Abstract
Background
Regulating meal timing may have efficacy for improving metabolic health for preventing or managing chronic disease. However, the reliability of measuring meal timing with commonly used dietary assessment tools needs characterization prior to investigating meal timing and health outcomes in epidemiologic studies.
Objectives
To evaluate the reliability of estimating meal timing parameters, including overnight fasting duration, the midpoint of overnight fasting time, the number of daily eating episodes, the period with the largest percentage of daily caloric intake, and late last eating episode (> 09:00 pm) from repeated 24-h dietary recalls (24HRs).
Methods
Intraclass correlation coefficients (ICC), Light’s Kappa estimates, and 95% CIs were calculated from repeated 24HR administered in 3 epidemiologic studies: The United States-based Interactive Diet and Activity Tracking in AARP (IDATA) study (n = 996, 6 24HR collected over 12-mo), German EPIC-Potsdam Validation Study (European Prospective Investigation into Cancer and Nutrition Potsdam Germany cohort) (n = 134, 12 24HR collected over 12-mo) and EPIC-Potsdam BMBF-II Study (Federal Ministry of Education and Research, “Bundesministerium für Bildung und Forschung”) (n = 725, 4 24HR collected over 36 mo).
Results
Measurement reliability of overnight fasting duration based on a single 24HR was “poor” in all studies [ICC range: 0.27; 95% CI: 0.23, 0.32 – 0.46; 95% CI: 0.43, 0.50]. Reliability was “moderate” with 3 24HR (ICC range: 0.53; 95% CI: 0.47, 0.58 in IDATA, 0.62; 95% CI: 0.52, 0.69 in the EPIC-Potsdam Validation Study, and 0.72; 95% CI: 0.70–0.75 in the EPIC-Potsdam BMBF-II Study). Results were similar for the midpoint of overnight fasting time and the number of eating episodes. Reliability of measuring late eating was “fair” in IDATA (Light’s Kappa: 0.30; 95% CI: 0.21, 0.39) and “slight” in the EPIC-Potsdam Validation study and the EPIC-Potsdam BMBF-II study (Light’s Kappa: 0.19; 95% CI: 0.15, 0.25 and 0.09; 95% CI: 0.06, 0.12, respectively). Reliability estimates differed by sex, BMI, weekday, and season of 24HR administration in some studies.
Conclusions
Our results show that ≥ 3 24HR over a 1–3-y period are required for reliable estimates of meal timing variables.
Keywords: meal timing, intermittent fasting, 24-hour dietary recall, reliability, nutrition, epidemiology
Introduction
The daily timing of when people eat could be important for managing or preventing metabolic diseases, independent of diet quality [1]. Irregular mealtimes have been linked to poor glycemic control, dyslipidemia, and hypertension [[2], [3], [4], [5]]. On the other hand, meal timing patterns like duration of overnight fasting, skipping breakfast, and late-night eating episodes have been associated with metabolic health parameters like blood glucose and blood pressure via circadian regulation of metabolic tissues, gut microbial characteristics, and health behaviors (e.g., food intake, physical activity, and sleep) [1,3,[6], [7], [8], [9], [10], [11]]. In addition, meal timing may play a role in energy balance and, thus, body weight and adipose tissue distribution [3,8]. Consequently, modifying the timing of food consumption could be leveraged as a tool to improve metabolic health with the goal of preventing, postponing, or managing diet and obesity-related chronic diseases [4]. Clinical studies (e.g., randomized trials) and longitudinal cohort studies are now testing the feasibility and efficacy of a range of meal timing modification regimes, including various forms of intermittent fasting, for their effects on metabolic parameters, body weight, and various health outcomes such as diabetes, cardiovascular disease, and cancer [3,4,[12], [13], [14], [15]].
Despite the promise of meal timing as a dietary strategy for improving metabolic health and/or disease prevention from animal and small clinical studies, few large observational studies have evaluated the association of meal timing with metabolic health or disease outcomes [3,[16], [17], [18], [19]]. Large studies often administer food frequency questionnaires (FFQs) that estimate habitual dietary intake time (e.g., intake over the past 6-mo or 1-y) to account for day-to-day and seasonal fluctuations. However, FFQs typically do not capture the daily timing of eating occasions [4,20]. On the other hand, 24-h dietary recalls (24HR) inquire about all foods, beverages, and often dietary supplements and the clock time of their consumption in the prior 24-h [21]. Although multiple administrations of 24HR and food records may produce more accurate and less biased estimates of nutrient intakes than FFQs, [21] their use in large observational studies has been considered impractical because of the cost and resources required to collect and code the data [20], and a single administration does not capture day-to-day or seasonal dietary intake variation [22,23]. However, this can be mitigated with repeated measures over time [21]. In addition, 24HR may be administered to a representative sub-sample of a cohort to account for random measurement errors in the FFQ via dietary calibration [24,25]. When implemented with a sufficient sample size, unique opportunities exist to conduct meal timing studies at the population level [[16], [17], [18]].
Several studies have investigated the reliability of 24HR for estimating intakes of energy and nutrients [22,26,27] but not for measuring meal timing parameters. Given that technology is now enabling the completion of 24HR via computer and telephone applications, their use in large-scale epidemiologic studies is likely to grow. The purpose of this study was to evaluate the reliability of 24HR to estimate meal timing [overnight fasting duration, the midpoint of overnight fasting time, number of eating episodes per day, daily period of greatest percentage caloric intake, and late last eating episode (> 09:00 pm)]. These variables were selected to enable comparison with the small number of previous studies on meal timing and health outcomes. We leveraged data from epidemiologic sub-studies that administered repeated 24HR over 12–36 mo.
Methods
The interactive diet and activity tracking in the NIH-AARP study
The Interactive Diet and Activity Tracking in AARP (IDATA) study was designed to evaluate measurement errors of self-reported dietary intake and physical activity against objective dietary reference biomarkers and physical activity measures among adults in the United States [28]. Invitations to participate were mailed to AARP (formerly known as the American Association of Retired Persons) members living in Pittsburgh, PA, in 2012. Eligibility criteria included having the ability to speak and read English, not recently following a weight loss diet, being free of health conditions affecting metabolism, having general mobility, and having access to the internet. Interested individuals were directed to a study website, and eligible individuals visited the study center to provide informed consent. The IDATA study was approved by the NCI special studies institutional review board (NCT03268577) [29]. A total of 1082 males and females aged 50–74 y completed the study. Participants were assigned to 1 of 4 groups. Groups 1 and 3 had reference biomarkers measured from blood samples taken in the first month of the study, and groups 2 and 4 measured reference biomarkers in the sixth month of the study. Assessment of biomarkers, physical activity, and dietary intake was identical for each assigned group, but individuals entered and exited the study on different timelines [30]. Participants were asked to complete 6 automated self-administered 24HR (ASA24), ∼2 mo apart. Each participant was provided with ≤ 3 attempts to complete a single 24HR. A total of 996 participants completed the recalls, 797 of whom completed all 6 administrations [30].
The European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam
The European Prospective Investigation into Cancer and Nutrition (EPIC) is a multi-center, prospective cohort study aimed at investigating the relationships between diet, metabolic characteristics, lifestyle, genetic and environmental factors, and the risk of chronic disease in 10 European countries (Denmark, France, Germany, Greece, Italy, Spain, the Netherlands, Sweden Norway, and the United Kingdom) [24]. The EPIC-Potsdam cohort is 1 of 2 German EPIC centers, including 27,548 participants aged 35–65 y who were recruited between 1994 and 1998. Two validation sub-studies within the EPIC-Potsdam cohort collected multiple 24HRs. At the baseline examination from 1995 to 1997, 160 participants were asked to participate in the EPIC-Potsdam Validation Study, a sub-study to examine the reliability and validity of the baseline FFQ. Participants were asked to complete a second FFQ after 1 y in addition to 12 24HR in monthly intervals. A total of 134 people completed ≤ 12 (≥ 10) repeated 24HR over a 36 mo period [31]. A second validation and calibration sub-study [the EPIC-Potsdam BMBF-II Study (Federal Ministry of Education and Research, “Bundesministerium für Bildung und Forschung”)] was designed to improve the assessment of exposures, including diet. A gender- and age-stratified random sample from the EPIC-Potsdam study (n = 815) were invited to participate. From this sample, 725 participants completed ≤ 4 24HR in 2 waves (BMBF-I 2010–2012; BMBF-II 2013). Although the first and fourth 24HRs were conducted during visits to the examination center, the second and third 24HR were conducted as telephone interviews on randomly chosen days, so weekdays were represented in equal proportions [32]. The mean time interval between the first and second 24HR was 107 (15–224) days, between the second and third 104 (13–450) days, and between the third and fourth 395 (31–825) days.
Dietary assessment
As part of the IDATA dietary assessment, participants completed 6 ASA24 dietary recalls [33]. Each 24-h recall (“recall”) captures dietary intake for a single day. Each recall was unscheduled on a randomly assigned day, approximately every other month over 1 y (i.e., months 1, 3, 5, 7, 9, and 11) covering all seasons [34]. A single recall was collected at each interval. The ASA24 is an online dietary assessment tool accessible via a website link [35]. Participants were asked to recall all foods and drinks they consumed the previous day from midnight to midnight. Initial questions inquired about whether participants consumed items from a list of foods or beverages the previous day. Subsequent questions collected information on food and beverage type and preparation and confirmed portion sizes using images depicting various examples. In the analytic cohort, 94% of participants completed ≥3 recalls, and 67% completed all 6 recalls [34].
In both EPIC-Potsdam sub-studies, participants completed computer-assisted and interviewer-based 24HR using EPIC-SOFT software (German version, software was developed specifically for this study), which was developed to standardize the 24HR interviews collected as part of the main EPIC study [36,37]. The EPIC-SOFT 24HR defined the 24-h period as the time between waking up on the day before the interview to the time upon waking on the interview day (mean 24-h) [36]. The interview involved a detailed assessment and description of the foods consumed per meal. Meals were referred to as “food consumption occasions” common to all EPIC countries. Eleven food consumption occasions covered the entire eating period from before breakfast to during the night [36,38]. The EPIC-Potsdam Validation Study includes the collection of 12 computer-assisted and interviewer-based 24HR (3 in each season) that were collectively conducted on all weekdays and weekend days [31]. Among the 4 24HR in the EPIC-Potsdam BMBF-II Study, the first recall was collected during a study center visit. Recalls 2 and 3 were collected via telephone interview between August 2010 and December 2012. The fourth 24HR was collected in person at another study center visit (February 2013–November 2013) [32].
Dietary data preparation
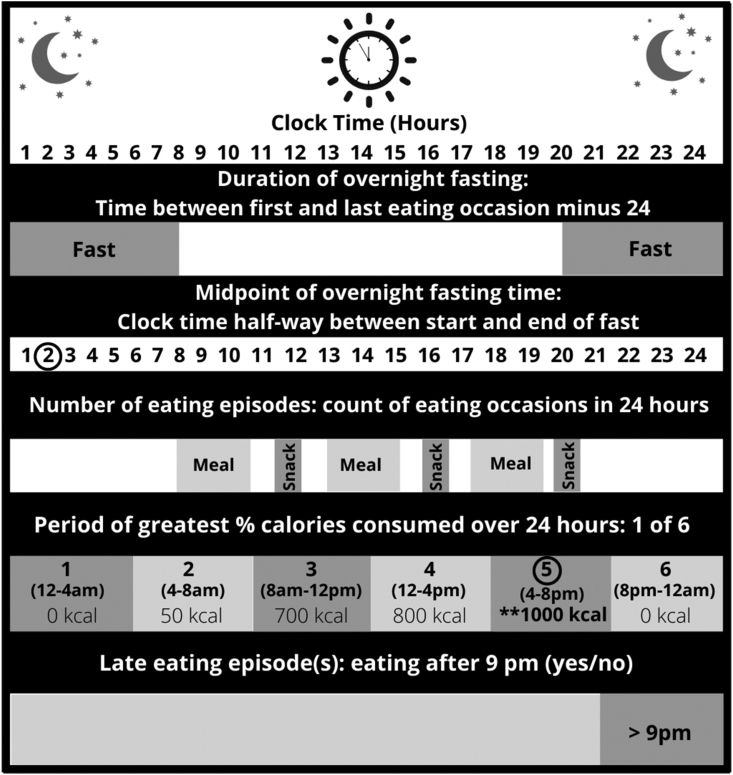
Meal timing variables were calculated using data from the 24HR in both IDATA and the 2 EPIC-Potsdam sub-studies. To enable a comparison of our findings with those from prior studies, 5 meal timing variables were generated for analysis [16,17,[39], [40], [41], [42]]. An overview of the meal timing variables is presented in Figure 1. The duration of overnight fasting (in h) was estimated by calculating the time between the first and the last eating episode of the day and subtracting it from 24-h. In IDATA, since intake was measured from midnight to midnight, a secondary fasting variable was also calculated to account for eating episodes occurring after midnight that were part of the prior days’ intake (i.e., presleep). We assessed the longest fasting duration in a 24-h period, which was > 5-h. The midpoint of the overnight fasting period (i.e., time on a 24-h clock) was calculated as the duration of overnight fasting divided by 2 and added to the start time of the overnight fast (i.e., the fasting period started either earlier or later in the day) [43]. The number of eating episodes was calculated as an ordinal variable and included any time-stamped eating episode of a calorie-containing food or beverage (≥ 25 calories) as defined in prior studies [17,40]. The period of greatest percentage daily calories was calculated as a 6-category variable, assigning participants to the category with the greatest caloric intake: 1) 00:00–04:00; 2) 04:00–08:00 ; 3) 08:00–12:00; 4) 12:00–16:00; 5) 16:00–20:00; 6) 20:00–24:00 h [44]. The late last eating episode was calculated as a binary variable to evaluate whether the last eating episode in the 24-h period was prior to or at 09:00 pm or after 09:00 pm [17,40,45]. We excluded recalls that were labeled as incomplete in the data set and those with implausible caloric intake estimates (< 500 kcals/d or > 4500 kcal/d) [46].
FIGURE 1.
Description of meal timing variables calculated from 24-h dietary recalls. Five meal timing variables were calculated based on 24-h dietary recall data from the Interactive Diet and Activity Tracking in Association of Retired Persons study, the EPIC-Potsdam Validation Study (European Prospective Investigation into Cancer and Nutrition Potsdam Germany cohort), and the EPIC-Potsdam BMBF-II Study (Federal Ministry of Education and Research “Bundesministerium für Bildung und Forschung”). The duration of overnight fasting (in h) was estimated by calculating the time between the first and the last eating episode of the day and subtracting it from 24-h. The midpoint of overnight fasting time was calculated as the duration of overnight fasting divided by 2 and added to the start time of the overnight fasting based on clock time. A number of eating episodes included any eating episode associated with calorie-containing food or beverage consumption > 25 calories. The period of greatest percentage caloric intake over 24-h was calculated as the percent of total daily caloric intake allocated to 6 4-h periods (1) 00:00–04:00; 2) 04:00–08:00 ; 3) 08:00–12:00; 4) 12:00–16:00; 5) 16:00–20:00; 6) 20:00–24:00 h) over the measurement day. Late eating was calculated as a binary variable (yes/no) to evaluate whether the last eating episode in the 24-h period was before or after 9 pm.
Covariates
Age, sex, and race/ethnicity were self-reported in IDATA. Age and sex were self-reported for EPIC-Potsdam, which includes mostly non-Hispanic White participants. Other demographic covariates, such as education, income, and smoking, were not available for IDATA. BMI (in kg/m2) was calculated from objectively measured height and weight taken at clinical visits in both IDATA and EPIC-Potsdam. In IDATA, the mean of all BMI calculations collected at 6 clinical visits over 1 y was used for the current analysis. In EPIC-Potsdam, BMI was measured at baseline by trained study personnel [47]. BMI was categorized according to WHO criteria for analysis: < 25 kg/m2 (normal weight), 25 to < 30 kg/m2 (overweight), 30 to < 35 kg/m2 (obese class 1), and > 35 kg/m2 (obese class 2+) [48,49].
Statistical analysis
Continuous variables
Intraclass correlation coefficients (ICC) were calculated to evaluate the reliability of repeated continuous or ordinal meal timing variables (i.e., overnight fasting duration, the midpoint of overnight fasting time on a 24-h clock, and the number of eating episodes) across repeated 24HR measurements. The ICC is calculated as the ratio of between-person variance (to total variance () in the meal timing variable value. The larger the ICC, the greater the proportion of the variability that is explained by between-person compared with within-person variation in meal timing values. Larger values represent a more reliable recall of meal timing over a specified period of time. Between- and within-person variation ( were estimated using linear mixed-effects regression in R using the lmer function (lme4 package, Rversion 4.1.2, R Foundation for Statistical Computing) [50]. We calculated the reliability of estimating meal timing variables based on multiple recalls, on average. The ICC of multiple () meal timing measurements (i.e., from different 24HR) is calculated by Equation 1:
| (1) |
For example, if we estimated = 0.1 and = 0.05, the ICC of 1 recall is 0.67 (Equation 2), the ICC of 2 recalls is 0.80 (Equation 3), and the ICC of 6 recalls is 0.92 (Equation 4).
| (2) |
| (3) |
| (4) |
Models were analyzed with and without adjustment for age, sex, BMI, and caloric intake. Four different models were fit: 1) unadjusted; 2) adjusted for sex and age; 3) model 2 plus BMI; 4) model 3 plus total daily caloric intake. All participants with at least the number of 24HR of interest were included in the model. Participant-wise bootstrapping with 10,000 iterations was used to calculate a 95% CI for the ICC. Results are presented for model 4, where ICC values were attenuated by ∼0.02 from the other models for continuous variables and were similar for the midpoint of overnight fasting time. ICC values closer to 1 imply greater reliability, whereas an ICC closer to 0 indicates lower reliability. The level of reliability was defined using the following criteria: “poor” (ICC <0.5), “moderate” (0.5 to <0.75), “good” (0.75 to <0.9), and “excellent” (ICC ≥0.9) [51].
Categorical variables
To estimate the reliability of 24HR for measuring categorical meal timing variables (i.e., the period of greatest percentage caloric intake in 24-h [6-level categorical variable defined above) and late last eating episode (> 09:00 pm, yes/no)], we calculated the Light’s Kappa measure of agreement. Light’s Kappa is an extension of Cohen’s Kappa for multiple raters (i.e., multiple 24HR) that equals computing the mean of Cohen’s Kappa for all pairs (i.e., it is an overall index of agreement). Light’s Kappa was computed for each categorical meal timing variable as a single reliability index based on the mean of all possible Cohen’s Kappa for any 2 measures [52]. Participant-wise bootstrapping with 10,000 iterations was used to generate 95% CI on the Light’s Kappa estimates. The strength of agreement was defined as “poor” (≤ 0), “slight” (0.01–0.20), “fair” (0.21–0.40), “moderate” (0.41– 0.60), “substantial” (0.61–0.80), and “almost perfect” (0.81–1.00) [53]. All analyses were conducted using R (iir package, R version 4.1.1, R Foundation for Statistical Computing) [50].
Sensitivity analyses
We re-categorized the distribution of caloric intake to evaluate 3 8-h time periods to determine whether an alternative cut-point would yield a more or less reliable estimate than 6, 4-h time periods. Being a late eater was also re-categorized using different cut-points (i.e., 19:00, 20:00, 21:00, and 22:00 h). Analyses were also stratified by weekday compared with weekend 24HR, sex, and BMI (< 25 or ≥ 25 kg/m2). Seasonal variation was investigated by stratifying estimates according to Autumn/Winter (September through February) and Spring/Summer (March through August) 24HR measurement. Differences in reliability estimates between subgroups were compared using 2-sample t-tests. Finally, to provide context for comparing the reliability of 24HR to measure meal timing with other commonly measured dietary variables in nutritional epidemiology, we calculated ICCs for daily calorie and protein intake in IDATA. Pearson correlation coefficients were computed for the correlation of meal timing variables between time points. We provide these coefficients to enable comparisons with other published literature on this topic.
Results
Study participant characteristics are detailed in Table 1. A total of 996 participants from IDATA, 134 participants from the EPIC-Potsdam Validation Study, and 725 participants from the EPIC-Potsdam BMBF-II study were included in our analyses (see study flow chart in Supplemental Figure 1). IDATA participants had equal proportions of male and female participants. On average, they were aged 63 ± 6 y, overweight (BMI 28 ± 5 kg/m2), and predominantly non-Hispanic White (91%). The EPIC-Potsdam Validation Study had a slightly higher proportion of male (56%) than female participants, the mean age at recall was 55 ± 8 y, and participants were slightly overweight (BMI of 26 ± 4 kg/m2), on average. The EPIC-Potsdam BMBF-II study had equal proportions of males and females with a mean age of 66 ± 8 y and a mean BMI in the overweight range (27 ± 4 kg/m2).
TABLE 1.
Baseline characteristics of The Interactive Diet and Activity Tracking in AARP (IDATA) study, the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Validation and EPIC-Potsdam BMBF-II sub-study participants
| Study | IDATA (n = 996)1 | EPIC-Potsdam validation study (n = 134)2 | EPIC-Potsdam BMBF-II study (n = 725)3 |
|---|---|---|---|
| Participant characteristics | N (%) or mean ± SD | ||
| Age, (y) | |||
| Mean ± SD | 63.10 ± 6.0 | 55.24 ± 8.16 | 65.73 ± 8.29 |
| <50 | 0 (0%) | 7 (5.2%) | 17 (2.4%) |
| 50–59 | 319 (29.5%) | 27 (20.2%) | 206 (28.4%) |
| 60–69 | 582 (53.8%) | 56 (41.8%) | 235 (32.4%) |
| >70 | 181 (16.7%) | 44 (32.8) | 267 (36.8%) |
| Sex | |||
| Male | 541 (50%) | 75 (56%) | 372 (51%) |
| Female | 541 (50%) | 59 (44%) | 353 (49%) |
| BMI (kg/m2) | |||
| Mean ± SD | 27.66 ± 4.72 | 26.44 ± 4.05 | 27.39 ± 4.21 |
| <25 | 294 (27.7%) | 49 (36.6%) | 227 (31.3%) |
| 25 to <30 | 437 (41.1%) | 63 (47.0%) | 336 (46.3%) |
| 30 to <35 | 230 (21.7%) | 18 (13.4%) | 123 (17.0%) |
| 35+ | 101 (9.5%) | 4 (3.0%) | 39 (5.4%) |
| Caloric intake (kcal/d) | 2095 (745) | ||
BMBF-II Study, Federal Ministry of Education and Research (German: Bundesministerium für Bildung und Forschung); BMI, body mass index; EPIC-Potsdam, European Prospective Investigation into Cancer and Nutrition; IDATA, The Interactive Diet and Activity Tracking in AARP; SD, standard deviation.
A total of 6 recalls were completed by 797 of the IDATA participants; 75 people completed 5 recalls, 13 people completed 4 recalls, and 111 people completed between 1–3 recalls.
A total of 134 participants of the EPIC-Potsdam Validation study completed ≤12 recalls.
A total of 725 participants of the EPIC- Potsdam BMBF-II study completed 4 recalls.
Overnight fasting duration
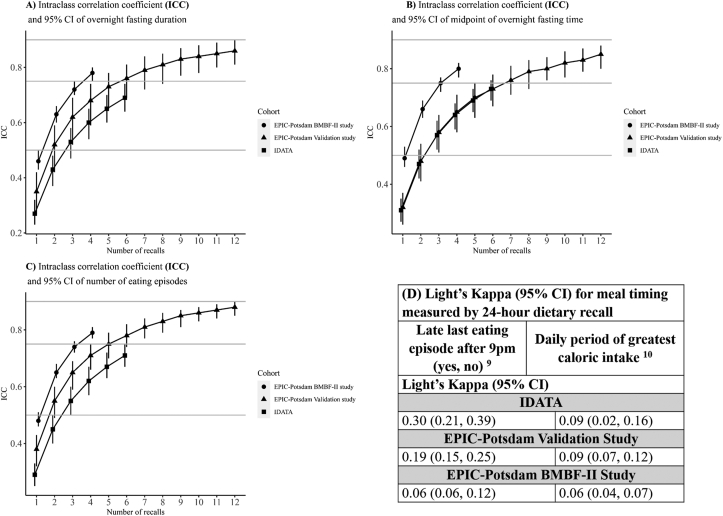
ICCs for overnight fasting duration are presented in Supplemental Table 1. A comparison across studies of ICCs for overnight fasting duration measured with ≤ 12 24HR is presented in Figure 2. Pearson correlation coefficients for overnight fasting duration between the repeated recalls in IDATA are presented in Table 2. Reliability of overnight fasting duration was “poor” based on a single 24HR for both IDATA (ICC: 0.27; 95% CI: 0.23, 0.32) and the EPIC-Potsdam Validation Study (ICC: 0.35; 95% CI: 0.27, 0.42) (Supplemental Table 1). In IDATA, “moderate” reliability was achieved with 3, 4, 5, or 6 24HR (ICC: 0.53; 95% CI: 0.47, 0.58 and ICC: 0.69; 95% CI: 0.64, 0.74 for 3 and 6 24HR, respectively). The EPIC-Potsdam Validation Study demonstrated “moderate” reliability for measuring overnight fasting duration with 2 24HR (ICC: 0.52; 95% CI: 0.42, 0.59) and “good” with 6 24HR (ICC: 0.76; 95% CI: 0.69, 0.81). Reliability continued to improve from 7 to 12 recalls (ICC: 0.86: 95% CI: 0.81, 0.90 for 12 24HR). Results from the EPIC-Potsdam BMBF-II study exhibited slightly higher reliability for measuring overnight fasting duration with a single 24HR, although the ICC was still “poor” (ICC: 0.46; 95% CI: 0.43, 0.50) and “moderate” reliability was attained with 2, 3, or 4 24HR measurements (ICC: 0.63; 95% CI: 0.60, 0.66 and ICC: 0.78; 95% CI: 0.75, 0.80 respectively). Reliability based on ICC increased in all groups with a larger number of recalls.
FIGURE 2.
Bootstrap mean and 95% CIs of intraclass correlation coefficients (ICC) and Light’s Kappa for meal timing variables measured by 24-h dietary recall in the IDATA, EPIC-Potsdam Validation, and EPIC-Potsdam BMBF-II studies. Shapes denoting the different studies indicate the level of reliability as defined using the following criteria: “poor” (ICC <0.50), “moderate” (0.50–0.75), and “good” (0.75–0.90). (A) Overnight fasting duration; (B) Midpoint of overnight fasting time; (C) Number of eating episodes. Intraclass correlation coefficients were calculated using a 1-way linear mixed effects model with and without fixed covariates [51]. Kappa statistics were calculated based on the formula for multiple raters proposed by Light [52]. IDATA participants (n = 996) completed ≤ 6 24-h dietary recalls. EPIC-Potsdam Validation study participants (n = 134) completed ≤ 12 24-h dietary recalls. EPIC-Potsdam BMBF-II study participants (n = 725) completed ≤ 4 24-h dietary recalls. The duration of overnight fasting (in h) was estimated by calculating the time between the first and the last eating episode of the day and subtracting it from 24-h. The midpoint of the overnight fasting period was calculated as the duration of overnight fasting divided by 2 and added to the start time of the overnight fasting. The number of eating episodes was calculated as an ordinal variable and included any time-stamped eating episode associated with calorie-containing food or beverage consumption (≥ 25 calories). Late eating was calculated as a binary variable (yes/no) indicating whether the last eating episode in the 24-h period was prior to 9 pm or at/after 9 pm. The daily period of greatest percentage caloric intake was calculated by assigning individuals to the period of greatest daily percentage caloric intake in the 24-h period: 1) 00:00–04:00; 2) 04:00–08:00 ; 3) 08:00–12:00; 4) 12:00–16:00; 5) 16:00–20:00; 6) 20:00–24:00 h. BMBF, Federal Ministry of Education and Research (German: Bundesministerium für Bildung und Forschung); EPIC, European Prospective Investigation into Cancer and Nutrition; IDATA, The Interactive Diet and Activity Tracking in AARP.
TABLE 2.
Pearson correlation matrix for overnight fasting duration1 measured with ≤ 6 automated self-administered 24-h dietary recalls measured over 12-mo in the Interactive Diet and Activity Tracking in AARP (IDATA) study
| Number of recalls | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 1.00 | |||||
| 2 | 0.21 | 1.00 | ||||
| 3 | 0.29 | 0.33 | 1.00 | |||
| 4 | 0.24 | 0.30 | 0.32 | 1.00 | ||
| 5 | 0.22 | 0.28 | 0.36 | 0.33 | 1.00 | |
| 6 | 0.24 | 0.23 | 0.27 | 0.28 | 0.34 | 1.00 |
Duration of overnight fasting (in h) was estimated by calculating the time between the first and the last eating episode of the day and subtracting from 24-h.
In IDATA, since dietary intake was evaluated from midnight to midnight and some participants may have eaten after midnight, but before bedtime, we generated an alternative variable for overnight fasting duration. We measured the longest time period without food or beverage intake ≥ 25 kcal. The reliability of overnight fasting duration was similar to the originally computed variable, with an ICC: 0.30; 95% CI: 0.26, 0.35 for 1 24HR and ICC: 0.72; 95% CI: 0.68, 0.76 for 6 24HR.
Midpoint of overnight fasting time
The ICC for the midpoint of overnight fasting time in IDATA showed “poor” reliability based on 1 24HR (ICC: 0.31; 95% CI: 0.27, 0.35) and was “moderate” for 3 24HR (ICC: 0.58; 95% CI: 0.53, 0.62) and ≤ 6 24HR (ICC: 0.73; 95% CI: 0.69, 0.77) (Supplemental Table 1 and Figure 2). Moreover, the reliability of measuring the midpoint of overnight fasting time increased when considering the window of the longest time period without food as opposed to the first compared with the last eating episode in the day from midnight to midnight. The midpoint of overnight fasting time in the EPIC-Potsdam Validation study showed “poor” reliability for 1–2 24HR and “moderate” reliability with 3 (ICC: 0.58; 95% CI: 0.51, 0.64) to 6 recalls (ICC: 0.73; 95% CI: 0.67, 0.78). Reliability was “good” when considering 7 24HR (ICC: 0.76; 95% CI: 0.71, 0.81). The EPIC-Potsdam BMBF-II study showed similar reliability with fewer recalls, “poor” when measured with a single 24HR (ICC: 0.49; 95% CI: 0.46, 0.53), and “good” with 3 24HR (ICC: 0.75; 95% CI: 0.72, 0.77).
Number of eating episodes
The reliability of measuring the number of eating episodes was similar to the midpoint of overnight fasting time. In IDATA, reliability was “poor” with 1 24HR measure of the number of eating episodes (ICC: 0.29; 95% CI: 0.25, 0.33) (Supplemental Table 1 and Figure 2). There was “moderate” reliability using 2–5 24HR and “good” reliability with 6 24HR in IDATA. In the EPIC-Potsdam Validation study, reliability was “moderate” with 2–4 24HR and “good” with 5 24HR (ICC: 0.75; 95% CI: 0.70, 0.79). Reliability continued to increase when administering ≤ 12 24HR, which had “good” reliability (ICC: 0.88; 95% CI: 0.85, 0.90). In the EPIC-Potsdam BMBF-II study, the number of eating episodes measured with 2 24HR had “moderate” reliability (ICC: 0.65; 95% CI: 0.63, 0.68), progressively improving ≤ 4 24HR, which had “good” reliability (ICC: 0.79; 95% CI: 0.77, 0.81).
Late last eating episode
Agreement between measures of late last eating episode after 21:00 h (yes, no) was “fair” in IDATA (Light’s Kappa: 0.30; 95% CI: 0.21, 0.39). The agreement was “slight” for both the EPIC-Potsdam Validation study (Light’s Kappa: 0.19; 95% CI: 0.15, 0.25) and the EPIC-Potsdam BMBF-II study (Light’s Kappa: 0.09; 95% CI: 0.06, 0.12). In IDATA, the late last eating episode was also re-categorized using different cut-points (i.e., 19:00, 20:00, and 22:00 h). There was a slight variation in consistency according to the cut-point (Light’s Kappa: 0.21; 95% CI: 0.18, 0.25 for 19:00 h; Light’s Kappa: 0.26; 95% CI: 0.23, 0.30 for 20:00 h; Light’s Kappa: 0.30; 95% CI: 0.21, 0.39 for 22:00 h).
Daily period of greatest percentage caloric intake
Light’s Kappa values for measuring the period in the day with the greatest proportion of caloric intake are presented for each study in Supplemental Table 1 and Figure 2. Results were similar across studies. IDATA showed a “slight” agreement between any 2 measures of the period with the largest percentage daily caloric intake (Light’s Kappa: 0.09; 95% CI: 0.02, 0.16). In addition, we conducted analyses using alternative cut-points of 3, 8-h periods of time across the day [i.e., 1) 21:00 h–05:00 h; 2) 05:00 h–13:00 h; and 3) 13:00–21:00 h], which also showed “slight” agreement between any 2 measures (Light’s Kappa: 0.12; 95% CI: 0.09, 0.14). The period of the greatest percentage caloric intake in the EPIC-Potsdam Validation study also showed “slight” agreement between any 2 measures (Light’s Kappa: 0.09; 95% CI: 0.07, 0.12), similar to results from the EPIC-Potsdam BMBF-II study (Light’s Kappa: 0.06; 95% CI: 0.04, 0.07).
Reliability by subgroup
Analyses of meal timing variables stratified by sex, BMI (< or ≥ 25 kg/m2), weekend compared with weekday, and season (Spring/Summer compared with Autumn/Winter) are presented in Table 3 for IDATA and Supplemental Tables 2–4 for both EPIC-Potsdam studies. Differences in reliability estimates were observed between days of the week, season, and sex. We did not stratify by weekend compared with weekday or season of Light’s Kappa because of the limited sample size for estimates by subgroup. We observed evidence of differences in reliability estimates from 24HR measurements of meal timing variables according to sex, day of the week, and season. Overnight fasting duration was more reliably measured in Autumn/Winter than in Spring/Summer in the IDATA and EPIC-Potsdam BMBF-II studies. Overnight fasting was also more reliably measured on the weekend than on weekdays in the EPIC-Potsdam Validation Study and EPIC-Potsdam BMBF-II study. There was suggestive evidence that fasting duration was measured more reliability among females than males in the EPIC-Potsdam Validation Study, and strong evidence that reliability is greater among males than females in IDATA. The midpoint of overnight fasting time was more reliably measured on weekdays than weekend days and in Autumn/Winter in the EPIC-Potsdam BMBF-II study. Lastly, the number of eating episodes was more reliably measured on weekdays compared with weekend days in the EPIC-Potsdam Validation Study and the EPIC-Potsdam BMBF-II study. In the IDATA and EPIC-Potsdam BMBF-II study, the reliability of measuring the number of easting episodes was greater among males than females. In the EPIC-Potsdam BMBF-II study, there was suggestive evidence of differences in the reliability of measuring the number of eating episodes according to BMI, with better reliability among individuals in the normal weight range compared to those with overweight or obesity. Autumn/Winter measures of the number of eating episodes were significantly more reliable compared to Spring/Summer in the EPIC-Potsdam BMBF-II study.
TABLE 3.
Estimated bootstrap mean and 95% CIs of intra-rater reliability 1 for meal timing variables measured by 24-h dietary recall in the Interactive Diet and Activity Tracking in AARP2 dietary calibration sub-study, stratified by biological sex, BMI, day of recall, and season
| Subgroup | Sex – ICC (95% CI) |
BMI – ICC (95% CI) |
Day of recall – ICC (95% CI) |
Season – ICC (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | P value | <25 kg/m2 | >25 kg/m2 | P value | Weekday | Weekend | P value | Spring/Summer | Autumn/Winter | P value | |
| Overnight fasting duration3 | ||||||||||||
| Mean ± SD | 11.40 ± 2.89 | 11.76 ± 2.59 | 11.58 ± 1.74 | 11.57 ± 2.75 | 11.44 ±2.73 | 11.91 ±2.78 | 11.59 ± 2.70 | 11.57 ± 2.81 | ||||
| 1 recall | 0.33 (0.26, 0.39) | 0.24 (0.18, 0.29) | 0.05 | 0.33 (0.24, 0.43) | 0.28 (0.22, 0.33) | 0.29 | 0.31 (0.26, 0.37) | 0.35 (0.24, 0.45) | 0.61 | 0.25 (0.19, 0.32) | 0.36 (0.28, 0.43) | 0.04 |
| 2 recalls | 0.49 (0.41, 0.57) | 0.38 (0.31, 0.45) | 0.04 | 0.50 (0.39, 0.60) | 0.43 (0.36, 0.49) | 0.29 | 0.48 (0.41, 0.54) | 0.51 (0.39, 0.62) | 0.62 | 0.40 (0.31, 0.48) | 0.53 (0.44, 0.60) | 0.04 |
| 3 recalls | 0.59 (0.51, 0.66) | 0.48 (0.40, 0.56) | 0.05 | 0.60 (0.48, 0.69) | 0.53 (0.46, 0.59) | 0.30 | 0.58 (0.51, 0.64) | 0.61 (0.48, 0.71) | 0.63 | 0.50 (0.41, 0.58) | 0.62 (0.54, 0.70) | 0.04 |
| 4 recalls | 0.66 (0.58, 0.72) | 0.55 (0.47, 0.62) | 0.05 | 0.66 (0.56, 0.75) | 0.60 (0.53, 0.66) | 0.30 | 0.65 (0.58, 0.70) | 0.67 (0.56, 0.77) | 0.64 | 0.57 (0.48, 0.65) | 0.69 (0.61, 0.75) | 0.04 |
| 5 recalls | 0.71 (0.64, 0.77) | 0.61 (0.53, 0.68) | 0.05 | 0.71 (0.61, 0.79) | 0.65 (0.59, 0.71) | 0.30 | 0.70 (0.64, 0.75) | 0.72 (0.61, 0.81) | 0.65 | 0.62 (0.53, 0.70) | 0.73 (0.66, 0.79) | 0.04 |
| 6 recalls | 0.74 (0.68, 0.88) | 0.65 (0.57, 0.71) | 0.05 | 0.75 (0.65, 0.82) | 0.69 (0.63, 0.74) | 0.30 | 0.73 (0.68, 0.78) | 0.76 (0.65, 0.83) | 0.65 | 0.66 (0.58, 0.74) | 0.77 (0.70, 0.82) | 0.04 |
| Midpoint of overnight fasting time4 | ||||||||||||
| Mean ± SD | 3.56 ± 4.89 | 3.26 ± 4.32 | 3.41 ± 5.06 | 3.41 ± 4.52 | 3.39 ± 4.75 | 3.46 ± 4.48 | 3.44 ± 4.69 | 3.38 ± 4.65 | ||||
| 1 recall | 0.33 (0.27, 0.38) | 0.29 (0.24, 0.35) | 0.38 | 0.36 (0.27, 0.44) | 0.29 (0.25, 0.34) | 0.20 | 0.18 (0.12, 0.25) | 0.11 (0.06, 0.17) | 0.13 | 0.30 (0.25, 0.36) | 0.35 (0.28, 0.42) | 0.30 |
| 2 recalls | 0.49 (0.43, 0.56) | 0.45 (0.38, 0.52) | 0.38 | 0.52 (0.43, 0.61) | 0.45 (0.40, 0.51) | 0.20 | 0.31 (0.21, 0.41) | 0.21 (0.12, 0.29) | 0.13 | 0.46 (0.40, 0.53) | 0.52 (0.44, 0.59) | 0.30 |
| 3 recalls | 0.59 (0.53, 0.65) | 0.55 (0.48, 0.62) | 0.38 | 0.62 (0.53, 0.70) | 0.55 (0.50, 0.60) | 0.20 | 0.40 (0.28, 0.51) | 0.28 (0.17, 0.38) | 0.13 | 0.56 (0.50, 0.63) | 0.61 (0.54, 0.68) | 0.30 |
| 4 recalls | 0.66 (0.60, 0.71) | 0.62 (0.55, 0.68) | 0.38 | 0.69 (0.60, 0.76) | 0.62 (0.57, 0.67) | 0.20 | 0.47 (0.34, 0.58) | 0.34 (0.21, 0.45) | 0.13 | 0.63 (0.57, 0.69) | 0.68 (0.61, 0.74) | 0.30 |
| 5 recalls | 0.71 (0.65, 0.76) | 0.67 (0.61, 0.73) | 0.38 | 0.73 (0.65, 0.80) | 0.67 (0.62, 0.72) | 0.20 | 0.52 (0.39, 0.63) | 0.39 (0.25, 0.50) | 0.13 | 0.68 (0.62, 0.74) | 0.73 (0.66, 0.78) | 0.30 |
| recalls | 0.74 (0.69, 0.79) | 0.71 (0.65, 0.76) | 0.38 | 0.77 (0.69, 0.83) | 0.71 (0.67, 0.75) | 0.20 | 0.57 (0.44, 0.67) | 0.43 (0.29, 0.55) | 0.13 | 0.72 (0.66, 0.77) | 0.76 (0.70, 0.81) | 0.31 |
| Number of eating episodes per day5 | ||||||||||||
| Mean ± SD | 3.72 ± 0.73 | 3.78 ± 0.68 | 3.79 ± 0.69 | 3.74 ± 0.71 | 3.78 ± 0.71 | 3.68 ± 0.70 | 3.74 ± 0.71 | 3.76 ± 0.70 | ||||
| 1 recalls | 0.34 (0.28, 0.40) | 0.23 (0.18, 0.29) | 0.01 | 0.29 (0.20, 0.39) | 0.29 (0.24, 0.34) | 0.98 | 0.31 (0.27, 0.36) | 0.25 (0.16, 0.33) | 0.18 | 0.27 (0.22, 0.33) | 0.33 (0.27, 0.39) | 0.17 |
| 2 recalls | 0.50 (0.43, 0.57) | 0.38 (0.31, 0.44) | 0.01 | 0.45 (0.34, 0.56) | 0.45 (0.39, 0.50) | 1.00 | 0.47 (0.42, 0.53) | 0.39 (0.27, 0.50) | 0.19 | 0.43 (0.36, 0.50) | 0.50 (0.43, 0.56) | 0.17 |
| 3 recalls | 0.60 (0.54, 0.66) | 0.48 (0.40, 0.55) | 0.01 | 0.55 (0.43, 0.65) | 0.55 (0.49, 0.60) | 0.99 | 0.58 (0.52, 0.63) | 0.49 (0.36, 0.60) | 0.19 | 0.53 (0.45, 0.60) | 0.60 (0.53, 0.66) | 0.18 |
| 4 recalls | 0.67 (0.61, 0.73) | 0.55 (0.47, 0.62) | 0.01 | 0.62 (0.50, 0.72) | 0.62 (0.56, 0.67) | 0.98 | 0.64 (0.59, 0.69) | 0.56 (0.43, 0.66) | 0.20 | 0.60 (0.53, 0.67) | 0.66 (0.60, 0.72) | 0.18 |
| 5 recalls | 0.72 (0.66, 0.77) | 0.60 (0.53, 0.67) | 0.01 | 0.67 (0.56, 0.76) | 0.67 (0.62, 0.72) | 0.98 | 0.69 (0.64, 0.74) | 0.61 (0.48, 0.71) | 0.21 | 0.65 (0.58 ,0.71) | 0.71 (0.65, 0.76) | 0.18 |
| 6 recalls | 0.75 (0.70, 0.80) | 0.65 (0.57, 0.71) | 0.01 | 0.71 (0.60, 0.79) | 0.71 (0.66, 0.75) | 0.97 | 0.73 (0.68, 0.77) | 0.66 (0.53, 0.75) | 0.21 | 0.69 (0.63, 0.75) | 0.75 (0.69, 0.79) | 0.18 |
ICC, intraclass correlation coefficient; IDATA, the Interactive Diet and Activity Tracking in AARP.
Intraclass correlation coefficients were calculated using a 1-way linear mixed effects model with and without fixed covariates.
IDATA participants completed ≤ 6 24-h dietary recalls.
Duration of overnight fasting (in h) was estimated by calculating the time between the first and the last eating episode of the day and subtracting from 24-h.
Midpoint of the overnight fasting period was calculated as the duration of overnight fasting divided by 2 and added to the start time of the overnight fasting.
The number of eating episodes was calculated as an ordinal variable and included any time-stamped eating episode associated with calorie-containing food or beverage consumption (≥25 calories).
Comparison of reliability estimates for meal timing variables and nutrients
Protein and caloric intake are commonly used variables in epidemiologic studies. We, therefore, compared the reliability of measuring meal timing variables with 24HR with these commonly used variables as a benchmark. The ICCs for overnight fasting duration in IDATA were comparable to ICCs for total protein intake (g/d), but overnight fasting duration showed a slightly lower reliability than daily caloric intake (kcal/d) for any combination of repeated 24HR (Table 4).
TABLE 4.
Comparison of estimated intra-rater reliability1 of 24-h dietary recall measurement of calories, protein, and overnight fasting duration in the Interactive Diet and Activity Tracking in AARP study (n = 996)
| Intraclass correlation coefficient | ||||||
|---|---|---|---|---|---|---|
| Dietary exposure/Number of recalls | 1 | 2 | 3 | 4 | 5 | 6 |
| Total calories (kcal/d) | 0.41 | 0.58 | 0.67 | 0.73 | 0.77 | 0.80 |
| Total protein (g/d) | 0.32 | 0.49 | 0.59 | 0.65 | 0.70 | 0.74 |
| Overnight fasting duration (h)2 | 0.29 | 0.45 | 0.55 | 0.62 | 0.67 | 0.71 |
Intraclass correlation coefficients were calculated using a 1-way linear mixed effects model with and without fixed covariates.
Duration of overnight fasting (in h) was estimated by calculating the time between the first and the last eating episode of the day and subtracting from 24-h.
Discussion
In 3 dietary calibration studies within prospective cohorts in both the United States and Germany, we evaluated the reliability over time (≤ 36-m) for 24HR estimates of meal timing. Three 24HR per individual showed “moderate” to “good” reliability for estimating overnight fasting duration, the midpoint of overnight fasting time, and the number of eating episodes in all cohorts. There was slight to the fair agreement between any 2 measures of late last eating episode and period of greatest percentage caloric intake. The reliability of the different meal timing variables was comparable, except for the number of eating episodes that were most reliably measured among German participants. There was poor reliability of single measures of meal timing. However, moderate to good reliability over 1–3 y of meal timing parameters was achieved with ≥ 3 24HR, which was similar to our estimates for calorie and protein intakes and those of other studies [20,22,27,[54], [55], [56], [57]]. Finally, we observed differences in reliability estimates according to the day of the week, the season of recall, and sex.
Since the 24HRs administered in IDATA and the EPIC-Potsdam sub-studies were conducted over time rather than on consecutive days, it is possible that meal timing variation over the year is a natural phenomenon rather than dietary recall being imprecise, per se. Since the 24HRs were nonconsecutive, the overnight fasting duration calculation assumed a similar day-to-day distribution of meal timing. Assessing the day-to-day reliability of meal timing using 24HR in conjunction with an alternative method, such as food records, would further inform meal timing measurement errors. Moreover, reliability estimates were calculated based on repeated recalls over a period of time. Reliability may be influenced by both day-to-day variability and changes over longer periods of time, potentially leading to lower reliability when implementing a single 24HR for estimating habitual meal timing. In our analyses, covariates were not modeled as being time-varying since we did not have measures at every recall. A time-varying (longitudinal) model may have inflated within-subject variability estimates, deflating the ICCs. Therefore, our observed ICC estimates are likely conservative.
Nonetheless, the current methodological assessment could be strengthened by studies that administer multiple 24HR within the same week. For example, 4 self-reported 24HR administered within 10-d yielded ICCs for energy and nutrients ranging from 0.31; 95% CI: 0.25, 0.37 – 0.43; 95% CI: 0.37, 0.49 for 1 24HR and 0.36; 95% CI: 0.27, 0.46 – 0.63; 95% CI: 0.55, 0.69 for paired 24HR [54]. Nonetheless, evaluating longitudinal 24HR provides information on habitual dietary intake, which could be considered a more relevant exposure period in the etiology of disease.
Sex differences were observed in meal timing reliability. Other studies have observed that habitual calorie and nutrient intakes can be estimated with fewer 24HR among males than females [26], which we observed in IDATA. A recent meta-analysis showed similar caloric underestimation by sex [58]. There was suggestive evidence that BMI modified reliability estimates of meal timing in our study, with estimates being more reliably measured among individuals in the normal weight than overweight/obese range. On the contrary, adults with overweight or obesity showed higher stability in meal timing than lean young adults in 1 study [59], although meal timing was estimated with food photograph time stamps, was small in size, and was conducted among college-age adults. Studies support that individuals with overweight or obesity may misreport dietary intakes more than those with a BMI < 25 kg/m2 [60,61]. In an analysis of data from US adults in the National Health and Nutrition Examination Survey (NHANES), the snacking frequency was not associated with the odds of being overweight or obese, although the odds trended downward with higher snack frequency [62]. Meal timing reliability estimates differed by day and season of 24HR administration. Other studies have reported variation in dietary intake comparing weekend days to weekdays, but no studies have investigated meal timing [[63], [64], [65]]. Additional research may be warranted to evaluate differences in meal timing based on geography, including north-to-south gradients that associate with sunlight exposure.
Meal timing reliability over time was similar in the United States and Germany, but the included studies lacked age and racial/ethnic diversity. Meal timing and frequency may differ for stages of life or other characteristics such as cultural meal patterns, social influences, marital status, attending college, or shift work [[66], [67], [68]]. There is limited research investigating meal timing among children and adolescents. One study among Hispanic adolescents showed that 65% of the intake of calories, carbohydrates, and added sugars occurred between 11:00 and 19:00 [69], but more studies are needed in younger populations.
Our findings lay the groundwork for future studies of meal timing in relation to disease outcomes. The 24HR and food records are the only commonly used, validated dietary assessment tools that include the timing of meals as an integral part of the data collection process [21]. Emerging alternatives include time-stamped food photograph technology [70], and 24-h meal timing grid [71]. Meal timing may be an important disease risk exposure given its links in preclinical and clinical studies with cardiometabolic disease risk factors [3]. However, chronic diseases evolve over long periods of time [72]; thus, prospective observational studies provide a unique resource to investigate dietary exposures in relation to disease outcomes. As with other dietary exposures, implementing ≥ 3 24HR may measure meal timing with an adequate level of reliability in epidemiologic studies [20,22,27]. A caveat is that prospective cohorts that conducted dietary calibration sub-studies with 24HR often administer 1 or 2 measures, which could attenuate or distort the magnitude of association with disease outcomes [73,74]. Methods to apply de-attenuation factors because of the suboptimal reliability have been proposed [73,75], which may be useful for future meal timing studies that leverage existing data from prospective cohorts.
Recalling foods and beverages consumed from waking to bedtime may provide different reliability estimates than recalling from midnight to midnight since foods may be consumed after 00:00 h. Therefore, estimates of overnight fasting duration may need to consider the timing of sleep in relation to the last eating episode. Nonetheless, we did not observe material differences in overnight fasting duration in IDATA when taking this into consideration. However, the pattern of meal timing in IDATA, a cohort of older adults, could differ in other populations. Recently, the ASA24 added an optional sleep assessment that could be informative when determining how sleep aligns with dietary intake [76]. Moreover, the timing of dietary intake may be influenced by sleep patterns, which have also been linked to metabolic health [[77], [78], [79]]. Thus, measuring sleep may be an important covariate to consider in studies of meal timing and health outcomes.
Strengths of our study include the large, international study sample with heterogeneity in meal timing behaviors and serial 24HR administration over time. Our robust sample size allowed for the stratification of reliability estimates by participant and 24HR measurement characteristics. The different approaches to collecting 24HR (midnight to midnight or waking to bedtime) enabled a comparison of approaches for estimating overnight fasting duration at its midpoint. Limitations exist, however. Since the 24HRs were nonconsecutive, the overnight fasting duration calculation assumed a similar day-to-day distribution of meal timing, and the reliability estimates reflect meal timing over time rather than day-to-day. Assessing the day-to-day reliability of meal timing using 24HR in conjunction with an alternative method, such as food records, would further inform meal timing measurement errors. Incorporating information on sleep timing would further validate meal timing self-report. Since our data contains 24HR collected from individuals within a timeframe (e.g., 1 y), our reliability estimates reflect the collection of recalls within the same time frame. The ICC calculation does not incorporate time. Therefore, it cannot be determined whether repeated recalls collected within different time frames have different levels of reliability. Since the ICC estimates do not consider the duration of time between measurements, we did not estimate the “optimal” timing of 24HR over months or years for measuring meal timing variables. The current 24HR methodology does not account for the duration of the eating occasion, which can modulate meal timing estimation [80]. Moreover, replication in more age and racial/ethnically-diverse populations is warranted.
In summary, meal timing variables are measured with moderate reliability over periods of 1–3 y with ≥ 3 24HR, and reliability estimates increase with more recalls. Different meal timing variables have different levels of reliability that should be considered in future analyses of meal timing in relation to health or disease outcomes. Reliability estimates showed some differences according to participant characteristics and the day or season of 24HR administration. Future meal timing methodology studies will benefit from leveraging consecutive 24HR recalls and more heterogeneous populations.
Author contribution
The authors’ responsibilities were as follows– LMP, IH, BH, and MCP: designed the research; LMP, HL, IH, CB, BK, and MCP: analyzed the data; LMP and MCP: wrote the paper and had primary responsibility for the final content. In addition, all authors: provided critical intellectual content to review and edit the paper; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest. Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policies, or views of the International Agency for Research on Cancer/WHO.
Data Availability
Data described in the manuscript, codebook, and analytic code will not be made available because the data supporting this study's findings are not publicly available. In accordance with the German Federal and State data protection regulations, epidemiological data analyses of The European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam may be initiated upon an information inquiry addressed to the PI of the EPIC-Potsdam study, who is a co-author of this manuscript (MBS). Data from the Interactive Diet and Activity Tracking in AARP Study may be available from the NCI Cancer Data Access System upon request.
Funding
This study was supported by the National Cancer Institute 5R00CA218694-03 and the Huntsman Cancer Institute Cancer Center P30CA040214 (to MCP).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.02.026.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Patterson R.E., Laughlin G.A., LaCroix A.Z., Hartman S.J., Natarajan L., Senger C.M., et al. Intermittent fasting and human metabolic health. J Acad Nutr Diet. 2015;115(8):1203–1212. doi: 10.1016/j.jand.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manoogian E.N.C., Chaix A., Panda S. When to eat: the importance of eating patterns in health and disease. J Biol Rhythms. 2019;34(6):579–581. doi: 10.1177/0748730419892105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabel K., Varady K.A. Current research: effect of time restricted eating on weight and cardiometabolic health. J Physiol. 2022;600(6):1313–1326. doi: 10.1113/JP280542. [DOI] [PubMed] [Google Scholar]
- 4.Chaix A., Manoogian E.N.C., Melkani G.C., Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291–315. doi: 10.1146/annurev-nutr-082018-124320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson M.J., Manoogian E.N.C., Zadourian A., Lo H., Fakhouri S., Shoghi A., et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92–104. doi: 10.1016/j.cmet.2019.11.004. e5 doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wehrens S.M.T., Christou S., Isherwood C., Middleton B., Gibbs M.A., Archer S.N., et al. Meal timing regulates the human circadian system. Curr Biol CB. 2017;27(12):1768–1775.e3. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarrinpar A., Chaix A., Panda S. Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab. 2016;27(2):69–83. doi: 10.1016/j.tem.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Q., Bauer C., Layne T., Playdon M. The association between overnight fasting and body mass index in older adults: the interaction between duration and timing. Int J Obes (Lond) 2021;45(3):555–564. doi: 10.1038/s41366-020-00715-z. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshmukh-Taskar P., Nicklas T.A., Radcliffe J.D., O’Neil C.E., Liu Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The national health and nutrition examination survey (NHANES): 1999-2006. Public Health Nutr. 2013;16(11):2073–2082. doi: 10.1017/S1368980012004296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutsuma A., Nakajima K., Suwa K. Potential association between breakfast skipping and concomitant late-night-dinner eating with metabolic syndrome and proteinuria in the Japanese population. Scientifica. 2014;2014:253581. doi: 10.1155/2014/253581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHill A.W., Phillips A.J., Czeisler C.A., Keating L., Yee K., Barger L.K., et al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106(5):1213–1219. doi: 10.3945/ajcn.117.161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson R.E., Sears D.D. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37:371–393. doi: 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- 13.Anton S.D., Lee S.A., Donahoo W.T., McLaren C., Manini T., Leeuwenburgh C., et al. The effects of time restricted feeding on overweight, older adults: A pilot study. Nutrients. 2019;11(7) doi: 10.3390/nu11071500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamshed H., Beyl R.A., Della Manna D.L., Yang E.S., Ravussin E., Peterson C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6) doi: 10.3390/nu11061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchison A.T., Regmi P., Manoogian E.N.C., Fleischer J.G., Wittert G.A., Panda S., et al. Time-restricted feeding improves glucose tolerance in men at risk for Type 2 diabetes: A randomized crossover trial. Obesity (Silver Spring) 2019;27(5):724–732. doi: 10.1002/oby.22449. [DOI] [PubMed] [Google Scholar]
- 16.Marinac C.R., Natarajan L., Sears D.D., Gallo L.C., Hartman S.J., Arredondo E., et al. Prolonged nightly fasting and breast cancer risk: findings from NHANES (2009-2010) Cancer Epidemiol Biomarkers Prev. 2015;24(5):783–789. doi: 10.1158/1055-9965.EPI-14-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marinac C.R., Nelson S.H., Breen C.I., Hartman S.J., Natarajan L., Pierce J.P., et al. Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol. 2016;2(8):1049–1055. doi: 10.1001/jamaoncol.2016.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kogevinas M., Espinosa A., Castelló A., Gómez-Acebo I., Guevara M., Martin V., et al. Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain Study) Int J Cancer. 2018;143(10):2380–2389. doi: 10.1002/ijc.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capron J.P., Delamarre J., Herve M.A., Dupas J.L., Poulain P., Descombes P. Meal frequency and duration of overnight fast: a role in gall-stone formation? Br Med J (Clin Res Ed) 1981;283(6304):1435. doi: 10.1136/bmj.283.6304.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim J.S., Oh K., Kim H.C. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36 doi: 10.4178/epih/e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Dietary Assessment Primer Internet. Accessed June 12 2021. https://dietassessmentprimer.cancer.gov/. National Institutes of Health.
- 22.Beaton G.H., Milner J., McGuire V., Feather T.E., Little J.A. Source of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Carbohydrate sources, vitamins, and minerals. Am J Clin Nutr. 1983;37(6):986–995. doi: 10.1093/ajcn/37.6.986. [DOI] [PubMed] [Google Scholar]
- 23.Tarasuk V., Beaton G.H. The nature and individuality of within-subject variation in energy intake. Am J Clin Nutr. 1991;54(3):464–470. doi: 10.1093/ajcn/54.3.464. [DOI] [PubMed] [Google Scholar]
- 24.Riboli E., Hunt K.J., Slimani N., Ferrari P., Norat T., Fahey M., et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6b):1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 25.Slimani N., Kaaks R., Ferrari P., Casagrande C., Clavel-Chapelon F., Lotze G., et al. European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study: rationale, design and population characteristics. Public Health Nutr. 2002;5(6b):1125–1145. doi: 10.1079/PHN2002395. [DOI] [PubMed] [Google Scholar]
- 26.Stote K.S., Radecki S.V., Moshfegh A.J., Ingwersen L.A., Baer D.J. The number of 24 h dietary recalls using the US Department of Agriculture’s automated multiple-pass method required to estimate nutrient intake in overweight and obese adults. Public Health Nutr. 2011;14(10):1736–1742. doi: 10.1017/S1368980011000358. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y., Olendzki B.C., Pagoto S.L., Hurley T.G., Magner R.P., Ockene I.S., et al. Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol. 2009;19(8):553–559. doi: 10.1016/j.annepidem.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park Y., Dodd K.W., Kipnis V., Thompson F.E., Potischman N., Schoeller D.A., et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am J Clin Nutr. 2018;107(1):80–93. doi: 10.1093/ajcn/nqx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Cancer Institute . National Institutes of Health; 2013. Internet-based and self-reports in assessing diet and physical activity within AARP Internet.https://clinicaltrials.gov/ct2/show/NCT03268577 [Internet] Available from: [Google Scholar]
- 30.National Cancer Institute. Cancer data access system. National Cancer Institute: IDATA study summary [Internet]. Available from: https://cdas.cancer.gov/learn/idata/study-summary/. Accessed 20 August 2021
- 31.Jannasch F., Nickel D., Schulze M.B. The reliability and relative validity of predefined dietary patterns were higher than that of exploratory dietary patterns in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam population. Br J Nutr. 2021;125(11):1270–1280. doi: 10.1017/S0007114520003517. [DOI] [PubMed] [Google Scholar]
- 32.Schwedhelm C., Knüppel S., Schwingshackl L., Boeing H., Iqbal K. Meal and habitual dietary networks identified through Semiparametric Gaussian copula Graphical Models in a German adult population. PLOS ONE. 2018;13(8) doi: 10.1371/journal.pone.0202936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Cancer Institute . Epidemiology and Genomics Research Program; 2010. Diet History Questionnaire.https://epi.grants.cancer.gov/dhq2/ National Institutes of Health. [Google Scholar]
- 34.Subar A.F., Potischman N., Dodd K.W., Thompson F.E., Baer D.J., Schoeller D.A., et al. Performance and feasibility of recalls completed using the automated self-administered 24-hour dietary assessment tool in relation to other self-report tools and biomarkers in the interactive diet and activity tracking in AARP (IDATA) study. J Acad Nutr Diet. 2020;120(11):1805–1820. doi: 10.1016/j.jand.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Cancer Institute . National Institutes of Health; 2021. ASA24, the Automated Self-Administered Recall System [Internet]https://asa24.nci.nih.gov/ Available from: [Google Scholar]
- 36.Slimani N., Ferrari P., Ocké M., Welch A., Boeing H., Liere M., et al. Standardization of the 24-hour diet recall calibration method used in the European prospective investigation into cancer and nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr. 2000;54(12):900–917. doi: 10.1038/sj.ejcn.1601107. [DOI] [PubMed] [Google Scholar]
- 37.Voss S., Charrondiere U.R., Slimani N., Kroke A., Riboli E., Wahrendorf J., et al. EPIC-SOFT a European computer program for 24-hour dietary protocols. Z Ernahrungswiss. 1998;37(3):227–233. doi: 10.1007/s003940050021. [DOI] [PubMed] [Google Scholar]
- 38.Crispim S.P., Nicolas G., Casagrande C., Knaze V., Illner A.K., Huybrechts I., et al. Quality assurance of the international computerised 24 h dietary recall method (EPIC-Soft) Br J Nutr. 2014;111(3):506–515. doi: 10.1017/S0007114513002766. [DOI] [PubMed] [Google Scholar]
- 39.Marinac C.R., Sears D.D., Natarajan L., Gallo L.C., Breen C.I., Patterson R.E. Frequency and circadian timing of eating may influence biomarkers of inflammation and insulin resistance associated with breast cancer risk. PLOS ONE. 2015;10(8) doi: 10.1371/journal.pone.0136240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srour B., Plancoulaine S., Andreeva V.A., Fassier P., Julia C., Galan P., et al. Circadian nutritional behaviours and cancer risk: new insights from the NutriNet-santé prospective cohort study: disclaimers. Int J Cancer. 2018;143(10):2369–2379. doi: 10.1002/ijc.31584. [DOI] [PubMed] [Google Scholar]
- 41.Park M.K., Freisling H., Huseinovic E., Winkvist A., Huybrechts I., Crispim S.P., et al. Comparison of meal patterns across five European countries using standardized 24-h recall (GloboDiet) data from the EFCOVAL project. Eur J Nutr. 2018;57(3):1045–1057. doi: 10.1007/s00394-017-1388-0. [DOI] [PubMed] [Google Scholar]
- 42.Sichieri R., Everhart J.E., Roth H. A prospective study of hospitalization with gallstone disease among women: role of dietary factors, fasting period, and dieting. Am J Public Health. 1991;81(7):880–884. doi: 10.2105/ajph.81.7.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reid K.J., Baron K.G., Zee P.C. Meal timing influences daily caloric intake in healthy adults. Nutr Res. 2014;34(11):930–935. doi: 10.1016/j.nutres.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Castro J.M. The time of day of food intake influences overall intake in humans. J Nutr. 2004;134(1):104–111. doi: 10.1093/jn/134.1.104. [DOI] [PubMed] [Google Scholar]
- 45.Baron K.G., Reid K.J., Horn L.V., Zee P.C. Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite. 2013;60(1):246–251. doi: 10.1016/j.appet.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhee J.J., Sampson L., Cho E., Hughes M.D., Hu F.B., Willett W.C. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am J Epidemiol. 2015;181(4):225–233. doi: 10.1093/aje/kwu308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroke A., Bergmann M.M., Lotze G., Jeckel A., Klipstein-Grobusch K., Boeing H. Measures of quality control in the German component of the EPIC study. European prospective investigation into cancer and nutrition. Ann Nutr Metab. 1999;43(4):216–224. doi: 10.1159/000012788. [DOI] [PubMed] [Google Scholar]
- 48.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A., et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wharton S., Lau D.C.W., Vallis M., Sharma A.M., Biertho L., Campbell-Scherer D., et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–e891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R Core Team . R Foundation for Statistical Computing; 2021. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 51.Liljequist D., Elfving B., Roaldsen K.S. Intraclass correlation – A discussion and demonstration of basic features. PLOS ONE. 2019;14(7) doi: 10.1371/journal.pone.0219854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Light R.J. Measures of response agreement for qualitative data: some generalizations and alternatives. Psychol Bull. 1971;76(5):365–377. doi: 10.1037/h0031643. [DOI] [Google Scholar]
- 53.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 54.Foster E., Lee C., Imamura F., Hollidge S.E., Westgate K.L., Venables M.C., et al. Validity and reliability of an online self-report 24-h dietary recall method (Intake24): a doubly labelled water study and repeated-measures analysis. J Nutr Sci. 2019;8:e29. doi: 10.1017/jns.2019.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St George S.M., Van Horn M.L., Lawman H.G., Wilson D.K. Reliability of 24-hour dietary recalls as a measure of diet in African-American youth. J Acad Nutr Diet. 2016;116(10):1551–1559. doi: 10.1016/j.jand.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preis S.R., Spiegelman D., Zhao B.B., Moshfegh A., Baer D.J., Willett W.C. Application of a repeat-measure biomarker measurement error model to 2 validation studies: examination of the effect of within-person variation in biomarker measurements. Am J Epidemiol. 2011;173(6):683–694. doi: 10.1093/aje/kwq415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mertens E., Kuijsten A., Geleijnse J.M., Boshuizen H.C., Feskens E.J.M., Van’t Veer P. FFQ versus repeated 24-h recalls for estimating diet-related environmental impact. Nutr J. 2019;18(1):2. doi: 10.1186/s12937-018-0425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKenzie B.L., Coyle D.H., Santos J.A., Burrows T., Rosewarne E., Peters S.A.E., et al. Investigating sex differences in the accuracy of dietary assessment methods to measure energy intake in adults: a systematic review and meta-analysis. Am J Clin Nutr. 2021;113(5):1241–1255. doi: 10.1093/ajcn/nqaa370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McHill A.W., Hilditch C.J., Fischer D., Czeisler C.A., Garaulet M., Scheer F., et al. Stability of the timing of food intake at daily and monthly timescales in young adults. Sci Rep. 2020;10(1):20849. doi: 10.1038/s41598-020-77851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wehling H., Lusher J J. People with a body mass index ⩾30 under-report their dietary intake: A systematic review. J Health Psychol. 2019;24(14):2042–2059. doi: 10.1177/1359105317714318. [DOI] [PubMed] [Google Scholar]
- 61.Heitmann B.L., Lissner L. Dietary underreporting by obese individuals—is it specific or non-specific? BMJ (Clin Res Ed) 1995;311(7011):986–989. doi: 10.1136/bmj.311.7011.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cowan A.E., Higgins K.A., Fisher J.O., Tripicchio G.L., Mattes R.D., Zou P., et al. Examination of different definitions of snacking frequency and associations with weight status among U.S. adults. PLOS ONE. 2020;15(6) doi: 10.1371/journal.pone.0234355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Castro JM J.M. Weekly rhythms of spontaneous nutrient intake and meal pattern of humans. Physiol Behav. 1991;50(4):729–738. doi: 10.1016/0031-9384(91)90010-l. [DOI] [PubMed] [Google Scholar]
- 64.Haines P.S., Hama M.Y., Guilkey D.K., Popkin B.M. Weekend eating in the United States is linked with greater energy, fat, and alcohol intake. Obes Res. 2003;11(8):945–949. doi: 10.1038/oby.2003.130. [DOI] [PubMed] [Google Scholar]
- 65.Jula A., Seppänen R., Alanen E. Influence of days of the week on reported food, macronutrient and alcohol intake among an adult population in south western Finland. Eur J Clin Nutr. 1999;53(10):808–812. doi: 10.1038/sj.ejcn.1600853. [DOI] [PubMed] [Google Scholar]
- 66.Gupta C.C., Coates A.M., Dorrian J., Banks S. The factors influencing the eating behaviour of shiftworkers: what, when, where and why. Ind Health. 2019;57(4):419–453. doi: 10.2486/indhealth.2018-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.James D.C. Factors influencing food choices, dietary intake, and nutrition-related attitudes among African Americans: application of a culturally sensitive model. Ethn Health. 2004;9(4):349–367. doi: 10.1080/1355785042000285375. [DOI] [PubMed] [Google Scholar]
- 68.Deliens T., Clarys P., De Bourdeaudhuij I., Deforche B. Determinants of eating behaviour in university students: a qualitative study using focus group discussions. BMC Public Health. 2014;14:53. doi: 10.1186/1471-2458-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vidmar A.P., Jones R.B., Wee C.P., Berger P.K., Plows J.F., Claudia Rios R.D., et al. Timing of food consumption in Hispanic adolescents with obesity. Pediatr Obes. 2021;16(7) doi: 10.1111/ijpo.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gill S., Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartman T.J., Masters M., Flanders W.D., Wang Y., Li M., Mitchell D.C., Guinter M., Patel A.V. Self-reported eating-occasion frequency and timing are reproducible and relatively valid in the American Cancer Society Cancer Prevention Study-3 Diet Assessment Substudy. J Nutr. 2023;152(12):2827–2836. doi: 10.1093/jn/nxac206. [DOI] [PubMed] [Google Scholar]
- 72.Maki K.C., Slavin J.L., Rains T.M., Kris-Etherton P.M. Limitations of observational evidence: implications for evidence-based dietary recommendations. Adv Nutr. 2014;5(1):7–15. doi: 10.3945/an.113.004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White E., Armstrong B.K., Saracci R. In: Principles of Exposure Measurement in Epidemiology: Collecting, Evaluating, and Improving Measures of Disease Risk Factors. 2nd Ed. White E., Armstrong B.K., Saracci R., editors. Oxford University Press; Oxford: 2008. Exposure measurement error and its effects. Chapter 3. [Google Scholar]
- 74.Bennett D.A., Landry D., Little J., Minelli C. Systematic review of statistical approaches to quantify, or correct for, measurement error in a continuous exposure in nutritional epidemiology. BMC Med Res Methodol. 2017;17(1):146. doi: 10.1186/s12874-017-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Klerk N.H., English D.R., Armstrong B.K. A review of the effects of random measurement error on relative risk estimates in epidemiological studies. Int J Epidemiol. 1989;18(3):705–712. doi: 10.1093/ije/18.3.705. [DOI] [PubMed] [Google Scholar]
- 76.Shams-White M., O’Connor L., O’Connor S., Miller A., Mittl B., Nicholson T., et al. Development of a sleep assessment module in the automated self-administered 24-hour (ASA24) dietary assessment tool: new research opportunities. Curr Dev Nutr. 2021;5(suppl 2):474. doi: 10.1093/cdn/nzab039_010. [DOI] [Google Scholar]
- 77.Dashti H.S., Scheer F.A., Jacques P.F., Lamon-Fava S., Ordovás J.M. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6(6):648–659. doi: 10.3945/an.115.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smiley A., King D., Bidulescu A. The association between sleep duration and metabolic syndrome: the NHANES 2013/2014. Nutrients. 2019;11(11) doi: 10.3390/nu11112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stone K.L., Xiao Q. Impact of poor sleep on physical and mental health in older women. Sleep Med Clin. 2018;13(3):457–465. doi: 10.1016/j.jsmc.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hill R.J., Davies P.S. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr. 2001;85(4):415–430. doi: 10.1079/bjn2000281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, codebook, and analytic code will not be made available because the data supporting this study's findings are not publicly available. In accordance with the German Federal and State data protection regulations, epidemiological data analyses of The European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam may be initiated upon an information inquiry addressed to the PI of the EPIC-Potsdam study, who is a co-author of this manuscript (MBS). Data from the Interactive Diet and Activity Tracking in AARP Study may be available from the NCI Cancer Data Access System upon request.