Abstract
Circulating IgM present in the body prior to any apparent Ag exposure is referred to as natural IgM. Natural IgM provides protective immunity against a variety of pathogens. Salmonella enterica serovar Typhi (S. Typhi) is the causative agent of typhoid fever in humans. Because mice are not permissive to S. Typhi infection, we employed a murine model of typhoid using S. enterica serovar Typhimurium expressing the Vi polysaccharide (ViPS) of S. Typhi (S. Typhimurium strain Typhi RC60) to evaluate the role of natural IgM in pathogenesis. We found that natural mouse IgM binds to S. and S. Typhimurium. The severity of S. Typhimurium infection in mice is dependent on presence of the natural resistance-associated macrophage protein 1 (Nramp1) allele; therefore, we infected mice deficient in secreted form of IgM (sIgM) on either a Nramp1-resistant (129S) or -susceptible (C57BL/6J) background. We found that the lack of natural IgM results in a significantly increased susceptibility and an exaggerated liver pathology regardless of the route of infection or the Nramp1 allele. Reconstitution of sIgM−/− mice with normal mouse serum or purified polyclonal IgM restored the resistance to that of sIgM+/+ mice. Furthermore, immunization of sIgM−/− mice with heat-killed S. Typhi induced a significantly reduced anti-ViPS IgG and complement-dependent bactericidal activity against S. Typhi in vitro, compared with that of sIgM+/+ mice. These findings indicate that natural IgM is an important factor in reducing the typhoid severity and inducing an optimal anti-ViPS IgG response to vaccination.
INTRODUCTION
Salmonella enterica serovar Typhi (S. Typhi) is the causative agent of typhoid fever in humans. Global estimates reported by the Centers for Disease Control and Prevention indicate that 21.6 million cases of typhoid fever occur each year resulting in 226,000 deaths (1). The rapid emergence of multiple drug-resistant strains of S. Typhi now complicates the treatment of typhoid (2). Typhoid is a vaccine-preventable disease, and vaccination of high-risk populations such as infants and young children is considered the most promising strategy for control (3). Vi polysaccharide (ViPS) is a target for immune responses, and anti-ViPS Abs correlate with protection against typhoid fever (3–5). Three types of vaccines are currently available (5): 1) a live attenuated vaccine (Vivotif), 2) a subunit vaccine composed of plain ViPS (Typhim Vi or Typherix) (5, 6), and 3) ViPS conjugated to a variety of carrier proteins such as a recombinant exoprotein A from P. aeruginosa (7), CRM197, a nontoxic mutant of diphtheria toxin (8), tetanus toxoid (9), or diphtheria toxoid (10). The efficacy of live-attenuated oral vaccine is ~60% (6, 7), and the plain ViPS, an injectable vaccine, is ~55% in older children and adults (5, 11). Importantly, plain ViPS vaccines do not induce optimal Ab responses in children <2 y of age. ViPS conjugated to recombinant exoprotein A, as well as tetanus toxoid, can induce anti-ViPS responses in infants and young children with 80–90% efficacy (7, 12, 13). ViPS conjugated to CRM197 was not very immunogenic in multinational clinical trials in the Philippines, Pakistan, and India (8, 14). Surprisingly, the secondary and tertiary boosters of this ViPS conjugate vaccine in these typhoid-endemic countries did not yield any increase in the IgG titers to ViPS (8, 14). The reasons for this lack of an optimal anti-ViPS IgG response are not understood.
One of the barriers to advancing the treatment and prevention of typhoid is the lack of a suitable animal model to study S. Typhi infection (6). Because S. Typhi is strictly human adapted, S. enterica serovar Typhimurium (S. Typhimurium), a natural pathogen of mice causing a “typhoid-like” systemic disease in mice, became the most widely used bacterium to understand pathogenesis and immunity in inbred mice (e.g., C57BL6/J and 129Sv strains). Organs of the mononuclear phagocytic system, such as liver and spleen, are the major sites of replication of S. Typhimurium during infection in mice (15, 16). The relative susceptibility or resistance to S. Typhimurium is dependent on a single nucleotide polymorphism in the Slc11a1 gene that encodes an ion transporter commonly referred to as natural resistance-associated macrophage protein 1 (Nramp1) (17). Humans and 129S mice have a functional Nramp1 allele, whereas C57BL/6 mice have the Nramp1 mutant allele that encodes for a nonfunctional protein. Consequently, C57BL/6J mice are more susceptible to S. Typhimurium infection than is the 129S strain (15). S. Typhimurium shares 90% of its genes with S. Typhi (18). However, ~600 S. Typhi genes, including those encoding for the ViPS biogenesis, a well-known virulence factor, are absent in S. Typhimurium, and several genes found in S. Typhimurium are pseudogenes in S. Typhi (18, 19). To understand the function of ViPS in vivo, Bäumler and colleagues (20) introduced all of the S. Typhi genes required for ViPS biogenesis in S. Typhimurium. Using this “chimeric” S. Typhimurium strain TH170 (20), roles for ViPS in resisting C3 deposition and complement receptor 3–mediated clearance (21), evading host TLR4 recognition (22, 23), and microbe-guided neutrophil chemotaxis (24) were identified. The length of LPS of S. Typhimurium is significantly elongated compared with S. Typhi. In S. Typhimurium the length of LPS is controlled by FepE gene product, which is a pseudogene in S. Typhi (19). To modify the surface characteristics of S. Typhimurium to resemble those of S. Typhi, the S. Typhimurium strain TH170 was further engineered by deleting the FepE gene (25). This strain of S. Typhimurium, referred to as RC60, exhibits cell surface characteristics of S. Typhi and captures certain aspects of S. Typhi pathology in mice (25). Using the S. Typhimurium strain RC60, we were able to identify several features of the anti-ViPS Ab repertoire required for protective immunity in vivo (16, 26, 27).
Circulating IgM present in the body prior to any apparent Ag exposure is referred to as natural IgM (28–30). Natural IgM in mice is produced mainly by B1a cells and marginal zone B (MZB) cells, can bind to evolutionarily conserved Ags on pathogens, and plays an important role in protective immunity (28–33). In contrast, Ab responses to a variety of bacterial Ags, including ViPS, are generated from B1b cells (34–38). Mice deficient in activation-induced cytidine deaminase (AID) have Abs that can neither undergo Ig isotype switching nor somatic hypermutation of the variable regions (39). The absence of functional AID in humans leads to significantly increased basal serum IgM levels, also known as hyper-IgM syndrome type 2 (40). Similarly, AID−/− mice have a 2- to 3-fold increase in basal levels of natural IgM (39). Using AID−/− mice, we have shown that unmutated IgM Abs against ViPS can confer protective immunity against S. Typhimurium RC60 infection (16). A striking observation was that the necrotic regions of the liver of unimmunized AID−/− mice infected with S. Typhimurium RC60 were significantly smaller than those in unimmunized wildtype mice (16). We hypothesized that this decrease in pathology is attributable to having 2- to 3-fold higher levels of natural IgM in the AID−/− mice than in naive wild-type mice (39). This finding is consistent with the fact that passive immunization of mice with high doses of polyclonal human IgM can reduce S. Typhimurium burden in various tissues (41). In the current study, we determined the role of natural IgM in typhoid susceptibility in a murine model by infecting mice deficient in the secreted form of IgM (sIgM−/−) with S. Typhimurium RC60 and the impact of natural IgM on the anti-ViPS IgG response to immunization.
MATERIALS AND METHODS
Mice
The Thomas Jefferson University Institutional Animal Care and Use Committee has approved these studies. Mice were housed in microisolator cages with free access to food and water and were maintained in a specific pathogen-free facility. Mice that lack sIgM but have the membrane-bound form of IgM (sIgM−/−) have been previously described (42). The sIgM−/− mice used are on a 129Sv (129Sv.sIgM−/−) or C57BL/6J (B6.sIgM−/−) background. Control wild-type (129S1/SvImJ; stock no. 002448) and (C57BL/6J; stock no. 000664) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The sIgM genotype/phenotype is confirmed either by PCR or IgM ELISA (42). To determine the variation in the Nramp1 allele in the 129Sv.sIgM−/− and B6.sIgM−/− mice, a 514-bp fragment of the Nramp1 gene was amplified by PCR using primers 5′-AAGTGACATCTCGCCATAGGTGCC-3′ and 5′-TTCTCTCACCATAGTTATCCAAG AAG-3′ (forward and reverse, respectively). The purified PCR product was then sequenced using the primer 5′-CCCCCATCTATGTTATCACCC-3′ (43). Sequencing for the point mutation that results in a glycine to aspartate coding change at position 169 within Nramp1 was as described (44). Age-matched (8- to 12-wk-old) mice of both sexes were used for all experiments.
Infections
Mice were infected with a chimeric strain of S. Typhimurium (strain RC60) that expresses the S. Typhi genes necessary for ViPS synthesis, export, and regulation as in S. Typhi (25). Strain RC60 was grown to an OD600 of ~1.0 in Luria–Bertani (LB) broth with 10 mM NaCl. The expression of ViPS was assessed by a slide agglutination test using a commercial Vi mAb reagent (Statens Serum Institute Diagnostica, Copenhagen, Denmark; lot 188L-8). Bacteria were washed twice in Dulbecco’s PBS (DPBS), and 100 μl of DPBS containing 3 × 104 CFU was injected i.p. or i.v. At 3 d postinfection liver and spleen were collected, and the tissues were processed using a Minilys tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). The bacterial burdens in the liver and spleen homogenates, as well as blood collected into anticoagulant, were determined by plating serial 10-fold dilutions on LB agar plates followed by counting CFU (16, 26, 27).
Histopathology analysis
Liver tissues obtained on day 3 postinfection were fixed in 10% buffered formalin, and 4 μM paraffin-embedded sections were stained with H&E. The specimen slides were scanned at ×20 magnification on an Aperio CS2 ScanScope (Leica Biosystems), and total, necrotic, and infiltration areas composed of lymphocytes and other mononuclear cells in the entire specimen were quantified using Aperio ImageScope software (Leica Biosystems) (16, 26).
Reconstitution of mice with naive mouse serum or IgM
Mice sufficient or deficient in IgM (i.e., 129Sv.sIgM+/+ or 129Sv.sIgM−/−) were injected i.p. with 150 μl of sterile-filtered serum obtained from naive 129S1/SvImJ, 129Sv.sIgM−/− mice or 200 μl of PBS containing 200 μg of purified polyclonal IgM purchased from Rockland Immunochemicals (Pottstown, PA). The commercial IgM preparation was purified from normal serum and contains 0.1% azide and 0.5 M NaCl and 0.1 M Tris (item no. 010–0107; Rockland Immunochemicals, Pottstown, PA). Therefore, the IgM preparation was dialyzed against PBS (1000 ml) twice in a 10,000 Da molecular mass cutoff Slide-ALyzer MINI dialysis device (Thermo Fisher Scientific). Because IgM has the shortest half-life among all of the Ab isotypes (45), blood was sampled a day after transfer to confirm IgM levels by ELISA and at the same time the mice were challenged with strain RC60 i.p.
Depletion of neutrophils
One day before infection, mice were injected i.p. with 300 μg of anti-mouse Ly6G/Ly6C (Gr-1) mAbs in 200 μl of PBS (clone RB6-8C5; Bio X Cell, Lebanon, NH) or PBS alone. As previously reported, this treatment results in neutrophil depletion for 4 d (46).
Immunization
For whole bacterial immunization, mice were injected i.p. with 3 × 108 CFU of heat-killed S. Typhi strain Ty2 (grown in LB broth containing 10 mM NaCl) in 100 μl of DPBS (16). Blood samples were obtained 0, 7, 14, 21, or 28 d following immunization and stored at −20°C.
ELISA
ViPS-specific IgM and IgG in the blood were measured by coating 96-well microtiter plates (Nunc MaxiSorp; Invitrogen, Carlsbad, CA) with 2 μg/ml ViPS purified from S. Typhi clinical isolate C6524 (47) in DPBS overnight at room temperature. All plates were washed and blocked with 1% BSA in PBS (pH 7.2) (blocking buffer) for 1 h at room temperature. Blood samples from immunized mice were diluted to 1:25 for IgG detection and 1:50 for IgM detection in blocking buffer, and samples were centrifuged (800 × g for 10 min) and cell-free supernatant was used. The dilutions, 1:25 for IgG and 1:50 for IgM, were chosen after evaluating various serum dilutions within the linear range by ELISA. Bound IgM or IgG was measured using HRP-conjugated goat anti-mouse IgM or IgG (Bethyl Laboratories, Montgomery, TX). Because ViPS-specific mouse IgM and IgG reference standards are not available, and the ViPS-specific Abs in mice are likely to be of oligoclonal nature with varying affinities, the Ag-specific Ab levels in the current study were interpreted as nanogram per microliter “equivalents” using normal mouse serum IgM or IgG standards (Bethyl Laboratories, Montgomery, TX).
Serum bactericidal assay
A serum bactericidal assay was performed as previously described (48). In brief, log-phase cultures (OD600 of 0.5 at 37°C) of S. Typhi strain Ty2 were prepared in LB broth with 10 mM NaCl. Bacterial cells were washed in DPBS, and the bacterial cell density was adjusted to 2.5–5.0 × 104 CFU/ml in DPBS. The expression of ViPS was assessed by a slide agglutination test using a commercial Vi mAb reagent (Statens Serum Institute Diagnostica, Copenhagen, Denmark; lot 188L-8). Serum samples were heat-inactivated by incubating at 56°C for 30 min prior to use in the assay. Ten microliters of S. Typhi strain Ty2 in DPBS (250–500 CFU) was added to each well of a round-bottom polypropylene 96-well plate containing 50 μl of heat-inactivated serum in serial dilutions, 12.5 μl of baby rabbit complement (Pel-Freez Biologicals, Rogers, AR), and 27.5 μl of DPBS. Triplicate samples of each dilution were incubated for 120 min at 37°C with gentle rocking, and 10 μl of this mixture were plated on LB agar plates for counting CFU. Serum bactericidal Ab titers are defined as the reciprocal of the highest dilution that produced 50% killing in relationship to control wells containing complement, but no mouse serum. Naive mouse serum served as a negative control, and serum from either mice immunized with heat-killed Escherichia coli strain W3110 expressing pDC5 plasmid, which contains the genes necessary for the synthesis and export of ViPS (49), or S. Typhi anti-Vi human IgG standard (lot R1, 2011; U.S. Food and Drug Administration, Silver Spring, MD) served as two independent positive controls.
Natural IgM binding assay
S. Typhimurium strains IR715 and RC60 and S. Typhi strain Ty2 were grown in LB broth. Overnight bacterial cultures were washed twice in PBS, then resuspended in 25% normal mouse serum (from C57BL/6 mice) in PBS to achieve a final concentration 1 × 109 CFU/ml. After a 1-h incubation at room temperature, the bacterial cells were washed three times in PBS by centrifugation (16,000 × g for 8 min). Bound serum proteins were eluted by resuspending the bacterial pellet in 100 μl of 0.1 M glycine-HCl for 30 min. Following centrifugation (16,000 × g for 8 min), 80-μl supernatants were collected and neutralized with 20 μl of 1 M Tris-HCl buffer (pH 8.0). Two microliters of these samples was mixed with 48 μl of Laemmli buffer containing 2-ME and boiled for 10 min. Five microliters of the samples was subjected to SDS-PAGE and Western blot analyses. After electrotransfer, the polyvinylidene difluoride membranes (Merck Millipore) were blocked for 1 h in 2% BSA in PBS containing 0.25% Tween 20. The blots were probed either with HRP-conjugated goat anti-mouse IgM Ab or with HRP-conjugated goat anti-mouse IgG Ab (Bethyl Laboratories, Montgomery, TX). After a 1-h incubation, the blots were washed with PBS containing 0.25% Tween 20, developed using Super-Signal West Dura substrate (Thermo Scientific, Rockford, IL), and images were obtained on an imager, Protein Simple FluorChemR (Biotechne, Minneapolis, MN).
Statistical analysis
Data presented throughout depict pooled data from at least two independent experiments. Statistics were performed using the Prism 5 software program (GraphPad Software, La Jolla, CA), and the statistical tests are indicated in the figure legends.
RESULTS
Normal serum IgM binds efficiently to S. Typhi and S. Typhimurium
Natural IgM binds to evolutionarily conserved Ags of bacteria and other microorganisms (31, 32). This property of natural IgM plays an important role in the host defense. To test whether natural IgM binds to S. Typhi and S. Typhimurium, we performed a mouse IgM binding assay using serum obtained from naive C57BL6/J mice. This assay is identical to the one developed for human IgM binding to Salmonella, except we used mouse serum instead of human serum (50). We found that normal mouse serum IgM adsorbed more efficiently than any other serum proteins to S. Typhi and S. Typhimurium, as evidenced by the appearance of a major band at the 65 kDa position in the Coomassie gel, which corresponds to the molecular mass of the IgM H chain under reducing conditions (Fig. 1). Immunoblot analysis indeed confirmed that this band is reactive to anti-mouse IgM (Fig. 1). To test whether natural mouse IgG also binds to Salmonella, we incubated an identical blot with goat-anti mouse IgG Abs. Although the band is very faint in the Coomassie gel, we observed a 50-kDa band with molecular mass of the IgG H chain under reducing conditions, reactive with anti-mouse IgG (Fig. 1). A comparable reactivity to S. Typhimurium strain IR715, which does not express ViPS, indicates that natural IgM and IgG bind to the surface of these bacteria, and to Ags other than ViPS Ag (Fig. 1). These data demonstrate that natural IgM and to a small extent natural IgG bind to S. Typhi and S. Typhimurium.
FIGURE 1. Normal serum IgM binds to S. Typhi and S. Typhimurium.
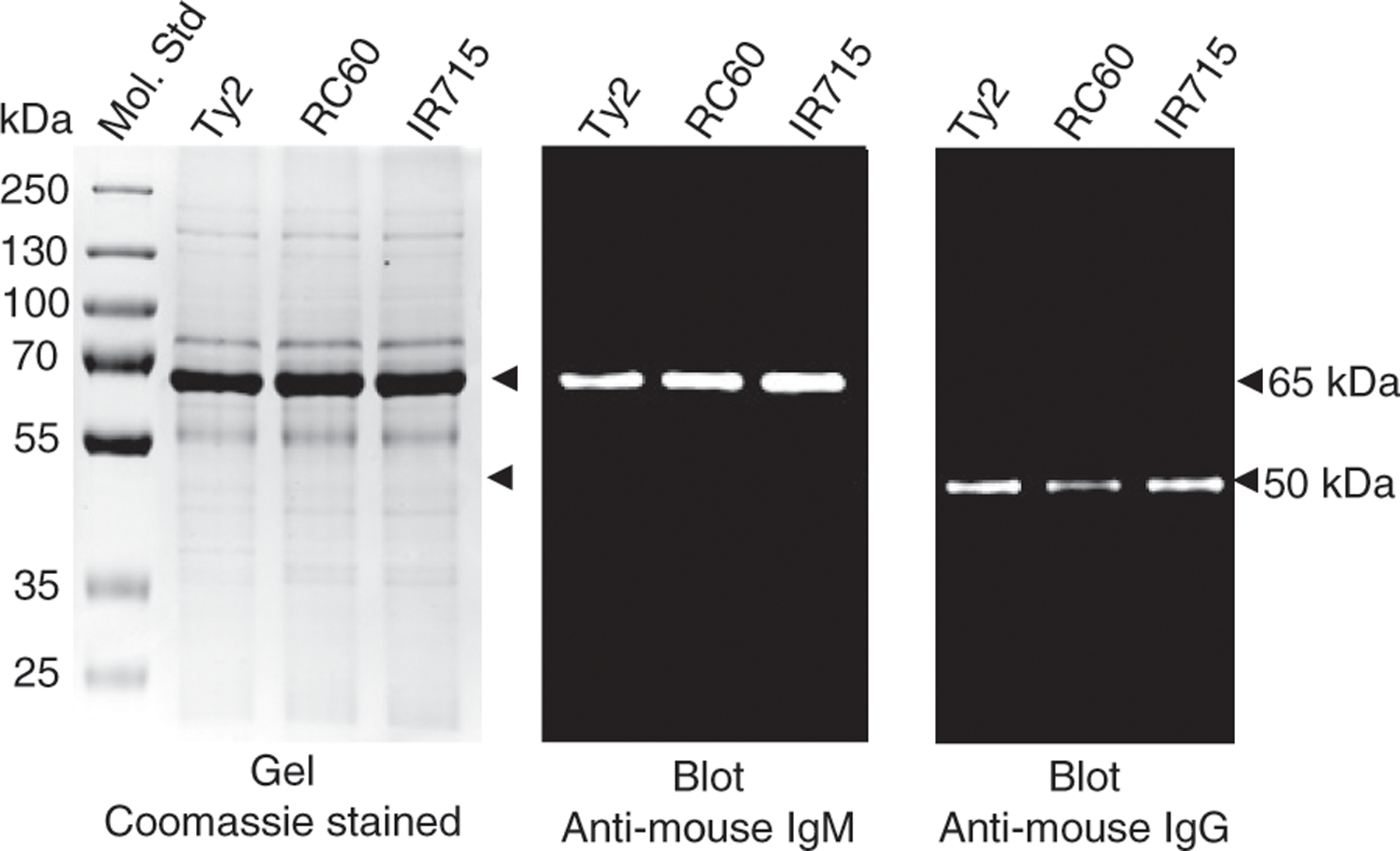
Normal mouse serum from C57BL/6 mice was incubated with indicated bacterial strains. Serum proteins bound to bacteria were eluted and subjected to SDS-PAGE and Western blot analyses. The gel was stained with Coomassie Blue, and the blots were developed using HRP-conjugated anti-mouse IgM or anti-mouse IgG. Arrowheads at 65 and 50 kDa correspond to IgM and IgG H chains under reducing conditions.
Mice deficient in sIgM are hypersusceptible to typhoid
To test whether natural IgM contributes to the control of S. Typhimurium in vivo, we infected wild-type 129S1/SvImJ mice (129S.sIgM+/+) or mice deficient in sIgM (129S.sIgM−/−) with S. Typhimurium strain RC60 either i.v. or i.p. Compared with 129S.sIgM+/+ mice, the 129S.sIgM−/− mice had a significantly increased bacterial burden in the blood, liver, and spleen regardless of the route of infection (Fig. 2A). S. Typhimurium susceptibility in mice is determined by the type of Nramp1 allele (17, 51–53). 129S1/SvImJ mice have the Nramp1-resistant alleles as in humans, and S. Typhimurium infection causes a chronic infection (15, 54). Conversely, the C57BL/6J mouse strain has the Nramp1-susceptible alleles, and S. Typhimurium infection causes an acute and lethal infection (15, 54). To test whether the Nramp1-resistant or -susceptible allele has an impact on the role of natural IgM-mediated protective immunity, we also infected C57BL/6J mice sufficient or deficient in sIgM (B6.sIgM+/+ or B6.sIgM−/−) with S. Typhimurium strain RC60. As previously shown for the S. Typhimurium strain SL1344 (15), C57BL/6J mice are more susceptible than 129S1/SvImJ mice to S. Typhimurium strain RC60 infection, indicated by 100- to 1000-fold higher bacterial burden (Fig. 2). Importantly, B6.sIgM−/− mice had a significantly increased susceptibility compared with the B6.sIgM+/+ mice (Fig. 2B). These data indicate that natural IgM contributes to the control of murine typhoid.
FIGURE 2. Increased bacterial burden in mice deficient in preimmune or natural IgM Abs.
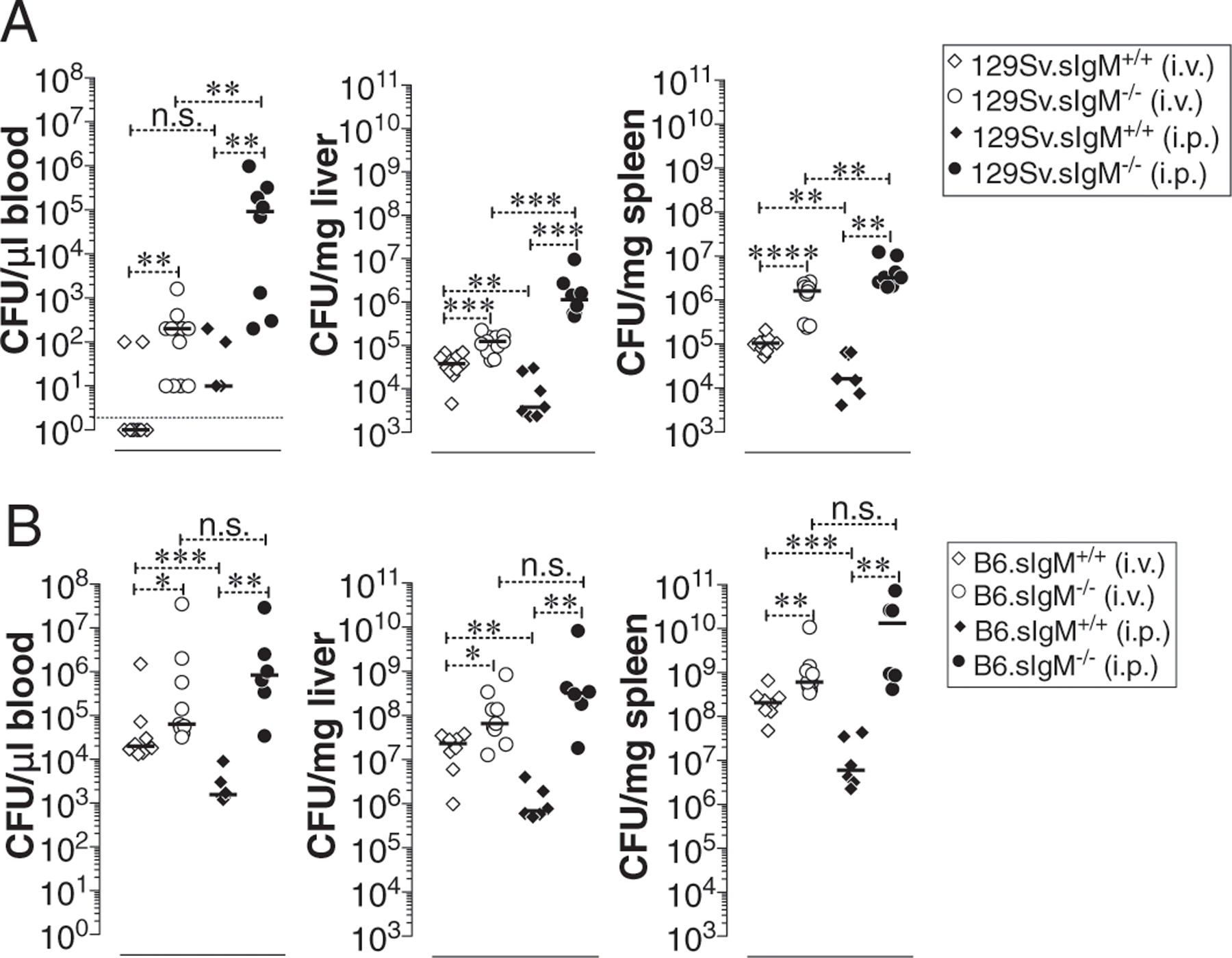
(A and B) Mice deficient in sIgM on 129S (Nramp1 r allele) (A) or on C57BL/6J (B6; Nramp1 s allele) (B) background were infected with 3 × 104 CFU of ViPS expressing S. Typhimurium strain RC60 either i.v. or i.p. At 3 d postinfection mice were sacrificed and bacterial burden in the liver and spleen was determined by plating serial 10-fold dilutions of tissue homogenates followed by colony counting. Each dot represents an individual mouse, and the black bar represents the mean. Dotted line in (A) indicates the limit of detection in blood. The data represent a pool of two independent experiments. Statistical differences were determined by a Mann–Whitney U test. *p < 0.05, **p < 0.01, ***p < 0.001. n.s., not significant.
Mice deficient in sIgM exhibit increased liver pathology
The murine typhoid-induced liver lesions consist of areas of mononuclear cell infiltrates and/or necrosis (16, 26) akin to the lesions described in the liver biopsies of human typhoid patients (55–57). Compared to the i.v. route of infection, the i.p. route of systemic infection resulted in higher bacterial burden in the absence of sIgM in both B6 and 129S backgrounds (Fig. 2). To test whether the increased susceptibility also reflects an exacerbated liver pathology, H&E-stained tissues specimens were evaluated by an observer-blind histopathological analysis. As expected, the relatively resistant 129S.sIgM+/+ mice showed smaller areas of mononuclear cell infiltration that increased significantly in 129S.sIgM−/− mice (Fig. 3A versus Fig. 3B) without a significant difference in the size of necrotic areas (Fig. 3E). In contrast, the necrotic areas in the livers of B6.sIgM−/− mice were significantly larger compared with B6.sIgM+/+ mice, whereas the areas of mononuclear cell infiltration were similar (Fig. 3B, 3D, 3F). These data indicate that natural IgM is a factor controlling the extent of liver pathology during the early stages of typhoid.
FIGURE 3. Increased liver pathology in mice deficient in sIgM.
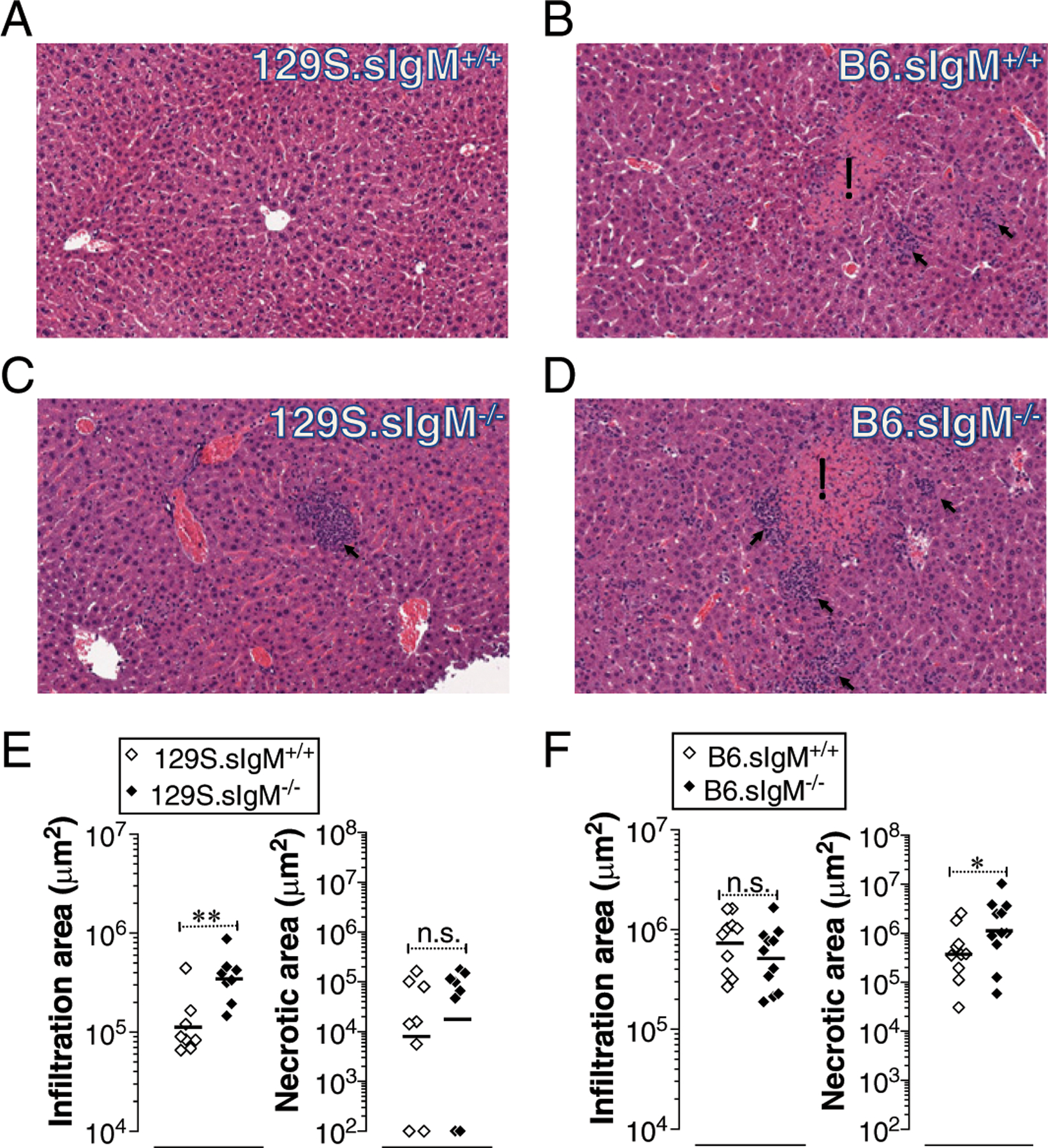
(A–F) Mice deficient in sIgM either on 129S (Nramp1 r allele) (A, C, and E) or on C57BL/6 (B6; Nramp1 s allele) (B, D, and F) background were infected with 3 × 104 CFU of ViPS expressing S. Typhimurium strain RC60 i.p. and H&E-stained liver sections of 3-d postinfected mice were analyzed. In (A)–(D), necrotic lesions are indicated with an asterisk and mononuclear cellular infiltration areas are indicated with arrows (original magnification ×20). (E and F) Quantification of liver pathology. Each dot represents an individual mouse, and the bar represents the mean. The data represent a pool of two independent experiments. Statistical differences were determined by a Mann–Whitney U test. *p < 0.05, **p < 0.01. n.s., not significant.
Reconstitution with naive wild-type mouse serum or polyclonal IgM prevents the hypersusceptibility of 129S.sIgM−/− mice
To test whether the increased susceptibility of sIgM−/− mice to S. Typhimurium strain RC60 infection is attributable to natural IgM deficiency, we transferred either naive wild-type or sIgM−/− mouse serum, or purified IgM to 129S.sIgM+/+ and 129S.sIgM−/− mice, prior to bacterial challenge. The serum of wild-type mice contains ~300 ng/μl IgM. By injecting 150 μl of wild-type mouse serum we restored the serum IgM levels of the 129S.sIgM−/− mice to ~30 ng/μl (Fig. 4A). Remarkably, even at this level of IgM reconstitution, the susceptibility of 129S.sIgM−/− mice to S. Typhimurium strain RC60 infection was significantly reduced as evident by a decrease in the bacterial burden in the blood, liver, and spleen (Fig. 4B). To confirm that the decreased bacterial burden in 129S.sIgM−/− mice was due to natural IgM alone, we transferred 200 μg of commercially available purified IgM from normal mouse serum. Coincidentally, we found similar levels of IgM (~30 ng/μl) reconstitution when we transferred 200 μg of purified IgM (Fig. 4C) as we found with 150 μl of whole serum transfer (which contains ~45 μg of IgM) (Fig. 4A). This level of purified polyclonal IgM reconstitution was sufficient in reducing the 129S.sIgM−/− mice hypersusceptibility to that of 129S.sIgM+/+ mice as evident by a corresponding decrease in the bacterial burden in blood, liver, and spleen (Fig. 4D). These data indicate that natural IgM in naive mouse serum plays a significant role in controlling the severity of typhoid.
FIGURE 4. Transfer of naive wild-type serum or purified polyclonal IgM reduces the bacterial burden in mice deficient in sIgM.
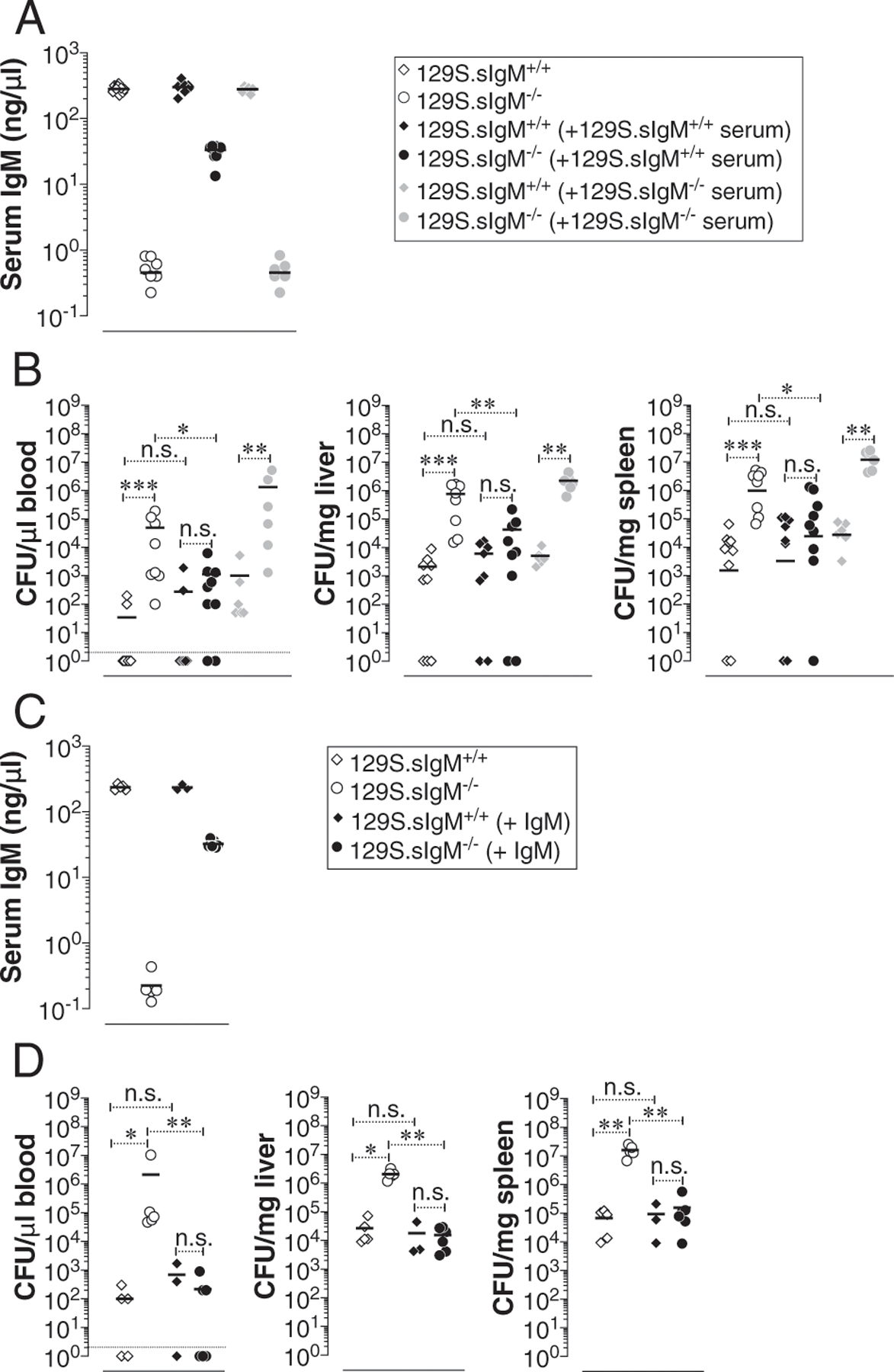
(A and C) 129Sv mice deficient or sufficient in sIgM were injected with 150 μl of wild-type or sIgM−/− serum or 150 μg of purified polyclonal IgM i.p. and a day later total IgM levels of recipient mice were determined by ELISA. (B and D) Mice were infected i.p. with 3 × 104 CFU of S. Typhimurium strain RC60. At 3 d postinfection, bacterial burden was determined as in Fig. 1. Each dot represents an individual mouse, and the bar represents the median. The data represent a pool of two independent experiments. Statistical differences were determined by a Mann–Whitney U test. *p < 0.05, **p < 0.01, ***p < 0.001. n.s., not significant.
Neutrophils play an important role in the protective immunity during early phases of Salmonella infection (58, 59). Depletion of neutrophils in mice increases the bacterial load in the blood, liver, and spleen (58, 59). FcμR (also known as TOSO), an Fc receptor for IgM, is expressed on neutrophils (60). To test the extent of protection mediated by natural IgM in the absence of neutrophils, we depleted neutrophils in mice sufficient or deficient in IgM. Depletion of neutrophils increased the bacterial burden in the livers more than in the blood and spleen of both 129S.sIgM+/+ and 129S.sIgM−/− mice (Fig. 5). Interestingly, reconstitution of IgM (by transferring wild-type mouse serum) in 129S.sIgM−/− mice significantly reduced the bacterial burden in the blood and spleen, but not in the liver. These data suggest that natural IgM contributes to protective immunity additively with that of the protection conferred by neutrophils (Fig. 5).
FIGURE 5. Natural IgM does not rescue the hypersusceptibility in neutrophil-depleted IgM-deficient mice.
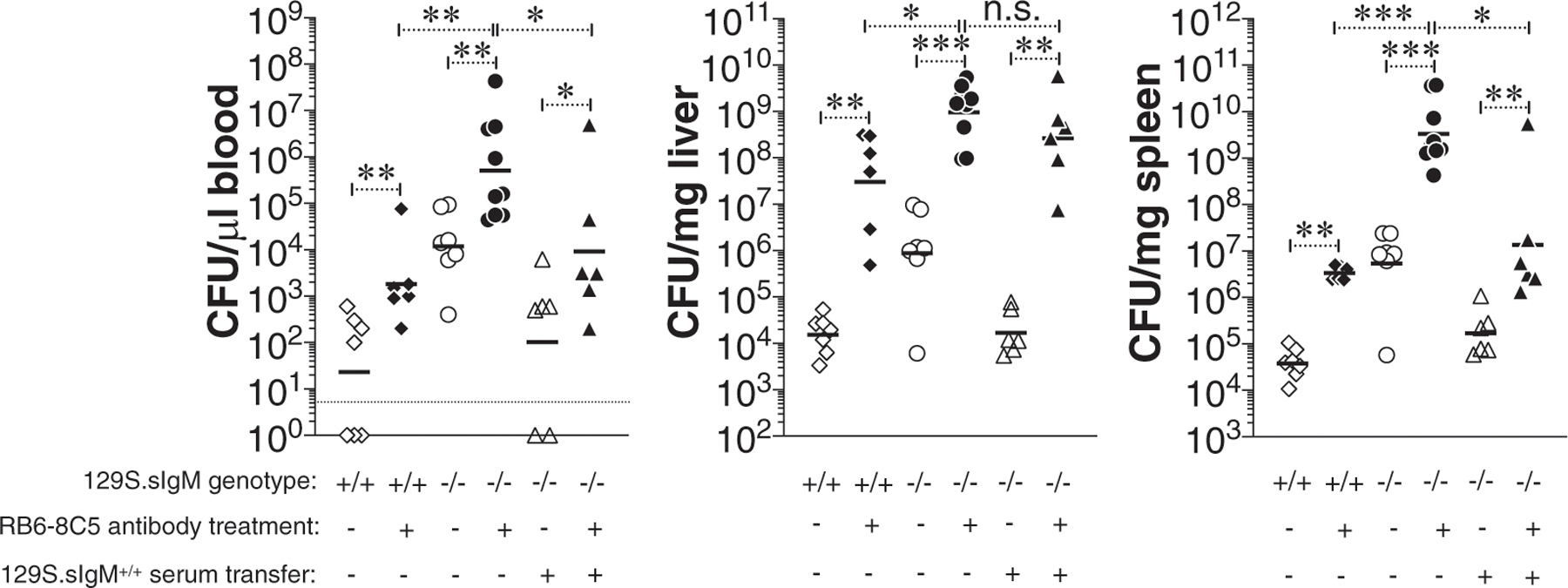
129S.sIgM+/+ or 129S.sIgM−/− mice were treated with anti–GR-1 mAbs i.p. to deplete neutrophils. On the same day some 129S.sIgM−/− mice were also given 129S.sIgM+/+ serum as in Fig. 3A. A day later, all mice were infected i.p. with 3 × 104 CFU of S. Typhimurium strain RC60. At 3 d postinfection, bacterial burden was determined as in Fig. 1. Each dot represents an individual mouse, and the bar represents the median. The data represent a pool of two independent experiments. Statistical differences were determined by a Mann–Whitney U test. *p < 0.05, **p < 0.01, ***p < 0.001. n.s., not significant.
IgM-deficient mice show impaired ViPS-specific IgG responses to vaccination
sIgM−/− mice express all IgG isotypes with baseline levels of IgG1, IgG2a, IgG2b, and IgG3 comparable to those of wild-type mice (42). Immunization with NP-Ficoll, a widely used synthetic polysaccharide model Ag, induces a comparable overall IgG response in wild-type and 129S.sIgM−/− mice (42). However, in a lupus mouse model, the absence of sIgM causes an accelerated development of autoreactive IgG Abs (61). To test whether the lack of sIgM impacts Ab responses to ViPS, the target Ag in all typhoid subunit vaccines, we compared anti-IgG responses to ViPS using heat-killed S. Typhi as an immunogen. We found that mice deficient in sIgM generated a significantly lower anti-ViPS IgG compared with that in wild-type mice, regardless of the mouse strain background (Fig. 6A, 6C). To compare the functionality of the immune serum, we performed a complement-dependent serum bactericidal assay against live S. Typhi. We found that compared with the sIgM+/+ mouse serum obtained either on day 7 or day 28 postimmunization, the serum of sIgM−/− mice obtained at those time points were significantly less potent in killing S. Typhi (Fig. 6B, 6D). These data indicate that natural IgM is also required for the generation of high titers of bactericidal anti-ViPS IgG responses.
FIGURE 6. Mice deficient in sIgM generate reduced anti-ViPS and bactericidal IgG Abs.
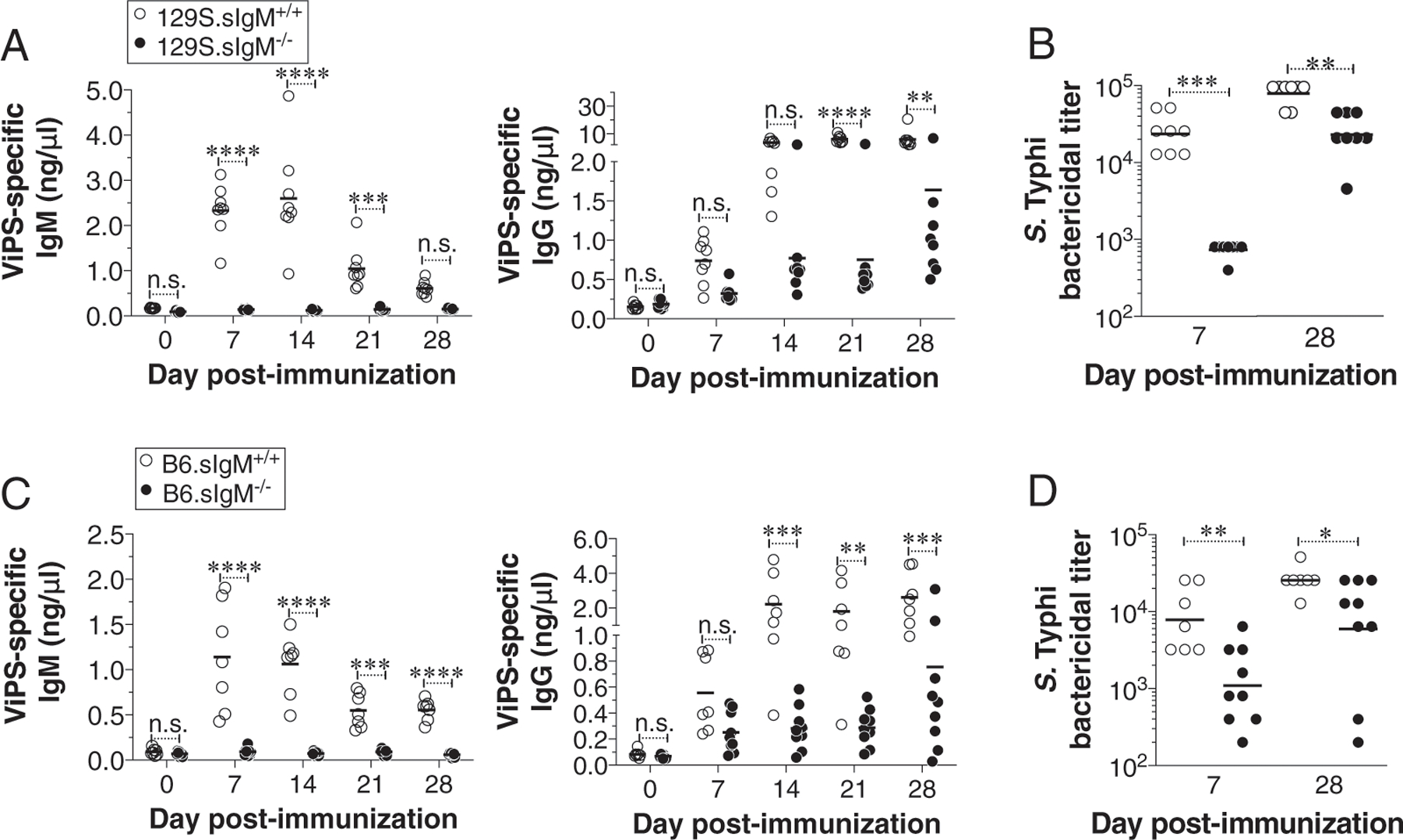
(A and C) Wild-type or mice deficient in sIgM on 129S or B6 backgrounds were immunized i.p. with heat-killed S. Typhi strain Ty2 (3 × 108 bacterial cells), and levels of ViPS-specific IgM and IgG in the blood were measured by ELISA. Each dot represents an individual mouse, and the bar represents the mean. The data represent a pool of two independent experiments. Statistical differences were determined using a two-way ANOVA with a Bonferroni posttest. (B and D) Serum bactericidal Ab titers against S. Typhi strain Ty2 were determined using serum obtained from 7 and 28 d postimmunization. Each dot represents an individual mouse, and the bar represents the geometric mean. The data represent a pool of two independent experiments. Statistical differences were determined by a Mann–Whitney U test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s., not significant.
DISCUSSION
In mice, follicular B, MZB, B1a, and B1b cell subsets are phenotypically, developmentally, and functionally distinct (32, 37, 62, 63). A division of labor exists between B1a, MZB, and B1b cells in their ability to provide protective immunity against pathogens by producing IgM. For example, B1a and MZB cells generate most of the natural IgM that binds phosphorylcholine present on the cell wall of Streptococcus pneumoniae, thereby providing protection (31, 64). In contrast, B1b cells generate the Ab responses to serotype 3 polysaccharide (PPS3) of S. pneumoniae that can also confer protection in a serotype-specific manner (37). Human natural IgM binding to the LPS of S. Typhi or S. Typhimurium promotes respiratory burst in neutrophils in vitro (50). In the current study, we show that mouse natural IgM also binds to S. Typhimurium RC60 (Fig. 1) and plays an important role in protection against S. Typhimurium RC60 in vivo (Fig. 2). In mice, B1b cells generate most of the anti–S. Typhi ViPS response, which controls S. Typhimurium expressing ViPS in vivo (34). Our data are consistent with a nonredundant role for B1a or MZB cell–derived natural IgM and B1b-derived Ag-specific Ab responses in providing immunity against typhoid, similar to the immunity described for S. pneumoniae and the influenza virus (37, 65).
IgM deficiency can impact the development of the immune system in various ways in humans and mice (33, 42, 66–68). We show that reconstitution with IgM in sIgM−/− mice 1 d prior to the infection prevented the hypersusceptibility (Fig. 4). Therefore, it is unlikely that an altered immune system of sIgM−/− mice accounted for the hypersusceptibility to S. Typhimurium RC60 infection. Furthermore, the suboptimal anti-ViPS IgG responses (Fig. 6) are unlikely due to a deficiency in B1b cells, as 129S.sIgM−/− and 129S.sIgM+/+ mice have an increased frequency of B1 cell subsets and the ratio of B1a and B1b cells is not altered (Supplemental Fig. 1) (42).
Natural IgM controls the early phases of viral and bacterial infections (69). Natural human IgM binds to LPS of S. Typhi and S. Typhimurium (50). Although IgM is an efficient isotype in activating the classical pathway of the complement system, the process requires a stable and multivalent binding on the surface of the bacterium. Using a complement-dependent bactericidal assay, we found that even at high concentrations, natural IgM does not exert any bactericidal activity (Supplemental Fig. 2). For the recruitment of C1qrs to initiate the classical pathway leading to membrane attack complex–mediated killing of the bacteria requires a stable engagement of at least two Abs with close proximity. We speculate the natural IgM binding to Ags with one of the five arms of the IgM pentamer, but the accessibility for the second arm to a nearby second epitope, might not be occurring. This explains why neither natural IgM nor natural IgG can exert any bactericidal activity. In contrast, ViPS has repetitive epitopes where Ag-specific IgM or IgG is expected to be in close proximity to recruit C1qrs and activate the classical pathway and membrane attack complex–mediated killing of the bacteria. Therefore, the mechanism of control of S. Typhimurium RC60 by natural IgM is independent of the classical pathway of complement activation.
Certain ViPS-conjugated vaccines do not induce optimal anti-ViPS IgG responses in typhoid disease–endemic areas (8), and it is possible that the natural IgM levels or natural IgM repertoire may be a factor to be considered in some populations. It was previously shown that natural IgM plays an important role in the initiation of Ag trapping and delivery of Ag immune complexes to the follicular dendritic cells in the spleen to initiate an optimal primary IgG Ab response (70–72). Natural IgM of humans agglutinates S. Typhimurium and generates immune complexes (41). In support of this, we found a robust binding of natural IgM to S. Typhi and S. Typhimurium (Fig. 1). In addition to promoting agglutination, the natural IgM may also facilitate an IgM Fc receptor–mediated opsonophagocytosis to control S. Typhimurium RC60 in vivo. Humans and mice express two distinct Fc receptors specific to IgM, namely Fcα/μR and FcμR (60) (73). Fcα/μR is expressed on B cells and macrophages and promote opsonophagocytosis of IgM-coated microbes (73). In contrast, FcμR is expressed on B cells and neutrophils but not macrophages (60). These cells in a Fc receptor–mediated manner may help clear Ags captured by natural IgM via opsonophagocytosis and/or transport natural IgM-complexed bacteria to the lymphoid follicles to enhance ViPS-specific IgG responses. Consistent with this, we found that the IgG responses to ViPS in the context of heat-killed S. Typhi immunization was significantly impaired in the absence of natural IgM (Fig. 6). Further studies are needed to understand whether these processes are mediated by natural IgM binding to its Fc receptors on neutrophils, macrophages, and/or B cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Sudeep Kothari for providing purified ViPS, and Darren Dougharty for critical reading of the manuscript. We also thank Dr. Emad Alnemri and Genevieve Lewis for help with SDS-PAGE and western blot.
This work was supported by the National Institutes of Health Grants AI121270 and AI159798 (to K.R.A.), AI127389 (to G.F.D.), and AI044170 (to A.J.B.). This work was also supported by Sidney Kimmel Cancer Center’s NCI core Grant P30 CA056036.
Abbreviations used in this article:
- AID
activation-induced cytidine deaminase
- DPBS
Dulbecco’s PBS
- LB
Luria–Bertani
- MZB
marginal zone B
- Nramp1
natural resistance-associated macrophage protein 1
- sIgM
secreted form of IgM
- S. Typhi
Salmonella enterica serovar Typhi
- S. Typhimurium
Salmonella enterica serovar Typhimurium
- ViPS
Vi polysaccharide
Footnotes
The online version of this article contains supplemental material.
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Crump JA, and Mintz ED. 2010. Global trends in typhoid and paratyphoid Fever. Clin. Infect. Dis 50: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britto CD, Wong VK, Dougan G, and Pollard AJ. 2018. A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid. PLoS Negl. Trop. Dis 12: e0006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkhold M, Mwisongo A, Pollard AJ, and Neuzil KM. 2021. Typhoid conjugate vaccines: advancing the research and public health agendas. J. Infect. Dis 224(12 Suppl. 2): S781–S787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin C, Hill J, Gunn BM, Yu WH, Dahora LC, Jones E, Johnson M, Gibani MM, Spreng RL, Alam SM, et al. 2021. Vi-specific serological correlates of protection for typhoid fever. J. Exp. Med 218: e20201116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine MM 2008. Typhoid fever vaccine. In Vaccines, 5th Ed. Plotkin S, Orenstein W, and Offit P, eds. Saunders, Philadelphia, p. 887–914. [Google Scholar]
- 6.Yang YA, Chong A, and Song J. 2018. Why is eradicating typhoid fever so challenging: implications for vaccine and therapeutic design. Vaccines (Basel) 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, et al. 2001. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N. Engl. J. Med 344: 1263–1269. [DOI] [PubMed] [Google Scholar]
- 8.Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, Anemona A, Habib MA, Alberto E, Juvekar S, et al. 2014. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect. Dis 14: 119–129. [DOI] [PubMed] [Google Scholar]
- 9.Mohan VK, Varanasi V, Singh A, Pasetti MF, Levine MM, Venkatesan R, and Ella KM. 2015. Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: a multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin. Infect. Dis 61: 393–402. [DOI] [PubMed] [Google Scholar]
- 10.Medise BE, Soedjatmiko S, Gunardi H, Sekartini R, Satari HI, Hadinegoro SR, Wirahmadi A, Puspita M, Sari RM, Yang JS, et al. 2020. A novel Vi-diphtheria toxoid typhoid conjugate vaccine is safe and can induce immunogenicity in healthy Indonesian children 2–11 years: a phase II preliminary report. BMC Pediatr 20: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantele A, Pakkanen SH, Karttunen R, and Kantele JM. 2013. Head-to-head comparison of humoral immune responses to Vi capsular polysaccharide and Salmonella Typhi Ty21a typhoid vaccines–a randomized trial. PLoS One 8: e60583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qadri F, Khanam F, Liu X, Theiss-Nyland K, Biswas PK, Bhuiyan AI, Ahmmed F, Colin-Jones R, Smith N, Tonks S, et al. 2021. Protection by vaccination of children against typhoid fever with a Vi-tetanus toxoid conjugate vaccine in urban Bangladesh: a cluster-randomised trial. Lancet 398: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel PD, Patel P, Liang Y, Meiring JE, Misiri T, Mwakiseghile F, Tracy JK, Masesa C, Msuku H, Banda D, et al. ; TyVAC Malawi Team. 2021. Safety and efficacy of a typhoid conjugate vaccine in Malawian children. N. Engl. J. Med 385: 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SE, and Marks F. 2014. A conjugate vaccine against typhoid fever. Lancet Infect. Dis 14: 90–91. [DOI] [PubMed] [Google Scholar]
- 15.Loomis WP, Johnson ML, Brasfield A, Blanc MP, Yi J, Miller SI, Cookson BT, and Hajjar AM. 2014. Temporal and anatomical host resistance to chronic Salmonella infection is quantitatively dictated by Nramp1 and influenced by host genetic background. PLoS One 9: e111763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandya KD, Palomo-Caturla I, Walker JA, K Sandilya V, Zhong Z, and Alugupalli KR. 2018. An unmutated IgM response to the Vi polysaccharide of Salmonella Typhi contributes to protective immunity in a murine model of typhoid. J. Immunol 200: 4078–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien AD, Rosenstreich DL, and Taylor BA. 1980. Control of natural resistance to Salmonella typhimurium and Leishmania donovani in mice by closely linked but distinct genetic loci. Nature 287: 440–442. [DOI] [PubMed] [Google Scholar]
- 18.Sabbagh SC, Forest CG, Lepage C, Leclerc JM, and Daigle F. 2010. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol. Lett 305: 1–13. [DOI] [PubMed] [Google Scholar]
- 19.Nuccio SP, and Bäumler AJ. 2014. Comparative analysis of Salmonella genomes identifies a metabolic network for escalating growth in the inflamed gut. MBio 5: e00929–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haneda T, Winter SE, Butler BP, Wilson RP, Tükel C, Winter MG, Godinez I, Tsolis RM, and Bäumler AJ. 2009. The capsule-encoding viaB locus reduces intestinal inflammation by a Salmonella pathogenicity island 1-independent mechanism. Infect. Immun 77: 2932–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RP, Winter SE, Spees AM, Winter MG, Nishimori JH, Sanchez JF, Nuccio SP, Crawford RW, Tükel Ç, and Bäumler AJ. 2011. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect. Immun 79: 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson RP, Raffatellu M, Chessa D, Winter SE, Tükel C, and Bäumler AJ. 2008. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell. Microbiol 10: 876–890. [DOI] [PubMed] [Google Scholar]
- 23.Raffatellu M, Chessa D, Wilson RP, Dusold R, Rubino S, and Bäumler AJ. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun 73: 3367–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wangdi T, Lee CY, Spees AM, Yu C, Kingsbury DD, Winter SE, Hastey CJ, Wilson RP, Heinrich V, and Bäumler AJ. 2014. The Vi capsular polysaccharide enables Salmonella enterica serovar Typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog 10: e1004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawford RW, Wangdi T, Spees AM, Xavier MN, Tsolis RM, and Bäumler AJ. 2013. Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi. MBio 4: e00232–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belde V, Cravens MP, Gulandijany D, Walker JA, Palomo-Caturla I, Alugupalli AS, Sandilya VK, Mahmoud T, Bäumler AJ, Kearney JF, and Alugupalli KR. 2018. Terminal deoxynucleotidyl transferase is not required for antibody response to polysaccharide vaccines against Streptococcus pneumoniae and Salmonella enterica serovar Typhi. Infect. Immun 86: e00211–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickinson GS, Levenson EA, Walker JA, Kearney JF, and Alugupalli KR. 2018. IL-7 enables antibody responses to bacterial polysaccharides by promoting B cell receptor diversity. J. Immunol 201: 1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boes M 2000. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol 37: 1141–1149. [DOI] [PubMed] [Google Scholar]
- 29.Panda S, and Ding JL. 2015. Natural antibodies bridge innate and adaptive immunity. J. Immunol 194: 13–20. [DOI] [PubMed] [Google Scholar]
- 30.Grönwall C, Vas J, and Silverman GJ. 2012. Protective roles of natural IgM antibodies. Front. Immunol 3: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin F, and Kearney JF. 2000. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol. Rev 175: 70–79. [PubMed] [Google Scholar]
- 32.Martin F, and Kearney JF. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol 13: 195–201. [DOI] [PubMed] [Google Scholar]
- 33.Ehrenstein MR, and Notley CA. 2010. The importance of natural IgM: scavenger, protector and regulator. Nat. Rev. Immunol 10: 778–786. [DOI] [PubMed] [Google Scholar]
- 34.Marshall JL, Flores-Langarica A, Kingsley RA, Hitchcock JR, Ross EA, López-Macías C, Lakey J, Martin LB, Toellner KM, MacLennan CA, et al. 2012. The capsular polysaccharide Vi from Salmonella typhi is a B1b antigen. J. Immunol 189: 5527–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, Henderson IR, Leyton DL, Coughlan RE, Khan M, Jensen KT, et al. 2009. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc. Natl. Acad. Sci. USA 106: 9803–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas KM, Poe JC, Steeber DA, and FColombo T, M. J., and Alugupalli KR. 2008. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J. Immunol 180: 4858–4864. [DOI] [PubMed] [Google Scholar]
- 37.Tedder. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23: 7–18. [DOI] [PubMed] [Google Scholar]
- 38.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, and Gerstein RM. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21: 379–390. [DOI] [PubMed] [Google Scholar]
- 39.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, and Honjo T. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102: 553–563. [DOI] [PubMed] [Google Scholar]
- 40.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, et al. 2000. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2). Cell 102: 565–575. [DOI] [PubMed] [Google Scholar]
- 41.Bioley G, Monnerat J, Lötscher M, Vonarburg C, Zuercher A, and Corthésy B. 2017. Plasma-derived polyreactive secretory-like IgA and IgM opsonizing Salmonella enterica Typhimurium reduces invasion and gut tissue inflammation through agglutination. Front. Immunol 8: 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, and Chen J. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol 160: 4776–4787. [PubMed] [Google Scholar]
- 43.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, and Galán JE. 2006. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med 203: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arpaia N, Godec J, Lau L, Sivick KE, McLaughlin LM, Jones MB, Dracheva T, Peterson SN, Monack DM, and Barton GM. 2011. TLR signaling is required for Salmonella typhimurium virulence. Cell 144: 675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vieira P, and Rajewsky K. 1988. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol 18: 313–316. [DOI] [PubMed] [Google Scholar]
- 46.Vassiloyanakopoulos AP, Okamoto S, and Fierer J. 1998. The crucial role of polymorphonuclear leukocytes in resistance to Salmonella dublin infections in genetically susceptible and resistant mice. Proc. Natl. Acad. Sci. USA 95: 7676–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kothari S, Kothari N, Kim JA, Lee E, Yoon YK, An SJ, Jones C, Choe WS, and Carbis R. 2013. A novel method for purification of Vi capsular polysaccharide produced by Salmonella enterica subspecies enterica serovar Typhi. [Published erratum appears in 2014 Vaccine 32: 3877.] Vaccine 31: 4714–4719. [DOI] [PubMed] [Google Scholar]
- 48.Boyd MA, Tennant SM, Saague VA, Simon R, Muhsen K, Ramachandran G, Cross AS, Galen JE, Pasetti MF, and Levine MM. 2014. Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines. Clin. Vaccine Immunol 21: 712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raffatellu M, Santos RL, Chessa D, Wilson RP, Winter SE, Rossetti CA, Lawhon SD, Chu H, Lau T, Bevins CL, et al. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun 75: 4342–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiyoshi H, Wangdi T, Lock G, Saechao C, Raffatellu M, Cobb BA, and Bäumler AJ. 2018. Mechanisms to evade the phagocyte respiratory burst arose by convergent evolution in typhoidal Salmonella serovars. Cell Rep 22: 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidal S, Gros P, and Skamene E. 1995. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J. Leukoc. Biol 58: 382–390. [DOI] [PubMed] [Google Scholar]
- 52.Lissner CR, Swanson RN, and O’Brien AD. 1983. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J. Immunol 131: 3006–3013. [PubMed] [Google Scholar]
- 53.Plant JE, Blackwell JM, O’Brien AD, Bradley DJ, and Glynn AA. 1982. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature 297: 510–511. [DOI] [PubMed] [Google Scholar]
- 54.Scoggin K, Gupta J, Lynch R, Nagarajan A, Aminian M, Peterson A, Adams LG, Kirby M, Threadgill DW, and Andrews-Polymenis HL. 2022. Elucidating mechanisms of tolerance to Salmonella Typhimurium across long-term infections using the collaborative cross. MBio 13: e0112022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramachandran S, Godfrey JJ, and Perera MV. 1974. Typhoid hepatitis. JAMA 230: 236–240. [PubMed] [Google Scholar]
- 56.Mert A, Tabak F, Ozaras R, Ozturk R, Aki H, and Aktuglu Y. 2004. Typhoid fever as a rare cause of hepatic, splenic, and bone marrow granulomas. Intern. Med 43: 436–439. [DOI] [PubMed] [Google Scholar]
- 57.Narechania S, Duran M, Karivedu V, and Gopalakrishna KV. 2015. A case of typhoid fever with hepatic granulomas and enteritis. Case Rep. Pathol 2015: 745461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheminay C, Chakravortty D, and Hensel M. 2004. Role of neutrophils in murine salmonellosis. Infect. Immun 72: 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dejager L, Pinheiro I, Bogaert P, Huys L, and Libert C. 2010. Role for neutrophils in host immune responses and genetic factors that modulate resistance to Salmonella enterica serovar typhimurium in the inbred mouse strain SPRET/Ei. Infect. Immun 78: 3848–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang KS, Lang PA, Meryk A, Pandyra AA, Boucher LM, Pozdeev VI, Tusche MW, Göthert JR, Haight J, Wakeham A, et al. 2013. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc. Natl. Acad. Sci. USA 110: 2593–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, and Chen J. 2000. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc. Natl. Acad. Sci. USA 97: 1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alugupalli KR, and Gerstein RM. 2005. Divide and conquer: division of labor by B-1 B cells. Immunity 23: 1–2. [DOI] [PubMed] [Google Scholar]
- 63.Alugupalli KR 2008. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr. Top. Microbiol. Immunol 319: 105–130. [DOI] [PubMed] [Google Scholar]
- 64.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, and Barletta R. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med 153: 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, and Chen J. 2000. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med 192: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mensen A, Krause T, Hanitsch LG, Meisel C, Kleint ME, Volk HD, Na IK, and Scheibenbogen C. 2015. Altered B-cell subsets and functional B-cell defects in selective IgM deficiency. Clin. Immunol 161: 96–102. [DOI] [PubMed] [Google Scholar]
- 67.Notley CA, Brown MA, Wright GP, and Ehrenstein MR. 2011. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J. Immunol 186: 4967–4972. [DOI] [PubMed] [Google Scholar]
- 68.Notley CA, Baker N, and Ehrenstein MR. 2010. Secreted IgM enhances B cell receptor signaling and promotes splenic but impairs peritoneal B cell survival. J. Immunol 184: 3386–3393. [DOI] [PubMed] [Google Scholar]
- 69.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, and Zinkernagel RM. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286: 2156–2159. [DOI] [PubMed] [Google Scholar]
- 70.Ferguson AR, Youd ME, and Corley RB. 2004. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int. Immunol 16: 1411–1422. [DOI] [PubMed] [Google Scholar]
- 71.Youd ME, Ferguson AR, and Corley RB. 2002. Synergistic roles of IgM and complement in antigen trapping and follicular localization. Eur. J. Immunol 32: 2328–2337. [DOI] [PubMed] [Google Scholar]
- 72.Corley RB, Morehouse EM, and Ferguson AR. 2005. IgM accelerates affinity maturation. Scand. J. Immunol 62(Suppl. 1): 55–61. [DOI] [PubMed] [Google Scholar]
- 73.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, Eyre HJ, Sutherland GR, Endo Y, Fujita T, et al. 2000. Fcα/μ receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol 1: 441–446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


