Abstract
Background
The glycoprotein D (gD)/AS04 vaccine failed to prevent herpes simplex virus (HSV) 2 in clinical trials. Failure was recapitulated in mice, in which the vaccine elicited neutralizing antibody but not antibody-dependent cell-mediated cytotoxicity (ADCC) responses. Preclinical findings suggest that ADCC is important for protection, but the clinical data are limited. We hypothesized that gD/AS04 and acute HSV-2 infection elicit primarily neutralizing antibodies, whereas ADCC emerges over time.
Methods
HSV-specific immunoglobulin G, subclass, function (neutralization, C1q binding and ADCC), and antigenic targets were compared (paired t test or Mann-Whitney U test) at enrollment and after gD/AS04 vaccination, before and after HSV-2 acquisition in vaccine controls, and in an independent cohort with chronic HSV-2 infection.
Results
Vaccination elicited only a neutralizing antibody response, whereas acute infection elicited neutralizing and C1q-binding antibodies but not a significant ADCC response. Antibodies to gD were exclusively immunoglobulin G1 and only neutralizing. In contrast, women with chronic HSV-2 infection had significantly greater ADCC responses and targeted a broader range of viral antigens compared with acutely infected or gD/AS04 vaccine recipients (P < .001).
Conclusions
Results from gD/AS04 vaccinated or acutely infected women recapitulate murine findings of limited functional antibody responses, supporting the speculation that vaccines that generate polyfunctional and specifically ADCC responses may be required to prevent HSV-2 acquisition and limit recurrences.
Keywords: Herpes simplex virus, antibody-dependent cell-mediated cytotoxicity, vaccines, HIV
The glycoprotein D/AS04 vaccine, which failed to prevent herpes simplex virus (HSV) 2 in a large field trial, elicited only neutralizing antibodies. Significant antibody-dependent cell-mediated cytotoxicity responses were detected in women with longstanding HSV-2 infection but not within 6 months of acute infection.
(See the Editorial Commentary by Bradfute and Mertz, on pages 1485–8.)
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2) are significant global health problems, disproportionately affecting developing countries and fueling the human immunodeficiency virus (HIV) epidemic [1–4]. HSV-2 is the leading cause of primary and recurrent genital disease worldwide, although HSV-1 has emerged as a common cause of primary genital infection in developed countries [2, 3]. Perinatal transmission, which is most often associated with unrecognized viral shedding of either serotype, can result in severe infant disease or death. Acyclovir and related drugs effectively treat disease and suppress clinical recurrences but do not prevent viral reactivation or eliminate latent viral reservoirs, highlighting the need for vaccines.
HSV vaccine efforts have been predicated on the presumption that neutralizing antibodies that target glycoprotein D (gD) are protective. However, results clinical trials with a vaccine comprising HSV-2 gD and the Toll-like receptor 4 agonist 3-O-desacyl-4’-monophosphoryl lipid A and aluminum salt (gD/AS04), which elicited neutralizing antibodies, were disappointing [5]. Two trials in serodiscordant couples in which the infected partner had a history of recurrent genital herpes resulted in overall efficacy against genital disease of 38% (95% confidence interval [CI], −18% to 68%) and 42% (−31% to 74%), respectively [5]. Subset analyses suggested that gD/AS04 was approximately 70% protective in doubly seronegative (HSV-1–seronegative/HSV-2–seronegative) women but provided no protection in HSV-1–seropositive women or in men regardless of serostatus. However, in the subsequent field study, which enrolled only HSV-1–uninfected/HSV-2–uninfected women, the vaccine did not protect against genital disease (efficacy, 20% [95% CI, −29% to 50%]) [6].
Subset analyses demonstrated some protection against HSV-1 infection (efficacy, 35% [95% CI, 13%–52%]) and symptomatic genital HSV-1 disease (58% [12%–80%]) but no protection against HSV-2 infection or disease (−8% [−59% to 26%]) [6]. Protection against HSV-1 was correlated with gD binding and neutralizing antibodies but not with cellular immune responses. Neutralizing antibody titers (without complement) were 3.5-fold higher against HSV-1 compared with HSV-2 in a substudy with serum samples from 30 vaccinated participants [7], although a different substudy using a microneutralization assay with 10% guinea pig complement showed no significant serotype difference in neutralization titers [8]. Other antibody-mediated immune mechanisms including antibody-dependent cell-mediated cytotoxicity (ADCC), C1q binding and complement-dependent cytolysis, were not assessed in any of the gD/AS04 trials. Notably, an earlier trial with an adjuvanted gD and glycoprotein B (gB) subunit vaccine (gD-gB/MF59), which generated neutralizing antibody but no ADCC responses, also failed to protect against HSV-2 [9]. These findings support the hypothesis that polyfunctional antibody responses, specifically ADCC, may be required for protection against HSV-2 and for optimal protection against HSV-1 [10].
The potential importance of ADCC is further suggested by preclinical studies comparing gD/AS04 with a single-cycle candidate vaccine deleted in HSV-2 gD (designated ΔgD-2) [11, 12]. In studies with male and female mice, ΔgD-2 provided significantly greater protection than gD/AS04 against lethal challenge with clinical isolates of HSV-1 or HSV-2 [13–16]. The gD/AS04 vaccine as well as primary sublethal HSV-1 or HSV-2 infection elicited neutralizing antibody but little or no ADCC response [13, 17]. In contrast, ΔgD-2 elicited high titer HSV-specific antibodies that activated Fc gamma receptors (FcγRs) and bound C1q to mediate ADCC and complement dependent cytolysis, respectively [11, 12, 15–18]. Mechanistic studies designed to determine why ΔgD-2 but not gD protein vaccines or primary infection elicited a vigorous ADCC response identified an unrecognized immune evasion strategy. Specifically, gD blocks interactions between herpesvirus entry mediator (HVEM), an immunomodulatory molecule expressed by immune cells, and its ligands to disrupt the generation and function of ADCC responses [14].
To explore how well mouse models recapitulate clinical responses to gD/AS04 and to better define the response to acute and chronic HSV-2 infection, we quantified total, neutralizing, ADCC, and C1q-binding antibodies in a subset of women who participated in the Herpevac clinical trial [6]. We compared the response in women who received gD/AS04 and did not acquire HSV (vaccine response) with that in women who received the control hepatitis A virus (HAV) vaccine and subsequently acquired genital HSV-2 disease (acute infection). We hypothesized that gD/AS04 and acute HSV-2 infection would elicit a predominantly neutralizing antibody response with little or no ADCC. However, we speculated that, over time, the magnitude, breadth, and functionality of the humoral response would expand with a boost in ADCC responses, reflecting ongoing antigenic stimulation from repeated exposures to reactivating virus as well as the ability of gD-binding antibodies, generated in response to acute infection, to overcome the gD-HVEM immune evasion strategy. Because longitudinal samples were not available beyond 20 months after enrollment in the Herpevac participants, we took advantage of the Bronx Women’s Interagency Study (WIHS) biorepository to assess antibody responses in chronic HSV-2 infection. This also provided the opportunity to compare HIV-uninfected and HIV-infected women, as HIV may affect quantitative and functional antibody responses.
METHODS
Study Populations
HSV-1–uninfected/HSV-2–uninfected women 18–30 years of age participated in the Herpevac field trial and were randomly assigned to receive gD/AS04 or HAV vaccine at months 0, 1, and 6 [6]. For the current study, serum samples from enrollment and month 7 were obtained from gD/AS04 vaccine recipients who remained HSV-1 or /HSV-2 seronegative by Western blot (except for vaccine-induced gD antibodies) (n = 15 in total; 5 paired samples and 10 additional month 7 samples). Serum samples were also obtained at enrollment and approximately 6 months after acquisition of HSV-2 genital disease (acute infection) in 18 women in the HAV vaccine arm (5 paired samples and 13 additional acute infection samples). Samples were selected at random based on availability. Acute HSV-2 was documented by a positive genital swab culture and detection of HSV-2 glycoprotein G antibodies on Western blots [6].
To assess immune responses to chronic HSV-2, samples were also obtained from the Bronx WIHS biorepository at visits 46–48 (2017–2018) from 18 HIV-uninfected and 30 HIV-infected women. HSV-2 seropositivity was confirmed using the HerpeSelect type-specific HSV immunoglobulin (Ig) G qualitative assay (Focus Diagnostics) with plasma from visits 44 (2016). Data extracted from the WIHS database included demographics, recent history of genital herpes recurrences or genital lesions and, for HIV-infected participants, HIV plasma viral load and CD4 cell count. The study was approved by the Albert Einstein College of Medicine Institutional Review Board.
IgG Responses
HSV-specific IgG, IgG1, and IgG3 were quantified by means enzyme-linked immunosorbent assay (ELISA) as described elsewhere, with species-specific modifications [12, 16]. ELISA plates were coated overnight with Vero cell lysates harvested 24 hours after infection with HSV-2 (4674) (clinical isolate) at a multiplicity of infection of 0.1 plaque-forming unit per cell or uninfected Vero cell lysates. Wells were blocked with 5% bovine serum albumin and then incubated with serial dilutions of serum in duplicate wells. Bound human IgG, IgG1, or Ig3 was quantified using specific horseradish peroxidase (HRP)–labeled secondary antibodies (Thermo Fisher Scientific). The dilution that yielded a 50% reduction in maximal optical densitometry units after subtracting the units obtained for uninfected cell lysates was determined for the study population and results reported as optical densitometry units at that dilution (1:10 000 for anti-HSV IgG and 1:1000 for IgG1 and IgG3). To quantify gD-specific antibodies, the plates were coated with recombinant gD-2 protein [16]. Total IgG concentration was quantified using a commercial ELISA (IgG [Total] Human ELISA kit; Thermo Fisher Scientific).
Functional Assays
Neutralization was assessed by plaque reduction assay. HSV-2 (4674) (75–100 plaque-forming units) was incubated with 2-fold serial dilutions of heat-inactivated serum for 1 hour before inoculating Vero cells. Plaques were counted after 48 hours, and the dilution that yielded a 50% reduction in plaque-forming units relative to cells infected without serum was determined. Neutralization titers for a subset of Herpevac trial participants were determined using a microneutralization assay described elsewhere [8]. ADCC was assayed (1:5 dilution of serum in duplicate) using the ADCC FcγRIIIa (human) Reporter Bioassay (Promeg) with HSV-2 (4674)–infected Vero cells as targets. Fold induction was calculated relative to luciferase activity in the absence of serum after subtracting the background for uninfected cells. C1q binding was assayed by means of ELISA [18]. Plates were coated with HSV-2 (4674)–infected (or uninfected) lysates, blocked, and then incubated with 5-fold serial dilutions of heat inactivated serum followed by 2-hour incubation with 1 μg/mL of human C1q (Complement Technology), and bound C1q was then quantified with 1 μg/mL of HRP-labeled anti-C1q antibodies (Complement Technology). The dilution that yielded a 50% reduction in maximal optical densitometry units after subtracting the unit for uninfected cell lysates for the study population was 1:25, and results are reported as optical densitometry units at that dilution.
Western Blots
Western blots were performed with 5 μg of gD protein or 10 µg of HSV-2 (4674)–infected or uninfected Vero cell lysates per lane as the immunogen. Blots were blocked for 2 hours with 5% milk in phosphate-buffered saline–Tween-20, incubated with serum samples containing 10 µg of IgG in blocking buffer overnight, and then incubated with HRP-labeled anti-human IgG-HRP (1:500) (BioRad no. 1721033) and scanned using a ChemiDoc imaging system equipped with GelDOC2000 software Image Lab 6.0.
Statistical Analysis
Student paired or unpaired t tests, Mann-Whitney U tests, and Spearman correlation coefficients (SCCs) were determined using GraphPad Prism version 9.1.2 software (GraphPad Software). Differences were considered statistically significant at P < .05. A forward stepwise method was performed to build logistic regression models using Stata software, version 15.1.
RESULTS
IgG1 Neutralizing Antibody Response to gD/AS04 Vaccine
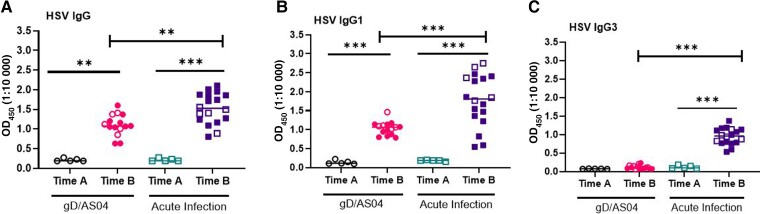
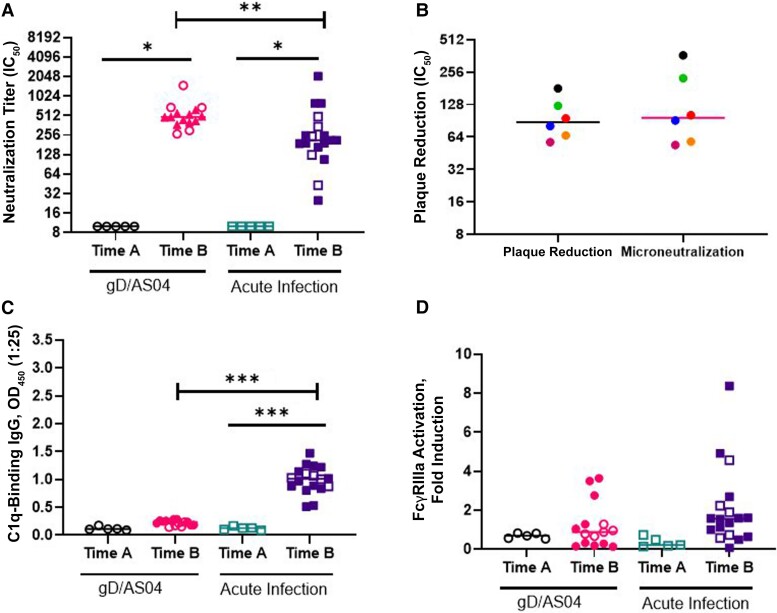
Women in the gD/AS04 arm were slightly older than those in the HAV vaccine arm who acquired acute HSV-2 infection (mean ± standard deviation), 22.53 ± 3.62 vs 20.5 ± 1.86 years, respectively) and more likely to report being white (13 of 15 vs 8 of 18) (Table 1). gD/AS04 elicited a significant increase in HSV-specific IgG and IgG1 but not IgG3 compared with enrollment serum samples (P < .001; paired t test) (Figure 1A–1C). The neutralizing antibody titer (microneutralization assay) increased from a baseline of 10 to 683 [284-1091] (median [IQR]) (P < .005 [paired t test]; n = 5) (Figure 2A). A subset of neutralization assays was also performed using a plaque reduction assay against HSV-2 (4674), but this did not alter the findings, and there were no significant differences in neutralization titers comparing samples tested in both assays (Figure 2B). In contrast to the robust neutralizing antibody response, the vaccine did not elicit any significant increase in C1q (P = .10) or human FcγRIIIa activation, a biomarker of ADCC (P = .08) (Figures 2C and D).
Table 1.
Demographic Characteristics of Study Participants
| Characteristic | gD/AS04 Vaccine (n = 15) |
Acute HSV Infection (n = 18) |
Chronic HSV Infection | |
|---|---|---|---|---|
| HIV Uninfected (n = 18) | HIV Infected (n = 30) | |||
| Age, mean (SD), ya | 22.53 (3.62) | 20.5 (1.86) | 40.45 (4.54) | 43.35 (4.05) |
| Race/ethnicity | ||||
| White | 13 | 8 | 0 | 1 |
| Nonwhite | 2 | 10 | 15 | 18 |
| Hispanic | 0 | 1 | 3 | 10 |
| Other | 0 | 0 | 0 | 1 |
| HSV seropositivity | 0 | 18 HSV-2+ | 16 HSV-1+/HSV-2+; 2 HSV-2+ | 18 HSV-1+/HSV-2+; 12 HSV-2+ |
Abbreviations: gD, glycoprotein D; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HSV-1+, HSV-1 seropositive; HSV-2+, HSV-2 seropositive; SD, standard deviation.
Significant difference for age across all groups (P < .001); no significant difference for group 1 versus 2 or group 3 versus 4.
Figure 1.
Herpes simplex virus (HSV)–specific antibody responses in glycoprotein D (gD)/ASO4 vaccinated women who remained uninfected and controls who subsequently acquired HSV-2 genital infection (primary infection). HSV-specific immunoglobulin (Ig) G (A), IgG1 (B), and IgG3 (C) were measured at study enrollment (time A; n = 5) and at 7 months (1 month after the third vaccine dose, time B; n = 15) in gD/AS04 vaccine recipients or at enrollment (time A; n = 5) and approximately 6 months after acquisition of HSV-2 genital herpes (time B; n = 18). Results are shown as optical densitometry units read at 450 nm (OD450) at 1:10 000 dilution for IgG and at 1:1000 dilution for IgG1 and IgG3. Abbreviation: OD450, optical density at 450 nm. The subset of paired samples (open symbols) was compared using paired t test, and responses to gD/AS04 vaccine versus acute infection for all samples (open and closed symbols) were compared using Mann-Whitney U test. **P < .01; ***P < .001.
Figure 2.
Glycoprotein D (gD/)ASO4 vaccination and primary infection elicit neutralizing but not antibody-dependent cell-mediated cytotoxicity (ADCC) responses. A, Herpes simplex virus (HSV)–specific neutralization was performed using a microneutralization or plaque reduction assay (triangles only), and results are presented as the serum dilution that inhibited 50% of infection (IC50). (See the legend to Figure 1 for definitions of time A and time B.) B, Neutralization titers for the 2 assays were compared for a subset of samples (n = 6). C, C1q-binding immunoglobulin (Ig) G was quantified by enzyme-linked immunosorbent assay. Abbreviation: OD450, optical density at 450 nm. D, Human Fc gamma receptor (FcγR) IIIa activation was measured using a Promega human FcγRIIIa ADCC Reporter Bioassay with cells infected with HSV-2 as targets and serum diluted 1:5. Paired t tests were performed on the subset of paired samples as in Figure 1 (open symbols; n = 5), and Mann-Whitney U tests were performed to compare responses to gD/AS04 versus acute infection at time B for all samples (open and closed symbols). *P < .05; **P < .01; ***P < .001.
Failure of Acute HSV-2 to Elicit a Significant ADCC Response
Next, we assessed the response to acute HSV-2 infection in 18 participants who received an HAV vaccine and subsequently acquired primary HSV-2 genital disease. Serum samples were obtained 6.47±3.72 (mean±SD) months after HSV-2 diagnosis and were compared with enrollment samples from 5 of these participants. Acute infection elicited a significant increase in HSV-specific IgG, IgG1, and IgG3 responses relative to enrollment serum samples (P < .001; paired t test) (Figure 1A–C).There was a significant increase in neutralizing (P = .04) and C1q-binding (P < .001) antibodies in response to acute infection, but little increase in ADCC (P = .10; paired t test) (Figure 2A–D). Acute infection elicited lower neutralizing antibody titers (P < .01) and higher C1q-binding antibody titers (P < .001) but no differences in ADCC responses (P = .10) compared with the vaccine (Mann-Whitney U test). The HSV-specific IgG (P < .01), IgG1 (P < .001), and IgG3 (P < .001) levels were also significantly higher in response to acute infection vs gDAS04 vaccination (Figure 1). These differences presumably reflect exposure to other viral antigens that are not present in the gD subunit protein vaccine.
gD Antibody Restrictions in Subclass and Function
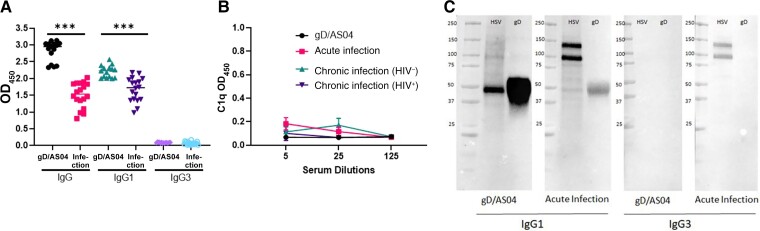
The observation that acute infection also elicited IgG3 and C1q-binding antibodies whereas vaccination only elicited IgG1 neutralizing antibodies suggests that gD responses may be restricted. To directly test this, ELISAs were conducted with recombinant gD-2 protein as the capture antigen. The vaccine elicited higher gD-specific IgG and IgG1 compared with acute infection (P < .001; Mann-Whitney U test), but neither the vaccine nor infection elicited an IgG3 gD response (Figure 3A). Moreover, gD-specific antibodies failed to bind C1q; the C1q gD-binding ELISAs were conducted with pooled (n = 5) serum samples obtained from vaccinated, acutely or chronically HSV-2–infected women (Figure 3B). Western blots with virally infected cell lysates or recombinant gD protein as the antigen- and subclass-specific secondary antibodies confirmed this. The gD band was detected only with an anti-IgG1 (but not anti-IgG3) secondary antibody. In contrast, two additional bands between 100 and 150 kD were detected with serum from acutely infected women as the primary and either anti-IgG1 or anti-IgG3 as secondary antibodies (Figure 3C).
Figure 3.
Anti–glycoprotein D (gD) antibodies are predominantly immunoglobulin (Ig) G1 subclass and do not bind to C1q. A, Anti-gD IgG1, IgG1, and IgG3 were quantified by means of enzyme-linked immunosorbent assay (ELISA) using plates coated with gD-2 protein (1:10 000 dilution for IgG and 1:1000 for IgG1 and IgG3). ***P < .001 (t test comparing gD/AS04 vaccine vs acute infection). Abbreviation: OD450, optical density at 450 nm. B, gD-specific C1q-binding antibodies were measured by ELISA, with 5 samples from each group at dilutions of 1:5, 1:25, and 1:125. Abbreviation: HIV−, human immunodeficiency virus (HIV) uninfected; HIV+, HIV infected. Results are means with standard deviation C, Western blots were performed with 10 µg of herpes simplex virus (HSV) 2 (4674)–infected Vero cell lysates (HSV) or 5 μg of gD-2 protein (gD) per lane as the antigen and probed with pooled immune serum (n = 3) containing 10 μg/mL of IgG from gD/ASO4 vaccine recipients or participants with acute infection using anti-IgG1 or anti-IgG3 secondary antibodies.
Detection of ADCC Responses in Chronic HSV-2 Infection
We hypothesized that repeated exposure to virus during episodes of asymptomatic or clinical reactivation coupled with gD-specific antibodies elicited in response to acute infection, which might overcome the gD-HVEM interference with generating ADCC [14], would result in increased ADCCs over time. We took advantage of the Bronx WIHS biobank and characterized HSV antibody responses in HSV-2–seropositive women. HSV serostatus is not routinely assessed at enrollment in WIHS, and thus the duration of HSV-2 infection is not known, but based on other studies with this cohort it was likely >5 years [19, 20].
The WIHS biobank included samples from HIV-uninfected and HIV-infected women, and because HIV may modify immune responses, we compared HSV humoral responses in the HIV-uninfected WIHS women (chronic infection) with those in the acutely infected control-vaccinated women (n = 18 each). The WIHS cohort was significantly older and differed in race/ethnicity (Table 1). There were no significant differences in total HSV-specific IgG, neutralizing, or C1q-binding antibodies but the ADCC responses were significantly greater with chronic versus acute HSV-2 infection (Table 2 and Figure 4). Differences in the proportion of IgG1 and IgG3 were also observed, with higher levels of IgG1 and lower levels of IgG3 with chronic infection.
Table 2.
Comparison of Humoral Responses in Women With Acute Versus Chronic Herpes Simplex Virus 2 Infection
| Response | Acute HSV-2 Infection | Chronic HSV-2 Infectiona | P Valueb |
|---|---|---|---|
| HSV IgG, OD450 (1:10 000 dilution) | 1.5 (1.2–1.9) | 1.5 (1.3–1.8) | .95 |
| HSV IgG1, OD450 (1:1000 dilution) |
1.8 (1.3–2.4) | 2.6 (2.1–2.7) | .01 |
| HSV IgG3, OD450 (1:1000 dilution) |
1.0 (0.8–1.1) | 0.7 (0.4–0.9) | .03 |
| Neutralization titerc | 214 (156–381) | 124 (74–230) | .054 |
| C1q binding Antibodies, OD450 (1:25 dilution) |
1.0 (0.9–1.2) | 0.9 (0.4–1.4) | .33 |
| ADCC, fold induction | 1.6 (0.7–2.3) | 5.7 (4.9–9.1) | <.001 |
Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity; Ig, immunoglobulin; HSV, herpes simplex virus; OD450, optical densitometry units read at 450 nm. Data shown as median (IQR).
Including only participants without human immunodeficiency virus infection.
P values determined using Mann-Whitney U test.
Neutralization titer is defined as the dilution of serum that inhibits 50% of viral plaques.
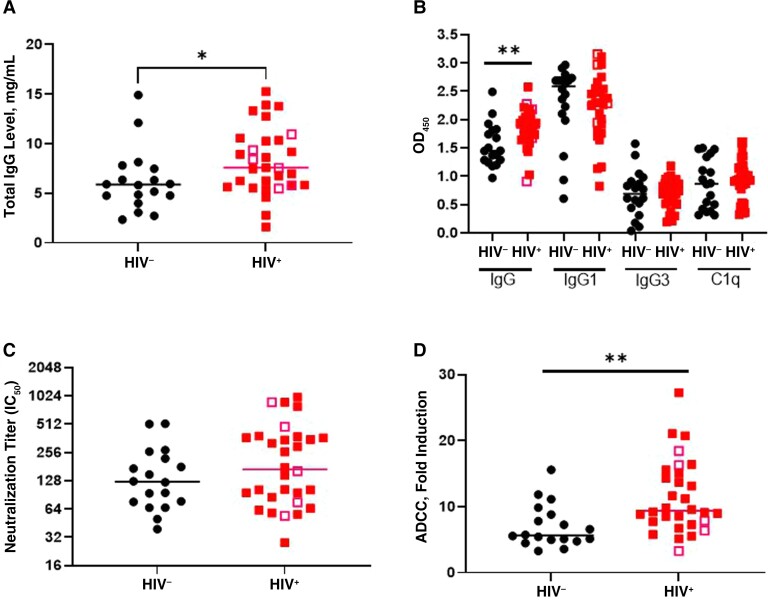
Figure 4.
Antibody responses in chronic herpes simplex virus (HSV) 2 infection in human immunodeficiency virus (HIV)–uninfected (HIV−) and HIV-infected (HIV+) women. A, Samples from HIV− and HIV+ women were assayed for total IgG. B, HSV-specific immunoglobulin (Ig) G, IgG1, IgG3, and C1q-binding antibodies assayed by enzyme-linked immunosorbent assay. Abbreviation: OD450, optical density at 450 nm C, Neutralization titers assayed by plaque reduction assay. Abbreviation: IC50, serum dilution that inhibited 50% of infection. D, Antibody-dependent cell-mediated cytotoxicity (ADCC) assessed using a Promega human FcγRIIIa (Fc gamma receptor IIIa) ADCC Reporter Bioassay and expressed as fold induction. Open symbols represent women who reported HSV-2 recurrences within the previous 12 months. *P < 0.5; **P < 0.01 (Mann-Whitney U test).
We also compared HSV antibodies in the HIV-infected and the HIV-uninfected women to explore whether HIV affected the response to chronic HSV-2 infection. There were no significant differences in age, race, or ethnicity between the 2 groups (Table 1). The mean peripheral blood CD4 cell count in the HIV-infected participants was 599.8/μL (standard error of the mean, 46.95/μL). HIV RNA was detected in plasma in 9 of 30 (30%) of the HIV-infected women (mean level, 15 056 copies/mL; range, 39–75 300 copies/mL). The majority (27 of 30 [90%]) were receiving antiretroviral therapy, and 25 of 27 (93%) reported >95% medication adherence. None of the HIV-uninfected women reported a history of genital herpes, compared with 7 of 30 (23%) of the HIV-infected women (P = .04; Fisher exact test). Five reported ≥1 outbreak within the past year (3 reported 1 outbreak, 1 reported 2, and 1 reported 5). None were receiving suppressive anti-herpes therapy, and genital HSV lesions were not documented in any of the women at semiannual physical examinations between visits 44 and 48 (2016–2018).
Because HIV is associated with hypergammaglobulinemia [21, 22], we measured total IgG as well as HSV-specific antibodies. HIV-infected women had higher total IgG levels than HIV-uninfected women, with median (IQR) levels of 7.6 (5.7–10.4) versus 5.8 (4.6–7.6) mg/mL, respectively (P < .05) (Figure 4A). There was also a significant increase in HSV-specific IgG but not in IgG1, IgG3, or C1q-binding antibodies in HIV-infected compared with HIV-uninfected women (Figure 4B). There was no significant difference in the neutralizing antibody titer (Figure 4C), but the ADCC response was significantly greater in HIV-infected than in HIV-uninfected participants (P < .01) (Figure 4D). The 5 HIV-infected women who reported HSV-2 recurrences within the past year (Figure 4, open symbols) did not differ significantly from the others. The increases in HSV-specific IgG and ADCC in HIV-infected versus HIV-uninfected women persisted when total IgG concentration, age, and race/ethnicity were included in a logistic regression model. ADCC fold induction was correlated significantly with total and HSV-specific IgG (SCC, 0.3 and 0.37, respectively; P = .04 and P = .002, respectively). C1q binding was correlated with HSV-specific IgG3 (SCC, 0.39; P < .001), but neutralizing antibody titers did not correlate with any parameters.
Association of Chronic HSV-2 Infection With Increased Breadth of Antigenic Targets
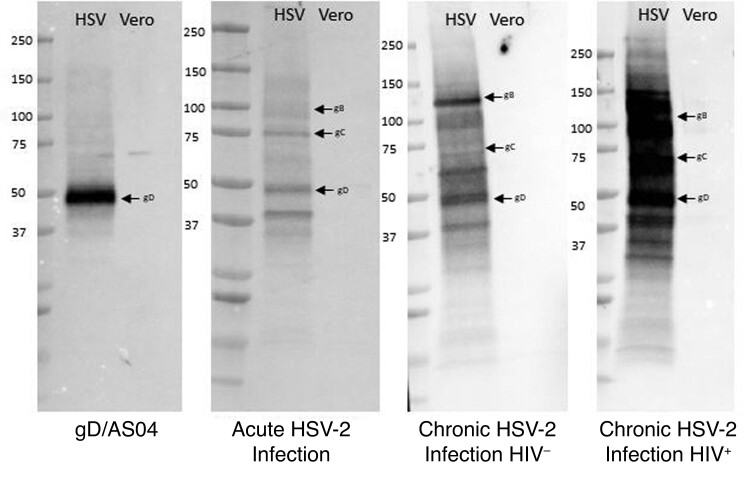
Repeated exposure to HSV-2 during episodes of viral reactivation might also expand the antigenic repertoire. To assess this, we performed additional Western blots with serum from a subset (n = 3) of participants with acute or chronic HSV-2 infection. There was a clear increase in number and intensity of bands identified on Western blots with immune serum (10 μg of total IgG) from chronic (HIV-uninfected or HIV-infected) versus acute HSV-2 infection or gD/AS04 vaccine recipients (Figure 5). Immunoprecipitation of infected cell lysates with commercially available monoclonal antibodies confirmed the identify of gD, gB, and glycoprotein C (gC) with a marked increase in anti-gB and anti-gC antibodies in chronically infected patients. The intensity of the gD band was strongest in the gD/AS04 recipients.
Figure 5.
Chronic herpes simplex virus (HSV) 2 infection is associated with an increase in breadth of antigenic targets. Western blots were performed with HSV-2–infected or HSV-2–uninfected Vero cell lysates as the antigen and probed with pooled immune serum (n = 3) from glycoprotein D (gD)/ASO4 vaccinated and acutely or chronically HSV-2–infected (human immunodeficiency virus [HIV]–negative [HIV−] and HIV-positive [HIV+]) women. Molecular weight markers are indicated on the left, and arrows denote gD (55 KDa), glycoprotein C (gC; 78 KDa) and glycoprotein B (gB; 116 KDa).
DISCUSSION
The results with clinical trial samples recapitulate findings from mouse studies and demonstrate that the response to gD/AS04 is restricted to the generation of gD neutralizing antibodies with no C1q binding or ADCC response. The neutralizing antibody titer was greater in magnitude in the vaccine recipients than in women with acute or chronic infection indicating that gD/AS04 achieved its goal. Thus, the lack of efficacy against HSV-2 supports the notion that gD- neutralizing antibodies are not sufficient for protection. This may be attributed in part to the ability of HSV to spread across intercellular junctions and thereby escape neutralization as well as other immune evasion strategies [23, 24]. In addition, a recent study noted that the neutralizing antibody response to gD/AS04 was short-lived [25]. We found that neutralizing antibodies persist, although there was a nonsignificant decrease in the neutralization titer (P = .054) in samples from chronically infected compared with acutely infected women.
The notion that neutralizing antibodies are not sufficient for HSV-2 prevention is supported by the clinical trial outcomes with gD-gB/MF59 and gDAS04 vaccines. Both vaccines elicited significant neutralizing antibody but no ADCC response and failed to prevent HSV-2 infection [9, 10]. ADCC also has been suggested to play a role in limiting neonatal disease. In a study of 47 infants with HSV disease, higher ADCC antibody levels were associated with protection against disseminated HSV disease, even after controlling for the level of neutralizing antibody [26].
Notably the gD/AS04 vaccine elicited IgG1 but not IgG3 antibodies. The IgG1 response appears to be a characteristic of the antigen, since gD antibodies generated by infection were also restricted to the IgG1 subclass and did not bind C1q. In addition to IgG gD-specific antibodies, acute infection elicited additional IgG1 and IgG3 responses that recognized other viral antigens and bound C1q. We focused on IgG1 and IgG3 subclasses because they have significantly greater affinity for C1q and FcγRs compared with IgG2 and IgG4. IgG1 accounts for the majority of total serum immunoglobulin, but monomeric IgG3 binds more efficiently than IgG1 to FcγRIIa, FcγRIIIa, and FcγRIIIb, which mediate antibody-dependent killing. Moreover, complexed IgG3 binds more efficiently than IgG1 to neonatal FcRs, which play an important role in transport of IgG into the female genital tract and across the placenta [27]. We speculate that the IgG3 antibodies contributed to C1q binding, as suggested by the significant positive correlation between HSV IgG3 and C1q.
There was a small and nonsignificant ADCC response to acute infection, which recapitulates results in mice [13, 17]. However, higher ADCC responses and an increase in the breadth of antigenic targets were detected in samples from chronically HSV-2–infected women. The generation of ADCC may have been facilitated by the presence of gD antibodies, which could overcome the ability of gD to block the generation of subclass switched ADCC antibodies through engagement of HVEM [14]. This notion is supported by the observation that sublethal infection of mice with a virus expressing a gD that is deleted for the HVEM-binding domain elicited more ADCC than in mice infected with the repaired virus [14]. The precise targets of the ADCC response in humans have not yet been determined, although mouse studies identified gB as one of several ADCC targets [14], and Western blots showed an increase in gB-specific antibodies in chronic infection.
Possibly, the generation of ADCC, combined with maturation of T-cell responses, contributes to the decrease in clinical recurrences and viral shedding that is observed after the first year following HSV-2 infection [20, 28]. Determining how protective ADCC is, however, will require further study. Protection will depend not only on the quantity of ADCC antibodies but also the ability of effector cells, typically natural killer cells, to mediate cytolysis. Natural killer cell function may be impaired in HIV infection [29] and this (along with other differences in HIV-infected and HIV-uninfected women) may contribute to the observation that despite higher ADCC responses, the HIV-infected women reported more clinical recurrences than the HIV-uninfected women. More frequent recurrences in HIV-infected individuals may also reflect impaired T-cell function, which play important roles in maintaining HSV latency [30]. Boosting of antibodies to other viral proteins such as gC and glycoprotein E (gE) may also contribute to increased protection over time, as both have immune evasion properties interfering with complement activation (gC) and binding to Fc regions of antibodies (gE) [31–33].
Limitations of the current study include the use of 2 cohorts, which differed in age and other demographics, sample size, and the absence of prospective data on viral shedding and clinical recurrences. More precisely defining the kinetics of the development of different functional antibody responses will require future study. However, the findings that gD/AS04 vaccine elicited only a neutralizing antibody response and that ADCC takes time to develop after acute infection support the contention that vaccine strategies that generate polyfunctional and specifically ADCC responses may prove more effective in preventing HSV-2 acquisition, limiting viral spread, and decreasing the frequency of clinical recurrences.
Notes
Acknowledgments. The authors thank the Einstein Macromolecular Therapeutics Development Facility for producing recombinant glycoprotein D.
Financial support. This work was supported by the National Institutes of Health (grants R01AI134367, R21AI147992, R01HD098977, U01AI03004, U01-HL146204, and P30AI124414), X-Vax, the Price Family Foundation, and Einstein-Montefiore Institute for Clinical and Translational Research (Clinical and Translational Science Awards training grant TL1 TR002557 to A. M. M.).
Contributor Information
Aakash Mahant Mahant, Department of Microbiology-Immunology, Albert Einstein College of Medicine, Bronx, New York, USA.
Sandra Guerguis, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, New York, USA.
Tamara P Blevins, Department of Internal Medicine, Saint Louis University School of Medicine, St Louis, Missouri, USA.
Natalia Cheshenko, Department of Microbiology-Immunology, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Pediatrics, Albert Einstein College of Medicine, Bronx, New York, USA.
Wei Gao, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA.
Kathryn Anastos, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA.
Robert B Belshe, Department of Internal Medicine, Saint Louis University School of Medicine, St Louis, Missouri, USA.
Betsy C Herold, Department of Microbiology-Immunology, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Pediatrics, Albert Einstein College of Medicine, Bronx, New York, USA.
References
- 1. Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Correction: global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PloS One 2015; 10:e0128615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PloS One 2015; 10:e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Looker KJ, Magaret AS, May MT, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PloS One 2015; 10:e0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernstein DI, Bellamy AR, Hook EW III, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type;1, and type 2 in young women. Clin Infect Dis 2013; 56:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stanberry LR, Spruance SL, Cunningham AL, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002; 347:1652–61. [DOI] [PubMed] [Google Scholar]
- 6. Belshe RB, Leone PA, Bernstein DI, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 2012; 366:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belshe RB, Heineman TC, Bernstein DI, et al. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 2014; 209:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blevins TP, Mitchell MC, Korom M, et al. Higher throughput quantification of neutralizing antibody to herpes simplex viruses. PloS One 2015; 10:e0144738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corey L, Langenberg AG, Ashley R, et al. ; Chiron HSV vaccine study group . Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 1999; 282:331–40. [DOI] [PubMed] [Google Scholar]
- 10. Kohl S, Charlebois ED, Sigouroudinia M, et al. Limited antibody-dependent cellular cytotoxicity antibody response induced by a herpes simplex virus type 2 subunit vaccine. J Infect Dis 2000; 181:335–9. [DOI] [PubMed] [Google Scholar]
- 11. Petro C, Gonzalez PA, Cheshenko N, et al. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petro CD, Weinrick B, Khajoueinejad N, et al. HSV-2 ΔD elicits FcγR-effector antibodies that protect against clinical isolates. JCI Insight 2016; 1:e88529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aschner CB, Knipe DM, Herold BC. Model of vaccine efficacy against HSV-2 superinfection of HSV-1 seropositive mice demonstrates protection by antibodies mediating cellular cytotoxicity. NPJ Vaccines 2020; 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aschner C B, Loh LN, Galen B, et al. HVEM signaling promotes protective antibody-dependent cellular cytotoxicity (ADCC) vaccine responses to herpes simplex viruses. Sci Immunol 2020; 5:eaax2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aschner C B, Pierce C, Knipe DM, Herold BC. Vaccination route as a determinant of protective antibody responses against herpes simplex virus. Vaccines 2020; 8:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burn C, Ramsey N, Garforth SJ, Almo S, Jacobs WR, Jr., Herold BC. A herpes simplex virus (HSV)-2 single-cycle candidate vaccine deleted in glycoprotein D protects male mice from lethal skin challenge with clinical isolates of HSV-1 and HSV-2. J Infect Dis 2018; 217:754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kao CM, Goymer J, Loh LN, Mahant A, Burn Aschner C, Herold BC. Murine model of maternal immunization demonstrates protective role for antibodies that mediate antibody-dependent cellular cytotoxicity in protecting neonates from herpes simplex virus type 1 and type 2. J Infect Dis 2020; 221:729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Visciano ML, Mahant AM, Pierce C, Hunte R, Herold BC. Antibodies elicited in response to a single cycle glycoprotein D deletion viral vaccine candidate bind C1q and activate complement mediated neutralization and cytolysis. Viruses 2021; 13:1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ameli N, Bacchetti P, Morrow RA, et al. Herpes simplex virus infection in women in the WIHS: epidemiology and effect of antiretroviral therapy on clinical manifestations. AIDS 2006; 20:1051–8. [DOI] [PubMed] [Google Scholar]
- 20. Aumakhan B, Gange SJ, Beyrer C, et al. Quantitative and qualitative correlates of cervicovaginal herpes simplex virus type 2 shedding among HIV-infected women in the Women’s Interagency HIV Study. Int J STD AIDS 2011; 22:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med 1983; 309:453–8. [DOI] [PubMed] [Google Scholar]
- 22. Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev 2013; 254:207–24. [DOI] [PubMed] [Google Scholar]
- 23. Cifuentes-Munoz N, El Najjar F, Dutch RE. Viral cell-to-cell spread: conventional and non-conventional ways. Adv Virus Res 2020; 108:85–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Awasthi S, Belshe RB, Friedman HM. Better neutralization of herpes simplex virus type 1 (HSV-1) than HSV-2 by antibody from recipients of GlaxoSmithKline HSV-2 glycoprotein D2 subunit vaccine. J Infect Dis 2014; 210:571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belshe RB, Blevins TP, Yu Y, et al. Neutralizing antibody kinetics and immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 2022: jiac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kohl S, West MS, Prober CG, Sullender WM, Loo LS, Arvin AM. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis 1989; 160:770–6. [DOI] [PubMed] [Google Scholar]
- 27. Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A 2011; 108:4388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis 2011; 203:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao WJ, Zhang XC, Wan LY, et al. Immune dysfunctions of CD56(neg) NK cells are associated with HIV-1 disease progression. Front Immunol 2021; 12:811091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verjans GM, Hintzen RQ, van Dun JM, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A 2007; 104:3496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Awasthi S, Huang J, Shaw C, Friedman HM. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J Virol 2014; 88:8421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerber SI, Belval BJ, Herold BC. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology 1995; 214:29–39. [DOI] [PubMed] [Google Scholar]
- 33. Hook LM, Lubinski JM, Jiang M, Pangburn MK, Friedman HM. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J Virol 2006; 80:4038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]