Abstract
Purpose
Severe traumatic brain injury is a leading cause of mortality and morbidity, and these patients are frequently intubated in the prehospital setting. Cerebral perfusion and intracranial pressure are influenced by the arterial partial pressure of CO2 and derangements might induce further brain damage. We investigated which lower and upper limits of prehospital end-tidal CO2 levels are associated with increased mortality in patients with severe traumatic brain injury.
Methods
The BRAIN-PROTECT study is an observational multicenter study. Patients with severe traumatic brain injury, treated by Dutch Helicopter Emergency Medical Services between February 2012 and December 2017, were included. Follow-up continued for 1 year after inclusion. End-tidal CO2 levels were measured during prehospital care and their association with 30-day mortality was analyzed with multivariable logistic regression.
Results
A total of 1776 patients were eligible for analysis. An L-shaped association between end-tidal CO2 levels and 30-day mortality was observed (p = 0.01), with a sharp increase in mortality with values below 35 mmHg. End-tidal CO2 values between 35 and 45 mmHg were associated with better survival rates compared to < 35 mmHg. No association between hypercapnia and mortality was observed. The odds ratio for the association between hypocapnia (< 35 mmHg) and mortality was 1.89 (95% CI 1.53–2.34, p < 0.001) and for hypercapnia (≥ 45 mmHg) 0.83 (0.62–1.11, p = 0.212).
Conclusion
A safe zone of 35–45 mmHg for end-tidal CO2 guidance seems reasonable during prehospital care. Particularly, end-tidal partial pressures of less than 35 mmHg were associated with a significantly increased mortality.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-023-07012-z.
Keywords: Ventilation, Traumatic brain injury, Critical care, Carbon dioxide, Endotracheal intubation
Take-home message
|
In this prospective observational multicenter study, post-intubation ventilation of patients with severe traumatic brain injury revealed a significant L-shaped association between end-tidal CO2 levels and 30-day mortality. Particularly, end-tidal partial pressures of CO2 of less than 35 mmHg are associated with a significantly increased mortality. |
Introduction
Severe traumatic brain injury (TBI) is a leading cause of mortality and morbidity worldwide [1, 2]. Patients are at a substantial risk of developing secondary brain injury before even reaching the hospital, and prehospital treatment is considered a pivotal contributor to patient outcome [1, 3, 4]. Prehospital care commonly includes endotracheal intubation and ventilation to prevent airway obstruction and hypoxemia [5]. However, ventilation affects the arterial partial pressure of carbon dioxide (PaCO2), which in turn modulates cerebral blood flow. Hypercapnia results in cerebral vasodilation and increased cerebral blood flow and may increase intracranial pressure, whereas hypocapnia causes cerebral vasoconstriction and a reduction of cerebral blood flow which may lead to cerebral ischemia [6]. Both cerebral vasodilatation and vasoconstriction might therefore aggravate the brain injury in TBI.
A recent systematic review as well as the international guideline on the prehospital management of patients with TBI recommend “normoventilation” [5, 7], but the evidence supporting this recommendation is weak. While various research groups have addressed the effects of hypo- and hypercapnia on the outcome of patients with TBI, results are conflicting and a clear understanding of the association between the CO2 levels and outcomes is lacking [8]. Previous studies categorized CO2 levels using inconsistent and arbitrary cut-offs or failed to adjust for key confounders [7, 9]. Moreover, blood gas analyses and measurements of PaCO2 to guide “normoventilation” are generally not available during prehospital care, which is why end-tidal CO2 (ETCO2) concentrations are commonly used to guide ventilation. To account for the physiological gradient between end-tidal and arterial concentrations and bearing in mind that the gap can be markedly increased in trauma patients, prehospital providers often target ETCO2 values below the “normal” range of 35–45 mmHg to maintain normocapnia [10]. Emergency medical services’ (EMS) protocols also commonly specify target ETCO2 values of ≤ 35 mmHg, typically in the range of 30–35 mmHg [11]. While in some protocols, the use of hyperventilation is relegated to cases of suspected cerebral herniation, other protocols do not specify such restrictions [11]. This common practice and existing protocols, however, may put the patient at risk of hypocarbia, and it is not yet clear which range of prehospital ETCO2 values is associated with best outcomes.
To investigate the association between ETCO2 levels and mortality, we performed analyses on the BRAIN-PROTECT data [12], a large observational cohort study in the Netherlands.
Methods
BRAIN-PROTECT is a multicenter prospective observational study focusing on the prehospital treatment of patients with severe TBI in the Netherlands. Data from patients with suspected severe TBI (trauma mechanism or clinical findings suggestive of severe TBI and a prehospital Glasgow Coma Scale [GCS] score of 8 or lower) treated by any one of the four Dutch physician-staffed Helicopter Emergency Medical Services (HEMS) were included. HEMS services started patient inclusion between February 2012 and April 2014, and included patients until December 2017. Inclusion was based on suspected severe TBI rather than confirmed TBI, because prehospital treatment, including airway management and ventilation, is based on the suspected rather than the definite diagnosis [13]. After prehospital care, patients were transferred to one of the nine participating trauma centers. In-hospital and outcome data were collected up to 1 year after inclusion. Patients were excluded from the analysis if they were transported to a non-participating hospital (no follow-up data), if they underwent prehospital traumatic cardiopulmonary resuscitation (very high mortality regardless of treatment, with low ETCO2 values), or if they did not receive prehospital advanced airway management. All other patients were considered for further analysis. Data were collected on the basis of the Utstein template for uniform reporting of major trauma data [14]. These data included patient and trauma characteristics, injuries, vital parameters, as well as prehospital treatments and interventions as described previously [12].
After advanced airway management, patients were usually mechanically ventilated with a respirator or manually ventilated with a self-inflating bag, depending on the availability of a mechanical ventilator in the ambulance, distance to hospital, and preference of the treating physician. ETCO2 was routinely measured after airway management, and measurements were recorded from available capnography modules included in patient monitors provided by the regional ambulance medical services, such as Lifepak 15 (Physio-Control), Tempus Pro (Philips), Zoll X (Zoll), or Corpuls 3 (Corpuls) monitors. All-cause mortality was assessed at 30 days (primary outcome), and the functional neurologic outcome (Glasgow outcome scale) at discharge was recorded [15, 16]. Long-term mortality data up to 1 year after inclusion were collected from hospital records and the Dutch Personal Records Database.
A detailed protocol has been previously published [12]. The Medical Research Ethics Committees of the Amsterdam University Medical Center, location VUmc, and Erasmus MC Rotterdam concluded that this research project did not fall under the Dutch Medical Research in Human Subjects Act. Prehospital vital parameters, including ETCO2, were recorded at three time points: after HEMS arrival, after initial stabilization and airway management, and before arriving at the emergency department. For this study, only ETCO2 values measured at the second and third time point were considered, because most patients had not undergone advanced airway management before HEMS arrival, and ETCO2 measurements were most often not available at the first time point.
Sample size considerations of the BRAIN-PROTECT project are comprehensively discussed in the study protocol and were based on the ability to detect an absolute 5% reduction in mortality for a binary exposure with 80% power [12]. In the present study, the exposure variable is continuous. At the given sample size and assuming a normal distribution of the exposure variable, logistic regression achieves > 80% power at a 0.05 alpha level to detect an odds ratio of 1.2.
Statistical analysis
Data were analyzed using Stata 17.0 (StataCorp, College Station, TX). The association between prehospital ETCO2 levels and 30-day mortality was investigated using logistic regression. To allow for a non-linear association between ETCO2 and the logit of mortality, ETCO2 was modeled as a restricted cubic spline with four knots. The number of knots was based on Akaike’s Information Criterion. After an initial explorative analysis (adjusting only for different ETCO2 measurement timepoints), a multivariable model was built to account for potential confounders. Covariates were simultaneously forced into the model based on theoretical considerations and included demographic factors (age, sex, and American Society of Anesthesiologists [ASA] Physical Status Classification System score), vital parameters measured during prehospital care (systolic blood pressure, heart rate and oxygen saturation measured at the same time as ETCO2, time point of ETCO2 measurement), injury severity (Injury Severity Score and first GCS), and operational factors (HEMS provider involved in the treatment, distance between incident scene and trauma hospital). In addition to these analyses, in which ETCO2 was considered a continuous variable, we performed analyses in which ETCO2 was categorized into hypocapnia (< 35 mmHg), normocapnia (35–44 mmHg), and hypercapnia (≥ 45 mmHg). As planned subgroup analyses, these analyses were repeated for patients with confirmed TBI (head AIS ≥ 3) and isolated TBI (head AIS ≥ 3, all other AIS ≤ 2). Post hoc analyses were performed for patients with symptoms of intracerebral herniation, i.e., (A) patients with abnormal pupils (unequal or not reacting to light) and (B) patients with signs of elevated intracranial pressure on the initial CT scan (midline shift > 5 mm or compressed/absent basal cisterns). Moreover, we performed analyses in which only each patient’s (A) first (B) second (C) lowest and (D) highest ETCO2 value were considered. In addition to the logistic regression analyses that all model 30-day mortality, the relationship between ETCO2 and the actual survival time (up to 1 year after the trauma) was modeled with Cox proportional hazards regression with cluster-robust standard errors and graphed with Kaplan–Meier curves [17].
Analyses were primarily performed as complete-case analyses, i.e., in patients with non-missing ETCO2 data and non-missing mortality data (and in multivariable models, additionally with non-missing data for all independent variables in the model). To gauge whether missing data affect our conclusions, 20 data sets were imputed using chained equations with an imputation model including the outcome variables, all independent variables included in the analysis models, as well as auxiliary variables. In all regression models, standard errors were adjusted for clustering of ETCO2 values measured at two time points within the same patients using generalized estimating equations (GEE) with independent correlation structure and robust Huber–White standard error estimates. Two-sided P values < 0.05 were considered statistically significant.
Results
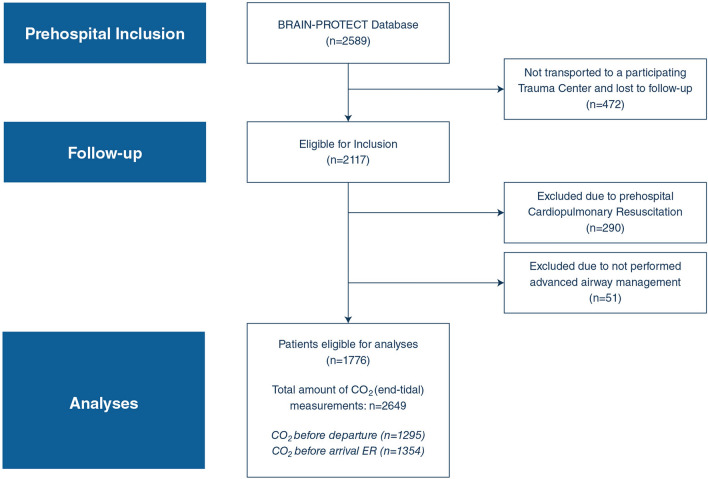
Of the 2589 patients with suspected severe traumatic brain injury included in the BRAIN-PROTECT database, 1776 were eligible for analysis (Fig. 1). The remainder were excluded due to transport to a non-participating trauma center (n = 472), due to prehospital cardiopulmonary resuscitation (n = 290) or due to absence of prehospital advanced airway management (n = 51).
Fig. 1.
Patient flow diagram
Of the patients eligible for further analysis, the majority were male (70%), had a median age of 45 [23, 65] years and an initial GCS of 4 [3, 7] (Table 1). Thirty days after injury, 66.8% of patients were still alive, of which 40.6% recovered with moderate or good recovery at discharge (GOS 4 & 5).
Table 1.
Patient characteristics
| Characteristics (n = 1776) | median [ quartiles] / n (percentage) | N missing |
|---|---|---|
| Demographic data and Injury Severity | ||
| Age (years) | 45 [23, 65] | 20 (1.1%) |
| Male sex [N (%)] | 1247 (70.3%) | 3 (0.2%) |
| Mechanism of injury | 29 (1.6%) | |
| Traffic—motor vehicle | 311 (17.8%) | |
| Traffic—motorcycle | 163 (9.3%) | |
| Traffic—bicycle | 389 (22.3%) | |
| Traffic—pedestrian | 123 (7%) | |
| Traffic—other | 53 (3%) | |
| Fall from height | 600 (34.3%) | |
| Gunshot or stab injury | 34 (2%) | |
| Other | 74 (4.2%) | |
| Injury Severity Score | 26 [19, 35] | 202 (11.4%) |
| Glasgow Coma Scale (GCS) at arrival of HEMS | 4 [3, 7] | 0 (0%) |
| Motor response component of the CGS | 2 [1, 4] | 0 (0%) |
| Pupils equal and reactive to light (N (%)) | 756 (49.8%) | 258 (14.5%) |
| Abbreviated Injury Scale Score—Head | 202 (11.4%) | |
| No head injury | 74 (4.7%) | |
| 1 | 68 (4.3%) | |
| 2 | 92 (5.8%) | |
| 3 | 222 (14.1%) | |
| 4 | 456 (29%) | |
| 5 | 650 (41.3%) | |
| 6 | 12 (0.8%) | |
| Abbreviated Injury Scale Score—Face | ||
| < 3 or no injury | 1460 (92.8%) | |
| ≥ 3 | 114 (7.2%) | |
| Abbreviated Injury Scale Score—Neck | ||
| < 3 or no injury | 1547 (98.3%) | |
| ≥ 3 | 27 (1.7%) | |
| Abbreviated Injury Scale Score—Spine | ||
| < 3 or no injury | 1418 (90.1%) | |
| ≥ 3 | 156 (9.9%) | |
| Abbreviated Injury Scale Score—Thorax | ||
| < 3 or no injury | 974 (61.9%) | |
| ≥ 3 | 600 (38.1%) | |
| Abbreviated Injury Scale Score—Abdomen | ||
| < 3 or no injury | 1461 (92.8%) | |
| ≥ 3 | 113 (7.2%) | |
| Abbreviated Injury Scale Score—Upper extremity | ||
| < 3 or no injury | 1513 (96.1%) | |
| ≥ 3 | 61 (3.9%) | |
| Abbreviated Injury Scale Score—Lower extremity | ||
| < 3 or no injury | 1344 (85.4%) | |
| ≥ 3 | 230 (14.6%) |
| Prehospital observations and Airway management | ||
|---|---|---|
| Respiratory rate at primary survey | 101 (5.7%) | |
| Normal | 1242 (74.2%) | |
| Tachypnea (> 29/min) | 68 (4.1%) | |
| Bradypnea (6–9/min) | 296 (17.7%) | |
| Gasping (1–5/min) | 56 (3.3%) | |
| No breathing | 13 (0.8%) | |
| Type of airway management | 23 (1.3%) | |
| Endotracheal intubation | 1733 (98.9%) | |
| Laryngeal mask | 13 (0.7%) | |
| Supraglottic device other than LMA | 1 (0.1%) | |
| Coniotomy | 6 (0.3%) | |
| Prehospital systolic blood pressure < 90 mmHg | 187 (10.9%) | 67 (3.8%) |
| Prehospital SpO2 < 90% or documented cyanosis | 332 (19.6%) | 79 (4.5%) |
| Distance from incident scene to hospital (km) | 24.6 [14.2, 40.4] | 289 (16.3%) |
| Time from HEMS dispatch to patient arrival at the hospital (minutes) | 56 [46, 69] | 94 (5.3%) |
| In-hospital data | ||
|---|---|---|
| First arterial pCO2 (mmHg) | 45 [40, 51] | 203 (11.4%) |
| Systolic blood pressure < 90 mmHg at hospital arrival | 144 (8.9%) | 166 (9.4%) |
| SpO2 < 90% at hospital arrival | 67 (4.5%) | 294 (16.6%) |
| First cerebral CT scan, Rotterdam classification | 351 (19.8%) | |
| Basal cisterns | ||
| Normal | 911 (63.9%) | |
| Compressed | 270 (19%) | |
| Absent | 244 (17.1%) | |
| Midline shift | ||
| No shift or ≤ 5 mm | 1106 (77.6%) | |
| > 5 mm | 319 (22.4%) | |
| Epidural mass lesion | ||
| Present | 200 (14%) | |
| Absent | 1225 (86%) | |
| Intraventricular blood or traumatic SAH | ||
| Present | 885 (62.2%) | |
| Absent | 539 (37.9%) | |
| Emergency neurosurgical intervention (first 48 h) | 521 (36.4%) | 343 (19.3%) |
| Emergency extracranial surgery (first 48 h) | 241 (16.8%) | 345 (19.4%) |
| Hospital LOS (days)* | 16 [6, 33]* | 10 (1%)* |
| Follow-up and outcome | ||
|---|---|---|
| Follow-up time (days) | 365 [5; 365] (range 1–365) | 109 (6.1%)‡ |
| Death at 30 days | 554 (33.2%) | 109 (6.1%)$ |
| Death at 1 year | 610 (40.2%) | 258 (14.5%) |
| GOS at discharge [N(%)] | ||
| Death | 564 (35%) | 166 (9.3%) |
| Vegetative state | 38 (2.4%) | |
| Severe disability | 583 (36.2%) | |
| Moderate disability | 170 (10.6%) | |
| Good recovery | 255 (15.8%) | |
Demographic, treatment, and outcome data of included patients. Numbers presented as median [quartiles] or n (percentage). For the calculation of percentages, the number of non-missing cases was used as the denominator. * Of surviving patients past hospital discharge (see GOS at discharge for the number of patients known to have survived past discharge). The total number of missing data for LOS was 134/1776 = 7.5%. ‡These patients could not be followed up, and thus, the follow-up time was coded as 0 in the database. Here, these patients are considered to have a missing follow-up time, and the follow-up time is reported only for those patients who were actually followed up. $The 109 patients with missing data for 30-day mortality are the same as those with also missing follow-up and thus are also missing all mortality data including survival time
In total, the results of 2649 CO2 measurements were available for further analyses (n = 1295 after initial stabilization and advanced airway management, n = 1354 before arriving at the emergency department of the participating hospitals). The measurements at these two time points showed a moderate correlation, and correlation between the last ETCO2 measurement and first in-hospital PaO2 was weak (Supplemental Figs 1 and 2) [18]. The distribution of ETCO2 measurements is shown in Supplemental Fig. 3.
Most patients were endotracheally intubated, with only few patients receiving a supraglottic airway device or coniotomy (Table 1). Of those patients with available information about how they were ventilated after airway management (n = 941), 61.4% were mechanically ventilated with a respirator and 35.4% were manually ventilated with a self-inflating bag.
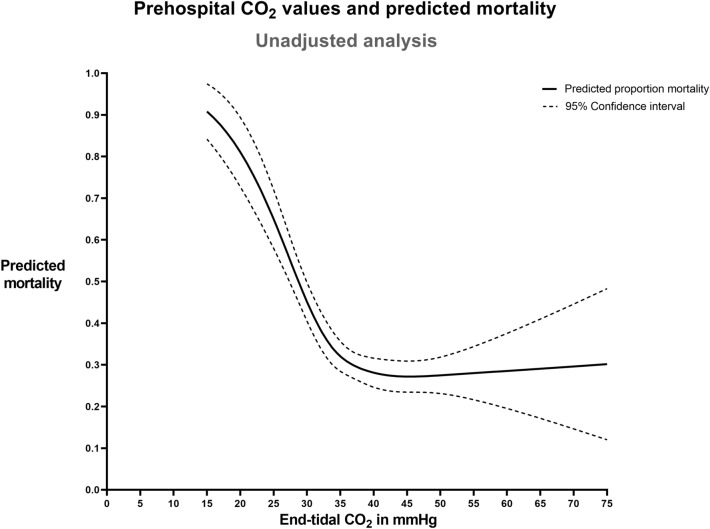
For a total of 1342 patients, 30-day mortality data as well as at least one ETCO2 measurement were available to study the relationship between ETCO2 levels and 30-day mortality. Analyses of the unadjusted association between ETCO2 levels and predicted mortality revealed an L-shaped association, with a marked increase in mortality with ETCO2 levels below 35 mmHg and a flat portion with ETCO2 levels above 35 mmHg (p < 0.001 for the overall association between ETCO2 and mortality, Fig. 2).
Fig. 2.
Prehospital ETCO2 values and predicted mortality—unadjusted analysis. Association between prehospital end-tidal CO2 values (regression model based on 2505 ETCO2 values observed after advanced airway management at two time points in 1342 patients; see text for details) and predicted 30-day mortality. This analysis does not adjust for confounders but does adjust for the time point of the end-tidal CO2 measurement. The figure can be interpreted as the expected probability of mortality given the first recorded ETCO2 value during prehospital ventilation. The curve for the second measurement time point is virtually identical (not shown)
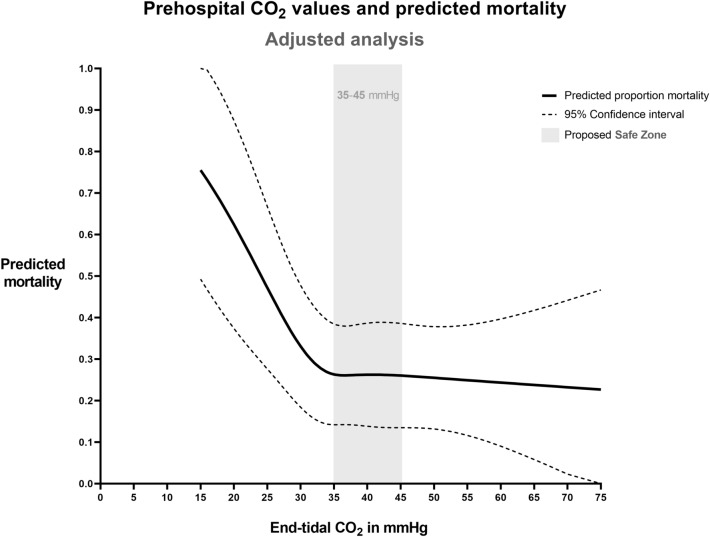
After adjusting for confounders in multivariable logistic regression, the L-shaped association between hypocapnia and predicted mortality persisted (p = 0.01 for the overall association, Fig. 3, Supplemental Table 1). As in the unadjusted analysis, ETCO2 levels below 35 mmHg were associated with markedly increased mortality, whereas values above 35 mmHg were associated with lower and rather constant mortality, with no evident increase in mortality for high ETCO2 levels. However, the precision of the estimated association declined above 45 mmHg, such that the uncertainty on how mortality changes with high ETCO2 values increases.
Fig. 3.
Prehospital ETCO2 values and predicted mortality—multivariable analysis. Prehospital end-tidal CO2 values measured after advanced airway management versus predicted 30-day mortality, after multivariable correction. The multivariable model is based on 1504 ETCO2 measurements in 836 patients and included the time point of the end-tidal CO2 measurement, age (spline variable), sex, ASA score, systolic blood pressure (spline variable), heart rate (spline variable), oxygen saturation (spline variable), Injury Severity Score (spline variable), first Glasgow Coma Scale score, HEMS service involved in the treatment, and distance between incident scene and trauma hospital (spline variable). As the plotted curve depends on the levels or values of all covariates in the model, this figure is just an example in which covariates were fixed at their mode (categorical variables) or mean (continuous variables) to obtain an estimate for the “average” patient, namely male gender, age 45 years, ISS 27, systolic blood pressure 131 mmHg, heart rate 95 beats per minute, ASA I, GCS 3, distance to hospital 28.6 km, and treated by the HEMS service that contributed most patients to the dataset. Moreover, as the time point of ETCO2 measurement was also included in the model, the figure shows the expected adjusted probability of mortality given the first recorded ETCO2 value after airway management. The curve for the second measurement time point is virtually identical (not shown)
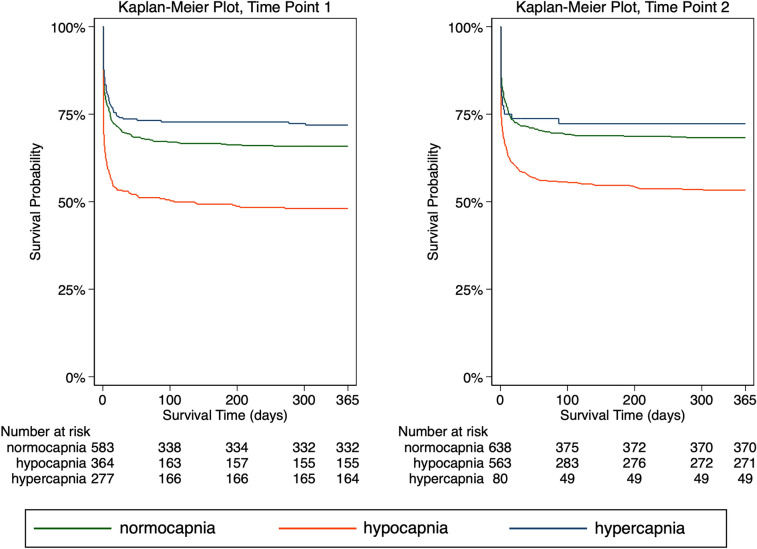
After stratification into the three categories normocapnia (1302 measurements), hypocapnia (976 measurements), and hypercapnia (371 measurements), hypocapnia was associated with an approximately 90% increased odds of mortality (OR 1.89, 95% CI 1.53–2.34, p < 0.001, Table 2) compared to normocapnia. No significant association between hypercapnia and mortality was observed (OR 0.83, 95% CI 0.62–1.11, p = 0.212). Likewise, survival analysis demonstrated an increased hazard of mortality with hypocapnia (HR 1.65, 95% CI 1.42–1.92, p < 0.001), whereas no relationship between hypercapnia and mortality was observed compared to normocapnia (HR 0.82, 95% CI 0.65–1.04, p = 0.095, Table 3, Fig. 4). Considering only the lowest and highest ETCO2 values per patient, nadir values < 35 mmHg (hypocapnia observed at least once) were associated with a higher mortality in logistic regression and survival analyses (both p < 0.001), whereas no association between peak values ≥ 45 mmHg (hypercapnia observed at least once) and mortality was observed (both p > 0.05, Tables 2, 3).
Table 2.
Logistic regression analyses for the association between hypo- and hypercapnia on 30-day mortality
| Hypocapnia (< 35 mmHg) | Hypercapnia (≥ 45 mmHg) | Number of ETCO2 measurements/patients/mortality events | |||
|---|---|---|---|---|---|
| Complete case analysis | OR | 95% CI | OR | 95% CI | |
| Overall | 1.89 | 1.53–2.34 | 0.83 | 0.62–1.11 | 2505/1342/459 |
| Confirmed TBI | 1.90 | 1.51–2.39 | 0.91 | 0.66–1.24 | 2051/1094/424 |
| Isolated TBI | 2.14 | 1.55–2.97 | 0.92 | 0.60–1.41 | 1069/567/231 |
| Patients with symptoms of cerebral herniation | |||||
| Absence of PEARL± | 1.72 | 1.27–2.32 | 0.81 | 0.54–1.20 | 1113/598/296 |
| Signs of elevated ICP on CT$ | 1.87 | 1.31–2.67 | 0.96 | 0.60–1.54 | 871/461/297 |
| Only considering a single CO2 value per patient | |||||
| First ETCO2 | 2.05 | 1.56–2.69 | 0.83 | 0.60–1.14 | 1224/1224/420 |
| Second ETCO2 | 1.78 | 1.40–2.27 | 0.90 | 0.53–1.52 | 1281/1281/435 |
| Nadir ETCO2 | 1.86 | 1.47–2.36 | 1.00 | 0.58–1.71 | 1342/1342/459 |
| Peak ETCO2 | 1.86 | 1.43–2.42 | 0.87 | 0.64–1.18 | 1342/1342/459 |
| After multiple imputation | OR | 95% CI | OR | 95% CI | (NA) |
|---|---|---|---|---|---|
| Overall | 1.89 | 1.57–2.29 | 0.78 | 0.59–1.03 | |
| Confirmed TBI | 1.83 | 1.50–2.24 | 0.84 | 0.63–1.13 | |
| Isolated TBI | 2.00 | 1.49–2.60 | 0.85 | 0.56–1.30 | |
| Patients with symptoms of cerebral herniation | |||||
| Absence of PEARL± | 1.70 | 1.33–2.17 | 0.79 | 0.55–1.13 | |
| Signs of elevated ICP on CT$ | 1.83 | 1.34–2.48 | 0.89 | 0.59–1.35 | |
| Only considering a single CO2 value per patient | |||||
| First ETCO2 | 2.02 | 1.57–2.60 | 0.77 | 0.57–1.04 | |
| Second ETCO2 | 1.80 | 1.44–2.26 | 0.86 | 0.51–1.45 | |
| Nadir ETCO2 | 1.93 | 1.52–2.45 | 0.63 | 0.33–1-22 | |
| Peak ETCO2 | 2.06 | 1.58–2.68 | 0.77 | 0.57–1.03 | |
| Confounder adjusted | OR | 95% CI | OR | 95% CI | (NA) |
|---|---|---|---|---|---|
| Overall | 1.57 | 1.22–2.03 | 0.85 | 0.61–1.19 | |
| Confirmed TBI | 1.54 | 1.19–2.00 | 0.87 | 0.61–1.22 | |
| Isolated TBI | 1.59 | 1.03–2.45 | 0.79 | 0.46–1.37 | |
| Patients with symptoms of cerebral herniation | |||||
| Absence of PEARL± | 1.64 | 1.17–2.30 | 0.87 | 0.55–1.37 | |
| Signs of elevated ICP on CT$ | 1.46 | 1.00–2.12 | 0.79 | 0.49–1.26 | |
| Only considering a single CO2 value per patient | |||||
| First ETCO2 | 1.53 | 1.09–2.17 | 0.84 | 0.57–1.25 | |
| Second ETCO2 | 1.62 | 1.19–2.23 | 0.85 | 0.44–1.65 | |
| Nadir ETCO2 | 1.50 | 1.10–2.06 | 0.77 | 0.36–1.64 | |
| Peak ETCO2 | 1.81 | 1.26–2.60 | 0.86 | 0.59–1.25 | |
Logistic regression analyses (complete-case analyses as well as after multiple imputation performed on all 1776 patients that had been selected from the BRAIN-PROTECT database) on hypo- and hypercapnia versus normocapnia for the overall population, as well as in subgroups of patients with confirmed and isolated TBI as well as in patients with signs of cerebral herniation. Additional sensitivity analyses consider only one measurement per patient, i.e., either the first or second measurement, as well as nadir and peak ETCO2 values per patient. The outcome variable was 30-day mortality for all analyses. ±At arrival of HEMS; $midline shift > 5 mm or compressed/absent basal cisterns. All analyses including multiple ETCO2 values per patient were adjusted for the measurement time point. Confounder adjusted analyses adjust for age (spline variable), sex, ASA score, systolic blood pressure (spline variable), heart rate (spline variable), oxygen saturation (spline variable), Injury Severity Score (spline variable), first Glasgow Coma Scale score, HEMS service involved in the treatment and distance between incident scene and trauma hospital (spline variable) after multiple imputation of missing variables
Table 3.
Survival analyses for the association between hypo- and hypercapnia on mortality
| Hypocapnia (< 35 mmHg) | Hypercapnia (≥ 45 mmHg) | Number of ETCO2 measurements/patients/mortality events | |||
|---|---|---|---|---|---|
| Complete case analysis | HR | 95% CI | HR | 95% CI | |
| Overall | 1.65 | 1.42–1.92 | 0.82 | 0.65–1.04 | 2505/1342/506 |
| Confirmed TBI | 1.64 | 1.40–1.92 | 0.89 | 0.70–1.13 | 2051/1094/462 |
| Isolated TBI | 1.77 | 1.43–2.19 | 0.93 | 0.67–1.28 | 1069/567/246 |
| Patients with symptoms of cerebral herniation | |||||
| Absence of PEARL± | 1.44 | 1.21–1.73 | 0.82 | 0.62–1.09 | 1113/598/317 |
| Signs of elevated ICP on CT$ | 1.44 | 1.22–1.71 | 0.99 | 0.76–1.29 | 871/461/319 |
| Only considering a single CO2 value per patient | |||||
| First ETCO2 | 1.71 | 1.42–2.07 | 0.81 | 0.63–1.04 | 1224/1224/460 |
| Second ETCO2 | 1.60 | 1.35–1.91 | 0.89 | 0.58–1.37 | 1281/1281/481 |
| Nadir ETCO2 | 1.68 | 1.42–2.00 | 0.96 | 0.62–1.49 | 1342/1342/506 |
| Peak ETCO2 | 1.58 | 1.32–1.89 | 0.83 | 0.66–1.06 | 1342/1342/506 |
| After multiple imputation | HR | 95% CI | HR | 95% CI | NA |
|---|---|---|---|---|---|
| Overall | 1.63 | 1.42–1.88 | 0.78 | 0.62–0.97 | |
| Confirmed TBI | 1.58 | 1.37–1.82 | 0.85 | 0.67–1.06 | |
| Isolated TBI | 1.68 | 1.38–2.06 | 0.88 | 0.64–1.21 | |
| Patients with symptoms of cerebral herniation | |||||
| Absence of PEARL± | 1.43 | 1.22–1.67 | 0.81 | 0.63–1.04 | |
| Signs of elevated ICP on CT$ | 1.40 | 1.21–1.63 | 0.95 | 0.75–1.19 | |
| Only considering a single CO2 value per patient | |||||
| First ETCO2 | 1.68 | 1.41–2.01 | 0.77 | 0.60–0.98 | |
| Second ETCO2 | 1.59 | 1.34–1.89 | 0.84 | 0.55–1.30 | |
| Nadir ETCO2 | 1.72 | 1.44–2.06 | 0.65 | 0.36–1.17 | |
| Peak ETCO2 | 1.69 | 1.41–2.02 | 0.77 | 0.61–0.96 | |
| Confounder adjusted | HR | 95% CI | HR | 95% CI | NA |
|---|---|---|---|---|---|
| Overall | 1.28 | 1.12–1.46 | 0.90 | 0.74–1.10 | |
| Confirmed TBI | 1.27 | 1.11–1.45 | 0.92 | 0.75–1.13 | |
| Isolated TBI | 1.23 | 1.01–1.51 | 0.88 | 0.67–1.16 | |
| Patients with symptoms of cerebral herniation | |||||
| Absence of PEARL± | 1.28 | 1.10–1.49 | 0.93 | 0.73–1.17 | |
| Signs of elevated ICP on CT$ | 1.19 | 1.03–1.37 | 0.94 | 0.77–1.14 | |
| Only considering a single CO2 value per patient | |||||
| First ETCO2 | 1.21 | 1.01–1.47 | 0.87 | 0.69–1.09 | |
| Second ETCO2 | 1.34 | 1.13–1.58 | 0.94 | 0.64–1.38 | |
| Nadir ETCO2 | 1.31 | 1.11–1.56 | 0.86 | 0.51–1.46 | |
| Peak ETCO2 | 1.33 | 1.10–1.61 | 0.88 | 0.71–1.09 | |
Cox regression analyses (complete-case analyses as well as after multiple imputation performed on all 1776 patients that had been selected from the BRAIN-PROTECT database) on hypo- and hypercapnia versus normocapnia for the overall population, as well as in subgroups of patients with confirmed and isolated TBI as well as in patients with signs of cerebral herniation. Additional sensitivity analyses consider only one measurement per patient, i.e., either the first or second measurement, as well as nadir and peak ETCO2 values per patient. ±At arrival of HEMS; $midline shift > 5 mm or compressed/absent basal cisterns. All analyses including multiple ETCO2 values per patient were adjusted for the measurement time point. Confounder adjusted analyses adjust for age (spline variable), sex, ASA score, systolic blood pressure (spline variable), heart rate (spline variable), oxygen saturation (spline variable), Injury Severity Score (spline variable), first Glasgow Coma Scale score, HEMS service involved in the treatment and distance between incident scene and trauma hospital (spline variable) after multiple imputation of missing variables. In all Cox regression models, mortality up to 1 year after the trauma was modeled
Fig. 4.
Kaplan–Meier plots of the estimated survival function (up to 1 year after the trauma) per ETCO2 category based on values measured after advanced airway management at two time points (time point 1 is the first recorded ETCO2 value after advanced airway management; time point 2 is before arrival at the emergency department); see text for details
In subgroup analyses, ETCO2 values < 35 mmHg were associated with increased mortality in patients with confirmed TBI (OR 1.90 versus normocapnia, 95% CI 1.51–2.39, p < 0.001) and with isolated TBI (OR 2.14, 95% CI 1.55–2.97, p < 0.001). Likewise, ETCO2 values < 35 mmHg were associated with an increased mortality in patients with signs of cerebral herniation. Consistent results were found in survival analyses and after multiple imputation (Tables 2, 3).
Discussion
The BRAIN-PROTECT study prospectively addressed the prehospital care of patients with severe TBI in the Netherlands. We observed an L-shaped association between ETCO2 levels and 30-day mortality, and that a “safe zone” of 35–45 mmHg CO2 seems to be a reasonable target range for prehospital ventilation.
Prehospital care of patients with severe TBI focuses on the prevention of secondary brain injury, and often involves advanced airway management and ventilation [19]. While guidelines recommend “normoventilation”, evidence for specific ventilation targets is lacking and clinical practice regarding ventilation of patients with TBI broadly varies, both in the prehospital setting [11] as well as in hospital [20]. “Normoventilation” implies normocapnia, commonly defined as an arterial CO2 partial pressure in the range between 35 and 45 mmHg. Notably, a previous study observed that only 36% of ventilated patients present to the emergency department with normocapnia [21], suggesting that prehospital normoventilation is often not delivered.
Previous data on the association between arterial CO2 partial pressures and cerebral oxygenation/metabolism or clinical outcomes have been conflicting and often derive from studies performed in the intensive-care unit rather than in the prehospital setting. On the one hand, for example, Brandi et al. did not find negative effects of hypocapnia on cerebral metabolism [22], whereas Coles et al. report adverse effects of hyperventilation [23]. With respect to clinical outcomes, Citerio et al. did not observe worse outcomes in patients treated in centers that use profound hyperventilation more often [20]. In contrast, in the only randomized trial to date performed more than 30 years ago, hyperventilation adversely affected the neurologic outcome at 3 and 6 months in a subgroup of patients with relatively good motor scores (4—5) of the GCS score [24].
While it is thus not even clear what PaCO2 values are optimal for TBI patients, prehospital ventilation is further complicated by the fact that PaCO2 measurements are generally not available to guide (normo-)ventilation, such that ETCO2 levels are commonly used as a surrogate. Several studies have addressed the correlation between end-tidal and arterial CO2 levels in severe TBI [10, 25–27]. Overall, they reported mixed correlations, from good to poor. The physiological gap between PaCO2 and ETCO2 can be markedly increased in trauma patients [10], for example due to thoracic injury or circulatory shock. Therefore, guiding ventilation by ETCO2 is challenging, and prehospital healthcare personnel often target low-normal or sub-normal ETCO2 values to account for this potentially increased PaCO2-ETCO2 gap, to achieve normoventilation. A recent study that observed a substantial gap between PaCO2 and ETCO2 values (mean difference was 12.8 mmHg) concluded that even lower ETCO2 targets than currently recommended may be safe and appropriate. Notably, however, neither this nor other studies provide clinical outcome data to support any specific ETCO2 target range.
In the context of clinical outcome data, Howard et al. recently summarized the effects of CO2 (ETCO2 or PCO2, depending on included studies) levels during the initial treatment of patients with TBI in a systematic review [7]. The authors identified six retrospective observational studies, of which five reported clinical outcomes [28–32]. None of these studies considered CO2 on its continuous scale, but rather used inconsistent and arbitrary thresholds to categorize CO2 values. Moreover, most had a rather small sample size [28, 30, 31], poorly controlled for confounding [28, 30, 31], assessed a bundle of care rather than (hyper-)ventilation in isolation [32], or did not actually consider prehospital ETCO2 values but rather used first documented in-hospital PaCO2 values as a surrogate for prehospital ventilation [29–31]. While these studies consistently suggest worse outcomes with hypocapnia, potential detrimental effects of hypercapnia as well as the range of reasonable ETCO2 values still remain unclear.
In contrast to previous studies, we considered ETCO2 on the continuous scale and allowed for a non-linear association between ETCO2 and the (logit of) mortality. The association between ETCO2 levels and mortality was L-shaped, with a profound increase in mortality in ETCO2 values < 35 mmHg, i.e., in hypocapnia. We did not observe an association between high ETCO2 values and increased mortality, but the precision of the estimated association declined above 45 mmHg. Our data therefore do not allow the conclusion that ETCO2 values > 45 mmHg are safe, and therefore, a “safe zone” of 35—45 mmHg ETCO2 seems a reasonable target to guide prehospital ventilation in patients with severe TBI. Notably, this ETCO2 range is higher than that currently recommended by many EMS protocols in the United States [11]. The increase in mortality with ETCO2 values below 35 mmHg was observed across all subgroup analyses, including patients with isolated TBI and patients with signs of cerebral herniation. While these subgroup analyses must be interpreted with care, the data also do not support the unproven paradigm of temporary hyperventilation in patients with signs of cerebral herniation. [33]
Limitations
BRAIN-PROTECT is a prospective observational study project, and the inherent limitations of observational research such as risk of selection bias and information bias have to be considered. The steps taken to minimize such bias have been described previously [12]. Importantly, observational data are subject to confounding, and we therefore emphasize that we observed an association but not necessarily a causal relationship between ETCO2 values and 30-day mortality. Decreases in ETCO2 levels are often due to hyperventilation, but may also have other causes, such as low cardiac output during circulatory shock, excessive blood loss, or tension pneumothorax. However, we thoroughly adjusted for potential confounders in the multivariable regression model. In plain language, for patients with everything else being held constant (i.e., same age, same injury severity score, same GCS score, same blood pressure, same heart rate, etc.), an ETCO2 value < 35 mmHg is independently associated with a markedly increased mortality. Moreover, in our subgroup analysis of patients with isolated TBI—i.e., patients without other significant injuries that could bias the association—we also found a profound association between hypocapnia and mortality. Nonetheless, residual confounding cannot be excluded, and clinical recommendations are not directly supported by the data. However, accumulating evidence suggests detrimental effects of hyperventilation, and our data provide the best available evidence for a specific ventilation target.
Missing data are also a limitation of our study. However, analyses produced consistent results after multiple imputation, [34] suggesting that missing data did not bias our results to a relevant degree. Moreover, data were collected in the Netherlands, a country with a high population density and highly developed emergency care infrastructure with short distances to trauma centers. The results may not necessarily generalize to other healthcare systems.
Our analysis focuses on 30-day mortality, which is clearly a clinically relevant endpoint. Nonetheless, neurologic recovery, e.g. as measured by the extended Glasgow Outcome Scale [15], is also of great importance and should be addressed in future studies. We had initially planned to analyze and show such data in the current study but refrained from doing so, because these data were incomplete and could not validly be imputed given the assumed mechanism of missingness.
Conclusions
We found an L-shaped association between ETCO2 levels and 30-day mortality in patients with severe TBI. The range between 35 and 45 mmHg seems a reasonable target as lower ETCO2 levels were significantly associated with increased 30-day mortality. These results suggest that the use of hyperventilation in prehospital treatment of severe TBI should be discouraged.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (EPS 459 KB) Supplemental Fig. 1 Correlation between the ETCO2 values (mmHg) measured after initial stabilization / airway management and before hospital arrival. r = 0.60, 95% CI 0.53 to 0.68, p<0.001
Supplementary file2 (EPS 457 KB) Supplemental Fig. 2 Correlation between the ETCO2 values (mmHg) measured before hospital arrival and PaCO2 values (mmHg) in the first blood gas analysis at the hospital. r = 0.17, 95% CI 0.10 to 0.23, p<0.001
Supplementary file3 (EPS 362 KB) Supplemental Fig. 3 Histogram showing the distribution of ETCO2 values at all 3 measurement time points: 1. after HEMS arrival (n=394); 2. after initial stabilization and airway management (n=1295) and 3. before arriving at the emergency department (n=1354). For the analysis of the relationship between ETCO2 and mortality, only the second and third time point were considered; see text for details
Acknowledgements
BRAIN-PROTECT collaborators: Anne Boer, de: MSc. SpoedZorgNet, Amsterdam, The Netherlands, Site facilitation. Johannes C. Goslings: MD, PhD, OLVG, Amsterdam, The Netherlands, Site facilitation. Sven H. Helden, van: MD, PhD, Isala Zwolle, The Netherlands, Site facilitation. Danique Hesselink: MSc, Netwerk Acute Zorg Zwolle, Zwolle, The Netherlands, Site facilitation. Gijs Aken, van: Netwerk Acute Zorg Zwolle, Zwolle, The Netherlands,Site facilitation. Albertus Beishuizen: MD, PhD, Medisch Spectrum Twente, Enschede, The Netherlands, Site facilitation. Rolf E. Egberink: MSc, Acute Zorg Euregio, Enschede, the Netherlands, Site facilitation. Nancy Bogt, ter: PhD, Acute Zorg Euregio, Enschede, The Netherlands, Site facilitation. Mariska A.C. Jongh, de: PhD, Netwerk Acute Zorg Brabant, Tilburg, The Netherlands, Site facilitation. Koen Lansink: MD, PhD, Elisabeth-TweeSteden ziekenhuis, Tilburg, The Netherlands, Site facilitation. Gerwin Roks: MD, PhD, Elisabeth-TweeSteden ziekenhuis, Tilburg, The Netherlands, Site facilitation. Pieter Joosse: MD, PhD, Noordwest Ziekenhuisgroep, Alkmaar, The Netherlands, Site facilitation: Kees J. Ponsen: MD, PhD, Noordwest Ziekenhuisgroep, Alkmaar, The Netherlands, Site facilitation. Lukas L. van Spengler: MSc, Traumazorgnetwerk Midden-Nederland, Utrecht, The Netherlands, Site facilitation. Stasja Aspers: Traumazorgnetwerk Midden-Nederland, Utrecht, The Netherlands, Site facilitation. Saskia M. Peerdeman: MD, PhD, AUMC, Amsterdam, The Netherlands, Study design, Steering Committee member. Robert J. Houmes: MD, PhD, Erasmus MC, Rotterdam, The Netherlands, Site facilitation. Jan Ditshuizen, van: MSc. Traumacentrum Zuidwest-Nederland, Rotterdam, The Netherlands, Site facilitation.Tea Voorden, van: MSc, Traumacentrum Zuidwest-Nederland, Rotterdam, The Netherlands, Site facilitation. Michael J.R. Edwards: MD, PhD, Radboudumc, Nijmegen, The Netherlands, Site facilitation. Bert Dercksen: MD, UMCG, Groningen, The Netherlands, Site facilitation. Rob Spanjersberg: UMCG, Groningen, The Netherlands, Site facilitation. Lieneke Venema: MD, UMCG, Groningen, The Netherlands, Site facilitation. Ellen Weelink: MD. UMCG, Groningen, The Netherlands, Site facilitation. Inge H. F. Reininga: PhD, Acute Zorgnetwerk Noord Nederland, Groningen, The Netherlands, Site facilitation. Gerard Innemee: MD, PhD, RAV Gooi & Vechtstreek, Hilversum, The Netherlands, Study design, Steering Committee member. Matthijs Visser, de: MSc, RAV Hollands Midden, Leiden, The Netherlands, Study design,Steering Committee member. Marcel A. Leeuw, de: MD, PhD, AUMC & Lifeliner 1, Amsterdam, The Netherlands, Study design. Fabian O Kooij: MD, PhD, AUMC & Lifeliner 1, Amsterdam, The Netherlands, Site facilitation.
Abbreviations
- AIS
Abbreviated Injury Score
- CI
Confidence Interval
- EMS
Emergency Medical Services
- GCS
Glasgow Coma Scale
- HAIS
Head Abbreviated Injury Score
- HEMS
Helicopter Emergency Medical Service
- ISS
Injury Severity Score
- LTR
Dutch National Trauma Registry ("Landelijke Trauma Registratie")
- TBI
Traumatic Brain Injury
- CBF
Cerebral Blood Flow
- ICP
Intracranial Pressure
- LMA
Laryngeal Mask Airway
- PEARL
Pupils Equal And Reacting to Light
Author contributions
SMB and PS had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis and take responsibility for this article as a whole. Concept and design: SMB, FM, SAL, AB, FWB, EMMVL, DDH, NH, JvdN, ARA, LAS, JWRT and PS. Acquisition, analysis, or interpretation of data: SMB, FM, SAL, AB, FWB, EMMVL, DDH, NH, JvdN, ARA, LAS, JWRT and PS. Drafting of the manuscript: SMB, FM, SAL and PS. Critical revision of the manuscript for important intellectual content: CB, FWB, EMMVL, DDH, NH, JvdN, ARA, LAS and JWRT. Statistical analysis: SMB, SAL, JWRT and PS. Obtained funding: SMB and PS. Administrative, technical, or material support: SMB, FM, SAL, LAS and PS. Supervision: SAL, CB, FWB, EMMVL, DDH, NH, JvdN, ARA, LAS, JWRT and PS.
Funding
This work was supported by the Dutch Brain Foundation (“Hersenstichting”) under Grant F2010(1)-14, and Achmea Healthcare Foundation (“Stichting Achmea Gezondheidszorg”) under Grant Z644. The funding sources did not have a role in the design or execution of this study and did not have any role during analysis and interpretation of the data, or in the decision to submit results.
Data sharing statement
Completely de-identified participant data as well as the data dictionary will be shared upon reasonable request, after approval by the scientific steering committee of the BRAIN-PROTECT study group. The study protocol has been published and is publicly available. Data will be available from 12 months following article publication and proposals may be submitted to the corresponding author (s.bossers@amsterdamumc.nl) up to 36 months following article publication.
Declarations
Conflicts of interest
SMB reported receiving grants from Achmea Healthcare Foundation during the conduct of the study. ARA reported receiving grants and personal fees from Becton Dickson and The Medicines Company; grants from Draeger; sponsor-initiated and funded phase 1 research from Rigel; and personal fees from PAION, Janssen Pharma, Ever Pharma, and Philips outside the submitted work. PS reported receiving grants from Dutch Brain Foundation and Achmea Healthcare Foundation during the conduct of the study. No other disclosures were reported.
Footnotes
The BRAINPROTECT Collaborators are listed in the Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sebastiaan M. Bossers, Email: s.bossers@amsterdamumc.nl
Floor Mansvelder, Email: f.j.mansvelder@amsterdamumc.nl.
Stephan A. Loer, Email: s.loer@amsterdamumc.nl
Christa Boer, Email: c.boer@amsterdamumc.nl.
Frank W. Bloemers, Email: fw.bloemers@amsterdamumc.nl
Esther M. M. Van Lieshout, Email: e.vanlieshout@erasmusmc.nl
Dennis Den Hartog, Email: d.denhartog@erasmusmc.nl.
Nico Hoogerwerf, Email: nico.hoogerwerf@radboudumc.nl.
Joukje van der Naalt, Email: j.van.der.naalt@umcg.nl.
Anthony R. Absalom, Email: a.r.absalom@umcg.nl
Lothar A. Schwarte, Email: l.schwarte@amsterdamumc.nl
Jos W. R. Twisk, Email: jwr.twisk@amsterdamumc.nl
Patrick Schober, Email: p.schober@amsterdamumc.nl.
the BRAIN-PROTECT Collaborators:
Anne de Boer, Johannes C. Goslings, Sven H. van Helden, Danique Hesselink, Gijs van Aken, Albertus Beishuizen, Rolf E. Egberink, Nancy ter Bogt, Mariska A. C. de Jongh, Koen Lansink, Koen Lansink, Gerwin Roks, Pieter Joosse, Kees J. Ponsen, Lukas L. van Spengler, Stasja Asper, Saskia M. Peerdeman, Robert J. Houmes, Jan van Ditshuizen, Tea van Voorden, Michael J. R. Edwards, Bert Dercksen, Rob Spanjersberg, Lieneke Venema, Ellen Weelink, Inge H. F. Reininga, Gerard Innemee, Matthijs de Visser, Marcel A. de Leeuw, and Fabian O. Kooij
References
- 1.Bossers SM, Boer C, Bloemers FW, Van Lieshout EMM, Den Hartog D, Hoogerwerf N, Innemee G, van der Naalt J, Absalom AR, Peerdeman SM, de Visser M, de Leeuw MA, Schwarte LA, Loer SA, Schober P, (2020) Epidemiology, Prehospital Characteristics and Outcomes of Severe Traumatic Brain Injury in The Netherlands: The BRAIN-PROTECT Study. Prehospital emergency care: official journal of the National Association of EMS Physicians and the National Association of State EMS Directors: 1–12
- 2.GBD Traumatic brain injury and spinal cord injury Collaborators, (2019) Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2016;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravesteijn BY, Sewalt CA, Stocchetti N, Citerio G, Ercole A, Lingsma HF, von Steinbüchel N, Steyerberg EW, Wilson L, Maas AIR, Menon DK, Lecky FE. Prehospital Management of Traumatic Brain Injury across Europe: A CENTER-TBI Study. Prehospital Emerg Care. 2021;25:629–643. doi: 10.1080/10903127.2020.1817210. [DOI] [PubMed] [Google Scholar]
- 4.Boer C, Franschman G, Loer SA. Prehospital management of severe traumatic brain injury: concepts and ongoing controversies. Curr Opin Anaesthesiol. 2012;25:556–562. doi: 10.1097/ACO.0b013e328357225c. [DOI] [PubMed] [Google Scholar]
- 5.Badjatia N, Carney N, Crocco TJ, Fallat ME, Hennes HM, Jagoda AS, Jernigan S, Letarte PB, Lerner EB, Moriarty TM, Pons PT, Sasser S, Scalea T, Schleien CL, Wright DW, (2008) Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehospital emergency care : official journal of the National Association of EMS Physicians and the National Association of State EMS Directors 12 Suppl 1: S1–52 [DOI] [PubMed]
- 6.Harper AM, Glass HI. Effect of alterations in the arterial carbon dioxide tension on the blood flow through the cerebral cortex at normal and low arterial blood pressures. J Neurol Neurosurg Psychiatry. 1965;28:449–452. doi: 10.1136/jnnp.28.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard MB, McCollum N, Alberto EC, Kotler H, Mottla ME, Tiusaba L, Keller S, Marsic I, Sarcevic A, Burd RS, O'Connell KJ. Association of ventilation during initial trauma resuscitation for traumatic brain injury and post-traumatic outcomes: a systematic review. Prehosp Disaster Med. 2021;36:460–465. doi: 10.1017/S1049023X21000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Guo Q, Wang E. Hyperventilation in neurological patients: from physiology to outcome evidence. Curr Opin Anaesthesiol. 2019;32:568–573. doi: 10.1097/ACO.0000000000000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts BW, Karagiannis P, Coletta M, Kilgannon JH, Chansky ME, Trzeciak S. Effects of PaCO2 derangements on clinical outcomes after cerebral injury: a systematic review. Resuscitation. 2015;91:32–41. doi: 10.1016/j.resuscitation.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Price J, Sandbach DD, Ercole A, Wilson A, Barnard EBG. End-tidal and arterial carbon dioxide gradient in serious traumatic brain injury after prehospital emergency anaesthesia: a retrospective observational study. Emerg Med J. 2020;37:674–679. doi: 10.1136/emermed-2019-209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuck CC, Martin TJ, Kalagara R, Shaaya E, Kheirbek T, Cielo D. Emergency medical services protocols for traumatic brain injury in the United States: a call for standardization. Injury. 2021;52:1145–1150. doi: 10.1016/j.injury.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Bossers SM, Boer C, Greuters S, Bloemers FW, Den Hartog D, Van Lieshout EMM, Hoogerwerf N, Innemee G, van der Naalt J, Absalom AR, Peerdeman SM, de Visser M, Loer S, Schober P, collaborators B-P, Dutch Prospective Observational Study on Prehospital Treatment of Severe Traumatic Brain Injury: The BRAIN-PROTECT Study Protocol. Prehospital Emerg Care. 2019;23:820–827. doi: 10.1080/10903127.2019.1587126. [DOI] [PubMed] [Google Scholar]
- 13.Bossers SM, Pol KM, Oude Ophuis EPA, Jacobs B, Visser MC, Loer SA, Boer C, van der Naalt J, Schober P. Discrepancy between the initial assessment of injury severity and post hoc determination of injury severity in patients with apparently mild traumatic brain injury: a retrospective multicenter cohort analysis. Eur J Trauma Emerg Surg. 2018;44:889–896. doi: 10.1007/s00068-017-0861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringdal KG, Coats TJ, Lefering R, Di Bartolomeo S, Steen PA, Roise O, Handolin L, Lossius HM. The Utstein template for uniform reporting of data following major trauma: a joint revision by SCANTEM, TARN, DGU-TR and RITG. Scand J Trauma Resusc Emerg Med. 2008;16:7. doi: 10.1186/1757-7241-16-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bossers SM, van der Naalt J, Jacobs B, Schwarte LA, Verheul R, Schober P. Face-to-face versus telephonic extended glasgow outcome score testing after traumatic brain injury. J Head Trauma Rehabil. 2021;36:E134–e138. doi: 10.1097/HTR.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 17.Schober P, Vetter TR. Kaplan-Meier Curves, Log-Rank Tests, and Cox Regression for Time-to-Event Data. Anesth Analg. 2021;132:969–970. doi: 10.1213/ANE.0000000000005358. [DOI] [PubMed] [Google Scholar]
- 18.Schober P, Mascha EJ, Vetter TR. Statistics from A (Agreement) to Z (z Score): a guide to interpreting common measures of association, agreement, diagnostic accuracy, effect size, heterogeneity, and reliability in medical research. Anesth Analg. 2021;133:1633–1641. doi: 10.1213/ANE.0000000000005773. [DOI] [PubMed] [Google Scholar]
- 19.Bossers SM, Schwarte LA, Loer SA, Twisk JW, Boer C, Schober P. Experience in prehospital endotracheal intubation significantly influences mortality of patients with severe traumatic brain injury: a systematic review and meta-analysis. PLoS ONE. 2015;10:e0141034. doi: 10.1371/journal.pone.0141034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Citerio G, Robba C, Rebora P, Petrosino M, Rossi E, Malgeri L, Stocchetti N, Galimberti S, Menon DK. Management of arterial partial pressure of carbon dioxide in the first week after traumatic brain injury: results from the CENTER-TBI study. Intens Care Med. 2021;47:961–973. doi: 10.1007/s00134-021-06470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curry BW, Ward S, Lindsell CJ, Hart KW, McMullan JT. Mechanical ventilation of severe traumatic brain injury patients in the prehospital setting. Air Med J. 2020;39:410–413. doi: 10.1016/j.amj.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Brandi G, Stocchetti N, Pagnamenta A, Stretti F, Steiger P, Klinzing S. Cerebral metabolism is not affected by moderate hyperventilation in patients with traumatic brain injury. Crit Care. 2019;23:45. doi: 10.1186/s13054-018-2304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coles JP, Fryer TD, Coleman MR, Smielewski P, Gupta AK, Minhas PS, Aigbirhio F, Chatfield DA, Williams GB, Boniface S, Carpenter TA, Clark JC, Pickard JD, Menon DK. Hyperventilation following head injury: effect on ischemic burden and cerebral oxidative metabolism. Crit Care Med. 2007;35:568–578. doi: 10.1097/01.CCM.0000254066.37187.88. [DOI] [PubMed] [Google Scholar]
- 24.Muizelaar JP, Marmarou A, Ward JD, Kontos HA, Choi SC, Becker DP, Gruemer H, Young HF. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731–739. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- 25.Doppmann P, Meuli L, Sollid SJM, Filipovic M, Knapp J, Exadaktylos A, Albrecht R, Pietsch U. End-tidal to arterial carbon dioxide gradient is associated with increased mortality in patients with traumatic brain injury: a retrospective observational study. Sci Rep. 2021;11:10391. doi: 10.1038/s41598-021-89913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SW, Hong YS, Han C, Kim SJ, Moon SW, Shin JH, Baek KJ. Concordance of end-tidal carbon dioxide and arterial carbon dioxide in severe traumatic brain injury. J Trauma. 2009;67:526–530. doi: 10.1097/TA.0b013e3181866432. [DOI] [PubMed] [Google Scholar]
- 27.Yang JT, Erickson SL, Killien EY, Mills B, Lele AV, Vavilala MS. Agreement between arterial carbon dioxide levels with end-tidal carbon dioxide levels and associated factors in children hospitalized with traumatic brain injury. JAMA Netw Open. 2019;2:e199448. doi: 10.1001/jamanetworkopen.2019.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caulfield EV, Dutton RP, Floccare DJ, Stansbury LG, Scalea TM, (2009) Prehospital hypocapnia and poor outcome after severe traumatic brain injury. J Trauma 66: 1577–1582; discussion 1583 [DOI] [PubMed]
- 29.Davis DP, Peay J, Sise MJ, Kennedy F, Simon F, Tominaga G, Steele J, Coimbra R. Prehospital airway and ventilation management: a trauma score and injury severity score-based analysis. J Trauma. 2010;69:294–301. doi: 10.1097/TA.0b013e3181dc6c7f. [DOI] [PubMed] [Google Scholar]
- 30.Warner KJ, Cuschieri J, Copass MK, Jurkovich GJ, Bulger EM, (2007) The impact of prehospital ventilation on outcome after severe traumatic brain injury. J Trauma 62: 1330–1336; discussion 1336–1338 [DOI] [PubMed]
- 31.Dumont TM, Visioni AJ, Rughani AI, Tranmer BI, Crookes B. Inappropriate prehospital ventilation in severe traumatic brain injury increases in-hospital mortality. J Neurotrauma. 2010;27:1233–1241. doi: 10.1089/neu.2009.1216. [DOI] [PubMed] [Google Scholar]
- 32.Spaite DW, Bobrow BJ, Keim SM, Barnhart B, Chikani V, Gaither JB, Sherrill D, Denninghoff KR, Mullins T, Adelson PD, Rice AD, Viscusi C, Hu C. Association of statewide implementation of the prehospital traumatic brain injury treatment guidelines with patient survival following traumatic brain injury: The excellence in prehospital injury care (EPIC) Study. JAMA Surg. 2019;154:e191152. doi: 10.1001/jamasurg.2019.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gouvea Bogossian E, Peluso L, Creteur J, Taccone FS. Hyperventilation in adult TBI patients: how to approach it? Front Neurol. 2020;11:580859. doi: 10.3389/fneur.2020.580859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schober P, Vetter TR. Missing data and imputation methods. Anesth Analg. 2020;131:1419–1420. doi: 10.1213/ANE.0000000000005068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (EPS 459 KB) Supplemental Fig. 1 Correlation between the ETCO2 values (mmHg) measured after initial stabilization / airway management and before hospital arrival. r = 0.60, 95% CI 0.53 to 0.68, p<0.001
Supplementary file2 (EPS 457 KB) Supplemental Fig. 2 Correlation between the ETCO2 values (mmHg) measured before hospital arrival and PaCO2 values (mmHg) in the first blood gas analysis at the hospital. r = 0.17, 95% CI 0.10 to 0.23, p<0.001
Supplementary file3 (EPS 362 KB) Supplemental Fig. 3 Histogram showing the distribution of ETCO2 values at all 3 measurement time points: 1. after HEMS arrival (n=394); 2. after initial stabilization and airway management (n=1295) and 3. before arriving at the emergency department (n=1354). For the analysis of the relationship between ETCO2 and mortality, only the second and third time point were considered; see text for details
Data Availability Statement
Completely de-identified participant data as well as the data dictionary will be shared upon reasonable request, after approval by the scientific steering committee of the BRAIN-PROTECT study group. The study protocol has been published and is publicly available. Data will be available from 12 months following article publication and proposals may be submitted to the corresponding author (s.bossers@amsterdamumc.nl) up to 36 months following article publication.