Abstract
Chemotherapy is one of the main therapeutic modalities for cancer patients. Cisplatin (CDDP), as one of the first-line drugs, is of great importance in the chemotherapy of various tumors. However, a significant percentage of cancer patients are resistant to CDDP treatment. Due to the CDDP side effects on normal tissues, the diagnosis of CDDP resistance is required to suggest the most efficient therapeutic strategies for cancer patients. Several molecular mechanisms and signaling pathways are associated with CDDP response. The PI3K/AKT signaling pathway has a pivotal role in the transmission of extracellular signals into the cells to regulate various pathophysiological processes such as cell proliferation, migration, and drug resistance. In the present review, we summarized all of the studies which have been reported on the role of PI3K/AKT pathway in regulation of CDDP response. It was shown that the PI3K/AKT pathway is mainly involved in CDDP response in lung, ovarian, and gastrointestinal cancers. It was also observed that the non-coding RNAs have a key role in CDDP response by regulation of PI3K/AKT pathway. This review paves the way for suggesting a PI3K/AKT-related panel marker for the prediction of CDDP response in different cancer patients.
Keywords: PI3K/AKT, Cancer, Chemoresistance, Cisplatin, Chemotherapy
Introduction
Cisplatin (CDDP) is a common first-line chemotherapeutic drug for different cancers [1]. However, there are some limitations to the application of cisplatin, such as drug resistance and side effects. Therefore, combination therapy can reduce the CDDP drug resistance and side effects [2]. Cisplatin binds with purine bases in DNA to interfere with DNA repair and replication result in tumor cell apoptosis [3]. CDDP also promotes the reactive oxygen species (ROS) production that induces cell death through apoptosis and necrosis. Cisplatin disturbs calcium homeostasis that affects the function of mitochondrial enzymes to promote cell death [2]. Although Cancer patients are initially CDDP responsive, CDDP resistance and tumor relapse are eventually observed in the majority of patients [4]. Various molecular and cellular mechanisms are involved in CDDP resistance, including increased drug efflux, increased DNA repair, and upregulation of anti-apoptotic factors [5]. Therefore, it is required to clarify the molecular mechanisms of cisplatin resistance to improve the clinical outcome in cancer patients. PI3K/AKT signaling pathway is frequently deregulated in different cancers [6]. PI3K can be activated by tyrosine kinase receptors, and G-protein coupled receptors to produce PIP3 that activates AKT. AKT also activates various effectors such as the Mammalian target of rapamycin (mTOR) and GSK3 to regulate cell metabolism, proliferation, and motility [7]. The mTOR as a serine/threonine kinase is considered the best-characterized AKT substrate, which can be activated by phosphorylation. Subsequently, mTOR activates ribosomal protein S6 kinases (S6K) and suppresses 4E-BP, that results in elevated protein translation [8]. PI3K/AKT/mTOR pathway is involved in the sensitivity of tumor cells toward cisplatin [9,10]. It has been reported that inhibition of AKT/mTOR promoted CDDP-induced apoptosis in resistant cells [11]. AKT promotes CDDP resistance via negative regulation of P53 [12]. P13K inhibitor also increases CDDP sensitivity by Bax upregulation and cytC release [13]. PI3K/AKT activates GSK-3β that promotes β-catenin transfer to the nucleus to upregulate target genes associated with multi-drug resistance (MDR) [14]. ABC transporters such as ABCB1, ABCC1, and ABCG2 can also be upregulated by PI3K/AKT pathway to promote CDDP efflux and resistance [15,16]. PI3K/AKT pathway regulates aerobic glycolysis to prepare energy and increase the efficiency of ABC transporters for drug efflux [17]. PI3K/AKT is a promising therapeutic target for clinical tumor therapy. mTOR is one of the main effectors in PI3K/AKT pathway that regulates various cellular processes. Rapamycin analogs such as everolimus and ridaforolimus are the mTOR inhibitors that are widely used as anti-cancer agents [18]. In the present review, we have summarized all of the studies that have reported on the role of PI3K/AKT pathway in the regulation of CDDP response in tumor cells (Fig. 1). It was observed that there is a complex network of non-coding RNAs (Table 1) and protein-protein interactions (Table 2) which are responsible for CDDP response through the regulation of PI3K/AKT signaling pathway.
Figure 1. All of the molecular mechanisms that affect the Cisplatin response via PI3K/AKT signaling pathway (Created with BioRender.com).
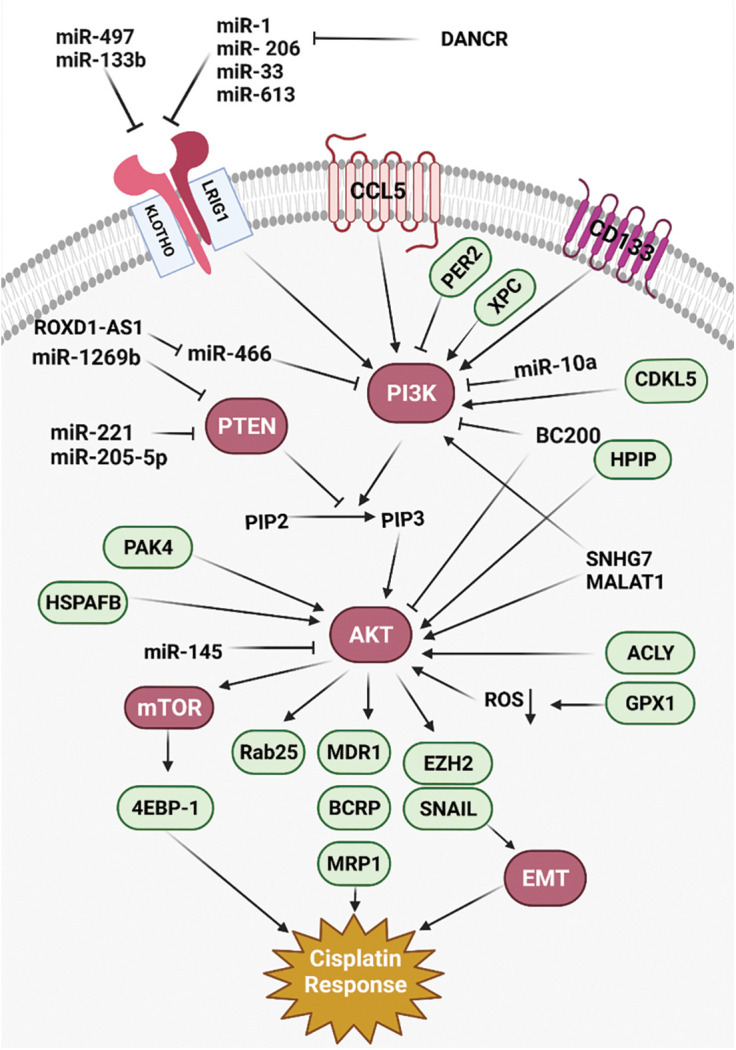
Table 1. All of the non-coding RNAs that affect Cisplatin response using the regulation of PI3K/AKT pathway.
| Gene | Mechanism | Target gene | Effect on the tumor cells | Clinical application | Samples | Study | Year |
|---|---|---|---|---|---|---|---|
| miR-181 | Downregulation | PTEN | Increased CDDP resistance | Diagnosis | 6 patients A549 cell line | Liu et al. [19] | 2018 |
| miR-1269b | Upregulation | PTEN | Increased CDDP resistance | Diagnosis | 32 patients HA549, SPCA1, H1299 H358, PC9, A549 cell lines | Yang et al. [20] | 2020 |
| miR-10a | Upregulation | PIK3CA | Increased CDDP resistance | Diagnosis and prognosis | 6 patients A549, H1299 cell lines | Huang et al. [21] | 2020 |
| miR-133b | Upregulation | EGFR | Increased CDDP sensitivity | Diagnosis | 24 patients A549 cell line | Li et al. [22] | 2018 |
| miR-221/222 | Downregulation | PTEN | Increased CDDP sensitivity | Diagnosis | A2780 cell line | Amini-Farsani et al. [23] | 2018 |
| miR-654-3p | Upregulation | PI3K/AKT | Increased CDDP sensitivity | Diagnosis | 20 patients IGROV-1, 293T cell lines | Niu et al. [24] | 2020 |
| miR-21 | Upregulation | PTEN | Increased CDDP resistance | Diagnosis | SGC7901 cell line | Yang et al. [25] | 2013 |
| miR-95-3p | Upregulation | EMP1 | Increased CDDP resistance | Diagnosis and prognosis | 92 patients SGC7901, AGS cell lines | Ni et al. [26] | 2021 |
| HOTAIR | Downregulation Upregulation |
miR-34a | Increased CDDP sensitivity | Diagnosis and prognosis | 27 patients SGC7901, MGC803 cell lines | Cheng et al. [27] | 2018 |
| FOXD1-AS1 | Upregulation | miR-466 | Increased CDDP resistance | Diagnosis | BGC-823, MKN28, MGC803, MKN45, AGS cell lines | Wu et al. [28] | 2021 |
| miR-4295 | Upregulation | LRIG1 | Increased CDDP resistance | Diagnosis | MKN-28, NCI-N87, SGC-7901, MKN-45, BGC-823 cell lines | Yan et al. [29] | 2018 |
| OIP5-AS1 | Upregulation | miR-340-5p | Increased CDDP resistance | Diagnosis | MG63-CR and SAOS2-CR cell lines | Song et al. [30] | 2019 |
| miR-497 | Upregulation | VEGFA | Increased CDDP sensitivity | Diagnosis | 74 patients SAOS-2 cell line | Shao et al. [31] | 2015 |
| miR-221 | Downregulation | PTEN | Increased CDDP sensitivity | Diagnosis | 60 patients SOSP-9607, SOSP-9901, MG63, SAOS-2, U20S, hFOB1.19 cell lines | Zhao et al. [32] | 2013 |
| miR-145 | Upregulation | AKT3 | Increased CDDP sensitivity | Diagnosis and prognosis | 30 patients EC109, EC9706, KYSE-150, KYSE-30, TE1 cell lines | Zheng et al. [33] | 2019 |
| MALAT1 | Downregulation | BRWD1 | Increased CDDP sensitivity | Diagnosis | Hela and C-33A cell lines | Wang et al. [34] | 2018 |
| miR-205-5p | Downregulation | PTEN | Increased CDDP sensitivity | Diagnosis | MG63, SAOS2 cell lines | Zhang et al. [35] | 2019 |
| DANCR | Upregulation | miR-33b5p, miR-33a-5p, miR-206, miR-1-3p, and miR-613 | Increased CDDP resistance | Diagnosis | U87MG, U251MG, LN18, U138MG cell lines | Ma et al. [36] | 2018 |
Table 2. All of the proteins that affect Cisplatin response using the regulation of PI3K/AKT pathway.
| Gene | Mechanism | Effect on the tumor cells | Clinical application | Samples | Study | Year |
|---|---|---|---|---|---|---|
| BC200 | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | 76 patients H1299, A549, SKMES1, PC9, H358, SPCA1 cell lines | Gao et al. [37] | 2019 |
| Survivin | Upregulation | Increased CDDP resistance | Diagnosis | SW2, H82, U1285, A549, MPM cell lines | Belyanskaya et al. [38] | 2005 |
| Klotho | Upregulation | Increased CDDP sensitivity | Diagnosis | A549, H460 cell lines | Wang et al. [39] | 2013 |
| XPC | Downregulation | Increased CDDP sensitivity | Diagnosis | A549 cell line | Teng et al. [40] | 2019 |
| KLF5 | Downregulation | Increased CDDP sensitivity | Diagnosis | H1299, A549 cell lines | Gong et al. [41] | 2018 |
| PAX6 | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | 92 patients A549, SPC-A-1 cell lines | Wu et al. [42] | 2019 |
| GPX1 | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | A549, H1975, H460, H1650 cell lines | Chen et al. [43] | 2019 |
| USP17 | Upregulation | Increased CDDP resistance | Diagnosis | A549, H1299 cell lines | Zhang et al. [44] | 2020 |
| HSPA12B | Upregulation | Increased CDDP resistance | Diagnosis | A549 cell line | Chen et al. [45] | 2018 |
| HPIP | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | OAW42, PA-1, SKOV3 cell lines | Bugide et al. [46] | 2017 |
| HOXB4 | Downregulation | Increased CDDP sensitivity | Diagnosis and prognosis | MKN-28, NCI-N87, SGC-7901, MKN-45, BGC-823 cell lines | Li et al. [47] | 2018 |
| Rab25 | Upregulation | Increased CDDP resistance | Diagnosis | SKOV-3, ES-2 cell lines | Fan et al. [48] | 2015 |
| CCL5 | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | 62 patients SKOV3 cell line | Zhou et al. [49] | 2016 |
| PER2 | Upregulation | Increased CDDP sensitivity | Diagnosis and prognosis | SKOV3 cell line | Wang et al. [50] | 2020 |
| ACLY | Downregulation | Increased CDDP sensitivity | Diagnosis and prognosis | 47 patients A2780, SKOV3, HEY cell lines | Wei et al. [51] | 2021 |
| CD133 | Downregulation | Increased CDDP sensitivity | Diagnosis | KATO-111 Cell line | Lu et al. [52] | 2019 |
| Neogenin-1 | Upregulation | Increased CDDP resistance | Diagnosis | MKN-28, BGC-823, MGC-803, SGC-7901, MKN-45 cell lines | Qu et al. [53] | 2018 |
| PAK4 | Downregulation | Increased CDDP sensitivity | Diagnosis | 49 patients AGS, MKN-45 cell lines | Fu et al. [54] | 2014 |
| HtrA1 | Downregulation | Increased CDDP resistance | Diagnosis | SW480 cell line | Xiong et al. [55] | 2017 |
| MNAT1 | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | 78 Patients MG63, U2OS, Well5, 143B cell lines | Qiu et al. [56] | 2020 |
| XPD | Upregulation | Increased CDDP sensitivity | Diagnosis and prognosis | 20 patients EC9706, EC109 cell lines | Jian et al. [57] | 2020 |
| ALC1 | Downregulation | Increased CDDP sensitivity | Diagnosis | TE1, TE13, ECA109, and EC9706 cell lines | Li et al. [58] | 2019 |
| RACK1 | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | Eca109, EC9706 cell lines | Liu et al. [59] | 2018 |
| PAK4 | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | 93 patients Hela, CaSki cell lines | Shu et al. [60] | 2015 |
| PRRX1 | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | MCF-7 cell line | Luo et al. [61] | 2020 |
| Ghrelin | Upregulation | Increased CDDP resistance | Diagnosis | MDA-MB-231 cell line | Zhang et al. [62] | 2020 |
| SPP1 | Downregulation | Increased CDDP sensitivity | Diagnosis | 16 patients C-33A (Hela and CaSki) cell line | Chen et al. [63] | 2019 |
| Par-4 | Downregulation | Increased CDDP resistance | Diagnosis | BXPC-3 cell line | Tan et al. [64] | 2014 |
| mTOR | Upregulation | Increased CDDP sensitivity | Diagnosis | SW1990 cell line | Li et al. [65] | 2019 |
| CDKL5 | Upregulation | Increased CDDP resistance | Diagnosis | 27 patients U87, U251 cell lines | Jiang et al. [66] | 2020 |
| SDC1 | Upregulation | Increased CDDP resistance | Diagnosis and prognosis | 30 patients HepG2 cell line | Yu et al. [67] | 2020 |
| HMGN5 | Downregulation | Increased CDDP sensitivity | Diagnosis | UBC5637, UM-UC-3, T24 cell lines | Gan et al. [68] | 2017 |
| Derlin-1 | Downregulation | Increased CDDP sensitivity | Diagnosis and prognosis | 150 patients SV-HUC-1, BIU-87, J82, 5637 cell lines | Dong et al. [69] | 2017 |
| TDRG1 | Upregulation | Increased CDDP resistance | Diagnosis | 35 patients TCam-2 cell line | Gan et al. [70] | 2016 |
Lung Cancer
PI3K/AKT/mTOR pathway has pivotal role in tumor progression, drug resistance, and poor prognosis in various cancers [71]. PTEN also induces autophagosome formation by PI3K/AKT inhibition [72]. MicroRNAs (miRNAs) are a class of non-coding RNAs that have pivotal roles in post-transcriptional regulation by mRNA degradation or translational inhibition [73,74]. There was miR-181 downregulation in CDDP -resistant Non-small-cell lung carcinoma (NSCLC) patients compared with controls. MiR-181 reduced cell growth and invasion, while promoting autophagy in lung tumor cells via PTEN/PI3K/AKT/mTOR axis [19]. MiR-1269b induced cell proliferation and CDDP resistance by PTEN targeting that resulted in PI3K/AKT signaling activation in lung tumor cells. There was also significant miR-1269b up-regulation in NSCLC patients that was correlated with CDDP resistance and survival [20]. PIK3CA as a subunit of PI3K has a pivotal role in PI3K/AKT/mTOR activation. There was miR-10a upregulation in circulating lung tumor cells that were associated with poor prognosis by induction of CDDP resistance. MiR-10a promoted CDDP resistance through PIK3CA targeting in circulating lung tumor cells [21]. There was a direct association between the levels of BC200 expression and clinicopathological features of the tumor, including tumor stage and lymph node involvement in NSCLC patients. BC200 knockdown significantly downregulated the PI3K, AKT, and STAT3. It induced NSCLC metastasis and regulated the cisplatin-induced apoptosis via PI3K/AKT pathway [37]. MiR-133b reduced lung tumor cell proliferation, while promoting apoptosis by CCND1 downregulation and Bax upregulation, respectively. It also downregulated the EGFR, p-STAT3, and p-JAK2 in cisplatin-induced lung tumor cells. Therefore, miR-133b reduced cell proliferation by EGFR targeting that mediated PI3K/AKT in cisplatin-induced NSCLC cells [22].
Apoptosis consists of two converging cascades, including extrinsic and intrinsic pathways [75]. Death ligands are initiators of the extrinsic pathway by a death-inducing signaling complex that results in caspase-8 activation. However, the intrinsic pathway is associated with the release of cytochrome c from mitochondria that activates caspases. Inhibitors of apoptosis proteins (IAP) are endogenous caspases inhibitors in intrinsic and extrinsic pathways [76]. Survivin belongs to the IAPs that regulate cell proliferation and suppress apoptosis [77]. AKT has a pivotal role in regulation of PI3K signaling, which is implicated in cell proliferation and metabolism [78]. It has been shown that cisplatin resistance can be associated with cisplatin-induced AKT activation that results in survivin induction in SCLC. There was survivin upregulation in SCLC cells following the cisplatin treatment [38].
Klotho is a transmembrane protein that functions as an inhibitor of the IGF-1 pathway [79]. PI3K/AKT is also a downstream cascade of IGF-1 pathway with pivotal roles in regulation of apoptosis and CDDP response [80,81]. There was significant klotho downregulation and p-AKT upregulation in CDDP-resistant lung tumor cells. Klotho upregulation significantly increased the Bax/Bcl-2 ratio in CDDP resistant cells. Therefore, klotho alleviated the CDDP resistance by regulation of PI3K/AKT pathway and Bax/Bcl-2 expression ratio in lung tumor cells [39].
Nucleotide excision repair is involved in the repair of DNA damages caused by CDDP [82]. Xeroderma pigmentosum complementation group C (XPC) is a key detector of DNA damage [83]. It has been reported that XPC silencing significantly reduced cell proliferation while promoting apoptosis in A549/CDDP cells. XPC silencing also significantly increased the Bax/Bcl-2 ratios in A549/CDDP cells, while downregulated the p-AKT. Therefore, XPC inhibition significantly induced apoptosis in CDDP-resistant lung tumor cells [40].
ATP-binding cassette proteins (ABC) are trans-membrane proteins involved in multi-drug resistance [84]. BCRP belongs to the ABC protein family that pumps intracellular drug to reduce intracellular concentration and increase drug resistance [85]. Long noncoding RNAs (lncRNAs) are a class of none-coding RNAs involved in regulation of miRNAs as competing endogenous RNAs (ceRNAs) [86]. Deregulation of lncRNAs is involved in cell proliferation, apoptosis, migration, and drug resistance [87]. SNHG7 knockdown increased cisplatin-sensitivity by downregulations of MRD1 and BCRP in NSCLC. There was also SNHG7 upregulation in NSCLC tissues compared with normal margins which was correlated with advanced-stage and CDDP-resistant. Since SNHG7 silencing significantly downregulated the PI3K, p-AKT, and p-mTOR in cisplatin-resistant NSCLC cells, SNHG7 promoted cisplatin-resistance via MRD1 and BCRP upregulations through PI3K/AKT pathway in NSCLC [88].
Hypoxia is commonly observed in solid tumors that affects pathophysiological processes such as angiogenesis and drug resistance [89]. Hypoxia inducible factor-1α (HIF-1α) is a pivotal regulator of cell proliferation and glycolysis during hypoxia response [90]. Krüppel-like factor 5 (KLF5) is a developmental transcription factor that regulates the levels of HIF-1α expression [91]. There was a significant KLF5 upregulation in hypoxic NSCLC cells. KLF5 knockdown reduced the levels of P-gp and HIF-1α expressions in hypoxic NSCLC cells. KLF5 knockdown also suppressed HIF-1α-dependent glycolysis through PI3K/AKT/mTOR inactivation that resulted in reduced hypoxia-induced cisplatin resistance [41].
Epithelial-to-mesenchymal transition (EMT) is a pathophysiological process in which the tumor cells lose their epithelial properties to obtain the mesenchymal phenotype [92,93]. Following the EMT, mesenchymal tumor cells obtain invasive and metastatic features [94]. It has been shown that there was PAX6 upregulation in NSCLC, which was significantly associated with lower overall survival (OS). PAX6 was involved in regulation of ZEB2 as a critical factor in EMT-mediated self renewal, thereby promoting cisplatin resistance in NSCLC. PAX6-ZEB2 also induced tumor invasion via PI3K/AKT dependent downregulation of CDH1 in NSCLC [42]. AFAP1-AS1 is an oncogenic lncRNA that encodes AFAP1 gene antisense [95]. There was AFAP1-AS1 upregulation in CDDP-resistant NSCLC tissues and cells. AFAP1-AS1 silencing reduced cell proliferation and migration via regulation of EMT process and PI3K/AKT pathway. It significantly promoted EMT in CDDP-resistant NSCLC cells. AFAP1-AS1 also inhibited apoptosis by regulation of EZH2 to activate PI3K/AKT pathway which increased CDDP resistance in NSCLC cells [96].
ROS is normally produced during cellular metabolism that regulates cell proliferation and apoptosis. Apoptosis-related ROS is a fundamental mechanism of CDDP [97]. ROS is produced by mitochondria due to the cisplatin treatment [98]. Glutathione peroxidase 1 (GPX1) is an antioxidant enzyme involved in ROS metabolizing. It has been reported that there was GPX1 upregulation in NSCLC cells. GPX1 inhibited cisplatin-induced ROS accumulation that promoted PI3K-AKT pathway. GPX1 downregulation promoted apoptosis via increased ROS accumulation and AKT suppression in CDDP-resistant NSCLC cells [43].
Deubiquitinating enzymes (DUBs) are the inhibitors of protein degradation by removing the ubiquitin chains [99]. Ubiquitin-specific protease 17 (USP17) belongs to the DUB protein family that regulates cell migration, inflammation, and tumor progression. USP17 regulates the Ras pathway to affect cell migration and proliferation [100,101]. It has been reported that CDDP increased the levels of USP17 expression. USP17 also induced the NSCLC cell proliferation through PI3K/AKT activation [44]. HSPA12B belongs to the HSP70 protein family that is directly associated with CDDP resistance through p-IκBα and p-AKT upregulations and caspase-3 downregulation in lung tumor cells [45].
Ovarian Cancer
Cisplatin resistance can be developed by increased drug efflux, increased detoxification, induced DNA repair, and reduced drug-induced apoptosis [102]. Cancer stem cells (CSCs) and EMT process are contributed with the chemoresistance and tumor relapse in ovarian cancer patients [103]. PI3K/AKT/mTOR signaling has a pivotal role in regulation of cell cycle, cell proliferation, and chemoresistance [104]. There was E-cadherin downregulation, while N-cadherin and Vimentin upregulations in cisplatin resistant ovarian tumor cells compared with sensitive cells. EMT process was along with increased levels of CSC markers in CDDP-resistant ovarian tumor cells. Suppression of PI3K/AKT/mTOR axis significantly inhibited the EMT and CSC features [105]. The hematopoietic PBX interaction protein (HPIP) is an oncogene that activates PI3K/AKT, MAPK, and SHH signaling pathways to promote tumor progression and metastasis [106–108]. HPIP silencing inhibited AKT and MAPK in ovarian tumor cells. HPIP promoted cisplatin resistance of ovarian cancer cells. There was a direct association between the levels of HPIP expression and higher tumor grades. HPIP activated the PI3K/AKT pathway, which resulted in E-cadherin downregulation, while stabilized Snail in ovarian tumor cells [46]. The Homeobox (HOX) family of transcription factors are pivotal regulators of developmental processes and tumor progressions [109]. There was HOXB4 upregulation in CDDP-resistant ovarian cancer cells. Silencing of HOXB4 increased CDDP sensitivity by PI3K/AKT suppression that resulted in ABC transporters downregulations in ovrian tumor cells [47].
Deregulation of miRNAs is one of the main reasons of chemotherapeutic resistance by targeting the drug response genes in various cancers [110]. As a tumor suppressor, PTEN is involved in regulation of cell growth, migration, and apoptosis [111]. It has been reported that there was a significant miR-221/222 upregulation in ovarian tumor cells. MiR-221/222 downregulation promoted apoptosis and cisplatin sensitivity through PTEN targeting and subsequent PI3K/AKT activation in ovarian cancer [23]. There was significant miR-654-3p downregulation in CDDP-resistant ovarian tumor cells. MiR-654-3p suppressed cell proliferation and migration while promoting the CDDP sensitivity by inactivation of PI3K/AKT pathway. It also suppressed P-gp via QPRT targeting which may associate with CDDP sensitivity in ovarian tumor cells [24]. Rab25 belongs to the GTPase protein family that has key roles in cell proliferation, signal transduction, and cytoskeletal organization [112]. PI3K/AKT signaling upregulated the Rab25 to induce CDDP resistance in ovarian tumor cells [48].
Autophagy is associated with drug response via different signaling pathways in tumor cells [113,114]. STAT3 has a dual function in regulation of autophagy in which the nuclear STAT3 positively regulates the transcription of autophagy-related genes, while cytoplasmic STAT3 has a negative role by interacting with FOXO1 and FOXO3 transcription factors. The mitochondrial STAT3 also inhibits the oxidative stress that is promoted by autophagy and maintains the mitochondria from mitophagy-related degradation [115]. It has been reported that STAT3 induced EMT while inhibited the autophagy that resulted in tumor cell proliferation, invasion, and cisplatin resistance through Slug and MAPK in ovarian cancer. STAT3 also suppressed the autophagy that resulted in cisplatin resistance following the activation of the PI3K/AKT and MEK/ERK pathways in ovarian tumor cells [116].
Cancer-associated fibroblasts (CAFs) as the main stromal cells in the tumor microenvironment can also be affected by the cisplatin treatment that can interfere with the normal tissue homeostasis. CCL5 belongs to the CC-chemokine family that has pivotal roles in drug resistance and metastasis. STAT3 is a transcriptional modulator of chemokines responses and growth factors that regulates cell invasion and chemotherapy resistance [117]. PI3K/AKT signaling pathway can also be activated by CCL5 [118]. It has been reported that CAFs promoted CDDP resistance via CCL5 production in ovarian tumor cells. CCL5 induced CDDP-resistance through the p-AKT and p-STAT3 pathways [49]. PER2, as a circadian factor, has critical role during tumor progression by apoptosis induction [119,120]. It has been shown that PER2 increased CDDP sensitivity in ovarian tumor cells by PI3K and AKT downregulations. It also increased the levels of caspase 3 expressions that resulted in apoptosis induction. There were significant serum TNF-α and IL-6 downregulations in PER2 upregulated tumors before the CDDP treatment. Therefore, PER2 reduced systemic inflammation to promote CDDP sensitivity in ovarian cancer patients [50].
Growth hormone secretagogue receptor (GHSR) is activated by Ghrelin that has a key role in tumor progression [121]. Ghrelin is observed in acylated and unacetylated forms in blood. The acetylated gherlin is the most active while less abundant form that activates secretagogue receptor type 1a (GHS-R1a). The unacylated ghrelin is the most abundant while less active form that functions independently of GHS-R1a [122]. Acetylated gherlin promoted the cisplatin resistance and cell proliferation in ovarian tumor cells. Gherlin mediates its role through GHS-R1a and activation of PI3K/AKT pathway that results in p53 and PUMA downregulations in ovarian cancer [123]. ATP citrate lyase (ACLY) catalyzes the conversion of the citrate and coenzyme A to acetyl CoA and oxaloacetate (OAA) [124]. Acetyl-CoA has a critical role in transcriptional regulation by histones acetylation. OAA is also an important substrate for aspartate production that is required for nucleotide synthesis and redox reactions [125]. There was significant ACLY upregulation in CDDP-resistant ovarian tumor cells. ACLY knockdown reduced cell proliferation, while promoted apoptosis in ovarian tumor cells via CCND1 and CDK4 downregulations and P16 upregulation. Knockdown of ACLY reduced the cell proliferation and CDDP resistance via p-AKT suppression and P16–CCND1–CDK4 axis regulation in ovarian cancer [51].
Gastric and Colorectal Cancers
Cancer stem cells are a subpopulation of tumor cells involved in chemotherapeutic resistance and tumor relapse [126]. They obtain drug resistance by upregulation of extrusion pumps and DNA repair proteins. Therefore, conventional anti-tumor agents are not effective in the elimination of CSCs and they cause recurrence [127]. CD133 is a glycoprotein involved in activation of the PI3K/AKT pathway through PI3K-p85 [128]. It has been reported that there were CD133 upregulations in cisplatin-resistant gastric tumor cells compared with sensitive cells. CD133 increased CDDP resistance via PI3K/AKT/mTOR activation in gastric tumor cells [52].
MiRNAs and lncRNAs have diverse functions in cellular processes such as cell differentiation, proliferation, and apoptosis. PTEN is a negative regulator of PI3K/AKT by dephosphorylation of PIP3. MiR-22-3p promoted cisplatin sensitivity though PTEN upregulation and PI3K/AKT inhibition in gastrointestinal tumors [129]. There was a significant miR-21 upregulation in cisplatin resistant gastric tumor cells in comparison with sensitive cells. MiR-21 regulated the PTEN expression as a pivotal factor in drug resistance [25]. There was a significant miR-95-3p upregulation in CDDP-resistant compared with sensitive gastric tumor cells. MiR-95-3p induced CDDP resistance, cell proliferation, and migration through EMP1 targeting that activated PI3K/AKT pathway in gastric tumor cells [26]. Knockdown of HOTAIR reduced cisplatin resistance and tumor growth through miR-34a upregulation that resulted in suppression of the PI3K/AKT and WNT signaling pathways in gastric tumor cells. There was also HOTAIR upregulation in GC tissues compared with normal margins [27]. MALAT1 induced cisplatin resistance of GC cells. There was a significant correlation between the levels of MALAT1 expression and poor survival in GC patients. MALAT1 promoted GC proliferation and invasion by p-PI3K and p-AKT upregulations and activation of PI3K/AKT pathway in gastric tumor cells. MALAT1 was also involved in CDDP resistance by regulation of Bcl-2 [130].
ZEB1 and ZEB2 are zinc finger EMT-transcription factors [94]. Neogenin-1 (Neo1) is a transmembrane receptor and member of the immunoglobulin superfamily [131]. Neo1 promoted cell migration and cisplatin resistance in gastric tumor cells. It also induced EMT process by activation of the Rac1/PI3K/AKT axis that resulted in ZEB1, CDH2, and VIM upregulations, while CDH1 downregulation in gastric tumor cells [53]. P21-activated kinases (PAKs) have critical roles in regulation of angiogenesis, EMT, and metabolic processes [132,133]. PAK4 is a key regulator of tumor cell migration that is down stream effector of Met receptor [134]. It has been reported that PAK4 promoted CDDP resistance by activation of PI3K/AKT pathways in gastric tumor cells [54]. There was significant UCA1 upregulation in gastric tumor tissues compared with normal margins which was correlated with lymph node involvement, distant metastasis, and advanced tumor stage. UCA1 also induced CDDP resistance through EZH2 targeting and PI3K/AKT activation in gastric tumor cells [135].
4E-BP1 is a translational inhibitor and down stream target of the PI3K/AKT/mTOR pathway [136,137]. FOXD1-AS1 activated the PI3K/AKT/mTOR signaling via miR-466 sponging and subsequent PIK3CA release that resulted in 4E-BP1 hyperphosphorylation. Activation of 4E-BP1 also induced eIF4E and eIF4G interaction that upregulated the FOXD1 protein to promote CDDP resistance in gastric tumor cells. Therefore, FOXD1-AS1 increased CDDP resistance via translational regulation of FOXD1 in gastric cancer [28]. EGFR is a key signaling pathway during tumor progression that functions by various intracellular cascades, such as MAPK and PI3K/AKT to promote cell proliferation and invasion [138,139]. LRIG1 is a negative regulator of the EGFR [140]. It has been shown that miR-4295 induced cell proliferation, while suppressed the CDDP-induced apoptosis in gastric tumor cells through LRIG1 targeting and subsequent activation of EGFR/PI3K/AKT axis [29].
HtrA1 belongs to the serine protease protein family involved in cell proliferation, apoptosis, and embryogenesis [141]. XIAP is an anti apoptotic factor that inhibits caspase-3, caspase-7, and caspase-9 [142]. HtrA1 has a critical role in XIAP targeting and degradation during the chemotherapeutic responses [143]. It has been reported that HtrA1 downregulation increased CDDP resistance through XIAP and PI3K/AKT activation in colon tumor cells [55].
Osteosarcoma
MRP1 and P-gp belong to the ATP-binding cassette (ABC) transporters that are involved in drug resistance [144,145]. There was a significant OIP5-AS1 upregulation in the cisplatin-resistant osteosarcoma (OS) cells compared with sensitive cells. OIP5-AS1 silencing inhibited cell proliferation and reduced cisplatin resistance in OS cells. Reduced levels of OIP5-AS1 expression significantly inhibited the PI3K/AKT/mTOR axis. OIP5-AS1 regulated the cisplatin resistance through the PI3K/AKT/mTOR pathway via miR-340-5p targeting and subsequent LPAATβ upregulation in OS cells [30]. Cyclin-dependent kinase-activating kinase (CAK) complex contains CDK7, Cyclin H, and MNAT1 [146]. It has been shown that there were MNAT1 upregulations in osteosarcoma tissues that were correlated with poor prognosis. MNAT1 downregulation suppressed osteosarcoma cell proliferation, invasion, and in-vivo growth. It also promoted cisplatin resistance through PI3K/AKT/mTOR activation [56].
There is a close correlation between the autophagy and chemoresistance. Autophagy maintains the cellular homeostasis by removing the damaged cellular components via autophagosomes. Therefore, it maintains a balance between the synthesis and degradation to protect the cells during nutrient deprivation [147,148]. However, it can also promote apoptosis due to the excessive proteins lack [149]. It has been reported that miR-22 regulated CDDP-resistance via autophagy inhibition in osteosarcoma cells. MiR-22 suppressed CDDP induced autophagy and reduced drug resistance through PI3K/AKT/mTOR axis in osteosarcoma [150]. There was a significant miR-497 downregulation in osteosarcoma tissues compared with normal margins. MiR-497 downregulation promoted cisplatin resistance via PI3K/AKT pathway by VEGFA targeting in osteosarcoma [31]. PTEN dephosphorylates PIP3 to suppress AKT activity [151]. It has been shown that miR-221 promoted cell proliferation and CDDP resistance, while reduced apoptosis via PTEN targeting and subsequent PI3K/AKT activation in osteosarcoma cells. MiR-221 also regulated the levels of Bcl-2, CCND1, and p27. There was also miR-221 upregulation in osteosarcoma tissues [32].
Esophageal Squamous Cell Carcinoma
Tumor malignancy is associated with genome instability which is the result of aberrant DNA repair, replication, and chromosome segregation. Xeroderma pigmentosum complementation group D (XPD) is a DNA helicase involved in DNA repair that is caused by oxidative stress [152]. There was a significant XPD downregulation in esophageal squamous cell carcinoma (ESCC) tissue compared with normal margins. XPD upregulation significantly suppressed the cell proliferation and migration, while increased CDDP sensitivity by suppression of the PI3K/AKT pathway [57]. ABC transporters are among the most important proteins involved in efflux of anticancer drugs which reduce the chemotherapeutics efficacy. Multidrug resistance-associated protein 1 (MRP1) is closely associated with opposed chemotherapeutic outcomes [153]. It has been observed that miR-145 increased CDDP sensitivity of ESCC cells through MRP1 and P-gp downregulations following the AKT3 targeting and subsequent PI3K/AKT inhibition. MiR-145 suppressed anti-apoptotic factors including CCND1 and Bcl-2, while induced Bax through PI3K/AKT inhibition in ESCC cells [33].
Amplified in liver cancer 1 gene (ALC1) belongs to the chromatin remodeling enzymes involved in tumor cell proliferation and metastasis [154]. There was ALC1 upregulation in ESCC cells. ALC1 knockdown reduced cell growth and increased CDDP sensitivity in ESCC cells through inactivation of PI3K/AKT pathway and subsequent glycolysis suppression. ALC1 knockdown also upregulated the caspase-3/7 and increased apoptosis in ESCC cells that induced CDDP sensitivity in ESCC cells [58]. RACK1 belongs to the WD40 repeat protein family that functions in protein anchoring and shuttling between cellular compartments. It is also involved in transcriptional and translational regulations [155]. As a scaffold protein, RACK1 binds with kinases and membrane-bound receptors to regulate cell proliferation, adhesion, and migration [156]. It has been observed that RACK1 increased cell proliferation and resistance toward the CDDP induced apoptosis in ESCC. RACK1 promoted CDDP-resistance by PI3K/AKT activation and subsequent Bcl-2 upregulation in ESCC cells [59].
Cervical and Breast Cancers
PAKs are serine/threonine kinases characterized as the effectors of Rac and Cdc42 [157]. They have pivotal roles in tumor progression by regulation of the Ras-induced metabolism, cell proliferation, angiogenesis, and EMT [133,158]. PAK4 belongs to the PAKs family which is involved in cervical cancer progression and cisplatin resistance. There was a significant PAK4 upregulation in cervical tumor tissues compared with normal margins which were correlated with FIGO stage, grade, and lymph node involvement. PAK4 also promotes the cisplatin resistance in cervical tumor cells through PI3K/AKT pathway [60]. P-glycoprotein (P-gp) is encoded by ABCB1 which induces chemoresistance [159]. Paired-related homeobox 1 (PRRX1) is a transcriptional co-activator that promotes EMT process during tumor progressions [160]. It was shown that PRRX1 reduced chemosensitivity of breast tumor cells by P-gp upregulation. PRRX1 also increased PI3K and AKT phosphorylations following the PTEN suppression in breast cancer [61].
Ghrelin is a hormone produced by the stomach that is involved in energy homeostasis via regulation of adipocyte function and glucose metabolism. Ghrelin reduced the CDDP sensitivity by activation of PI3K/AKT/mTOR signaling, Bcl-2 upregulation, and caspase-3 downregulation in breast tumor cells. PI3K/AKT/mTOR axis regulated the ghrelin-induced anti-apoptosis in breast tumor cells treated with cisplatin [62]. Secreted phosphoprotein 1 (SPP1) is a cytokine and cell-matrix adherence protein associated with regulation of cell proliferation, apoptosis, and migration. It has been observed that there was SPP1 upregulation in cervical cancer tissues. SPP1 suppression reduced CDDP resistance through inhibition of PI3K/AKT pathway [63]. BRWD1 is a developmental transcriptional factor that has a pivotal role in chromatin remodeling and transcriptional regulation. It has been shown that MALAT1 downregulation induced the cisplatin sensitivity in cervical tumor cells through regulating BRWD1 and cell apoptosis. The p-PI3K and p-AKT were also up-regulated following MALAT1 over expression [34].
Nasopharyngeal and Pancreatic Cancers
EMT is a cellular process that mediates differentiation of epithelial to mesenchymal cells via suppression of adhesion molecules, while upregulation of mesenchymal proteins. This process facilitates separation of tumor cells from primary tumors and invasion to the secondary sites [161]. EMT is orchestrated by E-cadherin (epithelial factor) downregulation, while Vimentin and N-cadherin (mesenchymal factors) upregulations [162]. EMT has a critical role in regulation of drug resistance [163]. PI3K/AKT signaling pathway is involved in regulation of tumor invasion and drug resistance [164]. PTEN is considered as a negative regulator of PI3K/AKT pathways in which it dephosphorylates the PIP3 which results in AKT inactivation and PI3K/AKT suppression [165]. MiR-205-5p promoted the ECM degradation by MMP-9 and MMP-2 up-regulations. It downregulated Ecadherin, while upregulated the Vimentin, N-cadherin, Slug, and Snail in nasopharyngeal tumor cells. Therefore, miR-205-5p induced EMT through PTEN inhibition in cisplatin-resistant NPC cells [35]. Prostate apoptosis response-4 (Par-4) is a pro-apoptotic factor highly expressed in apoptosis-induced prostate tumor cells [166]. It has been shown that Par-4 downregulation promoted CDDP resistance through stimulation of PI3K/AKT-dependent EMT in pancreatic tumor cells [64]. mTOR is one of the main effectors of PI3K/AKT that integrates the inputs from growth factors [167]. It is also a sensor of nutrients and oxygen [168]. It has been shown that the mTOR inhibited AKT phosphorylation to increase the cisplatin sensitivity in pancreatic tumor cells [65].
Other Cancers
AXL belongs to the receptor tyrosine kinase (RTK) protein family that activates PI3K/AKT pathway to promote cell proliferation and regulate tumor drug resistance [169,170]. It has been reported that there was an inverse association between the levels of DANCR expression and CDDP sensitivity in glioma cells. DNACR promoted cisplatin resistance via miR-33b-5p, miR-33a-5p, miR-206, miR-1-3p, and miR-613 sponging and AXL upregulation. DANCR activated the PI3K/AKT/NF-κB axis by increased AKT and IκBα phosphorylations [36]. Cigarette smoking increases urothelial tumor aggressiveness by promoting tumor proliferation and angiogenesis. Nicotine functions through different receptors, such as nicotinic acetylcholine receptors and/or EGF receptors [171]. It regulates PI3K/AKT pathway in different cancer cells [172,173]. It has been reported that nicotine promoted the CDDP resistance by PI3K/AKT/mTOR activation [174].
Cyclin-dependent kinase-like 5 (CDKL5) is a positive regulator of PI3K. There was CDKL5 upregulation in glioma tissues compared with normal samples. CDKL5 upregulation promoted CDDP drug resistance, cell migration, and proliferation in glioma cells via phosphorylation of PI3K and AKT and subsequent PI3K/AKT activation [66]. Syndecan 1 (SDC1) is a heparan sulfate proteoglycan involved in cell attachment, signaling, and cytoskeletal organization [175]. It has been reported that there was SDC1 upregulation in advanced stage and drug resistant liver tumors. There was also a correlation between AKT activation and SDC1 upregulation, which resulted in cisplatin resistance [67].
HMGN5 is a developmental transcriptional regulator and chromatin remodeler [176]. There was an inverse association between the levels of HMGN5 expressions and CDDP sensitivity in urothelial bladder tumor cells. HMGN5 knockdown induced CDDP sensitivity through suppression of PI3K/AKT signaling and subsequent cytochrome c, caspase-3, and PARP upregulations that confirmed the activation of the intrinsic apoptosis pathway [68]. Derlin-1 is a pivotal factor involved in the elimination and the retrotranslocation of misfolded proteins from endoplasmic reticulum [177]. There was Derlin-1 upregulation in bladder tumor tissues and cells. Derlin-1 also promoted chemoresistance through activation of PI3K/AKT/Bcl-2 axis in bladder tumor cells [69]. Testis developmental related gene 1 (TDRG1) induces seminoma cell proliferation and invasion via activation of PI3K/AKT pathway [178]. It positively regulated the p-mTOR and affected the cell cycle progression in seminoma cells during CDDP treatment. TDRG1 regulated the CDDP sensitivity through the PI3K/AKT/mTOR signaling and intrinsic apoptosis pathway in seminoma cells [70].
Conclusions
CDDP is a widely used first-line anti-cancer drug in various cancers. Since, CDDP has severe side effects on different normal organs and tissues in cancer patients; it is required to determine the CDDP-resistant from sensitive tumors. Therefore, clarification of the molecular mechanisms involved in CDDP response provides novel therapeutic strategies in chemo-resistant patients. In the present review, we summarized all of the studies that have been assessed the role of PI3K/AKT pathway in Cisplatin response. It was shown that the PI3K/AKT signaling pathway regulates the Cisplatin response via different cellular mechanisms such as apoptosis, autophagy, DNA repair, ABC transporters, and EMT process. The PI3K/AKT was mainly involved in regulation of CDDP response in lung, ovarian, and gastrointestinal tumors. It was also observed that the non-coding RNAs are the pivotal regulators of Cisplatin response via PI3K/AKT pathway. Non-coding RNAs mainly affected the PI3K/AKT pathway through the RTK and PTEN regulations. This review paves the way of suggesting a PI3K/AKT related panel marker for the prediction of Cisplatin response in cancer patients. Since PI3K/AKT mainly promotes the Cisplatin resistance in different tumors, suppression of this pathway through the RTK, PI3K, and mTOR inhibitors or specific miRNAs can be efficient methods to overcome the CDDP resistance and improve the quality of life in cancer patients. Moreover, the microRNAs that are involved in regulation of CDDP response by targeting the PI3K/AKT signaling can be introduced as the non-invasive markers for the prediction of CDDP response among cancer patients. The clinical non-invasive application of PI3K/AKT related miRNAs also reduces the CDDP side effects and paves the way to select the efficient therapeutic methods in a personalized medicine that significantly improves the quality of life and patient’s survival. A combination of PI3K/AKT inhibitors with CDDP can also be a promising therapeutic modality among the cancer patients who show the CDDP resistance through PI3K/AKT signaling pathway.
Ethics Approval
This study does not use animals or human subjects and therefore does not require ethical approval.
Author Contribution
The authors confirm contribution to the paper as follows: study conception, design, and manuscript edition: Meysam MOGHBELI; draft manuscript preparation: Zahra NASRPOUR NAVAEI, Ghazaleh KHALILI-TANHA, Amir Sadra ZANGOUEI, Mohammad Reza ABBASZADEGAN; study supervision: Meysam MOGHBELI. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that they have no conflicts of interest to report regarding the present study.
References
- 1.Cao, L., Chen, J., Ou, B., Liu, C., Zou, Y.et al. (2017). GAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axis. Biomedicine and Pharmacotherapy , 93(3), 570–579. DOI 10.1016/j.biopha.2017.06.089. [DOI] [PubMed] [Google Scholar]
- 2.Dasari, S., Tchounwou, P. B. (2014). Cisplatin in cancer therapy: Molecular mechanisms of action. European Journal of Pharmacology , 740, 364–378. DOI 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, B., Huang, Z., Gao, R., Zeng, Z., Yang, W.et al. (2017). Expression of long noncoding RNA Urothelial cancer associated 1 promotes cisplatin resistance in cervical cancer. Cancer Biother Radiopharm , 32(3), 101–110. DOI 10.1089/cbr.2016.2156. [DOI] [PubMed] [Google Scholar]
- 4.Cho, H. J., Kim, I. K., Park, S. M., Baek, K. E., Nam, I. K.et al. (2014). VEGF-C mediates RhoGDI2-induced gastric cancer cell metastasis and cisplatin resistance. International Journal of Cancer , 135(7), 1553–1563. DOI 10.1002/ijc.28801. [DOI] [PubMed] [Google Scholar]
- 5.Marin, J. J., Al-Abdulla, R., Lozano, E., Briz, O., Bujanda, L.et al. (2016). Mechanisms of resistance to chemotherapy in gastric cancer. Anti-Cancer Agents in Medicinal Chemistry , 16(3), 318–334. DOI 10.2174/1871520615666150803125121. [DOI] [PubMed] [Google Scholar]
- 6.Carnero, A., Blanco-Aparicio, C., Renner, O., Link, W., Leal, J. F. (2008). The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Current Cancer Drug Targets , 8(3), 187–198. DOI 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 7.Faried, L. S., Faried, A., Kanuma, T., Nakazato, T., Tamura, T.et al. (2006). Inhibition of the mammalian target of rapamycin (mTOR) by rapamycin increases chemosensitivity of CaSki cells to paclitaxel. European Journal of Cancer , 42(7), 934–947. DOI 10.1016/j.ejca.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Hay, N. (2005). The AKT-mTOR tango and its relevance to cancer. Cancer Cell , 8(3), 179–183. DOI 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Cai, Y., Tan, X., Liu, J., Shen, Y., Wu, D.et al. (2014). Inhibition of PI3K/AKT/mTOR signaling pathway enhances the sensitivity of the SKOV3/DDP ovarian cancer cell line to cisplatin in vitro. Chinese Journal of Cancer Research , 26(5), 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gohr, K., Hamacher, A., Engelke, L. H., Kassack, M. U. (2017). Inhibition of PI3K/AKT/mTOR overcomes cisplatin resistance in the triple negative breast cancer cell line HCC38. BMC Cancer , 17(1), 711. DOI 10.1186/s12885-017-3695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng, D. J., Wang, J., Zhou, J. Y., Wu, G. S. (2010). Role of the AKT/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochemical and Biophysical Research Communications , 394(3), 600–605. DOI 10.1016/j.bbrc.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, M., Leung, B. M., Yan, X., Dan, H. C., Cheng, J. Q.et al. (2003). p53 is a determinant of X-linked inhibitor of apoptosis protein/AKT-mediated chemoresistance in human ovarian cancer cells. Cancer Research , 63(21), 7081–7088. [PubMed] [Google Scholar]
- 13.Lee, S., Choi, E. J., Jin, C., Kim, D. H. (2005). Activation of PI3K/AKT pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecologic Oncology , 97(1), 26–34. DOI 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 14.Oh, S., Kim, H., Nam, K., Shin, I. (2017). Silencing of Glut1 induces chemoresistance via modulation of AKT/GSK-3beta/beta-catenin/survivin signaling pathway in breast cancer cells. Archives of Biochemistry and Biophysics , 636(2), 110–122. DOI 10.1016/j.abb.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Lampada, A., O’Prey, J., Szabadkai, G., Ryan, K. M., Hochhauser, D.et al. (2017). mTORC1-independent autophagy regulates receptor tyrosine kinase phosphorylation in colorectal cancer cells via an mTORC2-mediated mechanism. Cell Death and Differentiation , 24(6), 1045–1062. DOI 10.1038/cdd.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tazzari, P. L., Cappellini, A., Ricci, F., Evangelisti, C., Papa, V.et al. (2007). Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/AKT signal transduction network in human acute myelogenous leukemia blasts. Leukemia , 21(3), 427–438. DOI 10.1038/sj.leu.2404523. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto, T., Takano, N., Ishiwata, K., Ohmura, M., Nagahata, Y.et al. (2014). Reduced methylation of PFKFB3 in cancer cells shunts glucose towards the pentose phosphate pathway. Nature Communications , 5(1), 3480. DOI 10.1038/ncomms4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porta, C., Paglino, C., Mosca, A. (2014). Targeting PI3K/AKT/mTOR signaling in cancer. Frontiers in Oncology , 4, 64. DOI 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, J., Xing, Y., Rong, L. (2018). miR-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the PTEN/PI3K/AKT pathway. Oncology Reports , 39(4), 1631–1639. DOI 10.3892/or.2018.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, W., Xiao, W., Cai, Z., Jin, S., Li, T. (2020). miR-1269b drives cisplatin resistance of human non-small cell lung cancer via modulating the PTEN/PI3K/AKT signaling pathway. OncoTargets and Therapy , 13, 109–118. DOI 10.2147/OTT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, T., Ren, K., Ding, G., Yang, L., Wen, Y.et al. (2020). miR10a increases the cisplatin resistance of lung adenocarcinoma circulating tumor cells via targeting PIK3CA in the PI3K/AKT pathway. Oncology Reports , 43(6), 1906–1914. DOI 10.3892/or.2020.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, B., Ding, C. M., Li, Y. X., Peng, J. C., Geng, N.et al. (2018). Over-regulation of microRNA-133b inhibits cell proliferation of cisplatin-induced non-small cell lung cancer cells through PI3K/AKT and JAK2/STAT3 signaling pathway by targeting EGFR. Oncology Reports , 39(3), 1227–1234. DOI 10.3892/or.2018.6215. [DOI] [PubMed] [Google Scholar]
- 23.Amini-Farsani, Z., Sangtarash, M. H., Shamsara, M., Teimori, H. (2018). MiR-221/222 promote chemoresistance to cisplatin in ovarian cancer cells by targeting PTEN/PI3K/AKT signaling pathway. Cytotechnology , 70(1), 203–213. DOI 10.1007/s10616-017-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu, Y. C., Tong, J., Shi, X. F., Zhang, T. (2020). MicroRNA-654-3p enhances cisplatin sensitivity by targeting QPRT and inhibiting the PI3K/AKT signaling pathway in ovarian cancer cells. Experimental and Therapeutic Medicine , 20(2), 1467–1479. DOI 10.3892/etm.2020.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang, S. M., Huang, C., Li, X. F., Yu, M. Z., He, Y.et al. (2013). miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology , 306(9), 162–168. DOI 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Ni, Q., Zhang, Y., Tao, R., Li, X., Zhu, J. (2021). MicroRNA-95-3p serves as a contributor to cisplatin resistance in human gastric cancer cells by targeting EMP1/PI3K/AKT signaling. Aging , 13(6), 8665–8687. DOI 10.18632/aging.202679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng, C., Qin, Y., Zhi, Q., Wang, J., Qin, C. (2018). Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/AKT and Wnt/beta-catenin signaling pathways by up-regulating miR-34a. International Journal of Biological Macromolecules , 107, 2620–2629. DOI 10.1016/j.ijbiomac.2017.10.154. [DOI] [PubMed] [Google Scholar]
- 28.Wu, Q., Ma, J., Wei, J., Meng, W., Wang, Y.et al. (2021). FOXD1-AS1 regulates FOXD1 translation and promotes gastric cancer progression and chemoresistance by activating the PI3K/AKT/mTOR pathway. Molecular Oncology , 15(1), 299–316. DOI 10.1002/1878-0261.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan, R., Li, K., Yuan, D. W., Wang, H. N., Zhang, Y.et al. (2018). Downregulation of microRNA-4295 enhances cisplatin-induced gastric cancer cell apoptosis through the EGFR/PI3K/AKT signaling pathway by targeting LRIG1. International Journal of Oncology , 53(6), 2566–2578. DOI 10.3892/ijo.2018.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song, L., Zhou, Z., Gan, Y., Li, P., Xu, Y.et al. (2019). Long noncoding RNA OIP5-AS1 causes cisplatin resistance in osteosarcoma through inducing the LPAATbeta/PI3K/AKT/mTOR signaling pathway by sponging the miR-340-5p. Journal of Cellular Biochemistry , 120(6), 9656–9666. DOI 10.1002/jcb.28244. [DOI] [PubMed] [Google Scholar]
- 31.Shao, X. J., Miao, M. H., Xue, J., Xue, J., Ji, X. Q.et al. (2015). The down-regulation of MicroRNA-497 contributes to cell growth and cisplatin resistance through PI3K/AKT pathway in Osteosarcoma. Cellular Physiology and Biochemistry , 36(5), 2051–2062. DOI 10.1159/000430172. [DOI] [PubMed] [Google Scholar]
- 32.Zhao, G., Cai, C., Yang, T., Qiu, X., Liao, B.et al. (2013). MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/AKT pathway in human osteosarcoma. PLoS One , 8(1), e53906. DOI 10.1371/journal.pone.0053906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng, T. L., Li, D. P., He, Z. F., Zhao, S. (2019). miR-145 sensitizes esophageal squamous cell carcinoma to cisplatin through directly inhibiting PI3K/AKT signaling pathway. Cancer Cell International , 19(1), 250. DOI 10.1186/s12935-019-0943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, N., Hou, M. S., Zhan, Y., Shen, X. B., Xue, H. Y. (2018). MALAT1 promotes cisplatin resistance in cervical cancer by activating the PI3K/AKT pathway. European Review for Medical and Pharmacological Sciences , 22(22), 7653–7659. DOI 10.26355/eurrev_201811_16382. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, P., Lu, X., Shi, Z., Li, X., Zhang, Y.et al. (2019). miR-205-5p regulates epithelial-mesenchymal transition by targeting PTEN via PI3K/AKT signaling pathway in cisplatin-resistant nasopharyngeal carcinoma cells. Gene , 710, 103–113. DOI 10.1016/j.gene.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 36.Ma, Y., Zhou, G., Li, M., Hu, D., Zhang, L.et al. (2018). Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/AKT/NF-kappaB signaling pathway. Neurochemistry International , 118, 233–241. DOI 10.1016/j.neuint.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Gao, B. B., Wang, S. X. (2019). LncRNA BC200 regulates the cell proliferation and cisplatin resistance in non-small cell lung cancer via PI3K/AKT pathway. European Review for Medical and Pharmacological Sciences , 23(3), 1093–1101. DOI 10.26355/eurrev_201902_16999. [DOI] [PubMed] [Google Scholar]
- 38.Belyanskaya, L. L., Hopkins-Donaldson, S., Kurtz, S., Simoes-Wust, A. P., Yousefi, S.et al. (2005). Cisplatin activates AKT in small cell lung cancer cells and attenuates apoptosis by survivin upregulation. International Journal of Cancer , 117(5), 755–763. DOI 10.1002/(ISSN)1097-0215. [DOI] [PubMed] [Google Scholar]
- 39.Wang, Y., Chen, L., Huang, G., He, D., He, J.et al. (2013). Klotho sensitizes human lung cancer cell line to cisplatin via PI3k/AKT pathway. PLoS One , 8(2), e57391. DOI 10.1371/journal.pone.0057391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teng, X., Fan, X. F., Li, Q., Liu, S., Wu, D. Y.et al. (2019). XPC inhibition rescues cisplatin resistance via the AKT/mTOR signaling pathway in A549/DDP lung adenocarcinoma cells. Oncology Reports , 41(3), 1875–1882. DOI 10.3892/or.2019.6959. [DOI] [PubMed] [Google Scholar]
- 41.Gong, T., Cui, L., Wang, H., Wang, H., Han, N. (2018). Knockdown of KLF5 suppresses hypoxia-induced resistance to cisplatin in NSCLC cells by regulating HIF-1α-dependent glycolysis through inactivation of the PI3K/AKT/mTOR pathway. Journal of Translational Medicine , 16(1), 164. DOI 10.1186/s12967-018-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, D. M., Zhang, T., Liu, Y. B., Deng, S. H., Han, R.et al. (2019). The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling. Cell Death and Disease , 10(5), 349. DOI 10.1038/s41419-019-1591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen, B., Shen, Z., Wu, D., Xie, X., Xu, X.et al. (2019). Glutathione peroxidase 1 promotes NSCLC resistance to cisplatin via ROS-induced activation of PI3K/AKT pathway. Biomed Research International , 2019(1), 7640547. DOI 10.1155/2019/7640547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, S., Xu, Z., Yuan, J., Chen, H. (2020). Ubiquitin-specific peptidase 17 promotes cisplatin resistance via PI3K/AKT activation in non-small cell lung cancer. Oncology Letters , 20(1), 67–74. DOI 10.3892/ol.2020.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen, W., Liu, X., Yuan, S., Qiao, T. (2018). HSPA12B overexpression induces cisplatin resistance in non-small-cell lung cancer by regulating the PI3K/AKT/NF-kappaB signaling pathway. Oncology Letters , 15(3), 3883–3889. DOI 10.3892/ol.2018.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bugide, S., Gonugunta, V. K., Penugurti, V., Malisetty, V. L., Vadlamudi, R. K.et al. (2017). HPIP promotes epithelial-mesenchymal transition and cisplatin resistance in ovarian cancer cells through PI3K/AKT pathway activation. Cellular Oncology , 40(2), 133–144. DOI 10.1007/s13402-016-0308-2. [DOI] [PubMed] [Google Scholar]
- 47.Li, Y., Sun, J., Gao, S., Hu, H., Xie, P. (2018). HOXB4 knockdown enhances the cytotoxic effect of paclitaxel and cisplatin by downregulating ABC transporters in ovarian cancer cells. Gene , 663, 9–16. DOI 10.1016/j.gene.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 48.Fan, Y., Wang, L., Han, X., Liu, X., Ma, H. (2015). Rab25 is responsible for phosphoinositide 3-kinase/AKT mediated cisplatin resistance in human epithelial ovarian cancer cells. Molecular Medicine Reports , 11(3), 2173–2178. DOI 10.3892/mmr.2014.2963. [DOI] [PubMed] [Google Scholar]
- 49.Zhou, B., Sun, C., Li, N., Shan, W., Lu, H.et al. (2016). Cisplatin-induced CCL5 secretion from CAFs promotes cisplatin-resistance in ovarian cancer via regulation of the STAT3 and PI3K/AKT signaling pathways. International Journal of Oncology , 48(5), 2087–2097. DOI 10.3892/ijo.2016.3442. [DOI] [PubMed] [Google Scholar]
- 50.Wang, Z., Li, F., Wei, M., Zhang, S., Wang, T. (2020). Circadian clock protein PERIOD2 suppresses the PI3K/AKT pathway and promotes cisplatin sensitivity in ovarian cancer. Cancer Management and Research , 12, 11897–11908. DOI 10.2147/CMAR.S278903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei, X., Shi, J., Lin, Q., Ma, X., Pang, Y.et al. (2021). Targeting ACLY attenuates tumor growth and acquired cisplatin resistance in ovarian cancer by inhibiting the PI3K-AKT pathway and activating the AMPK-ROS pathway. Frontiers in Oncology , 11, 642229. DOI 10.3389/fonc.2021.642229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu, R., Zhao, G., Yang, Y., Jiang, Z., Cai, J.et al. (2019). Inhibition of CD133 overcomes cisplatin resistance through inhibiting PI3K/AKT/mTOR signaling pathway and autophagy in CD133-positive gastric cancer cells. Technology in cancer Research & Treatment , 18(3), 1533033819864311. DOI 10.1177/1533033819864311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qu, H., Sun, H., Wang, X. (2018). Neogenin-1 promotes cell proliferation, motility, and adhesion by up-regulation of zinc finger E-box binding homeobox 1 via activating the Rac1/PI3K/AKT pathway in gastric cancer cells. Cellular Physiology and Biochemistry , 48(4), 1457–1467. DOI 10.1159/000492255. [DOI] [PubMed] [Google Scholar]
- 54.Fu, X., Feng, J., Zeng, D., Ding, Y., Yu, C.et al. (2014). PAK4 confers cisplatin resistance in gastric cancer cells via PI3K/AKT- and MEK/ERK-dependent pathways. Bioscience Reports , 34(2), e00094. DOI 10.1042/BSR20130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong, Z., Fu, Z., Shi, J., Jiang, X., Wan, H. (2017). HtrA1 down-regulation induces cisplatin resistance in colon cancer by increasing XIAP and activating PI3K/AKT pathway. Annals of Clinical and Laboratory Science , 47(3), 264–270. [PubMed] [Google Scholar]
- 56.Qiu, C., Su, W., Shen, N., Qi, X., Wu, X.et al. (2020). MNAT1 promotes proliferation and the chemo-resistance of osteosarcoma cell to cisplatin through regulating PI3K/AKT/mTOR pathway. BMC Cancer , 20(1), 1187. DOI 10.1186/s12885-020-07687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jian, J., Li, S., Liu, L. Z., Zhen, L., Yao, L.et al. (2020). XPD inhibits cell growth and invasion and enhances chemosensitivity in esophageal squamous cell carcinoma by regulating the PI3K/AKT signaling pathway. International Journal of Molecular Medicine , 46(1), 201–210. DOI 10.3892/ijmm.2020.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li, F., Zhang, Z., Wang, P., Wen, P., Xu, Q.et al. (2019). ALC1 knockdown enhances cisplatin cytotoxicity of esophageal squamous cell carcinoma cells by inhibition of glycolysis through PI3K/AKT pathway. Life Sciences , 232, 116679. DOI 10.1016/j.lfs.2019.116679. [DOI] [PubMed] [Google Scholar]
- 59.Liu, B., Wang, C., Chen, P., Cheng, B., Cheng, Y. (2018). RACKI induces chemotherapy resistance in esophageal carcinoma by upregulating the PI3K/AKT pathway and Bcl-2 expression. OncoTargets and Therapy , 11, 211–220. DOI 10.2147/OTT.S152818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shu, X. R., Wu, J., Sun, H., Chi, L. Q., Wang, J. H. (2015). PAK4 confers the malignance of cervical cancers and contributes to the cisplatin-resistance in cervical cancer cells via PI3K/AKT pathway. Diagnostic Pathology , 10(1), 177. DOI 10.1186/s13000-015-0404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo, H., Cong, S., Dong, J., Jin, L., Jiang, D.et al. (2020). Pairedrelated homeobox 1 overexpression promotes multidrug resistance via PTEN/PI3K/AKT signaling in MCF7 breast cancer cells. Molecular Medicine Reports , 22(4), 3183–3190. DOI 10.3892/mmr.2020.11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, J., Xie, T. (2020). Ghrelin inhibits cisplatin-induced MDA-MB-231 breast cancer cell apoptosis via PI3K/AKT/mTOR signaling. Experimental and Therapeutic Medicine , 19(3), 1633–1640. DOI 10.3892/etm.2019.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen, X., Xiong, D., Ye, L., Yang, H., Mei, S.et al. (2019). SPP1 inhibition improves the cisplatin chemo-sensitivity of cervical cancer cell lines. Cancer Chemother Pharmacol , 83(4), 603–613. DOI 10.1007/s00280-018-3759-5. [DOI] [PubMed] [Google Scholar]
- 64.Tan, J., You, Y., Xu, T., Yu, P., Wu, D.et al. (2014). Par-4 downregulation confers cisplatin resistance in pancreatic cancer cells via PI3K/AKT pathway-dependent EMT. Toxicology Letters , 224(1), 7–15. DOI 10.1016/j.toxlet.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Li, B., Yang, J., Lu, Z., Liu, B., Liu, F. (2019). A study on the mechanism of rapamycin mediating the sensitivity of pancreatic cancer cells to cisplatin through PI3K/AKT/mTOR signaling pathway. Journal of Balkan Union of Oncology , 24(2), 739–745. [PubMed] [Google Scholar]
- 66.Jiang, Z., Gong, T., Wei, H. (2019). CDKL5 promotes proliferation, migration, and chemotherapeutic drug resistance of glioma cells via activation of the PI3K/AKT signaling pathway. FEBS Open Bio , 10(2), 268–277. DOI 10.1002/2211-5463.12780. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Yu, L., Xu, H., Zhang, S., Chen, J., Yu, Z. (2020). SDC1 promotes cisplatin resistance in hepatic carcinoma cells via PI3K-AKT pathway. Human Cell , 33(3), 721–729. DOI 10.1007/s13577-020-00362-6. [DOI] [PubMed] [Google Scholar]
- 68.Gan, Y., He, L., Yao, K., Tan, J., Zeng, Q.et al. (2017). Knockdown of HMGN5 increases the chemosensitivity of human urothelial bladder cancer cells to cisplatin by targeting PI3K/AKT signaling. Oncology Letters , 14(6), 6463–6470. DOI 10.3892/ol.2017.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong, Q., Fu, L., Zhao, Y., Tan, S., Wang, E. (2017). Derlin-1 overexpression confers poor prognosis in muscle invasive bladder cancer and contributes to chemoresistance and invasion through PI3K/AKT and ERK/MMP signaling. Oncotarget , 8(10), 17059–17069. DOI 10.18632/oncotarget.15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gan, Y., Wang, Y., Tan, Z., Zhou, J., Kitazawa, R.et al. (2016). TDRG1 regulates chemosensitivity of seminoma TCam-2 cells to cisplatin via PI3K/AKT/mTOR signaling pathway and mitochondria-mediated apoptotic pathway. Cancer Biology & Therapy , 17(7), 741–750. DOI 10.1080/15384047.2016.1178425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu, Q., Liang, X., Dai, J., Guan, X. (2015). Prostaglandin transporter, SLCO2A1, mediates the invasion and apoptosis of lung cancer cells via PI3K/AKT/mTOR pathway. International Journal of Clinical and Experimental Pathology , 8(8), 9175–9181. [PMC free article] [PubMed] [Google Scholar]
- 72.Guo, Y., Chang, H., Li, J., Xu, X. Y., Shen, L.et al. (2015). Thymosin alpha 1 suppresses proliferation and induces apoptosis in breast cancer cells through PTEN-mediated inhibition of PI3K/AKT/mTOR signaling pathway. Apoptosis , 20(8), 1109–1121. DOI 10.1007/s10495-015-1138-9. [DOI] [PubMed] [Google Scholar]
- 73.Moghbeli, M. (2021). Molecular interactions of miR-338 during tumor progression and metastasis. Cellular & Molecular Biology Letters , 26(1), 13. DOI 10.1186/s11658-021-00257-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zangouei, A. S., Rahimi, H. R., Mojarrad, M., Moghbeli, M. (2020). Non coding RNAs as the critical factors in chemo resistance of bladder tumor cells. Diagnostic Pathology , 15(1), 136. DOI 10.1186/s13000-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Igney, F. H., Krammer, P. H. (2002). Death and anti-death: Tumour resistance to apoptosis. Nature Reviews Cancer , 2(4), 277–288. DOI 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 76.Reed, J. C. (2001). The survivin saga goes in vivo. The Journal of Clinical Investigation , 108(7), 965–969. DOI 10.1172/JCI14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Altieri, D. C. (2003). Validating survivin as a cancer therapeutic target. Nature Reviews Cancer , 3(1), 46–54. DOI 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 78.Lawlor, M. A., Alessi, D. R. (2001). PKB/AKT: A key mediator of cell proliferation, survival and insulin responses? Journal of Cell Science , 114(16), 2903–2910. DOI 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 79.Wolf, I., Levanon-Cohen, S., Bose, S., Ligumsky, H., Sredni, B.et al. (2008). Klotho: A tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene , 27(56), 7094–7105. DOI 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 80.Hayakawa, J., Ohmichi, M., Kurachi, H., Kanda, Y., Hisamoto, K.et al. (2000). Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via AKT cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Research , 60(21), 5988–5994. [PubMed] [Google Scholar]
- 81.Shibata, T., Kokubu, A., Tsuta, K., Hirohashi, S. (2009). Oncogenic mutation of PIK3CA in small cell lung carcinoma: A potential therapeutic target pathway for chemotherapy-resistant lung cancer. Cancer Letters , 283(2), 203–211. DOI 10.1016/j.canlet.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 82.de Silva, I. U., McHugh, P. J., Clingen, P. H., Hartley, J. A. (2000). Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand cross-links in mammalian cells. Molecular and Cellular Biology , 20(21), 7980–7990. DOI 10.1128/MCB.20.21.7980-7990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shell, S. M., Hawkins, E. K., Tsai, M. S., Hlaing, A. S., Rizzo, C. J.et al. (2013). Xeroderma pigmentosum complementation group C protein (XPC) serves as a general sensor of damaged DNA. DNA Repair , 12(11), 947–953. DOI 10.1016/j.dnarep.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ross, D. D., Nakanishi, T. (2010). Impact of breast cancer resistance protein on cancer treatment outcomes. Methods in Molecular Biology , 596, 251–290. DOI 10.1007/978-1-60761-416-6_12. [DOI] [PubMed] [Google Scholar]
- 85.Wu, P. C., Lin, Y. H., Chang, J. S., Huang, Y. B., Tsai, Y. H. (2010). The effect of component of microemulsion for transdermal delivery of nicardipine hydrochloride. Drug Development and Industrial Pharmacy , 36(12), 1398–1403. DOI 10.3109/03639045.2010.485277. [DOI] [PubMed] [Google Scholar]
- 86.Khalili-Tanha, G., Moghbeli, M. (2021). Long non-coding RNAs as the critical regulators of doxorubicin resistance in tumor cells. Cellular & Molecular Biology Letters , 26(1), 39. DOI 10.1186/s11658-021-00282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rahmani, Z., Mojarrad, M., Moghbeli, M. (2020). Long non-coding RNAs as the critical factors during tumor progressions among Iranian population: An overview. Cell and Biosciense , 10(1), 6. DOI 10.1186/s13578-020-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen, K., Abuduwufuer, A., Zhang, H., Luo, L., Suotesiyali, M.et al. (2019). SNHG7 mediates cisplatin-resistance in non-small cell lung cancer by activating PI3K/AKT pathway. European Review for Medical and Pharmacological Sciences , 23(16), 6935–6943. DOI 10.26355/eurrev_201908_18733. [DOI] [PubMed] [Google Scholar]
- 89.Wang, G. L., Jiang, B. H., Rue, E. A., Semenza, G. L. (1995). Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. PNAS , 92(12), 5510–5514. DOI 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gu, J., Li, Y., Zeng, J., Wang, B., Ji, K.et al. (2017). Knockdown of HIF-1α by siRNA-expressing plasmid delivered by attenuated Salmonella enhances the antitumor effects of cisplatin on prostate cancer. Scientific Reports , 7(1), 7546. DOI 10.1038/s41598-017-07973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li, X., He, Y., Xu, Y., Huang, X., Liu, J.et al. (2016). KLF5 mediates vascular remodeling via HIF-1α in hypoxic pulmonary hypertension. American Journal of Physiology-Lung Cellular and Molecular Physiology , 310(4), L299–310. DOI 10.1152/ajplung.00189.2015. [DOI] [PubMed] [Google Scholar]
- 92.Forghanifard, M. M., Rad, A., Farshchian, M., Khaleghizadeh, M., Gholamin, M.et al. (2017). TWIST1 upregulates the MAGEA4 oncogene. Molecular Carcinogenesis , 56(3), 877–885. DOI 10.1002/mc.22541. [DOI] [PubMed] [Google Scholar]
- 93.Hamidi, A. A., Khalili-Tanha, G., Nasrpour Navaei, Z., Moghbeli, M. (2022). Long non-coding RNAs as the critical regulators of epithelial mesenchymal transition in colorectal tumor cells: An overview. Cancer Cell International , 22(1), 71. DOI 10.1186/s12935-022-02501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thiery, J. P., Acloque, H., Huang, R. Y., Nieto, M. A. (2009). Epithelial-mesenchymal transitions in development and disease. Cell , 139(5), 871–890. DOI 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Zeng, Z., Bo, H., Gong, Z., Lian, Y., Li, X.et al. (2016). AFAP1-AS1, a long noncoding RNA upregulated in lung cancer and promotes invasion and metastasis. Tumour Biology , 37(1), 729–737. DOI 10.1007/s13277-015-3860-x. [DOI] [PubMed] [Google Scholar]
- 96.Liu, Y., Hu, Q., Wang, X. (2020). AFAP1-AS1 induces cisplatin resistance in non-small cell lung cancer through PI3K/AKT pathway. Oncology Letters , 19(1), 1024–1030. DOI 10.3892/ol.2019.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang, Y., Zheng, S., Zheng, J. S., Wong, K. H., Huang, Z.et al. (2014). Synergistic induction of apoptosis by methylseleninic acid and cisplatin, the role of ROS-ERK/AKT-p53 pathway. Molecular Pharmaceutics , 11(4), 1282–1293. DOI 10.1021/mp400749f. [DOI] [PubMed] [Google Scholar]
- 98.Melendez-Zajgla, J., Cruz, E., Maldonado, V., Espinoza, A. M. (1999). Mitochondrial changes during the apoptotic process of HeLa cells exposed to cisplatin. Biochemistry and Molecular Biology International , 47(5), 765–771. DOI 10.1080/15216549900201853. [DOI] [PubMed] [Google Scholar]
- 99.Nijman, S. M., Luna-Vargas, M. P., Velds, A., Brummelkamp, T. R., Dirac, A. M.et al. (2005). A genomic and functional inventory of deubiquitinating enzymes. Cell , 123(5), 773–786. DOI 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 100.de la Vega, M.,Kelvin, A. A., Dunican, D. J., McFarlane, C., Burrows, J. F.et al. (2011). The deubiquitinating enzyme USP17 is essential for GTPase subcellular localization and cell motility. Nature Communications , 2(1), 259. DOI 10.1038/ncomms1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McFarlane, C., Kelvin, A. A., de la Vega, M.,Govender, U., Scott, C. J.et al. (2010). The deubiquitinating enzyme USP17 is highly expressed in tumor biopsies, is cell cycle regulated, and is required for G1-S progression. Cancer Research , 70(8), 3329–3339. DOI 10.1158/0008-5472.CAN-09-4152. [DOI] [PubMed] [Google Scholar]
- 102.Gillet, J. P., Gottesman, M. M. (2010). Mechanisms of multidrug resistance in cancer. Methods in Molecular Biology , 596, 47–76. DOI 10.1007/978-1-60761-416-6. [DOI] [PubMed] [Google Scholar]
- 103.Deng, J., Wang, L., Chen, H., Hao, J., Ni, J.et al. (2016). Targeting epithelial-mesenchymal transition and cancer stem cells for chemoresistant ovarian cancer. Oncotarget , 7(34), 55771–55788. DOI 10.18632/oncotarget.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen, J., Shao, R., Li, F., Monteiro, M., Liu, J. P.et al. (2015). PI3K/AKT/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cells. Clinical and Experimental Pharmacology & Physiology , 42(12), 1317–1326. DOI 10.1111/1440-1681.12493. [DOI] [PubMed] [Google Scholar]
- 105.Deng, J., Bai, X., Feng, X., Ni, J., Beretov, J.et al. (2019). Inhibition of PI3K/AKT/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer , 19(1), 618. DOI 10.1186/s12885-019-5824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manavathi, B., Acconcia, F., Rayala, S. K., Kumar, R. (2006). An inherent role of microtubule network in the action of nuclear receptor. PNAS , 103(43), 15981–15986. DOI 10.1073/pnas.0607445103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pan, J., Qin, Y., Zhang, M. (2016). HPIP promotes non-small cell lung cancer cell proliferation, migration and invasion through regulation of the Sonic hedgehog signaling pathway. Biomedicine & Pharmacotherapy , 77, 176–181. DOI 10.1016/j.biopha.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 108.Shostak, K., Patrascu, F., Goktuna, S. I., Close, P., Borgs, L.et al. (2014). MDM2 restrains estrogen-mediated AKT activation by promoting TBK1-dependent HPIP degradation. Cell Death and Differentiation , 21(5), 811–824. DOI 10.1038/cdd.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abate-Shen, C. (2002). Deregulated homeobox gene expression in cancer: Cause or consequence? Nature Reviews Cancer , 2(10), 777–785. DOI 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 110.Chatterjee, A., Chattopadhyay, D., Chakrabarti, G. (2014). miR-17-5p downregulation contributes to paclitaxel resistance of lung cancer cells through altering beclin1 expression. PLoS One , 9(4), e95716. DOI 10.1371/journal.pone.0095716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hafsi, S., Pezzino, F. M., Candido, S., Ligresti, G., Spandidos, D. A.et al. (2012). Gene alterations in the PI3K/PTEN/AKT pathway as a mechanism of drug-resistance (review). International Journal of Oncology , 40(3), 639–644. DOI 10.3892/ijo.2011.1312. [DOI] [PubMed] [Google Scholar]
- 112.Agarwal, R., Jurisica, I., Mills, G. B., Cheng, K. W. (2009). The emerging role of the RAB25 small GTPase in cancer. Traffic , 10(11), 1561–1568. DOI 10.1111/j.1600-0854.2009.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ji, M. M., Wang, L., Zhan, Q., Xue, W., Zhao, Y.et al. (2015). Induction of autophagy by valproic acid enhanced lymphoma cell chemosensitivity through HDAC-independent and IP3-mediated PRKAA activation. Autophagy , 11(12), 2160–2171. DOI 10.1080/15548627.2015.1082024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shteingauz, A., Boyango, I., Naroditsky, I., Hammond, E., Gruber, M.et al. (2015). Heparanase enhances tumor growth and chemoresistance by promoting autophagy. Cancer Research , 75(18), 3946–3957. DOI 10.1158/0008-5472.CAN-15-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.You, L., Wang, Z., Li, H., Shou, J., Jing, Z.et al. (2015). The role of STAT3 in autophagy. Autophagy , 11(5), 729–739. DOI 10.1080/15548627.2015.1017192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liang, F., Ren, C., Wang, J., Wang, S., Yang, L.et al. (2019). The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis , 8(10), 59. DOI 10.1038/s41389-019-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnston, P. A., Grandis, J. R. (2011). STAT3 signaling: Anticancer strategies and challenges. Molecular Interventions , 11(1), 18–26. DOI 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang, C. Y., Fong, Y. C., Lee, C. Y., Chen, M. Y., Tsai, H. C.et al. (2009). CCL5 increases lung cancer migration via PI3K, AKT and NF-kappaB pathways. Biochemical Pharmacology , 77(5), 794–803. DOI 10.1016/j.bcp.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 119.Sun, C. M., Huang, S. F., Zeng, J. M., Liu, D. B., Xiao, Q.et al. (2010). Per2 inhibits k562 leukemia cell growth in vitro and in vivo through cell cycle arrest and apoptosis induction. Pathology Oncology Research , 16(3), 403–411. DOI 10.1007/s12253-009-9227-0. [DOI] [PubMed] [Google Scholar]
- 120.Niu, Z. F., Wang, C. Q., Xia, H. C., Wang, J., Zhang, L. J.et al. (2016). Period2 downregulation inhibits glioma cell apoptosis by activating the MDM2-TP53 pathway. Oncotarget , 7(19), 27350–27362. DOI 10.18632/oncotarget.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nikolopoulos, D., Theocharis, S., Kouraklis, G. (2010). Ghrelin: A potential therapeutic target for cancer. Regulatory Peptides , 163(1–3), 7–17. DOI 10.1016/j.regpep.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 122.Muller, T. D., Nogueiras, R., Andermann, M. L., Andrews, Z. B., Anker, S. D.et al. (2015). Ghrelin. Molecular Metabolism , 4(6), 437–460. DOI 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.El-Kott, A. F., Shati, A. A., Al-Kahtani, M. A., Alqahtani, S. (2019). Acylated ghrelin renders chemosensitive ovarian cancer cells resistant to cisplatin chemotherapy via activation of the PI3K/AKT/mTOR survival pathway. Analytical Cellular Pathology , 2019(2), 9627810. DOI 10.1155/2019/9627810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bauer, D. E., Hatzivassiliou, G., Zhao, F., Andreadis, C., Thompson, C. B. (2005). ATP citrate lyase is an important component of cell growth and transformation. Oncogene , 24(41), 6314–6322. DOI 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 125.Icard, P., Lincet, H. (2012). A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochimica et Biophysica Acta , 1826(2), 423–433. DOI 10.1016/j.bbcan.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 126.Moghbeli, M., Mosannen Mozaffari, H., Memar, B., Forghanifard, M. M., Gholamin, M.et al. (2019). Role of MAML1 in targeted therapy against the esophageal cancer stem cells. Journal of Translational Medicine , 17(1), 126. DOI 10.1186/s12967-019-1876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rivas, S., Gomez-Oro, C., Anton, I. M., Wandosell, F. (2018). Role of AKT isoforms controlling cancer stem cell survival, phenotype and self-renewal. Biomedicines , 6(1), 29. DOI 10.3390/biomedicines6010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Song, S., Pei, G., Du, Y., Wu, J., Ni, X.et al. (2018). Interaction between CD133 and PI3K-p85 promotes chemoresistance in gastric cancer cells. American Journal of Translational Research , 10(1), 304–314. [PMC free article] [PubMed] [Google Scholar]
- 129.Xu, Y., Cheng, M., Mi, L., Qiu, Y., Hao, W.et al. (2018). Mir-22-3p enhances the chemosensitivity of gastrointestinal stromal tumor cell lines to cisplatin through PTEN/PI3K/AKT pathway. Iranian Journal of Allergy, Asthma, and Immunology , 17(4), 318–325. DOI 10.18502/ijaai.v17i4.91. [DOI] [PubMed] [Google Scholar]
- 130.Dai, Q., Zhang, T., Li, C. (2020). LncRNA MALAT1 regulates the cell proliferation and cisplatin resistance in gastric cancer via PI3K/AKT pathway. Cancer Management and Research , 12, 1929–1939. DOI 10.2147/CMAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilson, N. H., Key, B. (2007). Neogenin: One receptor, many functions. The International Journal of Biochemistry & Cell Biology , 39(5), 874–878. DOI 10.1016/j.biocel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 132.Gururaj, A., Barnes, C. J., Vadlamudi, R. K., Kumar, R. (2004). Regulation of phosphoglucomutase 1 phosphorylation and activity by a signaling kinase. Oncogene , 23(49), 8118–8127. DOI 10.1038/sj.onc.1207969. [DOI] [PubMed] [Google Scholar]
- 133.Yang, Z., Rayala, S., Nguyen, D., Vadlamudi, R. K., Chen, S.et al. (2005). Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Research , 65(8), 3179–3184. DOI 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 134.Paliouras, G. N., Naujokas, M. A., Park, M. (2009). Pak4, a novel Gab1 binding partner, modulates cell migration and invasion by the Met receptor. Molecular and Cellular Biology , 29(11), 3018–3032. DOI 10.1128/MCB.01286-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dai, Q., Zhang, T., Pan, J., Li, C. (2020). LncRNA UCA1 promotes cisplatin resistance in gastric cancer via recruiting EZH2 and activating PI3K/AKT pathway. Journal of Cancer , 11(13), 3882–3892. DOI 10.7150/jca.43446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fruman, D. A., Rommel, C. (2014). PI3K and cancer: Lessons, challenges and opportunities. Nature Reviews Drug Discovery , 13(2), 140–156. DOI 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tapia, O., Riquelme, I., Leal, P., Sandoval, A., Aedo, S.et al. (2014). The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Archiv , 465(1), 25–33. DOI 10.1007/s00428-014-1588-4. [DOI] [PubMed] [Google Scholar]
- 138.Abbaszadegan, M. R., Riahi, A., Forghanifard, M. M., Moghbeli, M. (2018). WNT and NOTCH signaling pathways as activators for epidermal growth factor receptor in esophageal squamous cell carcinoma. Cell and Molecular Biology Letters , 23(1), 42. DOI 10.1186/s11658-018-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moghbeli, M., Makhdoumi, Y., Soltani Delgosha, M., Aarabi, A., Dadkhah, E.et al. (2019). ErbB1 and ErbB3 co-over expression as a prognostic factor in gastric cancer. Biological Research , 52(1), 2. DOI 10.1186/s40659-018-0208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang, X., Song, Q., Wei, C., Qu, J. (2015). LRIG1 inhibits hypoxia-induced vasculogenic mimicry formation via suppression of the EGFR/PI3K/AKT pathway and epithelial-to-mesenchymal transition in human glioma SHG-44 cells. Cell Stress Chaperones , 20(4), 631–641. DOI 10.1007/s12192-015-0587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chien, J., Campioni, M., Shridhar, V., Baldi, A. (2009). HtrA serine proteases as potential therapeutic targets in cancer. Current Cancer Drug Targets , 9(4), 451–468. DOI 10.2174/156800909788486704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schimmer, A. D., Dalili, S., Batey, R. A., Riedl, S. J. (2006). Targeting XIAP for the treatment of malignancy. Cell Death and Differentiation , 13(2), 179–188. DOI 10.1038/sj.cdd.4401826. [DOI] [PubMed] [Google Scholar]
- 143.He, X., Khurana, A., Maguire, J. L., Chien, J., Shridhar, V. (2012). HtrA1 sensitizes ovarian cancer cells to cisplatin-induced cytotoxicity by targeting XIAP for degradation. International Journal of Cancer , 130(5), 1029–1035. DOI 10.1002/ijc.26044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chang, X. B. (2007). A molecular understanding of ATP-dependent solute transport by multidrug resistance-associated protein MRP1. Cancer Metastasis Reviews , 26(1), 15–37. DOI 10.1007/s10555-007-9041-7. [DOI] [PubMed] [Google Scholar]
- 145.Donnenberg, V. S., Burckart, G. J., Griffith, B. P., Jain, A. B., Zeevi, A.et al. (2001). P-glycoprotein (P-gp) is upregulated in peripheral T-cell subsets from solid organ transplant recipients. Journal of Clinical Pharmacology , 41(12), 1271–1279. DOI 10.1177/00912700122012850. [DOI] [PubMed] [Google Scholar]
- 146.Sano, M., Izumi, Y., Helenius, K., Asakura, M., Rossi, D. J.et al. (2007). Menage-a-trois 1 is critical for the transcriptional function of PPARgamma coactivator 1. Cell Metabolism , 5(2), 129–142. DOI 10.1016/j.cmet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 147.Mizushima, N., Levine, B., Cuervo, A. M., Klionsky, D. J. (2008). Autophagy fights disease through cellular self-digestion. Nature , 451(7182), 1069–1075. DOI 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Singh, R., Kaushik, S., Wang, Y., Xiang, Y., Novak, I.et al. (2009). Autophagy regulates lipid metabolism. Nature , 458(7242), 1131–1135. DOI 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.He, C., Klionsky, D. J. (2009). Regulation mechanisms and signaling pathways of autophagy. Annual Review of Genetics , 43(1), 67–93. DOI 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meng, C. Y., Zhao, Z. Q., Bai, R., Zhao, W., Wang, Y. X.et al. (2020). MicroRNA22 mediates the cisplatin resistance of osteosarcoma cells by inhibiting autophagy via the PI3K/AKT/mTOR pathway. Oncology Reports , 43(4), 1169–1186. DOI 10.3892/or.2020.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun, H., Lesche, R., Li, D. M., Liliental, J., Zhang, H.et al. (1999). PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and AKT/protein kinase B signaling pathway. PNAS , 96(11), 6199–6204. DOI 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lerner, L. K., Moreno, N. C., Rocha, C. R. R., Munford, V., Santos, V.et al. (2019). XPD/ERCC2 mutations interfere in cellular responses to oxidative stress. Mutagenesis , 34(4), 341–354. DOI 10.1093/mutage/gez020. [DOI] [PubMed] [Google Scholar]
- 153.Schaich, M., Soucek, S., Thiede, C., Ehninger, G., Illmer, T.et al. (2005). MDR1 and MRP1 gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemia. British Journal of Haematology , 128(3), 324–332. DOI 10.1111/j.1365-2141.2004.05319.x. [DOI] [PubMed] [Google Scholar]
- 154.He, L. R., Ma, N. F., Chen, J. W., Li, B. K., Guan, X. Y.et al. (2015). Overexpression of CHD1L is positively associated with metastasis of lung adenocarcinoma and predicts patients poor survival. Oncotarget , 6(31), 31181–31190. DOI 10.18632/oncotarget.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li, J. J., Xie, D. (2015). RACK1, a versatile hub in cancer. Oncogene , 34(15), 1890–1898. DOI 10.1038/onc.2014.127. [DOI] [PubMed] [Google Scholar]
- 156.McCahill, A., Warwicker, J., Bolger, G. B., Houslay, M. D., Yarwood, S. J. (2002). The RACK1 scaffold protein: A dynamic cog in cell response mechanisms. Molecular Pharmacology , 62(6), 1261–1273. DOI 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 157.Bokoch, G. M. (2003). Biology of the p21-activated kinases. Annual Review of Biochemistry , 72(1), 743–781. DOI 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 158.Nheu, T., He, H., Hirokawa, Y., Walker, F., Wood, J.et al. (2004). PAK is essential for RAS-induced upregulation of cyclin D1 during the G1 to S transition. Cell Cycle , 3(1), 71–74. DOI 10.4161/cc.3.1.593. [DOI] [PubMed] [Google Scholar]
- 159.Robey, R. W., Pluchino, K. M., Hall, M. D., Fojo, A. T., Bates, S. E.et al. (2018). Revisiting the role of ABC transporters in multidrug-resistant cancer. Nature Reviews Cancer , 18(7), 452–464. DOI 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takahashi, Y., Sawada, G., Kurashige, J., Uchi, R., Matsumura, T.et al. (2013). Paired related homoeobox 1, a new EMT inducer, is involved in metastasis and poor prognosis in colorectal cancer. British Journal of Cancer , 109(2), 307–311. DOI 10.1038/bjc.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gulei, D., Magdo, L., Jurj, A., Raduly, L., Cojocneanu-Petric, R.et al. (2018). The silent healer: miR-205-5p up-regulation inhibits epithelial to mesenchymal transition in colon cancer cells by indirectly up-regulating E-cadherin expression. Cell Death and Disease , 9(2), 66. DOI 10.1038/s41419-017-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiang, M., Zhong, T., Zhang, W., Xiao, Z., Hu, G.et al. (2017). Reduced expression of miR2055p promotes apoptosis and inhibits proliferation and invasion in lung cancer A549 cells by upregulation of ZEB2 and downregulation of erbB3. Molecular Medicine Reports , 15(5), 3231–3238. DOI 10.3892/mmr.2017.6398. [DOI] [PubMed] [Google Scholar]
- 163.Dongre, A., Weinberg, R. A. (2019). New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nature Reviews Molecular Cell Biology , 20(2), 69–84. DOI 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 164.Liu, J., Pan, C., Guo, L., Wu, M., Guo, J.et al. (2016). A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. Journal of Hematol and Oncology , 9(1), 76. DOI 10.1186/s13045-016-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Das, S., Dixon, J. E., Cho, W. (2003). Membrane-binding and activation mechanism of PTEN. PNAS , 100(13), 7491–7496. DOI 10.1073/pnas.0932835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cantore, M., Fiorentini, G., Luppi, G., Rosati, G., Caudana, R.et al. (2003). Randomised trial of gemcitabine versus flec regimen given intra-arterially for patients with unresectable pancreatic cancer. Journal of Experimental & Clinical Cancer Research , 22(4 Suppl), 51–57. [PubMed] [Google Scholar]
- 167.Hay, N., Sonenberg, N. (2004). Upstream and downstream of mTOR. Genes & Development , 18(16), 1926–1945. DOI 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 168.Tokunaga, C., Yoshino, K., Yonezawa, K. (2004). mTOR integrates amino acid- and energy-sensing pathways. Biochemical and Biophysical Research Communications , 313(2), 443–446. DOI 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 169.Giampazolias, E., Zunino, B., Dhayade, S., Bock, F., Cloix, C.et al. (2017). Mitochondrial permeabilization engages NF-kappaB-dependent anti-tumour activity under caspase deficiency. Nature Cell Biology , 19(9), 1116–1129. DOI 10.1038/ncb3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang, Y., Kwok-Shing Ng, P., Kucherlapati, M., Chen, F., Liu, Y.et al. (2017). A pan-cancer proteogenomic atlas of PI3K/AKT/mTOR pathway alterations. Cancer Cell , 31(6), 820–832 e823. DOI 10.1016/j.ccell.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Laag, E., Majidi, M., Cekanova, M., Masi, T., Takahashi, T.et al. (2006). NNK activates ERK1/2 and CREB/ATF-1 via beta-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. International Journal of Cancer , 119(7), 1547–1552. DOI 10.1002/(ISSN)1097-0215. [DOI] [PubMed] [Google Scholar]
- 172.Nishioka, T., Kim, H. S., Luo, L. Y., Huang, Y., Guo, J.et al. (2011). Sensitization of epithelial growth factor receptors by nicotine exposure to promote breast cancer cell growth. Breast Cancer Research , 13(6), R113. DOI 10.1186/bcr3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu, J., Huang, H., Pan, C., Zhang, B., Liu, X.et al. (2007). Nicotine inhibits apoptosis induced by cisplatin in human oral cancer cells. International Journal of Oral and Maxillofacial Surgery , 36(8), 739–744. DOI 10.1016/j.ijom.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 174.Yuge, K., Kikuchi, E., Hagiwara, M., Yasumizu, Y., Tanaka, N.et al. (2015). Nicotine Induces tumor growth and chemoresistance through activation of the PI3K/AKT/mTOR pathway in bladder cancer. Molecular Cancer Therapeutics , 14(9), 2112–2120. DOI 10.1158/1535-7163.MCT-15-0140. [DOI] [PubMed] [Google Scholar]
- 175.Stepp, M. A., Daley, W. P., Bernstein, A. M., Pal-Ghosh, S., Tadvalkar, G.et al. (2010). Syndecan-1 regulates cell migration and fibronectin fibril assembly. Experimental Cell Research , 316(14), 2322–2339. DOI 10.1016/j.yexcr.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rochman, M., Malicet, C., Bustin, M. (2010). HMGN5/NSBP1: A new member of the HMGN protein family that affects chromatin structure and function. Biochimica et Biophysica Acta , 1799(1–2), 86–92. DOI 10.1016/j.bbagrm.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lilley, B. N., Ploegh, H. L. (2004). A membrane protein required for dislocation of misfolded proteins from the ER. Nature , 429(6994), 834–840. DOI 10.1038/nature02592. [DOI] [PubMed] [Google Scholar]
- 178.Wang, Y., Gan, Y., Tan, Z., Zhou, J., Kitazawa, R.et al. (2016). TDRG1 functions in testicular seminoma are dependent on the PI3K/AKT/mTOR signaling pathway. OncoTargets and Therapy , 9, 409–420. DOI 10.2147/OTT.S97294. [DOI] [PMC free article] [PubMed] [Google Scholar]


