 and the KAT5 inhibitor ketamine promote ferroptosis by epigenetic regulation of SIRT1/G9a and GPX4 respectively, and the former is also effective in LUAD. 69 , 72 Additionally, there are three HDAC inhibitors which show promise for treating cancer. In colon adenocarcinoma, BEBT‐908 promotes ferroptosis by hyperacetylating p53. 75 Quisinostat and vorinostat inhibit tumor growth in tongue squamous cell carcinoma and lung adenocarcinoma, respectively, with unclear epigenetic regulation mechanism. 76 , 77 In addition to histone acetylation modification, DNA methylation is a potential target of epigenetic drugs for cancer treatment. In head and neck cancer, the DNMT1 inhibitor 5‐aza‐CdR inhibits ferroptosis by the epigenetic reprogramming of EMT, 65 which provides a therapeutic approach for therapy‐resistant cancer. Moreover, epigenetic drugs have shown their clinical value not only in cancer, but also in AD. The antiferroptosis effects of BRD4770 by inhibiting H3K9me1/2/3 may reveal a potential therapeutic strategy for targeting VSMCs ferroptosis in AD. 96
In conclusion, epigenetic drugs targeting ferroptosis provide more treatment options for related diseases. However, there are only a few epigenetic regulators that have clinical translational significance with diverse epigenetic modifications that efficiently regulate ferroptosis. Further research is needed to identify and develop more effective epigenetic drugs that can specifically target ferroptosis and improve treatment outcomes for related diseases.
5. CONCLUSIONS AND PERSPECTIVES
Since the concept of ferroptosis was first proposed, epigenetic regulation has become a hot topic in this field. Here, we summarize several types of epigenetic regulatory mechanisms in ferroptosis, such as histone modifications, DNA methylation, ncRNA, and RNA modifications. Undeniably, the epigenetic regulatory mechanisms in ferroptosis are complex and diverse, and targeting epigenetic marks is a promising strategy for the treatment of ferroptosis‐related diseases. However, before this possibility can be realized, many questions in epigenetic studies related to ferroptosis must be answered in the future.
First, recent research has been limited to a few key ferroptosis‐related molecules, such as GPX4 and SLC7A11. To determine whether epigenetic mechanisms regulate other FRGs, further exploration is needed. Organelle‐specific ferroptosis regulators are a valuable target. It has been found that ATF4 (a key molecular in the ER stress) and GLS2 (a key molecular in the mitochondria tricarboxylic acid cycle) are regulated by miRNA. 120 , 126 It is worth studying more interaction between epigenetics and organelle‐specific ferroptosis regulators. Additionally, the vast majority of ferroptosis‐related epigenetic mechanisms elucidated to date have been shown to regulate the expression of FRGs. However, the posttranslational modifications of these related genes including methylation, acetylation, and ubiquitination, which affect the activity, stability, and cellular localization of proteins, remain to be elucidated by more study. The dearth of investigation into these factors has limited our development of drugs targeting epigenetic modifications, which are important for interfering with ferroptosis and the treatment of ferroptosis‐related diseases.
Second, in ferroptosis, a single epigenetic regulation pathway is rarely active alone; instead, multiple pathways work together to regulate ferroptosis. On the one hand, multiple epigenetic modifications may regulate the same molecule. For example, high expression levels of GPX4 in tumor cells are simultaneously regulated by DNA methylation, histone methylation, and histone acetylation. 85 On the other hand, a single epigenetic regulator such as BRD4770 and miR‐7‐5p can target multiple ferroptosis‐related molecules. 96 , 139 Thus, epigenetic regulatory pathways form a complex network to regulate ferroptosis. However, how different epigenetic modifications affect each other and the mechanisms that jointly participate in ferroptosis are largely unknown. In addition, a study has shown that histone acetylation mediated by PCAF is more likely to be involved in the regulation of transcription factors, serving as a fine‐tuning mechanism, not a switch. 204 Whether this conclusion can be extended to other epigenetic regulatory mechanisms remains to be determined. Therefore, it is necessary to identify the major epigenetic regulatory mechanisms in ferroptosis, which may help us identify appropriate drugs with better targeting.
Finally, although some epigenetic regulators mentioned in this paper, such as, quisinostat, BRD4770, 5‐aza‐CdR, and miRNA nanomedicine, have shown excellent effects in the treatment of ferroptosis‐related diseases, each study has been limited to one disease model. Indeed, regulation of ferroptosis with the treatment of the same epigenetic modification can show opposite effects in different tissues or diseases. 78 , 87 , 88 , 89 , 96 , 97 The reasons for cell‐specific biological effects of epigenetic modifications are not clear, which may be influenced by multiple factors. In addition to the cell‐specific expression of ferroptosis‐related protein and cell microenvironment aforementioned, there is another factor to consider, which is the difference of the type and number of organelles in different cells, for example, mitochondria are most abundant in myocardial cells. The occurrence of ferroptosis depends on the integration of signals from different organelles including mitochondria, lysosomes, ER, and so on. Epigenetic modifications may lead to cell‐specific biological effects by regulating organelle‐specific ferroptosis‐related proteins. Overall, the cell‐specific biological effects of epigenetic modifications will be a valuable research direction. Furthermore, since the induction or inhibition of ferroptosis by epigenetic marks affects nontargeted cells, including normal cells, research on the cell‐specificity of ferroptosis mediated by epigenetic modulators may help prevent the side effects of drugs used in the clinic. Obviously, the exploration of these problems will be of great help for guiding drug use in different diseases.
AUTHOR CONTRIBUTIONS
The contribution of the article confirmed as follows: M. Y. and H. L. wrote and edited the manuscript; X. Y. searched and collected literatures; X. W. and D. J. provided ideas and funding support. All authors approved the article for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
Not applicable.
FUNDING INFORMATION
This work was supported by grants from the National Natural Science Foundation of China (No. 82170502 and No. 82070488).
ACKNOWLEDGMENTS
We apologize to the authors whose papers could not be cited due to space restrictions.
Yang M, Luo H, Yi X, Wei X, Jiang D‐S. The epigenetic regulatory mechanisms of ferroptosis and its implications for biological processes and diseases. MedComm. 2023;4:e267. 10.1002/mco2.267
Contributor Information
Xiang Wei, Email: xiangwei@tjh.tjmu.edu.cn.
Ding‐Sheng Jiang, Email: jds@hust.edu.cn.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron‐dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zou Y, Li H, Graham ET, et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol. 2020;16(3):302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hadian K, Stockwell BR. SnapShot: ferroptosis. Cell. 2020;181(5):1188‐1188. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell. 2015;59(2):298‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1‐2):317‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12(7):497‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione‐independent ferroptosis suppressor. Nature. 2019;575(7784):693‐698. [DOI] [PubMed] [Google Scholar]
- 10. Jiang L, Kon N, Li T, et al. Ferroptosis as a p53‐mediated activity during tumour suppression. Nature. 2015;520(7545):57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaramillo MC, Zhang DD. The emerging role of the Nrf2‐Keap1 signaling pathway in cancer. Genes Dev. 2013;27(20):2179‐2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen D, Tavana O, Chu B, et al. NRF2 is a major target of ARF in p53‐independent tumor suppression. Mol Cell. 2017;68(1):224‐232. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu J, Minikes AM, Gao M, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2‐YAP signalling. Nature. 2019;572(7769):402‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chu B, Kon N, Chen D, et al. ALOX12 is required for p53‐mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21(5):579‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cavalli G, Heard E. Advances in epigenetics link genetics to the environment and disease. Nature. 2019;571(7766):489‐499. [DOI] [PubMed] [Google Scholar]
- 16. Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12‐27. [DOI] [PubMed] [Google Scholar]
- 17. Cao J, Yan Q. Cancer epigenetics, tumor immunity, and immunotherapy. Trends Cancer. 2020;6(7):580‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radic Biol Med. 2002;33(8):1037‐1046. [DOI] [PubMed] [Google Scholar]
- 19. Fang X, Wang H, Han D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672‐2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron‐dependent, nonapoptotic cell death in oncogenic‐RAS‐harboring cancer cells. Chem Biol. 2008;15(3):234‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suttner DM, Dennery PA. Reversal of HO‐1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13(13):1800‐1809. [DOI] [PubMed] [Google Scholar]
- 22. Kwon MY, Park E, Lee SJ, Chung SW. Heme oxygenase‐1 accelerates erastin‐induced ferroptotic cell death. Oncotarget. 2015;6(27):24393‐24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu W, Geng Z, Bai H, Liu T, Zhang B. Ammonium ferric citrate induced ferroptosis in non‐small‐cell lung carcinoma through the inhibition of GPX4‐GSS/GSR‐GGT axis activity. Int J Med Sci. 2021;18(8):1899‐1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Liu Y, Liu J, Kang R, Tang D. NEDD4L‐mediated LTF protein degradation limits ferroptosis. Biochem Biophys Res Commun. 2020;531(4):581‐587. [DOI] [PubMed] [Google Scholar]
- 25. Hong X, Roh W, Sullivan RJ, et al. The lipogenic regulator SREBP2 induces transferrin in circulating melanoma cells and suppresses ferroptosis. Cancer Discov. 2021;11(3):678‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu Y, Jiang L, Wang H, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136(6):726‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fuhrmann DC, Mondorf A, Beifuss J, Jung M, Brune B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36:101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park E, Chung SW. ROS‐mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10(11):822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang Y, Chen X, Tan Q, Zhou H, Xu J, Gu Q. Inhibiting ferroptosis through disrupting the NCOA4‐FTH1 interaction: a new mechanism of action. ACS Cent Sci. 2021;7(6):980‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9‐17. [DOI] [PubMed] [Google Scholar]
- 32. Wu Y, Jiao H, Yue Y, et al. Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury. Cell Death Differ. 2022;29(9):1705‐1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30(6):478‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113(34):E4966‐E4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magtanong L, Ko PJ, To M, et al. Exogenous monounsaturated fatty acids promote a ferroptosis‐resistant cell state. Cell Chem Biol. 2019;26(3):420‐432. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tesfay L, Paul BT, Konstorum A, et al. Stearoyl‐CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 2019;79(20):5355‐5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kagan VE, Mao G, Qu F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stockwell BR, Jiang X. The chemistry and biology of ferroptosis. Cell Chem Biol. 2020;27(4):365‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ingold I, Berndt C, Schmitt S, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide‐induced ferroptosis. Cell. 2018;172(3):409‐422. e21. [DOI] [PubMed] [Google Scholar]
- 42. Alim I, Caulfield JT, Chen Y, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177(5):1262‐1279. e25. [DOI] [PubMed] [Google Scholar]
- 43. Ekoue DN, He C, Diamond AM, Bonini MG. Manganese superoxide dismutase and glutathione peroxidase‐1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim Biophys Acta Bioenerg. 2017;1858(8):628‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cao JY, Poddar A, Magtanong L, et al. A genome‐wide haploid genetic screen identifies regulators of glutathione abundance and ferroptosis sensitivity. Cell Rep. 2019;26(6):1544‐1556. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489‐492. [DOI] [PubMed] [Google Scholar]
- 46. Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274(17):11455‐11458. [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y, Swanda RV, Nie L, et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat Commun. 2021;12(1):1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu J, Berisa M, Schwörer S, Qin W, Cross JR, Thompson CB. Transsulfuration activity can support cell growth upon extracellular cysteine limitation. Cell Metab. 2019;30(5):865‐876. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu N, Lin X, Huang C. Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin‐induced ferroptosis resistance. Br J Cancer. 2020;122(2):279‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl‐tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23(2):270‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yao X, Xie R, Cao Y, et al. Simvastatin induced ferroptosis for triple‐negative breast cancer therapy. J Nanobiotechnology. 2021;19(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mishima E, Ito J, Wu Z, et al. A non‐canonical vitamin K cycle is a potent ferroptosis suppressor. Nature. 2022;608(7924):778‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kraft VAN, Bezjian CT, Pfeiffer S, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6(1):41‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mao C, Liu X, Zhang Y, et al. DHODH‐mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593(7860):586‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gao M, Yi J, Zhu J, et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73(2):354‐363. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wei X, Yi X, Zhu XH, Jiang DS. Posttranslational modifications in ferroptosis. Oxid Med Cell Longev. 2020;2020:8832043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang SJ, Li D, Ou Y, et al. Acetylation is crucial for p53‐mediated ferroptosis and tumor suppression. Cell Rep. 2016;17(2):366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53‐mediated ferroptotic responses. Proc Natl Acad Sci U S A. 2016;113(44):E6806‐E6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A. 2010;107(16):7455‐7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Niu Y, Zhang J, Tong Y, Li J, Liu B. Physcion 8‐O‐beta‐glucopyranoside induced ferroptosis via regulating miR‐103a‐3p/GLS2 axis in gastric cancer. Life Sci. 2019;237:116893. [DOI] [PubMed] [Google Scholar]
- 61. Xie Y, Zhu S, Song X, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20(7):1692‐1704. [DOI] [PubMed] [Google Scholar]
- 62. Chen D, Chu B, Yang X, et al. iPLA2β‐mediated lipid detoxification controls p53‐driven ferroptosis independent of GPX4. Nat Commun. 2021;12(1):3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fan Z, Wirth AK, Chen D, et al. Nrf2‐Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6(8):e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun X, Ou Z, Chen R, et al. Activation of the p62‐Keap1‐NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee J, You JH, Kim MS, Roh JL. Epigenetic reprogramming of epithelial‐mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020;37:101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sabari BR, Zhang D, Allis CD, Zhao Y. Metabolic regulation of gene expression through histone acylations. Nat Rev Mol Cell Biol. 2017;18(2):90‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guo X, Fang ZM, Wei X, et al. HDAC6 is associated with the formation of aortic dissection in human. Mol Med. 2019;25(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang Z, Guo M, Shen M, et al. The BRD7‐P53‐SLC25A28 axis regulates ferroptosis in hepatic stellate cells. Redox Biol. 2020;36:101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sui S, Zhang J, Xu S, Wang Q, Wang P, Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)‐JQ1 in cancer cells. Cell Death Dis. 2019;10(5):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barneda‐Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6(6):579‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chung S, Kim S, Son M, et al. Inhibition of p300/CBP‐associated factor attenuates renal tubulointerstitial fibrosis through modulation of NF‐kB and Nrf2. Int J Mol Sci. 2019;20(7):1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li H, Liu W, Zhang X, Wu F, Sun D, Wang Z. Ketamine suppresses proliferation and induces ferroptosis and apoptosis of breast cancer cells by targeting KAT5/GPX4 axis. Biochem Biophys Res Commun. 2021;585:111‐116. [DOI] [PubMed] [Google Scholar]
- 73. Zhang X, Du L, Qiao Y, et al. Ferroptosis is governed by differential regulation of transcription in liver cancer. Redox Biol. 2019;24:101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu L, Li Y, Cao D, et al. SIRT3 inhibits gallbladder cancer by induction of AKT‐dependent ferroptosis and blockade of epithelial‐mesenchymal transition. Cancer Lett. 2021;510:93‐104. [DOI] [PubMed] [Google Scholar]
- 75. Fan F, Liu P, Bao R, et al. A dual PI3K/HDAC inhibitor induces immunogenic ferroptosis to potentiate cancer immune checkpoint therapy. Cancer Res. 2021;81(24):6233‐6245. [DOI] [PubMed] [Google Scholar]
- 76. Wang X, Liu K, Gong H, et al. Death by histone deacetylase inhibitor quisinostat in tongue squamous cell carcinoma via apoptosis, pyroptosis, and ferroptosis. Toxicol Appl Pharmacol. 2021;410:115363. [DOI] [PubMed] [Google Scholar]
- 77. Zhang T, Sun B, Zhong C, et al. Targeting histone deacetylase enhances the therapeutic effect of erastin‐induced ferroptosis in EGFR‐activating mutant lung adenocarcinoma. Transl Lung Cancer Res. 2021;10(4):1857‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zille M, Kumar A, Kundu N, et al. Ferroptosis in neurons and cancer cells is similar but differentially regulated by histone deacetylase inhibitors. eNeuro. 2019;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yi X, Jiang X, Li X, Jiang DS. Histone lysine methylation and congenital heart disease: from bench to bedside (Review). Int J Mol Med. 2017;40(4):953‐964. [DOI] [PubMed] [Google Scholar]
- 80. Yi X, Tao Y, Lin X, et al. Histone methyltransferase Setd2 is critical for the proliferation and differentiation of myoblasts. Biochim Biophys Acta Mol Cell Res. 2017;1864(4):697‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jiang DS, Yi X, Li R, et al. The histone methyltransferase mixed lineage leukemia (MLL) 3 may play a potential role on clinical dilated cardiomyopathy. Mol Med. 2017;23:196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yi X, Jiang XJ, Li XY, Jiang DS. Histone methyltransferases: novel targets for tumor and developmental defects. Am J Transl Res. 2015;7(11):2159‐2175. [PMC free article] [PubMed] [Google Scholar]
- 83. Wei X, Yi X, Zhu XH, Jiang DS. Histone methylation and vascular biology. Clin Epigenetics. 2020;12(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li R, Wei X, Jiang DS. Protein methylation functions as the posttranslational modification switch to regulate autophagy. Cell Mol Life Sci. 2019;76(19):3711‐3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang X, Sui S, Wang L, et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J Cell Physiol. 2020;235(4):3425‐3437. [DOI] [PubMed] [Google Scholar]
- 86. Ma M, Kong P, Huang Y, et al. Activation of MAT2A‐ACSL3 pathway protects cells from ferroptosis in gastric cancer. Free Radic Biol Med. 2022;181:288‐299. [DOI] [PubMed] [Google Scholar]
- 87. Hasegawa M, Takahashi H, Rajabi H, et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1‐C oncoprotein in triple‐negative breast cancer cells. Oncotarget. 2016;7(11):11756‐11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu T, Xu P, Ke S, et al. Histone methyltransferase SETDB1 inhibits TGF‐beta‐induced epithelial‐mesenchymal transition in pulmonary fibrosis by regulating SNAI1 expression and the ferroptosis signaling pathway. Arch Biochem Biophys. 2022;715:109087. [DOI] [PubMed] [Google Scholar]
- 89. Chen M, Jiang Y, Sun Y. KDM4A‐mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma. Biochem Biophys Res Commun. 2021;550:77‐83. [DOI] [PubMed] [Google Scholar]
- 90. Chen TQ, Hu N, Huo B, et al. EHMT2/G9a inhibits aortic smooth muscle cell death by suppressing autophagy activation. Int J Biol Sci. 2020;16(7):1252‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li R, Yi X, Wei X, et al. EZH2 inhibits autophagic cell death of aortic vascular smooth muscle cells to affect aortic dissection. Cell Death Dis. 2018;9(2):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lu P, Feng X, Li R, et al. A novel serum biomarker model to discriminate aortic dissection from coronary artery disease. Dis Markers. 2022;2022:9716424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen Y, He Y, Wei X, Jiang DS. Targeting regulated cell death in aortic aneurysm and dissection therapy. Pharmacol Res. 2022;176:106048. [DOI] [PubMed] [Google Scholar]
- 94. Fang ZM, Feng X, Chen Y, Luo H, Jiang DS, Yi X. Targeting autophagy in aortic aneurysm and dissection. Biomed Pharmacother. 2022;153:113547. [DOI] [PubMed] [Google Scholar]
- 95. He Y, Yi X, Zhang Z, et al. JIB‐04, a histone demethylase Jumonji C domain inhibitor, regulates phenotypic switching of vascular smooth muscle cells. Clin Epigenetics. 2022;14(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen Y, Yi X, Huo B, et al. BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharmacol Res. 2022;177:106122. [DOI] [PubMed] [Google Scholar]
- 97. Wang J, Yin X, He W, Xue W, Zhang J, Huang Y. SUV39H1 deficiency suppresses clear cell renal cell carcinoma growth by inducing ferroptosis. Acta Pharm Sin B. 2021;11(2):406‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang Y, Shi J, Liu X, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20(10):1181‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang Y, Yang L, Zhang X, et al. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep. 2019;20(7):e47563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang Y, Koppula P, Gan B. Regulation of H2A ubiquitination and SLC7A11 expression by BAP1 and PRC1. Cell Cycle. 2019;18(8):773‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang J, Yang C, Wu C, Cui W, Wang L. DNA methyltransferases in cancer: biology, paradox, aberrations, and targeted therapy. Cancers (Basel). 2020;12(8):2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lee JY, Nam M, Son HY, et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A. 2020;117(51):32433‐32442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jiang Y, Mao C, Yang R, et al. EGLN1/c‐Myc induced lymphoid‐specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics. 2017;7(13):3293‐3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Huang D, Li Q, Sun X, et al. CRL4(DCAF8) dependent opposing stability control over the chromatin remodeler LSH orchestrates epigenetic dynamics in ferroptosis. Cell Death Differ. 2021;28(5):1593‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ling H, Li M, Yang C, et al. Glycine increased ferroptosis via SAM‐mediated GPX4 promoter methylation in rheumatoid arthritis. Rheumatology (Oxford). 2022;61(11):4521‐4534. [DOI] [PubMed] [Google Scholar]
- 106. Zhang J, Gao M, Niu Y, Sun J. From DNMT1 degrader to ferroptosis promoter: drug repositioning of 6‐Thioguanine as a ferroptosis inducer in gastric cancer. Biochem Biophys Res Commun. 2022;603:75‐81. [DOI] [PubMed] [Google Scholar]
- 107. Cao LL, Liu H, Yue Z, et al. Iron chelation inhibits cancer cell growth and modulates global histone methylation status in colorectal cancer. BioMetals. 2018;31(5):797‐805. [DOI] [PubMed] [Google Scholar]
- 108. Kawai K, Li YS, Song MF, Kasai H. DNA methylation by dimethyl sulfoxide and methionine sulfoxide triggered by hydroxyl radical and implications for epigenetic modifications. Bioorg Med Chem Lett. 2010;20(1):260‐265. [DOI] [PubMed] [Google Scholar]
- 109. Logie E, Van Puyvelde B, Cuypers B, et al. Ferroptosis induction in multiple myeloma cells triggers DNA methylation and histone modification changes associated with cellular senescence. Int J Mol Sci. 2021;22(22):12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pogribny IP, Tryndyak VP, Pogribna M, et al. Modulation of intracellular iron metabolism by iron chelation affects chromatin remodeling proteins and corresponding epigenetic modifications in breast cancer cells and increases their sensitivity to chemotherapeutic agents. Int J Oncol. 2013;42(5):1822‐1832. [DOI] [PubMed] [Google Scholar]
- 111. Horniblow RD, Pathak P, Balacco DL, et al. Iron‐mediated epigenetic activation of NRF2 targets. J Nutr Biochem. 2022;101:108929. [DOI] [PubMed] [Google Scholar]
- 112. Zhang F, Liu H. Identification of ferroptosis‐associated genes exhibiting altered expression in pulmonary arterial hypertension. Math Biosci Eng. 2021;18(6):7619‐7630. [DOI] [PubMed] [Google Scholar]
- 113. Gao J, Luo T, Wang J. Gene interfered‐ferroptosis therapy for cancers. Nat Commun. 2021;12(1):5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Luo Y, Niu G, Yi H, et al. Nanomedicine promotes ferroptosis to inhibit tumour proliferation in vivo. Redox Biol. 2021;42:101908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wei D, Ke YQ, Duan P, Zhou L, Wang CY, Cao P. MicroRNA‐302a‐3p induces ferroptosis of non‐small cell lung cancer cells via targeting ferroportin. Free Radic Res. 2021;55(7):821‐830. [DOI] [PubMed] [Google Scholar]
- 116. Yadav P, Sharma P, Sundaram S, Venkatraman G, Bera AK, Karunagaran D. SLC7A11/xCT is a target of miR‐5096 and its restoration partially rescues miR‐5096‐mediated ferroptosis and anti‐tumor effects in human breast cancer cells. Cancer Lett. 2021;522:211‐224. [DOI] [PubMed] [Google Scholar]
- 117. Li X, Si W, Li Z, et al. miR‑335 promotes ferroptosis by targeting ferritin heavy chain 1 in in vivo and in vitro models of Parkinson's disease. Int J Mol Med. 2021;47(4):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ding C, Ding X, Zheng J, et al. miR‐182‐5p and miR‐378a‐3p regulate ferroptosis in I/R‐induced renal injury. Cell Death Dis. 2020;11(10):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fan K, Huang W, Qi H, et al. The Egr‐1/miR‐15a‐5p/GPX4 axis regulates ferroptosis in acute myocardial infarction. Eur J Pharmacol. 2021;909:174403. [DOI] [PubMed] [Google Scholar]
- 120. Zhou X, Zhuo M, Zhang Y, Shi E, Ma X, Li H. miR‐190a‐5p regulates cardiomyocytes response to ferroptosis via directly targeting GLS2. Biochem Biophys Res Commun. 2021;566:9‐15. [DOI] [PubMed] [Google Scholar]
- 121. Xia J, Song X, Meng J, Lou D. Endothelial progenitor cells‐derived exosomes transfer microRNA‐30e‐5p to regulate Erastin‐induced ferroptosis in human umbilical vein endothelial cells via the specificity protein 1/adenosine monophosphate‐activated protein kinase axis. Bioengineered. 2022;13(2):3566‐3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Xiao FJ, Zhang D, Wu Y, et al. miRNA‐17‐92 protects endothelial cells from erastin‐induced ferroptosis through targeting the A20‐ACSL4 axis. Biochem Biophys Res Commun. 2019;515(3):448‐454. [DOI] [PubMed] [Google Scholar]
- 123. Ni H, Qin H, Sun C, et al. MiR‐375 reduces the stemness of gastric cancer cells through triggering ferroptosis. Stem Cell Res Ther. 2021;12(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Deng SH, Wu DM, Li L, et al. miR‐324‐3p reverses cisplatin resistance by inducing GPX4‐mediated ferroptosis in lung adenocarcinoma cell line A549. Biochem Biophys Res Commun. 2021;549:54‐60. [DOI] [PubMed] [Google Scholar]
- 125. Xu Q, Zhou L, Yang G, et al. CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR‐541‐3p/GPX4 axis. Cell Biol Int. 2020;44(11):2344‐2356. [DOI] [PubMed] [Google Scholar]
- 126. Bai T, Liang R, Zhu R, Wang W, Zhou L, Sun Y. MicroRNA‐214‐3p enhances erastin‐induced ferroptosis by targeting ATF4 in hepatoma cells. J Cell Physiol. 2020;235(7‐8):5637‐5648. [DOI] [PubMed] [Google Scholar]
- 127. Bi M, Naczki C, Koritzinsky M, et al. ER stress‐regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24(19):3470‐3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chen D, Fan Z, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT‐dependent manner. Oncogene. 2017;36(40):5593‐5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen MS, Wang SF, Hsu CY, et al. CHAC1 degradation of glutathione enhances cystine‐starvation‐induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2‐eIF2α‐ATF4 pathway. Oncotarget. 2017;8(70):114588‐114602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bao C, Zhang J, Xian SY, Chen F. MicroRNA‐670‐3p suppresses ferroptosis of human glioblastoma cells through targeting ACSL4. Free Radic Res. 2021;55(7):853‐864. [DOI] [PubMed] [Google Scholar]
- 131. Ma LL, Liang L, Zhou D, Wang SW. Tumor suppressor miR‐424‐5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma. 2021;68(1):165‐173. [DOI] [PubMed] [Google Scholar]
- 132. Zhao H, Li X, Yang L, et al. Isorhynchophylline relieves ferroptosis‐induced nerve damage after intracerebral hemorrhage via miR‐122‐5p/TP53/SLC7A11 pathway. Neurochem Res. 2021;46(8):1981‐1994. [DOI] [PubMed] [Google Scholar]
- 133. Liao Y, Jia X, Ren Y, et al. Suppressive role of microRNA‐130b‐3p in ferroptosis in melanoma cells correlates with DKK1 inhibition and Nrf2‐HO‐1 pathway activation. Hum Cell. 2021;34(5):1532‐1544. [DOI] [PubMed] [Google Scholar]
- 134. Zheng S, Hu L, Song Q, et al. miR‐545 promotes colorectal cancer by inhibiting transferring in the non‐normal ferroptosis signaling. Aging (Albany NY). 2021;13(24):26137‐26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Luo M, Wu L, Zhang K, et al. miR‐137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25(8):1457‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Song Y, Wang B, Zhu X, et al. Human umbilical cord blood‐derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol. 2021;37(1):51‐64. [DOI] [PubMed] [Google Scholar]
- 137. Xiao X, Jiang Y, Liang W, et al. miR‐212‐5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol Brain. 2019;12(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Li X, Zhang X, Liu Y, et al. Exosomes derived from mesenchyml stem cells ameliorate oxygen‐glucose deprivation/reoxygenation‐induced neuronal injury via transferring MicroRNA‐194 and targeting Bach1. Tissue Cell. 2021;73:101651. [DOI] [PubMed] [Google Scholar]
- 139. Tomita K, Nagasawa T, Kuwahara Y, et al. MiR‐7‐5p is involved in ferroptosis signaling and radioresistance thru the generation of ROS in radioresistant HeLa and SAS cell lines. Int J Mol Sci. 2021;22(15):8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hao R, Ge J, Song X, Li F, Sun‐Waterhouse D, Li D. Cadmium induces ferroptosis and apoptosis by modulating miR‐34a‐5p/Sirt1axis in PC12 cells. Environ Toxicol. 2022;37(1):41‐51. [DOI] [PubMed] [Google Scholar]
- 141. Zhang H, Deng T, Liu R, et al. CAF secreted miR‐522 suppresses ferroptosis and promotes acquired chemo‐resistance in gastric cancer. Mol Cancer. 2020;19(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang H, Ge Z, Wang Z, Gao Y, Wang Y, Qu X. Circular RNA RHOT1 promotes progression and inhibits ferroptosis via mir‐106a‐5p/STAT3 axis in breast cancer. Aging (Albany NY). 2021;13(6):8115‐8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Li C, Tian Y, Liang Y, Li Q. Retraction Note to: circ_0008035 contributes to cell proliferation and inhibits apoptosis and ferroptosis in gastric cancer via miR‐599/EIF4A1 axis. Cancer Cell Int. 2021;21(1):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Shanshan W, Hongying M, Jingjing F, Yiming Y, Yu R, Rui Y. CircDTL functions as an oncogene and regulates both apoptosis and ferroptosis in non‐small cell lung cancer cells. Front Genet. 2021;12:743505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chen W, Fu J, Chen Y, et al. Circular RNA circKIF4A facilitates the malignant progression and suppresses ferroptosis by sponging miR‐1231 and upregulating GPX4 in papillary thyroid cancer. Aging (Albany NY). 2021;13(12):16500‐16512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wu P, Li C, Ye DM, et al. Circular RNA circEPSTI1 accelerates cervical cancer progression via miR‐375/409‐3P/515‐5p‐SLC7A11 axis. Aging (Albany NY). 2021;13(3):4663‐4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Liu Z, Wang Q, Wang X, Xu Z, Wei X, Li J. Circular RNA cIARS regulates ferroptosis in HCC cells through interacting with RNA binding protein ALKBH5. Cell Death Discov. 2020;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhang M, Huang N, Yang X, et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37(13):1805‐1814. [DOI] [PubMed] [Google Scholar]
- 149. Zheng X, Chen L, Zhou Y, et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo‐YAP signaling. Mol Cancer. 2019;18(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhu Z, Duan P, Song H, Zhou R, Chen T. Downregulation of Circular RNA PSEN1 ameliorates ferroptosis of the high glucose treated retinal pigment epithelial cells via miR‐200b‐3p/cofilin‐2 axis. Bioengineered. 2021;12(2):12555‐12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Jin J, Wang Y, Zheng D, Liang M, He Q. A novel identified circular RNA, mmu_mmu_circRNA_0000309, involves in germacrone‐mediated improvement of diabetic nephropathy through regulating ferroptosis by targeting miR‐188‐3p/GPX4 signaling axis. Antioxid Redox Signal. 2022;36(10‐12):740‐759. [DOI] [PubMed] [Google Scholar]
- 152. Li RL, Fan CH, Gong SY, Kang S. Effect and mechanism of LRP6 on cardiac myocyte ferroptosis in myocardial infarction. Oxid Med Cell Longev. 2021;2021:8963987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wu C, Du M, Yu R, et al. A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR‐351‐5p/5‐LOX signaling in melatonin‐mediated treatment of traumatic brain injury. Free Radic Biol Med. 2022;178:271‐294. [DOI] [PubMed] [Google Scholar]
- 154. Zheng Q, Bao C, Guo W, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tan YT, Lin JF, Li T, Li JJ, Xu RH, Ju HQ. LncRNA‐mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun (Lond). 2021;41(2):109‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965‐3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wan P, Su W, Zhang Y, et al. LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death Differ. 2020;27(1):176‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lu J, Xu F, Lu H. LncRNA PVT1 regulates ferroptosis through miR‐214‐mediated TFR1 and p53. Life Sci. 2020;260:118305. [DOI] [PubMed] [Google Scholar]
- 159. Yao J, Chen X, Liu X, Li R, Zhou X, Qu Y. Characterization of a ferroptosis and iron‐metabolism related lncRNA signature in lung adenocarcinoma. Cancer Cell Int. 2021;21(1):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cai HJ, Zhuang ZC, Wu Y, et al. Development and validation of a ferroptosis‐related lncRNAs prognosis signature in colon cancer. Bosn J Basic Med Sci. 2021;21(5):569‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zhang K, Ping L, Du T, et al. A ferroptosis‐related lncRNAs signature predicts prognosis and immune microenvironment for breast cancer. Front Mol Biosci. 2021;8:678877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chen M, Nie Z, Li Y, et al. A New Ferroptosis‐Related lncRNA Signature Predicts the Prognosis of Bladder Cancer Patients. Front Cell Dev Biol. 2021;9:699804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zhang Y, Guo S, Wang S, et al. LncRNA OIP5‐AS1 inhibits ferroptosis in prostate cancer with long‐term cadmium exposure through miR‐128‐3p/SLC7A11 signaling. Ecotoxicol Environ Saf. 2021;220:112376. [DOI] [PubMed] [Google Scholar]
- 164. Li YZ, Zhu HC, Du Y, Zhao HC, Wang L. Silencing lncRNA SLC16A1‐AS1 induced ferroptosis in renal cell carcinoma through miR‐143‐3p/SLC7A11 signaling. Technol Cancer Res Treat. 2022;21:15330338221077803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yang Y, Tai W, Lu N, et al. lncRNA ZFAS1 promotes lung fibroblast‐to‐myofibroblast transition and ferroptosis via functioning as a ceRNA through miR‐150‐5p/SLC38A1 axis. Aging (Albany NY). 2020;12(10):9085‐9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang M, Mao C, Ouyang L, et al. Correction to: long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2020;27(4):1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Luo W, Wang J, Xu W, et al. LncRNA RP11‐89 facilitates tumorigenesis and ferroptosis resistance through PROM2‐activated iron export by sponging miR‐129‐5p in bladder cancer. Cell Death Dis. 2021;12(11):1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wei XB, Jiang WQ, Zeng JH, et al. Exosome‐derived lncRNA NEAT1 exacerbates sepsis‐associated encephalopathy by promoting ferroptosis through regulating miR‐9‐5p/TFRC and GOT1 axis. Mol Neurobiol. 2022;59(3):1954‐1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Gao M, Li C, Xu M, Liu Y, Cong M, Liu S. LncRNA MT1DP aggravates cadmium‐induced oxidative stress by repressing the function of Nrf2 and is dependent on interaction with miR‐365. Adv Sci (Weinh). 2018;5(7):1800087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gai C, Liu C, Wu X, et al. MT1DP loaded by folate‐modified liposomes sensitizes erastin‐induced ferroptosis via regulating miR‐365a‐3p/NRF2 axis in non‐small cell lung cancer cells. Cell Death Dis. 2020;11(9):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Qi W, Li Z, Xia L, et al. LncRNA GABPB1‐AS1 and GABPB1 regulate oxidative stress during erastin‐induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep. 2019;9(1):16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Zhang JK, Zhang Z, Guo ZA, et al. The BMSC‐derived exosomal lncRNA Mir9‐3hg suppresses cardiomyocyte ferroptosis in ischemia‐reperfusion mice via the Pum2/PRDX6 axis. Nutr Metab Cardiovasc Dis. 2022;32(2):515‐527. [DOI] [PubMed] [Google Scholar]
- 173. Sui X, Hu N, Zhang Z, Wang Y, Wang P, Xiu G. ASMTL‐AS1 impedes the malignant progression of lung adenocarcinoma by regulating SAT1 to promote ferroptosis. Pathol Int. 2021;71(11):741‐751. [DOI] [PubMed] [Google Scholar]
- 174. Chen C, Huang Y, Xia P, et al. Long noncoding RNA Meg3 mediates ferroptosis induced by oxygen and glucose deprivation combined with hyperglycemia in rat brain microvascular endothelial cells, through modulating the p53/GPX4 axis. Eur J Histochem. 2021;65(3):3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mao C, Wang X, Liu Y, et al. A G3BP1‐interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res. 2018;78(13):3484‐3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wang Z, Chen X, Liu N, et al. A nuclear long non‐coding RNA LINC00618 accelerates ferroptosis in a manner dependent upon apoptosis. Mol Ther. 2021;29(1):263‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Niu Y, Zhao X, Wu YS, Li MM, Wang XJ, Yang YG. N6‐methyl‐adenosine (m6A) in RNA: an old modification with a novel epigenetic function. Genomics Proteomics Bioinformatics. 2013;11(1):8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Chen J, Wei X, Yi X, Jiang DS. RNA modification by m(6)A methylation in cardiovascular disease. Oxid Med Cell Longev. 2021;2021:8813909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Song Z, Jia G, Ma P, Cang S. Exosomal miR‐4443 promotes cisplatin resistance in non‐small cell lung carcinoma by regulating FSP1 m6A modification‐mediated ferroptosis. Life Sci. 2021;276:119399. [DOI] [PubMed] [Google Scholar]
- 180. Li N, Yi X, He Y, et al. Targeting ferroptosis as a novel approach to alleviate aortic dissection. research paper. Int J Biol Sci. 2022;18(10):4118‐4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Liu L, He J, Sun G, et al. The N6‐methyladenosine modification enhances ferroptosis resistance through inhibiting SLC7A11 mRNA deadenylation in hepatoblastoma. Clin Transl Med. 2022;12(5):e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Xu Y, Lv D, Yan C, et al. METTL3 promotes lung adenocarcinoma tumor growth and inhibits ferroptosis by stabilizing SLC7A11 m(6)A modification. Cancer Cell Int. 2022;22(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Shi H, Zhang X, Weng YL, et al. A facilitates hippocampus‐dependent learning and memory through YTHDF1. Nature. 2018;563(7730):249‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Li Q, Ni Y, Zhang L, et al. HIF‐1alpha‐induced expression of m6A reader YTHDF1 drives hypoxia‐induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther. 2021;6(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Lin X, Chai G, Wu Y, et al. RNA m(6)A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat Commun. 2019;10(1):2065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 186. Shen M, Li Y, Wang Y, et al. N(6)‐methyladenosine modification regulates ferroptosis through autophagy signaling pathway in hepatic stellate cells. Redox Biol. 2021;47:102151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ruan F, Zeng J, Yin H, et al. RNA m6A modification alteration by black phosphorus quantum dots regulates cell ferroptosis: implications for nanotoxicological assessment. Small Methods. 2021;5(3):e2001045. [DOI] [PubMed] [Google Scholar]
- 188. Fan Z, Yang G, Zhang W, et al. Hypoxia blocks ferroptosis of hepatocellular carcinoma via suppression of METTL14 triggered YTHDF2‐dependent silencing of SLC7A11. J Cell Mol Med. 2021;25(21):10197‐10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ma L, Zhang X, Yu K, et al. Targeting SLC3A2 subunit of system XC(‐) is essential for m(6)A reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radic Biol Med. 2021;168:25‐43. [DOI] [PubMed] [Google Scholar]
- 190. Ye J, Chen X, Jiang X, Dong Z, Hu S, Xiao M. RNA demethylase ALKBH5 regulates hypopharyngeal squamous cell carcinoma ferroptosis by posttranscriptionally activating NFE2L2/NRF2 in an m(6) A‐IGF2BP2‐dependent manner. J Clin Lab Anal. 2022;36(7):e24514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ji FH, Fu XH, Li GQ, He Q, Qiu XG. FTO prevents thyroid cancer progression by SLC7A11 m6A methylation in a ferroptosis‐dependent manner. Front Endocrinol (Lausanne). 2022;13:857765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zhuang S, Ma Y, Zeng Y, et al. METTL14 promotes doxorubicin‐induced cardiomyocyte ferroptosis by regulating the KCNQ1OT1‐miR‐7‐5p‐TFRC axis. Cell Biol Toxicol. 2021. [DOI] [PubMed] [Google Scholar]
- 193. Sato H, Fujiwara K, Sagara J, Bannai S. Induction of cystine transport activity in mouse peritoneal macrophages by bacterial lipopolysaccharide. Biochem J. 1995;310:547‐551. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Schnurr K, Borchert A, Kuhn H. Inverse regulation of lipid‐peroxidizing and hydroperoxyl lipid‐reducing enzymes by interleukins 4 and 13. FASEB J. 1999;13(1):143‐154. [DOI] [PubMed] [Google Scholar]
- 195. Zheng H, Jiang L, Tsuduki T, Conrad M, Toyokuni S. Embryonal erythropoiesis and aging exploit ferroptosis. Redox Biol. 2021;48:102175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Wu W, Li D, Feng X, et al. A pan‐cancer study of selenoprotein genes as promising targets for cancer therapy. BMC Med Genomics. 2021;14(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Dai E, Han L, Liu J, et al. Autophagy‐dependent ferroptosis drives tumor‐associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16(11):2069‐2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Su L, Jiang X, Yang C, et al. Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J Biol Chem. 2019;294(50):19395‐19404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhang Z, Yao Z, Wang L, et al. Activation of ferritinophagy is required for the RNA‐binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14(12):2083‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Hu CL, Nydes M, Shanley KL, Morales Pantoja IE, Howard TA, Bizzozero OA. Reduced expression of the ferroptosis inhibitor glutathione peroxidase‐4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurochem. 2019;148(3):426‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Seiler A, Schneider M, Förster H, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15‐lipoxygenase dependent‐ and AIF‐mediated cell death. Cell Metab. 2008;8(3):237‐248. [DOI] [PubMed] [Google Scholar]
- 203. Chen L, Hambright WS, Na R, Ran Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J Biol Chem. 2015;290(47):28097‐28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter‐specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29(10):2658‐2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


