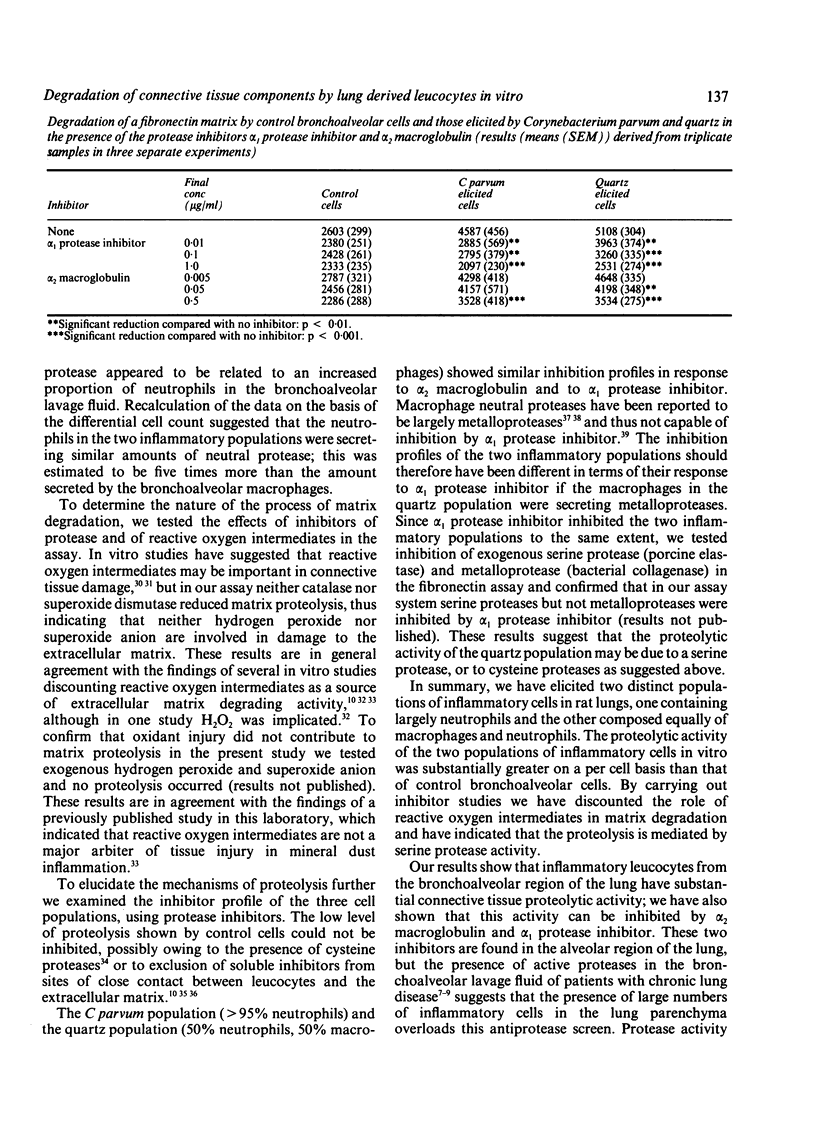
Abstract
Inflammatory leucocytes are implicated in connective tissue damage during chronic inflammatory lung disease. In an investigation of the role of leucocytes in connective tissue derangements in the lung, inflammatory leucocytes were generated in rat lungs by intratracheal instillation of inflammatory agents and retrieved by bronchoalveolar lavage. The proteolytic activities of control macrophages and of two inflammatory cell populations were compared; iodinated collagen, laminin, and fibronectin matrices were used. The inflammatory cells caused consistently and substantially more degradation of the matrices than the controls on a per cell basis. The oxidant scavengers superoxide dismutase and catalase did not inhibit matrix degradation, but alpha 1 protease inhibitor and alpha 2 macroglobulin were inhibitory. It is concluded that matrix damage in this assay is enhanced by inflammatory cells and is mediated principally by serine protease activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L. Comparison of live human neutrophil and alveolar macrophage elastolytic activity in vitro. Relative resistance of macrophage elastolytic activity to serum and alveolar proteinase inhibitors. J Clin Invest. 1984 Nov;74(5):1693–1700. doi: 10.1172/JCI111586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christner P., Fein A., Goldberg S., Lippmann M., Abrams W., Weinbaum G. Collagenase in the lower respiratory tract of patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1985 May;131(5):690–695. doi: 10.1164/arrd.1985.131.5.690. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Crystal R. G. Role of connective tissue proteases in the pathogenesis of chronic inflammatory lung disease. Environ Health Perspect. 1984 Apr;55:297–306. doi: 10.1289/ehp.8455297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek J. E., Kelman J. A., Fells G., Weinberger S. E., Horwitz A. L., Reynolds H. Y., Fulmer J. D., Crystal R. G. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979 Oct 4;301(14):737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Crystal R. G. The connective tissue of lung. Am Rev Respir Dis. 1975 Nov;112(5):657–711. doi: 10.1164/arrd.1975.112.5.657. [DOI] [PubMed] [Google Scholar]
- Holderbaum D., Ehrhart L. A. Substratum influence on collagen and fibronectin biosynthesis by arterial smooth muscle cells in vitro. J Cell Physiol. 1986 Feb;126(2):216–224. doi: 10.1002/jcp.1041260210. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Janoff A., White R., Carp H., Harel S., Dearing R., Lee D. Lung injury induced by leukocytic proteases. Am J Pathol. 1979 Oct;97(1):111–136. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Varani J. Substrate hydrolysis by immune complex-activated neutrophils: effect of physical presentation of complexes and protease inhibitors. J Immunol. 1981 Nov;127(5):1875–1879. [PubMed] [Google Scholar]
- Jones P. A., Werb Z. Degradation of connective tissue matrices by macrophages. II. Influence of matrix composition on proteolysis of glycoproteins, elastin, and collagen by macrophages in culture. J Exp Med. 1980 Dec 1;152(6):1527–1536. doi: 10.1084/jem.152.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh B. A., Crystal R. G. Alveolitis: the key to the interstitial lung disorders. Thorax. 1982 Jan;37(1):1–10. doi: 10.1136/thx.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Ingbar D. H., Madri J. A. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res. 1986 Feb;162(2):423–435. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Kleinman H. K., Terranova V. P., Ledbetter S., Hassell J. R. The regulation of basement membrane formation and cell-matrix interactions by defined supramolecular complexes. Ciba Found Symp. 1984;108:197–212. doi: 10.1002/9780470720899.ch13. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Baum B. J., Rosenberg D. M., Kelman J. A., Brin S. C., Crystal R. G. Destruction of a major extracellular adhesive glycoprotein (fibronectin) of human fibroblasts by neutral proteases from polymorphonuclear leukocyte granules. Lab Invest. 1979 Mar;40(3):350–357. [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976 Jun 1;143(6):1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. J., Kerr J. S. Oxidant injury of the extracellular matrix: potential role in the pathogenesis of pulmonary emphysema. Lung. 1985;163(1):1–13. doi: 10.1007/BF02713801. [DOI] [PubMed] [Google Scholar]
- Shah S. V., Baricos W. H., Basci A. Degradation of human glomerular basement membrane by stimulated neutrophils. Activation of a metalloproteinase(s) by reactive oxygen metabolites. J Clin Invest. 1987 Jan;79(1):25–31. doi: 10.1172/JCI112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille Y., Lwebuga-Mukasa J. S., Polomski L., Merrill W. W., Ingbar D. H., Gee J. B. An in vitro model for polymorphonuclear-leukocyte-induced injury to an extracellular matrix. Relative contribution of oxidants and elastase to fibronectin release from amnionic membranes. Am Rev Respir Dis. 1986 Jul;134(1):134–140. doi: 10.1164/arrd.1986.134.1.134. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Lucey E. C., Stone P. J. Animal models of emphysema. Am Rev Respir Dis. 1986 Jan;133(1):149–169. doi: 10.1164/arrd.1986.133.1.149. [DOI] [PubMed] [Google Scholar]
- Turino G. M. The lung parenchyma--a dynamic matrix. J. Burns Amberson lecture. Am Rev Respir Dis. 1985 Dec;132(6):1324–1334. doi: 10.1164/arrd.1985.132.6.1324. [DOI] [PubMed] [Google Scholar]
- Vracko R. Significance of basal lamina for regeneration of injured lung. Virchows Arch A Pathol Pathol Anat. 1972;355(3):264–274. doi: 10.1007/BF00551062. [DOI] [PubMed] [Google Scholar]
- Weiland J. E., Davis W. B., Holter J. F., Mohammed J. R., Dorinsky P. M., Gadek J. E. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986 Feb;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]