Abstract
The aquaculture industry is growing rapidly to meet the needs for global protein consumption. Viral diseases in aquaculture are quite challenging due to lack of treatment options as well as limited injection-delivery vaccines, which are costly. Thus, water-immersion antiviral treatments are highly desirable. This study focused on broad-spectrum, light-activated antivirals that target the viral membrane (envelope) of viruses to prevent viral-cell membrane fusion, ultimately blocking viral entry into cells. Of the tested small-molecules, JL122, a new broad-spectrum antiviral previously unexplored against aquatic viruses, blocked infection of three aquatic rhabdoviruses (IHNV, VHSV and SVCV) in cell culture and in two live fish challenge models. Importantly, JL122 inhibited transmission of IHNV from infected to uninfected rainbow trout. Further, the effective antiviral concentrations were not toxic to cells or susceptible fish. These results show promise for JL122 to become an immersion treatment option for outbreaks of aquatic enveloped viral infections.
Keywords: antivirals, rhabdovirus, JL122, fish viruses, enveloped, immersion
INTRODUCTION
The aquaculture industry is growing at a rapid pace globally, while the human population and its demand for protein consumption steadily increases. This industry is vulnerable to substantial losses associated with viral diseases for which there are no treatments. In addition, there are currently only two available vaccines for aquatic viruses in the US2. One is a DNA vaccine for infectious hematopoietic necrosis (IHN) that is injected intramuscularly (Anderson et al., 1996), and the other is a recombinant, killed vaccine for infectious salmon anemia (ISA) that is delivered via intraperitoneal injection (Falk, 2014). There is continual work on therapeutic and vaccine development and alternate vaccine delivery methods to aquatic species (Adomako et al., 2012; Mutoloki et al., 2015). Further, there is a consensus in the aquaculture industry that oral or immersion delivery of vaccines or treatments would be easier to administer, less stressful to fish, and faster than injection delivery methods, particularly when vaccinating thousands of fish at a time. The high cost of vaccines is also a hurdle for many producers in the industry.
Sixteen viral diseases that affect aquatic species are reportable to the World Organization of Animal Health (OIE)3 because they cause high mortality rates and great economic losses in the aquaculture industry. Three of the most important of these aquatic viruses are infectious hematopoietic necrosis virus (IHNV), viral hemorrhagic septicemia virus (VHSV) and spring viremia of carp virus (SVCV). These three viruses are enveloped rhabdoviruses (Wagner, 1987) that cause severe disease in a variety of important farmed fish species, including salmonids (IHNV, VHSV) (Walker and Winton, 2010), turbot (Ross et al., 1995) and Japanese flounder (VHSV) (Isshik et al., 2001), and cyprinids (SVCV) (Ahne et al., 2002). Aquatic rhabdoviruses, particularly IHNV, VHSV and SVCV, cause significant morbidity and mortality in wild and farmed fish (Kurath and Winton, 2008; Woo et al., 2011), with mortality rates reaching up to 100% in certain disease outbreaks (Baudouy et al., 1980; Olesen, 1998; Winton, 1991). As examples, epizootic outbreaks of IHNV in Atlantic salmon from 1992–1996 and from 2001–2003 resulted in estimated economic losses of $40 million dollars in inventory, which represented approximately $200 million dollars in lost sales (Garver et al., 2013). Due to viral hemorrhagic septicemia (VHS), European fish farmers estimated losses of approximately £40 million pounds in 1991 (Skall et al., 2005). In addition to the direct economic impact of rhabdovirus disease in susceptible fish, secondary environmental and ecosystem consequences are equally as important (Walker and Winton, 2010). Pathogenicity of disease for these viruses depends on multiple factors, including fish species and age, viral strain or genogroup, and water temperature, among others (Ahne et al., 2002; Ashraf et al., 2016; Lapatra, 1998; Purcell et al., 2012).
Our previously published work with LJ001, a broad-spectrum antiviral, demonstrated inhibition of IHNV infection in cell culture and in pre-incubation studies with rainbow trout (Balmer et al., 2017). LJ001 is a small molecule, lipophilic thiazolidine derivative that inhibits membrane fusion of enveloped viruses with their host cells, and thus viral entry. The mechanism of action of LJ001 involves production of singlet oxygen (1O2) free radicals by the compound in the lipid membrane/envelope when activated by light, and such free radicals damage membrane lipids to stabilize positive membrane curvature and prevent virus-host cell fusion/viral entry (Hollmann et al., 2014; Vigant et al., 2013; Wolf et al., 2010). Enveloped viruses lack the machinery for lipid synthesis and repair of their host-derived lipid bilayer/envelope, whereas host cells possess such machinery and multiple endogenous cytoprotective mechanisms, capable of fixing damaged membranes (Girotti, 2008; van Meer et al., 2008). Therefore, LJ001 was shown to have no overt toxicity at antiviral doses, but toxicity can be induced in the presence of fatty acid synthesis inhibitors (Wolf et al., 2010).
Recently developed structural homologs of LJ001, designated JL122 and JL118, had improved pharmacokinetic parameters in mouse models in vivo and ex vivo as compared to LJ001 (Vigant et al., 2013). Both JL122 and JL118 are oxazolidine-2,4-dithione compounds that are lipophilic, intercalate into lipid membranes, absorb light at longer (red-shifted) wavelengths (545 nm for JL122; 610 nm for JL118) as compared to 455 nm for LJ001 (all within the natural visible light spectrum), and are 1O2-generating photosensitizers (same mechanism of action as LJ001). These compounds were considered more suitable for in vivo treatment over LJ001 based on greater in vitro potency (IC50<10 nM), better tissue penetration (attributed to the red-shifted absorption spectra), increased quantum yield (efficiency of 1O2 generation), and improved bioavailability. However, practical applications for use of these compounds as treatments in a mammalian system are still considered limited due to light-dependency for activation (once the virus is inside the body and no longer exposed to light) (Wolf et al., 2010).
Additionally, the approximate biological half-life for JL001 was 4 hours at 37°C (Wolf et al., 2010), but our previous work demonstrated that LJ001 had a longer (~1.9 days) inhibitory half-life in 15°C hatchery water (Balmer et al., 2017). This was attributed to decreased chemical degradation at lower temperatures. Importantly, the preferential temperature that IHNV (and most fish viruses) infects aquacultured salmonids is ~15°C (Garver et al., 2013; Mulcahy et al., 1984). Transmission of aquatic viruses occurs in water, which is transparent to light, and conveniently, these compounds require minimal amounts of visible light, achievable in aquaculture settings. This environment gives the drug, light, and virus the opportunity to interact after leaving an infected fish and prior to entering naïve fish. Based on this information, we chose to pursue these new drugs to determine if they could inhibit better than LJ001 both aquatic enveloped viral infections and viral transmission through water.
MATERIALS AND METHODS
JL122, LJ001, and JL118
These antiviral compounds (Vigant et al., 2013; Wolf et al., 2010) were produced at the University of California, Los Angeles (UCLA) by Dr. Michael Jung’s group. In powder form, the compounds are stable for years if protected from light. Each compound was reconstituted in 100% DMSO, protected from light, stored at room temperature, and used within 6 months of reconstitution.
Cells and cell viability assay
Epithelioma papulosum cyprini (EPC) cells (Fijan et al., 1983; Winton et al., 2010) are a rhabdovirus-permissive fish cell line that was obtained from the Washington Animal Disease Diagnostic Laboratory (WADDL): Aquaculture section (ATCC CRL 2872). EPC cells were seeded in a 96-well plate and incubated at 22°C until confluent. Up to 10 μM JL122 or up to 0.1% DMSO were added to wells in triplicate for 24 or 72 h. Cytotoxicity was assessed as previously described (Balmer et al., 2017). Briefly, the CCK-8 kit quantifies WST-8 formazan produced by the inherent dehydrogenase enzyme activity of living cells.
Viruses and viral propagation
In vitro studies:
Stock concentrations of IHNV (ATCC VR1392 (039–82 [WRAC strain])), American Type Culture Collection, Rockville, MD), VHSV IVa (ATCC-1387VR), and SVCV (WADDL case # 2004–5061) were obtained from the WADDL Aquaculture laboratory. Viruses were amplified using Chinook salmon embryo-214 (CHSE-214) cells.
In vivo studies:
IHNV (isolate 220–90; M genogroup) was propagated in EPC cells as previously described (Batts and Winton, 1989; Purcell et al., 2013). VHSV (IVb, type strain MI03) was propagated in EPC cells as previously described (Elsayed et al., 2006; Winton et al., 2010).
In vitro inhibition
EPC cells were plated as previously described (Balmer et al., 2017). IHNV, VHSV, and SVCV (final viral titer of 1×104 PFU/mL) were pre-incubated in the presence of ambient light with up to 1 μM JL122, LJ001 or JL118. Viral titer was determined by plaque assay as previously described (Batts and Winton, 1989). Positive control (PC) virus was pre-incubated with vehicle control only (0.01% DMSO).
Fish
Rainbow trout (Oncorhynchus mykiss) studies with IHNV were performed at the Western Fisheries Research Center (WFRC); all experiments were approved under WFRC IACUC protocol 2008–50. Trout were obtained as fry from a commercial producer with a known negative viral status based on testing in accordance with the OIE Manual of Diagnostic Tests for Aquatic Animals4. Largemouth bass (Micropterus salmoides) studies with VHSV IVb and fathead minnow toxicity studies were performed at Cornell University, and experiments were approved under IACUC protocol 2007–063. The largemouth bass were cultured in New York and accompanied by a Fish Health Certification Report following standard procedures identified in the American Fisheries Society Fish Health Section (AFS-FHS) Blue Book. The report stated the fish were free of the diseases viral hemorrhagic septicemia, spring viremia of carp, furunculosis, enteric red mouth, and infectious pancreatic necrosis. Fathead minnows (Pimephales promelas) were used as an additional animal model to assess toxicity of JL122. The minnows were purchased from I.F. Anderson Farms, Inc. in Arkansas, and a Fish Health Certification Report also accompanied these fish.
Pre-incubation (immersion) efficacy
IHNV (final viral titer of 1×104 PFU/mL) was pre-incubated with 0.1, 1, 5 and 10 μM JL122 or vehicle control (DMSO; 0.01% final concentration) in challenge containers (500 mL of static water and continuous air supply) in the presence of light. Similarly, VHSV (1×104 PFU/mL) was pre-incubated with 0.01, 0.1, 1, 5 and 10 μM JL122 or 0.01% DMSO. After 15 min, eight randomized naïve rainbow trout fry (IHNV) or largemouth bass (VHSV) were added to the challenge containers. The fish remained together in batch for 12 h, at which time each fish was separated into an individual beaker containing 500 mL of static laboratory water until 72 h post infection. Mock-infected fish were used as negative controls (0.01% DMSO in MEM), and to assess for potential toxicity (fish exposed to 5 and 10 μM JL122 doses). At 72 h, fish were euthanized with buffered tricaine methanesulfonate (MS-222) at a final concentration of 240 mg/L. Five fish in each treatment group were frozen at −80°C until processed to assess for viral titer/quantity. The three remaining fish in each group were fixed in 10% neutral buffered formalin for histologic evaluation.
Cohabitation efficacy
Anesthetized donor fish were fin clipped 4 days prior to infection. Donor fish were immersed in 2×105 PFU/mL IHNV or minimal essential medium (MEM) only (mock infected) in static water for 1 h, then water flow was resumed. At 24 h post-exposure, three donor fish were placed in each challenge container (700 mL of static water and continuous air supply), and then JL122 (5 μM final concentration) or carrier reagent (DMSO; 0.005% final concentration) were added to challenge containers in the presence of light. After 15 min, nine naïve recipient fish were added to each challenge container. At 24 h and 48 h, the water was exchanged and re-dosed with JL122 (5 μM) or DMSO (0.005%); a total of 3 doses were delivered during the 72 h cohabitation period. At 72 h, fish were euthanized with buffered MS-222 and frozen at −80°C.
Histopathologic evaluation of fish
Following fixation of rainbow trout and fathead minnows (immersion), seven transverse sections of the head and abdominal cavity, and a single longitudinal section of the tail were processed, embedded in paraffin, sectioned at 4 μm and examined with hematoxylin and eosin (H&E) stain using standard methods (Carson, 2015). Histologic scores of lesions were defined prior to evaluation as follows: 0 = no histologic abnormalities; 1 = mild, multifocal epithelial hypertrophy and/or hyperplasia in the gills and/or skin; 2 = moderate epithelial hypertrophy and hyperplasia ± mild inflammation; 3 = moderate epithelial hyperplasia, evident epithelial degeneration or individual cell necrosis and/or mild to moderate inflammation; 4 = severe epithelial hyperplasia and individual cell necrosis and/or moderate to severe inflammation; 5 = significant necrosis in any organs or skin ulceration. Two veterinary pathologists blinded to the treatment groups independently scored lesions, and agreed on final scores.
VHSV qPCR
Pooled organs (liver, kidney, heart, and spleen) and separate brain samples were collected from each largemouth bass in the immersion experiment (described above) and samples were homogenized (Minibeater-16, BioSpec Products) with 500 μL HMEM (Minimal Essential Medium with Hank’s balanced salts solution) and a 1.3 mm chrome bead for 60 s. Homogenate was spun (centrifuged for 2 min at 8000 rpm) and supernatant was used for RNA extraction (Life Technologies Viral Isolation Kit and KingFisher MagMax instrument). Total RNA was quantified using a NanoVue spectrophotometer (GE Healthcare). The presence of VHSV was measured with a method of RT-qPCR assay as previously described (Hope et al., 2010) and modified (Cornwell et al., 2012).
Viral titer (plaque assays)
Whole rainbow trout were processed and analyzed for IHNV titer via a plaque assay as previously described (Balmer et al., 2017; Purcell et al., 2013). VHSV viral titer was determined from pooled organ homogenates from largemouth bass (described above) via plaque assay, in which 24-well plates were pre-treated with polyethylene glycol (PEG) (Batts and Winton, 1989), incubated for 30 min with duplicate viral samples, and overlain with methylcellulose until plaques appeared.
Statistical analyses
We conducted statistical analyses using GraphPad PRISM 5 software. Virus titer was log10 transformed prior to statistical analyses. An ANOVA and Bonferroni’s post-test with comparison of LJ001 to JL122 treatments were used for the in vitro (n=3) pre-incubation studies. ANOVA and Tukey’s Multiple Comparison Test methods were applied to in vitro cytotoxicity data as well as transmission study comparison of donors (JL122-treated groups vs DMSO controls; 12 donors per treatment) and comparison of recipients (JL122 vs. DMSO, 36 recipients per treatment). Additionally, all donors and recipients in the transmission study (48 fish total per treatment group: JL122 vs. DMSO) were compared using Student’s t-test. An ANOVA and Dunnett’s post-test with comparison to positive control were used for the in vivo (5 fish per group) immersion inhibition studies. Histologic scores were compared using a Mann Whitney U, non-parametric test.
RESULTS
JL122 was an effective aquatic antiviral in vitro
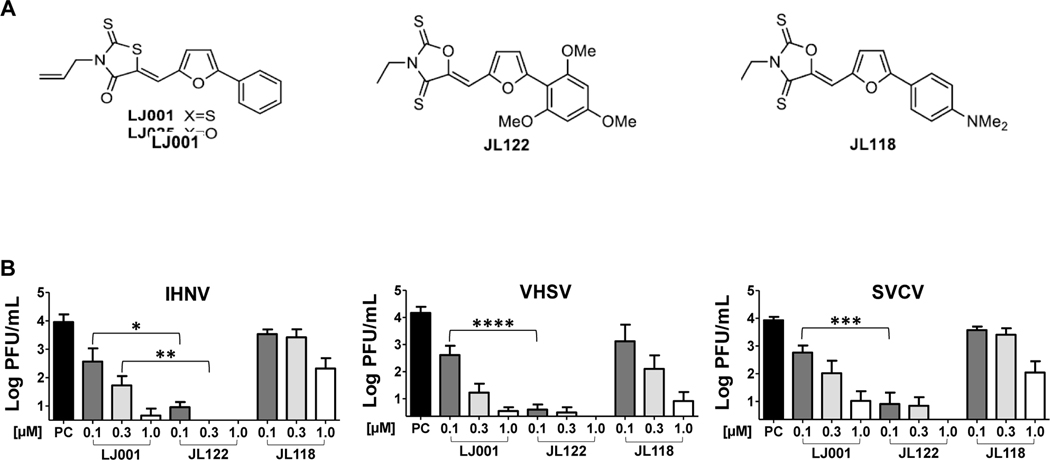
The efficacy of LJ001 and two structurally similar compounds, JL122 and JL118 (Fig. 1A) (Vigant et al., 2013), was compared against IHNV, VHSV and SVCV (Fig. 1B). For all three viruses, JL122 was consistently the best inhibitor in vitro, using the rhabdovirus-permissive fish cell line Epithelioma papulosum cyprini (EPC), yielding complete inhibition of 1 × 104 PFU/mL virus when pre-incubated with 1 μM JL122. Inhibition trends for the three aquatic viruses were similar for each drug, where JL122 was the most efficacious antiviral, most noticeable when 0.1 μM JL122 was compared to 0.1 μM LJ001 for each virus (P<0.05 for IHNV, P<0.0001 for VHSV, and P<0.001 for SVCV). JL118 was the least effective at inhibiting viral infection of the three viruses. These promising in vitro results obtained with JL122 directed focus on this compound for the remainder of the study.
Fig. 1. In vitro efficacy of three antiviral compounds.
(A) Chemical structures of LJ001, JL122, and JL118 (Vigant et al., 2013). (B) Concentrations up to 1 μM of each compound (LJ001, JL122 and JL118) were pre-incubated with 1×104 PFU/mL IHNV, VHSV or SVCV for 30 min while exposed to light. Viral titer was determined by plaque assay. Antiviral inhibition was compared between compounds. For all three viruses, JL122 completely blocked infection at 1.0 μM concentrations. JL122 was the most efficacious antiviral, followed by LJ001, and JL118 was least effective. Positive controls (PC) were virus pre-incubated with 0.01% DMSO (vehicle control). Data represents mean viral titer ± SE (n=3; experiments repeated 3 times). *: P<0.05, **: P<0.01, ***: P<0.001, ****: P<0.0001, ANOVA, Bonferroni’s Multiple Comparison Test.
Effective antiviral concentrations of JL122 were not cytotoxic in vitro
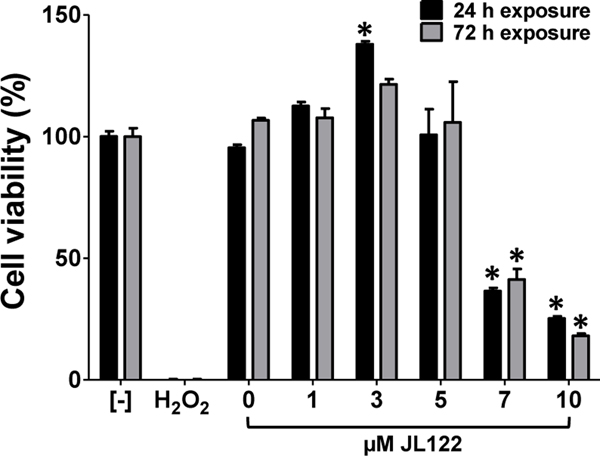
EPC cell viability was quantitatively assessed at 24 and 72 h post-exposure to up to 10 μM JL122 (CCK-8 cell counting kit). Acquired values were normalized to the control, which had no drug or vehicle exposure (RPMI medium only). EPC cells had no cytotoxicity when exposed to concentrations up to 5 μM JL122 for 72 h (Fig. 2). There was a hyperplastic response, most evident when cells were exposed to 3 μM JL122 and evaluated at the 24 h time point (P<0.001), which had higher cell viability values relative to negative controls. This hyperplastic pattern was observed in five repeated experiments. Death occurred in EPC cells exposed to concentrations higher than 5 μM JL122 (P<0.001). Based on this data, the compound dose used for in vivo work was ≤5 μM JL122, aside from additional toxicity evaluations (next section).
Fig. 2. EPC cell viability at 24 and 48 h post-exposure to JL122.
EPC cells were exposed to up to 10 μM JL122, vehicle control only (0.1% DMSO; 0 μM JL122), or hydrogen peroxide (40 mM final concentration) for 24 or 72 h in the presence of light. Formazan dye absorbance was measured at 450 nm and used to assess % cell viability. Negative control [-] = no compound. DMSO concentration varied for each JL122 dose depending on dilution: 10 μM JL122 had 0.1% DMSO; 1 μM JL122 had 0.01% DMSO. Cytotoxicity was absent when JL122 concentrations were ≤ 5 μM. Toxicity to cells was present when exposed to ≥7 μM JL122. A hyperplastic response occurred with 3 μM JL122 at 24 h post-exposure. Data represents mean cell viability ± SE (n=5; triplicate samples are shown and a similar trend was observed in 5 repeated experiments) when normalized to no treatment (negative control). *: P<0.001, ANOVA, Tukey’s Multiple Comparison Test.
Effective antiviral concentrations of JL122 were not overtly toxic in vivo
Additionally, fish were evaluated clinically during in vivo fish experiments, for which toxicity was not obvious in association with JL122 exposure. Rainbow trout (Oncorhynchus mykiss) and fathead minnows (Pimephales promelas) immersion-exposed to up to 10 μM JL122 did not display any signs of toxicity, such as overt mortality or morbidity (Table 1: survival). We also explored whether JL122 would have any adverse effects in fish with high parasite loads (common in pond-raised fish). In the pond-raised largemouth bass (Micropterus salmoides) immersion experiment, an effective antiviral concentration of 1 μM JL122 was not associated with any mortality or obvious toxic effects. However, the bass exposed to concentrations ≥ 5 μM JL122 experienced irregular mortality, and it is uncertain whether the mortality was due to the compound. For instance, 3 out of 8 (37.5%) bass exposed to 10 μM JL122 died during the 72 h holding period. However, a second group of bass exposed to VHSV pre-incubated with 10 μM JL122 suffered no morality (0/8). A single bass died in a group exposed to VHSV pre-incubated with 5 μM JL122 (1/8; 12.5%).
Table 1. Treatment groups and survival for immersion exposure experiments.
Rainbow trout were immersion exposed to 1×104 PFU/mL IHNV pre-incubated with 0–10 μM JL122 for 15 min exposed to ambient room lighting. Each group of 8 fish were left in batch for 12 h, then separated into individual beakers for a total of 72 h post-exposure. Similarly, fathead minnows and largemouth bass were immersion exposed to 1×104 PFU/mL VHSV pre-incubated with 0–10 μM JL122 for 15 minutes and held for 72 h post-exposure. Fish survival is shown, and the few mortalities are discussed in the text.
| Treatment Group | Rainbow Trout | Fathead Minnows | Largemouth Bass |
|---|---|---|---|
| No Virus/0 μM JL122 | 8/8 | 8/8 | 8/8 |
| No Virus/10 μM JL122 | 8/8 | 8/8 | 5/8 |
| Virus/0 μM JL122 | 8/8 | 8/8 | 8/8 |
| Virus/0.01 μM JL122 | 8/8 | 8/8 | 8/8 |
| Virus/0.1 μM JL122 | 8/8 | 8/8 | 8/8 |
| Virus/1 μM JL122 | 8/8 | 8/8 | 8/8 |
| Virus/5 μM JL122 | 8/8 | 8/8 | 7/8 |
| Virus/10 μM JL122 | 8/8 | 8/8 | 8/8 |
JL122-exposed fish displayed no histologic lesions of toxicity
There was no histologic difference between negative control rainbow trout with histologic scores of 1, 0, 1, and those immersed in 10 μM JL122, which had histologic scores of 0, 0, 1 (P=0.62, Mann-Whitney Test). The scores of 1 (mild epithelial hypertrophy or hyperplasia in skin or gills) were attributed to small numbers of associated Ichthyobodo necator (‘Costia’) parasites on the surface of the skin and gill epithelium. Similarly, the fathead minnows had no lesions in either the negative control fish or the fish exposed to 10 μM JL122 (histologic scores=0, 0, 0 for both groups). Our results found no evidence of JL122 toxicity to rainbow trout or fathead minnows up to 10 μM concentrations. Histologic evaluation of the largemouth bass identified significant trematode parasitism in many internal organs, especially within the liver and anterior kidney, as well as in the skeletal muscle and skin. These parasites were associated with large granulomas and moderate to severe eosinophilic granular cell inflammation. The gills and skin from the bass exposed to 10 μM JL122, which are the organs most apt to show signs of toxicity from immersion exposure, were not histologically different from the negative control fish. Importantly, although largemouth bass may be sensitive to JL122 doses between 5–10 μM, effective antiviral concentrations five and 50 times lower, applied in the next two sections below (specifically 1.0 and 0.1 μM) do not overlap with the concentrations (5–10 μM) we report were associated with mortality.
JL122 blocked IHNV and VHSV infection in vivo
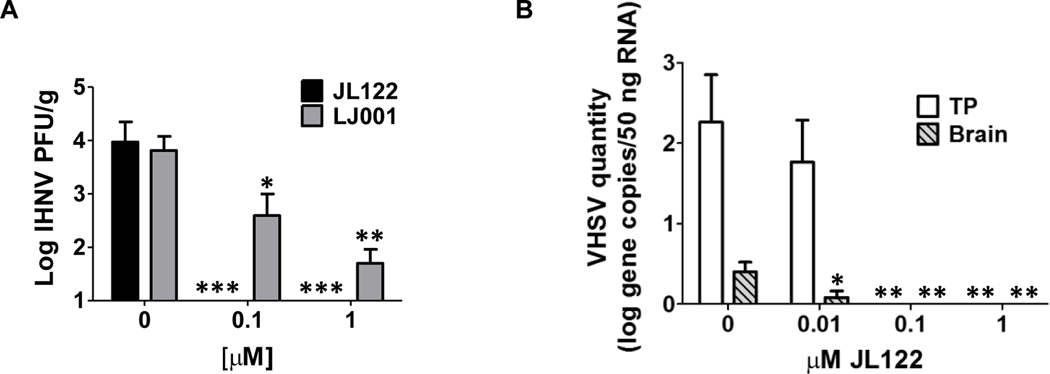
When JL122 was pre-incubated with IHNV and delivered by immersion to rainbow trout fry, inhibition of infection was significantly better (P<0.001) than results for LJ001 (P<0.05) [Fig. 3A; current JL122 results are displayed with previously published LJ001 data (Balmer et al., 2017)]. Even at the 0.1 μM concentration, JL122 completely blocked IHNV infection in rainbow trout. Additionally, trout were histologically evaluated; the group exposed to IHNV pre-incubated with 1 μM JL122 had no histologic evidence of IHNV infection (histologic scores=1, 0, 0). This is in contrast to positive control fish infected with IHNV (no treatment) that displayed necrosis and inflammation in the anterior and posterior kidney (histologic scores=3, 5, 4).
Fig. 3. In vivo efficacy of JL122 against IHNV and VHSV.
(A) 1×104 PFU/mL IHNV was pre-incubated with up to 1.0 μM JL122 or LJ001 for 15 min, while exposed to light. Eight naïve rainbow trout (5/8 fish used for viral titer) were added to each challenge container and held for 72 h. Homogenate supernatant from whole trout was used for plaque assay to determine IHNV titer. Positive control: IHNV and vehicle control (0.0001% DMSO, final concentration). IHNV infection was completely blocked with 0.1 and 1.0 μM JL122. JL122 inhibition is displayed with previously published LJ001 data (Balmer et al., 2017) that showed significant (but not complete) inhibition of IHNV infection with 0.1 and 1.0 μM LJ001. (B) Largemouth bass were similarly immersion infected with VHSV pre-incubated with up to 1.0 μM JL122. Homogenate supernatant from pooled tissues (liver, kidney, heart and spleen = TP) and brain from bass was used for RT-qPCR to quantify VHSV RNA. Positive control: VHSV and 0.0001% DMSO. VHSV infection was also completely blocked with 0.1 μM and 1.0 μM JL122. Plaque assay data from the pooled tissues (not shown) confirms and correlates with the qPCR data. There was a significant decrease in brain viral load even at the 0.01 μM JL122 dose. Data represents mean viral titer ± SE (n=5 fish per treatment group). Mock controls had MEM and vehicle control only; all mock infected fish had negative titers (data not shown). *: P<0.05, **: P<0.01, ***: P<0.001, ANOVA, Dunnett’s post-test.
Similarly, when JL122 was pre-incubated with VHSV in largemouth bass, infection was completely blocked using a 0.1 μM dose (Fig. 3B). Remarkably, although total tissue pool (TP) viral levels were not significantly different from positive controls at the 0.01 μM concentration of JL122 by qPCR (shown) and plaque assay (data not shown), viral quantities in the brain were significantly reduced even at this low drug dose (Fig. 3B).
JL122 inhibited horizontal transmission of IHNV
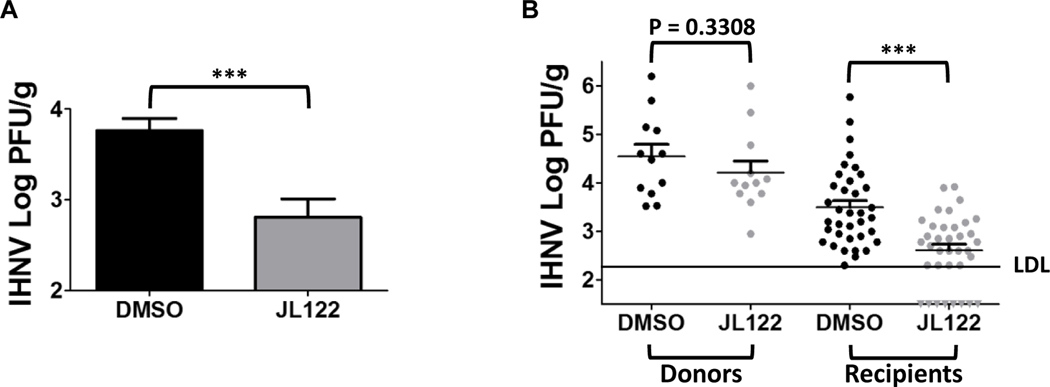
In the cohabitation challenge, which was used to demonstrate the ability of JL122 to inhibit transmission of IHNV from infected to unexposed fish, we dosed the cohabitated water with 5 μM JL122. This dose was chosen based on the in vitro cytotoxicity data in Fig. 2, and on the lack of histopathologic signs or mortality in vivo for rainbow trout at this concentration (described above and in Table 1), and to compare the efficacy to that of previous LJ001 data (Balmer et al., 2017). JL122-treated groups (48 trout) had a significantly lower viral titer (Fig. 4A, P=0.0003) as compared to the 48 positive control fish (DMSO vehicle only). Further, when separated into donor and recipient groups, the JL122-treated recipient fish viral titer was significantly decreased compared to the DMSO control recipient fish (Fig. 4B, right, P<0.001). Notably, 8 of the 36 trout (22.2%) in the JL122-treated recipient fish had titers below the detection limit (negative titers), whereas all 36 DMSO-only recipient fish had detectable infectious virus by plaque assay. These results confirm that JL122 can both decrease viral infection and transmission overall, and block infection completely in a subset of fish at this antiviral concentration.
Fig. 4. Horizontal transmission inhibition of IHNV by JL122.
Rainbow trout were immersion infected with 2×105 PFU/mL IHNV (donor fish) or MEM medium (mock) and remained in flow-through for 24 h. Experimental groups: IHNV- or mock-infected donor fish (three) were placed in static challenge containers, followed by addition of JL122 (5 μM final concentration) or 0.005% DMSO (vehicle/positive control); after 15 min, nine naïve recipient fish were added to each challenge container for cohabitation (n=1 mock-DMSO group [12 fish]; n=1 mock-JL122 group [12 fish], n=4 IHNV-DMSO groups [48 fish] and n=4 IHNV-JL122 groups [48 fish]). A total of 3 doses of JL122 or DMSO (dosed every 24 h) were delivered during the 72 h cohabitation period. All mock-infected fish (not shown) had negative titers (below detection). (A) Comparison of DMSO (control; black bar) vs. JL122-treated fish (gray bar). There was a significantly (***: P=0.0003, Student’s t-test) lower mean IHNV titer in the JL122-treated groups compared to the positive control fish. (B) Separation of donor (fin-clipped for identification) and recipient fish to determine inhibition of transmission. For immersion infected IHNV donor fish, there was a slight and non-significant decrease in viral load (P=0.3308) for the JL122-treated donor fish (left gray dots; 12 fish) compared to DMSO-treated (control) donor fish (left black dots; 12 fish). There was a highly significant decrease in viral load for JL122-treated IHNV recipient fish (right gray dots; 36 fish) with 8 fish below detection (negative titers; gray triangles) compared to DMSO-treated (control) IHNV recipient fish (right black dots; 36 fish). LDL = lower detection limit. ***: P<0.001, ANOVA, Tukey’s Multiple Comparison Test. Data represents mean IHNV titer ± SE.
DISCUSSION
Our results revealed that JL122 was consistently the most efficacious viral inhibitor of those tested for three fish rhabdoviruses in vitro (Fig. 1). We attribute these results to the increased efficiency of free radical production (1O2) in the lipid bilayer by JL122 (Vigant et al., 2013), which likely leads to more effective stabilization of positive membrane curvature to prevent fusion and viral entry. Although this improved efficiency had some associated increased cytotoxicity in vitro at >5 μM JL122 (Fig. 2), and possible toxic effects in largemouth bass between 5–10 μM JL122. However, toxicity was not observed in rainbow trout fry (Oncorhynchus mykiss) or fathead minnows (Pimephales promelas) exposed to up to 10 μM JL122 (lacked overt morbidity or histologic lesions). The histologic findings in the bass are inconclusive as to the cause of mortality in the fish exposed to 10 μM JL122 (3/8 bass died) because there was extensive parasitism found throughout the body of all the pond-raised bass analyzed. It is possible that the drug affected fish that were more heavily parasitized, that the drug interacted with parasites in some way to cause a host response, or that bass may be more sensitive to handling and/or JL122. Ultimately, toxicity can be reached at higher concentrations of drug in vitro and in vivo, but effective antiviral doses used in this study (up to 5 μM JL122) lacked toxicity. These results indicate that the optimal concentration of LJ122 needs to be determined for clinical use for each fish species-virus combination.
In vivo reparative capacity of lipid membranes appears to be more efficient in some fish species than in vitro lipid replacement, since EPC cells exposed to 10 μM JL122 had cytotoxicity, whereas rainbow trout and fathead minnows exposed to 10 μM JL122 had no evidence of cell damage or necrosis. However, some fish species, such as heavily parasitized largemouth bass, may yield different results. Additionally, cultured cells exposed to 3 μM JL122 had a noticeable hyperplastic response at 24hr, exhibited as an increased number of living cells. Hyperplasia is one of the responses to cell injury, and demonstrates the repair and regenerative capacity of living cells following mild, sub-lethal damage or reversible injury (Zachary, 2017). This is consistent with host cells having the biogenic capacity to synthesize and replace damaged lipids (Holthuis and Levine, 2005; van Meer et al., 2008), a capacity that viruses lack (Lorizate and Kräusslich, 2011). Most importantly, effective antiviral concentrations of JL122 in the low micromolar range were confirmed to lack toxicity in host cells and tissues, again, confirming that finding the right in vivo dose is key and dependent on the target fish-virus species combination.
When IHNV was pre-incubated with JL122, rainbow trout were protected at lower doses than with LJ001 during immersion-exposure challenge studies, and similar protection occurred in largemouth bass with VHSV (Fig. 3). There was also a difference in the viral load of the tissue pool compared to the brain in bass. The significantly lower viral titer in the brain with 0.01 μM JL122, and the lack of a significant difference in the tissue pool at the same concentration suggest that the blood brain barrier may deter a certain viral load until penetration and viremia in the brain occurs.
These immersion experiments only tackle part of the disease process because epizootics often have two peaks of mortality (Garver et al., 2013; Hershberger et al., 2011). Typically, a small proportion of fish initially become infected, and these fish shed large amounts of virus (reported to be approximately 3.2 × 107 PFU/fish/h for IHNV in Atlantic salmon) capable of infecting cohabiting uninfected fish. The higher efficiency of JL122 at lower concentrations was encouraging for possible application of this drug in the early stages of an epizootic outbreak.
We consider the most promising and clinically applicable data from this study to be that JL122 inhibited transmission of IHNV from infected donor fish to highly susceptible naïve rainbow trout fry during 72 h of cohabitation (Fig. 4). Notably, JL122 treatment completely blocked horizontal transmission of IHNV to 22.2% of naïve fish during the cohabitation period. The efficacy for JL122 inhibiting horizontal transmission was markedly enhanced as compared to our previous LJ001 data (Balmer et al., 2017), in which viral load was reduced overall in LJ001-treated groups, but all fish had positive viral titers (no fish were completely protected). Treatment with JL122 partially overcame the high shedding rate potential of IHNV. Since rhabdoviruses are shed in excretions (Wolf, 1988), the virus should generally be available to interact with drug in the water. However, the drug will likely bind to membranes in feces and other organic matter, and it is possible that some viral particles may be protected by fecal material or mucus. The peak shedding rate of IHNV and these other environmental factors could explain why not all JL122-treated fish were protected.
We believe JL122 could be used as an immersion delivery therapeutic antiviral in aquatic viral outbreak situations to decrease or potentially block transmission to, and infection of, naïve/susceptible fish. This is highly significant since aquaculture viral infections are predominantly caused by enveloped viruses. As mentioned above, immersion delivery has many advantages over injection methods, including lowered financial costs and time spent performing treatments. This drug could be applied in controlled/closed environments, such as raceways, aquariums (private and commercial), ponds, and short-term static conditions when fish are transported, tagged, held in quarantine or vaccinated, in which the depth of the water should be adequately penetrated by light, allowing for activation of the drug. Although the compounds in this study are within the umbrella of pan-assay interference compounds (PAINS), based on their singlet-oxygen production, fluorescent effects, redox activity and membrane disruption (Dahlin et al., 2015), the assays performed in this study (plaque assay, cytotoxicity and PCR) do not appear altered by these affects. In particular, the cytotoxicity assay measures absorbance at 450 nm, which is substantially lower than the emission wavelength for LJ001 at 515 nm, and the JL compounds have even higher emission wavelengths (Hollmann et al., 2014). Further, the in vivo efficacy of these drugs far outweighs and nullifies these in vitro concerns.
The economic impact and potential revenue saved if this drug were to become commercially available are difficult to predict, particularly since the use of these drugs can be expanded to any enveloped virus in aquaculture. Uncertainty could be due to factors such as age of the animals, susceptibility of aquatic species to various viruses, viral infectious dose, fish stocking density, amount of organic material present in the water that the compound may bind to, and other factors more challenging to account for. However, if death rates can be reduced in any way, as demonstrated in this study, economic losses would be decreased based on the percentages of animals that do not succumb to viral disease.
ACKNOWLEDGEMENTS
We are grateful to the WADDL aquaculture section, particularly Katie McMenamin-Snekvik and Andrew Vo, for providing EPC cells and media for in vitro experiments. The authors wish to thank Rebecca Johnson, Thomas Wootton, and Cesar Escobedo Bonilla for their technical support, and Benhur Lee for helpful discussions.
FUNDING INFORMATION
This work was supported by the McCleary Endowment (WSU-VMP, to KRS) and National Institutes of Health/NIAID Grant RO1 AI109022 (to HAC). Use of any trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Competing Interests
The authors have no competing interests to declare.
http://www.aphis.usda.gov/animal_health/vet_biologics/publications/aqua_products.pdf accessed 5/24/2018
http://www.oie.int/index.php?id=171&L=0&htmfile=chapitre_diseases_listed.htm accessed 5/24/2018
http://www.oie.int/doc/ged/D6505.pdf accessed 5/24/2018
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that they have no conflicts of interest to disclose.
REFERENCES
- 1.Adomako M, St-Hilaire S, Zheng Y, Eley J, Marcum RD, Sealey W, Donahower BC, Lapatra S, Sheridan PP. 2012. Oral DNA vaccination of rainbow trout, Oncorhynchus mykiss (Walbaum), against infectious haematopoietic necrosis virus using PLGA [Poly(D,L-Lactic-Co-Glycolic Acid)] nanoparticles. J Fish Dis 35:203–214. doi: 10.1111/j.1365-2761.2011.01338.x [DOI] [PubMed] [Google Scholar]
- 2.Ahne W, Bjorklund HV, Essbauer S, Fijan N, Kurath G, Winton JR. 2002. Spring viremia of carp (SVC). Dis Aquat Organ 52:261–272. doi: 10.3354/dao052261 [DOI] [PubMed] [Google Scholar]
- 3.Anderson ED, Mourich DV, Fahrenkrug SC, LaPatra S, Shepherd J, Leong JA. 1996. Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol Mar Biol Biotechnol 5:114–122. [PubMed] [Google Scholar]
- 4.Ashraf U, Lu Y, Wang M, Lin L, Liu X, Yuan J. 2016. Spring viremia of carp virus: recent advances. J Gen Virol 97:1037–1051. doi: 10.1099/jgv.0.000436 [DOI] [PubMed] [Google Scholar]
- 5.Balmer BF, Powers RL, Zhang TH, Lee J, Vigant F, Lee B, Jung ME, Purcell MK, Snekvik K, Aguilar HC. 2017. Inhibition of an aquatic rhabdovirus demonstrates promise of a broad-spectrum antiviral for use in aquaculture. J Virol 91:e02181–16. doi: 10.1128/JVI.02181-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batts WN, Winton JR. 1989. Enhanced detection of infectious hematopoietic necrosis virus and other fish viruses by pretreatment of cell monolayers with polyethylene glycol. J Aquat Anim Health 1:284–290. 10.1577/1548-8667(1989)001_0284:EDOIHN_2.3.CO;2. [DOI] [Google Scholar]
- 7.Baudouy AM, Danton M, Merle G. 1980. [SVCV infection of Carp (author’s transl)]. Ann Rech Vet 11:245–249. [PubMed] [Google Scholar]
- 8.Carson FL. 2015. Histotechnology: a self instructional text. American Society Of Clinical Pathology, Chicago, IL. [Google Scholar]
- 9.Cornwell ER, Eckerlin GE, Thompson TM, Batts WN, Getchell RG, Groocock GH, Kurath G, Winton JR, Casey RN, Casey JW, Bain MB, Bowser PR. 2012. Predictive factors and viral genetic diversity for viral hemorrhagic septicemia virus infection in Lake Ontario and the St. Lawrence River. J Great Lakes Res 38:278–288. [Google Scholar]
- 10.Dahlin JL, Nissink JW, Strasser JM, Francis S, Higgins L, Zhou H, Zhang Z, Walters MA, 2015. PAINS in the assay: chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS. J Med Chem 58:2091–2113. doi: 10.1021/jm5019093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsayed E, Faisal M, Thomas M, Whelan G, Batts W, Winton J. 2006. Isolation of viral haemorrhagic septicaemia virus from muskellunge, Esox masquinongy (Mitchill), in Lake St Clair, Michigan, USA reveals a new sublineage of the North American genotype. J Fish Dis 29:611–619. doi: 10.1111/j.1365-2761.2006.00755.x [DOI] [PubMed] [Google Scholar]
- 12.Falk K. 2014. Vaccination against Infectious Salmon Anemia, p 313–320, Fish Vaccination doi: 10.1002/9781118806913.ch26. John Wiley & Sons, Ltd. doi: [DOI] [Google Scholar]
- 13.Fijan N, Sulimanović D, Bearzotti M, Muzinić D, Zwillenberg LO, Chilmonczyk S, Vautherot JF, de Kinkelin P. 1983. Some properties of the Epithelioma papulosum cyprini (EPC) cell line from carp Cyprinus carpio. Ann Inst Pasteur Virol 134:207–220. 10.1016/S07692617(83)80060-4 [DOI] [Google Scholar]
- 14.Garver KA, Mahony AA, Stucchi D, Richard J, Van Woensel C, Foreman M. 2013. Estimation of parameters influencing waterborne transmission of infectious hematopoietic necrosis virus (IHNV) in Atlantic salmon (Salmo salar). PLoS One 8:e82296. 10.1371/journal.pone.0082296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girotti AW. 2008. Translocation as a means of disseminating lipid hydroperoxide-induced oxidative damage and effector action. Free Radic Biol Med 44:956–968. doi: 10.1016/j.freeradbiomed.2007.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hershberger PK, Gregg JL, Grady CA, Hart LM, Roon SR, Winton JR, 2011. Factors controlling the early stages of viral haemorrhagic septicaemia epizootics: low exposure levels, virus amplification and fish-to-fish transmission. J Fish Dis 34, 893–899. doi: 10.1111/j.1365-2761.2011.01305.x [DOI] [PubMed] [Google Scholar]
- 17.Hollmann A, Castanho MA, Lee B, Santos NC. 2014. Singlet oxygen effects on lipid membranes: implications for the mechanism of action of broad-spectrum viral fusion inhibitors. Biochem J 459:161–170. 10.1042/BJ20131058. [DOI] [PubMed] [Google Scholar]
- 18.Holthuis JC, Levine TP. 2005. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol 6:209–220. 10.1038/nrm1591 [DOI] [PubMed] [Google Scholar]
- 19.Hope KM, Casey RN, Groocock GH, Getchell RG, Bowser PR, Casey JW. 2010. Comparison of quantitative RT-PCR with cell culture to detect viral hemorrhagic septicemia virus (VHSV) IVb infections in the Great Lakes. J Aquat Anim Health 22:50–61. doi: 10.1577/H09-028.1 [DOI] [PubMed] [Google Scholar]
- 20.Isshiki T, Nishizawa T, Kobayashi T, Nagano T, Miyazaki T. 2001. An outbreak of VHSV (viral hemorrhagic septicemia virus) infection in farmed Japanese flounder Paralichthys olivaceus in Japan. Dis Aquat Organ 47:87–99. doi: 10.3354/dao047087 [DOI] [PubMed] [Google Scholar]
- 21.Kurath G, Winton J. 2008. Fish Rhabdoviruses, p 221–227 In Mahy BWJ, van Regenmortel MHVV (eds), Encyclopedia of Virology, 3rd ed. Academic Press, Oxford, United Kingdom. 10.1016/B978-012374410-4.00493-3. [DOI] [Google Scholar]
- 22.LaPatra SE. 1998. Factors affecting pathogenicity of infectious hematopoietic necrosis virus (IHNV) for salmonid fish. J Aquat Anim Health 10:121–131. [Google Scholar]
- 23.Lorizate M, Kräusslich H-G. 2011. Role of Lipids in Virus Replication. Cold Spring Harb Perspect Biol 3:a004820. doi: 10.1101/cshperspect.a004820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulcahy D, Pascho R, Jenes CK. 1984. Comparison of in vitro growth characteristics of ten isolates of infectious haematopoietic necrosis virus. J Gen Virol 65 (Pt 12):2199–2207. 10.1099/0022-1317-65-12-2199. [DOI] [PubMed] [Google Scholar]
- 25.Mutoloki S, Munang’andu HM, Evensen Ø. 2015. Oral vaccination of fish – antigen preparations, uptake, and immune induction. Front Immunol 6:519. doi: 10.3389/fimmu.2015.00519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olesen NJ. 1998. Sanitation of viral haemorrhagic septicaemia (VHS). J Appl Ichthyol 14:173–177. [Google Scholar]
- 27.Purcell MK, Laing KJ, Winton JR. 2012. Immunity to fish rhabdoviruses. Viruses 4:140–166. 10.3390/v4010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purcell MK, Thompson RL, Garver KA, Hawley LM, Batts WN, Sprague L, Sampson C, Winton JR. 2013. Universal reverse-transcriptase real-time PCR for infectious hematopoietic necrosis virus (IHNV). Dis Aquat Organ 106:103–115. 10.3354/dao02644. [DOI] [PubMed] [Google Scholar]
- 29.Ross K, McCarthy U, Huntly PJ, Wood BP, Stuart D, Rough EI, Smail DA, Bruno DW. 1995. An outbreak of viral haemorrhagic septicaemia (VHS) in turbot (Scophthalmus maximus) in Scotland. Bull Eur Assoc Fish Pathol 14:213–214. [Google Scholar]
- 30.Skall HF, Olesen NJ, Mellergaard S. 2005. Viral haemorrhagic septicaemia virus in marine fish and its implications for fish farming – a review. J Fish Dis 28:509–529. doi: 10.1111/j.1365-2761.2005.00654.x [DOI] [PubMed] [Google Scholar]
- 31.van Meer G, Voelker DR, Feigenson GW. 2008. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124. doi: 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigant F, Lee J, Hollmann A, Tanner LB, Akyol Ataman Z, Yun T, Shui G, Aguilar HC, Zhang D, Meriwether D, Roman-Sosa G, Robinson LR, Juelich TL, Buczkowski H, Chou S, Castanho MA, Wolf MC, Smith JK, Banyard A, Kielian M, Reddy S, Wenk MR, Selke M, Santos NC, Freiberg AN, Jung ME, Lee B. 2013. A mechanistic paradigm for broad-spectrum antivirals that target virus-cell fusion. PLoS Pathog 9:e1003297. 10.1371/journal.ppat.1003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner R. 1987. Rhabdovirus biology and infection, p 9–74. In Wagner R (ed), The rhabdoviruses. Springer, New York, NY. 10.1007/978-1-4684-7032-1_2. [DOI] [Google Scholar]
- 34.Walker PJ, Winton JR. 2010. Emerging viral diseases of fish and shrimp. Vet Res 41:51. doi: 10.1051/vetres/2010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winton J, Batts W, deKinkelin P, LeBerre M, Bremont M, Fijan N. 2010. Current lineages of the epithelioma papulosum cyprini (EPC) cell line are contaminated with fathead minnow, Pimephales promelas, cells. J Fish Dis 33:701–704. 10.1111/j.1365-2761.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- 36.Winton JR. 1991. Recent advances in detection and control of infectious hematopoietic necrosis virus in aquaculture. Annu Rev Fish Dis 1:83–93. 10.1016/0959-8030(91)90024-E. [DOI] [Google Scholar]
- 37.Wolf K, 1988. Fish viruses and fish viral diseases. Cornell University Press, Ithaca, NY. [Google Scholar]
- 38.Wolf MC, Freiberg AN, Zhang T, Akyol-Ataman Z, Grock A, Hong PW, Li J, Watson NF, Fang AQ, Aguilar HC, Porotto M, Honko AN, Damoiseaux R, Miller JP, Woodson SE, Chantasirivisal S, Fontanes V, Negrete OA, Krogstad P, Dasgupta A, Moscona A, Hensley LE, Whelan SP, Faull KF, Holbrook MR, Jung ME, Lee B. 2010. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci U S A 107:3157–3162. 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zachary JF, 2017. Pathologic basis of veterinary disease, Sixth edition. Elsevier, St. Louis, Missouri. [Google Scholar]