Abstract
Intracerebral hemorrhage (ICH) is a neurological disease with high mortality and disability. Recent studies showed that white matter injury (WMI) plays an important role in motor dysfunction after ICH. WMI includes WMI proximal to the lesion and WMI distal to the lesion, such as corticospinal tract injury located at the cervical enlargement of the spinal cord after ICH. Previous studies have tended to focus only on gray matter (GM) injury after ICH, and fewer studies have paid attention to WMI, which may be one of the reasons for the poor outcome of previous drug treatments. Microglia and astrocyte-mediated neuroinflammation are significant mechanisms responsible for secondary WMI following ICH. The NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome activation, has been shown to exacerbate neuroinflammation and brain injury after ICH. Moreover, NLRP3 inflammasome is activated in microglia and astrocytes and exerts a vital role in microglia and astrocytes-mediated neuroinflammation. We speculate that NLRP3 inflammasome activation is closely related to the polarization of microglia and astrocytes and that NLRP3 inflammasome activation may exacerbate WMI by polarizing microglia and astrocytes to the pro-inflammatory phenotype after ICH, while NLRP3 inflammasome inhibition may attenuate WMI by polarizing microglia and astrocytes to the anti-inflammatory phenotype following ICH. Therefore, NLRP3 inflammasome may act as leveraged regulatory fulcrums for microglia and astrocytes polarization to modulate WMI and WM repair after ICH. This review summarized the possible mechanisms by which neuroinflammation mediated by NLRP3 inflammasome exacerbates secondary WMI after ICH and discussed the potential therapeutic targets.
Keywords: Intracerebral hemorrhage, secondary white matter injury, neuroinflammation, NLRP3 inflammasome, microglia, astrocyte
1. INTRODUCTION
Intracerebral hemorrhage (ICH) is a neurological disease caused by vascular rupture of the cerebral parenchyma, which can cause severe neurological dysfunction that is closely related to the damage of gray matter (GM) and white matter (WM) after ICH [1, 2]. White matter tracts are composed of axons and glial cells, and multiple white matter tracts intertwine to form networks and are responsible for signal transduction, enabling humans to participate in daily activities [3]. Some studies have shown that more than 77% of patients with ICH are accompanied by WM injury (WMI), and a better understanding of WMI and promoting WM repair may provide a new perspective for the treatment of ICH [4]. In fact, contralateral limb hemiplegia caused by the corticospinal tracts (CST) and corticonuclear tracts damage, hemidysesthesia caused by central thalamic radiations damage and hemianopia caused by optic radiation damage is the major sequelae resulting from WMI after ICH [5, 6]. Therefore, WMI is an important contributor to the neurological dysfunction after ICH, but most previous studies have paid more attention to GM injury than WMI, which may be one of the reasons for the ineffectiveness of drugs targeting damaged neurons [7]. Hence, reducing WMI or promoting WMI repair after ICH is particularly important for the neurological recovery of ICH patients.
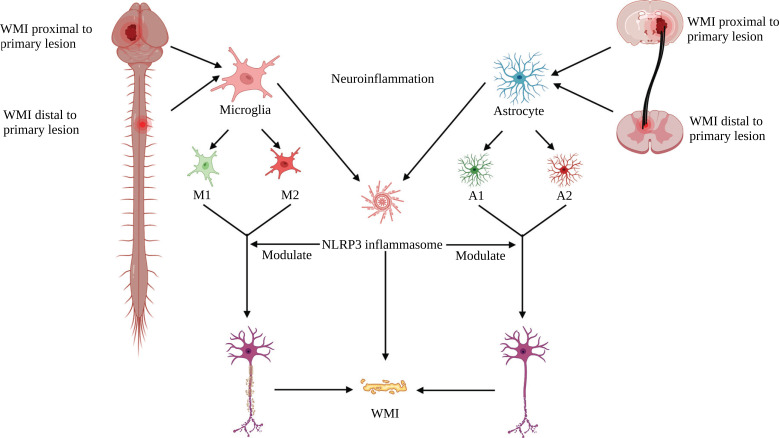
WMI is divided into primary and secondary injury after ICH. The mass effect and barotrauma due to hematoma can cause primary injury to WM [8]; the mass effect of the hematoma can be reduced after the evacuation of the hematoma. However, small hematoma fragments that cannot be surgically removed can cause secondary injury to WMI. And the pathophysiological mechanisms of secondary WMI after ICH are complicated, including neuroinflammation, oxidative stress, and neuroexcitoxiticty [8, 9]. Notably, neuroinflammation contributes to secondary injury in WMI [8, 9]. Unfortunately, these pathophysiological processes that lead to secondary WMI, such as neuroinflammation, cannot be surgically blocked. Therefore, the development of pharmacological treatments for secondary WMI is particularly important. Recently, the NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome, has been identified as a crucial player in neuroinflammation and is a pivotal contributor to the acceleration of pro-inflammatory cytokines secretion and subsequent inflammatory responses [10-13]. Most importantly, our recent study showed that selective NLRP3 inflammasome inhibitors attenuate CST injury after ICH [14], and CST is a type of WM projection fiber. This suggests that activation of NLRP3 inflammasome exerts an important role in WMI following ICH, and inhibition of NLRP3 inflammasome may be a promising therapeutic strategy to mitigate WMI [15]. Since WMI can cause multiple neurological deficits after ICH, there is an urgent need for new treatment strategies to reduce WMI and promote WM repair in ICH. This is particularly important for the recovery of motor function in patients with ICH. Thus, this review summarized the important role of NLRP3 inflammasome in secondary WMI and promising therapeutic strategies for targeting NLRP3 inflammasome for WMI after ICH (Fig. 1).
Fig. (1).
Schematic representation of NLRP3 inflammasome modulating secondary WMI after ICH. (Created with BioRender.com).
2. MAIN COMPONENTS AND FUNCTIONS OF WM
WM accounts for more than 40% of the total volume of adult brain tissue and plays an integral role in the distributed neural networks responsible for neurobehavioral management [16, 17], and the WM is composed mainly of myelinated axon tracts and supporting glial cells (including oligodendrocytes (OLs), astrocytes, and microglia). Axons are surrounded by multiple dense myelin membranes generated by OLs, which are mature [18]. The structural integrity of the myelin sheath isolates axons from each other and effectively prevents crosstalk between different signals, thus ensuring fast and efficient action potential signaling and protecting nerve fibers from damage [19].
WM fiber bundles can be divided into a projection, association, and commissural tracts [20]. The projection tracts are divided into two types of fibers: upward and downward, whose function is mainly responsible for transmitting neural signals between the cerebral cortex and subcortical structures. For instance, the corticospinal tract (CST) is the main pathway responsible for transmitting descending information from the cerebral cortex to the spinal cord. The association fibers establish connections between cortical areas within the ipsilateral hemisphere. Commissural tracts are responsible for communication between the left and right hemispheres. All these WM fiber bundles form complicated neural networks between different brain regions and are each responsible for different functions. Many studies have revealed WM-related brain functions, such as cognitive function, motor function, reading and practice abilities [21, 22].
3. ACTIVATION OF NLRP3 INFLAMMASOME AFTER ICH
Inflammasomes are oligomeric multiprotein complexes distributed in the cytoplasm that play a vital role in the innate immune response to central nervous system (CNS) disease [23, 24]. NLRP3 inflammasome is the most widely studied in CNS among all inflammasomes. The NLRP3 inflammasome complex consists of the NLRP3 scaffold, apoptosis-associated speckle-like protein (ASC) adaptor, and caspase-1 effector [25]. In recent years, NLRP3 inflammasome has been closely associated with the inflammatory response induced by ICH [26, 27]. And it has been shown that NLRP3 signaling expression is progressively upregulated in peri-hematoma tissue from 1 to 5 days after ICH [28]. Various upstream signals after ICH can stimulate the activation of the NLRP3 inflammasome, such as heme released from hemoglobin catabolism, which is an activating signal upstream of the NLRP3 inflammasome [29]. Moreover, there are other widely accepted upstream stimulatory signals of NLRP3 inflammasome such as activation of purinergic 2X7 receptor (P2X7R), K+ efflux, generation of reactive oxygen species (ROS), lysosomal disruption causing leakage of cathepsin B, the release of mitochondrial DNA or mitochondrial phospholipids cardiolipin and Ca2+ influx [30-33].
4. IMMUNE CELLS THAT MEDIATE NEUROINFLAMMATION ASSOCIATED WITH SECONDARY WMI AFTER ICH
The complicated immune cascade response is an important driving factor of brain injury after ICH, especially for secondary WMI [9, 34]. Multiple immune cells have been shown to participate and synergistically regulate the inflammatory response in the CNS after ICH. Herein, we introduce several immune cells associated with neuroinflammation-mediated secondary WMI after ICH (Figs. 2 and 3).
Fig. (2).
Schematic representation of the modulation of microglia phenotypic polarization by NLRP3 inflammasome after ICH and potential therapeutic strategies for targeting NLRP3 inflammasome for secondary WMI. (Created with BioRender.com).
Fig. (3).
Schematic representation of the modulation of astrocyte phenotypic polarization by NLRP3 inflammasome after ICH and potential therapeutic strategies for targeting NLRP3 inflammasome for secondary WMI. (Created with BioRender.com).
4.1. Microglia
Microglia are believed to be the first innate immune cells to respond to acute brain injury, including ICH [35]. Previous studies suggest that activated hyper-reactive microglia can release high levels of pro-inflammatory mediators (such as cytokines and chemokines) and cytotoxic mediators, leading to dysfunction and death of neurons [35-39]. Activated microglia can develop into two different states: pro-inflammatory phenotype (M1 phenotype) and anti-inflammatory phenotype (M2 phenotype), a process known as polarization [40, 41]. M1 microglia can secrete pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, which in turn impede axonal regeneration and OLs maturation [42-44], while M2 microglia can generate anti-inflammatory cytokines (such as IL-4 and IL-10) and growth factors to remove tissue debris via phagocytosis and facilitate remyelination [45-48]. And microglia have been shown to switch dynamically and temporarily between M1 and M2 phenotypes to respond to acute brain injury [49, 50]. It has been shown that fine-tuned M1/M2 polarization of microglia can decrease the detrimental effects of neuroinflammation in various neurological diseases such as ischemic brain injury, traumatic brain injury (TBI), and spinal cord injury (SCI) while boosting neuroprotective potential [50-52]. Crucially, it has been shown that activated microglia can polarize to pro-inflammatory phenotype and participate in the process of WMI after ICH and that with the use of P2X4R inhibitors, microglia are converted to an anti-inflammatory phenotype and attenuate WMI by promoting the production of brain-derived neurotrophic factor (BDNF) [53]. In addition, there is evidence that IL-33 can drive the transformation of microglia from the M1 phenotype to the M2 phenotype, thereby alleviating neuronal damage and WMI after ICH and improving neurological function [54]. Moreover, HDAC inhibitors and VK-28 have been shown to polarize microglia to M2 phenotype, thereby attenuating WMI following ICH [55, 56]. All of the above studies suggest that microglia-associated neuroinflammation after ICH is closely associated with WMI. Therefore, based on the prominent role of microglia in WMI after ICH, there is a pressing demand for therapeutic strategies to intervene in microglia to alleviate WMI after ICH. And the modulation of polarization of microglial phenotype could be one of the potential therapeutic strategies.
Notably, NLRP3 inflammasome has been shown to play a crucial role in microglia-associated neuroinflammation after ICH, and many studies have demonstrated that activation of NLRP3 inflammasome amplifies neuroinflammation and worsens neurological function following ICH [27, 35, 57, 58], and NLRP3 inflammasome is mainly expressed in microglia [12]. Most importantly, it has been shown that the selective NLRP3 inflammasome inhibitor MCC950 decreases microglia-associated pro-inflammatory cytokine secretion, increases microglia-associated anti-inflammatory cytokine production, and shifts the phenotype of microglia towards an anti-inflammatory state [10]. Furthermore, Chen et al. [59] demonstrated that mitoquinone (MitoQ) could reduce brain injury by inhibiting the NLRP3 inflammasome from polarizing microglia to the M2 phenotype after ICH. The above results suggest that NLRP3 inflammasome may be a leveraged regulatory fulcrum regulating microglia phenotypic transition after ICH (Fig. 2). Therefore, it is reasonable to speculate that activation of NLRP3 inflammasome after ICH may exacerbate WMI by promoting microglia polarization to the M1 phenotype, whereas when NLRP3 inflammasome is inhibited will accelerate the microglial transition of M1 phenotype to the M2 phenotype, thereby alleviating WMI. Unfortunately, however, direct evidence for attenuation of WMI after inhibition of NLRP3 inflammasome following ICH remains scarce at this time. Given the strong association between NLRP3 inflammasome and WMI mediated by neuroinflammation associated with microglia, we hypothesize that NLRP3 inflammasome may be a potential key target for mitigating further exacerbation of WMI mediated by neuroinflammation associated with microglia after ICH and promoting neurological recovery, but more experimental studies are still needed to confirm it, which is one of our future research directions.
4.2. Astrocyte
There is growing evidence that astrocytes are also closely associated with neuroinflammation in the CNS. Activated microglia can stimulate the activation of astrocytes, and astrocytes can secrete pro-inflammatory factors (such as IL-1β, IL-6) and anti-inflammatory cytokines, which regulate the activation and function of microglia [60, 61]. It has been shown that astrocytes accumulate around hematoma within 1 to 3 days after ICH [62]; hemoglobin can trigger oxidative stress in the brain parenchyma and induce the expression of matrix metalloproteinase-9 (MMP-9) in astrocytes, thereby damaging the BBB [63, 64]. When the activity of astrocytes is inhibited, it reduces hematoma volume and decreases BBB disruption [65]. These results suggest a close association between astrocytes and neuroinflammation after ICH. In addition, there is early evidence that various molecules expressed in astrocytes can inhibit axonal regeneration [66]. This shows that astrocytes and WM are also very closely related.
Interestingly, astrocytes are similar to microglia in terms of phenotype; some scholars believe that they can differentiate into A1 phenotype or A2 phenotype under specific conditions [67, 68]. A1 astrocytes can secrete pro-inflammatory factors and chemokines [69], leading to apoptosis of neurons and surrounding OLs [70]. It is known that OLs are responsible for participating in myelination and are an important component of WM in the CNS [71, 72]. Thus, A1 astrocytes are closely associated with WMI. In contrast, A2 astrocytes are considered to have a protective effect on the CNS because they accelerate the upregulation of neurotrophic factors. Reactive astrocytes promote CNS recovery and repair under ischemia-induced conditions [68, 73-75]. Therefore, phenotypic regulation of astrocytes may also be an important regulatory target to attenuate WMI and promote WM repair after ICH.
Notably, NLRP3 inflammasome is also expressed in astrocytes [76-80]. Lu et al. [80] demonstrated that deficiency of uncoupling protein 2 (UCP2) aggravates endoplasmic reticulum stress and cleavage of caspase-12 and exacerbates neuroinflammation by activating NLRP3 in astrocytes. Furthermore, it has been shown that in a model of chronic mild stress/depression, deficiency of mitochondrial UCP2 leads to enhanced oxidative, enhanced expression of the thioredoxin-interaction protein (TXNIP), and activation of NLRP3 inflammasome in astrocytes [81]. Most critically, Liu and his colleagues showed that adiponectin peptide (APNp) might inhibit the activation of NLRP3 inflammasome in astrocytes treated by oxygen-glucose deprivation and reintroduction (OGD-R) via the AMPK phosphorylation-dependent pathway [82]. The above studies suggest that astrocytes are closely related to neuroinflammation mediated by the NLRP3 inflammasome. Although fewer studies are related to NLRP3 inflammasome in astrocytes after ICH, some scholars have already started gradually conducting studies on NLRP3 in OGD-R-treated astrocytes. OGD-R can mimic cerebral ischemia-reperfusion injury in vitro, and both ischemic stroke and ICH can be categorized as stroke. Despite the pathophysiology of different types of strokes that may differ greatly, there may still be many commonalities. This suggests that NLRP3 inflammasome in astrocytes is of great potential research value in ICH. Therefore, NLRP3 inflammasome in astrocytes may also be one of the potential targets for the treatment of secondary WMI after ICH. In the future, if we can promote axonal regeneration by modulating the phenotype of astrocytes or altering the function of astrocytes through NLRP3 inflammasome, it will provide a new therapeutic idea for alleviating secondary WMI and promoting WM repair after ICH (Fig. 3).
4.3. T Lymphocytes
Previous studies have shown that T lymphocytes are also important players in neuroinflammation. In autologous blood or collagenase-induced ICH models, CD4+ T lymphocytes dominate the lymphocyte population, while CD8+ T lymphocytes constitute a very small number of infiltrating leukocyte populations [9]. It is generally believed that T lymphocytes rarely enter the brain parenchyma, and when the stroke occurs, T lymphocytes migrate in large numbers and infiltrate the lesion site after microglia activation [9, 83]. A recent study has shown that it is regulatory T lymphocytes (Tregs) in the brain that inhibit neurotoxic astrogliosis by generating epidermal growth factor receptor (EGFR) ligands, thus exerting a powerful neuronal protective effect in ischemic stroke [83]. This finding reveals that the function of T lymphocytes in the brain should also be given high priority during the repair of ICH processes. Moreover, Tregs-derived IL-10 can trigger a shift of hemoglobin-activated microglia/macrophages to the M2 phenotype. Similarly, it has been shown that Tregs regulate microglia/macrophage polarization via the IL-10/GSK3β/ PTEN axis, thereby protecting against ICH-induced inflammatory injury, which may play a pivotal role in Tregs-induced microglia polarization [84]. Tregs also altered microglia polarization, downregulated the expression of MHC-II, IL-6 and TNF-α, and upregulated the expression of CD206 when co-cultured in vitro [9]. Most importantly, Sen et al. [85] found that T lymphocyte infiltration exacerbates WMI after TBI. This shows that T lymphocytes and secondary WMI are closely related. Interestingly, both Th17 and Tregs have effects similar to microglia's M1/M2 phenotype. Th17 cells can cause autoimmunity and inflammation, while Treg cells can suppress these phenomena and maintain immune homeostasis [86]. Therefore, the modulation of Th17/Treg cells balance also appears to be crucial for secondary WMI and could be one of the key research directions in the future.
5. CELLS ASSOCIATED WITH WMI REPAIR
5.1. Oligodendrocytes (OLs)
OLs are a vital component of the WM, accounting for approximately 75% of subcortical WM glial cells [87]. OLs are susceptible to damage by neuroinflammation, oxidative stress, excitotoxicity and apoptotic pathways [88, 89]. OLs are reduced, and microglia are activated after ICH. Microglia promote myelin regeneration by removing damaged cells and damaged myelin sheaths [90]. In this process, microglia are converted from M1 phenotype to M2 phenotype [90]. M1 microglia can kill oligodendrocyte precursor cells (OPCs) via the TLR4 signaling pathway, whereas the M2 microglia have different functions [15]. Han et al. [91] showed that rosiglitazone improved the long-term integrity of WMI after stroke, at least in part by promoting the formation of OLs and facilitating the differentiation of microglia to the M2 phenotype. In addition, evidence showed that neural stem cells (NSC) or neural progenitor cells (NPC) in the subventricular zone (SVZ) provide newly formed oligodendrocytes to the damaged region of the WM when demyelination occurs [92]. They also demonstrated that focal demyelination in the corpus callosum caused microglia activation not only at the site of demyelination but also in the SVZ and significantly increased the production of OPCs in the SVZ [92]. This situation is obviously beneficial to the repair of WMI. In a word, OLs play an indispensable role in the repair process after WMI.
5.2. Microvascular Endothelial Cells
The nutrients provided by the blood are essential for the repair of the damaged WM. The expression of vascular endothelial growth factors (VEGF) receptors such as Flt-1 and Flk-1 are upregulated after ICH during the body's repair process, which implies the formation of neovascularization [15]. There is evidence of increased expression of VEGF, Flt-1 and Flk-1 in endothelial cells in the basal ganglia at the hemorrhagic region [93]. This suggests that regulating the expression of VEGF and related receptors could be a potential research direction to promote microvascular regeneration and facilitate WMI repair. Moreover, it has been shown that OPCs can induce excessive postnatal WM angiogenesis in vivo, directly stimulate endothelial cell proliferation in vitro, and promote vascular regeneration and coordinate myelin formation to ensure axonal and WM integrity [94]. Furthermore, in addition to supplying nutrients, microvascular endothelial cells play an important role in the phagocytosis of myelin debris. It is well known that removing damaged myelin is crucial to ensure functional recovery after neural injury. A recent study showed that endothelial cells could exert crucial functions beyond myelin clearance by modulating macrophage infiltration, pathological angiogenesis and fibrosis in SCI and experimental autoimmune encephalomyelitis (EAE) [95]. Chen et al. [96] found that knock-in miR-126 in primary cultured brain endothelial cells (BECs) significantly increased the capillary-like tube formation and axonal outgrowth in primary cultured cortical neurons. The above studies suggest that microvascular endothelial cells are essential for the repair of WMI.
6. ASSOCIATION OF NLRP3 INFLAMMASOME WITH SECONDARY WMI FOLLOWING ICH
6.1. Role of NLRP3 Inflammasome in Secondary WMI Proximal to the Primary Lesion after ICH
It is well known that blood vessels are distributed all over the CNS so that ICH may occur in all areas of the brain. However, different brain regions have different neurological functions, so the symptoms and severity after ICH are closely related to the location of the hemorrhage. In human cases of ICH, the putamen and the thalamus are particularly prone to hemorrhage [2]. It is worth noting that the symptoms and severity of hemorrhage in the putamen and thalamus appear to be associated with the severity of the internal capsule [97-99]. The internal capsule is a white matter area made up of several groups of fiber tracts that connect the cerebral cortex and the lower brain region. These fiber tracts are located between the putamen and the thalamus and are responsible for transmitting ascending (sensory) and descending (motor) information. When there is hemorrhage in the putamen or thalamus, the hematoma usually expands at the initial bleeding site, thus invading the internal capsule, damaging the axonal tracts near the original bleeding site, interrupting the transmission of information between the upper brain regions and the lower brain areas, leading to neurological dysfunction [1, 97-99]. Wasserman and Schlichter [7] observed axonal injury without demyelination at the edge of hematoma and axonal injury accompanied by demyelination inside and at the edge of hematoma in collagenase-induced ICH model in rats. Hijioka et al. [100] found that corticospinal axons were fragmented following ICH induced by local injection of collagenase into the internal capsule. These facts suggest that protecting axonal fiber tracts may be a potential strategy to effectively alleviate the neurological symptoms associated with ICH.
Interestingly, the accumulated evidence suggests that the expression of NLRP3 signaling in perihematomal tissue increases gradually from 1 to 5 days after ICH [28]. The activation of NLRP3 inflammasome amplifies the inflammatory response and deteriorates neurological function after ICH [27]. Hemorrhage in areas of denser WM trajection, such as the internal capsule, makes the WM in this region highly vulnerable to compression of the hematoma and secondary inflammatory injury so that activation of NLRP3 inflammasome around the hematoma inevitably will cause damage to the WM. Chen and his colleagues [101] found that mitochondrial ROS are involved in WMI proximal to the lesion after ICH in mice and that the clearance of mitochondrial ROS alleviates WMI and Chen et al. [59] also demonstrated that selective mitochondrial ROS scavengers can reduce brain injury by inhibiting NLRP3 inflammasome. This evidence is sufficient to speculate that the ROS/NLRP3 pathway may be involved in WMI proximal to the lesion after ICH. In addition, there is evidence that curcumin suppresses microglia/macrophage pyroptosis through inhibition of NF-κB and NLRP3 inflammasome and attenuates WMI after ischemic stroke [102]. This also suggests the involvement of NLRP3 inflammasome in post-stroke WMI. Moreover, it has been shown that inhibition of P2X purinoreceptor 4 (P2X4R) can be directly involved in the proximal WMI of the primary lesion by converting microglia to a proinflammatory phenotype after ICH [53]. Furthermore, P2X7R, a member of the same family as P2X4R, has been shown to activate NLRP3 inflammasome after ICH, and inhibition of P2X7R alleviates NLRP3 inflammasome-mediated neuroinflammation, thereby reducing brain injury after ICH [26, 103]. Since P2X4R and P2X7R are members of the P2X purine receptor family, they might have the same structure and motif or form heteromers with each other [104], there may be some degree of similarity in the pathophysiological role of them following ICH. Therefore, given that inhibition of P2X7R after ICH can suppress NLRP3 inflammasome to attenuate brain injury and that inhibition of P2X4R can attenuate WMI proximal to the primary lesion, members of the P2X purine receptor family may be an important target for the treatment of NLRP3 inflammasome-mediated WMI after ICH, but more studies are needed to confirm this. In summary, since the tissues studied above were all proximal to the primary lesion, we speculate that activation of NLRP3 inflammasome after ICH may directly cause damage to WM proximal to the primary lesion.
6.2. Relationship between NLRP3 Inflammasome and Secondary WMI Distal to the Primary Lesion after ICH
Interestingly, axons would experience degeneration and loss after injury in the original site, and the spread of the degeneration of these damaged neurons can occur in distal brain regions, which are anatomically connected to the proximal part of the initial infarction by axonal projection [105]. This view is supported in the results of studies on ICH; in addition to the proximal WMI of the primary lesion, the distal axons away from the hematoma are also damaged after ICH. It has been shown that significant corticospinal tract (CST) demyelination and axonal degeneration were observed in the cervical spinal cord in the mice model of ICH [3], but the exact mechanism has not been elucidated. Indeed, it is not uncommon for axonal injury proximal to the primary lesion to be followed by distal axonal injury, and this transaxonal injury may be closely associated with the NLRP3 inflammasome. For example, our recent study has shown that selective NLRP3 inflammasome inhibitors can alleviate CST injury located in cervical enlargement of the spinal cord distal to the hematoma and attenuate neurobehavioral deficits following ICH [14]. Zhang et al. [106] also supported this view by establishing a mouse model of unilateral optic nerve crush (ONC). Their results showed that the expression of NLRP3 in the primary visual cortex (V1) was significantly increased. The NLRP3 inflammasome was activated in the contralateral visual cortex (V1) from 1 to 14 days after ONC. Nevertheless, trans-neuronal degeneration was significantly alleviated within 14 days after the NLRP3 gene was knocked out, and the visual electrophysiological function was effectively improved [106]. Moreover, Li and his colleagues [107] demonstrated that indirect traumatic optic neuropathy (ITON) could promote JNK/c-jun signaling, which further activates the NLRP3 inflammasome in microglia and promotes axonal degeneration and retinal ganglion cells death. Furthermore, another study showed that after SCI, neuronal pyroptosis lasts longer and occurs farther away from the center of injury compared to neuronal apoptosis, and this neuronal pyroptosis is mediated by NLRP3 inflammasome [108].
Given the above results, we speculate that the NLRP3 inflammasome may mediate the CST injury in the cervical spinal cord after ICH. Notably, this distal axonal injury does not seem to occur immediately after the damage to the proximal axons of the lesion but takes some time. The mechanism may be that some inflammatory factors or NLRP3 inflammasome in the proximal part of the lesion are transported through the axon to the distal part, thus causing damage to the distal axon or the activation of NLRP3 inflammasome in situ in the distal part of the lesion may occur. No study has yet elucidated the exact mechanism of this injury. Regardless of the mechanism of injury, this suggests that NLPR3 inflammasome and WMI distal to the primary lesion are closely related after ICH. In future studies, it needs to be further explored whether NLRP3 is activated by trans-synaptic or trans-axonal activation to elucidate the mechanism of distal axonal injury after ICH. Together, NLRP3 inflammasome may be a promising therapeutic target to protect the distal axonal injury of the lesion following ICH.
7. NLRP3 INFLAMMASOME-MEDIATED POTENTIAL MECHANISMS THAT ARE ASSOCIATED WITH SECONDARY WMI AFTER ICH
7.1. P2X Receptor Family Members and Mitochondrial ROS-mediated Activation of NLRP3 Inflammasome
There is increasing evidence that some P2X receptor family members are involved in brain injury, including WMI after ICH. P2X4R is directly involved in WMI after ICH by polarizing microglia to a pro-inflammatory phenotype, and inhibition of P2X4R can polarize pro-inflammatory microglia to anti-inflammatory microglia, promote BDNF production, and it can attenuate WMI and improve neurological function via the BDNF/TrkB pathway [53]. And it has been shown that P2X7R, another member of the P2X receptor family, is a key regulator of NLRP3 inflammasome activation [109]. P2X7R is located upstream of the NLRP3 inflammasome, directly interacts with NLRP3 inflammasome scaffold protein, and is responsible for NLRP3 recruitment and activation [110]. In the case of ICH, levels of both P2X7R and NLRP3 inflammasome components were significantly elevated and peaked on 1 day after ICH, and the P2X7R/NLRP3 inflammasome axis participates in neuroinflammation and brain injury after ICH [26]. Moreover, Chen et al. [101] showed that mitochondrial ROS are involved in WMI after ICH, and that clearance of mitochondrial ROS attenuates WMI. At the same time, Chen and his colleagues [59] demonstrated that selective mitochondrial ROS scavengers could reduce brain injury by repressing the NLRP3 inflammasome to promote microglia polarization toward the M2 phenotype. We can therefore speculate that the mitochondrial ROS/NLRP3 inflammasome signaling pathway may also be an inflammatory pathway that promotes secondary WMI after ICH. Thus, activation of NLRP3 inflammasome mediated by some P2X receptor family members and mitochondrial ROS is closely related to post-ICH secondary WMI.
7.2. GSK-3β is Involved in Regulating NLRP3 Inflammasome
In recent years, it has been shown that glycogen synthase kinase 3β (GSK-3β) was shown to be associated with WMI after ICH, and it can regulate the NLRP3 inflammasome. Li et al. [111] intervened in the mouse model of ICH induced by autologous blood with lithium chloride. The results showed that glycogen synthase kinase-3β (GSK-3β) was inactivated. The expression of brain-derived neurotrophic factor (BDNF), located in nerve fibers, was up-regulated, thereby producing a protective effect on the damaged white matter after ICH. Several studies support the view that inhibition of GSK-3β can increase the expression of BDNF [111]. A previous study has shown that GSK-3β could be inhibited by suppressing the activation of the NLRP3 inflammasome, thus effectively attenuating lupus nephritis [112]. Another study of cerebral ischemia/reperfusion injury also showed that inhibition of GSK-3β attenuated cerebral ischemia/reperfusion injury; it may achieve by inhibiting the activation of NLRP3 inflammasome through autophagy [113]. Moreover, Liu et al. [82] demonstrated that adiponectin peptide could attenuate oxidative stress and activation of NLRP3 inflammasome after cerebral ischemia-reperfusion injury by modulating the AMPK/GSK-3β pathway. Therefore, it is reasonable to speculate that GSK-3β is inhibited after the NLRP3 inflammasome is suppressed and then upregulates the expression of BDNF, thereby protecting the brain's white matter and promoting nerve repair. Furthermore, it is reported that the reduction of BDNF after sleep deprivation (SD) requires the activation of the NLRP3 inflammasome. They suggest the downregulation of BDNF by activating NLRP3 inflammasome in astrocytes is a crucial pathological process of depression-like behavior induced by SD [114]. Hence, we hypothesize that NLRP3 inflammasome may not only indirectly affect the expression of BDNF, similar to the above study but also directly affect the production of BDNF. Unfortunately, there is no direct evidence to show that the expression of BDNF increases after the inhibition of NLRP3 inflammasome. Only Ward et al. [115] showed that selective NLRP3 inflammasome inhibitor MCC950 improved cognitive function and vascular integrity after stroke in diabetic animals and avoided hypoxia-induced reduction of BDNF secretion. Likewise, the findings of Fu et al. [116] also supported this point of view. They applied the selective NLRP3 inflammasome inhibitor MCC950 to perioperative aged mice, and the results showed that the inhibitor could inhibit the increase of NLRP3, apoptosis-associated speck-like protein (ASC), and caspase-1 and counteract the decrease of BDNF expression, thereby improving the neurocognitive impairment of perioperative aged mice. It is reported that although BDNF has a good protective effect on WM, its poor pharmacokinetics of BDNF and rapid half-life in blood circulation make it difficult for clinical application [117, 118]. In any case, it may be a great therapeutic strategy for white matter damage by enhancing the endogenous expression of BDNF with drugs after ICH. Consequently, the direct and indirect effects of NLRP3 inflammasome on BDNF need to be further investigated to promote clinical transformation. Of course, some researchers hold different views on the upstream and downstream relationship between the NLRP3 inflammasome and GSK-3β. They believe that GSK-3β is first inhibited, NLRP3 inflammasome is next suppressed, and then neuroinflammation is alleviated, rather than after NLRP3 inflammasome is suppressed, GSK-3β is inhibited [119-121]. No matter what their upstream and downstream relationship is, the final results are beneficial to the body. But more evidence is needed to clarify the relationship. Moreover, Li et al. [122] found that GSK-3β inhibitor lithium chloride can accelerate hematoma resolution by promoting microglia phagocytosis and M2-phenotype differentiation in the early stage of ICH. In the chronic phase of ICH, it can promote angiogenesis and neurogenesis and play a neuroprotective role. This echoes what we mentioned earlier about microglia polarization and provides data support for our hypothesis. In a word, both expressions of GSK-3β and BDNF can be regulated by NLRP3 inflammasome, thus affecting brain function. Therefore, the inhibition of NLRP3 inflammasome may be a potential therapeutic strategy to protect axonal fiber tracts and reduce neurological dysfunction after ICH, but the existing evidence is insufficient and still needs to be further explored.
7.3. Other Signaling Pathways Associated with NLRP3 Inflammasome
In addition to the above signaling pathways, the NFκB/ NLRP3 inflammasome axis has also been shown to be involved in WMI in ischemic stroke, and when NFκB/NLRP3 is inhibited, WMI caused by ischemic stroke is alleviated [102]. Moreover, there is evidence that activation of the Drp1-HK1-NLRP3 signaling axis is a key mechanism and therapeutic target for WM degeneration of Alzheimer's disease (AD) [123]. Furthermore, Shao et al. [124] demonstrated that transient receptor potential melastatin 2 (TRPM2) is involved in neuroinflammation and cognitive dysfunction via NLRP3 inflammasome in a cuprizone-induced model of multiple sclerosis (MS), and MS is a CNS disease characterized by demyelination and axonal injury, so the TRPM2/NLRP3 inflammasome axis may also be a potential inflammatory signaling pathway involved in secondary WMI after ICH.
Although the mechanisms mentioned above are not post-ICH mechanisms of secondary WMI, there may be similar mechanisms of WMI in different CNS diseases, especially the role of NFκB/NLRP3 inflammasome signaling pathway on secondary WMI in ischemic stroke has been verified, and both ischemic stroke and ICH can be categorized as stroke. Therefore, the above inflammatory pathways may be closely related to secondary WMI caused by ICH and deserve further investigation.
8. TARGETING NLRP3 IN THE PATHOGENESIS OF SECONDARY WMI AFTER ICH
There is growing evidence that NLRP3 inflammasome is strongly correlated with secondary WMI after ICH, and many researchers have developed many new drugs targeting NLRP3 inflammasome. Herein, we summarize some potential drugs targeting NLRP3 for secondary WMI after ICH (Table 1, Figs. 2 and 3).
Table 1.
Potential therapeutic strategies of WMI after ICH based on NLRP3 inflammasome inhibition.
| Potential Inhibitor of NLRP3 | Mechanism of Action | References | |
|---|---|---|---|
| Compounds targeting specific pathways |
BBG | Inhibiting P2X7R/NLRP3 axis. | [26] |
| H2S | Attenuating NLRP3 inflammasome-mediated neuroinflammation by inhibiting P2X7R. | [103] | |
| 5-BDBD | Alleviating WMI after ICH by M2 microglial polarization and increasing BDNF. | [53] | |
| MitoQ | Ameliorating WMI after ICH by suppressing mitochondrial ROS. Inhibiting the NLRP3 inflammasome to promote microglia polarization to the M2 phenotype. |
[59, 101] | |
| Lithium | GSK-3β-mediated microglia phagocytosis and M2 phenotypic differentiation. Attenuating WMI following ICH through BDNF signaling. |
[111, 128] | |
| APNp | Suppressing NLRP3 inflammasome activation in astrocytes via the AMPK/GSK-3β pathway. | [82] | |
| Scriptaid | Alleviating WMI following ICH by inhibiting HDAC through modulating microglial polarization. |
[55, 129] | |
| Curcumin | Attenuating WMI caused by stroke through inhibiting NF-κB/NLRP3 inflammasome axis. | [102] | |
| Small-molecule inhibitor |
MCC950 | Blocking ASC oligomerization, Suppressing of canonical and non-canonical NLRP3 inflammasome. |
[10, 115, 116] |
| OLT1177 | Promoting myelin preservation by inhibiting NLRP3 inflammasome. | [132-135] | |
| ZJU-37 | Promoting myelination by suppressing NLRP3 inflammasome. | [136] | |
| Novel materials and cell therapy | PHBV/PLA/Col membrane | Reducing glial scar formation and promoting axonal regeneration by suppressing NLRP3 inflammasome activation and M1 macrophage polarization. |
[139] |
| CeNP | Attenuate WMI after ICH, and the mechanisms may be related to the involvement of microglia and astrocytes in myelin regeneration. |
[71] | |
| Schwann cells | Inhibiting NLRP3 and NLRP1 inflammasome activation and promoting remyelination. | [140] | |
| hOPCs | Inhibiting NLRP3 inflammasome combined with ZJU-37 treatment. | [136] | |
| Knock-out of certain genes | Drp1 | Abolishing NLRP3 inflammasome activation and correcting myelin loss. | [123] |
| TRPM2 | Protecting from cuprizone-induced demyelination, activation of microglia, and NLRP3 inflammasome activation. |
[124] | |
| UCP2 | Activating NLRP3 inflammasome in astrocytes. | [80, 81] | |
| Other compounds | MLT | Inhibiting NLRP3-induced apoptosis in OLs. | [141] |
| 17β-Estradiol | Reducing demyelination and promoting myelin regeneration by promoting M2 microglia polarization and inhibiting NLRP3 inflammasome. |
[142] | |
| Sinomenine | Alleviating demyelination and axonal injury by inhibiting NLRP3 inflammasome. | [143] | |
| VK-28 | Attenuating WMI and promoting microglial polarization to the M2 phenotype. | [144] | |
| IL-33 | Ameliorating WMI after ICH by promoting microglial polarization to the M2 phenotype. | [54] | |
| Deferoxamine | Attenuating WMI. | [147] | |
| Minocycline | Attenuating WMI. | [148] | |
Note: NLRP3, the NOD-like receptor family, pyrin domain containing 3; WMI, white matter injury; MitoQ, mitoquinone; BDNF, brain-derived neurotrophic factor; ASC, apoptosis-associated speck-like protein; interleukin-33; IL-18, interleukin-33; NF-κB, nuclear factor kappa B; P2X7R, purinergic 2X7 receptor; P2X4R, purinergic 2X4 receptor; ROS, reactive oxygen species; GSK-3β, glycogen synthase kinase 3β; HDAC, histone deacetylase; PHBV/PLA/Col, poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/collagen; CeNP, ceria nanoparticle; Drp1, dynamin-related protein 1; TRPM2, transient receptor potential melastatin 2; MLT, melatonin; APNp, adiponectin peptide; BBG, blue brilliant G; UCP2, uncoupling protein 2; hOPCs, human neural stem cells.
8.1. Compounds Targeting Specific Pathways
As mentioned previously, P2X4R and P2X7R, members of the P2X receptor family, may be involved in NLRP3 inflammasome-mediated WMI after ICH, and therefore blocking them may be a therapeutic strategy for WMI. The selective P2X7R inhibitor blue brilliant G (BBG) has been shown to reduce neuroinflammation by inhibiting the P2X7R/ NLRP3 axis after ICH [26]. In addition, Zhao et al. [103] demonstrated that endogenous hydrogen sulphide (H2S) attenuated NLRP3 inflammasome-mediated neuroinflammation and alleviated brain injury by inhibiting P2X7R in rats after ICH. Moreover, the P2X4R antagonist 5-BDBD attenuated WMI after ICH by promoting microglia polarization to the M2 phenotype and increasing BDNF production [53]. This suggests that inhibitors of P2X receptor family members have great potential value for ameliorating neuroinflammation-mediated, especially NLRP3 inflammasome-mediated, WMI after ICH and deserve further development and investigation. However, it is worth mentioning that the application of P2X7R inhibitors is controversial because these receptors are located in different types of cells under pathological conditions and may result in undesirable off-target effects [125].
In addition, it has also been mentioned previously that the ROS/NLRP3 inflammasome axis exerts a vital role in WMI after ICH, and therefore ROS scavengers could be candidates for attenuating WMI after ICH. For example, Chen et al. [59] found that the selective ROS scavenger MitoQ could reduce brain injury by inhibiting the NLRP3 inflammasome from promoting microglia polarization to the M2 phenotype. And MitoQ also can attenuate WMI and improve neurological function by suppressing mitochondrial ROS after ICH [53]. Moreover, lithium is an inorganic salt used for many years as a treatment for bipolar disorder [126, 127]. However, in recent years, it has been shown that lithium treatment can promote hematoma resolution after ICH through GSK-3β-mediated microglia phagocytosis and M2 phenotypic differentiation, angiogenesis, and neurogenesis in rats [115]. Most importantly, lithium treatment can counteract the deleterious effects of bipolar disorder on WM by repressing GSK-3β [128], and it can also attenuate WMI after ICH in mice through BDNF signaling [111]. Furthermore, Liu and his colleagues found that adiponectin peptide (APNp) can inhibit NLRP3 inflammasome activation in astrocytes via the AMPK/GSK-3β pathway [82]. Scriptaid is a histone deacetylase (HDAC) inhibitor. Yang et al. [55] found that scriptaid can alleviate ICH-mediated neuroinflammation and WMI by inhibiting HDAC through modulating microglial polarization. HDAC inhibition has also been found to prevent WMI by regulating microglia/macrophage polarization by inhibiting the GSK3β/PTEN/AKT axis [129]. In summary, NLRP3 inflammasome and GSK-3β are closely related. Lithium, APNp, and scriptaid may indirectly affect NLRP3 inflammasome through their effects on GSK-3β, thereby reducing WMI after ICH. Curcumin (1,7-bis [4-hydroxy-3-methoxyphenyl]-1, 6heptadiene-3,5-dione) is the predominant curcuminoid in Curcuma longa. And it has been shown that WMI caused by a stroke can be alleviated by inhibiting NF-κB/NLRP3 inflammasome axis [102].
Altogether, inhibitors of specific pathways located upstream of NLRP3 inflammasome and associated with WMI are expected to be future drug candidates for treating secondary WMI after ICH.
8.2. Small-Molecule Inhibitors Targeting NLRP3 Inflammasome
It is reported that MCC950, a selective NLRP3 inflammasome inhibitor, is a small molecular compound similar to sulfonylurea, which can blockade the oligomerization of ASC in the NLRP3 inflammasome complex, and it is a highly effective NLRP3 inflammasome inhibitor. Crucially, MCC950 has been shown to attenuate neuroinflammation and induce a shift in microglia phenotype to an anti-inflammatory state in a mouse model of ICH, but the exact mechanism has not been fully elucidated [10]. In addition, Zhao et al. [130] showed that MCC950 could inhibit NLRP3 inflammasome and attenuate axonal damage in the rat model of diffuse axonal injury (DAI). This indicates the potential value of the MCC950 in protecting WMI after ICH. Moreover, OLT1177 (rINN: Dapansutrile) is a β-sulfonitrile nitrile synthetic compound with a molecular weight of 133.17 KDa, selectively inhibiting the NLRP3 inflammasome [131]. This compound has ameliorated disease severity in models of joint inflammation, myocardial ischemia-reperfusion injury, MS, and AD [132-134]. Most importantly, OLT1177 was shown to promote myelin preservation by inhibiting NLRP3 inflammasome in the model of SCI [135]. Furthermore, ZJU-37, a novel inhibitor with dual targeting of RIP1 and RIP3, has been shown to exert a pivotal role in cell death and inhibit the inflammatory response without inducing apoptosis [136]. And that ZJU-37 was shown to promote oligodendrocyte precursor cell (OPC) survival, differentiation and myelination by suppressing NLRP3 inflammasome activation in a neonatal rat model of WMI after transplantation of human neural stem cells (hOPCs) [136]. Compared with other macromolecular drugs, these small-molecular compounds are easier to penetrate the BBB to reach the brain tissue to exert their pharmacological effects, so these small-molecular compounds targeting NLRP3 inflammasome mentioned above should be one of the key research directions in the future.
8.3. Novel Materials and Cell Therapy
In recent years, many newly developed materials have been shown to positively affect WMI. For example, it has been shown that poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/ collagen (PHBV/Col) nanofibers are promising substrates as bioengineered grafts for the regeneration of neural tissue [137, 138]. Zhao and his colleagues found that the use of poly(hydroxybutyrate-co-hydroxyvalerate)/polylactic acid/ collagen (PHBV/PLA/Col) membranes for duralplasty reduced glial scar formation and promoted axonal regeneration after acute SCI by suppressing NLRP3 inflammasome activation and M1 macrophage polarization [139]. In addition, ceria nanoparticle (CeNP) has been shown to attenuate WMI after ICH, and the mechanisms may be related to the involvement of microglia and astrocytes in myelin regeneration [71]. This suggests that some novel materials may also ameliorate secondary WMI or promote WMI repair in addition to traditional drugs.
Interestingly, recent studies have shown that cell therapy may also exert a protective effect on WMI by inhibiting the NLRP3 inflammasome. For instance, Mousavi et al. [140] found that Schwann cell transplantation could be neuroprotective in a rat model of SCI by inhibiting NLRP3 and NLRP1 inflammasome activation and promoting motor recovery and remyelination. Moreover, hOPCs as a neural stem cell, its transplantation combined with ZJU-37 treatment can have a positive neuroprotective effect on neonatal WMI by inhibiting NLRP3 inflammasome [136]. The above studies suggest that partial kinds of cell therapy may also attenuate WMI by inhibiting NLRP3 inflammasome, and the effect of cell therapy on secondary WMI after ICH deserves further investigation.
8.4. Knock-Out of Certain Genes Modulates NLRP3 Inflammasome
In addition to the above treatment, the knockout of some genes can also interfere with NLRP3 inflammasome, thereby affecting WM. Zhang and his colleagues found that knockdown of dynamin-related protein 1 (Drp1), a mitochondrial fission guanosine triphosphatase, in OLs abolished NLRP3 inflammasome activation and corrected myelin loss, thereby improving cognitive function in a mouse model of AD [123]. At the same time, they also demonstrated that the Drp1-HK1-NLRP3 signal axis might be a key mechanism and therapeutic target of WM degeneration in AD [123]. Moreover, a study on MS showed that deletion of transient receptor potential melastatin 2 (TRPM2) protects from cuprizone-induced demyelination, activation of microglia, and NLRP3 inflammasome activation [124]. Demyelination and cognitive dysfunction are improved in TRPM2-deficient mice when NLRP3 inflammasome is inhibited [124]. The WMI involved in the above studies were all associated with NLRP3 inflammasome, suggesting that these genes may have a regulatory role in NLRP3 inflammasome-mediated WMI and deserve further investigation. Notably, one study found that deletion of UCP2 activated NLRP3 inflammasome in astrocytes [80, 81], but no study has yet reported the relationship between NLRP3 inflammasome and WMI after UCP2 deletion. Conversely, we speculate that activation of UCP2 may inhibit NLRP3 inflammasome activation. This may be one of the future directions that need to be studied in depth. In any case, these genes may be important regulatory targets for the future treatment of secondary WMI after ICH.
8.5. Other Compounds that May Ameliorate WMI by Inhibiting NLRP3 Inflammasome
Previous studies have shown that melatonin (MLT) can attenuate WMI in a mouse model of subarachnoid hemorrhage (SAH) by inhibiting NLRP3-induced apoptosis in OLs [141]. As previously mentioned, OLs are an important component of WM, and since SAH and ICH are both hemorrhagic strokes with strong similarities in the effects of blood on brain tissue and associated pathophysiology, MLT may attenuate WMI after ICH by suppressing NLRP3 inflammasome. In addition, Aryanpour et al. [142] found that 17β-Estradiol reduced demyelination and promoted myelin regeneration in cuprizone-fed mice by promoting M2 microglia polarization and inhibiting NLRP3 inflammasome. Moreover, sinomenine has been shown to alleviate demyelination and axonal injury in a mouse model of EAE by inhibiting NLRP3 inflammasome and inhibiting mobilization of microglia and astrocytes and suppressing neuroinflammation [143].
In addition, Li and his colleagues showed that VK-28 (5-[4-(2-hydroxyethyl) piperazine-1-ylmethyl]quinoline-8-ol), a brain-permeable iron chelator, has been shown to provide significant neuroprotection and a marked reduction in iron deposition in some models of neurodegenerative diseases such as Parkinson's disease (PD) [144], AD [145], and amyotrophic lateral sclerosis (ALS) [146]. Most importantly, VK-28 promotes microglial polarization to the M2 phenotype and attenuates WMI in the ICH model [56]. Furthermore, IL-33 ameliorated WMI after ICH by promoting microglial polarization to the M2 phenotype [54]. Deferoxamine and minocycline have been shown to attenuate WMI following ICH in piglets [147, 148]. Unfortunately, however, several of these studies above did not validate the relationship of the compounds with NLRP3 inflammasome. Given the potential role of NLRP3 inflammasome in microglial polarization, whether the protective effect of these compounds on WMI is achieved through inhibition of NLRP3 inflammasome needs to be verified by more experiments in the future. In conclusion, all of the above compounds may be valuable for further research and development by modulating NLRP3 inflammasome to attenuate secondary WMI after ICH.
CONCLUSION AND FUTURE DIRECTIONS
ICH is a very devastating neurological disease. Although there has been considerable progress in mechanistic research in the field of ICH over the past decades [149-153], it is only in recent years that it has been recognized that secondary WMI after ICH is closely associated with cognitive dysfunction and motor dysfunction. Therefore, the repair of secondary WMI after ICH is particularly important. We need to pay attention not only to the repair of GM damage but also to the repair of WMI so that real and lasting neural repair can be achieved. NLRP3 inflammasome-mediated neuroinflammation plays a vital role in secondary WMI after ICH. It is not difficult to understand that the damage to WM proximal to the primary lesion caused by NLRP3 inflammasome activation in the peri-hematoma tissue after ICH, but the mechanism of axonal damage distal to the primary lesion has not been reported. We speculate that NLRP3 itself may be a “transmitter” or can be activated by other stimuli such as high-mobility group box 1 (HMGB1) [154], ROS [155], and damage-associated molecular patterns (DAMPs) [156], and then delivered trans-synaptically, resulting in anterograde neurodegeneration. However, there is no research evidence to support this conjecture. The cellular and molecular mechanisms of axonal injury away from the primary lesion need further study to determine reasonable drug targets for treating ICH. Given the above theories, the treatment of ICH should not only be limited to the hematoma evacuation and the suppression of the inflammation around the hematoma but also include the early protection of the relevant distal nervous tissues. Therefore, it is imperative to seek effective treatment methods to meet this clinical need.
It is well known that neuroinflammation is a double-edged sword, and reasonable regulation of neuroinflammation can achieve the purpose of treating the disease. Both microglia and astrocytes play a critical role in the demyelination process [136]; NLRP3 inflammasome can be expressed in microglia and astrocytes. Suppression of NLRP3 inflammasome can attenuate CST injury, and inhibition of NLRP3 inflammasome activation in astrocytes and microglia combined with hOPCs can protect against ischemia and hypoxia caused neonatal WMI [14, 136]. It is, therefore, reasonable to assume that NLRP3 inflammasome may be an important target of action in reducing secondary WMI. Furthermore, M1 phenotype microglia and A1 astrocytes are thought to be one of the factors that may aggravate WMI, while M2 phenotype microglia and A2 astrocytes may have a protective effect on WMI. NLRP3 inflammasome may serve as leveraged regulatory fulcrums regulating the interconversion between these cell phenotypes.
In addition, a study showed that preconditioning ischemia is a classic example of hormesis, and cells exposed to moderate transient stress can protect them from more severe stress [157, 158]. Different intensities of exercise preconditioning can modulate the TXNIP/Trx/NF- Bp65/NLRP3 inflammatory signal pathway [159], which indicates that preconditioning signal plays an important role in inflammation and is one of the research directions in the future. Also, other studies have shown that the changes in the plasma membrane redox system (PMRS) in stress response may also make cells adapt to potentially destructive conditions [157, 158]. The study revealed that the mitochondria of cells are dysfunctional, and cells can survive because of the compensatory upregulation of PMRS activity [157, 158]. Neuroinflammatory injury plays an important role, so mitochondrial targeting therapy may also become one of the most promising research directions in treating secondary WMI after ICH.
Moreover, vitagene also shows great research potential. Some studies have shown that curcumin can mediate its anti-inflammatory activity by down-regulating a variety of inflammatory transcription factors and up-regulating cyprotective vitagenes, and it also can interact with members of the vitagenes family [157, 160-162]. As mentioned earlier, curcumin can reduce WMI after stroke by inhibiting NLRP3 inflammasome, but whether curcumin participates in the regulation of NLRP3 inflammasome through vitagene has not been reported, which may become one of the promising research directions in the future.
In conclusion, whether it is regulating the balance of different phenotypes of microglia or modulating the balance of different phenotypes of astrocytes to attenuate secondary WMI proximal to the primary lesion or distal to the primary lesion after ICH, NLRP3 inflammasome can be a suitable entry point. Early suppression of NLRP3 inflammasome may be a potential therapeutic strategy to protect secondary WMI and promote WM repair following ICH.
ACKNOWLEDGEMENTS
The support from 135 Project of Outstanding Development of West China Hospital, Sichuan University, Guangdong Basic and Applied Basic Research Foundation (2020A1515010038) and residential Foundation of Zhujiang Hospital of Southern Medical University is gratefully acknowledged.
LIST OF ABBREVIATIONS
- AD
Alzheimer's Disease
- ALS
Amyotrophic Lateral Sclerosis
- APNp
Adiponectin Peptide
- ASC
Apoptosis-Associated Speck Like Protein
- BBB
Blood-Brain Barrier
- BDNF
Brain-Derived Neurotrophic Factor
- CeNP
Ceria Nanoparticle
- CNS
Central Nervous System
- CST
Corticospinal Tracts
- DAI
Diffuse Axonal Injury
- DAMPs
Damage-Associated Molecular Patterns
- Drp1
Dynamin-Related Protein 1
- EAE
Experimental Autoimmune Encephalomyelitis
- GM
Gray Matter
- GSK-3β
Glycogen Synthase Kinase 3β
- H2S
Hydrogen Sulphide
- HDAC
Histone Deacetylase
- HMGB1
High-Mobility Group Box 1
- hOPCs
Human Neural Stem Cells
- ICH
Intracerebral Hemorrhage
- IL
Interleukin
- ITON
Indirect Traumatic Optic Neuropathy
- MitoQ
Mitoquinone
- MLT
Melatonin
- MMP-9
Matrix Metalloproteinase-9
- NLRP3
The NOD-Like Receptor Family, Pyrin Domain-Containing 3
- OGD-R
Oxygen-Glucose Deprivation and Reintroduction
- OLs
Oligodendrocytes
- ONC
Optic Nerve Crush
- OPC
Oligodendrocyte Precursor Cell
- P2X7R
Purinergic 2X7 Receptor
- PD
Parkinson's Disease
- PHBV/Col
Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/collagen
- PHBV/PLA/Col
Poly(hydroxybutyrate-co-hydroxyvalerate)/polylactic acid/collagen
- PMRS
Plasma Membrane Redox System
- ROS
Reactive Oxygen Species
- SAH
Subarachnoid Hemorrhage
- SCI
Spinal Cord Injury
- SD
Sleep Deprivation
- TBI
Traumatic Brain Injury
- TNF
Tumor Necrosis Factor
- TRPM2
Transient Receptor Potential Melastatin 2
- TXNIP
Thioredoxin-Interaction Protein
- UCP2
Uncoupling Protein 2
- WMI
White Matter Injury
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by grants from 135 Project of Outstanding Development of West China Hospital, Sichuan University (Grant no. ZY2017307), by Guangdong Basic and Applied Basic Research Foundation (Grant no. 2020A1515010038) and residential Foundation of Zhujiang Hospital of Southern Medical University (Grant no. yzjj2018rc03).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Katsuki H., Hijioka M. Intracerebral hemorrhage as an axonal tract injury disorder with inflammatory reactions. Biol. Pharm. Bull. 2017;40(5):564–568. doi: 10.1248/bpb.b16-01013. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi A.I., Mendelow A.D., Hanley D.F. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng A.C.K., Yao M., Cheng S.Y., Li J., Huang J.D., Wu W., Leung G.K.K., Sun H. Protracted morphological changes in the corticospinal tract within the cervical spinal cord after intracerebral hemorrhage in the right striatum of mice. Front. Neurosci. 2020;14:506. doi: 10.3389/fnins.2020.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith E.E., Gurol M.E., Eng J.A., Engel C.R., Nguyen T.N., Rosand J., Greenberg S.M. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63(9):1606–1612. doi: 10.1212/01.WNL.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 5.Chung C.S., Caplan L.R., Yamamoto Y., Chang H.M., Lee S.J., Song H.J., Lee H.S., Shin H.K., Yoo K.M. Striatocapsular haemorrhage. Brain. 2000;123(9):1850–1862. doi: 10.1093/brain/123.9.1850. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi A.I., Tuhrim S., Broderick J.P., Batjer H.H., Hondo H., Hanley D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 7.Wasserman J.K., Schlichter L.C. White matter injury in young and aged rats after intracerebral hemorrhage. Exp. Neurol. 2008;214(2):266–275. doi: 10.1016/j.expneurol.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Tao C., Hu X., Li H., You C. White matter injury after intracerebral hemorrhage: Pathophysiology and therapeutic strategies. Front. Hum. Neurosci. 2017;11:422. doi: 10.3389/fnhum.2017.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Xiao L., He D., Luo Y., Sun H. Mechanism of white matter injury and promising therapeutic strategies of MSCs after intracerebral hemorrhage. Front. Aging Neurosci. 2021;13:632054. doi: 10.3389/fnagi.2021.632054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren H., Kong Y., Liu Z., Zang D., Yang X., Wood K., Li M., Liu Q. Selective NLRP3 (pyrin domain–containing protein 3) inflammasome inhibitor reduces brain injury after intracerebral hemorrhage. Stroke. 2018;49(1):184–192. doi: 10.1161/STROKEAHA.117.018904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang S.J., Shao G.F., Chen J.L., Gong J. The NLRP3 inflammasome: An important driver of neuroinflammation in hemorrhagic stroke. Cell. Mol. Neurobiol. 2018;38(3):595–603. doi: 10.1007/s10571-017-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heneka M.T., McManus R.M., Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018;19(10):610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 13.Luo Y., Reis C., Chen S. NLRP3 inflammasome in the pathophysiology of hemorrhagic stroke: A review. Curr. Neuropharmacol. 2019;17(7):582–589. doi: 10.2174/1570159X17666181227170053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao L., Zheng H., Li J., Zeng M., He D., Liang J., Sun K., Luo Y., Li F., Ping B., Yuan W., Zhou H., Wang Q., Sun H. Targeting NLRP3 inflammasome modulates gut microbiota, attenuates corticospinal tract injury and ameliorates neurobehavioral deficits after intracerebral hemorrhage in mice. Biomed. Pharmacother. 2022;149:112797. doi: 10.1016/j.biopha.2022.112797. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y.B., Wei K.Y., Zhang X.Y., Feng H., Hu R. White matter repair and treatment strategy after intracerebral hemorrhage. CNS Neurosci. Ther. 2019;25(10):1113–1125. doi: 10.1111/cns.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedell B.J., Narayana P.A. Volumetric analysis of white matter, gray matter, and CSF using fractional volume analysis. Magn. Reson. Med. 1998;39(6):961–969. doi: 10.1002/mrm.1910390614. [DOI] [PubMed] [Google Scholar]
- 17.Herndon R.C., Lancaster J.L., Giedd J.N., Fox P.T. Quantification of white matter and gray matter volumes from three-dimensional magnetic resonance volume studies using fuzzy classifiers. J. Magn. Reson. Imaging. 1998;8(5):1097–1105. doi: 10.1002/jmri.1880080515. [DOI] [PubMed] [Google Scholar]
- 18.Kang M., Yao Y. Oligodendrocytes in intracerebral hemorrhage. CNS Neurosci. Ther. 2019;25(10):1075–1084. doi: 10.1111/cns.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roncagliolo M., Schlageter C., León C., Couve E., Bonansco C., Eguibar J.R. Developmental impairment of compound action potential in the optic nerve of myelin mutant taiep rats. Brain Res. 2006;1067(1):78–84. doi: 10.1016/j.brainres.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Gerrish A.C., Thomas A.G., Dineen R.A. Brain white matter tracts: Functional anatomy and clinical relevance. Semin. Ultrasound CT MR. 2014;35(5):432–444. doi: 10.1053/j.sult.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Carreiras M., Seghier M.L., Baquero S., Estévez A., Lozano A., Devlin J.T., Price C.J. An anatomical signature for literacy. Nature. 2009;461(7266):983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- 22.Schmahmann J.D., Smith E.E., Eichler F.S., Filley C.M. Cerebral white matter: Neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann. N. Y. Acad. Sci. 2008;1142(1):266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchi L., Eigenbrod T., Muñoz-Planillo R., Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009;10(3):241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heneka M.T., Kummer M.P., Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14(7):463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 25.Zaki M.H., Lamkanfi M., Kanneganti T.D. The Nlrp3 inflammasome: Contributions to intestinal homeostasis. Trends Immunol. 2011;32(4):171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng L., Chen Y., Ding R., Fu Z., Yang S., Deng X., Zeng J. P2X7R blockade prevents NLRP3 inflammasome activation and brain injury in a rat model of intracerebral hemorrhage: Involvement of peroxynitrite. J. Neuroinflammation. 2015;12(1):190. doi: 10.1186/s12974-015-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Q., Chen S., Hu Q., Feng H., Zhang J.H., Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann. Neurol. 2014;75(2):209–219. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao S.T., Cao F., Chen J.L., Chen W., Fan R.M., Li G., Zeng Y.C., Jiao S., Xia X.P., Han C., Ran Q.S. NLRP3 is required for complement-mediated caspase-1 and IL-1beta activation in ICH. J. Mol. Neurosci. 2017;61(3):385–395. doi: 10.1007/s12031-016-0874-9. [DOI] [PubMed] [Google Scholar]
- 29.Dutra F.F., Alves L.S., Rodrigues D., Fernandez P.L., de Oliveira R.B., Golenbock D.T., Zamboni D.S., Bozza M.T. Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl. Acad. Sci. USA. 2014;111(39):E4110–E4118. doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L., Fitzgerald K.A., Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muñoz-Planillo R., Kuffa P., Martínez-Colón G., Smith B.L., Rajendiran T.M., Núñez G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 33.Iyer S.S., He Q., Janczy J.R., Elliott E.I., Zhong Z., Olivier A.K., Sadler J.J., Knepper-Adrian V., Han R., Qiao L., Eisenbarth S.C., Nauseef W.M., Cassel S.L., Sutterwala F.S. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39(2):311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan X., Han X., Li Q., Yang Q.W., Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 2017;13(7):420–433. doi: 10.1038/nrneurol.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 2010;92(4):463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Lin J., Chen Q.Z., Zhu N., Jiang D.Q., Li M.X., Wang Y. Overexpression of mitochondrial Hsp75 protects neural stem cells against microglia-derived soluble factor-induced neurotoxicity by regulating mitochondrial permeability transition pore opening in vitro. Int. J. Mol. Med. 2015;36(6):1487–1496. doi: 10.3892/ijmm.2015.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yue X., Qiao D., Wang A., Tan X., Li Y., Liu C., Wang H. CD200 attenuates methamphetamine-induced microglial activation and dopamine depletion. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2012;32(3):415–421. doi: 10.1007/s11596-012-0072-0. [DOI] [PubMed] [Google Scholar]
- 38.Zhu N., Lin J., Wang K., Wei M., Chen Q., Wang Y. Huperzine A protects neural stem cells against Aβ-induced apoptosis in a neural stem cells and microglia co-culture system. Int. J. Clin. Exp. Pathol. 2015;8(6):6425–6433. [PMC free article] [PubMed] [Google Scholar]
- 39.Brown G.C., Vilalta A. How microglia kill neurons. Brain Res. 2015;1628(Pt B):288-297. doi: 10.1016/j.brainres.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 40.Xiong X.Y., Liu L., Yang Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Fumagalli S., Perego C., Pischiutta F., Zanier E.R., De Simoni M.G. The ischemic environment drives microglia and macrophage function. Front. Neurol. 2015;6:81. doi: 10.3389/fneur.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampron A., Larochelle A., Laflamme N., Préfontaine P., Plante M.M., Sánchez M.G., Yong V.W., Stys P.K., Tremblay M.È., Rivest S. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J. Exp. Med. 2015;212(4):481–495. doi: 10.1084/jem.20141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y., Tian H., Yao E., Tian Y., Zhang H., Xu L., Yu Z., Fang Y., Wang W., Du P., Xie M. Soluble epoxide hydrolase inhibition Promotes White Matter Integrity and Long-Term Functional Recovery after chronic hypoperfusion in mice. Sci. Rep. 2017;7(1):7758. doi: 10.1038/s41598-017-08227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin C., Fan W.H., Liu Q., Shang K., Murugan M., Wu L.J., Wang W., Tian D.S. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke. 2017;48(12):3336–3346. doi: 10.1161/STROKEAHA.117.018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olah M., Amor S., Brouwer N., Vinet J., Eggen B., Biber K., Boddeke H.W.G.M. Identification of a microglia phenotype supportive of remyelination. Glia. 2012;60(2):306–321. doi: 10.1002/glia.21266. [DOI] [PubMed] [Google Scholar]
- 46.Zhao X., Wang H., Sun G., Zhang J., Edwards N.J., Aronowski J. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J. Neurosci. 2015;35(32):11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouhlel M.A., Derudas B., Rigamonti E., Dièvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., Staels B., Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6(2):137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Tentillier N., Etzerodt A., Olesen M.N., Rizalar F.S., Jacobsen J., Bender D., Moestrup S.K., Romero-Ramos M. Anti-inflammatory modulation of microglia via CD163-targeted glucocorticoids protects dopaminergic neurons in the 6-OHDA Parkinson’s disease model. J. Neurosci. 2016;36(36):9375–9390. doi: 10.1523/JNEUROSCI.1636-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu X., Li P., Guo Y., Wang H., Leak R.K., Chen S., Gao Y., Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 50.Wang G., Zhang J., Hu X., Zhang L., Mao L., Jiang X., Liou A.K.F., Leak R.K., Gao Y., Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J. Cereb. Blood Flow Metab. 2013;33(12):1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y., Liu H., Zhang H., Ye Q., Wang J., Yang B., Mao L., Zhu W., Leak R.K., Xiao B., Lu B., Chen J., Hu X. ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J. Neurosci. 2017;37(18):4692–4704. doi: 10.1523/JNEUROSCI.3233-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Francos-Quijorna I., Amo-Aparicio J., Martinez-Muriana A., López-Vales R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. 2016;64(12):2079–2092. doi: 10.1002/glia.23041. [DOI] [PubMed] [Google Scholar]
- 53.Fu X., Zhou G., Wu X., Xu C., Zhou H., Zhuang J., Peng Y., Cao Y., Zeng H., Li Y., Li J., Gao L., Chen G., Wang L., Yan F. Inhibition of P2X4R attenuates white matter injury in mice after intracerebral hemorrhage by regulating microglial phenotypes. J. Neuroinflammation. 2021;18(1):184. doi: 10.1186/s12974-021-02239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z., Xu N., Dai X., Zhao C., Wu X., Shankar S., Huang H., Wang Z. Interleukin-33 reduces neuronal damage and white matter injury via selective microglia M2 polarization after intracerebral hemorrhage in rats. Brain Res. Bull. 2019;150:127–135. doi: 10.1016/j.brainresbull.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 55.Yang H., Ni W., Wei P., Li S., Gao X., Su J., Jiang H., Lei Y., Zhou L., Gu Y. HDAC inhibition reduces white matter injury after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2021;41(5):958–974. doi: 10.1177/0271678X20942613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Q., Wan J., Lan X., Han X., Wang Z., Wang J. Neuroprotection of brain-permeable iron chelator VK-28 against intracerebral hemorrhage in mice. J. Cereb. Blood Flow Metab. 2017;37(9):3110–3123. doi: 10.1177/0271678X17709186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Z., Zhong L., Xian R., Yuan B. MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol. Immunol. 2015;65(2):267–276. doi: 10.1016/j.molimm.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 58.Xiao L., Zheng H., Li J., Wang Q., Sun H. Neuroinflammation mediated by NLRP3 inflammasome after intracerebral hemorrhage and potential therapeutic targets. Mol. Neurobiol. 2020;57(12):5130–5149. doi: 10.1007/s12035-020-02082-2. [DOI] [PubMed] [Google Scholar]
- 59.Chen W., Guo C., Huang S., Jia Z., Wang J., Zhong J., Ge H., Yuan J., Chen T., Liu X., Hu R., Yin Y., Feng H. MitoQ attenuates brain damage by polarizing microglia towards the M2 phenotype through inhibition of the NLRP3 inflammasome after ICH. Pharmacol. Res. 2020;161:105122. doi: 10.1016/j.phrs.2020.105122. [DOI] [PubMed] [Google Scholar]
- 60.Scimemi A. Astrocytes and the warning signs of intracerebral hemorrhagic stroke. Neural Plast. 2018;2018:1–11. doi: 10.1155/2018/7301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tschoe C., Bushnell C.D., Duncan P.W., Alexander-Miller M.A., Wolfe S.Q. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J. Stroke. 2020;22(1):29–46. doi: 10.5853/jos.2019.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lively S., Schlichter L.C. Age-related comparisons of evolution of the inflammatory response after intracerebral hemorrhage in rats. Transl. Stroke Res. 2012;3(S1) Suppl. 1:132–146. doi: 10.1007/s12975-012-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tejima E., Zhao B.Q., Tsuji K., Rosell A., van Leyen K., Gonzalez R.G., Montaner J., Wang X., Lo E.H. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J. Cereb. Blood Flow Metab. 2007;27(3):460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- 64.Wang J., Tsirka S.E. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128(7):1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- 65.Chiu C.D., Yao N.W., Guo J.H., Shen C.C., Lee H.T., Chiu Y.P., Ji H.R., Chen X., Chen C.C., Chang C. Inhibition of astrocytic activity alleviates sequela in acute stages of intracerebral hemorrhage. Oncotarget. 2017;8(55):94850–94861. doi: 10.18632/oncotarget.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKeon R.J., Schreiber R.C., Rudge J.S., Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11(11):3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zamanian J.L., Xu L., Foo L.C., Nouri N., Zhou L., Giffard R.G., Barres B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012;32(18):6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.S., Peterson T.C., Wilton D.K., Frouin A., Napier B.A., Panicker N., Kumar M., Buckwalter M.S., Rowitch D.H., Dawson V.L., Dawson T.M., Stevens B., Barres B.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jha M.K., Lee W.H., Suk K. Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochem. Pharmacol. 2016;103:1–16. doi: 10.1016/j.bcp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Jha M.K., Jo M., Kim J.H., Suk K. Microglia-astrocyte crosstalk: An intimate molecular conversation. Neuroscientist. 2019;25(3):227–240. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- 71.Zheng J., Lu J., Mei S., Wu H., Sun Z., Fang Y., Xu S., Wang X., Shi L., Xu W., Chen S., Yu J., Liang F., Zhang J. Ceria nanoparticles ameliorate white matter injury after intracerebral hemorrhage: microglia-astrocyte involvement in remyelination. J. Neuroinflammation. 2021;18(1):43. doi: 10.1186/s12974-021-02101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mekhail M., Almazan G., Tabrizian M. Oligodendrocyte-protection and remyelination post-spinal cord injuries: A review. Prog. Neurobiol. 2012;96(3):322–339. doi: 10.1016/j.pneurobio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Sofroniew M.V., Vinters H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bush T.G., Puvanachandra N., Horner C.H., Polito A., Ostenfeld T., Svendsen C.N., Mucke L., Johnson M.H., Sofroniew M.V. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23(2):297–308. doi: 10.1016/S0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 75.Zador Z., Stiver S., Wang V., Manley G.T. Role of aquaporin-4 in cerebral edema and stroke. Handb. Exp. Pharmacol. 2009;190(190):159–170. doi: 10.1007/978-3-540-79885-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johann S., Heitzer M., Kanagaratnam M., Goswami A., Rizo T., Weis J., Troost D., Beyer C. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia. 2015;63(12):2260–2273. doi: 10.1002/glia.22891. [DOI] [PubMed] [Google Scholar]
- 77.Liu H.D., Li W., Chen Z.R., Hu Y.C., Zhang D.D., Shen W., Zhou M.L., Zhu L., Hang C.H. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem. Res. 2013;38(10):2072–2083. doi: 10.1007/s11064-013-1115-z. [DOI] [PubMed] [Google Scholar]
- 78.Cho M.H., Cho K., Kang H.J., Jeon E.Y., Kim H.S., Kwon H.J., Kim H.M., Kim D.H., Yoon S.Y. Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy. 2014;10(10):1761–1775. doi: 10.4161/auto.29647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zendedel A., Johann S., Mehrabi S., Joghataei M., Hassanzadeh G., Kipp M., Beyer C. Activation and regulation of NLRP3 inflammasome by intrathecal application of SDF-1a in a spinal cord injury model. Mol. Neurobiol. 2016;53(5):3063–3075. doi: 10.1007/s12035-015-9203-5. [DOI] [PubMed] [Google Scholar]
- 80.Lu M., Sun X.L., Qiao C., Liu Y., Ding J.H., Hu G. Uncoupling protein 2 deficiency aggravates astrocytic endoplasmic reticulum stress and nod-like receptor protein 3 inflammasome activation. Neurobiol. Aging. 2014;35(2):421–430. doi: 10.1016/j.neurobiolaging.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 81.Du R.H., Wu F.F., Lu M., Shu X., Ding J.H., Wu G., Hu G. Uncoupling protein 2 modulation of the NLRP3 inflammasome in astrocytes and its implications in depression. Redox Biol. 2016;9:178–187. doi: 10.1016/j.redox.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu H., Wu X., Luo J., Zhao L., Li X., Guo H., Bai H., Cui W., Guo W., Feng D., Qu Y. Adiponectin peptide alleviates oxidative stress and NLRP3 inflammasome activation after cerebral ischemia-reperfusion injury by regulating AMPK/GSK-3β. Exp. Neurol. 2020;329:113302. doi: 10.1016/j.expneurol.2020.113302. [DOI] [PubMed] [Google Scholar]
- 83.Ito M., Komai K., Mise-Omata S., Iizuka-Koga M., Noguchi Y., Kondo T., Sakai R., Matsuo K., Nakayama T., Yoshie O., Nakatsukasa H., Chikuma S., Shichita T., Yoshimura A. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565(7738):246–250. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- 84.Zhou K., Zhong Q., Wang Y.C., Xiong X.Y., Meng Z.Y., Zhao T., Zhu W.Y., Liao M.F., Wu L.R., Yang Y.R., Liu J., Duan C.M., Li J., Gong Q.W., Liu L., Yang M.H., Xiong A., Wang J., Yang Q.W. Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3β/PTEN axis. J. Cereb. Blood Flow Metab. 2017;37(3):967–979. doi: 10.1177/0271678X16648712. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Sen T., Saha P., Gupta R., Foley L.M., Jiang T., Abakumova O.S., Hitchens T.K., Sen N., Aberrant E.R. Aberrant ER stress induced neuronal-IFNβ elicits white matter injury due to microglial activation and T-cell infiltration after TBI. J. Neurosci. 2020;40(2):424–446. doi: 10.1523/JNEUROSCI.0718-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee G. The balance of Th17 versus treg cells in autoimmunity. Int. J. Mol. Sci. 2018;19(3):730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mori S., Leblond C.P. Electron microscopic identification of three classes of oligodendrocytes and a preliminary study of their proliferative activity in the corpus callosum of young rats. J. Comp. Neurol. 1970;139(1):1–29. doi: 10.1002/cne.901390102. [DOI] [PubMed] [Google Scholar]
- 88.Arai K., Lo E.H. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp. Transl. Stroke Med. 2009;1(1):6. doi: 10.1186/2040-7378-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bakiri Y., Burzomato V., Frugier G., Hamilton N.B., Káradóttir R., Attwell D. Glutamatergic signaling in the brain’s white matter. Neuroscience. 2009;158(1):266–274. doi: 10.1016/j.neuroscience.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 90.Miron V.E., Boyd A., Zhao J.W., Yuen T.J., Ruckh J.M., Shadrach J.L., van Wijngaarden P., Wagers A.J., Williams A., Franklin R.J.M. ffrench-Constant, C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013;16(9):1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han L., Cai W., Mao L., Liu J., Li P., Leak R.K., Xu Y., Hu X., Chen J. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke. 2015;46(9):2628–2636. doi: 10.1161/STROKEAHA.115.010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naruse M., Shibasaki K., Shimauchi-Ohtaki H., Ishizaki Y. Microglial activation induces generation of oligodendrocyte progenitor cells from the subventricular zone after focal demyelination in the corpus callosum. Dev. Neurosci. 2018;40(1):54–63. doi: 10.1159/000486332. [DOI] [PubMed] [Google Scholar]
- 93.Tang T., Liu X.J., Zhang Z.Q., Zhou H.J., Luo J.K., Huang J.F., Yang Q.D., Li X.Q. Cerebral angiogenesis after collagenase-induced intracerebral hemorrhage in rats. Brain Res. 2007;1175:134–142. doi: 10.1016/j.brainres.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 94.Yuen T.J., Silbereis J.C., Griveau A., Chang S.M., Daneman R., Fancy S.P.J., Zahed H., Maltepe E., Rowitch D.H. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158(2):383–396. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou T., Zheng Y., Sun L., Badea S.R., Jin Y., Liu Y., Rolfe A.J., Sun H., Wang X., Cheng Z., Huang Z., Zhao N., Sun X., Li J., Fan J., Lee C., Megraw T.L., Wu W., Wang G., Ren Y. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat. Neurosci. 2019;22(3):421–435. doi: 10.1038/s41593-018-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J., Ning R., Zacharek A., Cui C., Cui X., Yan T., Venkat P., Zhang Y., Chopp M. MiR-126 contributes to human umbilical cord blood cell-induced neurorestorative effects after stroke in type-2 diabetic mice. Stem Cells. 2016;34(1):102–113. doi: 10.1002/stem.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arboix A., Comes E., García-Eroles L., Massons J., Oliveres M., Balcells M., Targa C. Site of bleeding and early outcome in primary intracerebral hemorrhage. Acta Neurol. Scand. 2002;105(4):282–288. doi: 10.1034/j.1600-0404.2002.1o170.x. [DOI] [PubMed] [Google Scholar]
- 98.Mori E., Tabuchi M., Yamadori A. Lacunar syndrome due to intracerebral hemorrhage. Stroke. 1985;16(3):454–459. doi: 10.1161/01.STR.16.3.454. [DOI] [PubMed] [Google Scholar]
- 99.Tapia J.F., Kase C.S., Sawyer R.H., Mohr J.P. Hypertensive putaminal hemorrhage presenting as pure motor hemiparesis. Stroke. 1983;14(4):505–506. doi: 10.1161/01.STR.14.4.505. [DOI] [PubMed] [Google Scholar]
- 100.Hijioka M., Anan J., Matsushita H., Ishibashi H., Kurauchi Y., Hisatsune A., Seki T., Katsuki H. Axonal dysfunction in internal capsule is closely associated with early motor deficits after intracerebral hemorrhage in mice. Neurosci. Res. 2016;106:38–46. doi: 10.1016/j.neures.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 101.Chen W., Guo C., Jia Z., Wang J., Xia M., Li C., Li M., Yin Y., Tang X., Chen T., Hu R., Chen Y., Liu X., Feng H. Inhibition of mitochondrial ROS by MitoQ alleviates white matter injury and improves outcomes after intracerebral haemorrhage in mice. Oxid. Med. Cell. Longev. 2020;2020:1–12. doi: 10.1155/2020/8285065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ran Y., Su W., Gao F., Ding Z., Yang S., Ye L., Chen X., Tian G., Xi J., Liu Z. Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage pyroptosis through NF-κB suppression and NLRP3 inflammasome inhibition. Oxid. Med. Cell. Longev. 2021;2021:1–25. doi: 10.1155/2021/1552127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao H., Pan P., Yang Y., Ge H., Chen W., Qu J., Shi J., Cui G., Liu X., Feng H., Chen Y. Endogenous hydrogen sulphide attenuates NLRP3 inflammasome-mediated neuroinflammation by suppressing the P2X7 receptor after intracerebral haemorrhage in rats. J. Neuroinflammation. 2017;14(1):163. doi: 10.1186/s12974-017-0940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calzaferri F., Ruiz-Ruiz C., Diego A.M.G., Pascual R., Méndez-López I., Cano-Abad M.F., Maneu V., Ríos C., Gandía L., García A.G. The purinergic P2X7 receptor as a potential drug target to combat neuroinflammation in neurodegenerative diseases. Med. Res. Rev. 2020;40(6):2427–2465. doi: 10.1002/med.21710. [DOI] [PubMed] [Google Scholar]
- 105.Block F., Dihné M., Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog. Neurobiol. 2005;75(5):342–365. doi: 10.1016/j.pneurobio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Z., Liu W., Huang Y., Luo L., Cai X., Liu Y., Ai L., Yan J., Lin S., Ye J. NLRP3 deficiency attenuates secondary degeneration of visual cortical neurons following optic nerve injury. Neurosci. Bull. 2020;36(3):277–288. doi: 10.1007/s12264-019-00445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chu X., Wang C., Wu Z., Fan L., Tao C., Lin J., Chen S., Lin Y., Ge Y. JNK/c-Jun-driven NLRP3 inflammasome activation in microglia contributed to retinal ganglion cells degeneration induced by indirect traumatic optic neuropathy. Exp. Eye Res. 2021;202:108335. doi: 10.1016/j.exer.2020.108335. [DOI] [PubMed] [Google Scholar]
- 108.Li X., Yu Z., Zong W., Chen P., Li J., Wang M., Ding F., Xie M., Wang W., Luo X. Deficiency of the microglial Hv1 proton channel attenuates neuronal pyroptosis and inhibits inflammatory reaction after spinal cord injury. J. Neuroinflammation. 2020;17(1):263. doi: 10.1186/s12974-020-01942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Virgilio F. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol. Sci. 2007;28(9):465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 110.Franceschini A., Capece M., Chiozzi P., Falzoni S., Sanz J.M., Sarti A.C., Bonora M., Pinton P., Di Virgilio F. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. 2015;29(6):2450–2461. doi: 10.1096/fj.14-268714. [DOI] [PubMed] [Google Scholar]
- 111.Li M., Xia M., Chen W., Wang J., Yin Y., Guo C., Li C., Tang X., Zhao H., Tan Q., Chen Y., Jia Z., Liu X., Feng H. Lithium treatment mitigates white matter injury after intracerebral hemorrhage through brain-derived neurotrophic factor signaling in mice. Transl. Res. 2020;217:61–74. doi: 10.1016/j.trsl.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 112.Zhao J., Wang H., Huang Y., Zhang H., Wang S., Gaskin F., Yang N., Fu S.M. Lupus nephritis: Glycogen synthase kinase 3β promotion of renal damage through activation of the NLRP3 inflammasome in lupus-prone mice. Arthritis Rheumatol. 2015;67(4):1036–1044. doi: 10.1002/art.38993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y., Meng C., Zhang J., Wu J., Zhao J. Inhibition of GSK-3β alleviates cerebral ischemia/reperfusion injury in rats by suppressing NLRP3 inflammasome activation through autophagy. Int. Immunopharmacol. 2019;68:234–241. doi: 10.1016/j.intimp.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 114.Li X., Liang S., Li Z., Li S., Xia M., Verkhratsky A., Li B. Leptin increases expression of 5-HT2B receptors in astrocytes thus enhancing action of fluoxetine on the depressive behavior induced by sleep deprivation. Front. Psychiatry. 2019;9:734. doi: 10.3389/fpsyt.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ward R., Li W., Abdul Y., Jackson L., Dong G., Jamil S., Filosa J., Fagan S.C., Ergul A. NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol. Res. 2019;142:237–250. doi: 10.1016/j.phrs.2019.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fu Q., Li J., Qiu L., Ruan J., Mao M., Li S., Mao Q. Inhibiting NLRP3 inflammasome with MCC950 ameliorates perioperative neurocognitive disorders, suppressing neuroinflammation in the hippocampus in aged mice. Int. Immunopharmacol. 2020;82:106317. doi: 10.1016/j.intimp.2020.106317. [DOI] [PubMed] [Google Scholar]
- 117.Fletcher J., Murray S., Xiao J. Brain-derived neurotrophic factor in central nervous system myelination: A new mechanism to promote myelin plasticity and repair. Int. J. Mol. Sci. 2018;19(12):4131. doi: 10.3390/ijms19124131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Poduslo J.F., Curran G.L. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res. Mol. Brain Res. 1996;36(2):280–286. doi: 10.1016/0169-328X(95)00250-V. [DOI] [PubMed] [Google Scholar]
- 119.Zhang C.Y.Y., Zeng M.J., Zhou L.P., Li Y.Q., Zhao F., Shang Z.Y., Deng X.Y., Ma Z.Q., Fu Q., Ma S.P., Qu R. Baicalin exerts neuroprotective effects via inhibiting activation of GSK3β/NF- κB/NLRP3 signal pathway in a rat model of depression. Int. Immunopharmacol. 2018;64:175–182. doi: 10.1016/j.intimp.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 120.An P., Xie J., Qiu S., Liu Y., Wang J., Xiu X., Li L., Tang M. Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sci. 2019;232:116599. doi: 10.1016/j.lfs.2019.116599. [DOI] [PubMed] [Google Scholar]
- 121.Liu W., Wang H., Wang Y., Li H., Ji L. Metabolic factors-triggered inflammatory response drives antidepressant effects of exercise in CUMS rats. Psychiatry Res. 2015;228(3):257–264. doi: 10.1016/j.psychres.2015.05.102. [DOI] [PubMed] [Google Scholar]
- 122.Li R., Liu Z., Wu X., Yu Z., Zhao S., Tang X. Lithium chloride promoted hematoma resolution after intracerebral hemorrhage through GSK-3β-mediated pathways-dependent microglia phagocytosis and M2-phenotype differentiation, angiogenesis and neurogenesis in a rat model. Brain Res. Bull. 2019;152:117–127. doi: 10.1016/j.brainresbull.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 123.Zhang X., Wang R., Hu D., Sun X., Fujioka H., Lundberg K., Chan E.R., Wang Q., Xu R., Flanagan M.E., Pieper A.A., Qi X. Oligodendroglial glycolytic stress triggers inflammasome activation and neuropathology in Alzheimer’s disease. Sci. Adv. 2020;6(49):eabb8680. doi: 10.1126/sciadv.abb8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shao Y., Chen C., Zhu T., Sun Z., Li S., Gong L., Dong X., Shen W., Zeng L., Xie Y., Jiang P. TRPM2 contributes to neuroinflammation and cognitive deficits in a cuprizone-induced multiple sclerosis model via NLRP3 inflammasome. Neurobiol. Dis. 2021;160:105534. doi: 10.1016/j.nbd.2021.105534. [DOI] [PubMed] [Google Scholar]
- 125.Franke H., Günther A., Grosche J., Schmidt R., Rossner S., Reinhardt R., Faber-Zuschratter H., Schneider D., Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J. Neuropathol. Exp. Neurol. 2004;63(7):686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 126.Kilts C.D. The ups and downs of oral lithium dosing. J. Clin. Psychiatry. 1998;59(Suppl. 6):21–26. [PubMed] [Google Scholar]
- 127.Müller-Oerlinghausen B., Berghöfer A., Bauer M. Bipolar disorder. Lancet. 2002;359(9302):241–247. doi: 10.1016/S0140-6736(02)07450-0. [DOI] [PubMed] [Google Scholar]
- 128.Benedetti F., Bollettini I., Barberi I., Radaelli D., Poletti S., Locatelli C., Pirovano A., Lorenzi C., Falini A., Colombo C., Smeraldi E. Lithium and GSK3-β promoter gene variants influence white matter microstructure in bipolar disorder. Neuropsychopharmacology. 2013;38(2):313–327. doi: 10.1038/npp.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang G., Shi Y., Jiang X., Leak R.K., Hu X., Wu Y., Pu H., Li W.W., Tang B., Wang Y., Gao Y., Zheng P., Bennett M.V.L., Chen J. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc. Natl. Acad. Sci. USA. 2015;112(9):2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao J., Guo X., Wang B., Yang Z., Huang T., Guo D., Zhang M., Song J. MCC950 inhibits NLRP3 inflammasome and alleviates axonal injures in early stages of diffuse axonal injury in rats. Neurochem. Res. 2020;45(9):2020–2031. doi: 10.1007/s11064-020-03063-6. [DOI] [PubMed] [Google Scholar]
- 131.Marchetti C., Swartzwelter B., Gamboni F., Neff C.P., Richter K., Azam T., Carta S., Tengesdal I., Nemkov T., D’Alessandro A., Henry C., Jones G.S., Goodrich S.A., St Laurent J.P., Jones T.M., Scribner C.L., Barrow R.B., Altman R.D., Skouras D.B., Gattorno M., Grau V., Janciauskiene S., Rubartelli A., Joosten L.A.B., Dinarello C.A. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. USA. 2018;115(7):E1530–E1539. doi: 10.1073/pnas.1716095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toldo S., Mauro A.G., Cutter Z., Van Tassell B.W., Mezzaroma E., Del Buono M.G., Prestamburgo A., Potere N., Abbate A. The NLRP3 inflammasome inhibitor, OLT1177 (dapansutrile), reduces infarct size and preserves contractile function after ischemia reperfusion injury in the mouse. J. Cardiovasc. Pharmacol. 2019;73(4):215–222. doi: 10.1097/FJC.0000000000000658. [DOI] [PubMed] [Google Scholar]
- 133.Marchetti C., Swartzwelter B., Koenders M.I., Azam T., Tengesdal I.W., Powers N., de Graaf D.M., Dinarello C.A., Joosten L.A.B. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res. Ther. 2018;20(1):169. doi: 10.1186/s13075-018-1664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lonnemann N., Hosseini S., Marchetti C., Skouras D.B., Stefanoni D., D’Alessandro A., Dinarello C.A., Korte M. The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2020;117(50):32145–32154. doi: 10.1073/pnas.2009680117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Amo-Aparicio J., Garcia-Garcia J., Puigdomenech M., Francos-Quijorna I., Skouras D.B., Dinarello C.A., Lopez-Vales R. Inhibition of the NLRP3 inflammasome by OLT1177 induces functional protection and myelin preservation after spinal cord injury. Exp. Neurol. 2022;347:113889. doi: 10.1016/j.expneurol.2021.113889. [DOI] [PubMed] [Google Scholar]
- 136.Zhang C., Guan Q., Shi H., Cao L., Liu J., Gao Z., Zhu W., Yang Y., Luan Z., Yao R. A novel RIP1/RIP3 dual inhibitor promoted OPC survival and myelination in a rat neonatal white matter injury model with hOPC graft. Stem Cell Res. Ther. 2021;12(1):462. doi: 10.1186/s13287-021-02532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.He Y., Hu Z., Ren M., Ding C., Chen P., Gu Q., Wu Q. Evaluation of PHBHHx and PHBV/PLA fibers used as medical sutures. J. Mater. Sci. Mater. Med. 2014;25(2):561–571. doi: 10.1007/s10856-013-5073-4. [DOI] [PubMed] [Google Scholar]
- 138.Li H., Chang J. Fabrication and characterization of bioactive wollastonite/PHBV composite scaffolds. Biomaterials. 2004;25(24):5473–5480. doi: 10.1016/j.biomaterials.2003.12.052. [DOI] [PubMed] [Google Scholar]
- 139.Zhao T., Xu K., Wu Q., Wang C., Xiao S., Li H., He T., Wang L., Li F., Chen Q. Duraplasty of PHBV/PLA/Col membranes promotes axonal regeneration by inhibiting NLRP3 complex and M1 macrophage polarization in rats with spinal cord injury. FASEB J. 2020;34(9):12147–12162. doi: 10.1096/fj.202000190RR. [DOI] [PubMed] [Google Scholar]
- 140.Mousavi M., Hedayatpour A., Mortezaee K., Mohamadi Y., Abolhassani F., Hassanzadeh G. Schwann cell transplantation exerts neuroprotective roles in rat model of spinal cord injury by combating inflammasome activation and improving motor recovery and remyelination. Metab. Brain Dis. 2019;34(4):1117–1130. doi: 10.1007/s11011-019-00433-0. [DOI] [PubMed] [Google Scholar]
- 141.Liu D., Dong Y., Li G., Zou Z., Hao G., Feng H., Pan P., Liang G. Melatonin attenuates white matter injury via reducing oligodendrocyte apoptosis after subarachnoid hemorrhage in mice. Turk Neurosurg. 2020;30(5):685–692. doi: 10.5137/1019-5149.JTN.27986-19.3. [DOI] [PubMed] [Google Scholar]
- 142.Aryanpour R., Zibara K., Pasbakhsh P., Jame’ei S.B., Namjoo Z., Ghanbari A., Mahmoudi R., Amani S., Kashani I.R. 17β-Estradiol reduces demyelination in cuprizone-fed mice by promoting M2 microglia polarity and regulating NLRP3 inflammasome. Neuroscience. 2021;463:116–127. doi: 10.1016/j.neuroscience.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 143.Kiasalari Z., Afshin-Majd S., Baluchnejadmojarad T., Azadi-Ahmadabadi E., Fakour M., Ghasemi-Tarie R., Jalalzade-Ogvar S., Khodashenas V., Tashakori-Miyanroudi M., Roghani M. Sinomenine alleviates murine experimental autoimmune encephalomyelitis model of multiple sclerosis through inhibiting NLRP3 inflammasome. J. Mol. Neurosci. 2021;71(2):215–224. doi: 10.1007/s12031-020-01637-1. [DOI] [PubMed] [Google Scholar]
- 144.Youdim M.B.H., Stephenson G., Shachar D.B. Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: A lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann. N. Y. Acad. Sci. 2004;1012(1):306–325. doi: 10.1196/annals.1306.025. [DOI] [PubMed] [Google Scholar]
- 145.Bandyopadhyay S., Huang X., Cho H., Greig N.H., Youdim M.B., Rogers J.T. Metal specificity of an iron-responsive element in Alzheimer’s APP mRNA 5′untranslated region, tolerance of SH-SY5Y and H4 neural cells to desferrioxamine, clioquinol, VK-28, and a piperazine chelator. J. Neural Transm. Suppl. 2006;71(71):237–247. doi: 10.1007/978-3-211-33328-0_25. [DOI] [PubMed] [Google Scholar]
- 146.Wang Q., Zhang X., Chen S., Zhang X., Zhang S., Youdium M., Le W. Prevention of motor neuron degeneration by novel iron chelators in SOD1(G93A) transgenic mice of amyotrophic lateral sclerosis. Neurodegener. Dis. 2011;8(5):310–321. doi: 10.1159/000323469. [DOI] [PubMed] [Google Scholar]
- 147.Xie Q., Gu Y., Hua Y., Liu W., Keep R.F., Xi G. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke. 2014;45(1):290–292. doi: 10.1161/STROKEAHA.113.003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang H. Gao, X.J.; Li, Y.J.; Su, J.B.; e, T.Z.; Zhang, X.; Ni, W.; Gu, Y.X. Minocycline reduces intracerebral hemorrhage–induced white matter injury in piglets. CNS Neurosci. Ther. 2019;25(10):1195–1206. doi: 10.1111/cns.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Balami J.S., Buchan A.M. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11(1):101–118. doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- 150.Fang H., Wang P.F., Zhou Y., Wang Y.C., Yang Q.W. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J. Neuroinflammation. 2013;10(1):794. doi: 10.1186/1742-2094-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen S., Yang Q., Chen G., Zhang J.H. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl. Stroke Res. 2015;6(1):4–8. doi: 10.1007/s12975-014-0384-4. [DOI] [PubMed] [Google Scholar]
- 152.Duan X., Wen Z., Shen H., Shen M., Chen G. Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid. Med. Cell. Longev. 2016;2016:1–17. doi: 10.1155/2016/1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bobinger T., Burkardt P.B., Huttner H., Manaenko A. Programmed cell death after intracerebral hemorrhage. Curr. Neuropharmacol. 2018;16(9):1267–1281. doi: 10.2174/1570159X15666170602112851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frank M.G., Weber M.D., Fonken L.K., Hershman S.A., Watkins L.R., Maier S.F. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav. Immun. 2016;55:215–224. doi: 10.1016/j.bbi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Minutoli L., Puzzolo D., Rinaldi M., Irrera N., Marini H., Arcoraci V., Bitto A., Crea G., Pisani A., Squadrito F., Trichilo V., Bruschetta D., Micali A., Altavilla D. ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis Ischemia/Reperfusion injury. Oxid. Med. Cell. Longev. 2016;2016:1–10. doi: 10.1155/2016/2183026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rubartelli A. DAMP-mediated activation of NLRP3-inflammasome in brain sterile inflammation: The fine line between healing and neurodegeneration. Front. Immunol. 2014;5:99. doi: 10.3389/fimmu.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perluigi M., Di Domenico F., Giorgi A., Schininà M.E., Coccia R., Cini C., Bellia F., Cambria M.T., Cornelius C., Butterfield D.A., Calabrese V. Redox proteomics in aging rat brain: Involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J. Neurosci. Res. 2010;88(16):3498–3507. doi: 10.1002/jnr.22500. [DOI] [PubMed] [Google Scholar]
- 159.Li Y., Xu P., Wang Y., Zhang J., Yang M., Chang Y., Zheng P., Huang H., Cao X. Different intensity exercise preconditions affect cardiac function of exhausted rats through regulating TXNIP/] TRX/NF-ĸBp65/NLRP3 inflammatory pathways. Evid. Based Complement. Alternat. Med. 2020;2020:1–9. doi: 10.1155/2020/5809298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mancuso C., Pani G., Calabrese V. Bilirubin: An endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006;11(5):207–213. doi: 10.1179/135100006X154978. [DOI] [PubMed] [Google Scholar]
- 161.Drake J., Sultana R., Aksenova M., Calabrese V., Butterfield D.A. Elevation of mitochondrial glutathione by? -glutamylcysteine ethyl ester protects mitochondria against peroxynitrite-induced oxidative stress. J. Neurosci. Res. 2003;74(6):917–927. doi: 10.1002/jnr.10810. [DOI] [PubMed] [Google Scholar]
- 162.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Giuffrida Stella A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]